User login
AGA remembers former AGA President Marvin Sleisenger, MD, AGAF
Marvin H. Sleisenger, MD, AGAF, of Kentfield, Calif., died at age 93 on Thursday, Oct. 19, 2017. Sleisenger served as editor of Gastroenterology from 1965 to 1970, and as president of AGA in 1976.
Sleisenger attended Harvard College and Harvard Medical School. He trained at Harvard, the University of Pennsylvania, and Cornell Medical School. During the Korean War, he served in the U.S. Naval Medical Corps. He was a member of the faculty at Cornell Medical School and in 1954, was appointed as chief of the division of gastroenterology. In 1968, he became professor and vice chairman of the department of medicine of the University of California, San Francisco and chief of the medical service at the Veterans Administration Hospital. His achievements as an outstanding educator were recognized in 1994 when he became the recipient of the AGA Distinguished Educator Award.
Sleisenger’s full obituary was published in the SFGate. Members, colleagues, and friends posted remembrances in the Community.
Memorial services were held on Sunday, Oct. 29, 2017, at 11 a.m., at the Chapel of the Mt. Tamalpais Cemetery, 2500 Fifth Avenue, San Rafael, Calif.
Marvin H. Sleisenger, MD, AGAF, of Kentfield, Calif., died at age 93 on Thursday, Oct. 19, 2017. Sleisenger served as editor of Gastroenterology from 1965 to 1970, and as president of AGA in 1976.
Sleisenger attended Harvard College and Harvard Medical School. He trained at Harvard, the University of Pennsylvania, and Cornell Medical School. During the Korean War, he served in the U.S. Naval Medical Corps. He was a member of the faculty at Cornell Medical School and in 1954, was appointed as chief of the division of gastroenterology. In 1968, he became professor and vice chairman of the department of medicine of the University of California, San Francisco and chief of the medical service at the Veterans Administration Hospital. His achievements as an outstanding educator were recognized in 1994 when he became the recipient of the AGA Distinguished Educator Award.
Sleisenger’s full obituary was published in the SFGate. Members, colleagues, and friends posted remembrances in the Community.
Memorial services were held on Sunday, Oct. 29, 2017, at 11 a.m., at the Chapel of the Mt. Tamalpais Cemetery, 2500 Fifth Avenue, San Rafael, Calif.
Marvin H. Sleisenger, MD, AGAF, of Kentfield, Calif., died at age 93 on Thursday, Oct. 19, 2017. Sleisenger served as editor of Gastroenterology from 1965 to 1970, and as president of AGA in 1976.
Sleisenger attended Harvard College and Harvard Medical School. He trained at Harvard, the University of Pennsylvania, and Cornell Medical School. During the Korean War, he served in the U.S. Naval Medical Corps. He was a member of the faculty at Cornell Medical School and in 1954, was appointed as chief of the division of gastroenterology. In 1968, he became professor and vice chairman of the department of medicine of the University of California, San Francisco and chief of the medical service at the Veterans Administration Hospital. His achievements as an outstanding educator were recognized in 1994 when he became the recipient of the AGA Distinguished Educator Award.
Sleisenger’s full obituary was published in the SFGate. Members, colleagues, and friends posted remembrances in the Community.
Memorial services were held on Sunday, Oct. 29, 2017, at 11 a.m., at the Chapel of the Mt. Tamalpais Cemetery, 2500 Fifth Avenue, San Rafael, Calif.
A letter from Dr. Robert S. Sandler, MPH, AGAF
Dear Colleagues,
Where would clinical practice be today without GI research?
The way we diagnose and treat patients is thanks to years of research. But as you know, federal research funding is at risk. Promising, early-stage investigators find it increasingly difficult to secure funding and many leave the field because they are unable to sustain a research career.
This is bad news for digestive health patients and the clinicians who care for them.
As a member of the GI community, you understand the need to continually advance the science and practice of gastroenterology. You understand the physical, emotional, and financial costs of digestive diseases. And you understand the tremendous value of research to advance patient care.
At a time when we are on the brink of major scientific breakthroughs, there is a growing gap in federal funding for research. Many well-qualified young investigators cannot get government funding. Gifts to the AGA Research Foundation this year directly supported 52 talented investigators. Despite this success, over 200 other innovative and promising research ideas went unfunded.
I am asking you to support a cause important to me and equally important to you. You can help fill the funding gap and protect the next generation of investigators by joining me in supporting the AGA Research Foundation through a personal gift.
Every dollar is a step forward...to new treatments. To cures impacting patients’ lives. To new generations of talented investigators in digestive disease research.
Please help us continue our efforts by making your tax-deductible donation. Donate today at www.gastro.org/donate.
Thank you in advance for your support and best wishes for a happy, healthy holiday season and successful New Year.
