User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Warning: Watch out for ‘medication substitution reaction’
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Psychotropic medications for chronic pain
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.
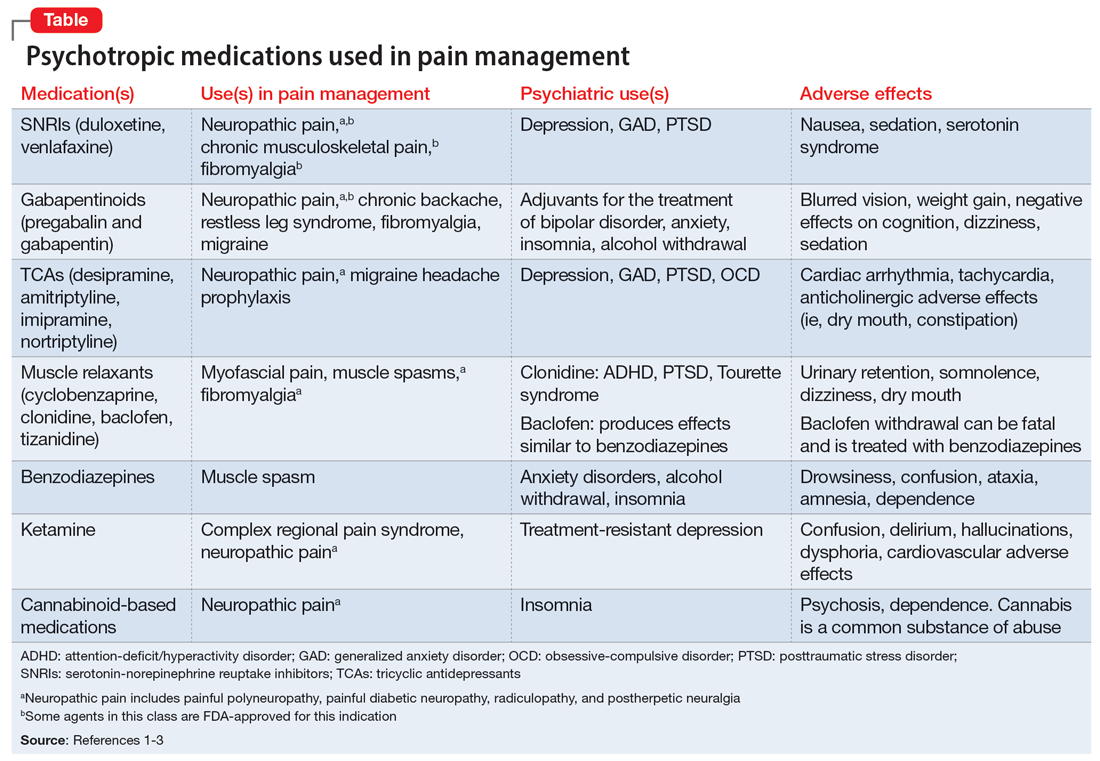
In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.

In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.

In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
The light at the end of the tunnel: Reflecting on a 7-year training journey
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
Lamotrigine for bipolar depression?
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
In reading Dr. Nasrallah's August 2022 editorial (“Reversing depression: A plethora of therapeutic strategies and mechanisms,”
Dr. Nasrallah responds
Thanks for your message. Lamotrigine is not FDA-approved for bipolar or unipolar depression, either as monotherapy or as an adjunctive therapy. It has never been approved for mania, either (no efficacy at all). Its only FDA-approved psychiatric indication is maintenance therapy after a patient with bipolar I disorder emerges from mania with the help of one of the antimanic drugs. Yet many clinicians may perceive lamotrigine as useful for bipolar depression because more than 20 years ago the manufacturer sponsored several small studies (not FDA trials). Two studies that showed efficacy were published, but 4 other studies that failed to show efficacy were not published. As a result, many clinicians got the false impression that lamotrigine is an effective antidepressant. I hope this explains why lamotrigine was not included in the list of antidepressants in my editorial.
Patients with schizophrenia may be twice as likely to develop dementia
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a review and meta-analysis of almost 13 million total participants from nine countries showed that, across multiple different psychotic disorders, there was a 2.5-fold higher risk of developing dementia later in life compared with individuals who did not have a disorder. This was regardless of the age at which the patients first developed the mental illness.
Moreover, participants with a psychotic disorder tended to be younger than average when diagnosed with dementia. Two studies showed that those with psychotic disorders were more likely to be diagnosed with dementia as early as in their 60s.
“The findings add to a growing body of evidence linking psychiatric disorders with later cognitive decline and dementia,” senior investigator Jean Stafford, PhD, a research fellow at MRC Unit for Lifelong Health and Ageing, University College London, told this news organization.
Dr. Stafford noted that the results highlight the importance of being aware of and watchful for symptoms of cognitive decline in patients with psychotic disorders in mid- and late life.
“In addition, given that people with psychotic disorders are at higher risk of experiencing multiple health conditions, including dementia, managing overall physical and mental health in this group is crucial,” she said.
The findings were published online in Psychological Medicine.
Bringing the evidence together
There is increasing evidence that multiple psychiatric symptoms and diagnoses are associated with cognitive decline and dementia, with particularly strong evidence for late-life depression, Dr. Stafford said.
“However, the relationship between psychotic disorders and dementia is less well-established,” she added.
Last year, her team published a study showing a strong association between very late onset psychotic disorders, defined as first diagnosed after age 60 years, and increased risk for dementia in Swedish population register data.
“We also became aware of several other large studies on the topic published in the last few years and realized that an up-to-date systematic review and meta-analysis was needed to bring together the evidence, specifically focusing on longitudinal studies,” Dr. Stafford said.
The researchers searched four databases of prospective and retrospective longitudinal studies published through March 2022. Studies were required to focus on adults aged 18 years or older with a clinical diagnosis of a nonaffective psychotic disorder and a comparison group consisting of adults without a nonaffective psychotic disorder.
Of 9,496 papers, the investigators selected 11 published from 2003 to 2022 that met criteria for inclusion in their meta-analysis (12,997,101 participants), with follow-up periods ranging from 1.57 to 33 years.
The studies hailed from Denmark, Finland, Sweden, the United Kingdom, the United States, Australia, Taiwan, New Zealand, and Israel.
Random-effects meta-analyses were used to pool estimates across studies. The researchers assessed the risk of bias for each study. They also included two additional studies in the review, but not the meta-analysis, that focused specifically on late-onset acute and transient psychosis and late-onset delusional disorder.
The other studies focused on late-onset schizophrenia and/or very late onset schizophrenia-like psychoses, schizophrenia, psychotic disorders, and schizophrenia in older people.
