User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
‘Baby TAM’ effective, tolerable for breast cancer prevention
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
FROM SABCS 2023
2023 Update on minimally invasive gynecologic surgery
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15
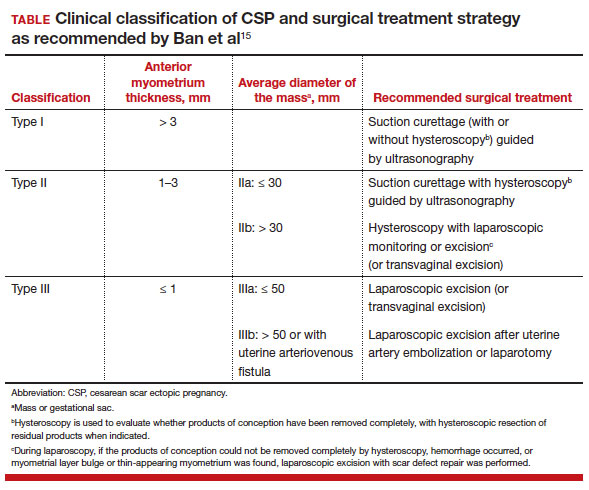
Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
It has been an incredible year for complex gynecology and minimally invasive gynecologic surgery (MIGS), with several outstanding new findings and reviews in 2023. The surgical community continues to push the envelope and emphasize the value of this specialty for women’s health.
Endometriosis and adenomyosis were at the center of several large cohort studies and systematic reviews that reassessed what we know about how to evaluate and treat these challenging diseases, including both surgical and nonsurgical approaches, with an emphasis on fertility-sparing modalities.1-8 In addition, a focus on quality of life, patient-centered care, and racial biases allowed us to reflect on our own practice patterns and keep the patient at the center of care models.9-13 Finally, there was a clear expansion in the use of technologies such as artificial intelligence (AI) and machine learning for care and novel minimally invasive tools.14
In this Update, we highlight and expand on how several particularly important developments are likely to make a difference in our clinical management.
New classification system for cesarean scar ectopic pregnancy with defined surgical guidance has 97% treatment success rate
Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097/AOG.0000000000005113
A large multiarmed study by Ban and colleagues used multivariable modeling to formulate and test a classification system and recommended surgical treatment strategies for patients with a cesarean scar ectopic pregnancy (CSP).15 In the study, 273 patients were included in the predictive modeling group, 118 in the internal validation group, and 564 within the model testing cohort. Classifications were based on 2 independent risk factors for intraoperative hemorrhage: anterior myometrial thickness and mean diameter of gestational sac (MSD).
Classification types
The 3 main CSP types were defined based on the anterior myometrial thickness at the cesarean section scar (type I, > 3 mm; type II, 1–3 mm; type III, ≤ 1 mm) and subtyped based on the MSD (type IIa, MSD ≤ 30 mm; type IIb, MSD > 30 mm; type IIIa, MSD ≤ 50 mm; type IIIb, MSD > 50 mm).
The subgroups were matched with recommended surgical strategy using expert opinion: Type I CSP was treated with suction dilation and aspiration (D&A) under ultrasound guidance, with or without hysteroscopy. Type IIa CSP was treated with suction D&A with hysteroscopy under ultrasound guidance. Type IIb CSP was treated with hysteroscopy with laparoscopic monitoring or excision, or transvaginal excision. Type IIIa CSP was treated with laparoscopic excision or transvaginal excision. Type IIIb CSP was treated with laparoscopic excision after uterine artery embolization or laparotomy (TABLE).15

Treatment outcomes
These guidelines were tested on a cohort of 564 patients between 2014 and 2022. Using these treatment guidelines, the overall treatment success rate was 97.5%; 85% of patients had a negative serum ß-human chorionic gonadotropin (ß-hCG) level within 3 weeks, and 95.2% of patients resumed menstrual cycles within 8 weeks. Successful treatment was defined as:
- complete resection of the products of conception
- no need to shift to a second-line surgical strategy
- no major complications
- no readmission for additional treatment
- serum ß-hCG levels that returned to normal within 4 weeks.
Although the incidence of CSP is reported to be around 1:2,000 pregnancies, these rare findings frequently cause a clinical conundrum.16 This thoughtful study by Ban and colleagues provides guidance with the creation of a classification system aimed at decreasing the severe morbidity that can come from mismanagement of these problematic pregnancies using predictive quantitative measures. In our own practice, we have used classification (type 1 endogenic or type 2 exogenic), mean gestational sac diameter, and overlying myometrial thickness when weighing options for treatment. However, decisions have been made on a case-by-case basis and expert opinion without specific cutoffs. Having defined parameters to more accurately classify the type of ectopic pregnancy is essential for communicating risk factors with all team members and for research purposes. The treatment algorithm proposed and tested in this study is logical with good outcomes in the test group. We applaud the authors of this study on a rare but potentially morbid pregnancy outcome. Of note, this study does not discuss nonsurgical alternatives for treatment, such as intra-sac methotrexate injection, which is another option used in select patients at our institution.
Continue to: Pre-op hormonal treatment of endometriosis found to be protective against post-op complications...
Pre-op hormonal treatment of endometriosis found to be protective against post-op complications
Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
In a large European multicenter retrospective cohort study, Casarin and colleagues evaluated perioperative complications during laparoscopic hysterectomy for endometriosis or adenomyosis in 995 patients treated from 2010 to 2020.2
Reported intraoperative data included the frequency of ureterolysis (26.8%), deep nodule resection (30%) and posterior adhesiolysis (38.9%), unilateral salpingo-oophorectomy (15.1%), bilateral salpingo-oophorectomy (26.8%), estimated blood loss (mean, 100 mL), and adverse events. Intraoperative complications occurred in 3% of cases (including bladder/bowel injury or need for transfusion).
Postoperative complications occurred in 13.8% of cases, and 9.3% had a major event, including vaginal cuff dehiscence, fever, abscess, and fistula.
Factors associated with postoperative complications
In a multivariate analysis, the authors found that increased operative time, younger age at surgery, previous surgery for endometriosis, and occurrence of intraoperative complications were associated with Clavien-Dindo score grade 2 or greater postoperative complications.
Medical treatment for endometriosis with estro-progestin or progestin medications, however, was found to be protective, with an odds ratio of 0.50 (95% confidence interval, 0.31–0.81).
It is well known that endometriosis is a risk factor for surgical complications. The reported complication rates in this cohort were relatively high, with nearly 10% of patients sustaining a major event postoperatively. While surgical risk is multifactorial and includes factors that are difficult to capture, including surgeon experience and patient population baseline risk, the relatively high incidence reported should be cause for pause and be incorporated in patient counseling. Of note, this cohort did undergo a large number of higher order dissections and a high number of bilateral salpingo-oophorectomies (26.8%), which suggests a high-risk population.
What we found most interesting, however, was the positive finding that medication administration was protective against complications. The authors suggested that the antiinflammatory effects of hormone suppressive medications may be the key. Although this was a retrospective cohort study, the significant risk reduction seen is extremely compelling. A randomized clinical trial corroborating these findings would be instrumental. Endometriosis acts similarly to cancer in its progressive spread and destruction of surrounding tissues. As is increasingly supported in the oncologic literature, perhaps neoadjuvant therapy should be the standard for our “benign” high-risk endometriosis surgeries, with hormonal suppression serving as our chemotherapy. In our own practices, we may be more likely to encourage preoperative medication management, citing this added benefit to patients.
Diaphragmatic endometriosis prevalence higher than previously reported
Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016/j.jmig.2023.01.006
Pagano and colleagues conducted an impressive large prospective cohort study that included more than 1,300 patients with histologically proven endometriosis.1 Each patient underwent a systematic evaluation and reporting of intraoperative findings, including bilateral evaluation for diaphragmatic endometriosis (DE).
Patients with DE had high rates of infertility and high-stage disease
In this cohort, 4.7% of patients were found to have diaphragmatic disease; 92.3% of these cases had DE involving the right diaphragm. Patients with DE had a higher rate of infertility than those without DE (nearly 50%), but otherwise they had no difference in typical endometriosis symptoms (dysmenorrhea, dyspareunia, dyschezia, dysuria). In this cohort, 27.4% had diaphragmatic symptoms (right shoulder pain, cough, cyclic dyspnea).
Patients found to have DE had higher rates of stage III/IV disease (78.4%), and the left pelvis was affected in more patients (73.8%).
The prevalence of DE in this large cohort evaluated by endometriosis surgeons was far higher than previously reported rates of DE (0.19%–1.5% for abdominal endometriosis cases).17,18 Although admittedly this center cares for a larger portion of women with high-stage disease than many nonspecialty centers do, it still begs the question: Are we as a specialty underdiagnosing diaphragmatic endometriosis, especially in our patients with more severe endometriosis? Because nearly 5% of endometriosis patients could have DE, a thoughtful and systematic approach to the abdominal survey and diaphragm should be performed for each case. Adding questions about diaphragmatic symptoms to our preoperative evaluation may help to identify about one-quarter of these complicated patients preoperatively to aid in counseling and surgical planning. Patients to be specifically mindful about include those with high-stage disease, especially left-sided disease, and those with infertility (although this could be a secondary association given the larger proportion of patients with stage III/IV disease with infertility, and no multivariate analysis was performed). This study serves as a thoughtful reminder of this important subject.
A word on fertility-sparing treatments for adenomyosis
Several interesting and thoughtful studies were published on the fertility-sparing management of adenomyosis.6-8 These included a comparison of fertility outcomes following excisional and nonexcisional therapies,6 a systematic review of the literature that compared recurrence rates following procedural and surgical treatments,8 and outcomes after use of a novel therapy (percutaneous microwave ablation) for the treatment of adenomyosis.7
Although our critical evaluation of these studies found that they are not robust enough to yet change our practice, we want to applaud the authors on their discerning questions and on taking the initial steps to answer critical questions, including:
- What is the best uterine-sparing method for treatment of diffuse adenomyosis?
- Are radiofrequency or microwave ablation procedures the future of adenomyosis care?
- How do we counsel patients about fertility potential following procedural treatments?
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
- Pagano F, Schwander A, Vaineau C, et al. True prevalence of diaphragmatic endometriosis and its association with severe endometriosis: a call for awareness and investigation. J Minim Invasive Gynecol. 2023;30:329-334. doi:10.1016 /j.jmig.2023.01.006
- Casarin J, Ghezzi F, Mueller M, et al. Surgical outcomes and complications of laparoscopic hysterectomy for endometriosis: a multicentric cohort study. J Minim Invasive Gynecol. 2023;30:587-592. doi:1016/j.jmig.2023.03.018
- Abrao MS, Andres MP, Gingold JA, et al. Preoperative ultrasound scoring of endometriosis by AAGL 2021 endometriosis classification is concordant with laparoscopic surgical findings and distinguishes early from advanced stages. J Minim Invasive Gynecol. 2023;30:363-373. doi:10.1016 /j.jmig.2022.11.003
- Meyer R, Siedhoff M, Truong M, et al. Risk factors for major complications following minimally invasive surgeries for endometriosis in the United States. J Minim Invasive Gynecol. 2023;30:820-826. doi:10.1016/j.jmig.2023.06.002
- Davenport S, Smith D, Green DJ. Barriers to a timely diagnosis of endometriosis. Obstet Gynecol. 2023;142:571-583. doi:10.1097/AOG.0000000000005255
- Jiang L, Han Y, Song Z, et al. Pregnancy outcomes after uterus-sparing operative treatment for adenomyosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2023:30:543-554. doi:10.1016/j.jmig.2023.03.015
- Li S, Li Z, Lin M, et al. Efficacy of transabdominal ultrasoundguided percutaneous microwave ablation in the treatment of symptomatic adenomyosis: a retrospective cohort study. J Minim Invasive Gynecol. 2023;30:137-146. doi:10.1016/j.jmig.2022.11.004
- Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and metaanalysis. Obstet Gynecol. 2023;141:711-723. doi:10.1097 /AOG.0000000000005080
- Chang OH, Tewari S, Yao M, et al. Who places high value on the uterus? A cross-sectional survey study evaluating predictors for uterine preservation. J Minim Invasive Gynecol. 2023;30:131-136. doi:10.1016/j.jmig.2022.10.012
- Carey ET, Moore KJ, McClurg AB, et al. Racial disparities in hysterectomy route for benign disease: examining trends and perioperative complications from 2007 to 2018 using the NSQIP database. J Minim Invasive Gynecol. 2023;30:627-634. doi:10.1016/j.jmig.2023.03.024
- Frisch EH, Mitchell J, Yao M, et al. The impact of fertility goals on long-term quality of life in reproductive-aged women who underwent myomectomy versus hysterectomy for uterine fibroids. J Minim Invasive Gynecol. 2023;30:642-651. doi:10.1016/j.jmig.2023.04.003 1
- Robinson WR, Mathias JG, Wood ME, et al. Ethnoracial differences in premenopausal hysterectomy: the role of symptom severity. Obstet Gynecol. 2023;142:350-359. doi:10.1097 /AOG.0000000000005225
- Harris HR, Peres LC, Johnson CE, et al. Racial differences in the association of endometriosis and uterine leiomyomas with the risk of ovarian cancer. Obstet Gynecol. 2023;141:11241138. doi:10.1097/AOG.0000000000005191
- Atia O, Hazan E, Rotem R, et al. A scoring system developed by a machine learning algorithm to better predict adnexal torsion. J Minim Invasive Gynecol. 2023;30:486-493. doi:10.1016/j.jmig.2023.02.008
- Ban Y, Shen J, Wang X, et al. Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy. Obstet Gynecol. 2023;141:927-936. doi:10.1097 /AOG.0000000000005113
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies. Obstet Gynecol. 2006;107:1373-1381. doi:10.1097/01.AOG.0000218690.24494.ce
- Scioscia M, Bruni F, Ceccaroni M, et al. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet Gynecol Scand. 2011;90:136-139. doi:10.1111/j.1600-0412.2010.01008.x
- Wetzel A, Philip C-A, Golfier F, et al. Surgical management of diaphragmatic and thoracic endometriosis: a French multicentric descriptive study. J Gynecol Obstet Hum Reprod. 2021;50:102147. doi:10.1016/j.jogoh.2021.102147
Is migraine really a female disorder?
