User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
OSHA revisited
Time for my periodic reminder about your Occupational Health and Safety Administration (OSHA) obligations. The health care field still has the most work-related illness and injury reports of any industry in the United States, and OSHA standards are in place to minimize potential workplace incidents.
Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain sight, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s website or order one at no charge by calling 800-321-OSHA.
The poster discusses the “general standards” that all workplaces must comply with to avoid work-related illnesses and injuries. The standards most applicable to medical offices are those dealing with personal protective equipment (PPE), bloodborne pathogens, hazard communication, and – increasingly, of late – ionizing radiation.
Physicians have become all too familiar with the PPE standard as a result of the COVID pandemic. Ironically, OSHA considers PPE a less acceptable means of employee protection than the other standards, as safe work practices should always supersede safety equipment. Nevertheless, employers must have a PPE program in place to train employees on what equipment is necessary, and under which conditions.
You also need a written exposure control plan for bloodborne pathogens. It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology. You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at that decision, and why you feel that your current protocol is as good or better.
The hazard communication (or right-to-know) standard involves compiling a list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
If your office has x-ray equipment, or you are considering one of the new image-guided superficial radiotherapy machines, you must be in compliance with the ionizing radiation standard, which entails shielding, radiation monitors, and clear labeling of equipment.
Other, more general regulations include the physical setup of your office. Everyone must be able to evacuate quickly in case of fire or other emergencies. At a minimum, you (or the owner of the office building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it, and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars. How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Time for my periodic reminder about your Occupational Health and Safety Administration (OSHA) obligations. The health care field still has the most work-related illness and injury reports of any industry in the United States, and OSHA standards are in place to minimize potential workplace incidents.
Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain sight, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s website or order one at no charge by calling 800-321-OSHA.
The poster discusses the “general standards” that all workplaces must comply with to avoid work-related illnesses and injuries. The standards most applicable to medical offices are those dealing with personal protective equipment (PPE), bloodborne pathogens, hazard communication, and – increasingly, of late – ionizing radiation.
Physicians have become all too familiar with the PPE standard as a result of the COVID pandemic. Ironically, OSHA considers PPE a less acceptable means of employee protection than the other standards, as safe work practices should always supersede safety equipment. Nevertheless, employers must have a PPE program in place to train employees on what equipment is necessary, and under which conditions.
You also need a written exposure control plan for bloodborne pathogens. It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology. You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at that decision, and why you feel that your current protocol is as good or better.
The hazard communication (or right-to-know) standard involves compiling a list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
If your office has x-ray equipment, or you are considering one of the new image-guided superficial radiotherapy machines, you must be in compliance with the ionizing radiation standard, which entails shielding, radiation monitors, and clear labeling of equipment.
Other, more general regulations include the physical setup of your office. Everyone must be able to evacuate quickly in case of fire or other emergencies. At a minimum, you (or the owner of the office building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it, and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars. How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Time for my periodic reminder about your Occupational Health and Safety Administration (OSHA) obligations. The health care field still has the most work-related illness and injury reports of any industry in the United States, and OSHA standards are in place to minimize potential workplace incidents.
Even if you hold regular safety meetings (which all too often is not the case), the occasional comprehensive review is always a good idea, and could save you a bundle in fines.
For starters, do you have an official OSHA poster, enumerating employee rights and explaining how to file complaints? Every office must have one posted in plain sight, and it is the first thing an OSHA inspector will look for. You can download one from OSHA’s website or order one at no charge by calling 800-321-OSHA.
The poster discusses the “general standards” that all workplaces must comply with to avoid work-related illnesses and injuries. The standards most applicable to medical offices are those dealing with personal protective equipment (PPE), bloodborne pathogens, hazard communication, and – increasingly, of late – ionizing radiation.
Physicians have become all too familiar with the PPE standard as a result of the COVID pandemic. Ironically, OSHA considers PPE a less acceptable means of employee protection than the other standards, as safe work practices should always supersede safety equipment. Nevertheless, employers must have a PPE program in place to train employees on what equipment is necessary, and under which conditions.
You also need a written exposure control plan for bloodborne pathogens. It should document your use of such protective equipment as gloves, face and eye protection, needle guards, and gowns, and your implementation of universal precautions – and it is supposed to be updated annually, to reflect changes in technology. You must provide all at-risk employees with hepatitis B vaccine at no cost to them. You also must provide and pay for appropriate medical treatment and follow-up after any exposure to a dangerous pathogen.
You need not adopt every new safety device as it comes on the market, but you should document which ones you are using – and which you pass up – and why. For example, you and your employees may decide not to purchase a new safety needle because you don’t think it will improve safety, or that it will be more trouble than it’s worth; but you should document how you arrived at that decision, and why you feel that your current protocol is as good or better.
The hazard communication (or right-to-know) standard involves compiling a list of hazardous substances, which all employees have a right to know about. Keep in mind that OSHA’s list includes alcohol, hydrogen peroxide, acetone, liquid nitrogen, and other substances that you might not consider particularly dangerous, but are nevertheless classified as “hazardous.” For each substance, your employees must have access to the manufacturer-supplied Material Safety Data Sheet, which outlines the proper procedures for working with a specific material, and for handling and containing it in a spill or other emergency.
If your office has x-ray equipment, or you are considering one of the new image-guided superficial radiotherapy machines, you must be in compliance with the ionizing radiation standard, which entails shielding, radiation monitors, and clear labeling of equipment.
Other, more general regulations include the physical setup of your office. Everyone must be able to evacuate quickly in case of fire or other emergencies. At a minimum, you (or the owner of the office building) are expected to establish exit routes to accommodate all employees and to post easily visible evacuation diagrams.
Examine all electrical devices and their power sources. All electrically powered equipment – medical, clerical, or anything else in the office – must operate safely. Pay particular attention to the way wall outlets are set up. Make sure each outlet has sufficient power to run the equipment plugged into it, and that circuit breakers are present and functioning. And beware the common situation of too many gadgets running off a single circuit.
Other components of the rule include proper containment of regulated medical waste, identification of regulated-waste containers, sharps disposal boxes, and periodic employee training regarding all of these things.
Federal OSHA regulations do not require medical and dental offices to keep an injury and illness log, as other businesses must; but your state may have a requirement that supersedes the federal law. Check with your state, or with your local OSHA office, regarding any such requirements.
It is a mistake to take OSHA regulations lightly; failure to comply with them can result in stiff penalties running into many thousands of dollars. How can you be certain you are complying with all the rules? The easiest and cheapest way is to call your local OSHA office and request an inspection. Why would you do that? Because OSHA issues no citations during voluntary inspections as long as you agree to remedy any violations they find.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
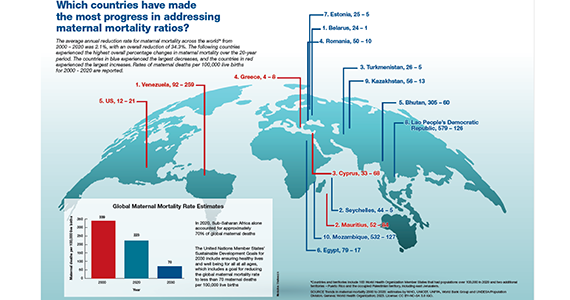
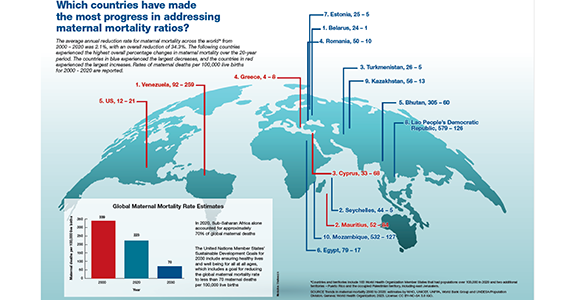
Which countries have made the most progress in addressing maternal mortality ratios?


Is azithromycin prophylaxis appropriate for vaginal delivery in low- and middle-resource populations?
Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
EXPERT COMMENTARY
Maternal peripartum infection is 1 of the top 5 causes of maternal death, accounting for about 10% of cases of maternal mortality. Cesarean delivery (CD), of course, is the most important risk factor for puerperal infection. However, even vaginal delivery, particularly in low- to middle-resource countries, where deliveries often occur under less-than-optimal conditions, may be associated with a surprisingly high frequency of both maternal and neonatal infections. The beneficial effect of prophylactic antibiotics for CD is well established. An important remaining question is whether similar benefit can be achieved with prophylaxis for women planning to have a vaginal birth.
In 2017, Oluwalana and colleagues conducted a prospective, randomized, double-blind, placebo-controlled trial of a single 2-g oral dose of azithromycin in Gambian women undergoing labor.1 During the 8 weeks after delivery, maternal infections were lower in the azithromycin group, 3.6% versus 9.2% (relative risk [RR], 0.40; 95% confidence interval [CI], 0.22–0.71; P=.002). Infections also were lower in the newborns, 18.1% versus 23.8% (RR, 0.76; 95% CI, 0.58–0.99; P=.052), delivered to mothers who received azithromycin. The greatest impact on neonatal infections was the reduced frequency of skin infections.
In 2021, Subramaniam and colleagues evaluated the effect of a single dose of oral azithromycin with, or without, amoxicillin on the prevalence of peripartum infection in laboring women in Cameroon.2 Patients and their newborns were followed for 6 weeks after delivery. Unlike the previous investigation, the authors were unable to show a protective effect of prophylaxis on either maternal or neonatal infection.
Against this backdrop, Tita and colleagues conducted a remarkably large, well-designed, randomized, placebo-controlled study of azithromycin prophylaxis in women at 8 different sites in 7 low- or middle-income countries (the A-PLUS investigation).3
Details of the study
The investigators randomly assigned 29,278 patients at or beyond 28 weeks’ gestation to receive either a 2-g oral dose of azithromycin or placebo during labor. This particular drug was chosen because it is readily available, inexpensive, well tolerated, and has a broad range of activity against many important pelvic pathogens, including genital mycoplasmas. Some patients also received other antibiotics, for example, for group B streptococcal (GBS) prophylaxis or for CD prophylaxis if abdominal delivery was indicated.
The 2 primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis within 4 weeks of delivery. Secondary outcomes included individual components of the primary outcomes.
Results. The results of the investigation were compelling, and the data safety monitoring committee recommended stopping the trial early because of clear maternal benefit. The groups were well balanced with respect to important characteristics, such as incidence of CD, receipt of other prophylactic antibiotics, and median time between randomization and delivery.
The incidence of maternal sepsis or death was lower in the azithromycin group (1.6% vs 2.4%; RR, 0.67; 95% CI, 0.56–0.79; P<.001). The key effect was on the frequency of maternal sepsis because the incidence of maternal death was very low in both groups, 0.1%. With respect to secondary outcomes, prophylaxis was effective in reducing the frequency of endometritis (RR, 0.66; 95% CI, 0.55–0.79) and perineal and incisional infection (RR, 0.71; 95% CI, 0.56–0.85).
There was no difference in the frequency of neonatal sepsis or death. There also was no difference in the frequency of adverse drug effects in either group. Of note, more cases of neonatal pyloric stenosis were observed in the azithromycin group, but the overall incidence was lower than the expected background rate. This possible “signal” is important because this effect has been noted with increased frequency in neonates who received this antibiotic. ●
I believe that Tita and colleagues are quite correct in concluding that the simple, inexpensive intervention of azithromycin prophylaxis should be used routinely in patient populations similar to those included in this investigation and that the intervention can be invaluable in advancing the World Health Organization’s campaign to reduce the rate of maternal mortality in low- and middleresource nations.
What is not clear, however, is whether this same intervention would be effective in high-resource countries in which the level of skill of the obstetric providers is higher and more uniform; deliveries occur under more optimal sanitary conditions; treatment and prophylaxis for infections such as gonorrhea, chlamydia, chorioamnionitis, and GBS is more consistent; and early neonatal care is more robust. A similar large trial in wellresourced nations would indeed be welcome, particularly if the trial also addressed the possibility of an adverse effect on the neonatal microbiome if a policy of nearly universal antibiotic prophylaxis was adopted.
In the interim, we should focus our attention on the key interventions that are of proven value in decreasing the risk of peripartum maternal and neonatal infection:
- consistently screening for GBS colonization and administering intrapartum antibiotic prophylaxis to patients who test positive
- consistently screening for gonococcal and chlamydia infection in the antepartum period and treating infected patients with appropriate antibiotics
- minimizing the number of internal vaginal examinations during labor, particularly following rupture of membranes
- promptly identifying patients with chorioamnionitis and treating with antibiotics that specifically target GBS and Escherichia coli, the 2 most likely causes of neonatal sepsis, pneumonia, and meningitis
- administering preoperative prophylactic antibiotics (cefazolin plus azithromycin) to women who require CD.
PATRICK DUFF, MD
- Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139:e20162281. doi:10.1542/peds.2016-2281.
- Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138:703-713. doi:10.1097/AOG.0000000000004565.
- Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.

Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
EXPERT COMMENTARY
Maternal peripartum infection is 1 of the top 5 causes of maternal death, accounting for about 10% of cases of maternal mortality. Cesarean delivery (CD), of course, is the most important risk factor for puerperal infection. However, even vaginal delivery, particularly in low- to middle-resource countries, where deliveries often occur under less-than-optimal conditions, may be associated with a surprisingly high frequency of both maternal and neonatal infections. The beneficial effect of prophylactic antibiotics for CD is well established. An important remaining question is whether similar benefit can be achieved with prophylaxis for women planning to have a vaginal birth.
In 2017, Oluwalana and colleagues conducted a prospective, randomized, double-blind, placebo-controlled trial of a single 2-g oral dose of azithromycin in Gambian women undergoing labor.1 During the 8 weeks after delivery, maternal infections were lower in the azithromycin group, 3.6% versus 9.2% (relative risk [RR], 0.40; 95% confidence interval [CI], 0.22–0.71; P=.002). Infections also were lower in the newborns, 18.1% versus 23.8% (RR, 0.76; 95% CI, 0.58–0.99; P=.052), delivered to mothers who received azithromycin. The greatest impact on neonatal infections was the reduced frequency of skin infections.
In 2021, Subramaniam and colleagues evaluated the effect of a single dose of oral azithromycin with, or without, amoxicillin on the prevalence of peripartum infection in laboring women in Cameroon.2 Patients and their newborns were followed for 6 weeks after delivery. Unlike the previous investigation, the authors were unable to show a protective effect of prophylaxis on either maternal or neonatal infection.
Against this backdrop, Tita and colleagues conducted a remarkably large, well-designed, randomized, placebo-controlled study of azithromycin prophylaxis in women at 8 different sites in 7 low- or middle-income countries (the A-PLUS investigation).3
Details of the study
The investigators randomly assigned 29,278 patients at or beyond 28 weeks’ gestation to receive either a 2-g oral dose of azithromycin or placebo during labor. This particular drug was chosen because it is readily available, inexpensive, well tolerated, and has a broad range of activity against many important pelvic pathogens, including genital mycoplasmas. Some patients also received other antibiotics, for example, for group B streptococcal (GBS) prophylaxis or for CD prophylaxis if abdominal delivery was indicated.
