User login
Market Watch
Discontinued Products
- Amoxicillin powder for oral suspension and pediatric drops for oral suspension1
- Amoxicillin powder for oral suspension (Amoxil brand usually for adults), 250mg/5mL (100mL and 150mL sizes)2
- Insulin isophane suspension (Humulin 50/50), due to limited use.3 Current patient demand and existing inventory note product availability through April 2010. There are about 3,000 patients in the U.S. who will be affected by this action.
- Phenytoin 30 mg (Dilantin Kapseals brand) are being reformulated in a new, extended-release formulation, but Kapseals will be discontinued.4
New Generics
- Tacrolimus (generic Prograf) capsules5
New Drugs, Indications, and Dosage Forms
- Asenapine tablets (Saphris) have been approved by the Food and Drug Administration (FDA) to treat adults with schizophrenia and bipolar I disorder. The most common adverse effects in trials were akathisia, oral hypoesthesia, and somnolence. The most common adverse effects that were reported in the bipolar disorder trials were somnolence, dizziness, movement disorders other than akathisia, and weight gain.6
- Colchicine 0.6 mg tablets (Colcrys) have been approved by the FDA to treat gout flares and familial Mediterranean fever.7 Colchicine has been used for many years but has not received FDA approval until recently. The FDA is re-evaluating some older drugs and drug classes. For example, the pancrelipase products fall under a similar ruling. Now that colchicine is approved, the manufacturer has shown that it meets modern standards for safety, effectiveness, quality, and labeling. Historically, physicians have administered colchicine hourly to treat acute gout flares until symptoms subsided or the patient developed adverse gastrointestinal symptoms. A dosing study determined that one 1.2-mg dose of this formulation followed by 0.6 mg one hour later was as effective as hourly dosing in patients without renal or hepatic dysfunction. This two-dose regimen was less toxic than prior dosing regimens and, therefore, it received the FDA’s approval.8
- Fentanyl buccal soluble film (Onsolis) has been approved by the FDA as an opioid for managing breakthrough cancer pain in patients 18 years and older who already are receiving and are tolerant to opioid therapy.9 It is available in 200-, 400-, 600-, 800- and 1,200-mcg strengths. A Risk Evaluation and Mitigation Strategies (REMS) will be available with dispensing.
- Insulin aspart injection (NovoLog) has undergone a label change. NovoLog can now be used in an insulin pump for up to six days. The infusion set should be changed at least every three days. The updated label includes information about discarding the drug if temperatures exceed 37oC (98.6oF).10
- Interferon beta-1b injection (Extavia): A new brand of interferon has been approved by the FDA for treating relapsing forms of multiple sclerosis (MS), as well as for patients who have experienced a first clinical episode of MS with magnetic resonance imaging (MRI) features consistent of the disease.11
- Morphine/naltrexone capsules (Embeda), a long-acting opioid designed to reduce drug euphoria, have been approved by the FDA to treat moderate to severe chronic pain. It was developed with the abuse-deterrent drug naltrexone, which reduces euphoria when crushed or chewed.12
- Pitavastatin 4 mg (Livalo) has been approved by the FDA to treat hypercholesterolemia and combined dyslipidemia.13 It’s a potent statin with a new base structure. Additionally, it is only minimally metabolized by the cytochrome P450 (CYP) pathway. It will be available in early 2010 in 1-, 2- and 4-mg strengths. Only time will tell whether this is truly a benefit for this new agent.
- Saxagliptin (Onglyza), a new oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been approved by the FDA to treat Type 2 diabetes mellitus as an adjunct to diet and exercise.14 It is administered once daily at a starting dose of 2.5 mg or 5 mg, without regard to meal.15 The lower dose is recommended in patients with moderate to severe renal impairment or end-stage renal disease (CrCL < 50 mL/min). The lower dose (2.5 mg) also is recommended for patients taking strong CYP3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). The most common adverse effects in clinical trials were respiratory tract infection, urinary tract infection, and headache.
Pipeline
- Roflumilast (Daxas), a phosphodiesterase 4 enzyme inhibitor, has been submitted to the FDA. It is a once-daily oral treatment for patients with symptomatic chronic obstructive pulmonary disease (COPD).16
- Tocilizumab (Actemra), an interleukin-6 receptor-inhibiting monoclonal antibody to treat rheumatoid arthritis (RA), has been approved for use in Europe. Its manufacturer has announced that the FDA has accepted its reapplication for treating moderate to severe RA. It is available in Japan for treating RA, juvenile idiopathic arthritis, and Castleman’s disease.17
- TZP-102, an investigational oral prokinetic agent for treating diabetic gastroparesis, has received FDA fast-track status. A multinational study is under way for this ghrelin receptor agonist.18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
Discontinued Products
- Amoxicillin powder for oral suspension and pediatric drops for oral suspension1
- Amoxicillin powder for oral suspension (Amoxil brand usually for adults), 250mg/5mL (100mL and 150mL sizes)2
- Insulin isophane suspension (Humulin 50/50), due to limited use.3 Current patient demand and existing inventory note product availability through April 2010. There are about 3,000 patients in the U.S. who will be affected by this action.
- Phenytoin 30 mg (Dilantin Kapseals brand) are being reformulated in a new, extended-release formulation, but Kapseals will be discontinued.4
New Generics
- Tacrolimus (generic Prograf) capsules5
New Drugs, Indications, and Dosage Forms
- Asenapine tablets (Saphris) have been approved by the Food and Drug Administration (FDA) to treat adults with schizophrenia and bipolar I disorder. The most common adverse effects in trials were akathisia, oral hypoesthesia, and somnolence. The most common adverse effects that were reported in the bipolar disorder trials were somnolence, dizziness, movement disorders other than akathisia, and weight gain.6
- Colchicine 0.6 mg tablets (Colcrys) have been approved by the FDA to treat gout flares and familial Mediterranean fever.7 Colchicine has been used for many years but has not received FDA approval until recently. The FDA is re-evaluating some older drugs and drug classes. For example, the pancrelipase products fall under a similar ruling. Now that colchicine is approved, the manufacturer has shown that it meets modern standards for safety, effectiveness, quality, and labeling. Historically, physicians have administered colchicine hourly to treat acute gout flares until symptoms subsided or the patient developed adverse gastrointestinal symptoms. A dosing study determined that one 1.2-mg dose of this formulation followed by 0.6 mg one hour later was as effective as hourly dosing in patients without renal or hepatic dysfunction. This two-dose regimen was less toxic than prior dosing regimens and, therefore, it received the FDA’s approval.8
- Fentanyl buccal soluble film (Onsolis) has been approved by the FDA as an opioid for managing breakthrough cancer pain in patients 18 years and older who already are receiving and are tolerant to opioid therapy.9 It is available in 200-, 400-, 600-, 800- and 1,200-mcg strengths. A Risk Evaluation and Mitigation Strategies (REMS) will be available with dispensing.
- Insulin aspart injection (NovoLog) has undergone a label change. NovoLog can now be used in an insulin pump for up to six days. The infusion set should be changed at least every three days. The updated label includes information about discarding the drug if temperatures exceed 37oC (98.6oF).10
- Interferon beta-1b injection (Extavia): A new brand of interferon has been approved by the FDA for treating relapsing forms of multiple sclerosis (MS), as well as for patients who have experienced a first clinical episode of MS with magnetic resonance imaging (MRI) features consistent of the disease.11
- Morphine/naltrexone capsules (Embeda), a long-acting opioid designed to reduce drug euphoria, have been approved by the FDA to treat moderate to severe chronic pain. It was developed with the abuse-deterrent drug naltrexone, which reduces euphoria when crushed or chewed.12
- Pitavastatin 4 mg (Livalo) has been approved by the FDA to treat hypercholesterolemia and combined dyslipidemia.13 It’s a potent statin with a new base structure. Additionally, it is only minimally metabolized by the cytochrome P450 (CYP) pathway. It will be available in early 2010 in 1-, 2- and 4-mg strengths. Only time will tell whether this is truly a benefit for this new agent.
- Saxagliptin (Onglyza), a new oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been approved by the FDA to treat Type 2 diabetes mellitus as an adjunct to diet and exercise.14 It is administered once daily at a starting dose of 2.5 mg or 5 mg, without regard to meal.15 The lower dose is recommended in patients with moderate to severe renal impairment or end-stage renal disease (CrCL < 50 mL/min). The lower dose (2.5 mg) also is recommended for patients taking strong CYP3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). The most common adverse effects in clinical trials were respiratory tract infection, urinary tract infection, and headache.
Pipeline
- Roflumilast (Daxas), a phosphodiesterase 4 enzyme inhibitor, has been submitted to the FDA. It is a once-daily oral treatment for patients with symptomatic chronic obstructive pulmonary disease (COPD).16
- Tocilizumab (Actemra), an interleukin-6 receptor-inhibiting monoclonal antibody to treat rheumatoid arthritis (RA), has been approved for use in Europe. Its manufacturer has announced that the FDA has accepted its reapplication for treating moderate to severe RA. It is available in Japan for treating RA, juvenile idiopathic arthritis, and Castleman’s disease.17
- TZP-102, an investigational oral prokinetic agent for treating diabetic gastroparesis, has received FDA fast-track status. A multinational study is under way for this ghrelin receptor agonist.18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
Discontinued Products
- Amoxicillin powder for oral suspension and pediatric drops for oral suspension1
- Amoxicillin powder for oral suspension (Amoxil brand usually for adults), 250mg/5mL (100mL and 150mL sizes)2
- Insulin isophane suspension (Humulin 50/50), due to limited use.3 Current patient demand and existing inventory note product availability through April 2010. There are about 3,000 patients in the U.S. who will be affected by this action.
- Phenytoin 30 mg (Dilantin Kapseals brand) are being reformulated in a new, extended-release formulation, but Kapseals will be discontinued.4
New Generics
- Tacrolimus (generic Prograf) capsules5
New Drugs, Indications, and Dosage Forms
- Asenapine tablets (Saphris) have been approved by the Food and Drug Administration (FDA) to treat adults with schizophrenia and bipolar I disorder. The most common adverse effects in trials were akathisia, oral hypoesthesia, and somnolence. The most common adverse effects that were reported in the bipolar disorder trials were somnolence, dizziness, movement disorders other than akathisia, and weight gain.6
- Colchicine 0.6 mg tablets (Colcrys) have been approved by the FDA to treat gout flares and familial Mediterranean fever.7 Colchicine has been used for many years but has not received FDA approval until recently. The FDA is re-evaluating some older drugs and drug classes. For example, the pancrelipase products fall under a similar ruling. Now that colchicine is approved, the manufacturer has shown that it meets modern standards for safety, effectiveness, quality, and labeling. Historically, physicians have administered colchicine hourly to treat acute gout flares until symptoms subsided or the patient developed adverse gastrointestinal symptoms. A dosing study determined that one 1.2-mg dose of this formulation followed by 0.6 mg one hour later was as effective as hourly dosing in patients without renal or hepatic dysfunction. This two-dose regimen was less toxic than prior dosing regimens and, therefore, it received the FDA’s approval.8
- Fentanyl buccal soluble film (Onsolis) has been approved by the FDA as an opioid for managing breakthrough cancer pain in patients 18 years and older who already are receiving and are tolerant to opioid therapy.9 It is available in 200-, 400-, 600-, 800- and 1,200-mcg strengths. A Risk Evaluation and Mitigation Strategies (REMS) will be available with dispensing.
- Insulin aspart injection (NovoLog) has undergone a label change. NovoLog can now be used in an insulin pump for up to six days. The infusion set should be changed at least every three days. The updated label includes information about discarding the drug if temperatures exceed 37oC (98.6oF).10
- Interferon beta-1b injection (Extavia): A new brand of interferon has been approved by the FDA for treating relapsing forms of multiple sclerosis (MS), as well as for patients who have experienced a first clinical episode of MS with magnetic resonance imaging (MRI) features consistent of the disease.11
- Morphine/naltrexone capsules (Embeda), a long-acting opioid designed to reduce drug euphoria, have been approved by the FDA to treat moderate to severe chronic pain. It was developed with the abuse-deterrent drug naltrexone, which reduces euphoria when crushed or chewed.12
- Pitavastatin 4 mg (Livalo) has been approved by the FDA to treat hypercholesterolemia and combined dyslipidemia.13 It’s a potent statin with a new base structure. Additionally, it is only minimally metabolized by the cytochrome P450 (CYP) pathway. It will be available in early 2010 in 1-, 2- and 4-mg strengths. Only time will tell whether this is truly a benefit for this new agent.
- Saxagliptin (Onglyza), a new oral dipeptidyl peptidase-4 (DPP-4) inhibitor, has been approved by the FDA to treat Type 2 diabetes mellitus as an adjunct to diet and exercise.14 It is administered once daily at a starting dose of 2.5 mg or 5 mg, without regard to meal.15 The lower dose is recommended in patients with moderate to severe renal impairment or end-stage renal disease (CrCL < 50 mL/min). The lower dose (2.5 mg) also is recommended for patients taking strong CYP3A4/5 inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). The most common adverse effects in clinical trials were respiratory tract infection, urinary tract infection, and headache.
Pipeline
- Roflumilast (Daxas), a phosphodiesterase 4 enzyme inhibitor, has been submitted to the FDA. It is a once-daily oral treatment for patients with symptomatic chronic obstructive pulmonary disease (COPD).16
- Tocilizumab (Actemra), an interleukin-6 receptor-inhibiting monoclonal antibody to treat rheumatoid arthritis (RA), has been approved for use in Europe. Its manufacturer has announced that the FDA has accepted its reapplication for treating moderate to severe RA. It is available in Japan for treating RA, juvenile idiopathic arthritis, and Castleman’s disease.17
- TZP-102, an investigational oral prokinetic agent for treating diabetic gastroparesis, has received FDA fast-track status. A multinational study is under way for this ghrelin receptor agonist.18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
What is the appropriate use of chronic medications in the perioperative setting?
Case
A 72-year-old female with multiple medical problems is admitted with a hip fracture. Surgery is scheduled in 48 hours. The patient’s home medications include aspirin, carbidopa/levodopa, celecoxib, clonidine, estradiol, ginkgo, lisinopril, NPH insulin, sulfasalazine, and prednisone 10 mg a day, which she has been taking for years. How should these and other medications be managed in the perioperative period?
Background
Perioperative management of chronic medications is a complex issue, as physicians are required to balance the beneficial and harmful effects of the individual drugs prescribed to their patients. On one hand, cessation of medications can result in decompensation of disease or withdrawal. On the other hand, continuation of drugs can alter metabolism of anesthetic agents, cause perioperative hemodynamic instability, or result in such post-operative complications as acute renal failure, bleeding, infection, and impaired wound healing.
Certain traits make it reasonable to continue medications during the perioperative period. A long elimination half-life or duration of action makes stopping some medications impractical as it takes four to five half-lives to completely clear the drug from the body; holding the drug for a few days around surgery will not appreciably affect its concentration. Stopping drugs that carry severe withdrawal symptoms can be impractical because of the need for lengthy tapers, which can delay surgery and result in decompensation of underlying disease.
Drugs with no significant interactions with anesthesia or risk of perioperative complications should be continued in order to avoid deterioration of the underlying disease. Conversely, drugs that interact with anesthesia or increase risk for complications should be stopped if this can be accomplished safely. Patient-specific factors should receive consideration, as the risk of complications has to be balanced against the danger of exacerbating the underlying disease.
Overview of the Data
The challenge in providing recommendations on perioperative medication management lies in a dearth of high-quality clinical trials. Thus, much of the information comes from case reports, expert opinion, and sound application of pharmacology.
Antiplatelet therapy: Nuances of perioperative antiplatelet therapy are beyond the scope of this review, but some general principles can be elucidated from the American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines.1 Management of antiplatelet therapy should be done in conjunction with the surgical team, as cardiovascular risk has to be weighed against bleeding risk.
Aspirin therapy should be continued in all patients with a history of coronary artery disease (CAD), balloon angioplasty, or percutaneous coronary intervention (PCI), unless the risk of bleeding complications is felt to exceed the cardioprotective benefits—for example, in some neurosurgical patients.1
Clopidogrel therapy is crucial for prevention of in-stent thrombosis (IST) following PCI because patients who experience IST suffer catastrophic myocardial infarctions with high mortality. Ideally, surgery should be delayed to permit completion of clopidogrel therapy—30 to 45 days after implantation of a bare-metal stent and 365 days after a drug-eluting stent. If surgery has to be performed sooner, guidelines recommend operating on dual antiplatelet therapy with aspirin and clopidogrel.1 Again, this course of treatment has to be balanced against the risk of hemorrhagic complications from surgery.
Both aspirin and clopidogrel irreversibly inhibit platelet aggregation. The recovery of normal coagulation involves formation of new platelets, which necessitates cessation of therapy for seven to 10 days before surgery. Platelet inhibition begins within minutes of restarting aspirin and within hours of taking clopidogrel, although attaining peak clopidogrel effect takes three to seven days, unless a loading dose is used.
Cardiovascular Drugs
Beta-blockers in the perioperative setting are a focus of an ongoing debate beyond the scope of this review (see “What Pre-Operative Cardiac Evaluation of Patients Undergoing Intermediate-Risk Surgery Is Most Effective?,” February 2008, p. 26). Given the current evidence and the latest ACC/AHA guidelines, it is still reasonable to continue therapy in patients who are already taking them to avoid precipitating cardiovascular events by withdrawal. Patients with increased cardiac risk, demonstrated by a Revised Cardiac Risk Index (RCRI) score of ≥2 (see Table 1, p. 12), should be considered for beta-blocker therapy before surgery.1 In either case, the dose should be titrated to a heart rate <65 for optimal cardiac protection.1
Statins should be continued if the patient is taking them, especially because preoperative withdrawal has been associated with a 4.6-fold increase in troponin release and a 7.5-fold increased risk of myocardial infarction (MI) and cardiovascular death following major vascular surgery.2 Patients with increased cardiac risk— RCRI ≥1—can be considered for initiation of statin therapy before surgery, although the benefit of this intervention has not been examined in prospective studies.1
Amiodarone has an exceptionally long half-life of up to 142 days. It should be continued in the perioperative period.
Calcium channel blockers (CCBs) can be continued with no unpleasant perioperative hemodynamic effects.1 CCBs have potential cardioprotective benefits.
Clonidine withdrawal can result in severe rebound hypertension with reports of encephalopathy, stroke, and death. These effects are exacerbated by concomitant beta-blocker therapy. For this reason, if a patient is expected to be NPO for more than 12 hours, they should be converted to a clonidine patch 48-72 hours before surgery with concurrent tapering of the oral dose.3
Digoxin has a long half-life (up to 48 hours) and should be continued with monitoring of levels if there is a change in renal function.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs) have been associated with a 50% increased risk of hypotension requiring vasopressors during induction of anesthesia.4 However, it is worth mentioning that this finding has not been corroborated in other studies. A large retrospective cohort of cardiothoracic surgical patients found a 28% increased risk of post-operative acute renal failure (ARF) with both drug classes, although another cardiothoracic report published the same year demonstrated a 50% reduction in risk with ACEIs.5,6 Although the evidence of harm is not unequivocal, perioperative blood-pressure control can be achieved with other drugs without hemodynamic or renal risk, such as CCBs, and in most cases ACEIs/ARBs should be stopped one day before surgery.
Diuretics carry a risk of volume depletion and electrolyte derangements, and should be stopped once a patient becomes NPO. Excess volume is managed with as-needed intravenous formulations.
Drugs Acting on the Central Nervous System
The majority of central nervous system (CNS)-active drugs, including antiepileptics, antipsychotics, benzodiazepines, bupropion, gabapentin, lithium, mirtazapine, selective serotonin and norepinephrine reuptake inhibitors (SSRIs and SNRIs), tricyclic antidepressants (TCAs), and valproic acid, balance a low risk of perioperative complications against a significant potential for withdrawal and disease decompensation. Therefore, these medications should be continued.
Carbidopa/Levodopa should be continued because abrupt cessation can precipitate systemic withdrawal resembling serotonin syndrome and rapid deterioration of Parkinson’s symptoms.
Monoamine oxidase inhibitor (MAOI) therapy usually indicates refractory psychiatric illness, so these drugs should be continued to avoid decompensation. Importantly, MAOI-safe anesthesia without dextromethorphan, meperidine, epinephrine, or norepinephrine has to be used due to the risk of cardiovascular instability.7
Diabetic Drugs
Insulin therapy should be continued with adjustments. Glargine basal insulin has no peak and can be continued without changing the dose. Likewise, patients with insulin pumps can continue the usual basal rate. Short-acting insulin or such insulin mixes as 70/30 should be stopped four hours before surgery to avoid hypoglycemia. Intermediate-acting insulin (e.g., NPH) can be administered at half the usual dose the day of surgery with a perioperative 5% dextrose infusion. NPH should not be given the day of surgery if the dextrose infusion cannot be used.8
Incretins (exenatide, sitagliptin) rarely cause hypoglycemia in the absence of insulin and may be beneficial in controlling intraoperative hyperglycemia. Therefore, these medications can be continued.8
Thiazolidinediones (TZDs; pioglitazone, rosiglitazone) alter gene transcription with biological duration of action on the order of weeks and low risk of hypoglycemia, and should be continued.
Metformin carries an FDA black-box warning to discontinue therapy before any intravascular radiocontrast studies or surgical procedures due to the risk of severe lactic acidosis if renal failure develops. It should be stopped 24 hours before surgery and restarted at least 48-72 hours after. Normal renal function should be confirmed before restarting therapy.8
Sulfonylureas (glimepiride, glipizide, glyburide) carry a significant risk of hypoglycemia in a patient who is NPO; they should be stopped the night before surgery or before commencement of NPO status.
Hormones
Antithyroid drugs (methimazole, propylthiouracil) and levothyroxine should be continued, as they have no perioperative contraindications.
Oral contraceptives (OCPs), hormone replacement therapy (HRT), and raloxifene can increase the risk of DVT. The largest study on the topic was the HERS trial of postmenopausal women on estrogen/progesterone HRT. The authors reported a 4.9-fold increased risk of DVT for 90 days after surgery.9 Unfortunately, no information was provided on the types of surgery, or whether appropriate and consistent DVT prophylaxis was utilized. HERS authors also reported a 2.5-fold increased risk of DVT for at least 30 days after cessation of HRT.9
Given the data, it is reasonable to stop hormone therapy four weeks before surgery when prolonged immobilization is anticipated and patients are able to tolerate hormone withdrawal, especially if other DVT risk factors are present. If hormone therapy cannot be stopped, strong consideration should be given to higher-intensity DVT prophylaxis (e.g., chemoprophylaxis as opposed to mechanical measures) of longer duration—up to 28 days following general surgery and up to 35 days after orthopedic procedures.10
Perioperative Corticosteroids
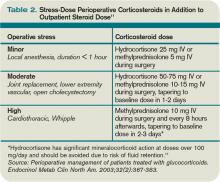
Corticosteroid therapy in excess of prednisone 5 mg/day or equivalent for more than five days in the 30 days preceding surgery might predispose patients to acute adrenal insufficiency in the perioperative period. Surgical procedures typically result in cortisol release of 50-150 mg/day, which returns to baseline within 48 hours.11 Therefore, the recommendation is to continue a patient’s baseline steroid dose and supplement it with stress-dose steroids tailored to the severity of operative stress (see Table 2, above).
Mineralocorticoid supplementation is not necessary, because endogenous production is not suppressed by corticosteroid therapy.11 Although a recent systematic review suggests that routine stress-dose steroids might not be indicated, high-quality prospective data are needed before abandoning this strategy due to complications of acute adrenal insufficiency compared to the risk of a brief corticosteroid burst.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Nonselective cyclooxygenase (COX) inhibitors reversibly decrease platelet aggregation only while the drug is present in the circulation and should be stopped one to three days before surgery due to risk of bleeding.
Selective COX-2 inhibitors do not significantly alter platelet aggregation and can be continued for opioid-sparing perioperative pain control.
Both COX-2-selective and nonselective inhibitors should be held if there are concerns for impaired renal function.
Disease-Modifying Antirheumatic Drugs (DMARDs) and Biological Response Modifiers (BRMs)
Methotrexate increases the risk of wound infections and dehiscence. However, this is offset by a decreased risk of post-operative disease flares with continued use. It can be continued unless the patient has medical comorbidities, advanced age, or chronic therapy with more than 10 mg/day of prednisone, in which case the drug should be stopped two weeks before surgery.12
Azathioprine, leflunomide, and sulfasalazine are renally cleared with a risk of myelosuppression; all of these medications should be stopped. Long half-life of leflunomide necessitates stopping it two weeks before surgery; azathioprine and sulfasalazine can be stopped one day in advance. The drugs can be restarted three days after surgery, assuming stable renal function.13
Anti-TNF-α (adalimumab, etanercept, infliximab), IL1 antagonist (anakinra), and anti-CD20 (rituximab) agents should be stopped one week before surgery and resumed 1-2 weeks afterward, unless risk of complications from disease flareup outweighs the concern for wound infections and dehiscence.14
Herbal Medicines
It is estimated that as much as a third of the U.S. population uses herbal medicines. These substances can result in perioperative hemodynamic instability (ephedra, ginseng, ma huang), hypoglycemia (ginseng), immunosuppression (echinacea, when taken for more than eight weeks), abnormal bleeding (garlic, ginkgo, ginseng), and prolongation of anesthesia (kava, St. John’s wort, valerian). All of these herbal medicine should be stopped one to two weeks before surgery.15,16
Back to the Case
The patient’s Carbidopa/Levodopa should be continued. Celecoxib can be continued if her renal function in stable. If aspirin is taken for a history of coronary artery disease or percutaneous coronary intervention, it should be continued, if possible. Clonidine should be continued or changed to a patch if an extended NPO period is anticipated. Ginkgo, lisinopril, and sulfasalazine should be stopped.
Hospitalization does not provide the luxury of stopping estradiol in advance, so it might be continued with chemical DVT prophylaxis for up to 35 days after surgery. The patient should receive 50-75 mg of IV hydrocortisone during surgery and an additional 25 mg the following day, in addition to her usual prednisone 10 mg/day. She can either receive half her usual NPH dose the morning of surgery with a 5% dextrose infusion in the operating room, or the NPH should be held altogether.
Bottom Line
Perioperative medication use should be tailored to each patient, balancing the risks and benefits of individual drugs. High-quality trials are needed to provide more robust clinical guidelines. TH
Dr. Levin is a hospitalist at the University of Colorado Denver.
References
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):1971-1996.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316-320.
- Spell NO III. Stopping and restarting medications in the perioperative period. Med Clin North Am. 2001;85(5):1117-1128.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325.
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266-1273.
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg. 2008;86(4):1160-1165.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm. 2004;61(9):899-914.
- Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93(5):1031-1047.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689-696.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):71S-109S.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am. 2003;32(2):367-383.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg. 2008;143(12):1222-1226.
- Rosandich PA, Kelley JT III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208-216.
- Hodges PJ, Kam PC. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57(9):889-899.
Case
A 72-year-old female with multiple medical problems is admitted with a hip fracture. Surgery is scheduled in 48 hours. The patient’s home medications include aspirin, carbidopa/levodopa, celecoxib, clonidine, estradiol, ginkgo, lisinopril, NPH insulin, sulfasalazine, and prednisone 10 mg a day, which she has been taking for years. How should these and other medications be managed in the perioperative period?
Background
Perioperative management of chronic medications is a complex issue, as physicians are required to balance the beneficial and harmful effects of the individual drugs prescribed to their patients. On one hand, cessation of medications can result in decompensation of disease or withdrawal. On the other hand, continuation of drugs can alter metabolism of anesthetic agents, cause perioperative hemodynamic instability, or result in such post-operative complications as acute renal failure, bleeding, infection, and impaired wound healing.
Certain traits make it reasonable to continue medications during the perioperative period. A long elimination half-life or duration of action makes stopping some medications impractical as it takes four to five half-lives to completely clear the drug from the body; holding the drug for a few days around surgery will not appreciably affect its concentration. Stopping drugs that carry severe withdrawal symptoms can be impractical because of the need for lengthy tapers, which can delay surgery and result in decompensation of underlying disease.
Drugs with no significant interactions with anesthesia or risk of perioperative complications should be continued in order to avoid deterioration of the underlying disease. Conversely, drugs that interact with anesthesia or increase risk for complications should be stopped if this can be accomplished safely. Patient-specific factors should receive consideration, as the risk of complications has to be balanced against the danger of exacerbating the underlying disease.
