User login
Lithium, valproate, and suicide risk: Analysis of 98,831 cases
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
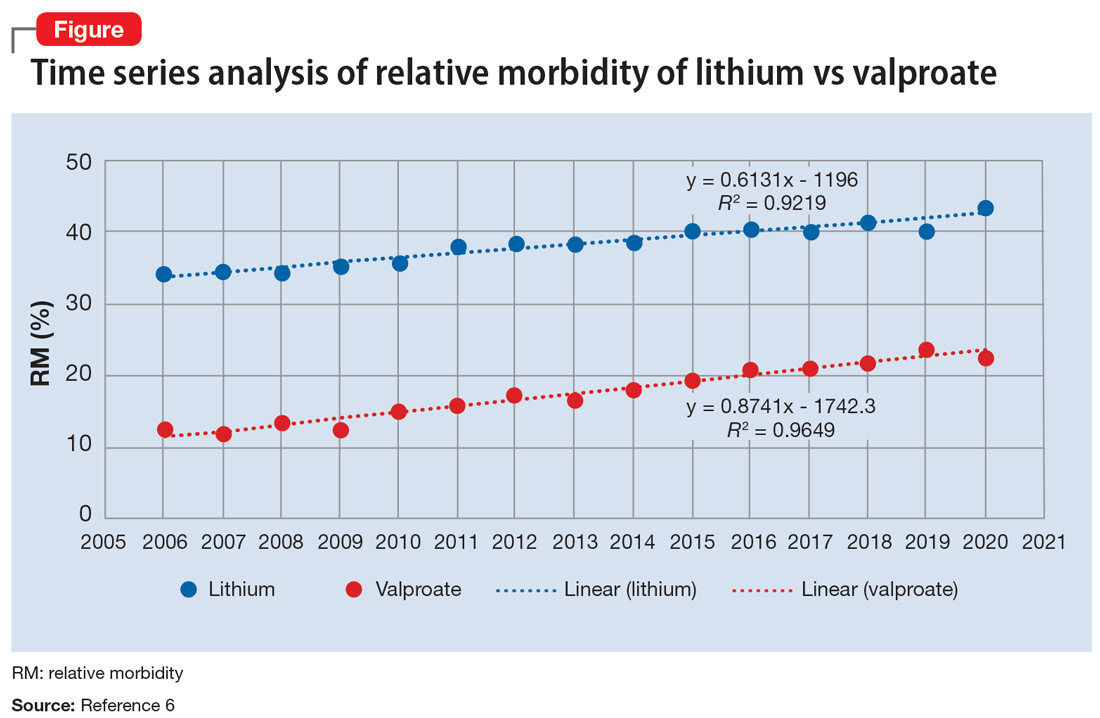
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12
Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
Hold or not to hold: Navigating involuntary commitment
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
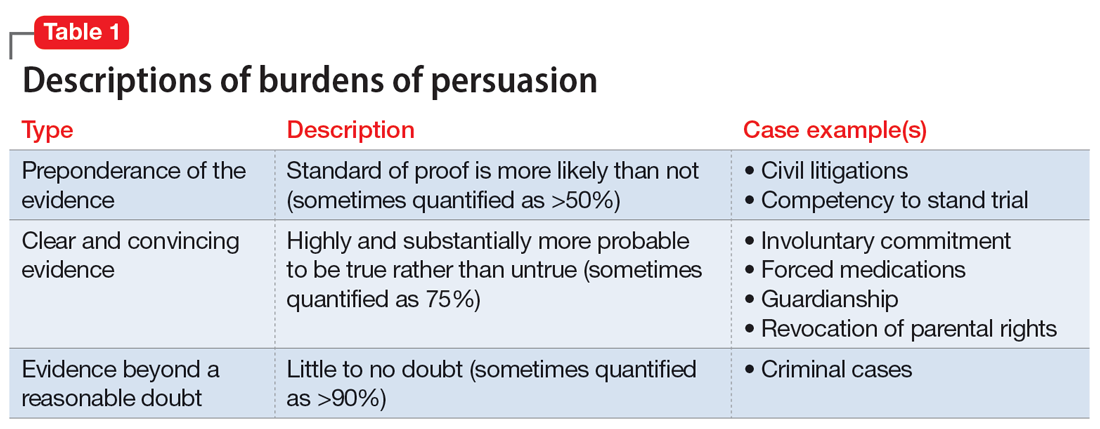
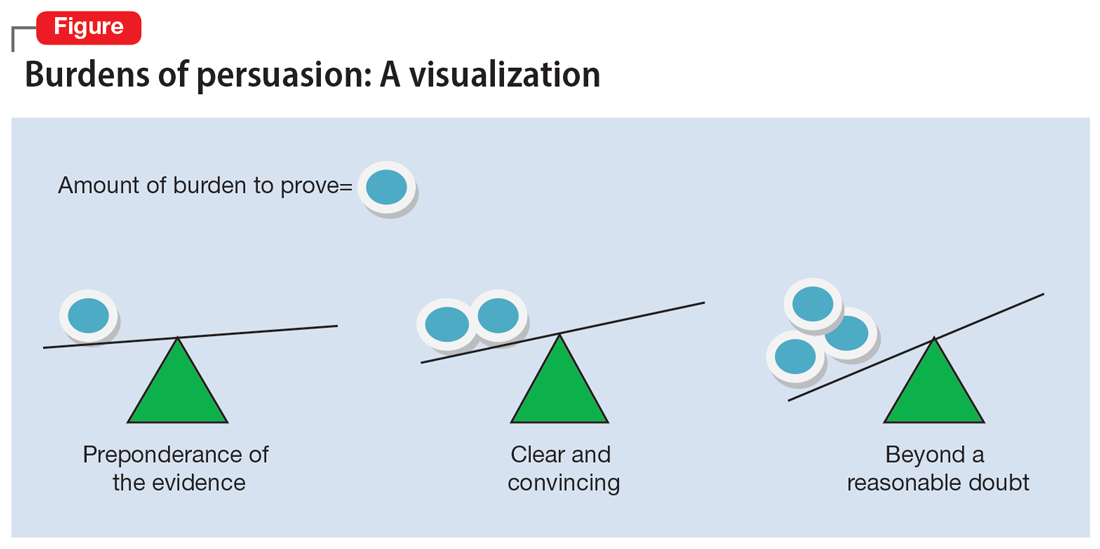
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.
TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.
[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.

TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.

[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
CASE Depressed and suicidal
Police arrive at the home of Mr. H, age 50, after his wife calls 911. She reports he has depression and she saw him in bed brandishing a firearm as if he wanted to hurt himself. Upon arrival, the officers enter the house and find Mr. H in bed without a firearm. Mr. H says little to the officers about the alleged events, but acknowledges he has depression and is willing to go the hospital for further evaluation. Neither his wife nor the officers locate a firearm in the home.
EVALUATION Emergency detention
In the emergency department (ED), Mr. H’s laboratory results and physical examination findings are normal. He acknowledges feeling depressed over the past 2 weeks. Though he cannot identify any precipitants, he says he has experienced anhedonia, lack of appetite, decreased energy, and changes in his sleep patterns. When asked about the day’s events concerning the firearm, Mr. H becomes guarded and does not give a clear answer regarding having thoughts of suicide.
The evaluating psychiatrist obtains collateral from Mr. H’s wife and reviews his medical records. There are no active prescriptions on file and the psychiatrist notices that last year there was a suicide attempt involving a firearm. Following that episode, Mr. H was hospitalized, treated with sertraline 50 mg/d, and discharged with a diagnosis of major depressive disorder. There was no legal or substance abuse history.
In the ED, the psychiatrist conducts a psychiatric evaluation, including a suicide risk assessment, and determines Mr. H is at imminent risk of ending his life. Mr. H’s psychiatrist explains there are 2 treatment options: to be admitted to the hospital or to be discharged. The psychiatrist recommends hospital admission to Mr. H for his safety and stabilization. Mr. H says he prefers to return home.