The 2 primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis within 4 weeks of delivery. Secondary outcomes included individual components of the primary outcomes.
Results. The results of the investigation were compelling, and the data safety monitoring committee recommended stopping the trial early because of clear maternal benefit. The groups were well balanced with respect to important characteristics, such as incidence of CD, receipt of other prophylactic antibiotics, and median time between randomization and delivery.
The incidence of maternal sepsis or death was lower in the azithromycin group (1.6% vs 2.4%; RR, 0.67; 95% CI, 0.56–0.79; P<.001). The key effect was on the frequency of maternal sepsis because the incidence of maternal death was very low in both groups, 0.1%. With respect to secondary outcomes, prophylaxis was effective in reducing the frequency of endometritis (RR, 0.66; 95% CI, 0.55–0.79) and perineal and incisional infection (RR, 0.71; 95% CI, 0.56–0.85).
There was no difference in the frequency of neonatal sepsis or death. There also was no difference in the frequency of adverse drug effects in either group. Of note, more cases of neonatal pyloric stenosis were observed in the azithromycin group, but the overall incidence was lower than the expected background rate. This possible “signal” is important because this effect has been noted with increased frequency in neonates who received this antibiotic. ●
I believe that Tita and colleagues are quite correct in concluding that the simple, inexpensive intervention of azithromycin prophylaxis should be used routinely in patient populations similar to those included in this investigation and that the intervention can be invaluable in advancing the World Health Organization’s campaign to reduce the rate of maternal mortality in low- and middleresource nations.
What is not clear, however, is whether this same intervention would be effective in high-resource countries in which the level of skill of the obstetric providers is higher and more uniform; deliveries occur under more optimal sanitary conditions; treatment and prophylaxis for infections such as gonorrhea, chlamydia, chorioamnionitis, and GBS is more consistent; and early neonatal care is more robust. A similar large trial in wellresourced nations would indeed be welcome, particularly if the trial also addressed the possibility of an adverse effect on the neonatal microbiome if a policy of nearly universal antibiotic prophylaxis was adopted.
In the interim, we should focus our attention on the key interventions that are of proven value in decreasing the risk of peripartum maternal and neonatal infection:
- consistently screening for GBS colonization and administering intrapartum antibiotic prophylaxis to patients who test positive
- consistently screening for gonococcal and chlamydia infection in the antepartum period and treating infected patients with appropriate antibiotics
- minimizing the number of internal vaginal examinations during labor, particularly following rupture of membranes
- promptly identifying patients with chorioamnionitis and treating with antibiotics that specifically target GBS and Escherichia coli, the 2 most likely causes of neonatal sepsis, pneumonia, and meningitis
- administering preoperative prophylactic antibiotics (cefazolin plus azithromycin) to women who require CD.
PATRICK DUFF, MD

Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
EXPERT COMMENTARY
Maternal peripartum infection is 1 of the top 5 causes of maternal death, accounting for about 10% of cases of maternal mortality. Cesarean delivery (CD), of course, is the most important risk factor for puerperal infection. However, even vaginal delivery, particularly in low- to middle-resource countries, where deliveries often occur under less-than-optimal conditions, may be associated with a surprisingly high frequency of both maternal and neonatal infections. The beneficial effect of prophylactic antibiotics for CD is well established. An important remaining question is whether similar benefit can be achieved with prophylaxis for women planning to have a vaginal birth.
In 2017, Oluwalana and colleagues conducted a prospective, randomized, double-blind, placebo-controlled trial of a single 2-g oral dose of azithromycin in Gambian women undergoing labor.1 During the 8 weeks after delivery, maternal infections were lower in the azithromycin group, 3.6% versus 9.2% (relative risk [RR], 0.40; 95% confidence interval [CI], 0.22–0.71; P=.002). Infections also were lower in the newborns, 18.1% versus 23.8% (RR, 0.76; 95% CI, 0.58–0.99; P=.052), delivered to mothers who received azithromycin. The greatest impact on neonatal infections was the reduced frequency of skin infections.
In 2021, Subramaniam and colleagues evaluated the effect of a single dose of oral azithromycin with, or without, amoxicillin on the prevalence of peripartum infection in laboring women in Cameroon.2 Patients and their newborns were followed for 6 weeks after delivery. Unlike the previous investigation, the authors were unable to show a protective effect of prophylaxis on either maternal or neonatal infection.
Against this backdrop, Tita and colleagues conducted a remarkably large, well-designed, randomized, placebo-controlled study of azithromycin prophylaxis in women at 8 different sites in 7 low- or middle-income countries (the A-PLUS investigation).3
Details of the study
The investigators randomly assigned 29,278 patients at or beyond 28 weeks’ gestation to receive either a 2-g oral dose of azithromycin or placebo during labor. This particular drug was chosen because it is readily available, inexpensive, well tolerated, and has a broad range of activity against many important pelvic pathogens, including genital mycoplasmas. Some patients also received other antibiotics, for example, for group B streptococcal (GBS) prophylaxis or for CD prophylaxis if abdominal delivery was indicated.
The 2 primary outcomes were a composite of maternal sepsis or death and a composite of stillbirth or neonatal death or sepsis within 4 weeks of delivery. Secondary outcomes included individual components of the primary outcomes.
Results. The results of the investigation were compelling, and the data safety monitoring committee recommended stopping the trial early because of clear maternal benefit. The groups were well balanced with respect to important characteristics, such as incidence of CD, receipt of other prophylactic antibiotics, and median time between randomization and delivery.
The incidence of maternal sepsis or death was lower in the azithromycin group (1.6% vs 2.4%; RR, 0.67; 95% CI, 0.56–0.79; P<.001). The key effect was on the frequency of maternal sepsis because the incidence of maternal death was very low in both groups, 0.1%. With respect to secondary outcomes, prophylaxis was effective in reducing the frequency of endometritis (RR, 0.66; 95% CI, 0.55–0.79) and perineal and incisional infection (RR, 0.71; 95% CI, 0.56–0.85).
There was no difference in the frequency of neonatal sepsis or death. There also was no difference in the frequency of adverse drug effects in either group. Of note, more cases of neonatal pyloric stenosis were observed in the azithromycin group, but the overall incidence was lower than the expected background rate. This possible “signal” is important because this effect has been noted with increased frequency in neonates who received this antibiotic. ●
I believe that Tita and colleagues are quite correct in concluding that the simple, inexpensive intervention of azithromycin prophylaxis should be used routinely in patient populations similar to those included in this investigation and that the intervention can be invaluable in advancing the World Health Organization’s campaign to reduce the rate of maternal mortality in low- and middleresource nations.
What is not clear, however, is whether this same intervention would be effective in high-resource countries in which the level of skill of the obstetric providers is higher and more uniform; deliveries occur under more optimal sanitary conditions; treatment and prophylaxis for infections such as gonorrhea, chlamydia, chorioamnionitis, and GBS is more consistent; and early neonatal care is more robust. A similar large trial in wellresourced nations would indeed be welcome, particularly if the trial also addressed the possibility of an adverse effect on the neonatal microbiome if a policy of nearly universal antibiotic prophylaxis was adopted.
In the interim, we should focus our attention on the key interventions that are of proven value in decreasing the risk of peripartum maternal and neonatal infection:
- consistently screening for GBS colonization and administering intrapartum antibiotic prophylaxis to patients who test positive
- consistently screening for gonococcal and chlamydia infection in the antepartum period and treating infected patients with appropriate antibiotics
- minimizing the number of internal vaginal examinations during labor, particularly following rupture of membranes
- promptly identifying patients with chorioamnionitis and treating with antibiotics that specifically target GBS and Escherichia coli, the 2 most likely causes of neonatal sepsis, pneumonia, and meningitis
- administering preoperative prophylactic antibiotics (cefazolin plus azithromycin) to women who require CD.
PATRICK DUFF, MD
- Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139:e20162281. doi:10.1542/peds.2016-2281.
- Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138:703-713. doi:10.1097/AOG.0000000000004565.
- Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
- Oluwalana C, Camara B, Bottomley C, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double-blind trial. Pediatrics. 2017;139:e20162281. doi:10.1542/peds.2016-2281.
- Subramaniam A, Ye Y, Mbah R, et al. Single dose of oral azithromycin with or without amoxicillin to prevent peripartum infection in laboring, high-risk women in Cameroon: a randomized controlled trial. Obstet Gynecol. 2021;138:703-713. doi:10.1097/AOG.0000000000004565.
- Tita ATN, Carlo WA, McClure EM, et al; for the A-PLUS Trial Group. Azithromycin to prevent sepsis or death in women planning a vaginal birth. N Engl J Med. 2023;388:1161-1170. doi:10:1056/NEJMoa2212111.
What is the most effective management of first trimester miscarriage?
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
First trimester miscarriage, the presence of a nonviable intrauterine pregnancy before 13 weeks’ gestation, is a common complication occurring in approximately 15% of clinical pregnancies.1,2 The goals for the holistic management of first-trimester miscarriage are to 1) reduce the risk of complications such as excessive bleeding and infection, 2) ensure that the patient is supported during a time of great distress, and 3) optimally counsel the patient about treatment options and elicit the patient’s preferences for care.3 To resolve a miscarriage, the intrauterine pregnancy tissue must be expelled, restoring normal reproductive function.
The options for the management of a nonviable intrauterine pregnancy include expectant management, medication treatment with mifepristone plus misoprostol or misoprostol-alone, or uterine aspiration. In the absence of uterine hemorrhage, infection, or another severe complication of miscarriage, the patient’s preferences should guide the choice of treatment. Many patients with miscarriage prioritize avoiding medical interventions and may prefer expectant management. A patient who prefers rapid and reliable completion of the pregnancy loss process may prefer uterine aspiration. If the patient prefers to avoid uterine aspiration but desires control over the time and location of the expulsion process, medication treatment may be optimal. Many other factors influence a patient’s choice of miscarriage treatment, including balancing work and childcare issues and the ease of scheduling a uterine aspiration. In counseling patients about the options for miscarriage treatment it is helpful to know the success rate of each treatment option.4 This editorial reviews miscarriage treatment outcomes as summarized in a recent Cochrane network meta-analysis.5
Uterine aspiration versus mifepristone-misoprostol
In 2 clinical trials that included 899 patients with miscarriage, successful treatment with uterine aspira-tion versus mifepristone-misoprostolwas reported in 95% and 66% of cases, respectively.6,7
In the largest clinical trial comparing uterine aspiration to mifepristone-misoprostol, 801 patients with first-trimester miscarriage were randomly assigned to uterine aspiration or mifepristone-misoprostol.6 Uterine aspiration and mifepristone-misoprostol were associated with successful miscarriage treatment in 95% and 64% of cases, respectively. In the uterine aspiration group, a second uterine aspiration occurred in 5% of patients. Two patients in the uterine aspiration group needed a third uterine aspiration to resolve the miscarriage. In the mifepristone-misoprostol group, 36% of patients had a uterine aspiration. It should be noted that the trial protocol guided patients having a medication abortion to uterine aspiration if expulsion of miscarriage tissue had not occurred within 8 hours of receiving misoprostol. If the trial protocol permitted 1 to 4 weeks of monitoring after mifepristone-misoprostol treatment, the success rate with medication treatment would be greater. Six to 8 weeks following miscarriage treatment, patient-reported anxiety and depression symptoms were similar in both groups.6
Uterine aspiration versus misoprostol
Among 3 clinical trials that limited enrollment to patients with missed miscarriage, involving 308 patients, the success rates for uterine aspiration and misoprostol treatment was 95% and 62%, respectively.5
In a study sponsored by the National Institutes of Health, 652 patients with missed miscarriage or incomplete miscarriage were randomly assigned in a 1:3 ratioto uterine aspiration or misoprostol treatment (800 µg vaginally). After 8 days of follow-up, successful treatment rates among the patients treated with uterine evacuation or misoprostol was 97% and 84%, respectively.8 Of note, with misoprostol treatment the success rate increased from day 3 to day 8 of follow-up—from 71% to 84%.8
Continue to: Mifepristone-misoprostol versus misoprostol...
Mifepristone-misoprostol versus misoprostol
The combined results of 7 clinical trials of medication management of missed miscarriage that included 1,812 patients showed that successful treatment with mifepristone-misoprostol or misoprostol alone occurred in 80% and 70% of cases, respectively.5
Schreiber and colleagues9 reported a study of 300 patients with an anembryonic gestation or embryonic demise that were between 5 and 12 completed weeks of gestation and randomly assigned to treatment with mifepristone (200 mg) plus vaginal misoprostol (800 µg) administered 24 to 48 hours after mifepristone or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% CI, 1.09–1.43). Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment (9%) than with misoprostol monotherapy (24%) (RR, 0.37; 95% CI, 0.21 ̶ 0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors, including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.10 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.11Chu and colleagues12 reporteda study of medication treatmentof missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study, the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route; 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. At 7 days of follow-up, the success rates in the mifepristone-misoprostol and misoprostol-alone groups were 83% and 76%, respectively. Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53 ̶ 0.95; P=.021).12 A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostolalone for the management of missed miscarriages.13
 Photo: Getty Images
Photo: Getty Images

Expectant management versus uterine aspiration
The combined results of 7 clinical trials that included a total of 1,693 patients showed that successful treatment of miscarriage with expectant management or uterine aspiration occurred in 68% and 93% of cases, respectively.5 In one study, 700 patients with miscarriage were randomly assigned to expectant management or uterine aspiration. Treatment was successful for 56% and 95% of patients in the expectant management and uterine aspiration groups, respectively.6
The Cochrane network meta-analysis concluded that cervical preparation followed by uterine aspiration may be more effective than expectant management, with a reported risk ratio (RR) of 2.12 (95% CI, 1.41–3.20) with low-certainty evidence.5 In addition, uterine aspiration compared with expectant management may reduce the risk of serious complications (RR, 0.55; 95% CI, 0.23–1.32), with a wide range of treatment effects in reported trials and low-certainty evidence.5
In the treatment of miscarriage, the efficacy of expectant management may vary by the type of miscarriage. In one study, following the identification of a miscarriage, the percent of patients who have completed the expulsion of pregnancy tissue by 14 days was reported to be 84% for incomplete miscarriage, 59% for pregnancy loss with no expulsion of tissue, and 52% with ultrasound detection of a nonviable pregnancy with a gestational sac.14
Expectant management versus mifepristone-misoprostol
Aggregated data from 3 clinical trials that included a total of 910 patients showed that successful treatment with expectant management or mifepristone-misoprostol was reported in 48% and 68% of cases, respectively.5 The Cochrane network meta-analysis concluded that mifepristone-misoprostol may be more effective than expectant management, with a risk ratio of 1.42 (95% CI, 1.22–1.66) with low-certainty evidence. In addition, mifepristone-misoprostol compared with expectant management may reduce the risk for serious complications (RR, 0.76; 95% CI, 0.31–1.84) with wide range of treatment effects and low-certainty evidence.5
Continue to: Expectant management versus misoprostol...