Overview of the Data
The challenge in providing recommendations on perioperative medication management lies in a dearth of high-quality clinical trials. Thus, much of the information comes from case reports, expert opinion, and sound application of pharmacology.
Antiplatelet therapy: Nuances of perioperative antiplatelet therapy are beyond the scope of this review, but some general principles can be elucidated from the American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines.1 Management of antiplatelet therapy should be done in conjunction with the surgical team, as cardiovascular risk has to be weighed against bleeding risk.
Aspirin therapy should be continued in all patients with a history of coronary artery disease (CAD), balloon angioplasty, or percutaneous coronary intervention (PCI), unless the risk of bleeding complications is felt to exceed the cardioprotective benefits—for example, in some neurosurgical patients.1
Clopidogrel therapy is crucial for prevention of in-stent thrombosis (IST) following PCI because patients who experience IST suffer catastrophic myocardial infarctions with high mortality. Ideally, surgery should be delayed to permit completion of clopidogrel therapy—30 to 45 days after implantation of a bare-metal stent and 365 days after a drug-eluting stent. If surgery has to be performed sooner, guidelines recommend operating on dual antiplatelet therapy with aspirin and clopidogrel.1 Again, this course of treatment has to be balanced against the risk of hemorrhagic complications from surgery.
Both aspirin and clopidogrel irreversibly inhibit platelet aggregation. The recovery of normal coagulation involves formation of new platelets, which necessitates cessation of therapy for seven to 10 days before surgery. Platelet inhibition begins within minutes of restarting aspirin and within hours of taking clopidogrel, although attaining peak clopidogrel effect takes three to seven days, unless a loading dose is used.
Cardiovascular Drugs
Beta-blockers in the perioperative setting are a focus of an ongoing debate beyond the scope of this review (see “What Pre-Operative Cardiac Evaluation of Patients Undergoing Intermediate-Risk Surgery Is Most Effective?,” February 2008, p. 26). Given the current evidence and the latest ACC/AHA guidelines, it is still reasonable to continue therapy in patients who are already taking them to avoid precipitating cardiovascular events by withdrawal. Patients with increased cardiac risk, demonstrated by a Revised Cardiac Risk Index (RCRI) score of ≥2 (see Table 1, p. 12), should be considered for beta-blocker therapy before surgery.1 In either case, the dose should be titrated to a heart rate <65 for optimal cardiac protection.1
Statins should be continued if the patient is taking them, especially because preoperative withdrawal has been associated with a 4.6-fold increase in troponin release and a 7.5-fold increased risk of myocardial infarction (MI) and cardiovascular death following major vascular surgery.2 Patients with increased cardiac risk— RCRI ≥1—can be considered for initiation of statin therapy before surgery, although the benefit of this intervention has not been examined in prospective studies.1
Amiodarone has an exceptionally long half-life of up to 142 days. It should be continued in the perioperative period.
Calcium channel blockers (CCBs) can be continued with no unpleasant perioperative hemodynamic effects.1 CCBs have potential cardioprotective benefits.
Clonidine withdrawal can result in severe rebound hypertension with reports of encephalopathy, stroke, and death. These effects are exacerbated by concomitant beta-blocker therapy. For this reason, if a patient is expected to be NPO for more than 12 hours, they should be converted to a clonidine patch 48-72 hours before surgery with concurrent tapering of the oral dose.3
Digoxin has a long half-life (up to 48 hours) and should be continued with monitoring of levels if there is a change in renal function.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs) have been associated with a 50% increased risk of hypotension requiring vasopressors during induction of anesthesia.4 However, it is worth mentioning that this finding has not been corroborated in other studies. A large retrospective cohort of cardiothoracic surgical patients found a 28% increased risk of post-operative acute renal failure (ARF) with both drug classes, although another cardiothoracic report published the same year demonstrated a 50% reduction in risk with ACEIs.5,6 Although the evidence of harm is not unequivocal, perioperative blood-pressure control can be achieved with other drugs without hemodynamic or renal risk, such as CCBs, and in most cases ACEIs/ARBs should be stopped one day before surgery.
Diuretics carry a risk of volume depletion and electrolyte derangements, and should be stopped once a patient becomes NPO. Excess volume is managed with as-needed intravenous formulations.
Drugs Acting on the Central Nervous System
The majority of central nervous system (CNS)-active drugs, including antiepileptics, antipsychotics, benzodiazepines, bupropion, gabapentin, lithium, mirtazapine, selective serotonin and norepinephrine reuptake inhibitors (SSRIs and SNRIs), tricyclic antidepressants (TCAs), and valproic acid, balance a low risk of perioperative complications against a significant potential for withdrawal and disease decompensation. Therefore, these medications should be continued.
Carbidopa/Levodopa should be continued because abrupt cessation can precipitate systemic withdrawal resembling serotonin syndrome and rapid deterioration of Parkinson’s symptoms.
Monoamine oxidase inhibitor (MAOI) therapy usually indicates refractory psychiatric illness, so these drugs should be continued to avoid decompensation. Importantly, MAOI-safe anesthesia without dextromethorphan, meperidine, epinephrine, or norepinephrine has to be used due to the risk of cardiovascular instability.7
Diabetic Drugs
Insulin therapy should be continued with adjustments. Glargine basal insulin has no peak and can be continued without changing the dose. Likewise, patients with insulin pumps can continue the usual basal rate. Short-acting insulin or such insulin mixes as 70/30 should be stopped four hours before surgery to avoid hypoglycemia. Intermediate-acting insulin (e.g., NPH) can be administered at half the usual dose the day of surgery with a perioperative 5% dextrose infusion. NPH should not be given the day of surgery if the dextrose infusion cannot be used.8
Incretins (exenatide, sitagliptin) rarely cause hypoglycemia in the absence of insulin and may be beneficial in controlling intraoperative hyperglycemia. Therefore, these medications can be continued.8
Thiazolidinediones (TZDs; pioglitazone, rosiglitazone) alter gene transcription with biological duration of action on the order of weeks and low risk of hypoglycemia, and should be continued.
Metformin carries an FDA black-box warning to discontinue therapy before any intravascular radiocontrast studies or surgical procedures due to the risk of severe lactic acidosis if renal failure develops. It should be stopped 24 hours before surgery and restarted at least 48-72 hours after. Normal renal function should be confirmed before restarting therapy.8
Sulfonylureas (glimepiride, glipizide, glyburide) carry a significant risk of hypoglycemia in a patient who is NPO; they should be stopped the night before surgery or before commencement of NPO status.
Hormones
Antithyroid drugs (methimazole, propylthiouracil) and levothyroxine should be continued, as they have no perioperative contraindications.
Oral contraceptives (OCPs), hormone replacement therapy (HRT), and raloxifene can increase the risk of DVT. The largest study on the topic was the HERS trial of postmenopausal women on estrogen/progesterone HRT. The authors reported a 4.9-fold increased risk of DVT for 90 days after surgery.9 Unfortunately, no information was provided on the types of surgery, or whether appropriate and consistent DVT prophylaxis was utilized. HERS authors also reported a 2.5-fold increased risk of DVT for at least 30 days after cessation of HRT.9
Given the data, it is reasonable to stop hormone therapy four weeks before surgery when prolonged immobilization is anticipated and patients are able to tolerate hormone withdrawal, especially if other DVT risk factors are present. If hormone therapy cannot be stopped, strong consideration should be given to higher-intensity DVT prophylaxis (e.g., chemoprophylaxis as opposed to mechanical measures) of longer duration—up to 28 days following general surgery and up to 35 days after orthopedic procedures.10
Perioperative Corticosteroids
Corticosteroid therapy in excess of prednisone 5 mg/day or equivalent for more than five days in the 30 days preceding surgery might predispose patients to acute adrenal insufficiency in the perioperative period. Surgical procedures typically result in cortisol release of 50-150 mg/day, which returns to baseline within 48 hours.11 Therefore, the recommendation is to continue a patient’s baseline steroid dose and supplement it with stress-dose steroids tailored to the severity of operative stress (see Table 2, above).
Mineralocorticoid supplementation is not necessary, because endogenous production is not suppressed by corticosteroid therapy.11 Although a recent systematic review suggests that routine stress-dose steroids might not be indicated, high-quality prospective data are needed before abandoning this strategy due to complications of acute adrenal insufficiency compared to the risk of a brief corticosteroid burst.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Nonselective cyclooxygenase (COX) inhibitors reversibly decrease platelet aggregation only while the drug is present in the circulation and should be stopped one to three days before surgery due to risk of bleeding.
Selective COX-2 inhibitors do not significantly alter platelet aggregation and can be continued for opioid-sparing perioperative pain control.
Both COX-2-selective and nonselective inhibitors should be held if there are concerns for impaired renal function.
Disease-Modifying Antirheumatic Drugs (DMARDs) and Biological Response Modifiers (BRMs)
Methotrexate increases the risk of wound infections and dehiscence. However, this is offset by a decreased risk of post-operative disease flares with continued use. It can be continued unless the patient has medical comorbidities, advanced age, or chronic therapy with more than 10 mg/day of prednisone, in which case the drug should be stopped two weeks before surgery.12
Azathioprine, leflunomide, and sulfasalazine are renally cleared with a risk of myelosuppression; all of these medications should be stopped. Long half-life of leflunomide necessitates stopping it two weeks before surgery; azathioprine and sulfasalazine can be stopped one day in advance. The drugs can be restarted three days after surgery, assuming stable renal function.13
Anti-TNF-α (adalimumab, etanercept, infliximab), IL1 antagonist (anakinra), and anti-CD20 (rituximab) agents should be stopped one week before surgery and resumed 1-2 weeks afterward, unless risk of complications from disease flareup outweighs the concern for wound infections and dehiscence.14
Herbal Medicines
It is estimated that as much as a third of the U.S. population uses herbal medicines. These substances can result in perioperative hemodynamic instability (ephedra, ginseng, ma huang), hypoglycemia (ginseng), immunosuppression (echinacea, when taken for more than eight weeks), abnormal bleeding (garlic, ginkgo, ginseng), and prolongation of anesthesia (kava, St. John’s wort, valerian). All of these herbal medicine should be stopped one to two weeks before surgery.15,16
Back to the Case
The patient’s Carbidopa/Levodopa should be continued. Celecoxib can be continued if her renal function in stable. If aspirin is taken for a history of coronary artery disease or percutaneous coronary intervention, it should be continued, if possible. Clonidine should be continued or changed to a patch if an extended NPO period is anticipated. Ginkgo, lisinopril, and sulfasalazine should be stopped.
Hospitalization does not provide the luxury of stopping estradiol in advance, so it might be continued with chemical DVT prophylaxis for up to 35 days after surgery. The patient should receive 50-75 mg of IV hydrocortisone during surgery and an additional 25 mg the following day, in addition to her usual prednisone 10 mg/day. She can either receive half her usual NPH dose the morning of surgery with a 5% dextrose infusion in the operating room, or the NPH should be held altogether.
Bottom Line
Perioperative medication use should be tailored to each patient, balancing the risks and benefits of individual drugs. High-quality trials are needed to provide more robust clinical guidelines. TH
Dr. Levin is a hospitalist at the University of Colorado Denver.
References
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):1971-1996.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316-320.
- Spell NO III. Stopping and restarting medications in the perioperative period. Med Clin North Am. 2001;85(5):1117-1128.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325.
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266-1273.
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg. 2008;86(4):1160-1165.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm. 2004;61(9):899-914.
- Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93(5):1031-1047.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689-696.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):71S-109S.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am. 2003;32(2):367-383.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg. 2008;143(12):1222-1226.
- Rosandich PA, Kelley JT III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208-216.
- Hodges PJ, Kam PC. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57(9):889-899.
Case
A 72-year-old female with multiple medical problems is admitted with a hip fracture. Surgery is scheduled in 48 hours. The patient’s home medications include aspirin, carbidopa/levodopa, celecoxib, clonidine, estradiol, ginkgo, lisinopril, NPH insulin, sulfasalazine, and prednisone 10 mg a day, which she has been taking for years. How should these and other medications be managed in the perioperative period?
Background
Perioperative management of chronic medications is a complex issue, as physicians are required to balance the beneficial and harmful effects of the individual drugs prescribed to their patients. On one hand, cessation of medications can result in decompensation of disease or withdrawal. On the other hand, continuation of drugs can alter metabolism of anesthetic agents, cause perioperative hemodynamic instability, or result in such post-operative complications as acute renal failure, bleeding, infection, and impaired wound healing.
Certain traits make it reasonable to continue medications during the perioperative period. A long elimination half-life or duration of action makes stopping some medications impractical as it takes four to five half-lives to completely clear the drug from the body; holding the drug for a few days around surgery will not appreciably affect its concentration. Stopping drugs that carry severe withdrawal symptoms can be impractical because of the need for lengthy tapers, which can delay surgery and result in decompensation of underlying disease.
Drugs with no significant interactions with anesthesia or risk of perioperative complications should be continued in order to avoid deterioration of the underlying disease. Conversely, drugs that interact with anesthesia or increase risk for complications should be stopped if this can be accomplished safely. Patient-specific factors should receive consideration, as the risk of complications has to be balanced against the danger of exacerbating the underlying disease.
Overview of the Data
The challenge in providing recommendations on perioperative medication management lies in a dearth of high-quality clinical trials. Thus, much of the information comes from case reports, expert opinion, and sound application of pharmacology.
Antiplatelet therapy: Nuances of perioperative antiplatelet therapy are beyond the scope of this review, but some general principles can be elucidated from the American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines.1 Management of antiplatelet therapy should be done in conjunction with the surgical team, as cardiovascular risk has to be weighed against bleeding risk.
Aspirin therapy should be continued in all patients with a history of coronary artery disease (CAD), balloon angioplasty, or percutaneous coronary intervention (PCI), unless the risk of bleeding complications is felt to exceed the cardioprotective benefits—for example, in some neurosurgical patients.1
Clopidogrel therapy is crucial for prevention of in-stent thrombosis (IST) following PCI because patients who experience IST suffer catastrophic myocardial infarctions with high mortality. Ideally, surgery should be delayed to permit completion of clopidogrel therapy—30 to 45 days after implantation of a bare-metal stent and 365 days after a drug-eluting stent. If surgery has to be performed sooner, guidelines recommend operating on dual antiplatelet therapy with aspirin and clopidogrel.1 Again, this course of treatment has to be balanced against the risk of hemorrhagic complications from surgery.
Both aspirin and clopidogrel irreversibly inhibit platelet aggregation. The recovery of normal coagulation involves formation of new platelets, which necessitates cessation of therapy for seven to 10 days before surgery. Platelet inhibition begins within minutes of restarting aspirin and within hours of taking clopidogrel, although attaining peak clopidogrel effect takes three to seven days, unless a loading dose is used.
Cardiovascular Drugs
Beta-blockers in the perioperative setting are a focus of an ongoing debate beyond the scope of this review (see “What Pre-Operative Cardiac Evaluation of Patients Undergoing Intermediate-Risk Surgery Is Most Effective?,” February 2008, p. 26). Given the current evidence and the latest ACC/AHA guidelines, it is still reasonable to continue therapy in patients who are already taking them to avoid precipitating cardiovascular events by withdrawal. Patients with increased cardiac risk, demonstrated by a Revised Cardiac Risk Index (RCRI) score of ≥2 (see Table 1, p. 12), should be considered for beta-blocker therapy before surgery.1 In either case, the dose should be titrated to a heart rate <65 for optimal cardiac protection.1
Statins should be continued if the patient is taking them, especially because preoperative withdrawal has been associated with a 4.6-fold increase in troponin release and a 7.5-fold increased risk of myocardial infarction (MI) and cardiovascular death following major vascular surgery.2 Patients with increased cardiac risk— RCRI ≥1—can be considered for initiation of statin therapy before surgery, although the benefit of this intervention has not been examined in prospective studies.1
Amiodarone has an exceptionally long half-life of up to 142 days. It should be continued in the perioperative period.
Calcium channel blockers (CCBs) can be continued with no unpleasant perioperative hemodynamic effects.1 CCBs have potential cardioprotective benefits.
Clonidine withdrawal can result in severe rebound hypertension with reports of encephalopathy, stroke, and death. These effects are exacerbated by concomitant beta-blocker therapy. For this reason, if a patient is expected to be NPO for more than 12 hours, they should be converted to a clonidine patch 48-72 hours before surgery with concurrent tapering of the oral dose.3
Digoxin has a long half-life (up to 48 hours) and should be continued with monitoring of levels if there is a change in renal function.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARBs) have been associated with a 50% increased risk of hypotension requiring vasopressors during induction of anesthesia.4 However, it is worth mentioning that this finding has not been corroborated in other studies. A large retrospective cohort of cardiothoracic surgical patients found a 28% increased risk of post-operative acute renal failure (ARF) with both drug classes, although another cardiothoracic report published the same year demonstrated a 50% reduction in risk with ACEIs.5,6 Although the evidence of harm is not unequivocal, perioperative blood-pressure control can be achieved with other drugs without hemodynamic or renal risk, such as CCBs, and in most cases ACEIs/ARBs should be stopped one day before surgery.
Diuretics carry a risk of volume depletion and electrolyte derangements, and should be stopped once a patient becomes NPO. Excess volume is managed with as-needed intravenous formulations.
Drugs Acting on the Central Nervous System
The majority of central nervous system (CNS)-active drugs, including antiepileptics, antipsychotics, benzodiazepines, bupropion, gabapentin, lithium, mirtazapine, selective serotonin and norepinephrine reuptake inhibitors (SSRIs and SNRIs), tricyclic antidepressants (TCAs), and valproic acid, balance a low risk of perioperative complications against a significant potential for withdrawal and disease decompensation. Therefore, these medications should be continued.
Carbidopa/Levodopa should be continued because abrupt cessation can precipitate systemic withdrawal resembling serotonin syndrome and rapid deterioration of Parkinson’s symptoms.
Monoamine oxidase inhibitor (MAOI) therapy usually indicates refractory psychiatric illness, so these drugs should be continued to avoid decompensation. Importantly, MAOI-safe anesthesia without dextromethorphan, meperidine, epinephrine, or norepinephrine has to be used due to the risk of cardiovascular instability.7
Diabetic Drugs
Insulin therapy should be continued with adjustments. Glargine basal insulin has no peak and can be continued without changing the dose. Likewise, patients with insulin pumps can continue the usual basal rate. Short-acting insulin or such insulin mixes as 70/30 should be stopped four hours before surgery to avoid hypoglycemia. Intermediate-acting insulin (e.g., NPH) can be administered at half the usual dose the day of surgery with a perioperative 5% dextrose infusion. NPH should not be given the day of surgery if the dextrose infusion cannot be used.8
Incretins (exenatide, sitagliptin) rarely cause hypoglycemia in the absence of insulin and may be beneficial in controlling intraoperative hyperglycemia. Therefore, these medications can be continued.8
Thiazolidinediones (TZDs; pioglitazone, rosiglitazone) alter gene transcription with biological duration of action on the order of weeks and low risk of hypoglycemia, and should be continued.
Metformin carries an FDA black-box warning to discontinue therapy before any intravascular radiocontrast studies or surgical procedures due to the risk of severe lactic acidosis if renal failure develops. It should be stopped 24 hours before surgery and restarted at least 48-72 hours after. Normal renal function should be confirmed before restarting therapy.8
Sulfonylureas (glimepiride, glipizide, glyburide) carry a significant risk of hypoglycemia in a patient who is NPO; they should be stopped the night before surgery or before commencement of NPO status.
Hormones
Antithyroid drugs (methimazole, propylthiouracil) and levothyroxine should be continued, as they have no perioperative contraindications.
Oral contraceptives (OCPs), hormone replacement therapy (HRT), and raloxifene can increase the risk of DVT. The largest study on the topic was the HERS trial of postmenopausal women on estrogen/progesterone HRT. The authors reported a 4.9-fold increased risk of DVT for 90 days after surgery.9 Unfortunately, no information was provided on the types of surgery, or whether appropriate and consistent DVT prophylaxis was utilized. HERS authors also reported a 2.5-fold increased risk of DVT for at least 30 days after cessation of HRT.9
Given the data, it is reasonable to stop hormone therapy four weeks before surgery when prolonged immobilization is anticipated and patients are able to tolerate hormone withdrawal, especially if other DVT risk factors are present. If hormone therapy cannot be stopped, strong consideration should be given to higher-intensity DVT prophylaxis (e.g., chemoprophylaxis as opposed to mechanical measures) of longer duration—up to 28 days following general surgery and up to 35 days after orthopedic procedures.10
Perioperative Corticosteroids
Corticosteroid therapy in excess of prednisone 5 mg/day or equivalent for more than five days in the 30 days preceding surgery might predispose patients to acute adrenal insufficiency in the perioperative period. Surgical procedures typically result in cortisol release of 50-150 mg/day, which returns to baseline within 48 hours.11 Therefore, the recommendation is to continue a patient’s baseline steroid dose and supplement it with stress-dose steroids tailored to the severity of operative stress (see Table 2, above).
Mineralocorticoid supplementation is not necessary, because endogenous production is not suppressed by corticosteroid therapy.11 Although a recent systematic review suggests that routine stress-dose steroids might not be indicated, high-quality prospective data are needed before abandoning this strategy due to complications of acute adrenal insufficiency compared to the risk of a brief corticosteroid burst.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Nonselective cyclooxygenase (COX) inhibitors reversibly decrease platelet aggregation only while the drug is present in the circulation and should be stopped one to three days before surgery due to risk of bleeding.
Selective COX-2 inhibitors do not significantly alter platelet aggregation and can be continued for opioid-sparing perioperative pain control.
Both COX-2-selective and nonselective inhibitors should be held if there are concerns for impaired renal function.
Disease-Modifying Antirheumatic Drugs (DMARDs) and Biological Response Modifiers (BRMs)
Methotrexate increases the risk of wound infections and dehiscence. However, this is offset by a decreased risk of post-operative disease flares with continued use. It can be continued unless the patient has medical comorbidities, advanced age, or chronic therapy with more than 10 mg/day of prednisone, in which case the drug should be stopped two weeks before surgery.12
Azathioprine, leflunomide, and sulfasalazine are renally cleared with a risk of myelosuppression; all of these medications should be stopped. Long half-life of leflunomide necessitates stopping it two weeks before surgery; azathioprine and sulfasalazine can be stopped one day in advance. The drugs can be restarted three days after surgery, assuming stable renal function.13
Anti-TNF-α (adalimumab, etanercept, infliximab), IL1 antagonist (anakinra), and anti-CD20 (rituximab) agents should be stopped one week before surgery and resumed 1-2 weeks afterward, unless risk of complications from disease flareup outweighs the concern for wound infections and dehiscence.14
Herbal Medicines
It is estimated that as much as a third of the U.S. population uses herbal medicines. These substances can result in perioperative hemodynamic instability (ephedra, ginseng, ma huang), hypoglycemia (ginseng), immunosuppression (echinacea, when taken for more than eight weeks), abnormal bleeding (garlic, ginkgo, ginseng), and prolongation of anesthesia (kava, St. John’s wort, valerian). All of these herbal medicine should be stopped one to two weeks before surgery.15,16
Back to the Case
The patient’s Carbidopa/Levodopa should be continued. Celecoxib can be continued if her renal function in stable. If aspirin is taken for a history of coronary artery disease or percutaneous coronary intervention, it should be continued, if possible. Clonidine should be continued or changed to a patch if an extended NPO period is anticipated. Ginkgo, lisinopril, and sulfasalazine should be stopped.
Hospitalization does not provide the luxury of stopping estradiol in advance, so it might be continued with chemical DVT prophylaxis for up to 35 days after surgery. The patient should receive 50-75 mg of IV hydrocortisone during surgery and an additional 25 mg the following day, in addition to her usual prednisone 10 mg/day. She can either receive half her usual NPH dose the morning of surgery with a 5% dextrose infusion in the operating room, or the NPH should be held altogether.
Bottom Line
Perioperative medication use should be tailored to each patient, balancing the risks and benefits of individual drugs. High-quality trials are needed to provide more robust clinical guidelines. TH
Dr. Levin is a hospitalist at the University of Colorado Denver.
References
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):1971-1996.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol. 2007;100(2):316-320.
- Spell NO III. Stopping and restarting medications in the perioperative period. Med Clin North Am. 2001;85(5):1117-1128.
- Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3(4):319-325.
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3(5):1266-1273.
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative Angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg. 2008;86(4):1160-1165.
- Pass SE, Simpson RW. Discontinuation and reinstitution of medications during the perioperative period. Am J Health Syst Pharm. 2004;61(9):899-914.
- Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93(5):1031-1047.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689-696.
- Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6 Suppl):71S-109S.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin North Am. 2003;32(2):367-383.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Arch Surg. 2008;143(12):1222-1226.
- Rosandich PA, Kelley JT III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198.
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-784.
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286(2):208-216.
- Hodges PJ, Kam PC. The peri-operative implications of herbal medicines. Anaesthesia. 2002;57(9):889-899.
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- CPOE and quality outcomes
- Outcomes of standardized management of endocarditis
- Effect of tPA three to 4.5 hours after stroke onset
- Failure to notify patients of significant test results
- PFO repair and stroke rate
- Predictors of delay in defibrillation for in-hospital arrest
- H. pylori eradication and risk of future gastric cancer
- Bleeding risk with fondaparinux vs. enoxaparin in ACS
- Perceptions of physician ability to predict medical futility
CPOE Is Associated with Improvement in Quality Measures
Clinical question: Is computerized physician order entry (CPOE) associated with improved outcomes across a large, nationally representative sample of hospitals?
Background: Several single-institution studies suggest CPOE leads to better outcomes in quality measures for heart failure, acute myocardial infarction, and pneumonia as defined by the Hospital Quality Alliance (HQA) initiative, led by the Centers for Medicare and Medicaid Services (CMS). Little systematic information is known about the effects of CPOE on quality of care.
Study design: Cross-sectional study.
Setting: The Health Information Management System Society (HIMSS) analytics database of 3,364 hospitals throughout the U.S.
Synopsis: Of the hospitals that reported CPOE utilization to HIMSS, 264 (7.8%) fully implement CPOE throughout their institutions. These CPOE hospitals outperformed their peers on five of 11 quality measures related to ordering medications, and in one of nine non-medication-related measures. No difference was noted in the other measures, except CPOE hospitals were less effective at providing antibiotics within four hours of pneumonia diagnosis. Hospitals that utilized CPOE were generally academic, larger, and nonprofit. After adjusting for these differences, benefits were still preserved.
The authors indicate that the lack of systematic outperformance by CPOE hospitals in all 20 of the quality categories inherently suggests that other factors (e.g., concomitant QI efforts) are not affecting these results. Given the observational nature of this study, no causal relationship can be established between CPOE and the observed benefits. CPOE might represent the commitment of certain hospitals to quality measures, but further study is needed.
Bottom line: Enhanced compliance in several CMS-established quality measures is seen in hospitals that utilize CPOE throughout their institutions.
Citation: Yu FB, Menachemi N, Berner ES, Allison JJ, Weissman NW, Houston TK. Full implementation of computerized physician order entry and medication-related quality outcomes: a study of 3,364 hospitals. Am J Med Qual. 2009;24(4):278-286.
Standardized Management of Endocarditis Leads to Significant Mortality Benefit
Clinical question: Does a standardized approach to the treatment of infective endocarditis reduce mortality and morbidity?
Background: Despite epidemiological changes to the inciting bacteria and improvements in available antibiotics, mortality and morbidity associated with endocarditis remain high. The contribution of inconsistent or inaccurate treatment of endocarditis is unclear.
Study design: Case series with historical controls from 1994 to 2001, compared with protocolized patients from 2002 to 2006.
Setting: Single teaching tertiary-care hospital in France.
Synopsis: The authors established a diagnostic protocol for infectious endocarditis from 1994 to 2001 (period 1) and established a treatment protocol from 2002 to 2006 (period 2). Despite a statistically significant sicker population (older, higher comorbidities, higher coagulase-negative staphylococcal infections, and fewer healthy valves), the period-2 patients had a dramatically lower mortality rate of 8.2% (P<0.001), compared with 18.5% in period-1 patients. Fewer episodes of renal failure, organ failure, and deaths associated with embolism were noted in period 2.
Whether these results are due to more frequent care, more aggressive care (patients were “summoned” if they did not show for appointments), standardized medication and surgical options, or the effects of long-term collaboration, these results appear durable, remarkable, and reproducible.
This study is limited by its lack of randomization and extensive time frame, with concomitant changes in medical treatment and observed infectious organisms.