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Migraine is widely considered a predominantly female disorder. Its frequency, duration, and severity tend to be higher in women, and women are also more likely than men to receive a migraine diagnosis. However, gender expectations, differences in the likelihood of self-reporting, and problems with how migraine is classified make it difficult to estimate its true prevalence in men and women.
Different Symptoms
Headache disorders are estimated to affect 50% of the general population ; tension-type headache and migraine are the two most common. According to epidemiologic studies, migraine is more prevalent in women, with a female-to-male ratio of 3:1. There are numerous studies of why this might be, most of which focus largely on female-related factors, such as hormones and the menstrual cycle.
“Despite many years of research, there isn’t one clear factor explaining this substantial difference between women and men,” said Tobias Kurth of Charité – Universitätsmedizin Berlin, Germany. “So the question is: Are we missing something else?”
One factor in these perceived sex differences in migraine is that women seem to report their migraines differently from men, and they also have different symptoms. For example, women are more likely than men to report severe pain, and their migraine attacks are more often accompanied by photophobia, phonophobia, and nausea, whereas men’s migraines are more often accompanied by aura.
“By favoring female symptoms, the classification system may not be picking up male symptoms because they’re not being classified in the right way,” Dr. Kurth said, with one consequence being that migraine is underdiagnosed in men. “Before trying to understand the biological and behavioral reasons for these sex differences, we first need to consider these methodological challenges that we all apply knowingly or unknowingly.”
Christian Lampl, professor of neurology at Konventhospital der Barmherzigen Brüder Linz, Austria, and president of the European Headache Federation, said in an interview, “I’m convinced that this 3:1 ratio which has been stated for decades is wrong, but we still don’t have the data. The criteria we have [for classifying migraine] are useful for clinical trials, but they are useless for determining the male-to-female ratio.
“We need a new definition of migraine,” he added. “Migraine is an episode, not an attack. Attacks have a sudden onset, and migraine onset is not sudden — it is an episode with a headache attack.”
Inadequate Menopause Services
Professor Anne MacGregor of St. Bartholomew’s Hospital in London, United Kingdom, specializes in migraine and women’s health. She presented data showing that migraine is underdiagnosed in women; one reason being that the disorder receives inadequate attention from healthcare professionals at specialist menopause services.
Menopause is associated with an increased prevalence of migraine, but women do not discuss headache symptoms at specialist menopause services, Dr. MacGregor said.
She then described unpublished results from a survey of 117 women attending the specialist menopause service at St. Bartholomew’s Hospital. Among the respondents, 34% reported experiencing episodic migraine and an additional 8% reported having chronic migraine.
“Within this population of women who were not reporting headache as a symptom [to the menopause service until asked in the survey], 42% of them were positive for a diagnosis of migraine,” said Dr. MacGregor. “They were mostly relying on prescribed paracetamol and codeine, or buying it over the counter, and only 22% of them were receiving triptans.
“They are clearly being undertreated,” she added. “Part of this issue is that they didn’t spontaneously report headache as a menopause symptom, so they weren’t consulting for headache to their primary care physicians.”
Correct diagnosis by a consultant is a prerequisite for receiving appropriate migraine treatment. Yet, according to a US study published in 2012, only 45.5% of women with episodic migraine consulted a prescribing healthcare professional. Of those who consulted, 89% were diagnosed correctly, and only 68% of those received the appropriate treatment.
A larger, more recent study confirmed that there is a massive unmet need for improving care in this patient population. The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, which analyzed data from nearly 90,000 participants, showed that just 4.8% of people with chronic migraine received consultation, correct diagnosis, and treatment, with 89% of women with chronic migraine left undiagnosed.
The OVERCOME Study further revealed that although many people with migraine were repeat consulters, they were consulting their physicians for other health problems.
“This makes it very clear that people in other specialties need to be more aware about picking up and diagnosing headache,” said MacGregor. “That’s where the real need is in managing headache. We have the treatments, but if the patients can’t access them, they’re not much good to them.”
A version of this article appeared on Medscape.com.
FROM EHC 2023
Is there a new role for metformin in the management of gestational diabetes?
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
Dunne F, Newman C, Alvarez-Iglesia A, et al. Early metformin in gestational diabetes: a randomized clinical trial. JAMA. 2023;330:1547-1556. doi:10.1001/jama .2023.19869
EXPERT COMMENTARY
Gestational diabetes mellitus occurs in 4% to 7% of pregnancies, and the prevalence is likely to continue to increase given the rising rates of hypertension, obesity, advanced maternal age, and other medical comorbidities in pregnant persons in the United States.1,2 Uncontrolled hyperglycemia in pregnancy is associated swith many adverse perinatal outcomes, including stillbirth, macrosomia, admission to the neonatal intensive care unit (NICU), development of hypertensive disorders, and cesarean deliveries. Hence, it is important to investigate and identify the optimal management of gestational diabetes.
Metformin, an oral biguanide, although studied for gestational diabetes treatment in phase 3 randomized clinical open-label trials, often is avoided in patients who are pregnant (with the exception of patients who have needle aversions, are financially unable to use insulin, or are unable to administer insulin safely).1,2 Metformin is a highly effective first-line agent in the management of both prediabetes and type 2 diabetes, which begs us to question if there is a role for it in the management of gestational diabetes.
Details about the study
The study by Dunne and colleagues was a randomized controlled trial (RCT) conducted in a 1:1 parallel fashion at two institutions in Ireland from 2017–2022. The primary outcome assessed if treatment with metformin would reduce fasting blood glucose levels and the initiation of insulin among women diagnosed with gestational diabetes. A total of 510 participants enrolled in the study, with 268 receiving metformin (up to a maximum dose of 2,500 mg) at diagnosis and 267 receiving an identical placebo. Blood sugar levels were monitored 7 times a day, and medication adherence was assessed every 4 weeks.
Results. At 32 or 38 weeks’ gestation, 56.8% of patients in the metformin arm, and 63.7% of patients in the placebo arm required insulin or had fasting blood glucose levels above 5.1 mmol/L (91.8mg/dL), which was a statistically insignificant difference (P = .13). Although there was similarly no difference in the total amount of insulin used in each study group, the percentage of patients who required insulin initiation was decreased in the metformin arm (38.4% vs 51.1%; P = .004).
Study strengths and weaknesses
The authors conducted a well-designed double-blinded RCT—in both rural and tertiary care settings. Additionally, the study had an impressive 90% patient adherence rate for home blood glucose monitoring 7 times per day. The study arms were balanced for body mass index, as obesity is a known contributor to the development of gestational diabetes and response to insulin.
This study findings’ generalizability is limited across subpopulations given the lack of ethnic and racial diversity—the study population was 80% White. Additionally, utilization of the World Health Organization guidelines for diagnosing gestational diabetes, although adopted by most associations across the world, limits its application to areas of the world that use the National Diabetes Data Group or the Carpenter-Coustan diagnosis guidelines.3,4 Furthermore, the diagnosis of gestational diabetes, which was based on 1 elevated value of a 2-hour glucose tolerance test, has limited scientific support, has not been proven to improve obstetric outcomes, and may increase health care costs when compared with the 2-step method.5 The criteria for insulin initiation in the trial was based on having 2 elevated measures of blood glucose during home glucose monitoring, a criteria that is much stricter than what is used in other countries or clinical practice. The trial authors concluded that use of metformin had a statistically significant reduction in neonates weighing > 4,000 g and > 90th% of weight, but they did not assess study group differences in neonatal skin fold thickness or anthropometric measurements, as reported in the Metformin in Gestational Diabetes trials.6 ●
The study findings by Dunne and colleagues reinforce the current standard practice for the management of gestational diabetes: prescribe medical nutrition therapy and exercise followed by insulin initiation in the setting of persistently elevated blood glucose levels. Knowing that metformin crosses the placenta, future studies should also address the long-term metabolic and health outcomes of fetuses exposed to metformin.
NKECHINYELUM OGU, MD; CHARLOTTE NIZNIK, APRN; MICHELLE A. KOMINIAREK, MD, MS
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
- Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015. doi: 10.1056/NEJMoa0707193
- American College of Obstetricians and Gynecologists. Gestational diabetes mellitus: Practice Bulletin No. 180. Obstet Gynecol. 2017;130:e17-31. doi: 10.1097/AOG.0000000000002159
- Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. doi: 10.2337 /diab.28.12.1039
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
- Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1-31.
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) body composition at 2 years of age. Diabetes Care. 2011;34:2279-2284. https://doi.org/10.2337/dc11-0660
Product Update
LUTECH LT-300 HD FOR COLPOSCOPY
Background. In March 1924, the colposcope was introduced to evaluate the portio of the cervix by Hans Hinselmann in Germany after years of work with the famous lens manufacturer Leitz.1 Although its adoption as a standard tool for evaluating lower genital tract neoplasia was protracted, today it sits as a cornerstone technology in gynecology, and every ObGyn provider has been trained to perform colposcopic exams that include visualizing the cervix, vagina, and vulva as well as taking biopsies. In December 2000, after 75 years of glass lens technology, Welch-Allyn (Skaneateles Falls, New York) introduced the first video colposcope, shepherding the field into the 21st century with only limited traction. Now, Lutech is entering the fray hoping to further nudge traditionalists into the digital age.
Design/Functionality. The Lutech LT-300 HD works off of a Sony Exmor CMOS (complementary metaloxide semiconductor) camera with 2.13 megapixels to provide high-definition optical magnification of 1-30X illuminated by a circular cool LED array that offers 3000 lx of white light with an adjustable green filter to allow for contrast at working distances between 5.1 and 15.7 inches. The colposcope comes with either a vertical stand or a swing arm stand and has both HDMI and USB 3.0 video output so that the system can be attached to either a stand-alone monitor or a computer (not included). The colposcope also comes in a standard definition configuration (LT-300 SD), but I did not trial that model because the price difference did not seem to justify the potentially lower resolution.
In my experience with its use, the Lutech LT-300 HD was pretty excellent. Being a man and a doctor, I refused the online training session that comes free with the colposcope, assuming I could figure it out on my own. My assumption was mostly true, but there were definitely some tips and tricks that would have made my life easier had I not been so stiff-necked. That said, the biggest adjustment is getting used to looking at a screen and not having to look through eyepieces. The picture output is great and, as a patient (or student) teaching tool, it is phenomenal. Also, because it is digital, the image capture features allow for image importation into notes (although it is clunky and requires work arounds when using Epic).
Innovation. From an innovation point of view, I am not sure that Lutech re-invented fire since, in essence, the LT-300 HD is a modified CMOS video camera. But the company did do a nice job bringing together a lot of existing technologies into a highly functional product. I would love to see better integration with some of the larger electronic medical records (EMRs), but I suspect the barriers lie with the EMR companies rather than with Lutech, so I am giving them a pass on that front.
Summary. At its core, a colposcope is simply a tool with which to obtain a magnified view of the cervix, vagina, and/or vulva. Prior to advent and proliferation of CMOS camera technology, the most readily available means of accomplishing this was to employ glass lenses. But that was then, and this is now; CMOS technology is just better, cheaper, and more versatile. I no longer turn my head to look over my shoulder while backing up my car—I use the back-up camera. My Kodak instamatic has given way to my iPhone. And now, my incredibly heavy, unwieldy Leisegang colposcope has been replaced by a light-weight camera on a stand that I can easily move from room to room. I won’t lie, though,…it still seems weird to not look through eyepieces and work the focus knobs, but I am happy with the change. My patients can now see what I am looking at and better understand their diagnosis (if they want), and my notes are prettier. Onward march of progress.
