User login
Lessons From Statin Failure in COPD
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN DIEGO – The failure of statins in the STATCOPE trial to prevent exacerbations of chronic obstructive pulmonary disease isn’t the only important message of the trial, Dr. Gerard J. Criner said in an interview after he presented the findings at an international conference of the American Thoracic Society.
Previous observational studies of statins in COPD that suggested survival benefits from the drugs probably didn’t screen out patients with indications for statin therapy, as STATCOPE (Statins in COPD Exacerbations) did, for a more pristine assessment, said Dr. Criner, professor of medicine and director of the medical intensive care unit and the ventilator rehabilitation unit at Temple University, Philadelphia. The real message may be that clinicians are missing patients who need statins but aren’t getting them, he suggested.
Dr. Criner also shared his take on other important statin trials presented at the meeting. Take a look.
The National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research funded the STATCOPE trial. The investigators reported financial associations with dozens of companies, including five of Dr. Criner’s coinvestigators who had financial associations with Merck, which makes a brand name formulation of simvastatin.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
Should You Consider Antibiotics for Exacerbations of Mild COPD?
PRACTICE CHANGER
Consider antibiotics for patients with exacerbations of mild to moderate chronic obstructive pulmonary disease (COPD).1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done multicenter randomized controlled trial (RCT) with quality evidence.1
ILLUSTRATIVE CASE
A 45-year-old man with a history of mild COPD seeks treatment for worsening dyspnea and increased (nonpurulent) sputum production. He denies fever or chills. On exam, he has coarse breath sounds and scattered wheezes. Should you add antibiotics to his treatment?
COPD exacerbations—a worsening of symptoms beyond day-to-day variations that leads to a medication change—are part of the disease course and can accelerate lung function decline, decrease quality of life, and, when severe, increase mortality.2 Infections cause an estimated 50% to 70% of COPD exacerbations.2-4
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using antibiotics to treat exacerbations in patients with moderate or severe COPD who
• Have increased dyspnea, sputum volume, and sputum purulence;
• Have two of these symptoms if increased sputum purulence is one of them; or
• Require mechanical ventilation.2
According to the GOLD guidelines, the choice of antibiotic should be based on local antibiograms; common options include amoxicillin, amoxicillin/clavulanate, azithromycin, and doxycycline.2 Although the GOLD guidelines cover use of antibiotics for COPD exacerbations, this recommendation is based on analyses of studies that focused on patients with moderate or severe COPD.2 There has been little research on using antibiotics for exacerbations of mild COPD.
STUDY SUMMARY
Using antibiotics often resolves symptoms
Llor et al1 conducted a multicenter, double-blind, placebo-controlled RCT to examine the effectiveness of antibiotic treatment for COPD exacerbations. Participants (ages 40 and older) had mild to moderate COPD, defined as 10 or more pack-years of smoking, an FEV1 greater than 50%, and an FEV1/FVC ratio lower than 0.7. An exacerbation was defined as at least one of the following: increased dyspnea, increased sputum volume, or sputum purulence.
Patients were randomly assigned to receive amoxicillin/clavulanate 500/125 mg or placebo three times a day for eight days. Primary endpoints were clinical cure (resolution of symptoms) and clinical success (resolution or improvement of symptoms) at days 9 to 11, as determined by physician assessment. Secondary measures included cure and clinical success at day 20 and time until next exacerbation. Patients were monitored for one year after the exacerbation.
There were 162 patients in the antibiotic group and 156 in the placebo group; the two groups were demographically similar. In each group, four patients withdrew consent and were removed from analysis. By the 9-to-11-day follow-up visit, 74.1% of patients in the antibiotic group had clinical cure, compared with 59.9% in the placebo group (number needed to treat [NNT] = 7). Clinical success also was significantly greater with antibiotics compared with placebo (90.5% vs 80.9%).
The clinical cure rate at day 20 also was significantly greater in patients on antibiotics compared with placebo (81.6% vs 67.8%; NNT = 7). During the one-year follow-up, 58% of patients in the antibiotic group and 73.2% of those in the placebo group experienced additional exacerbations. Time to next exacerbation was significantly longer in patients taking antibiotics (233 days vs 160 days).
Can CRP level help determine who should receive antibiotics?
Previous studies have identified biomarkers, including C-reactive protein (CRP), that indicate COPD exacerbation but have not linked them to clinical course.5-7 In this study, researchers measured CRP in patients receiving placebo to determine if this biomarker could predict clinical outcomes.
The researchers found that the clinical success rate among patients with a CRP lower than 40 mg/L was 87.6%, while only 34.5% of patients with a CRP greater than 40 mg/L experienced clinical success (sensitivity and specificity for clinical success at this cutoff were 0.655 and 0.876, respectively). This suggests that antibiotics might be appropriate for patients with an exacerbation of mild or moderate COPD who have a CRP greater than 40 mg/L.
There were 35 adverse events: 23 in the antibiotics group and 12 in the placebo group. Two patients in the antibiotics group discontinued treatment as a result. Most adverse events involved mild gastrointestinal problems.
Continued on next page >>
WHAT’S NEW?
Evidence supports antibiotics for mild to moderate COPD
Few placebo-controlled trials have addressed antibiotic use for exacerbations in patients with mild to moderate COPD.2,8,9 This study demonstrated that, compared with placebo, symptom resolution and clinical success is greater with amoxicillin/clavulanate and that antibiotic treatment also may increase time until next exacerbation.
The study also looked at the relationship of CRP and exacerbations in the placebo group. Higher spontaneous clinical cure rates were noted when the CRP was lower than 40 mg/L.
CAVEATS
Effects of concomitant medications are unclear
In both the placebo and antibiotic groups, patients were taking other medications (including short- and long-acting β-agonists, anticholinergics, theophyllines, and oral or inhaled corticosteroids). Roughly the same number of patients in each group took additional medications, but researchers did not conduct a subgroup analysis to see if patients treated with these medications responded differently from those who received antibiotics alone.
GOLD guidelines already suggest antibiotics for exacerbations in patients with moderate COPD.2 In this study, 89% of patients met criteria for moderate COPD and 11% for mild COPD. Though the percentage of patients who had mild COPD was small, we believe the results of this study warrant consideration of antibiotic use in patients with mild disease. Local antibiograms may show increased resistance to amoxicillin/clavulanate; this study did not address the use of other antibiotics.
CHALLENGES TO IMPLEMENTATION
Antibiotic overuse may be a concern
Concerns about antibiotic resistance may make clinicians reluctant to prescribe the drugs for those with mild to moderate COPD.
REFERENCES
1. Llor C, Moragas A, Hernández S, et al. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(8):716-723.
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. January 2014. www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed April 15, 2014.
3. Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847-852.
4. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-931.
5. Vollenweider DJ, Jarrett H, Steurer-Stey CA, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257.
6. Bartlett, JG, Sethi S. Management of infection in acute exacerbations of chronic obstructive pulmonary disease. In: Basow DS, ed. UpToDate. www.uptodate.com. Last updated March 27, 2012. Accessed January 2, 2013.
7. Lacoma A, Prat C, Andreo F, et al. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:157-169.
8. Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med. 2009;15(2):120-125.
9. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353-2361.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(4):E11-E13.
PRACTICE CHANGER
Consider antibiotics for patients with exacerbations of mild to moderate chronic obstructive pulmonary disease (COPD).1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done multicenter randomized controlled trial (RCT) with quality evidence.1
ILLUSTRATIVE CASE
A 45-year-old man with a history of mild COPD seeks treatment for worsening dyspnea and increased (nonpurulent) sputum production. He denies fever or chills. On exam, he has coarse breath sounds and scattered wheezes. Should you add antibiotics to his treatment?
COPD exacerbations—a worsening of symptoms beyond day-to-day variations that leads to a medication change—are part of the disease course and can accelerate lung function decline, decrease quality of life, and, when severe, increase mortality.2 Infections cause an estimated 50% to 70% of COPD exacerbations.2-4
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using antibiotics to treat exacerbations in patients with moderate or severe COPD who
• Have increased dyspnea, sputum volume, and sputum purulence;
• Have two of these symptoms if increased sputum purulence is one of them; or
• Require mechanical ventilation.2
According to the GOLD guidelines, the choice of antibiotic should be based on local antibiograms; common options include amoxicillin, amoxicillin/clavulanate, azithromycin, and doxycycline.2 Although the GOLD guidelines cover use of antibiotics for COPD exacerbations, this recommendation is based on analyses of studies that focused on patients with moderate or severe COPD.2 There has been little research on using antibiotics for exacerbations of mild COPD.
STUDY SUMMARY
Using antibiotics often resolves symptoms
Llor et al1 conducted a multicenter, double-blind, placebo-controlled RCT to examine the effectiveness of antibiotic treatment for COPD exacerbations. Participants (ages 40 and older) had mild to moderate COPD, defined as 10 or more pack-years of smoking, an FEV1 greater than 50%, and an FEV1/FVC ratio lower than 0.7. An exacerbation was defined as at least one of the following: increased dyspnea, increased sputum volume, or sputum purulence.
Patients were randomly assigned to receive amoxicillin/clavulanate 500/125 mg or placebo three times a day for eight days. Primary endpoints were clinical cure (resolution of symptoms) and clinical success (resolution or improvement of symptoms) at days 9 to 11, as determined by physician assessment. Secondary measures included cure and clinical success at day 20 and time until next exacerbation. Patients were monitored for one year after the exacerbation.
There were 162 patients in the antibiotic group and 156 in the placebo group; the two groups were demographically similar. In each group, four patients withdrew consent and were removed from analysis. By the 9-to-11-day follow-up visit, 74.1% of patients in the antibiotic group had clinical cure, compared with 59.9% in the placebo group (number needed to treat [NNT] = 7). Clinical success also was significantly greater with antibiotics compared with placebo (90.5% vs 80.9%).
The clinical cure rate at day 20 also was significantly greater in patients on antibiotics compared with placebo (81.6% vs 67.8%; NNT = 7). During the one-year follow-up, 58% of patients in the antibiotic group and 73.2% of those in the placebo group experienced additional exacerbations. Time to next exacerbation was significantly longer in patients taking antibiotics (233 days vs 160 days).
Can CRP level help determine who should receive antibiotics?
Previous studies have identified biomarkers, including C-reactive protein (CRP), that indicate COPD exacerbation but have not linked them to clinical course.5-7 In this study, researchers measured CRP in patients receiving placebo to determine if this biomarker could predict clinical outcomes.
The researchers found that the clinical success rate among patients with a CRP lower than 40 mg/L was 87.6%, while only 34.5% of patients with a CRP greater than 40 mg/L experienced clinical success (sensitivity and specificity for clinical success at this cutoff were 0.655 and 0.876, respectively). This suggests that antibiotics might be appropriate for patients with an exacerbation of mild or moderate COPD who have a CRP greater than 40 mg/L.
There were 35 adverse events: 23 in the antibiotics group and 12 in the placebo group. Two patients in the antibiotics group discontinued treatment as a result. Most adverse events involved mild gastrointestinal problems.
Continued on next page >>
WHAT’S NEW?
Evidence supports antibiotics for mild to moderate COPD
Few placebo-controlled trials have addressed antibiotic use for exacerbations in patients with mild to moderate COPD.2,8,9 This study demonstrated that, compared with placebo, symptom resolution and clinical success is greater with amoxicillin/clavulanate and that antibiotic treatment also may increase time until next exacerbation.
The study also looked at the relationship of CRP and exacerbations in the placebo group. Higher spontaneous clinical cure rates were noted when the CRP was lower than 40 mg/L.
CAVEATS
Effects of concomitant medications are unclear
In both the placebo and antibiotic groups, patients were taking other medications (including short- and long-acting β-agonists, anticholinergics, theophyllines, and oral or inhaled corticosteroids). Roughly the same number of patients in each group took additional medications, but researchers did not conduct a subgroup analysis to see if patients treated with these medications responded differently from those who received antibiotics alone.
GOLD guidelines already suggest antibiotics for exacerbations in patients with moderate COPD.2 In this study, 89% of patients met criteria for moderate COPD and 11% for mild COPD. Though the percentage of patients who had mild COPD was small, we believe the results of this study warrant consideration of antibiotic use in patients with mild disease. Local antibiograms may show increased resistance to amoxicillin/clavulanate; this study did not address the use of other antibiotics.
CHALLENGES TO IMPLEMENTATION
Antibiotic overuse may be a concern
Concerns about antibiotic resistance may make clinicians reluctant to prescribe the drugs for those with mild to moderate COPD.
REFERENCES
1. Llor C, Moragas A, Hernández S, et al. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(8):716-723.
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. January 2014. www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed April 15, 2014.
3. Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847-852.
4. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-931.
5. Vollenweider DJ, Jarrett H, Steurer-Stey CA, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257.
6. Bartlett, JG, Sethi S. Management of infection in acute exacerbations of chronic obstructive pulmonary disease. In: Basow DS, ed. UpToDate. www.uptodate.com. Last updated March 27, 2012. Accessed January 2, 2013.
7. Lacoma A, Prat C, Andreo F, et al. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:157-169.
8. Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med. 2009;15(2):120-125.
9. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353-2361.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(4):E11-E13.
PRACTICE CHANGER
Consider antibiotics for patients with exacerbations of mild to moderate chronic obstructive pulmonary disease (COPD).1
STRENGTH OF RECOMMENDATION
B: Based on a single well-done multicenter randomized controlled trial (RCT) with quality evidence.1
ILLUSTRATIVE CASE
A 45-year-old man with a history of mild COPD seeks treatment for worsening dyspnea and increased (nonpurulent) sputum production. He denies fever or chills. On exam, he has coarse breath sounds and scattered wheezes. Should you add antibiotics to his treatment?
COPD exacerbations—a worsening of symptoms beyond day-to-day variations that leads to a medication change—are part of the disease course and can accelerate lung function decline, decrease quality of life, and, when severe, increase mortality.2 Infections cause an estimated 50% to 70% of COPD exacerbations.2-4
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using antibiotics to treat exacerbations in patients with moderate or severe COPD who
• Have increased dyspnea, sputum volume, and sputum purulence;
• Have two of these symptoms if increased sputum purulence is one of them; or
• Require mechanical ventilation.2
According to the GOLD guidelines, the choice of antibiotic should be based on local antibiograms; common options include amoxicillin, amoxicillin/clavulanate, azithromycin, and doxycycline.2 Although the GOLD guidelines cover use of antibiotics for COPD exacerbations, this recommendation is based on analyses of studies that focused on patients with moderate or severe COPD.2 There has been little research on using antibiotics for exacerbations of mild COPD.
STUDY SUMMARY
Using antibiotics often resolves symptoms
Llor et al1 conducted a multicenter, double-blind, placebo-controlled RCT to examine the effectiveness of antibiotic treatment for COPD exacerbations. Participants (ages 40 and older) had mild to moderate COPD, defined as 10 or more pack-years of smoking, an FEV1 greater than 50%, and an FEV1/FVC ratio lower than 0.7. An exacerbation was defined as at least one of the following: increased dyspnea, increased sputum volume, or sputum purulence.
