User login
Routine Replacement of Peripheral Intravenous Catheters
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
The “Things We Do for No Reason” (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Hospitals and health systems worldwide have adopted policies for routine replacement of peripheral intravenous catheters (PIVCs) at prespecified time intervals (range, 48-96 hours). This practice accounts for a large number of PIVC reinsertions and places a significant cost burden on the healthcare infrastructure. The authors of this article examine the evidence that has been used to support this practice.
CASE PRESENTATION
A 67-year-old man with metastatic lung cancer presents to a hospital for pain control and “failure to thrive.” In the emergency department, a left antecubital peripheral intravenous catheter (PIVC) is placed. On admission, a prerenal acute kidney injury is noted. During the patient’s entire hospitalization, normal saline with parenteral hydromorphone is administered. On hospital day 4, the pain is still not adequately controlled, and the intravenous opioid is continued. On morning rounds, an intern notes that the PIVC is functioning well, and there are no signs of irritation. However, the nursing staff reminds the team that the PIVC should be changed because it has been in place for 4 days and is “due for replacement.” The patient does not want to receive another skin puncture for routine venous access. Does the PIVC need to be replaced, per routine?
WHY YOU MIGHT THINK ROUTINE PIVC REPLACEMENT IS HELPFUL
PIVC placement is easily the most common procedure performed in the United States. An estimated 200 million PIVCs are placed each year.1 Given the number of inpatient hospital stays per year in the United States alone—more than 37 million1,2—data regarding the care, maintenance, and complications of PIVCs are essential to the healthcare infrastructure.
The recommendation to routinely replace PIVCs dates to 1981, when the Centers for Disease Control and Prevention3 (CDC) issued a guideline that calls for replacing PIVCs every 24 to 48 hours. Most of the data and studies that established that recommendation originated in the 1970s, when catheters varied in length and material, and precise definitions of complications, such as phlebitis—localized vein inflammation characterized by pain, erythema, tenderness, swelling, and a palpable cord4,5—were not standardized across trials. Research at the time suggested higher rates of complications from IVCs dwelling longer than 48 to 72 hours. The latest (2011) CDC guidelines6,7 softened the recommendation but still concluded, “There is no need to replace peripheral catheters more frequently than every 72-96 hours.”
The 2011 recommendation6,7 is based on findings of a 1983 prospective observational study,8 a 1991 randomized controlled trial (RCT),9 and a 1998 prospective observational study.2 The 1983 and 1991 studies found higher rates of PIVC complications after day 2 of cannulation.8,9 The 1998 study found no increase in the rate of complications after day 3 of catheterization, and its authors, recommending a reevaluation of the need to routinely replace PIVCs, wrote, “[The] hazard for catheter-related complications, phlebitis, catheter-related infections, and mechanical complications did not increase during prolonged catheterization.”2
Results of RCTs conducted by Barker et al.10 (2004) and Nishanth et al.11 (2009) supported the claim that routine replacement of PIVCs leads to lower rates of thrombophlebitis. Nishanth et al. also included site pain and cannula dislodgement in their definition of phlebitis. Neither study compared blood stream infection rates, but both found higher rates of phlebitis between day 2.5 and day 3. However, Cochrane reviewers Webster et al.12 questioned the findings of these 2 trials, given their missing data and possibly biased results and conclusions. In the Barker study, patient numbers (screened, eligible, dropout) were unclear; each patient group was unbalanced; protocol deviations were not reported (possibly a result of incomplete data reporting or inappropriate randomization); and varied definitions of phlebitis were allowed, which may have resulted in more events being included. In the Nishanth study, the 100% phlebitis rate for the clinically indicated replacement group seemed extreme, which suggested confounding by an unknown bias or chance. Last, both samples were small: 47 patients (Barker) and 42 patients (Nishanth). Given all these concerns, the 2 trials were excluded from the Cochrane meta-analysis on the subject.12
In the 1980s and early 1990s, routine removal and exchange of PIVCs were supported by limited evidence. Current well-designed trial data cast doubt on the need for such a practice.
WHY YOU SHOULD NOT ROUTINELY REPLACE PIVCs
According to the CDC,6,7 the issue of routine PIVC replacement remains unresolved: “No recommendation is made regarding replacement of peripheral catheters in adults only when clinically indicated.”
Whereas earlier data showed a higher risk of complications with longer dwelling IVs, the majority of contemporary data has failed to support this conclusion. The recent (2015) Cochrane meta-analysis comparing routine with clinically indicated IVC replacement found “no evidence to support changing catheters every 72-96 hours.”12 Of the 7 studies that fulfilled the criteria for qualitative analysis, only 5 were included (the studies by Barker et al.10 and Nishanth et al.11 were excluded). The included studies assessed the endpoints of catheter-related blood stream infection (CRBSI), phlebitis, phlebitis per device-days, mortality, cost, and infiltration. Statistically significant differences were found only for cost (favoring clinically indicated replacement) and infiltration (occurring less with routine replacement).
The largest and most robust RCT in the meta-analysis12 was conducted by Rickard et al.13 (2012). Their nonblinded, intention-to-treat study of 3283 patients used concealed allocation to randomly assign patients to either clinically indicated or routine PIVC replacement in order to evaluate a primary endpoint, phlebitis. Secondary endpoints were CRBSI, venous port infection, IVC tip colonization, infusion failure, number of IVCs needed per patient, IV therapy duration, cost, and mortality. Need for PIVC replacement was methodically monitored (Table) with extensive nursing education and interrater validation. The study found no difference in the groups’ phlebitis rates; the rate was 7% for both routine and clinically indicated replacement (13.08% and 13.11%, respectively, adjusted for phlebitis per 1000 IVC days). In addition, there was no difference in the secondary outcome measures, except cost and number of catheters used, both of which favored clinically indicated replacement. The most serious complication, CRBSI, occurred at essentially the same rate in the 2 replacement arms: 0.11% (routine) and 0% (clinically indicated). Per-patient cost for the entire course of treatment was A$69.24 in the routine group and A$61.66 in the clinically indicated group; the difference was A$7.58 (P < 0.0001). Mean number of catheters used was 1.9 in the routine group and 1.7 in the clinically indicated group; the difference was 0.21 catheter per patient for the treatment course (P < 0.0001). Overall, the study found no important difference in significant outcomes between the 2 study arms.
The other 4 studies in the meta-analysis12 duplicated these results, with none finding a higher rate of major adverse events.14-17 All 4 showed virtually equivalent rates of phlebitis, the primary outcome; 3 also examined the secondary outcome measure of blood stream infection, and results were similar, with identical rates of complications. Only 1 trial identified any bloodstream infections (1 per group).15 The meta-analysis did find that routine catheter replacement resulted in less catheter infiltration.
Most of the data on PIVC exchange involves phlebitis and other local complications. A prospective study by Stuart et al.18 and commentary by Collignon et al.19 underscore the need for further research targeting blood stream infections (sepsis and severe sepsis in particular) as a primary outcome. Blood stream infections, especially those related to PIVC use, are rare entities overall, with most recent data yielding an estimated rate of 0.5 per 1000 catheter-days.20 Given this epidemiologic finding, researchers trying to acquire meaningful data on PIVC-related blood stream infections and subsequent complications would need to have tens of thousands of patients in routine and clinically indicated replacement arms to sufficiently power their studies.20 As they are infeasible, such trials cannot be found in the scientific literature.
Stuart et al.18 tried addressing the question. Prospectively examining more than 5 million occupied-bed days and the incidence of bloodstream infections by type of intravascular device over a 5-year period, they found that 137 (23.5%) of 583 healthcare-associated Staphylococcus aureus bacteremia (SAB) cases were attributed to PIVC use. PIVC insertions were performed equally (39.6%) in emergency departments and medical wards. About 45% of PIVCs remained in place 4 days or longer. Stuart et al. noted the “significant issue of PIVC-associated SAB” and favored routine removal of PIVCs within 96 hours (4 days). However, 55% of patients in their PIVC-related SAB group had the device in place less than 4 days. In addition, overall incidence of SAB was low: 0.3 per 10,000 occupied-bed days. Further, their study did not adjust device-specific SAB incidence for frequency of device use. For example, the rate of healthcare-acquired SAB was 19.7% for central venous catheters and 23.5% for PIVCs, despite PIVCs being used significantly more often than central lines. Device-specific adjustments would show a vastly different absolute risk of SAB in relation to individual devices. Nevertheless, the overall benefit of and need for routine PIVC replacement must be questioned. The percentage of PIVC-associated SAB in their study and the need for more research in this area should be noted. Given current information, their study and others in the literature underscore the need for selective use, appropriate maintenance, and timely removal of PIVCs.
Pure clinical outcomes are important, but procedural costs are as well. Clinically indicated replacement helps patients avoid an unpleasant procedure and saves money.21 If one third of the 37 million annual inpatient admissions require a PIVC for more than 3 days, then a strategy of “replacement when clinically indicated” could prevent almost 2.5 million unnecessary PIVC insertions each year. Equipment cost savings combined with savings of nearly 1 million staff hours could yield an estimated $400 million in savings over a 5-year period.22 Given current data suggesting no harm from clinically indicated PIVC replacement and clear evidence that routine replacement increases needle sticks and costs, it seems time to end the practice of routine PIVC replacement.
RECOMMENDATIONS
Compared with clinically indicated catheter replacement, routine replacement in the absence of a clinical indication (eg, infiltration, phlebitis, infection) provides no added benefit. Studies have consistently found that rates of phlebitis and SAB are not affected by scheduled replacement, though the largest RCT may not have been powered to show a difference in SAB. The present authors’ recommendations for PIVC care are:
- Scrutinize each patient’s need for PIVCs and remove each PIVC as soon as possible.
- Do not make routine replacement of otherwise well-functioning, well-appearing clinically necessary PIVCs the standard of care.
- Regularly examine PIVC sites for signs and symptoms of infection.
- Remove a PIVC immediately on recognition of any clinical sign of a complication (eg, infiltration, phlebitis, localized infection, blood stream infection) and replace the PIVC only if there is a clinical need.
- If replacing PIVCs on a clinical basis, establish protocols for frequency of evaluation for complications; these protocols might mirror those from prior studies (Table).10,22
- Replace as soon as possible any PIVC inserted during an urgent or emergent situation in which proper insertion technique could not be guaranteed.
- Conduct real-world observational studies to ensure that the switch to clinically driven replacement is safe and develop standardized definitions of complications.
Given the literature findings and the preceding recommendations, the authors conclude that the patient in the case example does not need routine PIVC replacement. His PIVC may remain in place as long as evaluation for local complications is routinely and methodically performed and the device is removed as soon as it is deemed unnecessary (transition to oral opioid therapy).
CONCLUSION
The long-standing practice of routinely replacing PIVCs every 72 to 96 hours during a hospital stay does not affect any meaningful clinical outcome. Specifically, data do not show that routine replacement prevents phlebitis or blood stream infections. Furthermore, routine PIVC replacement increases patient discomfort, uses resources unnecessarily, and raises hospital costs. Most of the PIVC research has involved phlebitis and other local complications; more research on PIVC use and bloodstream infections is needed. Given the findings in the current literature, routine PIVC replacement should be considered a Thing We Do For No Reason.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
1. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
2. Bregenzer T, Conen D, Sakmann P, Widmer AF. Is routine replacement of peripheral intravenous catheters necessary? Arch Intern Med. 1998;158(2):151-156. PubMed
3. Centers for Disease Control Working Group. Guidelines for prevention of intravenous therapy-related infections. Infect Control. 1981;3:62-79.
4. Hershey CO, Tomford JW, McLaren CE, Porter DK, Cohen DI. The natural history of intravenous catheter-associated phlebitis. Arch Intern Med. 1984;144(7):1373-1375. PubMed
5. Widmer AF. IV-related infections. In: Wenzel RP, ed. Prevention and Control of Nosocomial Infections. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997:556-579.
6. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Centers for Disease Control and Prevention website. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Published April 1, 2011. Accessed November 5, 2016. PubMed
7. O’Grady NP, Alexander M, Burns LA, et al; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. PubMed
8. Rhode Island Nosocomial Infection Consortium; Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118(6):839-851. PubMed
9. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. PubMed
10. Barker P, Anderson AD, MacFie J. Randomised clinical trial of elective re-siting of intravenous cannulae. Ann R Coll Surg Engl. 2004;86(4):281-283. PubMed
11. Nishanth S, Sivaram G, Kalayarasan R, Kate V, Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Int Med J India. 2009;22(2):60-62. PubMed
12. Webster J, Osborne S, Rickard CM, New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2015;(8):CD007798. PubMed
13. Rickard CM, Webster J, Wallis MC, et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380(9847):1066-1074. PubMed
14. Webster J, Lloyd S, Hopkins T, Osborne S, Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud. 2007;44(5):664-671. PubMed
15. Webster J, Clarke S, Paterson D, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ. 2008;337:a339. PubMed
16. Van Donk P, Rickard CM, McGrail MR, Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(9):915-917. PubMed
17. Rickard CM, McCann D, Munnings J, McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med. 2010;8:53. PubMed
18. Stuart RL, Cameron DR, Scott C, et al. Peripheral intravenous catheter-associated Staphylococcus aureus bacteraemia: more than 5 years of prospective data from two tertiary health services. Med J Aust. 2013;198(10):551-553. PubMed
19. Collignon PJ, Kimber FJ, Beckingham WD, Roberts JL. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for routine replacement [letter]. Med J Aust. 2013;199(11):750-751. PubMed
20. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006:81(9):1159-1171. PubMed
21. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy. 2014;12(1):51-58. PubMed
22. Rickard CM, Webster J, Playford EG. Prevention of peripheral intravenous catheter-related bloodstream infections: the need for a new focus. Med J Aust. 2013;198(10):519-520. PubMed
© 2017 Society of Hospital Medicine
Inherited Thrombophilia Testing
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of venous thromboembolism (VTE). This disorder is prevalent in approximately 7% of the population and includes mutations such as factor V Leiden, prothrombin 20210, protein C deficiency, protein S deficiency, antithrombin deficiency, and methylene tetrahydrofolate reductase. The relative risk of VTE is 3‐ to 20‐fold greater in patients with inherited thrombophilia compared with the general population. Is testing for inherited thrombophilia recommended? The available evidence suggests that testing for inherited thrombophilia is not recommended in most clinical settings. In patients without a personal history of VTE, thrombophilia results do not change management, as there is no evidence to support thromboprophylaxis in this setting. In patients with a personal history of provoked or unprovoked VTE, inpatient testing is not indicated, as results do not influence management, testing is not cost‐effective, and a positive test result may lead to unnecessary patient anxiety or may result in unnecessary involvement of consultants. Testing in hospitalized patients has even more limitations because many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation.
CASE PRESENTATION
A 23‐year‐old man presents to the emergency room with pleuritic chest pain and new oxygen requirement of 2 L nasal cannula. He has a history of unprovoked lower extremity deep venous thrombosis (DVT) diagnosed at age 20 and completed 3 months of systemic anticoagulation without complications. He reports no family history of clotting disorders or venous thromboembolism (VTE) and no reversible risk factors for VTE such as prolonged immobility, recent surgery, or high‐risk medications. A computed tomogram pulmonary embolism protocol shows multiple right lower lobe, segmental pulmonary emboli. Anticoagulation is initiated, and the patient is admitted to the hospital. Will inpatient inherited thrombophilia testing impact management for this case?
WHY MAY INHERITED THROMBOPHILIA TESTING PROVE HELPFUL?
The annual incidence rate of a first VTE event is estimated as 117 per 100,000 individuals per year.[1] The most common presentations are symptomatic DVT of the leg (annual incidence approximately 48 per 100,000 people), or a pulmonary embolism (annual incidence approximately 69 per 100,000 people).[1] Pulmonary embolism results in death in up to 30% of untreated patients and 2.5% of patients who receive systemic anticoagulation.[2] Principal in the pathogenesis of VTE are factors described by Virchow's triad: venous stasis, endothelial injury, and systemic hypercoagulability. By identifying a mutation in 1 or more of the factors in the clotting pathway, an evaluation for inherited thrombophilia theoretically may unearth factors that drive systemic hypercoagulability and inform decision making so as to prevent future events.
Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of VTE.[3] Approximately 7% of the general population has inherited thrombophilia, which includes factor V Leiden (FVL) mutation, prothrombin 20210 mutation (PT20210), protein C deficiency, protein S deficiency, antithrombin III (ATIII) deficiency, and methylene tetrahydrofolate reductase mutation (MTHFR).[4] Of note, the definition does not include acquired etiologies, such as antiphospholipid antibody syndrome. Depending on the underlying condition and expression of the genetic abnormality, the relative risk of VTE in patients with inherited thrombophilia is 3‐ to 20‐fold greater than that of the general population.[5] Therefore, it is logical to consider that testing for inherited thrombophilia might be clinically useful. However, the evidence for doing so is very limited.
DOES INHERITED THROMBOPHILIA TESTING CHANGE MANAGEMENT?
An inherited thrombophilia evaluation is unlikely to affect management in most clinical settings. There is no current evidence to support primary prophylaxis[6] nor is there evidence that management of patients with recurrent VTE should be altered in the setting of inherited thrombophilia.
To date, no prospective trials have evaluated the efficacy of anticoagulant use for primary prevention of VTE in patients with inherited thrombophilia.[6] Given the limited evidence for thromboprophylaxis and risks of anticoagulation, primary prevention for patients with inherited thrombophilia that remain asymptomatic is not recommended by the current American College of Chest Physicians guidelines.[7, 8]
Similarly, in patients with a first VTE or recurrent VTE, diagnosis of inherited thrombophilia is often not associated with recurrent events, which suggests that other nongenetic factors may be just as important, if not more important, in determining the risk of recurrence.[9] Although no randomized controlled or controlled clinical trials have evaluated the effects of testing for inherited thrombophilia on recurrent VTE,[10, 11] several prospective studies have assessed risk factors for recurrence. Data from these studies suggest that recurrence rates after unprovoked VTE are only weakly correlated with inherited thrombophilia status.[12, 13] Rather, it is postulated that patients with recurrent VTE may exhibit a prothrombotic tendency regardless of underlying genetic predisposition. In this case, decisions regarding anticoagulation do not vary by thrombophilia status. Instead, thrombophilia testing may divert attention away from the management of more prevalent, potentially modifiable risk factors such as immobility, oral contraceptive use, or malignancy, all of which are associated with recurrent VTE.[14] These provoking factors are the most important determinants of the chance of VTE recurrence as well as the most significant factors to take into account when deciding duration of anticoagulation.
Christiansen et al. performed a prospective study evaluating the association between recurrent VTE and thrombophilia status. After following 474 patients with confirmed first episode VTE for a mean of 7.3 years, no statistically significant risk of VTE was found for patients with FVL (hazard ratio [HR]: 1.2, 95% confidence interval [CI]: 0.7‐1.9), PT20210 (HR: 0.7, 95% CI: 0.3‐2.0), or an anticoagulant (protein C, protein S or ATIII) deficiency (HR: 1.8, 95% CI: 0.9‐3.7).[15] Although unexplained VTE was statistically associated with VTE recurrence, heritable thrombophilia status was not.
In a systematic review and meta‐analysis investigating the association of FVL and PT20210 with recurrent VTE, Ho and colleagues found a statistically significant risk of recurrent VTE in patients with inherited thrombophilia due to FVL (odds ratio [OR]: 1.41, 95% CI: 1.14‐1.75) and PT20210 (OR: 1.72, 95% CI: 1.27‐2.31), and reported that at most, only up to 1 in 6 recurrent VTEs may be attributable to these mutations.[16] Based on this relatively modest effect, the authors question the utility of testing for inherited thrombophilia, as thrombophilia status is unlikely to warrant a change in type or duration of treatment.
Regardless of whether an underlying inherited thrombophilia is identified, patients with history of recurrent VTE are often candidates for long‐term anticoagulation. Testing for inherited thrombophilia in patients with prior VTE events will therefore not influence decisions regarding clinical management. Additionally, such testing may be confounded by ongoing disease or treatment (Table 1). For example, protein C, protein S antigen, and ATIII levels are low in the setting of acute VTE.[17, 18] Likewise, protein C and S (vitamin Kdependent proteins) will be low in the setting of anticoagulation with warfarin.[19] Moreover, ATIII activity and antigen levels are low in the setting of heparin use.[20] Lack of provider awareness regarding these interactions may have important negative consequences, including a spurious diagnosis of thrombophilia,[21, 22] unnecessary hematology consultation, and psychological distress to patients in the form of ongoing unwarranted testing or apprehension regarding recurrence.[23]
| Acute VTE | Anticoagulation With Warfarin | Anticoagulation With NOACs | Anticoagulation With Heparin/LMWH | |
|---|---|---|---|---|
| ||||
| FVL/PT20210/MTHFR gene mutations | No Impact | No Impact | No Impact | No Impact |
| Protein C* | Decreased | Decreased | No impact | No impact |
| Protein S* | Decreased | Decreased | No impact | No impact |
| ATIII activity | Decreased | Slight increase | Slight increase | Decreased |
| ATIII antigen | Decreased | Slight increase | Slight increase | Decreased |
Additionally, this expensive evaluation has estimated direct costs of $1100 to $2400 per thrombophilia panel based on estimation of charges billed by a large commercial laboratory.[24, 25] In 2014, over 280,000 claims were submitted under Medicare Part B across all care settings for a thrombophilia analysis including FVL, PT20210, and MTHFR gene mutations,[24] which would equate to between $300 million to $672 million.[26] Unfortunately, there have been no large‐scale trials to assess cost‐effectiveness. However, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group stated that cost‐effectiveness modeling studies in this area require updating with current VTE risk estimates but are suggestive that routine FVL/PT20210 testing is not cost‐effective.[27]
ARE THERE CIRCUMSTANCES IN WHICH INPATIENT INHERITED THROMBOPHILIA TESTING PROVES BENEFICIAL?
The evidence for when to test for inherited thrombophilia is very limited and is often based on individualized risk. The current EGAPP guidelines acknowledge this limitation, specifically noting that there is a paucity of data evaluating management or prophylaxis of patients with homozygous or compound heterozygous FVL or P20210 mutation, and a lack of data surrounding whether or not knowledge of thrombophilia mutation should affect anticoagulation treatment.[27] This is why an individualized approach is deemed necessary. For example, the decision to prescribe hormone replacement therapy in women with a family history of inherited thrombophilia may be better informed by testing prior to treatment. Similarly, pregnant women with a family history or personal history of VTE may also benefit from inherited thrombophilia testing, as this may influence antepartum or postpartum management.[28, 29] The National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of testing for hereditary thrombophilia in patients with unprovoked VTE and a first‐degree relative with VTE, if stopping anticoagulation treatment is planned; however, these recommendations are based solely on Guideline Development Group's experience and opinion.[30] Regardless, testing for inherited thrombophilia has significant potential consequences. Patients at risk should meet with an outpatient hematologist and/or a genetic counselor, if available, to determine the risks and benefits of testing.
WHAT DO GUIDELINES SAY ABOUT INHERITED THROMBOPHILIA TESTING?
The most recent NICE guidelines recommend against offering inherited thrombophilia testing to patients presenting with a provoked VTE in any clinical setting.[30] In patients diagnosed with unprovoked VTE, testing should not be considered unless a first degree relative with a history of VTE exists.[30] The NICE guidelines also recommend against routinely offering thrombophilia testing to asymptomatic first‐degree relatives of patients with a history of VTE or known inherited thrombophilia. This recommendation is reflected in the American Society of Hematology's Choosing Wisely recommendations since 2013.[31] Further, The American College of Medical Genetics and Genomics' Choosing Wisely recommendations from 2015 state that MTHFR mutations should never be included in any thrombophilia workup, as recent meta‐analyses have disproven an association between the presence of these variants and venous thromboembolism.[32]
The EGAPP Working Group recommends against routine testing for FVL or PT20210 in patients who present with an idiopathic VTE, as longer‐term anticoagulation offers similar benefits to patients with or without these mutations.[27] EGAPP also recommends against testing asymptomatic adult family members of patients with VTE and/or an FVL or PT20210 mutation for the purpose of considering primary prophylactic anticoagulation. In these circumstances, it is felt that the potential risks of thrombophilia testing outweigh any potential benefits.
HOW SHOULD HOSPITALISTS APPROACH TESTING OF INHERITED THROMBOPHILIA?
The providers in our case presentation are challenged with determining whether inpatient thrombophilia evaluation will add value to the evaluation of patients with unprovoked VTE. The available evidence suggests that clinicians should avoid ordering thrombophilia testing for hospitalized patients with unprovoked VTE because (1) many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation, (2) results of testing often do not influence management, (3) testing is not cost‐effective, (4) a positive test result may lead to unnecessary patient anxiety, and (5) testing may result in inappropriately prolonged anticoagulation courses or unnecessary involvement of inpatient consultants. For these reasons, the patient in our case presentation should not be tested for inherited thrombophilia. In patients with personal or family histories of recurrent thromboembolism, modifiable clinical risk factors should be addressed, as these are more likely to influence treatment decisions compared to genetic testing. Finally, patients may be referred to an outpatient hematologist or geneticist for individualized discussions of risks and benefits of testing for inherited thrombophilia.
CONCLUSION
Inpatient evaluation for inherited thrombophilia for VTE is not clinically useful, cost‐effective, or reliable in the setting of VTE. The result of such testing does not affect management of acute primary or recurrent VTE. Testing should only be considered using an individualized approach in the outpatient setting with appropriate genetic counseling.
Disclosure: Christopher M. Petrilli, MD, and Lauren Heidemann, MD, contributed equally to this work. The authors report no conflicts of interest.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , , , , . Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585–593.
- , , , et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245.
- , . Hereditary thrombophilia. Thromb J. 2006;4:15.
- , , , . Deep‐vein thrombosis. Lancet. 1999;353(9151):479–485.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S–e736S.
- , . Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2009;(1):CD007069.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:CD007069.
- , , , . Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526.
- , , , et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436.
- , . Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699–704.
- , , , , . Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361.
- , , , . Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736.
- , , , . Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986;77(2):416–425.
- , . Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–1239.
- , , , . Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb Res. 1987;45(6):783–790.
- . Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135.
- , , . Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89(12):1147–1150.
- , . Quantitation of human protein S in the plasma of normal and warfarin‐treated individuals by radioimmunoassay. Thromb Res. 1984;36(6):527–535.
- , , , . Social aspects of genetic testing for factor V Leiden mutation in healthy individuals and their importance for daily practice. Thromb Res. 2004;113(1):7–12.
- , . Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013–1020.
- , , . An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120–127.
- CodeMap. Available at: https://www.codemap.com. Accessed January 18, 2016.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67–76.
- , , , et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444.
- , , , et al. Frequency of pregnancy‐related venous thromboembolism in anticoagulant factor‐deficient women: implications for prophylaxis. Ann Intern Med. 1996;125(12):955–960.
- , , , ; Guideline Development Group. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979.
- American Society of Hematology. Ten things physicians and patients should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐society‐of‐hematology. Published December 4, 2013. Accessed January 18, 2016.
- American College of Medical Genetics and Genomics. Five Things patients and providers should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐college‐of‐medical‐genetics‐and‐genomics. Published July 10, 2015. Accessed March 13, 2016.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of venous thromboembolism (VTE). This disorder is prevalent in approximately 7% of the population and includes mutations such as factor V Leiden, prothrombin 20210, protein C deficiency, protein S deficiency, antithrombin deficiency, and methylene tetrahydrofolate reductase. The relative risk of VTE is 3‐ to 20‐fold greater in patients with inherited thrombophilia compared with the general population. Is testing for inherited thrombophilia recommended? The available evidence suggests that testing for inherited thrombophilia is not recommended in most clinical settings. In patients without a personal history of VTE, thrombophilia results do not change management, as there is no evidence to support thromboprophylaxis in this setting. In patients with a personal history of provoked or unprovoked VTE, inpatient testing is not indicated, as results do not influence management, testing is not cost‐effective, and a positive test result may lead to unnecessary patient anxiety or may result in unnecessary involvement of consultants. Testing in hospitalized patients has even more limitations because many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation.
