User login
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
What’s Eating You? Carpet Beetles (Dermestidae)
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
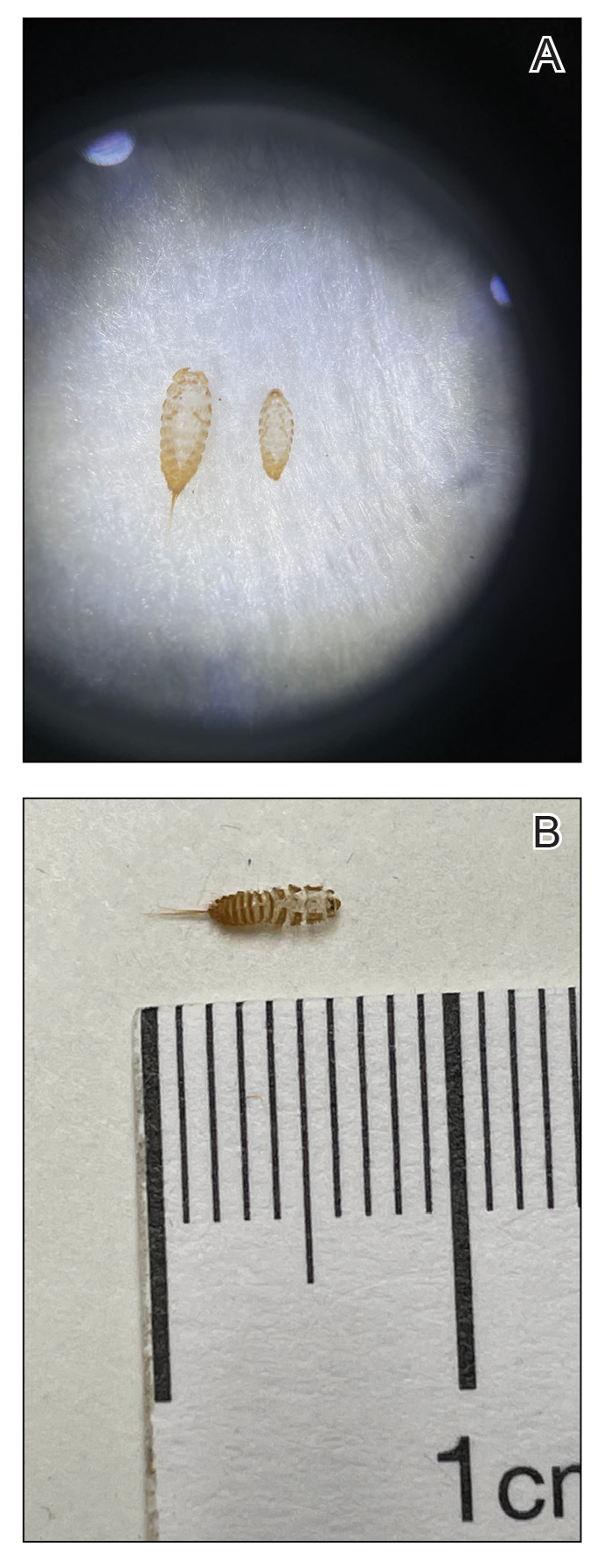
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13
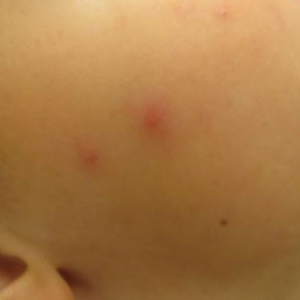
Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13

Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13

Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
Practice Points
- Given their ubiquity, dermatologists should be aware of the potential for hypersensitivity reactions to carpet beetles (Dermestidae).
- Pruritic erythematous papules, pustules, and vesicles are the most common manifestations of exposure to larval hairs.
- Treatment is symptom based, and future exposure can be greatly diminished with thorough cleaning of the patient’s environment.
Psilocybin Poison Control Calls Spike in Teens, Young Adults
Calls to US poison centers related to psilocybin more than tripled among teens and more than doubled in young adults between 2019 and 2022, new research suggests. Investigators say the increase may be linked to decriminalization efforts in US cities and states.
METHODOLOGY:
- Investigators used data from the National Poison Data System (NPDS) to identify calls involving psilocybin between January 2013 and December 2022.
- Researchers focused on calls about individuals between the ages of 13 and 25 years.
- Exposures to psilocybin were examined based on demographics, clinical effects, level of care, and medical outcome.
TAKEAWAY:
- During the entire 10-year study period, 4055 psilocybin-involved exposures were reported in the age groups studied, with 66% being single-substance exposures and close to three quarters receiving medical attention.
- Psilocybin’s most common effects were hallucinations or delusions (37% of calls), agitation (28%), tachycardia (20%), and confusion (16%).
- The number of psilocybin-related calls to poison control centers for youth were largely unchanged from 2013 to 2018 but more than tripled among adolescents (aged 13-19 years) from 2019 and 2022 and more than doubled among young adults (aged 20-25 years) between 2018 and 2022 (P < .0001).
IN PRACTICE:
The increase in poison center calls coincides with psilocybin decriminalization efforts in several states in 2019, the authors noted. However, because those efforts only legalized use in adults aged 21 years and older, the rise among younger people is concerning, they added. “As psilocybin may become more widely available, it is important for parents to be aware that psilocybin is also available in edible forms such as chocolate and gummies. And we learned from our experience with edible cannabis that young children can mistake edibles for candy,” lead author Rita Farah, PharmD, MPH, PhD, Blue Ridge Poison Center epidemiologist, said in a news release.
SOURCE:
Christopher Holstege, MD, director of UVA Health’s Blue Ridge Poison Center and chief of the Division of Medical Toxicology at the UVA School of Medicine was the senior and corresponding author of the study. It was published online on February 26 in the Journal of Adolescent Health.
LIMITATIONS:
NPDS data are not designed to assess potential risk factors leading to increases in psilocybin-related cases. Moreover, because reports to poison control centers are voluntary and don’t capture all exposures, NPDS data likely under-represent cases of hallucinogenic mushroom poisonings. Lastly, NPDS data are susceptible to reporting and misclassification biases.
DISCLOSURES:
Funding source was not disclosed. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Calls to US poison centers related to psilocybin more than tripled among teens and more than doubled in young adults between 2019 and 2022, new research suggests. Investigators say the increase may be linked to decriminalization efforts in US cities and states.
METHODOLOGY:
- Investigators used data from the National Poison Data System (NPDS) to identify calls involving psilocybin between January 2013 and December 2022.
- Researchers focused on calls about individuals between the ages of 13 and 25 years.
- Exposures to psilocybin were examined based on demographics, clinical effects, level of care, and medical outcome.
TAKEAWAY:
- During the entire 10-year study period, 4055 psilocybin-involved exposures were reported in the age groups studied, with 66% being single-substance exposures and close to three quarters receiving medical attention.
- Psilocybin’s most common effects were hallucinations or delusions (37% of calls), agitation (28%), tachycardia (20%), and confusion (16%).
- The number of psilocybin-related calls to poison control centers for youth were largely unchanged from 2013 to 2018 but more than tripled among adolescents (aged 13-19 years) from 2019 and 2022 and more than doubled among young adults (aged 20-25 years) between 2018 and 2022 (P < .0001).
IN PRACTICE:
The increase in poison center calls coincides with psilocybin decriminalization efforts in several states in 2019, the authors noted. However, because those efforts only legalized use in adults aged 21 years and older, the rise among younger people is concerning, they added. “As psilocybin may become more widely available, it is important for parents to be aware that psilocybin is also available in edible forms such as chocolate and gummies. And we learned from our experience with edible cannabis that young children can mistake edibles for candy,” lead author Rita Farah, PharmD, MPH, PhD, Blue Ridge Poison Center epidemiologist, said in a news release.
SOURCE:
Christopher Holstege, MD, director of UVA Health’s Blue Ridge Poison Center and chief of the Division of Medical Toxicology at the UVA School of Medicine was the senior and corresponding author of the study. It was published online on February 26 in the Journal of Adolescent Health.
LIMITATIONS:
NPDS data are not designed to assess potential risk factors leading to increases in psilocybin-related cases. Moreover, because reports to poison control centers are voluntary and don’t capture all exposures, NPDS data likely under-represent cases of hallucinogenic mushroom poisonings. Lastly, NPDS data are susceptible to reporting and misclassification biases.
DISCLOSURES:
Funding source was not disclosed. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Calls to US poison centers related to psilocybin more than tripled among teens and more than doubled in young adults between 2019 and 2022, new research suggests. Investigators say the increase may be linked to decriminalization efforts in US cities and states.
METHODOLOGY:
- Investigators used data from the National Poison Data System (NPDS) to identify calls involving psilocybin between January 2013 and December 2022.
- Researchers focused on calls about individuals between the ages of 13 and 25 years.
- Exposures to psilocybin were examined based on demographics, clinical effects, level of care, and medical outcome.
TAKEAWAY:
- During the entire 10-year study period, 4055 psilocybin-involved exposures were reported in the age groups studied, with 66% being single-substance exposures and close to three quarters receiving medical attention.
- Psilocybin’s most common effects were hallucinations or delusions (37% of calls), agitation (28%), tachycardia (20%), and confusion (16%).
- The number of psilocybin-related calls to poison control centers for youth were largely unchanged from 2013 to 2018 but more than tripled among adolescents (aged 13-19 years) from 2019 and 2022 and more than doubled among young adults (aged 20-25 years) between 2018 and 2022 (P < .0001).
IN PRACTICE:
The increase in poison center calls coincides with psilocybin decriminalization efforts in several states in 2019, the authors noted. However, because those efforts only legalized use in adults aged 21 years and older, the rise among younger people is concerning, they added. “As psilocybin may become more widely available, it is important for parents to be aware that psilocybin is also available in edible forms such as chocolate and gummies. And we learned from our experience with edible cannabis that young children can mistake edibles for candy,” lead author Rita Farah, PharmD, MPH, PhD, Blue Ridge Poison Center epidemiologist, said in a news release.
SOURCE:
Christopher Holstege, MD, director of UVA Health’s Blue Ridge Poison Center and chief of the Division of Medical Toxicology at the UVA School of Medicine was the senior and corresponding author of the study. It was published online on February 26 in the Journal of Adolescent Health.
LIMITATIONS:
NPDS data are not designed to assess potential risk factors leading to increases in psilocybin-related cases. Moreover, because reports to poison control centers are voluntary and don’t capture all exposures, NPDS data likely under-represent cases of hallucinogenic mushroom poisonings. Lastly, NPDS data are susceptible to reporting and misclassification biases.
