User login
Transcervical ablation of symptomatic uterine fibroids under US guidance
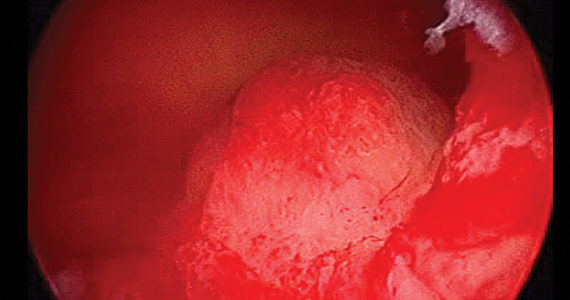
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
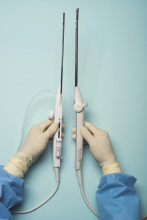
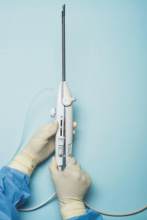
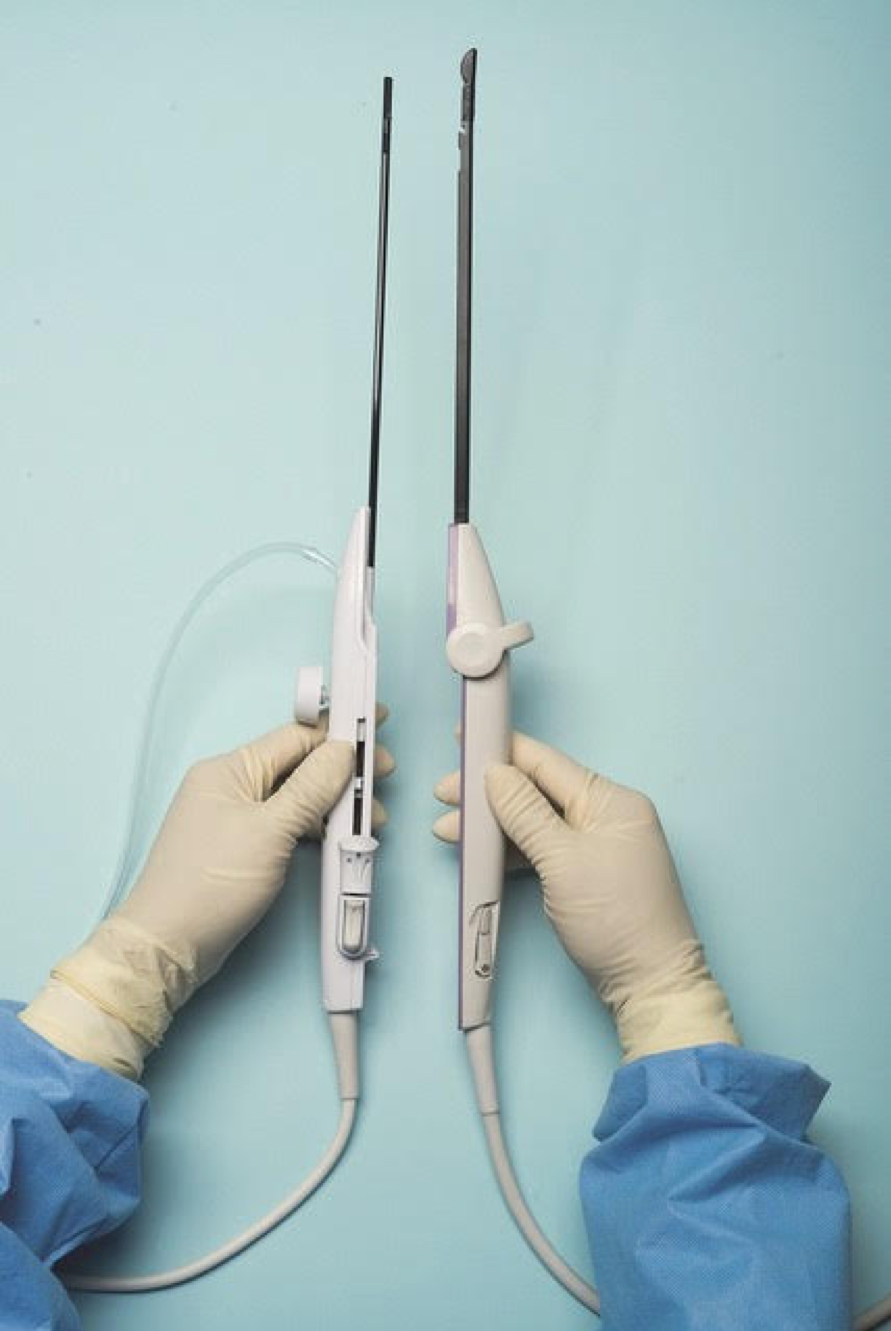
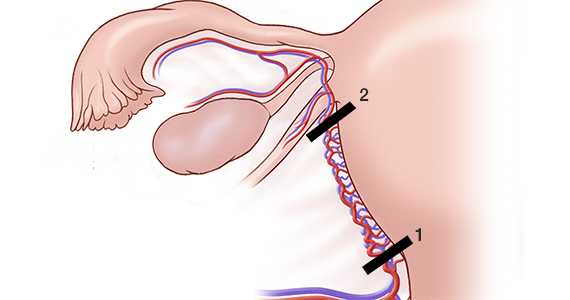
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
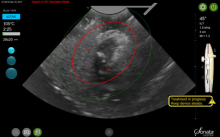
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
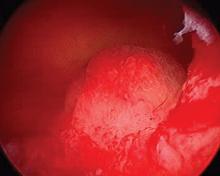
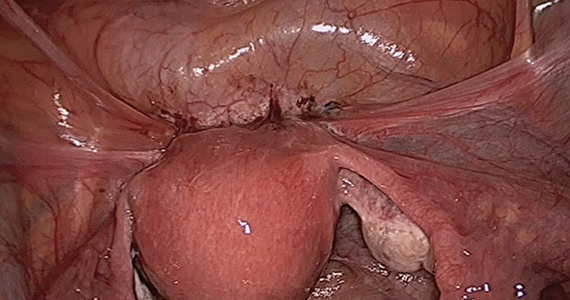
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
Treating uterine fibroids