Expectant management versus misoprostol
The combined results of 10 clinical trials that included a total of 838 patients with miscarriage, showed that successful treatment with expectant management or misoprostol-alone occurred in 44% and 75% of cases, respectively.5 Among 3 studies limiting enrollment to patients with missed miscarriage, successful treatment with expectant management or misoprostol-alone occurred in 32% and 70%, respectively.5
The Cochrane analysis concluded that misoprostol-alone may be more effective than expectant management, with a reported risk ratio of 1.30 (95% CI, 1.16–1.46) with low-certainty evidence. In addition, misoprostol-alone compared with expectant management may reduce the risk of serious complications (RR, 0.50; 95% CI, 0.22–1.15) with a wide range of treatment effects and low-certainty evidence.5
Patient experience of miscarriage care
Pregnancy loss is often a distressing experience, which is associated with grief, anxiety, depression, and guilt, lasting up to 2 years for some patients.15,16 Patient dissatisfaction with miscarriage care often focuses on 4 issues: a perceived lack of emotional support, failure to elicit patient preferences for treatment, insufficient provision of information, and inconsistent posttreatment follow-up.17-19 When caring for patients with miscarriage, key goals are to communicate medical information with empathy and to provide emotional support. In the setting of a miscarriage, it is easy for patients to perceive that the clinician is insensitive and cold.15 Expressions of sympathy, compassion, and condolence help build an emotional connection and improve trust with the patient. Communications that may be helpful include: “I am sorry for your loss,” “I wish the outcome could be different,” “Our clinical team wants to provide you the best care possible,” and “May I ask how you are feeling?” Many patients report that they would like to have been offered mental health services as part of their miscarriage care.15
The Cochrane network meta-analysis of miscarriage concluded that uterine aspiration, misoprostol-mifepristone, and misoprostol-alone were likely more effective in resolving a miscarriage than expectant management.5 The strength of the conclusion was limited because of significant heterogeneity among studies, including different inclusion criteria, definition of success, and length of follow-up. Clinical trials with follow-up intervals more than 7 days generally reported greater success rates with expectant14 and medication management8 than studies with short follow-up intervals. Generally, expectant or medication management treatment is more likely to be successful in cases of incomplete abortion than in cases of missed miscarriage.5
In a rank analysis of treatment efficacy, uterine aspiration was top-ranked, followed by medication management. Expectant management had the greatest probability of being associated with unplanned uterine aspiration. Based on my analysis of available miscarriage studies, I estimate that the treatment success rates are approximately:
- uterine aspiration (93% to 99%)
- misoprostol-mifepristone (66% to 84%)
- misoprostol-alone (62% to 76%)
- expectant management (32% to 68%).
Although there may be significant differences in efficacy among the treatment options, offering patients all available approaches to treatment, providing information about the relative success of each approach, and eliciting the patient preference for care ensures an optimal patient experience during a major life event. ●
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. Br Med J. 1997;315:32-34.
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189-194.
- Wallace R, DiLaura A, Dehlendorf C. “Every person’s just different”: women’s experiences with counseling for early pregnancy loss management. Womens Health Issues. 2017;27:456-462.
- Early pregnancy loss. ACOG Practice Bulletin No. 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132: E197-E207.
- Ghosh J, Papadopoulou A, Devall AJ, et al. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst Rev. 2021;CD012602.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical or surgical? Br Med J. 2006;332:1235-1240.
- Niinimaki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367-372.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:21612170.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-7.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:E201594.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128:1534-1545.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. Br Med J. 2002;324:873-875.
- Smith LF, Frost J, Levitas R, et al. Women’s experience of three early miscarriage options. Br J Gen Pract. 2006;56:198-205.
- Leppert PC, Pahlka BS. Grieving characteristics after spontaneous abortion: a management approach. Obstet Gynecol. 1984;64:119-122.
- Ho AL, Hernandez A, Robb JM, et al. Spontaneous miscarriage management experience: a systematic review. Cureus. 2022;14:E24269. 1
- Geller PA, Psaros C, Levine Kornfield S. Satisfaction with pregnancy loss aftercare: are women getting what they want? Arch Women’s Ment Health. 2010;13:111-124.
- Miller CA, Roe AH, McAllister A, et al. Patient experiences with miscarriage management in the emergency and ambulatory settings. Obstet Gynecol. 2019;134:1285-1292.
Insurers refusing MRI for women at high risk for breast cancer
Women harboring BRCA1/2 gene mutations are at high risk for breast cancer, and thus it’s recommended they undergo annual breast MRI screening in addition to mammogram screening.
However, some women are finding that their insurer is refusing to cover the cost of the MRI.
A new study exploring this issue was presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“Despite guidelines supporting annual breast MRI for screening in patients with gBRCA1/2, insurance denials were present in 11% of patients,” said lead author Sushmita Gordhandas, MD, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “In a high-resource setting, up to 14% of patients who were denied coverage did not undergo recommended MRI screening.”
She also pointed out that the rate of denials was rising. “Compared to 2020, there were significantly more denials, and denials on appeal, in 2021,” Dr. Gordhandas said. “This suggested worsening barriers and added burden on health care systems.”
The addition of MRI to mammography is a standard recommendation for women with BRCA mutations, she pointed out, as it has been shown improve detection of early disease and decrease interval cancer development.
An expert not involved in the study noted that the recommendation for annual MRI screening in women at high risk for breast cancer is “substantiated by many publications, including multiple prospective clinical trials.”
Linda Moy, MD, a radiologist at NYU Langone’s Perlmutter Cancer Center and professor of radiology at NYU Grossman School of Medicine, both in New York, noted that the American Cancer Society’s Guidelines for screening breast MRI recommends annual breast MRI in women with a lifetime risk of greater than 20% – which includes women who are BRCA carriers – and recommends the screening begins at age 30.
“The lifetime breast cancer risk is 72% among BRCA1 and 69% among BRCA2 carriers,” she said, adding that the “American College of Radiology also recommends for BRCA carriers to undergo annual screening MRI at age 30.”
The National Comprehensive Cancer Network recommends that women at high risk for breast cancer undergo a mammogram and breast MRI every year starting at age 25 to 40, depending on the type of gene mutation, noted Dr. Gordhandas. “These guidelines are consistent with those from American College of Obstetricians and Gynecologists, the American Cancer Society, and the American College of Radiology.”
Denials increased over time
For the study, Dr. Gordhandas and colleagues looked at the frequency of insurance denials for indicated breast MRI screening in women with germline BRCA1/2 pathogenic variants, and also looked at recent trends in denials over time.
The cohort comprised 682 women with BRCA1/2 gene mutations who were followed in a specialized high-risk breast cancer clinic, and who had breast MRIs ordered from 2020 to 2021. They were then cross-referenced with a database of insurance denials. Radiology records were also accessed to determine if screening breast MRIs had been performed in 2020 and 2021, and rates of MRI denials and results after appeals were determined. The rates between the 2 years were then compared.
The team found that overall, 73 women (11%) had an MRI denied. The median age of women who received a denial was 38 years, whereas those who had it approved was 44 years. “Patients with denials were significantly younger and more likely to be in the Medicaid population,” said Dr. Gordhandas.
In 2020, 29 breast MRIs (5%) were denied, and on appeal, 8 (28%) were denied and 21 (72%) approved. The number of denials rose in 2021 but approvals remained the same; 45 breast MRIs were denied (8%); on appeal, 23 (51%) were denied, and 22 (49%) approved.
Thus, noted the authors, there were significantly more denials in 2021 as compared with 2020 (P = .044), and the denials in 2021 denials were statistically more likely to be denied on appeal (P = .045).
Among the women whose coverage was denied, four (14%) in 2020 and five (11%) in 2021 did not have an MRI screening performed. And within this group, 17 women (2.5%) received a diagnosis of cancer; 12 (1.8%) had invasive carcinoma, and 5 (0.7%) had ductal carcinoma in situ (DCIS). One patient with DCIS had an MRI denial prior to receiving her diagnosis.
“The top reasons given for denials were that they were outside the approved time frame, authorization on file for a similar study, and that the clinician failed to show medical necessity,” she explained.
Additional data are needed to establish a trend. “We are working to increase the approval time frame, which is currently 45 days, and provide resources for the patient to deal with denials,” Dr. Gordhandas added. “We also have to advocate for updates to [U.S. Preventive Services Task Force] screening recommendations in high-risk patients.”
Dr. Gordhandas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women harboring BRCA1/2 gene mutations are at high risk for breast cancer, and thus it’s recommended they undergo annual breast MRI screening in addition to mammogram screening.
However, some women are finding that their insurer is refusing to cover the cost of the MRI.
A new study exploring this issue was presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“Despite guidelines supporting annual breast MRI for screening in patients with gBRCA1/2, insurance denials were present in 11% of patients,” said lead author Sushmita Gordhandas, MD, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “In a high-resource setting, up to 14% of patients who were denied coverage did not undergo recommended MRI screening.”
She also pointed out that the rate of denials was rising. “Compared to 2020, there were significantly more denials, and denials on appeal, in 2021,” Dr. Gordhandas said. “This suggested worsening barriers and added burden on health care systems.”
The addition of MRI to mammography is a standard recommendation for women with BRCA mutations, she pointed out, as it has been shown improve detection of early disease and decrease interval cancer development.
An expert not involved in the study noted that the recommendation for annual MRI screening in women at high risk for breast cancer is “substantiated by many publications, including multiple prospective clinical trials.”
Linda Moy, MD, a radiologist at NYU Langone’s Perlmutter Cancer Center and professor of radiology at NYU Grossman School of Medicine, both in New York, noted that the American Cancer Society’s Guidelines for screening breast MRI recommends annual breast MRI in women with a lifetime risk of greater than 20% – which includes women who are BRCA carriers – and recommends the screening begins at age 30.
“The lifetime breast cancer risk is 72% among BRCA1 and 69% among BRCA2 carriers,” she said, adding that the “American College of Radiology also recommends for BRCA carriers to undergo annual screening MRI at age 30.”
The National Comprehensive Cancer Network recommends that women at high risk for breast cancer undergo a mammogram and breast MRI every year starting at age 25 to 40, depending on the type of gene mutation, noted Dr. Gordhandas. “These guidelines are consistent with those from American College of Obstetricians and Gynecologists, the American Cancer Society, and the American College of Radiology.”
Denials increased over time
For the study, Dr. Gordhandas and colleagues looked at the frequency of insurance denials for indicated breast MRI screening in women with germline BRCA1/2 pathogenic variants, and also looked at recent trends in denials over time.
The cohort comprised 682 women with BRCA1/2 gene mutations who were followed in a specialized high-risk breast cancer clinic, and who had breast MRIs ordered from 2020 to 2021. They were then cross-referenced with a database of insurance denials. Radiology records were also accessed to determine if screening breast MRIs had been performed in 2020 and 2021, and rates of MRI denials and results after appeals were determined. The rates between the 2 years were then compared.
The team found that overall, 73 women (11%) had an MRI denied. The median age of women who received a denial was 38 years, whereas those who had it approved was 44 years. “Patients with denials were significantly younger and more likely to be in the Medicaid population,” said Dr. Gordhandas.
In 2020, 29 breast MRIs (5%) were denied, and on appeal, 8 (28%) were denied and 21 (72%) approved. The number of denials rose in 2021 but approvals remained the same; 45 breast MRIs were denied (8%); on appeal, 23 (51%) were denied, and 22 (49%) approved.
Thus, noted the authors, there were significantly more denials in 2021 as compared with 2020 (P = .044), and the denials in 2021 denials were statistically more likely to be denied on appeal (P = .045).
Among the women whose coverage was denied, four (14%) in 2020 and five (11%) in 2021 did not have an MRI screening performed. And within this group, 17 women (2.5%) received a diagnosis of cancer; 12 (1.8%) had invasive carcinoma, and 5 (0.7%) had ductal carcinoma in situ (DCIS). One patient with DCIS had an MRI denial prior to receiving her diagnosis.
“The top reasons given for denials were that they were outside the approved time frame, authorization on file for a similar study, and that the clinician failed to show medical necessity,” she explained.
Additional data are needed to establish a trend. “We are working to increase the approval time frame, which is currently 45 days, and provide resources for the patient to deal with denials,” Dr. Gordhandas added. “We also have to advocate for updates to [U.S. Preventive Services Task Force] screening recommendations in high-risk patients.”
Dr. Gordhandas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women harboring BRCA1/2 gene mutations are at high risk for breast cancer, and thus it’s recommended they undergo annual breast MRI screening in addition to mammogram screening.
However, some women are finding that their insurer is refusing to cover the cost of the MRI.
A new study exploring this issue was presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“Despite guidelines supporting annual breast MRI for screening in patients with gBRCA1/2, insurance denials were present in 11% of patients,” said lead author Sushmita Gordhandas, MD, a gynecologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York. “In a high-resource setting, up to 14% of patients who were denied coverage did not undergo recommended MRI screening.”
She also pointed out that the rate of denials was rising. “Compared to 2020, there were significantly more denials, and denials on appeal, in 2021,” Dr. Gordhandas said. “This suggested worsening barriers and added burden on health care systems.”
The addition of MRI to mammography is a standard recommendation for women with BRCA mutations, she pointed out, as it has been shown improve detection of early disease and decrease interval cancer development.
An expert not involved in the study noted that the recommendation for annual MRI screening in women at high risk for breast cancer is “substantiated by many publications, including multiple prospective clinical trials.”
Linda Moy, MD, a radiologist at NYU Langone’s Perlmutter Cancer Center and professor of radiology at NYU Grossman School of Medicine, both in New York, noted that the American Cancer Society’s Guidelines for screening breast MRI recommends annual breast MRI in women with a lifetime risk of greater than 20% – which includes women who are BRCA carriers – and recommends the screening begins at age 30.
“The lifetime breast cancer risk is 72% among BRCA1 and 69% among BRCA2 carriers,” she said, adding that the “American College of Radiology also recommends for BRCA carriers to undergo annual screening MRI at age 30.”
The National Comprehensive Cancer Network recommends that women at high risk for breast cancer undergo a mammogram and breast MRI every year starting at age 25 to 40, depending on the type of gene mutation, noted Dr. Gordhandas. “These guidelines are consistent with those from American College of Obstetricians and Gynecologists, the American Cancer Society, and the American College of Radiology.”
Denials increased over time
For the study, Dr. Gordhandas and colleagues looked at the frequency of insurance denials for indicated breast MRI screening in women with germline BRCA1/2 pathogenic variants, and also looked at recent trends in denials over time.
The cohort comprised 682 women with BRCA1/2 gene mutations who were followed in a specialized high-risk breast cancer clinic, and who had breast MRIs ordered from 2020 to 2021. They were then cross-referenced with a database of insurance denials. Radiology records were also accessed to determine if screening breast MRIs had been performed in 2020 and 2021, and rates of MRI denials and results after appeals were determined. The rates between the 2 years were then compared.