DISCLOSURES:
Funding source was not disclosed. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seladelpar Could ‘Raise the Bar’ in Primary Biliary Cholangitis Treatment
, according to the full results of the RESPONSE phase 3 study.
“At a dose of 10 mg daily, 1 in 4 patients normalize their alkaline phosphatase level,” chief investigator Gideon Hirschfield, PhD, BM BChir, with the Toronto Center for Liver Disease at Toronto General Hospital, Toronto, Ontario, Canada, said in an interview.
The study data are “genuinely exciting...and support the potential for seladelpar to raise the bar in PBC treatment,” Dr. Hirschfield added in a news release.
Seladelpar is being developed by CymaBay Therapeutics, which funded the study.
The results were published online in The New England Journal of Medicine.
Topline data from the study were presented in November at The Liver Meeting 2023: American Association for the Study of Liver Diseases.
‘Unequivocal’ Progress
Up to 40% of patients with PBC have an inadequate response to first-line therapy with ursodeoxycholic acid (UDCA) and are at a high risk for disease progression. More than half of patients with the disease fail to respond to second-line therapy with obeticholic acid.
Seladelpar, and the dual PPAR-alpha and PPAR-delta agonist elafibranor, are an “unequivocal sign of progress, marking the arrival of a new era in which PBC treatment is expected to provide both biochemical benefits and amelioration of symptoms for patients,” David N. Assis, MD, with the Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut, wrote in a linked editorial.
In the RESPONSE study, 193 patients with PBC who had an inadequate response to or a history of unacceptable side effects with UDCA were randomly allocated to either oral seladelpar 10 mg daily or placebo for 12 months. The vast majority (93.8%) continued UDCA as standard-of-care background therapy.
The primary endpoint was a biochemical response, which was defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a decrease of 15% or more from baseline, and a normal total bilirubin level at 12 months.
After 12 months, 61.7% of patients taking seladelpar met the primary endpoint vs 20% of patients taking placebo.
In addition, significantly more patients taking seladelpar than placebo had normalization of the ALP level (25% vs 0%). The average decrease in ALP from baseline was 42.4% in the seladelpar group vs 4.3% in the placebo group.
At 12 months, alanine aminotransferase and gamma-glutamyl transferase levels were reduced by 23.5% and 39.1%, respectively, in the seladelpar group compared with 6.5% and 11.4%, respectively, in the placebo group.
“In PBC, we use target endpoints, so the trial was not powered or able to show yet clinical outcomes because the pace of the disease is quite slow. But we believe that the normalization of liver tests and improvement in quality of life will change the disease trajectory over time,” Dr. Hirschfield said.
Significant Reduction in Pruritus
A key secondary endpoint was change in patient-reported pruritus.
At baseline, 38.3% of patients in the seladelpar group and 35.4% of those in the placebo group had moderate to severe pruritus, with a daily numerical rating scale (NRS) score of 4 or higher out of 10.
Among these patients, the reduction from baseline in the pruritus NRS score at month 6 was significantly greater with seladelpar than with placebo (change from baseline, −3.2 points vs −1.7 points). These improvements were sustained through 12 months.
Improvements on the 5-D Itch Scale in both the moderate to severe pruritus population and the overall population also favored seladelpar over placebo for itch relief, which had a positive impact on sleep. Similar results demonstrating reductions in itch and improvements in sleep were observed using the PBC-40 questionnaire.
Adverse events that led to discontinuation of seladelpar or placebo were rare, and there was no between-group difference in the incidence of serious adverse events.
“No worrisome adverse events affecting the muscles were observed, including among patients receiving statins. Certain gastrointestinal events — abdominal pain, abdominal distention, and nausea — were reported more frequently in the seladelpar group than in the placebo group,” the study authors wrote.
The most common adverse events that occurred in ≥ 5% of patients in either group were COVID-19 and pruritus. A greater percentage of patients treated with placebo reported pruritus (15.4% vs 4.7%) as an adverse event — a finding consistent with the positive effect of seladelpar on reducing pruritus.
The researchers noted that 96.4% of patients who participated in the RESPONSE trial chose to enroll in the extension trial to evaluate long-term safety and the side-effect profile of seladelpar.
Potential First-Line Treatment?
In Dr. Assis’ view, the RESPONSE trial, coupled with the recently reported ELATIVE trial of the dual PPAR-alpha and PPAR-delta agonist elafibranor in PBC, “cement the role of PPAR agonists as the preferred second-line treatment in primary biliary cholangitis.”
“The reduction in serum cholestatic markers and the safety profiles of elafibranor and seladelpar offer clear advantages beyond what was previously shown with obeticholic acid. These trials also cement a new treatment goal for primary biliary cholangitis in which a reduction in pruritus should be expected as part of anticholestatic treatment,” Dr. Assis wrote.
“The results of these trials suggest that the use of PPAR agonists in primary biliary cholangitis could improve treatment outcomes while also improving quality of life, which is a highly desirable alignment of clinician and patient goals,” Dr. Assis added.
Looking ahead, Dr. Hirschfield sees a potential role for seladelpar earlier in the course of PBC treatment, he said in an interview.
“Over time, the way we treat patients will not be to wait to fail. It will be treat to target and treat to success,” Dr. Hirschfield said.
Earlier this month, the US Food and Drug Administration accepted CymaBay Therapeutics’ new drug application for seladelpar for the treatment of PBC, including pruritus in adults without cirrhosis or with compensated cirrhosis (Child Pugh A) who fail to respond adequately or cannot tolerate UDCA. Seladelpar for PBC was granted breakthrough designation in October 2023.
The study was funded by CymaBay Therapeutics. Disclosures for authors and editorialist are available at NEJM.org.
A version of this article appeared on Medscape.com.
, according to the full results of the RESPONSE phase 3 study.
“At a dose of 10 mg daily, 1 in 4 patients normalize their alkaline phosphatase level,” chief investigator Gideon Hirschfield, PhD, BM BChir, with the Toronto Center for Liver Disease at Toronto General Hospital, Toronto, Ontario, Canada, said in an interview.
The study data are “genuinely exciting...and support the potential for seladelpar to raise the bar in PBC treatment,” Dr. Hirschfield added in a news release.
Seladelpar is being developed by CymaBay Therapeutics, which funded the study.
The results were published online in The New England Journal of Medicine.
Topline data from the study were presented in November at The Liver Meeting 2023: American Association for the Study of Liver Diseases.
‘Unequivocal’ Progress
Up to 40% of patients with PBC have an inadequate response to first-line therapy with ursodeoxycholic acid (UDCA) and are at a high risk for disease progression. More than half of patients with the disease fail to respond to second-line therapy with obeticholic acid.
Seladelpar, and the dual PPAR-alpha and PPAR-delta agonist elafibranor, are an “unequivocal sign of progress, marking the arrival of a new era in which PBC treatment is expected to provide both biochemical benefits and amelioration of symptoms for patients,” David N. Assis, MD, with the Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut, wrote in a linked editorial.
In the RESPONSE study, 193 patients with PBC who had an inadequate response to or a history of unacceptable side effects with UDCA were randomly allocated to either oral seladelpar 10 mg daily or placebo for 12 months. The vast majority (93.8%) continued UDCA as standard-of-care background therapy.
The primary endpoint was a biochemical response, which was defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a decrease of 15% or more from baseline, and a normal total bilirubin level at 12 months.
After 12 months, 61.7% of patients taking seladelpar met the primary endpoint vs 20% of patients taking placebo.
In addition, significantly more patients taking seladelpar than placebo had normalization of the ALP level (25% vs 0%). The average decrease in ALP from baseline was 42.4% in the seladelpar group vs 4.3% in the placebo group.
At 12 months, alanine aminotransferase and gamma-glutamyl transferase levels were reduced by 23.5% and 39.1%, respectively, in the seladelpar group compared with 6.5% and 11.4%, respectively, in the placebo group.
“In PBC, we use target endpoints, so the trial was not powered or able to show yet clinical outcomes because the pace of the disease is quite slow. But we believe that the normalization of liver tests and improvement in quality of life will change the disease trajectory over time,” Dr. Hirschfield said.
Significant Reduction in Pruritus
A key secondary endpoint was change in patient-reported pruritus.
At baseline, 38.3% of patients in the seladelpar group and 35.4% of those in the placebo group had moderate to severe pruritus, with a daily numerical rating scale (NRS) score of 4 or higher out of 10.
Among these patients, the reduction from baseline in the pruritus NRS score at month 6 was significantly greater with seladelpar than with placebo (change from baseline, −3.2 points vs −1.7 points). These improvements were sustained through 12 months.
Improvements on the 5-D Itch Scale in both the moderate to severe pruritus population and the overall population also favored seladelpar over placebo for itch relief, which had a positive impact on sleep. Similar results demonstrating reductions in itch and improvements in sleep were observed using the PBC-40 questionnaire.
Adverse events that led to discontinuation of seladelpar or placebo were rare, and there was no between-group difference in the incidence of serious adverse events.
“No worrisome adverse events affecting the muscles were observed, including among patients receiving statins. Certain gastrointestinal events — abdominal pain, abdominal distention, and nausea — were reported more frequently in the seladelpar group than in the placebo group,” the study authors wrote.
The most common adverse events that occurred in ≥ 5% of patients in either group were COVID-19 and pruritus. A greater percentage of patients treated with placebo reported pruritus (15.4% vs 4.7%) as an adverse event — a finding consistent with the positive effect of seladelpar on reducing pruritus.
The researchers noted that 96.4% of patients who participated in the RESPONSE trial chose to enroll in the extension trial to evaluate long-term safety and the side-effect profile of seladelpar.
Potential First-Line Treatment?
In Dr. Assis’ view, the RESPONSE trial, coupled with the recently reported ELATIVE trial of the dual PPAR-alpha and PPAR-delta agonist elafibranor in PBC, “cement the role of PPAR agonists as the preferred second-line treatment in primary biliary cholangitis.”
“The reduction in serum cholestatic markers and the safety profiles of elafibranor and seladelpar offer clear advantages beyond what was previously shown with obeticholic acid. These trials also cement a new treatment goal for primary biliary cholangitis in which a reduction in pruritus should be expected as part of anticholestatic treatment,” Dr. Assis wrote.
“The results of these trials suggest that the use of PPAR agonists in primary biliary cholangitis could improve treatment outcomes while also improving quality of life, which is a highly desirable alignment of clinician and patient goals,” Dr. Assis added.
