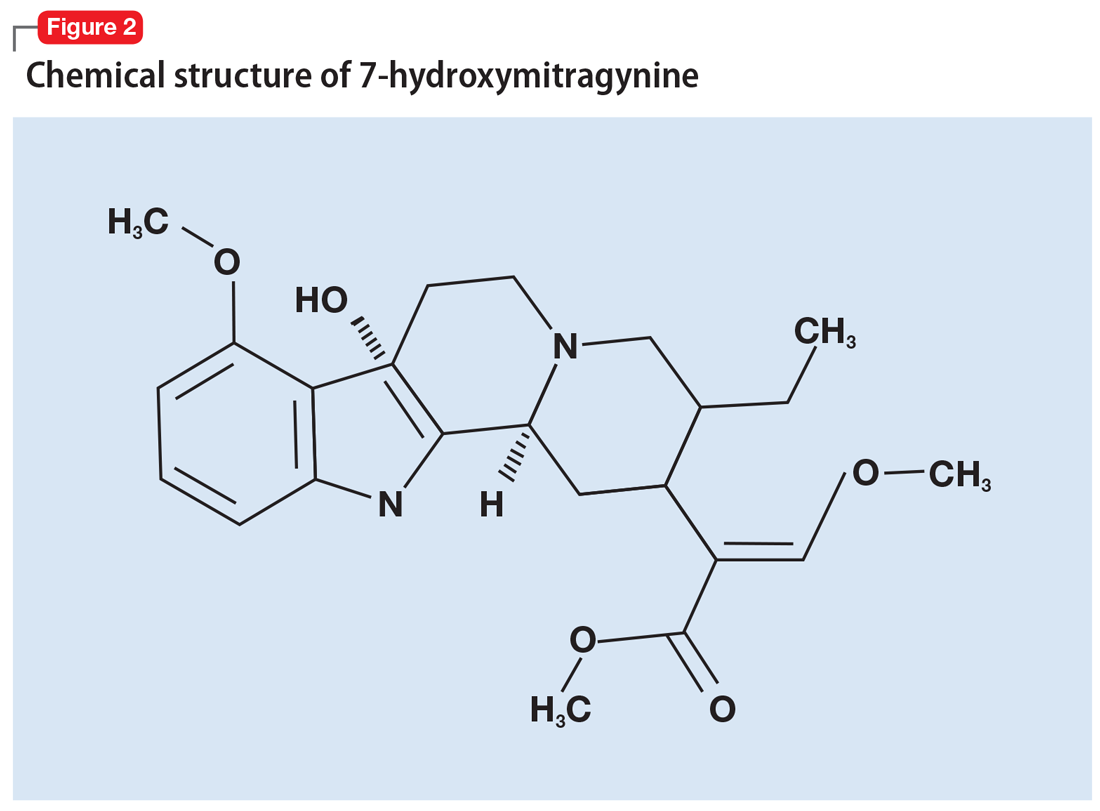
User login
Is psychosis toxic to the brain?
Schizophrenia has been described as the “worst disease” to afflict mankind.1 It causes psychosis, which is an abnormal state of mind marked by hyperarousal, overactivation of brain circuits, and emotional distress. An untreated episode of psychosis can result in structural brain damage due to neurotoxicity. Patients who experience psychosis may be affected by inflammatory processes, oxidative and nitrosative reactions, mitochondrial dysfunction, decreased synaptic plasticity and neurogenesis, demyelination, and autoimmune attacks—all of which can contribute to cell necrosis and irreversible neuronal atrophy.2-4
The impacts of untreated psychosis
First-episode psychosis (FEP) can result in a loss of up to 1% of total brain volume and up to 3% of cortical gray matter.4,5 When FEP goes untreated, approximately 10 to 12 cc of brain tissue—basically a tablespoon of cells and myelin—could be permanently damaged.2,6,7 This explains why enlarged ventricles are a common radiologic finding in patients with schizophrenia.2 In such patients, imaging of the brain will show these as hollow, fluid-filled spaces that appear expanded.
Repeated episodes of untreated psychosis could result in progressively lower levels of baseline functioning, and patients may require longer hospitalizations to achieve stabilization and higher doses of medications to achieve remission.4,7 Greater brain volume losses are associated with poorer outcomes.3 Brain volume loss is also detectable in patients with untreated major depressive episodes,8 and recurrent episodes of bipolar I disorder can also result in the loss of gray matter and structural brain damage.9 The progressive decline in cognitive and functional outcomes and eventual development of treatment resistance are likely due to a kindling phenomenon or receptor sensitization.10
Act fast to prevent brain damage
Since it was first identified, schizophrenia has been recognized as a degenerative disease. However, the progression to structural brain damage is not inevitable, and can be arrested with expeditious, decisive treatment. Some agents, such as certain antipsychotic medications10 and omega-3 fatty acids, can be neuroprotective.11 The early use of a long-acting injectable antipsychotic also may help prevent relapse and additional psychotic episodes.5
Psychosis requires expedient and competent intervention to improve outcomes and reduce disease burden. Timely psychiatric treatment can improve not only immediate functioning, but also long-term prognosis. Because untreated psychosis can result in irreversible structural brain damage, clinicians must act swiftly to provide assertive treatment.
1. Where next with psychiatric illness? Nature. 1998;336(6195):95-96.
2. Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521-529.
3. van Haren NE, Hulshoff HE, Shnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
4. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606.
9. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519.
10. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
11. Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934.
Schizophrenia has been described as the “worst disease” to afflict mankind.1 It causes psychosis, which is an abnormal state of mind marked by hyperarousal, overactivation of brain circuits, and emotional distress. An untreated episode of psychosis can result in structural brain damage due to neurotoxicity. Patients who experience psychosis may be affected by inflammatory processes, oxidative and nitrosative reactions, mitochondrial dysfunction, decreased synaptic plasticity and neurogenesis, demyelination, and autoimmune attacks—all of which can contribute to cell necrosis and irreversible neuronal atrophy.2-4
The impacts of untreated psychosis
First-episode psychosis (FEP) can result in a loss of up to 1% of total brain volume and up to 3% of cortical gray matter.4,5 When FEP goes untreated, approximately 10 to 12 cc of brain tissue—basically a tablespoon of cells and myelin—could be permanently damaged.2,6,7 This explains why enlarged ventricles are a common radiologic finding in patients with schizophrenia.2 In such patients, imaging of the brain will show these as hollow, fluid-filled spaces that appear expanded.
Repeated episodes of untreated psychosis could result in progressively lower levels of baseline functioning, and patients may require longer hospitalizations to achieve stabilization and higher doses of medications to achieve remission.4,7 Greater brain volume losses are associated with poorer outcomes.3 Brain volume loss is also detectable in patients with untreated major depressive episodes,8 and recurrent episodes of bipolar I disorder can also result in the loss of gray matter and structural brain damage.9 The progressive decline in cognitive and functional outcomes and eventual development of treatment resistance are likely due to a kindling phenomenon or receptor sensitization.10
Act fast to prevent brain damage
Since it was first identified, schizophrenia has been recognized as a degenerative disease. However, the progression to structural brain damage is not inevitable, and can be arrested with expeditious, decisive treatment. Some agents, such as certain antipsychotic medications10 and omega-3 fatty acids, can be neuroprotective.11 The early use of a long-acting injectable antipsychotic also may help prevent relapse and additional psychotic episodes.5
Psychosis requires expedient and competent intervention to improve outcomes and reduce disease burden. Timely psychiatric treatment can improve not only immediate functioning, but also long-term prognosis. Because untreated psychosis can result in irreversible structural brain damage, clinicians must act swiftly to provide assertive treatment.
Schizophrenia has been described as the “worst disease” to afflict mankind.1 It causes psychosis, which is an abnormal state of mind marked by hyperarousal, overactivation of brain circuits, and emotional distress. An untreated episode of psychosis can result in structural brain damage due to neurotoxicity. Patients who experience psychosis may be affected by inflammatory processes, oxidative and nitrosative reactions, mitochondrial dysfunction, decreased synaptic plasticity and neurogenesis, demyelination, and autoimmune attacks—all of which can contribute to cell necrosis and irreversible neuronal atrophy.2-4
The impacts of untreated psychosis
First-episode psychosis (FEP) can result in a loss of up to 1% of total brain volume and up to 3% of cortical gray matter.4,5 When FEP goes untreated, approximately 10 to 12 cc of brain tissue—basically a tablespoon of cells and myelin—could be permanently damaged.2,6,7 This explains why enlarged ventricles are a common radiologic finding in patients with schizophrenia.2 In such patients, imaging of the brain will show these as hollow, fluid-filled spaces that appear expanded.
Repeated episodes of untreated psychosis could result in progressively lower levels of baseline functioning, and patients may require longer hospitalizations to achieve stabilization and higher doses of medications to achieve remission.4,7 Greater brain volume losses are associated with poorer outcomes.3 Brain volume loss is also detectable in patients with untreated major depressive episodes,8 and recurrent episodes of bipolar I disorder can also result in the loss of gray matter and structural brain damage.9 The progressive decline in cognitive and functional outcomes and eventual development of treatment resistance are likely due to a kindling phenomenon or receptor sensitization.10
Act fast to prevent brain damage
Since it was first identified, schizophrenia has been recognized as a degenerative disease. However, the progression to structural brain damage is not inevitable, and can be arrested with expeditious, decisive treatment. Some agents, such as certain antipsychotic medications10 and omega-3 fatty acids, can be neuroprotective.11 The early use of a long-acting injectable antipsychotic also may help prevent relapse and additional psychotic episodes.5
Psychosis requires expedient and competent intervention to improve outcomes and reduce disease burden. Timely psychiatric treatment can improve not only immediate functioning, but also long-term prognosis. Because untreated psychosis can result in irreversible structural brain damage, clinicians must act swiftly to provide assertive treatment.
1. Where next with psychiatric illness? Nature. 1998;336(6195):95-96.
2. Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521-529.
3. van Haren NE, Hulshoff HE, Shnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
4. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606.
9. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519.
10. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
11. Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934.
1. Where next with psychiatric illness? Nature. 1998;336(6195):95-96.
2. Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521-529.
3. van Haren NE, Hulshoff HE, Shnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
4. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606.
9. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519.
10. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
11. Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934.
Does your patient have the right to refuse medications?
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
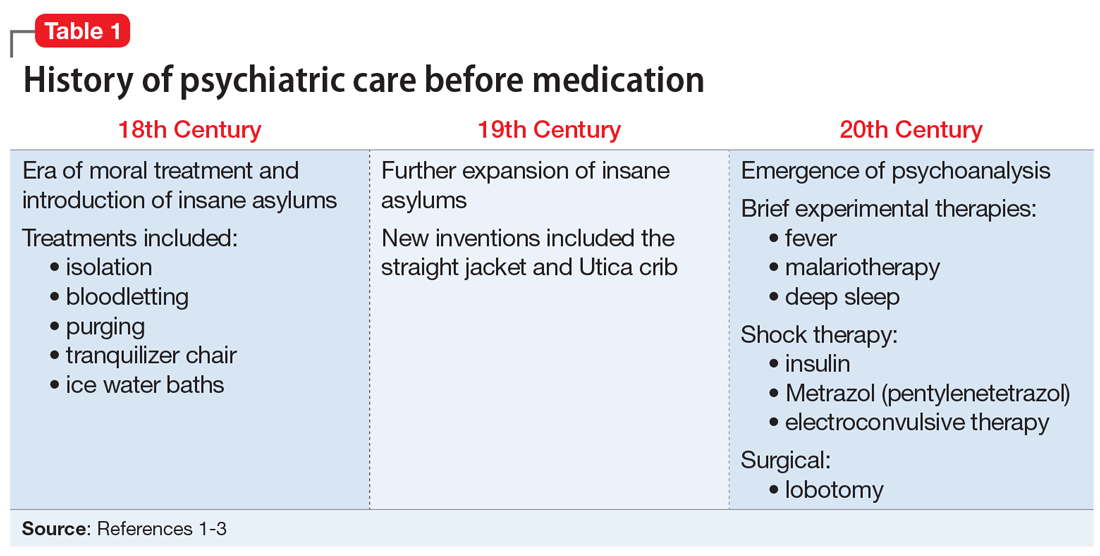
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
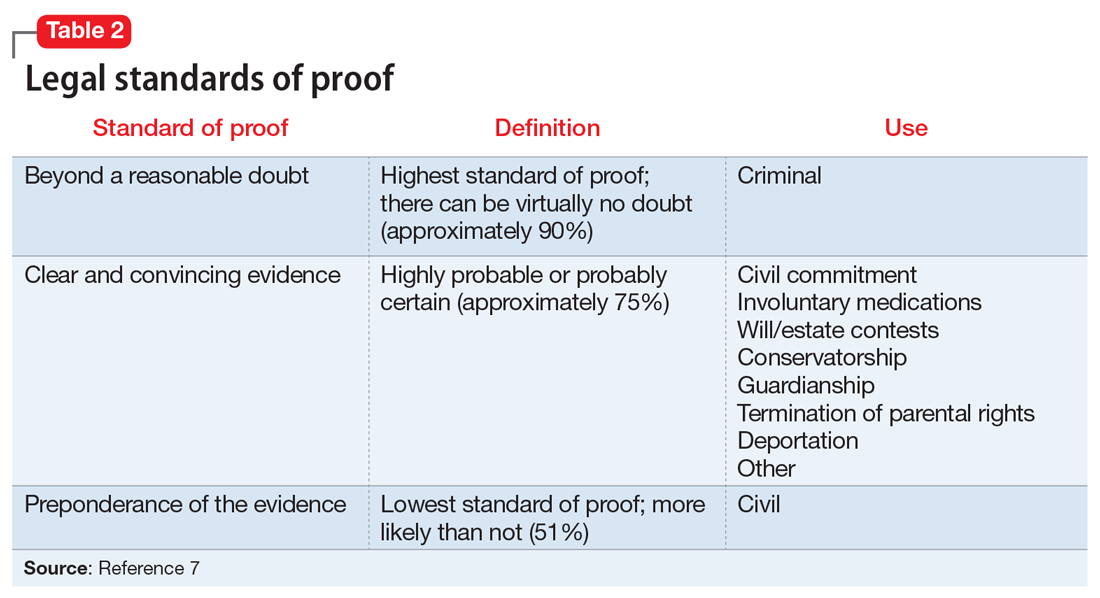
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
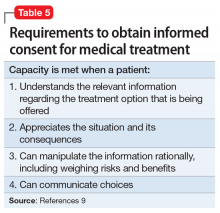
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
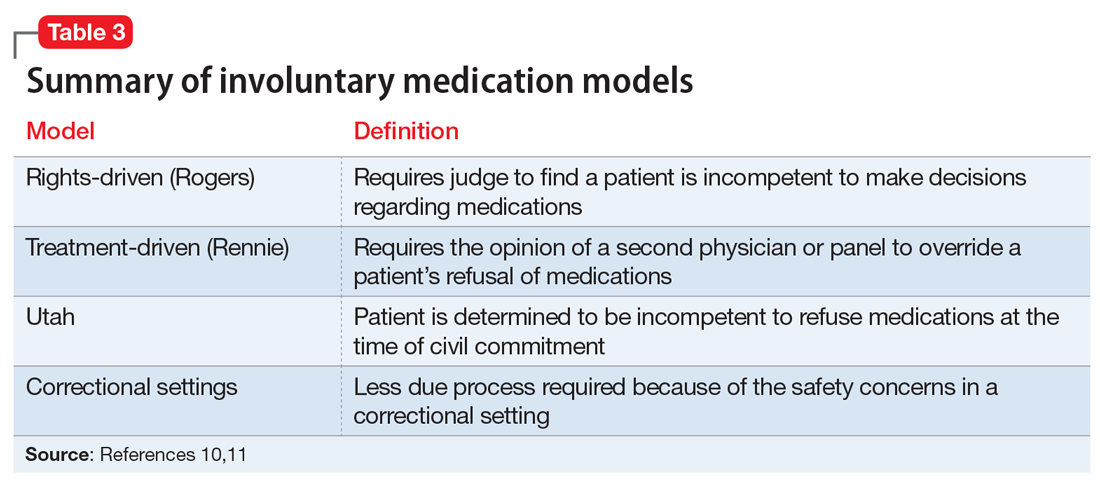
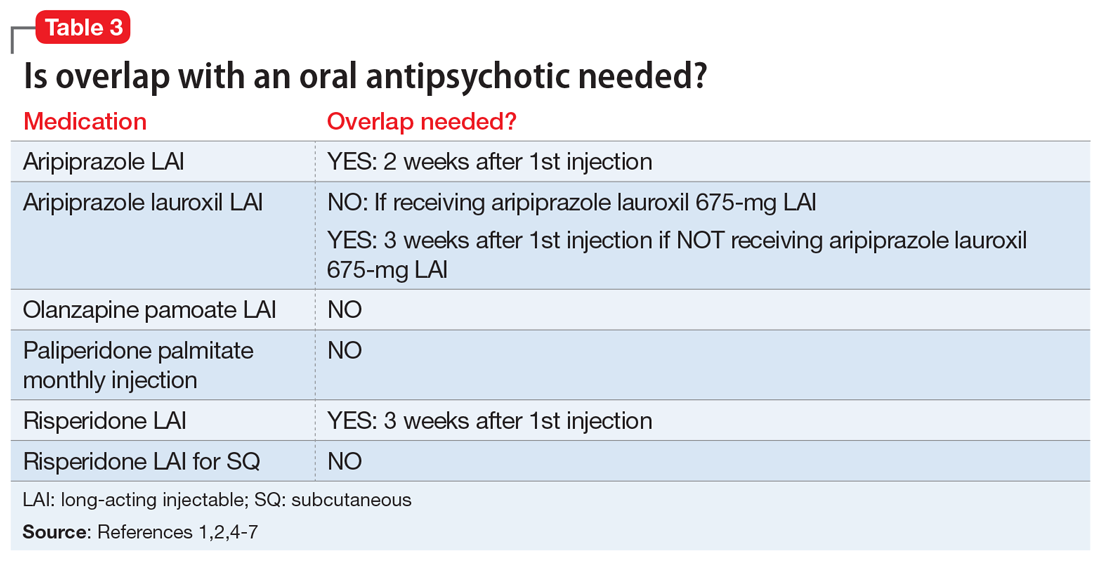
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
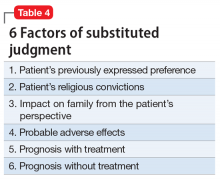
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
Ms. T, age 48, is brought to the psychiatric emergency department after the police find her walking along the highway at 3:00
Once involuntarily committed, does Ms. T have the right to refuse treatment?
Every psychiatrist has faced the predicament of a patient who refuses treatment. This creates an ethical dilemma between respecting the patient’s autonomy vs forcing treatment to ameliorate symptoms and reduce suffering. This article addresses case law related to the models for administering psychiatric medications over objection. We also discuss case law regarding court-appointed guardianship, and treating medical issues without consent. While this article provides valuable information on these scenarios, it is crucial to remember that the legal processes required to administer medications over patient objection are state-specific. In order to ensure the best practice and patient care, you must research the legal procedures specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
History of involuntary treatment
Prior to the 1960s, Ms. T would likely have been unable to refuse treatment. All patients were considered involuntary, and the course of treatment was decided solely by the psychiatric institution. Well into the 20th century, patients with psychiatric illness remained feared and stigmatized, which led to potent and potentially harsh methods of treatment. Some patients experienced extreme isolation, whipping, bloodletting, experimental use of chemicals, and starvation (Table 11-3).
With the advent of psychotropic medications and a focus on civil liberties, the psychiatric mindset began to change from hospital-based treatment to a community-based approach. The value of psychotherapy was recognized, and by the 1960s, the establishment of community mental health centers was gaining momentum.
In the context of these changes, the civil rights movement pressed for stronger legislation regarding autonomy and the quality of treatment available to patients with psychiatric illness. In the 1960s and 1970s, Rouse v Cameron4 and Wyatt v Stickney5 dealt with a patient’s right to receive treatment while involuntarily committed. However, it was not until the 1980s that the courts addressed the issue of a patient’s right to refuse treatment.
The judicial system: A primer
When reviewing case law and its applicability to your patients, it is important to understand the various court systems. The judicial system is divided into state and federal courts, which are subdivided into trial, appellate, and supreme courts. When decisions at either the state or federal level require an ultimate decision maker, the US Supreme Court can choose to hear the case, or grant certiorari, and make a ruling, which is then binding law.6 Decisions made by any court are based on various degrees of stringency, called standards of proof (Table 27).
Continue to: For Ms. T's case...
For Ms. T’s case, civil commitment and involuntary medication hearings are held in probate court, which is a civil (not criminal) court. In addition to overseeing civil commitment and involuntary medications, probate courts adjudicate will and estate contests, conservatorship, and guardianship. Conservatorship hearings deal with financial issues, and guardianship cases encompass personal and health-related needs. Regardless of the court, an individual is guaranteed due process under the 5th Amendment (federal) and 14th Amendment (state).
Individuals are presumed competent to make their own decisions, but a court may call this into question. Competencies are specific to a variety of areas, such as criminal proceedings, medical decision making, writing a will (testimonial capacity), etc. Because each field applies its own standard of competence, an individual may be competent in one area but incompetent in another. Competence in medical decision making varies by state but generally consists of being able to communicate a choice, understand relevant information, appreciate one’s illness and its likely consequences, and rationally manipulate information.8
Box
Administering medications despite a patient’s objection differs from situations in which medications are provided during a psychiatric emergency. In an emergency, courts do not have time to weigh in. Instead, emergency medications (most often given as IM injections) are administered based on the physician’s clinical judgment. The criteria for psychiatric emergencies are delineated at the state level, but typically are defined as when a person with a mental illness creates an imminent risk of harm to self or others. Alternative approaches to resolving the emergency may include verbal de-escalation, quiet time in a room devoid of stimuli, locked seclusion, or physical restraints. These measures are often exhausted before emergency medications are administered.
Source: Reference 9
It is important to note that the legal process required before administering involuntary medications is distinct from situations in which medication needs to be provided during a psychiatric emergency. The Box9 outlines the difference between these 2 scenarios.
4 Legal models
There are several legal models used to determine when a patient can be administered psychiatric medications over objection. Table 310,11 summarizes these models.
Rights-driven (Rogers) model. If Ms. T was involuntarily hospitalized in Massachusetts or another state that adopted the rights-driven model, she would retain the right to refuse treatment. These states require an external judicial review, and court approval is necessary before imposing any therapy. This model was established in Rogers v Commissioner,12 where 7 patients at the Boston State Hospital filed a lawsuit regarding their right to refuse medications. The Massachusetts Supreme Judicial Court ruled that, despite being involuntarily committed, a patient is considered competent to refuse treatment until found specifically incompetent to do so by the court. If a patient is found incompetent, the judge, using a full adversarial hearing, decides what the incompetent patient would have wanted if he/she were competent. The judge reaches a conclusion based on the substituted judgment model (Table 410). In Rogers v Commissioner,12 the court ruled that the right to decision making is not lost after becoming a patient at a mental health facility. The right is lost only if the patient is found incompetent by the judge. Thus, every individual has the right to “manage his own person” and “take care of himself.”
Continue to: An update to the rights-driven (Rogers) model
An update to the rights-driven (Rogers) model. Other states, such as Ohio, have adopted the Rogers model and addressed issues that arose subsequent to the aforementioned case. In Steele v Hamilton County,13 Jeffrey Steele was admitted and later civilly committed to the hospital. After 2 months, an involuntary medication hearing was completed in which 3 psychiatrists concluded that, although Mr. Steele was not a danger to himself or others while in the hospital, he would ultimately benefit from medications.
The probate court acknowledged that Mr. Steele lacked capacity and required hospitalization. However, because he was not imminently dangerous, medication should not be used involuntarily. After a series of appeals, the Ohio Supreme Court ruled that a court may authorize the administration of an antipsychotic medication against a patient’s wishes without a finding of dangerousness when clear and convincing evidence exists that:
- the patient lacks the capacity to give or withhold informed consent regarding treatment
- the proposed medication is in the patient’s best interest
- no less intrusive treatment will be as effective in treating the mental illness.
This ruling set a precedent that dangerousness is not a requirement for involuntary medications.
Treatment-driven (Rennie) model. As in the rights-driven model, in the treatment-driven model, Ms. T would retain the constitutional right to refuse treatment. However, the models differ in the amount of procedural due process required. The treatment-driven model derives from Rennie v Klein,14 in which John Rennie, a patient at Ancora State Psychiatric Hospital in New Jersey, filed a suit regarding the right of involuntarily committed patients to refuse antipsychotic medications. The Third Circuit Court of Appeals ruled that, if professional judgment deems a patient to be a danger to himself or others, then antipsychotics may be administered over individual objection. This professional judgment is typically based on the opinion of the treating physician, along with a second physician or panel.
Utah model. This model is based on A.E. and R.R. v Mitchell,15 in which the Utah District Court ruled that a civilly committed patient has no right to refuse treatment. This Utah model was created after state legislature determined that, in order to civilly commit a patient, hospitalization must be the least restrictive alternative and the patient is incompetent to consent to treatment. Unlike the 2 previous models, competency to refuse medications is not separated from a previous finding of civil commitment, but rather, they occur simultaneously.
Continue to: Rights in unique situations
Rights in unique situations
Correctional settings. If Ms. T was an inmate, would her right to refuse psychiatric medication change? This was addressed in the case of Washington v Harper.16 Walter Harper, serving time for a robbery conviction, filed a claim that his civil rights were being violated when he received involuntary medications based on the decision of a 3-person panel consisting of a psychiatrist, psychologist, and prison official. The US Supreme Court ruled that this process provided sufficient due process to mandate providing psychotropic medications against a patient’s will. This reduction in required procedures is related to the unique nature of the correctional environment and an increased need to maintain safety. This need was felt to outweigh an individual’s right to refuse medication.
Incompetent to stand trial. In Sell v U.S.,17 Charles Sell, a dentist, was charged with fraud and attempted murder. He underwent a competency evaluation and was found incompetent to stand trial because of delusional thinking. Mr. Sell was hospitalized for restorability but refused medications. The hospital held an administrative hearing to proceed with involuntary antipsychotic medications; however, Mr. Sell filed an order with the court to prevent this. Eventually, the US Supreme Court ruled that non-dangerous, incompetent defendants may be involuntarily medicated even if they do not pose a risk to self or others on the basis that it furthers the state’s interest in bringing to trial those charged with serious crimes. However, the following conditions must be met before involuntary medication can be administered:
- an important government issue must be at stake (determined case-by-case)
- a substantial probability must exist that the medication will enable the defendant to become competent without significant adverse effects
- the medication must be medically appropriate and necessary to restore competency, with no less restrictive alternative available.
This case suggests that, before one attempts to forcibly medicate a defendant for the purpose of competency restoration, one should exhaust the same judicial remedies one uses for civil patients first.
Court-appointed guardianship
In the case of Ms. T, what if her father requested to become her guardian? This question was explored in the matter of Guardianship of Richard Roe III.18 Mr. Roe was admitted to the Northampton State Hospital in Massachusetts, where he refused antipsychotic medications. Prior to his release, his father asked to be his guardian. The probate court obliged the request. However, Mr. Roe’s lawyer and guardian ad litem (a neutral temporary guardian often appointed when legal issues are pending) challenged the ruling, arguing the probate court cannot empower the guardian to consent to involuntary medication administration. On appeal, the court ruled:
- the guardianship was justified
- the standard of proof for establishment of a guardianship is preponderance of the evidence (Table 27)
- the guardian must seek from a court a “substituted judgment” to authorize forcible administration of antipsychotic medication.
The decision to establish the court as the final decision maker is based on the view that a patient’s relatives may be biased. Courts should take an objective approach that considers
- patient preference stated during periods of competency
- medication adverse effects
- consequences if treatment is refused
- prognosis with treatment
- religious beliefs
- impact on the patient’s family.
Continue to: This case set the stage for...
This case set the stage for later decisions that placed antipsychotic medications in the same category as electroconvulsive therapy and psychosurgery. This could mean a guardian would need specialized authorization to request antipsychotic treatment but could consent to an appendectomy without legal issue.
Fortunately, now most jurisdictions have remedied this cumbersome solution by requiring a higher standard of proof, clear and convincing evidence (Table 27), to establish guardianship but allowing the guardian more latitude to make decisions for their wards (such as those involving hospital admission or medications) without further court involvement.
Involuntary medical treatment
In order for a patient to consent for medical treatment, he/she must have the capacity to do so (Table 59). How do the courts handle the patient’s right to refuse medical treatment? This was addressed in the case of Georgetown College v Jones.19 Mrs. Jones, a 25-year-old Jehovah’s Witness and mother of a 7-month-old baby, suffered a ruptured ulcer and lost a life-threatening amount of blood. Due to her religious beliefs, Mrs. Jones refused a blood transfusion. The hospital quickly appealed to the court, who ruled the woman was help-seeking by going to the hospital, did not want to die, was in distress, and lacked capacity to make medical decisions. Acting in a parens patriae manner (when the government steps in to make decisions for its citizens who cannot), the court ordered the hospital to administer blood transfusions.
Proxy decision maker. When the situation is less emergent, a proxy decision maker can be appointed by the court. This was addressed in the case of Superintendent of Belchertown v Saikewicz.20 Mr. Saikewicz, a 67-year-old man with intellectual disability, was diagnosed with cancer and given weeks to months to live without treatment. However, treatment was only 50% effective and could potentially cause severe adverse effects. A guardian ad litem was appointed and recommended nontreatment, which the court upheld. The court ruled that the right to accept or reject medical treatment applies to both incompetent and competent persons. With incompetent persons, a “substituted judgment” analysis is used over the “best interest of the patient” doctrine.20 This falls in line with the Guardianship of Richard Roe III ruling,18 in which the court’s substituted judgment standard is enacted in an effort to respect patient autonomy.
