User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
COVID-19 again the third-leading cause of U.S. deaths
the Centers for Disease Control and Prevention said April 22.
About 693,000 people died of heart disease in 2021, with 605,000 dying of cancer and 415,000 of COVID, the CDC said, citing provisional data that might be updated later.
Unintentional injuries were the fourth-leading cause of death, increasing to 219,000 in 2021 from 201,000 in 2020. Influenza and pneumonia dropped out of the top 10 leading causes of death and suicide moved into 10th place.
Overall, about 3,458,697 deaths were reported in the United States in 2021. The age-adjusted death rate was 841.6 deaths per 100,000 people, an increase of 0.7% from 2020. The 2021 death rate was the highest since 2003, the CDC said.
The overall number of COVID deaths in 2021 increased around 20% over 2020, when around 384,000 people died from the virus, the CDC said. COVID deaths in 2021 peaked for the weeks ending Jan. 16 and Sept. 11, following holiday periods.
The demographics of COVID mortality changed slightly, the CDC said in a second report.
Blacks accounted for 13.3% of COVID deaths in 2021 and Hispanics 16.5%, down several percentage points from 2020, the CDC said. Asians made up 3.1% of COVID deaths for 2021, a drop from 3.6% in 2020. White people accounted for 65.2% of COVID deaths in 2021, an increase from 59.6% in 2020.
Non-Hispanic American Indian/Alaskan Native and non-Hispanic Black or African American had the highest overall death rates for COVID, the CDC said.
Breaking the data down by age, the number of COVID deaths among people aged 75 years and older dropped to 178,000 in 2021 from around 207,000 in 2020. The numbers went up in other age groups. Among people aged 65-75, about 101,000 died of COVID in 2021, up from around 76,000 in 2020.
“The results of both studies highlight the need for greater effort to implement effective interventions,” the CDC said in a statement. “We must work to ensure equal treatment in all communities in proportion to their need for effective interventions that can prevent excess COVID-19 deaths.”
Since the pandemic began, about 991,000 people in the United States have died from COVID-related causes, the most among all nations in the world.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Prevention said April 22.
About 693,000 people died of heart disease in 2021, with 605,000 dying of cancer and 415,000 of COVID, the CDC said, citing provisional data that might be updated later.
Unintentional injuries were the fourth-leading cause of death, increasing to 219,000 in 2021 from 201,000 in 2020. Influenza and pneumonia dropped out of the top 10 leading causes of death and suicide moved into 10th place.
Overall, about 3,458,697 deaths were reported in the United States in 2021. The age-adjusted death rate was 841.6 deaths per 100,000 people, an increase of 0.7% from 2020. The 2021 death rate was the highest since 2003, the CDC said.
The overall number of COVID deaths in 2021 increased around 20% over 2020, when around 384,000 people died from the virus, the CDC said. COVID deaths in 2021 peaked for the weeks ending Jan. 16 and Sept. 11, following holiday periods.
The demographics of COVID mortality changed slightly, the CDC said in a second report.
Blacks accounted for 13.3% of COVID deaths in 2021 and Hispanics 16.5%, down several percentage points from 2020, the CDC said. Asians made up 3.1% of COVID deaths for 2021, a drop from 3.6% in 2020. White people accounted for 65.2% of COVID deaths in 2021, an increase from 59.6% in 2020.
Non-Hispanic American Indian/Alaskan Native and non-Hispanic Black or African American had the highest overall death rates for COVID, the CDC said.
Breaking the data down by age, the number of COVID deaths among people aged 75 years and older dropped to 178,000 in 2021 from around 207,000 in 2020. The numbers went up in other age groups. Among people aged 65-75, about 101,000 died of COVID in 2021, up from around 76,000 in 2020.
“The results of both studies highlight the need for greater effort to implement effective interventions,” the CDC said in a statement. “We must work to ensure equal treatment in all communities in proportion to their need for effective interventions that can prevent excess COVID-19 deaths.”
Since the pandemic began, about 991,000 people in the United States have died from COVID-related causes, the most among all nations in the world.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Prevention said April 22.
About 693,000 people died of heart disease in 2021, with 605,000 dying of cancer and 415,000 of COVID, the CDC said, citing provisional data that might be updated later.
Unintentional injuries were the fourth-leading cause of death, increasing to 219,000 in 2021 from 201,000 in 2020. Influenza and pneumonia dropped out of the top 10 leading causes of death and suicide moved into 10th place.
Overall, about 3,458,697 deaths were reported in the United States in 2021. The age-adjusted death rate was 841.6 deaths per 100,000 people, an increase of 0.7% from 2020. The 2021 death rate was the highest since 2003, the CDC said.
The overall number of COVID deaths in 2021 increased around 20% over 2020, when around 384,000 people died from the virus, the CDC said. COVID deaths in 2021 peaked for the weeks ending Jan. 16 and Sept. 11, following holiday periods.
The demographics of COVID mortality changed slightly, the CDC said in a second report.
Blacks accounted for 13.3% of COVID deaths in 2021 and Hispanics 16.5%, down several percentage points from 2020, the CDC said. Asians made up 3.1% of COVID deaths for 2021, a drop from 3.6% in 2020. White people accounted for 65.2% of COVID deaths in 2021, an increase from 59.6% in 2020.
Non-Hispanic American Indian/Alaskan Native and non-Hispanic Black or African American had the highest overall death rates for COVID, the CDC said.
Breaking the data down by age, the number of COVID deaths among people aged 75 years and older dropped to 178,000 in 2021 from around 207,000 in 2020. The numbers went up in other age groups. Among people aged 65-75, about 101,000 died of COVID in 2021, up from around 76,000 in 2020.
“The results of both studies highlight the need for greater effort to implement effective interventions,” the CDC said in a statement. “We must work to ensure equal treatment in all communities in proportion to their need for effective interventions that can prevent excess COVID-19 deaths.”
Since the pandemic began, about 991,000 people in the United States have died from COVID-related causes, the most among all nations in the world.
A version of this article first appeared on WebMD.com.
FROM THE MMWR
Three in four U.S. doctors are employed by hospitals, corporate entities: Report
Marcus Welby, MD, was a fictitious hometown doctor featured in a TV drama with the same name that was shown on ABC from 1969 to 1976. Played by actor Robert Young, Dr. Welby treated his patients through their bouts with breast cancer, impotence, and Alzheimer’s disease.
, according to a recent report sponsored by the Physicians Advocacy Institute and prepared by consulting firm Avalere Health.
“COVID-19 drove physicians to leave private practice for employment at an even more rapid pace than we’ve seen in recent years, and these trends continued to accelerate in 2021,” Kelly Kenney, chief executive officer of Physicians Advocacy Institute, said in an announcement. “This study underscores the fact that physicians across the nation are facing severe burnout and strain. The pressures of the pandemic forced many independent physicians to make difficult decisions to sell their practices, health insurers, or other corporate entities.”
Corporate entities are defined in the report as health insurers, private equity firms, and umbrella corporate entities that own multiple physician practices.
“The pandemic has been just brutal ... for nurses and physicians who are caring for patients,” Ms. Kenney told this news organization. “Between the financial stress that the pandemic certainly had on practices, because they certainly had little revenue for a while, and then also we know that the stress that physicians have felt mentally, you can’t overstate that.”
More than half of physician practices owned by hospitals, corporate entities
The Physicians Advocacy Institute has tracked changes in physician employment consistently since 2012, said Ms. Kenney. In 2012, 25% of physicians were employed; that has jumped to nearly 74%, which means the past decade has brought a world of change to the nation’s physicians.
“These are essentially small-business people ... and they were primarily trained to care for patients,” said Ms. Kenney, referring to physicians in independent practice. Still, she understands why physicians would seek employment in the face of “the crushing kind of pressure of having to deal with 20 different payers, pay overhead, and keep the lights on [at the practice].”
According to the report, 108,700 physicians left independent practice to enter employment with hospitals or other corporate entities in the 3-year period that ended in 2021. Seventy-six percent of that shift to employed status among physicians has occurred since the start of the COVID-19 pandemic in March 2020.
From a regional perspective, the report found continued growth among employed physicians across all U.S. regions in the last half of 2020. Hospital- or corporate-owned physician practices increased between 28% and 44%, while the percentage of hospital- or corporate-employed physicians increased between 13% and 24%.
Eighty percent of physicians in the Midwest are employed by hospitals or corporations, which leads the rest of the country, per the report. That’s followed by the Northeast, the West, and the South. Overall, the number of physicians working for such entities increased in all regions.
The report revealed that physician employment by corporations such as health insurers and venture capital firms grew from 92,400 in January 2019 to 142,900 in January 2022.
Hospitals and corporate entities acquired 36,200 physician practices (representing 38% growth) between 2019 and 2021, and the majority of these moves occurred since the pandemic’s start, according to the report.
Value-based care, venture capital firms driving change
Ms. Kenney pointed to value-based care as driving much of this activity by hospitals. “We all embrace [value-based payment], because we need to get a handle on cost, and we want better quality [but] those trends tend to favor integrated systems and systems that can handle a lot of risk and populations of patients.”
