User login
Peri-Operative Phlebotomy Might Cause Significant Blood Loss in Cardiac Surgery Patients
Clinical question: What are the frequency of laboratory testing, the average total blood volume drawn per patient, and the resulting transfusion utilization in patients undergoing cardiac surgery?
Background: Healthcare providers seldom recognize the amount of phlebotomy, and, therefore, its consequences and possible solutions have not been fully evaluated.
Study design: Retrospective, cohort study.
Setting: Major U.S. academic medical center.
Synopsis: The authors examined 1,894 patients undergoing cardiac surgery over a six-month period. They determined the number and type of lab tests drawn on each patient during hospitalization, as well as the estimated total blood volume drawn on each patient.
Patients averaged 115 lab tests during their hospitalization and had cumulative median phlebotomy volume of 454 ml (equivalent to one to two units of red blood cells). They also found that increasing total phlebotomy volume correlated with increased blood product use and that increasing length of stay correlated with higher levels of both.
During an average patient day in the ICU, of the average 116 ml of blood drawn, 80 ml was discarded at the bedside.
Limitations include the broad applicability of this study, which focused on cardiac surgery patients, all of whom stayed in an ICU and had central lines as the source of the majority of their blood draws. Appropriateness of lab testing and transfusions were not examined in this study.
Bottom line: Blood volumes equivalent to one to two units of red blood cells are drawn for lab tests on patients undergoing cardiac surgery, with a large portion of that blood being wasted at the bedside. Initiatives to reduce blood draw volume may help to reduce resource utilization related to such high rates of blood loss from phlebotomy.
Citation: Koch CG, Reinecks EZ, Tang AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg. 2015;99(3):779-784.
Clinical question: What are the frequency of laboratory testing, the average total blood volume drawn per patient, and the resulting transfusion utilization in patients undergoing cardiac surgery?
Background: Healthcare providers seldom recognize the amount of phlebotomy, and, therefore, its consequences and possible solutions have not been fully evaluated.
Study design: Retrospective, cohort study.
Setting: Major U.S. academic medical center.
Synopsis: The authors examined 1,894 patients undergoing cardiac surgery over a six-month period. They determined the number and type of lab tests drawn on each patient during hospitalization, as well as the estimated total blood volume drawn on each patient.
Patients averaged 115 lab tests during their hospitalization and had cumulative median phlebotomy volume of 454 ml (equivalent to one to two units of red blood cells). They also found that increasing total phlebotomy volume correlated with increased blood product use and that increasing length of stay correlated with higher levels of both.
During an average patient day in the ICU, of the average 116 ml of blood drawn, 80 ml was discarded at the bedside.
Limitations include the broad applicability of this study, which focused on cardiac surgery patients, all of whom stayed in an ICU and had central lines as the source of the majority of their blood draws. Appropriateness of lab testing and transfusions were not examined in this study.
Bottom line: Blood volumes equivalent to one to two units of red blood cells are drawn for lab tests on patients undergoing cardiac surgery, with a large portion of that blood being wasted at the bedside. Initiatives to reduce blood draw volume may help to reduce resource utilization related to such high rates of blood loss from phlebotomy.
Citation: Koch CG, Reinecks EZ, Tang AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg. 2015;99(3):779-784.
Clinical question: What are the frequency of laboratory testing, the average total blood volume drawn per patient, and the resulting transfusion utilization in patients undergoing cardiac surgery?
Background: Healthcare providers seldom recognize the amount of phlebotomy, and, therefore, its consequences and possible solutions have not been fully evaluated.
Study design: Retrospective, cohort study.
Setting: Major U.S. academic medical center.
Synopsis: The authors examined 1,894 patients undergoing cardiac surgery over a six-month period. They determined the number and type of lab tests drawn on each patient during hospitalization, as well as the estimated total blood volume drawn on each patient.
Patients averaged 115 lab tests during their hospitalization and had cumulative median phlebotomy volume of 454 ml (equivalent to one to two units of red blood cells). They also found that increasing total phlebotomy volume correlated with increased blood product use and that increasing length of stay correlated with higher levels of both.
During an average patient day in the ICU, of the average 116 ml of blood drawn, 80 ml was discarded at the bedside.
Limitations include the broad applicability of this study, which focused on cardiac surgery patients, all of whom stayed in an ICU and had central lines as the source of the majority of their blood draws. Appropriateness of lab testing and transfusions were not examined in this study.
Bottom line: Blood volumes equivalent to one to two units of red blood cells are drawn for lab tests on patients undergoing cardiac surgery, with a large portion of that blood being wasted at the bedside. Initiatives to reduce blood draw volume may help to reduce resource utilization related to such high rates of blood loss from phlebotomy.
Citation: Koch CG, Reinecks EZ, Tang AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg. 2015;99(3):779-784.
Clostridium Difficile Infection Rates in the U.S. in 2011
Clinical question: What are the incidence, recurrence rate, and mortality rate of Clostridium difficile infection (CDI) in the U.S. in 2011?
Background: CDI has continued to change, and its impact on healthcare has continued to increase.
Study design: Cross-sectional analysis.
Setting: U.S.
