User login
Pathologic superstition
When you believe in things that you don’t understand
Then you suffer
Superstition ain’t the way
– Stevie Wonder
I have always found it odd that airplanes don’t have a 13th row and hotels don’t have a 13th floor. Well, of course they do, but they are not labeled that way. Many people would hesitate to sit in the 13th row of an airplane since 13 is such an unlucky number. At least many people in the United States think the number 13 is unlucky. Thirteen is just a number in much of Asia. There, the number 4 is just as threatening as 13 is to us.
Superstitions like these are familiar to all of us.
One of my favorites is the belief that vacuum cups attached to the skin will somehow draw out toxins and generally improve health. “Cupping,” as the practice is known, is endorsed by several celebrities and famous athletes. After the treatment, a cupped patient exhibits circles of hyperemia, and no other apparent harm. I suspect that about a third of cupped patients truly think they have benefited from a good cupping, about the same number that would benefit from an orally administered placebo.
Superstitions are everywhere. Whether it is a black cat in the United States, infinite reflecting mirrors in Mexico, going back to your house after a wake in the Philippines, or whistling indoors in Lithuania, superstitions are pervasive, deeply held, and generally harmless. They are good for a good laugh as we recognize how ludicrous these unfounded fears are.
Some superstitions, though, are no laughing matter. They can be quite harmful. They are pathologic superstitions.
For example, some people believe vaccines cause autism in children. That pathologic superstition has consequences. A recent CDC report revealed that the population of unvaccinated children in the United States has quadrupled since 2001. This comes as no surprise as we hear about more measles outbreaks – and the deaths associated with them – in populations of unvaccinated children every year. A similar and pervasive pathologic superstition is the fear that an influenza vaccine will cause the flu. I wonder how many people die from this misconception.
Other people believe that their cancer can be treated, if not cured, with unproven, unconventional treatments. I cannot understand how this pathologic superstition developed. The purveyors of unconventional treatment hold much of the blame, but gullibility and ignorance may play a larger role. The consequences are tragic. A recent report demonstrated an approximately twofold increased risk of death in patients who used complementary therapies, compared with those who did not (JAMA Oncol. 2018 Oct 1;4[10]:1375-81).
These are sobering data for those of us who have in the past relented when our patients asked if they could take this or that supplement because we did not think they would cause significant harm.
Superstitions apparently are part of the human condition, evolved to attribute causation and provide order. They are a learned phenomenon. They are learned by reasonable people with normal intelligence and rational thinking. A superstition is born when someone is exposed to a false statement by someone or something they trust – a trusted other.
Trusted others exude certainty. Once established, superstitions are regrettably difficult to remove by those who are less certain, like physicians. How willing are we to say that the flu vaccine is 100% safe? Without certainty, how can a physician debunk a superstition? The techniques that we have been taught usually work, but not when faced with a pathologic superstition.
Science and experience teach us that firmly held superstitions cannot be broken with logical, stepwise reasoning. Jonathan Haidt provides a useful metaphor for this problem in his book “The Happiness Hypothesis” (Basic Books, 2006). He describes a rider on an elephant. The rider represents our rational thought and the elephant represents our emotional foundation. The rider thinks he controls the elephant, but the opposite is more likely true. In order to move the elephant in a certain direction, the rider needs to make the elephant want to turn in that direction. Otherwise, all the cajoling and arguing in the world won’t make the elephant turn. A rational argument made to someone emotionally invested in the counter argument will fail. That is why we cannot convince antivaccine parents to vaccinate their children by trying to persuade them with facts. Neither can we convince global warming skeptics to stop burning coal, gun advocates to vote for restrictions on gun ownership, or cancer patients to accept curative treatment if their values and morals are being challenged.
In a later book, “The Righteous Mind: Why Good People Are Divided by Politics and Religion” (Vintage Books, 2012), Mr. Haidt expands his hypothesis to declare that to change minds, we must appeal to underlying moral values. The challenge is to identify those moral underpinnings in our patients in order to develop an appeal likely to resonate with their emotions and values.
Superstition derives from something people learn either from trusted others or from personal experience. It does no good for physicians to deride patient beliefs and denigrate their agency in an attempt to persuade them to abandon what we consider irrational beliefs. For physicians to penetrate pathologic superstitions, they will have to become the trusted other, to understand moral foundations, to emotionally connect. That does not usually happen the first day we meet a new patient, especially a skeptical one. It takes time, and effort, to reach out and bond with the patient and their family. Only then can pathologic superstitions dissolve and a better patient-doctor relationship evolve.
During this season rife with superstition, remember that your patient’s own superstitions are part of their belief system, and your belief system may be threatening to them. Make your beliefs less threatening, become a trusted other, and appeal to their foundational values, and you can successfully break a pathologic superstition.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When you believe in things that you don’t understand
Then you suffer
Superstition ain’t the way
– Stevie Wonder
I have always found it odd that airplanes don’t have a 13th row and hotels don’t have a 13th floor. Well, of course they do, but they are not labeled that way. Many people would hesitate to sit in the 13th row of an airplane since 13 is such an unlucky number. At least many people in the United States think the number 13 is unlucky. Thirteen is just a number in much of Asia. There, the number 4 is just as threatening as 13 is to us.
Superstitions like these are familiar to all of us.
One of my favorites is the belief that vacuum cups attached to the skin will somehow draw out toxins and generally improve health. “Cupping,” as the practice is known, is endorsed by several celebrities and famous athletes. After the treatment, a cupped patient exhibits circles of hyperemia, and no other apparent harm. I suspect that about a third of cupped patients truly think they have benefited from a good cupping, about the same number that would benefit from an orally administered placebo.
Superstitions are everywhere. Whether it is a black cat in the United States, infinite reflecting mirrors in Mexico, going back to your house after a wake in the Philippines, or whistling indoors in Lithuania, superstitions are pervasive, deeply held, and generally harmless. They are good for a good laugh as we recognize how ludicrous these unfounded fears are.
Some superstitions, though, are no laughing matter. They can be quite harmful. They are pathologic superstitions.
For example, some people believe vaccines cause autism in children. That pathologic superstition has consequences. A recent CDC report revealed that the population of unvaccinated children in the United States has quadrupled since 2001. This comes as no surprise as we hear about more measles outbreaks – and the deaths associated with them – in populations of unvaccinated children every year. A similar and pervasive pathologic superstition is the fear that an influenza vaccine will cause the flu. I wonder how many people die from this misconception.
Other people believe that their cancer can be treated, if not cured, with unproven, unconventional treatments. I cannot understand how this pathologic superstition developed. The purveyors of unconventional treatment hold much of the blame, but gullibility and ignorance may play a larger role. The consequences are tragic. A recent report demonstrated an approximately twofold increased risk of death in patients who used complementary therapies, compared with those who did not (JAMA Oncol. 2018 Oct 1;4[10]:1375-81).
These are sobering data for those of us who have in the past relented when our patients asked if they could take this or that supplement because we did not think they would cause significant harm.
Superstitions apparently are part of the human condition, evolved to attribute causation and provide order. They are a learned phenomenon. They are learned by reasonable people with normal intelligence and rational thinking. A superstition is born when someone is exposed to a false statement by someone or something they trust – a trusted other.
Trusted others exude certainty. Once established, superstitions are regrettably difficult to remove by those who are less certain, like physicians. How willing are we to say that the flu vaccine is 100% safe? Without certainty, how can a physician debunk a superstition? The techniques that we have been taught usually work, but not when faced with a pathologic superstition.
Science and experience teach us that firmly held superstitions cannot be broken with logical, stepwise reasoning. Jonathan Haidt provides a useful metaphor for this problem in his book “The Happiness Hypothesis” (Basic Books, 2006). He describes a rider on an elephant. The rider represents our rational thought and the elephant represents our emotional foundation. The rider thinks he controls the elephant, but the opposite is more likely true. In order to move the elephant in a certain direction, the rider needs to make the elephant want to turn in that direction. Otherwise, all the cajoling and arguing in the world won’t make the elephant turn. A rational argument made to someone emotionally invested in the counter argument will fail. That is why we cannot convince antivaccine parents to vaccinate their children by trying to persuade them with facts. Neither can we convince global warming skeptics to stop burning coal, gun advocates to vote for restrictions on gun ownership, or cancer patients to accept curative treatment if their values and morals are being challenged.
In a later book, “The Righteous Mind: Why Good People Are Divided by Politics and Religion” (Vintage Books, 2012), Mr. Haidt expands his hypothesis to declare that to change minds, we must appeal to underlying moral values. The challenge is to identify those moral underpinnings in our patients in order to develop an appeal likely to resonate with their emotions and values.
Superstition derives from something people learn either from trusted others or from personal experience. It does no good for physicians to deride patient beliefs and denigrate their agency in an attempt to persuade them to abandon what we consider irrational beliefs. For physicians to penetrate pathologic superstitions, they will have to become the trusted other, to understand moral foundations, to emotionally connect. That does not usually happen the first day we meet a new patient, especially a skeptical one. It takes time, and effort, to reach out and bond with the patient and their family. Only then can pathologic superstitions dissolve and a better patient-doctor relationship evolve.
During this season rife with superstition, remember that your patient’s own superstitions are part of their belief system, and your belief system may be threatening to them. Make your beliefs less threatening, become a trusted other, and appeal to their foundational values, and you can successfully break a pathologic superstition.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
When you believe in things that you don’t understand
Then you suffer
Superstition ain’t the way
– Stevie Wonder
I have always found it odd that airplanes don’t have a 13th row and hotels don’t have a 13th floor. Well, of course they do, but they are not labeled that way. Many people would hesitate to sit in the 13th row of an airplane since 13 is such an unlucky number. At least many people in the United States think the number 13 is unlucky. Thirteen is just a number in much of Asia. There, the number 4 is just as threatening as 13 is to us.
Superstitions like these are familiar to all of us.
One of my favorites is the belief that vacuum cups attached to the skin will somehow draw out toxins and generally improve health. “Cupping,” as the practice is known, is endorsed by several celebrities and famous athletes. After the treatment, a cupped patient exhibits circles of hyperemia, and no other apparent harm. I suspect that about a third of cupped patients truly think they have benefited from a good cupping, about the same number that would benefit from an orally administered placebo.
Superstitions are everywhere. Whether it is a black cat in the United States, infinite reflecting mirrors in Mexico, going back to your house after a wake in the Philippines, or whistling indoors in Lithuania, superstitions are pervasive, deeply held, and generally harmless. They are good for a good laugh as we recognize how ludicrous these unfounded fears are.
Some superstitions, though, are no laughing matter. They can be quite harmful. They are pathologic superstitions.
For example, some people believe vaccines cause autism in children. That pathologic superstition has consequences. A recent CDC report revealed that the population of unvaccinated children in the United States has quadrupled since 2001. This comes as no surprise as we hear about more measles outbreaks – and the deaths associated with them – in populations of unvaccinated children every year. A similar and pervasive pathologic superstition is the fear that an influenza vaccine will cause the flu. I wonder how many people die from this misconception.
