User login
Comparing Efficacy and Safety of Atogepant and Rimegepant for Migraine Prevention
Key clinical point: Atogepant led to greater improvements in efficacy and quality of life than rimegepant in patients with episodic migraine (EM), although both treatments had comparable safety profiles.
Major finding: Atogepant vs rimegepant led to significantly greater reductions in mean monthly migraine days (mean difference [MD] −1.65; P < .001) and acute medication use days (MD −2.08; P < .0001) across 1-12 weeks and significantly greater improvements in Migraine-Specific Quality of Life questionnaire version 2.1 Role Function-Restrictive domain scores at 12 weeks (MD 7.36; P < .01). Both treatments had comparable safety or tolerability profiles.
Study details: This anchored matching-adjusted indirect comparison analysis compared the efficacy and safety or tolerability of once-daily atogepant (60 mg) and once-every-other-day rimegepant (75 mg) for EM prevention using data from two phase 3 trials for atogepant (ADVANCE and PROGRESS) and one phase 2/3 trial for rimegepant.
Disclosures: This study was funded by AbbVie. Four authors declared being employees of or holding stocks in AbbVie at the time of the study. Two authors are employees of Luminity, which was paid by AbbVie for the statistical analyses in this study. The other authors declared ties with various sources, including AbbVie. One author is an associate editor of Cephalalgia.
Source: Tassorelli C, Onishchenko K, Halker Singh RB, et al. Comparative efficacy, quality of life, safety, and tolerability of atogepant and rimegepant in migraine prevention: A matching-adjusted indirect comparison analysis. Cephalalgia. 2024 (Feb 27). doi: 10.1177/03331024241235156 Source
Key clinical point: Atogepant led to greater improvements in efficacy and quality of life than rimegepant in patients with episodic migraine (EM), although both treatments had comparable safety profiles.
Major finding: Atogepant vs rimegepant led to significantly greater reductions in mean monthly migraine days (mean difference [MD] −1.65; P < .001) and acute medication use days (MD −2.08; P < .0001) across 1-12 weeks and significantly greater improvements in Migraine-Specific Quality of Life questionnaire version 2.1 Role Function-Restrictive domain scores at 12 weeks (MD 7.36; P < .01). Both treatments had comparable safety or tolerability profiles.
Study details: This anchored matching-adjusted indirect comparison analysis compared the efficacy and safety or tolerability of once-daily atogepant (60 mg) and once-every-other-day rimegepant (75 mg) for EM prevention using data from two phase 3 trials for atogepant (ADVANCE and PROGRESS) and one phase 2/3 trial for rimegepant.
Disclosures: This study was funded by AbbVie. Four authors declared being employees of or holding stocks in AbbVie at the time of the study. Two authors are employees of Luminity, which was paid by AbbVie for the statistical analyses in this study. The other authors declared ties with various sources, including AbbVie. One author is an associate editor of Cephalalgia.
Source: Tassorelli C, Onishchenko K, Halker Singh RB, et al. Comparative efficacy, quality of life, safety, and tolerability of atogepant and rimegepant in migraine prevention: A matching-adjusted indirect comparison analysis. Cephalalgia. 2024 (Feb 27). doi: 10.1177/03331024241235156 Source
Key clinical point: Atogepant led to greater improvements in efficacy and quality of life than rimegepant in patients with episodic migraine (EM), although both treatments had comparable safety profiles.
Major finding: Atogepant vs rimegepant led to significantly greater reductions in mean monthly migraine days (mean difference [MD] −1.65; P < .001) and acute medication use days (MD −2.08; P < .0001) across 1-12 weeks and significantly greater improvements in Migraine-Specific Quality of Life questionnaire version 2.1 Role Function-Restrictive domain scores at 12 weeks (MD 7.36; P < .01). Both treatments had comparable safety or tolerability profiles.