Three easy ways to give
Online: www.gastro.org/donateThrough the mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
Over the phone: 301-222-4002
All gifts are tax-deductible to the fullest extent of U.S. law.
Dear Colleagues,
Where would clinical practice be today without GI research?
The way we diagnose and treat patients is thanks to years of research. But as you know, federal research funding is at risk. Promising, early-stage investigators find it increasingly difficult to secure funding and many leave the field because they are unable to sustain a research career.
This is bad news for digestive health patients and the clinicians who care for them.
As a member of the GI community, you understand the need to continually advance the science and practice of gastroenterology. You understand the physical, emotional, and financial costs of digestive diseases. And you understand the tremendous value of research to advance patient care.
At a time when we are on the brink of major scientific breakthroughs, there is a growing gap in federal funding for research. Many well-qualified young investigators cannot get government funding. Gifts to the AGA Research Foundation this year directly supported 52 talented investigators. Despite this success, over 200 other innovative and promising research ideas went unfunded.
I am asking you to support a cause important to me and equally important to you. You can help fill the funding gap and protect the next generation of investigators by joining me in supporting the AGA Research Foundation through a personal gift.
Every dollar is a step forward...to new treatments. To cures impacting patients’ lives. To new generations of talented investigators in digestive disease research.
Please help us continue our efforts by making your tax-deductible donation. Donate today at www.gastro.org/donate.
Thank you in advance for your support and best wishes for a happy, healthy holiday season and successful New Year.
Three easy ways to give
Online: www.gastro.org/donateThrough the mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
Over the phone: 301-222-4002
All gifts are tax-deductible to the fullest extent of U.S. law.
Dear Colleagues,
Where would clinical practice be today without GI research?
The way we diagnose and treat patients is thanks to years of research. But as you know, federal research funding is at risk. Promising, early-stage investigators find it increasingly difficult to secure funding and many leave the field because they are unable to sustain a research career.
This is bad news for digestive health patients and the clinicians who care for them.
As a member of the GI community, you understand the need to continually advance the science and practice of gastroenterology. You understand the physical, emotional, and financial costs of digestive diseases. And you understand the tremendous value of research to advance patient care.
At a time when we are on the brink of major scientific breakthroughs, there is a growing gap in federal funding for research. Many well-qualified young investigators cannot get government funding. Gifts to the AGA Research Foundation this year directly supported 52 talented investigators. Despite this success, over 200 other innovative and promising research ideas went unfunded.
I am asking you to support a cause important to me and equally important to you. You can help fill the funding gap and protect the next generation of investigators by joining me in supporting the AGA Research Foundation through a personal gift.
Every dollar is a step forward...to new treatments. To cures impacting patients’ lives. To new generations of talented investigators in digestive disease research.
Please help us continue our efforts by making your tax-deductible donation. Donate today at www.gastro.org/donate.
Thank you in advance for your support and best wishes for a happy, healthy holiday season and successful New Year.
Three easy ways to give
Online: www.gastro.org/donateThrough the mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
Over the phone: 301-222-4002
All gifts are tax-deductible to the fullest extent of U.S. law.
DDW® 2018 abstract submission now open
The Digestive Disease Week® (DDW) 2018 call for abstracts is now open. The deadline to submit an abtsract is Dec. 1, 2017, at 9 p.m. ET.
Abstracts will be selected for oral, poster, or video presentation at DDW 2018. If accepted, your abstract will also be published in the online supplement to Gastroenterology or GIE: Gastrointestinal Endoscopy, two of the top-ranked journals in gastroenterology.
Abstracts presented at national meetings held outside of the United States may be submitted to DDW. Abstracts presented at national meetings held within the United States are not eligible, with the exceptions of the ACS Surgical Forum, the NASPGHAN Annual Meeting, and the Crohn’s & Colitis Congress.™ Authors of abstracts presented at those three meetings are welcome to submit to DDW. Please note that authors do not have to be members of one of the four sponsoring societies, AGA, AASLD, ASGE, and SSAT, in order to submit an abstract.
Easily navigate the online abstract submission process with a video tutorial, step-by-step guide, and full abstract submission guidelines at www.ddw.org/abstracts.
Join the effort in providing the latest clinical translational, and basic research to health care professionals in gastroenterology, hepatology, GI endoscopy, and GI surgery. Submit an abstract today.
Travel awards available
DDW will award up to 20 young investigators with the Basic Science Travel Award. Selected authors of basic science abstracts will receive a $1,000 travel grant along with recognition at a reception during DDW 2017. Residents and fellows are encouraged to apply.
The AGA Research Foundation also offers various travel awards:
- AGA-GRG Fellow Abstract Award
- AGA-Moti L. & Kamla Rustgi International Travel Awards
- AGA Student Abstract Award
For more information on travel awards and other DDW-related grants, please visit the DDW website, www.ddw.org.