Bottom line: Implementation of a standardized approach to endocarditis has significant benefit on mortality and morbidity.
Citation: Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298.
Treatment with tPA in the Three- to 4.5-Hour Time Window after Stroke Is Beneficial
Clinical question: What is the effect of tissue plasminogen activator (tPA) on outcomes in patients treated in the three- to 4.5-hour window after stroke?
Background: The third European Cooperative Acute Stroke Study 3 (ECASS-3) demonstrated benefit of treatment of acute stroke with tPA in the three- to 4.5-hour time window. Prior studies, however, did not show superiority of tPA over placebo, and there is a lack of a confirmatory randomized, controlled trial of tPA in this time frame.
Study design: Meta-analysis of randomized, controlled trials.
Setting: Four studies involving 1,622 patients who were treated with intravenous tPA for acute ischemic stroke from three to 4.5 hours after stroke compared with placebo.
Synopsis: Of the randomized, controlled trials of intravenous tPA for treatment of acute ischemic stroke from three to 4.5 hours after stroke, four trials (ECASS-1, ECASS-2, ECASS-3, and ATLANTIS) were included in the analysis. Treatment with tPA in the three- to 4.5-hour time window is associated with increased favorable outcomes based on the global outcome measure (OR 1.31; 95% CI: 1.10-1.56, P=0.002) and the modified Rankin Scale (OR 1.31; 95% CI: 1.07-1.59, P=0.01), compared with placebo. The 90-day mortality rate was not significantly different between the treatment and placebo groups (OR 1.04; 95% CI 0.75-1.43, P=0.83).
Due to the relatively high dose of tPA (1.1 mg/kg) administered in the ECASS-1 trial, a separate meta-analysis looking at the other three trials (tPA dose of 0.9 mg/kg) was conducted, and the favorable outcome with tPA remained.
Bottom line: Treatment of acute ischemic stroke with tPA in the three- to 4.5-hour time window results in an increased rate of favorable functional outcomes without a significant difference in mortality.
Citation: Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
Outpatients Often Are Not Notified of Clinically Significant Test Results
Clinical question: How frequently do primary-care physicians (PCPs) fail to inform patients of clinically significant outpatient test results?
Background: Diagnostic errors are the most common cause of malpractice claims in the U.S. It is unclear how often providers fail to either inform patients of abnormal test results or document that patients have been notified.
Study design: Retrospective chart review.
Setting: Twenty-three primary-care practices: 19 private, four academic.
Synopsis: More than 5,400 charts were reviewed, and 1,889 abnormal test results were identified in this study. Failure to inform or document notification was identified in 135 cases (7.1%). The failure rates in the practices ranged from 0.0% to 26.2%. Practices with the best processes for managing test results had the lowest failure rates; these processes included: all results being routed to the responsible physician; the physician signing off on all results; the practice informing patients of all results, both normal and abnormal; documenting when the patient is informed; and instructing patients to call if not notified of test results within a certain time interval.
Limitations of this study include the potential of over- or underreporting of failures to inform as a chart review was used, and only practices that agreed to participate were included.
Bottom line: Failure to notify outpatients of test results is common but can be minimized by creating a systematic management of test results that include best practices.
Citation: Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169(12):1123-1129.
Repair of Incidental PFO Discovered During Cardiothoracic Surgery Repair Increases Postoperative Stroke Risk
Clinical question: What is the impact of closing incidentally discovered patent foramen ovale (PFO) defects during cardiothoracic surgery?
Background: PFO’s role in cryptogenic stroke remains controversial. Incidental PFO is commonly detected by transesophageal echocardiography (TEE) during cardiothoracic surgery. Routine PFO closure has been recommended when almost no alteration of the surgical plan is required.
Study design: Retrospective chart review.
Setting: The Cleveland Clinic.
Synopsis: Between 1995 and 2006, 13,092 patients undergoing cardiothoracic surgery had TEE data with no previous diagnosis of PFO, but the review found that 2,277 (17%) had PFO discovered intraoperatively. Of these, 639 (28%) had the PFO repaired.
Patients with an intraoperative diagnosis of PFO had similar rates of in-hospital stroke and hospital death compared with those without PFO. Patients who had their PFO repaired had a greater in-hospital stroke risk (2.8% vs. 1.2%; P=0.04) compared with those with a non-repaired PFO, representing nearly 2.5 times greater odds of having an in-hospital stroke. No other difference was noted in perioperative outcomes for patients who underwent intraoperative repair compared with those who did not, including risk of in-hospital death, hospital length of stay, ICU length of stay, and time on cardiopulmonary bypass. Long-term analysis demonstrated that PFO repair was associated with no survival difference.
The study is limited by its retrospective nature.
Bottom line: Routine surgical closure of incidental PFO detected during intraoperative imaging should be discouraged.
Citation: Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of interoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297.
Hospital-Level Differences Are Strong Predictors of Time to Defibrillation Delay In Cardiac Arrest
Clinical question: What are the predictors of delay in the time to defibrillation after in-hospital cardiac arrest?
Background: Thirty percent of in-hospital cardiac arrests from ventricular arrhythmias are not treated within the American Heart Association’s recommendation of two minutes. This delay is associated with a 50% lower rate of in-hospital survival. Exploring the hospital-level variation in delays to defibrillation is a critical step toward sharing the best practices.
Study design: Retrospective review of registry data.
Setting: The National Registry of Cardiopulmonary Resuscitation (NRCPR) survey of 200 acute-care, nonpediatric hospitals.
Synopsis: The registry identified 7,479 patients who experienced cardiac arrest from ventricular tachycardia or pulseless ventricular fibrillation. The primary outcome was the hospital rate of delayed defibrillation (time to defibrillation > two minutes), which ranged from 2% to 51%.
Time to defibrillation was found to be a major predictor of survival after a cardiac arrest. Only bed volume and arrest location were associated with differences in rates of delayed defibrillation (lower rates in larger hospitals and in ICUs). The variability was not due to differences in patient characteristics, but was due to hospital-level effects. Academic status, geographical location, arrest volume, and daily admission volume did not affect the time to defibrillation.
The study was able to identify only a few facility characteristics that account for the variability between hospitals in the rate of delayed defibrillation. The study emphasizes the need for new approaches to identifying hospital innovations in process-of-care measures that are associated with improved performance in defibrillation times.
Bottom Line: Future research is needed to better understand the reason for the wide variation between hospitals in the rate of delayed defibrillation after in-hospital cardiac arrest.
Citation: Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265-1273.
Treating for H. Pylori Reduces the Risk for Developing Gastric Cancer in High-Risk Patients
Clinical question: In patients with high-baseline incidence of gastric cancer, does H. pylori eradication reduce the risk for developing gastric cancer?
Background: Gastric cancer remains a major health problem in Asia. The link of H. pylori and gastric cancer has been established, but it remains unclear whether treatment for H. pylori is effective primary prevention for the development of gastric cancer.
Study design: Meta-analysis of six studies.
Setting: All but one trial was performed in Asia.
Synopsis: Seven studies met inclusion criteria, one of which was excluded due to heterogeneity. The six remaining studies were pooled, with 37 of 3,388 (1.1%) treated patients developing a new gastric cancer, compared with 56 of 3,307 (1.7%) patients who received placebo or were in the control group (RR 0.65; 0.43-0.98). Most patients received gastric biopsy prior to enrollment, and most of those demonstrated gastric atrophy or intestinal metaplasia.
These patients, despite more advanced precancerous pathology findings, still benefited from eradication. The seventh study, which was excluded, enrolled patients with early gastric cancer; these patients still benefited from H. pylori eradication and, when included in the meta-analysis, the RR was even lower, 0.57 (0.49-0.81).
Only two trials were double-blinded, but all of the studies employed the same definition of gastric cancer and held to excellent data reporting standards. This study encourages screening and treatment in high-risk patients given the widespread incidence of H. pylori.
Bottom Line: Treatment of H. pylori reduces the risk of gastric cancer in high-risk patients.
Citation: Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121-128.
Patients on Anti-Platelet Agents with Acute Coronary Syndrome Have a Lower Bleeding Risk When Treated with Fondaparinux
Clinical question: Is there a difference in bleeding risk with fondaparinux and enoxaparin when used with GPIIb/IIIa inhibitors or thienopyridines in NSTEMI-ACS?
Background: The OASIS 5 study reported a 50% reduction in severe bleeding when comparing fondaparinux to enoxaparin in ACS while maintaining a similar efficacy. This subgroup analysis was performed to evaluate whether reduced bleeding risk with fondaparinux remains in patients treated with additional anti-platelet agents.
Study design: Subgroup analysis of a large, multicenter, randomized, double-blind trial.
Setting: Acute-care hospitals in North America, Eastern and Western Europe, Latin America, Australia, and Asia.
Synopsis: Patients with NSTE-ACS received either fondaparinux or enoxaparin and were treated with GPIIb/IIIa inhibitors or thienopyridines at the discretion of their physician. At 30 days, the fondaparinux group had similar efficacy and decreased bleeding risk in both the GPIIb/IIIa and the thienopyridine groups. Of the 3,630 patients in the GPIIb/IIIa group, the risk for major bleeding with fondaparinux was 5.2%, whereas the risk with enoxaparin was 8.3% (HR 0.61; P<0.001) compared with enoxaparin. Of the 1,352 patients treated with thienopyridines, the risk for major bleeding with fondaparinux was 3.4%, whereas the risk with enoxaparin was 5.4% (HR 0.62; P<0.001).
Bottom Line: This subgroup analysis suggests there are less-severe bleeding complications in patients treated with fondaparinux when compared with enoxaparin in the setting of cotreatment with GPIIb/IIIa inhibitors, thienopyridines, or both.
Citation: Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors of thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54(5):468-476.
Surrogate Decision-Makers Frequently Doubt Clinicians’ Ability to Predict Medical Futility
Clinical question: What attitudes do surrogate decision-makers hold toward clinicians’ predictions of medical futility in critically-ill patients?
Background: The clinical judgment of medical futility leading to the withdrawal of life-sustaining treatment—despite the objections of surrogate decision-makers—is controversial. Very little is known about how surrogate decision-makers view the futility rationale when physicians suggest limiting the use of life-sustaining treatment.
Study design: Multicenter, mixed, qualitative and quantitative study.
Setting: Three ICUs in three different California hospitals from 2006 to 2007.
Synopsis: Semi-structured interviews of surrogate decision-makers for 50 incapacitated, critically-ill patients were performed to ascertain their beliefs about medical futility in response to hypothetical situations. Of the surrogates surveyed, 64% expressed doubt about physicians’ futility predictions.
The interviewees gave four main reasons for their doubts. Two reasons not previously described were doubts about the accuracy of physicians’ predictions and the need for surrogates to see futility themselves. Previously described sources of conflict included a misunderstanding about prognosis and religious-based objections. Surrogates with religious objections were more likely to request continuation of life-sustaining treatments than those with secular or experiential objections (OR 4; 95% CI 1.2-14.0; P=0.03). Nearly a third (32%) of surrogates elected to continue life support with a <1% survival estimate; 18% elected to continue life support when physicians thought there was no chance of survival.
This study has several limitations: a small sample size, the use of hypothetical situations, and the inability to assess attitudes as they change over time.
Bottom line: The nature of surrogate decision-makers’ doubts about medical futility can help predict whether they accept predictions of medical futility from physicians.
Citation: Zier LS, Burack JH, Micco G, Chipman AK, Frank JA, White DB. Surrogate decision makers’ responses to physicians’ predictions of medical futility. Chest. 2009;136:110-117. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- CPOE and quality outcomes
- Outcomes of standardized management of endocarditis
- Effect of tPA three to 4.5 hours after stroke onset
- Failure to notify patients of significant test results
- PFO repair and stroke rate
- Predictors of delay in defibrillation for in-hospital arrest
- H. pylori eradication and risk of future gastric cancer
- Bleeding risk with fondaparinux vs. enoxaparin in ACS
- Perceptions of physician ability to predict medical futility
CPOE Is Associated with Improvement in Quality Measures
Clinical question: Is computerized physician order entry (CPOE) associated with improved outcomes across a large, nationally representative sample of hospitals?
Background: Several single-institution studies suggest CPOE leads to better outcomes in quality measures for heart failure, acute myocardial infarction, and pneumonia as defined by the Hospital Quality Alliance (HQA) initiative, led by the Centers for Medicare and Medicaid Services (CMS). Little systematic information is known about the effects of CPOE on quality of care.
Study design: Cross-sectional study.
Setting: The Health Information Management System Society (HIMSS) analytics database of 3,364 hospitals throughout the U.S.
Synopsis: Of the hospitals that reported CPOE utilization to HIMSS, 264 (7.8%) fully implement CPOE throughout their institutions. These CPOE hospitals outperformed their peers on five of 11 quality measures related to ordering medications, and in one of nine non-medication-related measures. No difference was noted in the other measures, except CPOE hospitals were less effective at providing antibiotics within four hours of pneumonia diagnosis. Hospitals that utilized CPOE were generally academic, larger, and nonprofit. After adjusting for these differences, benefits were still preserved.
The authors indicate that the lack of systematic outperformance by CPOE hospitals in all 20 of the quality categories inherently suggests that other factors (e.g., concomitant QI efforts) are not affecting these results. Given the observational nature of this study, no causal relationship can be established between CPOE and the observed benefits. CPOE might represent the commitment of certain hospitals to quality measures, but further study is needed.
Bottom line: Enhanced compliance in several CMS-established quality measures is seen in hospitals that utilize CPOE throughout their institutions.
Citation: Yu FB, Menachemi N, Berner ES, Allison JJ, Weissman NW, Houston TK. Full implementation of computerized physician order entry and medication-related quality outcomes: a study of 3,364 hospitals. Am J Med Qual. 2009;24(4):278-286.
Standardized Management of Endocarditis Leads to Significant Mortality Benefit
Clinical question: Does a standardized approach to the treatment of infective endocarditis reduce mortality and morbidity?
Background: Despite epidemiological changes to the inciting bacteria and improvements in available antibiotics, mortality and morbidity associated with endocarditis remain high. The contribution of inconsistent or inaccurate treatment of endocarditis is unclear.
Study design: Case series with historical controls from 1994 to 2001, compared with protocolized patients from 2002 to 2006.
Setting: Single teaching tertiary-care hospital in France.
Synopsis: The authors established a diagnostic protocol for infectious endocarditis from 1994 to 2001 (period 1) and established a treatment protocol from 2002 to 2006 (period 2). Despite a statistically significant sicker population (older, higher comorbidities, higher coagulase-negative staphylococcal infections, and fewer healthy valves), the period-2 patients had a dramatically lower mortality rate of 8.2% (P<0.001), compared with 18.5% in period-1 patients. Fewer episodes of renal failure, organ failure, and deaths associated with embolism were noted in period 2.
Whether these results are due to more frequent care, more aggressive care (patients were “summoned” if they did not show for appointments), standardized medication and surgical options, or the effects of long-term collaboration, these results appear durable, remarkable, and reproducible.
This study is limited by its lack of randomization and extensive time frame, with concomitant changes in medical treatment and observed infectious organisms.
Bottom line: Implementation of a standardized approach to endocarditis has significant benefit on mortality and morbidity.
Citation: Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298.
Treatment with tPA in the Three- to 4.5-Hour Time Window after Stroke Is Beneficial
Clinical question: What is the effect of tissue plasminogen activator (tPA) on outcomes in patients treated in the three- to 4.5-hour window after stroke?
Background: The third European Cooperative Acute Stroke Study 3 (ECASS-3) demonstrated benefit of treatment of acute stroke with tPA in the three- to 4.5-hour time window. Prior studies, however, did not show superiority of tPA over placebo, and there is a lack of a confirmatory randomized, controlled trial of tPA in this time frame.
Study design: Meta-analysis of randomized, controlled trials.
Setting: Four studies involving 1,622 patients who were treated with intravenous tPA for acute ischemic stroke from three to 4.5 hours after stroke compared with placebo.
Synopsis: Of the randomized, controlled trials of intravenous tPA for treatment of acute ischemic stroke from three to 4.5 hours after stroke, four trials (ECASS-1, ECASS-2, ECASS-3, and ATLANTIS) were included in the analysis. Treatment with tPA in the three- to 4.5-hour time window is associated with increased favorable outcomes based on the global outcome measure (OR 1.31; 95% CI: 1.10-1.56, P=0.002) and the modified Rankin Scale (OR 1.31; 95% CI: 1.07-1.59, P=0.01), compared with placebo. The 90-day mortality rate was not significantly different between the treatment and placebo groups (OR 1.04; 95% CI 0.75-1.43, P=0.83).
Due to the relatively high dose of tPA (1.1 mg/kg) administered in the ECASS-1 trial, a separate meta-analysis looking at the other three trials (tPA dose of 0.9 mg/kg) was conducted, and the favorable outcome with tPA remained.
Bottom line: Treatment of acute ischemic stroke with tPA in the three- to 4.5-hour time window results in an increased rate of favorable functional outcomes without a significant difference in mortality.
Citation: Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
Outpatients Often Are Not Notified of Clinically Significant Test Results
Clinical question: How frequently do primary-care physicians (PCPs) fail to inform patients of clinically significant outpatient test results?
Background: Diagnostic errors are the most common cause of malpractice claims in the U.S. It is unclear how often providers fail to either inform patients of abnormal test results or document that patients have been notified.
Study design: Retrospective chart review.
Setting: Twenty-three primary-care practices: 19 private, four academic.
Synopsis: More than 5,400 charts were reviewed, and 1,889 abnormal test results were identified in this study. Failure to inform or document notification was identified in 135 cases (7.1%). The failure rates in the practices ranged from 0.0% to 26.2%. Practices with the best processes for managing test results had the lowest failure rates; these processes included: all results being routed to the responsible physician; the physician signing off on all results; the practice informing patients of all results, both normal and abnormal; documenting when the patient is informed; and instructing patients to call if not notified of test results within a certain time interval.
Limitations of this study include the potential of over- or underreporting of failures to inform as a chart review was used, and only practices that agreed to participate were included.
Bottom line: Failure to notify outpatients of test results is common but can be minimized by creating a systematic management of test results that include best practices.
Citation: Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169(12):1123-1129.
Repair of Incidental PFO Discovered During Cardiothoracic Surgery Repair Increases Postoperative Stroke Risk
Clinical question: What is the impact of closing incidentally discovered patent foramen ovale (PFO) defects during cardiothoracic surgery?
Background: PFO’s role in cryptogenic stroke remains controversial. Incidental PFO is commonly detected by transesophageal echocardiography (TEE) during cardiothoracic surgery. Routine PFO closure has been recommended when almost no alteration of the surgical plan is required.
Study design: Retrospective chart review.
Setting: The Cleveland Clinic.
Synopsis: Between 1995 and 2006, 13,092 patients undergoing cardiothoracic surgery had TEE data with no previous diagnosis of PFO, but the review found that 2,277 (17%) had PFO discovered intraoperatively. Of these, 639 (28%) had the PFO repaired.
Patients with an intraoperative diagnosis of PFO had similar rates of in-hospital stroke and hospital death compared with those without PFO. Patients who had their PFO repaired had a greater in-hospital stroke risk (2.8% vs. 1.2%; P=0.04) compared with those with a non-repaired PFO, representing nearly 2.5 times greater odds of having an in-hospital stroke. No other difference was noted in perioperative outcomes for patients who underwent intraoperative repair compared with those who did not, including risk of in-hospital death, hospital length of stay, ICU length of stay, and time on cardiopulmonary bypass. Long-term analysis demonstrated that PFO repair was associated with no survival difference.
The study is limited by its retrospective nature.
Bottom line: Routine surgical closure of incidental PFO detected during intraoperative imaging should be discouraged.
Citation: Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of interoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297.
Hospital-Level Differences Are Strong Predictors of Time to Defibrillation Delay In Cardiac Arrest
Clinical question: What are the predictors of delay in the time to defibrillation after in-hospital cardiac arrest?
Background: Thirty percent of in-hospital cardiac arrests from ventricular arrhythmias are not treated within the American Heart Association’s recommendation of two minutes. This delay is associated with a 50% lower rate of in-hospital survival. Exploring the hospital-level variation in delays to defibrillation is a critical step toward sharing the best practices.
Study design: Retrospective review of registry data.
Setting: The National Registry of Cardiopulmonary Resuscitation (NRCPR) survey of 200 acute-care, nonpediatric hospitals.
Synopsis: The registry identified 7,479 patients who experienced cardiac arrest from ventricular tachycardia or pulseless ventricular fibrillation. The primary outcome was the hospital rate of delayed defibrillation (time to defibrillation > two minutes), which ranged from 2% to 51%.
Time to defibrillation was found to be a major predictor of survival after a cardiac arrest. Only bed volume and arrest location were associated with differences in rates of delayed defibrillation (lower rates in larger hospitals and in ICUs). The variability was not due to differences in patient characteristics, but was due to hospital-level effects. Academic status, geographical location, arrest volume, and daily admission volume did not affect the time to defibrillation.
The study was able to identify only a few facility characteristics that account for the variability between hospitals in the rate of delayed defibrillation. The study emphasizes the need for new approaches to identifying hospital innovations in process-of-care measures that are associated with improved performance in defibrillation times.
Bottom Line: Future research is needed to better understand the reason for the wide variation between hospitals in the rate of delayed defibrillation after in-hospital cardiac arrest.
Citation: Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265-1273.
Treating for H. Pylori Reduces the Risk for Developing Gastric Cancer in High-Risk Patients
Clinical question: In patients with high-baseline incidence of gastric cancer, does H. pylori eradication reduce the risk for developing gastric cancer?
Background: Gastric cancer remains a major health problem in Asia. The link of H. pylori and gastric cancer has been established, but it remains unclear whether treatment for H. pylori is effective primary prevention for the development of gastric cancer.
Study design: Meta-analysis of six studies.
Setting: All but one trial was performed in Asia.
Synopsis: Seven studies met inclusion criteria, one of which was excluded due to heterogeneity. The six remaining studies were pooled, with 37 of 3,388 (1.1%) treated patients developing a new gastric cancer, compared with 56 of 3,307 (1.7%) patients who received placebo or were in the control group (RR 0.65; 0.43-0.98). Most patients received gastric biopsy prior to enrollment, and most of those demonstrated gastric atrophy or intestinal metaplasia.
These patients, despite more advanced precancerous pathology findings, still benefited from eradication. The seventh study, which was excluded, enrolled patients with early gastric cancer; these patients still benefited from H. pylori eradication and, when included in the meta-analysis, the RR was even lower, 0.57 (0.49-0.81).
Only two trials were double-blinded, but all of the studies employed the same definition of gastric cancer and held to excellent data reporting standards. This study encourages screening and treatment in high-risk patients given the widespread incidence of H. pylori.
Bottom Line: Treatment of H. pylori reduces the risk of gastric cancer in high-risk patients.
Citation: Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121-128.
Patients on Anti-Platelet Agents with Acute Coronary Syndrome Have a Lower Bleeding Risk When Treated with Fondaparinux
Clinical question: Is there a difference in bleeding risk with fondaparinux and enoxaparin when used with GPIIb/IIIa inhibitors or thienopyridines in NSTEMI-ACS?
Background: The OASIS 5 study reported a 50% reduction in severe bleeding when comparing fondaparinux to enoxaparin in ACS while maintaining a similar efficacy. This subgroup analysis was performed to evaluate whether reduced bleeding risk with fondaparinux remains in patients treated with additional anti-platelet agents.
Study design: Subgroup analysis of a large, multicenter, randomized, double-blind trial.
Setting: Acute-care hospitals in North America, Eastern and Western Europe, Latin America, Australia, and Asia.
Synopsis: Patients with NSTE-ACS received either fondaparinux or enoxaparin and were treated with GPIIb/IIIa inhibitors or thienopyridines at the discretion of their physician. At 30 days, the fondaparinux group had similar efficacy and decreased bleeding risk in both the GPIIb/IIIa and the thienopyridine groups. Of the 3,630 patients in the GPIIb/IIIa group, the risk for major bleeding with fondaparinux was 5.2%, whereas the risk with enoxaparin was 8.3% (HR 0.61; P<0.001) compared with enoxaparin. Of the 1,352 patients treated with thienopyridines, the risk for major bleeding with fondaparinux was 3.4%, whereas the risk with enoxaparin was 5.4% (HR 0.62; P<0.001).
Bottom Line: This subgroup analysis suggests there are less-severe bleeding complications in patients treated with fondaparinux when compared with enoxaparin in the setting of cotreatment with GPIIb/IIIa inhibitors, thienopyridines, or both.
Citation: Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors of thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54(5):468-476.
Surrogate Decision-Makers Frequently Doubt Clinicians’ Ability to Predict Medical Futility
Clinical question: What attitudes do surrogate decision-makers hold toward clinicians’ predictions of medical futility in critically-ill patients?
Background: The clinical judgment of medical futility leading to the withdrawal of life-sustaining treatment—despite the objections of surrogate decision-makers—is controversial. Very little is known about how surrogate decision-makers view the futility rationale when physicians suggest limiting the use of life-sustaining treatment.
Study design: Multicenter, mixed, qualitative and quantitative study.
Setting: Three ICUs in three different California hospitals from 2006 to 2007.
Synopsis: Semi-structured interviews of surrogate decision-makers for 50 incapacitated, critically-ill patients were performed to ascertain their beliefs about medical futility in response to hypothetical situations. Of the surrogates surveyed, 64% expressed doubt about physicians’ futility predictions.
The interviewees gave four main reasons for their doubts. Two reasons not previously described were doubts about the accuracy of physicians’ predictions and the need for surrogates to see futility themselves. Previously described sources of conflict included a misunderstanding about prognosis and religious-based objections. Surrogates with religious objections were more likely to request continuation of life-sustaining treatments than those with secular or experiential objections (OR 4; 95% CI 1.2-14.0; P=0.03). Nearly a third (32%) of surrogates elected to continue life support with a <1% survival estimate; 18% elected to continue life support when physicians thought there was no chance of survival.
This study has several limitations: a small sample size, the use of hypothetical situations, and the inability to assess attitudes as they change over time.
Bottom line: The nature of surrogate decision-makers’ doubts about medical futility can help predict whether they accept predictions of medical futility from physicians.
Citation: Zier LS, Burack JH, Micco G, Chipman AK, Frank JA, White DB. Surrogate decision makers’ responses to physicians’ predictions of medical futility. Chest. 2009;136:110-117. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- CPOE and quality outcomes
- Outcomes of standardized management of endocarditis
- Effect of tPA three to 4.5 hours after stroke onset
- Failure to notify patients of significant test results
- PFO repair and stroke rate
- Predictors of delay in defibrillation for in-hospital arrest
- H. pylori eradication and risk of future gastric cancer
- Bleeding risk with fondaparinux vs. enoxaparin in ACS
- Perceptions of physician ability to predict medical futility
CPOE Is Associated with Improvement in Quality Measures
Clinical question: Is computerized physician order entry (CPOE) associated with improved outcomes across a large, nationally representative sample of hospitals?
Background: Several single-institution studies suggest CPOE leads to better outcomes in quality measures for heart failure, acute myocardial infarction, and pneumonia as defined by the Hospital Quality Alliance (HQA) initiative, led by the Centers for Medicare and Medicaid Services (CMS). Little systematic information is known about the effects of CPOE on quality of care.
Study design: Cross-sectional study.
Setting: The Health Information Management System Society (HIMSS) analytics database of 3,364 hospitals throughout the U.S.
Synopsis: Of the hospitals that reported CPOE utilization to HIMSS, 264 (7.8%) fully implement CPOE throughout their institutions. These CPOE hospitals outperformed their peers on five of 11 quality measures related to ordering medications, and in one of nine non-medication-related measures. No difference was noted in the other measures, except CPOE hospitals were less effective at providing antibiotics within four hours of pneumonia diagnosis. Hospitals that utilized CPOE were generally academic, larger, and nonprofit. After adjusting for these differences, benefits were still preserved.
The authors indicate that the lack of systematic outperformance by CPOE hospitals in all 20 of the quality categories inherently suggests that other factors (e.g., concomitant QI efforts) are not affecting these results. Given the observational nature of this study, no causal relationship can be established between CPOE and the observed benefits. CPOE might represent the commitment of certain hospitals to quality measures, but further study is needed.
Bottom line: Enhanced compliance in several CMS-established quality measures is seen in hospitals that utilize CPOE throughout their institutions.
Citation: Yu FB, Menachemi N, Berner ES, Allison JJ, Weissman NW, Houston TK. Full implementation of computerized physician order entry and medication-related quality outcomes: a study of 3,364 hospitals. Am J Med Qual. 2009;24(4):278-286.
