User login
Bova Risk Model Predicts 30-Day Pulmonary Embolism-Related Complications
Clinical question: Can the Bova risk model stratify patients with acute PE into stages of increasing risk for 30-day pulmonary embolism (PE)-related complications?
Background: The Bova score is based on four variables assessed at the time of PE diagnosis: heart rate, systolic blood pressure, cardiac troponin, and a marker of right ventricular (RV) dysfunction. In the original study, the Bova risk model was derived from 2,874 normotensive patients with PE. This study performed a retrospective validation of this model on a different cohort of patients.
Study design: Retrospective cohort study.
Setting: Academic urban ED in Madrid, Spain.
Synopsis: Investigators included 1,083 patients with normotensive PE, and the Bova risk score classified 80% into class I, 15% into class II, and 5% into class III—correlating 30-day PE-related complication rates were 4.4%, 18%, and 42%, respectively. When dichotomized into low risk (class I and II) versus intermediate to high risk (class III), the model had a specificity of 97%, a positive predictive value of 42%, and a positive likelihood ratio of 7.9 for predicting 30-day PE-related complications.
The existing risk assessment models, the pulmonary embolism severity index (PESI) and the simplified PESI (sPESI), have been extensively validated but were specifically developed to identity patients with low risk for mortality. The Bova risk model could be used in a stepwise fashion, with the PESI or sPESI model, to further assess intermediate-risk patients.
This model was derived and validated at one single center, so the results may not be generalizable. Additionally, the variables were collected prospectively, but this validation analysis was performed retrospectively.
Bottom line: The Bova risk model accurately stratifies patients with normotensive PE into stages of increasing risk for developing 30-day PE-related complications.
Citation: Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism [published online ahead of print January 29, 2015]. Chest.
Clinical question: Can the Bova risk model stratify patients with acute PE into stages of increasing risk for 30-day pulmonary embolism (PE)-related complications?
Background: The Bova score is based on four variables assessed at the time of PE diagnosis: heart rate, systolic blood pressure, cardiac troponin, and a marker of right ventricular (RV) dysfunction. In the original study, the Bova risk model was derived from 2,874 normotensive patients with PE. This study performed a retrospective validation of this model on a different cohort of patients.
Study design: Retrospective cohort study.
Setting: Academic urban ED in Madrid, Spain.
Synopsis: Investigators included 1,083 patients with normotensive PE, and the Bova risk score classified 80% into class I, 15% into class II, and 5% into class III—correlating 30-day PE-related complication rates were 4.4%, 18%, and 42%, respectively. When dichotomized into low risk (class I and II) versus intermediate to high risk (class III), the model had a specificity of 97%, a positive predictive value of 42%, and a positive likelihood ratio of 7.9 for predicting 30-day PE-related complications.
The existing risk assessment models, the pulmonary embolism severity index (PESI) and the simplified PESI (sPESI), have been extensively validated but were specifically developed to identity patients with low risk for mortality. The Bova risk model could be used in a stepwise fashion, with the PESI or sPESI model, to further assess intermediate-risk patients.
This model was derived and validated at one single center, so the results may not be generalizable. Additionally, the variables were collected prospectively, but this validation analysis was performed retrospectively.
Bottom line: The Bova risk model accurately stratifies patients with normotensive PE into stages of increasing risk for developing 30-day PE-related complications.
Citation: Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism [published online ahead of print January 29, 2015]. Chest.
Clinical question: Can the Bova risk model stratify patients with acute PE into stages of increasing risk for 30-day pulmonary embolism (PE)-related complications?
Background: The Bova score is based on four variables assessed at the time of PE diagnosis: heart rate, systolic blood pressure, cardiac troponin, and a marker of right ventricular (RV) dysfunction. In the original study, the Bova risk model was derived from 2,874 normotensive patients with PE. This study performed a retrospective validation of this model on a different cohort of patients.
Study design: Retrospective cohort study.
Setting: Academic urban ED in Madrid, Spain.
Synopsis: Investigators included 1,083 patients with normotensive PE, and the Bova risk score classified 80% into class I, 15% into class II, and 5% into class III—correlating 30-day PE-related complication rates were 4.4%, 18%, and 42%, respectively. When dichotomized into low risk (class I and II) versus intermediate to high risk (class III), the model had a specificity of 97%, a positive predictive value of 42%, and a positive likelihood ratio of 7.9 for predicting 30-day PE-related complications.
The existing risk assessment models, the pulmonary embolism severity index (PESI) and the simplified PESI (sPESI), have been extensively validated but were specifically developed to identity patients with low risk for mortality. The Bova risk model could be used in a stepwise fashion, with the PESI or sPESI model, to further assess intermediate-risk patients.
This model was derived and validated at one single center, so the results may not be generalizable. Additionally, the variables were collected prospectively, but this validation analysis was performed retrospectively.
Bottom line: The Bova risk model accurately stratifies patients with normotensive PE into stages of increasing risk for developing 30-day PE-related complications.
Citation: Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism [published online ahead of print January 29, 2015]. Chest.
Intracranial Bleeding Risk for Head Injury Patients on Warfarin
Clinical question: Do minor and minimal head injuries in patients on warfarin lead to significant intracranial bleed?
Background: Warfarin use is common, and many of these patients sustain minor and minimal head injuries. When presenting to the ED, these patients pose a clinical dilemma regarding whether to obtain neuroimaging and/or admit.
Study design: Retrospective cohort study.
Setting: Two urban tertiary care EDs in Ottawa, Canada, over a two-year period.
