User login
CCSC issues five Choosing Wisely recommendations
Overutilization of tests, treatments, and procedures is an important example of low-value care that adds to the high cost of health care and provides little to no benefit for patients. To combat this problem, the American Board of Internal Medicine Foundation developed the Choosing Wisely Campaign, tasking professional societies to develop lists of the top five medical services that patients should question.
The Critical Care Societies Collaborative (CCSC), which comprises the four major U.S. professional and scientific societies – the American Association of Critical-Care Nurses, the American College of Chest Physicians, the American Thoracic Society, and the Society of Critical Care Medicine – participated by creating a task force that addressed this task to focus on critical care delivery.
Five CCSC recommendations were formulated:
1. Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.
2. Don’t transfuse red blood cells in hemodynamically stable, nonbleeding patients with a hemoglobin concentration greater than 7 mg/dL.
3. Don’t use parenteral nutrition in adequately nourished critically ill patients within the first 7 days of an ICU stay.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort.
The CCSC is tracking use/implementation of the Choosing Wisely recommendations among its four member organizations. Please complete this short survey at https://redcap.rush.edu/redcap/surveys/?s. Please click submit when finished.
Overutilization of tests, treatments, and procedures is an important example of low-value care that adds to the high cost of health care and provides little to no benefit for patients. To combat this problem, the American Board of Internal Medicine Foundation developed the Choosing Wisely Campaign, tasking professional societies to develop lists of the top five medical services that patients should question.
The Critical Care Societies Collaborative (CCSC), which comprises the four major U.S. professional and scientific societies – the American Association of Critical-Care Nurses, the American College of Chest Physicians, the American Thoracic Society, and the Society of Critical Care Medicine – participated by creating a task force that addressed this task to focus on critical care delivery.
Five CCSC recommendations were formulated:
1. Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.
2. Don’t transfuse red blood cells in hemodynamically stable, nonbleeding patients with a hemoglobin concentration greater than 7 mg/dL.
3. Don’t use parenteral nutrition in adequately nourished critically ill patients within the first 7 days of an ICU stay.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort.
The CCSC is tracking use/implementation of the Choosing Wisely recommendations among its four member organizations. Please complete this short survey at https://redcap.rush.edu/redcap/surveys/?s. Please click submit when finished.
Overutilization of tests, treatments, and procedures is an important example of low-value care that adds to the high cost of health care and provides little to no benefit for patients. To combat this problem, the American Board of Internal Medicine Foundation developed the Choosing Wisely Campaign, tasking professional societies to develop lists of the top five medical services that patients should question.
The Critical Care Societies Collaborative (CCSC), which comprises the four major U.S. professional and scientific societies – the American Association of Critical-Care Nurses, the American College of Chest Physicians, the American Thoracic Society, and the Society of Critical Care Medicine – participated by creating a task force that addressed this task to focus on critical care delivery.
Five CCSC recommendations were formulated:
1. Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.
2. Don’t transfuse red blood cells in hemodynamically stable, nonbleeding patients with a hemoglobin concentration greater than 7 mg/dL.
3. Don’t use parenteral nutrition in adequately nourished critically ill patients within the first 7 days of an ICU stay.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort.
The CCSC is tracking use/implementation of the Choosing Wisely recommendations among its four member organizations. Please complete this short survey at https://redcap.rush.edu/redcap/surveys/?s. Please click submit when finished.
CHEST Foundation can give more than $500,000 in grants
Every year, the CHEST Foundation awards more than a half-million dollars in grants to the next generation of lung health champions. February 2017 marks the start of the foundation’s next grant cycle, and we are excited to announce a new clinical research grant in Cystic Fibrosis, among many other disease-state topics. In 2016, the foundation awarded 11 CHEST members for their innovative and inspiring research proposals and community service programs.
“I am very proud to have been awarded a CHEST Foundation grant and pleased that clinical research and real-world evidence are a priority to the foundation,” stated Alice Turner, MBChB, PhD. Dr. Turner was awarded the 2016 CHEST Foundation and the Alpha-1 Foundation Clinical Research Grant in Alpha-1 Antitrypsin Deficiency. “This award means that my patients can now see publicly the efforts that are being made to reduce inequities in care and ensure that the best treatments are made available in the UK.”

The award will allow Dr. Turner to compare patients who are being treated in the United States with those who are untreated in the United Kingdom and then analyze the effects on mortality, hospitalization, and quality of life to make inferences about whether or not the treatment should be implemented in the United Kingdom. Currently, the type of treatment used to treat patients with alpha-1 antitrypsin deficiency in the United States is not available in the United Kingdom, and the results of this study will be provided to the National Health Service in England to help overcome the barriers of legalizing the treatment in the United Kingdom.
Sydney Montesi, MD, was awarded the CHEST Foundation Research Grant in Pulmonary Fibrosis for her work on using noninvasive lung imaging to see how contrast agents can be used to measure disease activity and progression.
“As a provider, it can be very difficult when we first meet a patient to know what disease course they will take, but if we had this information, it would help us in determining earlier lung transplant referrals, choosing the best therapies and treatments, and ultimately lowering the mortality rate of idiopathic pulmonary fibrosis,” Dr. Montesi said of her research. “Receiving this grant is essential because it will allow us to test our hypothesis that vascular leakage is increased in patients with pulmonary fibrosis, and we will also be able to look more in depth at the comparison of patients with stable disease and those with progressive disease.”
These grants help advance the work of young investigators all over the globe. Over the last 20 years, thousands of researchers and community service volunteers have received more than $10 million in funding.
Beginning in February 2017, the Foundation will have more than a half-million dollars available in funding toward the next generation of lung health champions.
Learn more about the CHEST Foundation grant application process at chestnet.org/grants or e-mail the foundation at [email protected].
Every year, the CHEST Foundation awards more than a half-million dollars in grants to the next generation of lung health champions. February 2017 marks the start of the foundation’s next grant cycle, and we are excited to announce a new clinical research grant in Cystic Fibrosis, among many other disease-state topics. In 2016, the foundation awarded 11 CHEST members for their innovative and inspiring research proposals and community service programs.
“I am very proud to have been awarded a CHEST Foundation grant and pleased that clinical research and real-world evidence are a priority to the foundation,” stated Alice Turner, MBChB, PhD. Dr. Turner was awarded the 2016 CHEST Foundation and the Alpha-1 Foundation Clinical Research Grant in Alpha-1 Antitrypsin Deficiency. “This award means that my patients can now see publicly the efforts that are being made to reduce inequities in care and ensure that the best treatments are made available in the UK.”

The award will allow Dr. Turner to compare patients who are being treated in the United States with those who are untreated in the United Kingdom and then analyze the effects on mortality, hospitalization, and quality of life to make inferences about whether or not the treatment should be implemented in the United Kingdom. Currently, the type of treatment used to treat patients with alpha-1 antitrypsin deficiency in the United States is not available in the United Kingdom, and the results of this study will be provided to the National Health Service in England to help overcome the barriers of legalizing the treatment in the United Kingdom.
Sydney Montesi, MD, was awarded the CHEST Foundation Research Grant in Pulmonary Fibrosis for her work on using noninvasive lung imaging to see how contrast agents can be used to measure disease activity and progression.
“As a provider, it can be very difficult when we first meet a patient to know what disease course they will take, but if we had this information, it would help us in determining earlier lung transplant referrals, choosing the best therapies and treatments, and ultimately lowering the mortality rate of idiopathic pulmonary fibrosis,” Dr. Montesi said of her research. “Receiving this grant is essential because it will allow us to test our hypothesis that vascular leakage is increased in patients with pulmonary fibrosis, and we will also be able to look more in depth at the comparison of patients with stable disease and those with progressive disease.”
These grants help advance the work of young investigators all over the globe. Over the last 20 years, thousands of researchers and community service volunteers have received more than $10 million in funding.
Beginning in February 2017, the Foundation will have more than a half-million dollars available in funding toward the next generation of lung health champions.
Learn more about the CHEST Foundation grant application process at chestnet.org/grants or e-mail the foundation at [email protected].
Every year, the CHEST Foundation awards more than a half-million dollars in grants to the next generation of lung health champions. February 2017 marks the start of the foundation’s next grant cycle, and we are excited to announce a new clinical research grant in Cystic Fibrosis, among many other disease-state topics. In 2016, the foundation awarded 11 CHEST members for their innovative and inspiring research proposals and community service programs.
“I am very proud to have been awarded a CHEST Foundation grant and pleased that clinical research and real-world evidence are a priority to the foundation,” stated Alice Turner, MBChB, PhD. Dr. Turner was awarded the 2016 CHEST Foundation and the Alpha-1 Foundation Clinical Research Grant in Alpha-1 Antitrypsin Deficiency. “This award means that my patients can now see publicly the efforts that are being made to reduce inequities in care and ensure that the best treatments are made available in the UK.”

The award will allow Dr. Turner to compare patients who are being treated in the United States with those who are untreated in the United Kingdom and then analyze the effects on mortality, hospitalization, and quality of life to make inferences about whether or not the treatment should be implemented in the United Kingdom. Currently, the type of treatment used to treat patients with alpha-1 antitrypsin deficiency in the United States is not available in the United Kingdom, and the results of this study will be provided to the National Health Service in England to help overcome the barriers of legalizing the treatment in the United Kingdom.
