User login
Managing psychiatric illness in patients with epilepsy
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
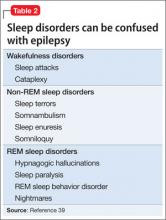
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
The late effects of cancer and cancer treatment: a rapid review
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Mechanical Ventilation in Hypoxemia
The indications for endotracheal intubation and mechanical ventilation in acutely hypoxemic patients depend on the severity of respiratory failure as well as the patient's hemodynamic and neurologic status. Once intubated, however, how a patient is ventilated can have a significant impact on the subsequent hospital course and ultimate outcome. Regardless of whether the hospitalist manages the ventilator directly, comanages patients in the intensive care unit (ICU), or merely transfers a hypoxemic patient into or out of an intensivist‐run unit, a basic familiarity with the evidence supporting various mechanical ventilation strategies will enhance the care provided. It is also helpful to understand the goals of mechanical ventilation in acute hypoxemic respiratory failure, such as minimizing the risk of ventilator‐induced lung injury, enhancing recovery from the underlying cause of respiratory failure, and limiting the duration of mechanical ventilation.[1, 2, 3] With these objectives in mind, this review will examine the evidence that supports specific ventilator strategies in common clinical conditions that cause acute hypoxemia.
First, we will discuss the evidence supporting the use of low tidal volume ventilation in patients with the acute respiratory distress syndrome (ARDS), as well as several novel ventilator modes that have been proposed as alternatives to low tidal volume ventilation in ARDS. We will also briefly review adjunctive therapies that may enhance the efficacy of lung‐protective ventilation in ARDS. We will then discuss emerging evidence regarding the use of lung‐protective ventilation strategies in patients without ARDS, as well as potential contraindications to this approach. Finally, we will cover rescue strategies for refractory hypoxemia, as well as an evidence‐based approach to weaning from mechanical ventilation.
LUNG‐PROTECTIVE VENTILATION IN ARDS
Low Tidal Volume Ventilation
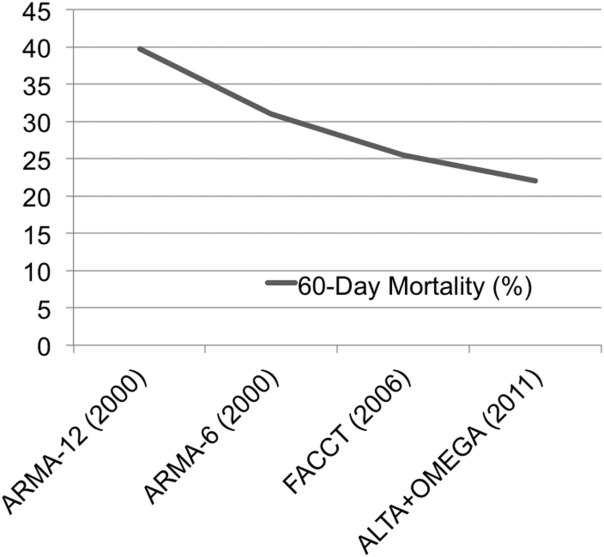
Over a decade following the original ARDS Clinical Network trial of lower versus traditional tidal volume ventilation, it is broadly accepted that ventilation with tidal volumes 6 mL/kg predicted body weight, targeting a plateau pressure 30 cm H2O, reduces mortality and increases ventilator‐free days in patients with ARDS.[4, 5, 6] Moreover, lung‐protective ventilation appears to reduce mortality in all patients with ARDS, regardless of the associated clinical disorder.[7] The substantial decline in mortality in ARDS observed over the past decade (Figure 1) is due in part to the broader use of lung‐protective ventilation.[8, 9]
Despite the strong evidence supporting the value of lung‐protective ventilation for decreasing mortality in ARDS, adherence to low tidal volume strategies in ARDS patients remains variable.[10, 11] This may be due to several reasons, including (1) mistakenly using actual instead of predicted body weight to determine appropriate tidal volume, (2) lack of awareness of the changes made by the most recent consensus‐based definition of ARDS (Table 1),[12] (3) under‐recognition of the heterogeneity of chest radiograph findings in ARDS (Figure 2), and (4) underdiagnosis of ARDS by providers.[13] Thus, prompt recognition of ARDS and the immediate initiation of lung‐protective ventilation strategies should be a high priority in caring for all patients with ARDS. Table 2 summarizes how to implement the ARDS network lung‐protective strategy, including how to determine the correct tidal volume based on predicted body weight, calculated from the patient's sex and height. Although a full discussion of the relative merits of pressure control versus volume control ventilation is outside the scope of this review, it is worth noting that either mode can be used to achieve low tidal volumes, and which mode is selected is often determined by individual patient factors and institutional or provider preference.
| |
| Timing | Within 7 days of known clinical insult or new/worsening respiratory symptoms. |
| Chest imaging | Chest radiograph or CT: bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis, or nodules. |
| Cause of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment (eg, echocardiography) required to exclude hydrostaticedema if no ARDS risk factor present. |
| Oxygenation deficit | Mild: PaO2/FiO2< 300 but >200 mm Hg, on 5 cm H2O PEEP/CPAPa |
| Moderate: PaO2/FiO2 200 but >100 mm Hg, on 5 cm H2O PEEP/CPAP | |
| Severe: PaO2/FiO2 100 mm Hg on 5 cm H2O PEEP/CPAP | |
| |||||||||||||
| To calculate predicted body weight: | |||||||||||||
| Male PBW: 50 + 2.3 (height in inches 60) or 50 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Female PBW: 45.5 + 2.3 (height in inches 60) or 45.5 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Select assist control mode | |||||||||||||
| Set initial VT at 8 mL/kg PBW | |||||||||||||
| Reduce VT by 1 mL/kg at intervals < 2 hours until VT = 6 mL/kg PBW | |||||||||||||
| Set initial RR to approximate baseline minute ventilation (maximum RR = 35/minute) | |||||||||||||
| Adjust VT and RR further to achieve Pplat and pH goals | |||||||||||||
| If Pplat> 30 cm H2O: decrease VT by 1 mL/kg PBW (minimum = 4 mL/kg PBW) | |||||||||||||
| If pH 7.30, increase RR (maximum = 35) | |||||||||||||
| If pH < 7.15, increase RR to 35; consider sodium bicarbonate administration or increase VT | |||||||||||||
| FiO2/PEEP combinations | |||||||||||||
| FiO2 | |||||||||||||
| 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP (cm H2O) | |||||||||||||
| 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18, 22, 24 |

Positive End‐Expiratory Pressure and Recruitment Maneuvers
The application of positive end‐expiratory pressure (PEEP) can prevent alveolar derecruitment and atelectrauma; too much PEEP, however, can cause alveolar overdistension or hemodynamic compromise due to high intrathoracic pressures and decreased venous return. Likewise, recruitment maneuvers, in which a high PEEP is applied for a brief interval, may improve oxygenation by opening up atelectatic alveoli, but can also cause barotrauma or hemodynamic compromise. Thus, in addition to research into the effects of low tidal volume ventilation, 3 additional trials have tested the potential value of higher versus lower PEEP in ARDS.[14, 15, 16] Although none of these trials showed a significant reduction in mortality with a higher PEEP strategy, a recent meta‐analysis of the data from all 3 trials reported a statistically significant mortality benefit for ARDS patients with a higher‐PEEP strategy versus a lower‐PEEP strategy (adjusted relative risk [RR], 0.90; 95% confidence interval [CI], 0.81‐1.00; P = 0.049).[17] Because of differences in trial design and patient selection, however, a change of practice cannot be reasonably based on this meta‐analysis alone. Current research is focused on whether there is a subset of ARDS patients who may benefit from a higher PEEP strategy, and how best to determine optimal PEEP more generally.[18, 19] In addition to these ongoing questions about PEEP, the value of recruitment maneuvers remains uncertain.[1, 20]
High‐Frequency Oscillating Ventilation
High‐frequency oscillating ventilation (HFOV) is a technique in which very small tidal volumes are delivered at high frequency (315 breaths per second) at high mean airway pressures. Until recently, trials of HFOV in ARDS have been inconclusive due to small size or inappropriate control arms that did not utilize low tidal volume ventilation.[21] However, 2 recent large, multicenter, randomized trials comparing HFOV to low tidal volume ventilation in ARDS have shown that there is no benefit (and perhaps even harm) associated with HFOV. The Oscillation in ARDS (OSCAR) trial reported no change in mortality, whereas the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trial found that HFOV was associated with increased risk of death.[22, 23] As such, HFOV is no longer recommended in ARDS.
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a mode of ventilation, in which a relatively high level of continuous positive airway pressure (P high) is applied for a large portion of the respiratory cycle. During the time spent at P high (T high), the patient can take small spontaneous breaths, with or without the assistance of additional pressure support. At the end of T high, the applied pressure releases to a lower level (P low) for a brief time (T low) to allow CO2 clearance (Figure 3).

Theoretically, the long inflation time in APRV allows for more uniform recruitment of alveoli and raises mean airway pressure without increasing barotrauma. APRV also allows for spontaneous breathing even at high levels of support. Despite preclinical and observational data suggesting that APRV may reduce the development or progression of lung injury,[24, 25, 26, 27] prospective clinical trials comparing APRV to low tidal volume ventilation have yet to support any clear benefit, and 1 trial has demonstrated a trend toward more days of mechanical ventilation.[28, 29] Multiple clinical trials are ongoing (NCT01901354, NCT01339533), but in the interim, the use of APRV instead of conventional low tidal volume ventilation is not supported by high‐level evidence.
ADJUNCTIVE THERAPIES IN ARDS
Although a full discussion of the numerous nonventilatory therapies that have been tested for ARDS is beyond the scope of this focused review, several of these strategies have been shown to improve outcomes and deserve mention here.
Fluid Management
The first such therapy is the implementation of a fluid conservative strategy. This approach is based on the ARDS network Fluid and Catheter Treatment Trial (FACTT), which demonstrated that in the absence of shock or oliguria, a fluid‐conservative strategy improves lung function and decreases the duration of mechanical ventilation in ARDS patients.[30] Indeed, multiple studies have found that a positive fluid balance is associated with worsened multiorgan dysfunction and poor outcomes in patients with ARDS.[31] In terms of translating this evidence into practice, the ARDS Network has published a simplified algorithm for conservative fluid management based on the results of FACTT.[32]
Prone Positioning
Although prone positioning during mechanical ventilation improves oxygenation by improving lung recruitment and ventilation‐perfusion matching, several early trials of prone positioning did not demonstrate a mortality benefit. Although a 2010 meta‐analysis of 10 previous trials did find a mortality benefit in the most hypoxemic patients, there was also an increased risk of pressure ulcers and endotracheal tube obstruction.[33] Thus, the indications for prone positioning in ARDS remained uncertain until 2013, when Guerin et al. reported the results of a large, multicenter, randomized trial that demonstrated a major reduction in mortality in ARDS patients treated with prone positioning.[34] The trial included 466 patients with early ARDS, in whom the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) was < 150 mm Hg on an FiO2 of at least 0.6 and PEEP of at least 5 cm H2O. Of note, all the sites involved in the trial (26 centers in France, 1 in Spain) had extensive experience with prone positioning prior to the trial. The rate of death at 28 days was 33% in the supine group and 16% in the prone group (hazard ratio 0.39 [95% CI, 0.25‐0.63]; P < 0.001); this mortality reduction persisted at 90 days, and after adjustment for Sequential Organ Failure Assessment (SOFA) score, use of vasopressors, and use of neuromuscular blockade. Finally, there was no difference in adverse events (such as unplanned extubation) between groups. Implementation of prone‐positioning protocols in less experienced centers with higher rates of obesity will be challenging, and additional confirmatory trials would be ideal. Nevertheless, this trial will prompt broader application of prone positioning in patients with moderate to severe ARDS.
Neuromuscular Blockade
In addition to conservative fluid management, early consideration of neuromuscular blockade (NMB) in patients with moderate‐to‐severe ARDS likely improves outcomes. NMB may enhance the protective effects of low tidal volume ventilation in the most hypoxemic ARDS patients, because it removes the resistance of the chest wall and the diaphragm, and more importantly, reduces dyssynchrony between the patient and the ventilator. Although previous studies of NMB in ARDS yielded conflicting results, a more recent well‐done randomized clinical trial showed a mortality benefit. In this trial, 340 patients with a PaO2/FiO2 ratio of <150 mm Hg were randomized to receive a 48‐hour infusion of cisatracurium (a nondepolarizing neuromuscular blocking agent) or placebo within 48 hours of ARDS onset.[35] Both groups were deeply sedated and ventilated with low tidal volumes, but mortality was lower in patients treated with NMB compared to patients who did not receive NMB. Although there are understandable concerns that NMB will mask the ability to detect important changes in the patient's clinical exam and increase risk of ICU‐acquired weakness, the results of this trial suggest that clinicians should strongly consider early, short‐term NMB with cisatracurium in patients with moderate‐to‐severe ARDS.
Other Pharmacotherapies
Although several other pharmacologic interventions for ARDS have been studied (eg, glucocorticoids, exogenous surfactant, activated protein C, inhaled ‐agonists), none has demonstrated a mortality benefit.[9]
BEYOND ARDS: LUNG‐PROTECTIVE VENTILATION FOR ALL?
Low Tidal Volume Ventilation Strategies in Patients Without ARDS
Given concerns about ventilator‐induced lung injury and the known benefits of lung‐protective ventilation in patients with ARDS, there is growing interest in determining whether low tidal volume ventilation may be beneficial to mechanically ventilated patients who do not have ARDS. In 2010, Serpa Neto et al. published a meta‐analysis of 20 studies (mixed population of >2800 ICU and operating room patients) comparing lower versus higher tidal volume ventilation in patients without ARDS.[36] They found that low tidal volume ventilation (mean tidal volume of 6.5 mL/kg) was associated with significantly decreased mortality and risk of lung injury compared to ventilation with higher tidal volumes (mean tidal volume 10.6 mL/kg). This investigation has been followed by a randomized, double‐blind trial of intraoperative low tidal volume ventilation in 400 patients at intermediate or high risk for pulmonary complications after major abdominal surgery.[37] Remarkably, lower tidal volume ventilation was associated with a decreased risk of both pulmonary and extrapulmonary complications in the first week following surgery. These studies are in line with preclinical animal studies that show an association between higher tidal volume ventilation and development of lung injury.[38] Although this evidence does not warrant indiscriminate low tidal volume ventilation in all critically ill patients, it certainly suggests that clinicians should strongly consider lung protective ventilation in patients at high risk for ARDS (eg, patients with pneumonia, aspiration, sepsis, or massive transfusion), and points to an urgent need for more randomized clinical trials of low tidal volume and lung‐protective ventilation in various groups of patients who do not have ARDS.
Potential Contraindications to Lower Tidal Volume, Higher PEEP Ventilation
Despite speculation that a lower tidal volume ventilation strategy may be superior to conventional ventilation in most mechanically ventilated patients, there are some clinical scenarios in which typical lung‐protective ventilation protocols are not appropriate. First, there are some patients (eg, patients with neurologic injury or pulmonary hypertension) in whom the lower oxygenation and permissive hypercapnia targeted by lung‐protective ventilation protocols may be harmful. Second, higher PEEP protocols may be dangerous for patients with pneumothorax or who are at risk for bronchopleural fistula. Third, patients with airway obstruction often require lower respiratory rates to permit maximization of expiratory time; if tidal volume is lowered aggressively as part of a lung‐protective ventilation protocol, higher respiratory rates may be required to achieve PaCO2/arterial pH goals, leading to decreased expiratory time and worsening air trapping. Finally, because mandatory low tidal volumes may be poorly tolerated in some patients, allowing low‐risk patients to transition directly to a spontaneous breathing mode may have benefits that outweigh those of lung‐protective ventilation protocols, including decreased need for sedating medications, less muscle atrophy, shorter duration of intubation and mechanical ventilation, and a lower incidence of delirium.[39]
RESCUE THERAPIES FOR REFRACTORY HYPOXEMIA
Despite treatment with lung‐protective ventilation and the best adjunctive strategies, some patients may progress to develop life‐threatening, refractory hypoxemia. Beyond the therapies already discussed (ie, prone positioning or neuromuscular blockade), there are additional interventions that should be considered in such cases.
Inhaled Vasodilator
Inhaled vasodilators may improve ventilation‐perfusion matching and improve pulmonary hypertension by selectively causing local vasodilation in well‐ventilated areas of the lung. Although there are several inhaled vasodilators available, including inhaled nitric oxide (iNO), inhaled prostacyclin, and inhaled prostaglandin E1, the best studied in ARDS is iNO. Although multiple studies have found transient improvement in oxygenation with iNO therapy in ARDS, a mortality benefit has never been demonstrated.[40] In addition, concerns about high cost, sophisticated equipment requirements, the risk of methemoglobinemia, and the potential increased risk of renal failure found in a 2007 meta‐analysis have limited the use of iNO in ARDS.[41] Thus, inhaled vasodilators should be considered only for patients with preexisting pulmonary hypertension or as a true rescue therapy in refractory hypoxemia cases, where the transient oxygenation could act as a bridge to other therapies.[40]
Extracorporeal Membrane Oxygenation
The use of extracorporeal membrane oxygenation (ECMO) in refractory acute hypoxemic respiratory failure in adults is an evolving therapy for which evidence is still emerging. During ECMO, blood is removed from the body, circulated by a mechanical pump through a membrane oxygenator, and then returned to the body. Observational studies have shown improved survival with ECMO compared to historic survival rates, and a study of 75 matched pairs of patients with severe influenza A (H1N1)‐related ARDS comparing mortality between patients transferred to an ECMO center and those who continued to receive conventional care, found improved survival in transferred patients compared to matched, nonreferred patients.[42] The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was a multicenter trial in which 180 patients with severe but potentially reversible respiratory failure were randomized to receive either conventional management or referral for consideration of ECMO to a major referral center in the United Kingdom.[43] Of the 90 patients referred for ECMO consideration, 76% actually received ECMO. Death or severe disability at 6 months occurred in 37% of the ECMO‐referred patients versus 53% of the conventional therapy patients (RR, 0.69; 95% CI, 0.05‐0.97; P = 0.03). Whether the benefit observed in the CESAR trial was due to ECMO itself or due to cointerventions and expert management at the referral ECMO center remains unclear. The exact indications, timing, titration, optimal cointerventions, and end points of ECMO therapy are likewise unsettled, and further trials are ongoing in Europe (NCT01470703). Nonetheless, based on the findings of the CESAR trial, consideration of transfer to an experienced ECMO center is recommended for patients with refractory hypoxemia who fail aggressive conventional therapy, and have potentially reversible disease or are possible candidates for lung transplant.[44]
LIBERATION FROM MECHANICAL VENTILATION
Once the underlying cause of respiratory failure is resolved and the patient demonstrates improvement, clinicians' attention must turn to decreasing the duration of mechanical ventilation. Some argue that the phrase weaning from mechanical ventilation is not always appropriate, as it implies a protracted, gradual process that is often not required; liberation from mechanical ventilation has been offered as a better description of the task of transitioning a patient back to normal breathing after they demonstrate readiness for spontaneous breathing and extubation.[3] Regardless of the terminology, the same principle applies: once ready, patients should be extubated as expeditiously as possible.
In addition to evidence‐based management strategies aimed at limiting the time a patient requires mechanical ventilation (such as lung‐protective ventilation, a fluid conservative strategy, and ventilator‐associated pneumonia prevention bundles), there is also the question of how to best assess whether a patient is ready for transition back to normal breathing, and how to operationalize that transition. This process may account for more than half of the total duration of mechanical ventilation in some cases.[3] Based on evidence from trials assessing various weaning protocols published in the 1990s, daily spontaneous breathing trials (in which the ventilator provides zero or minimal support during patient triggered breaths) are favored over slow weaning of pressure support or intermittent mandatory ventilation.[45] Although several novel ventilator modes aimed at improving patient‐ventilator interaction (eg, adaptive support ventilation, proportional assist ventilation, and neurally adjusted ventilatory assistance) have been proposed as optimal weaning modes, their benefit is theoretical, and data demonstrating improved outcomes are lacking.[28]
In addition to evidence supporting daily spontaneous breathing trials (SBTs), a Cochrane Database systematic review and meta‐analysis published in 2011 found that protocolized weaning was associated with shorter duration of mechanical ventilation than usual care.[2] Although the specifics of what constitutes the optimal weaning protocol remain unclear, there is general agreement that a standardized approach involving prespecified criteria and daily assessment for readiness for spontaneous breathing and potential extubation improves patient outcomes.[3] If the SBT is well tolerated hemodynamically, respiratory mechanics and gas exchange remain adequate, and airway factors and mental status permit, the patient should be extubated.
As emphasized in an excellent recent review by McConville and Kress, patients who fail 3 or more SBTs, or remain mechanically ventilated for 7 or more days following their first failed SBT, as well as patients who require reintubation after failed extubation, are at increased risk of in‐hospital mortality and prolonged hospital stay.[3, 46] For patients who fall into these categories without a clearly reversible cause, clinicians should consider initiating discussions about tracheostomy and goals of care. It should be noted, however, that multiple trials have failed to demonstrate the benefit of early tracheostomy, and the optimal timing of this intervention remains uncertain.[47]
CONCLUSIONS
When hypoxemic respiratory failure requires endotracheal intubation and mechanical ventilation, the clinician's management of the ventilator can have a profound impact on patient outcomes. Prompt recognition of ARDS and use of a lung‐protective ventilation strategy, as well as evidence‐based adjunctive therapies, remain the cornerstones of caring for patients with ARDS. Based on 2 recent large trials, HFOV is no longer recommended in ARDS. APRV in ARDS is also not supported by current evidence, though clinical trials are ongoing. In contrast, certain adjunctive therapies in ARDS, such as a conservative fluid strategy, early neuromuscular blockade, and prone positioning for moderate‐to‐severe cases, improve outcomes. There is also preliminary evidence to support the use of a lung‐protective strategy in selected non‐ARDS patients, especially in patients at high risk for developing ARDS. In cases of refractory hypoxemia and potentially survivable disease, extracorporeal membrane oxygenation should be considered. Finally, once the patient demonstrates signs of recovery, the best approach to liberation from mechanical ventilation involves daily protocolized, spontaneous breathing trials and assessment of readiness for extubation.
Acknowledgements
The authors thank Andrew Manies for his invaluable assistance in preparing this article.
Disclosures
Dr. Wilson was supported by National Institutes of Health grant U01 HL108713, and Dr. Matthay was supported by National Institutes of Health grants U01 HL108713 and R37 HL051856. The authors report no conflicts of interest.
- , . Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- , , , , , . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta‐analysis. BMJ. 2011;342:c7237.
- , . Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–2239.
- , , , et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354.
- , , , . A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308.
- , , , et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236.
- , . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127.
- , , . The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740.
- , , , et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468.
- , , , et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
- , , , et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- , , , et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234.
- , , , et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- , , , et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- , , , et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336.
- , , , et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865–873.
- , , , et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104.
- , , , et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423.
- , , . Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20(1):63–68.
- , . Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 suppl):S170–S174.
- , , , et al. High‐frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805.
- . High‐frequency oscillation for ARDS. N Engl J Med. 2013;368(23):2234.
- , , , et al. Early airway pressure release ventilation prevents ARDS‐a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
- , , , et al. Airway pressure release ventilation prevents ventilator‐induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012.
- , , , et al. Early application of airway pressure release ventilation may reduce mortality in high‐risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641.
- , , , et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49.
- , . Novel modes of mechanical ventilation. Semin Respir Crit Care Med. 2013;34(4):499–507.
- , , , et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma. 2010;69(3):501–510; discussion 511.
- , , , et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- , , , et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra‐abdominal pressure: a pilot study looking at the effects of PAL‐treatment. Ann Intensive Care. 2012;(2 suppl 1):S15.
- , . Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920.
- , , , et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Med. 2010;36(4):585–599.
- , , , et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- , , , et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116.
- , , , et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA. 2012;308(16):1651–1659.
- , , , et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437.
- , , . Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta‐analysis. Curr Opin Crit Care. 2014;20(1):25–32.
- . Low tidal volumes for all? JAMA. 2012;308(16):1689–1690.
- , . Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304(22):2521–2527.
- , , , , , . Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ. 2007;334(7597):779.
- , , , et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–1668.
- , , , et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–350.
- , , . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302.
- , , , . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129.
- , , , , , . Enteral omega‐3 fatty acid, gamma‐linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; , , , et al. Randomized, placebo‐controlled clinical trial of an aerosolized beta‐agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568.
The indications for endotracheal intubation and mechanical ventilation in acutely hypoxemic patients depend on the severity of respiratory failure as well as the patient's hemodynamic and neurologic status. Once intubated, however, how a patient is ventilated can have a significant impact on the subsequent hospital course and ultimate outcome. Regardless of whether the hospitalist manages the ventilator directly, comanages patients in the intensive care unit (ICU), or merely transfers a hypoxemic patient into or out of an intensivist‐run unit, a basic familiarity with the evidence supporting various mechanical ventilation strategies will enhance the care provided. It is also helpful to understand the goals of mechanical ventilation in acute hypoxemic respiratory failure, such as minimizing the risk of ventilator‐induced lung injury, enhancing recovery from the underlying cause of respiratory failure, and limiting the duration of mechanical ventilation.[1, 2, 3] With these objectives in mind, this review will examine the evidence that supports specific ventilator strategies in common clinical conditions that cause acute hypoxemia.
First, we will discuss the evidence supporting the use of low tidal volume ventilation in patients with the acute respiratory distress syndrome (ARDS), as well as several novel ventilator modes that have been proposed as alternatives to low tidal volume ventilation in ARDS. We will also briefly review adjunctive therapies that may enhance the efficacy of lung‐protective ventilation in ARDS. We will then discuss emerging evidence regarding the use of lung‐protective ventilation strategies in patients without ARDS, as well as potential contraindications to this approach. Finally, we will cover rescue strategies for refractory hypoxemia, as well as an evidence‐based approach to weaning from mechanical ventilation.
LUNG‐PROTECTIVE VENTILATION IN ARDS
Low Tidal Volume Ventilation
Over a decade following the original ARDS Clinical Network trial of lower versus traditional tidal volume ventilation, it is broadly accepted that ventilation with tidal volumes 6 mL/kg predicted body weight, targeting a plateau pressure 30 cm H2O, reduces mortality and increases ventilator‐free days in patients with ARDS.[4, 5, 6] Moreover, lung‐protective ventilation appears to reduce mortality in all patients with ARDS, regardless of the associated clinical disorder.[7] The substantial decline in mortality in ARDS observed over the past decade (Figure 1) is due in part to the broader use of lung‐protective ventilation.[8, 9]

Despite the strong evidence supporting the value of lung‐protective ventilation for decreasing mortality in ARDS, adherence to low tidal volume strategies in ARDS patients remains variable.[10, 11] This may be due to several reasons, including (1) mistakenly using actual instead of predicted body weight to determine appropriate tidal volume, (2) lack of awareness of the changes made by the most recent consensus‐based definition of ARDS (Table 1),[12] (3) under‐recognition of the heterogeneity of chest radiograph findings in ARDS (Figure 2), and (4) underdiagnosis of ARDS by providers.[13] Thus, prompt recognition of ARDS and the immediate initiation of lung‐protective ventilation strategies should be a high priority in caring for all patients with ARDS. Table 2 summarizes how to implement the ARDS network lung‐protective strategy, including how to determine the correct tidal volume based on predicted body weight, calculated from the patient's sex and height. Although a full discussion of the relative merits of pressure control versus volume control ventilation is outside the scope of this review, it is worth noting that either mode can be used to achieve low tidal volumes, and which mode is selected is often determined by individual patient factors and institutional or provider preference.
| |
| Timing | Within 7 days of known clinical insult or new/worsening respiratory symptoms. |
| Chest imaging | Chest radiograph or CT: bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis, or nodules. |
| Cause of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment (eg, echocardiography) required to exclude hydrostaticedema if no ARDS risk factor present. |
| Oxygenation deficit | Mild: PaO2/FiO2< 300 but >200 mm Hg, on 5 cm H2O PEEP/CPAPa |
| Moderate: PaO2/FiO2 200 but >100 mm Hg, on 5 cm H2O PEEP/CPAP | |
| Severe: PaO2/FiO2 100 mm Hg on 5 cm H2O PEEP/CPAP | |
| |||||||||||||
| To calculate predicted body weight: | |||||||||||||
| Male PBW: 50 + 2.3 (height in inches 60) or 50 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Female PBW: 45.5 + 2.3 (height in inches 60) or 45.5 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Select assist control mode | |||||||||||||
| Set initial VT at 8 mL/kg PBW | |||||||||||||
| Reduce VT by 1 mL/kg at intervals < 2 hours until VT = 6 mL/kg PBW | |||||||||||||
| Set initial RR to approximate baseline minute ventilation (maximum RR = 35/minute) | |||||||||||||
| Adjust VT and RR further to achieve Pplat and pH goals | |||||||||||||
| If Pplat> 30 cm H2O: decrease VT by 1 mL/kg PBW (minimum = 4 mL/kg PBW) | |||||||||||||
| If pH 7.30, increase RR (maximum = 35) | |||||||||||||
| If pH < 7.15, increase RR to 35; consider sodium bicarbonate administration or increase VT | |||||||||||||
| FiO2/PEEP combinations | |||||||||||||
| FiO2 | |||||||||||||
| 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP (cm H2O) | |||||||||||||
| 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18, 22, 24 |

Positive End‐Expiratory Pressure and Recruitment Maneuvers
The application of positive end‐expiratory pressure (PEEP) can prevent alveolar derecruitment and atelectrauma; too much PEEP, however, can cause alveolar overdistension or hemodynamic compromise due to high intrathoracic pressures and decreased venous return. Likewise, recruitment maneuvers, in which a high PEEP is applied for a brief interval, may improve oxygenation by opening up atelectatic alveoli, but can also cause barotrauma or hemodynamic compromise. Thus, in addition to research into the effects of low tidal volume ventilation, 3 additional trials have tested the potential value of higher versus lower PEEP in ARDS.[14, 15, 16] Although none of these trials showed a significant reduction in mortality with a higher PEEP strategy, a recent meta‐analysis of the data from all 3 trials reported a statistically significant mortality benefit for ARDS patients with a higher‐PEEP strategy versus a lower‐PEEP strategy (adjusted relative risk [RR], 0.90; 95% confidence interval [CI], 0.81‐1.00; P = 0.049).[17] Because of differences in trial design and patient selection, however, a change of practice cannot be reasonably based on this meta‐analysis alone. Current research is focused on whether there is a subset of ARDS patients who may benefit from a higher PEEP strategy, and how best to determine optimal PEEP more generally.[18, 19] In addition to these ongoing questions about PEEP, the value of recruitment maneuvers remains uncertain.[1, 20]
High‐Frequency Oscillating Ventilation
High‐frequency oscillating ventilation (HFOV) is a technique in which very small tidal volumes are delivered at high frequency (315 breaths per second) at high mean airway pressures. Until recently, trials of HFOV in ARDS have been inconclusive due to small size or inappropriate control arms that did not utilize low tidal volume ventilation.[21] However, 2 recent large, multicenter, randomized trials comparing HFOV to low tidal volume ventilation in ARDS have shown that there is no benefit (and perhaps even harm) associated with HFOV. The Oscillation in ARDS (OSCAR) trial reported no change in mortality, whereas the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trial found that HFOV was associated with increased risk of death.[22, 23] As such, HFOV is no longer recommended in ARDS.
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a mode of ventilation, in which a relatively high level of continuous positive airway pressure (P high) is applied for a large portion of the respiratory cycle. During the time spent at P high (T high), the patient can take small spontaneous breaths, with or without the assistance of additional pressure support. At the end of T high, the applied pressure releases to a lower level (P low) for a brief time (T low) to allow CO2 clearance (Figure 3).

Theoretically, the long inflation time in APRV allows for more uniform recruitment of alveoli and raises mean airway pressure without increasing barotrauma. APRV also allows for spontaneous breathing even at high levels of support. Despite preclinical and observational data suggesting that APRV may reduce the development or progression of lung injury,[24, 25, 26, 27] prospective clinical trials comparing APRV to low tidal volume ventilation have yet to support any clear benefit, and 1 trial has demonstrated a trend toward more days of mechanical ventilation.[28, 29] Multiple clinical trials are ongoing (NCT01901354, NCT01339533), but in the interim, the use of APRV instead of conventional low tidal volume ventilation is not supported by high‐level evidence.
ADJUNCTIVE THERAPIES IN ARDS
Although a full discussion of the numerous nonventilatory therapies that have been tested for ARDS is beyond the scope of this focused review, several of these strategies have been shown to improve outcomes and deserve mention here.
Fluid Management
The first such therapy is the implementation of a fluid conservative strategy. This approach is based on the ARDS network Fluid and Catheter Treatment Trial (FACTT), which demonstrated that in the absence of shock or oliguria, a fluid‐conservative strategy improves lung function and decreases the duration of mechanical ventilation in ARDS patients.[30] Indeed, multiple studies have found that a positive fluid balance is associated with worsened multiorgan dysfunction and poor outcomes in patients with ARDS.[31] In terms of translating this evidence into practice, the ARDS Network has published a simplified algorithm for conservative fluid management based on the results of FACTT.[32]
Prone Positioning
Although prone positioning during mechanical ventilation improves oxygenation by improving lung recruitment and ventilation‐perfusion matching, several early trials of prone positioning did not demonstrate a mortality benefit. Although a 2010 meta‐analysis of 10 previous trials did find a mortality benefit in the most hypoxemic patients, there was also an increased risk of pressure ulcers and endotracheal tube obstruction.[33] Thus, the indications for prone positioning in ARDS remained uncertain until 2013, when Guerin et al. reported the results of a large, multicenter, randomized trial that demonstrated a major reduction in mortality in ARDS patients treated with prone positioning.[34] The trial included 466 patients with early ARDS, in whom the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) was < 150 mm Hg on an FiO2 of at least 0.6 and PEEP of at least 5 cm H2O. Of note, all the sites involved in the trial (26 centers in France, 1 in Spain) had extensive experience with prone positioning prior to the trial. The rate of death at 28 days was 33% in the supine group and 16% in the prone group (hazard ratio 0.39 [95% CI, 0.25‐0.63]; P < 0.001); this mortality reduction persisted at 90 days, and after adjustment for Sequential Organ Failure Assessment (SOFA) score, use of vasopressors, and use of neuromuscular blockade. Finally, there was no difference in adverse events (such as unplanned extubation) between groups. Implementation of prone‐positioning protocols in less experienced centers with higher rates of obesity will be challenging, and additional confirmatory trials would be ideal. Nevertheless, this trial will prompt broader application of prone positioning in patients with moderate to severe ARDS.
Neuromuscular Blockade
In addition to conservative fluid management, early consideration of neuromuscular blockade (NMB) in patients with moderate‐to‐severe ARDS likely improves outcomes. NMB may enhance the protective effects of low tidal volume ventilation in the most hypoxemic ARDS patients, because it removes the resistance of the chest wall and the diaphragm, and more importantly, reduces dyssynchrony between the patient and the ventilator. Although previous studies of NMB in ARDS yielded conflicting results, a more recent well‐done randomized clinical trial showed a mortality benefit. In this trial, 340 patients with a PaO2/FiO2 ratio of <150 mm Hg were randomized to receive a 48‐hour infusion of cisatracurium (a nondepolarizing neuromuscular blocking agent) or placebo within 48 hours of ARDS onset.[35] Both groups were deeply sedated and ventilated with low tidal volumes, but mortality was lower in patients treated with NMB compared to patients who did not receive NMB. Although there are understandable concerns that NMB will mask the ability to detect important changes in the patient's clinical exam and increase risk of ICU‐acquired weakness, the results of this trial suggest that clinicians should strongly consider early, short‐term NMB with cisatracurium in patients with moderate‐to‐severe ARDS.
Other Pharmacotherapies
Although several other pharmacologic interventions for ARDS have been studied (eg, glucocorticoids, exogenous surfactant, activated protein C, inhaled ‐agonists), none has demonstrated a mortality benefit.[9]
BEYOND ARDS: LUNG‐PROTECTIVE VENTILATION FOR ALL?
Low Tidal Volume Ventilation Strategies in Patients Without ARDS
Given concerns about ventilator‐induced lung injury and the known benefits of lung‐protective ventilation in patients with ARDS, there is growing interest in determining whether low tidal volume ventilation may be beneficial to mechanically ventilated patients who do not have ARDS. In 2010, Serpa Neto et al. published a meta‐analysis of 20 studies (mixed population of >2800 ICU and operating room patients) comparing lower versus higher tidal volume ventilation in patients without ARDS.[36] They found that low tidal volume ventilation (mean tidal volume of 6.5 mL/kg) was associated with significantly decreased mortality and risk of lung injury compared to ventilation with higher tidal volumes (mean tidal volume 10.6 mL/kg). This investigation has been followed by a randomized, double‐blind trial of intraoperative low tidal volume ventilation in 400 patients at intermediate or high risk for pulmonary complications after major abdominal surgery.[37] Remarkably, lower tidal volume ventilation was associated with a decreased risk of both pulmonary and extrapulmonary complications in the first week following surgery. These studies are in line with preclinical animal studies that show an association between higher tidal volume ventilation and development of lung injury.[38] Although this evidence does not warrant indiscriminate low tidal volume ventilation in all critically ill patients, it certainly suggests that clinicians should strongly consider lung protective ventilation in patients at high risk for ARDS (eg, patients with pneumonia, aspiration, sepsis, or massive transfusion), and points to an urgent need for more randomized clinical trials of low tidal volume and lung‐protective ventilation in various groups of patients who do not have ARDS.
Potential Contraindications to Lower Tidal Volume, Higher PEEP Ventilation
Despite speculation that a lower tidal volume ventilation strategy may be superior to conventional ventilation in most mechanically ventilated patients, there are some clinical scenarios in which typical lung‐protective ventilation protocols are not appropriate. First, there are some patients (eg, patients with neurologic injury or pulmonary hypertension) in whom the lower oxygenation and permissive hypercapnia targeted by lung‐protective ventilation protocols may be harmful. Second, higher PEEP protocols may be dangerous for patients with pneumothorax or who are at risk for bronchopleural fistula. Third, patients with airway obstruction often require lower respiratory rates to permit maximization of expiratory time; if tidal volume is lowered aggressively as part of a lung‐protective ventilation protocol, higher respiratory rates may be required to achieve PaCO2/arterial pH goals, leading to decreased expiratory time and worsening air trapping. Finally, because mandatory low tidal volumes may be poorly tolerated in some patients, allowing low‐risk patients to transition directly to a spontaneous breathing mode may have benefits that outweigh those of lung‐protective ventilation protocols, including decreased need for sedating medications, less muscle atrophy, shorter duration of intubation and mechanical ventilation, and a lower incidence of delirium.[39]
RESCUE THERAPIES FOR REFRACTORY HYPOXEMIA
Despite treatment with lung‐protective ventilation and the best adjunctive strategies, some patients may progress to develop life‐threatening, refractory hypoxemia. Beyond the therapies already discussed (ie, prone positioning or neuromuscular blockade), there are additional interventions that should be considered in such cases.
Inhaled Vasodilator
Inhaled vasodilators may improve ventilation‐perfusion matching and improve pulmonary hypertension by selectively causing local vasodilation in well‐ventilated areas of the lung. Although there are several inhaled vasodilators available, including inhaled nitric oxide (iNO), inhaled prostacyclin, and inhaled prostaglandin E1, the best studied in ARDS is iNO. Although multiple studies have found transient improvement in oxygenation with iNO therapy in ARDS, a mortality benefit has never been demonstrated.[40] In addition, concerns about high cost, sophisticated equipment requirements, the risk of methemoglobinemia, and the potential increased risk of renal failure found in a 2007 meta‐analysis have limited the use of iNO in ARDS.[41] Thus, inhaled vasodilators should be considered only for patients with preexisting pulmonary hypertension or as a true rescue therapy in refractory hypoxemia cases, where the transient oxygenation could act as a bridge to other therapies.[40]
Extracorporeal Membrane Oxygenation
The use of extracorporeal membrane oxygenation (ECMO) in refractory acute hypoxemic respiratory failure in adults is an evolving therapy for which evidence is still emerging. During ECMO, blood is removed from the body, circulated by a mechanical pump through a membrane oxygenator, and then returned to the body. Observational studies have shown improved survival with ECMO compared to historic survival rates, and a study of 75 matched pairs of patients with severe influenza A (H1N1)‐related ARDS comparing mortality between patients transferred to an ECMO center and those who continued to receive conventional care, found improved survival in transferred patients compared to matched, nonreferred patients.[42] The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was a multicenter trial in which 180 patients with severe but potentially reversible respiratory failure were randomized to receive either conventional management or referral for consideration of ECMO to a major referral center in the United Kingdom.[43] Of the 90 patients referred for ECMO consideration, 76% actually received ECMO. Death or severe disability at 6 months occurred in 37% of the ECMO‐referred patients versus 53% of the conventional therapy patients (RR, 0.69; 95% CI, 0.05‐0.97; P = 0.03). Whether the benefit observed in the CESAR trial was due to ECMO itself or due to cointerventions and expert management at the referral ECMO center remains unclear. The exact indications, timing, titration, optimal cointerventions, and end points of ECMO therapy are likewise unsettled, and further trials are ongoing in Europe (NCT01470703). Nonetheless, based on the findings of the CESAR trial, consideration of transfer to an experienced ECMO center is recommended for patients with refractory hypoxemia who fail aggressive conventional therapy, and have potentially reversible disease or are possible candidates for lung transplant.[44]
LIBERATION FROM MECHANICAL VENTILATION
Once the underlying cause of respiratory failure is resolved and the patient demonstrates improvement, clinicians' attention must turn to decreasing the duration of mechanical ventilation. Some argue that the phrase weaning from mechanical ventilation is not always appropriate, as it implies a protracted, gradual process that is often not required; liberation from mechanical ventilation has been offered as a better description of the task of transitioning a patient back to normal breathing after they demonstrate readiness for spontaneous breathing and extubation.[3] Regardless of the terminology, the same principle applies: once ready, patients should be extubated as expeditiously as possible.
In addition to evidence‐based management strategies aimed at limiting the time a patient requires mechanical ventilation (such as lung‐protective ventilation, a fluid conservative strategy, and ventilator‐associated pneumonia prevention bundles), there is also the question of how to best assess whether a patient is ready for transition back to normal breathing, and how to operationalize that transition. This process may account for more than half of the total duration of mechanical ventilation in some cases.[3] Based on evidence from trials assessing various weaning protocols published in the 1990s, daily spontaneous breathing trials (in which the ventilator provides zero or minimal support during patient triggered breaths) are favored over slow weaning of pressure support or intermittent mandatory ventilation.[45] Although several novel ventilator modes aimed at improving patient‐ventilator interaction (eg, adaptive support ventilation, proportional assist ventilation, and neurally adjusted ventilatory assistance) have been proposed as optimal weaning modes, their benefit is theoretical, and data demonstrating improved outcomes are lacking.[28]
In addition to evidence supporting daily spontaneous breathing trials (SBTs), a Cochrane Database systematic review and meta‐analysis published in 2011 found that protocolized weaning was associated with shorter duration of mechanical ventilation than usual care.[2] Although the specifics of what constitutes the optimal weaning protocol remain unclear, there is general agreement that a standardized approach involving prespecified criteria and daily assessment for readiness for spontaneous breathing and potential extubation improves patient outcomes.[3] If the SBT is well tolerated hemodynamically, respiratory mechanics and gas exchange remain adequate, and airway factors and mental status permit, the patient should be extubated.