Right to die. When does a patient have the right to die and what is the standard of proof? The US Supreme Court case Cruzan v Director21 addressed this. Nancy Cruzan was involved in a car crash, which left her in a persistent vegetative state with no significant cognitive function. She remained this way for 6 years before her parents sought to terminate life support. The hospital refused. The Missouri Supreme Court ruled that a standard of clear and convincing evidence (Table 27) is required to withdraw treatment, and in a 5-to-4 decision, the US Supreme Court upheld Missouri’s decision. This set the national standard for withdrawal of life-sustaining treatment. The moderate standard of proof is based on the court’s ruling that the decision to terminate life is a particularly important one.
Continue to: CASE
CASE CONTINUED
After having been civilly committed to your inpatient psychiatric facility, Ms. T’s paranoia and disorganized behavior persist. She continues to refuse medications.
There are 3 options: respect her decision, negotiate with her, or attempt to force medications through due process.11 In negotiating a compromise, it is best to understand the barriers to treatment. A patient may refuse medications due to poor insight into his/her illness, medication adverse effects, a preference for an alternative treatment, delusional concerns over contamination and/or poisoning, interpersonal conflicts with the treatment staff, a preference for symptoms (eg, mania) over wellness, medication ineffectiveness, length of treatment course, or stigma.22,23 However, a patient’s unwillingness to compromise creates the dilemma of autonomy vs treatment.
For Ms. T, the treatment team felt initiating involuntary medication was the best option for her quality of life and safety. Because she resides in Ohio, a Rogers-like model was applied. The probate court was petitioned and found her incompetent to make medical decisions. The court accepted the physician’s recommendation of treatment with antipsychotic medications. If this scenario took place in New Jersey, a Rennie model would apply, requiring due process through the second opinion of another physician. Lastly, if Ms. T lived in Utah, she would have been unable to refuse medications once civilly committed.
Pros and cons of each model
Over the years, various concerns about each of these models have been raised. Given the slow-moving wheels of justice, one concern was that perhaps patients would be left “rotting with their rights on,” or lingering in a psychotic state out of respect for their civil liberties.19 While court hearings do not always happen quickly, more often than not, a judge will agree with the psychiatrist seeking treatment because the judge likely has little experience with mental illness and will defer to the physician’s expertise. This means the Rogers model may be more likely to produce the desired outcome, just more slowly. With respect to the Rennie model, although it is often more expeditious, the second opinion of an independent psychiatrist may contradict that of the original physician because the consultant will rely on his/her own expertise. Finally, some were concerned that psychiatrists would view the Utah model as carte blanche to start whatever medications they wanted with no respect for patient preference. Based on our clinical experience, none of these concerns have come to fruition over time, and patients safely receive medications over objection in hospitals every day.
Consider why the patient refuses medication
Regardless of which involuntary medication model is employed, it is important to consider the underlying cause for medication refusal, because it may affect future compliance. If the refusal is the result of a religious belief, history of adverse effects, or other rational motive, then it may be reasonable to respect the patient’s autonomy.24 However, if the refusal is secondary to symptoms of mental illness, it is appropriate to move forward with an involuntary medication hearing and treat the underlying condition.
Continue to: In the case of Ms. T...
In the case of Ms. T, she appeared to be refusing medications because of her psychotic symptoms, which could be effectively treated with antipsychotic medications. Therefore, Ms. T’s current lack of capacity is hopefully a transient phenomenon that can be ameliorated by initiating medication. Typically, antipsychotic medications begin to reduce psychotic symptoms within the first week, with further improvement over time.25 The value of the inpatient psychiatric setting is that it allows for daily monitoring of a patient’s response to treatment. As capacity is regained, patient autonomy over medical decisions is reinstated.
Bottom Line
The legal processes required to administer medications over a patient’s objection are state-specific, and multiple models are used. In general, a patient’s right to refuse treatment can be overruled by obtaining adjudication through the courts (Rogers model) or the opinion of a second physician (Rennie model). In order to ensure the best practice and patient care, research the legal procedure specific to your jurisdiction, consult your clinic/hospital attorney, and/or contact your state’s mental health board for further clarification.
Related Resources
- Miller D. Is forced treatment in our outpatients’ best interests? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/80277/forced-treatment-our-outpatients-best-interests.
- Miller D, Hanson A. Committed: The battle over involuntary psychiatric care. Baltimore, MD: Johns Hopkins University Press; 2016.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
1. Laffey P. Psychiatric therapy in Georgian Britain. Psychol Med. 2003;33(7):1285-1297.
2. Porter R. Madness: a brief history. New York, NY: Oxford Press; 2002.
3. Stetka B, Watson J. Odd and outlandish psychiatric treatments throughout history. Medscape Psychiatry. https://www.medscape.com/features/slideshow/odd-psychiatric-treatments. Published April 13, 2016. Accessed February 26, 2020.
4. Rouse v Cameron, 373, F2d 451 (DC Cir 1966).
5. Wyatt v Stickney, 325 F Supp 781 (MD Ala 1971).
6. Administrative Office of the US Courts. Comparing federal and state Courts. United States Courts. https://www.uscourts.gov/about-federal-courts/court-role-and-structure/comparing-federal-state-courts. Accessed February 26, 2020.
7. Drogin E, Williams C. Introduction to the Legal System. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:80-83.
8. Appelbaum P, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
9. Kambam P. Informed consent and competence. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:115-121.
10. Wall B, Anfang S. Legal regulation of psychiatric treatment. In: Gold L, Frierson R, eds. Textbook of forensic psychiatry, 3rd ed. Arlington, VA: American Psychiatric Association Publishing; 2018:306-333.
11. Pinals D, Nesbit A, Hoge S. Treatment refusal in psychiatric practice. In: Rosnar R, Scott C, eds. Principles and practice of forensic psychiatry, 3rd ed. Boca Raton, FL: CRC press; 2017:155-163.
12. Rogers v Commissioner, 390 489 (Mass 1983).
13. Steele v Hamilton County, 90 Ohio St3d 176 (Ohio 2000).
14. Rennie v Klein, 462 F Supp 1131 (D NJ 1978).
15. AE and RR v Mitchell, 724 F.2d 864 (10th Cir 1983).
16. Washington v Harper, 494 US 210 (1990).
17. Sell v US, 539 US 166 (2003).
18. Guardianship of Richard Roe III, 383 415, 435 (Mass 1981).
19. Georgetown College v Jones, 331 F2d 1010 (DC Cir 1964).
20. Superintendent of Belchertown v Saikewicz, 370 NE 2d 417 (1977).
21. Cruzan v Director, 497 US 261 (1990).
22. Owiti J, Bowers L. A literature review: refusal of psychotropic medication in acute inpatient psychiatric care. J Psychiatr Ment Health Nurs. 2011;18(7):637-647.
23. Appelbaum P, Gutheil T. “Rotting with their rights on”: constitutional theory and clinical reality in drug refusal by psychiatric patients. Bull Am Acad Psychiatry Law. 1979;7(3):306-315.
24. Adelugba OO, Mela M, Haq IU. Psychotropic medication refusal: reasons and patients’ perception at a secure forensic psychiatric treatment centre. J Forensic Sci Med. 2016;2(1):12-17.
25. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228.
Second-generation long-acting injectable antipsychotics: A practical guide
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
Kratom: What we know, what to tell your patients
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
Mitragyna speciosa, better known as kratom, is a tropical evergreen tree that is native to Southeast Asia. Botanically, it is a member of the Rubiaceae family, as is the coffee plant, and physical laborers among indigenous populations have historically chewed the leaves or brewed them as a tea to improve endurance and reduce fatigue.1 Kratom is psychoactive; small amounts (up to 5 g of plant material) possess stimulant properties, while larger doses (>5 g) produce opioid-like, sedative, euphoric, and antinociceptive effects.2
In recent years, kratom has gained popularity in Western parts of the world due to its unique properties and perceived safety as a botanical product. Individuals may use kratom to boost their energy, relieve pain, or treat a wide range of physical or mood problems. Increasingly, kratom is being used by people who abuse opioids to self-manage opioid withdrawal, or for its euphoric effects. But kratom carries several important risks, including addiction, serious adverse effects, and possibly death. In this article, we review the epidemiology and pharmacology of kratom, and provide some guidance for educating patients about this substance.
Widely used but not FDA approved
Although kratom is not regulated or approved by the FDA, 3 to 5 million Americans use it regularly.3 According to an internet survey, kratom users are mostly college-educated, employed white men, age 31 to 50, who take the substance to manage pain or to treat general anxiety and mood disorders.4 Some individuals use kratom as an opioid substitute to reduce symptoms of opioid withdrawal.4
Kratom is available from a wide range of manufacturers in various formulations, including powders, tablets, liquids, and gum. It is sometimes sold in combination with other agents as a single product. Low-cost, over-the-counter kratom products are available as “dietary supplements” in retail stores or online. Although the product packaging sometimes recommends a specific dose, the amount of active ingredients (as well as other agents) is unknown. Kratom is illegal in several states (Box5).
Box
The use and sale of kratom is illegal in several countries, including Australia, Poland, Denmark, Sweden, Malaysia, and Vietnam. In the United States, kratom was legal to grow and purchase in all 50 states until 2015, when the Drug Enforcement Administration (DEA) identified kratom as a “substance of concern.” In August 2016, the DEA submitted a notice of intent to place mitragynine and 7-hydroxymitragynine, 2 alkaloids of kratom that have opioid-like properties, into Schedule I of the Controlled Substance Act; however, due to significant public pressure, the DEA withdrew the request in October 2016.
As of February 2020, kratom was illegal to buy, sell, or use in Wisconsin, Rhode Island, Vermont, Indiana, Arkansas, Alabama, specific counties of some states, and the District of Columbia. Legislation was pending in New York, Missouri, and Louisiana.
Source: Reference 5
The 2 alkaloids of interest
More than 40 alkaloids have been isolated from kratom leaves. The proportions of these alkaloids vary significantly depending on the environment in which the plant is grown, the breeding and harvesting techniques, and the age of the plant.6 Two alkaloids of significant interest are mitragynine (Figure 1) and 7-hydroxymitragynine (Figure 2), both of which are unique to M. speciosa and have opioid-like properties. Administering these alkaloids to morphine-dependent rats resulted in cross-tolerance and precipitated withdrawal when the rats were given naloxone.7 The potency of kratom at the mu opioid receptor has been found to exceed that of morphine.
Competitive binding studies that examined the affinity of mitragynine and 7-hydroxymitragynine at the various opioid receptor subtypes found a preference for the kappa receptors (antagonism), followed by mu (partial agonism), and lastly delta. This profile of mitragynine is very similar to that of buprenorphine.8 The affinity of 7-hydroxymitragynine for the mu receptor (agonism) is significantly greater than that of mitragynine.9 Mitragynine also interacts with noradrenergic and serotonergic pathways by stimulating postsynaptic alpha-2 adrenergic receptors and inhibiting 5-HT2A receptors.9 These properties are responsible for kratom’s ability to manage opioid withdrawal symptoms, which are generally attributed to a hyperactive noradrenergic system. There also is evidence that the hepatic metabolite 7-hydroxymitragynine is important in mediating the analgesic component of mitragynine.10
The initial effects of kratom typically begin within 10 to 20 minutes of consumption, and the full effects are experienced in 30 to 60 minutes.1 The half-life of mitragynine in humans has not yet been determined, but is believed to be relatively short.11 In rats, the half-life of mitragynine is 2 to 3 hours.12 Individuals who use kratom to prevent opioid withdrawal have reported taking it as often as every 6 to 12 hours.13
Continue to: Metabolism of mitragynine...
Metabolism of mitragynine is predominantly carried out through cytochrome P450 (CYP) 3A4, with minor contributions by 2D6 and 2C9. A total of 13 metabolites are produced, including 7-hydroxymitragynine.14 Kratom’s constituents also interact with the CYP system, inhibiting 2C9, 2D6, and 3A4 isoenzymes, and to some extent, 1A2.
Adverse effects can be fatal
An animal study revealed that when administered intravenously, mitragynine and 7-hydroxymitragynine have a similar toxicity profile to heroin.15 When these alkaloids were administered in ascending doses, increases in blood pressure and elevations in liver function tests and creatinine levels from baseline were observed.
Chronic kratom use can result in weight loss, insomnia, constipation, dehydration, skin hyperpigmentation, and extreme fatigue.16 There have also been reports of seizures, delusions, hallucinations, respiratory depression, hepatotoxicity, coma, and death.17,18 An emerging concern is the potential development of fatty liver infiltrates leading to cholestatic liver damage.19-25 One case report described a young man who developed a serum aspartate aminotransferase level of 1,300 IU/L (reference range: 5 to 45 IU/L) and a serum alanine aminotransaminase level of 3,700 IU/L (reference range: 5 to 60 IU/L) after he ingested a kratom product.26 Histologically, the pattern of liver injury mimics primary biliary cholangitis.27
In recent years, calls to poison control centers in the United States related to kratom exposure have risen. Between 2011 and 2017, the number of calls increased from 1 a month to 2 each day.28 The US National Poison Data System has also noted an increase in the number of calls in reference to kratom. It received 2,312 calls from January 2011 through July 2018, with 18 calls occurring in 2011, and 357 within the first 7 months of 2018.29
As of February 2018, the FDA had received reports of 44 deaths associated with kratom.30 There have been reports of fatal overdoses involving kratom, particularly when kratom is co-ingested or used with adulterated and/or combination agents, including one case that involved quetiapine.31-33 There have been reports of deaths believed to be attributed to the use of kratom alone; in one such case, a 35-year-old man experienced a fatal cardiac arrest due to kratom use with no other coingestants.34 Among the reports of deaths in which kratom was the only substance consumed, the mitragynine blood levels of the deceased individuals were found to be higher than the levels associated with individuals who had consumed traditional kratom teas.29
Continue to: There is a lack of quality control...
There is a lack of quality control of commercially available kratom preparations. The FDA has found kratom products that exceeded the level of safe exposure to nickel and lead.35 There have also been reports of Salmonella outbreaks associated with kratom products.36
Detecting kratom use
Mitragynine is a lipophilic alkaloid that is poorly soluble in water37 and eliminated primarily in urine.12 Based on data from treatment center admissions, kratom can be detected in urine samples for 5 to 6 days after use.24,38,39 However, kratom is not detectable by a standard urine toxicology screen; therefore, a high degree of suspicion and special confirmatory testing are necessary. The breakdown products of mitragynine can be detected through gas chromatography coupled with mass spectrometry (GC/MS), liquid chromatography with linear ion trap mass spectrometry, or electrospray tandem mass spectrometry.40-42
A familiar withdrawal syndrome
Abrupt discontinuation of high-dose, long-term kratom use can produce withdrawal symptoms.13 Symptoms of kratom withdrawal resemble those of opioid withdrawal. These include physiological symptoms (mydriasis, nausea, sweating and chills, muscle and body aches, tremors and twitches, diarrhea, rhinorrhea, and lacrimation) and psychological symptoms (insomnia, restlessness, irritability/hostility, fatigue, anxiety, mood disturbances, and hallucinations).13 Symptoms are first noted starting 12 hours after the last use of kratom, and can last up to 7 days.43 Withdrawal intensity has been positively correlated with the daily amount of kratom consumed, as well as the duration and frequency of use.13,16
In 2 case reports, the newborns of women who used kratom during pregnancy experienced neonatal abstinence syndrome.44,45 In these 2 reports, symptoms such as jitteriness, irritability, feeding intolerance, and vomiting emerged on postpartum Day 2. The newborns were admitted to a neonatal ICU and started on a standard opioid protocol with IV morphine and subsequently tapered with an oral formulation over 5 days.44,45
Helping patients who use kratom
The best approach to treating a patient who is experiencing kratom withdrawal is symptomatic management, as would be appropriate for a patient experiencing opioid withdrawal.13 However, the use of agents such as methadone or buprenorphine for patients undergoing kratom withdrawal has not been thoroughly evaluated; very few reports have been published.46,47
Continue to: Similarly, while the standard of care...
Similarly, while the standard of care for treating a patient with opioid use disorder is medication-assisted treatment in combination with counseling and behavioral therapies, there is little evidence on the efficacy of such treatments for patients who use kratom. There are no specific guidelines, and the risk of relapsing to kratom use is high.48,49 Nonetheless, some clinicians have used the same protocol for patients with opioid use disorder to treat patients using kratom, and several published case reports describe this approach.50,51 Because administering buprenorphine/naltrexone to a patient who is dependent on kratom can precipitate withdrawal, clinicians should follow a similar initiation protocol as for opioid dependence when starting a patient on these agents (ie, a washout period with a challenge test would be prudent prior to starting naltrexone).
In cases of kratom overdose, naloxone has been shown to reverse the analgesic effects of mitragynine in rats. However, in a case report of an individual who accidently overdosed on a kratom product, naloxone had a modest effect.52
Bottom Line
Kratom is a botanical substance that acts like a stimulant at low doses and an opioid at higher doses. Patients might use it to treat mood-related symptoms, relieve pain, or manage opioid withdrawal. Kratom use has been associated with the development of addiction as well as a multitude of serious adverse effects, including hepatotoxicity and overdose. Long-term management may be required for a patient who uses kratom.
Related Resources
- White CM. Pharmacologic and clinical assessment of kratom: an update. Am J Health Syst Pharm. 2019;76(23):1915-1925.
- Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340-348.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naltrexone • Suboxone
Methadone • Methadose
Naltrexone • Revia
Naloxone • Narcan
Quetiapine • Seroquel
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
1. Henningfield JE, Fant RV, Wang DW. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology (Berl). 2018;235(2):573-589.
2. Chang-Chien GC, Odonkor CA, Amorapanth P, et al. Is kratom the new ‘legal high’ on the block?: the case of an emerging opioid receptor agonist with substance abuse potential. Pain Physician. 2017;20(1):E195-E198.
3. Penders T, Jones WB. Kratom, a substance of increasing concern [PCSS webinar]. Providers Clinical Support System. November 28, 2018. https://pcssnow.org/event/kratom-a-substance-of-increasing-concern. Accessed January 29, 2020.
4. Grundmann O. Patterns of kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 2017;176:63-70.
5. US Drug Enforcement Administration. Drugs of concern. https://www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=84. Updated June 16, 2017. Accessed January 29, 2020.
6. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
7. Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull (Tokyo). 2004;52(8):916-928.
8. Suhaimi FW, Yusoff NH, Hassan R, et al. Neurobiology of kratom and its main alkaloid mitragynine. Brain Res Bull. 2016;126(pt 1):29-40.
9. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
10. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992-1001.
11. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015:9:2421-2429.
12. Warner ML, Kaufman NC, Grundmann O, et al. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Intl J Legal Med. 2016;130(1):127-138.
13. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12-18.
14. Kamble SH, Sharma A, King TI, et al. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica. 2019;49(11):1279-1288.
15. Smith LC, Lin L, Hwang CS, et al. Lateral flow assessment and unanticipated toxicity of kratom. Chem Res Toxicol. 2019;32(1):113-121.
16. Saingam D, Assanangkornchai S, Geater AF, et al. Factor analytical investigation of Krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J Psychoactive Drugs. 2016;48(2):76-85.
17. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283-288.
18. Saingam D, Assanangkornchai S, Geater AF, et al. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351-358.
19. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. doi: 10.1177/2324709619836138.
20. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086-1087.
21. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. doi: 10.1177/2324709619826167.
22. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550-e551.
23. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterology Res. 2018;11(1):79-82.
24. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227-231.
25. Antony A, Lee TP. Herb-induced liver injury with cholestasis and renal injury secondary to short-term use of kratom (Mitragyna speciosa). Am J Ther. 2019;26(4):e546-e547.
26. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28-30.
27. Aldyab M, Ells PF, Bui R, et al. Kratom-induced cholestatic liver injury mimicking anti-mitochondrial antibody-negative primary biliary cholangitis: a case report and review of literature. Gastroenterology Res. 2019;12(4):211-215.
28. Post S, Spiller HA, Chounthirath T. Kratom exposures reported to United States poison control centers: 2011-2017. Clinical Toxicol (Phila). 2019;57(10):847-854.
29. Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacotherapy. 2019;39(7):775-777.
30. US Food & Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom , underscoring its potential for abuse. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-scientific-evidence-presence-opioid-compounds. Published February 6, 2019. Accessed January 29, 2020.
31. Gershman K, Timm K, Frank M, et al. Deaths in Colorado attributed to kratom. N Engl J Med. 2019;380(1):97-98.
32. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242-247.
33. Hughes RL. Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol. 2019;15(1):110-113.
34. Abdullah HMA, Haq I, Lamfers R. Cardiac arrest in a young healthy male patient secondary to kratom ingestion: is this ‘legal high’ substance more dangerous than initially thought? BMJ Case Rep. 2019;12(7):pii: e229778. doi: 10.1136/bcr-2019-229778.
35. Laboratory analysis of kratom products for heavy metals. US FDA. https://www.fda.gov/news-events/public-health-focus/laboratory-analysis-kratom-products-heavy-metals. Updated April 3, 2019. Accessed January 29, 2020.
36. FDA investigated multistate outbreak of salmonella infections linked to products reported to contain kratom. US FDA. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. Updated June 29, 2018. Accessed January 14, 2020.
37. Aggarwal G, Robertson E, McKinlay J, et a., Death from kratom toxicity and the possible role of intralipid. J Intensive Care Soc. 2018;19(1):61-63.
38. Drug Facts. Kratom. Confirm Biosciences. https://www.confirmbiosciences.com/knowledge/drug-facts/kratom/. Accessed January 14, 2020.
39. Grinspoon P. How long does kratom stay in the system? Addiction Resource. https://addictionresource.com/drugs/kratom/how-long-kratom-stay-in-your-system/. Updated December 18, 2019. Accessed January 29, 2020.
40. Kaewklum D, Kaewklum M, Pootrakronchai R, et al. Detection of mitragynine and its metaboilite in urine following ingestion of leaves of Mitragyna speciosa korth. Recent Advances in Doping Analysis (13). Proceedings of the Manfred Donike Workshop, 23rd Cologne Workshop on Dope Analysis. 2005:403-406.
41. Lu S, Tran BN, Nelsen JL, et al. Quantitative analysis of mitragynine in human urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(24):2499-2505.
42. Philipp AA, Wissenbach DK, Zoerntlein SW, et al. Studies on the metabolism of mitragynine, the main alkaloid of the herbal drug kratom, in rat and human urine using liquid chromatography-linear ion trap mass spectrometry. J Mass Spectrom. 2009;44(8):1249-1261.
43. Manda VK, Bharathi A, Ali Z, et al. Evaluation of in vitro absorption, distribution, metabolism, and excretion (ADME) properties of mitragynine, 7-hydroxymitragynine, and mitraphylline. Planta Med. 2014;80(7):568-576.
44. Davidson L, Rawat M, Stojanovski S, et al. Natural drugs, not so natural effects: neonatal abstinence syndrome secondary to ‘kratom‘. J Neonatal Perinatal Med. 2019;12(1):109-112.
45. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121-122.
46. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
47. Galbis-Reig David. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49-52; quiz 53.
48. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciose) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132-137.
49. Singh D, Müller CP, Vicknasingam, et al. Social functioning of kratom (Mitragyna speciosa) users in Malaysia. J Psychoactive Drugs. 2015;47(2):125-131.
50. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493-495.
51. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481-483.
52. Overbeek DL, Abraham J, Munzer BW. Kratom (mitragynine) ingestion requiring naloxone reversal. Clin Pract Cases Emerg Med. 2019;3(1):24-26.
Meeting the mental health needs of American Muslim patients
Nearly 3.5 million adherents to the religion of Islam (Muslims) live in the United States, and this number is projected to more than double to 8.1 million by 2050.1 As of 2017, an estimated 58% of American Muslims were immigrants, but nearly 25% were born in the United States to parents who also were US-born, including many African American Muslims.1 American Muslims face a rise of Islamophobia, hate crimes, and discrimination at school, work, and the communities in which they live.2 This population may experience feelings of anger as well as rejection and abandonment by the country they call home. They may also wrestle with conforming to American social norms while maintaining their Muslim identity.
When providing psychiatric care to American Muslim patients, clinicians need to understand these patients’ specific stressors. American Muslim patients may experience a variety of mental health conditions, including posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, panic attacks, adjustment disorder, and somatization.2 Unfortunately, American Muslims may be less likely to seek psychiatric help because the stigma of mental illness remains a barrier to care in this community.3 In addition, entrenched cultural beliefs about mental illness may discourage many from seeking treatment. Because of a paucity of data on the psychiatric care of American Muslim patients, there is a great need to understand how to treat this vulnerable, often marginalized population.
Suggestions for improving care
Based on my clinical experience, I recommend the following practices for clinicians to consider when caring for American Muslim patients:
- Ensure that your assessment accounts for religious and cultural factors to help understand your patient’s perception of his/her illness.4 Consider using the DSM-IV Outline for Culture Formulation,5 DSM-5 Cultural Formulation Interview,6 or the American Academy of Child and Adolescent Psychiatry Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice.7
- Be sensitive to your patient’s family hierarchy and history. Often, extended family members will accompany an American Muslim patient for input and support during a clinical visit.
- Provide appropriate psychoeducation, because some patients may shun medication, especially those who think that medication should be reserved for severe illness that requires long-term inpatient stays.
- Listen for somatic symptoms that may mask distress.8
- Be mindful of your patient’s preferences regarding gender roles. For example, a female patient may prefer to receive care from a female clinician.
- Align therapy with your patient’s religious and cultural beliefs. Some research shows that for Muslim patients, modified short-term psychodynamic therapy fares better than classic long-term psychoanalysis.9 Therapy should focus on family dynamics, conflicts, and relationships.9
- Consider employing cognitive-behavioral therapy, solution-focused therapy, modeling, and behavioral techniques such as behavioral modification, systemic desensitization, and flooding because these may be effective for Muslim patients.9,10
- Suggest guided imagery and relaxation techniques because some Muslim patients may find that these could be a natural extension of prayer and meditation.9
- Work with local Imams (Muslim spiritual leaders) to recognize mental illness, overcome stigma in the community, dispel misinformation, and refer patients.11
- If the patient has a “traditional healer,” such as a Sheikh or Imam, understand the extent of this individual’s involvement. Some healers may tell patients that mental illness is a problem of faith or being possessed by a spirit (ie, “jinn” or Satan-like spirits).
- Use an interpreter if English is not the patient’s primary language.
- Respect the patient’s religious practices. When possible, offer alternative treatments to medication formulations that include pork (most gelatins). Also, patients may want to adjust their medication schedule during Ramadan, when many Muslims fast from dawn to dusk.
Future goals: Increasing access, reducing stigma
More research on the mental health needs of American Muslim patients is needed, especially as discrimination and hate crimes against this population continue to rise. Clinicians should tailor their assessments and recommended treatments to these patients’ preferences, and address how religious and cultural connectedness may impact their patient’s mental health. Increasing access to services and reducing stigma associated with mental health care are critical for improving outcomes among Muslim patients.
1. Pew Research Center. Demographic portrait of Muslim Americans. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans. Published November 9, 2017. Accessed January 15 , 2019.
2. Moffic HS, Peteet J, Hankir AZ, eds. Islamophobia and psychiatry: recognition, prevention, and treatment. Cham, Switzerland: Springer; 2019:171-181,335-345.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. Journal of Muslim Mental Health. 2013;7(1). doi: 10.3998/jmmh.10381607.0007.102.
4. Ahmed SR, Amer MM, Killawi A. The ecosystems perspective in social work: Implications for culturally competent practice with American Muslims. J Relig Spiritual Soc Work. 2017;36(1-2):48-72.
5. Outline for Cultural Formulation. In: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:845-846.
6. Lewis-Fernández R, Aggarwal NK, Hinton L, et al, eds. DSM-5 handbook on the Cultural Formulation Interview. Washington, DC: American Psychiatric Association Publishing; 2016.
7. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
8. Basit A, Hamid M. Mental health issues of American Muslims. J IMA. 2010;42(3):106-110.
9. Amer MM, Jalal B. Individual psychotherapy/counseling. In: Ahmed S, Amer MM, eds. Counseling Muslims: handbook of mental health issues and interventions. New York, NY: Routledge; 2013:87-117.
10. Chaudhry S, Li C. Is solution-focused brief therapy culturally appropriate for Muslim American counselees? J Contemp Psychotherapy. 2011;41(2):109-113.
11. Ali OM. The Imam and the mental health of Muslims: learning from research with other clergy. Journal of Muslim Mental Health. 2016;10(1). doi: 10.3998/jmmh.10381607.0010.106.
Nearly 3.5 million adherents to the religion of Islam (Muslims) live in the United States, and this number is projected to more than double to 8.1 million by 2050.1 As of 2017, an estimated 58% of American Muslims were immigrants, but nearly 25% were born in the United States to parents who also were US-born, including many African American Muslims.1 American Muslims face a rise of Islamophobia, hate crimes, and discrimination at school, work, and the communities in which they live.2 This population may experience feelings of anger as well as rejection and abandonment by the country they call home. They may also wrestle with conforming to American social norms while maintaining their Muslim identity.
When providing psychiatric care to American Muslim patients, clinicians need to understand these patients’ specific stressors. American Muslim patients may experience a variety of mental health conditions, including posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, panic attacks, adjustment disorder, and somatization.2 Unfortunately, American Muslims may be less likely to seek psychiatric help because the stigma of mental illness remains a barrier to care in this community.3 In addition, entrenched cultural beliefs about mental illness may discourage many from seeking treatment. Because of a paucity of data on the psychiatric care of American Muslim patients, there is a great need to understand how to treat this vulnerable, often marginalized population.
Suggestions for improving care
Based on my clinical experience, I recommend the following practices for clinicians to consider when caring for American Muslim patients:
- Ensure that your assessment accounts for religious and cultural factors to help understand your patient’s perception of his/her illness.4 Consider using the DSM-IV Outline for Culture Formulation,5 DSM-5 Cultural Formulation Interview,6 or the American Academy of Child and Adolescent Psychiatry Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice.7
- Be sensitive to your patient’s family hierarchy and history. Often, extended family members will accompany an American Muslim patient for input and support during a clinical visit.
- Provide appropriate psychoeducation, because some patients may shun medication, especially those who think that medication should be reserved for severe illness that requires long-term inpatient stays.