The Digestive Disease Week® (DDW) 2018 call for abstracts is now open. The deadline to submit an abtsract is Dec. 1, 2017, at 9 p.m. ET.
Abstracts will be selected for oral, poster, or video presentation at DDW 2018. If accepted, your abstract will also be published in the online supplement to Gastroenterology or GIE: Gastrointestinal Endoscopy, two of the top-ranked journals in gastroenterology.
Abstracts presented at national meetings held outside of the United States may be submitted to DDW. Abstracts presented at national meetings held within the United States are not eligible, with the exceptions of the ACS Surgical Forum, the NASPGHAN Annual Meeting, and the Crohn’s & Colitis Congress.™ Authors of abstracts presented at those three meetings are welcome to submit to DDW. Please note that authors do not have to be members of one of the four sponsoring societies, AGA, AASLD, ASGE, and SSAT, in order to submit an abstract.
Easily navigate the online abstract submission process with a video tutorial, step-by-step guide, and full abstract submission guidelines at www.ddw.org/abstracts.
Join the effort in providing the latest clinical translational, and basic research to health care professionals in gastroenterology, hepatology, GI endoscopy, and GI surgery. Submit an abstract today.
Travel awards available
DDW will award up to 20 young investigators with the Basic Science Travel Award. Selected authors of basic science abstracts will receive a $1,000 travel grant along with recognition at a reception during DDW 2017. Residents and fellows are encouraged to apply.
The AGA Research Foundation also offers various travel awards:
- AGA-GRG Fellow Abstract Award
- AGA-Moti L. & Kamla Rustgi International Travel Awards
- AGA Student Abstract Award
For more information on travel awards and other DDW-related grants, please visit the DDW website, www.ddw.org.
The Digestive Disease Week® (DDW) 2018 call for abstracts is now open. The deadline to submit an abtsract is Dec. 1, 2017, at 9 p.m. ET.
Abstracts will be selected for oral, poster, or video presentation at DDW 2018. If accepted, your abstract will also be published in the online supplement to Gastroenterology or GIE: Gastrointestinal Endoscopy, two of the top-ranked journals in gastroenterology.
Abstracts presented at national meetings held outside of the United States may be submitted to DDW. Abstracts presented at national meetings held within the United States are not eligible, with the exceptions of the ACS Surgical Forum, the NASPGHAN Annual Meeting, and the Crohn’s & Colitis Congress.™ Authors of abstracts presented at those three meetings are welcome to submit to DDW. Please note that authors do not have to be members of one of the four sponsoring societies, AGA, AASLD, ASGE, and SSAT, in order to submit an abstract.
Easily navigate the online abstract submission process with a video tutorial, step-by-step guide, and full abstract submission guidelines at www.ddw.org/abstracts.
Join the effort in providing the latest clinical translational, and basic research to health care professionals in gastroenterology, hepatology, GI endoscopy, and GI surgery. Submit an abstract today.
Travel awards available
DDW will award up to 20 young investigators with the Basic Science Travel Award. Selected authors of basic science abstracts will receive a $1,000 travel grant along with recognition at a reception during DDW 2017. Residents and fellows are encouraged to apply.
The AGA Research Foundation also offers various travel awards:
- AGA-GRG Fellow Abstract Award
- AGA-Moti L. & Kamla Rustgi International Travel Awards
- AGA Student Abstract Award
For more information on travel awards and other DDW-related grants, please visit the DDW website, www.ddw.org.
AGA launches new registry to track patient outcomes
The AGA Center for GI Innovation and Technology is excited to announce a new clinical research registry to track and evaluate patient outcomes after trans-oral endoscopic suturing procedures.
The Prospective Registry for Trans-Oral Suturing Applications (“Endoscopic Suturing Registry”) will collect real-world data related to the safety and effectiveness of procedures done with Apollo Endosurgery’s OverStitch™ Endoscopic Suturing System. Jennifer Maranki, MD, director of endoscopy, Penn State Milton S. Hershey School of Medicine, and Brian Dunkin, MD, head of endoscopic surgery and medical director, Houston Methodist Institute for Technology, Innovation and Education, will serve as principal investigators for the Endoscopic Suturing Registry. The Registry will begin collecting patient data in early 2018.
We asked Michael Kochman, MD, AGAF, past chair of the AGA Center for GI Innovation and Technology and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, to weigh in on the value of this new registry.
“Flexible endoscopic suturing is an important tool for the treatment of a number of GI disorders. As these procedures become more routine in GI and surgery practices across the country, the real-world data AGA will collect through the Endoscopic Suturing Registry will guide all stakeholders in making informed decisions around the continued adoption of these procedures in clinical practice.”
Learn more about AGA’s registry initiative at www.gastro.org/patient-care/registries-studies.