Still, the moves by private equity firms and health insurers in this space is relatively new, said Ms. Kenney, who added that her organization started tracking this trend 3 years ago. She pointed to a “marked acceleration” in the trend toward employing physicians and the sale of practices in the 18 months following the pandemic’s start; nonhospital corporate entities drove that steep increase, she said.
Ms. Kenney calls for further study and “guardrails” to respond to “that force in the health care system,” referring to the acquisition of practices by entities such as private equity firms. “Are these big [health care] systems going to continue to see patients in underserved areas, rural areas, and Medicaid patients if it doesn’t make sense financially to do so?
“That’s what we’re teeing up with this research,” added Ms. Kenney. “We are providing information that starts some conversations around what we might want to think about in terms of policies to ensure that we don’t impact patients’ access to care.”
The Physicians Advocacy Institute represents more than 170,000 physicians and medical students. Avalere Health used the IQVIA OneKey database for the report. The researchers studied the 3-year period from Jan. 1, 2019, to Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
Marcus Welby, MD, was a fictitious hometown doctor featured in a TV drama with the same name that was shown on ABC from 1969 to 1976. Played by actor Robert Young, Dr. Welby treated his patients through their bouts with breast cancer, impotence, and Alzheimer’s disease.
, according to a recent report sponsored by the Physicians Advocacy Institute and prepared by consulting firm Avalere Health.
“COVID-19 drove physicians to leave private practice for employment at an even more rapid pace than we’ve seen in recent years, and these trends continued to accelerate in 2021,” Kelly Kenney, chief executive officer of Physicians Advocacy Institute, said in an announcement. “This study underscores the fact that physicians across the nation are facing severe burnout and strain. The pressures of the pandemic forced many independent physicians to make difficult decisions to sell their practices, health insurers, or other corporate entities.”
Corporate entities are defined in the report as health insurers, private equity firms, and umbrella corporate entities that own multiple physician practices.
“The pandemic has been just brutal ... for nurses and physicians who are caring for patients,” Ms. Kenney told this news organization. “Between the financial stress that the pandemic certainly had on practices, because they certainly had little revenue for a while, and then also we know that the stress that physicians have felt mentally, you can’t overstate that.”
More than half of physician practices owned by hospitals, corporate entities
The Physicians Advocacy Institute has tracked changes in physician employment consistently since 2012, said Ms. Kenney. In 2012, 25% of physicians were employed; that has jumped to nearly 74%, which means the past decade has brought a world of change to the nation’s physicians.
“These are essentially small-business people ... and they were primarily trained to care for patients,” said Ms. Kenney, referring to physicians in independent practice. Still, she understands why physicians would seek employment in the face of “the crushing kind of pressure of having to deal with 20 different payers, pay overhead, and keep the lights on [at the practice].”
According to the report, 108,700 physicians left independent practice to enter employment with hospitals or other corporate entities in the 3-year period that ended in 2021. Seventy-six percent of that shift to employed status among physicians has occurred since the start of the COVID-19 pandemic in March 2020.
From a regional perspective, the report found continued growth among employed physicians across all U.S. regions in the last half of 2020. Hospital- or corporate-owned physician practices increased between 28% and 44%, while the percentage of hospital- or corporate-employed physicians increased between 13% and 24%.
Eighty percent of physicians in the Midwest are employed by hospitals or corporations, which leads the rest of the country, per the report. That’s followed by the Northeast, the West, and the South. Overall, the number of physicians working for such entities increased in all regions.
The report revealed that physician employment by corporations such as health insurers and venture capital firms grew from 92,400 in January 2019 to 142,900 in January 2022.
Hospitals and corporate entities acquired 36,200 physician practices (representing 38% growth) between 2019 and 2021, and the majority of these moves occurred since the pandemic’s start, according to the report.
Value-based care, venture capital firms driving change
Ms. Kenney pointed to value-based care as driving much of this activity by hospitals. “We all embrace [value-based payment], because we need to get a handle on cost, and we want better quality [but] those trends tend to favor integrated systems and systems that can handle a lot of risk and populations of patients.”
Still, the moves by private equity firms and health insurers in this space is relatively new, said Ms. Kenney, who added that her organization started tracking this trend 3 years ago. She pointed to a “marked acceleration” in the trend toward employing physicians and the sale of practices in the 18 months following the pandemic’s start; nonhospital corporate entities drove that steep increase, she said.
Ms. Kenney calls for further study and “guardrails” to respond to “that force in the health care system,” referring to the acquisition of practices by entities such as private equity firms. “Are these big [health care] systems going to continue to see patients in underserved areas, rural areas, and Medicaid patients if it doesn’t make sense financially to do so?
“That’s what we’re teeing up with this research,” added Ms. Kenney. “We are providing information that starts some conversations around what we might want to think about in terms of policies to ensure that we don’t impact patients’ access to care.”
The Physicians Advocacy Institute represents more than 170,000 physicians and medical students. Avalere Health used the IQVIA OneKey database for the report. The researchers studied the 3-year period from Jan. 1, 2019, to Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
Marcus Welby, MD, was a fictitious hometown doctor featured in a TV drama with the same name that was shown on ABC from 1969 to 1976. Played by actor Robert Young, Dr. Welby treated his patients through their bouts with breast cancer, impotence, and Alzheimer’s disease.
, according to a recent report sponsored by the Physicians Advocacy Institute and prepared by consulting firm Avalere Health.
“COVID-19 drove physicians to leave private practice for employment at an even more rapid pace than we’ve seen in recent years, and these trends continued to accelerate in 2021,” Kelly Kenney, chief executive officer of Physicians Advocacy Institute, said in an announcement. “This study underscores the fact that physicians across the nation are facing severe burnout and strain. The pressures of the pandemic forced many independent physicians to make difficult decisions to sell their practices, health insurers, or other corporate entities.”
Corporate entities are defined in the report as health insurers, private equity firms, and umbrella corporate entities that own multiple physician practices.
“The pandemic has been just brutal ... for nurses and physicians who are caring for patients,” Ms. Kenney told this news organization. “Between the financial stress that the pandemic certainly had on practices, because they certainly had little revenue for a while, and then also we know that the stress that physicians have felt mentally, you can’t overstate that.”
More than half of physician practices owned by hospitals, corporate entities
The Physicians Advocacy Institute has tracked changes in physician employment consistently since 2012, said Ms. Kenney. In 2012, 25% of physicians were employed; that has jumped to nearly 74%, which means the past decade has brought a world of change to the nation’s physicians.
“These are essentially small-business people ... and they were primarily trained to care for patients,” said Ms. Kenney, referring to physicians in independent practice. Still, she understands why physicians would seek employment in the face of “the crushing kind of pressure of having to deal with 20 different payers, pay overhead, and keep the lights on [at the practice].”
According to the report, 108,700 physicians left independent practice to enter employment with hospitals or other corporate entities in the 3-year period that ended in 2021. Seventy-six percent of that shift to employed status among physicians has occurred since the start of the COVID-19 pandemic in March 2020.
From a regional perspective, the report found continued growth among employed physicians across all U.S. regions in the last half of 2020. Hospital- or corporate-owned physician practices increased between 28% and 44%, while the percentage of hospital- or corporate-employed physicians increased between 13% and 24%.
Eighty percent of physicians in the Midwest are employed by hospitals or corporations, which leads the rest of the country, per the report. That’s followed by the Northeast, the West, and the South. Overall, the number of physicians working for such entities increased in all regions.
The report revealed that physician employment by corporations such as health insurers and venture capital firms grew from 92,400 in January 2019 to 142,900 in January 2022.
Hospitals and corporate entities acquired 36,200 physician practices (representing 38% growth) between 2019 and 2021, and the majority of these moves occurred since the pandemic’s start, according to the report.
Value-based care, venture capital firms driving change
Ms. Kenney pointed to value-based care as driving much of this activity by hospitals. “We all embrace [value-based payment], because we need to get a handle on cost, and we want better quality [but] those trends tend to favor integrated systems and systems that can handle a lot of risk and populations of patients.”
Still, the moves by private equity firms and health insurers in this space is relatively new, said Ms. Kenney, who added that her organization started tracking this trend 3 years ago. She pointed to a “marked acceleration” in the trend toward employing physicians and the sale of practices in the 18 months following the pandemic’s start; nonhospital corporate entities drove that steep increase, she said.
Ms. Kenney calls for further study and “guardrails” to respond to “that force in the health care system,” referring to the acquisition of practices by entities such as private equity firms. “Are these big [health care] systems going to continue to see patients in underserved areas, rural areas, and Medicaid patients if it doesn’t make sense financially to do so?
“That’s what we’re teeing up with this research,” added Ms. Kenney. “We are providing information that starts some conversations around what we might want to think about in terms of policies to ensure that we don’t impact patients’ access to care.”
The Physicians Advocacy Institute represents more than 170,000 physicians and medical students. Avalere Health used the IQVIA OneKey database for the report. The researchers studied the 3-year period from Jan. 1, 2019, to Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
Mediterranean diet linked to lower risk for preeclampsia
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Myocarditis higher with Moderna COVID vax in young men
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
One of the largest studies to date on myocarditis after COVID-19 vaccination confirms an increased risk with both the Pfizer and Moderna vaccines in young men and shows that the risk is higher with the Moderna than with the Pfizer vaccine.
