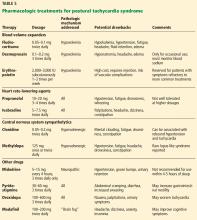
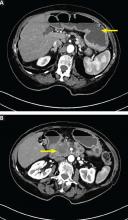
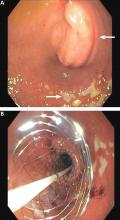
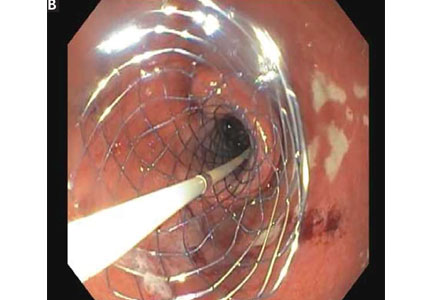
User login
SPIRITT: What does ‘spirituality’ mean?
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
Both patients and clinicians alike have shown increasing interest in spirituality as a component of physical and mental well-being.1 However, there’s no clear consensus on what spirituality actually means. The Merriam-Webster dictionary defines it “affecting the spirit, relating to sacred matters, concerned with religious issues.”2 Spirituality is sometimes defined in broadly secular terms, such as the feeling of “being part of something greater than ourselves,” or in connection to ideas rooted in a specific belief system, such as “aligning oneself with the Will of God.”
I prefer to think of the word “spiritual” as encompassing multiple practices and beliefs that have the common goal of helping us deepen our capacity for self-awareness, joy, compassion, love, freedom, justice, and mutual cooperation, not only for our own benefit, but also to create a better world. To help clinicians better understand what the term spirituality implies, whether for themselves or for their patients, I offer the acronym SPIRITT to describe core components of varied spiritual perspectives, beliefs, and practices.
Sacred. Considering certain aspects of life, time, or place as non-ordinary and worthy of reverence and awe.
Presence. Cultivating an inner presence that is open, accepting, compassionate, and loving toward others. During a spiritual experience, some may feel embraced in this way by a presence outside of themselves, such as an encounter with a spiritual teacher or an experience of feeling held lovingly by a transcendent power.
Interconnection. Understanding that we are not separate entities but are interconnected beings existing in interdependent unity, starting with our families and extending out universally. According to this perspective, harming anything or anyone is doing harm to ourself.
Rest. Taking a Sabbath or unplugging. Dedicating time each week for resting your mind and body. Spending quality time with family. Decreasing excessive stimulation and loosening the grip of consumerism.
Introspection. Looking inwardly. Eastern traditions emphasize deepening self-awareness through mindful meditation practices, while Western traditions include taking a personal inventory through self-examination or confessional practices.
Continue to: Traditions
Traditions. Studying sacred texts, participating in communal prayer, meditating, or engaging in rituals. This requires sorting through outmoded beliefs and ways of thinking while updating beliefs that are compatible with our lived experiences.
Transcendence. Experiencing moments, whether through nature, music, dance, ritual, prayer, art, etc., in which the narrow sense of being a separate self fades away and there is a deeper sense of a larger connection and belonging that is transpersonal, timeless, and expansive.
The components of SPIRITT have helped me to think about and pursue the physical, emotional, and social benefits of adopting a spiritual practice for my well-being as well as for the benefit of my patients.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
1. Koenig HG. Religion, spirituality, and health: a review and update. Adv Mind Body Med. 2015;29(3):19-26.
2. Spiritual. Miriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/spiritual. Accessed May 9, 2019.
When your patient is a physician: Overcoming the challenges
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
Physicians’ physical and mental well-being has become a major concern in health care. In the United States, an estimated 300 to 400 physicians die from suicide each year.1 Compared with the general population, the suicide rates for male and female physicians are 1.41 and 2.27 times higher, respectively.2 As psychiatrists, we can play an instrumental role in preserving our colleagues’ mental health. While treating a fellow physician can be rewarding, these situations also can be challenging. Here we describe a few of the challenges of treating physicians, and solutions we can employ to minimize potential pitfalls.
Challenges: How our relationship can affect care
We may view physician-patients as “VIPs” because of their profession, which might lead us to assume they are more knowledgeable than the average patient.1,3 This mindset could result in taking an inadequate history, having an incomplete informed-consent discussion, avoiding or limiting educational discussions, performing an inadequate suicide risk assessment, or underestimating the need for higher levels of care (eg, psychiatric hospitalization).1
We may have difficulty maintaining appropriate professional boundaries due to the relationship (eg, friend, colleague, or mentor) we have established with a physician-patient.3 It may be difficult to establish the usual roles of patient and physician, particularly if we have a professional relationship with a physician-patient that requires routine contact at work. The issue of boundaries can become compounded if there is an emotional component to the relationship, which may make it difficult to discuss sensitive topics.3 A physician-patient may be reluctant to discuss sensitive information due to concerns about the confidentiality of their medical record.3 They also might obtain our personal contact information through work-related networks and use it to contact us about their care.
Solutions: Treat them as you would any other patient
Although physician-patients may have more medical knowledge than other patients, we should avoid showing deference and making assumptions about their knowledge of psychiatric illnesses and treatment. As we would with other patients, we should always1:
- conduct a thorough evaluation
- develop a comprehensive treatment plan
- provide appropriate informed consent
- adequately assess suicide risk.
We should also maintain boundaries as best we can, while understanding that our professional relationships might complicate this.
We should ask our physician-patients if they have been self-prescribing and/or self-treating.1 We shouldn’t shy away from considering inpatient treatment for physician-patients (when clinically indicated) because of our concern that such treatment might jeopardize their ability to practice medicine. Also, to help decrease barriers to and enhance engagement in treatment, consider recommending treatment options that can take place outside of the physician-patient’s work environment.3
Continue to: We should provide...
We should provide the same confidentiality considerations to physician-patients as we do to other patients. However, at times, we may need to break confidentiality for safety concerns or reporting that is required by law. We may have to contact a state licensing board if a physician-patient continues to practice while impaired despite engaging in treatment.1 We should understand the procedures for reporting; have referral resources available for these patients, such as recovering physician programs; and know whom to contact for further counsel, such as risk management or legal teams.1
The best way to provide optimal psychiatric care to a physician colleague is to acknowledge the potential challenges at the onset of treatment, and work collaboratively to avoid the potential pitfalls during the course of treatment.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
1. Fischer-Sanchez D. Risk management considerations when treating fellow physicians. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.7a21. Published July 3, 2018. Accessed May 9, 2019.
Caring for patients on probation or parole
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Agitation in children and adolescents: Diagnostic and treatment considerations
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
Unipolar vs bipolar depression: A clinician’s perspective
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Preventing Delirium Takes a Village: Systematic Review and Meta-Analysis of Delirium Preventive Models of Care
Delirium presents as an acute change in mentation characterized by reduced attention, clouding of awareness, and typically an altered level of arousal. It can be caused by a host of medical conditions, medications, or other psychoactive substances and is therefore encountered primarily in acute and postacute medical settings.1 More than a quarter of all hospitalized patients develop delirium,2 with rates up to 80% in the critically ill.3 Similarly, delirium occurs in more than one-third of patients who transition to postacute care.4 These high prevalence rates are alarming, especially because delirium is a risk factor for mortality, prolonged hospitalization, institutionalization, and overall higher cost of care.5 However, more than a quarter of delirium is preventable.6 Evidence-based guidelines for delirium uniformly call for multicomponent prevention strategies,7 and these are best delivered through collaborative models of care. In short, delirium impacts healthcare systems; therefore, interventions aimed at preventing delirium and its consequences ought to be systems-based.
Since the Institute of Medicine issued its 1999 report highlighting the critical role of medical errors in healthcare, healthcare systems have increasingly become team-based.8 “Medical care is inherently interdependent,”9 and this implies that delirium prevention rests not only on individuals but also on broader systems of care. Although nonpharmacological interventions are efficacious at preventing delirium,10 previous reviews have focused on specific interventions or multiple interventions rather than the systems of care needed to deliver them. Indeed, teams and the quality of their teamwork impact outcomes.11
Herein, we provide a systematic review and meta-analysis of integrated models of care designed to prevent delirium. What distinguishes this review from previous reviews of nonpharmacological interventions to prevent delirium is our focus on discrete models of care that involve collaboration among clinicians. Our goal is to identify the most promising models that deserve further development, investigation, and dissemination. Viewing delirium prevention through a collaborative care lens is consistent with efforts to achieve value-based care and may encourage drawing from the expanding literature outlining the benefits of mental healthcare integration.12,13 Specifically, a systems perspective highlights the potential for system-wide benefits such as reducing readmissions14,15 and cost savings.16
METHODS
This systematic review and meta-analysis follows PRISMA guidelines. A search of OVID, MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, EMBASE, and PsycINFO was completed by a medical librarian for clinical studies in which models of care were implemented to prevent delirium using PICO (P patient, problem or population; I, intervention; C, comparison, control or comparator; O, outcome) inquiries. Search terms included delirium, acute confusional state, altered mental status, prevention, and control (“delirium”/exp OR “acute confusion”/exp OR “altered mental status”/exp) AND “prevention and control”/exp AND [English]/lim AND [embase]/lim).
One researcher (AK) screened articles by title for relevance. Relevant articles were then divided among four authors (AK, MO, NF, and OB), and the abstracts were screened for eligibility. The authors reviewed the full texts of any potentially eligible studies. Each full text was assigned to two authors for full review. Discrepancies were adjudicated by conference among all authors. In addition, references within all full-text publications were scanned for potential additional articles.
The inclusion criteria for review of full-text articles required English-language description of a model of care with multiple interventions, delirium reported as an outcome, and presence of a comparator group.
“Model of care” was defined by the Cochrane Effective Practice and Organization of Care Review Group as follows: (1) revision of professional roles, including shifting of professional roles or expansion of roles to new tasks; (2) creation of clinical multidisciplinary teams or addition of new members to the team who collaborate inpatient care; (3) delivery of multiple interventions across multiple domains (ie, studies involving a single intervention such as physical therapy or targeting a single domain such as sleep were excluded); and (4) formal integration of services whereby teams work together in collaboration with existing services to enhance care.17 For this review, we required that studies include a comparator group so that effectiveness of the intervention could be assessed. Quality improvement studies that lacked a comparator group were excluded.
Delirium incidence was the primary outcome and was evaluated by meta-analysis. Heterogeneity was assessed using I2 and visual inspection of forest plots. I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The studies were pooled according to study type as follows: randomized controlled trials, pre–post design, and other nonrandomized prospective studies. Random effects models were used to calculate estimates using the Comprehensive Meta-Analysis software (Version 3, Biostat, Englewood, New Jersey), which also generated forest plots.
Risk of bias was assessed using criteria established by the Cochrane Collaborative Review Criteria, which lists six categories of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.17 Each study was assessed by two authors (either MO and AK or MO-P. and OB) for bias and a numerical value was assigned to each of the six categories as follows: 1 = low risk, 2 = unknown/moderate risk, and 3 = high risk. Where scorers disagreed, all authors jointly conferred, and a consensus score was given. The values for each of these six categories were added to create a composite risk-of-bias score for each study, with 6 being the lowest possible score and 18 the highest. Overall risk was classified as follows: <9 = low risk, 9-12 = moderate risk, and >12 = high risk.
RESULTS
Study Selection Process
An initial literature search identified 352 articles. After reviewing the titles, 308 articles were excluded for irrelevance, and 44 abstracts were screened for eligibility. We excluded 27 articles upon abstract review, and the full texts of 17 were obtained for detailed review. In addition, we identified another 10 potentially eligible articles through review of references and obtained full texts of these as well. Of the 27 full-text articles reviewed, 15 were included in this systematic review, 10 of which were suitable for meta-analysis. The Figure shows the PRISMA flow chart.
Study Characteristics
The 15 studies that met the inclusion criteria are summarized in the Table.18-32 Delirium prevention was among the primary outcomes of 13 studies; delirium outcomes were reported in the other two studies as well, which were primarily designed to assess feasibility.26,27 Six studies were conducted in the United States, three in Sweden, two in Spain, two in the United Kingdom, and one each conducted in Korea and Canada. Healthcare settings among the included studies involved the intensive care unit (six studies), medical floors (four studies), surgical floors (three studies), a long-term care unit (one study), and
Outcomes Reported
All but one of the studies reported delirium incidence. The most commonly used delirium screening instrument was the Confusion Assessment Method (CAM) or its modified version, the CAM-ICU (11 studies).33,34 Other methods used to assess mentation included the Richmond Agitation Sedation Scale,35 the Organic Brain Syndrome scale,36 the revised Delirium Rating Scale,37 the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,38 and the Confusion Rating Scale.39 (Details regarding delirium screening tools can be found in the systematic review by De and Wand.40) Researchers performed delirium assessment in nine studies, whereas assessments were performed by clinical staff in the remaining studies. Other outcomes reported included length of stay (LOS), mortality, number of days ventilated, and functional decline. None of the included studies reported cost effectiveness.
Risk of Bias Assessment
Risk of bias assessment identified only two studies—both randomized controlled trials—as low risk (Table). The remaining studies had moderate (four studies) or high risk (nine studies).
Results from Individual Studies
Of the 15 studies, nine reported a statistically significant reduction in delirium incidence, and another two reported a statistically insignificant reduction. In addition, seven of the eight studies that assessed delirium duration found reduced duration in the intervention cohort, and two of the three studies that reported delirium severity found a reduction in the intervention group.
Results of Meta-Analysis
Random effects models were created to meta-analyze groups of studies based on design as follows: randomized controlled trials (three studies18,19,25), pre–post intervention studies (four of six studies included28-31), and other nonrandomized studies (three of four studies included21-23). Meta-analysis was not completed for the two feasibility studies26,27 because delirium outcome data were limited due to the feasibility study design. The study of Dale et al.32 was excluded from the meta-analysis because the rates of CAM-ICU completion differed substantially between control and intervention groups (0.35 vs 1.49 per 24 hours, respectively), leading to imbalanced between-group sensitivity in delirium detection and Needham et al.20 was also excluded because it reported only days of delirium, not delirium incidence. The study by Lundström et al.24 was also excluded from the meta-analysis because delirium incidence was measured on days 1, 3, and 5, whereas the other studies reported delirium daily.
Meta-analysis of the three randomized controlled trials revealed a pooled odds ratio of 0.56 (95% CI: 0.37-0.85; P = .006) for delirium incidence among intervention group subjects relative to those in comparator groups. The heterogeneity across studies was low (I2 = 29%). Pooling data from four pre–post studies found that the odds ratio for delirium incidence was 0.63 (95% CI: 0.37-1.07; P = .09). The heterogeneity across these studies was moderate (I2 = 65%). Results from the three eligible, nonrandomized prospective studies were also pooled. The odds ratio for developing delirium among study subjects was 0.79 (95% CI: 0.46-1.37; P = .40), and the heterogeneity among these studies was high (I2 = 85%).
DISCUSSION
We provide a systematic review and meta-analysis of delirium preventive models of care. Meta-analysis of the three randomized controlled trials found that these models of care led to a statistically significant reduction in delirium incidence; study subjects had an 11.5% reduction in absolute delirium incidence. The pooled odds ratios for both of the other sets of nonrandomized studies favored the intervention group but were not significant, each because of one included study. The pre–post meta-analysis failed to reach significance as one of the included studies found a trend toward higher delirium incidence; however, interestingly, in that same study, the overall delirium-free days were significantly reduced overall (24 vs 27, P = .002). Similarly, meta-analysis of the three additional nonrandomized prospective studies failed to reach significance because the largest included study found higher rates of delirium among intervention group subjects. Despite considerable risk of bias in several of these studies, their findings were broadly consistent; all but one study (Gagnon 201221) reported a trend or a significant reduction in delirium incidence, duration, severity, or number of delirium episodes. Moreover, the value of such models of care extended beyond preventing delirium; for instance, other positive outcomes included reduced LOS and fewer medical complications.
Models of care ranged widely with respect to specific interventions, though several common elements highlighted their relevance for delirium care and as potential delirium prevention strategies in future studies. For example, two of the randomized controlled trials18,19 employed early mobilization, enhanced nutrition, sleep hygiene, early reduction of invasive procedures (eg, urinary catheterization), and pain control in their multicomponent models. Five additional studies also incorporated early mobilization,20,22,23,31,32 and three sought to improve sleep quality.22,28,30 Among other important strategies were delirium screening,18,20,22,30,31 monitoring medication,18,20,22,26,28,30,32 orientation,18,21,23,28 addressing vision and hearing impairment,18,22,23,32 hydration,18,22,23 avoiding hypoxia,18,20,30 and staff, patient, and caretaker education.19,21,23,27-30 Unique strategies were implemented in certain studies. For instance, one study used massage therapy,28 preventing delays in transfer logistics in another,30 and a third addressed psychosocial problems.25 Overall, the selection of strategies depended on the patient setting; thus, no one care bundle should be expected to emerge as a universal model for delirium prevention. Rather, these results should be interpreted within their specific care contexts and judged on the quality of evidence (eg, effect size and statistically significant findings, low risk of bias, sound experimental design). The one study that failed to find any positive effect on delirium, that of Gagnon et al.,21 was conducted on an inpatient palliative care service in Canada, and its negative finding may reflect the unique delirium risk factors in patients who are nearing end of life.
This current review differs from previous delirium prevention reviews in operationally defining a “model of care.” We identified a great deal of variation in specific models and team composition. For example, some interventions were carried out by nurses18-20,31 and physicians,20,21,25,32 whereas others involved physical therapists,20,22,28 medical residents,23 geriatricians,22,23,25 pharmacists,26 researchers,18 and trained volunteers.22 In all cases, the staff roles were expanded to include new tasks, and the clinical team worked collaboratively to administer interventions across multiple domains. Team-related considerations are critical because modern medical care is inherently interdependent.9 These broad differences in team composition across studies demonstrate the number of potential options for team structure and function. They also highlight the number of “moving parts” to be considered when designing and implementing delirium care bundles.
Most of the delirium prevention studies implementing models of care are characterized by a substantial risk of bias. We evaluated risk of bias along six categories of potential sources, including random assignment to groups, ability to foresee future group allocation, blinding of participants and personnel to group assignment, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias.17 Two of the three studies that used randomization had a low risk of bias, and four additional studies had a moderate risk of bias. Allocation concealment was accomplished only in randomized controlled trials, whereas blinding of both subjects and study personnel was not implemented in any of the studies. Although some studies relied on data analysis by research personnel blinded to group membership or the nature of the intervention, others failed to do so or failed to describe data analysis in sufficient detail. Studies also failed to report the percentage of unscorable or otherwise omitted delirium assessments necessary to calculate attrition rates or to understand the comprehensiveness of outcome assessment in a systematic manner. Other potential sources of bias included systematic differences between the intervention and control groups (such as differences in gender composition, age, or delirium risk) at study outset.
A primary limitation of this review is the heterogeneity of settings, interventions, and models of care across included studies. We excluded several studies from this review for being delivered by a single individual or service line (eg, introduction of a geriatric consult service, physical therapy, or volunteers), for providing a single intervention (eg, early ambulation alone), or for multiple interventions targeting a single domain (eg, sleep). We did so because the future of value-based care lies in collaboration of providers and services, and in a way the complexity across and within these studies ultimately reflects the complexity of medical settings as well as the multifactorial nature of delirium. The broader message is a call for increasing the integration of delirium-related care services. As discussed earlier, the high risk of bias across these studies is a limitation of our findings; high-quality evidence on the value of delirium prevention models of care remains limited. Thus, although our review suggests that there are multicomponent models of care that hold promise in mitigating delirium and its outcomes, additional randomized studies are required to confirm the efficacy of such models of care and to test which services, interventions, and clinical domains deserve priority.
CONCLUSION
To our knowledge, this is the first systematic review and meta-analysis of delirium preventive models of care. Models of care, as defined here, necessarily included a multidisciplinary team in which traditional staff roles had been revised to implement a multicomponent, multidomain intervention. Other recent reviews are available for multicomponent pharmacological and nonpharmacological interventions to prevent and manage delirium,41-49 but just as important as which interventions are being delivered is the team that delivers them. Care delivery in a complex medical system is more than handing a patient a medication or facilitating ambulation; it requires a choreographed dance of teamwork and integration across services. This review identifies promising models of care that deserve further recognition, refinement, and ultimately widespread implementation.
Acknowledgments
The authors comprise a writing group created through the Delirium Boot Camp, an annual meeting originally sponsored by the Center of Excellence for Delirium in Aging: Research, Training, and Educational Enhancement (CEDARTREE, Boston, Massachusetts); it is currently supported by the Network for Investigation of Delirium: Unifying Scientists (NIDUS, Boston, Massachusetts). The authors would like to thank medical librarian Rita Mitchell (Aurora Health Care, Milwaukee, Wisconsin) for the literature search, senior scientific writer and editor Joe Grundle (Aurora Research Institute, Milwaukee, Wisconsin) for editorial assistance, and graphics specialist Brian Miller (Aurora Research Institute, Milwaukee, Wisconsin) for help with the figures.
Disclosures
The authors report no relevant conflicts of interest.
Funding
No funding was dedicated to the conduct of this review.
1. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc.
2. Schubert M, Schürch R, Boettger S, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - A cohort study. BMC Health Serv Res. 2018;18(1):550. https://doi.org/10.1186/s12913-018-3345-x.
3. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the Intensive Care Unit. JAMA. 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753.
4. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1-2):121-129. https://doi.org/10.1159/000485794.
5. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1.
7. Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: A systematic appraisal. BMJ Open. 2017;7(3):e013809. https://doi.org/10.1136/bmjopen-2016-013809.
8. Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
9. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am Psychol. 2018;73(4):433-450. https://doi.org/10.1037/amp0000298.
10. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLOS ONE. 2015;10(6):e0123090. https://doi.org/10.1371/journal.pone.0123090.
11. Thomas EJ. Improving teamwork in healthcare: current approaches and the path forward. BMJ Qual Saf. 2011;20(8):647-650. https://doi.org/10.1136/bmjqs-2011-000117.
12. Sledge W, Bozzo J, White-McCullum B, Lee H. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome -Res. 2016;2:2-6.
13. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. PubMed
14. Lee E, Kim J. Cost-benefit analysis of a delirium prevention strategy in the intensive care unit. Nurs Crit Care. 2014;21:367-373. https://doi.org/10.1111/nicc.12124.
15. Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the hospital elder life program on risk of 30-day readmission. J Am Geriatr Soc. 2018;66(1):145-149. https://doi.org/10.1111/jgs.15132.
16. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219-226. https://doi.org/10.1016/j.psym.2013.01.010.
17. Cochrane effective practice and organisation of Care Group (EPOC). Data collection Checklistist. Chochrane Effective Practice and Organisation of Care Group (EPOC) Methods Papers. . https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC Data Collection Checklist.pdf. Accessed May 27, 2014.
18. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: A randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423-1432. https://doi.org/10.1016/j.ijnurstu.2015.04.021.
19. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res-. 2007;19(3):178-186. https://doi.org/10.1007/BF03324687.
20. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients With acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. https://doi.org/10.1016/j.apmr.2010.01.002.
21. Gagnon P, Allard P, Gagnon B, Mérette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187-194. https://doi.org/10.1002/pon.1881.
22. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
23. Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029-2036. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
24. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
25. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
26. Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32(1):E1-E10. https://doi.org/10.1097/JCN.0000000000000356.
27. Siddiqi N, Cheater F, Collinson M, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652-661. https://doi.org/10.1093/ageing/afw091.
28. Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit (SICU) improved the outcomes of older adults. J Surg Res. 2014;186:519. https://doi.org/10.1016/j.jss.2013.11.352
29. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721-727. https://doi.org/10.1093/ageing/aft120.
30. Björkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol-Scand. 2010;54(6):678-688. https://doi.org/10.1111/j.1399-6576.2010.02232.x.
31. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility (ABCDE) bundle. Crit Care Med. 2014;42(5):1024-1036. https://doi.org/10.1097/CCM.0000000000000129.
32. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367-374. https://doi.org/10.1513/AnnalsATS.201306-210OC.
33. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941.
34. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. https://doi.org/10.1097/00003246-200107000-00012.
35. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. https://doi.org/10.1164/rccm.2107138.
36. Jensen E, Dehlin O, Gustafson L. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry. 1993;8(3):215-229. https://doi.org/10.1002/gps.930080305.
37. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13(2):229-242. https://doi.org/10.1176/jnp.13.2.229.
38. American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc.
39. Williams MA. Delirium/acute confusional states: evaluation devices in nursing. Int Psychogeriatr. 1991;3(2):301-308. PubMed
40. De J, Wand APF. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist-. 2015;55(6):1079-1099. https://doi.org/10.1093/geront/gnv100.
41. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological,
multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. https://doi.org/10.1093/ageing/afu173.
42. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):375-380. https://doi.org/10.7326/0003-4819-158-5-201303051-00003.
43. Rivosecchi RM, Smithburger PL, Svec S, Campbell S, Kane-Gill SL. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35(1):39-50; quiz 51. https://doi.org/10.4037/ccn2015423.
44. Trogrlić Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. https://doi.org/10.1186/s13054-015-0886-9.
45. Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017;96(26):e7361. https://doi.org/10.1097/MD.0000000000007361.
46. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium After hip fracture: A systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559-1565. https://doi.org/10.1111/jgs.14846.
47. Oberai T, Lizarondo L, Ruurd J. Effectiveness of multi-component interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2017;15(2):259-268. https://doi.org/10.11124/JBISRIR-2016-002943.
48. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016;31(2):127-141. https://doi.org/10.1177/0885066614553925.
49. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological
delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779.
Delirium presents as an acute change in mentation characterized by reduced attention, clouding of awareness, and typically an altered level of arousal. It can be caused by a host of medical conditions, medications, or other psychoactive substances and is therefore encountered primarily in acute and postacute medical settings.1 More than a quarter of all hospitalized patients develop delirium,2 with rates up to 80% in the critically ill.3 Similarly, delirium occurs in more than one-third of patients who transition to postacute care.4 These high prevalence rates are alarming, especially because delirium is a risk factor for mortality, prolonged hospitalization, institutionalization, and overall higher cost of care.5 However, more than a quarter of delirium is preventable.6 Evidence-based guidelines for delirium uniformly call for multicomponent prevention strategies,7 and these are best delivered through collaborative models of care. In short, delirium impacts healthcare systems; therefore, interventions aimed at preventing delirium and its consequences ought to be systems-based.
Since the Institute of Medicine issued its 1999 report highlighting the critical role of medical errors in healthcare, healthcare systems have increasingly become team-based.8 “Medical care is inherently interdependent,”9 and this implies that delirium prevention rests not only on individuals but also on broader systems of care. Although nonpharmacological interventions are efficacious at preventing delirium,10 previous reviews have focused on specific interventions or multiple interventions rather than the systems of care needed to deliver them. Indeed, teams and the quality of their teamwork impact outcomes.11
Herein, we provide a systematic review and meta-analysis of integrated models of care designed to prevent delirium. What distinguishes this review from previous reviews of nonpharmacological interventions to prevent delirium is our focus on discrete models of care that involve collaboration among clinicians. Our goal is to identify the most promising models that deserve further development, investigation, and dissemination. Viewing delirium prevention through a collaborative care lens is consistent with efforts to achieve value-based care and may encourage drawing from the expanding literature outlining the benefits of mental healthcare integration.12,13 Specifically, a systems perspective highlights the potential for system-wide benefits such as reducing readmissions14,15 and cost savings.16
METHODS
This systematic review and meta-analysis follows PRISMA guidelines. A search of OVID, MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, EMBASE, and PsycINFO was completed by a medical librarian for clinical studies in which models of care were implemented to prevent delirium using PICO (P patient, problem or population; I, intervention; C, comparison, control or comparator; O, outcome) inquiries. Search terms included delirium, acute confusional state, altered mental status, prevention, and control (“delirium”/exp OR “acute confusion”/exp OR “altered mental status”/exp) AND “prevention and control”/exp AND [English]/lim AND [embase]/lim).
One researcher (AK) screened articles by title for relevance. Relevant articles were then divided among four authors (AK, MO, NF, and OB), and the abstracts were screened for eligibility. The authors reviewed the full texts of any potentially eligible studies. Each full text was assigned to two authors for full review. Discrepancies were adjudicated by conference among all authors. In addition, references within all full-text publications were scanned for potential additional articles.
The inclusion criteria for review of full-text articles required English-language description of a model of care with multiple interventions, delirium reported as an outcome, and presence of a comparator group.
“Model of care” was defined by the Cochrane Effective Practice and Organization of Care Review Group as follows: (1) revision of professional roles, including shifting of professional roles or expansion of roles to new tasks; (2) creation of clinical multidisciplinary teams or addition of new members to the team who collaborate inpatient care; (3) delivery of multiple interventions across multiple domains (ie, studies involving a single intervention such as physical therapy or targeting a single domain such as sleep were excluded); and (4) formal integration of services whereby teams work together in collaboration with existing services to enhance care.17 For this review, we required that studies include a comparator group so that effectiveness of the intervention could be assessed. Quality improvement studies that lacked a comparator group were excluded.
Delirium incidence was the primary outcome and was evaluated by meta-analysis. Heterogeneity was assessed using I2 and visual inspection of forest plots. I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The studies were pooled according to study type as follows: randomized controlled trials, pre–post design, and other nonrandomized prospective studies. Random effects models were used to calculate estimates using the Comprehensive Meta-Analysis software (Version 3, Biostat, Englewood, New Jersey), which also generated forest plots.
Risk of bias was assessed using criteria established by the Cochrane Collaborative Review Criteria, which lists six categories of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.17 Each study was assessed by two authors (either MO and AK or MO-P. and OB) for bias and a numerical value was assigned to each of the six categories as follows: 1 = low risk, 2 = unknown/moderate risk, and 3 = high risk. Where scorers disagreed, all authors jointly conferred, and a consensus score was given. The values for each of these six categories were added to create a composite risk-of-bias score for each study, with 6 being the lowest possible score and 18 the highest. Overall risk was classified as follows: <9 = low risk, 9-12 = moderate risk, and >12 = high risk.
RESULTS
Study Selection Process
An initial literature search identified 352 articles. After reviewing the titles, 308 articles were excluded for irrelevance, and 44 abstracts were screened for eligibility. We excluded 27 articles upon abstract review, and the full texts of 17 were obtained for detailed review. In addition, we identified another 10 potentially eligible articles through review of references and obtained full texts of these as well. Of the 27 full-text articles reviewed, 15 were included in this systematic review, 10 of which were suitable for meta-analysis. The Figure shows the PRISMA flow chart.