Looking ahead, Dr. Hirschfield sees a potential role for seladelpar earlier in the course of PBC treatment, he said in an interview.
“Over time, the way we treat patients will not be to wait to fail. It will be treat to target and treat to success,” Dr. Hirschfield said.
Earlier this month, the US Food and Drug Administration accepted CymaBay Therapeutics’ new drug application for seladelpar for the treatment of PBC, including pruritus in adults without cirrhosis or with compensated cirrhosis (Child Pugh A) who fail to respond adequately or cannot tolerate UDCA. Seladelpar for PBC was granted breakthrough designation in October 2023.
The study was funded by CymaBay Therapeutics. Disclosures for authors and editorialist are available at NEJM.org.
A version of this article appeared on Medscape.com.
, according to the full results of the RESPONSE phase 3 study.
“At a dose of 10 mg daily, 1 in 4 patients normalize their alkaline phosphatase level,” chief investigator Gideon Hirschfield, PhD, BM BChir, with the Toronto Center for Liver Disease at Toronto General Hospital, Toronto, Ontario, Canada, said in an interview.
The study data are “genuinely exciting...and support the potential for seladelpar to raise the bar in PBC treatment,” Dr. Hirschfield added in a news release.
Seladelpar is being developed by CymaBay Therapeutics, which funded the study.
The results were published online in The New England Journal of Medicine.
Topline data from the study were presented in November at The Liver Meeting 2023: American Association for the Study of Liver Diseases.
‘Unequivocal’ Progress
Up to 40% of patients with PBC have an inadequate response to first-line therapy with ursodeoxycholic acid (UDCA) and are at a high risk for disease progression. More than half of patients with the disease fail to respond to second-line therapy with obeticholic acid.
Seladelpar, and the dual PPAR-alpha and PPAR-delta agonist elafibranor, are an “unequivocal sign of progress, marking the arrival of a new era in which PBC treatment is expected to provide both biochemical benefits and amelioration of symptoms for patients,” David N. Assis, MD, with the Section of Digestive Diseases, Yale School of Medicine, New Haven, Connecticut, wrote in a linked editorial.
In the RESPONSE study, 193 patients with PBC who had an inadequate response to or a history of unacceptable side effects with UDCA were randomly allocated to either oral seladelpar 10 mg daily or placebo for 12 months. The vast majority (93.8%) continued UDCA as standard-of-care background therapy.
The primary endpoint was a biochemical response, which was defined as an alkaline phosphatase (ALP) level < 1.67 times the upper limit of the normal range, with a decrease of 15% or more from baseline, and a normal total bilirubin level at 12 months.
After 12 months, 61.7% of patients taking seladelpar met the primary endpoint vs 20% of patients taking placebo.
In addition, significantly more patients taking seladelpar than placebo had normalization of the ALP level (25% vs 0%). The average decrease in ALP from baseline was 42.4% in the seladelpar group vs 4.3% in the placebo group.
At 12 months, alanine aminotransferase and gamma-glutamyl transferase levels were reduced by 23.5% and 39.1%, respectively, in the seladelpar group compared with 6.5% and 11.4%, respectively, in the placebo group.
“In PBC, we use target endpoints, so the trial was not powered or able to show yet clinical outcomes because the pace of the disease is quite slow. But we believe that the normalization of liver tests and improvement in quality of life will change the disease trajectory over time,” Dr. Hirschfield said.
Significant Reduction in Pruritus
A key secondary endpoint was change in patient-reported pruritus.
At baseline, 38.3% of patients in the seladelpar group and 35.4% of those in the placebo group had moderate to severe pruritus, with a daily numerical rating scale (NRS) score of 4 or higher out of 10.
Among these patients, the reduction from baseline in the pruritus NRS score at month 6 was significantly greater with seladelpar than with placebo (change from baseline, −3.2 points vs −1.7 points). These improvements were sustained through 12 months.
Improvements on the 5-D Itch Scale in both the moderate to severe pruritus population and the overall population also favored seladelpar over placebo for itch relief, which had a positive impact on sleep. Similar results demonstrating reductions in itch and improvements in sleep were observed using the PBC-40 questionnaire.
Adverse events that led to discontinuation of seladelpar or placebo were rare, and there was no between-group difference in the incidence of serious adverse events.
“No worrisome adverse events affecting the muscles were observed, including among patients receiving statins. Certain gastrointestinal events — abdominal pain, abdominal distention, and nausea — were reported more frequently in the seladelpar group than in the placebo group,” the study authors wrote.
The most common adverse events that occurred in ≥ 5% of patients in either group were COVID-19 and pruritus. A greater percentage of patients treated with placebo reported pruritus (15.4% vs 4.7%) as an adverse event — a finding consistent with the positive effect of seladelpar on reducing pruritus.
The researchers noted that 96.4% of patients who participated in the RESPONSE trial chose to enroll in the extension trial to evaluate long-term safety and the side-effect profile of seladelpar.
Potential First-Line Treatment?
In Dr. Assis’ view, the RESPONSE trial, coupled with the recently reported ELATIVE trial of the dual PPAR-alpha and PPAR-delta agonist elafibranor in PBC, “cement the role of PPAR agonists as the preferred second-line treatment in primary biliary cholangitis.”
“The reduction in serum cholestatic markers and the safety profiles of elafibranor and seladelpar offer clear advantages beyond what was previously shown with obeticholic acid. These trials also cement a new treatment goal for primary biliary cholangitis in which a reduction in pruritus should be expected as part of anticholestatic treatment,” Dr. Assis wrote.
“The results of these trials suggest that the use of PPAR agonists in primary biliary cholangitis could improve treatment outcomes while also improving quality of life, which is a highly desirable alignment of clinician and patient goals,” Dr. Assis added.
Looking ahead, Dr. Hirschfield sees a potential role for seladelpar earlier in the course of PBC treatment, he said in an interview.
“Over time, the way we treat patients will not be to wait to fail. It will be treat to target and treat to success,” Dr. Hirschfield said.
Earlier this month, the US Food and Drug Administration accepted CymaBay Therapeutics’ new drug application for seladelpar for the treatment of PBC, including pruritus in adults without cirrhosis or with compensated cirrhosis (Child Pugh A) who fail to respond adequately or cannot tolerate UDCA. Seladelpar for PBC was granted breakthrough designation in October 2023.
The study was funded by CymaBay Therapeutics. Disclosures for authors and editorialist are available at NEJM.org.
A version of this article appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
What’s Changed in Asthma Treatment? Quite a Bit
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik, and today I am going to talk about the 2023 update to the Global Strategy for Asthma Management and Prevention. We treat a lot of asthma, and there are some important changes, particularly around the use of albuterol. There are two main guidelines when it comes to asthma, the Global Initiative for Asthma (GINA) guideline and the US National Heart, Lung, and Blood Institute Guidelines. While I had the privilege of serving on the expert working group for the US guidelines, what I like about the GINA guidelines is that they are updated annually, and so they really help us keep up with rapid changes in the field.
Today, I’m going to focus on assessment and treatment.
Four Questions to Assess Asthma Control
Because over half of patients with asthma are not well controlled, it is important to assess control at every asthma visit. Asthma control has two domains: symptom control and the risk for future exacerbations. It is not enough to simply ask, “How is your asthma?” because many patients overrate their control and live with ongoing symptoms. There are many assessment tools; the Asthma Control Test (ACT) focuses on symptoms, and the new Asthma Impairment and Risk Questionnaire (AIRQ) assesses both symptoms and risk for exacerbations. The GINA assessment is probably the easiest to implement, with just four questions relevant to the past 4 weeks:
- Have you had daytime symptoms more than twice in one week?
- Have you had any night waking due to asthma?
- Have you needed short-acting beta-agonist (SABA), such as albuterol, rescue more than twice in one week?
- Have you had any activity limitation due to asthma?
Well-controlled asthma is defined as a negative response to all four of these questions, partly controlled asthma is one or two “yes” answers, and uncontrolled asthma is three to four positive responses. You can’t modify a patient’s therapy if you don’t know whether their asthma is well or poorly controlled. You’ll notice that these questions focus on symptom control. It is important also to ask about risk factors for exacerbations, particularly previous exacerbations.
Asthma Treatment Changes
The goals of treatment are control of symptoms and avoidance of exacerbations. The GINA guidelines emphasize that even patients with mild asthma can have severe or fatal exacerbations.
GINA recommends two management tracks. The preferred track uses inhaled corticosteroid (ICS)-formoterol as both maintenance and reliever therapy (MART). Track 2, without the use of ICS-formoterol for MART, is also offered, recognizing that the use of ICS-formoterol for MART is not approved by the US Food and Drug Administration. There is an easy-to-follow stepped-care diagram that is worth looking at; it’s on page 66 of the GINA guideline PDF.
For patients who have symptoms less than twice a month, begin with Step 1 therapy:
- Track 1: as-needed low-dose ICS-formoterol.
- Track 2: treatment with albuterol; also use ICS whenever albuterol is used.
For patients with symptoms more than twice a month (but not most days of the week) treatment can start with Step 2 therapy:
- Track 1: as-needed low-dose ICS-formoterol
- Track 2: daily low-dose ICS plus as-needed SABA
An option for rescue therapy for Track 2 across all steps of therapy is to use an ICS whenever a SABA is used for rescue to reduce the likelihood of exacerbation.
For patients with more severe asthma symptoms most days of the week, or whose asthma is waking them from sleep one or more times weekly, then you can start with Step 3 therapy as follows:
- Track 1: low dose ICS-formoterol as MART
- Track 2: low-dose ICS with long-acting beta-agonist (LABA) for maintenance, plus as needed SABA or as needed ICS-SABA
That’s going to cover most of our patients. As we see people back, if escalation of therapy is needed, then Step 4 therapy is:
- Track 1: medium-dose ICS-formoterol as MART
- Track 2: medium-dose ICS-LABA plus as needed SABA or as-needed ICS-SABA
For patients who remain uncontrolled, it’s important to realize that Step 5 gives you the option to add a long-acting muscarinic antagonist (LAMA). In my experience this can be very helpful. We can also consider going to high-dose ICS-LABS for maintenance. At this step, the patient usually has pretty severe, uncontrolled asthma and we can think about checking eosinophil counts, ordering pulmonary function tests, and referring to our specialist colleagues for consideration of biologic therapy.