Study details: This anchored matching-adjusted indirect comparison analysis compared the efficacy and safety or tolerability of once-daily atogepant (60 mg) and once-every-other-day rimegepant (75 mg) for EM prevention using data from two phase 3 trials for atogepant (ADVANCE and PROGRESS) and one phase 2/3 trial for rimegepant.
Disclosures: This study was funded by AbbVie. Four authors declared being employees of or holding stocks in AbbVie at the time of the study. Two authors are employees of Luminity, which was paid by AbbVie for the statistical analyses in this study. The other authors declared ties with various sources, including AbbVie. One author is an associate editor of Cephalalgia.
Source: Tassorelli C, Onishchenko K, Halker Singh RB, et al. Comparative efficacy, quality of life, safety, and tolerability of atogepant and rimegepant in migraine prevention: A matching-adjusted indirect comparison analysis. Cephalalgia. 2024 (Feb 27). doi: 10.1177/03331024241235156 Source
OnabotulinumtoxinA Reduces Interictal Burden and Allodynia Severity in Chronic Migraine
Key clinical point: OnabotulinumtoxinA (BoNTA) had beneficial effects in reducing frequency or severity of interictal burden and cutaneous allodynia in patients with chronic migraine (CM).
Major finding: After three courses of BoNTA treatment, median Migraine Interictal Burden Scale-4 scores significantly reduced from 9 at baseline to 2 (z −7.222), median Allodynia Symptom Checklist-12 scores significantly reduced from 6 at baseline to 1 (z −5.393), and median pain intensity numerical rating scale scores for hair brushing significantly reduced from 5 at baseline to 1 (z −5.398; all P < .001).
Study details: This prospective open-label study included 70 BoNTA-naive patients with CM who received three consecutive cycles of BoNTA.
Disclosures: This study did not receive any funding. Several authors declared receiving investigator fees or travel grants from or serving as advisory board members or consultants for various sources.
Source: Argyriou AA, Dermitzakis EV, Rikos D, et al. Effects of onabotulinumtoxinA on allodynia and interictal burden of patients with chronic migraine. Toxins (Basel). 2024;16(2):106 (Feb 15). doi: 10.3390/toxins16020106 Source
Key clinical point: OnabotulinumtoxinA (BoNTA) had beneficial effects in reducing frequency or severity of interictal burden and cutaneous allodynia in patients with chronic migraine (CM).
Major finding: After three courses of BoNTA treatment, median Migraine Interictal Burden Scale-4 scores significantly reduced from 9 at baseline to 2 (z −7.222), median Allodynia Symptom Checklist-12 scores significantly reduced from 6 at baseline to 1 (z −5.393), and median pain intensity numerical rating scale scores for hair brushing significantly reduced from 5 at baseline to 1 (z −5.398; all P < .001).
Study details: This prospective open-label study included 70 BoNTA-naive patients with CM who received three consecutive cycles of BoNTA.
Disclosures: This study did not receive any funding. Several authors declared receiving investigator fees or travel grants from or serving as advisory board members or consultants for various sources.
Source: Argyriou AA, Dermitzakis EV, Rikos D, et al. Effects of onabotulinumtoxinA on allodynia and interictal burden of patients with chronic migraine. Toxins (Basel). 2024;16(2):106 (Feb 15). doi: 10.3390/toxins16020106 Source
Key clinical point: OnabotulinumtoxinA (BoNTA) had beneficial effects in reducing frequency or severity of interictal burden and cutaneous allodynia in patients with chronic migraine (CM).
Major finding: After three courses of BoNTA treatment, median Migraine Interictal Burden Scale-4 scores significantly reduced from 9 at baseline to 2 (z −7.222), median Allodynia Symptom Checklist-12 scores significantly reduced from 6 at baseline to 1 (z −5.393), and median pain intensity numerical rating scale scores for hair brushing significantly reduced from 5 at baseline to 1 (z −5.398; all P < .001).