Because the psychiatrist believes Mr. H is at imminent risk of ending his life and there is no less restrictive setting for treatment, he implements an emergency detention. In Ohio, this allows Mr. H to be held in the hospital for no more than 3 court days in accordance with state law. Before Mr. H’s emergency detention periods ends, the psychiatrist will need to decide whether Mr. H can be safely discharged. If the psychiatrist determines that Mr. H still needs treatment, the court will be petitioned for a civil commitment hearing.
[polldaddy:11189291]
The author’s observations
In some cases, courts allow information a psychiatrist does not directly obtain from a patient to be admitted as testimony in a civil commitment hearing. However, some jurisdictions consider sources of information not obtained directly from the patient as hearsay and thus inadmissible.1 Though each source listed may provide credible information that could be presented at a hearing, the psychiatrist should discuss with the patient the information obtained from these sources to ensure it is admissable.2 A discussion with Mr. H about the factors that led to his hospital arrival will avoid the psychiatrist’s reliance on what another person has heard or seen when providing testimony. Even when a psychiatrist is not faced with an issue of admissibility, caution must be taken with third-party reports.3
TREATMENT Civil commitment hearing
Before the emergency detention period expires, Mr. H’s psychiatrist determines that he remains at imminent risk of self-harm. To continue hospitalization, the psychiatrist files a petition for civil commitment and testifies at the commitment hearing. He reports that Mr. H suffers from a substantial mood disorder that grossly impairs his judgment and behavior. The psychiatrist also testifies that the least restrictive environment for treatment continues to be inpatient hospitalization, because Mr. H is still at imminent risk of harming himself.
Continue to: Following the psychiatrist's...
Following the psychiatrist’s testimony, the magistrate finds that Mr. H is a mentally ill person subject to hospitalization given his mood disorder that grossly impairs his judgment and behavior. The magistrate orders that Mr. H be civilly committed to the hospital.
[polldaddy:11189293]
The author’s observations
The psychiatrist’s testimony mirrors the language regarding civil commitment in the Ohio Revised Code.4 Other elements considered for mental illness in Ohio are a substantial disorder of memory, thought, orientation, or perception that grossly impairs one’s capacity to recognize reality or meet the demands of life.4 The definition of what constitutes a mental disorder varies by state, but the burden of persuasion—the standard by which the court must be convinced—is generally uniform.5 In the 1979 case Addington v Texas, the United States Supreme Court concluded that in a civil commitment hearing, the minimum standard of proof for involuntary commitment must be clear and convincing evidence.6 Neither medical certainty nor substantial probability are burdens of persuasions.6 Instead, these terms may be presented in a forensic report when an examiner outlines their opinion. Table 1 and the Figure provide more detail on burdens of persuasion.

TREATMENT Civil commitment and patient rights
At a regularly scheduled treatment team meeting, the team informs Mr. H that he has been civilly committed for further treatment. Mr. H becomes upset and tells the team the decision is a complete violation of his rights. After a long rant, Mr. H walks out of the room, saying, “I did not even know when this hearing was.” A member of the treatment team becomes concerned that Mr. H may not have been notified of the hearing.

[polldaddy:11189294]
The author’s observations
It is not clear if Mr. H had been notified of his civil commitment hearing. If Mr. H had not been notified, his rights would have been compromised. Lessard v Schmidt (1972) outlined that individuals involved in a civil commitment hearing should be afforded the same rights as those involved in criminal proceedings.7 Mr. H should have been notified of his hearing and afforded the opportunity to have the assignment of counsel, the right to appear, the right to testify, the right to present witnesses and other evidence, and the right to confront witnesses.
Without notification of the hearing, the only right that would have remained intact for Mr. H would have been the assignment of counsel in his absence and without his knowledge. If Mr. H had been notified of the hearing and did not want to attend, yet still desired legal counsel, he could have waived his presence voluntarily after discussing this option with his attorney.8,9
Continue to: OUTCOME Stabilization and discharge
OUTCOME Stabilization and discharge
During his 10-day stay, Mr. H is treated with sertraline 50 mg/d and engages in individual and group therapy. He shows noticeable improvement in his depressive symptoms and reports having no thoughts of suicide or self-harm. The treatment team determines it is appropriate to discharge him home (the firearm was never found) and involves his wife in safety planning and follow-up care. On the day of his discharge, Mr. H reflects on his treatment and civil commitment. He says, “I did not know a judge could order me to be hospitalized.”
[polldaddy:11189297]
The author’s observations
The physician’s decision to pursue civil commitment is best described by the legal doctrines of police powers and parens patriae. Other relevant ethical principles are described in Table 2.10

Though ethical principles may play a role in civil commitment, parens patriae and police powers is the answer with respect to the State.11Parens patriae is Latin for the “parent of the country” and grants the State the power to protect those residents who are most vulnerable. Police power is the authority of the State to enact and enforce rules that limit the rights of individuals for the greater good of ensuring health, safety, and welfare of all citizens.
Bottom Line
Psychiatrists are entrusted with recognizing when a patient, due to mental illness, is a danger to themselves or others and in need of treatment. After an emergency detention period, if the patient remains a danger to themselves or others and does not want to voluntarily receive treatment, a court hearing is required. As an expert witness, the treating psychiatrist should know the factors of law in their jurisdiction that determine civil commitment.
Related Resources
- Extreme Risk Protection Orders. Johns Hopkins Bloomberg School of Public Health. https://www.jhsph.edu/research/ centers-and-institutes/johns-hopkins-center-for-gun-violenceprevention-and-policy/research/extreme-risk-protectionorders/
- Gutheil TG. The Psychiatrist as Expert Witness. 2nd ed. American Psychiatric Association Publishing; 2009.
- Landmark Cases 2014. American Academy of Psychiatry and the Law. https://www.aapl.org/landmark-cases
Drug Brand Names
Sertraline • Zoloft
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
1. Pinals DA, Mossman D. Evaluation for Civil Commitment. Oxford University Press; 2012.
2. Thatcher BT, Mossman D. Testifying for civil commitment: help unwilling patients get the treatment they need. Current Psychiatry. 2009;8(11):51-56.
3. Marett CP, Mossman D. What is your liability for involuntary commitment based on faulty information? Current Psychiatry. 2017;16(3):21-25,33.
4. Ohio Rev Code § 5122.01 (2018).
5. The Burden of Proof. University of Minnesota. Accessed January 23, 2022. https://open.lib.umn.edu/criminallaw/chapter/2-4-the-burden-of-proof/
6. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Forensic Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2018.
7. Gold LH, Frierson RL, eds. The American Psychiatric Association Publishing Textbook of Suicide Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
8. Cook J. Good lawyering and bad role models: the role of respondent’s counsel in a civil commitment hearing. Georgetown Journal of Legal Ethics. 2000;14(1):179-195.
9. Ferris CE. The search for due process in civil commitment hearings: how procedural realities have altered substantive standards. Vanderbilt Law Rev. 2008;61(3):959-981.
10. Substance Abuse and Mental Health Services Administration. Civil Commitment and the Mental Health Care Continuum: Historical Trends and Principles for Law and Practice. 2019. Accessed January 23, 2022. https://www.samhsa.gov/resource/ebp/civil-commitment-mental-health-care-continuum-historical-trends-principles-law
11. Melton GB, Petrila J, Poythress NG, et al. Psychological Evaluations for the Courts: A Handbook for Mental Health Profession. 4th ed. Guilford Press; 2018.
Preparing patients with serious mental illness for extreme HEAT
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Lithium for bipolar disorder: Which patients will respond?
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.