CASE PRESENTATION
A 23‐year‐old man presents to the emergency room with pleuritic chest pain and new oxygen requirement of 2 L nasal cannula. He has a history of unprovoked lower extremity deep venous thrombosis (DVT) diagnosed at age 20 and completed 3 months of systemic anticoagulation without complications. He reports no family history of clotting disorders or venous thromboembolism (VTE) and no reversible risk factors for VTE such as prolonged immobility, recent surgery, or high‐risk medications. A computed tomogram pulmonary embolism protocol shows multiple right lower lobe, segmental pulmonary emboli. Anticoagulation is initiated, and the patient is admitted to the hospital. Will inpatient inherited thrombophilia testing impact management for this case?
WHY MAY INHERITED THROMBOPHILIA TESTING PROVE HELPFUL?
The annual incidence rate of a first VTE event is estimated as 117 per 100,000 individuals per year.[1] The most common presentations are symptomatic DVT of the leg (annual incidence approximately 48 per 100,000 people), or a pulmonary embolism (annual incidence approximately 69 per 100,000 people).[1] Pulmonary embolism results in death in up to 30% of untreated patients and 2.5% of patients who receive systemic anticoagulation.[2] Principal in the pathogenesis of VTE are factors described by Virchow's triad: venous stasis, endothelial injury, and systemic hypercoagulability. By identifying a mutation in 1 or more of the factors in the clotting pathway, an evaluation for inherited thrombophilia theoretically may unearth factors that drive systemic hypercoagulability and inform decision making so as to prevent future events.
Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of VTE.[3] Approximately 7% of the general population has inherited thrombophilia, which includes factor V Leiden (FVL) mutation, prothrombin 20210 mutation (PT20210), protein C deficiency, protein S deficiency, antithrombin III (ATIII) deficiency, and methylene tetrahydrofolate reductase mutation (MTHFR).[4] Of note, the definition does not include acquired etiologies, such as antiphospholipid antibody syndrome. Depending on the underlying condition and expression of the genetic abnormality, the relative risk of VTE in patients with inherited thrombophilia is 3‐ to 20‐fold greater than that of the general population.[5] Therefore, it is logical to consider that testing for inherited thrombophilia might be clinically useful. However, the evidence for doing so is very limited.
DOES INHERITED THROMBOPHILIA TESTING CHANGE MANAGEMENT?
An inherited thrombophilia evaluation is unlikely to affect management in most clinical settings. There is no current evidence to support primary prophylaxis[6] nor is there evidence that management of patients with recurrent VTE should be altered in the setting of inherited thrombophilia.
To date, no prospective trials have evaluated the efficacy of anticoagulant use for primary prevention of VTE in patients with inherited thrombophilia.[6] Given the limited evidence for thromboprophylaxis and risks of anticoagulation, primary prevention for patients with inherited thrombophilia that remain asymptomatic is not recommended by the current American College of Chest Physicians guidelines.[7, 8]
Similarly, in patients with a first VTE or recurrent VTE, diagnosis of inherited thrombophilia is often not associated with recurrent events, which suggests that other nongenetic factors may be just as important, if not more important, in determining the risk of recurrence.[9] Although no randomized controlled or controlled clinical trials have evaluated the effects of testing for inherited thrombophilia on recurrent VTE,[10, 11] several prospective studies have assessed risk factors for recurrence. Data from these studies suggest that recurrence rates after unprovoked VTE are only weakly correlated with inherited thrombophilia status.[12, 13] Rather, it is postulated that patients with recurrent VTE may exhibit a prothrombotic tendency regardless of underlying genetic predisposition. In this case, decisions regarding anticoagulation do not vary by thrombophilia status. Instead, thrombophilia testing may divert attention away from the management of more prevalent, potentially modifiable risk factors such as immobility, oral contraceptive use, or malignancy, all of which are associated with recurrent VTE.[14] These provoking factors are the most important determinants of the chance of VTE recurrence as well as the most significant factors to take into account when deciding duration of anticoagulation.
Christiansen et al. performed a prospective study evaluating the association between recurrent VTE and thrombophilia status. After following 474 patients with confirmed first episode VTE for a mean of 7.3 years, no statistically significant risk of VTE was found for patients with FVL (hazard ratio [HR]: 1.2, 95% confidence interval [CI]: 0.7‐1.9), PT20210 (HR: 0.7, 95% CI: 0.3‐2.0), or an anticoagulant (protein C, protein S or ATIII) deficiency (HR: 1.8, 95% CI: 0.9‐3.7).[15] Although unexplained VTE was statistically associated with VTE recurrence, heritable thrombophilia status was not.
In a systematic review and meta‐analysis investigating the association of FVL and PT20210 with recurrent VTE, Ho and colleagues found a statistically significant risk of recurrent VTE in patients with inherited thrombophilia due to FVL (odds ratio [OR]: 1.41, 95% CI: 1.14‐1.75) and PT20210 (OR: 1.72, 95% CI: 1.27‐2.31), and reported that at most, only up to 1 in 6 recurrent VTEs may be attributable to these mutations.[16] Based on this relatively modest effect, the authors question the utility of testing for inherited thrombophilia, as thrombophilia status is unlikely to warrant a change in type or duration of treatment.
Regardless of whether an underlying inherited thrombophilia is identified, patients with history of recurrent VTE are often candidates for long‐term anticoagulation. Testing for inherited thrombophilia in patients with prior VTE events will therefore not influence decisions regarding clinical management. Additionally, such testing may be confounded by ongoing disease or treatment (Table 1). For example, protein C, protein S antigen, and ATIII levels are low in the setting of acute VTE.[17, 18] Likewise, protein C and S (vitamin Kdependent proteins) will be low in the setting of anticoagulation with warfarin.[19] Moreover, ATIII activity and antigen levels are low in the setting of heparin use.[20] Lack of provider awareness regarding these interactions may have important negative consequences, including a spurious diagnosis of thrombophilia,[21, 22] unnecessary hematology consultation, and psychological distress to patients in the form of ongoing unwarranted testing or apprehension regarding recurrence.[23]
| Acute VTE | Anticoagulation With Warfarin | Anticoagulation With NOACs | Anticoagulation With Heparin/LMWH | |
|---|---|---|---|---|
| ||||
| FVL/PT20210/MTHFR gene mutations | No Impact | No Impact | No Impact | No Impact |
| Protein C* | Decreased | Decreased | No impact | No impact |
| Protein S* | Decreased | Decreased | No impact | No impact |
| ATIII activity | Decreased | Slight increase | Slight increase | Decreased |
| ATIII antigen | Decreased | Slight increase | Slight increase | Decreased |
Additionally, this expensive evaluation has estimated direct costs of $1100 to $2400 per thrombophilia panel based on estimation of charges billed by a large commercial laboratory.[24, 25] In 2014, over 280,000 claims were submitted under Medicare Part B across all care settings for a thrombophilia analysis including FVL, PT20210, and MTHFR gene mutations,[24] which would equate to between $300 million to $672 million.[26] Unfortunately, there have been no large‐scale trials to assess cost‐effectiveness. However, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group stated that cost‐effectiveness modeling studies in this area require updating with current VTE risk estimates but are suggestive that routine FVL/PT20210 testing is not cost‐effective.[27]
ARE THERE CIRCUMSTANCES IN WHICH INPATIENT INHERITED THROMBOPHILIA TESTING PROVES BENEFICIAL?
The evidence for when to test for inherited thrombophilia is very limited and is often based on individualized risk. The current EGAPP guidelines acknowledge this limitation, specifically noting that there is a paucity of data evaluating management or prophylaxis of patients with homozygous or compound heterozygous FVL or P20210 mutation, and a lack of data surrounding whether or not knowledge of thrombophilia mutation should affect anticoagulation treatment.[27] This is why an individualized approach is deemed necessary. For example, the decision to prescribe hormone replacement therapy in women with a family history of inherited thrombophilia may be better informed by testing prior to treatment. Similarly, pregnant women with a family history or personal history of VTE may also benefit from inherited thrombophilia testing, as this may influence antepartum or postpartum management.[28, 29] The National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of testing for hereditary thrombophilia in patients with unprovoked VTE and a first‐degree relative with VTE, if stopping anticoagulation treatment is planned; however, these recommendations are based solely on Guideline Development Group's experience and opinion.[30] Regardless, testing for inherited thrombophilia has significant potential consequences. Patients at risk should meet with an outpatient hematologist and/or a genetic counselor, if available, to determine the risks and benefits of testing.
WHAT DO GUIDELINES SAY ABOUT INHERITED THROMBOPHILIA TESTING?
The most recent NICE guidelines recommend against offering inherited thrombophilia testing to patients presenting with a provoked VTE in any clinical setting.[30] In patients diagnosed with unprovoked VTE, testing should not be considered unless a first degree relative with a history of VTE exists.[30] The NICE guidelines also recommend against routinely offering thrombophilia testing to asymptomatic first‐degree relatives of patients with a history of VTE or known inherited thrombophilia. This recommendation is reflected in the American Society of Hematology's Choosing Wisely recommendations since 2013.[31] Further, The American College of Medical Genetics and Genomics' Choosing Wisely recommendations from 2015 state that MTHFR mutations should never be included in any thrombophilia workup, as recent meta‐analyses have disproven an association between the presence of these variants and venous thromboembolism.[32]
The EGAPP Working Group recommends against routine testing for FVL or PT20210 in patients who present with an idiopathic VTE, as longer‐term anticoagulation offers similar benefits to patients with or without these mutations.[27] EGAPP also recommends against testing asymptomatic adult family members of patients with VTE and/or an FVL or PT20210 mutation for the purpose of considering primary prophylactic anticoagulation. In these circumstances, it is felt that the potential risks of thrombophilia testing outweigh any potential benefits.
HOW SHOULD HOSPITALISTS APPROACH TESTING OF INHERITED THROMBOPHILIA?
The providers in our case presentation are challenged with determining whether inpatient thrombophilia evaluation will add value to the evaluation of patients with unprovoked VTE. The available evidence suggests that clinicians should avoid ordering thrombophilia testing for hospitalized patients with unprovoked VTE because (1) many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation, (2) results of testing often do not influence management, (3) testing is not cost‐effective, (4) a positive test result may lead to unnecessary patient anxiety, and (5) testing may result in inappropriately prolonged anticoagulation courses or unnecessary involvement of inpatient consultants. For these reasons, the patient in our case presentation should not be tested for inherited thrombophilia. In patients with personal or family histories of recurrent thromboembolism, modifiable clinical risk factors should be addressed, as these are more likely to influence treatment decisions compared to genetic testing. Finally, patients may be referred to an outpatient hematologist or geneticist for individualized discussions of risks and benefits of testing for inherited thrombophilia.
CONCLUSION
Inpatient evaluation for inherited thrombophilia for VTE is not clinically useful, cost‐effective, or reliable in the setting of VTE. The result of such testing does not affect management of acute primary or recurrent VTE. Testing should only be considered using an individualized approach in the outpatient setting with appropriate genetic counseling.
Disclosure: Christopher M. Petrilli, MD, and Lauren Heidemann, MD, contributed equally to this work. The authors report no conflicts of interest.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of venous thromboembolism (VTE). This disorder is prevalent in approximately 7% of the population and includes mutations such as factor V Leiden, prothrombin 20210, protein C deficiency, protein S deficiency, antithrombin deficiency, and methylene tetrahydrofolate reductase. The relative risk of VTE is 3‐ to 20‐fold greater in patients with inherited thrombophilia compared with the general population. Is testing for inherited thrombophilia recommended? The available evidence suggests that testing for inherited thrombophilia is not recommended in most clinical settings. In patients without a personal history of VTE, thrombophilia results do not change management, as there is no evidence to support thromboprophylaxis in this setting. In patients with a personal history of provoked or unprovoked VTE, inpatient testing is not indicated, as results do not influence management, testing is not cost‐effective, and a positive test result may lead to unnecessary patient anxiety or may result in unnecessary involvement of consultants. Testing in hospitalized patients has even more limitations because many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation.
CASE PRESENTATION
A 23‐year‐old man presents to the emergency room with pleuritic chest pain and new oxygen requirement of 2 L nasal cannula. He has a history of unprovoked lower extremity deep venous thrombosis (DVT) diagnosed at age 20 and completed 3 months of systemic anticoagulation without complications. He reports no family history of clotting disorders or venous thromboembolism (VTE) and no reversible risk factors for VTE such as prolonged immobility, recent surgery, or high‐risk medications. A computed tomogram pulmonary embolism protocol shows multiple right lower lobe, segmental pulmonary emboli. Anticoagulation is initiated, and the patient is admitted to the hospital. Will inpatient inherited thrombophilia testing impact management for this case?
WHY MAY INHERITED THROMBOPHILIA TESTING PROVE HELPFUL?
The annual incidence rate of a first VTE event is estimated as 117 per 100,000 individuals per year.[1] The most common presentations are symptomatic DVT of the leg (annual incidence approximately 48 per 100,000 people), or a pulmonary embolism (annual incidence approximately 69 per 100,000 people).[1] Pulmonary embolism results in death in up to 30% of untreated patients and 2.5% of patients who receive systemic anticoagulation.[2] Principal in the pathogenesis of VTE are factors described by Virchow's triad: venous stasis, endothelial injury, and systemic hypercoagulability. By identifying a mutation in 1 or more of the factors in the clotting pathway, an evaluation for inherited thrombophilia theoretically may unearth factors that drive systemic hypercoagulability and inform decision making so as to prevent future events.
Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of VTE.[3] Approximately 7% of the general population has inherited thrombophilia, which includes factor V Leiden (FVL) mutation, prothrombin 20210 mutation (PT20210), protein C deficiency, protein S deficiency, antithrombin III (ATIII) deficiency, and methylene tetrahydrofolate reductase mutation (MTHFR).[4] Of note, the definition does not include acquired etiologies, such as antiphospholipid antibody syndrome. Depending on the underlying condition and expression of the genetic abnormality, the relative risk of VTE in patients with inherited thrombophilia is 3‐ to 20‐fold greater than that of the general population.[5] Therefore, it is logical to consider that testing for inherited thrombophilia might be clinically useful. However, the evidence for doing so is very limited.
DOES INHERITED THROMBOPHILIA TESTING CHANGE MANAGEMENT?
An inherited thrombophilia evaluation is unlikely to affect management in most clinical settings. There is no current evidence to support primary prophylaxis[6] nor is there evidence that management of patients with recurrent VTE should be altered in the setting of inherited thrombophilia.
To date, no prospective trials have evaluated the efficacy of anticoagulant use for primary prevention of VTE in patients with inherited thrombophilia.[6] Given the limited evidence for thromboprophylaxis and risks of anticoagulation, primary prevention for patients with inherited thrombophilia that remain asymptomatic is not recommended by the current American College of Chest Physicians guidelines.[7, 8]
Similarly, in patients with a first VTE or recurrent VTE, diagnosis of inherited thrombophilia is often not associated with recurrent events, which suggests that other nongenetic factors may be just as important, if not more important, in determining the risk of recurrence.[9] Although no randomized controlled or controlled clinical trials have evaluated the effects of testing for inherited thrombophilia on recurrent VTE,[10, 11] several prospective studies have assessed risk factors for recurrence. Data from these studies suggest that recurrence rates after unprovoked VTE are only weakly correlated with inherited thrombophilia status.[12, 13] Rather, it is postulated that patients with recurrent VTE may exhibit a prothrombotic tendency regardless of underlying genetic predisposition. In this case, decisions regarding anticoagulation do not vary by thrombophilia status. Instead, thrombophilia testing may divert attention away from the management of more prevalent, potentially modifiable risk factors such as immobility, oral contraceptive use, or malignancy, all of which are associated with recurrent VTE.[14] These provoking factors are the most important determinants of the chance of VTE recurrence as well as the most significant factors to take into account when deciding duration of anticoagulation.
Christiansen et al. performed a prospective study evaluating the association between recurrent VTE and thrombophilia status. After following 474 patients with confirmed first episode VTE for a mean of 7.3 years, no statistically significant risk of VTE was found for patients with FVL (hazard ratio [HR]: 1.2, 95% confidence interval [CI]: 0.7‐1.9), PT20210 (HR: 0.7, 95% CI: 0.3‐2.0), or an anticoagulant (protein C, protein S or ATIII) deficiency (HR: 1.8, 95% CI: 0.9‐3.7).[15] Although unexplained VTE was statistically associated with VTE recurrence, heritable thrombophilia status was not.
In a systematic review and meta‐analysis investigating the association of FVL and PT20210 with recurrent VTE, Ho and colleagues found a statistically significant risk of recurrent VTE in patients with inherited thrombophilia due to FVL (odds ratio [OR]: 1.41, 95% CI: 1.14‐1.75) and PT20210 (OR: 1.72, 95% CI: 1.27‐2.31), and reported that at most, only up to 1 in 6 recurrent VTEs may be attributable to these mutations.[16] Based on this relatively modest effect, the authors question the utility of testing for inherited thrombophilia, as thrombophilia status is unlikely to warrant a change in type or duration of treatment.
Regardless of whether an underlying inherited thrombophilia is identified, patients with history of recurrent VTE are often candidates for long‐term anticoagulation. Testing for inherited thrombophilia in patients with prior VTE events will therefore not influence decisions regarding clinical management. Additionally, such testing may be confounded by ongoing disease or treatment (Table 1). For example, protein C, protein S antigen, and ATIII levels are low in the setting of acute VTE.[17, 18] Likewise, protein C and S (vitamin Kdependent proteins) will be low in the setting of anticoagulation with warfarin.[19] Moreover, ATIII activity and antigen levels are low in the setting of heparin use.[20] Lack of provider awareness regarding these interactions may have important negative consequences, including a spurious diagnosis of thrombophilia,[21, 22] unnecessary hematology consultation, and psychological distress to patients in the form of ongoing unwarranted testing or apprehension regarding recurrence.[23]
| Acute VTE | Anticoagulation With Warfarin | Anticoagulation With NOACs | Anticoagulation With Heparin/LMWH | |
|---|---|---|---|---|
| ||||
| FVL/PT20210/MTHFR gene mutations | No Impact | No Impact | No Impact | No Impact |
| Protein C* | Decreased | Decreased | No impact | No impact |
| Protein S* | Decreased | Decreased | No impact | No impact |
| ATIII activity | Decreased | Slight increase | Slight increase | Decreased |
| ATIII antigen | Decreased | Slight increase | Slight increase | Decreased |
Additionally, this expensive evaluation has estimated direct costs of $1100 to $2400 per thrombophilia panel based on estimation of charges billed by a large commercial laboratory.[24, 25] In 2014, over 280,000 claims were submitted under Medicare Part B across all care settings for a thrombophilia analysis including FVL, PT20210, and MTHFR gene mutations,[24] which would equate to between $300 million to $672 million.[26] Unfortunately, there have been no large‐scale trials to assess cost‐effectiveness. However, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group stated that cost‐effectiveness modeling studies in this area require updating with current VTE risk estimates but are suggestive that routine FVL/PT20210 testing is not cost‐effective.[27]
ARE THERE CIRCUMSTANCES IN WHICH INPATIENT INHERITED THROMBOPHILIA TESTING PROVES BENEFICIAL?
The evidence for when to test for inherited thrombophilia is very limited and is often based on individualized risk. The current EGAPP guidelines acknowledge this limitation, specifically noting that there is a paucity of data evaluating management or prophylaxis of patients with homozygous or compound heterozygous FVL or P20210 mutation, and a lack of data surrounding whether or not knowledge of thrombophilia mutation should affect anticoagulation treatment.[27] This is why an individualized approach is deemed necessary. For example, the decision to prescribe hormone replacement therapy in women with a family history of inherited thrombophilia may be better informed by testing prior to treatment. Similarly, pregnant women with a family history or personal history of VTE may also benefit from inherited thrombophilia testing, as this may influence antepartum or postpartum management.[28, 29] The National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of testing for hereditary thrombophilia in patients with unprovoked VTE and a first‐degree relative with VTE, if stopping anticoagulation treatment is planned; however, these recommendations are based solely on Guideline Development Group's experience and opinion.[30] Regardless, testing for inherited thrombophilia has significant potential consequences. Patients at risk should meet with an outpatient hematologist and/or a genetic counselor, if available, to determine the risks and benefits of testing.
WHAT DO GUIDELINES SAY ABOUT INHERITED THROMBOPHILIA TESTING?
The most recent NICE guidelines recommend against offering inherited thrombophilia testing to patients presenting with a provoked VTE in any clinical setting.[30] In patients diagnosed with unprovoked VTE, testing should not be considered unless a first degree relative with a history of VTE exists.[30] The NICE guidelines also recommend against routinely offering thrombophilia testing to asymptomatic first‐degree relatives of patients with a history of VTE or known inherited thrombophilia. This recommendation is reflected in the American Society of Hematology's Choosing Wisely recommendations since 2013.[31] Further, The American College of Medical Genetics and Genomics' Choosing Wisely recommendations from 2015 state that MTHFR mutations should never be included in any thrombophilia workup, as recent meta‐analyses have disproven an association between the presence of these variants and venous thromboembolism.[32]
The EGAPP Working Group recommends against routine testing for FVL or PT20210 in patients who present with an idiopathic VTE, as longer‐term anticoagulation offers similar benefits to patients with or without these mutations.[27] EGAPP also recommends against testing asymptomatic adult family members of patients with VTE and/or an FVL or PT20210 mutation for the purpose of considering primary prophylactic anticoagulation. In these circumstances, it is felt that the potential risks of thrombophilia testing outweigh any potential benefits.
HOW SHOULD HOSPITALISTS APPROACH TESTING OF INHERITED THROMBOPHILIA?
The providers in our case presentation are challenged with determining whether inpatient thrombophilia evaluation will add value to the evaluation of patients with unprovoked VTE. The available evidence suggests that clinicians should avoid ordering thrombophilia testing for hospitalized patients with unprovoked VTE because (1) many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation, (2) results of testing often do not influence management, (3) testing is not cost‐effective, (4) a positive test result may lead to unnecessary patient anxiety, and (5) testing may result in inappropriately prolonged anticoagulation courses or unnecessary involvement of inpatient consultants. For these reasons, the patient in our case presentation should not be tested for inherited thrombophilia. In patients with personal or family histories of recurrent thromboembolism, modifiable clinical risk factors should be addressed, as these are more likely to influence treatment decisions compared to genetic testing. Finally, patients may be referred to an outpatient hematologist or geneticist for individualized discussions of risks and benefits of testing for inherited thrombophilia.
CONCLUSION
Inpatient evaluation for inherited thrombophilia for VTE is not clinically useful, cost‐effective, or reliable in the setting of VTE. The result of such testing does not affect management of acute primary or recurrent VTE. Testing should only be considered using an individualized approach in the outpatient setting with appropriate genetic counseling.
Disclosure: Christopher M. Petrilli, MD, and Lauren Heidemann, MD, contributed equally to this work. The authors report no conflicts of interest.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , , , , . Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585–593.
- , , , et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245.
- , . Hereditary thrombophilia. Thromb J. 2006;4:15.
- , , , . Deep‐vein thrombosis. Lancet. 1999;353(9151):479–485.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S–e736S.
- , . Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2009;(1):CD007069.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:CD007069.
- , , , . Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526.
- , , , et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436.
- , . Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699–704.
- , , , , . Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361.
- , , , . Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736.
- , , , . Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986;77(2):416–425.
- , . Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–1239.
- , , , . Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb Res. 1987;45(6):783–790.
- . Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135.
- , , . Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89(12):1147–1150.
- , . Quantitation of human protein S in the plasma of normal and warfarin‐treated individuals by radioimmunoassay. Thromb Res. 1984;36(6):527–535.
- , , , . Social aspects of genetic testing for factor V Leiden mutation in healthy individuals and their importance for daily practice. Thromb Res. 2004;113(1):7–12.
- , . Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013–1020.
- , , . An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120–127.
- CodeMap. Available at: https://www.codemap.com. Accessed January 18, 2016.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67–76.
- , , , et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444.
- , , , et al. Frequency of pregnancy‐related venous thromboembolism in anticoagulant factor‐deficient women: implications for prophylaxis. Ann Intern Med. 1996;125(12):955–960.
- , , , ; Guideline Development Group. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979.
- American Society of Hematology. Ten things physicians and patients should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐society‐of‐hematology. Published December 4, 2013. Accessed January 18, 2016.
- American College of Medical Genetics and Genomics. Five Things patients and providers should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐college‐of‐medical‐genetics‐and‐genomics. Published July 10, 2015. Accessed March 13, 2016.
- , , , , , . Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585–593.
- , , , et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245.
- , . Hereditary thrombophilia. Thromb J. 2006;4:15.
- , , , . Deep‐vein thrombosis. Lancet. 1999;353(9151):479–485.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S–e736S.
- , . Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2009;(1):CD007069.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:CD007069.
- , , , . Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526.
- , , , et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436.
- , . Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699–704.
- , , , , . Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361.
- , , , . Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736.
- , , , . Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986;77(2):416–425.
- , . Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–1239.
- , , , . Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb Res. 1987;45(6):783–790.
- . Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135.
- , , . Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89(12):1147–1150.
- , . Quantitation of human protein S in the plasma of normal and warfarin‐treated individuals by radioimmunoassay. Thromb Res. 1984;36(6):527–535.
- , , , . Social aspects of genetic testing for factor V Leiden mutation in healthy individuals and their importance for daily practice. Thromb Res. 2004;113(1):7–12.
- , . Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013–1020.
- , , . An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120–127.
- CodeMap. Available at: https://www.codemap.com. Accessed January 18, 2016.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67–76.
- , , , et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444.
- , , , et al. Frequency of pregnancy‐related venous thromboembolism in anticoagulant factor‐deficient women: implications for prophylaxis. Ann Intern Med. 1996;125(12):955–960.
- , , , ; Guideline Development Group. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979.
- American Society of Hematology. Ten things physicians and patients should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐society‐of‐hematology. Published December 4, 2013. Accessed January 18, 2016.
- American College of Medical Genetics and Genomics. Five Things patients and providers should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐college‐of‐medical‐genetics‐and‐genomics. Published July 10, 2015. Accessed March 13, 2016.
© 2016 Society of Hospital Medicine
Overtreatment of Nonpurulent Cellulitis
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.
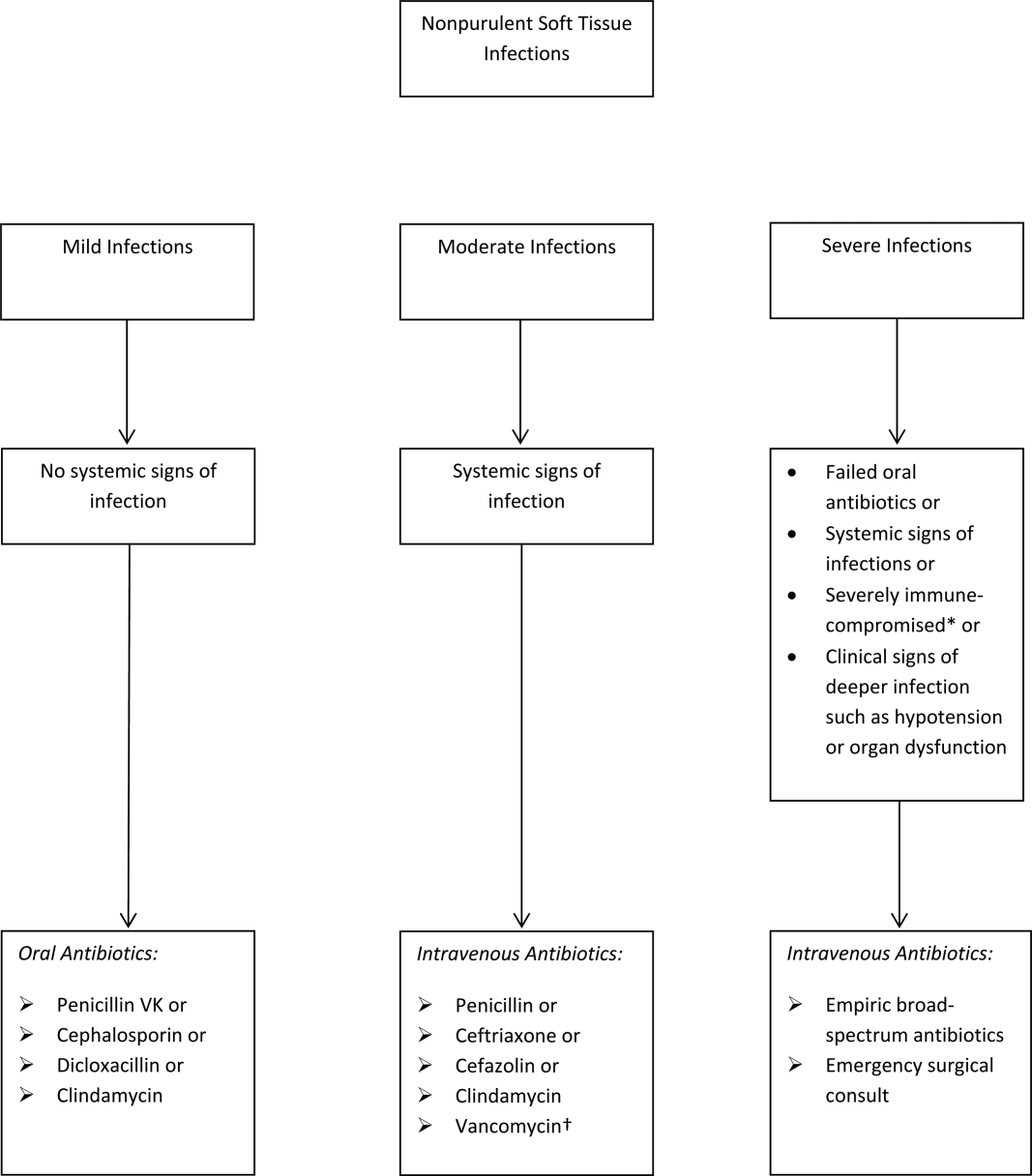
THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.

THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.

THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
© 2016 Society of Hospital Medicine
Prolonged IV Instead of Oral Antibiotics
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A previously healthy 6‐year‐old boy presented to the emergency room with 3 days of right lower leg pain and fevers up to 102F. The leg pain had progressed until he refused to walk. The patient and family did not recall any trauma to the leg. In the emergency department, he had a blood culture drawn. Because he had elevated inflammatory markers and a negative x‐ray of his right leg, a magnetic resonance imaging scan of the right leg was obtained that revealed right tibial osteomyelitis. He was taken to the operating room for debridement. After obtaining blood and bone cultures, he was started on intravenous (IV) vancomycin. His blood and surgical cultures grew methicillin‐resistant Staphylococcus aureus, sensitive to clindamycin. Subsequent blood cultures were negative, and his inflammatory markers trended down shortly after starting therapy. As he clinically improved, a peripherally inserted central catheter (PICC) was placed, and he was discharged home to complete a 6‐week course of IV vancomycin.
BACKGROUND
Osteoarticular infections (osteomyelitis and septic arthritis) are common problems in the pediatric population, affecting 1/2000 children annually and accounting for approximately 1% of all pediatric hospitalizations.[1, 2] Osteomyelitis can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. The most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis), particularly in young children, due to the highly vascular nature of the developing bone. Long bones, such as the femur, tibia, and humerus, are most commonly involved. Treatment of acute osteomyelitis requires prolonged administration of antimicrobial agents. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone.[3]
WHY YOU MIGHT THINK PARENTERAL ANTIBIOTICS AT DISCHARGE IS SUPERIOR TO ENTERAL THERAPY
In the United States, a large proportion of children with hematogenous osteomyelitis are discharged from the hospital with long‐term parenteral intravenous antibiotics through a PICC line.[3] The medical community historically favored parenteral therapy for young children with serious bacterial infections given concerns regarding impaired enteral absorption. As a result, children with osteomyelitis were initially stabilized in the hospital and discharged with parenteral therapy through a PICC line to continue or complete care, even when the organism was susceptible to a viable oral alternative such as clindamycin or cephalexin. Recommendations regarding the safety and timing to transition to oral antibiotics have been lacking. There is also extreme variation in practice in route of administration (oral vs prolonged IV therapy) in patients being discharged from the hospital with osteomyelitis.[3, 4] The most recent Infectious Diseases Society of America (IDSA) guidelines do not clearly state when transition to oral antibiotics may be safe. Specifically, they state that if patients are stable and without ongoing bacteremia, they can transition to oral therapy to complete a 4‐ to 6‐week course.[5]
WHY LONG‐TERM PARENTERAL ANTIBIOTICS MAY NOT BE SUPERIOR
The use of PICC lines has increased substantially in recent years. This has led to an increasing awareness of complications associated with PICC lines. As a result, guidelines for the appropriate use of PICC lines have been established in adults by collaborators at the University of Michigan.[6] Mounting evidence has called into question whether longer parenteral therapy is truly a more conservative or safer approach for the treatment of osteomyelitis.[3, 4, 7, 8, 9] Providing antibiotics via a PICC line in both the inpatient and outpatient settings may not be as benign as once accepted and may not improve outcomes in osteomyelitis as expected.
Costs and Potential Harms Associated With PICC Lines
PICC lines are known to have complications in the hospital including infection and thrombotic events,[10] but these events are not isolated to the hospital setting. Multiple studies have shown outpatient PICC line complication rates ranging from 29% to 41% depending on the type of catheter, the population, and the indication for use.[8, 10, 11, 12, 13] In a recently published study by Keren et al. looking specifically at children with osteomyelitis, emergency department visits and readmissions for PICC line complications occurred in 15% of patients discharged with a PICC line.[4] Given the potential complications and complexity that are inherent in outpatient parenteral therapy, the ISDA has even published guidelines regarding its use.[9] In addition, the cost of IV antibiotics, including administration costs, need for sedation in some children for line placement, and cost of the antibiotic itself, is significantly higher compared to oral therapy. In studies looking at early conversion to oral antibiotics versus prolonged intravenous antibiotics for complicated skin and soft tissue infections, as well as perforated appendicitis, oral antibiotics were more cost effective with an average savings of 30% to 50% and >$4000 respectively.[14, 15]
Patient Outcomes Are Similar When Comparing Parenteral and Enteral Therapy
In addition to increased costs and medication‐related complications, treatment of osteomyelitis with parenteral antibiotics through a PICC line does not improve clinical outcomes. As early as 1997, evidence emerged that an early transition to enteral therapy for osteomyelitis in children may be safe.[16] In 2010, the same group published a larger randomized study with the intent of determining overall treatment duration for osteomyelitis. This study involved 131 culture‐positive cases of osteomyelitis randomized to either a short‐term (20 days) or long‐term (30 days) oral antibiotics following 2 to 4 days of parenteral therapy. In this study, outcomes were favorable and similar despite such a short course of parenteral antibiotics and regardless of the overall treatment duration.[17] Although the aim of this study was not to compare oral and parenteral antibiotics, all patients in this large cohort were treated successfully with early transition to oral therapy.
In 2009, Zaoutis et al. published a large, multicenter, retrospective study of 1969 children with culture‐positive osteomyelitis treated with either prolonged IV therapy (defined as a central line placed before discharge) or oral therapy (no central line placed). They found a 4% incidence of treatment failure in the oral therapy group compared to a 5% incidence in the prolonged IV therapy group. They concluded that early transition to oral therapy was not associated with an increased risk of treatment failure.[3]
More recently, Keren et al. published a comparative effectiveness study using propensity scorebased matching to adjust for confounding variables. This retrospective study included 2060 children without comorbid conditions, ages 2 months to 18 years, with both culture‐positive and culture‐negative acute hematogenous osteomyelitis. Propensity‐based matching used logistic regression to compare patient‐level characteristics including age, race, insurance, length of stay, location of infection, surgical procedures, and isolation of causative pathogens. The rates of treatment failure were nearly identical in the oral therapy (5.0%) and PICC line (6.0%) groups. Similarly, in across‐hospital (risk difference, 0.3% [95% confidence interval {CI}: 0.1% to 2.5%]) and within‐hospital (risk difference, 0.6% [95% CI: 0.2% to 3.0%]) matched analyses, children in the oral therapy group did not have more treatment failures than those in the PICC line group. In the same comparisons, both adverse drug reactions and all treatment‐related events were significantly more likely to occur in children treated with long‐term parenteral antibiotics.[4]
Other studies have looked at the treatment of culture‐negative osteoarticular infections in children and have similarly found favorable outcomes in transitioning to oral therapy after a short course of parenteral treatment.[18]
In short, enteral therapy has similar treatment outcomes for culture‐positive and culture‐negative osteomyelitis without the complications associated with parenteral treatment via a PICC line.
WHEN TO CONSIDER PROLONGED PARENTERAL ANTIBIOTICS
The studies indicating the safe transition to oral antibiotics discussed above all excluded children with certain comorbid conditions. Although this varied from study to study, exclusions were as general in some as not previously healthy, and others were as specific as hematologic malignancies, immunocompromised states, sickle cell disease, malabsorption, and penetrating injuries. Also, although we know blood cultures obtained in children with osteomyelitis are positive in only approximately half of the patients,[19] the studies cited do not contain information for their study populations regarding the duration of bacteremia or endovascular complications, such as septic thrombophlebitis, which are well described in the literature.[20, 21] There are limited data on optimal treatment of children with prolonged bacteremia and endovascular complications. Because studies generally involved previously healthy children and do not specifically address these potential complications, the safety of early oral transition in complicated cases is not clear. The current IDSA and Red Book Committee on Infectious Diseases recommend intravenous therapy for bacteremia and endovascular infections with methicillin‐resistant S aureus.[5, 22] Clinical judgement should be used when treating children with comorbid illnesses who experience persistent bacteremia >48 hours, or who have endovascular complications.
WHAT YOU SHOULD DO INSTEAD
For children with acute hematogenous osteomyelitis who are either culture negative and improve on empiric therapy, or who have culture results (blood or tissue) that are susceptible to a reasonable oral antibiotic agent and who have clinical improvement on initial IV antibiotic therapy, a growing body of evidence indicates that the benefit of early transition to oral antibiotics outweighs the risks of continuing with parenteral therapy. Discharging children on oral antibiotics does not increase their risk of treatment failure but seems to decrease the risk of therapy‐associated complications, including increased healthcare utilization with return visits to the emergency department or the hospital. The possible exceptions to early transition to enteral antibiotics are prolonged bacteremia or endovascular infection, though there are insufficient data in the literature indicating benefits or risks of one administration route over the other.
RECOMMENDATIONS
- Previously healthy children with acute hematogenous osteomyelitis, without endovascular complications, should be transitioned to enteral antibiotics when they are showing signs of clinical improvement, as defined by: resolution of fever, improving physical exam, ability to take oral medications, and decreasing C‐reactive protein.
- The choice of oral antibiotics should be based on the organism's antibiotic susceptibility. If cultures are negative and the child has improved on empiric IV therapy, transition to an oral regimen with similar spectrum is acceptable.
- Patients with acute osteomyelitis should have close follow‐up after discharge from the hospital, within 1 to 2 weeks, to ensure continued improvement on therapy.
CONCLUSION
Early transition to oral antibiotics should be used in children with acute, uncomplicated osteomyelitis. A growing body of evidence shows that early transition to oral antibiotics does not increase the risk of treatment failure and can obviate the need for an outpatient PICC line. Oral antibiotics do not carry the risk of potential complications and complexity that are inherent in outpatient parenteral therapy. The transition to oral therapy should occur prior to discharge from the hospital after clinical improvement. Close follow‐up is essential to ensure successful treatment in children with acute osteomyelitis.
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . Osteomyelitis. In: Feigin RD, Cherry JD, Kaplan, SL, Demmler‐Harrison, GJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders Elsevier; 2009.
- . Osteomyelitis in children. Curr Opin Pediatr. 2002;14:112–115.
- , , , , , . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642.
- , , , et al.; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292.
- , , , et al.; Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , . Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9(9):604–609.
- , , , , , . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117:1210–1215.
- , , , et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651–1672.
- , , , . Frequency of Peripherally Inserted Central Catheter Complications in Children. Pediatr Infect Dis J. 2012;31(5):519–521.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , , . Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2007;46:247–251.
- , , . Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Pediatr Infect Dis J. 2008;27:1069–1072.
- , , , , , . Economic burden of inpatient and outpatient antibiotic treatment for methicillin‐resistant Staphylococcus aureus complicated skin and soft‐tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- , , , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–143.
- , , . Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850.
- , , , ; Osteomyelitis‐Septic Arthritis Study Group. Short‐ versus long‐term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture‐positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128.
- , , , . Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Ped Infect Dis. 2013;2(2):119–125.
- , . Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435.
- , , , . Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr. 2006;149(4):537–541.
- , , , et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679.
- , , , . Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A previously healthy 6‐year‐old boy presented to the emergency room with 3 days of right lower leg pain and fevers up to 102F. The leg pain had progressed until he refused to walk. The patient and family did not recall any trauma to the leg. In the emergency department, he had a blood culture drawn. Because he had elevated inflammatory markers and a negative x‐ray of his right leg, a magnetic resonance imaging scan of the right leg was obtained that revealed right tibial osteomyelitis. He was taken to the operating room for debridement. After obtaining blood and bone cultures, he was started on intravenous (IV) vancomycin. His blood and surgical cultures grew methicillin‐resistant Staphylococcus aureus, sensitive to clindamycin. Subsequent blood cultures were negative, and his inflammatory markers trended down shortly after starting therapy. As he clinically improved, a peripherally inserted central catheter (PICC) was placed, and he was discharged home to complete a 6‐week course of IV vancomycin.
BACKGROUND
Osteoarticular infections (osteomyelitis and septic arthritis) are common problems in the pediatric population, affecting 1/2000 children annually and accounting for approximately 1% of all pediatric hospitalizations.[1, 2] Osteomyelitis can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. The most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis), particularly in young children, due to the highly vascular nature of the developing bone. Long bones, such as the femur, tibia, and humerus, are most commonly involved. Treatment of acute osteomyelitis requires prolonged administration of antimicrobial agents. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone.[3]
WHY YOU MIGHT THINK PARENTERAL ANTIBIOTICS AT DISCHARGE IS SUPERIOR TO ENTERAL THERAPY
In the United States, a large proportion of children with hematogenous osteomyelitis are discharged from the hospital with long‐term parenteral intravenous antibiotics through a PICC line.[3] The medical community historically favored parenteral therapy for young children with serious bacterial infections given concerns regarding impaired enteral absorption. As a result, children with osteomyelitis were initially stabilized in the hospital and discharged with parenteral therapy through a PICC line to continue or complete care, even when the organism was susceptible to a viable oral alternative such as clindamycin or cephalexin. Recommendations regarding the safety and timing to transition to oral antibiotics have been lacking. There is also extreme variation in practice in route of administration (oral vs prolonged IV therapy) in patients being discharged from the hospital with osteomyelitis.[3, 4] The most recent Infectious Diseases Society of America (IDSA) guidelines do not clearly state when transition to oral antibiotics may be safe. Specifically, they state that if patients are stable and without ongoing bacteremia, they can transition to oral therapy to complete a 4‐ to 6‐week course.[5]
WHY LONG‐TERM PARENTERAL ANTIBIOTICS MAY NOT BE SUPERIOR
The use of PICC lines has increased substantially in recent years. This has led to an increasing awareness of complications associated with PICC lines. As a result, guidelines for the appropriate use of PICC lines have been established in adults by collaborators at the University of Michigan.[6] Mounting evidence has called into question whether longer parenteral therapy is truly a more conservative or safer approach for the treatment of osteomyelitis.[3, 4, 7, 8, 9] Providing antibiotics via a PICC line in both the inpatient and outpatient settings may not be as benign as once accepted and may not improve outcomes in osteomyelitis as expected.
Costs and Potential Harms Associated With PICC Lines
PICC lines are known to have complications in the hospital including infection and thrombotic events,[10] but these events are not isolated to the hospital setting. Multiple studies have shown outpatient PICC line complication rates ranging from 29% to 41% depending on the type of catheter, the population, and the indication for use.[8, 10, 11, 12, 13] In a recently published study by Keren et al. looking specifically at children with osteomyelitis, emergency department visits and readmissions for PICC line complications occurred in 15% of patients discharged with a PICC line.[4] Given the potential complications and complexity that are inherent in outpatient parenteral therapy, the ISDA has even published guidelines regarding its use.[9] In addition, the cost of IV antibiotics, including administration costs, need for sedation in some children for line placement, and cost of the antibiotic itself, is significantly higher compared to oral therapy. In studies looking at early conversion to oral antibiotics versus prolonged intravenous antibiotics for complicated skin and soft tissue infections, as well as perforated appendicitis, oral antibiotics were more cost effective with an average savings of 30% to 50% and >$4000 respectively.[14, 15]
Patient Outcomes Are Similar When Comparing Parenteral and Enteral Therapy
In addition to increased costs and medication‐related complications, treatment of osteomyelitis with parenteral antibiotics through a PICC line does not improve clinical outcomes. As early as 1997, evidence emerged that an early transition to enteral therapy for osteomyelitis in children may be safe.[16] In 2010, the same group published a larger randomized study with the intent of determining overall treatment duration for osteomyelitis. This study involved 131 culture‐positive cases of osteomyelitis randomized to either a short‐term (20 days) or long‐term (30 days) oral antibiotics following 2 to 4 days of parenteral therapy. In this study, outcomes were favorable and similar despite such a short course of parenteral antibiotics and regardless of the overall treatment duration.[17] Although the aim of this study was not to compare oral and parenteral antibiotics, all patients in this large cohort were treated successfully with early transition to oral therapy.
In 2009, Zaoutis et al. published a large, multicenter, retrospective study of 1969 children with culture‐positive osteomyelitis treated with either prolonged IV therapy (defined as a central line placed before discharge) or oral therapy (no central line placed). They found a 4% incidence of treatment failure in the oral therapy group compared to a 5% incidence in the prolonged IV therapy group. They concluded that early transition to oral therapy was not associated with an increased risk of treatment failure.[3]
More recently, Keren et al. published a comparative effectiveness study using propensity scorebased matching to adjust for confounding variables. This retrospective study included 2060 children without comorbid conditions, ages 2 months to 18 years, with both culture‐positive and culture‐negative acute hematogenous osteomyelitis. Propensity‐based matching used logistic regression to compare patient‐level characteristics including age, race, insurance, length of stay, location of infection, surgical procedures, and isolation of causative pathogens. The rates of treatment failure were nearly identical in the oral therapy (5.0%) and PICC line (6.0%) groups. Similarly, in across‐hospital (risk difference, 0.3% [95% confidence interval {CI}: 0.1% to 2.5%]) and within‐hospital (risk difference, 0.6% [95% CI: 0.2% to 3.0%]) matched analyses, children in the oral therapy group did not have more treatment failures than those in the PICC line group. In the same comparisons, both adverse drug reactions and all treatment‐related events were significantly more likely to occur in children treated with long‐term parenteral antibiotics.[4]
Other studies have looked at the treatment of culture‐negative osteoarticular infections in children and have similarly found favorable outcomes in transitioning to oral therapy after a short course of parenteral treatment.[18]
In short, enteral therapy has similar treatment outcomes for culture‐positive and culture‐negative osteomyelitis without the complications associated with parenteral treatment via a PICC line.
WHEN TO CONSIDER PROLONGED PARENTERAL ANTIBIOTICS
The studies indicating the safe transition to oral antibiotics discussed above all excluded children with certain comorbid conditions. Although this varied from study to study, exclusions were as general in some as not previously healthy, and others were as specific as hematologic malignancies, immunocompromised states, sickle cell disease, malabsorption, and penetrating injuries. Also, although we know blood cultures obtained in children with osteomyelitis are positive in only approximately half of the patients,[19] the studies cited do not contain information for their study populations regarding the duration of bacteremia or endovascular complications, such as septic thrombophlebitis, which are well described in the literature.[20, 21] There are limited data on optimal treatment of children with prolonged bacteremia and endovascular complications. Because studies generally involved previously healthy children and do not specifically address these potential complications, the safety of early oral transition in complicated cases is not clear. The current IDSA and Red Book Committee on Infectious Diseases recommend intravenous therapy for bacteremia and endovascular infections with methicillin‐resistant S aureus.[5, 22] Clinical judgement should be used when treating children with comorbid illnesses who experience persistent bacteremia >48 hours, or who have endovascular complications.
WHAT YOU SHOULD DO INSTEAD
For children with acute hematogenous osteomyelitis who are either culture negative and improve on empiric therapy, or who have culture results (blood or tissue) that are susceptible to a reasonable oral antibiotic agent and who have clinical improvement on initial IV antibiotic therapy, a growing body of evidence indicates that the benefit of early transition to oral antibiotics outweighs the risks of continuing with parenteral therapy. Discharging children on oral antibiotics does not increase their risk of treatment failure but seems to decrease the risk of therapy‐associated complications, including increased healthcare utilization with return visits to the emergency department or the hospital. The possible exceptions to early transition to enteral antibiotics are prolonged bacteremia or endovascular infection, though there are insufficient data in the literature indicating benefits or risks of one administration route over the other.
RECOMMENDATIONS
- Previously healthy children with acute hematogenous osteomyelitis, without endovascular complications, should be transitioned to enteral antibiotics when they are showing signs of clinical improvement, as defined by: resolution of fever, improving physical exam, ability to take oral medications, and decreasing C‐reactive protein.
- The choice of oral antibiotics should be based on the organism's antibiotic susceptibility. If cultures are negative and the child has improved on empiric IV therapy, transition to an oral regimen with similar spectrum is acceptable.
- Patients with acute osteomyelitis should have close follow‐up after discharge from the hospital, within 1 to 2 weeks, to ensure continued improvement on therapy.
CONCLUSION
Early transition to oral antibiotics should be used in children with acute, uncomplicated osteomyelitis. A growing body of evidence shows that early transition to oral antibiotics does not increase the risk of treatment failure and can obviate the need for an outpatient PICC line. Oral antibiotics do not carry the risk of potential complications and complexity that are inherent in outpatient parenteral therapy. The transition to oral therapy should occur prior to discharge from the hospital after clinical improvement. Close follow‐up is essential to ensure successful treatment in children with acute osteomyelitis.
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A previously healthy 6‐year‐old boy presented to the emergency room with 3 days of right lower leg pain and fevers up to 102F. The leg pain had progressed until he refused to walk. The patient and family did not recall any trauma to the leg. In the emergency department, he had a blood culture drawn. Because he had elevated inflammatory markers and a negative x‐ray of his right leg, a magnetic resonance imaging scan of the right leg was obtained that revealed right tibial osteomyelitis. He was taken to the operating room for debridement. After obtaining blood and bone cultures, he was started on intravenous (IV) vancomycin. His blood and surgical cultures grew methicillin‐resistant Staphylococcus aureus, sensitive to clindamycin. Subsequent blood cultures were negative, and his inflammatory markers trended down shortly after starting therapy. As he clinically improved, a peripherally inserted central catheter (PICC) was placed, and he was discharged home to complete a 6‐week course of IV vancomycin.
BACKGROUND
Osteoarticular infections (osteomyelitis and septic arthritis) are common problems in the pediatric population, affecting 1/2000 children annually and accounting for approximately 1% of all pediatric hospitalizations.[1, 2] Osteomyelitis can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. The most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis), particularly in young children, due to the highly vascular nature of the developing bone. Long bones, such as the femur, tibia, and humerus, are most commonly involved. Treatment of acute osteomyelitis requires prolonged administration of antimicrobial agents. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone.[3]
WHY YOU MIGHT THINK PARENTERAL ANTIBIOTICS AT DISCHARGE IS SUPERIOR TO ENTERAL THERAPY
In the United States, a large proportion of children with hematogenous osteomyelitis are discharged from the hospital with long‐term parenteral intravenous antibiotics through a PICC line.[3] The medical community historically favored parenteral therapy for young children with serious bacterial infections given concerns regarding impaired enteral absorption. As a result, children with osteomyelitis were initially stabilized in the hospital and discharged with parenteral therapy through a PICC line to continue or complete care, even when the organism was susceptible to a viable oral alternative such as clindamycin or cephalexin. Recommendations regarding the safety and timing to transition to oral antibiotics have been lacking. There is also extreme variation in practice in route of administration (oral vs prolonged IV therapy) in patients being discharged from the hospital with osteomyelitis.[3, 4] The most recent Infectious Diseases Society of America (IDSA) guidelines do not clearly state when transition to oral antibiotics may be safe. Specifically, they state that if patients are stable and without ongoing bacteremia, they can transition to oral therapy to complete a 4‐ to 6‐week course.[5]
WHY LONG‐TERM PARENTERAL ANTIBIOTICS MAY NOT BE SUPERIOR
The use of PICC lines has increased substantially in recent years. This has led to an increasing awareness of complications associated with PICC lines. As a result, guidelines for the appropriate use of PICC lines have been established in adults by collaborators at the University of Michigan.[6] Mounting evidence has called into question whether longer parenteral therapy is truly a more conservative or safer approach for the treatment of osteomyelitis.[3, 4, 7, 8, 9] Providing antibiotics via a PICC line in both the inpatient and outpatient settings may not be as benign as once accepted and may not improve outcomes in osteomyelitis as expected.
Costs and Potential Harms Associated With PICC Lines
PICC lines are known to have complications in the hospital including infection and thrombotic events,[10] but these events are not isolated to the hospital setting. Multiple studies have shown outpatient PICC line complication rates ranging from 29% to 41% depending on the type of catheter, the population, and the indication for use.[8, 10, 11, 12, 13] In a recently published study by Keren et al. looking specifically at children with osteomyelitis, emergency department visits and readmissions for PICC line complications occurred in 15% of patients discharged with a PICC line.[4] Given the potential complications and complexity that are inherent in outpatient parenteral therapy, the ISDA has even published guidelines regarding its use.[9] In addition, the cost of IV antibiotics, including administration costs, need for sedation in some children for line placement, and cost of the antibiotic itself, is significantly higher compared to oral therapy. In studies looking at early conversion to oral antibiotics versus prolonged intravenous antibiotics for complicated skin and soft tissue infections, as well as perforated appendicitis, oral antibiotics were more cost effective with an average savings of 30% to 50% and >$4000 respectively.[14, 15]
Patient Outcomes Are Similar When Comparing Parenteral and Enteral Therapy
In addition to increased costs and medication‐related complications, treatment of osteomyelitis with parenteral antibiotics through a PICC line does not improve clinical outcomes. As early as 1997, evidence emerged that an early transition to enteral therapy for osteomyelitis in children may be safe.[16] In 2010, the same group published a larger randomized study with the intent of determining overall treatment duration for osteomyelitis. This study involved 131 culture‐positive cases of osteomyelitis randomized to either a short‐term (20 days) or long‐term (30 days) oral antibiotics following 2 to 4 days of parenteral therapy. In this study, outcomes were favorable and similar despite such a short course of parenteral antibiotics and regardless of the overall treatment duration.[17] Although the aim of this study was not to compare oral and parenteral antibiotics, all patients in this large cohort were treated successfully with early transition to oral therapy.
In 2009, Zaoutis et al. published a large, multicenter, retrospective study of 1969 children with culture‐positive osteomyelitis treated with either prolonged IV therapy (defined as a central line placed before discharge) or oral therapy (no central line placed). They found a 4% incidence of treatment failure in the oral therapy group compared to a 5% incidence in the prolonged IV therapy group. They concluded that early transition to oral therapy was not associated with an increased risk of treatment failure.[3]
More recently, Keren et al. published a comparative effectiveness study using propensity scorebased matching to adjust for confounding variables. This retrospective study included 2060 children without comorbid conditions, ages 2 months to 18 years, with both culture‐positive and culture‐negative acute hematogenous osteomyelitis. Propensity‐based matching used logistic regression to compare patient‐level characteristics including age, race, insurance, length of stay, location of infection, surgical procedures, and isolation of causative pathogens. The rates of treatment failure were nearly identical in the oral therapy (5.0%) and PICC line (6.0%) groups. Similarly, in across‐hospital (risk difference, 0.3% [95% confidence interval {CI}: 0.1% to 2.5%]) and within‐hospital (risk difference, 0.6% [95% CI: 0.2% to 3.0%]) matched analyses, children in the oral therapy group did not have more treatment failures than those in the PICC line group. In the same comparisons, both adverse drug reactions and all treatment‐related events were significantly more likely to occur in children treated with long‐term parenteral antibiotics.[4]
Other studies have looked at the treatment of culture‐negative osteoarticular infections in children and have similarly found favorable outcomes in transitioning to oral therapy after a short course of parenteral treatment.[18]
In short, enteral therapy has similar treatment outcomes for culture‐positive and culture‐negative osteomyelitis without the complications associated with parenteral treatment via a PICC line.