Synopsis: Using the Canadian National Ambulatory Care Reporting System database and the associated coding data, 259 patients were identified that fit the inclusion criteria GCS ≥13 and INR >1.5. This study showed that the rate of intracranial bleeds in this group of patients was high (15.9%); for minor and minimal head injury groups, the rate was 21.9% and 4.8%, respectively. Additionally, loss of consciousness was associated with higher rates of intracranial bleeding.
The risk of intracranial bleed after a head injury while on warfarin is considerably high, particularly for those patients with minor head injury (21.9%), which is about three times the rate previously reported. Hospitalists evaluating these patients should consider obtaining neuroimaging.
Nonetheless, these rates may be overestimating the true prevalence due to the following: 1) Coding data may overlook minor and minimal head injuries in the presence of more serious injuries, and 2) patients with minimal head injuries may not seek medical care.
Bottom line: Patients sustaining minor head injury while on warfarin have a high rate of intracranial bleed.
Reference: Alrajhi KN, Perry JJ, Forster AJ. Intracranial bleeds after minor and minimal head injury in patients on warfarin. J Emer Med. 2015;48(2):137-142.
Clinical question: Do minor and minimal head injuries in patients on warfarin lead to significant intracranial bleed?
Background: Warfarin use is common, and many of these patients sustain minor and minimal head injuries. When presenting to the ED, these patients pose a clinical dilemma regarding whether to obtain neuroimaging and/or admit.
Study design: Retrospective cohort study.
Setting: Two urban tertiary care EDs in Ottawa, Canada, over a two-year period.
Synopsis: Using the Canadian National Ambulatory Care Reporting System database and the associated coding data, 259 patients were identified that fit the inclusion criteria GCS ≥13 and INR >1.5. This study showed that the rate of intracranial bleeds in this group of patients was high (15.9%); for minor and minimal head injury groups, the rate was 21.9% and 4.8%, respectively. Additionally, loss of consciousness was associated with higher rates of intracranial bleeding.
The risk of intracranial bleed after a head injury while on warfarin is considerably high, particularly for those patients with minor head injury (21.9%), which is about three times the rate previously reported. Hospitalists evaluating these patients should consider obtaining neuroimaging.
Nonetheless, these rates may be overestimating the true prevalence due to the following: 1) Coding data may overlook minor and minimal head injuries in the presence of more serious injuries, and 2) patients with minimal head injuries may not seek medical care.
Bottom line: Patients sustaining minor head injury while on warfarin have a high rate of intracranial bleed.
Reference: Alrajhi KN, Perry JJ, Forster AJ. Intracranial bleeds after minor and minimal head injury in patients on warfarin. J Emer Med. 2015;48(2):137-142.
Clinical question: Do minor and minimal head injuries in patients on warfarin lead to significant intracranial bleed?
Background: Warfarin use is common, and many of these patients sustain minor and minimal head injuries. When presenting to the ED, these patients pose a clinical dilemma regarding whether to obtain neuroimaging and/or admit.
Study design: Retrospective cohort study.
Setting: Two urban tertiary care EDs in Ottawa, Canada, over a two-year period.
Synopsis: Using the Canadian National Ambulatory Care Reporting System database and the associated coding data, 259 patients were identified that fit the inclusion criteria GCS ≥13 and INR >1.5. This study showed that the rate of intracranial bleeds in this group of patients was high (15.9%); for minor and minimal head injury groups, the rate was 21.9% and 4.8%, respectively. Additionally, loss of consciousness was associated with higher rates of intracranial bleeding.
The risk of intracranial bleed after a head injury while on warfarin is considerably high, particularly for those patients with minor head injury (21.9%), which is about three times the rate previously reported. Hospitalists evaluating these patients should consider obtaining neuroimaging.
Nonetheless, these rates may be overestimating the true prevalence due to the following: 1) Coding data may overlook minor and minimal head injuries in the presence of more serious injuries, and 2) patients with minimal head injuries may not seek medical care.
Bottom line: Patients sustaining minor head injury while on warfarin have a high rate of intracranial bleed.
Reference: Alrajhi KN, Perry JJ, Forster AJ. Intracranial bleeds after minor and minimal head injury in patients on warfarin. J Emer Med. 2015;48(2):137-142.
Enriched Nutritional Formulas Help Heal Pressure Ulcers
Clinical question: Does a high-calorie, high-protein formula enriched with supplements of arginine, zinc, and antioxidants improve pressure ulcer healing?
Background: Malnutrition is thought to be a major factor in the development and poor healing of pressure ulcers. Trials evaluating whether or not the addition of antioxidants, arginine, and zinc to nutritional formulas improves pressure ulcer healing have been small and inconsistent.
Study design: Multicenter, randomized, controlled, blinded trial.
Setting: Long-term care facilities and patients receiving home care services.
Synopsis: Two hundred patients with stage II, III, or IV pressure ulcers receiving standardized wound care were randomly assigned to a control formula or an experimental formula enriched with arginine, zinc, and antioxidants. At eight weeks, the experimental formula group had an 18.7% (CI, 5.7% to 31.8%, P=0.017) mean reduction in pressure ulcer size compared with the control formula group, although both groups showed efficacy in wound healing.
Nutrition is an important part of wound healing and should be incorporated into the plan of care for the hospitalized patient with pressure ulcers. Hospitalists should be mindful that this study was conducted in non-acute settings, with a chronically ill patient population; more research needs to be done to investigate the effect of these specific immune-modulating nutritional supplements in acutely ill hospitalized patients, given the inconclusive safety profile of certain nutrients such as arginine in severe sepsis.
Bottom line: Enhanced nutritional support with an oral nutritional formula enriched with arginine, zinc, and antioxidants improves pressure ulcer healing in malnourished patients already receiving standard wound care.