Study details: This prospective open-label study included 70 BoNTA-naive patients with CM who received three consecutive cycles of BoNTA.
Disclosures: This study did not receive any funding. Several authors declared receiving investigator fees or travel grants from or serving as advisory board members or consultants for various sources.
Source: Argyriou AA, Dermitzakis EV, Rikos D, et al. Effects of onabotulinumtoxinA on allodynia and interictal burden of patients with chronic migraine. Toxins (Basel). 2024;16(2):106 (Feb 15). doi: 10.3390/toxins16020106 Source
Atogepant Effective and Safe in Pretreated Episodic Migraine, Shows Phase 3 Study
Key clinical point: Atogepant (dosage 60 mg/day) may be an effective and safe treatment option in patients with difficult to treat episodic migraine who have previously failed 2-4 conventional oral preventive treatments.
Major finding: Patients receiving atogepant vs placebo had a significantly greater reduction in monthly migraine days across 12 weeks (adjusted least squares mean difference −2.4 days; P < .0001). Constipation was the most common treatment-emergent adverse event (TEAE) in the atogepant group (10%), with TEAE leading to treatment discontinuation in only 2% vs 1% of patients receiving atogepant vs placebo.
Study details: Findings are from the phase 3b ELEVATE trial including 315 patients with episodic migraine who had previously failed 2-4 classes of conventional oral migraine prevention treatments and were randomly assigned to receive 60 mg/day atogepant or placebo.
Disclosures: This study was funded by AbbVie. Five authors declared being employees of or holding stocks in AbbVie. The other authors declared ties with various sources, including AbbVie.
Source: Tassorelli C, Nagy K, Pozo-Rosich P, et al. Safety and efficacy of atogepant for the preventive treatment of episodic migraine in adults for whom conventional oral preventive treatments have failed (ELEVATE): A randomised, placebo-controlled, phase 3b trial. Lancet Neurol. 2024 (Feb 13). doi: 10.1016/S1474-4422(24)00025-5 Source
Key clinical point: Atogepant (dosage 60 mg/day) may be an effective and safe treatment option in patients with difficult to treat episodic migraine who have previously failed 2-4 conventional oral preventive treatments.
Major finding: Patients receiving atogepant vs placebo had a significantly greater reduction in monthly migraine days across 12 weeks (adjusted least squares mean difference −2.4 days; P < .0001). Constipation was the most common treatment-emergent adverse event (TEAE) in the atogepant group (10%), with TEAE leading to treatment discontinuation in only 2% vs 1% of patients receiving atogepant vs placebo.
Study details: Findings are from the phase 3b ELEVATE trial including 315 patients with episodic migraine who had previously failed 2-4 classes of conventional oral migraine prevention treatments and were randomly assigned to receive 60 mg/day atogepant or placebo.
Disclosures: This study was funded by AbbVie. Five authors declared being employees of or holding stocks in AbbVie. The other authors declared ties with various sources, including AbbVie.
Source: Tassorelli C, Nagy K, Pozo-Rosich P, et al. Safety and efficacy of atogepant for the preventive treatment of episodic migraine in adults for whom conventional oral preventive treatments have failed (ELEVATE): A randomised, placebo-controlled, phase 3b trial. Lancet Neurol. 2024 (Feb 13). doi: 10.1016/S1474-4422(24)00025-5 Source
Key clinical point: Atogepant (dosage 60 mg/day) may be an effective and safe treatment option in patients with difficult to treat episodic migraine who have previously failed 2-4 conventional oral preventive treatments.
Major finding: Patients receiving atogepant vs placebo had a significantly greater reduction in monthly migraine days across 12 weeks (adjusted least squares mean difference −2.4 days; P < .0001). Constipation was the most common treatment-emergent adverse event (TEAE) in the atogepant group (10%), with TEAE leading to treatment discontinuation in only 2% vs 1% of patients receiving atogepant vs placebo.