Patients were randomly assigned to receive amoxicillin/clavulanate 500/125 mg or placebo three times a day for eight days. Primary endpoints were clinical cure (resolution of symptoms) and clinical success (resolution or improvement of symptoms) at days 9 to 11, as determined by physician assessment. Secondary measures included cure and clinical success at day 20 and time until next exacerbation. Patients were monitored for one year after the exacerbation.
There were 162 patients in the antibiotic group and 156 in the placebo group; the two groups were demographically similar. In each group, four patients withdrew consent and were removed from analysis. By the 9-to-11-day follow-up visit, 74.1% of patients in the antibiotic group had clinical cure, compared with 59.9% in the placebo group (number needed to treat [NNT] = 7). Clinical success also was significantly greater with antibiotics compared with placebo (90.5% vs 80.9%).
The clinical cure rate at day 20 also was significantly greater in patients on antibiotics compared with placebo (81.6% vs 67.8%; NNT = 7). During the one-year follow-up, 58% of patients in the antibiotic group and 73.2% of those in the placebo group experienced additional exacerbations. Time to next exacerbation was significantly longer in patients taking antibiotics (233 days vs 160 days).
Can CRP level help determine who should receive antibiotics?
Previous studies have identified biomarkers, including C-reactive protein (CRP), that indicate COPD exacerbation but have not linked them to clinical course.5-7 In this study, researchers measured CRP in patients receiving placebo to determine if this biomarker could predict clinical outcomes.
The researchers found that the clinical success rate among patients with a CRP lower than 40 mg/L was 87.6%, while only 34.5% of patients with a CRP greater than 40 mg/L experienced clinical success (sensitivity and specificity for clinical success at this cutoff were 0.655 and 0.876, respectively). This suggests that antibiotics might be appropriate for patients with an exacerbation of mild or moderate COPD who have a CRP greater than 40 mg/L.
There were 35 adverse events: 23 in the antibiotics group and 12 in the placebo group. Two patients in the antibiotics group discontinued treatment as a result. Most adverse events involved mild gastrointestinal problems.
Continued on next page >>
WHAT’S NEW?
Evidence supports antibiotics for mild to moderate COPD
Few placebo-controlled trials have addressed antibiotic use for exacerbations in patients with mild to moderate COPD.2,8,9 This study demonstrated that, compared with placebo, symptom resolution and clinical success is greater with amoxicillin/clavulanate and that antibiotic treatment also may increase time until next exacerbation.
The study also looked at the relationship of CRP and exacerbations in the placebo group. Higher spontaneous clinical cure rates were noted when the CRP was lower than 40 mg/L.
CAVEATS
Effects of concomitant medications are unclear
In both the placebo and antibiotic groups, patients were taking other medications (including short- and long-acting β-agonists, anticholinergics, theophyllines, and oral or inhaled corticosteroids). Roughly the same number of patients in each group took additional medications, but researchers did not conduct a subgroup analysis to see if patients treated with these medications responded differently from those who received antibiotics alone.
GOLD guidelines already suggest antibiotics for exacerbations in patients with moderate COPD.2 In this study, 89% of patients met criteria for moderate COPD and 11% for mild COPD. Though the percentage of patients who had mild COPD was small, we believe the results of this study warrant consideration of antibiotic use in patients with mild disease. Local antibiograms may show increased resistance to amoxicillin/clavulanate; this study did not address the use of other antibiotics.
CHALLENGES TO IMPLEMENTATION
Antibiotic overuse may be a concern
Concerns about antibiotic resistance may make clinicians reluctant to prescribe the drugs for those with mild to moderate COPD.
REFERENCES
1. Llor C, Moragas A, Hernández S, et al. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(8):716-723.
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. January 2014. www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed April 15, 2014.
3. Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847-852.
4. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-931.
5. Vollenweider DJ, Jarrett H, Steurer-Stey CA, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257.
6. Bartlett, JG, Sethi S. Management of infection in acute exacerbations of chronic obstructive pulmonary disease. In: Basow DS, ed. UpToDate. www.uptodate.com. Last updated March 27, 2012. Accessed January 2, 2013.
7. Lacoma A, Prat C, Andreo F, et al. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:157-169.
8. Antonescu-Turcu AL, Tomic R. C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med. 2009;15(2):120-125.
9. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353-2361.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(4):E11-E13.
Aspirin Sensitivity Signals Asthma Severity
MADRID – Aspirin sensitivity was strongly associated with asthma severity and the presence of chronic rhinosinusitis with nasal polyps in a prospective, multicenter study.
"Aspirin sensitivity may be considered a clinical marker for severe asthma and for the presence of chronic rhinosinusitis with nasal polyps, and a potential marker for united airway disease," Dr. José Antonio Castillo reported at the world congress of the American College of Chest Physicians.
Aspirin-exacerbated respiratory disease is commonly associated with chronic rhinosinusitis (CRS) with nasal polyps, but little information is available on the correlation between aspirin sensitivity and severe asthma.
To evaluate the presence of aspirin sensitivity and CRS with nasal polyps in a cohort of asthmatic patients, pulmonologists and ear, nose, and throat specialists at 23 hospitals in Spain and Latin America recruited 492 patients, aged 18-70 years, attending outpatient clinics with the diagnosis of asthma for at least 1 year. Aspirin sensitivity was assessed by clinical history and/or aspirin challenge, and CRS with nasal polyps was assessed by nasal symptoms, nasal endoscopy, and sinus computed tomography (CT) scan.
Among 473 evaluable patients, 72 (15%) were aspirin sensitive, 14.6% had no nasosinal disease, 12.6% nonallergic rhinitis, 36.8% allergic rhinitis, 16.6% CRS without nasal polyps, and 19.4% CRS with nasal polyps.
*Aspirin-intolerant asthma was strongly related to asthma severity. In all, 3 of the 72 (4.2%) aspirin-intolerant patients were classified as having intermittent asthma (odds ratio, 1); 17 (23.6%) as mild persistent (OR, 4.3); 21 (29.2%) as moderate persistent (OR, 4.3); and 31 (43%) as severe persistent asthma, which was statistically significant (OR, 7.8; P less than .05), reported Dr. Castillo, with the pneumology service at Chiron Dexeus University Hospital, Barcelona.
The presence of CRS with nasal polyps was also significantly associated (38.9%; 28/72 patients) with aspirin sensitivity (OR, 9.05; P less than .001).
Aspirin sensitivity was present in 4.5% of patients with no nasosinal disease, 18.6% of those with nonallergic rhinitis, 9.2% with allergic rhinitis, 17.5% with CRS with no nasal polyps, and 29.8% with CRS and nasal polyps.
Further, patients with aspirin-intolerant asthma showed significantly higher Lund & McKay CT scores than aspirin-tolerant asthmatic patients, according to the poster presentation.
The current results perhaps could be validated by matching aspirin sensitivity with a biomarker of severe asthma, that is, periostin, but are such that they already use aspirin sensitivity as a clinical marker of severe asthma, Dr. Castillo said in an interview.
Patients in the study had a mean age of 45 years and a mean body mass index of 26.9 kg/m2 (range, 16.8-49.8 kg/m2); 70.5% were female, and 9.6% were smokers.
Asthma was intermittent in 85 patients, mild persistent in 122, moderate persistent in 154, and severe persistent in 131, according to Global Initiative for Asthma (GINA) severity criteria.
Dr. Castillo and his coauthors reported no financial disclosures.
*This article was updated 4/7/14
MADRID – Aspirin sensitivity was strongly associated with asthma severity and the presence of chronic rhinosinusitis with nasal polyps in a prospective, multicenter study.
"Aspirin sensitivity may be considered a clinical marker for severe asthma and for the presence of chronic rhinosinusitis with nasal polyps, and a potential marker for united airway disease," Dr. José Antonio Castillo reported at the world congress of the American College of Chest Physicians.
Aspirin-exacerbated respiratory disease is commonly associated with chronic rhinosinusitis (CRS) with nasal polyps, but little information is available on the correlation between aspirin sensitivity and severe asthma.
To evaluate the presence of aspirin sensitivity and CRS with nasal polyps in a cohort of asthmatic patients, pulmonologists and ear, nose, and throat specialists at 23 hospitals in Spain and Latin America recruited 492 patients, aged 18-70 years, attending outpatient clinics with the diagnosis of asthma for at least 1 year. Aspirin sensitivity was assessed by clinical history and/or aspirin challenge, and CRS with nasal polyps was assessed by nasal symptoms, nasal endoscopy, and sinus computed tomography (CT) scan.
Among 473 evaluable patients, 72 (15%) were aspirin sensitive, 14.6% had no nasosinal disease, 12.6% nonallergic rhinitis, 36.8% allergic rhinitis, 16.6% CRS without nasal polyps, and 19.4% CRS with nasal polyps.
*Aspirin-intolerant asthma was strongly related to asthma severity. In all, 3 of the 72 (4.2%) aspirin-intolerant patients were classified as having intermittent asthma (odds ratio, 1); 17 (23.6%) as mild persistent (OR, 4.3); 21 (29.2%) as moderate persistent (OR, 4.3); and 31 (43%) as severe persistent asthma, which was statistically significant (OR, 7.8; P less than .05), reported Dr. Castillo, with the pneumology service at Chiron Dexeus University Hospital, Barcelona.
The presence of CRS with nasal polyps was also significantly associated (38.9%; 28/72 patients) with aspirin sensitivity (OR, 9.05; P less than .001).
Aspirin sensitivity was present in 4.5% of patients with no nasosinal disease, 18.6% of those with nonallergic rhinitis, 9.2% with allergic rhinitis, 17.5% with CRS with no nasal polyps, and 29.8% with CRS and nasal polyps.
Further, patients with aspirin-intolerant asthma showed significantly higher Lund & McKay CT scores than aspirin-tolerant asthmatic patients, according to the poster presentation.
The current results perhaps could be validated by matching aspirin sensitivity with a biomarker of severe asthma, that is, periostin, but are such that they already use aspirin sensitivity as a clinical marker of severe asthma, Dr. Castillo said in an interview.
Patients in the study had a mean age of 45 years and a mean body mass index of 26.9 kg/m2 (range, 16.8-49.8 kg/m2); 70.5% were female, and 9.6% were smokers.
Asthma was intermittent in 85 patients, mild persistent in 122, moderate persistent in 154, and severe persistent in 131, according to Global Initiative for Asthma (GINA) severity criteria.
Dr. Castillo and his coauthors reported no financial disclosures.
*This article was updated 4/7/14
MADRID – Aspirin sensitivity was strongly associated with asthma severity and the presence of chronic rhinosinusitis with nasal polyps in a prospective, multicenter study.
"Aspirin sensitivity may be considered a clinical marker for severe asthma and for the presence of chronic rhinosinusitis with nasal polyps, and a potential marker for united airway disease," Dr. José Antonio Castillo reported at the world congress of the American College of Chest Physicians.
Aspirin-exacerbated respiratory disease is commonly associated with chronic rhinosinusitis (CRS) with nasal polyps, but little information is available on the correlation between aspirin sensitivity and severe asthma.
To evaluate the presence of aspirin sensitivity and CRS with nasal polyps in a cohort of asthmatic patients, pulmonologists and ear, nose, and throat specialists at 23 hospitals in Spain and Latin America recruited 492 patients, aged 18-70 years, attending outpatient clinics with the diagnosis of asthma for at least 1 year. Aspirin sensitivity was assessed by clinical history and/or aspirin challenge, and CRS with nasal polyps was assessed by nasal symptoms, nasal endoscopy, and sinus computed tomography (CT) scan.
Among 473 evaluable patients, 72 (15%) were aspirin sensitive, 14.6% had no nasosinal disease, 12.6% nonallergic rhinitis, 36.8% allergic rhinitis, 16.6% CRS without nasal polyps, and 19.4% CRS with nasal polyps.
*Aspirin-intolerant asthma was strongly related to asthma severity. In all, 3 of the 72 (4.2%) aspirin-intolerant patients were classified as having intermittent asthma (odds ratio, 1); 17 (23.6%) as mild persistent (OR, 4.3); 21 (29.2%) as moderate persistent (OR, 4.3); and 31 (43%) as severe persistent asthma, which was statistically significant (OR, 7.8; P less than .05), reported Dr. Castillo, with the pneumology service at Chiron Dexeus University Hospital, Barcelona.
The presence of CRS with nasal polyps was also significantly associated (38.9%; 28/72 patients) with aspirin sensitivity (OR, 9.05; P less than .001).
Aspirin sensitivity was present in 4.5% of patients with no nasosinal disease, 18.6% of those with nonallergic rhinitis, 9.2% with allergic rhinitis, 17.5% with CRS with no nasal polyps, and 29.8% with CRS and nasal polyps.
Further, patients with aspirin-intolerant asthma showed significantly higher Lund & McKay CT scores than aspirin-tolerant asthmatic patients, according to the poster presentation.
The current results perhaps could be validated by matching aspirin sensitivity with a biomarker of severe asthma, that is, periostin, but are such that they already use aspirin sensitivity as a clinical marker of severe asthma, Dr. Castillo said in an interview.
Patients in the study had a mean age of 45 years and a mean body mass index of 26.9 kg/m2 (range, 16.8-49.8 kg/m2); 70.5% were female, and 9.6% were smokers.
Asthma was intermittent in 85 patients, mild persistent in 122, moderate persistent in 154, and severe persistent in 131, according to Global Initiative for Asthma (GINA) severity criteria.
Dr. Castillo and his coauthors reported no financial disclosures.
*This article was updated 4/7/14
What Are the Benefits and Risks of Inhaled Corticosteroids for COPD?
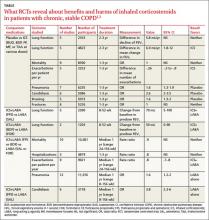
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.
Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
Recommendations
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.
Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
Recommendations
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.
Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
Recommendations
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
What are the benefits and risks of inhaled corticosteroids for COPD?
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.
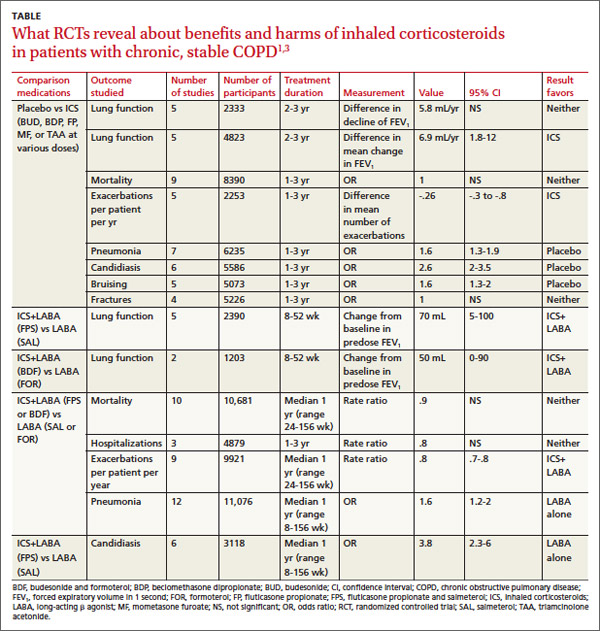
Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.

Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.

Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Evidence-based answers from the Family Physicians Inquiries Network
It’s Time to Use an Age-based Approach to D-dimer
PRACTICE CHANGER
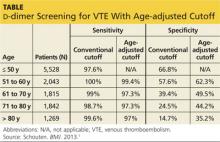
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
Unusual Case of Chest and Left Arm Pain
A 37-year-old white man presented to his primary care provider’s office for follow-up after a visit to the emergency department (ED). He had been evaluated at a local ED a week earlier for atypical chest pain and left arm pain. At the ED, blood work was done, along with an ECG, chest x-ray, and chest CT scan, but the results of these evaluations were not available during his initial primary care visit. On discharge from the ED, he was told that his heart was not the cause of his pain and that he should follow up with his primary care provider.
In the office, the patient reported that for the past several months he had been experiencing pain in his left arm when doing heavy or continuous physical labor; he noted that his job as a laborer required vigorous activity. Rest seemed to make his pain go away. He denied pain in the right arm or being awakened by the pain at night. Review of systems was unremarkable, and medical and surgical history was negative.
On physical exam, inspection of his torso and upper and lower extremities did not reveal any apparent abnormalities. Left shoulder and neck exams were normal. Cardiac auscultation was unremarkable, but palpation of the left upper extremity revealed neither a brachial, radial, nor ulnar pulse. Pulses in his right upper extremity were within normal limits. No bruits were appreciated over the carotids or either subclavian artery. Basic Doppler ultrasound over the left upper extremity at the brachial, radial, and ulnar sites showed symmetrical Doppler sounds. The remainder of his exam was unremarkable.
The patient’s ED documents and imaging results were received later in the day, after his office visit. The ECG, blood work results, and chest x-ray were normal. The chest CT results showed no evidence of pulmonary embolism. The radiologist did note mild narrowing at the left subclavian artery secondary to nonspecific surrounding soft tissue, which was noted to possibly represent intramural hemorrhage or atherosclerotic changes. No intimal flap was identified.
Because the diagnosis remained unclear, the patient was asked to bring the disc containing his chest CT images to the office. The radiologist, who was informed about the patient’s history and exam findings by phone, reviewed the CT images and felt there were changes surrounding the three branches off the aortic arch suggestive of inflammation, in addition to the stenosis at the left subclavian artery (see Figure 1 and Figure 2).
Based on the radiologist’s interpretation, additional lab tests were ordered. A complete blood count, comprehensive metabolic panel, prothrombin time/partial thromboplastin time, and lipid panel all yielded results within normal limits. Erythrocyte sedimentation rate (ESR) was 12 mm/h (reference range, 0 to 15 mm/h) and C-reactive protein (CRP) level was 4.9 mg/dL (reference range, 0.1 to 4.9 mg/dL). These laboratory results were essentially unremarkable, and therefore made his diagnosis more elusive.
The patient was referred to a vascular surgeon because of his immediate symptoms. The surgeon performed a thoracic outlet study in which Doppler waveform analysis of the left brachial, radial, and ulnar arteries of the thoracic outlet were analyzed during range-of-motion testing. Results suggested the possibility of thoracic outlet syndrome involving the left upper extremity, with significant baseline arterial insufficiency. A CT angiogram showed critical stenosis of the left subclavian artery and arterial wall thickening. Inflammatory changes were noted as well, and concern for “an inflammatory vasculitis” was described on the CT angiogram. The patient underwent left carotid-to-axillary bypass grafting, after which his left arm pain improved.
Following surgery, the patient returned to the primary care office for evaluation. Although the surgery was successful, the diagnosis was still not clear, requiring additional medical evaluation. The physical exam showed normal pulses in his left upper extremity. Lab tests revealed an elevated ESR of 54 mm/h and a CRP level of 4 mg/dL (reference range, 0.1 to 0.8 mg/dL; a different lab testing site was used, which accounts for the different reference range). In light of the patient’s lab test results, premature arterial vascular disease, and imaging studies suggesting inflammation, Takayasu arteritis (TA) was arrived at as a working diagnosis.
The patient was referred to a rheumatologist, who ordered a repeat ESR and CRP, antineutrophil cytoplasmic antibodies, and a magnetic resonance angiography study of the right brachial artery and major aortic branches to rule out other types of arteritis. Based on the test results, the patient was diagnosed with TA. He was placed on high-dose corticosteroid therapy (prednisone 60 mg/d). Methotrexate 10 mg/wk po was added three months after initiation of the prednisone.
Since being diagnosed with TA, the patient has presented with complaints related to the adverse effects of high-dose corticosteroids (ie, insomnia, weight gain, elevated blood pressure).
Continued on the next page >>
DISCUSSION
The first description of TA is credited to Japanese ophthalmologist Mikito Takayasu, who in 1908 described a wreathlike arteriovenous anastomosis around the optic disc of a 21-year-old woman who had experienced acute vision loss.1-3 Much earlier, in 1761, Italian anatomist Giovanni Battista Morgagni described large-vessel aneurysms and stenosis on a postmortem exam of a 40-year-old woman.2,4 However, TA was not formally labeled a disease until 1975.
TA is a chronic large vessel vasculitis of unknown origin, mainly involving the aorta and its primary branches: the left common carotid, brachiocephalic, and left subclavian arteries. Ongoing inflammation of affected vessels causes fibrotic changes, stenosis, and eventual occlusion and may lead to aneurysm formation.5,6 TA is rare, with an annual incidence in North America of 2.6 cases per million population.6 It occurs most frequently in Asian countries but has been reported in a wide range of ethnic groups.5,7 TA has been characterized as a disease of young women: Between 80% and 97% of patients are women,6,8 and the average age at diagnosis is 25 to 30.8-10
The process of vascular injury in TA begins with inflammation in the vasa vasorum of the aortic vessels. This inflammation, thought to be triggered by an as-yet-unknown antigen, leads to an initial inflammatory cellular infiltration of the aortic media and adventitia; the infiltrate is comprised predominantly of macrophages and T cells.5,9 Inflammatory infiltration causes myointimal proliferation, thickening of the blood vessel wall, and eventual luminal stenosis.5 Cytokines, interleukin 6, interferon , and other chemokines released by infiltrating inflammatory cells within the injured tissue also contribute to the inflammatory response and tissue damage.5,11
Histologically, granulomatous inflammation and giant cells are found in the media.12,13 Destruction of the elastic lamina and the muscular media results in the aneurysmal dilation seen in TA, while dense scarring and continued inflammation of the arterial vasculature results in arterial stenosis.12
Continued on the next page >>
CLINICAL PRESENTATION
Presentation of TA varies widely and can range from asymptomatic disease identified by pulse deficits or impalpable pulses to severe neurologic impairment. The early or prepulseless phase of TA is characterized by inflammatory changes.14 Signs and symptoms are frequently vague and nonspecific, particularly in this early phase, when fatigue, weight loss, and low-grade fever may be seen.12 Headache is another common symptom at the time of disease onset.5
In the later or chronic phase of the disease, individuals will begin to demonstrate signs and symptoms of vascular insufficiency.14 More common physical signs reflect the underlying arterial occlusive disease and include diminished or absent arterial pulses, asymmetrical arm blood pressures, bruits, extremity claudication, and hypertension.5,9,10 Hypertension, generally reflecting renal artery stenosis,10 is present in approximately 40% of cases in the United States and Europe.5,7,15 Neurologic features secondary to hypertension or ischemia affect more than half of patients; in addition to headache, these may include dizziness, syncope, vertigo, transient ischemic attack, and stroke.5
TA can also present with eye, lung, and skin manifestations; however, these features are less common. Although ocular involvement, including amaurosis fugax, has been reported in up to 26% of patients in TA series,5,7,16 permanent loss of vision in North American patients is uncommon.5,7 Pulmonary involvement affecting the large- or medium-sized pulmonary arteries has been reported to occur in approximately 55% of cases5; however, there is uncertainty regarding the prevalence of angiographically demonstrated pulmonary artery involvement, as studies have reported rates ranging from 14.3% to 70%.9,17-20 Pulmonary involvement is often asymptomatic, but features can include dyspnea, cough, and chest pain.5 Skin lesions are seen in up to 28% of cases, most commonly erythema nodosum, erythema induratum, tuberculoidlike eruptions, pyoderma gangrenosum, and cutaneous signs of necrotizing or granulomatous vasculitis.5,21
Continued on the next page >>
DIAGNOSIS
The American College of Rheumatology (ACR) has developed classification criteria for the diagnosis of TA.22 The presence of three or more of the six criteria (age of onset ≤ 40, claudication of the extremities, decreased brachial artery pulse, > 10 mm Hg difference in systolic blood pressure between the arms, bruit over subclavian arteries or aorta, and arteriographic abnormalities) yields a sensitivity of 90.5% and a specificity of 97.8%. Although the ACR classification remains the most widely applied for TA, a limitation of its diagnostic criteria is its failure to distinguish patients with early nonocclusive disease.23
In 1988, the Ishikawa classification criteria were developed, with a modified version subsequently published in 1996.23 Considered superior to the original Ishikawa and ACR criteria based on its application in 106 patients with angiographically proven TA, the modified version has a reported sensitivity and specificity of 92.5% and 95%, respectively.23
With the modified Ishikawa diagnostic criteria, the presence of two major or one major and two or four minor criteria suggests a high probability of TA. The three major criteria consist of lesions of the left mid-subclavian artery and the right mid-subclavian artery and characteristic signs and symptoms of at least 1 mo duration. The 10 minor criteria are high ESR (> 20 mm/h); carotid artery tenderness; hypertension; aortic regurgitation or annuloaortic ectasia; and lesions of pulmonary artery, left mid-common carotid, distal brachiocephalic trunk, descending thoracic aorta, abdominal aorta, and coronary artery.
The diagnosis of TA is based on recognition of clinical findings suggestive of large-vessel vasculitis. Imaging of the arterial tree with CT, MRI, or angiography also demonstrates findings consistent with TA, typically including early-onset vascular wall thickening/enhancement.24 Late imaging studies may reveal arterial stenoses, occlusions, and aneurysms.
Several types of imaging modalities have been used in the diagnosis and management of TA, each with strengths and limitations. Traditional angiography is invasive and requires an arterial puncture. Large doses of radiation are used, exposing the patient to iodinated contrast material, which may be dangerous in patients with poor renal function. However, the primary advantage of traditional angiography is that it allows for interventions such as stent placement and/or angioplasty to be performed.24 Findings on angiography often include long, smooth, tapered stenoses ranging from mild to severe or frank occlusions, as well as collateral vessels or the subclavian steal phenomenon.24
CT imaging is very useful for assessing thickening of the arterial wall. In early TA disease, evaluation of vessel wall thickness may be identified prior to frank stenosis of the artery(s).24 The spectrum of findings on CT angiography includes stenoses; occlusions; aneurysms; and concentric arterial wall thickening affecting the aorta and its branches, the pulmonary arteries, and occasionally the coronary arteries.24
MRI does not require the use of iodinated contrast, nor is there radiation exposure. MRI also has the advantage of evaluating arterial wall thickening, which is often present prior to stenosis (similar to CT imaging).24 Findings of TA on MRI include mural thrombi, signal alterations within and surrounding inflamed vessels, fusiform vascular dilation, thickened aortic valvular cusps, multifocal stenoses, and concentric thickening of the aortic wall.24
Laboratory testing is neither specific nor sensitive. Hoffman and Ahmed studied multiple serologic tests and found that no test reliably distinguishes between patients with active TA and healthy volunteers.25 Increases in the acute phase reactants (ESR and CRP) support the presence of an underlying inflammatory process, and these laboratory tests may be useful in disease monitoring. The ESR and CRP often do not correlate with systemic symptoms or disease progression but are used in conjunction with the clinical exam and serial imaging to gauge treatment success and to monitor disease progression.5,25 Biopsy material typically is not available in the initial diagnosis of TA, but histologic examination at the time of a surgery or procedure is often undertaken to confirm the diagnosis.5
The differential diagnosis of TA includes connective tissue diseases associated with the formation of multiple aneurysms, such as Marfan syndrome and Ehlers-Danlos syndrome.5 However, these diseases do not manifest with large vessel stenosis, the hallmark of TA. Infections known to cause aneurysms of the aorta should also be considered; these include bacterial, fungal, syphilitic, mycotic, and mycobacterial pathogens.5 Blood cultures are used to rule out bacterial agents. Rapid plasma reagin (RPR) and venereal disease research laboratory tests (VDRL) will identify a syphilitic etiology. Fungal cultures or fungal serology will help to rule out a mycotic pathogen.
Autoimmune diseases that can mimic TA include Behçet’s disease, Cogan syndrome, the spondyloarthropathies, and systemic lupus erythematosus. These diseases are not associated with stenosis of large vessels, which differentiates them from TA.5 Giant cell (temporal) arteritis (GCA) may present very similarly to TA, as both diseases affect large arteries.12 The table provides distinguishing features of TA and GCA.26
Continued on the next page >>
TREATMENT
Active phase TA is initially treated with high-dose glucocorticoid therapy (prednisone or methylprednisolone). Typical prednisone doses are 0.5 to 1 mg/kg/d.5 Clinical improvement is seen in almost all patients with glucocorticoid therapy,6,10,23 but relapse is common when prednisone is tapered to less than 20 mg/d.5 The corticosteroid dose is gradually tapered depending on patient response. Common side effects of corticosteroids may include weight gain, elevations in blood glucose, insomnia, increased infection risk, osteoporosis, and slowing of wound healing.
Because nearly half of all patients treated with glucocorticoids alone demonstrate chronic active disease, immunosuppressive therapies are almost always used concomitantly.27 Immune-suppressing drugs that may be used include methotrexate (15 to 25 mg/wk), azathioprine (2 mg/kg/d), and cyclophosphamide (1 to 2 mg/kg/d orally).5,28 Tumor necrosis factor (TNF)–blocking agents used to treat TA include etanercept, infliximab, or adalimumab.28,29 Adverse effects associated with immunosuppressive therapies and TNF-blocking agents include an increased risk for infection(s) and malignancy, bone marrow suppression, and hepatitis B reactivation. Although data are limited on anti-TNF agents, this class of drug has shown promise when used in conjunction with corticosteroids.28
In one open-label study by Hoffman and colleagues, remission rates with methotrexate plus steroids were 81%. Relapse occurred in 44% of study participants when the steroid dose was tapered or decreased to near discontinuation.27 More recently, in an uncontrolled study series involving 15 TA patients from India who were treated with azathioprine plus steroids, remission was achieved following 12 weeks of therapy. Angiographically, there was no progression of arterial disease after one year.30
Surgical and endovascular procedures used to return blood flow in stenotic or occluded vessels include synthetic or autologous vessel bypass, endarterectomy, and percutaneous transluminal angioplasty.5 When aortic insufficiency is present, aortic root replacement or repair is undertaken.5 These procedures are performed by vascular or cardiovascular surgeons and interventional radiologists. Rheumatologists are the medical specialists most involved in the direct care and management of TA patients. Cardiologists are sometimes consulted as well.
