User login
Hospital-acquired VTE with high risk of recurrence
Clinical question: Is the risk of recurrence for a venous thromboembolism (VTE) acquired as an inpatient higher than other reversible risk factors?
Background: In patients with acute VTE, transient provoking factors place patients at lower risks for recurrent VTE, while persistent factors (that is, cancer) increase risk for recurrence. Unprovoked VTE places patients at intermediate to high risk, but few data are present for VTE experienced while in the hospital.
Study design: Single-center, population-based, prospective cohort study.
Setting: Tromso, Norway.
Synopsis: Using repeat health surveys from 1994 to 2012, researchers followed 822 patients with a validated, first-lifetime VTE. Hospital-related VTE was defined as a VTE within 8 weeks of hospitalization related to medical illness, surgery, or in patients with active cancer.
This global definition of hospital-related VTE was not associated with an increased risk of recurrent VTE (hazard ratio, 0.99; 0.69-1.41). However, in separate groups, the cumulative risk of recurrence after 5 years in hospital-related VTE due to medical illness was similar to nonhospital-related VTE (20.1% vs. 18.4%), higher than VTE related to surgery (11%), and lower than VTE related to cancer (27.4%).
Risk-adjusted analyses maintained these differences in recurrence risk dependent on reason for hospitalization (cancer, medical illness, surgery). When compared with non-hospital VTE, however, hospital-related VTE was associated with a threefold higher risk of death.
Study limitations included being from a single center, possibly underpowered due to low number of events.
Bottom line: Hospital-related VTE has a high risk of recurrence, but risk level is variable and dependent on the reason for hospitalization.
Citation: Bjøri E, Arshad N, Johnsen HS, Hansen J-B, Brækkan SK. Hospital-related first venous thromboembolism and risk of recurrence [published online ahead of print, Sept. 2, 2016]. J Thromb Haemost. doi: 10.1111/jth.13492
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Is the risk of recurrence for a venous thromboembolism (VTE) acquired as an inpatient higher than other reversible risk factors?
Background: In patients with acute VTE, transient provoking factors place patients at lower risks for recurrent VTE, while persistent factors (that is, cancer) increase risk for recurrence. Unprovoked VTE places patients at intermediate to high risk, but few data are present for VTE experienced while in the hospital.
Study design: Single-center, population-based, prospective cohort study.
Setting: Tromso, Norway.
Synopsis: Using repeat health surveys from 1994 to 2012, researchers followed 822 patients with a validated, first-lifetime VTE. Hospital-related VTE was defined as a VTE within 8 weeks of hospitalization related to medical illness, surgery, or in patients with active cancer.
This global definition of hospital-related VTE was not associated with an increased risk of recurrent VTE (hazard ratio, 0.99; 0.69-1.41). However, in separate groups, the cumulative risk of recurrence after 5 years in hospital-related VTE due to medical illness was similar to nonhospital-related VTE (20.1% vs. 18.4%), higher than VTE related to surgery (11%), and lower than VTE related to cancer (27.4%).
Risk-adjusted analyses maintained these differences in recurrence risk dependent on reason for hospitalization (cancer, medical illness, surgery). When compared with non-hospital VTE, however, hospital-related VTE was associated with a threefold higher risk of death.
Study limitations included being from a single center, possibly underpowered due to low number of events.
Bottom line: Hospital-related VTE has a high risk of recurrence, but risk level is variable and dependent on the reason for hospitalization.
Citation: Bjøri E, Arshad N, Johnsen HS, Hansen J-B, Brækkan SK. Hospital-related first venous thromboembolism and risk of recurrence [published online ahead of print, Sept. 2, 2016]. J Thromb Haemost. doi: 10.1111/jth.13492
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Is the risk of recurrence for a venous thromboembolism (VTE) acquired as an inpatient higher than other reversible risk factors?
Background: In patients with acute VTE, transient provoking factors place patients at lower risks for recurrent VTE, while persistent factors (that is, cancer) increase risk for recurrence. Unprovoked VTE places patients at intermediate to high risk, but few data are present for VTE experienced while in the hospital.
Study design: Single-center, population-based, prospective cohort study.