The study also suggests for the first time that in young men 16 to 24 years of age, the risk for myocarditis after vaccination with either the Pfizer or Moderna vaccine is higher than the risk for myocarditis after COVID-19 infection.
The population-based study involved data on 23.1 million residents across four Scandinavian countries – Denmark, Finland, Norway, and Sweden – 74% of whom had received two vaccine doses and 7% of whom had received one dose.
By linking data from high-quality nationwide health registers on COVID-19 vaccination, infection rates, and myocarditis diagnoses, the researchers were able to evaluate the risk for myocarditis by vaccine product, vaccination dose number, sex, and age.
The study was published online in JAMA Cardiology.
The results confirm that the risk for myocarditis after COVID-19 mRNA vaccines is highest in young men 16 to 24 years of age after the second dose.
For men in this age group who received two doses of the same vaccine, data were compatible, with between four and seven excess myocarditis events in 28 days per 100,000 individuals after the second dose of the Pfizer vaccine, and between nine and 28 per 100,000 individuals after the second dose of the Moderna vaccine.
“This is one of the largest studies on this topic to date. The first population studies were in Israel, with 5 million individuals, and looked at just the Pfizer vaccine. We have data on 23 million people from Scandinavia that include both the Pfizer and Moderna vaccines,” senior author Rickard Ljung, MD, Swedish Medical Products Agency, told this news organization.
“We show a clearly higher risk of myocarditis after the Moderna vaccine than after the Pfizer vaccine. This has been suggested before, but our data confirm definitively that the Moderna vaccine has a higher risk of myocarditis than the Pfizer vaccine,” he added.
“In the group at highest risk of myocarditis after COVID vaccination – young men aged 16 to 24 – the Pfizer vaccine shows a five times higher risk of myocarditis versus the unvaccinated cohort, while the Moderna vaccine shows a 15 times higher risk,” Dr. Ljung noted.
After seeing these data, the Swedish regulatory authority is no longer recommending use of the Moderna vaccine for people younger than 30 years, Dr. Ljung said. Similar recommendations have been made in Norway and Finland.
The researchers report that their finding of a higher risk for myocarditis after the Moderna vaccine than after the Pfizer vaccine in young men is in line with data from the Canada, France, the United Kingdom, and the United States. But they point out that, compared with previous studies, the current study had the advantage of data analyzed according to a common protocol from four different countries and that showed similar directions of associations, despite considerable differences in previous COVID-19 infection levels and lockdown policies.
Risk higher with vaccination than infection?
For what is believed to be the first time, the Scandinavian data also suggest a higher risk for myocarditis after COVID-19 vaccination with both the Pfizer and Moderna vaccines than after COVID-19 infection in young men 16 to 24 years.
Although previous studies have shown that males in this age group have the highest risk for myocarditis after vaccination, it has always been suggested that the risk after vaccination is lower than the risk after infection. The Scandinavian data suggest otherwise for this age group.
Dr. Ljung explained that the myocarditis risk after COVID infection is very hard to study.
“It is highly dependent on the testing strategy,” he said. “For example, in the first half of 2020, the only people being tested were those admitted to hospital, so studies would have included the sickest patients and would therefore likely have found a higher rate of myocarditis. But this current Scandinavian dataset only included individuals with a positive COVID test after August 2020, reflecting a broader range of people.”
The researchers found an excess rate of myocarditis of 3.26 per 100,000 individuals within 28 days of a positive COVID-19 test among all males, and 1.37 per 100,000 individuals among males 16 to 24 years of age.
“We show that the risk of myocarditis after COVID infection is lower in younger people and higher in older people, but the opposite is true after COVID vaccination, where the risk of myocarditis is higher in younger people and lower in older people,” Dr. Ljung said.
The study was not able to look at severity of myocarditis but did record length of hospital stay, which was similar in patients who developed myocarditis after vaccination and those in the unvaccinated cohort (4 to 5 days). Deaths were rare, with no deaths in people younger than 40 years.
“I think we can say that in people aged over 40, the risk of myocarditis is greater with infection than with vaccination, but in those under 40, it is not so clear. And our data suggest that for young men aged 16 to 24 years, the risk of myocarditis after COVID vaccination with either the Pfizer or Moderna vaccine is higher than after COVID infection,” Dr. Ljung commented.
Although the Swedish regulatory agency has already stopped recommending use of Moderna vaccine in those younger than 30 years on the basis of these data, Dr. Ljung was reluctant to make any recommendations regarding the use of the Pfizer vaccine in young males, saying it was up to individual public-health agencies to makes these decisions.
But he pointed out that the current study only looked at myocarditis, and COVID infection can result in many other complications that can lead to hospitalization and death, which needs to be taken into account when assessing the risk and benefit of vaccination.
Dr. Ljung noted that the current data only applied to the first two doses of the vaccines; data after booster injections have not been included, although the researchers are looking at that now.
What to advise patients?
In an accompanying Editor’s Note, Ann Marie Navar, MD, University of Texas Southwestern Medical Center, Dallas, who is editor of JAMA Cardiology, and Robert Bonow, MD, Northwestern University Feinberg School of Medicine, Chicago, who is deputy editor of JAMA Cardiology, try to explain how these data can inform the way health care professionals communicate with their patients about vaccination.
They point out the “good news,” that older adults who are at highest risk for COVID-19 complications appear to be at extremely low risk for vaccine-associated myocarditis.
They note that for both men and women older than 40 years, the excess number of cases of myocarditis after vaccination was fewer than two in 100,000 vaccinees across all vaccines studied, and the death toll from COVID-19 in the United States as of March was more than 200 per 100,000 population.
“Given the high rates of morbidity and mortality from COVID-19 infection in older adults and the efficacy of the vaccine in preventing severe infection and death, the benefits of immunization in those older than 40 years clearly outweigh the risks,” the editors say.
But given these data in young men, they suggest that health care professionals consider recommending the Pfizer vaccine over the Moderna vaccine for certain populations, including young men and other individuals for whom concerns about myocarditis present a barrier to immunization.
The editors also point out that although the risk for myocarditis after COVID-19 immunization is real, this low risk must be considered in the context of the overall benefit of the vaccine.
“At the individual level, immunization prevents not only COVID-19-related myocarditis but also severe disease, hospitalization, long-term complications after COVID-19 infection, and death. At the population level, immunization helps to decrease community spread, decrease the chances of new variants emerging, protect people who are immunocompromised, and ensure our health care system can continue to provide for our communities,” they conclude.
Dr. Ljung reports grants from Sanofi Aventis paid to his institution outside the submitted work and personal fees from Pfizer outside the submitted work. Dr. Navar reports personal fees from Pfizer and AstraZeneca, outside the scope of this work.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
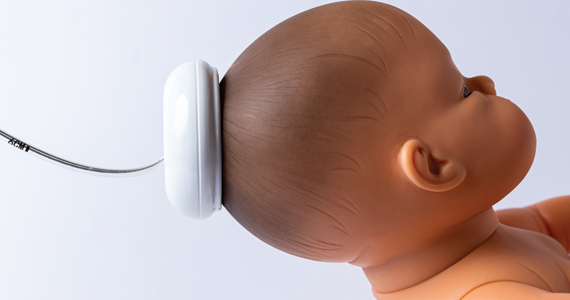
How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery?
Muraca GM, Boutin A, Razaz N, et al. Maternal and neonatal trauma following operative vaginal delivery. CMAJ. 2022;194:E1-E12. doi: 10.1503/cmaj.210841.
EXPERT COMMENTARY
Operative vaginal delivery is used to achieve and expedite safe vaginal birth while avoiding CD and its associated morbidities.1,2 Despite support from the American College of Obstetricians and Gynecologists (ACOG) for the use of OVD as an alternative to CD, OVD was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987.1,3 Reported complications of OVD are biased by the level of experience of the operator, changes in practice, and by misinterpretation of the counterfactual.1
Outcomes of OVD should be compared with appropriate reference groups, namely, with second-stage CD births rather than with spontaneous vaginal births.4 With decreasing rates of OVD, evidence of contemporary data is needed on appropriately compared perinatal outcomes.4
Details of the study
Muraca and colleagues conducted an observational cohort study of births in Canada between 2013 and 2019 to assess the incidence of maternal and neonatal trauma following OVD. They used composites defined a priori— stratified by instrument, region, level of obstetric care, and institutional OVD volume.
Results. Among 1,326,191 live or stillbirths, 2.9% were attempted forceps deliveries and 8.4% were attempted vacuum deliveries. Following forceps delivery, the maternal trauma rate was 25.3% (95% confidence interval [CI], 24.8%–25.7%), and the neonatal trauma rate was 9.6 per 1,000 live births (95% CI, 8.6–10.6). Following vacuum delivery, maternal and neonatal trauma rates were 13.2% (95% CI, 13.0%–13.4%) and 9.6 per 1,000 live births (95% CI, 9.0–10.2), respectively. Maternal trauma was driven by higher order perineal lacerations. Some association was seen between increased forceps volume and decreased maternal trauma rates.