Synopsis: The incidence, rate of recurrence, and rate of mortality of C. diff were estimated using 10 sites from the CDC Emerging Infections Program. C. diff incidence was estimated at 453,000 cases, with higher rates among females, whites, and those over age 65. One-third of the cases were community associated. There were an estimated 83,000 first-time recurrent infections and 29,300 estimated deaths within 30 days of diagnosis, with half of those deaths attributable to CDI itself.
This study was limited by the reliance of the case definition solely on positive test results and the trend of labs transitioning to nucleic acid amplification testing (NAAT), both of which can lead to inclusion of colonization (not just actual disease). Also, the recurrence and mortality rates were underestimated, because the study only included first-time recurrences and deaths that were documented in the medical record.
Bottom line: C. diff caused nearly half a million infections and was associated with roughly 29,000 deaths in the U.S. in 2011.
Citation: Less FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825-834.
Clinical question: What are the incidence, recurrence rate, and mortality rate of Clostridium difficile infection (CDI) in the U.S. in 2011?
Background: CDI has continued to change, and its impact on healthcare has continued to increase.
Study design: Cross-sectional analysis.
Setting: U.S.
Synopsis: The incidence, rate of recurrence, and rate of mortality of C. diff were estimated using 10 sites from the CDC Emerging Infections Program. C. diff incidence was estimated at 453,000 cases, with higher rates among females, whites, and those over age 65. One-third of the cases were community associated. There were an estimated 83,000 first-time recurrent infections and 29,300 estimated deaths within 30 days of diagnosis, with half of those deaths attributable to CDI itself.
This study was limited by the reliance of the case definition solely on positive test results and the trend of labs transitioning to nucleic acid amplification testing (NAAT), both of which can lead to inclusion of colonization (not just actual disease). Also, the recurrence and mortality rates were underestimated, because the study only included first-time recurrences and deaths that were documented in the medical record.
Bottom line: C. diff caused nearly half a million infections and was associated with roughly 29,000 deaths in the U.S. in 2011.
Citation: Less FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825-834.
Clinical question: What are the incidence, recurrence rate, and mortality rate of Clostridium difficile infection (CDI) in the U.S. in 2011?
Background: CDI has continued to change, and its impact on healthcare has continued to increase.
Study design: Cross-sectional analysis.
Setting: U.S.
Synopsis: The incidence, rate of recurrence, and rate of mortality of C. diff were estimated using 10 sites from the CDC Emerging Infections Program. C. diff incidence was estimated at 453,000 cases, with higher rates among females, whites, and those over age 65. One-third of the cases were community associated. There were an estimated 83,000 first-time recurrent infections and 29,300 estimated deaths within 30 days of diagnosis, with half of those deaths attributable to CDI itself.
This study was limited by the reliance of the case definition solely on positive test results and the trend of labs transitioning to nucleic acid amplification testing (NAAT), both of which can lead to inclusion of colonization (not just actual disease). Also, the recurrence and mortality rates were underestimated, because the study only included first-time recurrences and deaths that were documented in the medical record.
Bottom line: C. diff caused nearly half a million infections and was associated with roughly 29,000 deaths in the U.S. in 2011.
Citation: Less FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825-834.
CT Angiography Effect on Outcome for Patients with Symptomatic Chest Pain
Clinical question: Does CT angiography (CTA) improve clinical outcomes in patients with new-onset stable chest pain more than functional testing?
Background: Chest pain is a common clinical problem, and multiple noninvasive tests are available to detect coronary artery disease (CAD). CT angiography is more accurate than noninvasive testing and may decrease unnecessary invasive testing and improve outcomes in patients with new-onset stable chest pain.
Study design: Pragmatic, comparative-effectiveness design.
Setting: One hundred ninety-three North American sites.
Synopsis: Ten thousand three symptomatic outpatients, mean age 60 years, with at least one cardiovascular risk factor, were randomized to CTA or functional testing to detect CAD. Primary endpoints including death, myocardial infarction, hospitalization for unstable angina, or major procedural complication occurred in 3.3% of CTA patients and 3.0% of functional testing patients (adjusted hazard ratio, 1.04; 95% confidence interval, 0.83 to 1.29; P=0.75). CTA patients received fewer catheterizations showing nonobstructive CAD (3.4% of versus 4.3%, P=0.02).
More CTA patients underwent catheterization within 90 days after randomization (12.2% vs 8.1%), however. Patients in the CTA group had higher exposures to radiation overall, but, per patient, their mean cumulative radiation dose was lower than that of the functional testing group (10.0 mSv vs. 11.3 mSv).
Interestingly, 6.2% of CTA patients versus 3.2% of functional testing patients underwent revascularization, but the study was not powered to assess invasive catheterization or revascularization rates on outcomes.
This study is interesting because results are generalizable to real-world settings; CTA did not improve outcomes compared to functional testing in patients undergoing testing for CAD.
Bottom line: No improvement was seen in clinical outcomes for symptomatic patients undergoing evaluation for CAD with CTA compared with those receiving functional testing.
Citation: Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-1300.
Clinical question: Does CT angiography (CTA) improve clinical outcomes in patients with new-onset stable chest pain more than functional testing?
Background: Chest pain is a common clinical problem, and multiple noninvasive tests are available to detect coronary artery disease (CAD). CT angiography is more accurate than noninvasive testing and may decrease unnecessary invasive testing and improve outcomes in patients with new-onset stable chest pain.