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Morcellation use in gynecologic surgery: Current clinical recommendations and cautions
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
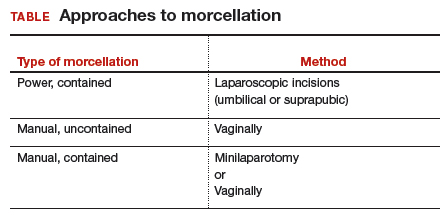
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7
Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
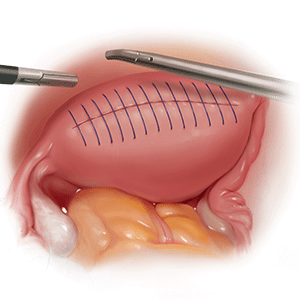
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Office hysteroscopic evaluation of postmenopausal bleeding
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
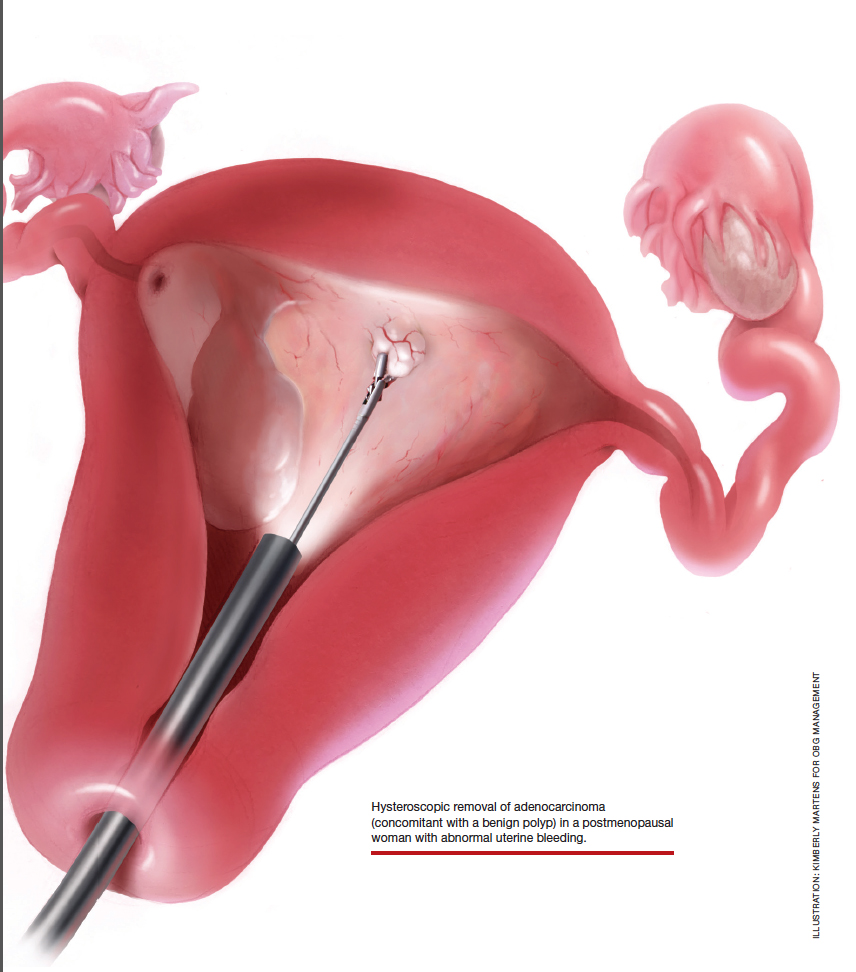
Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
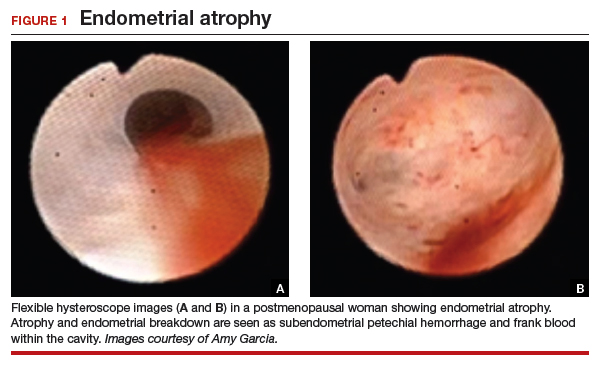
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
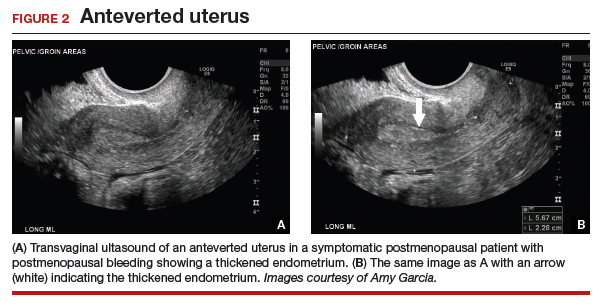