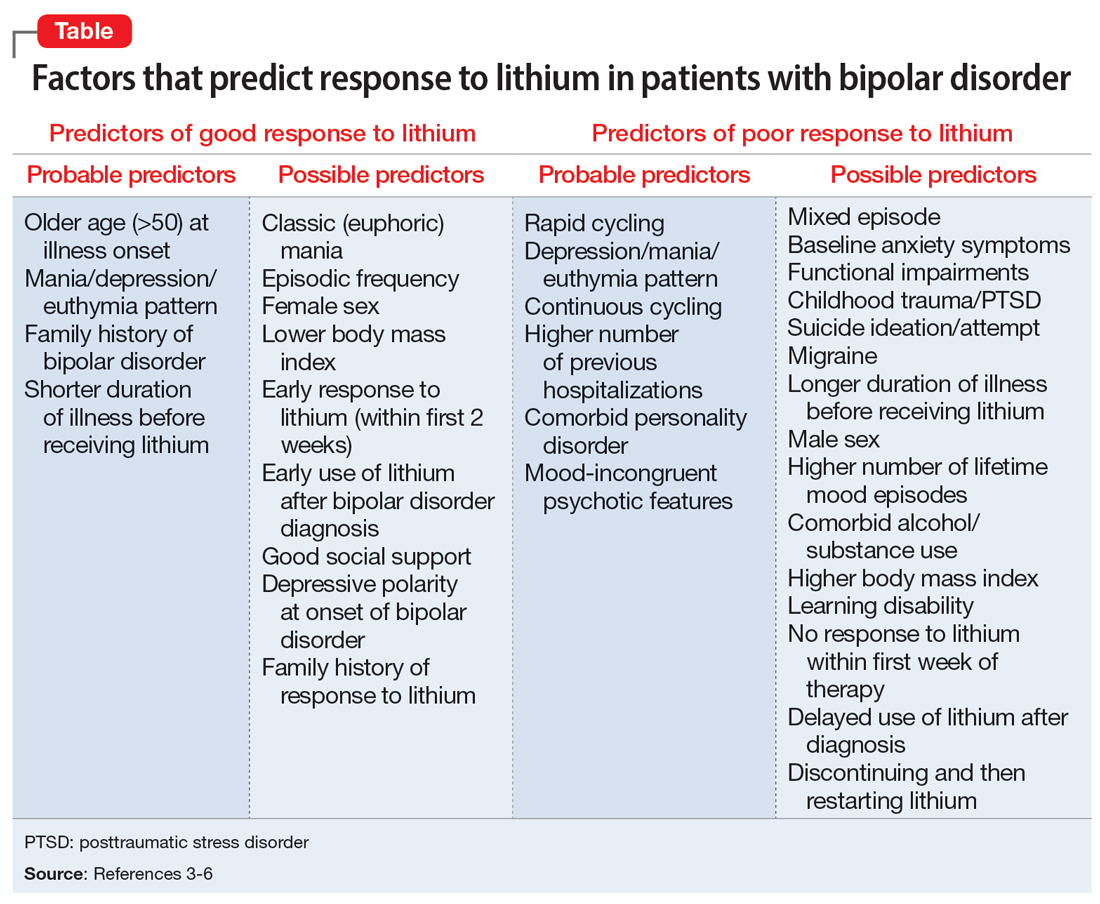
Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Melatonin as a sleep aid: Are you prescribing it correctly?
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
Risk factors for nonsuicidal self-injury: A review of the evidence
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.
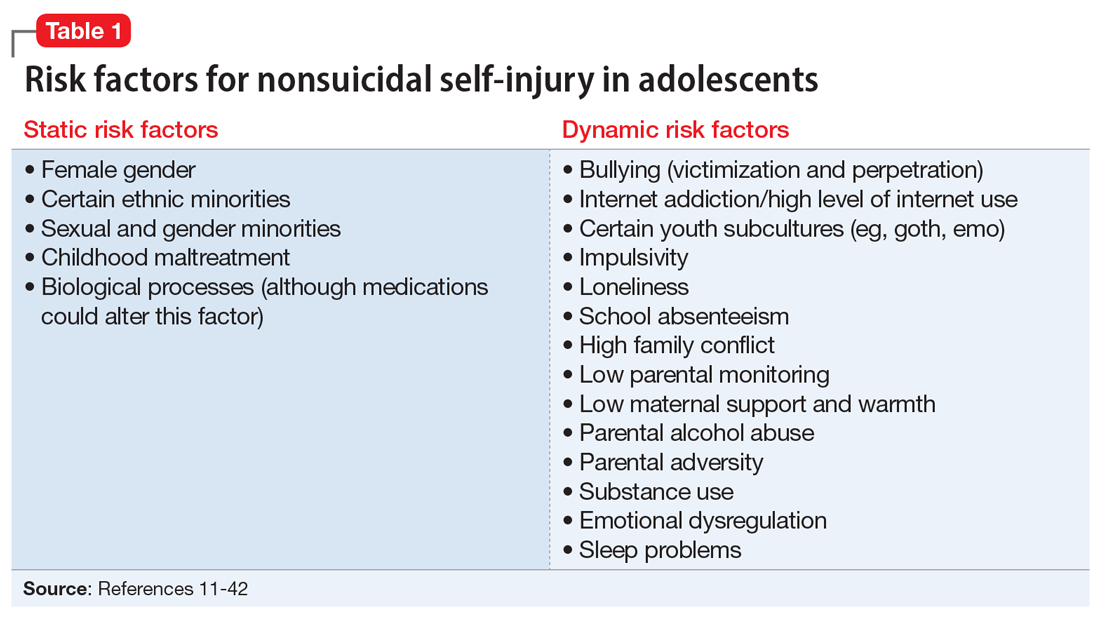
NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.
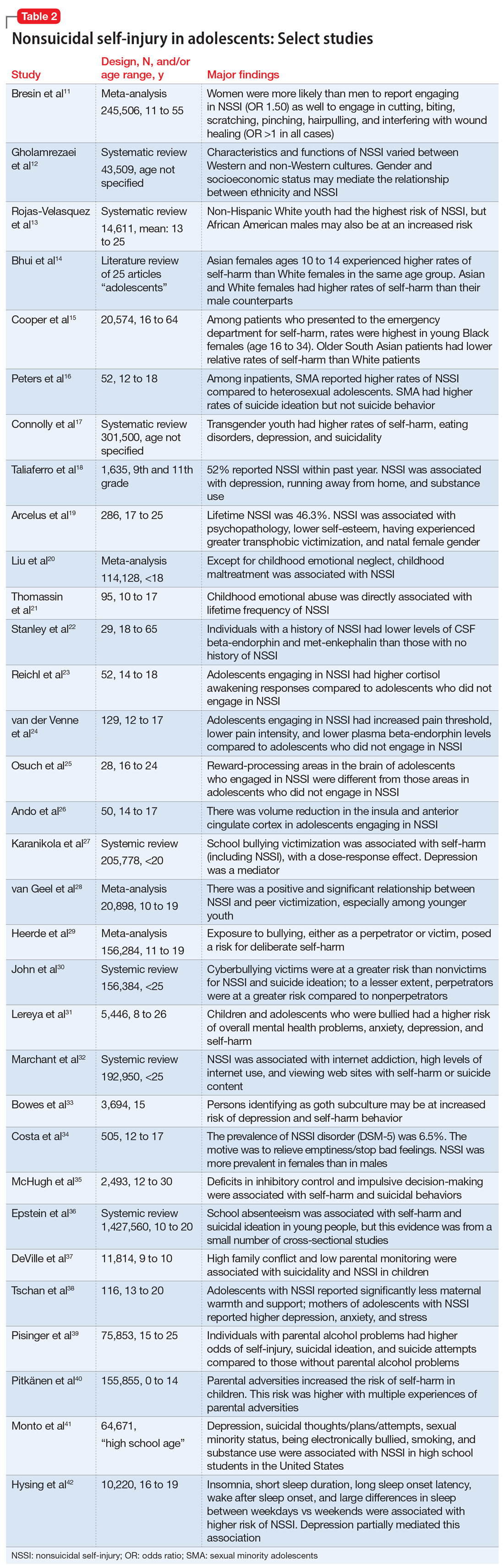
Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.