As emphasized in an excellent recent review by McConville and Kress, patients who fail 3 or more SBTs, or remain mechanically ventilated for 7 or more days following their first failed SBT, as well as patients who require reintubation after failed extubation, are at increased risk of in‐hospital mortality and prolonged hospital stay.[3, 46] For patients who fall into these categories without a clearly reversible cause, clinicians should consider initiating discussions about tracheostomy and goals of care. It should be noted, however, that multiple trials have failed to demonstrate the benefit of early tracheostomy, and the optimal timing of this intervention remains uncertain.[47]
CONCLUSIONS
When hypoxemic respiratory failure requires endotracheal intubation and mechanical ventilation, the clinician's management of the ventilator can have a profound impact on patient outcomes. Prompt recognition of ARDS and use of a lung‐protective ventilation strategy, as well as evidence‐based adjunctive therapies, remain the cornerstones of caring for patients with ARDS. Based on 2 recent large trials, HFOV is no longer recommended in ARDS. APRV in ARDS is also not supported by current evidence, though clinical trials are ongoing. In contrast, certain adjunctive therapies in ARDS, such as a conservative fluid strategy, early neuromuscular blockade, and prone positioning for moderate‐to‐severe cases, improve outcomes. There is also preliminary evidence to support the use of a lung‐protective strategy in selected non‐ARDS patients, especially in patients at high risk for developing ARDS. In cases of refractory hypoxemia and potentially survivable disease, extracorporeal membrane oxygenation should be considered. Finally, once the patient demonstrates signs of recovery, the best approach to liberation from mechanical ventilation involves daily protocolized, spontaneous breathing trials and assessment of readiness for extubation.
Acknowledgements
The authors thank Andrew Manies for his invaluable assistance in preparing this article.
Disclosures
Dr. Wilson was supported by National Institutes of Health grant U01 HL108713, and Dr. Matthay was supported by National Institutes of Health grants U01 HL108713 and R37 HL051856. The authors report no conflicts of interest.
The indications for endotracheal intubation and mechanical ventilation in acutely hypoxemic patients depend on the severity of respiratory failure as well as the patient's hemodynamic and neurologic status. Once intubated, however, how a patient is ventilated can have a significant impact on the subsequent hospital course and ultimate outcome. Regardless of whether the hospitalist manages the ventilator directly, comanages patients in the intensive care unit (ICU), or merely transfers a hypoxemic patient into or out of an intensivist‐run unit, a basic familiarity with the evidence supporting various mechanical ventilation strategies will enhance the care provided. It is also helpful to understand the goals of mechanical ventilation in acute hypoxemic respiratory failure, such as minimizing the risk of ventilator‐induced lung injury, enhancing recovery from the underlying cause of respiratory failure, and limiting the duration of mechanical ventilation.[1, 2, 3] With these objectives in mind, this review will examine the evidence that supports specific ventilator strategies in common clinical conditions that cause acute hypoxemia.
First, we will discuss the evidence supporting the use of low tidal volume ventilation in patients with the acute respiratory distress syndrome (ARDS), as well as several novel ventilator modes that have been proposed as alternatives to low tidal volume ventilation in ARDS. We will also briefly review adjunctive therapies that may enhance the efficacy of lung‐protective ventilation in ARDS. We will then discuss emerging evidence regarding the use of lung‐protective ventilation strategies in patients without ARDS, as well as potential contraindications to this approach. Finally, we will cover rescue strategies for refractory hypoxemia, as well as an evidence‐based approach to weaning from mechanical ventilation.
LUNG‐PROTECTIVE VENTILATION IN ARDS
Low Tidal Volume Ventilation
Over a decade following the original ARDS Clinical Network trial of lower versus traditional tidal volume ventilation, it is broadly accepted that ventilation with tidal volumes 6 mL/kg predicted body weight, targeting a plateau pressure 30 cm H2O, reduces mortality and increases ventilator‐free days in patients with ARDS.[4, 5, 6] Moreover, lung‐protective ventilation appears to reduce mortality in all patients with ARDS, regardless of the associated clinical disorder.[7] The substantial decline in mortality in ARDS observed over the past decade (Figure 1) is due in part to the broader use of lung‐protective ventilation.[8, 9]

Despite the strong evidence supporting the value of lung‐protective ventilation for decreasing mortality in ARDS, adherence to low tidal volume strategies in ARDS patients remains variable.[10, 11] This may be due to several reasons, including (1) mistakenly using actual instead of predicted body weight to determine appropriate tidal volume, (2) lack of awareness of the changes made by the most recent consensus‐based definition of ARDS (Table 1),[12] (3) under‐recognition of the heterogeneity of chest radiograph findings in ARDS (Figure 2), and (4) underdiagnosis of ARDS by providers.[13] Thus, prompt recognition of ARDS and the immediate initiation of lung‐protective ventilation strategies should be a high priority in caring for all patients with ARDS. Table 2 summarizes how to implement the ARDS network lung‐protective strategy, including how to determine the correct tidal volume based on predicted body weight, calculated from the patient's sex and height. Although a full discussion of the relative merits of pressure control versus volume control ventilation is outside the scope of this review, it is worth noting that either mode can be used to achieve low tidal volumes, and which mode is selected is often determined by individual patient factors and institutional or provider preference.
| |
| Timing | Within 7 days of known clinical insult or new/worsening respiratory symptoms. |
| Chest imaging | Chest radiograph or CT: bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis, or nodules. |
| Cause of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Objective assessment (eg, echocardiography) required to exclude hydrostaticedema if no ARDS risk factor present. |
| Oxygenation deficit | Mild: PaO2/FiO2< 300 but >200 mm Hg, on 5 cm H2O PEEP/CPAPa |
| Moderate: PaO2/FiO2 200 but >100 mm Hg, on 5 cm H2O PEEP/CPAP | |
| Severe: PaO2/FiO2 100 mm Hg on 5 cm H2O PEEP/CPAP | |
| |||||||||||||
| To calculate predicted body weight: | |||||||||||||
| Male PBW: 50 + 2.3 (height in inches 60) or 50 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Female PBW: 45.5 + 2.3 (height in inches 60) or 45.5 + 0.91 (height in centimeters 152.4) | |||||||||||||
| Select assist control mode | |||||||||||||
| Set initial VT at 8 mL/kg PBW | |||||||||||||
| Reduce VT by 1 mL/kg at intervals < 2 hours until VT = 6 mL/kg PBW | |||||||||||||
| Set initial RR to approximate baseline minute ventilation (maximum RR = 35/minute) | |||||||||||||
| Adjust VT and RR further to achieve Pplat and pH goals | |||||||||||||
| If Pplat> 30 cm H2O: decrease VT by 1 mL/kg PBW (minimum = 4 mL/kg PBW) | |||||||||||||
| If pH 7.30, increase RR (maximum = 35) | |||||||||||||
| If pH < 7.15, increase RR to 35; consider sodium bicarbonate administration or increase VT | |||||||||||||
| FiO2/PEEP combinations | |||||||||||||
| FiO2 | |||||||||||||
| 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 | 0.7 | 0.8 | 0.9 | 0.9 | 0.9 | 1.0 |
| PEEP (cm H2O) | |||||||||||||
| 5 | 5 | 8 | 8 | 10 | 10 | 10 | 12 | 14 | 14 | 14 | 16 | 18 | 18, 22, 24 |

Positive End‐Expiratory Pressure and Recruitment Maneuvers
The application of positive end‐expiratory pressure (PEEP) can prevent alveolar derecruitment and atelectrauma; too much PEEP, however, can cause alveolar overdistension or hemodynamic compromise due to high intrathoracic pressures and decreased venous return. Likewise, recruitment maneuvers, in which a high PEEP is applied for a brief interval, may improve oxygenation by opening up atelectatic alveoli, but can also cause barotrauma or hemodynamic compromise. Thus, in addition to research into the effects of low tidal volume ventilation, 3 additional trials have tested the potential value of higher versus lower PEEP in ARDS.[14, 15, 16] Although none of these trials showed a significant reduction in mortality with a higher PEEP strategy, a recent meta‐analysis of the data from all 3 trials reported a statistically significant mortality benefit for ARDS patients with a higher‐PEEP strategy versus a lower‐PEEP strategy (adjusted relative risk [RR], 0.90; 95% confidence interval [CI], 0.81‐1.00; P = 0.049).[17] Because of differences in trial design and patient selection, however, a change of practice cannot be reasonably based on this meta‐analysis alone. Current research is focused on whether there is a subset of ARDS patients who may benefit from a higher PEEP strategy, and how best to determine optimal PEEP more generally.[18, 19] In addition to these ongoing questions about PEEP, the value of recruitment maneuvers remains uncertain.[1, 20]
High‐Frequency Oscillating Ventilation
High‐frequency oscillating ventilation (HFOV) is a technique in which very small tidal volumes are delivered at high frequency (315 breaths per second) at high mean airway pressures. Until recently, trials of HFOV in ARDS have been inconclusive due to small size or inappropriate control arms that did not utilize low tidal volume ventilation.[21] However, 2 recent large, multicenter, randomized trials comparing HFOV to low tidal volume ventilation in ARDS have shown that there is no benefit (and perhaps even harm) associated with HFOV. The Oscillation in ARDS (OSCAR) trial reported no change in mortality, whereas the Oscillation for Acute Respiratory Distress Syndrome Treated Early (OSCILLATE) trial found that HFOV was associated with increased risk of death.[22, 23] As such, HFOV is no longer recommended in ARDS.
Airway Pressure Release Ventilation
Airway pressure release ventilation (APRV) is a mode of ventilation, in which a relatively high level of continuous positive airway pressure (P high) is applied for a large portion of the respiratory cycle. During the time spent at P high (T high), the patient can take small spontaneous breaths, with or without the assistance of additional pressure support. At the end of T high, the applied pressure releases to a lower level (P low) for a brief time (T low) to allow CO2 clearance (Figure 3).

Theoretically, the long inflation time in APRV allows for more uniform recruitment of alveoli and raises mean airway pressure without increasing barotrauma. APRV also allows for spontaneous breathing even at high levels of support. Despite preclinical and observational data suggesting that APRV may reduce the development or progression of lung injury,[24, 25, 26, 27] prospective clinical trials comparing APRV to low tidal volume ventilation have yet to support any clear benefit, and 1 trial has demonstrated a trend toward more days of mechanical ventilation.[28, 29] Multiple clinical trials are ongoing (NCT01901354, NCT01339533), but in the interim, the use of APRV instead of conventional low tidal volume ventilation is not supported by high‐level evidence.
ADJUNCTIVE THERAPIES IN ARDS
Although a full discussion of the numerous nonventilatory therapies that have been tested for ARDS is beyond the scope of this focused review, several of these strategies have been shown to improve outcomes and deserve mention here.
Fluid Management
The first such therapy is the implementation of a fluid conservative strategy. This approach is based on the ARDS network Fluid and Catheter Treatment Trial (FACTT), which demonstrated that in the absence of shock or oliguria, a fluid‐conservative strategy improves lung function and decreases the duration of mechanical ventilation in ARDS patients.[30] Indeed, multiple studies have found that a positive fluid balance is associated with worsened multiorgan dysfunction and poor outcomes in patients with ARDS.[31] In terms of translating this evidence into practice, the ARDS Network has published a simplified algorithm for conservative fluid management based on the results of FACTT.[32]
Prone Positioning
Although prone positioning during mechanical ventilation improves oxygenation by improving lung recruitment and ventilation‐perfusion matching, several early trials of prone positioning did not demonstrate a mortality benefit. Although a 2010 meta‐analysis of 10 previous trials did find a mortality benefit in the most hypoxemic patients, there was also an increased risk of pressure ulcers and endotracheal tube obstruction.[33] Thus, the indications for prone positioning in ARDS remained uncertain until 2013, when Guerin et al. reported the results of a large, multicenter, randomized trial that demonstrated a major reduction in mortality in ARDS patients treated with prone positioning.[34] The trial included 466 patients with early ARDS, in whom the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) was < 150 mm Hg on an FiO2 of at least 0.6 and PEEP of at least 5 cm H2O. Of note, all the sites involved in the trial (26 centers in France, 1 in Spain) had extensive experience with prone positioning prior to the trial. The rate of death at 28 days was 33% in the supine group and 16% in the prone group (hazard ratio 0.39 [95% CI, 0.25‐0.63]; P < 0.001); this mortality reduction persisted at 90 days, and after adjustment for Sequential Organ Failure Assessment (SOFA) score, use of vasopressors, and use of neuromuscular blockade. Finally, there was no difference in adverse events (such as unplanned extubation) between groups. Implementation of prone‐positioning protocols in less experienced centers with higher rates of obesity will be challenging, and additional confirmatory trials would be ideal. Nevertheless, this trial will prompt broader application of prone positioning in patients with moderate to severe ARDS.
Neuromuscular Blockade
In addition to conservative fluid management, early consideration of neuromuscular blockade (NMB) in patients with moderate‐to‐severe ARDS likely improves outcomes. NMB may enhance the protective effects of low tidal volume ventilation in the most hypoxemic ARDS patients, because it removes the resistance of the chest wall and the diaphragm, and more importantly, reduces dyssynchrony between the patient and the ventilator. Although previous studies of NMB in ARDS yielded conflicting results, a more recent well‐done randomized clinical trial showed a mortality benefit. In this trial, 340 patients with a PaO2/FiO2 ratio of <150 mm Hg were randomized to receive a 48‐hour infusion of cisatracurium (a nondepolarizing neuromuscular blocking agent) or placebo within 48 hours of ARDS onset.[35] Both groups were deeply sedated and ventilated with low tidal volumes, but mortality was lower in patients treated with NMB compared to patients who did not receive NMB. Although there are understandable concerns that NMB will mask the ability to detect important changes in the patient's clinical exam and increase risk of ICU‐acquired weakness, the results of this trial suggest that clinicians should strongly consider early, short‐term NMB with cisatracurium in patients with moderate‐to‐severe ARDS.
Other Pharmacotherapies
Although several other pharmacologic interventions for ARDS have been studied (eg, glucocorticoids, exogenous surfactant, activated protein C, inhaled ‐agonists), none has demonstrated a mortality benefit.[9]
BEYOND ARDS: LUNG‐PROTECTIVE VENTILATION FOR ALL?
Low Tidal Volume Ventilation Strategies in Patients Without ARDS
Given concerns about ventilator‐induced lung injury and the known benefits of lung‐protective ventilation in patients with ARDS, there is growing interest in determining whether low tidal volume ventilation may be beneficial to mechanically ventilated patients who do not have ARDS. In 2010, Serpa Neto et al. published a meta‐analysis of 20 studies (mixed population of >2800 ICU and operating room patients) comparing lower versus higher tidal volume ventilation in patients without ARDS.[36] They found that low tidal volume ventilation (mean tidal volume of 6.5 mL/kg) was associated with significantly decreased mortality and risk of lung injury compared to ventilation with higher tidal volumes (mean tidal volume 10.6 mL/kg). This investigation has been followed by a randomized, double‐blind trial of intraoperative low tidal volume ventilation in 400 patients at intermediate or high risk for pulmonary complications after major abdominal surgery.[37] Remarkably, lower tidal volume ventilation was associated with a decreased risk of both pulmonary and extrapulmonary complications in the first week following surgery. These studies are in line with preclinical animal studies that show an association between higher tidal volume ventilation and development of lung injury.[38] Although this evidence does not warrant indiscriminate low tidal volume ventilation in all critically ill patients, it certainly suggests that clinicians should strongly consider lung protective ventilation in patients at high risk for ARDS (eg, patients with pneumonia, aspiration, sepsis, or massive transfusion), and points to an urgent need for more randomized clinical trials of low tidal volume and lung‐protective ventilation in various groups of patients who do not have ARDS.
Potential Contraindications to Lower Tidal Volume, Higher PEEP Ventilation
Despite speculation that a lower tidal volume ventilation strategy may be superior to conventional ventilation in most mechanically ventilated patients, there are some clinical scenarios in which typical lung‐protective ventilation protocols are not appropriate. First, there are some patients (eg, patients with neurologic injury or pulmonary hypertension) in whom the lower oxygenation and permissive hypercapnia targeted by lung‐protective ventilation protocols may be harmful. Second, higher PEEP protocols may be dangerous for patients with pneumothorax or who are at risk for bronchopleural fistula. Third, patients with airway obstruction often require lower respiratory rates to permit maximization of expiratory time; if tidal volume is lowered aggressively as part of a lung‐protective ventilation protocol, higher respiratory rates may be required to achieve PaCO2/arterial pH goals, leading to decreased expiratory time and worsening air trapping. Finally, because mandatory low tidal volumes may be poorly tolerated in some patients, allowing low‐risk patients to transition directly to a spontaneous breathing mode may have benefits that outweigh those of lung‐protective ventilation protocols, including decreased need for sedating medications, less muscle atrophy, shorter duration of intubation and mechanical ventilation, and a lower incidence of delirium.[39]
RESCUE THERAPIES FOR REFRACTORY HYPOXEMIA
Despite treatment with lung‐protective ventilation and the best adjunctive strategies, some patients may progress to develop life‐threatening, refractory hypoxemia. Beyond the therapies already discussed (ie, prone positioning or neuromuscular blockade), there are additional interventions that should be considered in such cases.
Inhaled Vasodilator
Inhaled vasodilators may improve ventilation‐perfusion matching and improve pulmonary hypertension by selectively causing local vasodilation in well‐ventilated areas of the lung. Although there are several inhaled vasodilators available, including inhaled nitric oxide (iNO), inhaled prostacyclin, and inhaled prostaglandin E1, the best studied in ARDS is iNO. Although multiple studies have found transient improvement in oxygenation with iNO therapy in ARDS, a mortality benefit has never been demonstrated.[40] In addition, concerns about high cost, sophisticated equipment requirements, the risk of methemoglobinemia, and the potential increased risk of renal failure found in a 2007 meta‐analysis have limited the use of iNO in ARDS.[41] Thus, inhaled vasodilators should be considered only for patients with preexisting pulmonary hypertension or as a true rescue therapy in refractory hypoxemia cases, where the transient oxygenation could act as a bridge to other therapies.[40]
Extracorporeal Membrane Oxygenation
The use of extracorporeal membrane oxygenation (ECMO) in refractory acute hypoxemic respiratory failure in adults is an evolving therapy for which evidence is still emerging. During ECMO, blood is removed from the body, circulated by a mechanical pump through a membrane oxygenator, and then returned to the body. Observational studies have shown improved survival with ECMO compared to historic survival rates, and a study of 75 matched pairs of patients with severe influenza A (H1N1)‐related ARDS comparing mortality between patients transferred to an ECMO center and those who continued to receive conventional care, found improved survival in transferred patients compared to matched, nonreferred patients.[42] The Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial was a multicenter trial in which 180 patients with severe but potentially reversible respiratory failure were randomized to receive either conventional management or referral for consideration of ECMO to a major referral center in the United Kingdom.[43] Of the 90 patients referred for ECMO consideration, 76% actually received ECMO. Death or severe disability at 6 months occurred in 37% of the ECMO‐referred patients versus 53% of the conventional therapy patients (RR, 0.69; 95% CI, 0.05‐0.97; P = 0.03). Whether the benefit observed in the CESAR trial was due to ECMO itself or due to cointerventions and expert management at the referral ECMO center remains unclear. The exact indications, timing, titration, optimal cointerventions, and end points of ECMO therapy are likewise unsettled, and further trials are ongoing in Europe (NCT01470703). Nonetheless, based on the findings of the CESAR trial, consideration of transfer to an experienced ECMO center is recommended for patients with refractory hypoxemia who fail aggressive conventional therapy, and have potentially reversible disease or are possible candidates for lung transplant.[44]
LIBERATION FROM MECHANICAL VENTILATION
Once the underlying cause of respiratory failure is resolved and the patient demonstrates improvement, clinicians' attention must turn to decreasing the duration of mechanical ventilation. Some argue that the phrase weaning from mechanical ventilation is not always appropriate, as it implies a protracted, gradual process that is often not required; liberation from mechanical ventilation has been offered as a better description of the task of transitioning a patient back to normal breathing after they demonstrate readiness for spontaneous breathing and extubation.[3] Regardless of the terminology, the same principle applies: once ready, patients should be extubated as expeditiously as possible.
In addition to evidence‐based management strategies aimed at limiting the time a patient requires mechanical ventilation (such as lung‐protective ventilation, a fluid conservative strategy, and ventilator‐associated pneumonia prevention bundles), there is also the question of how to best assess whether a patient is ready for transition back to normal breathing, and how to operationalize that transition. This process may account for more than half of the total duration of mechanical ventilation in some cases.[3] Based on evidence from trials assessing various weaning protocols published in the 1990s, daily spontaneous breathing trials (in which the ventilator provides zero or minimal support during patient triggered breaths) are favored over slow weaning of pressure support or intermittent mandatory ventilation.[45] Although several novel ventilator modes aimed at improving patient‐ventilator interaction (eg, adaptive support ventilation, proportional assist ventilation, and neurally adjusted ventilatory assistance) have been proposed as optimal weaning modes, their benefit is theoretical, and data demonstrating improved outcomes are lacking.[28]
In addition to evidence supporting daily spontaneous breathing trials (SBTs), a Cochrane Database systematic review and meta‐analysis published in 2011 found that protocolized weaning was associated with shorter duration of mechanical ventilation than usual care.[2] Although the specifics of what constitutes the optimal weaning protocol remain unclear, there is general agreement that a standardized approach involving prespecified criteria and daily assessment for readiness for spontaneous breathing and potential extubation improves patient outcomes.[3] If the SBT is well tolerated hemodynamically, respiratory mechanics and gas exchange remain adequate, and airway factors and mental status permit, the patient should be extubated.
As emphasized in an excellent recent review by McConville and Kress, patients who fail 3 or more SBTs, or remain mechanically ventilated for 7 or more days following their first failed SBT, as well as patients who require reintubation after failed extubation, are at increased risk of in‐hospital mortality and prolonged hospital stay.[3, 46] For patients who fall into these categories without a clearly reversible cause, clinicians should consider initiating discussions about tracheostomy and goals of care. It should be noted, however, that multiple trials have failed to demonstrate the benefit of early tracheostomy, and the optimal timing of this intervention remains uncertain.[47]
CONCLUSIONS
When hypoxemic respiratory failure requires endotracheal intubation and mechanical ventilation, the clinician's management of the ventilator can have a profound impact on patient outcomes. Prompt recognition of ARDS and use of a lung‐protective ventilation strategy, as well as evidence‐based adjunctive therapies, remain the cornerstones of caring for patients with ARDS. Based on 2 recent large trials, HFOV is no longer recommended in ARDS. APRV in ARDS is also not supported by current evidence, though clinical trials are ongoing. In contrast, certain adjunctive therapies in ARDS, such as a conservative fluid strategy, early neuromuscular blockade, and prone positioning for moderate‐to‐severe cases, improve outcomes. There is also preliminary evidence to support the use of a lung‐protective strategy in selected non‐ARDS patients, especially in patients at high risk for developing ARDS. In cases of refractory hypoxemia and potentially survivable disease, extracorporeal membrane oxygenation should be considered. Finally, once the patient demonstrates signs of recovery, the best approach to liberation from mechanical ventilation involves daily protocolized, spontaneous breathing trials and assessment of readiness for extubation.
Acknowledgements
The authors thank Andrew Manies for his invaluable assistance in preparing this article.
Disclosures
Dr. Wilson was supported by National Institutes of Health grant U01 HL108713, and Dr. Matthay was supported by National Institutes of Health grants U01 HL108713 and R37 HL051856. The authors report no conflicts of interest.
- , . Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- , , , , , . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta‐analysis. BMJ. 2011;342:c7237.
- , . Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–2239.
- , , , et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354.
- , , , . A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308.
- , , , et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236.
- , . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127.
- , , . The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740.
- , , , et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468.
- , , , et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
- , , , et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- , , , et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234.
- , , , et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- , , , et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- , , , et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336.
- , , , et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865–873.
- , , , et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104.
- , , , et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423.
- , , . Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20(1):63–68.
- , . Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 suppl):S170–S174.
- , , , et al. High‐frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805.
- . High‐frequency oscillation for ARDS. N Engl J Med. 2013;368(23):2234.
- , , , et al. Early airway pressure release ventilation prevents ARDS‐a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
- , , , et al. Airway pressure release ventilation prevents ventilator‐induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012.
- , , , et al. Early application of airway pressure release ventilation may reduce mortality in high‐risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641.
- , , , et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49.
- , . Novel modes of mechanical ventilation. Semin Respir Crit Care Med. 2013;34(4):499–507.
- , , , et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma. 2010;69(3):501–510; discussion 511.
- , , , et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- , , , et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra‐abdominal pressure: a pilot study looking at the effects of PAL‐treatment. Ann Intensive Care. 2012;(2 suppl 1):S15.
- , . Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920.
- , , , et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Med. 2010;36(4):585–599.
- , , , et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- , , , et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116.
- , , , et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA. 2012;308(16):1651–1659.
- , , , et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437.
- , , . Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta‐analysis. Curr Opin Crit Care. 2014;20(1):25–32.
- . Low tidal volumes for all? JAMA. 2012;308(16):1689–1690.
- , . Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304(22):2521–2527.
- , , , , , . Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ. 2007;334(7597):779.
- , , , et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–1668.
- , , , et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–350.
- , , . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302.
- , , , . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129.
- , , , , , . Enteral omega‐3 fatty acid, gamma‐linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; , , , et al. Randomized, placebo‐controlled clinical trial of an aerosolized beta‐agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568.
- , . Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136.
- , , , , , . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta‐analysis. BMJ. 2011;342:c7237.
- , . Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–2239.
- , , , et al. Effect of a protective‐ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354.
- , , , . A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308.
- , , , et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236.
- , . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127.
- , , . The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740.
- , , , et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468.
- , , , et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124.
- , , , et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533.
- , , , et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234.
- , , , et al. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- , , , et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- , , , et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336.
- , , , et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865–873.
- , , , et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104.
- , , , et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423.
- , , . Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20(1):63–68.
- , . Clinical use of high‐frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 suppl):S170–S174.
- , , , et al. High‐frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805.
- . High‐frequency oscillation for ARDS. N Engl J Med. 2013;368(23):2234.
- , , , et al. Early airway pressure release ventilation prevents ARDS‐a novel preventive approach to lung injury. Shock. 2013;39(1):28–38.
- , , , et al. Airway pressure release ventilation prevents ventilator‐induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012.
- , , , et al. Early application of airway pressure release ventilation may reduce mortality in high‐risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641.
- , , , et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49.
- , . Novel modes of mechanical ventilation. Semin Respir Crit Care Med. 2013;34(4):499–507.
- , , , et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma. 2010;69(3):501–510; discussion 511.
- , , , et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- , , , et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra‐abdominal pressure: a pilot study looking at the effects of PAL‐treatment. Ann Intensive Care. 2012;(2 suppl 1):S15.
- , . Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920.
- , , , et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta‐analysis. Intensive Care Med. 2010;36(4):585–599.
- , , , et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- , , , et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116.
- , , , et al. Association between use of lung‐protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta‐analysis. JAMA. 2012;308(16):1651–1659.
- , , , et al. A trial of intraoperative low‐tidal‐volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437.
- , , . Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta‐analysis. Curr Opin Crit Care. 2014;20(1):25–32.
- . Low tidal volumes for all? JAMA. 2012;308(16):1689–1690.
- , . Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010;304(22):2521–2527.
- , , , , , . Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta‐analysis. BMJ. 2007;334(7597):779.
- , , , et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659–1668.
- , , , et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363.
- , . Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–1914.
- , , , et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–350.
- , , . The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302.
- , , , . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129.
- , , , , , . Enteral omega‐3 fatty acid, gamma‐linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; , , , et al. Randomized, placebo‐controlled clinical trial of an aerosolized beta‐agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568.
Novel Anticoagulants in Atrial Fibrillation
Approximately 2.3 million people in the United States and 4.5 million people in Europe have atrial fibrillation (AF), with an increase in prevalence with age to 8% among patients aged 80 years and older.[1] The most feared and potentially preventable complications of AF are stroke or systemic thromboembolism, and stroke in particular is increased approximately 5‐fold in patients with nonvalvular atrial fibrillation (NVAF).[2] For over 50 years, warfarin and similar vitamin K antagonists have been the principal anticoagulants used for preventing stroke in NVAF, with consistent reductions in systemic thromboembolic events when compared with placebo or aspirin.[2, 3] However, because of its narrow therapeutic window and related management difficulties (ie, frequent monitoring of international normalized ratio [INR] levels, dietary and medication restrictions, interindividual variability in dosing), many patients with NVAF do not receive warfarin or are inadequately treated.[4]
In response to the need for antithrombotic agents with better efficacy, patient tolerance, and convenience, the US Food and Drug Administration (FDA) recently approved 3 novel oral anticoagulants (NOACs) as alternatives to warfarin for NVAF: dabigatran, rivaroxaban, and apixaban. In this review, we evaluated the pharmacologic properties and clinical studies of these NOACs, including the continued role of warfarin in many patients requiring systemic anticoagulation, to guide practicing clinicians in providing individualized, patient‐centered care to each of their patients with NVAF.
PHARMACOLOGY
Mechanisms of Action
Whereas warfarin inhibits the formation of multiple vitamin K‐dependent coagulation factors (II, VII, IX, and X),[5] the NOACs are competitive and reversible inhibitors of more distal targets in the coagulation pathway. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban and apixaban directly inhibit factor Xa, ultimately resulting in the inhibition of fibrin formation and thrombosis.
Clinical Pathways and Drug Interactions
Key aspects of the pharmacokinetic profiles of the 3 NOACs are summarized in Table 1. In addition to these baseline properties of each medication, drug interactions play an important role in the effectiveness and potential toxicities of the NOACs. For example, dabigatran is almost exclusively excreted via glomerular filtration, resulting in a terminal half‐life of 12 to 17 hours in normal volunteers and a significantly higher half‐life in moderate and severe renal dysfunction (18 and 27 hours, respectively). In phase II and III trials, there was a 30% decrease in bioavailability when dabigatran was administered with pantoprazole, but no comparable effect was noted when coadministered with histamine receptor blockers like ranitidine.[6] In addition, although dabigatran has no significant interaction with hepatic P450 enzymes, its prodrug is excreted by the intestinal efflux transporter p‐glycoprotein. As a result, dabigatran's bioavailability is increased by coadministration with potent p‐glycoprotein inhibitors such as dronedarone, amiodarone, verapamil, diltiazem, or ketoconazole.[6, 7] According to FDA labeling, the only drug contraindicated with concomitant dabigatran administration is rifampin, which reduces serum concentration of dabigatran by 66%.
| Characteristic | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| |||
| Target | Factor IIa | Factor Xa | Factor Xa |
| Reversible binding | Yes | Yes | Yes |
| Half‐life, h | 1217 | 59 | 815 |
| Time to peak serum concentration, h | 13 | 24 | 34 |
| Protein binding, % | 35 | 9295 | 87 |
| Renal excretion, % | 80 | 66 | 2527 |
| Primary hepatic clearance pathway | Does not interact with CYP enzymes | CYP‐3A4 | CYP‐3A4 |
Unlike dabigatran, the absorption of rivaroxaban has significant variability between individuals, but the bioavailability of the 20‐mg dose increases by 39% and is significantly less variable when taken with food.[8] Phase I studies of rivaroxaban demonstrated that elderly patients had 50% higher serum concentrations when compared with younger patients.[7, 9] Also of note, rivaroxaban has 50% higher bioavailability in Japanese patients as compared with other ethnicities, including Chinese ethnicity, resulting in higher exposure to the drug and potentially explaining higher bleeding rates in Japan when using this drug.[9] The primary mechanisms for metabolism of rivaroxaban are the CYP‐3A4 and CYP‐2C8 pathways in the liver,[10] so other drugs metabolized through these pathways (eg, azole antifungals, protease inhibitors, clarithromycin) may have significant drug‐drug interactions.
Like the other NOACs, apixaban achieves its maximal concentration within 3 to 4 hours,[11] and like rivaroxaban, apixaban is metabolized by the CYP‐3A4 hepatic pathway. However, apixaban does not induce or inhibit hepatic cytochrome P450 (CYP) enzymes, so the potential for drug‐drug interactions is considered minimal.[12] Important exceptions include coadministration with ketoconazole or clarithromycin, each of which increases the bioavailability of apixaban up to 1.5‐fold, so a dose reduction to 2.5 mg twice‐daily (BID) is recommended.[11]
CLINICAL STUDIES
Randomized trials evaluating warfarin against placebo or aspirin for NVAF have spanned more than 3 decades, encompassing a variety of study designs, patient populations, and analytic techniques.[2, 3] Despite differences between trials, these studies have provided the framework for contemporary AF management, with consistent reductions in thromboembolic events with systemic anticoagulation, most notably among patients with multiple risk factors for stroke. Current professional guidelines recommend risk assessment of patients with NVAF, using the CHADS2 (1 point each for Congestive heart failure, Hypertension, Age 75 years, Diabetes, and 2 points for prior Stroke) or similar risk scores, to identify patients most likely to benefit from systemic anticoagulation.[1, 13] As a result of this extensive background literature, the 3 NOACs have primarily been evaluated against warfarin (instead of aspirin or placebo) as potential alternatives for reducing thromboembolic events in patients with NVAF. The 1 exception is a prematurely terminated trial of apixaban in warfarin‐ineligible patients with NVAF, in which apixaban reduced stroke or systemic embolism by 55% compared with aspirin after only 1.1 years of follow‐up, with no significant difference in major bleeding.[14]
Pivotal Clinical Trials
The 3 principal trials evaluating the NOACs against warfarin for NVAF are summarized in Table 2. In the Randomized Evaluation of Long‐term anticoagulation Therapy (RE‐LY) trial, dabigatran was compared with warfarin in 18,113 patients recruited from 951 clinical centers in 44 countries using a noninferiority study design.[15] Two different doses of dabigatran were studied, but only the 150‐mg BID dose was approved by the FDA. As a result, only the findings from the clinically approved 150‐mg dose are summarized in this review. Although RE‐LY was considered a semiblinded randomized trial, patients enrolled in the warfarin control arm underwent regular INR surveillance by their treating physicians, leaving the trial open to potential reporting biases. The authors tried to minimize bias by providing a standardized protocol for INR management, and by assigning 2 independent investigators blinded to the treatment assignments to adjudicate each event.
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Drug | Dabigatran | Rivaroxaban | Apixaban |
| Dosing | 150 mg BID (110 mg BID also tested) | 20 mg daily (15 mg for creatinine clearance 3049 mL/min) | 5 mg BID (2.5 mg for patients at higher risk of bleeding)a |
| Total population | 18,113 | 14,264 | 18,201 |
| Randomization | Semiblinded | Double blinded | Double blinded |
| Primary analytic approach | Noninferiority, intention‐to‐treat | Noninferiority, both intention‐to‐treat and on‐treatment | Noninferiority, intention‐to‐treat |
| Primary efficacy end point | Stroke or systemic embolism | Stroke or systemic embolism | Stroke or systemic embolism |
| Primary safety end point | Major bleeding | Major and clinically relevant nonmajor bleeding | Major bleeding |
| Key inclusion criteria | |||
| Documented atrial fibrillation | At screening or within 6 months | Within 30 days prior to randomization and within past year | At least 2 episodes recorded 2 weeks apart in past year |
| Eligible CHADS[2] scores | 1 | 2 | 1 |
| Selected exclusion criteria | |||
| Valvular heart disease | Any hemodynamically relevant or prosthetic valve | Severe mitral stenosis or any mechanical prosthetic valve | Moderate or severe mitral stenosis, or any mechanical prosthetic valve |
| Stroke | Severe 6 months or mild/moderate 14 days | Severe 3 months, any stroke 14 days, TIA 3 days | Stroke 7 days |
| Bleeding | Surgery 30 days, gastrointestinal bleed 12 months, any prior intracranial bleed, severe hypertension | Surgery 30 days, gastrointestinal bleed 6 months, active internal bleeding, any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension, platelets 90,000/L | Any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension |
| Renal | Creatinine clearance <30 mL/min | Creatinine clearance <30 mL/min | Creatinine clearance <25 mL/min |
The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) study involved 14,264 patients from 1178 participating sites in 45 countries.[16] Again, a noninferiority design was used to evaluate 20‐mg daily rivaroxaban against warfarin, but the 2 arms were compared in double‐blinded, double‐dummy fashion (thus eliminating the reporting bias related to the warfarin control arm in RE‐LY). In addition, whereas RE‐LY randomized patients to fixed doses of dabigatran within their respective treatment arms, ROCKET‐AF required a lower dose of rivaroxaban (15 mg daily) for patients with moderately reduced creatinine clearance (3049 mL/min). Also of note, ROCKET‐AF reported both intention‐to‐treat and on‐treatment analyses, with outcomes listed as number of events per 100 patient‐years (instead of percent per year). To facilitate comparisons between trials, only the intention‐to‐treat data are reported in this review.
Like ROCKET‐AF, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized patients using a double‐blind, double‐dummy, noninferiority design to therapy with apixaban 5 mg BID versus warfarin, ultimately enrolling 18,201 patients at 1034 clinical sites in 39 countries.[17] ARISTOTLE also provided a lower dose of apixaban (2.5 mg BID) for patients at higher risk of bleeding, defined by the authors as patients with 2 of the following characteristics: age 80 years and older, weight 60 kg, or serum creatinine 1.5 mg/dL. However, <5% of all patients in ARISTOTLE met these criteria and received the lower dose of apixaban.
Patient Populations and Study End Points
All 3 trials used relatively similar criteria for enrolling and following patients, with individual thromboembolic risk calculated using the CHADS2 definition, where higher scores are associated with incrementally higher risk of stroke.[18] However, ROCKET‐AF required a minimum CHADS2 score of 2 and permitted patients with lower left ventricular ejection fractions (35%), thus enrolling a higher‐risk patient population than RE‐LY and ARISTOTLE (where ejection fraction 40% was considered a risk factor for thromboembolism). As a result, more patients in ROCKET‐AF had prior stroke or systemic embolism than the other 2 trials (55% vs 20% in RE‐LY and 19% in ARISTOTLE) and more patients had significant heart failure (63%,vs 32% in RE‐LY and 36% in ARISTOTLE). These differences in enrollment ultimately translated into a higher overall risk profile in ROCKET‐AF (Table 3), which may have impacted some of the study results. In addition, patients requiring dual antiplatelet therapy (ie, clopidogrel and aspirin) were permitted in RE‐LY (5% of the final randomized population) but were excluded from the other 2 trials. The primary outcome for all 3 trials was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding (RE‐LY and ARISTOTLE), or combined major and clinically relevant nonmajor bleeding events (ROCKET‐AF).
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Age, y | 72 | 73 | 70 |
| Male sex, % | 63 | 60 | 65 |
| Type of atrial fibrillation, % | |||
| Paroxysmal | 33 | 18 | 15 |
| Persistent/permanent | 67 | 82 | 85 |
| Comorbidities, % | |||
| Hypertension | 79 | 90 | 87 |
| Previous stroke or systemic embolism | 20 | 55 | 19 |
| Diabetes | 23 | 40 | 25 |
| Congestive heart failure | 32 | 63 | 36 |
| Prior myocardial infarction | 17 | 17 | 15 |
| CHADS2 score, % | |||
| 01 | 32 | 0 | 34 |
| 2 | 35 | 13 | 36 |
| 3 | 33 | 87 | 30 |
| Medications, % | |||
| ACE inhibitor or angiotensin receptor blocker | 67 | 55 | 71 |
| ‐Blockers | 64 | 65 | 64 |
| Digoxin | 29 | 39 | 32 |
| Amiodarone | 11 | Not reported | 11 |
| Aspirin | 39 | 36 | 31 |
| Aspirin and clopidogrel | 5 | 0 | 0 |
| Prior long‐term warfarin or other vitamin K antagonist | 50 | 62 | 57 |
| Creatinine clearance, % | |||
| >80 mL/min | 32 | 32 | 41 |
| >5080 mL/min | 48 | 47 | 42 |
| >3050 mL/min | 20 | 21 | 15 |
| <30 mL/min | <1 | None reported | 2 |
| Mean time in therapeutic range among warfarin‐treated patients, % | 64 | 55 | 66 |
Clinical Outcomes
As illustrated in Table 4, the dabigatran 150‐mg BID dose was both noninferior and superior to warfarin for reducing the composite primary end point. Patients randomized to this arm of the RE‐LY study experienced fewer ischemic strokes, fewer hemorrhagic strokes, and a strong trend toward lower all‐cause mortality despite higher rates of myocardial infarction. There was no difference in overall major bleeding, although a significant reduction in intracranial hemorrhage was offset by a higher rate of gastrointestinal bleeding.
| Clinical Outcome | RE‐LY | ROCKET‐AF | ARISTOTLE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatran, 150 mg BID, %/y | Warfarin, %/y | Hazard Ratio | P Valuea | Rivaroxaban, 20 mg QD, No./100 Patient‐Years | Warfarin, No./100 Patient‐Years | Hazard Ratio | P Valuea | Apixaban 5 mg BID, %/y | Warfarin, %/yr | Hazard Ratio | P Valuea | |
| ||||||||||||
| Stroke or systemic embolism | 1.11 | 1.69 | 0.66 | <0.001 | 2.1 | 2.4 | 0.88 | <0.001 | 1.27 | 1.60 | 0.79 | 0.01 |
| Any stroke | 1.01 | 1.57 | 0.64 | <0.001 | 1.65 | 1.96 | 0.85 | 0.092 | 1.19 | 1.51 | 0.79 | 0.01 |
| Ischemic | 0.92 | 1.20 | 0.76 | 0.03 | 1.34 | 1.42 | 0.94 | 0.581 | 0.97 | 1.05 | 0.92 | 0.42 |
| Hemorrhagic | 0.10 | 0.38 | 0.26 | <0.001 | 0.26 | 0.44 | 0.59 | 0.024 | 0.24 | 0.47 | 0.51 | <0.001 |
| Myocardial infarction | 0.74 | 0.53 | 1.38 | 0.048 | 0.91 | 1.12 | 0.81 | 0.121 | 0.53 | 0.61 | 0.88 | 0.37 |
| All‐cause mortality | 3.64 | 4.13 | 0.88 | 0.051 | 1.87 | 2.21 | 0.85 | 0.073 | 3.52 | 3.94 | 0.89 | 0.047 |
| Major bleeds | 3.11 | 3.36 | 0.93 | 0.31 | 3.6 | 3.4 | 1.04 | 0.58 | 2.13 | 3.09 | 0.69 | <0.001 |
| Intracranial | 0.30 | 0.74 | 0.40 | <0.001 | 0.5 | 0.7 | 0.67 | 0.02 | 0.33 | 0.80 | 0.42 | <0.001 |
| Gastrointestinal | 1.51 | 1.02 | 1.50 | <0.001 | 3.15b | 2.16b | <0.001 | 0.76 | 0.86 | 0.89 | 0.37 | |
In the intention‐to‐treat analyses from ROCKET‐AF, rivaroxaban was noninferior to warfarin for reducing the primary end point, and there was a significant reduction in hemorrhagic stroke by rivaroxaban. Again, a strong trend toward lower mortality was seen, and like RE‐LY, an equivocal bleeding end point was largely driven by the combination of lower intracranial hemorrhage but higher gastrointestinal bleeding rates. Of note, the on‐treatment analysis from ROCKET‐AF demonstrated both noninferiority and superiority to warfarin, and there was no signal for higher rates of myocardial infarction as seen in RE‐LY.
In ARISTOTLE, apixaban was both noninferior and superior to warfarin, with stroke reduction largely driven by lower rates of intracranial hemorrhage. Unlike the prior studies of dabigatran and rivaroxaban, ARISTOTLE demonstrated a statistically significant reduction in all‐cause mortality and a significant reduction in major bleeding with apixaban therapy, with no increase in gastrointestinal bleeding.