Study design: Pragmatic, comparative-effectiveness design.
Setting: One hundred ninety-three North American sites.
Synopsis: Ten thousand three symptomatic outpatients, mean age 60 years, with at least one cardiovascular risk factor, were randomized to CTA or functional testing to detect CAD. Primary endpoints including death, myocardial infarction, hospitalization for unstable angina, or major procedural complication occurred in 3.3% of CTA patients and 3.0% of functional testing patients (adjusted hazard ratio, 1.04; 95% confidence interval, 0.83 to 1.29; P=0.75). CTA patients received fewer catheterizations showing nonobstructive CAD (3.4% of versus 4.3%, P=0.02).
More CTA patients underwent catheterization within 90 days after randomization (12.2% vs 8.1%), however. Patients in the CTA group had higher exposures to radiation overall, but, per patient, their mean cumulative radiation dose was lower than that of the functional testing group (10.0 mSv vs. 11.3 mSv).
Interestingly, 6.2% of CTA patients versus 3.2% of functional testing patients underwent revascularization, but the study was not powered to assess invasive catheterization or revascularization rates on outcomes.
This study is interesting because results are generalizable to real-world settings; CTA did not improve outcomes compared to functional testing in patients undergoing testing for CAD.
Bottom line: No improvement was seen in clinical outcomes for symptomatic patients undergoing evaluation for CAD with CTA compared with those receiving functional testing.
Citation: Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-1300.
Clinical question: Does CT angiography (CTA) improve clinical outcomes in patients with new-onset stable chest pain more than functional testing?
Background: Chest pain is a common clinical problem, and multiple noninvasive tests are available to detect coronary artery disease (CAD). CT angiography is more accurate than noninvasive testing and may decrease unnecessary invasive testing and improve outcomes in patients with new-onset stable chest pain.
Study design: Pragmatic, comparative-effectiveness design.
Setting: One hundred ninety-three North American sites.
Synopsis: Ten thousand three symptomatic outpatients, mean age 60 years, with at least one cardiovascular risk factor, were randomized to CTA or functional testing to detect CAD. Primary endpoints including death, myocardial infarction, hospitalization for unstable angina, or major procedural complication occurred in 3.3% of CTA patients and 3.0% of functional testing patients (adjusted hazard ratio, 1.04; 95% confidence interval, 0.83 to 1.29; P=0.75). CTA patients received fewer catheterizations showing nonobstructive CAD (3.4% of versus 4.3%, P=0.02).
More CTA patients underwent catheterization within 90 days after randomization (12.2% vs 8.1%), however. Patients in the CTA group had higher exposures to radiation overall, but, per patient, their mean cumulative radiation dose was lower than that of the functional testing group (10.0 mSv vs. 11.3 mSv).
Interestingly, 6.2% of CTA patients versus 3.2% of functional testing patients underwent revascularization, but the study was not powered to assess invasive catheterization or revascularization rates on outcomes.
This study is interesting because results are generalizable to real-world settings; CTA did not improve outcomes compared to functional testing in patients undergoing testing for CAD.
Bottom line: No improvement was seen in clinical outcomes for symptomatic patients undergoing evaluation for CAD with CTA compared with those receiving functional testing.
Citation: Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-1300.
Early Goal-Directed Therapy for Sepsis Offers No Benefit Over Usual Care
Background: Recent trials (ARISE, ProCESS) showed EGDT provided no mortality benefit over usual care. Questions remain about the effectiveness of intensive monitoring protocols, as well as the evolution of what constitutes usual care. The ProMISe trial seeks to test the hypothesis that EGDT impacts mortality in a cost-effective way.
Study design: Pragmatic, open, multicenter, parallel group RCT.
Setting: English National Health Service hospitals that did not routinely use EGDT that included continuous ScvO2 monitoring.
Synopsis: The authors enrolled 1,260 adult patients with early severe sepsis or septic shock; they randomized patients to either usual care or EGDT for six hours. Data was collected prospectively on the EGDT group and retrospectively on the usual care group.
By intention-to-treat analysis, all-cause mortality at 90 days was not significantly different (unadjusted RR 1.01, 95% CI, 0.85-1.20; adjusted OR 0.95, 95% CI, 0.74-1.24, P=0.73). EGDT patients received more intensive therapy, their quality of life scores were similar, and their average costs were higher, though not statistically significant. The probability that EGDT was cost effective was calculated to be below 20%.
Usual care patients had lower-than-expected mortality (29% vs. 40%), limiting the treatment effect of EGDT and limiting extrapolation to groups with higher mortality. Comparison to older studies is limited by the evolution in usual care for sepsis, with earlier recognition and antibiotic administration and greater use of vasoactive drugs. This study adds significant information about quality of life and cost to the discussion about EGDT.
Bottom line: The ProMISe study completes a powerful trio of papers suggesting that EGDT might be an expensive option that offers no clinical benefit over usual care.
Citation: Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301-1311.
Background: Recent trials (ARISE, ProCESS) showed EGDT provided no mortality benefit over usual care. Questions remain about the effectiveness of intensive monitoring protocols, as well as the evolution of what constitutes usual care. The ProMISe trial seeks to test the hypothesis that EGDT impacts mortality in a cost-effective way.