Continued on the next page >>
PROGNOSIS
Disability is common in TA. In a National Institutes of Health cohort study, 74% of TA patients reported experiencing functional effects from their disease, and 47% were fully disabled.2,8 In their retrospective review of 107 cases of TA, Lupi-Herrera and colleagues reported a 14% mortality rate.31 Half the deaths in this study were attributed to congestive heart failure (CHF). A cohort study in India that included 88 patients with TA reported cumulative 5- and 10-year survival rates of 91% and 84%, respectively. Of the 10 deaths in this cohort, four were due to CHF.2,32
CONCLUSION
Signs and symptoms of rheumatologic diseases such as TA are often vague, and diagnosis may prove difficult and elusive. Repeat office visits at short intervals may prove to be helpful in making the diagnosis. Referral for radiology and/or rheumatology consultation (face-to-face, if possible) is often necessary.
In cases such as this, completing a personal review of documents and test results done elsewhere, particularly ED/inpatient hospital data, is necessary; relying on the patient’s word that “they told me everything was fine” is insufficient. Clinicians should implement a system that works best for obtaining test results and other documents, follow their instincts, and if the correct diagnosis is not arrived at immediately, keep looking.
References >>
REFERENCES
1. Takayasu M. A case with peculiar changes of the retinal central vessels. Acta Soc Ophthalmol Jpn. 1908;12:554-555.
2. Maksimowicz-McKinnon K, Hoffman GS. Takayasu arteritis: what is the long-term prognosis? Rheum Dis Clin North Am. 2007;33:777-786.
3. Numano F. The story of Takayasu arteritis. Rheumatology. 2002;41:103-106.
4. Morgagni GB. De sedibus et causis morborum per anatomen indagatis.
(Letter 30).1761. Article 12.
5. Hernandez-Rodriguez J, Maksimowicz-McKinnon K, Hoffman GS. Takayasu’s arteritis. In: Carey WD, ed. Current Clinical Medicine. 2nd ed. Philadelphia: Saunders Elsevier; 2010:1195-1199.
6. Hall S, Barr W, Lie JT, et al. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore). 1985;64:89-99.
7. Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000-1009.
8. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994:120:919-929.
9. Gornik HL, Creager MA. Aortic diseases: aortitis. Circulation. 2008;117:
3039-3051.
10. Mwipatayi BP, Jeffery PC, Beningfield SJ, et al. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg. 2005;75:110-117.
11. Noris M. Pathogenesis of Takayasu’s arteritis. J Nephrol. 2001;14:506-513.
12. Hunder GG, Stone JH, Ramirez MP. Clinical features and diagnosis of Takayasu arteritis (2013). www.uptodate.com/contents/clinical-features-and-
diagnosis-of-takayasu-arteritis. Accessed March 24, 2014.
13. Nasu T. Takayasu’s truncoarteritis. Pulseless disease or aortitis syndrome. Acta Pathol Jpn. 1982;32 (suppl 1):117.
14. Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol. 2002;55:481-486.
15. Vanoli M, Daina E, Salvarani C, et al. Takayasu’s arteritis: a study of 104 Italian patients. Arthritis Rheum. 2005;53:100-107.
16. Chun YS, Park SJ, Chung H, Lee J. The clinical and ocular manifestations of Takayasu’s arteritis. Retina. 2001;21:132-140.
17. Liu YQ, Jin BL, Ling J. Pulmonary artery involvement in aortoarteritis: an angiographic study. Cardiovasc Intervent Radiol. 1994;17:2-6.
18. Yamada I, Shibuya H, Matsubara O, et al. Pulmonary artery disease in Takayasu’s arteritis: angiographic findings. AJR Am J Roentgenol. 1992;159:
263-269.
19. Sharma S, Kamalakar T, Rajani M, et al. The incidence and patterns of pulmonary artery involvement in Takayasu’s arteritis. Clin Radiol. 1990;42:177-181.
20. He NS, Liu F, Wu EH, et al. Pulmonary artery involvement in aorto-arteritis: an analysis of DSA. Chin Med J (Engl). 1990;103:666-672.
21. Werfel T, Kuipers JG, Zeidler H, et al. Cutaneous manifestations of Takayasu arteritis. Acta Derm Venereol. 1996;76:496-497.
22. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129-1134.
23. Andrews J, Mason JC. Takayasu’s arteritis—recent advances in imaging offer promise. Rheumatology (Oxford). 2007;46:6-15.
24. Gotway MB, Araoz PA, Macedo TA, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005;184:1945-1950.
25. Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with Takayasu arteritis. A preliminary report from The International Network for the Study of the Systemic Vasculitides (INSSYS). Int J Cardiol. 1998;66 (suppl 1):S191-S194.
26. Michel BA, Arend WP, Hunder GG. Clinical differentiation between giant cell (temporal) arteritis and Takayasu’s arteritis. J Rheumatol. 1996;23:106-111.
27. Hoffman GS, Leavitt RY, Kerr GS, et al. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578-582.
28. Hunder GG, Stone JH, Ramirez MP. Treatment of Takayasu arteritis (2013). www.uptodate.com/contents/treatment-of-takayasu-arteritis. Accessed March 24, 2014.
29. Schmidt J, Kerman TA, Bacani AK, et al. Tumor necrosis factor inhibitors in patients with Takayasu arteritis: experience from a referral center with long-term followup. Arthritis Care Res (Hoboken). 2012;64:1079-1083.
30. Valsakumar AK, Valappil UC, Jorapur V, et al. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu’s arteritis. J Rheumatol. 2003;30:1793-1798.
31. Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, et al. Takayasu’s arteritis: clinical study of 107 cases. Am Heart J. 1977;93:94-103.
32. Subramanyan R, Joy J, Balakrishnan KG. Natural history of aortoarteritis (Takayasu’s disease). Circulation. 1989;80:429-437.
A 37-year-old white man presented to his primary care provider’s office for follow-up after a visit to the emergency department (ED). He had been evaluated at a local ED a week earlier for atypical chest pain and left arm pain. At the ED, blood work was done, along with an ECG, chest x-ray, and chest CT scan, but the results of these evaluations were not available during his initial primary care visit. On discharge from the ED, he was told that his heart was not the cause of his pain and that he should follow up with his primary care provider.
In the office, the patient reported that for the past several months he had been experiencing pain in his left arm when doing heavy or continuous physical labor; he noted that his job as a laborer required vigorous activity. Rest seemed to make his pain go away. He denied pain in the right arm or being awakened by the pain at night. Review of systems was unremarkable, and medical and surgical history was negative.
On physical exam, inspection of his torso and upper and lower extremities did not reveal any apparent abnormalities. Left shoulder and neck exams were normal. Cardiac auscultation was unremarkable, but palpation of the left upper extremity revealed neither a brachial, radial, nor ulnar pulse. Pulses in his right upper extremity were within normal limits. No bruits were appreciated over the carotids or either subclavian artery. Basic Doppler ultrasound over the left upper extremity at the brachial, radial, and ulnar sites showed symmetrical Doppler sounds. The remainder of his exam was unremarkable.
The patient’s ED documents and imaging results were received later in the day, after his office visit. The ECG, blood work results, and chest x-ray were normal. The chest CT results showed no evidence of pulmonary embolism. The radiologist did note mild narrowing at the left subclavian artery secondary to nonspecific surrounding soft tissue, which was noted to possibly represent intramural hemorrhage or atherosclerotic changes. No intimal flap was identified.
Because the diagnosis remained unclear, the patient was asked to bring the disc containing his chest CT images to the office. The radiologist, who was informed about the patient’s history and exam findings by phone, reviewed the CT images and felt there were changes surrounding the three branches off the aortic arch suggestive of inflammation, in addition to the stenosis at the left subclavian artery (see Figure 1 and Figure 2).
Based on the radiologist’s interpretation, additional lab tests were ordered. A complete blood count, comprehensive metabolic panel, prothrombin time/partial thromboplastin time, and lipid panel all yielded results within normal limits. Erythrocyte sedimentation rate (ESR) was 12 mm/h (reference range, 0 to 15 mm/h) and C-reactive protein (CRP) level was 4.9 mg/dL (reference range, 0.1 to 4.9 mg/dL). These laboratory results were essentially unremarkable, and therefore made his diagnosis more elusive.
The patient was referred to a vascular surgeon because of his immediate symptoms. The surgeon performed a thoracic outlet study in which Doppler waveform analysis of the left brachial, radial, and ulnar arteries of the thoracic outlet were analyzed during range-of-motion testing. Results suggested the possibility of thoracic outlet syndrome involving the left upper extremity, with significant baseline arterial insufficiency. A CT angiogram showed critical stenosis of the left subclavian artery and arterial wall thickening. Inflammatory changes were noted as well, and concern for “an inflammatory vasculitis” was described on the CT angiogram. The patient underwent left carotid-to-axillary bypass grafting, after which his left arm pain improved.
Following surgery, the patient returned to the primary care office for evaluation. Although the surgery was successful, the diagnosis was still not clear, requiring additional medical evaluation. The physical exam showed normal pulses in his left upper extremity. Lab tests revealed an elevated ESR of 54 mm/h and a CRP level of 4 mg/dL (reference range, 0.1 to 0.8 mg/dL; a different lab testing site was used, which accounts for the different reference range). In light of the patient’s lab test results, premature arterial vascular disease, and imaging studies suggesting inflammation, Takayasu arteritis (TA) was arrived at as a working diagnosis.
The patient was referred to a rheumatologist, who ordered a repeat ESR and CRP, antineutrophil cytoplasmic antibodies, and a magnetic resonance angiography study of the right brachial artery and major aortic branches to rule out other types of arteritis. Based on the test results, the patient was diagnosed with TA. He was placed on high-dose corticosteroid therapy (prednisone 60 mg/d). Methotrexate 10 mg/wk po was added three months after initiation of the prednisone.
Since being diagnosed with TA, the patient has presented with complaints related to the adverse effects of high-dose corticosteroids (ie, insomnia, weight gain, elevated blood pressure).
Continued on the next page >>
DISCUSSION
The first description of TA is credited to Japanese ophthalmologist Mikito Takayasu, who in 1908 described a wreathlike arteriovenous anastomosis around the optic disc of a 21-year-old woman who had experienced acute vision loss.1-3 Much earlier, in 1761, Italian anatomist Giovanni Battista Morgagni described large-vessel aneurysms and stenosis on a postmortem exam of a 40-year-old woman.2,4 However, TA was not formally labeled a disease until 1975.
TA is a chronic large vessel vasculitis of unknown origin, mainly involving the aorta and its primary branches: the left common carotid, brachiocephalic, and left subclavian arteries. Ongoing inflammation of affected vessels causes fibrotic changes, stenosis, and eventual occlusion and may lead to aneurysm formation.5,6 TA is rare, with an annual incidence in North America of 2.6 cases per million population.6 It occurs most frequently in Asian countries but has been reported in a wide range of ethnic groups.5,7 TA has been characterized as a disease of young women: Between 80% and 97% of patients are women,6,8 and the average age at diagnosis is 25 to 30.8-10
The process of vascular injury in TA begins with inflammation in the vasa vasorum of the aortic vessels. This inflammation, thought to be triggered by an as-yet-unknown antigen, leads to an initial inflammatory cellular infiltration of the aortic media and adventitia; the infiltrate is comprised predominantly of macrophages and T cells.5,9 Inflammatory infiltration causes myointimal proliferation, thickening of the blood vessel wall, and eventual luminal stenosis.5 Cytokines, interleukin 6, interferon , and other chemokines released by infiltrating inflammatory cells within the injured tissue also contribute to the inflammatory response and tissue damage.5,11
Histologically, granulomatous inflammation and giant cells are found in the media.12,13 Destruction of the elastic lamina and the muscular media results in the aneurysmal dilation seen in TA, while dense scarring and continued inflammation of the arterial vasculature results in arterial stenosis.12
Continued on the next page >>
CLINICAL PRESENTATION
Presentation of TA varies widely and can range from asymptomatic disease identified by pulse deficits or impalpable pulses to severe neurologic impairment. The early or prepulseless phase of TA is characterized by inflammatory changes.14 Signs and symptoms are frequently vague and nonspecific, particularly in this early phase, when fatigue, weight loss, and low-grade fever may be seen.12 Headache is another common symptom at the time of disease onset.5
In the later or chronic phase of the disease, individuals will begin to demonstrate signs and symptoms of vascular insufficiency.14 More common physical signs reflect the underlying arterial occlusive disease and include diminished or absent arterial pulses, asymmetrical arm blood pressures, bruits, extremity claudication, and hypertension.5,9,10 Hypertension, generally reflecting renal artery stenosis,10 is present in approximately 40% of cases in the United States and Europe.5,7,15 Neurologic features secondary to hypertension or ischemia affect more than half of patients; in addition to headache, these may include dizziness, syncope, vertigo, transient ischemic attack, and stroke.5
TA can also present with eye, lung, and skin manifestations; however, these features are less common. Although ocular involvement, including amaurosis fugax, has been reported in up to 26% of patients in TA series,5,7,16 permanent loss of vision in North American patients is uncommon.5,7 Pulmonary involvement affecting the large- or medium-sized pulmonary arteries has been reported to occur in approximately 55% of cases5; however, there is uncertainty regarding the prevalence of angiographically demonstrated pulmonary artery involvement, as studies have reported rates ranging from 14.3% to 70%.9,17-20 Pulmonary involvement is often asymptomatic, but features can include dyspnea, cough, and chest pain.5 Skin lesions are seen in up to 28% of cases, most commonly erythema nodosum, erythema induratum, tuberculoidlike eruptions, pyoderma gangrenosum, and cutaneous signs of necrotizing or granulomatous vasculitis.5,21
Continued on the next page >>
DIAGNOSIS
The American College of Rheumatology (ACR) has developed classification criteria for the diagnosis of TA.22 The presence of three or more of the six criteria (age of onset ≤ 40, claudication of the extremities, decreased brachial artery pulse, > 10 mm Hg difference in systolic blood pressure between the arms, bruit over subclavian arteries or aorta, and arteriographic abnormalities) yields a sensitivity of 90.5% and a specificity of 97.8%. Although the ACR classification remains the most widely applied for TA, a limitation of its diagnostic criteria is its failure to distinguish patients with early nonocclusive disease.23
In 1988, the Ishikawa classification criteria were developed, with a modified version subsequently published in 1996.23 Considered superior to the original Ishikawa and ACR criteria based on its application in 106 patients with angiographically proven TA, the modified version has a reported sensitivity and specificity of 92.5% and 95%, respectively.23
With the modified Ishikawa diagnostic criteria, the presence of two major or one major and two or four minor criteria suggests a high probability of TA. The three major criteria consist of lesions of the left mid-subclavian artery and the right mid-subclavian artery and characteristic signs and symptoms of at least 1 mo duration. The 10 minor criteria are high ESR (> 20 mm/h); carotid artery tenderness; hypertension; aortic regurgitation or annuloaortic ectasia; and lesions of pulmonary artery, left mid-common carotid, distal brachiocephalic trunk, descending thoracic aorta, abdominal aorta, and coronary artery.
The diagnosis of TA is based on recognition of clinical findings suggestive of large-vessel vasculitis. Imaging of the arterial tree with CT, MRI, or angiography also demonstrates findings consistent with TA, typically including early-onset vascular wall thickening/enhancement.24 Late imaging studies may reveal arterial stenoses, occlusions, and aneurysms.