Most studies investigated the incidence of all-cause dementia, although one study focused on the incidence of Alzheimer’s disease.
Potential mechanisms
The narrative review showed that most studies (n = 10) were of high methodological quality, although two were rated as fair and one as poor.
Almost all studies accounted for basic sociodemographic confounders. Several also adjusted for comorbidities, alcohol/substance use disorders, medications, smoking status, and income/education level.
Pooled estimates from the meta-analyzed studies showed that only one showed no significant association between psychotic disorders and dementia, whereas 10 reported increased risk (pooled risk ratio, 2.52; 95% confidence interval, 1.67-3.80; I2, 99.7%).
Subgroup analyses showed higher risk in participants with typical and late-onset psychotic disorders (pooled RR, 2.10; 95% CI, 2.33-4.14; I2, 77.5%; P = .004) vs. those with very late onset schizophrenia-like psychoses (pooled RR, 2.77; 95% CI, 1.74-4.40 I2, 98.9%; P < .001).
The effect was larger in studies with a follow-up of less than 10 years vs. those with a follow-up of 10 years or more, and it was also greater in studies conducted in non-European vs. European countries (all P < .001).
Studies with more female participants (≥ 60%) showed higher risk compared with those that had a lower percentage of female participants. Studies published during or after 2020 showed a stronger association than those published before 2020 (all P < .001).
There was also a higher risk for dementia in studies investigating broader nonaffective psychotic disorders compared with studies investigating only schizophrenia, in prospective vs. retrospective studies, and in studies with a minimum age of less than 60 years at baseline vs. a minimum age of 60 or older (all P < .001).
“Several possible mechanisms could underlie these findings, although we were not able to directly test these in our review,” Dr. Stafford said. She noted that psychotic disorders and other psychiatric diagnoses may cause dementia.
“People with psychotic disorders such as schizophrenia are also at higher risk of health conditions including cardiovascular disease and diabetes, which are known risk factors for dementia and could underpin these associations,” said Dr. Stafford.
It is also possible “that psychotic symptoms could be early markers of dementia for some people, rather than causes,” she added.
Neuroimaging evidence lacking
Commenting on the study, Dilip V. Jeste, MD, former senior associate dean for healthy aging and senior care and distinguished professor of psychiatry and neurosciences at the University of California, San Diego, complimented the investigators for “an excellent article on an important but difficult topic.”
Limitations “pertain not to the meta-analysis but to the original studies,” said Dr. Jeste, who was not involved with the review. Diagnosing dementia in individuals with psychotic disorders is “challenging because cognitive deficits and behavioral symptoms in psychotic disorders may be misdiagnosed as dementia in some individuals – and vice versa,” he added.
Moreover, the studies did not specify the type of dementia, such as Alzheimer’s disease, vascular, Lewy body, frontotemporal, or mixed. Together, “they account for 90% of the dementias, and most patients with these dementias have brain abnormalities that can clearly be seen on MRI,” Dr. Jeste said.
However, patients with schizophrenia who are diagnosed with dementia “rarely show severe brain atrophy, even in specific regions commonly observed in nonpsychotic people with these dementias,” Dr. Jeste noted.
Thus, objective neuroimaging-based evidence for dementia and its subtype “is lacking in most of the published studies of persons with psychotic disorders diagnosed as having dementia,” he said.
There is a “clear need for comprehensive studies of dementia in people with psychotic disorders to understand the significance of the results,” Dr. Jeste concluded.
The review did not receive any funding. Dr. Stafford was supported by an NIHR-UCLH BRC Postdoctoral Bridging Fellowship and the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust. Dr. Stafford was also the principal investigator in one of the studies meeting the inclusion criteria of the review. The other investigators and Dr. Jeste reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL MEDICINE
Menopause an independent risk factor for schizophrenia relapse
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCHIZOPHRENIA BULLETIN
Younger doctors call for more attention to patients with disabilities
As an undergraduate student at Northeastern University in Boston, Meghan Chin spent her summers working for a day program in Rhode Island. Her charges were adults with various forms of intellectual and developmental disabilities (IDD).
“I was very much a caretaker,” Ms. Chin, now 29, said. “It was everything from helping them get dressed in the morning to getting them to medical appointments.”
During one such visit Ms. Chin got a lesson about how health care looks from the viewpoint of someone with an IDD.
The patient was a woman in her 60s and she was having gastrointestinal issues; symptoms she could have articulated, if asked. “She was perfectly capable of telling a clinician where it hurt, how long she had experienced the problem, and what she had done or not done to alleviate it,” Ms. Chin said.
And of comprehending a response. But she was not given the opportunity.
“She would explain what was going on to the clinician,” Ms. Chin recalled. “And the clinician would turn to me and answer. It was this weird three-way conversation – as if she wasn’t even there in the room with us.”
Ms. Chin was incensed at the rude and disrespectful way the patient had been treated. But her charge didn’t seem upset or surprised. Just resigned. “Sadly, she had become used to this,” Ms. Chin said.
For the young aide, however, the experience was searing. “It didn’t seem right to me,” Ms. Chin said. “That’s why, when I went to medical school, I knew I wanted to do better for this population.”
Serendipity led her to Georgetown University, Washington, where she met Kim Bullock, MD, one of the country’s leading advocates for improved health care delivery to those with IDDs.
Dr. Bullock, an associate professor of family medicine, seeks to create better training and educational opportunities for medical students who will likely encounter patients with these disabilities in their practices.
When Dr. Bullock heard Ms. Chin’s story about the patient being ignored, she was not surprised.
“This is not an unusual or unique situation,” said Dr. Bullock, who is also director of Georgetown’s community health division and a faculty member of the university’s Center for Excellence for Developmental Disabilities. “In fact, it’s quite common and is part of what spurred my own interest in educating pre-med and medical students about effective communication techniques, particularly when addressing neurodiverse patients.”
More than 13% of Americans, or roughly 44 million people, have some form of disability, according to the National Institute on Disability at the University of New Hampshire, a figure that does not include those who are institutionalized. The Centers for Disease Control and Prevention estimates that 17% of children aged 3-17 years have a developmental disability.
Even so, many physicians feel ill-prepared to care for disabled patients. A survey of physicians, published in the journal Health Affairs, found that some lacked the resources and training to properly care for patients with disabilities, or that they struggled to coordinate care for such individuals. Some said they did not know which types of accessible equipment, like adjustable tables and chair scales, were needed or how to use them. And some said they actively try to avoid treating patients with disabilities.