INR Control
In prior randomized trials and observational registries of patients with AF, INR control has been highly variable, and better clinical outcomes were observed among patients consistently achieving INR levels between 2 and 3.[3, 19] For all 3 randomized trials of the NOACs summarized in this review, the warfarin control arms were analyzed using the Rosendaal method of evaluating total time in therapeutic range (TTR), reflecting the percent of time the patient had an INR between 2 and 3.[20] Overall, the mean TTR was 64% to 66% in the RE‐LY and ARISTOTLE trials, but only 55% in ROCKET‐AF. This has led to considerable criticism of the ROCKET‐AF trial, given concerns for a less robust comparator arm for rivaroxaban (and thus the potential for inflated efficacy of rivaroxaban over warfarin).[21, 22] However, these TTR levels are similar to those reported in prior studies of warfarin and may better represent real‐world INR management across multiple countries.[23]
Of note, the heterogeneity of INR management also appeared to impact clinical outcomes. For example, in RE‐LY, the INR control for warfarin was particularly poor in countries from east and southeast Asia, which may explain the more robust performance of dabigatran in these regions (vs Western and Central Europe, where TTR was >64%).[24] In the same analysis of variability within the RE‐LY trial, center‐specific TTRs demonstrated higher rates of cardiovascular events and major bleeds in centers with TTR <57%.[24] A different issue was noted in ROCKET‐AF, where TTR was relatively low in the overall trial and clinical outcomes were more equivalent between rivaroxaban and warfarin, when compared with the superiority of the new drugs in RE‐LY and ARISTOTLE. However, in ROCKET‐AF centers with mean TTR >68%, rivaroxaban was associated with higher rates of stroke and systemic embolism, and in the US subgroup (where TTR was 64%), rivaroxaban had a higher bleeding rate than warfarin.[9] Taken together, these findings highlight the potential for net clinical benefit among patients and populations with poor INR control during warfarin therapy, and conversely, the loss of benefit (and even potential harm) if replacing good INR management with the newer antithrombotic drugs.
To further explore these questions regarding NOAC efficacy and safety, the FDA review of rivaroxaban included a calculation of the major bleeds incurred per embolic event prevented.[9] Using this risk‐benefit ratio, the FDA confirmed that the advantage of using rivaroxaban over warfarin in ROCKET‐AF occurred among patients with difficult INR control, whereas patients with better INR management did not experience this net clinical benefit. As a result, in the absence of carefully managed INR levels in randomized trials (where INRs are managed through an intensive protocol), careful selection of patients with poor INR control may be prudent when considering rivaroxaban or other NOACs over warfarin.
Patient‐Centered Selection of Therapy
Although none of the NOACs have been compared with each other, several important drug and trial characteristics may help identify patients most likely to benefit from a specific drug choice for preventing thromboembolism in NVAF (Figure 1). For example, the modest increase in myocardial infarction noted among patients randomized to dabigatran in RE‐LY remains inadequately understood, and may lead some practitioners to favor using rivaroxaban or apixaban for NVAF patients at risk for coronary events. Others may point to the mortality reduction and lower rates of bleeding, including no increase in gastrointestinal hemorrhage, among patients receiving apixaban in ARISTOTLE. Concerns about reversibility also may impact drug selection, as none of the NOACs can be easily reversed for major life‐threatening bleeding, although potential antidotes are in development and may hopefully address this concern in the near future.[25] Other considerations include patient adherence to the twice‐daily dosing regimen of dabigatran or apixaban, comorbid conditions such as bleeding risk, drug‐drug interactions, outcomes reported during postmarketing surveillance, and cost. Overall, the noninferiority of these new agents compared with warfarin, plus their superiority in reducing the risk of important clinical events like intracranial hemorrhage, has led some professional societies to recommend the NOACs over warfarin in patients with NVAF whose CHADS2 scores are 1 or greater.[26]
Limitations
Several important limitations to these agents and their principal clinical trials should be noted. First, all 3 NOACs were compared with warfarin (or aspirin in the 1 prematurely halted apixaban trial), so comparisons between each drug and comparisons with placebo cannot be extrapolated from the data available. Second, the importance of remaining on label and using the NOACs appropriately for NVAF cannot be overemphasized, as recent experience with the NOACs among patients with mechanical heart valves or other clinical scenarios outside of the patient populations from the pivotal clinical trials (eg, severe renal dysfunction) will likely result in adverse patient outcomes.[27] Third, despite greater reliability in drug effects between patients and lack of need for intensive INR monitoring, more than 1 in 5 patients treated with the NOACs in these trials prematurely stopped therapy before reaching a study end point. Some of this premature discontinuation may be related to the more consistent degree of systemic anticoagulation with NOACs when compared with warfarin, thus resulting in higher bleeding rates (major, minor, or nuisance) than those reported in older trials using aspirin or placebo as the comparator. For each new antithrombotic medication, annual rates of major bleeding were higher than annual thromboembolic event rates (3.1% vs 1.1% in RE‐LY, 3.6% vs 2.1% in ROCKET‐AF, and 2.1% vs 1.3% in ARISTOTLE, respectively), although similar trends were noted for patients treated with warfarin. Nonetheless, because the average thromboembolic event may have more devastating consequences than the average bleeding event,[28] these clinical considerations must be carefully weighed for each patient when expanding the use of all 3 new drugs to the general population with NVAF. Further evaluation of the NOACs in real‐world populations, including an assessment of these drugs among patients taking dual antiplatelet therapy, is clearly warranted.
CONCLUSIONS
The recent development of alternative anticoagulation strategies to warfarin represents an exciting new opportunity for preventing the devastating consequences of stroke or systemic thromboembolism in patients with NVAF. However, despite the limitations of chronic warfarin therapy, it remains highly effective for a large proportion of patients with good INR control. Future studies will allow clinicians to better understand the advantages and disadvantages of each NOAC, so that ultimately an individualized, patient‐centered plan of care may be developed for each patient with NVAF.
ACKNOWLEDGMENTS
Disclosures: No funding support was used for the preparation of this review. Parts of these data were previously presented at the annual meeting of the Midwest Stroke Network (October 2013). Dr. Patel has no disclosures to report. Dr. Mehdirad serves on the speaker's bureau for Johnson & Johnson and Bristol‐Myers Squibb. Dr. Lim receives grant support from Astellas and InfraReDx, is a consultant for Acist Medical and Astra Zeneca, and serves on the speaker's bureau for Boehringer Ingelheim, Boston Scientific, St. Jude Medical, Abiomed, and Volcano Corporation. Dr. Ferreira reports consulting for St. Jude Medical. Dr. Mikolajczak reports grant support from Medtronic. Dr. Stolker receives grant support from GE Healthcare; is a consultant for Cordis Corp, and serves on the speaker's bureau for Astra Zeneca, Astellas, and InfraReDx. Dr Ferreira also reports serving on the speaker's bureau for Medtronic.
- , , , et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354.
- , , , . Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501.
- , , . Current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta‐analysis. Arch Intern Med. 2012;172:623–631.
- , , , et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort‐study on Atrial Fibrillation (SCAF‐study). Eur Heart J. 2006;27:1954–1964.
- , , , et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5 suppl):445S–469S.
- U.S. Food and Drug Administration. Boehringer Ingelheim advisory committee briefing document for dabigatran. Available at: http://www.fda.gov/downloads/advisorycommittees/committeemeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm226009.pdf. Accessed May 20, 2013.
- , , , et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis‐task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425.
- Rivaroxaban (Xarelto rivaroxaban tablets) prescribing information [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc.; 2011.
- U.S. Food and Drug Administration. Johnson 48:1–22.
- Apixaban (Eliquis apixaban tablets) prescribing information [package insert]. Princeton, NJ: Bristol‐Myers Squibb Co.; 2012.
- , , , et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- , , , et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–575S.
- , , , et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- , , , et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- , , , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- , , , et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- , , , et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292.
- , , , et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non‐valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477.
- , , , . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- , . Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag. 2012;8:525–531.
- , . Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–1559.
- , , , . Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77.
- , , , et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983.
- , , , et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: WHO, 2004. Available at: www.who.int/healthinfo/global_burden_disease/gbd2004_disabilityweights.pdf. Accessed February 24, 2013.
Approximately 2.3 million people in the United States and 4.5 million people in Europe have atrial fibrillation (AF), with an increase in prevalence with age to 8% among patients aged 80 years and older.[1] The most feared and potentially preventable complications of AF are stroke or systemic thromboembolism, and stroke in particular is increased approximately 5‐fold in patients with nonvalvular atrial fibrillation (NVAF).[2] For over 50 years, warfarin and similar vitamin K antagonists have been the principal anticoagulants used for preventing stroke in NVAF, with consistent reductions in systemic thromboembolic events when compared with placebo or aspirin.[2, 3] However, because of its narrow therapeutic window and related management difficulties (ie, frequent monitoring of international normalized ratio [INR] levels, dietary and medication restrictions, interindividual variability in dosing), many patients with NVAF do not receive warfarin or are inadequately treated.[4]
In response to the need for antithrombotic agents with better efficacy, patient tolerance, and convenience, the US Food and Drug Administration (FDA) recently approved 3 novel oral anticoagulants (NOACs) as alternatives to warfarin for NVAF: dabigatran, rivaroxaban, and apixaban. In this review, we evaluated the pharmacologic properties and clinical studies of these NOACs, including the continued role of warfarin in many patients requiring systemic anticoagulation, to guide practicing clinicians in providing individualized, patient‐centered care to each of their patients with NVAF.
PHARMACOLOGY
Mechanisms of Action
Whereas warfarin inhibits the formation of multiple vitamin K‐dependent coagulation factors (II, VII, IX, and X),[5] the NOACs are competitive and reversible inhibitors of more distal targets in the coagulation pathway. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban and apixaban directly inhibit factor Xa, ultimately resulting in the inhibition of fibrin formation and thrombosis.
Clinical Pathways and Drug Interactions
Key aspects of the pharmacokinetic profiles of the 3 NOACs are summarized in Table 1. In addition to these baseline properties of each medication, drug interactions play an important role in the effectiveness and potential toxicities of the NOACs. For example, dabigatran is almost exclusively excreted via glomerular filtration, resulting in a terminal half‐life of 12 to 17 hours in normal volunteers and a significantly higher half‐life in moderate and severe renal dysfunction (18 and 27 hours, respectively). In phase II and III trials, there was a 30% decrease in bioavailability when dabigatran was administered with pantoprazole, but no comparable effect was noted when coadministered with histamine receptor blockers like ranitidine.[6] In addition, although dabigatran has no significant interaction with hepatic P450 enzymes, its prodrug is excreted by the intestinal efflux transporter p‐glycoprotein. As a result, dabigatran's bioavailability is increased by coadministration with potent p‐glycoprotein inhibitors such as dronedarone, amiodarone, verapamil, diltiazem, or ketoconazole.[6, 7] According to FDA labeling, the only drug contraindicated with concomitant dabigatran administration is rifampin, which reduces serum concentration of dabigatran by 66%.
| Characteristic | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| |||
| Target | Factor IIa | Factor Xa | Factor Xa |
| Reversible binding | Yes | Yes | Yes |
| Half‐life, h | 1217 | 59 | 815 |
| Time to peak serum concentration, h | 13 | 24 | 34 |
| Protein binding, % | 35 | 9295 | 87 |
| Renal excretion, % | 80 | 66 | 2527 |
| Primary hepatic clearance pathway | Does not interact with CYP enzymes | CYP‐3A4 | CYP‐3A4 |
Unlike dabigatran, the absorption of rivaroxaban has significant variability between individuals, but the bioavailability of the 20‐mg dose increases by 39% and is significantly less variable when taken with food.[8] Phase I studies of rivaroxaban demonstrated that elderly patients had 50% higher serum concentrations when compared with younger patients.[7, 9] Also of note, rivaroxaban has 50% higher bioavailability in Japanese patients as compared with other ethnicities, including Chinese ethnicity, resulting in higher exposure to the drug and potentially explaining higher bleeding rates in Japan when using this drug.[9] The primary mechanisms for metabolism of rivaroxaban are the CYP‐3A4 and CYP‐2C8 pathways in the liver,[10] so other drugs metabolized through these pathways (eg, azole antifungals, protease inhibitors, clarithromycin) may have significant drug‐drug interactions.
Like the other NOACs, apixaban achieves its maximal concentration within 3 to 4 hours,[11] and like rivaroxaban, apixaban is metabolized by the CYP‐3A4 hepatic pathway. However, apixaban does not induce or inhibit hepatic cytochrome P450 (CYP) enzymes, so the potential for drug‐drug interactions is considered minimal.[12] Important exceptions include coadministration with ketoconazole or clarithromycin, each of which increases the bioavailability of apixaban up to 1.5‐fold, so a dose reduction to 2.5 mg twice‐daily (BID) is recommended.[11]
CLINICAL STUDIES
Randomized trials evaluating warfarin against placebo or aspirin for NVAF have spanned more than 3 decades, encompassing a variety of study designs, patient populations, and analytic techniques.[2, 3] Despite differences between trials, these studies have provided the framework for contemporary AF management, with consistent reductions in thromboembolic events with systemic anticoagulation, most notably among patients with multiple risk factors for stroke. Current professional guidelines recommend risk assessment of patients with NVAF, using the CHADS2 (1 point each for Congestive heart failure, Hypertension, Age 75 years, Diabetes, and 2 points for prior Stroke) or similar risk scores, to identify patients most likely to benefit from systemic anticoagulation.[1, 13] As a result of this extensive background literature, the 3 NOACs have primarily been evaluated against warfarin (instead of aspirin or placebo) as potential alternatives for reducing thromboembolic events in patients with NVAF. The 1 exception is a prematurely terminated trial of apixaban in warfarin‐ineligible patients with NVAF, in which apixaban reduced stroke or systemic embolism by 55% compared with aspirin after only 1.1 years of follow‐up, with no significant difference in major bleeding.[14]
Pivotal Clinical Trials
The 3 principal trials evaluating the NOACs against warfarin for NVAF are summarized in Table 2. In the Randomized Evaluation of Long‐term anticoagulation Therapy (RE‐LY) trial, dabigatran was compared with warfarin in 18,113 patients recruited from 951 clinical centers in 44 countries using a noninferiority study design.[15] Two different doses of dabigatran were studied, but only the 150‐mg BID dose was approved by the FDA. As a result, only the findings from the clinically approved 150‐mg dose are summarized in this review. Although RE‐LY was considered a semiblinded randomized trial, patients enrolled in the warfarin control arm underwent regular INR surveillance by their treating physicians, leaving the trial open to potential reporting biases. The authors tried to minimize bias by providing a standardized protocol for INR management, and by assigning 2 independent investigators blinded to the treatment assignments to adjudicate each event.
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Drug | Dabigatran | Rivaroxaban | Apixaban |
| Dosing | 150 mg BID (110 mg BID also tested) | 20 mg daily (15 mg for creatinine clearance 3049 mL/min) | 5 mg BID (2.5 mg for patients at higher risk of bleeding)a |
| Total population | 18,113 | 14,264 | 18,201 |
| Randomization | Semiblinded | Double blinded | Double blinded |
| Primary analytic approach | Noninferiority, intention‐to‐treat | Noninferiority, both intention‐to‐treat and on‐treatment | Noninferiority, intention‐to‐treat |
| Primary efficacy end point | Stroke or systemic embolism | Stroke or systemic embolism | Stroke or systemic embolism |
| Primary safety end point | Major bleeding | Major and clinically relevant nonmajor bleeding | Major bleeding |
| Key inclusion criteria | |||
| Documented atrial fibrillation | At screening or within 6 months | Within 30 days prior to randomization and within past year | At least 2 episodes recorded 2 weeks apart in past year |
| Eligible CHADS[2] scores | 1 | 2 | 1 |
| Selected exclusion criteria | |||
| Valvular heart disease | Any hemodynamically relevant or prosthetic valve | Severe mitral stenosis or any mechanical prosthetic valve | Moderate or severe mitral stenosis, or any mechanical prosthetic valve |
| Stroke | Severe 6 months or mild/moderate 14 days | Severe 3 months, any stroke 14 days, TIA 3 days | Stroke 7 days |
| Bleeding | Surgery 30 days, gastrointestinal bleed 12 months, any prior intracranial bleed, severe hypertension | Surgery 30 days, gastrointestinal bleed 6 months, active internal bleeding, any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension, platelets 90,000/L | Any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension |
| Renal | Creatinine clearance <30 mL/min | Creatinine clearance <30 mL/min | Creatinine clearance <25 mL/min |
The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) study involved 14,264 patients from 1178 participating sites in 45 countries.[16] Again, a noninferiority design was used to evaluate 20‐mg daily rivaroxaban against warfarin, but the 2 arms were compared in double‐blinded, double‐dummy fashion (thus eliminating the reporting bias related to the warfarin control arm in RE‐LY). In addition, whereas RE‐LY randomized patients to fixed doses of dabigatran within their respective treatment arms, ROCKET‐AF required a lower dose of rivaroxaban (15 mg daily) for patients with moderately reduced creatinine clearance (3049 mL/min). Also of note, ROCKET‐AF reported both intention‐to‐treat and on‐treatment analyses, with outcomes listed as number of events per 100 patient‐years (instead of percent per year). To facilitate comparisons between trials, only the intention‐to‐treat data are reported in this review.
Like ROCKET‐AF, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized patients using a double‐blind, double‐dummy, noninferiority design to therapy with apixaban 5 mg BID versus warfarin, ultimately enrolling 18,201 patients at 1034 clinical sites in 39 countries.[17] ARISTOTLE also provided a lower dose of apixaban (2.5 mg BID) for patients at higher risk of bleeding, defined by the authors as patients with 2 of the following characteristics: age 80 years and older, weight 60 kg, or serum creatinine 1.5 mg/dL. However, <5% of all patients in ARISTOTLE met these criteria and received the lower dose of apixaban.
Patient Populations and Study End Points
All 3 trials used relatively similar criteria for enrolling and following patients, with individual thromboembolic risk calculated using the CHADS2 definition, where higher scores are associated with incrementally higher risk of stroke.[18] However, ROCKET‐AF required a minimum CHADS2 score of 2 and permitted patients with lower left ventricular ejection fractions (35%), thus enrolling a higher‐risk patient population than RE‐LY and ARISTOTLE (where ejection fraction 40% was considered a risk factor for thromboembolism). As a result, more patients in ROCKET‐AF had prior stroke or systemic embolism than the other 2 trials (55% vs 20% in RE‐LY and 19% in ARISTOTLE) and more patients had significant heart failure (63%,vs 32% in RE‐LY and 36% in ARISTOTLE). These differences in enrollment ultimately translated into a higher overall risk profile in ROCKET‐AF (Table 3), which may have impacted some of the study results. In addition, patients requiring dual antiplatelet therapy (ie, clopidogrel and aspirin) were permitted in RE‐LY (5% of the final randomized population) but were excluded from the other 2 trials. The primary outcome for all 3 trials was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding (RE‐LY and ARISTOTLE), or combined major and clinically relevant nonmajor bleeding events (ROCKET‐AF).
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Age, y | 72 | 73 | 70 |
| Male sex, % | 63 | 60 | 65 |
| Type of atrial fibrillation, % | |||
| Paroxysmal | 33 | 18 | 15 |
| Persistent/permanent | 67 | 82 | 85 |
| Comorbidities, % | |||
| Hypertension | 79 | 90 | 87 |
| Previous stroke or systemic embolism | 20 | 55 | 19 |
| Diabetes | 23 | 40 | 25 |
| Congestive heart failure | 32 | 63 | 36 |
| Prior myocardial infarction | 17 | 17 | 15 |
| CHADS2 score, % | |||
| 01 | 32 | 0 | 34 |
| 2 | 35 | 13 | 36 |
| 3 | 33 | 87 | 30 |
| Medications, % | |||
| ACE inhibitor or angiotensin receptor blocker | 67 | 55 | 71 |
| ‐Blockers | 64 | 65 | 64 |
| Digoxin | 29 | 39 | 32 |
| Amiodarone | 11 | Not reported | 11 |
| Aspirin | 39 | 36 | 31 |
| Aspirin and clopidogrel | 5 | 0 | 0 |
| Prior long‐term warfarin or other vitamin K antagonist | 50 | 62 | 57 |
| Creatinine clearance, % | |||
| >80 mL/min | 32 | 32 | 41 |
| >5080 mL/min | 48 | 47 | 42 |
| >3050 mL/min | 20 | 21 | 15 |
| <30 mL/min | <1 | None reported | 2 |
| Mean time in therapeutic range among warfarin‐treated patients, % | 64 | 55 | 66 |
Clinical Outcomes
As illustrated in Table 4, the dabigatran 150‐mg BID dose was both noninferior and superior to warfarin for reducing the composite primary end point. Patients randomized to this arm of the RE‐LY study experienced fewer ischemic strokes, fewer hemorrhagic strokes, and a strong trend toward lower all‐cause mortality despite higher rates of myocardial infarction. There was no difference in overall major bleeding, although a significant reduction in intracranial hemorrhage was offset by a higher rate of gastrointestinal bleeding.
| Clinical Outcome | RE‐LY | ROCKET‐AF | ARISTOTLE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatran, 150 mg BID, %/y | Warfarin, %/y | Hazard Ratio | P Valuea | Rivaroxaban, 20 mg QD, No./100 Patient‐Years | Warfarin, No./100 Patient‐Years | Hazard Ratio | P Valuea | Apixaban 5 mg BID, %/y | Warfarin, %/yr | Hazard Ratio | P Valuea | |
| ||||||||||||
| Stroke or systemic embolism | 1.11 | 1.69 | 0.66 | <0.001 | 2.1 | 2.4 | 0.88 | <0.001 | 1.27 | 1.60 | 0.79 | 0.01 |
| Any stroke | 1.01 | 1.57 | 0.64 | <0.001 | 1.65 | 1.96 | 0.85 | 0.092 | 1.19 | 1.51 | 0.79 | 0.01 |
| Ischemic | 0.92 | 1.20 | 0.76 | 0.03 | 1.34 | 1.42 | 0.94 | 0.581 | 0.97 | 1.05 | 0.92 | 0.42 |
| Hemorrhagic | 0.10 | 0.38 | 0.26 | <0.001 | 0.26 | 0.44 | 0.59 | 0.024 | 0.24 | 0.47 | 0.51 | <0.001 |
| Myocardial infarction | 0.74 | 0.53 | 1.38 | 0.048 | 0.91 | 1.12 | 0.81 | 0.121 | 0.53 | 0.61 | 0.88 | 0.37 |
| All‐cause mortality | 3.64 | 4.13 | 0.88 | 0.051 | 1.87 | 2.21 | 0.85 | 0.073 | 3.52 | 3.94 | 0.89 | 0.047 |
| Major bleeds | 3.11 | 3.36 | 0.93 | 0.31 | 3.6 | 3.4 | 1.04 | 0.58 | 2.13 | 3.09 | 0.69 | <0.001 |
| Intracranial | 0.30 | 0.74 | 0.40 | <0.001 | 0.5 | 0.7 | 0.67 | 0.02 | 0.33 | 0.80 | 0.42 | <0.001 |
| Gastrointestinal | 1.51 | 1.02 | 1.50 | <0.001 | 3.15b | 2.16b | <0.001 | 0.76 | 0.86 | 0.89 | 0.37 | |
In the intention‐to‐treat analyses from ROCKET‐AF, rivaroxaban was noninferior to warfarin for reducing the primary end point, and there was a significant reduction in hemorrhagic stroke by rivaroxaban. Again, a strong trend toward lower mortality was seen, and like RE‐LY, an equivocal bleeding end point was largely driven by the combination of lower intracranial hemorrhage but higher gastrointestinal bleeding rates. Of note, the on‐treatment analysis from ROCKET‐AF demonstrated both noninferiority and superiority to warfarin, and there was no signal for higher rates of myocardial infarction as seen in RE‐LY.
In ARISTOTLE, apixaban was both noninferior and superior to warfarin, with stroke reduction largely driven by lower rates of intracranial hemorrhage. Unlike the prior studies of dabigatran and rivaroxaban, ARISTOTLE demonstrated a statistically significant reduction in all‐cause mortality and a significant reduction in major bleeding with apixaban therapy, with no increase in gastrointestinal bleeding.
INR Control
In prior randomized trials and observational registries of patients with AF, INR control has been highly variable, and better clinical outcomes were observed among patients consistently achieving INR levels between 2 and 3.[3, 19] For all 3 randomized trials of the NOACs summarized in this review, the warfarin control arms were analyzed using the Rosendaal method of evaluating total time in therapeutic range (TTR), reflecting the percent of time the patient had an INR between 2 and 3.[20] Overall, the mean TTR was 64% to 66% in the RE‐LY and ARISTOTLE trials, but only 55% in ROCKET‐AF. This has led to considerable criticism of the ROCKET‐AF trial, given concerns for a less robust comparator arm for rivaroxaban (and thus the potential for inflated efficacy of rivaroxaban over warfarin).[21, 22] However, these TTR levels are similar to those reported in prior studies of warfarin and may better represent real‐world INR management across multiple countries.[23]
Of note, the heterogeneity of INR management also appeared to impact clinical outcomes. For example, in RE‐LY, the INR control for warfarin was particularly poor in countries from east and southeast Asia, which may explain the more robust performance of dabigatran in these regions (vs Western and Central Europe, where TTR was >64%).[24] In the same analysis of variability within the RE‐LY trial, center‐specific TTRs demonstrated higher rates of cardiovascular events and major bleeds in centers with TTR <57%.[24] A different issue was noted in ROCKET‐AF, where TTR was relatively low in the overall trial and clinical outcomes were more equivalent between rivaroxaban and warfarin, when compared with the superiority of the new drugs in RE‐LY and ARISTOTLE. However, in ROCKET‐AF centers with mean TTR >68%, rivaroxaban was associated with higher rates of stroke and systemic embolism, and in the US subgroup (where TTR was 64%), rivaroxaban had a higher bleeding rate than warfarin.[9] Taken together, these findings highlight the potential for net clinical benefit among patients and populations with poor INR control during warfarin therapy, and conversely, the loss of benefit (and even potential harm) if replacing good INR management with the newer antithrombotic drugs.
To further explore these questions regarding NOAC efficacy and safety, the FDA review of rivaroxaban included a calculation of the major bleeds incurred per embolic event prevented.[9] Using this risk‐benefit ratio, the FDA confirmed that the advantage of using rivaroxaban over warfarin in ROCKET‐AF occurred among patients with difficult INR control, whereas patients with better INR management did not experience this net clinical benefit. As a result, in the absence of carefully managed INR levels in randomized trials (where INRs are managed through an intensive protocol), careful selection of patients with poor INR control may be prudent when considering rivaroxaban or other NOACs over warfarin.
Patient‐Centered Selection of Therapy
Although none of the NOACs have been compared with each other, several important drug and trial characteristics may help identify patients most likely to benefit from a specific drug choice for preventing thromboembolism in NVAF (Figure 1). For example, the modest increase in myocardial infarction noted among patients randomized to dabigatran in RE‐LY remains inadequately understood, and may lead some practitioners to favor using rivaroxaban or apixaban for NVAF patients at risk for coronary events. Others may point to the mortality reduction and lower rates of bleeding, including no increase in gastrointestinal hemorrhage, among patients receiving apixaban in ARISTOTLE. Concerns about reversibility also may impact drug selection, as none of the NOACs can be easily reversed for major life‐threatening bleeding, although potential antidotes are in development and may hopefully address this concern in the near future.[25] Other considerations include patient adherence to the twice‐daily dosing regimen of dabigatran or apixaban, comorbid conditions such as bleeding risk, drug‐drug interactions, outcomes reported during postmarketing surveillance, and cost. Overall, the noninferiority of these new agents compared with warfarin, plus their superiority in reducing the risk of important clinical events like intracranial hemorrhage, has led some professional societies to recommend the NOACs over warfarin in patients with NVAF whose CHADS2 scores are 1 or greater.[26]

Limitations
Several important limitations to these agents and their principal clinical trials should be noted. First, all 3 NOACs were compared with warfarin (or aspirin in the 1 prematurely halted apixaban trial), so comparisons between each drug and comparisons with placebo cannot be extrapolated from the data available. Second, the importance of remaining on label and using the NOACs appropriately for NVAF cannot be overemphasized, as recent experience with the NOACs among patients with mechanical heart valves or other clinical scenarios outside of the patient populations from the pivotal clinical trials (eg, severe renal dysfunction) will likely result in adverse patient outcomes.[27] Third, despite greater reliability in drug effects between patients and lack of need for intensive INR monitoring, more than 1 in 5 patients treated with the NOACs in these trials prematurely stopped therapy before reaching a study end point. Some of this premature discontinuation may be related to the more consistent degree of systemic anticoagulation with NOACs when compared with warfarin, thus resulting in higher bleeding rates (major, minor, or nuisance) than those reported in older trials using aspirin or placebo as the comparator. For each new antithrombotic medication, annual rates of major bleeding were higher than annual thromboembolic event rates (3.1% vs 1.1% in RE‐LY, 3.6% vs 2.1% in ROCKET‐AF, and 2.1% vs 1.3% in ARISTOTLE, respectively), although similar trends were noted for patients treated with warfarin. Nonetheless, because the average thromboembolic event may have more devastating consequences than the average bleeding event,[28] these clinical considerations must be carefully weighed for each patient when expanding the use of all 3 new drugs to the general population with NVAF. Further evaluation of the NOACs in real‐world populations, including an assessment of these drugs among patients taking dual antiplatelet therapy, is clearly warranted.
CONCLUSIONS
The recent development of alternative anticoagulation strategies to warfarin represents an exciting new opportunity for preventing the devastating consequences of stroke or systemic thromboembolism in patients with NVAF. However, despite the limitations of chronic warfarin therapy, it remains highly effective for a large proportion of patients with good INR control. Future studies will allow clinicians to better understand the advantages and disadvantages of each NOAC, so that ultimately an individualized, patient‐centered plan of care may be developed for each patient with NVAF.
ACKNOWLEDGMENTS
Disclosures: No funding support was used for the preparation of this review. Parts of these data were previously presented at the annual meeting of the Midwest Stroke Network (October 2013). Dr. Patel has no disclosures to report. Dr. Mehdirad serves on the speaker's bureau for Johnson & Johnson and Bristol‐Myers Squibb. Dr. Lim receives grant support from Astellas and InfraReDx, is a consultant for Acist Medical and Astra Zeneca, and serves on the speaker's bureau for Boehringer Ingelheim, Boston Scientific, St. Jude Medical, Abiomed, and Volcano Corporation. Dr. Ferreira reports consulting for St. Jude Medical. Dr. Mikolajczak reports grant support from Medtronic. Dr. Stolker receives grant support from GE Healthcare; is a consultant for Cordis Corp, and serves on the speaker's bureau for Astra Zeneca, Astellas, and InfraReDx. Dr Ferreira also reports serving on the speaker's bureau for Medtronic.
Approximately 2.3 million people in the United States and 4.5 million people in Europe have atrial fibrillation (AF), with an increase in prevalence with age to 8% among patients aged 80 years and older.[1] The most feared and potentially preventable complications of AF are stroke or systemic thromboembolism, and stroke in particular is increased approximately 5‐fold in patients with nonvalvular atrial fibrillation (NVAF).[2] For over 50 years, warfarin and similar vitamin K antagonists have been the principal anticoagulants used for preventing stroke in NVAF, with consistent reductions in systemic thromboembolic events when compared with placebo or aspirin.[2, 3] However, because of its narrow therapeutic window and related management difficulties (ie, frequent monitoring of international normalized ratio [INR] levels, dietary and medication restrictions, interindividual variability in dosing), many patients with NVAF do not receive warfarin or are inadequately treated.[4]
In response to the need for antithrombotic agents with better efficacy, patient tolerance, and convenience, the US Food and Drug Administration (FDA) recently approved 3 novel oral anticoagulants (NOACs) as alternatives to warfarin for NVAF: dabigatran, rivaroxaban, and apixaban. In this review, we evaluated the pharmacologic properties and clinical studies of these NOACs, including the continued role of warfarin in many patients requiring systemic anticoagulation, to guide practicing clinicians in providing individualized, patient‐centered care to each of their patients with NVAF.
PHARMACOLOGY
Mechanisms of Action
Whereas warfarin inhibits the formation of multiple vitamin K‐dependent coagulation factors (II, VII, IX, and X),[5] the NOACs are competitive and reversible inhibitors of more distal targets in the coagulation pathway. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban and apixaban directly inhibit factor Xa, ultimately resulting in the inhibition of fibrin formation and thrombosis.
Clinical Pathways and Drug Interactions
Key aspects of the pharmacokinetic profiles of the 3 NOACs are summarized in Table 1. In addition to these baseline properties of each medication, drug interactions play an important role in the effectiveness and potential toxicities of the NOACs. For example, dabigatran is almost exclusively excreted via glomerular filtration, resulting in a terminal half‐life of 12 to 17 hours in normal volunteers and a significantly higher half‐life in moderate and severe renal dysfunction (18 and 27 hours, respectively). In phase II and III trials, there was a 30% decrease in bioavailability when dabigatran was administered with pantoprazole, but no comparable effect was noted when coadministered with histamine receptor blockers like ranitidine.[6] In addition, although dabigatran has no significant interaction with hepatic P450 enzymes, its prodrug is excreted by the intestinal efflux transporter p‐glycoprotein. As a result, dabigatran's bioavailability is increased by coadministration with potent p‐glycoprotein inhibitors such as dronedarone, amiodarone, verapamil, diltiazem, or ketoconazole.[6, 7] According to FDA labeling, the only drug contraindicated with concomitant dabigatran administration is rifampin, which reduces serum concentration of dabigatran by 66%.
| Characteristic | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| |||
| Target | Factor IIa | Factor Xa | Factor Xa |
| Reversible binding | Yes | Yes | Yes |
| Half‐life, h | 1217 | 59 | 815 |
| Time to peak serum concentration, h | 13 | 24 | 34 |
| Protein binding, % | 35 | 9295 | 87 |
| Renal excretion, % | 80 | 66 | 2527 |
| Primary hepatic clearance pathway | Does not interact with CYP enzymes | CYP‐3A4 | CYP‐3A4 |
Unlike dabigatran, the absorption of rivaroxaban has significant variability between individuals, but the bioavailability of the 20‐mg dose increases by 39% and is significantly less variable when taken with food.[8] Phase I studies of rivaroxaban demonstrated that elderly patients had 50% higher serum concentrations when compared with younger patients.[7, 9] Also of note, rivaroxaban has 50% higher bioavailability in Japanese patients as compared with other ethnicities, including Chinese ethnicity, resulting in higher exposure to the drug and potentially explaining higher bleeding rates in Japan when using this drug.[9] The primary mechanisms for metabolism of rivaroxaban are the CYP‐3A4 and CYP‐2C8 pathways in the liver,[10] so other drugs metabolized through these pathways (eg, azole antifungals, protease inhibitors, clarithromycin) may have significant drug‐drug interactions.
Like the other NOACs, apixaban achieves its maximal concentration within 3 to 4 hours,[11] and like rivaroxaban, apixaban is metabolized by the CYP‐3A4 hepatic pathway. However, apixaban does not induce or inhibit hepatic cytochrome P450 (CYP) enzymes, so the potential for drug‐drug interactions is considered minimal.[12] Important exceptions include coadministration with ketoconazole or clarithromycin, each of which increases the bioavailability of apixaban up to 1.5‐fold, so a dose reduction to 2.5 mg twice‐daily (BID) is recommended.[11]
CLINICAL STUDIES
Randomized trials evaluating warfarin against placebo or aspirin for NVAF have spanned more than 3 decades, encompassing a variety of study designs, patient populations, and analytic techniques.[2, 3] Despite differences between trials, these studies have provided the framework for contemporary AF management, with consistent reductions in thromboembolic events with systemic anticoagulation, most notably among patients with multiple risk factors for stroke. Current professional guidelines recommend risk assessment of patients with NVAF, using the CHADS2 (1 point each for Congestive heart failure, Hypertension, Age 75 years, Diabetes, and 2 points for prior Stroke) or similar risk scores, to identify patients most likely to benefit from systemic anticoagulation.[1, 13] As a result of this extensive background literature, the 3 NOACs have primarily been evaluated against warfarin (instead of aspirin or placebo) as potential alternatives for reducing thromboembolic events in patients with NVAF. The 1 exception is a prematurely terminated trial of apixaban in warfarin‐ineligible patients with NVAF, in which apixaban reduced stroke or systemic embolism by 55% compared with aspirin after only 1.1 years of follow‐up, with no significant difference in major bleeding.[14]
Pivotal Clinical Trials
The 3 principal trials evaluating the NOACs against warfarin for NVAF are summarized in Table 2. In the Randomized Evaluation of Long‐term anticoagulation Therapy (RE‐LY) trial, dabigatran was compared with warfarin in 18,113 patients recruited from 951 clinical centers in 44 countries using a noninferiority study design.[15] Two different doses of dabigatran were studied, but only the 150‐mg BID dose was approved by the FDA. As a result, only the findings from the clinically approved 150‐mg dose are summarized in this review. Although RE‐LY was considered a semiblinded randomized trial, patients enrolled in the warfarin control arm underwent regular INR surveillance by their treating physicians, leaving the trial open to potential reporting biases. The authors tried to minimize bias by providing a standardized protocol for INR management, and by assigning 2 independent investigators blinded to the treatment assignments to adjudicate each event.
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Drug | Dabigatran | Rivaroxaban | Apixaban |
| Dosing | 150 mg BID (110 mg BID also tested) | 20 mg daily (15 mg for creatinine clearance 3049 mL/min) | 5 mg BID (2.5 mg for patients at higher risk of bleeding)a |
| Total population | 18,113 | 14,264 | 18,201 |
| Randomization | Semiblinded | Double blinded | Double blinded |
| Primary analytic approach | Noninferiority, intention‐to‐treat | Noninferiority, both intention‐to‐treat and on‐treatment | Noninferiority, intention‐to‐treat |
| Primary efficacy end point | Stroke or systemic embolism | Stroke or systemic embolism | Stroke or systemic embolism |
| Primary safety end point | Major bleeding | Major and clinically relevant nonmajor bleeding | Major bleeding |
| Key inclusion criteria | |||
| Documented atrial fibrillation | At screening or within 6 months | Within 30 days prior to randomization and within past year | At least 2 episodes recorded 2 weeks apart in past year |
| Eligible CHADS[2] scores | 1 | 2 | 1 |
| Selected exclusion criteria | |||
| Valvular heart disease | Any hemodynamically relevant or prosthetic valve | Severe mitral stenosis or any mechanical prosthetic valve | Moderate or severe mitral stenosis, or any mechanical prosthetic valve |
| Stroke | Severe 6 months or mild/moderate 14 days | Severe 3 months, any stroke 14 days, TIA 3 days | Stroke 7 days |
| Bleeding | Surgery 30 days, gastrointestinal bleed 12 months, any prior intracranial bleed, severe hypertension | Surgery 30 days, gastrointestinal bleed 6 months, active internal bleeding, any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension, platelets 90,000/L | Any prior intracranial bleed, chronic dual antiplatelet therapy, severe hypertension |
| Renal | Creatinine clearance <30 mL/min | Creatinine clearance <30 mL/min | Creatinine clearance <25 mL/min |
The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) study involved 14,264 patients from 1178 participating sites in 45 countries.[16] Again, a noninferiority design was used to evaluate 20‐mg daily rivaroxaban against warfarin, but the 2 arms were compared in double‐blinded, double‐dummy fashion (thus eliminating the reporting bias related to the warfarin control arm in RE‐LY). In addition, whereas RE‐LY randomized patients to fixed doses of dabigatran within their respective treatment arms, ROCKET‐AF required a lower dose of rivaroxaban (15 mg daily) for patients with moderately reduced creatinine clearance (3049 mL/min). Also of note, ROCKET‐AF reported both intention‐to‐treat and on‐treatment analyses, with outcomes listed as number of events per 100 patient‐years (instead of percent per year). To facilitate comparisons between trials, only the intention‐to‐treat data are reported in this review.
Like ROCKET‐AF, the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) study randomized patients using a double‐blind, double‐dummy, noninferiority design to therapy with apixaban 5 mg BID versus warfarin, ultimately enrolling 18,201 patients at 1034 clinical sites in 39 countries.[17] ARISTOTLE also provided a lower dose of apixaban (2.5 mg BID) for patients at higher risk of bleeding, defined by the authors as patients with 2 of the following characteristics: age 80 years and older, weight 60 kg, or serum creatinine 1.5 mg/dL. However, <5% of all patients in ARISTOTLE met these criteria and received the lower dose of apixaban.
Patient Populations and Study End Points
All 3 trials used relatively similar criteria for enrolling and following patients, with individual thromboembolic risk calculated using the CHADS2 definition, where higher scores are associated with incrementally higher risk of stroke.[18] However, ROCKET‐AF required a minimum CHADS2 score of 2 and permitted patients with lower left ventricular ejection fractions (35%), thus enrolling a higher‐risk patient population than RE‐LY and ARISTOTLE (where ejection fraction 40% was considered a risk factor for thromboembolism). As a result, more patients in ROCKET‐AF had prior stroke or systemic embolism than the other 2 trials (55% vs 20% in RE‐LY and 19% in ARISTOTLE) and more patients had significant heart failure (63%,vs 32% in RE‐LY and 36% in ARISTOTLE). These differences in enrollment ultimately translated into a higher overall risk profile in ROCKET‐AF (Table 3), which may have impacted some of the study results. In addition, patients requiring dual antiplatelet therapy (ie, clopidogrel and aspirin) were permitted in RE‐LY (5% of the final randomized population) but were excluded from the other 2 trials. The primary outcome for all 3 trials was the composite of stroke or systemic embolism, and the primary safety end point was major bleeding (RE‐LY and ARISTOTLE), or combined major and clinically relevant nonmajor bleeding events (ROCKET‐AF).
| Characteristic | RE‐LY | ROCKET‐AF | ARISTOTLE |
|---|---|---|---|
| |||
| Age, y | 72 | 73 | 70 |
| Male sex, % | 63 | 60 | 65 |
| Type of atrial fibrillation, % | |||
| Paroxysmal | 33 | 18 | 15 |
| Persistent/permanent | 67 | 82 | 85 |
| Comorbidities, % | |||
| Hypertension | 79 | 90 | 87 |
| Previous stroke or systemic embolism | 20 | 55 | 19 |
| Diabetes | 23 | 40 | 25 |
| Congestive heart failure | 32 | 63 | 36 |
| Prior myocardial infarction | 17 | 17 | 15 |
| CHADS2 score, % | |||
| 01 | 32 | 0 | 34 |
| 2 | 35 | 13 | 36 |
| 3 | 33 | 87 | 30 |
| Medications, % | |||
| ACE inhibitor or angiotensin receptor blocker | 67 | 55 | 71 |
| ‐Blockers | 64 | 65 | 64 |
| Digoxin | 29 | 39 | 32 |
| Amiodarone | 11 | Not reported | 11 |
| Aspirin | 39 | 36 | 31 |
| Aspirin and clopidogrel | 5 | 0 | 0 |
| Prior long‐term warfarin or other vitamin K antagonist | 50 | 62 | 57 |
| Creatinine clearance, % | |||
| >80 mL/min | 32 | 32 | 41 |
| >5080 mL/min | 48 | 47 | 42 |
| >3050 mL/min | 20 | 21 | 15 |
| <30 mL/min | <1 | None reported | 2 |
| Mean time in therapeutic range among warfarin‐treated patients, % | 64 | 55 | 66 |
Clinical Outcomes
As illustrated in Table 4, the dabigatran 150‐mg BID dose was both noninferior and superior to warfarin for reducing the composite primary end point. Patients randomized to this arm of the RE‐LY study experienced fewer ischemic strokes, fewer hemorrhagic strokes, and a strong trend toward lower all‐cause mortality despite higher rates of myocardial infarction. There was no difference in overall major bleeding, although a significant reduction in intracranial hemorrhage was offset by a higher rate of gastrointestinal bleeding.
| Clinical Outcome | RE‐LY | ROCKET‐AF | ARISTOTLE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dabigatran, 150 mg BID, %/y | Warfarin, %/y | Hazard Ratio | P Valuea | Rivaroxaban, 20 mg QD, No./100 Patient‐Years | Warfarin, No./100 Patient‐Years | Hazard Ratio | P Valuea | Apixaban 5 mg BID, %/y | Warfarin, %/yr | Hazard Ratio | P Valuea | |
| ||||||||||||
| Stroke or systemic embolism | 1.11 | 1.69 | 0.66 | <0.001 | 2.1 | 2.4 | 0.88 | <0.001 | 1.27 | 1.60 | 0.79 | 0.01 |
| Any stroke | 1.01 | 1.57 | 0.64 | <0.001 | 1.65 | 1.96 | 0.85 | 0.092 | 1.19 | 1.51 | 0.79 | 0.01 |
| Ischemic | 0.92 | 1.20 | 0.76 | 0.03 | 1.34 | 1.42 | 0.94 | 0.581 | 0.97 | 1.05 | 0.92 | 0.42 |
| Hemorrhagic | 0.10 | 0.38 | 0.26 | <0.001 | 0.26 | 0.44 | 0.59 | 0.024 | 0.24 | 0.47 | 0.51 | <0.001 |
| Myocardial infarction | 0.74 | 0.53 | 1.38 | 0.048 | 0.91 | 1.12 | 0.81 | 0.121 | 0.53 | 0.61 | 0.88 | 0.37 |
| All‐cause mortality | 3.64 | 4.13 | 0.88 | 0.051 | 1.87 | 2.21 | 0.85 | 0.073 | 3.52 | 3.94 | 0.89 | 0.047 |
| Major bleeds | 3.11 | 3.36 | 0.93 | 0.31 | 3.6 | 3.4 | 1.04 | 0.58 | 2.13 | 3.09 | 0.69 | <0.001 |
| Intracranial | 0.30 | 0.74 | 0.40 | <0.001 | 0.5 | 0.7 | 0.67 | 0.02 | 0.33 | 0.80 | 0.42 | <0.001 |
| Gastrointestinal | 1.51 | 1.02 | 1.50 | <0.001 | 3.15b | 2.16b | <0.001 | 0.76 | 0.86 | 0.89 | 0.37 | |
In the intention‐to‐treat analyses from ROCKET‐AF, rivaroxaban was noninferior to warfarin for reducing the primary end point, and there was a significant reduction in hemorrhagic stroke by rivaroxaban. Again, a strong trend toward lower mortality was seen, and like RE‐LY, an equivocal bleeding end point was largely driven by the combination of lower intracranial hemorrhage but higher gastrointestinal bleeding rates. Of note, the on‐treatment analysis from ROCKET‐AF demonstrated both noninferiority and superiority to warfarin, and there was no signal for higher rates of myocardial infarction as seen in RE‐LY.