Several types of imaging modalities have been used in the diagnosis and management of TA, each with strengths and limitations. Traditional angiography is invasive and requires an arterial puncture. Large doses of radiation are used, exposing the patient to iodinated contrast material, which may be dangerous in patients with poor renal function. However, the primary advantage of traditional angiography is that it allows for interventions such as stent placement and/or angioplasty to be performed.24 Findings on angiography often include long, smooth, tapered stenoses ranging from mild to severe or frank occlusions, as well as collateral vessels or the subclavian steal phenomenon.24
CT imaging is very useful for assessing thickening of the arterial wall. In early TA disease, evaluation of vessel wall thickness may be identified prior to frank stenosis of the artery(s).24 The spectrum of findings on CT angiography includes stenoses; occlusions; aneurysms; and concentric arterial wall thickening affecting the aorta and its branches, the pulmonary arteries, and occasionally the coronary arteries.24
MRI does not require the use of iodinated contrast, nor is there radiation exposure. MRI also has the advantage of evaluating arterial wall thickening, which is often present prior to stenosis (similar to CT imaging).24 Findings of TA on MRI include mural thrombi, signal alterations within and surrounding inflamed vessels, fusiform vascular dilation, thickened aortic valvular cusps, multifocal stenoses, and concentric thickening of the aortic wall.24
Laboratory testing is neither specific nor sensitive. Hoffman and Ahmed studied multiple serologic tests and found that no test reliably distinguishes between patients with active TA and healthy volunteers.25 Increases in the acute phase reactants (ESR and CRP) support the presence of an underlying inflammatory process, and these laboratory tests may be useful in disease monitoring. The ESR and CRP often do not correlate with systemic symptoms or disease progression but are used in conjunction with the clinical exam and serial imaging to gauge treatment success and to monitor disease progression.5,25 Biopsy material typically is not available in the initial diagnosis of TA, but histologic examination at the time of a surgery or procedure is often undertaken to confirm the diagnosis.5
The differential diagnosis of TA includes connective tissue diseases associated with the formation of multiple aneurysms, such as Marfan syndrome and Ehlers-Danlos syndrome.5 However, these diseases do not manifest with large vessel stenosis, the hallmark of TA. Infections known to cause aneurysms of the aorta should also be considered; these include bacterial, fungal, syphilitic, mycotic, and mycobacterial pathogens.5 Blood cultures are used to rule out bacterial agents. Rapid plasma reagin (RPR) and venereal disease research laboratory tests (VDRL) will identify a syphilitic etiology. Fungal cultures or fungal serology will help to rule out a mycotic pathogen.
Autoimmune diseases that can mimic TA include Behçet’s disease, Cogan syndrome, the spondyloarthropathies, and systemic lupus erythematosus. These diseases are not associated with stenosis of large vessels, which differentiates them from TA.5 Giant cell (temporal) arteritis (GCA) may present very similarly to TA, as both diseases affect large arteries.12 The table provides distinguishing features of TA and GCA.26
Continued on the next page >>
TREATMENT
Active phase TA is initially treated with high-dose glucocorticoid therapy (prednisone or methylprednisolone). Typical prednisone doses are 0.5 to 1 mg/kg/d.5 Clinical improvement is seen in almost all patients with glucocorticoid therapy,6,10,23 but relapse is common when prednisone is tapered to less than 20 mg/d.5 The corticosteroid dose is gradually tapered depending on patient response. Common side effects of corticosteroids may include weight gain, elevations in blood glucose, insomnia, increased infection risk, osteoporosis, and slowing of wound healing.
Because nearly half of all patients treated with glucocorticoids alone demonstrate chronic active disease, immunosuppressive therapies are almost always used concomitantly.27 Immune-suppressing drugs that may be used include methotrexate (15 to 25 mg/wk), azathioprine (2 mg/kg/d), and cyclophosphamide (1 to 2 mg/kg/d orally).5,28 Tumor necrosis factor (TNF)–blocking agents used to treat TA include etanercept, infliximab, or adalimumab.28,29 Adverse effects associated with immunosuppressive therapies and TNF-blocking agents include an increased risk for infection(s) and malignancy, bone marrow suppression, and hepatitis B reactivation. Although data are limited on anti-TNF agents, this class of drug has shown promise when used in conjunction with corticosteroids.28
In one open-label study by Hoffman and colleagues, remission rates with methotrexate plus steroids were 81%. Relapse occurred in 44% of study participants when the steroid dose was tapered or decreased to near discontinuation.27 More recently, in an uncontrolled study series involving 15 TA patients from India who were treated with azathioprine plus steroids, remission was achieved following 12 weeks of therapy. Angiographically, there was no progression of arterial disease after one year.30
Surgical and endovascular procedures used to return blood flow in stenotic or occluded vessels include synthetic or autologous vessel bypass, endarterectomy, and percutaneous transluminal angioplasty.5 When aortic insufficiency is present, aortic root replacement or repair is undertaken.5 These procedures are performed by vascular or cardiovascular surgeons and interventional radiologists. Rheumatologists are the medical specialists most involved in the direct care and management of TA patients. Cardiologists are sometimes consulted as well.
Continued on the next page >>
PROGNOSIS
Disability is common in TA. In a National Institutes of Health cohort study, 74% of TA patients reported experiencing functional effects from their disease, and 47% were fully disabled.2,8 In their retrospective review of 107 cases of TA, Lupi-Herrera and colleagues reported a 14% mortality rate.31 Half the deaths in this study were attributed to congestive heart failure (CHF). A cohort study in India that included 88 patients with TA reported cumulative 5- and 10-year survival rates of 91% and 84%, respectively. Of the 10 deaths in this cohort, four were due to CHF.2,32
CONCLUSION
Signs and symptoms of rheumatologic diseases such as TA are often vague, and diagnosis may prove difficult and elusive. Repeat office visits at short intervals may prove to be helpful in making the diagnosis. Referral for radiology and/or rheumatology consultation (face-to-face, if possible) is often necessary.
In cases such as this, completing a personal review of documents and test results done elsewhere, particularly ED/inpatient hospital data, is necessary; relying on the patient’s word that “they told me everything was fine” is insufficient. Clinicians should implement a system that works best for obtaining test results and other documents, follow their instincts, and if the correct diagnosis is not arrived at immediately, keep looking.
References >>
REFERENCES
1. Takayasu M. A case with peculiar changes of the retinal central vessels. Acta Soc Ophthalmol Jpn. 1908;12:554-555.
2. Maksimowicz-McKinnon K, Hoffman GS. Takayasu arteritis: what is the long-term prognosis? Rheum Dis Clin North Am. 2007;33:777-786.
3. Numano F. The story of Takayasu arteritis. Rheumatology. 2002;41:103-106.
4. Morgagni GB. De sedibus et causis morborum per anatomen indagatis.
(Letter 30).1761. Article 12.
5. Hernandez-Rodriguez J, Maksimowicz-McKinnon K, Hoffman GS. Takayasu’s arteritis. In: Carey WD, ed. Current Clinical Medicine. 2nd ed. Philadelphia: Saunders Elsevier; 2010:1195-1199.
6. Hall S, Barr W, Lie JT, et al. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore). 1985;64:89-99.
7. Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000-1009.
8. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994:120:919-929.
9. Gornik HL, Creager MA. Aortic diseases: aortitis. Circulation. 2008;117:
3039-3051.
10. Mwipatayi BP, Jeffery PC, Beningfield SJ, et al. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg. 2005;75:110-117.
11. Noris M. Pathogenesis of Takayasu’s arteritis. J Nephrol. 2001;14:506-513.
12. Hunder GG, Stone JH, Ramirez MP. Clinical features and diagnosis of Takayasu arteritis (2013). www.uptodate.com/contents/clinical-features-and-
diagnosis-of-takayasu-arteritis. Accessed March 24, 2014.
13. Nasu T. Takayasu’s truncoarteritis. Pulseless disease or aortitis syndrome. Acta Pathol Jpn. 1982;32 (suppl 1):117.
14. Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol. 2002;55:481-486.
15. Vanoli M, Daina E, Salvarani C, et al. Takayasu’s arteritis: a study of 104 Italian patients. Arthritis Rheum. 2005;53:100-107.
16. Chun YS, Park SJ, Chung H, Lee J. The clinical and ocular manifestations of Takayasu’s arteritis. Retina. 2001;21:132-140.
17. Liu YQ, Jin BL, Ling J. Pulmonary artery involvement in aortoarteritis: an angiographic study. Cardiovasc Intervent Radiol. 1994;17:2-6.
18. Yamada I, Shibuya H, Matsubara O, et al. Pulmonary artery disease in Takayasu’s arteritis: angiographic findings. AJR Am J Roentgenol. 1992;159:
263-269.
19. Sharma S, Kamalakar T, Rajani M, et al. The incidence and patterns of pulmonary artery involvement in Takayasu’s arteritis. Clin Radiol. 1990;42:177-181.
20. He NS, Liu F, Wu EH, et al. Pulmonary artery involvement in aorto-arteritis: an analysis of DSA. Chin Med J (Engl). 1990;103:666-672.
21. Werfel T, Kuipers JG, Zeidler H, et al. Cutaneous manifestations of Takayasu arteritis. Acta Derm Venereol. 1996;76:496-497.
22. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129-1134.
23. Andrews J, Mason JC. Takayasu’s arteritis—recent advances in imaging offer promise. Rheumatology (Oxford). 2007;46:6-15.
24. Gotway MB, Araoz PA, Macedo TA, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005;184:1945-1950.
25. Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with Takayasu arteritis. A preliminary report from The International Network for the Study of the Systemic Vasculitides (INSSYS). Int J Cardiol. 1998;66 (suppl 1):S191-S194.
26. Michel BA, Arend WP, Hunder GG. Clinical differentiation between giant cell (temporal) arteritis and Takayasu’s arteritis. J Rheumatol. 1996;23:106-111.
27. Hoffman GS, Leavitt RY, Kerr GS, et al. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578-582.
28. Hunder GG, Stone JH, Ramirez MP. Treatment of Takayasu arteritis (2013). www.uptodate.com/contents/treatment-of-takayasu-arteritis. Accessed March 24, 2014.
29. Schmidt J, Kerman TA, Bacani AK, et al. Tumor necrosis factor inhibitors in patients with Takayasu arteritis: experience from a referral center with long-term followup. Arthritis Care Res (Hoboken). 2012;64:1079-1083.
30. Valsakumar AK, Valappil UC, Jorapur V, et al. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu’s arteritis. J Rheumatol. 2003;30:1793-1798.
31. Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, et al. Takayasu’s arteritis: clinical study of 107 cases. Am Heart J. 1977;93:94-103.
32. Subramanyan R, Joy J, Balakrishnan KG. Natural history of aortoarteritis (Takayasu’s disease). Circulation. 1989;80:429-437.
A 37-year-old white man presented to his primary care provider’s office for follow-up after a visit to the emergency department (ED). He had been evaluated at a local ED a week earlier for atypical chest pain and left arm pain. At the ED, blood work was done, along with an ECG, chest x-ray, and chest CT scan, but the results of these evaluations were not available during his initial primary care visit. On discharge from the ED, he was told that his heart was not the cause of his pain and that he should follow up with his primary care provider.
In the office, the patient reported that for the past several months he had been experiencing pain in his left arm when doing heavy or continuous physical labor; he noted that his job as a laborer required vigorous activity. Rest seemed to make his pain go away. He denied pain in the right arm or being awakened by the pain at night. Review of systems was unremarkable, and medical and surgical history was negative.
On physical exam, inspection of his torso and upper and lower extremities did not reveal any apparent abnormalities. Left shoulder and neck exams were normal. Cardiac auscultation was unremarkable, but palpation of the left upper extremity revealed neither a brachial, radial, nor ulnar pulse. Pulses in his right upper extremity were within normal limits. No bruits were appreciated over the carotids or either subclavian artery. Basic Doppler ultrasound over the left upper extremity at the brachial, radial, and ulnar sites showed symmetrical Doppler sounds. The remainder of his exam was unremarkable.
The patient’s ED documents and imaging results were received later in the day, after his office visit. The ECG, blood work results, and chest x-ray were normal. The chest CT results showed no evidence of pulmonary embolism. The radiologist did note mild narrowing at the left subclavian artery secondary to nonspecific surrounding soft tissue, which was noted to possibly represent intramural hemorrhage or atherosclerotic changes. No intimal flap was identified.
Because the diagnosis remained unclear, the patient was asked to bring the disc containing his chest CT images to the office. The radiologist, who was informed about the patient’s history and exam findings by phone, reviewed the CT images and felt there were changes surrounding the three branches off the aortic arch suggestive of inflammation, in addition to the stenosis at the left subclavian artery (see Figure 1 and Figure 2).
Based on the radiologist’s interpretation, additional lab tests were ordered. A complete blood count, comprehensive metabolic panel, prothrombin time/partial thromboplastin time, and lipid panel all yielded results within normal limits. Erythrocyte sedimentation rate (ESR) was 12 mm/h (reference range, 0 to 15 mm/h) and C-reactive protein (CRP) level was 4.9 mg/dL (reference range, 0.1 to 4.9 mg/dL). These laboratory results were essentially unremarkable, and therefore made his diagnosis more elusive.
The patient was referred to a vascular surgeon because of his immediate symptoms. The surgeon performed a thoracic outlet study in which Doppler waveform analysis of the left brachial, radial, and ulnar arteries of the thoracic outlet were analyzed during range-of-motion testing. Results suggested the possibility of thoracic outlet syndrome involving the left upper extremity, with significant baseline arterial insufficiency. A CT angiogram showed critical stenosis of the left subclavian artery and arterial wall thickening. Inflammatory changes were noted as well, and concern for “an inflammatory vasculitis” was described on the CT angiogram. The patient underwent left carotid-to-axillary bypass grafting, after which his left arm pain improved.
Following surgery, the patient returned to the primary care office for evaluation. Although the surgery was successful, the diagnosis was still not clear, requiring additional medical evaluation. The physical exam showed normal pulses in his left upper extremity. Lab tests revealed an elevated ESR of 54 mm/h and a CRP level of 4 mg/dL (reference range, 0.1 to 0.8 mg/dL; a different lab testing site was used, which accounts for the different reference range). In light of the patient’s lab test results, premature arterial vascular disease, and imaging studies suggesting inflammation, Takayasu arteritis (TA) was arrived at as a working diagnosis.
The patient was referred to a rheumatologist, who ordered a repeat ESR and CRP, antineutrophil cytoplasmic antibodies, and a magnetic resonance angiography study of the right brachial artery and major aortic branches to rule out other types of arteritis. Based on the test results, the patient was diagnosed with TA. He was placed on high-dose corticosteroid therapy (prednisone 60 mg/d). Methotrexate 10 mg/wk po was added three months after initiation of the prednisone.
Since being diagnosed with TA, the patient has presented with complaints related to the adverse effects of high-dose corticosteroids (ie, insomnia, weight gain, elevated blood pressure).