Study details: Findings are from the phase 3b ELEVATE trial including 315 patients with episodic migraine who had previously failed 2-4 classes of conventional oral migraine prevention treatments and were randomly assigned to receive 60 mg/day atogepant or placebo.
Disclosures: This study was funded by AbbVie. Five authors declared being employees of or holding stocks in AbbVie. The other authors declared ties with various sources, including AbbVie.
Source: Tassorelli C, Nagy K, Pozo-Rosich P, et al. Safety and efficacy of atogepant for the preventive treatment of episodic migraine in adults for whom conventional oral preventive treatments have failed (ELEVATE): A randomised, placebo-controlled, phase 3b trial. Lancet Neurol. 2024 (Feb 13). doi: 10.1016/S1474-4422(24)00025-5 Source
Do Migraine and Vasomotor Symptoms Raise CVD Risk in Women?
Key clinical point: Persistent vasomotor symptoms (VMS) and a history of migraine were associated with an increased risk for cardiovascular disease (CVD) and stroke; however, these associations were attenuated after adjustment for CVD risk factors, such as blood pressure and glucose and cholesterol levels.
Major finding: Women with vs without persistent VMS and a history of migraine had over two times higher risk for CVD (adjusted HR [aHR] 2.25; 95% CI 1.15-4.38) and three times higher risk for stroke (aHR 3.15; 95% CI 1.35-7.34; both P < .05). These associations, however, diminished after adjustment for cigarette use and levels of glucose, cholesterol, and blood pressure.
Study details: This secondary analysis of a subset of the CARDIA study included 1954 women with 15-year follow-up data.
Disclosures: This study was supported by the US National Heart, Lung, and Blood Institute. The authors declared no conflicts of interest.
Source: Kim C, Schreiner PJ, Yin Z, et al. Migraines, vasomotor symptoms, and cardiovascular disease in the Coronary Artery Risk Development in Young Adults study. Menopause. 2024;31(3):202-208 (Feb 13). doi: 10.1097/GME.0000000000002311 Source
Key clinical point: Persistent vasomotor symptoms (VMS) and a history of migraine were associated with an increased risk for cardiovascular disease (CVD) and stroke; however, these associations were attenuated after adjustment for CVD risk factors, such as blood pressure and glucose and cholesterol levels.
Major finding: Women with vs without persistent VMS and a history of migraine had over two times higher risk for CVD (adjusted HR [aHR] 2.25; 95% CI 1.15-4.38) and three times higher risk for stroke (aHR 3.15; 95% CI 1.35-7.34; both P < .05). These associations, however, diminished after adjustment for cigarette use and levels of glucose, cholesterol, and blood pressure.
Study details: This secondary analysis of a subset of the CARDIA study included 1954 women with 15-year follow-up data.
Disclosures: This study was supported by the US National Heart, Lung, and Blood Institute. The authors declared no conflicts of interest.
Source: Kim C, Schreiner PJ, Yin Z, et al. Migraines, vasomotor symptoms, and cardiovascular disease in the Coronary Artery Risk Development in Young Adults study. Menopause. 2024;31(3):202-208 (Feb 13). doi: 10.1097/GME.0000000000002311 Source
Key clinical point: Persistent vasomotor symptoms (VMS) and a history of migraine were associated with an increased risk for cardiovascular disease (CVD) and stroke; however, these associations were attenuated after adjustment for CVD risk factors, such as blood pressure and glucose and cholesterol levels.
Major finding: Women with vs without persistent VMS and a history of migraine had over two times higher risk for CVD (adjusted HR [aHR] 2.25; 95% CI 1.15-4.38) and three times higher risk for stroke (aHR 3.15; 95% CI 1.35-7.34; both P < .05). These associations, however, diminished after adjustment for cigarette use and levels of glucose, cholesterol, and blood pressure.