Standardized Management of Endocarditis Leads to Significant Mortality Benefit
Clinical question: Does a standardized approach to the treatment of infective endocarditis reduce mortality and morbidity?
Background: Despite epidemiological changes to the inciting bacteria and improvements in available antibiotics, mortality and morbidity associated with endocarditis remain high. The contribution of inconsistent or inaccurate treatment of endocarditis is unclear.
Study design: Case series with historical controls from 1994 to 2001, compared with protocolized patients from 2002 to 2006.
Setting: Single teaching tertiary-care hospital in France.
Synopsis: The authors established a diagnostic protocol for infectious endocarditis from 1994 to 2001 (period 1) and established a treatment protocol from 2002 to 2006 (period 2). Despite a statistically significant sicker population (older, higher comorbidities, higher coagulase-negative staphylococcal infections, and fewer healthy valves), the period-2 patients had a dramatically lower mortality rate of 8.2% (P<0.001), compared with 18.5% in period-1 patients. Fewer episodes of renal failure, organ failure, and deaths associated with embolism were noted in period 2.
Whether these results are due to more frequent care, more aggressive care (patients were “summoned” if they did not show for appointments), standardized medication and surgical options, or the effects of long-term collaboration, these results appear durable, remarkable, and reproducible.
This study is limited by its lack of randomization and extensive time frame, with concomitant changes in medical treatment and observed infectious organisms.
Bottom line: Implementation of a standardized approach to endocarditis has significant benefit on mortality and morbidity.
Citation: Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298.
Treatment with tPA in the Three- to 4.5-Hour Time Window after Stroke Is Beneficial
Clinical question: What is the effect of tissue plasminogen activator (tPA) on outcomes in patients treated in the three- to 4.5-hour window after stroke?
Background: The third European Cooperative Acute Stroke Study 3 (ECASS-3) demonstrated benefit of treatment of acute stroke with tPA in the three- to 4.5-hour time window. Prior studies, however, did not show superiority of tPA over placebo, and there is a lack of a confirmatory randomized, controlled trial of tPA in this time frame.
Study design: Meta-analysis of randomized, controlled trials.
Setting: Four studies involving 1,622 patients who were treated with intravenous tPA for acute ischemic stroke from three to 4.5 hours after stroke compared with placebo.
Synopsis: Of the randomized, controlled trials of intravenous tPA for treatment of acute ischemic stroke from three to 4.5 hours after stroke, four trials (ECASS-1, ECASS-2, ECASS-3, and ATLANTIS) were included in the analysis. Treatment with tPA in the three- to 4.5-hour time window is associated with increased favorable outcomes based on the global outcome measure (OR 1.31; 95% CI: 1.10-1.56, P=0.002) and the modified Rankin Scale (OR 1.31; 95% CI: 1.07-1.59, P=0.01), compared with placebo. The 90-day mortality rate was not significantly different between the treatment and placebo groups (OR 1.04; 95% CI 0.75-1.43, P=0.83).
Due to the relatively high dose of tPA (1.1 mg/kg) administered in the ECASS-1 trial, a separate meta-analysis looking at the other three trials (tPA dose of 0.9 mg/kg) was conducted, and the favorable outcome with tPA remained.
Bottom line: Treatment of acute ischemic stroke with tPA in the three- to 4.5-hour time window results in an increased rate of favorable functional outcomes without a significant difference in mortality.
Citation: Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
Outpatients Often Are Not Notified of Clinically Significant Test Results
Clinical question: How frequently do primary-care physicians (PCPs) fail to inform patients of clinically significant outpatient test results?
Background: Diagnostic errors are the most common cause of malpractice claims in the U.S. It is unclear how often providers fail to either inform patients of abnormal test results or document that patients have been notified.
Study design: Retrospective chart review.
Setting: Twenty-three primary-care practices: 19 private, four academic.
Synopsis: More than 5,400 charts were reviewed, and 1,889 abnormal test results were identified in this study. Failure to inform or document notification was identified in 135 cases (7.1%). The failure rates in the practices ranged from 0.0% to 26.2%. Practices with the best processes for managing test results had the lowest failure rates; these processes included: all results being routed to the responsible physician; the physician signing off on all results; the practice informing patients of all results, both normal and abnormal; documenting when the patient is informed; and instructing patients to call if not notified of test results within a certain time interval.
Limitations of this study include the potential of over- or underreporting of failures to inform as a chart review was used, and only practices that agreed to participate were included.
Bottom line: Failure to notify outpatients of test results is common but can be minimized by creating a systematic management of test results that include best practices.
Citation: Casalino LP, Dunham D, Chin MH, et al. Frequency of failure to inform patients of clinically significant outpatient test results. Arch Intern Med. 2009;169(12):1123-1129.
Repair of Incidental PFO Discovered During Cardiothoracic Surgery Repair Increases Postoperative Stroke Risk
Clinical question: What is the impact of closing incidentally discovered patent foramen ovale (PFO) defects during cardiothoracic surgery?
Background: PFO’s role in cryptogenic stroke remains controversial. Incidental PFO is commonly detected by transesophageal echocardiography (TEE) during cardiothoracic surgery. Routine PFO closure has been recommended when almost no alteration of the surgical plan is required.
Study design: Retrospective chart review.
Setting: The Cleveland Clinic.
Synopsis: Between 1995 and 2006, 13,092 patients undergoing cardiothoracic surgery had TEE data with no previous diagnosis of PFO, but the review found that 2,277 (17%) had PFO discovered intraoperatively. Of these, 639 (28%) had the PFO repaired.
Patients with an intraoperative diagnosis of PFO had similar rates of in-hospital stroke and hospital death compared with those without PFO. Patients who had their PFO repaired had a greater in-hospital stroke risk (2.8% vs. 1.2%; P=0.04) compared with those with a non-repaired PFO, representing nearly 2.5 times greater odds of having an in-hospital stroke. No other difference was noted in perioperative outcomes for patients who underwent intraoperative repair compared with those who did not, including risk of in-hospital death, hospital length of stay, ICU length of stay, and time on cardiopulmonary bypass. Long-term analysis demonstrated that PFO repair was associated with no survival difference.
The study is limited by its retrospective nature.
Bottom line: Routine surgical closure of incidental PFO detected during intraoperative imaging should be discouraged.
Citation: Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of interoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA. 2009;302(3):290-297.
Hospital-Level Differences Are Strong Predictors of Time to Defibrillation Delay In Cardiac Arrest
Clinical question: What are the predictors of delay in the time to defibrillation after in-hospital cardiac arrest?
Background: Thirty percent of in-hospital cardiac arrests from ventricular arrhythmias are not treated within the American Heart Association’s recommendation of two minutes. This delay is associated with a 50% lower rate of in-hospital survival. Exploring the hospital-level variation in delays to defibrillation is a critical step toward sharing the best practices.
Study design: Retrospective review of registry data.
Setting: The National Registry of Cardiopulmonary Resuscitation (NRCPR) survey of 200 acute-care, nonpediatric hospitals.
Synopsis: The registry identified 7,479 patients who experienced cardiac arrest from ventricular tachycardia or pulseless ventricular fibrillation. The primary outcome was the hospital rate of delayed defibrillation (time to defibrillation > two minutes), which ranged from 2% to 51%.
Time to defibrillation was found to be a major predictor of survival after a cardiac arrest. Only bed volume and arrest location were associated with differences in rates of delayed defibrillation (lower rates in larger hospitals and in ICUs). The variability was not due to differences in patient characteristics, but was due to hospital-level effects. Academic status, geographical location, arrest volume, and daily admission volume did not affect the time to defibrillation.
The study was able to identify only a few facility characteristics that account for the variability between hospitals in the rate of delayed defibrillation. The study emphasizes the need for new approaches to identifying hospital innovations in process-of-care measures that are associated with improved performance in defibrillation times.
Bottom Line: Future research is needed to better understand the reason for the wide variation between hospitals in the rate of delayed defibrillation after in-hospital cardiac arrest.
Citation: Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265-1273.
Treating for H. Pylori Reduces the Risk for Developing Gastric Cancer in High-Risk Patients
Clinical question: In patients with high-baseline incidence of gastric cancer, does H. pylori eradication reduce the risk for developing gastric cancer?
Background: Gastric cancer remains a major health problem in Asia. The link of H. pylori and gastric cancer has been established, but it remains unclear whether treatment for H. pylori is effective primary prevention for the development of gastric cancer.
Study design: Meta-analysis of six studies.
Setting: All but one trial was performed in Asia.
Synopsis: Seven studies met inclusion criteria, one of which was excluded due to heterogeneity. The six remaining studies were pooled, with 37 of 3,388 (1.1%) treated patients developing a new gastric cancer, compared with 56 of 3,307 (1.7%) patients who received placebo or were in the control group (RR 0.65; 0.43-0.98). Most patients received gastric biopsy prior to enrollment, and most of those demonstrated gastric atrophy or intestinal metaplasia.
These patients, despite more advanced precancerous pathology findings, still benefited from eradication. The seventh study, which was excluded, enrolled patients with early gastric cancer; these patients still benefited from H. pylori eradication and, when included in the meta-analysis, the RR was even lower, 0.57 (0.49-0.81).
Only two trials were double-blinded, but all of the studies employed the same definition of gastric cancer and held to excellent data reporting standards. This study encourages screening and treatment in high-risk patients given the widespread incidence of H. pylori.
Bottom Line: Treatment of H. pylori reduces the risk of gastric cancer in high-risk patients.
Citation: Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121-128.
Patients on Anti-Platelet Agents with Acute Coronary Syndrome Have a Lower Bleeding Risk When Treated with Fondaparinux
Clinical question: Is there a difference in bleeding risk with fondaparinux and enoxaparin when used with GPIIb/IIIa inhibitors or thienopyridines in NSTEMI-ACS?
Background: The OASIS 5 study reported a 50% reduction in severe bleeding when comparing fondaparinux to enoxaparin in ACS while maintaining a similar efficacy. This subgroup analysis was performed to evaluate whether reduced bleeding risk with fondaparinux remains in patients treated with additional anti-platelet agents.
Study design: Subgroup analysis of a large, multicenter, randomized, double-blind trial.
Setting: Acute-care hospitals in North America, Eastern and Western Europe, Latin America, Australia, and Asia.
Synopsis: Patients with NSTE-ACS received either fondaparinux or enoxaparin and were treated with GPIIb/IIIa inhibitors or thienopyridines at the discretion of their physician. At 30 days, the fondaparinux group had similar efficacy and decreased bleeding risk in both the GPIIb/IIIa and the thienopyridine groups. Of the 3,630 patients in the GPIIb/IIIa group, the risk for major bleeding with fondaparinux was 5.2%, whereas the risk with enoxaparin was 8.3% (HR 0.61; P<0.001) compared with enoxaparin. Of the 1,352 patients treated with thienopyridines, the risk for major bleeding with fondaparinux was 3.4%, whereas the risk with enoxaparin was 5.4% (HR 0.62; P<0.001).
Bottom Line: This subgroup analysis suggests there are less-severe bleeding complications in patients treated with fondaparinux when compared with enoxaparin in the setting of cotreatment with GPIIb/IIIa inhibitors, thienopyridines, or both.
Citation: Jolly SS, Faxon DP, Fox KA, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors of thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54(5):468-476.
Surrogate Decision-Makers Frequently Doubt Clinicians’ Ability to Predict Medical Futility
Clinical question: What attitudes do surrogate decision-makers hold toward clinicians’ predictions of medical futility in critically-ill patients?
Background: The clinical judgment of medical futility leading to the withdrawal of life-sustaining treatment—despite the objections of surrogate decision-makers—is controversial. Very little is known about how surrogate decision-makers view the futility rationale when physicians suggest limiting the use of life-sustaining treatment.
Study design: Multicenter, mixed, qualitative and quantitative study.
Setting: Three ICUs in three different California hospitals from 2006 to 2007.
Synopsis: Semi-structured interviews of surrogate decision-makers for 50 incapacitated, critically-ill patients were performed to ascertain their beliefs about medical futility in response to hypothetical situations. Of the surrogates surveyed, 64% expressed doubt about physicians’ futility predictions.
The interviewees gave four main reasons for their doubts. Two reasons not previously described were doubts about the accuracy of physicians’ predictions and the need for surrogates to see futility themselves. Previously described sources of conflict included a misunderstanding about prognosis and religious-based objections. Surrogates with religious objections were more likely to request continuation of life-sustaining treatments than those with secular or experiential objections (OR 4; 95% CI 1.2-14.0; P=0.03). Nearly a third (32%) of surrogates elected to continue life support with a <1% survival estimate; 18% elected to continue life support when physicians thought there was no chance of survival.
This study has several limitations: a small sample size, the use of hypothetical situations, and the inability to assess attitudes as they change over time.
Bottom line: The nature of surrogate decision-makers’ doubts about medical futility can help predict whether they accept predictions of medical futility from physicians.
Citation: Zier LS, Burack JH, Micco G, Chipman AK, Frank JA, White DB. Surrogate decision makers’ responses to physicians’ predictions of medical futility. Chest. 2009;136:110-117. TH
Mentored Implementation
When Kendall Rogers, MD, signed up for his first mentored implementation project, he remembers being skeptical. After all, it seemed too good to be true. “I wanted to ask, ‘What’s the catch? Are you trying to get us to adopt a certain practice?’ ” says Dr. Rogers, a hospitalist at the University of New Mexico Health Science Center School of Medicine in Albuquerque.
Now, after participating in SHM’s Venous Thromboembolism (VTE) Prevention Collaborative and later mentoring other hospitalists in SHM’s Glycemic Control Mentored Implemen-tation (GCMI) program, he understands the motivation.
“Mentored implementation is unique in that it accomplishes two goals,” he says. “It improves the nuts and bolts of a project, and it also creates new hospitalist leaders and quality-improvement [QI] experts.”
Prior to his work in the VTE Prevention Collaborative, Dr. Rogers had little exposure to QI programs. He has since implemented a VTE prevention program at his hospital, and his mentorship of hospitalists in the GCMI program is helping to create custom programs to optimize glycemic control protocols. He also is a faculty member for SHM’s QI and patient-safety pre-course and is leading SHM training sessions on VTE prevention.
The mentored implementation model, he says, is an effective way to get over many of the daunting roadblocks that can stand in the way of a hospitalist-led QI program. “Many people need that spark,” Dr. Rogers says. “This is a highly effective way to be that spark. I’ve seen too many people get disillusioned and frustrated with quality-improvement programs and give up. In these programs, the mentor can help identify and address roadblocks.”
What is Mentored Implementation?
In theory, mentored implementation is a unique and simple approach to both education and QI in healthcare. At its core, mentored implementation is the pairing of a program participant with a subject-matter expert who already has been involved in similar programs and will help the participant implement a QI program of their own.
The concept is new to QI initiatives. Although SHM has embraced the idea, mentored implementation programs first started at the Center to Advance Palliative Care in New York City, says Kathleen Kerr, SHM’s program manager for mentored implementation programs and senior research analyst in the Department of Medicine at the University of California at San Francisco. The model is an alternative to more traditional educational approaches that rely exclusively on lectures or educational sessions.
“You could sit in a session and it’s very valuable, but also very different from actually doing it,” Kerr says. “It’s hard to process so much information in a session. You don’t understand the complexity of something like gathering data until you’re actually doing it. The mentor can tailor what they’re teaching to the exact stage of the project.”
In practice, the most effective mentored implementation projects create multiple layers of support for both the mentor and the participant. SHM’s mentored implementation programs include online resource rooms on the topic (e.g., glycemic control or hospital discharge) and collaboration between participants. Rather than being just repositories of information on the subject, SHM’s resource rooms are roadmaps for new programs.
“SHM’s resource rooms define an intervention that can be implemented,” says Geri Barnes, SHM’s senior director of education and meetings.
Those resources, plus ongoing guidance from mentors, help hospitalists implement QI programs at their hospitals. Many hospitalists are early in their careers and benefit from all of the resources available. The energy that early-career hospitalists bring to QI is one of the key components the program harnesses, Kerr says.
“Junior staff are really motivated to do things in their scope, but there aren’t really a lot of mid-career local mentors” who can provide the guidance they need, Kerr says.
Training Days
Given SHM’s focus on QI and the relative youth of both HM as a specialty and hospitalists in relation to their peers, the mentored implementation model seems particularly suited to hospitalists. Launched in 2007, the VTE Prevention Collaborative was SHM’s first mentored implementation program. It was designed to help hospitalists create custom programs to prevent VTE. The collaborative included mentors, an online resource room, and on-site consultations with experts.

—Kendall Rogers, MD, University of New Mexico Health Science Center School of Medicine, Albuquerque
SHM created Project BOOST (Better Outcomes for Older adults through Safe Transitions) in 2008. Project BOOST began with six pilot sites and has now expanded to 30 sites. Each hospital site can participate in daylong training sessions and yearlong mentorships. Sites also receive the Project BOOST implementation guide from SHM’s resource room. Since it was posted in July 2008, more than 250 hospitals have downloaded the guide.
In 2009, SHM and hospitalists are teaming up in 30 different sites across the country to improve early detection and treatment of hyperglycemia in hospitalized patients through the Glycemic Control Mentored Implementation program. Each participant in the two-year program receives a toolkit, access to Web-based resources, and is assigned a mentor to guide implementation.
MI 2.0
Despite early successes with SHM’s mentored implementation programs, those closest to them acknowledge there is room for improvement. Among a host of factors is the success of the next generation of programs, which will hinge on the idea’s scalability.
“We’re looking at testing models where we have a one-to-one mentoring program, compared to a one-to-five mentoring program,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives.
Kerr also sees opportunities to expand the scope without sacrificing the customized approach. “We are looking for ways to expand the reach of each individual effort. Right now, customization means that mentored implementation is more like building a Ferrari than a Ford,” she says. “We need to do some ‘train the trainer’ models and explore ways to reach more hospitals simultaneously.”
For Dr. Rogers, his experience with mentored implementation and QI has strengthened his resolve to help hospitalists get it right.
“We have a lot to learn to do this effectively. We have 5,000 hospitals out there and hospitalists are naturally looked at as leaders within the institution,” he says. “The failure of one hospitalist quality-improvement program affects all of us, so success is key. This is one of the most effective tools for doing it.” TH
Brandon Shank is a freelance writer based in Philadelphia.
Letters
The Unique Potential of Hospitalists as Leaders in Healthcare Reform
The usual first response when a physician is asked, “Why do you practice medicine?” is “to help people.” This is especially true for younger practitioners. A frequent second response is “I like the independence.” As physicians, we enjoy being our own boss and calling the shots.
Therein rests the cultural healthcare quandary. Physicians need to accept the fact that standardization of medicine is going to happen, as it allows for improved efficiencies with a resultant decrease in healthcare expenditures. Yet the independent and entrepreneurial nature of physicians has caused them to resist the standardization of medicine for years. After all, while one fellow physician might treat a disease or perform a procedure differently than another, as long as it is efficacious, we all believe our peers should be able to practice the way they want.
Hospitalists are no different, as they are independent, too. They are simply working under the hospital umbrella. This relationship of working in hospitals positions HM practitioners, as a group, to be central players in the healthcare reform debate. This truly is a unique opportunity.
Looking demographically at the generational makeup of all physicians, we have four familiar groups represented: baby boomers, Gen X’ers, Gen Y’ers, and millennials. There are certain broad yet defining characteristics of these four generational groups. The baby boomers, being the offspring of the World War II generation, the generation that rebuilt the world and kept their “nose to the grindstone,” are defined by their work ethic. Simply put, boomers live to work. As children and students of the 1960s, they also value individuality.
Gen X’ers focus more on themselves, and often are referred to as the “me generation.” They expect to have a range of choices within their expression of individuality.
Gen Y’ers have a different work ethic, one their managers often find alarming. They are defined by the adage “work to live.” This dilemma, while difficult for their managers, allows Gen Y’ers to adapt to workplace practices, as their individuality is no longer of primary concern. After all, “it is only work.”
Millennials, having been brought up in the digital age, are bombarded with information and entertainment 24 hours a day. From birth on, they have heard that the future is uncertain. Demographically, they are more aligned with the work ethic of their great-grandparents, the World War II generation, and they are more willing to serve the common good. Thus, millennials, like Generation Y, are less individualistic and more willing to adapt to the work environment.
In considering hospitalists and their roles in the current healthcare debate and medical standards, this young specialty is uniquely poised to implement the upcoming standardizations required for three reasons. First, HM has an unusually large representation of Gen Y’ers and millennials—more than other medical specialties. These younger physicians, with their adaptability for the common good, are less resistant to the standardization of medicine.
Second, unlike most practitioners, hospitalists tend to practice in larger medical groups. Thus, they are familiar with standardization and the uniformity necessary for the group to practice effectively.
Third, with the Centers for Medicare and Medicaid Services (CMS) adopting the experimental payment mechanism known as value-based purchasing, hospitals will insist on standardization to maximize reimbursement.
The benefits to HM practitioners are twofold. The hospitalist will share in reimbursement of pay-for-performance, thereby gaining a financial incentive for the greater efficiencies that standardization yields. This is evidenced by the trend that hospitalist contracts are increasingly based on pay-for-performance, rather than payment based on relative value units.
The second benefit, and perhaps the most important, is that the influence and power of hospitalists will greatly increase, particularly in formulating the standards of medical treatment, procedures, and, more importantly, QI and patient safety.
As the practice of HM matures from infancy into adolescence, recognizing the opportunity at hand and deciding how to proceed is paramount to its future position and existence.
Michael G. Cassatly, DMD
Certified business coach,
American Board of Oral and Maxillofacial Surgery diplomate
When Kendall Rogers, MD, signed up for his first mentored implementation project, he remembers being skeptical. After all, it seemed too good to be true. “I wanted to ask, ‘What’s the catch? Are you trying to get us to adopt a certain practice?’ ” says Dr. Rogers, a hospitalist at the University of New Mexico Health Science Center School of Medicine in Albuquerque.
Now, after participating in SHM’s Venous Thromboembolism (VTE) Prevention Collaborative and later mentoring other hospitalists in SHM’s Glycemic Control Mentored Implemen-tation (GCMI) program, he understands the motivation.
“Mentored implementation is unique in that it accomplishes two goals,” he says. “It improves the nuts and bolts of a project, and it also creates new hospitalist leaders and quality-improvement [QI] experts.”
Prior to his work in the VTE Prevention Collaborative, Dr. Rogers had little exposure to QI programs. He has since implemented a VTE prevention program at his hospital, and his mentorship of hospitalists in the GCMI program is helping to create custom programs to optimize glycemic control protocols. He also is a faculty member for SHM’s QI and patient-safety pre-course and is leading SHM training sessions on VTE prevention.
The mentored implementation model, he says, is an effective way to get over many of the daunting roadblocks that can stand in the way of a hospitalist-led QI program. “Many people need that spark,” Dr. Rogers says. “This is a highly effective way to be that spark. I’ve seen too many people get disillusioned and frustrated with quality-improvement programs and give up. In these programs, the mentor can help identify and address roadblocks.”
What is Mentored Implementation?
In theory, mentored implementation is a unique and simple approach to both education and QI in healthcare. At its core, mentored implementation is the pairing of a program participant with a subject-matter expert who already has been involved in similar programs and will help the participant implement a QI program of their own.
The concept is new to QI initiatives. Although SHM has embraced the idea, mentored implementation programs first started at the Center to Advance Palliative Care in New York City, says Kathleen Kerr, SHM’s program manager for mentored implementation programs and senior research analyst in the Department of Medicine at the University of California at San Francisco. The model is an alternative to more traditional educational approaches that rely exclusively on lectures or educational sessions.
“You could sit in a session and it’s very valuable, but also very different from actually doing it,” Kerr says. “It’s hard to process so much information in a session. You don’t understand the complexity of something like gathering data until you’re actually doing it. The mentor can tailor what they’re teaching to the exact stage of the project.”
In practice, the most effective mentored implementation projects create multiple layers of support for both the mentor and the participant. SHM’s mentored implementation programs include online resource rooms on the topic (e.g., glycemic control or hospital discharge) and collaboration between participants. Rather than being just repositories of information on the subject, SHM’s resource rooms are roadmaps for new programs.
“SHM’s resource rooms define an intervention that can be implemented,” says Geri Barnes, SHM’s senior director of education and meetings.
Those resources, plus ongoing guidance from mentors, help hospitalists implement QI programs at their hospitals. Many hospitalists are early in their careers and benefit from all of the resources available. The energy that early-career hospitalists bring to QI is one of the key components the program harnesses, Kerr says.
“Junior staff are really motivated to do things in their scope, but there aren’t really a lot of mid-career local mentors” who can provide the guidance they need, Kerr says.
Training Days
Given SHM’s focus on QI and the relative youth of both HM as a specialty and hospitalists in relation to their peers, the mentored implementation model seems particularly suited to hospitalists. Launched in 2007, the VTE Prevention Collaborative was SHM’s first mentored implementation program. It was designed to help hospitalists create custom programs to prevent VTE. The collaborative included mentors, an online resource room, and on-site consultations with experts.

—Kendall Rogers, MD, University of New Mexico Health Science Center School of Medicine, Albuquerque
SHM created Project BOOST (Better Outcomes for Older adults through Safe Transitions) in 2008. Project BOOST began with six pilot sites and has now expanded to 30 sites. Each hospital site can participate in daylong training sessions and yearlong mentorships. Sites also receive the Project BOOST implementation guide from SHM’s resource room. Since it was posted in July 2008, more than 250 hospitals have downloaded the guide.
In 2009, SHM and hospitalists are teaming up in 30 different sites across the country to improve early detection and treatment of hyperglycemia in hospitalized patients through the Glycemic Control Mentored Implementation program. Each participant in the two-year program receives a toolkit, access to Web-based resources, and is assigned a mentor to guide implementation.
MI 2.0
Despite early successes with SHM’s mentored implementation programs, those closest to them acknowledge there is room for improvement. Among a host of factors is the success of the next generation of programs, which will hinge on the idea’s scalability.
“We’re looking at testing models where we have a one-to-one mentoring program, compared to a one-to-five mentoring program,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives.
Kerr also sees opportunities to expand the scope without sacrificing the customized approach. “We are looking for ways to expand the reach of each individual effort. Right now, customization means that mentored implementation is more like building a Ferrari than a Ford,” she says. “We need to do some ‘train the trainer’ models and explore ways to reach more hospitals simultaneously.”
For Dr. Rogers, his experience with mentored implementation and QI has strengthened his resolve to help hospitalists get it right.
“We have a lot to learn to do this effectively. We have 5,000 hospitals out there and hospitalists are naturally looked at as leaders within the institution,” he says. “The failure of one hospitalist quality-improvement program affects all of us, so success is key. This is one of the most effective tools for doing it.” TH
Brandon Shank is a freelance writer based in Philadelphia.
Letters
The Unique Potential of Hospitalists as Leaders in Healthcare Reform
The usual first response when a physician is asked, “Why do you practice medicine?” is “to help people.” This is especially true for younger practitioners. A frequent second response is “I like the independence.” As physicians, we enjoy being our own boss and calling the shots.
Therein rests the cultural healthcare quandary. Physicians need to accept the fact that standardization of medicine is going to happen, as it allows for improved efficiencies with a resultant decrease in healthcare expenditures. Yet the independent and entrepreneurial nature of physicians has caused them to resist the standardization of medicine for years. After all, while one fellow physician might treat a disease or perform a procedure differently than another, as long as it is efficacious, we all believe our peers should be able to practice the way they want.
Hospitalists are no different, as they are independent, too. They are simply working under the hospital umbrella. This relationship of working in hospitals positions HM practitioners, as a group, to be central players in the healthcare reform debate. This truly is a unique opportunity.
Looking demographically at the generational makeup of all physicians, we have four familiar groups represented: baby boomers, Gen X’ers, Gen Y’ers, and millennials. There are certain broad yet defining characteristics of these four generational groups. The baby boomers, being the offspring of the World War II generation, the generation that rebuilt the world and kept their “nose to the grindstone,” are defined by their work ethic. Simply put, boomers live to work. As children and students of the 1960s, they also value individuality.
Gen X’ers focus more on themselves, and often are referred to as the “me generation.” They expect to have a range of choices within their expression of individuality.
Gen Y’ers have a different work ethic, one their managers often find alarming. They are defined by the adage “work to live.” This dilemma, while difficult for their managers, allows Gen Y’ers to adapt to workplace practices, as their individuality is no longer of primary concern. After all, “it is only work.”