Citation: Cereda E, Klersy C, Serioli M, Crespi A, D’Andrea F, OligoElement Sore Trial Study Group. A nutritional formula enriched with arginine, zinc, and antioxidants for the healing of pressure ulcers: a randomized trial. Ann Intern Med. 2015;162(3):167-174.
Clinical question: Does a high-calorie, high-protein formula enriched with supplements of arginine, zinc, and antioxidants improve pressure ulcer healing?
Background: Malnutrition is thought to be a major factor in the development and poor healing of pressure ulcers. Trials evaluating whether or not the addition of antioxidants, arginine, and zinc to nutritional formulas improves pressure ulcer healing have been small and inconsistent.
Study design: Multicenter, randomized, controlled, blinded trial.
Setting: Long-term care facilities and patients receiving home care services.
Synopsis: Two hundred patients with stage II, III, or IV pressure ulcers receiving standardized wound care were randomly assigned to a control formula or an experimental formula enriched with arginine, zinc, and antioxidants. At eight weeks, the experimental formula group had an 18.7% (CI, 5.7% to 31.8%, P=0.017) mean reduction in pressure ulcer size compared with the control formula group, although both groups showed efficacy in wound healing.
Nutrition is an important part of wound healing and should be incorporated into the plan of care for the hospitalized patient with pressure ulcers. Hospitalists should be mindful that this study was conducted in non-acute settings, with a chronically ill patient population; more research needs to be done to investigate the effect of these specific immune-modulating nutritional supplements in acutely ill hospitalized patients, given the inconclusive safety profile of certain nutrients such as arginine in severe sepsis.
Bottom line: Enhanced nutritional support with an oral nutritional formula enriched with arginine, zinc, and antioxidants improves pressure ulcer healing in malnourished patients already receiving standard wound care.
Citation: Cereda E, Klersy C, Serioli M, Crespi A, D’Andrea F, OligoElement Sore Trial Study Group. A nutritional formula enriched with arginine, zinc, and antioxidants for the healing of pressure ulcers: a randomized trial. Ann Intern Med. 2015;162(3):167-174.
Clinical question: Does a high-calorie, high-protein formula enriched with supplements of arginine, zinc, and antioxidants improve pressure ulcer healing?
Background: Malnutrition is thought to be a major factor in the development and poor healing of pressure ulcers. Trials evaluating whether or not the addition of antioxidants, arginine, and zinc to nutritional formulas improves pressure ulcer healing have been small and inconsistent.
Study design: Multicenter, randomized, controlled, blinded trial.
Setting: Long-term care facilities and patients receiving home care services.
Synopsis: Two hundred patients with stage II, III, or IV pressure ulcers receiving standardized wound care were randomly assigned to a control formula or an experimental formula enriched with arginine, zinc, and antioxidants. At eight weeks, the experimental formula group had an 18.7% (CI, 5.7% to 31.8%, P=0.017) mean reduction in pressure ulcer size compared with the control formula group, although both groups showed efficacy in wound healing.
Nutrition is an important part of wound healing and should be incorporated into the plan of care for the hospitalized patient with pressure ulcers. Hospitalists should be mindful that this study was conducted in non-acute settings, with a chronically ill patient population; more research needs to be done to investigate the effect of these specific immune-modulating nutritional supplements in acutely ill hospitalized patients, given the inconclusive safety profile of certain nutrients such as arginine in severe sepsis.
Bottom line: Enhanced nutritional support with an oral nutritional formula enriched with arginine, zinc, and antioxidants improves pressure ulcer healing in malnourished patients already receiving standard wound care.
Citation: Cereda E, Klersy C, Serioli M, Crespi A, D’Andrea F, OligoElement Sore Trial Study Group. A nutritional formula enriched with arginine, zinc, and antioxidants for the healing of pressure ulcers: a randomized trial. Ann Intern Med. 2015;162(3):167-174.
High-Volume Hospitals Have Higher Readmission Rates
Clinical question: Is there an association between hospital volume and hospital readmission rates?
Background: There is an established association between high patient volume and reduced complications or mortality after surgical procedures; however, readmission represents a different type of quality metric than mortality or complications. Studies on the association between hospital patient volume and readmission rates have been controversial.
Study design: Retrospective, cross-sectional study.
Setting: Acute care hospitals.
Synopsis: The study included 6,916,644 admissions to 4,651 hospitals, where patients were assigned to one of five cohorts: medicine, surgery/gynecology, cardiorespiratory, cardiovascular, and neurology. The hospital with the highest volume group had a hospital-wide mean standardized readmission rate of 15.9%, while the hospital with the lowest volume group had a readmission rate of 14.7%. This was a 1.2 percentage point absolute difference between the two hospitals (95% confidence interval 0.9 to 1.5). This trend continued when specialty cohorts were examined, with the exception of the procedure-heavy cardiovascular cohort.
Results showed a trend toward decreased readmission rates in lower-volume hospitals; however, it is unclear why this trend exists. Possible reasons include different patient populations and different practitioner-to-patient ratios in low-volume hospitals.
Limitations of this study are the inclusion of only patients 65 years and older and the fact that all admissions per patient were included, which may bias the results against hospitals with many frequently admitted patients.
Bottom line: Hospitals with high patient volumes are associated with higher readmission rates, except in procedure-heavy patient groups.
Citation: Horwitz LI, Lin Z, Herrin J, et al.Association of hospital volume with readmission rates: a retrospective cross-sectional study. BMJ. 2015;350:h447.
Clinical question: Is there an association between hospital volume and hospital readmission rates?