Continued on the next page >>
DISCUSSION
The first description of TA is credited to Japanese ophthalmologist Mikito Takayasu, who in 1908 described a wreathlike arteriovenous anastomosis around the optic disc of a 21-year-old woman who had experienced acute vision loss.1-3 Much earlier, in 1761, Italian anatomist Giovanni Battista Morgagni described large-vessel aneurysms and stenosis on a postmortem exam of a 40-year-old woman.2,4 However, TA was not formally labeled a disease until 1975.
TA is a chronic large vessel vasculitis of unknown origin, mainly involving the aorta and its primary branches: the left common carotid, brachiocephalic, and left subclavian arteries. Ongoing inflammation of affected vessels causes fibrotic changes, stenosis, and eventual occlusion and may lead to aneurysm formation.5,6 TA is rare, with an annual incidence in North America of 2.6 cases per million population.6 It occurs most frequently in Asian countries but has been reported in a wide range of ethnic groups.5,7 TA has been characterized as a disease of young women: Between 80% and 97% of patients are women,6,8 and the average age at diagnosis is 25 to 30.8-10
The process of vascular injury in TA begins with inflammation in the vasa vasorum of the aortic vessels. This inflammation, thought to be triggered by an as-yet-unknown antigen, leads to an initial inflammatory cellular infiltration of the aortic media and adventitia; the infiltrate is comprised predominantly of macrophages and T cells.5,9 Inflammatory infiltration causes myointimal proliferation, thickening of the blood vessel wall, and eventual luminal stenosis.5 Cytokines, interleukin 6, interferon , and other chemokines released by infiltrating inflammatory cells within the injured tissue also contribute to the inflammatory response and tissue damage.5,11
Histologically, granulomatous inflammation and giant cells are found in the media.12,13 Destruction of the elastic lamina and the muscular media results in the aneurysmal dilation seen in TA, while dense scarring and continued inflammation of the arterial vasculature results in arterial stenosis.12
Continued on the next page >>
CLINICAL PRESENTATION
Presentation of TA varies widely and can range from asymptomatic disease identified by pulse deficits or impalpable pulses to severe neurologic impairment. The early or prepulseless phase of TA is characterized by inflammatory changes.14 Signs and symptoms are frequently vague and nonspecific, particularly in this early phase, when fatigue, weight loss, and low-grade fever may be seen.12 Headache is another common symptom at the time of disease onset.5
In the later or chronic phase of the disease, individuals will begin to demonstrate signs and symptoms of vascular insufficiency.14 More common physical signs reflect the underlying arterial occlusive disease and include diminished or absent arterial pulses, asymmetrical arm blood pressures, bruits, extremity claudication, and hypertension.5,9,10 Hypertension, generally reflecting renal artery stenosis,10 is present in approximately 40% of cases in the United States and Europe.5,7,15 Neurologic features secondary to hypertension or ischemia affect more than half of patients; in addition to headache, these may include dizziness, syncope, vertigo, transient ischemic attack, and stroke.5
TA can also present with eye, lung, and skin manifestations; however, these features are less common. Although ocular involvement, including amaurosis fugax, has been reported in up to 26% of patients in TA series,5,7,16 permanent loss of vision in North American patients is uncommon.5,7 Pulmonary involvement affecting the large- or medium-sized pulmonary arteries has been reported to occur in approximately 55% of cases5; however, there is uncertainty regarding the prevalence of angiographically demonstrated pulmonary artery involvement, as studies have reported rates ranging from 14.3% to 70%.9,17-20 Pulmonary involvement is often asymptomatic, but features can include dyspnea, cough, and chest pain.5 Skin lesions are seen in up to 28% of cases, most commonly erythema nodosum, erythema induratum, tuberculoidlike eruptions, pyoderma gangrenosum, and cutaneous signs of necrotizing or granulomatous vasculitis.5,21
Continued on the next page >>
DIAGNOSIS
The American College of Rheumatology (ACR) has developed classification criteria for the diagnosis of TA.22 The presence of three or more of the six criteria (age of onset ≤ 40, claudication of the extremities, decreased brachial artery pulse, > 10 mm Hg difference in systolic blood pressure between the arms, bruit over subclavian arteries or aorta, and arteriographic abnormalities) yields a sensitivity of 90.5% and a specificity of 97.8%. Although the ACR classification remains the most widely applied for TA, a limitation of its diagnostic criteria is its failure to distinguish patients with early nonocclusive disease.23
In 1988, the Ishikawa classification criteria were developed, with a modified version subsequently published in 1996.23 Considered superior to the original Ishikawa and ACR criteria based on its application in 106 patients with angiographically proven TA, the modified version has a reported sensitivity and specificity of 92.5% and 95%, respectively.23
With the modified Ishikawa diagnostic criteria, the presence of two major or one major and two or four minor criteria suggests a high probability of TA. The three major criteria consist of lesions of the left mid-subclavian artery and the right mid-subclavian artery and characteristic signs and symptoms of at least 1 mo duration. The 10 minor criteria are high ESR (> 20 mm/h); carotid artery tenderness; hypertension; aortic regurgitation or annuloaortic ectasia; and lesions of pulmonary artery, left mid-common carotid, distal brachiocephalic trunk, descending thoracic aorta, abdominal aorta, and coronary artery.
The diagnosis of TA is based on recognition of clinical findings suggestive of large-vessel vasculitis. Imaging of the arterial tree with CT, MRI, or angiography also demonstrates findings consistent with TA, typically including early-onset vascular wall thickening/enhancement.24 Late imaging studies may reveal arterial stenoses, occlusions, and aneurysms.
Several types of imaging modalities have been used in the diagnosis and management of TA, each with strengths and limitations. Traditional angiography is invasive and requires an arterial puncture. Large doses of radiation are used, exposing the patient to iodinated contrast material, which may be dangerous in patients with poor renal function. However, the primary advantage of traditional angiography is that it allows for interventions such as stent placement and/or angioplasty to be performed.24 Findings on angiography often include long, smooth, tapered stenoses ranging from mild to severe or frank occlusions, as well as collateral vessels or the subclavian steal phenomenon.24
CT imaging is very useful for assessing thickening of the arterial wall. In early TA disease, evaluation of vessel wall thickness may be identified prior to frank stenosis of the artery(s).24 The spectrum of findings on CT angiography includes stenoses; occlusions; aneurysms; and concentric arterial wall thickening affecting the aorta and its branches, the pulmonary arteries, and occasionally the coronary arteries.24
MRI does not require the use of iodinated contrast, nor is there radiation exposure. MRI also has the advantage of evaluating arterial wall thickening, which is often present prior to stenosis (similar to CT imaging).24 Findings of TA on MRI include mural thrombi, signal alterations within and surrounding inflamed vessels, fusiform vascular dilation, thickened aortic valvular cusps, multifocal stenoses, and concentric thickening of the aortic wall.24
Laboratory testing is neither specific nor sensitive. Hoffman and Ahmed studied multiple serologic tests and found that no test reliably distinguishes between patients with active TA and healthy volunteers.25 Increases in the acute phase reactants (ESR and CRP) support the presence of an underlying inflammatory process, and these laboratory tests may be useful in disease monitoring. The ESR and CRP often do not correlate with systemic symptoms or disease progression but are used in conjunction with the clinical exam and serial imaging to gauge treatment success and to monitor disease progression.5,25 Biopsy material typically is not available in the initial diagnosis of TA, but histologic examination at the time of a surgery or procedure is often undertaken to confirm the diagnosis.5
The differential diagnosis of TA includes connective tissue diseases associated with the formation of multiple aneurysms, such as Marfan syndrome and Ehlers-Danlos syndrome.5 However, these diseases do not manifest with large vessel stenosis, the hallmark of TA. Infections known to cause aneurysms of the aorta should also be considered; these include bacterial, fungal, syphilitic, mycotic, and mycobacterial pathogens.5 Blood cultures are used to rule out bacterial agents. Rapid plasma reagin (RPR) and venereal disease research laboratory tests (VDRL) will identify a syphilitic etiology. Fungal cultures or fungal serology will help to rule out a mycotic pathogen.
Autoimmune diseases that can mimic TA include Behçet’s disease, Cogan syndrome, the spondyloarthropathies, and systemic lupus erythematosus. These diseases are not associated with stenosis of large vessels, which differentiates them from TA.5 Giant cell (temporal) arteritis (GCA) may present very similarly to TA, as both diseases affect large arteries.12 The table provides distinguishing features of TA and GCA.26
Continued on the next page >>
TREATMENT
Active phase TA is initially treated with high-dose glucocorticoid therapy (prednisone or methylprednisolone). Typical prednisone doses are 0.5 to 1 mg/kg/d.5 Clinical improvement is seen in almost all patients with glucocorticoid therapy,6,10,23 but relapse is common when prednisone is tapered to less than 20 mg/d.5 The corticosteroid dose is gradually tapered depending on patient response. Common side effects of corticosteroids may include weight gain, elevations in blood glucose, insomnia, increased infection risk, osteoporosis, and slowing of wound healing.
Because nearly half of all patients treated with glucocorticoids alone demonstrate chronic active disease, immunosuppressive therapies are almost always used concomitantly.27 Immune-suppressing drugs that may be used include methotrexate (15 to 25 mg/wk), azathioprine (2 mg/kg/d), and cyclophosphamide (1 to 2 mg/kg/d orally).5,28 Tumor necrosis factor (TNF)–blocking agents used to treat TA include etanercept, infliximab, or adalimumab.28,29 Adverse effects associated with immunosuppressive therapies and TNF-blocking agents include an increased risk for infection(s) and malignancy, bone marrow suppression, and hepatitis B reactivation. Although data are limited on anti-TNF agents, this class of drug has shown promise when used in conjunction with corticosteroids.28
In one open-label study by Hoffman and colleagues, remission rates with methotrexate plus steroids were 81%. Relapse occurred in 44% of study participants when the steroid dose was tapered or decreased to near discontinuation.27 More recently, in an uncontrolled study series involving 15 TA patients from India who were treated with azathioprine plus steroids, remission was achieved following 12 weeks of therapy. Angiographically, there was no progression of arterial disease after one year.30
Surgical and endovascular procedures used to return blood flow in stenotic or occluded vessels include synthetic or autologous vessel bypass, endarterectomy, and percutaneous transluminal angioplasty.5 When aortic insufficiency is present, aortic root replacement or repair is undertaken.5 These procedures are performed by vascular or cardiovascular surgeons and interventional radiologists. Rheumatologists are the medical specialists most involved in the direct care and management of TA patients. Cardiologists are sometimes consulted as well.
Continued on the next page >>
PROGNOSIS
Disability is common in TA. In a National Institutes of Health cohort study, 74% of TA patients reported experiencing functional effects from their disease, and 47% were fully disabled.2,8 In their retrospective review of 107 cases of TA, Lupi-Herrera and colleagues reported a 14% mortality rate.31 Half the deaths in this study were attributed to congestive heart failure (CHF). A cohort study in India that included 88 patients with TA reported cumulative 5- and 10-year survival rates of 91% and 84%, respectively. Of the 10 deaths in this cohort, four were due to CHF.2,32
CONCLUSION
Signs and symptoms of rheumatologic diseases such as TA are often vague, and diagnosis may prove difficult and elusive. Repeat office visits at short intervals may prove to be helpful in making the diagnosis. Referral for radiology and/or rheumatology consultation (face-to-face, if possible) is often necessary.
In cases such as this, completing a personal review of documents and test results done elsewhere, particularly ED/inpatient hospital data, is necessary; relying on the patient’s word that “they told me everything was fine” is insufficient. Clinicians should implement a system that works best for obtaining test results and other documents, follow their instincts, and if the correct diagnosis is not arrived at immediately, keep looking.
References >>
REFERENCES
1. Takayasu M. A case with peculiar changes of the retinal central vessels. Acta Soc Ophthalmol Jpn. 1908;12:554-555.
2. Maksimowicz-McKinnon K, Hoffman GS. Takayasu arteritis: what is the long-term prognosis? Rheum Dis Clin North Am. 2007;33:777-786.
3. Numano F. The story of Takayasu arteritis. Rheumatology. 2002;41:103-106.
4. Morgagni GB. De sedibus et causis morborum per anatomen indagatis.
(Letter 30).1761. Article 12.
5. Hernandez-Rodriguez J, Maksimowicz-McKinnon K, Hoffman GS. Takayasu’s arteritis. In: Carey WD, ed. Current Clinical Medicine. 2nd ed. Philadelphia: Saunders Elsevier; 2010:1195-1199.
6. Hall S, Barr W, Lie JT, et al. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore). 1985;64:89-99.
7. Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000-1009.
8. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994:120:919-929.
9. Gornik HL, Creager MA. Aortic diseases: aortitis. Circulation. 2008;117:
3039-3051.
10. Mwipatayi BP, Jeffery PC, Beningfield SJ, et al. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg. 2005;75:110-117.
11. Noris M. Pathogenesis of Takayasu’s arteritis. J Nephrol. 2001;14:506-513.
12. Hunder GG, Stone JH, Ramirez MP. Clinical features and diagnosis of Takayasu arteritis (2013). www.uptodate.com/contents/clinical-features-and-
diagnosis-of-takayasu-arteritis. Accessed March 24, 2014.
13. Nasu T. Takayasu’s truncoarteritis. Pulseless disease or aortitis syndrome. Acta Pathol Jpn. 1982;32 (suppl 1):117.
14. Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol. 2002;55:481-486.
15. Vanoli M, Daina E, Salvarani C, et al. Takayasu’s arteritis: a study of 104 Italian patients. Arthritis Rheum. 2005;53:100-107.
16. Chun YS, Park SJ, Chung H, Lee J. The clinical and ocular manifestations of Takayasu’s arteritis. Retina. 2001;21:132-140.
17. Liu YQ, Jin BL, Ling J. Pulmonary artery involvement in aortoarteritis: an angiographic study. Cardiovasc Intervent Radiol. 1994;17:2-6.
18. Yamada I, Shibuya H, Matsubara O, et al. Pulmonary artery disease in Takayasu’s arteritis: angiographic findings. AJR Am J Roentgenol. 1992;159:
263-269.
19. Sharma S, Kamalakar T, Rajani M, et al. The incidence and patterns of pulmonary artery involvement in Takayasu’s arteritis. Clin Radiol. 1990;42:177-181.
20. He NS, Liu F, Wu EH, et al. Pulmonary artery involvement in aorto-arteritis: an analysis of DSA. Chin Med J (Engl). 1990;103:666-672.
21. Werfel T, Kuipers JG, Zeidler H, et al. Cutaneous manifestations of Takayasu arteritis. Acta Derm Venereol. 1996;76:496-497.
22. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129-1134.
23. Andrews J, Mason JC. Takayasu’s arteritis—recent advances in imaging offer promise. Rheumatology (Oxford). 2007;46:6-15.
24. Gotway MB, Araoz PA, Macedo TA, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005;184:1945-1950.
25. Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with Takayasu arteritis. A preliminary report from The International Network for the Study of the Systemic Vasculitides (INSSYS). Int J Cardiol. 1998;66 (suppl 1):S191-S194.
26. Michel BA, Arend WP, Hunder GG. Clinical differentiation between giant cell (temporal) arteritis and Takayasu’s arteritis. J Rheumatol. 1996;23:106-111.