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
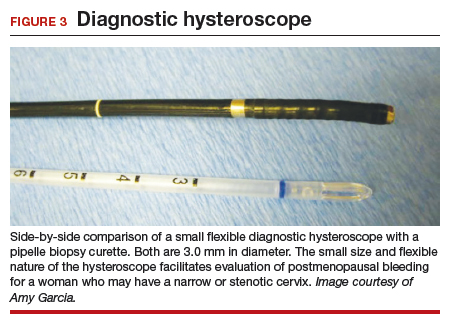
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
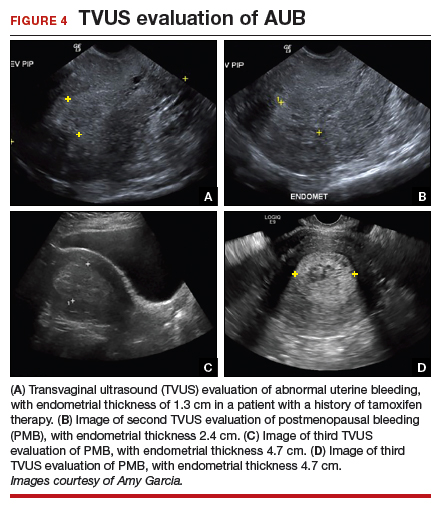
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

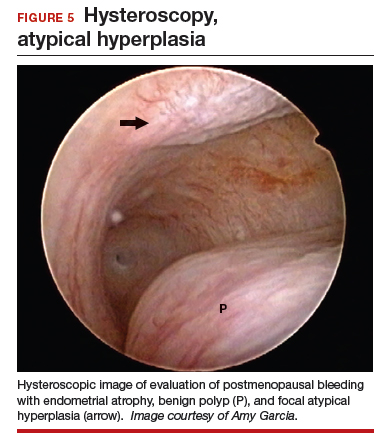
This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

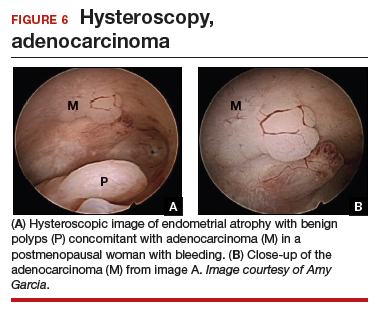
This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Uterus-sparing interventions to treat postpartum hemorrhage during cesarean delivery surgery
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.
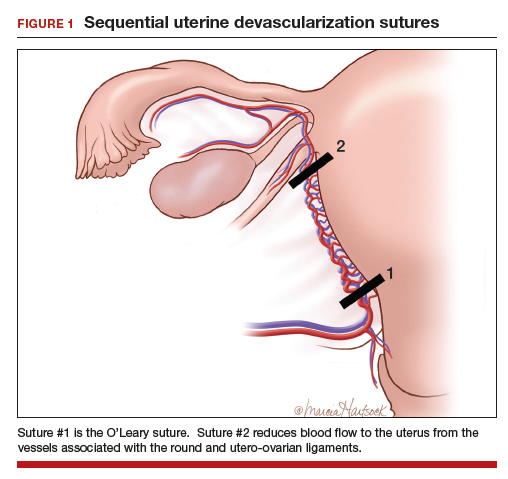
In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.