Study design: Pragmatic, open, multicenter, parallel group RCT.
Setting: English National Health Service hospitals that did not routinely use EGDT that included continuous ScvO2 monitoring.
Synopsis: The authors enrolled 1,260 adult patients with early severe sepsis or septic shock; they randomized patients to either usual care or EGDT for six hours. Data was collected prospectively on the EGDT group and retrospectively on the usual care group.
By intention-to-treat analysis, all-cause mortality at 90 days was not significantly different (unadjusted RR 1.01, 95% CI, 0.85-1.20; adjusted OR 0.95, 95% CI, 0.74-1.24, P=0.73). EGDT patients received more intensive therapy, their quality of life scores were similar, and their average costs were higher, though not statistically significant. The probability that EGDT was cost effective was calculated to be below 20%.
Usual care patients had lower-than-expected mortality (29% vs. 40%), limiting the treatment effect of EGDT and limiting extrapolation to groups with higher mortality. Comparison to older studies is limited by the evolution in usual care for sepsis, with earlier recognition and antibiotic administration and greater use of vasoactive drugs. This study adds significant information about quality of life and cost to the discussion about EGDT.
Bottom line: The ProMISe study completes a powerful trio of papers suggesting that EGDT might be an expensive option that offers no clinical benefit over usual care.
Citation: Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301-1311.
Background: Recent trials (ARISE, ProCESS) showed EGDT provided no mortality benefit over usual care. Questions remain about the effectiveness of intensive monitoring protocols, as well as the evolution of what constitutes usual care. The ProMISe trial seeks to test the hypothesis that EGDT impacts mortality in a cost-effective way.
Study design: Pragmatic, open, multicenter, parallel group RCT.
Setting: English National Health Service hospitals that did not routinely use EGDT that included continuous ScvO2 monitoring.
Synopsis: The authors enrolled 1,260 adult patients with early severe sepsis or septic shock; they randomized patients to either usual care or EGDT for six hours. Data was collected prospectively on the EGDT group and retrospectively on the usual care group.
By intention-to-treat analysis, all-cause mortality at 90 days was not significantly different (unadjusted RR 1.01, 95% CI, 0.85-1.20; adjusted OR 0.95, 95% CI, 0.74-1.24, P=0.73). EGDT patients received more intensive therapy, their quality of life scores were similar, and their average costs were higher, though not statistically significant. The probability that EGDT was cost effective was calculated to be below 20%.
Usual care patients had lower-than-expected mortality (29% vs. 40%), limiting the treatment effect of EGDT and limiting extrapolation to groups with higher mortality. Comparison to older studies is limited by the evolution in usual care for sepsis, with earlier recognition and antibiotic administration and greater use of vasoactive drugs. This study adds significant information about quality of life and cost to the discussion about EGDT.
Bottom line: The ProMISe study completes a powerful trio of papers suggesting that EGDT might be an expensive option that offers no clinical benefit over usual care.
Citation: Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301-1311.
NSAID Use by Patients on Antithrombotic Therapy Has Bleeding, Cardiovascular Risks
Clinical question: Is there increased risk of bleeding or cardiovascular events when using NSAIDs while on antithrombotic therapy for secondary cardiovascular prevention?
Background: NSAIDs are among the most commonly used medications, despite the fact that individual NSAIDs have been associated with increased cardiovascular risk, and despite guidelines recommending against the use of NSAIDs in patients with cardiovascular disease. The risk of using NSAIDs with antithrombotic medications after first MI has not yet been examined.
Study design: Retrospective registry study.
Setting: Patients registered in official medical, pharmacy, and civil databases in Denmark, with unique individual identifier numbers allowing for database cross-reference.
Synopsis: The authors enrolled 61,971 patients of a possible 88,662 who were 30 years or older and admitted for a first-time MI starting 30 days following discharge, and tracked them for endpoint events and prescriptions. NSAID prescriptions were identified for 20,931 patients. Patients were placed in cohorts by their specific antithrombotic regimen (monotherapy, or combination therapy with aspirin, clopidogrel, or vitamin K antagonist) and specific NSAID use, accounting for changes in prescription combinations for a given individual.
Antithrombotic use between the NSAID and non-NSAID groups was equal. NSAID use, regardless of duration, was associated with increased risk of admission or death from bleeding (HR 2.02, 95% CI 1.81-2.26). NSAID use was also associated with increased cardiovascular endpoints (HR 1.40, 95% CI 1.30-1.49), including with the most common antithrombotic regimens.
This study is limited by its observational design, lack of more detailed database information, and use of prescription data. Differences in mortality were not separately presented. This study implies that even short exposures to NSAIDs while on antithrombotic therapy may be problematic.
Bottom line: NSAID use is associated with significant bleeding and cardiovascular events in patients who are on antithrombotic medications following their first MI.
Citation: Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Clinical question: Is there increased risk of bleeding or cardiovascular events when using NSAIDs while on antithrombotic therapy for secondary cardiovascular prevention?
Background: NSAIDs are among the most commonly used medications, despite the fact that individual NSAIDs have been associated with increased cardiovascular risk, and despite guidelines recommending against the use of NSAIDs in patients with cardiovascular disease. The risk of using NSAIDs with antithrombotic medications after first MI has not yet been examined.