The authors concluded that in Canada, rates of maternal and neonatal trauma following OVD are higher than previously reported in consensus statements.
Study strengths and limitations
This large contemporary study uniquely stratified perinatal outcomes following OVD. The outcomes are well defined and meaningful, but some limitations affect the generalizability of the findings.
First, stillbirths were included for the maternal composite outcome, yet the incidence of this within the study population is not reported. Operative vaginal deliveries that involve stillbirths can be complex; a subgroup analysis excluding these would aid in interpretation.
Second, complicated OVDs, including sequential use of forceps and vacuum and OVDs from midpelvic station, were included; ACOG recommends against both these practices in routine circumstances due to known increases in maternal and neonatal morbidity.1 As such, the inclusion of these OVDs may bias results away from the null.
Finally, despite discussing the role of episiotomy, the episiotomy rate in this cohort is not reported.
Despite these limitations, the study by Muraca and colleagues is a positive step forward toward understanding the role of OVD in contemporary obstetric practice, and it uniquely ascertains the impact of OVD volume outcomes that previously had been an elusive exposure ●
While it is important to understand perinatal outcomes following OVD in a contemporary cohort, utilizing the correct cohort and reference group is critical.4 Risks for maternal and neonatal trauma follow OVD; however, outcomes vary based on appropriate selection of OVD candidates and adherence to recommended national guidelines.1,4 The infrequency of OVD raises concerns regarding adequate training for obstetricians, which should be prioritized so that they can offer OVD as a safe alternative to CD birth.3
HAYLEY E. MILLER, MD, AND DANIELLE M. PANELLI, MD
- American College of Obstetricians and Gynecologists. Operative vaginal birth: ACOG practice bulletin, number 219. Obstet Gynecol. 2020;135:e149-e159.
- Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. Obstet Gynecol. 2012;120:1181-1193.
- Zahniser SC, Kendrick JS, Franks AL, et al. Trends in obstetric operative procedures, 1980 to 1987. Am J Public Health. 1992;82:1340-1344.
- Panelli DM, Leonard SA, Joudi N, et al. Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Am J Obstet Gynecol MFM. 2021;3: 100339.
Muraca GM, Boutin A, Razaz N, et al. Maternal and neonatal trauma following operative vaginal delivery. CMAJ. 2022;194:E1-E12. doi: 10.1503/cmaj.210841.
EXPERT COMMENTARY
Operative vaginal delivery is used to achieve and expedite safe vaginal birth while avoiding CD and its associated morbidities.1,2 Despite support from the American College of Obstetricians and Gynecologists (ACOG) for the use of OVD as an alternative to CD, OVD was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987.1,3 Reported complications of OVD are biased by the level of experience of the operator, changes in practice, and by misinterpretation of the counterfactual.1
Outcomes of OVD should be compared with appropriate reference groups, namely, with second-stage CD births rather than with spontaneous vaginal births.4 With decreasing rates of OVD, evidence of contemporary data is needed on appropriately compared perinatal outcomes.4
Details of the study
Muraca and colleagues conducted an observational cohort study of births in Canada between 2013 and 2019 to assess the incidence of maternal and neonatal trauma following OVD. They used composites defined a priori— stratified by instrument, region, level of obstetric care, and institutional OVD volume.
Results. Among 1,326,191 live or stillbirths, 2.9% were attempted forceps deliveries and 8.4% were attempted vacuum deliveries. Following forceps delivery, the maternal trauma rate was 25.3% (95% confidence interval [CI], 24.8%–25.7%), and the neonatal trauma rate was 9.6 per 1,000 live births (95% CI, 8.6–10.6). Following vacuum delivery, maternal and neonatal trauma rates were 13.2% (95% CI, 13.0%–13.4%) and 9.6 per 1,000 live births (95% CI, 9.0–10.2), respectively. Maternal trauma was driven by higher order perineal lacerations. Some association was seen between increased forceps volume and decreased maternal trauma rates.
The authors concluded that in Canada, rates of maternal and neonatal trauma following OVD are higher than previously reported in consensus statements.
Study strengths and limitations
This large contemporary study uniquely stratified perinatal outcomes following OVD. The outcomes are well defined and meaningful, but some limitations affect the generalizability of the findings.
First, stillbirths were included for the maternal composite outcome, yet the incidence of this within the study population is not reported. Operative vaginal deliveries that involve stillbirths can be complex; a subgroup analysis excluding these would aid in interpretation.
Second, complicated OVDs, including sequential use of forceps and vacuum and OVDs from midpelvic station, were included; ACOG recommends against both these practices in routine circumstances due to known increases in maternal and neonatal morbidity.1 As such, the inclusion of these OVDs may bias results away from the null.
Finally, despite discussing the role of episiotomy, the episiotomy rate in this cohort is not reported.
Despite these limitations, the study by Muraca and colleagues is a positive step forward toward understanding the role of OVD in contemporary obstetric practice, and it uniquely ascertains the impact of OVD volume outcomes that previously had been an elusive exposure ●
While it is important to understand perinatal outcomes following OVD in a contemporary cohort, utilizing the correct cohort and reference group is critical.4 Risks for maternal and neonatal trauma follow OVD; however, outcomes vary based on appropriate selection of OVD candidates and adherence to recommended national guidelines.1,4 The infrequency of OVD raises concerns regarding adequate training for obstetricians, which should be prioritized so that they can offer OVD as a safe alternative to CD birth.3
HAYLEY E. MILLER, MD, AND DANIELLE M. PANELLI, MD
Muraca GM, Boutin A, Razaz N, et al. Maternal and neonatal trauma following operative vaginal delivery. CMAJ. 2022;194:E1-E12. doi: 10.1503/cmaj.210841.
EXPERT COMMENTARY
Operative vaginal delivery is used to achieve and expedite safe vaginal birth while avoiding CD and its associated morbidities.1,2 Despite support from the American College of Obstetricians and Gynecologists (ACOG) for the use of OVD as an alternative to CD, OVD was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987.1,3 Reported complications of OVD are biased by the level of experience of the operator, changes in practice, and by misinterpretation of the counterfactual.1
Outcomes of OVD should be compared with appropriate reference groups, namely, with second-stage CD births rather than with spontaneous vaginal births.4 With decreasing rates of OVD, evidence of contemporary data is needed on appropriately compared perinatal outcomes.4
Details of the study
Muraca and colleagues conducted an observational cohort study of births in Canada between 2013 and 2019 to assess the incidence of maternal and neonatal trauma following OVD. They used composites defined a priori— stratified by instrument, region, level of obstetric care, and institutional OVD volume.
Results. Among 1,326,191 live or stillbirths, 2.9% were attempted forceps deliveries and 8.4% were attempted vacuum deliveries. Following forceps delivery, the maternal trauma rate was 25.3% (95% confidence interval [CI], 24.8%–25.7%), and the neonatal trauma rate was 9.6 per 1,000 live births (95% CI, 8.6–10.6). Following vacuum delivery, maternal and neonatal trauma rates were 13.2% (95% CI, 13.0%–13.4%) and 9.6 per 1,000 live births (95% CI, 9.0–10.2), respectively. Maternal trauma was driven by higher order perineal lacerations. Some association was seen between increased forceps volume and decreased maternal trauma rates.
The authors concluded that in Canada, rates of maternal and neonatal trauma following OVD are higher than previously reported in consensus statements.
Study strengths and limitations
This large contemporary study uniquely stratified perinatal outcomes following OVD. The outcomes are well defined and meaningful, but some limitations affect the generalizability of the findings.
First, stillbirths were included for the maternal composite outcome, yet the incidence of this within the study population is not reported. Operative vaginal deliveries that involve stillbirths can be complex; a subgroup analysis excluding these would aid in interpretation.
Second, complicated OVDs, including sequential use of forceps and vacuum and OVDs from midpelvic station, were included; ACOG recommends against both these practices in routine circumstances due to known increases in maternal and neonatal morbidity.1 As such, the inclusion of these OVDs may bias results away from the null.
Finally, despite discussing the role of episiotomy, the episiotomy rate in this cohort is not reported.
Despite these limitations, the study by Muraca and colleagues is a positive step forward toward understanding the role of OVD in contemporary obstetric practice, and it uniquely ascertains the impact of OVD volume outcomes that previously had been an elusive exposure ●
While it is important to understand perinatal outcomes following OVD in a contemporary cohort, utilizing the correct cohort and reference group is critical.4 Risks for maternal and neonatal trauma follow OVD; however, outcomes vary based on appropriate selection of OVD candidates and adherence to recommended national guidelines.1,4 The infrequency of OVD raises concerns regarding adequate training for obstetricians, which should be prioritized so that they can offer OVD as a safe alternative to CD birth.3
HAYLEY E. MILLER, MD, AND DANIELLE M. PANELLI, MD
- American College of Obstetricians and Gynecologists. Operative vaginal birth: ACOG practice bulletin, number 219. Obstet Gynecol. 2020;135:e149-e159.
- Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. Obstet Gynecol. 2012;120:1181-1193.
- Zahniser SC, Kendrick JS, Franks AL, et al. Trends in obstetric operative procedures, 1980 to 1987. Am J Public Health. 1992;82:1340-1344.