Other people believe that their cancer can be treated, if not cured, with unproven, unconventional treatments. I cannot understand how this pathologic superstition developed. The purveyors of unconventional treatment hold much of the blame, but gullibility and ignorance may play a larger role. The consequences are tragic. A recent report demonstrated an approximately twofold increased risk of death in patients who used complementary therapies, compared with those who did not (JAMA Oncol. 2018 Oct 1;4[10]:1375-81).
These are sobering data for those of us who have in the past relented when our patients asked if they could take this or that supplement because we did not think they would cause significant harm.
Superstitions apparently are part of the human condition, evolved to attribute causation and provide order. They are a learned phenomenon. They are learned by reasonable people with normal intelligence and rational thinking. A superstition is born when someone is exposed to a false statement by someone or something they trust – a trusted other.
Trusted others exude certainty. Once established, superstitions are regrettably difficult to remove by those who are less certain, like physicians. How willing are we to say that the flu vaccine is 100% safe? Without certainty, how can a physician debunk a superstition? The techniques that we have been taught usually work, but not when faced with a pathologic superstition.
Science and experience teach us that firmly held superstitions cannot be broken with logical, stepwise reasoning. Jonathan Haidt provides a useful metaphor for this problem in his book “The Happiness Hypothesis” (Basic Books, 2006). He describes a rider on an elephant. The rider represents our rational thought and the elephant represents our emotional foundation. The rider thinks he controls the elephant, but the opposite is more likely true. In order to move the elephant in a certain direction, the rider needs to make the elephant want to turn in that direction. Otherwise, all the cajoling and arguing in the world won’t make the elephant turn. A rational argument made to someone emotionally invested in the counter argument will fail. That is why we cannot convince antivaccine parents to vaccinate their children by trying to persuade them with facts. Neither can we convince global warming skeptics to stop burning coal, gun advocates to vote for restrictions on gun ownership, or cancer patients to accept curative treatment if their values and morals are being challenged.
In a later book, “The Righteous Mind: Why Good People Are Divided by Politics and Religion” (Vintage Books, 2012), Mr. Haidt expands his hypothesis to declare that to change minds, we must appeal to underlying moral values. The challenge is to identify those moral underpinnings in our patients in order to develop an appeal likely to resonate with their emotions and values.
Superstition derives from something people learn either from trusted others or from personal experience. It does no good for physicians to deride patient beliefs and denigrate their agency in an attempt to persuade them to abandon what we consider irrational beliefs. For physicians to penetrate pathologic superstitions, they will have to become the trusted other, to understand moral foundations, to emotionally connect. That does not usually happen the first day we meet a new patient, especially a skeptical one. It takes time, and effort, to reach out and bond with the patient and their family. Only then can pathologic superstitions dissolve and a better patient-doctor relationship evolve.
During this season rife with superstition, remember that your patient’s own superstitions are part of their belief system, and your belief system may be threatening to them. Make your beliefs less threatening, become a trusted other, and appeal to their foundational values, and you can successfully break a pathologic superstition.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Finding that sweet spot where science, practice, and best-possible outcomes come together
The practice of oncology and the science driving it have undergone substantial change in recent years, so it was particularly exciting when this year’s Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuko Honjo for their discovery that the body’s immune system can be harnessed to fight cancer. The advent of immunotherapy has expanded our therapeutic options, especially for patients whose previous treatments have failed, and in some patients, improvement in overall survival and safety profiles have been encouraging. But we still have a way to go with immunotherapies: not all patients respond to them and they are a costly therapeutic option. In addition, while chemotherapy supresses the immune system, immune-checkpoint inhibitors can hyperactivate it, and patients can experience serious immune-related adverse events that can result in life-threatening toxicities. Among the many things we grapple with in our daily practice is pairing these new and thrilling findings with our patients on a case-by-case basis to ensure the best-possible outcomes at every level – clinical, psychosocial, financial.
In recent years, we have seen an uptick in the number of FDA approvals, and as our therapeutic options have expanded, we have been able to refine and microtarget our treatment approaches, with encouraging clinical and quality-of-life outcomes. Our approach to practice has changed as well – our care is more patient focused, and we work more as part of a team, rather than individually, to ensure that our patients’ clinical and supportive needs are met. We hope our content reflects these shifts. For example, on page e188, Ibrahimi and colleagues looked at the time from admission to treatment initiation (TAT) in patients who were newly diagnosed with acute myeloid leukemia to see if it had an impact on overall survival (OS) and event-free survival. They obtained retrospective data over 5 years, focusing on patients with a TAT of 0-4 days and those with a TAT of >4 days, and found that the median OS in the 0-4 days group was almost double that of the <4 days group (1.3 years and 0.57 years, respectively). Median event-free survival for the groups was 1.21 years and 0.57 years, respectively. Moreover, that association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Marriage and survival
Does marital status have a prognostic bearing on outcomes in patients with cancer? Vyfhuis and colleagues addressed that question in their study of patients with stage III non–small-cell lung cancer (NSCLC) who had been treated uniformly with curative intent (p. e194). Specifically, they looked at OS and freedom from recurrence and they adjusted for patient-, disease-, and treatment-specific factors, as well as the interaction with racial, nutritional, and immunologic status.
In all, 52% of patients in the study were married, and were more likely to self-identify as white; live in areas with a higher household median income; undergo surgery; and have insurance, an ECOG of 0, and higher pretreatment albumin. The authors report that on multivariate analysis, marital status remained an independent predictor of survival and was associated with a 40% decreased risk of death, further stratifying outcomes beyond gender and stage grouping. Freedom from recurrence was comparable between the married and not-married patients. These findings suggest that in a cancer such as NSCLC, for which survival is modest despite therapeutic advances and which is associated with considerable treatment-related toxicities, marital status might be an independent predictor for survival. The authors suggest that marriage is likely a surrogate for better psychosocial support, and that the survival improvements might justify investment in supportive care interventional strategies to help advance overall outcomes.
Cancer in children and AYAs
Two articles in this issue examine cancers in pediatric patients and in adolescents and young adults (AYAs), and by doing so, demonstrate the importance of having evidence-based research findings to help us refine and deliver better-quality, patient-focused care. On page e217, Sharon Worcester documents the growing efforts by researchers and clinicians to understand and address the disparities in survival outcomes between AYAs with cancer and their pediatric and adult counterparts.
It has been known for a while that some cancers are more common among AYAs compared with the other 2 populations, and others are less common. More recent findings suggest that the biology and molecular make-up of AYA cancers might also be different and therefore necessitate different therapeutic protocols, and that the social and psychological needs unique to this population also require specifically tailored supportive care. What about treatment setting for AYAs with cancer – would outcomes be better in a pediatric or adult care center? There is evidence that the pediatric setting might have some advantage, but a recent study from Canada suggests that the cost of care in that setting might be higher. Despite these encouraging findings, there are very few trials designed specifically for the AYA cancer population, and the “pediatric-versus-adult” question also applies to AYA participation in trials. Worcester’s comprehensive article weaves together these issues and offers insights and useful explanations from a number of experts who study or care for AYAs with cancers.
Pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, but they are the second leading cause of death in children aged 1 to 14 years and therefore warrant attention, writes Jane de Lartigue in an article on page e210. She echoes Worcester’s point that better understanding of cancers in this younger population has brought to light their unique molecular drivers and challenged the assumption that drugs developed for adults can be used in children and young adults. Dr de Lartigue drills down into the science behind the unique biology and molecular aberrations in pediatric cancers and provides a useful list of ongoing clinical trials of targeted therapies in this population. She notes that because of their rarity, pediatric cancers are difficult to study and adequate enrollment in trials is challenging, although that is changing with researchers’ greater awareness of the uniqueness of these cancers and need for age-specific trials.
Also included in this issue are Community Translation articles on the approval of an immunotherapy combination – nivolumab plus ipilimumab – for the treatment of advanced RCC (p. e182), and for venetoclax as a therapy for patients with chronic lymphocytic leukemia, regardless of genotype (p. e185); and 2 Case Reports, one describing a diagnostic dilemma relating to a patient eventually diagnosed with primary renal synovial sarcoma (p. e202), and another detailing prolonged survival in a patient with adenocarcinoma of unknown primary who was treated with chemoradiotherapy (p. e206).
The practice of oncology and the science driving it have undergone substantial change in recent years, so it was particularly exciting when this year’s Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuko Honjo for their discovery that the body’s immune system can be harnessed to fight cancer. The advent of immunotherapy has expanded our therapeutic options, especially for patients whose previous treatments have failed, and in some patients, improvement in overall survival and safety profiles have been encouraging. But we still have a way to go with immunotherapies: not all patients respond to them and they are a costly therapeutic option. In addition, while chemotherapy supresses the immune system, immune-checkpoint inhibitors can hyperactivate it, and patients can experience serious immune-related adverse events that can result in life-threatening toxicities. Among the many things we grapple with in our daily practice is pairing these new and thrilling findings with our patients on a case-by-case basis to ensure the best-possible outcomes at every level – clinical, psychosocial, financial.
In recent years, we have seen an uptick in the number of FDA approvals, and as our therapeutic options have expanded, we have been able to refine and microtarget our treatment approaches, with encouraging clinical and quality-of-life outcomes. Our approach to practice has changed as well – our care is more patient focused, and we work more as part of a team, rather than individually, to ensure that our patients’ clinical and supportive needs are met. We hope our content reflects these shifts. For example, on page e188, Ibrahimi and colleagues looked at the time from admission to treatment initiation (TAT) in patients who were newly diagnosed with acute myeloid leukemia to see if it had an impact on overall survival (OS) and event-free survival. They obtained retrospective data over 5 years, focusing on patients with a TAT of 0-4 days and those with a TAT of >4 days, and found that the median OS in the 0-4 days group was almost double that of the <4 days group (1.3 years and 0.57 years, respectively). Median event-free survival for the groups was 1.21 years and 0.57 years, respectively. Moreover, that association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Marriage and survival
Does marital status have a prognostic bearing on outcomes in patients with cancer? Vyfhuis and colleagues addressed that question in their study of patients with stage III non–small-cell lung cancer (NSCLC) who had been treated uniformly with curative intent (p. e194). Specifically, they looked at OS and freedom from recurrence and they adjusted for patient-, disease-, and treatment-specific factors, as well as the interaction with racial, nutritional, and immunologic status.