Don’t assume
The first step at correcting the problem, Dr. Bullock said, is to not assume that all IDD patients are incapable of communicating. By talking not to the patient but to their caregiver or spouse or child, as the clinician did with Ms. Chin years ago, “we are taking away their agency, their autonomy to speak for and about themselves.”
Change involves altering physicians’ attitudes and assumptions toward this population, through education. But how?
“The medical school curriculum is tight as it is,” Dr. Bullock acknowledged. “There’s a lot of things students have to learn. People wonder: where we will add this?”
Her suggestion: Incorporate IDD all along the way, through programs or experiences that will enable medical students to see such patients “not as something separate, but as people that have special needs just as other populations have.”
Case in point: Operation House Call, a program in Massachusetts designed to support young health care professionals, by building “confidence, interest, and sensitivity” toward individuals with IDD.
Eight medical and allied health schools, including those at Harvard Medical School and Yale School of Nursing, participate in the program, the centerpiece of which is time spent by teams of medical students in the homes of families with neurodiverse members. “It’s transformational,” said Susan Feeney, DNP, NP-C, director of adult gerontology and family nurse practitioner programs at the graduate school of nursing at the University of Massachusetts, Worcester. “They spend a few hours at the homes of these families, have this interaction with them, and journal about their experiences.”
Dr. Feeney described as “transformational” the experience of the students after getting to know these families. “They all come back profoundly changed,” she told this news organization. “As a medical or health care professional, you meet people in an artificial environment of the clinic and hospital. Here, they become human, like you. It takes the stigma away.”
One area of medicine in which this is an exception is pediatrics, where interaction with children with IDD and their families is common – and close. “They’re going to be much more attuned to this,” Dr. Feeney said. “The problem is primary care or internal medicine. Once these children get into their mid and later 20s, and they need a practitioner to talk to about adult concerns.”
And with adulthood come other medical needs, as the physical demands of age fall no less heavily on individuals with IDDs than those without. For example: “Neurodiverse people get pregnant,” Dr. Bullock said. They also can get heart disease as they age; or require the care of a rheumatologist, a neurologist, an orthopedic surgeon, or any other medical specialty.
Generation gap
Fortunately, the next generation of physicians may be more open to this more inclusionary approach toward a widely misunderstood population.
Like Ms. Chin, Sarah Bdeir had experience with this population prior to beginning her training in medicine. She had volunteered at a school for people with IDD.
“It was one of the best experiences I’ve ever had,” Ms. Bdeir, now 23 and a first-year medical student at Wayne State University, Detroit, said. She found that the neurodiverse individuals she worked with had as many abilities as disabilities. “They are capable of learning, but they do it differently,” she said. “You have to adjust to the way they learn. And you have to step out of your own box.”
Ms. Bdeir also heard about Dr. Bullock’s work and is assisting her in a research project on how to better improve nutritional education for people with IDDs. And although she said it may take time for curriculum boards at medical schools to integrate this kind of training into their programs, she believes they will, in part because the rising cohort of medical students today have an eagerness to engage with and learn more about IDD patients.
As does Ms. Chin.
“When I talk to my peers about this, they’re very receptive,” Ms. Chin said. “They want to learn how to better support the IDD population. And they will learn. I believe in my generation of future doctors.”
A version of this article first appeared on Medscape.com.
As an undergraduate student at Northeastern University in Boston, Meghan Chin spent her summers working for a day program in Rhode Island. Her charges were adults with various forms of intellectual and developmental disabilities (IDD).
“I was very much a caretaker,” Ms. Chin, now 29, said. “It was everything from helping them get dressed in the morning to getting them to medical appointments.”
During one such visit Ms. Chin got a lesson about how health care looks from the viewpoint of someone with an IDD.
The patient was a woman in her 60s and she was having gastrointestinal issues; symptoms she could have articulated, if asked. “She was perfectly capable of telling a clinician where it hurt, how long she had experienced the problem, and what she had done or not done to alleviate it,” Ms. Chin said.
And of comprehending a response. But she was not given the opportunity.
“She would explain what was going on to the clinician,” Ms. Chin recalled. “And the clinician would turn to me and answer. It was this weird three-way conversation – as if she wasn’t even there in the room with us.”
Ms. Chin was incensed at the rude and disrespectful way the patient had been treated. But her charge didn’t seem upset or surprised. Just resigned. “Sadly, she had become used to this,” Ms. Chin said.
For the young aide, however, the experience was searing. “It didn’t seem right to me,” Ms. Chin said. “That’s why, when I went to medical school, I knew I wanted to do better for this population.”
Serendipity led her to Georgetown University, Washington, where she met Kim Bullock, MD, one of the country’s leading advocates for improved health care delivery to those with IDDs.
Dr. Bullock, an associate professor of family medicine, seeks to create better training and educational opportunities for medical students who will likely encounter patients with these disabilities in their practices.
When Dr. Bullock heard Ms. Chin’s story about the patient being ignored, she was not surprised.
“This is not an unusual or unique situation,” said Dr. Bullock, who is also director of Georgetown’s community health division and a faculty member of the university’s Center for Excellence for Developmental Disabilities. “In fact, it’s quite common and is part of what spurred my own interest in educating pre-med and medical students about effective communication techniques, particularly when addressing neurodiverse patients.”
More than 13% of Americans, or roughly 44 million people, have some form of disability, according to the National Institute on Disability at the University of New Hampshire, a figure that does not include those who are institutionalized. The Centers for Disease Control and Prevention estimates that 17% of children aged 3-17 years have a developmental disability.
Even so, many physicians feel ill-prepared to care for disabled patients. A survey of physicians, published in the journal Health Affairs, found that some lacked the resources and training to properly care for patients with disabilities, or that they struggled to coordinate care for such individuals. Some said they did not know which types of accessible equipment, like adjustable tables and chair scales, were needed or how to use them. And some said they actively try to avoid treating patients with disabilities.
Don’t assume
The first step at correcting the problem, Dr. Bullock said, is to not assume that all IDD patients are incapable of communicating. By talking not to the patient but to their caregiver or spouse or child, as the clinician did with Ms. Chin years ago, “we are taking away their agency, their autonomy to speak for and about themselves.”
Change involves altering physicians’ attitudes and assumptions toward this population, through education. But how?
“The medical school curriculum is tight as it is,” Dr. Bullock acknowledged. “There’s a lot of things students have to learn. People wonder: where we will add this?”
Her suggestion: Incorporate IDD all along the way, through programs or experiences that will enable medical students to see such patients “not as something separate, but as people that have special needs just as other populations have.”
Case in point: Operation House Call, a program in Massachusetts designed to support young health care professionals, by building “confidence, interest, and sensitivity” toward individuals with IDD.