Sydney Montesi, MD, was awarded the CHEST Foundation Research Grant in Pulmonary Fibrosis for her work on using noninvasive lung imaging to see how contrast agents can be used to measure disease activity and progression.
“As a provider, it can be very difficult when we first meet a patient to know what disease course they will take, but if we had this information, it would help us in determining earlier lung transplant referrals, choosing the best therapies and treatments, and ultimately lowering the mortality rate of idiopathic pulmonary fibrosis,” Dr. Montesi said of her research. “Receiving this grant is essential because it will allow us to test our hypothesis that vascular leakage is increased in patients with pulmonary fibrosis, and we will also be able to look more in depth at the comparison of patients with stable disease and those with progressive disease.”
These grants help advance the work of young investigators all over the globe. Over the last 20 years, thousands of researchers and community service volunteers have received more than $10 million in funding.
Beginning in February 2017, the Foundation will have more than a half-million dollars available in funding toward the next generation of lung health champions.
Learn more about the CHEST Foundation grant application process at chestnet.org/grants or e-mail the foundation at [email protected].
This Month in CHEST: Editor’s Picks
E
GOLD 2017: A New Report
By Dr. P. J. Barnes
Original Research
Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. By Dr. S. Sawada, et al.
ICU Telemedicine Program Financial Outcomes. By Dr. C. M. Lilly et al.
Accuracy of Lung Ultrasonography in the Diagnosis of Pneumonia in Adults: Systematic Review and Meta-Analysis. By Dr. A. M. Llamas-Álvarez, et al.
Evidence-based Medicine
Cough in the Athlete: CHEST Guideline and Expert Panel Report. By Dr. L-P Boulet, et al, on behalf of the CHEST Expert Cough Panel.
E
GOLD 2017: A New Report
By Dr. P. J. Barnes
Original Research
Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. By Dr. S. Sawada, et al.
ICU Telemedicine Program Financial Outcomes. By Dr. C. M. Lilly et al.
Accuracy of Lung Ultrasonography in the Diagnosis of Pneumonia in Adults: Systematic Review and Meta-Analysis. By Dr. A. M. Llamas-Álvarez, et al.
Evidence-based Medicine
Cough in the Athlete: CHEST Guideline and Expert Panel Report. By Dr. L-P Boulet, et al, on behalf of the CHEST Expert Cough Panel.
E
GOLD 2017: A New Report
By Dr. P. J. Barnes
Original Research
Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. By Dr. S. Sawada, et al.
ICU Telemedicine Program Financial Outcomes. By Dr. C. M. Lilly et al.
Accuracy of Lung Ultrasonography in the Diagnosis of Pneumonia in Adults: Systematic Review and Meta-Analysis. By Dr. A. M. Llamas-Álvarez, et al.
Evidence-based Medicine
Cough in the Athlete: CHEST Guideline and Expert Panel Report. By Dr. L-P Boulet, et al, on behalf of the CHEST Expert Cough Panel.
Meet the CHEST President-Designate
Clayton T. Cowl, MD, FCCP, is the CHEST President-Designate and sits as a member of the Board of Regents. Dr. Cowl’s presidential term will be 2018-2019. He currently is the Chair of the Division of Preventive, Occupational, and Aerospace Medicine with a joint appointment in the Division of Pulmonary and Critical Care Medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Cowl is triple board-certified in Pulmonary and Critical Care Medicine, Occupational Medicine, and Internal Medicine, with an interest in airway disorders, occupational-related respiratory health, toxicology, altitude physiology, and transportation medicine.
His research focus has included projects in altitude physiology at Mayo Clinic’s altitude chamber and testing for the emergency oxygen passenger mask in the Boeing 787 airliner. He has also published in the areas of occupational asthma and toxic inhalations.
He is currently the President of the Civil Aviation Medical Association and is a Senior Aviation Medical Examiner designated by the Federal Aviation Administration.
Dr. Cowl has been a recipient of the Innovation in Education Award from the Mayo School of Continuous Professional Development, and the Laureate Award in the Mayo Clinic Department of Medicine.
Clayton T. Cowl, MD, FCCP, is the CHEST President-Designate and sits as a member of the Board of Regents. Dr. Cowl’s presidential term will be 2018-2019. He currently is the Chair of the Division of Preventive, Occupational, and Aerospace Medicine with a joint appointment in the Division of Pulmonary and Critical Care Medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Cowl is triple board-certified in Pulmonary and Critical Care Medicine, Occupational Medicine, and Internal Medicine, with an interest in airway disorders, occupational-related respiratory health, toxicology, altitude physiology, and transportation medicine.
His research focus has included projects in altitude physiology at Mayo Clinic’s altitude chamber and testing for the emergency oxygen passenger mask in the Boeing 787 airliner. He has also published in the areas of occupational asthma and toxic inhalations.
He is currently the President of the Civil Aviation Medical Association and is a Senior Aviation Medical Examiner designated by the Federal Aviation Administration.
Dr. Cowl has been a recipient of the Innovation in Education Award from the Mayo School of Continuous Professional Development, and the Laureate Award in the Mayo Clinic Department of Medicine.
Clayton T. Cowl, MD, FCCP, is the CHEST President-Designate and sits as a member of the Board of Regents. Dr. Cowl’s presidential term will be 2018-2019. He currently is the Chair of the Division of Preventive, Occupational, and Aerospace Medicine with a joint appointment in the Division of Pulmonary and Critical Care Medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Cowl is triple board-certified in Pulmonary and Critical Care Medicine, Occupational Medicine, and Internal Medicine, with an interest in airway disorders, occupational-related respiratory health, toxicology, altitude physiology, and transportation medicine.
His research focus has included projects in altitude physiology at Mayo Clinic’s altitude chamber and testing for the emergency oxygen passenger mask in the Boeing 787 airliner. He has also published in the areas of occupational asthma and toxic inhalations.
He is currently the President of the Civil Aviation Medical Association and is a Senior Aviation Medical Examiner designated by the Federal Aviation Administration.
Dr. Cowl has been a recipient of the Innovation in Education Award from the Mayo School of Continuous Professional Development, and the Laureate Award in the Mayo Clinic Department of Medicine.
Critical Care Commentary: Highlights from the 2016 hospital-acquired and ventilator-associated pneumonia guideline
The 2016 hospital-acquired and ventilator-associated pneumonia guidelines, sponsored by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS), and endorsed by the American College of Chest Physicians (CHEST), Society of Critical Care Medicine (SCCM), and the Society for Healthcare Epidemiology, was published recently (Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63[5]:575-82).
This Critical Care Commentary aims to provide the highlights of the new guideline and to motivate readers to read the complete report that best represents the primary intent of the guideline panelists.
The new guideline was written using the Grading of Recommendations Assessment, Development, and Evaluation methodology. This was the framework to address all clinical questions referred to as PICOs (patient; intervention; comparator; outcome), which can be explicitly seen in the published guideline. For every PICO question, the wording “we suggest” was used for a weak recommendation (lack of high confidence; further evidence could change it), and “we recommend” was used for a strong recommendation (high confidence; further evidence is unlikely to change it). Also, part of the panel framework was the requirement to disclose any actual, potential, or perceived conflicts of interest for each panelist to be accepted to participate, as well as to remain in the panel for the duration of the process. The cochairs remained free of any financial conflicts during the entire process.
Choosing an empiric antibiotic regimen for patients with HAP and VAP requires balancing the potentially competing goals of ensuring that likely infecting pathogens are covered while avoiding excess antibiotic use. In order to guide clinicians on empiric antibiotic therapy, the panel performed a comprehensive review of the potential risk factors for HAP and VAP. For VAP, three factors associated with disease severity (septic shock at time of VAP, ARDS preceding VAP, and acute renal replacement prior to VAP onset) and two epidemiologic factors (prior use of IV antibiotic use within 90 days, and 5 or more days of hospitalization prior to the occurrence of VAP) made the final risk factors list. For HAP, only the prior use of IV antibiotics within 90 days was associated with risk for MDR. However, because of the limitations and small number of studies on HAP only, the panel decided to add risk factors for mortality (ventilator support for HAP and septic shock) as surrogates for MDR risk factors in patients with HAP, as these factors presumably increase the risk of poor outcomes if there is initial inadequate empiric therapy.
In conjunction with the bedside evaluation of risk factors for MDR, the guideline recommends the use of local antibiograms not only to guide empiric therapy but also to decide if antibiotic coverage for MDR is needed. Ideally, the antibiogram should be based on the specific ICU, but if this is not feasible, or the hospital is of small size, an institutional antibiogram can also be helpful. The first benefit of local antibiograms is derived from the knowledge gained regarding the prevalence of each microorganism; for example, if only 3% of all VAP or HAP in a given unit or hospital is caused by Pseudomonas aeruginosa, it is likely that an empiric coverage for this microorganism will neither be necessary nor appropriate for most patients. The second benefit is derived from the knowledge concerning the frequency of MDR microorganisms within the unit or hospital: for example, patients with VAP in units where 10%-20% of Staphylococcus aureus isolates are resistant to methicillin, or greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy, should receive antibiotics for MDR infections. With these two critical pieces of information, the clinician will have a higher probability of starting the correct empiric antibiotics, and, consequently, improve the survival outcomes of patients with HAP and VAP.