In all, 52% of patients in the study were married, and were more likely to self-identify as white; live in areas with a higher household median income; undergo surgery; and have insurance, an ECOG of 0, and higher pretreatment albumin. The authors report that on multivariate analysis, marital status remained an independent predictor of survival and was associated with a 40% decreased risk of death, further stratifying outcomes beyond gender and stage grouping. Freedom from recurrence was comparable between the married and not-married patients. These findings suggest that in a cancer such as NSCLC, for which survival is modest despite therapeutic advances and which is associated with considerable treatment-related toxicities, marital status might be an independent predictor for survival. The authors suggest that marriage is likely a surrogate for better psychosocial support, and that the survival improvements might justify investment in supportive care interventional strategies to help advance overall outcomes.
Cancer in children and AYAs
Two articles in this issue examine cancers in pediatric patients and in adolescents and young adults (AYAs), and by doing so, demonstrate the importance of having evidence-based research findings to help us refine and deliver better-quality, patient-focused care. On page e217, Sharon Worcester documents the growing efforts by researchers and clinicians to understand and address the disparities in survival outcomes between AYAs with cancer and their pediatric and adult counterparts.
It has been known for a while that some cancers are more common among AYAs compared with the other 2 populations, and others are less common. More recent findings suggest that the biology and molecular make-up of AYA cancers might also be different and therefore necessitate different therapeutic protocols, and that the social and psychological needs unique to this population also require specifically tailored supportive care. What about treatment setting for AYAs with cancer – would outcomes be better in a pediatric or adult care center? There is evidence that the pediatric setting might have some advantage, but a recent study from Canada suggests that the cost of care in that setting might be higher. Despite these encouraging findings, there are very few trials designed specifically for the AYA cancer population, and the “pediatric-versus-adult” question also applies to AYA participation in trials. Worcester’s comprehensive article weaves together these issues and offers insights and useful explanations from a number of experts who study or care for AYAs with cancers.
Pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, but they are the second leading cause of death in children aged 1 to 14 years and therefore warrant attention, writes Jane de Lartigue in an article on page e210. She echoes Worcester’s point that better understanding of cancers in this younger population has brought to light their unique molecular drivers and challenged the assumption that drugs developed for adults can be used in children and young adults. Dr de Lartigue drills down into the science behind the unique biology and molecular aberrations in pediatric cancers and provides a useful list of ongoing clinical trials of targeted therapies in this population. She notes that because of their rarity, pediatric cancers are difficult to study and adequate enrollment in trials is challenging, although that is changing with researchers’ greater awareness of the uniqueness of these cancers and need for age-specific trials.
Also included in this issue are Community Translation articles on the approval of an immunotherapy combination – nivolumab plus ipilimumab – for the treatment of advanced RCC (p. e182), and for venetoclax as a therapy for patients with chronic lymphocytic leukemia, regardless of genotype (p. e185); and 2 Case Reports, one describing a diagnostic dilemma relating to a patient eventually diagnosed with primary renal synovial sarcoma (p. e202), and another detailing prolonged survival in a patient with adenocarcinoma of unknown primary who was treated with chemoradiotherapy (p. e206).
The practice of oncology and the science driving it have undergone substantial change in recent years, so it was particularly exciting when this year’s Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuko Honjo for their discovery that the body’s immune system can be harnessed to fight cancer. The advent of immunotherapy has expanded our therapeutic options, especially for patients whose previous treatments have failed, and in some patients, improvement in overall survival and safety profiles have been encouraging. But we still have a way to go with immunotherapies: not all patients respond to them and they are a costly therapeutic option. In addition, while chemotherapy supresses the immune system, immune-checkpoint inhibitors can hyperactivate it, and patients can experience serious immune-related adverse events that can result in life-threatening toxicities. Among the many things we grapple with in our daily practice is pairing these new and thrilling findings with our patients on a case-by-case basis to ensure the best-possible outcomes at every level – clinical, psychosocial, financial.
In recent years, we have seen an uptick in the number of FDA approvals, and as our therapeutic options have expanded, we have been able to refine and microtarget our treatment approaches, with encouraging clinical and quality-of-life outcomes. Our approach to practice has changed as well – our care is more patient focused, and we work more as part of a team, rather than individually, to ensure that our patients’ clinical and supportive needs are met. We hope our content reflects these shifts. For example, on page e188, Ibrahimi and colleagues looked at the time from admission to treatment initiation (TAT) in patients who were newly diagnosed with acute myeloid leukemia to see if it had an impact on overall survival (OS) and event-free survival. They obtained retrospective data over 5 years, focusing on patients with a TAT of 0-4 days and those with a TAT of >4 days, and found that the median OS in the 0-4 days group was almost double that of the <4 days group (1.3 years and 0.57 years, respectively). Median event-free survival for the groups was 1.21 years and 0.57 years, respectively. Moreover, that association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Marriage and survival
Does marital status have a prognostic bearing on outcomes in patients with cancer? Vyfhuis and colleagues addressed that question in their study of patients with stage III non–small-cell lung cancer (NSCLC) who had been treated uniformly with curative intent (p. e194). Specifically, they looked at OS and freedom from recurrence and they adjusted for patient-, disease-, and treatment-specific factors, as well as the interaction with racial, nutritional, and immunologic status.
In all, 52% of patients in the study were married, and were more likely to self-identify as white; live in areas with a higher household median income; undergo surgery; and have insurance, an ECOG of 0, and higher pretreatment albumin. The authors report that on multivariate analysis, marital status remained an independent predictor of survival and was associated with a 40% decreased risk of death, further stratifying outcomes beyond gender and stage grouping. Freedom from recurrence was comparable between the married and not-married patients. These findings suggest that in a cancer such as NSCLC, for which survival is modest despite therapeutic advances and which is associated with considerable treatment-related toxicities, marital status might be an independent predictor for survival. The authors suggest that marriage is likely a surrogate for better psychosocial support, and that the survival improvements might justify investment in supportive care interventional strategies to help advance overall outcomes.
Cancer in children and AYAs
Two articles in this issue examine cancers in pediatric patients and in adolescents and young adults (AYAs), and by doing so, demonstrate the importance of having evidence-based research findings to help us refine and deliver better-quality, patient-focused care. On page e217, Sharon Worcester documents the growing efforts by researchers and clinicians to understand and address the disparities in survival outcomes between AYAs with cancer and their pediatric and adult counterparts.
It has been known for a while that some cancers are more common among AYAs compared with the other 2 populations, and others are less common. More recent findings suggest that the biology and molecular make-up of AYA cancers might also be different and therefore necessitate different therapeutic protocols, and that the social and psychological needs unique to this population also require specifically tailored supportive care. What about treatment setting for AYAs with cancer – would outcomes be better in a pediatric or adult care center? There is evidence that the pediatric setting might have some advantage, but a recent study from Canada suggests that the cost of care in that setting might be higher. Despite these encouraging findings, there are very few trials designed specifically for the AYA cancer population, and the “pediatric-versus-adult” question also applies to AYA participation in trials. Worcester’s comprehensive article weaves together these issues and offers insights and useful explanations from a number of experts who study or care for AYAs with cancers.
Pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, but they are the second leading cause of death in children aged 1 to 14 years and therefore warrant attention, writes Jane de Lartigue in an article on page e210. She echoes Worcester’s point that better understanding of cancers in this younger population has brought to light their unique molecular drivers and challenged the assumption that drugs developed for adults can be used in children and young adults. Dr de Lartigue drills down into the science behind the unique biology and molecular aberrations in pediatric cancers and provides a useful list of ongoing clinical trials of targeted therapies in this population. She notes that because of their rarity, pediatric cancers are difficult to study and adequate enrollment in trials is challenging, although that is changing with researchers’ greater awareness of the uniqueness of these cancers and need for age-specific trials.
Also included in this issue are Community Translation articles on the approval of an immunotherapy combination – nivolumab plus ipilimumab – for the treatment of advanced RCC (p. e182), and for venetoclax as a therapy for patients with chronic lymphocytic leukemia, regardless of genotype (p. e185); and 2 Case Reports, one describing a diagnostic dilemma relating to a patient eventually diagnosed with primary renal synovial sarcoma (p. e202), and another detailing prolonged survival in a patient with adenocarcinoma of unknown primary who was treated with chemoradiotherapy (p. e206).
This is a drill
She had fallen on a garden implement, lacerating her superficial femoral artery. She used her cell phone to call 911. Thanks to an alert EMS crew and the Stop the Bleed training they recently received, a tourniquet was placed without delay. She got to our trauma bay in about 15 minutes after the tourniquet was applied. Although the patient made it abundantly clear that she was in pain, she was stable with moderate tachycardia and a good blood pressure.
Our trauma team leaped into action. The leader took report from the EMS while two nurses and a second surgeon assessed the patient, got her clothes cut off, and applied monitors. A third nurse got a second IV going. Primary survey was done in less than 90 seconds. The patient then underwent a focused exam including a log roll for back injuries.
The leg wound was still oozing a bit, so a second tourniquet was called for and pressure applied until it could be acquired. The patient was given 5 mg of morphine sulfate, which calmed her down a bit. Labs and x-rays were done quickly. The nursing staff suggested a tetanus booster, and the second surgeon who had gotten a basic past medical history suggested vancomycin since the patient said she was allergic to penicillin. Fifteen minutes after she hit the trauma bay, she was on her way to the OR for exploration, debridement, and vascular repair of her injury.
This was all done by four M2 medical students and five N4 nursing students, none of whom had had previous experience with this type of trauma patient
The students were managing this trauma situation in the simulation center of their medical school with four staff watching. This was their second run through for the afternoon. At debriefing, they compared their work on the first trauma of the day (a stab wound to the right chest) to their second attempt. They were satisfied with their efforts and so were we, the faculty who ran the simulation. Comparing their response to those I’ve seen in real life, I’d say these students understood their roles and responsibilities as well as the sort of thrown-together teams I’ve seen at places where trauma is not the main focus. While these young men and women are in the early stage of training and not ready for a real-world trauma emergency, they have gained knowledge about this kind of situation that I didn’t see until I was in residency and beyond. The times they are a-changing.
A couple of days later I was in Rochester, Minn., attending an American College of Surgeons Advanced Education Institute (ACS/AEI) course on simulations. At the end of that course, we participants were challenged by a manikin in extremis. Everyone there was an expert, had an advanced degree, had some experience in simulation, or were surgeons interested in simulation. I found that, even though this was a simulation and the patient was only a pretend human being, my adrenal cortex performed almost as if I were doing a real resuscitation. Previous training I’d had on teamwork, crew resource management, and ACLS all kicked in, and we got it done. But interestingly, we weren’t perfect. We debriefed and found that, even at our level of experience and training, a simple simulation could be very instructive. Seeing/doing is believing.
High-tech skills in high-risk occupations are well served by simulation training. Much of the airline piloting training is done by simulation. It works well for aviation, nuclear reactors, high voltage line work, and medicine. Most of these disciplines have embraced simulation as an essential part of training. Simulation is part of many surgical training programs, but it has other uses.