In ARISTOTLE, apixaban was both noninferior and superior to warfarin, with stroke reduction largely driven by lower rates of intracranial hemorrhage. Unlike the prior studies of dabigatran and rivaroxaban, ARISTOTLE demonstrated a statistically significant reduction in all‐cause mortality and a significant reduction in major bleeding with apixaban therapy, with no increase in gastrointestinal bleeding.
INR Control
In prior randomized trials and observational registries of patients with AF, INR control has been highly variable, and better clinical outcomes were observed among patients consistently achieving INR levels between 2 and 3.[3, 19] For all 3 randomized trials of the NOACs summarized in this review, the warfarin control arms were analyzed using the Rosendaal method of evaluating total time in therapeutic range (TTR), reflecting the percent of time the patient had an INR between 2 and 3.[20] Overall, the mean TTR was 64% to 66% in the RE‐LY and ARISTOTLE trials, but only 55% in ROCKET‐AF. This has led to considerable criticism of the ROCKET‐AF trial, given concerns for a less robust comparator arm for rivaroxaban (and thus the potential for inflated efficacy of rivaroxaban over warfarin).[21, 22] However, these TTR levels are similar to those reported in prior studies of warfarin and may better represent real‐world INR management across multiple countries.[23]
Of note, the heterogeneity of INR management also appeared to impact clinical outcomes. For example, in RE‐LY, the INR control for warfarin was particularly poor in countries from east and southeast Asia, which may explain the more robust performance of dabigatran in these regions (vs Western and Central Europe, where TTR was >64%).[24] In the same analysis of variability within the RE‐LY trial, center‐specific TTRs demonstrated higher rates of cardiovascular events and major bleeds in centers with TTR <57%.[24] A different issue was noted in ROCKET‐AF, where TTR was relatively low in the overall trial and clinical outcomes were more equivalent between rivaroxaban and warfarin, when compared with the superiority of the new drugs in RE‐LY and ARISTOTLE. However, in ROCKET‐AF centers with mean TTR >68%, rivaroxaban was associated with higher rates of stroke and systemic embolism, and in the US subgroup (where TTR was 64%), rivaroxaban had a higher bleeding rate than warfarin.[9] Taken together, these findings highlight the potential for net clinical benefit among patients and populations with poor INR control during warfarin therapy, and conversely, the loss of benefit (and even potential harm) if replacing good INR management with the newer antithrombotic drugs.
To further explore these questions regarding NOAC efficacy and safety, the FDA review of rivaroxaban included a calculation of the major bleeds incurred per embolic event prevented.[9] Using this risk‐benefit ratio, the FDA confirmed that the advantage of using rivaroxaban over warfarin in ROCKET‐AF occurred among patients with difficult INR control, whereas patients with better INR management did not experience this net clinical benefit. As a result, in the absence of carefully managed INR levels in randomized trials (where INRs are managed through an intensive protocol), careful selection of patients with poor INR control may be prudent when considering rivaroxaban or other NOACs over warfarin.
Patient‐Centered Selection of Therapy
Although none of the NOACs have been compared with each other, several important drug and trial characteristics may help identify patients most likely to benefit from a specific drug choice for preventing thromboembolism in NVAF (Figure 1). For example, the modest increase in myocardial infarction noted among patients randomized to dabigatran in RE‐LY remains inadequately understood, and may lead some practitioners to favor using rivaroxaban or apixaban for NVAF patients at risk for coronary events. Others may point to the mortality reduction and lower rates of bleeding, including no increase in gastrointestinal hemorrhage, among patients receiving apixaban in ARISTOTLE. Concerns about reversibility also may impact drug selection, as none of the NOACs can be easily reversed for major life‐threatening bleeding, although potential antidotes are in development and may hopefully address this concern in the near future.[25] Other considerations include patient adherence to the twice‐daily dosing regimen of dabigatran or apixaban, comorbid conditions such as bleeding risk, drug‐drug interactions, outcomes reported during postmarketing surveillance, and cost. Overall, the noninferiority of these new agents compared with warfarin, plus their superiority in reducing the risk of important clinical events like intracranial hemorrhage, has led some professional societies to recommend the NOACs over warfarin in patients with NVAF whose CHADS2 scores are 1 or greater.[26]

Limitations
Several important limitations to these agents and their principal clinical trials should be noted. First, all 3 NOACs were compared with warfarin (or aspirin in the 1 prematurely halted apixaban trial), so comparisons between each drug and comparisons with placebo cannot be extrapolated from the data available. Second, the importance of remaining on label and using the NOACs appropriately for NVAF cannot be overemphasized, as recent experience with the NOACs among patients with mechanical heart valves or other clinical scenarios outside of the patient populations from the pivotal clinical trials (eg, severe renal dysfunction) will likely result in adverse patient outcomes.[27] Third, despite greater reliability in drug effects between patients and lack of need for intensive INR monitoring, more than 1 in 5 patients treated with the NOACs in these trials prematurely stopped therapy before reaching a study end point. Some of this premature discontinuation may be related to the more consistent degree of systemic anticoagulation with NOACs when compared with warfarin, thus resulting in higher bleeding rates (major, minor, or nuisance) than those reported in older trials using aspirin or placebo as the comparator. For each new antithrombotic medication, annual rates of major bleeding were higher than annual thromboembolic event rates (3.1% vs 1.1% in RE‐LY, 3.6% vs 2.1% in ROCKET‐AF, and 2.1% vs 1.3% in ARISTOTLE, respectively), although similar trends were noted for patients treated with warfarin. Nonetheless, because the average thromboembolic event may have more devastating consequences than the average bleeding event,[28] these clinical considerations must be carefully weighed for each patient when expanding the use of all 3 new drugs to the general population with NVAF. Further evaluation of the NOACs in real‐world populations, including an assessment of these drugs among patients taking dual antiplatelet therapy, is clearly warranted.
CONCLUSIONS
The recent development of alternative anticoagulation strategies to warfarin represents an exciting new opportunity for preventing the devastating consequences of stroke or systemic thromboembolism in patients with NVAF. However, despite the limitations of chronic warfarin therapy, it remains highly effective for a large proportion of patients with good INR control. Future studies will allow clinicians to better understand the advantages and disadvantages of each NOAC, so that ultimately an individualized, patient‐centered plan of care may be developed for each patient with NVAF.
ACKNOWLEDGMENTS
Disclosures: No funding support was used for the preparation of this review. Parts of these data were previously presented at the annual meeting of the Midwest Stroke Network (October 2013). Dr. Patel has no disclosures to report. Dr. Mehdirad serves on the speaker's bureau for Johnson & Johnson and Bristol‐Myers Squibb. Dr. Lim receives grant support from Astellas and InfraReDx, is a consultant for Acist Medical and Astra Zeneca, and serves on the speaker's bureau for Boehringer Ingelheim, Boston Scientific, St. Jude Medical, Abiomed, and Volcano Corporation. Dr. Ferreira reports consulting for St. Jude Medical. Dr. Mikolajczak reports grant support from Medtronic. Dr. Stolker receives grant support from GE Healthcare; is a consultant for Cordis Corp, and serves on the speaker's bureau for Astra Zeneca, Astellas, and InfraReDx. Dr Ferreira also reports serving on the speaker's bureau for Medtronic.
- , , , et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354.
- , , , . Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501.
- , , . Current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta‐analysis. Arch Intern Med. 2012;172:623–631.
- , , , et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort‐study on Atrial Fibrillation (SCAF‐study). Eur Heart J. 2006;27:1954–1964.
- , , , et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5 suppl):445S–469S.
- U.S. Food and Drug Administration. Boehringer Ingelheim advisory committee briefing document for dabigatran. Available at: http://www.fda.gov/downloads/advisorycommittees/committeemeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm226009.pdf. Accessed May 20, 2013.
- , , , et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis‐task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425.
- Rivaroxaban (Xarelto rivaroxaban tablets) prescribing information [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc.; 2011.
- U.S. Food and Drug Administration. Johnson 48:1–22.
- Apixaban (Eliquis apixaban tablets) prescribing information [package insert]. Princeton, NJ: Bristol‐Myers Squibb Co.; 2012.
- , , , et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- , , , et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–575S.
- , , , et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- , , , et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- , , , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- , , , et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- , , , et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292.
- , , , et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non‐valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477.
- , , , . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- , . Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag. 2012;8:525–531.
- , . Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–1559.
- , , , . Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77.
- , , , et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983.
- , , , et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: WHO, 2004. Available at: www.who.int/healthinfo/global_burden_disease/gbd2004_disabilityweights.pdf. Accessed February 24, 2013.
- , , , et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354.
- , , , . Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131:492–501.
- , , . Current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta‐analysis. Arch Intern Med. 2012;172:623–631.
- , , , et al. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort‐study on Atrial Fibrillation (SCAF‐study). Eur Heart J. 2006;27:1954–1964.
- , , , et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5 suppl):445S–469S.
- U.S. Food and Drug Administration. Boehringer Ingelheim advisory committee briefing document for dabigatran. Available at: http://www.fda.gov/downloads/advisorycommittees/committeemeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm226009.pdf. Accessed May 20, 2013.
- , , , et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis‐task force on anticoagulants in heart disease position paper. J Am Coll Cardiol. 2012;59:1413–1425.
- Rivaroxaban (Xarelto rivaroxaban tablets) prescribing information [package insert]. Titusville, NJ: Janssen Pharmaceuticals Inc.; 2011.
- U.S. Food and Drug Administration. Johnson 48:1–22.
- Apixaban (Eliquis apixaban tablets) prescribing information [package insert]. Princeton, NJ: Bristol‐Myers Squibb Co.; 2012.
- , , , et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
- , , , et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S–575S.
- , , , et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817.
- , , , et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- , , , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- , , , et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- , , , et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292.
- , , , et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non‐valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477.
- , , , . A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239.
- , . Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag. 2012;8:525–531.
- , . Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–1559.
- , , , . Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb Thrombolysis. 2006;21:73–77.
- , , , et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983.
- , , , et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214.
- World Health Organization. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneva: WHO, 2004. Available at: www.who.int/healthinfo/global_burden_disease/gbd2004_disabilityweights.pdf. Accessed February 24, 2013.
Don't forget non-Alzheimer dementias
Dementia is not always due to Alzheimer disease. An accurate diagnosis is important, as the various causative conditions can differ in their course and treatment.
Dementia refers to cognitive impairment severe enough to interfere with the ability to independently perform activities of daily living. It can occur at any age but is most common after age 60. Some studies estimate that 13.9% of people age 71 and older have some form of dementia.1 The prevalence increases with age, ranging from 5% at age 70 to 79 to 37% at age 90 and older.1
Alzheimer disease accounts for about 60% to 80% of cases,2 or an estimated 4.7 million people age 65 and older in the United States, a number anticipated to climb to 13.8 million by 2050.3
Other types of dementia are less often considered and are challenging to recognize, although many have distinct characteristics. This article summarizes the features and management of the more common non-Alzheimer dementias:
- Vascular dementia
- Dementia with Lewy bodies
- Progressive supranuclear palsy
- Corticobasal degeneration
- Multiple system atrophy
- Parkinson disease dementia
- Frontotemporal dementia
- Primary progressive aphasia
- Normal-pressure hydrocephalus
- Rapidly progressive dementia (ie, Creutzfeld-Jakob disease, autoimmune disease).
VASCULAR DEMENTIA
After Alzheimer disease, vascular dementia is the most common dementia, accounting for about 20% to 30% of cases. Clinical criteria have not been widely accepted, although several have been published, including those in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and the National Institute of Neurological and Communicative Diseases and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Risk factors for vascular dementia include cerebrovascular disease (hypertension, diabetes, hyperlipidemia) and coexisting conditions related to atherosclerosis (coronary artery disease, peripheral artery disease).
The Hachinski Ischemic Score is a good bedside tool to help differentiate Alzheimer dementia from vascular dementia.5
Sudden onset and stepwise decline
Vascular dementia often presents as a sudden and stepwise progression of cognitive deficits that stabilize and that are caused by vascular insults (Table 1).6–10 Some patients have continuous decline after a vascular event, indicating that Alzheimer dementia may also be present. Dementia is then defined as a mixed type.
Behavioral problems such as physical aggression, hallucinations, paranoia, and mood fluctuations are common.11
Deficits depend on vascular areas affected
Cognitive deficits are heterogeneous and are often related to the location of the vascular insult. Involvement of subcortical areas may result in executive dysfunction, slowed processing speed, and behavioral changes.12
Executive dysfunction may be identified using the Trail Making Test (Part B) or the Executive Interview (EXIT25). Office-based tools such as the Folstein Mini-Mental State Examination, the Montreal Cognitive Assessment, or the St. Louis University Mental Status Examination may also uncover these deficits.
Focal neurologic deficits may be found on clinical examination.
Structural neuroimaging may identify small strokes in areas of the brain affecting cognitive function or occlusion of a larger vessel associated with more profound neurologic deficits. Neuroimaging findings may not correlate with any significant decline noted by the patient, suggesting “silent” strokes.
Treat symptoms and manage risk factors
Although the US Food and Drug Administration (FDA) has not approved any pharmacotherapy for vascular dementia, commonly prescribed cognitive enhancers have demonstrated some benefit.13
Behavioral problems such as aggression can be disturbing to the patient and the caregiver. Nonpharmacologic methods (eg, redirection, rescheduling care activities to avoid conflict, avoiding issues that lead to agitation) should be tried first to address these problems.
Drug therapy may be used off-label for neuropsychiatric symptoms such as hallucinations, delusions, and combativeness, but clinical trials of these agents for this purpose have shown mixed results,14 and their use is often associated with significant risk.15 Antipsychotic drugs are associated with a risk of death and pneumonia when prescribed for dementia. Many also carry a risk of QT prolongation, which is particularly concerning for patients with coronary artery disease or rhythm disturbances.
The key to reducing further decline is to optimize management of vascular risk factors to reduce stroke risk.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33
CORTICOBASAL DEGENERATION
Corticobasal degeneration is a progressive, asymmetric movement disorder often manifesting initially with cognitive or behavioral impairment. It is associated with abnormality of the cytoskeleton protein tau. Onset is usually after age 60.
Asymmetric movement disorder with cognitive dysfunction
This diagnosis is clinical. Diagnostic criteria proposed in 2003 include the following core features34:
- Insidious onset and progressive course
- No identifiable cause
- Cortical dysfunction with at least one of the following: apraxia, alien limb phenomenon (one limb moves involuntarily with complex movements, eg, grabbing the other hand), cortical sensory loss, visual hemineglect, nonfluent aphasia
- Extrapyramidal dysfunction: focal rigidity unresponsive to levodopa, asymmetric dystonia.
An international consortium has developed more specific clinical research criteria for probable and possible corticobasal degeneration.35 In a series of 147 patients, the following clinical features were found: parkinsonism (100%), higher cortical dysfunction (93%), dyspraxia (82%), gait disorder (80%), unilateral limb dystonia (71%), tremor (55%), and dementia (25%).36
Behavioral problems commonly include depression; apathy, irritability, and agitation are also reported.37
Cognitive testing may reveal deficits in frontal-parietal cognitive domains including attention and concentration, executive function, verbal fluency, and visuospatial skills.38 Learning disabilities may be improved with verbal cueing (in contrast to Alzheimer disease). Patients may also have impaired graphesthesia (the ability to recognize writing on the skin only by the sensation of touch).39,40
Motor examination may reveal marked asymmetry. Hand, limb, speech, and gait apraxias are common. Gait is typically slow, with short steps and shuffling, and a wide-based or freezing gait. Arm swing may be absent on one side.
Asymmetric cortical atrophy
Early on, MRI may be normal. As the disease progresses, asymmetric cortical atrophy may be seen, especially in the posterior frontal and parietal lobes.
Levodopa ineffective in corticobasal degeneration
Corticobasal degeneration responds poorly to levodopa. Botulinum toxin has been used to help with dystonia and limb pain.
MULTIPLE SYSTEM ATROPHY
Multiple system atrophy is another atypical parkinsonian disorder, most often diagnosed in men over age 60. It is characterized by sporadic parkinsonism, cerebellar signs (involving balance and coordination), pyramidal tract dysfunction, and autonomic insufficiency in varying combinations. Two major subtypes are recognized, depending on whether the predominating presenting features are cerebellar signs or parkinsonism. In contrast to dementia with Lewy bodies, psychiatric symptoms are not a major feature, except possibly depression.41
Diagnosis requires a sporadic progressive disorder that has features of autonomic failure and poor response of parkinsonism or cerebellar ataxia to levodopa.42
Multiple system atrophy is usually not associated with dementia in the early stages, but patients develop deficits in learning, recognition, memory, and verbal fluency as the disease progresses.43 Rapid-eye-movement sleep behavior disorder has been reported in more than half of patients.44
A neurologic examination provides clues
Parkinsonian features are usually symmetric, in contrast to idiopathic Parkinson disease. These signs may include akinesia with rigidity, postural instability, hypokinetic speech, and tremor.
Cerebellar signs include nystagmus and dysarthria (speech disturbance), and gait and limb ataxia.
Pyramidal features include extensor plantar responses and hyperreflexia.
Autonomic dysfunction includes orthostatic hypotension, bladder and rectal atony, loss of sweating, urinary or fecal incontinence, and erectile dysfunction.
Electromyography may demonstrate decreased anal sphincter tone.
MRI shows atrophy of putamen and pons
Brain MRI may show atrophy of the putamen (hypointensity of the putamen with a hyperintense rim). Pons atrophy may also be present, revealing a “hot cross bun” sign in axial images. These combined findings have specificity above 90% but limited sensitivity. These signs are useful to distinguish multiple system atrophy from Parkinson dementia, but their absence does not exclude the diagnosis of multiple system atrophy.45,46
Multiple system atrophy typically responds poorly to levodopa
Levodopa may improve movement and rigidity, but many respond poorly to treatment or lose response after a few years. Fludrocortisone (Florinef) or vasoconstrictors such as midodrine (Orvaten, Proamatine) may help with orthostatic hypotension.47,48
PARKINSON DISEASE DEMENTIA
Dementia eventually develops in most patients with Parkinson disease. Older age and the akinetic rigid form of the disease are associated with higher risk. Diagnosis of idiopathic Parkinson disease before the development of dementia is essential for the diagnosis.
The Movement Disorder Society Task Force has developed new diagnostic criteria.49 Deficits must be present in at least two of the four core cognitive domains (attention, memory, executive, and visuospatial functions) and must be severe enough to affect daily functioning.
Behavioral symptoms such as affective changes, hallucinations, and apathy are common.
MRI shows characteristic brain atrophy in Parkinson disease dementia
MRI shows reduced gray matter volume in the frontal lobe in patients with Parkinson disease without dementia compared with controls. In Parkinson disease dementia, reduced volume extends to temporal, occipital, and subcortical areas. No significant volumetric differences have been observed in Parkinson dementia compared with dementia with Lewy bodies.50 A greater decrease of glucose metabolism has been found in the inferior parietal and occipital lobes in Parkinson disease dementia than in Parkinson disease without dementia.51
Rivastigmine effective for dementia
A Cochrane review supports the use of acetylcholinesterase inhibitors in patients with Parkinson disease dementia, with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales.19 At this time, rivastigmine is the only FDA-approved cholinesterase inhibitor for treating Parkinson disease dementia. In clinical trials, memantine did not improve global clinical status or behavioral symptoms of dementia of Parkinson disease.51
FRONTOTEMPORAL DEMENTIA
Frontotemporal dementia frequently starts before age 65 and accounts for 20% to 50% of dementias in this age group.52 Recognition of the condition in older patients is also growing.53 Frontotemporal dementia encompasses a spectrum of dementias, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.54
Gradual onset of uncharacteristic behaviors
Accepted diagnostic criteria include core features of gradual onset, early decline in social and interpersonal conduct, early impairment of self-regulation, emotional blunting, and loss of insight. Many patients are diagnosed with psychiatric conditions. Changes reported by family and caregivers typically deviate substantially from the person’s usual behavior, such as impulsive and inappropriate behaviors or complete withdrawal and apathy.
Language sometimes affected in frontotemporal dementia
Language impairment may be present in some variants. Behavioral and language changes often accompany other forms of dementia (Alzheimer disease, vascular dementia, primary progressive aphasia), making diagnosis more challenging. Office-based testing often does not reveal any deficits, although the Frontal Behavioral Inventory may help.55 A referral to a clinical neuropsychologist may help identify and quantify cognitive impairments.
MRI shows frontotemporal lobes affected
Structural neuroimaging may not reveal abnormalities initially, but with progression, atrophy may be seen in the frontal and temporal lobes. Functional neuroimaging (positron emission tomography, brain SPECT, functional MRI) show hypometabolism in the same areas.
Treat symptoms
There are no specific FDA-approved therapies for frontotemporal dementia. Acetylcholinesterase inhibitors can help progressive nonfluent aphasia in some cases. Selective serotonin reuptake inhibitors may alleviate depressive symptoms, and low doses of atypical antipsychotic medications may help with impulsivity, disinhibition, and aggressive or disruptive behaviors.56
PRIMARY PROGRESSIVE APHASIA
Language impairment predominates
Primary progressive aphasia is a rare form of dementia in which symptoms typically develop around age 60. Pathology is varied. In a study of 60 patients with initial clinical symptoms of primary progressive aphasia, postmortem histology of brain tissue revealed various findings, including those consistent with Alzheimer pathology and motor neuron diseasetype inclusions.57
Patients typically present with expressive language problems as the primary deficit for the first 2 years of the disease, with preservation in other cognitive areas such as memory, visuospatial skills, and executive function.58 Office-based testing may overstate the severity of the dementia, given the dependence of performance on intact language.
It is important to distinguish primary progressive aphasia from other dementias that also affect language. In the frontal variant of frontotemporal dementia, the primary language problem is anomia (inability to name objects) or diminished speech output, which may be accompanied by behavioral problems. Semantic dementia affects word recognition as well as comprehension. In Alzheimer disease, language may be affected along with memory and other areas of cognitive function.
Imaging shows focal degeneration in the left hemisphere
Structural neuroimaging does not initially reveal any deficits, but later it may reveal atrophy in the frontal, perisylvian complex, and temporal areas of the left hemisphere, reflecting the focal nature of the degeneration.59 Functional neuroimaging (positron emission tomography, SPECT) may reveal hypometabolism or diminished blood flow in these areas prior to changes in structural neuroimaging.60
Other communication methods may help
There are no FDA-approved therapies for primary progressive aphasia. Off-label use of some agents (eg, selective serotonin reuptake inhibitors and small doses of antipsychotic medications) has been found useful in small trials.56 Patients may benefit from learning other forms of communication, such as using sign language, laminated cards with printed words or pictures, or artificial voice synthesizers, to express their needs.
NORMAL-PRESSURE HYDROCEPHALUS
Classic triad: Gait, cognition, incontinence
With the onset of symptoms in the sixth or seventh decade, normal-pressure hydrocephalus affects less than 1% of people age 65 and older. It represents up to 5% of dementias, although estimates are influenced by the varied criteria for diagnosis.61 It is characterized by the classic triad of gait impairment, cognitive impairment, and urinary frequency or incontinence.62
Symptoms progress over a period of years, with gait impairment often predominating. As this triad is common in the geriatric population, identifying other explanations is important. Gait impairment caused by spinal stenosis, peripheral neuropathy, or parkinsonism should be explored. Cognitive impairment could be due to depression, Alzheimer disease, or other forms of dementia. Urinary symptoms may be related to detrusor instability or an enlarged prostate.
Gait impairment initially manifests as slowing of gait, but progresses to difficulty with gait initiation. Gait tends to be wide-based (stance more than 1 foot wide).
Cognitive impairment is typically subcortical, manifested as slowed processing speed and impaired executive function. Recall and working memory may be impaired.
Enlarged ventricles seen on imaging in normal-pressure hydrocephalus
Structural neuroimaging reveals enlarged ventricles (Evan’s ratio > 0.358). This can be difficult to distinguish from ventriculomegaly due to cerebral atrophy; assessing the callosal angle on MRI may distinguish the two.63,64 Diagnosis of normal-pressure hydrocephalus can be confirmed using a cerebrospinal fluid infusion test to assess resistance of fluid to resorption.65
Treat with cerebrospinal fluid drainage
Specific tests should be performed to determine candidacy for surgery. These include a high-volume lumbar puncture (40 to 50 mL) or a trial of external lumbar drainage (10 mL per hour for 48 to 72 hours).65 Definitive treatment is surgical placement of a shunt to allow cerebrospinal fluid to drain into the atria or peritoneal cavity.
Surgery may improve gait, but cognitive symptoms often remain,66 and clinical decline may occur after the shunt is placed. Once gait dysfunction is resolved, other explanations for cognitive impairment or residual gait impairment should be considered. An underlying reason for progression of normal-pressure hydrocephalus symptoms after surgical intervention should be identified.67
RAPIDLY PROGRESSIVE DEMENTIAS
Rapidly progressive dementias are among the most challenging of dementing illnesses. They are characterized by a subacute course and an accelerated rate of decline, developing in less than 2 years. Evaluation should typically be more comprehensive than for other types of dementia. The main goal is to diagnose potentially treatable conditions, such as Hashimoto encephalopathy or paraneoplastic limbic encephalitis, and to distinguish these conditions from diseases with a very poor prognosis, such as Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease is a fatal prion-related neurodegenerative illness. Sporadic disease is most common, but variant, familial, and iatrogenic types have been reported. The most common initial symptoms in sporadic disease are cognitive (39%), cerebellar (21%), behavioral (20%), constitutional (20%), sensory (11%), motor (9%), and visual (7%).68
Chronic neurodegenerative diseases can be misdiagnosed as Creutzfeldt-Jakob disease because of an atypical time course and multi-system neurologic findings.
The US Centers for Disease Control and Prevention has adopted criteria for diagnosing probable Creutzfeldt-Jakob disease (Table 3). Routine investigations should also not suggest an alternative diagnosis.69
Autoimmune diseases
Autoimmune conditions may present as a rapidly progressive dementia, including Hashimoto encephalopathy and antibody-mediated limbic encephalitis, either associated with cancer (paraneoplastic) or without cancer (nonparaneoplastic).
Paraneoplastic limbic encephalitis is a group of inflammatory conditions involving antibodies produced within the cerebrospinal fluid and serum resulting in neurologic symptoms. These antibodies react against proteins expressed mostly by a tumor somewhere else in the body.70
Hashimoto encephalitis is a subacute to chronic encephalopathy that may present as dementia with abnormally high levels of thyroid antibodies. The symptoms can vary from confusion to psychosis. There are two main presentations: one involves a relapsing-remitting course with stroke-like episodes (27% of patients) and the second consists of insidious onset of seizures (66% of patients).
Diagnosis involves testing for elevated anti-thyroid peroxidase and thyroglobulin antibodies. MRI findings are nonspecific. Hashimoto encephalitis responds to treatment with corticosteroids, plasmapheresis, or immunosuppressive therapy.71
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–132.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed February 3, 2014.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Hou CE, Carlin D, Miller BL. Non-Alzheimer’s disease dementias: anatomic, clinical, and molecular correlates. Can J Psychiatry 2004; 49:164–171.
- Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637.
- Fereshtehnejad SM, Religa D, Westman E, Aarsland D, Lökk J, Eriksdotter M. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish Dementia Quality Registry (SveDem). Neuropsychiatr Dis Treat 2013; 9:927–935.
- Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18:394–396.
- Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progrssive supranuclear palsy. Neurology 1988; 38:1546–1552.
- Tousi B, Schuele SU, Subramanian T. A 46-year-old woman with rigidity and frequent falls. Cleve Clin J Med 2005; 72:57–63.
- Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord 2009; 15:59–61.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study0. JAMA 2002; 288:1475–1483.
- Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006; 81:223–230.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210.
- McKeith IG, Dickson DW, Lowe J, et al; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics 2011; 8:82–92.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Emre M, Tsolaki M, Bonucelli U, et al; on behalf of the 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977.
- Kurlan R, Cummings J, Raman R, Thal L; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68:1356–1363.
- Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611.
- Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003; 60:917–922.
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105:117–124.
- Fahn S, Jankovic J, Hallett M, editors. Principles and Practice of Movement Disorders. 2nd ed. New York, NY: Elsevier/Saunders; 2011.
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996; 60:615–620.
- Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61:40–45.
- Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 2001; 56:957–963.
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 2010; 25:357–362.
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary parkinsonisms. Mov Disord 2011; 26:1083–1095.
- Gallucci M, Limbucci N, Catalucci A, Caulo M. Neurodegenerative diseases. Radiol Clin North Am 2008; 46:799–817.
- Stamelou M, de Silva R, Arias-Carrión O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133:1578–1590.
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat 2012; 8:85–93.
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15–S19.
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503.
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998; 55:957–961.
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721.
- Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology 1995; 45:1477–1483.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer’s disease.” J Neurol Neurosurg Psychiatry 2003; 74:389.
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003; 61:1134–1135.
- Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000; 54:507–511.
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676.
- Berent S, Giordani B, Gilman S, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn 2002; 50:194–206.
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 72:798–800.
- Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998; 65:65–71.
- Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 2012; 27:1754–1762.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Mathias CJ, Kimber JR. Postural hypotension: causes, clinical features, investigation, and management. Annu Rev Med 1999; 50:317–336.
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707.
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800.
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson’s disease. J Nucl Med 1996; 37:1115–1122.
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621.
- Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal dementia in elderly individuals. Arch Neurol 2012; 69:1052–1960.
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468.
- Mendez MF, Lauterbach EC, Sampson SM; ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci 2008; 20:130–149.
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med 2003; 349:1535–1542.
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39:166–173.
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997; 39:556–559.
- Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:1017–1022.
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965; 2:307–327.
- Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol 2000; 247:5–14.
- Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57(suppl 3):S17–S28.
- Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery 2008; 62(suppl 2):643–659.
- Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013; 10:22.
- Rabinovici GD, Wang PN, Levin J, et al. First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287.
- Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol 2003; 60:164–171.
Dementia is not always due to Alzheimer disease. An accurate diagnosis is important, as the various causative conditions can differ in their course and treatment.
Dementia refers to cognitive impairment severe enough to interfere with the ability to independently perform activities of daily living. It can occur at any age but is most common after age 60. Some studies estimate that 13.9% of people age 71 and older have some form of dementia.1 The prevalence increases with age, ranging from 5% at age 70 to 79 to 37% at age 90 and older.1
Alzheimer disease accounts for about 60% to 80% of cases,2 or an estimated 4.7 million people age 65 and older in the United States, a number anticipated to climb to 13.8 million by 2050.3
Other types of dementia are less often considered and are challenging to recognize, although many have distinct characteristics. This article summarizes the features and management of the more common non-Alzheimer dementias:
- Vascular dementia
- Dementia with Lewy bodies
- Progressive supranuclear palsy
- Corticobasal degeneration
- Multiple system atrophy
- Parkinson disease dementia
- Frontotemporal dementia
- Primary progressive aphasia
- Normal-pressure hydrocephalus
- Rapidly progressive dementia (ie, Creutzfeld-Jakob disease, autoimmune disease).
VASCULAR DEMENTIA
After Alzheimer disease, vascular dementia is the most common dementia, accounting for about 20% to 30% of cases. Clinical criteria have not been widely accepted, although several have been published, including those in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and the National Institute of Neurological and Communicative Diseases and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Risk factors for vascular dementia include cerebrovascular disease (hypertension, diabetes, hyperlipidemia) and coexisting conditions related to atherosclerosis (coronary artery disease, peripheral artery disease).
The Hachinski Ischemic Score is a good bedside tool to help differentiate Alzheimer dementia from vascular dementia.5
Sudden onset and stepwise decline
Vascular dementia often presents as a sudden and stepwise progression of cognitive deficits that stabilize and that are caused by vascular insults (Table 1).6–10 Some patients have continuous decline after a vascular event, indicating that Alzheimer dementia may also be present. Dementia is then defined as a mixed type.
Behavioral problems such as physical aggression, hallucinations, paranoia, and mood fluctuations are common.11
Deficits depend on vascular areas affected
Cognitive deficits are heterogeneous and are often related to the location of the vascular insult. Involvement of subcortical areas may result in executive dysfunction, slowed processing speed, and behavioral changes.12
Executive dysfunction may be identified using the Trail Making Test (Part B) or the Executive Interview (EXIT25). Office-based tools such as the Folstein Mini-Mental State Examination, the Montreal Cognitive Assessment, or the St. Louis University Mental Status Examination may also uncover these deficits.
Focal neurologic deficits may be found on clinical examination.
Structural neuroimaging may identify small strokes in areas of the brain affecting cognitive function or occlusion of a larger vessel associated with more profound neurologic deficits. Neuroimaging findings may not correlate with any significant decline noted by the patient, suggesting “silent” strokes.
Treat symptoms and manage risk factors
Although the US Food and Drug Administration (FDA) has not approved any pharmacotherapy for vascular dementia, commonly prescribed cognitive enhancers have demonstrated some benefit.13
Behavioral problems such as aggression can be disturbing to the patient and the caregiver. Nonpharmacologic methods (eg, redirection, rescheduling care activities to avoid conflict, avoiding issues that lead to agitation) should be tried first to address these problems.
Drug therapy may be used off-label for neuropsychiatric symptoms such as hallucinations, delusions, and combativeness, but clinical trials of these agents for this purpose have shown mixed results,14 and their use is often associated with significant risk.15 Antipsychotic drugs are associated with a risk of death and pneumonia when prescribed for dementia. Many also carry a risk of QT prolongation, which is particularly concerning for patients with coronary artery disease or rhythm disturbances.
The key to reducing further decline is to optimize management of vascular risk factors to reduce stroke risk.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33
CORTICOBASAL DEGENERATION
Corticobasal degeneration is a progressive, asymmetric movement disorder often manifesting initially with cognitive or behavioral impairment. It is associated with abnormality of the cytoskeleton protein tau. Onset is usually after age 60.
Asymmetric movement disorder with cognitive dysfunction
This diagnosis is clinical. Diagnostic criteria proposed in 2003 include the following core features34:
- Insidious onset and progressive course
- No identifiable cause
- Cortical dysfunction with at least one of the following: apraxia, alien limb phenomenon (one limb moves involuntarily with complex movements, eg, grabbing the other hand), cortical sensory loss, visual hemineglect, nonfluent aphasia
- Extrapyramidal dysfunction: focal rigidity unresponsive to levodopa, asymmetric dystonia.
An international consortium has developed more specific clinical research criteria for probable and possible corticobasal degeneration.35 In a series of 147 patients, the following clinical features were found: parkinsonism (100%), higher cortical dysfunction (93%), dyspraxia (82%), gait disorder (80%), unilateral limb dystonia (71%), tremor (55%), and dementia (25%).36
Behavioral problems commonly include depression; apathy, irritability, and agitation are also reported.37
Cognitive testing may reveal deficits in frontal-parietal cognitive domains including attention and concentration, executive function, verbal fluency, and visuospatial skills.38 Learning disabilities may be improved with verbal cueing (in contrast to Alzheimer disease). Patients may also have impaired graphesthesia (the ability to recognize writing on the skin only by the sensation of touch).39,40
Motor examination may reveal marked asymmetry. Hand, limb, speech, and gait apraxias are common. Gait is typically slow, with short steps and shuffling, and a wide-based or freezing gait. Arm swing may be absent on one side.
Asymmetric cortical atrophy
Early on, MRI may be normal. As the disease progresses, asymmetric cortical atrophy may be seen, especially in the posterior frontal and parietal lobes.
Levodopa ineffective in corticobasal degeneration
Corticobasal degeneration responds poorly to levodopa. Botulinum toxin has been used to help with dystonia and limb pain.
MULTIPLE SYSTEM ATROPHY
Multiple system atrophy is another atypical parkinsonian disorder, most often diagnosed in men over age 60. It is characterized by sporadic parkinsonism, cerebellar signs (involving balance and coordination), pyramidal tract dysfunction, and autonomic insufficiency in varying combinations. Two major subtypes are recognized, depending on whether the predominating presenting features are cerebellar signs or parkinsonism. In contrast to dementia with Lewy bodies, psychiatric symptoms are not a major feature, except possibly depression.41
Diagnosis requires a sporadic progressive disorder that has features of autonomic failure and poor response of parkinsonism or cerebellar ataxia to levodopa.42
Multiple system atrophy is usually not associated with dementia in the early stages, but patients develop deficits in learning, recognition, memory, and verbal fluency as the disease progresses.43 Rapid-eye-movement sleep behavior disorder has been reported in more than half of patients.44
A neurologic examination provides clues
Parkinsonian features are usually symmetric, in contrast to idiopathic Parkinson disease. These signs may include akinesia with rigidity, postural instability, hypokinetic speech, and tremor.
Cerebellar signs include nystagmus and dysarthria (speech disturbance), and gait and limb ataxia.
Pyramidal features include extensor plantar responses and hyperreflexia.
Autonomic dysfunction includes orthostatic hypotension, bladder and rectal atony, loss of sweating, urinary or fecal incontinence, and erectile dysfunction.
Electromyography may demonstrate decreased anal sphincter tone.
MRI shows atrophy of putamen and pons
Brain MRI may show atrophy of the putamen (hypointensity of the putamen with a hyperintense rim). Pons atrophy may also be present, revealing a “hot cross bun” sign in axial images. These combined findings have specificity above 90% but limited sensitivity. These signs are useful to distinguish multiple system atrophy from Parkinson dementia, but their absence does not exclude the diagnosis of multiple system atrophy.45,46
Multiple system atrophy typically responds poorly to levodopa
Levodopa may improve movement and rigidity, but many respond poorly to treatment or lose response after a few years. Fludrocortisone (Florinef) or vasoconstrictors such as midodrine (Orvaten, Proamatine) may help with orthostatic hypotension.47,48
PARKINSON DISEASE DEMENTIA
Dementia eventually develops in most patients with Parkinson disease. Older age and the akinetic rigid form of the disease are associated with higher risk. Diagnosis of idiopathic Parkinson disease before the development of dementia is essential for the diagnosis.
The Movement Disorder Society Task Force has developed new diagnostic criteria.49 Deficits must be present in at least two of the four core cognitive domains (attention, memory, executive, and visuospatial functions) and must be severe enough to affect daily functioning.
Behavioral symptoms such as affective changes, hallucinations, and apathy are common.
MRI shows characteristic brain atrophy in Parkinson disease dementia
MRI shows reduced gray matter volume in the frontal lobe in patients with Parkinson disease without dementia compared with controls. In Parkinson disease dementia, reduced volume extends to temporal, occipital, and subcortical areas. No significant volumetric differences have been observed in Parkinson dementia compared with dementia with Lewy bodies.50 A greater decrease of glucose metabolism has been found in the inferior parietal and occipital lobes in Parkinson disease dementia than in Parkinson disease without dementia.51
Rivastigmine effective for dementia
A Cochrane review supports the use of acetylcholinesterase inhibitors in patients with Parkinson disease dementia, with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales.19 At this time, rivastigmine is the only FDA-approved cholinesterase inhibitor for treating Parkinson disease dementia. In clinical trials, memantine did not improve global clinical status or behavioral symptoms of dementia of Parkinson disease.51
FRONTOTEMPORAL DEMENTIA
Frontotemporal dementia frequently starts before age 65 and accounts for 20% to 50% of dementias in this age group.52 Recognition of the condition in older patients is also growing.53 Frontotemporal dementia encompasses a spectrum of dementias, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.54
Gradual onset of uncharacteristic behaviors
Accepted diagnostic criteria include core features of gradual onset, early decline in social and interpersonal conduct, early impairment of self-regulation, emotional blunting, and loss of insight. Many patients are diagnosed with psychiatric conditions. Changes reported by family and caregivers typically deviate substantially from the person’s usual behavior, such as impulsive and inappropriate behaviors or complete withdrawal and apathy.
Language sometimes affected in frontotemporal dementia
Language impairment may be present in some variants. Behavioral and language changes often accompany other forms of dementia (Alzheimer disease, vascular dementia, primary progressive aphasia), making diagnosis more challenging. Office-based testing often does not reveal any deficits, although the Frontal Behavioral Inventory may help.55 A referral to a clinical neuropsychologist may help identify and quantify cognitive impairments.
MRI shows frontotemporal lobes affected
Structural neuroimaging may not reveal abnormalities initially, but with progression, atrophy may be seen in the frontal and temporal lobes. Functional neuroimaging (positron emission tomography, brain SPECT, functional MRI) show hypometabolism in the same areas.
Treat symptoms
There are no specific FDA-approved therapies for frontotemporal dementia. Acetylcholinesterase inhibitors can help progressive nonfluent aphasia in some cases. Selective serotonin reuptake inhibitors may alleviate depressive symptoms, and low doses of atypical antipsychotic medications may help with impulsivity, disinhibition, and aggressive or disruptive behaviors.56
PRIMARY PROGRESSIVE APHASIA
Language impairment predominates
Primary progressive aphasia is a rare form of dementia in which symptoms typically develop around age 60. Pathology is varied. In a study of 60 patients with initial clinical symptoms of primary progressive aphasia, postmortem histology of brain tissue revealed various findings, including those consistent with Alzheimer pathology and motor neuron diseasetype inclusions.57
Patients typically present with expressive language problems as the primary deficit for the first 2 years of the disease, with preservation in other cognitive areas such as memory, visuospatial skills, and executive function.58 Office-based testing may overstate the severity of the dementia, given the dependence of performance on intact language.