Study Characteristics
The 15 studies that met the inclusion criteria are summarized in the Table.18-32 Delirium prevention was among the primary outcomes of 13 studies; delirium outcomes were reported in the other two studies as well, which were primarily designed to assess feasibility.26,27 Six studies were conducted in the United States, three in Sweden, two in Spain, two in the United Kingdom, and one each conducted in Korea and Canada. Healthcare settings among the included studies involved the intensive care unit (six studies), medical floors (four studies), surgical floors (three studies), a long-term care unit (one study), and
Outcomes Reported
All but one of the studies reported delirium incidence. The most commonly used delirium screening instrument was the Confusion Assessment Method (CAM) or its modified version, the CAM-ICU (11 studies).33,34 Other methods used to assess mentation included the Richmond Agitation Sedation Scale,35 the Organic Brain Syndrome scale,36 the revised Delirium Rating Scale,37 the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,38 and the Confusion Rating Scale.39 (Details regarding delirium screening tools can be found in the systematic review by De and Wand.40) Researchers performed delirium assessment in nine studies, whereas assessments were performed by clinical staff in the remaining studies. Other outcomes reported included length of stay (LOS), mortality, number of days ventilated, and functional decline. None of the included studies reported cost effectiveness.
Risk of Bias Assessment
Risk of bias assessment identified only two studies—both randomized controlled trials—as low risk (Table). The remaining studies had moderate (four studies) or high risk (nine studies).
Results from Individual Studies
Of the 15 studies, nine reported a statistically significant reduction in delirium incidence, and another two reported a statistically insignificant reduction. In addition, seven of the eight studies that assessed delirium duration found reduced duration in the intervention cohort, and two of the three studies that reported delirium severity found a reduction in the intervention group.
Results of Meta-Analysis
Random effects models were created to meta-analyze groups of studies based on design as follows: randomized controlled trials (three studies18,19,25), pre–post intervention studies (four of six studies included28-31), and other nonrandomized studies (three of four studies included21-23). Meta-analysis was not completed for the two feasibility studies26,27 because delirium outcome data were limited due to the feasibility study design. The study of Dale et al.32 was excluded from the meta-analysis because the rates of CAM-ICU completion differed substantially between control and intervention groups (0.35 vs 1.49 per 24 hours, respectively), leading to imbalanced between-group sensitivity in delirium detection and Needham et al.20 was also excluded because it reported only days of delirium, not delirium incidence. The study by Lundström et al.24 was also excluded from the meta-analysis because delirium incidence was measured on days 1, 3, and 5, whereas the other studies reported delirium daily.
Meta-analysis of the three randomized controlled trials revealed a pooled odds ratio of 0.56 (95% CI: 0.37-0.85; P = .006) for delirium incidence among intervention group subjects relative to those in comparator groups. The heterogeneity across studies was low (I2 = 29%). Pooling data from four pre–post studies found that the odds ratio for delirium incidence was 0.63 (95% CI: 0.37-1.07; P = .09). The heterogeneity across these studies was moderate (I2 = 65%). Results from the three eligible, nonrandomized prospective studies were also pooled. The odds ratio for developing delirium among study subjects was 0.79 (95% CI: 0.46-1.37; P = .40), and the heterogeneity among these studies was high (I2 = 85%).
DISCUSSION
We provide a systematic review and meta-analysis of delirium preventive models of care. Meta-analysis of the three randomized controlled trials found that these models of care led to a statistically significant reduction in delirium incidence; study subjects had an 11.5% reduction in absolute delirium incidence. The pooled odds ratios for both of the other sets of nonrandomized studies favored the intervention group but were not significant, each because of one included study. The pre–post meta-analysis failed to reach significance as one of the included studies found a trend toward higher delirium incidence; however, interestingly, in that same study, the overall delirium-free days were significantly reduced overall (24 vs 27, P = .002). Similarly, meta-analysis of the three additional nonrandomized prospective studies failed to reach significance because the largest included study found higher rates of delirium among intervention group subjects. Despite considerable risk of bias in several of these studies, their findings were broadly consistent; all but one study (Gagnon 201221) reported a trend or a significant reduction in delirium incidence, duration, severity, or number of delirium episodes. Moreover, the value of such models of care extended beyond preventing delirium; for instance, other positive outcomes included reduced LOS and fewer medical complications.
Models of care ranged widely with respect to specific interventions, though several common elements highlighted their relevance for delirium care and as potential delirium prevention strategies in future studies. For example, two of the randomized controlled trials18,19 employed early mobilization, enhanced nutrition, sleep hygiene, early reduction of invasive procedures (eg, urinary catheterization), and pain control in their multicomponent models. Five additional studies also incorporated early mobilization,20,22,23,31,32 and three sought to improve sleep quality.22,28,30 Among other important strategies were delirium screening,18,20,22,30,31 monitoring medication,18,20,22,26,28,30,32 orientation,18,21,23,28 addressing vision and hearing impairment,18,22,23,32 hydration,18,22,23 avoiding hypoxia,18,20,30 and staff, patient, and caretaker education.19,21,23,27-30 Unique strategies were implemented in certain studies. For instance, one study used massage therapy,28 preventing delays in transfer logistics in another,30 and a third addressed psychosocial problems.25 Overall, the selection of strategies depended on the patient setting; thus, no one care bundle should be expected to emerge as a universal model for delirium prevention. Rather, these results should be interpreted within their specific care contexts and judged on the quality of evidence (eg, effect size and statistically significant findings, low risk of bias, sound experimental design). The one study that failed to find any positive effect on delirium, that of Gagnon et al.,21 was conducted on an inpatient palliative care service in Canada, and its negative finding may reflect the unique delirium risk factors in patients who are nearing end of life.
This current review differs from previous delirium prevention reviews in operationally defining a “model of care.” We identified a great deal of variation in specific models and team composition. For example, some interventions were carried out by nurses18-20,31 and physicians,20,21,25,32 whereas others involved physical therapists,20,22,28 medical residents,23 geriatricians,22,23,25 pharmacists,26 researchers,18 and trained volunteers.22 In all cases, the staff roles were expanded to include new tasks, and the clinical team worked collaboratively to administer interventions across multiple domains. Team-related considerations are critical because modern medical care is inherently interdependent.9 These broad differences in team composition across studies demonstrate the number of potential options for team structure and function. They also highlight the number of “moving parts” to be considered when designing and implementing delirium care bundles.
Most of the delirium prevention studies implementing models of care are characterized by a substantial risk of bias. We evaluated risk of bias along six categories of potential sources, including random assignment to groups, ability to foresee future group allocation, blinding of participants and personnel to group assignment, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias.17 Two of the three studies that used randomization had a low risk of bias, and four additional studies had a moderate risk of bias. Allocation concealment was accomplished only in randomized controlled trials, whereas blinding of both subjects and study personnel was not implemented in any of the studies. Although some studies relied on data analysis by research personnel blinded to group membership or the nature of the intervention, others failed to do so or failed to describe data analysis in sufficient detail. Studies also failed to report the percentage of unscorable or otherwise omitted delirium assessments necessary to calculate attrition rates or to understand the comprehensiveness of outcome assessment in a systematic manner. Other potential sources of bias included systematic differences between the intervention and control groups (such as differences in gender composition, age, or delirium risk) at study outset.
A primary limitation of this review is the heterogeneity of settings, interventions, and models of care across included studies. We excluded several studies from this review for being delivered by a single individual or service line (eg, introduction of a geriatric consult service, physical therapy, or volunteers), for providing a single intervention (eg, early ambulation alone), or for multiple interventions targeting a single domain (eg, sleep). We did so because the future of value-based care lies in collaboration of providers and services, and in a way the complexity across and within these studies ultimately reflects the complexity of medical settings as well as the multifactorial nature of delirium. The broader message is a call for increasing the integration of delirium-related care services. As discussed earlier, the high risk of bias across these studies is a limitation of our findings; high-quality evidence on the value of delirium prevention models of care remains limited. Thus, although our review suggests that there are multicomponent models of care that hold promise in mitigating delirium and its outcomes, additional randomized studies are required to confirm the efficacy of such models of care and to test which services, interventions, and clinical domains deserve priority.
CONCLUSION
To our knowledge, this is the first systematic review and meta-analysis of delirium preventive models of care. Models of care, as defined here, necessarily included a multidisciplinary team in which traditional staff roles had been revised to implement a multicomponent, multidomain intervention. Other recent reviews are available for multicomponent pharmacological and nonpharmacological interventions to prevent and manage delirium,41-49 but just as important as which interventions are being delivered is the team that delivers them. Care delivery in a complex medical system is more than handing a patient a medication or facilitating ambulation; it requires a choreographed dance of teamwork and integration across services. This review identifies promising models of care that deserve further recognition, refinement, and ultimately widespread implementation.
Acknowledgments
The authors comprise a writing group created through the Delirium Boot Camp, an annual meeting originally sponsored by the Center of Excellence for Delirium in Aging: Research, Training, and Educational Enhancement (CEDARTREE, Boston, Massachusetts); it is currently supported by the Network for Investigation of Delirium: Unifying Scientists (NIDUS, Boston, Massachusetts). The authors would like to thank medical librarian Rita Mitchell (Aurora Health Care, Milwaukee, Wisconsin) for the literature search, senior scientific writer and editor Joe Grundle (Aurora Research Institute, Milwaukee, Wisconsin) for editorial assistance, and graphics specialist Brian Miller (Aurora Research Institute, Milwaukee, Wisconsin) for help with the figures.
Disclosures
The authors report no relevant conflicts of interest.
Funding
No funding was dedicated to the conduct of this review.
Delirium presents as an acute change in mentation characterized by reduced attention, clouding of awareness, and typically an altered level of arousal. It can be caused by a host of medical conditions, medications, or other psychoactive substances and is therefore encountered primarily in acute and postacute medical settings.1 More than a quarter of all hospitalized patients develop delirium,2 with rates up to 80% in the critically ill.3 Similarly, delirium occurs in more than one-third of patients who transition to postacute care.4 These high prevalence rates are alarming, especially because delirium is a risk factor for mortality, prolonged hospitalization, institutionalization, and overall higher cost of care.5 However, more than a quarter of delirium is preventable.6 Evidence-based guidelines for delirium uniformly call for multicomponent prevention strategies,7 and these are best delivered through collaborative models of care. In short, delirium impacts healthcare systems; therefore, interventions aimed at preventing delirium and its consequences ought to be systems-based.
Since the Institute of Medicine issued its 1999 report highlighting the critical role of medical errors in healthcare, healthcare systems have increasingly become team-based.8 “Medical care is inherently interdependent,”9 and this implies that delirium prevention rests not only on individuals but also on broader systems of care. Although nonpharmacological interventions are efficacious at preventing delirium,10 previous reviews have focused on specific interventions or multiple interventions rather than the systems of care needed to deliver them. Indeed, teams and the quality of their teamwork impact outcomes.11
Herein, we provide a systematic review and meta-analysis of integrated models of care designed to prevent delirium. What distinguishes this review from previous reviews of nonpharmacological interventions to prevent delirium is our focus on discrete models of care that involve collaboration among clinicians. Our goal is to identify the most promising models that deserve further development, investigation, and dissemination. Viewing delirium prevention through a collaborative care lens is consistent with efforts to achieve value-based care and may encourage drawing from the expanding literature outlining the benefits of mental healthcare integration.12,13 Specifically, a systems perspective highlights the potential for system-wide benefits such as reducing readmissions14,15 and cost savings.16
METHODS
This systematic review and meta-analysis follows PRISMA guidelines. A search of OVID, MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, EMBASE, and PsycINFO was completed by a medical librarian for clinical studies in which models of care were implemented to prevent delirium using PICO (P patient, problem or population; I, intervention; C, comparison, control or comparator; O, outcome) inquiries. Search terms included delirium, acute confusional state, altered mental status, prevention, and control (“delirium”/exp OR “acute confusion”/exp OR “altered mental status”/exp) AND “prevention and control”/exp AND [English]/lim AND [embase]/lim).
One researcher (AK) screened articles by title for relevance. Relevant articles were then divided among four authors (AK, MO, NF, and OB), and the abstracts were screened for eligibility. The authors reviewed the full texts of any potentially eligible studies. Each full text was assigned to two authors for full review. Discrepancies were adjudicated by conference among all authors. In addition, references within all full-text publications were scanned for potential additional articles.
The inclusion criteria for review of full-text articles required English-language description of a model of care with multiple interventions, delirium reported as an outcome, and presence of a comparator group.
“Model of care” was defined by the Cochrane Effective Practice and Organization of Care Review Group as follows: (1) revision of professional roles, including shifting of professional roles or expansion of roles to new tasks; (2) creation of clinical multidisciplinary teams or addition of new members to the team who collaborate inpatient care; (3) delivery of multiple interventions across multiple domains (ie, studies involving a single intervention such as physical therapy or targeting a single domain such as sleep were excluded); and (4) formal integration of services whereby teams work together in collaboration with existing services to enhance care.17 For this review, we required that studies include a comparator group so that effectiveness of the intervention could be assessed. Quality improvement studies that lacked a comparator group were excluded.
Delirium incidence was the primary outcome and was evaluated by meta-analysis. Heterogeneity was assessed using I2 and visual inspection of forest plots. I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The studies were pooled according to study type as follows: randomized controlled trials, pre–post design, and other nonrandomized prospective studies. Random effects models were used to calculate estimates using the Comprehensive Meta-Analysis software (Version 3, Biostat, Englewood, New Jersey), which also generated forest plots.
Risk of bias was assessed using criteria established by the Cochrane Collaborative Review Criteria, which lists six categories of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.17 Each study was assessed by two authors (either MO and AK or MO-P. and OB) for bias and a numerical value was assigned to each of the six categories as follows: 1 = low risk, 2 = unknown/moderate risk, and 3 = high risk. Where scorers disagreed, all authors jointly conferred, and a consensus score was given. The values for each of these six categories were added to create a composite risk-of-bias score for each study, with 6 being the lowest possible score and 18 the highest. Overall risk was classified as follows: <9 = low risk, 9-12 = moderate risk, and >12 = high risk.
RESULTS
Study Selection Process
An initial literature search identified 352 articles. After reviewing the titles, 308 articles were excluded for irrelevance, and 44 abstracts were screened for eligibility. We excluded 27 articles upon abstract review, and the full texts of 17 were obtained for detailed review. In addition, we identified another 10 potentially eligible articles through review of references and obtained full texts of these as well. Of the 27 full-text articles reviewed, 15 were included in this systematic review, 10 of which were suitable for meta-analysis. The Figure shows the PRISMA flow chart.
Study Characteristics
The 15 studies that met the inclusion criteria are summarized in the Table.18-32 Delirium prevention was among the primary outcomes of 13 studies; delirium outcomes were reported in the other two studies as well, which were primarily designed to assess feasibility.26,27 Six studies were conducted in the United States, three in Sweden, two in Spain, two in the United Kingdom, and one each conducted in Korea and Canada. Healthcare settings among the included studies involved the intensive care unit (six studies), medical floors (four studies), surgical floors (three studies), a long-term care unit (one study), and
Outcomes Reported
All but one of the studies reported delirium incidence. The most commonly used delirium screening instrument was the Confusion Assessment Method (CAM) or its modified version, the CAM-ICU (11 studies).33,34 Other methods used to assess mentation included the Richmond Agitation Sedation Scale,35 the Organic Brain Syndrome scale,36 the revised Delirium Rating Scale,37 the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,38 and the Confusion Rating Scale.39 (Details regarding delirium screening tools can be found in the systematic review by De and Wand.40) Researchers performed delirium assessment in nine studies, whereas assessments were performed by clinical staff in the remaining studies. Other outcomes reported included length of stay (LOS), mortality, number of days ventilated, and functional decline. None of the included studies reported cost effectiveness.
Risk of Bias Assessment
Risk of bias assessment identified only two studies—both randomized controlled trials—as low risk (Table). The remaining studies had moderate (four studies) or high risk (nine studies).
Results from Individual Studies
Of the 15 studies, nine reported a statistically significant reduction in delirium incidence, and another two reported a statistically insignificant reduction. In addition, seven of the eight studies that assessed delirium duration found reduced duration in the intervention cohort, and two of the three studies that reported delirium severity found a reduction in the intervention group.
Results of Meta-Analysis
Random effects models were created to meta-analyze groups of studies based on design as follows: randomized controlled trials (three studies18,19,25), pre–post intervention studies (four of six studies included28-31), and other nonrandomized studies (three of four studies included21-23). Meta-analysis was not completed for the two feasibility studies26,27 because delirium outcome data were limited due to the feasibility study design. The study of Dale et al.32 was excluded from the meta-analysis because the rates of CAM-ICU completion differed substantially between control and intervention groups (0.35 vs 1.49 per 24 hours, respectively), leading to imbalanced between-group sensitivity in delirium detection and Needham et al.20 was also excluded because it reported only days of delirium, not delirium incidence. The study by Lundström et al.24 was also excluded from the meta-analysis because delirium incidence was measured on days 1, 3, and 5, whereas the other studies reported delirium daily.
Meta-analysis of the three randomized controlled trials revealed a pooled odds ratio of 0.56 (95% CI: 0.37-0.85; P = .006) for delirium incidence among intervention group subjects relative to those in comparator groups. The heterogeneity across studies was low (I2 = 29%). Pooling data from four pre–post studies found that the odds ratio for delirium incidence was 0.63 (95% CI: 0.37-1.07; P = .09). The heterogeneity across these studies was moderate (I2 = 65%). Results from the three eligible, nonrandomized prospective studies were also pooled. The odds ratio for developing delirium among study subjects was 0.79 (95% CI: 0.46-1.37; P = .40), and the heterogeneity among these studies was high (I2 = 85%).
DISCUSSION
We provide a systematic review and meta-analysis of delirium preventive models of care. Meta-analysis of the three randomized controlled trials found that these models of care led to a statistically significant reduction in delirium incidence; study subjects had an 11.5% reduction in absolute delirium incidence. The pooled odds ratios for both of the other sets of nonrandomized studies favored the intervention group but were not significant, each because of one included study. The pre–post meta-analysis failed to reach significance as one of the included studies found a trend toward higher delirium incidence; however, interestingly, in that same study, the overall delirium-free days were significantly reduced overall (24 vs 27, P = .002). Similarly, meta-analysis of the three additional nonrandomized prospective studies failed to reach significance because the largest included study found higher rates of delirium among intervention group subjects. Despite considerable risk of bias in several of these studies, their findings were broadly consistent; all but one study (Gagnon 201221) reported a trend or a significant reduction in delirium incidence, duration, severity, or number of delirium episodes. Moreover, the value of such models of care extended beyond preventing delirium; for instance, other positive outcomes included reduced LOS and fewer medical complications.
Models of care ranged widely with respect to specific interventions, though several common elements highlighted their relevance for delirium care and as potential delirium prevention strategies in future studies. For example, two of the randomized controlled trials18,19 employed early mobilization, enhanced nutrition, sleep hygiene, early reduction of invasive procedures (eg, urinary catheterization), and pain control in their multicomponent models. Five additional studies also incorporated early mobilization,20,22,23,31,32 and three sought to improve sleep quality.22,28,30 Among other important strategies were delirium screening,18,20,22,30,31 monitoring medication,18,20,22,26,28,30,32 orientation,18,21,23,28 addressing vision and hearing impairment,18,22,23,32 hydration,18,22,23 avoiding hypoxia,18,20,30 and staff, patient, and caretaker education.19,21,23,27-30 Unique strategies were implemented in certain studies. For instance, one study used massage therapy,28 preventing delays in transfer logistics in another,30 and a third addressed psychosocial problems.25 Overall, the selection of strategies depended on the patient setting; thus, no one care bundle should be expected to emerge as a universal model for delirium prevention. Rather, these results should be interpreted within their specific care contexts and judged on the quality of evidence (eg, effect size and statistically significant findings, low risk of bias, sound experimental design). The one study that failed to find any positive effect on delirium, that of Gagnon et al.,21 was conducted on an inpatient palliative care service in Canada, and its negative finding may reflect the unique delirium risk factors in patients who are nearing end of life.
This current review differs from previous delirium prevention reviews in operationally defining a “model of care.” We identified a great deal of variation in specific models and team composition. For example, some interventions were carried out by nurses18-20,31 and physicians,20,21,25,32 whereas others involved physical therapists,20,22,28 medical residents,23 geriatricians,22,23,25 pharmacists,26 researchers,18 and trained volunteers.22 In all cases, the staff roles were expanded to include new tasks, and the clinical team worked collaboratively to administer interventions across multiple domains. Team-related considerations are critical because modern medical care is inherently interdependent.9 These broad differences in team composition across studies demonstrate the number of potential options for team structure and function. They also highlight the number of “moving parts” to be considered when designing and implementing delirium care bundles.
Most of the delirium prevention studies implementing models of care are characterized by a substantial risk of bias. We evaluated risk of bias along six categories of potential sources, including random assignment to groups, ability to foresee future group allocation, blinding of participants and personnel to group assignment, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias.17 Two of the three studies that used randomization had a low risk of bias, and four additional studies had a moderate risk of bias. Allocation concealment was accomplished only in randomized controlled trials, whereas blinding of both subjects and study personnel was not implemented in any of the studies. Although some studies relied on data analysis by research personnel blinded to group membership or the nature of the intervention, others failed to do so or failed to describe data analysis in sufficient detail. Studies also failed to report the percentage of unscorable or otherwise omitted delirium assessments necessary to calculate attrition rates or to understand the comprehensiveness of outcome assessment in a systematic manner. Other potential sources of bias included systematic differences between the intervention and control groups (such as differences in gender composition, age, or delirium risk) at study outset.
A primary limitation of this review is the heterogeneity of settings, interventions, and models of care across included studies. We excluded several studies from this review for being delivered by a single individual or service line (eg, introduction of a geriatric consult service, physical therapy, or volunteers), for providing a single intervention (eg, early ambulation alone), or for multiple interventions targeting a single domain (eg, sleep). We did so because the future of value-based care lies in collaboration of providers and services, and in a way the complexity across and within these studies ultimately reflects the complexity of medical settings as well as the multifactorial nature of delirium. The broader message is a call for increasing the integration of delirium-related care services. As discussed earlier, the high risk of bias across these studies is a limitation of our findings; high-quality evidence on the value of delirium prevention models of care remains limited. Thus, although our review suggests that there are multicomponent models of care that hold promise in mitigating delirium and its outcomes, additional randomized studies are required to confirm the efficacy of such models of care and to test which services, interventions, and clinical domains deserve priority.
CONCLUSION
To our knowledge, this is the first systematic review and meta-analysis of delirium preventive models of care. Models of care, as defined here, necessarily included a multidisciplinary team in which traditional staff roles had been revised to implement a multicomponent, multidomain intervention. Other recent reviews are available for multicomponent pharmacological and nonpharmacological interventions to prevent and manage delirium,41-49 but just as important as which interventions are being delivered is the team that delivers them. Care delivery in a complex medical system is more than handing a patient a medication or facilitating ambulation; it requires a choreographed dance of teamwork and integration across services. This review identifies promising models of care that deserve further recognition, refinement, and ultimately widespread implementation.
Acknowledgments
The authors comprise a writing group created through the Delirium Boot Camp, an annual meeting originally sponsored by the Center of Excellence for Delirium in Aging: Research, Training, and Educational Enhancement (CEDARTREE, Boston, Massachusetts); it is currently supported by the Network for Investigation of Delirium: Unifying Scientists (NIDUS, Boston, Massachusetts). The authors would like to thank medical librarian Rita Mitchell (Aurora Health Care, Milwaukee, Wisconsin) for the literature search, senior scientific writer and editor Joe Grundle (Aurora Research Institute, Milwaukee, Wisconsin) for editorial assistance, and graphics specialist Brian Miller (Aurora Research Institute, Milwaukee, Wisconsin) for help with the figures.
Disclosures
The authors report no relevant conflicts of interest.
Funding
No funding was dedicated to the conduct of this review.
1. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc.
2. Schubert M, Schürch R, Boettger S, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - A cohort study. BMC Health Serv Res. 2018;18(1):550. https://doi.org/10.1186/s12913-018-3345-x.
3. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the Intensive Care Unit. JAMA. 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753.
4. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1-2):121-129. https://doi.org/10.1159/000485794.
5. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1.
7. Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: A systematic appraisal. BMJ Open. 2017;7(3):e013809. https://doi.org/10.1136/bmjopen-2016-013809.
8. Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
9. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am Psychol. 2018;73(4):433-450. https://doi.org/10.1037/amp0000298.
10. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLOS ONE. 2015;10(6):e0123090. https://doi.org/10.1371/journal.pone.0123090.
11. Thomas EJ. Improving teamwork in healthcare: current approaches and the path forward. BMJ Qual Saf. 2011;20(8):647-650. https://doi.org/10.1136/bmjqs-2011-000117.
12. Sledge W, Bozzo J, White-McCullum B, Lee H. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome -Res. 2016;2:2-6.
13. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. PubMed
14. Lee E, Kim J. Cost-benefit analysis of a delirium prevention strategy in the intensive care unit. Nurs Crit Care. 2014;21:367-373. https://doi.org/10.1111/nicc.12124.
15. Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the hospital elder life program on risk of 30-day readmission. J Am Geriatr Soc. 2018;66(1):145-149. https://doi.org/10.1111/jgs.15132.
16. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219-226. https://doi.org/10.1016/j.psym.2013.01.010.
17. Cochrane effective practice and organisation of Care Group (EPOC). Data collection Checklistist. Chochrane Effective Practice and Organisation of Care Group (EPOC) Methods Papers. . https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC Data Collection Checklist.pdf. Accessed May 27, 2014.
18. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: A randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423-1432. https://doi.org/10.1016/j.ijnurstu.2015.04.021.
19. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res-. 2007;19(3):178-186. https://doi.org/10.1007/BF03324687.
20. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients With acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. https://doi.org/10.1016/j.apmr.2010.01.002.
21. Gagnon P, Allard P, Gagnon B, Mérette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187-194. https://doi.org/10.1002/pon.1881.
22. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
23. Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029-2036. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
24. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
25. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
26. Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32(1):E1-E10. https://doi.org/10.1097/JCN.0000000000000356.
27. Siddiqi N, Cheater F, Collinson M, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652-661. https://doi.org/10.1093/ageing/afw091.
28. Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit (SICU) improved the outcomes of older adults. J Surg Res. 2014;186:519. https://doi.org/10.1016/j.jss.2013.11.352
29. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721-727. https://doi.org/10.1093/ageing/aft120.
30. Björkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol-Scand. 2010;54(6):678-688. https://doi.org/10.1111/j.1399-6576.2010.02232.x.
31. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility (ABCDE) bundle. Crit Care Med. 2014;42(5):1024-1036. https://doi.org/10.1097/CCM.0000000000000129.
32. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367-374. https://doi.org/10.1513/AnnalsATS.201306-210OC.
33. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941.
34. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. https://doi.org/10.1097/00003246-200107000-00012.
35. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. https://doi.org/10.1164/rccm.2107138.
36. Jensen E, Dehlin O, Gustafson L. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry. 1993;8(3):215-229. https://doi.org/10.1002/gps.930080305.
37. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13(2):229-242. https://doi.org/10.1176/jnp.13.2.229.
38. American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc.
39. Williams MA. Delirium/acute confusional states: evaluation devices in nursing. Int Psychogeriatr. 1991;3(2):301-308. PubMed
40. De J, Wand APF. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist-. 2015;55(6):1079-1099. https://doi.org/10.1093/geront/gnv100.
41. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological,
multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. https://doi.org/10.1093/ageing/afu173.
42. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):375-380. https://doi.org/10.7326/0003-4819-158-5-201303051-00003.
43. Rivosecchi RM, Smithburger PL, Svec S, Campbell S, Kane-Gill SL. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35(1):39-50; quiz 51. https://doi.org/10.4037/ccn2015423.
44. Trogrlić Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. https://doi.org/10.1186/s13054-015-0886-9.
45. Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017;96(26):e7361. https://doi.org/10.1097/MD.0000000000007361.
46. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium After hip fracture: A systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559-1565. https://doi.org/10.1111/jgs.14846.
47. Oberai T, Lizarondo L, Ruurd J. Effectiveness of multi-component interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2017;15(2):259-268. https://doi.org/10.11124/JBISRIR-2016-002943.
48. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016;31(2):127-141. https://doi.org/10.1177/0885066614553925.
49. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological
delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779.
1. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc.
2. Schubert M, Schürch R, Boettger S, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - A cohort study. BMC Health Serv Res. 2018;18(1):550. https://doi.org/10.1186/s12913-018-3345-x.
3. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the Intensive Care Unit. JAMA. 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753.
4. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1-2):121-129. https://doi.org/10.1159/000485794.
5. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1.
7. Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: A systematic appraisal. BMJ Open. 2017;7(3):e013809. https://doi.org/10.1136/bmjopen-2016-013809.
8. Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
9. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am Psychol. 2018;73(4):433-450. https://doi.org/10.1037/amp0000298.
10. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLOS ONE. 2015;10(6):e0123090. https://doi.org/10.1371/journal.pone.0123090.
11. Thomas EJ. Improving teamwork in healthcare: current approaches and the path forward. BMJ Qual Saf. 2011;20(8):647-650. https://doi.org/10.1136/bmjqs-2011-000117.
12. Sledge W, Bozzo J, White-McCullum B, Lee H. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome -Res. 2016;2:2-6.
13. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. PubMed
14. Lee E, Kim J. Cost-benefit analysis of a delirium prevention strategy in the intensive care unit. Nurs Crit Care. 2014;21:367-373. https://doi.org/10.1111/nicc.12124.
15. Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the hospital elder life program on risk of 30-day readmission. J Am Geriatr Soc. 2018;66(1):145-149. https://doi.org/10.1111/jgs.15132.
16. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219-226. https://doi.org/10.1016/j.psym.2013.01.010.
17. Cochrane effective practice and organisation of Care Group (EPOC). Data collection Checklistist. Chochrane Effective Practice and Organisation of Care Group (EPOC) Methods Papers. . https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC Data Collection Checklist.pdf. Accessed May 27, 2014.
18. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: A randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423-1432. https://doi.org/10.1016/j.ijnurstu.2015.04.021.
19. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res-. 2007;19(3):178-186. https://doi.org/10.1007/BF03324687.
20. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients With acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. https://doi.org/10.1016/j.apmr.2010.01.002.
21. Gagnon P, Allard P, Gagnon B, Mérette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187-194. https://doi.org/10.1002/pon.1881.
22. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
23. Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029-2036. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
24. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
25. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
26. Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32(1):E1-E10. https://doi.org/10.1097/JCN.0000000000000356.
27. Siddiqi N, Cheater F, Collinson M, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652-661. https://doi.org/10.1093/ageing/afw091.
28. Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit (SICU) improved the outcomes of older adults. J Surg Res. 2014;186:519. https://doi.org/10.1016/j.jss.2013.11.352
29. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721-727. https://doi.org/10.1093/ageing/aft120.
30. Björkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol-Scand. 2010;54(6):678-688. https://doi.org/10.1111/j.1399-6576.2010.02232.x.
31. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility (ABCDE) bundle. Crit Care Med. 2014;42(5):1024-1036. https://doi.org/10.1097/CCM.0000000000000129.
32. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367-374. https://doi.org/10.1513/AnnalsATS.201306-210OC.
33. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941.
34. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. https://doi.org/10.1097/00003246-200107000-00012.
35. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. https://doi.org/10.1164/rccm.2107138.
36. Jensen E, Dehlin O, Gustafson L. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry. 1993;8(3):215-229. https://doi.org/10.1002/gps.930080305.
37. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13(2):229-242. https://doi.org/10.1176/jnp.13.2.229.
38. American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc.
39. Williams MA. Delirium/acute confusional states: evaluation devices in nursing. Int Psychogeriatr. 1991;3(2):301-308. PubMed
40. De J, Wand APF. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist-. 2015;55(6):1079-1099. https://doi.org/10.1093/geront/gnv100.
41. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological,
multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. https://doi.org/10.1093/ageing/afu173.
42. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):375-380. https://doi.org/10.7326/0003-4819-158-5-201303051-00003.
43. Rivosecchi RM, Smithburger PL, Svec S, Campbell S, Kane-Gill SL. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35(1):39-50; quiz 51. https://doi.org/10.4037/ccn2015423.
44. Trogrlić Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. https://doi.org/10.1186/s13054-015-0886-9.
45. Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017;96(26):e7361. https://doi.org/10.1097/MD.0000000000007361.
46. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium After hip fracture: A systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559-1565. https://doi.org/10.1111/jgs.14846.
47. Oberai T, Lizarondo L, Ruurd J. Effectiveness of multi-component interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2017;15(2):259-268. https://doi.org/10.11124/JBISRIR-2016-002943.
48. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016;31(2):127-141. https://doi.org/10.1177/0885066614553925.
49. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological
delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779.
© 2019 Society of Hospital Medicine
Inpatient Management of Acute Severe Ulcerative Colitis
Ulcerative colitis (UC) is a chronic inflammatory condition of the colonic mucosa. Classically, it starts in the rectum and can extend continuously from the distal to the proximal colon. The defining clinical symptom of UC is bloody diarrhea, typically accompanied by rectal urgency and mucus discharge. The natural history of this disease includes periods of exacerbations and remissions occurring spontaneously or in response to medical treatment.1
Acute severe ulcerative colitis (ASUC) is a potentially life-threatening complication of UC that typically requires hospitalization and interdisciplinary care between hospitalists, gastroenterologists, and colorectal or general surgeons. The risk of a patient with UC requiring hospitalization for ASUC ranges from 15%-25%2,3 and, in total, UC accounts for 30,000 hospital visits annually.4 The direct medical costs exceed $4 billion annually, with hospital costs of over $960 million.5 Historically, mortality from ASUC was as high as 24% but decreased substantially to 7% after the introduction of systemic corticosteroid therapy.6 Further advances in care have reduced mortality to approximately 1% or less.7,8 Nonetheless, up to 20% of patients admitted with ASUC have a colectomy on their first admission, and this rate rises to 40% after two admissions.2
DEFINING ACUTE SEVERE ULCERATIVE COLITIS
To categorize UC severity, assess patients using the Truelove and Witt’s criteria. The system classifies patients as having mild, moderate, severe, or fulminant disease. Severe disease by these criteria includes patients with >6 bloody bowel movements per day and at least one of the following clinical features: fever (>37.8°C), tachycardia (>90 bpm), anemia (hemoglobin <10.5 g/dl), or elevated inflammatory markers (traditionally, erythrocyte sedimentation rate greater than 30 mm/h or, more recently, C-reactive protein (CRP) greater than 30 mg/L. (Table 1).6,9
Fulminant colitis refers to a subgroup of patients with more than 10 stools per day, continuous bleeding, abdominal pain, colonic dilatation on abdominal X-ray film, and severe toxic symptoms including fever and anorexia. Such patients are at risk of progressing to toxic megacolon and bowel perforation.10
INDICATIONS FOR HOSPITALIZATION AND INPATIENT LEVEL OF CARE
Patients with ASUC almost always require hospitalization for their disease management. In many cases, these patients have been receiving outpatient oral prednisone 40-60 mg daily but continue to have ongoing disease activity.11 Most patients will require close clinical monitoring, frequent blood testing, endoscopic or radiologic evaluation, as well as administration of intravenous corticosteroids. The average length of stay (LOS) ranges from 4.6 to 12.5 days, depending on disease severity.12 Not surprisingly, Kelso et al. reported that predictors of hospital LOS greater than four days include initiating a biologic drug in the hospital, undergoing two or more imaging modalities and treatment with intravenous steroids,13 and so it is rare that patients do not meet billing requirements for an inpatient level of care.
INITIAL EVALUATION
The multifaceted initial inpatient evaluation of patients with ASUC aims to assess disease severity, identify and prevent potential complications, and initiate planning for potential failure of first-line pharmacologic therapy. Due to the accumulating evidence that involving physicians with expertise in managing ASUC improves outcomes, gastroenterologists should be involved in the care of patients with ASUC from the time of their admission.14,15 Additionally, creating standardized care pathways for the management of ASUC can reduce cost, LOS, and improve quality.16
History and Physical Examination
Patients should be asked about fever, abdominal pain, nausea, emesis, bloating, weight loss, and bowel movements (frequency, consistency, the presence of blood, urgency, nighttime awakenings). The number of bowel movements over a 24-hour period should be quantified as this helps assess the overall disease severity (Table 1).
The patient’s initial inflammatory bowel disease (IBD) history is also essential. The review of pertinent information regarding the patient’s initial diagnosis of UC includes the severity and anatomic extent of disease, extraintestinal manifestations, previous medical therapies, and surgical interventions. Exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) or antibiotics should be identified as they may precipitate flares.17 Travel history may be pertinent as travel increases the risk of infections with food-borne or parasitic pathogens.18
Physical examination begins with an assessment of vital signs and volume status. Abdominal examination should include evaluation of bowel sounds, an assessment of distention, location, the extent of abdominal tenderness, and peritoneal signs. The abdominal exam should be interpreted in the context of the patient’s medications, as the use of steroid or analgesic therapies may affect the sensitivity for detecting complications. An external rectal exam evaluating perianal disease should be performed, as perianal disease raises concern for Crohn’s, a disease whose surgical management differs from UC.
A careful exam for extraintestinal manifestations is also essential. The skin should be evaluated for any new rashes, especially on the anterior shin consistent with erythema nodosum or ulcerated lesions on the skin suggestive of pyoderma gangrenosum. The peripheral joints should also be examined for any synovitis. Additional examinations should be performed based on any reported symptoms (eg, the ophthalmic exam for uveitis or scleritis if visual changes or eye pain are reported). Some extraintestinal manifestations require subspecialty consultation and comanagement to guide disease therapy. Patients with underlying pyoderma gangrenosum may require a dermatology consultation to guide management. Ocular inflammation requires ophthalmology involvement, and inflammatory arthritis is best comanaged with rheumatology.19
Laboratory Testing
Initial testing should include a complete blood count with differential, basic metabolic panel, and liver chemistries including alkaline phosphatase and albumin. When relevant, pregnancy testing should be performed. Measure CRP on admission so that its trajectory can be followed during therapy. However, a normal CRP does not exclude the presence of a UC flare as a subset of patients with ASUC will have a normal CRP despite severe mucosal inflammation.20
Since one-third of patients do not respond to intravenous corticosteroids and will require rescue therapy during the hospitalization with infliximab or cyclosporine, anticipatory testing for these medications should be performed on admission to avoid delays in the administration of rescue therapy.6,21 This should include an interferon-gamma release assay (eg, quantiferon gold) to test for latent tuberculosis and hepatitis B serologies in anticipation of possible treatment with infliximab. An interferon-gamma release assay is preferred to a tuberculin skin test because patients may be anergic, and a skin test does not provide a control to determine whether a negative test is due to anergy. In contrast, although a quantiferon gold test can be indeterminate in ASUC due to disease activity and systemic steroids, the results indicate if the patient is anergic so that one will not rely on a false-negative result. In the event of an equivocal result, a careful clinical assessment for risks of TB exposures should be elicited, and a chest radiograph should be obtained.22 In patients with prior high risk of tuberculosis exposures or a positive test for tuberculosis, an infectious disease specialist should be consulted early to advise if therapy should be started in preparation for the potential use of infliximab.23 In cases where cyclosporine may be considered, magnesium and total cholesterol level should be checked. Sending thiopurine methyltranferase (TPMT) enzyme activity should be considered as well, in case of a need for future thiopurine use for maintenance of disease activity.24
Infectious diarrhea may be indistinguishable from ASUC and may also be the trigger of a flare; thus, it is important to rule out infection with stool microbiologic studies. Most importantly, Clostridium difficile infection must be ruled out in all patients with ASUC. Although patients with IBD, especially those with UC, have significantly higher rates of asymptomatic C. difficile carriage than the general population, a positive polymerase chain reaction test for C. difficile in a patient with ASUC should prompt treatment with oral vancomycin.25 However, if carriage if suspected and a subsequent enzyme-linked immunoassay for C. difficile toxin is negative, treatment can be discontinued. Active C. difficile infection in patients with IBD is associated with increased disease severity, greater length of hospital stay, and increased the likelihood of colectomy and mortality.26,27 Other bacterial infections including Escherichia coli, Campylobacter, Shigella, Salmonella, Yersinia, Entamoeba histolytica, as well as other parasitic infestations may mimic UC. Testing should be considered in cases of foreign travel, immunosuppression or contact with other persons with diarrhea.7,28 Routine testing of these other enteric infections without a clear exposure risk is of little benefit and may raise costs.23,29
Radiologic Evaluation
A plain X-ray film of the abdomen should be obtained in all patients on admission to evaluate for evolving colonic dilation or undiagnosed free air. Small bowel distension >3 cm may predict an increased risk of colectomy.30 Clinicians must be mindful that steroids can mask peritoneal signs and that retroperitoneal perforations may not be apparent on plain X-ray films. Nonetheless, a CT of the abdomen is usually not necessary and should be reserved for cases with severe abdominal pain out of proportion to clinical signs in which a plain X-ray film is unrevealing. Judicious use of CT imaging is especially important in younger patients, as there is growing concern that patients with IBD may be exposed to potentially harmful cumulative levels of radiation in their lifetime from repeated CT imaging.31
Endoscopic Evaluation
Flexible sigmoidoscopy aids in the assessment of disease severity and extent and biopsies can assist in ruling out a diagnosis of cytomegalovirus (CMV) colitis in patients already on immunosuppression. For this reason, many clinicians prefer to perform a sigmoidoscopy on admission.23 If one is not performed on admission, a sigmoidoscopy is advised in all patients who are not responding adequately after 72 hours of intravenous steroid therapy in order to rule out superimposed CMV colitis.28
Sigmoidoscopy should be avoided in patients with toxic megacolon and when there is a concern for peritonitis. A complete colonoscopy is rarely indicated in the acute setting and carries a theoretical risk of colonic perforation.7
INITIAL THERAPY
The first therapeutic steps aim to reduce inflammation with the use of systemic corticosteroids, avoid colonic and extraintestinal complications, and plan for the potential need for rescue therapy.
Intravenous Corticosteroids
The cornerstone of ASUC management is treatment with intravenous corticosteroids. Their initiation should not be delayed in patients with an established diagnosis of UC while waiting for results of evaluations for infectious colitis. Even among patients who have failed oral steroids, a meta-regression analysis showed that two-thirds of patients will still respond to intravenous corticosteroids.21,32 Methylprednisolone 20 mg IV three times daily (or hydrocortisone 100 mg IV three times daily) is a standard regimen; higher doses do not provide additional benefit.21 Patients’ response to intravenous steroids should be assessed with repeat labs including CRP and an assessment of the total number of bowel movements over a 24-hour period, with special attention to their overall response after three days of treatment.33-36
Intravenous Fluids
Many patients admitted with ASUC will have significant volume depletion, and intravenous fluids should be administered in a manner like other volume-depleted or oral-intake-restricted patients.
Venous Thromboembolism Prophylaxis
The risk of VTE in hospitalized patients with IBD exceeds that of inpatients without IBD, approximately 2%, a risk similar to patients with respiratory failure.37 Additionally, VTE in hospitalized patients with IBD is associated with a 2.5-fold increase in mortality.38,39 Therefore, all patients hospitalized with ASUC should receive subcutaneous unfractionated or low molecular weight heparin or fondaparinux for VTE prophylaxis. Rectal bleeding, expected in ASUC, is not a contraindication to chemo-prophylaxis. Additionally, it is important to check if patients are receiving the ordered VTE prophylaxis.40,41 Pleet et al. found that only 7% of patients at a tertiary center had adequate prophylaxis for greater than 80% of their hospitalization.41
Unnecessary or Potentially Harmful Medications
Several medications have the potential for misuse in patients hospitalized with UC.
Antimotility Agents
Loperamide, diphenoxylate, and opiate antidiarrheals should not be used as they may provoke toxic megacolon.42 Similarly, drugs with antimotility side effects (eg, anticholinergics) should be avoided.
Opiates
In addition to their undesirable antimotility effect, the use of opiates has been associated with poor outcomes among inpatients and outpatients with IBD, including increased morbidity and mortality.43,44 Pain severe enough to require opiates should raise suspicion for toxic megacolon, perforation, or a noninflammatory etiology. If opiates are utilized, they should be ordered as one-time doses and the patient should be reassessed for each dose.
Nonsteroidal Anti-inflammatory Drugs
These drugs, which include oral NSAIDs, intravenous ketorolac, and topic diclofenac gels, may increase disease activity in inflammatory bowel disease and should be avoided.17
5-aminosalicylates (5-ASA)
A small proportion of patients experience a paradoxical worsening of diarrhea due to the use of 5-ASA agents such as mesalamine. It is reasonable to discontinue or avoid the use of 5-ASA agents in hospitalized patients, especially as there is little to no benefit from combining a 5-ASA with a biologic or immunosuppressive drug.45
Antibiotics
There is no role for the routine use of antibiotics in patients hospitalized with ASUC. 23,46,47 Inappropriate use of antibiotics raises the risk of C. difficile infection and antibiotic resistance. However, in cases of suspected toxic megacolon or perforation, antibiotics should be administered. In situations in which a patient is treated with triple immunosuppression (ie, steroids plus two other agents, cyclosporine and mercaptopurine) antibiotic prophylaxis for Pneumocystis jiroveci is advisable.48 Using a large insurance database, Long et al. reported a low absolute incidence of Pneumocystis jiroveci in IBD patients but noted that the risk in patients with IBD was still significantly higher than matched controls. While it can be considered, we typically refrain from using prophylaxis in patients on double immunosuppression (for example, steroids plus infliximab) due to the potential adverse effects of antibiotics in this population, though many advocate using prophylaxis for all patients on cyclosporine even if this is only double immunosuppressive therapy.23
Surgical Consultation
Involving a surgeon early in an ASUC patient’s care—before needing urgent colectomy—is critical. As part of the consultation, a surgeon experienced in IBD should meet with patients to discuss multistage colectomy with ileostomy and potential future J-pouch (ileal pouch-anal anastomosis) formation. Patients should be given ample opportunity to ask questions before surgery may become urgent. Also, patients should be counseled on realistic expectations of ostomy and pouch function and, ideally, meet with an ostomy nurse.23
At some centers, surgical consultation is requested on the first hospital day, but this can result in consultations for patients who ultimately respond to intravenous steroids. Therefore, some centers advocate for surgical consultation only after a patient has failed treatment with intravenous steroids (ie, day three to four) when the risk of needing surgical management increases.23
Nutrition
Bowel rest with parenteral nutrition does not improve outcomes in ASUC versus an oral diet, and there is no contraindication to allowing patients to continue on a regular diet unless they have toxic megacolon or other signs of fulminant colitis.49,50 However, patients may feel better eating less, as this will reduce their bowel movement frequency. Unfortunately, this can give a false sense of reassurance that the patient is improving. Therefore, it remains important to evaluate a patient’s symptoms in the context of their food intake.
Assessing Response to Steroids
Patients who do not respond adequately to the first-line intravenous steroid therapy will require medical or surgical rescue therapy; therefore, deciding whether a patient has responded is essential. Patients should have less than four bowel movements per day – ideally just one to two – with no blood to indicate a complete response. For more ambiguous situations, although there is no strict definition of steroid responsiveness, multiple prediction indices have attempted to identify patients who will require rescue therapy. One of the simplest, the Oxford index, illustrates two of the most critical parameters to follow, stool frequency and CRP.51 In a preinfliximab cohort, Oxford index predicted an 85% likelihood of colectomy in patients with eight or more daily bowel movements or with three to eight daily bowel movements and a CRP greater than 45 mg/L after three days of intravenous steroid treatment.52 To assist with assessing responsiveness to therapy, we ask patients to log their bowel movements – either on paper or on a whiteboard in the hospital room – so that we can review their progress daily. Other predictors of colectomy include hypoalbuminemia, scoring of endoscopic severity, and colonic dilation.53
Patients who fail to respond to intravenous corticosteroids after three days33,35 of treatment should be started on rescue therapy with infliximab or cyclosporine or undergo colectomy. A common pitfall in the treatment of ASUC is waiting for a response to steroids beyond this time frame, after which patients are unlikely to benefit.34,36 Furthermore, patients for whom surgical rescue therapy is delayed have higher operative morbidity and mortality.54,55 Because timely decision making regarding rescue therapy is crucial to optimizing outcomes, patient education efforts regarding potential rescue therapy should take place on admission or soon after, rather than waiting to ascertain steroid responsiveness.
RESCUE THERAPY FOR STEROID-REFRACTORY DISEASE
Medical options for rescue therapy include the antitumor necrosis factor (anti-TNF) agent infliximab or the calcineurin inhibitor cyclosporine. In general, infliximab and cyclosporine have been found to be roughly equivalent in efficacy in clinical trials regarding response, remission, and colectomy at 12 months.56,57 However, many clinicians prefer infliximab due to its relative ease of use, familiarity with the agent from outpatient experience, and ability to continue to use long term for maintenance of disease remission.58 In contrast to infliximab, intravenous cyclosporine requires closer monitoring and labs to assess the therapeutic trough level. The decision regarding which drug to use should be made on a case-by-case basis in conjunction with a gastroenterologist experienced in their use, and if no such specialist is available, transfer to a specialized center should be considered. Generally, successive treatment with cyclosporine or infliximab followed by third-line salvage therapy with the other drug should be avoided due to low rates of response and high rates of adverse events.59
Infliximab
Infliximab is an intravenously-administered anti-TNF monoclonal chimeric antibody that is effective both for outpatient treatment of moderate to severe UC and inpatient treatment of ASUC.1 It is relatively contraindicated in patients with untreated latent tuberculosis, demyelinating disease, advanced congestive heart failure, or uncontrolled infection.
The optimal dosing strategy for infliximab in ASUC is unknown. Infliximab clearance in the setting of ASUC is increased, partly because it is bound to albumin, which is often low in ASUC, and partly because it is excreted in the stool.60,61 As a result, accelerated loading doses may be more successful than a typical loading schedule,62 and most clinicians use alternative dosing strategies.63 Our typical approach for ASUC is an initial dose of 10 mg/kg rather than 5 mg/kg, with an additional 10 mg/kg dose 48-72 hours later if an adequate clinical response is lacking. Patients who respond to infliximab can continue to use the drug as an outpatient for maintenance of remission.
Cyclosporine
Cyclosporine is a fast-acting immunosuppressive agent that acts primarily via T-cell inhibition. Although older literature used a dose of 4 mg/kg per day, a randomized trial demonstrated similar response rates to a dose of 2 mg/kg per day.64 Patients receiving treatment with cyclosporine, which is given as a continuous infusion, must be monitored for toxicities. These can include potentially severe infection, seizures (often associated with low total cholesterol or hypomagnesemia), electrolyte abnormalities, renal impairment, hypertension, hypertrichosis, tremor, and others.65
Before initiation of treatment, serum cholesterol levels should be obtained to screen for low total cholesterol that may portend risk of seizures on the drug. Additionally, baseline creatinine and magnesium should be established. While on treatment, daily serum cyclosporine levels and electrolytes including magnesium should be measured. Patients who respond to intravenous cyclosporine must be transitioned to oral cyclosporine and have stable drug levels before discharge. Unfortunately, oral cyclosporine has not been shown to be as effective as long-term maintenance therapy. Therefore, cyclosporine can only be used as a “bridge” to another therapy. Historically, thiopurines like azathioprine or mercaptopurine have been used for this purpose because they are effective for the treatment of UC but may require months to have a full therapeutic effect. There have been promising reports of using vedolizumab similarly.66,67 Vedolizumab is a monoclonal antibody that selectively blocks lymphocyte trafficking to the gut that, like thiopurines, has an onset of action that is significantly longer than calcineurin and TNF inhibitors.
COLECTOMY
Colectomy should be considered as a second- or third-line therapy for patients who fail to respond to intravenous corticosteroids. In an analysis of 10 years of data from the Nationwide Inpatient Sample, mortality rates for colectomy in this setting varied from 0.7% at high volume centers to 4% at low volume centers.68 Therefore, if a patient is not hospitalized at a center with expertise in colectomy for UC, transfer to a specialized center should be considered. Colectomy should be performed promptly in all the patients who have failed rescue therapy with infliximab or cyclosporine or have opted against medical rescue therapy. Surgery should be performed emergently in patients with toxic megacolon, uncontrolled colonic hemorrhage or perforation.
QUALITY OF CARE AND THE USE OF CARE PATHWAYS
Physician and center-level characteristics are associated with the quality of care and outcomes in ASUC. Gastroenterologists with expertise in IBD are more likely than other gastroenterologists to request appropriate surgical consultation for steroid-refractory patients,69 and inpatients with ASUC primarily cared by gastroenterologists rather than nongastroenterologists have lower in-hospital and one-year mortality.14 Moreover, surgical outcomes differ based on center volume, with higher volume centers having lower rates of postoperative mortality.68,70 However, even at referral centers, key metrics of care quality such as rates of VTE prophylaxis, testing for C. difficile, and timely rescue therapy for steroid-refractory UC patients are suboptimal, with only 70%-82% of patients with IBD hospitalized at four referral centers in Canada meeting these metrics.71
Inpatient clinical pathways reduce LOS, reduce hospital costs, and likely reduce complications.72 For this reason, a consensus group recommended the use of care pathways for the management of ASUC and, although there is little data on the use of pathways for ASUC specifically, the use of such a pathway in the United Kingdom was associated with improved metrics including LOS, time to VTE prophylaxis, testing of stool for infection, CRP measurement, and timely gastroenterologist consultation.16,18
DISCHARGE CRITERIA AND FOLLOW UP
In general, patients should enter clinical remission, defined as resolution of rectal bleeding and diarrhea or altered bowel habits,73 before discharge, and achieving this may require a relatively prolonged hospitalization. Most patients should have one to two bowel movements a day without blood but, at a minimum, all should have less than four nonbloody bowel movements per day. Patients are candidates for discharge if they remain well after transitioning to oral prednisone at a dose of 40-60 mg daily and tolerate a regular diet.
For patients who initiated infliximab during their admission, plans for outpatient infusions including insurance approval should be made before discharge, and patients who started cyclosporine should be transitioned to oral dosing and have stable serum concentrations before leaving the hospital. Patients should leave with a preliminary plan for a steroid taper, which may vary depending on their clinical presentation. Usually, gastroenterology follow-up should be arranged after two weeks following discharge, but patients on cyclosporine need sooner laboratory monitoring.
CONCLUSION
The care of patients with ASUC requires an interdisciplinary team and close collaboration between hospitalists, gastroenterologists, and surgeons. Patients should be treated with intravenous corticosteroids and monitored carefully for response and need for rescue therapy. Establishing algorithms for the management of patients with ASUC can further improve the care of these complex patients.
Disclosures
Drs. Feuerstein, Fudman, and Sattler report no potential conflict of interest.
Funding
This work was not supported by any grant.
1. Sands BE. Mount Sinai Expert Guides: Gastroenterology. Hoboken, NJ: John Wiley & Sons; 2014.
2. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4(4):431-437. https://doi.org/10.1016/j.crohns.2010.02.001.
3. Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299-315. https://doi.org/10.1136/gut.4.4.299.
4. Sonnenberg A, Chang J. Time trends of physician visits for Crohn’s disease and ulcerative colitis in the United States, 1960-2006. 2007;14(2):249-252. https://doi.org/10.1002/ibd.20273.
5. Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13(12):1529-1535. https://doi.org/10.1002/ibd.20250.
6. Truelove S, Witts L. Cortisone in ulcerative colitis. Br Med J. 1955;2:104-108.
7. Jakobovits SL, Travis S. Management of acute severe colitis. Br Med Bull. 2005;75(1):131-144. https://doi.org/10.1093/bmb/ldl001.
8. Lynch R, Lowe D, Protheroe A, Driscoll R, Rhodes J, Arnott I. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther. 2013;38(8):935-945. https://doi.org/10.1111/apt.12473.
9. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileoanal pouch disorders. J Crohns Colitis. 2017;11(6):649-670. https://doi.org/10.1093/ecco-jcc/jjx008.
10. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501. https://doi.org/10.1038/ajg.2009.727.
11. Dassopoulos T, Cohen RD, Scherl EJ, Schwartz RM, Kosinski L, Regueiro MD. Ulcerative colitis care pathway. Gastroenterology. 2015;149(1):238-245. https://doi.org/10.1053/j.gastro.2015.05.036.
12. Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A nationwide analysis of changes in severity and outcomes of inflammatory bowel disease hospitalizations. J Gastrointest Surg. 2011;15(2):267-276. https://doi.org/10.1007/s11605-010-1396-3.
13. Kelso M, Weideman RA, Cipher DJ, Feagins LA. Factors associated with length of stay in veterans with inflammatory bowel disease hospitalized for an acute flare. Inflamm Bowel Dis. 2017;24(1):5-11. https://doi.org/10.1093/ibd/izx020.
14. Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Nguyen GC. Impact of gastroenterologist care on health outcomes of hospitalized ulcerative colitis patients. Gut. 2012;61(10):1410-1416. https://doi.org/10.1136/gutjnl-2011-301978.
15. Lee NS, Pola S, Groessl EJ, Rivera-Nieves J, Ho SB. Opportunities for improvement in the care of patients hospitalized for inflammatory bowel disease-related colitis. Dig Dis Sci. 2016;61(4):1003-1012. https://doi.org/10.1007/s10620-016-4046-0.
16. Neary BP, Doherty GA. A structured care pathway improves quality of care for acute severe ulcerative colitis. Gastroenterology. 2017;152(5):S218. https://doi.org/10.1016/S0016-5085(17)31028-4.
17. Klein A, Eliakim R. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals. 2010;3(4):1084-1092. https://doi.org/10.3390/ph3041084.
18. Chen JH, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44(2):127-144. https://doi.org/10.1111/apt.13670.
19. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982-1992. https://doi.org/10.1097/MIB.0000000000000392.
20. Solem CA, Loftus EV, Jr., Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707-712. https://doi.org/10.1097/01.MIB.0000173271.18319.53.
21. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5(1):103-110. https://doi.org/10.1016/j.cgh.2006.09.033.
22. Kaur M, Singapura P, Kalakota N, et al. Factors that contribute to indeterminate results from the QuantiFERON-TB Gold in-tube test in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018;16(10):1616-1621.e1. https://doi.org/10.1016/j.cgh.2017.11.038.
23. Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107(2):179-194. https://doi.org/10.1038/ajg.2011.386.
24. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Clinical Guidelines committee. American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827-834.
25. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
26. Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104(5):1162-1169. https://doi.org/10.1038/ajg.2009.4.
27. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(6):1443-1450. https://doi.org/10.1111/j.1572-0241.2007.01780.x.
28. Rahier J-F, Yazdanpanah Y, Colombel J-F, Travis S. The European (ECCO) Consensus on infection in IBD: what does it change for the clinician? Gut. 2009;58(10). https://doi.org/10.1136/gut.2008.175950.
29. Meyer AM, Ramzan NN, Loftus EV, Jr., Heigh RI, Leighton JA. The diagnostic yield of stool pathogen studies during relapses of inflammatory bowel disease. J Clin Gastroenterol. 2004;38(9):772-775. https://doi.org/10.1097/01.mcg.0000139057.05297.d6.
30. Chew C, Nolan D, Jewell D. Small bowel gas in severe ulcerative colitis. Gut. 1991;32(12):1535-1537. https://doi.org/10.1136/gut.32.12.1535.
31. Zakeri N, Pollok RC. Diagnostic imaging and radiation exposure in inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2165-2178. https://doi.org/10.3748/wjg.v22.i7.2165.
32. Llaó J, Naves JE, Ruiz-Cerulla A, et al. Intravenous corticosteroids in moderately active ulcerative colitis refractory to oral corticosteroids. J Crohns Colitis. 2014;8(11):1523-1528. https://doi.org/10.1016/j.crohns.2014.06.010.
33. Seo M, Okada M, Yao T, Matake H, Maeda K. Evaluation of the clinical course of acute attacks in patients with ulcerative colitis through the use of an activity index. Journal of Gastroenterology. 2002;37(1):29-34. https://doi.org/10.1007/s535-002-8129-2.
34. Meyers S, Sachar DB, Goldberg JD, Janowitz HD. Corticotropin versus hydrocortisone in the intravenous treatment of ulcerative colitis: a prospective, randomized, double-blind clinical trial. Gastroenterology. 1983;85(2):351-357.
35. Ho G, Mowat C, Goddard C, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second‐line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19(10):1079-1087. https://doi.org/10.1111/j.1365-2036.2004.01945.x.
36. Järnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89(5):1005-1013. https://doi.org/10.1016/0016-5085(85)90201-X.
37. Wang JY, Terdiman JP, Vittinghoff E, Minichiello T, Varma MG. Hospitalized ulcerative colitis patients have an elevated risk of thromboembolic events. World J Gastroenterol. 2009;15(8):927-935. https://doi.org/10.3748/wjg.15.927.
38. Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835-848. https://doi.org/10.1053/j.gastro.2014.01.042.
39. Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(9):2272-2280. https://doi.org/10.1111/j.1572-0241.2008.02052.x.
40. Tinsley A, Naymagon S, Enomoto LM, Hollenbeak CS, Sands BE, Ullman TA. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7(12):e635-e640. https://doi.org/10.1016/j.crohns.2013.05.002.