Eight medical and allied health schools, including those at Harvard Medical School and Yale School of Nursing, participate in the program, the centerpiece of which is time spent by teams of medical students in the homes of families with neurodiverse members. “It’s transformational,” said Susan Feeney, DNP, NP-C, director of adult gerontology and family nurse practitioner programs at the graduate school of nursing at the University of Massachusetts, Worcester. “They spend a few hours at the homes of these families, have this interaction with them, and journal about their experiences.”
Dr. Feeney described as “transformational” the experience of the students after getting to know these families. “They all come back profoundly changed,” she told this news organization. “As a medical or health care professional, you meet people in an artificial environment of the clinic and hospital. Here, they become human, like you. It takes the stigma away.”
One area of medicine in which this is an exception is pediatrics, where interaction with children with IDD and their families is common – and close. “They’re going to be much more attuned to this,” Dr. Feeney said. “The problem is primary care or internal medicine. Once these children get into their mid and later 20s, and they need a practitioner to talk to about adult concerns.”
And with adulthood come other medical needs, as the physical demands of age fall no less heavily on individuals with IDDs than those without. For example: “Neurodiverse people get pregnant,” Dr. Bullock said. They also can get heart disease as they age; or require the care of a rheumatologist, a neurologist, an orthopedic surgeon, or any other medical specialty.
Generation gap
Fortunately, the next generation of physicians may be more open to this more inclusionary approach toward a widely misunderstood population.
Like Ms. Chin, Sarah Bdeir had experience with this population prior to beginning her training in medicine. She had volunteered at a school for people with IDD.
“It was one of the best experiences I’ve ever had,” Ms. Bdeir, now 23 and a first-year medical student at Wayne State University, Detroit, said. She found that the neurodiverse individuals she worked with had as many abilities as disabilities. “They are capable of learning, but they do it differently,” she said. “You have to adjust to the way they learn. And you have to step out of your own box.”
Ms. Bdeir also heard about Dr. Bullock’s work and is assisting her in a research project on how to better improve nutritional education for people with IDDs. And although she said it may take time for curriculum boards at medical schools to integrate this kind of training into their programs, she believes they will, in part because the rising cohort of medical students today have an eagerness to engage with and learn more about IDD patients.
As does Ms. Chin.
“When I talk to my peers about this, they’re very receptive,” Ms. Chin said. “They want to learn how to better support the IDD population. And they will learn. I believe in my generation of future doctors.”
A version of this article first appeared on Medscape.com.
As an undergraduate student at Northeastern University in Boston, Meghan Chin spent her summers working for a day program in Rhode Island. Her charges were adults with various forms of intellectual and developmental disabilities (IDD).
“I was very much a caretaker,” Ms. Chin, now 29, said. “It was everything from helping them get dressed in the morning to getting them to medical appointments.”
During one such visit Ms. Chin got a lesson about how health care looks from the viewpoint of someone with an IDD.
The patient was a woman in her 60s and she was having gastrointestinal issues; symptoms she could have articulated, if asked. “She was perfectly capable of telling a clinician where it hurt, how long she had experienced the problem, and what she had done or not done to alleviate it,” Ms. Chin said.
And of comprehending a response. But she was not given the opportunity.
“She would explain what was going on to the clinician,” Ms. Chin recalled. “And the clinician would turn to me and answer. It was this weird three-way conversation – as if she wasn’t even there in the room with us.”
Ms. Chin was incensed at the rude and disrespectful way the patient had been treated. But her charge didn’t seem upset or surprised. Just resigned. “Sadly, she had become used to this,” Ms. Chin said.
For the young aide, however, the experience was searing. “It didn’t seem right to me,” Ms. Chin said. “That’s why, when I went to medical school, I knew I wanted to do better for this population.”
Serendipity led her to Georgetown University, Washington, where she met Kim Bullock, MD, one of the country’s leading advocates for improved health care delivery to those with IDDs.
Dr. Bullock, an associate professor of family medicine, seeks to create better training and educational opportunities for medical students who will likely encounter patients with these disabilities in their practices.
When Dr. Bullock heard Ms. Chin’s story about the patient being ignored, she was not surprised.
“This is not an unusual or unique situation,” said Dr. Bullock, who is also director of Georgetown’s community health division and a faculty member of the university’s Center for Excellence for Developmental Disabilities. “In fact, it’s quite common and is part of what spurred my own interest in educating pre-med and medical students about effective communication techniques, particularly when addressing neurodiverse patients.”
More than 13% of Americans, or roughly 44 million people, have some form of disability, according to the National Institute on Disability at the University of New Hampshire, a figure that does not include those who are institutionalized. The Centers for Disease Control and Prevention estimates that 17% of children aged 3-17 years have a developmental disability.
Even so, many physicians feel ill-prepared to care for disabled patients. A survey of physicians, published in the journal Health Affairs, found that some lacked the resources and training to properly care for patients with disabilities, or that they struggled to coordinate care for such individuals. Some said they did not know which types of accessible equipment, like adjustable tables and chair scales, were needed or how to use them. And some said they actively try to avoid treating patients with disabilities.
Don’t assume
The first step at correcting the problem, Dr. Bullock said, is to not assume that all IDD patients are incapable of communicating. By talking not to the patient but to their caregiver or spouse or child, as the clinician did with Ms. Chin years ago, “we are taking away their agency, their autonomy to speak for and about themselves.”
Change involves altering physicians’ attitudes and assumptions toward this population, through education. But how?
“The medical school curriculum is tight as it is,” Dr. Bullock acknowledged. “There’s a lot of things students have to learn. People wonder: where we will add this?”
Her suggestion: Incorporate IDD all along the way, through programs or experiences that will enable medical students to see such patients “not as something separate, but as people that have special needs just as other populations have.”
Case in point: Operation House Call, a program in Massachusetts designed to support young health care professionals, by building “confidence, interest, and sensitivity” toward individuals with IDD.
Eight medical and allied health schools, including those at Harvard Medical School and Yale School of Nursing, participate in the program, the centerpiece of which is time spent by teams of medical students in the homes of families with neurodiverse members. “It’s transformational,” said Susan Feeney, DNP, NP-C, director of adult gerontology and family nurse practitioner programs at the graduate school of nursing at the University of Massachusetts, Worcester. “They spend a few hours at the homes of these families, have this interaction with them, and journal about their experiences.”
Dr. Feeney described as “transformational” the experience of the students after getting to know these families. “They all come back profoundly changed,” she told this news organization. “As a medical or health care professional, you meet people in an artificial environment of the clinic and hospital. Here, they become human, like you. It takes the stigma away.”