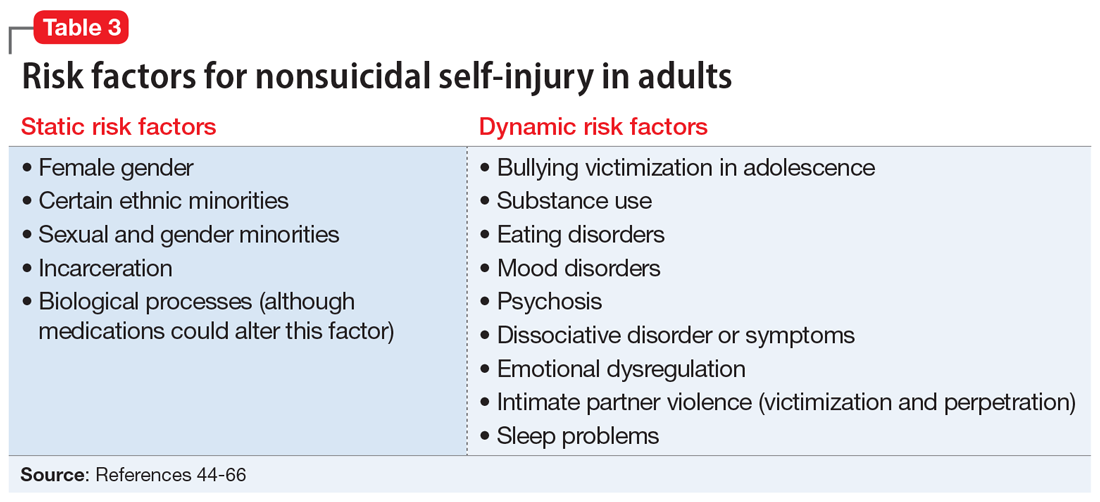
Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11
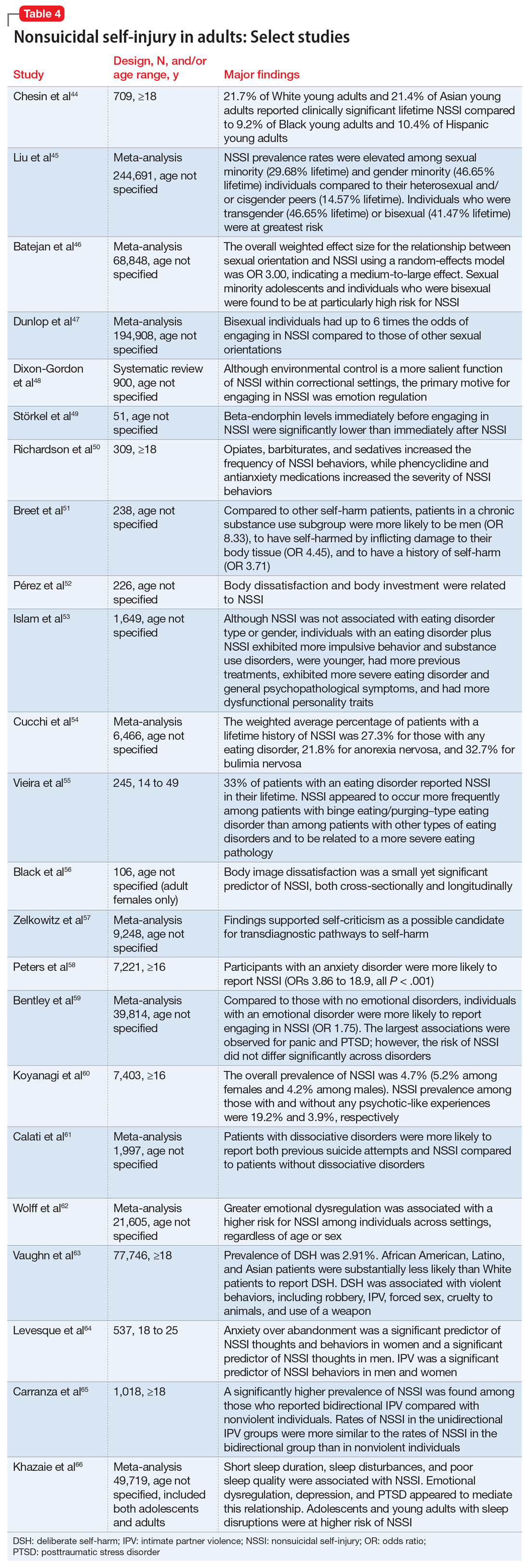
As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
How bariatric surgery affects psychotropic drug absorption
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5
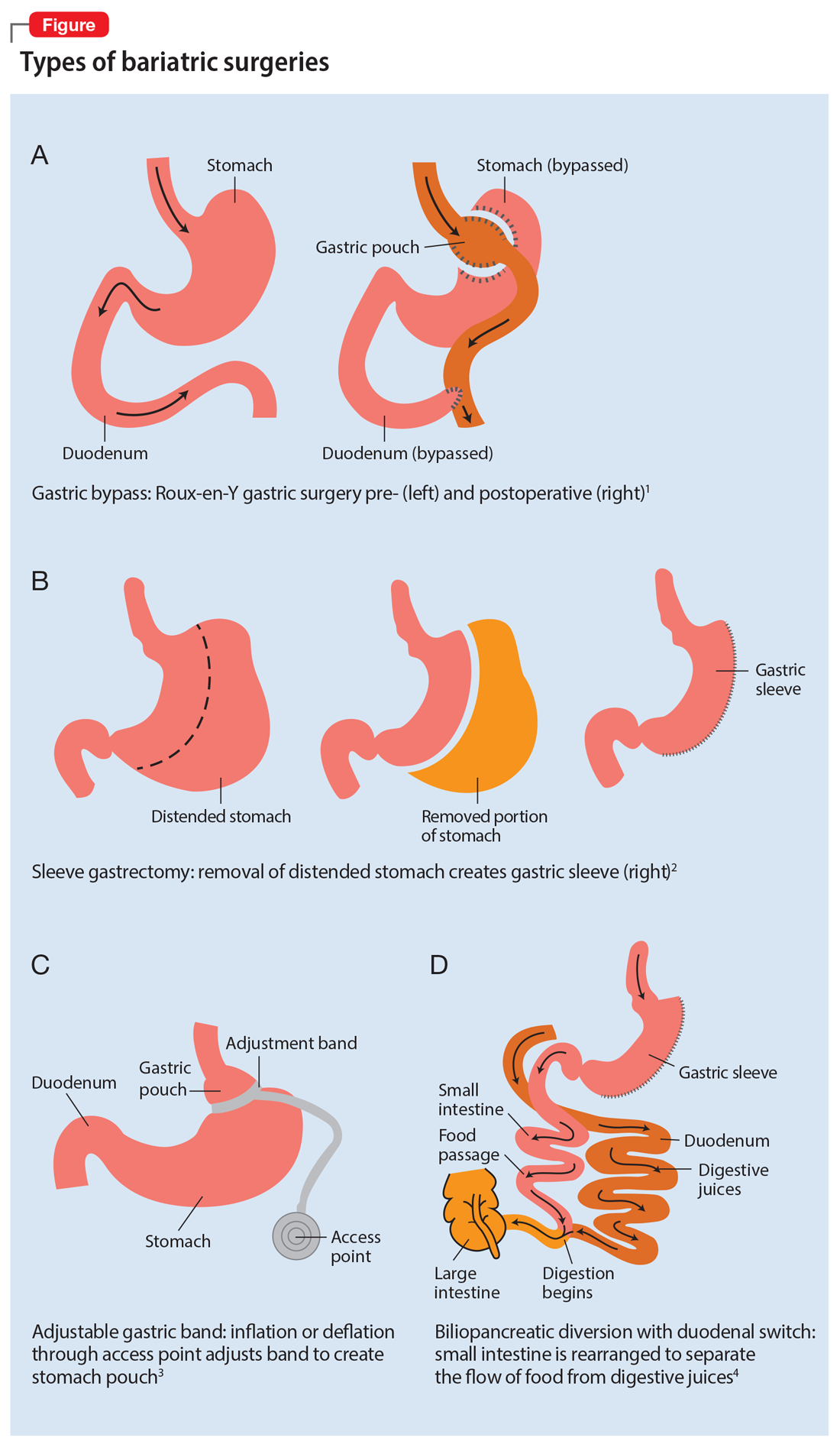
Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.