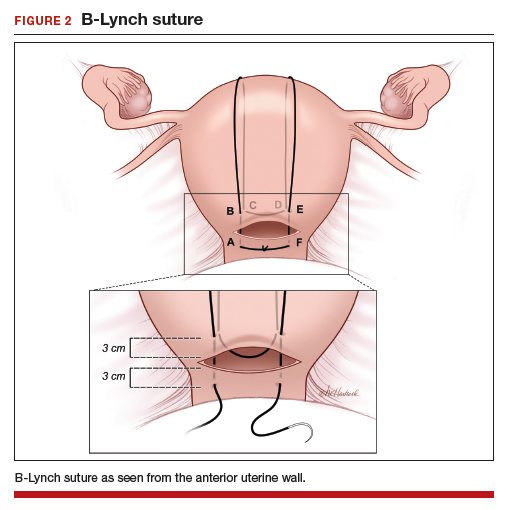
The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4
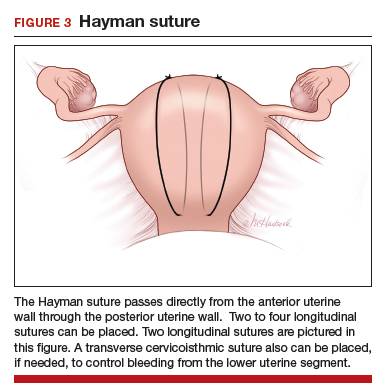
The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.
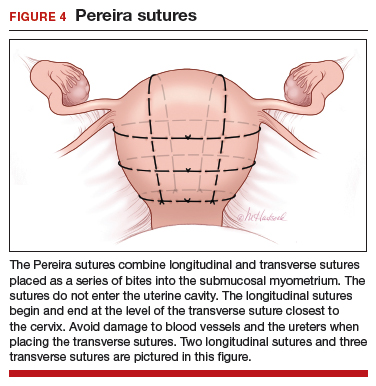
Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
Postpartum blood loss greater than 1,000 mL occurs in approximately 7% of cesarean delivery (CD) procedures with the administration of oxytocin alone or oxytocin plus misoprostol.1 Rapid identification and control of hemorrhage is essential to avoid escalating coagulopathy and maternal instability. In cases of excess blood loss, clinicians request assistance from colleagues, endeavor to identify the cause of the bleeding, utilize additional uterotonics (methylergonovine, carboprost, misoprostol), perform uterine massage, warm the uterus, repair lacerations and replace blood products. If blood loss continues after these initial measures, obstetricians may consider uterine artery embolization (UAE) or hysterectomy. While UAE is a highly effective measure to control postpartum hemorrhage, it is not available at all obstetric hospitals. Even when available, there may be a significant time delay from the decision to consult an interventional radiologist to completion of the embolization procedure.
To avoid the permanent sterilization of a hysterectomy, or to obtain time for UAE or correction of coagulopathy, additional uterus-sparing surgical interventions should be considered. These include: 1) progressive uterine devascularization, 2) uterine compression sutures, and 3) intrauterine balloon tamponade. One caveat is that there is very little high-quality evidence from randomized trials to compare the efficacy or outcome of these uterine-sparing surgical interventions. Most of our evidence is based on limited case series and expert recommendations.
Uterine devascularization
Many techniques have been described for performing progressive uterine devascularization. Most experts recommend first performing an O’Leary suture, ligating both ascending uterine arteries and accompanying veins at a point approximately 2 cm closer to the cervix than the uterine incision (FIGURE 1). An absorbable suture is passed through the myometrium, being sure to remain medial to the ascending uterine vessels. Clear visualization of the vessels posteriorly is essential, usually necessitating exteriorization of the uterus. The needle is then driven through an avascular space in the broad ligament close to the uterine vessels, and the suture is tied down. Ureteral injury can be avoided by extending the bladder flap laterally to the level of the round ligament and mobilizing the vesicouterine peritoneum inferiorly, with the suture placed directly on endopelvic fascia. If necessary, the utero-ovarian ligament can be ligated in a second step, just below the uterine-tubal junction. The progressive devascularization intervention can be limited to the first or second steps if bleeding is well controlled.

In our experience, bilateral O’Leary sutures are highly effective at controlling ongoing uterine bleeding, particularly from the lower uterine segment. In the event that they are not successful, placement does not preclude later use of UAE.
Uterine compression sutures
Compression sutures are most often used in the setting of refractory uterine atony. They also may be helpful for controlling focal atony or bleeding from a placental implantation site. More than a dozen different types of uterine compression sutures have been reported in the literature; the B-Lynch, Hyman, and Pereira sutures are most commonly performed.2
Continue to: The B-Lynch suture3 is performed with...
The B-Lynch suture3 is performed with a long, rapidly absorbable suture on a large needle (FIGURE 2). We use a 60-inch #1 or #2 chromic suture on a TP-1 needle in the following steps:
- Take bites on either side of the right edge of the hysterotomy incision (A and B). Place these bites approximately 3 cm from the edge of the hysterotomy incision.