The AGA Center for GI Innovation and Technology is excited to announce a new clinical research registry to track and evaluate patient outcomes after trans-oral endoscopic suturing procedures.
The Prospective Registry for Trans-Oral Suturing Applications (“Endoscopic Suturing Registry”) will collect real-world data related to the safety and effectiveness of procedures done with Apollo Endosurgery’s OverStitch™ Endoscopic Suturing System. Jennifer Maranki, MD, director of endoscopy, Penn State Milton S. Hershey School of Medicine, and Brian Dunkin, MD, head of endoscopic surgery and medical director, Houston Methodist Institute for Technology, Innovation and Education, will serve as principal investigators for the Endoscopic Suturing Registry. The Registry will begin collecting patient data in early 2018.
We asked Michael Kochman, MD, AGAF, past chair of the AGA Center for GI Innovation and Technology and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, to weigh in on the value of this new registry.
“Flexible endoscopic suturing is an important tool for the treatment of a number of GI disorders. As these procedures become more routine in GI and surgery practices across the country, the real-world data AGA will collect through the Endoscopic Suturing Registry will guide all stakeholders in making informed decisions around the continued adoption of these procedures in clinical practice.”
Learn more about AGA’s registry initiative at www.gastro.org/patient-care/registries-studies.
The AGA Center for GI Innovation and Technology is excited to announce a new clinical research registry to track and evaluate patient outcomes after trans-oral endoscopic suturing procedures.
The Prospective Registry for Trans-Oral Suturing Applications (“Endoscopic Suturing Registry”) will collect real-world data related to the safety and effectiveness of procedures done with Apollo Endosurgery’s OverStitch™ Endoscopic Suturing System. Jennifer Maranki, MD, director of endoscopy, Penn State Milton S. Hershey School of Medicine, and Brian Dunkin, MD, head of endoscopic surgery and medical director, Houston Methodist Institute for Technology, Innovation and Education, will serve as principal investigators for the Endoscopic Suturing Registry. The Registry will begin collecting patient data in early 2018.
We asked Michael Kochman, MD, AGAF, past chair of the AGA Center for GI Innovation and Technology and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, to weigh in on the value of this new registry.
“Flexible endoscopic suturing is an important tool for the treatment of a number of GI disorders. As these procedures become more routine in GI and surgery practices across the country, the real-world data AGA will collect through the Endoscopic Suturing Registry will guide all stakeholders in making informed decisions around the continued adoption of these procedures in clinical practice.”
Learn more about AGA’s registry initiative at www.gastro.org/patient-care/registries-studies.
Closing the colonoscopy loophole
What is the colonoscopy loophole?
The Affordable Care Act covers screening colonoscopies at no cost to patients as long as no polyps are found. As Dr. Siddique explains in her article, finding a polyp changes the billing code to a therapeutic colonoscopy, a reclassification that changes the procedure from a diagnostic screening to an intervention. And this means a bill is generated. This reclassification directly affects those covered by Medicare and not commercial insurers.
AGA leaders urge Congress to correct this problem
Dr. Siddique – a member of the AGA Trainee and Early Career Committee and AGA Clinical Guidelines Committee – joined other AGA leaders for AGA Advocacy Day in late September where they spoke directly to lawmakers about patients who are blindsided by this regulation. AGA supports closing this loophole to ensure patients continue to have access to quality care and preventative screenings. We encourage all members to continue to share their patient stories, like Dr. Siddique has, to help raise awareness of this issue.
AGA can help you advocate for GI
Watch an AGA webinar at www.gastro.org/webinars/CongressionalMeeting (login required) to learn more about how to set up congressional meetings in your district, or contact Navneet Buttar, AGA government and political affairs manager, at [email protected] or 240-482-3221.
What is the colonoscopy loophole?
The Affordable Care Act covers screening colonoscopies at no cost to patients as long as no polyps are found. As Dr. Siddique explains in her article, finding a polyp changes the billing code to a therapeutic colonoscopy, a reclassification that changes the procedure from a diagnostic screening to an intervention. And this means a bill is generated. This reclassification directly affects those covered by Medicare and not commercial insurers.
AGA leaders urge Congress to correct this problem
Dr. Siddique – a member of the AGA Trainee and Early Career Committee and AGA Clinical Guidelines Committee – joined other AGA leaders for AGA Advocacy Day in late September where they spoke directly to lawmakers about patients who are blindsided by this regulation. AGA supports closing this loophole to ensure patients continue to have access to quality care and preventative screenings. We encourage all members to continue to share their patient stories, like Dr. Siddique has, to help raise awareness of this issue.
AGA can help you advocate for GI
Watch an AGA webinar at www.gastro.org/webinars/CongressionalMeeting (login required) to learn more about how to set up congressional meetings in your district, or contact Navneet Buttar, AGA government and political affairs manager, at [email protected] or 240-482-3221.