- Panelli DM, Leonard SA, Joudi N, et al. Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Am J Obstet Gynecol MFM. 2021;3: 100339.
- American College of Obstetricians and Gynecologists. Operative vaginal birth: ACOG practice bulletin, number 219. Obstet Gynecol. 2020;135:e149-e159.
- Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. Obstet Gynecol. 2012;120:1181-1193.
- Zahniser SC, Kendrick JS, Franks AL, et al. Trends in obstetric operative procedures, 1980 to 1987. Am J Public Health. 1992;82:1340-1344.
- Panelli DM, Leonard SA, Joudi N, et al. Severe maternal and neonatal morbidity after attempted operative vaginal delivery. Am J Obstet Gynecol MFM. 2021;3: 100339.
Infectious disease pop quiz: Clinical challenge #24 for the ObGyn
What are the 2 most likely causes for persistent fever in a patient who is being treated with antibiotics for postcesarean endometritis?
Continue to the answer...
The 2 most likely causes of a poor response to treatment for postcesarean endometritis are a resistant microorganism and wound infection. Less common causes of persistent postoperative fever include septic pelvic vein thrombophlebitis, pelvic abscess, retained products of conception, reactivation of a connective tissue disorder, and drug fever.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the 2 most likely causes for persistent fever in a patient who is being treated with antibiotics for postcesarean endometritis?
Continue to the answer...
The 2 most likely causes of a poor response to treatment for postcesarean endometritis are a resistant microorganism and wound infection. Less common causes of persistent postoperative fever include septic pelvic vein thrombophlebitis, pelvic abscess, retained products of conception, reactivation of a connective tissue disorder, and drug fever.
What are the 2 most likely causes for persistent fever in a patient who is being treated with antibiotics for postcesarean endometritis?
Continue to the answer...
The 2 most likely causes of a poor response to treatment for postcesarean endometritis are a resistant microorganism and wound infection. Less common causes of persistent postoperative fever include septic pelvic vein thrombophlebitis, pelvic abscess, retained products of conception, reactivation of a connective tissue disorder, and drug fever.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Recurrent DCIS can be genetically distinct from primary lesion
according to a study presented at the annual meeting of the American Association for Cancer Research.
If these findings of de novo tumor recurrences hold true, “it should change how you should treat the patients in the clinic,” commented lead author Tanjina Kader, PhD, a postdoctoral researcher in the department of oncology at the Peter MacCallum Cancer Centre in the University of Melbourne.
Up to a quarter of cases of DCIS recur, and half of those cases emerge in the form of invasive breast cancer. Currently, all recurrent tumor patients are provided the same treatment on the assumption that all recurrences arise from the primary lesion, Dr. Kader commented.
But the new findings could change this practice. If a patient with DCIS returns to the clinic with a tumor independent of the primary lesion, physicians should consider preventive therapies, such as mastectomy or genetic counseling, she said in an interview.
For their study, Dr. Kader and colleagues gathered patient samples and extracted 67 pairs of primary DCIS and their recurrences from the same breast. They also collected 32 samples from nonrecurrent cases of DCIS.
They then used advanced DNA sequencing methods to conduct detailed molecular analyses in order to determine whether the recurrences were genetically distinct from the original lesion.
The team found that 82% of cases appeared to be clonal – derived from the same ancestral cell as the original tumor – and 18% were nonclonal – arose independently of the original DCIS.
The researchers also identified specific genetic changes, including a mutation in the TP53 gene, that were present in recurrences of DCIS but not in nonrecurrent or nonclonal cases.
“It was surprising to see that nonclonal tumors have a similar genetic profile as nonrecurrent tumors,” Dr. Kader said. This means that, if these genetic changes are used as biomarkers to predict the recurrence of DCIS, they could lead to the undertreatment of patients who could develop nonclonal tumors, since these individuals may be categorized as having a low risk of recurrence, she explained.
“For the last 10 years, everyone has been trying their best to find a biomarker without actually taking into account that independent tumors can actually arise on the same breast independently,” Dr. Kader said.
The main limitation of this study was the lack of DNA from matched healthy cells to compare to the patient samples, said Dr. Kader. Because of the lack of these samples, the study focused only on chromosomal changes.
This study is “highly relevant, as it adds to our knowledge to what extent DCIS can be considered a precursor lesion as well as a risk lesion,” said Jelle Wesseling, MD, PhD, a breast pathologist at the Netherlands Cancer Institute. He was not involved in this research, but his team has also found that primary DCIS lesions and their subsequent events can be clonally unrelated.
Dr. Wesseling said there are still many questions, such as whether inherited genetic variants or the tumor microenvironment contribute to DCIS recurrences. “There is a lot more work to be done here to tease this out in more detail.”
The study was funded by grants from the National Breast Cancer Foundation, the Cancer Council Victoria, and the Victorian Cancer Agency.
A version of this article first appeared on Medscape.com.
according to a study presented at the annual meeting of the American Association for Cancer Research.
If these findings of de novo tumor recurrences hold true, “it should change how you should treat the patients in the clinic,” commented lead author Tanjina Kader, PhD, a postdoctoral researcher in the department of oncology at the Peter MacCallum Cancer Centre in the University of Melbourne.
Up to a quarter of cases of DCIS recur, and half of those cases emerge in the form of invasive breast cancer. Currently, all recurrent tumor patients are provided the same treatment on the assumption that all recurrences arise from the primary lesion, Dr. Kader commented.
But the new findings could change this practice. If a patient with DCIS returns to the clinic with a tumor independent of the primary lesion, physicians should consider preventive therapies, such as mastectomy or genetic counseling, she said in an interview.
For their study, Dr. Kader and colleagues gathered patient samples and extracted 67 pairs of primary DCIS and their recurrences from the same breast. They also collected 32 samples from nonrecurrent cases of DCIS.
They then used advanced DNA sequencing methods to conduct detailed molecular analyses in order to determine whether the recurrences were genetically distinct from the original lesion.
The team found that 82% of cases appeared to be clonal – derived from the same ancestral cell as the original tumor – and 18% were nonclonal – arose independently of the original DCIS.
The researchers also identified specific genetic changes, including a mutation in the TP53 gene, that were present in recurrences of DCIS but not in nonrecurrent or nonclonal cases.
“It was surprising to see that nonclonal tumors have a similar genetic profile as nonrecurrent tumors,” Dr. Kader said. This means that, if these genetic changes are used as biomarkers to predict the recurrence of DCIS, they could lead to the undertreatment of patients who could develop nonclonal tumors, since these individuals may be categorized as having a low risk of recurrence, she explained.
“For the last 10 years, everyone has been trying their best to find a biomarker without actually taking into account that independent tumors can actually arise on the same breast independently,” Dr. Kader said.
The main limitation of this study was the lack of DNA from matched healthy cells to compare to the patient samples, said Dr. Kader. Because of the lack of these samples, the study focused only on chromosomal changes.
This study is “highly relevant, as it adds to our knowledge to what extent DCIS can be considered a precursor lesion as well as a risk lesion,” said Jelle Wesseling, MD, PhD, a breast pathologist at the Netherlands Cancer Institute. He was not involved in this research, but his team has also found that primary DCIS lesions and their subsequent events can be clonally unrelated.
Dr. Wesseling said there are still many questions, such as whether inherited genetic variants or the tumor microenvironment contribute to DCIS recurrences. “There is a lot more work to be done here to tease this out in more detail.”
The study was funded by grants from the National Breast Cancer Foundation, the Cancer Council Victoria, and the Victorian Cancer Agency.
A version of this article first appeared on Medscape.com.
according to a study presented at the annual meeting of the American Association for Cancer Research.
If these findings of de novo tumor recurrences hold true, “it should change how you should treat the patients in the clinic,” commented lead author Tanjina Kader, PhD, a postdoctoral researcher in the department of oncology at the Peter MacCallum Cancer Centre in the University of Melbourne.
Up to a quarter of cases of DCIS recur, and half of those cases emerge in the form of invasive breast cancer. Currently, all recurrent tumor patients are provided the same treatment on the assumption that all recurrences arise from the primary lesion, Dr. Kader commented.
But the new findings could change this practice. If a patient with DCIS returns to the clinic with a tumor independent of the primary lesion, physicians should consider preventive therapies, such as mastectomy or genetic counseling, she said in an interview.
For their study, Dr. Kader and colleagues gathered patient samples and extracted 67 pairs of primary DCIS and their recurrences from the same breast. They also collected 32 samples from nonrecurrent cases of DCIS.
They then used advanced DNA sequencing methods to conduct detailed molecular analyses in order to determine whether the recurrences were genetically distinct from the original lesion.
The team found that 82% of cases appeared to be clonal – derived from the same ancestral cell as the original tumor – and 18% were nonclonal – arose independently of the original DCIS.
The researchers also identified specific genetic changes, including a mutation in the TP53 gene, that were present in recurrences of DCIS but not in nonrecurrent or nonclonal cases.
“It was surprising to see that nonclonal tumors have a similar genetic profile as nonrecurrent tumors,” Dr. Kader said. This means that, if these genetic changes are used as biomarkers to predict the recurrence of DCIS, they could lead to the undertreatment of patients who could develop nonclonal tumors, since these individuals may be categorized as having a low risk of recurrence, she explained.