- Loop the suture around the fundus and reenter the uterus through the posterior uterine wall at point C, which is directly posterior to point B.
- Exit the posterior wall of the uterus through point D.
- Loop the suture over the uterine fundus.
- Anchor the suture in the lower uterine segment by taking bites on either side of the left edge of the uterine hysterotomy incision (points E and F).
- Pull the two ends of the suture tight while an assistant squeezes the uterus to aid compression.
- Place a surgical knot to secure the suture.
- Close the hysterotomy incision.
The B-Lynch suture was described with an open hysterotomy incision,3 which avoids closing off the lower uterine segment. We have successfully performed a modific tion on a closed uterus, taking care to not drive the lower uterine sutures through both the anterior and posterior walls.

The Hayman suture4 was proposed with two important modifications: The suture is placed through-and-through the lower uterine segment with a closed hysterotomy, and the suture can be fixed to the uterine fundus to avoid slippage. This vertical compression suture (FIGURE 3) is performed by placing two to four vertical #2 chromic sutures directly through the anterior to posterior uterine wall, tying the suture on the fundus using a 3-throw technique to minimize slippage of the first knot. In the original description, Hayman also described injecting carboprost into the uterine fundus to stimulate uterine contraction and regularly inspecting the vagina to evaluate the extent of continued bleeding.4

The Pereira sutures,5 also described on a closed uterus, combine vertical and horizontal sutures placed as a series of bites into the submucosal myometrium using #1 polyglactin 910 (Vicryl) sutures (FIGURE 4). The sutures do not enter the uterine cavity. Two to three transverse sutures are initially placed followed by two vertical sutures. When placing the transverse sutures, it is important to cross the broad ligament in an avascular area and avoid trauma to blood vessels, ureters, gonadal vessels and fallopian tubes. The vertical sutures begin and end at the level of the transverse suture closest to the cervix.

Intrauterine balloon tamponade
Many types of balloon tamponade devices have been developed, ranging from the humble condom tied to a Foley urinary catheter to the sophisticated Bakri6,7 and Belfort-Dildy8 balloon tamponade devices. Intrauterine balloon tamponade is highly effective in controlling excess bleeding following vaginal delivery and less effective when used following a CD. In one study of 226 women with postpartum hemorrhage treated with a Bakri balloon the success rate was 89% and 66% following vaginal delivery and CD, respectively.9
Continue to: When using balloon tamponade during a CD...
When using balloon tamponade during a CD, some experts recommend partially closing the transverse hysterotomy incision by placing sutures to close edges of the hysterotomy, followed by insertion of the balloon into the uterus and the stem through the cervix into the vagina. Attachment of the stem to a collection bag should help to quickly assess the rate of blood loss. The balloon is inflated after the hysterotomy is closed. Following inflation of an intrauterine balloon, blood loss should decrease almost immediately.10 If excessive blood loss continues for more than 10 minutes, additional uterus-sparing interventions or hysterectomy may be required. Following successful balloon tamponade, the balloon may be deflated 12 to 24 hours postpartum when maternal stabilization and normal coagulation have been achieved. If bleeding resumes, the balloon may be reinflated and UAE should be considered.
Combined interventions: Uterine devascularization plus uterine compression sutures
There are no high-quality randomized trials comparing the devascularization plus compression sutures versus a single intervention alone, and case series and case reports on this topic are lacking. If uterine devascularization alone does not sufficiently control bleeding, adding a uterine compression stitch might resolve the hemorrhage. Both procedures require only suture material, which is immediately available in all operating rooms. Hence, this combination of interventions can be executed quickly.
Uterine sandwich: Intrauterine balloon tamponade plus uterine compression sutures
CD for placenta previa is associated with an increased risk of postpartum hemorrhage, with bleeding from the lower uterine segment greatly contributing to total blood loss. While O’Leary sutures can stem the flow of bleeding in this area, the use of both an intrauterine balloon tamponade plus uterine compression sutures—a so-called uterine sandwich—may result in maximal reduction in blood loss.11,12
In one randomized trial, 106 women undergoing CD for a placenta previa were randomly assigned to uterine devascularization alone or double transverse compression suture at the lower uterine segment plus intrauterine Foley catheter balloon. Compared with women receiving devascularization alone, the combination of compression suture plus intrauterine balloon significantly reduced blood loss (1,350 mL vs 750 mL, respectively; P = .0001).13
Underutilization of uterine-sparing interventions
In a nationwide study of 50 consecutive Danish peripartum hysterectomy cases, an audit committee concluded that 24% of the hysterectomies could have been avoided, and an additional 30% of hysterectomies might have been avoided, if uterine-sparing surgical interventions had been utilized.14 In a recent survey of senior ObGyn residents in France, greater than 70% of respondents reported that they had not mastered uterine-sparing techniques of uterine devascularization and compression sutures, nor peripartum hysterectomy.15 Together, these studies suggest that uterine-sparing interventions are underutilized and that with more training and practice clinicians would become facile with these interventions.
