User login
Therapeutic hypothermia for newborns who suffer hypoxic–ischemic birth injury
CASE: Risky decision to let labor continue
The baby was born blue and limp, after a long labor complicated by maternal fever and a prolonged fetal heart rate deceleration.
Earlier, just before the mother was raced to the OR for emergency cesarean delivery, the fetal heart rate had increased sufficiently for the OB to decide to permit labor to continue— the goal being rapid vaginal delivery.
In hindsight, that decision appears to have been potentially fateful for this newborn: Apgar scores were 1 at 5 minutes and 3 at 10 minutes. Umbilical artery pH was 6.98; base deficit, –13 mmol/L. The pediatricians made a preliminary diagnosis of hypoxic-ischemic injury and recommended whole-body cooling of the newborn to limit neural damage.
The frightened parents prayed the treatment would work. Later, recalling news reports that they had read about spinal cord-injured football players and survivors of cardiac arrest who received therapeutic cooling with apparent success, they grew more optimistic about the potential benefit of this treatment for their baby.
In animal models of hypoxic-ischemic neural injury, reducing core body temperature minimizes long-term neural consequences of the injury. Experiments have demonstrated that the optimal course is to induce hypothermia immediately after the neural injury, but initiating hypothermia even as long as 6 hours after injury has a protective effect.
Reducing core temperature appears to limit neural injury by decreasing oxygen requirements and suppressing the accumulation of harmful cytotoxic amino acids, cytokines, and free radicals. In contrast to the beneficial effect of hypothermia, raising body temperature by only 1°C or 2°C in experimental animals that have undergone an isolated hypoxic-ischemic event makes neural damage worse.
Clinical trials in newborns
The beneficial effect of whole-body cooling for newborns who have hypoxic-ischemic encephalopathy has been reported in several randomized clinical trials.
In a multicenter trial, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, 208 newborns in severe acidosis at birth were randomized to whole-body cooling or usual care.1 Whole-body cooling, initiated within 6 hours of birth, was achieved by:
- placing subjects on a cooling blanket precooled to 5°C
- inserting an esophageal temperature probe
- adjusting the temperature of the cooling blanket to achieve an esophageal temperature of 33.5°C for 72 hours
- slow rewarming.
Abdominal-wall skin temperature was also monitored in these newborns.
Subjects in the usual care group were nursed in an incubator, with the radiant heat element adjusted to maintain skin temperature of 36.5°C to 37°C.
Investigators determined that the composite end-point of risk of death or disability at 20 months of life was significantly reduced by whole-body cooling (relative risk, 0.72; 95% confidence interval [CI], 0.54–0.95; P=.01).
Death occurred in 24% of subjects in the hypothermia group and in 37% of subjects in the usual care group (risk ratio, 0.68; 95% CI, 0.44–1.05; P=.08). The rate of cerebral palsy was 19% in the hypothermia group and 30% in the usual care group (risk ratio, 0.68; 95% CI, 0.38–1.22; P=.20).
The rate of blindness was 7% in the hypothermia group and 14% in usual care group. The rate of hearing impairment was 4% and 6% percent (P - not significant).1
In a second clinical trial, the Total Body Hypothermia for Neonatal Encephalopathy (TOBY) trial of whole-body cooling, 325 newborns with hypoxic-ischemic encephalopathy were randomized to whole-body cooling or usual care.2 Whole-body cooling was achieved by nursing the infant in an incubator on a cooling blanket set at 25°C to 30°C, with the goal of maintaining a rectal temperature in the newborn of 33°C to 34°C. In this group, the heat element in the incubator was turned off.
Newborns assigned to the usual care group were nursed in an incubator with the heat element turned on and set to maintain a rectal temperature of 37°C.
The rate of death was similar in the two groups, and the rate of severe neurodevelopmental disability in each of the groups was not significantly different. Among surviving infants, however, whole-body cooling was associated with a higher rate of intact neurologic function and a diminished risk of cerebral palsy (compared to what was seen in the usual-care group). Among surviving newborns, cerebral palsy occurred in 28% of those treated with whole-body cooling and in 41% of those receiving usual care (relative risk 0.67; 95% CI, 0.47–0.96; P=.03).
No major adverse effects were observed with whole-body cooling, when compare d against usual care. A 2007 meta-analysis of four trials of therapeutic hypothermia in newborns reported a significant reduction in the composite endpoint of death or in moderate or severe neurodevelopmental disability (relative risk 0.76; 95% CI, 0.65–0.88). The number that needed to be treated to prevent one endpoint event was 6 (95% CI, 4–14).3
Note that some pediatricians remain cautious about using therapeutic hypothermia, because long-term data on the safety and efficacy of the practice have not been reported.
A devastating complication of cardiac arrest is coma and long-term neurologic dysfunction. Randomized trials have reported that therapeutic hypothermia is beneficial for comatose survivors of cardiac arrest.
In one study, 275 comatose survivors of cardiac arrest were randomized to therapeutic hypothermia (core temperature, 32°C to 34°C) or usual care. At 6 months, mortality was 41% in the hypothermia group and 55% in the usual care group (risk ratio 0.74; 95% Ci, 0.58–0.95). At 6 months, the rate of favorable neurologic recovery among survivors was 93% in those who had been treated with hypothermia and 87% in those given usual care.1
One protocol for inducing moderate hypothermia that has been studied in adults is to infuse, intravenously, approximately 2 L of cold (4°C) lactated Ringer’s solution and to cover the body with refrigerated cooling pads. The typical target is a core temperature of 32°C to 34°C, maintained for 24 hours.
Reference
1. The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556.
Impact on your practice
For decades, newborns in neonatal intensive care units received their care in warm incubators designed to maintain a body temperature of approximately 37°C. Could the standard practice of warming newborns have contributed to neurologic problems in those who suffered hypoxic-ischemic injury?
Whole-body cooling appears to reduce the rate of cerebral palsy in newborns after hypoxic-ischemic injury. For OBs, this treatment could significantly reduce their exposure to litigation that is based on a theory of hypoxic-ischemic birth injury—by reducing the number of surviving infants who have cerebral palsy.
Does your nursery have a protocol for rapidly instituting therapeutic cooling?
How does the partial pressure of arterial oxygen (PaO2) in a newborn compare with that of an adult breathing ambient air near the summit of Mount Everest?
Take the INSTANT QUIZ.
We want to hear from you! Tell us what you think.
1. Shankaran S, Laptook AR, Ehrenkranz RA, et al. for National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584.
2. Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349-1358.
3. Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic-ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161(10):951-958.
CASE: Risky decision to let labor continue
The baby was born blue and limp, after a long labor complicated by maternal fever and a prolonged fetal heart rate deceleration.
Earlier, just before the mother was raced to the OR for emergency cesarean delivery, the fetal heart rate had increased sufficiently for the OB to decide to permit labor to continue— the goal being rapid vaginal delivery.
In hindsight, that decision appears to have been potentially fateful for this newborn: Apgar scores were 1 at 5 minutes and 3 at 10 minutes. Umbilical artery pH was 6.98; base deficit, –13 mmol/L. The pediatricians made a preliminary diagnosis of hypoxic-ischemic injury and recommended whole-body cooling of the newborn to limit neural damage.
The frightened parents prayed the treatment would work. Later, recalling news reports that they had read about spinal cord-injured football players and survivors of cardiac arrest who received therapeutic cooling with apparent success, they grew more optimistic about the potential benefit of this treatment for their baby.
In animal models of hypoxic-ischemic neural injury, reducing core body temperature minimizes long-term neural consequences of the injury. Experiments have demonstrated that the optimal course is to induce hypothermia immediately after the neural injury, but initiating hypothermia even as long as 6 hours after injury has a protective effect.
Reducing core temperature appears to limit neural injury by decreasing oxygen requirements and suppressing the accumulation of harmful cytotoxic amino acids, cytokines, and free radicals. In contrast to the beneficial effect of hypothermia, raising body temperature by only 1°C or 2°C in experimental animals that have undergone an isolated hypoxic-ischemic event makes neural damage worse.
Clinical trials in newborns
The beneficial effect of whole-body cooling for newborns who have hypoxic-ischemic encephalopathy has been reported in several randomized clinical trials.
In a multicenter trial, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, 208 newborns in severe acidosis at birth were randomized to whole-body cooling or usual care.1 Whole-body cooling, initiated within 6 hours of birth, was achieved by:
- placing subjects on a cooling blanket precooled to 5°C
- inserting an esophageal temperature probe
- adjusting the temperature of the cooling blanket to achieve an esophageal temperature of 33.5°C for 72 hours
- slow rewarming.
Abdominal-wall skin temperature was also monitored in these newborns.
Subjects in the usual care group were nursed in an incubator, with the radiant heat element adjusted to maintain skin temperature of 36.5°C to 37°C.
Investigators determined that the composite end-point of risk of death or disability at 20 months of life was significantly reduced by whole-body cooling (relative risk, 0.72; 95% confidence interval [CI], 0.54–0.95; P=.01).
Death occurred in 24% of subjects in the hypothermia group and in 37% of subjects in the usual care group (risk ratio, 0.68; 95% CI, 0.44–1.05; P=.08). The rate of cerebral palsy was 19% in the hypothermia group and 30% in the usual care group (risk ratio, 0.68; 95% CI, 0.38–1.22; P=.20).
The rate of blindness was 7% in the hypothermia group and 14% in usual care group. The rate of hearing impairment was 4% and 6% percent (P - not significant).1
In a second clinical trial, the Total Body Hypothermia for Neonatal Encephalopathy (TOBY) trial of whole-body cooling, 325 newborns with hypoxic-ischemic encephalopathy were randomized to whole-body cooling or usual care.2 Whole-body cooling was achieved by nursing the infant in an incubator on a cooling blanket set at 25°C to 30°C, with the goal of maintaining a rectal temperature in the newborn of 33°C to 34°C. In this group, the heat element in the incubator was turned off.
Newborns assigned to the usual care group were nursed in an incubator with the heat element turned on and set to maintain a rectal temperature of 37°C.
The rate of death was similar in the two groups, and the rate of severe neurodevelopmental disability in each of the groups was not significantly different. Among surviving infants, however, whole-body cooling was associated with a higher rate of intact neurologic function and a diminished risk of cerebral palsy (compared to what was seen in the usual-care group). Among surviving newborns, cerebral palsy occurred in 28% of those treated with whole-body cooling and in 41% of those receiving usual care (relative risk 0.67; 95% CI, 0.47–0.96; P=.03).
No major adverse effects were observed with whole-body cooling, when compare d against usual care. A 2007 meta-analysis of four trials of therapeutic hypothermia in newborns reported a significant reduction in the composite endpoint of death or in moderate or severe neurodevelopmental disability (relative risk 0.76; 95% CI, 0.65–0.88). The number that needed to be treated to prevent one endpoint event was 6 (95% CI, 4–14).3
Note that some pediatricians remain cautious about using therapeutic hypothermia, because long-term data on the safety and efficacy of the practice have not been reported.
A devastating complication of cardiac arrest is coma and long-term neurologic dysfunction. Randomized trials have reported that therapeutic hypothermia is beneficial for comatose survivors of cardiac arrest.
In one study, 275 comatose survivors of cardiac arrest were randomized to therapeutic hypothermia (core temperature, 32°C to 34°C) or usual care. At 6 months, mortality was 41% in the hypothermia group and 55% in the usual care group (risk ratio 0.74; 95% Ci, 0.58–0.95). At 6 months, the rate of favorable neurologic recovery among survivors was 93% in those who had been treated with hypothermia and 87% in those given usual care.1
One protocol for inducing moderate hypothermia that has been studied in adults is to infuse, intravenously, approximately 2 L of cold (4°C) lactated Ringer’s solution and to cover the body with refrigerated cooling pads. The typical target is a core temperature of 32°C to 34°C, maintained for 24 hours.
Reference
1. The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556.
Impact on your practice
For decades, newborns in neonatal intensive care units received their care in warm incubators designed to maintain a body temperature of approximately 37°C. Could the standard practice of warming newborns have contributed to neurologic problems in those who suffered hypoxic-ischemic injury?
Whole-body cooling appears to reduce the rate of cerebral palsy in newborns after hypoxic-ischemic injury. For OBs, this treatment could significantly reduce their exposure to litigation that is based on a theory of hypoxic-ischemic birth injury—by reducing the number of surviving infants who have cerebral palsy.
Does your nursery have a protocol for rapidly instituting therapeutic cooling?
How does the partial pressure of arterial oxygen (PaO2) in a newborn compare with that of an adult breathing ambient air near the summit of Mount Everest?
Take the INSTANT QUIZ.
We want to hear from you! Tell us what you think.
CASE: Risky decision to let labor continue
The baby was born blue and limp, after a long labor complicated by maternal fever and a prolonged fetal heart rate deceleration.
Earlier, just before the mother was raced to the OR for emergency cesarean delivery, the fetal heart rate had increased sufficiently for the OB to decide to permit labor to continue— the goal being rapid vaginal delivery.
In hindsight, that decision appears to have been potentially fateful for this newborn: Apgar scores were 1 at 5 minutes and 3 at 10 minutes. Umbilical artery pH was 6.98; base deficit, –13 mmol/L. The pediatricians made a preliminary diagnosis of hypoxic-ischemic injury and recommended whole-body cooling of the newborn to limit neural damage.
The frightened parents prayed the treatment would work. Later, recalling news reports that they had read about spinal cord-injured football players and survivors of cardiac arrest who received therapeutic cooling with apparent success, they grew more optimistic about the potential benefit of this treatment for their baby.
In animal models of hypoxic-ischemic neural injury, reducing core body temperature minimizes long-term neural consequences of the injury. Experiments have demonstrated that the optimal course is to induce hypothermia immediately after the neural injury, but initiating hypothermia even as long as 6 hours after injury has a protective effect.
Reducing core temperature appears to limit neural injury by decreasing oxygen requirements and suppressing the accumulation of harmful cytotoxic amino acids, cytokines, and free radicals. In contrast to the beneficial effect of hypothermia, raising body temperature by only 1°C or 2°C in experimental animals that have undergone an isolated hypoxic-ischemic event makes neural damage worse.
Clinical trials in newborns
The beneficial effect of whole-body cooling for newborns who have hypoxic-ischemic encephalopathy has been reported in several randomized clinical trials.
In a multicenter trial, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, 208 newborns in severe acidosis at birth were randomized to whole-body cooling or usual care.1 Whole-body cooling, initiated within 6 hours of birth, was achieved by:
- placing subjects on a cooling blanket precooled to 5°C
- inserting an esophageal temperature probe
- adjusting the temperature of the cooling blanket to achieve an esophageal temperature of 33.5°C for 72 hours
- slow rewarming.
Abdominal-wall skin temperature was also monitored in these newborns.
Subjects in the usual care group were nursed in an incubator, with the radiant heat element adjusted to maintain skin temperature of 36.5°C to 37°C.
Investigators determined that the composite end-point of risk of death or disability at 20 months of life was significantly reduced by whole-body cooling (relative risk, 0.72; 95% confidence interval [CI], 0.54–0.95; P=.01).
Death occurred in 24% of subjects in the hypothermia group and in 37% of subjects in the usual care group (risk ratio, 0.68; 95% CI, 0.44–1.05; P=.08). The rate of cerebral palsy was 19% in the hypothermia group and 30% in the usual care group (risk ratio, 0.68; 95% CI, 0.38–1.22; P=.20).
The rate of blindness was 7% in the hypothermia group and 14% in usual care group. The rate of hearing impairment was 4% and 6% percent (P - not significant).1
In a second clinical trial, the Total Body Hypothermia for Neonatal Encephalopathy (TOBY) trial of whole-body cooling, 325 newborns with hypoxic-ischemic encephalopathy were randomized to whole-body cooling or usual care.2 Whole-body cooling was achieved by nursing the infant in an incubator on a cooling blanket set at 25°C to 30°C, with the goal of maintaining a rectal temperature in the newborn of 33°C to 34°C. In this group, the heat element in the incubator was turned off.
Newborns assigned to the usual care group were nursed in an incubator with the heat element turned on and set to maintain a rectal temperature of 37°C.
The rate of death was similar in the two groups, and the rate of severe neurodevelopmental disability in each of the groups was not significantly different. Among surviving infants, however, whole-body cooling was associated with a higher rate of intact neurologic function and a diminished risk of cerebral palsy (compared to what was seen in the usual-care group). Among surviving newborns, cerebral palsy occurred in 28% of those treated with whole-body cooling and in 41% of those receiving usual care (relative risk 0.67; 95% CI, 0.47–0.96; P=.03).
No major adverse effects were observed with whole-body cooling, when compare d against usual care. A 2007 meta-analysis of four trials of therapeutic hypothermia in newborns reported a significant reduction in the composite endpoint of death or in moderate or severe neurodevelopmental disability (relative risk 0.76; 95% CI, 0.65–0.88). The number that needed to be treated to prevent one endpoint event was 6 (95% CI, 4–14).3
Note that some pediatricians remain cautious about using therapeutic hypothermia, because long-term data on the safety and efficacy of the practice have not been reported.
A devastating complication of cardiac arrest is coma and long-term neurologic dysfunction. Randomized trials have reported that therapeutic hypothermia is beneficial for comatose survivors of cardiac arrest.
In one study, 275 comatose survivors of cardiac arrest were randomized to therapeutic hypothermia (core temperature, 32°C to 34°C) or usual care. At 6 months, mortality was 41% in the hypothermia group and 55% in the usual care group (risk ratio 0.74; 95% Ci, 0.58–0.95). At 6 months, the rate of favorable neurologic recovery among survivors was 93% in those who had been treated with hypothermia and 87% in those given usual care.1
One protocol for inducing moderate hypothermia that has been studied in adults is to infuse, intravenously, approximately 2 L of cold (4°C) lactated Ringer’s solution and to cover the body with refrigerated cooling pads. The typical target is a core temperature of 32°C to 34°C, maintained for 24 hours.
Reference
1. The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556.
Impact on your practice
For decades, newborns in neonatal intensive care units received their care in warm incubators designed to maintain a body temperature of approximately 37°C. Could the standard practice of warming newborns have contributed to neurologic problems in those who suffered hypoxic-ischemic injury?
Whole-body cooling appears to reduce the rate of cerebral palsy in newborns after hypoxic-ischemic injury. For OBs, this treatment could significantly reduce their exposure to litigation that is based on a theory of hypoxic-ischemic birth injury—by reducing the number of surviving infants who have cerebral palsy.
Does your nursery have a protocol for rapidly instituting therapeutic cooling?