Millennials, having been brought up in the digital age, are bombarded with information and entertainment 24 hours a day. From birth on, they have heard that the future is uncertain. Demographically, they are more aligned with the work ethic of their great-grandparents, the World War II generation, and they are more willing to serve the common good. Thus, millennials, like Generation Y, are less individualistic and more willing to adapt to the work environment.
In considering hospitalists and their roles in the current healthcare debate and medical standards, this young specialty is uniquely poised to implement the upcoming standardizations required for three reasons. First, HM has an unusually large representation of Gen Y’ers and millennials—more than other medical specialties. These younger physicians, with their adaptability for the common good, are less resistant to the standardization of medicine.
Second, unlike most practitioners, hospitalists tend to practice in larger medical groups. Thus, they are familiar with standardization and the uniformity necessary for the group to practice effectively.
Third, with the Centers for Medicare and Medicaid Services (CMS) adopting the experimental payment mechanism known as value-based purchasing, hospitals will insist on standardization to maximize reimbursement.
The benefits to HM practitioners are twofold. The hospitalist will share in reimbursement of pay-for-performance, thereby gaining a financial incentive for the greater efficiencies that standardization yields. This is evidenced by the trend that hospitalist contracts are increasingly based on pay-for-performance, rather than payment based on relative value units.
The second benefit, and perhaps the most important, is that the influence and power of hospitalists will greatly increase, particularly in formulating the standards of medical treatment, procedures, and, more importantly, QI and patient safety.
As the practice of HM matures from infancy into adolescence, recognizing the opportunity at hand and deciding how to proceed is paramount to its future position and existence.
Michael G. Cassatly, DMD
Certified business coach,
American Board of Oral and Maxillofacial Surgery diplomate
When Kendall Rogers, MD, signed up for his first mentored implementation project, he remembers being skeptical. After all, it seemed too good to be true. “I wanted to ask, ‘What’s the catch? Are you trying to get us to adopt a certain practice?’ ” says Dr. Rogers, a hospitalist at the University of New Mexico Health Science Center School of Medicine in Albuquerque.
Now, after participating in SHM’s Venous Thromboembolism (VTE) Prevention Collaborative and later mentoring other hospitalists in SHM’s Glycemic Control Mentored Implemen-tation (GCMI) program, he understands the motivation.
“Mentored implementation is unique in that it accomplishes two goals,” he says. “It improves the nuts and bolts of a project, and it also creates new hospitalist leaders and quality-improvement [QI] experts.”
Prior to his work in the VTE Prevention Collaborative, Dr. Rogers had little exposure to QI programs. He has since implemented a VTE prevention program at his hospital, and his mentorship of hospitalists in the GCMI program is helping to create custom programs to optimize glycemic control protocols. He also is a faculty member for SHM’s QI and patient-safety pre-course and is leading SHM training sessions on VTE prevention.
The mentored implementation model, he says, is an effective way to get over many of the daunting roadblocks that can stand in the way of a hospitalist-led QI program. “Many people need that spark,” Dr. Rogers says. “This is a highly effective way to be that spark. I’ve seen too many people get disillusioned and frustrated with quality-improvement programs and give up. In these programs, the mentor can help identify and address roadblocks.”
What is Mentored Implementation?
In theory, mentored implementation is a unique and simple approach to both education and QI in healthcare. At its core, mentored implementation is the pairing of a program participant with a subject-matter expert who already has been involved in similar programs and will help the participant implement a QI program of their own.
The concept is new to QI initiatives. Although SHM has embraced the idea, mentored implementation programs first started at the Center to Advance Palliative Care in New York City, says Kathleen Kerr, SHM’s program manager for mentored implementation programs and senior research analyst in the Department of Medicine at the University of California at San Francisco. The model is an alternative to more traditional educational approaches that rely exclusively on lectures or educational sessions.
“You could sit in a session and it’s very valuable, but also very different from actually doing it,” Kerr says. “It’s hard to process so much information in a session. You don’t understand the complexity of something like gathering data until you’re actually doing it. The mentor can tailor what they’re teaching to the exact stage of the project.”
In practice, the most effective mentored implementation projects create multiple layers of support for both the mentor and the participant. SHM’s mentored implementation programs include online resource rooms on the topic (e.g., glycemic control or hospital discharge) and collaboration between participants. Rather than being just repositories of information on the subject, SHM’s resource rooms are roadmaps for new programs.
“SHM’s resource rooms define an intervention that can be implemented,” says Geri Barnes, SHM’s senior director of education and meetings.
Those resources, plus ongoing guidance from mentors, help hospitalists implement QI programs at their hospitals. Many hospitalists are early in their careers and benefit from all of the resources available. The energy that early-career hospitalists bring to QI is one of the key components the program harnesses, Kerr says.
“Junior staff are really motivated to do things in their scope, but there aren’t really a lot of mid-career local mentors” who can provide the guidance they need, Kerr says.
Training Days
Given SHM’s focus on QI and the relative youth of both HM as a specialty and hospitalists in relation to their peers, the mentored implementation model seems particularly suited to hospitalists. Launched in 2007, the VTE Prevention Collaborative was SHM’s first mentored implementation program. It was designed to help hospitalists create custom programs to prevent VTE. The collaborative included mentors, an online resource room, and on-site consultations with experts.

—Kendall Rogers, MD, University of New Mexico Health Science Center School of Medicine, Albuquerque
SHM created Project BOOST (Better Outcomes for Older adults through Safe Transitions) in 2008. Project BOOST began with six pilot sites and has now expanded to 30 sites. Each hospital site can participate in daylong training sessions and yearlong mentorships. Sites also receive the Project BOOST implementation guide from SHM’s resource room. Since it was posted in July 2008, more than 250 hospitals have downloaded the guide.
In 2009, SHM and hospitalists are teaming up in 30 different sites across the country to improve early detection and treatment of hyperglycemia in hospitalized patients through the Glycemic Control Mentored Implementation program. Each participant in the two-year program receives a toolkit, access to Web-based resources, and is assigned a mentor to guide implementation.
MI 2.0
Despite early successes with SHM’s mentored implementation programs, those closest to them acknowledge there is room for improvement. Among a host of factors is the success of the next generation of programs, which will hinge on the idea’s scalability.
“We’re looking at testing models where we have a one-to-one mentoring program, compared to a one-to-five mentoring program,” says Jane Kelly-Cummings, RN, CPHQ, SHM’s senior director of quality initiatives.
Kerr also sees opportunities to expand the scope without sacrificing the customized approach. “We are looking for ways to expand the reach of each individual effort. Right now, customization means that mentored implementation is more like building a Ferrari than a Ford,” she says. “We need to do some ‘train the trainer’ models and explore ways to reach more hospitals simultaneously.”
For Dr. Rogers, his experience with mentored implementation and QI has strengthened his resolve to help hospitalists get it right.
“We have a lot to learn to do this effectively. We have 5,000 hospitals out there and hospitalists are naturally looked at as leaders within the institution,” he says. “The failure of one hospitalist quality-improvement program affects all of us, so success is key. This is one of the most effective tools for doing it.” TH
Brandon Shank is a freelance writer based in Philadelphia.
Letters
The Unique Potential of Hospitalists as Leaders in Healthcare Reform
The usual first response when a physician is asked, “Why do you practice medicine?” is “to help people.” This is especially true for younger practitioners. A frequent second response is “I like the independence.” As physicians, we enjoy being our own boss and calling the shots.
Therein rests the cultural healthcare quandary. Physicians need to accept the fact that standardization of medicine is going to happen, as it allows for improved efficiencies with a resultant decrease in healthcare expenditures. Yet the independent and entrepreneurial nature of physicians has caused them to resist the standardization of medicine for years. After all, while one fellow physician might treat a disease or perform a procedure differently than another, as long as it is efficacious, we all believe our peers should be able to practice the way they want.
Hospitalists are no different, as they are independent, too. They are simply working under the hospital umbrella. This relationship of working in hospitals positions HM practitioners, as a group, to be central players in the healthcare reform debate. This truly is a unique opportunity.
Looking demographically at the generational makeup of all physicians, we have four familiar groups represented: baby boomers, Gen X’ers, Gen Y’ers, and millennials. There are certain broad yet defining characteristics of these four generational groups. The baby boomers, being the offspring of the World War II generation, the generation that rebuilt the world and kept their “nose to the grindstone,” are defined by their work ethic. Simply put, boomers live to work. As children and students of the 1960s, they also value individuality.
Gen X’ers focus more on themselves, and often are referred to as the “me generation.” They expect to have a range of choices within their expression of individuality.
Gen Y’ers have a different work ethic, one their managers often find alarming. They are defined by the adage “work to live.” This dilemma, while difficult for their managers, allows Gen Y’ers to adapt to workplace practices, as their individuality is no longer of primary concern. After all, “it is only work.”
Millennials, having been brought up in the digital age, are bombarded with information and entertainment 24 hours a day. From birth on, they have heard that the future is uncertain. Demographically, they are more aligned with the work ethic of their great-grandparents, the World War II generation, and they are more willing to serve the common good. Thus, millennials, like Generation Y, are less individualistic and more willing to adapt to the work environment.
In considering hospitalists and their roles in the current healthcare debate and medical standards, this young specialty is uniquely poised to implement the upcoming standardizations required for three reasons. First, HM has an unusually large representation of Gen Y’ers and millennials—more than other medical specialties. These younger physicians, with their adaptability for the common good, are less resistant to the standardization of medicine.
Second, unlike most practitioners, hospitalists tend to practice in larger medical groups. Thus, they are familiar with standardization and the uniformity necessary for the group to practice effectively.
Third, with the Centers for Medicare and Medicaid Services (CMS) adopting the experimental payment mechanism known as value-based purchasing, hospitals will insist on standardization to maximize reimbursement.
The benefits to HM practitioners are twofold. The hospitalist will share in reimbursement of pay-for-performance, thereby gaining a financial incentive for the greater efficiencies that standardization yields. This is evidenced by the trend that hospitalist contracts are increasingly based on pay-for-performance, rather than payment based on relative value units.
The second benefit, and perhaps the most important, is that the influence and power of hospitalists will greatly increase, particularly in formulating the standards of medical treatment, procedures, and, more importantly, QI and patient safety.
As the practice of HM matures from infancy into adolescence, recognizing the opportunity at hand and deciding how to proceed is paramount to its future position and existence.
Michael G. Cassatly, DMD
Certified business coach,
American Board of Oral and Maxillofacial Surgery diplomate
Did Patient Require Clearance for Surgery?
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Did Patient Require Clearance for Surgery?
A 36-year-old man was admitted to the defendant hospital with congestive heart failure and shortness of breath. CT angiography revealed cardiomegaly and several enlarged mediastinal lymph nodes. His medical history included cigarette smoking and ongoing treatment for tuberculosis and diabetes.
Echocardiography performed soon after the man's admission revealed diffuse hypokinesis consistent with cardiomyopathy and an ejection fraction of 30% to 35%. Two days later, a differential diagnosis was formed for the enlarged lymph nodes which included disseminated tuberculosis, infection (including the possibility of HIV), and neoplasm. A lymph node biopsy by way of mediastinoscopy was recommended.
The following day, the patient was seen by a cardiothoracic surgeon, who also recommended a mediastinoscopy and obtained consent from the patient for the procedure. That same day, an adenosine Cardiolite stress test was performed, with neither ischemia nor chest pain reported. The imaging portion of the study indicated several areas of prior infarction and an ejection fraction of 20%.
The following day, the man was seen by the defendant cardiologist, who added two cardiac agents to his medications. A plan was formulated to await results of a lymph node biopsy, scheduled for a few hours later, and to continue to follow the patient.
No complications occurred during the mediastinoscopy, but immediately after extubation, the man went into cardiopulmonary arrest. He was resuscitated but never regained consciousness. Two days later, he experience a second arrest and died.
Autopsy revealed triple-vessel coronary artery disease and a hemorrhagic septal infarction. The plaintiffs claimed that the decedent's presentation and coronary artery risk factors required that he not be cleared for surgery without undergoing coronary angiography. Angiography results, the plaintiff claimed, would have prompted bypass surgery and ensured the decedent's survival.
The defendant claimed that the treatment was proper, that a presurgery cardiac catheterization was not required, and that she had not been consulted to clear the decedent for surgery. The defendant further argued that the decedent's postsurgical cardiopulmonary arrest resulted from an adverse reaction to epinephrine and administration of 700 cc of IV fluid.
According to a published account, a defense verdict was returned.
Peritoneal Abscess, Colonic Rupture Missed
Sudden-onset abdominal pain brought a 69-year-old woman to the emergency department, where she was seen by a surgeon. He ordered abdominal CT, which was read by the defendant radiologist as showing free air. The next day a pelvic CT was read by the same radiologist, who concluded that the patient's colon appeared healthy. After undergoing a cholecystectomy the next day, the patient was soon released.
She returned to the hospital three days later with persistent pain, and a third CT scan showed free air. Exploratory laparotomy revealed a colonic rupture related to diverticulitis. In spite of aggressive intervention, the woman died several weeks later due to complications of sepsis.
The plaintiff alleged that the radiologist had misread the second CT, which had shown evidence of peritoneal abscess. The plaintiff claimed that a proper reading of this CT would have led to repair of the perforation before sepsis became fulminant.
The defense claimed that the reading of the pelvic CT was reasonable and that the woman's death was the result of comorbidities and complications from several previous surgeries.
A defense verdict was returned.
Infant Injured During (or After?) Cesarean Delivery
After a nonemergent cesarean delivery by a physician from a women's medical group in June 2001, the plaintiff infant was noted to be jittery and hypertonic, and stridor was developing, so she was admitted to a special care unit. Several hours later, the child's father noticed that her right hand was clenched in a fist except for the middle finger, which had a gash at the base.
He brought this to the attention of a nurse, who said it was probably just dried blood from the delivery. When she wiped the area, however, it began to bleed. The nurse then discovered a laceration, about 1.0 cm long and 0.5 cm deep, which extended into the bone of the finger. Two flexor tendons, the tendon pulleys, and a portion of the digital nerve had all been severed. Reconstructive surgery was performed two days later to reattach the tendons, rebuild the pulleys, and repair the nerve.
Because the infant could not participate actively in physical therapy, scar tissue developed. A second surgery was performed in March 2003 to release the tendons and nerve from the scar tissue.
By age 7, the girl had very limited use of her right middle finger and will need a third surgery to release additional scar tissue. This surgery has a success rate of only 65%, and function of the patient's finger will never be completely normal. The finger is also slightly shorter than the ring and index fingers. The plaintiff alleged res ipsa loquitur ("the thing speaks for itself").
Defense for the hospital argued that the laceration occurred during the cesarean delivery and was a known risk for this procedure. A resident obstetrician testified that she remembered the baby's hands being in the surgical field and "fighting" to come out as soon as the uterus was surgically entered. This, she claimed, was "the strangest thing she had ever seen."
Defense for the women's medical group argued that the laceration could not have occurred during the surgery based on the infant's face-down position at the time of delivery. It was alleged that the laceration must have occurred after the baby was handed off to a hospital staff member.
According to a published report, a $2,756,442 verdict was returned against the hospital. The women's medical group received a defense verdict.
Failure to Treat Ascending Cholangitis
In December 2002, a 45-year-old woman presented to the hospital with right upper quadrant abdominal pain, a urinary tract infection, and a pelvic infection. She also had a history of irritable bowel syndrome. The defendant family doctor, who had provided the patient's care for 10 years, admitted her and prescribed oral antibiotics to treat the urinary tract infection and the pelvic infection. Ultrasonography and magnetic resonance cholangiopancreatography showed a 3.0- to 4.0-cm mass at the head of the pancreas, a dilated common bile duct, and gallstones.
Lab test results included elevations in bilirubin, white blood cell count, and liver enzymes. The defendant gastroenterologist was called in for a consult.
Over the next few days, the woman's abdominal pain waxed and waned and shifted in location; her temperature dropped. After four days, her bilirubin level and white blood cell count spiked, so IV antibiotics were started. Two days later, the patient arrested and coded and remained in a coma until her death in May 2003.
The plaintiff claimed that the defendants failed to diagnose and treat ascending cholangitis from the time of the patient's admission until her cardiac arrest, resulting in sepsis and death.
The defendants claimed that the decedent did not have ascending cholangitis until two days before she went into arrest, at which time the condition was properly addressed. The defendants also claimed that there were no signs or symptoms of sepsis before that time and that the decedent had refused to consent to endoscopic retrograde cholangiopancreatography (ERCP) or surgical intervention. The defendants contended that a CT-guided biopsy was not performed because of the decedent's obesity.
The plaintiff denied that an ERCP or surgical intervention had been recommended and refused, since there was no record of this in the medical chart.
A defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Did Patient Require Clearance for Surgery?
A 36-year-old man was admitted to the defendant hospital with congestive heart failure and shortness of breath. CT angiography revealed cardiomegaly and several enlarged mediastinal lymph nodes. His medical history included cigarette smoking and ongoing treatment for tuberculosis and diabetes.
Echocardiography performed soon after the man's admission revealed diffuse hypokinesis consistent with cardiomyopathy and an ejection fraction of 30% to 35%. Two days later, a differential diagnosis was formed for the enlarged lymph nodes which included disseminated tuberculosis, infection (including the possibility of HIV), and neoplasm. A lymph node biopsy by way of mediastinoscopy was recommended.
The following day, the patient was seen by a cardiothoracic surgeon, who also recommended a mediastinoscopy and obtained consent from the patient for the procedure. That same day, an adenosine Cardiolite stress test was performed, with neither ischemia nor chest pain reported. The imaging portion of the study indicated several areas of prior infarction and an ejection fraction of 20%.
The following day, the man was seen by the defendant cardiologist, who added two cardiac agents to his medications. A plan was formulated to await results of a lymph node biopsy, scheduled for a few hours later, and to continue to follow the patient.
No complications occurred during the mediastinoscopy, but immediately after extubation, the man went into cardiopulmonary arrest. He was resuscitated but never regained consciousness. Two days later, he experience a second arrest and died.
Autopsy revealed triple-vessel coronary artery disease and a hemorrhagic septal infarction. The plaintiffs claimed that the decedent's presentation and coronary artery risk factors required that he not be cleared for surgery without undergoing coronary angiography. Angiography results, the plaintiff claimed, would have prompted bypass surgery and ensured the decedent's survival.
The defendant claimed that the treatment was proper, that a presurgery cardiac catheterization was not required, and that she had not been consulted to clear the decedent for surgery. The defendant further argued that the decedent's postsurgical cardiopulmonary arrest resulted from an adverse reaction to epinephrine and administration of 700 cc of IV fluid.
According to a published account, a defense verdict was returned.
Peritoneal Abscess, Colonic Rupture Missed
Sudden-onset abdominal pain brought a 69-year-old woman to the emergency department, where she was seen by a surgeon. He ordered abdominal CT, which was read by the defendant radiologist as showing free air. The next day a pelvic CT was read by the same radiologist, who concluded that the patient's colon appeared healthy. After undergoing a cholecystectomy the next day, the patient was soon released.
She returned to the hospital three days later with persistent pain, and a third CT scan showed free air. Exploratory laparotomy revealed a colonic rupture related to diverticulitis. In spite of aggressive intervention, the woman died several weeks later due to complications of sepsis.
The plaintiff alleged that the radiologist had misread the second CT, which had shown evidence of peritoneal abscess. The plaintiff claimed that a proper reading of this CT would have led to repair of the perforation before sepsis became fulminant.
The defense claimed that the reading of the pelvic CT was reasonable and that the woman's death was the result of comorbidities and complications from several previous surgeries.
A defense verdict was returned.
Infant Injured During (or After?) Cesarean Delivery
After a nonemergent cesarean delivery by a physician from a women's medical group in June 2001, the plaintiff infant was noted to be jittery and hypertonic, and stridor was developing, so she was admitted to a special care unit. Several hours later, the child's father noticed that her right hand was clenched in a fist except for the middle finger, which had a gash at the base.
He brought this to the attention of a nurse, who said it was probably just dried blood from the delivery. When she wiped the area, however, it began to bleed. The nurse then discovered a laceration, about 1.0 cm long and 0.5 cm deep, which extended into the bone of the finger. Two flexor tendons, the tendon pulleys, and a portion of the digital nerve had all been severed. Reconstructive surgery was performed two days later to reattach the tendons, rebuild the pulleys, and repair the nerve.
Because the infant could not participate actively in physical therapy, scar tissue developed. A second surgery was performed in March 2003 to release the tendons and nerve from the scar tissue.
By age 7, the girl had very limited use of her right middle finger and will need a third surgery to release additional scar tissue. This surgery has a success rate of only 65%, and function of the patient's finger will never be completely normal. The finger is also slightly shorter than the ring and index fingers. The plaintiff alleged res ipsa loquitur ("the thing speaks for itself").
Defense for the hospital argued that the laceration occurred during the cesarean delivery and was a known risk for this procedure. A resident obstetrician testified that she remembered the baby's hands being in the surgical field and "fighting" to come out as soon as the uterus was surgically entered. This, she claimed, was "the strangest thing she had ever seen."
Defense for the women's medical group argued that the laceration could not have occurred during the surgery based on the infant's face-down position at the time of delivery. It was alleged that the laceration must have occurred after the baby was handed off to a hospital staff member.
According to a published report, a $2,756,442 verdict was returned against the hospital. The women's medical group received a defense verdict.
Failure to Treat Ascending Cholangitis
In December 2002, a 45-year-old woman presented to the hospital with right upper quadrant abdominal pain, a urinary tract infection, and a pelvic infection. She also had a history of irritable bowel syndrome. The defendant family doctor, who had provided the patient's care for 10 years, admitted her and prescribed oral antibiotics to treat the urinary tract infection and the pelvic infection. Ultrasonography and magnetic resonance cholangiopancreatography showed a 3.0- to 4.0-cm mass at the head of the pancreas, a dilated common bile duct, and gallstones.
Lab test results included elevations in bilirubin, white blood cell count, and liver enzymes. The defendant gastroenterologist was called in for a consult.
Over the next few days, the woman's abdominal pain waxed and waned and shifted in location; her temperature dropped. After four days, her bilirubin level and white blood cell count spiked, so IV antibiotics were started. Two days later, the patient arrested and coded and remained in a coma until her death in May 2003.
The plaintiff claimed that the defendants failed to diagnose and treat ascending cholangitis from the time of the patient's admission until her cardiac arrest, resulting in sepsis and death.
The defendants claimed that the decedent did not have ascending cholangitis until two days before she went into arrest, at which time the condition was properly addressed. The defendants also claimed that there were no signs or symptoms of sepsis before that time and that the decedent had refused to consent to endoscopic retrograde cholangiopancreatography (ERCP) or surgical intervention. The defendants contended that a CT-guided biopsy was not performed because of the decedent's obesity.
The plaintiff denied that an ERCP or surgical intervention had been recommended and refused, since there was no record of this in the medical chart.
A defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Did Patient Require Clearance for Surgery?
A 36-year-old man was admitted to the defendant hospital with congestive heart failure and shortness of breath. CT angiography revealed cardiomegaly and several enlarged mediastinal lymph nodes. His medical history included cigarette smoking and ongoing treatment for tuberculosis and diabetes.
Echocardiography performed soon after the man's admission revealed diffuse hypokinesis consistent with cardiomyopathy and an ejection fraction of 30% to 35%. Two days later, a differential diagnosis was formed for the enlarged lymph nodes which included disseminated tuberculosis, infection (including the possibility of HIV), and neoplasm. A lymph node biopsy by way of mediastinoscopy was recommended.
The following day, the patient was seen by a cardiothoracic surgeon, who also recommended a mediastinoscopy and obtained consent from the patient for the procedure. That same day, an adenosine Cardiolite stress test was performed, with neither ischemia nor chest pain reported. The imaging portion of the study indicated several areas of prior infarction and an ejection fraction of 20%.
The following day, the man was seen by the defendant cardiologist, who added two cardiac agents to his medications. A plan was formulated to await results of a lymph node biopsy, scheduled for a few hours later, and to continue to follow the patient.
No complications occurred during the mediastinoscopy, but immediately after extubation, the man went into cardiopulmonary arrest. He was resuscitated but never regained consciousness. Two days later, he experience a second arrest and died.
Autopsy revealed triple-vessel coronary artery disease and a hemorrhagic septal infarction. The plaintiffs claimed that the decedent's presentation and coronary artery risk factors required that he not be cleared for surgery without undergoing coronary angiography. Angiography results, the plaintiff claimed, would have prompted bypass surgery and ensured the decedent's survival.
The defendant claimed that the treatment was proper, that a presurgery cardiac catheterization was not required, and that she had not been consulted to clear the decedent for surgery. The defendant further argued that the decedent's postsurgical cardiopulmonary arrest resulted from an adverse reaction to epinephrine and administration of 700 cc of IV fluid.
According to a published account, a defense verdict was returned.
Peritoneal Abscess, Colonic Rupture Missed
Sudden-onset abdominal pain brought a 69-year-old woman to the emergency department, where she was seen by a surgeon. He ordered abdominal CT, which was read by the defendant radiologist as showing free air. The next day a pelvic CT was read by the same radiologist, who concluded that the patient's colon appeared healthy. After undergoing a cholecystectomy the next day, the patient was soon released.
She returned to the hospital three days later with persistent pain, and a third CT scan showed free air. Exploratory laparotomy revealed a colonic rupture related to diverticulitis. In spite of aggressive intervention, the woman died several weeks later due to complications of sepsis.
The plaintiff alleged that the radiologist had misread the second CT, which had shown evidence of peritoneal abscess. The plaintiff claimed that a proper reading of this CT would have led to repair of the perforation before sepsis became fulminant.
The defense claimed that the reading of the pelvic CT was reasonable and that the woman's death was the result of comorbidities and complications from several previous surgeries.
A defense verdict was returned.
Infant Injured During (or After?) Cesarean Delivery
After a nonemergent cesarean delivery by a physician from a women's medical group in June 2001, the plaintiff infant was noted to be jittery and hypertonic, and stridor was developing, so she was admitted to a special care unit. Several hours later, the child's father noticed that her right hand was clenched in a fist except for the middle finger, which had a gash at the base.
He brought this to the attention of a nurse, who said it was probably just dried blood from the delivery. When she wiped the area, however, it began to bleed. The nurse then discovered a laceration, about 1.0 cm long and 0.5 cm deep, which extended into the bone of the finger. Two flexor tendons, the tendon pulleys, and a portion of the digital nerve had all been severed. Reconstructive surgery was performed two days later to reattach the tendons, rebuild the pulleys, and repair the nerve.
Because the infant could not participate actively in physical therapy, scar tissue developed. A second surgery was performed in March 2003 to release the tendons and nerve from the scar tissue.
By age 7, the girl had very limited use of her right middle finger and will need a third surgery to release additional scar tissue. This surgery has a success rate of only 65%, and function of the patient's finger will never be completely normal. The finger is also slightly shorter than the ring and index fingers. The plaintiff alleged res ipsa loquitur ("the thing speaks for itself").
Defense for the hospital argued that the laceration occurred during the cesarean delivery and was a known risk for this procedure. A resident obstetrician testified that she remembered the baby's hands being in the surgical field and "fighting" to come out as soon as the uterus was surgically entered. This, she claimed, was "the strangest thing she had ever seen."
Defense for the women's medical group argued that the laceration could not have occurred during the surgery based on the infant's face-down position at the time of delivery. It was alleged that the laceration must have occurred after the baby was handed off to a hospital staff member.
According to a published report, a $2,756,442 verdict was returned against the hospital. The women's medical group received a defense verdict.
Failure to Treat Ascending Cholangitis
In December 2002, a 45-year-old woman presented to the hospital with right upper quadrant abdominal pain, a urinary tract infection, and a pelvic infection. She also had a history of irritable bowel syndrome. The defendant family doctor, who had provided the patient's care for 10 years, admitted her and prescribed oral antibiotics to treat the urinary tract infection and the pelvic infection. Ultrasonography and magnetic resonance cholangiopancreatography showed a 3.0- to 4.0-cm mass at the head of the pancreas, a dilated common bile duct, and gallstones.
Lab test results included elevations in bilirubin, white blood cell count, and liver enzymes. The defendant gastroenterologist was called in for a consult.
Over the next few days, the woman's abdominal pain waxed and waned and shifted in location; her temperature dropped. After four days, her bilirubin level and white blood cell count spiked, so IV antibiotics were started. Two days later, the patient arrested and coded and remained in a coma until her death in May 2003.