Background: There is an established association between high patient volume and reduced complications or mortality after surgical procedures; however, readmission represents a different type of quality metric than mortality or complications. Studies on the association between hospital patient volume and readmission rates have been controversial.
Study design: Retrospective, cross-sectional study.
Setting: Acute care hospitals.
Synopsis: The study included 6,916,644 admissions to 4,651 hospitals, where patients were assigned to one of five cohorts: medicine, surgery/gynecology, cardiorespiratory, cardiovascular, and neurology. The hospital with the highest volume group had a hospital-wide mean standardized readmission rate of 15.9%, while the hospital with the lowest volume group had a readmission rate of 14.7%. This was a 1.2 percentage point absolute difference between the two hospitals (95% confidence interval 0.9 to 1.5). This trend continued when specialty cohorts were examined, with the exception of the procedure-heavy cardiovascular cohort.
Results showed a trend toward decreased readmission rates in lower-volume hospitals; however, it is unclear why this trend exists. Possible reasons include different patient populations and different practitioner-to-patient ratios in low-volume hospitals.
Limitations of this study are the inclusion of only patients 65 years and older and the fact that all admissions per patient were included, which may bias the results against hospitals with many frequently admitted patients.
Bottom line: Hospitals with high patient volumes are associated with higher readmission rates, except in procedure-heavy patient groups.
Citation: Horwitz LI, Lin Z, Herrin J, et al.Association of hospital volume with readmission rates: a retrospective cross-sectional study. BMJ. 2015;350:h447.
Clinical question: Is there an association between hospital volume and hospital readmission rates?
Background: There is an established association between high patient volume and reduced complications or mortality after surgical procedures; however, readmission represents a different type of quality metric than mortality or complications. Studies on the association between hospital patient volume and readmission rates have been controversial.
Study design: Retrospective, cross-sectional study.
Setting: Acute care hospitals.
Synopsis: The study included 6,916,644 admissions to 4,651 hospitals, where patients were assigned to one of five cohorts: medicine, surgery/gynecology, cardiorespiratory, cardiovascular, and neurology. The hospital with the highest volume group had a hospital-wide mean standardized readmission rate of 15.9%, while the hospital with the lowest volume group had a readmission rate of 14.7%. This was a 1.2 percentage point absolute difference between the two hospitals (95% confidence interval 0.9 to 1.5). This trend continued when specialty cohorts were examined, with the exception of the procedure-heavy cardiovascular cohort.
Results showed a trend toward decreased readmission rates in lower-volume hospitals; however, it is unclear why this trend exists. Possible reasons include different patient populations and different practitioner-to-patient ratios in low-volume hospitals.
Limitations of this study are the inclusion of only patients 65 years and older and the fact that all admissions per patient were included, which may bias the results against hospitals with many frequently admitted patients.
Bottom line: Hospitals with high patient volumes are associated with higher readmission rates, except in procedure-heavy patient groups.
Citation: Horwitz LI, Lin Z, Herrin J, et al.Association of hospital volume with readmission rates: a retrospective cross-sectional study. BMJ. 2015;350:h447.
Hospital Testing Overuse Done to Reassure Patients, Families
Clinical Question: What is the extent of, and factors associated with, testing overuse in U.S. hospitals for pre-operative evaluation and syncope.
Background: Little is known about the extent and drivers of overuse by hospitalists.
Study design: Two vignettes (pre-operative evaluation and syncope) were mailed to hospitalists. They were asked to identify what most hospitalists at their institution would recommend and “the most likely primary driver of the hospitalist’s decision.”
Setting: Random selection of hospitalists from SHM member database and SHM national meeting attendees.
Synopsis: Investigators mailed 1,753 surveys and received a 68% response rate. For the pre-operative evaluation vignette, 52% of hospitalists reported overuse of pre-operative testing. When a family member was a physician and requested further testing, overuse increased significantly to 65%. For the syncope vignette, any choice involving admission was considered overuse.
Eighty-two percent of respondents reported overuse; when the wife was a lawyer or requested further testing, overuse remained the same. Overuse in both cases was more frequent due to a hospitalist’s desire to reassure patients or themselves, rather than a belief that it was clinically indicated (pre-operative evaluation, 63% vs. 37%; syncope, 69% vs. 31%, P<0.001).
The survey responses do not necessarily represent actual clinical choices, and the hospitalist sample may not be representative of all hospitalists; however, this study shows that efforts to reduce overuse in hospitals need to move beyond financial incentives and/or informing providers of evidence-based recommendations.
Bottom line: A survey of hospitalists showed substantial overuse in two common clinical situations, syncope and pre-operative evaluation, mostly driven by a desire to reassure patients, families, or themselves.
Citation: Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108.
Clinical Question: What is the extent of, and factors associated with, testing overuse in U.S. hospitals for pre-operative evaluation and syncope.
Background: Little is known about the extent and drivers of overuse by hospitalists.
Study design: Two vignettes (pre-operative evaluation and syncope) were mailed to hospitalists. They were asked to identify what most hospitalists at their institution would recommend and “the most likely primary driver of the hospitalist’s decision.”
Setting: Random selection of hospitalists from SHM member database and SHM national meeting attendees.
Synopsis: Investigators mailed 1,753 surveys and received a 68% response rate. For the pre-operative evaluation vignette, 52% of hospitalists reported overuse of pre-operative testing. When a family member was a physician and requested further testing, overuse increased significantly to 65%. For the syncope vignette, any choice involving admission was considered overuse.
Eighty-two percent of respondents reported overuse; when the wife was a lawyer or requested further testing, overuse remained the same. Overuse in both cases was more frequent due to a hospitalist’s desire to reassure patients or themselves, rather than a belief that it was clinically indicated (pre-operative evaluation, 63% vs. 37%; syncope, 69% vs. 31%, P<0.001).