One area of medicine in which this is an exception is pediatrics, where interaction with children with IDD and their families is common – and close. “They’re going to be much more attuned to this,” Dr. Feeney said. “The problem is primary care or internal medicine. Once these children get into their mid and later 20s, and they need a practitioner to talk to about adult concerns.”
And with adulthood come other medical needs, as the physical demands of age fall no less heavily on individuals with IDDs than those without. For example: “Neurodiverse people get pregnant,” Dr. Bullock said. They also can get heart disease as they age; or require the care of a rheumatologist, a neurologist, an orthopedic surgeon, or any other medical specialty.
Generation gap
Fortunately, the next generation of physicians may be more open to this more inclusionary approach toward a widely misunderstood population.
Like Ms. Chin, Sarah Bdeir had experience with this population prior to beginning her training in medicine. She had volunteered at a school for people with IDD.
“It was one of the best experiences I’ve ever had,” Ms. Bdeir, now 23 and a first-year medical student at Wayne State University, Detroit, said. She found that the neurodiverse individuals she worked with had as many abilities as disabilities. “They are capable of learning, but they do it differently,” she said. “You have to adjust to the way they learn. And you have to step out of your own box.”
Ms. Bdeir also heard about Dr. Bullock’s work and is assisting her in a research project on how to better improve nutritional education for people with IDDs. And although she said it may take time for curriculum boards at medical schools to integrate this kind of training into their programs, she believes they will, in part because the rising cohort of medical students today have an eagerness to engage with and learn more about IDD patients.
As does Ms. Chin.
“When I talk to my peers about this, they’re very receptive,” Ms. Chin said. “They want to learn how to better support the IDD population. And they will learn. I believe in my generation of future doctors.”
A version of this article first appeared on Medscape.com.
Viagra, Cialis, and Alzheimer’s risk: New data
The findings contradict results from a previous study that suggested that individuals who take sildenafil (Viagra) were significantly less likely to develop Alzheimer’s.
The new research, part of a larger effort to identify existing medications that could be repurposed to treat ADRD, employed a study design that reduced the risk for potential bias that may have influenced the earlier findings, the investigators note.
“That study came out last fall and was widely covered in the media, and we thought there were some methodological shortcomings that might have explained the results,” lead investigator Rishi Desai, PhD, assistant professor of medicine at Harvard Medical School and an associate epidemiologist at Brigham and Women’s Hospital, both in Boston, said in an interview.
The new study was published online in Brain Communications.
Not the final word?
Animal studies suggest that phosphodiesterase-5 (PDE5) inhibitors, a drug class that includes the ED drugs sildenafil and tadalafil (Cialis), improve memory and cognitive function and reduce amyloid burden. But studies in humans have yielded conflicting results.*
Although the new research and the work published last year both drew on Medicare data, they examined different patient populations.
The first study compared those who took sildenafil for any reason to those who did not take it. That design likely resulted in an analysis of a comparison of individuals with ED – the most common indication for sildenafil – to generally older individuals with diabetes or hypertension, Dr. Desai said.
In contrast, the current study included only those with pulmonary arterial hypertension (PAH), which is also an indication for PDE5 inhibitors. The researchers compared ADRD incidence in those who took PDE5 inhibitors with the incidence among those who took a different medication to treat their PAH. They used propensity matching to create two groups with similar characteristics and examined the data using four analytic strategies.
The investigators found no significant difference between groups in the incidence of ADRD, regardless of the strategy they used. Cell culture studies also revealed no protective effect from PDE5 inhibitors.
“No study of this kind should claim the final word,” Dr. Desai said. “It is extremely difficult to nail down causality from these types of data sources.”
Impressive study design
Commenting on the findings, David Knopman, MD, professor of neurology at Mayo Clinic, Rochester, Minn., described the study design as “impressive” for its efforts to minimize bias, a key limitation in the previous study.
“It was always the case that the claims about sildenafil needed further developmental work prior to testing the drug in randomized controlled trials,” Dr. Knopman said. “The evidence for the use of the drug was never sufficient for clinicians to use it in their patients.”
The study was funded by National Institute on Aging. Dr. Desai is an investigator who receives research grants from Bayer, Vertex, and Novartis that were given to the Brigham and Women’s Hospital for unrelated projects. Dr. Knopman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 11/3/22: An earlier version of this article misstated the abbreviation for phosphodiesterase-5. It is PDE-5.
The findings contradict results from a previous study that suggested that individuals who take sildenafil (Viagra) were significantly less likely to develop Alzheimer’s.
The new research, part of a larger effort to identify existing medications that could be repurposed to treat ADRD, employed a study design that reduced the risk for potential bias that may have influenced the earlier findings, the investigators note.
“That study came out last fall and was widely covered in the media, and we thought there were some methodological shortcomings that might have explained the results,” lead investigator Rishi Desai, PhD, assistant professor of medicine at Harvard Medical School and an associate epidemiologist at Brigham and Women’s Hospital, both in Boston, said in an interview.
The new study was published online in Brain Communications.
Not the final word?
Animal studies suggest that phosphodiesterase-5 (PDE5) inhibitors, a drug class that includes the ED drugs sildenafil and tadalafil (Cialis), improve memory and cognitive function and reduce amyloid burden. But studies in humans have yielded conflicting results.*
Although the new research and the work published last year both drew on Medicare data, they examined different patient populations.
The first study compared those who took sildenafil for any reason to those who did not take it. That design likely resulted in an analysis of a comparison of individuals with ED – the most common indication for sildenafil – to generally older individuals with diabetes or hypertension, Dr. Desai said.
In contrast, the current study included only those with pulmonary arterial hypertension (PAH), which is also an indication for PDE5 inhibitors. The researchers compared ADRD incidence in those who took PDE5 inhibitors with the incidence among those who took a different medication to treat their PAH. They used propensity matching to create two groups with similar characteristics and examined the data using four analytic strategies.
The investigators found no significant difference between groups in the incidence of ADRD, regardless of the strategy they used. Cell culture studies also revealed no protective effect from PDE5 inhibitors.
“No study of this kind should claim the final word,” Dr. Desai said. “It is extremely difficult to nail down causality from these types of data sources.”
Impressive study design
Commenting on the findings, David Knopman, MD, professor of neurology at Mayo Clinic, Rochester, Minn., described the study design as “impressive” for its efforts to minimize bias, a key limitation in the previous study.
“It was always the case that the claims about sildenafil needed further developmental work prior to testing the drug in randomized controlled trials,” Dr. Knopman said. “The evidence for the use of the drug was never sufficient for clinicians to use it in their patients.”