The cornerstones of uterine-sparing surgical interventions are simplicity, safety, and efficacy. If a combination of pharmacologic and multiple uterine-sparing surgical interventions do not control the bleeding, the patient may need an emergency hysterectomy or, if stable, a UAE. While devascularization and compression sutures are described during CD, it is reasonable to use them after vaginal delivery if the next reasonable step would be a laparotomy. When you next face the clinical challenge of a postpartum hemorrhage, rapid recognition of excess blood loss, early identification of the cause, swift pharmacologic treatment, and timely escalation of surgical interventions will help you reduce the risk of hysterectomy and severe maternal morbidity.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
- Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database of Syst Rev. 2018;12:CD011689.
- Li GT, Li XF, Wu BP, et al. Three cornerstones of uterine compression sutures: simplicity, safety, and efficacy. Arch Gynecol Obstet. 2015;292:949-952.
- B-Lynch C, Coker A, Lawal AH, et al. The B-Lynch surgical technique for the control of massive postpartum hemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372-375.
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502-506.
- Pereira A, Nunes F, Pedroso S, et al. Compressive sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569-572.
- Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet. 1992;37:302-303.
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001;74:139-142.
- Dildy GA, Belfort MA, Adair CD, et al; ebb Surveillance Study Team. Initial experience with a dual-balloon catheter for the management of postpartum hemorrhage. Am J Obstet Gynecol. 2014;210:136.e1-e6.
- Revert M, Cottenet J, Raynal P, et al. Intrauterine balloon tamponade for management of severe postpartum hemorrhage in a perinatal network: a prospective cohort study. BJOG. 2017;124:1255-1262.
- Condous GS, Arulkumaran S, Symonds I, et al. The “tamponade test” in the management of massive postpartum hemorrhage. Obstet Gynecol. 2003;101:767-772.
- Nelson WL, O’Brien JM. The uterine sandwich for persistent uterine atony: combining the B-Lynch compression suture and an intrauterine Bakri balloon. Am J Obstet Gynecol. 2007;196:e9-e10.
- Matsubara S, Kuwata T, Baba Y, et al. A novel “uterine sandwich” for haemorrhage at cesarean section for placenta praevia. Aust N Z J Obstet Gynaecol. 2014;54:283-286.
- Sallam HF, Shady NW. A sandwich technique (N&H variation technique) to reduce blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-8.
- Colmorn LB, Krebs L, Langhoff-Roos J; NOSS study group. Potentially avoidable peripartum hysterectomies in Denmark: a population based clinical audit. PLoS One. 2016;11:e0161302.
- Bouet PE, Madar H, Froeliger A, et al. Surgical treatment of postpartum haemorrhage: national survey of French residents in obstetrics and gynecology. BMC Pregnancy Childbirth. 2019;19:91.
Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?
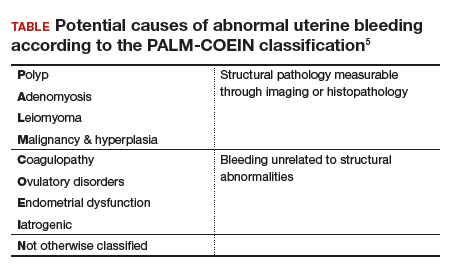
Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2019 Update on abnormal uterine bleeding
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
Keeping current with causes of and treatments for abnormal uterine bleeding (AUB) is important. AUB can have a major impact on women’s lives in terms of health care expenses, productivity, and quality of life. The focus of this Update is on information that has been published over the past year that is helpful for clinicians who counsel and treat women with AUB. First, we focus on new data on endometrial polyps, which are a common cause of AUB. For the first time, a meta-analysis has examined polyp-associated cancer risk. In addition, does a causal relationship exist between endometrial polyps and chronic endometritis? We also address the first published report of successful treatment of endometrial intraepithelial neoplasia (EIN, formerly complex endometrial hyperplasia with atypia) using the etonogestrel subdermal implant. Last, we discuss efficacy data for a new device for endometrial ablation, which has new features to consider.
What is the risk of malignancy with endometrial polyps?
Sasaki LM, Andrade KR, Figeuiredo AC, et al. Factors associated with malignancy in hysteroscopically resected endometrial polyps: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:777-785.
In the past year, 2 studies have contributed to our understanding of endometrial polyps, with one published as the first ever meta-analysis on polyp risk of malignancy.