The survey responses do not necessarily represent actual clinical choices, and the hospitalist sample may not be representative of all hospitalists; however, this study shows that efforts to reduce overuse in hospitals need to move beyond financial incentives and/or informing providers of evidence-based recommendations.
Bottom line: A survey of hospitalists showed substantial overuse in two common clinical situations, syncope and pre-operative evaluation, mostly driven by a desire to reassure patients, families, or themselves.
Citation: Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108.
Clinical Question: What is the extent of, and factors associated with, testing overuse in U.S. hospitals for pre-operative evaluation and syncope.
Background: Little is known about the extent and drivers of overuse by hospitalists.
Study design: Two vignettes (pre-operative evaluation and syncope) were mailed to hospitalists. They were asked to identify what most hospitalists at their institution would recommend and “the most likely primary driver of the hospitalist’s decision.”
Setting: Random selection of hospitalists from SHM member database and SHM national meeting attendees.
Synopsis: Investigators mailed 1,753 surveys and received a 68% response rate. For the pre-operative evaluation vignette, 52% of hospitalists reported overuse of pre-operative testing. When a family member was a physician and requested further testing, overuse increased significantly to 65%. For the syncope vignette, any choice involving admission was considered overuse.
Eighty-two percent of respondents reported overuse; when the wife was a lawyer or requested further testing, overuse remained the same. Overuse in both cases was more frequent due to a hospitalist’s desire to reassure patients or themselves, rather than a belief that it was clinically indicated (pre-operative evaluation, 63% vs. 37%; syncope, 69% vs. 31%, P<0.001).
The survey responses do not necessarily represent actual clinical choices, and the hospitalist sample may not be representative of all hospitalists; however, this study shows that efforts to reduce overuse in hospitals need to move beyond financial incentives and/or informing providers of evidence-based recommendations.
Bottom line: A survey of hospitalists showed substantial overuse in two common clinical situations, syncope and pre-operative evaluation, mostly driven by a desire to reassure patients, families, or themselves.
Citation: Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100-108.
Functional Impairment Boosts Readmission for Medicare Seniors
Clinical question: Is functional impairment associated with an increased risk of 30-day readmission?
Background: Many Medicare seniors suffer from some level of impairment in functional status, which, in turn, has been linked to high healthcare utilization. Studies that examine the role of functional impairment with readmission rates are limited.
Study design: Prospective, cohort study.
Setting: Seniors enrolled in the Health and Retirement Study (HRS) with Medicare hospitalizations from Jan. 1, 2000, to Dec. 31, 2010.
Synopsis: The primary outcome was readmissions within 30 days of discharge. Activities of daily living (ADL) scale and instrumental ADL were used as measures of functional impairment.
Overall, 48.3% of patients had preadmission functional impairments with a readmission rate of 15.5%. There was a progressive increase in the adjusted risk of readmission as the degree of functional impairment increased: 13.5% with no functional impairment, 14.3% with difficulty in one or more instrumental ADLs (OR 1.06; 95% CI 0.94-1.20), 14.4% with difficulty in one or more ADLs (OR 1.08; 95% CI 0.96-1.21), 16.5% with dependency in one or two ADLs (OR, 1.26; 95% CI 1.11-1.44), and 18.2% with dependency in three or more ADLs (OR 1.42; 95% CI 1.20-1.69).
This observation was more pronounced in patients admitted for heart failure, MI, and pneumonia (16.9% readmission rate for no impairment vs. 25.7% dependency in three or more ADLs, OR 1.70; 95% CI 1.04-2.78).
Although the study is limited by reliance on survey data and Medicare claim data, functional status may be an important variable in calculating readmission risk and a potential target for intervention.
Bottom line: Functional impairment is associated with an increased risk of 30-day readmission, especially in patients admitted for heart failure, MI, and pneumonia.
Citation: Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565.
Clinical question: Is functional impairment associated with an increased risk of 30-day readmission?
Background: Many Medicare seniors suffer from some level of impairment in functional status, which, in turn, has been linked to high healthcare utilization. Studies that examine the role of functional impairment with readmission rates are limited.
Study design: Prospective, cohort study.
Setting: Seniors enrolled in the Health and Retirement Study (HRS) with Medicare hospitalizations from Jan. 1, 2000, to Dec. 31, 2010.
Synopsis: The primary outcome was readmissions within 30 days of discharge. Activities of daily living (ADL) scale and instrumental ADL were used as measures of functional impairment.
Overall, 48.3% of patients had preadmission functional impairments with a readmission rate of 15.5%. There was a progressive increase in the adjusted risk of readmission as the degree of functional impairment increased: 13.5% with no functional impairment, 14.3% with difficulty in one or more instrumental ADLs (OR 1.06; 95% CI 0.94-1.20), 14.4% with difficulty in one or more ADLs (OR 1.08; 95% CI 0.96-1.21), 16.5% with dependency in one or two ADLs (OR, 1.26; 95% CI 1.11-1.44), and 18.2% with dependency in three or more ADLs (OR 1.42; 95% CI 1.20-1.69).
This observation was more pronounced in patients admitted for heart failure, MI, and pneumonia (16.9% readmission rate for no impairment vs. 25.7% dependency in three or more ADLs, OR 1.70; 95% CI 1.04-2.78).
Although the study is limited by reliance on survey data and Medicare claim data, functional status may be an important variable in calculating readmission risk and a potential target for intervention.
Bottom line: Functional impairment is associated with an increased risk of 30-day readmission, especially in patients admitted for heart failure, MI, and pneumonia.