- Listen for somatic symptoms that may mask distress.8
- Be mindful of your patient’s preferences regarding gender roles. For example, a female patient may prefer to receive care from a female clinician.
- Align therapy with your patient’s religious and cultural beliefs. Some research shows that for Muslim patients, modified short-term psychodynamic therapy fares better than classic long-term psychoanalysis.9 Therapy should focus on family dynamics, conflicts, and relationships.9
- Consider employing cognitive-behavioral therapy, solution-focused therapy, modeling, and behavioral techniques such as behavioral modification, systemic desensitization, and flooding because these may be effective for Muslim patients.9,10
- Suggest guided imagery and relaxation techniques because some Muslim patients may find that these could be a natural extension of prayer and meditation.9
- Work with local Imams (Muslim spiritual leaders) to recognize mental illness, overcome stigma in the community, dispel misinformation, and refer patients.11
- If the patient has a “traditional healer,” such as a Sheikh or Imam, understand the extent of this individual’s involvement. Some healers may tell patients that mental illness is a problem of faith or being possessed by a spirit (ie, “jinn” or Satan-like spirits).
- Use an interpreter if English is not the patient’s primary language.
- Respect the patient’s religious practices. When possible, offer alternative treatments to medication formulations that include pork (most gelatins). Also, patients may want to adjust their medication schedule during Ramadan, when many Muslims fast from dawn to dusk.
Future goals: Increasing access, reducing stigma
More research on the mental health needs of American Muslim patients is needed, especially as discrimination and hate crimes against this population continue to rise. Clinicians should tailor their assessments and recommended treatments to these patients’ preferences, and address how religious and cultural connectedness may impact their patient’s mental health. Increasing access to services and reducing stigma associated with mental health care are critical for improving outcomes among Muslim patients.
Nearly 3.5 million adherents to the religion of Islam (Muslims) live in the United States, and this number is projected to more than double to 8.1 million by 2050.1 As of 2017, an estimated 58% of American Muslims were immigrants, but nearly 25% were born in the United States to parents who also were US-born, including many African American Muslims.1 American Muslims face a rise of Islamophobia, hate crimes, and discrimination at school, work, and the communities in which they live.2 This population may experience feelings of anger as well as rejection and abandonment by the country they call home. They may also wrestle with conforming to American social norms while maintaining their Muslim identity.
When providing psychiatric care to American Muslim patients, clinicians need to understand these patients’ specific stressors. American Muslim patients may experience a variety of mental health conditions, including posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, panic attacks, adjustment disorder, and somatization.2 Unfortunately, American Muslims may be less likely to seek psychiatric help because the stigma of mental illness remains a barrier to care in this community.3 In addition, entrenched cultural beliefs about mental illness may discourage many from seeking treatment. Because of a paucity of data on the psychiatric care of American Muslim patients, there is a great need to understand how to treat this vulnerable, often marginalized population.
Suggestions for improving care
Based on my clinical experience, I recommend the following practices for clinicians to consider when caring for American Muslim patients:
- Ensure that your assessment accounts for religious and cultural factors to help understand your patient’s perception of his/her illness.4 Consider using the DSM-IV Outline for Culture Formulation,5 DSM-5 Cultural Formulation Interview,6 or the American Academy of Child and Adolescent Psychiatry Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice.7
- Be sensitive to your patient’s family hierarchy and history. Often, extended family members will accompany an American Muslim patient for input and support during a clinical visit.
- Provide appropriate psychoeducation, because some patients may shun medication, especially those who think that medication should be reserved for severe illness that requires long-term inpatient stays.
- Listen for somatic symptoms that may mask distress.8
- Be mindful of your patient’s preferences regarding gender roles. For example, a female patient may prefer to receive care from a female clinician.
- Align therapy with your patient’s religious and cultural beliefs. Some research shows that for Muslim patients, modified short-term psychodynamic therapy fares better than classic long-term psychoanalysis.9 Therapy should focus on family dynamics, conflicts, and relationships.9
- Consider employing cognitive-behavioral therapy, solution-focused therapy, modeling, and behavioral techniques such as behavioral modification, systemic desensitization, and flooding because these may be effective for Muslim patients.9,10
- Suggest guided imagery and relaxation techniques because some Muslim patients may find that these could be a natural extension of prayer and meditation.9
- Work with local Imams (Muslim spiritual leaders) to recognize mental illness, overcome stigma in the community, dispel misinformation, and refer patients.11
- If the patient has a “traditional healer,” such as a Sheikh or Imam, understand the extent of this individual’s involvement. Some healers may tell patients that mental illness is a problem of faith or being possessed by a spirit (ie, “jinn” or Satan-like spirits).
- Use an interpreter if English is not the patient’s primary language.
- Respect the patient’s religious practices. When possible, offer alternative treatments to medication formulations that include pork (most gelatins). Also, patients may want to adjust their medication schedule during Ramadan, when many Muslims fast from dawn to dusk.
Future goals: Increasing access, reducing stigma
More research on the mental health needs of American Muslim patients is needed, especially as discrimination and hate crimes against this population continue to rise. Clinicians should tailor their assessments and recommended treatments to these patients’ preferences, and address how religious and cultural connectedness may impact their patient’s mental health. Increasing access to services and reducing stigma associated with mental health care are critical for improving outcomes among Muslim patients.
1. Pew Research Center. Demographic portrait of Muslim Americans. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans. Published November 9, 2017. Accessed January 15 , 2019.
2. Moffic HS, Peteet J, Hankir AZ, eds. Islamophobia and psychiatry: recognition, prevention, and treatment. Cham, Switzerland: Springer; 2019:171-181,335-345.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. Journal of Muslim Mental Health. 2013;7(1). doi: 10.3998/jmmh.10381607.0007.102.
4. Ahmed SR, Amer MM, Killawi A. The ecosystems perspective in social work: Implications for culturally competent practice with American Muslims. J Relig Spiritual Soc Work. 2017;36(1-2):48-72.
5. Outline for Cultural Formulation. In: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:845-846.
6. Lewis-Fernández R, Aggarwal NK, Hinton L, et al, eds. DSM-5 handbook on the Cultural Formulation Interview. Washington, DC: American Psychiatric Association Publishing; 2016.
7. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
8. Basit A, Hamid M. Mental health issues of American Muslims. J IMA. 2010;42(3):106-110.
9. Amer MM, Jalal B. Individual psychotherapy/counseling. In: Ahmed S, Amer MM, eds. Counseling Muslims: handbook of mental health issues and interventions. New York, NY: Routledge; 2013:87-117.
10. Chaudhry S, Li C. Is solution-focused brief therapy culturally appropriate for Muslim American counselees? J Contemp Psychotherapy. 2011;41(2):109-113.
11. Ali OM. The Imam and the mental health of Muslims: learning from research with other clergy. Journal of Muslim Mental Health. 2016;10(1). doi: 10.3998/jmmh.10381607.0010.106.
1. Pew Research Center. Demographic portrait of Muslim Americans. https://www.pewforum.org/2017/07/26/demographic-portrait-of-muslim-americans. Published November 9, 2017. Accessed January 15 , 2019.
2. Moffic HS, Peteet J, Hankir AZ, eds. Islamophobia and psychiatry: recognition, prevention, and treatment. Cham, Switzerland: Springer; 2019:171-181,335-345.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. Journal of Muslim Mental Health. 2013;7(1). doi: 10.3998/jmmh.10381607.0007.102.
4. Ahmed SR, Amer MM, Killawi A. The ecosystems perspective in social work: Implications for culturally competent practice with American Muslims. J Relig Spiritual Soc Work. 2017;36(1-2):48-72.
5. Outline for Cultural Formulation. In: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:845-846.
6. Lewis-Fernández R, Aggarwal NK, Hinton L, et al, eds. DSM-5 handbook on the Cultural Formulation Interview. Washington, DC: American Psychiatric Association Publishing; 2016.
7. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for Cultural Competence in Child and Adolescent Psychiatric Practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
8. Basit A, Hamid M. Mental health issues of American Muslims. J IMA. 2010;42(3):106-110.
9. Amer MM, Jalal B. Individual psychotherapy/counseling. In: Ahmed S, Amer MM, eds. Counseling Muslims: handbook of mental health issues and interventions. New York, NY: Routledge; 2013:87-117.
10. Chaudhry S, Li C. Is solution-focused brief therapy culturally appropriate for Muslim American counselees? J Contemp Psychotherapy. 2011;41(2):109-113.
11. Ali OM. The Imam and the mental health of Muslims: learning from research with other clergy. Journal of Muslim Mental Health. 2016;10(1). doi: 10.3998/jmmh.10381607.0010.106.
Interacting with colleagues on social media: Tips for avoiding trouble
As clinicians, we increasingly communicate with each other via social media platforms to network, collaborate on research, find professional and personal support, refer patients, or hold academic conversations.1,2 Although it is convenient, this form of online communication directly and indirectly breaks down barriers between our professional and personal lives, which can make it challenging to avoid behavior that could negatively affect one’s professional image.2
Although some medical organizations have offered guidelines on health care professionals’ use of social media,2,3 these are subjective and not evidence-based. Here I offer some suggestions for appropriately interacting with your colleagues (ie, individuals who are not your personal friends) on social media.
Think before you post. Consider the potential professional ramifications of what you are about to post, and don’t post anything that may have adverse consequences on your image or that of psychiatry as a whole.
Don’t post derogatory or defamatory statements about colleagues. Such actions may violate professional codes of conduct. Disagreeing with colleagues on social media is common, but your posts should be respectful, friendly, and reflect positively on the profession.2 Avoid making negative statements—even in jest—about your colleagues’ skills or professional experience, because such communication is not appropriate for public dissemination.2
If you notice colleagues posting unprofessional content that could negatively affect their careers or the public’s trust in psychiatry, tactfully express your concerns to them, and suggest that they take appropriate measures to rectify the situation.2 Be aware of your state’s laws and regulations about mandated reporting if you discover a colleague’s online content violates the scope of clinical practice or ethical standards.1 If the content is in violation of the law or medical board regulations, you may have a legal obligation to report that colleague to law enforcement, the licensing board, and/or his/her employer.1,2
Avoid online snooping into the personal lives of colleagues. Respect their privacy when viewing their posts about personal activities that are not germane to their professional services.1 If you find videos, images, or messages that reveal private, confidential, or sensitive information about a colleague, do not distribute that information without the colleague’s consent.1
Be careful when accepting friend requests. Conflicts could arise if you accept friend requests from some but not all of your colleagues; this could be interpreted as favoritism and potentially create problematic work relationships.2 Be consistent in accepting or rejecting colleagues’ friend requests. Consider using an employment-oriented social media platform to connect with colleagues outside of the workplace.2
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
3. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499,520.
As clinicians, we increasingly communicate with each other via social media platforms to network, collaborate on research, find professional and personal support, refer patients, or hold academic conversations.1,2 Although it is convenient, this form of online communication directly and indirectly breaks down barriers between our professional and personal lives, which can make it challenging to avoid behavior that could negatively affect one’s professional image.2
Although some medical organizations have offered guidelines on health care professionals’ use of social media,2,3 these are subjective and not evidence-based. Here I offer some suggestions for appropriately interacting with your colleagues (ie, individuals who are not your personal friends) on social media.
Think before you post. Consider the potential professional ramifications of what you are about to post, and don’t post anything that may have adverse consequences on your image or that of psychiatry as a whole.
Don’t post derogatory or defamatory statements about colleagues. Such actions may violate professional codes of conduct. Disagreeing with colleagues on social media is common, but your posts should be respectful, friendly, and reflect positively on the profession.2 Avoid making negative statements—even in jest—about your colleagues’ skills or professional experience, because such communication is not appropriate for public dissemination.2
If you notice colleagues posting unprofessional content that could negatively affect their careers or the public’s trust in psychiatry, tactfully express your concerns to them, and suggest that they take appropriate measures to rectify the situation.2 Be aware of your state’s laws and regulations about mandated reporting if you discover a colleague’s online content violates the scope of clinical practice or ethical standards.1 If the content is in violation of the law or medical board regulations, you may have a legal obligation to report that colleague to law enforcement, the licensing board, and/or his/her employer.1,2
Avoid online snooping into the personal lives of colleagues. Respect their privacy when viewing their posts about personal activities that are not germane to their professional services.1 If you find videos, images, or messages that reveal private, confidential, or sensitive information about a colleague, do not distribute that information without the colleague’s consent.1
Be careful when accepting friend requests. Conflicts could arise if you accept friend requests from some but not all of your colleagues; this could be interpreted as favoritism and potentially create problematic work relationships.2 Be consistent in accepting or rejecting colleagues’ friend requests. Consider using an employment-oriented social media platform to connect with colleagues outside of the workplace.2
As clinicians, we increasingly communicate with each other via social media platforms to network, collaborate on research, find professional and personal support, refer patients, or hold academic conversations.1,2 Although it is convenient, this form of online communication directly and indirectly breaks down barriers between our professional and personal lives, which can make it challenging to avoid behavior that could negatively affect one’s professional image.2
Although some medical organizations have offered guidelines on health care professionals’ use of social media,2,3 these are subjective and not evidence-based. Here I offer some suggestions for appropriately interacting with your colleagues (ie, individuals who are not your personal friends) on social media.
Think before you post. Consider the potential professional ramifications of what you are about to post, and don’t post anything that may have adverse consequences on your image or that of psychiatry as a whole.
Don’t post derogatory or defamatory statements about colleagues. Such actions may violate professional codes of conduct. Disagreeing with colleagues on social media is common, but your posts should be respectful, friendly, and reflect positively on the profession.2 Avoid making negative statements—even in jest—about your colleagues’ skills or professional experience, because such communication is not appropriate for public dissemination.2
If you notice colleagues posting unprofessional content that could negatively affect their careers or the public’s trust in psychiatry, tactfully express your concerns to them, and suggest that they take appropriate measures to rectify the situation.2 Be aware of your state’s laws and regulations about mandated reporting if you discover a colleague’s online content violates the scope of clinical practice or ethical standards.1 If the content is in violation of the law or medical board regulations, you may have a legal obligation to report that colleague to law enforcement, the licensing board, and/or his/her employer.1,2
Avoid online snooping into the personal lives of colleagues. Respect their privacy when viewing their posts about personal activities that are not germane to their professional services.1 If you find videos, images, or messages that reveal private, confidential, or sensitive information about a colleague, do not distribute that information without the colleague’s consent.1
Be careful when accepting friend requests. Conflicts could arise if you accept friend requests from some but not all of your colleagues; this could be interpreted as favoritism and potentially create problematic work relationships.2 Be consistent in accepting or rejecting colleagues’ friend requests. Consider using an employment-oriented social media platform to connect with colleagues outside of the workplace.2
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
3. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499,520.
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
3. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499,520.
Hospital Care of Opioid-Exposed Newborns: Clinical and Psychosocial Challenges
In the past two decades, the incidence rate of opioid use disorder (OUD) among pregnant women has increased by more than 400%, constituting a United States public health crisis.1 Newborns exposed to intrauterine opioids are at risk for the postnatal withdrawal syndrome known as neonatal abstinence syndrome (NAS), which requires increased hospital resources, such as neonatal intensive care unit (NICU) admission and prolonged length of stay.2 Given the medical and psychosocial challenges associated with maternal OUD and NAS, a multidisciplinary, patient-centered approach to hospital care for affected newborns and their mothers is warranted. A large and growing body of research has focused on the epidemiology of NAS and approaches for its prevention, screening, and management. This review appraises updates to the literature within the past five years, with an emphasis on considerations for newborn discharge to promote optimal care for this population.
DEFINITION
NAS is a complex disorder arising from the abrupt cessation of placental transfer of opioids after birth, although other maternal substances, including benzodiazepines and other antidepressants, have been less commonly implicated.2 The term neonatal opioid withdrawal syndrome is sometimes used to indicate withdrawal from opioids specifically.3 The central and autonomic nervous systems and the gastrointestinal system (eg, tremors, increased muscle tone, high-pitched crying, feeding difficulties) are affected in NAS, with most newborns demonstrating symptoms within the first few days of life.4 Previously reported factors associated with NAS include opioid type, timing of exposure during pregnancy, maternal tobacco use, infant sex, and gestational age.5 Literature demonstrates that concurrent exposure to other prenatal substances, particularly antidepressants, benzodiazepines, and gabapentin, is significantly associated with increased risk of NAS.6 Recent studies also suggest that expression of NAS may relate to newborn genetic variations, particularly at the OPRM1, COMT, and CYP2B6 gene sites.7, 8
State health departments have increasingly deemed NAS as a reportable diagnosis for public health surveillance, which relies on the accurate diagnosis and documentation of NAS during birth hospitalization.9 The diagnosis codes for NAS include the International Classification of Diseases, Ninth Revision, Clinical Modification code (ICD-9-CM) 779.5 and the International Classification of Diseases, Tenth Revision, Clinical Modification ICD-10-CM code P96.1.10 However, given the variation in the presentation and severity of NAS, no consensus has been established with regard to a standardized case definition for reporting across hospitals and states.9 In fact, NAS should be conceptualized as a continuum of withdrawal symptoms along which every infant with intrauterine opioid exposure resides; this continuum ranges from minor findings, which do not affect the infant’s ability to grow and develop, to severe withdrawal, resulting in excessive weight loss, dehydration, or seizures.3,11 Ultimately, the diagnosis of NAS is made clinically based on cardinal symptoms in the setting of known or highly suspected opioid exposure. In a recent study of Tennessee Medicaid claims data, >25% of infants with a confirmed diagnosis code for NAS did not receive pharmacotherapy.10 Pharmacologic treatment of NAS, therefore, may be more appropriately considered as a marker of disease severity, rather than a requirement for diagnosis.
EPIDEMIOLOGY
Although the opioid crisis and resulting rise in NAS have affected communities across the US, substantial statewide variation exists, with extremes ranging from 0.7 per 1,000 births affected by NAS in Hawaii to 33.4 per 1,000 births in West Virginia.12 Within states, increased maternal OUD and NAS rates have also disproportionately affected rural communities possibly due to reduced access to healthcare and mental health services and poor economic conditions.13 A recent national study demonstrated that the proportion of newborns with NAS who were born in rural hospitals increased from 12.9% to 21.2% over the past decade; these rural newborns with NAS are more likely to be publicly insured and to require transfer after birth than newborns in urban hospitals.14 These data suggest a particular need among rural communities for increased resources targeting NAS care as well as maternal OUD prevention and treatment.
RISK IDENTIFICATION
The American College of Obstetricians and Gynecologists (ACOG) recommends early universal screening for maternal substance use at the first prenatal visit with a validated screening tool; examples include the 4Ps (parents, partners, past, and pregnancy), CRAFFT (car, relax, alone, forget, friends, trouble), and the National Institute on Drug Abuse quick screen, which have all been well studied and have a high sensitivity for detecting substance use and misuse.15
Toxicology Screening
Toxicology testing for both mother and newborn is helpful in identifying or confirming intrauterine exposures, particularly in cases of polysubstance use or when a newborn manifests signs of NAS but whose mother denies opioid use. All toxicology testing should be performed with the mother’s consent, and any potential legal or mandatory ramifications of a positive test should be considered. Although universal maternal toxicology testing improves the identification of newborns at risk for NAS, this approach remains controversial; most hospitals use a risk-based approach for maternal toxicology testing.16,17
Newborn toxicology testing can be performed from samples of hair, urine, meconium, and umbilical cord. Although frequently used, newborn urine testing has the shortest window of detection, ie, the last few days prior to delivery (Table 1). Meconium drug testing has been considered the gold standard and can detect exposures from the last 20 weeks of gestation, providing information on chronic exposures.18 In a recent survey, 10% of hospitals reported using umbilical cord toxicology as the primary method for detecting intrauterine exposures.17 This approach allows greater ease of specimen collection but may not yield results that are exactly equivalent to meconium testing.19
MONITORING AND EVALUATION
Close monitoring for development of NAS after birth is indicated for all newborns with confirmed or suspected intrauterine opioid exposure. Although the current American Academy of Pediatrics (AAP) guidelines recommend three days of newborn observation for exposure to short-acting opioids and five to seven days for longer acting opioids, substantial variation has been described across US hospitals in policies related to newborn length of stay for NAS observation.17, 20 In most hospitals, monitoring for NAS occurs in the routine postpartum unit (ie, level 1 nursery), with transfer to the NICU if pharmacologic treatment is indicated.17
The most widely used assessment tool for NAS is the Finnegan Neonatal Abstinence Scoring System (FNASS) or the modified FNASS, which assigns points for the 21 most common opioid withdrawal symptoms based on perceived severity.17, 21 This tool allows for assessment of symptoms, helps determine need for pharmacologic intervention, and can guide monitoring of symptoms and weaning of therapy. A commonly used score cutoff of 8 is based on prior research validating scores >8 as indicative of withdrawal symptoms as opposed to normal newborn findings.22 Despite its popularity and widespread usage, FNASS has limitations, including the need for the newborn to be stimulated or disturbed to produce an accurate assessment and scoring for nonspecific signs of withdrawal, including sneezing, yawning, and stuffiness. Recent work has attempted to simplify and shorten the FNASS to elements that are unique and specific for withdrawal.23,24 Further research is needed to establish the validity of common scoring practices (ie, use of 8 as a cutoff) to determine the need for pharmacologic treatment.25
Recent studies suggest that simple, function-based assessments, such as the Eat, Sleep, Console (ESC) approach developed by Grossman and colleagues, may serve as an alternative to the FNASS for evaluating withdrawal.26,27 With ESC, the need for pharmacotherapy is evaluated by the newborn’s ability to (1) eat (breastfeed successfully or eat at least 1oz per feed), (2) sleep uninterrupted for at least one hour, and (3) be consoled within 10 minutes. To date, research on the implementation of ESC has primarily focused on reducing length of stay and need for pharmacologic treatment in the context of quality improvement initiatives.26,27 Further prospective studies are warranted that compare ESC to traditional approaches involving the FNASS, and that evaluate post-discharge outcomes including newborn weight gain, ongoing withdrawal symptoms at home, and readmission.
NONPHARMACOLOGIC TREATMENT
In recent years, research increasingly supports the critical role of nonpharmacological care in management of all opioid-exposed newborns, regardless of NAS severity.11,27, 28 Rooming-in of mothers or caregivers has been shown to decrease the need for pharmacologic treatment, shorten the length of stay, and reduce hospital costs.28,29 Other well-established practices include maintaining a low stimulus environment for infants with low lighting and sound, swaddling, maximizing caregiver contact with kangaroo care and skin to skin, and minimizing interventions. Therapeutic modalities, such as massage and music therapy, have been used for infants with NAS, but no evidence has supported their use. Recent studies have increasingly supported the use of acupuncture as an emerging modality in treating NAS. 30
Feeding
Breastfeeding is encouraged for mothers who are stable on their methadone or buprenorphine maintenance treatment, are not using heroin or other illicit drugs, and have no other contraindications to breastfeeding, such as human immunodeficiency virus.31 Despite the known benefits of breastfeeding, which include decreased NAS severity, decreased need for pharmacological treatment, and shortened length of hospital stay, breastfeeding rates among mothers with OUD are low.31 Hospital policies that can promote maternal success in breastfeeding include tailored breastfeeding support, rooming in, and early, consistent maternal education on the benefits and safety of breastfeeding.32 A small percentage of hospitals use donor breastmilk for this population, although data on outcomes are limited.17 For formula-fed newborns, emerging research suggests that early initiation of high-calorie (22-24 kcal/ounce) formula may be beneficial to prevent excessive weight loss and poor weight gain after intrauterine opioid exposure.33
PHARMACOLOGIC TREATMENT
When supportive therapy fails to adequately control symptoms of withdrawal, pharmacological management is initiated to improve infant discomfort, allow for adequate feeding and nutrition, and facilitate parental bonding (Table 2).11 Opioids are the primary agent used for pharmacologic treatment, and morphine is the most commonly utilized.17 Morphine is a short-acting opioid and can be prescribed either as a weight-based weaning protocol or symptom-based regimen. Methadone is also widely used, and as a long-acting opioid, it has the advantage of twice daily dosing after the initial loading dose. Recently, buprenorphine, a partial mu opioid agonist with a long half-life, has emerged as a promising primary opioid treatment agent and has been shown to reduce the length of stay and the number of opioid treatment days compared with morphine and methadone.36
When the signs and symptoms of NAS are not effectively controlled with a primary opioid or in the case of polysubstance exposure, adjunctive agents are often used, with phenobarbital and clonidine being the most common (Table 3).11 Regardless of opioid agent used, multicenter quality improvement initiatives demonstrate that having a standardized weaning protocol is critical to minimizing the overall length of stay and reducing the need for adjunctive agents.38,39 Additionally, modeling tools such as pharmacometrics for methadone and buprenorphine have shown promise in optimizing dose selection.40,41 Modeling may include pharmacodynamic data (ie, clinical response to treatment), pharmacokinetics (ie, measures of drug distribution and clearance), and other factors, such as patient demographics, intrauterine exposure type, and symptom severity. Future studies should examine weight versus symptom-based dosing regimens as well as compare weaning schedules versus “as needed” dosing regimens.11
PSYCHOSOCIAL CONSIDERATIONS
The need for comprehensive medical and psychosocial supports for mothers with OUD cannot be overstated, given the high rates of concurrent illicit or other substance use, comorbid depression and anxiety, physical and sexual trauma, poverty and homelessness, intravenous drug use, and sex-related risk patterns.15 Significant issues of healthcare-associated stigma and criminality also affect this population. As of 2019, 23 states and the District of Columbia classify substance use during pregnancy as child abuse under civil child-welfare statutes, potentially resulting in termination of parental rights.42 Studies of mothers with OUD have demonstrated that they often experience guilt, shame, and fear of loss of custody, all of which can impede their trust in hospital providers and future engagement in care.43 They also report frustration with and mistrust of NAS scoring assessments, which they can perceive to be disruptive and potentially biased.44 Multiple approaches should be considered to standardize and improve the hospital experience for this population, in a way that emphasizes the mother’s role as a capable, respected participant in her newborn’s care.
Maternal Support
A coordinated, multidisciplinary approach to comprehensively support mothers with OUD should involve team members from pediatrics, neonatology, obstetrics, nursing, social work, case management, and lactation.35 This support includes screening for adequate resources and a safe, supportive, drug-free home environment as well as evaluating co-occurring mental health conditions. Referrals should be provided as needed to social services, postpartum psychiatry or behavioral health services, OUD treatment and relapse-prevention programs, and harm reduction services (eg, naloxone training). In addition to the healthcare team, other community members can be enlisted to serve as a trusted, consistent, and nonjudgmental support during the hospitalization; examples may include a peer support (another mother with OUD), an OUD program caseworker, or a doula.44
Clinical Pathways
Hospitals should establish clinical pathways for women with OUD to standardize care and communication across the continuum of care for themselves and their newborn, with input from all healthcare team members involved (prenatal, intrapartum, and postpartum).35 Early, consistent information should be provided regarding expected newborn hospital course, including toxicology testing, NAS monitoring, possible NICU admission, and involvement of social work.
Provider Training
Educational opportunities in the form of continuing medical education, in-service trainings, etc., should be provided for clinical staff who care for mothers with OUD and their newborns, regarding issues of substance use, stigma, bias, and trauma-informed care.35 Online training resources are available through the American Society of Addiction Medicine, the ACOG, AAP, the Centers for Disease Control and Prevention, and the Substance Abuse and Mental Health Services Administration.
DISCHARGE PLANNING
Regardless of whether or not NAS is treated pharmacologically, newborns with opioid exposure may experience residual symptoms of withdrawal that persist for months.4 Current research suggests increased risk for morbidity, emergency department utilization, and rehospitalization after discharge in this population as well as difficulty in accessing and engaging with pediatric preventative care.45, 46
A clear plan should be established upon discharge to ensure optimal newborn care and follow-up. A complete record of the newborn’s hospital stay, including maternal toxicology screenings and summary of any social work documentation, should be communicated to the primary care provider upon discharge. Close postdischarge monitoring involves addressing parenting knowledge gaps, assessing illness and injury risk, and evaluating for the presence of ongoing withdrawal symptoms.4 Primary care providers can also play a key role in assessing maternal stress, coping, and parenting skills as well as helping families connect to resources. Further research is warranted on how pediatric primary care systems can better build maternal trust, address parenting needs, and engage this population in routine well-child care.47
Child Welfare, Early Intervention, and Other Services
In general, newborn safety and keeping families intact should be prioritized, with disposition into foster care only in cases of concern for child maltreatment or neglect. Under the Child Abuse Prevention and Treatment Act (CAPTA), states are required to develop Plans of Safe Care for women and newborns affected by OUD, with the goal of fostering collaboration between healthcare and social service organizations around care of these families.48 Given the variable interpretation of Plans of Safe Care across the U.S., providers should be knowledgeable about state and local statutes and reporting requirements related to parental substance use.
As part of Plans of Safe Care, providers may be well-positioned to initiate referrals for early intervention, home visiting, and other programs designed to provide developmental or wrap-around support for families. Under Part C of the Individuals with Disabilities Education Act, many states offer early intervention on the basis of NAS as an automatic qualifying diagnosis; however, attrition of eligible families along the referral and enrollment process is substantial.49 A standardized approach to discharging opioid-exposed newborns includes referrals to available resources and discussion of their importance with families and may increase utilization and decrease variation in care.50
CONCLUSION
Maternal OUD presents a unique combination of medical and psychosocial challenges that affect hospital care for mothers and their newborns. Optimal care for this population warrants a multidisciplinary team of providers who are knowledgeable, collaborative, and mindful of the important role of the mother as a key participant in her newborn’s care. Despite a large and growing body of research focused on NAS prevention, screening, and treatment, ongoing efforts are needed to create hospital policies and clinical pathways that are responsive to the healthcare needs of this population, navigate sensitive issues of criminality and stigma, and ultimately support maternal parenting success.
Disclosures
The authors have no financial relationships and conflicts of interest relevant to this article to disclose.