It is important to see patients back regularly, and to assess asthma control. If a patient is not well controlled or has had exacerbations, consider stepping up therapy, or changing from albuterol alone as rescue to albuterol plus ICS for rescue. If they have been well controlled for a long time, consider de-escalation of therapy among patients on one of the higher therapy steps.
Dr. Skolnik has disclosed the following relevant financial relationships: Serve(d) on the advisory board for AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck; and Bayer; serve(d) as a speaker or a member of a speakers bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline. Received research grant from Sanofi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Bayer; and received income in an amount equal to or greater than $250 from AstraZeneca, Teva, Eli Lilly and Company, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article appeared on Medscape.com.
Is Primary Tumor Resection Beneficial in Stage IV CRC?
TOPLINE:
not amenable to curative therapy, new data showed.
METHODOLOGY:
- Chemotherapy is the primary treatment in patients with stage IV (CRC) and unresectable metastases. It’s unclear whether primary tumor resection before chemotherapy prolongs survival.
- Among 393 patients with stage IV colon cancer and unresectable metastases enrolled in the and trials, 187 were randomly allocated to undergo primary tumor resection and 206 to upfront chemotherapy.
- The chemotherapy regimen was left up to the treating physician. Overall survival was the primary endpoint. Median follow-up time was 36.7 months.
TAKEAWAY:
- Median overall survival was 16.7 months with primary tumor resection and 18.6 months with upfront chemotherapy (P = .191).
- Comparable overall survival between the study groups was further confirmed on multivariate analysis (hazard ratio, 0.944; P = .65) and across all subgroups.
- Serious adverse events were more common with upfront chemo than surgery (18% vs 10%; P = .027), due mainly to a significantly higher incidence of GI-related events (11% vs 5%; P = .031).
- Overall, 24% of the primary tumor resection group did not receive any chemotherapy.
IN PRACTICE:
“The results of our study provide compelling data that upfront primary tumor resection in treatment-naive stage IV CRC not amenable for curative treatment does not prolong [overall survival]. A relatively low incidence of serious adverse events in patients with an intact primary tumor together with a considerable number of patients who did not receive any chemotherapy in the primary tumor resection group provides further arguments against resection of the primary tumor in this group of patients,” the authors of the combined analysis concluded.
SOURCE:
The study, with first author Nuh N. Rahbari, MD, University of Ulm, Ulm, Germany, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Neither study completed their planned patient accrual. Although both trials are nearly identical, differences in the individual study cohorts and trial implementation could have introduced bias. Tumor molecular profiling was not performed.
DISCLOSURES:
The study had no commercial funding. Disclosures for authors are available with the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
not amenable to curative therapy, new data showed.
METHODOLOGY:
- Chemotherapy is the primary treatment in patients with stage IV (CRC) and unresectable metastases. It’s unclear whether primary tumor resection before chemotherapy prolongs survival.
- Among 393 patients with stage IV colon cancer and unresectable metastases enrolled in the and trials, 187 were randomly allocated to undergo primary tumor resection and 206 to upfront chemotherapy.
- The chemotherapy regimen was left up to the treating physician. Overall survival was the primary endpoint. Median follow-up time was 36.7 months.
TAKEAWAY:
- Median overall survival was 16.7 months with primary tumor resection and 18.6 months with upfront chemotherapy (P = .191).
- Comparable overall survival between the study groups was further confirmed on multivariate analysis (hazard ratio, 0.944; P = .65) and across all subgroups.
- Serious adverse events were more common with upfront chemo than surgery (18% vs 10%; P = .027), due mainly to a significantly higher incidence of GI-related events (11% vs 5%; P = .031).
- Overall, 24% of the primary tumor resection group did not receive any chemotherapy.
IN PRACTICE:
“The results of our study provide compelling data that upfront primary tumor resection in treatment-naive stage IV CRC not amenable for curative treatment does not prolong [overall survival]. A relatively low incidence of serious adverse events in patients with an intact primary tumor together with a considerable number of patients who did not receive any chemotherapy in the primary tumor resection group provides further arguments against resection of the primary tumor in this group of patients,” the authors of the combined analysis concluded.
SOURCE:
The study, with first author Nuh N. Rahbari, MD, University of Ulm, Ulm, Germany, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Neither study completed their planned patient accrual. Although both trials are nearly identical, differences in the individual study cohorts and trial implementation could have introduced bias. Tumor molecular profiling was not performed.
DISCLOSURES:
The study had no commercial funding. Disclosures for authors are available with the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
not amenable to curative therapy, new data showed.
METHODOLOGY:
- Chemotherapy is the primary treatment in patients with stage IV (CRC) and unresectable metastases. It’s unclear whether primary tumor resection before chemotherapy prolongs survival.
- Among 393 patients with stage IV colon cancer and unresectable metastases enrolled in the and trials, 187 were randomly allocated to undergo primary tumor resection and 206 to upfront chemotherapy.
- The chemotherapy regimen was left up to the treating physician. Overall survival was the primary endpoint. Median follow-up time was 36.7 months.
TAKEAWAY:
- Median overall survival was 16.7 months with primary tumor resection and 18.6 months with upfront chemotherapy (P = .191).
- Comparable overall survival between the study groups was further confirmed on multivariate analysis (hazard ratio, 0.944; P = .65) and across all subgroups.
- Serious adverse events were more common with upfront chemo than surgery (18% vs 10%; P = .027), due mainly to a significantly higher incidence of GI-related events (11% vs 5%; P = .031).
- Overall, 24% of the primary tumor resection group did not receive any chemotherapy.
IN PRACTICE:
“The results of our study provide compelling data that upfront primary tumor resection in treatment-naive stage IV CRC not amenable for curative treatment does not prolong [overall survival]. A relatively low incidence of serious adverse events in patients with an intact primary tumor together with a considerable number of patients who did not receive any chemotherapy in the primary tumor resection group provides further arguments against resection of the primary tumor in this group of patients,” the authors of the combined analysis concluded.
SOURCE:
The study, with first author Nuh N. Rahbari, MD, University of Ulm, Ulm, Germany, was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Neither study completed their planned patient accrual. Although both trials are nearly identical, differences in the individual study cohorts and trial implementation could have introduced bias. Tumor molecular profiling was not performed.
DISCLOSURES:
The study had no commercial funding. Disclosures for authors are available with the original article.
A version of this article appeared on Medscape.com.
What’s Next for the World’s First HIV Vaccine?
When the world needed a COVID vaccine, leading HIV investigators answered the call to intervene in the coronavirus pandemic. Now, efforts to discover the world’s first HIV vaccine are revitalized.
“The body is capable of making antibodies to protect us from HIV,” says Yunda Huang, PhD, from the Fred Hutchinson Cancer Center in Seattle, Washington, who sat down with me before her talk today at the Conference on Retroviruses & Opportunistic Infections.
Dr. Huang spoke about the path forward for neutralizing antibody protection after the last attempt in a generation of HIV vaccine development ended in disappointment.
The past two decades marked the rise in HIV broadly neutralizing antibodies, with vaccine strategies to induce them. Promising advances include germline approaches, mRNA, and nanoparticle technologies.
The PrEP vaccine trial testing two experimental prevention regimens in Africa was stopped after investigators reported there is “little to no chance” the trial will show the vaccines are effective.
A Shape-Shifting Virus
HIV has been called the shape-shifting virus because it disguises itself so that even when people are able to make antibodies to it, the virus changes to escape.
But Dr. Huang and others are optimistic that an effective vaccine is still possible.
“We cannot and will not lose hope that the world will have an effective HIV vaccine that is accessible by all who need it, anywhere,” International AIDS Society (IAS) Executive Director Birgit Poniatowski said in a statement in December, when the trial was stopped.
HIV is a still persistent problem in the United States, according to the Centers for Disease Control and Prevention that reports it has affected an estimated 1.2 million people.
With new people infected every day around the globe, Dr. Huang says she feels a sense of urgency to help. “I think about all the people around the globe and the large number of young girls being hurt and I know our big pool of talent can intervene to change what we see happening.”
Dr. Huang says the clinical trial failures we’ve seen so far will help guide next steps in HIV research as much as successes typically do.
Advances in the Field
With significant advances in protein nanoparticle science, mRNA technology, adjuvant development, and B-cell and antibody analyses, a new wave of clinical trials are on the way.
And with so many new approaches in the works, the HIV Vaccine Trials Network is retooling how it operates to navigate a burgeoning field and identify the most promising regimens.
A new Discovery Medicine Program will help the network assess new vaccine candidates. It will also aim to rule out others earlier on.
For COVID-19 and the flu, multimeric nanoparticles are an important alternative under investigation that could also be adapted for HIV.
Dr. Huang says she is particularly excited to watch the progress in cocktails of combination monoclonals. “I’ve been working in this field for 20 years now and there is a misconception that with pre-exposure prophylaxis, our job is done, but HIV is so far from away from being solved.”
But you just never know, Dr. Huang says. With new research, “we could bump on something at any point that changes everything.”
A version of this article appeared on Medscape.com.
When the world needed a COVID vaccine, leading HIV investigators answered the call to intervene in the coronavirus pandemic. Now, efforts to discover the world’s first HIV vaccine are revitalized.
“The body is capable of making antibodies to protect us from HIV,” says Yunda Huang, PhD, from the Fred Hutchinson Cancer Center in Seattle, Washington, who sat down with me before her talk today at the Conference on Retroviruses & Opportunistic Infections.
Dr. Huang spoke about the path forward for neutralizing antibody protection after the last attempt in a generation of HIV vaccine development ended in disappointment.
The past two decades marked the rise in HIV broadly neutralizing antibodies, with vaccine strategies to induce them. Promising advances include germline approaches, mRNA, and nanoparticle technologies.
The PrEP vaccine trial testing two experimental prevention regimens in Africa was stopped after investigators reported there is “little to no chance” the trial will show the vaccines are effective.
A Shape-Shifting Virus
HIV has been called the shape-shifting virus because it disguises itself so that even when people are able to make antibodies to it, the virus changes to escape.
But Dr. Huang and others are optimistic that an effective vaccine is still possible.
“We cannot and will not lose hope that the world will have an effective HIV vaccine that is accessible by all who need it, anywhere,” International AIDS Society (IAS) Executive Director Birgit Poniatowski said in a statement in December, when the trial was stopped.
HIV is a still persistent problem in the United States, according to the Centers for Disease Control and Prevention that reports it has affected an estimated 1.2 million people.
With new people infected every day around the globe, Dr. Huang says she feels a sense of urgency to help. “I think about all the people around the globe and the large number of young girls being hurt and I know our big pool of talent can intervene to change what we see happening.”