The study was funded by National Institute on Aging. Dr. Desai is an investigator who receives research grants from Bayer, Vertex, and Novartis that were given to the Brigham and Women’s Hospital for unrelated projects. Dr. Knopman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 11/3/22: An earlier version of this article misstated the abbreviation for phosphodiesterase-5. It is PDE-5.
The findings contradict results from a previous study that suggested that individuals who take sildenafil (Viagra) were significantly less likely to develop Alzheimer’s.
The new research, part of a larger effort to identify existing medications that could be repurposed to treat ADRD, employed a study design that reduced the risk for potential bias that may have influenced the earlier findings, the investigators note.
“That study came out last fall and was widely covered in the media, and we thought there were some methodological shortcomings that might have explained the results,” lead investigator Rishi Desai, PhD, assistant professor of medicine at Harvard Medical School and an associate epidemiologist at Brigham and Women’s Hospital, both in Boston, said in an interview.
The new study was published online in Brain Communications.
Not the final word?
Animal studies suggest that phosphodiesterase-5 (PDE5) inhibitors, a drug class that includes the ED drugs sildenafil and tadalafil (Cialis), improve memory and cognitive function and reduce amyloid burden. But studies in humans have yielded conflicting results.*
Although the new research and the work published last year both drew on Medicare data, they examined different patient populations.
The first study compared those who took sildenafil for any reason to those who did not take it. That design likely resulted in an analysis of a comparison of individuals with ED – the most common indication for sildenafil – to generally older individuals with diabetes or hypertension, Dr. Desai said.
In contrast, the current study included only those with pulmonary arterial hypertension (PAH), which is also an indication for PDE5 inhibitors. The researchers compared ADRD incidence in those who took PDE5 inhibitors with the incidence among those who took a different medication to treat their PAH. They used propensity matching to create two groups with similar characteristics and examined the data using four analytic strategies.
The investigators found no significant difference between groups in the incidence of ADRD, regardless of the strategy they used. Cell culture studies also revealed no protective effect from PDE5 inhibitors.
“No study of this kind should claim the final word,” Dr. Desai said. “It is extremely difficult to nail down causality from these types of data sources.”
Impressive study design
Commenting on the findings, David Knopman, MD, professor of neurology at Mayo Clinic, Rochester, Minn., described the study design as “impressive” for its efforts to minimize bias, a key limitation in the previous study.
“It was always the case that the claims about sildenafil needed further developmental work prior to testing the drug in randomized controlled trials,” Dr. Knopman said. “The evidence for the use of the drug was never sufficient for clinicians to use it in their patients.”
The study was funded by National Institute on Aging. Dr. Desai is an investigator who receives research grants from Bayer, Vertex, and Novartis that were given to the Brigham and Women’s Hospital for unrelated projects. Dr. Knopman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 11/3/22: An earlier version of this article misstated the abbreviation for phosphodiesterase-5. It is PDE-5.
FROM BRAIN COMMUNICATIONS
Listen up: Birdsong may calm anxiety, paranoia
Investigators found that people who listened to recordings of birds singing experienced a significant reduction in anxiety and paranoia. In contrast, the researchers also found that recordings of traffic noises, including car engines, sirens, and construction, increased depressive states.
“The results suggest that it may be worthwhile to investigate the targeted use of natural sounds such as birdsong in a clinical setting – for example, in hospital waiting rooms or in psychiatric settings,” study investigator Emil Stobbe, MSc, a predoctoral fellow at the Max Planck Institute for Human Development, Berlin, said in an interview.
“If someone is seeking an easily accessible intervention to lower distress, listening to an audio clip of birds singing might be a great option,” he added.
The study was published online in Scientific Reports.
Nature’s calming effect
The aim of the research was “to investigate how the physical environment impact brain and mental health,” Mr. Stobbe said.
Mr. Stobbe said that there is significantly more research examining visual properties of the physical environment but that the auditory domain is not as well researched, although, he added, that the beneficial effects of interactions with nature are “well studied.”
He noted that anxiety and paranoia can be experienced by many individuals even though they may be unaware that they are experiencing these states.
“We wanted to investigate if the beneficial effects of nature can also exert their impact on these states. In theory, birds can be representational for natural and vital environment, which, in turn, transfer the positive effects of nature on birdsong listeners,” he said.
A previous study compared nature versus city soundscape conditions and showed that the nature soundscape improved participants’ cognitive performance but did not improve mood. The present study added diversity to the soundscapes and focused not only on cognition and general mood but also on state paranoia, “which can be measured in a change-sensitive manner” and “has been shown to increase in response to traffic noise.”
The researchers hypothesized that birdsong would have a greater beneficial effect on mood and paranoia and on cognitive performance compared with traffic noise. They also investigated whether greater versus lower diversity of bird species or noise sources within the soundscapes “would be a relevant factor modulating the effects.”
The researchers recruited participants (n = 295) from a crowdsourcing platform. Participants’ mean age was late 20s (standard deviations ranged from 6.30 to 7.72), with a greater proportion of male versus female participants.
To be included, participants were required to have no history of mental illness, hearing difficulties, substance/drug intake, or suicidal thoughts/tendencies.
The outcomes of interest (mood, paranoia, cognitive performance) were measured before and after soundscape exposure and each soundscape had a low- versus high-diversity version. This resulted in several analyses that compared two types of sounds (birdsongs vs. traffic noise) x two levels of diversity (low vs. high diversity) and two time points (pre- vs. post exposure).
The exposure to sounds lasted for 6 minutes, after which they were asked to report (on a 0-100 visual scale) how diverse/monotone, beautiful, and pleasant they perceived the soundscape to be.
Reduction in depressive symptoms
Participants were divided into four groups: low-diversity traffic noise soundscape (n = 83), high-diversity traffic noise soundscape (n = 60), low-diversity birdsong soundscape (n = 63), and high-diversity birdsong soundscape (n = 80)
In addition to listening to the sounds, participants completed questionnaires measuring mood (depression and anxiety) and paranoia as well as a test of digit span cognitive performance (both the forward and the backward versions).
The type, diversity, and type x diversity all revealed significant effect sizes (F[3, 276] = 78.6; P < .001; eta-squared = 0.461; F[3, 276] = 3.16; P = .025; eta-squared = 0.033; and F[3, 276] = 2.66; P = .028, respectively), “suggesting that all of these factors, as well as their interaction, had a significant impact on the perception of soundscapes (that is, ratings on monotony/diversity, beauty, and pleasantness).”
A post hoc examination showed that depressive symptoms significantly increased within the low- and high-diversity urban soundscapes but decreased significantly in the high-diversity birdsong soundscapes (T[1, 60] = –2.57; P = .012; d = –0.29).