WHEN TO CONSIDER PROLONGED PARENTERAL ANTIBIOTICS
The studies indicating the safe transition to oral antibiotics discussed above all excluded children with certain comorbid conditions. Although this varied from study to study, exclusions were as general in some as not previously healthy, and others were as specific as hematologic malignancies, immunocompromised states, sickle cell disease, malabsorption, and penetrating injuries. Also, although we know blood cultures obtained in children with osteomyelitis are positive in only approximately half of the patients,[19] the studies cited do not contain information for their study populations regarding the duration of bacteremia or endovascular complications, such as septic thrombophlebitis, which are well described in the literature.[20, 21] There are limited data on optimal treatment of children with prolonged bacteremia and endovascular complications. Because studies generally involved previously healthy children and do not specifically address these potential complications, the safety of early oral transition in complicated cases is not clear. The current IDSA and Red Book Committee on Infectious Diseases recommend intravenous therapy for bacteremia and endovascular infections with methicillin‐resistant S aureus.[5, 22] Clinical judgement should be used when treating children with comorbid illnesses who experience persistent bacteremia >48 hours, or who have endovascular complications.
WHAT YOU SHOULD DO INSTEAD
For children with acute hematogenous osteomyelitis who are either culture negative and improve on empiric therapy, or who have culture results (blood or tissue) that are susceptible to a reasonable oral antibiotic agent and who have clinical improvement on initial IV antibiotic therapy, a growing body of evidence indicates that the benefit of early transition to oral antibiotics outweighs the risks of continuing with parenteral therapy. Discharging children on oral antibiotics does not increase their risk of treatment failure but seems to decrease the risk of therapy‐associated complications, including increased healthcare utilization with return visits to the emergency department or the hospital. The possible exceptions to early transition to enteral antibiotics are prolonged bacteremia or endovascular infection, though there are insufficient data in the literature indicating benefits or risks of one administration route over the other.
RECOMMENDATIONS
- Previously healthy children with acute hematogenous osteomyelitis, without endovascular complications, should be transitioned to enteral antibiotics when they are showing signs of clinical improvement, as defined by: resolution of fever, improving physical exam, ability to take oral medications, and decreasing C‐reactive protein.
- The choice of oral antibiotics should be based on the organism's antibiotic susceptibility. If cultures are negative and the child has improved on empiric IV therapy, transition to an oral regimen with similar spectrum is acceptable.
- Patients with acute osteomyelitis should have close follow‐up after discharge from the hospital, within 1 to 2 weeks, to ensure continued improvement on therapy.
CONCLUSION
Early transition to oral antibiotics should be used in children with acute, uncomplicated osteomyelitis. A growing body of evidence shows that early transition to oral antibiotics does not increase the risk of treatment failure and can obviate the need for an outpatient PICC line. Oral antibiotics do not carry the risk of potential complications and complexity that are inherent in outpatient parenteral therapy. The transition to oral therapy should occur prior to discharge from the hospital after clinical improvement. Close follow‐up is essential to ensure successful treatment in children with acute osteomyelitis.
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . Osteomyelitis. In: Feigin RD, Cherry JD, Kaplan, SL, Demmler‐Harrison, GJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders Elsevier; 2009.
- . Osteomyelitis in children. Curr Opin Pediatr. 2002;14:112–115.
- , , , , , . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642.
- , , , et al.; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292.
- , , , et al.; Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , . Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9(9):604–609.
- , , , , , . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117:1210–1215.
- , , , et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651–1672.
- , , , . Frequency of Peripherally Inserted Central Catheter Complications in Children. Pediatr Infect Dis J. 2012;31(5):519–521.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , , . Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2007;46:247–251.
- , , . Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Pediatr Infect Dis J. 2008;27:1069–1072.
- , , , , , . Economic burden of inpatient and outpatient antibiotic treatment for methicillin‐resistant Staphylococcus aureus complicated skin and soft‐tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- , , , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–143.
- , , . Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850.
- , , , ; Osteomyelitis‐Septic Arthritis Study Group. Short‐ versus long‐term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture‐positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128.
- , , , . Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Ped Infect Dis. 2013;2(2):119–125.
- , . Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435.
- , , , . Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr. 2006;149(4):537–541.
- , , , et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679.
- , , , . Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
- . Osteomyelitis. In: Feigin RD, Cherry JD, Kaplan, SL, Demmler‐Harrison, GJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders Elsevier; 2009.
- . Osteomyelitis in children. Curr Opin Pediatr. 2002;14:112–115.
- , , , , , . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642.
- , , , et al.; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292.
- , , , et al.; Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , . Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9(9):604–609.
- , , , , , . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117:1210–1215.
- , , , et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651–1672.
- , , , . Frequency of Peripherally Inserted Central Catheter Complications in Children. Pediatr Infect Dis J. 2012;31(5):519–521.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , , . Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2007;46:247–251.
- , , . Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Pediatr Infect Dis J. 2008;27:1069–1072.
- , , , , , . Economic burden of inpatient and outpatient antibiotic treatment for methicillin‐resistant Staphylococcus aureus complicated skin and soft‐tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- , , , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–143.
- , , . Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850.
- , , , ; Osteomyelitis‐Septic Arthritis Study Group. Short‐ versus long‐term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture‐positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128.
- , , , . Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Ped Infect Dis. 2013;2(2):119–125.
- , . Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435.
- , , , . Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr. 2006;149(4):537–541.
- , , , et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679.
- , , , . Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
© 2016 Society of Hospital Medicine
Shellfish Allergies and CT Scans
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 55‐year‐old patient with a history of chronic obstructive pulmonary disease and diabetes mellitus presented to the emergency room with acute shortness of breath and right leg swelling that began 1 week after lumbar disk surgery. The emergency department team decides against ordering a chest CT scan with contrast to evaluate for a possible pulmonary embolism after noting that the patient's allergies include shellfish, which cause urticaria and facial edema. A ventilation‐perfusion scan reveals heterogeneous perfusion defects consistent with an intermediate probability (20%80%) for pulmonary embolism. The treating physicians consider starting the patient on a steroid regimen to prepare him for a CT scan with IV contrast, while presumptively anticoagulating the patient for 24 hours in order for the steroids to provide maximal protective effect before obtaining the scan. Should a history of shellfish allergy affect decision making regarding whether to administer IV contrast?
WHY YOU MIGHT THINK ASKING ABOUT SHELLFISH ALLERGIES BEFORE PERFORMING CONTRAST‐ENHANCED CT SCANS IS HELPFUL
Fish and shellfish contain iodine, and allergic reactions to seafood are quite common, with a prevalence ranging anywhere between 2% and 6% of the population.[1] As a result, patients with suspected shellfish allergies are often told by providers that they are allergic to iodine. In 1 study, nearly 92% of patients presenting to a pediatrics clinic with a suspected seafood or shellfish allergy cited iodine as the culprit.[2] As contrast‐enhanced CT scans utilize a variety of iodine‐based agents, patients are often told to avoid CT scans with iodinated contrast agents or receive corticosteroid/antihistamine premedications prior to undergoing CT scans to mitigate potentially life‐threatening allergic reactions. A survey of radiologists and interventional cardiologists revealed that 65.3% and 88.9%, respectively, asked about seafood or shellfish allergies prior to administering contrast enhanced CT scans, and 34.7% and 50.0%, respectively, stated that they would withhold contrast media or recommend premedication with corticosteroid/antihistamines for patients with seafood or shellfish allergy.[2]
WHY ASKING ABOUT SHELLFISH ALLERGIES BEFORE IV CONTRAST CT SCANS DOES NOT REDUCE THE RISK OF CONTRAST REACTIONS
What Causes Allergic‐Like Reactions to Fish and Shellfish?
Allergic reactions are inappropriate or exaggerated immune response (hypersensitivity reaction). Four types of hypersensitivity reactions have been described (type IIV)[3]; allergic reactions mediated by immunoglobulin E (IgE) represent type I hypersensitivity reactions.
Although fish and shellfish contain iodine, so too do a wide variety of commonly consumed foods (eg, yogurt, milk, bread). In addition, our bodies contain and require sufficient quantities of iodine for basic functions, making immune reactions to such an essential ingredient of life unlikely. Instead, fish and shellfish contain proteins (parvalbumin and tropomyosins, respectively), which act as the major allergens, not iodine.[4]
What Causes Reactions to IV Contrast Media?
Around the world, tens of millions of injections occur every year for contrast‐enhanced scans.[5] Reactions to IV contrast media are not uncommon, occurring anywhere between 0.6% and 17% of the time, with severe reactions occurring between 0.02% and 0.5% of the time.[6] Higher reaction rates were associated with the use of higher‐osmolarity contrast agents. A review of research studies found a lower rate of reactions to IV contrast in eras in which low‐osmolarity agents were exclusively used (0.2% after 1991) versus eras in which high‐osmolarity agents were exclusively used (7.0% between 1985 and 1986).[7]
Reactions to contrast include allergic‐like reactions as well as a variety of other reactions (eg, arrhythmias, vasovagal reactions, flushing), which are thought to be related to the dose and concentration of contrast media.[8]
Allergic‐like, or anaphylactoid, reactions related to contrast are largely thought to have a fundamentally different molecular mechanism than true classic allergic reactions. Anaphylactoid reactions are caused by direct release of histamine into the bloodstream in response to interacting with chemicals. These reactions are not related to or mediated by IgE antibodies and do not require prior exposure.
True classic allergic reactions, on the other hand, are mediated by IgE antibodies in which initial exposure to an allergen (antigen) is followed by subsequent exposure and production of IgE antibodies.[9] The allergenIgE antibody complex causes the degranulation of mast cells and basophils, leading to the release of histamines.
Reactions to IV contrast are likely related to some component of the contrast media instead of the iodine itself. It is thought that the majority of these reactions are anaphylactoid reactions instead of true classic allergic reactions, given that IgE antibodies are not consistently elevated in patients who exhibit these reactions.[8] Nevertheless, the symptoms of these 2 types of reactions (anaphylactoid and allergic reactions) are similar and require comparable treatment to prevent life‐threatening anaphylaxis.
What Are the Major Risk Factors for Allergic‐Like Contrast Reactions?
Previous studies on risk factors for allergic‐like contrast reactions suggest that the strongest predictor of future contrast reactions is a history of prior contrast reaction (5‐fold higher risk), with an estimated 10% to 35% recurrence risk of contrast reactions.[8] Patients with a history of atopy, asthma, and food allergies (including seafood) are at approximately 2 to 3 times greater risk of contrast reactions.[9]
Do Shellfish Allergies Place Patients at Higher Risk for Contrast Reactions Than Other Allergies?
In 1 of the few studies evaluating seafood allergies specifically, Witten et al. compared the frequency of contrast reactions in patients with histories of seafood allergy, food allergy, asthma, hay fever, hives, and contrast medium.[10] Using their results, we compared the frequency of reactions in patients with histories of seafood allergy (6.3%, 4/64) to patients with any other type of allergy or atopic state (9.2%, 212/2304) and found no statistically significant differences (P = 0.418). Similarly, Shehadi evaluated seafood as well as asthma, hay fever, common medications (eg, penicillin, aspirin, morphine), and others.[11] A reanalysis of the results found no statistically significant differences comparing the frequencies of contrast reactions in patients with seafood allergy (15.0%) compared with other allergens (eggs, milk and chocolate, 14.6%; general allergies, 13.1%; fruit allergies, 12.9%; asthma, 11.2%; P values ranging between 0.2 and 0.6).[6] Overall, the results suggest that patients with seafood allergy are at no higher risk for having a contrast reaction compared with patients with other food allergies or other forms of atopy.
Additionally, seafood and other food allergies should be distinguished from food intolerances in which the ingestion of histamine‐rich materials in conjunction with histamine inhibitors (drugs or alcohol) leads to symptoms that can mimic allergic‐like reactions (urticaria, pruritus, diarrhea, asthma).[12]
What Do the Guidelines Recommend?
For patients who require IV contrast media for CT scans, the American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering lowiso‐osmolar radiocontrast media or pretreating with corticosteroids and antihistamines for patients with a history of seafood allergy.[13] The American College of Radiology recommends pretreatment with corticosteroids only for those patients who have previously experienced moderate to severe reactions to IV contrast.[8]
WHAT YOU SHOULD DO INSTEAD: ASK ABOUT PRIOR CONTRAST REACTIONS BEFORE ADMINISTERING CONTRAST
When a patient presents for a contrast‐enhanced CT scan, patients should be asked if they have experienced reactions to contrast and the severity and type of the associated reactions. Providers and support staff should not ask specifically about shellfish allergies, as they have not been found to be associated with an elevated risk of contrast reactions compared with other allergens. Although all allergies seem to increase the likelihood of having a reaction to contrast, only a history of previous contrast reactions will prompt a change in management. Asking specifically about seafood allergies before performing an IV contrast CT scan is a Thing We Do for No Reason.
RECOMMENDATIONS
- Before performing contrast‐enhanced CT scans, patients should be asked if they have experienced reactions to IV contrast. There is no reason for providers and support staff to specifically inquire about seafood allergies.
- Patients with seafood and other food allergies do not require premedication prior to CT scans. Seafood and other food allergies do not represent contraindications to obtaining contrast‐enhanced CT scans and should not prompt a change in management.
Disclosures
The authors do not have any relevant financial disclosures to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 55‐year‐old patient with a history of chronic obstructive pulmonary disease and diabetes mellitus presented to the emergency room with acute shortness of breath and right leg swelling that began 1 week after lumbar disk surgery. The emergency department team decides against ordering a chest CT scan with contrast to evaluate for a possible pulmonary embolism after noting that the patient's allergies include shellfish, which cause urticaria and facial edema. A ventilation‐perfusion scan reveals heterogeneous perfusion defects consistent with an intermediate probability (20%80%) for pulmonary embolism. The treating physicians consider starting the patient on a steroid regimen to prepare him for a CT scan with IV contrast, while presumptively anticoagulating the patient for 24 hours in order for the steroids to provide maximal protective effect before obtaining the scan. Should a history of shellfish allergy affect decision making regarding whether to administer IV contrast?
WHY YOU MIGHT THINK ASKING ABOUT SHELLFISH ALLERGIES BEFORE PERFORMING CONTRAST‐ENHANCED CT SCANS IS HELPFUL
Fish and shellfish contain iodine, and allergic reactions to seafood are quite common, with a prevalence ranging anywhere between 2% and 6% of the population.[1] As a result, patients with suspected shellfish allergies are often told by providers that they are allergic to iodine. In 1 study, nearly 92% of patients presenting to a pediatrics clinic with a suspected seafood or shellfish allergy cited iodine as the culprit.[2] As contrast‐enhanced CT scans utilize a variety of iodine‐based agents, patients are often told to avoid CT scans with iodinated contrast agents or receive corticosteroid/antihistamine premedications prior to undergoing CT scans to mitigate potentially life‐threatening allergic reactions. A survey of radiologists and interventional cardiologists revealed that 65.3% and 88.9%, respectively, asked about seafood or shellfish allergies prior to administering contrast enhanced CT scans, and 34.7% and 50.0%, respectively, stated that they would withhold contrast media or recommend premedication with corticosteroid/antihistamines for patients with seafood or shellfish allergy.[2]
WHY ASKING ABOUT SHELLFISH ALLERGIES BEFORE IV CONTRAST CT SCANS DOES NOT REDUCE THE RISK OF CONTRAST REACTIONS
What Causes Allergic‐Like Reactions to Fish and Shellfish?
Allergic reactions are inappropriate or exaggerated immune response (hypersensitivity reaction). Four types of hypersensitivity reactions have been described (type IIV)[3]; allergic reactions mediated by immunoglobulin E (IgE) represent type I hypersensitivity reactions.
Although fish and shellfish contain iodine, so too do a wide variety of commonly consumed foods (eg, yogurt, milk, bread). In addition, our bodies contain and require sufficient quantities of iodine for basic functions, making immune reactions to such an essential ingredient of life unlikely. Instead, fish and shellfish contain proteins (parvalbumin and tropomyosins, respectively), which act as the major allergens, not iodine.[4]
What Causes Reactions to IV Contrast Media?
Around the world, tens of millions of injections occur every year for contrast‐enhanced scans.[5] Reactions to IV contrast media are not uncommon, occurring anywhere between 0.6% and 17% of the time, with severe reactions occurring between 0.02% and 0.5% of the time.[6] Higher reaction rates were associated with the use of higher‐osmolarity contrast agents. A review of research studies found a lower rate of reactions to IV contrast in eras in which low‐osmolarity agents were exclusively used (0.2% after 1991) versus eras in which high‐osmolarity agents were exclusively used (7.0% between 1985 and 1986).[7]
Reactions to contrast include allergic‐like reactions as well as a variety of other reactions (eg, arrhythmias, vasovagal reactions, flushing), which are thought to be related to the dose and concentration of contrast media.[8]
Allergic‐like, or anaphylactoid, reactions related to contrast are largely thought to have a fundamentally different molecular mechanism than true classic allergic reactions. Anaphylactoid reactions are caused by direct release of histamine into the bloodstream in response to interacting with chemicals. These reactions are not related to or mediated by IgE antibodies and do not require prior exposure.
True classic allergic reactions, on the other hand, are mediated by IgE antibodies in which initial exposure to an allergen (antigen) is followed by subsequent exposure and production of IgE antibodies.[9] The allergenIgE antibody complex causes the degranulation of mast cells and basophils, leading to the release of histamines.
Reactions to IV contrast are likely related to some component of the contrast media instead of the iodine itself. It is thought that the majority of these reactions are anaphylactoid reactions instead of true classic allergic reactions, given that IgE antibodies are not consistently elevated in patients who exhibit these reactions.[8] Nevertheless, the symptoms of these 2 types of reactions (anaphylactoid and allergic reactions) are similar and require comparable treatment to prevent life‐threatening anaphylaxis.
What Are the Major Risk Factors for Allergic‐Like Contrast Reactions?
Previous studies on risk factors for allergic‐like contrast reactions suggest that the strongest predictor of future contrast reactions is a history of prior contrast reaction (5‐fold higher risk), with an estimated 10% to 35% recurrence risk of contrast reactions.[8] Patients with a history of atopy, asthma, and food allergies (including seafood) are at approximately 2 to 3 times greater risk of contrast reactions.[9]
Do Shellfish Allergies Place Patients at Higher Risk for Contrast Reactions Than Other Allergies?
In 1 of the few studies evaluating seafood allergies specifically, Witten et al. compared the frequency of contrast reactions in patients with histories of seafood allergy, food allergy, asthma, hay fever, hives, and contrast medium.[10] Using their results, we compared the frequency of reactions in patients with histories of seafood allergy (6.3%, 4/64) to patients with any other type of allergy or atopic state (9.2%, 212/2304) and found no statistically significant differences (P = 0.418). Similarly, Shehadi evaluated seafood as well as asthma, hay fever, common medications (eg, penicillin, aspirin, morphine), and others.[11] A reanalysis of the results found no statistically significant differences comparing the frequencies of contrast reactions in patients with seafood allergy (15.0%) compared with other allergens (eggs, milk and chocolate, 14.6%; general allergies, 13.1%; fruit allergies, 12.9%; asthma, 11.2%; P values ranging between 0.2 and 0.6).[6] Overall, the results suggest that patients with seafood allergy are at no higher risk for having a contrast reaction compared with patients with other food allergies or other forms of atopy.
Additionally, seafood and other food allergies should be distinguished from food intolerances in which the ingestion of histamine‐rich materials in conjunction with histamine inhibitors (drugs or alcohol) leads to symptoms that can mimic allergic‐like reactions (urticaria, pruritus, diarrhea, asthma).[12]
What Do the Guidelines Recommend?
For patients who require IV contrast media for CT scans, the American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering lowiso‐osmolar radiocontrast media or pretreating with corticosteroids and antihistamines for patients with a history of seafood allergy.[13] The American College of Radiology recommends pretreatment with corticosteroids only for those patients who have previously experienced moderate to severe reactions to IV contrast.[8]
WHAT YOU SHOULD DO INSTEAD: ASK ABOUT PRIOR CONTRAST REACTIONS BEFORE ADMINISTERING CONTRAST
When a patient presents for a contrast‐enhanced CT scan, patients should be asked if they have experienced reactions to contrast and the severity and type of the associated reactions. Providers and support staff should not ask specifically about shellfish allergies, as they have not been found to be associated with an elevated risk of contrast reactions compared with other allergens. Although all allergies seem to increase the likelihood of having a reaction to contrast, only a history of previous contrast reactions will prompt a change in management. Asking specifically about seafood allergies before performing an IV contrast CT scan is a Thing We Do for No Reason.
RECOMMENDATIONS
- Before performing contrast‐enhanced CT scans, patients should be asked if they have experienced reactions to IV contrast. There is no reason for providers and support staff to specifically inquire about seafood allergies.
- Patients with seafood and other food allergies do not require premedication prior to CT scans. Seafood and other food allergies do not represent contraindications to obtaining contrast‐enhanced CT scans and should not prompt a change in management.
Disclosures
The authors do not have any relevant financial disclosures to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 55‐year‐old patient with a history of chronic obstructive pulmonary disease and diabetes mellitus presented to the emergency room with acute shortness of breath and right leg swelling that began 1 week after lumbar disk surgery. The emergency department team decides against ordering a chest CT scan with contrast to evaluate for a possible pulmonary embolism after noting that the patient's allergies include shellfish, which cause urticaria and facial edema. A ventilation‐perfusion scan reveals heterogeneous perfusion defects consistent with an intermediate probability (20%80%) for pulmonary embolism. The treating physicians consider starting the patient on a steroid regimen to prepare him for a CT scan with IV contrast, while presumptively anticoagulating the patient for 24 hours in order for the steroids to provide maximal protective effect before obtaining the scan. Should a history of shellfish allergy affect decision making regarding whether to administer IV contrast?
WHY YOU MIGHT THINK ASKING ABOUT SHELLFISH ALLERGIES BEFORE PERFORMING CONTRAST‐ENHANCED CT SCANS IS HELPFUL
Fish and shellfish contain iodine, and allergic reactions to seafood are quite common, with a prevalence ranging anywhere between 2% and 6% of the population.[1] As a result, patients with suspected shellfish allergies are often told by providers that they are allergic to iodine. In 1 study, nearly 92% of patients presenting to a pediatrics clinic with a suspected seafood or shellfish allergy cited iodine as the culprit.[2] As contrast‐enhanced CT scans utilize a variety of iodine‐based agents, patients are often told to avoid CT scans with iodinated contrast agents or receive corticosteroid/antihistamine premedications prior to undergoing CT scans to mitigate potentially life‐threatening allergic reactions. A survey of radiologists and interventional cardiologists revealed that 65.3% and 88.9%, respectively, asked about seafood or shellfish allergies prior to administering contrast enhanced CT scans, and 34.7% and 50.0%, respectively, stated that they would withhold contrast media or recommend premedication with corticosteroid/antihistamines for patients with seafood or shellfish allergy.[2]
WHY ASKING ABOUT SHELLFISH ALLERGIES BEFORE IV CONTRAST CT SCANS DOES NOT REDUCE THE RISK OF CONTRAST REACTIONS
What Causes Allergic‐Like Reactions to Fish and Shellfish?
Allergic reactions are inappropriate or exaggerated immune response (hypersensitivity reaction). Four types of hypersensitivity reactions have been described (type IIV)[3]; allergic reactions mediated by immunoglobulin E (IgE) represent type I hypersensitivity reactions.
Although fish and shellfish contain iodine, so too do a wide variety of commonly consumed foods (eg, yogurt, milk, bread). In addition, our bodies contain and require sufficient quantities of iodine for basic functions, making immune reactions to such an essential ingredient of life unlikely. Instead, fish and shellfish contain proteins (parvalbumin and tropomyosins, respectively), which act as the major allergens, not iodine.[4]
What Causes Reactions to IV Contrast Media?
Around the world, tens of millions of injections occur every year for contrast‐enhanced scans.[5] Reactions to IV contrast media are not uncommon, occurring anywhere between 0.6% and 17% of the time, with severe reactions occurring between 0.02% and 0.5% of the time.[6] Higher reaction rates were associated with the use of higher‐osmolarity contrast agents. A review of research studies found a lower rate of reactions to IV contrast in eras in which low‐osmolarity agents were exclusively used (0.2% after 1991) versus eras in which high‐osmolarity agents were exclusively used (7.0% between 1985 and 1986).[7]
Reactions to contrast include allergic‐like reactions as well as a variety of other reactions (eg, arrhythmias, vasovagal reactions, flushing), which are thought to be related to the dose and concentration of contrast media.[8]
Allergic‐like, or anaphylactoid, reactions related to contrast are largely thought to have a fundamentally different molecular mechanism than true classic allergic reactions. Anaphylactoid reactions are caused by direct release of histamine into the bloodstream in response to interacting with chemicals. These reactions are not related to or mediated by IgE antibodies and do not require prior exposure.
True classic allergic reactions, on the other hand, are mediated by IgE antibodies in which initial exposure to an allergen (antigen) is followed by subsequent exposure and production of IgE antibodies.[9] The allergenIgE antibody complex causes the degranulation of mast cells and basophils, leading to the release of histamines.
Reactions to IV contrast are likely related to some component of the contrast media instead of the iodine itself. It is thought that the majority of these reactions are anaphylactoid reactions instead of true classic allergic reactions, given that IgE antibodies are not consistently elevated in patients who exhibit these reactions.[8] Nevertheless, the symptoms of these 2 types of reactions (anaphylactoid and allergic reactions) are similar and require comparable treatment to prevent life‐threatening anaphylaxis.
What Are the Major Risk Factors for Allergic‐Like Contrast Reactions?
Previous studies on risk factors for allergic‐like contrast reactions suggest that the strongest predictor of future contrast reactions is a history of prior contrast reaction (5‐fold higher risk), with an estimated 10% to 35% recurrence risk of contrast reactions.[8] Patients with a history of atopy, asthma, and food allergies (including seafood) are at approximately 2 to 3 times greater risk of contrast reactions.[9]
Do Shellfish Allergies Place Patients at Higher Risk for Contrast Reactions Than Other Allergies?
In 1 of the few studies evaluating seafood allergies specifically, Witten et al. compared the frequency of contrast reactions in patients with histories of seafood allergy, food allergy, asthma, hay fever, hives, and contrast medium.[10] Using their results, we compared the frequency of reactions in patients with histories of seafood allergy (6.3%, 4/64) to patients with any other type of allergy or atopic state (9.2%, 212/2304) and found no statistically significant differences (P = 0.418). Similarly, Shehadi evaluated seafood as well as asthma, hay fever, common medications (eg, penicillin, aspirin, morphine), and others.[11] A reanalysis of the results found no statistically significant differences comparing the frequencies of contrast reactions in patients with seafood allergy (15.0%) compared with other allergens (eggs, milk and chocolate, 14.6%; general allergies, 13.1%; fruit allergies, 12.9%; asthma, 11.2%; P values ranging between 0.2 and 0.6).[6] Overall, the results suggest that patients with seafood allergy are at no higher risk for having a contrast reaction compared with patients with other food allergies or other forms of atopy.
Additionally, seafood and other food allergies should be distinguished from food intolerances in which the ingestion of histamine‐rich materials in conjunction with histamine inhibitors (drugs or alcohol) leads to symptoms that can mimic allergic‐like reactions (urticaria, pruritus, diarrhea, asthma).[12]
What Do the Guidelines Recommend?
For patients who require IV contrast media for CT scans, the American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering lowiso‐osmolar radiocontrast media or pretreating with corticosteroids and antihistamines for patients with a history of seafood allergy.[13] The American College of Radiology recommends pretreatment with corticosteroids only for those patients who have previously experienced moderate to severe reactions to IV contrast.[8]
WHAT YOU SHOULD DO INSTEAD: ASK ABOUT PRIOR CONTRAST REACTIONS BEFORE ADMINISTERING CONTRAST
When a patient presents for a contrast‐enhanced CT scan, patients should be asked if they have experienced reactions to contrast and the severity and type of the associated reactions. Providers and support staff should not ask specifically about shellfish allergies, as they have not been found to be associated with an elevated risk of contrast reactions compared with other allergens. Although all allergies seem to increase the likelihood of having a reaction to contrast, only a history of previous contrast reactions will prompt a change in management. Asking specifically about seafood allergies before performing an IV contrast CT scan is a Thing We Do for No Reason.