Study design: Retrospective registry study.
Setting: Patients registered in official medical, pharmacy, and civil databases in Denmark, with unique individual identifier numbers allowing for database cross-reference.
Synopsis: The authors enrolled 61,971 patients of a possible 88,662 who were 30 years or older and admitted for a first-time MI starting 30 days following discharge, and tracked them for endpoint events and prescriptions. NSAID prescriptions were identified for 20,931 patients. Patients were placed in cohorts by their specific antithrombotic regimen (monotherapy, or combination therapy with aspirin, clopidogrel, or vitamin K antagonist) and specific NSAID use, accounting for changes in prescription combinations for a given individual.
Antithrombotic use between the NSAID and non-NSAID groups was equal. NSAID use, regardless of duration, was associated with increased risk of admission or death from bleeding (HR 2.02, 95% CI 1.81-2.26). NSAID use was also associated with increased cardiovascular endpoints (HR 1.40, 95% CI 1.30-1.49), including with the most common antithrombotic regimens.
This study is limited by its observational design, lack of more detailed database information, and use of prescription data. Differences in mortality were not separately presented. This study implies that even short exposures to NSAIDs while on antithrombotic therapy may be problematic.
Bottom line: NSAID use is associated with significant bleeding and cardiovascular events in patients who are on antithrombotic medications following their first MI.
Citation: Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Clinical question: Is there increased risk of bleeding or cardiovascular events when using NSAIDs while on antithrombotic therapy for secondary cardiovascular prevention?
Background: NSAIDs are among the most commonly used medications, despite the fact that individual NSAIDs have been associated with increased cardiovascular risk, and despite guidelines recommending against the use of NSAIDs in patients with cardiovascular disease. The risk of using NSAIDs with antithrombotic medications after first MI has not yet been examined.
Study design: Retrospective registry study.
Setting: Patients registered in official medical, pharmacy, and civil databases in Denmark, with unique individual identifier numbers allowing for database cross-reference.
Synopsis: The authors enrolled 61,971 patients of a possible 88,662 who were 30 years or older and admitted for a first-time MI starting 30 days following discharge, and tracked them for endpoint events and prescriptions. NSAID prescriptions were identified for 20,931 patients. Patients were placed in cohorts by their specific antithrombotic regimen (monotherapy, or combination therapy with aspirin, clopidogrel, or vitamin K antagonist) and specific NSAID use, accounting for changes in prescription combinations for a given individual.
Antithrombotic use between the NSAID and non-NSAID groups was equal. NSAID use, regardless of duration, was associated with increased risk of admission or death from bleeding (HR 2.02, 95% CI 1.81-2.26). NSAID use was also associated with increased cardiovascular endpoints (HR 1.40, 95% CI 1.30-1.49), including with the most common antithrombotic regimens.
This study is limited by its observational design, lack of more detailed database information, and use of prescription data. Differences in mortality were not separately presented. This study implies that even short exposures to NSAIDs while on antithrombotic therapy may be problematic.
Bottom line: NSAID use is associated with significant bleeding and cardiovascular events in patients who are on antithrombotic medications following their first MI.
Citation: Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814.
Treatment of Patients with Atrial Fibrillation, Low CHA2DS2-VASc Scores
Clinical question: Is anticoagulation beneficial for patients with atrial fibrillation (Afib) and low CHA2DS2-VASc score (0 for men, 1 for women) or for those with one additional stroke risk factor?
Background: Guidelines nearly universally recommend anticoagulation for patients with a CHA2DS2-VASc of >2, but differ on recommendation for patients with a CHA2DS2-VASc of 1.
Study design: Cohort study.
Setting: Multiple national registries in Denmark.
Synopsis: Based on analysis, patients with very low stroke risk using the CHA2DS2-VASc score (0 for men, 1 for women) had particularly low stroke risk and did not appear to benefit from additional therapy with aspirin or warfarin, both at one year and at full follow-up (mean 5.9 years).
The addition of one stroke risk factor increased stroke risk without treatment significantly (three-fold increase). Hazard ratios favored treatment with warfarin in these patients, most notably with a reduction in all-cause mortality (though this was more significant at one year than at full follow-up).
Bottom line: Although guidelines differ on treatment strategy for patients with Afib and one stroke risk factor (i.e., CHA2DS2-VASc score of 1 for men, 2 for women), this study supports treatment with warfarin.
Citation: Lip GY, Skjöth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394.
Clinical question: Is anticoagulation beneficial for patients with atrial fibrillation (Afib) and low CHA2DS2-VASc score (0 for men, 1 for women) or for those with one additional stroke risk factor?
Background: Guidelines nearly universally recommend anticoagulation for patients with a CHA2DS2-VASc of >2, but differ on recommendation for patients with a CHA2DS2-VASc of 1.
Study design: Cohort study.
Setting: Multiple national registries in Denmark.
Synopsis: Based on analysis, patients with very low stroke risk using the CHA2DS2-VASc score (0 for men, 1 for women) had particularly low stroke risk and did not appear to benefit from additional therapy with aspirin or warfarin, both at one year and at full follow-up (mean 5.9 years).
The addition of one stroke risk factor increased stroke risk without treatment significantly (three-fold increase). Hazard ratios favored treatment with warfarin in these patients, most notably with a reduction in all-cause mortality (though this was more significant at one year than at full follow-up).