41. Pleet JL, Vaughn BP, Morris JA, Moss AC, Cheifetz AS. The use of pharmacological prophylaxis against venous thromboembolism in hospitalized patients with severe active ulcerative colitis. Aliment Pharmacol Ther. 2014;39(9):940-948. https://doi.org/10.1111/apt.12691.
42. Gan SI, Beck PL. A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98(11):2363-2371 https://doi.org/10.1111/j.1572-0241.2003.07696.x.
43. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621-630. https://doi.org/10.1016/j.cgh.2006.03.002.
44. Docherty MJ, Jones III RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol. 2011;7(9):592-601.
45. Singh S, Proudfoot JA, Dulai PS, et al. No benefit of concomitant 5-aminosalicylates in patients with ulcerative colitis escalated to biologic therapy: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018;113(8):1197-1205. https://doi.org/10.1038/s41395-018-0144-2.
46. Mantzaris GJ, Hatzis A, Kontogiannis P, Triadaphyllou G. Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol. 1994;89(1):43-46.
47. Mantzaris GJ, Petraki K, Archavlis E, et al. A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol. 2001;36(9):971-974.
48. Rahier J-F, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443-468. https://doi.org/10.1016/j.crohns.2013.12.013.
49. Dickinson RJ, Ashton MG, Axon AT, Smith RC, Yeung CK, Hill GL. Controlled trial of intravenous hyperalimentation and total bowel rest as an adjunct to the routine therapy of acute colitis. Gastroenterology. 1980;79(6):1199-1204.
50. McIntyre P, Powell-Tuck J, Wood S, et al. Controlled trial of bowel rest in the treatment of severe acute colitis. Gut. 1986;27(5):481-485. https://doi.org/10.1136/gut.27.5.481.
51. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38(6):905-910. https://doi.org/10.1136/gut.38.6.905.
52. Bernardo S, Fernandes SR, Goncalves AR, et al. Predicting the course of disease in hospitalized patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2018;25(3):541-546. https://doi.org/10.1093/ibd/izy256.
53. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769-784. https://doi.org/10.1093/ecco-jcc/jjx009.
54. Randall J, Singh B, Warren B, Travis S, Mortensen N, George B. Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. Br J Surg. 2010;97(3):404-409. https://doi.org/10.1002/bjs.6874.
55. Bartels S, Gardenbroek T, Ubbink D, Buskens C, Tanis P, Bemelman W. Systematic review and meta‐analysis of laparoscopic versus open colectomy with end ileostomy for non‐toxic colitis. Br J Surg. 2013;100(6):726-733. https://doi.org/10.1002/bjs.9061.
56. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomized controlled trial. Lancet. 2012;380(9857):1909-1915. https://doi.org/10.1016/S0140-6736(12)61084-8.
57. Leblanc S, Allez M, Seksik P, et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol. 2011;106(4):771-777. https://doi.org/10.1038/ajg.2011.62.
58. Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111(4):477-491. https://doi.org/10.1038/ajg.2016.7.
59. Feuerstein JD, Akbari M, Tapper EB, Cheifetz AS. Systematic review and meta-analysis of third-line salvage therapy with infliximab or cyclosporine in severe ulcerative colitis. Ann Gastroenterol. 2016;29(3):341-347. https://doi.org/10.20524/aog.2016.0032.
60. Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associated with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14(2):251-258. https://doi.org/10.1016/j.cgh.2015.10.029.
61. Hindryckx P, Novak G, Vande Casteele N, et al. Review article: dose optimization of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017;45(5):617-630. https://doi.org/10.1111/apt.13913.
62. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(2):330-335. https://doi.org/10.1016/j.cgh.2014.07.041.
63. Herfarth HH, Rogler G, Higgins PD. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol. 2015;13(2):336-338. https://doi.org/10.1016/j.cgh.2014.09.045.
64. Van Assche G, D’haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125(4):1025-1031.
65. Arts J, D’haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10(2):73-78.
66. Tarabar D, El Jurdi K, Yvellez O, et al. 330-combination therapy of cyclosporine and vedolizumab is effective and safe for severe, steroid-resistant ulcerative colitis patients: a prospective study. Gastroenterology. 2018;154(6):S-82-S-83.https://doi.org/10.1016/S0016-5085(18)30725-X.
67. Szántó K, Molnár T, Farkas K. New promising combo therapy in inflammatory bowel diseases refractory to anti-TNF agents: cyclosporine plus vedolizumab. J Crohns Colitis. 2018;12(5):629. https://doi.org/10.1093/ecco-jcc/jjx179.
68. Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134(3):680-687. https://doi.org/10.1053/j.gastro.2008.01.004.
69. Spiegel BM, Ho W, Esrailian E, et al. Controversies in ulcerative colitis: a survey comparing decision making of experts versus community gastroenterologists. Clin Gastroenterol Hepatol. 2009;7(2):168-174. https://doi.org/10.1016/j.cgh.2008.08.029.
70. Ananthakrishnan AN, Issa M, Beaulieu DB, et al. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15(2):176-181. https://doi.org/10.1002/ibd.20639.
71. Nguyen GC, Murthy SK, Bressler B, et al. Quality of care and outcomes among hospitalized inflammatory bowel disease patients: a multicenter retrospective study. Inflamm Bowel Dis. 2017;23(5):695-701. https://doi.org/10.1097/MIB.0000000000001068.
72. Rotter T, Kugler J, Koch R, et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs, and patient outcomes. BMC Health Serv Res. 2008;8:265. https://doi.org/10.1186/1472-6963-8-265.
73. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (stride): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338. https://doi.org/10.1038/ajg.2015.233.
Ulcerative colitis (UC) is a chronic inflammatory condition of the colonic mucosa. Classically, it starts in the rectum and can extend continuously from the distal to the proximal colon. The defining clinical symptom of UC is bloody diarrhea, typically accompanied by rectal urgency and mucus discharge. The natural history of this disease includes periods of exacerbations and remissions occurring spontaneously or in response to medical treatment.1
Acute severe ulcerative colitis (ASUC) is a potentially life-threatening complication of UC that typically requires hospitalization and interdisciplinary care between hospitalists, gastroenterologists, and colorectal or general surgeons. The risk of a patient with UC requiring hospitalization for ASUC ranges from 15%-25%2,3 and, in total, UC accounts for 30,000 hospital visits annually.4 The direct medical costs exceed $4 billion annually, with hospital costs of over $960 million.5 Historically, mortality from ASUC was as high as 24% but decreased substantially to 7% after the introduction of systemic corticosteroid therapy.6 Further advances in care have reduced mortality to approximately 1% or less.7,8 Nonetheless, up to 20% of patients admitted with ASUC have a colectomy on their first admission, and this rate rises to 40% after two admissions.2
DEFINING ACUTE SEVERE ULCERATIVE COLITIS
To categorize UC severity, assess patients using the Truelove and Witt’s criteria. The system classifies patients as having mild, moderate, severe, or fulminant disease. Severe disease by these criteria includes patients with >6 bloody bowel movements per day and at least one of the following clinical features: fever (>37.8°C), tachycardia (>90 bpm), anemia (hemoglobin <10.5 g/dl), or elevated inflammatory markers (traditionally, erythrocyte sedimentation rate greater than 30 mm/h or, more recently, C-reactive protein (CRP) greater than 30 mg/L. (Table 1).6,9
Fulminant colitis refers to a subgroup of patients with more than 10 stools per day, continuous bleeding, abdominal pain, colonic dilatation on abdominal X-ray film, and severe toxic symptoms including fever and anorexia. Such patients are at risk of progressing to toxic megacolon and bowel perforation.10
INDICATIONS FOR HOSPITALIZATION AND INPATIENT LEVEL OF CARE
Patients with ASUC almost always require hospitalization for their disease management. In many cases, these patients have been receiving outpatient oral prednisone 40-60 mg daily but continue to have ongoing disease activity.11 Most patients will require close clinical monitoring, frequent blood testing, endoscopic or radiologic evaluation, as well as administration of intravenous corticosteroids. The average length of stay (LOS) ranges from 4.6 to 12.5 days, depending on disease severity.12 Not surprisingly, Kelso et al. reported that predictors of hospital LOS greater than four days include initiating a biologic drug in the hospital, undergoing two or more imaging modalities and treatment with intravenous steroids,13 and so it is rare that patients do not meet billing requirements for an inpatient level of care.
INITIAL EVALUATION
The multifaceted initial inpatient evaluation of patients with ASUC aims to assess disease severity, identify and prevent potential complications, and initiate planning for potential failure of first-line pharmacologic therapy. Due to the accumulating evidence that involving physicians with expertise in managing ASUC improves outcomes, gastroenterologists should be involved in the care of patients with ASUC from the time of their admission.14,15 Additionally, creating standardized care pathways for the management of ASUC can reduce cost, LOS, and improve quality.16
History and Physical Examination
Patients should be asked about fever, abdominal pain, nausea, emesis, bloating, weight loss, and bowel movements (frequency, consistency, the presence of blood, urgency, nighttime awakenings). The number of bowel movements over a 24-hour period should be quantified as this helps assess the overall disease severity (Table 1).
The patient’s initial inflammatory bowel disease (IBD) history is also essential. The review of pertinent information regarding the patient’s initial diagnosis of UC includes the severity and anatomic extent of disease, extraintestinal manifestations, previous medical therapies, and surgical interventions. Exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) or antibiotics should be identified as they may precipitate flares.17 Travel history may be pertinent as travel increases the risk of infections with food-borne or parasitic pathogens.18
Physical examination begins with an assessment of vital signs and volume status. Abdominal examination should include evaluation of bowel sounds, an assessment of distention, location, the extent of abdominal tenderness, and peritoneal signs. The abdominal exam should be interpreted in the context of the patient’s medications, as the use of steroid or analgesic therapies may affect the sensitivity for detecting complications. An external rectal exam evaluating perianal disease should be performed, as perianal disease raises concern for Crohn’s, a disease whose surgical management differs from UC.
A careful exam for extraintestinal manifestations is also essential. The skin should be evaluated for any new rashes, especially on the anterior shin consistent with erythema nodosum or ulcerated lesions on the skin suggestive of pyoderma gangrenosum. The peripheral joints should also be examined for any synovitis. Additional examinations should be performed based on any reported symptoms (eg, the ophthalmic exam for uveitis or scleritis if visual changes or eye pain are reported). Some extraintestinal manifestations require subspecialty consultation and comanagement to guide disease therapy. Patients with underlying pyoderma gangrenosum may require a dermatology consultation to guide management. Ocular inflammation requires ophthalmology involvement, and inflammatory arthritis is best comanaged with rheumatology.19
Laboratory Testing
Initial testing should include a complete blood count with differential, basic metabolic panel, and liver chemistries including alkaline phosphatase and albumin. When relevant, pregnancy testing should be performed. Measure CRP on admission so that its trajectory can be followed during therapy. However, a normal CRP does not exclude the presence of a UC flare as a subset of patients with ASUC will have a normal CRP despite severe mucosal inflammation.20
Since one-third of patients do not respond to intravenous corticosteroids and will require rescue therapy during the hospitalization with infliximab or cyclosporine, anticipatory testing for these medications should be performed on admission to avoid delays in the administration of rescue therapy.6,21 This should include an interferon-gamma release assay (eg, quantiferon gold) to test for latent tuberculosis and hepatitis B serologies in anticipation of possible treatment with infliximab. An interferon-gamma release assay is preferred to a tuberculin skin test because patients may be anergic, and a skin test does not provide a control to determine whether a negative test is due to anergy. In contrast, although a quantiferon gold test can be indeterminate in ASUC due to disease activity and systemic steroids, the results indicate if the patient is anergic so that one will not rely on a false-negative result. In the event of an equivocal result, a careful clinical assessment for risks of TB exposures should be elicited, and a chest radiograph should be obtained.22 In patients with prior high risk of tuberculosis exposures or a positive test for tuberculosis, an infectious disease specialist should be consulted early to advise if therapy should be started in preparation for the potential use of infliximab.23 In cases where cyclosporine may be considered, magnesium and total cholesterol level should be checked. Sending thiopurine methyltranferase (TPMT) enzyme activity should be considered as well, in case of a need for future thiopurine use for maintenance of disease activity.24
Infectious diarrhea may be indistinguishable from ASUC and may also be the trigger of a flare; thus, it is important to rule out infection with stool microbiologic studies. Most importantly, Clostridium difficile infection must be ruled out in all patients with ASUC. Although patients with IBD, especially those with UC, have significantly higher rates of asymptomatic C. difficile carriage than the general population, a positive polymerase chain reaction test for C. difficile in a patient with ASUC should prompt treatment with oral vancomycin.25 However, if carriage if suspected and a subsequent enzyme-linked immunoassay for C. difficile toxin is negative, treatment can be discontinued. Active C. difficile infection in patients with IBD is associated with increased disease severity, greater length of hospital stay, and increased the likelihood of colectomy and mortality.26,27 Other bacterial infections including Escherichia coli, Campylobacter, Shigella, Salmonella, Yersinia, Entamoeba histolytica, as well as other parasitic infestations may mimic UC. Testing should be considered in cases of foreign travel, immunosuppression or contact with other persons with diarrhea.7,28 Routine testing of these other enteric infections without a clear exposure risk is of little benefit and may raise costs.23,29
Radiologic Evaluation
A plain X-ray film of the abdomen should be obtained in all patients on admission to evaluate for evolving colonic dilation or undiagnosed free air. Small bowel distension >3 cm may predict an increased risk of colectomy.30 Clinicians must be mindful that steroids can mask peritoneal signs and that retroperitoneal perforations may not be apparent on plain X-ray films. Nonetheless, a CT of the abdomen is usually not necessary and should be reserved for cases with severe abdominal pain out of proportion to clinical signs in which a plain X-ray film is unrevealing. Judicious use of CT imaging is especially important in younger patients, as there is growing concern that patients with IBD may be exposed to potentially harmful cumulative levels of radiation in their lifetime from repeated CT imaging.31
Endoscopic Evaluation
Flexible sigmoidoscopy aids in the assessment of disease severity and extent and biopsies can assist in ruling out a diagnosis of cytomegalovirus (CMV) colitis in patients already on immunosuppression. For this reason, many clinicians prefer to perform a sigmoidoscopy on admission.23 If one is not performed on admission, a sigmoidoscopy is advised in all patients who are not responding adequately after 72 hours of intravenous steroid therapy in order to rule out superimposed CMV colitis.28
Sigmoidoscopy should be avoided in patients with toxic megacolon and when there is a concern for peritonitis. A complete colonoscopy is rarely indicated in the acute setting and carries a theoretical risk of colonic perforation.7
INITIAL THERAPY
The first therapeutic steps aim to reduce inflammation with the use of systemic corticosteroids, avoid colonic and extraintestinal complications, and plan for the potential need for rescue therapy.
Intravenous Corticosteroids
The cornerstone of ASUC management is treatment with intravenous corticosteroids. Their initiation should not be delayed in patients with an established diagnosis of UC while waiting for results of evaluations for infectious colitis. Even among patients who have failed oral steroids, a meta-regression analysis showed that two-thirds of patients will still respond to intravenous corticosteroids.21,32 Methylprednisolone 20 mg IV three times daily (or hydrocortisone 100 mg IV three times daily) is a standard regimen; higher doses do not provide additional benefit.21 Patients’ response to intravenous steroids should be assessed with repeat labs including CRP and an assessment of the total number of bowel movements over a 24-hour period, with special attention to their overall response after three days of treatment.33-36
Intravenous Fluids
Many patients admitted with ASUC will have significant volume depletion, and intravenous fluids should be administered in a manner like other volume-depleted or oral-intake-restricted patients.
Venous Thromboembolism Prophylaxis
The risk of VTE in hospitalized patients with IBD exceeds that of inpatients without IBD, approximately 2%, a risk similar to patients with respiratory failure.37 Additionally, VTE in hospitalized patients with IBD is associated with a 2.5-fold increase in mortality.38,39 Therefore, all patients hospitalized with ASUC should receive subcutaneous unfractionated or low molecular weight heparin or fondaparinux for VTE prophylaxis. Rectal bleeding, expected in ASUC, is not a contraindication to chemo-prophylaxis. Additionally, it is important to check if patients are receiving the ordered VTE prophylaxis.40,41 Pleet et al. found that only 7% of patients at a tertiary center had adequate prophylaxis for greater than 80% of their hospitalization.41
Unnecessary or Potentially Harmful Medications
Several medications have the potential for misuse in patients hospitalized with UC.
Antimotility Agents
Loperamide, diphenoxylate, and opiate antidiarrheals should not be used as they may provoke toxic megacolon.42 Similarly, drugs with antimotility side effects (eg, anticholinergics) should be avoided.
Opiates
In addition to their undesirable antimotility effect, the use of opiates has been associated with poor outcomes among inpatients and outpatients with IBD, including increased morbidity and mortality.43,44 Pain severe enough to require opiates should raise suspicion for toxic megacolon, perforation, or a noninflammatory etiology. If opiates are utilized, they should be ordered as one-time doses and the patient should be reassessed for each dose.
Nonsteroidal Anti-inflammatory Drugs
These drugs, which include oral NSAIDs, intravenous ketorolac, and topic diclofenac gels, may increase disease activity in inflammatory bowel disease and should be avoided.17
5-aminosalicylates (5-ASA)
A small proportion of patients experience a paradoxical worsening of diarrhea due to the use of 5-ASA agents such as mesalamine. It is reasonable to discontinue or avoid the use of 5-ASA agents in hospitalized patients, especially as there is little to no benefit from combining a 5-ASA with a biologic or immunosuppressive drug.45
Antibiotics
There is no role for the routine use of antibiotics in patients hospitalized with ASUC. 23,46,47 Inappropriate use of antibiotics raises the risk of C. difficile infection and antibiotic resistance. However, in cases of suspected toxic megacolon or perforation, antibiotics should be administered. In situations in which a patient is treated with triple immunosuppression (ie, steroids plus two other agents, cyclosporine and mercaptopurine) antibiotic prophylaxis for Pneumocystis jiroveci is advisable.48 Using a large insurance database, Long et al. reported a low absolute incidence of Pneumocystis jiroveci in IBD patients but noted that the risk in patients with IBD was still significantly higher than matched controls. While it can be considered, we typically refrain from using prophylaxis in patients on double immunosuppression (for example, steroids plus infliximab) due to the potential adverse effects of antibiotics in this population, though many advocate using prophylaxis for all patients on cyclosporine even if this is only double immunosuppressive therapy.23
Surgical Consultation
Involving a surgeon early in an ASUC patient’s care—before needing urgent colectomy—is critical. As part of the consultation, a surgeon experienced in IBD should meet with patients to discuss multistage colectomy with ileostomy and potential future J-pouch (ileal pouch-anal anastomosis) formation. Patients should be given ample opportunity to ask questions before surgery may become urgent. Also, patients should be counseled on realistic expectations of ostomy and pouch function and, ideally, meet with an ostomy nurse.23
At some centers, surgical consultation is requested on the first hospital day, but this can result in consultations for patients who ultimately respond to intravenous steroids. Therefore, some centers advocate for surgical consultation only after a patient has failed treatment with intravenous steroids (ie, day three to four) when the risk of needing surgical management increases.23
Nutrition
Bowel rest with parenteral nutrition does not improve outcomes in ASUC versus an oral diet, and there is no contraindication to allowing patients to continue on a regular diet unless they have toxic megacolon or other signs of fulminant colitis.49,50 However, patients may feel better eating less, as this will reduce their bowel movement frequency. Unfortunately, this can give a false sense of reassurance that the patient is improving. Therefore, it remains important to evaluate a patient’s symptoms in the context of their food intake.
Assessing Response to Steroids
Patients who do not respond adequately to the first-line intravenous steroid therapy will require medical or surgical rescue therapy; therefore, deciding whether a patient has responded is essential. Patients should have less than four bowel movements per day – ideally just one to two – with no blood to indicate a complete response. For more ambiguous situations, although there is no strict definition of steroid responsiveness, multiple prediction indices have attempted to identify patients who will require rescue therapy. One of the simplest, the Oxford index, illustrates two of the most critical parameters to follow, stool frequency and CRP.51 In a preinfliximab cohort, Oxford index predicted an 85% likelihood of colectomy in patients with eight or more daily bowel movements or with three to eight daily bowel movements and a CRP greater than 45 mg/L after three days of intravenous steroid treatment.52 To assist with assessing responsiveness to therapy, we ask patients to log their bowel movements – either on paper or on a whiteboard in the hospital room – so that we can review their progress daily. Other predictors of colectomy include hypoalbuminemia, scoring of endoscopic severity, and colonic dilation.53
Patients who fail to respond to intravenous corticosteroids after three days33,35 of treatment should be started on rescue therapy with infliximab or cyclosporine or undergo colectomy. A common pitfall in the treatment of ASUC is waiting for a response to steroids beyond this time frame, after which patients are unlikely to benefit.34,36 Furthermore, patients for whom surgical rescue therapy is delayed have higher operative morbidity and mortality.54,55 Because timely decision making regarding rescue therapy is crucial to optimizing outcomes, patient education efforts regarding potential rescue therapy should take place on admission or soon after, rather than waiting to ascertain steroid responsiveness.
RESCUE THERAPY FOR STEROID-REFRACTORY DISEASE
Medical options for rescue therapy include the antitumor necrosis factor (anti-TNF) agent infliximab or the calcineurin inhibitor cyclosporine. In general, infliximab and cyclosporine have been found to be roughly equivalent in efficacy in clinical trials regarding response, remission, and colectomy at 12 months.56,57 However, many clinicians prefer infliximab due to its relative ease of use, familiarity with the agent from outpatient experience, and ability to continue to use long term for maintenance of disease remission.58 In contrast to infliximab, intravenous cyclosporine requires closer monitoring and labs to assess the therapeutic trough level. The decision regarding which drug to use should be made on a case-by-case basis in conjunction with a gastroenterologist experienced in their use, and if no such specialist is available, transfer to a specialized center should be considered. Generally, successive treatment with cyclosporine or infliximab followed by third-line salvage therapy with the other drug should be avoided due to low rates of response and high rates of adverse events.59
Infliximab
Infliximab is an intravenously-administered anti-TNF monoclonal chimeric antibody that is effective both for outpatient treatment of moderate to severe UC and inpatient treatment of ASUC.1 It is relatively contraindicated in patients with untreated latent tuberculosis, demyelinating disease, advanced congestive heart failure, or uncontrolled infection.
The optimal dosing strategy for infliximab in ASUC is unknown. Infliximab clearance in the setting of ASUC is increased, partly because it is bound to albumin, which is often low in ASUC, and partly because it is excreted in the stool.60,61 As a result, accelerated loading doses may be more successful than a typical loading schedule,62 and most clinicians use alternative dosing strategies.63 Our typical approach for ASUC is an initial dose of 10 mg/kg rather than 5 mg/kg, with an additional 10 mg/kg dose 48-72 hours later if an adequate clinical response is lacking. Patients who respond to infliximab can continue to use the drug as an outpatient for maintenance of remission.
Cyclosporine
Cyclosporine is a fast-acting immunosuppressive agent that acts primarily via T-cell inhibition. Although older literature used a dose of 4 mg/kg per day, a randomized trial demonstrated similar response rates to a dose of 2 mg/kg per day.64 Patients receiving treatment with cyclosporine, which is given as a continuous infusion, must be monitored for toxicities. These can include potentially severe infection, seizures (often associated with low total cholesterol or hypomagnesemia), electrolyte abnormalities, renal impairment, hypertension, hypertrichosis, tremor, and others.65
Before initiation of treatment, serum cholesterol levels should be obtained to screen for low total cholesterol that may portend risk of seizures on the drug. Additionally, baseline creatinine and magnesium should be established. While on treatment, daily serum cyclosporine levels and electrolytes including magnesium should be measured. Patients who respond to intravenous cyclosporine must be transitioned to oral cyclosporine and have stable drug levels before discharge. Unfortunately, oral cyclosporine has not been shown to be as effective as long-term maintenance therapy. Therefore, cyclosporine can only be used as a “bridge” to another therapy. Historically, thiopurines like azathioprine or mercaptopurine have been used for this purpose because they are effective for the treatment of UC but may require months to have a full therapeutic effect. There have been promising reports of using vedolizumab similarly.66,67 Vedolizumab is a monoclonal antibody that selectively blocks lymphocyte trafficking to the gut that, like thiopurines, has an onset of action that is significantly longer than calcineurin and TNF inhibitors.
COLECTOMY
Colectomy should be considered as a second- or third-line therapy for patients who fail to respond to intravenous corticosteroids. In an analysis of 10 years of data from the Nationwide Inpatient Sample, mortality rates for colectomy in this setting varied from 0.7% at high volume centers to 4% at low volume centers.68 Therefore, if a patient is not hospitalized at a center with expertise in colectomy for UC, transfer to a specialized center should be considered. Colectomy should be performed promptly in all the patients who have failed rescue therapy with infliximab or cyclosporine or have opted against medical rescue therapy. Surgery should be performed emergently in patients with toxic megacolon, uncontrolled colonic hemorrhage or perforation.
QUALITY OF CARE AND THE USE OF CARE PATHWAYS
Physician and center-level characteristics are associated with the quality of care and outcomes in ASUC. Gastroenterologists with expertise in IBD are more likely than other gastroenterologists to request appropriate surgical consultation for steroid-refractory patients,69 and inpatients with ASUC primarily cared by gastroenterologists rather than nongastroenterologists have lower in-hospital and one-year mortality.14 Moreover, surgical outcomes differ based on center volume, with higher volume centers having lower rates of postoperative mortality.68,70 However, even at referral centers, key metrics of care quality such as rates of VTE prophylaxis, testing for C. difficile, and timely rescue therapy for steroid-refractory UC patients are suboptimal, with only 70%-82% of patients with IBD hospitalized at four referral centers in Canada meeting these metrics.71
Inpatient clinical pathways reduce LOS, reduce hospital costs, and likely reduce complications.72 For this reason, a consensus group recommended the use of care pathways for the management of ASUC and, although there is little data on the use of pathways for ASUC specifically, the use of such a pathway in the United Kingdom was associated with improved metrics including LOS, time to VTE prophylaxis, testing of stool for infection, CRP measurement, and timely gastroenterologist consultation.16,18
DISCHARGE CRITERIA AND FOLLOW UP
In general, patients should enter clinical remission, defined as resolution of rectal bleeding and diarrhea or altered bowel habits,73 before discharge, and achieving this may require a relatively prolonged hospitalization. Most patients should have one to two bowel movements a day without blood but, at a minimum, all should have less than four nonbloody bowel movements per day. Patients are candidates for discharge if they remain well after transitioning to oral prednisone at a dose of 40-60 mg daily and tolerate a regular diet.
For patients who initiated infliximab during their admission, plans for outpatient infusions including insurance approval should be made before discharge, and patients who started cyclosporine should be transitioned to oral dosing and have stable serum concentrations before leaving the hospital. Patients should leave with a preliminary plan for a steroid taper, which may vary depending on their clinical presentation. Usually, gastroenterology follow-up should be arranged after two weeks following discharge, but patients on cyclosporine need sooner laboratory monitoring.
CONCLUSION
The care of patients with ASUC requires an interdisciplinary team and close collaboration between hospitalists, gastroenterologists, and surgeons. Patients should be treated with intravenous corticosteroids and monitored carefully for response and need for rescue therapy. Establishing algorithms for the management of patients with ASUC can further improve the care of these complex patients.
Disclosures
Drs. Feuerstein, Fudman, and Sattler report no potential conflict of interest.
Funding
This work was not supported by any grant.
Ulcerative colitis (UC) is a chronic inflammatory condition of the colonic mucosa. Classically, it starts in the rectum and can extend continuously from the distal to the proximal colon. The defining clinical symptom of UC is bloody diarrhea, typically accompanied by rectal urgency and mucus discharge. The natural history of this disease includes periods of exacerbations and remissions occurring spontaneously or in response to medical treatment.1
Acute severe ulcerative colitis (ASUC) is a potentially life-threatening complication of UC that typically requires hospitalization and interdisciplinary care between hospitalists, gastroenterologists, and colorectal or general surgeons. The risk of a patient with UC requiring hospitalization for ASUC ranges from 15%-25%2,3 and, in total, UC accounts for 30,000 hospital visits annually.4 The direct medical costs exceed $4 billion annually, with hospital costs of over $960 million.5 Historically, mortality from ASUC was as high as 24% but decreased substantially to 7% after the introduction of systemic corticosteroid therapy.6 Further advances in care have reduced mortality to approximately 1% or less.7,8 Nonetheless, up to 20% of patients admitted with ASUC have a colectomy on their first admission, and this rate rises to 40% after two admissions.2
DEFINING ACUTE SEVERE ULCERATIVE COLITIS
To categorize UC severity, assess patients using the Truelove and Witt’s criteria. The system classifies patients as having mild, moderate, severe, or fulminant disease. Severe disease by these criteria includes patients with >6 bloody bowel movements per day and at least one of the following clinical features: fever (>37.8°C), tachycardia (>90 bpm), anemia (hemoglobin <10.5 g/dl), or elevated inflammatory markers (traditionally, erythrocyte sedimentation rate greater than 30 mm/h or, more recently, C-reactive protein (CRP) greater than 30 mg/L. (Table 1).6,9
Fulminant colitis refers to a subgroup of patients with more than 10 stools per day, continuous bleeding, abdominal pain, colonic dilatation on abdominal X-ray film, and severe toxic symptoms including fever and anorexia. Such patients are at risk of progressing to toxic megacolon and bowel perforation.10
INDICATIONS FOR HOSPITALIZATION AND INPATIENT LEVEL OF CARE
Patients with ASUC almost always require hospitalization for their disease management. In many cases, these patients have been receiving outpatient oral prednisone 40-60 mg daily but continue to have ongoing disease activity.11 Most patients will require close clinical monitoring, frequent blood testing, endoscopic or radiologic evaluation, as well as administration of intravenous corticosteroids. The average length of stay (LOS) ranges from 4.6 to 12.5 days, depending on disease severity.12 Not surprisingly, Kelso et al. reported that predictors of hospital LOS greater than four days include initiating a biologic drug in the hospital, undergoing two or more imaging modalities and treatment with intravenous steroids,13 and so it is rare that patients do not meet billing requirements for an inpatient level of care.