RECOMMENDATIONS
- Before performing contrast‐enhanced CT scans, patients should be asked if they have experienced reactions to IV contrast. There is no reason for providers and support staff to specifically inquire about seafood allergies.
- Patients with seafood and other food allergies do not require premedication prior to CT scans. Seafood and other food allergies do not represent contraindications to obtaining contrast‐enhanced CT scans and should not prompt a change in management.
Disclosures
The authors do not have any relevant financial disclosures to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
© 2015 Society of Hospital Medicine
Amylase Testing for Acute Pancreatitis
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
© 2016 Society of Hospital Medicine
Carotid Artery Ultrasound for Syncope
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 66‐year‐old man with a history of hypertension is hospitalized for a transient loss of consciousness while shopping at a farmers market with his wife on a hot summer day. He recalls feeling lightheaded seconds before he lost consciousness. He had no chest pain, diaphoresis, dyspnea, shaking movements, slurred speech, or head trauma. He felt mildly fatigued following the episode, but has since returned to his baseline. Physical examination, including a thorough cardiac and neurological examination, is normal. The hospitalist ponders whether to order a carotid artery ultrasound as part of a syncope evaluation.
BRIEF OVERVIEW
Syncope is defined as a rapid onset loss of consciousness of short duration as a result of global cerebral hypoperfusion with loss of postural tone, which is followed by spontaneous and complete recovery.[1] This definition describes syncope as a symptom rather than a disease. The challenge for providers is to determine the etiology of the syncope along with its attendant risk of morbidity and mortality. Given the wide variety of etiologies and concern over potentially missing an important etiology, diagnostic testing can become elaborate, expensive, and frequently low yield.
In the adult population, it is believed that approximately 35% of individuals will experience a syncopal episode in their lifetime.[2] As a result, syncope accounts for 1% to 3% of all emergency department visits and 1% to 6% of hospital admissions from emergency departments in the United States.[3, 4] The incidence and rate of hospitalization increase with age, as does the risk of mortality.[5, 6] There are 3 main types of syncope: cardiac, neurocardiogenic (vasovagal), and orthostatic. The presence of associated signs or symptoms with the syncope helps to differentiate the type and complexity of the syncope, while helping guide the diagnostic evaluation. Simple syncope is defined as the absence of focal neurological deficits or other signs or symptoms suggestive of transient ischemic attack (TIA) or cerebrovascular accident (CVA).[7] A differential diagnosis for a transient loss of consciousness that includes TIA and CVA will prompt a very different evaluation.
WHY YOU MIGHT THINK ORDERING CAROTID ARTERY ULTRASOUNDS FOR SYNCOPE EVALUATION ARE HELPFUL
Carotid artery ultrasounds are used to assess the extracranial carotid arteries for the presence of stenosis and to determine the direction of blood flow. The use of carotid artery ultrasound as a diagnostic tool in the evaluation of syncope can be traced to multiple articles from the 1980s. These articles noted the utility of screening patients with dizziness, lightheadedness, or syncope using carotid artery ultrasound due to possible decreased flow in the carotid artery circulation affecting cerebral perfusion.[8, 9] An association was noted between these symptoms and the presence of carotid artery stenosis. Further, a 1997 position paper from the American College of Physicians recommended that carotid artery or transcranial ultrasonography be reserved for syncope patients with carotid artery bruits or a history of neurovascular signs or symptoms.[10] More recent studies reveal carotid artery ultrasounds are still being performed regularly in syncope patients. In 2 studies evaluating syncope in the elderly, approximately 13% to 16% of syncope patients had a carotid artery ultrasound performed in an effort to identify an etiology.[7, 11]
Additionally, practitioners sometimes choose to perform carotid artery ultrasound in the evaluation of carotid sinus hypersensitivity. The carotid artery ultrasound can assess for the presence of stenosis or atheroma prior to performing carotid sinus massage, although the rate of persistent neurological complications from carotid sinus massage is estimated to occur in 1:1000 patients.[12]
WHY THERE IS NO REASON TO ORDER CAROTID ARTERY ULTRASOUNDS FOR THE EVALUATION OF SIMPLE SYNCOPE
Carotid artery ultrasounds are unlikely to determine the etiology of the syncope. We should expect a high‐value test to reveal an etiology for the syncope episodes at a significant rate. In the 2009 study by Mendu et al. at YaleNew Haven Hospital, 267 ultrasounds were performed on 2106 syncope admissions of high‐risk elderly patients (1920 total patients).[11] Of the 267 ultrasounds, only 2 of the tests (0.8%) helped to determine an etiology. Although 46% of the ultrasounds had abnormal findings, the measuring stick for these studies should be whether they uncover the etiology for syncope, not whether they find other unrelated vascular disease. In contrast, performing postural blood pressures helped to determine an etiology 15% to 21% of the time, depending on the criteria used to define an abnormal drop in postural blood pressures.
Similarly, in the 2005 study by Schnipper et al. at Massachusetts General Hospital, only 140 of 4199 adult patients (3.3%) who presented as either inpatients or outpatients for syncope or presyncope were referred for neurovascular testing.[13] Carotid artery ultrasound was performed in 109 of these patients, and the study neurologist could invoke cerebrovascular lesions as potential factors for syncope in only 2 patients, both of whom had syncope and focal neurologic symptoms. Moreover, both of the patients had severe cardiovascular disease (severe ischemic cardiomyopathy with complete heart block following coronary bypass surgery in 1 and aortic stenosis with decreased left ventricular ejection fraction in the other). It is quite possible that the ultrasounds did not find the etiology for any of the 140 high‐risk patients with syncope in the study.
In addition, the 2014 study by Scott et al. at Brigham and Women's Hospital analyzed carotid artery duplex ultrasounds performed on 313 inpatients and outpatients with syncope over a 5‐year period, excluding those with focal neurological deficits or carotid bruits.[7] Although 48 of the 313 patients (15.4%) were diagnosed with carotid stenosis of greater than 50%, the carotid artery ultrasound did not reveal a causal diagnosis in any patients. On the other hand, 7 patients had a change in medical management, and 1 patient underwent carotid endarterectomy following the carotid artery ultrasound, which was incidental to what prompted the evaluation.
Mendu et al. calculated the cost per test affecting the diagnosis or management of syncope (although diagnosis is the only important parameter). The cost per test was calculated as the charge per test multiplied by the cost‐to‐charge ratio of 0.34, based on the 2007 YaleNew Haven Hospital cost‐to‐charge ratio.[11] For carotid artery ultrasound, the cost per test was $19,580 to affect diagnosis or management as compared to $23 to $33 for postural blood pressures. Combining these findings with the results from the Schnipper et al. and Scott et al. articles, where carotid artery ultrasounds may not have found the cause of syncope in any of the patients, it seems clear that obtaining a carotid artery ultrasound in the evaluation of simple syncope is a low‐value proposition.
Many low‐value tests, like carotid artery ultrasounds, suffer from both upfront costs, as calculated in the Mendu et al. study, and downstream costs triggered by the testing itself. Performing carotid artery ultrasounds in elderly high‐risk syncope populations is likely to reveal asymptomatic carotid artery vascular disease, which may lead to more unwarranted testing or treatments in light of the initial indication for the test. In the Mendu et al. article, 122 (46%) of the 267 carotid artery ultrasounds performed on elderly patients admitted with syncope were abnormal. Abnormal findings were defined as any abnormality that was not seen on prior testing as written in the test reports. Similarly, Schnipper et al. found that 40% of the 140 highly selected patients had mild‐to‐severe carotid vascular disease.
National guideline recommendations are aligned with these findings. The National Institute for Health and Clinical Excellence Guideline for the Management of Transient Loss of Consciousness does not include carotid artery ultrasound in the summary of clinical recommendations.[14] Furthermore, the American Academy of Neurology Choosing Wisely campaign's recommendation 2 is: Do not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms.[15]
WHAT YOU SHOULD DO INSTEAD: CHECK POSTURAL BLOOD PRESSURES
As is true for most of medicine, greater focus should be paid to the history and physical examination during the initial evaluation of the patient with syncope. Take great care to determine which patients have a history or symptoms concerning for neurologic or cardiac etiologies. Use this information to guide further diagnostic testing. Additionally, orthostatic testing is too often overlooked as an important diagnostic study. As described in the Mendu et al. study, orthostatic testing is inexpensive and effective, helping to determine an etiology 15% to 21% of the time. Carotid artery ultrasounds should be reserved for those patients with transient or permanent focal neurological symptoms.
RECOMMENDATIONS
- In patients suspected of syncope in the absence of other neurologic symptoms, carotid artery ultrasound should not be included in the diagnostic evaluation.
- Utilize postural blood pressures in the initial evaluation of syncope as an inexpensive and high‐value component of the physical examination.
- For patients with acute neurological findings in the setting of possible syncope, evaluate the patient for stroke.
- Use the history and physical examination to guide further evaluation.
CONCLUSION
Carotid artery ultrasounds should not be used to evaluate the cause of syncope in an effort to find incident symptomatic carotid vascular disease. Carotid artery ultrasounds rarely help determine the etiology of the syncopal episode and are more likely to find asymptomatic carotid vascular disease in the elderly population. The identification of carotid vascular disease can lead to further inappropriate testing and treatments unrelated to the indication for testing.
Acknowledgment
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . Syncope. N Engl J Med. 2000;313(25):1856–1862.
- , , , , , . Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol. 2006;17(11):1172–1176.
- . Evaluation and management of patients with syncope. JAMA. 1992;268(18):2553–2560.
- , , . Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992 to 2000. Acad Emerg Med. 2004;11:1029–1034.
- , . An approach to the evaluation and management of syncope in adults. BMJ. 2010;340:c880.
- , , , et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878–885.
- , , , , . Choosing wisely for syncope: low‐value carotid ultrasound use. J Am Heart Assoc. 2014;3(4):e001063.
- , , . Hemodynamics of the carotid‐artery circulation in the elderly “dizzy” patient. J Am Geriatr Soc. 1981;29(9):402–406.
- . Clinical applications of noninvasive carotid artery testing. J Am Coll Cardiol. 1985;5(1):137–148.
- , , , , , . Diagnosing syncope. Part 2: unexplained syncope. Clinical efficacy assessment project of the American College of Physicians. Ann Intern Med. 1997;127(1):76–86.
- , , , , . Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299–1305.
- , , , , , . Complications of carotid sinus massage—a prospective series of older patients. Age Ageing. 2000;29(5):413–417.
- , , , . Diagnostic yield and utility of neurovascular ultrasonography in the evaluation of patients with syncope. Mayo Clin Proc. 2005;80(4):480–488.
- , , , et al. Transient Loss of Consciousness (‘Blackouts’) Management in Adults and Young People. NICE Clinical Guidelines, No. 109. London, UK: National Clinical Guideline Centre for Acute and Chronic Conditions, Royal College of Physicians; 2010.
- , , , et al. The American Academy of Neurology's top five choosing wisely recommendations. Neurology. 2013;81(11):1004–1011.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 66‐year‐old man with a history of hypertension is hospitalized for a transient loss of consciousness while shopping at a farmers market with his wife on a hot summer day. He recalls feeling lightheaded seconds before he lost consciousness. He had no chest pain, diaphoresis, dyspnea, shaking movements, slurred speech, or head trauma. He felt mildly fatigued following the episode, but has since returned to his baseline. Physical examination, including a thorough cardiac and neurological examination, is normal. The hospitalist ponders whether to order a carotid artery ultrasound as part of a syncope evaluation.
BRIEF OVERVIEW
Syncope is defined as a rapid onset loss of consciousness of short duration as a result of global cerebral hypoperfusion with loss of postural tone, which is followed by spontaneous and complete recovery.[1] This definition describes syncope as a symptom rather than a disease. The challenge for providers is to determine the etiology of the syncope along with its attendant risk of morbidity and mortality. Given the wide variety of etiologies and concern over potentially missing an important etiology, diagnostic testing can become elaborate, expensive, and frequently low yield.
In the adult population, it is believed that approximately 35% of individuals will experience a syncopal episode in their lifetime.[2] As a result, syncope accounts for 1% to 3% of all emergency department visits and 1% to 6% of hospital admissions from emergency departments in the United States.[3, 4] The incidence and rate of hospitalization increase with age, as does the risk of mortality.[5, 6] There are 3 main types of syncope: cardiac, neurocardiogenic (vasovagal), and orthostatic. The presence of associated signs or symptoms with the syncope helps to differentiate the type and complexity of the syncope, while helping guide the diagnostic evaluation. Simple syncope is defined as the absence of focal neurological deficits or other signs or symptoms suggestive of transient ischemic attack (TIA) or cerebrovascular accident (CVA).[7] A differential diagnosis for a transient loss of consciousness that includes TIA and CVA will prompt a very different evaluation.
WHY YOU MIGHT THINK ORDERING CAROTID ARTERY ULTRASOUNDS FOR SYNCOPE EVALUATION ARE HELPFUL
Carotid artery ultrasounds are used to assess the extracranial carotid arteries for the presence of stenosis and to determine the direction of blood flow. The use of carotid artery ultrasound as a diagnostic tool in the evaluation of syncope can be traced to multiple articles from the 1980s. These articles noted the utility of screening patients with dizziness, lightheadedness, or syncope using carotid artery ultrasound due to possible decreased flow in the carotid artery circulation affecting cerebral perfusion.[8, 9] An association was noted between these symptoms and the presence of carotid artery stenosis. Further, a 1997 position paper from the American College of Physicians recommended that carotid artery or transcranial ultrasonography be reserved for syncope patients with carotid artery bruits or a history of neurovascular signs or symptoms.[10] More recent studies reveal carotid artery ultrasounds are still being performed regularly in syncope patients. In 2 studies evaluating syncope in the elderly, approximately 13% to 16% of syncope patients had a carotid artery ultrasound performed in an effort to identify an etiology.[7, 11]
Additionally, practitioners sometimes choose to perform carotid artery ultrasound in the evaluation of carotid sinus hypersensitivity. The carotid artery ultrasound can assess for the presence of stenosis or atheroma prior to performing carotid sinus massage, although the rate of persistent neurological complications from carotid sinus massage is estimated to occur in 1:1000 patients.[12]
WHY THERE IS NO REASON TO ORDER CAROTID ARTERY ULTRASOUNDS FOR THE EVALUATION OF SIMPLE SYNCOPE
Carotid artery ultrasounds are unlikely to determine the etiology of the syncope. We should expect a high‐value test to reveal an etiology for the syncope episodes at a significant rate. In the 2009 study by Mendu et al. at YaleNew Haven Hospital, 267 ultrasounds were performed on 2106 syncope admissions of high‐risk elderly patients (1920 total patients).[11] Of the 267 ultrasounds, only 2 of the tests (0.8%) helped to determine an etiology. Although 46% of the ultrasounds had abnormal findings, the measuring stick for these studies should be whether they uncover the etiology for syncope, not whether they find other unrelated vascular disease. In contrast, performing postural blood pressures helped to determine an etiology 15% to 21% of the time, depending on the criteria used to define an abnormal drop in postural blood pressures.
Similarly, in the 2005 study by Schnipper et al. at Massachusetts General Hospital, only 140 of 4199 adult patients (3.3%) who presented as either inpatients or outpatients for syncope or presyncope were referred for neurovascular testing.[13] Carotid artery ultrasound was performed in 109 of these patients, and the study neurologist could invoke cerebrovascular lesions as potential factors for syncope in only 2 patients, both of whom had syncope and focal neurologic symptoms. Moreover, both of the patients had severe cardiovascular disease (severe ischemic cardiomyopathy with complete heart block following coronary bypass surgery in 1 and aortic stenosis with decreased left ventricular ejection fraction in the other). It is quite possible that the ultrasounds did not find the etiology for any of the 140 high‐risk patients with syncope in the study.
In addition, the 2014 study by Scott et al. at Brigham and Women's Hospital analyzed carotid artery duplex ultrasounds performed on 313 inpatients and outpatients with syncope over a 5‐year period, excluding those with focal neurological deficits or carotid bruits.[7] Although 48 of the 313 patients (15.4%) were diagnosed with carotid stenosis of greater than 50%, the carotid artery ultrasound did not reveal a causal diagnosis in any patients. On the other hand, 7 patients had a change in medical management, and 1 patient underwent carotid endarterectomy following the carotid artery ultrasound, which was incidental to what prompted the evaluation.
Mendu et al. calculated the cost per test affecting the diagnosis or management of syncope (although diagnosis is the only important parameter). The cost per test was calculated as the charge per test multiplied by the cost‐to‐charge ratio of 0.34, based on the 2007 YaleNew Haven Hospital cost‐to‐charge ratio.[11] For carotid artery ultrasound, the cost per test was $19,580 to affect diagnosis or management as compared to $23 to $33 for postural blood pressures. Combining these findings with the results from the Schnipper et al. and Scott et al. articles, where carotid artery ultrasounds may not have found the cause of syncope in any of the patients, it seems clear that obtaining a carotid artery ultrasound in the evaluation of simple syncope is a low‐value proposition.
Many low‐value tests, like carotid artery ultrasounds, suffer from both upfront costs, as calculated in the Mendu et al. study, and downstream costs triggered by the testing itself. Performing carotid artery ultrasounds in elderly high‐risk syncope populations is likely to reveal asymptomatic carotid artery vascular disease, which may lead to more unwarranted testing or treatments in light of the initial indication for the test. In the Mendu et al. article, 122 (46%) of the 267 carotid artery ultrasounds performed on elderly patients admitted with syncope were abnormal. Abnormal findings were defined as any abnormality that was not seen on prior testing as written in the test reports. Similarly, Schnipper et al. found that 40% of the 140 highly selected patients had mild‐to‐severe carotid vascular disease.
National guideline recommendations are aligned with these findings. The National Institute for Health and Clinical Excellence Guideline for the Management of Transient Loss of Consciousness does not include carotid artery ultrasound in the summary of clinical recommendations.[14] Furthermore, the American Academy of Neurology Choosing Wisely campaign's recommendation 2 is: Do not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms.[15]
WHAT YOU SHOULD DO INSTEAD: CHECK POSTURAL BLOOD PRESSURES
As is true for most of medicine, greater focus should be paid to the history and physical examination during the initial evaluation of the patient with syncope. Take great care to determine which patients have a history or symptoms concerning for neurologic or cardiac etiologies. Use this information to guide further diagnostic testing. Additionally, orthostatic testing is too often overlooked as an important diagnostic study. As described in the Mendu et al. study, orthostatic testing is inexpensive and effective, helping to determine an etiology 15% to 21% of the time. Carotid artery ultrasounds should be reserved for those patients with transient or permanent focal neurological symptoms.
RECOMMENDATIONS
- In patients suspected of syncope in the absence of other neurologic symptoms, carotid artery ultrasound should not be included in the diagnostic evaluation.
- Utilize postural blood pressures in the initial evaluation of syncope as an inexpensive and high‐value component of the physical examination.
- For patients with acute neurological findings in the setting of possible syncope, evaluate the patient for stroke.
- Use the history and physical examination to guide further evaluation.
CONCLUSION
Carotid artery ultrasounds should not be used to evaluate the cause of syncope in an effort to find incident symptomatic carotid vascular disease. Carotid artery ultrasounds rarely help determine the etiology of the syncopal episode and are more likely to find asymptomatic carotid vascular disease in the elderly population. The identification of carotid vascular disease can lead to further inappropriate testing and treatments unrelated to the indication for testing.
Acknowledgment
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 66‐year‐old man with a history of hypertension is hospitalized for a transient loss of consciousness while shopping at a farmers market with his wife on a hot summer day. He recalls feeling lightheaded seconds before he lost consciousness. He had no chest pain, diaphoresis, dyspnea, shaking movements, slurred speech, or head trauma. He felt mildly fatigued following the episode, but has since returned to his baseline. Physical examination, including a thorough cardiac and neurological examination, is normal. The hospitalist ponders whether to order a carotid artery ultrasound as part of a syncope evaluation.
BRIEF OVERVIEW
Syncope is defined as a rapid onset loss of consciousness of short duration as a result of global cerebral hypoperfusion with loss of postural tone, which is followed by spontaneous and complete recovery.[1] This definition describes syncope as a symptom rather than a disease. The challenge for providers is to determine the etiology of the syncope along with its attendant risk of morbidity and mortality. Given the wide variety of etiologies and concern over potentially missing an important etiology, diagnostic testing can become elaborate, expensive, and frequently low yield.
In the adult population, it is believed that approximately 35% of individuals will experience a syncopal episode in their lifetime.[2] As a result, syncope accounts for 1% to 3% of all emergency department visits and 1% to 6% of hospital admissions from emergency departments in the United States.[3, 4] The incidence and rate of hospitalization increase with age, as does the risk of mortality.[5, 6] There are 3 main types of syncope: cardiac, neurocardiogenic (vasovagal), and orthostatic. The presence of associated signs or symptoms with the syncope helps to differentiate the type and complexity of the syncope, while helping guide the diagnostic evaluation. Simple syncope is defined as the absence of focal neurological deficits or other signs or symptoms suggestive of transient ischemic attack (TIA) or cerebrovascular accident (CVA).[7] A differential diagnosis for a transient loss of consciousness that includes TIA and CVA will prompt a very different evaluation.
WHY YOU MIGHT THINK ORDERING CAROTID ARTERY ULTRASOUNDS FOR SYNCOPE EVALUATION ARE HELPFUL
Carotid artery ultrasounds are used to assess the extracranial carotid arteries for the presence of stenosis and to determine the direction of blood flow. The use of carotid artery ultrasound as a diagnostic tool in the evaluation of syncope can be traced to multiple articles from the 1980s. These articles noted the utility of screening patients with dizziness, lightheadedness, or syncope using carotid artery ultrasound due to possible decreased flow in the carotid artery circulation affecting cerebral perfusion.[8, 9] An association was noted between these symptoms and the presence of carotid artery stenosis. Further, a 1997 position paper from the American College of Physicians recommended that carotid artery or transcranial ultrasonography be reserved for syncope patients with carotid artery bruits or a history of neurovascular signs or symptoms.[10] More recent studies reveal carotid artery ultrasounds are still being performed regularly in syncope patients. In 2 studies evaluating syncope in the elderly, approximately 13% to 16% of syncope patients had a carotid artery ultrasound performed in an effort to identify an etiology.[7, 11]
Additionally, practitioners sometimes choose to perform carotid artery ultrasound in the evaluation of carotid sinus hypersensitivity. The carotid artery ultrasound can assess for the presence of stenosis or atheroma prior to performing carotid sinus massage, although the rate of persistent neurological complications from carotid sinus massage is estimated to occur in 1:1000 patients.[12]
WHY THERE IS NO REASON TO ORDER CAROTID ARTERY ULTRASOUNDS FOR THE EVALUATION OF SIMPLE SYNCOPE
Carotid artery ultrasounds are unlikely to determine the etiology of the syncope. We should expect a high‐value test to reveal an etiology for the syncope episodes at a significant rate. In the 2009 study by Mendu et al. at YaleNew Haven Hospital, 267 ultrasounds were performed on 2106 syncope admissions of high‐risk elderly patients (1920 total patients).[11] Of the 267 ultrasounds, only 2 of the tests (0.8%) helped to determine an etiology. Although 46% of the ultrasounds had abnormal findings, the measuring stick for these studies should be whether they uncover the etiology for syncope, not whether they find other unrelated vascular disease. In contrast, performing postural blood pressures helped to determine an etiology 15% to 21% of the time, depending on the criteria used to define an abnormal drop in postural blood pressures.
Similarly, in the 2005 study by Schnipper et al. at Massachusetts General Hospital, only 140 of 4199 adult patients (3.3%) who presented as either inpatients or outpatients for syncope or presyncope were referred for neurovascular testing.[13] Carotid artery ultrasound was performed in 109 of these patients, and the study neurologist could invoke cerebrovascular lesions as potential factors for syncope in only 2 patients, both of whom had syncope and focal neurologic symptoms. Moreover, both of the patients had severe cardiovascular disease (severe ischemic cardiomyopathy with complete heart block following coronary bypass surgery in 1 and aortic stenosis with decreased left ventricular ejection fraction in the other). It is quite possible that the ultrasounds did not find the etiology for any of the 140 high‐risk patients with syncope in the study.
In addition, the 2014 study by Scott et al. at Brigham and Women's Hospital analyzed carotid artery duplex ultrasounds performed on 313 inpatients and outpatients with syncope over a 5‐year period, excluding those with focal neurological deficits or carotid bruits.[7] Although 48 of the 313 patients (15.4%) were diagnosed with carotid stenosis of greater than 50%, the carotid artery ultrasound did not reveal a causal diagnosis in any patients. On the other hand, 7 patients had a change in medical management, and 1 patient underwent carotid endarterectomy following the carotid artery ultrasound, which was incidental to what prompted the evaluation.
Mendu et al. calculated the cost per test affecting the diagnosis or management of syncope (although diagnosis is the only important parameter). The cost per test was calculated as the charge per test multiplied by the cost‐to‐charge ratio of 0.34, based on the 2007 YaleNew Haven Hospital cost‐to‐charge ratio.[11] For carotid artery ultrasound, the cost per test was $19,580 to affect diagnosis or management as compared to $23 to $33 for postural blood pressures. Combining these findings with the results from the Schnipper et al. and Scott et al. articles, where carotid artery ultrasounds may not have found the cause of syncope in any of the patients, it seems clear that obtaining a carotid artery ultrasound in the evaluation of simple syncope is a low‐value proposition.
Many low‐value tests, like carotid artery ultrasounds, suffer from both upfront costs, as calculated in the Mendu et al. study, and downstream costs triggered by the testing itself. Performing carotid artery ultrasounds in elderly high‐risk syncope populations is likely to reveal asymptomatic carotid artery vascular disease, which may lead to more unwarranted testing or treatments in light of the initial indication for the test. In the Mendu et al. article, 122 (46%) of the 267 carotid artery ultrasounds performed on elderly patients admitted with syncope were abnormal. Abnormal findings were defined as any abnormality that was not seen on prior testing as written in the test reports. Similarly, Schnipper et al. found that 40% of the 140 highly selected patients had mild‐to‐severe carotid vascular disease.
National guideline recommendations are aligned with these findings. The National Institute for Health and Clinical Excellence Guideline for the Management of Transient Loss of Consciousness does not include carotid artery ultrasound in the summary of clinical recommendations.[14] Furthermore, the American Academy of Neurology Choosing Wisely campaign's recommendation 2 is: Do not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms.[15]
WHAT YOU SHOULD DO INSTEAD: CHECK POSTURAL BLOOD PRESSURES
As is true for most of medicine, greater focus should be paid to the history and physical examination during the initial evaluation of the patient with syncope. Take great care to determine which patients have a history or symptoms concerning for neurologic or cardiac etiologies. Use this information to guide further diagnostic testing. Additionally, orthostatic testing is too often overlooked as an important diagnostic study. As described in the Mendu et al. study, orthostatic testing is inexpensive and effective, helping to determine an etiology 15% to 21% of the time. Carotid artery ultrasounds should be reserved for those patients with transient or permanent focal neurological symptoms.
RECOMMENDATIONS
- In patients suspected of syncope in the absence of other neurologic symptoms, carotid artery ultrasound should not be included in the diagnostic evaluation.
- Utilize postural blood pressures in the initial evaluation of syncope as an inexpensive and high‐value component of the physical examination.
- For patients with acute neurological findings in the setting of possible syncope, evaluate the patient for stroke.
- Use the history and physical examination to guide further evaluation.
CONCLUSION
Carotid artery ultrasounds should not be used to evaluate the cause of syncope in an effort to find incident symptomatic carotid vascular disease. Carotid artery ultrasounds rarely help determine the etiology of the syncopal episode and are more likely to find asymptomatic carotid vascular disease in the elderly population. The identification of carotid vascular disease can lead to further inappropriate testing and treatments unrelated to the indication for testing.
Acknowledgment
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . Syncope. N Engl J Med. 2000;313(25):1856–1862.
- , , , , , . Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol. 2006;17(11):1172–1176.
- . Evaluation and management of patients with syncope. JAMA. 1992;268(18):2553–2560.
- , , . Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992 to 2000. Acad Emerg Med. 2004;11:1029–1034.
- , . An approach to the evaluation and management of syncope in adults. BMJ. 2010;340:c880.
- , , , et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878–885.
- , , , , . Choosing wisely for syncope: low‐value carotid ultrasound use. J Am Heart Assoc. 2014;3(4):e001063.
- , , . Hemodynamics of the carotid‐artery circulation in the elderly “dizzy” patient. J Am Geriatr Soc. 1981;29(9):402–406.
- . Clinical applications of noninvasive carotid artery testing. J Am Coll Cardiol. 1985;5(1):137–148.
- , , , , , . Diagnosing syncope. Part 2: unexplained syncope. Clinical efficacy assessment project of the American College of Physicians. Ann Intern Med. 1997;127(1):76–86.
- , , , , . Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299–1305.
- , , , , , . Complications of carotid sinus massage—a prospective series of older patients. Age Ageing. 2000;29(5):413–417.
- , , , . Diagnostic yield and utility of neurovascular ultrasonography in the evaluation of patients with syncope. Mayo Clin Proc. 2005;80(4):480–488.
- , , , et al. Transient Loss of Consciousness (‘Blackouts’) Management in Adults and Young People. NICE Clinical Guidelines, No. 109. London, UK: National Clinical Guideline Centre for Acute and Chronic Conditions, Royal College of Physicians; 2010.
- , , , et al. The American Academy of Neurology's top five choosing wisely recommendations. Neurology. 2013;81(11):1004–1011.
- . Syncope. N Engl J Med. 2000;313(25):1856–1862.
- , , , , , . Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol. 2006;17(11):1172–1176.
- . Evaluation and management of patients with syncope. JAMA. 1992;268(18):2553–2560.
- , , . Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992 to 2000. Acad Emerg Med. 2004;11:1029–1034.
- , . An approach to the evaluation and management of syncope in adults. BMJ. 2010;340:c880.
- , , , et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347(12):878–885.
- , , , , . Choosing wisely for syncope: low‐value carotid ultrasound use. J Am Heart Assoc. 2014;3(4):e001063.
- , , . Hemodynamics of the carotid‐artery circulation in the elderly “dizzy” patient. J Am Geriatr Soc. 1981;29(9):402–406.
- . Clinical applications of noninvasive carotid artery testing. J Am Coll Cardiol. 1985;5(1):137–148.
- , , , , , . Diagnosing syncope. Part 2: unexplained syncope. Clinical efficacy assessment project of the American College of Physicians. Ann Intern Med. 1997;127(1):76–86.
- , , , , . Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169(14):1299–1305.
- , , , , , . Complications of carotid sinus massage—a prospective series of older patients. Age Ageing. 2000;29(5):413–417.
- , , , . Diagnostic yield and utility of neurovascular ultrasonography in the evaluation of patients with syncope. Mayo Clin Proc. 2005;80(4):480–488.
- , , , et al. Transient Loss of Consciousness (‘Blackouts’) Management in Adults and Young People. NICE Clinical Guidelines, No. 109. London, UK: National Clinical Guideline Centre for Acute and Chronic Conditions, Royal College of Physicians; 2010.
- , , , et al. The American Academy of Neurology's top five choosing wisely recommendations. Neurology. 2013;81(11):1004–1011.
© 2015 Society of Hospital Medicine
Urinary Excretion Indices in AKI
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 70‐year‐old woman with a history of diabetes mellitus type 2 and hypertension was admitted with abdominal pain following 2 days of nausea and diarrhea. Initial laboratory studies revealed blood urea nitrogen (BUN) 25 mg/dL and serum creatinine 1.3 mg/dL. Computed tomography of the abdomen and pelvis with nonionic, low osmolar intravenous and oral contrast demonstrated acute diverticulitis with an associated small abscess. She was administered intravenous 0.9% sodium chloride solution and antibiotics. Blood pressure on admission was 92/55 mm Hg, and 24 hours later, her BUN and serum creatinine increased to 33 mg/dL and 1.9 mg/dL, respectively. Her urine output during the preceding 24 hours was 500 mL.
In the evaluation of acute kidney injury (AKI), is the measurement of fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUr) of value?
WHY YOU MIGHT THINK ORDERING FeNa AND/OR FeUr IN THE EVALUATION OF AKI IS HELPFUL
The proper maintenance of sodium balance is paramount to regulating the size of body fluid compartments. Through the interaction of multiple physiologic processes, the kidney regulates tubular reabsorption (or lack thereof) of sodium chloride to match excretion to intake. In normal health, FeNa is typically 1%, although it may vary depending on the dietary sodium intake. The corollary is that 99% of filtered sodium is reabsorbed. Acute tubular injury (ATI) that impairs the tubular resorptive capacity for sodium may increase FeNa to >3%. In addition, during states of water conservation, urea is reabsorbed from the medullary collecting duct, explaining the discrepant rise in BUN relative to creatinine in prerenal azotemia. FeUr falls progressively as water is reabsorbed and urine flow declines, and FeUr less than 35% to 40% may result during prerenal azotemia versus >50% in health or ATI. Theoretically, FeUr is largely unaffected by diuretics, whereas FeNa is increased by diuretics.
In 1976, Espinel reported on the use of FeNa in 17 oliguric patients to discriminate prerenal azotemia from ATI.[1] Establishing what are now familiar indices, FeNa <1% was deemed consistent with prerenal physiology versus >3% indicating ATI. Notably, the study excluded patients who had received diuretics or in whom chronic kidney disease (CKD), glomerulonephritis, or urinary obstruction was suspected.
Given the limitations of FeNa in the context of diuretic use, many physicians instead use FeUr to distinguish prerenal versus ATI causes of AKI. Carvounis et al. reported FeUr and FeNa in 50 patients with prerenal azotemia, 27 with prerenal azotemia receiving diuretics and 25 patients with ATI.[2] Patients with interstitial nephritis, glomerulonephritis, and obstruction were excluded. In the entire cohort, the authors reported sensitivity of 90% and specificity of 96% for FeUr <35% in identifying prerenal azotemia (Table 1). FeNa <1% was slightly less sensitive for prerenal azotemia in the entire cohort at 77%, and this fell to 48% in the presence of diuretics as compared to 89% for FeUr. Naturally, the specificity of FeNa for ATI will fall with the use of diuretics. As shown in Table 1, FeUr <35% has an excellent positive likelihood ratio (LR+) of 22 for prerenal azotemia and a moderate LR+ of 9 for FeUr 35% being consistent with ATI, regardless of the presence of diuretics. This contrasts with FeNa, which if 1% in the presence of diuretics, lacked utility in the diagnosis of ATI. Of note, diuretic use was reported only in the prerenal azotemia group and not specifically in the ATI group. Thus, these comparisons assume diuretics have no effect on test characteristics in ATI. This assumption, however, may not be valid.
| FeUr | FeNa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | LR+ | LR | Sens | Spec | PPV | NPV | LR+ | LR | |||
| ||||||||||||||
| Carvounis[2] | Prerenal | Overall | 0.90 | 0.96 | 0.99 | 0.75 | 22.4 | 0.1 | 0.77 | 0.96 | 0.98 | 0.57 | 19.2 | 0.2 |
| No diuretics | 0.90 | 0.96 | 0.98 | 0.83 | 22.5 | 0.1 | 0.92 | 0.96 | 0.98 | 0.86 | 23.0 | 0.1 | ||
| Diuretics | 0.89 | 0.96 | 0.96 | 0.89 | 22.2 | 0.1 | 0.48 | 0.96 | 0.93 | 0.63 | 12.0 | 0.5 | ||
| ATI | Overall | 0.96 | 0.90 | 0.75 | 0.99 | 9.2 | 0.0 | 0.96 | 0.77 | 0.57 | 0.98 | 4.1 | 0.1 | |
| No diuretics* | 0.96 | 0.90 | 0.83 | 0.98 | 9.6 | 0.0 | 0.96 | 0.92 | 0.86 | 0.98 | 12.0 | 0.0 | ||
| Diuretics | 0.96 | 0.89 | 0.89 | 0.96 | 8.6 | 0.0 | 0.96 | 0.48 | 0.63 | 0.93 | 1.9 | 0.1 | ||
| Diskin[8] | Prerenal | Overall | 0.97 | 0.85 | 0.96 | 0.89 | 6.5 | 0.0 | 0.44 | 0.75 | 0.88 | 0.25 | 1.8 | 0.7 |
| No diuretics | 0.91 | 0.89 | 0.95 | 0.80 | 8.2 | 0.1 | 0.83 | 0.67 | 0.86 | 0.60 | 2.5 | 0.3 | ||
| Diuretics | 1.00 | 0.82 | 0.97 | 1.00 | 5.5 | 0.0 | 0.29 | 0.82 | 0.89 | 0.18 | 1.6 | 0.9 | ||
| ATI | Overall | 0.85 | 0.97 | 0.89 | 0.96 | 33.6 | 0.2 | 0.75 | 0.44 | 0.25 | 0.88 | 1.3 | 0.6 | |
| No diuretics | 0.89 | 0.91 | 0.80 | 0.95 | 10.2 | 0.1 | 0.67 | 0.83 | 0.60 | 0.86 | 3.8 | 0.4 | ||
| Diuretics | 0.82 | 1.00 | 1.00 | 0.97 | N/A | 0.2 | 0.82 | 0.29 | 0.18 | 0.89 | 1.1 | 0.6 | ||
WHY THERE IS LITTLE REASON TO ROUTINELY ORDER FeNa AND FeUr IN PATIENTS WITH AKI
The argument against FeNa and FeUr is not primarily financial. FeNa and FeUr testing on all Medicare patients discharged with AKI in 2013 would have cost US$6 million.[3] Although a tiny fraction of annual healthcare expenditure, it would nevertheless be wasteful spending, and its true harm lays in the application of flawed diagnostic reasoning.
That flaw in our conceptual approach to AKI is the broad categorization of patients into either a prerenal or intrinsic etiology of AKI. In reality, renal injury is often multifactorial, and significant prerenal injury may progress to or coexist with intrinsic disease that is commonly ATI. Measurement of a urinary index at a single point in time will often fail to capture this spectrum of causes for AKI. Unfortunately, accurately assessing volume status through physical examination is difficult.[4] Considering FeNa and FeUr may be low in both hemorrhage as well as congestive heart failure, the measurement of these variables adds little to body volume assessment.
It cannot be overemphasized that application of FeNa and FeUr is predicated on the provider already knowing the diagnosis is either prerenal azotemia or ATI. Studies have generally excluded patients >65 years old and those with CKD or notable comorbid renal processes apart from prerenal azotemia or ATI. It is important to recall that a third of kidney biopsies may yield a diagnosis different than the prebiopsy clinical diagnosis, and the gold standard for ATI in studies of FeNa and FeUr was simply a failure of kidney function to improve promptly.[5] Why send a test that is predicated on largely already knowing the answer?
Fractional Excretion of Sodium for Diagnosis
Unfortunately, FeNa is neither sensitive nor specific enough in the general inpatient population to inform important clinical decisions regarding the etiology of AKI. Miller et al. examined 30 patients with oliguric prerenal azotemia, 55 with ATI (oliguric and nonoliguric), 10 with obstructive uropathy, and 7 with glomerulonephritis.[6] None of the patients had received diuretics within 24 hours of study entry. A FeNa <1% was present in 90% of prerenal patients and 4% of oliguric ATI. Importantly, of nonoliguric patients with ATI, 10% had a false positive FeNa <1%. Many subsequent studies have similarly documented the existence of FeNa <1% or otherwise indeterminate in ATI, particularly, but not exclusively, in nonoliguric states.[7] Diskin et al. evaluated FeNa in 100 prospective oliguric AKI patients (80 with prerenal azotemia and 20 with ATI) without CKD, with FeNa <1% being consistent with prerenal azotemia, 1% to 3% indeterminate, and >3% ATI.[8] The derived LR for FeNa for both prerenal azotemia and ATI are unlikely to alter pretest probability (Table 1). In part, this may be due to Diskin et al.'s incorporation of indeterminate FeNa, consistent with clinical reality. Carvounis et al. did not account for indeterminate values, and consequently the LR were likely overinflated in that study. It is now well‐recognized that glomerulonephritis may also result in FeNa <1% despite absence of identifiable prerenal physiology, as can intravenous iodinated contrast administration and rhabdomyolysis. Moreover, diuretic administration, polyuria due to osmotic diuresis, increased excretion of anions such as ketone bodies in diabetic ketoacidosis, the presence of CKD, and increased age, among others, can produce an FeNa that is indeterminate or >3% in the absence of ATI. Regarding diuretics, although the duration of action of furosemide is approximately 6 hours, longer‐acting loop diuretics such as torsemide or thiazide diuretics such as chlorthalidone may result in natriuresis for 24 hours.
Fractional Excretion of Urea for Diagnosis
Despite the potential superiority of FeUr to FeNa in supporting a diagnosis of prerenal azotemia in the setting of diuretic administration, FeUr nevertheless will only moderately increase the post‐test probability of prerenal azotemia under ideal conditions. In the study by Diskin et al., FeUr <40% was deemed consistent with prerenal azotemia and 40% with ATI. In the diagnosis of prerenal azotemia, the LR+ were 5.5 and 8.2 in the presence and absence of diuretics, respectively. Although the LR+ for the diagnosis of ATI was impressive, this was based on only 9 patients in the ATI‐no diuretic and 11 patients in the ATI‐diuretic groups. Carvounis, moreover, demonstrated considerably lower LR+ of approximately 9 for the diagnosis of ATI, and this study was unable to account for diuretic use specifically within the ATI group. Four of the 5 prerenal patients in Diskin et al.'s study misdiagnosed by FeUr had infection, and each were properly diagnosed by FeNa. Experimental data suggest endotoxemia may downregulate urea transporters as does aging, thereby increasing FeUr in sepsis and the elderly even in times of prerenal azotemia.[9, 10] Moreover, osmotic diuresis, such as with hyperglycemia or sickle cell nephropathy with medullary injury, may result in a falsely negative FeUr during prerenal states. In summary, these data suggest FeUr less than 35% to 40%, with the noted caveats, is most applicable to an oliguric patient in whom the pretest probability of prerenal azotemia is high, and it may be superior in the context of diuretics to the use of FeNa. Nonetheless, the impact on posttest probability is marginal. Of note, the diagnostic categories lack gold standards in these studies, and in the Carvounis study, FeNa (index under study) was 1 of several criteria actually used to categorize patients as either prerenal or ATI (outcomes under study). It is important to recognize these datasets contained very small numbers of patients with ATI, limiting the strength and generalizability of the scientific evidence. Other studies have failed to consistently demonstrate any utility to FeUr, particularly in those with CKD or critical illness.[11, 12, 13, 14, 15]
WHAT YOU SHOULD DO INSTEAD: DECIDE IF VOLUME MANIPULATION IS APPROPRIATE
The gold standard for diagnosis, as in many of the above studies, is the prompt improvement of prerenal azotemia with correction of renal hypoperfusion. Ultimately, the decision to administer intravenous fluids or diuretics in the management of AKI will often be independent of both FeNa and FeUr. In considering, for example, the case described above, it is not possible to realistically dichotomize the patient into either a prerenal or ATI category; both are quite likely present. If the clinical assessment supports a component of prerenal azotemia, a low FeNa and/or FeUr will not change the intervention. An elevated FeNa and/or FeUr, however, has at best moderate and potentially no impact on the likelihood for ATI. A patient, moreover, may still require volume manipulation in the context of established ATI. As such, these indices should not alter therapeutic decisions. There may be value in utilizing and identifying new approaches to determining a priori which patients will be fluid responsive, such as inferior vena cava ultrasound.[16] Lastly, evaluation of the urine sediment is an underutilized tool that may prove more useful in discriminating prerenal azotemia from ATI. It also helps to exclude other etiologies of AKI, such as glomerulonephritis and acute interstitial nephritis, which are typical exclusion criteria in studies of FeNa and FeUr.[17, 18]
WHEN IS FeNa AND/OR FeUr USEFUL IN DEFINING THE ETIOLOGY OF AKI?
FeNa and FeUr at best only support a clinical impression of prerenal azotemia or ATI in oliguric AKI, and the accuracy of these metrics is questionable in the setting of CKD, older age, and a variety of comorbidities. There is, however, a setting in which FeNa may be helpful. In practice, FeNa is useful in the evaluation of hepatorenal syndrome, a disorder characterized by oliguria and intense renal sodium reabsorption with resultant spot urine sodium <10 mEq/L and FeNa <1%.[19]
CONCLUSION
The evidence base supporting the use of FeNa and FeUr is limited and often not generalizable to many patients with AKI. The small sample sizes of the studies do not permit adequate capture of diverse mechanisms for renal injury, and these studies are of patients referred for Nephrology consultation and may not be representative of the larger population of patients with less severe AKI. Ultimately, the true etiology will be proven by time and response to therapy. Apart from a supportive role in the diagnosis of hepatorenal syndrome, there is little practical utility to FeNa and FeUr measurement, and these indices should not alter therapeutic decisions when inconsistent with the clinical impression. The evaluation of AKI requires thoughtful clinical assessment, and the gold standard still remains the judicious decision of when to manipulate the intravascular volume status of a patient. In regard to the presented case, urine chemistries are unhelpful due to the combined vasoconstrictive and tubulotoxic effects of the administered intravenous contrast. The ongoing hypotension further contributes to both pre‐renal as well as ischemic tubular injury.
RECOMMENDATIONS
- FeNa can aid in the diagnosis of hepatorenal syndrome. Otherwise, the routine use of FeNa and FeUr in the diagnosis and management of AKI should be avoided.
- In pre‐renal azotemia, therapeutic intervention is guided by etiology of the disorder (e.g., intravenous crystalloid support based on a history of hypovolemia and ongoing hypoperfusion, diuresis and/or inotropic support in setting of decompensated heart failure, etc.), without regard to baseline FeNa and FeUr.
- In ATI, fluid administration is appropriate if hypovolemia is present. FeNa and FeUr cannot diagnose hypovolemia.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236(6):579–581.
- , , . Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229.
- Centers for Medicare 275(8):630–634.
- , , , . Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35(3):433–447.
- , , , et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89(1):47–50.
- , . Urinary indices and chemistries in the differential diagnosis of prerenal failure and acute tubular necrosis. Semin Nephrol. 1985;5(3):224–233.
- , , , , . The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114(2):c145–c150.
- MachasNúñez JF, Cameron JS, Oreopoulos DG, eds. The Aging Kidney in Health and Disease. New York, NY: Springer Science + Business Media, LLC; 2008.
- , , . Cytokine‐mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292(5):F1479–F1489.
- , , . Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705.
- , , , et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
- , , , et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care. 2012;27(5):505–510.
- , , , . Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50(4):566–573.
- , , , et al. Transient versus persistent acute kidney injury and the diagnostic performance of fractional excretion of urea in critically ill patients. Nephron Clin Pract. 2014;126(1):8–13.
- , , , , . Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290–295.
- , , , , , . Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408.
- , , , , . Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619.
- , , , et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 70‐year‐old woman with a history of diabetes mellitus type 2 and hypertension was admitted with abdominal pain following 2 days of nausea and diarrhea. Initial laboratory studies revealed blood urea nitrogen (BUN) 25 mg/dL and serum creatinine 1.3 mg/dL. Computed tomography of the abdomen and pelvis with nonionic, low osmolar intravenous and oral contrast demonstrated acute diverticulitis with an associated small abscess. She was administered intravenous 0.9% sodium chloride solution and antibiotics. Blood pressure on admission was 92/55 mm Hg, and 24 hours later, her BUN and serum creatinine increased to 33 mg/dL and 1.9 mg/dL, respectively. Her urine output during the preceding 24 hours was 500 mL.
In the evaluation of acute kidney injury (AKI), is the measurement of fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUr) of value?
WHY YOU MIGHT THINK ORDERING FeNa AND/OR FeUr IN THE EVALUATION OF AKI IS HELPFUL
The proper maintenance of sodium balance is paramount to regulating the size of body fluid compartments. Through the interaction of multiple physiologic processes, the kidney regulates tubular reabsorption (or lack thereof) of sodium chloride to match excretion to intake. In normal health, FeNa is typically 1%, although it may vary depending on the dietary sodium intake. The corollary is that 99% of filtered sodium is reabsorbed. Acute tubular injury (ATI) that impairs the tubular resorptive capacity for sodium may increase FeNa to >3%. In addition, during states of water conservation, urea is reabsorbed from the medullary collecting duct, explaining the discrepant rise in BUN relative to creatinine in prerenal azotemia. FeUr falls progressively as water is reabsorbed and urine flow declines, and FeUr less than 35% to 40% may result during prerenal azotemia versus >50% in health or ATI. Theoretically, FeUr is largely unaffected by diuretics, whereas FeNa is increased by diuretics.
In 1976, Espinel reported on the use of FeNa in 17 oliguric patients to discriminate prerenal azotemia from ATI.[1] Establishing what are now familiar indices, FeNa <1% was deemed consistent with prerenal physiology versus >3% indicating ATI. Notably, the study excluded patients who had received diuretics or in whom chronic kidney disease (CKD), glomerulonephritis, or urinary obstruction was suspected.
Given the limitations of FeNa in the context of diuretic use, many physicians instead use FeUr to distinguish prerenal versus ATI causes of AKI. Carvounis et al. reported FeUr and FeNa in 50 patients with prerenal azotemia, 27 with prerenal azotemia receiving diuretics and 25 patients with ATI.[2] Patients with interstitial nephritis, glomerulonephritis, and obstruction were excluded. In the entire cohort, the authors reported sensitivity of 90% and specificity of 96% for FeUr <35% in identifying prerenal azotemia (Table 1). FeNa <1% was slightly less sensitive for prerenal azotemia in the entire cohort at 77%, and this fell to 48% in the presence of diuretics as compared to 89% for FeUr. Naturally, the specificity of FeNa for ATI will fall with the use of diuretics. As shown in Table 1, FeUr <35% has an excellent positive likelihood ratio (LR+) of 22 for prerenal azotemia and a moderate LR+ of 9 for FeUr 35% being consistent with ATI, regardless of the presence of diuretics. This contrasts with FeNa, which if 1% in the presence of diuretics, lacked utility in the diagnosis of ATI. Of note, diuretic use was reported only in the prerenal azotemia group and not specifically in the ATI group. Thus, these comparisons assume diuretics have no effect on test characteristics in ATI. This assumption, however, may not be valid.
| FeUr | FeNa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | LR+ | LR | Sens | Spec | PPV | NPV | LR+ | LR | |||
| ||||||||||||||
| Carvounis[2] | Prerenal | Overall | 0.90 | 0.96 | 0.99 | 0.75 | 22.4 | 0.1 | 0.77 | 0.96 | 0.98 | 0.57 | 19.2 | 0.2 |
| No diuretics | 0.90 | 0.96 | 0.98 | 0.83 | 22.5 | 0.1 | 0.92 | 0.96 | 0.98 | 0.86 | 23.0 | 0.1 | ||
| Diuretics | 0.89 | 0.96 | 0.96 | 0.89 | 22.2 | 0.1 | 0.48 | 0.96 | 0.93 | 0.63 | 12.0 | 0.5 | ||
| ATI | Overall | 0.96 | 0.90 | 0.75 | 0.99 | 9.2 | 0.0 | 0.96 | 0.77 | 0.57 | 0.98 | 4.1 | 0.1 | |
| No diuretics* | 0.96 | 0.90 | 0.83 | 0.98 | 9.6 | 0.0 | 0.96 | 0.92 | 0.86 | 0.98 | 12.0 | 0.0 | ||
| Diuretics | 0.96 | 0.89 | 0.89 | 0.96 | 8.6 | 0.0 | 0.96 | 0.48 | 0.63 | 0.93 | 1.9 | 0.1 | ||
| Diskin[8] | Prerenal | Overall | 0.97 | 0.85 | 0.96 | 0.89 | 6.5 | 0.0 | 0.44 | 0.75 | 0.88 | 0.25 | 1.8 | 0.7 |
| No diuretics | 0.91 | 0.89 | 0.95 | 0.80 | 8.2 | 0.1 | 0.83 | 0.67 | 0.86 | 0.60 | 2.5 | 0.3 | ||
| Diuretics | 1.00 | 0.82 | 0.97 | 1.00 | 5.5 | 0.0 | 0.29 | 0.82 | 0.89 | 0.18 | 1.6 | 0.9 | ||
| ATI | Overall | 0.85 | 0.97 | 0.89 | 0.96 | 33.6 | 0.2 | 0.75 | 0.44 | 0.25 | 0.88 | 1.3 | 0.6 | |
| No diuretics | 0.89 | 0.91 | 0.80 | 0.95 | 10.2 | 0.1 | 0.67 | 0.83 | 0.60 | 0.86 | 3.8 | 0.4 | ||
| Diuretics | 0.82 | 1.00 | 1.00 | 0.97 | N/A | 0.2 | 0.82 | 0.29 | 0.18 | 0.89 | 1.1 | 0.6 | ||
WHY THERE IS LITTLE REASON TO ROUTINELY ORDER FeNa AND FeUr IN PATIENTS WITH AKI
The argument against FeNa and FeUr is not primarily financial. FeNa and FeUr testing on all Medicare patients discharged with AKI in 2013 would have cost US$6 million.[3] Although a tiny fraction of annual healthcare expenditure, it would nevertheless be wasteful spending, and its true harm lays in the application of flawed diagnostic reasoning.
That flaw in our conceptual approach to AKI is the broad categorization of patients into either a prerenal or intrinsic etiology of AKI. In reality, renal injury is often multifactorial, and significant prerenal injury may progress to or coexist with intrinsic disease that is commonly ATI. Measurement of a urinary index at a single point in time will often fail to capture this spectrum of causes for AKI. Unfortunately, accurately assessing volume status through physical examination is difficult.[4] Considering FeNa and FeUr may be low in both hemorrhage as well as congestive heart failure, the measurement of these variables adds little to body volume assessment.
It cannot be overemphasized that application of FeNa and FeUr is predicated on the provider already knowing the diagnosis is either prerenal azotemia or ATI. Studies have generally excluded patients >65 years old and those with CKD or notable comorbid renal processes apart from prerenal azotemia or ATI. It is important to recall that a third of kidney biopsies may yield a diagnosis different than the prebiopsy clinical diagnosis, and the gold standard for ATI in studies of FeNa and FeUr was simply a failure of kidney function to improve promptly.[5] Why send a test that is predicated on largely already knowing the answer?
Fractional Excretion of Sodium for Diagnosis
Unfortunately, FeNa is neither sensitive nor specific enough in the general inpatient population to inform important clinical decisions regarding the etiology of AKI. Miller et al. examined 30 patients with oliguric prerenal azotemia, 55 with ATI (oliguric and nonoliguric), 10 with obstructive uropathy, and 7 with glomerulonephritis.[6] None of the patients had received diuretics within 24 hours of study entry. A FeNa <1% was present in 90% of prerenal patients and 4% of oliguric ATI. Importantly, of nonoliguric patients with ATI, 10% had a false positive FeNa <1%. Many subsequent studies have similarly documented the existence of FeNa <1% or otherwise indeterminate in ATI, particularly, but not exclusively, in nonoliguric states.[7] Diskin et al. evaluated FeNa in 100 prospective oliguric AKI patients (80 with prerenal azotemia and 20 with ATI) without CKD, with FeNa <1% being consistent with prerenal azotemia, 1% to 3% indeterminate, and >3% ATI.[8] The derived LR for FeNa for both prerenal azotemia and ATI are unlikely to alter pretest probability (Table 1). In part, this may be due to Diskin et al.'s incorporation of indeterminate FeNa, consistent with clinical reality. Carvounis et al. did not account for indeterminate values, and consequently the LR were likely overinflated in that study. It is now well‐recognized that glomerulonephritis may also result in FeNa <1% despite absence of identifiable prerenal physiology, as can intravenous iodinated contrast administration and rhabdomyolysis. Moreover, diuretic administration, polyuria due to osmotic diuresis, increased excretion of anions such as ketone bodies in diabetic ketoacidosis, the presence of CKD, and increased age, among others, can produce an FeNa that is indeterminate or >3% in the absence of ATI. Regarding diuretics, although the duration of action of furosemide is approximately 6 hours, longer‐acting loop diuretics such as torsemide or thiazide diuretics such as chlorthalidone may result in natriuresis for 24 hours.