The team found that overall, 73 women (11%) had an MRI denied. The median age of women who received a denial was 38 years, whereas those who had it approved was 44 years. “Patients with denials were significantly younger and more likely to be in the Medicaid population,” said Dr. Gordhandas.
In 2020, 29 breast MRIs (5%) were denied, and on appeal, 8 (28%) were denied and 21 (72%) approved. The number of denials rose in 2021 but approvals remained the same; 45 breast MRIs were denied (8%); on appeal, 23 (51%) were denied, and 22 (49%) approved.
Thus, noted the authors, there were significantly more denials in 2021 as compared with 2020 (P = .044), and the denials in 2021 denials were statistically more likely to be denied on appeal (P = .045).
Among the women whose coverage was denied, four (14%) in 2020 and five (11%) in 2021 did not have an MRI screening performed. And within this group, 17 women (2.5%) received a diagnosis of cancer; 12 (1.8%) had invasive carcinoma, and 5 (0.7%) had ductal carcinoma in situ (DCIS). One patient with DCIS had an MRI denial prior to receiving her diagnosis.
“The top reasons given for denials were that they were outside the approved time frame, authorization on file for a similar study, and that the clinician failed to show medical necessity,” she explained.
Additional data are needed to establish a trend. “We are working to increase the approval time frame, which is currently 45 days, and provide resources for the patient to deal with denials,” Dr. Gordhandas added. “We also have to advocate for updates to [U.S. Preventive Services Task Force] screening recommendations in high-risk patients.”
Dr. Gordhandas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SGO 2023
Misoprostol only for abortion: Viable option but not ‘the best’
With a federal judge’s recent ruling clouding the future availability of mifepristone for terminating pregnancies, attention has shifted to the efficacy of another abortion drug, misoprostol.
A misoprostol-only approach also comes with more pronounced side effects.
“Misoprostol only is a good alternative; it’s not the best alternative,” Beverly Gray, MD, associate professor in the department of obstetrics and Gynecology at Duke University, Durham, N.C., said during a video conference on April 12. “The best medication would be to use mifepristone and misoprostol together because they’re efficacious with fewer side effects.”
To medically terminate a pregnancy using the two-drug regimen, patients first take the progesterone blocker mifepristone, which ends the pregnancy. That is followed 24-48 hours later with misoprostol, which causes the uterus to expel the pregnancy tissue. Used in combination, the two drugs have an efficacy rate of 98% in terminating a pregnancy.
An alternative approach is a misoprostol-only regimen. Patients take multiple doses of the drug over the course of hours until the pregnancy passes. This method is considered effective and safe, although patients may experience more nausea, vomiting, diarrhea, bleeding, and cramping.
“It’s effective, but not as effective as the combination treatment,” said Mitchell D. Creinin, MD, professor in the department of obstetrics and gynecology at University of California, Davis. “It also requires much higher doses. To get misoprostol by itself to have relatively high efficacy, you have to use multiple doses. It causes significantly more side effects, and it’s less effective.”
Dr. Creinin was part of a team that earlier this year conducted a study of misoprostol-only medical abortions. In that study, which was published in the journal Contraception, the investigators found that the misoprostol-only regimen was 78% effective at aborting completely without a procedure or unplanned additional medications. The investigators concluded that prohibiting the use of mifepristone was “senseless” but that offering misoprostol-only abortions would be a “safe, effective, and patient-centered approach.”
Both drug regimens are intended to be used during the first trimester of pregnancy, and their effectiveness is influenced by the gestation period.
Medical abortions have grown in popularity. They now account for more than half of all abortions. Last year’s U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization overturned the court’s 1973 ruling in Roe v. Wade, leaving it up to states to regulate abortion. Currently, nearly half of states have banned or are considering some sort of ban on the procedure, according to the Guttmacher Institute, a reproductive health advocacy group.
“Medication abortion is good for privacy in many ways,” Jolynn Dellinger, JD, a visiting lecturer at Duke Law School, said during the conference call with Dr. Gray. “It’s incredibly safe and effective and can be the very best choice for people.”
On April 7, a federal judge in Texas suspended the Food and Drug Administration approval of mifepristone. The drug has been on the market for 23 years. A federal judge in Washington State issued a competing ruling, and the Biden Administration has appealed the Texas decision.
The future of the use of mifepristone is now in the courts, but not that of misoprostol – for now. The latter is used to prevent ulcers; its use in medical abortions is secondary. Dr. Creinin said that that will make it much more difficult for antiabortion advocates to challenge.
While courts, lawmakers, and regulators at the state and federal levels work through what is allowable for medical abortions, the medical community sits and waits.
“We’re working out a variety of scenarios,” Dr. Gray said. “I think right now we’re just hoping that the legislative dust will settle enough so that we’ll have a better understanding. In the meantime, we’re creating protocols and trying to be as prepared as we can.”
A version of this article first appeared on Medscape.com.
With a federal judge’s recent ruling clouding the future availability of mifepristone for terminating pregnancies, attention has shifted to the efficacy of another abortion drug, misoprostol.
A misoprostol-only approach also comes with more pronounced side effects.
“Misoprostol only is a good alternative; it’s not the best alternative,” Beverly Gray, MD, associate professor in the department of obstetrics and Gynecology at Duke University, Durham, N.C., said during a video conference on April 12. “The best medication would be to use mifepristone and misoprostol together because they’re efficacious with fewer side effects.”
To medically terminate a pregnancy using the two-drug regimen, patients first take the progesterone blocker mifepristone, which ends the pregnancy. That is followed 24-48 hours later with misoprostol, which causes the uterus to expel the pregnancy tissue. Used in combination, the two drugs have an efficacy rate of 98% in terminating a pregnancy.
An alternative approach is a misoprostol-only regimen. Patients take multiple doses of the drug over the course of hours until the pregnancy passes. This method is considered effective and safe, although patients may experience more nausea, vomiting, diarrhea, bleeding, and cramping.
“It’s effective, but not as effective as the combination treatment,” said Mitchell D. Creinin, MD, professor in the department of obstetrics and gynecology at University of California, Davis. “It also requires much higher doses. To get misoprostol by itself to have relatively high efficacy, you have to use multiple doses. It causes significantly more side effects, and it’s less effective.”
Dr. Creinin was part of a team that earlier this year conducted a study of misoprostol-only medical abortions. In that study, which was published in the journal Contraception, the investigators found that the misoprostol-only regimen was 78% effective at aborting completely without a procedure or unplanned additional medications. The investigators concluded that prohibiting the use of mifepristone was “senseless” but that offering misoprostol-only abortions would be a “safe, effective, and patient-centered approach.”
Both drug regimens are intended to be used during the first trimester of pregnancy, and their effectiveness is influenced by the gestation period.
Medical abortions have grown in popularity. They now account for more than half of all abortions. Last year’s U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization overturned the court’s 1973 ruling in Roe v. Wade, leaving it up to states to regulate abortion. Currently, nearly half of states have banned or are considering some sort of ban on the procedure, according to the Guttmacher Institute, a reproductive health advocacy group.
“Medication abortion is good for privacy in many ways,” Jolynn Dellinger, JD, a visiting lecturer at Duke Law School, said during the conference call with Dr. Gray. “It’s incredibly safe and effective and can be the very best choice for people.”
On April 7, a federal judge in Texas suspended the Food and Drug Administration approval of mifepristone. The drug has been on the market for 23 years. A federal judge in Washington State issued a competing ruling, and the Biden Administration has appealed the Texas decision.
The future of the use of mifepristone is now in the courts, but not that of misoprostol – for now. The latter is used to prevent ulcers; its use in medical abortions is secondary. Dr. Creinin said that that will make it much more difficult for antiabortion advocates to challenge.
While courts, lawmakers, and regulators at the state and federal levels work through what is allowable for medical abortions, the medical community sits and waits.
“We’re working out a variety of scenarios,” Dr. Gray said. “I think right now we’re just hoping that the legislative dust will settle enough so that we’ll have a better understanding. In the meantime, we’re creating protocols and trying to be as prepared as we can.”
A version of this article first appeared on Medscape.com.
With a federal judge’s recent ruling clouding the future availability of mifepristone for terminating pregnancies, attention has shifted to the efficacy of another abortion drug, misoprostol.
A misoprostol-only approach also comes with more pronounced side effects.
“Misoprostol only is a good alternative; it’s not the best alternative,” Beverly Gray, MD, associate professor in the department of obstetrics and Gynecology at Duke University, Durham, N.C., said during a video conference on April 12. “The best medication would be to use mifepristone and misoprostol together because they’re efficacious with fewer side effects.”
To medically terminate a pregnancy using the two-drug regimen, patients first take the progesterone blocker mifepristone, which ends the pregnancy. That is followed 24-48 hours later with misoprostol, which causes the uterus to expel the pregnancy tissue. Used in combination, the two drugs have an efficacy rate of 98% in terminating a pregnancy.
An alternative approach is a misoprostol-only regimen. Patients take multiple doses of the drug over the course of hours until the pregnancy passes. This method is considered effective and safe, although patients may experience more nausea, vomiting, diarrhea, bleeding, and cramping.
“It’s effective, but not as effective as the combination treatment,” said Mitchell D. Creinin, MD, professor in the department of obstetrics and gynecology at University of California, Davis. “It also requires much higher doses. To get misoprostol by itself to have relatively high efficacy, you have to use multiple doses. It causes significantly more side effects, and it’s less effective.”
Dr. Creinin was part of a team that earlier this year conducted a study of misoprostol-only medical abortions. In that study, which was published in the journal Contraception, the investigators found that the misoprostol-only regimen was 78% effective at aborting completely without a procedure or unplanned additional medications. The investigators concluded that prohibiting the use of mifepristone was “senseless” but that offering misoprostol-only abortions would be a “safe, effective, and patient-centered approach.”
Both drug regimens are intended to be used during the first trimester of pregnancy, and their effectiveness is influenced by the gestation period.
Medical abortions have grown in popularity. They now account for more than half of all abortions. Last year’s U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization overturned the court’s 1973 ruling in Roe v. Wade, leaving it up to states to regulate abortion. Currently, nearly half of states have banned or are considering some sort of ban on the procedure, according to the Guttmacher Institute, a reproductive health advocacy group.
“Medication abortion is good for privacy in many ways,” Jolynn Dellinger, JD, a visiting lecturer at Duke Law School, said during the conference call with Dr. Gray. “It’s incredibly safe and effective and can be the very best choice for people.”
On April 7, a federal judge in Texas suspended the Food and Drug Administration approval of mifepristone. The drug has been on the market for 23 years. A federal judge in Washington State issued a competing ruling, and the Biden Administration has appealed the Texas decision.
The future of the use of mifepristone is now in the courts, but not that of misoprostol – for now. The latter is used to prevent ulcers; its use in medical abortions is secondary. Dr. Creinin said that that will make it much more difficult for antiabortion advocates to challenge.
While courts, lawmakers, and regulators at the state and federal levels work through what is allowable for medical abortions, the medical community sits and waits.
“We’re working out a variety of scenarios,” Dr. Gray said. “I think right now we’re just hoping that the legislative dust will settle enough so that we’ll have a better understanding. In the meantime, we’re creating protocols and trying to be as prepared as we can.”
A version of this article first appeared on Medscape.com.
Should you recommend e-cigs to help patients quit smoking?
In 2014, after smoking cigarettes for 40 years, Kati Markowitz decided to switch to vaping. She had heard the newer electronic cigarettes might be less harmful. And, at the time, she said, she wasn’t aware of other options to try to quit smoking.
For 7 years, she vaped every day.
Then Ms. Markowitz received news she’d hoped never to hear: She had lung cancer. A nodule detected in a CT scan had grown. She was scheduled for treatment – the removal of an entire lobe from her right lung. But first, she said, her surgeon told her she had to quit vaping, which reduces the risk for post-operative complications and enables a healthy recovery.
Ms. Markowitz had thought switching to vaping would be less harmful than smoking cigarettes. Now, she no longer believes that’s true.
“Did I fool myself by hoping to get lucky and not have any bad repercussions? Yes, I did,” Ms. Markowitz said, adding that she wonders if vaping contributed to her lung cancer or if she’ll experience other negative health effects in the future.
Researchers are divided on if e-cigarettes are as effective in smoking cessation as other nicotine replacement therapies like gums and lozenges. They also say more research is needed on the long-term health impacts of vaping to ultimately determine if vapes are a safe replacement for cigarettes.
“There is scientific research to support vaping as a cessation tool, but we wouldn’t use it as a first line of defense because we still need longitudinal studies to understand the long-term risk of e-cigarettes,” said Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery, Eatontown, N.J. “We also need research to understand exactly how we could use e-cigarettes as a cessation device.”
Vaping to quit
The first prototypes of e-cigarettes were developed in the 1930s, although what are now known as vapes weren’t sold by manufacturers until the 2000s in the United States, following an invention by a former health official in China. The vape was touted by both researchers and manufacturers over the years of development as a way to quit smoking cigarettes.
The Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping and accepts donations from the e-cigarette industry, has compiled more than 13,000 testimonials from people who say vaping helped them give up smoking.
Studies show mixed results that using vapes can help traditional smokers quit.
A November 2022 Cochrane review showed a “high certainty of evidence that people are more likely to stop smoking traditional cigarettes for at least 6 months using e-cigarettes, or ‘vapes,’ than using nicotine replacement therapies, such as patches and gums.” The meta-analysis examined 78 studies with more than 22,000 participants. And a 2019 study with 886 participants, published in the New England Journal Medicine, found smokers who tried vaping to quit were twice as likely after a year to have stopped smoking cigarettes than those who used nicotine replacement therapy.
“In terms of the global research, it’s pretty clear that vaping can help smokers quit,” said Peter Shields, MD, a professor in the department of internal medicine at The Ohio State University College of Medicine, Columbus, who specializes in the treatment of lung cancer.
But a 2013 study published in the Lancet, and another from the Lancet in 2019, found only a modest improvement in cessation outcomes when participants used e-cigarettes paired with patches, compared with patches alone.
“For a disruptive technology that was supposed to end combustible tobacco use, there seems very little large-scale disruption,” said Thomas Eissenberg, PhD, co-director of Virginia Commonwealth University’s Center for the Study of Tobacco Products, Richmond.
Michael Joseph Blaha, MD, MPH, director of clinical research at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, pointed to research that shows a portion of people who start vaping to quit smoking end up using both products – or become so-called “dual” users.
“I do think there is fairly high-quality evidence that vaping can lead to more cessation, but at the tradeoff of more long-term dual users and more overall nicotine addiction,” Dr. Blaha said. “Vaping remains a third-line clinical tool after nicotine replacement therapy and FDA-approved cessation medications.”
The U.S. Food and Drug Administration has not approved any e-cigarette or vaping device for smoking cessation, like it has for patches and gums, which means manufacturers cannot market their products as helping tobacco smokers quit.