The choice of the empirical treatment of VAP and HAP becomes a natural derivation of the three main factors discussed above: (1) epidemiologic history of antibiotics’ use and prior hospitalization length, (2) local antibiogram for the prevalence and resistance of microorganisms, and (3) disease severity and risk of mortality by the identification of septic shock, ARDS, and acute renal replacement therapy. For example, if 17% of all VAPs in your unit is from P aeruginosa (which is the national prevalence in patients with VAP), and 8% of these strains are resistant to an agent being considered for gram-negative monotherapy, not prescribing double coverage for P aeruginosa would still result in initial appropriate therapy in 98.6% (derived from 1-[0.17 x 0.08]) of cases. The reason why the panelists chose the threshold of 10% for P aeruginosa, and 10%-20% for S aureus, was based on the national prevalence rates reported by the Centers for Disease Control and Prevention, with the goal of limiting the initial inappropriate antibiotic therapy decision to less than 5% of all cases. We strongly believe that this “epidemiologic/antibiogram/disease severity” approach to select the empiric therapy is both clinically intuitive and essential to improve patients’ outcomes. Further, this approach will substantially reduce the unnecessary use of double antibiotic therapy in patients with VAP or HAP.
This guideline suggests that the use of inhaled antibiotic therapy in conjunction with IV antibiotics may benefit patients with VAP or HAP from MDR microorganisms that are sensitive to only polymyxins or aminoglycosides. The panel also suggested that the use of pharmacokinetic and pharmacodynamics should be used to optimize the administration of antibiotic therapy for all patients with HAP or VAP.
Last, after an extensive review and multiple analyses of all available evidence, the panel concluded that the majority of patients with HAP or VAP should be treated with 7 days of therapy, independent of the microorganism causing the pneumonia. In several meta-analyses performed by the panelists to evaluate all patients with VAP, as well as only patients with VAP caused by nonfermenting gram-negative organisms such as Pseudomonas species, Stenotrophomonas species, and Acinetobacter species, the panel did not find differences between short and long courses of antibiotics regarding mortality, clinical cure, pneumonia recurrence, and mechanical ventilation duration. In recognition of the individual needs of each patient, we made a remark that shorter or longer duration of antibiotics may be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters. Several adjunctive methods of deescalation were assessed, but only procalcitonin was suggested to aid health care providers to shorten the course of antibiotic therapy.
In conclusion, the authors of this 2016 HAP/VAP IDSA/ATS guideline hope to achieve the ultimate goal of improving the treatment and outcomes of patients with HAP and VAP and reducing unnecessary antibiotic use.
Dr. Kalil is with the department of internal medicine, division of infectious diseases, University of Nebraska Medical Center, Omaha; Dr. Metersky is with the division of pulmonary and critical care medicine, University of Connecticut, Farmington.
The 2016 hospital-acquired and ventilator-associated pneumonia guidelines, sponsored by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS), and endorsed by the American College of Chest Physicians (CHEST), Society of Critical Care Medicine (SCCM), and the Society for Healthcare Epidemiology, was published recently (Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63[5]:575-82).
This Critical Care Commentary aims to provide the highlights of the new guideline and to motivate readers to read the complete report that best represents the primary intent of the guideline panelists.
The new guideline was written using the Grading of Recommendations Assessment, Development, and Evaluation methodology. This was the framework to address all clinical questions referred to as PICOs (patient; intervention; comparator; outcome), which can be explicitly seen in the published guideline. For every PICO question, the wording “we suggest” was used for a weak recommendation (lack of high confidence; further evidence could change it), and “we recommend” was used for a strong recommendation (high confidence; further evidence is unlikely to change it). Also, part of the panel framework was the requirement to disclose any actual, potential, or perceived conflicts of interest for each panelist to be accepted to participate, as well as to remain in the panel for the duration of the process. The cochairs remained free of any financial conflicts during the entire process.
Choosing an empiric antibiotic regimen for patients with HAP and VAP requires balancing the potentially competing goals of ensuring that likely infecting pathogens are covered while avoiding excess antibiotic use. In order to guide clinicians on empiric antibiotic therapy, the panel performed a comprehensive review of the potential risk factors for HAP and VAP. For VAP, three factors associated with disease severity (septic shock at time of VAP, ARDS preceding VAP, and acute renal replacement prior to VAP onset) and two epidemiologic factors (prior use of IV antibiotic use within 90 days, and 5 or more days of hospitalization prior to the occurrence of VAP) made the final risk factors list. For HAP, only the prior use of IV antibiotics within 90 days was associated with risk for MDR. However, because of the limitations and small number of studies on HAP only, the panel decided to add risk factors for mortality (ventilator support for HAP and septic shock) as surrogates for MDR risk factors in patients with HAP, as these factors presumably increase the risk of poor outcomes if there is initial inadequate empiric therapy.
In conjunction with the bedside evaluation of risk factors for MDR, the guideline recommends the use of local antibiograms not only to guide empiric therapy but also to decide if antibiotic coverage for MDR is needed. Ideally, the antibiogram should be based on the specific ICU, but if this is not feasible, or the hospital is of small size, an institutional antibiogram can also be helpful. The first benefit of local antibiograms is derived from the knowledge gained regarding the prevalence of each microorganism; for example, if only 3% of all VAP or HAP in a given unit or hospital is caused by Pseudomonas aeruginosa, it is likely that an empiric coverage for this microorganism will neither be necessary nor appropriate for most patients. The second benefit is derived from the knowledge concerning the frequency of MDR microorganisms within the unit or hospital: for example, patients with VAP in units where 10%-20% of Staphylococcus aureus isolates are resistant to methicillin, or greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy, should receive antibiotics for MDR infections. With these two critical pieces of information, the clinician will have a higher probability of starting the correct empiric antibiotics, and, consequently, improve the survival outcomes of patients with HAP and VAP.
The choice of the empirical treatment of VAP and HAP becomes a natural derivation of the three main factors discussed above: (1) epidemiologic history of antibiotics’ use and prior hospitalization length, (2) local antibiogram for the prevalence and resistance of microorganisms, and (3) disease severity and risk of mortality by the identification of septic shock, ARDS, and acute renal replacement therapy. For example, if 17% of all VAPs in your unit is from P aeruginosa (which is the national prevalence in patients with VAP), and 8% of these strains are resistant to an agent being considered for gram-negative monotherapy, not prescribing double coverage for P aeruginosa would still result in initial appropriate therapy in 98.6% (derived from 1-[0.17 x 0.08]) of cases. The reason why the panelists chose the threshold of 10% for P aeruginosa, and 10%-20% for S aureus, was based on the national prevalence rates reported by the Centers for Disease Control and Prevention, with the goal of limiting the initial inappropriate antibiotic therapy decision to less than 5% of all cases. We strongly believe that this “epidemiologic/antibiogram/disease severity” approach to select the empiric therapy is both clinically intuitive and essential to improve patients’ outcomes. Further, this approach will substantially reduce the unnecessary use of double antibiotic therapy in patients with VAP or HAP.
This guideline suggests that the use of inhaled antibiotic therapy in conjunction with IV antibiotics may benefit patients with VAP or HAP from MDR microorganisms that are sensitive to only polymyxins or aminoglycosides. The panel also suggested that the use of pharmacokinetic and pharmacodynamics should be used to optimize the administration of antibiotic therapy for all patients with HAP or VAP.
Last, after an extensive review and multiple analyses of all available evidence, the panel concluded that the majority of patients with HAP or VAP should be treated with 7 days of therapy, independent of the microorganism causing the pneumonia. In several meta-analyses performed by the panelists to evaluate all patients with VAP, as well as only patients with VAP caused by nonfermenting gram-negative organisms such as Pseudomonas species, Stenotrophomonas species, and Acinetobacter species, the panel did not find differences between short and long courses of antibiotics regarding mortality, clinical cure, pneumonia recurrence, and mechanical ventilation duration. In recognition of the individual needs of each patient, we made a remark that shorter or longer duration of antibiotics may be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters. Several adjunctive methods of deescalation were assessed, but only procalcitonin was suggested to aid health care providers to shorten the course of antibiotic therapy.
In conclusion, the authors of this 2016 HAP/VAP IDSA/ATS guideline hope to achieve the ultimate goal of improving the treatment and outcomes of patients with HAP and VAP and reducing unnecessary antibiotic use.
Dr. Kalil is with the department of internal medicine, division of infectious diseases, University of Nebraska Medical Center, Omaha; Dr. Metersky is with the division of pulmonary and critical care medicine, University of Connecticut, Farmington.
The 2016 hospital-acquired and ventilator-associated pneumonia guidelines, sponsored by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS), and endorsed by the American College of Chest Physicians (CHEST), Society of Critical Care Medicine (SCCM), and the Society for Healthcare Epidemiology, was published recently (Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63[5]:575-82).
This Critical Care Commentary aims to provide the highlights of the new guideline and to motivate readers to read the complete report that best represents the primary intent of the guideline panelists.