Fractional Excretion of Urea for Diagnosis
Despite the potential superiority of FeUr to FeNa in supporting a diagnosis of prerenal azotemia in the setting of diuretic administration, FeUr nevertheless will only moderately increase the post‐test probability of prerenal azotemia under ideal conditions. In the study by Diskin et al., FeUr <40% was deemed consistent with prerenal azotemia and 40% with ATI. In the diagnosis of prerenal azotemia, the LR+ were 5.5 and 8.2 in the presence and absence of diuretics, respectively. Although the LR+ for the diagnosis of ATI was impressive, this was based on only 9 patients in the ATI‐no diuretic and 11 patients in the ATI‐diuretic groups. Carvounis, moreover, demonstrated considerably lower LR+ of approximately 9 for the diagnosis of ATI, and this study was unable to account for diuretic use specifically within the ATI group. Four of the 5 prerenal patients in Diskin et al.'s study misdiagnosed by FeUr had infection, and each were properly diagnosed by FeNa. Experimental data suggest endotoxemia may downregulate urea transporters as does aging, thereby increasing FeUr in sepsis and the elderly even in times of prerenal azotemia.[9, 10] Moreover, osmotic diuresis, such as with hyperglycemia or sickle cell nephropathy with medullary injury, may result in a falsely negative FeUr during prerenal states. In summary, these data suggest FeUr less than 35% to 40%, with the noted caveats, is most applicable to an oliguric patient in whom the pretest probability of prerenal azotemia is high, and it may be superior in the context of diuretics to the use of FeNa. Nonetheless, the impact on posttest probability is marginal. Of note, the diagnostic categories lack gold standards in these studies, and in the Carvounis study, FeNa (index under study) was 1 of several criteria actually used to categorize patients as either prerenal or ATI (outcomes under study). It is important to recognize these datasets contained very small numbers of patients with ATI, limiting the strength and generalizability of the scientific evidence. Other studies have failed to consistently demonstrate any utility to FeUr, particularly in those with CKD or critical illness.[11, 12, 13, 14, 15]
WHAT YOU SHOULD DO INSTEAD: DECIDE IF VOLUME MANIPULATION IS APPROPRIATE
The gold standard for diagnosis, as in many of the above studies, is the prompt improvement of prerenal azotemia with correction of renal hypoperfusion. Ultimately, the decision to administer intravenous fluids or diuretics in the management of AKI will often be independent of both FeNa and FeUr. In considering, for example, the case described above, it is not possible to realistically dichotomize the patient into either a prerenal or ATI category; both are quite likely present. If the clinical assessment supports a component of prerenal azotemia, a low FeNa and/or FeUr will not change the intervention. An elevated FeNa and/or FeUr, however, has at best moderate and potentially no impact on the likelihood for ATI. A patient, moreover, may still require volume manipulation in the context of established ATI. As such, these indices should not alter therapeutic decisions. There may be value in utilizing and identifying new approaches to determining a priori which patients will be fluid responsive, such as inferior vena cava ultrasound.[16] Lastly, evaluation of the urine sediment is an underutilized tool that may prove more useful in discriminating prerenal azotemia from ATI. It also helps to exclude other etiologies of AKI, such as glomerulonephritis and acute interstitial nephritis, which are typical exclusion criteria in studies of FeNa and FeUr.[17, 18]
WHEN IS FeNa AND/OR FeUr USEFUL IN DEFINING THE ETIOLOGY OF AKI?
FeNa and FeUr at best only support a clinical impression of prerenal azotemia or ATI in oliguric AKI, and the accuracy of these metrics is questionable in the setting of CKD, older age, and a variety of comorbidities. There is, however, a setting in which FeNa may be helpful. In practice, FeNa is useful in the evaluation of hepatorenal syndrome, a disorder characterized by oliguria and intense renal sodium reabsorption with resultant spot urine sodium <10 mEq/L and FeNa <1%.[19]
CONCLUSION
The evidence base supporting the use of FeNa and FeUr is limited and often not generalizable to many patients with AKI. The small sample sizes of the studies do not permit adequate capture of diverse mechanisms for renal injury, and these studies are of patients referred for Nephrology consultation and may not be representative of the larger population of patients with less severe AKI. Ultimately, the true etiology will be proven by time and response to therapy. Apart from a supportive role in the diagnosis of hepatorenal syndrome, there is little practical utility to FeNa and FeUr measurement, and these indices should not alter therapeutic decisions when inconsistent with the clinical impression. The evaluation of AKI requires thoughtful clinical assessment, and the gold standard still remains the judicious decision of when to manipulate the intravascular volume status of a patient. In regard to the presented case, urine chemistries are unhelpful due to the combined vasoconstrictive and tubulotoxic effects of the administered intravenous contrast. The ongoing hypotension further contributes to both pre‐renal as well as ischemic tubular injury.
RECOMMENDATIONS
- FeNa can aid in the diagnosis of hepatorenal syndrome. Otherwise, the routine use of FeNa and FeUr in the diagnosis and management of AKI should be avoided.
- In pre‐renal azotemia, therapeutic intervention is guided by etiology of the disorder (e.g., intravenous crystalloid support based on a history of hypovolemia and ongoing hypoperfusion, diuresis and/or inotropic support in setting of decompensated heart failure, etc.), without regard to baseline FeNa and FeUr.
- In ATI, fluid administration is appropriate if hypovolemia is present. FeNa and FeUr cannot diagnose hypovolemia.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 70‐year‐old woman with a history of diabetes mellitus type 2 and hypertension was admitted with abdominal pain following 2 days of nausea and diarrhea. Initial laboratory studies revealed blood urea nitrogen (BUN) 25 mg/dL and serum creatinine 1.3 mg/dL. Computed tomography of the abdomen and pelvis with nonionic, low osmolar intravenous and oral contrast demonstrated acute diverticulitis with an associated small abscess. She was administered intravenous 0.9% sodium chloride solution and antibiotics. Blood pressure on admission was 92/55 mm Hg, and 24 hours later, her BUN and serum creatinine increased to 33 mg/dL and 1.9 mg/dL, respectively. Her urine output during the preceding 24 hours was 500 mL.
In the evaluation of acute kidney injury (AKI), is the measurement of fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUr) of value?
WHY YOU MIGHT THINK ORDERING FeNa AND/OR FeUr IN THE EVALUATION OF AKI IS HELPFUL
The proper maintenance of sodium balance is paramount to regulating the size of body fluid compartments. Through the interaction of multiple physiologic processes, the kidney regulates tubular reabsorption (or lack thereof) of sodium chloride to match excretion to intake. In normal health, FeNa is typically 1%, although it may vary depending on the dietary sodium intake. The corollary is that 99% of filtered sodium is reabsorbed. Acute tubular injury (ATI) that impairs the tubular resorptive capacity for sodium may increase FeNa to >3%. In addition, during states of water conservation, urea is reabsorbed from the medullary collecting duct, explaining the discrepant rise in BUN relative to creatinine in prerenal azotemia. FeUr falls progressively as water is reabsorbed and urine flow declines, and FeUr less than 35% to 40% may result during prerenal azotemia versus >50% in health or ATI. Theoretically, FeUr is largely unaffected by diuretics, whereas FeNa is increased by diuretics.
In 1976, Espinel reported on the use of FeNa in 17 oliguric patients to discriminate prerenal azotemia from ATI.[1] Establishing what are now familiar indices, FeNa <1% was deemed consistent with prerenal physiology versus >3% indicating ATI. Notably, the study excluded patients who had received diuretics or in whom chronic kidney disease (CKD), glomerulonephritis, or urinary obstruction was suspected.
Given the limitations of FeNa in the context of diuretic use, many physicians instead use FeUr to distinguish prerenal versus ATI causes of AKI. Carvounis et al. reported FeUr and FeNa in 50 patients with prerenal azotemia, 27 with prerenal azotemia receiving diuretics and 25 patients with ATI.[2] Patients with interstitial nephritis, glomerulonephritis, and obstruction were excluded. In the entire cohort, the authors reported sensitivity of 90% and specificity of 96% for FeUr <35% in identifying prerenal azotemia (Table 1). FeNa <1% was slightly less sensitive for prerenal azotemia in the entire cohort at 77%, and this fell to 48% in the presence of diuretics as compared to 89% for FeUr. Naturally, the specificity of FeNa for ATI will fall with the use of diuretics. As shown in Table 1, FeUr <35% has an excellent positive likelihood ratio (LR+) of 22 for prerenal azotemia and a moderate LR+ of 9 for FeUr 35% being consistent with ATI, regardless of the presence of diuretics. This contrasts with FeNa, which if 1% in the presence of diuretics, lacked utility in the diagnosis of ATI. Of note, diuretic use was reported only in the prerenal azotemia group and not specifically in the ATI group. Thus, these comparisons assume diuretics have no effect on test characteristics in ATI. This assumption, however, may not be valid.
| FeUr | FeNa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | LR+ | LR | Sens | Spec | PPV | NPV | LR+ | LR | |||
| ||||||||||||||
| Carvounis[2] | Prerenal | Overall | 0.90 | 0.96 | 0.99 | 0.75 | 22.4 | 0.1 | 0.77 | 0.96 | 0.98 | 0.57 | 19.2 | 0.2 |
| No diuretics | 0.90 | 0.96 | 0.98 | 0.83 | 22.5 | 0.1 | 0.92 | 0.96 | 0.98 | 0.86 | 23.0 | 0.1 | ||
| Diuretics | 0.89 | 0.96 | 0.96 | 0.89 | 22.2 | 0.1 | 0.48 | 0.96 | 0.93 | 0.63 | 12.0 | 0.5 | ||
| ATI | Overall | 0.96 | 0.90 | 0.75 | 0.99 | 9.2 | 0.0 | 0.96 | 0.77 | 0.57 | 0.98 | 4.1 | 0.1 | |
| No diuretics* | 0.96 | 0.90 | 0.83 | 0.98 | 9.6 | 0.0 | 0.96 | 0.92 | 0.86 | 0.98 | 12.0 | 0.0 | ||
| Diuretics | 0.96 | 0.89 | 0.89 | 0.96 | 8.6 | 0.0 | 0.96 | 0.48 | 0.63 | 0.93 | 1.9 | 0.1 | ||
| Diskin[8] | Prerenal | Overall | 0.97 | 0.85 | 0.96 | 0.89 | 6.5 | 0.0 | 0.44 | 0.75 | 0.88 | 0.25 | 1.8 | 0.7 |
| No diuretics | 0.91 | 0.89 | 0.95 | 0.80 | 8.2 | 0.1 | 0.83 | 0.67 | 0.86 | 0.60 | 2.5 | 0.3 | ||
| Diuretics | 1.00 | 0.82 | 0.97 | 1.00 | 5.5 | 0.0 | 0.29 | 0.82 | 0.89 | 0.18 | 1.6 | 0.9 | ||
| ATI | Overall | 0.85 | 0.97 | 0.89 | 0.96 | 33.6 | 0.2 | 0.75 | 0.44 | 0.25 | 0.88 | 1.3 | 0.6 | |
| No diuretics | 0.89 | 0.91 | 0.80 | 0.95 | 10.2 | 0.1 | 0.67 | 0.83 | 0.60 | 0.86 | 3.8 | 0.4 | ||
| Diuretics | 0.82 | 1.00 | 1.00 | 0.97 | N/A | 0.2 | 0.82 | 0.29 | 0.18 | 0.89 | 1.1 | 0.6 | ||
WHY THERE IS LITTLE REASON TO ROUTINELY ORDER FeNa AND FeUr IN PATIENTS WITH AKI
The argument against FeNa and FeUr is not primarily financial. FeNa and FeUr testing on all Medicare patients discharged with AKI in 2013 would have cost US$6 million.[3] Although a tiny fraction of annual healthcare expenditure, it would nevertheless be wasteful spending, and its true harm lays in the application of flawed diagnostic reasoning.
That flaw in our conceptual approach to AKI is the broad categorization of patients into either a prerenal or intrinsic etiology of AKI. In reality, renal injury is often multifactorial, and significant prerenal injury may progress to or coexist with intrinsic disease that is commonly ATI. Measurement of a urinary index at a single point in time will often fail to capture this spectrum of causes for AKI. Unfortunately, accurately assessing volume status through physical examination is difficult.[4] Considering FeNa and FeUr may be low in both hemorrhage as well as congestive heart failure, the measurement of these variables adds little to body volume assessment.
It cannot be overemphasized that application of FeNa and FeUr is predicated on the provider already knowing the diagnosis is either prerenal azotemia or ATI. Studies have generally excluded patients >65 years old and those with CKD or notable comorbid renal processes apart from prerenal azotemia or ATI. It is important to recall that a third of kidney biopsies may yield a diagnosis different than the prebiopsy clinical diagnosis, and the gold standard for ATI in studies of FeNa and FeUr was simply a failure of kidney function to improve promptly.[5] Why send a test that is predicated on largely already knowing the answer?
Fractional Excretion of Sodium for Diagnosis
Unfortunately, FeNa is neither sensitive nor specific enough in the general inpatient population to inform important clinical decisions regarding the etiology of AKI. Miller et al. examined 30 patients with oliguric prerenal azotemia, 55 with ATI (oliguric and nonoliguric), 10 with obstructive uropathy, and 7 with glomerulonephritis.[6] None of the patients had received diuretics within 24 hours of study entry. A FeNa <1% was present in 90% of prerenal patients and 4% of oliguric ATI. Importantly, of nonoliguric patients with ATI, 10% had a false positive FeNa <1%. Many subsequent studies have similarly documented the existence of FeNa <1% or otherwise indeterminate in ATI, particularly, but not exclusively, in nonoliguric states.[7] Diskin et al. evaluated FeNa in 100 prospective oliguric AKI patients (80 with prerenal azotemia and 20 with ATI) without CKD, with FeNa <1% being consistent with prerenal azotemia, 1% to 3% indeterminate, and >3% ATI.[8] The derived LR for FeNa for both prerenal azotemia and ATI are unlikely to alter pretest probability (Table 1). In part, this may be due to Diskin et al.'s incorporation of indeterminate FeNa, consistent with clinical reality. Carvounis et al. did not account for indeterminate values, and consequently the LR were likely overinflated in that study. It is now well‐recognized that glomerulonephritis may also result in FeNa <1% despite absence of identifiable prerenal physiology, as can intravenous iodinated contrast administration and rhabdomyolysis. Moreover, diuretic administration, polyuria due to osmotic diuresis, increased excretion of anions such as ketone bodies in diabetic ketoacidosis, the presence of CKD, and increased age, among others, can produce an FeNa that is indeterminate or >3% in the absence of ATI. Regarding diuretics, although the duration of action of furosemide is approximately 6 hours, longer‐acting loop diuretics such as torsemide or thiazide diuretics such as chlorthalidone may result in natriuresis for 24 hours.
Fractional Excretion of Urea for Diagnosis
Despite the potential superiority of FeUr to FeNa in supporting a diagnosis of prerenal azotemia in the setting of diuretic administration, FeUr nevertheless will only moderately increase the post‐test probability of prerenal azotemia under ideal conditions. In the study by Diskin et al., FeUr <40% was deemed consistent with prerenal azotemia and 40% with ATI. In the diagnosis of prerenal azotemia, the LR+ were 5.5 and 8.2 in the presence and absence of diuretics, respectively. Although the LR+ for the diagnosis of ATI was impressive, this was based on only 9 patients in the ATI‐no diuretic and 11 patients in the ATI‐diuretic groups. Carvounis, moreover, demonstrated considerably lower LR+ of approximately 9 for the diagnosis of ATI, and this study was unable to account for diuretic use specifically within the ATI group. Four of the 5 prerenal patients in Diskin et al.'s study misdiagnosed by FeUr had infection, and each were properly diagnosed by FeNa. Experimental data suggest endotoxemia may downregulate urea transporters as does aging, thereby increasing FeUr in sepsis and the elderly even in times of prerenal azotemia.[9, 10] Moreover, osmotic diuresis, such as with hyperglycemia or sickle cell nephropathy with medullary injury, may result in a falsely negative FeUr during prerenal states. In summary, these data suggest FeUr less than 35% to 40%, with the noted caveats, is most applicable to an oliguric patient in whom the pretest probability of prerenal azotemia is high, and it may be superior in the context of diuretics to the use of FeNa. Nonetheless, the impact on posttest probability is marginal. Of note, the diagnostic categories lack gold standards in these studies, and in the Carvounis study, FeNa (index under study) was 1 of several criteria actually used to categorize patients as either prerenal or ATI (outcomes under study). It is important to recognize these datasets contained very small numbers of patients with ATI, limiting the strength and generalizability of the scientific evidence. Other studies have failed to consistently demonstrate any utility to FeUr, particularly in those with CKD or critical illness.[11, 12, 13, 14, 15]
WHAT YOU SHOULD DO INSTEAD: DECIDE IF VOLUME MANIPULATION IS APPROPRIATE
The gold standard for diagnosis, as in many of the above studies, is the prompt improvement of prerenal azotemia with correction of renal hypoperfusion. Ultimately, the decision to administer intravenous fluids or diuretics in the management of AKI will often be independent of both FeNa and FeUr. In considering, for example, the case described above, it is not possible to realistically dichotomize the patient into either a prerenal or ATI category; both are quite likely present. If the clinical assessment supports a component of prerenal azotemia, a low FeNa and/or FeUr will not change the intervention. An elevated FeNa and/or FeUr, however, has at best moderate and potentially no impact on the likelihood for ATI. A patient, moreover, may still require volume manipulation in the context of established ATI. As such, these indices should not alter therapeutic decisions. There may be value in utilizing and identifying new approaches to determining a priori which patients will be fluid responsive, such as inferior vena cava ultrasound.[16] Lastly, evaluation of the urine sediment is an underutilized tool that may prove more useful in discriminating prerenal azotemia from ATI. It also helps to exclude other etiologies of AKI, such as glomerulonephritis and acute interstitial nephritis, which are typical exclusion criteria in studies of FeNa and FeUr.[17, 18]
WHEN IS FeNa AND/OR FeUr USEFUL IN DEFINING THE ETIOLOGY OF AKI?
FeNa and FeUr at best only support a clinical impression of prerenal azotemia or ATI in oliguric AKI, and the accuracy of these metrics is questionable in the setting of CKD, older age, and a variety of comorbidities. There is, however, a setting in which FeNa may be helpful. In practice, FeNa is useful in the evaluation of hepatorenal syndrome, a disorder characterized by oliguria and intense renal sodium reabsorption with resultant spot urine sodium <10 mEq/L and FeNa <1%.[19]
CONCLUSION
The evidence base supporting the use of FeNa and FeUr is limited and often not generalizable to many patients with AKI. The small sample sizes of the studies do not permit adequate capture of diverse mechanisms for renal injury, and these studies are of patients referred for Nephrology consultation and may not be representative of the larger population of patients with less severe AKI. Ultimately, the true etiology will be proven by time and response to therapy. Apart from a supportive role in the diagnosis of hepatorenal syndrome, there is little practical utility to FeNa and FeUr measurement, and these indices should not alter therapeutic decisions when inconsistent with the clinical impression. The evaluation of AKI requires thoughtful clinical assessment, and the gold standard still remains the judicious decision of when to manipulate the intravascular volume status of a patient. In regard to the presented case, urine chemistries are unhelpful due to the combined vasoconstrictive and tubulotoxic effects of the administered intravenous contrast. The ongoing hypotension further contributes to both pre‐renal as well as ischemic tubular injury.
RECOMMENDATIONS
- FeNa can aid in the diagnosis of hepatorenal syndrome. Otherwise, the routine use of FeNa and FeUr in the diagnosis and management of AKI should be avoided.
- In pre‐renal azotemia, therapeutic intervention is guided by etiology of the disorder (e.g., intravenous crystalloid support based on a history of hypovolemia and ongoing hypoperfusion, diuresis and/or inotropic support in setting of decompensated heart failure, etc.), without regard to baseline FeNa and FeUr.
- In ATI, fluid administration is appropriate if hypovolemia is present. FeNa and FeUr cannot diagnose hypovolemia.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236(6):579–581.
- , , . Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229.
- Centers for Medicare 275(8):630–634.
- , , , . Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35(3):433–447.
- , , , et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89(1):47–50.
- , . Urinary indices and chemistries in the differential diagnosis of prerenal failure and acute tubular necrosis. Semin Nephrol. 1985;5(3):224–233.
- , , , , . The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114(2):c145–c150.
- MachasNúñez JF, Cameron JS, Oreopoulos DG, eds. The Aging Kidney in Health and Disease. New York, NY: Springer Science + Business Media, LLC; 2008.
- , , . Cytokine‐mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292(5):F1479–F1489.
- , , . Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705.
- , , , et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
- , , , et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care. 2012;27(5):505–510.
- , , , . Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50(4):566–573.
- , , , et al. Transient versus persistent acute kidney injury and the diagnostic performance of fractional excretion of urea in critically ill patients. Nephron Clin Pract. 2014;126(1):8–13.
- , , , , . Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290–295.
- , , , , , . Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408.
- , , , , . Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619.
- , , , et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176.
- . The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236(6):579–581.
- , , . Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229.
- Centers for Medicare 275(8):630–634.
- , , , . Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35(3):433–447.
- , , , et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89(1):47–50.
- , . Urinary indices and chemistries in the differential diagnosis of prerenal failure and acute tubular necrosis. Semin Nephrol. 1985;5(3):224–233.
- , , , , . The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114(2):c145–c150.
- MachasNúñez JF, Cameron JS, Oreopoulos DG, eds. The Aging Kidney in Health and Disease. New York, NY: Springer Science + Business Media, LLC; 2008.
- , , . Cytokine‐mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292(5):F1479–F1489.
- , , . Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705.
- , , , et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
- , , , et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care. 2012;27(5):505–510.
- , , , . Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50(4):566–573.
- , , , et al. Transient versus persistent acute kidney injury and the diagnostic performance of fractional excretion of urea in critically ill patients. Nephron Clin Pract. 2014;126(1):8–13.
- , , , , . Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290–295.
- , , , , , . Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408.
- , , , , . Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619.
- , , , et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176.
© 2015 Society of Hospital Medicine
CK‐MB for Chest Pain and Suspected ACS
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 45‐year‐old man with medically controlled hypertension and a 40‐pack‐year smoking history presents to the emergency room complaining of intermittent chest pain for several days. He first noticed a sharp, knifelike sensation in the center of his chest when he reached for a glass in his kitchen a few days ago. The pain lasted for 30 seconds and resolved spontaneously. Since this time, he has had 2 subsequent episodes unrelated to exertion or rest. His physical exam is unremarkable, except for a body mass index of 29. An initial electrocardiogram shows no ischemic changes and no evidence of prior myocardial infarction.
He is currently chest‐painfree and admitted to the inpatient telemetry floor. Is ordering serial sets of creatine kinase (CK), creatine kinase‐myocardial band (CK‐MB), and troponin the most high‐value method to evaluate him for acute coronary syndrome (ACS)?
WHY YOU MIGHT THINK CK‐MB TESTING IS HELPFUL
CK‐MB has been used for 4 decades in the diagnostic evaluation of patients with chest pain and suspected ACS. Despite the advent of a more sensitive and specific test for myocardial injurythe cardiac troponinnearly 3 decades ago, 75% of US clinical pathology laboratories perform both CK‐MB and troponin assays, suggesting that many US physicians continue to order both tests in evaluating patients with chest pain.[1] There are several clinical scenarios in which physicians generally regard CK‐MB testing as useful in addition to troponin. These scenarios include CK‐MB testing (1) for the diagnosis of ACS in special patient populations, like those with acute or chronic renal disease, who are thought to have chronically elevated troponins as a function of their renal disease and not myocardial disease; (2) for additional prognostic information in the setting of a minimally elevated troponin; (3) for the detection of reinfarction, in which troponin is thought to be inferior to CK‐MB; and (4) for estimation of infarct size.
WHY CK‐MB TESTING ADDS NO ADDITIONAL VALUE TO TROPONIN TESTING IN DIAGNOSIS OF ACS
Is CK‐MB More Accurate Than Troponin in the Diagnosis of ACS?
Numerous studies have established that CK‐MB is not as specific as troponin for detecting myocardial injury and will result in more false‐positive tests.[2, 3] CK‐MB can be elevated in the setting of acute muscle injury (in 60% of patients), as well as chronic muscle disease (in 80% of patients). In contrast, troponin (I or T), a protein exclusively found in cardiac myocytes, is only elevated due to myocardial injury and is therefore more specific for ACS than CK‐MB.[2] In a study of patients with both skeletal muscle injury and suspected ACS, the respective specificities of troponin and CK‐MB were 94% and 63%, respectively.[3] In special patient populations, like those with chronic renal disease, both troponin and CK‐MB can be elevated in the absence of ACS; the mechanism for cardiac enzyme elevation is unclear. Importantly, there is no evidence to support the incremental value of CK‐MB over troponin alone in this population.[4, 5] Despite chronic troponin and CK‐MB elevations in some patients with chronic renal failure, it is still possible for these patients to have acute changes from baseline that represent myocardial injury. In these patients, cardiac biomarker results must be considered in the context of other clinical features (ie, the patient history, physical exam, and electrocardiogram findings) in making or excluding the diagnosis of ACS.
Does CK‐MB Diagnose ACS More Rapidly Than Troponin?
In patients with myocardial injury, both troponin and CK‐MB typically are detectable in the bloodstream within 2 to 4 hours of symptom onset and peak within 12 to 18 hours; neither has been established as a more rapid biomarker for the detection of myocardial infarction.[6] Furthermore, a systemic review of point‐of‐care cardiac enzyme testing reported that troponin and CK‐MB had similar positive and negative predictive values for diagnosing acute myocardial infarction (AMI) within the first 6 hours of symptom onset.[7]
Does CK‐MB Add Prognostic Information in Addition to Troponin in Patients With ACS?
If CK‐MB adds additional prognostic information in patients with suspected ACS and normal troponin values, then we should continue using it. Based on several large registries of patients with chest pain and/or ACS, approximately 8% to 28% of patients have discordant CK‐MB and troponin values, where 1 value is normal while the other value is abnormal. Several studies have examined whether an abnormal CK‐MB, in the setting of a normal troponin, offers additional prognostic information in comparison with normal values of both biomarkers.
In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) registry, a cohort of 29,357 patients with ACS was retrospectively divided into 4 groups: (1) patients with abnormal CK‐MB (CK‐MB+) and troponin (Tn+) values (ie, double‐positive group); (2) patients with normal CK‐MB (CK‐MB) and troponin (Tn) values (ie, double‐negative group); (3) patients with CK‐MB+/Tn; and (4) patients with CK‐MB/Tn+ values. Among the 4 groups, the rate of in‐hospital mortality was not significantly different between CK‐MB+/Tn (group 3) and patients with double‐negative (ie, normal) values. However, the presence of an abnormal troponin, regardless of CK‐MB status, was associated with an increased risk of in‐hospital death. The authors concluded that in clinical practice, there is little advantage of simultaneous CK‐MB and cTn testing for risk stratification in patients with high‐risk ACS presentations.[8]
In addition to the CRUSADE registry, 2 smaller registries, involving different patient populations, have reported similar results. An analysis of the Global Registry of Acute Coronary Events (GRACE) registry of 10,719 patients with ACS reported no difference between CK‐MB+/Tn patients and double‐negative patients with respect to in‐hospital mortality, as well as 6‐month mortality.[9] In the Internet Tracking Registry of Acute Coronary Syndromes (ITRACS) registry, 8769 patients presenting to emergency rooms with chest pain were analyzed. A minority (18.4%) were ultimately diagnosed with ACS. The authors found that an abnormal troponin, irrespective of CK‐MB status, was associated with an increased in‐hospital mortality rate. In‐hospital death rates were similar between CK‐MB+/Tn and double‐negative patients.[10]
In summary, troponin offers important prognostic information regardless of the CK‐MB result.
Is CK‐MB More Accurate for Diagnosing Reinfarction (Repeat Infarction in Patients With Recent Acute Myocardial Infarction)?