Funding
Funding for this work was provided by Cincinnati Children’s Hospital Medical Center and Nemours/AI duPont Hospital for Children.
2. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126. https://doi.org/10.1056/NEJMsa1500439.
3. Devlin LA, Davis JM. A practical approach to neonatal opiate withdrawal syndrome. Am J Perinatol. 2018;35(4):324-330. https://doi.org/10.1055/s-0037-1608630.
4. Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547-e561. https://doi.org/10.1542/peds.2013-3524.
5. Kaltenbach K, Holbrook AM, Coyle MG, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction. 2012;107 Supplement 1:45-52. https://doi.org/10.1111/j.1360-0443.2012.04038.x.
6. Huybrechts KF, Bateman BT, Desai RJ, et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: cohort study. BMJ. 2017;358:j3326. https://doi.org/10.1136/bmj.j3326.
7. Wachman EM, Hayes MJ, Brown MS, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309(17):1821-1827. https://doi.org/10.1001/jama.2013.3411.
8. Mactier H, McLaughlin P, Gillis C, Osselton MD. Variations in infant CYP2B6 genotype associated with the need for pharmacological treatment for neonatal abstinence syndrome in infants of methadone-maintained opioid-dependent mothers. Am J Perinatol. 2017;34(9):918–921. https://doi.org/10.1055/s-0037-1600917.
9. Jilani SM, Frey MT, Pepin D, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome - six states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(1):6-10. https://doi.org/10.15585/mmwr.mm6801a2.
10. Maalouf FI, Cooper WO, Stratton SM, et al. Positive predictive value of administrative data for neonatal abstinence syndrome. Pediatrics. 2019;143(1). https://doi.org/10.1542/peds.2017-4183.
11. Mangat AK, Schmölzer GM, Kraft WK. Pharmacological and non-pharmacological treatments for the Neonatal Abstinence Syndrome (NAS). Semin Fetal Neonat Med. 2019;24(2):133-141. https://doi.org/10.1016/j.siny.2019.01.009.
12. Ko JY, Wolicki S, Barfield WD, et al. CDC Grand Rounds: public health strategies to prevent neonatal abstinence syndrome. MMWR Morb Mortal Wkly Rep. 2017;66(9):242-245. https://doi.org/10.15585/mmwr.mm6609a2.
13. Patrick SW, Faherty LJ, Dick AW, et al. Association Among County-Level economic factors, clinician supply, metropolitan or rural location, and neonatal abstinence syndrome. JAMA. 2019;321(4):385-393. https://doi.org/10.1001/jama.2018.20851.
14. Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW. Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr. 2017;171(2):194-196. https://doi.org/10.1001/jamapediatrics.2016.3750.
15. Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet Gynecol. 2017;130(2):e81-e94. https://doi.org/10.1097/AOG.0000000000002235.
16. Wexelblatt SL, Ward LP, Torok K, et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
17. Bogen DL, Whalen BL, Kair LR, Vining M, King BA. Wide variation found in care of opioid-exposed newborns. Acad Pediatr. 2017;17(4):374-380. https://doi.org/10.1016/j.acap.2016.10.003.
18. Cotten SW. Drug testing in the neonate. Clin Lab Med. 2012;32(3):449-466. https://doi.org/10.1016/j.cll.2012.06.008.
19. Colby JM, Adams BC, Morad A, Presley LD, Patrick SW. Umbilical cord tissue and meconium may not be equivalent for confirming in utero substance exposure. J Pediatr. 2019;205:277-280. https://doi.org/10.1016/j.jpeds.2018.09.046.
20. Hudak ML, Tan RC, COMMITTEE ON DRUGS, COMMITTEE ON FETUS AND NEWBORN, American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560. https://doi.org/10.1542/peds.2011-3212.
21. Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1-2):141-158.
22. Zimmermann-Baer U, Nötzli U, Rentsch K, Bucher HU. Finnegan neonatal abstinence scoring system: normal values for first 3 days and weeks 5-6 in non-addicted infants. Addiction. 2010;105(3):524-528. https://doi.org/10.1111/j.1360-0443.2009.02802.x.
23. Jones HE, Seashore C, Johnson E, et al. Measurement of neonatal abstinence syndrome: evaluation of short forms. J Opioid Manag. 2016;12(1):19-23. https://doi.org/10.5055/jom.2016.0308.
24. Isemann BT, Stoeckle EC, Taleghani AA, Mueller EW. Early prediction tool to identify the need for pharmacotherapy in infants at risk of neonatal abstinence syndrome. Pharmacotherapy. 2017;37(7):840-848. https://doi.org/10.1002/phar.1948.
25. Schiff DM, Grossman MR. Beyond the Finnegan scoring system: novel assessment and diagnostic techniques for the opioid-exposed infant. Semin Fetal Neonat Med. 2019;24(2):115-120. https://doi.org/10.1016/j.siny.2019.01.003.
26. Grossman MR, Berkwitt AK, Osborn RR, et al. An initiative to improve the quality of care of infants With neonatal abstinence syndrome. Pediatrics. 2017;139(6). https://doi.org/10.1542/peds.2016-3360.
27. Wachman EM, Grossman M, Schiff DM, et al. Quality improvement initiative to improve inpatient outcomes for Neonatal Abstinence Syndrome. J Perinatol. 2018;38(8):1114-1122. https://doi.org/10.1038/s41372-018-0109-8.
28. Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6). https://doi.org/10.1542/peds.2015-2929.
29. MacMillan KDL, Rendon CP, Verma K, et al. Association of rooming-in With outcomes for neonatal abstinence syndrome: A systematic review and meta-analysis. JAMA Pediatr. 2018 Apr 1;172(4):345-351. https://doi.org/10.1001/jamapediatrics.2017.5195.
30. Jackson HJ, Lopez C, Miller S, Engelhardt B. A scoping review of acupuncture as a potential intervention for neonatal abstinence syndrome. Med Acupunct. 2019;31(2):69-84. https://doi.org/10.1089/acu.2018.1323.
31. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: Guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. https://doi.org/10.1089/bfm.2015.9992.
32. Krans EE, Campopiano M, Cleveland LM, et al. National partnership for maternal safety: consensus bundle on obstetric care for women With opioid use disorder. Obstet Gynecol. 2019;134(2):365-375. https://doi.org/10.1097/AOG.0000000000003381.
33. Bogen DL, Hanusa BH, Baker R, Medoff-Cooper B, Cohlan B. Randomized clinical trial of standard- Versus high-calorie formula for methadone-exposed infants: A feasibility study. Hosp Pediatr. 2018;8(1):7-14. https://doi.org/10.1542/hpeds.2017-0114.
34. Lexicomp. Opioids, Urine, Screen and Confirmation. https://online.lexi.com/lco/action/doc/retrieve/docid/lthdph/382929. Accessed September 4, 2019.
35. Mayo Clinic Laboratories. Opiates. https://www.mayocliniclabs.com/test-info/drug-book/opiates.html. Accessed Sept 4, 2019.
36. Kraft WK, Adeniyi-Jones SC, Chervoneva I, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med. 2017;376(24):2341-2348. https://doi.org/10.1056/NEJMoa1614835.
37. Lexicomp. https://online.lexi.com/lco/action/home. Accessed September 4, 2019
38. Hall ES, Wexelblatt SL, Crowley M, et al. Implementation of a neonatal abstinence syndrome weaning protocol: A multicenter cohort study. Pediatrics. 2015;136(4):e803-e810. https://doi.org/10.1542/peds.2015-1141.
39. Patrick SW, Schumacher RE, Horbar JD, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016;137(5):38. https://doi.org/10.1542/peds.2015-3835.
40. Wiles JR, Isemann B, Mizuno T, et al. Pharmacokinetics of oral methadone in the treatment of neonatal abstinence syndrome: A pilot study. J Pediatr. 2015;167(6):1214–20.e3. https://doi.org/10.1016/j.jpeds.2015.08.032.
41. Ng CM, Dombrowsky E, Lin H, et al. Population pharmacokinetic model of sublingual buprenorphine in neonatal abstinence syndrome. Pharmacotherapy. 2015;35(7):670-680. https://doi.org/10.1002/phar.1610.
42. The Guttmacher Institute. Substance abuse During pregnancy. https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy. Accessed November 20, 2019; Updated November 1, 2019.
43. Cleveland LM, Bonugli R. Experiences of mothers of infants with neonatal abstinence syndrome in the neonatal intensive care unit. J Obstet Gynecol Neonat Nurs. 2014;43(3):318-329. https://doi.org/10.1111/1552-6909.12306.
44. Rockefeller K, Macken LC, Craig A. Trying to do what is best: A qualitative study of maternal-infant bonding and neonatal abstinence syndrome. Adv Neonat Care. 2019;19(5):E3-E15. https://doi.org/10.1097/ANC.0000000000000616.
45. Liu G, Kong L, Leslie DL, Corr TE. A longitudinal healthcare use profile of children with a history of neonatal abstinence syndrome. J Pediatr. 2019;204:111-117. https://doi.org/10.1016/j.jpeds.2018.08.032.
46. Goyal NK, Rhode JF, Short V, et al. Well child care adherence during the first 2 years of life after intrauterine opioid exposure. Pediatrics. In press.
47. Short VL, Goyal NK, Chung EK, Hand DJ, Abatemarco DJ. Perceptions of pediatric primary care among mothers in treatment for opioid use disorder. J Commun Health. 2019 Dec;44(6):1127-1134. https://doi.org/10.1007/s10900-019-00701-1.
48. Plans of Safe Care. Administration for Children and Families. https://www.acf.hhs.gov/sites/default/files/cb/pi1702.pdf. Accessed September 1, 2019.
49. Peacock-Chambers E, Leyenaar JK, Foss S, et al. Early Intervention referral and enrollment among infants with neonatal abstinence syndrome. J Dev Behav Pediatr. 2019;40(6):441-450. https://doi.org/10.1097/DBP.0000000000000679.
50. Crook TW, Munn EK, Scott TA, et al. Improving the discharge process for opioid-exposed neonates. Hosp Pediatr. 2019;9(8):643-648. https://doi.org/10.1542/hpeds.2019-0088.
In the past two decades, the incidence rate of opioid use disorder (OUD) among pregnant women has increased by more than 400%, constituting a United States public health crisis.1 Newborns exposed to intrauterine opioids are at risk for the postnatal withdrawal syndrome known as neonatal abstinence syndrome (NAS), which requires increased hospital resources, such as neonatal intensive care unit (NICU) admission and prolonged length of stay.2 Given the medical and psychosocial challenges associated with maternal OUD and NAS, a multidisciplinary, patient-centered approach to hospital care for affected newborns and their mothers is warranted. A large and growing body of research has focused on the epidemiology of NAS and approaches for its prevention, screening, and management. This review appraises updates to the literature within the past five years, with an emphasis on considerations for newborn discharge to promote optimal care for this population.
DEFINITION
NAS is a complex disorder arising from the abrupt cessation of placental transfer of opioids after birth, although other maternal substances, including benzodiazepines and other antidepressants, have been less commonly implicated.2 The term neonatal opioid withdrawal syndrome is sometimes used to indicate withdrawal from opioids specifically.3 The central and autonomic nervous systems and the gastrointestinal system (eg, tremors, increased muscle tone, high-pitched crying, feeding difficulties) are affected in NAS, with most newborns demonstrating symptoms within the first few days of life.4 Previously reported factors associated with NAS include opioid type, timing of exposure during pregnancy, maternal tobacco use, infant sex, and gestational age.5 Literature demonstrates that concurrent exposure to other prenatal substances, particularly antidepressants, benzodiazepines, and gabapentin, is significantly associated with increased risk of NAS.6 Recent studies also suggest that expression of NAS may relate to newborn genetic variations, particularly at the OPRM1, COMT, and CYP2B6 gene sites.7, 8
State health departments have increasingly deemed NAS as a reportable diagnosis for public health surveillance, which relies on the accurate diagnosis and documentation of NAS during birth hospitalization.9 The diagnosis codes for NAS include the International Classification of Diseases, Ninth Revision, Clinical Modification code (ICD-9-CM) 779.5 and the International Classification of Diseases, Tenth Revision, Clinical Modification ICD-10-CM code P96.1.10 However, given the variation in the presentation and severity of NAS, no consensus has been established with regard to a standardized case definition for reporting across hospitals and states.9 In fact, NAS should be conceptualized as a continuum of withdrawal symptoms along which every infant with intrauterine opioid exposure resides; this continuum ranges from minor findings, which do not affect the infant’s ability to grow and develop, to severe withdrawal, resulting in excessive weight loss, dehydration, or seizures.3,11 Ultimately, the diagnosis of NAS is made clinically based on cardinal symptoms in the setting of known or highly suspected opioid exposure. In a recent study of Tennessee Medicaid claims data, >25% of infants with a confirmed diagnosis code for NAS did not receive pharmacotherapy.10 Pharmacologic treatment of NAS, therefore, may be more appropriately considered as a marker of disease severity, rather than a requirement for diagnosis.
EPIDEMIOLOGY
Although the opioid crisis and resulting rise in NAS have affected communities across the US, substantial statewide variation exists, with extremes ranging from 0.7 per 1,000 births affected by NAS in Hawaii to 33.4 per 1,000 births in West Virginia.12 Within states, increased maternal OUD and NAS rates have also disproportionately affected rural communities possibly due to reduced access to healthcare and mental health services and poor economic conditions.13 A recent national study demonstrated that the proportion of newborns with NAS who were born in rural hospitals increased from 12.9% to 21.2% over the past decade; these rural newborns with NAS are more likely to be publicly insured and to require transfer after birth than newborns in urban hospitals.14 These data suggest a particular need among rural communities for increased resources targeting NAS care as well as maternal OUD prevention and treatment.
RISK IDENTIFICATION
The American College of Obstetricians and Gynecologists (ACOG) recommends early universal screening for maternal substance use at the first prenatal visit with a validated screening tool; examples include the 4Ps (parents, partners, past, and pregnancy), CRAFFT (car, relax, alone, forget, friends, trouble), and the National Institute on Drug Abuse quick screen, which have all been well studied and have a high sensitivity for detecting substance use and misuse.15
Toxicology Screening
Toxicology testing for both mother and newborn is helpful in identifying or confirming intrauterine exposures, particularly in cases of polysubstance use or when a newborn manifests signs of NAS but whose mother denies opioid use. All toxicology testing should be performed with the mother’s consent, and any potential legal or mandatory ramifications of a positive test should be considered. Although universal maternal toxicology testing improves the identification of newborns at risk for NAS, this approach remains controversial; most hospitals use a risk-based approach for maternal toxicology testing.16,17
Newborn toxicology testing can be performed from samples of hair, urine, meconium, and umbilical cord. Although frequently used, newborn urine testing has the shortest window of detection, ie, the last few days prior to delivery (Table 1). Meconium drug testing has been considered the gold standard and can detect exposures from the last 20 weeks of gestation, providing information on chronic exposures.18 In a recent survey, 10% of hospitals reported using umbilical cord toxicology as the primary method for detecting intrauterine exposures.17 This approach allows greater ease of specimen collection but may not yield results that are exactly equivalent to meconium testing.19
MONITORING AND EVALUATION
Close monitoring for development of NAS after birth is indicated for all newborns with confirmed or suspected intrauterine opioid exposure. Although the current American Academy of Pediatrics (AAP) guidelines recommend three days of newborn observation for exposure to short-acting opioids and five to seven days for longer acting opioids, substantial variation has been described across US hospitals in policies related to newborn length of stay for NAS observation.17, 20 In most hospitals, monitoring for NAS occurs in the routine postpartum unit (ie, level 1 nursery), with transfer to the NICU if pharmacologic treatment is indicated.17
The most widely used assessment tool for NAS is the Finnegan Neonatal Abstinence Scoring System (FNASS) or the modified FNASS, which assigns points for the 21 most common opioid withdrawal symptoms based on perceived severity.17, 21 This tool allows for assessment of symptoms, helps determine need for pharmacologic intervention, and can guide monitoring of symptoms and weaning of therapy. A commonly used score cutoff of 8 is based on prior research validating scores >8 as indicative of withdrawal symptoms as opposed to normal newborn findings.22 Despite its popularity and widespread usage, FNASS has limitations, including the need for the newborn to be stimulated or disturbed to produce an accurate assessment and scoring for nonspecific signs of withdrawal, including sneezing, yawning, and stuffiness. Recent work has attempted to simplify and shorten the FNASS to elements that are unique and specific for withdrawal.23,24 Further research is needed to establish the validity of common scoring practices (ie, use of 8 as a cutoff) to determine the need for pharmacologic treatment.25
Recent studies suggest that simple, function-based assessments, such as the Eat, Sleep, Console (ESC) approach developed by Grossman and colleagues, may serve as an alternative to the FNASS for evaluating withdrawal.26,27 With ESC, the need for pharmacotherapy is evaluated by the newborn’s ability to (1) eat (breastfeed successfully or eat at least 1oz per feed), (2) sleep uninterrupted for at least one hour, and (3) be consoled within 10 minutes. To date, research on the implementation of ESC has primarily focused on reducing length of stay and need for pharmacologic treatment in the context of quality improvement initiatives.26,27 Further prospective studies are warranted that compare ESC to traditional approaches involving the FNASS, and that evaluate post-discharge outcomes including newborn weight gain, ongoing withdrawal symptoms at home, and readmission.
NONPHARMACOLOGIC TREATMENT
In recent years, research increasingly supports the critical role of nonpharmacological care in management of all opioid-exposed newborns, regardless of NAS severity.11,27, 28 Rooming-in of mothers or caregivers has been shown to decrease the need for pharmacologic treatment, shorten the length of stay, and reduce hospital costs.28,29 Other well-established practices include maintaining a low stimulus environment for infants with low lighting and sound, swaddling, maximizing caregiver contact with kangaroo care and skin to skin, and minimizing interventions. Therapeutic modalities, such as massage and music therapy, have been used for infants with NAS, but no evidence has supported their use. Recent studies have increasingly supported the use of acupuncture as an emerging modality in treating NAS. 30
Feeding
Breastfeeding is encouraged for mothers who are stable on their methadone or buprenorphine maintenance treatment, are not using heroin or other illicit drugs, and have no other contraindications to breastfeeding, such as human immunodeficiency virus.31 Despite the known benefits of breastfeeding, which include decreased NAS severity, decreased need for pharmacological treatment, and shortened length of hospital stay, breastfeeding rates among mothers with OUD are low.31 Hospital policies that can promote maternal success in breastfeeding include tailored breastfeeding support, rooming in, and early, consistent maternal education on the benefits and safety of breastfeeding.32 A small percentage of hospitals use donor breastmilk for this population, although data on outcomes are limited.17 For formula-fed newborns, emerging research suggests that early initiation of high-calorie (22-24 kcal/ounce) formula may be beneficial to prevent excessive weight loss and poor weight gain after intrauterine opioid exposure.33
PHARMACOLOGIC TREATMENT
When supportive therapy fails to adequately control symptoms of withdrawal, pharmacological management is initiated to improve infant discomfort, allow for adequate feeding and nutrition, and facilitate parental bonding (Table 2).11 Opioids are the primary agent used for pharmacologic treatment, and morphine is the most commonly utilized.17 Morphine is a short-acting opioid and can be prescribed either as a weight-based weaning protocol or symptom-based regimen. Methadone is also widely used, and as a long-acting opioid, it has the advantage of twice daily dosing after the initial loading dose. Recently, buprenorphine, a partial mu opioid agonist with a long half-life, has emerged as a promising primary opioid treatment agent and has been shown to reduce the length of stay and the number of opioid treatment days compared with morphine and methadone.36
When the signs and symptoms of NAS are not effectively controlled with a primary opioid or in the case of polysubstance exposure, adjunctive agents are often used, with phenobarbital and clonidine being the most common (Table 3).11 Regardless of opioid agent used, multicenter quality improvement initiatives demonstrate that having a standardized weaning protocol is critical to minimizing the overall length of stay and reducing the need for adjunctive agents.38,39 Additionally, modeling tools such as pharmacometrics for methadone and buprenorphine have shown promise in optimizing dose selection.40,41 Modeling may include pharmacodynamic data (ie, clinical response to treatment), pharmacokinetics (ie, measures of drug distribution and clearance), and other factors, such as patient demographics, intrauterine exposure type, and symptom severity. Future studies should examine weight versus symptom-based dosing regimens as well as compare weaning schedules versus “as needed” dosing regimens.11
PSYCHOSOCIAL CONSIDERATIONS
The need for comprehensive medical and psychosocial supports for mothers with OUD cannot be overstated, given the high rates of concurrent illicit or other substance use, comorbid depression and anxiety, physical and sexual trauma, poverty and homelessness, intravenous drug use, and sex-related risk patterns.15 Significant issues of healthcare-associated stigma and criminality also affect this population. As of 2019, 23 states and the District of Columbia classify substance use during pregnancy as child abuse under civil child-welfare statutes, potentially resulting in termination of parental rights.42 Studies of mothers with OUD have demonstrated that they often experience guilt, shame, and fear of loss of custody, all of which can impede their trust in hospital providers and future engagement in care.43 They also report frustration with and mistrust of NAS scoring assessments, which they can perceive to be disruptive and potentially biased.44 Multiple approaches should be considered to standardize and improve the hospital experience for this population, in a way that emphasizes the mother’s role as a capable, respected participant in her newborn’s care.
Maternal Support
A coordinated, multidisciplinary approach to comprehensively support mothers with OUD should involve team members from pediatrics, neonatology, obstetrics, nursing, social work, case management, and lactation.35 This support includes screening for adequate resources and a safe, supportive, drug-free home environment as well as evaluating co-occurring mental health conditions. Referrals should be provided as needed to social services, postpartum psychiatry or behavioral health services, OUD treatment and relapse-prevention programs, and harm reduction services (eg, naloxone training). In addition to the healthcare team, other community members can be enlisted to serve as a trusted, consistent, and nonjudgmental support during the hospitalization; examples may include a peer support (another mother with OUD), an OUD program caseworker, or a doula.44
Clinical Pathways
Hospitals should establish clinical pathways for women with OUD to standardize care and communication across the continuum of care for themselves and their newborn, with input from all healthcare team members involved (prenatal, intrapartum, and postpartum).35 Early, consistent information should be provided regarding expected newborn hospital course, including toxicology testing, NAS monitoring, possible NICU admission, and involvement of social work.
Provider Training
Educational opportunities in the form of continuing medical education, in-service trainings, etc., should be provided for clinical staff who care for mothers with OUD and their newborns, regarding issues of substance use, stigma, bias, and trauma-informed care.35 Online training resources are available through the American Society of Addiction Medicine, the ACOG, AAP, the Centers for Disease Control and Prevention, and the Substance Abuse and Mental Health Services Administration.
DISCHARGE PLANNING
Regardless of whether or not NAS is treated pharmacologically, newborns with opioid exposure may experience residual symptoms of withdrawal that persist for months.4 Current research suggests increased risk for morbidity, emergency department utilization, and rehospitalization after discharge in this population as well as difficulty in accessing and engaging with pediatric preventative care.45, 46
A clear plan should be established upon discharge to ensure optimal newborn care and follow-up. A complete record of the newborn’s hospital stay, including maternal toxicology screenings and summary of any social work documentation, should be communicated to the primary care provider upon discharge. Close postdischarge monitoring involves addressing parenting knowledge gaps, assessing illness and injury risk, and evaluating for the presence of ongoing withdrawal symptoms.4 Primary care providers can also play a key role in assessing maternal stress, coping, and parenting skills as well as helping families connect to resources. Further research is warranted on how pediatric primary care systems can better build maternal trust, address parenting needs, and engage this population in routine well-child care.47
Child Welfare, Early Intervention, and Other Services
In general, newborn safety and keeping families intact should be prioritized, with disposition into foster care only in cases of concern for child maltreatment or neglect. Under the Child Abuse Prevention and Treatment Act (CAPTA), states are required to develop Plans of Safe Care for women and newborns affected by OUD, with the goal of fostering collaboration between healthcare and social service organizations around care of these families.48 Given the variable interpretation of Plans of Safe Care across the U.S., providers should be knowledgeable about state and local statutes and reporting requirements related to parental substance use.
As part of Plans of Safe Care, providers may be well-positioned to initiate referrals for early intervention, home visiting, and other programs designed to provide developmental or wrap-around support for families. Under Part C of the Individuals with Disabilities Education Act, many states offer early intervention on the basis of NAS as an automatic qualifying diagnosis; however, attrition of eligible families along the referral and enrollment process is substantial.49 A standardized approach to discharging opioid-exposed newborns includes referrals to available resources and discussion of their importance with families and may increase utilization and decrease variation in care.50
CONCLUSION
Maternal OUD presents a unique combination of medical and psychosocial challenges that affect hospital care for mothers and their newborns. Optimal care for this population warrants a multidisciplinary team of providers who are knowledgeable, collaborative, and mindful of the important role of the mother as a key participant in her newborn’s care. Despite a large and growing body of research focused on NAS prevention, screening, and treatment, ongoing efforts are needed to create hospital policies and clinical pathways that are responsive to the healthcare needs of this population, navigate sensitive issues of criminality and stigma, and ultimately support maternal parenting success.
Disclosures
The authors have no financial relationships and conflicts of interest relevant to this article to disclose.
Funding
Funding for this work was provided by Cincinnati Children’s Hospital Medical Center and Nemours/AI duPont Hospital for Children.
In the past two decades, the incidence rate of opioid use disorder (OUD) among pregnant women has increased by more than 400%, constituting a United States public health crisis.1 Newborns exposed to intrauterine opioids are at risk for the postnatal withdrawal syndrome known as neonatal abstinence syndrome (NAS), which requires increased hospital resources, such as neonatal intensive care unit (NICU) admission and prolonged length of stay.2 Given the medical and psychosocial challenges associated with maternal OUD and NAS, a multidisciplinary, patient-centered approach to hospital care for affected newborns and their mothers is warranted. A large and growing body of research has focused on the epidemiology of NAS and approaches for its prevention, screening, and management. This review appraises updates to the literature within the past five years, with an emphasis on considerations for newborn discharge to promote optimal care for this population.
DEFINITION
NAS is a complex disorder arising from the abrupt cessation of placental transfer of opioids after birth, although other maternal substances, including benzodiazepines and other antidepressants, have been less commonly implicated.2 The term neonatal opioid withdrawal syndrome is sometimes used to indicate withdrawal from opioids specifically.3 The central and autonomic nervous systems and the gastrointestinal system (eg, tremors, increased muscle tone, high-pitched crying, feeding difficulties) are affected in NAS, with most newborns demonstrating symptoms within the first few days of life.4 Previously reported factors associated with NAS include opioid type, timing of exposure during pregnancy, maternal tobacco use, infant sex, and gestational age.5 Literature demonstrates that concurrent exposure to other prenatal substances, particularly antidepressants, benzodiazepines, and gabapentin, is significantly associated with increased risk of NAS.6 Recent studies also suggest that expression of NAS may relate to newborn genetic variations, particularly at the OPRM1, COMT, and CYP2B6 gene sites.7, 8
State health departments have increasingly deemed NAS as a reportable diagnosis for public health surveillance, which relies on the accurate diagnosis and documentation of NAS during birth hospitalization.9 The diagnosis codes for NAS include the International Classification of Diseases, Ninth Revision, Clinical Modification code (ICD-9-CM) 779.5 and the International Classification of Diseases, Tenth Revision, Clinical Modification ICD-10-CM code P96.1.10 However, given the variation in the presentation and severity of NAS, no consensus has been established with regard to a standardized case definition for reporting across hospitals and states.9 In fact, NAS should be conceptualized as a continuum of withdrawal symptoms along which every infant with intrauterine opioid exposure resides; this continuum ranges from minor findings, which do not affect the infant’s ability to grow and develop, to severe withdrawal, resulting in excessive weight loss, dehydration, or seizures.3,11 Ultimately, the diagnosis of NAS is made clinically based on cardinal symptoms in the setting of known or highly suspected opioid exposure. In a recent study of Tennessee Medicaid claims data, >25% of infants with a confirmed diagnosis code for NAS did not receive pharmacotherapy.10 Pharmacologic treatment of NAS, therefore, may be more appropriately considered as a marker of disease severity, rather than a requirement for diagnosis.
EPIDEMIOLOGY
Although the opioid crisis and resulting rise in NAS have affected communities across the US, substantial statewide variation exists, with extremes ranging from 0.7 per 1,000 births affected by NAS in Hawaii to 33.4 per 1,000 births in West Virginia.12 Within states, increased maternal OUD and NAS rates have also disproportionately affected rural communities possibly due to reduced access to healthcare and mental health services and poor economic conditions.13 A recent national study demonstrated that the proportion of newborns with NAS who were born in rural hospitals increased from 12.9% to 21.2% over the past decade; these rural newborns with NAS are more likely to be publicly insured and to require transfer after birth than newborns in urban hospitals.14 These data suggest a particular need among rural communities for increased resources targeting NAS care as well as maternal OUD prevention and treatment.