The new guideline was written using the Grading of Recommendations Assessment, Development, and Evaluation methodology. This was the framework to address all clinical questions referred to as PICOs (patient; intervention; comparator; outcome), which can be explicitly seen in the published guideline. For every PICO question, the wording “we suggest” was used for a weak recommendation (lack of high confidence; further evidence could change it), and “we recommend” was used for a strong recommendation (high confidence; further evidence is unlikely to change it). Also, part of the panel framework was the requirement to disclose any actual, potential, or perceived conflicts of interest for each panelist to be accepted to participate, as well as to remain in the panel for the duration of the process. The cochairs remained free of any financial conflicts during the entire process.
Choosing an empiric antibiotic regimen for patients with HAP and VAP requires balancing the potentially competing goals of ensuring that likely infecting pathogens are covered while avoiding excess antibiotic use. In order to guide clinicians on empiric antibiotic therapy, the panel performed a comprehensive review of the potential risk factors for HAP and VAP. For VAP, three factors associated with disease severity (septic shock at time of VAP, ARDS preceding VAP, and acute renal replacement prior to VAP onset) and two epidemiologic factors (prior use of IV antibiotic use within 90 days, and 5 or more days of hospitalization prior to the occurrence of VAP) made the final risk factors list. For HAP, only the prior use of IV antibiotics within 90 days was associated with risk for MDR. However, because of the limitations and small number of studies on HAP only, the panel decided to add risk factors for mortality (ventilator support for HAP and septic shock) as surrogates for MDR risk factors in patients with HAP, as these factors presumably increase the risk of poor outcomes if there is initial inadequate empiric therapy.
In conjunction with the bedside evaluation of risk factors for MDR, the guideline recommends the use of local antibiograms not only to guide empiric therapy but also to decide if antibiotic coverage for MDR is needed. Ideally, the antibiogram should be based on the specific ICU, but if this is not feasible, or the hospital is of small size, an institutional antibiogram can also be helpful. The first benefit of local antibiograms is derived from the knowledge gained regarding the prevalence of each microorganism; for example, if only 3% of all VAP or HAP in a given unit or hospital is caused by Pseudomonas aeruginosa, it is likely that an empiric coverage for this microorganism will neither be necessary nor appropriate for most patients. The second benefit is derived from the knowledge concerning the frequency of MDR microorganisms within the unit or hospital: for example, patients with VAP in units where 10%-20% of Staphylococcus aureus isolates are resistant to methicillin, or greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy, should receive antibiotics for MDR infections. With these two critical pieces of information, the clinician will have a higher probability of starting the correct empiric antibiotics, and, consequently, improve the survival outcomes of patients with HAP and VAP.
The choice of the empirical treatment of VAP and HAP becomes a natural derivation of the three main factors discussed above: (1) epidemiologic history of antibiotics’ use and prior hospitalization length, (2) local antibiogram for the prevalence and resistance of microorganisms, and (3) disease severity and risk of mortality by the identification of septic shock, ARDS, and acute renal replacement therapy. For example, if 17% of all VAPs in your unit is from P aeruginosa (which is the national prevalence in patients with VAP), and 8% of these strains are resistant to an agent being considered for gram-negative monotherapy, not prescribing double coverage for P aeruginosa would still result in initial appropriate therapy in 98.6% (derived from 1-[0.17 x 0.08]) of cases. The reason why the panelists chose the threshold of 10% for P aeruginosa, and 10%-20% for S aureus, was based on the national prevalence rates reported by the Centers for Disease Control and Prevention, with the goal of limiting the initial inappropriate antibiotic therapy decision to less than 5% of all cases. We strongly believe that this “epidemiologic/antibiogram/disease severity” approach to select the empiric therapy is both clinically intuitive and essential to improve patients’ outcomes. Further, this approach will substantially reduce the unnecessary use of double antibiotic therapy in patients with VAP or HAP.
This guideline suggests that the use of inhaled antibiotic therapy in conjunction with IV antibiotics may benefit patients with VAP or HAP from MDR microorganisms that are sensitive to only polymyxins or aminoglycosides. The panel also suggested that the use of pharmacokinetic and pharmacodynamics should be used to optimize the administration of antibiotic therapy for all patients with HAP or VAP.
Last, after an extensive review and multiple analyses of all available evidence, the panel concluded that the majority of patients with HAP or VAP should be treated with 7 days of therapy, independent of the microorganism causing the pneumonia. In several meta-analyses performed by the panelists to evaluate all patients with VAP, as well as only patients with VAP caused by nonfermenting gram-negative organisms such as Pseudomonas species, Stenotrophomonas species, and Acinetobacter species, the panel did not find differences between short and long courses of antibiotics regarding mortality, clinical cure, pneumonia recurrence, and mechanical ventilation duration. In recognition of the individual needs of each patient, we made a remark that shorter or longer duration of antibiotics may be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters. Several adjunctive methods of deescalation were assessed, but only procalcitonin was suggested to aid health care providers to shorten the course of antibiotic therapy.
In conclusion, the authors of this 2016 HAP/VAP IDSA/ATS guideline hope to achieve the ultimate goal of improving the treatment and outcomes of patients with HAP and VAP and reducing unnecessary antibiotic use.
Dr. Kalil is with the department of internal medicine, division of infectious diseases, University of Nebraska Medical Center, Omaha; Dr. Metersky is with the division of pulmonary and critical care medicine, University of Connecticut, Farmington.
Bronchoscopy sedation changes in 2017
A major change in coding for bronchoscopy occurred on January 1, 2017, as moderate (conscious) sedation is now separately identified from the work relative value units (wRVUs) for the bronchoscopy codes. While traditionally the bronchoscopist provided moderate sedation, in recent clinical practice, other individuals often provide the sedation. CMS mandated refinement of separate Current Procedural Terminology (CPT®) codes to account for the work of moderate procedural sedation. In the final rule published in November 2016, CMS removed 0.25 wRVUs from many of the bronchoscopy codes to account for the work of moderate sedation. To be reimbursed appropriately, include a moderate sedation CPT code with all bronchoscopy procedures.
Use codes 99151 and 99155 for patients younger than 5 years. For a patient 5 years or older, when the bronchoscopist provides moderate sedation, report code 99152 for the initial 15 minutes and 99153 for subsequent time in 15-minute increments. For a patient 5 years or older, when a provider other than the bronchoscopist provides moderate sedation, use code 99156 for the initial 15 minutes and 99157 for subsequent time in 15-minute increments. Utilize codes 99156 and 99157 only when a second provider (other than the bronchoscopist) performs moderate sedation in the facility setting (eg, hospital, outpatient hospital/ambulatory surgery center, skilled nursing facility). When the second provider performs these services in the nonfacility setting (eg, physician office, freestanding imaging center), do not report codes 99155, 99156, or 99157. Moderate sedation does not include minimal sedation (anxiolysis), deep sedation, or monitored anesthesia care (00100-01999).
Do not use a moderate sedation code (99151-2 or 99155-6) if providing less than 10 minutes of moderate sedation. As with other time-based codes, use the subsequent codes 99153 and 99157 when moderate sedation lasts 8 minutes or longer than the initial 15 minutes. The time for moderate sedation begins with the administration of the sedating agent and concludes when the continuous face-to-face presence of the bronchoscopist ends after completion of the procedure. Intermittent, re-evaluation of the patient afterward is postservice work and is not included in the time for moderate sedation. For example, if the bronchoscopist provides moderate sedation for 25 minutes in a 65-year-old man, report 99152 (for the initial 15 minutes) and 99153 (for the subsequent 10 minutes). If an individual other than the bronchoscopist provides moderate sedation for 41 minutes in a 57-year-old woman, use 99156 (for the initial 15 minutes) and two units of 99157 (for the subsequent 26 minutes). If a bronchoscopist provides moderate sedation and reports the appropriate codes after January 1, the 0.25 wRVU change will have no financial impact compared with 2016. If a second provider performs the moderate sedation, expect an approximately $8.72 drop in reimbursement per procedure.
A major change in coding for bronchoscopy occurred on January 1, 2017, as moderate (conscious) sedation is now separately identified from the work relative value units (wRVUs) for the bronchoscopy codes. While traditionally the bronchoscopist provided moderate sedation, in recent clinical practice, other individuals often provide the sedation. CMS mandated refinement of separate Current Procedural Terminology (CPT®) codes to account for the work of moderate procedural sedation. In the final rule published in November 2016, CMS removed 0.25 wRVUs from many of the bronchoscopy codes to account for the work of moderate sedation. To be reimbursed appropriately, include a moderate sedation CPT code with all bronchoscopy procedures.
Use codes 99151 and 99155 for patients younger than 5 years. For a patient 5 years or older, when the bronchoscopist provides moderate sedation, report code 99152 for the initial 15 minutes and 99153 for subsequent time in 15-minute increments. For a patient 5 years or older, when a provider other than the bronchoscopist provides moderate sedation, use code 99156 for the initial 15 minutes and 99157 for subsequent time in 15-minute increments. Utilize codes 99156 and 99157 only when a second provider (other than the bronchoscopist) performs moderate sedation in the facility setting (eg, hospital, outpatient hospital/ambulatory surgery center, skilled nursing facility). When the second provider performs these services in the nonfacility setting (eg, physician office, freestanding imaging center), do not report codes 99155, 99156, or 99157. Moderate sedation does not include minimal sedation (anxiolysis), deep sedation, or monitored anesthesia care (00100-01999).