For anxiety, the post hoc within-group analyses found no effects within low- and high-diversity traffic noise conditions (T[1, 82] = –1.37; P = .174; d = –0.15 and T[1, 68] = 0.49; P = .629; d = 0.06, respectively). By contrast, there were significant declines in both birdsong conditions (low diversity: T[1, 62] = –6.13; P < .001; d = –0.77; high diversity: T[1, 60] = –6.32; P < .001; d = –0.70).
Similarly, there were no changes in participants with paranoia when they listened to either low- or high-diversity traffic noises (T[1, 82] = –0.55; P = .583; d = –0.06 and T[1, 68] = 0.67; P = .507; d = 0.08, respectively). On the other hand, both birdsong conditions yielded reductions in paranoia (low diversity: T[1, 62] = –5.90; P < .001; d = –0.74; high diversity: T[1, 60] = –4.11; P < .001; d = –0.46).
None of the soundscapes had any effect on cognition.
“In theory, birds can be representational for natural and vital environments which, in turn, transfer the positive effects of nature on birdsong listeners,” said Mr. Stobbe.
“Taken together, the findings of the current study provide another facet of why interactions with nature can be beneficial for our mental health, and it is highly important to preserve nature,” he added.
Mr. Stobbe said that future research should focus on investigating mixed soundscapes including examining whether the presence of natural sounds in urban settings lower stressors such as traffic noise.
An understudied area
Commenting for this article, Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness called the study “interesting but limited.”
Dr. Duckworth, who was not involved in the research said that the “benefits of nature are understudied” and agreed with the investigators that it is potentially important to study the use of birdsongs in psychiatric facilities. “Future studies could also correlate the role of birdsong with the mental health benefits/aspects of ‘being in nature,’ which has been found to have some effect.”
Open Access funding was enabled and organized by Projekt DEAL. The authors and Dr. Duckworth declared no competing interests.
A version of this article first appeared on Medscape.com.
Investigators found that people who listened to recordings of birds singing experienced a significant reduction in anxiety and paranoia. In contrast, the researchers also found that recordings of traffic noises, including car engines, sirens, and construction, increased depressive states.
“The results suggest that it may be worthwhile to investigate the targeted use of natural sounds such as birdsong in a clinical setting – for example, in hospital waiting rooms or in psychiatric settings,” study investigator Emil Stobbe, MSc, a predoctoral fellow at the Max Planck Institute for Human Development, Berlin, said in an interview.
“If someone is seeking an easily accessible intervention to lower distress, listening to an audio clip of birds singing might be a great option,” he added.
The study was published online in Scientific Reports.
Nature’s calming effect
The aim of the research was “to investigate how the physical environment impact brain and mental health,” Mr. Stobbe said.
Mr. Stobbe said that there is significantly more research examining visual properties of the physical environment but that the auditory domain is not as well researched, although, he added, that the beneficial effects of interactions with nature are “well studied.”
He noted that anxiety and paranoia can be experienced by many individuals even though they may be unaware that they are experiencing these states.
“We wanted to investigate if the beneficial effects of nature can also exert their impact on these states. In theory, birds can be representational for natural and vital environment, which, in turn, transfer the positive effects of nature on birdsong listeners,” he said.
A previous study compared nature versus city soundscape conditions and showed that the nature soundscape improved participants’ cognitive performance but did not improve mood. The present study added diversity to the soundscapes and focused not only on cognition and general mood but also on state paranoia, “which can be measured in a change-sensitive manner” and “has been shown to increase in response to traffic noise.”
The researchers hypothesized that birdsong would have a greater beneficial effect on mood and paranoia and on cognitive performance compared with traffic noise. They also investigated whether greater versus lower diversity of bird species or noise sources within the soundscapes “would be a relevant factor modulating the effects.”
The researchers recruited participants (n = 295) from a crowdsourcing platform. Participants’ mean age was late 20s (standard deviations ranged from 6.30 to 7.72), with a greater proportion of male versus female participants.
To be included, participants were required to have no history of mental illness, hearing difficulties, substance/drug intake, or suicidal thoughts/tendencies.
The outcomes of interest (mood, paranoia, cognitive performance) were measured before and after soundscape exposure and each soundscape had a low- versus high-diversity version. This resulted in several analyses that compared two types of sounds (birdsongs vs. traffic noise) x two levels of diversity (low vs. high diversity) and two time points (pre- vs. post exposure).
The exposure to sounds lasted for 6 minutes, after which they were asked to report (on a 0-100 visual scale) how diverse/monotone, beautiful, and pleasant they perceived the soundscape to be.
Reduction in depressive symptoms
Participants were divided into four groups: low-diversity traffic noise soundscape (n = 83), high-diversity traffic noise soundscape (n = 60), low-diversity birdsong soundscape (n = 63), and high-diversity birdsong soundscape (n = 80)
In addition to listening to the sounds, participants completed questionnaires measuring mood (depression and anxiety) and paranoia as well as a test of digit span cognitive performance (both the forward and the backward versions).
The type, diversity, and type x diversity all revealed significant effect sizes (F[3, 276] = 78.6; P < .001; eta-squared = 0.461; F[3, 276] = 3.16; P = .025; eta-squared = 0.033; and F[3, 276] = 2.66; P = .028, respectively), “suggesting that all of these factors, as well as their interaction, had a significant impact on the perception of soundscapes (that is, ratings on monotony/diversity, beauty, and pleasantness).”
A post hoc examination showed that depressive symptoms significantly increased within the low- and high-diversity urban soundscapes but decreased significantly in the high-diversity birdsong soundscapes (T[1, 60] = –2.57; P = .012; d = –0.29).
For anxiety, the post hoc within-group analyses found no effects within low- and high-diversity traffic noise conditions (T[1, 82] = –1.37; P = .174; d = –0.15 and T[1, 68] = 0.49; P = .629; d = 0.06, respectively). By contrast, there were significant declines in both birdsong conditions (low diversity: T[1, 62] = –6.13; P < .001; d = –0.77; high diversity: T[1, 60] = –6.32; P < .001; d = –0.70).
Similarly, there were no changes in participants with paranoia when they listened to either low- or high-diversity traffic noises (T[1, 82] = –0.55; P = .583; d = –0.06 and T[1, 68] = 0.67; P = .507; d = 0.08, respectively). On the other hand, both birdsong conditions yielded reductions in paranoia (low diversity: T[1, 62] = –5.90; P < .001; d = –0.74; high diversity: T[1, 60] = –4.11; P < .001; d = –0.46).
None of the soundscapes had any effect on cognition.
“In theory, birds can be representational for natural and vital environments which, in turn, transfer the positive effects of nature on birdsong listeners,” said Mr. Stobbe.