What is the colonoscopy loophole?
The Affordable Care Act covers screening colonoscopies at no cost to patients as long as no polyps are found. As Dr. Siddique explains in her article, finding a polyp changes the billing code to a therapeutic colonoscopy, a reclassification that changes the procedure from a diagnostic screening to an intervention. And this means a bill is generated. This reclassification directly affects those covered by Medicare and not commercial insurers.
AGA leaders urge Congress to correct this problem
Dr. Siddique – a member of the AGA Trainee and Early Career Committee and AGA Clinical Guidelines Committee – joined other AGA leaders for AGA Advocacy Day in late September where they spoke directly to lawmakers about patients who are blindsided by this regulation. AGA supports closing this loophole to ensure patients continue to have access to quality care and preventative screenings. We encourage all members to continue to share their patient stories, like Dr. Siddique has, to help raise awareness of this issue.
AGA can help you advocate for GI
Watch an AGA webinar at www.gastro.org/webinars/CongressionalMeeting (login required) to learn more about how to set up congressional meetings in your district, or contact Navneet Buttar, AGA government and political affairs manager, at [email protected] or 240-482-3221.
GIs take on Capitol Hill
During AGA’s annual Joint Committee weekend, 55 AGA members collectively attended 79 meetings with staff from the offices of their House representatives and senators, lobbying for the top concerns of gastroenterologists across the country, including:
- Continued coverage of patients through either the Affordable Care Act or another bill that has the patient’s best interests in mind. More specifically, one that provides coverage for those with pre-existing conditions and for children under their parents’ plan until 26 years of age, among many other important provisions.
- Changes in health care language that label a colonoscopy for cancer screening as “therapeutic,” which renders a large copay for patients.
- Increased funding for the NIH.
Participants shared experiences from their time on Capitol Hill in the AGA Community forum, and encouraged others to get involved. Here are some of their reasons why.
- Your voice matters: You are constituents – which translates to votes in the minds of representatives and senators – and providing face-to-face conversation with their staffers shows them that you care about your patients and their needs, explains Siddharth Singh, MD.
- Being consistent gets your foot in the door: Some staffers recognized and remembered previous Advocacy Day participants, like Peter Liang, MD, MPH. Personally connecting could lead to follow-up communication and advocacy efforts, says Sarah Streett, MD, AGAF.
- You’re indirectly (and sometimes directly) connecting with decision makers: Staff members from these offices work closely with the legislators who evaluate which policies to support or oppose. “So it’s important to come to Washington, build relationships, and make the case for our science, our specialty, and our patients,” says Kim Barrett, PhD, AGAF.
- Others could be advocating against you on the same issues: “I very strongly believe that it is important to keep letting our legislators know how we feel and what we believe in,” shares Deborah Proctor, MD, AGAF.
- It’s a rewarding experience: “Voice [your] concerns to your representatives who embrace the stories of how their decisions and policies affect your patients, practice, research, and institution,” explains Susan Ramdhaney, MD, AGAF.
- It’s a critical time to take action: With the current health care environment, gastroenterologists need to express the needs of their patients and profession, Dr. Streett explains.
View the full discussion and read updates from colleagues who visited with legislative staffers from California, New York, North Carolina, and Oregon in the forum, community.gastro.org.
Please contact [email protected].
During AGA’s annual Joint Committee weekend, 55 AGA members collectively attended 79 meetings with staff from the offices of their House representatives and senators, lobbying for the top concerns of gastroenterologists across the country, including:
- Continued coverage of patients through either the Affordable Care Act or another bill that has the patient’s best interests in mind. More specifically, one that provides coverage for those with pre-existing conditions and for children under their parents’ plan until 26 years of age, among many other important provisions.
- Changes in health care language that label a colonoscopy for cancer screening as “therapeutic,” which renders a large copay for patients.
- Increased funding for the NIH.
Participants shared experiences from their time on Capitol Hill in the AGA Community forum, and encouraged others to get involved. Here are some of their reasons why.
- Your voice matters: You are constituents – which translates to votes in the minds of representatives and senators – and providing face-to-face conversation with their staffers shows them that you care about your patients and their needs, explains Siddharth Singh, MD.
- Being consistent gets your foot in the door: Some staffers recognized and remembered previous Advocacy Day participants, like Peter Liang, MD, MPH. Personally connecting could lead to follow-up communication and advocacy efforts, says Sarah Streett, MD, AGAF.
- You’re indirectly (and sometimes directly) connecting with decision makers: Staff members from these offices work closely with the legislators who evaluate which policies to support or oppose. “So it’s important to come to Washington, build relationships, and make the case for our science, our specialty, and our patients,” says Kim Barrett, PhD, AGAF.