Do not use a moderate sedation code (99151-2 or 99155-6) if providing less than 10 minutes of moderate sedation. As with other time-based codes, use the subsequent codes 99153 and 99157 when moderate sedation lasts 8 minutes or longer than the initial 15 minutes. The time for moderate sedation begins with the administration of the sedating agent and concludes when the continuous face-to-face presence of the bronchoscopist ends after completion of the procedure. Intermittent, re-evaluation of the patient afterward is postservice work and is not included in the time for moderate sedation. For example, if the bronchoscopist provides moderate sedation for 25 minutes in a 65-year-old man, report 99152 (for the initial 15 minutes) and 99153 (for the subsequent 10 minutes). If an individual other than the bronchoscopist provides moderate sedation for 41 minutes in a 57-year-old woman, use 99156 (for the initial 15 minutes) and two units of 99157 (for the subsequent 26 minutes). If a bronchoscopist provides moderate sedation and reports the appropriate codes after January 1, the 0.25 wRVU change will have no financial impact compared with 2016. If a second provider performs the moderate sedation, expect an approximately $8.72 drop in reimbursement per procedure.
A major change in coding for bronchoscopy occurred on January 1, 2017, as moderate (conscious) sedation is now separately identified from the work relative value units (wRVUs) for the bronchoscopy codes. While traditionally the bronchoscopist provided moderate sedation, in recent clinical practice, other individuals often provide the sedation. CMS mandated refinement of separate Current Procedural Terminology (CPT®) codes to account for the work of moderate procedural sedation. In the final rule published in November 2016, CMS removed 0.25 wRVUs from many of the bronchoscopy codes to account for the work of moderate sedation. To be reimbursed appropriately, include a moderate sedation CPT code with all bronchoscopy procedures.
Use codes 99151 and 99155 for patients younger than 5 years. For a patient 5 years or older, when the bronchoscopist provides moderate sedation, report code 99152 for the initial 15 minutes and 99153 for subsequent time in 15-minute increments. For a patient 5 years or older, when a provider other than the bronchoscopist provides moderate sedation, use code 99156 for the initial 15 minutes and 99157 for subsequent time in 15-minute increments. Utilize codes 99156 and 99157 only when a second provider (other than the bronchoscopist) performs moderate sedation in the facility setting (eg, hospital, outpatient hospital/ambulatory surgery center, skilled nursing facility). When the second provider performs these services in the nonfacility setting (eg, physician office, freestanding imaging center), do not report codes 99155, 99156, or 99157. Moderate sedation does not include minimal sedation (anxiolysis), deep sedation, or monitored anesthesia care (00100-01999).
Do not use a moderate sedation code (99151-2 or 99155-6) if providing less than 10 minutes of moderate sedation. As with other time-based codes, use the subsequent codes 99153 and 99157 when moderate sedation lasts 8 minutes or longer than the initial 15 minutes. The time for moderate sedation begins with the administration of the sedating agent and concludes when the continuous face-to-face presence of the bronchoscopist ends after completion of the procedure. Intermittent, re-evaluation of the patient afterward is postservice work and is not included in the time for moderate sedation. For example, if the bronchoscopist provides moderate sedation for 25 minutes in a 65-year-old man, report 99152 (for the initial 15 minutes) and 99153 (for the subsequent 10 minutes). If an individual other than the bronchoscopist provides moderate sedation for 41 minutes in a 57-year-old woman, use 99156 (for the initial 15 minutes) and two units of 99157 (for the subsequent 26 minutes). If a bronchoscopist provides moderate sedation and reports the appropriate codes after January 1, the 0.25 wRVU change will have no financial impact compared with 2016. If a second provider performs the moderate sedation, expect an approximately $8.72 drop in reimbursement per procedure.
Sleep strategies: Sleep-disordered breathing and pregnancy complications: Emerging data and future directions
Background
Sleep-disordered breathing (SDB) conditions are characterized by abnormal respiratory patterns and abnormal gas exchange during sleep.1-3 Obstructive sleep apnea (OSA), the most common type of SDB, is characterized by repetitive episodes of airway narrowing during sleep that lead to respiratory disruption, hypoxia, and sleep fragmentation. In reproductive-aged women, epidemiologic studies suggest a 2% to 13% prevalence of OSA.4-6 Pregnancy is associated with changes that promote OSA, such as weight gain and edema of the upper airway.7 Frequent snoring, a common symptom of OSA, is endorsed by 15% to 25% of pregnant women.8-10 Health outcomes that have been linked to SDB in the nonpregnant population, such as hypertension and insulin-resistant diabetes, have clinically relevant correlates in pregnancy (preeclampsia, gestational diabetes).11-13
While several retrospective and cross-sectional studies suggest that SDB may increase the risk of developing hypertensive disorders and gestational diabetes during pregnancy,16-18 up until recently, there were limited and conflicting data from prospective observational cohorts in which SDB exposure and pregnancy outcomes have been methodically measured and confounding variables carefully considered.19-21 Louis et al.19 reported on a cohort of 175 obese women and demonstrated that women with SDB (apnea-hypopnea index greater than or equal to 5) were more likely to develop preeclampsia (adjusted odds ratio, 3.5; 95% CI, 1.3, 9.9). However, two other small studies failed to demonstrate a positive association between SDB and pregnancy-related hypertension, but one suggested a relationship between SDB and gestational diabetes.20,21
Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Sleep-Disordered Breathing Substudy
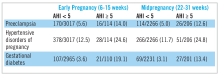
The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Sleep-Disordered Breathing Substudy (nuMoM2b-SDB) was a prospective cohort study.22,23 Level 3 home sleep tests were performed using a six-channel monitor that was self-applied by the participant twice during pregnancy, first between 60 and 150 weeks of pregnancy and then again between 220 and 310 weeks. An apnea-hypopnea index (AHI) of at least 5 was used to define SDB. The study was powered to test the primary hypothesis that SDB occurring in pregnancy is associated with an increased incidence of preeclampsia. Secondary outcomes were rates of hypertensive disorders of pregnancy, defined as preeclampsia and prenatal gestational hypertension, and gestational diabetes. Crude and adjusted odds ratios and 95% confidence intervals were calculated from univariate and multivariate logistic regression models. Adjustment covariates included maternal age (less than or equal to 21, 22-35, and over 35 years), body mass index (less than 25, 25 to less than 30, greater than or equal to 30 kg/m2), chronic hypertension (yes, no), and, for midpregnancy, rate of weight gain per week between early and midpregnancy assessments, treated as a continuous variable.
Conclusions and future directions
The nuMoM2b data are provocative because sleep apnea is a potentially modifiable risk factor for adverse pregnancy outcomes. While a majority of SDB cases identified during pregnancy were mild, the nuMoM2b data demonstrate that even modest elevations of AHI in pregnancy are associated with an increased risk of developing hypertensive disorders and an increased incidence of gestational diabetes.
Pregnancy is conceivably an ideal scenario in which to better understand the role of SDB treatment as a preventive strategy for reducing cardiometabolic morbidity as the time frame needed to measure incident outcomes after initiating therapy is significantly contracted. However, data regarding the role of OSA treatment with continuous positive airway pressure (CPAP) during pregnancy, both regarding its acceptability to patients and its therapeutic benefit, are extremely limited. Further research is needed to establish whether universal screening for and treating of SDB in pregnancy can mitigate the risks and consequences of hypertensive disorders of pregnancy and gestational diabetes. However, in the meantime, we have to recognize that as our obstetric patient population is becoming more obese, we will encounter more women with symptomatic SDB in pregnancy. It is well documented that patients with symptomatic SDB, those who report that their snoring leads to chronic sleep disruption and excessive daytime sleepiness, can benefit from CPAP in terms of sleep quality and daytime function. Therefore, in addition to encouraging women already prescribed CPAP to continue their therapy during pregnancy, obstetricians who encounter a patient reporting severe SDB symptoms should refer her to a sleep specialist for further evaluation.
Dr. Facco is assistant professor, department of obstetrics and gynecology, University of Pittsburgh, Magee-Women’s Hospital, Magee Women’s Research Institute.
References
1. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619.
2. Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86(6):549-54; quiz, 554-5.
3. Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, Ill.: American Academy of Sleep Medicine, 2007.
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 May 1;177(9):1006-14.
5. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-5.
6. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181-5.
7. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405-17.
8. Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers’ sleep. Sleep Med. 2002;3(1):37-42.
9. Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299-1305.
10. Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77-83.
11. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378-84.
12. Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521-30.
13. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med. 2005;172(12):1590-5.
14. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112.
15. Romero R, Badr MS. A role for sleep disorders in pregnancy complications: Challenges and opportunities. Am J Obstet Gynecol. 2014;210(1):3-11.
16. O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: Prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487.e1-9
17. Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136.e1-5.
18. Bourjeily G, Raker CA, Chalhoub M, Miller MA, et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849-55.
19. Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085-92.
20. Facco FL, Ouyang DW, Zee PC, et al. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2014 Jun;210(6):559.e1-6.
21. Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371-7.
22. Facco FL, Parker CB, Reddy UM, et al. NuMoM2b Sleep-Disordered Breathing study: Objectives and methods. Am J Obstet Gynecol. 2015 April;212(4):542.e1–542.e127.
23. Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregn ancy and gestational diabetes mellitus. Obstet Gynecol. ePub. 2016 Dec 2.
Background
Sleep-disordered breathing (SDB) conditions are characterized by abnormal respiratory patterns and abnormal gas exchange during sleep.1-3 Obstructive sleep apnea (OSA), the most common type of SDB, is characterized by repetitive episodes of airway narrowing during sleep that lead to respiratory disruption, hypoxia, and sleep fragmentation. In reproductive-aged women, epidemiologic studies suggest a 2% to 13% prevalence of OSA.4-6 Pregnancy is associated with changes that promote OSA, such as weight gain and edema of the upper airway.7 Frequent snoring, a common symptom of OSA, is endorsed by 15% to 25% of pregnant women.8-10 Health outcomes that have been linked to SDB in the nonpregnant population, such as hypertension and insulin-resistant diabetes, have clinically relevant correlates in pregnancy (preeclampsia, gestational diabetes).11-13
While several retrospective and cross-sectional studies suggest that SDB may increase the risk of developing hypertensive disorders and gestational diabetes during pregnancy,16-18 up until recently, there were limited and conflicting data from prospective observational cohorts in which SDB exposure and pregnancy outcomes have been methodically measured and confounding variables carefully considered.19-21 Louis et al.19 reported on a cohort of 175 obese women and demonstrated that women with SDB (apnea-hypopnea index greater than or equal to 5) were more likely to develop preeclampsia (adjusted odds ratio, 3.5; 95% CI, 1.3, 9.9). However, two other small studies failed to demonstrate a positive association between SDB and pregnancy-related hypertension, but one suggested a relationship between SDB and gestational diabetes.20,21
Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Sleep-Disordered Breathing Substudy
The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Sleep-Disordered Breathing Substudy (nuMoM2b-SDB) was a prospective cohort study.22,23 Level 3 home sleep tests were performed using a six-channel monitor that was self-applied by the participant twice during pregnancy, first between 60 and 150 weeks of pregnancy and then again between 220 and 310 weeks. An apnea-hypopnea index (AHI) of at least 5 was used to define SDB. The study was powered to test the primary hypothesis that SDB occurring in pregnancy is associated with an increased incidence of preeclampsia. Secondary outcomes were rates of hypertensive disorders of pregnancy, defined as preeclampsia and prenatal gestational hypertension, and gestational diabetes. Crude and adjusted odds ratios and 95% confidence intervals were calculated from univariate and multivariate logistic regression models. Adjustment covariates included maternal age (less than or equal to 21, 22-35, and over 35 years), body mass index (less than 25, 25 to less than 30, greater than or equal to 30 kg/m2), chronic hypertension (yes, no), and, for midpregnancy, rate of weight gain per week between early and midpregnancy assessments, treated as a continuous variable.
Conclusions and future directions
The nuMoM2b data are provocative because sleep apnea is a potentially modifiable risk factor for adverse pregnancy outcomes. While a majority of SDB cases identified during pregnancy were mild, the nuMoM2b data demonstrate that even modest elevations of AHI in pregnancy are associated with an increased risk of developing hypertensive disorders and an increased incidence of gestational diabetes.
Pregnancy is conceivably an ideal scenario in which to better understand the role of SDB treatment as a preventive strategy for reducing cardiometabolic morbidity as the time frame needed to measure incident outcomes after initiating therapy is significantly contracted. However, data regarding the role of OSA treatment with continuous positive airway pressure (CPAP) during pregnancy, both regarding its acceptability to patients and its therapeutic benefit, are extremely limited. Further research is needed to establish whether universal screening for and treating of SDB in pregnancy can mitigate the risks and consequences of hypertensive disorders of pregnancy and gestational diabetes. However, in the meantime, we have to recognize that as our obstetric patient population is becoming more obese, we will encounter more women with symptomatic SDB in pregnancy. It is well documented that patients with symptomatic SDB, those who report that their snoring leads to chronic sleep disruption and excessive daytime sleepiness, can benefit from CPAP in terms of sleep quality and daytime function. Therefore, in addition to encouraging women already prescribed CPAP to continue their therapy during pregnancy, obstetricians who encounter a patient reporting severe SDB symptoms should refer her to a sleep specialist for further evaluation.
Dr. Facco is assistant professor, department of obstetrics and gynecology, University of Pittsburgh, Magee-Women’s Hospital, Magee Women’s Research Institute.
References
1. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619.
2. Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86(6):549-54; quiz, 554-5.
3. Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, Ill.: American Academy of Sleep Medicine, 2007.
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 May 1;177(9):1006-14.
5. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-5.
6. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181-5.
7. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405-17.
8. Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers’ sleep. Sleep Med. 2002;3(1):37-42.
9. Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299-1305.
10. Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77-83.
11. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378-84.
12. Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521-30.
13. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med. 2005;172(12):1590-5.
14. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112.
15. Romero R, Badr MS. A role for sleep disorders in pregnancy complications: Challenges and opportunities. Am J Obstet Gynecol. 2014;210(1):3-11.
16. O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: Prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487.e1-9
17. Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136.e1-5.
18. Bourjeily G, Raker CA, Chalhoub M, Miller MA, et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849-55.
19. Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085-92.
20. Facco FL, Ouyang DW, Zee PC, et al. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2014 Jun;210(6):559.e1-6.
21. Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371-7.
22. Facco FL, Parker CB, Reddy UM, et al. NuMoM2b Sleep-Disordered Breathing study: Objectives and methods. Am J Obstet Gynecol. 2015 April;212(4):542.e1–542.e127.
23. Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregn ancy and gestational diabetes mellitus. Obstet Gynecol. ePub. 2016 Dec 2.
Background
Sleep-disordered breathing (SDB) conditions are characterized by abnormal respiratory patterns and abnormal gas exchange during sleep.1-3 Obstructive sleep apnea (OSA), the most common type of SDB, is characterized by repetitive episodes of airway narrowing during sleep that lead to respiratory disruption, hypoxia, and sleep fragmentation. In reproductive-aged women, epidemiologic studies suggest a 2% to 13% prevalence of OSA.4-6 Pregnancy is associated with changes that promote OSA, such as weight gain and edema of the upper airway.7 Frequent snoring, a common symptom of OSA, is endorsed by 15% to 25% of pregnant women.8-10 Health outcomes that have been linked to SDB in the nonpregnant population, such as hypertension and insulin-resistant diabetes, have clinically relevant correlates in pregnancy (preeclampsia, gestational diabetes).11-13
While several retrospective and cross-sectional studies suggest that SDB may increase the risk of developing hypertensive disorders and gestational diabetes during pregnancy,16-18 up until recently, there were limited and conflicting data from prospective observational cohorts in which SDB exposure and pregnancy outcomes have been methodically measured and confounding variables carefully considered.19-21 Louis et al.19 reported on a cohort of 175 obese women and demonstrated that women with SDB (apnea-hypopnea index greater than or equal to 5) were more likely to develop preeclampsia (adjusted odds ratio, 3.5; 95% CI, 1.3, 9.9). However, two other small studies failed to demonstrate a positive association between SDB and pregnancy-related hypertension, but one suggested a relationship between SDB and gestational diabetes.20,21
Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Sleep-Disordered Breathing Substudy
The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be Sleep-Disordered Breathing Substudy (nuMoM2b-SDB) was a prospective cohort study.22,23 Level 3 home sleep tests were performed using a six-channel monitor that was self-applied by the participant twice during pregnancy, first between 60 and 150 weeks of pregnancy and then again between 220 and 310 weeks. An apnea-hypopnea index (AHI) of at least 5 was used to define SDB. The study was powered to test the primary hypothesis that SDB occurring in pregnancy is associated with an increased incidence of preeclampsia. Secondary outcomes were rates of hypertensive disorders of pregnancy, defined as preeclampsia and prenatal gestational hypertension, and gestational diabetes. Crude and adjusted odds ratios and 95% confidence intervals were calculated from univariate and multivariate logistic regression models. Adjustment covariates included maternal age (less than or equal to 21, 22-35, and over 35 years), body mass index (less than 25, 25 to less than 30, greater than or equal to 30 kg/m2), chronic hypertension (yes, no), and, for midpregnancy, rate of weight gain per week between early and midpregnancy assessments, treated as a continuous variable.
Conclusions and future directions
The nuMoM2b data are provocative because sleep apnea is a potentially modifiable risk factor for adverse pregnancy outcomes. While a majority of SDB cases identified during pregnancy were mild, the nuMoM2b data demonstrate that even modest elevations of AHI in pregnancy are associated with an increased risk of developing hypertensive disorders and an increased incidence of gestational diabetes.
Pregnancy is conceivably an ideal scenario in which to better understand the role of SDB treatment as a preventive strategy for reducing cardiometabolic morbidity as the time frame needed to measure incident outcomes after initiating therapy is significantly contracted. However, data regarding the role of OSA treatment with continuous positive airway pressure (CPAP) during pregnancy, both regarding its acceptability to patients and its therapeutic benefit, are extremely limited. Further research is needed to establish whether universal screening for and treating of SDB in pregnancy can mitigate the risks and consequences of hypertensive disorders of pregnancy and gestational diabetes. However, in the meantime, we have to recognize that as our obstetric patient population is becoming more obese, we will encounter more women with symptomatic SDB in pregnancy. It is well documented that patients with symptomatic SDB, those who report that their snoring leads to chronic sleep disruption and excessive daytime sleepiness, can benefit from CPAP in terms of sleep quality and daytime function. Therefore, in addition to encouraging women already prescribed CPAP to continue their therapy during pregnancy, obstetricians who encounter a patient reporting severe SDB symptoms should refer her to a sleep specialist for further evaluation.