Citation: Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565.
Clinical question: Is functional impairment associated with an increased risk of 30-day readmission?
Background: Many Medicare seniors suffer from some level of impairment in functional status, which, in turn, has been linked to high healthcare utilization. Studies that examine the role of functional impairment with readmission rates are limited.
Study design: Prospective, cohort study.
Setting: Seniors enrolled in the Health and Retirement Study (HRS) with Medicare hospitalizations from Jan. 1, 2000, to Dec. 31, 2010.
Synopsis: The primary outcome was readmissions within 30 days of discharge. Activities of daily living (ADL) scale and instrumental ADL were used as measures of functional impairment.
Overall, 48.3% of patients had preadmission functional impairments with a readmission rate of 15.5%. There was a progressive increase in the adjusted risk of readmission as the degree of functional impairment increased: 13.5% with no functional impairment, 14.3% with difficulty in one or more instrumental ADLs (OR 1.06; 95% CI 0.94-1.20), 14.4% with difficulty in one or more ADLs (OR 1.08; 95% CI 0.96-1.21), 16.5% with dependency in one or two ADLs (OR, 1.26; 95% CI 1.11-1.44), and 18.2% with dependency in three or more ADLs (OR 1.42; 95% CI 1.20-1.69).
This observation was more pronounced in patients admitted for heart failure, MI, and pneumonia (16.9% readmission rate for no impairment vs. 25.7% dependency in three or more ADLs, OR 1.70; 95% CI 1.04-2.78).
Although the study is limited by reliance on survey data and Medicare claim data, functional status may be an important variable in calculating readmission risk and a potential target for intervention.
Bottom line: Functional impairment is associated with an increased risk of 30-day readmission, especially in patients admitted for heart failure, MI, and pneumonia.
Citation: Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559-565.
Delirium, Falls Reduced by Nonpharmacological Intervention
Clinical question: Are multicomponent, nonpharmacological interventions effective in decreasing delirium and falls?
Background: Delirium is prevalent among elderly hospitalized patients and is associated with increased morbidity, length of stay, healthcare costs, and risk of institutionalization. Multicomponent nonpharmacologic interventions have been used to prevent incident delirium in the elderly, but data regarding their effectiveness and impact on preventing poor outcomes are lacking.
Study design: Systematic literature review and meta-analysis.
Setting: Review of medical databases from Jan. 1, 1999, to Dec. 31, 2013.
Synopsis: Fourteen studies were included involving 4,267 elderly patients from 12 acute medical and surgical sites from around the world. There was a 53% reduction in delirium incidence associated with multicomponent, nonpharmacological interventions (OR, 0.47; 95% CI, 0.38-0.58). The odds of falling were 62% lower among intervention patients compared with controls (2.79 vs. 7.05 falls per 1,000 patient-days). The intervention group also showed a decrease in length of stay, with a mean difference of -0.16 (95% CI, -0.97 to 0.64) days and a 5% lower chance of institutionalization (95% CI, 0.71 to 1.26); however, the differences were not statistically significant.
Although the small number and heterogeneity of the studies included limited the analysis, the use of nonpharmacologic interventions appears to be a low-risk, low-cost strategy to prevent delirium. The challenge for the hospitalist in developing a nonpharmacological protocol is to determine which interventions to include; the study did not look at which interventions were most effective.
Bottom line: The use of multicomponent nonpharmacological interventions in older patients can lower the risk of delirium and falls.
Citation: Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520.
Clinical question: Are multicomponent, nonpharmacological interventions effective in decreasing delirium and falls?
Background: Delirium is prevalent among elderly hospitalized patients and is associated with increased morbidity, length of stay, healthcare costs, and risk of institutionalization. Multicomponent nonpharmacologic interventions have been used to prevent incident delirium in the elderly, but data regarding their effectiveness and impact on preventing poor outcomes are lacking.
Study design: Systematic literature review and meta-analysis.
Setting: Review of medical databases from Jan. 1, 1999, to Dec. 31, 2013.
Synopsis: Fourteen studies were included involving 4,267 elderly patients from 12 acute medical and surgical sites from around the world. There was a 53% reduction in delirium incidence associated with multicomponent, nonpharmacological interventions (OR, 0.47; 95% CI, 0.38-0.58). The odds of falling were 62% lower among intervention patients compared with controls (2.79 vs. 7.05 falls per 1,000 patient-days). The intervention group also showed a decrease in length of stay, with a mean difference of -0.16 (95% CI, -0.97 to 0.64) days and a 5% lower chance of institutionalization (95% CI, 0.71 to 1.26); however, the differences were not statistically significant.
Although the small number and heterogeneity of the studies included limited the analysis, the use of nonpharmacologic interventions appears to be a low-risk, low-cost strategy to prevent delirium. The challenge for the hospitalist in developing a nonpharmacological protocol is to determine which interventions to include; the study did not look at which interventions were most effective.
Bottom line: The use of multicomponent nonpharmacological interventions in older patients can lower the risk of delirium and falls.
Citation: Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520.
Clinical question: Are multicomponent, nonpharmacological interventions effective in decreasing delirium and falls?
Background: Delirium is prevalent among elderly hospitalized patients and is associated with increased morbidity, length of stay, healthcare costs, and risk of institutionalization. Multicomponent nonpharmacologic interventions have been used to prevent incident delirium in the elderly, but data regarding their effectiveness and impact on preventing poor outcomes are lacking.
Study design: Systematic literature review and meta-analysis.
Setting: Review of medical databases from Jan. 1, 1999, to Dec. 31, 2013.