INITIAL EVALUATION
The multifaceted initial inpatient evaluation of patients with ASUC aims to assess disease severity, identify and prevent potential complications, and initiate planning for potential failure of first-line pharmacologic therapy. Due to the accumulating evidence that involving physicians with expertise in managing ASUC improves outcomes, gastroenterologists should be involved in the care of patients with ASUC from the time of their admission.14,15 Additionally, creating standardized care pathways for the management of ASUC can reduce cost, LOS, and improve quality.16
History and Physical Examination
Patients should be asked about fever, abdominal pain, nausea, emesis, bloating, weight loss, and bowel movements (frequency, consistency, the presence of blood, urgency, nighttime awakenings). The number of bowel movements over a 24-hour period should be quantified as this helps assess the overall disease severity (Table 1).
The patient’s initial inflammatory bowel disease (IBD) history is also essential. The review of pertinent information regarding the patient’s initial diagnosis of UC includes the severity and anatomic extent of disease, extraintestinal manifestations, previous medical therapies, and surgical interventions. Exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) or antibiotics should be identified as they may precipitate flares.17 Travel history may be pertinent as travel increases the risk of infections with food-borne or parasitic pathogens.18
Physical examination begins with an assessment of vital signs and volume status. Abdominal examination should include evaluation of bowel sounds, an assessment of distention, location, the extent of abdominal tenderness, and peritoneal signs. The abdominal exam should be interpreted in the context of the patient’s medications, as the use of steroid or analgesic therapies may affect the sensitivity for detecting complications. An external rectal exam evaluating perianal disease should be performed, as perianal disease raises concern for Crohn’s, a disease whose surgical management differs from UC.
A careful exam for extraintestinal manifestations is also essential. The skin should be evaluated for any new rashes, especially on the anterior shin consistent with erythema nodosum or ulcerated lesions on the skin suggestive of pyoderma gangrenosum. The peripheral joints should also be examined for any synovitis. Additional examinations should be performed based on any reported symptoms (eg, the ophthalmic exam for uveitis or scleritis if visual changes or eye pain are reported). Some extraintestinal manifestations require subspecialty consultation and comanagement to guide disease therapy. Patients with underlying pyoderma gangrenosum may require a dermatology consultation to guide management. Ocular inflammation requires ophthalmology involvement, and inflammatory arthritis is best comanaged with rheumatology.19
Laboratory Testing
Initial testing should include a complete blood count with differential, basic metabolic panel, and liver chemistries including alkaline phosphatase and albumin. When relevant, pregnancy testing should be performed. Measure CRP on admission so that its trajectory can be followed during therapy. However, a normal CRP does not exclude the presence of a UC flare as a subset of patients with ASUC will have a normal CRP despite severe mucosal inflammation.20
Since one-third of patients do not respond to intravenous corticosteroids and will require rescue therapy during the hospitalization with infliximab or cyclosporine, anticipatory testing for these medications should be performed on admission to avoid delays in the administration of rescue therapy.6,21 This should include an interferon-gamma release assay (eg, quantiferon gold) to test for latent tuberculosis and hepatitis B serologies in anticipation of possible treatment with infliximab. An interferon-gamma release assay is preferred to a tuberculin skin test because patients may be anergic, and a skin test does not provide a control to determine whether a negative test is due to anergy. In contrast, although a quantiferon gold test can be indeterminate in ASUC due to disease activity and systemic steroids, the results indicate if the patient is anergic so that one will not rely on a false-negative result. In the event of an equivocal result, a careful clinical assessment for risks of TB exposures should be elicited, and a chest radiograph should be obtained.22 In patients with prior high risk of tuberculosis exposures or a positive test for tuberculosis, an infectious disease specialist should be consulted early to advise if therapy should be started in preparation for the potential use of infliximab.23 In cases where cyclosporine may be considered, magnesium and total cholesterol level should be checked. Sending thiopurine methyltranferase (TPMT) enzyme activity should be considered as well, in case of a need for future thiopurine use for maintenance of disease activity.24
Infectious diarrhea may be indistinguishable from ASUC and may also be the trigger of a flare; thus, it is important to rule out infection with stool microbiologic studies. Most importantly, Clostridium difficile infection must be ruled out in all patients with ASUC. Although patients with IBD, especially those with UC, have significantly higher rates of asymptomatic C. difficile carriage than the general population, a positive polymerase chain reaction test for C. difficile in a patient with ASUC should prompt treatment with oral vancomycin.25 However, if carriage if suspected and a subsequent enzyme-linked immunoassay for C. difficile toxin is negative, treatment can be discontinued. Active C. difficile infection in patients with IBD is associated with increased disease severity, greater length of hospital stay, and increased the likelihood of colectomy and mortality.26,27 Other bacterial infections including Escherichia coli, Campylobacter, Shigella, Salmonella, Yersinia, Entamoeba histolytica, as well as other parasitic infestations may mimic UC. Testing should be considered in cases of foreign travel, immunosuppression or contact with other persons with diarrhea.7,28 Routine testing of these other enteric infections without a clear exposure risk is of little benefit and may raise costs.23,29
Radiologic Evaluation
A plain X-ray film of the abdomen should be obtained in all patients on admission to evaluate for evolving colonic dilation or undiagnosed free air. Small bowel distension >3 cm may predict an increased risk of colectomy.30 Clinicians must be mindful that steroids can mask peritoneal signs and that retroperitoneal perforations may not be apparent on plain X-ray films. Nonetheless, a CT of the abdomen is usually not necessary and should be reserved for cases with severe abdominal pain out of proportion to clinical signs in which a plain X-ray film is unrevealing. Judicious use of CT imaging is especially important in younger patients, as there is growing concern that patients with IBD may be exposed to potentially harmful cumulative levels of radiation in their lifetime from repeated CT imaging.31
Endoscopic Evaluation
Flexible sigmoidoscopy aids in the assessment of disease severity and extent and biopsies can assist in ruling out a diagnosis of cytomegalovirus (CMV) colitis in patients already on immunosuppression. For this reason, many clinicians prefer to perform a sigmoidoscopy on admission.23 If one is not performed on admission, a sigmoidoscopy is advised in all patients who are not responding adequately after 72 hours of intravenous steroid therapy in order to rule out superimposed CMV colitis.28
Sigmoidoscopy should be avoided in patients with toxic megacolon and when there is a concern for peritonitis. A complete colonoscopy is rarely indicated in the acute setting and carries a theoretical risk of colonic perforation.7
INITIAL THERAPY
The first therapeutic steps aim to reduce inflammation with the use of systemic corticosteroids, avoid colonic and extraintestinal complications, and plan for the potential need for rescue therapy.
Intravenous Corticosteroids
The cornerstone of ASUC management is treatment with intravenous corticosteroids. Their initiation should not be delayed in patients with an established diagnosis of UC while waiting for results of evaluations for infectious colitis. Even among patients who have failed oral steroids, a meta-regression analysis showed that two-thirds of patients will still respond to intravenous corticosteroids.21,32 Methylprednisolone 20 mg IV three times daily (or hydrocortisone 100 mg IV three times daily) is a standard regimen; higher doses do not provide additional benefit.21 Patients’ response to intravenous steroids should be assessed with repeat labs including CRP and an assessment of the total number of bowel movements over a 24-hour period, with special attention to their overall response after three days of treatment.33-36
Intravenous Fluids
Many patients admitted with ASUC will have significant volume depletion, and intravenous fluids should be administered in a manner like other volume-depleted or oral-intake-restricted patients.
Venous Thromboembolism Prophylaxis
The risk of VTE in hospitalized patients with IBD exceeds that of inpatients without IBD, approximately 2%, a risk similar to patients with respiratory failure.37 Additionally, VTE in hospitalized patients with IBD is associated with a 2.5-fold increase in mortality.38,39 Therefore, all patients hospitalized with ASUC should receive subcutaneous unfractionated or low molecular weight heparin or fondaparinux for VTE prophylaxis. Rectal bleeding, expected in ASUC, is not a contraindication to chemo-prophylaxis. Additionally, it is important to check if patients are receiving the ordered VTE prophylaxis.40,41 Pleet et al. found that only 7% of patients at a tertiary center had adequate prophylaxis for greater than 80% of their hospitalization.41
Unnecessary or Potentially Harmful Medications
Several medications have the potential for misuse in patients hospitalized with UC.
Antimotility Agents
Loperamide, diphenoxylate, and opiate antidiarrheals should not be used as they may provoke toxic megacolon.42 Similarly, drugs with antimotility side effects (eg, anticholinergics) should be avoided.
Opiates
In addition to their undesirable antimotility effect, the use of opiates has been associated with poor outcomes among inpatients and outpatients with IBD, including increased morbidity and mortality.43,44 Pain severe enough to require opiates should raise suspicion for toxic megacolon, perforation, or a noninflammatory etiology. If opiates are utilized, they should be ordered as one-time doses and the patient should be reassessed for each dose.
Nonsteroidal Anti-inflammatory Drugs
These drugs, which include oral NSAIDs, intravenous ketorolac, and topic diclofenac gels, may increase disease activity in inflammatory bowel disease and should be avoided.17
5-aminosalicylates (5-ASA)
A small proportion of patients experience a paradoxical worsening of diarrhea due to the use of 5-ASA agents such as mesalamine. It is reasonable to discontinue or avoid the use of 5-ASA agents in hospitalized patients, especially as there is little to no benefit from combining a 5-ASA with a biologic or immunosuppressive drug.45
Antibiotics
There is no role for the routine use of antibiotics in patients hospitalized with ASUC. 23,46,47 Inappropriate use of antibiotics raises the risk of C. difficile infection and antibiotic resistance. However, in cases of suspected toxic megacolon or perforation, antibiotics should be administered. In situations in which a patient is treated with triple immunosuppression (ie, steroids plus two other agents, cyclosporine and mercaptopurine) antibiotic prophylaxis for Pneumocystis jiroveci is advisable.48 Using a large insurance database, Long et al. reported a low absolute incidence of Pneumocystis jiroveci in IBD patients but noted that the risk in patients with IBD was still significantly higher than matched controls. While it can be considered, we typically refrain from using prophylaxis in patients on double immunosuppression (for example, steroids plus infliximab) due to the potential adverse effects of antibiotics in this population, though many advocate using prophylaxis for all patients on cyclosporine even if this is only double immunosuppressive therapy.23
Surgical Consultation
Involving a surgeon early in an ASUC patient’s care—before needing urgent colectomy—is critical. As part of the consultation, a surgeon experienced in IBD should meet with patients to discuss multistage colectomy with ileostomy and potential future J-pouch (ileal pouch-anal anastomosis) formation. Patients should be given ample opportunity to ask questions before surgery may become urgent. Also, patients should be counseled on realistic expectations of ostomy and pouch function and, ideally, meet with an ostomy nurse.23
At some centers, surgical consultation is requested on the first hospital day, but this can result in consultations for patients who ultimately respond to intravenous steroids. Therefore, some centers advocate for surgical consultation only after a patient has failed treatment with intravenous steroids (ie, day three to four) when the risk of needing surgical management increases.23
Nutrition
Bowel rest with parenteral nutrition does not improve outcomes in ASUC versus an oral diet, and there is no contraindication to allowing patients to continue on a regular diet unless they have toxic megacolon or other signs of fulminant colitis.49,50 However, patients may feel better eating less, as this will reduce their bowel movement frequency. Unfortunately, this can give a false sense of reassurance that the patient is improving. Therefore, it remains important to evaluate a patient’s symptoms in the context of their food intake.
Assessing Response to Steroids
Patients who do not respond adequately to the first-line intravenous steroid therapy will require medical or surgical rescue therapy; therefore, deciding whether a patient has responded is essential. Patients should have less than four bowel movements per day – ideally just one to two – with no blood to indicate a complete response. For more ambiguous situations, although there is no strict definition of steroid responsiveness, multiple prediction indices have attempted to identify patients who will require rescue therapy. One of the simplest, the Oxford index, illustrates two of the most critical parameters to follow, stool frequency and CRP.51 In a preinfliximab cohort, Oxford index predicted an 85% likelihood of colectomy in patients with eight or more daily bowel movements or with three to eight daily bowel movements and a CRP greater than 45 mg/L after three days of intravenous steroid treatment.52 To assist with assessing responsiveness to therapy, we ask patients to log their bowel movements – either on paper or on a whiteboard in the hospital room – so that we can review their progress daily. Other predictors of colectomy include hypoalbuminemia, scoring of endoscopic severity, and colonic dilation.53
Patients who fail to respond to intravenous corticosteroids after three days33,35 of treatment should be started on rescue therapy with infliximab or cyclosporine or undergo colectomy. A common pitfall in the treatment of ASUC is waiting for a response to steroids beyond this time frame, after which patients are unlikely to benefit.34,36 Furthermore, patients for whom surgical rescue therapy is delayed have higher operative morbidity and mortality.54,55 Because timely decision making regarding rescue therapy is crucial to optimizing outcomes, patient education efforts regarding potential rescue therapy should take place on admission or soon after, rather than waiting to ascertain steroid responsiveness.
RESCUE THERAPY FOR STEROID-REFRACTORY DISEASE
Medical options for rescue therapy include the antitumor necrosis factor (anti-TNF) agent infliximab or the calcineurin inhibitor cyclosporine. In general, infliximab and cyclosporine have been found to be roughly equivalent in efficacy in clinical trials regarding response, remission, and colectomy at 12 months.56,57 However, many clinicians prefer infliximab due to its relative ease of use, familiarity with the agent from outpatient experience, and ability to continue to use long term for maintenance of disease remission.58 In contrast to infliximab, intravenous cyclosporine requires closer monitoring and labs to assess the therapeutic trough level. The decision regarding which drug to use should be made on a case-by-case basis in conjunction with a gastroenterologist experienced in their use, and if no such specialist is available, transfer to a specialized center should be considered. Generally, successive treatment with cyclosporine or infliximab followed by third-line salvage therapy with the other drug should be avoided due to low rates of response and high rates of adverse events.59
Infliximab
Infliximab is an intravenously-administered anti-TNF monoclonal chimeric antibody that is effective both for outpatient treatment of moderate to severe UC and inpatient treatment of ASUC.1 It is relatively contraindicated in patients with untreated latent tuberculosis, demyelinating disease, advanced congestive heart failure, or uncontrolled infection.
The optimal dosing strategy for infliximab in ASUC is unknown. Infliximab clearance in the setting of ASUC is increased, partly because it is bound to albumin, which is often low in ASUC, and partly because it is excreted in the stool.60,61 As a result, accelerated loading doses may be more successful than a typical loading schedule,62 and most clinicians use alternative dosing strategies.63 Our typical approach for ASUC is an initial dose of 10 mg/kg rather than 5 mg/kg, with an additional 10 mg/kg dose 48-72 hours later if an adequate clinical response is lacking. Patients who respond to infliximab can continue to use the drug as an outpatient for maintenance of remission.
Cyclosporine
Cyclosporine is a fast-acting immunosuppressive agent that acts primarily via T-cell inhibition. Although older literature used a dose of 4 mg/kg per day, a randomized trial demonstrated similar response rates to a dose of 2 mg/kg per day.64 Patients receiving treatment with cyclosporine, which is given as a continuous infusion, must be monitored for toxicities. These can include potentially severe infection, seizures (often associated with low total cholesterol or hypomagnesemia), electrolyte abnormalities, renal impairment, hypertension, hypertrichosis, tremor, and others.65
Before initiation of treatment, serum cholesterol levels should be obtained to screen for low total cholesterol that may portend risk of seizures on the drug. Additionally, baseline creatinine and magnesium should be established. While on treatment, daily serum cyclosporine levels and electrolytes including magnesium should be measured. Patients who respond to intravenous cyclosporine must be transitioned to oral cyclosporine and have stable drug levels before discharge. Unfortunately, oral cyclosporine has not been shown to be as effective as long-term maintenance therapy. Therefore, cyclosporine can only be used as a “bridge” to another therapy. Historically, thiopurines like azathioprine or mercaptopurine have been used for this purpose because they are effective for the treatment of UC but may require months to have a full therapeutic effect. There have been promising reports of using vedolizumab similarly.66,67 Vedolizumab is a monoclonal antibody that selectively blocks lymphocyte trafficking to the gut that, like thiopurines, has an onset of action that is significantly longer than calcineurin and TNF inhibitors.
COLECTOMY
Colectomy should be considered as a second- or third-line therapy for patients who fail to respond to intravenous corticosteroids. In an analysis of 10 years of data from the Nationwide Inpatient Sample, mortality rates for colectomy in this setting varied from 0.7% at high volume centers to 4% at low volume centers.68 Therefore, if a patient is not hospitalized at a center with expertise in colectomy for UC, transfer to a specialized center should be considered. Colectomy should be performed promptly in all the patients who have failed rescue therapy with infliximab or cyclosporine or have opted against medical rescue therapy. Surgery should be performed emergently in patients with toxic megacolon, uncontrolled colonic hemorrhage or perforation.
QUALITY OF CARE AND THE USE OF CARE PATHWAYS
Physician and center-level characteristics are associated with the quality of care and outcomes in ASUC. Gastroenterologists with expertise in IBD are more likely than other gastroenterologists to request appropriate surgical consultation for steroid-refractory patients,69 and inpatients with ASUC primarily cared by gastroenterologists rather than nongastroenterologists have lower in-hospital and one-year mortality.14 Moreover, surgical outcomes differ based on center volume, with higher volume centers having lower rates of postoperative mortality.68,70 However, even at referral centers, key metrics of care quality such as rates of VTE prophylaxis, testing for C. difficile, and timely rescue therapy for steroid-refractory UC patients are suboptimal, with only 70%-82% of patients with IBD hospitalized at four referral centers in Canada meeting these metrics.71
Inpatient clinical pathways reduce LOS, reduce hospital costs, and likely reduce complications.72 For this reason, a consensus group recommended the use of care pathways for the management of ASUC and, although there is little data on the use of pathways for ASUC specifically, the use of such a pathway in the United Kingdom was associated with improved metrics including LOS, time to VTE prophylaxis, testing of stool for infection, CRP measurement, and timely gastroenterologist consultation.16,18
DISCHARGE CRITERIA AND FOLLOW UP
In general, patients should enter clinical remission, defined as resolution of rectal bleeding and diarrhea or altered bowel habits,73 before discharge, and achieving this may require a relatively prolonged hospitalization. Most patients should have one to two bowel movements a day without blood but, at a minimum, all should have less than four nonbloody bowel movements per day. Patients are candidates for discharge if they remain well after transitioning to oral prednisone at a dose of 40-60 mg daily and tolerate a regular diet.
For patients who initiated infliximab during their admission, plans for outpatient infusions including insurance approval should be made before discharge, and patients who started cyclosporine should be transitioned to oral dosing and have stable serum concentrations before leaving the hospital. Patients should leave with a preliminary plan for a steroid taper, which may vary depending on their clinical presentation. Usually, gastroenterology follow-up should be arranged after two weeks following discharge, but patients on cyclosporine need sooner laboratory monitoring.
CONCLUSION
The care of patients with ASUC requires an interdisciplinary team and close collaboration between hospitalists, gastroenterologists, and surgeons. Patients should be treated with intravenous corticosteroids and monitored carefully for response and need for rescue therapy. Establishing algorithms for the management of patients with ASUC can further improve the care of these complex patients.
Disclosures
Drs. Feuerstein, Fudman, and Sattler report no potential conflict of interest.
Funding
This work was not supported by any grant.
1. Sands BE. Mount Sinai Expert Guides: Gastroenterology. Hoboken, NJ: John Wiley & Sons; 2014.
2. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4(4):431-437. https://doi.org/10.1016/j.crohns.2010.02.001.
3. Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299-315. https://doi.org/10.1136/gut.4.4.299.
4. Sonnenberg A, Chang J. Time trends of physician visits for Crohn’s disease and ulcerative colitis in the United States, 1960-2006. 2007;14(2):249-252. https://doi.org/10.1002/ibd.20273.
5. Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13(12):1529-1535. https://doi.org/10.1002/ibd.20250.
6. Truelove S, Witts L. Cortisone in ulcerative colitis. Br Med J. 1955;2:104-108.
7. Jakobovits SL, Travis S. Management of acute severe colitis. Br Med Bull. 2005;75(1):131-144. https://doi.org/10.1093/bmb/ldl001.
8. Lynch R, Lowe D, Protheroe A, Driscoll R, Rhodes J, Arnott I. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther. 2013;38(8):935-945. https://doi.org/10.1111/apt.12473.
9. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileoanal pouch disorders. J Crohns Colitis. 2017;11(6):649-670. https://doi.org/10.1093/ecco-jcc/jjx008.
10. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501. https://doi.org/10.1038/ajg.2009.727.
11. Dassopoulos T, Cohen RD, Scherl EJ, Schwartz RM, Kosinski L, Regueiro MD. Ulcerative colitis care pathway. Gastroenterology. 2015;149(1):238-245. https://doi.org/10.1053/j.gastro.2015.05.036.
12. Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A nationwide analysis of changes in severity and outcomes of inflammatory bowel disease hospitalizations. J Gastrointest Surg. 2011;15(2):267-276. https://doi.org/10.1007/s11605-010-1396-3.
13. Kelso M, Weideman RA, Cipher DJ, Feagins LA. Factors associated with length of stay in veterans with inflammatory bowel disease hospitalized for an acute flare. Inflamm Bowel Dis. 2017;24(1):5-11. https://doi.org/10.1093/ibd/izx020.
14. Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Nguyen GC. Impact of gastroenterologist care on health outcomes of hospitalized ulcerative colitis patients. Gut. 2012;61(10):1410-1416. https://doi.org/10.1136/gutjnl-2011-301978.
15. Lee NS, Pola S, Groessl EJ, Rivera-Nieves J, Ho SB. Opportunities for improvement in the care of patients hospitalized for inflammatory bowel disease-related colitis. Dig Dis Sci. 2016;61(4):1003-1012. https://doi.org/10.1007/s10620-016-4046-0.
16. Neary BP, Doherty GA. A structured care pathway improves quality of care for acute severe ulcerative colitis. Gastroenterology. 2017;152(5):S218. https://doi.org/10.1016/S0016-5085(17)31028-4.
17. Klein A, Eliakim R. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals. 2010;3(4):1084-1092. https://doi.org/10.3390/ph3041084.
18. Chen JH, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44(2):127-144. https://doi.org/10.1111/apt.13670.
19. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982-1992. https://doi.org/10.1097/MIB.0000000000000392.
20. Solem CA, Loftus EV, Jr., Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707-712. https://doi.org/10.1097/01.MIB.0000173271.18319.53.
21. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5(1):103-110. https://doi.org/10.1016/j.cgh.2006.09.033.
22. Kaur M, Singapura P, Kalakota N, et al. Factors that contribute to indeterminate results from the QuantiFERON-TB Gold in-tube test in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018;16(10):1616-1621.e1. https://doi.org/10.1016/j.cgh.2017.11.038.
23. Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107(2):179-194. https://doi.org/10.1038/ajg.2011.386.
24. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Clinical Guidelines committee. American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827-834.
25. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
26. Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104(5):1162-1169. https://doi.org/10.1038/ajg.2009.4.
27. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(6):1443-1450. https://doi.org/10.1111/j.1572-0241.2007.01780.x.
28. Rahier J-F, Yazdanpanah Y, Colombel J-F, Travis S. The European (ECCO) Consensus on infection in IBD: what does it change for the clinician? Gut. 2009;58(10). https://doi.org/10.1136/gut.2008.175950.
29. Meyer AM, Ramzan NN, Loftus EV, Jr., Heigh RI, Leighton JA. The diagnostic yield of stool pathogen studies during relapses of inflammatory bowel disease. J Clin Gastroenterol. 2004;38(9):772-775. https://doi.org/10.1097/01.mcg.0000139057.05297.d6.
30. Chew C, Nolan D, Jewell D. Small bowel gas in severe ulcerative colitis. Gut. 1991;32(12):1535-1537. https://doi.org/10.1136/gut.32.12.1535.
31. Zakeri N, Pollok RC. Diagnostic imaging and radiation exposure in inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2165-2178. https://doi.org/10.3748/wjg.v22.i7.2165.
32. Llaó J, Naves JE, Ruiz-Cerulla A, et al. Intravenous corticosteroids in moderately active ulcerative colitis refractory to oral corticosteroids. J Crohns Colitis. 2014;8(11):1523-1528. https://doi.org/10.1016/j.crohns.2014.06.010.
33. Seo M, Okada M, Yao T, Matake H, Maeda K. Evaluation of the clinical course of acute attacks in patients with ulcerative colitis through the use of an activity index. Journal of Gastroenterology. 2002;37(1):29-34. https://doi.org/10.1007/s535-002-8129-2.
34. Meyers S, Sachar DB, Goldberg JD, Janowitz HD. Corticotropin versus hydrocortisone in the intravenous treatment of ulcerative colitis: a prospective, randomized, double-blind clinical trial. Gastroenterology. 1983;85(2):351-357.
35. Ho G, Mowat C, Goddard C, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second‐line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19(10):1079-1087. https://doi.org/10.1111/j.1365-2036.2004.01945.x.
36. Järnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89(5):1005-1013. https://doi.org/10.1016/0016-5085(85)90201-X.
37. Wang JY, Terdiman JP, Vittinghoff E, Minichiello T, Varma MG. Hospitalized ulcerative colitis patients have an elevated risk of thromboembolic events. World J Gastroenterol. 2009;15(8):927-935. https://doi.org/10.3748/wjg.15.927.
38. Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835-848. https://doi.org/10.1053/j.gastro.2014.01.042.
39. Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(9):2272-2280. https://doi.org/10.1111/j.1572-0241.2008.02052.x.
40. Tinsley A, Naymagon S, Enomoto LM, Hollenbeak CS, Sands BE, Ullman TA. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7(12):e635-e640. https://doi.org/10.1016/j.crohns.2013.05.002.
41. Pleet JL, Vaughn BP, Morris JA, Moss AC, Cheifetz AS. The use of pharmacological prophylaxis against venous thromboembolism in hospitalized patients with severe active ulcerative colitis. Aliment Pharmacol Ther. 2014;39(9):940-948. https://doi.org/10.1111/apt.12691.
42. Gan SI, Beck PL. A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98(11):2363-2371 https://doi.org/10.1111/j.1572-0241.2003.07696.x.
43. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621-630. https://doi.org/10.1016/j.cgh.2006.03.002.
44. Docherty MJ, Jones III RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol. 2011;7(9):592-601.
45. Singh S, Proudfoot JA, Dulai PS, et al. No benefit of concomitant 5-aminosalicylates in patients with ulcerative colitis escalated to biologic therapy: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018;113(8):1197-1205. https://doi.org/10.1038/s41395-018-0144-2.
46. Mantzaris GJ, Hatzis A, Kontogiannis P, Triadaphyllou G. Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol. 1994;89(1):43-46.
47. Mantzaris GJ, Petraki K, Archavlis E, et al. A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol. 2001;36(9):971-974.
48. Rahier J-F, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443-468. https://doi.org/10.1016/j.crohns.2013.12.013.
49. Dickinson RJ, Ashton MG, Axon AT, Smith RC, Yeung CK, Hill GL. Controlled trial of intravenous hyperalimentation and total bowel rest as an adjunct to the routine therapy of acute colitis. Gastroenterology. 1980;79(6):1199-1204.
50. McIntyre P, Powell-Tuck J, Wood S, et al. Controlled trial of bowel rest in the treatment of severe acute colitis. Gut. 1986;27(5):481-485. https://doi.org/10.1136/gut.27.5.481.
51. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38(6):905-910. https://doi.org/10.1136/gut.38.6.905.
52. Bernardo S, Fernandes SR, Goncalves AR, et al. Predicting the course of disease in hospitalized patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2018;25(3):541-546. https://doi.org/10.1093/ibd/izy256.
53. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769-784. https://doi.org/10.1093/ecco-jcc/jjx009.
54. Randall J, Singh B, Warren B, Travis S, Mortensen N, George B. Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. Br J Surg. 2010;97(3):404-409. https://doi.org/10.1002/bjs.6874.
55. Bartels S, Gardenbroek T, Ubbink D, Buskens C, Tanis P, Bemelman W. Systematic review and meta‐analysis of laparoscopic versus open colectomy with end ileostomy for non‐toxic colitis. Br J Surg. 2013;100(6):726-733. https://doi.org/10.1002/bjs.9061.
56. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomized controlled trial. Lancet. 2012;380(9857):1909-1915. https://doi.org/10.1016/S0140-6736(12)61084-8.
57. Leblanc S, Allez M, Seksik P, et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol. 2011;106(4):771-777. https://doi.org/10.1038/ajg.2011.62.
58. Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111(4):477-491. https://doi.org/10.1038/ajg.2016.7.
59. Feuerstein JD, Akbari M, Tapper EB, Cheifetz AS. Systematic review and meta-analysis of third-line salvage therapy with infliximab or cyclosporine in severe ulcerative colitis. Ann Gastroenterol. 2016;29(3):341-347. https://doi.org/10.20524/aog.2016.0032.
60. Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associated with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14(2):251-258. https://doi.org/10.1016/j.cgh.2015.10.029.
61. Hindryckx P, Novak G, Vande Casteele N, et al. Review article: dose optimization of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017;45(5):617-630. https://doi.org/10.1111/apt.13913.
62. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(2):330-335. https://doi.org/10.1016/j.cgh.2014.07.041.
63. Herfarth HH, Rogler G, Higgins PD. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol. 2015;13(2):336-338. https://doi.org/10.1016/j.cgh.2014.09.045.
64. Van Assche G, D’haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125(4):1025-1031.
65. Arts J, D’haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10(2):73-78.
66. Tarabar D, El Jurdi K, Yvellez O, et al. 330-combination therapy of cyclosporine and vedolizumab is effective and safe for severe, steroid-resistant ulcerative colitis patients: a prospective study. Gastroenterology. 2018;154(6):S-82-S-83.https://doi.org/10.1016/S0016-5085(18)30725-X.
67. Szántó K, Molnár T, Farkas K. New promising combo therapy in inflammatory bowel diseases refractory to anti-TNF agents: cyclosporine plus vedolizumab. J Crohns Colitis. 2018;12(5):629. https://doi.org/10.1093/ecco-jcc/jjx179.
68. Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134(3):680-687. https://doi.org/10.1053/j.gastro.2008.01.004.
69. Spiegel BM, Ho W, Esrailian E, et al. Controversies in ulcerative colitis: a survey comparing decision making of experts versus community gastroenterologists. Clin Gastroenterol Hepatol. 2009;7(2):168-174. https://doi.org/10.1016/j.cgh.2008.08.029.
70. Ananthakrishnan AN, Issa M, Beaulieu DB, et al. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15(2):176-181. https://doi.org/10.1002/ibd.20639.
71. Nguyen GC, Murthy SK, Bressler B, et al. Quality of care and outcomes among hospitalized inflammatory bowel disease patients: a multicenter retrospective study. Inflamm Bowel Dis. 2017;23(5):695-701. https://doi.org/10.1097/MIB.0000000000001068.