Dr. Facco is assistant professor, department of obstetrics and gynecology, University of Pittsburgh, Magee-Women’s Hospital, Magee Women’s Research Institute.
References
1. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619.
2. Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86(6):549-54; quiz, 554-5.
3. Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, Ill.: American Academy of Sleep Medicine, 2007.
4. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 May 1;177(9):1006-14.
5. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230-5.
6. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181-5.
7. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405-17.
8. Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers’ sleep. Sleep Med. 2002;3(1):37-42.
9. Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299-1305.
10. Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77-83.
11. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378-84.
12. Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521-30.
13. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med. 2005;172(12):1590-5.
14. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47-112.
15. Romero R, Badr MS. A role for sleep disorders in pregnancy complications: Challenges and opportunities. Am J Obstet Gynecol. 2014;210(1):3-11.
16. O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: Prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487.e1-9
17. Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136.e1-5.
18. Bourjeily G, Raker CA, Chalhoub M, Miller MA, et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849-55.
19. Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085-92.
20. Facco FL, Ouyang DW, Zee PC, et al. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2014 Jun;210(6):559.e1-6.
21. Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371-7.
22. Facco FL, Parker CB, Reddy UM, et al. NuMoM2b Sleep-Disordered Breathing study: Objectives and methods. Am J Obstet Gynecol. 2015 April;212(4):542.e1–542.e127.
23. Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregn ancy and gestational diabetes mellitus. Obstet Gynecol. ePub. 2016 Dec 2.
NETWORKS: Pneumonia Day, evaluating inhalers, tobacco taxes Chest Infections Clinical Pulmonary Medicine Interprofessional Team
Pneumonia Day: Today is the day to act!
This past November 12, we celebrated “Pneumonia Day,” named for a disease that has little connotation in the real world, because of the perception that we need only a short course of antibiotics to get better. Such is the origin of the term “walking pneumonia,” which emphasizes that we can still walk even while sick with pneumonia.
However, we recently experienced the most important moment of awareness related to this condition, when one of the U.S. presidential candidates became sick with that disease known as “pneumonia.”
Suddenly, the media devoted great interest to explore this condition, as if it were a new outbreak or a rare disease that could potentially kill someone. Even the health-care providers seem to believe that “pneumonia” is not a big deal, ignoring the fact that it is the most common infectious cause of death overall, and that it not only affects children but also the elderly and patients with poor immune systems.
One out of nine patients who are admitted to the hospital for pneumonia may die during the hospitalization, and one out of four patients who get admitted to an ICU may not survive the event.
However, it also highlights that pneumonia is more than just an acute disease, compromising the brain, heart, and kidneys. In the long run, even after surviving the hospitalization for pneumonia, it can kill and cause other well-known complications leading to death, such as myocardial infarction, arrhythmias, heart failure, and sudden cardiac death.
Please, stop for one moment and ask yourself about your role in preventing pneumonia and pneumonia-related deaths in your communities. The Chest Infections NetWork is here to help you advocate for the common goal of solving this problem.
Steering Committee Member
Delivery makes a difference: Providing inhaled medication to your patients
One might ask why CHEST (American College of Chest Physicians) and Sunovion developed a steering committee of experts in the field of obstructive lung disease to evaluate the knowledge, attitudes, beliefs, and practices of physicians and other health-care professionals related to inhalational medicines and devices. While inhalers are approved by the FDA Center for Drug Evaluation Research (CEDER) as drug and device combination, the process assesses reproducibility and shelf‐life but does not address the real‐world situation that each of us face with individual patients. How often do clinicians consider the characteristics of each delivery system, as well as the medication being delivered? One might be surprised at the answer.
Patients are frequently prescribed several types of devices with different instructions for optimal use. For example, dry powder inhalers often require high flow rates (30-90 L/min) to deaggregate powder pellets into particles less than 5 mcm, while metereddose inhalers require a slow inspiratory flow (less than 30 L/min). Patients who use both types of devices often confuse which inspiratory flow rate to use with which devices, despite proper education and training. This does not even take into consideration the variable number of steps required by various inhalational devices (which can be as few as 3 steps to as many as 12 steps). Additionally, studies demonstrate that peak inspiratory flow rates, inspiratory volumes, and drug deposition in the lungs may be influenced by gender, height, and weight; as well as by the degree of pulmonary reserve and hyperinflation.
Are there data to suggest that these questions impact the care of patients with severe asthma or COPD? I eagerly await the results of the survey.
Steering Committee Member
A California victory for tobacco control
Californians approved Proposition 56, “Cigarette Tax to Fund Healthcare, Tobacco Use Prevention, Research, and Law Enforcement.” This measure increases the excise tax on all forms of tobacco by $2.00. For the first time, it applies to electronic products that vaporize nicotine that were previously only subject to sales tax. This is in addition to federal excise taxes ($1.01) and state and local sales taxes ($0.50 to $0.60). (https://ballotpedia.org/California_Proposition_56,_Tobacco _Tax_Increase_(2016)
When Prop 56 goes into effect April 1, 2017, the average price of a package of cigarettes will increase to at least $7.89. Based on data from the Surgeon General’s report on “Preventing Tobacco Use Among Youth and Young Adults,” this tax increase should equate with a fall in smoking rates by about 12%. Youth and young adults are particularly susceptible to price increases, which helps prevent smoking initiation or continuation.
Tobacco-related health-care costs Californians $3.5 billion dollars annually (Official Voter Information Guide, 2016). Funds raised by Prop 56 will be used by state and local health programs such as Medi-Cal to defray the costs of smoking prevention pro
Prop 56 expands on tougher laws implemented in 2016 that expanded the workplace prohibition of smoking, increased fees for tobacco retailers and wholesalers, broadened the definition of smoking to include e-cigarettes, and increased the minimum age to purchase tobacco to 21 years old. Combined, these measures are expected to result in a further decline in tobacco usage in California.
Alan Roth, RRT, MS, FCCP
Steering Committee Member
Pneumonia Day: Today is the day to act!
This past November 12, we celebrated “Pneumonia Day,” named for a disease that has little connotation in the real world, because of the perception that we need only a short course of antibiotics to get better. Such is the origin of the term “walking pneumonia,” which emphasizes that we can still walk even while sick with pneumonia.
However, we recently experienced the most important moment of awareness related to this condition, when one of the U.S. presidential candidates became sick with that disease known as “pneumonia.”
Suddenly, the media devoted great interest to explore this condition, as if it were a new outbreak or a rare disease that could potentially kill someone. Even the health-care providers seem to believe that “pneumonia” is not a big deal, ignoring the fact that it is the most common infectious cause of death overall, and that it not only affects children but also the elderly and patients with poor immune systems.
One out of nine patients who are admitted to the hospital for pneumonia may die during the hospitalization, and one out of four patients who get admitted to an ICU may not survive the event.
However, it also highlights that pneumonia is more than just an acute disease, compromising the brain, heart, and kidneys. In the long run, even after surviving the hospitalization for pneumonia, it can kill and cause other well-known complications leading to death, such as myocardial infarction, arrhythmias, heart failure, and sudden cardiac death.
Please, stop for one moment and ask yourself about your role in preventing pneumonia and pneumonia-related deaths in your communities. The Chest Infections NetWork is here to help you advocate for the common goal of solving this problem.
Steering Committee Member
Delivery makes a difference: Providing inhaled medication to your patients
One might ask why CHEST (American College of Chest Physicians) and Sunovion developed a steering committee of experts in the field of obstructive lung disease to evaluate the knowledge, attitudes, beliefs, and practices of physicians and other health-care professionals related to inhalational medicines and devices. While inhalers are approved by the FDA Center for Drug Evaluation Research (CEDER) as drug and device combination, the process assesses reproducibility and shelf‐life but does not address the real‐world situation that each of us face with individual patients. How often do clinicians consider the characteristics of each delivery system, as well as the medication being delivered? One might be surprised at the answer.
Patients are frequently prescribed several types of devices with different instructions for optimal use. For example, dry powder inhalers often require high flow rates (30-90 L/min) to deaggregate powder pellets into particles less than 5 mcm, while metereddose inhalers require a slow inspiratory flow (less than 30 L/min). Patients who use both types of devices often confuse which inspiratory flow rate to use with which devices, despite proper education and training. This does not even take into consideration the variable number of steps required by various inhalational devices (which can be as few as 3 steps to as many as 12 steps). Additionally, studies demonstrate that peak inspiratory flow rates, inspiratory volumes, and drug deposition in the lungs may be influenced by gender, height, and weight; as well as by the degree of pulmonary reserve and hyperinflation.
Are there data to suggest that these questions impact the care of patients with severe asthma or COPD? I eagerly await the results of the survey.