When was the last time you practiced a trauma resuscitation, an ultrasound fine-needle biopsy, laparoscopic maneuvers, or an unusual technique that you seldom perform, but when needed, must be pulled off very well? Most of us taking this simulation course agreed that time, money, and ego may get in the way of maintaining those skills for those rare instances when they are needed. Surgeons might want to consider simulation to keep some of our rarely used skills from getting rusty.
If you’re going to make a costly error, I would very much like you to do it on a piece of plastic, not on a patient. There are no consequences for messing up a procedure on a manikin and this kind of practice might teach you something critical. Practicing reduces stress and improves the performance of those placed on the spot by real-life events. Do you think Captain “Sully” Sullenberger could have landed that airliner in the Hudson River safely if he hadn’t practiced with countless mind-numbingly complex simulations? Sure, luck plays a part, and innate ability plays a part. But skill, knowledge, and practice are your best bet when all the eyes in the room swivel to you in a moment of crisis.
You may think that simulators have to cost $100,000 and be completely realistic to do the job. That’s not true. A banana, orange, or stick of butter can be fabulous sims for a med student. Felt and cardboard can make a realistic cricothyroidotomy model.
Surgeons all over the country are using simulation training to learn how to be better without getting real blood on their shoes. If you haven’t participated in a training simulation recently, I double-dog dare you to try it and tell me you found it without merit. The ACS Surgical Simulation Summit is being held in March 2019 in Chicago. You might want to check that out.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the University of Kansas School of Medicine, Salina, and Coeditor of ACS Surgery News.
She had fallen on a garden implement, lacerating her superficial femoral artery. She used her cell phone to call 911. Thanks to an alert EMS crew and the Stop the Bleed training they recently received, a tourniquet was placed without delay. She got to our trauma bay in about 15 minutes after the tourniquet was applied. Although the patient made it abundantly clear that she was in pain, she was stable with moderate tachycardia and a good blood pressure.
Our trauma team leaped into action. The leader took report from the EMS while two nurses and a second surgeon assessed the patient, got her clothes cut off, and applied monitors. A third nurse got a second IV going. Primary survey was done in less than 90 seconds. The patient then underwent a focused exam including a log roll for back injuries.
The leg wound was still oozing a bit, so a second tourniquet was called for and pressure applied until it could be acquired. The patient was given 5 mg of morphine sulfate, which calmed her down a bit. Labs and x-rays were done quickly. The nursing staff suggested a tetanus booster, and the second surgeon who had gotten a basic past medical history suggested vancomycin since the patient said she was allergic to penicillin. Fifteen minutes after she hit the trauma bay, she was on her way to the OR for exploration, debridement, and vascular repair of her injury.
This was all done by four M2 medical students and five N4 nursing students, none of whom had had previous experience with this type of trauma patient
The students were managing this trauma situation in the simulation center of their medical school with four staff watching. This was their second run through for the afternoon. At debriefing, they compared their work on the first trauma of the day (a stab wound to the right chest) to their second attempt. They were satisfied with their efforts and so were we, the faculty who ran the simulation. Comparing their response to those I’ve seen in real life, I’d say these students understood their roles and responsibilities as well as the sort of thrown-together teams I’ve seen at places where trauma is not the main focus. While these young men and women are in the early stage of training and not ready for a real-world trauma emergency, they have gained knowledge about this kind of situation that I didn’t see until I was in residency and beyond. The times they are a-changing.
A couple of days later I was in Rochester, Minn., attending an American College of Surgeons Advanced Education Institute (ACS/AEI) course on simulations. At the end of that course, we participants were challenged by a manikin in extremis. Everyone there was an expert, had an advanced degree, had some experience in simulation, or were surgeons interested in simulation. I found that, even though this was a simulation and the patient was only a pretend human being, my adrenal cortex performed almost as if I were doing a real resuscitation. Previous training I’d had on teamwork, crew resource management, and ACLS all kicked in, and we got it done. But interestingly, we weren’t perfect. We debriefed and found that, even at our level of experience and training, a simple simulation could be very instructive. Seeing/doing is believing.
High-tech skills in high-risk occupations are well served by simulation training. Much of the airline piloting training is done by simulation. It works well for aviation, nuclear reactors, high voltage line work, and medicine. Most of these disciplines have embraced simulation as an essential part of training. Simulation is part of many surgical training programs, but it has other uses.
When was the last time you practiced a trauma resuscitation, an ultrasound fine-needle biopsy, laparoscopic maneuvers, or an unusual technique that you seldom perform, but when needed, must be pulled off very well? Most of us taking this simulation course agreed that time, money, and ego may get in the way of maintaining those skills for those rare instances when they are needed. Surgeons might want to consider simulation to keep some of our rarely used skills from getting rusty.
If you’re going to make a costly error, I would very much like you to do it on a piece of plastic, not on a patient. There are no consequences for messing up a procedure on a manikin and this kind of practice might teach you something critical. Practicing reduces stress and improves the performance of those placed on the spot by real-life events. Do you think Captain “Sully” Sullenberger could have landed that airliner in the Hudson River safely if he hadn’t practiced with countless mind-numbingly complex simulations? Sure, luck plays a part, and innate ability plays a part. But skill, knowledge, and practice are your best bet when all the eyes in the room swivel to you in a moment of crisis.
You may think that simulators have to cost $100,000 and be completely realistic to do the job. That’s not true. A banana, orange, or stick of butter can be fabulous sims for a med student. Felt and cardboard can make a realistic cricothyroidotomy model.
Surgeons all over the country are using simulation training to learn how to be better without getting real blood on their shoes. If you haven’t participated in a training simulation recently, I double-dog dare you to try it and tell me you found it without merit. The ACS Surgical Simulation Summit is being held in March 2019 in Chicago. You might want to check that out.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the University of Kansas School of Medicine, Salina, and Coeditor of ACS Surgery News.
She had fallen on a garden implement, lacerating her superficial femoral artery. She used her cell phone to call 911. Thanks to an alert EMS crew and the Stop the Bleed training they recently received, a tourniquet was placed without delay. She got to our trauma bay in about 15 minutes after the tourniquet was applied. Although the patient made it abundantly clear that she was in pain, she was stable with moderate tachycardia and a good blood pressure.
Our trauma team leaped into action. The leader took report from the EMS while two nurses and a second surgeon assessed the patient, got her clothes cut off, and applied monitors. A third nurse got a second IV going. Primary survey was done in less than 90 seconds. The patient then underwent a focused exam including a log roll for back injuries.
The leg wound was still oozing a bit, so a second tourniquet was called for and pressure applied until it could be acquired. The patient was given 5 mg of morphine sulfate, which calmed her down a bit. Labs and x-rays were done quickly. The nursing staff suggested a tetanus booster, and the second surgeon who had gotten a basic past medical history suggested vancomycin since the patient said she was allergic to penicillin. Fifteen minutes after she hit the trauma bay, she was on her way to the OR for exploration, debridement, and vascular repair of her injury.
This was all done by four M2 medical students and five N4 nursing students, none of whom had had previous experience with this type of trauma patient
The students were managing this trauma situation in the simulation center of their medical school with four staff watching. This was their second run through for the afternoon. At debriefing, they compared their work on the first trauma of the day (a stab wound to the right chest) to their second attempt. They were satisfied with their efforts and so were we, the faculty who ran the simulation. Comparing their response to those I’ve seen in real life, I’d say these students understood their roles and responsibilities as well as the sort of thrown-together teams I’ve seen at places where trauma is not the main focus. While these young men and women are in the early stage of training and not ready for a real-world trauma emergency, they have gained knowledge about this kind of situation that I didn’t see until I was in residency and beyond. The times they are a-changing.
A couple of days later I was in Rochester, Minn., attending an American College of Surgeons Advanced Education Institute (ACS/AEI) course on simulations. At the end of that course, we participants were challenged by a manikin in extremis. Everyone there was an expert, had an advanced degree, had some experience in simulation, or were surgeons interested in simulation. I found that, even though this was a simulation and the patient was only a pretend human being, my adrenal cortex performed almost as if I were doing a real resuscitation. Previous training I’d had on teamwork, crew resource management, and ACLS all kicked in, and we got it done. But interestingly, we weren’t perfect. We debriefed and found that, even at our level of experience and training, a simple simulation could be very instructive. Seeing/doing is believing.
High-tech skills in high-risk occupations are well served by simulation training. Much of the airline piloting training is done by simulation. It works well for aviation, nuclear reactors, high voltage line work, and medicine. Most of these disciplines have embraced simulation as an essential part of training. Simulation is part of many surgical training programs, but it has other uses.
When was the last time you practiced a trauma resuscitation, an ultrasound fine-needle biopsy, laparoscopic maneuvers, or an unusual technique that you seldom perform, but when needed, must be pulled off very well? Most of us taking this simulation course agreed that time, money, and ego may get in the way of maintaining those skills for those rare instances when they are needed. Surgeons might want to consider simulation to keep some of our rarely used skills from getting rusty.
If you’re going to make a costly error, I would very much like you to do it on a piece of plastic, not on a patient. There are no consequences for messing up a procedure on a manikin and this kind of practice might teach you something critical. Practicing reduces stress and improves the performance of those placed on the spot by real-life events. Do you think Captain “Sully” Sullenberger could have landed that airliner in the Hudson River safely if he hadn’t practiced with countless mind-numbingly complex simulations? Sure, luck plays a part, and innate ability plays a part. But skill, knowledge, and practice are your best bet when all the eyes in the room swivel to you in a moment of crisis.
You may think that simulators have to cost $100,000 and be completely realistic to do the job. That’s not true. A banana, orange, or stick of butter can be fabulous sims for a med student. Felt and cardboard can make a realistic cricothyroidotomy model.
Surgeons all over the country are using simulation training to learn how to be better without getting real blood on their shoes. If you haven’t participated in a training simulation recently, I double-dog dare you to try it and tell me you found it without merit. The ACS Surgical Simulation Summit is being held in March 2019 in Chicago. You might want to check that out.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the University of Kansas School of Medicine, Salina, and Coeditor of ACS Surgery News.