27. Hoffman GS, Leavitt RY, Kerr GS, et al. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578-582.
28. Hunder GG, Stone JH, Ramirez MP. Treatment of Takayasu arteritis (2013). www.uptodate.com/contents/treatment-of-takayasu-arteritis. Accessed March 24, 2014.
29. Schmidt J, Kerman TA, Bacani AK, et al. Tumor necrosis factor inhibitors in patients with Takayasu arteritis: experience from a referral center with long-term followup. Arthritis Care Res (Hoboken). 2012;64:1079-1083.
30. Valsakumar AK, Valappil UC, Jorapur V, et al. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu’s arteritis. J Rheumatol. 2003;30:1793-1798.
31. Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, et al. Takayasu’s arteritis: clinical study of 107 cases. Am Heart J. 1977;93:94-103.
32. Subramanyan R, Joy J, Balakrishnan KG. Natural history of aortoarteritis (Takayasu’s disease). Circulation. 1989;80:429-437.
Asthma May Increase Risk of Cardiovascular Events
SAN DIEGO – Having asthma appears to significantly increase your risk for cardiovascular events, while having allergic rhinitis appears to protect your risk for such events, results from a large cohort study demonstrated.
Studies of mouse models have suggested that Th1 inflammation "is associated with atherosclerosis and plaque development, while the Th2 or general allergic response seems to be protective against atherosclerosis," Dr. Angelina Crans Yoon said during a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. At the same time, results from human studies regarding the association between allergic rhinitis and cardiovascular events are mixed, said Dr. Crans Yoon, a first-year allergy fellow at Kaiser Permanente Los Angeles Medical Center.
In an effort to assess the relationship between cardiovascular disease and allergic rhinitis, she and her associates used the Kaiser Permanente Southern California regional database and ICD-9 codes to compare the incidence of cardiovascular and cerebrovascular events and all-cause mortality in a cohort of 109,229 allergic rhinitis patients and 92,775 asthma patients who were seen between Jan. 1, 1995, and Dec. 31, 2012. The cohorts were matched by age, sex, and ethnicity to reference cohorts and followed for a median of 8 years.
Dr. Crans Yoon reported that patients with allergic rhinitis had significantly lower risk for myocardial infarction (hazard ratio, 0.75), cerebrovascular disease (HR, 0.81), and all-cause mortality (HR, 0.51), yet their risk of all cardiovascular events was equal to that of the control cohort (HR, 0.97). At the same time, patients with asthma had a significantly higher risk of all cardiovascular disease (HR, 1.36), yet no significantly higher risk of cerebrovascular disease (HR, 1.03) or all-cause mortality (HR, 1.00).
The findings "led us to think of more questions," Dr. Crans Yoon said. "Why is there this decreased risk of events in patients with allergic rhinitis? What explains the risk of cardiovascular events in patients with asthma? Is atopy related to these differences? We started some secondary analyses looking at medication use. It looks like if you use any medications for allergic rhinitis or asthma, you have a decreased risk of some of these events, except for long-acting beta-agonists, which is consistent with previous reports. We’re also starting to look at specific IgE data on these patients. It looks like positive IgE testing may be associated with a decreased risk of all these events."
She speculated that asthma physiology may explain why patients with asthma had significantly higher risk of cardiovascular disease but not cerebrovascular disease. "The interesting point is that potentially, atopic asthmatics may not have the same increased risk," she said.
Dr. Crans Yoon said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Having asthma appears to significantly increase your risk for cardiovascular events, while having allergic rhinitis appears to protect your risk for such events, results from a large cohort study demonstrated.
Studies of mouse models have suggested that Th1 inflammation "is associated with atherosclerosis and plaque development, while the Th2 or general allergic response seems to be protective against atherosclerosis," Dr. Angelina Crans Yoon said during a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. At the same time, results from human studies regarding the association between allergic rhinitis and cardiovascular events are mixed, said Dr. Crans Yoon, a first-year allergy fellow at Kaiser Permanente Los Angeles Medical Center.
In an effort to assess the relationship between cardiovascular disease and allergic rhinitis, she and her associates used the Kaiser Permanente Southern California regional database and ICD-9 codes to compare the incidence of cardiovascular and cerebrovascular events and all-cause mortality in a cohort of 109,229 allergic rhinitis patients and 92,775 asthma patients who were seen between Jan. 1, 1995, and Dec. 31, 2012. The cohorts were matched by age, sex, and ethnicity to reference cohorts and followed for a median of 8 years.
Dr. Crans Yoon reported that patients with allergic rhinitis had significantly lower risk for myocardial infarction (hazard ratio, 0.75), cerebrovascular disease (HR, 0.81), and all-cause mortality (HR, 0.51), yet their risk of all cardiovascular events was equal to that of the control cohort (HR, 0.97). At the same time, patients with asthma had a significantly higher risk of all cardiovascular disease (HR, 1.36), yet no significantly higher risk of cerebrovascular disease (HR, 1.03) or all-cause mortality (HR, 1.00).
The findings "led us to think of more questions," Dr. Crans Yoon said. "Why is there this decreased risk of events in patients with allergic rhinitis? What explains the risk of cardiovascular events in patients with asthma? Is atopy related to these differences? We started some secondary analyses looking at medication use. It looks like if you use any medications for allergic rhinitis or asthma, you have a decreased risk of some of these events, except for long-acting beta-agonists, which is consistent with previous reports. We’re also starting to look at specific IgE data on these patients. It looks like positive IgE testing may be associated with a decreased risk of all these events."
She speculated that asthma physiology may explain why patients with asthma had significantly higher risk of cardiovascular disease but not cerebrovascular disease. "The interesting point is that potentially, atopic asthmatics may not have the same increased risk," she said.
Dr. Crans Yoon said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Having asthma appears to significantly increase your risk for cardiovascular events, while having allergic rhinitis appears to protect your risk for such events, results from a large cohort study demonstrated.
Studies of mouse models have suggested that Th1 inflammation "is associated with atherosclerosis and plaque development, while the Th2 or general allergic response seems to be protective against atherosclerosis," Dr. Angelina Crans Yoon said during a press briefing at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. At the same time, results from human studies regarding the association between allergic rhinitis and cardiovascular events are mixed, said Dr. Crans Yoon, a first-year allergy fellow at Kaiser Permanente Los Angeles Medical Center.
In an effort to assess the relationship between cardiovascular disease and allergic rhinitis, she and her associates used the Kaiser Permanente Southern California regional database and ICD-9 codes to compare the incidence of cardiovascular and cerebrovascular events and all-cause mortality in a cohort of 109,229 allergic rhinitis patients and 92,775 asthma patients who were seen between Jan. 1, 1995, and Dec. 31, 2012. The cohorts were matched by age, sex, and ethnicity to reference cohorts and followed for a median of 8 years.
Dr. Crans Yoon reported that patients with allergic rhinitis had significantly lower risk for myocardial infarction (hazard ratio, 0.75), cerebrovascular disease (HR, 0.81), and all-cause mortality (HR, 0.51), yet their risk of all cardiovascular events was equal to that of the control cohort (HR, 0.97). At the same time, patients with asthma had a significantly higher risk of all cardiovascular disease (HR, 1.36), yet no significantly higher risk of cerebrovascular disease (HR, 1.03) or all-cause mortality (HR, 1.00).
The findings "led us to think of more questions," Dr. Crans Yoon said. "Why is there this decreased risk of events in patients with allergic rhinitis? What explains the risk of cardiovascular events in patients with asthma? Is atopy related to these differences? We started some secondary analyses looking at medication use. It looks like if you use any medications for allergic rhinitis or asthma, you have a decreased risk of some of these events, except for long-acting beta-agonists, which is consistent with previous reports. We’re also starting to look at specific IgE data on these patients. It looks like positive IgE testing may be associated with a decreased risk of all these events."
She speculated that asthma physiology may explain why patients with asthma had significantly higher risk of cardiovascular disease but not cerebrovascular disease. "The interesting point is that potentially, atopic asthmatics may not have the same increased risk," she said.
Dr. Crans Yoon said that she had no relevant financial conflicts to disclose.
AT THE 2014 AAAAI ANNUAL MEETING
Purpuric lesions in an elderly woman
A 68-year-old woman presented with a 5-day history of extensive pruritic purpuric skin lesions of varying sizes on her trunk and extremities (FIGURE 1A and 1B). In addition, the patient had a few nonblanching, erythematous macules on her extremities.
The patient had no neurological complaints and her family history was negative for a similar condition. We performed a punch biopsy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Churg-Strauss syndrome
Churg-Strauss syndrome (CSS)—also known as allergic granulomatosis and angiitis—is a rare multisystemic vasculitis of small- to medium-sized vessels characterized by asthma, chronic rhinosinusitis, and prominent peripheral blood eosinophilia.1,2 Mean diagnosis age is 50 years with no gender predilection.2 Any organ system can be affected, although the lungs are most commonly involved, followed by the skin.1,2
Based on criteria from the American College of Rheumatology, the diagnosis of CSS can be made if 4 of the following 6 criteria are met: (1) asthma, (2) eosinophilia >10% on a differential white blood cell (WBC) count, (3) paranasal sinus abnormalities, (4) a transient pulmonary infiltrate detected on chest x-ray, (5) mono- or polyneuropathy, and (6) a biopsy specimen showing extravascular accumulation of eosinophils.2
Skin biopsy specimen from our patient showed leukocytoclastic vasculitis with prominent tissue eosinophilia. Laboratory studies showed an elevated WBC count of 12,300/mcL (reference range, 4500-11,000/mcL), and eosinophilia of 40% (reference range, 1%-4%). A serologic test for perinuclear pattern antineutrophil cytoplasmic antibodies (p-ANCA) was positive. (More on this in a moment.) Radiography of the chest showed transient pulmonary infiltrates.
Based on the clinical and laboratory findings, the patient was positive for 4 of 6 criteria and given a diagnosis of CSS.
What we know—and don’t know—about CSS
The exact etiopathogenesis of CSS is unknown.2-4 Although ANCAs are detected in about 40% to 60% of CSS patients, it is not yet known whether ANCAs have a pathogenic role.2-3 Abnormalities in immunologic function also occur, including heightened Th1 and Th2 lymphocyte function, increased recruitment of eosinophils, and decreased eosinophil apoptosis. Genetic factors, including certain interleukin-10 polymorphisms and HLA classes such as HLA-DRB4, may also contribute to CSS pathogenesis.4
Three distinct sequential phases have been described, although these are not always clearly distinguishable.2,5
• The first is the prodromal or allergic phase, which is characterized by the onset of asthma later in life in patients with no family history of atopy. There may or may not be an associated allergic rhinitis.
• In the eosinophilic phase, peripheral blood eosinophilia and eosinophilic infiltration of multiple organs (especially the lungs and gastrointestinal [GI] tract) occur.
• The vasculitis phase is characterized by life-threatening systemic vasculitis of the small and medium vessels that is often associated with vascular and extravascular granulomatosis.
Cutaneous and extracutaneous findings
One-half to two-thirds of patients with CSS have cutaneous manifestations that typically present in the vasculitis phase.2,5 The most common skin finding is palpable purpura on the lower extremities. Macular or papular erythematous eruption, urticaria, subcutaneous skin-colored or erythematous nodules, livedo reticularis, and erythema multiforme–like eruption may also be seen.2,5,6 Skin biopsies will show numerous eosinophils with either leukocytoclastic vasculitis or extravascular necrotizing granuloma.5
Extracutaneous manifestations of CSS include renal, cardiac, GI tract, and nervous system involvement.2,7
To identify patients with a poor prognosis, the 5-factor score (FFS) can be used. This score assigns 1 point each to GI tract involvement, renal insufficiency, proteinuria, central nervous system involvement, and cardiomyopathy.7 CSS patients with an FFS ≥2 have a considerably greater risk of mortality.7
Treatment involves corticosteroids
Systemic corticosteroids (prednisone, 1 mg/kg/day) are the primary treatment for patients with CSS; most patients improve dramatically with therapy.2 Adjunctive therapy with immunosuppressive agents such as cyclophosphamide, methotrexate (10-15 mg per week), chlorambucil, or azathioprine may be needed if a patient does not respond adequately to steroids alone.2
Prednisone for our patient
We started our patient on prednisone 1 mg/kg/d. Her skin lesions resolved and subsequent laboratory tests, including eosinophil counts, normalized. Prednisone therapy was gradually tapered over several months to attain the lowest dose required for control of symptoms—in this case, 5 mg/d.
CORRESPONDENCE
Ossama Abbas, MD, Associate Professor, Department of Dermatology, American University of Beirut Medical Center, PO Box 11-0236, Riad El Solh, Beirut 1107 2020, Beirut, Lebanon; [email protected]
1. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis and periarteritis nodosa. Am J Pathol. 1951;27:277-301.
2. Sinico RA, Bottero P. Churg-Strauss angiitis. Best Pract Res Clin Rheumatol. 2009;23:355-366.
3. Zwerina J, Axmann R, Jatzwauk M, et al. Pathogenesis of Churg-Strauss syndrome: recent insights. Autoimmunity. 2009;42:376-379.
4. Vaglio A, Martorana D, Maggiore U, et al; Secondary and Primary Vasculitis Study Group. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 2007;56:3159-3166.
5. Davis MD, Daoud MS, McEvoy MT, et al. Cutaneous manifestations of Churg-Strauss syndrome: a clinicopathologic correlation. J Am Acad Dermatol. 1997;37(2 pt 1):199-203.
6. Tlacuilo-Parra A, Soto-Ortíz JA, Guevara-Gutiérrez E. Churg-Strauss syndrome manifested by urticarial plaques. Int J Dermatol. 2003;42:386-388.
7. Guillevin L, Lhote F, Gayraud M, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996;75:17-28.
A 68-year-old woman presented with a 5-day history of extensive pruritic purpuric skin lesions of varying sizes on her trunk and extremities (FIGURE 1A and 1B). In addition, the patient had a few nonblanching, erythematous macules on her extremities.
The patient had no neurological complaints and her family history was negative for a similar condition. We performed a punch biopsy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Churg-Strauss syndrome
Churg-Strauss syndrome (CSS)—also known as allergic granulomatosis and angiitis—is a rare multisystemic vasculitis of small- to medium-sized vessels characterized by asthma, chronic rhinosinusitis, and prominent peripheral blood eosinophilia.1,2 Mean diagnosis age is 50 years with no gender predilection.2 Any organ system can be affected, although the lungs are most commonly involved, followed by the skin.1,2
Based on criteria from the American College of Rheumatology, the diagnosis of CSS can be made if 4 of the following 6 criteria are met: (1) asthma, (2) eosinophilia >10% on a differential white blood cell (WBC) count, (3) paranasal sinus abnormalities, (4) a transient pulmonary infiltrate detected on chest x-ray, (5) mono- or polyneuropathy, and (6) a biopsy specimen showing extravascular accumulation of eosinophils.2
Skin biopsy specimen from our patient showed leukocytoclastic vasculitis with prominent tissue eosinophilia. Laboratory studies showed an elevated WBC count of 12,300/mcL (reference range, 4500-11,000/mcL), and eosinophilia of 40% (reference range, 1%-4%). A serologic test for perinuclear pattern antineutrophil cytoplasmic antibodies (p-ANCA) was positive. (More on this in a moment.) Radiography of the chest showed transient pulmonary infiltrates.