How does the partial pressure of arterial oxygen (PaO2) in a newborn compare with that of an adult breathing ambient air near the summit of Mount Everest?
Take the INSTANT QUIZ.
We want to hear from you! Tell us what you think.
1. Shankaran S, Laptook AR, Ehrenkranz RA, et al. for National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584.
2. Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349-1358.
3. Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic-ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161(10):951-958.
1. Shankaran S, Laptook AR, Ehrenkranz RA, et al. for National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574-1584.
2. Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349-1358.
3. Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic-ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161(10):951-958.
Chronic pain after vaginal wall repair…and more
What caused chronic pain after repair of the vaginal wall?
A WOMAN IN HER THIRTIES underwent anterior and posterior repair of the vaginal wall, including repair of a cystocele and a rectocystocele. Postoperatively, the patient developed a chronic pain syndrome.
PATIENT’S CLAIM The ObGyn failed to properly perform the surgery, and damaged the pudendal nerve, which causes chronic pain. The ObGyn moved the levator ani muscle; the muscle shifted into the vaginal canal and damaged the pudendal nerve. Informed consent was not obtained.
PHYSICIAN’S DEFENSE The patient was fully informed of all the procedure’s risks. The injury could not have been from displacement of the levator ani muscle because the muscle cannot reach the vaginal canal. Pain is from scar formation that is entrapping a nerve.
VERDICT A New York defense verdict was returned.
DVT + estrogen-based contraception=stroke?
AFTER A DEEP VENOUS THROMBOSIS (DVT) in her leg at age 29, a woman was told by her family physician to avoid birth control that contained estrogen. She claimed she told her ObGyn of the history of DVT and the no-estrogen advice, but he prescribed and inserted a Nuva Ring, which contains ethinyl estradiol. A few months later, the woman was hospitalized with a severe headache, and suffered a stroke that affected her speech and cognitive functions.
PATIENT’S CLAIM The ObGyn was negligent in prescribing a contraceptive that contained estrogen, knowing the patient’s history of blood clot.
PHYSICIAN’S DEFENSE An injury caused the first clot; the Nuva Ring did not cause the second clot or stroke.
VERDICT A $523,000 Georgia verdict was returned.
New mother dies; was preeclampsia treated properly?
AT HER SEVENTH-MONTH VISIT to her ObGyn (Dr. A), a woman began to show signs of preeclampsia. Two weeks later, she went to the emergency department (ED) with chest pain, cough, and shortness of breath; she was found to have hypertension and tachycardia. She was examined by an emergency medicine physician (Dr. B), and discharged with a diagnosis of bronchitis and a finding of dyspnea.
At a scheduled prenatal visit 2 days later, she was hypertensive. Dr. A sent her to the ED, where a physician assistant noted signs of edema in her extremities. Attempts to draw arterial blood were unsuccessful, and crackles were heard in her lungs. She was diagnosed as having worsening preeclampsia with pulmonary edema, and admitted.
Dr. C, another ObGyn, decided to perform a cesarean delivery, but on the way to the OR, the patient became unresponsive. After delivery, she went into cardiopulmonary arrest and sustained anoxic brain injury. She died after life support was removed. An autopsy determined cause of death was anoxic encephalopathy due to respiratory arrest caused by preeclampsia.
ESTATE’S CLAIM Dr. A failed to provide proper prenatal care, and failed to recognize preeclampsia. Dr. B failed to recognize preeclampsia, failed to contact a specialist, and failed to immediately admit the patient for monitoring and treatment. Dr. C negligently administered a bolus of IV fluids when the patient showed signs of preeclampsia. He failed to administer medication to reduce fluid retention, and failed to timely admit the patient to the hospital.
PHYSICIANS’ DEFENSE All three physicians denied negligence.
VERDICT A $1.5 million Michigan settlement was reached.
Did resident use forceful traction with shoulder dystocia?
SHOULDER DYSTOCIA was encountered during vaginal delivery, and managed by a resident. The child suffered a brachial plexus injury.
PATIENT’S CLAIM The attending physician failed to 1) properly supervise the resident who was delivering the infant, and 2) prevent the use of traction after it was determined that shoulder dystocia was present.
PHYSICIANS’ DEFENSE The resident, under full supervision of the attending physician, utilized traction after the baby’s head was delivered and shoulder dystocia became evident—but traction was gentle. The maternal forces of labor caused the injury.
VERDICT A $950,000 Virginia settlement was reached.
Was patient informed that tubal ligation had not been performed?
PREGNANT WITH HER FOURTH CHILD despite birth control, a woman and her husband told the ObGyn that they did not want, nor could they afford, a fifth child. They requested bilateral tubal ligation during cesarean delivery. Two days before the scheduled birth, the mother went into labor. Her prenatal records could not be found, and the ObGyn’s office was closed. The ObGyn delivered the baby, but did not perform tubal ligation. She claimed she was never told that the tubal ligation had not been completed, even at the 6-week postpartum visit. She did not take precautions to prevent pregnancy, and later conceived a fifth child.
PATIENT’S CLAIM The ObGyn was negligent in not performing the tubal ligation and in not telling the patient until after the fifth child’s conception.
PHYSICIAN’S DEFENSE The mother was told that tubal ligation had not been performed at the 6-week visit. She was advised to use birth control until she recovered from the cesarean delivery and could undergo a tubal ligation procedure. The ObGyn acknowledged he had forgotten to perform the tubal ligation at delivery, but insisted there was no negligence under the circumstances.
VERDICT A California defense verdict was returned.
Patient claims stomach injury caused GERD
DUE TO PELVIC PAIN, a woman underwent laparoscopy by her ObGyn. During the procedure, a trocar punctured her stomach. The injury was discovered, the procedure converted to a laparotomy with a vertical incision, and the injury repaired.
PATIENT’S CLAIM She developed gastroesophageal reflux disease (GERD) because of the puncture wound, and anxiety because of the scar.
PHYSICIAN’S DEFENSE Gastric perforation is a rare but recognized complication of abdominal laparoscopy, and can occur without negligence. Her GERD is either due to a hiatal hernia or pychosomatic disorder.
VERDICT A Virginia defense verdict was returned.
Physicians not responsible for stroke
SEVERAL DAYS AFTER GIVING BIRTH, a 33-year-old woman visited the ED with chest pain, headache, and abdominal pain. An emergency medicine physician and an ObGyn ordered a chest CT scan and administered anticoagulants. By the time the CT scan was completed, the woman denied having chest pain. No pulmonary emboli (PE) were detected on chest CT, and she was discharged.
The next day, she went to another hospital’s ED with a headache and right-side weakness. A CT scan revealed a large left parietal-lobe intracerebral hematoma. A ventricular catheter was placed and she underwent a stereotactic craniotomy for evacuation of the hematoma. She was transferred to a rehabilitation facility a month later.
She suffers permanent neurologic damage, including short-term memory loss and an inability to lift or walk for any great distance.
PATIENT’S CLAIM The ED physicians failed to diagnose and treat an acute neurologic event in a timely manner, and did not obtain specialist consults. Administration of anticoagulants was negligent; protamine therapy should have been started to reverse the anticoagulant effects. Laboratory testing of clotting times and a ventilation-perfusion lung scan should have been conducted to confirm the presence of PE.
PHYSICIANS’ DEFENSE The patient’s condition was appropriately diagnosed and treated in the ED. Administration of anticoagulants was necessary because of suspected PE. There is no evidence that the heparin given to the plaintiff the day before her stroke was related to the stroke.
VERDICT A Florida defense verdict was returned.
Did failure to diagnose preeclampsia lead to infant’s death?
AT 38-WEEKS’ GESTATION, a 21-year-old woman was seen at a hospital’s obstetric clinic, and sent to the ED with complaints of leaking fluid and lack of fetal movement. She claimed she showed signs of preeclampsia, pregnancy-induced hypertension, and oligohydramnios, but was not admitted to the hospital. The baby was born 2 days later with persistent pulmonary hypertension (PPH), which led to the child’s death at 33 days of age.
PATIENT’S CLAIM There was negligence in failing to diagnose preeclampsia, pregnancy-induced hypertension, and oligohydramnios, which caused the baby to be born with PPH.
PHYSICIAN’S DEFENSE The cause of the infant’s PPH was unknown, and most likely arose in utero prior to birth. An earlier delivery would not have resulted in a different outcome.
VERDICT A Illinois defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
What caused chronic pain after repair of the vaginal wall?
A WOMAN IN HER THIRTIES underwent anterior and posterior repair of the vaginal wall, including repair of a cystocele and a rectocystocele. Postoperatively, the patient developed a chronic pain syndrome.
PATIENT’S CLAIM The ObGyn failed to properly perform the surgery, and damaged the pudendal nerve, which causes chronic pain. The ObGyn moved the levator ani muscle; the muscle shifted into the vaginal canal and damaged the pudendal nerve. Informed consent was not obtained.
PHYSICIAN’S DEFENSE The patient was fully informed of all the procedure’s risks. The injury could not have been from displacement of the levator ani muscle because the muscle cannot reach the vaginal canal. Pain is from scar formation that is entrapping a nerve.
VERDICT A New York defense verdict was returned.
DVT + estrogen-based contraception=stroke?
AFTER A DEEP VENOUS THROMBOSIS (DVT) in her leg at age 29, a woman was told by her family physician to avoid birth control that contained estrogen. She claimed she told her ObGyn of the history of DVT and the no-estrogen advice, but he prescribed and inserted a Nuva Ring, which contains ethinyl estradiol. A few months later, the woman was hospitalized with a severe headache, and suffered a stroke that affected her speech and cognitive functions.
PATIENT’S CLAIM The ObGyn was negligent in prescribing a contraceptive that contained estrogen, knowing the patient’s history of blood clot.
PHYSICIAN’S DEFENSE An injury caused the first clot; the Nuva Ring did not cause the second clot or stroke.
VERDICT A $523,000 Georgia verdict was returned.
New mother dies; was preeclampsia treated properly?
AT HER SEVENTH-MONTH VISIT to her ObGyn (Dr. A), a woman began to show signs of preeclampsia. Two weeks later, she went to the emergency department (ED) with chest pain, cough, and shortness of breath; she was found to have hypertension and tachycardia. She was examined by an emergency medicine physician (Dr. B), and discharged with a diagnosis of bronchitis and a finding of dyspnea.
At a scheduled prenatal visit 2 days later, she was hypertensive. Dr. A sent her to the ED, where a physician assistant noted signs of edema in her extremities. Attempts to draw arterial blood were unsuccessful, and crackles were heard in her lungs. She was diagnosed as having worsening preeclampsia with pulmonary edema, and admitted.
Dr. C, another ObGyn, decided to perform a cesarean delivery, but on the way to the OR, the patient became unresponsive. After delivery, she went into cardiopulmonary arrest and sustained anoxic brain injury. She died after life support was removed. An autopsy determined cause of death was anoxic encephalopathy due to respiratory arrest caused by preeclampsia.
ESTATE’S CLAIM Dr. A failed to provide proper prenatal care, and failed to recognize preeclampsia. Dr. B failed to recognize preeclampsia, failed to contact a specialist, and failed to immediately admit the patient for monitoring and treatment. Dr. C negligently administered a bolus of IV fluids when the patient showed signs of preeclampsia. He failed to administer medication to reduce fluid retention, and failed to timely admit the patient to the hospital.
PHYSICIANS’ DEFENSE All three physicians denied negligence.
VERDICT A $1.5 million Michigan settlement was reached.
Did resident use forceful traction with shoulder dystocia?
SHOULDER DYSTOCIA was encountered during vaginal delivery, and managed by a resident. The child suffered a brachial plexus injury.
PATIENT’S CLAIM The attending physician failed to 1) properly supervise the resident who was delivering the infant, and 2) prevent the use of traction after it was determined that shoulder dystocia was present.
PHYSICIANS’ DEFENSE The resident, under full supervision of the attending physician, utilized traction after the baby’s head was delivered and shoulder dystocia became evident—but traction was gentle. The maternal forces of labor caused the injury.
VERDICT A $950,000 Virginia settlement was reached.
Was patient informed that tubal ligation had not been performed?
PREGNANT WITH HER FOURTH CHILD despite birth control, a woman and her husband told the ObGyn that they did not want, nor could they afford, a fifth child. They requested bilateral tubal ligation during cesarean delivery. Two days before the scheduled birth, the mother went into labor. Her prenatal records could not be found, and the ObGyn’s office was closed. The ObGyn delivered the baby, but did not perform tubal ligation. She claimed she was never told that the tubal ligation had not been completed, even at the 6-week postpartum visit. She did not take precautions to prevent pregnancy, and later conceived a fifth child.
PATIENT’S CLAIM The ObGyn was negligent in not performing the tubal ligation and in not telling the patient until after the fifth child’s conception.
PHYSICIAN’S DEFENSE The mother was told that tubal ligation had not been performed at the 6-week visit. She was advised to use birth control until she recovered from the cesarean delivery and could undergo a tubal ligation procedure. The ObGyn acknowledged he had forgotten to perform the tubal ligation at delivery, but insisted there was no negligence under the circumstances.
VERDICT A California defense verdict was returned.
Patient claims stomach injury caused GERD
DUE TO PELVIC PAIN, a woman underwent laparoscopy by her ObGyn. During the procedure, a trocar punctured her stomach. The injury was discovered, the procedure converted to a laparotomy with a vertical incision, and the injury repaired.
PATIENT’S CLAIM She developed gastroesophageal reflux disease (GERD) because of the puncture wound, and anxiety because of the scar.
PHYSICIAN’S DEFENSE Gastric perforation is a rare but recognized complication of abdominal laparoscopy, and can occur without negligence. Her GERD is either due to a hiatal hernia or pychosomatic disorder.
VERDICT A Virginia defense verdict was returned.
Physicians not responsible for stroke
SEVERAL DAYS AFTER GIVING BIRTH, a 33-year-old woman visited the ED with chest pain, headache, and abdominal pain. An emergency medicine physician and an ObGyn ordered a chest CT scan and administered anticoagulants. By the time the CT scan was completed, the woman denied having chest pain. No pulmonary emboli (PE) were detected on chest CT, and she was discharged.
The next day, she went to another hospital’s ED with a headache and right-side weakness. A CT scan revealed a large left parietal-lobe intracerebral hematoma. A ventricular catheter was placed and she underwent a stereotactic craniotomy for evacuation of the hematoma. She was transferred to a rehabilitation facility a month later.
She suffers permanent neurologic damage, including short-term memory loss and an inability to lift or walk for any great distance.
PATIENT’S CLAIM The ED physicians failed to diagnose and treat an acute neurologic event in a timely manner, and did not obtain specialist consults. Administration of anticoagulants was negligent; protamine therapy should have been started to reverse the anticoagulant effects. Laboratory testing of clotting times and a ventilation-perfusion lung scan should have been conducted to confirm the presence of PE.
PHYSICIANS’ DEFENSE The patient’s condition was appropriately diagnosed and treated in the ED. Administration of anticoagulants was necessary because of suspected PE. There is no evidence that the heparin given to the plaintiff the day before her stroke was related to the stroke.
VERDICT A Florida defense verdict was returned.
Did failure to diagnose preeclampsia lead to infant’s death?
AT 38-WEEKS’ GESTATION, a 21-year-old woman was seen at a hospital’s obstetric clinic, and sent to the ED with complaints of leaking fluid and lack of fetal movement. She claimed she showed signs of preeclampsia, pregnancy-induced hypertension, and oligohydramnios, but was not admitted to the hospital. The baby was born 2 days later with persistent pulmonary hypertension (PPH), which led to the child’s death at 33 days of age.
PATIENT’S CLAIM There was negligence in failing to diagnose preeclampsia, pregnancy-induced hypertension, and oligohydramnios, which caused the baby to be born with PPH.
PHYSICIAN’S DEFENSE The cause of the infant’s PPH was unknown, and most likely arose in utero prior to birth. An earlier delivery would not have resulted in a different outcome.
VERDICT A Illinois defense verdict was returned.
What caused chronic pain after repair of the vaginal wall?
A WOMAN IN HER THIRTIES underwent anterior and posterior repair of the vaginal wall, including repair of a cystocele and a rectocystocele. Postoperatively, the patient developed a chronic pain syndrome.
PATIENT’S CLAIM The ObGyn failed to properly perform the surgery, and damaged the pudendal nerve, which causes chronic pain. The ObGyn moved the levator ani muscle; the muscle shifted into the vaginal canal and damaged the pudendal nerve. Informed consent was not obtained.
PHYSICIAN’S DEFENSE The patient was fully informed of all the procedure’s risks. The injury could not have been from displacement of the levator ani muscle because the muscle cannot reach the vaginal canal. Pain is from scar formation that is entrapping a nerve.
VERDICT A New York defense verdict was returned.
DVT + estrogen-based contraception=stroke?
AFTER A DEEP VENOUS THROMBOSIS (DVT) in her leg at age 29, a woman was told by her family physician to avoid birth control that contained estrogen. She claimed she told her ObGyn of the history of DVT and the no-estrogen advice, but he prescribed and inserted a Nuva Ring, which contains ethinyl estradiol. A few months later, the woman was hospitalized with a severe headache, and suffered a stroke that affected her speech and cognitive functions.
PATIENT’S CLAIM The ObGyn was negligent in prescribing a contraceptive that contained estrogen, knowing the patient’s history of blood clot.
PHYSICIAN’S DEFENSE An injury caused the first clot; the Nuva Ring did not cause the second clot or stroke.
VERDICT A $523,000 Georgia verdict was returned.
New mother dies; was preeclampsia treated properly?
AT HER SEVENTH-MONTH VISIT to her ObGyn (Dr. A), a woman began to show signs of preeclampsia. Two weeks later, she went to the emergency department (ED) with chest pain, cough, and shortness of breath; she was found to have hypertension and tachycardia. She was examined by an emergency medicine physician (Dr. B), and discharged with a diagnosis of bronchitis and a finding of dyspnea.
At a scheduled prenatal visit 2 days later, she was hypertensive. Dr. A sent her to the ED, where a physician assistant noted signs of edema in her extremities. Attempts to draw arterial blood were unsuccessful, and crackles were heard in her lungs. She was diagnosed as having worsening preeclampsia with pulmonary edema, and admitted.
Dr. C, another ObGyn, decided to perform a cesarean delivery, but on the way to the OR, the patient became unresponsive. After delivery, she went into cardiopulmonary arrest and sustained anoxic brain injury. She died after life support was removed. An autopsy determined cause of death was anoxic encephalopathy due to respiratory arrest caused by preeclampsia.