“Taken together, the findings of the current study provide another facet of why interactions with nature can be beneficial for our mental health, and it is highly important to preserve nature,” he added.
Mr. Stobbe said that future research should focus on investigating mixed soundscapes including examining whether the presence of natural sounds in urban settings lower stressors such as traffic noise.
An understudied area
Commenting for this article, Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness called the study “interesting but limited.”
Dr. Duckworth, who was not involved in the research said that the “benefits of nature are understudied” and agreed with the investigators that it is potentially important to study the use of birdsongs in psychiatric facilities. “Future studies could also correlate the role of birdsong with the mental health benefits/aspects of ‘being in nature,’ which has been found to have some effect.”
Open Access funding was enabled and organized by Projekt DEAL. The authors and Dr. Duckworth declared no competing interests.
A version of this article first appeared on Medscape.com.
Investigators found that people who listened to recordings of birds singing experienced a significant reduction in anxiety and paranoia. In contrast, the researchers also found that recordings of traffic noises, including car engines, sirens, and construction, increased depressive states.
“The results suggest that it may be worthwhile to investigate the targeted use of natural sounds such as birdsong in a clinical setting – for example, in hospital waiting rooms or in psychiatric settings,” study investigator Emil Stobbe, MSc, a predoctoral fellow at the Max Planck Institute for Human Development, Berlin, said in an interview.
“If someone is seeking an easily accessible intervention to lower distress, listening to an audio clip of birds singing might be a great option,” he added.
The study was published online in Scientific Reports.
Nature’s calming effect
The aim of the research was “to investigate how the physical environment impact brain and mental health,” Mr. Stobbe said.
Mr. Stobbe said that there is significantly more research examining visual properties of the physical environment but that the auditory domain is not as well researched, although, he added, that the beneficial effects of interactions with nature are “well studied.”
He noted that anxiety and paranoia can be experienced by many individuals even though they may be unaware that they are experiencing these states.
“We wanted to investigate if the beneficial effects of nature can also exert their impact on these states. In theory, birds can be representational for natural and vital environment, which, in turn, transfer the positive effects of nature on birdsong listeners,” he said.
A previous study compared nature versus city soundscape conditions and showed that the nature soundscape improved participants’ cognitive performance but did not improve mood. The present study added diversity to the soundscapes and focused not only on cognition and general mood but also on state paranoia, “which can be measured in a change-sensitive manner” and “has been shown to increase in response to traffic noise.”
The researchers hypothesized that birdsong would have a greater beneficial effect on mood and paranoia and on cognitive performance compared with traffic noise. They also investigated whether greater versus lower diversity of bird species or noise sources within the soundscapes “would be a relevant factor modulating the effects.”
The researchers recruited participants (n = 295) from a crowdsourcing platform. Participants’ mean age was late 20s (standard deviations ranged from 6.30 to 7.72), with a greater proportion of male versus female participants.
To be included, participants were required to have no history of mental illness, hearing difficulties, substance/drug intake, or suicidal thoughts/tendencies.
The outcomes of interest (mood, paranoia, cognitive performance) were measured before and after soundscape exposure and each soundscape had a low- versus high-diversity version. This resulted in several analyses that compared two types of sounds (birdsongs vs. traffic noise) x two levels of diversity (low vs. high diversity) and two time points (pre- vs. post exposure).
The exposure to sounds lasted for 6 minutes, after which they were asked to report (on a 0-100 visual scale) how diverse/monotone, beautiful, and pleasant they perceived the soundscape to be.
Reduction in depressive symptoms
Participants were divided into four groups: low-diversity traffic noise soundscape (n = 83), high-diversity traffic noise soundscape (n = 60), low-diversity birdsong soundscape (n = 63), and high-diversity birdsong soundscape (n = 80)
In addition to listening to the sounds, participants completed questionnaires measuring mood (depression and anxiety) and paranoia as well as a test of digit span cognitive performance (both the forward and the backward versions).
The type, diversity, and type x diversity all revealed significant effect sizes (F[3, 276] = 78.6; P < .001; eta-squared = 0.461; F[3, 276] = 3.16; P = .025; eta-squared = 0.033; and F[3, 276] = 2.66; P = .028, respectively), “suggesting that all of these factors, as well as their interaction, had a significant impact on the perception of soundscapes (that is, ratings on monotony/diversity, beauty, and pleasantness).”
A post hoc examination showed that depressive symptoms significantly increased within the low- and high-diversity urban soundscapes but decreased significantly in the high-diversity birdsong soundscapes (T[1, 60] = –2.57; P = .012; d = –0.29).
For anxiety, the post hoc within-group analyses found no effects within low- and high-diversity traffic noise conditions (T[1, 82] = –1.37; P = .174; d = –0.15 and T[1, 68] = 0.49; P = .629; d = 0.06, respectively). By contrast, there were significant declines in both birdsong conditions (low diversity: T[1, 62] = –6.13; P < .001; d = –0.77; high diversity: T[1, 60] = –6.32; P < .001; d = –0.70).
Similarly, there were no changes in participants with paranoia when they listened to either low- or high-diversity traffic noises (T[1, 82] = –0.55; P = .583; d = –0.06 and T[1, 68] = 0.67; P = .507; d = 0.08, respectively). On the other hand, both birdsong conditions yielded reductions in paranoia (low diversity: T[1, 62] = –5.90; P < .001; d = –0.74; high diversity: T[1, 60] = –4.11; P < .001; d = –0.46).
None of the soundscapes had any effect on cognition.
“In theory, birds can be representational for natural and vital environments which, in turn, transfer the positive effects of nature on birdsong listeners,” said Mr. Stobbe.
“Taken together, the findings of the current study provide another facet of why interactions with nature can be beneficial for our mental health, and it is highly important to preserve nature,” he added.
Mr. Stobbe said that future research should focus on investigating mixed soundscapes including examining whether the presence of natural sounds in urban settings lower stressors such as traffic noise.
An understudied area
Commenting for this article, Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness called the study “interesting but limited.”
Dr. Duckworth, who was not involved in the research said that the “benefits of nature are understudied” and agreed with the investigators that it is potentially important to study the use of birdsongs in psychiatric facilities. “Future studies could also correlate the role of birdsong with the mental health benefits/aspects of ‘being in nature,’ which has been found to have some effect.”
Open Access funding was enabled and organized by Projekt DEAL. The authors and Dr. Duckworth declared no competing interests.
A version of this article first appeared on Medscape.com.
FROM SCIENTIFIC REPORTS
I’m a physician battling long COVID. I can assure you it’s real
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.