Bottom line: Although guidelines differ on treatment strategy for patients with Afib and one stroke risk factor (i.e., CHA2DS2-VASc score of 1 for men, 2 for women), this study supports treatment with warfarin.
Citation: Lip GY, Skjöth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394.
Clinical question: Is anticoagulation beneficial for patients with atrial fibrillation (Afib) and low CHA2DS2-VASc score (0 for men, 1 for women) or for those with one additional stroke risk factor?
Background: Guidelines nearly universally recommend anticoagulation for patients with a CHA2DS2-VASc of >2, but differ on recommendation for patients with a CHA2DS2-VASc of 1.
Study design: Cohort study.
Setting: Multiple national registries in Denmark.
Synopsis: Based on analysis, patients with very low stroke risk using the CHA2DS2-VASc score (0 for men, 1 for women) had particularly low stroke risk and did not appear to benefit from additional therapy with aspirin or warfarin, both at one year and at full follow-up (mean 5.9 years).
The addition of one stroke risk factor increased stroke risk without treatment significantly (three-fold increase). Hazard ratios favored treatment with warfarin in these patients, most notably with a reduction in all-cause mortality (though this was more significant at one year than at full follow-up).
Bottom line: Although guidelines differ on treatment strategy for patients with Afib and one stroke risk factor (i.e., CHA2DS2-VASc score of 1 for men, 2 for women), this study supports treatment with warfarin.
Citation: Lip GY, Skjöth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65(14):1385-1394.
Research Review: Ticagrelor for Post-Myocardial Infarction
Clinical question: Is ticagrelor for secondary prevention indicated for more than one year after myocardial infarction (MI)?
Background: The efficacy and safety of ticagrelor combined with low-dose aspirin more than one year after MI for secondary prevention has not previously been established.
Study design: Randomized, double-blinded, placebo-controlled, clinical trial.
Setting: Multi-center across 31 countries.
Synopsis: Investigators randomized 21,162 patients one to three years after first MI to a 90 mg, twice daily dose; a 60 mg, twice daily dose; or placebo. Patients also received low-dose aspirin (75 mg to 100 mg). Interestingly, a number of patients had been off dual antiplatelet therapy prior to the start of the trial, because most patients were enrolled closer to two years after primary MI. The manufacturer of ticagrelor sponsored the trial.
The study authors’ analysis showed that treating 10,000 patients with the 90 mg dose would prevent 40 cardiac events (cardiovascular death, MI, or stroke), while the 60 mg dose would prevent 42 events; however, the 90 mg dose would cause 41 major bleeding events and the 60 mg dose 31 major bleeding events. Fatal bleeding was less than 1% in all groups, though patients with increased bleeding risk were excluded.
In addition, patients on either dose of ticagrelor had a significantly higher rate of dyspnea, which resulted in increases in drug discontinuation.
Bottom line: Use of ticagrelor with aspirin for secondary prevention greater than one year after myocardial infarction reduced rates of cardiovascular death, MI, and stroke but increased the risk of major bleeding.
Citation: Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
Clinical question: Is ticagrelor for secondary prevention indicated for more than one year after myocardial infarction (MI)?
Background: The efficacy and safety of ticagrelor combined with low-dose aspirin more than one year after MI for secondary prevention has not previously been established.
Study design: Randomized, double-blinded, placebo-controlled, clinical trial.
Setting: Multi-center across 31 countries.
Synopsis: Investigators randomized 21,162 patients one to three years after first MI to a 90 mg, twice daily dose; a 60 mg, twice daily dose; or placebo. Patients also received low-dose aspirin (75 mg to 100 mg). Interestingly, a number of patients had been off dual antiplatelet therapy prior to the start of the trial, because most patients were enrolled closer to two years after primary MI. The manufacturer of ticagrelor sponsored the trial.
The study authors’ analysis showed that treating 10,000 patients with the 90 mg dose would prevent 40 cardiac events (cardiovascular death, MI, or stroke), while the 60 mg dose would prevent 42 events; however, the 90 mg dose would cause 41 major bleeding events and the 60 mg dose 31 major bleeding events. Fatal bleeding was less than 1% in all groups, though patients with increased bleeding risk were excluded.
In addition, patients on either dose of ticagrelor had a significantly higher rate of dyspnea, which resulted in increases in drug discontinuation.
Bottom line: Use of ticagrelor with aspirin for secondary prevention greater than one year after myocardial infarction reduced rates of cardiovascular death, MI, and stroke but increased the risk of major bleeding.
Citation: Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
Clinical question: Is ticagrelor for secondary prevention indicated for more than one year after myocardial infarction (MI)?
Background: The efficacy and safety of ticagrelor combined with low-dose aspirin more than one year after MI for secondary prevention has not previously been established.
Study design: Randomized, double-blinded, placebo-controlled, clinical trial.
Setting: Multi-center across 31 countries.
Synopsis: Investigators randomized 21,162 patients one to three years after first MI to a 90 mg, twice daily dose; a 60 mg, twice daily dose; or placebo. Patients also received low-dose aspirin (75 mg to 100 mg). Interestingly, a number of patients had been off dual antiplatelet therapy prior to the start of the trial, because most patients were enrolled closer to two years after primary MI. The manufacturer of ticagrelor sponsored the trial.