Reference
1. Fusco E, Padula F, Mancini E, et al. History of colposcopy: a brief biography of Hinselmann. J Prenat Med. 2008;2:19-23.
Continue to: DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH...
DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH
The single-use DTR Medical Cervical Rotating Biopsy Punch from Innovia Medical (Swansea, United Kingdom) “works great” and “is reasonably cost-effective to replace reusables.”
Background. Integral to every colposcopic examination is the potential need to biopsy abnormal appearing tissues. To accomplish this latter task, numerous punch-style biopsy devices have been developed in a variety of jaw shapes and styles, crafted from materials ranging from stainless steel to titanium to ceramic, with the ultimate goal the same—get a piece of tissue from the cervix as easily as possible.
Design/Functionality. DTR Medical Cervical Rotating Biopsy Punch is a single-use sterile device that comes packaged as 10 per box. It features Kevorkian-style “stronger than Titanium” jaws that yield a 3.0 mm x 7.5 mm sample attached to a metal shaft that can rotate 360°. The shaft inserts into a lightweight plastic pistol-grip style handle. From tip to handle, the device measures 36.5 cm (14.125 in).
In my experience with its use, the DTR Medical Cervical Rotating Biopsy Punch performed flawlessly. Its relatively low-profile jaws allowed for unobstructed access to biopsy sites and the ability to rotate the jaws was a big plus. The “stronger than Titanium” jaws consistently yielded the exact biopsies I wanted, like a knife going through butter.
Innovation. From an innovation standpoint, the DTR Medical Cervical Rotating Biopsy Punch is more of an engineering “duh” than “wow,” but it works great so who cares that it’s not a fusion reactor. That said, the innovative part from Innovia Medical is their ability to make such a high-quality biopsy device and sell it at a price that makes it reasonably cost-effective to replace reusables.
Summary. Whether it is a Tischler, Kevorkian, or Burke tip, the real question before any gynecologist uses the cervical biopsy device she/he/they has in her/his/ their hand is, will it cut? Because all reusable surgical instruments are in fact reusable, those edges that are designed to cut invariably become dull with reuse. And, unless they are meticulously maintained and routinely sharpened (spoiler alert, they never are), providers are not infrequently chagrinned by the gnawing rather than cutting that these instruments deliver. Thinking back, I could not remember the last time I had made an incision with a surgical scalpel blade that had previously been used then sharpened and re-sterilized. Then I did remember…never. Reflecting on this, I wondered why I was doing this with my cervical biopsy devices. While I really do not like the environmental waste created by single-use devices, reusable instruments that require re-processing do have an environmental impact and a significant cost. Considering this, I do not think that environmental reasons are enough of a barrier to justify using dull biopsy tools if it can be done cost-effectively with a minimal carbon footprint. All-in-all, I like this product, and I plan to use it. ●
LUTECH LT-300 HD FOR COLPOSCOPY
Background. In March 1924, the colposcope was introduced to evaluate the portio of the cervix by Hans Hinselmann in Germany after years of work with the famous lens manufacturer Leitz.1 Although its adoption as a standard tool for evaluating lower genital tract neoplasia was protracted, today it sits as a cornerstone technology in gynecology, and every ObGyn provider has been trained to perform colposcopic exams that include visualizing the cervix, vagina, and vulva as well as taking biopsies. In December 2000, after 75 years of glass lens technology, Welch-Allyn (Skaneateles Falls, New York) introduced the first video colposcope, shepherding the field into the 21st century with only limited traction. Now, Lutech is entering the fray hoping to further nudge traditionalists into the digital age.
Design/Functionality. The Lutech LT-300 HD works off of a Sony Exmor CMOS (complementary metaloxide semiconductor) camera with 2.13 megapixels to provide high-definition optical magnification of 1-30X illuminated by a circular cool LED array that offers 3000 lx of white light with an adjustable green filter to allow for contrast at working distances between 5.1 and 15.7 inches. The colposcope comes with either a vertical stand or a swing arm stand and has both HDMI and USB 3.0 video output so that the system can be attached to either a stand-alone monitor or a computer (not included). The colposcope also comes in a standard definition configuration (LT-300 SD), but I did not trial that model because the price difference did not seem to justify the potentially lower resolution.
In my experience with its use, the Lutech LT-300 HD was pretty excellent. Being a man and a doctor, I refused the online training session that comes free with the colposcope, assuming I could figure it out on my own. My assumption was mostly true, but there were definitely some tips and tricks that would have made my life easier had I not been so stiff-necked. That said, the biggest adjustment is getting used to looking at a screen and not having to look through eyepieces. The picture output is great and, as a patient (or student) teaching tool, it is phenomenal. Also, because it is digital, the image capture features allow for image importation into notes (although it is clunky and requires work arounds when using Epic).
Innovation. From an innovation point of view, I am not sure that Lutech re-invented fire since, in essence, the LT-300 HD is a modified CMOS video camera. But the company did do a nice job bringing together a lot of existing technologies into a highly functional product. I would love to see better integration with some of the larger electronic medical records (EMRs), but I suspect the barriers lie with the EMR companies rather than with Lutech, so I am giving them a pass on that front.
Summary. At its core, a colposcope is simply a tool with which to obtain a magnified view of the cervix, vagina, and/or vulva. Prior to advent and proliferation of CMOS camera technology, the most readily available means of accomplishing this was to employ glass lenses. But that was then, and this is now; CMOS technology is just better, cheaper, and more versatile. I no longer turn my head to look over my shoulder while backing up my car—I use the back-up camera. My Kodak instamatic has given way to my iPhone. And now, my incredibly heavy, unwieldy Leisegang colposcope has been replaced by a light-weight camera on a stand that I can easily move from room to room. I won’t lie, though,…it still seems weird to not look through eyepieces and work the focus knobs, but I am happy with the change. My patients can now see what I am looking at and better understand their diagnosis (if they want), and my notes are prettier. Onward march of progress.
Reference
1. Fusco E, Padula F, Mancini E, et al. History of colposcopy: a brief biography of Hinselmann. J Prenat Med. 2008;2:19-23.
Continue to: DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH...
DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH
The single-use DTR Medical Cervical Rotating Biopsy Punch from Innovia Medical (Swansea, United Kingdom) “works great” and “is reasonably cost-effective to replace reusables.”
Background. Integral to every colposcopic examination is the potential need to biopsy abnormal appearing tissues. To accomplish this latter task, numerous punch-style biopsy devices have been developed in a variety of jaw shapes and styles, crafted from materials ranging from stainless steel to titanium to ceramic, with the ultimate goal the same—get a piece of tissue from the cervix as easily as possible.
Design/Functionality. DTR Medical Cervical Rotating Biopsy Punch is a single-use sterile device that comes packaged as 10 per box. It features Kevorkian-style “stronger than Titanium” jaws that yield a 3.0 mm x 7.5 mm sample attached to a metal shaft that can rotate 360°. The shaft inserts into a lightweight plastic pistol-grip style handle. From tip to handle, the device measures 36.5 cm (14.125 in).
In my experience with its use, the DTR Medical Cervical Rotating Biopsy Punch performed flawlessly. Its relatively low-profile jaws allowed for unobstructed access to biopsy sites and the ability to rotate the jaws was a big plus. The “stronger than Titanium” jaws consistently yielded the exact biopsies I wanted, like a knife going through butter.
Innovation. From an innovation standpoint, the DTR Medical Cervical Rotating Biopsy Punch is more of an engineering “duh” than “wow,” but it works great so who cares that it’s not a fusion reactor. That said, the innovative part from Innovia Medical is their ability to make such a high-quality biopsy device and sell it at a price that makes it reasonably cost-effective to replace reusables.
Summary. Whether it is a Tischler, Kevorkian, or Burke tip, the real question before any gynecologist uses the cervical biopsy device she/he/they has in her/his/ their hand is, will it cut? Because all reusable surgical instruments are in fact reusable, those edges that are designed to cut invariably become dull with reuse. And, unless they are meticulously maintained and routinely sharpened (spoiler alert, they never are), providers are not infrequently chagrinned by the gnawing rather than cutting that these instruments deliver. Thinking back, I could not remember the last time I had made an incision with a surgical scalpel blade that had previously been used then sharpened and re-sterilized. Then I did remember…never. Reflecting on this, I wondered why I was doing this with my cervical biopsy devices. While I really do not like the environmental waste created by single-use devices, reusable instruments that require re-processing do have an environmental impact and a significant cost. Considering this, I do not think that environmental reasons are enough of a barrier to justify using dull biopsy tools if it can be done cost-effectively with a minimal carbon footprint. All-in-all, I like this product, and I plan to use it. ●
LUTECH LT-300 HD FOR COLPOSCOPY
Background. In March 1924, the colposcope was introduced to evaluate the portio of the cervix by Hans Hinselmann in Germany after years of work with the famous lens manufacturer Leitz.1 Although its adoption as a standard tool for evaluating lower genital tract neoplasia was protracted, today it sits as a cornerstone technology in gynecology, and every ObGyn provider has been trained to perform colposcopic exams that include visualizing the cervix, vagina, and vulva as well as taking biopsies. In December 2000, after 75 years of glass lens technology, Welch-Allyn (Skaneateles Falls, New York) introduced the first video colposcope, shepherding the field into the 21st century with only limited traction. Now, Lutech is entering the fray hoping to further nudge traditionalists into the digital age.
Design/Functionality. The Lutech LT-300 HD works off of a Sony Exmor CMOS (complementary metaloxide semiconductor) camera with 2.13 megapixels to provide high-definition optical magnification of 1-30X illuminated by a circular cool LED array that offers 3000 lx of white light with an adjustable green filter to allow for contrast at working distances between 5.1 and 15.7 inches. The colposcope comes with either a vertical stand or a swing arm stand and has both HDMI and USB 3.0 video output so that the system can be attached to either a stand-alone monitor or a computer (not included). The colposcope also comes in a standard definition configuration (LT-300 SD), but I did not trial that model because the price difference did not seem to justify the potentially lower resolution.
In my experience with its use, the Lutech LT-300 HD was pretty excellent. Being a man and a doctor, I refused the online training session that comes free with the colposcope, assuming I could figure it out on my own. My assumption was mostly true, but there were definitely some tips and tricks that would have made my life easier had I not been so stiff-necked. That said, the biggest adjustment is getting used to looking at a screen and not having to look through eyepieces. The picture output is great and, as a patient (or student) teaching tool, it is phenomenal. Also, because it is digital, the image capture features allow for image importation into notes (although it is clunky and requires work arounds when using Epic).
Innovation. From an innovation point of view, I am not sure that Lutech re-invented fire since, in essence, the LT-300 HD is a modified CMOS video camera. But the company did do a nice job bringing together a lot of existing technologies into a highly functional product. I would love to see better integration with some of the larger electronic medical records (EMRs), but I suspect the barriers lie with the EMR companies rather than with Lutech, so I am giving them a pass on that front.
Summary. At its core, a colposcope is simply a tool with which to obtain a magnified view of the cervix, vagina, and/or vulva. Prior to advent and proliferation of CMOS camera technology, the most readily available means of accomplishing this was to employ glass lenses. But that was then, and this is now; CMOS technology is just better, cheaper, and more versatile. I no longer turn my head to look over my shoulder while backing up my car—I use the back-up camera. My Kodak instamatic has given way to my iPhone. And now, my incredibly heavy, unwieldy Leisegang colposcope has been replaced by a light-weight camera on a stand that I can easily move from room to room. I won’t lie, though,…it still seems weird to not look through eyepieces and work the focus knobs, but I am happy with the change. My patients can now see what I am looking at and better understand their diagnosis (if they want), and my notes are prettier. Onward march of progress.
Reference
1. Fusco E, Padula F, Mancini E, et al. History of colposcopy: a brief biography of Hinselmann. J Prenat Med. 2008;2:19-23.
Continue to: DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH...
DTR MEDICAL CERVICAL ROTATING BIOPSY PUNCH
The single-use DTR Medical Cervical Rotating Biopsy Punch from Innovia Medical (Swansea, United Kingdom) “works great” and “is reasonably cost-effective to replace reusables.”
Background. Integral to every colposcopic examination is the potential need to biopsy abnormal appearing tissues. To accomplish this latter task, numerous punch-style biopsy devices have been developed in a variety of jaw shapes and styles, crafted from materials ranging from stainless steel to titanium to ceramic, with the ultimate goal the same—get a piece of tissue from the cervix as easily as possible.