Synopsis: Fourteen studies were included involving 4,267 elderly patients from 12 acute medical and surgical sites from around the world. There was a 53% reduction in delirium incidence associated with multicomponent, nonpharmacological interventions (OR, 0.47; 95% CI, 0.38-0.58). The odds of falling were 62% lower among intervention patients compared with controls (2.79 vs. 7.05 falls per 1,000 patient-days). The intervention group also showed a decrease in length of stay, with a mean difference of -0.16 (95% CI, -0.97 to 0.64) days and a 5% lower chance of institutionalization (95% CI, 0.71 to 1.26); however, the differences were not statistically significant.
Although the small number and heterogeneity of the studies included limited the analysis, the use of nonpharmacologic interventions appears to be a low-risk, low-cost strategy to prevent delirium. The challenge for the hospitalist in developing a nonpharmacological protocol is to determine which interventions to include; the study did not look at which interventions were most effective.
Bottom line: The use of multicomponent nonpharmacological interventions in older patients can lower the risk of delirium and falls.
Citation: Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520.
Bridging Anticoagulation for Patients with Atrial Fibrillation
Clinical question: Is bridging anticoagulation for procedures associated with a higher bleeding risk and increased adverse outcomes compared to no bridging?
Background: Practice guidelines have been published to determine when, how, and on whom to bridge anticoagulation for procedures; however, uncertainty remains as to whether or not bridging changes outcomes.
Study design: Prospective, observational study.
Setting: Outcomes Registry for Better Informed treatment of Atrial Fibrillation (ORBIT-AF) study.
Synopsis: Investigators included 10,132 patients who were 18 years and older, with a baseline EKG documenting atrial fibrillation (Afib) and undergoing procedures. Interruptions of oral anticoagulation for a procedure, as well as the use and type of bridging method, were recorded. Six hundred sixty-five patients (24%) used bridging anticoagulation (73% low molecular weight heparin, 15% unfractionated heparin) prior to a procedure. Bridged patients were more likely to have had a mechanical valve replacement (9.6% vs. 2.4%, P<0.0001) and prior stroke (22% vs. 15%, P=0.0003).
Multivariate adjusted analysis showed that bridged patients, compared with non-bridged patients, had higher rates of bleeding (5.0% vs. 1.3%, adjusted odds ratio (OR) 3.84, P<0.0001) and an increased risk for adverse events, including the composite of myocardial infarction (MI), bleeding, stroke or systemic embolism, hospitalization, or death within 30 days (OR 1.94, 95% CI 1.38-271, P=0.0001). Rates of CHADS2 ≥2 or CHA2DS2-VASc score ≥2 were similar between bridged and nonbridged patients.
These results are observational and, therefore, a causal relationship cannot be established; however, the Effectiveness of Bridging Anticoagulation for Surgery (BRIDGE) study will give us more insight and answers.
Bottom line: Bridging anticoagulation prior to procedures is associated with a higher risk of bleeding and adverse outcomes.
Citation: Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: Findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488-494.
Clinical question: Is bridging anticoagulation for procedures associated with a higher bleeding risk and increased adverse outcomes compared to no bridging?
Background: Practice guidelines have been published to determine when, how, and on whom to bridge anticoagulation for procedures; however, uncertainty remains as to whether or not bridging changes outcomes.
Study design: Prospective, observational study.
Setting: Outcomes Registry for Better Informed treatment of Atrial Fibrillation (ORBIT-AF) study.
Synopsis: Investigators included 10,132 patients who were 18 years and older, with a baseline EKG documenting atrial fibrillation (Afib) and undergoing procedures. Interruptions of oral anticoagulation for a procedure, as well as the use and type of bridging method, were recorded. Six hundred sixty-five patients (24%) used bridging anticoagulation (73% low molecular weight heparin, 15% unfractionated heparin) prior to a procedure. Bridged patients were more likely to have had a mechanical valve replacement (9.6% vs. 2.4%, P<0.0001) and prior stroke (22% vs. 15%, P=0.0003).
Multivariate adjusted analysis showed that bridged patients, compared with non-bridged patients, had higher rates of bleeding (5.0% vs. 1.3%, adjusted odds ratio (OR) 3.84, P<0.0001) and an increased risk for adverse events, including the composite of myocardial infarction (MI), bleeding, stroke or systemic embolism, hospitalization, or death within 30 days (OR 1.94, 95% CI 1.38-271, P=0.0001). Rates of CHADS2 ≥2 or CHA2DS2-VASc score ≥2 were similar between bridged and nonbridged patients.
These results are observational and, therefore, a causal relationship cannot be established; however, the Effectiveness of Bridging Anticoagulation for Surgery (BRIDGE) study will give us more insight and answers.
Bottom line: Bridging anticoagulation prior to procedures is associated with a higher risk of bleeding and adverse outcomes.
Citation: Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: Findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488-494.
Clinical question: Is bridging anticoagulation for procedures associated with a higher bleeding risk and increased adverse outcomes compared to no bridging?
Background: Practice guidelines have been published to determine when, how, and on whom to bridge anticoagulation for procedures; however, uncertainty remains as to whether or not bridging changes outcomes.
Study design: Prospective, observational study.
Setting: Outcomes Registry for Better Informed treatment of Atrial Fibrillation (ORBIT-AF) study.
Synopsis: Investigators included 10,132 patients who were 18 years and older, with a baseline EKG documenting atrial fibrillation (Afib) and undergoing procedures. Interruptions of oral anticoagulation for a procedure, as well as the use and type of bridging method, were recorded. Six hundred sixty-five patients (24%) used bridging anticoagulation (73% low molecular weight heparin, 15% unfractionated heparin) prior to a procedure. Bridged patients were more likely to have had a mechanical valve replacement (9.6% vs. 2.4%, P<0.0001) and prior stroke (22% vs. 15%, P=0.0003).