ESTATE’S CLAIM Dr. A failed to provide proper prenatal care, and failed to recognize preeclampsia. Dr. B failed to recognize preeclampsia, failed to contact a specialist, and failed to immediately admit the patient for monitoring and treatment. Dr. C negligently administered a bolus of IV fluids when the patient showed signs of preeclampsia. He failed to administer medication to reduce fluid retention, and failed to timely admit the patient to the hospital.
PHYSICIANS’ DEFENSE All three physicians denied negligence.
VERDICT A $1.5 million Michigan settlement was reached.
Did resident use forceful traction with shoulder dystocia?
SHOULDER DYSTOCIA was encountered during vaginal delivery, and managed by a resident. The child suffered a brachial plexus injury.
PATIENT’S CLAIM The attending physician failed to 1) properly supervise the resident who was delivering the infant, and 2) prevent the use of traction after it was determined that shoulder dystocia was present.
PHYSICIANS’ DEFENSE The resident, under full supervision of the attending physician, utilized traction after the baby’s head was delivered and shoulder dystocia became evident—but traction was gentle. The maternal forces of labor caused the injury.
VERDICT A $950,000 Virginia settlement was reached.
Was patient informed that tubal ligation had not been performed?
PREGNANT WITH HER FOURTH CHILD despite birth control, a woman and her husband told the ObGyn that they did not want, nor could they afford, a fifth child. They requested bilateral tubal ligation during cesarean delivery. Two days before the scheduled birth, the mother went into labor. Her prenatal records could not be found, and the ObGyn’s office was closed. The ObGyn delivered the baby, but did not perform tubal ligation. She claimed she was never told that the tubal ligation had not been completed, even at the 6-week postpartum visit. She did not take precautions to prevent pregnancy, and later conceived a fifth child.
PATIENT’S CLAIM The ObGyn was negligent in not performing the tubal ligation and in not telling the patient until after the fifth child’s conception.
PHYSICIAN’S DEFENSE The mother was told that tubal ligation had not been performed at the 6-week visit. She was advised to use birth control until she recovered from the cesarean delivery and could undergo a tubal ligation procedure. The ObGyn acknowledged he had forgotten to perform the tubal ligation at delivery, but insisted there was no negligence under the circumstances.
VERDICT A California defense verdict was returned.
Patient claims stomach injury caused GERD
DUE TO PELVIC PAIN, a woman underwent laparoscopy by her ObGyn. During the procedure, a trocar punctured her stomach. The injury was discovered, the procedure converted to a laparotomy with a vertical incision, and the injury repaired.
PATIENT’S CLAIM She developed gastroesophageal reflux disease (GERD) because of the puncture wound, and anxiety because of the scar.
PHYSICIAN’S DEFENSE Gastric perforation is a rare but recognized complication of abdominal laparoscopy, and can occur without negligence. Her GERD is either due to a hiatal hernia or pychosomatic disorder.
VERDICT A Virginia defense verdict was returned.
Physicians not responsible for stroke
SEVERAL DAYS AFTER GIVING BIRTH, a 33-year-old woman visited the ED with chest pain, headache, and abdominal pain. An emergency medicine physician and an ObGyn ordered a chest CT scan and administered anticoagulants. By the time the CT scan was completed, the woman denied having chest pain. No pulmonary emboli (PE) were detected on chest CT, and she was discharged.
The next day, she went to another hospital’s ED with a headache and right-side weakness. A CT scan revealed a large left parietal-lobe intracerebral hematoma. A ventricular catheter was placed and she underwent a stereotactic craniotomy for evacuation of the hematoma. She was transferred to a rehabilitation facility a month later.
She suffers permanent neurologic damage, including short-term memory loss and an inability to lift or walk for any great distance.
PATIENT’S CLAIM The ED physicians failed to diagnose and treat an acute neurologic event in a timely manner, and did not obtain specialist consults. Administration of anticoagulants was negligent; protamine therapy should have been started to reverse the anticoagulant effects. Laboratory testing of clotting times and a ventilation-perfusion lung scan should have been conducted to confirm the presence of PE.
PHYSICIANS’ DEFENSE The patient’s condition was appropriately diagnosed and treated in the ED. Administration of anticoagulants was necessary because of suspected PE. There is no evidence that the heparin given to the plaintiff the day before her stroke was related to the stroke.
VERDICT A Florida defense verdict was returned.
Did failure to diagnose preeclampsia lead to infant’s death?
AT 38-WEEKS’ GESTATION, a 21-year-old woman was seen at a hospital’s obstetric clinic, and sent to the ED with complaints of leaking fluid and lack of fetal movement. She claimed she showed signs of preeclampsia, pregnancy-induced hypertension, and oligohydramnios, but was not admitted to the hospital. The baby was born 2 days later with persistent pulmonary hypertension (PPH), which led to the child’s death at 33 days of age.
PATIENT’S CLAIM There was negligence in failing to diagnose preeclampsia, pregnancy-induced hypertension, and oligohydramnios, which caused the baby to be born with PPH.
PHYSICIAN’S DEFENSE The cause of the infant’s PPH was unknown, and most likely arose in utero prior to birth. An earlier delivery would not have resulted in a different outcome.
VERDICT A Illinois defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
What Should I Do If I Get a Needlestick?
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
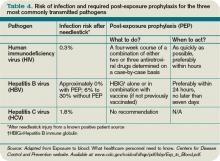
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Malpractice Chronicle
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Ischemic Colitis Leads to Septic Shock
A Massachusetts woman, age 59, presented to an emergency department (ED) with a week-long history of progressively worsening abdominal pain. She also complained of epigastric pain, left shoulder and breast pain, dizziness, nausea, and diarrhea. The emergency physician noted that her abdomen was soft, with diffuse tenderness to palpation. The patient was given 4.0 mg of morphine for pain. Laboratory studies revealed an elevated white blood cell count (16,200 cells/mL).
The patient’s blood pressure remained elevated and her pain persisted. She was given an additional 4.0 mg of morphine two-and-one-half hours after the initial dose. Nursing notes indicate that she was sitting on the edge of the stretcher, moaning with pain. An hour later, she was given a third dose of morphine.
Forty minutes later, the woman underwent abdominal CT, which revealed diverticulosis. The patient continued to complain of severe abdominal pain, which relented somewhat after acetaminophen was administered. She was discharged home with instructions to return to the ED in the event of worsening pain, fever, or other concerns; otherwise, she should follow up with her primary care physician the following day.
The woman returned to the ED the next day with continuing severe abdominal pain. She was determined to be experiencing septic shock at that time. She underwent abdominal/pelvic CT, which revealed new development of several areas of free air within the peritoneal cavity, compared with the previous study. She was immediately taken to surgery and was found to have gangrene of the small bowel and perforation of the cecum, with free stool in the area. Resection of the small bowel was performed. The woman remained acidotic with low blood pressure despite various interventions. She went into cardiac arrest and died of septic shock secondary to ischemic colitis.
The plaintiff claimed that the radiologist who reviewed the first abdominal CT failed to identify and report findings of impaired blood flow on the later abdominal/pelvic CT and failed to recommend an urgent surgical consultation.
The radiologist contended that the CT did not show evidence of impaired blood flow.
A $1.5 million settlement was reached.
Failure to Contact Patient With Pathology Results
An Illinois woman, age 31, presented to the defendant dermatologist, Dr. K., in September 2002 with concerns about a mole. The dermatologist thought the mole in question was of no concern but recommended removal of a second mole on the back of her arm. After removing that mole in December 2002, Dr. K. scheduled a return visit in two weeks to assess the healing of the area, remove the sutures, review the pathology results, and discuss further plans.
The patient did not keep the scheduled appointment because she thought she was returning simply to have the stitches removed, and they had already fallen out. The pathology report indicated some architectural disorder with one peripheral margin involved and suggested a further wide excision in the area of the removed mole, with histologic tissue analysis and definitive diagnosis. Neither Dr. K. nor his office staff made any attempt to contact the patient to urge her to come in or to inform her of the pathology results.
The patient later returned to Dr. K.’s office to be seen for acne, but saw a physician assistant instead.
Three years after her procedure, the patient developed melanoma near the site of the mole removal. During a wide excision procedure she underwent in September 2005, a residual melanoma was found. A sentinel lymph node biopsy was also performed. Results were negative, but hypertrophic scarring resulted. At several visits since that time, no additional melanoma has been found.
The plaintiff claimed that the diagnosis of melanoma caused substantial alteration of her lifestyle and a personality change. She claimed that the original arm lesion the defendant removed was premalignant and that he had a duty to contact her, explain the pathology result, and recommend that she return for a wide excision.
The defendant claimed that the lesion he removed was benign, that there was no duty to contact the plaintiff regarding a benign pathology report, and that the proper recommendation would be to watch the area and return if any problems developed. The defendant further claimed that it could not be determined with certainty whether the melanoma evolved from a microscopic portion of the mole or was a new development. The defendant claimed that the plaintiff needed to alter her lifestyle and protect herself from the sun in any event because she had a biogenetic predisposition for melanoma. She was also at future risk for developing new melanomas, unrelated to his treatment.
According to a published report, a defense verdict was returned.
Infant’s Bacterial Infection Missed
The plaintiff child, age 20 months, was brought to the emergency department (ED) with trouble breathing and a low-grade fever. The emergency physician, Dr. S., made a diagnosis of viral croup and discharged her.
The next day, the girl was brought back to the ED, where she was seen by a second emergency physician, Dr. G. Her temperature had risen to 106°F, and she had developed a croupy cough. Following administration of acetaminophen and other medications, the child’s temperature was reduced to 100°F. A test for streptococcal infection yielded negative results, and the infant was discharged.
The infant did well the following day, but that night her parents noticed that she was lethargic and breathing with difficulty. She was taken to the ED again and seen by Dr. G. This time, a diagnosis of pneumonia was quickly made, and the patient was transferred to a children’s hospital.
The child died that evening. Autopsy revealed group A streptococcal infection, which had led to toxic shock syndrome.
The plaintiff claimed that Dr. G. was negligent in his failure to diagnose the infection and to begin antibiotics when he first saw the infant. The plaintiffs claimed that the medications used included steroids, which suppressed the child’s immune system, making it more difficult for her to fight infection.
Dr. G. claimed that the infant’s presentation was inconsistent with a bacterial infection and that his diagnosis was reasonable. The defendant also claimed that the infant had an infection that would not have responded to antibiotics.
A defense verdict was returned.
Change of Procedure, Surgical Instrument Retained
In New York City, a 54-year-old man underwent a sleeve gastrectomy, performed by Dr. M. and Dr. S. Three days later, the patient was informed that a surgical tool had been left in his body, and he returned to the hospital.
CT revealed no foreign object. The patient’s large size made MRI impossible, and he was discharged. The man died three days later of peritonitis, which the plaintiff for the decedent claimed resulted from damage inflicted by a surgical instrument that had not been removed. The plaintiff claimed that the patient had consented to a Roux-en-Y bypass and had undergone presurgical preparation for that procedure, but that plan was abandoned shortly before the surgery. The plaintiff claimed that the problems resulting from the surgery performed were related to the procedure itself—specifically, that the cutting and stapling tool used in the gastrectomy had severed the esophageal stethoscope used, after which the severed remnant of the stethoscope was inadvertently stapled into the patient’s stomach.
The plaintiff for the decedent claimed that the remnant caused leakage of bile, which led to fatal peritonitis. The plaintiff claimed that the surgeons should not have concluded the surgery until it was confirmed that the stomach was properly sealed. The plaintiff also claimed that the defendants failed to address the decedent’s postsurgical symptoms of fever and severe pain.
The defendants countered that the decedent had reported no such symptoms and that there were no indications of infection. The defendants also claimed that neither CT nor intraoperative testing revealed any leakage in the decedent’s stomach. The defendants claimed that the decedent had signed a consent to undergo a gastrectomy, and that a gastrectomy had always been the intended procedure. The defendants additionally claimed that the foreign object in question was a probe, which the anesthesiologist inserted to measure the patient’s esophageal temperature without the surgeons’ knowledge.
According to a published account, a settlement of $675,000 was reached.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Ischemic Colitis Leads to Septic Shock
A Massachusetts woman, age 59, presented to an emergency department (ED) with a week-long history of progressively worsening abdominal pain. She also complained of epigastric pain, left shoulder and breast pain, dizziness, nausea, and diarrhea. The emergency physician noted that her abdomen was soft, with diffuse tenderness to palpation. The patient was given 4.0 mg of morphine for pain. Laboratory studies revealed an elevated white blood cell count (16,200 cells/mL).
The patient’s blood pressure remained elevated and her pain persisted. She was given an additional 4.0 mg of morphine two-and-one-half hours after the initial dose. Nursing notes indicate that she was sitting on the edge of the stretcher, moaning with pain. An hour later, she was given a third dose of morphine.
Forty minutes later, the woman underwent abdominal CT, which revealed diverticulosis. The patient continued to complain of severe abdominal pain, which relented somewhat after acetaminophen was administered. She was discharged home with instructions to return to the ED in the event of worsening pain, fever, or other concerns; otherwise, she should follow up with her primary care physician the following day.
The woman returned to the ED the next day with continuing severe abdominal pain. She was determined to be experiencing septic shock at that time. She underwent abdominal/pelvic CT, which revealed new development of several areas of free air within the peritoneal cavity, compared with the previous study. She was immediately taken to surgery and was found to have gangrene of the small bowel and perforation of the cecum, with free stool in the area. Resection of the small bowel was performed. The woman remained acidotic with low blood pressure despite various interventions. She went into cardiac arrest and died of septic shock secondary to ischemic colitis.
The plaintiff claimed that the radiologist who reviewed the first abdominal CT failed to identify and report findings of impaired blood flow on the later abdominal/pelvic CT and failed to recommend an urgent surgical consultation.
The radiologist contended that the CT did not show evidence of impaired blood flow.
A $1.5 million settlement was reached.
Failure to Contact Patient With Pathology Results
An Illinois woman, age 31, presented to the defendant dermatologist, Dr. K., in September 2002 with concerns about a mole. The dermatologist thought the mole in question was of no concern but recommended removal of a second mole on the back of her arm. After removing that mole in December 2002, Dr. K. scheduled a return visit in two weeks to assess the healing of the area, remove the sutures, review the pathology results, and discuss further plans.
The patient did not keep the scheduled appointment because she thought she was returning simply to have the stitches removed, and they had already fallen out. The pathology report indicated some architectural disorder with one peripheral margin involved and suggested a further wide excision in the area of the removed mole, with histologic tissue analysis and definitive diagnosis. Neither Dr. K. nor his office staff made any attempt to contact the patient to urge her to come in or to inform her of the pathology results.
The patient later returned to Dr. K.’s office to be seen for acne, but saw a physician assistant instead.
Three years after her procedure, the patient developed melanoma near the site of the mole removal. During a wide excision procedure she underwent in September 2005, a residual melanoma was found. A sentinel lymph node biopsy was also performed. Results were negative, but hypertrophic scarring resulted. At several visits since that time, no additional melanoma has been found.
The plaintiff claimed that the diagnosis of melanoma caused substantial alteration of her lifestyle and a personality change. She claimed that the original arm lesion the defendant removed was premalignant and that he had a duty to contact her, explain the pathology result, and recommend that she return for a wide excision.
The defendant claimed that the lesion he removed was benign, that there was no duty to contact the plaintiff regarding a benign pathology report, and that the proper recommendation would be to watch the area and return if any problems developed. The defendant further claimed that it could not be determined with certainty whether the melanoma evolved from a microscopic portion of the mole or was a new development. The defendant claimed that the plaintiff needed to alter her lifestyle and protect herself from the sun in any event because she had a biogenetic predisposition for melanoma. She was also at future risk for developing new melanomas, unrelated to his treatment.
According to a published report, a defense verdict was returned.
Infant’s Bacterial Infection Missed
The plaintiff child, age 20 months, was brought to the emergency department (ED) with trouble breathing and a low-grade fever. The emergency physician, Dr. S., made a diagnosis of viral croup and discharged her.
The next day, the girl was brought back to the ED, where she was seen by a second emergency physician, Dr. G. Her temperature had risen to 106°F, and she had developed a croupy cough. Following administration of acetaminophen and other medications, the child’s temperature was reduced to 100°F. A test for streptococcal infection yielded negative results, and the infant was discharged.
The infant did well the following day, but that night her parents noticed that she was lethargic and breathing with difficulty. She was taken to the ED again and seen by Dr. G. This time, a diagnosis of pneumonia was quickly made, and the patient was transferred to a children’s hospital.
The child died that evening. Autopsy revealed group A streptococcal infection, which had led to toxic shock syndrome.
The plaintiff claimed that Dr. G. was negligent in his failure to diagnose the infection and to begin antibiotics when he first saw the infant. The plaintiffs claimed that the medications used included steroids, which suppressed the child’s immune system, making it more difficult for her to fight infection.
Dr. G. claimed that the infant’s presentation was inconsistent with a bacterial infection and that his diagnosis was reasonable. The defendant also claimed that the infant had an infection that would not have responded to antibiotics.
A defense verdict was returned.
Change of Procedure, Surgical Instrument Retained
In New York City, a 54-year-old man underwent a sleeve gastrectomy, performed by Dr. M. and Dr. S. Three days later, the patient was informed that a surgical tool had been left in his body, and he returned to the hospital.
CT revealed no foreign object. The patient’s large size made MRI impossible, and he was discharged. The man died three days later of peritonitis, which the plaintiff for the decedent claimed resulted from damage inflicted by a surgical instrument that had not been removed. The plaintiff claimed that the patient had consented to a Roux-en-Y bypass and had undergone presurgical preparation for that procedure, but that plan was abandoned shortly before the surgery. The plaintiff claimed that the problems resulting from the surgery performed were related to the procedure itself—specifically, that the cutting and stapling tool used in the gastrectomy had severed the esophageal stethoscope used, after which the severed remnant of the stethoscope was inadvertently stapled into the patient’s stomach.
The plaintiff for the decedent claimed that the remnant caused leakage of bile, which led to fatal peritonitis. The plaintiff claimed that the surgeons should not have concluded the surgery until it was confirmed that the stomach was properly sealed. The plaintiff also claimed that the defendants failed to address the decedent’s postsurgical symptoms of fever and severe pain.
The defendants countered that the decedent had reported no such symptoms and that there were no indications of infection. The defendants also claimed that neither CT nor intraoperative testing revealed any leakage in the decedent’s stomach. The defendants claimed that the decedent had signed a consent to undergo a gastrectomy, and that a gastrectomy had always been the intended procedure. The defendants additionally claimed that the foreign object in question was a probe, which the anesthesiologist inserted to measure the patient’s esophageal temperature without the surgeons’ knowledge.