Design/Functionality. DTR Medical Cervical Rotating Biopsy Punch is a single-use sterile device that comes packaged as 10 per box. It features Kevorkian-style “stronger than Titanium” jaws that yield a 3.0 mm x 7.5 mm sample attached to a metal shaft that can rotate 360°. The shaft inserts into a lightweight plastic pistol-grip style handle. From tip to handle, the device measures 36.5 cm (14.125 in).
In my experience with its use, the DTR Medical Cervical Rotating Biopsy Punch performed flawlessly. Its relatively low-profile jaws allowed for unobstructed access to biopsy sites and the ability to rotate the jaws was a big plus. The “stronger than Titanium” jaws consistently yielded the exact biopsies I wanted, like a knife going through butter.
Innovation. From an innovation standpoint, the DTR Medical Cervical Rotating Biopsy Punch is more of an engineering “duh” than “wow,” but it works great so who cares that it’s not a fusion reactor. That said, the innovative part from Innovia Medical is their ability to make such a high-quality biopsy device and sell it at a price that makes it reasonably cost-effective to replace reusables.
Summary. Whether it is a Tischler, Kevorkian, or Burke tip, the real question before any gynecologist uses the cervical biopsy device she/he/they has in her/his/ their hand is, will it cut? Because all reusable surgical instruments are in fact reusable, those edges that are designed to cut invariably become dull with reuse. And, unless they are meticulously maintained and routinely sharpened (spoiler alert, they never are), providers are not infrequently chagrinned by the gnawing rather than cutting that these instruments deliver. Thinking back, I could not remember the last time I had made an incision with a surgical scalpel blade that had previously been used then sharpened and re-sterilized. Then I did remember…never. Reflecting on this, I wondered why I was doing this with my cervical biopsy devices. While I really do not like the environmental waste created by single-use devices, reusable instruments that require re-processing do have an environmental impact and a significant cost. Considering this, I do not think that environmental reasons are enough of a barrier to justify using dull biopsy tools if it can be done cost-effectively with a minimal carbon footprint. All-in-all, I like this product, and I plan to use it. ●
Norgestrel for nonprescription contraception: What you and your patients need to know
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.
How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8
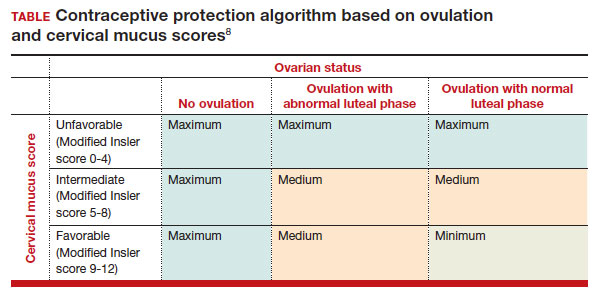
In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.

How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8

In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
On July 13, 2023, the US Food and Drug Administration (FDA) approved norgestrel 0.075 mg (Opill, HRA Pharma, Paris, France) as the first nonprescription oral contraceptive pill (FIGURE). This progestin-only pill was originally FDA approved in 1973, with prescription required, and was available as Ovrette until 2005, when product distribution ceased for marketing reasons and not for safety or effectiveness concerns.1 In recent years, studies have been conducted to support converted approval from prescription to nonprescription to increase access to safe and effective contraception. Overall, norgestrel is more effective than other currently available nonprescription contraceptive options when used as directed, and widespread accessibility to this method has the potential to decrease the risk of unintended pregnancies. This product is expected to be available in drugstores, convenience stores, grocery stores, and online in 2024.

How it works
The indication for norgestrel 0.075 mg is pregnancy prevention in people with the capacity to become pregnant; this product is not intended for emergency contraception. Norgestrel is a racemic mixture of 2 isomers, of which only levonorgestrel is bioactive. The mechanism of action for contraception is primarily through cervical mucus thickening, which inhibits sperm movement through the cervix. About 50% of users also have an additional contraceptive effect of ovulation suppression.2
Instructions for use. In the package label, users are instructed to take the norgestrel 0.075 mg pill daily, preferably at the same time each day and no more than 3 hours from the time taken on the previous day. This method can be started on any day of the cycle, and backup contraception (a barrier method) should be used for the first 48 hours after starting the method if it has been more than 5 days since menstrual bleeding started.3 Product instructions indicate that, if users miss a dose, they should take the next dose as soon as possible. If a pill is taken 3 hours or more later than the usual time, they should take a pill immediately and then resume the next pill at the usual time. In addition, backup contraception is recommended for 48 hours.2
Based on the Centers for Disease Control and Prevention (CDC) Selected Practice Recommendations for Contraceptive Use, no examinations or tests are required prior to initiation of progestin-only pills for safe and effective use.3
Efficacy
The product label indicates that the pregnancy rate is approximately 2 per 100 women-years based on over 21,000 28-day exposure cycles from 8 US clinical studies.2 In a recent review by Glasier and colleagues, the authors identified 13 trials that assessed the efficacy of the norgestrel 0.075 mg pill, all published several decades ago.4 Given that breastfeeding can have contraceptive impact through ovulation inhibition, studies that included breastfeeding participants were evaluated separately. Six studies without breastfeeding participants included 3,184 women who provided more than 35,000 months of use. The overall failure rates ranged from 0 to 2.4 per hundred woman-years with typical use; an aggregate Pearl Index was calculated to be 2.2 based on the total numbers of pregnancies and cycles. The remaining 7 studies included individuals who were breastfeeding for at least part of their study participation. These studies included 5,445 women, and the 12-month life table cumulative pregnancy rates in this group ranged from 0.0% to 3.4%. This review noted that the available studies are limited by incomplete descriptions of study participant information and differences in reporting of failure rates; however, the overall data support the effectiveness of the norgestrel 0.075 mg pill for pregnancy prevention.
Continue to: Norgestrel’s mechanism of action on ovarian activity and cervical mucus...
Norgestrel’s mechanism of action on ovarian activity and cervical mucus
More recently, a prospective, multicenter randomized, crossover study was performed to better understand this pill’s impact on cervical mucus and ovulation during preparation for nonprescription approval. In this study, participants were evaluated with frequent transvaginal ultrasonography, cervical mucus, and blood assessments (including levels of follicular-stimulating hormone, luteinizing hormone, progesterone, and estradiol) for three 28-day cycles. Cervical mucus was scored on a modified Insler scale to indicate if the mucus was favorable (Insler score ≥9), intermediate (Insler score 5-8), or unfavorable to fertility (Insler score ≤4).5
In the first cycle, participants were instructed to use the pills as prescribed (described as “correct use”). During this cycle, most participants (n = 34/51; 67%) did not ovulate, confirming that norgestrel 0.075 mg does impact ovulation.6 Most participants also had unfavorable cervical mucus (n = 39/51; 76%).6 Overall, 94% had full protection against pregnancy, either through lack of ovulation (n = 9), unfavorable mucus (n = 14), or both (n = 25). The remaining 3 participants ovulated and had intermediate mucus scores; ultimately, these participants were considered to have medium protection against pregnancy.7,8 (See the contraceptive protection algorithm [TABLE]).8

In the second and third cycles, the investigators evaluated ovulation and cervical mucus changes in the setting of either a delayed (by 6 hours) or missed dose midcycle.8 Of the 46 participants with evaluable data during the intervention cycles, 32 (70%) did not ovulate in each of the delayed- and missed-dose cycles. Most participants (n = 27; 59%) also demonstrated unfavorable mucus scores (modified Insler score ≤4) over the entire cycle despite delaying or missing a pill. There was no significant change to the cervical mucus score when comparing the scores on the days before, during, and after the delayed or missed pills (P = .26), nor when comparing between delayed pill use and missed pill use (P = .45). With the delayed pill intervention, 4 (9%) had reduced contraceptive protection (ie, medium protection) based on ovulation with intermediate mucus scores. With the missed pill intervention, 5 (11%) had reduced protection, of whom 3 had medium protection and 2 had minimum protection with ovulation and favorable mucus scores. Overall, this study shows that delaying or missing one pill may not impact contraceptive efficacy as much as previously thought given the strict 3-hour window for progestin-only pills. However, these findings are theoretical as information about pregnancy outcomes with delaying or missing pills are lacking.
Safety
Progestin-only methods are one of the safest options for contraception, with few contraindications to use; those listed include known or suspected pregnancy, known or suspected carcinoma of the breast or other progestinsensitive cancer, undiagnosed abnormal uterine bleeding, hypersensitivity to any component of the product, benign or malignant liver tumors, and acute liver disease.2
The CDC Medical Eligibility Criteria for Contraceptive Use guidelines offer guidance for progestin-only pills, indicating a category 3 (theoretical or proven risks usually outweigh the advantages) or category 4 (unacceptable health risk, method not to be used) for only a select number of additional conditions. These conditions include a history of malabsorptive bariatric surgery (category 3) and concurrent use of medications that induce hepatic enzyme activity (category 3)— such as phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine, rifampin, and rifabutin.9 These conditions are included primarily due to concerns of decreased effectivenessof the contraception and not necessarily because of evidence of harm with use.
The prevalence of consumers with contraindications to progestin-only pills appears to be low. In a large database study, only 4.36% seeking preventive care and 2.29% seeking both preventive and contraceptive services had a contraindication to progestin-only pills.10 Therefore, candidates for norgestrel use include individuals who have commonly encountered conditions, including those who9:
- have recently given birth
- are breastfeeding
- have a history of venous thromboembolism
- smoke
- have cardiovascular disease, hypertension, migraines with aura, or longstanding diabetes.
Adverse effects
The most common adverse effects (AEs) related to norgestrel use are bleeding changes.2 In the initial clinical studies for FDA approval, about half of enrolled participants reported a change in bleeding; about 9% discontinued the contraceptive due to bleeding. Breakthrough bleeding and spotting were reported by 48.6% and 47.3% of participants, respectively. About 6.1% had amenorrhea in their first cycle; 28.7% of participants had amenorrhea overall. Other reported AEs were headache, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating, breast tenderness, and acne.
- Brand name: Opill
- Class: Progestin-only contraception
- Indication: Pregnancy prevention
- Approval date: Initial approval in 1973, nonprescription approval on July 13, 2023
- Availability date: 2024
- Manufacturer: Perrigo Company, HRA Pharma, Paris, France
- Dosage forms: 0.075 mg tablet
Continue to: FDA approval required determining appropriate direct-to-patient classification...
FDA approval required determining appropriate direct-to-patient classification
As part of the process for obtaining nonprescription approval, studies needed to determine that patients can safely and effectively use norgestrel without talking to a health care provider first. As part of that process, label comprehension, self-selection, and actualuse studies were required to demonstrate that consumers can use the package information to determine their eligibility and take the medication appropriately.
The ACCESS study Research Q: Do patients appropriately determine if the contraceptive is right for them?
Study A: Yes, 99% of the time. In the Adherence with Continuous-dose Oral Contraceptive: Evaluation of Self-Selection and Use (ACCESS) pivotal study, which evaluated prescription to nonprescription approval, participants were asked to review the label and determine whether the product was appropriate for them to use based on their health history.11 Approximately 99% of participants (n = 1,234/1,246) were able to correctly self-select whether norgestrel was appropriate for their own use.12
Research Q: After beginning the contraceptive, do patients adhere to correct use?
Study A: Yes, more than 90% of the time (and that remained true for subpopulations).
In the next phase of the ACCESS study, eligible participants from the self-selection population who purchased norgestrel and reported using the product at least once in their e-diary over a 6-month study period comprised the “User Population.”12 The overall adherence to daily pill intake was 92.5% (95% confidence interval [CI], 92.3–92.6%) among the 883 participants who contributed more than 90,000 days of study participation, and adherence was similarly high in subpopulations of individuals with low health literacy (92.6%; 95% CI, 92.1–93.0), adolescents aged 12–14 years (91.8%; 95% CI, 91.0–92.5%), and adolescents aged 15–17 years (91.9%; 95% CI, 91.4%–92.3%).
Research Q: When a pill was missed, did patients use backup contraception?
Study A: Yes, 97% of the time.
When including whether participants followed label instructions for mitigating behaviors when the pill was missed (eg, take a pill as soon as they remember, use backup contraception for 2 days after restarting the pill), adherence was 97.1% (95% CI, 97.0–97.2%). Most participants missed a single day of taking pills, and the most common reported reason for missing pills was issues with resupply as participants needed to get new packs from their enrolled research site, which should be less of a barrier when these pills are available over the counter.