Setting: Tromso, Norway.
Synopsis: Using repeat health surveys from 1994 to 2012, researchers followed 822 patients with a validated, first-lifetime VTE. Hospital-related VTE was defined as a VTE within 8 weeks of hospitalization related to medical illness, surgery, or in patients with active cancer.
This global definition of hospital-related VTE was not associated with an increased risk of recurrent VTE (hazard ratio, 0.99; 0.69-1.41). However, in separate groups, the cumulative risk of recurrence after 5 years in hospital-related VTE due to medical illness was similar to nonhospital-related VTE (20.1% vs. 18.4%), higher than VTE related to surgery (11%), and lower than VTE related to cancer (27.4%).
Risk-adjusted analyses maintained these differences in recurrence risk dependent on reason for hospitalization (cancer, medical illness, surgery). When compared with non-hospital VTE, however, hospital-related VTE was associated with a threefold higher risk of death.
Study limitations included being from a single center, possibly underpowered due to low number of events.
Bottom line: Hospital-related VTE has a high risk of recurrence, but risk level is variable and dependent on the reason for hospitalization.
Citation: Bjøri E, Arshad N, Johnsen HS, Hansen J-B, Brækkan SK. Hospital-related first venous thromboembolism and risk of recurrence [published online ahead of print, Sept. 2, 2016]. J Thromb Haemost. doi: 10.1111/jth.13492
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Do not use steroids in patients with severe sepsis without shock
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Dabigatran has less bleeding than rivaroxaban in atrial fibrillation
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
High-flow oxygen noninferior to noninvasive ventilation postextubation
Clinical Question: Is high-flow oxygen noninferior to noninvasive ventilation (NIV) in preventing postextubation respiratory failure and reintubation?
Background: Studies that suggest NIV usage following extubation reduces the risk of postextubation respiratory failure have led to an increase in use of this practice. Compared with NIV, high-flow, conditioned oxygen therapy has many advantages and fewer adverse effects, suggesting it might be a useful alternative.
Study design: Randomized clinical trial.
Setting: Three ICUs in Spain.
Rates of most secondary outcomes, including infection, mortality, and hospital length of stay (LOS) were similar between the two groups. ICU LOS was significantly less in the high-flow oxygen group (3d vs. 4d; 95% CI, –6.8 to –0.8).
Additionally, every patient tolerated high-flow oxygen therapy, while 40% of patients in the NIV arm required withdrawal of therapy for at least 6 hours due to adverse effects (P less than .001).
Bottom line: High-flow oxygen immediately following extubation may be a useful alternative to NIV in preventing postextubation respiratory failure.
Citation: Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-61.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical Question: Is high-flow oxygen noninferior to noninvasive ventilation (NIV) in preventing postextubation respiratory failure and reintubation?
Background: Studies that suggest NIV usage following extubation reduces the risk of postextubation respiratory failure have led to an increase in use of this practice. Compared with NIV, high-flow, conditioned oxygen therapy has many advantages and fewer adverse effects, suggesting it might be a useful alternative.
Study design: Randomized clinical trial.
Setting: Three ICUs in Spain.
Rates of most secondary outcomes, including infection, mortality, and hospital length of stay (LOS) were similar between the two groups. ICU LOS was significantly less in the high-flow oxygen group (3d vs. 4d; 95% CI, –6.8 to –0.8).
Additionally, every patient tolerated high-flow oxygen therapy, while 40% of patients in the NIV arm required withdrawal of therapy for at least 6 hours due to adverse effects (P less than .001).
Bottom line: High-flow oxygen immediately following extubation may be a useful alternative to NIV in preventing postextubation respiratory failure.
Citation: Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-61.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical Question: Is high-flow oxygen noninferior to noninvasive ventilation (NIV) in preventing postextubation respiratory failure and reintubation?
Background: Studies that suggest NIV usage following extubation reduces the risk of postextubation respiratory failure have led to an increase in use of this practice. Compared with NIV, high-flow, conditioned oxygen therapy has many advantages and fewer adverse effects, suggesting it might be a useful alternative.
Study design: Randomized clinical trial.
Setting: Three ICUs in Spain.