According to a published account, a settlement of $675,000 was reached.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Ischemic Colitis Leads to Septic Shock
A Massachusetts woman, age 59, presented to an emergency department (ED) with a week-long history of progressively worsening abdominal pain. She also complained of epigastric pain, left shoulder and breast pain, dizziness, nausea, and diarrhea. The emergency physician noted that her abdomen was soft, with diffuse tenderness to palpation. The patient was given 4.0 mg of morphine for pain. Laboratory studies revealed an elevated white blood cell count (16,200 cells/mL).
The patient’s blood pressure remained elevated and her pain persisted. She was given an additional 4.0 mg of morphine two-and-one-half hours after the initial dose. Nursing notes indicate that she was sitting on the edge of the stretcher, moaning with pain. An hour later, she was given a third dose of morphine.
Forty minutes later, the woman underwent abdominal CT, which revealed diverticulosis. The patient continued to complain of severe abdominal pain, which relented somewhat after acetaminophen was administered. She was discharged home with instructions to return to the ED in the event of worsening pain, fever, or other concerns; otherwise, she should follow up with her primary care physician the following day.
The woman returned to the ED the next day with continuing severe abdominal pain. She was determined to be experiencing septic shock at that time. She underwent abdominal/pelvic CT, which revealed new development of several areas of free air within the peritoneal cavity, compared with the previous study. She was immediately taken to surgery and was found to have gangrene of the small bowel and perforation of the cecum, with free stool in the area. Resection of the small bowel was performed. The woman remained acidotic with low blood pressure despite various interventions. She went into cardiac arrest and died of septic shock secondary to ischemic colitis.
The plaintiff claimed that the radiologist who reviewed the first abdominal CT failed to identify and report findings of impaired blood flow on the later abdominal/pelvic CT and failed to recommend an urgent surgical consultation.
The radiologist contended that the CT did not show evidence of impaired blood flow.
A $1.5 million settlement was reached.
Failure to Contact Patient With Pathology Results
An Illinois woman, age 31, presented to the defendant dermatologist, Dr. K., in September 2002 with concerns about a mole. The dermatologist thought the mole in question was of no concern but recommended removal of a second mole on the back of her arm. After removing that mole in December 2002, Dr. K. scheduled a return visit in two weeks to assess the healing of the area, remove the sutures, review the pathology results, and discuss further plans.
The patient did not keep the scheduled appointment because she thought she was returning simply to have the stitches removed, and they had already fallen out. The pathology report indicated some architectural disorder with one peripheral margin involved and suggested a further wide excision in the area of the removed mole, with histologic tissue analysis and definitive diagnosis. Neither Dr. K. nor his office staff made any attempt to contact the patient to urge her to come in or to inform her of the pathology results.
The patient later returned to Dr. K.’s office to be seen for acne, but saw a physician assistant instead.
Three years after her procedure, the patient developed melanoma near the site of the mole removal. During a wide excision procedure she underwent in September 2005, a residual melanoma was found. A sentinel lymph node biopsy was also performed. Results were negative, but hypertrophic scarring resulted. At several visits since that time, no additional melanoma has been found.
The plaintiff claimed that the diagnosis of melanoma caused substantial alteration of her lifestyle and a personality change. She claimed that the original arm lesion the defendant removed was premalignant and that he had a duty to contact her, explain the pathology result, and recommend that she return for a wide excision.
The defendant claimed that the lesion he removed was benign, that there was no duty to contact the plaintiff regarding a benign pathology report, and that the proper recommendation would be to watch the area and return if any problems developed. The defendant further claimed that it could not be determined with certainty whether the melanoma evolved from a microscopic portion of the mole or was a new development. The defendant claimed that the plaintiff needed to alter her lifestyle and protect herself from the sun in any event because she had a biogenetic predisposition for melanoma. She was also at future risk for developing new melanomas, unrelated to his treatment.
According to a published report, a defense verdict was returned.
Infant’s Bacterial Infection Missed
The plaintiff child, age 20 months, was brought to the emergency department (ED) with trouble breathing and a low-grade fever. The emergency physician, Dr. S., made a diagnosis of viral croup and discharged her.
The next day, the girl was brought back to the ED, where she was seen by a second emergency physician, Dr. G. Her temperature had risen to 106°F, and she had developed a croupy cough. Following administration of acetaminophen and other medications, the child’s temperature was reduced to 100°F. A test for streptococcal infection yielded negative results, and the infant was discharged.
The infant did well the following day, but that night her parents noticed that she was lethargic and breathing with difficulty. She was taken to the ED again and seen by Dr. G. This time, a diagnosis of pneumonia was quickly made, and the patient was transferred to a children’s hospital.
The child died that evening. Autopsy revealed group A streptococcal infection, which had led to toxic shock syndrome.
The plaintiff claimed that Dr. G. was negligent in his failure to diagnose the infection and to begin antibiotics when he first saw the infant. The plaintiffs claimed that the medications used included steroids, which suppressed the child’s immune system, making it more difficult for her to fight infection.
Dr. G. claimed that the infant’s presentation was inconsistent with a bacterial infection and that his diagnosis was reasonable. The defendant also claimed that the infant had an infection that would not have responded to antibiotics.
A defense verdict was returned.
Change of Procedure, Surgical Instrument Retained
In New York City, a 54-year-old man underwent a sleeve gastrectomy, performed by Dr. M. and Dr. S. Three days later, the patient was informed that a surgical tool had been left in his body, and he returned to the hospital.
CT revealed no foreign object. The patient’s large size made MRI impossible, and he was discharged. The man died three days later of peritonitis, which the plaintiff for the decedent claimed resulted from damage inflicted by a surgical instrument that had not been removed. The plaintiff claimed that the patient had consented to a Roux-en-Y bypass and had undergone presurgical preparation for that procedure, but that plan was abandoned shortly before the surgery. The plaintiff claimed that the problems resulting from the surgery performed were related to the procedure itself—specifically, that the cutting and stapling tool used in the gastrectomy had severed the esophageal stethoscope used, after which the severed remnant of the stethoscope was inadvertently stapled into the patient’s stomach.
The plaintiff for the decedent claimed that the remnant caused leakage of bile, which led to fatal peritonitis. The plaintiff claimed that the surgeons should not have concluded the surgery until it was confirmed that the stomach was properly sealed. The plaintiff also claimed that the defendants failed to address the decedent’s postsurgical symptoms of fever and severe pain.
The defendants countered that the decedent had reported no such symptoms and that there were no indications of infection. The defendants also claimed that neither CT nor intraoperative testing revealed any leakage in the decedent’s stomach. The defendants claimed that the decedent had signed a consent to undergo a gastrectomy, and that a gastrectomy had always been the intended procedure. The defendants additionally claimed that the foreign object in question was a probe, which the anesthesiologist inserted to measure the patient’s esophageal temperature without the surgeons’ knowledge.
According to a published account, a settlement of $675,000 was reached.
Large prolapsed fibroid left untreated—despite surgery...and more
Large prolapsed fibroid left untreated—despite surgery
A 48-YEAR-OLD WOMAN PRESENTED to the emergency department (ED) with vaginal pain. A large, prolapsed uterine fibroid was diagnosed. Because she was scheduled for an ObGyn visit 2 days later, she was discharged without any treatment.
The next day, she returned to the ED with vaginal bleeding. Ultrasonography (US) showed multiple fibroids. Physical exam confirmed a prolapsed uterine fibroid extending into the vaginal vault. Her ObGyn performed an open myomectomy a few days later.
She called her ObGyn’s office prior to her scheduled postoperative visit because she still felt something in her vagina, and had pelvic pain and vaginal bleeding. She also reported this at the office visit, where she met with a nurse practitioner.
Two months later, she called the ObGyn’s office to complain of vaginal bleeding, and described passing large clots.
A month later, she saw a surgeon, who determined that the large prolapsed fibroid had never been removed. Surgery was scheduled, during which her uterus was removed. The patient was hospitalized for 11 days.
PATIENT’S CLAIM The ObGyn was negligent in failing to surgically remove the fibroid, perform postoperative US, and properly examine, diagnose, and treat her postoperatively. The ObGyn’s office staff failed to relay her telephone and in-person complaints to the physician.
DEFENDANTS’ DEFENSE The ObGyn and his group denied negligence.
VERDICT A $248,160 Georgia verdict was returned against the group.
Woman claims she was never told mammogram results
AFTER MANY NORMAL MAMMOGRAMS, a woman had an abnormal annual result. However, she claimed the physician did not inform her of the reported results. A year later, she was diagnosed with breast cancer.
PATIENT’S CLAIM The physician was negligent in failing to follow-up on the abnormal mammogram and make a correct diagnosis.
PHYSICIAN’S DEFENSE The woman had refused a recommended biopsy after the abnormal mammogram, and later refused mastectomy and radiation therapy. The patient’s outcome would have been the same even if treatment had begun shortly after the abnormal mammogram.
VERDICT A $175,000 verdict was returned in Indiana.
5,386-g newborn has Erb’s palsy
OXYTOCIN WAS ADMINISTERED after a woman’s labor slowed. During vaginal delivery, the ObGyn encountered and managed shoulder dystocia. The 11-lb, 14-oz infant was later given a diagnosis of Erb’s palsy.
PLAINTIFF’S CLAIM Excessive force during the ObGyn’s management of shoulder dystocia caused the Erb’s palsy. US should have been performed prior to delivery to determine fetal weight. Cesarean section may have prevented the injury.
PHYSICIAN’S DEFENSE Fetal weight was calculated at a time when vaginal delivery could not be safely discontinued. Excessive traction was not used; if it had been used, the injury would have been more significant.
VERDICT A New York jury returned a $485,000 verdict.
Was there delay in recognizing necrotizing fasciitis?
PREGNANT WITH TWINS, a 24-year-old woman was hospitalized at 33 weeks’ gestation, and remained there until delivery. There was no clinical evidence of fever or intrauterine infection during her hospitalization. Her anogenital culture for group B Streptococcus was positive. Clindamycin was begun 11 days prior to delivery, and continued after a successful cesarean delivery by her ObGyn.
Three days later, the mother suffered a high fever and marked elevation of her white blood cell count. The ObGyn reopened and drained the wound incision. Surgical debridement was not performed. The woman continued to deteriorate.
She developed extensive necrosis of the tissue around the abdominal wound, extending to the pannus and mons pubis. The ObGyn performed wide excision of the tissue. Necrotizing fasciitis was confirmed by pathology.
The woman was diagnosed with sepsis, multi-system organ failure, disseminated intravascular coagulopathy, and respiratory dependence. She was transferred to another hospital, where she remained until her death 3 months after delivery.
ESTATE’S CLAIM The ObGyn failed to diagnose and treat the necrotizing fasciitis in a timely manner. He failed to perform emergency surgical debridement when the lesions were first identified.
PHYSICIAN’S DEFENSE Antibiotics were ordered at the first sign of the vaginal strep infection and continued due to postsurgical wound infection.
Consultations with infectious disease specialists were obtained because of the patient’s history of extreme medication reaction and numerous antibiotic allergies. Although testing reported negative results for other infection sources, the patient failed to respond to treatment. Surgical debridement was performed when necessary, and as often as the patient was deemed able to tolerate the procedure.
VERDICT A Georgia verdict of $4,317,495 was returned.
ObGyn at fault for child’s brain injury and vision loss?
AT 22 WEEKS’ GESTATION, a woman presented to the ED with cramping and bleeding. A nurse called the woman’s ObGyn, who was not at the hospital; he ordered monitoring and laboratory tests. Two hours later, the bleeding and pain increased. The ObGyn was notified, although whether he was told about the excessive bleeding or not is in dispute. He ordered morphine. The patient was sent home without being seen by a physician, with instructions to follow-up with her ObGyn.
The woman claimed she called the hospital the next morning to report continued pain and bleeding, and was told to take a bath. She returned to the hospital the next evening. US revealed a dilated cervix with hour-glassing membranes. The child was delivered at 23-weeks’ gestation and suffers from a brain injury and vision loss.
PLAINTIFF’S CLAIM Premature delivery was due to an incompetent cervix, which could have been treated with cerclage. Diagnostic US and a physical examination by the ObGyn were never performed.
DEFENDANT’S DEFENSE Postdelivery evaluation of the placenta indicated that the mother had chorioamnionitis. Cerclage would have been contraindicated; delivery would have occurred despite any efforts to prolong the pregnancy.
VERDICT A Utah defense verdict was returned.
Did untreated hypertension cause mother’s blindness?
A 34-YEAR-OLD PREGNANT WOMAN was admitted to the hospital with new onset hypertension. Three days later her BP increased to 170/98 mm Hg; her ObGyn performed an emergent cesarean delivery. During the procedure, the woman’s BP rose to 203/120, and remained high in recovery. When she awoke, she reported blurred vision, and was later declared to be legally blind.
PATIENT’S CLAIM The physician failed to properly monitor her BP. Failure to use antihypertensive drugs led to an ischemic event, resulting in vision loss.
PHYSICIAN’S DEFENSE The woman’s BP was properly monitored at all times. She had been diagnosed with Purtscher’s retinopathy syndrome, which predisposed her to pregnancy-related vision loss. Her blindness was not BP-related.
VERDICT A Tennessee defense verdict was returned.
Ruptured ectopic pregnancy not treated properly in ED
WHEN BROUGHT TO THE EMERGENCY DEPARTMENT, a 25-year-old woman was found to be in hemorrhagic shock following a ruptured ectopic pregnancy. Her BP was 42/19 mm Hg. She was taken to surgery, where the ruptured fallopian tube was removed.
After surgery, she complained of tremors in her legs and torso, and had difficulty walking unassisted. She was diagnosed with hypoxic ischemic encephalopathy and transferred to a rehabilitation facility.
PATIENT’S CLAIM She was not properly resuscitated in the ED; intravenous fluids and transfusions should have been given immediately. Delayed treatment in the ED caused hypoxic ischemic encephalopathy or a conversion disorder.
PHYSICIAN’S DEFENSE Intravenous fluids and transfusions were started appropriately and promptly in the ED. The patient did not suffer hypoxic ischemic encephalopathy; a conversion disorder could have occurred from the stress of the ruptured ectopic pregnancy.
VERDICT An Illinois jury returned a defense verdict.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
Large prolapsed fibroid left untreated—despite surgery
A 48-YEAR-OLD WOMAN PRESENTED to the emergency department (ED) with vaginal pain. A large, prolapsed uterine fibroid was diagnosed. Because she was scheduled for an ObGyn visit 2 days later, she was discharged without any treatment.
The next day, she returned to the ED with vaginal bleeding. Ultrasonography (US) showed multiple fibroids. Physical exam confirmed a prolapsed uterine fibroid extending into the vaginal vault. Her ObGyn performed an open myomectomy a few days later.
She called her ObGyn’s office prior to her scheduled postoperative visit because she still felt something in her vagina, and had pelvic pain and vaginal bleeding. She also reported this at the office visit, where she met with a nurse practitioner.
Two months later, she called the ObGyn’s office to complain of vaginal bleeding, and described passing large clots.
A month later, she saw a surgeon, who determined that the large prolapsed fibroid had never been removed. Surgery was scheduled, during which her uterus was removed. The patient was hospitalized for 11 days.
PATIENT’S CLAIM The ObGyn was negligent in failing to surgically remove the fibroid, perform postoperative US, and properly examine, diagnose, and treat her postoperatively. The ObGyn’s office staff failed to relay her telephone and in-person complaints to the physician.
DEFENDANTS’ DEFENSE The ObGyn and his group denied negligence.
VERDICT A $248,160 Georgia verdict was returned against the group.
Woman claims she was never told mammogram results
AFTER MANY NORMAL MAMMOGRAMS, a woman had an abnormal annual result. However, she claimed the physician did not inform her of the reported results. A year later, she was diagnosed with breast cancer.
PATIENT’S CLAIM The physician was negligent in failing to follow-up on the abnormal mammogram and make a correct diagnosis.
PHYSICIAN’S DEFENSE The woman had refused a recommended biopsy after the abnormal mammogram, and later refused mastectomy and radiation therapy. The patient’s outcome would have been the same even if treatment had begun shortly after the abnormal mammogram.
VERDICT A $175,000 verdict was returned in Indiana.
5,386-g newborn has Erb’s palsy
OXYTOCIN WAS ADMINISTERED after a woman’s labor slowed. During vaginal delivery, the ObGyn encountered and managed shoulder dystocia. The 11-lb, 14-oz infant was later given a diagnosis of Erb’s palsy.
PLAINTIFF’S CLAIM Excessive force during the ObGyn’s management of shoulder dystocia caused the Erb’s palsy. US should have been performed prior to delivery to determine fetal weight. Cesarean section may have prevented the injury.
PHYSICIAN’S DEFENSE Fetal weight was calculated at a time when vaginal delivery could not be safely discontinued. Excessive traction was not used; if it had been used, the injury would have been more significant.
VERDICT A New York jury returned a $485,000 verdict.
Was there delay in recognizing necrotizing fasciitis?
PREGNANT WITH TWINS, a 24-year-old woman was hospitalized at 33 weeks’ gestation, and remained there until delivery. There was no clinical evidence of fever or intrauterine infection during her hospitalization. Her anogenital culture for group B Streptococcus was positive. Clindamycin was begun 11 days prior to delivery, and continued after a successful cesarean delivery by her ObGyn.
Three days later, the mother suffered a high fever and marked elevation of her white blood cell count. The ObGyn reopened and drained the wound incision. Surgical debridement was not performed. The woman continued to deteriorate.
She developed extensive necrosis of the tissue around the abdominal wound, extending to the pannus and mons pubis. The ObGyn performed wide excision of the tissue. Necrotizing fasciitis was confirmed by pathology.
The woman was diagnosed with sepsis, multi-system organ failure, disseminated intravascular coagulopathy, and respiratory dependence. She was transferred to another hospital, where she remained until her death 3 months after delivery.
ESTATE’S CLAIM The ObGyn failed to diagnose and treat the necrotizing fasciitis in a timely manner. He failed to perform emergency surgical debridement when the lesions were first identified.
PHYSICIAN’S DEFENSE Antibiotics were ordered at the first sign of the vaginal strep infection and continued due to postsurgical wound infection.
Consultations with infectious disease specialists were obtained because of the patient’s history of extreme medication reaction and numerous antibiotic allergies. Although testing reported negative results for other infection sources, the patient failed to respond to treatment. Surgical debridement was performed when necessary, and as often as the patient was deemed able to tolerate the procedure.