Clinical implications of expanded access
Opportunities to expand access to effective contraception have become more critical in the increasingly restrictive environment for abortion care in the post-Dobbs era, and the availability of norgestrel to patients without prescription can advance contraceptive equity. Patients encounter many barriers to accessing prescription contraception, such as lack of insurance; difficulty with scheduling an appointment or getting to a clinic; not having a regular clinician or clinic; or health care providers requiring a visit, exam, or test prior to prescribing contraception.13,14 For patients who face these challenges, an alternative option is to use a nonprescription contraceptive, such as barrier or fertility awareness–based methods, which are typically associated with higher failure rates. With the introduction of norgestrel as a nonprescription contraceptive product, people can have direct access to a more effective contraceptive option.
A follow-up study of participants who had participated in the ACCESS actual-use study demonstrated that most (83%) would be likely to use the nonprescription method if available in the future for many reasons, including convenience, ease of access, ability to save time and money, not needing to visit a clinic, and flexibility of accessing the pills while traveling or having someone else get their pills for them.14 Furthermore, a nonprescription method could be beneficial for people who have concerns about privacy, such as adolescents or individuals affected by contraception sabotage (an act that can intentionally limit or prohibit a person's contraception access or use, ie, damaging condoms or hiding a person’s contraception method). This expansion of access can ultimately lead to a decrease in unintended pregnancies. In a model using the ACCESS actual-use data, about 1,500 to 34,000 unintended pregnancies would be prevented per year based on varying model parameters, with all scenarios demonstrating a benefit to nonprescription access to norgestrel.15
After norgestrel is available, where will patients be able to seek more information?
Patients who have questions or concerns about starting or taking norgestrel should talk to their clinician or a pharmacist for additional information (FIGURE 2). Examples of situations when additional clinical evaluation or counseling are recommended include:
- when a person is taking any medications with possible drug-drug interactions
- if a person is starting norgestrel after taking an emergency contraceptive in the last 5 days
- if there is a concern about pregnancy
- when there are any questions about adverse effects while taking norgestrel.
Bottom line
The nonprescription approval of norgestrel, a progestin-only pill, has the potential to greatly expand patient access to a safe and effective contraceptive method and advance contraceptive equity. The availability of informational materials for consumers about potential issues that may arise (for instance, changes in bleeding) will be important for initiation and continuation of this method. As this product is not yet available for purchase, several unknown factors remain, such as the cost and ease of accessibility in stores or online, that will ultimately determine its public health impact on unintended pregnancies. ●
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
- US Food and Drug Administration. 82 FR 49380. Determination that Ovrette (norgestrel) tablet, 0.075 milligrams, was not withdrawn from sale for reasons of safety or effectiveness. October 25, 2017. Accessed December 5, 2023. https://www.federalregister.gov/d/2017-23125
- US Food and Drug Administration. Opill tablets (norgestrel tablets) package label. August 2017. Accessed December 5, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label /2017/017031s035s036lbl.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No. RR-4):1-66.
- Glasier A, Sober S, Gasloli R, et al. A review of the effectiveness of a progestogen-only pill containing norgestrel 75 µg/day. Contraception. 2022;105:1-6.
- Edelman A, Hemon A, Creinin M, et al. Assessing the pregnancy protective impact of scheduled nonadherence to a novel progestin-only pill: protocol for a prospective, multicenter, randomized, crossover study. JMIR Res Protoc. 2021;10:e292208.
- Glasier A, Edelman A, Creinin MD, et al. Mechanism of action of norgestrel 0.075 mg a progestogen-only pill. I. Effect on ovarian activity. Contraception. 2022;112:37-42.
- Han L, Creinin MD, Hemon A, et al. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception. 2022;112:43-47.
- Glasier A, Edelman A, Creinin MD, et al. The effect of deliberate non-adherence to a norgestrel progestin-only pill: a randomized, crossover study. Contraception. 2023;117:1-6.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(No RR-3):1-104.
- Dutton C, Kim R, Janiak E. Prevalence of contraindications to progestin-only contraceptive pills in a multi-institution patient database. Contraception. 2021;103:367-370.
- Clinicaltrials.gov. Adherence with Continuous-dose Oral Contraceptive Evaluation of Self-Selection and Use (ACCESS). Accessed December 5, 2023. https://clinicaltrials.gov/study /NCT04112095
- HRA Pharma. Opill (norgestrel 0.075 mg tablets) for Rx-toOTC switch. Sponsor Briefing Documents. Joint Meeting of the Nonprescription Drugs Advisory Committee and the Obstetrics, Reproductive, and Urology Drugs Advisory Committee. Meeting dates: 9-10 May 2023. Accessed December 5, 2023. https://www.fda.gov/media/167893 /download
- American College of Obstetricians and Gynecologists. Committee Opinion No. 788: Over-the-counter access to hormonal contraception. Obstet Gynecol. 2019;134:e96-105.
- Grindlay K, Key K, Zuniga C, et al. Interest in continued use after participation in a study of over-the-counter progestin-only pills in the United States. Womens Health Rep. 2022;3:904-914.
- Guillard H, Laurora I, Sober S, et al. Modeling the potential benefit of an over-the-counter progestin-only pill in preventing unintended pregnancies in the U.S. Contraception. 2023;117:7-12.
Recruiting ObGyns: Starting salary considerations
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4
Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4


Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
Evidence continues to show that the number of practicing ObGyns lags the growing and diverse US population of women.1 Furthermore, approximately 1 in every 3 ObGyns will move usually once or twice every 10 years.2 Knowing what to expect in being recruited requires a better understanding of your needs and capabilities and what they may be worth in real time. Some ObGyns elect to use a recruitment firm to begin their search to more objectively assess what is fair and equitable.
Understanding physician compensation involves many factors, such as patient composition, sources of reimbursement, impact of health care systems, and geography.3 Several sources report trends in annual physician compensation, most notably the American Medical Association, medical specialty organizations, and recruitment firms. Sources such as the Medical Group Management Association (MGMA), the American Medical Group Association (AMGA), and Medscape report total compensation.
Determining salaries for new positions
A standard and comprehensive benchmarking resource for salaries in new positions has been the annual review of physician and advanced practitioner recruiting incentives by AMN Healthcare (formerly Merritt Hawkins) Physician Solutions.4 This resource is used by hospitals, medical groups, academics, other health care systems, and others who track trends in physician supply, demand, and compensation. Their 2023 report considered starting salaries for more than 20 medical or surgical specialties.
Specialists’ revenue-generating potential is tracked by annual billings to commercial payers. The average annual billing by a full-time ObGyn ($3.8 million) is about the same as that of other specialties combined.5 As in the past, ObGyns are among the most consistently requested specialists in searches. In 2023, ObGyns were ranked the third most common physician specialists being recruited and tenth as the percentage of physicians per specialty (TABLE).4
Full-time salaries for ObGyns have remained within the middle third of all specialties. They consistently have been higher than primary care physicians’ salaries but remain among the lowest of the surgical specialties. This impression is reinforced by 2023 data shown in FIGURE 1.4 In the past, salaries remained flat compared with other surgical specialties. As with other specialties, starting salaries decreased during the peak 2020 and 2021 COVID-19 years. It is encouraging that averaged full-time salaries for recruiting ObGyns increased by 14.1% from 2020–2021 to 2021–2022 and by 10.5% from 2021–2022 to 2022–2023 (FIGURE 2).4


Special considerations
Incomes tended to be highest for ObGyns practicing in metropolitan areas with population sizes less than 1 million rather than in larger metropolitan areas.3 However, differences in reported incomes do not control for cost of living and other determinants of income (for example, surgeries, deliveries, patient care hours worked). Averaged salaries can vary regionally in the following order from highest to lowest: Midwest/Great Plains, West, Southwest, and Northeast and Southeast.4
Differences in starting salaries between male and female ObGyns are often not reported, although they are a very important consideration.6,7 Both men and women desire “controllable lifestyles” with more flexibility and working in shifts. Sex-based differences in physician salary and compensation can be complex. Explanations may deal with the number of patients seen, number of procedures and surgeries performed, and frequency of after-hours duties. Women constitute most ObGyns, and their salary being at any lower end of the income spectrum may be partially explained by fewer desired work hours or less seniority.
Annual earnings can vary and are positively related to the number of working hours, being in the middle of one’s career (aged 42–51 years), working in a moderately large practice rather than in a solo or self-employed practice, and being board certified.3 A lower starting salary would be anticipated for a recent graduate. However, the resident going into a hard-to-fill position may be offered a higher salary than an experienced ObGyn who takes a relatively easy-to-fill position in a popular location. Practices would be more desirable in which patient volume is sufficient to invest in nonphysician clinicians and revenue-generating ancillary services that do not require costly layers of administration.
Information on physician salaries for new positions from individual recruiting or research firms can serve as a starting point for negotiation, although it may not entirely be representative. Sample sizes can be small, and information in some specialties may not separate salaries of physicians in academic versus nonacademic positions and generalists versus subspecialists. The information in this article reflects the average salaries offered to attract physicians to new practice settings rather than what they might earn and report on their tax return.
Continue to: Incentives...
Incentives
Negotiations involve incentives along with a starting salary. Signing bonuses, movingallowances, continuing education time and allowances, and medical education loan repayments are important incentives. Recent signing bonuses (average, $37,472) likely reflect efforts to bring physicians back to health care facilities post-COVID-19 or, more commonly, when candidates are considering multiple opportunities.4 It is important to clarify at the beginning any coverage for health insurance and professional liability insurance.
Relocation allowances are for those being recruited outside their current area of residence. The average continuing medical education allowance was $3,840 in 2023.4 Medical school debt is common, being approximately $200,000 at graduation for many. An educational loan repayment (average, $98,665) is typically an exchange for a commitment to stay in the community for a given period.
Starting employment contracts with hospitals or large medical groups often feature a production bonus to reward additional clinical work performed or an adherence to quality protocol or guidelines, rather than income guarantees alone. Metrics are usually volume driven (for example, relative value units, net collections, gross billings, patients seen). Initiatives by payers and health care organizations have included quality metrics, such as high patient satisfaction scores, low morbidity rates, and low readmission rates. Production-based formulas are straightforward, while use of quality-based formulas (up to 14% of total compensation) can be less clear to define.4 ●
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
- Rayburn WF, Xierali IM. Expanded fellowship training and residency graduates’ availability for women’s general health needs. Obstet Gynecol. 2021;137:1119-1121.
- Xierali IM, Nivett MA, Rayburn WF. Relocation of obstetriciangynecologists in the United States, 2005-2015. Obstet Gynecol. 2017;129:543-550.
- Rayburn WF. The Obstetrician-Gynecologist Workforce in the United States: Facts, Figures, and Implications. 2nd ed. American College of Obstetricians and Gynecologists; 2017.
- AMN Healthcare. 2023 Review of physician and advanced practitioner recruiting incentives. July 24, 2023. Accessed October 3, 2023. https://www.amnhealthcare.com/amn -insights/physician/surveys/2023-physician-and-ap -recruiting-incentives/
- AMN Healthcare. 2023 Physician billing report. March 21, 2023. Accessed October 7, 2023. https://www.amnhealthcare. com/amn-insights/physician/whitepapers/2023-physician -billing-report/
- Bravender T, Selkie E, Sturza J, et al. Association of salary differences between medical specialties with sex distribution. JAMA Pediatr. 2021;175:524-525.
- Lo Sasso AT, Armstrong D, Forte G, et al. Differences in starting pay for male and female physicians persist; explanations for the gender gap remain elusive. Health Aff. 2020;39:256-263.
Patient counseling for breast cancer screening: Taking changes to USPSTF recommendations into account
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Focus on long-COVID: Perimenopause and post-COVID chronic fatigue
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.