The study authors’ analysis showed that treating 10,000 patients with the 90 mg dose would prevent 40 cardiac events (cardiovascular death, MI, or stroke), while the 60 mg dose would prevent 42 events; however, the 90 mg dose would cause 41 major bleeding events and the 60 mg dose 31 major bleeding events. Fatal bleeding was less than 1% in all groups, though patients with increased bleeding risk were excluded.
In addition, patients on either dose of ticagrelor had a significantly higher rate of dyspnea, which resulted in increases in drug discontinuation.
Bottom line: Use of ticagrelor with aspirin for secondary prevention greater than one year after myocardial infarction reduced rates of cardiovascular death, MI, and stroke but increased the risk of major bleeding.
Citation: Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
How Should a Patient with Pulmonary Hypertension Be Evaluated, Managed?
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
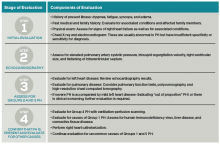
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
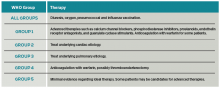
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
D-Dimer Not Reliable Marker to Stop Anticoagulation Therapy
Clinical question: In patients with a first unprovoked VTE, is it safe to use a normalized D-dimer test to stop anticoagulation therapy?
Background: The risk of VTE recurrence after stopping anticoagulation is higher in patients who have elevated D-dimer levels after treatment. It is unknown whether we can use normalized D-dimer levels to guide the decision about whether or not to stop anticoagulation.
Study design: Prospective cohort study.
Setting: Thirteen university-affiliated centers.
Synopsis: Study authors screened 410 adult patients who had a first unprovoked VTE and completed three to seven months of anticoagulation therapy with D-dimer tests. In patients with negative D-dimer tests, anticoagulation was stopped, and D-dimer tests were repeated after a month. In those with two consecutive negative D-dimer tests, anticoagulation was stopped indefinitely; these patients were followed for an average of 2.2 years. Among those 319 patients, there was an overall recurrent VTE rate of 6.7% per patient year. Subgroup analysis was performed among men, women not on estrogen therapy, and women on estrogen therapy; recurrence rates per patient year were 9.7%, 5.4%, and 0%, respectively.
This study used a point-of-care D-dimer test that was either positive or negative; it is unclear if the results can be generalized to all D-dimer tests. Additionally, although the study found a lower recurrence VTE rate among women, the study was not powered for the subgroups.
Bottom line: The high rate of recurrent VTE among men makes the D-dimer test an unsafe marker to use in deciding whether or not to stop anticoagulation for an unprovoked VTE. Among women, D-dimer test can potentially be used to guide length of treatment, but, given the limitations of the study, more evidence is needed.
Citation: Kearon C, Spencer FA, O’Keeffe D, et al. D-Dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy. Ann Intern Med. 2015;162(1):27-34.
Clinical question: In patients with a first unprovoked VTE, is it safe to use a normalized D-dimer test to stop anticoagulation therapy?
Background: The risk of VTE recurrence after stopping anticoagulation is higher in patients who have elevated D-dimer levels after treatment. It is unknown whether we can use normalized D-dimer levels to guide the decision about whether or not to stop anticoagulation.
Study design: Prospective cohort study.
Setting: Thirteen university-affiliated centers.
Synopsis: Study authors screened 410 adult patients who had a first unprovoked VTE and completed three to seven months of anticoagulation therapy with D-dimer tests. In patients with negative D-dimer tests, anticoagulation was stopped, and D-dimer tests were repeated after a month. In those with two consecutive negative D-dimer tests, anticoagulation was stopped indefinitely; these patients were followed for an average of 2.2 years. Among those 319 patients, there was an overall recurrent VTE rate of 6.7% per patient year. Subgroup analysis was performed among men, women not on estrogen therapy, and women on estrogen therapy; recurrence rates per patient year were 9.7%, 5.4%, and 0%, respectively.
This study used a point-of-care D-dimer test that was either positive or negative; it is unclear if the results can be generalized to all D-dimer tests. Additionally, although the study found a lower recurrence VTE rate among women, the study was not powered for the subgroups.
Bottom line: The high rate of recurrent VTE among men makes the D-dimer test an unsafe marker to use in deciding whether or not to stop anticoagulation for an unprovoked VTE. Among women, D-dimer test can potentially be used to guide length of treatment, but, given the limitations of the study, more evidence is needed.
Citation: Kearon C, Spencer FA, O’Keeffe D, et al. D-Dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy. Ann Intern Med. 2015;162(1):27-34.
Clinical question: In patients with a first unprovoked VTE, is it safe to use a normalized D-dimer test to stop anticoagulation therapy?
Background: The risk of VTE recurrence after stopping anticoagulation is higher in patients who have elevated D-dimer levels after treatment. It is unknown whether we can use normalized D-dimer levels to guide the decision about whether or not to stop anticoagulation.
Study design: Prospective cohort study.
Setting: Thirteen university-affiliated centers.