Based on the clinical and laboratory findings, the patient was positive for 4 of 6 criteria and given a diagnosis of CSS.
What we know—and don’t know—about CSS
The exact etiopathogenesis of CSS is unknown.2-4 Although ANCAs are detected in about 40% to 60% of CSS patients, it is not yet known whether ANCAs have a pathogenic role.2-3 Abnormalities in immunologic function also occur, including heightened Th1 and Th2 lymphocyte function, increased recruitment of eosinophils, and decreased eosinophil apoptosis. Genetic factors, including certain interleukin-10 polymorphisms and HLA classes such as HLA-DRB4, may also contribute to CSS pathogenesis.4
Three distinct sequential phases have been described, although these are not always clearly distinguishable.2,5
• The first is the prodromal or allergic phase, which is characterized by the onset of asthma later in life in patients with no family history of atopy. There may or may not be an associated allergic rhinitis.
• In the eosinophilic phase, peripheral blood eosinophilia and eosinophilic infiltration of multiple organs (especially the lungs and gastrointestinal [GI] tract) occur.
• The vasculitis phase is characterized by life-threatening systemic vasculitis of the small and medium vessels that is often associated with vascular and extravascular granulomatosis.
Cutaneous and extracutaneous findings
One-half to two-thirds of patients with CSS have cutaneous manifestations that typically present in the vasculitis phase.2,5 The most common skin finding is palpable purpura on the lower extremities. Macular or papular erythematous eruption, urticaria, subcutaneous skin-colored or erythematous nodules, livedo reticularis, and erythema multiforme–like eruption may also be seen.2,5,6 Skin biopsies will show numerous eosinophils with either leukocytoclastic vasculitis or extravascular necrotizing granuloma.5
Extracutaneous manifestations of CSS include renal, cardiac, GI tract, and nervous system involvement.2,7
To identify patients with a poor prognosis, the 5-factor score (FFS) can be used. This score assigns 1 point each to GI tract involvement, renal insufficiency, proteinuria, central nervous system involvement, and cardiomyopathy.7 CSS patients with an FFS ≥2 have a considerably greater risk of mortality.7
Treatment involves corticosteroids
Systemic corticosteroids (prednisone, 1 mg/kg/day) are the primary treatment for patients with CSS; most patients improve dramatically with therapy.2 Adjunctive therapy with immunosuppressive agents such as cyclophosphamide, methotrexate (10-15 mg per week), chlorambucil, or azathioprine may be needed if a patient does not respond adequately to steroids alone.2
Prednisone for our patient
We started our patient on prednisone 1 mg/kg/d. Her skin lesions resolved and subsequent laboratory tests, including eosinophil counts, normalized. Prednisone therapy was gradually tapered over several months to attain the lowest dose required for control of symptoms—in this case, 5 mg/d.
CORRESPONDENCE
Ossama Abbas, MD, Associate Professor, Department of Dermatology, American University of Beirut Medical Center, PO Box 11-0236, Riad El Solh, Beirut 1107 2020, Beirut, Lebanon; [email protected]
A 68-year-old woman presented with a 5-day history of extensive pruritic purpuric skin lesions of varying sizes on her trunk and extremities (FIGURE 1A and 1B). In addition, the patient had a few nonblanching, erythematous macules on her extremities.
The patient had no neurological complaints and her family history was negative for a similar condition. We performed a punch biopsy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Churg-Strauss syndrome
Churg-Strauss syndrome (CSS)—also known as allergic granulomatosis and angiitis—is a rare multisystemic vasculitis of small- to medium-sized vessels characterized by asthma, chronic rhinosinusitis, and prominent peripheral blood eosinophilia.1,2 Mean diagnosis age is 50 years with no gender predilection.2 Any organ system can be affected, although the lungs are most commonly involved, followed by the skin.1,2
Based on criteria from the American College of Rheumatology, the diagnosis of CSS can be made if 4 of the following 6 criteria are met: (1) asthma, (2) eosinophilia >10% on a differential white blood cell (WBC) count, (3) paranasal sinus abnormalities, (4) a transient pulmonary infiltrate detected on chest x-ray, (5) mono- or polyneuropathy, and (6) a biopsy specimen showing extravascular accumulation of eosinophils.2
Skin biopsy specimen from our patient showed leukocytoclastic vasculitis with prominent tissue eosinophilia. Laboratory studies showed an elevated WBC count of 12,300/mcL (reference range, 4500-11,000/mcL), and eosinophilia of 40% (reference range, 1%-4%). A serologic test for perinuclear pattern antineutrophil cytoplasmic antibodies (p-ANCA) was positive. (More on this in a moment.) Radiography of the chest showed transient pulmonary infiltrates.
Based on the clinical and laboratory findings, the patient was positive for 4 of 6 criteria and given a diagnosis of CSS.
What we know—and don’t know—about CSS
The exact etiopathogenesis of CSS is unknown.2-4 Although ANCAs are detected in about 40% to 60% of CSS patients, it is not yet known whether ANCAs have a pathogenic role.2-3 Abnormalities in immunologic function also occur, including heightened Th1 and Th2 lymphocyte function, increased recruitment of eosinophils, and decreased eosinophil apoptosis. Genetic factors, including certain interleukin-10 polymorphisms and HLA classes such as HLA-DRB4, may also contribute to CSS pathogenesis.4
Three distinct sequential phases have been described, although these are not always clearly distinguishable.2,5
• The first is the prodromal or allergic phase, which is characterized by the onset of asthma later in life in patients with no family history of atopy. There may or may not be an associated allergic rhinitis.
• In the eosinophilic phase, peripheral blood eosinophilia and eosinophilic infiltration of multiple organs (especially the lungs and gastrointestinal [GI] tract) occur.
• The vasculitis phase is characterized by life-threatening systemic vasculitis of the small and medium vessels that is often associated with vascular and extravascular granulomatosis.
Cutaneous and extracutaneous findings
One-half to two-thirds of patients with CSS have cutaneous manifestations that typically present in the vasculitis phase.2,5 The most common skin finding is palpable purpura on the lower extremities. Macular or papular erythematous eruption, urticaria, subcutaneous skin-colored or erythematous nodules, livedo reticularis, and erythema multiforme–like eruption may also be seen.2,5,6 Skin biopsies will show numerous eosinophils with either leukocytoclastic vasculitis or extravascular necrotizing granuloma.5
Extracutaneous manifestations of CSS include renal, cardiac, GI tract, and nervous system involvement.2,7
To identify patients with a poor prognosis, the 5-factor score (FFS) can be used. This score assigns 1 point each to GI tract involvement, renal insufficiency, proteinuria, central nervous system involvement, and cardiomyopathy.7 CSS patients with an FFS ≥2 have a considerably greater risk of mortality.7
Treatment involves corticosteroids
Systemic corticosteroids (prednisone, 1 mg/kg/day) are the primary treatment for patients with CSS; most patients improve dramatically with therapy.2 Adjunctive therapy with immunosuppressive agents such as cyclophosphamide, methotrexate (10-15 mg per week), chlorambucil, or azathioprine may be needed if a patient does not respond adequately to steroids alone.2
Prednisone for our patient
We started our patient on prednisone 1 mg/kg/d. Her skin lesions resolved and subsequent laboratory tests, including eosinophil counts, normalized. Prednisone therapy was gradually tapered over several months to attain the lowest dose required for control of symptoms—in this case, 5 mg/d.
CORRESPONDENCE
Ossama Abbas, MD, Associate Professor, Department of Dermatology, American University of Beirut Medical Center, PO Box 11-0236, Riad El Solh, Beirut 1107 2020, Beirut, Lebanon; [email protected]
1. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis and periarteritis nodosa. Am J Pathol. 1951;27:277-301.
2. Sinico RA, Bottero P. Churg-Strauss angiitis. Best Pract Res Clin Rheumatol. 2009;23:355-366.
3. Zwerina J, Axmann R, Jatzwauk M, et al. Pathogenesis of Churg-Strauss syndrome: recent insights. Autoimmunity. 2009;42:376-379.
4. Vaglio A, Martorana D, Maggiore U, et al; Secondary and Primary Vasculitis Study Group. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 2007;56:3159-3166.
5. Davis MD, Daoud MS, McEvoy MT, et al. Cutaneous manifestations of Churg-Strauss syndrome: a clinicopathologic correlation. J Am Acad Dermatol. 1997;37(2 pt 1):199-203.
6. Tlacuilo-Parra A, Soto-Ortíz JA, Guevara-Gutiérrez E. Churg-Strauss syndrome manifested by urticarial plaques. Int J Dermatol. 2003;42:386-388.
7. Guillevin L, Lhote F, Gayraud M, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996;75:17-28.
1. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis and periarteritis nodosa. Am J Pathol. 1951;27:277-301.
2. Sinico RA, Bottero P. Churg-Strauss angiitis. Best Pract Res Clin Rheumatol. 2009;23:355-366.
3. Zwerina J, Axmann R, Jatzwauk M, et al. Pathogenesis of Churg-Strauss syndrome: recent insights. Autoimmunity. 2009;42:376-379.
4. Vaglio A, Martorana D, Maggiore U, et al; Secondary and Primary Vasculitis Study Group. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 2007;56:3159-3166.
5. Davis MD, Daoud MS, McEvoy MT, et al. Cutaneous manifestations of Churg-Strauss syndrome: a clinicopathologic correlation. J Am Acad Dermatol. 1997;37(2 pt 1):199-203.
6. Tlacuilo-Parra A, Soto-Ortíz JA, Guevara-Gutiérrez E. Churg-Strauss syndrome manifested by urticarial plaques. Int J Dermatol. 2003;42:386-388.
7. Guillevin L, Lhote F, Gayraud M, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996;75:17-28.
Steroids for Acute COPD—But for How Long?
PRACTICE CHANGER
Prescribe a five-day regimen of glucocorticoid therapy for acute exacerbations of chronic obstructive pulmonary disease (COPD); the shorter course of treatment appears to be as effective as a 14-day regimen.1
Strength of recommendation
B: Based on a single well-designed randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
A 55-year-old man with COPD presents to the emergency department (ED) with progressive shortness of breath, cough, and sputum production in the past four days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk for early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids versus a longer (eight-week) duration come from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to 14-day regimen (30 to 40 mg/d) but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (three to seven days) with a longer regimen (10 to 15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing five-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
Continue reading for the study summary...
STUDY SUMMARY
Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a five- and a 14-day course of prednisone 40 mg/d to treat patients with COPD exacerbations. A patient was considered to have an exacerbation if he or she had a change from baseline in two or more of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who presented to the EDs of five Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 or older and have at least 20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (FEV1/FVC > 70%), pneumonia, an estimated survival of less than six months, pregnancy, and lactation.
All the participants (N = 311) received 40 mg methylprednisolone intravenously on day 1, followed by prednisone 40 mg orally on days 2 through 5. The researchers then randomly divided participants into two groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional nine days. Participants in both groups also received antibiotics for seven days, twice-daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the five-day steroid regimen versus 57 days for those on the
14-day regimen in the intention-to-treat analysis (HR, 0.95). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took more than 400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg, respectively).
WHAT’S NEW?
Now we know: five days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for more than five days.3 This trial clearly demonstrated that 40-mg prednisone for five days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS
Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION
Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a five-day regimen may be appropriate. For those in whom prior attempts at short-course treatment have failed, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which patients previously treated with a longer regimen will find the shorter course of treatment unsuccessful.
Continue for references...
REFERENCES
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org. Accessed January 9, 2014.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(1):29-30, 32.
PRACTICE CHANGER
Prescribe a five-day regimen of glucocorticoid therapy for acute exacerbations of chronic obstructive pulmonary disease (COPD); the shorter course of treatment appears to be as effective as a 14-day regimen.1
Strength of recommendation
B: Based on a single well-designed randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
A 55-year-old man with COPD presents to the emergency department (ED) with progressive shortness of breath, cough, and sputum production in the past four days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk for early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids versus a longer (eight-week) duration come from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to 14-day regimen (30 to 40 mg/d) but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (three to seven days) with a longer regimen (10 to 15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing five-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
Continue reading for the study summary...
STUDY SUMMARY
Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a five- and a 14-day course of prednisone 40 mg/d to treat patients with COPD exacerbations. A patient was considered to have an exacerbation if he or she had a change from baseline in two or more of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who presented to the EDs of five Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 or older and have at least 20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (FEV1/FVC > 70%), pneumonia, an estimated survival of less than six months, pregnancy, and lactation.
All the participants (N = 311) received 40 mg methylprednisolone intravenously on day 1, followed by prednisone 40 mg orally on days 2 through 5. The researchers then randomly divided participants into two groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional nine days. Participants in both groups also received antibiotics for seven days, twice-daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the five-day steroid regimen versus 57 days for those on the
14-day regimen in the intention-to-treat analysis (HR, 0.95). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took more than 400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg, respectively).
WHAT’S NEW?
Now we know: five days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for more than five days.3 This trial clearly demonstrated that 40-mg prednisone for five days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS
Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION
Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a five-day regimen may be appropriate. For those in whom prior attempts at short-course treatment have failed, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which patients previously treated with a longer regimen will find the shorter course of treatment unsuccessful.
Continue for references...
REFERENCES
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org. Accessed January 9, 2014.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(1):29-30, 32.
PRACTICE CHANGER
Prescribe a five-day regimen of glucocorticoid therapy for acute exacerbations of chronic obstructive pulmonary disease (COPD); the shorter course of treatment appears to be as effective as a 14-day regimen.1
Strength of recommendation
B: Based on a single well-designed randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
A 55-year-old man with COPD presents to the emergency department (ED) with progressive shortness of breath, cough, and sputum production in the past four days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk for early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids versus a longer (eight-week) duration come from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to 14-day regimen (30 to 40 mg/d) but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (three to seven days) with a longer regimen (10 to 15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing five-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
Continue reading for the study summary...
STUDY SUMMARY
Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a five- and a 14-day course of prednisone 40 mg/d to treat patients with COPD exacerbations. A patient was considered to have an exacerbation if he or she had a change from baseline in two or more of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who presented to the EDs of five Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 or older and have at least 20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (FEV1/FVC > 70%), pneumonia, an estimated survival of less than six months, pregnancy, and lactation.
All the participants (N = 311) received 40 mg methylprednisolone intravenously on day 1, followed by prednisone 40 mg orally on days 2 through 5. The researchers then randomly divided participants into two groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional nine days. Participants in both groups also received antibiotics for seven days, twice-daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the five-day steroid regimen versus 57 days for those on the
14-day regimen in the intention-to-treat analysis (HR, 0.95). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took more than 400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg, respectively).
WHAT’S NEW?
Now we know: five days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for more than five days.3 This trial clearly demonstrated that 40-mg prednisone for five days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS
Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION
Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a five-day regimen may be appropriate. For those in whom prior attempts at short-course treatment have failed, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which patients previously treated with a longer regimen will find the shorter course of treatment unsuccessful.
Continue for references...
REFERENCES
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org. Accessed January 9, 2014.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(1):29-30, 32.