Postpartum hemorrhage: Aortic compression to reduce pelvic bleeding
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11
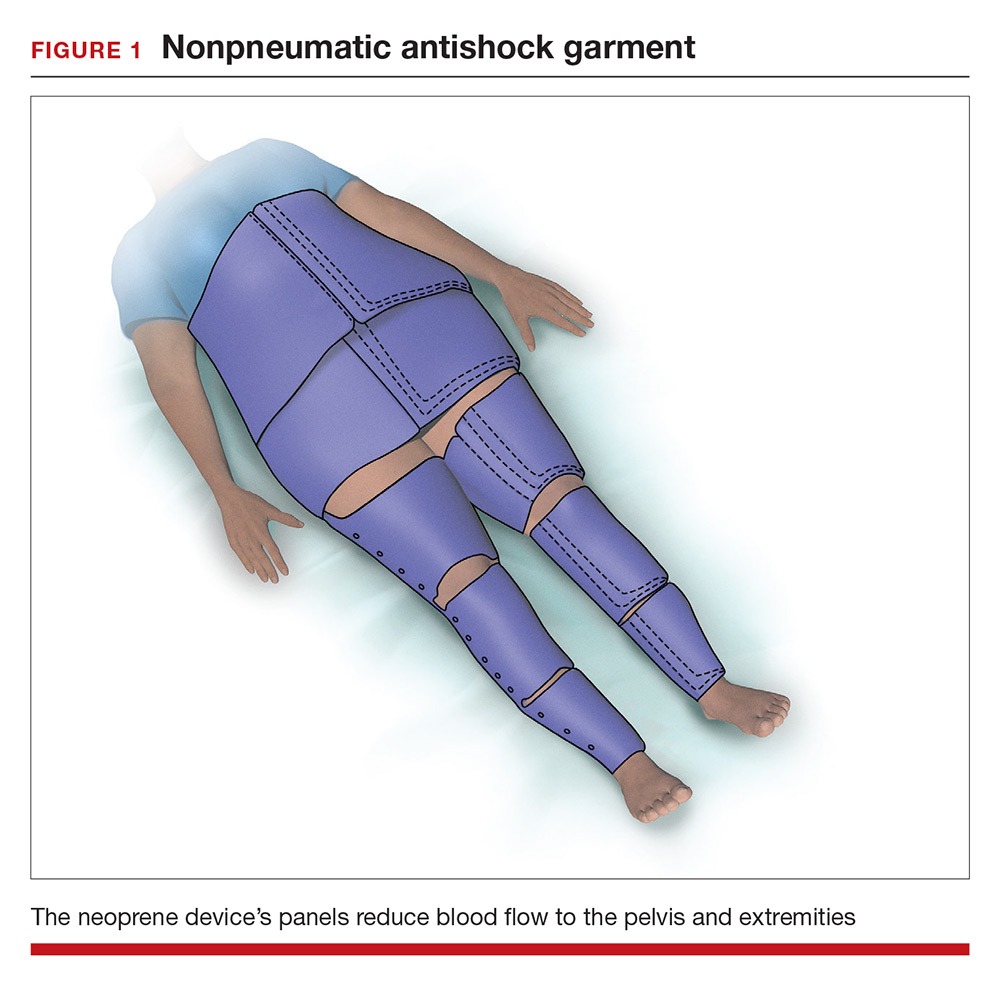
In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
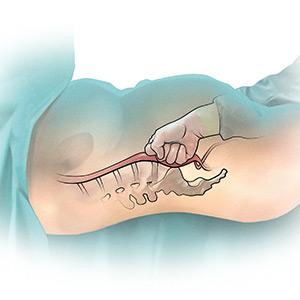
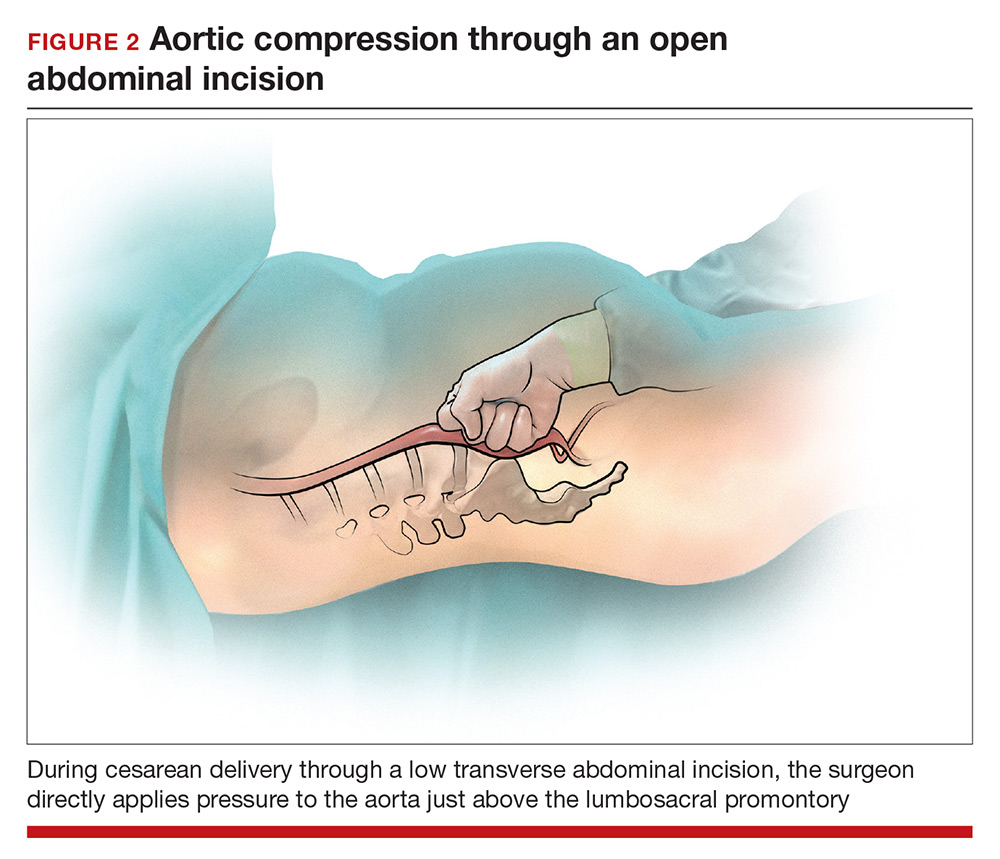
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.
Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
Small fibers, large impact
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
The details about an individual’s search for information tell us a lot about healthcare concerns and uncertainty across the medical universe. For nearly a decade, one of the most “clicked on” papers we have published in the Journal has been a review of small fiber neuropathy—a clinical entity with a prevalence of perhaps 1 in 1,000 to 2,000 people and, to my knowledge, no associated walkathons or arm bracelets. Yet it certainly piques the interest of clinicians from many specialties far broader than neurology. In this issue of the Journal, Dr. Jinny Tavee updates her 2009 review and provides us with a clinical overview of the disorder and the opportunity to assess how much further we need to more fully understand its management and associated comorbid conditions.
The wide interest in this disorder plugs into our current seeming epidemic of patients with chronic pain. It seems that almost half of my new patients have issues related to chronic pain that are not directly explained by active inflammation or anatomic damage. Many of these patients have diffuse body pains with associated fatigue and sleep disorders and are diagnosed with fibromyalgia. But others describe pain with a burning and tingling quality that seems of neurologic origin, yet their neurologic examination is normal. A few describe a predominantly distal symmetric stocking-and-glove distribution, but most do not. In some patients these pains are spatially random and evanescent, which to me are usually the hardest to fathom. Nerve conduction studies, when performed, are unrevealing.
A number of systemic autoimmune disorders, as discussed by Dr. Tavee in her article, are suggested to have an association with these symptoms. Given the chronicity and the frustrating nature of the symptoms, it is no surprise that a panoply of immune serologies are frequently ordered. Invariably, since serologies (eg, ANA, SSA, SSB, rheumatoid factor) are not specific for any single entity, some will return as positive. The strength of many of these associations is weak; even when the clinical diagnosis of lupus, for example, is definite, treatment of the underlying disease does not necessarily improve the dysesthetic pain. In an alternative scenario, the small fiber neuropathy is ascribed to a systemic autoimmune disorder that has been diagnosed because an autoantibody has been detected, but this rarely helps the patient and may in fact worsen symptoms by increasing anxiety and concern over having a systemic disease such as Sjögren syndrome or lupus (both of which sound terrible when reviewed on the Internet).
Some patients describe autonomic symptoms. Given the biologic basis that has been defined for this entity, it is no surprise that some patients have marked symptoms of decreased exocrine gland function, gastrointestinal dysmotility, and orthostasis. These symptoms may not be recognized unless specifically sought out when interviewing the patient.
Given the chronicity and sometimes the vagaries of symptoms, it is often comforting for patients to get an actual diagnosis. Dr. Tavee notes the relative simplicity of diagnostic procedures. But determining the clinical implications of the results may not be straightforward, and devising a fully and uniformly effective therapeutic approach eludes us still. As she points out, a multidisciplinary approach to therapy and diagnosis can be quite helpful.
Neuropolitics in the age of extremism: Brain regions involved in hatred
We psychiatrists encounter a wide variety of intense negative emotions in our patients on a daily basis, whether in the clinic or on an inpatient unit. These include rage, irritability, hostility, paranoia, loathing, and unadulterated hatred.
We evaluate, diagnose, and treat the underlying psychiatric brain disorders that generate such maladaptive emotions, and have our patients regain their baseline functioning by resolving the psychopathology that ignited their amygdala and their limbic circuitry.
But while we can manage the microcosm of one patient’s mental state, we are unable to intervene in the macrocosm of an entire society ravaged by extreme hyper-partisanship and naked bidirectional hatred. It is literally impossible for even the most skillful psychiatrists to repair a nation caught up in poisonous emotional turmoil, irreconcilable political differences, and a veritable war of belief systems that mimic religious fanaticism, which history tells us led to so many tragic wars over the centuries and millennia.
Ideally, politics is supposed to be an elegant cerebral process, a debate of ideas across disparate ideologies, the product of which is expected to be the advancement of the welfare of the nation and its citizens. But what we are currently witnessing is a distressing degeneration of politics into personal hatred and ad hominem attacks, with partisans frothing at the mouth as they describe the utter stupidity and dangerousness of their despised political opponents-cum-bitter enemies. They even declare each other “mentally ill,” which is an absurd explanation of why other people do not agree with their belief system. Neither side can find an iota of redeeming value in the political views of the “other side” and hurl insults and epithets verbally and in writing via dueling books that become instant best sellers among the partisan aficionados on both sides.
This disastrous political “climate change” may have ominous repercussions for the brains of the political combatants themselves, and even for those on the sidelines who are subjected to the relentless stress of witnessing a social train wreck in the making. As a neuropsychiatrist, I wonder if the collective national amygdala of the country is on fire, and the national prefrontal cortex is being corroded by the pervasive and ugly negativity that engulfs us all, with social media that incites its users night and day, adding gasoline to the fire. Chronic stress and its associated hypercortisolemia are known to be neurotoxic to the hippocampus and eventuate in clinical depression and its grave consequences.
Continued to: I think I sensed this odious scenario coming...
I think I sensed this odious scenario coming 2 years ago during the bizarre presidential election, when I wrote an editorial describing the “fear and loathing” that permeated the political process and the unusual behavior of the candidates.1 A year after the election, I commented about the toxic zeitgeist of political extremism from a psychiatric perspective.2 The situation appears to be getting worse, and the folie en masse is intensifying and its hateful cacophony is deafening to our sensibilities.