It is important to distinguish primary progressive aphasia from other dementias that also affect language. In the frontal variant of frontotemporal dementia, the primary language problem is anomia (inability to name objects) or diminished speech output, which may be accompanied by behavioral problems. Semantic dementia affects word recognition as well as comprehension. In Alzheimer disease, language may be affected along with memory and other areas of cognitive function.
Imaging shows focal degeneration in the left hemisphere
Structural neuroimaging does not initially reveal any deficits, but later it may reveal atrophy in the frontal, perisylvian complex, and temporal areas of the left hemisphere, reflecting the focal nature of the degeneration.59 Functional neuroimaging (positron emission tomography, SPECT) may reveal hypometabolism or diminished blood flow in these areas prior to changes in structural neuroimaging.60
Other communication methods may help
There are no FDA-approved therapies for primary progressive aphasia. Off-label use of some agents (eg, selective serotonin reuptake inhibitors and small doses of antipsychotic medications) has been found useful in small trials.56 Patients may benefit from learning other forms of communication, such as using sign language, laminated cards with printed words or pictures, or artificial voice synthesizers, to express their needs.
NORMAL-PRESSURE HYDROCEPHALUS
Classic triad: Gait, cognition, incontinence
With the onset of symptoms in the sixth or seventh decade, normal-pressure hydrocephalus affects less than 1% of people age 65 and older. It represents up to 5% of dementias, although estimates are influenced by the varied criteria for diagnosis.61 It is characterized by the classic triad of gait impairment, cognitive impairment, and urinary frequency or incontinence.62
Symptoms progress over a period of years, with gait impairment often predominating. As this triad is common in the geriatric population, identifying other explanations is important. Gait impairment caused by spinal stenosis, peripheral neuropathy, or parkinsonism should be explored. Cognitive impairment could be due to depression, Alzheimer disease, or other forms of dementia. Urinary symptoms may be related to detrusor instability or an enlarged prostate.
Gait impairment initially manifests as slowing of gait, but progresses to difficulty with gait initiation. Gait tends to be wide-based (stance more than 1 foot wide).
Cognitive impairment is typically subcortical, manifested as slowed processing speed and impaired executive function. Recall and working memory may be impaired.
Enlarged ventricles seen on imaging in normal-pressure hydrocephalus
Structural neuroimaging reveals enlarged ventricles (Evan’s ratio > 0.358). This can be difficult to distinguish from ventriculomegaly due to cerebral atrophy; assessing the callosal angle on MRI may distinguish the two.63,64 Diagnosis of normal-pressure hydrocephalus can be confirmed using a cerebrospinal fluid infusion test to assess resistance of fluid to resorption.65
Treat with cerebrospinal fluid drainage
Specific tests should be performed to determine candidacy for surgery. These include a high-volume lumbar puncture (40 to 50 mL) or a trial of external lumbar drainage (10 mL per hour for 48 to 72 hours).65 Definitive treatment is surgical placement of a shunt to allow cerebrospinal fluid to drain into the atria or peritoneal cavity.
Surgery may improve gait, but cognitive symptoms often remain,66 and clinical decline may occur after the shunt is placed. Once gait dysfunction is resolved, other explanations for cognitive impairment or residual gait impairment should be considered. An underlying reason for progression of normal-pressure hydrocephalus symptoms after surgical intervention should be identified.67
RAPIDLY PROGRESSIVE DEMENTIAS
Rapidly progressive dementias are among the most challenging of dementing illnesses. They are characterized by a subacute course and an accelerated rate of decline, developing in less than 2 years. Evaluation should typically be more comprehensive than for other types of dementia. The main goal is to diagnose potentially treatable conditions, such as Hashimoto encephalopathy or paraneoplastic limbic encephalitis, and to distinguish these conditions from diseases with a very poor prognosis, such as Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease is a fatal prion-related neurodegenerative illness. Sporadic disease is most common, but variant, familial, and iatrogenic types have been reported. The most common initial symptoms in sporadic disease are cognitive (39%), cerebellar (21%), behavioral (20%), constitutional (20%), sensory (11%), motor (9%), and visual (7%).68
Chronic neurodegenerative diseases can be misdiagnosed as Creutzfeldt-Jakob disease because of an atypical time course and multi-system neurologic findings.
The US Centers for Disease Control and Prevention has adopted criteria for diagnosing probable Creutzfeldt-Jakob disease (Table 3). Routine investigations should also not suggest an alternative diagnosis.69
Autoimmune diseases
Autoimmune conditions may present as a rapidly progressive dementia, including Hashimoto encephalopathy and antibody-mediated limbic encephalitis, either associated with cancer (paraneoplastic) or without cancer (nonparaneoplastic).
Paraneoplastic limbic encephalitis is a group of inflammatory conditions involving antibodies produced within the cerebrospinal fluid and serum resulting in neurologic symptoms. These antibodies react against proteins expressed mostly by a tumor somewhere else in the body.70
Hashimoto encephalitis is a subacute to chronic encephalopathy that may present as dementia with abnormally high levels of thyroid antibodies. The symptoms can vary from confusion to psychosis. There are two main presentations: one involves a relapsing-remitting course with stroke-like episodes (27% of patients) and the second consists of insidious onset of seizures (66% of patients).
Diagnosis involves testing for elevated anti-thyroid peroxidase and thyroglobulin antibodies. MRI findings are nonspecific. Hashimoto encephalitis responds to treatment with corticosteroids, plasmapheresis, or immunosuppressive therapy.71
Dementia is not always due to Alzheimer disease. An accurate diagnosis is important, as the various causative conditions can differ in their course and treatment.
Dementia refers to cognitive impairment severe enough to interfere with the ability to independently perform activities of daily living. It can occur at any age but is most common after age 60. Some studies estimate that 13.9% of people age 71 and older have some form of dementia.1 The prevalence increases with age, ranging from 5% at age 70 to 79 to 37% at age 90 and older.1
Alzheimer disease accounts for about 60% to 80% of cases,2 or an estimated 4.7 million people age 65 and older in the United States, a number anticipated to climb to 13.8 million by 2050.3
Other types of dementia are less often considered and are challenging to recognize, although many have distinct characteristics. This article summarizes the features and management of the more common non-Alzheimer dementias:
- Vascular dementia
- Dementia with Lewy bodies
- Progressive supranuclear palsy
- Corticobasal degeneration
- Multiple system atrophy
- Parkinson disease dementia
- Frontotemporal dementia
- Primary progressive aphasia
- Normal-pressure hydrocephalus
- Rapidly progressive dementia (ie, Creutzfeld-Jakob disease, autoimmune disease).
VASCULAR DEMENTIA
After Alzheimer disease, vascular dementia is the most common dementia, accounting for about 20% to 30% of cases. Clinical criteria have not been widely accepted, although several have been published, including those in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and the National Institute of Neurological and Communicative Diseases and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Risk factors for vascular dementia include cerebrovascular disease (hypertension, diabetes, hyperlipidemia) and coexisting conditions related to atherosclerosis (coronary artery disease, peripheral artery disease).
The Hachinski Ischemic Score is a good bedside tool to help differentiate Alzheimer dementia from vascular dementia.5
Sudden onset and stepwise decline
Vascular dementia often presents as a sudden and stepwise progression of cognitive deficits that stabilize and that are caused by vascular insults (Table 1).6–10 Some patients have continuous decline after a vascular event, indicating that Alzheimer dementia may also be present. Dementia is then defined as a mixed type.
Behavioral problems such as physical aggression, hallucinations, paranoia, and mood fluctuations are common.11
Deficits depend on vascular areas affected
Cognitive deficits are heterogeneous and are often related to the location of the vascular insult. Involvement of subcortical areas may result in executive dysfunction, slowed processing speed, and behavioral changes.12
Executive dysfunction may be identified using the Trail Making Test (Part B) or the Executive Interview (EXIT25). Office-based tools such as the Folstein Mini-Mental State Examination, the Montreal Cognitive Assessment, or the St. Louis University Mental Status Examination may also uncover these deficits.
Focal neurologic deficits may be found on clinical examination.
Structural neuroimaging may identify small strokes in areas of the brain affecting cognitive function or occlusion of a larger vessel associated with more profound neurologic deficits. Neuroimaging findings may not correlate with any significant decline noted by the patient, suggesting “silent” strokes.
Treat symptoms and manage risk factors
Although the US Food and Drug Administration (FDA) has not approved any pharmacotherapy for vascular dementia, commonly prescribed cognitive enhancers have demonstrated some benefit.13
Behavioral problems such as aggression can be disturbing to the patient and the caregiver. Nonpharmacologic methods (eg, redirection, rescheduling care activities to avoid conflict, avoiding issues that lead to agitation) should be tried first to address these problems.
Drug therapy may be used off-label for neuropsychiatric symptoms such as hallucinations, delusions, and combativeness, but clinical trials of these agents for this purpose have shown mixed results,14 and their use is often associated with significant risk.15 Antipsychotic drugs are associated with a risk of death and pneumonia when prescribed for dementia. Many also carry a risk of QT prolongation, which is particularly concerning for patients with coronary artery disease or rhythm disturbances.
The key to reducing further decline is to optimize management of vascular risk factors to reduce stroke risk.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33
CORTICOBASAL DEGENERATION
Corticobasal degeneration is a progressive, asymmetric movement disorder often manifesting initially with cognitive or behavioral impairment. It is associated with abnormality of the cytoskeleton protein tau. Onset is usually after age 60.
Asymmetric movement disorder with cognitive dysfunction
This diagnosis is clinical. Diagnostic criteria proposed in 2003 include the following core features34:
- Insidious onset and progressive course
- No identifiable cause
- Cortical dysfunction with at least one of the following: apraxia, alien limb phenomenon (one limb moves involuntarily with complex movements, eg, grabbing the other hand), cortical sensory loss, visual hemineglect, nonfluent aphasia
- Extrapyramidal dysfunction: focal rigidity unresponsive to levodopa, asymmetric dystonia.
An international consortium has developed more specific clinical research criteria for probable and possible corticobasal degeneration.35 In a series of 147 patients, the following clinical features were found: parkinsonism (100%), higher cortical dysfunction (93%), dyspraxia (82%), gait disorder (80%), unilateral limb dystonia (71%), tremor (55%), and dementia (25%).36
Behavioral problems commonly include depression; apathy, irritability, and agitation are also reported.37
Cognitive testing may reveal deficits in frontal-parietal cognitive domains including attention and concentration, executive function, verbal fluency, and visuospatial skills.38 Learning disabilities may be improved with verbal cueing (in contrast to Alzheimer disease). Patients may also have impaired graphesthesia (the ability to recognize writing on the skin only by the sensation of touch).39,40
Motor examination may reveal marked asymmetry. Hand, limb, speech, and gait apraxias are common. Gait is typically slow, with short steps and shuffling, and a wide-based or freezing gait. Arm swing may be absent on one side.
Asymmetric cortical atrophy
Early on, MRI may be normal. As the disease progresses, asymmetric cortical atrophy may be seen, especially in the posterior frontal and parietal lobes.
Levodopa ineffective in corticobasal degeneration
Corticobasal degeneration responds poorly to levodopa. Botulinum toxin has been used to help with dystonia and limb pain.
MULTIPLE SYSTEM ATROPHY
Multiple system atrophy is another atypical parkinsonian disorder, most often diagnosed in men over age 60. It is characterized by sporadic parkinsonism, cerebellar signs (involving balance and coordination), pyramidal tract dysfunction, and autonomic insufficiency in varying combinations. Two major subtypes are recognized, depending on whether the predominating presenting features are cerebellar signs or parkinsonism. In contrast to dementia with Lewy bodies, psychiatric symptoms are not a major feature, except possibly depression.41
Diagnosis requires a sporadic progressive disorder that has features of autonomic failure and poor response of parkinsonism or cerebellar ataxia to levodopa.42
Multiple system atrophy is usually not associated with dementia in the early stages, but patients develop deficits in learning, recognition, memory, and verbal fluency as the disease progresses.43 Rapid-eye-movement sleep behavior disorder has been reported in more than half of patients.44
A neurologic examination provides clues
Parkinsonian features are usually symmetric, in contrast to idiopathic Parkinson disease. These signs may include akinesia with rigidity, postural instability, hypokinetic speech, and tremor.
Cerebellar signs include nystagmus and dysarthria (speech disturbance), and gait and limb ataxia.
Pyramidal features include extensor plantar responses and hyperreflexia.
Autonomic dysfunction includes orthostatic hypotension, bladder and rectal atony, loss of sweating, urinary or fecal incontinence, and erectile dysfunction.
Electromyography may demonstrate decreased anal sphincter tone.
MRI shows atrophy of putamen and pons
Brain MRI may show atrophy of the putamen (hypointensity of the putamen with a hyperintense rim). Pons atrophy may also be present, revealing a “hot cross bun” sign in axial images. These combined findings have specificity above 90% but limited sensitivity. These signs are useful to distinguish multiple system atrophy from Parkinson dementia, but their absence does not exclude the diagnosis of multiple system atrophy.45,46
Multiple system atrophy typically responds poorly to levodopa
Levodopa may improve movement and rigidity, but many respond poorly to treatment or lose response after a few years. Fludrocortisone (Florinef) or vasoconstrictors such as midodrine (Orvaten, Proamatine) may help with orthostatic hypotension.47,48
PARKINSON DISEASE DEMENTIA
Dementia eventually develops in most patients with Parkinson disease. Older age and the akinetic rigid form of the disease are associated with higher risk. Diagnosis of idiopathic Parkinson disease before the development of dementia is essential for the diagnosis.
The Movement Disorder Society Task Force has developed new diagnostic criteria.49 Deficits must be present in at least two of the four core cognitive domains (attention, memory, executive, and visuospatial functions) and must be severe enough to affect daily functioning.
Behavioral symptoms such as affective changes, hallucinations, and apathy are common.
MRI shows characteristic brain atrophy in Parkinson disease dementia
MRI shows reduced gray matter volume in the frontal lobe in patients with Parkinson disease without dementia compared with controls. In Parkinson disease dementia, reduced volume extends to temporal, occipital, and subcortical areas. No significant volumetric differences have been observed in Parkinson dementia compared with dementia with Lewy bodies.50 A greater decrease of glucose metabolism has been found in the inferior parietal and occipital lobes in Parkinson disease dementia than in Parkinson disease without dementia.51
Rivastigmine effective for dementia
A Cochrane review supports the use of acetylcholinesterase inhibitors in patients with Parkinson disease dementia, with a positive impact on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales.19 At this time, rivastigmine is the only FDA-approved cholinesterase inhibitor for treating Parkinson disease dementia. In clinical trials, memantine did not improve global clinical status or behavioral symptoms of dementia of Parkinson disease.51
FRONTOTEMPORAL DEMENTIA
Frontotemporal dementia frequently starts before age 65 and accounts for 20% to 50% of dementias in this age group.52 Recognition of the condition in older patients is also growing.53 Frontotemporal dementia encompasses a spectrum of dementias, including behavioral variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia.54
Gradual onset of uncharacteristic behaviors
Accepted diagnostic criteria include core features of gradual onset, early decline in social and interpersonal conduct, early impairment of self-regulation, emotional blunting, and loss of insight. Many patients are diagnosed with psychiatric conditions. Changes reported by family and caregivers typically deviate substantially from the person’s usual behavior, such as impulsive and inappropriate behaviors or complete withdrawal and apathy.
Language sometimes affected in frontotemporal dementia
Language impairment may be present in some variants. Behavioral and language changes often accompany other forms of dementia (Alzheimer disease, vascular dementia, primary progressive aphasia), making diagnosis more challenging. Office-based testing often does not reveal any deficits, although the Frontal Behavioral Inventory may help.55 A referral to a clinical neuropsychologist may help identify and quantify cognitive impairments.
MRI shows frontotemporal lobes affected
Structural neuroimaging may not reveal abnormalities initially, but with progression, atrophy may be seen in the frontal and temporal lobes. Functional neuroimaging (positron emission tomography, brain SPECT, functional MRI) show hypometabolism in the same areas.
Treat symptoms
There are no specific FDA-approved therapies for frontotemporal dementia. Acetylcholinesterase inhibitors can help progressive nonfluent aphasia in some cases. Selective serotonin reuptake inhibitors may alleviate depressive symptoms, and low doses of atypical antipsychotic medications may help with impulsivity, disinhibition, and aggressive or disruptive behaviors.56
PRIMARY PROGRESSIVE APHASIA
Language impairment predominates
Primary progressive aphasia is a rare form of dementia in which symptoms typically develop around age 60. Pathology is varied. In a study of 60 patients with initial clinical symptoms of primary progressive aphasia, postmortem histology of brain tissue revealed various findings, including those consistent with Alzheimer pathology and motor neuron diseasetype inclusions.57
Patients typically present with expressive language problems as the primary deficit for the first 2 years of the disease, with preservation in other cognitive areas such as memory, visuospatial skills, and executive function.58 Office-based testing may overstate the severity of the dementia, given the dependence of performance on intact language.
It is important to distinguish primary progressive aphasia from other dementias that also affect language. In the frontal variant of frontotemporal dementia, the primary language problem is anomia (inability to name objects) or diminished speech output, which may be accompanied by behavioral problems. Semantic dementia affects word recognition as well as comprehension. In Alzheimer disease, language may be affected along with memory and other areas of cognitive function.
Imaging shows focal degeneration in the left hemisphere
Structural neuroimaging does not initially reveal any deficits, but later it may reveal atrophy in the frontal, perisylvian complex, and temporal areas of the left hemisphere, reflecting the focal nature of the degeneration.59 Functional neuroimaging (positron emission tomography, SPECT) may reveal hypometabolism or diminished blood flow in these areas prior to changes in structural neuroimaging.60
Other communication methods may help
There are no FDA-approved therapies for primary progressive aphasia. Off-label use of some agents (eg, selective serotonin reuptake inhibitors and small doses of antipsychotic medications) has been found useful in small trials.56 Patients may benefit from learning other forms of communication, such as using sign language, laminated cards with printed words or pictures, or artificial voice synthesizers, to express their needs.
NORMAL-PRESSURE HYDROCEPHALUS
Classic triad: Gait, cognition, incontinence
With the onset of symptoms in the sixth or seventh decade, normal-pressure hydrocephalus affects less than 1% of people age 65 and older. It represents up to 5% of dementias, although estimates are influenced by the varied criteria for diagnosis.61 It is characterized by the classic triad of gait impairment, cognitive impairment, and urinary frequency or incontinence.62
Symptoms progress over a period of years, with gait impairment often predominating. As this triad is common in the geriatric population, identifying other explanations is important. Gait impairment caused by spinal stenosis, peripheral neuropathy, or parkinsonism should be explored. Cognitive impairment could be due to depression, Alzheimer disease, or other forms of dementia. Urinary symptoms may be related to detrusor instability or an enlarged prostate.
Gait impairment initially manifests as slowing of gait, but progresses to difficulty with gait initiation. Gait tends to be wide-based (stance more than 1 foot wide).
Cognitive impairment is typically subcortical, manifested as slowed processing speed and impaired executive function. Recall and working memory may be impaired.
Enlarged ventricles seen on imaging in normal-pressure hydrocephalus
Structural neuroimaging reveals enlarged ventricles (Evan’s ratio > 0.358). This can be difficult to distinguish from ventriculomegaly due to cerebral atrophy; assessing the callosal angle on MRI may distinguish the two.63,64 Diagnosis of normal-pressure hydrocephalus can be confirmed using a cerebrospinal fluid infusion test to assess resistance of fluid to resorption.65
Treat with cerebrospinal fluid drainage
Specific tests should be performed to determine candidacy for surgery. These include a high-volume lumbar puncture (40 to 50 mL) or a trial of external lumbar drainage (10 mL per hour for 48 to 72 hours).65 Definitive treatment is surgical placement of a shunt to allow cerebrospinal fluid to drain into the atria or peritoneal cavity.
Surgery may improve gait, but cognitive symptoms often remain,66 and clinical decline may occur after the shunt is placed. Once gait dysfunction is resolved, other explanations for cognitive impairment or residual gait impairment should be considered. An underlying reason for progression of normal-pressure hydrocephalus symptoms after surgical intervention should be identified.67
RAPIDLY PROGRESSIVE DEMENTIAS
Rapidly progressive dementias are among the most challenging of dementing illnesses. They are characterized by a subacute course and an accelerated rate of decline, developing in less than 2 years. Evaluation should typically be more comprehensive than for other types of dementia. The main goal is to diagnose potentially treatable conditions, such as Hashimoto encephalopathy or paraneoplastic limbic encephalitis, and to distinguish these conditions from diseases with a very poor prognosis, such as Creutzfeldt-Jakob disease.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease is a fatal prion-related neurodegenerative illness. Sporadic disease is most common, but variant, familial, and iatrogenic types have been reported. The most common initial symptoms in sporadic disease are cognitive (39%), cerebellar (21%), behavioral (20%), constitutional (20%), sensory (11%), motor (9%), and visual (7%).68
Chronic neurodegenerative diseases can be misdiagnosed as Creutzfeldt-Jakob disease because of an atypical time course and multi-system neurologic findings.
The US Centers for Disease Control and Prevention has adopted criteria for diagnosing probable Creutzfeldt-Jakob disease (Table 3). Routine investigations should also not suggest an alternative diagnosis.69
Autoimmune diseases
Autoimmune conditions may present as a rapidly progressive dementia, including Hashimoto encephalopathy and antibody-mediated limbic encephalitis, either associated with cancer (paraneoplastic) or without cancer (nonparaneoplastic).
Paraneoplastic limbic encephalitis is a group of inflammatory conditions involving antibodies produced within the cerebrospinal fluid and serum resulting in neurologic symptoms. These antibodies react against proteins expressed mostly by a tumor somewhere else in the body.70
Hashimoto encephalitis is a subacute to chronic encephalopathy that may present as dementia with abnormally high levels of thyroid antibodies. The symptoms can vary from confusion to psychosis. There are two main presentations: one involves a relapsing-remitting course with stroke-like episodes (27% of patients) and the second consists of insidious onset of seizures (66% of patients).
Diagnosis involves testing for elevated anti-thyroid peroxidase and thyroglobulin antibodies. MRI findings are nonspecific. Hashimoto encephalitis responds to treatment with corticosteroids, plasmapheresis, or immunosuppressive therapy.71
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–132.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed February 3, 2014.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Hou CE, Carlin D, Miller BL. Non-Alzheimer’s disease dementias: anatomic, clinical, and molecular correlates. Can J Psychiatry 2004; 49:164–171.
- Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637.
- Fereshtehnejad SM, Religa D, Westman E, Aarsland D, Lökk J, Eriksdotter M. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish Dementia Quality Registry (SveDem). Neuropsychiatr Dis Treat 2013; 9:927–935.
- Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18:394–396.
- Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progrssive supranuclear palsy. Neurology 1988; 38:1546–1552.
- Tousi B, Schuele SU, Subramanian T. A 46-year-old woman with rigidity and frequent falls. Cleve Clin J Med 2005; 72:57–63.
- Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord 2009; 15:59–61.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study0. JAMA 2002; 288:1475–1483.
- Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006; 81:223–230.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210.
- McKeith IG, Dickson DW, Lowe J, et al; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics 2011; 8:82–92.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Emre M, Tsolaki M, Bonucelli U, et al; on behalf of the 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977.
- Kurlan R, Cummings J, Raman R, Thal L; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68:1356–1363.
- Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611.
- Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003; 60:917–922.
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105:117–124.
- Fahn S, Jankovic J, Hallett M, editors. Principles and Practice of Movement Disorders. 2nd ed. New York, NY: Elsevier/Saunders; 2011.
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996; 60:615–620.
- Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61:40–45.
- Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 2001; 56:957–963.
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 2010; 25:357–362.
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary parkinsonisms. Mov Disord 2011; 26:1083–1095.
- Gallucci M, Limbucci N, Catalucci A, Caulo M. Neurodegenerative diseases. Radiol Clin North Am 2008; 46:799–817.
- Stamelou M, de Silva R, Arias-Carrión O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133:1578–1590.
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat 2012; 8:85–93.
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15–S19.
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503.
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998; 55:957–961.
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721.
- Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology 1995; 45:1477–1483.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer’s disease.” J Neurol Neurosurg Psychiatry 2003; 74:389.
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003; 61:1134–1135.
- Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000; 54:507–511.
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676.
- Berent S, Giordani B, Gilman S, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn 2002; 50:194–206.
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 72:798–800.
- Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998; 65:65–71.
- Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 2012; 27:1754–1762.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Mathias CJ, Kimber JR. Postural hypotension: causes, clinical features, investigation, and management. Annu Rev Med 1999; 50:317–336.
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707.
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800.
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson’s disease. J Nucl Med 1996; 37:1115–1122.
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621.
- Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal dementia in elderly individuals. Arch Neurol 2012; 69:1052–1960.
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468.
- Mendez MF, Lauterbach EC, Sampson SM; ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci 2008; 20:130–149.
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med 2003; 349:1535–1542.
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39:166–173.
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997; 39:556–559.
- Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:1017–1022.
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965; 2:307–327.
- Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol 2000; 247:5–14.
- Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57(suppl 3):S17–S28.
- Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery 2008; 62(suppl 2):643–659.
- Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013; 10:22.
- Rabinovici GD, Wang PN, Levin J, et al. First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287.
- Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol 2003; 60:164–171.
- Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29:125–132.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed February 3, 2014.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Hou CE, Carlin D, Miller BL. Non-Alzheimer’s disease dementias: anatomic, clinical, and molecular correlates. Can J Psychiatry 2004; 49:164–171.
- Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32:632–637.
- Fereshtehnejad SM, Religa D, Westman E, Aarsland D, Lökk J, Eriksdotter M. Demography, diagnostics, and medication in dementia with Lewy bodies and Parkinson’s disease with dementia: data from the Swedish Dementia Quality Registry (SveDem). Neuropsychiatr Dis Treat 2013; 9:927–935.
- Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord 2012; 18:394–396.
- Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progrssive supranuclear palsy. Neurology 1988; 38:1546–1552.
- Tousi B, Schuele SU, Subramanian T. A 46-year-old woman with rigidity and frequent falls. Cleve Clin J Med 2005; 72:57–63.
- Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord 2009; 15:59–61.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study0. JAMA 2002; 288:1475–1483.
- Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc 2006; 81:223–230.
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359:1283–1290.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:191–210.
- McKeith IG, Dickson DW, Lowe J, et al; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Tartaglia MC, Rosen HJ, Miller BL. Neuroimaging in dementia. Neurotherapeutics 2011; 8:82–92.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Emre M, Tsolaki M, Bonucelli U, et al; on behalf of the 11018 Study Investigators. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977.
- Kurlan R, Cummings J, Raman R, Thal L; Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68:1356–1363.
- Ballard C, Aarsland D, Francis P, Corbett A. Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611.
- Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003; 60:917–922.
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105:117–124.
- Fahn S, Jankovic J, Hallett M, editors. Principles and Practice of Movement Disorders. 2nd ed. New York, NY: Elsevier/Saunders; 2011.
- Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996; 60:615–620.
- Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61:40–45.
- Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 2001; 56:957–963.
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 2010; 25:357–362.
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary parkinsonisms. Mov Disord 2011; 26:1083–1095.
- Gallucci M, Limbucci N, Catalucci A, Caulo M. Neurodegenerative diseases. Radiol Clin North Am 2008; 46:799–817.
- Stamelou M, de Silva R, Arias-Carrión O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain 2010; 133:1578–1590.
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat 2012; 8:85–93.
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003; 54(suppl 5):S15–S19.
- Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503.
- Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol 1998; 55:957–961.
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998; 65:717–721.
- Pillon B, Blin J, Vidailhet M, et al. The neuropsychological pattern of corticobasal degeneration: comparison with progressive supranuclear palsy and Alzheimer’s disease. Neurology 1995; 45:1477–1483.
- Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer’s disease.” J Neurol Neurosurg Psychiatry 2003; 74:389.
- Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003; 61:1134–1135.
- Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000; 54:507–511.
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676.
- Berent S, Giordani B, Gilman S, et al. Patterns of neuropsychological performance in multiple system atrophy compared to sporadic and hereditary olivopontocerebellar atrophy. Brain Cogn 2002; 50:194–206.
- Ghorayeb I, Yekhlef F, Chrysostome V, Balestre E, Bioulac B, Tison F. Sleep disorders and their determinants in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 72:798–800.
- Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998; 65:65–71.
- Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 2012; 27:1754–1762.
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. JAMA 1997; 277:1046–1051.
- Mathias CJ, Kimber JR. Postural hypotension: causes, clinical features, investigation, and management. Annu Rev Med 1999; 50:317–336.
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22:1689–1707.
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800.
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson’s disease. J Nucl Med 1996; 37:1115–1122.
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621.
- Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal dementia in elderly individuals. Arch Neurol 2012; 69:1052–1960.
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468.
- Mendez MF, Lauterbach EC, Sampson SM; ANPA Committee on Research. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci 2008; 20:130–149.
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med 2003; 349:1535–1542.
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39:166–173.
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology 1997; 39:556–559.
- Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly. Prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:1017–1022.
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965; 2:307–327.
- Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol 2000; 247:5–14.
- Ishii K, Kanda T, Harada A, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 2008; 18:2678–2683.
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005; 57(suppl 3):S17–S28.
- Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery 2008; 62(suppl 2):643–659.
- Malm J, Graff-Radford NR, Ishikawa M, et al. Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013; 10:22.
- Rabinovici GD, Wang PN, Levin J, et al. First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287.
- Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7:327–340.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol 2003; 60:164–171.
KEY POINTS
- Vascular dementia presents as a sudden, stepwise progression of cognitive deficits.
- Lewy body dementia often involves prominent visual hallucinations.
- Progressive supranuclear palsy starts with gait and balance problems caused by downward-gaze palsy.
- Many neurodegenerative conditions involve parkinsonism, but unlike Parkinson disease, they do not tend to respond well to levodopa, and dementia develops early.
- Corticobasal degeneration involves markedly asymmetric parkinsonism.
- Frontotemporal dementia involves dramatic behavior changes, including inappropriate impulsivity and complete apathy.
- Patients with rapidly progressive dementia should be evaluated for a treatable condition such as antibody-mediated encephalitis.
Recognizing, managing medical consequences of eating disorders in primary care
Eating disorders are debilitating biopsychosocial illnesses associated with serious medical illness and a high risk of death.1
Primary care physicians are often the first to see young women who have these problems, diagnose them, and start their evaluation and treatment.2–4 Many patients require acute medical interventions as well as long-term care for chronic medical issues. Therefore, primary care physicians play essential front-line and long-term roles in the multidisciplinary treatment team.
DEFINITIONS OF EATING DISORDERS HAVE CHANGED
Several problems existed in the category of eating disorders in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) and in the DSM-4 Text Revision (DSM-4-TR). These problems have been addressed in the fifth edition (DSM-5), released in 2013.5
One problem in the earlier editions was that many patients referred for treatment of eating disorders—more than 50% in one study6—did not meet the criteria for anorexia nervosa or bulimia nervosa and thus had to be categorized as having “eating disorder not otherwise specified.” Further, the earlier editions did not recognize that young children and adolescent males can be affected.7
Eating disorders are now recognized as an equal-opportunity disease, with all ethnic and socioeconomic groups affected. Children can run into medical trouble with even a small amount of weight loss or falling off the growth curve. Moreover, children and adolescents do not “experience” their bodies in the same way adults do; they may lack the vocabulary for eating-disorder thoughts.
For these reasons, the definitions of eating disorders have changed in the DSM-5.5
Anorexia nervosa. Older editions of the DSM listed amenorrhea as a criterion. This has been eliminated in DSM-5, since amenorrhea does not necessarily predict medical risk or treatment outcome; also, it is not applicable to males or premenorrheal girls and postmenopausal women.8 In addition, the requirement of low weight is now defined in the context of “age, sex, developmental trajectory, and physical health,” rather than the old threshold of 85% of expected weight.9
What remains unchanged is that anorexia nervosa is still characterized by self-starvation in order to maintain an abnormally low body weight, along with an intense fear of being fat and a disturbed self-image.
Bulimia nervosa. In both the old and the new editions of the DSM, bulimia nervosa is characterized by episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain, such as vomiting, laxative abuse, diuretic abuse, and overexercise. In DSM-5, bulimia nervosa no longer has subtypes and requires only one binge per week with compensatory behavior, for at least 3 months. This change was based on the finding that there is no clear difference in psychopathology or treatment outcome between patients with one and two binge-purge episodes a week.10
“Eating disorder not otherwise specified” was a wastebasket category, lumping all those who did not meet the criteria for anorexia nervosa or bulimia nervosa or who did not neatly fit into a specific category.10 In DSM-5, subcategories were designed to help distinguish different treatment needs and outcomes between various subtypes.
Binge-eating disorder, one of the new subcategories, is characterized by binge eating without inappropriate compensatory behaviors.9 Patients with binge-eating disorder are often obese, have greater functional impairment, and are more likely to develop components of metabolic syndrome than obese patients without eating disorders.11
Avoidant/restrictive food intake disorder is another new DSM-5 diagnosis, characterized by failure to meet nutritional needs for reasons other than weight control. Reasons include disinterest in eating, dislike of sensory characteristics of food, or avoidance of consequences of eating. This disorder replaces the category “feeding disorder of infancy or early childhood,” since the condition can also occur in adolescents and adults.12
Other new diagnoses are:
- Atypical anorexia nervosa (if the patient is not underweight)
- Purging disorder
- Subthreshold bulimia nervosa (if the patient has < 1 episode per week or has had them for < 3 months)
- Subthreshold binge eating disorder (< 1 time a week or < 3 months)
- Night eating syndrome
- Pica and rumination disorder.
Regardless of the diagnostic label, the medical evaluation and treatment of anyone with an eating disorder should be tailored to the specific behaviors of the eating disorder. Medical complications can be subdivided into those from starvation, from purging, and from refeeding.
MEDICAL COMPLICATIONS OF STARVATION
Cardiovascular effects of starvation
Malnutrition and starvation have multiple adverse effects on the heart.
Electrophysiologic effects. Sinus bradycardia (< 60 bpm) and hypotension are common cardiac manifestations of starvation.13 Bradycardia has been attributed to an adaptive increase in parasympathetic vagal tone.14 QTc prolongation is also seen in patients with malnutrition.15
Together, these electrocardiographic abnormalities predispose the patient to ventricular arrhythmia and sudden cardiac death.16 The risk of ventricular arrhythmia is particularly relevant when treating psychiatric symptoms, since antipsychotics and tricyclic antidepressants are among several drug classes that can cause further QTc prolongation (Table 1).17,18
In patients with QTc prolongation, bradycardia, or both, the standard of care involves acute hospitalization for refeeding using continuous telemetric monitoring until normal rhythm is restored and the heart rate is above 40 at night and 50 by day.4,19
Structural changes. Starvation also causes structural changes in the heart. Loss of lean body mass can reduce cardiac muscle mass, compromise cardiac output, and lead to mitral valve prolapse.20 These changes are fully reversible with restored nutrition and regaining of heart mass.21,22
Effects of starvation on the brain
Starvation can affect brain structure and cognitive function. Undernourished patients have reduced volumes of white and gray matter, a change that can occur within months. Cortical volumes may increase with weight gain, but a reduction in gray matter volume may not be completely reversible.23
Furthermore, starvation impairs cognitive functions that are needed to stop eating-disorder behaviors; namely, decision-making, emotional control, regulation of appetite, and reward path-ways. Therefore, undernourished patients may not have sufficient insight into the disease to be able to make the best choices for recovery. This finding lends support for using the Maudsley method in adolescents, in which parents take control of their child’s eating until the child can maintain a healthy weight.24
Gastrointestinal consequences of starvation
Patients with malnutrition have prolonged gastric emptying and colonic transit time with solid foods.25 They often complain of early satiety, abdominal pain, bloating, and constipation, all symptoms that complicate the refeeding process. A prokinetic such as metoclopramide (Reglan), given 1 hour before meals and at bedtime, may provide some relief from gastrointestinal symptoms.26
Patients may also experience transient lactose or fructose intolerance after prolonged starvation. Taking a lactase supplement (eg, Lactaid 1–10 tabs) before consuming dairy products and dextrose (contained in candies such as Smarties) before eating fruit or fructose-containing foods can sometimes partially relieve symptoms. In general, gastrointestinal function returns over time as nutritional status improves.
Patients with severe or prolonged starvation can develop steatosis accompanied by elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In reports of starvation-induced steatosis, liver enzyme levels rapidly normalize with nutritional rehabilitation.27
Endocrine consequences of starvation
Amenorrhea. Dysregulation of the hypothalamic-pituitary-gonadal axis is a major endocrine complication of nutritional in-sufficiency. Weight loss disrupts the normal pulsatile secretion of gonadotropin-releasing hormone, reduces secretion of luteinizing hormone and follicle-stimulating hormone, and decreases estrogen levels.28 Leptin deficiency likely plays a role in suppressing gonadotropin secretion with subsequent development of amenorrhea. With weight gain, levels of leptin and gonadotropins normalize and menstruation eventually returns.29,30
Hypothyroidism. Starvation can also lead to dysregulation of the hypothalamic-pituitary-thyroid axis. Typically, the concentration of triiodothyronine (T3) is reduced, the ratio of thyroxine (T4) to T3 is elevated, and thyroid-stimulating hormone (TSH) is close to or within the normal range, creating a euthyroid sick syndrome. In eating disorders, this thyroid disturbance is a result of starvation and resolves with weight restoration. Therefore, thyroid hormone replacement therapy is not medically indicated.28
Osteoporosis. Amenorrhea resulting from low estrogen levels in undernourished patients can raise the risk of osteoporosis and fractures, particularly in patients with a low body mass index. Osteopenia results from a negative balance between bone deposition and resorption.
Lack of bone deposition can be especially problematic when disordered eating occurs during peak bone mass development, ie, ages 11 to 14 for girls, and ages 15 to 17 for boys.31,32 Even a 5% to 10% decrease in bone deposition can result in significant risk of osteopenia.33 However, after age 30, bone resorption is a greater contributor.34
Does hormone therapy correct bone loss? Given the association between estrogen deficiency and bone loss, estrogen supplementation was expected to be an effective treatment for bone loss in patients with eating disorders.35 Also, the restoration of menses through hormone replacement may give underweight patients a false sense of achieving a “healthy” weight.36
Golden et al37 prospectively studied 50 adolescents and found no significant difference in bone mineral density at 1 year of follow-up between patients treated with estrogen and those who received only standard nutritional therapy. However, increased bone mineral density was achieved in adolescents with anorexia nervosa treated with transdermally administered estrogen dosed to mimic physiologic pubertal levels.38
Klibanski et al39 found that hormone therapy resulted in a 4% gain in bone density in an extremely low-weight subset of women with anorexia nervosa (< 70% of ideal body weight), whereas similar patients in the control group lost 20%. However, in all groups, only weight gain correlated with bone gain in women who were within 70% of their ideal body weight.
Divasta et al40 evaluated 60 girls and women ages 13 to 27 with anorexia nervosa, randomized to receive either placebo or dehydroepiandrosterone combined with an estrogen-progestin oral contraceptive, and followed for 18 months. As in the study by Klibanski et al,39 bone loss was prevented in the treatment group, but significant bone gain occurred only in the context of weight gain.
The bottom line is that only weight gain has resulted in significant increases in bone density in patients with anorexia nervosa, and hormone therapy without weight gain has not been shown to increase bone density effectively in this population. Although calcium and vitamin D in oral therapeutic doses through foods or through supplementation are required for bone gain, the combination is not enough to augment bone density in the absence of weight gain.37 Although not curative, weight gain is currently the best option for treating bone loss, and no single pharmacologic treatment is effective.
COMPLICATIONS OF PURGING
Oral complications of purging
Patients who purge by vomiting are at risk of complications from exposure of the esophagus, pharynx, and mouth to acidic gastric contents.
Dental problems. Over time, contact with gastric acid wears down enamel on the lingual and occlusal surfaces of teeth, resulting in dental caries and periodontal disease. Until they can give up purging, patients should be instructed to rinse with mouthwash or water immediately after vomiting to reduce the acidity in the mouth.41,42 We recommend that patients not brush their teeth after vomiting, because brushing can deliver acid to otherwise unreachable surfaces and thus worsen tooth erosion. For patients who are determined to brush after vomiting, a bicarbonate toothpaste might mitigate harm.42
Sialadenosis (hypertrophy of the salivary glands) is another consequence of repeated vomiting, with elevated salivary amylase. Both the size of the glands and the salivary amylase level generally normalize on their own after vomiting is stopped, but parotitis can take up to a year to resolve. Similar to smoker’s cough, parotitis may acutely worsen when the patient abruptly stops vomiting and may worsen before it improves.
To reduce discomfort, patients can use hot compresses or sugarless hard candies.44 However, the latter should not be substituted as a chronic habit in a patient with disordered eating. Patients need to be reassured that the swelling is not permanent, since they often interpret it as having fat cheeks (the “chipmunk sign”).
Hypokalemia, metabolic alkalosis, renal dysfunction
Chronic vomiting can cause electrolyte and acid-base imbalances, the most worrisome of which is hypokalemia. With repeated vomiting, loss of potassium and gastric acid causes metabolic alkalosis with hypokalemia, hypochloremia, and hypomagnesemia. Loss of water and the resultant volume contraction activates the renin-angiotensin-aldosterone system, and elevated aldosterone further decreases serum potassium.
In patients with eating disorders, who often have other factors contributing to electrolyte imbalance, vomiting-induced hypokalemia heightens the risk of cardiac arrhythmias.43
Hypokalemia can also cause rhabdomyolysis and kidney damage.41,43 Prolonged hypokalemia and reduced kidney perfusion in the setting of volume depletion causes acute kidney injury and impaired concentrating ability of the renal tubules. Hypovolemia can cause prerenal azotemia and increases the risk for nephrolithiasis and nephrocalcinosis.44,45
When a patient stops vomiting, elevated aldosterone from prior hypovolemia results in water retention and can manifest in significant edema associated with hypochloremic alkalosis. This condition, known as pseudo-Bartter syndrome, usually resolves without treatment. In the meantime, salt restriction and leg elevation can help reduce edema.26
Laxative abuse: A mode of purging
Many patients with eating disorders abuse laxatives to lose weight or to prevent weight gain. Believing that laxatives will prevent calorie absorption, patients commonly take them to compensate for caloric intake (eg, during a binge episode). The immediate weight loss, albeit artificial, is highly reinforcing for an eating-disorder patient. In some cases, patients with eating disorders also abuse laxatives to self-treat the constipation that results from chronic starvation.46
Over time, tolerance to laxatives develops, and patients use increasingly larger doses. This can lead to activation of the renin-angiotensin-aldosterone system.47 Patients interpret the resultant edema as true weight gain and again take laxatives to get rid of it. If laxatives are stopped abruptly, the patient may need inpatient and outpatient support for the resultant fluid shifts.
Gastrointestinal complications of laxative abuse include reflex hypofunction of the bowel, malabsorption, steatorrhea, and gastrointestinal bleeding.47 Reflex hypofunction during laxative withdrawal is a consequence of the bowel becoming tolerant of laxatives.48 Cathartic colon syndrome is a rare complication characterized by loss of the normal haustral markings and slowed or absent peristalsis in segments of the colon.49
Systemically, the major risk of laxative abuse relates to electrolyte and acid-base imbalance. Loss of potassium and water in the stool can cause hypokalemia and metabolic alkalosis.48 The disturbances caused by laxative abuse are similar to those caused by vomiting and diuretic use and have the same treatment.