72. Rotter T, Kugler J, Koch R, et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs, and patient outcomes. BMC Health Serv Res. 2008;8:265. https://doi.org/10.1186/1472-6963-8-265.
73. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (stride): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338. https://doi.org/10.1038/ajg.2015.233.
1. Sands BE. Mount Sinai Expert Guides: Gastroenterology. Hoboken, NJ: John Wiley & Sons; 2014.
2. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4(4):431-437. https://doi.org/10.1016/j.crohns.2010.02.001.
3. Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963;4:299-315. https://doi.org/10.1136/gut.4.4.299.
4. Sonnenberg A, Chang J. Time trends of physician visits for Crohn’s disease and ulcerative colitis in the United States, 1960-2006. 2007;14(2):249-252. https://doi.org/10.1002/ibd.20273.
5. Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13(12):1529-1535. https://doi.org/10.1002/ibd.20250.
6. Truelove S, Witts L. Cortisone in ulcerative colitis. Br Med J. 1955;2:104-108.
7. Jakobovits SL, Travis S. Management of acute severe colitis. Br Med Bull. 2005;75(1):131-144. https://doi.org/10.1093/bmb/ldl001.
8. Lynch R, Lowe D, Protheroe A, Driscoll R, Rhodes J, Arnott I. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther. 2013;38(8):935-945. https://doi.org/10.1111/apt.12473.
9. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileoanal pouch disorders. J Crohns Colitis. 2017;11(6):649-670. https://doi.org/10.1093/ecco-jcc/jjx008.
10. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501. https://doi.org/10.1038/ajg.2009.727.
11. Dassopoulos T, Cohen RD, Scherl EJ, Schwartz RM, Kosinski L, Regueiro MD. Ulcerative colitis care pathway. Gastroenterology. 2015;149(1):238-245. https://doi.org/10.1053/j.gastro.2015.05.036.
12. Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A nationwide analysis of changes in severity and outcomes of inflammatory bowel disease hospitalizations. J Gastrointest Surg. 2011;15(2):267-276. https://doi.org/10.1007/s11605-010-1396-3.
13. Kelso M, Weideman RA, Cipher DJ, Feagins LA. Factors associated with length of stay in veterans with inflammatory bowel disease hospitalized for an acute flare. Inflamm Bowel Dis. 2017;24(1):5-11. https://doi.org/10.1093/ibd/izx020.
14. Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Nguyen GC. Impact of gastroenterologist care on health outcomes of hospitalized ulcerative colitis patients. Gut. 2012;61(10):1410-1416. https://doi.org/10.1136/gutjnl-2011-301978.
15. Lee NS, Pola S, Groessl EJ, Rivera-Nieves J, Ho SB. Opportunities for improvement in the care of patients hospitalized for inflammatory bowel disease-related colitis. Dig Dis Sci. 2016;61(4):1003-1012. https://doi.org/10.1007/s10620-016-4046-0.
16. Neary BP, Doherty GA. A structured care pathway improves quality of care for acute severe ulcerative colitis. Gastroenterology. 2017;152(5):S218. https://doi.org/10.1016/S0016-5085(17)31028-4.
17. Klein A, Eliakim R. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals. 2010;3(4):1084-1092. https://doi.org/10.3390/ph3041084.
18. Chen JH, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis - evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44(2):127-144. https://doi.org/10.1111/apt.13670.
19. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982-1992. https://doi.org/10.1097/MIB.0000000000000392.
20. Solem CA, Loftus EV, Jr., Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707-712. https://doi.org/10.1097/01.MIB.0000173271.18319.53.
21. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5(1):103-110. https://doi.org/10.1016/j.cgh.2006.09.033.
22. Kaur M, Singapura P, Kalakota N, et al. Factors that contribute to indeterminate results from the QuantiFERON-TB Gold in-tube test in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018;16(10):1616-1621.e1. https://doi.org/10.1016/j.cgh.2017.11.038.
23. Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107(2):179-194. https://doi.org/10.1038/ajg.2011.386.
24. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute Clinical Guidelines committee. American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827-834.
25. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. https://doi.org/10.1093/cid/cix1085.
26. Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104(5):1162-1169. https://doi.org/10.1038/ajg.2009.4.
27. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(6):1443-1450. https://doi.org/10.1111/j.1572-0241.2007.01780.x.
28. Rahier J-F, Yazdanpanah Y, Colombel J-F, Travis S. The European (ECCO) Consensus on infection in IBD: what does it change for the clinician? Gut. 2009;58(10). https://doi.org/10.1136/gut.2008.175950.
29. Meyer AM, Ramzan NN, Loftus EV, Jr., Heigh RI, Leighton JA. The diagnostic yield of stool pathogen studies during relapses of inflammatory bowel disease. J Clin Gastroenterol. 2004;38(9):772-775. https://doi.org/10.1097/01.mcg.0000139057.05297.d6.
30. Chew C, Nolan D, Jewell D. Small bowel gas in severe ulcerative colitis. Gut. 1991;32(12):1535-1537. https://doi.org/10.1136/gut.32.12.1535.
31. Zakeri N, Pollok RC. Diagnostic imaging and radiation exposure in inflammatory bowel disease. World J Gastroenterol. 2016;22(7):2165-2178. https://doi.org/10.3748/wjg.v22.i7.2165.
32. Llaó J, Naves JE, Ruiz-Cerulla A, et al. Intravenous corticosteroids in moderately active ulcerative colitis refractory to oral corticosteroids. J Crohns Colitis. 2014;8(11):1523-1528. https://doi.org/10.1016/j.crohns.2014.06.010.
33. Seo M, Okada M, Yao T, Matake H, Maeda K. Evaluation of the clinical course of acute attacks in patients with ulcerative colitis through the use of an activity index. Journal of Gastroenterology. 2002;37(1):29-34. https://doi.org/10.1007/s535-002-8129-2.
34. Meyers S, Sachar DB, Goldberg JD, Janowitz HD. Corticotropin versus hydrocortisone in the intravenous treatment of ulcerative colitis: a prospective, randomized, double-blind clinical trial. Gastroenterology. 1983;85(2):351-357.
35. Ho G, Mowat C, Goddard C, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second‐line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19(10):1079-1087. https://doi.org/10.1111/j.1365-2036.2004.01945.x.
36. Järnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89(5):1005-1013. https://doi.org/10.1016/0016-5085(85)90201-X.
37. Wang JY, Terdiman JP, Vittinghoff E, Minichiello T, Varma MG. Hospitalized ulcerative colitis patients have an elevated risk of thromboembolic events. World J Gastroenterol. 2009;15(8):927-935. https://doi.org/10.3748/wjg.15.927.
38. Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835-848. https://doi.org/10.1053/j.gastro.2014.01.042.
39. Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(9):2272-2280. https://doi.org/10.1111/j.1572-0241.2008.02052.x.
40. Tinsley A, Naymagon S, Enomoto LM, Hollenbeak CS, Sands BE, Ullman TA. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7(12):e635-e640. https://doi.org/10.1016/j.crohns.2013.05.002.
41. Pleet JL, Vaughn BP, Morris JA, Moss AC, Cheifetz AS. The use of pharmacological prophylaxis against venous thromboembolism in hospitalized patients with severe active ulcerative colitis. Aliment Pharmacol Ther. 2014;39(9):940-948. https://doi.org/10.1111/apt.12691.
42. Gan SI, Beck PL. A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98(11):2363-2371 https://doi.org/10.1111/j.1572-0241.2003.07696.x.
43. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621-630. https://doi.org/10.1016/j.cgh.2006.03.002.
44. Docherty MJ, Jones III RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol. 2011;7(9):592-601.
45. Singh S, Proudfoot JA, Dulai PS, et al. No benefit of concomitant 5-aminosalicylates in patients with ulcerative colitis escalated to biologic therapy: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018;113(8):1197-1205. https://doi.org/10.1038/s41395-018-0144-2.
46. Mantzaris GJ, Hatzis A, Kontogiannis P, Triadaphyllou G. Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol. 1994;89(1):43-46.
47. Mantzaris GJ, Petraki K, Archavlis E, et al. A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol. 2001;36(9):971-974.
48. Rahier J-F, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443-468. https://doi.org/10.1016/j.crohns.2013.12.013.
49. Dickinson RJ, Ashton MG, Axon AT, Smith RC, Yeung CK, Hill GL. Controlled trial of intravenous hyperalimentation and total bowel rest as an adjunct to the routine therapy of acute colitis. Gastroenterology. 1980;79(6):1199-1204.
50. McIntyre P, Powell-Tuck J, Wood S, et al. Controlled trial of bowel rest in the treatment of severe acute colitis. Gut. 1986;27(5):481-485. https://doi.org/10.1136/gut.27.5.481.
51. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38(6):905-910. https://doi.org/10.1136/gut.38.6.905.
52. Bernardo S, Fernandes SR, Goncalves AR, et al. Predicting the course of disease in hospitalized patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2018;25(3):541-546. https://doi.org/10.1093/ibd/izy256.
53. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769-784. https://doi.org/10.1093/ecco-jcc/jjx009.
54. Randall J, Singh B, Warren B, Travis S, Mortensen N, George B. Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. Br J Surg. 2010;97(3):404-409. https://doi.org/10.1002/bjs.6874.
55. Bartels S, Gardenbroek T, Ubbink D, Buskens C, Tanis P, Bemelman W. Systematic review and meta‐analysis of laparoscopic versus open colectomy with end ileostomy for non‐toxic colitis. Br J Surg. 2013;100(6):726-733. https://doi.org/10.1002/bjs.9061.
56. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomized controlled trial. Lancet. 2012;380(9857):1909-1915. https://doi.org/10.1016/S0140-6736(12)61084-8.
57. Leblanc S, Allez M, Seksik P, et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol. 2011;106(4):771-777. https://doi.org/10.1038/ajg.2011.62.
58. Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111(4):477-491. https://doi.org/10.1038/ajg.2016.7.
59. Feuerstein JD, Akbari M, Tapper EB, Cheifetz AS. Systematic review and meta-analysis of third-line salvage therapy with infliximab or cyclosporine in severe ulcerative colitis. Ann Gastroenterol. 2016;29(3):341-347. https://doi.org/10.20524/aog.2016.0032.
60. Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associated with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14(2):251-258. https://doi.org/10.1016/j.cgh.2015.10.029.
61. Hindryckx P, Novak G, Vande Casteele N, et al. Review article: dose optimization of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017;45(5):617-630. https://doi.org/10.1111/apt.13913.
62. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(2):330-335. https://doi.org/10.1016/j.cgh.2014.07.041.
63. Herfarth HH, Rogler G, Higgins PD. Pushing the pedal to the metal: should we accelerate infliximab therapy for patients with severe ulcerative colitis? Clin Gastroenterol Hepatol. 2015;13(2):336-338. https://doi.org/10.1016/j.cgh.2014.09.045.
64. Van Assche G, D’haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125(4):1025-1031.
65. Arts J, D’haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10(2):73-78.
66. Tarabar D, El Jurdi K, Yvellez O, et al. 330-combination therapy of cyclosporine and vedolizumab is effective and safe for severe, steroid-resistant ulcerative colitis patients: a prospective study. Gastroenterology. 2018;154(6):S-82-S-83.https://doi.org/10.1016/S0016-5085(18)30725-X.
67. Szántó K, Molnár T, Farkas K. New promising combo therapy in inflammatory bowel diseases refractory to anti-TNF agents: cyclosporine plus vedolizumab. J Crohns Colitis. 2018;12(5):629. https://doi.org/10.1093/ecco-jcc/jjx179.
68. Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134(3):680-687. https://doi.org/10.1053/j.gastro.2008.01.004.
69. Spiegel BM, Ho W, Esrailian E, et al. Controversies in ulcerative colitis: a survey comparing decision making of experts versus community gastroenterologists. Clin Gastroenterol Hepatol. 2009;7(2):168-174. https://doi.org/10.1016/j.cgh.2008.08.029.
70. Ananthakrishnan AN, Issa M, Beaulieu DB, et al. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15(2):176-181. https://doi.org/10.1002/ibd.20639.
71. Nguyen GC, Murthy SK, Bressler B, et al. Quality of care and outcomes among hospitalized inflammatory bowel disease patients: a multicenter retrospective study. Inflamm Bowel Dis. 2017;23(5):695-701. https://doi.org/10.1097/MIB.0000000000001068.
72. Rotter T, Kugler J, Koch R, et al. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs, and patient outcomes. BMC Health Serv Res. 2008;8:265. https://doi.org/10.1186/1472-6963-8-265.
73. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (stride): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338. https://doi.org/10.1038/ajg.2015.233.
© 2019 Society of Hospital Medicine
Evaluating and managing postural tachycardia syndrome
Some people, most of them relatively young women, experience lightheadedness, a racing heart, and other symptoms (but not hypotension) when they stand up, in a condition known as postural tachycardia syndrome (POTS).1 Although not known to shorten life,1 it can be physically and mentally debilitating.2,3 Therapy rarely cures it, but a multifaceted approach can substantially improve quality of life.
This review outlines the evaluation and diagnosis of POTS and provides guidance for a therapy regimen.
HOW IS POTS DEFINED?
POTS is a multifactorial syndrome rather than a specific disease. It is characterized by all of the following1,4–6:
- An increase in heart rate of ≥ 30 bpm, or ≥ 40 bpm for those under age 19, within 10 minutes of standing from a supine position
- Sustained tachycardia (> 30 seconds)
- Absence of orthostatic hypotension (a fall in blood pressure of ≥ 20/10 mm Hg)
- Frequent and chronic duration (≥ 6 months).
These features are critical to diagnosis. Hemodynamic criteria in isolation may describe postural tachycardia but are not sufficient to diagnose POTS.
The prevalence of POTS is estimated to be between 0.2% and 1.0%,7 affecting up to 3 million people in the United States. Most cases arise between ages 13 and 50, with a female-to-male ratio of 5:1.8
MANY NAMES, SAME CONDITION
In 1871, Da Costa9 described a condition he called “irritable heart syndrome” that had characteristics similar to those of POTS, including extreme fatigue and exercise intolerance. Decades later, Lewis10 and Wood11 provided more detailed descriptions of the disorder, renaming it “soldier’s heart” or “Da Costa syndrome.” As other cases were documented, more terms arose, including “effort syndrome” and “mitral valve prolapse syndrome.”
In 1982, Rosen and Cryer12 were the first to use the term “postural tachycardia syndrome” for patients with disabling tachycardia upon standing without orthostatic hypotension. In 1986, Fouad et al13 described patients with postural tachycardia, orthostatic intolerance, and a small degree of hypotension as having “idiopathic hypovolemia.”
In 1993, Schondorf and Low14 established the current definition of POTS, leading to increased awareness and research efforts to understand its pathophysiology.
MULTIFACTORIAL PATHOPHYSIOLOGY
During the last 2 decades, several often-overlapping forms of POTS have been recognized, all of which share a final common pathway of sustained orthostatic tachycardia.15–19 In addition, a number of common comorbidities were identified through review of large clinic populations of POTS.20,21
Hypovolemic POTS
Up to 70% of patients with POTS have hypovolemia. The average plasma volume deficit is about 13%, which typically causes only insignificant changes in heart rate and norepinephrine levels while a patient is supine. However, blood pooling associated with upright posture further compromises cardiac output and consequently increases sympathetic nerve activity. Abnormalities in the renin-angiotensin-aldosterone volume regulation system are also suspected to impair sodium retention, contributing to hypovolemia.1,22
Neuropathic POTS
About half of patients with POTS have partial sympathetic denervation (particularly in the lower limbs) and inadequate vasoconstriction upon standing, leading to reduced venous return and stroke volume.17,23 A compensatory increase in sympathetic tone results in tachycardia to maintain cardiac output and blood pressure.
Hyperadrenergic POTS
Up to 50% of patients with POTS have high norepinephrine levels (≥ 600 pg/mL) when upright. This subtype, hyperadrenergic POTS, is characterized by an increase in systolic blood pressure of at least 10 mm Hg within 10 minutes of standing, with concomitant tachycardia that can be similar to or greater than that seen in nonhyperadrenergic POTS. Patients with hyperadrenergic POTS tend to report more prominent symptoms of sympathetic activation, such as palpitations, anxiety, and tremulousness.24,25
Norepinephrine transporter deficiency
The norepinephrine transporter (NET) is on the presynaptic cleft of sympathetic neurons and serves to clear synaptic norepinephrine. NET deficiency leads to a hyperadrenergic state and elevated sympathetic nerve activation.18 NET deficiency may be induced by common antidepressants (eg, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors) and attention-deficit disorder medications.4
Mast cell activation syndrome
The relationship between mast cell activation syndrome and POTS is poorly understood.4,26 Mast cell activation syndrome has been described in a subset of patients with POTS who have sinus tachycardia accompanied by severe episodic flushing. Patients with this subtype have a hyperadrenergic response to postural change and elevated urine methylhistamine during flushing episodes.
Patients with mast cell activation syndrome tend to have strong allergic symptoms and may also have severe gastrointestinal problems, food sensitivities, dermatographism, and neuropathy. Diagnosis can be difficult, as the condition is associated with numerous markers with varying sensitivity and specificity.
Autoimmune origin
A significant minority of patients report a viral-like illness before the onset of POTS symptoms, suggesting a possible autoimmune-mediated or inflammatory cause. Also, some autoimmune disorders (eg, Sjögren syndrome) can present with a POTS-like manifestation.
Research into the role of autoantibodies in the pathophysiology of POTS offers the potential to develop novel therapeutic targets. Autoantibodies that have been reported in POTS include those against M1 to M3 muscarinic receptors (present in over 87% of patients with POTS),27 cardiac lipid raft-associated proteins,28 adrenergic G-protein coupled receptors, alpha-1-adrenergic receptors, and beta-1- and beta-2-adrenergic receptors.29 Although commercial enzyme-linked immunosorbent assays can assess for these antibody fragments, it is not known whether targeting the antibodies improves outcomes. At this time, antibody testing for POTS should be confined to the research setting.
LINKS TO OTHER SYNDROMES
POTS is often associated with other conditions whose symptoms cannot be explained by postural intolerance or tachycardia.
Ehlers-Danlos syndromes are a group of inherited heterogeneous disorders involving joint hypermobility, skin hyperextensibility, and tissue fragility.30 The hypermobile subtype is most commonly associated with POTS, with patients often having symptoms of autonomic dysregulation and autonomic test abnormalities.31–33 Patients with POTS may have a history of joint subluxations, joint pain, cervical instability, and spontaneous epidural leaks. The reason for the overlap between the two syndromes is not clear.
Chronic fatigue syndrome is characterized by persistent fatigue that does not resolve with rest and is not necessarily associated with orthostatic changes. More than 75% of patients with POTS report general fatigue as a major complaint, and up to 23% meet the full criteria for chronic fatigue syndrome.34
DIAGNOSTIC STRATEGY
A patient presenting with symptoms suggestive of POTS should first undergo a detailed history and physical examination. Other causes of sinus tachycardia should be considered.
Detailed history, symptom review
The history should focus on determining symptom burden, including tachycardia onset, frequency, severity, and triggers; the presence of syncope; and the impact of symptoms on daily function and quality of life.
Presyncope and its associated symptoms occur in less than one-third of patients with POTS, and syncope is not a principal feature.4 If syncope is the predominant complaint, alternative causes should be investigated. The usual cause of syncope in the general population is thought to be vasovagal.
In addition to orthostatic intolerance, gastrointestinal disturbances are common in POTS, presenting as abdominal pain, heartburn, irregular bowel movements, diarrhea, or constipation. Symptoms of gastroparesis are less common. Gastrointestinal symptoms tend to be prolonged, lasting hours and occurring multiple times a week. They tend not to improve in the supine position.35
POTS-associated symptoms may develop insidiously, but patients often report onset after an acute stressor such as pregnancy, major surgery, or a presumed viral illness.4 Whether these putative triggers are causative or coincidental is unknown. Symptoms of orthostatic intolerance tend to be exacerbated by dehydration, heat, alcohol, exercise, and menstruation.36,37
Consider the family history: 1 in 8 patients with POTS reports familial orthostatic intolerance,38 suggesting a genetic role in some patients. Inquire about symptoms or a previous diagnosis of Ehlers-Danlos syndrome and mast cell activation syndrome.
Consider other conditions
Pheochromocytoma causes hyperadrenergic symptoms (eg, palpitations, lightheadedness) like those in POTS, but patients with pheochromocytoma typically have these symptoms while supine. Pheochromocytoma is also characterized by plasma norepinephrine levels much higher than in POTS.4 Plasma metanephrine testing helps diagnose or rule out pheochromocytoma.5
Inappropriate sinus tachycardia, like pheochromocytoma, also has clinical features similar to those of POTS, as well as tachycardia present when supine. It involves higher sympathetic tone and lower parasympathetic tone compared with POTS; patients commonly have a daytime resting heart rate of at least 100 bpm or a 24-hour mean heart rate of at least 90 bpm.1,42 While the intrinsic heart rate is heightened in inappropriate sinus tachycardia, it is not different between POTS patients and healthy individuals.42,43 Distinguishing POTS from inappropriate sinus tachycardia is further complicated by the broad inclusion criteria of most studies of inappropriate sinus tachycardia, which failed to exclude patients with POTS.44 The Heart Rhythm Society recently adopted distinct definitions for the 2 conditions.1
Physical examination: Focus on vital signs
Dependent acrocyanosis—dark red-blue discoloration of the lower legs that is cold to the touch—occurs in about half of patients with POTS upon standing.4 Dependent acrocyanosis is associated with joint hypermobility and Ehlers-Danlos syndrome, so these conditions should also be considered if findings are positive.
Laboratory testing for other causes
Laboratory testing is used mainly to detect primary causes of sinus tachycardia. Tests should include:
- Complete blood cell count with hematocrit (for severe anemia)
- Thyroid-stimulating hormone level (for hyperthyroidism)
- Electrolyte panel (for significant electrolyte disturbances).
Evidence is insufficient to support routinely measuring the vitamin B12 level, iron indices, and serum markers for celiac disease, although these may be done if the history or physical examination suggests related problems.4 Sicca symptoms (severe dry eye or dry mouth) should trigger evaluation for Sjögren syndrome.
Electrocardiography needed
Electrocardiography should be performed to investigate for cardiac conduction abnormalities as well as for resting markers of a supraventricular tachyarrhythmia. Extended ambulatory (Holter) monitoring may be useful to evaluate for a transient reentrant tachyarrhythmia4; however, it does not record body position, so it can be difficult to determine if detected episodes of tachycardia are related to posture.
Additional testing for select cases
Further investigation is usually not needed to diagnose POTS but should be considered in some cases. Advanced tests are typically performed at a tertiary care referral center and include:
- Quantitative sensory testing to evaluate for small-fiber neuropathy (ie, Quantitative Sudomotor Axon Reflex Test, or QSART), which occurs in the neuropathic POTS subtype
- Formal autonomic function testing to characterize neurovascular responsiveness
- Supine and standing plasma norepinephrine levels (fractionated catecholamines) to characterize the net activation of the sympathetic nervous system
- Blood volume assessments to assess hypovolemia
- Formal exercise testing to objectively quantify exercise capacity.
GRADED MANAGEMENT
No single universal gold-standard therapy exists for POTS, and management should be individually determined with the primary goals of treating symptoms and restoring function. A graded approach should be used, starting with conservative nonpharmacologic therapies and adding medications as needed.
While the disease course varies substantially from patient to patient, proper management is strongly associated with eventual symptom improvement.1
NONPHARMACOLOGIC STEPS FIRST
Education
Patients should be informed of the nature of their condition and referred to appropriate healthcare personnel. POTS is a chronic illness requiring individualized coping strategies, intensive physician interaction, and support of a multidisciplinary team. Patients and family members can be reassured that most symptoms improve over time with appropriate diagnosis and treatment.1 Patients should be advised to avoid aggravating triggers and activities.
Exercise
Exercise programs are encouraged but should be introduced gradually, as physical activity can exacerbate symptoms, especially at the outset. Several studies have reported benefits from a short-term (3-month) program, in which the patient gradually progresses from non-upright exercise (eg, rowing machine, recumbent cycle, swimming) to upright endurance exercises. At the end of these programs, significant cardiac remodeling, improved quality of life, and reduced heart rate responses to standing have been reported, and benefits have been reported to persist in patients who continued exercising after the 3-month study period.46,47
Despite the benefits of exercise interventions, compliance is low.46,47 To prevent early discouragement, patients should be advised that it can take 4 to 6 weeks of continued exercise before benefits appear. Patients are encouraged to exercise every other day for 30 minutes or more. Regimens should primarily focus on aerobic conditioning, but resistance training, concentrating on thigh muscles, can also help. Exercise is a treatment and not a cure, and benefits can rapidly disappear if regular activity (at least 3 times per week) is stopped.48
Compression stockings
Compression stockings help reduce peripheral venous pooling and enhance venous return to the heart. Waist-high stockings with compression of at least 30 to 40 mm Hg offer the best results.
Diet
Increased fluid and salt intake is advisable for patients with suspected hypovolemia. At least 2 to 3 L of water accompanied by 10 to 12 g of daily sodium intake is recommended.1 This can usually be accomplished with diet and salt added to food, but salt tablets can be used if the patient prefers. The resultant plasma volume expansion may help reduce the reflex tachycardia upon standing.49
Check medications
Rescue therapy with saline infusion
Intravenous saline infusion can augment blood volume in patients who are clinically decompensated and present with severe symptoms.1 Intermittent infusion of 1 L of normal saline has been found to significantly reduce orthostatic tachycardia and related symptoms in patients with POTS, contributing to improved quality of life.51,52
Chronic saline infusions are not recommended for long-term care because of the risk of access complications and infection.1 Moak et al53 reported a high rate of bacteremia in a cohort of children with POTS with regular saline infusions, most of whom had a central line. On the other hand, Ruzieh et al54 reported significantly improved symptoms with regular saline infusions without a high rate of complications, but patients in this study received infusions for only a few months and through a peripheral intravenous catheter.
DRUG THERAPY
No medications are approved by the US Food and Drug Administration (FDA) or Health Canada specifically for treating POTS, making all pharmacologic recommendations off-label. Although the drugs discussed below have been evaluated for POTS in controlled laboratory settings, they have yet to be tested in robust clinical trials.
Blood volume expansion
Several drugs expand blood volume, which may reduce orthostatic tachycardia.
Fludrocortisone is a synthetic aldosterone analogue that enhances sodium and water retention. Although one observational study found that it normalizes hemodynamic changes in response to orthostatic stress, no high-level evidence exists for its effectiveness for POTS.55 It is generally well tolerated, although possible adverse effects include hyperkalemia, hypertension, fatigue, nausea, headache, and edema.5,56
Desmopressin is a synthetic version of a natural antidiuretic hormone that increases kidney-mediated free-water reabsorption without sodium retention. It significantly reduces upright heart rate in patients with POTS and improves symptom burden. Although potential adverse effects include edema and headache, hyponatremia is the primary concern with daily use, especially with the increased water intake advised for POTS.57 Patients should be advised to use desmopressin no more than once a week for the acute improvement of symptoms. Intermittent monitoring of serum sodium levels is recommended for safety.
Erythropoietin replacement has been suggested for treating POTS to address the significant deficit in red blood cell volume. Although erythropoietin therapy has a direct vasoconstrictive effect and largely improves red blood cell volume in patients with POTS, it does not expand plasma volume, so orthostatic tachycardia is not itself reduced.22 Nevertheless, it may significantly improve POTS symptoms refractory to more common methods of treatment, and it should be reserved for such cases. In addition to the lack of effect on orthostatic tachycardia, drawbacks to using erythropoietin include its high cost, the need for subcutaneous administration, and the risk of life-threatening complications such as myocardial infarction and stroke.58,59
Heart rate-lowering agents
Propranolol, a nonselective beta-adrenergic antagonist, can significantly reduce standing heart rate and improve symptoms at low dosages (10–20 mg). Higher dosages can further restrain orthostatic tachycardia but are not as well tolerated, mainly due to hypotension and worsening of existing symptoms such as fatigue.60 Regular-acting propranolol works for about 4 to 5 hours per dose, so full-day coverage often requires dosing 4 times per day.
Ivabradine is a selective blocker of the “funny” (If) channel that reduces the sinus node firing rate without affecting blood pressure, so it slows heart rate without causing supine hypertension or orthostatic hypotension.
A retrospective case series found that 60% of patients with POTS treated with ivabradine reported symptomatic improvement, and all patients experienced reduced tachycardia with continued use.61 Ivabradine has not been compared with placebo or propranolol in a randomized controlled trial, and it has not been well studied in pregnancy and so should be avoided because of potential teratogenic effects.
When prescribing ivabradine for women of childbearing age, a negative pregnancy test may be documented prior to initiation of therapy, and the use of highly effective methods of contraception is recommended. Ivabradine should be avoided in women contemplating pregnancy. Insurance coverage can limit access to ivabradine in the United States.
Central nervous system sympatholytics
Patients with prominent hyperadrenergic features may benefit from central sympatholytic agents. However, these drugs may not be well tolerated in patients with neuropathic POTS because of the effects of reduced systemic vascular resistance5 and the possible exacerbation of drowsiness, fatigue, and mental clouding.4 Patients can be extremely sensitive to these medications, so they should initially be prescribed at the lowest dose, then gradually increased as tolerated.
Clonidine, an alpha-2-adrenergic agonist, decreases central sympathetic tone. In hyperadrenergic patients, clonidine can stabilize heart rate and blood pressure, thereby reducing orthostatic symptoms.62
Methyldopa has effects similar to those of clonidine but is easier to titrate owing to its longer half-life.63 Methyldopa is typically started at 125 mg at bedtime and increased to 125 mg twice daily, if tolerated.
Other agents
Midodrine is a prodrug. The active form, an alpha-1-adrenergic agonist, constricts peripheral veins and arteries to increase vascular resistance and venous return, thereby reducing orthostatic tachycardia.52 It is most useful in patients with impaired peripheral vasoconstriction (eg, neuropathic POTS) and may be less effective in those with hyperadrenergic POTS.64 Major limitations of midodrine include worsening supine hypertension and possible urinary retention.39
Because of midodrine’s short half-life, frequent dosing is required during daytime hours (eg, 8 AM, noon, and 4 PM), but it should not be taken within 4 to 5 hours of sleep because of the risk of supine hypertension. Midodrine is typically started at 2.5 to 5 mg per dose and can be titrated up to 15 mg per dose.