Study details: This secondary analysis of a subset of the CARDIA study included 1954 women with 15-year follow-up data.
Disclosures: This study was supported by the US National Heart, Lung, and Blood Institute. The authors declared no conflicts of interest.
Source: Kim C, Schreiner PJ, Yin Z, et al. Migraines, vasomotor symptoms, and cardiovascular disease in the Coronary Artery Risk Development in Young Adults study. Menopause. 2024;31(3):202-208 (Feb 13). doi: 10.1097/GME.0000000000002311 Source
Meta-analysis Shows Link Between Shift Work and Risk for Headache and Migraine
Key clinical point: Shift work was associated with significantly increased risks for headache and migraine, whereas night shifts only increased the risk for headaches.
Major finding: Shift work was significantly associated with an increased risk for headaches (hazard ratio [HR] 1.32; P < .001) and migraine (HR 1.63; P < .001), with the risk for headaches being further elevated in individuals who worked night shifts (HR 1.44; P = .011).
Study details: Findings are from a meta-analysis of seven cross-sectional studies that included 422,869 participants.
Disclosures: This study was supported by the Sichuan Youth Science and Technology Innovation Research Team. The authors declared no conflicts of interest.
Source: Wang Z, Zhu T, Gong M, et al. Relationship between shift work, night work, and headache and migraine risk: A meta-analysis of observational studies. Sleep Med. 2024;115:218-225 (Feb 19). doi: 10.1016/j.sleep.2024.02.011 Source
Key clinical point: Shift work was associated with significantly increased risks for headache and migraine, whereas night shifts only increased the risk for headaches.
Major finding: Shift work was significantly associated with an increased risk for headaches (hazard ratio [HR] 1.32; P < .001) and migraine (HR 1.63; P < .001), with the risk for headaches being further elevated in individuals who worked night shifts (HR 1.44; P = .011).
Study details: Findings are from a meta-analysis of seven cross-sectional studies that included 422,869 participants.
Disclosures: This study was supported by the Sichuan Youth Science and Technology Innovation Research Team. The authors declared no conflicts of interest.
Source: Wang Z, Zhu T, Gong M, et al. Relationship between shift work, night work, and headache and migraine risk: A meta-analysis of observational studies. Sleep Med. 2024;115:218-225 (Feb 19). doi: 10.1016/j.sleep.2024.02.011 Source
Key clinical point: Shift work was associated with significantly increased risks for headache and migraine, whereas night shifts only increased the risk for headaches.
Major finding: Shift work was significantly associated with an increased risk for headaches (hazard ratio [HR] 1.32; P < .001) and migraine (HR 1.63; P < .001), with the risk for headaches being further elevated in individuals who worked night shifts (HR 1.44; P = .011).
Study details: Findings are from a meta-analysis of seven cross-sectional studies that included 422,869 participants.
Disclosures: This study was supported by the Sichuan Youth Science and Technology Innovation Research Team. The authors declared no conflicts of interest.
Source: Wang Z, Zhu T, Gong M, et al. Relationship between shift work, night work, and headache and migraine risk: A meta-analysis of observational studies. Sleep Med. 2024;115:218-225 (Feb 19). doi: 10.1016/j.sleep.2024.02.011 Source
Studies Reinforce JAK Inhibitor Efficacy for Most Challenging Alopecia Types
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
Cognitive Deficits After Most Severe COVID Cases Associated With 9-Point IQ Drop
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
Consider These Factors in an Academic Radiation Oncology Position
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
— and accept an offer if the practice is “great” in at least two of those areas and “good” in the third, experts say in a recent editorial.
METHODOLOGY:
- Many physicians choose to go into academic medicine because they want to stay involved in research and education while still treating patients.
- However, graduating radiation oncology residents often lack or have limited guidance on what to look for in a prospective job and how to assess their contract.
- This recent editorial provides guidance to radiation oncologists seeking academic positions. The authors advise prospective employees to evaluate three main factors — compensation, daily duties, and location — as well as provide tips for identifying red flags in each category.