What can information from more than 21,000 patients with polyps teach us about the risk factors associated with endometrial malignancy? For instance, with concern over balancing health care costs with potential surgical risks, should all patients with endometrial polyps undergo routine surgical removal, or should we stratify risks and offer surgery to only selected patients? This is the first meta-analysis to evaluate the risk factors for endometrial cancer (such as obesity, parity, tamoxifen use, and hormonal therapy use) in patients with endometrial polyps.
Risk factors for and prevalence of malignancy
Sasaki and colleagues found that about 3 of every 100 patients with recognized polyps will harbor a premalignant or malignant lesion (3.4%; 716 of 21,057 patients). The identified risk factors for a cancerous polyp included: menopausal status, age greater than 60 years, presence of AUB, diabetes mellitus, hypertension, obesity, and tamoxifen use. The risk for cancer was 2-fold greater in women older than 60 years compared with those younger than age 60 (prevalence ratio, 2.41). The authors found no risk association with use of combination hormone therapy, parity, breast cancer, or polyp size.
The investigators advised caution with using their conclusions, as there was high heterogeneity for some of the factors studied (including age, AUB, parity, and hypertension).
The study takeaways regarding clinical and demographic risk factors suggest that menopausal status, age greater than 60 years, the presence of AUB, diabetes, hypertension, obesity, and tamoxifen use have an increased risk for premalignant and malignant lesions.
This study is important because its findings will better enable physicians to inform and counsel patients about the risks for malignancy associated with endometrial polyps, which will better foster discussion and joint decision-making about whether or not surgery should be performed.
New evidence associates endometrial polyps with chronic endometritis
The second important study published this year on polyps was conducted by Cicinelli and colleagues and suggests that inflammation may be part of the pathophysiology behind the common problem of polyps. The authors cite a recent study that showed that abnormal expression of "local" paracrine inflammatory mediators, such as interferon-gamma, may enhance the proliferation of endometrial mucosa.1 Building on this possibility further, they hypothesized that chronic endometrial inflammation may affect the pathogenesis of endometrial polyps.
Details of the study
To investigate the possible correlation between polyps and chronic endometritis, Cicinelli and colleagues compared the endometrial biopsies of 240 women with AUB and hysteroscopically and histologically diagnosed endometrial polyps with 240 women with AUB and no polyp seen on hysteroscopy. The tissue samples were evaluated with immunohistochemistry for CD-138 for plasma cell identification.
The study authors found a significantly higher prevalence of chronic endometritis in the group with endometrial polyps than in the group without polyps (61.7% vs 24.2%, respectively; P <.0001). They suggest that this evidence supports the hypothesis that endometrial polyps may be a result of endometrial proliferation and vasculopathy triggered by chronic endometritis.
The significance of this study is that there is a possible causal relationship between endometrial polyps and chronic endometritis, which may expand the options for endometrial polyp therapy beyond surgical management in the future.
Continue to: Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Can endometrial intraepithelial neoplasia be treated with the etonogestrel subdermal implant?
Wong S, Naresh A. Etonogestrel subdermal implant-associated regression of endometrial intraepithelial neoplasia. Obstet Gynecol. 2019;133:780-782.
Recently, Wong and Naresh gave us the first case report of successful treatment of EIN using the etonogestrel subdermal implant. With so many other options available to treat EIN, some of which have been studied extensively, why should we take note of this study? First, the authors point out the risk of endometrial cancer development among patients with EIN, and they acknowledge the standard recommendation of hysterectomy in women with EIN who have finished childbearing and are appropriate candidates for a surgical approach. There is also concern about lower serum etonogestrel levels in obese patients. In this case, the patient (aged 36 with obesity) had been nonadherent with oral progestin therapy and stated that she would not adhere to daily oral therapy. She also declined hysterectomy, levonorgestrel-releasing intrauterine device therapy, and injectable progestin therapy after being counseled about the risk of malignancy development. She consented to subdermal etonogestrel as an alternative to no therapy.
EIN regressed. Endometrial biopsies at 4 and 8 months showed regression of EIN, and at 16 months after implantation (as well as a dilation and curettage at 9 months) demonstrated an inactive endometrium with no sign of hyperplasia.
The authors remain cautious about recommending the etonogestrel subdermal implant as a first-line therapy for EIN, but the implant was reported to be effective in this case that involved a patient with obesity. In cases in which surgery or other medical options for EIN are not feasible, the etonogestrel subdermal implant is reasonable to consider. Its routine use for EIN management warrants future study.
New endometrial ablation technology shows promising benefits
Do we need another endometrial ablation device? Are there improvements that can be made to our existing technology? There already are several endometrial ablation devices, using varying technology, that currently are approved by the US Food and Drug Administration (FDA) for treatment of AUB. The devices use bipolar radiofrequency, cryotherapy, circulating hot fluid, and combined thermal and radiofrequency modalities. Additional devices, employing heated balloon and microwaves, are no longer used. Data on a new device, approved by the FDA in 2017 (the AEGEA Vapor System, called Mara), were recently published.