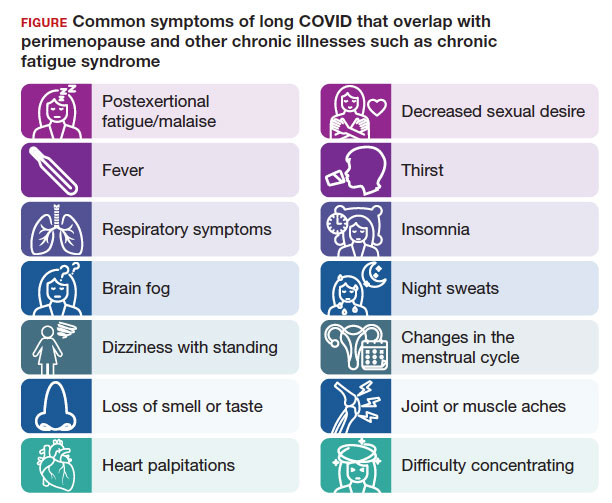
Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Long COVID (postacute sequelae of SARS-CoV-2 infection, or PASC) is an emerging syndrome that affects 50% to 70% of people who survive COVID-19 for up to 3 months or longer after acute disease.1 It is a multisystem condition that causes dysfunction of respiratory, cardiac, and nervous tissue, at least in part likely due to alterations in cellular energy metabolism and reduced oxygen supply to tissue.3 Patients who have had SARS-CoV-2 infection report persistent symptoms and signs that affect their quality of life. These may include neurocognitive, cardiorespiratory, gastrointestinal, and musculoskeletal symptoms; loss of taste and smell; and constitutional symptoms.2 There is no one test to determine if symptoms are due to COVID-19.3
Acute COVID-19 mortality risk factors include increasing age, chronic comorbidities, and male sex. However, long COVID risk factors are quite different. A meta-analysis and review of 20 articles that met inclusion criteria (n = 13,340 study participants), limited by pooling of crude estimates, found that risk factors were female sex and severity of acute disease.4 A second meta-analysis of 37 studies with 1 preprint found that female sex and comorbidities such as pulmonary disease, diabetes, and obesity were risk factors for long COVID.5 Qualitative analysis of single studies (n = 18 study participants) suggested that older adults can develop more long COVID symptoms than younger adults, but this association between advancing age and long COVID was not supported when data were pooled into a meta-analysis.3 However, both single studies (n = 16 study participants) and the meta-analysis (n = 7 study participants) did support female sex as a risk factor for long COVID, along with single studies suggesting increased risk with medical comorbidities for pulmonary disease, diabetes, and organ transplantation.
Perimenopause
Perimenopause: A temporary disruption to physiologic ovarian steroid hormone production following COVID could acutely exacerbate symptoms of perimenopause and menopause.
JoAnn V. Pinkerton, MD, MSCP
The higher prevalence of long COVID in women younger than 50 years6 supports the overlap that studies have shown between symptoms of long COVID and perimenopause,7 as the median age of natural menopause is 51 years. Thus, health care providers need to differentiate between long COVID and other conditions, such as perimenopause, which share similar symptoms (FIGURE). Perimenopause might be diagnosed as long COVID, or the 2 might affect each other.

Symptoms of long COVID include fatigue, brain fog, and increased heart rate after recovering from COVID-19 and may continue or increase after an initial infection.8 Common symptoms of perimenopause and menopause, which also could be seen with long COVID, include typical menopausal symptoms such as hot flashes, night sweats, or disrupted sleep; changes in mood including dysthymia, depression, anxiety, or emotional lability; cognitive concerns such as brain fog or decreased concentration; and decreased stamina, fatigue, joint and muscle pains, or more frequent headaches. Therefore, women in their 40s or 50s with persistent symptoms after having COVID-19 without an alternative diagnosis, and who present with menstrual irregularity,9hot flashes, or night sweats, could be having an exacerbation of perimenopausal symptoms, or they could be experiencing a combination of long COVID and perimenopausal symptoms.
- Consider long COVID, versus perimenopause, or both, in women aged younger than 50 years
- Estradiol, which has been shown to alleviate perimenopausal and menopausal symptoms, also has been shown to have beneficial effects during acute COVID-19 infection
- Hormone therapy could improve symptoms of perimenopause and long COVID if some of the symptoms are due to changes in ovary function
Continue to: Potential pathophysiology...
Potential pathophysiology
Inflammation is likely to be critical in the pathogenesis of postacute sequelae of SARS-CoV-2 infection, or PASC. Individuals with long COVID have elevated inflammatory markers for several months.10 The chronic inflammation associated with long COVID could cause disturbances in the ovary and ovarian hormone production.2,10,11
During perimenopause, the ovary is more sensitive to illnesses such as COVID-19and to stress. The current theory is that COVID-19 affects the ovary with declines in ovarian reserve and ovarian function7 and with potential disruptions to the menstrual cycle, gonadal function, and ovarian sufficiency that lead to issues with menopause or fertility, as well as symptom exacerbation around menstruation.12 Another theory is that SARS-CoV-2 infection affects ovary hormone production, as there is an abundance of angiotensin-converting enzyme-2 receptors on ovarian and endometrial tissue.11 Thus, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely or lengthen the duration of perimenopausal symptoms.
Perimenopause is the transitional period prior to menopause, when the ovaries gradually produce fewer hormones and is associated with erratic hormonal fluctuations. The length of this transitional period varies from 4 to 10 years. Ethnic variations in the duration of hot flashes have been found, noting that Black and Hispanic women have them for an average of 8 to 10 years (longer), White women for an average of 7 years, and Asian, Japanese, and Chinese women for an average of 5 to 6 years (shorter).17
What should health care providers ask?
Distinguishing perimenopause from long COVID. It is important to try to differentiate between perimenopause and long COVID, and it is possible to have both, with long COVID exacerbating the menopausal symptoms.7,8 Health care providers should be alert to consider perimenopause if women present with shorter or longer cycles (21-40 days), missed periods (particularly 60 days or 2 months), or worsening perimenopausal mood, migraines, insomnia, or hot flashes. Clinicians should actively enquire about all of these symptoms.
Moreover, if a perimenopausal woman reports acutely worsening symptoms after COVID-19, health care providers should address the perimenopausal symptoms and determine whether hormone therapy is appropriate and could improve their symptoms. Women do not need to wait until they go 1 year without a period to be treated with hormone therapy to improve perimenopausal and menopausal symptoms. If women with long COVID have perimenopause or menopause symptoms, they should have access to evidence-based information and discuss menopausal hormone therapy if appropriate. Hormone therapy could improve both perimenopausal symptoms and the long COVID symptoms if some of the symptoms are due to changes in ovary function. Health care providers could consider progesterone or antidepressants during the second half of the cycle (luteal phase) or estrogen combined with progesterone for the entire cycle.18
For health care providers working in long COVID clinics, in addition to asking when symptoms started, what makes symptoms worse, the frequency of symptoms, and which activities are affected, ask about perimenopausal and menopausal symptoms. If a woman has irregular periods, sleep disturbances, fatigue, or mood changes, consider that these could be related to long COVID, perimenopause, or both.8,18 Be able to offer treatment or refer patients to a women’s health specialist who can assess and offer treatment.
A role for vitamin D? A recent retrospective case-matched study found that 6 months after hospital discharge, patients with long COVID had lower levels of 25(OH) vitamin D with the most notable symptom being brain fog.19 Thus, there may be a role for vitamin D supplementation as a preventive strategy in those being discharged after hospitalization. Vitamin D levels and supplementation have not been otherwise evaluated to date.
Lifestyle strategies for women with perimenopause and long COVID
Lifestyle strategies should be encouraged for women during perimenopause and long COVID. This includes good nutrition (avoiding carbs and sweets, particularly before menses), getting at least 7 hours of sleep and using sleep hygiene (regular bedtimes, sleep regimen, no late screens), getting regular exercise 5 days per week, reducing stress, avoiding excess alcohol, and not smoking. All of these factors can help women and their ovarian function during this period of ovarian fluctuations.
The timing of menopause and COVID may coincide with midlife stressors, including relationship issues (separations or divorce), health issues for the individual or their partner, widowhood, parenting challenges (care of young children, struggles with adolescents, grown children returning home), being childless, concerns about aging parents and caregiving responsibilities, as well as midlife career, community, or education issues—all of which make both long COVID and perimenopause more challenging to navigate.
Need for research
There is a need for future research to understand the epidemiologic basis and underlying biological mechanisms of sex differences seen in women with long COVID. Studying the effects of COVID-19 on ovarian function could lead to a better understanding of perimenopause, what causes ovarian failure to speed up, and possibly ways to slow it down8 since there are health risks of early menopause.16
References
- Fernández-de-Las-Peñas C, Palacios-Ceña D, GómezMayordomo V, et al. Defining post-COVID symptoms (postacute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18:2621. doi: 10.3390/ijerph18052621
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. doi: 10.1038/s41591 -021-01283-z
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022-00846-2
- Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. J Clin Med. 2022;11:1541. doi: 10.3390 /jcm11061541
- Notarte KI, de Oliveira MHS, Peligro PJ, et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: a systematic review and meta-analysis. J Clin Med. 2022;11:7314. doi: 10.3390 /jcm11247314
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 2021;8:100186. doi: 10.1016/j.lanepe.2021.100186
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. doi: 10.1016 /j.lanepe.2021.100242
- Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260-267. doi: 10.1016 /j.rbmo.2020.09.020
- Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210216. doi: 10.1038/s41590-021-01113-x
- Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691-700. doi: 10.1093/ije/dyab239
- Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. doi: 10.3389/fmed.2021.635255
- Huang B, Cai Y, Li N, et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2
- Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi:10.1210/endocr/bqaa127
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi:10.1001 /jamainternmed.2014.8063
- Newson L, Lewis R, O’Hara M. Long COVID and menopause - the important role of hormones in long COVID must be considered. Maturitas. 2021;152:74. doi: 10.1016 /j.maturitas.2021.08.026
- di Filippo L, Frara S, Nannipieri F, et al. Low Vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. 2023;108:e1106-e1116. doi: 10.1210/clinem/dgad207
Continue to: Chronic fatigue syndrome...
Chronic fatigue syndrome
Chronic fatigue syndrome: A large number of patients have “post-COVID conditions” affecting everyday function, including depression/anxiety, insomnia, and chronic fatigue (with a 3:1 female predominance)
Alexandra Kadl, MD
After 3 years battling acute COVID-19 infections, we encounter now a large number of patients with PASC— also known as “long COVID,” “COVID long-hauler syndrome,” and “post-COVID conditions”—a persistent multisystem syndrome that impacts everyday function.1 As of October 2023, there are more than 100 million COVID-19 survivors reported in the United States; 10% to 85% of COVID survivors2-4 may show lingering, life-altering symptoms after recovery. Common reported symptoms include fatigue, depression/ anxiety, insomnia, and brain fog/difficulty concentrating, which are particularly high in women who often had experienced only mild acute COVID-19 disease and were not even hospitalized. More recently, chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as major component of PASC5 with a 3:1 female predominance.6 Up to 75% of patients with this diagnosis are not able to maintain their jobs and normal life, and up to 25% are so disabled that they are bedbound.6
Diagnosis
Although illnesses resembling CFS have been reported for more than 200 years,7 the diagnosis of CFS/ME remains difficult to make. There is a likely underreporting due to fear of being labeled as malingering when reaching out to health care providers, and there is a reporting bias toward higher socioeconomic groups due to better access to health care. The current criteria for the diagnosis of CFS/ME include the following 3 components8:
- substantial impairment in the ability to function for more than 6 months, accompanied by profound fatigue, not alleviated by rest
- post-exertional malaise (PEM; prolonged, disabling exacerbation of the patient’s baseline symptoms after exercise)
- non-refreshing sleep, PLUS either cognitive impairment or orthostatic intolerance.
Pathophysiology
Originally found to evolve in a small patient population with Epstein-Barr virus infection and Lyme disease, CFS/ME has moved to centerstage after the COVID-19 pandemic. While the diagnosis of COVID-19–related CFS/ME has advanced in the field, a clear mechanistic explanation of why it occurs is still missing. Certain risk factors have been identified for the development of CFS/ME, including female sex, reactivation of herpesviruses, and presence of connective tissue disorders; however, about one-third of patients with CFS/ME do not have identifiable risk factors.9,10 Persistence of viral particles11 and prolonged inflammatory states are speculated to affect the nervous system and mitochondrial function and metabolism. Interestingly, there is no correlation between severity of initial COVID-19 illness and the development of CFS/ME, similar to observations in non–COVID-19–related CFS/ME.
Proposed therapy
There is currently no proven therapy for CFS/ME. At this time, several immunomodulatory, antiviral, and neuromodulator drugs are being tested in clinical trial networks around the world.12 Usual physical therapy with near maximum intensity has been shown to exacerbate symptoms and often results in PEM, which is described as a “crash” or “full collapse” by patients. The time for recovery after such episodes can be several days.13
Instead, the focus should be on addressing “treatable” concomitant symptoms, such as sleep disorders, anxiety and depression, and chronic pain. Lifestyle changes, avoidance of triggers, and exercise without over exertion are currently recommended to avoid incapacitating PEM.
Gaps in knowledge
There is a large knowledge gap regarding the pathophysiology, prevention, and therapy for CFS/ME. Many health care practitioners are not familiar with the disease and have focused on measurable parameters of exercise limitations and fatigue, such as anemias and lung and cardiac impairments, thus treating CFS/ME as a form of deconditioning. Given the large number of patients who recovered from acute COVID-19 that are now disabled due to CFS/ME, a patient-centered research opportunity has arisen. Biomedical/mechanistic research is ongoing, and well-designed clinical trials evaluating pharmacologic intervention as well as tailored exercise programs are needed.