Rates of most secondary outcomes, including infection, mortality, and hospital length of stay (LOS) were similar between the two groups. ICU LOS was significantly less in the high-flow oxygen group (3d vs. 4d; 95% CI, –6.8 to –0.8).
Additionally, every patient tolerated high-flow oxygen therapy, while 40% of patients in the NIV arm required withdrawal of therapy for at least 6 hours due to adverse effects (P less than .001).
Bottom line: High-flow oxygen immediately following extubation may be a useful alternative to NIV in preventing postextubation respiratory failure.
Citation: Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-61.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
New guidelines for patients requiring red blood cell transfusion
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
How should urine electrolytes be ordered and interpreted in acute kidney injury and electrolyte abnormalities?
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.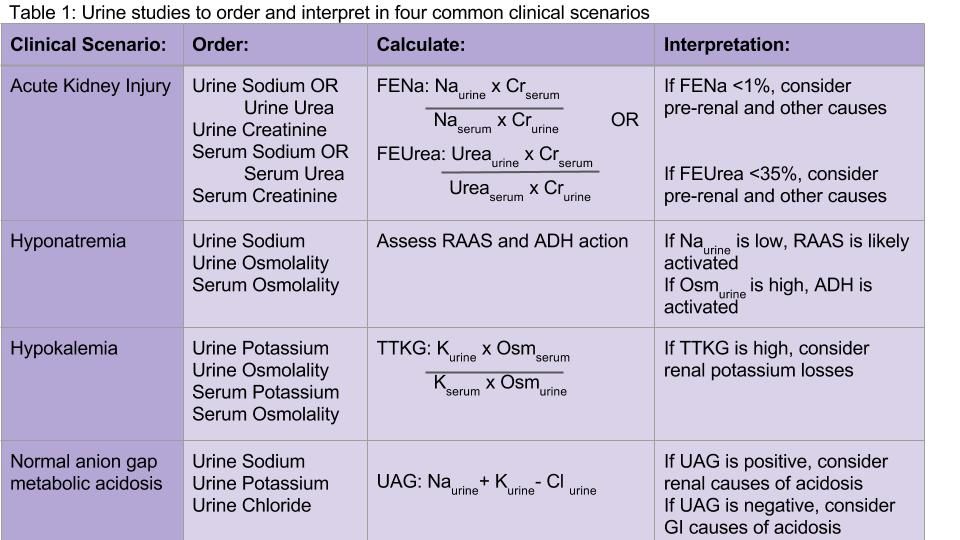
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
Sneak Peek: Journal of Hospital Medicine
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Do not overtreat febrile neutropenia
Clinical question: Does emergency department management of patients with febrile neutropenia (FN) follow current guidelines?
Background: Chemotherapy-related FN is an oncologic emergency frequently leading to hospitalization and intravenous antibiotics. Familiarity with FN guidelines allows risk stratification for inpatient versus outpatient therapy.
Study design: Single-center, retrospective, cohort study.
Setting: Large, urban, tertiary-care academic hospital.
Synopsis: Of 173 patient visits, 25% were risk stratified as eligible for outpatient treatment and 75% as inpatient care. All patient care was assessed for guideline concordance at the time of ED disposition and therapy.
Primary outcome analysis demonstrated management was guideline discordant in 98% of low-risk patients versus 7% of high-risk patients. Secondary 30-day clinical outcomes showed high-risk patients were more likely to have positive blood cultures (54%), sepsis-induced hypotension (9.3%), and death (5.4%). Seventeen percent of all patients who received IV antibiotics were prescribed vancomycin without guideline support.
Bottom line: Low-risk FN patients in the ED received more aggressive treatment than recommended. Further research is needed to strategize means of better aligning FN management with standards of care.
Citation: Baugh CW, Wang TJ, Caterino JM, et al. ED management of patients with febrile neutropenia: guideline concordant or overly aggressive [published online ahead of print Sept. 9, 2016]? Acad Emerg Med. doi: 10.1111/acem.13079.
Dr. Zuleta is an assistant professor and associate program director of the Jackson Memorial/University of Miami Internal Medicine residency training program and the site director of the program at University of Miami Hospital.