Aaron Beck, MD, the father of cognitive-behavioral therapy (CBT), wrote a book about hate.3 It may be a fantasy, but I wish the leaders on both sides would agree to a course of CBT to recognize the destructive path of intransigent hyper-partisanship. They might then transcend their egocentric attitudes and inspire millions of their followers to communicate rationally, instead of stoking the fires of resentment and enmity toward the “other side.”
Let’s get back to science: Where are the pathways of hate located in the brain? An interesting study was conducted to detect the neural circuits that mediate hate.4 The researchers obtained functional magnetic resonance imaging scans of participants while they were viewing the face of a person they hate compared with the face of an acquaintance toward whom they have neutral feelings. They also calculated a “hate score” for each participant for the analysis. They found that viewing a hated person increased the activity in several brain regions, including the medial frontal gyrus, right putamen, premotor cortex, frontal pole, and medial insula bilaterally. The activation in 3 areas correlated with the intensity of the hatred: right insula, right premotor cortex, and right frontal-medial gyrus. At the same time, the right superior frontal gyrus showed deactivation. Interestingly, hate and romantic love shared activation in 2 areas: the putamen and insula. This suggests that passionate love and passionate hate are 2 sides of the same neural coin! It prompts me to wonder what happens to the capacity to love among political extremists when their putamen and insula are filled up with hate. It also makes me wonder if unbridled hatred can be “enjoyable” and even addictive, as passionate romantic love is.
The bottom line: Consider the brain changes that are occurring on a large scale in at least a hundred million political partisans, and whether those neural circuits get even more intensely activated following the elections, regardless of the outcome.
Finally, we must remain cognizant of the epigenetic consequences of emotions and stress.5 There is solid scientific evidence that extremes of human experiences can modify gene expression in sperm and fetuses, resulting in a transgenerational effect upon the children of the extreme partisans, and also the children of nonpartisan observers, who experience unmitigated anxiety due to the inescapable cloud of negative affect shrouding their daily lives.6 So politicians should be cognizant that perpetuating a bitter war against each other may be detrimental to their progeny and future generations. I am frankly worried about the epigenetically disrupted emotional stability of voters circa 2035, born in these days of unprecedented and tumultuous hatred by their hyper-partisan parents.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Nasrallah HA. Fear and loathing abound in the ‘off-label’ presidential election of 2016. Current Psychiatry. 2016;15(7):21,26.
2. Nasrallah HA. The toxic zeitgeist of hyper-partisanship: a psychiatric perspective. Current Psychiatry. 2018;17(2):17-18.
3. Beck AT. Prisoners of hate: the cognitive basis of anger, hostility, and violence. New York, NY: Harper-Collins; 1999.
4. Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556.
5. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience. 2014;275:420-435.
6. Bartlett AA, Singh R, Hunter RG. Anxiety and epigenetics. Adv Exp Med Biol.2017;978:145-166.
We psychiatrists encounter a wide variety of intense negative emotions in our patients on a daily basis, whether in the clinic or on an inpatient unit. These include rage, irritability, hostility, paranoia, loathing, and unadulterated hatred.
We evaluate, diagnose, and treat the underlying psychiatric brain disorders that generate such maladaptive emotions, and have our patients regain their baseline functioning by resolving the psychopathology that ignited their amygdala and their limbic circuitry.
But while we can manage the microcosm of one patient’s mental state, we are unable to intervene in the macrocosm of an entire society ravaged by extreme hyper-partisanship and naked bidirectional hatred. It is literally impossible for even the most skillful psychiatrists to repair a nation caught up in poisonous emotional turmoil, irreconcilable political differences, and a veritable war of belief systems that mimic religious fanaticism, which history tells us led to so many tragic wars over the centuries and millennia.
Ideally, politics is supposed to be an elegant cerebral process, a debate of ideas across disparate ideologies, the product of which is expected to be the advancement of the welfare of the nation and its citizens. But what we are currently witnessing is a distressing degeneration of politics into personal hatred and ad hominem attacks, with partisans frothing at the mouth as they describe the utter stupidity and dangerousness of their despised political opponents-cum-bitter enemies. They even declare each other “mentally ill,” which is an absurd explanation of why other people do not agree with their belief system. Neither side can find an iota of redeeming value in the political views of the “other side” and hurl insults and epithets verbally and in writing via dueling books that become instant best sellers among the partisan aficionados on both sides.
This disastrous political “climate change” may have ominous repercussions for the brains of the political combatants themselves, and even for those on the sidelines who are subjected to the relentless stress of witnessing a social train wreck in the making. As a neuropsychiatrist, I wonder if the collective national amygdala of the country is on fire, and the national prefrontal cortex is being corroded by the pervasive and ugly negativity that engulfs us all, with social media that incites its users night and day, adding gasoline to the fire. Chronic stress and its associated hypercortisolemia are known to be neurotoxic to the hippocampus and eventuate in clinical depression and its grave consequences.
Continued to: I think I sensed this odious scenario coming...
I think I sensed this odious scenario coming 2 years ago during the bizarre presidential election, when I wrote an editorial describing the “fear and loathing” that permeated the political process and the unusual behavior of the candidates.1 A year after the election, I commented about the toxic zeitgeist of political extremism from a psychiatric perspective.2 The situation appears to be getting worse, and the folie en masse is intensifying and its hateful cacophony is deafening to our sensibilities.
Aaron Beck, MD, the father of cognitive-behavioral therapy (CBT), wrote a book about hate.3 It may be a fantasy, but I wish the leaders on both sides would agree to a course of CBT to recognize the destructive path of intransigent hyper-partisanship. They might then transcend their egocentric attitudes and inspire millions of their followers to communicate rationally, instead of stoking the fires of resentment and enmity toward the “other side.”
Let’s get back to science: Where are the pathways of hate located in the brain? An interesting study was conducted to detect the neural circuits that mediate hate.4 The researchers obtained functional magnetic resonance imaging scans of participants while they were viewing the face of a person they hate compared with the face of an acquaintance toward whom they have neutral feelings. They also calculated a “hate score” for each participant for the analysis. They found that viewing a hated person increased the activity in several brain regions, including the medial frontal gyrus, right putamen, premotor cortex, frontal pole, and medial insula bilaterally. The activation in 3 areas correlated with the intensity of the hatred: right insula, right premotor cortex, and right frontal-medial gyrus. At the same time, the right superior frontal gyrus showed deactivation. Interestingly, hate and romantic love shared activation in 2 areas: the putamen and insula. This suggests that passionate love and passionate hate are 2 sides of the same neural coin! It prompts me to wonder what happens to the capacity to love among political extremists when their putamen and insula are filled up with hate. It also makes me wonder if unbridled hatred can be “enjoyable” and even addictive, as passionate romantic love is.
The bottom line: Consider the brain changes that are occurring on a large scale in at least a hundred million political partisans, and whether those neural circuits get even more intensely activated following the elections, regardless of the outcome.
Finally, we must remain cognizant of the epigenetic consequences of emotions and stress.5 There is solid scientific evidence that extremes of human experiences can modify gene expression in sperm and fetuses, resulting in a transgenerational effect upon the children of the extreme partisans, and also the children of nonpartisan observers, who experience unmitigated anxiety due to the inescapable cloud of negative affect shrouding their daily lives.6 So politicians should be cognizant that perpetuating a bitter war against each other may be detrimental to their progeny and future generations. I am frankly worried about the epigenetically disrupted emotional stability of voters circa 2035, born in these days of unprecedented and tumultuous hatred by their hyper-partisan parents.
Henry A. Nasrallah, MD
Editor-in-Chief
We psychiatrists encounter a wide variety of intense negative emotions in our patients on a daily basis, whether in the clinic or on an inpatient unit. These include rage, irritability, hostility, paranoia, loathing, and unadulterated hatred.
We evaluate, diagnose, and treat the underlying psychiatric brain disorders that generate such maladaptive emotions, and have our patients regain their baseline functioning by resolving the psychopathology that ignited their amygdala and their limbic circuitry.
But while we can manage the microcosm of one patient’s mental state, we are unable to intervene in the macrocosm of an entire society ravaged by extreme hyper-partisanship and naked bidirectional hatred. It is literally impossible for even the most skillful psychiatrists to repair a nation caught up in poisonous emotional turmoil, irreconcilable political differences, and a veritable war of belief systems that mimic religious fanaticism, which history tells us led to so many tragic wars over the centuries and millennia.
Ideally, politics is supposed to be an elegant cerebral process, a debate of ideas across disparate ideologies, the product of which is expected to be the advancement of the welfare of the nation and its citizens. But what we are currently witnessing is a distressing degeneration of politics into personal hatred and ad hominem attacks, with partisans frothing at the mouth as they describe the utter stupidity and dangerousness of their despised political opponents-cum-bitter enemies. They even declare each other “mentally ill,” which is an absurd explanation of why other people do not agree with their belief system. Neither side can find an iota of redeeming value in the political views of the “other side” and hurl insults and epithets verbally and in writing via dueling books that become instant best sellers among the partisan aficionados on both sides.
This disastrous political “climate change” may have ominous repercussions for the brains of the political combatants themselves, and even for those on the sidelines who are subjected to the relentless stress of witnessing a social train wreck in the making. As a neuropsychiatrist, I wonder if the collective national amygdala of the country is on fire, and the national prefrontal cortex is being corroded by the pervasive and ugly negativity that engulfs us all, with social media that incites its users night and day, adding gasoline to the fire. Chronic stress and its associated hypercortisolemia are known to be neurotoxic to the hippocampus and eventuate in clinical depression and its grave consequences.
Continued to: I think I sensed this odious scenario coming...
I think I sensed this odious scenario coming 2 years ago during the bizarre presidential election, when I wrote an editorial describing the “fear and loathing” that permeated the political process and the unusual behavior of the candidates.1 A year after the election, I commented about the toxic zeitgeist of political extremism from a psychiatric perspective.2 The situation appears to be getting worse, and the folie en masse is intensifying and its hateful cacophony is deafening to our sensibilities.
Aaron Beck, MD, the father of cognitive-behavioral therapy (CBT), wrote a book about hate.3 It may be a fantasy, but I wish the leaders on both sides would agree to a course of CBT to recognize the destructive path of intransigent hyper-partisanship. They might then transcend their egocentric attitudes and inspire millions of their followers to communicate rationally, instead of stoking the fires of resentment and enmity toward the “other side.”