Conclusion
General practitioners and women’s health specialists need to be aware of CFS/ME, especially when managing patients with long COVID. They also need to know that typical physical therapy may worsen symptoms. Furthermore, clinicians should shy away from trial drugs with a theoretical benefit outside of a clinical trial. ●
- Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) has been recognized as a major component of PASC
- Typical physical therapy has been shown to exacerbate symptoms of CFS/ME
- Treatment should focus on addressing “treatable” concomitant symptoms, lifestyle changes, avoidance of triggers, and exercise without over exertion
References
- Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. doi: 10.1016 /S1473-3099(21)00703-9
- Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593-1607. doi: 10.1093/infdis/jiac136
- Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-146. doi: 10.1038/s41579-022 -00846-2
- Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575-581. doi: 10.1016/j.arcmed.2021.03.010
- Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:28612878. doi: 10.1016/j.mayocp.2021.07.004
- Wessely S. History of postviral fatigue syndrome. Br Med Bull. 1991;47:919-941. doi: 10.1093/oxfordjournals.bmb.a072521
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. doi: 10.17226/19012
- Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93135. doi: 10.1016/j.bbi.2021.12.020
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
- Hanson MR. The viral origin of myalgic encephalomyelitis/ chronic fatigue syndrome. PLoS Pathog. 2023;19:e1011523. doi: 10.1371/journal.ppat.1011523
- Scheibenbogen C, Bellmann-Strobl JT, Heindrich C, et al. Fighting post-COVID and ME/CFS—development of curative therapies. Front Med (Lausanne). 2023;10:1194754. doi: 10.3389/fmed.2023.1194754
- Stussman B, Williams A, Snow J, et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025
Time to rethink endometrial ablation: A gyn oncology perspective on the sequelae of an overused procedure
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.
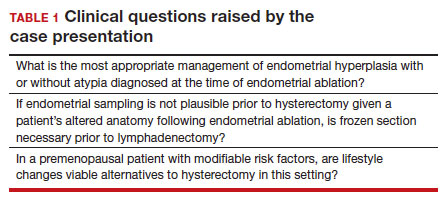
Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.
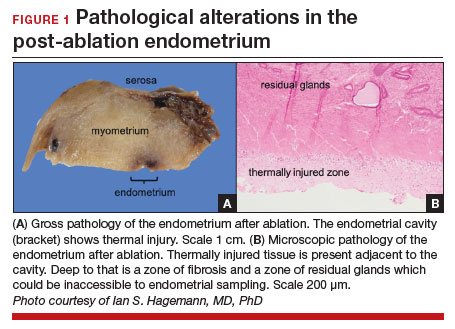
Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.
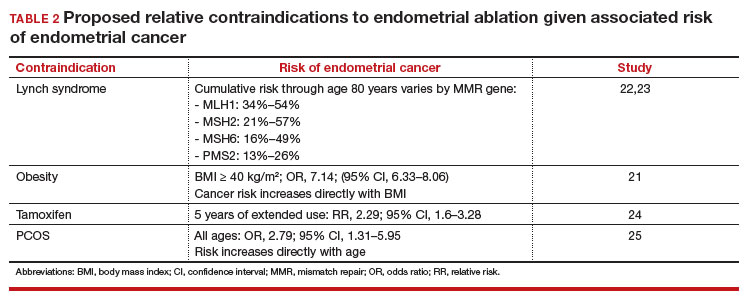
Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.
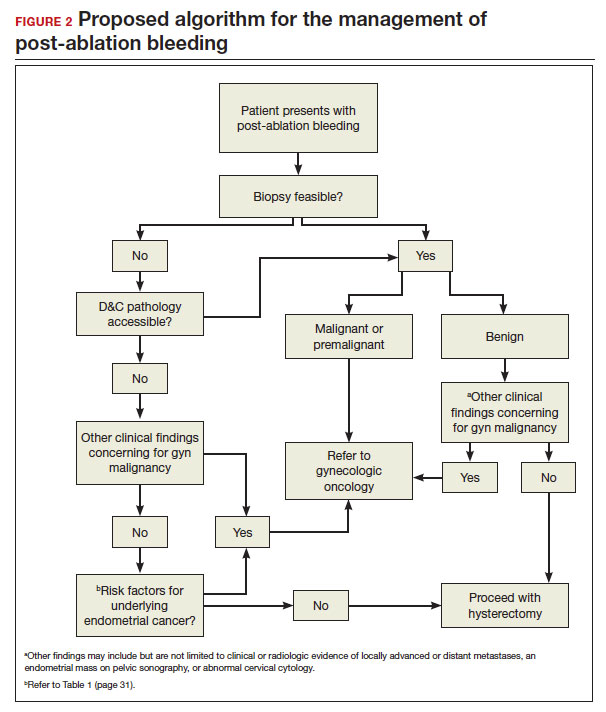
CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
CASE New patient presents with a history of endometrial hyperplasia
A 51-year-old patient (G2P2002) presents to a new gynecologist’s office after moving from a different state. In her medical history, the gynecologist notes that 5 years ago she underwent dilation and curettage and endometrial ablation procedures for heavy menstrual bleeding (HMB). Ultrasonography performed prior to those procedures showed a slightly enlarged uterus, a simple left ovarian cyst, and a non ̶ visualized right ovary. The patient had declined a 2-step procedure due to concerns with anesthesia, and surgical pathology at the time of ablation revealed hyperplasia without atypia. The patient’s medical history was otherwise notable for prediabetes (recent hemoglobin A1c [HbA1c] measurement, 6.0%) and obesity (body mass index, 43 kg/m2). Pertinent family history included her mother’s diagnosis of endometrial cancer at age 36. Given the patient’s diagnosis of endometrial hyperplasia, she was referred to gynecologic oncology, but she ultimately declined hysterectomy, stating that she was happy with the resolution of her abnormal bleeding. At the time of her initial gynecologic oncology consultation, the consultant suggested lifestyle changes to combat prediabetes and obesity to reduce the risk of endometrial cancer, as future signs of cancer, namely bleeding, may be masked by the endometrial ablation. The patient was prescribed metformin given these medical comorbidities.
At today’s appointment, the patient notes continued resolution of bleeding since the procedure. She does, however, note a 6-month history of vasomotor symptoms and one episode of spotting 3 months ago. Three years ago she was diagnosed with type 2 diabetes mellitus, and her current HbA1c is 6.9%. She has gained 10 lb since being diagnosed with endometrial cancer 5 years ago, and she has continued to take metformin.
An in-office endometrial biopsy is unsuccessful due to cervical stenosis. The treating gynecologist orders a transvaginal ultrasound, which reveals a small left ovarian cyst and a thickened endometrium (measuring 10 mm). Concerned that these findings could represent endometrial cancer, the gynecologist refers the patient to gynecologic oncology for further evaluation.
Sequelae and complications following endometrial ablation are often managed by a gynecologic oncologist. Indeed, a 2018 poll of Society of Gynecologic Oncology (SGO) members revealed that 93.8% of respondents had received such a referral, and almost 20% of respondents were managing more than 20 patients with post-ablation complications in their practices.1 These complications, including hematometra, post-ablation tubal sterilization syndrome, other pain syndromes associated with retrograde menstruation, and thickened endometrium with scarring leading to an inability to sample the endometrium to investigate post-ablation bleeding are symptoms and findings that often lead to further surgery, including hysterectomy.2 General gynecologists faced with these complications may refer patients to gynecologic oncology given an inability to sample the post-ablation endometrium or anticipated difficulties with hysterectomy. A recent meta-analysis revealed a 12.4% hysterectomy rate 5 years after endometrial ablation. Among these patients, the incidence of endometrial cancer ranged from 0% to 1.6%.3
In 2023, endometrial cancer incidence continues to increase, as does the incidence of obesity in women of all ages. Endometrial cancer mortality rates are also increasing, and these trends disproportionately affects non-Hispanic Black women.4 As providers and advocates work to narrow these disparities, gynecologic oncologists are simultaneously noting increased referrals for very likely benign conditions.5 Patients referred for post-ablation bleeding are a subset of these, as most patients who undergo endometrial ablation will not develop cancer. Considering the potential bottlenecks created en route to a gynecologic oncology evaluation, it seems prudent to minimize practices, like endometrial ablation, that may directly or indirectly prevent timely referral of patients with cancer to a gynecologic oncologist.
In this review we focus on the current use of endometrial ablation, associated complications, the incidence of treatment failure, and patient selection. Considering these issues in the context of the current endometrial cancer landscape, we posit best practices aimed at optimizing patient outcomes, and empowering general gynecologists to practice cancer prevention and to triage their surgical patients.
- Before performing endometrial ablation, consider whether alternatives such as hysterectomy or insertion of a progestin-containing IUD would be appropriate.
- Clinical management of patients with abnormal bleeding with indications for endometrial ablation should be guidelinedriven.
- Post-ablation bleeding or pain does not inherently require referral to oncology.
- General gynecologists can perform hysterectomy in this setting if appropriate.
- Patients with endometrial hyperplasia at endometrial ablation should be promptly offered hysterectomy. If atypia is not present, this hysterectomy, too, can be performed by a general gynecologist if appropriate, as the chance for malignancy is minimal.
Continue to: Current use of endometrial ablation in the US...
Current use of endometrial ablation in the US
In 2015, more than 500,000 endometrial ablations were performed in the United States.Given the ability to perform in-office ablation, this number is growing and potentially underestimated each year.6 In 2022, the global endometrial ablation market was valued at $3.4 billion, a figure projected to double in 10 years.7 The procedure has evolved as different devices and approaches have developed, offering patients different means to manage bleeding without hysterectomy. The minimally invasive procedure, performed in premenopausal patients with heavy menstrual bleeding (HMB) due to benign causes who have completed childbearing, has been associated with faster recovery times and fewer short-term complications compared with more invasive surgery.8 There are several non-resectoscope ablative devices approved by the US Food and Drug Administration (FDA), and each work to destroy the endometrial lining via thermal or cryoablation. Endometrial ablation can be performed in premenopausal patients with HMB due to benign causes who have completed childbearing.
Recently, promotional literature has begun to report on so-called overuse of hysterectomy, despite decreasing overall hysterectomy rates. This reporting proposes and applies “appropriateness criteria,” accounting for the rate of preoperative counseling regarding alternatives to hysterectomy, as well as the rate of “unsupportive” final pathology.9 The adoption of endometrial ablation and increasing market value of such vendors suggest that this campaign is having its desired effect. From the oncology perspective, we are concerned the pendulum could swing too far away from hysterectomy, a procedure that definitively cures abnormal uterine bleeding, toward endometrial ablation without explicit acknowledgement of the trade-offs involved.
Endometrial ablation complications: Late-onset procedure failure
A number of post-ablation syndromes may present at least 1 month following the procedure. Collectively known as late-onset endometrial ablation failure (LOEAF), these syndromes are characterized by recurrent vaginal bleeding, and/or new cyclic pelvic pain.10 It is difficult to measure the true incidence of LOEAF. Thomassee and colleagues examined a Canadian retrospective cohort of 437 patients who underwent endometrial ablation; 20.8% reported post-ablation pelvic pain after a median 301 days.11 The subsequent need for surgical intervention, often hysterectomy, is a surrogate for LOEAF.
It should be noted that LOEAF is distinct from post-ablation tubal sterilization syndrome (PATSS), which describes cornual menstrual bleeding impeded by the ligated proximal fallopian tube.12 Increased awareness of PATSS, along with the discontinuation of Essure (a permanent hysteroscopic sterilization device) in 2018, has led some surgeons to advocate for concomitant salpingectomy at the time of endometrial ablation.13 The role of opportunistic salpingectomy in primary prevention of epithelial ovarian cancer is well described, and while we strongly support this practice at the time of endometrial ablation, we do not feel that it effectively prevents LOEAF.14
The post-ablation inability to adequately sample the endometrium is also considered a LOEAF. A prospective study of 57 women who underwent endometrial ablation assessed post-ablation sampling feasibility via transvaginal ultrasonography, saline infusion sonohysterography (SIS), and in-office endometrial biopsies. In 23% of the cohort, endometrial sampling failed, and the authors noted decreased reliability of pathologic assessment.15 One systematic review, in which authors examined the incidence of endometrial cancer following endometrial ablation, characterized 38 cases of endometrial cancer and reported a post-ablation endometrial sampling success rate of 89%. This figure was based on a self-selected sample of 18 patients; cases in which endometrial sampling was thought to be impossible were excluded. The study also had a 30% missing data rate and several other biases.16
In the previously mentioned poll of SGO members,1 84% of the surveyed gynecologic oncologists managing post-ablation patients reported that endometrial sampling following endometrial ablation was “moderately” or “extremely” difficult. More than half of the survey respondents believed that hysterectomy was required for accurate diagnosis.1 While we acknowledge the likely sampling bias affecting the survey results, we are not comforted by any data that minimizes this diagnostic challenge.