Clinical question: Does emergency department management of patients with febrile neutropenia (FN) follow current guidelines?
Background: Chemotherapy-related FN is an oncologic emergency frequently leading to hospitalization and intravenous antibiotics. Familiarity with FN guidelines allows risk stratification for inpatient versus outpatient therapy.
Study design: Single-center, retrospective, cohort study.
Setting: Large, urban, tertiary-care academic hospital.
Synopsis: Of 173 patient visits, 25% were risk stratified as eligible for outpatient treatment and 75% as inpatient care. All patient care was assessed for guideline concordance at the time of ED disposition and therapy.
Primary outcome analysis demonstrated management was guideline discordant in 98% of low-risk patients versus 7% of high-risk patients. Secondary 30-day clinical outcomes showed high-risk patients were more likely to have positive blood cultures (54%), sepsis-induced hypotension (9.3%), and death (5.4%). Seventeen percent of all patients who received IV antibiotics were prescribed vancomycin without guideline support.
Bottom line: Low-risk FN patients in the ED received more aggressive treatment than recommended. Further research is needed to strategize means of better aligning FN management with standards of care.
Citation: Baugh CW, Wang TJ, Caterino JM, et al. ED management of patients with febrile neutropenia: guideline concordant or overly aggressive [published online ahead of print Sept. 9, 2016]? Acad Emerg Med. doi: 10.1111/acem.13079.
Dr. Zuleta is an assistant professor and associate program director of the Jackson Memorial/University of Miami Internal Medicine residency training program and the site director of the program at University of Miami Hospital.
Clinical question: Does emergency department management of patients with febrile neutropenia (FN) follow current guidelines?
Background: Chemotherapy-related FN is an oncologic emergency frequently leading to hospitalization and intravenous antibiotics. Familiarity with FN guidelines allows risk stratification for inpatient versus outpatient therapy.
Study design: Single-center, retrospective, cohort study.
Setting: Large, urban, tertiary-care academic hospital.
Synopsis: Of 173 patient visits, 25% were risk stratified as eligible for outpatient treatment and 75% as inpatient care. All patient care was assessed for guideline concordance at the time of ED disposition and therapy.
Primary outcome analysis demonstrated management was guideline discordant in 98% of low-risk patients versus 7% of high-risk patients. Secondary 30-day clinical outcomes showed high-risk patients were more likely to have positive blood cultures (54%), sepsis-induced hypotension (9.3%), and death (5.4%). Seventeen percent of all patients who received IV antibiotics were prescribed vancomycin without guideline support.
Bottom line: Low-risk FN patients in the ED received more aggressive treatment than recommended. Further research is needed to strategize means of better aligning FN management with standards of care.
Citation: Baugh CW, Wang TJ, Caterino JM, et al. ED management of patients with febrile neutropenia: guideline concordant or overly aggressive [published online ahead of print Sept. 9, 2016]? Acad Emerg Med. doi: 10.1111/acem.13079.
Dr. Zuleta is an assistant professor and associate program director of the Jackson Memorial/University of Miami Internal Medicine residency training program and the site director of the program at University of Miami Hospital.
What to do with isolated calf DVT
Clinical question: Does therapeutic anticoagulation of isolated calf deep vein thrombosis (DVT) decrease risk for proximal DVT or PE?
Background: Optimal management of isolated calf DVT lacks consensus.
Study design: Single-center, retrospective, cohort study.
Setting: Large academic hospital.
Nevertheless, 9.2% of control patients and 3.3% of exposure patients developed a proximal DVT or PE. The anticoagulation group was associated with lower likelihood of proximal DVT or PE (risk ratio 0.36; 95% CI, 0.15-0.84) but an increased risk of bleeding (8.6%), compared with the nonexposure group (2.2%). Sensitivity analysis did not alter the observed association.
Bottom line: Therapeutic anticoagulation for isolated calf DVT may be warranted to decrease the risk for proximal DVT or PE but with an increased risk of bleeding. Randomized trials are needed to clarify the risk versus benefit.
Citation: Utter GH, Dhillon TS, Salcedo ES, et al. Therapeutic anticoagulation for isolated calf deep vein thrombosis. JAMA Surg. 2016;151(9):e161770. doi: 10.1001/jamasurg.2016.1770.