- Others could be advocating against you on the same issues: “I very strongly believe that it is important to keep letting our legislators know how we feel and what we believe in,” shares Deborah Proctor, MD, AGAF.
- It’s a rewarding experience: “Voice [your] concerns to your representatives who embrace the stories of how their decisions and policies affect your patients, practice, research, and institution,” explains Susan Ramdhaney, MD, AGAF.
- It’s a critical time to take action: With the current health care environment, gastroenterologists need to express the needs of their patients and profession, Dr. Streett explains.
View the full discussion and read updates from colleagues who visited with legislative staffers from California, New York, North Carolina, and Oregon in the forum, community.gastro.org.
Please contact [email protected].
During AGA’s annual Joint Committee weekend, 55 AGA members collectively attended 79 meetings with staff from the offices of their House representatives and senators, lobbying for the top concerns of gastroenterologists across the country, including:
- Continued coverage of patients through either the Affordable Care Act or another bill that has the patient’s best interests in mind. More specifically, one that provides coverage for those with pre-existing conditions and for children under their parents’ plan until 26 years of age, among many other important provisions.
- Changes in health care language that label a colonoscopy for cancer screening as “therapeutic,” which renders a large copay for patients.
- Increased funding for the NIH.
Participants shared experiences from their time on Capitol Hill in the AGA Community forum, and encouraged others to get involved. Here are some of their reasons why.
- Your voice matters: You are constituents – which translates to votes in the minds of representatives and senators – and providing face-to-face conversation with their staffers shows them that you care about your patients and their needs, explains Siddharth Singh, MD.
- Being consistent gets your foot in the door: Some staffers recognized and remembered previous Advocacy Day participants, like Peter Liang, MD, MPH. Personally connecting could lead to follow-up communication and advocacy efforts, says Sarah Streett, MD, AGAF.
- You’re indirectly (and sometimes directly) connecting with decision makers: Staff members from these offices work closely with the legislators who evaluate which policies to support or oppose. “So it’s important to come to Washington, build relationships, and make the case for our science, our specialty, and our patients,” says Kim Barrett, PhD, AGAF.
- Others could be advocating against you on the same issues: “I very strongly believe that it is important to keep letting our legislators know how we feel and what we believe in,” shares Deborah Proctor, MD, AGAF.
- It’s a rewarding experience: “Voice [your] concerns to your representatives who embrace the stories of how their decisions and policies affect your patients, practice, research, and institution,” explains Susan Ramdhaney, MD, AGAF.
- It’s a critical time to take action: With the current health care environment, gastroenterologists need to express the needs of their patients and profession, Dr. Streett explains.
View the full discussion and read updates from colleagues who visited with legislative staffers from California, New York, North Carolina, and Oregon in the forum, community.gastro.org.
Please contact [email protected].
AGA releases new clinical guidance on opioids in gastroenterology
The U.S. is facing an opioid epidemic – 91 Americans die every day from an opioid overdose. While all health care professionals should remain up to date on the risks associated with opioids, it is as important for GIs to understand how opioids can affect diverse parts of the gastrointestinal tract. Patients can experience GI symptoms and side effects related to the intake of opioids, including opioid-induced constipation (OIC), esophageal dysmotility, and delayed gastric emptying, according to a new AGA Clinical Practice Update published in the September 2017 issue of Clinical Gastroenterology and Hepatology.
Because of the common use of opioid medications to treat chronic pain, the authors recommend that physicians should first consider whether any gastrointestinal symptoms are directly related to the intake of opioids. In acute administration of opioids, symptomatic remedies should be used to counter the pharmacologic effects. For OIC, the bowel function index – a clinician assessment tool to appraise severity and responsiveness to current treatment – should be used to identify chronic OIC that is not responding to first-line therapies.
The clinical practice update also outlines:
- Pharmacologic effects of opiates in different regions of the gastrointestinal tract.
- Therapeutic uses of opioid receptor agonists and antagonists in gastroenterology.
- Prevention and treatment of OIC.
The U.S. is facing an opioid epidemic – 91 Americans die every day from an opioid overdose. While all health care professionals should remain up to date on the risks associated with opioids, it is as important for GIs to understand how opioids can affect diverse parts of the gastrointestinal tract. Patients can experience GI symptoms and side effects related to the intake of opioids, including opioid-induced constipation (OIC), esophageal dysmotility, and delayed gastric emptying, according to a new AGA Clinical Practice Update published in the September 2017 issue of Clinical Gastroenterology and Hepatology.
Because of the common use of opioid medications to treat chronic pain, the authors recommend that physicians should first consider whether any gastrointestinal symptoms are directly related to the intake of opioids. In acute administration of opioids, symptomatic remedies should be used to counter the pharmacologic effects. For OIC, the bowel function index – a clinician assessment tool to appraise severity and responsiveness to current treatment – should be used to identify chronic OIC that is not responding to first-line therapies.