“ Ms. Hanna, from RWJBarnabas Health’s Institute for Prevention and Recovery, said.
Reducing harm and improving health?
Vapes have also been touted as a boon to individual and public health since cigarette smoking is the leading cause of preventable disease and disability in the United States, responsible for more than 480,000 deaths per year in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Quitting smoking lowers the risk of developing various cancers, heart disease, stroke, and other serious diseases. The aim of nicotine replacement therapy is to help smokers quit by gradually providing the body with smaller doses of nicotine over time, without exposing the body to toxic chemicals found in cigarettes.
“No one should say that e-cigarettes are safe, but compared to cigarettes, the data is consistent: They are not as harmful, and when a smoker switches, it’s better for them,” Dr. Shields said. “Like with other nicotine replacement therapies, if there is a risk that someone stops vaping and returns to smoking, I would rather have them as long-term vapers since it is generally considered to be less harmful than combustible tobacco.”
The FDA has allowed a handful of companies to market their electronic nicotine delivery systems as safer than traditional cigarettes by gaining approval through the Premarket Tobacco Product Applications process. In 2021, the agency announced its first PMTA authorization of an electronic cigarette to R.J. Reynolds for three of its tobacco-flavored vaping products. Regulators approved more products from three additional companies in 2022.
But the FDA has also denied others, including two products in 2023 from R.J. Reynolds, stating that, “the applications lacked sufficient evidence to demonstrate that permitting the marketing of the products would be appropriate for the protection of the public health.”
Questions remain among some researchers on the effects of vaping if used long term. Data on the health effects of vapes are just beginning to emerge and are mainly from studies of animals or cells. Measuring health effects among vape users will entail decades more of study, since Americans only gained access to the products in the 2000s.
Dr. Eissenberg said vaping likely does not cause the same diseases as cigarette smoking, but that does not mean they are not harmful. Ingredients found in e-cigarettes, such as heated propylene glycol, vegetable glycerin, and flavors, have only been used as food ingredients. The potential diseases caused by vapes are still unknown, because inhaling these heated ingredients is new. He also said he had “no issue” with an adult smoker vaping to help them quit smoking – as long they do so for a short period.
“I am very concerned that long-term use in adults could lead to considerable disease and death,” Dr. Eissenberg said. “Simply put, the human lung evolved for one purpose: gas exchange of oxygen in, carbon dioxide out. Anything else that enters the lung is a challenge to the organ.”
But Kenneth Warner, PhD, dean emeritus at the University of Michigan School of Public Health, Ann Arbor, said breaking the addiction to traditional cigarettes could reduce high rates of lung cancer in lower income communities where rates of smoking are comparatively high.
About three times as many Americans smoked (12.6%) than vaped (4.7%) in 2021, but those who live in households with lower incomes are more likely to smoke. According to the CDC, use of tobacco is higher among adults who were uninsured (27.3%) or who had Medicaid coverage (28.6%) than among those with private insurance (16.4%). People with annual family incomes of less than $12,500 also are more likely to be diagnosed with lung cancer than those with family incomes of $50,000 or more. Public health researchers have attributed those disparities in part to higher rates of smoking in lower-income households.
Dr. Warner said many lower-income and other Americans may never quit smoking cigarettes because they believe making the switch to e-cigarettes will not benefit their health. A 2022 study, published in the American Journal of Preventive Medicine, found that the percent of Americans who thought vaping was more harmful than smoking quadrupled between 2018 and 2020, from 6.8% to 28.3%. A third of respondents thought vaping was as harmful as smoking.
“We’ve convinced a large percentage of the American public that vaping is as harmful as smoking when it could be helping people quit smoking,” Dr. Warner said. “People are dying right now.”
Ms. Markowitz did quit smoking by taking up vaping. But now she questions if her lung cancer prognosis would have been delayed, or even avoided, if she’d tried a traditional method like a lozenge or gum instead. She vaped once an hour for most of her 7 years of using the devices.
“For people who are trying to stop smoking, I would recommend something like the patch instead,” Ms. Markowitz said.
The Consumer Advocates for Smoke-free Alternatives receives funding from the vaping industry. Dr. Blaha, Dr. Eissenberg, Ms. Hanna, Dr. Shields, and Dr. Warner reported no funding from the tobacco or e-cigarette industry. Dr. Blaha and Dr. Warner receive tobacco-related research funding from the FDA. Dr. Warner is a member of the FDA’s Tobacco Products Scientific Advisory Committee.
A version of this article first appeared on Medscape.com.
In 2014, after smoking cigarettes for 40 years, Kati Markowitz decided to switch to vaping. She had heard the newer electronic cigarettes might be less harmful. And, at the time, she said, she wasn’t aware of other options to try to quit smoking.
For 7 years, she vaped every day.
Then Ms. Markowitz received news she’d hoped never to hear: She had lung cancer. A nodule detected in a CT scan had grown. She was scheduled for treatment – the removal of an entire lobe from her right lung. But first, she said, her surgeon told her she had to quit vaping, which reduces the risk for post-operative complications and enables a healthy recovery.
Ms. Markowitz had thought switching to vaping would be less harmful than smoking cigarettes. Now, she no longer believes that’s true.
“Did I fool myself by hoping to get lucky and not have any bad repercussions? Yes, I did,” Ms. Markowitz said, adding that she wonders if vaping contributed to her lung cancer or if she’ll experience other negative health effects in the future.
Researchers are divided on if e-cigarettes are as effective in smoking cessation as other nicotine replacement therapies like gums and lozenges. They also say more research is needed on the long-term health impacts of vaping to ultimately determine if vapes are a safe replacement for cigarettes.
“There is scientific research to support vaping as a cessation tool, but we wouldn’t use it as a first line of defense because we still need longitudinal studies to understand the long-term risk of e-cigarettes,” said Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery, Eatontown, N.J. “We also need research to understand exactly how we could use e-cigarettes as a cessation device.”
Vaping to quit
The first prototypes of e-cigarettes were developed in the 1930s, although what are now known as vapes weren’t sold by manufacturers until the 2000s in the United States, following an invention by a former health official in China. The vape was touted by both researchers and manufacturers over the years of development as a way to quit smoking cigarettes.
The Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping and accepts donations from the e-cigarette industry, has compiled more than 13,000 testimonials from people who say vaping helped them give up smoking.
Studies show mixed results that using vapes can help traditional smokers quit.
A November 2022 Cochrane review showed a “high certainty of evidence that people are more likely to stop smoking traditional cigarettes for at least 6 months using e-cigarettes, or ‘vapes,’ than using nicotine replacement therapies, such as patches and gums.” The meta-analysis examined 78 studies with more than 22,000 participants. And a 2019 study with 886 participants, published in the New England Journal Medicine, found smokers who tried vaping to quit were twice as likely after a year to have stopped smoking cigarettes than those who used nicotine replacement therapy.
“In terms of the global research, it’s pretty clear that vaping can help smokers quit,” said Peter Shields, MD, a professor in the department of internal medicine at The Ohio State University College of Medicine, Columbus, who specializes in the treatment of lung cancer.
But a 2013 study published in the Lancet, and another from the Lancet in 2019, found only a modest improvement in cessation outcomes when participants used e-cigarettes paired with patches, compared with patches alone.
“For a disruptive technology that was supposed to end combustible tobacco use, there seems very little large-scale disruption,” said Thomas Eissenberg, PhD, co-director of Virginia Commonwealth University’s Center for the Study of Tobacco Products, Richmond.
Michael Joseph Blaha, MD, MPH, director of clinical research at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, pointed to research that shows a portion of people who start vaping to quit smoking end up using both products – or become so-called “dual” users.
“I do think there is fairly high-quality evidence that vaping can lead to more cessation, but at the tradeoff of more long-term dual users and more overall nicotine addiction,” Dr. Blaha said. “Vaping remains a third-line clinical tool after nicotine replacement therapy and FDA-approved cessation medications.”
The U.S. Food and Drug Administration has not approved any e-cigarette or vaping device for smoking cessation, like it has for patches and gums, which means manufacturers cannot market their products as helping tobacco smokers quit.
“ Ms. Hanna, from RWJBarnabas Health’s Institute for Prevention and Recovery, said.
Reducing harm and improving health?
Vapes have also been touted as a boon to individual and public health since cigarette smoking is the leading cause of preventable disease and disability in the United States, responsible for more than 480,000 deaths per year in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Quitting smoking lowers the risk of developing various cancers, heart disease, stroke, and other serious diseases. The aim of nicotine replacement therapy is to help smokers quit by gradually providing the body with smaller doses of nicotine over time, without exposing the body to toxic chemicals found in cigarettes.
“No one should say that e-cigarettes are safe, but compared to cigarettes, the data is consistent: They are not as harmful, and when a smoker switches, it’s better for them,” Dr. Shields said. “Like with other nicotine replacement therapies, if there is a risk that someone stops vaping and returns to smoking, I would rather have them as long-term vapers since it is generally considered to be less harmful than combustible tobacco.”
The FDA has allowed a handful of companies to market their electronic nicotine delivery systems as safer than traditional cigarettes by gaining approval through the Premarket Tobacco Product Applications process. In 2021, the agency announced its first PMTA authorization of an electronic cigarette to R.J. Reynolds for three of its tobacco-flavored vaping products. Regulators approved more products from three additional companies in 2022.
But the FDA has also denied others, including two products in 2023 from R.J. Reynolds, stating that, “the applications lacked sufficient evidence to demonstrate that permitting the marketing of the products would be appropriate for the protection of the public health.”
Questions remain among some researchers on the effects of vaping if used long term. Data on the health effects of vapes are just beginning to emerge and are mainly from studies of animals or cells. Measuring health effects among vape users will entail decades more of study, since Americans only gained access to the products in the 2000s.
Dr. Eissenberg said vaping likely does not cause the same diseases as cigarette smoking, but that does not mean they are not harmful. Ingredients found in e-cigarettes, such as heated propylene glycol, vegetable glycerin, and flavors, have only been used as food ingredients. The potential diseases caused by vapes are still unknown, because inhaling these heated ingredients is new. He also said he had “no issue” with an adult smoker vaping to help them quit smoking – as long they do so for a short period.
“I am very concerned that long-term use in adults could lead to considerable disease and death,” Dr. Eissenberg said. “Simply put, the human lung evolved for one purpose: gas exchange of oxygen in, carbon dioxide out. Anything else that enters the lung is a challenge to the organ.”
But Kenneth Warner, PhD, dean emeritus at the University of Michigan School of Public Health, Ann Arbor, said breaking the addiction to traditional cigarettes could reduce high rates of lung cancer in lower income communities where rates of smoking are comparatively high.
About three times as many Americans smoked (12.6%) than vaped (4.7%) in 2021, but those who live in households with lower incomes are more likely to smoke. According to the CDC, use of tobacco is higher among adults who were uninsured (27.3%) or who had Medicaid coverage (28.6%) than among those with private insurance (16.4%). People with annual family incomes of less than $12,500 also are more likely to be diagnosed with lung cancer than those with family incomes of $50,000 or more. Public health researchers have attributed those disparities in part to higher rates of smoking in lower-income households.
Dr. Warner said many lower-income and other Americans may never quit smoking cigarettes because they believe making the switch to e-cigarettes will not benefit their health. A 2022 study, published in the American Journal of Preventive Medicine, found that the percent of Americans who thought vaping was more harmful than smoking quadrupled between 2018 and 2020, from 6.8% to 28.3%. A third of respondents thought vaping was as harmful as smoking.
“We’ve convinced a large percentage of the American public that vaping is as harmful as smoking when it could be helping people quit smoking,” Dr. Warner said. “People are dying right now.”
Ms. Markowitz did quit smoking by taking up vaping. But now she questions if her lung cancer prognosis would have been delayed, or even avoided, if she’d tried a traditional method like a lozenge or gum instead. She vaped once an hour for most of her 7 years of using the devices.
“For people who are trying to stop smoking, I would recommend something like the patch instead,” Ms. Markowitz said.
The Consumer Advocates for Smoke-free Alternatives receives funding from the vaping industry. Dr. Blaha, Dr. Eissenberg, Ms. Hanna, Dr. Shields, and Dr. Warner reported no funding from the tobacco or e-cigarette industry. Dr. Blaha and Dr. Warner receive tobacco-related research funding from the FDA. Dr. Warner is a member of the FDA’s Tobacco Products Scientific Advisory Committee.
A version of this article first appeared on Medscape.com.
In 2014, after smoking cigarettes for 40 years, Kati Markowitz decided to switch to vaping. She had heard the newer electronic cigarettes might be less harmful. And, at the time, she said, she wasn’t aware of other options to try to quit smoking.
For 7 years, she vaped every day.
Then Ms. Markowitz received news she’d hoped never to hear: She had lung cancer. A nodule detected in a CT scan had grown. She was scheduled for treatment – the removal of an entire lobe from her right lung. But first, she said, her surgeon told her she had to quit vaping, which reduces the risk for post-operative complications and enables a healthy recovery.
Ms. Markowitz had thought switching to vaping would be less harmful than smoking cigarettes. Now, she no longer believes that’s true.
“Did I fool myself by hoping to get lucky and not have any bad repercussions? Yes, I did,” Ms. Markowitz said, adding that she wonders if vaping contributed to her lung cancer or if she’ll experience other negative health effects in the future.
Researchers are divided on if e-cigarettes are as effective in smoking cessation as other nicotine replacement therapies like gums and lozenges. They also say more research is needed on the long-term health impacts of vaping to ultimately determine if vapes are a safe replacement for cigarettes.
“There is scientific research to support vaping as a cessation tool, but we wouldn’t use it as a first line of defense because we still need longitudinal studies to understand the long-term risk of e-cigarettes,” said Monica Hanna, MPH, assistant director of the Nicotine and Tobacco Recovery Program at RWJBarnabas Health’s Institute for Prevention and Recovery, Eatontown, N.J. “We also need research to understand exactly how we could use e-cigarettes as a cessation device.”
Vaping to quit
The first prototypes of e-cigarettes were developed in the 1930s, although what are now known as vapes weren’t sold by manufacturers until the 2000s in the United States, following an invention by a former health official in China. The vape was touted by both researchers and manufacturers over the years of development as a way to quit smoking cigarettes.
The Consumer Advocates for Smoke-free Alternatives Association, a nonprofit group that supports vaping and accepts donations from the e-cigarette industry, has compiled more than 13,000 testimonials from people who say vaping helped them give up smoking.
Studies show mixed results that using vapes can help traditional smokers quit.
A November 2022 Cochrane review showed a “high certainty of evidence that people are more likely to stop smoking traditional cigarettes for at least 6 months using e-cigarettes, or ‘vapes,’ than using nicotine replacement therapies, such as patches and gums.” The meta-analysis examined 78 studies with more than 22,000 participants. And a 2019 study with 886 participants, published in the New England Journal Medicine, found smokers who tried vaping to quit were twice as likely after a year to have stopped smoking cigarettes than those who used nicotine replacement therapy.