Dr. Huang says the clinical trial failures we’ve seen so far will help guide next steps in HIV research as much as successes typically do.
Advances in the Field
With significant advances in protein nanoparticle science, mRNA technology, adjuvant development, and B-cell and antibody analyses, a new wave of clinical trials are on the way.
And with so many new approaches in the works, the HIV Vaccine Trials Network is retooling how it operates to navigate a burgeoning field and identify the most promising regimens.
A new Discovery Medicine Program will help the network assess new vaccine candidates. It will also aim to rule out others earlier on.
For COVID-19 and the flu, multimeric nanoparticles are an important alternative under investigation that could also be adapted for HIV.
Dr. Huang says she is particularly excited to watch the progress in cocktails of combination monoclonals. “I’ve been working in this field for 20 years now and there is a misconception that with pre-exposure prophylaxis, our job is done, but HIV is so far from away from being solved.”
But you just never know, Dr. Huang says. With new research, “we could bump on something at any point that changes everything.”
A version of this article appeared on Medscape.com.
When the world needed a COVID vaccine, leading HIV investigators answered the call to intervene in the coronavirus pandemic. Now, efforts to discover the world’s first HIV vaccine are revitalized.
“The body is capable of making antibodies to protect us from HIV,” says Yunda Huang, PhD, from the Fred Hutchinson Cancer Center in Seattle, Washington, who sat down with me before her talk today at the Conference on Retroviruses & Opportunistic Infections.
Dr. Huang spoke about the path forward for neutralizing antibody protection after the last attempt in a generation of HIV vaccine development ended in disappointment.
The past two decades marked the rise in HIV broadly neutralizing antibodies, with vaccine strategies to induce them. Promising advances include germline approaches, mRNA, and nanoparticle technologies.
The PrEP vaccine trial testing two experimental prevention regimens in Africa was stopped after investigators reported there is “little to no chance” the trial will show the vaccines are effective.
A Shape-Shifting Virus
HIV has been called the shape-shifting virus because it disguises itself so that even when people are able to make antibodies to it, the virus changes to escape.
But Dr. Huang and others are optimistic that an effective vaccine is still possible.
“We cannot and will not lose hope that the world will have an effective HIV vaccine that is accessible by all who need it, anywhere,” International AIDS Society (IAS) Executive Director Birgit Poniatowski said in a statement in December, when the trial was stopped.
HIV is a still persistent problem in the United States, according to the Centers for Disease Control and Prevention that reports it has affected an estimated 1.2 million people.
With new people infected every day around the globe, Dr. Huang says she feels a sense of urgency to help. “I think about all the people around the globe and the large number of young girls being hurt and I know our big pool of talent can intervene to change what we see happening.”
Dr. Huang says the clinical trial failures we’ve seen so far will help guide next steps in HIV research as much as successes typically do.
Advances in the Field
With significant advances in protein nanoparticle science, mRNA technology, adjuvant development, and B-cell and antibody analyses, a new wave of clinical trials are on the way.
And with so many new approaches in the works, the HIV Vaccine Trials Network is retooling how it operates to navigate a burgeoning field and identify the most promising regimens.
A new Discovery Medicine Program will help the network assess new vaccine candidates. It will also aim to rule out others earlier on.
For COVID-19 and the flu, multimeric nanoparticles are an important alternative under investigation that could also be adapted for HIV.
Dr. Huang says she is particularly excited to watch the progress in cocktails of combination monoclonals. “I’ve been working in this field for 20 years now and there is a misconception that with pre-exposure prophylaxis, our job is done, but HIV is so far from away from being solved.”
But you just never know, Dr. Huang says. With new research, “we could bump on something at any point that changes everything.”
A version of this article appeared on Medscape.com.
FROM CROI 2024
Doxy-PEP Cut STIs in San Francisco in Half
Syphilis and chlamydia infections were reduced by half among men who have sex with men and transgender women 1 year after San Francisco rolled out doxycycline postexposure prophylaxis (doxy-PEP), according to data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) this week.
After a clinical trial showed that doxy-PEP taken after sex reduced the chance of acquiring syphilis, gonorrhea, and chlamydia by about two-thirds, the San Francisco Department of Public Health released the first guidelines in the country in October 2022.
So far, more than 3700 people in San Francisco have been prescribed doxy-PEP, reports Stephanie Cohen, MD, director of HIV and sexually transmitted infection (STI) prevention in the Disease Prevention and Control Branch of Public Health.
Dr. Cohen and her colleagues spent a year monitoring the uptake of doxy-PEP and used a computer model to predict what the rates of sexually transmitted infection would have been without doxy-PEP.
In November 2023, 13 months after the guidelines were introduced, they found that monthly chlamydia and early syphilis infections were 50% and 51% lower, respectively, than what was predicted by the model.
Fewer Infections
The drop in infections is having a tangible effect on patients in San Francisco, and many clinicians are noting that they are seeing far fewer positive tests. “The results that we’re seeing on a city-wide level are absolutely being experienced by individual providers and patients,” Dr. Cohen said.
However, the analysis showed no effect on rates of gonorrhea. It’s not clear why, although Dr. Cohen points out that doxy-PEP was less effective against gonorrhea in the clinical trial. And “there could be other factors in play,” she added. “Adherence might matter more, or it could be affected by the prevalence of tetracycline resistance in the community.”
With rates of STIs, particularly syphilis, quickly rising in recent years, healthcare providers have been scrambling to find effective interventions. So far, doxy-PEP has shown the most promise. “We’ve known for a while that all of the strategies we’ve been employing don’t seem to be working,” noted Chase Cannon, MD, an infectious disease specialist at the University of Washington in Seattle. “That’s why doxy-PEP is important. We haven’t had anything that can deflect the curve in a long time.”
What About the Side Effects?
Some concerns remain, however, about the widespread prophylactic use of antibiotics. There are no long-term safety data on the potential side effects of doxy-PEP, and there is still a lot of stigma around interventions that allow people to have sex the way they want, said Dr. Cannon.
But perhaps, the biggest concern is that doxy-PEP could contribute to antibiotic resistance. Those fears are not misplaced, Dr. Cannon added. The results of one study, presented in a poster at CROI, showed that stool samples from people prescribed doxy-PEP had elevated levels of bacterial genes that can confer resistance to tetracyclines, the class of antibiotics to which doxycycline belongs. There was no change in resistance to other classes of antibiotics and no difference in bacterial diversity over the 6 months of the study.
Dr. Cannon cautioned, however, that we can’t extrapolate these results to clinical outcomes. “We can look for signals [of resistance], but we don’t know if this means someone will fail therapy for chlamydia or syphilis,” he said.
There are still many challenges to overcome before doxy-PEP can be rolled out widely, Dr. Cohen explained. There is a lack of consensus among healthcare professionals about who should be offered doxy-PEP. The clinical trial results and the San Fransisco guidelines only apply to men who have sex with men and to transgender women.
Some clinicians argue that the intervention should be provided to a broader population, whereas others want to see more research to ensure that unnecessary antibiotic use is minimized.
So far just one study has tested doxy-PEP in another population — in women in Kenya — and it was found to not be effective. But the data suggest that adherence to the protocol was poor in that study, so the results may not be reliable, Dr. Cohen said.
“We need effective prevention tools for all genders, especially cis women who bear most of the morbidity,” she said. “It stands to reason that this should work for them, but without high-quality evidence, there is insufficient information to make a recommendation for cis women.”
The US Centers for Disease Control and Prevention is currently reviewing public and expert comments and refining final guidelines for release in the coming months, which should alleviate some of the uncertainty. “Many providers are waiting for that guidance before they will feel confident moving forward,” Dr. Cohen noted.
But despite the risks and uncertainty, doxy-PEP looks set to be a major part of the fight against STIs going forward. “Doxy-PEP is essential for us as a nation to be dealing with the syphilis epidemic,” Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Disease, said in a video introduction to CROI.
A version of this article appeared on Medscape.com.
Syphilis and chlamydia infections were reduced by half among men who have sex with men and transgender women 1 year after San Francisco rolled out doxycycline postexposure prophylaxis (doxy-PEP), according to data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) this week.
After a clinical trial showed that doxy-PEP taken after sex reduced the chance of acquiring syphilis, gonorrhea, and chlamydia by about two-thirds, the San Francisco Department of Public Health released the first guidelines in the country in October 2022.
So far, more than 3700 people in San Francisco have been prescribed doxy-PEP, reports Stephanie Cohen, MD, director of HIV and sexually transmitted infection (STI) prevention in the Disease Prevention and Control Branch of Public Health.
Dr. Cohen and her colleagues spent a year monitoring the uptake of doxy-PEP and used a computer model to predict what the rates of sexually transmitted infection would have been without doxy-PEP.
In November 2023, 13 months after the guidelines were introduced, they found that monthly chlamydia and early syphilis infections were 50% and 51% lower, respectively, than what was predicted by the model.
Fewer Infections
The drop in infections is having a tangible effect on patients in San Francisco, and many clinicians are noting that they are seeing far fewer positive tests. “The results that we’re seeing on a city-wide level are absolutely being experienced by individual providers and patients,” Dr. Cohen said.
However, the analysis showed no effect on rates of gonorrhea. It’s not clear why, although Dr. Cohen points out that doxy-PEP was less effective against gonorrhea in the clinical trial. And “there could be other factors in play,” she added. “Adherence might matter more, or it could be affected by the prevalence of tetracycline resistance in the community.”
With rates of STIs, particularly syphilis, quickly rising in recent years, healthcare providers have been scrambling to find effective interventions. So far, doxy-PEP has shown the most promise. “We’ve known for a while that all of the strategies we’ve been employing don’t seem to be working,” noted Chase Cannon, MD, an infectious disease specialist at the University of Washington in Seattle. “That’s why doxy-PEP is important. We haven’t had anything that can deflect the curve in a long time.”
What About the Side Effects?
Some concerns remain, however, about the widespread prophylactic use of antibiotics. There are no long-term safety data on the potential side effects of doxy-PEP, and there is still a lot of stigma around interventions that allow people to have sex the way they want, said Dr. Cannon.
But perhaps, the biggest concern is that doxy-PEP could contribute to antibiotic resistance. Those fears are not misplaced, Dr. Cannon added. The results of one study, presented in a poster at CROI, showed that stool samples from people prescribed doxy-PEP had elevated levels of bacterial genes that can confer resistance to tetracyclines, the class of antibiotics to which doxycycline belongs. There was no change in resistance to other classes of antibiotics and no difference in bacterial diversity over the 6 months of the study.