The clinical practice update also outlines:
- Pharmacologic effects of opiates in different regions of the gastrointestinal tract.
- Therapeutic uses of opioid receptor agonists and antagonists in gastroenterology.
- Prevention and treatment of OIC.
The U.S. is facing an opioid epidemic – 91 Americans die every day from an opioid overdose. While all health care professionals should remain up to date on the risks associated with opioids, it is as important for GIs to understand how opioids can affect diverse parts of the gastrointestinal tract. Patients can experience GI symptoms and side effects related to the intake of opioids, including opioid-induced constipation (OIC), esophageal dysmotility, and delayed gastric emptying, according to a new AGA Clinical Practice Update published in the September 2017 issue of Clinical Gastroenterology and Hepatology.
Because of the common use of opioid medications to treat chronic pain, the authors recommend that physicians should first consider whether any gastrointestinal symptoms are directly related to the intake of opioids. In acute administration of opioids, symptomatic remedies should be used to counter the pharmacologic effects. For OIC, the bowel function index – a clinician assessment tool to appraise severity and responsiveness to current treatment – should be used to identify chronic OIC that is not responding to first-line therapies.
The clinical practice update also outlines:
- Pharmacologic effects of opiates in different regions of the gastrointestinal tract.
- Therapeutic uses of opioid receptor agonists and antagonists in gastroenterology.
- Prevention and treatment of OIC.
AGA members meet with Rep. Gene Green at Baylor College
In-district meetings with congressional representatives provide a great opportunity for AGA members to establish working relationships with legislators, and help make the voices of our profession and our patients heard.
Members of the Baylor College of Medicine gastroenterology division – Avi Ketwaroo, MD; Richa Shukla, MD; Yamini Natarajan, MD; and Jordan Shapiro, MD – had the opportunity to meet with U.S. Rep. Gene Green, a Democrat from Texas’ 29th Congressional District, as part of AGA’s efforts to link constituents with local representatives. The group discussed the importance of supporting increases in NIH funding to maintain similar levels based on biomedical research inflation, the importance of screening colonoscopy, and improving access to care by opposing the repeal of the Affordable Care Act.
Watch an AGA webinar, available in the AGA Community resource library for AGA members only (community.gastro.org) to learn more about how to set up congressional meetings in your district or contact Navneet Buttar, AGA government and political affairs manager, at [email protected] or 240-482-3221.
[email protected]
In-district meetings with congressional representatives provide a great opportunity for AGA members to establish working relationships with legislators, and help make the voices of our profession and our patients heard.
Members of the Baylor College of Medicine gastroenterology division – Avi Ketwaroo, MD; Richa Shukla, MD; Yamini Natarajan, MD; and Jordan Shapiro, MD – had the opportunity to meet with U.S. Rep. Gene Green, a Democrat from Texas’ 29th Congressional District, as part of AGA’s efforts to link constituents with local representatives. The group discussed the importance of supporting increases in NIH funding to maintain similar levels based on biomedical research inflation, the importance of screening colonoscopy, and improving access to care by opposing the repeal of the Affordable Care Act.
Watch an AGA webinar, available in the AGA Community resource library for AGA members only (community.gastro.org) to learn more about how to set up congressional meetings in your district or contact Navneet Buttar, AGA government and political affairs manager, at [email protected] or 240-482-3221.
[email protected]
In-district meetings with congressional representatives provide a great opportunity for AGA members to establish working relationships with legislators, and help make the voices of our profession and our patients heard.
Members of the Baylor College of Medicine gastroenterology division – Avi Ketwaroo, MD; Richa Shukla, MD; Yamini Natarajan, MD; and Jordan Shapiro, MD – had the opportunity to meet with U.S. Rep. Gene Green, a Democrat from Texas’ 29th Congressional District, as part of AGA’s efforts to link constituents with local representatives. The group discussed the importance of supporting increases in NIH funding to maintain similar levels based on biomedical research inflation, the importance of screening colonoscopy, and improving access to care by opposing the repeal of the Affordable Care Act.
Watch an AGA webinar, available in the AGA Community resource library for AGA members only (community.gastro.org) to learn more about how to set up congressional meetings in your district or contact Navneet Buttar, AGA government and political affairs manager, at [email protected] or 240-482-3221.
[email protected]
AGA comments on Quality Payment proposed rule
AGA provided comments on a proposed rule describing potential changes to the Quality Payment Program (QPP) established under the Medicare Access and CHIP Reauthorization Act (MACRA) for the 2018 performance year. AGA thanks the many members who also submitted comments to CMS to tell the agency how proposed changes will impact you.