“For the last 10 years, everyone has been trying their best to find a biomarker without actually taking into account that independent tumors can actually arise on the same breast independently,” Dr. Kader said.
The main limitation of this study was the lack of DNA from matched healthy cells to compare to the patient samples, said Dr. Kader. Because of the lack of these samples, the study focused only on chromosomal changes.
This study is “highly relevant, as it adds to our knowledge to what extent DCIS can be considered a precursor lesion as well as a risk lesion,” said Jelle Wesseling, MD, PhD, a breast pathologist at the Netherlands Cancer Institute. He was not involved in this research, but his team has also found that primary DCIS lesions and their subsequent events can be clonally unrelated.
Dr. Wesseling said there are still many questions, such as whether inherited genetic variants or the tumor microenvironment contribute to DCIS recurrences. “There is a lot more work to be done here to tease this out in more detail.”
The study was funded by grants from the National Breast Cancer Foundation, the Cancer Council Victoria, and the Victorian Cancer Agency.
A version of this article first appeared on Medscape.com.
FROM AACR 2022
Breast anatomy and augmentation in transfeminine individuals
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Which breast cancer surgery leads to better quality of life?
Women diagnosed with early breast cancer facing surgery often have a choice of having all of their breast or only a part of the breast removed.
A new study shows that a patient’s satisfaction with their breasts at 10 years after surgery is similar for both groups of women.
However, superior psychosocial and sexual well-being at 10 years after surgery was reported by women who underwent breast-conserving surgery and adjuvant radiation therapy (RT), compared with those who underwent mastectomy and reconstruction.
“These findings may inform preference-sensitive decision-making for women with early-stage breast cancer,” write the authors, led by Benjamin D. Smith, MD, department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
The study was published online in JAMA Surgery.
These findings have important implications for patient decision-making, given that more women eligible for breast-conserving surgery are opting for a mastectomy, say Sudheer Vemuru, MD, from the University of Colorado at Denver, Aurora, and colleagues, writing in an accompanying editorial.
“Overall, the preponderance of evidence suggests superior short-term and long-term patient-reported outcomes in patients with early-stage breast cancer undergoing breast conserving surgery compared with mastectomy,” they comment.
Study details
For their study, Dr. Smith and colleagues conducted a comparative effectiveness research study using data from the Texas Cancer Registry and identified women diagnosed with stage 0-II breast cancer and treated with breast-conserving surgery or mastectomy and reconstruction between 2006 and 2008.
A total of 647 patients were included in their analysis (40%; 356 had undergone breast-conserving surgery; 291 had undergone mastectomy and reconstruction), 551 (85.2%) confirmed treatment with breast-conserving surgery with RT (n = 315) or mastectomy and reconstruction without RT (n = 236).
The median age of the cohort was 53 years and the median time from diagnosis to survey was 10.3 years. Mastectomy and reconstruction were more common among women who were White, younger, node positive, had larger tumors, had bilateral breast cancer, received chemotherapy, and had higher income.
The primary outcome was patient satisfaction with their breasts, as measured with the BREAST-Q patient-reported outcome measure. Secondary outcomes included physical well-being, psychosocial well-being, and sexual well-being. The EuroQol Health-Related Quality of Life 5-Dimension, 3-Level gaged health utility, and local therapy decisional regret was measured via the Decisional Regret Scale.
Using breast-conserving surgery plus RT as the referent, the authors did not find any significant differences in breast satisfaction, physical well-being, health utility, or decisional regret among the study cohorts: breast satisfaction: effect size, 2.71 (P = .30); physical well-being: effect size, –1.80 (P = .36); health utility: effect size, –0.003 (P = .83); and decisional regret: effect size, 1.32 (P = .61).
However, psychosocial well-being (effect size, –8.61; P < .001) and sexual well-being (effect size, –10.68; P < .001) were significantly worse among women who had undergone mastectomy and reconstruction without RT.
They noted that interactions of race and ethnicity and age by treatment group were not significant for reported satisfaction with breast outcomes. But the findings “indicated that the burden of poor long-term QOL outcomes was greater among younger individuals, those with lower educational attainment and income, and certain racial and ethnic minority populations,” they write. “These findings suggest that opportunities exist to enhance equity in the long-term QOL of individuals with breast cancer.”
The editorialists note that previous studies have also found diminished quality of life following mastectomy compared with breast-conserving surgery. However, most of these prior studies included patients undergoing breast-conserving surgery without RT, patients undergoing mastectomy without reconstruction, and patients undergoing mastectomy with RT.
In contrast, this latest study “directly compared breast-conserving surgery with RT vs. mastectomy and reconstruction without RT to avoid those potential confounders,” they point out.
The study was supported by grants from the National Cancer Institute and other bodies. Several of the study authors disclosed relationships with industry and/or with nonprofit organizations. The full list can be found with the original article. Editorialist Clara Lee, MD, reported receiving grants from the Agency for Healthcare Research and Quality during the conduct of the study.
A version of this article first appeared on Medscape.com.
Women diagnosed with early breast cancer facing surgery often have a choice of having all of their breast or only a part of the breast removed.
A new study shows that a patient’s satisfaction with their breasts at 10 years after surgery is similar for both groups of women.
However, superior psychosocial and sexual well-being at 10 years after surgery was reported by women who underwent breast-conserving surgery and adjuvant radiation therapy (RT), compared with those who underwent mastectomy and reconstruction.
“These findings may inform preference-sensitive decision-making for women with early-stage breast cancer,” write the authors, led by Benjamin D. Smith, MD, department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
The study was published online in JAMA Surgery.
These findings have important implications for patient decision-making, given that more women eligible for breast-conserving surgery are opting for a mastectomy, say Sudheer Vemuru, MD, from the University of Colorado at Denver, Aurora, and colleagues, writing in an accompanying editorial.
“Overall, the preponderance of evidence suggests superior short-term and long-term patient-reported outcomes in patients with early-stage breast cancer undergoing breast conserving surgery compared with mastectomy,” they comment.
Study details
For their study, Dr. Smith and colleagues conducted a comparative effectiveness research study using data from the Texas Cancer Registry and identified women diagnosed with stage 0-II breast cancer and treated with breast-conserving surgery or mastectomy and reconstruction between 2006 and 2008.
A total of 647 patients were included in their analysis (40%; 356 had undergone breast-conserving surgery; 291 had undergone mastectomy and reconstruction), 551 (85.2%) confirmed treatment with breast-conserving surgery with RT (n = 315) or mastectomy and reconstruction without RT (n = 236).
The median age of the cohort was 53 years and the median time from diagnosis to survey was 10.3 years. Mastectomy and reconstruction were more common among women who were White, younger, node positive, had larger tumors, had bilateral breast cancer, received chemotherapy, and had higher income.
The primary outcome was patient satisfaction with their breasts, as measured with the BREAST-Q patient-reported outcome measure. Secondary outcomes included physical well-being, psychosocial well-being, and sexual well-being. The EuroQol Health-Related Quality of Life 5-Dimension, 3-Level gaged health utility, and local therapy decisional regret was measured via the Decisional Regret Scale.
Using breast-conserving surgery plus RT as the referent, the authors did not find any significant differences in breast satisfaction, physical well-being, health utility, or decisional regret among the study cohorts: breast satisfaction: effect size, 2.71 (P = .30); physical well-being: effect size, –1.80 (P = .36); health utility: effect size, –0.003 (P = .83); and decisional regret: effect size, 1.32 (P = .61).
However, psychosocial well-being (effect size, –8.61; P < .001) and sexual well-being (effect size, –10.68; P < .001) were significantly worse among women who had undergone mastectomy and reconstruction without RT.
They noted that interactions of race and ethnicity and age by treatment group were not significant for reported satisfaction with breast outcomes. But the findings “indicated that the burden of poor long-term QOL outcomes was greater among younger individuals, those with lower educational attainment and income, and certain racial and ethnic minority populations,” they write. “These findings suggest that opportunities exist to enhance equity in the long-term QOL of individuals with breast cancer.”
The editorialists note that previous studies have also found diminished quality of life following mastectomy compared with breast-conserving surgery. However, most of these prior studies included patients undergoing breast-conserving surgery without RT, patients undergoing mastectomy without reconstruction, and patients undergoing mastectomy with RT.
In contrast, this latest study “directly compared breast-conserving surgery with RT vs. mastectomy and reconstruction without RT to avoid those potential confounders,” they point out.
The study was supported by grants from the National Cancer Institute and other bodies. Several of the study authors disclosed relationships with industry and/or with nonprofit organizations. The full list can be found with the original article. Editorialist Clara Lee, MD, reported receiving grants from the Agency for Healthcare Research and Quality during the conduct of the study.
A version of this article first appeared on Medscape.com.
Women diagnosed with early breast cancer facing surgery often have a choice of having all of their breast or only a part of the breast removed.
A new study shows that a patient’s satisfaction with their breasts at 10 years after surgery is similar for both groups of women.
However, superior psychosocial and sexual well-being at 10 years after surgery was reported by women who underwent breast-conserving surgery and adjuvant radiation therapy (RT), compared with those who underwent mastectomy and reconstruction.