The plaintiff claimed that the defendants failed to diagnose and treat ascending cholangitis from the time of the patient's admission until her cardiac arrest, resulting in sepsis and death.
The defendants claimed that the decedent did not have ascending cholangitis until two days before she went into arrest, at which time the condition was properly addressed. The defendants also claimed that there were no signs or symptoms of sepsis before that time and that the decedent had refused to consent to endoscopic retrograde cholangiopancreatography (ERCP) or surgical intervention. The defendants contended that a CT-guided biopsy was not performed because of the decedent's obesity.
The plaintiff denied that an ERCP or surgical intervention had been recommended and refused, since there was no record of this in the medical chart.
A defense verdict was returned.
Covering Your (Professional) Assets
Regular readers of “Malpractice Chronicle” know that all types of cases—from instances of clear-cut negligence to those of obvious noncompliance by the patient—find their way into the legal system. It often seems that there is no rhyme or reason to how a verdict is decided or how high the judgment or settlement is. In light of this unpredictability, protecting yourself—with liability insurance acquired through your practice or on your own—seems like a no-brainer.
Always There for You
It’s hard to imagine any clinician “going bare” and having no liability coverage at all. At the very least, most will be covered by their employer’s policy. But there are advantages to having your own malpractice insurance.
For one thing, it’s portable—wherever you practice, the policy goes with you—and it covers all aspects of professional liability. “It protects you 24 hours a day, so if you’re moonlighting, if you’re volunteering, you’re protected,” says Rebecca S. Crosby, Vice President of Marsh, the insurance brokerage for the American Academy of Nurse Practitioners (AANP). “It also protects you if you give advice—friends and family sue, too.” The policies offered through AANP and the American Academy of Physician Assistants (AAPA) also provide coverage for policyholders who are called before their state licensing board.
The policy is also 100% yours. “You are the only named person on the policy, so the limits are not shared,” says Ellen Rathfon, Senior Director of Professional Affairs for AAPA. “If you’re covered by your employer, the limits may be shared among several people—or a lot of people, depending on what kind of policy it is.”
In the event of a lawsuit, you will have your own legal representation. “There can be conflicts of interest between an employer or a supervising physician and the PA; they could be pointing fingers at each other,” says Gary M. McCammon, President of Professional Risk Advisors, Inc, the AAPA’s insurance broker. “And when a case is ultimately settled, or maybe it even goes to trial, the apportionment of liability can be greater if the PA is not in a position to negotiate his own liability.”
This can be important, because settlement information is reported to the National Practitioner Data Bank. Cases are often settled because it is ultimately less costly than taking a case to trial. Even a successful defense costs money, after all. But if a group of providers is named in the settlement, and they are covered by a single employer policy, the individual clinician may not be in a position to decide what portion of the settlement is his or her responsibility.
“Now, it doesn’t come out of their pocket, it comes out of the insurance,” Rathfon acknowledges. “But in the Data Bank, that clinician might have a high payment value.”
ow Risk, High Cost?
You may think that PAs and NPs don’t get sued. Actually, they do. Not as often as physicians do, certainly, but even a small risk can carry a big price tag.
An analysis of data from the National Practitioner Data Bank, published earlier this year in the Journal of Medical Licensure and Discipline (a publication of the Federation of State Medical Boards), indicated that approximately 3% of active PAs and at least 1.5% of active APNs made a payment during the 17-year study period. However, while PAs and APNs accounted for 0.003% and 0.007%, respectively, of the $74 billion in malpractice payments made from 1991 to 2008, the mean/median payments for these providers were $173,128/$80,003 and $350,540/$190,898, respectively. The risk may be small, but is it one clinicians can afford?
“If you look at the spread of payments, some of them are really high,” Rathfon says. “And I think most people look at that and say, ‘I don’t want to take that risk.’”
In some cases, verdicts are exceeding even the limits of the defendant’s insurance, says Michele Kauffman, JD, PA-C, Chair of the Physician Assistant Department at Gannon University in Erie, Pennsylvania. This can open clinicians up to personal liability; while this hasn’t really happened yet, it’s a sobering possibility.
“Some states—Pennsylvania is one of them—now require PAs and NPs to have a minimum insurance, whether it’s through their employer or on their own,” Kauffman says. “In order to get their license, they have to prove that they have that insurance.”
Oh, and that old “deep pocket” argument—the idea that having liability insurance will just make you a target for litigation—is “a fallacy,” according to Crosby. “They’re going after everybody who even said ‘good morning’ to the patient. They don’t know if you have insurance until you’re in the discovery phase of the case.” By then, it’s too late to think about obtaining malpractice coverage.
Know Your Limits
Whether or not you opt for your own policy, know what coverage you do have. If you’re covered through your employer, make sure you have (at the very least) a copy of the declarations page, which will provide the name of the insurance company, the policy number, and the terms of coverage. McCammon suggests making it a condition of your employment that your employer will provide this information every year.
“People need to know their limits and know that they are individually named under those limits,” Rathfon says. “And they need to feel confident that there’s enough coverage, whatever the policy is, whether it’s their own or through their employer.”
Familiarize yourself with the type of policy you have: Is it occurrence-based or claims-made coverage? The former applies to any incident that occurs during the period of coverage, even if the claim is made years later. The latter covers claims filed during the life of the policy; people with this type of policy often purchase tail coverage to close any gaps that may occur. McCammon, who has authored a series of articles on liability insurance that can be found in the “Advocacy and Practice Resources” section on the AAPA Web site (www.aapa.org), says one type is not better than the other.
Occurrence-based coverage may be easier to manage; you file a copy for your records and hope you never need it. As Crosby says, “You can be retired and lying on the beach in Florida, and if there’s a lawsuit over something that happened in your last year of work, you would be covered.”
However, the economic downturn has highlighted one drawback to occurrence-based coverage: The company that covered you 10 or 20 years ago may not be in business by the time you have a claim. “Ten or 20 years ago, that was not a real issue,” McCammon says, “because companies just didn’t go under. But in this day and age, companies are getting out of the business or going out of business.”
That doesn’t mean you should avoid occurrence-based policies. But you might do a little extra homework on a company’s financial stability and longevity.
Rates depend on a number of factors, including geographic location and specialty of practice. AANP members can find information at www.proliability.com. AAPA members can contact AAPA Insurance Services at (877) 356-2272.
Regular readers of “Malpractice Chronicle” know that all types of cases—from instances of clear-cut negligence to those of obvious noncompliance by the patient—find their way into the legal system. It often seems that there is no rhyme or reason to how a verdict is decided or how high the judgment or settlement is. In light of this unpredictability, protecting yourself—with liability insurance acquired through your practice or on your own—seems like a no-brainer.
Always There for You
It’s hard to imagine any clinician “going bare” and having no liability coverage at all. At the very least, most will be covered by their employer’s policy. But there are advantages to having your own malpractice insurance.
For one thing, it’s portable—wherever you practice, the policy goes with you—and it covers all aspects of professional liability. “It protects you 24 hours a day, so if you’re moonlighting, if you’re volunteering, you’re protected,” says Rebecca S. Crosby, Vice President of Marsh, the insurance brokerage for the American Academy of Nurse Practitioners (AANP). “It also protects you if you give advice—friends and family sue, too.” The policies offered through AANP and the American Academy of Physician Assistants (AAPA) also provide coverage for policyholders who are called before their state licensing board.
The policy is also 100% yours. “You are the only named person on the policy, so the limits are not shared,” says Ellen Rathfon, Senior Director of Professional Affairs for AAPA. “If you’re covered by your employer, the limits may be shared among several people—or a lot of people, depending on what kind of policy it is.”
In the event of a lawsuit, you will have your own legal representation. “There can be conflicts of interest between an employer or a supervising physician and the PA; they could be pointing fingers at each other,” says Gary M. McCammon, President of Professional Risk Advisors, Inc, the AAPA’s insurance broker. “And when a case is ultimately settled, or maybe it even goes to trial, the apportionment of liability can be greater if the PA is not in a position to negotiate his own liability.”
This can be important, because settlement information is reported to the National Practitioner Data Bank. Cases are often settled because it is ultimately less costly than taking a case to trial. Even a successful defense costs money, after all. But if a group of providers is named in the settlement, and they are covered by a single employer policy, the individual clinician may not be in a position to decide what portion of the settlement is his or her responsibility.
“Now, it doesn’t come out of their pocket, it comes out of the insurance,” Rathfon acknowledges. “But in the Data Bank, that clinician might have a high payment value.”
ow Risk, High Cost?
You may think that PAs and NPs don’t get sued. Actually, they do. Not as often as physicians do, certainly, but even a small risk can carry a big price tag.
An analysis of data from the National Practitioner Data Bank, published earlier this year in the Journal of Medical Licensure and Discipline (a publication of the Federation of State Medical Boards), indicated that approximately 3% of active PAs and at least 1.5% of active APNs made a payment during the 17-year study period. However, while PAs and APNs accounted for 0.003% and 0.007%, respectively, of the $74 billion in malpractice payments made from 1991 to 2008, the mean/median payments for these providers were $173,128/$80,003 and $350,540/$190,898, respectively. The risk may be small, but is it one clinicians can afford?
“If you look at the spread of payments, some of them are really high,” Rathfon says. “And I think most people look at that and say, ‘I don’t want to take that risk.’”
In some cases, verdicts are exceeding even the limits of the defendant’s insurance, says Michele Kauffman, JD, PA-C, Chair of the Physician Assistant Department at Gannon University in Erie, Pennsylvania. This can open clinicians up to personal liability; while this hasn’t really happened yet, it’s a sobering possibility.
“Some states—Pennsylvania is one of them—now require PAs and NPs to have a minimum insurance, whether it’s through their employer or on their own,” Kauffman says. “In order to get their license, they have to prove that they have that insurance.”
Oh, and that old “deep pocket” argument—the idea that having liability insurance will just make you a target for litigation—is “a fallacy,” according to Crosby. “They’re going after everybody who even said ‘good morning’ to the patient. They don’t know if you have insurance until you’re in the discovery phase of the case.” By then, it’s too late to think about obtaining malpractice coverage.
Know Your Limits
Whether or not you opt for your own policy, know what coverage you do have. If you’re covered through your employer, make sure you have (at the very least) a copy of the declarations page, which will provide the name of the insurance company, the policy number, and the terms of coverage. McCammon suggests making it a condition of your employment that your employer will provide this information every year.
“People need to know their limits and know that they are individually named under those limits,” Rathfon says. “And they need to feel confident that there’s enough coverage, whatever the policy is, whether it’s their own or through their employer.”
Familiarize yourself with the type of policy you have: Is it occurrence-based or claims-made coverage? The former applies to any incident that occurs during the period of coverage, even if the claim is made years later. The latter covers claims filed during the life of the policy; people with this type of policy often purchase tail coverage to close any gaps that may occur. McCammon, who has authored a series of articles on liability insurance that can be found in the “Advocacy and Practice Resources” section on the AAPA Web site (www.aapa.org), says one type is not better than the other.
Occurrence-based coverage may be easier to manage; you file a copy for your records and hope you never need it. As Crosby says, “You can be retired and lying on the beach in Florida, and if there’s a lawsuit over something that happened in your last year of work, you would be covered.”
However, the economic downturn has highlighted one drawback to occurrence-based coverage: The company that covered you 10 or 20 years ago may not be in business by the time you have a claim. “Ten or 20 years ago, that was not a real issue,” McCammon says, “because companies just didn’t go under. But in this day and age, companies are getting out of the business or going out of business.”
That doesn’t mean you should avoid occurrence-based policies. But you might do a little extra homework on a company’s financial stability and longevity.
Rates depend on a number of factors, including geographic location and specialty of practice. AANP members can find information at www.proliability.com. AAPA members can contact AAPA Insurance Services at (877) 356-2272.
Regular readers of “Malpractice Chronicle” know that all types of cases—from instances of clear-cut negligence to those of obvious noncompliance by the patient—find their way into the legal system. It often seems that there is no rhyme or reason to how a verdict is decided or how high the judgment or settlement is. In light of this unpredictability, protecting yourself—with liability insurance acquired through your practice or on your own—seems like a no-brainer.
Always There for You
It’s hard to imagine any clinician “going bare” and having no liability coverage at all. At the very least, most will be covered by their employer’s policy. But there are advantages to having your own malpractice insurance.
For one thing, it’s portable—wherever you practice, the policy goes with you—and it covers all aspects of professional liability. “It protects you 24 hours a day, so if you’re moonlighting, if you’re volunteering, you’re protected,” says Rebecca S. Crosby, Vice President of Marsh, the insurance brokerage for the American Academy of Nurse Practitioners (AANP). “It also protects you if you give advice—friends and family sue, too.” The policies offered through AANP and the American Academy of Physician Assistants (AAPA) also provide coverage for policyholders who are called before their state licensing board.
The policy is also 100% yours. “You are the only named person on the policy, so the limits are not shared,” says Ellen Rathfon, Senior Director of Professional Affairs for AAPA. “If you’re covered by your employer, the limits may be shared among several people—or a lot of people, depending on what kind of policy it is.”
In the event of a lawsuit, you will have your own legal representation. “There can be conflicts of interest between an employer or a supervising physician and the PA; they could be pointing fingers at each other,” says Gary M. McCammon, President of Professional Risk Advisors, Inc, the AAPA’s insurance broker. “And when a case is ultimately settled, or maybe it even goes to trial, the apportionment of liability can be greater if the PA is not in a position to negotiate his own liability.”
This can be important, because settlement information is reported to the National Practitioner Data Bank. Cases are often settled because it is ultimately less costly than taking a case to trial. Even a successful defense costs money, after all. But if a group of providers is named in the settlement, and they are covered by a single employer policy, the individual clinician may not be in a position to decide what portion of the settlement is his or her responsibility.
“Now, it doesn’t come out of their pocket, it comes out of the insurance,” Rathfon acknowledges. “But in the Data Bank, that clinician might have a high payment value.”
ow Risk, High Cost?
You may think that PAs and NPs don’t get sued. Actually, they do. Not as often as physicians do, certainly, but even a small risk can carry a big price tag.
An analysis of data from the National Practitioner Data Bank, published earlier this year in the Journal of Medical Licensure and Discipline (a publication of the Federation of State Medical Boards), indicated that approximately 3% of active PAs and at least 1.5% of active APNs made a payment during the 17-year study period. However, while PAs and APNs accounted for 0.003% and 0.007%, respectively, of the $74 billion in malpractice payments made from 1991 to 2008, the mean/median payments for these providers were $173,128/$80,003 and $350,540/$190,898, respectively. The risk may be small, but is it one clinicians can afford?
“If you look at the spread of payments, some of them are really high,” Rathfon says. “And I think most people look at that and say, ‘I don’t want to take that risk.’”
In some cases, verdicts are exceeding even the limits of the defendant’s insurance, says Michele Kauffman, JD, PA-C, Chair of the Physician Assistant Department at Gannon University in Erie, Pennsylvania. This can open clinicians up to personal liability; while this hasn’t really happened yet, it’s a sobering possibility.
“Some states—Pennsylvania is one of them—now require PAs and NPs to have a minimum insurance, whether it’s through their employer or on their own,” Kauffman says. “In order to get their license, they have to prove that they have that insurance.”
Oh, and that old “deep pocket” argument—the idea that having liability insurance will just make you a target for litigation—is “a fallacy,” according to Crosby. “They’re going after everybody who even said ‘good morning’ to the patient. They don’t know if you have insurance until you’re in the discovery phase of the case.” By then, it’s too late to think about obtaining malpractice coverage.
Know Your Limits
Whether or not you opt for your own policy, know what coverage you do have. If you’re covered through your employer, make sure you have (at the very least) a copy of the declarations page, which will provide the name of the insurance company, the policy number, and the terms of coverage. McCammon suggests making it a condition of your employment that your employer will provide this information every year.
“People need to know their limits and know that they are individually named under those limits,” Rathfon says. “And they need to feel confident that there’s enough coverage, whatever the policy is, whether it’s their own or through their employer.”
Familiarize yourself with the type of policy you have: Is it occurrence-based or claims-made coverage? The former applies to any incident that occurs during the period of coverage, even if the claim is made years later. The latter covers claims filed during the life of the policy; people with this type of policy often purchase tail coverage to close any gaps that may occur. McCammon, who has authored a series of articles on liability insurance that can be found in the “Advocacy and Practice Resources” section on the AAPA Web site (www.aapa.org), says one type is not better than the other.
Occurrence-based coverage may be easier to manage; you file a copy for your records and hope you never need it. As Crosby says, “You can be retired and lying on the beach in Florida, and if there’s a lawsuit over something that happened in your last year of work, you would be covered.”
However, the economic downturn has highlighted one drawback to occurrence-based coverage: The company that covered you 10 or 20 years ago may not be in business by the time you have a claim. “Ten or 20 years ago, that was not a real issue,” McCammon says, “because companies just didn’t go under. But in this day and age, companies are getting out of the business or going out of business.”
That doesn’t mean you should avoid occurrence-based policies. But you might do a little extra homework on a company’s financial stability and longevity.
Rates depend on a number of factors, including geographic location and specialty of practice. AANP members can find information at www.proliability.com. AAPA members can contact AAPA Insurance Services at (877) 356-2272.
Revenue Essentials
As physicians take on more extensive roles outside of patient care (e.g., administrative, academic, and billing compliance), involvement in the revenue cycle might diminish or even fail to commence. It is crucial for physicians to keep abreast of revenue cycle issues, but more often than not, they go unnoticed until a physician’s bottom line is affected.
The risk of inappropriately billed claims and corresponding reimbursement is increased until the problem is identified and resolved. In an effort to prevent this from occurring, physicians should get involved with or oversee their billing service or staff. Some of the revenue cycle essentials that require physician attention are:1
- Periodic reports of claims billed on the physician’s behalf and data regarding payments;
- Changes in procedure codes, diagnosis codes, or other information furnished by the physician without the physician’s knowledge and consent; and
- Information received from Medicare and other payors.
Feedback
One of the most common billing-related physician complaints involves the lack of feedback. Most physicians want to receive information regarding their quarterly billings: the volume and frequency of specific reported services, and corresponding payments or denials. Physicians prefer to know how they rank as individuals and as a group. Although they might not be experts in coding and documentation, this information offers physicians a feeling of security, as it permits them to identify typical billing patterns or highlight outlier patterns.
Establish communication with the manager/coder/biller to better assist with feedback. Appoint a physician leader to spearhead this effort; ensure feedback is provided quarterly, at a minimum. If the coders/billers feel that they have an approachable contact, they’re more likely to offer feedback before formal reports are generated. A quick resolution of potential problems lessens the financial burden on the HM group, as well as the resource-intensive education process that ensues.
Discrepancy Notation
Physicians should be notified whenever coding changes take place. Discrepancies occur when the physician employs coders to select the service or diagnosis codes, and the selected codes differ from the physician-intended codes. Discrepancies also occur when billers change the original physician-selected codes to codes that are considered covered or medically necessary by the pay0r. Physicians need to instruct coders to only report codes that are supported by the documentation.
Physicians must be aware that delegating any portion of the billing to an employee or a billing company does not alleviate physicians’ personal responsibility for erroneously submitted claims or receipt of overpayments. Physicians should regularly review information submitted by the designated employee or billing service to ensure consistency with their own records, and also keep complete administrative records for the claims a billing service files on their behalf.1 Physicians also should meet with staff to resolve discrepancies and reinforce the billing education process. If biller/coder performance becomes a recurring problem, the physician should question the competency of the employee or company with whom the billing is entrusted.
Accounts Receivable
Physicians do not necessarily recognize the need for involvement in the accounts receivable (A/R) component of the revenue cycle. Physicians should be aware of denials, and the reasons for the denials. Some services are denied because of issues that can be easily corrected (e.g., truncated diagnoses, two physicians of the same specialty billing on the same date, missing modifiers). These denial types might require physician assistance in changing the codes originally submitted. If the denied services can be corrected with the appropriate information and resubmitted electronically, payment might be recovered quickly. Other types of denials require submission of the documentation to support the service billed.
Billers should know the difference between the types of denials and the required action for each denial type. Physicians should feel confident that such denials will be handled in the correct manner. Be mindful of billing staff that accepts denials and surrenders the reimbursement efforts without hesitation. As a physician, do not default to the idea that “no news is good news.” Do not assume the billing manager (physician employee or outsourced firm) will let the group know if there is a problem. Develop a standard that requires monthly feedback of denials.
Only a short window of time exists for the appeals process to occur. Do not lose the potential to recover monies because the information was not provided to the physician in a timely manner. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. Protecting Your Practice. CMS Web site. Available at: www.cms.hhs.gov/MLNProducts/downloads/Protectingpracbroch508-09.pdf. Accessed Aug. 2, 2009.
- Office of Inspector General. Work Plan Health Care Financing Administration Projects Fiscal Year 1999. Physicians: Billing Service Companies. Available at: http://oig.hhs.gov/publications/docs/workplan/1999/99hcfawp.pdf. Accessed Aug. 2, 2009.
- Office of Inspector General. OIG Compliance Program Guidance for Third-Party Medical Billing Companies in Federal Register, December 1998, Vol. 63; pages 70138-70152. Available at: http://oig.hhs.gov/fraud/docs/complianceguidance/thirdparty.pdf. Accessed Aug. 2, 2009.
As physicians take on more extensive roles outside of patient care (e.g., administrative, academic, and billing compliance), involvement in the revenue cycle might diminish or even fail to commence. It is crucial for physicians to keep abreast of revenue cycle issues, but more often than not, they go unnoticed until a physician’s bottom line is affected.
The risk of inappropriately billed claims and corresponding reimbursement is increased until the problem is identified and resolved. In an effort to prevent this from occurring, physicians should get involved with or oversee their billing service or staff. Some of the revenue cycle essentials that require physician attention are:1
- Periodic reports of claims billed on the physician’s behalf and data regarding payments;
- Changes in procedure codes, diagnosis codes, or other information furnished by the physician without the physician’s knowledge and consent; and
- Information received from Medicare and other payors.
Feedback
One of the most common billing-related physician complaints involves the lack of feedback. Most physicians want to receive information regarding their quarterly billings: the volume and frequency of specific reported services, and corresponding payments or denials. Physicians prefer to know how they rank as individuals and as a group. Although they might not be experts in coding and documentation, this information offers physicians a feeling of security, as it permits them to identify typical billing patterns or highlight outlier patterns.
Establish communication with the manager/coder/biller to better assist with feedback. Appoint a physician leader to spearhead this effort; ensure feedback is provided quarterly, at a minimum. If the coders/billers feel that they have an approachable contact, they’re more likely to offer feedback before formal reports are generated. A quick resolution of potential problems lessens the financial burden on the HM group, as well as the resource-intensive education process that ensues.
Discrepancy Notation
Physicians should be notified whenever coding changes take place. Discrepancies occur when the physician employs coders to select the service or diagnosis codes, and the selected codes differ from the physician-intended codes. Discrepancies also occur when billers change the original physician-selected codes to codes that are considered covered or medically necessary by the pay0r. Physicians need to instruct coders to only report codes that are supported by the documentation.
Physicians must be aware that delegating any portion of the billing to an employee or a billing company does not alleviate physicians’ personal responsibility for erroneously submitted claims or receipt of overpayments. Physicians should regularly review information submitted by the designated employee or billing service to ensure consistency with their own records, and also keep complete administrative records for the claims a billing service files on their behalf.1 Physicians also should meet with staff to resolve discrepancies and reinforce the billing education process. If biller/coder performance becomes a recurring problem, the physician should question the competency of the employee or company with whom the billing is entrusted.
Accounts Receivable
Physicians do not necessarily recognize the need for involvement in the accounts receivable (A/R) component of the revenue cycle. Physicians should be aware of denials, and the reasons for the denials. Some services are denied because of issues that can be easily corrected (e.g., truncated diagnoses, two physicians of the same specialty billing on the same date, missing modifiers). These denial types might require physician assistance in changing the codes originally submitted. If the denied services can be corrected with the appropriate information and resubmitted electronically, payment might be recovered quickly. Other types of denials require submission of the documentation to support the service billed.
Billers should know the difference between the types of denials and the required action for each denial type. Physicians should feel confident that such denials will be handled in the correct manner. Be mindful of billing staff that accepts denials and surrenders the reimbursement efforts without hesitation. As a physician, do not default to the idea that “no news is good news.” Do not assume the billing manager (physician employee or outsourced firm) will let the group know if there is a problem. Develop a standard that requires monthly feedback of denials.
Only a short window of time exists for the appeals process to occur. Do not lose the potential to recover monies because the information was not provided to the physician in a timely manner. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. Protecting Your Practice. CMS Web site. Available at: www.cms.hhs.gov/MLNProducts/downloads/Protectingpracbroch508-09.pdf. Accessed Aug. 2, 2009.
- Office of Inspector General. Work Plan Health Care Financing Administration Projects Fiscal Year 1999. Physicians: Billing Service Companies. Available at: http://oig.hhs.gov/publications/docs/workplan/1999/99hcfawp.pdf. Accessed Aug. 2, 2009.
- Office of Inspector General. OIG Compliance Program Guidance for Third-Party Medical Billing Companies in Federal Register, December 1998, Vol. 63; pages 70138-70152. Available at: http://oig.hhs.gov/fraud/docs/complianceguidance/thirdparty.pdf. Accessed Aug. 2, 2009.
As physicians take on more extensive roles outside of patient care (e.g., administrative, academic, and billing compliance), involvement in the revenue cycle might diminish or even fail to commence. It is crucial for physicians to keep abreast of revenue cycle issues, but more often than not, they go unnoticed until a physician’s bottom line is affected.
The risk of inappropriately billed claims and corresponding reimbursement is increased until the problem is identified and resolved. In an effort to prevent this from occurring, physicians should get involved with or oversee their billing service or staff. Some of the revenue cycle essentials that require physician attention are:1
- Periodic reports of claims billed on the physician’s behalf and data regarding payments;
- Changes in procedure codes, diagnosis codes, or other information furnished by the physician without the physician’s knowledge and consent; and
- Information received from Medicare and other payors.
Feedback
One of the most common billing-related physician complaints involves the lack of feedback. Most physicians want to receive information regarding their quarterly billings: the volume and frequency of specific reported services, and corresponding payments or denials. Physicians prefer to know how they rank as individuals and as a group. Although they might not be experts in coding and documentation, this information offers physicians a feeling of security, as it permits them to identify typical billing patterns or highlight outlier patterns.
Establish communication with the manager/coder/biller to better assist with feedback. Appoint a physician leader to spearhead this effort; ensure feedback is provided quarterly, at a minimum. If the coders/billers feel that they have an approachable contact, they’re more likely to offer feedback before formal reports are generated. A quick resolution of potential problems lessens the financial burden on the HM group, as well as the resource-intensive education process that ensues.
Discrepancy Notation
Physicians should be notified whenever coding changes take place. Discrepancies occur when the physician employs coders to select the service or diagnosis codes, and the selected codes differ from the physician-intended codes. Discrepancies also occur when billers change the original physician-selected codes to codes that are considered covered or medically necessary by the pay0r. Physicians need to instruct coders to only report codes that are supported by the documentation.
Physicians must be aware that delegating any portion of the billing to an employee or a billing company does not alleviate physicians’ personal responsibility for erroneously submitted claims or receipt of overpayments. Physicians should regularly review information submitted by the designated employee or billing service to ensure consistency with their own records, and also keep complete administrative records for the claims a billing service files on their behalf.1 Physicians also should meet with staff to resolve discrepancies and reinforce the billing education process. If biller/coder performance becomes a recurring problem, the physician should question the competency of the employee or company with whom the billing is entrusted.
Accounts Receivable
Physicians do not necessarily recognize the need for involvement in the accounts receivable (A/R) component of the revenue cycle. Physicians should be aware of denials, and the reasons for the denials. Some services are denied because of issues that can be easily corrected (e.g., truncated diagnoses, two physicians of the same specialty billing on the same date, missing modifiers). These denial types might require physician assistance in changing the codes originally submitted. If the denied services can be corrected with the appropriate information and resubmitted electronically, payment might be recovered quickly. Other types of denials require submission of the documentation to support the service billed.
Billers should know the difference between the types of denials and the required action for each denial type. Physicians should feel confident that such denials will be handled in the correct manner. Be mindful of billing staff that accepts denials and surrenders the reimbursement efforts without hesitation. As a physician, do not default to the idea that “no news is good news.” Do not assume the billing manager (physician employee or outsourced firm) will let the group know if there is a problem. Develop a standard that requires monthly feedback of denials.