RISK IDENTIFICATION
The American College of Obstetricians and Gynecologists (ACOG) recommends early universal screening for maternal substance use at the first prenatal visit with a validated screening tool; examples include the 4Ps (parents, partners, past, and pregnancy), CRAFFT (car, relax, alone, forget, friends, trouble), and the National Institute on Drug Abuse quick screen, which have all been well studied and have a high sensitivity for detecting substance use and misuse.15
Toxicology Screening
Toxicology testing for both mother and newborn is helpful in identifying or confirming intrauterine exposures, particularly in cases of polysubstance use or when a newborn manifests signs of NAS but whose mother denies opioid use. All toxicology testing should be performed with the mother’s consent, and any potential legal or mandatory ramifications of a positive test should be considered. Although universal maternal toxicology testing improves the identification of newborns at risk for NAS, this approach remains controversial; most hospitals use a risk-based approach for maternal toxicology testing.16,17
Newborn toxicology testing can be performed from samples of hair, urine, meconium, and umbilical cord. Although frequently used, newborn urine testing has the shortest window of detection, ie, the last few days prior to delivery (Table 1). Meconium drug testing has been considered the gold standard and can detect exposures from the last 20 weeks of gestation, providing information on chronic exposures.18 In a recent survey, 10% of hospitals reported using umbilical cord toxicology as the primary method for detecting intrauterine exposures.17 This approach allows greater ease of specimen collection but may not yield results that are exactly equivalent to meconium testing.19
MONITORING AND EVALUATION
Close monitoring for development of NAS after birth is indicated for all newborns with confirmed or suspected intrauterine opioid exposure. Although the current American Academy of Pediatrics (AAP) guidelines recommend three days of newborn observation for exposure to short-acting opioids and five to seven days for longer acting opioids, substantial variation has been described across US hospitals in policies related to newborn length of stay for NAS observation.17, 20 In most hospitals, monitoring for NAS occurs in the routine postpartum unit (ie, level 1 nursery), with transfer to the NICU if pharmacologic treatment is indicated.17
The most widely used assessment tool for NAS is the Finnegan Neonatal Abstinence Scoring System (FNASS) or the modified FNASS, which assigns points for the 21 most common opioid withdrawal symptoms based on perceived severity.17, 21 This tool allows for assessment of symptoms, helps determine need for pharmacologic intervention, and can guide monitoring of symptoms and weaning of therapy. A commonly used score cutoff of 8 is based on prior research validating scores >8 as indicative of withdrawal symptoms as opposed to normal newborn findings.22 Despite its popularity and widespread usage, FNASS has limitations, including the need for the newborn to be stimulated or disturbed to produce an accurate assessment and scoring for nonspecific signs of withdrawal, including sneezing, yawning, and stuffiness. Recent work has attempted to simplify and shorten the FNASS to elements that are unique and specific for withdrawal.23,24 Further research is needed to establish the validity of common scoring practices (ie, use of 8 as a cutoff) to determine the need for pharmacologic treatment.25
Recent studies suggest that simple, function-based assessments, such as the Eat, Sleep, Console (ESC) approach developed by Grossman and colleagues, may serve as an alternative to the FNASS for evaluating withdrawal.26,27 With ESC, the need for pharmacotherapy is evaluated by the newborn’s ability to (1) eat (breastfeed successfully or eat at least 1oz per feed), (2) sleep uninterrupted for at least one hour, and (3) be consoled within 10 minutes. To date, research on the implementation of ESC has primarily focused on reducing length of stay and need for pharmacologic treatment in the context of quality improvement initiatives.26,27 Further prospective studies are warranted that compare ESC to traditional approaches involving the FNASS, and that evaluate post-discharge outcomes including newborn weight gain, ongoing withdrawal symptoms at home, and readmission.
NONPHARMACOLOGIC TREATMENT
In recent years, research increasingly supports the critical role of nonpharmacological care in management of all opioid-exposed newborns, regardless of NAS severity.11,27, 28 Rooming-in of mothers or caregivers has been shown to decrease the need for pharmacologic treatment, shorten the length of stay, and reduce hospital costs.28,29 Other well-established practices include maintaining a low stimulus environment for infants with low lighting and sound, swaddling, maximizing caregiver contact with kangaroo care and skin to skin, and minimizing interventions. Therapeutic modalities, such as massage and music therapy, have been used for infants with NAS, but no evidence has supported their use. Recent studies have increasingly supported the use of acupuncture as an emerging modality in treating NAS. 30
Feeding
Breastfeeding is encouraged for mothers who are stable on their methadone or buprenorphine maintenance treatment, are not using heroin or other illicit drugs, and have no other contraindications to breastfeeding, such as human immunodeficiency virus.31 Despite the known benefits of breastfeeding, which include decreased NAS severity, decreased need for pharmacological treatment, and shortened length of hospital stay, breastfeeding rates among mothers with OUD are low.31 Hospital policies that can promote maternal success in breastfeeding include tailored breastfeeding support, rooming in, and early, consistent maternal education on the benefits and safety of breastfeeding.32 A small percentage of hospitals use donor breastmilk for this population, although data on outcomes are limited.17 For formula-fed newborns, emerging research suggests that early initiation of high-calorie (22-24 kcal/ounce) formula may be beneficial to prevent excessive weight loss and poor weight gain after intrauterine opioid exposure.33
PHARMACOLOGIC TREATMENT
When supportive therapy fails to adequately control symptoms of withdrawal, pharmacological management is initiated to improve infant discomfort, allow for adequate feeding and nutrition, and facilitate parental bonding (Table 2).11 Opioids are the primary agent used for pharmacologic treatment, and morphine is the most commonly utilized.17 Morphine is a short-acting opioid and can be prescribed either as a weight-based weaning protocol or symptom-based regimen. Methadone is also widely used, and as a long-acting opioid, it has the advantage of twice daily dosing after the initial loading dose. Recently, buprenorphine, a partial mu opioid agonist with a long half-life, has emerged as a promising primary opioid treatment agent and has been shown to reduce the length of stay and the number of opioid treatment days compared with morphine and methadone.36
When the signs and symptoms of NAS are not effectively controlled with a primary opioid or in the case of polysubstance exposure, adjunctive agents are often used, with phenobarbital and clonidine being the most common (Table 3).11 Regardless of opioid agent used, multicenter quality improvement initiatives demonstrate that having a standardized weaning protocol is critical to minimizing the overall length of stay and reducing the need for adjunctive agents.38,39 Additionally, modeling tools such as pharmacometrics for methadone and buprenorphine have shown promise in optimizing dose selection.40,41 Modeling may include pharmacodynamic data (ie, clinical response to treatment), pharmacokinetics (ie, measures of drug distribution and clearance), and other factors, such as patient demographics, intrauterine exposure type, and symptom severity. Future studies should examine weight versus symptom-based dosing regimens as well as compare weaning schedules versus “as needed” dosing regimens.11
PSYCHOSOCIAL CONSIDERATIONS
The need for comprehensive medical and psychosocial supports for mothers with OUD cannot be overstated, given the high rates of concurrent illicit or other substance use, comorbid depression and anxiety, physical and sexual trauma, poverty and homelessness, intravenous drug use, and sex-related risk patterns.15 Significant issues of healthcare-associated stigma and criminality also affect this population. As of 2019, 23 states and the District of Columbia classify substance use during pregnancy as child abuse under civil child-welfare statutes, potentially resulting in termination of parental rights.42 Studies of mothers with OUD have demonstrated that they often experience guilt, shame, and fear of loss of custody, all of which can impede their trust in hospital providers and future engagement in care.43 They also report frustration with and mistrust of NAS scoring assessments, which they can perceive to be disruptive and potentially biased.44 Multiple approaches should be considered to standardize and improve the hospital experience for this population, in a way that emphasizes the mother’s role as a capable, respected participant in her newborn’s care.
Maternal Support
A coordinated, multidisciplinary approach to comprehensively support mothers with OUD should involve team members from pediatrics, neonatology, obstetrics, nursing, social work, case management, and lactation.35 This support includes screening for adequate resources and a safe, supportive, drug-free home environment as well as evaluating co-occurring mental health conditions. Referrals should be provided as needed to social services, postpartum psychiatry or behavioral health services, OUD treatment and relapse-prevention programs, and harm reduction services (eg, naloxone training). In addition to the healthcare team, other community members can be enlisted to serve as a trusted, consistent, and nonjudgmental support during the hospitalization; examples may include a peer support (another mother with OUD), an OUD program caseworker, or a doula.44
Clinical Pathways
Hospitals should establish clinical pathways for women with OUD to standardize care and communication across the continuum of care for themselves and their newborn, with input from all healthcare team members involved (prenatal, intrapartum, and postpartum).35 Early, consistent information should be provided regarding expected newborn hospital course, including toxicology testing, NAS monitoring, possible NICU admission, and involvement of social work.
Provider Training
Educational opportunities in the form of continuing medical education, in-service trainings, etc., should be provided for clinical staff who care for mothers with OUD and their newborns, regarding issues of substance use, stigma, bias, and trauma-informed care.35 Online training resources are available through the American Society of Addiction Medicine, the ACOG, AAP, the Centers for Disease Control and Prevention, and the Substance Abuse and Mental Health Services Administration.
DISCHARGE PLANNING
Regardless of whether or not NAS is treated pharmacologically, newborns with opioid exposure may experience residual symptoms of withdrawal that persist for months.4 Current research suggests increased risk for morbidity, emergency department utilization, and rehospitalization after discharge in this population as well as difficulty in accessing and engaging with pediatric preventative care.45, 46
A clear plan should be established upon discharge to ensure optimal newborn care and follow-up. A complete record of the newborn’s hospital stay, including maternal toxicology screenings and summary of any social work documentation, should be communicated to the primary care provider upon discharge. Close postdischarge monitoring involves addressing parenting knowledge gaps, assessing illness and injury risk, and evaluating for the presence of ongoing withdrawal symptoms.4 Primary care providers can also play a key role in assessing maternal stress, coping, and parenting skills as well as helping families connect to resources. Further research is warranted on how pediatric primary care systems can better build maternal trust, address parenting needs, and engage this population in routine well-child care.47
Child Welfare, Early Intervention, and Other Services
In general, newborn safety and keeping families intact should be prioritized, with disposition into foster care only in cases of concern for child maltreatment or neglect. Under the Child Abuse Prevention and Treatment Act (CAPTA), states are required to develop Plans of Safe Care for women and newborns affected by OUD, with the goal of fostering collaboration between healthcare and social service organizations around care of these families.48 Given the variable interpretation of Plans of Safe Care across the U.S., providers should be knowledgeable about state and local statutes and reporting requirements related to parental substance use.
As part of Plans of Safe Care, providers may be well-positioned to initiate referrals for early intervention, home visiting, and other programs designed to provide developmental or wrap-around support for families. Under Part C of the Individuals with Disabilities Education Act, many states offer early intervention on the basis of NAS as an automatic qualifying diagnosis; however, attrition of eligible families along the referral and enrollment process is substantial.49 A standardized approach to discharging opioid-exposed newborns includes referrals to available resources and discussion of their importance with families and may increase utilization and decrease variation in care.50
CONCLUSION
Maternal OUD presents a unique combination of medical and psychosocial challenges that affect hospital care for mothers and their newborns. Optimal care for this population warrants a multidisciplinary team of providers who are knowledgeable, collaborative, and mindful of the important role of the mother as a key participant in her newborn’s care. Despite a large and growing body of research focused on NAS prevention, screening, and treatment, ongoing efforts are needed to create hospital policies and clinical pathways that are responsive to the healthcare needs of this population, navigate sensitive issues of criminality and stigma, and ultimately support maternal parenting success.
Disclosures
The authors have no financial relationships and conflicts of interest relevant to this article to disclose.
Funding
Funding for this work was provided by Cincinnati Children’s Hospital Medical Center and Nemours/AI duPont Hospital for Children.
2. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126. https://doi.org/10.1056/NEJMsa1500439.
3. Devlin LA, Davis JM. A practical approach to neonatal opiate withdrawal syndrome. Am J Perinatol. 2018;35(4):324-330. https://doi.org/10.1055/s-0037-1608630.
4. Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547-e561. https://doi.org/10.1542/peds.2013-3524.
5. Kaltenbach K, Holbrook AM, Coyle MG, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction. 2012;107 Supplement 1:45-52. https://doi.org/10.1111/j.1360-0443.2012.04038.x.
6. Huybrechts KF, Bateman BT, Desai RJ, et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: cohort study. BMJ. 2017;358:j3326. https://doi.org/10.1136/bmj.j3326.
7. Wachman EM, Hayes MJ, Brown MS, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309(17):1821-1827. https://doi.org/10.1001/jama.2013.3411.
8. Mactier H, McLaughlin P, Gillis C, Osselton MD. Variations in infant CYP2B6 genotype associated with the need for pharmacological treatment for neonatal abstinence syndrome in infants of methadone-maintained opioid-dependent mothers. Am J Perinatol. 2017;34(9):918–921. https://doi.org/10.1055/s-0037-1600917.
9. Jilani SM, Frey MT, Pepin D, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome - six states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(1):6-10. https://doi.org/10.15585/mmwr.mm6801a2.
10. Maalouf FI, Cooper WO, Stratton SM, et al. Positive predictive value of administrative data for neonatal abstinence syndrome. Pediatrics. 2019;143(1). https://doi.org/10.1542/peds.2017-4183.
11. Mangat AK, Schmölzer GM, Kraft WK. Pharmacological and non-pharmacological treatments for the Neonatal Abstinence Syndrome (NAS). Semin Fetal Neonat Med. 2019;24(2):133-141. https://doi.org/10.1016/j.siny.2019.01.009.
12. Ko JY, Wolicki S, Barfield WD, et al. CDC Grand Rounds: public health strategies to prevent neonatal abstinence syndrome. MMWR Morb Mortal Wkly Rep. 2017;66(9):242-245. https://doi.org/10.15585/mmwr.mm6609a2.
13. Patrick SW, Faherty LJ, Dick AW, et al. Association Among County-Level economic factors, clinician supply, metropolitan or rural location, and neonatal abstinence syndrome. JAMA. 2019;321(4):385-393. https://doi.org/10.1001/jama.2018.20851.
14. Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW. Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr. 2017;171(2):194-196. https://doi.org/10.1001/jamapediatrics.2016.3750.
15. Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet Gynecol. 2017;130(2):e81-e94. https://doi.org/10.1097/AOG.0000000000002235.
16. Wexelblatt SL, Ward LP, Torok K, et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
17. Bogen DL, Whalen BL, Kair LR, Vining M, King BA. Wide variation found in care of opioid-exposed newborns. Acad Pediatr. 2017;17(4):374-380. https://doi.org/10.1016/j.acap.2016.10.003.
18. Cotten SW. Drug testing in the neonate. Clin Lab Med. 2012;32(3):449-466. https://doi.org/10.1016/j.cll.2012.06.008.
19. Colby JM, Adams BC, Morad A, Presley LD, Patrick SW. Umbilical cord tissue and meconium may not be equivalent for confirming in utero substance exposure. J Pediatr. 2019;205:277-280. https://doi.org/10.1016/j.jpeds.2018.09.046.
20. Hudak ML, Tan RC, COMMITTEE ON DRUGS, COMMITTEE ON FETUS AND NEWBORN, American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560. https://doi.org/10.1542/peds.2011-3212.
21. Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1-2):141-158.
22. Zimmermann-Baer U, Nötzli U, Rentsch K, Bucher HU. Finnegan neonatal abstinence scoring system: normal values for first 3 days and weeks 5-6 in non-addicted infants. Addiction. 2010;105(3):524-528. https://doi.org/10.1111/j.1360-0443.2009.02802.x.
23. Jones HE, Seashore C, Johnson E, et al. Measurement of neonatal abstinence syndrome: evaluation of short forms. J Opioid Manag. 2016;12(1):19-23. https://doi.org/10.5055/jom.2016.0308.
24. Isemann BT, Stoeckle EC, Taleghani AA, Mueller EW. Early prediction tool to identify the need for pharmacotherapy in infants at risk of neonatal abstinence syndrome. Pharmacotherapy. 2017;37(7):840-848. https://doi.org/10.1002/phar.1948.
25. Schiff DM, Grossman MR. Beyond the Finnegan scoring system: novel assessment and diagnostic techniques for the opioid-exposed infant. Semin Fetal Neonat Med. 2019;24(2):115-120. https://doi.org/10.1016/j.siny.2019.01.003.
26. Grossman MR, Berkwitt AK, Osborn RR, et al. An initiative to improve the quality of care of infants With neonatal abstinence syndrome. Pediatrics. 2017;139(6). https://doi.org/10.1542/peds.2016-3360.
27. Wachman EM, Grossman M, Schiff DM, et al. Quality improvement initiative to improve inpatient outcomes for Neonatal Abstinence Syndrome. J Perinatol. 2018;38(8):1114-1122. https://doi.org/10.1038/s41372-018-0109-8.
28. Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6). https://doi.org/10.1542/peds.2015-2929.
29. MacMillan KDL, Rendon CP, Verma K, et al. Association of rooming-in With outcomes for neonatal abstinence syndrome: A systematic review and meta-analysis. JAMA Pediatr. 2018 Apr 1;172(4):345-351. https://doi.org/10.1001/jamapediatrics.2017.5195.
30. Jackson HJ, Lopez C, Miller S, Engelhardt B. A scoping review of acupuncture as a potential intervention for neonatal abstinence syndrome. Med Acupunct. 2019;31(2):69-84. https://doi.org/10.1089/acu.2018.1323.
31. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: Guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. https://doi.org/10.1089/bfm.2015.9992.
32. Krans EE, Campopiano M, Cleveland LM, et al. National partnership for maternal safety: consensus bundle on obstetric care for women With opioid use disorder. Obstet Gynecol. 2019;134(2):365-375. https://doi.org/10.1097/AOG.0000000000003381.
33. Bogen DL, Hanusa BH, Baker R, Medoff-Cooper B, Cohlan B. Randomized clinical trial of standard- Versus high-calorie formula for methadone-exposed infants: A feasibility study. Hosp Pediatr. 2018;8(1):7-14. https://doi.org/10.1542/hpeds.2017-0114.
34. Lexicomp. Opioids, Urine, Screen and Confirmation. https://online.lexi.com/lco/action/doc/retrieve/docid/lthdph/382929. Accessed September 4, 2019.
35. Mayo Clinic Laboratories. Opiates. https://www.mayocliniclabs.com/test-info/drug-book/opiates.html. Accessed Sept 4, 2019.
36. Kraft WK, Adeniyi-Jones SC, Chervoneva I, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med. 2017;376(24):2341-2348. https://doi.org/10.1056/NEJMoa1614835.
37. Lexicomp. https://online.lexi.com/lco/action/home. Accessed September 4, 2019
38. Hall ES, Wexelblatt SL, Crowley M, et al. Implementation of a neonatal abstinence syndrome weaning protocol: A multicenter cohort study. Pediatrics. 2015;136(4):e803-e810. https://doi.org/10.1542/peds.2015-1141.
39. Patrick SW, Schumacher RE, Horbar JD, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016;137(5):38. https://doi.org/10.1542/peds.2015-3835.
40. Wiles JR, Isemann B, Mizuno T, et al. Pharmacokinetics of oral methadone in the treatment of neonatal abstinence syndrome: A pilot study. J Pediatr. 2015;167(6):1214–20.e3. https://doi.org/10.1016/j.jpeds.2015.08.032.
41. Ng CM, Dombrowsky E, Lin H, et al. Population pharmacokinetic model of sublingual buprenorphine in neonatal abstinence syndrome. Pharmacotherapy. 2015;35(7):670-680. https://doi.org/10.1002/phar.1610.
42. The Guttmacher Institute. Substance abuse During pregnancy. https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy. Accessed November 20, 2019; Updated November 1, 2019.
43. Cleveland LM, Bonugli R. Experiences of mothers of infants with neonatal abstinence syndrome in the neonatal intensive care unit. J Obstet Gynecol Neonat Nurs. 2014;43(3):318-329. https://doi.org/10.1111/1552-6909.12306.
44. Rockefeller K, Macken LC, Craig A. Trying to do what is best: A qualitative study of maternal-infant bonding and neonatal abstinence syndrome. Adv Neonat Care. 2019;19(5):E3-E15. https://doi.org/10.1097/ANC.0000000000000616.
45. Liu G, Kong L, Leslie DL, Corr TE. A longitudinal healthcare use profile of children with a history of neonatal abstinence syndrome. J Pediatr. 2019;204:111-117. https://doi.org/10.1016/j.jpeds.2018.08.032.
46. Goyal NK, Rhode JF, Short V, et al. Well child care adherence during the first 2 years of life after intrauterine opioid exposure. Pediatrics. In press.
47. Short VL, Goyal NK, Chung EK, Hand DJ, Abatemarco DJ. Perceptions of pediatric primary care among mothers in treatment for opioid use disorder. J Commun Health. 2019 Dec;44(6):1127-1134. https://doi.org/10.1007/s10900-019-00701-1.
48. Plans of Safe Care. Administration for Children and Families. https://www.acf.hhs.gov/sites/default/files/cb/pi1702.pdf. Accessed September 1, 2019.
49. Peacock-Chambers E, Leyenaar JK, Foss S, et al. Early Intervention referral and enrollment among infants with neonatal abstinence syndrome. J Dev Behav Pediatr. 2019;40(6):441-450. https://doi.org/10.1097/DBP.0000000000000679.
50. Crook TW, Munn EK, Scott TA, et al. Improving the discharge process for opioid-exposed neonates. Hosp Pediatr. 2019;9(8):643-648. https://doi.org/10.1542/hpeds.2019-0088.
2. Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372(22):2118-2126. https://doi.org/10.1056/NEJMsa1500439.
3. Devlin LA, Davis JM. A practical approach to neonatal opiate withdrawal syndrome. Am J Perinatol. 2018;35(4):324-330. https://doi.org/10.1055/s-0037-1608630.
4. Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2):e547-e561. https://doi.org/10.1542/peds.2013-3524.
5. Kaltenbach K, Holbrook AM, Coyle MG, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction. 2012;107 Supplement 1:45-52. https://doi.org/10.1111/j.1360-0443.2012.04038.x.
6. Huybrechts KF, Bateman BT, Desai RJ, et al. Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: cohort study. BMJ. 2017;358:j3326. https://doi.org/10.1136/bmj.j3326.
7. Wachman EM, Hayes MJ, Brown MS, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309(17):1821-1827. https://doi.org/10.1001/jama.2013.3411.
8. Mactier H, McLaughlin P, Gillis C, Osselton MD. Variations in infant CYP2B6 genotype associated with the need for pharmacological treatment for neonatal abstinence syndrome in infants of methadone-maintained opioid-dependent mothers. Am J Perinatol. 2017;34(9):918–921. https://doi.org/10.1055/s-0037-1600917.
9. Jilani SM, Frey MT, Pepin D, et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome - six states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(1):6-10. https://doi.org/10.15585/mmwr.mm6801a2.
10. Maalouf FI, Cooper WO, Stratton SM, et al. Positive predictive value of administrative data for neonatal abstinence syndrome. Pediatrics. 2019;143(1). https://doi.org/10.1542/peds.2017-4183.
11. Mangat AK, Schmölzer GM, Kraft WK. Pharmacological and non-pharmacological treatments for the Neonatal Abstinence Syndrome (NAS). Semin Fetal Neonat Med. 2019;24(2):133-141. https://doi.org/10.1016/j.siny.2019.01.009.
12. Ko JY, Wolicki S, Barfield WD, et al. CDC Grand Rounds: public health strategies to prevent neonatal abstinence syndrome. MMWR Morb Mortal Wkly Rep. 2017;66(9):242-245. https://doi.org/10.15585/mmwr.mm6609a2.
13. Patrick SW, Faherty LJ, Dick AW, et al. Association Among County-Level economic factors, clinician supply, metropolitan or rural location, and neonatal abstinence syndrome. JAMA. 2019;321(4):385-393. https://doi.org/10.1001/jama.2018.20851.
14. Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW. Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr. 2017;171(2):194-196. https://doi.org/10.1001/jamapediatrics.2016.3750.
15. Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet Gynecol. 2017;130(2):e81-e94. https://doi.org/10.1097/AOG.0000000000002235.
16. Wexelblatt SL, Ward LP, Torok K, et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
17. Bogen DL, Whalen BL, Kair LR, Vining M, King BA. Wide variation found in care of opioid-exposed newborns. Acad Pediatr. 2017;17(4):374-380. https://doi.org/10.1016/j.acap.2016.10.003.
18. Cotten SW. Drug testing in the neonate. Clin Lab Med. 2012;32(3):449-466. https://doi.org/10.1016/j.cll.2012.06.008.
19. Colby JM, Adams BC, Morad A, Presley LD, Patrick SW. Umbilical cord tissue and meconium may not be equivalent for confirming in utero substance exposure. J Pediatr. 2019;205:277-280. https://doi.org/10.1016/j.jpeds.2018.09.046.
20. Hudak ML, Tan RC, COMMITTEE ON DRUGS, COMMITTEE ON FETUS AND NEWBORN, American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560. https://doi.org/10.1542/peds.2011-3212.
21. Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1-2):141-158.
22. Zimmermann-Baer U, Nötzli U, Rentsch K, Bucher HU. Finnegan neonatal abstinence scoring system: normal values for first 3 days and weeks 5-6 in non-addicted infants. Addiction. 2010;105(3):524-528. https://doi.org/10.1111/j.1360-0443.2009.02802.x.
23. Jones HE, Seashore C, Johnson E, et al. Measurement of neonatal abstinence syndrome: evaluation of short forms. J Opioid Manag. 2016;12(1):19-23. https://doi.org/10.5055/jom.2016.0308.
24. Isemann BT, Stoeckle EC, Taleghani AA, Mueller EW. Early prediction tool to identify the need for pharmacotherapy in infants at risk of neonatal abstinence syndrome. Pharmacotherapy. 2017;37(7):840-848. https://doi.org/10.1002/phar.1948.
25. Schiff DM, Grossman MR. Beyond the Finnegan scoring system: novel assessment and diagnostic techniques for the opioid-exposed infant. Semin Fetal Neonat Med. 2019;24(2):115-120. https://doi.org/10.1016/j.siny.2019.01.003.
26. Grossman MR, Berkwitt AK, Osborn RR, et al. An initiative to improve the quality of care of infants With neonatal abstinence syndrome. Pediatrics. 2017;139(6). https://doi.org/10.1542/peds.2016-3360.
27. Wachman EM, Grossman M, Schiff DM, et al. Quality improvement initiative to improve inpatient outcomes for Neonatal Abstinence Syndrome. J Perinatol. 2018;38(8):1114-1122. https://doi.org/10.1038/s41372-018-0109-8.
28. Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6). https://doi.org/10.1542/peds.2015-2929.
29. MacMillan KDL, Rendon CP, Verma K, et al. Association of rooming-in With outcomes for neonatal abstinence syndrome: A systematic review and meta-analysis. JAMA Pediatr. 2018 Apr 1;172(4):345-351. https://doi.org/10.1001/jamapediatrics.2017.5195.
30. Jackson HJ, Lopez C, Miller S, Engelhardt B. A scoping review of acupuncture as a potential intervention for neonatal abstinence syndrome. Med Acupunct. 2019;31(2):69-84. https://doi.org/10.1089/acu.2018.1323.
31. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: Guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. https://doi.org/10.1089/bfm.2015.9992.
32. Krans EE, Campopiano M, Cleveland LM, et al. National partnership for maternal safety: consensus bundle on obstetric care for women With opioid use disorder. Obstet Gynecol. 2019;134(2):365-375. https://doi.org/10.1097/AOG.0000000000003381.
33. Bogen DL, Hanusa BH, Baker R, Medoff-Cooper B, Cohlan B. Randomized clinical trial of standard- Versus high-calorie formula for methadone-exposed infants: A feasibility study. Hosp Pediatr. 2018;8(1):7-14. https://doi.org/10.1542/hpeds.2017-0114.
34. Lexicomp. Opioids, Urine, Screen and Confirmation. https://online.lexi.com/lco/action/doc/retrieve/docid/lthdph/382929. Accessed September 4, 2019.
35. Mayo Clinic Laboratories. Opiates. https://www.mayocliniclabs.com/test-info/drug-book/opiates.html. Accessed Sept 4, 2019.
36. Kraft WK, Adeniyi-Jones SC, Chervoneva I, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med. 2017;376(24):2341-2348. https://doi.org/10.1056/NEJMoa1614835.
37. Lexicomp. https://online.lexi.com/lco/action/home. Accessed September 4, 2019
38. Hall ES, Wexelblatt SL, Crowley M, et al. Implementation of a neonatal abstinence syndrome weaning protocol: A multicenter cohort study. Pediatrics. 2015;136(4):e803-e810. https://doi.org/10.1542/peds.2015-1141.
39. Patrick SW, Schumacher RE, Horbar JD, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016;137(5):38. https://doi.org/10.1542/peds.2015-3835.
40. Wiles JR, Isemann B, Mizuno T, et al. Pharmacokinetics of oral methadone in the treatment of neonatal abstinence syndrome: A pilot study. J Pediatr. 2015;167(6):1214–20.e3. https://doi.org/10.1016/j.jpeds.2015.08.032.
41. Ng CM, Dombrowsky E, Lin H, et al. Population pharmacokinetic model of sublingual buprenorphine in neonatal abstinence syndrome. Pharmacotherapy. 2015;35(7):670-680. https://doi.org/10.1002/phar.1610.
42. The Guttmacher Institute. Substance abuse During pregnancy. https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy. Accessed November 20, 2019; Updated November 1, 2019.
43. Cleveland LM, Bonugli R. Experiences of mothers of infants with neonatal abstinence syndrome in the neonatal intensive care unit. J Obstet Gynecol Neonat Nurs. 2014;43(3):318-329. https://doi.org/10.1111/1552-6909.12306.
44. Rockefeller K, Macken LC, Craig A. Trying to do what is best: A qualitative study of maternal-infant bonding and neonatal abstinence syndrome. Adv Neonat Care. 2019;19(5):E3-E15. https://doi.org/10.1097/ANC.0000000000000616.
45. Liu G, Kong L, Leslie DL, Corr TE. A longitudinal healthcare use profile of children with a history of neonatal abstinence syndrome. J Pediatr. 2019;204:111-117. https://doi.org/10.1016/j.jpeds.2018.08.032.
46. Goyal NK, Rhode JF, Short V, et al. Well child care adherence during the first 2 years of life after intrauterine opioid exposure. Pediatrics. In press.
47. Short VL, Goyal NK, Chung EK, Hand DJ, Abatemarco DJ. Perceptions of pediatric primary care among mothers in treatment for opioid use disorder. J Commun Health. 2019 Dec;44(6):1127-1134. https://doi.org/10.1007/s10900-019-00701-1.
48. Plans of Safe Care. Administration for Children and Families. https://www.acf.hhs.gov/sites/default/files/cb/pi1702.pdf. Accessed September 1, 2019.
49. Peacock-Chambers E, Leyenaar JK, Foss S, et al. Early Intervention referral and enrollment among infants with neonatal abstinence syndrome. J Dev Behav Pediatr. 2019;40(6):441-450. https://doi.org/10.1097/DBP.0000000000000679.