“These findings may inform preference-sensitive decision-making for women with early-stage breast cancer,” write the authors, led by Benjamin D. Smith, MD, department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
The study was published online in JAMA Surgery.
These findings have important implications for patient decision-making, given that more women eligible for breast-conserving surgery are opting for a mastectomy, say Sudheer Vemuru, MD, from the University of Colorado at Denver, Aurora, and colleagues, writing in an accompanying editorial.
“Overall, the preponderance of evidence suggests superior short-term and long-term patient-reported outcomes in patients with early-stage breast cancer undergoing breast conserving surgery compared with mastectomy,” they comment.
Study details
For their study, Dr. Smith and colleagues conducted a comparative effectiveness research study using data from the Texas Cancer Registry and identified women diagnosed with stage 0-II breast cancer and treated with breast-conserving surgery or mastectomy and reconstruction between 2006 and 2008.
A total of 647 patients were included in their analysis (40%; 356 had undergone breast-conserving surgery; 291 had undergone mastectomy and reconstruction), 551 (85.2%) confirmed treatment with breast-conserving surgery with RT (n = 315) or mastectomy and reconstruction without RT (n = 236).
The median age of the cohort was 53 years and the median time from diagnosis to survey was 10.3 years. Mastectomy and reconstruction were more common among women who were White, younger, node positive, had larger tumors, had bilateral breast cancer, received chemotherapy, and had higher income.
The primary outcome was patient satisfaction with their breasts, as measured with the BREAST-Q patient-reported outcome measure. Secondary outcomes included physical well-being, psychosocial well-being, and sexual well-being. The EuroQol Health-Related Quality of Life 5-Dimension, 3-Level gaged health utility, and local therapy decisional regret was measured via the Decisional Regret Scale.
Using breast-conserving surgery plus RT as the referent, the authors did not find any significant differences in breast satisfaction, physical well-being, health utility, or decisional regret among the study cohorts: breast satisfaction: effect size, 2.71 (P = .30); physical well-being: effect size, –1.80 (P = .36); health utility: effect size, –0.003 (P = .83); and decisional regret: effect size, 1.32 (P = .61).
However, psychosocial well-being (effect size, –8.61; P < .001) and sexual well-being (effect size, –10.68; P < .001) were significantly worse among women who had undergone mastectomy and reconstruction without RT.
They noted that interactions of race and ethnicity and age by treatment group were not significant for reported satisfaction with breast outcomes. But the findings “indicated that the burden of poor long-term QOL outcomes was greater among younger individuals, those with lower educational attainment and income, and certain racial and ethnic minority populations,” they write. “These findings suggest that opportunities exist to enhance equity in the long-term QOL of individuals with breast cancer.”
The editorialists note that previous studies have also found diminished quality of life following mastectomy compared with breast-conserving surgery. However, most of these prior studies included patients undergoing breast-conserving surgery without RT, patients undergoing mastectomy without reconstruction, and patients undergoing mastectomy with RT.
In contrast, this latest study “directly compared breast-conserving surgery with RT vs. mastectomy and reconstruction without RT to avoid those potential confounders,” they point out.
The study was supported by grants from the National Cancer Institute and other bodies. Several of the study authors disclosed relationships with industry and/or with nonprofit organizations. The full list can be found with the original article. Editorialist Clara Lee, MD, reported receiving grants from the Agency for Healthcare Research and Quality during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Are free lunches back? Docs start seeing drug reps again
In their heyday, drug reps had big expense budgets and would wine and dine physicians, golf with them, and give gifts to their potential physician clients.
But in 2002, pressure from Congress and increased scrutiny from the American Medical Association prompted the Pharmaceutical Research and Manufacturers of America to adopt a set of voluntary ethical codes to regulate the gifts given to physicians. Now, physicians must report even small gifts or meals to the National Practitioner Data Bank.
Before the restrictions, physician/pharmaceutical rep relationships relied on face-to-face meetings. These included lunches with a limited budget or sharing a cup of coffee during a morning visit to a practice. The parties got to know each other, which led to trust and long-term relationships.
During the COVID-19 pandemic, everything changed. “It was culture shock for us,” admitted Craig F, a career pharmaceutical rep. “We didn’t know what we were going to do.”
The pharmaceutical industry pivoted and quickly got up to speed with Zoom, Microsoft Teams, and the like. “We began by reaching out to doctors via email and cell phones to set up virtual meetings,” Craig said. “Most of the doctors were working from home, doing telehealth whenever possible. For new sales reps, this was particularly difficult, because they couldn’t visit offices and get to know doctors.”
Many physicians didn’t want to devote time to Zoom meetings with pharma reps. “We worked around their schedules, and sometimes this even looked like Sunday calls,” he said.
As vaccination levels increased and medical offices began to reopen, so too did some of the old-school, face-to-face pharma rep/doctor meetings. But most proceeded with caution. “Some pharmaceutical companies didn’t put reps back into the field until the fall of 2020,” said Craig. “If we weren’t welcome in an office, we didn’t push it.”
Once much of the population was vaccinated, the thaw began in earnest, although the drug reps continued to tread cautiously, mask up, and respect the wishes of physicians. Today, Craig estimated that about two-thirds of his appointments are in person.
Still, it’s unlikely that the drug rep–supplied “free staff lunch” will ever regain its former popularity. Medical office staff are still keeping distance, owing to COVID; office schedules may be more crowded and may not allow the time; and many physicians are still nervous about having to report “gifts” or “paid lunches” from pharma.
The post-COVID paradigm shift
The pandemic put a dent in the pharma rep/doctor relationship, said Suzy Jackson, managing director of life sciences at Accenture and an author of The “New” Rules of Healthcare Provider Engagement . “COVID started moving power away from reps because they lost the ability to simply wander into a building and have a conversation with a health care provider. We’re seeing the pandemic evolve the meeting model into a hybrid in-person and virtual.”
“Many doctors are operating in a slower fashion because they’re balancing a hybrid model with patients, as well,” said Craig. “Some of my visits now involve talking to nurses or front-office staff, not getting in to see the doctors.”
The push from some doctors to see reps virtually as opposed to in person is a challenge for the pharma companies. “We get more done in person, so virtual is not our favorite way to do business,” said Craig. “But we’re thankful for any time we can get with doctors, so when they ask to do virtual, we agree.”
Still, the Accenture survey offered good news for pharma reps: Only 4% of respondents didn’t want to continue with in-person meetings at all. “I think of this as a positive,” Ms. Jackson said. “It shows that physicians value these relationships, if they’re done in the right way.”
But a survey by Boston Consulting Group confirms that virtual visits are likely to continue. BCG’s Doctors’ Changing Expectations of Pharma Are Here to Stay revealed that three-quarters of respondent physicians prefer to maintain or increase the amount of virtual engagements with pharma reps after becoming accustomed to the practice during the pandemic.
Under these changing scenarios, said Ms. Jackson, pharma reps have to think about more meaningful ways to engage with doctors.
“I feel that doctors are more crunched for time now, managing hybrid environments,” Craig said. “They have less time and want more patient-specific information that leads to fewer calls back to their offices.”
More physicians now value webinars, virtual training, and speaker programs. Virtual channels, the survey found, “give physicians access to the information they need in an easy and convenient manner.”
Still, physicians have noted that the survey indicated that email communications from pharma reps had increased. Often, physicians found the useful information buried in irrelevant “clutter.”
Restrictions on drug reps became tighter
In the 20 years since the guidelines came into existence, PhRMA has continued to strengthen the codes. In 2009, PhRMA issued new recommendations surrounding noneducational gifts and placed a cap of $100 for meals, drug samples, and other items. In 2022, they added layers to the code that focus on speaker programs. For instance, while companies can provide “modest” meals to attendees as an incidental courtesy, pharma reps can no longer pay for or provide alcohol in conjunction with these programs.
The rules vary from state to state. In Minnesota, for instance, gifts from pharma companies cannot exceed $50 per year. Some institutions – such as the Cleveland Clinic – have even stricter rules. “When we have conventions, we put up signage reminding doctors from the strictest states that they can’t even accept a cup of coffee from a rep,” said Craig.
However, COVID hasn’t completely changed doctor/pharma relationships. In Ms. Jackson’s words, “In spite of the shift to a more hybrid model, this is a very human relationship yielding real human results.”
A version of this article first appeared on Medscape.com.
In their heyday, drug reps had big expense budgets and would wine and dine physicians, golf with them, and give gifts to their potential physician clients.
But in 2002, pressure from Congress and increased scrutiny from the American Medical Association prompted the Pharmaceutical Research and Manufacturers of America to adopt a set of voluntary ethical codes to regulate the gifts given to physicians. Now, physicians must report even small gifts or meals to the National Practitioner Data Bank.
Before the restrictions, physician/pharmaceutical rep relationships relied on face-to-face meetings. These included lunches with a limited budget or sharing a cup of coffee during a morning visit to a practice. The parties got to know each other, which led to trust and long-term relationships.
During the COVID-19 pandemic, everything changed. “It was culture shock for us,” admitted Craig F, a career pharmaceutical rep. “We didn’t know what we were going to do.”