Whereas CK‐MB typically returns to normal within 2 to 3 days, troponin can be elevated for up to 5 to 14 days. Consequently, some have argued that CK‐MB may be more accurate in detecting reinfarction. In the only study to date comparing CK‐MB and troponin patterns in 9 patients with reinfarction, the rise and fall of both biomarkers were similar. Furthermore, those patients with persistently elevated troponin values from baseline (after the initial infarction) experienced a significant rise in troponin with reinfarction.[11]
Is CK‐MB More Accurate for Estimating Infarct Size?
Some have argued that a peak CK‐MB value is more accurate than a peak troponin value for estimating infarct size. However, 2 comparative studies have reported that troponin is as good as and possibly superior to CK‐MB for estimating infarct size. In a study of 65 patients with AMI, a single troponin T measurement obtained 72 hours after coronary care unit admission significantly correlated with peak CK‐MB in estimating infarct size (r=0.76, P<0.001), using single‐photon emission computed tomography imaging as the gold standard.[12] In a similar study of 37 patients with AMI, a single troponin T value had a significantly higher correlation with infarct size than serial and peak CK‐MB. Unlike CK‐MB, the ability of troponin T to predict infarct size was independent of coronary reperfusion.[13]
What do Guidelines and Thought Leaders Say About Using CK‐MB?
The most recent Third Universal Definition of Myocardial Infarction states that troponin is the preferred (cardiac) biomarker‐overall and for each specific category of MI, and that CK‐MB should be considered an alternative if troponin is not available.[14] Several national guidelines endorse troponin as the primary cardiac biomarker for diagnosis of ACS.[15, 16, 17] Finally, several groups have called for the elimination of CK‐MB. In 2008, 2 experts in the field of cardiovascular laboratory medicine argued that CK‐MB test adds little to no incremental information but does add cost andconfusion. Their institution, the Mayo Clinic, removed CK‐MB from their cardiac biomarker panel without any discernible negative effects on clinical care.[6] In a more recent publication, a group of authors from the departments of pathology and laboratory medicine of 7 major US academic medical centers identified CK‐MB as part of a top 10 list of antiquated tests that no longer provide value.[18]
WHAT YOU SHOULD DO INSTEAD: ORDER TROPONIN ALONE
In all cases where a patient presents with chest pain and/or symptoms concerning for ACS, we recommend that troponin be ordered alone. CK‐MB is no longer necessary as an additional test. As healthcare providers, we aim to provide the highest healthcare valuedefined as clinical benefit divided by cost. Routine ordering of CK‐MB offers essentially no benefit but does come at a significant cost. Each CK‐MB costs roughly $40 to $50 a test. If CK‐MB is used in approximately 2 million patients annually diagnosed with ACS and a proportion of the 17 million patients annually evaluated for chest pain, the potential cost, without clear benefit, is substantial.[19]
RECOMMENDATIONS
- In patients suspected of having ACS, troponin should be measured in lieu of CK‐MB and serial CK testing to evaluate for myocardial injury.
- CK‐MB tests should not be ordered routinely for patients suspected of having ACS. Hospitals should remove CK‐MB from pathology lab catalogs or require specific permission to order it.
CONCLUSION
Because CK‐MB, as compared to troponin, is detectable in the bloodstream in a similar timeframe, adds no additional prognostic information, estimates infarct size no differently, and appears to diagnose reinfarction no differently (Table 1), the authors believe that CK‐MB should no longer be ordered for patients with suspected ACS, unless ordering troponin is not an option. Ordering CK‐MB and serial CK for the evaluation of ACS is a Thing We Do for No Reason.
| Test Characteristic | CK‐MB | Troponin |
|---|---|---|
| ||
| Sensitivity | Lower than troponin | Higher than CK‐MB |
| Specificity | 60% to 70% | >94% |
| Diagnostic accuracy in patients with chronic renal failure | Equivalent | Equivalent |
| Rapidity of diagnosis | 24 hours | 2‐4 hours |
| Estimation of infarct size | Equivalent or possibly inferior to troponin | Equivalent or possibly superior to CK‐MB |
| Diagnosis of reinfarction | Equivalent | Equivalent |
Disclosures
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing
- , Creatine kinase‐MB: the journey to obsolescence. Am J Clin Pathol. 2014;141:415–419.
- , , , et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912.
- , , , et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993:88:101–106.
- , Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60(2):281–290.
- , , , , Unmasking artifactual increases in creatine kinase isoenzymes in patients with renal failure. J Clin Lab Med. 1984;104:193–202.
- , Requiem for a heavyweight: the demise of creatine kinase‐MB. Circulation. 2008;118(21):2200–2206.
- , , , , Int J Cardiol. 2013;168(6):5355–5362.
- , , , et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312–318.
- , , , et al.; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2006;151(3):654–660.
- , , , et al.; EMCREG‐i*trACS Investigators. Discordant cardiac biomarkers: frequency and outcomes in emergency department patients with chest pain. Ann Emerg Med. 2006:48(6):660–665.
- , Cardiac troponin and creatine kinase MB monitoring during in‐hospital myocardial reinfarction. Clin Chem. 2005;51:460–463.
- , , , , , Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–1436
- , , , , , Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524.
- , , , et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.
- , , , et al. Institute for Clinical Systems Improvement. Diagnosis and Treatment of Chest Pain and Acute Coronary Syndrome (ACS). Available at: http://bit.ly.ACS1112. Updated November 2012.
- , , , et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–e347.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, , , , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.
- , , , , , Antiquated tests within the clinical pathology laboratory. Am J Manag Care. 2010;16(9):e220–e227.
- , , , , Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468–1474.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 45‐year‐old man with medically controlled hypertension and a 40‐pack‐year smoking history presents to the emergency room complaining of intermittent chest pain for several days. He first noticed a sharp, knifelike sensation in the center of his chest when he reached for a glass in his kitchen a few days ago. The pain lasted for 30 seconds and resolved spontaneously. Since this time, he has had 2 subsequent episodes unrelated to exertion or rest. His physical exam is unremarkable, except for a body mass index of 29. An initial electrocardiogram shows no ischemic changes and no evidence of prior myocardial infarction.
He is currently chest‐painfree and admitted to the inpatient telemetry floor. Is ordering serial sets of creatine kinase (CK), creatine kinase‐myocardial band (CK‐MB), and troponin the most high‐value method to evaluate him for acute coronary syndrome (ACS)?
WHY YOU MIGHT THINK CK‐MB TESTING IS HELPFUL
CK‐MB has been used for 4 decades in the diagnostic evaluation of patients with chest pain and suspected ACS. Despite the advent of a more sensitive and specific test for myocardial injurythe cardiac troponinnearly 3 decades ago, 75% of US clinical pathology laboratories perform both CK‐MB and troponin assays, suggesting that many US physicians continue to order both tests in evaluating patients with chest pain.[1] There are several clinical scenarios in which physicians generally regard CK‐MB testing as useful in addition to troponin. These scenarios include CK‐MB testing (1) for the diagnosis of ACS in special patient populations, like those with acute or chronic renal disease, who are thought to have chronically elevated troponins as a function of their renal disease and not myocardial disease; (2) for additional prognostic information in the setting of a minimally elevated troponin; (3) for the detection of reinfarction, in which troponin is thought to be inferior to CK‐MB; and (4) for estimation of infarct size.
WHY CK‐MB TESTING ADDS NO ADDITIONAL VALUE TO TROPONIN TESTING IN DIAGNOSIS OF ACS
Is CK‐MB More Accurate Than Troponin in the Diagnosis of ACS?
Numerous studies have established that CK‐MB is not as specific as troponin for detecting myocardial injury and will result in more false‐positive tests.[2, 3] CK‐MB can be elevated in the setting of acute muscle injury (in 60% of patients), as well as chronic muscle disease (in 80% of patients). In contrast, troponin (I or T), a protein exclusively found in cardiac myocytes, is only elevated due to myocardial injury and is therefore more specific for ACS than CK‐MB.[2] In a study of patients with both skeletal muscle injury and suspected ACS, the respective specificities of troponin and CK‐MB were 94% and 63%, respectively.[3] In special patient populations, like those with chronic renal disease, both troponin and CK‐MB can be elevated in the absence of ACS; the mechanism for cardiac enzyme elevation is unclear. Importantly, there is no evidence to support the incremental value of CK‐MB over troponin alone in this population.[4, 5] Despite chronic troponin and CK‐MB elevations in some patients with chronic renal failure, it is still possible for these patients to have acute changes from baseline that represent myocardial injury. In these patients, cardiac biomarker results must be considered in the context of other clinical features (ie, the patient history, physical exam, and electrocardiogram findings) in making or excluding the diagnosis of ACS.
Does CK‐MB Diagnose ACS More Rapidly Than Troponin?
In patients with myocardial injury, both troponin and CK‐MB typically are detectable in the bloodstream within 2 to 4 hours of symptom onset and peak within 12 to 18 hours; neither has been established as a more rapid biomarker for the detection of myocardial infarction.[6] Furthermore, a systemic review of point‐of‐care cardiac enzyme testing reported that troponin and CK‐MB had similar positive and negative predictive values for diagnosing acute myocardial infarction (AMI) within the first 6 hours of symptom onset.[7]
Does CK‐MB Add Prognostic Information in Addition to Troponin in Patients With ACS?
If CK‐MB adds additional prognostic information in patients with suspected ACS and normal troponin values, then we should continue using it. Based on several large registries of patients with chest pain and/or ACS, approximately 8% to 28% of patients have discordant CK‐MB and troponin values, where 1 value is normal while the other value is abnormal. Several studies have examined whether an abnormal CK‐MB, in the setting of a normal troponin, offers additional prognostic information in comparison with normal values of both biomarkers.
In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) registry, a cohort of 29,357 patients with ACS was retrospectively divided into 4 groups: (1) patients with abnormal CK‐MB (CK‐MB+) and troponin (Tn+) values (ie, double‐positive group); (2) patients with normal CK‐MB (CK‐MB) and troponin (Tn) values (ie, double‐negative group); (3) patients with CK‐MB+/Tn; and (4) patients with CK‐MB/Tn+ values. Among the 4 groups, the rate of in‐hospital mortality was not significantly different between CK‐MB+/Tn (group 3) and patients with double‐negative (ie, normal) values. However, the presence of an abnormal troponin, regardless of CK‐MB status, was associated with an increased risk of in‐hospital death. The authors concluded that in clinical practice, there is little advantage of simultaneous CK‐MB and cTn testing for risk stratification in patients with high‐risk ACS presentations.[8]
In addition to the CRUSADE registry, 2 smaller registries, involving different patient populations, have reported similar results. An analysis of the Global Registry of Acute Coronary Events (GRACE) registry of 10,719 patients with ACS reported no difference between CK‐MB+/Tn patients and double‐negative patients with respect to in‐hospital mortality, as well as 6‐month mortality.[9] In the Internet Tracking Registry of Acute Coronary Syndromes (ITRACS) registry, 8769 patients presenting to emergency rooms with chest pain were analyzed. A minority (18.4%) were ultimately diagnosed with ACS. The authors found that an abnormal troponin, irrespective of CK‐MB status, was associated with an increased in‐hospital mortality rate. In‐hospital death rates were similar between CK‐MB+/Tn and double‐negative patients.[10]
In summary, troponin offers important prognostic information regardless of the CK‐MB result.
Is CK‐MB More Accurate for Diagnosing Reinfarction (Repeat Infarction in Patients With Recent Acute Myocardial Infarction)?
Whereas CK‐MB typically returns to normal within 2 to 3 days, troponin can be elevated for up to 5 to 14 days. Consequently, some have argued that CK‐MB may be more accurate in detecting reinfarction. In the only study to date comparing CK‐MB and troponin patterns in 9 patients with reinfarction, the rise and fall of both biomarkers were similar. Furthermore, those patients with persistently elevated troponin values from baseline (after the initial infarction) experienced a significant rise in troponin with reinfarction.[11]
Is CK‐MB More Accurate for Estimating Infarct Size?
Some have argued that a peak CK‐MB value is more accurate than a peak troponin value for estimating infarct size. However, 2 comparative studies have reported that troponin is as good as and possibly superior to CK‐MB for estimating infarct size. In a study of 65 patients with AMI, a single troponin T measurement obtained 72 hours after coronary care unit admission significantly correlated with peak CK‐MB in estimating infarct size (r=0.76, P<0.001), using single‐photon emission computed tomography imaging as the gold standard.[12] In a similar study of 37 patients with AMI, a single troponin T value had a significantly higher correlation with infarct size than serial and peak CK‐MB. Unlike CK‐MB, the ability of troponin T to predict infarct size was independent of coronary reperfusion.[13]
What do Guidelines and Thought Leaders Say About Using CK‐MB?
The most recent Third Universal Definition of Myocardial Infarction states that troponin is the preferred (cardiac) biomarker‐overall and for each specific category of MI, and that CK‐MB should be considered an alternative if troponin is not available.[14] Several national guidelines endorse troponin as the primary cardiac biomarker for diagnosis of ACS.[15, 16, 17] Finally, several groups have called for the elimination of CK‐MB. In 2008, 2 experts in the field of cardiovascular laboratory medicine argued that CK‐MB test adds little to no incremental information but does add cost andconfusion. Their institution, the Mayo Clinic, removed CK‐MB from their cardiac biomarker panel without any discernible negative effects on clinical care.[6] In a more recent publication, a group of authors from the departments of pathology and laboratory medicine of 7 major US academic medical centers identified CK‐MB as part of a top 10 list of antiquated tests that no longer provide value.[18]
WHAT YOU SHOULD DO INSTEAD: ORDER TROPONIN ALONE
In all cases where a patient presents with chest pain and/or symptoms concerning for ACS, we recommend that troponin be ordered alone. CK‐MB is no longer necessary as an additional test. As healthcare providers, we aim to provide the highest healthcare valuedefined as clinical benefit divided by cost. Routine ordering of CK‐MB offers essentially no benefit but does come at a significant cost. Each CK‐MB costs roughly $40 to $50 a test. If CK‐MB is used in approximately 2 million patients annually diagnosed with ACS and a proportion of the 17 million patients annually evaluated for chest pain, the potential cost, without clear benefit, is substantial.[19]
RECOMMENDATIONS
- In patients suspected of having ACS, troponin should be measured in lieu of CK‐MB and serial CK testing to evaluate for myocardial injury.
- CK‐MB tests should not be ordered routinely for patients suspected of having ACS. Hospitals should remove CK‐MB from pathology lab catalogs or require specific permission to order it.
CONCLUSION
Because CK‐MB, as compared to troponin, is detectable in the bloodstream in a similar timeframe, adds no additional prognostic information, estimates infarct size no differently, and appears to diagnose reinfarction no differently (Table 1), the authors believe that CK‐MB should no longer be ordered for patients with suspected ACS, unless ordering troponin is not an option. Ordering CK‐MB and serial CK for the evaluation of ACS is a Thing We Do for No Reason.
| Test Characteristic | CK‐MB | Troponin |
|---|---|---|
| ||
| Sensitivity | Lower than troponin | Higher than CK‐MB |
| Specificity | 60% to 70% | >94% |
| Diagnostic accuracy in patients with chronic renal failure | Equivalent | Equivalent |
| Rapidity of diagnosis | 24 hours | 2‐4 hours |
| Estimation of infarct size | Equivalent or possibly inferior to troponin | Equivalent or possibly superior to CK‐MB |
| Diagnosis of reinfarction | Equivalent | Equivalent |
Disclosures
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 45‐year‐old man with medically controlled hypertension and a 40‐pack‐year smoking history presents to the emergency room complaining of intermittent chest pain for several days. He first noticed a sharp, knifelike sensation in the center of his chest when he reached for a glass in his kitchen a few days ago. The pain lasted for 30 seconds and resolved spontaneously. Since this time, he has had 2 subsequent episodes unrelated to exertion or rest. His physical exam is unremarkable, except for a body mass index of 29. An initial electrocardiogram shows no ischemic changes and no evidence of prior myocardial infarction.
He is currently chest‐painfree and admitted to the inpatient telemetry floor. Is ordering serial sets of creatine kinase (CK), creatine kinase‐myocardial band (CK‐MB), and troponin the most high‐value method to evaluate him for acute coronary syndrome (ACS)?
WHY YOU MIGHT THINK CK‐MB TESTING IS HELPFUL
CK‐MB has been used for 4 decades in the diagnostic evaluation of patients with chest pain and suspected ACS. Despite the advent of a more sensitive and specific test for myocardial injurythe cardiac troponinnearly 3 decades ago, 75% of US clinical pathology laboratories perform both CK‐MB and troponin assays, suggesting that many US physicians continue to order both tests in evaluating patients with chest pain.[1] There are several clinical scenarios in which physicians generally regard CK‐MB testing as useful in addition to troponin. These scenarios include CK‐MB testing (1) for the diagnosis of ACS in special patient populations, like those with acute or chronic renal disease, who are thought to have chronically elevated troponins as a function of their renal disease and not myocardial disease; (2) for additional prognostic information in the setting of a minimally elevated troponin; (3) for the detection of reinfarction, in which troponin is thought to be inferior to CK‐MB; and (4) for estimation of infarct size.
WHY CK‐MB TESTING ADDS NO ADDITIONAL VALUE TO TROPONIN TESTING IN DIAGNOSIS OF ACS
Is CK‐MB More Accurate Than Troponin in the Diagnosis of ACS?
Numerous studies have established that CK‐MB is not as specific as troponin for detecting myocardial injury and will result in more false‐positive tests.[2, 3] CK‐MB can be elevated in the setting of acute muscle injury (in 60% of patients), as well as chronic muscle disease (in 80% of patients). In contrast, troponin (I or T), a protein exclusively found in cardiac myocytes, is only elevated due to myocardial injury and is therefore more specific for ACS than CK‐MB.[2] In a study of patients with both skeletal muscle injury and suspected ACS, the respective specificities of troponin and CK‐MB were 94% and 63%, respectively.[3] In special patient populations, like those with chronic renal disease, both troponin and CK‐MB can be elevated in the absence of ACS; the mechanism for cardiac enzyme elevation is unclear. Importantly, there is no evidence to support the incremental value of CK‐MB over troponin alone in this population.[4, 5] Despite chronic troponin and CK‐MB elevations in some patients with chronic renal failure, it is still possible for these patients to have acute changes from baseline that represent myocardial injury. In these patients, cardiac biomarker results must be considered in the context of other clinical features (ie, the patient history, physical exam, and electrocardiogram findings) in making or excluding the diagnosis of ACS.
Does CK‐MB Diagnose ACS More Rapidly Than Troponin?
In patients with myocardial injury, both troponin and CK‐MB typically are detectable in the bloodstream within 2 to 4 hours of symptom onset and peak within 12 to 18 hours; neither has been established as a more rapid biomarker for the detection of myocardial infarction.[6] Furthermore, a systemic review of point‐of‐care cardiac enzyme testing reported that troponin and CK‐MB had similar positive and negative predictive values for diagnosing acute myocardial infarction (AMI) within the first 6 hours of symptom onset.[7]
Does CK‐MB Add Prognostic Information in Addition to Troponin in Patients With ACS?
If CK‐MB adds additional prognostic information in patients with suspected ACS and normal troponin values, then we should continue using it. Based on several large registries of patients with chest pain and/or ACS, approximately 8% to 28% of patients have discordant CK‐MB and troponin values, where 1 value is normal while the other value is abnormal. Several studies have examined whether an abnormal CK‐MB, in the setting of a normal troponin, offers additional prognostic information in comparison with normal values of both biomarkers.
In the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) registry, a cohort of 29,357 patients with ACS was retrospectively divided into 4 groups: (1) patients with abnormal CK‐MB (CK‐MB+) and troponin (Tn+) values (ie, double‐positive group); (2) patients with normal CK‐MB (CK‐MB) and troponin (Tn) values (ie, double‐negative group); (3) patients with CK‐MB+/Tn; and (4) patients with CK‐MB/Tn+ values. Among the 4 groups, the rate of in‐hospital mortality was not significantly different between CK‐MB+/Tn (group 3) and patients with double‐negative (ie, normal) values. However, the presence of an abnormal troponin, regardless of CK‐MB status, was associated with an increased risk of in‐hospital death. The authors concluded that in clinical practice, there is little advantage of simultaneous CK‐MB and cTn testing for risk stratification in patients with high‐risk ACS presentations.[8]
In addition to the CRUSADE registry, 2 smaller registries, involving different patient populations, have reported similar results. An analysis of the Global Registry of Acute Coronary Events (GRACE) registry of 10,719 patients with ACS reported no difference between CK‐MB+/Tn patients and double‐negative patients with respect to in‐hospital mortality, as well as 6‐month mortality.[9] In the Internet Tracking Registry of Acute Coronary Syndromes (ITRACS) registry, 8769 patients presenting to emergency rooms with chest pain were analyzed. A minority (18.4%) were ultimately diagnosed with ACS. The authors found that an abnormal troponin, irrespective of CK‐MB status, was associated with an increased in‐hospital mortality rate. In‐hospital death rates were similar between CK‐MB+/Tn and double‐negative patients.[10]
In summary, troponin offers important prognostic information regardless of the CK‐MB result.
Is CK‐MB More Accurate for Diagnosing Reinfarction (Repeat Infarction in Patients With Recent Acute Myocardial Infarction)?
Whereas CK‐MB typically returns to normal within 2 to 3 days, troponin can be elevated for up to 5 to 14 days. Consequently, some have argued that CK‐MB may be more accurate in detecting reinfarction. In the only study to date comparing CK‐MB and troponin patterns in 9 patients with reinfarction, the rise and fall of both biomarkers were similar. Furthermore, those patients with persistently elevated troponin values from baseline (after the initial infarction) experienced a significant rise in troponin with reinfarction.[11]
Is CK‐MB More Accurate for Estimating Infarct Size?
Some have argued that a peak CK‐MB value is more accurate than a peak troponin value for estimating infarct size. However, 2 comparative studies have reported that troponin is as good as and possibly superior to CK‐MB for estimating infarct size. In a study of 65 patients with AMI, a single troponin T measurement obtained 72 hours after coronary care unit admission significantly correlated with peak CK‐MB in estimating infarct size (r=0.76, P<0.001), using single‐photon emission computed tomography imaging as the gold standard.[12] In a similar study of 37 patients with AMI, a single troponin T value had a significantly higher correlation with infarct size than serial and peak CK‐MB. Unlike CK‐MB, the ability of troponin T to predict infarct size was independent of coronary reperfusion.[13]
What do Guidelines and Thought Leaders Say About Using CK‐MB?
The most recent Third Universal Definition of Myocardial Infarction states that troponin is the preferred (cardiac) biomarker‐overall and for each specific category of MI, and that CK‐MB should be considered an alternative if troponin is not available.[14] Several national guidelines endorse troponin as the primary cardiac biomarker for diagnosis of ACS.[15, 16, 17] Finally, several groups have called for the elimination of CK‐MB. In 2008, 2 experts in the field of cardiovascular laboratory medicine argued that CK‐MB test adds little to no incremental information but does add cost andconfusion. Their institution, the Mayo Clinic, removed CK‐MB from their cardiac biomarker panel without any discernible negative effects on clinical care.[6] In a more recent publication, a group of authors from the departments of pathology and laboratory medicine of 7 major US academic medical centers identified CK‐MB as part of a top 10 list of antiquated tests that no longer provide value.[18]
WHAT YOU SHOULD DO INSTEAD: ORDER TROPONIN ALONE
In all cases where a patient presents with chest pain and/or symptoms concerning for ACS, we recommend that troponin be ordered alone. CK‐MB is no longer necessary as an additional test. As healthcare providers, we aim to provide the highest healthcare valuedefined as clinical benefit divided by cost. Routine ordering of CK‐MB offers essentially no benefit but does come at a significant cost. Each CK‐MB costs roughly $40 to $50 a test. If CK‐MB is used in approximately 2 million patients annually diagnosed with ACS and a proportion of the 17 million patients annually evaluated for chest pain, the potential cost, without clear benefit, is substantial.[19]
RECOMMENDATIONS
- In patients suspected of having ACS, troponin should be measured in lieu of CK‐MB and serial CK testing to evaluate for myocardial injury.
- CK‐MB tests should not be ordered routinely for patients suspected of having ACS. Hospitals should remove CK‐MB from pathology lab catalogs or require specific permission to order it.
CONCLUSION
Because CK‐MB, as compared to troponin, is detectable in the bloodstream in a similar timeframe, adds no additional prognostic information, estimates infarct size no differently, and appears to diagnose reinfarction no differently (Table 1), the authors believe that CK‐MB should no longer be ordered for patients with suspected ACS, unless ordering troponin is not an option. Ordering CK‐MB and serial CK for the evaluation of ACS is a Thing We Do for No Reason.
| Test Characteristic | CK‐MB | Troponin |
|---|---|---|
| ||
| Sensitivity | Lower than troponin | Higher than CK‐MB |
| Specificity | 60% to 70% | >94% |
| Diagnostic accuracy in patients with chronic renal failure | Equivalent | Equivalent |
| Rapidity of diagnosis | 24 hours | 2‐4 hours |
| Estimation of infarct size | Equivalent or possibly inferior to troponin | Equivalent or possibly superior to CK‐MB |
| Diagnosis of reinfarction | Equivalent | Equivalent |
Disclosures
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing
- , Creatine kinase‐MB: the journey to obsolescence. Am J Clin Pathol. 2014;141:415–419.
- , , , et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912.
- , , , et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993:88:101–106.
- , Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60(2):281–290.
- , , , , Unmasking artifactual increases in creatine kinase isoenzymes in patients with renal failure. J Clin Lab Med. 1984;104:193–202.
- , Requiem for a heavyweight: the demise of creatine kinase‐MB. Circulation. 2008;118(21):2200–2206.
- , , , , Int J Cardiol. 2013;168(6):5355–5362.
- , , , et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312–318.
- , , , et al.; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2006;151(3):654–660.
- , , , et al.; EMCREG‐i*trACS Investigators. Discordant cardiac biomarkers: frequency and outcomes in emergency department patients with chest pain. Ann Emerg Med. 2006:48(6):660–665.
- , Cardiac troponin and creatine kinase MB monitoring during in‐hospital myocardial reinfarction. Clin Chem. 2005;51:460–463.
- , , , , , Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–1436
- , , , , , Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524.
- , , , et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.
- , , , et al. Institute for Clinical Systems Improvement. Diagnosis and Treatment of Chest Pain and Acute Coronary Syndrome (ACS). Available at: http://bit.ly.ACS1112. Updated November 2012.
- , , , et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–e347.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, , , , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.
- , , , , , Antiquated tests within the clinical pathology laboratory. Am J Manag Care. 2010;16(9):e220–e227.
- , , , , Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468–1474.
- , Creatine kinase‐MB: the journey to obsolescence. Am J Clin Pathol. 2014;141:415–419.
- , , , et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912.
- , , , et al. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993:88:101–106.
- , Cardiac troponin I in patients with chronic kidney disease stage 3 to 5 in conditions other than acute coronary syndrome. Clin Lab. 2014;60(2):281–290.
- , , , , Unmasking artifactual increases in creatine kinase isoenzymes in patients with renal failure. J Clin Lab Med. 1984;104:193–202.
- , Requiem for a heavyweight: the demise of creatine kinase‐MB. Circulation. 2008;118(21):2200–2206.
- , , , , Int J Cardiol. 2013;168(6):5355–5362.
- , , , et al. Frequency and clinical implications of discordant creatine kinase‐MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol. 2006;47(2):312–318.
- , , , et al.; GRACE Investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2006;151(3):654–660.
- , , , et al.; EMCREG‐i*trACS Investigators. Discordant cardiac biomarkers: frequency and outcomes in emergency department patients with chest pain. Ann Emerg Med. 2006:48(6):660–665.
- , Cardiac troponin and creatine kinase MB monitoring during in‐hospital myocardial reinfarction. Clin Chem. 2005;51:460–463.
- , , , , , Single‐point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–1436
- , , , , , Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524.
- , , , et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.
- , , , et al. Institute for Clinical Systems Improvement. Diagnosis and Treatment of Chest Pain and Acute Coronary Syndrome (ACS). Available at: http://bit.ly.ACS1112. Updated November 2012.
- , , , et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–e347.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions, , , , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.
- , , , , , Antiquated tests within the clinical pathology laboratory. Am J Manag Care. 2010;16(9):e220–e227.
- , , , , Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468–1474.
© 2015 Society of Hospital Medicine