50. Crook TW, Munn EK, Scott TA, et al. Improving the discharge process for opioid-exposed neonates. Hosp Pediatr. 2019;9(8):643-648. https://doi.org/10.1542/hpeds.2019-0088.
© 2020 Society of Hospital Medicine
Diagnosis and Management of UTI in Febrile Infants Age 0–2 Months: Applicability of the AAP Guideline
Urinary tract infections (UTIs) are the most common bacterial infection and one of the most common reasons for hospitalization in young infants.1,2 The American Academy of Pediatrics (AAP) has published several clinical practice guidelines for the evaluation and management of febrile children ages 2-24 months with first-time UTIs, most recently in 2011 and affirmed in 2016.3 These guidelines do not provide recommendations for infants aged <2 months, which leads to uncertainty regarding the diagnosis and management of UTIs for infants in this age group. We assess the applicability of the AAP UTI Guideline’s action statements for infants aged <2 months presenting with first-time UTIs, with an emphasis on recent evidence. Because the considerations for bacterial infections differ for febrile infants aged <2 months compared with older infants, we do not discuss action statements one and two (determination of the likelihood of UTIs and decision to test urine) and statement seven (medical evaluation for fever after first UTI).3 Additionally, because concomitant bacteremia and meningitis are more common in this age group than in older infants, we review some of the controversies surrounding the diagnosis and treatment of these disease entities.
DIAGNOSIS
“Action Statement 3: To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection (pyuria and/or bacteriuria) and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.”3
To distinguish asymptomatic bacteriuria or contamination from a true UTI, the AAP Guideline requires both a positive urinalysis (UA) and culture for a diagnosis of a UTI.3 Historically, the UA was considered to be poorly sensitive for infections in young infants, with older studies reporting sensitivities ranging from 40% to 82% using urine culture as the gold standard.4-7 Thus, infants aged <2 months with positive urine cultures and negative UAs are often treated as having true UTIs, though this practice varies by institution.8 Possible explanations for the low UA sensitivity in this population include rapid bladder emptying, immature immune systems, and inability to concentrate urine. However, a negative UA plus a positive urine culture could also represent a “true negative” UA and a “false positive” culture, a finding that may be more common in young infants in whom sterile urine obtainment is often challenging.
Two recent studies have addressed this issue by evaluating the UA sensitivity in patients with bacteremic UTIs, as growth of the same pathogenic organism from the blood and urine almost certainly represents true infection.9,10 In a retrospective study of 203 infants aged <3 months with bacteremic UTIs, the presence of any leukocyte esterase (LE) or pyuria (>3 white blood cells per high-powered field [WBC/HPF]) had a sensitivity of 99.5% (95% CI: 98.5%-100%) and specificity of 93.9% (95% CI: 87.8%-93.2%).9 In a prospective, multicenter study of 4,147 febrile infants aged ≤60 days, of whom 27 infants had bacteremic UTIs, a positive UA (any LE, >5 WBC/HPF, or nitrite) had a sensitivity and specificity of 1.00 (95% CI: 0.87-1.00) and 0.91 (95% CI: 0.90-0.91), respectively.10 Although screening tests may appear to have higher sensitivity in more severely diseased populations (“spectrum bias”),11 it is not clear that infants with bacteremic UTIs are definitively sicker than infants with nonbacteremic UTIs (see “bacteremic UTI” section below). Additionally, this study found similarly excellent sensitivity (0.94 [95% CI: 0.90-0.96]) and specificity (0.91 [95% CI: 0.90-0.91]) of the UA among infants with nonbacteremic UTIs, including infants <28 days old.10
UA sensitivity (using urine culture as the gold standard) may be lower for non-Escherichia coli UTIs.9,10,12 In a retrospective study that included 90 infants <2 months old with UTIs, urine cultures yielding Pseudomonas aeruginosa, Enterococcus, or Klebsiella species were significantly less likely (odds ratio [95% CI]: 0.19 [0.06-0.60]; 0.14 [0.07-0.28]; 0.34 [0.17-0.68], respectively) to have pyuria (≥5 WBC/HPF) or LE (1+ or greater) than urine cultures yielding E. coli.,12 though an alternative explanation for this finding is that these organisms may be more likely to cause asymptomatic bacteriuria or contamination.13
The appropriate CFU/mL threshold to define a UTI and the extent that this threshold should vary by urine collection methods are still unclear. In the aforementioned bacteremic UTI study,9 12 patients with E. coli bacteremia had urine cultures with <50,000 CFU/mL plus pyuria (WBC or LE) in the UA, indicating that true UTIs may occur with <50,000 CFU/mL.
Based on these recent studies, we believe that the recommendation to incorporate UA results into the diagnoses of UTIs can be applied to infants <2 months old, as well as consideration for a UTI for colony counts of ≥10,000 CFU/mL if the UA is positive. For infants with positive urine cultures and negative UAs who have not received antibiotics, we suggest repeating both studies if treatment is being considered. For those who have started antibiotics, the pretest probability of a UTI, initial illness severity, and risks and benefits of continuing treatment should be considered.
TREATMENT
“Action Statement 4a: When initiating treatment, the clinician should base the choice of route of administration on practical considerations. Initiating treatment orally or parenterally is equally efficacious. The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.”3
Most infants <2 months old with UTIs are hospitalized initially because of fever. Therefore, the decision point for most clinicians is not whether to hospitalize but for how long to hospitalize and treat with intravenous (IV) antibiotics prior to discharging home on oral antibiotics. Although all-oral antibiotic regimens are used to treat UTIs in older infants and children,14-18 to our knowledge, there are no randomized controlled trials (RCTs) comparing all-IV vs all-oral antibiotics or a longer vs shorter initial IV course that include infants <1 month old. In the trials that do include infants aged 1-2 months,14,18 the number of subjects in this age group is too small to draw conclusions, a finding supported by a 2014 Cochrane review.19 An adequately powered RCT of different IV antibiotic durations in this age group would be challenging. For example, nearly 1,000 subjects would be needed to demonstrate a statistically significant difference between a 5% and 10% relapse risk between groups, a difference that some may find clinically important.
The paucity of evidence in this age group may explain the considerable variability in the approach to IV antibiotic duration in young infants. Concerns about enteral absorption and underdeveloped immune systems may prompt some physicians to treat the youngest patients more aggressively. One study demonstrated that the proportion of patients <2 months old receiving prolonged courses (≥4 days) of IV antibiotics for UTIs in 46 U.S. children’s hospitals ranged from 0% to 67%.20 Similar variability across hospitals has been described in other observational studies21,22 and across subspecialties in one survey of pediatricians.23
Several observational studies provide additional evidence supporting shorter IV courses. In two studies that examined administrative databases, there was no difference in treatment failure rates between infants aged <2 months20 and <6 months21 receiving longer (≥4 days) vs shorter IV courses. In a study of 172 infants <1 month old with UTIs, the median IV duration was 4 days (range 2-12 days), and no subjects experienced treatment failure or relapse.24 In a multicenter study of 251 infants <3 months old with bacteremic UTIs, mean IV antibiotic durations ranged from 5.5–12 days, and no patient had a relapsed bacteremic UTI. Six infants (2.4%) had a relapsed UTI without bacteremia, with no association between IV antibiotic duration and relapse.22
Based on the available data and known risks of hospitalization and prolonged IV therapy, a reasonable approach for infants <1 month old would be to hospitalize for two to three days while awaiting blood and cerebral spinal fluid (CSF) culture results. Given the possibility of Enterococcus or Enterobacteriaceae that are resistant to third-generation cephalosporins, standard therapy of ampicillin and gentamicin for febrile neonates is reasonable, assuming there is no concern for meningitis. Antibiotics should be narrowed when susceptibilities are known. Once culture results return and signs and symptoms have resolved, discharge home on oral antibiotics is justifiable based on the available literature. For well-appearing infants aged 1-2 months with a presumptive UTI (based on UA results), if hospitalization is not warranted for other reasons, then we recommend outpatient treatment with oral or intramuscular therapy based on local susceptibilities (typically a cephalosporin) and close follow-up for one to two days while awaiting culture results. Although empiric cephalosporin therapy may not provide 100% coverage for all potential organisms, clinical deterioration is uncommon in infants and children receiving discordant therapy.25
“Action Statement 4b: The clinician should choose 7 to 14 days as the duration of antimicrobial therapy.”3
The AAP’s recommendation to provide antibiotics (by oral or parenteral route) for a minimum of seven days total stems from a 2002 meta-analysis comparing long (7-14 days) vs short (≤3 days) courses, where the pooled relative risk of treatment failure with short-course therapy was 1.94 (95% CI: 1.19-3.15).26 However, in this analysis, the trials that demonstrated inferiority with short courses were all trials that used single doses of antibiotics, and a similar Cochrane review comparing 2-4 days with 7-14 days demonstrated no differences in outcomes.27 Therefore, shorter total courses, but not a single dose, are probably appropriate for most UTIs in children. Although there are no obvious biologic reasons why longer total courses would be needed in young infants, there are unfortunately limited data comparing different total antibiotic durations in this age group. We believe that 7-14 days of total therapy is a reasonable recommendation for infants <2 months old, and that future studies should investigate shorter total courses.
IMAGING
“Action Statement 5: Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS).”3
The AAP Guideline acknowledges that the RBUS is a poor screening test for the detection of genitourinary abnormalities in infants.3 The RBUS can be normal in infants with vesicoureteral reflux (VUR) or show nonspecific findings of unclear clinical significance.28 In a prospective study of 220 infants <3 months old by Tsai et al, 9/39 infants (23%) with grade III-V VUR had normal RBUS.29 Studies that included older infants have found a similar false-negative rate of 0%-40% for detecting grade IV-V VUR by RBUS.28 Nonetheless, since a RBUS is safe and noninvasive, we feel that the benefits of screening for abnormalities such as hydronephrosis (that could indicate posterior urethral valves or ureteropelvic junction obstruction) outweigh the risks (eg, false positives, overdiagnosis, and cost) of performing a RBUS after a first-time UTI.
“Action Statement 6a: Voiding cystourethrography (VCUG) should not be performed routinely after the first febrile UTI; VCUG is indicated if RBUS reveals hydronephrosis, scarring, or other findings that would suggest either high-grade VUR or obstructive uropathy, as well as in other atypical or complex clinical circumstances.”3
“Action Statement 6b: Further evaluation should be conducted if there is a recurrence of febrile UTI.”3
The RBUS may be normal in infants with VUR. Therefore, the AAP’s recommendation to perform a VCUG only if the RBUS is abnormal or after a recurrent UTI concedes that there will be infants with VUR who are missed after the first UTI.3
The United Kingdom’s National Institute for Health and Care Excellence guideline recommends a VCUG for infants <6 months old with a bacteremic or non-E. coli UTI.30 Whether high-grade VUR is more common in young infants with bacteremic UTIs than nonbacteremic UTIs remains inconclusive. In the Honkinen et al. study that included 87 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR (10%) and obstruction (7%) was higher than that of the 88 nonbacteremic infants (2% grade IV-V VUR and 2% with obstruction). In the multicenter study of 251 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR was 12.1%.31 This is higher than that of the nonbacteremic infants in Honkinen et al.’s study32 but more similar to the prevalence of grade IV-V VUR found in Tsai et al. (8.2%) and Ismaili et al.’s (7.0%) studies of UTIs in general.29,33
There does appear to be a higher prevalence of urinary tract abnormalities in young infants with non-E. coli vs E. coli UTIs.31,32,34,35 The odds of an abnormal VCUG was 8.0 (95% CI: 2.3-28) times higher for non-E. coli than E. coli UTIs in the study of 251 bacteremic infants.31 In a study of 122 infants <3 months old, the odds of grade III-V VUR was 10 (95% CI 2.6-41) times higher for non-E. coli than E. coli UTIs.35
However, the need for early detection of VUR is controversial, and VCUGs are invasive, involve ionizing radiation, and may require sedation. Two recent trials (one which included only children with VUR and another in which 42% of subjects had VUR) demonstrated a modest effect of prophylactic antibiotics in preventing recurrent UTIs (>5,000 doses of antibiotics needed to prevent one UTI recurrence), but the effect size did not differ by the presence or degree of VUR, and neither demonstrated any benefit in reducing future renal scarring.36, 37 The benefit of surgical interventions for VUR also remains unclear, though studies are limited.38 Overall, there is no evidence suggesting that infants <2 months old require more vigilance for VUR detection than the 2-24 month age group.
SPECIAL CONSIDERATIONS
Bacteremic UTI
The prevalence of bacteremia in infants ≤60 days old with UTIs was 9% in a study conducted from 2008 to 2013 in 26 EDs and has ranged from 3% to 17% in older studies.10, 22 Many studies have described similar clinical and laboratory findings in young infants with bacteremic and nonbacteremic UTIs.39-41 Despite this, bacteremic UTIs have been associated with prolonged parenteral antibiotic courses, resulting in longer hospitalizations and increased costs.40 Two recent multicenter studies of infants with bacteremic UTIs (251 infants <3 months old22 and 115 infants ≤60 days old42) demonstrated variable IV courses and no association between IV duration and relapsed UTI. The latter study showed no risk difference in the adjusted 30-day UTI recurrence (risk difference 3%, 95% CI: −5.8 to 12.7) or all-cause reutilization (risk difference 3%, 95% CI: −14.5 to 20.6) between long and short IV groups.42 Neither study had patients with relapsed bacteremic UTIs or reported that patients suffered clinical deterioration while on oral antibiotics.22,42
Based on these data demonstrating that adverse outcomes are rare in infants with bacteremic UTIs and not associated with parenteral antibiotic duration, we recommend short parenteral courses (2-3 days) with conversion to oral therapy once infants have clinically improved.
Positive Urinalysis and Testing for Meningitis
Multiple risk stratification algorithms for febrile infants aged ≤60 days categorize infants with a positive UA (and therefore likely UTI) as high-risk for having concomitant bacteremia or meningitis, for which lumbar puncture (LP) is typically recommended.43-45 The risk of not testing CSF is the potential to insufficiently treat meningitis because treatment for UTIs and meningitis differ in dosing, route, and duration. Recent studies have challenged the practice of routine LPs for infants aged 1-2 months with a suspected UTI due to the low prevalence (0%-0.3%) of concomitant meningitis.39,46-48 A meta-analysis of 20 studies reporting rates of concomitant meningitis with UTI in infants aged 29-90 days found a pooled prevalence of 0.25% (95% CI: 0.09%-0.70%).49 Furthermore, a study of febrile infants ages 29-60 days found that the prevalence of meningitis did not differ between those with a positive vs negative UA (3/337 [0.9%] vs 5/498 [1.0%], respectively), suggesting that a positive UA alone should not modify the pretest probability of meningitis in this age group.50
Two studies have also examined the risk of delayed meningitis among infants ≤60 days old treated for UTIs without CSF testing. A northern California study that examined 345 episodes among 341 UA-positive infants aged 29-60 days found zero cases (95% CI: 0%-1.1%) of delayed meningitis within 30 days of evaluation.50 A multicenter study of well-appearing febrile infants aged 7-60 days found 0/505 cases (95% CI: 0%-0.6%) of delayed meningitis within 7 days of discharge; 407 (81%) were aged 31-60 days.51 In summary, studies have shown a low rate of concomitant meningitis and a low risk of delayed meningitis in infants aged 1-2 months treated for UTI without CSF testing. Given this, clinically targeted (eg, based on ill appearance and/or lethargy), rather than routine, CSF testing in this age group can be considered.
CONCLUSION
While the AAP UTI Guideline is directed toward 2-24-month-old infants, recent evidence suggests that action statements 3-6 apply to infants <2 months old. Incorporation of pyuria as a diagnostic criterion for UTIs, early transition to oral therapy, and selective VCUG testing are all warranted based on the available evidence and consideration of known risks and benefits. Future studies with larger sample sizes that include infants <2 months old would be beneficial to ensure that the available studies, which have relatively small cohorts, do not suffer from type II error. We propose that future studies examine shorter (<7 days) vs longer total antibiotic duration, shorter vs longer initial IV antibiotics (especially in infants <1 month old or with bacteremic UTIs), and whether RBUS can be performed in a targeted manner. RCTs comparing universal vs targeted imaging strategies would help ascertain whether the increased diagnostic yield that accompanies more aggressive imaging strategies translates into improved outcomes. Application of these AAP guidelines to the <2-month age group and enhancement of the evidence base can promote the high-value care of young infants with UTIs.
1. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. https://doi.org/10.1097/INF.0000000000000225.
2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. https://doi.org/10.1007/s00467-010-1625-8.
3. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):1-5. https://doi.org/10.1542/peds.2016-3026.
4. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363-367. https://doi.org/10.1542/peds.105.2.e20
5. Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants <or= 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12-14. https://doi.org/10.1097/00006565-200202000-00004.
6. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. https://doi.org/10.1001/archpedi.155.1.60.
7. Reardon JM, Carstairs KL, Rudinsky SL, Simon LV, Riffenburgh RH, Tanen DA. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27(8):930-932. https://doi.org/10.1016/j.ajem.2008.07.015.
8. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. https://doi.org/10.12788/jhm.3120.
9. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. https://doi.org/10.1542/peds.2015-0012.
10. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068.
11. Newman TB, Kohn MA. Evidence-based diagnosis. Practical Guides to Biostatistics and Epidemiology. Cambridge; New York: Cambridge University Press, 2009.
12. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association Between Uropathogen and Pyuria. Pediatrics. 2016;138(1):e20160087. https://doi.org/10.1542/peds.2016-0087.
13. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr. 2016;55(9):819-824. https://doi.org/10.1177/0009922815608278.
14. Bocquet N, Sergent Alaoui A, Jais JP, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129(2):e269-e275. https://doi.org/10.1542/peds.2011-0814.
15. Bouissou F, Munzer C, Decramer S, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics. 2008;121(3):e553-e560. https://doi.org/10.1542/peds.2006-3632.
16. Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2007(4):CD003772. https://doi.org/10.1002/14651858.CD003772.pub3.
17. Neuhaus TJ, Berger C, Buechner K, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. Eur J Pediatr. 2008;167(9):1037-1047. https://doi.org/10.1007/s00431-007-0638-1
18. Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. https://doi.org/10.1542/peds.104.1.79.
19. Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014(7):CD003772. https://doi.org/10.1002/14651858.CD003772.pub4.
20. Lewis-de Los Angeles WW, Thurm C, Hersh AL, et al. Trends in intravenous antibiotic duration for urinary tract infections in young infants. Pediatrics. 2017;140(6):e20171021. https://doi.org/10.1542/peds.2017-1021.
21. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203. https://doi.org/10.1542/peds.2009-2948.
22. Schroeder AR, Shen MW, Biondi EA, et al. Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. https://doi.org/10.1136/archdischild-2014-307997.
23. Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. https://doi.org/10.1542/hpeds.2017-0082.
24. Magin EC, Garcia-Garcia JJ, Sert SZ, Giralt AG, Cubells CL. Efficacy of short-term intravenous antibiotic in neonates with urinary tract infection. Pediatr Emerg Care. 2007;23(2):83-86. https://doi.org/10.1097/PEC.0b013e3180302c47.
25. Wang ME, Lee V, Greenhow TL, et al. Clinical response to discordant therapy in third-generation cephalosporin-resistant UTIs. Pediatrics. 2019; In press.
26. Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70. https://doi.org/10.1542/peds.109.5.e70.
27. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003(1):CD003966. https://doi.org/10.1002/14651858.CD003966.
28. Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract I. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749-e770. https://doi.org/10.1542/peds.2011-1332.
29. Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955-963. https://doi.org/10.1007/s00467-012-2104-1.
30. National Institue for Health and Care Excellence. Urinary Tract Infection in Children. http://www.nice.org.uk/guidance/cg54/evidence/cg54-urinary-tract-infection-in-children-full-guideline2. Published August 2007. Accessed August 2019.
31. Chang PW, Abidari JM, Shen MW, et al. Urinary imaging findings in young infants with bacteremic urinary tract infection. Hosp Pediatr. 2016;6(11):647-652. https://doi.org/10.1542/hpeds.2015-0229.
32. Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19(7):630-634. https://doi.org/10.1097/00006454-200007000-00009
33. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158(1):91-94. https://doi.org/10.1016/j.jpeds.2010.06.053.
34. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr. 2004;43(7):619-625. https://doi.org/10.1177/000992280404300706.
35. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. https://doi.org/10.1136/archdischild-2016-311587.
36. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748-1759. https://doi.org/10.1056/NEJMoa0902295.
37. Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367-2376. https://doi.org/10.1056/NEJMoa1401811.
38. Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2019;(2):CD001532. https://doi.org/10.1002/14651858.CD001532.pub4.
39. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479,
40. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. https://doi.org/10.1542/hpeds.2014-0051.
41. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. https://doi.org/10.1001/archpedi.156.1.44.
42. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844,
43. Gomez B, Mintegi S, Bressan S, et al. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381.
44. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501.
45. DePorre AG, Aronson PL, McCulloh RJ. Facing the ongoing challenge of the febrile young infant. Crit Care. 2017;21(1):68. https://doi.org/10.1186/s13054-017-1646-9,
46. Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6(11):e26576. https://doi.org/10.1371/journal.pone.0026576.
47. Thomson J, Cruz AT, Nigrovic LE, et al. Concomitant bacterial meningitis in infants with urinary tract infection. Pediatr Infect Dis J. 2017;36(9):908-910. https://doi.org/10.1097/INF.0000000000001626.
48. Wallace SS, Brown DN, Cruz AT. Prevalence of concomitant acute bacterial meningitis in neonates with febrile urinary tract infection: a retrospective cross-sectional study. J Pediatr. 2017;184:199-203. https://doi.org/10.1016/j.jpeds.2017.01.022.
49. Nugent J, Childers M, Singh-Miller N, Howard R, Allard R, Eberly M. Risk of meningitis in infants aged 29 to 90 days with urinary tract infection: a systematic review and meta-analysis. J Pediatr. 2019;212:102-110.e5. https://doi.org/10.1016/j.jpeds.2019.04.053.
50. Young BR, Nguyen THP, Alabaster A, Greenhow TL. The prevalence of bacterial meningitis in febrile infants 29-60 days with positive urinalysis. Hosp Pediatr. 2018;8(8):450-457. https
51. Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. https://doi.org/10.1542/peds.2018-3979.
Urinary tract infections (UTIs) are the most common bacterial infection and one of the most common reasons for hospitalization in young infants.1,2 The American Academy of Pediatrics (AAP) has published several clinical practice guidelines for the evaluation and management of febrile children ages 2-24 months with first-time UTIs, most recently in 2011 and affirmed in 2016.3 These guidelines do not provide recommendations for infants aged <2 months, which leads to uncertainty regarding the diagnosis and management of UTIs for infants in this age group. We assess the applicability of the AAP UTI Guideline’s action statements for infants aged <2 months presenting with first-time UTIs, with an emphasis on recent evidence. Because the considerations for bacterial infections differ for febrile infants aged <2 months compared with older infants, we do not discuss action statements one and two (determination of the likelihood of UTIs and decision to test urine) and statement seven (medical evaluation for fever after first UTI).3 Additionally, because concomitant bacteremia and meningitis are more common in this age group than in older infants, we review some of the controversies surrounding the diagnosis and treatment of these disease entities.
DIAGNOSIS
“Action Statement 3: To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection (pyuria and/or bacteriuria) and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.”3
To distinguish asymptomatic bacteriuria or contamination from a true UTI, the AAP Guideline requires both a positive urinalysis (UA) and culture for a diagnosis of a UTI.3 Historically, the UA was considered to be poorly sensitive for infections in young infants, with older studies reporting sensitivities ranging from 40% to 82% using urine culture as the gold standard.4-7 Thus, infants aged <2 months with positive urine cultures and negative UAs are often treated as having true UTIs, though this practice varies by institution.8 Possible explanations for the low UA sensitivity in this population include rapid bladder emptying, immature immune systems, and inability to concentrate urine. However, a negative UA plus a positive urine culture could also represent a “true negative” UA and a “false positive” culture, a finding that may be more common in young infants in whom sterile urine obtainment is often challenging.
Two recent studies have addressed this issue by evaluating the UA sensitivity in patients with bacteremic UTIs, as growth of the same pathogenic organism from the blood and urine almost certainly represents true infection.9,10 In a retrospective study of 203 infants aged <3 months with bacteremic UTIs, the presence of any leukocyte esterase (LE) or pyuria (>3 white blood cells per high-powered field [WBC/HPF]) had a sensitivity of 99.5% (95% CI: 98.5%-100%) and specificity of 93.9% (95% CI: 87.8%-93.2%).9 In a prospective, multicenter study of 4,147 febrile infants aged ≤60 days, of whom 27 infants had bacteremic UTIs, a positive UA (any LE, >5 WBC/HPF, or nitrite) had a sensitivity and specificity of 1.00 (95% CI: 0.87-1.00) and 0.91 (95% CI: 0.90-0.91), respectively.10 Although screening tests may appear to have higher sensitivity in more severely diseased populations (“spectrum bias”),11 it is not clear that infants with bacteremic UTIs are definitively sicker than infants with nonbacteremic UTIs (see “bacteremic UTI” section below). Additionally, this study found similarly excellent sensitivity (0.94 [95% CI: 0.90-0.96]) and specificity (0.91 [95% CI: 0.90-0.91]) of the UA among infants with nonbacteremic UTIs, including infants <28 days old.10
UA sensitivity (using urine culture as the gold standard) may be lower for non-Escherichia coli UTIs.9,10,12 In a retrospective study that included 90 infants <2 months old with UTIs, urine cultures yielding Pseudomonas aeruginosa, Enterococcus, or Klebsiella species were significantly less likely (odds ratio [95% CI]: 0.19 [0.06-0.60]; 0.14 [0.07-0.28]; 0.34 [0.17-0.68], respectively) to have pyuria (≥5 WBC/HPF) or LE (1+ or greater) than urine cultures yielding E. coli.,12 though an alternative explanation for this finding is that these organisms may be more likely to cause asymptomatic bacteriuria or contamination.13
The appropriate CFU/mL threshold to define a UTI and the extent that this threshold should vary by urine collection methods are still unclear. In the aforementioned bacteremic UTI study,9 12 patients with E. coli bacteremia had urine cultures with <50,000 CFU/mL plus pyuria (WBC or LE) in the UA, indicating that true UTIs may occur with <50,000 CFU/mL.
Based on these recent studies, we believe that the recommendation to incorporate UA results into the diagnoses of UTIs can be applied to infants <2 months old, as well as consideration for a UTI for colony counts of ≥10,000 CFU/mL if the UA is positive. For infants with positive urine cultures and negative UAs who have not received antibiotics, we suggest repeating both studies if treatment is being considered. For those who have started antibiotics, the pretest probability of a UTI, initial illness severity, and risks and benefits of continuing treatment should be considered.
TREATMENT
“Action Statement 4a: When initiating treatment, the clinician should base the choice of route of administration on practical considerations. Initiating treatment orally or parenterally is equally efficacious. The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.”3
Most infants <2 months old with UTIs are hospitalized initially because of fever. Therefore, the decision point for most clinicians is not whether to hospitalize but for how long to hospitalize and treat with intravenous (IV) antibiotics prior to discharging home on oral antibiotics. Although all-oral antibiotic regimens are used to treat UTIs in older infants and children,14-18 to our knowledge, there are no randomized controlled trials (RCTs) comparing all-IV vs all-oral antibiotics or a longer vs shorter initial IV course that include infants <1 month old. In the trials that do include infants aged 1-2 months,14,18 the number of subjects in this age group is too small to draw conclusions, a finding supported by a 2014 Cochrane review.19 An adequately powered RCT of different IV antibiotic durations in this age group would be challenging. For example, nearly 1,000 subjects would be needed to demonstrate a statistically significant difference between a 5% and 10% relapse risk between groups, a difference that some may find clinically important.
The paucity of evidence in this age group may explain the considerable variability in the approach to IV antibiotic duration in young infants. Concerns about enteral absorption and underdeveloped immune systems may prompt some physicians to treat the youngest patients more aggressively. One study demonstrated that the proportion of patients <2 months old receiving prolonged courses (≥4 days) of IV antibiotics for UTIs in 46 U.S. children’s hospitals ranged from 0% to 67%.20 Similar variability across hospitals has been described in other observational studies21,22 and across subspecialties in one survey of pediatricians.23
Several observational studies provide additional evidence supporting shorter IV courses. In two studies that examined administrative databases, there was no difference in treatment failure rates between infants aged <2 months20 and <6 months21 receiving longer (≥4 days) vs shorter IV courses. In a study of 172 infants <1 month old with UTIs, the median IV duration was 4 days (range 2-12 days), and no subjects experienced treatment failure or relapse.24 In a multicenter study of 251 infants <3 months old with bacteremic UTIs, mean IV antibiotic durations ranged from 5.5–12 days, and no patient had a relapsed bacteremic UTI. Six infants (2.4%) had a relapsed UTI without bacteremia, with no association between IV antibiotic duration and relapse.22
Based on the available data and known risks of hospitalization and prolonged IV therapy, a reasonable approach for infants <1 month old would be to hospitalize for two to three days while awaiting blood and cerebral spinal fluid (CSF) culture results. Given the possibility of Enterococcus or Enterobacteriaceae that are resistant to third-generation cephalosporins, standard therapy of ampicillin and gentamicin for febrile neonates is reasonable, assuming there is no concern for meningitis. Antibiotics should be narrowed when susceptibilities are known. Once culture results return and signs and symptoms have resolved, discharge home on oral antibiotics is justifiable based on the available literature. For well-appearing infants aged 1-2 months with a presumptive UTI (based on UA results), if hospitalization is not warranted for other reasons, then we recommend outpatient treatment with oral or intramuscular therapy based on local susceptibilities (typically a cephalosporin) and close follow-up for one to two days while awaiting culture results. Although empiric cephalosporin therapy may not provide 100% coverage for all potential organisms, clinical deterioration is uncommon in infants and children receiving discordant therapy.25
“Action Statement 4b: The clinician should choose 7 to 14 days as the duration of antimicrobial therapy.”3
The AAP’s recommendation to provide antibiotics (by oral or parenteral route) for a minimum of seven days total stems from a 2002 meta-analysis comparing long (7-14 days) vs short (≤3 days) courses, where the pooled relative risk of treatment failure with short-course therapy was 1.94 (95% CI: 1.19-3.15).26 However, in this analysis, the trials that demonstrated inferiority with short courses were all trials that used single doses of antibiotics, and a similar Cochrane review comparing 2-4 days with 7-14 days demonstrated no differences in outcomes.27 Therefore, shorter total courses, but not a single dose, are probably appropriate for most UTIs in children. Although there are no obvious biologic reasons why longer total courses would be needed in young infants, there are unfortunately limited data comparing different total antibiotic durations in this age group. We believe that 7-14 days of total therapy is a reasonable recommendation for infants <2 months old, and that future studies should investigate shorter total courses.