The most important component of treating laxative abuse is giving patients realistic expectations to help them tolerate temporary discomfort and to help manage the edema and fluid shifts that can happen acutely with shifting of fluid into the intracellular space. In extreme cases, this may need to be managed in the hospital. To help relieve the initial anxiety, doctors should emphasize that any bloating the patient experiences is not true weight gain and will go away within a few days to weeks. In addition, explaining that laxatives reduce nutrient absorption only minimally may lessen the temptation to resume taking them.48
Diuretic abuse: Another form of purging
Diuretic abuse is yet another mode of purging, with its own set of medical complications. Like laxatives, diuretics are not effective weight-loss agents, and the weight reduction they cause is only temporary.
As with vomiting, there is a compensatory activation of the renin-angiotensin-aldosterone system, and therefore subsequent fluid intake will lead to water retention, which encourages further diuretic use.41 Diuretics can also contribute to hypokalemia, hypomagnesemia, hypochloremia, and metabolic alkalosis.
Ipecac abuse can lead to heart failure
Ipecac syrup has long been used to induce vomiting, but this practice has become much less common since ipecac has become harder to obtain in the United States.50 The emetine base contained in ipecac binds irreversibly to cardiac and skeletal muscle. With continued use, irreversible cardiomyopathy develops and can lead to heart failure. Treatment should include supportive care and immediate cessation of ipecac use.
Diabetic patients may skip insulin to lose weight
Patients with diabetes, especially those with type 1 that begins in childhood, are at greater risk of eating disorders over time.51 They may withhold insulin to lose weight, a practice referred to in the nonmedical literature as “diabulimia,” and they seem particularly more likely to develop bulimia nervosa than those without diabetes.52
The medical prognosis is poor for patients with diabetes who develop eating disorders and do not receive intensive treatment.51 In addition, if a diabetic patient on an insulin pump becomes depressed in addition to having an eating disorder, careful monitoring for suicidal thoughts and a rapid follow-up with mental health services are in order.
REFEEDING SYNDROME
When refeeding is started, a high glucose load stimulates insulin secretion, resulting in cellular uptake of phosphorus along with potassium, magnesium, and glucose. In addition, total body phosphorus is depleted by the increased demand for adenosine triphosphate and 2,3-diphosphoglycerate for cellular metabolism.
When liver enzyme levels increase, the astute clinician will closely monitor the patient for evidence of refeeding syndrome. In a child, adolescent, or young adult, the standard of care is inpatient monitoring for acute stabilization.4,19
Hypophosphatemia is the hallmark of refeeding syndrome, although hypomagnesemia, hypokalemia, and hypoglycemia can also occur.53 In addition, sodium and water retention can lead to fluid overload, with shifting of fluid into the intracellular space, resulting in dependent edema.
Cardiovascular complications are the most worrisome manifestations of refeeding syndrome. Electrolyte shifts and increased fluid volume can cause arrhythmias and heart failure. Furthermore, severely undernourished patients may have reduced myocardial mass as well as electrocardiographic abnormalities associated with starvation, which further increase their vulnerability to electrolyte shifts and fluid retention during refeeding.15
Other manifestations of refeeding syndrome include delirium, seizures, rhabdomyolysis, and respiratory failure. In the most extreme cases, refeeding syndrome causes sudden death.53
Fortunately, refeeding syndrome is easily preventable and treatable when recognized early. Electrolytes and cardiovascular and renal function must be carefully monitored, especially during the first week of nutritional restoration.53 In patients with extremely low body mass (< 70% of ideal body weight) or with precipitous weight loss, close monitoring of the complete metabolic panel including electrolytes, AST, ALT, calcium, magnesium, and phosphorus may be required to detect changes that can affect cardiac status. Specific suggestions for refeeding are discussed below and in Table 2.45
ACUTE CARE OF PATIENTS WITH EATING DISORDERS
Refeeding in the inpatient setting
The decision to hospitalize an eating-disorder patient is based on the current or potential risk of serious medical complications and the likelihood of success at home. Medical criteria for hospital admission are outlined in Table 3.4,54
In refeeding undernourished patients, the challenge is to maximize weight gain while preventing refeeding syndrome. Undernourished patients are generally hypometabolic at baseline but become hypermetabolic once refeeding begins.
How many calories should refeeding start with? The traditional principle of “start low and go slow” has been recently challenged.55 Starting at 1,200 kcal/day or less in the typical patient can result in failure to gain weight or even in weight loss in the first week of refeeding.56 The goal is to achieve a weight gain of 0.2 kg/day while the patient is in the hospital. Thus, we start higher, and to date we have seen no cases of life-threatening refeeding syndrome. In all patients who need hospitalization or who are beginning the refeeding process as outpatients, caloric intake should be started at 1,500 to 2,000 kcal/day.45,57 However, for exceptionally low-weight patients, intake may be started lower.
In Australia, patients are started at 1,900 kcal/day.56 All patients in one program there receive nasogastric feeding initially in an intensive care unit and then are moved to a regular nursing floor where they graduate to full oral feeding as they improve cardiovascularly and behaviorally. In the United States, some programs use nasogastric feeding at night for caloric restoration; our program and others use nasogastric feeding as a behavioral modification strategy for patients who refuse food or supplements by mouth.
Phosphorus supplementation. Many centers give phosphorus supplements preventively. In our center, we give potassium phosphate (Neutra-Phos) 500 mg orally twice daily for 5 days, and we have seen no life-threatening cases of refeeding syndrome with that regimen. Other centers give phosphorus supplements in a dose of 250 mg orally twice a day for 5 days, while still others only supplement phosphorus reactively once a deficit has been identified. The latter method requires daily blood draws for monitoring and is reactive rather than proactive. Further studies can help clarify the optimal dosing and timing of phosphorus supplementation.
Managing fluid balance. Fluid-loading these patients may tip them over the edge into refeeding syndrome. Except in cases of shock, patients with eating disorders should not be given intravenous fluids, as it is safer to rehydrate and feed them orally. Electrolyte imbalances can be corrected orally with no need for intravenous supplementation. To avoid fluid overload, fluids can be started at 1,500 mL to 2,000 mL per day, with strict monitoring of intake and output. Fluids are liberalized if ALT and AST levels remain normal and to gradually correct orthostatic hypotension; caloric fluids are ideal to help address energy needs and improve bradycardia.
Laboratory monitoring. On admission, a urinalysis, complete blood cell count, complete metabolic panel, TSH, erythrocyte sedimentation rate, serum magnesium, and phosphorus should be obtained.26 In addition, continuous electrocardiographic recording should begin on admission.45 Inpatient use of a telemetry bed helps identify extreme tachycardia with arrhythmia, as well as profound bradycardia.45,56
Some protocols call for daily laboratory monitoring, although that degree of testing is less cost-effective. If initial results are normal, clinical judgment can be used on when to repeat laboratory evaluation. For instance, patients with edema require repeat complete metabolic panels to assess for elevated ALT and AST, electrolyte imbalances, and other abnormalities.
Signs of refeeding syndrome include tachycardia, hepatosplenomegaly, peripheral edema, altered mental status, and electrolyte disturbances, specifically, acute or severe hypophosphatemia or hypokalemia.26,45 If refeeding syndrome is suspected, the rate of caloric intake should be reduced or not advanced, fluid intake should be urgently reassessed for volume overload, and supportive care with close monitoring should be provided.
KNOWLEDGE SAVES LIVES
Eating disorders can lead to potentially life-threatening medical complications that require attentive care by the primary care clinician and subspecialist. Without thoughtful consideration, it is easy for even a caring medical team to unintentionally enable patients with these illnesses or to cause active harm in the case of underrecognized pathology.58
Acute medical stabilization on an inpatient unit trained to recognize pathology and treat sequelae can be lifesaving. Arming patients and families with medical knowledge, as provided in the Academy for Eating Disorders’ brochure, “Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders”59 can help save patients’ lives.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731.
- Walsh JM, Wheat ME, Freund K. Detection, evaluation, and treatment of eating disorders the role of the primary care physician. J Gen Intern Med 2000; 15:577–590.
- American Academy of Pediatrics; Committee on Adolescence. Identifying and treating eating disorders. Pediatrics 2003; 111:204–211.
- Rosen DS; American Academy of Pediatrics Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics 2010; 126:1240–1253.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Publishing, Incorporated; 2013.
- Eddy KT, Celio Doyle A, Hoste RR, Herzog DB, le Grange D. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47:156–164.
- Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health 2003; 33:427–435.
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 2009; 42:581–589.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. http://dsm.psychiatryonline.org/content.aspx?bookid=556§ionid=41101776#103439089. Accessed January 31, 2014.
- Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord 2007; 40:S123–S129.
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009; 42:687–705.
- Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health 2013: 53:303–305.
- Winston AP, Stafford PJ. Cardiovascular effects of anorexia nervosa. Eur Eat Disord Rev 2000; 8:117–125.
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health 2003; 32:416–421.
- McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord 2006; 14:73–80.
- Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation 1990; 82:1561–1573.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- The University of Arizona Center for Education and Research on Therapeutics. QT Drug Lists. http://crediblemeds.org/everyone/compos-ite-list-all-qtdrugs/?rf=US. Accessed January 31, 2014.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 2003; 77:308–312.
- Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health 2003; 32:73–77.
- Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 2003; 42:808–813.
- Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord 2011; 44:406–411.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011; 199:5–7.
- Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003; 2:1–9.
- Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med 2005; 353:1481–1488.
- De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 2006; 22:572–575.
- Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 2008; 4:407–414.
- Holtkamp K, Mika C, Grzella I, et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110:427–435.
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151:16–21.
- Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002; 87:4177–4185.
- Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Invest 2011; 34:324–332.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 1989; 68:548–554.
- Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol 1997; 176:1017–1025.
- Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 2010; 43:218–225.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26:2430–2438.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012; 61:1010–1020.
- Mehler PS. Medical complications of bulimia nervosa and their treatments. Int J Eat Disord 2011; 44:95–104.
- Milosevic A. Eating disorders and the dentist. Br Dent J 1999; 186:109–113.
- Greenfeld D, Mickley D, Quinlan DM, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152:60–63.
- Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis 2012; 60:299–307.
- Auron M, Rome E. Anorexia nervosa and bulimia nervosa: what the hospitalist needs to know about CPT 269.9, or nutritional insufficiency. ACP Hospitalist 2011 Sept:28–45.
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL. The eating disorders medicine cabinet revisited: a clinician’s guide to ipecac and laxatives. Int J Eat Disord 2007; 40:360–368.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70:1487–1503.
- Mitchell JE, Boutacoff LI. Laxative abuse complicating bulimia: medical and treatment implications. Int J Eat Disord 1986; 5:325–334.
- Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26:283–286.
- Drugs.com. Ipecac syrup. www.drugs.com/monograph/ipecac-syrup.html. Accessed January 31, 2014.
- Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005; 28:84–88.
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005; 28:417–419.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17:632–637.
- Fisher M, Golden NH, Katzman DK, et al. Eating disorders in adolescents: a background paper. J Adolesc Health 1995; 16:420–437.
- Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr 2011; 23:390–394.
- Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health 2012; 50:24–29.
- Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health 2010; 46:577–582.
- Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev 2011; 19:296–302.
- Academy for Eating Disorders (AED). Critical points for early recognition and medical risk management in the care of individuals with eating disorders. http://www.aedweb.org/AM/Template.cfm?Section=Medical_Care_Standards&Template=/CM/ContentDisplay.cfm&ContentID=2413. Accessed January 31, 2014.
Eating disorders are debilitating biopsychosocial illnesses associated with serious medical illness and a high risk of death.1
Primary care physicians are often the first to see young women who have these problems, diagnose them, and start their evaluation and treatment.2–4 Many patients require acute medical interventions as well as long-term care for chronic medical issues. Therefore, primary care physicians play essential front-line and long-term roles in the multidisciplinary treatment team.
DEFINITIONS OF EATING DISORDERS HAVE CHANGED
Several problems existed in the category of eating disorders in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) and in the DSM-4 Text Revision (DSM-4-TR). These problems have been addressed in the fifth edition (DSM-5), released in 2013.5
One problem in the earlier editions was that many patients referred for treatment of eating disorders—more than 50% in one study6—did not meet the criteria for anorexia nervosa or bulimia nervosa and thus had to be categorized as having “eating disorder not otherwise specified.” Further, the earlier editions did not recognize that young children and adolescent males can be affected.7
Eating disorders are now recognized as an equal-opportunity disease, with all ethnic and socioeconomic groups affected. Children can run into medical trouble with even a small amount of weight loss or falling off the growth curve. Moreover, children and adolescents do not “experience” their bodies in the same way adults do; they may lack the vocabulary for eating-disorder thoughts.
For these reasons, the definitions of eating disorders have changed in the DSM-5.5
Anorexia nervosa. Older editions of the DSM listed amenorrhea as a criterion. This has been eliminated in DSM-5, since amenorrhea does not necessarily predict medical risk or treatment outcome; also, it is not applicable to males or premenorrheal girls and postmenopausal women.8 In addition, the requirement of low weight is now defined in the context of “age, sex, developmental trajectory, and physical health,” rather than the old threshold of 85% of expected weight.9
What remains unchanged is that anorexia nervosa is still characterized by self-starvation in order to maintain an abnormally low body weight, along with an intense fear of being fat and a disturbed self-image.
Bulimia nervosa. In both the old and the new editions of the DSM, bulimia nervosa is characterized by episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain, such as vomiting, laxative abuse, diuretic abuse, and overexercise. In DSM-5, bulimia nervosa no longer has subtypes and requires only one binge per week with compensatory behavior, for at least 3 months. This change was based on the finding that there is no clear difference in psychopathology or treatment outcome between patients with one and two binge-purge episodes a week.10
“Eating disorder not otherwise specified” was a wastebasket category, lumping all those who did not meet the criteria for anorexia nervosa or bulimia nervosa or who did not neatly fit into a specific category.10 In DSM-5, subcategories were designed to help distinguish different treatment needs and outcomes between various subtypes.
Binge-eating disorder, one of the new subcategories, is characterized by binge eating without inappropriate compensatory behaviors.9 Patients with binge-eating disorder are often obese, have greater functional impairment, and are more likely to develop components of metabolic syndrome than obese patients without eating disorders.11
Avoidant/restrictive food intake disorder is another new DSM-5 diagnosis, characterized by failure to meet nutritional needs for reasons other than weight control. Reasons include disinterest in eating, dislike of sensory characteristics of food, or avoidance of consequences of eating. This disorder replaces the category “feeding disorder of infancy or early childhood,” since the condition can also occur in adolescents and adults.12
Other new diagnoses are:
- Atypical anorexia nervosa (if the patient is not underweight)
- Purging disorder
- Subthreshold bulimia nervosa (if the patient has < 1 episode per week or has had them for < 3 months)
- Subthreshold binge eating disorder (< 1 time a week or < 3 months)
- Night eating syndrome
- Pica and rumination disorder.
Regardless of the diagnostic label, the medical evaluation and treatment of anyone with an eating disorder should be tailored to the specific behaviors of the eating disorder. Medical complications can be subdivided into those from starvation, from purging, and from refeeding.
MEDICAL COMPLICATIONS OF STARVATION
Cardiovascular effects of starvation
Malnutrition and starvation have multiple adverse effects on the heart.
Electrophysiologic effects. Sinus bradycardia (< 60 bpm) and hypotension are common cardiac manifestations of starvation.13 Bradycardia has been attributed to an adaptive increase in parasympathetic vagal tone.14 QTc prolongation is also seen in patients with malnutrition.15
Together, these electrocardiographic abnormalities predispose the patient to ventricular arrhythmia and sudden cardiac death.16 The risk of ventricular arrhythmia is particularly relevant when treating psychiatric symptoms, since antipsychotics and tricyclic antidepressants are among several drug classes that can cause further QTc prolongation (Table 1).17,18
In patients with QTc prolongation, bradycardia, or both, the standard of care involves acute hospitalization for refeeding using continuous telemetric monitoring until normal rhythm is restored and the heart rate is above 40 at night and 50 by day.4,19
Structural changes. Starvation also causes structural changes in the heart. Loss of lean body mass can reduce cardiac muscle mass, compromise cardiac output, and lead to mitral valve prolapse.20 These changes are fully reversible with restored nutrition and regaining of heart mass.21,22
Effects of starvation on the brain
Starvation can affect brain structure and cognitive function. Undernourished patients have reduced volumes of white and gray matter, a change that can occur within months. Cortical volumes may increase with weight gain, but a reduction in gray matter volume may not be completely reversible.23
Furthermore, starvation impairs cognitive functions that are needed to stop eating-disorder behaviors; namely, decision-making, emotional control, regulation of appetite, and reward path-ways. Therefore, undernourished patients may not have sufficient insight into the disease to be able to make the best choices for recovery. This finding lends support for using the Maudsley method in adolescents, in which parents take control of their child’s eating until the child can maintain a healthy weight.24
Gastrointestinal consequences of starvation
Patients with malnutrition have prolonged gastric emptying and colonic transit time with solid foods.25 They often complain of early satiety, abdominal pain, bloating, and constipation, all symptoms that complicate the refeeding process. A prokinetic such as metoclopramide (Reglan), given 1 hour before meals and at bedtime, may provide some relief from gastrointestinal symptoms.26
Patients may also experience transient lactose or fructose intolerance after prolonged starvation. Taking a lactase supplement (eg, Lactaid 1–10 tabs) before consuming dairy products and dextrose (contained in candies such as Smarties) before eating fruit or fructose-containing foods can sometimes partially relieve symptoms. In general, gastrointestinal function returns over time as nutritional status improves.
Patients with severe or prolonged starvation can develop steatosis accompanied by elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In reports of starvation-induced steatosis, liver enzyme levels rapidly normalize with nutritional rehabilitation.27
Endocrine consequences of starvation
Amenorrhea. Dysregulation of the hypothalamic-pituitary-gonadal axis is a major endocrine complication of nutritional in-sufficiency. Weight loss disrupts the normal pulsatile secretion of gonadotropin-releasing hormone, reduces secretion of luteinizing hormone and follicle-stimulating hormone, and decreases estrogen levels.28 Leptin deficiency likely plays a role in suppressing gonadotropin secretion with subsequent development of amenorrhea. With weight gain, levels of leptin and gonadotropins normalize and menstruation eventually returns.29,30
Hypothyroidism. Starvation can also lead to dysregulation of the hypothalamic-pituitary-thyroid axis. Typically, the concentration of triiodothyronine (T3) is reduced, the ratio of thyroxine (T4) to T3 is elevated, and thyroid-stimulating hormone (TSH) is close to or within the normal range, creating a euthyroid sick syndrome. In eating disorders, this thyroid disturbance is a result of starvation and resolves with weight restoration. Therefore, thyroid hormone replacement therapy is not medically indicated.28
Osteoporosis. Amenorrhea resulting from low estrogen levels in undernourished patients can raise the risk of osteoporosis and fractures, particularly in patients with a low body mass index. Osteopenia results from a negative balance between bone deposition and resorption.
Lack of bone deposition can be especially problematic when disordered eating occurs during peak bone mass development, ie, ages 11 to 14 for girls, and ages 15 to 17 for boys.31,32 Even a 5% to 10% decrease in bone deposition can result in significant risk of osteopenia.33 However, after age 30, bone resorption is a greater contributor.34
Does hormone therapy correct bone loss? Given the association between estrogen deficiency and bone loss, estrogen supplementation was expected to be an effective treatment for bone loss in patients with eating disorders.35 Also, the restoration of menses through hormone replacement may give underweight patients a false sense of achieving a “healthy” weight.36
Golden et al37 prospectively studied 50 adolescents and found no significant difference in bone mineral density at 1 year of follow-up between patients treated with estrogen and those who received only standard nutritional therapy. However, increased bone mineral density was achieved in adolescents with anorexia nervosa treated with transdermally administered estrogen dosed to mimic physiologic pubertal levels.38
Klibanski et al39 found that hormone therapy resulted in a 4% gain in bone density in an extremely low-weight subset of women with anorexia nervosa (< 70% of ideal body weight), whereas similar patients in the control group lost 20%. However, in all groups, only weight gain correlated with bone gain in women who were within 70% of their ideal body weight.
Divasta et al40 evaluated 60 girls and women ages 13 to 27 with anorexia nervosa, randomized to receive either placebo or dehydroepiandrosterone combined with an estrogen-progestin oral contraceptive, and followed for 18 months. As in the study by Klibanski et al,39 bone loss was prevented in the treatment group, but significant bone gain occurred only in the context of weight gain.
The bottom line is that only weight gain has resulted in significant increases in bone density in patients with anorexia nervosa, and hormone therapy without weight gain has not been shown to increase bone density effectively in this population. Although calcium and vitamin D in oral therapeutic doses through foods or through supplementation are required for bone gain, the combination is not enough to augment bone density in the absence of weight gain.37 Although not curative, weight gain is currently the best option for treating bone loss, and no single pharmacologic treatment is effective.
COMPLICATIONS OF PURGING
Oral complications of purging
Patients who purge by vomiting are at risk of complications from exposure of the esophagus, pharynx, and mouth to acidic gastric contents.
Dental problems. Over time, contact with gastric acid wears down enamel on the lingual and occlusal surfaces of teeth, resulting in dental caries and periodontal disease. Until they can give up purging, patients should be instructed to rinse with mouthwash or water immediately after vomiting to reduce the acidity in the mouth.41,42 We recommend that patients not brush their teeth after vomiting, because brushing can deliver acid to otherwise unreachable surfaces and thus worsen tooth erosion. For patients who are determined to brush after vomiting, a bicarbonate toothpaste might mitigate harm.42
Sialadenosis (hypertrophy of the salivary glands) is another consequence of repeated vomiting, with elevated salivary amylase. Both the size of the glands and the salivary amylase level generally normalize on their own after vomiting is stopped, but parotitis can take up to a year to resolve. Similar to smoker’s cough, parotitis may acutely worsen when the patient abruptly stops vomiting and may worsen before it improves.
To reduce discomfort, patients can use hot compresses or sugarless hard candies.44 However, the latter should not be substituted as a chronic habit in a patient with disordered eating. Patients need to be reassured that the swelling is not permanent, since they often interpret it as having fat cheeks (the “chipmunk sign”).
Hypokalemia, metabolic alkalosis, renal dysfunction
Chronic vomiting can cause electrolyte and acid-base imbalances, the most worrisome of which is hypokalemia. With repeated vomiting, loss of potassium and gastric acid causes metabolic alkalosis with hypokalemia, hypochloremia, and hypomagnesemia. Loss of water and the resultant volume contraction activates the renin-angiotensin-aldosterone system, and elevated aldosterone further decreases serum potassium.
In patients with eating disorders, who often have other factors contributing to electrolyte imbalance, vomiting-induced hypokalemia heightens the risk of cardiac arrhythmias.43
Hypokalemia can also cause rhabdomyolysis and kidney damage.41,43 Prolonged hypokalemia and reduced kidney perfusion in the setting of volume depletion causes acute kidney injury and impaired concentrating ability of the renal tubules. Hypovolemia can cause prerenal azotemia and increases the risk for nephrolithiasis and nephrocalcinosis.44,45
When a patient stops vomiting, elevated aldosterone from prior hypovolemia results in water retention and can manifest in significant edema associated with hypochloremic alkalosis. This condition, known as pseudo-Bartter syndrome, usually resolves without treatment. In the meantime, salt restriction and leg elevation can help reduce edema.26
Laxative abuse: A mode of purging
Many patients with eating disorders abuse laxatives to lose weight or to prevent weight gain. Believing that laxatives will prevent calorie absorption, patients commonly take them to compensate for caloric intake (eg, during a binge episode). The immediate weight loss, albeit artificial, is highly reinforcing for an eating-disorder patient. In some cases, patients with eating disorders also abuse laxatives to self-treat the constipation that results from chronic starvation.46
Over time, tolerance to laxatives develops, and patients use increasingly larger doses. This can lead to activation of the renin-angiotensin-aldosterone system.47 Patients interpret the resultant edema as true weight gain and again take laxatives to get rid of it. If laxatives are stopped abruptly, the patient may need inpatient and outpatient support for the resultant fluid shifts.
Gastrointestinal complications of laxative abuse include reflex hypofunction of the bowel, malabsorption, steatorrhea, and gastrointestinal bleeding.47 Reflex hypofunction during laxative withdrawal is a consequence of the bowel becoming tolerant of laxatives.48 Cathartic colon syndrome is a rare complication characterized by loss of the normal haustral markings and slowed or absent peristalsis in segments of the colon.49
Systemically, the major risk of laxative abuse relates to electrolyte and acid-base imbalance. Loss of potassium and water in the stool can cause hypokalemia and metabolic alkalosis.48 The disturbances caused by laxative abuse are similar to those caused by vomiting and diuretic use and have the same treatment.
The most important component of treating laxative abuse is giving patients realistic expectations to help them tolerate temporary discomfort and to help manage the edema and fluid shifts that can happen acutely with shifting of fluid into the intracellular space. In extreme cases, this may need to be managed in the hospital. To help relieve the initial anxiety, doctors should emphasize that any bloating the patient experiences is not true weight gain and will go away within a few days to weeks. In addition, explaining that laxatives reduce nutrient absorption only minimally may lessen the temptation to resume taking them.48
Diuretic abuse: Another form of purging
Diuretic abuse is yet another mode of purging, with its own set of medical complications. Like laxatives, diuretics are not effective weight-loss agents, and the weight reduction they cause is only temporary.
As with vomiting, there is a compensatory activation of the renin-angiotensin-aldosterone system, and therefore subsequent fluid intake will lead to water retention, which encourages further diuretic use.41 Diuretics can also contribute to hypokalemia, hypomagnesemia, hypochloremia, and metabolic alkalosis.
Ipecac abuse can lead to heart failure
Ipecac syrup has long been used to induce vomiting, but this practice has become much less common since ipecac has become harder to obtain in the United States.50 The emetine base contained in ipecac binds irreversibly to cardiac and skeletal muscle. With continued use, irreversible cardiomyopathy develops and can lead to heart failure. Treatment should include supportive care and immediate cessation of ipecac use.
Diabetic patients may skip insulin to lose weight
Patients with diabetes, especially those with type 1 that begins in childhood, are at greater risk of eating disorders over time.51 They may withhold insulin to lose weight, a practice referred to in the nonmedical literature as “diabulimia,” and they seem particularly more likely to develop bulimia nervosa than those without diabetes.52
The medical prognosis is poor for patients with diabetes who develop eating disorders and do not receive intensive treatment.51 In addition, if a diabetic patient on an insulin pump becomes depressed in addition to having an eating disorder, careful monitoring for suicidal thoughts and a rapid follow-up with mental health services are in order.
REFEEDING SYNDROME
When refeeding is started, a high glucose load stimulates insulin secretion, resulting in cellular uptake of phosphorus along with potassium, magnesium, and glucose. In addition, total body phosphorus is depleted by the increased demand for adenosine triphosphate and 2,3-diphosphoglycerate for cellular metabolism.
When liver enzyme levels increase, the astute clinician will closely monitor the patient for evidence of refeeding syndrome. In a child, adolescent, or young adult, the standard of care is inpatient monitoring for acute stabilization.4,19
Hypophosphatemia is the hallmark of refeeding syndrome, although hypomagnesemia, hypokalemia, and hypoglycemia can also occur.53 In addition, sodium and water retention can lead to fluid overload, with shifting of fluid into the intracellular space, resulting in dependent edema.
Cardiovascular complications are the most worrisome manifestations of refeeding syndrome. Electrolyte shifts and increased fluid volume can cause arrhythmias and heart failure. Furthermore, severely undernourished patients may have reduced myocardial mass as well as electrocardiographic abnormalities associated with starvation, which further increase their vulnerability to electrolyte shifts and fluid retention during refeeding.15
Other manifestations of refeeding syndrome include delirium, seizures, rhabdomyolysis, and respiratory failure. In the most extreme cases, refeeding syndrome causes sudden death.53
Fortunately, refeeding syndrome is easily preventable and treatable when recognized early. Electrolytes and cardiovascular and renal function must be carefully monitored, especially during the first week of nutritional restoration.53 In patients with extremely low body mass (< 70% of ideal body weight) or with precipitous weight loss, close monitoring of the complete metabolic panel including electrolytes, AST, ALT, calcium, magnesium, and phosphorus may be required to detect changes that can affect cardiac status. Specific suggestions for refeeding are discussed below and in Table 2.45
ACUTE CARE OF PATIENTS WITH EATING DISORDERS
Refeeding in the inpatient setting
The decision to hospitalize an eating-disorder patient is based on the current or potential risk of serious medical complications and the likelihood of success at home. Medical criteria for hospital admission are outlined in Table 3.4,54
In refeeding undernourished patients, the challenge is to maximize weight gain while preventing refeeding syndrome. Undernourished patients are generally hypometabolic at baseline but become hypermetabolic once refeeding begins.
How many calories should refeeding start with? The traditional principle of “start low and go slow” has been recently challenged.55 Starting at 1,200 kcal/day or less in the typical patient can result in failure to gain weight or even in weight loss in the first week of refeeding.56 The goal is to achieve a weight gain of 0.2 kg/day while the patient is in the hospital. Thus, we start higher, and to date we have seen no cases of life-threatening refeeding syndrome. In all patients who need hospitalization or who are beginning the refeeding process as outpatients, caloric intake should be started at 1,500 to 2,000 kcal/day.45,57 However, for exceptionally low-weight patients, intake may be started lower.
In Australia, patients are started at 1,900 kcal/day.56 All patients in one program there receive nasogastric feeding initially in an intensive care unit and then are moved to a regular nursing floor where they graduate to full oral feeding as they improve cardiovascularly and behaviorally. In the United States, some programs use nasogastric feeding at night for caloric restoration; our program and others use nasogastric feeding as a behavioral modification strategy for patients who refuse food or supplements by mouth.
Phosphorus supplementation. Many centers give phosphorus supplements preventively. In our center, we give potassium phosphate (Neutra-Phos) 500 mg orally twice daily for 5 days, and we have seen no life-threatening cases of refeeding syndrome with that regimen. Other centers give phosphorus supplements in a dose of 250 mg orally twice a day for 5 days, while still others only supplement phosphorus reactively once a deficit has been identified. The latter method requires daily blood draws for monitoring and is reactive rather than proactive. Further studies can help clarify the optimal dosing and timing of phosphorus supplementation.
Managing fluid balance. Fluid-loading these patients may tip them over the edge into refeeding syndrome. Except in cases of shock, patients with eating disorders should not be given intravenous fluids, as it is safer to rehydrate and feed them orally. Electrolyte imbalances can be corrected orally with no need for intravenous supplementation. To avoid fluid overload, fluids can be started at 1,500 mL to 2,000 mL per day, with strict monitoring of intake and output. Fluids are liberalized if ALT and AST levels remain normal and to gradually correct orthostatic hypotension; caloric fluids are ideal to help address energy needs and improve bradycardia.
Laboratory monitoring. On admission, a urinalysis, complete blood cell count, complete metabolic panel, TSH, erythrocyte sedimentation rate, serum magnesium, and phosphorus should be obtained.26 In addition, continuous electrocardiographic recording should begin on admission.45 Inpatient use of a telemetry bed helps identify extreme tachycardia with arrhythmia, as well as profound bradycardia.45,56
Some protocols call for daily laboratory monitoring, although that degree of testing is less cost-effective. If initial results are normal, clinical judgment can be used on when to repeat laboratory evaluation. For instance, patients with edema require repeat complete metabolic panels to assess for elevated ALT and AST, electrolyte imbalances, and other abnormalities.
Signs of refeeding syndrome include tachycardia, hepatosplenomegaly, peripheral edema, altered mental status, and electrolyte disturbances, specifically, acute or severe hypophosphatemia or hypokalemia.26,45 If refeeding syndrome is suspected, the rate of caloric intake should be reduced or not advanced, fluid intake should be urgently reassessed for volume overload, and supportive care with close monitoring should be provided.
KNOWLEDGE SAVES LIVES
Eating disorders can lead to potentially life-threatening medical complications that require attentive care by the primary care clinician and subspecialist. Without thoughtful consideration, it is easy for even a caring medical team to unintentionally enable patients with these illnesses or to cause active harm in the case of underrecognized pathology.58
Acute medical stabilization on an inpatient unit trained to recognize pathology and treat sequelae can be lifesaving. Arming patients and families with medical knowledge, as provided in the Academy for Eating Disorders’ brochure, “Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders”59 can help save patients’ lives.
Eating disorders are debilitating biopsychosocial illnesses associated with serious medical illness and a high risk of death.1
Primary care physicians are often the first to see young women who have these problems, diagnose them, and start their evaluation and treatment.2–4 Many patients require acute medical interventions as well as long-term care for chronic medical issues. Therefore, primary care physicians play essential front-line and long-term roles in the multidisciplinary treatment team.
DEFINITIONS OF EATING DISORDERS HAVE CHANGED
Several problems existed in the category of eating disorders in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) and in the DSM-4 Text Revision (DSM-4-TR). These problems have been addressed in the fifth edition (DSM-5), released in 2013.5
One problem in the earlier editions was that many patients referred for treatment of eating disorders—more than 50% in one study6—did not meet the criteria for anorexia nervosa or bulimia nervosa and thus had to be categorized as having “eating disorder not otherwise specified.” Further, the earlier editions did not recognize that young children and adolescent males can be affected.7
Eating disorders are now recognized as an equal-opportunity disease, with all ethnic and socioeconomic groups affected. Children can run into medical trouble with even a small amount of weight loss or falling off the growth curve. Moreover, children and adolescents do not “experience” their bodies in the same way adults do; they may lack the vocabulary for eating-disorder thoughts.
For these reasons, the definitions of eating disorders have changed in the DSM-5.5
Anorexia nervosa. Older editions of the DSM listed amenorrhea as a criterion. This has been eliminated in DSM-5, since amenorrhea does not necessarily predict medical risk or treatment outcome; also, it is not applicable to males or premenorrheal girls and postmenopausal women.8 In addition, the requirement of low weight is now defined in the context of “age, sex, developmental trajectory, and physical health,” rather than the old threshold of 85% of expected weight.9
What remains unchanged is that anorexia nervosa is still characterized by self-starvation in order to maintain an abnormally low body weight, along with an intense fear of being fat and a disturbed self-image.
Bulimia nervosa. In both the old and the new editions of the DSM, bulimia nervosa is characterized by episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain, such as vomiting, laxative abuse, diuretic abuse, and overexercise. In DSM-5, bulimia nervosa no longer has subtypes and requires only one binge per week with compensatory behavior, for at least 3 months. This change was based on the finding that there is no clear difference in psychopathology or treatment outcome between patients with one and two binge-purge episodes a week.10
“Eating disorder not otherwise specified” was a wastebasket category, lumping all those who did not meet the criteria for anorexia nervosa or bulimia nervosa or who did not neatly fit into a specific category.10 In DSM-5, subcategories were designed to help distinguish different treatment needs and outcomes between various subtypes.
Binge-eating disorder, one of the new subcategories, is characterized by binge eating without inappropriate compensatory behaviors.9 Patients with binge-eating disorder are often obese, have greater functional impairment, and are more likely to develop components of metabolic syndrome than obese patients without eating disorders.11
Avoidant/restrictive food intake disorder is another new DSM-5 diagnosis, characterized by failure to meet nutritional needs for reasons other than weight control. Reasons include disinterest in eating, dislike of sensory characteristics of food, or avoidance of consequences of eating. This disorder replaces the category “feeding disorder of infancy or early childhood,” since the condition can also occur in adolescents and adults.12
Other new diagnoses are:
- Atypical anorexia nervosa (if the patient is not underweight)
- Purging disorder
- Subthreshold bulimia nervosa (if the patient has < 1 episode per week or has had them for < 3 months)
- Subthreshold binge eating disorder (< 1 time a week or < 3 months)
- Night eating syndrome
- Pica and rumination disorder.
Regardless of the diagnostic label, the medical evaluation and treatment of anyone with an eating disorder should be tailored to the specific behaviors of the eating disorder. Medical complications can be subdivided into those from starvation, from purging, and from refeeding.
MEDICAL COMPLICATIONS OF STARVATION
Cardiovascular effects of starvation
Malnutrition and starvation have multiple adverse effects on the heart.
Electrophysiologic effects. Sinus bradycardia (< 60 bpm) and hypotension are common cardiac manifestations of starvation.13 Bradycardia has been attributed to an adaptive increase in parasympathetic vagal tone.14 QTc prolongation is also seen in patients with malnutrition.15
Together, these electrocardiographic abnormalities predispose the patient to ventricular arrhythmia and sudden cardiac death.16 The risk of ventricular arrhythmia is particularly relevant when treating psychiatric symptoms, since antipsychotics and tricyclic antidepressants are among several drug classes that can cause further QTc prolongation (Table 1).17,18
In patients with QTc prolongation, bradycardia, or both, the standard of care involves acute hospitalization for refeeding using continuous telemetric monitoring until normal rhythm is restored and the heart rate is above 40 at night and 50 by day.4,19
Structural changes. Starvation also causes structural changes in the heart. Loss of lean body mass can reduce cardiac muscle mass, compromise cardiac output, and lead to mitral valve prolapse.20 These changes are fully reversible with restored nutrition and regaining of heart mass.21,22
Effects of starvation on the brain
Starvation can affect brain structure and cognitive function. Undernourished patients have reduced volumes of white and gray matter, a change that can occur within months. Cortical volumes may increase with weight gain, but a reduction in gray matter volume may not be completely reversible.23
Furthermore, starvation impairs cognitive functions that are needed to stop eating-disorder behaviors; namely, decision-making, emotional control, regulation of appetite, and reward path-ways. Therefore, undernourished patients may not have sufficient insight into the disease to be able to make the best choices for recovery. This finding lends support for using the Maudsley method in adolescents, in which parents take control of their child’s eating until the child can maintain a healthy weight.24
Gastrointestinal consequences of starvation
Patients with malnutrition have prolonged gastric emptying and colonic transit time with solid foods.25 They often complain of early satiety, abdominal pain, bloating, and constipation, all symptoms that complicate the refeeding process. A prokinetic such as metoclopramide (Reglan), given 1 hour before meals and at bedtime, may provide some relief from gastrointestinal symptoms.26
Patients may also experience transient lactose or fructose intolerance after prolonged starvation. Taking a lactase supplement (eg, Lactaid 1–10 tabs) before consuming dairy products and dextrose (contained in candies such as Smarties) before eating fruit or fructose-containing foods can sometimes partially relieve symptoms. In general, gastrointestinal function returns over time as nutritional status improves.
Patients with severe or prolonged starvation can develop steatosis accompanied by elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In reports of starvation-induced steatosis, liver enzyme levels rapidly normalize with nutritional rehabilitation.27
Endocrine consequences of starvation
Amenorrhea. Dysregulation of the hypothalamic-pituitary-gonadal axis is a major endocrine complication of nutritional in-sufficiency. Weight loss disrupts the normal pulsatile secretion of gonadotropin-releasing hormone, reduces secretion of luteinizing hormone and follicle-stimulating hormone, and decreases estrogen levels.28 Leptin deficiency likely plays a role in suppressing gonadotropin secretion with subsequent development of amenorrhea. With weight gain, levels of leptin and gonadotropins normalize and menstruation eventually returns.29,30
Hypothyroidism. Starvation can also lead to dysregulation of the hypothalamic-pituitary-thyroid axis. Typically, the concentration of triiodothyronine (T3) is reduced, the ratio of thyroxine (T4) to T3 is elevated, and thyroid-stimulating hormone (TSH) is close to or within the normal range, creating a euthyroid sick syndrome. In eating disorders, this thyroid disturbance is a result of starvation and resolves with weight restoration. Therefore, thyroid hormone replacement therapy is not medically indicated.28
Osteoporosis. Amenorrhea resulting from low estrogen levels in undernourished patients can raise the risk of osteoporosis and fractures, particularly in patients with a low body mass index. Osteopenia results from a negative balance between bone deposition and resorption.
Lack of bone deposition can be especially problematic when disordered eating occurs during peak bone mass development, ie, ages 11 to 14 for girls, and ages 15 to 17 for boys.31,32 Even a 5% to 10% decrease in bone deposition can result in significant risk of osteopenia.33 However, after age 30, bone resorption is a greater contributor.34
Does hormone therapy correct bone loss? Given the association between estrogen deficiency and bone loss, estrogen supplementation was expected to be an effective treatment for bone loss in patients with eating disorders.35 Also, the restoration of menses through hormone replacement may give underweight patients a false sense of achieving a “healthy” weight.36
Golden et al37 prospectively studied 50 adolescents and found no significant difference in bone mineral density at 1 year of follow-up between patients treated with estrogen and those who received only standard nutritional therapy. However, increased bone mineral density was achieved in adolescents with anorexia nervosa treated with transdermally administered estrogen dosed to mimic physiologic pubertal levels.38
Klibanski et al39 found that hormone therapy resulted in a 4% gain in bone density in an extremely low-weight subset of women with anorexia nervosa (< 70% of ideal body weight), whereas similar patients in the control group lost 20%. However, in all groups, only weight gain correlated with bone gain in women who were within 70% of their ideal body weight.
Divasta et al40 evaluated 60 girls and women ages 13 to 27 with anorexia nervosa, randomized to receive either placebo or dehydroepiandrosterone combined with an estrogen-progestin oral contraceptive, and followed for 18 months. As in the study by Klibanski et al,39 bone loss was prevented in the treatment group, but significant bone gain occurred only in the context of weight gain.
The bottom line is that only weight gain has resulted in significant increases in bone density in patients with anorexia nervosa, and hormone therapy without weight gain has not been shown to increase bone density effectively in this population. Although calcium and vitamin D in oral therapeutic doses through foods or through supplementation are required for bone gain, the combination is not enough to augment bone density in the absence of weight gain.37 Although not curative, weight gain is currently the best option for treating bone loss, and no single pharmacologic treatment is effective.
COMPLICATIONS OF PURGING
Oral complications of purging
Patients who purge by vomiting are at risk of complications from exposure of the esophagus, pharynx, and mouth to acidic gastric contents.
Dental problems. Over time, contact with gastric acid wears down enamel on the lingual and occlusal surfaces of teeth, resulting in dental caries and periodontal disease. Until they can give up purging, patients should be instructed to rinse with mouthwash or water immediately after vomiting to reduce the acidity in the mouth.41,42 We recommend that patients not brush their teeth after vomiting, because brushing can deliver acid to otherwise unreachable surfaces and thus worsen tooth erosion. For patients who are determined to brush after vomiting, a bicarbonate toothpaste might mitigate harm.42
Sialadenosis (hypertrophy of the salivary glands) is another consequence of repeated vomiting, with elevated salivary amylase. Both the size of the glands and the salivary amylase level generally normalize on their own after vomiting is stopped, but parotitis can take up to a year to resolve. Similar to smoker’s cough, parotitis may acutely worsen when the patient abruptly stops vomiting and may worsen before it improves.
To reduce discomfort, patients can use hot compresses or sugarless hard candies.44 However, the latter should not be substituted as a chronic habit in a patient with disordered eating. Patients need to be reassured that the swelling is not permanent, since they often interpret it as having fat cheeks (the “chipmunk sign”).
Hypokalemia, metabolic alkalosis, renal dysfunction
Chronic vomiting can cause electrolyte and acid-base imbalances, the most worrisome of which is hypokalemia. With repeated vomiting, loss of potassium and gastric acid causes metabolic alkalosis with hypokalemia, hypochloremia, and hypomagnesemia. Loss of water and the resultant volume contraction activates the renin-angiotensin-aldosterone system, and elevated aldosterone further decreases serum potassium.