Let’s get back to science: Where are the pathways of hate located in the brain? An interesting study was conducted to detect the neural circuits that mediate hate.4 The researchers obtained functional magnetic resonance imaging scans of participants while they were viewing the face of a person they hate compared with the face of an acquaintance toward whom they have neutral feelings. They also calculated a “hate score” for each participant for the analysis. They found that viewing a hated person increased the activity in several brain regions, including the medial frontal gyrus, right putamen, premotor cortex, frontal pole, and medial insula bilaterally. The activation in 3 areas correlated with the intensity of the hatred: right insula, right premotor cortex, and right frontal-medial gyrus. At the same time, the right superior frontal gyrus showed deactivation. Interestingly, hate and romantic love shared activation in 2 areas: the putamen and insula. This suggests that passionate love and passionate hate are 2 sides of the same neural coin! It prompts me to wonder what happens to the capacity to love among political extremists when their putamen and insula are filled up with hate. It also makes me wonder if unbridled hatred can be “enjoyable” and even addictive, as passionate romantic love is.
The bottom line: Consider the brain changes that are occurring on a large scale in at least a hundred million political partisans, and whether those neural circuits get even more intensely activated following the elections, regardless of the outcome.
Finally, we must remain cognizant of the epigenetic consequences of emotions and stress.5 There is solid scientific evidence that extremes of human experiences can modify gene expression in sperm and fetuses, resulting in a transgenerational effect upon the children of the extreme partisans, and also the children of nonpartisan observers, who experience unmitigated anxiety due to the inescapable cloud of negative affect shrouding their daily lives.6 So politicians should be cognizant that perpetuating a bitter war against each other may be detrimental to their progeny and future generations. I am frankly worried about the epigenetically disrupted emotional stability of voters circa 2035, born in these days of unprecedented and tumultuous hatred by their hyper-partisan parents.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Nasrallah HA. Fear and loathing abound in the ‘off-label’ presidential election of 2016. Current Psychiatry. 2016;15(7):21,26.
2. Nasrallah HA. The toxic zeitgeist of hyper-partisanship: a psychiatric perspective. Current Psychiatry. 2018;17(2):17-18.
3. Beck AT. Prisoners of hate: the cognitive basis of anger, hostility, and violence. New York, NY: Harper-Collins; 1999.
4. Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556.
5. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience. 2014;275:420-435.
6. Bartlett AA, Singh R, Hunter RG. Anxiety and epigenetics. Adv Exp Med Biol.2017;978:145-166.
1. Nasrallah HA. Fear and loathing abound in the ‘off-label’ presidential election of 2016. Current Psychiatry. 2016;15(7):21,26.
2. Nasrallah HA. The toxic zeitgeist of hyper-partisanship: a psychiatric perspective. Current Psychiatry. 2018;17(2):17-18.
3. Beck AT. Prisoners of hate: the cognitive basis of anger, hostility, and violence. New York, NY: Harper-Collins; 1999.
4. Zeki S, Romaya JP. Neural correlates of hate. PloS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556.
5. Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience. 2014;275:420-435.
6. Bartlett AA, Singh R, Hunter RG. Anxiety and epigenetics. Adv Exp Med Biol.2017;978:145-166.
From the Editor: An open letter to our hospital consultants
Dear <redacted>,
Just thought I would write and give you a quick update on our situation, not that you asked. As you recall, a few years ago we spent many hours discussing and planning the Heart and Vascular Service Line that you encouraged us to set up in our new hospital. These conversations were filled with the greatest hits of health care administrators. “Institutional silos,” “layers of integration,” and “financial dashboards” were crowd favorites. Personally, I enjoyed the endless timelines, flow charts to nowhere, and of course the countless hours spent crafting a vision statement. It’s a wonder we got any work done! At least one of us was getting paid by the hour.
When I asked you to provide concrete examples of fully integrated, functional Heart and Vascular Service Lines you initially deferred. Finally, you listed three examples. Of course, when I reached out to them, two had disbanded, and the third had no idea what I was talking about. Nevertheless, I pressed on. I tried to figure out how this would all work. What keeps the service line synchronous? My research kept turning up lines like: “alignment across departments and specialists is imperative for addressing the care delivery and business challenges facing cardiovascular providers.” OK sure, but huh?
The purpose of a Heart and Vascular Service Line is purportedly to improve the quality of care of cardiovascular patients. The concept of the service line has been in place for over 20 years, so where is the literature demonstrating the quality benefits? The improved outcomes? As far as I can tell it does not exist. So maybe that was never really the primary goal. Based on the vigor and financial capital hospitals pour into the creation of these lines, there must be a different game afoot. Looking deeper into the health care administration literature it seems the true benefit of a service line is that it keeps patients within the system. Once a patient is brought in by one specialty, the other specialties can converge to offer their services. It soon becomes an assembly line of atherosclerotic delights. The patient enjoys the theoretical advantage of having all of his or her specialists together, and the hospital enjoys the profits.
I would have been comfortable fighting the concept of the service line on the basis of access, practicality, or quality. The truth is, it isn’t about any of these things. If the service line were really about patient convenience, we would have put a vascular lab in the clinic as I requested. But that wouldn’t have been convenient for radiology or cardiology. In your model, which specialty does the work doesn’t matter because the hospital profits regardless. So without a financial map to describe how this all plays out, you just threw us into the same cage Thunderdome-style. Two specialists enter, one specialist leaves. The problem is that CMS is already looking at the volume of diagnostic studies as a factor in total cost. You helped us build a system that enables and encourages more testing. Cue Tina Turner, “We don’t need another ECHO…”
The turning point in our relationship should have come when you sent me the list of CPT codes for the procedures expected to be performed by vascular surgery. You got a few right. Lower extremity bypass, amputations, and even aneurysms were there. You seemed surprised, though, that we would be doing other leg interventions and thought the carotid endarterectomies would be done by neurosurgery. Here the problem was laid out. You were describing in detail the mechanisms for our new service line, but you didn’t really know what a vascular surgeon was. It’s a little late, but let me help you.
When I sent back the CPT list, even I forgot a few. Like 35251 (repair of intra-abdominal blood vessel), 27364 (radical resection of thigh sarcoma), or 35141 (repair of femoral pseudoaneurysm). You see, vascular surgeons are the great facilitators. Our expertise enables other specialties to perform at their highest levels. Comprehensive programs in orthopedics, neurosurgery, cardiology, cardiac surgery, surgical oncology, trauma, and urology would be essentially impossible without vascular surgery. A study conducted at Northwestern showed that 7% of their total volume of vascular surgeries were cases providing intraoperative assistance to other specialties.1 And this excluded trauma. While the hospital greatly benefits from this relationship, the vascular surgeons often do not. Emergently helping other physicians requires canceling our responsibilities, both at work and at home. CPT codes often severely undervalue our time spent assisting with large resections or waiting “on standby.”
The overall financial contributions of vascular surgeons to hospital systems are often overlooked. In a study performed at a tertiary care hospital in New Jersey, vascular surgeons were found to have the leading gross margin per FTE of any specialty, 66% more than cardiology. (And these were academic vascular surgeons, a famously lazy breed!)2 In 2002, Merritt, Hawkins & Associates found that the average vascular surgeon provides over $2 million in revenue to his or her hospital, third highest of any specialty.3 With the widespread adoption of endovascular procedures, this number is likely to be higher today.
So now, an update on our great experiment. Run by cardiology our service line treats heart disease, heart attacks, heart failure, and high blood pressure. At least according to the website. If you want to find vascular surgery, it is listed last, under “other services.” And no, sadly the list is not alphabetical. Around the country, there is a great shortage of vascular surgeons. There are two to three job openings for every graduate annually. Remarkably, our service line boasts ten board-certified vascular surgeons. But people always seem to want what they don’t have. In our service line director’s case, that was a TAVR program. So there was great effort and expense in creating one. When it came time to start our FEVAR program, I simply took my friend Andy Schanzer out to dinner and asked him how he did it at UMass. Then we just started doing cases. No fanfare, no press releases. No expensive hires. The dinner cost about $100 (Andy is a cheap date, but I ordered multiple apps).
Today, our service line is disintegrating. There is no animosity. It just didn’t work out. It never really made sense. Yes, our patients have heart disease. They also have lung cancer, diabetes, prostate disease, and spinal stenosis. They would benefit from wound care, smoking cessation, and certainly a comprehensive vascular lab. None of which were offered in our service line. We didn’t belong in the same silo as cardiology, and I certainly never believed they should be in one that treats PVD. I guess it is quaint to expect that a specialty’s scope of practice matches their ACGME and ABMS training requirements. Maybe I’m old-fashioned.
So <redacted>, looking back on our meetings you often took the tone of an adult explaining a difficult, but necessary thing to a child. Maybe the biggest lie we tell children is that adults know what they’re doing. Vascular surgery is an incredibly valuable asset to a health care system. One threatened by physician scarcity and one deserving of promotion and growth. It seems remarkably shortsighted to bury this asset on a service line under the direction of cardiology.
In the end, I have only one request. The next time you are in a meeting with a vascular surgeon who asks for an example of a successful Heart and Vascular Service Line don’t use us. It didn’t work. I don’t think it ever truly works.
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, division of vascular and endovascular surgery, Louisiana State University Health Sciences Center, New Orleans.
References
1. JAMA Surg. 2016;151(11):1032-8.
2. J. Vasc. Surg. 2012;55(1):281-5.
3. Merritt, Hawkins & Associates, 2002 Physician Inpatient/Outpatient Revenue Survey.
Dear <redacted>,
Just thought I would write and give you a quick update on our situation, not that you asked. As you recall, a few years ago we spent many hours discussing and planning the Heart and Vascular Service Line that you encouraged us to set up in our new hospital. These conversations were filled with the greatest hits of health care administrators. “Institutional silos,” “layers of integration,” and “financial dashboards” were crowd favorites. Personally, I enjoyed the endless timelines, flow charts to nowhere, and of course the countless hours spent crafting a vision statement. It’s a wonder we got any work done! At least one of us was getting paid by the hour.
When I asked you to provide concrete examples of fully integrated, functional Heart and Vascular Service Lines you initially deferred. Finally, you listed three examples. Of course, when I reached out to them, two had disbanded, and the third had no idea what I was talking about. Nevertheless, I pressed on. I tried to figure out how this would all work. What keeps the service line synchronous? My research kept turning up lines like: “alignment across departments and specialists is imperative for addressing the care delivery and business challenges facing cardiovascular providers.” OK sure, but huh?
The purpose of a Heart and Vascular Service Line is purportedly to improve the quality of care of cardiovascular patients. The concept of the service line has been in place for over 20 years, so where is the literature demonstrating the quality benefits? The improved outcomes? As far as I can tell it does not exist. So maybe that was never really the primary goal. Based on the vigor and financial capital hospitals pour into the creation of these lines, there must be a different game afoot. Looking deeper into the health care administration literature it seems the true benefit of a service line is that it keeps patients within the system. Once a patient is brought in by one specialty, the other specialties can converge to offer their services. It soon becomes an assembly line of atherosclerotic delights. The patient enjoys the theoretical advantage of having all of his or her specialists together, and the hospital enjoys the profits.