IMAGING
“Action Statement 5: Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS).”3
The AAP Guideline acknowledges that the RBUS is a poor screening test for the detection of genitourinary abnormalities in infants.3 The RBUS can be normal in infants with vesicoureteral reflux (VUR) or show nonspecific findings of unclear clinical significance.28 In a prospective study of 220 infants <3 months old by Tsai et al, 9/39 infants (23%) with grade III-V VUR had normal RBUS.29 Studies that included older infants have found a similar false-negative rate of 0%-40% for detecting grade IV-V VUR by RBUS.28 Nonetheless, since a RBUS is safe and noninvasive, we feel that the benefits of screening for abnormalities such as hydronephrosis (that could indicate posterior urethral valves or ureteropelvic junction obstruction) outweigh the risks (eg, false positives, overdiagnosis, and cost) of performing a RBUS after a first-time UTI.
“Action Statement 6a: Voiding cystourethrography (VCUG) should not be performed routinely after the first febrile UTI; VCUG is indicated if RBUS reveals hydronephrosis, scarring, or other findings that would suggest either high-grade VUR or obstructive uropathy, as well as in other atypical or complex clinical circumstances.”3
“Action Statement 6b: Further evaluation should be conducted if there is a recurrence of febrile UTI.”3
The RBUS may be normal in infants with VUR. Therefore, the AAP’s recommendation to perform a VCUG only if the RBUS is abnormal or after a recurrent UTI concedes that there will be infants with VUR who are missed after the first UTI.3
The United Kingdom’s National Institute for Health and Care Excellence guideline recommends a VCUG for infants <6 months old with a bacteremic or non-E. coli UTI.30 Whether high-grade VUR is more common in young infants with bacteremic UTIs than nonbacteremic UTIs remains inconclusive. In the Honkinen et al. study that included 87 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR (10%) and obstruction (7%) was higher than that of the 88 nonbacteremic infants (2% grade IV-V VUR and 2% with obstruction). In the multicenter study of 251 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR was 12.1%.31 This is higher than that of the nonbacteremic infants in Honkinen et al.’s study32 but more similar to the prevalence of grade IV-V VUR found in Tsai et al. (8.2%) and Ismaili et al.’s (7.0%) studies of UTIs in general.29,33
There does appear to be a higher prevalence of urinary tract abnormalities in young infants with non-E. coli vs E. coli UTIs.31,32,34,35 The odds of an abnormal VCUG was 8.0 (95% CI: 2.3-28) times higher for non-E. coli than E. coli UTIs in the study of 251 bacteremic infants.31 In a study of 122 infants <3 months old, the odds of grade III-V VUR was 10 (95% CI 2.6-41) times higher for non-E. coli than E. coli UTIs.35
However, the need for early detection of VUR is controversial, and VCUGs are invasive, involve ionizing radiation, and may require sedation. Two recent trials (one which included only children with VUR and another in which 42% of subjects had VUR) demonstrated a modest effect of prophylactic antibiotics in preventing recurrent UTIs (>5,000 doses of antibiotics needed to prevent one UTI recurrence), but the effect size did not differ by the presence or degree of VUR, and neither demonstrated any benefit in reducing future renal scarring.36, 37 The benefit of surgical interventions for VUR also remains unclear, though studies are limited.38 Overall, there is no evidence suggesting that infants <2 months old require more vigilance for VUR detection than the 2-24 month age group.
SPECIAL CONSIDERATIONS
Bacteremic UTI
The prevalence of bacteremia in infants ≤60 days old with UTIs was 9% in a study conducted from 2008 to 2013 in 26 EDs and has ranged from 3% to 17% in older studies.10, 22 Many studies have described similar clinical and laboratory findings in young infants with bacteremic and nonbacteremic UTIs.39-41 Despite this, bacteremic UTIs have been associated with prolonged parenteral antibiotic courses, resulting in longer hospitalizations and increased costs.40 Two recent multicenter studies of infants with bacteremic UTIs (251 infants <3 months old22 and 115 infants ≤60 days old42) demonstrated variable IV courses and no association between IV duration and relapsed UTI. The latter study showed no risk difference in the adjusted 30-day UTI recurrence (risk difference 3%, 95% CI: −5.8 to 12.7) or all-cause reutilization (risk difference 3%, 95% CI: −14.5 to 20.6) between long and short IV groups.42 Neither study had patients with relapsed bacteremic UTIs or reported that patients suffered clinical deterioration while on oral antibiotics.22,42
Based on these data demonstrating that adverse outcomes are rare in infants with bacteremic UTIs and not associated with parenteral antibiotic duration, we recommend short parenteral courses (2-3 days) with conversion to oral therapy once infants have clinically improved.
Positive Urinalysis and Testing for Meningitis
Multiple risk stratification algorithms for febrile infants aged ≤60 days categorize infants with a positive UA (and therefore likely UTI) as high-risk for having concomitant bacteremia or meningitis, for which lumbar puncture (LP) is typically recommended.43-45 The risk of not testing CSF is the potential to insufficiently treat meningitis because treatment for UTIs and meningitis differ in dosing, route, and duration. Recent studies have challenged the practice of routine LPs for infants aged 1-2 months with a suspected UTI due to the low prevalence (0%-0.3%) of concomitant meningitis.39,46-48 A meta-analysis of 20 studies reporting rates of concomitant meningitis with UTI in infants aged 29-90 days found a pooled prevalence of 0.25% (95% CI: 0.09%-0.70%).49 Furthermore, a study of febrile infants ages 29-60 days found that the prevalence of meningitis did not differ between those with a positive vs negative UA (3/337 [0.9%] vs 5/498 [1.0%], respectively), suggesting that a positive UA alone should not modify the pretest probability of meningitis in this age group.50
Two studies have also examined the risk of delayed meningitis among infants ≤60 days old treated for UTIs without CSF testing. A northern California study that examined 345 episodes among 341 UA-positive infants aged 29-60 days found zero cases (95% CI: 0%-1.1%) of delayed meningitis within 30 days of evaluation.50 A multicenter study of well-appearing febrile infants aged 7-60 days found 0/505 cases (95% CI: 0%-0.6%) of delayed meningitis within 7 days of discharge; 407 (81%) were aged 31-60 days.51 In summary, studies have shown a low rate of concomitant meningitis and a low risk of delayed meningitis in infants aged 1-2 months treated for UTI without CSF testing. Given this, clinically targeted (eg, based on ill appearance and/or lethargy), rather than routine, CSF testing in this age group can be considered.
CONCLUSION
While the AAP UTI Guideline is directed toward 2-24-month-old infants, recent evidence suggests that action statements 3-6 apply to infants <2 months old. Incorporation of pyuria as a diagnostic criterion for UTIs, early transition to oral therapy, and selective VCUG testing are all warranted based on the available evidence and consideration of known risks and benefits. Future studies with larger sample sizes that include infants <2 months old would be beneficial to ensure that the available studies, which have relatively small cohorts, do not suffer from type II error. We propose that future studies examine shorter (<7 days) vs longer total antibiotic duration, shorter vs longer initial IV antibiotics (especially in infants <1 month old or with bacteremic UTIs), and whether RBUS can be performed in a targeted manner. RCTs comparing universal vs targeted imaging strategies would help ascertain whether the increased diagnostic yield that accompanies more aggressive imaging strategies translates into improved outcomes. Application of these AAP guidelines to the <2-month age group and enhancement of the evidence base can promote the high-value care of young infants with UTIs.
Urinary tract infections (UTIs) are the most common bacterial infection and one of the most common reasons for hospitalization in young infants.1,2 The American Academy of Pediatrics (AAP) has published several clinical practice guidelines for the evaluation and management of febrile children ages 2-24 months with first-time UTIs, most recently in 2011 and affirmed in 2016.3 These guidelines do not provide recommendations for infants aged <2 months, which leads to uncertainty regarding the diagnosis and management of UTIs for infants in this age group. We assess the applicability of the AAP UTI Guideline’s action statements for infants aged <2 months presenting with first-time UTIs, with an emphasis on recent evidence. Because the considerations for bacterial infections differ for febrile infants aged <2 months compared with older infants, we do not discuss action statements one and two (determination of the likelihood of UTIs and decision to test urine) and statement seven (medical evaluation for fever after first UTI).3 Additionally, because concomitant bacteremia and meningitis are more common in this age group than in older infants, we review some of the controversies surrounding the diagnosis and treatment of these disease entities.
DIAGNOSIS
“Action Statement 3: To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection (pyuria and/or bacteriuria) and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.”3
To distinguish asymptomatic bacteriuria or contamination from a true UTI, the AAP Guideline requires both a positive urinalysis (UA) and culture for a diagnosis of a UTI.3 Historically, the UA was considered to be poorly sensitive for infections in young infants, with older studies reporting sensitivities ranging from 40% to 82% using urine culture as the gold standard.4-7 Thus, infants aged <2 months with positive urine cultures and negative UAs are often treated as having true UTIs, though this practice varies by institution.8 Possible explanations for the low UA sensitivity in this population include rapid bladder emptying, immature immune systems, and inability to concentrate urine. However, a negative UA plus a positive urine culture could also represent a “true negative” UA and a “false positive” culture, a finding that may be more common in young infants in whom sterile urine obtainment is often challenging.
Two recent studies have addressed this issue by evaluating the UA sensitivity in patients with bacteremic UTIs, as growth of the same pathogenic organism from the blood and urine almost certainly represents true infection.9,10 In a retrospective study of 203 infants aged <3 months with bacteremic UTIs, the presence of any leukocyte esterase (LE) or pyuria (>3 white blood cells per high-powered field [WBC/HPF]) had a sensitivity of 99.5% (95% CI: 98.5%-100%) and specificity of 93.9% (95% CI: 87.8%-93.2%).9 In a prospective, multicenter study of 4,147 febrile infants aged ≤60 days, of whom 27 infants had bacteremic UTIs, a positive UA (any LE, >5 WBC/HPF, or nitrite) had a sensitivity and specificity of 1.00 (95% CI: 0.87-1.00) and 0.91 (95% CI: 0.90-0.91), respectively.10 Although screening tests may appear to have higher sensitivity in more severely diseased populations (“spectrum bias”),11 it is not clear that infants with bacteremic UTIs are definitively sicker than infants with nonbacteremic UTIs (see “bacteremic UTI” section below). Additionally, this study found similarly excellent sensitivity (0.94 [95% CI: 0.90-0.96]) and specificity (0.91 [95% CI: 0.90-0.91]) of the UA among infants with nonbacteremic UTIs, including infants <28 days old.10
UA sensitivity (using urine culture as the gold standard) may be lower for non-Escherichia coli UTIs.9,10,12 In a retrospective study that included 90 infants <2 months old with UTIs, urine cultures yielding Pseudomonas aeruginosa, Enterococcus, or Klebsiella species were significantly less likely (odds ratio [95% CI]: 0.19 [0.06-0.60]; 0.14 [0.07-0.28]; 0.34 [0.17-0.68], respectively) to have pyuria (≥5 WBC/HPF) or LE (1+ or greater) than urine cultures yielding E. coli.,12 though an alternative explanation for this finding is that these organisms may be more likely to cause asymptomatic bacteriuria or contamination.13
The appropriate CFU/mL threshold to define a UTI and the extent that this threshold should vary by urine collection methods are still unclear. In the aforementioned bacteremic UTI study,9 12 patients with E. coli bacteremia had urine cultures with <50,000 CFU/mL plus pyuria (WBC or LE) in the UA, indicating that true UTIs may occur with <50,000 CFU/mL.
Based on these recent studies, we believe that the recommendation to incorporate UA results into the diagnoses of UTIs can be applied to infants <2 months old, as well as consideration for a UTI for colony counts of ≥10,000 CFU/mL if the UA is positive. For infants with positive urine cultures and negative UAs who have not received antibiotics, we suggest repeating both studies if treatment is being considered. For those who have started antibiotics, the pretest probability of a UTI, initial illness severity, and risks and benefits of continuing treatment should be considered.
TREATMENT
“Action Statement 4a: When initiating treatment, the clinician should base the choice of route of administration on practical considerations. Initiating treatment orally or parenterally is equally efficacious. The clinician should base the choice of agent on local antimicrobial sensitivity patterns (if available) and should adjust the choice according to sensitivity testing of the isolated uropathogen.”3
Most infants <2 months old with UTIs are hospitalized initially because of fever. Therefore, the decision point for most clinicians is not whether to hospitalize but for how long to hospitalize and treat with intravenous (IV) antibiotics prior to discharging home on oral antibiotics. Although all-oral antibiotic regimens are used to treat UTIs in older infants and children,14-18 to our knowledge, there are no randomized controlled trials (RCTs) comparing all-IV vs all-oral antibiotics or a longer vs shorter initial IV course that include infants <1 month old. In the trials that do include infants aged 1-2 months,14,18 the number of subjects in this age group is too small to draw conclusions, a finding supported by a 2014 Cochrane review.19 An adequately powered RCT of different IV antibiotic durations in this age group would be challenging. For example, nearly 1,000 subjects would be needed to demonstrate a statistically significant difference between a 5% and 10% relapse risk between groups, a difference that some may find clinically important.
The paucity of evidence in this age group may explain the considerable variability in the approach to IV antibiotic duration in young infants. Concerns about enteral absorption and underdeveloped immune systems may prompt some physicians to treat the youngest patients more aggressively. One study demonstrated that the proportion of patients <2 months old receiving prolonged courses (≥4 days) of IV antibiotics for UTIs in 46 U.S. children’s hospitals ranged from 0% to 67%.20 Similar variability across hospitals has been described in other observational studies21,22 and across subspecialties in one survey of pediatricians.23
Several observational studies provide additional evidence supporting shorter IV courses. In two studies that examined administrative databases, there was no difference in treatment failure rates between infants aged <2 months20 and <6 months21 receiving longer (≥4 days) vs shorter IV courses. In a study of 172 infants <1 month old with UTIs, the median IV duration was 4 days (range 2-12 days), and no subjects experienced treatment failure or relapse.24 In a multicenter study of 251 infants <3 months old with bacteremic UTIs, mean IV antibiotic durations ranged from 5.5–12 days, and no patient had a relapsed bacteremic UTI. Six infants (2.4%) had a relapsed UTI without bacteremia, with no association between IV antibiotic duration and relapse.22
Based on the available data and known risks of hospitalization and prolonged IV therapy, a reasonable approach for infants <1 month old would be to hospitalize for two to three days while awaiting blood and cerebral spinal fluid (CSF) culture results. Given the possibility of Enterococcus or Enterobacteriaceae that are resistant to third-generation cephalosporins, standard therapy of ampicillin and gentamicin for febrile neonates is reasonable, assuming there is no concern for meningitis. Antibiotics should be narrowed when susceptibilities are known. Once culture results return and signs and symptoms have resolved, discharge home on oral antibiotics is justifiable based on the available literature. For well-appearing infants aged 1-2 months with a presumptive UTI (based on UA results), if hospitalization is not warranted for other reasons, then we recommend outpatient treatment with oral or intramuscular therapy based on local susceptibilities (typically a cephalosporin) and close follow-up for one to two days while awaiting culture results. Although empiric cephalosporin therapy may not provide 100% coverage for all potential organisms, clinical deterioration is uncommon in infants and children receiving discordant therapy.25
“Action Statement 4b: The clinician should choose 7 to 14 days as the duration of antimicrobial therapy.”3
The AAP’s recommendation to provide antibiotics (by oral or parenteral route) for a minimum of seven days total stems from a 2002 meta-analysis comparing long (7-14 days) vs short (≤3 days) courses, where the pooled relative risk of treatment failure with short-course therapy was 1.94 (95% CI: 1.19-3.15).26 However, in this analysis, the trials that demonstrated inferiority with short courses were all trials that used single doses of antibiotics, and a similar Cochrane review comparing 2-4 days with 7-14 days demonstrated no differences in outcomes.27 Therefore, shorter total courses, but not a single dose, are probably appropriate for most UTIs in children. Although there are no obvious biologic reasons why longer total courses would be needed in young infants, there are unfortunately limited data comparing different total antibiotic durations in this age group. We believe that 7-14 days of total therapy is a reasonable recommendation for infants <2 months old, and that future studies should investigate shorter total courses.
IMAGING
“Action Statement 5: Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS).”3
The AAP Guideline acknowledges that the RBUS is a poor screening test for the detection of genitourinary abnormalities in infants.3 The RBUS can be normal in infants with vesicoureteral reflux (VUR) or show nonspecific findings of unclear clinical significance.28 In a prospective study of 220 infants <3 months old by Tsai et al, 9/39 infants (23%) with grade III-V VUR had normal RBUS.29 Studies that included older infants have found a similar false-negative rate of 0%-40% for detecting grade IV-V VUR by RBUS.28 Nonetheless, since a RBUS is safe and noninvasive, we feel that the benefits of screening for abnormalities such as hydronephrosis (that could indicate posterior urethral valves or ureteropelvic junction obstruction) outweigh the risks (eg, false positives, overdiagnosis, and cost) of performing a RBUS after a first-time UTI.
“Action Statement 6a: Voiding cystourethrography (VCUG) should not be performed routinely after the first febrile UTI; VCUG is indicated if RBUS reveals hydronephrosis, scarring, or other findings that would suggest either high-grade VUR or obstructive uropathy, as well as in other atypical or complex clinical circumstances.”3
“Action Statement 6b: Further evaluation should be conducted if there is a recurrence of febrile UTI.”3
The RBUS may be normal in infants with VUR. Therefore, the AAP’s recommendation to perform a VCUG only if the RBUS is abnormal or after a recurrent UTI concedes that there will be infants with VUR who are missed after the first UTI.3
The United Kingdom’s National Institute for Health and Care Excellence guideline recommends a VCUG for infants <6 months old with a bacteremic or non-E. coli UTI.30 Whether high-grade VUR is more common in young infants with bacteremic UTIs than nonbacteremic UTIs remains inconclusive. In the Honkinen et al. study that included 87 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR (10%) and obstruction (7%) was higher than that of the 88 nonbacteremic infants (2% grade IV-V VUR and 2% with obstruction). In the multicenter study of 251 infants <3 months old with bacteremic UTIs, the prevalence of grade IV-V VUR was 12.1%.31 This is higher than that of the nonbacteremic infants in Honkinen et al.’s study32 but more similar to the prevalence of grade IV-V VUR found in Tsai et al. (8.2%) and Ismaili et al.’s (7.0%) studies of UTIs in general.29,33
There does appear to be a higher prevalence of urinary tract abnormalities in young infants with non-E. coli vs E. coli UTIs.31,32,34,35 The odds of an abnormal VCUG was 8.0 (95% CI: 2.3-28) times higher for non-E. coli than E. coli UTIs in the study of 251 bacteremic infants.31 In a study of 122 infants <3 months old, the odds of grade III-V VUR was 10 (95% CI 2.6-41) times higher for non-E. coli than E. coli UTIs.35
However, the need for early detection of VUR is controversial, and VCUGs are invasive, involve ionizing radiation, and may require sedation. Two recent trials (one which included only children with VUR and another in which 42% of subjects had VUR) demonstrated a modest effect of prophylactic antibiotics in preventing recurrent UTIs (>5,000 doses of antibiotics needed to prevent one UTI recurrence), but the effect size did not differ by the presence or degree of VUR, and neither demonstrated any benefit in reducing future renal scarring.36, 37 The benefit of surgical interventions for VUR also remains unclear, though studies are limited.38 Overall, there is no evidence suggesting that infants <2 months old require more vigilance for VUR detection than the 2-24 month age group.
SPECIAL CONSIDERATIONS
Bacteremic UTI
The prevalence of bacteremia in infants ≤60 days old with UTIs was 9% in a study conducted from 2008 to 2013 in 26 EDs and has ranged from 3% to 17% in older studies.10, 22 Many studies have described similar clinical and laboratory findings in young infants with bacteremic and nonbacteremic UTIs.39-41 Despite this, bacteremic UTIs have been associated with prolonged parenteral antibiotic courses, resulting in longer hospitalizations and increased costs.40 Two recent multicenter studies of infants with bacteremic UTIs (251 infants <3 months old22 and 115 infants ≤60 days old42) demonstrated variable IV courses and no association between IV duration and relapsed UTI. The latter study showed no risk difference in the adjusted 30-day UTI recurrence (risk difference 3%, 95% CI: −5.8 to 12.7) or all-cause reutilization (risk difference 3%, 95% CI: −14.5 to 20.6) between long and short IV groups.42 Neither study had patients with relapsed bacteremic UTIs or reported that patients suffered clinical deterioration while on oral antibiotics.22,42
Based on these data demonstrating that adverse outcomes are rare in infants with bacteremic UTIs and not associated with parenteral antibiotic duration, we recommend short parenteral courses (2-3 days) with conversion to oral therapy once infants have clinically improved.
Positive Urinalysis and Testing for Meningitis
Multiple risk stratification algorithms for febrile infants aged ≤60 days categorize infants with a positive UA (and therefore likely UTI) as high-risk for having concomitant bacteremia or meningitis, for which lumbar puncture (LP) is typically recommended.43-45 The risk of not testing CSF is the potential to insufficiently treat meningitis because treatment for UTIs and meningitis differ in dosing, route, and duration. Recent studies have challenged the practice of routine LPs for infants aged 1-2 months with a suspected UTI due to the low prevalence (0%-0.3%) of concomitant meningitis.39,46-48 A meta-analysis of 20 studies reporting rates of concomitant meningitis with UTI in infants aged 29-90 days found a pooled prevalence of 0.25% (95% CI: 0.09%-0.70%).49 Furthermore, a study of febrile infants ages 29-60 days found that the prevalence of meningitis did not differ between those with a positive vs negative UA (3/337 [0.9%] vs 5/498 [1.0%], respectively), suggesting that a positive UA alone should not modify the pretest probability of meningitis in this age group.50
Two studies have also examined the risk of delayed meningitis among infants ≤60 days old treated for UTIs without CSF testing. A northern California study that examined 345 episodes among 341 UA-positive infants aged 29-60 days found zero cases (95% CI: 0%-1.1%) of delayed meningitis within 30 days of evaluation.50 A multicenter study of well-appearing febrile infants aged 7-60 days found 0/505 cases (95% CI: 0%-0.6%) of delayed meningitis within 7 days of discharge; 407 (81%) were aged 31-60 days.51 In summary, studies have shown a low rate of concomitant meningitis and a low risk of delayed meningitis in infants aged 1-2 months treated for UTI without CSF testing. Given this, clinically targeted (eg, based on ill appearance and/or lethargy), rather than routine, CSF testing in this age group can be considered.
CONCLUSION
While the AAP UTI Guideline is directed toward 2-24-month-old infants, recent evidence suggests that action statements 3-6 apply to infants <2 months old. Incorporation of pyuria as a diagnostic criterion for UTIs, early transition to oral therapy, and selective VCUG testing are all warranted based on the available evidence and consideration of known risks and benefits. Future studies with larger sample sizes that include infants <2 months old would be beneficial to ensure that the available studies, which have relatively small cohorts, do not suffer from type II error. We propose that future studies examine shorter (<7 days) vs longer total antibiotic duration, shorter vs longer initial IV antibiotics (especially in infants <1 month old or with bacteremic UTIs), and whether RBUS can be performed in a targeted manner. RCTs comparing universal vs targeted imaging strategies would help ascertain whether the increased diagnostic yield that accompanies more aggressive imaging strategies translates into improved outcomes. Application of these AAP guidelines to the <2-month age group and enhancement of the evidence base can promote the high-value care of young infants with UTIs.
1. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. https://doi.org/10.1097/INF.0000000000000225.
2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. https://doi.org/10.1007/s00467-010-1625-8.
3. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):1-5. https://doi.org/10.1542/peds.2016-3026.
4. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363-367. https://doi.org/10.1542/peds.105.2.e20
5. Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants <or= 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12-14. https://doi.org/10.1097/00006565-200202000-00004.
6. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. https://doi.org/10.1001/archpedi.155.1.60.
7. Reardon JM, Carstairs KL, Rudinsky SL, Simon LV, Riffenburgh RH, Tanen DA. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27(8):930-932. https://doi.org/10.1016/j.ajem.2008.07.015.
8. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. https://doi.org/10.12788/jhm.3120.
9. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. https://doi.org/10.1542/peds.2015-0012.
10. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068.
11. Newman TB, Kohn MA. Evidence-based diagnosis. Practical Guides to Biostatistics and Epidemiology. Cambridge; New York: Cambridge University Press, 2009.
12. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association Between Uropathogen and Pyuria. Pediatrics. 2016;138(1):e20160087. https://doi.org/10.1542/peds.2016-0087.
13. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr. 2016;55(9):819-824. https://doi.org/10.1177/0009922815608278.
14. Bocquet N, Sergent Alaoui A, Jais JP, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129(2):e269-e275. https://doi.org/10.1542/peds.2011-0814.
15. Bouissou F, Munzer C, Decramer S, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics. 2008;121(3):e553-e560. https://doi.org/10.1542/peds.2006-3632.
16. Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2007(4):CD003772. https://doi.org/10.1002/14651858.CD003772.pub3.
17. Neuhaus TJ, Berger C, Buechner K, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. Eur J Pediatr. 2008;167(9):1037-1047. https://doi.org/10.1007/s00431-007-0638-1
18. Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. https://doi.org/10.1542/peds.104.1.79.
19. Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014(7):CD003772. https://doi.org/10.1002/14651858.CD003772.pub4.
20. Lewis-de Los Angeles WW, Thurm C, Hersh AL, et al. Trends in intravenous antibiotic duration for urinary tract infections in young infants. Pediatrics. 2017;140(6):e20171021. https://doi.org/10.1542/peds.2017-1021.
21. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203. https://doi.org/10.1542/peds.2009-2948.
22. Schroeder AR, Shen MW, Biondi EA, et al. Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. https://doi.org/10.1136/archdischild-2014-307997.
23. Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. https://doi.org/10.1542/hpeds.2017-0082.
24. Magin EC, Garcia-Garcia JJ, Sert SZ, Giralt AG, Cubells CL. Efficacy of short-term intravenous antibiotic in neonates with urinary tract infection. Pediatr Emerg Care. 2007;23(2):83-86. https://doi.org/10.1097/PEC.0b013e3180302c47.
25. Wang ME, Lee V, Greenhow TL, et al. Clinical response to discordant therapy in third-generation cephalosporin-resistant UTIs. Pediatrics. 2019; In press.
26. Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70. https://doi.org/10.1542/peds.109.5.e70.
27. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003(1):CD003966. https://doi.org/10.1002/14651858.CD003966.
28. Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract I. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749-e770. https://doi.org/10.1542/peds.2011-1332.
29. Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955-963. https://doi.org/10.1007/s00467-012-2104-1.
30. National Institue for Health and Care Excellence. Urinary Tract Infection in Children. http://www.nice.org.uk/guidance/cg54/evidence/cg54-urinary-tract-infection-in-children-full-guideline2. Published August 2007. Accessed August 2019.
31. Chang PW, Abidari JM, Shen MW, et al. Urinary imaging findings in young infants with bacteremic urinary tract infection. Hosp Pediatr. 2016;6(11):647-652. https://doi.org/10.1542/hpeds.2015-0229.
32. Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19(7):630-634. https://doi.org/10.1097/00006454-200007000-00009
33. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158(1):91-94. https://doi.org/10.1016/j.jpeds.2010.06.053.
34. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr. 2004;43(7):619-625. https://doi.org/10.1177/000992280404300706.
35. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. https://doi.org/10.1136/archdischild-2016-311587.
36. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748-1759. https://doi.org/10.1056/NEJMoa0902295.
37. Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367-2376. https://doi.org/10.1056/NEJMoa1401811.
38. Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2019;(2):CD001532. https://doi.org/10.1002/14651858.CD001532.pub4.
39. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479,
40. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. https://doi.org/10.1542/hpeds.2014-0051.
41. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. https://doi.org/10.1001/archpedi.156.1.44.
42. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844,
43. Gomez B, Mintegi S, Bressan S, et al. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381.
44. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501.
45. DePorre AG, Aronson PL, McCulloh RJ. Facing the ongoing challenge of the febrile young infant. Crit Care. 2017;21(1):68. https://doi.org/10.1186/s13054-017-1646-9,
46. Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6(11):e26576. https://doi.org/10.1371/journal.pone.0026576.
47. Thomson J, Cruz AT, Nigrovic LE, et al. Concomitant bacterial meningitis in infants with urinary tract infection. Pediatr Infect Dis J. 2017;36(9):908-910. https://doi.org/10.1097/INF.0000000000001626.
48. Wallace SS, Brown DN, Cruz AT. Prevalence of concomitant acute bacterial meningitis in neonates with febrile urinary tract infection: a retrospective cross-sectional study. J Pediatr. 2017;184:199-203. https://doi.org/10.1016/j.jpeds.2017.01.022.
49. Nugent J, Childers M, Singh-Miller N, Howard R, Allard R, Eberly M. Risk of meningitis in infants aged 29 to 90 days with urinary tract infection: a systematic review and meta-analysis. J Pediatr. 2019;212:102-110.e5. https://doi.org/10.1016/j.jpeds.2019.04.053.