The pharmaceutical industry pivoted and quickly got up to speed with Zoom, Microsoft Teams, and the like. “We began by reaching out to doctors via email and cell phones to set up virtual meetings,” Craig said. “Most of the doctors were working from home, doing telehealth whenever possible. For new sales reps, this was particularly difficult, because they couldn’t visit offices and get to know doctors.”
Many physicians didn’t want to devote time to Zoom meetings with pharma reps. “We worked around their schedules, and sometimes this even looked like Sunday calls,” he said.
As vaccination levels increased and medical offices began to reopen, so too did some of the old-school, face-to-face pharma rep/doctor meetings. But most proceeded with caution. “Some pharmaceutical companies didn’t put reps back into the field until the fall of 2020,” said Craig. “If we weren’t welcome in an office, we didn’t push it.”
Once much of the population was vaccinated, the thaw began in earnest, although the drug reps continued to tread cautiously, mask up, and respect the wishes of physicians. Today, Craig estimated that about two-thirds of his appointments are in person.
Still, it’s unlikely that the drug rep–supplied “free staff lunch” will ever regain its former popularity. Medical office staff are still keeping distance, owing to COVID; office schedules may be more crowded and may not allow the time; and many physicians are still nervous about having to report “gifts” or “paid lunches” from pharma.
The post-COVID paradigm shift
The pandemic put a dent in the pharma rep/doctor relationship, said Suzy Jackson, managing director of life sciences at Accenture and an author of The “New” Rules of Healthcare Provider Engagement . “COVID started moving power away from reps because they lost the ability to simply wander into a building and have a conversation with a health care provider. We’re seeing the pandemic evolve the meeting model into a hybrid in-person and virtual.”
“Many doctors are operating in a slower fashion because they’re balancing a hybrid model with patients, as well,” said Craig. “Some of my visits now involve talking to nurses or front-office staff, not getting in to see the doctors.”
The push from some doctors to see reps virtually as opposed to in person is a challenge for the pharma companies. “We get more done in person, so virtual is not our favorite way to do business,” said Craig. “But we’re thankful for any time we can get with doctors, so when they ask to do virtual, we agree.”
Still, the Accenture survey offered good news for pharma reps: Only 4% of respondents didn’t want to continue with in-person meetings at all. “I think of this as a positive,” Ms. Jackson said. “It shows that physicians value these relationships, if they’re done in the right way.”
But a survey by Boston Consulting Group confirms that virtual visits are likely to continue. BCG’s Doctors’ Changing Expectations of Pharma Are Here to Stay revealed that three-quarters of respondent physicians prefer to maintain or increase the amount of virtual engagements with pharma reps after becoming accustomed to the practice during the pandemic.
Under these changing scenarios, said Ms. Jackson, pharma reps have to think about more meaningful ways to engage with doctors.
“I feel that doctors are more crunched for time now, managing hybrid environments,” Craig said. “They have less time and want more patient-specific information that leads to fewer calls back to their offices.”
More physicians now value webinars, virtual training, and speaker programs. Virtual channels, the survey found, “give physicians access to the information they need in an easy and convenient manner.”
Still, physicians have noted that the survey indicated that email communications from pharma reps had increased. Often, physicians found the useful information buried in irrelevant “clutter.”
Restrictions on drug reps became tighter
In the 20 years since the guidelines came into existence, PhRMA has continued to strengthen the codes. In 2009, PhRMA issued new recommendations surrounding noneducational gifts and placed a cap of $100 for meals, drug samples, and other items. In 2022, they added layers to the code that focus on speaker programs. For instance, while companies can provide “modest” meals to attendees as an incidental courtesy, pharma reps can no longer pay for or provide alcohol in conjunction with these programs.
The rules vary from state to state. In Minnesota, for instance, gifts from pharma companies cannot exceed $50 per year. Some institutions – such as the Cleveland Clinic – have even stricter rules. “When we have conventions, we put up signage reminding doctors from the strictest states that they can’t even accept a cup of coffee from a rep,” said Craig.
However, COVID hasn’t completely changed doctor/pharma relationships. In Ms. Jackson’s words, “In spite of the shift to a more hybrid model, this is a very human relationship yielding real human results.”
A version of this article first appeared on Medscape.com.
In their heyday, drug reps had big expense budgets and would wine and dine physicians, golf with them, and give gifts to their potential physician clients.
But in 2002, pressure from Congress and increased scrutiny from the American Medical Association prompted the Pharmaceutical Research and Manufacturers of America to adopt a set of voluntary ethical codes to regulate the gifts given to physicians. Now, physicians must report even small gifts or meals to the National Practitioner Data Bank.
Before the restrictions, physician/pharmaceutical rep relationships relied on face-to-face meetings. These included lunches with a limited budget or sharing a cup of coffee during a morning visit to a practice. The parties got to know each other, which led to trust and long-term relationships.
During the COVID-19 pandemic, everything changed. “It was culture shock for us,” admitted Craig F, a career pharmaceutical rep. “We didn’t know what we were going to do.”
The pharmaceutical industry pivoted and quickly got up to speed with Zoom, Microsoft Teams, and the like. “We began by reaching out to doctors via email and cell phones to set up virtual meetings,” Craig said. “Most of the doctors were working from home, doing telehealth whenever possible. For new sales reps, this was particularly difficult, because they couldn’t visit offices and get to know doctors.”
Many physicians didn’t want to devote time to Zoom meetings with pharma reps. “We worked around their schedules, and sometimes this even looked like Sunday calls,” he said.
As vaccination levels increased and medical offices began to reopen, so too did some of the old-school, face-to-face pharma rep/doctor meetings. But most proceeded with caution. “Some pharmaceutical companies didn’t put reps back into the field until the fall of 2020,” said Craig. “If we weren’t welcome in an office, we didn’t push it.”
Once much of the population was vaccinated, the thaw began in earnest, although the drug reps continued to tread cautiously, mask up, and respect the wishes of physicians. Today, Craig estimated that about two-thirds of his appointments are in person.
Still, it’s unlikely that the drug rep–supplied “free staff lunch” will ever regain its former popularity. Medical office staff are still keeping distance, owing to COVID; office schedules may be more crowded and may not allow the time; and many physicians are still nervous about having to report “gifts” or “paid lunches” from pharma.
The post-COVID paradigm shift
The pandemic put a dent in the pharma rep/doctor relationship, said Suzy Jackson, managing director of life sciences at Accenture and an author of The “New” Rules of Healthcare Provider Engagement . “COVID started moving power away from reps because they lost the ability to simply wander into a building and have a conversation with a health care provider. We’re seeing the pandemic evolve the meeting model into a hybrid in-person and virtual.”
“Many doctors are operating in a slower fashion because they’re balancing a hybrid model with patients, as well,” said Craig. “Some of my visits now involve talking to nurses or front-office staff, not getting in to see the doctors.”
The push from some doctors to see reps virtually as opposed to in person is a challenge for the pharma companies. “We get more done in person, so virtual is not our favorite way to do business,” said Craig. “But we’re thankful for any time we can get with doctors, so when they ask to do virtual, we agree.”
Still, the Accenture survey offered good news for pharma reps: Only 4% of respondents didn’t want to continue with in-person meetings at all. “I think of this as a positive,” Ms. Jackson said. “It shows that physicians value these relationships, if they’re done in the right way.”
But a survey by Boston Consulting Group confirms that virtual visits are likely to continue. BCG’s Doctors’ Changing Expectations of Pharma Are Here to Stay revealed that three-quarters of respondent physicians prefer to maintain or increase the amount of virtual engagements with pharma reps after becoming accustomed to the practice during the pandemic.
Under these changing scenarios, said Ms. Jackson, pharma reps have to think about more meaningful ways to engage with doctors.
“I feel that doctors are more crunched for time now, managing hybrid environments,” Craig said. “They have less time and want more patient-specific information that leads to fewer calls back to their offices.”
More physicians now value webinars, virtual training, and speaker programs. Virtual channels, the survey found, “give physicians access to the information they need in an easy and convenient manner.”
Still, physicians have noted that the survey indicated that email communications from pharma reps had increased. Often, physicians found the useful information buried in irrelevant “clutter.”
Restrictions on drug reps became tighter
In the 20 years since the guidelines came into existence, PhRMA has continued to strengthen the codes. In 2009, PhRMA issued new recommendations surrounding noneducational gifts and placed a cap of $100 for meals, drug samples, and other items. In 2022, they added layers to the code that focus on speaker programs. For instance, while companies can provide “modest” meals to attendees as an incidental courtesy, pharma reps can no longer pay for or provide alcohol in conjunction with these programs.
The rules vary from state to state. In Minnesota, for instance, gifts from pharma companies cannot exceed $50 per year. Some institutions – such as the Cleveland Clinic – have even stricter rules. “When we have conventions, we put up signage reminding doctors from the strictest states that they can’t even accept a cup of coffee from a rep,” said Craig.
However, COVID hasn’t completely changed doctor/pharma relationships. In Ms. Jackson’s words, “In spite of the shift to a more hybrid model, this is a very human relationship yielding real human results.”
A version of this article first appeared on Medscape.com.