Only a short window of time exists for the appeals process to occur. Do not lose the potential to recover monies because the information was not provided to the physician in a timely manner. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. Protecting Your Practice. CMS Web site. Available at: www.cms.hhs.gov/MLNProducts/downloads/Protectingpracbroch508-09.pdf. Accessed Aug. 2, 2009.
- Office of Inspector General. Work Plan Health Care Financing Administration Projects Fiscal Year 1999. Physicians: Billing Service Companies. Available at: http://oig.hhs.gov/publications/docs/workplan/1999/99hcfawp.pdf. Accessed Aug. 2, 2009.
- Office of Inspector General. OIG Compliance Program Guidance for Third-Party Medical Billing Companies in Federal Register, December 1998, Vol. 63; pages 70138-70152. Available at: http://oig.hhs.gov/fraud/docs/complianceguidance/thirdparty.pdf. Accessed Aug. 2, 2009.
Payment Purgatory
It happens every year: A new Medicare physician fee schedule recommends a painful reduction in the rates the government doles out for more than 7,000 types of medical services performed by hospitalists, primary-care doctors, and other healthcare providers. And then Congress intervenes to keep the reduction from going into effect.
This year, however, the fee updates and policy proposals have become intertwined in the larger debate over comprehensive healthcare reform and a push toward what SHM calls a “value-based health delivery system.”
Few think the whopping 21.5% reduction proposed for 2010 rates, which are due to be published Nov. 1, actually will go into effect in January. A combination of other proposed changes to the fee schedule could soften any reduction. But the larger issue of how fees are currently calculated has become a rallying point for SHM and other healthcare-reform advocates who’d like to see the entire formula scrapped in favor of a more equitable distribution that focuses on outcomes. SHM has expressed concern that the current fee-for-service payment system “fails to provide providers with incentives to improve efficiency or quality of care, and encourages overutilization of services.”
—J. James Rohack, MD, AMA president
The current fee formula, known as the sustainable growth rate (SGR) and stipulated by the Balanced Budget Act of 1997, has resulted in a proposed rate cut every year since it went into effect in 2002. Congress consistently overrides the reimbursement cuts in one way or another, usually enacting modest increases or, such as in 2006 and 2007, an across-the-board freeze.
Hospitalists and other physicians have greeted another high-profile proposal with more enthusiasm: the removal of physician-administered drugs from the definition of “physician services,” thereby eliminating a high-cost item from the SGR formula. A Centers for Medicare and Medicaid Services (CMS) spokeswoman says the agency previously felt it lacked the authority to propose the removal. But a review earlier this year reversed that position, leading to the change. The removal, she says, “wouldn’t change the 21.5% percent decline in 2010, but it could have an impact in future years.”
American Medical Association President J. James Rohack, MD, joined the chorus of approval with a statement that asserted, “The removal of physician-administered drugs from the broken Medicare physician payment formula is a major victory for America’s seniors and their physicians.”
Consultation Change
A separate physician fee schedule proposal would eliminate payments for consultation codes in both outpatient and inpatient settings in favor of codes for evaluation and management services that carry lower reimbursement rates. Doctors would have a new way to bill for tele-health consultations, however. Leslie Flores, director of SHM’s Practice Management Institute, said the proposal has generated a high level of misinformation and misunderstanding among hospitalists who fear CMS will no longer reimburse for inpatient consultations. “It’s not that they won’t allow the work to be done anymore,” Flores says. “They’re just instructing people to bill in a different way for that work.”
CMS notes that the savings would be redistributed to increase payments for existing evaluation and management services codes, making the change cost-neutral. But Flores says a lack of details about the “crosswalk” from the old codes to the new codes, especially as they relate to transfers of care, is fueling hospitalists’ confusion over the true impact on their reimbursements.
“Our biggest concern with this consultation proposal that is now on the table is that CMS has not done anything to clarify how these transfers of care would be treated,” she says. For example, would hospitalists bill an admission code the first time they see a patient after being asked by a surgeon to take over that patient’s diabetes care? If so, “this proposal could actually result in some pretty good revenue increases for a lot of hospitalist practices that are doing a lot of surgical comanagement,” she says.
If hospitalists are instead required to bill a work-intensive transition of care under the code for a subsequent visit, which CMS has enforced since a 2006 rule clarification, Flores explains, HM could lose anywhere from 1% to 18% of reimbursements, depending on the exact code used.
Quality Reporting
The proposed fee schedule also is generating disappointment about what will not be included in Medicare’s Physician Quality Reporting Initiative (PQRI) for 2010. The pay-for-performance PQRI, which rewards physicians for reporting on quality measures by paying them an additional 2% of their estimated charges for covered services, will streamline some reporting requirements and increase the overall number of reportable measures. But several care-transition measures sought by SHM were not endorsed in time and will not be among them. Those measures include:
- Reconciled medication lists received by discharged patients;
- Transition records with specified elements received by discharged patients (including one measure for an inpatient discharge to home or self-care, and another for an ED discharge to home, ambulatory, or self-care); and
- Timely transmission of transition records.
“It was really a question of timing,” says Jill Epstein, senior advisor of SHM’s Performance and Standards Committee. The National Quality Forum, the nonprofit organization charged with signing off on all new measures, was not able to fully vet the recommended additions by the July 1 deadline, Epstein says, postponing their inclusion. “Our hope is that the measures will be included for 2011, of course.”
Separately, the PQRI proposal seeks to add an electronic-health-record-based mechanism to the list of eligible reporting methods. Although that addition is welcome news, Epstein says, SHM is expressing its concern about the shift toward patient-registry-based reporting, including a proposal to lessen or perhaps even discontinue claims-based reporting after 2010. “The issue for hospitalists, as well as for any specialty,” she says, “is that not every group will have access to a qualified PQRI registry as early as 2011,” particularly rural-based groups with fewer resources.
A similar change would streamline the E-Prescribing Incentive Program’s rules for how often e-prescribing codes must be reported. It also will offer more choices for how to report them, including through qualified registries. In the past, the program had little direct impact on hospitalists, but the new proposal recommends adding reporting codes specific to nursing homes, where some hospitalists provide care and could benefit from the incentives.
Paul Fishman, an economist at the Group Health Center for Health Studies in Seattle, says the increased focus on incentives in Medicare’s fee schedule suggests a growing realization of how such incentives can drive the delivery of healthcare services. “We know with absolute certainty that physicians make choices based on how things are reimbursed,” he says. Developing good outcome measures, then, will be critical for establishing pay-for-performance standards as part of a fee package that he says should include a blend of capitation and service-based and outcome-based reimbursements to strike a fairer balance.
“In healthcare, we’ve incented people to do more and more stuff, whether they improve outcomes or not, but we have to figure out a way to incent improvements in outcomes, while still retaining the long-term incentive to keep people healthy,” Fishman says. If better transitions of care result in a healthier population that is rehospitalized less often, for example, how can outcome-based incentives prevent hospitals from losing money in the long run? “We want to create the incentives to make the improvements in health outcomes, but we don’t want to punish the better actors because they are consistently lowering costs and also lowering reimbursement levels,” he says.
Achieving that balance in both Medicare and the broader healthcare system, he and other observers agree, is still very much a work in progress. TH
Bryn Nelson is a freelance writer based in Seattle.
It happens every year: A new Medicare physician fee schedule recommends a painful reduction in the rates the government doles out for more than 7,000 types of medical services performed by hospitalists, primary-care doctors, and other healthcare providers. And then Congress intervenes to keep the reduction from going into effect.
This year, however, the fee updates and policy proposals have become intertwined in the larger debate over comprehensive healthcare reform and a push toward what SHM calls a “value-based health delivery system.”
Few think the whopping 21.5% reduction proposed for 2010 rates, which are due to be published Nov. 1, actually will go into effect in January. A combination of other proposed changes to the fee schedule could soften any reduction. But the larger issue of how fees are currently calculated has become a rallying point for SHM and other healthcare-reform advocates who’d like to see the entire formula scrapped in favor of a more equitable distribution that focuses on outcomes. SHM has expressed concern that the current fee-for-service payment system “fails to provide providers with incentives to improve efficiency or quality of care, and encourages overutilization of services.”
—J. James Rohack, MD, AMA president
The current fee formula, known as the sustainable growth rate (SGR) and stipulated by the Balanced Budget Act of 1997, has resulted in a proposed rate cut every year since it went into effect in 2002. Congress consistently overrides the reimbursement cuts in one way or another, usually enacting modest increases or, such as in 2006 and 2007, an across-the-board freeze.
Hospitalists and other physicians have greeted another high-profile proposal with more enthusiasm: the removal of physician-administered drugs from the definition of “physician services,” thereby eliminating a high-cost item from the SGR formula. A Centers for Medicare and Medicaid Services (CMS) spokeswoman says the agency previously felt it lacked the authority to propose the removal. But a review earlier this year reversed that position, leading to the change. The removal, she says, “wouldn’t change the 21.5% percent decline in 2010, but it could have an impact in future years.”
American Medical Association President J. James Rohack, MD, joined the chorus of approval with a statement that asserted, “The removal of physician-administered drugs from the broken Medicare physician payment formula is a major victory for America’s seniors and their physicians.”
Consultation Change
A separate physician fee schedule proposal would eliminate payments for consultation codes in both outpatient and inpatient settings in favor of codes for evaluation and management services that carry lower reimbursement rates. Doctors would have a new way to bill for tele-health consultations, however. Leslie Flores, director of SHM’s Practice Management Institute, said the proposal has generated a high level of misinformation and misunderstanding among hospitalists who fear CMS will no longer reimburse for inpatient consultations. “It’s not that they won’t allow the work to be done anymore,” Flores says. “They’re just instructing people to bill in a different way for that work.”
CMS notes that the savings would be redistributed to increase payments for existing evaluation and management services codes, making the change cost-neutral. But Flores says a lack of details about the “crosswalk” from the old codes to the new codes, especially as they relate to transfers of care, is fueling hospitalists’ confusion over the true impact on their reimbursements.
“Our biggest concern with this consultation proposal that is now on the table is that CMS has not done anything to clarify how these transfers of care would be treated,” she says. For example, would hospitalists bill an admission code the first time they see a patient after being asked by a surgeon to take over that patient’s diabetes care? If so, “this proposal could actually result in some pretty good revenue increases for a lot of hospitalist practices that are doing a lot of surgical comanagement,” she says.
If hospitalists are instead required to bill a work-intensive transition of care under the code for a subsequent visit, which CMS has enforced since a 2006 rule clarification, Flores explains, HM could lose anywhere from 1% to 18% of reimbursements, depending on the exact code used.
Quality Reporting
The proposed fee schedule also is generating disappointment about what will not be included in Medicare’s Physician Quality Reporting Initiative (PQRI) for 2010. The pay-for-performance PQRI, which rewards physicians for reporting on quality measures by paying them an additional 2% of their estimated charges for covered services, will streamline some reporting requirements and increase the overall number of reportable measures. But several care-transition measures sought by SHM were not endorsed in time and will not be among them. Those measures include:
- Reconciled medication lists received by discharged patients;
- Transition records with specified elements received by discharged patients (including one measure for an inpatient discharge to home or self-care, and another for an ED discharge to home, ambulatory, or self-care); and
- Timely transmission of transition records.
“It was really a question of timing,” says Jill Epstein, senior advisor of SHM’s Performance and Standards Committee. The National Quality Forum, the nonprofit organization charged with signing off on all new measures, was not able to fully vet the recommended additions by the July 1 deadline, Epstein says, postponing their inclusion. “Our hope is that the measures will be included for 2011, of course.”
Separately, the PQRI proposal seeks to add an electronic-health-record-based mechanism to the list of eligible reporting methods. Although that addition is welcome news, Epstein says, SHM is expressing its concern about the shift toward patient-registry-based reporting, including a proposal to lessen or perhaps even discontinue claims-based reporting after 2010. “The issue for hospitalists, as well as for any specialty,” she says, “is that not every group will have access to a qualified PQRI registry as early as 2011,” particularly rural-based groups with fewer resources.
A similar change would streamline the E-Prescribing Incentive Program’s rules for how often e-prescribing codes must be reported. It also will offer more choices for how to report them, including through qualified registries. In the past, the program had little direct impact on hospitalists, but the new proposal recommends adding reporting codes specific to nursing homes, where some hospitalists provide care and could benefit from the incentives.
Paul Fishman, an economist at the Group Health Center for Health Studies in Seattle, says the increased focus on incentives in Medicare’s fee schedule suggests a growing realization of how such incentives can drive the delivery of healthcare services. “We know with absolute certainty that physicians make choices based on how things are reimbursed,” he says. Developing good outcome measures, then, will be critical for establishing pay-for-performance standards as part of a fee package that he says should include a blend of capitation and service-based and outcome-based reimbursements to strike a fairer balance.
“In healthcare, we’ve incented people to do more and more stuff, whether they improve outcomes or not, but we have to figure out a way to incent improvements in outcomes, while still retaining the long-term incentive to keep people healthy,” Fishman says. If better transitions of care result in a healthier population that is rehospitalized less often, for example, how can outcome-based incentives prevent hospitals from losing money in the long run? “We want to create the incentives to make the improvements in health outcomes, but we don’t want to punish the better actors because they are consistently lowering costs and also lowering reimbursement levels,” he says.
Achieving that balance in both Medicare and the broader healthcare system, he and other observers agree, is still very much a work in progress. TH
Bryn Nelson is a freelance writer based in Seattle.
It happens every year: A new Medicare physician fee schedule recommends a painful reduction in the rates the government doles out for more than 7,000 types of medical services performed by hospitalists, primary-care doctors, and other healthcare providers. And then Congress intervenes to keep the reduction from going into effect.
This year, however, the fee updates and policy proposals have become intertwined in the larger debate over comprehensive healthcare reform and a push toward what SHM calls a “value-based health delivery system.”
Few think the whopping 21.5% reduction proposed for 2010 rates, which are due to be published Nov. 1, actually will go into effect in January. A combination of other proposed changes to the fee schedule could soften any reduction. But the larger issue of how fees are currently calculated has become a rallying point for SHM and other healthcare-reform advocates who’d like to see the entire formula scrapped in favor of a more equitable distribution that focuses on outcomes. SHM has expressed concern that the current fee-for-service payment system “fails to provide providers with incentives to improve efficiency or quality of care, and encourages overutilization of services.”
—J. James Rohack, MD, AMA president
The current fee formula, known as the sustainable growth rate (SGR) and stipulated by the Balanced Budget Act of 1997, has resulted in a proposed rate cut every year since it went into effect in 2002. Congress consistently overrides the reimbursement cuts in one way or another, usually enacting modest increases or, such as in 2006 and 2007, an across-the-board freeze.
Hospitalists and other physicians have greeted another high-profile proposal with more enthusiasm: the removal of physician-administered drugs from the definition of “physician services,” thereby eliminating a high-cost item from the SGR formula. A Centers for Medicare and Medicaid Services (CMS) spokeswoman says the agency previously felt it lacked the authority to propose the removal. But a review earlier this year reversed that position, leading to the change. The removal, she says, “wouldn’t change the 21.5% percent decline in 2010, but it could have an impact in future years.”
American Medical Association President J. James Rohack, MD, joined the chorus of approval with a statement that asserted, “The removal of physician-administered drugs from the broken Medicare physician payment formula is a major victory for America’s seniors and their physicians.”
Consultation Change
A separate physician fee schedule proposal would eliminate payments for consultation codes in both outpatient and inpatient settings in favor of codes for evaluation and management services that carry lower reimbursement rates. Doctors would have a new way to bill for tele-health consultations, however. Leslie Flores, director of SHM’s Practice Management Institute, said the proposal has generated a high level of misinformation and misunderstanding among hospitalists who fear CMS will no longer reimburse for inpatient consultations. “It’s not that they won’t allow the work to be done anymore,” Flores says. “They’re just instructing people to bill in a different way for that work.”
CMS notes that the savings would be redistributed to increase payments for existing evaluation and management services codes, making the change cost-neutral. But Flores says a lack of details about the “crosswalk” from the old codes to the new codes, especially as they relate to transfers of care, is fueling hospitalists’ confusion over the true impact on their reimbursements.
“Our biggest concern with this consultation proposal that is now on the table is that CMS has not done anything to clarify how these transfers of care would be treated,” she says. For example, would hospitalists bill an admission code the first time they see a patient after being asked by a surgeon to take over that patient’s diabetes care? If so, “this proposal could actually result in some pretty good revenue increases for a lot of hospitalist practices that are doing a lot of surgical comanagement,” she says.
If hospitalists are instead required to bill a work-intensive transition of care under the code for a subsequent visit, which CMS has enforced since a 2006 rule clarification, Flores explains, HM could lose anywhere from 1% to 18% of reimbursements, depending on the exact code used.
Quality Reporting
The proposed fee schedule also is generating disappointment about what will not be included in Medicare’s Physician Quality Reporting Initiative (PQRI) for 2010. The pay-for-performance PQRI, which rewards physicians for reporting on quality measures by paying them an additional 2% of their estimated charges for covered services, will streamline some reporting requirements and increase the overall number of reportable measures. But several care-transition measures sought by SHM were not endorsed in time and will not be among them. Those measures include:
- Reconciled medication lists received by discharged patients;
- Transition records with specified elements received by discharged patients (including one measure for an inpatient discharge to home or self-care, and another for an ED discharge to home, ambulatory, or self-care); and
- Timely transmission of transition records.
“It was really a question of timing,” says Jill Epstein, senior advisor of SHM’s Performance and Standards Committee. The National Quality Forum, the nonprofit organization charged with signing off on all new measures, was not able to fully vet the recommended additions by the July 1 deadline, Epstein says, postponing their inclusion. “Our hope is that the measures will be included for 2011, of course.”
Separately, the PQRI proposal seeks to add an electronic-health-record-based mechanism to the list of eligible reporting methods. Although that addition is welcome news, Epstein says, SHM is expressing its concern about the shift toward patient-registry-based reporting, including a proposal to lessen or perhaps even discontinue claims-based reporting after 2010. “The issue for hospitalists, as well as for any specialty,” she says, “is that not every group will have access to a qualified PQRI registry as early as 2011,” particularly rural-based groups with fewer resources.
A similar change would streamline the E-Prescribing Incentive Program’s rules for how often e-prescribing codes must be reported. It also will offer more choices for how to report them, including through qualified registries. In the past, the program had little direct impact on hospitalists, but the new proposal recommends adding reporting codes specific to nursing homes, where some hospitalists provide care and could benefit from the incentives.
Paul Fishman, an economist at the Group Health Center for Health Studies in Seattle, says the increased focus on incentives in Medicare’s fee schedule suggests a growing realization of how such incentives can drive the delivery of healthcare services. “We know with absolute certainty that physicians make choices based on how things are reimbursed,” he says. Developing good outcome measures, then, will be critical for establishing pay-for-performance standards as part of a fee package that he says should include a blend of capitation and service-based and outcome-based reimbursements to strike a fairer balance.
“In healthcare, we’ve incented people to do more and more stuff, whether they improve outcomes or not, but we have to figure out a way to incent improvements in outcomes, while still retaining the long-term incentive to keep people healthy,” Fishman says. If better transitions of care result in a healthier population that is rehospitalized less often, for example, how can outcome-based incentives prevent hospitals from losing money in the long run? “We want to create the incentives to make the improvements in health outcomes, but we don’t want to punish the better actors because they are consistently lowering costs and also lowering reimbursement levels,” he says.
Achieving that balance in both Medicare and the broader healthcare system, he and other observers agree, is still very much a work in progress. TH
Bryn Nelson is a freelance writer based in Seattle.
Physician Engagement
There is a lot of talk in HM circles right now about quantifying such nebulous medical phrases as “meaningful use” and “healthcare reform.” A phrase heard less often, but potentially just as critical to HM’s future, is “physician engagement.”
A prod to help define the latter comes from Kelly Caverzagie, MD, an academic hospitalist in the division of hospital medicine at Henry Ford Hospital in Detroit. Dr. Caverzagie and staff from the American Board of Internal Medicine (ABIM) set out to attach some sort of metric quantification to that engagement, then determine what impact it had on QI programs. Hence the aptly named study to be published in the October edition of the Journal of Hospital Medicine: “The Role of Physician Engagement on the Impact of the Hospital Based Practice Improvement Module.”
Before joining Henry Ford in 2007 as a hospitalist and evaluation research specialist, Dr. Caverzagie had worked as a contractor with ABIM, completing a two-year fellowship at the University of Pennsylvania in Philadelphia. He remains under contract with ABIM, which still pays a “small portion” of his salary. That relationship and Dr. Caverzagie’s hospitalist-tinged view of evaluation techniques piqued his interest in the engagement issue.
“The physician needs to engage in the process,” Dr. Caverzagie says, defining that engagement as “active enrollment and doing it for the right reason. Just enrolling in it doesn’t make quality improvement happen. You actually need to be engaged. And then … there is added value.”
The study focused on 21 physicians who completed their Maintenance of Certification (MOC) to remain current with ABIM guidelines. The hospital-based practice improvement module (PIM) is a Web-based platform developed by ABIM that “allows physicians to use nationally-approved, hospital-level performance data to complete the module” as part of attaining their MOC.
Each of the doctors in Dr. Caverzagie’s study completed their PIM by January 2007 and were interviewed anonymously about their experience. Interviews were recorded and transcribed to better verify responses. Nearly all of the subjects found the PIM useful (n=17, 81%). But with more questioning, the authors determined that how valuable the module was viewed depended on how involved the physician was in its completion.

—Kelly Caverzagie, MD, academic hospitalist, Henry Ford Hospital, Detroit
“The impact of completing the hospital PIM is mediated by the degree of physician engagement with the QI process,” the authors conclude. “Physicians who become engaged with the hospital PIM and QI process may be more likely to report successful experiences … than those who do not become engaged.”
Dr. Caverzagie and three ABIM staffers understood the limits of their effort, which breaks little new ground but piles on further evidence to prove the efficacy of getting hospitalists and other physicians more engaged in QI. A sample size of fewer than two dozen anonymous physicians allows for too many variables to consider the data indefatigable, so Dr. Caverzagie leaves it up to such regulatory and advocacy bodies as ABIM and SHM to determine whether and how to make systemic and process changes that encourage more involvement. “I don’t know if you can force engagement,” he adds.
His team created a set of definitions to showcase how different physicians with different attitudes experience hospital PIMs differently.
The most successful category is defined as “active engagers,” physicians who exhibited personal involvement. Eight of the 21 physicians, or 38% of the sample population, fell into this grouping. Ten physicians (48%) fell under the heading of “passive engagers,” a somewhat ironic category in which physicians reported negative experiences even as they documented what they felt was the knowledge gained from their hospital PIM. Finally, the authors tagged three (14%) “non-engagers” who “documented no evidence of QI learning and reported little impact from completing the PIM.”
Correspondingly, case studies highlighted in the study showed that “active engagers” took advantage of existing QI resources and staff at their respective institutions. They sought out staff leadership and fed off positive hospital cultures where they existed. One physician said it was “surprisingly easier to begin and initiate a quality improvement project than thought.”
One “passive engager” described their previous QI experience in terms of mandates handed down from administration, although several in the subcategory acknowledged they learned new skills or new information about how QI programs operate in their hospital. There also was some dissatisfaction in this category about the leadership shown by institutional staff.
Still, Dr. Caverzagie expresses optimism with this middle grouping, the largest statistically. “QI learning occurred despite the presence of multiple barriers,” the authors wrote.
In the least successful category—the non-engagers—several physicians interviewed said they didn’t need QI projects or were unsatisfied with a past experience, so they didn’t bother to try again. One physician declared, “We’re at a terrific level right now,” despite a hospital baseline performance measure of 5% compliance for percutaneous coronary intervention in less than 120 minutes.
To be sure, all of the groupings were at the mercy of internal and external factors—hospital culture, perceived relevance, institutional bias, and access to QI leaders among them. What remains to be studied is how to overcome those hurdles. Dr. Caverzagie says more work is needed to determine just how effective PIMs can be. He thinks the next stage for the modules could include more quantifiable metrics, which would be reported and then analyzed to “take doctors to the next level.”
“The vast majority of physicians, hospitalists in particular, are very interested in improving the care that they provide for their patients,” Dr. Caverzagie says. “They’re just not necessarily sure how to get it done. A challenge for our profession is to try to find a way to facilitate becoming involved in activities.”TH
Richard Quinn is a freelance writer based in New Jersey.
There is a lot of talk in HM circles right now about quantifying such nebulous medical phrases as “meaningful use” and “healthcare reform.” A phrase heard less often, but potentially just as critical to HM’s future, is “physician engagement.”
A prod to help define the latter comes from Kelly Caverzagie, MD, an academic hospitalist in the division of hospital medicine at Henry Ford Hospital in Detroit. Dr. Caverzagie and staff from the American Board of Internal Medicine (ABIM) set out to attach some sort of metric quantification to that engagement, then determine what impact it had on QI programs. Hence the aptly named study to be published in the October edition of the Journal of Hospital Medicine: “The Role of Physician Engagement on the Impact of the Hospital Based Practice Improvement Module.”
Before joining Henry Ford in 2007 as a hospitalist and evaluation research specialist, Dr. Caverzagie had worked as a contractor with ABIM, completing a two-year fellowship at the University of Pennsylvania in Philadelphia. He remains under contract with ABIM, which still pays a “small portion” of his salary. That relationship and Dr. Caverzagie’s hospitalist-tinged view of evaluation techniques piqued his interest in the engagement issue.
“The physician needs to engage in the process,” Dr. Caverzagie says, defining that engagement as “active enrollment and doing it for the right reason. Just enrolling in it doesn’t make quality improvement happen. You actually need to be engaged. And then … there is added value.”
The study focused on 21 physicians who completed their Maintenance of Certification (MOC) to remain current with ABIM guidelines. The hospital-based practice improvement module (PIM) is a Web-based platform developed by ABIM that “allows physicians to use nationally-approved, hospital-level performance data to complete the module” as part of attaining their MOC.
Each of the doctors in Dr. Caverzagie’s study completed their PIM by January 2007 and were interviewed anonymously about their experience. Interviews were recorded and transcribed to better verify responses. Nearly all of the subjects found the PIM useful (n=17, 81%). But with more questioning, the authors determined that how valuable the module was viewed depended on how involved the physician was in its completion.

—Kelly Caverzagie, MD, academic hospitalist, Henry Ford Hospital, Detroit
“The impact of completing the hospital PIM is mediated by the degree of physician engagement with the QI process,” the authors conclude. “Physicians who become engaged with the hospital PIM and QI process may be more likely to report successful experiences … than those who do not become engaged.”
Dr. Caverzagie and three ABIM staffers understood the limits of their effort, which breaks little new ground but piles on further evidence to prove the efficacy of getting hospitalists and other physicians more engaged in QI. A sample size of fewer than two dozen anonymous physicians allows for too many variables to consider the data indefatigable, so Dr. Caverzagie leaves it up to such regulatory and advocacy bodies as ABIM and SHM to determine whether and how to make systemic and process changes that encourage more involvement. “I don’t know if you can force engagement,” he adds.
His team created a set of definitions to showcase how different physicians with different attitudes experience hospital PIMs differently.
The most successful category is defined as “active engagers,” physicians who exhibited personal involvement. Eight of the 21 physicians, or 38% of the sample population, fell into this grouping. Ten physicians (48%) fell under the heading of “passive engagers,” a somewhat ironic category in which physicians reported negative experiences even as they documented what they felt was the knowledge gained from their hospital PIM. Finally, the authors tagged three (14%) “non-engagers” who “documented no evidence of QI learning and reported little impact from completing the PIM.”
Correspondingly, case studies highlighted in the study showed that “active engagers” took advantage of existing QI resources and staff at their respective institutions. They sought out staff leadership and fed off positive hospital cultures where they existed. One physician said it was “surprisingly easier to begin and initiate a quality improvement project than thought.”
One “passive engager” described their previous QI experience in terms of mandates handed down from administration, although several in the subcategory acknowledged they learned new skills or new information about how QI programs operate in their hospital. There also was some dissatisfaction in this category about the leadership shown by institutional staff.
Still, Dr. Caverzagie expresses optimism with this middle grouping, the largest statistically. “QI learning occurred despite the presence of multiple barriers,” the authors wrote.
In the least successful category—the non-engagers—several physicians interviewed said they didn’t need QI projects or were unsatisfied with a past experience, so they didn’t bother to try again. One physician declared, “We’re at a terrific level right now,” despite a hospital baseline performance measure of 5% compliance for percutaneous coronary intervention in less than 120 minutes.