Appropriate patient selection and contraindications
The ideal candidate for endometrial ablation is a premenopausal patient with HMB who does not desire future fertility. According to the FDA, absolute contraindications include pregnancy or desired fertility, prior ablation, current IUD in place, inadequate preoperative endometrial assessment, known or suspected malignancy, active infection, or unfavorable anatomy.17
What about patients who may be at increased risk for endometrial cancer?
There is a paucity of data regarding the safety of endometrial ablation in patients at increased risk for developing endometrial cancer in the future. The American College of Obstetricians and Gynecologists (ACOG) 2007 practice bulletin on endometrial ablation (no longer accessible online) alludes to this concern and other contraindications,18 but there are no established guidelines. Currently, no ACOG practice bulletin or committee opinion lists relative contraindications to endometrial ablation, long-term complications (except risks associated with future pregnancy), or risk of subsequent hysterectomy. The risk that “it may be harder to detect endometrial cancer after ablation” is noted on ACOG’s web page dedicated to frequently asked questions (FAQs) regarding abnormal uterine bleeding.19 It is not mentioned on their web page dedicated to the FAQs regarding endometrial ablation.20
In the absence of high-quality published data on established contraindications for endometrial ablation, we advocate for the increased awareness of possible relative contraindications—namely well-established risk factors for endometrial cancer (TABLE 1).For example, in a pooled analysis of 24 epidemiologic studies, authors found that the odds of developing endometrial cancer was 7 times higher among patients with a body mass index (BMI) ≥ 40 kg/m2, compared with controls (odds ratio [OR], 7.14; 95% confidence interval [CI], 6.33–8.06).21 Additionally, patients with Lynch syndrome, a history of extended tamoxifen use, or those with a history of chronic anovulation or polycystic ovary syndrome are at increased risk for endometrial cancer.22-24 If the presence of one or more of these factors does not dissuade general gynecologists from performing an endometrial ablation (even armed with a negative preoperative endometrial biopsy), we feel they should at least prompt thoughtful guideline-driven pause.

Continue to: Hysterectomy—A disincentivized option...
Hysterectomy—A disincentivized option
The annual number of hysterectomies performed by general gynecologists has declined over time. One study by Cadish and colleagues revealed that recent residency graduates performed only 3 to 4 annually.25 These numbers partly reflect the decreasing number of hysterectomies performed during residency training. Furthermore, other factors—including the increasing rate of placenta accreta spectrum, the focus on risk stratification of adnexal masses via the ovarian-adnexal reporting and data classification system (O-RADs), and the emphasis on minimally invasive approaches often acquired in subspecialty training—have likely contributed to referral patterns to such specialists as minimally invasive gynecologic surgeons and gynecologic oncologists.26 This trend is self-actualizing, as quality metrics funnel patients to high-volume surgeons, and general gynecologists risk losing hysterectomy privileges.
These factors lend themselves to a growing emphasis on endometrial ablation. Endometrial ablations can be performed in several settings, including in the hospital, in outpatient clinics, and more and more commonly, in ambulatory surgery centers. This increased access to endometrial ablation in the ambulatory surgery setting has corresponded with an annual endometrial ablation market value growth rate of 5% to 7%.27 These rates are likely compounded by payer reimbursement policies that promote endometrial ablation and other alternatives to hysterectomy that are cost savings in the short term.28 While the actual payer models are unavailable to review, they may not consider the costs of LOEAFs, including subsequent hysterectomy up to 5 years after initial ablation procedures. Provocatively, they almost certainly do not consider the costs of delayed care of patients with endometrial cancer vying for gynecologic oncology appointment slots occupied by post-ablation patients.
We urge providers, patients, and advocates to question who benefits from the uptake of ablation procedures: Patients? Payors? Providers? And how will the field of gynecology fare if hysterectomy skills and privileges are supplanted by ablation?
Post-ablation bleeding: Management by the gyn oncologist
Patients with post-ablation bleeding, either immediately or years later, are sometimes referred to a gynecologic oncologist given the possible risk for cancer and need for surgical staging if cancer is found on the hysterectomy specimen. In practice, assuming normal preoperative ultrasonography and no other clinical or radiologic findings suggestive of malignancy (eg, computed tomography findings concerning for metastases, abnormal cervical cytology, etc.), the presence of cancer is extremely unlikely to be determined at the time of surgery. Frozen section is not generally performed on the endometrium; intraoperative evaluation of even the unablated endometrium is notoriously unreliable; and histologic assessment of the ablated endometrium is limited by artifact (FIGURE 1). The abnormalities caused by ablation further impede selection of a representative focus, obfuscating any actionable result.

Some surgeons routinely bivalve the excised uterus prior to fixation to assess presence of tumor, tumor size, and the degree of myometrial invasion.29 A combination of factors may compel surgeons to perform lymphadenectomy if not already performed, or if sentinel lymph node mapping was unsuccessful. But this practice has not been studied in patients with post-ablation bleeding, and applying these principles relies on a preoperative diagnosis establishing the presence and grade of a cancer. Furthermore, the utility of frozen section and myometrial assessment to decide whether or not to proceed with lymphadenectomy is less relevant in the era of molecular classification guiding adjuvant therapy. In summary, assuming no pathologic or radiologic findings suggestive of cancer, gynecologic oncologists are unlikely to perform lymphadenectomy at the time of hysterectomy in these post-ablation cases, which therefore can safely be performed by general gynecologists.

Our recommendations
Consider the LNG-IUD as an alternative to ablation. A recent randomized controlled trial by Beelen and colleagues compared the effectiveness of LNG-releasing IUDs with endometrial ablation in patients with HMB. While the LNG-IUD was inferior to endometrial ablation, quality-of-life measures were similar up to 2 years.31 Realizing that the hysterectomy rate following endometrial ablation increases significantly beyond that time point (2 years), this narrative may be incomplete. A 5- to 10-year follow-up time-frame may be a more helpful gauge of long-term outcomes. This prolonged time-frame also may allow study of the LNG-IUD’s protective effects on the endometrium in the prevention of endometrial hyperplasia and cancer.
Consider hysterectomy. A 2021 Cochrane review revealed that, compared with endometrial ablation, minimally invasive hysterectomy is associated with higher quality-of-life metrics, higher self-reported patient satisfaction, and similar rates of adverse events.32 While patient autonomy is paramount, the developing step-wise approach from endometrial ablation to hysterectomy, and its potential effects on the health care system at a time when endometrial cancer incidence and mortality rates are rising, is troubling.
Postablation, consider hysterectomy by the general gynecologist. Current trends appear to disincentivize general gynecologists from performing hysterectomy either for HMB or LOEAF. We would offer reassurance that they can safely perform this procedure. Referral to oncology may not be necessary since, in the absence of an established diagnosis of cancer, a lymphadenectomy is not typically required. A shift away from referral for these patients can preserve access to oncology for those women, especially minority women, with an explicit need for oncologic care.
In FIGURE 2, we propose a management algorithm for the patient who presents with post–ablation bleeding. We acknowledge that the evidence base for our management recommendations is limited. Still, we hope providers, ACOG, and other guidelines-issuing organizations consider them as they adapt their own practices and recommendations. We believe this is one of many steps needed to improve outcomes for patients with gynecologic cancer, particularly those in marginalized communities disproportionately impacted by current trends.

CASE Resolution
After reviewing the relevant documentation and examining the patient, the gynecologic oncology consultant contacts the referring gynecologist. They review the low utility of frozen section and the overall low risk of cancer on the final hysterectomy specimen if the patient were to undergo hysterectomy. The consultant clarifies that there is no other concern for surgical complexity beyond the skill of the referring provider, and they discuss the possibility of referral to a minimally invasive specialist for the surgery.
Ultimately, the patient undergoes uncomplicated laparoscopic hysterectomy performed by the original referring gynecologist. Final pathology reveals inactive endometrium with ablative changes and cornual focus of endometrial hyperplasia without atypia. ●
Acknowledgement
The authors acknowledge Ian Hagemann, MD, PhD, for his review of the manuscript.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.
- Chen H, Saiz AM, McCausland AM, et al. Experience of gynecologic oncologists regarding endometrial cancer after endometrial ablation. J Clin Oncol. 2018;36:e17566-e.
- McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399-406.
- Oderkerk TJ, Beelen P, Bukkems ALA, et al. Risk of hysterectomy after endometrial ablation: a systematic review and meta-analysis. Obstet Gynecol. 2023;142:51-60.
- Clarke MA, Devesa SS, Hammer A, et al. Racial and ethnic differences in hysterectomy-corrected uterine corpus cancer mortality by stage and histologic subtype. JAMA Oncol. 2022;8:895-903.
- Barber EL, Rossi EC, Alexander A, et al. Benign hysterectomy performed by gynecologic oncologists: is selection bias altering our ability to measure surgical quality? Gynecol Oncol. 2018;151:141-144.
- Wortman M. Late-onset endometrial ablation failure. Case Rep Womens Health. 2017;15:11-28.
- Insights FM. Endometrial Ablation Market Outlook.Accessed July 26, 2023. https://www.futuremarketinsights.com/reports/endometrial-ablation -market
- Famuyide A. Endometrial ablation. J Minim Invasive Gynecol. 2018;25:299-307.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7.
- Wortman M, Cholkeri A, McCausland AM, et al. Late-onset endometrial ablation failure—etiology, treatment, and prevention. J Minim Invasive Gynecol. 2015;22:323-331.
- Thomassee MS, Curlin H, Yunker A, et al. Predicting pelvic pain after endometrial ablation: which preoperative patient characteristics are associated? J Minim Invasive Gynecol. 2013;20:642-647.
- Townsend DE, McCausland V, McCausland A, et al. Post-ablation-tubal sterilization syndrome. Obstet Gynecol. 1993;82:422-424.
- Greer Polite F, DeAgostino-Kelly M, Marchand GJ. Combination of laparoscopic salpingectomy and endometrial ablation: a potentially underused procedure. J Gynecol Surg. 2021;37:89-91.
- Hanley GE, Pearce CL, Talhouk A, et al. Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Network Open. 2022;5:e2147343-e.
- Ahonkallio SJ, Liakka AK, Martikainen HK, et al. Feasibility of endometrial assessment after thermal ablation. Eur J Obstet Gynecol Reprod Biol. 2009;147:69-71.
- Tamara JO, Mileen RDvdK, Karlijn MCC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555.
- US Food and Drug Administration. Endometrial ablation for heavy menstrual bleeding.Accessed July 26, 2023. https://www.fda.gov/medical-devices /surgery-devices/endometrial-ablation-heavy-menstrual-bleeding
- ACOG Practice Bulletin. Clinical management guidelines for obstetriciangynecologists. Number 81, May 2007. Obstet Gynecol. 2007;109:1233-1248.
- The American College of Obstetricians and Gynecologists. Abnormal uterine bleeding frequently asked questions. Accessed July 26, 2023. https://www.acog .org/womens-health/faqs/abnormal-uterine-bleeding
- The American College of Obstetricians and Gynecologists. Endometrial ablation frequently asked questions. Accessed November 28, 2023. https://www.acog. org/womens-health/faqs/endometrial-ablation#:~:text=Can%20I%20still%20 get%20pregnant,should%20not%20have%20this%20procedure
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31:2607-2618.
- National Comprehensive Cancer Network. Lynch Syndrome (Version 2.2023). Accessed November 15, 2023. https://www.nccn.org/professionals /physician_gls/pdf/genetics_colon.pdf
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305: 2304-2310.
- Fleming CA, Heneghan HM, O’Brien D, et al. Meta-analysis of the cumulative risk of endometrial malignancy and systematic review of endometrial surveillance in extended tamoxifen therapy. Br J Surg. 2018;105:1098-1106.
- Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748-758.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Urogynecology. 2021;27.
- Blank SV, Huh WK, Bell M, et al. Doubling down on the future of gynecologic oncology: the SGO future of the profession summit report. Gynecol Oncol. 2023;171:76-82.
- Reports MI. Global endometrial ablation market growth, trends and forecast 2023 to 2028 by types, by application, by regions and by key players like Boston Scientific, Hologic, Olympus, Minerva Surgical. Accessed July 30, 2023. https://www.marketinsightsreports.com/single-report/061612632440/global -endometrial-ablation-market-growth-trends-and-forecast-2023-to-2028-by -types-by-application-by-regions-and-by-key-players-like-boston-scientific -hologic-olympus-minerva-surgical
- London R, Holzman M, Rubin D, et al. Payer cost savings with endometrial ablation therapy. Am J Manag Care. 1999;5:889-897.
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-18.
- Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-e10.
- Bofill Rodriguez M, Lethaby A, Fergusson RJ. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2021;2:Cd000329.