In patients with eating disorders, who often have other factors contributing to electrolyte imbalance, vomiting-induced hypokalemia heightens the risk of cardiac arrhythmias.43
Hypokalemia can also cause rhabdomyolysis and kidney damage.41,43 Prolonged hypokalemia and reduced kidney perfusion in the setting of volume depletion causes acute kidney injury and impaired concentrating ability of the renal tubules. Hypovolemia can cause prerenal azotemia and increases the risk for nephrolithiasis and nephrocalcinosis.44,45
When a patient stops vomiting, elevated aldosterone from prior hypovolemia results in water retention and can manifest in significant edema associated with hypochloremic alkalosis. This condition, known as pseudo-Bartter syndrome, usually resolves without treatment. In the meantime, salt restriction and leg elevation can help reduce edema.26
Laxative abuse: A mode of purging
Many patients with eating disorders abuse laxatives to lose weight or to prevent weight gain. Believing that laxatives will prevent calorie absorption, patients commonly take them to compensate for caloric intake (eg, during a binge episode). The immediate weight loss, albeit artificial, is highly reinforcing for an eating-disorder patient. In some cases, patients with eating disorders also abuse laxatives to self-treat the constipation that results from chronic starvation.46
Over time, tolerance to laxatives develops, and patients use increasingly larger doses. This can lead to activation of the renin-angiotensin-aldosterone system.47 Patients interpret the resultant edema as true weight gain and again take laxatives to get rid of it. If laxatives are stopped abruptly, the patient may need inpatient and outpatient support for the resultant fluid shifts.
Gastrointestinal complications of laxative abuse include reflex hypofunction of the bowel, malabsorption, steatorrhea, and gastrointestinal bleeding.47 Reflex hypofunction during laxative withdrawal is a consequence of the bowel becoming tolerant of laxatives.48 Cathartic colon syndrome is a rare complication characterized by loss of the normal haustral markings and slowed or absent peristalsis in segments of the colon.49
Systemically, the major risk of laxative abuse relates to electrolyte and acid-base imbalance. Loss of potassium and water in the stool can cause hypokalemia and metabolic alkalosis.48 The disturbances caused by laxative abuse are similar to those caused by vomiting and diuretic use and have the same treatment.
The most important component of treating laxative abuse is giving patients realistic expectations to help them tolerate temporary discomfort and to help manage the edema and fluid shifts that can happen acutely with shifting of fluid into the intracellular space. In extreme cases, this may need to be managed in the hospital. To help relieve the initial anxiety, doctors should emphasize that any bloating the patient experiences is not true weight gain and will go away within a few days to weeks. In addition, explaining that laxatives reduce nutrient absorption only minimally may lessen the temptation to resume taking them.48
Diuretic abuse: Another form of purging
Diuretic abuse is yet another mode of purging, with its own set of medical complications. Like laxatives, diuretics are not effective weight-loss agents, and the weight reduction they cause is only temporary.
As with vomiting, there is a compensatory activation of the renin-angiotensin-aldosterone system, and therefore subsequent fluid intake will lead to water retention, which encourages further diuretic use.41 Diuretics can also contribute to hypokalemia, hypomagnesemia, hypochloremia, and metabolic alkalosis.
Ipecac abuse can lead to heart failure
Ipecac syrup has long been used to induce vomiting, but this practice has become much less common since ipecac has become harder to obtain in the United States.50 The emetine base contained in ipecac binds irreversibly to cardiac and skeletal muscle. With continued use, irreversible cardiomyopathy develops and can lead to heart failure. Treatment should include supportive care and immediate cessation of ipecac use.
Diabetic patients may skip insulin to lose weight
Patients with diabetes, especially those with type 1 that begins in childhood, are at greater risk of eating disorders over time.51 They may withhold insulin to lose weight, a practice referred to in the nonmedical literature as “diabulimia,” and they seem particularly more likely to develop bulimia nervosa than those without diabetes.52
The medical prognosis is poor for patients with diabetes who develop eating disorders and do not receive intensive treatment.51 In addition, if a diabetic patient on an insulin pump becomes depressed in addition to having an eating disorder, careful monitoring for suicidal thoughts and a rapid follow-up with mental health services are in order.
REFEEDING SYNDROME
When refeeding is started, a high glucose load stimulates insulin secretion, resulting in cellular uptake of phosphorus along with potassium, magnesium, and glucose. In addition, total body phosphorus is depleted by the increased demand for adenosine triphosphate and 2,3-diphosphoglycerate for cellular metabolism.
When liver enzyme levels increase, the astute clinician will closely monitor the patient for evidence of refeeding syndrome. In a child, adolescent, or young adult, the standard of care is inpatient monitoring for acute stabilization.4,19
Hypophosphatemia is the hallmark of refeeding syndrome, although hypomagnesemia, hypokalemia, and hypoglycemia can also occur.53 In addition, sodium and water retention can lead to fluid overload, with shifting of fluid into the intracellular space, resulting in dependent edema.
Cardiovascular complications are the most worrisome manifestations of refeeding syndrome. Electrolyte shifts and increased fluid volume can cause arrhythmias and heart failure. Furthermore, severely undernourished patients may have reduced myocardial mass as well as electrocardiographic abnormalities associated with starvation, which further increase their vulnerability to electrolyte shifts and fluid retention during refeeding.15
Other manifestations of refeeding syndrome include delirium, seizures, rhabdomyolysis, and respiratory failure. In the most extreme cases, refeeding syndrome causes sudden death.53
Fortunately, refeeding syndrome is easily preventable and treatable when recognized early. Electrolytes and cardiovascular and renal function must be carefully monitored, especially during the first week of nutritional restoration.53 In patients with extremely low body mass (< 70% of ideal body weight) or with precipitous weight loss, close monitoring of the complete metabolic panel including electrolytes, AST, ALT, calcium, magnesium, and phosphorus may be required to detect changes that can affect cardiac status. Specific suggestions for refeeding are discussed below and in Table 2.45
ACUTE CARE OF PATIENTS WITH EATING DISORDERS
Refeeding in the inpatient setting
The decision to hospitalize an eating-disorder patient is based on the current or potential risk of serious medical complications and the likelihood of success at home. Medical criteria for hospital admission are outlined in Table 3.4,54
In refeeding undernourished patients, the challenge is to maximize weight gain while preventing refeeding syndrome. Undernourished patients are generally hypometabolic at baseline but become hypermetabolic once refeeding begins.
How many calories should refeeding start with? The traditional principle of “start low and go slow” has been recently challenged.55 Starting at 1,200 kcal/day or less in the typical patient can result in failure to gain weight or even in weight loss in the first week of refeeding.56 The goal is to achieve a weight gain of 0.2 kg/day while the patient is in the hospital. Thus, we start higher, and to date we have seen no cases of life-threatening refeeding syndrome. In all patients who need hospitalization or who are beginning the refeeding process as outpatients, caloric intake should be started at 1,500 to 2,000 kcal/day.45,57 However, for exceptionally low-weight patients, intake may be started lower.
In Australia, patients are started at 1,900 kcal/day.56 All patients in one program there receive nasogastric feeding initially in an intensive care unit and then are moved to a regular nursing floor where they graduate to full oral feeding as they improve cardiovascularly and behaviorally. In the United States, some programs use nasogastric feeding at night for caloric restoration; our program and others use nasogastric feeding as a behavioral modification strategy for patients who refuse food or supplements by mouth.
Phosphorus supplementation. Many centers give phosphorus supplements preventively. In our center, we give potassium phosphate (Neutra-Phos) 500 mg orally twice daily for 5 days, and we have seen no life-threatening cases of refeeding syndrome with that regimen. Other centers give phosphorus supplements in a dose of 250 mg orally twice a day for 5 days, while still others only supplement phosphorus reactively once a deficit has been identified. The latter method requires daily blood draws for monitoring and is reactive rather than proactive. Further studies can help clarify the optimal dosing and timing of phosphorus supplementation.
Managing fluid balance. Fluid-loading these patients may tip them over the edge into refeeding syndrome. Except in cases of shock, patients with eating disorders should not be given intravenous fluids, as it is safer to rehydrate and feed them orally. Electrolyte imbalances can be corrected orally with no need for intravenous supplementation. To avoid fluid overload, fluids can be started at 1,500 mL to 2,000 mL per day, with strict monitoring of intake and output. Fluids are liberalized if ALT and AST levels remain normal and to gradually correct orthostatic hypotension; caloric fluids are ideal to help address energy needs and improve bradycardia.
Laboratory monitoring. On admission, a urinalysis, complete blood cell count, complete metabolic panel, TSH, erythrocyte sedimentation rate, serum magnesium, and phosphorus should be obtained.26 In addition, continuous electrocardiographic recording should begin on admission.45 Inpatient use of a telemetry bed helps identify extreme tachycardia with arrhythmia, as well as profound bradycardia.45,56
Some protocols call for daily laboratory monitoring, although that degree of testing is less cost-effective. If initial results are normal, clinical judgment can be used on when to repeat laboratory evaluation. For instance, patients with edema require repeat complete metabolic panels to assess for elevated ALT and AST, electrolyte imbalances, and other abnormalities.
Signs of refeeding syndrome include tachycardia, hepatosplenomegaly, peripheral edema, altered mental status, and electrolyte disturbances, specifically, acute or severe hypophosphatemia or hypokalemia.26,45 If refeeding syndrome is suspected, the rate of caloric intake should be reduced or not advanced, fluid intake should be urgently reassessed for volume overload, and supportive care with close monitoring should be provided.
KNOWLEDGE SAVES LIVES
Eating disorders can lead to potentially life-threatening medical complications that require attentive care by the primary care clinician and subspecialist. Without thoughtful consideration, it is easy for even a caring medical team to unintentionally enable patients with these illnesses or to cause active harm in the case of underrecognized pathology.58
Acute medical stabilization on an inpatient unit trained to recognize pathology and treat sequelae can be lifesaving. Arming patients and families with medical knowledge, as provided in the Academy for Eating Disorders’ brochure, “Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders”59 can help save patients’ lives.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731.
- Walsh JM, Wheat ME, Freund K. Detection, evaluation, and treatment of eating disorders the role of the primary care physician. J Gen Intern Med 2000; 15:577–590.
- American Academy of Pediatrics; Committee on Adolescence. Identifying and treating eating disorders. Pediatrics 2003; 111:204–211.
- Rosen DS; American Academy of Pediatrics Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics 2010; 126:1240–1253.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Publishing, Incorporated; 2013.
- Eddy KT, Celio Doyle A, Hoste RR, Herzog DB, le Grange D. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47:156–164.
- Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health 2003; 33:427–435.
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 2009; 42:581–589.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. http://dsm.psychiatryonline.org/content.aspx?bookid=556§ionid=41101776#103439089. Accessed January 31, 2014.
- Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord 2007; 40:S123–S129.
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009; 42:687–705.
- Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health 2013: 53:303–305.
- Winston AP, Stafford PJ. Cardiovascular effects of anorexia nervosa. Eur Eat Disord Rev 2000; 8:117–125.
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health 2003; 32:416–421.
- McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord 2006; 14:73–80.
- Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation 1990; 82:1561–1573.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- The University of Arizona Center for Education and Research on Therapeutics. QT Drug Lists. http://crediblemeds.org/everyone/compos-ite-list-all-qtdrugs/?rf=US. Accessed January 31, 2014.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 2003; 77:308–312.
- Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health 2003; 32:73–77.
- Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 2003; 42:808–813.
- Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord 2011; 44:406–411.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011; 199:5–7.
- Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003; 2:1–9.
- Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med 2005; 353:1481–1488.
- De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 2006; 22:572–575.
- Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 2008; 4:407–414.
- Holtkamp K, Mika C, Grzella I, et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110:427–435.
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151:16–21.
- Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002; 87:4177–4185.
- Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Invest 2011; 34:324–332.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 1989; 68:548–554.
- Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol 1997; 176:1017–1025.
- Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 2010; 43:218–225.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26:2430–2438.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012; 61:1010–1020.
- Mehler PS. Medical complications of bulimia nervosa and their treatments. Int J Eat Disord 2011; 44:95–104.
- Milosevic A. Eating disorders and the dentist. Br Dent J 1999; 186:109–113.
- Greenfeld D, Mickley D, Quinlan DM, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152:60–63.
- Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis 2012; 60:299–307.
- Auron M, Rome E. Anorexia nervosa and bulimia nervosa: what the hospitalist needs to know about CPT 269.9, or nutritional insufficiency. ACP Hospitalist 2011 Sept:28–45.
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL. The eating disorders medicine cabinet revisited: a clinician’s guide to ipecac and laxatives. Int J Eat Disord 2007; 40:360–368.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70:1487–1503.
- Mitchell JE, Boutacoff LI. Laxative abuse complicating bulimia: medical and treatment implications. Int J Eat Disord 1986; 5:325–334.
- Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26:283–286.
- Drugs.com. Ipecac syrup. www.drugs.com/monograph/ipecac-syrup.html. Accessed January 31, 2014.
- Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005; 28:84–88.
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005; 28:417–419.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17:632–637.
- Fisher M, Golden NH, Katzman DK, et al. Eating disorders in adolescents: a background paper. J Adolesc Health 1995; 16:420–437.
- Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr 2011; 23:390–394.
- Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health 2012; 50:24–29.
- Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health 2010; 46:577–582.
- Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev 2011; 19:296–302.
- Academy for Eating Disorders (AED). Critical points for early recognition and medical risk management in the care of individuals with eating disorders. http://www.aedweb.org/AM/Template.cfm?Section=Medical_Care_Standards&Template=/CM/ContentDisplay.cfm&ContentID=2413. Accessed January 31, 2014.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731.
- Walsh JM, Wheat ME, Freund K. Detection, evaluation, and treatment of eating disorders the role of the primary care physician. J Gen Intern Med 2000; 15:577–590.
- American Academy of Pediatrics; Committee on Adolescence. Identifying and treating eating disorders. Pediatrics 2003; 111:204–211.
- Rosen DS; American Academy of Pediatrics Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics 2010; 126:1240–1253.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Publishing, Incorporated; 2013.
- Eddy KT, Celio Doyle A, Hoste RR, Herzog DB, le Grange D. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47:156–164.
- Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health 2003; 33:427–435.
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 2009; 42:581–589.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. http://dsm.psychiatryonline.org/content.aspx?bookid=556§ionid=41101776#103439089. Accessed January 31, 2014.
- Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord 2007; 40:S123–S129.
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009; 42:687–705.
- Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health 2013: 53:303–305.
- Winston AP, Stafford PJ. Cardiovascular effects of anorexia nervosa. Eur Eat Disord Rev 2000; 8:117–125.
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health 2003; 32:416–421.
- McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord 2006; 14:73–80.
- Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation 1990; 82:1561–1573.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- The University of Arizona Center for Education and Research on Therapeutics. QT Drug Lists. http://crediblemeds.org/everyone/compos-ite-list-all-qtdrugs/?rf=US. Accessed January 31, 2014.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 2003; 77:308–312.
- Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health 2003; 32:73–77.
- Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 2003; 42:808–813.
- Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord 2011; 44:406–411.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011; 199:5–7.
- Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003; 2:1–9.
- Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med 2005; 353:1481–1488.
- De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 2006; 22:572–575.
- Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 2008; 4:407–414.
- Holtkamp K, Mika C, Grzella I, et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110:427–435.
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151:16–21.
- Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002; 87:4177–4185.
- Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Invest 2011; 34:324–332.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 1989; 68:548–554.
- Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol 1997; 176:1017–1025.
- Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 2010; 43:218–225.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26:2430–2438.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012; 61:1010–1020.
- Mehler PS. Medical complications of bulimia nervosa and their treatments. Int J Eat Disord 2011; 44:95–104.
- Milosevic A. Eating disorders and the dentist. Br Dent J 1999; 186:109–113.
- Greenfeld D, Mickley D, Quinlan DM, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152:60–63.
- Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis 2012; 60:299–307.
- Auron M, Rome E. Anorexia nervosa and bulimia nervosa: what the hospitalist needs to know about CPT 269.9, or nutritional insufficiency. ACP Hospitalist 2011 Sept:28–45.
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL. The eating disorders medicine cabinet revisited: a clinician’s guide to ipecac and laxatives. Int J Eat Disord 2007; 40:360–368.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70:1487–1503.
- Mitchell JE, Boutacoff LI. Laxative abuse complicating bulimia: medical and treatment implications. Int J Eat Disord 1986; 5:325–334.
- Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26:283–286.
- Drugs.com. Ipecac syrup. www.drugs.com/monograph/ipecac-syrup.html. Accessed January 31, 2014.
- Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005; 28:84–88.
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005; 28:417–419.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17:632–637.
- Fisher M, Golden NH, Katzman DK, et al. Eating disorders in adolescents: a background paper. J Adolesc Health 1995; 16:420–437.
- Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr 2011; 23:390–394.
- Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health 2012; 50:24–29.
- Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health 2010; 46:577–582.
- Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev 2011; 19:296–302.
- Academy for Eating Disorders (AED). Critical points for early recognition and medical risk management in the care of individuals with eating disorders. http://www.aedweb.org/AM/Template.cfm?Section=Medical_Care_Standards&Template=/CM/ContentDisplay.cfm&ContentID=2413. Accessed January 31, 2014.
KEY POINTS
- The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), released in 2013, has updated the criteria for some eating disorders and has added some new disorders.
- Starvation can cause cardiac, cerebral, gastrointestinal, and endocrine problems.
- Purging can lead to problems with oral health, electrolyte imbalances, and even renal failure.
- Refeeding poses the risk of refeeding syndrome, with fluid overload and electrolyte imbalances. Many patients undergoing refeeding are best managed in the hospital.
Using the Internet in your practice. Part 2: Generating new patients using social media
With this article, we intend to illustrate the value of having a social media presence and how you can use social media to attract new patients. One of us (NHB) has been using social media to promote his medical practice for 3 years and can be found on the first page of Google search results for several of the medical conditions he treats. As a result of these high search rankings, he is able to generate two to four new patient visits every day.
You can achieve the same results using the techniques described in this article. You certainly can buy banner ads and buy traffic to your page, but we want to show you how to get on the first page of Google using the natural, organic method.
PUSH VS PULL
Social media can be used in different ways to build your practice. What you employ depends on what you want to accomplish and the time and energy you want to devote to each of these social media opportunities.
By its very definition, social media is social engagement—and what is known as a “pull” technology. There are two ways to share your information with people on the Internet:
- “Pull” Web site surfers to your information
- “Push” your information to them.
Push occurs when you initiate the process by placing your information in front of the Web site surfer. They get it or see it because of the actions you have taken. Sending e-mails is one way to push information to your target audience, or potential patients, to your practice. Another way to push your Web site and its contents is to get listed on the first page of search engine results. You want to “push” your Web site in plain view of the person who has typed in keywords or keyword phrases that relate to your practice (ie, “OBGYN” plus “<your city>,” “tubal ligation” plus “<your city>,” or “loss of urine” plus “<your zip code>.” Push techniques are the best way to market your services and offer the best return on your marketing investment.
Using social media, you are able to “pull” your audience of potential patients to you and your practice. In other words, your target market of potential patients has to take the time and make the effort to type in your Web site address in order to come to you. The information or message you have on your social media sites has to be strong enough and of sufficient compelling interest that patients want to come to read what you have to say. Web surfers are looking for online relationships for information sharing. It is this interaction with your potential patients that makes social media unique. Using this pull technology, you have the opportunity to interact and develop a relationship with a patient before she picks up the phone to make an appointment, before she comes to the office to see you eyeball to eyeball.
FACEBOOK AND HOW IT RELATES TO YOUR PRACTICE
Originally, Facebook was developed as a way for people to see what was going on in each other’s lives, a method to stay in contact with one another. In the beginning, it was friends, family members, or groups of like-minded individuals frequenting each other’s Facebook pages. Typically, they would keep tabs on who was having a party or post pictures of their kids for family members to see.
Facebook has evolved. Today, companies, businesses, and, yes, medical practices are trying to “pull” more Web site visitors to their Facebook pages. To do this, they hold contests with prizes; offer great content, coupons, and videos; and provide special offers to get Web surfers to their site. Large companies and large group practices like the Mayo Clinic, Cleveland Clinic, and MD Anderson Cancer Center, have whole social media departments that post regularly, respond to comments left on their pages, and answer questions posted by those who “like” their page or site.
Individual practicing clinicians, and most smaller ObGyn practices, do not have the budget for a social media team. They also don’t have the time or the training to write effective copy that is so compelling that Web surfers are drawn or “pulled” to their Facebook page. The reality is, your patients expect you to have a Facebook page, and they expect you to have quality information that is helpful and relevant to their well-being. But, the question remains…
Related article: Four pillars of a successful practice: 1. Keep your current patients happy Neal H. Baum, MD (Practice Management, March 2013)
Can Facebook generate new patients?
You and your practice certainly can place a lot of information and pictures on Facebook, and potential patients can leave comments or ask questions easily. You can start a dialog with a patient without providing medical advice and motivate her to see that you are providing medical value before the doctor–patient relationship is established. Still, does a Facebook page generate new patients? It depends on the information you post and how you use Facebook to acquire new patients.
For instance, your practice is probably restricted to a local area—a few zip codes surrounding your office and hospital—which means you really only want patients who are in your area to visit your practice’s Facebook page because those are the only ones who are likely to call and make an appointment. Unless you are highly specialized in a particular field, such as fistula repair, robotic surgery, or the treatment of mesh complications, the Facebook surfer from New York isn’t likely to hop on a plane to come to your practice on the West Coast for gynecologic or obstetric care.
Related article: Four pillars of a successful practice: 2. Attract new patients Neal H. Baum, MD (Practice Management, May 2013)
On the surface, it appears that it is impossible to compete with larger practices and hospitals that have more dedicated staff to draw prospective patients to a practice through Facebook. However, the real, overarching challenge is to improve your Web site rankings on the major search engines, to be on the first page of Google, Bing, and Yahoo search results. And what we do know is that Google has placed a high value on Web site rankings through social media sites like Facebook, Twitter, and YouTube—that is, of course, as long as your Facebook page provides content that has keywords relevant to your target market and the content on your page links back to your Web site.
Therefore, it is not necessary to devote an inordinate amount of time to your social media presence to obtain results. You will, on the other hand, get more visitors to your Web site if it is found on the first page of search engine results because of your Facebook posts. Of course, if your Web site is not set up properly for easy visitor navigation and visitor conversion, you may not be able to obtain the desired result of gaining new patients even if they do find your site. You need to have a Web site with marketing and patient conversion systems built into it; don’t overlook the layout of your Web site. For more on this issue, see Part 1 of this series.
Related article: Using the Internet in your practice. Part 1: Why social media are important and how to get started Neal H. Baum, MD, and Ron Romano (Practice Management, February 2014)
YOUTUBE VIDEOS AND YOUR PRACTICE
YouTube has become a significant search engine for virtually every product and service you offer your patients. There are millions of videos on YouTube, and you can search topics simply by typing in any topic that your patients might be interested in, from birth control to cancer.
There are five ways your practice can benefit from a video posted on YouTube:
- Web site traffic driver. To achieve this “pull,” you must label your posted video correctly, with keyword phrases that are relevant to the type of patient or conditions you are looking for, and offer a description that would make a viewer want to see the video. You also must provide a link back to your Web site, which increases your chances of gaining a new patient from YouTube.
- Boost your search engine optimization. Google places a high-ranking factor on videos posted to YouTube that are keyword-relevant.
- A video library can position you as an expert in the field. You can create your own YouTube channel and keep adding videos. One of us (NHB) has more than
70 medical videos on his YouTube channel. If someone views one of these videos, they will have immediate access to the rest of the video collection even though they may be labelled with other keywords. This further positions you as the knowledgeable expert in your field. - Video embedding capability. Any video you have posted to YouTube can be placed on your Web site, in a format that keeps the viewer on your site. This means the viewer has less of a chance of getting distracted with other video offerings and landing on someone else’s Web site.
- Free video storage. Because you have stored the video on YouTube, you are not using the resources on your Web site when someone, or several people, view the video at the same time.
Getting started with YouTube
Making a video can be easier than you think. First, a video can simply be a PowerPoint presentation. Studies have demonstrated that it is more about the content of the video than a physician being in front of a camera. There are lots of Web sites you can use to record a presentation; one of the most popular and easy to use is http://www.GoToWebinar.com. There are computer programs that make it easy to record and then simply upload the recording to YouTube. Cam Studio (http://camstudio.org) is a free open-source program available that has a lot of flexibility for editing audio and video files, and it is easy to use. Camtasia (http://www.techsmith.com/camtasia.html) is a popular program that costs about $300 and has a lot of features for advanced editing. Camtasia also has a simple navigation system for the nontechnical person.
Content is key. You can select a few frequently asked questions (FAQs) that your patients regularly ask and simply record yourself giving the answers. Take a look at what is new, relevant, or controversial in regard to the procedures you perform. Or just look at all the pages on your Web site that have the procedures and services you provide and make a video on those topics. The ideal video is 3 to 5 minutes in length.
ATTRACTING PATIENTS VIA TWITTER
The most amazing example of social media and building a fan base is Twitter. Here’s a question: Who are the people that have the biggest following on Twitter? The answer: Celebrities, rock stars, and athletes. As a society, we are obsessed with these groups and want to know their every thought, what they like, what they had for lunch, what they think, and who they think about.
Now how, as a practicing ObGyn, do you expect to build a base of Web site surfers who want to know your every thought on urinary incontinence? The harsh reality is, if you think you are going to get new patients by making posts on Twitter of 140 characters or less every day, you will be disappointed.
However, the return from using Twitter is, similar to Facebook and YouTube, related to the fact that Twitter is one of the top accessed Web sites in the world. Linking your own content from such a Web site increases the search placement of your content when a potential patient performs a general Google search.
ARE SOCIAL MEDIA EFFECTIVE?
The effective use of social media can result in attracting new patients every day to your practice—if you post quality information on a regular basis that is helpful to your existing patients and especially to potential new patients. Overall, social media can help you get new patients through search engine rankings. Even if you don’t want to do any work on your social media sites, you can hire companies that will do it for you.
However, the return from using Twitter is, similar to Facebook and YouTube, related to the fact that Twitter is one of the top accessed Web sites in the world. Linking your own content from such a Web site increases the search placement of your content when a potential patient performs a general Google search.
The bottom line
There will be many ObGyns who will read this article, throw up their hands and say, “Makes sense, but this is over my head.” Because it sounds so technical, many clinicians will just ignore social media and hope it goes away. If your plans for the next 5 years include practicing medicine, we don’t recommend that you take that approach. The Internet and social media are the “places” in which patients of today are searching for their doctors. Trust us—potential new patients are no longer using the Yellow Pages.
The patients of tomorrow will be increasingly technologically sophisticated, and these social media techniques will continue to evolve. Don’t get left behind. And don’t let your competitors dominate one of the most important sources of new patients you have, along with patient referrals and physician referrals. Jump into this world yourself, and you will be richly rewarded. The social media train is leaving the station, and we hope that we have shown you how to hitch a ride. See you online!
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
With this article, we intend to illustrate the value of having a social media presence and how you can use social media to attract new patients. One of us (NHB) has been using social media to promote his medical practice for 3 years and can be found on the first page of Google search results for several of the medical conditions he treats. As a result of these high search rankings, he is able to generate two to four new patient visits every day.
You can achieve the same results using the techniques described in this article. You certainly can buy banner ads and buy traffic to your page, but we want to show you how to get on the first page of Google using the natural, organic method.
PUSH VS PULL
Social media can be used in different ways to build your practice. What you employ depends on what you want to accomplish and the time and energy you want to devote to each of these social media opportunities.
By its very definition, social media is social engagement—and what is known as a “pull” technology. There are two ways to share your information with people on the Internet:
- “Pull” Web site surfers to your information
- “Push” your information to them.
Push occurs when you initiate the process by placing your information in front of the Web site surfer. They get it or see it because of the actions you have taken. Sending e-mails is one way to push information to your target audience, or potential patients, to your practice. Another way to push your Web site and its contents is to get listed on the first page of search engine results. You want to “push” your Web site in plain view of the person who has typed in keywords or keyword phrases that relate to your practice (ie, “OBGYN” plus “<your city>,” “tubal ligation” plus “<your city>,” or “loss of urine” plus “<your zip code>.” Push techniques are the best way to market your services and offer the best return on your marketing investment.
Using social media, you are able to “pull” your audience of potential patients to you and your practice. In other words, your target market of potential patients has to take the time and make the effort to type in your Web site address in order to come to you. The information or message you have on your social media sites has to be strong enough and of sufficient compelling interest that patients want to come to read what you have to say. Web surfers are looking for online relationships for information sharing. It is this interaction with your potential patients that makes social media unique. Using this pull technology, you have the opportunity to interact and develop a relationship with a patient before she picks up the phone to make an appointment, before she comes to the office to see you eyeball to eyeball.
FACEBOOK AND HOW IT RELATES TO YOUR PRACTICE
Originally, Facebook was developed as a way for people to see what was going on in each other’s lives, a method to stay in contact with one another. In the beginning, it was friends, family members, or groups of like-minded individuals frequenting each other’s Facebook pages. Typically, they would keep tabs on who was having a party or post pictures of their kids for family members to see.
Facebook has evolved. Today, companies, businesses, and, yes, medical practices are trying to “pull” more Web site visitors to their Facebook pages. To do this, they hold contests with prizes; offer great content, coupons, and videos; and provide special offers to get Web surfers to their site. Large companies and large group practices like the Mayo Clinic, Cleveland Clinic, and MD Anderson Cancer Center, have whole social media departments that post regularly, respond to comments left on their pages, and answer questions posted by those who “like” their page or site.
Individual practicing clinicians, and most smaller ObGyn practices, do not have the budget for a social media team. They also don’t have the time or the training to write effective copy that is so compelling that Web surfers are drawn or “pulled” to their Facebook page. The reality is, your patients expect you to have a Facebook page, and they expect you to have quality information that is helpful and relevant to their well-being. But, the question remains…
Related article: Four pillars of a successful practice: 1. Keep your current patients happy Neal H. Baum, MD (Practice Management, March 2013)
Can Facebook generate new patients?
You and your practice certainly can place a lot of information and pictures on Facebook, and potential patients can leave comments or ask questions easily. You can start a dialog with a patient without providing medical advice and motivate her to see that you are providing medical value before the doctor–patient relationship is established. Still, does a Facebook page generate new patients? It depends on the information you post and how you use Facebook to acquire new patients.
For instance, your practice is probably restricted to a local area—a few zip codes surrounding your office and hospital—which means you really only want patients who are in your area to visit your practice’s Facebook page because those are the only ones who are likely to call and make an appointment. Unless you are highly specialized in a particular field, such as fistula repair, robotic surgery, or the treatment of mesh complications, the Facebook surfer from New York isn’t likely to hop on a plane to come to your practice on the West Coast for gynecologic or obstetric care.
Related article: Four pillars of a successful practice: 2. Attract new patients Neal H. Baum, MD (Practice Management, May 2013)
On the surface, it appears that it is impossible to compete with larger practices and hospitals that have more dedicated staff to draw prospective patients to a practice through Facebook. However, the real, overarching challenge is to improve your Web site rankings on the major search engines, to be on the first page of Google, Bing, and Yahoo search results. And what we do know is that Google has placed a high value on Web site rankings through social media sites like Facebook, Twitter, and YouTube—that is, of course, as long as your Facebook page provides content that has keywords relevant to your target market and the content on your page links back to your Web site.
Therefore, it is not necessary to devote an inordinate amount of time to your social media presence to obtain results. You will, on the other hand, get more visitors to your Web site if it is found on the first page of search engine results because of your Facebook posts. Of course, if your Web site is not set up properly for easy visitor navigation and visitor conversion, you may not be able to obtain the desired result of gaining new patients even if they do find your site. You need to have a Web site with marketing and patient conversion systems built into it; don’t overlook the layout of your Web site. For more on this issue, see Part 1 of this series.
Related article: Using the Internet in your practice. Part 1: Why social media are important and how to get started Neal H. Baum, MD, and Ron Romano (Practice Management, February 2014)
YOUTUBE VIDEOS AND YOUR PRACTICE
YouTube has become a significant search engine for virtually every product and service you offer your patients. There are millions of videos on YouTube, and you can search topics simply by typing in any topic that your patients might be interested in, from birth control to cancer.
There are five ways your practice can benefit from a video posted on YouTube:
- Web site traffic driver. To achieve this “pull,” you must label your posted video correctly, with keyword phrases that are relevant to the type of patient or conditions you are looking for, and offer a description that would make a viewer want to see the video. You also must provide a link back to your Web site, which increases your chances of gaining a new patient from YouTube.
- Boost your search engine optimization. Google places a high-ranking factor on videos posted to YouTube that are keyword-relevant.
- A video library can position you as an expert in the field. You can create your own YouTube channel and keep adding videos. One of us (NHB) has more than
70 medical videos on his YouTube channel. If someone views one of these videos, they will have immediate access to the rest of the video collection even though they may be labelled with other keywords. This further positions you as the knowledgeable expert in your field. - Video embedding capability. Any video you have posted to YouTube can be placed on your Web site, in a format that keeps the viewer on your site. This means the viewer has less of a chance of getting distracted with other video offerings and landing on someone else’s Web site.
- Free video storage. Because you have stored the video on YouTube, you are not using the resources on your Web site when someone, or several people, view the video at the same time.
Getting started with YouTube
Making a video can be easier than you think. First, a video can simply be a PowerPoint presentation. Studies have demonstrated that it is more about the content of the video than a physician being in front of a camera. There are lots of Web sites you can use to record a presentation; one of the most popular and easy to use is http://www.GoToWebinar.com. There are computer programs that make it easy to record and then simply upload the recording to YouTube. Cam Studio (http://camstudio.org) is a free open-source program available that has a lot of flexibility for editing audio and video files, and it is easy to use. Camtasia (http://www.techsmith.com/camtasia.html) is a popular program that costs about $300 and has a lot of features for advanced editing. Camtasia also has a simple navigation system for the nontechnical person.
Content is key. You can select a few frequently asked questions (FAQs) that your patients regularly ask and simply record yourself giving the answers. Take a look at what is new, relevant, or controversial in regard to the procedures you perform. Or just look at all the pages on your Web site that have the procedures and services you provide and make a video on those topics. The ideal video is 3 to 5 minutes in length.
ATTRACTING PATIENTS VIA TWITTER
The most amazing example of social media and building a fan base is Twitter. Here’s a question: Who are the people that have the biggest following on Twitter? The answer: Celebrities, rock stars, and athletes. As a society, we are obsessed with these groups and want to know their every thought, what they like, what they had for lunch, what they think, and who they think about.
Now how, as a practicing ObGyn, do you expect to build a base of Web site surfers who want to know your every thought on urinary incontinence? The harsh reality is, if you think you are going to get new patients by making posts on Twitter of 140 characters or less every day, you will be disappointed.
However, the return from using Twitter is, similar to Facebook and YouTube, related to the fact that Twitter is one of the top accessed Web sites in the world. Linking your own content from such a Web site increases the search placement of your content when a potential patient performs a general Google search.
ARE SOCIAL MEDIA EFFECTIVE?
The effective use of social media can result in attracting new patients every day to your practice—if you post quality information on a regular basis that is helpful to your existing patients and especially to potential new patients. Overall, social media can help you get new patients through search engine rankings. Even if you don’t want to do any work on your social media sites, you can hire companies that will do it for you.
However, the return from using Twitter is, similar to Facebook and YouTube, related to the fact that Twitter is one of the top accessed Web sites in the world. Linking your own content from such a Web site increases the search placement of your content when a potential patient performs a general Google search.
The bottom line
There will be many ObGyns who will read this article, throw up their hands and say, “Makes sense, but this is over my head.” Because it sounds so technical, many clinicians will just ignore social media and hope it goes away. If your plans for the next 5 years include practicing medicine, we don’t recommend that you take that approach. The Internet and social media are the “places” in which patients of today are searching for their doctors. Trust us—potential new patients are no longer using the Yellow Pages.
The patients of tomorrow will be increasingly technologically sophisticated, and these social media techniques will continue to evolve. Don’t get left behind. And don’t let your competitors dominate one of the most important sources of new patients you have, along with patient referrals and physician referrals. Jump into this world yourself, and you will be richly rewarded. The social media train is leaving the station, and we hope that we have shown you how to hitch a ride. See you online!
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
With this article, we intend to illustrate the value of having a social media presence and how you can use social media to attract new patients. One of us (NHB) has been using social media to promote his medical practice for 3 years and can be found on the first page of Google search results for several of the medical conditions he treats. As a result of these high search rankings, he is able to generate two to four new patient visits every day.
You can achieve the same results using the techniques described in this article. You certainly can buy banner ads and buy traffic to your page, but we want to show you how to get on the first page of Google using the natural, organic method.
PUSH VS PULL
Social media can be used in different ways to build your practice. What you employ depends on what you want to accomplish and the time and energy you want to devote to each of these social media opportunities.
By its very definition, social media is social engagement—and what is known as a “pull” technology. There are two ways to share your information with people on the Internet:
- “Pull” Web site surfers to your information
- “Push” your information to them.
Push occurs when you initiate the process by placing your information in front of the Web site surfer. They get it or see it because of the actions you have taken. Sending e-mails is one way to push information to your target audience, or potential patients, to your practice. Another way to push your Web site and its contents is to get listed on the first page of search engine results. You want to “push” your Web site in plain view of the person who has typed in keywords or keyword phrases that relate to your practice (ie, “OBGYN” plus “<your city>,” “tubal ligation” plus “<your city>,” or “loss of urine” plus “<your zip code>.” Push techniques are the best way to market your services and offer the best return on your marketing investment.
Using social media, you are able to “pull” your audience of potential patients to you and your practice. In other words, your target market of potential patients has to take the time and make the effort to type in your Web site address in order to come to you. The information or message you have on your social media sites has to be strong enough and of sufficient compelling interest that patients want to come to read what you have to say. Web surfers are looking for online relationships for information sharing. It is this interaction with your potential patients that makes social media unique. Using this pull technology, you have the opportunity to interact and develop a relationship with a patient before she picks up the phone to make an appointment, before she comes to the office to see you eyeball to eyeball.
FACEBOOK AND HOW IT RELATES TO YOUR PRACTICE
Originally, Facebook was developed as a way for people to see what was going on in each other’s lives, a method to stay in contact with one another. In the beginning, it was friends, family members, or groups of like-minded individuals frequenting each other’s Facebook pages. Typically, they would keep tabs on who was having a party or post pictures of their kids for family members to see.
Facebook has evolved. Today, companies, businesses, and, yes, medical practices are trying to “pull” more Web site visitors to their Facebook pages. To do this, they hold contests with prizes; offer great content, coupons, and videos; and provide special offers to get Web surfers to their site. Large companies and large group practices like the Mayo Clinic, Cleveland Clinic, and MD Anderson Cancer Center, have whole social media departments that post regularly, respond to comments left on their pages, and answer questions posted by those who “like” their page or site.
Individual practicing clinicians, and most smaller ObGyn practices, do not have the budget for a social media team. They also don’t have the time or the training to write effective copy that is so compelling that Web surfers are drawn or “pulled” to their Facebook page. The reality is, your patients expect you to have a Facebook page, and they expect you to have quality information that is helpful and relevant to their well-being. But, the question remains…
Related article: Four pillars of a successful practice: 1. Keep your current patients happy Neal H. Baum, MD (Practice Management, March 2013)
Can Facebook generate new patients?
You and your practice certainly can place a lot of information and pictures on Facebook, and potential patients can leave comments or ask questions easily. You can start a dialog with a patient without providing medical advice and motivate her to see that you are providing medical value before the doctor–patient relationship is established. Still, does a Facebook page generate new patients? It depends on the information you post and how you use Facebook to acquire new patients.
For instance, your practice is probably restricted to a local area—a few zip codes surrounding your office and hospital—which means you really only want patients who are in your area to visit your practice’s Facebook page because those are the only ones who are likely to call and make an appointment. Unless you are highly specialized in a particular field, such as fistula repair, robotic surgery, or the treatment of mesh complications, the Facebook surfer from New York isn’t likely to hop on a plane to come to your practice on the West Coast for gynecologic or obstetric care.
Related article: Four pillars of a successful practice: 2. Attract new patients Neal H. Baum, MD (Practice Management, May 2013)
On the surface, it appears that it is impossible to compete with larger practices and hospitals that have more dedicated staff to draw prospective patients to a practice through Facebook. However, the real, overarching challenge is to improve your Web site rankings on the major search engines, to be on the first page of Google, Bing, and Yahoo search results. And what we do know is that Google has placed a high value on Web site rankings through social media sites like Facebook, Twitter, and YouTube—that is, of course, as long as your Facebook page provides content that has keywords relevant to your target market and the content on your page links back to your Web site.
Therefore, it is not necessary to devote an inordinate amount of time to your social media presence to obtain results. You will, on the other hand, get more visitors to your Web site if it is found on the first page of search engine results because of your Facebook posts. Of course, if your Web site is not set up properly for easy visitor navigation and visitor conversion, you may not be able to obtain the desired result of gaining new patients even if they do find your site. You need to have a Web site with marketing and patient conversion systems built into it; don’t overlook the layout of your Web site. For more on this issue, see Part 1 of this series.
Related article: Using the Internet in your practice. Part 1: Why social media are important and how to get started Neal H. Baum, MD, and Ron Romano (Practice Management, February 2014)
YOUTUBE VIDEOS AND YOUR PRACTICE
YouTube has become a significant search engine for virtually every product and service you offer your patients. There are millions of videos on YouTube, and you can search topics simply by typing in any topic that your patients might be interested in, from birth control to cancer.
There are five ways your practice can benefit from a video posted on YouTube:
- Web site traffic driver. To achieve this “pull,” you must label your posted video correctly, with keyword phrases that are relevant to the type of patient or conditions you are looking for, and offer a description that would make a viewer want to see the video. You also must provide a link back to your Web site, which increases your chances of gaining a new patient from YouTube.
- Boost your search engine optimization. Google places a high-ranking factor on videos posted to YouTube that are keyword-relevant.
- A video library can position you as an expert in the field. You can create your own YouTube channel and keep adding videos. One of us (NHB) has more than
70 medical videos on his YouTube channel. If someone views one of these videos, they will have immediate access to the rest of the video collection even though they may be labelled with other keywords. This further positions you as the knowledgeable expert in your field. - Video embedding capability. Any video you have posted to YouTube can be placed on your Web site, in a format that keeps the viewer on your site. This means the viewer has less of a chance of getting distracted with other video offerings and landing on someone else’s Web site.
- Free video storage. Because you have stored the video on YouTube, you are not using the resources on your Web site when someone, or several people, view the video at the same time.
Getting started with YouTube
Making a video can be easier than you think. First, a video can simply be a PowerPoint presentation. Studies have demonstrated that it is more about the content of the video than a physician being in front of a camera. There are lots of Web sites you can use to record a presentation; one of the most popular and easy to use is http://www.GoToWebinar.com. There are computer programs that make it easy to record and then simply upload the recording to YouTube. Cam Studio (http://camstudio.org) is a free open-source program available that has a lot of flexibility for editing audio and video files, and it is easy to use. Camtasia (http://www.techsmith.com/camtasia.html) is a popular program that costs about $300 and has a lot of features for advanced editing. Camtasia also has a simple navigation system for the nontechnical person.
Content is key. You can select a few frequently asked questions (FAQs) that your patients regularly ask and simply record yourself giving the answers. Take a look at what is new, relevant, or controversial in regard to the procedures you perform. Or just look at all the pages on your Web site that have the procedures and services you provide and make a video on those topics. The ideal video is 3 to 5 minutes in length.
ATTRACTING PATIENTS VIA TWITTER
The most amazing example of social media and building a fan base is Twitter. Here’s a question: Who are the people that have the biggest following on Twitter? The answer: Celebrities, rock stars, and athletes. As a society, we are obsessed with these groups and want to know their every thought, what they like, what they had for lunch, what they think, and who they think about.
Now how, as a practicing ObGyn, do you expect to build a base of Web site surfers who want to know your every thought on urinary incontinence? The harsh reality is, if you think you are going to get new patients by making posts on Twitter of 140 characters or less every day, you will be disappointed.
However, the return from using Twitter is, similar to Facebook and YouTube, related to the fact that Twitter is one of the top accessed Web sites in the world. Linking your own content from such a Web site increases the search placement of your content when a potential patient performs a general Google search.