Synopsis: Study authors screened 410 adult patients who had a first unprovoked VTE and completed three to seven months of anticoagulation therapy with D-dimer tests. In patients with negative D-dimer tests, anticoagulation was stopped, and D-dimer tests were repeated after a month. In those with two consecutive negative D-dimer tests, anticoagulation was stopped indefinitely; these patients were followed for an average of 2.2 years. Among those 319 patients, there was an overall recurrent VTE rate of 6.7% per patient year. Subgroup analysis was performed among men, women not on estrogen therapy, and women on estrogen therapy; recurrence rates per patient year were 9.7%, 5.4%, and 0%, respectively.
This study used a point-of-care D-dimer test that was either positive or negative; it is unclear if the results can be generalized to all D-dimer tests. Additionally, although the study found a lower recurrence VTE rate among women, the study was not powered for the subgroups.
Bottom line: The high rate of recurrent VTE among men makes the D-dimer test an unsafe marker to use in deciding whether or not to stop anticoagulation for an unprovoked VTE. Among women, D-dimer test can potentially be used to guide length of treatment, but, given the limitations of the study, more evidence is needed.
Citation: Kearon C, Spencer FA, O’Keeffe D, et al. D-Dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy. Ann Intern Med. 2015;162(1):27-34.
Noninvasive Ventilation Improves Outcomes for COPD Inpatients
Clinical question: Do patients hospitalized with acute COPD exacerbations have improved outcomes with noninvasive ventilation (NIV) compared to those treated with invasive mechanical ventilation (IMV)?
Background: Previous studies have shown that in select patients, NIV has a mortality benefit over IMV for acute COPD exacerbations requiring hospitalization. NIV may also decrease complication rates and reduce length of stay; however, the previous prospective studies have been small.
Study design: Retrospective cohort study.
Setting: 420 structurally and geographically diverse U.S. hospitals.
Synopsis: Using the Premier Healthcare Informatics database, this study looked at 25,628 patients over 40 years old who were hospitalized with COPD exacerbations. Compared with patients who were initially treated with IMV, patients treated with NIV demonstrated lower mortality rates with an odds ratio of 0.54, lower risk of hospital-acquired pneumonia with an odds ratio of 0.53, and a 32% cost reduction. They also had shorter lengths of stay.
This was a retrospective study using a limited data set, and the authors did not have access to potentially confounding factors between the two groups, including vital signs and blood gases. Additionally, the advantages of NIV were attenuated among patients with pneumonia present on admission, patients with high burden of comorbid diseases, and patients older than 85 years.
Bottom line: Treatment of acute COPD exacerbations with NIV is associated with lower mortality, lower costs, and shorter length of stay as compared with IMV.
Citation: Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174(12):1982-1993.
Clinical question: Do patients hospitalized with acute COPD exacerbations have improved outcomes with noninvasive ventilation (NIV) compared to those treated with invasive mechanical ventilation (IMV)?
Background: Previous studies have shown that in select patients, NIV has a mortality benefit over IMV for acute COPD exacerbations requiring hospitalization. NIV may also decrease complication rates and reduce length of stay; however, the previous prospective studies have been small.
Study design: Retrospective cohort study.
Setting: 420 structurally and geographically diverse U.S. hospitals.
Synopsis: Using the Premier Healthcare Informatics database, this study looked at 25,628 patients over 40 years old who were hospitalized with COPD exacerbations. Compared with patients who were initially treated with IMV, patients treated with NIV demonstrated lower mortality rates with an odds ratio of 0.54, lower risk of hospital-acquired pneumonia with an odds ratio of 0.53, and a 32% cost reduction. They also had shorter lengths of stay.
This was a retrospective study using a limited data set, and the authors did not have access to potentially confounding factors between the two groups, including vital signs and blood gases. Additionally, the advantages of NIV were attenuated among patients with pneumonia present on admission, patients with high burden of comorbid diseases, and patients older than 85 years.
Bottom line: Treatment of acute COPD exacerbations with NIV is associated with lower mortality, lower costs, and shorter length of stay as compared with IMV.
Citation: Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174(12):1982-1993.
Clinical question: Do patients hospitalized with acute COPD exacerbations have improved outcomes with noninvasive ventilation (NIV) compared to those treated with invasive mechanical ventilation (IMV)?
Background: Previous studies have shown that in select patients, NIV has a mortality benefit over IMV for acute COPD exacerbations requiring hospitalization. NIV may also decrease complication rates and reduce length of stay; however, the previous prospective studies have been small.
Study design: Retrospective cohort study.
Setting: 420 structurally and geographically diverse U.S. hospitals.
Synopsis: Using the Premier Healthcare Informatics database, this study looked at 25,628 patients over 40 years old who were hospitalized with COPD exacerbations. Compared with patients who were initially treated with IMV, patients treated with NIV demonstrated lower mortality rates with an odds ratio of 0.54, lower risk of hospital-acquired pneumonia with an odds ratio of 0.53, and a 32% cost reduction. They also had shorter lengths of stay.
This was a retrospective study using a limited data set, and the authors did not have access to potentially confounding factors between the two groups, including vital signs and blood gases. Additionally, the advantages of NIV were attenuated among patients with pneumonia present on admission, patients with high burden of comorbid diseases, and patients older than 85 years.
Bottom line: Treatment of acute COPD exacerbations with NIV is associated with lower mortality, lower costs, and shorter length of stay as compared with IMV.
Citation: Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174(12):1982-1993.