50. Young BR, Nguyen THP, Alabaster A, Greenhow TL. The prevalence of bacterial meningitis in febrile infants 29-60 days with positive urinalysis. Hosp Pediatr. 2018;8(8):450-457. https
51. Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. https://doi.org/10.1542/peds.2018-3979.
1. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. https://doi.org/10.1097/INF.0000000000000225.
2. Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. https://doi.org/10.1007/s00467-010-1625-8.
3. Subcommittee On Urinary Tract Infection. Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):1-5. https://doi.org/10.1542/peds.2016-3026.
4. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363-367. https://doi.org/10.1542/peds.105.2.e20
5. Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine Gram stain in infants <or= 60 days of age with fever. Pediatr Emerg Care. 2002;18(1):12-14. https://doi.org/10.1097/00006565-200202000-00004.
6. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60-65. https://doi.org/10.1001/archpedi.155.1.60.
7. Reardon JM, Carstairs KL, Rudinsky SL, Simon LV, Riffenburgh RH, Tanen DA. Urinalysis is not reliable to detect a urinary tract infection in febrile infants presenting to the ED. Am J Emerg Med. 2009;27(8):930-932. https://doi.org/10.1016/j.ajem.2008.07.015.
8. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. https://doi.org/10.12788/jhm.3120.
9. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6):965-971. https://doi.org/10.1542/peds.2015-0012.
10. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068.
11. Newman TB, Kohn MA. Evidence-based diagnosis. Practical Guides to Biostatistics and Epidemiology. Cambridge; New York: Cambridge University Press, 2009.
12. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association Between Uropathogen and Pyuria. Pediatrics. 2016;138(1):e20160087. https://doi.org/10.1542/peds.2016-0087.
13. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clin Pediatr. 2016;55(9):819-824. https://doi.org/10.1177/0009922815608278.
14. Bocquet N, Sergent Alaoui A, Jais JP, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics. 2012;129(2):e269-e275. https://doi.org/10.1542/peds.2011-0814.
15. Bouissou F, Munzer C, Decramer S, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics. 2008;121(3):e553-e560. https://doi.org/10.1542/peds.2006-3632.
16. Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2007(4):CD003772. https://doi.org/10.1002/14651858.CD003772.pub3.
17. Neuhaus TJ, Berger C, Buechner K, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. Eur J Pediatr. 2008;167(9):1037-1047. https://doi.org/10.1007/s00431-007-0638-1
18. Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. https://doi.org/10.1542/peds.104.1.79.
19. Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014(7):CD003772. https://doi.org/10.1002/14651858.CD003772.pub4.
20. Lewis-de Los Angeles WW, Thurm C, Hersh AL, et al. Trends in intravenous antibiotic duration for urinary tract infections in young infants. Pediatrics. 2017;140(6):e20171021. https://doi.org/10.1542/peds.2017-1021.
21. Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203. https://doi.org/10.1542/peds.2009-2948.
22. Schroeder AR, Shen MW, Biondi EA, et al. Bacteraemic urinary tract infection: management and outcomes in young infants. Arch Dis Child. 2016;101(2):125-130. https://doi.org/10.1136/archdischild-2014-307997.
23. Joshi NS, Lucas BP, Schroeder AR. Physician preferences surrounding urinary tract infection management in neonates. Hosp Pediatr. 2018;8(1):21-27. https://doi.org/10.1542/hpeds.2017-0082.
24. Magin EC, Garcia-Garcia JJ, Sert SZ, Giralt AG, Cubells CL. Efficacy of short-term intravenous antibiotic in neonates with urinary tract infection. Pediatr Emerg Care. 2007;23(2):83-86. https://doi.org/10.1097/PEC.0b013e3180302c47.
25. Wang ME, Lee V, Greenhow TL, et al. Clinical response to discordant therapy in third-generation cephalosporin-resistant UTIs. Pediatrics. 2019; In press.
26. Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70. https://doi.org/10.1542/peds.109.5.e70.
27. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003(1):CD003966. https://doi.org/10.1002/14651858.CD003966.
28. Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract I. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749-e770. https://doi.org/10.1542/peds.2011-1332.
29. Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955-963. https://doi.org/10.1007/s00467-012-2104-1.
30. National Institue for Health and Care Excellence. Urinary Tract Infection in Children. http://www.nice.org.uk/guidance/cg54/evidence/cg54-urinary-tract-infection-in-children-full-guideline2. Published August 2007. Accessed August 2019.
31. Chang PW, Abidari JM, Shen MW, et al. Urinary imaging findings in young infants with bacteremic urinary tract infection. Hosp Pediatr. 2016;6(11):647-652. https://doi.org/10.1542/hpeds.2015-0229.
32. Honkinen O, Jahnukainen T, Mertsola J, Eskola J, Ruuskanen O. Bacteremic urinary tract infection in children. Pediatr Infect Dis J. 2000;19(7):630-634. https://doi.org/10.1097/00006454-200007000-00009
33. Ismaili K, Lolin K, Damry N, Alexander M, Lepage P, Hall M. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158(1):91-94. https://doi.org/10.1016/j.jpeds.2010.06.053.
34. Cleper R, Krause I, Eisenstein B, Davidovits M. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr. 2004;43(7):619-625. https://doi.org/10.1177/000992280404300706.
35. Pauchard JY, Chehade H, Kies CZ, Girardin E, Cachat F, Gehri M. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102(9):804-808. https://doi.org/10.1136/archdischild-2016-311587.
36. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748-1759. https://doi.org/10.1056/NEJMoa0902295.
37. Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367-2376. https://doi.org/10.1056/NEJMoa1401811.
38. Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2019;(2):CD001532. https://doi.org/10.1002/14651858.CD001532.pub4.
39. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479,
40. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. https://doi.org/10.1542/hpeds.2014-0051.
41. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. https://doi.org/10.1001/archpedi.156.1.44.
42. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844,
43. Gomez B, Mintegi S, Bressan S, et al. Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381.
44. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501.
45. DePorre AG, Aronson PL, McCulloh RJ. Facing the ongoing challenge of the febrile young infant. Crit Care. 2017;21(1):68. https://doi.org/10.1186/s13054-017-1646-9,
46. Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6(11):e26576. https://doi.org/10.1371/journal.pone.0026576.
47. Thomson J, Cruz AT, Nigrovic LE, et al. Concomitant bacterial meningitis in infants with urinary tract infection. Pediatr Infect Dis J. 2017;36(9):908-910. https://doi.org/10.1097/INF.0000000000001626.
48. Wallace SS, Brown DN, Cruz AT. Prevalence of concomitant acute bacterial meningitis in neonates with febrile urinary tract infection: a retrospective cross-sectional study. J Pediatr. 2017;184:199-203. https://doi.org/10.1016/j.jpeds.2017.01.022.
49. Nugent J, Childers M, Singh-Miller N, Howard R, Allard R, Eberly M. Risk of meningitis in infants aged 29 to 90 days with urinary tract infection: a systematic review and meta-analysis. J Pediatr. 2019;212:102-110.e5. https://doi.org/10.1016/j.jpeds.2019.04.053.
50. Young BR, Nguyen THP, Alabaster A, Greenhow TL. The prevalence of bacterial meningitis in febrile infants 29-60 days with positive urinalysis. Hosp Pediatr. 2018;8(8):450-457. https
51. Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. https://doi.org/10.1542/peds.2018-3979.
© 2020 Society of Hospital Medicine
Opioid use disorder in adolescents: An overview
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Ms. L, age 17, seeks treatment because she has an ongoing struggle with multiple substances, including benzodiazepines, heroin, alcohol, cannabis, and prescription opioids.
She reports that she was 13 when she first used a prescription opioid that was not prescribed for her. She also reports engaging in unsafe sexual practices while using these substances, and has been diagnosed and treated for a sexually transmitted disease. She dropped out of school and is estranged from her family. She says that for a long time she has felt depressed and that she uses drugs to “self-medicate my emotions.” She endorses high anxiety and lack of motivation. Ms. L also reports having several criminal charges for theft, assault, and exchanging sex for drugs. She has undergone 3 admissions for detoxification, but promptly resumed using drugs, primarily heroin and oxycodone, immediately after discharge. Ms. L meets DSM-5 criteria for opioid use disorder (OUD).
Ms. L’s case illustrates a disturbing trend in the current opioid epidemic in the United States. Nearly 11.8 million individuals age ≥12 reported misuse of opioids in the last year.1 Adolescents who misuse prescription or illicit opioids are more likely to be involved with the legal system due to truancy, running away from home, physical altercations, prostitution, exchanging sex for drugs, robbery, and gang involvement. Adolescents who use opioids may also struggle with academic decline, drop out of school early, be unable to maintain a job, and have relationship difficulties, especially with family members.
In this article, I describe the scope of OUD among adolescents, including epidemiology, clinical manifestations, screening tools, and treatment approaches.
Scope of the problem
According to the most recent Monitoring the Future survey of more than 42,500 8th, 10th, and 12th grade students, 2.7% of 12th graders reported prescription opioid misuse (reported in the survey as “narcotics other than heroin”) in the past year.2 In addition, 0.4% of 12th graders reported heroin use over the same period.2 Although the prevalence of opioid use among adolescents has been declining over the past 5 years,2 it still represents a serious health crisis.
Part of the issue may relate to easier access to more potent opioids. For example, heroin available today can be >4 times purer than it was in the past. In 2002, t
Between 1997 and 2012, the annual incidence of youth (age 15 to 19) hospitalizations for prescription opioid poisoning increased >170%.5 Approximately 6% to 9% of youth involved in risky opioid use develop OUD 6 to 12 months after s
Continue to: In recent years...
In recent years, deaths from drug overdose have increased for all age groups; however, limited data is available regarding adolescent overdose deaths. According to the Centers for Disease Control and Prevention (CDC), from 2015 to 2016, drug overdose death rates for persons age 15 to 24 increased to 28%.9
How opioids work
Opioids activate specific transmembrane neurotransmitter receptors, including mu, kappa, and delta, in the CNS and peripheral nervous system (PNS). This leads to activation of G protein–mediated intracellular signal transduction. Mainly it is activation of endogenous mu opioid receptors that mediates the reward, withdrawal, and analgesic effects of opioids. These effects depend on the location of mu receptors. In the CNS, activation of mu opioid receptors may cause miosis, respiratory depression, euphoria, and analgesia.10
Different opioids vary in terms of their half-life; for most opioids, the half-life ranges from 2 to 4 hours.10 Heroin has a half-life of 30 minutes, but due to active metabolites its duration of action is 4 to 5 hours. Opioid metabolites can be detected in urine toxicology within approximately 1 to 2 days since last use.10
Chronic opioid use is associated with neurologic effects that change the function of areas of the brain that control pleasure/reward, stress, decision-making, and more. This leads to cravings, continued substance use, and dependence.11 After continued long-term use, patients report decreased euphoria, but typically they continue to use opioids to avoid withdrawal symptoms or worsening mood.
Criteria for opioid use disorder
In DSM-5, substance use disorders (SUDs)are no longer categorized as abuse or dependence.12 For opioids, the diagnosis is OUD. The Table12 outlines the DSM-5 criteria for OUD. Craving opioids is included for the first time in the OUD diagnosis. Having problems with the legal system is no longer considered a diagnostic criterion for OUD.
Continue to: A vulnerable population
A vulnerable population
As defined by Erik Erikson’s psychosocial stages of development, adolescents struggle between establishing their own identity vs role confusion.13 In an attempt to relate to peers or give in to peer pressure, some adolescents start by experimenting with nicotine, alcohol, and/or marijuana; however, some may move on to using other illicit drugs.14 Risk factors for the development of SUDs include early onset of substance use and a rapid progression through stages of substance use from experimentation to regular use, risky use, and dependence.15 In our case study, Ms. L’s substance use followed a similar pattern. Further, the comorbidity of SUDs and other psychiatric disorders may add a layer of complexity when caring for adolescents. Box 116-20 describes the relationship between comorbid psychiatric disorders and SUDs in adolescents.
Box 1
Disruptive behavior disorders are the most common coexisting psychiatric disorders in an adolescent with a substance use disorder (SUD), including opioid use disorder. These individuals typically present with aggression and other conduct disorder symptoms, and have early involvement with the legal system. Conversely, patients with conduct disorder are at high risk of early initiation of illicit substance use, including opioids. Early onset of substance use is a strong risk factor for developing an SUD.16
Mood disorders, particularly depression, can either precede or occur as a result of heavy and prolonged substance use.17 The estimated prevalence of major depressive disorder in individuals with an SUD is 24% to 50%. Among adolescents, an SUD is also a risk factor for suicidal ideation, suicide attempts, and completed suicide.18-20
Anxiety disorders, especially social phobia, and posttraumatic stress disorder are common in individuals with SUD.
Adolescents with SUD should be carefully evaluated for comorbid psychiatric disorders and treated accordingly.
Clinical manifestations
Common clinical manifestations of opioid use vary depending on when the patient is seen. An individual with OUD may appear acutely intoxicated, be in withdrawal, or show no effects. Chronic/prolonged use can lead to tolerance, such that a user needs to ingest larger amounts of the opioid to produce the same effects.
Acute intoxication can cause sedation, slurring of speech, and pinpoint pupils. Fresh injection sites may be visible on physical examination of IV users. The effects of acute intoxication usually depend on the half-life of the specific opioid and the individual’s tolerance.10 Tolerance to heroin can occur in 10 days and withdrawal can manifest in 3 to 7 hours after last use, depending on dose and purity.3 Tolerance can lead to unintentional overdose and death.
Withdrawal. Individuals experiencing withdrawal from opioids present with flu-like physical symptoms, including generalized body ache, rhinorrhea, diarrhea, goose bumps, lacrimation, and vomiting. Individuals also may experience irritability, restlessness, insomnia, anxiety, and depression during withdrawal.
Other manifestations. Excessive and chronic/prolonged opioid use can adversely impact socio-occupational functioning and cause academic decline in adolescents and youth. Personal relationships are significantly affected. Opioid users may have legal difficulties as a result of committing crimes such as theft, prostitution, or robbery in order to obtain opioids.
Continue to: Screening for OUD
Screening for OUD
Several screening tools are available to assess adolescents for SUDs, including OUD.
CRAFFT is a 6-item, clinician-administered screening tool that has been approved by American Academy of Pediatrics’ Committee on Substance Abuse for adolescents and young adults age <21.21-23 This commonly used tool can assess for alcohol, cannabis, and other drug use. A score ≥2 is considered positive for drug use, indicating that the individual would require further evaluation and assessment22,23 (Figure). There is also a self-administered CRAFFT questionnaire that can be completed by the patient.
NIDA-modified ASSIST. The American Psychiatric Association has adapted the National Institute on Drug Abuse (NIDA)-modified ASSIST. One version is designated for parents/guardians to administer to their children (age 6 to 17), and one is designated for adolescents (age 11 to 17) to self-administer.24,25 Each screening tool has 2 levels: Level 1 screens for substance use and other mental health symptoms, and Level 2 is more specific for substance use alone.
Drug Use Screening Inventory (DUSI) is a self-report questionnaire that has 149 items that assess the use of numerous drugs. It is designed to quantify the severity of consequences associated with drug and alcohol use.26,27
Problem-Oriented Screening Instrument for Teenagers (PO
Continue to: Personal Experience Screening Questionnaire (PESQ)...
Personal Experience Screening Questionnaire (PESQ) is a brief, 40-item, cost-effective, self-report questionnaire that can help identify adolescents (age 12 to 18) who should be referred for further evaluation.30
Addressing treatment expectations
For an adolescent with OUD, treatment should begin in the least restrictive environment that is perceived as safe for the patient. An adolescent’s readiness and motivation to achieve and maintain abstinence are crucial. Treatment planning should include the adolescent as well as his/her family to ensure they are able to verbalize their expectations. Start with a definitive treatment plan that addresses an individual’s needs. The plan should provide structure and an understanding of treatment expectations. The treatment team should clarify the realistic plan and goals based on empirical and clinical evidence. Treatment goals should include interventions to strengthen interpersonal relationships and assist with rehabilitation, such as establishing academic and/or vocational goals. Addressing readiness and working on a patient’s motivation is extremely important for most of these interventions.
In order for any intervention to be successful, clinicians need to establish and foster rapport with the adolescent. By law, substance use or behaviors related to substance use are not allowed to be shared outside the patient-clinician relationship, unless the adolescent gives consent or there are concerns that such behaviors might put the patient or others at risk. It is important to prime the adolescent and help them understand that any information pertaining to their safety or the safety of others may need to be shared outside the patient-clinician relationship.
Choosing an intervention
Less than 50% of a nationally representative sample of 345 addiction treatment programs serving adolescents and adults offer medications for treating OUD.31 Even in programs that offer pharmacotherapy, medications are significantly underutilized. Fewer than 30% of patients in addiction treatment programs receive medication, compared with 74% of patients receiving treatment for other mental health disorders.31 A
Psychotherapy may be used to treat OUD in adolescents. Several family therapies have been studied and are considered as critical psychotherapeutic interventions for treating SUDs, including structural family treatment and functional family therapy approaches.34 An integrated behavioral and family therapy model is also recommended for adolescent patients with SUDs. Cognitive distortions and use of self-deprecatory statements are common among adolescents.35 Therefore, using approaches of cognitive-behavioral therapy (CBT), or CBT plus motivational enhancement therapy, also might be effective for this population.36 The adolescent community reinforcement approach (A-CRA) is a behavioral treatment designed to help adolescents and their families learn how to lead a healthy and happy life without the use of drugs or alcohol by increasing access to social, familial, and educational/vocational reinforcers. Support groups and peer and family support should be encouraged as adjuncts to other interventions. In some areas, sober housing options for adolescents are also available.
Continue to: Harm-reduction strategies
Harm-reduction strategies. Although the primary goal of treatment for adolescents with OUD is to achieve and maintain abstinence from opioid use, implicit and explicit goals can be set. Short-term implicit goals may include harm-reduction strategies that emphasize decreasing the duration, frequency, and amount of substance use and limiting the chances of adverse effects, while the long-term explicit goal should be abstinence from opioid use.
Naloxone nasal spray is used as a harm-reduction strategy. It is an FDA-approved formulation that can reverse the effects of unintentional opioid overdoses and potentially prevent death from respiratory depression.37 Other harm-reduction strategies include needle exchange programs, which provide sterile needles to individuals who inject drugs in an effort to prevent or reduce the transmission of human immunodeficiency virus and other bloodborne viruses that can be spread via shared injection equipment. Fentanyl testing strips allow opioid users to test for the presence fentanyl and fentanyl analogs in the unregulated “street” opioid supply.
Pharmacologic interventions. Because there is limited empirical evidence on the efficacy of medication-assisted treatment (MAT) for adolescents with OUD, clinicians need to rely on evidence from research and experience with adults. Unfortunately, MAT is offered to adolescents considerably less often than it is to adults. Feder et al38 reported that only 2.4% of adolescents received MAT for heroin use and only 0.4% of adolescents received MAT for prescription opioid use, compared with 26.3% and 12% of adults, respectively.
Detoxification. Medications available for detoxification from opioids include opiates (such as methadone or buprenorphine) and clonidine (a central sympathomimetic). If the patient has used heroin for a short period (<1 year) and has no history of detoxification, consider a detoxification strategy with a longer-term taper (90 to 180 days) to allow for stabilization.
Maintenance treatment. Consider maintenance treatment for adolescents with a history of long-term opioid use and at least 2 prior short-term detoxification attempts or nonpharmacotherapy-based treatment within 12 months. Be sure to receive consent from a legal guardian and the patient. Maintenance treatment is usually recommended to continue for 1 to 6 years. Maintenance programs with longer durations have shown higher rates of abstinence, improved engagement, and retention in treatment.39
Continue to: According to guidelines from...
According to guidelines from the American Society of Addiction Medicine (ASAM), adolescents age >16 should be offered MAT; the first-line treatment is buprenorphine.40 To avoid risks of abuse and diversion, a combination of buprenorphine/naloxone may be administered.
Maintenance with buprenorphine
In order to prescribe and dispense buprenorphine, clinicians need to obtain a waiver from the Substance Abuse and Mental Health Services Administration. Before initiating buprenorphine, consider the type of opioid the individual used (short- or long-acting), the severity of the OUD, and the last reported use. The 3 phases of buprenorphine treatment are41:
- Induction phase. Buprenorphine can be initiated at 2 to 4 mg/d. Some patients may require up to 8 mg/d on the first day, which can be administered in divided doses.42 Evaluate and monitor patients carefully during the first few hours after the first dose. Patients should be in early withdrawal; otherwise, the buprenorphine might precipitate withdrawal. The induction phase can be completed in 2 to 4 days by titrating the dose so that the signs and symptoms of opioid withdrawal are minimal, and the patient is able to continue treatment. It may be helpful to have the patient’s legal guardian nearby in case the patient does not tolerate the medication or experiences withdrawal. The initial target dose for buprenorphine is approximately 12 to 16 mg/d.
- Stabilization phase. Patients no longer experience withdrawal symptoms and no longer have cravings. This phase can last 6 to 8 weeks. During this phase, patients should be seen weekly and doses should be adjusted if necessary. As a partial mu agonist, buprenorphine does not activate mu receptors fully and reaches a ceiling effect. Hence, doses >24 mg/d have limited added agonist properties.
- Maintenance phase. Because discontinuation of buprenorphine is associated with high relapse rates, patients may need to be maintained long-term on their stabilization dose, and for some patients, the length of time could be indefinite.39 During this phase, patients continue to undergo follow-up, but do so less frequently.
Methadone maintenance is generally not recommended for individuals age <18.
Preventing opioid diversion
Prescription medications that are kept in the home are a substantial source of opioids for adolescents. In 2014, 56% of 12th graders who did not need medications for medical purposes were able to acquire them from their friends or relatives; 36% of 12th graders used their own prescriptions.21 Limiting adolescents’ access to prescription opioids is the first line of prevention. Box 2 describes interventions and strategies to limit adolescents’ access to opioids.
Box 2
Many adolescents obtain opioids for recreational use from medications that were legitimately prescribed to family or friends. Both clinicians and parents/ guardians can take steps to reduce or prevent this type of diversion
Health care facilities. Regulating the number of pills dispensed to patients is crucial. It is highly recommended to prescribe only the minimal number of opioids necessary. In most cases, 3 to 7 days’ worth of opioids at a time might be sufficient, especially after surgical procedures.
Home. Families can limit adolescents’ access to prescription opioids in the home by keeping all medications in a lock box.
Proper disposal. Various entities offer locations for patients to drop off their unused opioids and other medications for safe disposal. These include police or fire departments and retail pharmacies. The US Drug Enforcement Administration sponsors a National Prescription Drug Take Back Day; see https://www.deadiversion.usdoj.gov/drug_disposal/takeback/index.html. The FDA also offers information on where and how to dispose of unused medicines at https://www.fda.gov/consumers/consumer-updates/where-and-how-dispose-unused-medicines.
CASE CONTINUED
Ms. L is initially prescribed, clonidine, 0.1 mg every 6 hours, to address opioid withdrawal. Clonidine is then tapered and maintained at 0.1 mg twice a day for irritability and impulse control. She is also prescribed sertraline, 100 mg/d, for depression and anxiety, and trazodone, 75 mg as needed at night, to assist with sleep.
Continue to: Following inpatient hospitalization...
Following inpatient hospitalization, during 12 weeks of partial hospital treatment, Ms. L participates in individual psychotherapy sessions 5 days/week; family therapy sessions once a week; and experiential therapy along with group sessions with other peers. She undergoes medication evaluations and adjustments on a weekly basis. Ms. L is now working at a store and is pursuing a high school equivalency certificate. She manages to avoid high-risk behaviors, although she reports having occasional cravings. Ms. L is actively involved in Narcotics Anonymous and has a sponsor. She has reconciled with her mother and moved back home, so she can stay away from her former acquaintances who are still using.
Bottom Line
Adolescents with opioid use disorder can benefit from an individualized treatment plan that includes psychosocial interventions, pharmacotherapy, or a combination of the two. Treatment planning should include the adolescent and his/her family to ensure they are able to verbalize their expectations. Treatment should focus on interventions that strengthen interpersonal relationships and assist with rehabilitation. Ongoing follow-up care is necessary for maintaining abstinence.
Related Resource
- Patkar AA, Weisler RH. Opioid abuse and overdose: Keep your patients safe. Current Psychiatry. 2017;16(8):8-12,14-16.
Drug Brand Names
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone
Clonidine • Clorpres
Methadone • Methadose
Naloxone • Narcan
Oxycodone • OxyContin
Sertraline • Zoloft
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.
1. Davis JP, Prindle JJ, Eddie D, et al. Addressing the opioid epidemic with behavioral interventions for adolescents and young adults: a quasi-experimental design. J Consult Clin Psychol. 2019;87(10):941-951.
2. National Institute on Drug Abuse; National Institutes of Health; U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. https://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends. Updated December 2019. Accessed January 13, 2020.
3. Hopfer CJ, Khuri E, Crowley TJ. Treating adolescent heroin use. J Am Acad Child Adolesc Psychiatry. 2003;42(5):609-611.
4. US Department of Justice, Drug Enforcement Agency, Diversion Control Division. https://www.deadiversion.usdoj.gov/. Accessed January 21, 2020.
5. Gaither JR, Leventhal JM, Ryan SA, et al. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997-2012. JAMA Pediatr. 2016;170(12):1195-1201.
6. Parker MA, Anthony JC. Epidemiological evidence on extra-medical use of prescription pain relievers: transitions from newly incident use to dependence among 12-21 year olds in United States using meta-analysis, 2002-13. Peer J. 2015;3:e1340. doi: 10.7717/peerj.1340. eCollection 2015.
7. Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11(5):360-363.
8. Borodovsky JT, Levy S, Fishman M. Buprenorphine treatment for adolescents and young adults with opioid use disorders: a narrative review. J Addict Med. 2018;12(3):170-183.
9. Centers for Disease Control and Prevention: National Center for Health Statistics. Drug overdose deaths in the United States, 1999-2016. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published December 2017. Accessed January 15, 2020.
10. Strain E. Opioid use disorder: epidemiology, pharmacology, clinical manifestation, course, screening, assessment, diagnosis. https://www.uptodate.com/contents/opioid-use-disorder-epidemiology-pharmacology-clinical-manifestations-course-screening-assessment-and-diagnosis. Updated August 15, 2019. Accessed January 21, 2020.
11. American Academy of Pediatrics Committee on Substance Use and Prevention. Policy statement: medication-assisted treatment of adolescents with opioid use disorder. Pediatrics. 2016;138(3):e20161893. doi: https://doi.org/10.1542/peds.2016-1893.
12. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:514.
13. Sadock BJ, Sadock VA. Chapter 6: Theories of personality and psychopathology. In: Sadock BJ, Sadock VA, eds. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:209.
14. Kandel DB. Stages and pathways of drug involvement: examining the gateway hypothesis. Cambridge, United Kingdom: Cambridge University Press; 2002.
15. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990;182-204.
16. Hopfer C, Salomonsen-Sautel S, Mikulich-Gilbertson S, et al. Conduct disorder and initiation of substance use: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2013;52(5):511-518.e4.
17. Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224-1239.
18. Crumley FE. Substance abuse and adolescent suicidal behavior. JAMA. 1990;263(22):3051-3056.
19. Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clinical Psychology: Science and Practice. 1996;3(1):25-46.
20. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorder in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953-959.
21. Yule AM, Wilens TE, Rausch PK. The opioid epidemic: what a child psychiatrist is to do? J Am Acad Child Adolesc Psychiatry. 2017;56(7);541-543.
22. CRAFFT. https://crafft.org. Accessed January 21, 2020.
23. Knight JR, Sherritt L, Harris SK, et al. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27(1):67-73.
24. American Psychiatric Association. Online assessment measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures. Accessed January 15, 2020.
25. National Institute of Drug Abuse. American Psychiatric Association adapted NIDA modified ASSIST tools. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/american-psychiatric-association-adapted-nida. Updated November 15, 2015. Accessed January 21, 2020.
26. Canada’s Mental Health & Addiction Network. Drug Use Screening Inventory (DUSI). https://www.porticonetwork.ca/web/knowledgex-archive/amh-specialists/screening-for-cd-in-youth/screening-both-mh-sud/dusi. Published 2009. Accessed January 21, 2020.
27. Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1-2):1-46.
28. Klitzner M, Gruenwald PJ, Taff GA, et al. The adolescent assessment referral system-final report. National Institute on Drug Abuse; Rockville, MD: 1993. NIDA Contract No. 271-89-8252.
29. Slesnick N, Tonigan JS. Assessment of alcohol and other drug use by runaway youths: a test-retest study of the Form 90. Alcohol Treat Q. 2004;22(2):21-34.
30. Winters KC, Kaminer Y. Screening and assessing adolescent substance use disorders in clinical populations. J Am Acad Child Adolesc Psychiatry. 2008;47(7):740-744.
31. Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21-27.
32. Deas D, Thomas SE. An overview of controlled study of adolescent substance abuse treatment. Am J Addiction. 2001;10(2):178-189.
33. William RJ, Chang, SY. A comprehensive and comparative review of adolescent substance abuse treatment outcome. Clinical Psychology: Science and Practice. 2000;7(2):138-166.
34. Bukstein OG, Work Group on Quality Issues. Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(6):609-621.
35. Van Hasselt VB, Null JA, Kempton T, et al. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18(1):9-18.
36. Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197-213.
37. US Food and Drug Administration. Information about naloxone. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Updated December 19, 2019. Accessed January 21, 2020.
38. Feder KA, Krawcyzk N, Saloner, B. Medication-assisted treatment for adolescents in specialty treatment for opioid use disorder. J Adolesc Health. 2018;60(6):747-750.
39. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003-2011.
40. US Department of Health and Human Services. Substance Abuse and Mental Health Ser-vices Administration. Medication-assisted treatment for opioid addiction in opioid treatment programs: a treatment improvement protocol TIP 43. https://www.asam.org/docs/advocacy/samhsa_tip43_matforopioidaddiction.pdf?sfvrsn=0. Published 2005. Accessed January 15, 2020.
41. US Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated September 9, 2019. Accessed January 21, 2020.
42. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(suppl 2):S59-S77.