Steering Committee Member
A California victory for tobacco control
Californians approved Proposition 56, “Cigarette Tax to Fund Healthcare, Tobacco Use Prevention, Research, and Law Enforcement.” This measure increases the excise tax on all forms of tobacco by $2.00. For the first time, it applies to electronic products that vaporize nicotine that were previously only subject to sales tax. This is in addition to federal excise taxes ($1.01) and state and local sales taxes ($0.50 to $0.60). (https://ballotpedia.org/California_Proposition_56,_Tobacco _Tax_Increase_(2016)
When Prop 56 goes into effect April 1, 2017, the average price of a package of cigarettes will increase to at least $7.89. Based on data from the Surgeon General’s report on “Preventing Tobacco Use Among Youth and Young Adults,” this tax increase should equate with a fall in smoking rates by about 12%. Youth and young adults are particularly susceptible to price increases, which helps prevent smoking initiation or continuation.
Tobacco-related health-care costs Californians $3.5 billion dollars annually (Official Voter Information Guide, 2016). Funds raised by Prop 56 will be used by state and local health programs such as Medi-Cal to defray the costs of smoking prevention pro
Prop 56 expands on tougher laws implemented in 2016 that expanded the workplace prohibition of smoking, increased fees for tobacco retailers and wholesalers, broadened the definition of smoking to include e-cigarettes, and increased the minimum age to purchase tobacco to 21 years old. Combined, these measures are expected to result in a further decline in tobacco usage in California.
Alan Roth, RRT, MS, FCCP
Steering Committee Member
Pneumonia Day: Today is the day to act!
This past November 12, we celebrated “Pneumonia Day,” named for a disease that has little connotation in the real world, because of the perception that we need only a short course of antibiotics to get better. Such is the origin of the term “walking pneumonia,” which emphasizes that we can still walk even while sick with pneumonia.
However, we recently experienced the most important moment of awareness related to this condition, when one of the U.S. presidential candidates became sick with that disease known as “pneumonia.”
Suddenly, the media devoted great interest to explore this condition, as if it were a new outbreak or a rare disease that could potentially kill someone. Even the health-care providers seem to believe that “pneumonia” is not a big deal, ignoring the fact that it is the most common infectious cause of death overall, and that it not only affects children but also the elderly and patients with poor immune systems.
One out of nine patients who are admitted to the hospital for pneumonia may die during the hospitalization, and one out of four patients who get admitted to an ICU may not survive the event.
However, it also highlights that pneumonia is more than just an acute disease, compromising the brain, heart, and kidneys. In the long run, even after surviving the hospitalization for pneumonia, it can kill and cause other well-known complications leading to death, such as myocardial infarction, arrhythmias, heart failure, and sudden cardiac death.
Please, stop for one moment and ask yourself about your role in preventing pneumonia and pneumonia-related deaths in your communities. The Chest Infections NetWork is here to help you advocate for the common goal of solving this problem.
Steering Committee Member
Delivery makes a difference: Providing inhaled medication to your patients
One might ask why CHEST (American College of Chest Physicians) and Sunovion developed a steering committee of experts in the field of obstructive lung disease to evaluate the knowledge, attitudes, beliefs, and practices of physicians and other health-care professionals related to inhalational medicines and devices. While inhalers are approved by the FDA Center for Drug Evaluation Research (CEDER) as drug and device combination, the process assesses reproducibility and shelf‐life but does not address the real‐world situation that each of us face with individual patients. How often do clinicians consider the characteristics of each delivery system, as well as the medication being delivered? One might be surprised at the answer.
Patients are frequently prescribed several types of devices with different instructions for optimal use. For example, dry powder inhalers often require high flow rates (30-90 L/min) to deaggregate powder pellets into particles less than 5 mcm, while metereddose inhalers require a slow inspiratory flow (less than 30 L/min). Patients who use both types of devices often confuse which inspiratory flow rate to use with which devices, despite proper education and training. This does not even take into consideration the variable number of steps required by various inhalational devices (which can be as few as 3 steps to as many as 12 steps). Additionally, studies demonstrate that peak inspiratory flow rates, inspiratory volumes, and drug deposition in the lungs may be influenced by gender, height, and weight; as well as by the degree of pulmonary reserve and hyperinflation.
Are there data to suggest that these questions impact the care of patients with severe asthma or COPD? I eagerly await the results of the survey.
Steering Committee Member
A California victory for tobacco control
Californians approved Proposition 56, “Cigarette Tax to Fund Healthcare, Tobacco Use Prevention, Research, and Law Enforcement.” This measure increases the excise tax on all forms of tobacco by $2.00. For the first time, it applies to electronic products that vaporize nicotine that were previously only subject to sales tax. This is in addition to federal excise taxes ($1.01) and state and local sales taxes ($0.50 to $0.60). (https://ballotpedia.org/California_Proposition_56,_Tobacco _Tax_Increase_(2016)
When Prop 56 goes into effect April 1, 2017, the average price of a package of cigarettes will increase to at least $7.89. Based on data from the Surgeon General’s report on “Preventing Tobacco Use Among Youth and Young Adults,” this tax increase should equate with a fall in smoking rates by about 12%. Youth and young adults are particularly susceptible to price increases, which helps prevent smoking initiation or continuation.
Tobacco-related health-care costs Californians $3.5 billion dollars annually (Official Voter Information Guide, 2016). Funds raised by Prop 56 will be used by state and local health programs such as Medi-Cal to defray the costs of smoking prevention pro
Prop 56 expands on tougher laws implemented in 2016 that expanded the workplace prohibition of smoking, increased fees for tobacco retailers and wholesalers, broadened the definition of smoking to include e-cigarettes, and increased the minimum age to purchase tobacco to 21 years old. Combined, these measures are expected to result in a further decline in tobacco usage in California.
Alan Roth, RRT, MS, FCCP
Steering Committee Member
This month in CHEST : Editor’s picks
Editorial
Spread the Word About CHEST for 2017: Collaboration With Elsevier, Publishing of Guidelines, More Multimedia Content, and Changes for Reviewers and Authors. By Dr. Richard S. Irwin; Dr. John E. Heffner; Jean Rice; Dr. Cynthia T. French; on behalf of the Editorial Leadership Team.
Point Counterpoint Editorial
POINT: Will New Anti-eosinophilic Drugs Be Useful In Asthma Management?
Yes. Dr. P.M. O’Byrne
No. Dr. P. Barnes
Giants in Chest Medicine
Dr. Claude Lenfant. By Dr. E.J. Roccella.
Special Feature
The Eighth Edition Lung Cancer Stage Classification. By Dr. F.C. Detterbeck, et al.
Evidence-based MedicineLiberation From Mechanical Ventilation in Critically Ill Adults: Executive Summary of an Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. By Dr. G.A. Schmidt, et al.
Original ResearchEffect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States. By Dr. R.A. Balk, et al.
Use of Palliative Care in Patients With End-Stage COPD and Receiving Home Oxygen: National Trends and Barriers to Care in the United States. By Dr. B. Rush, et al.
Editorial
Spread the Word About CHEST for 2017: Collaboration With Elsevier, Publishing of Guidelines, More Multimedia Content, and Changes for Reviewers and Authors. By Dr. Richard S. Irwin; Dr. John E. Heffner; Jean Rice; Dr. Cynthia T. French; on behalf of the Editorial Leadership Team.
Point Counterpoint Editorial
POINT: Will New Anti-eosinophilic Drugs Be Useful In Asthma Management?
Yes. Dr. P.M. O’Byrne
No. Dr. P. Barnes
Giants in Chest Medicine
Dr. Claude Lenfant. By Dr. E.J. Roccella.
Special Feature
The Eighth Edition Lung Cancer Stage Classification. By Dr. F.C. Detterbeck, et al.
Evidence-based MedicineLiberation From Mechanical Ventilation in Critically Ill Adults: Executive Summary of an Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. By Dr. G.A. Schmidt, et al.
Original ResearchEffect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States. By Dr. R.A. Balk, et al.
Use of Palliative Care in Patients With End-Stage COPD and Receiving Home Oxygen: National Trends and Barriers to Care in the United States. By Dr. B. Rush, et al.
Editorial
Spread the Word About CHEST for 2017: Collaboration With Elsevier, Publishing of Guidelines, More Multimedia Content, and Changes for Reviewers and Authors. By Dr. Richard S. Irwin; Dr. John E. Heffner; Jean Rice; Dr. Cynthia T. French; on behalf of the Editorial Leadership Team.
Point Counterpoint Editorial
POINT: Will New Anti-eosinophilic Drugs Be Useful In Asthma Management?
Yes. Dr. P.M. O’Byrne
No. Dr. P. Barnes
Giants in Chest Medicine
Dr. Claude Lenfant. By Dr. E.J. Roccella.
Special Feature
The Eighth Edition Lung Cancer Stage Classification. By Dr. F.C. Detterbeck, et al.
Evidence-based MedicineLiberation From Mechanical Ventilation in Critically Ill Adults: Executive Summary of an Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. By Dr. G.A. Schmidt, et al.
Original ResearchEffect of Procalcitonin Testing on Health-care Utilization and Costs in Critically Ill Patients in the United States. By Dr. R.A. Balk, et al.
Use of Palliative Care in Patients With End-Stage COPD and Receiving Home Oxygen: National Trends and Barriers to Care in the United States. By Dr. B. Rush, et al.
Introducing our new Editorial Board Members