TAKEAWAY:
- Compensation: Prospective faculty should assess both direct compensation, that is, salary, and indirect compensation, which typically includes retirement contributions and other perks. For direct compensation, what is the base salary? Is extra work compensated? How does the salary offer measure up to salary data reported by national agencies? Also: Don’t overlook uncompensated duties, such as time in tumor boards or in meetings, which may be time-consuming, and make sure compensation terms are clearly delineated in a contract and equitable among physicians in a specific rank.
- Daily duties: When it comes to daily life on the job, a prospective employee should consider many factors, including the cancer center’s excitement to hire you, the reputation of the faculty and leaders at the organization, employee turnover rates, diversity among faculty, and the time line of career advancement.
- Location: The location of the job encompasses the geography — such as distance from home to work, the number of practices covered, cost of living, and the area itself — as well as the atmosphere for conducting research and publishing.
- Finally, carefully review the job contract. All the key aspects of the job, including compensation and benefits, should be clearly stated in the contract to “improve communication of expectations.”
IN PRACTICE:
“A prospective faculty member can ask 100 questions, but they can’t make 100 demands; consideration of the three domains can help to focus negotiation efforts where the efforts are needed,” the authors noted.
SOURCE:
This editorial, led by Nicholas G. Zaorsky from the Department of Radiation Oncology, University Hospitals Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, Ohio, was published online in Practical Radiation Oncology
DISCLOSURES:
The lead author declared being supported by the American Cancer Society and National Institutes of Health. He also reported having ties with many other sources.
A version of this article appeared on Medscape.com.
Essential Tremor Tied to a Threefold Increased Risk for Dementia
, new research showed.
In a prospective, longitudinal study, incidence of dementia was nearly 20% among older adults with ET. However, the rates were lower than those in adults with Parkinson’s disease.
The study is “the most complete exposition of the longitudinal trajectory of cognitive impairment in an ET cohort,” said the authors, led by Elan D. Louis, MD, MSc, from University of Texas Southwestern Medical Center in Dallas, Texas.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Mild Cognitive Impairment Prevalence Nearly Double
For the study, 222 adults with ET with an average age of 79 years at baseline underwent detailed cognitive assessments and were followed for an average of 5 years.
At baseline, 168 people had normal cognitive skills, 35 had mild cognitive impairment (MCI), and 19 had dementia. During the follow-up, 59 individuals developed MCI and 41 developed dementia.
During the follow-up, the cumulative prevalence of dementia was 18.5%, and the average annual conversion rate of MCI to dementia was 12.2% — nearly threefold higher than rates in the general population and roughly one-half the magnitude of those reported for adults with Parkinson’s disease.
The cumulative prevalence of MCI (26.6%) was nearly double that of the general population but less than that in patients with Parkinson’s disease.
“Our data indicate that the prevalence of and conversion rates to dementia in ET fall between those associated with the natural course of aging and the more pronounced rates observed in individuals with Parkinson’s disease,” the researchers wrote in their conference abstract.
Far From Trivial
Reached for comment, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, Florida, said, “The days of viewing ET as just a ‘nuisance tremor’ are over. This study shatters the notion that essential tremor is a trivial condition.”
“Moving forward, the research agenda must further elucidate the link between ET and dementia and develop neuroprotective strategies. But this study represents a seismic shift in how we understand essential tremor,” Dr. Lakhan said.
“The benign label no longer applies given the cognitive risks ET patients face. Our clinical practice and communication with patients must adapt accordingly,” he added.
The study was supported by the National Institutes of Health. Drs. Louis and Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
, new research showed.
In a prospective, longitudinal study, incidence of dementia was nearly 20% among older adults with ET. However, the rates were lower than those in adults with Parkinson’s disease.