Dr. Cannon cautioned, however, that we can’t extrapolate these results to clinical outcomes. “We can look for signals [of resistance], but we don’t know if this means someone will fail therapy for chlamydia or syphilis,” he said.
There are still many challenges to overcome before doxy-PEP can be rolled out widely, Dr. Cohen explained. There is a lack of consensus among healthcare professionals about who should be offered doxy-PEP. The clinical trial results and the San Fransisco guidelines only apply to men who have sex with men and to transgender women.
Some clinicians argue that the intervention should be provided to a broader population, whereas others want to see more research to ensure that unnecessary antibiotic use is minimized.
So far just one study has tested doxy-PEP in another population — in women in Kenya — and it was found to not be effective. But the data suggest that adherence to the protocol was poor in that study, so the results may not be reliable, Dr. Cohen said.
“We need effective prevention tools for all genders, especially cis women who bear most of the morbidity,” she said. “It stands to reason that this should work for them, but without high-quality evidence, there is insufficient information to make a recommendation for cis women.”
The US Centers for Disease Control and Prevention is currently reviewing public and expert comments and refining final guidelines for release in the coming months, which should alleviate some of the uncertainty. “Many providers are waiting for that guidance before they will feel confident moving forward,” Dr. Cohen noted.
But despite the risks and uncertainty, doxy-PEP looks set to be a major part of the fight against STIs going forward. “Doxy-PEP is essential for us as a nation to be dealing with the syphilis epidemic,” Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Disease, said in a video introduction to CROI.
A version of this article appeared on Medscape.com.
Syphilis and chlamydia infections were reduced by half among men who have sex with men and transgender women 1 year after San Francisco rolled out doxycycline postexposure prophylaxis (doxy-PEP), according to data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) this week.
After a clinical trial showed that doxy-PEP taken after sex reduced the chance of acquiring syphilis, gonorrhea, and chlamydia by about two-thirds, the San Francisco Department of Public Health released the first guidelines in the country in October 2022.
So far, more than 3700 people in San Francisco have been prescribed doxy-PEP, reports Stephanie Cohen, MD, director of HIV and sexually transmitted infection (STI) prevention in the Disease Prevention and Control Branch of Public Health.
Dr. Cohen and her colleagues spent a year monitoring the uptake of doxy-PEP and used a computer model to predict what the rates of sexually transmitted infection would have been without doxy-PEP.
In November 2023, 13 months after the guidelines were introduced, they found that monthly chlamydia and early syphilis infections were 50% and 51% lower, respectively, than what was predicted by the model.
Fewer Infections
The drop in infections is having a tangible effect on patients in San Francisco, and many clinicians are noting that they are seeing far fewer positive tests. “The results that we’re seeing on a city-wide level are absolutely being experienced by individual providers and patients,” Dr. Cohen said.
However, the analysis showed no effect on rates of gonorrhea. It’s not clear why, although Dr. Cohen points out that doxy-PEP was less effective against gonorrhea in the clinical trial. And “there could be other factors in play,” she added. “Adherence might matter more, or it could be affected by the prevalence of tetracycline resistance in the community.”
With rates of STIs, particularly syphilis, quickly rising in recent years, healthcare providers have been scrambling to find effective interventions. So far, doxy-PEP has shown the most promise. “We’ve known for a while that all of the strategies we’ve been employing don’t seem to be working,” noted Chase Cannon, MD, an infectious disease specialist at the University of Washington in Seattle. “That’s why doxy-PEP is important. We haven’t had anything that can deflect the curve in a long time.”
What About the Side Effects?
Some concerns remain, however, about the widespread prophylactic use of antibiotics. There are no long-term safety data on the potential side effects of doxy-PEP, and there is still a lot of stigma around interventions that allow people to have sex the way they want, said Dr. Cannon.
But perhaps, the biggest concern is that doxy-PEP could contribute to antibiotic resistance. Those fears are not misplaced, Dr. Cannon added. The results of one study, presented in a poster at CROI, showed that stool samples from people prescribed doxy-PEP had elevated levels of bacterial genes that can confer resistance to tetracyclines, the class of antibiotics to which doxycycline belongs. There was no change in resistance to other classes of antibiotics and no difference in bacterial diversity over the 6 months of the study.
Dr. Cannon cautioned, however, that we can’t extrapolate these results to clinical outcomes. “We can look for signals [of resistance], but we don’t know if this means someone will fail therapy for chlamydia or syphilis,” he said.
There are still many challenges to overcome before doxy-PEP can be rolled out widely, Dr. Cohen explained. There is a lack of consensus among healthcare professionals about who should be offered doxy-PEP. The clinical trial results and the San Fransisco guidelines only apply to men who have sex with men and to transgender women.
Some clinicians argue that the intervention should be provided to a broader population, whereas others want to see more research to ensure that unnecessary antibiotic use is minimized.
So far just one study has tested doxy-PEP in another population — in women in Kenya — and it was found to not be effective. But the data suggest that adherence to the protocol was poor in that study, so the results may not be reliable, Dr. Cohen said.
“We need effective prevention tools for all genders, especially cis women who bear most of the morbidity,” she said. “It stands to reason that this should work for them, but without high-quality evidence, there is insufficient information to make a recommendation for cis women.”
The US Centers for Disease Control and Prevention is currently reviewing public and expert comments and refining final guidelines for release in the coming months, which should alleviate some of the uncertainty. “Many providers are waiting for that guidance before they will feel confident moving forward,” Dr. Cohen noted.
But despite the risks and uncertainty, doxy-PEP looks set to be a major part of the fight against STIs going forward. “Doxy-PEP is essential for us as a nation to be dealing with the syphilis epidemic,” Carl Dieffenbach, PhD, director of the Division of AIDS at the National Institute of Allergy and Infectious Disease, said in a video introduction to CROI.
A version of this article appeared on Medscape.com.
A New Biomarker of Brain Injury?
Posttraumatic headache (PTH) is associated with an increase in iron accumulation in certain brain regions , notably those involved in the pain network, early research shows.
The findings come on the heels of previous research showing patients with iron accumulation in certain brain regions don’t respond as well to treatment, study investigator, Simona Nikolova, PhD, assistant professor of neurology, Mayo Clinic, Phoenix, Arizona, told this news organization.
“This is really important, and doctors need to be aware of it. If you have a patient who is not responding to treatment, then you know what to look at,” she said.
The findings (Abstract #3379) will be presented on April 15 at the American Academy of Neurology (AAN) 2024 Annual Meeting.
Dose Effect
The study included 60 people with acute PTH due to mTBI. Most were White, and almost half had sustained a concussion due to a fall, with about 30% injured in a vehicle accident and a smaller number injured during a fight.
The mean number of lifetime mTBIs was 2.4, although participants had sustained as many as five or six and as few as one. The mean time from the most recent mTBI was 25 days, and the mean score on the Sport Concussion Assessment Tool (SCAT), which measures postconcussion symptom severity, was 29.
Most in the mTBI group (43) had migraine or probable migraine, and 14 had tension-type headaches. Mean headache frequency was 81%.
Researchers matched these patients with 60 controls without concussion or headache. Because iron accumulation is age-related, they tried to eliminate this covariant by pairing each participant with mTBI with an age- and sex-matched control.
All participants underwent a type of brain MRI known as T2* weighted sequence that can identify brain iron accumulation, a marker of neural injury.
Investigators found that the PTH group had significantly higher levels of iron accumulation in several areas of the brain, most of which are part of a “pain network” that includes about 63 areas of the brain, Dr. Nikolova said.
The study wasn’t designed to determine how much more iron accumulation mTBI patients had vs controls.
“We can’t say it was twice as much or three times as much; we can only say it was significant. Measuring concentrations in PTH patients and comparing that with controls is something we haven’t don’t yet,” said Dr. Nikolova.
Areas of the brain with increased iron accumulation, included the periaqueductal gray (PAG), anterior cingulated cortex, and supramarginal gyrus.
Research suggests patients with migraine who have elevated levels of iron in the PAG have a poorer response to botulinum toxin treatment. An earlier study by the same team showed a poorer response to the calcitonin gene-related peptide inhibitor erenumab in migraine patients with elevated iron in the PAG.
Researchers discovered that those with more lifetime TBIs had higher iron accumulation in the right gyrus rectus and right putamen vs those with fewer injuries and that headache frequency was associated with iron accumulation in the posterior corona radiata, bilateral temporal, right frontal, bilateral supplemental motor area, left fusiform, right hippocampus, sagittal striatum, and left cerebellum.
Surprising Result
The investigators also found a link between time since the most recent mTBI and iron accumulation in the bilateral temporal, right hippocampus, posterior and superior corona radiata, bilateral thalamus, right precuneus and cuneus, right lingual, and right cerebellum.
“The more time that passed since the concussion occurred, the more likely that people had higher iron levels,” said Dr. Nikolova.
It’s perhaps to be expected that the length of time since injury is linked to iron accumulation in the brain as iron accumulates over time. But even those whose injury was relatively recent had higher amounts of iron, which Dr. Nikolova said was “surprising.”
“We thought iron accumulates over time so we were thinking maybe we should be doing a longitudinal study to see what happens, but we see definite iron accumulation due to injury shortly after the injury,” she said.
There was no association between iron accumulation and symptom severity as measured by SCAT scores.
Questions Remain
It’s unclear why iron accumulates after an injury or what the ramifications are of this accumulation, Dr. Nikolova noted.
The imaging used in the study doesn’t distinguish between “bound” iron found after a hemorrhage and “free” iron in the brain. The free iron type has been shown to be increased after TBI and is “the stuff you should be afraid of,” Dr. Nikolova said.
Iron’s role in the metabolic process is important, but must be closely regulated, she said. Even a small accumulation can lead to oxidative stress.
Researchers are investigating whether the findings would be similar in mTBI but no headache and want to increase the number of study participants. A larger, more diverse sample would allow them to probe other questions, including whether iron accumulation is different in men and women. More data could also eventually lead to iron accumulation becoming a biomarker for concussion and PTH, Dr. Nikolova said.
“If you know a certain person has that biomarker, you might be able to administer a drug or some therapeutic procedure to prevent that iron from continuing to accumulate in the brain.”
Chelation drugs and other therapies may clear iron from the body but not necessarily from the brain.
Commenting on the study for this news organization, Frank Conidi, MD, director, Florida Center for Headache and Sports Neurology, Port St. Lucie , said that the study supports the hypothesis that concussion “is not a benign process for the brain, and the cumulative effect of repetitive head injury can result in permanent brain injury.”