Midodrine is an FDA pregnancy category C drug (adverse effects in pregnancy seen in animal models, but evidence lacking in humans). While ideally it should be avoided, we have used it safely in pregnant women with disabling POTS symptoms.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cardiovagal tone and possibly sympathetic tone. It has been reported to significantly reduce standing heart rate and improve symptom burden in patients with POTS.65 However, pyridostigmine increases gastrointestinal mobility, leading to severe adverse effects in over 20% of patients, including abdominal cramps, nausea, and diarrhea.66
Droxidopa, a synthetic amino acid precursor of norepinephrine, improves dizziness and fatigue in POTS with minimal effects on blood pressure.67
Modafinil, a psychostimulant, may improve POTS-associated cognitive symptoms.4 It also raises upright blood pressure without significantly worsening standing heart rate or acute orthostatic symptoms.68
EFFECTS OF COMORBID DISORDERS ON MANAGEMENT
Ehlers-Danlos syndrome
Pharmacologic approaches to POTS should not be altered based on the presence of Ehlers-Danlos syndrome, but because many of these patients are prone to joint dislocation, exercise prescriptions may need adjusting.
A medical genetics consult is recommended for patients with Ehlers-Danlos syndrome. Although the hypermobile type (the form most commonly associated with POTS) is not associated with aortopathy, it can be confused with classical and vascular Ehlers-Danlos syndromes, which require serial aortic screening.30
Mast cell activation syndrome
Consultation with an allergist or immunologist may help patients with severe symptoms.
Autoantibodies and autoimmunity
Treatment of the underlying disorder is recommended and can result in significantly improved POTS symptoms.
SPECIALTY CARE REFERRAL
POTS can be challenging to manage. Given the range of physiologic, emotional, and functional distress patients experience, it often requires significant physician time and multidisciplinary care. Patients with continued severe or debilitating symptoms may benefit from referral to a tertiary-care center with experience in autonomic nervous system disorders.
PROGNOSIS
Limited data are available on the long-term prognosis of POTS, and more studies are needed in pediatric and adult populations. No deaths have been reported in the handful of published cases of POTS in patients older than 50.1 Some pediatric studies suggest that some teenagers “outgrow” their POTS. However, these data are not robust, and an alternative explanation is that as they get older, they see adult physicians for their POTS symptoms and so are lost to study follow-up.6,44,69
We have not often seen POTS simply resolve without ongoing treatment. However, in our experience, most patients have improved symptoms and function with multimodal treatment (ie, exercise, salt, water, stockings, and some medications) and time.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7(2):204–210. pmid:21509337
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77(6):531–537. doi:10.4065/77.6.531
- Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013; 127(23):2336–2342. doi:10.1161/CIRCULATIONAHA.112.144501
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006; 6(2):84–99. pmid:16943900
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012; 160(2):222–226. doi:10.1016/j.jpeds.2011.08.054
- Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol 2014; 5:220. doi:10.3389/fphys.2014.00220
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69(8):790–798. doi:10.1212/01.wnl.0000267663.05398.40
- Da Costa JM. On irritable heart: a clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci 1871; 61(121):2–52.
- Lewis T. The tolerance of physical exertion, as shown by soldiers suffering from so-called “irritable heart.” Br Med J 1918; 1(2987):363–365. pmid:20768980
- Wood P. Da Costa’s syndrome (or effort syndrome): lecture I. Br Med J 1941; 1(4194):767–772. pmid:20783672
- Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982; 72(5):847–850.
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986; 104(3):298–303. pmid:3511818
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993; 43(1):132–137. pmid:8423877
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007; 334(1):57–60. doi:10.1097/MAJ.0b013e318063c6c0
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med 2000; 343(14):1008–1014. doi:10.1056/NEJM200010053431404
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 342(8):541–549. doi:10.1056/NEJM200002243420803
- Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16(6):431–438. doi:10.1136/practneurol-2016-001405
- Gunning WT, Karabin BL, Blomquist TM, Grubb BP. Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine (Baltimore) 2016; 95(37):e4849. doi:10.1097/MD.0000000000004849
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011; 34(5):549–554. doi:10.1111/j.1540-8159.2010.02994.x
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111(13):1574–1582. doi:10.1161/01.CIR.0000160356.97313.5D
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013; 8(12):e84716. doi:10.1371/journal.pone.0084716
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20(3):352–358. doi:10.1111/j.1540-8167.2008.01407.x
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18(5):527–531. pmid:21947988
- Shibao C, Arzubiaga C, Roberts J, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45(3):385–390. doi:10.1161/01.HYP.0000158259.68614.40
- Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker [abstract]. J Clin Neuromuscul Dis 2016; 17(3):179S.
- Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013; 162(1):34–44. doi:10.1016/j.trsl.2013.03.002
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014; 3(1):e000755. doi:10.1161/JAHA.113.000755
- Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175(1):8–26. doi:10.1002/ajmg.c.31552
- Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340(1-2):99–102. doi:10.1016/j.jns.2014.03.002
- De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014; 44(3):353–361. doi:10.1016/j.semarthrit.2014.05.013
- Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med 2003; 115(1):33–40. pmid:12867232
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012; 122(4):183–192. doi:10.1042/CS20110200
- Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD. Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 2015; 359(1-2):193–196. doi:10.1016/j.jns.2015.10.052
- Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5(2):122–129. doi:10.15420/AER.2016.7.2
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012; 118(3):242–246. doi:10.1016/j.ijgo.2012.04.014
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82(3):308–313. doi:10.4065/82.3.308
- Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2015; 28(7):157–159. pmid:25829642
- Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci 2013; 9(5):944–952. doi:10.5114/aoms.2013.38685
- Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol. Br J Clin Pharmacol 1982; 13(suppl 2):345S–351S. pmid:6125187
- Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc 2014; 3(2):e000700. doi:10.1161/JAHA.113.000700
- Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of “inappropriate” sinus tachycardia. Role of sympathovagal balance. Circulation 1994; 90(2):873–877. pmid:7913886
- Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117(21):2814–2817. doi:10.1161/CIRCULATIONAHA.107.761643
- Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173:149–153. doi:10.1016/j.jpeds.2016.02.035
- George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016; 13(4):943–950. doi:10.1016/j.hrthm.2015.12.012
- Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55(25):2858–2868. doi:10.1016/j.jacc.2010.02.043
- Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13(4):951–952. doi:10.1016/j.hrthm.2015.12.039
- Celedonio JE, Garland EM, Nwazue VC, et al. Effects of high sodium intake on blood volume and catecholamines in patients with postural tachycardia syndrome and healthy females [abstract]. Clin Auton Res 2014; 24:211.
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15(9):60. doi:10.1007/s11910-015-0583-8
- Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000; 10:29–33. pmid:10750641
- Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997; 96(2):575–580. pmid:9244228
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol 2016; 37(2):278–282. doi:10.1007/s00246-015-1274-6
- Ruzieh M, Baugh A, Dasa O, et al. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol 2017; 48(3):255–260. doi:10.1007/s10840-017-0225-y
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res 2000; 10(5):293–299. pmid:11198485
- Lee AK, Krahn AD. Evaluation of syncope: focus on diagnosis and treatment of neurally mediated syncope. Expert Rev Cardiovasc Ther 2016; 14(6):725–736. doi:10.1586/14779072.2016.1164034
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012; 9(9):1484–1490. doi:10.1016/j.hrthm.2012.05.002
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2012; 19(2):92–95. doi:10.1097/MJT.0b013e3181ef621a
- Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med 1995; 99(5):525–529. pmid:7485211
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009; 120(9):725–734. doi:10.1161/CIRCULATIONAHA.108.846501
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011; 13(3):427–430. doi:10.1093/europace/euq390
- Gaffney FA, Lane LB, Pettinger W, Blomqvist G. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest 1983; 83(suppl 2):436–438. pmid:6295714
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999; 317(2):88–101. pmid:10037112
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014; 126(4):289–296. doi:10.1042/CS20130222
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005; 111(21):2734–2340. doi:10.1161/CIRCULATIONAHA.104.497594
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol 2011; 34(6):750–755. doi:10.1111/j.1540-8159.2011.03047.x
- Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017; 24(2):e157–e161. doi:10.1097/MJT.0000000000000468
- Kpaeyeh AG Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol 2014; 34(6):738–741. doi:10.1097/JCP.0000000000000221
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol 2009; 32(2):234–238. doi:10.1111/j.1540-8159.2008.02207.x
Some people, most of them relatively young women, experience lightheadedness, a racing heart, and other symptoms (but not hypotension) when they stand up, in a condition known as postural tachycardia syndrome (POTS).1 Although not known to shorten life,1 it can be physically and mentally debilitating.2,3 Therapy rarely cures it, but a multifaceted approach can substantially improve quality of life.
This review outlines the evaluation and diagnosis of POTS and provides guidance for a therapy regimen.
HOW IS POTS DEFINED?
POTS is a multifactorial syndrome rather than a specific disease. It is characterized by all of the following1,4–6:
- An increase in heart rate of ≥ 30 bpm, or ≥ 40 bpm for those under age 19, within 10 minutes of standing from a supine position
- Sustained tachycardia (> 30 seconds)
- Absence of orthostatic hypotension (a fall in blood pressure of ≥ 20/10 mm Hg)
- Frequent and chronic duration (≥ 6 months).
These features are critical to diagnosis. Hemodynamic criteria in isolation may describe postural tachycardia but are not sufficient to diagnose POTS.
The prevalence of POTS is estimated to be between 0.2% and 1.0%,7 affecting up to 3 million people in the United States. Most cases arise between ages 13 and 50, with a female-to-male ratio of 5:1.8
MANY NAMES, SAME CONDITION
In 1871, Da Costa9 described a condition he called “irritable heart syndrome” that had characteristics similar to those of POTS, including extreme fatigue and exercise intolerance. Decades later, Lewis10 and Wood11 provided more detailed descriptions of the disorder, renaming it “soldier’s heart” or “Da Costa syndrome.” As other cases were documented, more terms arose, including “effort syndrome” and “mitral valve prolapse syndrome.”
In 1982, Rosen and Cryer12 were the first to use the term “postural tachycardia syndrome” for patients with disabling tachycardia upon standing without orthostatic hypotension. In 1986, Fouad et al13 described patients with postural tachycardia, orthostatic intolerance, and a small degree of hypotension as having “idiopathic hypovolemia.”
In 1993, Schondorf and Low14 established the current definition of POTS, leading to increased awareness and research efforts to understand its pathophysiology.
MULTIFACTORIAL PATHOPHYSIOLOGY
During the last 2 decades, several often-overlapping forms of POTS have been recognized, all of which share a final common pathway of sustained orthostatic tachycardia.15–19 In addition, a number of common comorbidities were identified through review of large clinic populations of POTS.20,21
Hypovolemic POTS
Up to 70% of patients with POTS have hypovolemia. The average plasma volume deficit is about 13%, which typically causes only insignificant changes in heart rate and norepinephrine levels while a patient is supine. However, blood pooling associated with upright posture further compromises cardiac output and consequently increases sympathetic nerve activity. Abnormalities in the renin-angiotensin-aldosterone volume regulation system are also suspected to impair sodium retention, contributing to hypovolemia.1,22
Neuropathic POTS
About half of patients with POTS have partial sympathetic denervation (particularly in the lower limbs) and inadequate vasoconstriction upon standing, leading to reduced venous return and stroke volume.17,23 A compensatory increase in sympathetic tone results in tachycardia to maintain cardiac output and blood pressure.
Hyperadrenergic POTS
Up to 50% of patients with POTS have high norepinephrine levels (≥ 600 pg/mL) when upright. This subtype, hyperadrenergic POTS, is characterized by an increase in systolic blood pressure of at least 10 mm Hg within 10 minutes of standing, with concomitant tachycardia that can be similar to or greater than that seen in nonhyperadrenergic POTS. Patients with hyperadrenergic POTS tend to report more prominent symptoms of sympathetic activation, such as palpitations, anxiety, and tremulousness.24,25
Norepinephrine transporter deficiency
The norepinephrine transporter (NET) is on the presynaptic cleft of sympathetic neurons and serves to clear synaptic norepinephrine. NET deficiency leads to a hyperadrenergic state and elevated sympathetic nerve activation.18 NET deficiency may be induced by common antidepressants (eg, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors) and attention-deficit disorder medications.4
Mast cell activation syndrome
The relationship between mast cell activation syndrome and POTS is poorly understood.4,26 Mast cell activation syndrome has been described in a subset of patients with POTS who have sinus tachycardia accompanied by severe episodic flushing. Patients with this subtype have a hyperadrenergic response to postural change and elevated urine methylhistamine during flushing episodes.
Patients with mast cell activation syndrome tend to have strong allergic symptoms and may also have severe gastrointestinal problems, food sensitivities, dermatographism, and neuropathy. Diagnosis can be difficult, as the condition is associated with numerous markers with varying sensitivity and specificity.
Autoimmune origin
A significant minority of patients report a viral-like illness before the onset of POTS symptoms, suggesting a possible autoimmune-mediated or inflammatory cause. Also, some autoimmune disorders (eg, Sjögren syndrome) can present with a POTS-like manifestation.
Research into the role of autoantibodies in the pathophysiology of POTS offers the potential to develop novel therapeutic targets. Autoantibodies that have been reported in POTS include those against M1 to M3 muscarinic receptors (present in over 87% of patients with POTS),27 cardiac lipid raft-associated proteins,28 adrenergic G-protein coupled receptors, alpha-1-adrenergic receptors, and beta-1- and beta-2-adrenergic receptors.29 Although commercial enzyme-linked immunosorbent assays can assess for these antibody fragments, it is not known whether targeting the antibodies improves outcomes. At this time, antibody testing for POTS should be confined to the research setting.
LINKS TO OTHER SYNDROMES
POTS is often associated with other conditions whose symptoms cannot be explained by postural intolerance or tachycardia.
Ehlers-Danlos syndromes are a group of inherited heterogeneous disorders involving joint hypermobility, skin hyperextensibility, and tissue fragility.30 The hypermobile subtype is most commonly associated with POTS, with patients often having symptoms of autonomic dysregulation and autonomic test abnormalities.31–33 Patients with POTS may have a history of joint subluxations, joint pain, cervical instability, and spontaneous epidural leaks. The reason for the overlap between the two syndromes is not clear.
Chronic fatigue syndrome is characterized by persistent fatigue that does not resolve with rest and is not necessarily associated with orthostatic changes. More than 75% of patients with POTS report general fatigue as a major complaint, and up to 23% meet the full criteria for chronic fatigue syndrome.34
DIAGNOSTIC STRATEGY
A patient presenting with symptoms suggestive of POTS should first undergo a detailed history and physical examination. Other causes of sinus tachycardia should be considered.
Detailed history, symptom review
The history should focus on determining symptom burden, including tachycardia onset, frequency, severity, and triggers; the presence of syncope; and the impact of symptoms on daily function and quality of life.
Presyncope and its associated symptoms occur in less than one-third of patients with POTS, and syncope is not a principal feature.4 If syncope is the predominant complaint, alternative causes should be investigated. The usual cause of syncope in the general population is thought to be vasovagal.
In addition to orthostatic intolerance, gastrointestinal disturbances are common in POTS, presenting as abdominal pain, heartburn, irregular bowel movements, diarrhea, or constipation. Symptoms of gastroparesis are less common. Gastrointestinal symptoms tend to be prolonged, lasting hours and occurring multiple times a week. They tend not to improve in the supine position.35
POTS-associated symptoms may develop insidiously, but patients often report onset after an acute stressor such as pregnancy, major surgery, or a presumed viral illness.4 Whether these putative triggers are causative or coincidental is unknown. Symptoms of orthostatic intolerance tend to be exacerbated by dehydration, heat, alcohol, exercise, and menstruation.36,37
Consider the family history: 1 in 8 patients with POTS reports familial orthostatic intolerance,38 suggesting a genetic role in some patients. Inquire about symptoms or a previous diagnosis of Ehlers-Danlos syndrome and mast cell activation syndrome.
Consider other conditions
Pheochromocytoma causes hyperadrenergic symptoms (eg, palpitations, lightheadedness) like those in POTS, but patients with pheochromocytoma typically have these symptoms while supine. Pheochromocytoma is also characterized by plasma norepinephrine levels much higher than in POTS.4 Plasma metanephrine testing helps diagnose or rule out pheochromocytoma.5
Inappropriate sinus tachycardia, like pheochromocytoma, also has clinical features similar to those of POTS, as well as tachycardia present when supine. It involves higher sympathetic tone and lower parasympathetic tone compared with POTS; patients commonly have a daytime resting heart rate of at least 100 bpm or a 24-hour mean heart rate of at least 90 bpm.1,42 While the intrinsic heart rate is heightened in inappropriate sinus tachycardia, it is not different between POTS patients and healthy individuals.42,43 Distinguishing POTS from inappropriate sinus tachycardia is further complicated by the broad inclusion criteria of most studies of inappropriate sinus tachycardia, which failed to exclude patients with POTS.44 The Heart Rhythm Society recently adopted distinct definitions for the 2 conditions.1
Physical examination: Focus on vital signs
Dependent acrocyanosis—dark red-blue discoloration of the lower legs that is cold to the touch—occurs in about half of patients with POTS upon standing.4 Dependent acrocyanosis is associated with joint hypermobility and Ehlers-Danlos syndrome, so these conditions should also be considered if findings are positive.
Laboratory testing for other causes
Laboratory testing is used mainly to detect primary causes of sinus tachycardia. Tests should include:
- Complete blood cell count with hematocrit (for severe anemia)
- Thyroid-stimulating hormone level (for hyperthyroidism)
- Electrolyte panel (for significant electrolyte disturbances).
Evidence is insufficient to support routinely measuring the vitamin B12 level, iron indices, and serum markers for celiac disease, although these may be done if the history or physical examination suggests related problems.4 Sicca symptoms (severe dry eye or dry mouth) should trigger evaluation for Sjögren syndrome.
Electrocardiography needed
Electrocardiography should be performed to investigate for cardiac conduction abnormalities as well as for resting markers of a supraventricular tachyarrhythmia. Extended ambulatory (Holter) monitoring may be useful to evaluate for a transient reentrant tachyarrhythmia4; however, it does not record body position, so it can be difficult to determine if detected episodes of tachycardia are related to posture.
Additional testing for select cases
Further investigation is usually not needed to diagnose POTS but should be considered in some cases. Advanced tests are typically performed at a tertiary care referral center and include:
- Quantitative sensory testing to evaluate for small-fiber neuropathy (ie, Quantitative Sudomotor Axon Reflex Test, or QSART), which occurs in the neuropathic POTS subtype
- Formal autonomic function testing to characterize neurovascular responsiveness
- Supine and standing plasma norepinephrine levels (fractionated catecholamines) to characterize the net activation of the sympathetic nervous system
- Blood volume assessments to assess hypovolemia
- Formal exercise testing to objectively quantify exercise capacity.
GRADED MANAGEMENT
No single universal gold-standard therapy exists for POTS, and management should be individually determined with the primary goals of treating symptoms and restoring function. A graded approach should be used, starting with conservative nonpharmacologic therapies and adding medications as needed.
While the disease course varies substantially from patient to patient, proper management is strongly associated with eventual symptom improvement.1
NONPHARMACOLOGIC STEPS FIRST
Education
Patients should be informed of the nature of their condition and referred to appropriate healthcare personnel. POTS is a chronic illness requiring individualized coping strategies, intensive physician interaction, and support of a multidisciplinary team. Patients and family members can be reassured that most symptoms improve over time with appropriate diagnosis and treatment.1 Patients should be advised to avoid aggravating triggers and activities.
Exercise
Exercise programs are encouraged but should be introduced gradually, as physical activity can exacerbate symptoms, especially at the outset. Several studies have reported benefits from a short-term (3-month) program, in which the patient gradually progresses from non-upright exercise (eg, rowing machine, recumbent cycle, swimming) to upright endurance exercises. At the end of these programs, significant cardiac remodeling, improved quality of life, and reduced heart rate responses to standing have been reported, and benefits have been reported to persist in patients who continued exercising after the 3-month study period.46,47
Despite the benefits of exercise interventions, compliance is low.46,47 To prevent early discouragement, patients should be advised that it can take 4 to 6 weeks of continued exercise before benefits appear. Patients are encouraged to exercise every other day for 30 minutes or more. Regimens should primarily focus on aerobic conditioning, but resistance training, concentrating on thigh muscles, can also help. Exercise is a treatment and not a cure, and benefits can rapidly disappear if regular activity (at least 3 times per week) is stopped.48
Compression stockings
Compression stockings help reduce peripheral venous pooling and enhance venous return to the heart. Waist-high stockings with compression of at least 30 to 40 mm Hg offer the best results.
Diet
Increased fluid and salt intake is advisable for patients with suspected hypovolemia. At least 2 to 3 L of water accompanied by 10 to 12 g of daily sodium intake is recommended.1 This can usually be accomplished with diet and salt added to food, but salt tablets can be used if the patient prefers. The resultant plasma volume expansion may help reduce the reflex tachycardia upon standing.49
Check medications
Rescue therapy with saline infusion
Intravenous saline infusion can augment blood volume in patients who are clinically decompensated and present with severe symptoms.1 Intermittent infusion of 1 L of normal saline has been found to significantly reduce orthostatic tachycardia and related symptoms in patients with POTS, contributing to improved quality of life.51,52
Chronic saline infusions are not recommended for long-term care because of the risk of access complications and infection.1 Moak et al53 reported a high rate of bacteremia in a cohort of children with POTS with regular saline infusions, most of whom had a central line. On the other hand, Ruzieh et al54 reported significantly improved symptoms with regular saline infusions without a high rate of complications, but patients in this study received infusions for only a few months and through a peripheral intravenous catheter.
DRUG THERAPY
No medications are approved by the US Food and Drug Administration (FDA) or Health Canada specifically for treating POTS, making all pharmacologic recommendations off-label. Although the drugs discussed below have been evaluated for POTS in controlled laboratory settings, they have yet to be tested in robust clinical trials.
Blood volume expansion
Several drugs expand blood volume, which may reduce orthostatic tachycardia.
Fludrocortisone is a synthetic aldosterone analogue that enhances sodium and water retention. Although one observational study found that it normalizes hemodynamic changes in response to orthostatic stress, no high-level evidence exists for its effectiveness for POTS.55 It is generally well tolerated, although possible adverse effects include hyperkalemia, hypertension, fatigue, nausea, headache, and edema.5,56
Desmopressin is a synthetic version of a natural antidiuretic hormone that increases kidney-mediated free-water reabsorption without sodium retention. It significantly reduces upright heart rate in patients with POTS and improves symptom burden. Although potential adverse effects include edema and headache, hyponatremia is the primary concern with daily use, especially with the increased water intake advised for POTS.57 Patients should be advised to use desmopressin no more than once a week for the acute improvement of symptoms. Intermittent monitoring of serum sodium levels is recommended for safety.
Erythropoietin replacement has been suggested for treating POTS to address the significant deficit in red blood cell volume. Although erythropoietin therapy has a direct vasoconstrictive effect and largely improves red blood cell volume in patients with POTS, it does not expand plasma volume, so orthostatic tachycardia is not itself reduced.22 Nevertheless, it may significantly improve POTS symptoms refractory to more common methods of treatment, and it should be reserved for such cases. In addition to the lack of effect on orthostatic tachycardia, drawbacks to using erythropoietin include its high cost, the need for subcutaneous administration, and the risk of life-threatening complications such as myocardial infarction and stroke.58,59
Heart rate-lowering agents
Propranolol, a nonselective beta-adrenergic antagonist, can significantly reduce standing heart rate and improve symptoms at low dosages (10–20 mg). Higher dosages can further restrain orthostatic tachycardia but are not as well tolerated, mainly due to hypotension and worsening of existing symptoms such as fatigue.60 Regular-acting propranolol works for about 4 to 5 hours per dose, so full-day coverage often requires dosing 4 times per day.
Ivabradine is a selective blocker of the “funny” (If) channel that reduces the sinus node firing rate without affecting blood pressure, so it slows heart rate without causing supine hypertension or orthostatic hypotension.
A retrospective case series found that 60% of patients with POTS treated with ivabradine reported symptomatic improvement, and all patients experienced reduced tachycardia with continued use.61 Ivabradine has not been compared with placebo or propranolol in a randomized controlled trial, and it has not been well studied in pregnancy and so should be avoided because of potential teratogenic effects.
When prescribing ivabradine for women of childbearing age, a negative pregnancy test may be documented prior to initiation of therapy, and the use of highly effective methods of contraception is recommended. Ivabradine should be avoided in women contemplating pregnancy. Insurance coverage can limit access to ivabradine in the United States.
Central nervous system sympatholytics
Patients with prominent hyperadrenergic features may benefit from central sympatholytic agents. However, these drugs may not be well tolerated in patients with neuropathic POTS because of the effects of reduced systemic vascular resistance5 and the possible exacerbation of drowsiness, fatigue, and mental clouding.4 Patients can be extremely sensitive to these medications, so they should initially be prescribed at the lowest dose, then gradually increased as tolerated.
Clonidine, an alpha-2-adrenergic agonist, decreases central sympathetic tone. In hyperadrenergic patients, clonidine can stabilize heart rate and blood pressure, thereby reducing orthostatic symptoms.62
Methyldopa has effects similar to those of clonidine but is easier to titrate owing to its longer half-life.63 Methyldopa is typically started at 125 mg at bedtime and increased to 125 mg twice daily, if tolerated.
Other agents
Midodrine is a prodrug. The active form, an alpha-1-adrenergic agonist, constricts peripheral veins and arteries to increase vascular resistance and venous return, thereby reducing orthostatic tachycardia.52 It is most useful in patients with impaired peripheral vasoconstriction (eg, neuropathic POTS) and may be less effective in those with hyperadrenergic POTS.64 Major limitations of midodrine include worsening supine hypertension and possible urinary retention.39
Because of midodrine’s short half-life, frequent dosing is required during daytime hours (eg, 8 AM, noon, and 4 PM), but it should not be taken within 4 to 5 hours of sleep because of the risk of supine hypertension. Midodrine is typically started at 2.5 to 5 mg per dose and can be titrated up to 15 mg per dose.
Midodrine is an FDA pregnancy category C drug (adverse effects in pregnancy seen in animal models, but evidence lacking in humans). While ideally it should be avoided, we have used it safely in pregnant women with disabling POTS symptoms.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cardiovagal tone and possibly sympathetic tone. It has been reported to significantly reduce standing heart rate and improve symptom burden in patients with POTS.65 However, pyridostigmine increases gastrointestinal mobility, leading to severe adverse effects in over 20% of patients, including abdominal cramps, nausea, and diarrhea.66
Droxidopa, a synthetic amino acid precursor of norepinephrine, improves dizziness and fatigue in POTS with minimal effects on blood pressure.67
Modafinil, a psychostimulant, may improve POTS-associated cognitive symptoms.4 It also raises upright blood pressure without significantly worsening standing heart rate or acute orthostatic symptoms.68
EFFECTS OF COMORBID DISORDERS ON MANAGEMENT
Ehlers-Danlos syndrome
Pharmacologic approaches to POTS should not be altered based on the presence of Ehlers-Danlos syndrome, but because many of these patients are prone to joint dislocation, exercise prescriptions may need adjusting.
A medical genetics consult is recommended for patients with Ehlers-Danlos syndrome. Although the hypermobile type (the form most commonly associated with POTS) is not associated with aortopathy, it can be confused with classical and vascular Ehlers-Danlos syndromes, which require serial aortic screening.30
Mast cell activation syndrome
Consultation with an allergist or immunologist may help patients with severe symptoms.
Autoantibodies and autoimmunity
Treatment of the underlying disorder is recommended and can result in significantly improved POTS symptoms.
SPECIALTY CARE REFERRAL
POTS can be challenging to manage. Given the range of physiologic, emotional, and functional distress patients experience, it often requires significant physician time and multidisciplinary care. Patients with continued severe or debilitating symptoms may benefit from referral to a tertiary-care center with experience in autonomic nervous system disorders.
PROGNOSIS
Limited data are available on the long-term prognosis of POTS, and more studies are needed in pediatric and adult populations. No deaths have been reported in the handful of published cases of POTS in patients older than 50.1 Some pediatric studies suggest that some teenagers “outgrow” their POTS. However, these data are not robust, and an alternative explanation is that as they get older, they see adult physicians for their POTS symptoms and so are lost to study follow-up.6,44,69
We have not often seen POTS simply resolve without ongoing treatment. However, in our experience, most patients have improved symptoms and function with multimodal treatment (ie, exercise, salt, water, stockings, and some medications) and time.
Some people, most of them relatively young women, experience lightheadedness, a racing heart, and other symptoms (but not hypotension) when they stand up, in a condition known as postural tachycardia syndrome (POTS).1 Although not known to shorten life,1 it can be physically and mentally debilitating.2,3 Therapy rarely cures it, but a multifaceted approach can substantially improve quality of life.
This review outlines the evaluation and diagnosis of POTS and provides guidance for a therapy regimen.
HOW IS POTS DEFINED?
POTS is a multifactorial syndrome rather than a specific disease. It is characterized by all of the following1,4–6:
- An increase in heart rate of ≥ 30 bpm, or ≥ 40 bpm for those under age 19, within 10 minutes of standing from a supine position
- Sustained tachycardia (> 30 seconds)
- Absence of orthostatic hypotension (a fall in blood pressure of ≥ 20/10 mm Hg)
- Frequent and chronic duration (≥ 6 months).
These features are critical to diagnosis. Hemodynamic criteria in isolation may describe postural tachycardia but are not sufficient to diagnose POTS.
The prevalence of POTS is estimated to be between 0.2% and 1.0%,7 affecting up to 3 million people in the United States. Most cases arise between ages 13 and 50, with a female-to-male ratio of 5:1.8
MANY NAMES, SAME CONDITION
In 1871, Da Costa9 described a condition he called “irritable heart syndrome” that had characteristics similar to those of POTS, including extreme fatigue and exercise intolerance. Decades later, Lewis10 and Wood11 provided more detailed descriptions of the disorder, renaming it “soldier’s heart” or “Da Costa syndrome.” As other cases were documented, more terms arose, including “effort syndrome” and “mitral valve prolapse syndrome.”
In 1982, Rosen and Cryer12 were the first to use the term “postural tachycardia syndrome” for patients with disabling tachycardia upon standing without orthostatic hypotension. In 1986, Fouad et al13 described patients with postural tachycardia, orthostatic intolerance, and a small degree of hypotension as having “idiopathic hypovolemia.”
In 1993, Schondorf and Low14 established the current definition of POTS, leading to increased awareness and research efforts to understand its pathophysiology.