“In terms of the global research, it’s pretty clear that vaping can help smokers quit,” said Peter Shields, MD, a professor in the department of internal medicine at The Ohio State University College of Medicine, Columbus, who specializes in the treatment of lung cancer.
But a 2013 study published in the Lancet, and another from the Lancet in 2019, found only a modest improvement in cessation outcomes when participants used e-cigarettes paired with patches, compared with patches alone.
“For a disruptive technology that was supposed to end combustible tobacco use, there seems very little large-scale disruption,” said Thomas Eissenberg, PhD, co-director of Virginia Commonwealth University’s Center for the Study of Tobacco Products, Richmond.
Michael Joseph Blaha, MD, MPH, director of clinical research at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, pointed to research that shows a portion of people who start vaping to quit smoking end up using both products – or become so-called “dual” users.
“I do think there is fairly high-quality evidence that vaping can lead to more cessation, but at the tradeoff of more long-term dual users and more overall nicotine addiction,” Dr. Blaha said. “Vaping remains a third-line clinical tool after nicotine replacement therapy and FDA-approved cessation medications.”
The U.S. Food and Drug Administration has not approved any e-cigarette or vaping device for smoking cessation, like it has for patches and gums, which means manufacturers cannot market their products as helping tobacco smokers quit.
“ Ms. Hanna, from RWJBarnabas Health’s Institute for Prevention and Recovery, said.
Reducing harm and improving health?
Vapes have also been touted as a boon to individual and public health since cigarette smoking is the leading cause of preventable disease and disability in the United States, responsible for more than 480,000 deaths per year in the U.S., according to the U.S. Centers for Disease Control and Prevention.
Quitting smoking lowers the risk of developing various cancers, heart disease, stroke, and other serious diseases. The aim of nicotine replacement therapy is to help smokers quit by gradually providing the body with smaller doses of nicotine over time, without exposing the body to toxic chemicals found in cigarettes.
“No one should say that e-cigarettes are safe, but compared to cigarettes, the data is consistent: They are not as harmful, and when a smoker switches, it’s better for them,” Dr. Shields said. “Like with other nicotine replacement therapies, if there is a risk that someone stops vaping and returns to smoking, I would rather have them as long-term vapers since it is generally considered to be less harmful than combustible tobacco.”
The FDA has allowed a handful of companies to market their electronic nicotine delivery systems as safer than traditional cigarettes by gaining approval through the Premarket Tobacco Product Applications process. In 2021, the agency announced its first PMTA authorization of an electronic cigarette to R.J. Reynolds for three of its tobacco-flavored vaping products. Regulators approved more products from three additional companies in 2022.
But the FDA has also denied others, including two products in 2023 from R.J. Reynolds, stating that, “the applications lacked sufficient evidence to demonstrate that permitting the marketing of the products would be appropriate for the protection of the public health.”
Questions remain among some researchers on the effects of vaping if used long term. Data on the health effects of vapes are just beginning to emerge and are mainly from studies of animals or cells. Measuring health effects among vape users will entail decades more of study, since Americans only gained access to the products in the 2000s.
Dr. Eissenberg said vaping likely does not cause the same diseases as cigarette smoking, but that does not mean they are not harmful. Ingredients found in e-cigarettes, such as heated propylene glycol, vegetable glycerin, and flavors, have only been used as food ingredients. The potential diseases caused by vapes are still unknown, because inhaling these heated ingredients is new. He also said he had “no issue” with an adult smoker vaping to help them quit smoking – as long they do so for a short period.
“I am very concerned that long-term use in adults could lead to considerable disease and death,” Dr. Eissenberg said. “Simply put, the human lung evolved for one purpose: gas exchange of oxygen in, carbon dioxide out. Anything else that enters the lung is a challenge to the organ.”
But Kenneth Warner, PhD, dean emeritus at the University of Michigan School of Public Health, Ann Arbor, said breaking the addiction to traditional cigarettes could reduce high rates of lung cancer in lower income communities where rates of smoking are comparatively high.
About three times as many Americans smoked (12.6%) than vaped (4.7%) in 2021, but those who live in households with lower incomes are more likely to smoke. According to the CDC, use of tobacco is higher among adults who were uninsured (27.3%) or who had Medicaid coverage (28.6%) than among those with private insurance (16.4%). People with annual family incomes of less than $12,500 also are more likely to be diagnosed with lung cancer than those with family incomes of $50,000 or more. Public health researchers have attributed those disparities in part to higher rates of smoking in lower-income households.
Dr. Warner said many lower-income and other Americans may never quit smoking cigarettes because they believe making the switch to e-cigarettes will not benefit their health. A 2022 study, published in the American Journal of Preventive Medicine, found that the percent of Americans who thought vaping was more harmful than smoking quadrupled between 2018 and 2020, from 6.8% to 28.3%. A third of respondents thought vaping was as harmful as smoking.
“We’ve convinced a large percentage of the American public that vaping is as harmful as smoking when it could be helping people quit smoking,” Dr. Warner said. “People are dying right now.”
Ms. Markowitz did quit smoking by taking up vaping. But now she questions if her lung cancer prognosis would have been delayed, or even avoided, if she’d tried a traditional method like a lozenge or gum instead. She vaped once an hour for most of her 7 years of using the devices.
“For people who are trying to stop smoking, I would recommend something like the patch instead,” Ms. Markowitz said.
The Consumer Advocates for Smoke-free Alternatives receives funding from the vaping industry. Dr. Blaha, Dr. Eissenberg, Ms. Hanna, Dr. Shields, and Dr. Warner reported no funding from the tobacco or e-cigarette industry. Dr. Blaha and Dr. Warner receive tobacco-related research funding from the FDA. Dr. Warner is a member of the FDA’s Tobacco Products Scientific Advisory Committee.
A version of this article first appeared on Medscape.com.
Vaginal microbiome does not affect infant gut microbiome
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The findings suggest that practices such as vaginal seeding are ineffective.
A longitudinal, prospective cohort study of more than 600 pregnant Canadian women and their newborns showed significant differences in an infant’s stool composition by delivery mode at 10 days post partum, but the differences could not be explained by the mother’s vaginal microbiome, and they effectively disappeared by 3 months.
The findings were surprising, Scott Dos Santos, a PhD candidate at the University of Saskatchewan in Saskatoon, told this news organization. “The bacteria living in the maternal vagina are the first microbes that vaginally delivered infants are exposed to. … so it sounds intuitive that different kinds of vaginal microbiomes could end up influencing the development of a baby’s gut microbiome in different ways. But the maternal vaginal microbiome didn’t seem to have any role in predicting what the infant stool microbiome looked like.”
Therefore, women should not be concerned about cesarean delivery having an adverse effect on their baby’s gut microbiome, said Mr. Dos Santos. Moreover, “vaginal seeding is not safe or advised. Professional bodies, including the Society of Obstetricians and Gynecologists of Canada and the American College of Obstetricians and Gynecologists, strongly advise against this practice.”
The study was published online in Frontiers in Cellular and Infection Microbiology.
Independent communities
The investigators analyzed vaginal and stool microbiome profiles from 442 mother-infant dyads. The mothers were healthy, low-risk women who delivered at term. They were recruited into the Maternal Microbiome LEGACY Project from three hospitals in British Columbia.
The mean age of the mothers at delivery was 34.6 years, which is typical of the study hospitals’ delivery populations. Participants identified themselves as White (54.7%), Asian (21.2%), South Asian (8.3%), and of other ethnicities.
A nurse, midwife, or clinician collected maternal vaginal swabs of the posterior fornix and lateral vaginal wall at first presentation to the labor and delivery area. Neonatal meconium, which was defined as the first stool specimen collected within 72 hours of birth, and two infant stool samples were collected at follow-up visits at 10 days and 3 months post partum.
A principal component analysis of infant stool microbiomes showed no significant clustering of microbiome profiles at 10 days or 3 months by maternal community state types (that is, microbial species).
Correspondence analyses also showed no coclustering of maternal and infant clusters at either time. In addition, there were no differences in the distribution of maternal vaginal microbiome clusters among infant stool microbiome clusters, regardless of delivery mode.
Vaginal microbiome clusters were distributed across infant stool clusters in proportion to their frequency in the overall maternal population, indicating that the two communities were independent of each other.
Intrapartum antibiotic administration was identified as a confounder of infant stool microbiome differences and was associated with lower abundances of Escherichia coli, Bacteroides vulgatus, Bifidobacterium longum, and Parabacteroides distasonis.
“Our findings demonstrate that maternal vaginal microbiome composition at delivery does not affect infant stool microbiome composition and development, suggesting that practices to amend infant stool microbiome composition focus on factors other than maternal vaginal microbes,” the authors conclude.
More evidence needed
Commenting on the study, Emily H. Adhikari, MD, assistant professor of obstetrics and gynecology at UT Southwestern Medical Center in Dallas, and medical director of perinatal infectious diseases for the Parkland Health and Hospital System, said, “These findings contribute significantly more data to an understudied area of research into factors that affect the infant gut microbiome from the earliest hours of life. Prior studies have been small and often conflicting, and the authors reference recent larger studies, which corroborate their findings.”
The data regarding whether delivery mode or antibiotic-associated differences in infant microbiomes persist remain controversial, said Dr. Adhikari. “More evidence is needed involving a more ethnically diverse sampling of patients.” In addition, prospectively evaluating vaginal seeding in a rigorously designed clinical trial setting is “imperative to understand any potential benefit and certainly to understand the potential harms of the practice. To date, this does not exist.”
The study was funded by a Canadian Institutes of Health Research grant. Mr. Dos Santos and Dr. Adhikari have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM FRONTIERS IN CELLULAR AND INFECTION MICROBIOLOGY
Health care in America: Let that tapeworm grow
In my most recent column, “ ‘They All Laughed When I Spoke of Greedy Doctors,’ ” I attempted to provide a global understanding of some of the economic forces that have made American medicine what it is, how that happened, and why it is still happening.
I did not propose a fix. I have been proposing fixes for more than 30 years, on the pages of JAMA until 1999 and then for this news organization, most recently in 2019 with “Healthcare for All in a Land of Special Interests.”
Where you stand depends a lot on where you sit.
Is this good news or bad news? When William Hubbard was the dean of the University of Michigan School of Medicine in 1969, he said that “an academic medical center is the most efficient energy and resource trapping device that has ever been created” (personal communication, 1969).
To me as a faculty member of an academic medical center for many years, that was great news. We could grow faculty, erect buildings, take the best care of sick people, churn out research papers, mint new physicians and specialists, and get paid well in the process for doing “the Lord’s work.” What’s not to like? At that time, the proportion of the country’s gross national product expended for medical and health care was about 7%. And the predicted life span of an American at birth was 70.5 years.
Is this good news or bad news? In 2021, the proportion of our annual gross domestic product (GDP) consumed by health care was 18.3%, totaling $4.3 trillion, or $12,914 per person. For perspective, in 2021, the median income per capita was $37,638. Because quite a few Americans have very high incomes, the mean income per capita is much higher: $63,444. Predicted life span in 2021 was 76.4 years.
Thus, in a span of 53 years (1969-2022), only 5.9 years of life were gained per person born, for how many trillions of dollars expended? To me as a tax-paying citizen and payer of medical insurance premiums, that is bad news.
Is this good news or bad news? If we compare developed societies globally, our medical system does a whole lot of things very well indeed. But we spend a great deal more than any other country for health care and objectively achieve poorer outcomes. Thus, we are neither efficient nor effective. We keep a lot of workers very busy doing stuff, and they are generally well paid. As a worker, that’s good news; as a manager who values efficiency, it’s bad news indeed.
Is this good news or bad news? We’re the leader at finding money to pay people to do “health care work.” More Americans work in health care than any other field. In 2019, the United States employed some 21,000,000 people doing “health care and social assistance.” Among others, these occupations include physicians, dentists, dental hygienists and assistants, pharmacists, registered nurses, LVNs/LPNs, nursing aides, technologists and technicians, home health aides, respiratory therapists, occupational and speech therapists, social workers, childcare workers, and personal and home care aides. For a patient, parent, grandparent, and great-grandparent, it is good news to have all those folks available to take care of us when we need it.
So, while I have cringed at the frequent exposés from Roy Poses of what seem to me to be massive societal betrayals by American health care industry giants, it doesn’t have to be that way. Might it still be possible to do well while doing good?
A jobs program
Consider such common medical procedures as coronary artery stents or bypass grafts for stable angina (when optimal medical therapy is as good, or better than, and much less expensive); PSAs on asymptomatic men followed by unnecessary surgery for localized cancer; excess surgery for low back pain; and the jobs created by managing the people caught up in medical complications of the obesity epidemic.
Don’t forget the number of people employed simply to “follow the money” within our byzantine cockamamie medical billing system. In 2009, this prompted me to describe the bloated system as a “health care bubble” not unlike Enron, the submarket real estate financing debacle, or the dot-com boom and bust. I warned of the downside of bursting that bubble, particularly lost jobs.
The Affordable Care Act (ACA) provided health insurance to some 35 million Americans who had been uninsured. It retarded health care inflation. But it did nothing to trim administrative costs or very high pay for nonclinical executives, or shareholder profits in those companies that were for-profit, or drug and device prices. Without the support of all those groups, the ACA would never have passed Congress. The ACA has clearly been a mixed blessing.
If any large American constituency were ever serious about reducing the percentage of our GDP expended on health care, we have excellent ways to do that while improving the health and well-being of the American people. But remember, one person’s liability (unnecessary work) is another person’s asset (needed job).
The MBAization of medicine
Meanwhile, back at Dean Hubbard’s voracious academic medical center, the high intellect and driven nature of those who are attracted to medicine as a career has had other effects. The resulting organizations reflect not only the glorious calling of caring for the sick and the availability of lots of money to recruit and compensate leaders, but also the necessity to develop strong executive types who won’t be “eaten alive” by the high-powered workforce of demanding physicians and the surrounding environment.
Thus, it came as no great surprise that in its 2021 determination of America’s top 25 Best Large Employers, Forbes included five health care organizations and seven universities. Beating out such giants as NASA, Cisco, Microsoft, Netflix, and Google, the University of Alabama Birmingham Hospital was ranked first. Mayo Clinic and Yale University came in third and fifth, respectively, and at the other end of the list were Duke (23), MIT (24), and MD Anderson (25).
My goodness! Well done.
Yet, as a country attempting to be balanced, Warren Buffett’s descriptive entreaty on the 2021 failure of Haven, the Amazon-Chase-Berkshire Hathaway joint initiative, remains troubling. Calling upon Haven to change the U.S. health care system, Buffet said, “We learned a lot about the difficulty of changing around an industry that’s 17% of the GDP. We were fighting a tapeworm in the American economy, and the tapeworm won.” They had failed to tame the American health care cost beast.
I am on record as despising the “MBAization” of American medicine. Unfairly, I blamed a professional and technical discipline for what I considered misuse. I hereby repent and renounce my earlier condemnations.
Take it all over?
Here’s an idea: If you can’t beat them, join them.