The study is “the most complete exposition of the longitudinal trajectory of cognitive impairment in an ET cohort,” said the authors, led by Elan D. Louis, MD, MSc, from University of Texas Southwestern Medical Center in Dallas, Texas.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Mild Cognitive Impairment Prevalence Nearly Double
For the study, 222 adults with ET with an average age of 79 years at baseline underwent detailed cognitive assessments and were followed for an average of 5 years.
At baseline, 168 people had normal cognitive skills, 35 had mild cognitive impairment (MCI), and 19 had dementia. During the follow-up, 59 individuals developed MCI and 41 developed dementia.
During the follow-up, the cumulative prevalence of dementia was 18.5%, and the average annual conversion rate of MCI to dementia was 12.2% — nearly threefold higher than rates in the general population and roughly one-half the magnitude of those reported for adults with Parkinson’s disease.
The cumulative prevalence of MCI (26.6%) was nearly double that of the general population but less than that in patients with Parkinson’s disease.
“Our data indicate that the prevalence of and conversion rates to dementia in ET fall between those associated with the natural course of aging and the more pronounced rates observed in individuals with Parkinson’s disease,” the researchers wrote in their conference abstract.
Far From Trivial
Reached for comment, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, Florida, said, “The days of viewing ET as just a ‘nuisance tremor’ are over. This study shatters the notion that essential tremor is a trivial condition.”
“Moving forward, the research agenda must further elucidate the link between ET and dementia and develop neuroprotective strategies. But this study represents a seismic shift in how we understand essential tremor,” Dr. Lakhan said.
“The benign label no longer applies given the cognitive risks ET patients face. Our clinical practice and communication with patients must adapt accordingly,” he added.
The study was supported by the National Institutes of Health. Drs. Louis and Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
, new research showed.
In a prospective, longitudinal study, incidence of dementia was nearly 20% among older adults with ET. However, the rates were lower than those in adults with Parkinson’s disease.
The study is “the most complete exposition of the longitudinal trajectory of cognitive impairment in an ET cohort,” said the authors, led by Elan D. Louis, MD, MSc, from University of Texas Southwestern Medical Center in Dallas, Texas.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Mild Cognitive Impairment Prevalence Nearly Double
For the study, 222 adults with ET with an average age of 79 years at baseline underwent detailed cognitive assessments and were followed for an average of 5 years.
At baseline, 168 people had normal cognitive skills, 35 had mild cognitive impairment (MCI), and 19 had dementia. During the follow-up, 59 individuals developed MCI and 41 developed dementia.
During the follow-up, the cumulative prevalence of dementia was 18.5%, and the average annual conversion rate of MCI to dementia was 12.2% — nearly threefold higher than rates in the general population and roughly one-half the magnitude of those reported for adults with Parkinson’s disease.
The cumulative prevalence of MCI (26.6%) was nearly double that of the general population but less than that in patients with Parkinson’s disease.
“Our data indicate that the prevalence of and conversion rates to dementia in ET fall between those associated with the natural course of aging and the more pronounced rates observed in individuals with Parkinson’s disease,” the researchers wrote in their conference abstract.
Far From Trivial
Reached for comment, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, Florida, said, “The days of viewing ET as just a ‘nuisance tremor’ are over. This study shatters the notion that essential tremor is a trivial condition.”
“Moving forward, the research agenda must further elucidate the link between ET and dementia and develop neuroprotective strategies. But this study represents a seismic shift in how we understand essential tremor,” Dr. Lakhan said.
“The benign label no longer applies given the cognitive risks ET patients face. Our clinical practice and communication with patients must adapt accordingly,” he added.
The study was supported by the National Institutes of Health. Drs. Louis and Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAN 2024
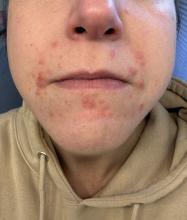
A 35-year-old female presented with a 1-day history of eroded papules and vesicles distributed periorally
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.