He said that he found the accumulation of iron in cortical structures particularly interesting. This, he said, differs from most current research that suggests head trauma mainly results in damage to white matter tracts.
He prefers the term “concussion” over “mild traumatic brain injury” which was used in the study. “Recent guidelines, including some that I’ve been involved with, have defined mild traumatic brain injury as a more permanent process,” he said.
The study was supported by the US Department of Defense and National Institutes of Health. No relevant conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
Posttraumatic headache (PTH) is associated with an increase in iron accumulation in certain brain regions , notably those involved in the pain network, early research shows.
The findings come on the heels of previous research showing patients with iron accumulation in certain brain regions don’t respond as well to treatment, study investigator, Simona Nikolova, PhD, assistant professor of neurology, Mayo Clinic, Phoenix, Arizona, told this news organization.
“This is really important, and doctors need to be aware of it. If you have a patient who is not responding to treatment, then you know what to look at,” she said.
The findings (Abstract #3379) will be presented on April 15 at the American Academy of Neurology (AAN) 2024 Annual Meeting.
Dose Effect
The study included 60 people with acute PTH due to mTBI. Most were White, and almost half had sustained a concussion due to a fall, with about 30% injured in a vehicle accident and a smaller number injured during a fight.
The mean number of lifetime mTBIs was 2.4, although participants had sustained as many as five or six and as few as one. The mean time from the most recent mTBI was 25 days, and the mean score on the Sport Concussion Assessment Tool (SCAT), which measures postconcussion symptom severity, was 29.
Most in the mTBI group (43) had migraine or probable migraine, and 14 had tension-type headaches. Mean headache frequency was 81%.
Researchers matched these patients with 60 controls without concussion or headache. Because iron accumulation is age-related, they tried to eliminate this covariant by pairing each participant with mTBI with an age- and sex-matched control.
All participants underwent a type of brain MRI known as T2* weighted sequence that can identify brain iron accumulation, a marker of neural injury.
Investigators found that the PTH group had significantly higher levels of iron accumulation in several areas of the brain, most of which are part of a “pain network” that includes about 63 areas of the brain, Dr. Nikolova said.
The study wasn’t designed to determine how much more iron accumulation mTBI patients had vs controls.
“We can’t say it was twice as much or three times as much; we can only say it was significant. Measuring concentrations in PTH patients and comparing that with controls is something we haven’t don’t yet,” said Dr. Nikolova.
Areas of the brain with increased iron accumulation, included the periaqueductal gray (PAG), anterior cingulated cortex, and supramarginal gyrus.
Research suggests patients with migraine who have elevated levels of iron in the PAG have a poorer response to botulinum toxin treatment. An earlier study by the same team showed a poorer response to the calcitonin gene-related peptide inhibitor erenumab in migraine patients with elevated iron in the PAG.
Researchers discovered that those with more lifetime TBIs had higher iron accumulation in the right gyrus rectus and right putamen vs those with fewer injuries and that headache frequency was associated with iron accumulation in the posterior corona radiata, bilateral temporal, right frontal, bilateral supplemental motor area, left fusiform, right hippocampus, sagittal striatum, and left cerebellum.
Surprising Result
The investigators also found a link between time since the most recent mTBI and iron accumulation in the bilateral temporal, right hippocampus, posterior and superior corona radiata, bilateral thalamus, right precuneus and cuneus, right lingual, and right cerebellum.
“The more time that passed since the concussion occurred, the more likely that people had higher iron levels,” said Dr. Nikolova.
It’s perhaps to be expected that the length of time since injury is linked to iron accumulation in the brain as iron accumulates over time. But even those whose injury was relatively recent had higher amounts of iron, which Dr. Nikolova said was “surprising.”
“We thought iron accumulates over time so we were thinking maybe we should be doing a longitudinal study to see what happens, but we see definite iron accumulation due to injury shortly after the injury,” she said.
There was no association between iron accumulation and symptom severity as measured by SCAT scores.
Questions Remain
It’s unclear why iron accumulates after an injury or what the ramifications are of this accumulation, Dr. Nikolova noted.
The imaging used in the study doesn’t distinguish between “bound” iron found after a hemorrhage and “free” iron in the brain. The free iron type has been shown to be increased after TBI and is “the stuff you should be afraid of,” Dr. Nikolova said.
Iron’s role in the metabolic process is important, but must be closely regulated, she said. Even a small accumulation can lead to oxidative stress.
Researchers are investigating whether the findings would be similar in mTBI but no headache and want to increase the number of study participants. A larger, more diverse sample would allow them to probe other questions, including whether iron accumulation is different in men and women. More data could also eventually lead to iron accumulation becoming a biomarker for concussion and PTH, Dr. Nikolova said.
“If you know a certain person has that biomarker, you might be able to administer a drug or some therapeutic procedure to prevent that iron from continuing to accumulate in the brain.”
Chelation drugs and other therapies may clear iron from the body but not necessarily from the brain.
Commenting on the study for this news organization, Frank Conidi, MD, director, Florida Center for Headache and Sports Neurology, Port St. Lucie , said that the study supports the hypothesis that concussion “is not a benign process for the brain, and the cumulative effect of repetitive head injury can result in permanent brain injury.”
He said that he found the accumulation of iron in cortical structures particularly interesting. This, he said, differs from most current research that suggests head trauma mainly results in damage to white matter tracts.
He prefers the term “concussion” over “mild traumatic brain injury” which was used in the study. “Recent guidelines, including some that I’ve been involved with, have defined mild traumatic brain injury as a more permanent process,” he said.
The study was supported by the US Department of Defense and National Institutes of Health. No relevant conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
Posttraumatic headache (PTH) is associated with an increase in iron accumulation in certain brain regions , notably those involved in the pain network, early research shows.
The findings come on the heels of previous research showing patients with iron accumulation in certain brain regions don’t respond as well to treatment, study investigator, Simona Nikolova, PhD, assistant professor of neurology, Mayo Clinic, Phoenix, Arizona, told this news organization.
“This is really important, and doctors need to be aware of it. If you have a patient who is not responding to treatment, then you know what to look at,” she said.
The findings (Abstract #3379) will be presented on April 15 at the American Academy of Neurology (AAN) 2024 Annual Meeting.
Dose Effect
The study included 60 people with acute PTH due to mTBI. Most were White, and almost half had sustained a concussion due to a fall, with about 30% injured in a vehicle accident and a smaller number injured during a fight.
The mean number of lifetime mTBIs was 2.4, although participants had sustained as many as five or six and as few as one. The mean time from the most recent mTBI was 25 days, and the mean score on the Sport Concussion Assessment Tool (SCAT), which measures postconcussion symptom severity, was 29.
Most in the mTBI group (43) had migraine or probable migraine, and 14 had tension-type headaches. Mean headache frequency was 81%.
Researchers matched these patients with 60 controls without concussion or headache. Because iron accumulation is age-related, they tried to eliminate this covariant by pairing each participant with mTBI with an age- and sex-matched control.
All participants underwent a type of brain MRI known as T2* weighted sequence that can identify brain iron accumulation, a marker of neural injury.
Investigators found that the PTH group had significantly higher levels of iron accumulation in several areas of the brain, most of which are part of a “pain network” that includes about 63 areas of the brain, Dr. Nikolova said.
The study wasn’t designed to determine how much more iron accumulation mTBI patients had vs controls.
“We can’t say it was twice as much or three times as much; we can only say it was significant. Measuring concentrations in PTH patients and comparing that with controls is something we haven’t don’t yet,” said Dr. Nikolova.
Areas of the brain with increased iron accumulation, included the periaqueductal gray (PAG), anterior cingulated cortex, and supramarginal gyrus.
Research suggests patients with migraine who have elevated levels of iron in the PAG have a poorer response to botulinum toxin treatment. An earlier study by the same team showed a poorer response to the calcitonin gene-related peptide inhibitor erenumab in migraine patients with elevated iron in the PAG.
Researchers discovered that those with more lifetime TBIs had higher iron accumulation in the right gyrus rectus and right putamen vs those with fewer injuries and that headache frequency was associated with iron accumulation in the posterior corona radiata, bilateral temporal, right frontal, bilateral supplemental motor area, left fusiform, right hippocampus, sagittal striatum, and left cerebellum.
Surprising Result
The investigators also found a link between time since the most recent mTBI and iron accumulation in the bilateral temporal, right hippocampus, posterior and superior corona radiata, bilateral thalamus, right precuneus and cuneus, right lingual, and right cerebellum.
“The more time that passed since the concussion occurred, the more likely that people had higher iron levels,” said Dr. Nikolova.
It’s perhaps to be expected that the length of time since injury is linked to iron accumulation in the brain as iron accumulates over time. But even those whose injury was relatively recent had higher amounts of iron, which Dr. Nikolova said was “surprising.”
“We thought iron accumulates over time so we were thinking maybe we should be doing a longitudinal study to see what happens, but we see definite iron accumulation due to injury shortly after the injury,” she said.
There was no association between iron accumulation and symptom severity as measured by SCAT scores.
Questions Remain
It’s unclear why iron accumulates after an injury or what the ramifications are of this accumulation, Dr. Nikolova noted.
The imaging used in the study doesn’t distinguish between “bound” iron found after a hemorrhage and “free” iron in the brain. The free iron type has been shown to be increased after TBI and is “the stuff you should be afraid of,” Dr. Nikolova said.
Iron’s role in the metabolic process is important, but must be closely regulated, she said. Even a small accumulation can lead to oxidative stress.
Researchers are investigating whether the findings would be similar in mTBI but no headache and want to increase the number of study participants. A larger, more diverse sample would allow them to probe other questions, including whether iron accumulation is different in men and women. More data could also eventually lead to iron accumulation becoming a biomarker for concussion and PTH, Dr. Nikolova said.
“If you know a certain person has that biomarker, you might be able to administer a drug or some therapeutic procedure to prevent that iron from continuing to accumulate in the brain.”
Chelation drugs and other therapies may clear iron from the body but not necessarily from the brain.
Commenting on the study for this news organization, Frank Conidi, MD, director, Florida Center for Headache and Sports Neurology, Port St. Lucie , said that the study supports the hypothesis that concussion “is not a benign process for the brain, and the cumulative effect of repetitive head injury can result in permanent brain injury.”
He said that he found the accumulation of iron in cortical structures particularly interesting. This, he said, differs from most current research that suggests head trauma mainly results in damage to white matter tracts.