VERDICT A Georgia verdict of $4,317,495 was returned.
ObGyn at fault for child’s brain injury and vision loss?
AT 22 WEEKS’ GESTATION, a woman presented to the ED with cramping and bleeding. A nurse called the woman’s ObGyn, who was not at the hospital; he ordered monitoring and laboratory tests. Two hours later, the bleeding and pain increased. The ObGyn was notified, although whether he was told about the excessive bleeding or not is in dispute. He ordered morphine. The patient was sent home without being seen by a physician, with instructions to follow-up with her ObGyn.
The woman claimed she called the hospital the next morning to report continued pain and bleeding, and was told to take a bath. She returned to the hospital the next evening. US revealed a dilated cervix with hour-glassing membranes. The child was delivered at 23-weeks’ gestation and suffers from a brain injury and vision loss.
PLAINTIFF’S CLAIM Premature delivery was due to an incompetent cervix, which could have been treated with cerclage. Diagnostic US and a physical examination by the ObGyn were never performed.
DEFENDANT’S DEFENSE Postdelivery evaluation of the placenta indicated that the mother had chorioamnionitis. Cerclage would have been contraindicated; delivery would have occurred despite any efforts to prolong the pregnancy.
VERDICT A Utah defense verdict was returned.
Did untreated hypertension cause mother’s blindness?
A 34-YEAR-OLD PREGNANT WOMAN was admitted to the hospital with new onset hypertension. Three days later her BP increased to 170/98 mm Hg; her ObGyn performed an emergent cesarean delivery. During the procedure, the woman’s BP rose to 203/120, and remained high in recovery. When she awoke, she reported blurred vision, and was later declared to be legally blind.
PATIENT’S CLAIM The physician failed to properly monitor her BP. Failure to use antihypertensive drugs led to an ischemic event, resulting in vision loss.
PHYSICIAN’S DEFENSE The woman’s BP was properly monitored at all times. She had been diagnosed with Purtscher’s retinopathy syndrome, which predisposed her to pregnancy-related vision loss. Her blindness was not BP-related.
VERDICT A Tennessee defense verdict was returned.
Ruptured ectopic pregnancy not treated properly in ED
WHEN BROUGHT TO THE EMERGENCY DEPARTMENT, a 25-year-old woman was found to be in hemorrhagic shock following a ruptured ectopic pregnancy. Her BP was 42/19 mm Hg. She was taken to surgery, where the ruptured fallopian tube was removed.
After surgery, she complained of tremors in her legs and torso, and had difficulty walking unassisted. She was diagnosed with hypoxic ischemic encephalopathy and transferred to a rehabilitation facility.
PATIENT’S CLAIM She was not properly resuscitated in the ED; intravenous fluids and transfusions should have been given immediately. Delayed treatment in the ED caused hypoxic ischemic encephalopathy or a conversion disorder.
PHYSICIAN’S DEFENSE Intravenous fluids and transfusions were started appropriately and promptly in the ED. The patient did not suffer hypoxic ischemic encephalopathy; a conversion disorder could have occurred from the stress of the ruptured ectopic pregnancy.
VERDICT An Illinois jury returned a defense verdict.
Large prolapsed fibroid left untreated—despite surgery
A 48-YEAR-OLD WOMAN PRESENTED to the emergency department (ED) with vaginal pain. A large, prolapsed uterine fibroid was diagnosed. Because she was scheduled for an ObGyn visit 2 days later, she was discharged without any treatment.
The next day, she returned to the ED with vaginal bleeding. Ultrasonography (US) showed multiple fibroids. Physical exam confirmed a prolapsed uterine fibroid extending into the vaginal vault. Her ObGyn performed an open myomectomy a few days later.
She called her ObGyn’s office prior to her scheduled postoperative visit because she still felt something in her vagina, and had pelvic pain and vaginal bleeding. She also reported this at the office visit, where she met with a nurse practitioner.
Two months later, she called the ObGyn’s office to complain of vaginal bleeding, and described passing large clots.
A month later, she saw a surgeon, who determined that the large prolapsed fibroid had never been removed. Surgery was scheduled, during which her uterus was removed. The patient was hospitalized for 11 days.
PATIENT’S CLAIM The ObGyn was negligent in failing to surgically remove the fibroid, perform postoperative US, and properly examine, diagnose, and treat her postoperatively. The ObGyn’s office staff failed to relay her telephone and in-person complaints to the physician.
DEFENDANTS’ DEFENSE The ObGyn and his group denied negligence.
VERDICT A $248,160 Georgia verdict was returned against the group.
Woman claims she was never told mammogram results
AFTER MANY NORMAL MAMMOGRAMS, a woman had an abnormal annual result. However, she claimed the physician did not inform her of the reported results. A year later, she was diagnosed with breast cancer.
PATIENT’S CLAIM The physician was negligent in failing to follow-up on the abnormal mammogram and make a correct diagnosis.
PHYSICIAN’S DEFENSE The woman had refused a recommended biopsy after the abnormal mammogram, and later refused mastectomy and radiation therapy. The patient’s outcome would have been the same even if treatment had begun shortly after the abnormal mammogram.
VERDICT A $175,000 verdict was returned in Indiana.
5,386-g newborn has Erb’s palsy
OXYTOCIN WAS ADMINISTERED after a woman’s labor slowed. During vaginal delivery, the ObGyn encountered and managed shoulder dystocia. The 11-lb, 14-oz infant was later given a diagnosis of Erb’s palsy.
PLAINTIFF’S CLAIM Excessive force during the ObGyn’s management of shoulder dystocia caused the Erb’s palsy. US should have been performed prior to delivery to determine fetal weight. Cesarean section may have prevented the injury.
PHYSICIAN’S DEFENSE Fetal weight was calculated at a time when vaginal delivery could not be safely discontinued. Excessive traction was not used; if it had been used, the injury would have been more significant.
VERDICT A New York jury returned a $485,000 verdict.
Was there delay in recognizing necrotizing fasciitis?
PREGNANT WITH TWINS, a 24-year-old woman was hospitalized at 33 weeks’ gestation, and remained there until delivery. There was no clinical evidence of fever or intrauterine infection during her hospitalization. Her anogenital culture for group B Streptococcus was positive. Clindamycin was begun 11 days prior to delivery, and continued after a successful cesarean delivery by her ObGyn.
Three days later, the mother suffered a high fever and marked elevation of her white blood cell count. The ObGyn reopened and drained the wound incision. Surgical debridement was not performed. The woman continued to deteriorate.
She developed extensive necrosis of the tissue around the abdominal wound, extending to the pannus and mons pubis. The ObGyn performed wide excision of the tissue. Necrotizing fasciitis was confirmed by pathology.
The woman was diagnosed with sepsis, multi-system organ failure, disseminated intravascular coagulopathy, and respiratory dependence. She was transferred to another hospital, where she remained until her death 3 months after delivery.
ESTATE’S CLAIM The ObGyn failed to diagnose and treat the necrotizing fasciitis in a timely manner. He failed to perform emergency surgical debridement when the lesions were first identified.
PHYSICIAN’S DEFENSE Antibiotics were ordered at the first sign of the vaginal strep infection and continued due to postsurgical wound infection.
Consultations with infectious disease specialists were obtained because of the patient’s history of extreme medication reaction and numerous antibiotic allergies. Although testing reported negative results for other infection sources, the patient failed to respond to treatment. Surgical debridement was performed when necessary, and as often as the patient was deemed able to tolerate the procedure.
VERDICT A Georgia verdict of $4,317,495 was returned.
ObGyn at fault for child’s brain injury and vision loss?
AT 22 WEEKS’ GESTATION, a woman presented to the ED with cramping and bleeding. A nurse called the woman’s ObGyn, who was not at the hospital; he ordered monitoring and laboratory tests. Two hours later, the bleeding and pain increased. The ObGyn was notified, although whether he was told about the excessive bleeding or not is in dispute. He ordered morphine. The patient was sent home without being seen by a physician, with instructions to follow-up with her ObGyn.
The woman claimed she called the hospital the next morning to report continued pain and bleeding, and was told to take a bath. She returned to the hospital the next evening. US revealed a dilated cervix with hour-glassing membranes. The child was delivered at 23-weeks’ gestation and suffers from a brain injury and vision loss.
PLAINTIFF’S CLAIM Premature delivery was due to an incompetent cervix, which could have been treated with cerclage. Diagnostic US and a physical examination by the ObGyn were never performed.
DEFENDANT’S DEFENSE Postdelivery evaluation of the placenta indicated that the mother had chorioamnionitis. Cerclage would have been contraindicated; delivery would have occurred despite any efforts to prolong the pregnancy.
VERDICT A Utah defense verdict was returned.
Did untreated hypertension cause mother’s blindness?
A 34-YEAR-OLD PREGNANT WOMAN was admitted to the hospital with new onset hypertension. Three days later her BP increased to 170/98 mm Hg; her ObGyn performed an emergent cesarean delivery. During the procedure, the woman’s BP rose to 203/120, and remained high in recovery. When she awoke, she reported blurred vision, and was later declared to be legally blind.
PATIENT’S CLAIM The physician failed to properly monitor her BP. Failure to use antihypertensive drugs led to an ischemic event, resulting in vision loss.
PHYSICIAN’S DEFENSE The woman’s BP was properly monitored at all times. She had been diagnosed with Purtscher’s retinopathy syndrome, which predisposed her to pregnancy-related vision loss. Her blindness was not BP-related.
VERDICT A Tennessee defense verdict was returned.
Ruptured ectopic pregnancy not treated properly in ED
WHEN BROUGHT TO THE EMERGENCY DEPARTMENT, a 25-year-old woman was found to be in hemorrhagic shock following a ruptured ectopic pregnancy. Her BP was 42/19 mm Hg. She was taken to surgery, where the ruptured fallopian tube was removed.
After surgery, she complained of tremors in her legs and torso, and had difficulty walking unassisted. She was diagnosed with hypoxic ischemic encephalopathy and transferred to a rehabilitation facility.
PATIENT’S CLAIM She was not properly resuscitated in the ED; intravenous fluids and transfusions should have been given immediately. Delayed treatment in the ED caused hypoxic ischemic encephalopathy or a conversion disorder.
PHYSICIAN’S DEFENSE Intravenous fluids and transfusions were started appropriately and promptly in the ED. The patient did not suffer hypoxic ischemic encephalopathy; a conversion disorder could have occurred from the stress of the ruptured ectopic pregnancy.
VERDICT An Illinois jury returned a defense verdict.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
Playground Politics
Baseball, kick the can, Russian roulette—pick your game. Chances are good that it has worked its way into a metaphor to illustrate the infuriating, perplexing, and altogether frustrating inability of Congress to step up to the plate and pass a long-term fix to the broken sustainable growth rate (SGR) formula used to determine Medicare reimbursement rates.
On June 24, legislators avoided catastrophe by temporarily rescinding a 21.3% rate cut that went into effect June 1. The after-the-fact patch meant that some Medicare claims had to be reprocessed to recoup the full value, creating an administrative mess. The accompanying 2.2% rate increase expires Nov. 30. The reimbursement cut could reach nearly 30% next year unless Congress intervenes again.
“Obviously, there’s a lot of frustration around the issue, especially on the membership side,” says Ron Greeno, MD, FACP, SFHM, a member of SHM’s Public Policy and Leadership committees, and chief medical officer for Brentwood, Tenn.-based Cogent Healthcare. For hospitalists in many small private practices, he says, a major percentage of income comes from Medicare. “It’s a tremendous headache,” he says of the uncertainty. “It’s very hard to plan for. You’re trying to budget and you don’t know what the policy is going to be literally from week to week.”
The Blame Game
Despite the widespread sentiment among doctors that a permanent reimbursement rate fix should have been included in the healthcare reform legislation, skittishness over the price tag led legislators to drop it from the package. Based on last fall’s estimates, the total cost of a reform bill that scrapped the SGR would have ballooned by roughly $250 billion over 10 years, which would have threatened the bill’s passage.
But Congress has since been unable to pass a permanent fix as standalone legislation amid mounting concern over the national debt, and the price of inaction continues to rise. On April 30, the Congressional Budget Office (CBO) estimated that the cost of jettisoning the SGR formula and freezing rates at current levels had grown to $276 billion over 10 years.
Any serious consideration of lasting alternatives has now been pushed back to the lame-duck session, after the midterm elections. The can has been kicked down the road so many times, Dr. Greeno and others say, that most Congressional members have boot marks all over them. “So now you have a bigger problem at a more crucial time, when money is tighter than ever in a poor economy,” Dr. Greeno says. “And I just think it’s been a failure of our politicians.”
Other healthcare industry leaders have been just as critical. “Delaying the problem is not a solution,” said AMA President Cecil B. Wilson, MD, in a prepared statement after Congress passed the latest six-month reprieve in June. “It doesn’t solve the Medicare mess Congress has created with a long series of short-term Medicare patches over the last decade—including four to avert the 2010 cut alone.”
AMA-sponsored print ads have reminded legislators that delaying a fix until 2013 will again increase its cost, to $396 billion over 10 years. And the association’s June press release asserted that “Congress is playing a dangerous game of Russian roulette with seniors’ healthcare.”
Perhaps a game of “chicken” would be more apt.
Republicans have dared Democrats to spend the billions for a more lasting solution—in the absence of any cuts elsewhere in the healthcare delivery system—and be labeled as fiscally irresponsible. In turn, Democrats have dared Republicans to let the rate cut take effect and be labeled heartless as Medicare beneficiaries lose access to their healthcare providers.
Both parties blinked, resorting to almost unanimous short-term fixes that have allowed legislators to save face while putting off politically risky votes until after the November elections.
Lynne M. Allen, MN, ARNP, who works as a part-time hospitalist in hematology-oncology at 188-bed Kadlec Regional Medical Center in Richland, Wash., says she and other colleagues were initially hopeful that the Obama administration would make Congress work together to find a lasting solution. “There’s a sense of frustration because instead of that happening from our legislators, they’re playing a lot of games with the funding,” says Allen, a member of Team Hospitalist. “They’re not willing to step up to the plate, as they say, and make a decision that will allow us to go forward smoothly.”
The result, Allen says, has been a “roller-coaster ride” of uncertainty over reimbursements. Because Washington’s Tri-Cities region has a relatively high percentage of patients with private insurance, her hospital is somewhat cushioned from a precipitous drop in Medicare fees. But if CMS is ever forced to cut back on its rates, she fully expects private insurers to follow the same downward track.
Practical Concerns
Barbara Hartley, MD, a part-time hospitalist at 22-bed Benson Hospital in Benson, Ariz., says the town’s healthcare facility is somewhat protected from potential Medicare rate cuts through its official status as a Critical Access Hospital. Instead of being reimbursed through diagnosis-related group (DRG) codes, the rural hospital is repaid by Medicare for its total cost per day per patient.
The arrangement is a stable one at the moment, but not enough to dispel Dr. Hartley’s uneasy question: If the economy worsens, will Medicare be able to retain its commitment to rural hospitals? If not, the pain might be felt acutely in communities like Benson, where Dr. Hartley estimates that as much as 75% of the hospital’s in-patient business is through either Medicare or a Medicare Advantage plan.
Kirk Mathews, CEO of St. Louis-based Inpatient Management Inc. and a member of SHM’s Public Policy and Practice Management committees, says Medicare rate cuts also could significantly reduce the leverage of hospitalists during contract negotiations.
“Even if we’re employed by the hospital, but our professional fees that the hospital can recoup for our services are dramatically affected, it will affect how those future contracts go,” Mathews says. “We might be insulated temporarily by the strength of our current contract. But if the formula—however that works out—dramatically impacts the hospitalist reimbursement on the professional fee side, the hospital will feel that, and then hospitalists will eventually feel that as well.” In other words, it could strengthen the bargaining hand of the hospital at the expense of the hospitalist. “Therein lies the long-term threat,” he points out.
Independent Solution?
Some of the authority over physician payments might eventually be depoliticized via language in the reform legislation that empowers a new entity, the Independent Payment Advisory Board, to create policy on such critical monetary issues as reimbursement rates. Congress could still override the board’s policy decisions, but only if the Congressional alternative saves just as much money.
In the meantime, the money for a fix still has to come from somewhere, and no consensus has emerged. Advocates likewise refuse to coalesce around any single alternative. Some experts favor a new formula based on the Medicare economic index, which measures inflation in healthcare delivery costs. But the CBO estimates that per-beneficiary spending under such a formula would be 30% more by 2016 than under the current formula. Other proposals call for temporarily increasing rates, then reverting to annual GDP growth, plus a bit more to cover physician costs.
No matter how the crisis is resolved, experts say, doctors almost certainly will have to make do with less. “When healthcare reform is finally fully implemented, there are going to be less dollars to pay for more services. It’s inevitable,” Mathews says. “And whether it takes the form of SGR or some other form, I’m afraid physicians are going to have to get used to having less money in the pool of money that’s allocated to pay providers.”
It could be a whole new ballgame. TH
Bryn Nelson, PhD, is a freelance medical writer based in Seattle.
Baseball, kick the can, Russian roulette—pick your game. Chances are good that it has worked its way into a metaphor to illustrate the infuriating, perplexing, and altogether frustrating inability of Congress to step up to the plate and pass a long-term fix to the broken sustainable growth rate (SGR) formula used to determine Medicare reimbursement rates.
On June 24, legislators avoided catastrophe by temporarily rescinding a 21.3% rate cut that went into effect June 1. The after-the-fact patch meant that some Medicare claims had to be reprocessed to recoup the full value, creating an administrative mess. The accompanying 2.2% rate increase expires Nov. 30. The reimbursement cut could reach nearly 30% next year unless Congress intervenes again.
“Obviously, there’s a lot of frustration around the issue, especially on the membership side,” says Ron Greeno, MD, FACP, SFHM, a member of SHM’s Public Policy and Leadership committees, and chief medical officer for Brentwood, Tenn.-based Cogent Healthcare. For hospitalists in many small private practices, he says, a major percentage of income comes from Medicare. “It’s a tremendous headache,” he says of the uncertainty. “It’s very hard to plan for. You’re trying to budget and you don’t know what the policy is going to be literally from week to week.”
The Blame Game
Despite the widespread sentiment among doctors that a permanent reimbursement rate fix should have been included in the healthcare reform legislation, skittishness over the price tag led legislators to drop it from the package. Based on last fall’s estimates, the total cost of a reform bill that scrapped the SGR would have ballooned by roughly $250 billion over 10 years, which would have threatened the bill’s passage.