Details of the study
Levie and colleagues conducted a prospective pivotal trial on Mara's safety and effectiveness. The benefits presented by the authors include that the device 1) does not require that an intrauterine array be deployed up to and abutting the fundus and cornu, 2) does not necessitate cervical dilatation, 3) is a free-flowing vapor system that can navigate differences in uterine contour and sizes (up to 12 cm in length), and 4) accomplishes ablation in 2 minutes. So there are indeed some novel features of this device.
This pivotal study was a multicenter trial using objective performance criterion (OPC), which is based on using the average success rates across the 5 FDA-approved ablation devices as historic controls. In the study an OPC of 66% correlated to the lower bound of the 95% confidence intervals. The primary outcome of the study was effectiveness in the reduction of blood loss using a pictorial blood loss assessment score (PBLAS) of less than 75. Of note, a PBLAS of 150 was a study entry criterion. FIGO types 2 through 6 fibroids were included in the trial. Secondary endpoints were quality of life and patient satisfaction as assessed by the Menorrhagia Impact Questionnaire and the Aberdeen Menorrhagia Severity Score, as well as the need to intervene medically or surgically to treat AUB in the first 12 months after ablation.
Efficacy, satisfaction, and quality of life results
At 12 months, the primary effectiveness end point was achieved in 78.7% of study participants. The satisfaction rate was 90.8% (satisfied or very satisfied), and 99% of participants showed improvement in quality of life scores. There were no reported serious adverse events.
The takeaway is that the AEGEA device appears to be effective for endometrial ablation and offers the novel features of not relying on an intrauterine array to be deployed up to and abutting the fundus and cornu, not necessitating cervical dilatation in all cases, and offering a free-flowing vapor system that can navigate differences in uterine contour and sizes quickly (approximately 2 minutes).
The fact that new devices for endometrial ablation are still being developed is encouraging, and it suggests that endometrial ablation technology can be improved. Although AEGEA's Mara system is not yet commercially available, it is anticipated that it will be available at the start of 2020. The ability to treat large uteri (up to 12-cm cavities) with FIGO type 2 to 6 fibroids with less cervical dilatation makes the device attractive and perhaps well suited for office use.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
- Mollo A, Stile A, Alviggi C, et al. Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril. 2011:96:1209-1212.
Genitourinary endometriosis: Diagnosis and management
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18
Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36
Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43
Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
A patient with severe adenomyosis requests uterine-sparing surgery
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.
Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25
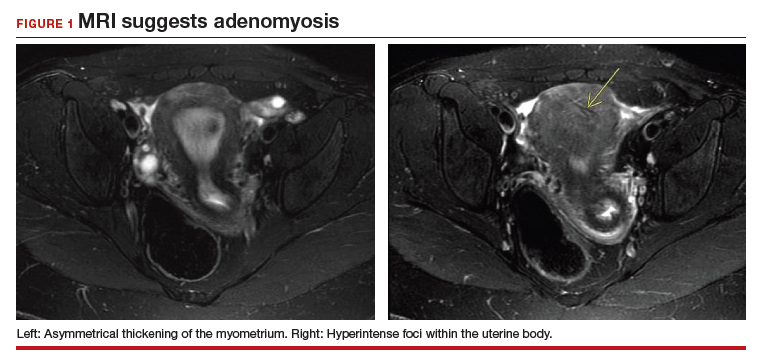
Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9
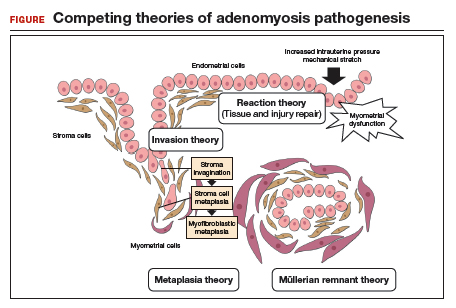
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.
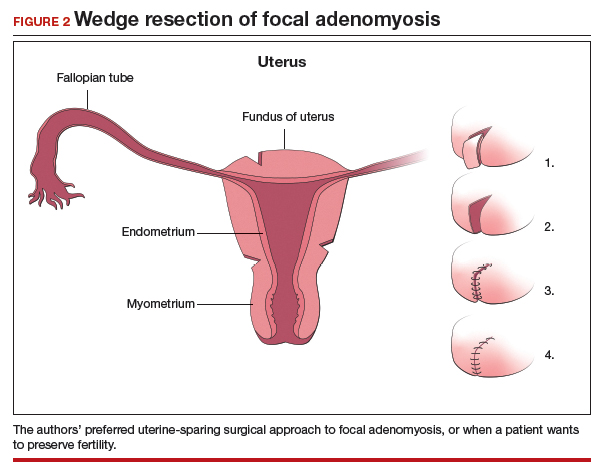
CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
Meaningful endometriosis treatment requires a holistic approach and an understanding of chronic pain
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.