Dr. Zuleta is an assistant professor and associate program director of the Jackson Memorial/University of Miami Internal Medicine residency training program and the site director of the program at University of Miami Hospital.
Clinical question: Does therapeutic anticoagulation of isolated calf deep vein thrombosis (DVT) decrease risk for proximal DVT or PE?
Background: Optimal management of isolated calf DVT lacks consensus.
Study design: Single-center, retrospective, cohort study.
Setting: Large academic hospital.
Nevertheless, 9.2% of control patients and 3.3% of exposure patients developed a proximal DVT or PE. The anticoagulation group was associated with lower likelihood of proximal DVT or PE (risk ratio 0.36; 95% CI, 0.15-0.84) but an increased risk of bleeding (8.6%), compared with the nonexposure group (2.2%). Sensitivity analysis did not alter the observed association.
Bottom line: Therapeutic anticoagulation for isolated calf DVT may be warranted to decrease the risk for proximal DVT or PE but with an increased risk of bleeding. Randomized trials are needed to clarify the risk versus benefit.
Citation: Utter GH, Dhillon TS, Salcedo ES, et al. Therapeutic anticoagulation for isolated calf deep vein thrombosis. JAMA Surg. 2016;151(9):e161770. doi: 10.1001/jamasurg.2016.1770.
Dr. Zuleta is an assistant professor and associate program director of the Jackson Memorial/University of Miami Internal Medicine residency training program and the site director of the program at University of Miami Hospital.
Clinical question: Does therapeutic anticoagulation of isolated calf deep vein thrombosis (DVT) decrease risk for proximal DVT or PE?
Background: Optimal management of isolated calf DVT lacks consensus.
Study design: Single-center, retrospective, cohort study.
Setting: Large academic hospital.
Nevertheless, 9.2% of control patients and 3.3% of exposure patients developed a proximal DVT or PE. The anticoagulation group was associated with lower likelihood of proximal DVT or PE (risk ratio 0.36; 95% CI, 0.15-0.84) but an increased risk of bleeding (8.6%), compared with the nonexposure group (2.2%). Sensitivity analysis did not alter the observed association.
Bottom line: Therapeutic anticoagulation for isolated calf DVT may be warranted to decrease the risk for proximal DVT or PE but with an increased risk of bleeding. Randomized trials are needed to clarify the risk versus benefit.
Citation: Utter GH, Dhillon TS, Salcedo ES, et al. Therapeutic anticoagulation for isolated calf deep vein thrombosis. JAMA Surg. 2016;151(9):e161770. doi: 10.1001/jamasurg.2016.1770.
Dr. Zuleta is an assistant professor and associate program director of the Jackson Memorial/University of Miami Internal Medicine residency training program and the site director of the program at University of Miami Hospital.
Vent bundles and ventilator-associated pneumonia outcomes
Clinical question: Are the components of the ventilator bundles (VBs) associated with better outcomes for patients?
Background: VBs have been shown to prevent ventilator-associated pneumonia (VAP). However, most of the studies have analyzed outcomes based on the whole bundle without considering each individual component.
Study design: Retrospective cohort study.
Setting: Brigham and Women’s Hospital in Boston.
Spontaneous breathing trials were associated with lower hazards for VAEs (HR, 0.55; 95% CI, 0.40-0.76; P less than .001) and infection-related ventilator-associated complications (IVACs) (HR, 0.60; 95% CI, 0.37-1.00; P = .05). Head-of-bed elevation (HR, 1.38; 95% CI, 1.14-1.68; P = 0.001) and thromboembolism prophylaxis (HR, 2.57; 95% CI, 1.80-3.66; P less than .001) were associated with less time to extubation.
Oral care with chlorhexidine was associated with lower hazards for IVACs (HR, 0.60; 95% CI 0.36-1.00; P = .05) and for VAPs (HR, 0.55; 95% CI, 0.27-1.14; P = .11) but an increased risk for ventilator mortality (HR, 1.63; 95% CI, 1.15-2.31; P = .006). Stress ulcer prophylaxis was associated with higher risk for VAP (HR, 7.69; 95% CI, 1.44-41.10; P = .02).