Medical care is important, especially for acute illnesses and injuries, early cancer therapy, and many chronic conditions. But the real determinants of health writ large are social: wealth, education, housing, nutritious food, childcare, climate, clean air and water, meaningful employment, safety from violence, exercise schemes, vaccinations, and so on.
Why doesn’t the American medical-industrial complex simply bestow the label of “health care” on all health-related social determinants? Take it all over. Good “health care” jobs for everyone. Medical professionals will still be blamed for the low health quality and poor outcome scores, the main social determinants of health over which we have no control or influence.
Let that tapeworm grow to encompass all social determinants of health, and measure results by length and quality of life, national human happiness, and, of course, jobs. We can do it. Let that bubble glow. Party time.
And that’s the way it is. That’s my opinion.
George Lundberg, MD, is editor-in-chief at Cancer Commons, president of the Lundberg Institute, executive advisor at Cureus, and a clinical professor of pathology at Northwestern University. Previously, he served as editor-in-chief of JAMA (including 10 specialty journals), American Medical News, and Medscape.
A version of this article first appeared on Medscape.com.
In my most recent column, “ ‘They All Laughed When I Spoke of Greedy Doctors,’ ” I attempted to provide a global understanding of some of the economic forces that have made American medicine what it is, how that happened, and why it is still happening.
I did not propose a fix. I have been proposing fixes for more than 30 years, on the pages of JAMA until 1999 and then for this news organization, most recently in 2019 with “Healthcare for All in a Land of Special Interests.”
Where you stand depends a lot on where you sit.
Is this good news or bad news? When William Hubbard was the dean of the University of Michigan School of Medicine in 1969, he said that “an academic medical center is the most efficient energy and resource trapping device that has ever been created” (personal communication, 1969).
To me as a faculty member of an academic medical center for many years, that was great news. We could grow faculty, erect buildings, take the best care of sick people, churn out research papers, mint new physicians and specialists, and get paid well in the process for doing “the Lord’s work.” What’s not to like? At that time, the proportion of the country’s gross national product expended for medical and health care was about 7%. And the predicted life span of an American at birth was 70.5 years.
Is this good news or bad news? In 2021, the proportion of our annual gross domestic product (GDP) consumed by health care was 18.3%, totaling $4.3 trillion, or $12,914 per person. For perspective, in 2021, the median income per capita was $37,638. Because quite a few Americans have very high incomes, the mean income per capita is much higher: $63,444. Predicted life span in 2021 was 76.4 years.
Thus, in a span of 53 years (1969-2022), only 5.9 years of life were gained per person born, for how many trillions of dollars expended? To me as a tax-paying citizen and payer of medical insurance premiums, that is bad news.
Is this good news or bad news? If we compare developed societies globally, our medical system does a whole lot of things very well indeed. But we spend a great deal more than any other country for health care and objectively achieve poorer outcomes. Thus, we are neither efficient nor effective. We keep a lot of workers very busy doing stuff, and they are generally well paid. As a worker, that’s good news; as a manager who values efficiency, it’s bad news indeed.
Is this good news or bad news? We’re the leader at finding money to pay people to do “health care work.” More Americans work in health care than any other field. In 2019, the United States employed some 21,000,000 people doing “health care and social assistance.” Among others, these occupations include physicians, dentists, dental hygienists and assistants, pharmacists, registered nurses, LVNs/LPNs, nursing aides, technologists and technicians, home health aides, respiratory therapists, occupational and speech therapists, social workers, childcare workers, and personal and home care aides. For a patient, parent, grandparent, and great-grandparent, it is good news to have all those folks available to take care of us when we need it.
So, while I have cringed at the frequent exposés from Roy Poses of what seem to me to be massive societal betrayals by American health care industry giants, it doesn’t have to be that way. Might it still be possible to do well while doing good?
A jobs program
Consider such common medical procedures as coronary artery stents or bypass grafts for stable angina (when optimal medical therapy is as good, or better than, and much less expensive); PSAs on asymptomatic men followed by unnecessary surgery for localized cancer; excess surgery for low back pain; and the jobs created by managing the people caught up in medical complications of the obesity epidemic.
Don’t forget the number of people employed simply to “follow the money” within our byzantine cockamamie medical billing system. In 2009, this prompted me to describe the bloated system as a “health care bubble” not unlike Enron, the submarket real estate financing debacle, or the dot-com boom and bust. I warned of the downside of bursting that bubble, particularly lost jobs.
The Affordable Care Act (ACA) provided health insurance to some 35 million Americans who had been uninsured. It retarded health care inflation. But it did nothing to trim administrative costs or very high pay for nonclinical executives, or shareholder profits in those companies that were for-profit, or drug and device prices. Without the support of all those groups, the ACA would never have passed Congress. The ACA has clearly been a mixed blessing.
If any large American constituency were ever serious about reducing the percentage of our GDP expended on health care, we have excellent ways to do that while improving the health and well-being of the American people. But remember, one person’s liability (unnecessary work) is another person’s asset (needed job).
The MBAization of medicine
Meanwhile, back at Dean Hubbard’s voracious academic medical center, the high intellect and driven nature of those who are attracted to medicine as a career has had other effects. The resulting organizations reflect not only the glorious calling of caring for the sick and the availability of lots of money to recruit and compensate leaders, but also the necessity to develop strong executive types who won’t be “eaten alive” by the high-powered workforce of demanding physicians and the surrounding environment.
Thus, it came as no great surprise that in its 2021 determination of America’s top 25 Best Large Employers, Forbes included five health care organizations and seven universities. Beating out such giants as NASA, Cisco, Microsoft, Netflix, and Google, the University of Alabama Birmingham Hospital was ranked first. Mayo Clinic and Yale University came in third and fifth, respectively, and at the other end of the list were Duke (23), MIT (24), and MD Anderson (25).
My goodness! Well done.
Yet, as a country attempting to be balanced, Warren Buffett’s descriptive entreaty on the 2021 failure of Haven, the Amazon-Chase-Berkshire Hathaway joint initiative, remains troubling. Calling upon Haven to change the U.S. health care system, Buffet said, “We learned a lot about the difficulty of changing around an industry that’s 17% of the GDP. We were fighting a tapeworm in the American economy, and the tapeworm won.” They had failed to tame the American health care cost beast.
I am on record as despising the “MBAization” of American medicine. Unfairly, I blamed a professional and technical discipline for what I considered misuse. I hereby repent and renounce my earlier condemnations.
Take it all over?
Here’s an idea: If you can’t beat them, join them.
Medical care is important, especially for acute illnesses and injuries, early cancer therapy, and many chronic conditions. But the real determinants of health writ large are social: wealth, education, housing, nutritious food, childcare, climate, clean air and water, meaningful employment, safety from violence, exercise schemes, vaccinations, and so on.
Why doesn’t the American medical-industrial complex simply bestow the label of “health care” on all health-related social determinants? Take it all over. Good “health care” jobs for everyone. Medical professionals will still be blamed for the low health quality and poor outcome scores, the main social determinants of health over which we have no control or influence.
Let that tapeworm grow to encompass all social determinants of health, and measure results by length and quality of life, national human happiness, and, of course, jobs. We can do it. Let that bubble glow. Party time.
And that’s the way it is. That’s my opinion.
George Lundberg, MD, is editor-in-chief at Cancer Commons, president of the Lundberg Institute, executive advisor at Cureus, and a clinical professor of pathology at Northwestern University. Previously, he served as editor-in-chief of JAMA (including 10 specialty journals), American Medical News, and Medscape.
A version of this article first appeared on Medscape.com.
In my most recent column, “ ‘They All Laughed When I Spoke of Greedy Doctors,’ ” I attempted to provide a global understanding of some of the economic forces that have made American medicine what it is, how that happened, and why it is still happening.
I did not propose a fix. I have been proposing fixes for more than 30 years, on the pages of JAMA until 1999 and then for this news organization, most recently in 2019 with “Healthcare for All in a Land of Special Interests.”
Where you stand depends a lot on where you sit.
Is this good news or bad news? When William Hubbard was the dean of the University of Michigan School of Medicine in 1969, he said that “an academic medical center is the most efficient energy and resource trapping device that has ever been created” (personal communication, 1969).
To me as a faculty member of an academic medical center for many years, that was great news. We could grow faculty, erect buildings, take the best care of sick people, churn out research papers, mint new physicians and specialists, and get paid well in the process for doing “the Lord’s work.” What’s not to like? At that time, the proportion of the country’s gross national product expended for medical and health care was about 7%. And the predicted life span of an American at birth was 70.5 years.
Is this good news or bad news? In 2021, the proportion of our annual gross domestic product (GDP) consumed by health care was 18.3%, totaling $4.3 trillion, or $12,914 per person. For perspective, in 2021, the median income per capita was $37,638. Because quite a few Americans have very high incomes, the mean income per capita is much higher: $63,444. Predicted life span in 2021 was 76.4 years.
Thus, in a span of 53 years (1969-2022), only 5.9 years of life were gained per person born, for how many trillions of dollars expended? To me as a tax-paying citizen and payer of medical insurance premiums, that is bad news.
Is this good news or bad news? If we compare developed societies globally, our medical system does a whole lot of things very well indeed. But we spend a great deal more than any other country for health care and objectively achieve poorer outcomes. Thus, we are neither efficient nor effective. We keep a lot of workers very busy doing stuff, and they are generally well paid. As a worker, that’s good news; as a manager who values efficiency, it’s bad news indeed.
Is this good news or bad news? We’re the leader at finding money to pay people to do “health care work.” More Americans work in health care than any other field. In 2019, the United States employed some 21,000,000 people doing “health care and social assistance.” Among others, these occupations include physicians, dentists, dental hygienists and assistants, pharmacists, registered nurses, LVNs/LPNs, nursing aides, technologists and technicians, home health aides, respiratory therapists, occupational and speech therapists, social workers, childcare workers, and personal and home care aides. For a patient, parent, grandparent, and great-grandparent, it is good news to have all those folks available to take care of us when we need it.
So, while I have cringed at the frequent exposés from Roy Poses of what seem to me to be massive societal betrayals by American health care industry giants, it doesn’t have to be that way. Might it still be possible to do well while doing good?
A jobs program
Consider such common medical procedures as coronary artery stents or bypass grafts for stable angina (when optimal medical therapy is as good, or better than, and much less expensive); PSAs on asymptomatic men followed by unnecessary surgery for localized cancer; excess surgery for low back pain; and the jobs created by managing the people caught up in medical complications of the obesity epidemic.
Don’t forget the number of people employed simply to “follow the money” within our byzantine cockamamie medical billing system. In 2009, this prompted me to describe the bloated system as a “health care bubble” not unlike Enron, the submarket real estate financing debacle, or the dot-com boom and bust. I warned of the downside of bursting that bubble, particularly lost jobs.
The Affordable Care Act (ACA) provided health insurance to some 35 million Americans who had been uninsured. It retarded health care inflation. But it did nothing to trim administrative costs or very high pay for nonclinical executives, or shareholder profits in those companies that were for-profit, or drug and device prices. Without the support of all those groups, the ACA would never have passed Congress. The ACA has clearly been a mixed blessing.
If any large American constituency were ever serious about reducing the percentage of our GDP expended on health care, we have excellent ways to do that while improving the health and well-being of the American people. But remember, one person’s liability (unnecessary work) is another person’s asset (needed job).
The MBAization of medicine
Meanwhile, back at Dean Hubbard’s voracious academic medical center, the high intellect and driven nature of those who are attracted to medicine as a career has had other effects. The resulting organizations reflect not only the glorious calling of caring for the sick and the availability of lots of money to recruit and compensate leaders, but also the necessity to develop strong executive types who won’t be “eaten alive” by the high-powered workforce of demanding physicians and the surrounding environment.
Thus, it came as no great surprise that in its 2021 determination of America’s top 25 Best Large Employers, Forbes included five health care organizations and seven universities. Beating out such giants as NASA, Cisco, Microsoft, Netflix, and Google, the University of Alabama Birmingham Hospital was ranked first. Mayo Clinic and Yale University came in third and fifth, respectively, and at the other end of the list were Duke (23), MIT (24), and MD Anderson (25).
My goodness! Well done.
Yet, as a country attempting to be balanced, Warren Buffett’s descriptive entreaty on the 2021 failure of Haven, the Amazon-Chase-Berkshire Hathaway joint initiative, remains troubling. Calling upon Haven to change the U.S. health care system, Buffet said, “We learned a lot about the difficulty of changing around an industry that’s 17% of the GDP. We were fighting a tapeworm in the American economy, and the tapeworm won.” They had failed to tame the American health care cost beast.
I am on record as despising the “MBAization” of American medicine. Unfairly, I blamed a professional and technical discipline for what I considered misuse. I hereby repent and renounce my earlier condemnations.
Take it all over?
Here’s an idea: If you can’t beat them, join them.
Medical care is important, especially for acute illnesses and injuries, early cancer therapy, and many chronic conditions. But the real determinants of health writ large are social: wealth, education, housing, nutritious food, childcare, climate, clean air and water, meaningful employment, safety from violence, exercise schemes, vaccinations, and so on.
Why doesn’t the American medical-industrial complex simply bestow the label of “health care” on all health-related social determinants? Take it all over. Good “health care” jobs for everyone. Medical professionals will still be blamed for the low health quality and poor outcome scores, the main social determinants of health over which we have no control or influence.
Let that tapeworm grow to encompass all social determinants of health, and measure results by length and quality of life, national human happiness, and, of course, jobs. We can do it. Let that bubble glow. Party time.
And that’s the way it is. That’s my opinion.
George Lundberg, MD, is editor-in-chief at Cancer Commons, president of the Lundberg Institute, executive advisor at Cureus, and a clinical professor of pathology at Northwestern University. Previously, he served as editor-in-chief of JAMA (including 10 specialty journals), American Medical News, and Medscape.
A version of this article first appeared on Medscape.com.
Previously unknown viral families hide in the darnedest places
You and me and baby makes 10,003
If you were a virus hunter, looking for your next big virus discovery, where would you go? The wholesale seafood market in Wuhan? A gathering of unmasked anti-vaxxers in the heartland of America? The frozen snot fields of northwest Siberia?
How about babies? Well, it’s too late now, because that’s what Dennis Sandris Nielsen, PhD, of the University of Copenhagen, and his associates did, and they hit the mother lode. Actually, it was more like the infant load, if we’re being honest here.
“We found an exceptional number of unknown viruses in the faeces of these babies,” Dr. Nielsen said in a written statement from the university. (The study was published in Nature Microbiology, so we get the English spelling of feces.)
The investigators mapped the gut “viromes” of 647 healthy Danish 1-year-old children over the course of 5 years and found 10,000 species of viruses distributed across 248 different viral families, of which only 16 were already known. Incredible stuff, but then things took a turn for the cute. “The researchers named the remaining 232 unknown viral families after the children whose diapers made the study possible. As a result, new viral families include names like Sylvesterviridae, Rigmorviridae and Tristanviridae,” the university said.