I would have been comfortable fighting the concept of the service line on the basis of access, practicality, or quality. The truth is, it isn’t about any of these things. If the service line were really about patient convenience, we would have put a vascular lab in the clinic as I requested. But that wouldn’t have been convenient for radiology or cardiology. In your model, which specialty does the work doesn’t matter because the hospital profits regardless. So without a financial map to describe how this all plays out, you just threw us into the same cage Thunderdome-style. Two specialists enter, one specialist leaves. The problem is that CMS is already looking at the volume of diagnostic studies as a factor in total cost. You helped us build a system that enables and encourages more testing. Cue Tina Turner, “We don’t need another ECHO…”
The turning point in our relationship should have come when you sent me the list of CPT codes for the procedures expected to be performed by vascular surgery. You got a few right. Lower extremity bypass, amputations, and even aneurysms were there. You seemed surprised, though, that we would be doing other leg interventions and thought the carotid endarterectomies would be done by neurosurgery. Here the problem was laid out. You were describing in detail the mechanisms for our new service line, but you didn’t really know what a vascular surgeon was. It’s a little late, but let me help you.
When I sent back the CPT list, even I forgot a few. Like 35251 (repair of intra-abdominal blood vessel), 27364 (radical resection of thigh sarcoma), or 35141 (repair of femoral pseudoaneurysm). You see, vascular surgeons are the great facilitators. Our expertise enables other specialties to perform at their highest levels. Comprehensive programs in orthopedics, neurosurgery, cardiology, cardiac surgery, surgical oncology, trauma, and urology would be essentially impossible without vascular surgery. A study conducted at Northwestern showed that 7% of their total volume of vascular surgeries were cases providing intraoperative assistance to other specialties.1 And this excluded trauma. While the hospital greatly benefits from this relationship, the vascular surgeons often do not. Emergently helping other physicians requires canceling our responsibilities, both at work and at home. CPT codes often severely undervalue our time spent assisting with large resections or waiting “on standby.”
The overall financial contributions of vascular surgeons to hospital systems are often overlooked. In a study performed at a tertiary care hospital in New Jersey, vascular surgeons were found to have the leading gross margin per FTE of any specialty, 66% more than cardiology. (And these were academic vascular surgeons, a famously lazy breed!)2 In 2002, Merritt, Hawkins & Associates found that the average vascular surgeon provides over $2 million in revenue to his or her hospital, third highest of any specialty.3 With the widespread adoption of endovascular procedures, this number is likely to be higher today.
So now, an update on our great experiment. Run by cardiology our service line treats heart disease, heart attacks, heart failure, and high blood pressure. At least according to the website. If you want to find vascular surgery, it is listed last, under “other services.” And no, sadly the list is not alphabetical. Around the country, there is a great shortage of vascular surgeons. There are two to three job openings for every graduate annually. Remarkably, our service line boasts ten board-certified vascular surgeons. But people always seem to want what they don’t have. In our service line director’s case, that was a TAVR program. So there was great effort and expense in creating one. When it came time to start our FEVAR program, I simply took my friend Andy Schanzer out to dinner and asked him how he did it at UMass. Then we just started doing cases. No fanfare, no press releases. No expensive hires. The dinner cost about $100 (Andy is a cheap date, but I ordered multiple apps).
Today, our service line is disintegrating. There is no animosity. It just didn’t work out. It never really made sense. Yes, our patients have heart disease. They also have lung cancer, diabetes, prostate disease, and spinal stenosis. They would benefit from wound care, smoking cessation, and certainly a comprehensive vascular lab. None of which were offered in our service line. We didn’t belong in the same silo as cardiology, and I certainly never believed they should be in one that treats PVD. I guess it is quaint to expect that a specialty’s scope of practice matches their ACGME and ABMS training requirements. Maybe I’m old-fashioned.
So <redacted>, looking back on our meetings you often took the tone of an adult explaining a difficult, but necessary thing to a child. Maybe the biggest lie we tell children is that adults know what they’re doing. Vascular surgery is an incredibly valuable asset to a health care system. One threatened by physician scarcity and one deserving of promotion and growth. It seems remarkably shortsighted to bury this asset on a service line under the direction of cardiology.
In the end, I have only one request. The next time you are in a meeting with a vascular surgeon who asks for an example of a successful Heart and Vascular Service Line don’t use us. It didn’t work. I don’t think it ever truly works.
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, division of vascular and endovascular surgery, Louisiana State University Health Sciences Center, New Orleans.
References
1. JAMA Surg. 2016;151(11):1032-8.
2. J. Vasc. Surg. 2012;55(1):281-5.
3. Merritt, Hawkins & Associates, 2002 Physician Inpatient/Outpatient Revenue Survey.
Dear <redacted>,
Just thought I would write and give you a quick update on our situation, not that you asked. As you recall, a few years ago we spent many hours discussing and planning the Heart and Vascular Service Line that you encouraged us to set up in our new hospital. These conversations were filled with the greatest hits of health care administrators. “Institutional silos,” “layers of integration,” and “financial dashboards” were crowd favorites. Personally, I enjoyed the endless timelines, flow charts to nowhere, and of course the countless hours spent crafting a vision statement. It’s a wonder we got any work done! At least one of us was getting paid by the hour.
When I asked you to provide concrete examples of fully integrated, functional Heart and Vascular Service Lines you initially deferred. Finally, you listed three examples. Of course, when I reached out to them, two had disbanded, and the third had no idea what I was talking about. Nevertheless, I pressed on. I tried to figure out how this would all work. What keeps the service line synchronous? My research kept turning up lines like: “alignment across departments and specialists is imperative for addressing the care delivery and business challenges facing cardiovascular providers.” OK sure, but huh?
The purpose of a Heart and Vascular Service Line is purportedly to improve the quality of care of cardiovascular patients. The concept of the service line has been in place for over 20 years, so where is the literature demonstrating the quality benefits? The improved outcomes? As far as I can tell it does not exist. So maybe that was never really the primary goal. Based on the vigor and financial capital hospitals pour into the creation of these lines, there must be a different game afoot. Looking deeper into the health care administration literature it seems the true benefit of a service line is that it keeps patients within the system. Once a patient is brought in by one specialty, the other specialties can converge to offer their services. It soon becomes an assembly line of atherosclerotic delights. The patient enjoys the theoretical advantage of having all of his or her specialists together, and the hospital enjoys the profits.
I would have been comfortable fighting the concept of the service line on the basis of access, practicality, or quality. The truth is, it isn’t about any of these things. If the service line were really about patient convenience, we would have put a vascular lab in the clinic as I requested. But that wouldn’t have been convenient for radiology or cardiology. In your model, which specialty does the work doesn’t matter because the hospital profits regardless. So without a financial map to describe how this all plays out, you just threw us into the same cage Thunderdome-style. Two specialists enter, one specialist leaves. The problem is that CMS is already looking at the volume of diagnostic studies as a factor in total cost. You helped us build a system that enables and encourages more testing. Cue Tina Turner, “We don’t need another ECHO…”
The turning point in our relationship should have come when you sent me the list of CPT codes for the procedures expected to be performed by vascular surgery. You got a few right. Lower extremity bypass, amputations, and even aneurysms were there. You seemed surprised, though, that we would be doing other leg interventions and thought the carotid endarterectomies would be done by neurosurgery. Here the problem was laid out. You were describing in detail the mechanisms for our new service line, but you didn’t really know what a vascular surgeon was. It’s a little late, but let me help you.
When I sent back the CPT list, even I forgot a few. Like 35251 (repair of intra-abdominal blood vessel), 27364 (radical resection of thigh sarcoma), or 35141 (repair of femoral pseudoaneurysm). You see, vascular surgeons are the great facilitators. Our expertise enables other specialties to perform at their highest levels. Comprehensive programs in orthopedics, neurosurgery, cardiology, cardiac surgery, surgical oncology, trauma, and urology would be essentially impossible without vascular surgery. A study conducted at Northwestern showed that 7% of their total volume of vascular surgeries were cases providing intraoperative assistance to other specialties.1 And this excluded trauma. While the hospital greatly benefits from this relationship, the vascular surgeons often do not. Emergently helping other physicians requires canceling our responsibilities, both at work and at home. CPT codes often severely undervalue our time spent assisting with large resections or waiting “on standby.”
The overall financial contributions of vascular surgeons to hospital systems are often overlooked. In a study performed at a tertiary care hospital in New Jersey, vascular surgeons were found to have the leading gross margin per FTE of any specialty, 66% more than cardiology. (And these were academic vascular surgeons, a famously lazy breed!)2 In 2002, Merritt, Hawkins & Associates found that the average vascular surgeon provides over $2 million in revenue to his or her hospital, third highest of any specialty.3 With the widespread adoption of endovascular procedures, this number is likely to be higher today.
So now, an update on our great experiment. Run by cardiology our service line treats heart disease, heart attacks, heart failure, and high blood pressure. At least according to the website. If you want to find vascular surgery, it is listed last, under “other services.” And no, sadly the list is not alphabetical. Around the country, there is a great shortage of vascular surgeons. There are two to three job openings for every graduate annually. Remarkably, our service line boasts ten board-certified vascular surgeons. But people always seem to want what they don’t have. In our service line director’s case, that was a TAVR program. So there was great effort and expense in creating one. When it came time to start our FEVAR program, I simply took my friend Andy Schanzer out to dinner and asked him how he did it at UMass. Then we just started doing cases. No fanfare, no press releases. No expensive hires. The dinner cost about $100 (Andy is a cheap date, but I ordered multiple apps).
Today, our service line is disintegrating. There is no animosity. It just didn’t work out. It never really made sense. Yes, our patients have heart disease. They also have lung cancer, diabetes, prostate disease, and spinal stenosis. They would benefit from wound care, smoking cessation, and certainly a comprehensive vascular lab. None of which were offered in our service line. We didn’t belong in the same silo as cardiology, and I certainly never believed they should be in one that treats PVD. I guess it is quaint to expect that a specialty’s scope of practice matches their ACGME and ABMS training requirements. Maybe I’m old-fashioned.
So <redacted>, looking back on our meetings you often took the tone of an adult explaining a difficult, but necessary thing to a child. Maybe the biggest lie we tell children is that adults know what they’re doing. Vascular surgery is an incredibly valuable asset to a health care system. One threatened by physician scarcity and one deserving of promotion and growth. It seems remarkably shortsighted to bury this asset on a service line under the direction of cardiology.
In the end, I have only one request. The next time you are in a meeting with a vascular surgeon who asks for an example of a successful Heart and Vascular Service Line don’t use us. It didn’t work. I don’t think it ever truly works.
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, division of vascular and endovascular surgery, Louisiana State University Health Sciences Center, New Orleans.
References
1. JAMA Surg. 2016;151(11):1032-8.
2. J. Vasc. Surg. 2012;55(1):281-5.
3. Merritt, Hawkins & Associates, 2002 Physician Inpatient/Outpatient Revenue Survey.