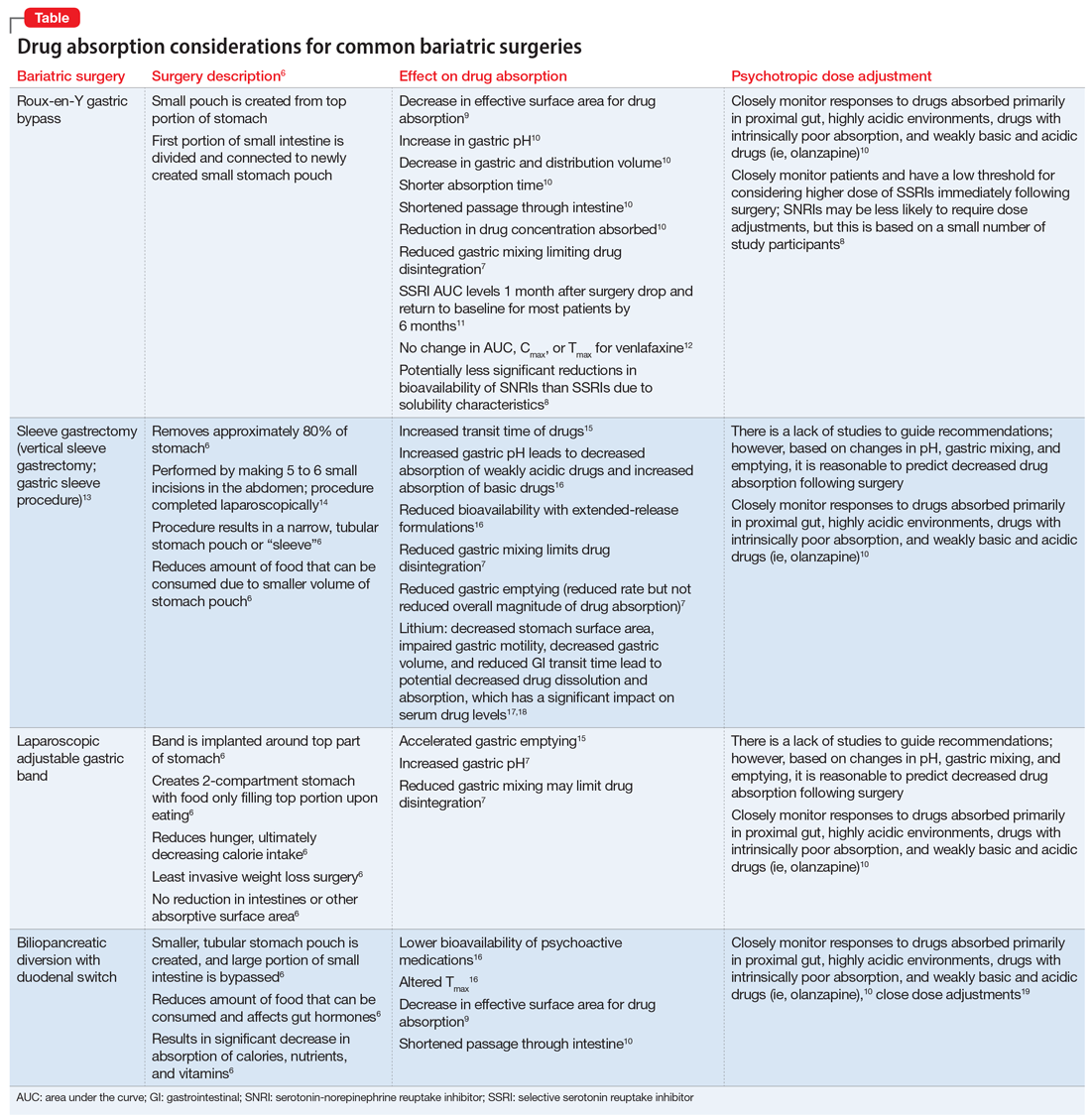
Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5

Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.

Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5

Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.

Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
Impaired cognition in a patient with schizophrenia and HIV
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.
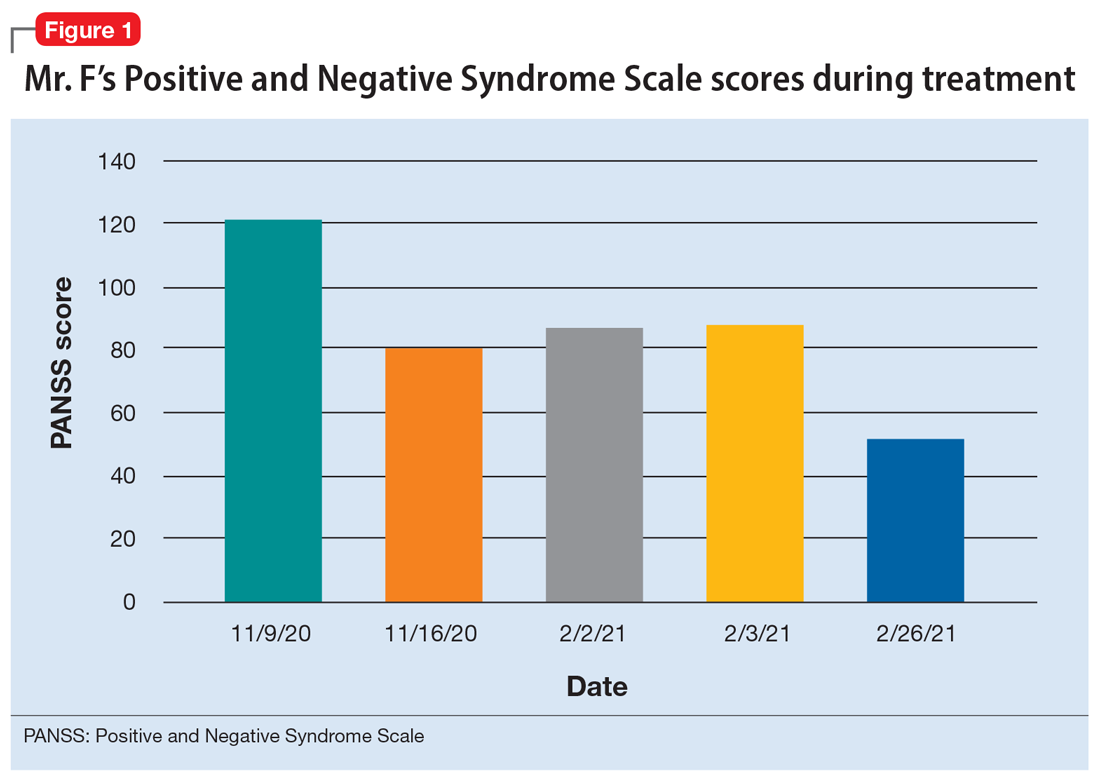
During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.