But Congress has since been unable to pass a permanent fix as standalone legislation amid mounting concern over the national debt, and the price of inaction continues to rise. On April 30, the Congressional Budget Office (CBO) estimated that the cost of jettisoning the SGR formula and freezing rates at current levels had grown to $276 billion over 10 years.
Any serious consideration of lasting alternatives has now been pushed back to the lame-duck session, after the midterm elections. The can has been kicked down the road so many times, Dr. Greeno and others say, that most Congressional members have boot marks all over them. “So now you have a bigger problem at a more crucial time, when money is tighter than ever in a poor economy,” Dr. Greeno says. “And I just think it’s been a failure of our politicians.”
Other healthcare industry leaders have been just as critical. “Delaying the problem is not a solution,” said AMA President Cecil B. Wilson, MD, in a prepared statement after Congress passed the latest six-month reprieve in June. “It doesn’t solve the Medicare mess Congress has created with a long series of short-term Medicare patches over the last decade—including four to avert the 2010 cut alone.”
AMA-sponsored print ads have reminded legislators that delaying a fix until 2013 will again increase its cost, to $396 billion over 10 years. And the association’s June press release asserted that “Congress is playing a dangerous game of Russian roulette with seniors’ healthcare.”
Perhaps a game of “chicken” would be more apt.
Republicans have dared Democrats to spend the billions for a more lasting solution—in the absence of any cuts elsewhere in the healthcare delivery system—and be labeled as fiscally irresponsible. In turn, Democrats have dared Republicans to let the rate cut take effect and be labeled heartless as Medicare beneficiaries lose access to their healthcare providers.
Both parties blinked, resorting to almost unanimous short-term fixes that have allowed legislators to save face while putting off politically risky votes until after the November elections.
Lynne M. Allen, MN, ARNP, who works as a part-time hospitalist in hematology-oncology at 188-bed Kadlec Regional Medical Center in Richland, Wash., says she and other colleagues were initially hopeful that the Obama administration would make Congress work together to find a lasting solution. “There’s a sense of frustration because instead of that happening from our legislators, they’re playing a lot of games with the funding,” says Allen, a member of Team Hospitalist. “They’re not willing to step up to the plate, as they say, and make a decision that will allow us to go forward smoothly.”
The result, Allen says, has been a “roller-coaster ride” of uncertainty over reimbursements. Because Washington’s Tri-Cities region has a relatively high percentage of patients with private insurance, her hospital is somewhat cushioned from a precipitous drop in Medicare fees. But if CMS is ever forced to cut back on its rates, she fully expects private insurers to follow the same downward track.
Practical Concerns
Barbara Hartley, MD, a part-time hospitalist at 22-bed Benson Hospital in Benson, Ariz., says the town’s healthcare facility is somewhat protected from potential Medicare rate cuts through its official status as a Critical Access Hospital. Instead of being reimbursed through diagnosis-related group (DRG) codes, the rural hospital is repaid by Medicare for its total cost per day per patient.
The arrangement is a stable one at the moment, but not enough to dispel Dr. Hartley’s uneasy question: If the economy worsens, will Medicare be able to retain its commitment to rural hospitals? If not, the pain might be felt acutely in communities like Benson, where Dr. Hartley estimates that as much as 75% of the hospital’s in-patient business is through either Medicare or a Medicare Advantage plan.
Kirk Mathews, CEO of St. Louis-based Inpatient Management Inc. and a member of SHM’s Public Policy and Practice Management committees, says Medicare rate cuts also could significantly reduce the leverage of hospitalists during contract negotiations.
“Even if we’re employed by the hospital, but our professional fees that the hospital can recoup for our services are dramatically affected, it will affect how those future contracts go,” Mathews says. “We might be insulated temporarily by the strength of our current contract. But if the formula—however that works out—dramatically impacts the hospitalist reimbursement on the professional fee side, the hospital will feel that, and then hospitalists will eventually feel that as well.” In other words, it could strengthen the bargaining hand of the hospital at the expense of the hospitalist. “Therein lies the long-term threat,” he points out.
Independent Solution?
Some of the authority over physician payments might eventually be depoliticized via language in the reform legislation that empowers a new entity, the Independent Payment Advisory Board, to create policy on such critical monetary issues as reimbursement rates. Congress could still override the board’s policy decisions, but only if the Congressional alternative saves just as much money.
In the meantime, the money for a fix still has to come from somewhere, and no consensus has emerged. Advocates likewise refuse to coalesce around any single alternative. Some experts favor a new formula based on the Medicare economic index, which measures inflation in healthcare delivery costs. But the CBO estimates that per-beneficiary spending under such a formula would be 30% more by 2016 than under the current formula. Other proposals call for temporarily increasing rates, then reverting to annual GDP growth, plus a bit more to cover physician costs.
No matter how the crisis is resolved, experts say, doctors almost certainly will have to make do with less. “When healthcare reform is finally fully implemented, there are going to be less dollars to pay for more services. It’s inevitable,” Mathews says. “And whether it takes the form of SGR or some other form, I’m afraid physicians are going to have to get used to having less money in the pool of money that’s allocated to pay providers.”
It could be a whole new ballgame. TH
Bryn Nelson, PhD, is a freelance medical writer based in Seattle.
Baseball, kick the can, Russian roulette—pick your game. Chances are good that it has worked its way into a metaphor to illustrate the infuriating, perplexing, and altogether frustrating inability of Congress to step up to the plate and pass a long-term fix to the broken sustainable growth rate (SGR) formula used to determine Medicare reimbursement rates.
On June 24, legislators avoided catastrophe by temporarily rescinding a 21.3% rate cut that went into effect June 1. The after-the-fact patch meant that some Medicare claims had to be reprocessed to recoup the full value, creating an administrative mess. The accompanying 2.2% rate increase expires Nov. 30. The reimbursement cut could reach nearly 30% next year unless Congress intervenes again.
“Obviously, there’s a lot of frustration around the issue, especially on the membership side,” says Ron Greeno, MD, FACP, SFHM, a member of SHM’s Public Policy and Leadership committees, and chief medical officer for Brentwood, Tenn.-based Cogent Healthcare. For hospitalists in many small private practices, he says, a major percentage of income comes from Medicare. “It’s a tremendous headache,” he says of the uncertainty. “It’s very hard to plan for. You’re trying to budget and you don’t know what the policy is going to be literally from week to week.”
The Blame Game
Despite the widespread sentiment among doctors that a permanent reimbursement rate fix should have been included in the healthcare reform legislation, skittishness over the price tag led legislators to drop it from the package. Based on last fall’s estimates, the total cost of a reform bill that scrapped the SGR would have ballooned by roughly $250 billion over 10 years, which would have threatened the bill’s passage.
But Congress has since been unable to pass a permanent fix as standalone legislation amid mounting concern over the national debt, and the price of inaction continues to rise. On April 30, the Congressional Budget Office (CBO) estimated that the cost of jettisoning the SGR formula and freezing rates at current levels had grown to $276 billion over 10 years.
Any serious consideration of lasting alternatives has now been pushed back to the lame-duck session, after the midterm elections. The can has been kicked down the road so many times, Dr. Greeno and others say, that most Congressional members have boot marks all over them. “So now you have a bigger problem at a more crucial time, when money is tighter than ever in a poor economy,” Dr. Greeno says. “And I just think it’s been a failure of our politicians.”
Other healthcare industry leaders have been just as critical. “Delaying the problem is not a solution,” said AMA President Cecil B. Wilson, MD, in a prepared statement after Congress passed the latest six-month reprieve in June. “It doesn’t solve the Medicare mess Congress has created with a long series of short-term Medicare patches over the last decade—including four to avert the 2010 cut alone.”
AMA-sponsored print ads have reminded legislators that delaying a fix until 2013 will again increase its cost, to $396 billion over 10 years. And the association’s June press release asserted that “Congress is playing a dangerous game of Russian roulette with seniors’ healthcare.”
Perhaps a game of “chicken” would be more apt.
Republicans have dared Democrats to spend the billions for a more lasting solution—in the absence of any cuts elsewhere in the healthcare delivery system—and be labeled as fiscally irresponsible. In turn, Democrats have dared Republicans to let the rate cut take effect and be labeled heartless as Medicare beneficiaries lose access to their healthcare providers.
Both parties blinked, resorting to almost unanimous short-term fixes that have allowed legislators to save face while putting off politically risky votes until after the November elections.
Lynne M. Allen, MN, ARNP, who works as a part-time hospitalist in hematology-oncology at 188-bed Kadlec Regional Medical Center in Richland, Wash., says she and other colleagues were initially hopeful that the Obama administration would make Congress work together to find a lasting solution. “There’s a sense of frustration because instead of that happening from our legislators, they’re playing a lot of games with the funding,” says Allen, a member of Team Hospitalist. “They’re not willing to step up to the plate, as they say, and make a decision that will allow us to go forward smoothly.”
The result, Allen says, has been a “roller-coaster ride” of uncertainty over reimbursements. Because Washington’s Tri-Cities region has a relatively high percentage of patients with private insurance, her hospital is somewhat cushioned from a precipitous drop in Medicare fees. But if CMS is ever forced to cut back on its rates, she fully expects private insurers to follow the same downward track.
Practical Concerns
Barbara Hartley, MD, a part-time hospitalist at 22-bed Benson Hospital in Benson, Ariz., says the town’s healthcare facility is somewhat protected from potential Medicare rate cuts through its official status as a Critical Access Hospital. Instead of being reimbursed through diagnosis-related group (DRG) codes, the rural hospital is repaid by Medicare for its total cost per day per patient.
The arrangement is a stable one at the moment, but not enough to dispel Dr. Hartley’s uneasy question: If the economy worsens, will Medicare be able to retain its commitment to rural hospitals? If not, the pain might be felt acutely in communities like Benson, where Dr. Hartley estimates that as much as 75% of the hospital’s in-patient business is through either Medicare or a Medicare Advantage plan.
Kirk Mathews, CEO of St. Louis-based Inpatient Management Inc. and a member of SHM’s Public Policy and Practice Management committees, says Medicare rate cuts also could significantly reduce the leverage of hospitalists during contract negotiations.
“Even if we’re employed by the hospital, but our professional fees that the hospital can recoup for our services are dramatically affected, it will affect how those future contracts go,” Mathews says. “We might be insulated temporarily by the strength of our current contract. But if the formula—however that works out—dramatically impacts the hospitalist reimbursement on the professional fee side, the hospital will feel that, and then hospitalists will eventually feel that as well.” In other words, it could strengthen the bargaining hand of the hospital at the expense of the hospitalist. “Therein lies the long-term threat,” he points out.
Independent Solution?
Some of the authority over physician payments might eventually be depoliticized via language in the reform legislation that empowers a new entity, the Independent Payment Advisory Board, to create policy on such critical monetary issues as reimbursement rates. Congress could still override the board’s policy decisions, but only if the Congressional alternative saves just as much money.
In the meantime, the money for a fix still has to come from somewhere, and no consensus has emerged. Advocates likewise refuse to coalesce around any single alternative. Some experts favor a new formula based on the Medicare economic index, which measures inflation in healthcare delivery costs. But the CBO estimates that per-beneficiary spending under such a formula would be 30% more by 2016 than under the current formula. Other proposals call for temporarily increasing rates, then reverting to annual GDP growth, plus a bit more to cover physician costs.
No matter how the crisis is resolved, experts say, doctors almost certainly will have to make do with less. “When healthcare reform is finally fully implemented, there are going to be less dollars to pay for more services. It’s inevitable,” Mathews says. “And whether it takes the form of SGR or some other form, I’m afraid physicians are going to have to get used to having less money in the pool of money that’s allocated to pay providers.”
It could be a whole new ballgame. TH
Bryn Nelson, PhD, is a freelance medical writer based in Seattle.
Malpractice Chronicle
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Too Young for a TIA?
In May 2004, a 41-year-old Wisconsin man presented to the defendant neurologist, reporting episodes of vertigo, headaches, double vision, and occasional hallucinations. MRI was performed with results reported as normal, and the neurologist told the patient that the cause of his symptoms was most likely nonorganic.
The symptoms abated for a time, but when they began to recur in November 2005, the man returned to the neurologist. MRI was performed again and interpreted by the defendant radiologist, who noted that the patient had experienced a stroke in the brain stem. He sent a report to that effect on a “stat” basis.
A few days later, the patient presented to a hospital emergency department (ED) with worsening symptoms. When the neurologist was called, he claimed to be learning of the MRI results for the first time. The radiologist reportedly acknowledged that he had not documented where the “stat” report was sent; nor did he follow up to confirm that it had been received.
At the time of the man’s admission through the ED, he was found to have had a full-blown brain-stem stroke with blockage of the basilar artery. His symptoms progressed to a condition of locked-in syndrome. His condition gradually improved, but he was left with permanent weakness in the arms and legs, blurred vision, and difficulty swallowing. His leg weakness makes it impossible to stand or walk. He also requires injections of onabotulinum toxin A every few months to control left arm and leg spasms.
The plaintiff claimed that the neurologist should have made a diagnosis of transient ischemic attacks (TIAs), and that another MRI and other testing should have been ordered. The plaintiff maintained that additional testing would have led to recognition of narrowing in his basilar artery and appropriate treatment, thereby preventing the stroke. The plaintiff claimed negligence on the radiologist’s part for failing to confirm that the report of the acute brain-stem stroke had been received and acted upon.
The defendants claimed that even an earlier diagnosis and treatment would not have led to a better result. The neurologist also claimed that the plaintiff’s symptoms were not consistent with TIAs, particularly in a person of his age.
According to a published account, a $4.5 million settlement was reached.
Suspected Cellulitis–C difficile Colitis Link
In July 2005, a 71-year-old New York man presented to the defendant orthopedic surgeon, Dr. F., because his prosthetic right knee was painful and swollen. The surgeon, who had inserted the prosthetic hardware, found a stress fracture of the patella. Aspirated fluid was bloody; Dr. F., suspecting an infection, prescribed cephalexin.
Almost a month later, the patient returned and reported continuing problems with the knee. X-rays revealed loosening of the tibial component of the prosthesis. Again, aspirated fluid contained blood, and the defendant orthopedist continued to treat the patient for what he believed was an infection.
Two weeks later, Dr. F. performed exploratory surgery on the man’s knee. The prosthesis was removed, and an antibiotic spacer was implanted. A culture revealed a small amount of cellulitis. During this hospitalization, the patient received a diagnosis of Clostridium difficile colitis, which led to pulmonary and renal system failure, sepsis, fungemia, and a stroke.
The plaintiff was hospitalized until December 2005, then was transferred to a nursing home. He remained there until June 2006, when a new prosthesis was placed. He was hospitalized for that surgery until August 2006.
The plaintiff claimed that his cellulitis should have been diagnosed in July 2005 and that the surgery and colitis and its effects resulted from Dr. F.’s failure to make a timely diagnosis of cellulitis. The plaintiff argued that the fluid aspirated in July should have been cultured, that the cephalexin dosage should have been increased, and that a different antibiotic should have been prescribed.
The defendant claimed that the plaintiff’s knee was not infected when he examined it in July and that any infection present during the surgery was unrelated to the plaintiff’s colitis. The defendant maintained that cephalexin was an appropriate treatment choice, and that cellulitis would not have been detected by any test that could have been performed in July.
According to a published account, a $1.9 million verdict was returned.
When Was the Bowel Perforated?
A 34-year-old woman underwent surgery at a Michigan hospital for removal of her left ovary. The defendant surgeon attempted to perform the procedure laparoscopically but encountered extensive scar tissue resulting from several previous surgeries. He found the patient’s ovary adhered to the sigmoid colon and pelvic sidewall. After separating the ovary, the surgeon abandoned the plan to remove it completely out of concern for the adjacent ureter.
The woman was discharged the next day, although she claimed to be in severe pain. Six days after the surgery, she called the defendant to report difficulty catching her breath. She was sent to her primary care clinician’s office, where she expressed additional complaints of fever and abdominal pain.
Three days later, the patient called the surgeon with continuing complaints of poor appetite and difficulty breathing and walking. She was directed to present to the emergency department (ED), where a 2.5-inch perforation of the sigmoid colon was discovered. The woman was required to undergo removal of a nine-inch segment of the bowel and primary reattachment of the remaining colon.
The plaintiff claimed that the colon was perforated during the attempted ovarectomy and that the defendant surgeon was negligent in failing to diagnose and repair the perforation at that time. The plaintiff alleged that the delay in diagnosis and treatment led to the subsequent surgery, loss of part of the bowel, and development of irritable bowel syndrome.
The defendant denied that any injury to the colon was detectable during the surgery he performed and claimed that the perforation occurred later.
A defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Too Young for a TIA?
In May 2004, a 41-year-old Wisconsin man presented to the defendant neurologist, reporting episodes of vertigo, headaches, double vision, and occasional hallucinations. MRI was performed with results reported as normal, and the neurologist told the patient that the cause of his symptoms was most likely nonorganic.
The symptoms abated for a time, but when they began to recur in November 2005, the man returned to the neurologist. MRI was performed again and interpreted by the defendant radiologist, who noted that the patient had experienced a stroke in the brain stem. He sent a report to that effect on a “stat” basis.
A few days later, the patient presented to a hospital emergency department (ED) with worsening symptoms. When the neurologist was called, he claimed to be learning of the MRI results for the first time. The radiologist reportedly acknowledged that he had not documented where the “stat” report was sent; nor did he follow up to confirm that it had been received.
At the time of the man’s admission through the ED, he was found to have had a full-blown brain-stem stroke with blockage of the basilar artery. His symptoms progressed to a condition of locked-in syndrome. His condition gradually improved, but he was left with permanent weakness in the arms and legs, blurred vision, and difficulty swallowing. His leg weakness makes it impossible to stand or walk. He also requires injections of onabotulinum toxin A every few months to control left arm and leg spasms.
The plaintiff claimed that the neurologist should have made a diagnosis of transient ischemic attacks (TIAs), and that another MRI and other testing should have been ordered. The plaintiff maintained that additional testing would have led to recognition of narrowing in his basilar artery and appropriate treatment, thereby preventing the stroke. The plaintiff claimed negligence on the radiologist’s part for failing to confirm that the report of the acute brain-stem stroke had been received and acted upon.
The defendants claimed that even an earlier diagnosis and treatment would not have led to a better result. The neurologist also claimed that the plaintiff’s symptoms were not consistent with TIAs, particularly in a person of his age.
According to a published account, a $4.5 million settlement was reached.