He prefers the term “concussion” over “mild traumatic brain injury” which was used in the study. “Recent guidelines, including some that I’ve been involved with, have defined mild traumatic brain injury as a more permanent process,” he said.
The study was supported by the US Department of Defense and National Institutes of Health. No relevant conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
Artificially Sweetened Drinks Linked to Increased AF Risk
TOPLINE:
(AF) in a new observational study.
METHODOLOGY:
- The population-based cohort study looked at the associations of sugar-sweetened beverages, artificial sweetened beverages, and pure fruit juice consumption with the risk for incident AF and evaluated whether genetic susceptibility modifies these associations.
- The authors analyzed data from the UK Biobank on 201,856 participants who were free of baseline AF, had genetic data available, and completed a 24-hour diet questionnaire. The diagnosis of AF was obtained by linkage from primary care, hospital inpatient, and death register records.
- The results were adjusted for a wide range of potential confounders including age, sex, ethnicity, education level, socioeconomic status, smoking, alcohol consumption, physical activity level, sleep duration, body mass index, blood pressure, kidney function, sleep apnea, coronary heart disease, diabetes, and the use of lipid-lowering or antihypertensive medication.
TAKEAWAY:
- During a median follow-up of 9.9 years, 9362 incident AF cases were documented.
- Compared with nonconsumers, individuals who consumed more than 2 L per week of artificially sweetened beverages had a 20% increased risk of developing AF (hazard ratio [HR], 1.20; 95% CI, 1.10-1.31).
- Those who drank more than 2 L per week of sugar-sweetened beverages had a 10% increased risk for AF (HR, 1.10; 95% CI, 1.01-1.20).
- Consumption of 1 L or less per week of pure fruit juice was associated with an 8% lower risk of developing AF (HR, 0.92; 95% CI, 0.87-0.97).
- The associations persisted after adjustment for genetic susceptibility for AF.
IN PRACTICE:
The study authors concluded that this study does not demonstrate that consumption of sugar-sweetened or artificially sweetened beverages alters AF risk but rather that the consumption of these drinks may predict AF risk beyond traditional risk factors. They added that intervention studies and basic research are warranted to confirm whether the observed associations are causal. Commenting on the study, Duane Mellor, MD, registered dietitian at Aston University, Birmingham, England, said it is unclear if the observations in this study are a chance finding as there is a lack of a clear biological link. Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow, Glasgow, Scotland, added that although the authors tried to adjust for many factors, there is a strong chance that other behavioral aspects linked to beverage choice could be more relevant as a cause of AF rather than the drinks themselves. Tom Sanders, MD, professor emeritus of nutrition and dietetics, King’s College London, London, England, pointed out that as this is the first study that has reported such an effect with artificially sweetened drinks, the finding needs replication before any conclusions can be drawn. “It remains good dietary advice to recommend the consumption of low-calorie artificially sweetened drink in place of sugar-sweetened drinks and alcohol,” he added.
SOURCE:
The study, led by Ying Sun, MD, Shanghai Jiao Tong University School of Medicine, Shanghai, China, was published online in Circulation: Arrhythmia and Electrophysiology.
LIMITATIONS:
The consumption of beverages was self-reported and based on only five separate single-day food intake recalls which were taken over the first 3 years of the study, which was extrapolated to estimate weekly intake. The researchers could not tell whether the sugar-sweetened and artificially sweetened drinks were caffeinated and could not rule out residual confounding by other unmeasured or unknown factors.
DISCLOSURES:
This study was supported by the National Natural Science Foundation of China, Shanghai Municipal Health Commission, Shanghai Municipal Human Resources and Social Security Bureau, Clinical Research Plan of Shanghai Hospital Development Center, Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, and Shanghai Jiao Tong University School of Medicine.
A version of this article appeared on Medscape.com.
TOPLINE:
(AF) in a new observational study.
METHODOLOGY:
- The population-based cohort study looked at the associations of sugar-sweetened beverages, artificial sweetened beverages, and pure fruit juice consumption with the risk for incident AF and evaluated whether genetic susceptibility modifies these associations.
- The authors analyzed data from the UK Biobank on 201,856 participants who were free of baseline AF, had genetic data available, and completed a 24-hour diet questionnaire. The diagnosis of AF was obtained by linkage from primary care, hospital inpatient, and death register records.
- The results were adjusted for a wide range of potential confounders including age, sex, ethnicity, education level, socioeconomic status, smoking, alcohol consumption, physical activity level, sleep duration, body mass index, blood pressure, kidney function, sleep apnea, coronary heart disease, diabetes, and the use of lipid-lowering or antihypertensive medication.
TAKEAWAY:
- During a median follow-up of 9.9 years, 9362 incident AF cases were documented.
- Compared with nonconsumers, individuals who consumed more than 2 L per week of artificially sweetened beverages had a 20% increased risk of developing AF (hazard ratio [HR], 1.20; 95% CI, 1.10-1.31).
- Those who drank more than 2 L per week of sugar-sweetened beverages had a 10% increased risk for AF (HR, 1.10; 95% CI, 1.01-1.20).
- Consumption of 1 L or less per week of pure fruit juice was associated with an 8% lower risk of developing AF (HR, 0.92; 95% CI, 0.87-0.97).
- The associations persisted after adjustment for genetic susceptibility for AF.
IN PRACTICE:
The study authors concluded that this study does not demonstrate that consumption of sugar-sweetened or artificially sweetened beverages alters AF risk but rather that the consumption of these drinks may predict AF risk beyond traditional risk factors. They added that intervention studies and basic research are warranted to confirm whether the observed associations are causal. Commenting on the study, Duane Mellor, MD, registered dietitian at Aston University, Birmingham, England, said it is unclear if the observations in this study are a chance finding as there is a lack of a clear biological link. Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow, Glasgow, Scotland, added that although the authors tried to adjust for many factors, there is a strong chance that other behavioral aspects linked to beverage choice could be more relevant as a cause of AF rather than the drinks themselves. Tom Sanders, MD, professor emeritus of nutrition and dietetics, King’s College London, London, England, pointed out that as this is the first study that has reported such an effect with artificially sweetened drinks, the finding needs replication before any conclusions can be drawn. “It remains good dietary advice to recommend the consumption of low-calorie artificially sweetened drink in place of sugar-sweetened drinks and alcohol,” he added.
SOURCE:
The study, led by Ying Sun, MD, Shanghai Jiao Tong University School of Medicine, Shanghai, China, was published online in Circulation: Arrhythmia and Electrophysiology.
LIMITATIONS:
The consumption of beverages was self-reported and based on only five separate single-day food intake recalls which were taken over the first 3 years of the study, which was extrapolated to estimate weekly intake. The researchers could not tell whether the sugar-sweetened and artificially sweetened drinks were caffeinated and could not rule out residual confounding by other unmeasured or unknown factors.
DISCLOSURES:
This study was supported by the National Natural Science Foundation of China, Shanghai Municipal Health Commission, Shanghai Municipal Human Resources and Social Security Bureau, Clinical Research Plan of Shanghai Hospital Development Center, Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, and Shanghai Jiao Tong University School of Medicine.
A version of this article appeared on Medscape.com.
TOPLINE:
(AF) in a new observational study.
METHODOLOGY:
- The population-based cohort study looked at the associations of sugar-sweetened beverages, artificial sweetened beverages, and pure fruit juice consumption with the risk for incident AF and evaluated whether genetic susceptibility modifies these associations.
- The authors analyzed data from the UK Biobank on 201,856 participants who were free of baseline AF, had genetic data available, and completed a 24-hour diet questionnaire. The diagnosis of AF was obtained by linkage from primary care, hospital inpatient, and death register records.
- The results were adjusted for a wide range of potential confounders including age, sex, ethnicity, education level, socioeconomic status, smoking, alcohol consumption, physical activity level, sleep duration, body mass index, blood pressure, kidney function, sleep apnea, coronary heart disease, diabetes, and the use of lipid-lowering or antihypertensive medication.
TAKEAWAY:
- During a median follow-up of 9.9 years, 9362 incident AF cases were documented.
- Compared with nonconsumers, individuals who consumed more than 2 L per week of artificially sweetened beverages had a 20% increased risk of developing AF (hazard ratio [HR], 1.20; 95% CI, 1.10-1.31).
- Those who drank more than 2 L per week of sugar-sweetened beverages had a 10% increased risk for AF (HR, 1.10; 95% CI, 1.01-1.20).
- Consumption of 1 L or less per week of pure fruit juice was associated with an 8% lower risk of developing AF (HR, 0.92; 95% CI, 0.87-0.97).
- The associations persisted after adjustment for genetic susceptibility for AF.
IN PRACTICE:
The study authors concluded that this study does not demonstrate that consumption of sugar-sweetened or artificially sweetened beverages alters AF risk but rather that the consumption of these drinks may predict AF risk beyond traditional risk factors. They added that intervention studies and basic research are warranted to confirm whether the observed associations are causal. Commenting on the study, Duane Mellor, MD, registered dietitian at Aston University, Birmingham, England, said it is unclear if the observations in this study are a chance finding as there is a lack of a clear biological link. Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow, Glasgow, Scotland, added that although the authors tried to adjust for many factors, there is a strong chance that other behavioral aspects linked to beverage choice could be more relevant as a cause of AF rather than the drinks themselves. Tom Sanders, MD, professor emeritus of nutrition and dietetics, King’s College London, London, England, pointed out that as this is the first study that has reported such an effect with artificially sweetened drinks, the finding needs replication before any conclusions can be drawn. “It remains good dietary advice to recommend the consumption of low-calorie artificially sweetened drink in place of sugar-sweetened drinks and alcohol,” he added.
SOURCE:
The study, led by Ying Sun, MD, Shanghai Jiao Tong University School of Medicine, Shanghai, China, was published online in Circulation: Arrhythmia and Electrophysiology.
LIMITATIONS:
The consumption of beverages was self-reported and based on only five separate single-day food intake recalls which were taken over the first 3 years of the study, which was extrapolated to estimate weekly intake. The researchers could not tell whether the sugar-sweetened and artificially sweetened drinks were caffeinated and could not rule out residual confounding by other unmeasured or unknown factors.
DISCLOSURES:
This study was supported by the National Natural Science Foundation of China, Shanghai Municipal Health Commission, Shanghai Municipal Human Resources and Social Security Bureau, Clinical Research Plan of Shanghai Hospital Development Center, Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, and Shanghai Jiao Tong University School of Medicine.
A version of this article appeared on Medscape.com.