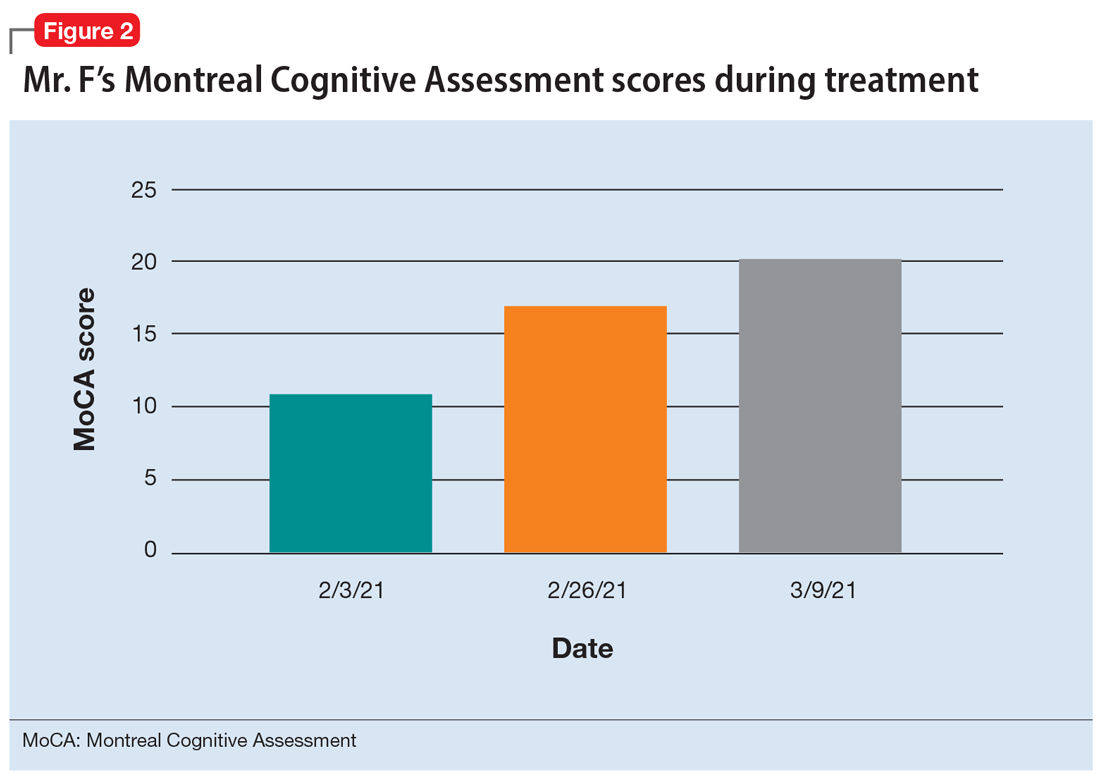
The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
Pregnancy termination: What psychiatrists need to know
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
Generalized anxiety disorder: 8 studies of biological interventions
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).
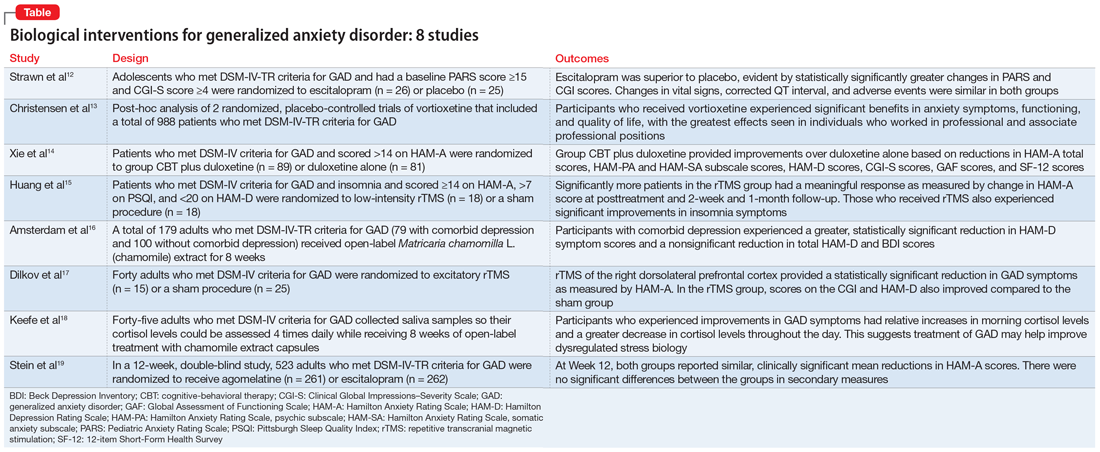
1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).

1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
Generalized anxiety disorder (GAD) typically begins in early adulthood and persists throughout life. Many individuals with GAD report they have felt anxious their entire lives. The essential symptom of GAD is excessive anxiety and worry about numerous events or activities. The intensity, duration, and/or frequency of the anxiety and worry are out of proportion to the actual likelihood or impact of the anticipated event. The individual finds it difficult to control their worry and prevent worrisome thoughts from interfering with attention to everyday tasks.1
Treatment of GAD typically consists of psychotherapy and pharmacotherapy. Several studies have suggested that concurrent psychotherapy amplifies the benefits of pharmacotherapy.2-5 Additionally, combined treatment may differentially target specific symptoms (eg, cognitive vs somatic). The addition of psychotherapy may also increase treatment adherence and decrease potential adverse effects of pharmacotherapy.
Multiple classes of medications are available for treating GAD. Current guidelines and evidence suggest that selective serotonin reuptake inhibitors (SSRIs) should be considered a first-line intervention, followed by serotonin-norepinephrine reuptake inhibitors.6-11 While the evidence supporting pharmacotherapy for GAD continues to expand, many patients with GAD do not respond to first-line treatment. There is limited data regarding second-line or augmentation strategies for treating these patients. Because current treatment options for GAD are commonly associated with suboptimal treatment outcomes, researchers are investigating the use of nonpharmacologic biological interventions, such as repetitive transcranial magnetic stimulation (rTMS), which was first cleared by the FDA to treat major depressive disorder (MDD) in 2008.
In Part 1 of this 2-part article, we review 8 randomized controlled trials (RCTs) of biological interventions for GAD that have been published within the last 5 years (Table12-19).

1. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
GAD is highly prevalent in adolescents, and SSRIs are often used as first-line agents. However, treatment response is often variable, and clinicians often use trial-and-error to identify an appropriate medication and dose that will result in meaningful improvement. Understanding an individual’s pharmacokinetic response may help predict response and guide therapy. Adult studies have shown cytochrome P450 (CYP) 2C19 metabolizes several SSRIs, including escitalopram, with faster CYP2C19 metabolism leading to decreased plasma concentrations. Strawn et al12 studied the effects of escitalopram in adolescents with GAD as well as the effects of CYP2C19 metabolism.
Study design
- A double-blind, placebo-controlled trial evaluated 51 adolescents (age 12 to 17) who met DSM-IV-TR criteria for GAD. They had a baseline Pediatric Anxiety Rating Scale (PARS) score ≥15 and a Clinical Global Impressions–Severity (CGI-S) Scale score ≥4.
- Participants were randomized to escitalopram (n = 26; scheduled titration to 15 mg/d, then flexible to 20 mg/d), or placebo (n = 25) and monitored for 8 weeks.
- Patients with panic disorder, agoraphobia, or social anxiety disorder were also enrolled, but GAD was the primary diagnosis.
- The primary outcome was change in PARS score and change from baseline in CGI-S and Clinical Global Impressions–Improvement (CGI-I) scale scores, with assessments completed at Week 1, Week 2, Week 4, Week 6, and Week 8, or at early termination.
- Genomic DNA was obtained via buccal swab to assess 9 alleles of CYP2C19. Plasma concentrations of escitalopram and its major metabolite, desmethylescitalopram, were collected to assess plasma escitalopram and desmethylescitalopram area under the curve for 24 hours (AUC0-24) and maximum plasma concentration (CMAX).