Bottom line: Standard VB components merit revision to increase emphasis on beneficial components and eliminate potentially harmful ones.
Citation: Klompas M, Li L, Kleinman K, Szumita PM, Massaro AF. Association between ventilator bundle components and outcomes. JAMA Intern Med. 2016;176(9):1277-1283.
Dr. Mosetti is an assistant professor at the University of Miami Miller School of Medicine and a hospitalist at University of Miami Hospital and Jackson Memorial Hospital.
Clinical question: Are the components of the ventilator bundles (VBs) associated with better outcomes for patients?
Background: VBs have been shown to prevent ventilator-associated pneumonia (VAP). However, most of the studies have analyzed outcomes based on the whole bundle without considering each individual component.
Study design: Retrospective cohort study.
Setting: Brigham and Women’s Hospital in Boston.
Spontaneous breathing trials were associated with lower hazards for VAEs (HR, 0.55; 95% CI, 0.40-0.76; P less than .001) and infection-related ventilator-associated complications (IVACs) (HR, 0.60; 95% CI, 0.37-1.00; P = .05). Head-of-bed elevation (HR, 1.38; 95% CI, 1.14-1.68; P = 0.001) and thromboembolism prophylaxis (HR, 2.57; 95% CI, 1.80-3.66; P less than .001) were associated with less time to extubation.
Oral care with chlorhexidine was associated with lower hazards for IVACs (HR, 0.60; 95% CI 0.36-1.00; P = .05) and for VAPs (HR, 0.55; 95% CI, 0.27-1.14; P = .11) but an increased risk for ventilator mortality (HR, 1.63; 95% CI, 1.15-2.31; P = .006). Stress ulcer prophylaxis was associated with higher risk for VAP (HR, 7.69; 95% CI, 1.44-41.10; P = .02).
Bottom line: Standard VB components merit revision to increase emphasis on beneficial components and eliminate potentially harmful ones.
Citation: Klompas M, Li L, Kleinman K, Szumita PM, Massaro AF. Association between ventilator bundle components and outcomes. JAMA Intern Med. 2016;176(9):1277-1283.
Dr. Mosetti is an assistant professor at the University of Miami Miller School of Medicine and a hospitalist at University of Miami Hospital and Jackson Memorial Hospital.
Clinical question: Are the components of the ventilator bundles (VBs) associated with better outcomes for patients?
Background: VBs have been shown to prevent ventilator-associated pneumonia (VAP). However, most of the studies have analyzed outcomes based on the whole bundle without considering each individual component.
Study design: Retrospective cohort study.
Setting: Brigham and Women’s Hospital in Boston.
Spontaneous breathing trials were associated with lower hazards for VAEs (HR, 0.55; 95% CI, 0.40-0.76; P less than .001) and infection-related ventilator-associated complications (IVACs) (HR, 0.60; 95% CI, 0.37-1.00; P = .05). Head-of-bed elevation (HR, 1.38; 95% CI, 1.14-1.68; P = 0.001) and thromboembolism prophylaxis (HR, 2.57; 95% CI, 1.80-3.66; P less than .001) were associated with less time to extubation.
Oral care with chlorhexidine was associated with lower hazards for IVACs (HR, 0.60; 95% CI 0.36-1.00; P = .05) and for VAPs (HR, 0.55; 95% CI, 0.27-1.14; P = .11) but an increased risk for ventilator mortality (HR, 1.63; 95% CI, 1.15-2.31; P = .006). Stress ulcer prophylaxis was associated with higher risk for VAP (HR, 7.69; 95% CI, 1.44-41.10; P = .02).
Bottom line: Standard VB components merit revision to increase emphasis on beneficial components and eliminate potentially harmful ones.
Citation: Klompas M, Li L, Kleinman K, Szumita PM, Massaro AF. Association between ventilator bundle components and outcomes. JAMA Intern Med. 2016;176(9):1277-1283.
Dr. Mosetti is an assistant professor at the University of Miami Miller School of Medicine and a hospitalist at University of Miami Hospital and Jackson Memorial Hospital.