Suspected Cellulitis–C difficile Colitis Link
In July 2005, a 71-year-old New York man presented to the defendant orthopedic surgeon, Dr. F., because his prosthetic right knee was painful and swollen. The surgeon, who had inserted the prosthetic hardware, found a stress fracture of the patella. Aspirated fluid was bloody; Dr. F., suspecting an infection, prescribed cephalexin.
Almost a month later, the patient returned and reported continuing problems with the knee. X-rays revealed loosening of the tibial component of the prosthesis. Again, aspirated fluid contained blood, and the defendant orthopedist continued to treat the patient for what he believed was an infection.
Two weeks later, Dr. F. performed exploratory surgery on the man’s knee. The prosthesis was removed, and an antibiotic spacer was implanted. A culture revealed a small amount of cellulitis. During this hospitalization, the patient received a diagnosis of Clostridium difficile colitis, which led to pulmonary and renal system failure, sepsis, fungemia, and a stroke.
The plaintiff was hospitalized until December 2005, then was transferred to a nursing home. He remained there until June 2006, when a new prosthesis was placed. He was hospitalized for that surgery until August 2006.
The plaintiff claimed that his cellulitis should have been diagnosed in July 2005 and that the surgery and colitis and its effects resulted from Dr. F.’s failure to make a timely diagnosis of cellulitis. The plaintiff argued that the fluid aspirated in July should have been cultured, that the cephalexin dosage should have been increased, and that a different antibiotic should have been prescribed.
The defendant claimed that the plaintiff’s knee was not infected when he examined it in July and that any infection present during the surgery was unrelated to the plaintiff’s colitis. The defendant maintained that cephalexin was an appropriate treatment choice, and that cellulitis would not have been detected by any test that could have been performed in July.
According to a published account, a $1.9 million verdict was returned.
When Was the Bowel Perforated?
A 34-year-old woman underwent surgery at a Michigan hospital for removal of her left ovary. The defendant surgeon attempted to perform the procedure laparoscopically but encountered extensive scar tissue resulting from several previous surgeries. He found the patient’s ovary adhered to the sigmoid colon and pelvic sidewall. After separating the ovary, the surgeon abandoned the plan to remove it completely out of concern for the adjacent ureter.
The woman was discharged the next day, although she claimed to be in severe pain. Six days after the surgery, she called the defendant to report difficulty catching her breath. She was sent to her primary care clinician’s office, where she expressed additional complaints of fever and abdominal pain.
Three days later, the patient called the surgeon with continuing complaints of poor appetite and difficulty breathing and walking. She was directed to present to the emergency department (ED), where a 2.5-inch perforation of the sigmoid colon was discovered. The woman was required to undergo removal of a nine-inch segment of the bowel and primary reattachment of the remaining colon.
The plaintiff claimed that the colon was perforated during the attempted ovarectomy and that the defendant surgeon was negligent in failing to diagnose and repair the perforation at that time. The plaintiff alleged that the delay in diagnosis and treatment led to the subsequent surgery, loss of part of the bowel, and development of irritable bowel syndrome.
The defendant denied that any injury to the colon was detectable during the surgery he performed and claimed that the perforation occurred later.
A defense verdict was returned.
Reprinted with permission from Medical Malpractice Verdicts, Settlements and Experts, Lewis Laska, Editor, (800) 298-6288.
Too Young for a TIA?
In May 2004, a 41-year-old Wisconsin man presented to the defendant neurologist, reporting episodes of vertigo, headaches, double vision, and occasional hallucinations. MRI was performed with results reported as normal, and the neurologist told the patient that the cause of his symptoms was most likely nonorganic.
The symptoms abated for a time, but when they began to recur in November 2005, the man returned to the neurologist. MRI was performed again and interpreted by the defendant radiologist, who noted that the patient had experienced a stroke in the brain stem. He sent a report to that effect on a “stat” basis.
A few days later, the patient presented to a hospital emergency department (ED) with worsening symptoms. When the neurologist was called, he claimed to be learning of the MRI results for the first time. The radiologist reportedly acknowledged that he had not documented where the “stat” report was sent; nor did he follow up to confirm that it had been received.
At the time of the man’s admission through the ED, he was found to have had a full-blown brain-stem stroke with blockage of the basilar artery. His symptoms progressed to a condition of locked-in syndrome. His condition gradually improved, but he was left with permanent weakness in the arms and legs, blurred vision, and difficulty swallowing. His leg weakness makes it impossible to stand or walk. He also requires injections of onabotulinum toxin A every few months to control left arm and leg spasms.
The plaintiff claimed that the neurologist should have made a diagnosis of transient ischemic attacks (TIAs), and that another MRI and other testing should have been ordered. The plaintiff maintained that additional testing would have led to recognition of narrowing in his basilar artery and appropriate treatment, thereby preventing the stroke. The plaintiff claimed negligence on the radiologist’s part for failing to confirm that the report of the acute brain-stem stroke had been received and acted upon.
The defendants claimed that even an earlier diagnosis and treatment would not have led to a better result. The neurologist also claimed that the plaintiff’s symptoms were not consistent with TIAs, particularly in a person of his age.
According to a published account, a $4.5 million settlement was reached.
Suspected Cellulitis–C difficile Colitis Link
In July 2005, a 71-year-old New York man presented to the defendant orthopedic surgeon, Dr. F., because his prosthetic right knee was painful and swollen. The surgeon, who had inserted the prosthetic hardware, found a stress fracture of the patella. Aspirated fluid was bloody; Dr. F., suspecting an infection, prescribed cephalexin.
Almost a month later, the patient returned and reported continuing problems with the knee. X-rays revealed loosening of the tibial component of the prosthesis. Again, aspirated fluid contained blood, and the defendant orthopedist continued to treat the patient for what he believed was an infection.
Two weeks later, Dr. F. performed exploratory surgery on the man’s knee. The prosthesis was removed, and an antibiotic spacer was implanted. A culture revealed a small amount of cellulitis. During this hospitalization, the patient received a diagnosis of Clostridium difficile colitis, which led to pulmonary and renal system failure, sepsis, fungemia, and a stroke.
The plaintiff was hospitalized until December 2005, then was transferred to a nursing home. He remained there until June 2006, when a new prosthesis was placed. He was hospitalized for that surgery until August 2006.
The plaintiff claimed that his cellulitis should have been diagnosed in July 2005 and that the surgery and colitis and its effects resulted from Dr. F.’s failure to make a timely diagnosis of cellulitis. The plaintiff argued that the fluid aspirated in July should have been cultured, that the cephalexin dosage should have been increased, and that a different antibiotic should have been prescribed.
The defendant claimed that the plaintiff’s knee was not infected when he examined it in July and that any infection present during the surgery was unrelated to the plaintiff’s colitis. The defendant maintained that cephalexin was an appropriate treatment choice, and that cellulitis would not have been detected by any test that could have been performed in July.
According to a published account, a $1.9 million verdict was returned.
When Was the Bowel Perforated?
A 34-year-old woman underwent surgery at a Michigan hospital for removal of her left ovary. The defendant surgeon attempted to perform the procedure laparoscopically but encountered extensive scar tissue resulting from several previous surgeries. He found the patient’s ovary adhered to the sigmoid colon and pelvic sidewall. After separating the ovary, the surgeon abandoned the plan to remove it completely out of concern for the adjacent ureter.
The woman was discharged the next day, although she claimed to be in severe pain. Six days after the surgery, she called the defendant to report difficulty catching her breath. She was sent to her primary care clinician’s office, where she expressed additional complaints of fever and abdominal pain.
Three days later, the patient called the surgeon with continuing complaints of poor appetite and difficulty breathing and walking. She was directed to present to the emergency department (ED), where a 2.5-inch perforation of the sigmoid colon was discovered. The woman was required to undergo removal of a nine-inch segment of the bowel and primary reattachment of the remaining colon.
The plaintiff claimed that the colon was perforated during the attempted ovarectomy and that the defendant surgeon was negligent in failing to diagnose and repair the perforation at that time. The plaintiff alleged that the delay in diagnosis and treatment led to the subsequent surgery, loss of part of the bowel, and development of irritable bowel syndrome.
The defendant denied that any injury to the colon was detectable during the surgery he performed and claimed that the perforation occurred later.
A defense verdict was returned.
Should you restrain yourself from ordering restraints?
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
The Coming Challenges—and Opportunities—of Value-Based Purchasing
The Coming Challenges—and Opportunities—of Value-Based Purchasing
The Patient Protection and Affordable Care Act was signed into law in March, furthering the federal government’s commitment to increasing the efficiency of the U.S. healthcare system by decreasing cost and improving quality. An expansion of the “value-based purchasing” model, this law mandates that ratings and reimbursements to physicians and hospitals be increasingly tied to measured quality of care.
In this system, a physician’s quality profile will be determined by a variety of factors, including reported quality data, severity-adjusted clinical outcome measures, patient-safety indicators, and hospital-acquired conditions (HACs). Since all of these are, to a large extent, documentation issues, physicians are now forced to pay close attention to how they identify and describe diagnoses and procedures.
Medicare will, in effect, attempt to determine: “Did the clinical team correctly identify and appropriately treat all relevant patient conditions—without causing any adverse conditions—and do so safely, efficiently, and with good outcomes?”
The patient chart must “tell the story” of the episode of care in the hospital. It must accurately describe all of the patient’s conditions and demonstrate the complexity of medical decision-making and establishment of risk. Upon discharge, this risk must “match up” with the diagnoses that are being coded and the Medicare Severity Diagnostic Related Group (MS-DRGs) being assigned. Often, the information is present in the chart but is inconsistent from provider to provider, or documented in a way that is misunderstood by hospital coders.
If successful in improving our documentation skills, the reward will be a higher rating and increased reimbursement.
Medicare also is ramping up its claims denial and recovery business to help “clean up” the system. This includes the national rollout of the Recovery Audit Contactor (RAC) initiative (see “Attention to Detail,” April 2010, p. 1), as well as the new Medicare administrative contractors (MACs). Both rely on accurate documentation. The RACs will penalize hospitals and physicians financially for documentation lacking in specificity and accuracy; the MACs will deny payment for claims erroneously submitted (technical errors) or lacking documentation of medical necessity.
What makes many physicians even more uncomfortable in this new environment is that Medicare is striving to better align the priorities and financial incentives of hospitals and physicians. Alliances between the two are strongly encouraged through such programs as the Acute Care Episode (ACE) Demonstration Project (the precursor to hospital-physician bundled payments), and the establishment of accountable care organizations (ACOs).
This emerging environment presents a unique set of challenges and opportunities to HM groups. Hospitalists are historically more aligned with hospital administrations, as compared with most other specialties. Hospitalists also are asked to participate in the care of an increasingly large percentage of patients across all specialties. This could well be an opportunity to be rewarded for embracing both of these trends.
Hospitalists are uniquely positioned to function as the documentation improvement clinical team leaders, working closely with other physicians across all specialties and the administration to fulfill all documentation requirements—and to be rewarded for doing so. Though hospitalists should have a general understanding of the language and rules of documentation, a system must be in place that helps them identify and capture all the pertinent aspects of the medical record without the need to become coders themselves. To this end, a clinical documentation improvement (CDI) program is critical.
But it might not be enough.
What is needed is a clinical integration program, an enhancement of traditional CDI. This approach requires participation from ED physicians, along with clinical integration specialists, to document accurately and completely from the start. The clinical integration specialist ensures that medical necessity for inpatient admission and patient risk is being addressed and established, conditions are appropriately identified as being present on admission (POA), and all diagnoses are properly recognized and documented thoroughly and accurately. Clinical integration through collaborative documentation then continues throughout the hospitalization, with diagnostic authority and oversight from the hospitalist, all the way through discharge.
Hospitalists should welcome and champion this type of program. As documentation becomes the key to survival, a complete medical record will stand up to any and all scrutiny by Medicare or others.
As for the negatives, there are none.
Andrew H. Dombro, MD, national medical director, and Paul Weygandt, MD, JD, vice president of physician services, J.A. Thomas & Associates, Atlanta
The Coming Challenges—and Opportunities—of Value-Based Purchasing
The Patient Protection and Affordable Care Act was signed into law in March, furthering the federal government’s commitment to increasing the efficiency of the U.S. healthcare system by decreasing cost and improving quality. An expansion of the “value-based purchasing” model, this law mandates that ratings and reimbursements to physicians and hospitals be increasingly tied to measured quality of care.
In this system, a physician’s quality profile will be determined by a variety of factors, including reported quality data, severity-adjusted clinical outcome measures, patient-safety indicators, and hospital-acquired conditions (HACs). Since all of these are, to a large extent, documentation issues, physicians are now forced to pay close attention to how they identify and describe diagnoses and procedures.
Medicare will, in effect, attempt to determine: “Did the clinical team correctly identify and appropriately treat all relevant patient conditions—without causing any adverse conditions—and do so safely, efficiently, and with good outcomes?”
The patient chart must “tell the story” of the episode of care in the hospital. It must accurately describe all of the patient’s conditions and demonstrate the complexity of medical decision-making and establishment of risk. Upon discharge, this risk must “match up” with the diagnoses that are being coded and the Medicare Severity Diagnostic Related Group (MS-DRGs) being assigned. Often, the information is present in the chart but is inconsistent from provider to provider, or documented in a way that is misunderstood by hospital coders.
If successful in improving our documentation skills, the reward will be a higher rating and increased reimbursement.
Medicare also is ramping up its claims denial and recovery business to help “clean up” the system. This includes the national rollout of the Recovery Audit Contactor (RAC) initiative (see “Attention to Detail,” April 2010, p. 1), as well as the new Medicare administrative contractors (MACs). Both rely on accurate documentation. The RACs will penalize hospitals and physicians financially for documentation lacking in specificity and accuracy; the MACs will deny payment for claims erroneously submitted (technical errors) or lacking documentation of medical necessity.
What makes many physicians even more uncomfortable in this new environment is that Medicare is striving to better align the priorities and financial incentives of hospitals and physicians. Alliances between the two are strongly encouraged through such programs as the Acute Care Episode (ACE) Demonstration Project (the precursor to hospital-physician bundled payments), and the establishment of accountable care organizations (ACOs).
This emerging environment presents a unique set of challenges and opportunities to HM groups. Hospitalists are historically more aligned with hospital administrations, as compared with most other specialties. Hospitalists also are asked to participate in the care of an increasingly large percentage of patients across all specialties. This could well be an opportunity to be rewarded for embracing both of these trends.
Hospitalists are uniquely positioned to function as the documentation improvement clinical team leaders, working closely with other physicians across all specialties and the administration to fulfill all documentation requirements—and to be rewarded for doing so. Though hospitalists should have a general understanding of the language and rules of documentation, a system must be in place that helps them identify and capture all the pertinent aspects of the medical record without the need to become coders themselves. To this end, a clinical documentation improvement (CDI) program is critical.
But it might not be enough.
What is needed is a clinical integration program, an enhancement of traditional CDI. This approach requires participation from ED physicians, along with clinical integration specialists, to document accurately and completely from the start. The clinical integration specialist ensures that medical necessity for inpatient admission and patient risk is being addressed and established, conditions are appropriately identified as being present on admission (POA), and all diagnoses are properly recognized and documented thoroughly and accurately. Clinical integration through collaborative documentation then continues throughout the hospitalization, with diagnostic authority and oversight from the hospitalist, all the way through discharge.
Hospitalists should welcome and champion this type of program. As documentation becomes the key to survival, a complete medical record will stand up to any and all scrutiny by Medicare or others.
As for the negatives, there are none.
Andrew H. Dombro, MD, national medical director, and Paul Weygandt, MD, JD, vice president of physician services, J.A. Thomas & Associates, Atlanta
The Coming Challenges—and Opportunities—of Value-Based Purchasing
The Patient Protection and Affordable Care Act was signed into law in March, furthering the federal government’s commitment to increasing the efficiency of the U.S. healthcare system by decreasing cost and improving quality. An expansion of the “value-based purchasing” model, this law mandates that ratings and reimbursements to physicians and hospitals be increasingly tied to measured quality of care.
In this system, a physician’s quality profile will be determined by a variety of factors, including reported quality data, severity-adjusted clinical outcome measures, patient-safety indicators, and hospital-acquired conditions (HACs). Since all of these are, to a large extent, documentation issues, physicians are now forced to pay close attention to how they identify and describe diagnoses and procedures.
Medicare will, in effect, attempt to determine: “Did the clinical team correctly identify and appropriately treat all relevant patient conditions—without causing any adverse conditions—and do so safely, efficiently, and with good outcomes?”
The patient chart must “tell the story” of the episode of care in the hospital. It must accurately describe all of the patient’s conditions and demonstrate the complexity of medical decision-making and establishment of risk. Upon discharge, this risk must “match up” with the diagnoses that are being coded and the Medicare Severity Diagnostic Related Group (MS-DRGs) being assigned. Often, the information is present in the chart but is inconsistent from provider to provider, or documented in a way that is misunderstood by hospital coders.
If successful in improving our documentation skills, the reward will be a higher rating and increased reimbursement.
Medicare also is ramping up its claims denial and recovery business to help “clean up” the system. This includes the national rollout of the Recovery Audit Contactor (RAC) initiative (see “Attention to Detail,” April 2010, p. 1), as well as the new Medicare administrative contractors (MACs). Both rely on accurate documentation. The RACs will penalize hospitals and physicians financially for documentation lacking in specificity and accuracy; the MACs will deny payment for claims erroneously submitted (technical errors) or lacking documentation of medical necessity.
What makes many physicians even more uncomfortable in this new environment is that Medicare is striving to better align the priorities and financial incentives of hospitals and physicians. Alliances between the two are strongly encouraged through such programs as the Acute Care Episode (ACE) Demonstration Project (the precursor to hospital-physician bundled payments), and the establishment of accountable care organizations (ACOs).
This emerging environment presents a unique set of challenges and opportunities to HM groups. Hospitalists are historically more aligned with hospital administrations, as compared with most other specialties. Hospitalists also are asked to participate in the care of an increasingly large percentage of patients across all specialties. This could well be an opportunity to be rewarded for embracing both of these trends.
Hospitalists are uniquely positioned to function as the documentation improvement clinical team leaders, working closely with other physicians across all specialties and the administration to fulfill all documentation requirements—and to be rewarded for doing so. Though hospitalists should have a general understanding of the language and rules of documentation, a system must be in place that helps them identify and capture all the pertinent aspects of the medical record without the need to become coders themselves. To this end, a clinical documentation improvement (CDI) program is critical.
But it might not be enough.