ARE SOCIAL MEDIA EFFECTIVE?
The effective use of social media can result in attracting new patients every day to your practice—if you post quality information on a regular basis that is helpful to your existing patients and especially to potential new patients. Overall, social media can help you get new patients through search engine rankings. Even if you don’t want to do any work on your social media sites, you can hire companies that will do it for you.
However, the return from using Twitter is, similar to Facebook and YouTube, related to the fact that Twitter is one of the top accessed Web sites in the world. Linking your own content from such a Web site increases the search placement of your content when a potential patient performs a general Google search.
The bottom line
There will be many ObGyns who will read this article, throw up their hands and say, “Makes sense, but this is over my head.” Because it sounds so technical, many clinicians will just ignore social media and hope it goes away. If your plans for the next 5 years include practicing medicine, we don’t recommend that you take that approach. The Internet and social media are the “places” in which patients of today are searching for their doctors. Trust us—potential new patients are no longer using the Yellow Pages.
The patients of tomorrow will be increasingly technologically sophisticated, and these social media techniques will continue to evolve. Don’t get left behind. And don’t let your competitors dominate one of the most important sources of new patients you have, along with patient referrals and physician referrals. Jump into this world yourself, and you will be richly rewarded. The social media train is leaving the station, and we hope that we have shown you how to hitch a ride. See you online!
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
THE SERIES: USING THE INTERNET IN YOUR PRACTICE
Part 1: Why social media are important and how to get started (February 2014)
Part 3: Search engine optimization
Part 4: Online reputation management
(Look for Parts 3 and 4 in 2014)
UTI, then massive hemorrhage
UTI, THEN MASSIVE HEMORRHAGE
A woman in her 60s was hospitalized with a urinary tract infection (UTI). She was treated with antibiotics and intravenous (IV) fluids but developed deep vein thrombosis (DVT) at the IV site. Enoxaparin sodium was ordered to treat the clot. After 3 days, she suffered a massive abdominal hemorrhage. When she woke from resuscitation, her weight had doubled. She developed a methicillin-resistant Staphylococcus aureus (MRSA) infection, then Clostridium difficile infection due to antibiotics, plus bedsores. Multiple surgeries left her with an abdominal wall defect that cannot be repaired, and a permanent hernia. She was hospitalized for 75 days.
PATIENT’S CLAIM The hemorrhage was caused when enoxaparin was given at 1.5 times the proper dosage because the patient’s weight was overestimated by 50%. Excessive blood, plasma, and fluids caused her weight to double after resuscitation. Her intestines were forced out of her abdominal cavity by the hemorrhage. A permanent hernia, visible underneath her skin, causes pain.
DEFENDANTS’ DEFENSE The patient’s preexisting diabetes, heart condition, high cholesterol levels, and orthopedic issues impacted her condition. She was not compliant in managing her diabetes, causing many of the current problems.
VERDICT A $9.3 million Connecticut verdict was returned.
Related article: Update: Pelvic floor dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
CESAREAN DELAYED UNNECESSARILY
At 37 weeks’ gestation, a mother reported decreased fetal movement. When the biophysical profile test scored 8/8 and the fetal heart rate was reassuring, the attending ObGyn discharged the patient. However, it was the middle of the night, and the nurse kept the mother in the emergency department (ED). At 8:30 am, the fetus began to show signs of fetal distress. Three ObGyns agreed to monitor labor, although one physician wanted delivery to occur that morning.
The next morning, a second biophysical profile scored 2/8, but the on-call ObGyn misunderstood the score as 6/8 and scheduled cesarean delivery for noon. Two hours after the second biophysical profile, the fetal heart rate crashed. A nurse called the ObGyn, who began an emergency cesarean 15 minutes later. The baby, born lifeless, was resuscitated. The child suffered permanent brain damage, and has cerebral palsy, severe cognitive deficits and speech deficits, and walks with an abnormal gait.
PARENTS’ CLAIM A physician did not see the patient for 24 hours, once the decision was made to monitor the mother, even though the fetal heart rate continued to decline. A biophysical profile test score of 2/8 indicates the need for immediate delivery. An earlier cesarean delivery could have reduced the child’s injuries.
DEFENDANTS’ DEFENSE After a settlement was reached with the hospital, the trial continued against the delivering ObGyn. He claimed that decreased fetal movement indicated that the brain injury had occurred 1 to 4 days before the mother came to the ED. The technician had manipulated the mother’s abdomen to wake the fetus before starting the first biophysical profile, which invalidated the score. The nurse miscommunicated the score of the second biophysical profile.
VERDICT A gross $29.8 million Illinois verdict was returned that included a $1.65 million settlement with the hospital.
WAS FACILITY ADEQUATELY STAFFED AFTER HURRICANE IKE?
A mother was admitted to a hospital for induction of labor in September 2008. After birth, the child was found to have cerebral palsy.
PARENTS’ CLAIM The mother should have been sent to another facility before delivery was induced because the hospital was short-staffed and low on resources due to Hurricane Ike. Too much oxytocin was used to induce contractions, which led to a lack of oxygen for the fetus. All prenatal testing had shown a healthy fetus. A cesarean delivery should have occurred when fetal distress was noted.
DEFENDANTS’ DEFENSE The mother had gastric bypass surgery 8 months before she became pregnant, and smoked during pregnancy, which accounted for the infant’s injuries. Treatment during labor and delivery was appropriate. Hospital staffing and resources were adequate.
VERDICT A $6.5 million Texas settlement was reached.
PLACENTA ACCRETA; MOTHER DIES
A 33-year-old woman became pregnant with her second child. A variety of conditions caused this to be high-risk pregnancy, so she saw a maternal-fetal medicine (MFM) specialist 2 months before delivery. The MFM reported that his examination and the ultrasonography (US) results were normal.
The ObGyn who provided prenatal care and delivered her first child scheduled cesarean delivery. During the procedure, the ObGyn noticed a 3- to 4-inch lesion where the placenta had penetrated the uterus. When the placenta was removed, the patient began to hemorrhage and a hysterectomy was performed. The hemorrhage created blood clots that led to gangrene in the patient’s extremities. She died 5 days after giving birth.
ESTATE’S CLAIM Both the MFM and the ObGyn failed to recognize placenta accreta on US prior to delivery. The ObGyn should have performed US prior to beginning cesarean delivery. The hospital’s protocols were not followed: the ObGyn should have stopped the procedure and called for extra surgical assistance and additional blood when he encountered placenta accreta, and again when the patient began to hemorrhage. Placenta accreta does not have to be fatal if detected and managed properly.
DEFENDANTS’ DEFENSE There was no negligence; the patient was treated properly.
VERDICT A $15.5 million Illinois verdict was returned against both physicians and the medical center.
Related article: Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence, December 2013)
ANTICONVULSANT AND MIGRAINE MEDS TAKEN DURING PREGNANCY
A woman was prescribed topiramate (Topamax) for migraine headaches and hand tremors during the first trimester of her pregnancy in 2007. With a history of seizures, she also took several anticonvulsants throughout her pregnancy. Her child was diagnosed with right unilateral cleft lip (cheiloschisis) in utero. The condition had not been surgically corrected at the time of trial.
PARENTS’ CLAIM The use of topiramate caused the child’s cleft lip. Janssen Pharmaceuticals, the manufacturer of Topamax, knew about the risk of birth defects associated with the drug in 2007, but failed to provide adequate warnings.
DEFENDANTS’ DEFENSE The mother received at least two warnings from her physician regarding the potential risks of anticonvulsant and antiepileptic drugs and the importance of not becoming pregnant while taking the medications. An action against the physician was barred by the applicable statute of limitations. The mother had taken topiramate prescribed to her mother for a time; such actions should release Janssen from liability.
VERDICT A $11 million Pennsylvania verdict was returned.
PID MASKS ECTOPIC PREGNANCY
A woman in her 40s became pregnant. On the first two prenatal diagnostic imaging studies, the ObGyn saw an intrauterine pregnancy. He later realized that the pregnancy was ectopic after beta human chorionic gonadotrophin (beta-hCG) blood levels were abnormal. During surgery to terminate the pregnancy, he found he had to perform a total hysterectomy because the patient had extensive pelvic inflammatory disease (PID) caused by a long history of sexually transmitted disease.
PATIENT’S CLAIM If the ectopic pregnancy had been diagnosed earlier, one of her ovaries could have been preserved, saving her from the symptoms of surgical menopause.
PHYSICIAN’S DEFENSE PID had caused the ovaries, numerous fibroid tumors, and the uterus to fuse into one mass. That was why the first two imaging studies appeared to show an intrauterine pregnancy. It was not possible to diagnose the extent of the problem until surgery. The patient did not have a true ectopic pregnancy.
The patient’s difficulties occurred during a 2-week time period in which she had one visit with him and another visit to an ED where two other physicians examined her and missed the diagnosis.
VERDICT A Michigan defense verdict was returned.
ILIAC ARTERY INJURED DURING LAPAROSCOPIC SURGERY; PATIENT DIES
A 40-year-old woman underwent laparoscopic gynecologic surgery performed by her ObGyn. During the procedure, the patient’s left internal iliac artery was punctured, but the injury was not recognized at the time. She was discharged the same day. The next morning, she went into hypovolemic shock due to internal bleeding. She was taken to the ED, where she died.
ESTATE’S CLAIM The ObGyn, anesthesiologist, and hospital staff were negligent in their postoperative care. The anesthesiologist prescribed pain medication that masked the injury; the patient was discharged from the postanesthesia unit too early and without proper examination. The nursing staff did not react to the patient’s reports of abdominal pain, nor did they properly assess her condition prior to discharge. The ObGyn failed to return a phone call the evening after the procedure.
DEFENDANTS’ DEFENSE The ObGyn settled before trial. The anesthesiologist and hospital denied negligence: care was proper and followed all protocols.
VERDICT A confidential California settlement was reached with the ObGyn. A defense verdict was returned for the anesthesiologist and hospital.
Related article: Anatomy for the laparoscopic surgeon Emad Mikhail, MD; Lauren Scott, MD; Stuart Hart, MD, MS (April 2014)
GENETIC TESTING MISSED A KEY DIAGNOSIS
A 40-year-old woman underwent genetic testing after she became pregnant. She was assured that there were no abnormalities that would impact her child.
The baby was born with Wolf-Hirschhorn syndrome, characterized by facial deformities, intellectual disabilities, delayed growth, and seizures. The child is nonverbal, deaf, and blind. She uses a feeding tube and requires 24-hour care.
PARENTS’ CLAIM The genetic testing was improperly conducted. The mother would have had an abortion if she’d known that the child was so disabled.
DEFENDANTS’ DEFENSE Settlements were mediated.
VERDICT A $6.15 million New Jersey settlement was reached on behalf of the hospital and two laboratory technicians, and a $1 million settlement was reached with the director of the genetic laboratory.
HEAT INJURY TO COLON: ABSCESSES, PERITONITIS
A 43-year-old patient had a history of symptomatic uterine fibroids and infertility. Her ObGyn performed a hysteroscopy because he suspected endometriosis, but found none. He then successfully removed a large uterine fibroid during laparoscopic myomectomy. The patient was discharged the same day.
Two days later, the patient developed abdominal pain, nausea, and fever. She went to the ED and was taken into emergency surgery after a CT scan showed free air and fluid in her abdomen. She suffered multiple abscesses and peritonitis.
PATIENT’S CLAIM The ObGyn was negligent in performing the surgery: the sigmoid colon sustained a thermal injury, which caused the abscesses and peritonitis.
PHYSICIAN’S DEFENSE There was no evidence of thermal injury during the original operation; heat damage can and does occur in the absence of negligence. The patient’s previously unknown diverticulitis contributed to the development of the recurrent abscesses and peritonitis.
VERDICT A Florida defense verdict was returned.
RUPTURED UTERUS IS UNDETECTED
During labor and delivery, a declining fetal heart rate was observed, but there was an hour’s delay before cesarean delivery was started. The child suffered a hypoxic brain injury. He has spastic quadriplegia, cannot speak, and requires a respirator and feeding tube.
PARENTS’ CLAIM The mother suffered a ruptured uterus during labor that was not recognized by the ObGyn or nursing staff.
DEFENDANTS’ DEFENSE A settlement was reached during trial.
VERDICT A $7.5 million New Jersey settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
UTI, THEN MASSIVE HEMORRHAGE
A woman in her 60s was hospitalized with a urinary tract infection (UTI). She was treated with antibiotics and intravenous (IV) fluids but developed deep vein thrombosis (DVT) at the IV site. Enoxaparin sodium was ordered to treat the clot. After 3 days, she suffered a massive abdominal hemorrhage. When she woke from resuscitation, her weight had doubled. She developed a methicillin-resistant Staphylococcus aureus (MRSA) infection, then Clostridium difficile infection due to antibiotics, plus bedsores. Multiple surgeries left her with an abdominal wall defect that cannot be repaired, and a permanent hernia. She was hospitalized for 75 days.
PATIENT’S CLAIM The hemorrhage was caused when enoxaparin was given at 1.5 times the proper dosage because the patient’s weight was overestimated by 50%. Excessive blood, plasma, and fluids caused her weight to double after resuscitation. Her intestines were forced out of her abdominal cavity by the hemorrhage. A permanent hernia, visible underneath her skin, causes pain.
DEFENDANTS’ DEFENSE The patient’s preexisting diabetes, heart condition, high cholesterol levels, and orthopedic issues impacted her condition. She was not compliant in managing her diabetes, causing many of the current problems.
VERDICT A $9.3 million Connecticut verdict was returned.
Related article: Update: Pelvic floor dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
CESAREAN DELAYED UNNECESSARILY
At 37 weeks’ gestation, a mother reported decreased fetal movement. When the biophysical profile test scored 8/8 and the fetal heart rate was reassuring, the attending ObGyn discharged the patient. However, it was the middle of the night, and the nurse kept the mother in the emergency department (ED). At 8:30 am, the fetus began to show signs of fetal distress. Three ObGyns agreed to monitor labor, although one physician wanted delivery to occur that morning.
The next morning, a second biophysical profile scored 2/8, but the on-call ObGyn misunderstood the score as 6/8 and scheduled cesarean delivery for noon. Two hours after the second biophysical profile, the fetal heart rate crashed. A nurse called the ObGyn, who began an emergency cesarean 15 minutes later. The baby, born lifeless, was resuscitated. The child suffered permanent brain damage, and has cerebral palsy, severe cognitive deficits and speech deficits, and walks with an abnormal gait.
PARENTS’ CLAIM A physician did not see the patient for 24 hours, once the decision was made to monitor the mother, even though the fetal heart rate continued to decline. A biophysical profile test score of 2/8 indicates the need for immediate delivery. An earlier cesarean delivery could have reduced the child’s injuries.
DEFENDANTS’ DEFENSE After a settlement was reached with the hospital, the trial continued against the delivering ObGyn. He claimed that decreased fetal movement indicated that the brain injury had occurred 1 to 4 days before the mother came to the ED. The technician had manipulated the mother’s abdomen to wake the fetus before starting the first biophysical profile, which invalidated the score. The nurse miscommunicated the score of the second biophysical profile.
VERDICT A gross $29.8 million Illinois verdict was returned that included a $1.65 million settlement with the hospital.
WAS FACILITY ADEQUATELY STAFFED AFTER HURRICANE IKE?
A mother was admitted to a hospital for induction of labor in September 2008. After birth, the child was found to have cerebral palsy.
PARENTS’ CLAIM The mother should have been sent to another facility before delivery was induced because the hospital was short-staffed and low on resources due to Hurricane Ike. Too much oxytocin was used to induce contractions, which led to a lack of oxygen for the fetus. All prenatal testing had shown a healthy fetus. A cesarean delivery should have occurred when fetal distress was noted.
DEFENDANTS’ DEFENSE The mother had gastric bypass surgery 8 months before she became pregnant, and smoked during pregnancy, which accounted for the infant’s injuries. Treatment during labor and delivery was appropriate. Hospital staffing and resources were adequate.
VERDICT A $6.5 million Texas settlement was reached.
PLACENTA ACCRETA; MOTHER DIES
A 33-year-old woman became pregnant with her second child. A variety of conditions caused this to be high-risk pregnancy, so she saw a maternal-fetal medicine (MFM) specialist 2 months before delivery. The MFM reported that his examination and the ultrasonography (US) results were normal.
The ObGyn who provided prenatal care and delivered her first child scheduled cesarean delivery. During the procedure, the ObGyn noticed a 3- to 4-inch lesion where the placenta had penetrated the uterus. When the placenta was removed, the patient began to hemorrhage and a hysterectomy was performed. The hemorrhage created blood clots that led to gangrene in the patient’s extremities. She died 5 days after giving birth.
ESTATE’S CLAIM Both the MFM and the ObGyn failed to recognize placenta accreta on US prior to delivery. The ObGyn should have performed US prior to beginning cesarean delivery. The hospital’s protocols were not followed: the ObGyn should have stopped the procedure and called for extra surgical assistance and additional blood when he encountered placenta accreta, and again when the patient began to hemorrhage. Placenta accreta does not have to be fatal if detected and managed properly.
DEFENDANTS’ DEFENSE There was no negligence; the patient was treated properly.
VERDICT A $15.5 million Illinois verdict was returned against both physicians and the medical center.
Related article: Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence, December 2013)
ANTICONVULSANT AND MIGRAINE MEDS TAKEN DURING PREGNANCY
A woman was prescribed topiramate (Topamax) for migraine headaches and hand tremors during the first trimester of her pregnancy in 2007. With a history of seizures, she also took several anticonvulsants throughout her pregnancy. Her child was diagnosed with right unilateral cleft lip (cheiloschisis) in utero. The condition had not been surgically corrected at the time of trial.
PARENTS’ CLAIM The use of topiramate caused the child’s cleft lip. Janssen Pharmaceuticals, the manufacturer of Topamax, knew about the risk of birth defects associated with the drug in 2007, but failed to provide adequate warnings.
DEFENDANTS’ DEFENSE The mother received at least two warnings from her physician regarding the potential risks of anticonvulsant and antiepileptic drugs and the importance of not becoming pregnant while taking the medications. An action against the physician was barred by the applicable statute of limitations. The mother had taken topiramate prescribed to her mother for a time; such actions should release Janssen from liability.
VERDICT A $11 million Pennsylvania verdict was returned.
PID MASKS ECTOPIC PREGNANCY
A woman in her 40s became pregnant. On the first two prenatal diagnostic imaging studies, the ObGyn saw an intrauterine pregnancy. He later realized that the pregnancy was ectopic after beta human chorionic gonadotrophin (beta-hCG) blood levels were abnormal. During surgery to terminate the pregnancy, he found he had to perform a total hysterectomy because the patient had extensive pelvic inflammatory disease (PID) caused by a long history of sexually transmitted disease.
PATIENT’S CLAIM If the ectopic pregnancy had been diagnosed earlier, one of her ovaries could have been preserved, saving her from the symptoms of surgical menopause.
PHYSICIAN’S DEFENSE PID had caused the ovaries, numerous fibroid tumors, and the uterus to fuse into one mass. That was why the first two imaging studies appeared to show an intrauterine pregnancy. It was not possible to diagnose the extent of the problem until surgery. The patient did not have a true ectopic pregnancy.
The patient’s difficulties occurred during a 2-week time period in which she had one visit with him and another visit to an ED where two other physicians examined her and missed the diagnosis.
VERDICT A Michigan defense verdict was returned.
ILIAC ARTERY INJURED DURING LAPAROSCOPIC SURGERY; PATIENT DIES
A 40-year-old woman underwent laparoscopic gynecologic surgery performed by her ObGyn. During the procedure, the patient’s left internal iliac artery was punctured, but the injury was not recognized at the time. She was discharged the same day. The next morning, she went into hypovolemic shock due to internal bleeding. She was taken to the ED, where she died.
ESTATE’S CLAIM The ObGyn, anesthesiologist, and hospital staff were negligent in their postoperative care. The anesthesiologist prescribed pain medication that masked the injury; the patient was discharged from the postanesthesia unit too early and without proper examination. The nursing staff did not react to the patient’s reports of abdominal pain, nor did they properly assess her condition prior to discharge. The ObGyn failed to return a phone call the evening after the procedure.
DEFENDANTS’ DEFENSE The ObGyn settled before trial. The anesthesiologist and hospital denied negligence: care was proper and followed all protocols.
VERDICT A confidential California settlement was reached with the ObGyn. A defense verdict was returned for the anesthesiologist and hospital.
Related article: Anatomy for the laparoscopic surgeon Emad Mikhail, MD; Lauren Scott, MD; Stuart Hart, MD, MS (April 2014)
GENETIC TESTING MISSED A KEY DIAGNOSIS
A 40-year-old woman underwent genetic testing after she became pregnant. She was assured that there were no abnormalities that would impact her child.
The baby was born with Wolf-Hirschhorn syndrome, characterized by facial deformities, intellectual disabilities, delayed growth, and seizures. The child is nonverbal, deaf, and blind. She uses a feeding tube and requires 24-hour care.
PARENTS’ CLAIM The genetic testing was improperly conducted. The mother would have had an abortion if she’d known that the child was so disabled.
DEFENDANTS’ DEFENSE Settlements were mediated.
VERDICT A $6.15 million New Jersey settlement was reached on behalf of the hospital and two laboratory technicians, and a $1 million settlement was reached with the director of the genetic laboratory.
HEAT INJURY TO COLON: ABSCESSES, PERITONITIS
A 43-year-old patient had a history of symptomatic uterine fibroids and infertility. Her ObGyn performed a hysteroscopy because he suspected endometriosis, but found none. He then successfully removed a large uterine fibroid during laparoscopic myomectomy. The patient was discharged the same day.
Two days later, the patient developed abdominal pain, nausea, and fever. She went to the ED and was taken into emergency surgery after a CT scan showed free air and fluid in her abdomen. She suffered multiple abscesses and peritonitis.
PATIENT’S CLAIM The ObGyn was negligent in performing the surgery: the sigmoid colon sustained a thermal injury, which caused the abscesses and peritonitis.
PHYSICIAN’S DEFENSE There was no evidence of thermal injury during the original operation; heat damage can and does occur in the absence of negligence. The patient’s previously unknown diverticulitis contributed to the development of the recurrent abscesses and peritonitis.
VERDICT A Florida defense verdict was returned.
RUPTURED UTERUS IS UNDETECTED
During labor and delivery, a declining fetal heart rate was observed, but there was an hour’s delay before cesarean delivery was started. The child suffered a hypoxic brain injury. He has spastic quadriplegia, cannot speak, and requires a respirator and feeding tube.
PARENTS’ CLAIM The mother suffered a ruptured uterus during labor that was not recognized by the ObGyn or nursing staff.
DEFENDANTS’ DEFENSE A settlement was reached during trial.
VERDICT A $7.5 million New Jersey settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
UTI, THEN MASSIVE HEMORRHAGE
A woman in her 60s was hospitalized with a urinary tract infection (UTI). She was treated with antibiotics and intravenous (IV) fluids but developed deep vein thrombosis (DVT) at the IV site. Enoxaparin sodium was ordered to treat the clot. After 3 days, she suffered a massive abdominal hemorrhage. When she woke from resuscitation, her weight had doubled. She developed a methicillin-resistant Staphylococcus aureus (MRSA) infection, then Clostridium difficile infection due to antibiotics, plus bedsores. Multiple surgeries left her with an abdominal wall defect that cannot be repaired, and a permanent hernia. She was hospitalized for 75 days.
PATIENT’S CLAIM The hemorrhage was caused when enoxaparin was given at 1.5 times the proper dosage because the patient’s weight was overestimated by 50%. Excessive blood, plasma, and fluids caused her weight to double after resuscitation. Her intestines were forced out of her abdominal cavity by the hemorrhage. A permanent hernia, visible underneath her skin, causes pain.
DEFENDANTS’ DEFENSE The patient’s preexisting diabetes, heart condition, high cholesterol levels, and orthopedic issues impacted her condition. She was not compliant in managing her diabetes, causing many of the current problems.
VERDICT A $9.3 million Connecticut verdict was returned.
Related article: Update: Pelvic floor dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
CESAREAN DELAYED UNNECESSARILY
At 37 weeks’ gestation, a mother reported decreased fetal movement. When the biophysical profile test scored 8/8 and the fetal heart rate was reassuring, the attending ObGyn discharged the patient. However, it was the middle of the night, and the nurse kept the mother in the emergency department (ED). At 8:30 am, the fetus began to show signs of fetal distress. Three ObGyns agreed to monitor labor, although one physician wanted delivery to occur that morning.
The next morning, a second biophysical profile scored 2/8, but the on-call ObGyn misunderstood the score as 6/8 and scheduled cesarean delivery for noon. Two hours after the second biophysical profile, the fetal heart rate crashed. A nurse called the ObGyn, who began an emergency cesarean 15 minutes later. The baby, born lifeless, was resuscitated. The child suffered permanent brain damage, and has cerebral palsy, severe cognitive deficits and speech deficits, and walks with an abnormal gait.
PARENTS’ CLAIM A physician did not see the patient for 24 hours, once the decision was made to monitor the mother, even though the fetal heart rate continued to decline. A biophysical profile test score of 2/8 indicates the need for immediate delivery. An earlier cesarean delivery could have reduced the child’s injuries.
DEFENDANTS’ DEFENSE After a settlement was reached with the hospital, the trial continued against the delivering ObGyn. He claimed that decreased fetal movement indicated that the brain injury had occurred 1 to 4 days before the mother came to the ED. The technician had manipulated the mother’s abdomen to wake the fetus before starting the first biophysical profile, which invalidated the score. The nurse miscommunicated the score of the second biophysical profile.
VERDICT A gross $29.8 million Illinois verdict was returned that included a $1.65 million settlement with the hospital.
WAS FACILITY ADEQUATELY STAFFED AFTER HURRICANE IKE?
A mother was admitted to a hospital for induction of labor in September 2008. After birth, the child was found to have cerebral palsy.
PARENTS’ CLAIM The mother should have been sent to another facility before delivery was induced because the hospital was short-staffed and low on resources due to Hurricane Ike. Too much oxytocin was used to induce contractions, which led to a lack of oxygen for the fetus. All prenatal testing had shown a healthy fetus. A cesarean delivery should have occurred when fetal distress was noted.
DEFENDANTS’ DEFENSE The mother had gastric bypass surgery 8 months before she became pregnant, and smoked during pregnancy, which accounted for the infant’s injuries. Treatment during labor and delivery was appropriate. Hospital staffing and resources were adequate.
VERDICT A $6.5 million Texas settlement was reached.
PLACENTA ACCRETA; MOTHER DIES
A 33-year-old woman became pregnant with her second child. A variety of conditions caused this to be high-risk pregnancy, so she saw a maternal-fetal medicine (MFM) specialist 2 months before delivery. The MFM reported that his examination and the ultrasonography (US) results were normal.
The ObGyn who provided prenatal care and delivered her first child scheduled cesarean delivery. During the procedure, the ObGyn noticed a 3- to 4-inch lesion where the placenta had penetrated the uterus. When the placenta was removed, the patient began to hemorrhage and a hysterectomy was performed. The hemorrhage created blood clots that led to gangrene in the patient’s extremities. She died 5 days after giving birth.
ESTATE’S CLAIM Both the MFM and the ObGyn failed to recognize placenta accreta on US prior to delivery. The ObGyn should have performed US prior to beginning cesarean delivery. The hospital’s protocols were not followed: the ObGyn should have stopped the procedure and called for extra surgical assistance and additional blood when he encountered placenta accreta, and again when the patient began to hemorrhage. Placenta accreta does not have to be fatal if detected and managed properly.
DEFENDANTS’ DEFENSE There was no negligence; the patient was treated properly.
VERDICT A $15.5 million Illinois verdict was returned against both physicians and the medical center.
Related article: Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence, December 2013)
ANTICONVULSANT AND MIGRAINE MEDS TAKEN DURING PREGNANCY
A woman was prescribed topiramate (Topamax) for migraine headaches and hand tremors during the first trimester of her pregnancy in 2007. With a history of seizures, she also took several anticonvulsants throughout her pregnancy. Her child was diagnosed with right unilateral cleft lip (cheiloschisis) in utero. The condition had not been surgically corrected at the time of trial.
PARENTS’ CLAIM The use of topiramate caused the child’s cleft lip. Janssen Pharmaceuticals, the manufacturer of Topamax, knew about the risk of birth defects associated with the drug in 2007, but failed to provide adequate warnings.
DEFENDANTS’ DEFENSE The mother received at least two warnings from her physician regarding the potential risks of anticonvulsant and antiepileptic drugs and the importance of not becoming pregnant while taking the medications. An action against the physician was barred by the applicable statute of limitations. The mother had taken topiramate prescribed to her mother for a time; such actions should release Janssen from liability.
VERDICT A $11 million Pennsylvania verdict was returned.
PID MASKS ECTOPIC PREGNANCY
A woman in her 40s became pregnant. On the first two prenatal diagnostic imaging studies, the ObGyn saw an intrauterine pregnancy. He later realized that the pregnancy was ectopic after beta human chorionic gonadotrophin (beta-hCG) blood levels were abnormal. During surgery to terminate the pregnancy, he found he had to perform a total hysterectomy because the patient had extensive pelvic inflammatory disease (PID) caused by a long history of sexually transmitted disease.
PATIENT’S CLAIM If the ectopic pregnancy had been diagnosed earlier, one of her ovaries could have been preserved, saving her from the symptoms of surgical menopause.
PHYSICIAN’S DEFENSE PID had caused the ovaries, numerous fibroid tumors, and the uterus to fuse into one mass. That was why the first two imaging studies appeared to show an intrauterine pregnancy. It was not possible to diagnose the extent of the problem until surgery. The patient did not have a true ectopic pregnancy.
The patient’s difficulties occurred during a 2-week time period in which she had one visit with him and another visit to an ED where two other physicians examined her and missed the diagnosis.
VERDICT A Michigan defense verdict was returned.
ILIAC ARTERY INJURED DURING LAPAROSCOPIC SURGERY; PATIENT DIES
A 40-year-old woman underwent laparoscopic gynecologic surgery performed by her ObGyn. During the procedure, the patient’s left internal iliac artery was punctured, but the injury was not recognized at the time. She was discharged the same day. The next morning, she went into hypovolemic shock due to internal bleeding. She was taken to the ED, where she died.
ESTATE’S CLAIM The ObGyn, anesthesiologist, and hospital staff were negligent in their postoperative care. The anesthesiologist prescribed pain medication that masked the injury; the patient was discharged from the postanesthesia unit too early and without proper examination. The nursing staff did not react to the patient’s reports of abdominal pain, nor did they properly assess her condition prior to discharge. The ObGyn failed to return a phone call the evening after the procedure.
DEFENDANTS’ DEFENSE The ObGyn settled before trial. The anesthesiologist and hospital denied negligence: care was proper and followed all protocols.
VERDICT A confidential California settlement was reached with the ObGyn. A defense verdict was returned for the anesthesiologist and hospital.
Related article: Anatomy for the laparoscopic surgeon Emad Mikhail, MD; Lauren Scott, MD; Stuart Hart, MD, MS (April 2014)
GENETIC TESTING MISSED A KEY DIAGNOSIS
A 40-year-old woman underwent genetic testing after she became pregnant. She was assured that there were no abnormalities that would impact her child.
The baby was born with Wolf-Hirschhorn syndrome, characterized by facial deformities, intellectual disabilities, delayed growth, and seizures. The child is nonverbal, deaf, and blind. She uses a feeding tube and requires 24-hour care.
PARENTS’ CLAIM The genetic testing was improperly conducted. The mother would have had an abortion if she’d known that the child was so disabled.
DEFENDANTS’ DEFENSE Settlements were mediated.
VERDICT A $6.15 million New Jersey settlement was reached on behalf of the hospital and two laboratory technicians, and a $1 million settlement was reached with the director of the genetic laboratory.
HEAT INJURY TO COLON: ABSCESSES, PERITONITIS
A 43-year-old patient had a history of symptomatic uterine fibroids and infertility. Her ObGyn performed a hysteroscopy because he suspected endometriosis, but found none. He then successfully removed a large uterine fibroid during laparoscopic myomectomy. The patient was discharged the same day.
Two days later, the patient developed abdominal pain, nausea, and fever. She went to the ED and was taken into emergency surgery after a CT scan showed free air and fluid in her abdomen. She suffered multiple abscesses and peritonitis.
PATIENT’S CLAIM The ObGyn was negligent in performing the surgery: the sigmoid colon sustained a thermal injury, which caused the abscesses and peritonitis.
PHYSICIAN’S DEFENSE There was no evidence of thermal injury during the original operation; heat damage can and does occur in the absence of negligence. The patient’s previously unknown diverticulitis contributed to the development of the recurrent abscesses and peritonitis.
VERDICT A Florida defense verdict was returned.
RUPTURED UTERUS IS UNDETECTED
During labor and delivery, a declining fetal heart rate was observed, but there was an hour’s delay before cesarean delivery was started. The child suffered a hypoxic brain injury. He has spastic quadriplegia, cannot speak, and requires a respirator and feeding tube.
PARENTS’ CLAIM The mother suffered a ruptured uterus during labor that was not recognized by the ObGyn or nursing staff.
DEFENDANTS’ DEFENSE A settlement was reached during trial.
VERDICT A $7.5 million New Jersey settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
Current therapeutic options in hairy cell leukemia
Hairy cell leukemia (HCL) is a B-cell chronic lymphoproliferative disorder that was initially described as leukemic reticuloendotheliosis. The disease is characterized by monocytopenia, organomegaly, constitutional symptoms, and bone marrow fibrosis. Significant advances have improved the diagnosis and management of HCL over the last 55 years. Although HCL has an indolent course, most patients will require treatment of the disease. Indications to initiate therapy include disease-related symptoms, signs of bone marrow failure, or frequent infections. Asymptomatic patients without cytopenias can be observed without the need for therapeutic interventions. Therapeutic options usually consist of chemotherapy, immunotherapy, biological agents, and surgery.
Click on the PDF icon at the top of this introduction to read the full article.
Hairy cell leukemia (HCL) is a B-cell chronic lymphoproliferative disorder that was initially described as leukemic reticuloendotheliosis. The disease is characterized by monocytopenia, organomegaly, constitutional symptoms, and bone marrow fibrosis. Significant advances have improved the diagnosis and management of HCL over the last 55 years. Although HCL has an indolent course, most patients will require treatment of the disease. Indications to initiate therapy include disease-related symptoms, signs of bone marrow failure, or frequent infections. Asymptomatic patients without cytopenias can be observed without the need for therapeutic interventions. Therapeutic options usually consist of chemotherapy, immunotherapy, biological agents, and surgery.
Click on the PDF icon at the top of this introduction to read the full article.
Hairy cell leukemia (HCL) is a B-cell chronic lymphoproliferative disorder that was initially described as leukemic reticuloendotheliosis. The disease is characterized by monocytopenia, organomegaly, constitutional symptoms, and bone marrow fibrosis. Significant advances have improved the diagnosis and management of HCL over the last 55 years. Although HCL has an indolent course, most patients will require treatment of the disease. Indications to initiate therapy include disease-related symptoms, signs of bone marrow failure, or frequent infections. Asymptomatic patients without cytopenias can be observed without the need for therapeutic interventions. Therapeutic options usually consist of chemotherapy, immunotherapy, biological agents, and surgery.
Click on the PDF icon at the top of this introduction to read the full article.
Bicytopenia: Adverse effect of risperidone
Hematologic abnormalities, such as leukopenia, agranulocytosis, and thrombocytopenia, can be life-threatening adverse reactions to atypical antipsychotics. Although clozapine has the highest risk of leukopenia and neutropenia, these side effects also have been associated with other atypical antipsychotics, including risperidone, olanzapine, ziprasidone, paliperdione, and quetiapine. Risperdone-induced leukopenia has been reported,1,2 but risperidone-
induced bicytopenia— that is, leukopenia/ thrombocytopenia—is rare.
Case
Mr. A, age 25, is an African American man admitted to an inpatient psychiatric unit for management of acute psychotic symptoms. He has been taking risperidone, 4 mg/d, for the past 6 months, although his adherence to the regimen is questionable. Baseline blood count shows a white blood cell (WBC) count of 4,400/μL with an absolute neutrophil count (ANC) of 1,900/μL and a platelet count
160×103/μL. A few days after restarting risperidone, repeat blood count shows a drop in the WBC count to 2,900/μL, with an ANC of 900/μL and a platelet count of 130×103/μL.
Mr. A’s physical examination is normal, he does not have any signs or symptoms of infection, and additional lab tests are negative. Risperidone is considered as a possible cause of bicytopenia and is discontinued. Mr. A agrees to start treatment with aripiprazole, 10 mg/d. In next 10 days, the WBC count increases to 6,000/μL. The ANC at 3,100/μL and platelets at 150×103/μL remain stable throughout hospitalization. The slowly increasing WBC count after stopping risperidone is highly suggestive that this agent caused Mr. A’s bicytopenia.
Differential diagnosis
Bone-marrow suppression is associated with first- and second-generation antipsychotics. Blood dyscrasia is a concern in clinical psychiatry because hematologic abnormalities can be life-threatening, requiring close monitoring of the blood count for patients taking an antipsychotic. It is important, therefore, to consider medication side effects in the differential diagnosis of >1 hematologic abnormalities in these patients.
Precise pathophysiologic understanding of the hematologic side effects of antipsychotics is lacking, although different mechanisms of action have been proposed.3 Possible mechanisms when a patient is taking clozapine or olanzapine include:
• direct toxic effect of the drug on bone marrow
• increased peripheral destruction
• oxidative stress induced by unstable metabolites.
There is not enough evidence, however, to identify risperidone’s mechanism of action on blood cells.
Aripiprazole might be a useful alternative when another antipsychotic causes leukopenia and neutropenia. In addition to regularly monitoring the blood cell count during antipsychotic treatment, the neutrophil and platelet counts should be monitored.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Manfredi G, Solfanelli A, Dimitri G, et al. Risperidone-induced leukopenia: a case report and brief review of literature. Gen Hosp Psychiatry. 2013;35(1):102.e3-102.e6.
2. Cosar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18 patients. J Clin Psychopharmacol. 2011;31(2):169-173.
3. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol. 2011;26(2):112-119.
Hematologic abnormalities, such as leukopenia, agranulocytosis, and thrombocytopenia, can be life-threatening adverse reactions to atypical antipsychotics. Although clozapine has the highest risk of leukopenia and neutropenia, these side effects also have been associated with other atypical antipsychotics, including risperidone, olanzapine, ziprasidone, paliperdione, and quetiapine. Risperdone-induced leukopenia has been reported,1,2 but risperidone-
induced bicytopenia— that is, leukopenia/ thrombocytopenia—is rare.
Case
Mr. A, age 25, is an African American man admitted to an inpatient psychiatric unit for management of acute psychotic symptoms. He has been taking risperidone, 4 mg/d, for the past 6 months, although his adherence to the regimen is questionable. Baseline blood count shows a white blood cell (WBC) count of 4,400/μL with an absolute neutrophil count (ANC) of 1,900/μL and a platelet count
160×103/μL. A few days after restarting risperidone, repeat blood count shows a drop in the WBC count to 2,900/μL, with an ANC of 900/μL and a platelet count of 130×103/μL.
Mr. A’s physical examination is normal, he does not have any signs or symptoms of infection, and additional lab tests are negative. Risperidone is considered as a possible cause of bicytopenia and is discontinued. Mr. A agrees to start treatment with aripiprazole, 10 mg/d. In next 10 days, the WBC count increases to 6,000/μL. The ANC at 3,100/μL and platelets at 150×103/μL remain stable throughout hospitalization. The slowly increasing WBC count after stopping risperidone is highly suggestive that this agent caused Mr. A’s bicytopenia.
Differential diagnosis
Bone-marrow suppression is associated with first- and second-generation antipsychotics. Blood dyscrasia is a concern in clinical psychiatry because hematologic abnormalities can be life-threatening, requiring close monitoring of the blood count for patients taking an antipsychotic. It is important, therefore, to consider medication side effects in the differential diagnosis of >1 hematologic abnormalities in these patients.
Precise pathophysiologic understanding of the hematologic side effects of antipsychotics is lacking, although different mechanisms of action have been proposed.3 Possible mechanisms when a patient is taking clozapine or olanzapine include:
• direct toxic effect of the drug on bone marrow
• increased peripheral destruction
• oxidative stress induced by unstable metabolites.
There is not enough evidence, however, to identify risperidone’s mechanism of action on blood cells.
Aripiprazole might be a useful alternative when another antipsychotic causes leukopenia and neutropenia. In addition to regularly monitoring the blood cell count during antipsychotic treatment, the neutrophil and platelet counts should be monitored.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Hematologic abnormalities, such as leukopenia, agranulocytosis, and thrombocytopenia, can be life-threatening adverse reactions to atypical antipsychotics. Although clozapine has the highest risk of leukopenia and neutropenia, these side effects also have been associated with other atypical antipsychotics, including risperidone, olanzapine, ziprasidone, paliperdione, and quetiapine. Risperdone-induced leukopenia has been reported,1,2 but risperidone-
induced bicytopenia— that is, leukopenia/ thrombocytopenia—is rare.
Case
Mr. A, age 25, is an African American man admitted to an inpatient psychiatric unit for management of acute psychotic symptoms. He has been taking risperidone, 4 mg/d, for the past 6 months, although his adherence to the regimen is questionable. Baseline blood count shows a white blood cell (WBC) count of 4,400/μL with an absolute neutrophil count (ANC) of 1,900/μL and a platelet count
160×103/μL. A few days after restarting risperidone, repeat blood count shows a drop in the WBC count to 2,900/μL, with an ANC of 900/μL and a platelet count of 130×103/μL.
Mr. A’s physical examination is normal, he does not have any signs or symptoms of infection, and additional lab tests are negative. Risperidone is considered as a possible cause of bicytopenia and is discontinued. Mr. A agrees to start treatment with aripiprazole, 10 mg/d. In next 10 days, the WBC count increases to 6,000/μL. The ANC at 3,100/μL and platelets at 150×103/μL remain stable throughout hospitalization. The slowly increasing WBC count after stopping risperidone is highly suggestive that this agent caused Mr. A’s bicytopenia.
Differential diagnosis
Bone-marrow suppression is associated with first- and second-generation antipsychotics. Blood dyscrasia is a concern in clinical psychiatry because hematologic abnormalities can be life-threatening, requiring close monitoring of the blood count for patients taking an antipsychotic. It is important, therefore, to consider medication side effects in the differential diagnosis of >1 hematologic abnormalities in these patients.
Precise pathophysiologic understanding of the hematologic side effects of antipsychotics is lacking, although different mechanisms of action have been proposed.3 Possible mechanisms when a patient is taking clozapine or olanzapine include:
• direct toxic effect of the drug on bone marrow
• increased peripheral destruction
• oxidative stress induced by unstable metabolites.
There is not enough evidence, however, to identify risperidone’s mechanism of action on blood cells.
Aripiprazole might be a useful alternative when another antipsychotic causes leukopenia and neutropenia. In addition to regularly monitoring the blood cell count during antipsychotic treatment, the neutrophil and platelet counts should be monitored.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Manfredi G, Solfanelli A, Dimitri G, et al. Risperidone-induced leukopenia: a case report and brief review of literature. Gen Hosp Psychiatry. 2013;35(1):102.e3-102.e6.
2. Cosar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18 patients. J Clin Psychopharmacol. 2011;31(2):169-173.
3. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol. 2011;26(2):112-119.
1. Manfredi G, Solfanelli A, Dimitri G, et al. Risperidone-induced leukopenia: a case report and brief review of literature. Gen Hosp Psychiatry. 2013;35(1):102.e3-102.e6.
2. Cosar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18 patients. J Clin Psychopharmacol. 2011;31(2):169-173.
3. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol. 2011;26(2):112-119.