About 90% of the viruses found in the feces are bacterial viruses, aka bacteriophages, which have bacteria as their hosts and don’t attack the children’s cells, so they don’t cause disease. The other 10%, however, are eukaryotic: They use human cells as hosts, so they can be either friend or foe. “It is thought-provoking that all children run around with 10-20 of these virus types that infect human cells. So, there is a constant viral infection taking place, which apparently doesn’t make them sick,” Dr. Nielsen said.
Doesn’t make them sick? Riiiight. The thought that this gives rise to now? People love babies. Everyone wants to pick up the baby. Now we know why. Because the viruses want us to! Well, those cute little faces aren’t fooling us anymore. No more babies for us. Everyone should stay away from babies and their evil little eukaryotic viruses. STOP THE BABIES!
[Editor’s note: After a short timeout, we explained to the staff that the human species actually needs babies for its survival. They calmed down, picked up their crayons, and quietly went back to work.]
Fooled them. Stop the babies!
At least someone out there appreciates hospital food
Life in Alaska is not for the meek. It’s dark half the year. Summer is 3 weeks in July. And somehow, there’s a moose in line ahead of you at the doctor’s office. To make matters worse, it’s arguing about insurance. “What do you mean, you’ve heard the Moo Cross Moo Shield joke before?”
One might expect that Providence Alaska Health Park, located near downtown Anchorage, the largest city in Alaska by a massive margin, might be safe from ungulate invasion. Nope. In recent days, a young moose has taken to hanging around Providence campus, and it just could not find anything to eat. Remember, it may be early April, but this is Alaska. It’s still winter there. The ground’s still covered in snow.
Eventually, the gears in our young moose friend’s mind turned and it settled on a course of action: “Hey, those are some nice-looking plants behind that door over there. …” And that’s how Providence Alaska Health ended up with a moose munching on decorative potted plants in the hospital lobby.
Funnily enough, the moose didn’t even make a big scene. It just walked through the automatic doors and started chowing down. Security only found out because a tenant called them. Naturally though, once security made the announcement that a massive wild animal had been spotted in the building, the lobby was evacuated. … What do you mean, half the hospital came around to see it? Apparently, even though Alaskans have to fight moose herds on their daily commute, a lot of people wanted to see our moose friend do its thing.
“That’s crazy,” a woman in scrubs said in a video as she snapped a photo with her phone.
“This is the best. Like, what’s the code for this?” asked another bystander.
Despite security’s best efforts to shoo the moose out with barricades and offers of tasty branches, our furry friend left of its own volition, presumably irritated that his breakfast had become a spectator sport. But it didn’t go far. It hung around the front drive for a while, then went around the back of the building for a nap. What has four hooves and still doesn’t give a crap? Bob Moose-o! How you doing?
That click sounded stressed
How can people tell that you’re stressed? Maybe you get irritable and a little snappy. Some people have an inability to concentrate or focus. Eating that muffin when you weren’t really hungry could be a sign you’re not relaxed.
Did you know that your computer can be an indicator of your stress levels?
We tend to be working when we’re using computers, right? That can be a stressor in itself. Well, some researchers at ETH Zürich decided to have a look at the situation. Surprisingly, at least to us, one in three Swiss employees experience workplace stress, which makes us wonder what the percentage is in this country.
The Swiss researchers developed a model that tells how stressed someone is just by the way they use their computer mouse or type. The results of their study showed that those who were stressed clicked and tapped differently than participants who were more relaxed.
Stressed people click “more often and less precisely and cover longer distances on the screen,” while the relaxed take “shorter, more direct routes to reach their destination and take more time doing so,” study author Mara Nägelin explained in a written statement from ETH (Eidgenössische Technische Hochschule, or Swiss Federal Institute of Technology) Zürich.
Ever find when you’re frustrated and in a rush you end up making more mistakes? Same deal. Coauthor Jasmine Kerr noted that “increased levels of stress negatively impact our brain’s ability to process information.” Which totally is going to affect how we move.
Hopefully, these results can give insight to companies on how stressed their employees are and the effect it has on their work performance, eventually leading to, guess what, more research on how to alleviate workplace stress in general, which can benefit us all.
So if you find yourself in the office working on your computer like it’s a game of Perfection and time is running out, take a beat. Maybe try a stress-relieving breathing technique. Nonstressed people, according to the study, take fewer and longer pauses on their computers. Perfection on the job may mean relaxing first.
You and me and baby makes 10,003
If you were a virus hunter, looking for your next big virus discovery, where would you go? The wholesale seafood market in Wuhan? A gathering of unmasked anti-vaxxers in the heartland of America? The frozen snot fields of northwest Siberia?
How about babies? Well, it’s too late now, because that’s what Dennis Sandris Nielsen, PhD, of the University of Copenhagen, and his associates did, and they hit the mother lode. Actually, it was more like the infant load, if we’re being honest here.
“We found an exceptional number of unknown viruses in the faeces of these babies,” Dr. Nielsen said in a written statement from the university. (The study was published in Nature Microbiology, so we get the English spelling of feces.)
The investigators mapped the gut “viromes” of 647 healthy Danish 1-year-old children over the course of 5 years and found 10,000 species of viruses distributed across 248 different viral families, of which only 16 were already known. Incredible stuff, but then things took a turn for the cute. “The researchers named the remaining 232 unknown viral families after the children whose diapers made the study possible. As a result, new viral families include names like Sylvesterviridae, Rigmorviridae and Tristanviridae,” the university said.
About 90% of the viruses found in the feces are bacterial viruses, aka bacteriophages, which have bacteria as their hosts and don’t attack the children’s cells, so they don’t cause disease. The other 10%, however, are eukaryotic: They use human cells as hosts, so they can be either friend or foe. “It is thought-provoking that all children run around with 10-20 of these virus types that infect human cells. So, there is a constant viral infection taking place, which apparently doesn’t make them sick,” Dr. Nielsen said.
Doesn’t make them sick? Riiiight. The thought that this gives rise to now? People love babies. Everyone wants to pick up the baby. Now we know why. Because the viruses want us to! Well, those cute little faces aren’t fooling us anymore. No more babies for us. Everyone should stay away from babies and their evil little eukaryotic viruses. STOP THE BABIES!
[Editor’s note: After a short timeout, we explained to the staff that the human species actually needs babies for its survival. They calmed down, picked up their crayons, and quietly went back to work.]
Fooled them. Stop the babies!
At least someone out there appreciates hospital food
Life in Alaska is not for the meek. It’s dark half the year. Summer is 3 weeks in July. And somehow, there’s a moose in line ahead of you at the doctor’s office. To make matters worse, it’s arguing about insurance. “What do you mean, you’ve heard the Moo Cross Moo Shield joke before?”
One might expect that Providence Alaska Health Park, located near downtown Anchorage, the largest city in Alaska by a massive margin, might be safe from ungulate invasion. Nope. In recent days, a young moose has taken to hanging around Providence campus, and it just could not find anything to eat. Remember, it may be early April, but this is Alaska. It’s still winter there. The ground’s still covered in snow.
Eventually, the gears in our young moose friend’s mind turned and it settled on a course of action: “Hey, those are some nice-looking plants behind that door over there. …” And that’s how Providence Alaska Health ended up with a moose munching on decorative potted plants in the hospital lobby.
Funnily enough, the moose didn’t even make a big scene. It just walked through the automatic doors and started chowing down. Security only found out because a tenant called them. Naturally though, once security made the announcement that a massive wild animal had been spotted in the building, the lobby was evacuated. … What do you mean, half the hospital came around to see it? Apparently, even though Alaskans have to fight moose herds on their daily commute, a lot of people wanted to see our moose friend do its thing.
“That’s crazy,” a woman in scrubs said in a video as she snapped a photo with her phone.
“This is the best. Like, what’s the code for this?” asked another bystander.
Despite security’s best efforts to shoo the moose out with barricades and offers of tasty branches, our furry friend left of its own volition, presumably irritated that his breakfast had become a spectator sport. But it didn’t go far. It hung around the front drive for a while, then went around the back of the building for a nap. What has four hooves and still doesn’t give a crap? Bob Moose-o! How you doing?
That click sounded stressed
How can people tell that you’re stressed? Maybe you get irritable and a little snappy. Some people have an inability to concentrate or focus. Eating that muffin when you weren’t really hungry could be a sign you’re not relaxed.
Did you know that your computer can be an indicator of your stress levels?
We tend to be working when we’re using computers, right? That can be a stressor in itself. Well, some researchers at ETH Zürich decided to have a look at the situation. Surprisingly, at least to us, one in three Swiss employees experience workplace stress, which makes us wonder what the percentage is in this country.
The Swiss researchers developed a model that tells how stressed someone is just by the way they use their computer mouse or type. The results of their study showed that those who were stressed clicked and tapped differently than participants who were more relaxed.
Stressed people click “more often and less precisely and cover longer distances on the screen,” while the relaxed take “shorter, more direct routes to reach their destination and take more time doing so,” study author Mara Nägelin explained in a written statement from ETH (Eidgenössische Technische Hochschule, or Swiss Federal Institute of Technology) Zürich.
Ever find when you’re frustrated and in a rush you end up making more mistakes? Same deal. Coauthor Jasmine Kerr noted that “increased levels of stress negatively impact our brain’s ability to process information.” Which totally is going to affect how we move.
Hopefully, these results can give insight to companies on how stressed their employees are and the effect it has on their work performance, eventually leading to, guess what, more research on how to alleviate workplace stress in general, which can benefit us all.
So if you find yourself in the office working on your computer like it’s a game of Perfection and time is running out, take a beat. Maybe try a stress-relieving breathing technique. Nonstressed people, according to the study, take fewer and longer pauses on their computers. Perfection on the job may mean relaxing first.
You and me and baby makes 10,003
If you were a virus hunter, looking for your next big virus discovery, where would you go? The wholesale seafood market in Wuhan? A gathering of unmasked anti-vaxxers in the heartland of America? The frozen snot fields of northwest Siberia?
How about babies? Well, it’s too late now, because that’s what Dennis Sandris Nielsen, PhD, of the University of Copenhagen, and his associates did, and they hit the mother lode. Actually, it was more like the infant load, if we’re being honest here.
“We found an exceptional number of unknown viruses in the faeces of these babies,” Dr. Nielsen said in a written statement from the university. (The study was published in Nature Microbiology, so we get the English spelling of feces.)
The investigators mapped the gut “viromes” of 647 healthy Danish 1-year-old children over the course of 5 years and found 10,000 species of viruses distributed across 248 different viral families, of which only 16 were already known. Incredible stuff, but then things took a turn for the cute. “The researchers named the remaining 232 unknown viral families after the children whose diapers made the study possible. As a result, new viral families include names like Sylvesterviridae, Rigmorviridae and Tristanviridae,” the university said.
About 90% of the viruses found in the feces are bacterial viruses, aka bacteriophages, which have bacteria as their hosts and don’t attack the children’s cells, so they don’t cause disease. The other 10%, however, are eukaryotic: They use human cells as hosts, so they can be either friend or foe. “It is thought-provoking that all children run around with 10-20 of these virus types that infect human cells. So, there is a constant viral infection taking place, which apparently doesn’t make them sick,” Dr. Nielsen said.
Doesn’t make them sick? Riiiight. The thought that this gives rise to now? People love babies. Everyone wants to pick up the baby. Now we know why. Because the viruses want us to! Well, those cute little faces aren’t fooling us anymore. No more babies for us. Everyone should stay away from babies and their evil little eukaryotic viruses. STOP THE BABIES!
[Editor’s note: After a short timeout, we explained to the staff that the human species actually needs babies for its survival. They calmed down, picked up their crayons, and quietly went back to work.]
Fooled them. Stop the babies!
At least someone out there appreciates hospital food
Life in Alaska is not for the meek. It’s dark half the year. Summer is 3 weeks in July. And somehow, there’s a moose in line ahead of you at the doctor’s office. To make matters worse, it’s arguing about insurance. “What do you mean, you’ve heard the Moo Cross Moo Shield joke before?”
One might expect that Providence Alaska Health Park, located near downtown Anchorage, the largest city in Alaska by a massive margin, might be safe from ungulate invasion. Nope. In recent days, a young moose has taken to hanging around Providence campus, and it just could not find anything to eat. Remember, it may be early April, but this is Alaska. It’s still winter there. The ground’s still covered in snow.
Eventually, the gears in our young moose friend’s mind turned and it settled on a course of action: “Hey, those are some nice-looking plants behind that door over there. …” And that’s how Providence Alaska Health ended up with a moose munching on decorative potted plants in the hospital lobby.
Funnily enough, the moose didn’t even make a big scene. It just walked through the automatic doors and started chowing down. Security only found out because a tenant called them. Naturally though, once security made the announcement that a massive wild animal had been spotted in the building, the lobby was evacuated. … What do you mean, half the hospital came around to see it? Apparently, even though Alaskans have to fight moose herds on their daily commute, a lot of people wanted to see our moose friend do its thing.
“That’s crazy,” a woman in scrubs said in a video as she snapped a photo with her phone.
“This is the best. Like, what’s the code for this?” asked another bystander.
Despite security’s best efforts to shoo the moose out with barricades and offers of tasty branches, our furry friend left of its own volition, presumably irritated that his breakfast had become a spectator sport. But it didn’t go far. It hung around the front drive for a while, then went around the back of the building for a nap. What has four hooves and still doesn’t give a crap? Bob Moose-o! How you doing?
That click sounded stressed
How can people tell that you’re stressed? Maybe you get irritable and a little snappy. Some people have an inability to concentrate or focus. Eating that muffin when you weren’t really hungry could be a sign you’re not relaxed.
Did you know that your computer can be an indicator of your stress levels?
We tend to be working when we’re using computers, right? That can be a stressor in itself. Well, some researchers at ETH Zürich decided to have a look at the situation. Surprisingly, at least to us, one in three Swiss employees experience workplace stress, which makes us wonder what the percentage is in this country.
The Swiss researchers developed a model that tells how stressed someone is just by the way they use their computer mouse or type. The results of their study showed that those who were stressed clicked and tapped differently than participants who were more relaxed.
Stressed people click “more often and less precisely and cover longer distances on the screen,” while the relaxed take “shorter, more direct routes to reach their destination and take more time doing so,” study author Mara Nägelin explained in a written statement from ETH (Eidgenössische Technische Hochschule, or Swiss Federal Institute of Technology) Zürich.
Ever find when you’re frustrated and in a rush you end up making more mistakes? Same deal. Coauthor Jasmine Kerr noted that “increased levels of stress negatively impact our brain’s ability to process information.” Which totally is going to affect how we move.
Hopefully, these results can give insight to companies on how stressed their employees are and the effect it has on their work performance, eventually leading to, guess what, more research on how to alleviate workplace stress in general, which can benefit us all.
So if you find yourself in the office working on your computer like it’s a game of Perfection and time is running out, take a beat. Maybe try a stress-relieving breathing technique. Nonstressed people, according to the study, take fewer and longer pauses on their computers. Perfection on the job may mean relaxing first.