Outcomes
- Escitalopram was superior to placebo, evident by statistically significantly greater changes in PARS and CGI scores.
- Greater improvement over time on PARS was correlated with intermediate CYP2C19 metabolizers, and greater response as measured by CGI-I was associated with having at least 1 long allele of SLC6A4 and being an intermediate CYP2C19 metabolizer.
- While plasma escitalopram exposure (AUC0-24) significantly decreased and desmethylcitalopram-to-escitalopram ratios increased with faster CYP2C19 metabolism at 15 mg/d, escitalopram exposure at the 15 mg/d dose and escitalopram-to-desmethylcitalopram ratios did not differ at Week 8 between responders and nonresponders. Patients with activation symptoms had higher CMAX and AUC0-24.
- Changes in vital signs, corrected QT interval, and adverse events were similar in both groups.
Conclusions/limitations
- For adolescents with GAD, escitalopram showed a benefit compared to placebo.
- Allelic differences in CYP2C19 metabolism may lead to variations in pharmacokinetics, and understanding a patient’s CYP2C19 phenotype may help guide dosing escitalopram and predicting adverse effects.
- This study enrolled a small, predominantly female, White, treatment-naïve sample, which may limit conclusions on allelic differences. Additionally, the sample included adolescents with severe anxiety and comorbid anxiety conditions, which may limit generalizability.
Continue to: #2
2. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
Vortioxetine, an FDA-approved antidepressant, has been shown to improve anxiety symptoms in patients with GAD. Additionally, vortioxetine has shown positive effects in patients with MDD, with greater improvement seen in the working and professional population. Due to the overlap between MDD and GAD, Christensen et al13 assessed the effectiveness of vortioxetine on anxiety symptoms in individuals who were working.
Study design
- Researchers conducted a post-hoc analysis of a previously completed randomized, placebo-controlled trial of 301 patients as well as a previously completed randomized, placebo-controlled relapse prevention study of 687 patients. Patients in both groups met DSM-IV-TR criteria for GAD.
- Inclusion criteria included a Hamilton Anxiety Rating Scale (HAM-A) score ≥20 with HAM-A scores ≥2 on items 1 (anxious mood), and 2 (tension), and a Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤16 at screening and baseline.
- Researchers compared participants who were working or pursuing an education vs the full study sample.
Outcomes
- Vortioxetine was significantly associated with benefits in anxiety symptoms, functioning, and quality of life in both working participants and the total population, with the greatest effects seen in professional (ie, managers, administrators) and associate professional (ie, technical, nursing, clerical workers, or secretarial) positions. Working participants who received placebo were more likely to relapse compared to those receiving vortioxetine.
- There did not appear to be a statistically significant benefit or increase in relapse among the skilled labor group (ie, building, electrical/factory worker, or services/sales) while receiving vortioxetine.
Conclusions/limitations
- Vortioxetine may have a more pronounced effect in patients who are working or pursuing an education vs the full GAD population, which suggests that targeting this medication at particular patient demographics may be beneficial.
- Working patients with GAD may also differ from nonworking patients by factors other than work, such as education, support system, motivation, and other personal factors.
- This study was a post-hoc analysis, which limits definitive conclusions but may help guide future studies.
Continue to: #3
3. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
Treatment of GAD should include nonmedication options such as psychotherapy to help enhance efficacy. Few studies have evaluated whether combined cognitive-behavioral therapy (CBT) plus medication has more benefit than medication monotherapy, specifically in patients with GAD. In this randomized trial, Xie et al14 examined how a study population undergoing CBT and receiving duloxetine differed from those receiving duloxetine monotherapy for GAD.
Study design
- In this randomized, open-label trial, adults who met DSM-IV criteria for GAD and had a HAM-A score >14 were randomized to group CBT plus duloxetine (n = 89) or duloxetine only (n = 81), with follow-up at Week 4, Week 8, and Month 3.
- The primary outcomes included response and remission rates based on HAM-A score. Secondary outcomes included HAM-A total score reductions, psychic anxiety (HAMA-PA) and somatic anxiety (HAMA-SA) subscale score reductions, Hamilton Depression Rating Scale score reductions, and reductions in overall illness severity as measured by CGI-S, the Global Assessment of Functioning Scale, and the 12-item Short-Form Health Survey.
Outcomes
- At Week 4, combined therapy was superior to duloxetine alone as evident by the primary and most secondary outcomes, with continued benefits but smaller effect size at Week 8.
- At Month 3, combined therapy was significantly better only in HAM-A total score and HAMA-PA score reductions.
Conclusions/limitations
- Patients who received group CBT plus duloxetine treatment experienced faster improvement of GAD symptoms compared to patients who received duloxetine monotherapy, though the difference reduced over time.
- The most benefit appeared to be for psychic anxiety symptoms, which suggests that group CBT can help change cognition style.
- This study had a short follow-up period, high dropout rates, and recruited patients from only 1 institution.
4. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
Insomnia and anxiety often present together. rTMS has demonstrated efficacy in various psychiatric illnesses, but there is limited research regarding its effectiveness in GAD. Additionally, little is known regarding the benefits of rTMS for patients with comorbid insomnia and GAD. Huang et al15 examined the therapeutic effects of rTMS in patients with comorbid insomnia and GAD.
Continue to: Study design
Study design
- Adults who met DSM-IV criteria for GAD and insomnia were randomized to receive 10 days of low-intensity rTMS on the right parietal lobe (n = 18) or a sham procedure (n = 18). Inclusion criteria also included a score ≥14 on HAM-A, ≥7 on the Pittsburgh Sleep Quality Index (PSQI), and <20 on the 24-item Hamilton Depression Rating Scale (HAM-D).
- rTMS settings included a frequency of 1 Hz, 90% intensity of the resting motor threshold, 3 trains of 500 pulses, and an intertrain interval of 10 minutes.
- Study measurements included HAM-A, PSQI, and HAM-D at baseline, posttreatment at Day 10, Week 2 follow-up, and Month 1 follow-up.
Outcomes
- Significantly more patients in the rTMS group had a meaningful response as measured by change in HAM-A score at posttreatment and both follow-up sessions.
- The rTMS group had significant remission compared to the sham group at posttreatment and Week 2 follow-up, but showed no significant difference at Month 1.
- There were significant improvements in insomnia symptoms in the rTMS group at the posttreatment and follow-up time points.
Conclusions/limitations
- Low-frequency rTMS over the right parietal cortex is an effective treatment option for patients with comorbid GAD and insomnia.
- This study had a small sample size consisting of participants from only 1 institution.
5. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
GAD often presents with comorbid depression. While antidepressants are the standard approach to treatment of both conditions, patients may seek alternative therapies. In previous studies,20Matricaria chamomilla L. (chamomile) has been shown to reduce GAD symptoms, and post-hoc analyses21 have shown its benefits in treating depression. Amsterdam et al16 assessed the effects of chamomile on patients with GAD with and without comorbid depression.
Study design
- As part of an RCT, 179 adults who met DSM-IV-TR criteria for GAD underwent an 8-week open-label phase of chamomile extract therapy (1,500 mg/d). Participants who responded were enrolled in a randomized, double-blind, placebo-control trial. Amsterdam et al16 specifically analyzed the 8-week open label portion of the study.
- Participants were divided into 2 groups: GAD without comorbid depression (n = 100), and GAD with comorbid depression (n = 79).
- Outcome measures included the 7-item generalized anxiety disorder scale (GAD-7), HAM-A, Beck Anxiety Inventory, 17-item HAM-D, 6-item HAM-D, and the Beck Depression Inventory (BDI).
Continue to: Outcomes
Outcomes
- Patients with comorbid depression experienced a greater, statistically significant reduction in HAM-D core symptom scores (depressed mood, guilt, suicide ideation, work and interest, retardation, and somatic symptoms general).