MULTIFACTORIAL PATHOPHYSIOLOGY
During the last 2 decades, several often-overlapping forms of POTS have been recognized, all of which share a final common pathway of sustained orthostatic tachycardia.15–19 In addition, a number of common comorbidities were identified through review of large clinic populations of POTS.20,21
Hypovolemic POTS
Up to 70% of patients with POTS have hypovolemia. The average plasma volume deficit is about 13%, which typically causes only insignificant changes in heart rate and norepinephrine levels while a patient is supine. However, blood pooling associated with upright posture further compromises cardiac output and consequently increases sympathetic nerve activity. Abnormalities in the renin-angiotensin-aldosterone volume regulation system are also suspected to impair sodium retention, contributing to hypovolemia.1,22
Neuropathic POTS
About half of patients with POTS have partial sympathetic denervation (particularly in the lower limbs) and inadequate vasoconstriction upon standing, leading to reduced venous return and stroke volume.17,23 A compensatory increase in sympathetic tone results in tachycardia to maintain cardiac output and blood pressure.
Hyperadrenergic POTS
Up to 50% of patients with POTS have high norepinephrine levels (≥ 600 pg/mL) when upright. This subtype, hyperadrenergic POTS, is characterized by an increase in systolic blood pressure of at least 10 mm Hg within 10 minutes of standing, with concomitant tachycardia that can be similar to or greater than that seen in nonhyperadrenergic POTS. Patients with hyperadrenergic POTS tend to report more prominent symptoms of sympathetic activation, such as palpitations, anxiety, and tremulousness.24,25
Norepinephrine transporter deficiency
The norepinephrine transporter (NET) is on the presynaptic cleft of sympathetic neurons and serves to clear synaptic norepinephrine. NET deficiency leads to a hyperadrenergic state and elevated sympathetic nerve activation.18 NET deficiency may be induced by common antidepressants (eg, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors) and attention-deficit disorder medications.4
Mast cell activation syndrome
The relationship between mast cell activation syndrome and POTS is poorly understood.4,26 Mast cell activation syndrome has been described in a subset of patients with POTS who have sinus tachycardia accompanied by severe episodic flushing. Patients with this subtype have a hyperadrenergic response to postural change and elevated urine methylhistamine during flushing episodes.
Patients with mast cell activation syndrome tend to have strong allergic symptoms and may also have severe gastrointestinal problems, food sensitivities, dermatographism, and neuropathy. Diagnosis can be difficult, as the condition is associated with numerous markers with varying sensitivity and specificity.
Autoimmune origin
A significant minority of patients report a viral-like illness before the onset of POTS symptoms, suggesting a possible autoimmune-mediated or inflammatory cause. Also, some autoimmune disorders (eg, Sjögren syndrome) can present with a POTS-like manifestation.
Research into the role of autoantibodies in the pathophysiology of POTS offers the potential to develop novel therapeutic targets. Autoantibodies that have been reported in POTS include those against M1 to M3 muscarinic receptors (present in over 87% of patients with POTS),27 cardiac lipid raft-associated proteins,28 adrenergic G-protein coupled receptors, alpha-1-adrenergic receptors, and beta-1- and beta-2-adrenergic receptors.29 Although commercial enzyme-linked immunosorbent assays can assess for these antibody fragments, it is not known whether targeting the antibodies improves outcomes. At this time, antibody testing for POTS should be confined to the research setting.
LINKS TO OTHER SYNDROMES
POTS is often associated with other conditions whose symptoms cannot be explained by postural intolerance or tachycardia.
Ehlers-Danlos syndromes are a group of inherited heterogeneous disorders involving joint hypermobility, skin hyperextensibility, and tissue fragility.30 The hypermobile subtype is most commonly associated with POTS, with patients often having symptoms of autonomic dysregulation and autonomic test abnormalities.31–33 Patients with POTS may have a history of joint subluxations, joint pain, cervical instability, and spontaneous epidural leaks. The reason for the overlap between the two syndromes is not clear.
Chronic fatigue syndrome is characterized by persistent fatigue that does not resolve with rest and is not necessarily associated with orthostatic changes. More than 75% of patients with POTS report general fatigue as a major complaint, and up to 23% meet the full criteria for chronic fatigue syndrome.34
DIAGNOSTIC STRATEGY
A patient presenting with symptoms suggestive of POTS should first undergo a detailed history and physical examination. Other causes of sinus tachycardia should be considered.
Detailed history, symptom review
The history should focus on determining symptom burden, including tachycardia onset, frequency, severity, and triggers; the presence of syncope; and the impact of symptoms on daily function and quality of life.
Presyncope and its associated symptoms occur in less than one-third of patients with POTS, and syncope is not a principal feature.4 If syncope is the predominant complaint, alternative causes should be investigated. The usual cause of syncope in the general population is thought to be vasovagal.
In addition to orthostatic intolerance, gastrointestinal disturbances are common in POTS, presenting as abdominal pain, heartburn, irregular bowel movements, diarrhea, or constipation. Symptoms of gastroparesis are less common. Gastrointestinal symptoms tend to be prolonged, lasting hours and occurring multiple times a week. They tend not to improve in the supine position.35
POTS-associated symptoms may develop insidiously, but patients often report onset after an acute stressor such as pregnancy, major surgery, or a presumed viral illness.4 Whether these putative triggers are causative or coincidental is unknown. Symptoms of orthostatic intolerance tend to be exacerbated by dehydration, heat, alcohol, exercise, and menstruation.36,37
Consider the family history: 1 in 8 patients with POTS reports familial orthostatic intolerance,38 suggesting a genetic role in some patients. Inquire about symptoms or a previous diagnosis of Ehlers-Danlos syndrome and mast cell activation syndrome.
Consider other conditions
Pheochromocytoma causes hyperadrenergic symptoms (eg, palpitations, lightheadedness) like those in POTS, but patients with pheochromocytoma typically have these symptoms while supine. Pheochromocytoma is also characterized by plasma norepinephrine levels much higher than in POTS.4 Plasma metanephrine testing helps diagnose or rule out pheochromocytoma.5
Inappropriate sinus tachycardia, like pheochromocytoma, also has clinical features similar to those of POTS, as well as tachycardia present when supine. It involves higher sympathetic tone and lower parasympathetic tone compared with POTS; patients commonly have a daytime resting heart rate of at least 100 bpm or a 24-hour mean heart rate of at least 90 bpm.1,42 While the intrinsic heart rate is heightened in inappropriate sinus tachycardia, it is not different between POTS patients and healthy individuals.42,43 Distinguishing POTS from inappropriate sinus tachycardia is further complicated by the broad inclusion criteria of most studies of inappropriate sinus tachycardia, which failed to exclude patients with POTS.44 The Heart Rhythm Society recently adopted distinct definitions for the 2 conditions.1
Physical examination: Focus on vital signs
Dependent acrocyanosis—dark red-blue discoloration of the lower legs that is cold to the touch—occurs in about half of patients with POTS upon standing.4 Dependent acrocyanosis is associated with joint hypermobility and Ehlers-Danlos syndrome, so these conditions should also be considered if findings are positive.
Laboratory testing for other causes
Laboratory testing is used mainly to detect primary causes of sinus tachycardia. Tests should include:
- Complete blood cell count with hematocrit (for severe anemia)
- Thyroid-stimulating hormone level (for hyperthyroidism)
- Electrolyte panel (for significant electrolyte disturbances).
Evidence is insufficient to support routinely measuring the vitamin B12 level, iron indices, and serum markers for celiac disease, although these may be done if the history or physical examination suggests related problems.4 Sicca symptoms (severe dry eye or dry mouth) should trigger evaluation for Sjögren syndrome.
Electrocardiography needed
Electrocardiography should be performed to investigate for cardiac conduction abnormalities as well as for resting markers of a supraventricular tachyarrhythmia. Extended ambulatory (Holter) monitoring may be useful to evaluate for a transient reentrant tachyarrhythmia4; however, it does not record body position, so it can be difficult to determine if detected episodes of tachycardia are related to posture.
Additional testing for select cases
Further investigation is usually not needed to diagnose POTS but should be considered in some cases. Advanced tests are typically performed at a tertiary care referral center and include:
- Quantitative sensory testing to evaluate for small-fiber neuropathy (ie, Quantitative Sudomotor Axon Reflex Test, or QSART), which occurs in the neuropathic POTS subtype
- Formal autonomic function testing to characterize neurovascular responsiveness
- Supine and standing plasma norepinephrine levels (fractionated catecholamines) to characterize the net activation of the sympathetic nervous system
- Blood volume assessments to assess hypovolemia
- Formal exercise testing to objectively quantify exercise capacity.
GRADED MANAGEMENT
No single universal gold-standard therapy exists for POTS, and management should be individually determined with the primary goals of treating symptoms and restoring function. A graded approach should be used, starting with conservative nonpharmacologic therapies and adding medications as needed.
While the disease course varies substantially from patient to patient, proper management is strongly associated with eventual symptom improvement.1
NONPHARMACOLOGIC STEPS FIRST
Education
Patients should be informed of the nature of their condition and referred to appropriate healthcare personnel. POTS is a chronic illness requiring individualized coping strategies, intensive physician interaction, and support of a multidisciplinary team. Patients and family members can be reassured that most symptoms improve over time with appropriate diagnosis and treatment.1 Patients should be advised to avoid aggravating triggers and activities.
Exercise
Exercise programs are encouraged but should be introduced gradually, as physical activity can exacerbate symptoms, especially at the outset. Several studies have reported benefits from a short-term (3-month) program, in which the patient gradually progresses from non-upright exercise (eg, rowing machine, recumbent cycle, swimming) to upright endurance exercises. At the end of these programs, significant cardiac remodeling, improved quality of life, and reduced heart rate responses to standing have been reported, and benefits have been reported to persist in patients who continued exercising after the 3-month study period.46,47
Despite the benefits of exercise interventions, compliance is low.46,47 To prevent early discouragement, patients should be advised that it can take 4 to 6 weeks of continued exercise before benefits appear. Patients are encouraged to exercise every other day for 30 minutes or more. Regimens should primarily focus on aerobic conditioning, but resistance training, concentrating on thigh muscles, can also help. Exercise is a treatment and not a cure, and benefits can rapidly disappear if regular activity (at least 3 times per week) is stopped.48
Compression stockings
Compression stockings help reduce peripheral venous pooling and enhance venous return to the heart. Waist-high stockings with compression of at least 30 to 40 mm Hg offer the best results.
Diet
Increased fluid and salt intake is advisable for patients with suspected hypovolemia. At least 2 to 3 L of water accompanied by 10 to 12 g of daily sodium intake is recommended.1 This can usually be accomplished with diet and salt added to food, but salt tablets can be used if the patient prefers. The resultant plasma volume expansion may help reduce the reflex tachycardia upon standing.49
Check medications
Rescue therapy with saline infusion
Intravenous saline infusion can augment blood volume in patients who are clinically decompensated and present with severe symptoms.1 Intermittent infusion of 1 L of normal saline has been found to significantly reduce orthostatic tachycardia and related symptoms in patients with POTS, contributing to improved quality of life.51,52
Chronic saline infusions are not recommended for long-term care because of the risk of access complications and infection.1 Moak et al53 reported a high rate of bacteremia in a cohort of children with POTS with regular saline infusions, most of whom had a central line. On the other hand, Ruzieh et al54 reported significantly improved symptoms with regular saline infusions without a high rate of complications, but patients in this study received infusions for only a few months and through a peripheral intravenous catheter.
DRUG THERAPY
No medications are approved by the US Food and Drug Administration (FDA) or Health Canada specifically for treating POTS, making all pharmacologic recommendations off-label. Although the drugs discussed below have been evaluated for POTS in controlled laboratory settings, they have yet to be tested in robust clinical trials.
Blood volume expansion
Several drugs expand blood volume, which may reduce orthostatic tachycardia.
Fludrocortisone is a synthetic aldosterone analogue that enhances sodium and water retention. Although one observational study found that it normalizes hemodynamic changes in response to orthostatic stress, no high-level evidence exists for its effectiveness for POTS.55 It is generally well tolerated, although possible adverse effects include hyperkalemia, hypertension, fatigue, nausea, headache, and edema.5,56
Desmopressin is a synthetic version of a natural antidiuretic hormone that increases kidney-mediated free-water reabsorption without sodium retention. It significantly reduces upright heart rate in patients with POTS and improves symptom burden. Although potential adverse effects include edema and headache, hyponatremia is the primary concern with daily use, especially with the increased water intake advised for POTS.57 Patients should be advised to use desmopressin no more than once a week for the acute improvement of symptoms. Intermittent monitoring of serum sodium levels is recommended for safety.
Erythropoietin replacement has been suggested for treating POTS to address the significant deficit in red blood cell volume. Although erythropoietin therapy has a direct vasoconstrictive effect and largely improves red blood cell volume in patients with POTS, it does not expand plasma volume, so orthostatic tachycardia is not itself reduced.22 Nevertheless, it may significantly improve POTS symptoms refractory to more common methods of treatment, and it should be reserved for such cases. In addition to the lack of effect on orthostatic tachycardia, drawbacks to using erythropoietin include its high cost, the need for subcutaneous administration, and the risk of life-threatening complications such as myocardial infarction and stroke.58,59
Heart rate-lowering agents
Propranolol, a nonselective beta-adrenergic antagonist, can significantly reduce standing heart rate and improve symptoms at low dosages (10–20 mg). Higher dosages can further restrain orthostatic tachycardia but are not as well tolerated, mainly due to hypotension and worsening of existing symptoms such as fatigue.60 Regular-acting propranolol works for about 4 to 5 hours per dose, so full-day coverage often requires dosing 4 times per day.
Ivabradine is a selective blocker of the “funny” (If) channel that reduces the sinus node firing rate without affecting blood pressure, so it slows heart rate without causing supine hypertension or orthostatic hypotension.
A retrospective case series found that 60% of patients with POTS treated with ivabradine reported symptomatic improvement, and all patients experienced reduced tachycardia with continued use.61 Ivabradine has not been compared with placebo or propranolol in a randomized controlled trial, and it has not been well studied in pregnancy and so should be avoided because of potential teratogenic effects.
When prescribing ivabradine for women of childbearing age, a negative pregnancy test may be documented prior to initiation of therapy, and the use of highly effective methods of contraception is recommended. Ivabradine should be avoided in women contemplating pregnancy. Insurance coverage can limit access to ivabradine in the United States.
Central nervous system sympatholytics
Patients with prominent hyperadrenergic features may benefit from central sympatholytic agents. However, these drugs may not be well tolerated in patients with neuropathic POTS because of the effects of reduced systemic vascular resistance5 and the possible exacerbation of drowsiness, fatigue, and mental clouding.4 Patients can be extremely sensitive to these medications, so they should initially be prescribed at the lowest dose, then gradually increased as tolerated.
Clonidine, an alpha-2-adrenergic agonist, decreases central sympathetic tone. In hyperadrenergic patients, clonidine can stabilize heart rate and blood pressure, thereby reducing orthostatic symptoms.62
Methyldopa has effects similar to those of clonidine but is easier to titrate owing to its longer half-life.63 Methyldopa is typically started at 125 mg at bedtime and increased to 125 mg twice daily, if tolerated.
Other agents
Midodrine is a prodrug. The active form, an alpha-1-adrenergic agonist, constricts peripheral veins and arteries to increase vascular resistance and venous return, thereby reducing orthostatic tachycardia.52 It is most useful in patients with impaired peripheral vasoconstriction (eg, neuropathic POTS) and may be less effective in those with hyperadrenergic POTS.64 Major limitations of midodrine include worsening supine hypertension and possible urinary retention.39
Because of midodrine’s short half-life, frequent dosing is required during daytime hours (eg, 8 AM, noon, and 4 PM), but it should not be taken within 4 to 5 hours of sleep because of the risk of supine hypertension. Midodrine is typically started at 2.5 to 5 mg per dose and can be titrated up to 15 mg per dose.
Midodrine is an FDA pregnancy category C drug (adverse effects in pregnancy seen in animal models, but evidence lacking in humans). While ideally it should be avoided, we have used it safely in pregnant women with disabling POTS symptoms.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cardiovagal tone and possibly sympathetic tone. It has been reported to significantly reduce standing heart rate and improve symptom burden in patients with POTS.65 However, pyridostigmine increases gastrointestinal mobility, leading to severe adverse effects in over 20% of patients, including abdominal cramps, nausea, and diarrhea.66
Droxidopa, a synthetic amino acid precursor of norepinephrine, improves dizziness and fatigue in POTS with minimal effects on blood pressure.67
Modafinil, a psychostimulant, may improve POTS-associated cognitive symptoms.4 It also raises upright blood pressure without significantly worsening standing heart rate or acute orthostatic symptoms.68
EFFECTS OF COMORBID DISORDERS ON MANAGEMENT
Ehlers-Danlos syndrome
Pharmacologic approaches to POTS should not be altered based on the presence of Ehlers-Danlos syndrome, but because many of these patients are prone to joint dislocation, exercise prescriptions may need adjusting.
A medical genetics consult is recommended for patients with Ehlers-Danlos syndrome. Although the hypermobile type (the form most commonly associated with POTS) is not associated with aortopathy, it can be confused with classical and vascular Ehlers-Danlos syndromes, which require serial aortic screening.30
Mast cell activation syndrome
Consultation with an allergist or immunologist may help patients with severe symptoms.
Autoantibodies and autoimmunity
Treatment of the underlying disorder is recommended and can result in significantly improved POTS symptoms.
SPECIALTY CARE REFERRAL
POTS can be challenging to manage. Given the range of physiologic, emotional, and functional distress patients experience, it often requires significant physician time and multidisciplinary care. Patients with continued severe or debilitating symptoms may benefit from referral to a tertiary-care center with experience in autonomic nervous system disorders.
PROGNOSIS
Limited data are available on the long-term prognosis of POTS, and more studies are needed in pediatric and adult populations. No deaths have been reported in the handful of published cases of POTS in patients older than 50.1 Some pediatric studies suggest that some teenagers “outgrow” their POTS. However, these data are not robust, and an alternative explanation is that as they get older, they see adult physicians for their POTS symptoms and so are lost to study follow-up.6,44,69
We have not often seen POTS simply resolve without ongoing treatment. However, in our experience, most patients have improved symptoms and function with multimodal treatment (ie, exercise, salt, water, stockings, and some medications) and time.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7(2):204–210. pmid:21509337
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77(6):531–537. doi:10.4065/77.6.531
- Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013; 127(23):2336–2342. doi:10.1161/CIRCULATIONAHA.112.144501
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006; 6(2):84–99. pmid:16943900
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012; 160(2):222–226. doi:10.1016/j.jpeds.2011.08.054
- Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol 2014; 5:220. doi:10.3389/fphys.2014.00220
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69(8):790–798. doi:10.1212/01.wnl.0000267663.05398.40
- Da Costa JM. On irritable heart: a clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci 1871; 61(121):2–52.
- Lewis T. The tolerance of physical exertion, as shown by soldiers suffering from so-called “irritable heart.” Br Med J 1918; 1(2987):363–365. pmid:20768980
- Wood P. Da Costa’s syndrome (or effort syndrome): lecture I. Br Med J 1941; 1(4194):767–772. pmid:20783672
- Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982; 72(5):847–850.
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986; 104(3):298–303. pmid:3511818
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993; 43(1):132–137. pmid:8423877
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007; 334(1):57–60. doi:10.1097/MAJ.0b013e318063c6c0
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med 2000; 343(14):1008–1014. doi:10.1056/NEJM200010053431404
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 342(8):541–549. doi:10.1056/NEJM200002243420803
- Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16(6):431–438. doi:10.1136/practneurol-2016-001405
- Gunning WT, Karabin BL, Blomquist TM, Grubb BP. Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine (Baltimore) 2016; 95(37):e4849. doi:10.1097/MD.0000000000004849
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011; 34(5):549–554. doi:10.1111/j.1540-8159.2010.02994.x
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111(13):1574–1582. doi:10.1161/01.CIR.0000160356.97313.5D
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013; 8(12):e84716. doi:10.1371/journal.pone.0084716
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20(3):352–358. doi:10.1111/j.1540-8167.2008.01407.x
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18(5):527–531. pmid:21947988
- Shibao C, Arzubiaga C, Roberts J, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45(3):385–390. doi:10.1161/01.HYP.0000158259.68614.40
- Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker [abstract]. J Clin Neuromuscul Dis 2016; 17(3):179S.
- Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013; 162(1):34–44. doi:10.1016/j.trsl.2013.03.002
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014; 3(1):e000755. doi:10.1161/JAHA.113.000755
- Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175(1):8–26. doi:10.1002/ajmg.c.31552
- Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340(1-2):99–102. doi:10.1016/j.jns.2014.03.002
- De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014; 44(3):353–361. doi:10.1016/j.semarthrit.2014.05.013
- Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med 2003; 115(1):33–40. pmid:12867232
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012; 122(4):183–192. doi:10.1042/CS20110200
- Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD. Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 2015; 359(1-2):193–196. doi:10.1016/j.jns.2015.10.052
- Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5(2):122–129. doi:10.15420/AER.2016.7.2
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012; 118(3):242–246. doi:10.1016/j.ijgo.2012.04.014
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82(3):308–313. doi:10.4065/82.3.308
- Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2015; 28(7):157–159. pmid:25829642
- Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci 2013; 9(5):944–952. doi:10.5114/aoms.2013.38685
- Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol. Br J Clin Pharmacol 1982; 13(suppl 2):345S–351S. pmid:6125187
- Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc 2014; 3(2):e000700. doi:10.1161/JAHA.113.000700
- Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of “inappropriate” sinus tachycardia. Role of sympathovagal balance. Circulation 1994; 90(2):873–877. pmid:7913886
- Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117(21):2814–2817. doi:10.1161/CIRCULATIONAHA.107.761643
- Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173:149–153. doi:10.1016/j.jpeds.2016.02.035
- George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016; 13(4):943–950. doi:10.1016/j.hrthm.2015.12.012
- Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55(25):2858–2868. doi:10.1016/j.jacc.2010.02.043
- Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13(4):951–952. doi:10.1016/j.hrthm.2015.12.039
- Celedonio JE, Garland EM, Nwazue VC, et al. Effects of high sodium intake on blood volume and catecholamines in patients with postural tachycardia syndrome and healthy females [abstract]. Clin Auton Res 2014; 24:211.
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15(9):60. doi:10.1007/s11910-015-0583-8
- Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000; 10:29–33. pmid:10750641
- Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997; 96(2):575–580. pmid:9244228
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol 2016; 37(2):278–282. doi:10.1007/s00246-015-1274-6
- Ruzieh M, Baugh A, Dasa O, et al. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol 2017; 48(3):255–260. doi:10.1007/s10840-017-0225-y
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res 2000; 10(5):293–299. pmid:11198485
- Lee AK, Krahn AD. Evaluation of syncope: focus on diagnosis and treatment of neurally mediated syncope. Expert Rev Cardiovasc Ther 2016; 14(6):725–736. doi:10.1586/14779072.2016.1164034
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012; 9(9):1484–1490. doi:10.1016/j.hrthm.2012.05.002
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2012; 19(2):92–95. doi:10.1097/MJT.0b013e3181ef621a
- Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med 1995; 99(5):525–529. pmid:7485211
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009; 120(9):725–734. doi:10.1161/CIRCULATIONAHA.108.846501
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011; 13(3):427–430. doi:10.1093/europace/euq390
- Gaffney FA, Lane LB, Pettinger W, Blomqvist G. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest 1983; 83(suppl 2):436–438. pmid:6295714
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999; 317(2):88–101. pmid:10037112
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014; 126(4):289–296. doi:10.1042/CS20130222
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005; 111(21):2734–2340. doi:10.1161/CIRCULATIONAHA.104.497594
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol 2011; 34(6):750–755. doi:10.1111/j.1540-8159.2011.03047.x
- Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017; 24(2):e157–e161. doi:10.1097/MJT.0000000000000468
- Kpaeyeh AG Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol 2014; 34(6):738–741. doi:10.1097/JCP.0000000000000221
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol 2009; 32(2):234–238. doi:10.1111/j.1540-8159.2008.02207.x
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7(2):204–210. pmid:21509337
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77(6):531–537. doi:10.4065/77.6.531
- Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013; 127(23):2336–2342. doi:10.1161/CIRCULATIONAHA.112.144501
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006; 6(2):84–99. pmid:16943900
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012; 160(2):222–226. doi:10.1016/j.jpeds.2011.08.054
- Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol 2014; 5:220. doi:10.3389/fphys.2014.00220
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69(8):790–798. doi:10.1212/01.wnl.0000267663.05398.40
- Da Costa JM. On irritable heart: a clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci 1871; 61(121):2–52.
- Lewis T. The tolerance of physical exertion, as shown by soldiers suffering from so-called “irritable heart.” Br Med J 1918; 1(2987):363–365. pmid:20768980
- Wood P. Da Costa’s syndrome (or effort syndrome): lecture I. Br Med J 1941; 1(4194):767–772. pmid:20783672
- Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982; 72(5):847–850.
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986; 104(3):298–303. pmid:3511818
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993; 43(1):132–137. pmid:8423877
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007; 334(1):57–60. doi:10.1097/MAJ.0b013e318063c6c0
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med 2000; 343(14):1008–1014. doi:10.1056/NEJM200010053431404
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 342(8):541–549. doi:10.1056/NEJM200002243420803
- Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16(6):431–438. doi:10.1136/practneurol-2016-001405
- Gunning WT, Karabin BL, Blomquist TM, Grubb BP. Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine (Baltimore) 2016; 95(37):e4849. doi:10.1097/MD.0000000000004849
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011; 34(5):549–554. doi:10.1111/j.1540-8159.2010.02994.x
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111(13):1574–1582. doi:10.1161/01.CIR.0000160356.97313.5D
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013; 8(12):e84716. doi:10.1371/journal.pone.0084716
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20(3):352–358. doi:10.1111/j.1540-8167.2008.01407.x
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18(5):527–531. pmid:21947988
- Shibao C, Arzubiaga C, Roberts J, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45(3):385–390. doi:10.1161/01.HYP.0000158259.68614.40
- Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker [abstract]. J Clin Neuromuscul Dis 2016; 17(3):179S.
- Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013; 162(1):34–44. doi:10.1016/j.trsl.2013.03.002
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014; 3(1):e000755. doi:10.1161/JAHA.113.000755
- Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175(1):8–26. doi:10.1002/ajmg.c.31552
- Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340(1-2):99–102. doi:10.1016/j.jns.2014.03.002
- De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014; 44(3):353–361. doi:10.1016/j.semarthrit.2014.05.013
- Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med 2003; 115(1):33–40. pmid:12867232
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012; 122(4):183–192. doi:10.1042/CS20110200
- Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD. Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 2015; 359(1-2):193–196. doi:10.1016/j.jns.2015.10.052
- Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5(2):122–129. doi:10.15420/AER.2016.7.2
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012; 118(3):242–246. doi:10.1016/j.ijgo.2012.04.014
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82(3):308–313. doi:10.4065/82.3.308
- Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2015; 28(7):157–159. pmid:25829642
- Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci 2013; 9(5):944–952. doi:10.5114/aoms.2013.38685
- Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol. Br J Clin Pharmacol 1982; 13(suppl 2):345S–351S. pmid:6125187
- Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc 2014; 3(2):e000700. doi:10.1161/JAHA.113.000700
- Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of “inappropriate” sinus tachycardia. Role of sympathovagal balance. Circulation 1994; 90(2):873–877. pmid:7913886
- Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117(21):2814–2817. doi:10.1161/CIRCULATIONAHA.107.761643
- Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173:149–153. doi:10.1016/j.jpeds.2016.02.035
- George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016; 13(4):943–950. doi:10.1016/j.hrthm.2015.12.012
- Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55(25):2858–2868. doi:10.1016/j.jacc.2010.02.043
- Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13(4):951–952. doi:10.1016/j.hrthm.2015.12.039
- Celedonio JE, Garland EM, Nwazue VC, et al. Effects of high sodium intake on blood volume and catecholamines in patients with postural tachycardia syndrome and healthy females [abstract]. Clin Auton Res 2014; 24:211.
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15(9):60. doi:10.1007/s11910-015-0583-8
- Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000; 10:29–33. pmid:10750641
- Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997; 96(2):575–580. pmid:9244228
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol 2016; 37(2):278–282. doi:10.1007/s00246-015-1274-6
- Ruzieh M, Baugh A, Dasa O, et al. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol 2017; 48(3):255–260. doi:10.1007/s10840-017-0225-y
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res 2000; 10(5):293–299. pmid:11198485
- Lee AK, Krahn AD. Evaluation of syncope: focus on diagnosis and treatment of neurally mediated syncope. Expert Rev Cardiovasc Ther 2016; 14(6):725–736. doi:10.1586/14779072.2016.1164034
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012; 9(9):1484–1490. doi:10.1016/j.hrthm.2012.05.002
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2012; 19(2):92–95. doi:10.1097/MJT.0b013e3181ef621a
- Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med 1995; 99(5):525–529. pmid:7485211
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009; 120(9):725–734. doi:10.1161/CIRCULATIONAHA.108.846501
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011; 13(3):427–430. doi:10.1093/europace/euq390
- Gaffney FA, Lane LB, Pettinger W, Blomqvist G. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest 1983; 83(suppl 2):436–438. pmid:6295714
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999; 317(2):88–101. pmid:10037112
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014; 126(4):289–296. doi:10.1042/CS20130222
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005; 111(21):2734–2340. doi:10.1161/CIRCULATIONAHA.104.497594
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol 2011; 34(6):750–755. doi:10.1111/j.1540-8159.2011.03047.x
- Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017; 24(2):e157–e161. doi:10.1097/MJT.0000000000000468
- Kpaeyeh AG Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol 2014; 34(6):738–741. doi:10.1097/JCP.0000000000000221
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol 2009; 32(2):234–238. doi:10.1111/j.1540-8159.2008.02207.x
KEY POINTS
- Several POTS subtypes have been recognized, including hypovolemic, neuropathic, and hyperadrenergic forms, overlapping with Ehlers-Danlos syndrome, mast cell activation, and autoimmune syndromes.
- Treatment should take a graded approach, beginning with increasing salt and water intake, exercise, and compression stockings.
- If needed, consider medications to expand blood volume, slow heart rate, or reduce central sympathetic tone.
- Certain medications, including venodilators, diuretics, and serotonin-norepinephrine reuptake inhibitors, can exacerbate symptoms and should be avoided.
Gastric outlet obstruction: A red flag, potentially manageable
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
KEY POINTS
- Causes of gastric outlet obstruction fall into 2 categories: benign and malignant. The cause should be presumed to be malignant until proven otherwise.
- Peptic ulcer disease, a benign cause, used to account for most cases of gastric outlet obstruction. It is still common but has declined in frequency with the development of acid-suppressing drugs.
- Gastric cancer used to be the most common malignant cause but has declined in frequency in Western countries with treatment for Helicobacter pylori infection. Now, pancreatic cancer predominates.
- Endoscopic stenting is an effective, minimally invasive treatment for patients with malignant gastric outlet obstruction and poor prognosis, allowing resumption of oral intake and improving quality of life.