Multivariate adjusted analysis showed that bridged patients, compared with non-bridged patients, had higher rates of bleeding (5.0% vs. 1.3%, adjusted odds ratio (OR) 3.84, P<0.0001) and an increased risk for adverse events, including the composite of myocardial infarction (MI), bleeding, stroke or systemic embolism, hospitalization, or death within 30 days (OR 1.94, 95% CI 1.38-271, P=0.0001). Rates of CHADS2 ≥2 or CHA2DS2-VASc score ≥2 were similar between bridged and nonbridged patients.
These results are observational and, therefore, a causal relationship cannot be established; however, the Effectiveness of Bridging Anticoagulation for Surgery (BRIDGE) study will give us more insight and answers.
Bottom line: Bridging anticoagulation prior to procedures is associated with a higher risk of bleeding and adverse outcomes.
Citation: Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: Findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488-494.
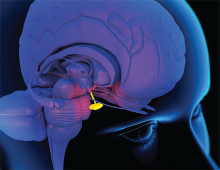
When Should Hypopituitarism Be Suspected?
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case Studies in Improving Patient Care – “Missing the Beat on Patient Experience”
Presenter: Elizabeth Harry, MD
Summation: Dr. Harry structured her talk on Jonathan Sweller’s theory of Cognitive Load. Representing 3 components; Intrinsic Load (acute focused analysis and problem solving), Extrinsic load (external forces affecting our focus) and Germane Load (that which we have already learned and/or automated to minimize acute effort)
This theory applies very well to what we do as physicians. Focus on this model can make the days better for our colleges, our patients and us. It also greatly affects the patient experience. This has application both on an individual level and a system level.
Key Points:
Improve our germane load capability.
- Develop automatic behaviors regarding patient interaction that help with patient ownership and engagement.
- Develop clinical facility with standards of care, necessary studies and labs regarding the most frequent diagnosis we see
- How can we use our IT systems and daily rounding schedules to help with germane load?
Minimize our extrinsic load.
- Organized structure for communication between colleagues and staff
- Make things automatic as part of our workflow during the day
- Set up work areas and expectations that minimize interruptions
Focus effort on our intrinsic load.
- Intrinsic load is the area where we make the most difference in clinical decisions.
- Focusing on the information and wishes that are patients are conveying
- Input from different members of our teams to coordinate care
- Intrinsic load is also where we provide great value for our patient’s experience
- Is the cognitive component that provides professional satisfaction for physicians
Negative interactions, short tempers and fatigue area main symptoms of cognitive overload
“The Watchman’s Rattle” By Rebecca D. Costa was a book Dr. Harry recommended
Presenter: Elizabeth Harry, MD
Summation: Dr. Harry structured her talk on Jonathan Sweller’s theory of Cognitive Load. Representing 3 components; Intrinsic Load (acute focused analysis and problem solving), Extrinsic load (external forces affecting our focus) and Germane Load (that which we have already learned and/or automated to minimize acute effort)
This theory applies very well to what we do as physicians. Focus on this model can make the days better for our colleges, our patients and us. It also greatly affects the patient experience. This has application both on an individual level and a system level.
Key Points:
Improve our germane load capability.
- Develop automatic behaviors regarding patient interaction that help with patient ownership and engagement.
- Develop clinical facility with standards of care, necessary studies and labs regarding the most frequent diagnosis we see
- How can we use our IT systems and daily rounding schedules to help with germane load?
Minimize our extrinsic load.
- Organized structure for communication between colleagues and staff
- Make things automatic as part of our workflow during the day
- Set up work areas and expectations that minimize interruptions
Focus effort on our intrinsic load.
- Intrinsic load is the area where we make the most difference in clinical decisions.
- Focusing on the information and wishes that are patients are conveying
- Input from different members of our teams to coordinate care
- Intrinsic load is also where we provide great value for our patient’s experience
- Is the cognitive component that provides professional satisfaction for physicians
Negative interactions, short tempers and fatigue area main symptoms of cognitive overload
“The Watchman’s Rattle” By Rebecca D. Costa was a book Dr. Harry recommended
Presenter: Elizabeth Harry, MD
Summation: Dr. Harry structured her talk on Jonathan Sweller’s theory of Cognitive Load. Representing 3 components; Intrinsic Load (acute focused analysis and problem solving), Extrinsic load (external forces affecting our focus) and Germane Load (that which we have already learned and/or automated to minimize acute effort)
This theory applies very well to what we do as physicians. Focus on this model can make the days better for our colleges, our patients and us. It also greatly affects the patient experience. This has application both on an individual level and a system level.
Key Points:
Improve our germane load capability.
- Develop automatic behaviors regarding patient interaction that help with patient ownership and engagement.
- Develop clinical facility with standards of care, necessary studies and labs regarding the most frequent diagnosis we see
- How can we use our IT systems and daily rounding schedules to help with germane load?
Minimize our extrinsic load.
- Organized structure for communication between colleagues and staff
- Make things automatic as part of our workflow during the day
- Set up work areas and expectations that minimize interruptions
Focus effort on our intrinsic load.
- Intrinsic load is the area where we make the most difference in clinical decisions.
- Focusing on the information and wishes that are patients are conveying
- Input from different members of our teams to coordinate care
- Intrinsic load is also where we provide great value for our patient’s experience
- Is the cognitive component that provides professional satisfaction for physicians
Negative interactions, short tempers and fatigue area main symptoms of cognitive overload
“The Watchman’s Rattle” By Rebecca D. Costa was a book Dr. Harry recommended