To be sure, all of the groupings were at the mercy of internal and external factors—hospital culture, perceived relevance, institutional bias, and access to QI leaders among them. What remains to be studied is how to overcome those hurdles. Dr. Caverzagie says more work is needed to determine just how effective PIMs can be. He thinks the next stage for the modules could include more quantifiable metrics, which would be reported and then analyzed to “take doctors to the next level.”
“The vast majority of physicians, hospitalists in particular, are very interested in improving the care that they provide for their patients,” Dr. Caverzagie says. “They’re just not necessarily sure how to get it done. A challenge for our profession is to try to find a way to facilitate becoming involved in activities.”TH
Richard Quinn is a freelance writer based in New Jersey.
There is a lot of talk in HM circles right now about quantifying such nebulous medical phrases as “meaningful use” and “healthcare reform.” A phrase heard less often, but potentially just as critical to HM’s future, is “physician engagement.”
A prod to help define the latter comes from Kelly Caverzagie, MD, an academic hospitalist in the division of hospital medicine at Henry Ford Hospital in Detroit. Dr. Caverzagie and staff from the American Board of Internal Medicine (ABIM) set out to attach some sort of metric quantification to that engagement, then determine what impact it had on QI programs. Hence the aptly named study to be published in the October edition of the Journal of Hospital Medicine: “The Role of Physician Engagement on the Impact of the Hospital Based Practice Improvement Module.”
Before joining Henry Ford in 2007 as a hospitalist and evaluation research specialist, Dr. Caverzagie had worked as a contractor with ABIM, completing a two-year fellowship at the University of Pennsylvania in Philadelphia. He remains under contract with ABIM, which still pays a “small portion” of his salary. That relationship and Dr. Caverzagie’s hospitalist-tinged view of evaluation techniques piqued his interest in the engagement issue.
“The physician needs to engage in the process,” Dr. Caverzagie says, defining that engagement as “active enrollment and doing it for the right reason. Just enrolling in it doesn’t make quality improvement happen. You actually need to be engaged. And then … there is added value.”
The study focused on 21 physicians who completed their Maintenance of Certification (MOC) to remain current with ABIM guidelines. The hospital-based practice improvement module (PIM) is a Web-based platform developed by ABIM that “allows physicians to use nationally-approved, hospital-level performance data to complete the module” as part of attaining their MOC.
Each of the doctors in Dr. Caverzagie’s study completed their PIM by January 2007 and were interviewed anonymously about their experience. Interviews were recorded and transcribed to better verify responses. Nearly all of the subjects found the PIM useful (n=17, 81%). But with more questioning, the authors determined that how valuable the module was viewed depended on how involved the physician was in its completion.

—Kelly Caverzagie, MD, academic hospitalist, Henry Ford Hospital, Detroit
“The impact of completing the hospital PIM is mediated by the degree of physician engagement with the QI process,” the authors conclude. “Physicians who become engaged with the hospital PIM and QI process may be more likely to report successful experiences … than those who do not become engaged.”
Dr. Caverzagie and three ABIM staffers understood the limits of their effort, which breaks little new ground but piles on further evidence to prove the efficacy of getting hospitalists and other physicians more engaged in QI. A sample size of fewer than two dozen anonymous physicians allows for too many variables to consider the data indefatigable, so Dr. Caverzagie leaves it up to such regulatory and advocacy bodies as ABIM and SHM to determine whether and how to make systemic and process changes that encourage more involvement. “I don’t know if you can force engagement,” he adds.
His team created a set of definitions to showcase how different physicians with different attitudes experience hospital PIMs differently.
The most successful category is defined as “active engagers,” physicians who exhibited personal involvement. Eight of the 21 physicians, or 38% of the sample population, fell into this grouping. Ten physicians (48%) fell under the heading of “passive engagers,” a somewhat ironic category in which physicians reported negative experiences even as they documented what they felt was the knowledge gained from their hospital PIM. Finally, the authors tagged three (14%) “non-engagers” who “documented no evidence of QI learning and reported little impact from completing the PIM.”
Correspondingly, case studies highlighted in the study showed that “active engagers” took advantage of existing QI resources and staff at their respective institutions. They sought out staff leadership and fed off positive hospital cultures where they existed. One physician said it was “surprisingly easier to begin and initiate a quality improvement project than thought.”
One “passive engager” described their previous QI experience in terms of mandates handed down from administration, although several in the subcategory acknowledged they learned new skills or new information about how QI programs operate in their hospital. There also was some dissatisfaction in this category about the leadership shown by institutional staff.
Still, Dr. Caverzagie expresses optimism with this middle grouping, the largest statistically. “QI learning occurred despite the presence of multiple barriers,” the authors wrote.
In the least successful category—the non-engagers—several physicians interviewed said they didn’t need QI projects or were unsatisfied with a past experience, so they didn’t bother to try again. One physician declared, “We’re at a terrific level right now,” despite a hospital baseline performance measure of 5% compliance for percutaneous coronary intervention in less than 120 minutes.
To be sure, all of the groupings were at the mercy of internal and external factors—hospital culture, perceived relevance, institutional bias, and access to QI leaders among them. What remains to be studied is how to overcome those hurdles. Dr. Caverzagie says more work is needed to determine just how effective PIMs can be. He thinks the next stage for the modules could include more quantifiable metrics, which would be reported and then analyzed to “take doctors to the next level.”
“The vast majority of physicians, hospitalists in particular, are very interested in improving the care that they provide for their patients,” Dr. Caverzagie says. “They’re just not necessarily sure how to get it done. A challenge for our profession is to try to find a way to facilitate becoming involved in activities.”TH
Richard Quinn is a freelance writer based in New Jersey.
Market Watch
In the Pipeline
- A new drug application (NDA) has been submitted to the Food and Drug Administration (FDA) for Acetavance, an intravenous (IV) form of acetaminophen used in the treatment of acute pain and fever in adults and children who cannot take oral medications.1
- An NDA for a low-dose aspirin and esomeprazole combination product has been submitted for risk reduction of low-dose-aspirin-associated gastric and/or duodenal ulcers. A supplemental NDA was submitted for esomeprazole for risk reduction of low-dose-aspirin-associated peptic ulcer disease.2
- An NDA for a new formulation of ondansetron, as an orally dissolving film strip, has been submitted to the FDA.3 The product has shown comparable bioequivalence to ondansetron (Zofran) as an anti-emetic for preventing chemotherapy-induced nausea and vomiting, nausea and vomiting associated with radiotherapy, and post-operative nausea and vomiting.
- An NDA for tesamorelin to treat HIV-lipodystrophy has been submitted to the FDA.4
New Generics
- Acarbose 25-, 50- and 100-mg tablets (generic precose)5
- Bicalutamide (Casodex)6
New Drugs, Indications and Dosage Forms
- Artemether and lumefantrine (Coartem) tablets have been approved by the FDA for the treatment of acute, uncomplicated malarial infections in adults and children who weigh at least 5 kg.7 Coartem should be taken with food, particularly food that contains fat, because it improves drug absorption. Common adverse effects include headache, anorexia, dizziness, asthenia, arthralgia, and myalgia.
- Besifloxacin 0.6% (Besivance) ophthalmic suspension has been approved by the FDA to treat bacterial conjunctivitis. Adverse effects include eye redness, blurred vision, eye pain, irritation, itching, and headache.8
- Dexamethasone intravitreal implant (Ozurdex) has been approved by the FDA for treating macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO).9 The implant is biodegradable and delivers dexamethasone via an extended-release mechanism over one to three months. Ozurdex is administered as an in-office procedure and is expected to be available soon.
- Dronedarone (Multaq) tablets have been approved by the FDA as an antiarrhythmic agent.10 It is indicated to reduce the risk of cardiovascular hospitalization in patients with atrial flutter, or paroxysmal or persistent atrial fibrillation, with a recent episode of atrial fibrillation/atrial flutter and associated cardiovascular risk, who are in sinus rhythm or who will be cardioverted.11 Risk factors include age >70, hypertension, diabetes, prior cerebrovascular accident, a left atrial diameter ≥50 mm, and left ventricular ejection fraction [LVEF] <40%.
- Hyoscyamine sulfate (NuLev) chewable antispasmodic orally disintegrating tablets (0.125 mg) have been reintroduced and are available to treat patients age 2 and older with gastrointestinal spasticity disorders.12
- Lacosamide (Vimpat) has been approved by the FDA for add-on therapy in the treatment of partial-onset seizures in epilepsy patients age 17 and older.13
- Lamotrigine extended-release (Lamictal XR) has been approved by the FDA as a once-daily add-on therapy for partial-onset seizures in epilepsy patients age 13 and older.14
- Pioglitazone/metformin (ACTOplus met XR) combination tablets have been approved by the FDA for treating Type 2 diabetes.15 It should be available later this year.
- Risperidone (Risperdal Consta) has received additional FDA approval as a biweekly injection as monotherapy, and as adjunctive therapy to lithium or valproate, in the maintenance of bipolar I disorder.16
- Tadalafil (Adcirc) has been approved by the FDA for improving exercise ability in patients with pulmonary arterial hypertension (PAH).17 Tadalafil already is approved as brand-name Cialis for treating erectile dysfunction. Tadalafil is the first once-daily phosphodiesterase type-5 inhibitor for treating PAH, and it is hoped it will gain some of the sildenafil (Revatio) market.
Update
As a followup to the August “Market Watch,” pancrelipase (Creon) has been approved by the FDA and is available in three FDA-approved prescription strengths based on the lipase content (6,000, 12,000, and 24,000).18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
- NDA submitted for acetavance, a treatment for acute pain and fever. Monthly Prescribing Reference Web site. Available at: www.empr.com/NDA-submitted-for-Acetavance-a-treatment-for-acute-pain-and-fever/article/136799/. Accessed July 8, 2009.
- AstraZeneca submits NDA for low dose aspirin/esomeprazole combination product. AstraZeneca Web site. Available at: www.astrazeneca-us.com/about-astrazeneca-us/newsroom/all/5762542?itemId=5762542. Accessed July 8, 2009.
- MonoSol Rx and Strativa Pharmaceuticals submit new drug application for ondansetron orally dissolving film strip. Available at: www.monosolrx.com/news_09/news_040909.html. Accessed July 8, 2009.
- Theratech shares jump 8 pct on FDA filing. Reuters Web site. Available at: www.reuters.com/article/idUSN0126728920090601. Accessed July 8, 2009.
- Impax receives FDA approval for generic precos tablets, 25 mg, 50 mg and 100 mg. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=67240&p=irol-newsArticle&ID=1289907&highlight=. Accessed July 8, 2009.
- Caraco: FDA approves generic prostate cancer drug. Forbes Web site. Available at: www.forbes.com/feeds/ap/2009/07/07/ap6626628.html. Accessed July 8, 2009.
- Walsh S. FDA approved coartem tablets to treat malaria. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149559.htm. Accessed July 8, 2009.
- Walsh S. FDA approves besivance to treat bacterial conjunctivitis. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm162486.htm. Accessed July 8, 2009.
- Ozurdex approved for treatment of macular edema. Monthly Prescribing Reference Web site. Available at: www.empr.com/Ozurdex-approved-for-treatment-of-macular-edema/article/138708/. Accessed July 8, 2009.
- Walsh S. FDA approves multaq to treat heart rhythm disorder. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170276.htm. Accessed July 21, 2009.
- Multaq label. FDA Web site: Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/022425lbl.pdf. Accessed July 21, 2009.
- NuLev chewable melt tablets available again. Monthly Prescribing Reference Web site. Available at: www.empr.com/NuLev-Chewable-Melt-Tablets-available-again/article/138396/?DCMP=EMC-MPR_WeeklyNewsbrief. Accessed July 8, 2009.
- UCB’s Vimpat (lacosamide) approved by FDA as add-on therapy for partial onset seizures in adults. Epilepsy Foundation Web site. Available at: www.epilepsyfoundation.org/epilepsyusa/news/UCB_Vimpat.cfm. Accessed July 8, 2009.
- Bratulic A. FDA approves GlaxoSmithKline’s lamictal XR. Available at: www.firstwordplus.com/Fws.do?articleid=62DD8DEA3F594BDC98DA97F80AB13ABB&logRowId=307825. Accessed July 8, 2009.
- FDA approves ACTOplus met XR (pioglitazone HCL and metformin HCl extended-release) tablets for the treatment of Type 2 diabetes. Available at: www.pipelinereview.com/index.php/2009051326959/Small-Molecules/FDA-Approves-ACTOplus-met-XR-pioglitazone-HCl-and-metformin-HCl-extended-release-Tablets-for-the-Treatment-of-Type-2-Diabetes.html. Accessed July 8, 2009.
- FDA approval letter for risperdal consta. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/021346s025,021346s028ltr.pdf. Accessed July 8, 2009.
- Todoruk M. United Therapeutics’ Adcirca approved for pulmonary arterial hypertension in US. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=E71E91953E9844D2A776B021E19AEE22&logRowId=306627. Accessed July 8, 2009.
- Creon delayed-release capsules available for exocrine pancreatic insufficiency. Monthly Prescribing Reference Web site. Available at: www.empr.com/Creon-delayed-release-capsules-available-for-exocrine-pancreatic-insufficiency/article/139668/. Accessed August 10, 2009.
In the Pipeline
- A new drug application (NDA) has been submitted to the Food and Drug Administration (FDA) for Acetavance, an intravenous (IV) form of acetaminophen used in the treatment of acute pain and fever in adults and children who cannot take oral medications.1
- An NDA for a low-dose aspirin and esomeprazole combination product has been submitted for risk reduction of low-dose-aspirin-associated gastric and/or duodenal ulcers. A supplemental NDA was submitted for esomeprazole for risk reduction of low-dose-aspirin-associated peptic ulcer disease.2
- An NDA for a new formulation of ondansetron, as an orally dissolving film strip, has been submitted to the FDA.3 The product has shown comparable bioequivalence to ondansetron (Zofran) as an anti-emetic for preventing chemotherapy-induced nausea and vomiting, nausea and vomiting associated with radiotherapy, and post-operative nausea and vomiting.
- An NDA for tesamorelin to treat HIV-lipodystrophy has been submitted to the FDA.4
New Generics
- Acarbose 25-, 50- and 100-mg tablets (generic precose)5
- Bicalutamide (Casodex)6
New Drugs, Indications and Dosage Forms
- Artemether and lumefantrine (Coartem) tablets have been approved by the FDA for the treatment of acute, uncomplicated malarial infections in adults and children who weigh at least 5 kg.7 Coartem should be taken with food, particularly food that contains fat, because it improves drug absorption. Common adverse effects include headache, anorexia, dizziness, asthenia, arthralgia, and myalgia.
- Besifloxacin 0.6% (Besivance) ophthalmic suspension has been approved by the FDA to treat bacterial conjunctivitis. Adverse effects include eye redness, blurred vision, eye pain, irritation, itching, and headache.8
- Dexamethasone intravitreal implant (Ozurdex) has been approved by the FDA for treating macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO).9 The implant is biodegradable and delivers dexamethasone via an extended-release mechanism over one to three months. Ozurdex is administered as an in-office procedure and is expected to be available soon.
- Dronedarone (Multaq) tablets have been approved by the FDA as an antiarrhythmic agent.10 It is indicated to reduce the risk of cardiovascular hospitalization in patients with atrial flutter, or paroxysmal or persistent atrial fibrillation, with a recent episode of atrial fibrillation/atrial flutter and associated cardiovascular risk, who are in sinus rhythm or who will be cardioverted.11 Risk factors include age >70, hypertension, diabetes, prior cerebrovascular accident, a left atrial diameter ≥50 mm, and left ventricular ejection fraction [LVEF] <40%.
- Hyoscyamine sulfate (NuLev) chewable antispasmodic orally disintegrating tablets (0.125 mg) have been reintroduced and are available to treat patients age 2 and older with gastrointestinal spasticity disorders.12
- Lacosamide (Vimpat) has been approved by the FDA for add-on therapy in the treatment of partial-onset seizures in epilepsy patients age 17 and older.13
- Lamotrigine extended-release (Lamictal XR) has been approved by the FDA as a once-daily add-on therapy for partial-onset seizures in epilepsy patients age 13 and older.14
- Pioglitazone/metformin (ACTOplus met XR) combination tablets have been approved by the FDA for treating Type 2 diabetes.15 It should be available later this year.
- Risperidone (Risperdal Consta) has received additional FDA approval as a biweekly injection as monotherapy, and as adjunctive therapy to lithium or valproate, in the maintenance of bipolar I disorder.16
- Tadalafil (Adcirc) has been approved by the FDA for improving exercise ability in patients with pulmonary arterial hypertension (PAH).17 Tadalafil already is approved as brand-name Cialis for treating erectile dysfunction. Tadalafil is the first once-daily phosphodiesterase type-5 inhibitor for treating PAH, and it is hoped it will gain some of the sildenafil (Revatio) market.
Update
As a followup to the August “Market Watch,” pancrelipase (Creon) has been approved by the FDA and is available in three FDA-approved prescription strengths based on the lipase content (6,000, 12,000, and 24,000).18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
- NDA submitted for acetavance, a treatment for acute pain and fever. Monthly Prescribing Reference Web site. Available at: www.empr.com/NDA-submitted-for-Acetavance-a-treatment-for-acute-pain-and-fever/article/136799/. Accessed July 8, 2009.
- AstraZeneca submits NDA for low dose aspirin/esomeprazole combination product. AstraZeneca Web site. Available at: www.astrazeneca-us.com/about-astrazeneca-us/newsroom/all/5762542?itemId=5762542. Accessed July 8, 2009.
- MonoSol Rx and Strativa Pharmaceuticals submit new drug application for ondansetron orally dissolving film strip. Available at: www.monosolrx.com/news_09/news_040909.html. Accessed July 8, 2009.
- Theratech shares jump 8 pct on FDA filing. Reuters Web site. Available at: www.reuters.com/article/idUSN0126728920090601. Accessed July 8, 2009.
- Impax receives FDA approval for generic precos tablets, 25 mg, 50 mg and 100 mg. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=67240&p=irol-newsArticle&ID=1289907&highlight=. Accessed July 8, 2009.
- Caraco: FDA approves generic prostate cancer drug. Forbes Web site. Available at: www.forbes.com/feeds/ap/2009/07/07/ap6626628.html. Accessed July 8, 2009.
- Walsh S. FDA approved coartem tablets to treat malaria. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149559.htm. Accessed July 8, 2009.
- Walsh S. FDA approves besivance to treat bacterial conjunctivitis. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm162486.htm. Accessed July 8, 2009.
- Ozurdex approved for treatment of macular edema. Monthly Prescribing Reference Web site. Available at: www.empr.com/Ozurdex-approved-for-treatment-of-macular-edema/article/138708/. Accessed July 8, 2009.
- Walsh S. FDA approves multaq to treat heart rhythm disorder. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170276.htm. Accessed July 21, 2009.
- Multaq label. FDA Web site: Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/022425lbl.pdf. Accessed July 21, 2009.
- NuLev chewable melt tablets available again. Monthly Prescribing Reference Web site. Available at: www.empr.com/NuLev-Chewable-Melt-Tablets-available-again/article/138396/?DCMP=EMC-MPR_WeeklyNewsbrief. Accessed July 8, 2009.
- UCB’s Vimpat (lacosamide) approved by FDA as add-on therapy for partial onset seizures in adults. Epilepsy Foundation Web site. Available at: www.epilepsyfoundation.org/epilepsyusa/news/UCB_Vimpat.cfm. Accessed July 8, 2009.
- Bratulic A. FDA approves GlaxoSmithKline’s lamictal XR. Available at: www.firstwordplus.com/Fws.do?articleid=62DD8DEA3F594BDC98DA97F80AB13ABB&logRowId=307825. Accessed July 8, 2009.
- FDA approves ACTOplus met XR (pioglitazone HCL and metformin HCl extended-release) tablets for the treatment of Type 2 diabetes. Available at: www.pipelinereview.com/index.php/2009051326959/Small-Molecules/FDA-Approves-ACTOplus-met-XR-pioglitazone-HCl-and-metformin-HCl-extended-release-Tablets-for-the-Treatment-of-Type-2-Diabetes.html. Accessed July 8, 2009.
- FDA approval letter for risperdal consta. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/021346s025,021346s028ltr.pdf. Accessed July 8, 2009.
- Todoruk M. United Therapeutics’ Adcirca approved for pulmonary arterial hypertension in US. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=E71E91953E9844D2A776B021E19AEE22&logRowId=306627. Accessed July 8, 2009.
- Creon delayed-release capsules available for exocrine pancreatic insufficiency. Monthly Prescribing Reference Web site. Available at: www.empr.com/Creon-delayed-release-capsules-available-for-exocrine-pancreatic-insufficiency/article/139668/. Accessed August 10, 2009.
In the Pipeline
- A new drug application (NDA) has been submitted to the Food and Drug Administration (FDA) for Acetavance, an intravenous (IV) form of acetaminophen used in the treatment of acute pain and fever in adults and children who cannot take oral medications.1
- An NDA for a low-dose aspirin and esomeprazole combination product has been submitted for risk reduction of low-dose-aspirin-associated gastric and/or duodenal ulcers. A supplemental NDA was submitted for esomeprazole for risk reduction of low-dose-aspirin-associated peptic ulcer disease.2
- An NDA for a new formulation of ondansetron, as an orally dissolving film strip, has been submitted to the FDA.3 The product has shown comparable bioequivalence to ondansetron (Zofran) as an anti-emetic for preventing chemotherapy-induced nausea and vomiting, nausea and vomiting associated with radiotherapy, and post-operative nausea and vomiting.
- An NDA for tesamorelin to treat HIV-lipodystrophy has been submitted to the FDA.4
New Generics
- Acarbose 25-, 50- and 100-mg tablets (generic precose)5
- Bicalutamide (Casodex)6
New Drugs, Indications and Dosage Forms
- Artemether and lumefantrine (Coartem) tablets have been approved by the FDA for the treatment of acute, uncomplicated malarial infections in adults and children who weigh at least 5 kg.7 Coartem should be taken with food, particularly food that contains fat, because it improves drug absorption. Common adverse effects include headache, anorexia, dizziness, asthenia, arthralgia, and myalgia.
- Besifloxacin 0.6% (Besivance) ophthalmic suspension has been approved by the FDA to treat bacterial conjunctivitis. Adverse effects include eye redness, blurred vision, eye pain, irritation, itching, and headache.8
- Dexamethasone intravitreal implant (Ozurdex) has been approved by the FDA for treating macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO).9 The implant is biodegradable and delivers dexamethasone via an extended-release mechanism over one to three months. Ozurdex is administered as an in-office procedure and is expected to be available soon.
- Dronedarone (Multaq) tablets have been approved by the FDA as an antiarrhythmic agent.10 It is indicated to reduce the risk of cardiovascular hospitalization in patients with atrial flutter, or paroxysmal or persistent atrial fibrillation, with a recent episode of atrial fibrillation/atrial flutter and associated cardiovascular risk, who are in sinus rhythm or who will be cardioverted.11 Risk factors include age >70, hypertension, diabetes, prior cerebrovascular accident, a left atrial diameter ≥50 mm, and left ventricular ejection fraction [LVEF] <40%.
- Hyoscyamine sulfate (NuLev) chewable antispasmodic orally disintegrating tablets (0.125 mg) have been reintroduced and are available to treat patients age 2 and older with gastrointestinal spasticity disorders.12
- Lacosamide (Vimpat) has been approved by the FDA for add-on therapy in the treatment of partial-onset seizures in epilepsy patients age 17 and older.13
- Lamotrigine extended-release (Lamictal XR) has been approved by the FDA as a once-daily add-on therapy for partial-onset seizures in epilepsy patients age 13 and older.14
- Pioglitazone/metformin (ACTOplus met XR) combination tablets have been approved by the FDA for treating Type 2 diabetes.15 It should be available later this year.
- Risperidone (Risperdal Consta) has received additional FDA approval as a biweekly injection as monotherapy, and as adjunctive therapy to lithium or valproate, in the maintenance of bipolar I disorder.16
- Tadalafil (Adcirc) has been approved by the FDA for improving exercise ability in patients with pulmonary arterial hypertension (PAH).17 Tadalafil already is approved as brand-name Cialis for treating erectile dysfunction. Tadalafil is the first once-daily phosphodiesterase type-5 inhibitor for treating PAH, and it is hoped it will gain some of the sildenafil (Revatio) market.
Update
As a followup to the August “Market Watch,” pancrelipase (Creon) has been approved by the FDA and is available in three FDA-approved prescription strengths based on the lipase content (6,000, 12,000, and 24,000).18 TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
- NDA submitted for acetavance, a treatment for acute pain and fever. Monthly Prescribing Reference Web site. Available at: www.empr.com/NDA-submitted-for-Acetavance-a-treatment-for-acute-pain-and-fever/article/136799/. Accessed July 8, 2009.
- AstraZeneca submits NDA for low dose aspirin/esomeprazole combination product. AstraZeneca Web site. Available at: www.astrazeneca-us.com/about-astrazeneca-us/newsroom/all/5762542?itemId=5762542. Accessed July 8, 2009.
- MonoSol Rx and Strativa Pharmaceuticals submit new drug application for ondansetron orally dissolving film strip. Available at: www.monosolrx.com/news_09/news_040909.html. Accessed July 8, 2009.
- Theratech shares jump 8 pct on FDA filing. Reuters Web site. Available at: www.reuters.com/article/idUSN0126728920090601. Accessed July 8, 2009.
- Impax receives FDA approval for generic precos tablets, 25 mg, 50 mg and 100 mg. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=67240&p=irol-newsArticle&ID=1289907&highlight=. Accessed July 8, 2009.
- Caraco: FDA approves generic prostate cancer drug. Forbes Web site. Available at: www.forbes.com/feeds/ap/2009/07/07/ap6626628.html. Accessed July 8, 2009.
- Walsh S. FDA approved coartem tablets to treat malaria. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149559.htm. Accessed July 8, 2009.
- Walsh S. FDA approves besivance to treat bacterial conjunctivitis. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm162486.htm. Accessed July 8, 2009.
- Ozurdex approved for treatment of macular edema. Monthly Prescribing Reference Web site. Available at: www.empr.com/Ozurdex-approved-for-treatment-of-macular-edema/article/138708/. Accessed July 8, 2009.
- Walsh S. FDA approves multaq to treat heart rhythm disorder. FDA Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170276.htm. Accessed July 21, 2009.
- Multaq label. FDA Web site: Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/022425lbl.pdf. Accessed July 21, 2009.
- NuLev chewable melt tablets available again. Monthly Prescribing Reference Web site. Available at: www.empr.com/NuLev-Chewable-Melt-Tablets-available-again/article/138396/?DCMP=EMC-MPR_WeeklyNewsbrief. Accessed July 8, 2009.
- UCB’s Vimpat (lacosamide) approved by FDA as add-on therapy for partial onset seizures in adults. Epilepsy Foundation Web site. Available at: www.epilepsyfoundation.org/epilepsyusa/news/UCB_Vimpat.cfm. Accessed July 8, 2009.
- Bratulic A. FDA approves GlaxoSmithKline’s lamictal XR. Available at: www.firstwordplus.com/Fws.do?articleid=62DD8DEA3F594BDC98DA97F80AB13ABB&logRowId=307825. Accessed July 8, 2009.
- FDA approves ACTOplus met XR (pioglitazone HCL and metformin HCl extended-release) tablets for the treatment of Type 2 diabetes. Available at: www.pipelinereview.com/index.php/2009051326959/Small-Molecules/FDA-Approves-ACTOplus-met-XR-pioglitazone-HCl-and-metformin-HCl-extended-release-Tablets-for-the-Treatment-of-Type-2-Diabetes.html. Accessed July 8, 2009.
- FDA approval letter for risperdal consta. FDA Web site. Available at: www.accessdata.fda.gov/drugsatfda_docs/appletter/2009/021346s025,021346s028ltr.pdf. Accessed July 8, 2009.
- Todoruk M. United Therapeutics’ Adcirca approved for pulmonary arterial hypertension in US. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=E71E91953E9844D2A776B021E19AEE22&logRowId=306627. Accessed July 8, 2009.
- Creon delayed-release capsules available for exocrine pancreatic insufficiency. Monthly Prescribing Reference Web site. Available at: www.empr.com/Creon-delayed-release-capsules-available-for-exocrine-pancreatic-insufficiency/article/139668/. Accessed August 10, 2009.