For year two, CMS proposed many policies that increase flexibility and incentives under the QPP. However, many proposals target solo practitioners, small practices, and other eligible clinicians with special circumstances. While we support these proposals, AGA’s comments to CMS also ask for changes that are needed to make the QPP work for all gastroenterologists, such as reducing the number of points needed to avoid a payment penalty.
CMS will finalize changes to the QPP during the fall of 2017. Final changes will take effect with the performance period that begins on Jan. 1, 2018. Performance during 2018 will impact payment for services in 2020. AGA members will be notified as soon as the rule is made available by CMS.
Still unsure how to participate in year one?
Make sure your practice is prepared for the 2017 performance year. If you are eligible to participate in 2017, but choose not to, your rates will decrease by 4% in 2019. AGA’s MACRA resource center provides customized advice based on your practice situation to get you on track. It’s not too late to start, but if you wait until Oct. 2, 2017, the deadline to start submitting claims, it will be. Get started now, http://www.gastro.org/macra.
AGA provided comments on a proposed rule describing potential changes to the Quality Payment Program (QPP) established under the Medicare Access and CHIP Reauthorization Act (MACRA) for the 2018 performance year. AGA thanks the many members who also submitted comments to CMS to tell the agency how proposed changes will impact you.
For year two, CMS proposed many policies that increase flexibility and incentives under the QPP. However, many proposals target solo practitioners, small practices, and other eligible clinicians with special circumstances. While we support these proposals, AGA’s comments to CMS also ask for changes that are needed to make the QPP work for all gastroenterologists, such as reducing the number of points needed to avoid a payment penalty.
CMS will finalize changes to the QPP during the fall of 2017. Final changes will take effect with the performance period that begins on Jan. 1, 2018. Performance during 2018 will impact payment for services in 2020. AGA members will be notified as soon as the rule is made available by CMS.
Still unsure how to participate in year one?
Make sure your practice is prepared for the 2017 performance year. If you are eligible to participate in 2017, but choose not to, your rates will decrease by 4% in 2019. AGA’s MACRA resource center provides customized advice based on your practice situation to get you on track. It’s not too late to start, but if you wait until Oct. 2, 2017, the deadline to start submitting claims, it will be. Get started now, http://www.gastro.org/macra.
AGA provided comments on a proposed rule describing potential changes to the Quality Payment Program (QPP) established under the Medicare Access and CHIP Reauthorization Act (MACRA) for the 2018 performance year. AGA thanks the many members who also submitted comments to CMS to tell the agency how proposed changes will impact you.
For year two, CMS proposed many policies that increase flexibility and incentives under the QPP. However, many proposals target solo practitioners, small practices, and other eligible clinicians with special circumstances. While we support these proposals, AGA’s comments to CMS also ask for changes that are needed to make the QPP work for all gastroenterologists, such as reducing the number of points needed to avoid a payment penalty.
CMS will finalize changes to the QPP during the fall of 2017. Final changes will take effect with the performance period that begins on Jan. 1, 2018. Performance during 2018 will impact payment for services in 2020. AGA members will be notified as soon as the rule is made available by CMS.
Still unsure how to participate in year one?
Make sure your practice is prepared for the 2017 performance year. If you are eligible to participate in 2017, but choose not to, your rates will decrease by 4% in 2019. AGA’s MACRA resource center provides customized advice based on your practice situation to get you on track. It’s not too late to start, but if you wait until Oct. 2, 2017, the deadline to start submitting claims, it will be. Get started now, http://www.gastro.org/macra.
AGA releases new clinical guideline on therapeutic drug monitoring in IBD
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:
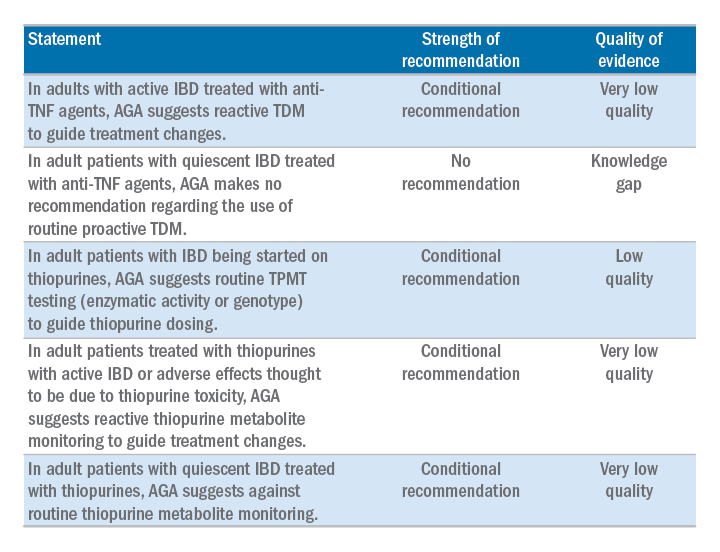
The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:

The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:

The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.