What is needed is a clinical integration program, an enhancement of traditional CDI. This approach requires participation from ED physicians, along with clinical integration specialists, to document accurately and completely from the start. The clinical integration specialist ensures that medical necessity for inpatient admission and patient risk is being addressed and established, conditions are appropriately identified as being present on admission (POA), and all diagnoses are properly recognized and documented thoroughly and accurately. Clinical integration through collaborative documentation then continues throughout the hospitalization, with diagnostic authority and oversight from the hospitalist, all the way through discharge.
Hospitalists should welcome and champion this type of program. As documentation becomes the key to survival, a complete medical record will stand up to any and all scrutiny by Medicare or others.
As for the negatives, there are none.
Andrew H. Dombro, MD, national medical director, and Paul Weygandt, MD, JD, vice president of physician services, J.A. Thomas & Associates, Atlanta
Financial Risk
When I started writing this, Congress hadn’t settled the issue of the 21% cut in Medicare reimbursement for services called for by the sustainable growth rate (SGR) formula. Fortunately, Congress stepped up and passed another extension with a 2.2% pay increase; however, the quick fix only lasts until November.
The process is all too routine: The deadline for these reimbursement cuts looms, Medicare instructs its fiscal intermediaries (the organizations that actually write the checks to providers) to hold claims rather than pay at the lower rate, and, within a few days of the deadline passing, Congress decides to pass an extension, which allows Medicare to continue paying the historical (higher) rate for the time being.
Imagine Medicare reimbursement rates dropping 21% overnight. I suspect it would be cataclysmic. But I hear remarkably little chatter about this possibility. In fact, while with 2,500 other hospitalists for several days at HM10 in April, I didn’t hear a single person bring up the SGR issue.
One reason there isn’t more handwringing about the looming, draconian cuts is that we’ve been there before. In fact, reimbursement cuts required by the SGR have come up every year since 2001. Each time, Congress has chosen not to implement the cuts; and in some years it has approved reimbursement increases instead. So most in healthcare circles basically have come to expect Congress to pass last-minute legislation to avoid the drastic cuts. (SHM and most other medical societies want a repeal of the flawed SGR formula. Visit SHM’s Legislative Action Center, http://capwiz.com/hospitalmedicine/home/, to write your legislators and urge repeal of the SGR. It only takes about two minutes, and you don’t even need to remember who your representatives are; you just need to know your ZIP code.)
Don’t Be Too Smug
There is another reason many hospitalists, and other doctors who are employed and salaried by a large entity like a hospital, might not be more concerned about proposed cuts: They probably think their own salaries will be unaffected by decreases in reimbursement from Medicare and other payors. My experience is that a lot of hospitalists are so unconcerned about payor reimbursement rates that they aren’t even aware of the threatened Medicare cuts.
Their thinking goes something like this: “I’m paid mostly via a fixed annual salary with a small productivity and quality incentive. None of this is connected to the payor mix or collection rates from the patients I see. So if the portion of uninsured patients I see goes up, my compensation is unaffected. Or if payors decrease their rates, my compensation is unaffected. So I don’t need to sweat the possibility of a 21% decrease in Medicare rates. The hospital will have to make up the difference, so my salary is unaffected, and it will be up to bean counters at the hospital to get the numbers to work out.”
In fact, this is true, in theory, for the majority of hospitalists. But I think it is a mistake to assume your salary is untouchable. If Medicare were to cut rates by 21%, you’d better run to your hospital CEO’s office right away, because a long line will form immediately. Every doctor who sees patients at your hospital will be in that line asking the CEO to provide some money to offset the Medicare cuts, and I doubt any hospital will be able to satisfy their doctors without spending so much money that the hospital goes bankrupt or out of business.
Even if you have a valid contract that calls for your compensation to be paid independent of the amount of professional fee collections, a dire shortage of money could lead a hospital to lay off hospitalists or cancel the contract (most contracts would allow the hospital to do this simply by giving a 90-day notice).
I suggest that no hospitalist feel too smug about how well their employment contract protects the group from broader market forces like reimbursement rates. I doubt we’ll ever see an overnight 21% reduction in Medicare rates, but over time, we could see ever-increasing pressure to limit the growth in our incomes.
I believe every hospitalist should spend at least a little time following broader financial issues like this one, and get involved in the political process to let your legislators know your thoughts. For the record, I think the financial underpinnings of our healthcare system are disastrously messed up and something has to be done. And I don’t think anyone’s salary, including mine, is untouchable. But I also believe the SGR is an ineffective way to make the system more financially sound. That said, you don’t need to agree with me; I only recommend that you have a reasonably informed opinion.
One approach might be for your HM group to appoint a “political” or “marketplace” watchdog. This person could be charged with following issues closely and reporting back to the whole group during regular meetings.
“Marketplace” Risk
Medicare rates are only one part of the complex financial ecosystem on which we depend. It is awfully common, and I think pretty reasonable, for hospitalists to have a contractual arrangement with hospitals. The majority of the time, the hospital has most—or all—of the risk for the financial performance of the practice. In fact, most prospective hospitalists, especially those seeking their first jobs out after residency, say one of the most attractive reasons for choosing work as a hospitalist is that many practices provide a salary that is nearly fixed. Any variable components to the salary, such as those based on production or quality, are typically very small.
A hospitalist might think, “I want a practice that pays a fixed salary so I don’t have to worry about any business and financial issues other than when to show up to work.” In fact, a lot of recruitment ads trumpet this very idea (i.e., “you handle the doctoring and get to enjoy the wonderful recreational opportunities and schools our locale provides, and we’ll worry about all the business issues”). That may sound nice, but I worry it is a little short-sighted.
Here is another point of view, which is only slightly more complicated. In most cases, you should try to negotiate a contract that insulates you from “payor risk” (e.g., changes in payor mix and rates paid by payors don’t flow through to your compensation). But you should think twice before asking your employer to assume all the risk for staffing and scheduling decisions, such as whether you get the work done with 10 hospitalists or 11, or whether you have an evening admitter (“swing”) shift. If the employer holds all the risk, then the hospitalists give up nearly all their autonomy to decide how hard they want to work and how they want to schedule themselves. This causes problems for many practices, and is the No. 1 reason I’m called in as a consultant. Contrary to being very risky and stressful, many hospitalists find it liberating to assume financial risk for their staffing and workload decisions.
You should realize that if your employer pays you a fixed compensation, then someone has to ensure that you do enough work to justify that compensation. This can mean that the employer “issues decrees” (i.e., “we won’t add another provide to the practice until we’ve averaged ‘X’ encounters per month for 6 months”). A hospitalist might see this as unreasonable, yet the group has limited recourse since the employer has already guaranteed the compensation.
If you’d rather have more autonomy in your staffing and workload, then you will need to connect your paycheck to these decisions. Although it might sound terribly risky, those who make the switch often say they wouldn’t have it any other way. Most importantly, it ensures hospitalists have much more say in big decisions. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
When I started writing this, Congress hadn’t settled the issue of the 21% cut in Medicare reimbursement for services called for by the sustainable growth rate (SGR) formula. Fortunately, Congress stepped up and passed another extension with a 2.2% pay increase; however, the quick fix only lasts until November.
The process is all too routine: The deadline for these reimbursement cuts looms, Medicare instructs its fiscal intermediaries (the organizations that actually write the checks to providers) to hold claims rather than pay at the lower rate, and, within a few days of the deadline passing, Congress decides to pass an extension, which allows Medicare to continue paying the historical (higher) rate for the time being.
Imagine Medicare reimbursement rates dropping 21% overnight. I suspect it would be cataclysmic. But I hear remarkably little chatter about this possibility. In fact, while with 2,500 other hospitalists for several days at HM10 in April, I didn’t hear a single person bring up the SGR issue.
One reason there isn’t more handwringing about the looming, draconian cuts is that we’ve been there before. In fact, reimbursement cuts required by the SGR have come up every year since 2001. Each time, Congress has chosen not to implement the cuts; and in some years it has approved reimbursement increases instead. So most in healthcare circles basically have come to expect Congress to pass last-minute legislation to avoid the drastic cuts. (SHM and most other medical societies want a repeal of the flawed SGR formula. Visit SHM’s Legislative Action Center, http://capwiz.com/hospitalmedicine/home/, to write your legislators and urge repeal of the SGR. It only takes about two minutes, and you don’t even need to remember who your representatives are; you just need to know your ZIP code.)
Don’t Be Too Smug
There is another reason many hospitalists, and other doctors who are employed and salaried by a large entity like a hospital, might not be more concerned about proposed cuts: They probably think their own salaries will be unaffected by decreases in reimbursement from Medicare and other payors. My experience is that a lot of hospitalists are so unconcerned about payor reimbursement rates that they aren’t even aware of the threatened Medicare cuts.
Their thinking goes something like this: “I’m paid mostly via a fixed annual salary with a small productivity and quality incentive. None of this is connected to the payor mix or collection rates from the patients I see. So if the portion of uninsured patients I see goes up, my compensation is unaffected. Or if payors decrease their rates, my compensation is unaffected. So I don’t need to sweat the possibility of a 21% decrease in Medicare rates. The hospital will have to make up the difference, so my salary is unaffected, and it will be up to bean counters at the hospital to get the numbers to work out.”
In fact, this is true, in theory, for the majority of hospitalists. But I think it is a mistake to assume your salary is untouchable. If Medicare were to cut rates by 21%, you’d better run to your hospital CEO’s office right away, because a long line will form immediately. Every doctor who sees patients at your hospital will be in that line asking the CEO to provide some money to offset the Medicare cuts, and I doubt any hospital will be able to satisfy their doctors without spending so much money that the hospital goes bankrupt or out of business.
Even if you have a valid contract that calls for your compensation to be paid independent of the amount of professional fee collections, a dire shortage of money could lead a hospital to lay off hospitalists or cancel the contract (most contracts would allow the hospital to do this simply by giving a 90-day notice).
I suggest that no hospitalist feel too smug about how well their employment contract protects the group from broader market forces like reimbursement rates. I doubt we’ll ever see an overnight 21% reduction in Medicare rates, but over time, we could see ever-increasing pressure to limit the growth in our incomes.
I believe every hospitalist should spend at least a little time following broader financial issues like this one, and get involved in the political process to let your legislators know your thoughts. For the record, I think the financial underpinnings of our healthcare system are disastrously messed up and something has to be done. And I don’t think anyone’s salary, including mine, is untouchable. But I also believe the SGR is an ineffective way to make the system more financially sound. That said, you don’t need to agree with me; I only recommend that you have a reasonably informed opinion.
One approach might be for your HM group to appoint a “political” or “marketplace” watchdog. This person could be charged with following issues closely and reporting back to the whole group during regular meetings.
“Marketplace” Risk
Medicare rates are only one part of the complex financial ecosystem on which we depend. It is awfully common, and I think pretty reasonable, for hospitalists to have a contractual arrangement with hospitals. The majority of the time, the hospital has most—or all—of the risk for the financial performance of the practice. In fact, most prospective hospitalists, especially those seeking their first jobs out after residency, say one of the most attractive reasons for choosing work as a hospitalist is that many practices provide a salary that is nearly fixed. Any variable components to the salary, such as those based on production or quality, are typically very small.
A hospitalist might think, “I want a practice that pays a fixed salary so I don’t have to worry about any business and financial issues other than when to show up to work.” In fact, a lot of recruitment ads trumpet this very idea (i.e., “you handle the doctoring and get to enjoy the wonderful recreational opportunities and schools our locale provides, and we’ll worry about all the business issues”). That may sound nice, but I worry it is a little short-sighted.
Here is another point of view, which is only slightly more complicated. In most cases, you should try to negotiate a contract that insulates you from “payor risk” (e.g., changes in payor mix and rates paid by payors don’t flow through to your compensation). But you should think twice before asking your employer to assume all the risk for staffing and scheduling decisions, such as whether you get the work done with 10 hospitalists or 11, or whether you have an evening admitter (“swing”) shift. If the employer holds all the risk, then the hospitalists give up nearly all their autonomy to decide how hard they want to work and how they want to schedule themselves. This causes problems for many practices, and is the No. 1 reason I’m called in as a consultant. Contrary to being very risky and stressful, many hospitalists find it liberating to assume financial risk for their staffing and workload decisions.
You should realize that if your employer pays you a fixed compensation, then someone has to ensure that you do enough work to justify that compensation. This can mean that the employer “issues decrees” (i.e., “we won’t add another provide to the practice until we’ve averaged ‘X’ encounters per month for 6 months”). A hospitalist might see this as unreasonable, yet the group has limited recourse since the employer has already guaranteed the compensation.
If you’d rather have more autonomy in your staffing and workload, then you will need to connect your paycheck to these decisions. Although it might sound terribly risky, those who make the switch often say they wouldn’t have it any other way. Most importantly, it ensures hospitalists have much more say in big decisions. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
When I started writing this, Congress hadn’t settled the issue of the 21% cut in Medicare reimbursement for services called for by the sustainable growth rate (SGR) formula. Fortunately, Congress stepped up and passed another extension with a 2.2% pay increase; however, the quick fix only lasts until November.
The process is all too routine: The deadline for these reimbursement cuts looms, Medicare instructs its fiscal intermediaries (the organizations that actually write the checks to providers) to hold claims rather than pay at the lower rate, and, within a few days of the deadline passing, Congress decides to pass an extension, which allows Medicare to continue paying the historical (higher) rate for the time being.
Imagine Medicare reimbursement rates dropping 21% overnight. I suspect it would be cataclysmic. But I hear remarkably little chatter about this possibility. In fact, while with 2,500 other hospitalists for several days at HM10 in April, I didn’t hear a single person bring up the SGR issue.
One reason there isn’t more handwringing about the looming, draconian cuts is that we’ve been there before. In fact, reimbursement cuts required by the SGR have come up every year since 2001. Each time, Congress has chosen not to implement the cuts; and in some years it has approved reimbursement increases instead. So most in healthcare circles basically have come to expect Congress to pass last-minute legislation to avoid the drastic cuts. (SHM and most other medical societies want a repeal of the flawed SGR formula. Visit SHM’s Legislative Action Center, http://capwiz.com/hospitalmedicine/home/, to write your legislators and urge repeal of the SGR. It only takes about two minutes, and you don’t even need to remember who your representatives are; you just need to know your ZIP code.)
Don’t Be Too Smug
There is another reason many hospitalists, and other doctors who are employed and salaried by a large entity like a hospital, might not be more concerned about proposed cuts: They probably think their own salaries will be unaffected by decreases in reimbursement from Medicare and other payors. My experience is that a lot of hospitalists are so unconcerned about payor reimbursement rates that they aren’t even aware of the threatened Medicare cuts.
Their thinking goes something like this: “I’m paid mostly via a fixed annual salary with a small productivity and quality incentive. None of this is connected to the payor mix or collection rates from the patients I see. So if the portion of uninsured patients I see goes up, my compensation is unaffected. Or if payors decrease their rates, my compensation is unaffected. So I don’t need to sweat the possibility of a 21% decrease in Medicare rates. The hospital will have to make up the difference, so my salary is unaffected, and it will be up to bean counters at the hospital to get the numbers to work out.”
In fact, this is true, in theory, for the majority of hospitalists. But I think it is a mistake to assume your salary is untouchable. If Medicare were to cut rates by 21%, you’d better run to your hospital CEO’s office right away, because a long line will form immediately. Every doctor who sees patients at your hospital will be in that line asking the CEO to provide some money to offset the Medicare cuts, and I doubt any hospital will be able to satisfy their doctors without spending so much money that the hospital goes bankrupt or out of business.
Even if you have a valid contract that calls for your compensation to be paid independent of the amount of professional fee collections, a dire shortage of money could lead a hospital to lay off hospitalists or cancel the contract (most contracts would allow the hospital to do this simply by giving a 90-day notice).
I suggest that no hospitalist feel too smug about how well their employment contract protects the group from broader market forces like reimbursement rates. I doubt we’ll ever see an overnight 21% reduction in Medicare rates, but over time, we could see ever-increasing pressure to limit the growth in our incomes.
I believe every hospitalist should spend at least a little time following broader financial issues like this one, and get involved in the political process to let your legislators know your thoughts. For the record, I think the financial underpinnings of our healthcare system are disastrously messed up and something has to be done. And I don’t think anyone’s salary, including mine, is untouchable. But I also believe the SGR is an ineffective way to make the system more financially sound. That said, you don’t need to agree with me; I only recommend that you have a reasonably informed opinion.
One approach might be for your HM group to appoint a “political” or “marketplace” watchdog. This person could be charged with following issues closely and reporting back to the whole group during regular meetings.
“Marketplace” Risk
Medicare rates are only one part of the complex financial ecosystem on which we depend. It is awfully common, and I think pretty reasonable, for hospitalists to have a contractual arrangement with hospitals. The majority of the time, the hospital has most—or all—of the risk for the financial performance of the practice. In fact, most prospective hospitalists, especially those seeking their first jobs out after residency, say one of the most attractive reasons for choosing work as a hospitalist is that many practices provide a salary that is nearly fixed. Any variable components to the salary, such as those based on production or quality, are typically very small.
A hospitalist might think, “I want a practice that pays a fixed salary so I don’t have to worry about any business and financial issues other than when to show up to work.” In fact, a lot of recruitment ads trumpet this very idea (i.e., “you handle the doctoring and get to enjoy the wonderful recreational opportunities and schools our locale provides, and we’ll worry about all the business issues”). That may sound nice, but I worry it is a little short-sighted.
Here is another point of view, which is only slightly more complicated. In most cases, you should try to negotiate a contract that insulates you from “payor risk” (e.g., changes in payor mix and rates paid by payors don’t flow through to your compensation). But you should think twice before asking your employer to assume all the risk for staffing and scheduling decisions, such as whether you get the work done with 10 hospitalists or 11, or whether you have an evening admitter (“swing”) shift. If the employer holds all the risk, then the hospitalists give up nearly all their autonomy to decide how hard they want to work and how they want to schedule themselves. This causes problems for many practices, and is the No. 1 reason I’m called in as a consultant. Contrary to being very risky and stressful, many hospitalists find it liberating to assume financial risk for their staffing and workload decisions.
You should realize that if your employer pays you a fixed compensation, then someone has to ensure that you do enough work to justify that compensation. This can mean that the employer “issues decrees” (i.e., “we won’t add another provide to the practice until we’ve averaged ‘X’ encounters per month for 6 months”). A hospitalist might see this as unreasonable, yet the group has limited recourse since the employer has already guaranteed the compensation.
If you’d rather have more autonomy in your staffing and workload, then you will need to connect your paycheck to these decisions. Although it might sound terribly risky, those who make the switch often say they wouldn’t have it any other way. Most importantly, it ensures hospitalists have much more say in big decisions. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.