- The comorbid depression group experienced a trend (but not significant) reduction in total HAM-D and BDI scores.
Conclusions/limitations
- Chamomile extract may help reduce depressive symptoms in patients with GAD who also have depression.
- This study was not powered to detect significant differences in depression outcome ratings between groups, was exploratory, and was not a controlled trial.
6. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
Nonpharmacologic modalities, including rTMS, may be effective alternatives for treating GAD. Dilkov et al17 examined whether excitatory rTMS is an effective treatment option for GAD.
Study design
- In this double-blind, sham-controlled trial, adults who met DSM-IV criteria for GAD were randomized to excitatory rTMS of the right dorsolateral prefrontal cortex therapy (n = 15) or a sham procedure (n = 25).
- rTMS settings included a frequency of 20 Hz, 110% intensity of resting motor threshold, 20 trains, 9 seconds/train, and 51-second intertrain intervals.
- Outcomes were measured by HAM-A, CGI, and 21-item HAM-D.
Outcomes
- At the conclusion of 25 treatments, the rTMS group experienced a statistically significant reduction in GAD symptoms as measured by HAM-A.
- Improvements were also noted in the CGI and HAM-D scores in the rTMS group compared to the sham group.
- The benefits continued at the Week 4 follow-up visit.
Conclusions/limitations
- Participants in the rTMS group experienced a significant decrease in anxiety symptoms, which suggests that rTMS may be an effective treatment for GAD.
- The benefits appear sustainable even after the conclusion of the rTMS sessions.
- This study had a small sample size and excluded patients with comorbid psychiatric conditions.
Continue to: #7
7. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
Dysregulated stress response has been proposed as a mechanism for anxiety.22,23 Patients with GAD have been reported to have alterations in cortisol levels, specifically lower morning cortisol levels and a less steep diurnal cortisol slope; however, it is not clear how treatment affects these levels. Keefe et al18 examined whether chamomile therapy in patients with GAD affects cortisol levels.
Study design
- In an 8-week, open-label study, 45 adults who met DSM-IV criteria for GAD received chamomile extract capsules 1,500 mg/d.
- Participants used at-home kits to collect their saliva so cortisol levels could be assessed at 8
am , 12pm , 4pm , and 8pm . - The GAD-7 was used to assess anxiety symptoms.
Outcomes
- Participants who experienced greater improvements in GAD symptoms had relative increases in morning cortisol levels compared to their baseline levels.
- Participants who experienced greater improvements in GAD symptoms had a greater decrease in cortisol levels throughout the day (ie, greater diurnal slope).
Conclusions/limitations
- Greater improvement in GAD symptoms after treatment with chamomile extract appeared to be correlated with increased morning cortisol levels and a steeper diurnal cortisol slope after awakening, which suggests that treatment of GAD may help improve dysregulated stress biology.
- This study had a small sample size and was not placebo-controlled.
Continue to: #8
8. Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
Compared to the medications that are FDA-approved for GAD, agomelatine has a different mechanism of action, and has shown to be efficacious and tolerable in previous studies.24-26 In this study, Stein et al19 compared agomelatine vs escitalopram for patients with severe GAD.
Study design
- In a 12-week, double-blind study, adults who met DSM-IV-TR criteria for GAD were randomized to agomelatine 25 to 50 mg/d (n = 261) or escitalopram 10 to 20 mg/d (n = 262).
- Participants had to meet specific criteria for severe anxiety, including a HAM-A total score ≥25.
- The primary outcome measure was the change in HAM-A score from baseline to Week 12. Secondary outcome measures included the rate of response as determined by change in scores on the HAM-PA, HAM-SA, CGI, Toronto Hospital Alertness Test, Snaith-Hamilton Pleasure Scale, and Leeds Sleep Evaluation Questionnaire.
Outcomes
- Participants in both the agomelatine and escitalopram groups reported similar, clinically significant mean reductions in HAM-A scores at Week 12.
- There were no significant differences in secondary measures between the 2 groups, and both groups experienced improvement in psychic and somatic symptoms, alertness, and sleep.
- Overall, the agomelatine group experienced fewer adverse events compared to the escitalopram group.
Conclusions/limitations
- Agomelatine may be an efficacious and well-tolerated treatment option for severe GAD.
- This study excluded individuals with comorbid conditions.
Bottom Line
Recent research suggests that escitalopram; vortioxetine; agomelatine; duloxetine plus group cognitive-behavioral therapy; repetitive transcranial magnetic stimulation; and chamomile extract can improve symptoms in patients with generalized anxiety disorder.
Related Resources
- Abell SR, El-Mallakh RS. Serotonin-mediated anxiety: how to recognize and treat it. Current Psychiatry. 2021;20(11):37-40. doi:10.12788/cp.0168
Drug Brand Names
Duloxetine • Cymbalta
Escitalopram • Lexapro
Vortioxetine • Trintellix
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., text revision. American Psychiatric Association; 2022.
2. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. doi:10.1056/NEJMoa0804633
3. Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):527-539. doi:10.1016/j.chc.2012.05.003
4. Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. doi:10.1097/chi.0b013e318154bb57
5. Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am J Psychiatry. 2013;170(7):782-789. doi:10.1176/app.ajp.2013.12081104
6. Stein DJ. Evidence-based pharmacotherapy of generalised anxiety disorder: focus on agomelatine. Adv Ther. 2021;38(Suppl 2):52-60. doi:10.1007/s12325-021-01860-1
7. Andrews G, Bell C, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Aust N Z J Psychiatry. 2018;52(12):1109-1172. doi:10.1177/0004867418799453
8. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-439. doi:10.1177/0269881114525674
9. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77-84. doi:10.3109/13651501.2012.667114
10. Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl 1):S1. doi:10.1186/1471-244X-14-S1-S1
11. Generalised anxiety disorder and panic disorder in adults: management. National Institute for Health and Care Excellence. January 26, 2011. Updated June 15, 2020. Accessed April 27, 2022. https://www.nice.org.uk/guidance/cg113
12. Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. doi:10.4088/JCP.20m13396
13. Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2019;24(2):249-257. doi:10.1017/S1092852917000761
14. Xie ZJ, Han N, Law S, et al. The efficacy of group cognitive-behavioural therapy plus duloxetine for generalised anxiety disorder versus duloxetine alone. Acta Neuropsychiatr. 2019;31(6):316-324. doi:10.1017/neu.2019.32
15. Huang Z, Li Y, Bianchi MT, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018;11(5):1103-1109. doi:10.1016/j.brs.2018.05.016
16. Amsterdam JD, Li QS, Xie SX, et al. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J Altern Complement Med. 2020;26(9):813-819. doi:10.1089/acm.2019.0252
17. Dilkov D, Hawken ER, Kaludiev E, et al. Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: a randomized, double-blind sham controlled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:61-65. doi:10.1016/j.pnpbp.2017.05.018
18. Keefe JR, Guo W, Li QS, et al. An exploratory study of salivary cortisol changes during chamomile extract therapy of moderate to severe generalized anxiety disorder. J Psychiatr Res. 2018;96:189-195. doi:10.1016/j.jpsychires.2017.10.011
19. Stein DJ, Khoo J, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970-979. doi:10.1016/j.euroneuro.2018.05.006
20. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(4):378-382. doi:10.1097/JCP.0b013e3181ac935c
21. Amsterdam JD, Shults J, Soeller I, et al. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern Ther Health Med. 2012;18(5):44-49.
22. Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214. doi:10.1080/15622975.2016.1190867
23. Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191-216. doi:10.1007/7854_2014_299
24. de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628-642. doi:10.1038/nrd3140
25. Guardiola-Lemaitre B, de Bodinat C, Delagrange P, et al. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171(15):3604-3619. doi:10.1111/bph.12720
26. Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526-537. doi:10.1016/j.euroneuro.2017.02.007