User login
Reconstruction Technique for Defects of the Cutaneous and Mucosal Lip: V-to-flying-Y Closure
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
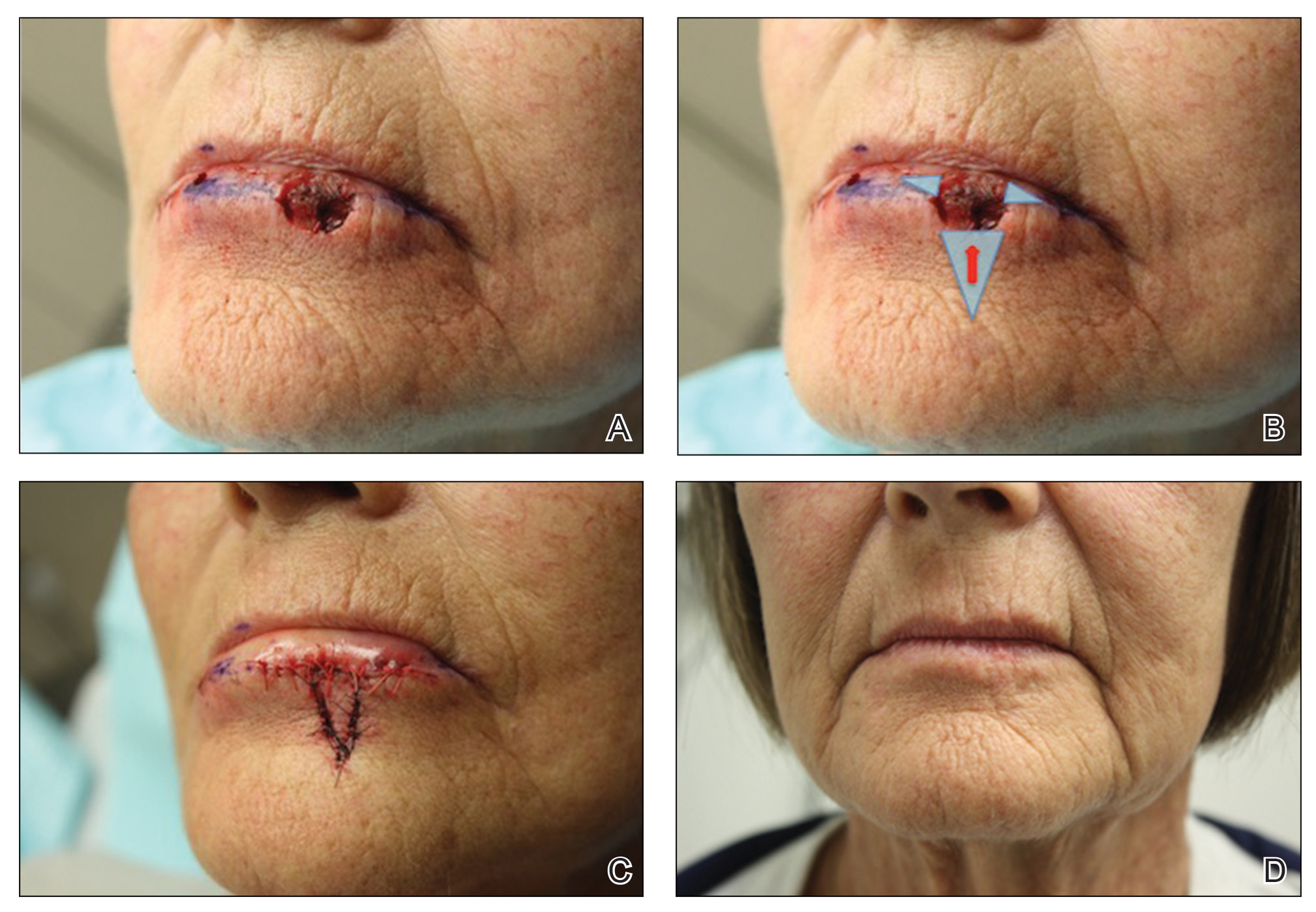
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?
Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?

Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?

Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
Tanning Attitudes and Behaviors in Adolescents and Young Adults
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.

Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
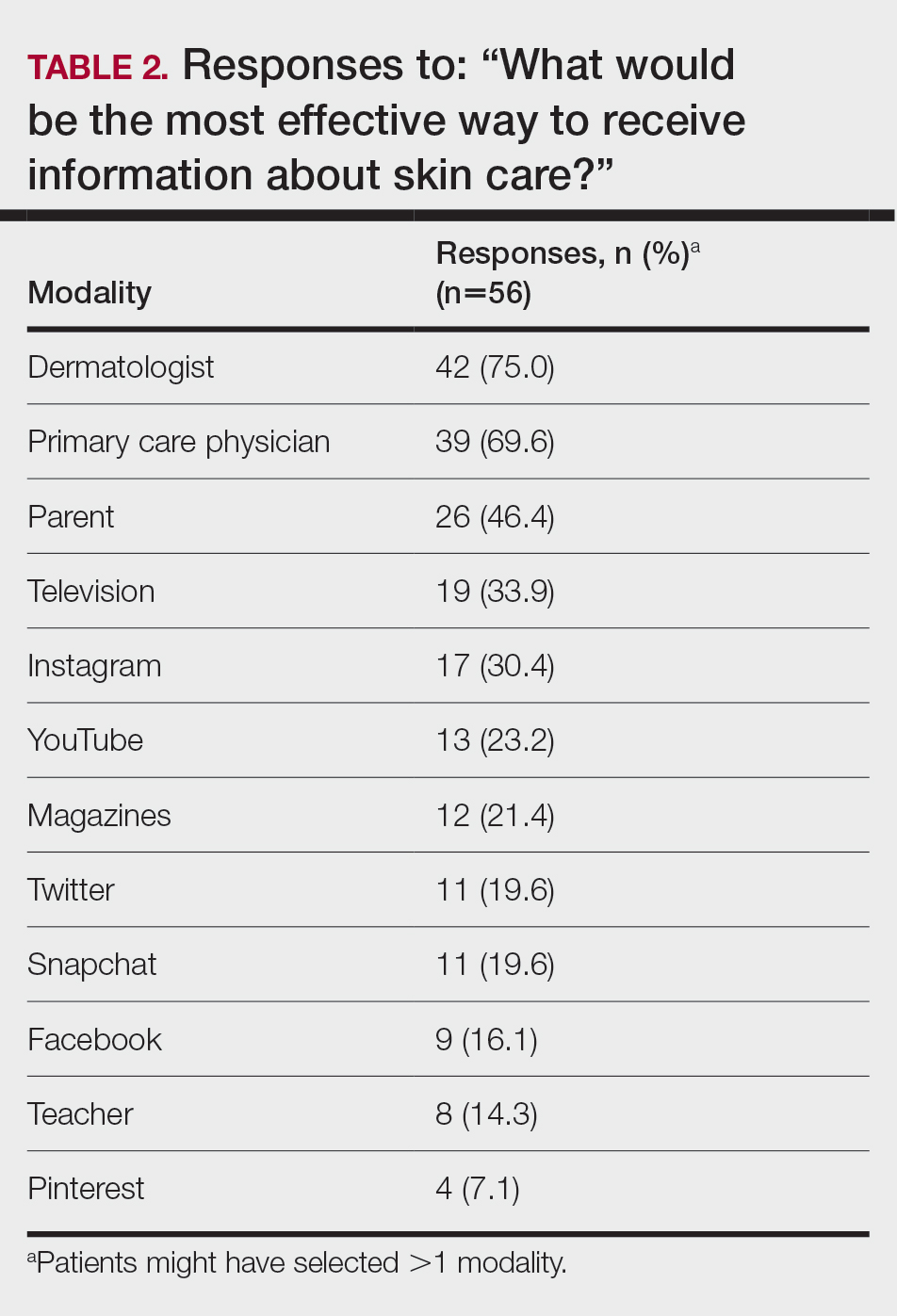
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.

Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).

Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.

Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).

Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
PRACTICE POINTS
- Dermatologists are the preferred educators of skin care for adolescents and young adults.
- Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist.
- Education of young people focusing on their concerns about maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Communication Strategies in Mohs Micrographic Surgery: A Survey of Methods, Time Savings, and Perceived Patient Satisfaction
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
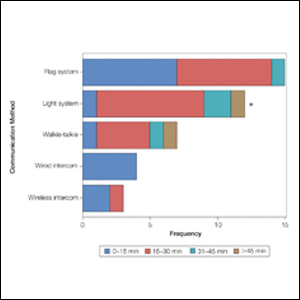
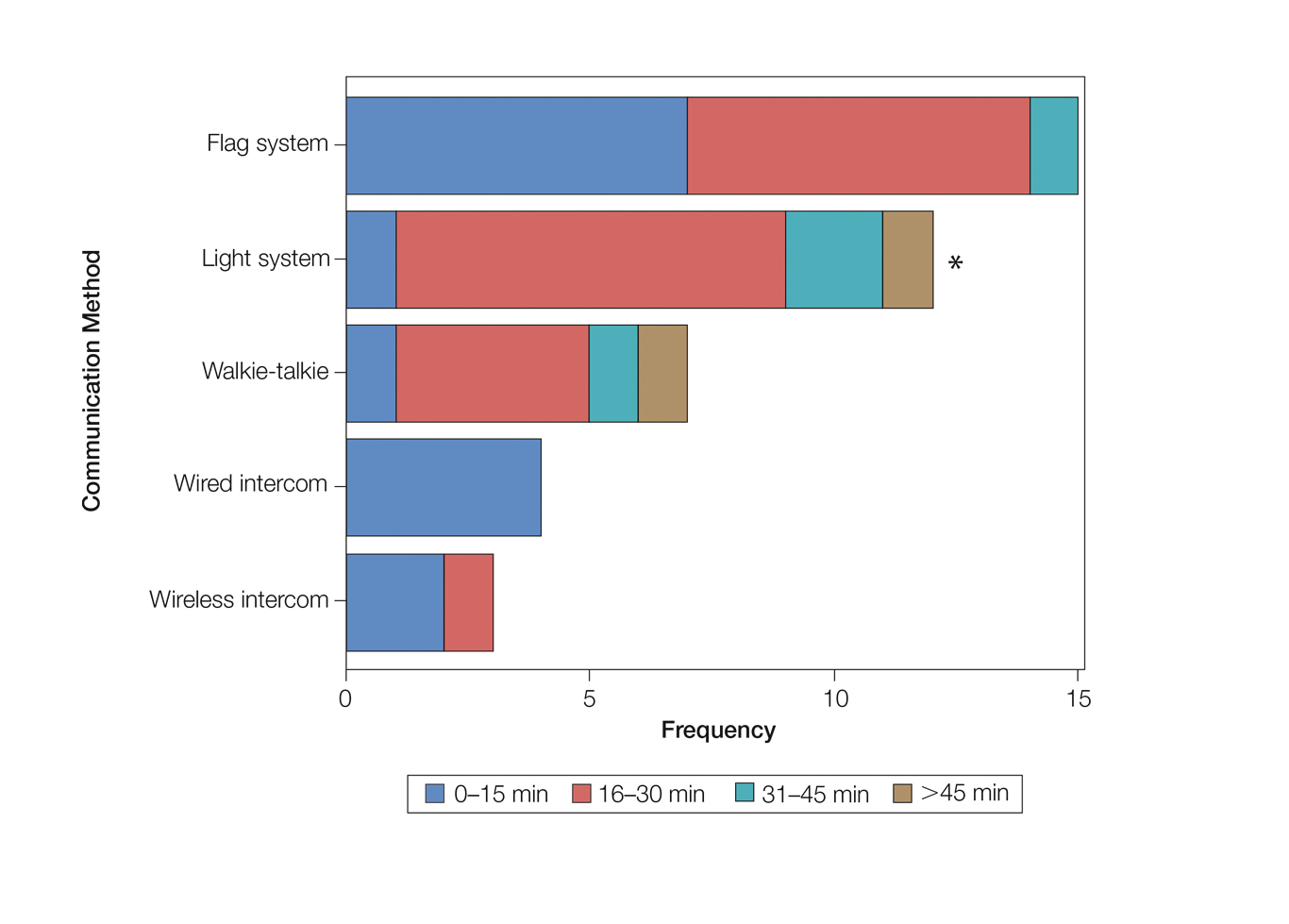
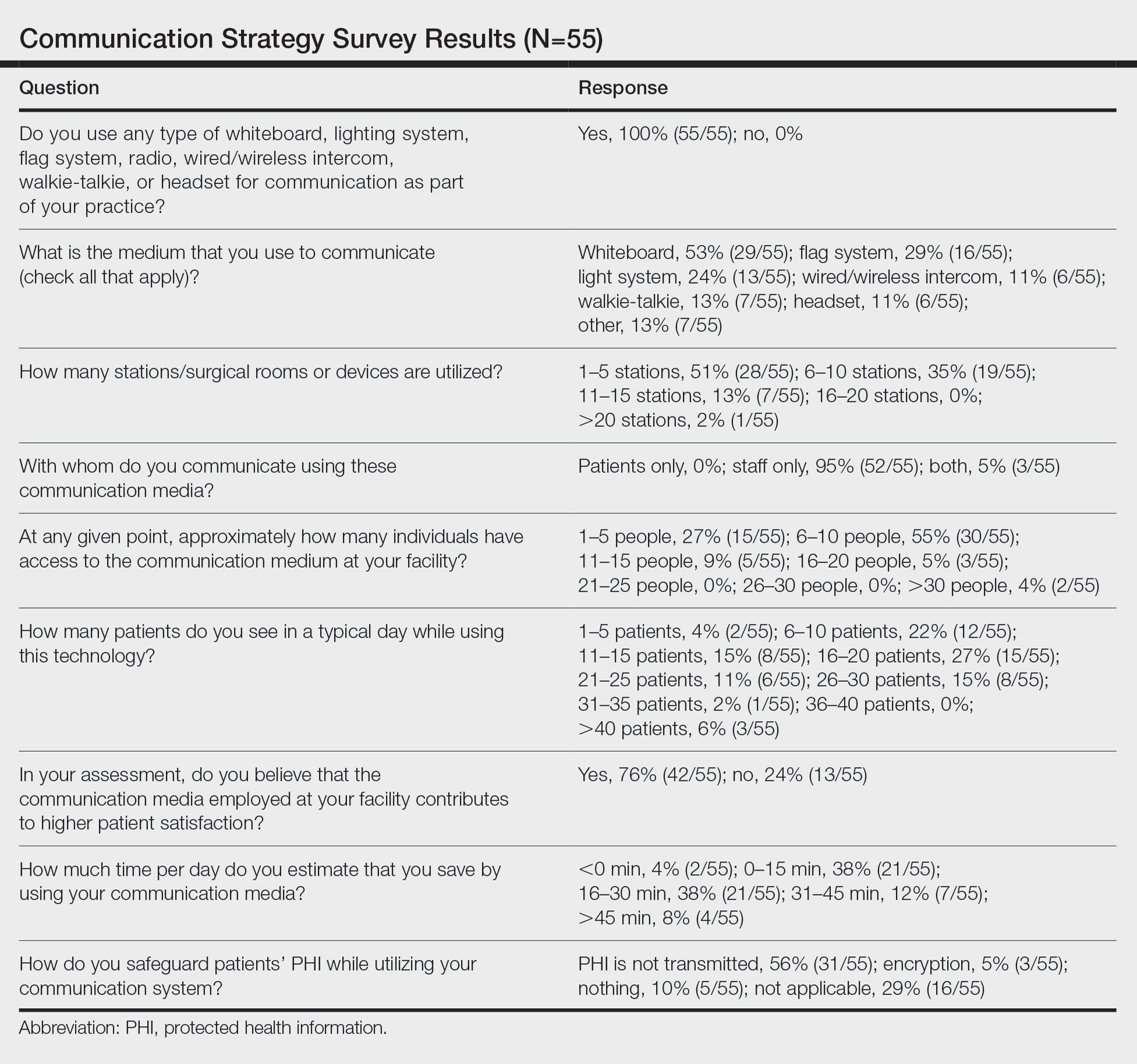
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Practice Points
- There are limited studies evaluating the efficacy of different communication methods in Mohs micrographic surgery (MMS) clinics.
- This study suggests that incorporation of a light-based system into an MMS clinic improves workplace efficiency.
AAD unveils new guidelines for actinic keratosis management
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
. They also conditionally recommend the use of photodynamic therapy (PDT) and diclofenac for the treatment of AK, both individually and as part of combination therapy regimens.
Those are two of 18 recommendations made by 14 members of the multidisciplinary work group that convened to assemble the AAD’s first-ever guidelines on the management of AKs, which were published online April 2 in the Journal of the American Academy of Dermatology. The group, cochaired by Daniel B. Eisen, MD, professor of clinical dermatology at the University of California, Davis, and Todd E. Schlesinger, MD, medical director of the Dermatology and Laser Center of Charleston, S.C., conducted a systematic review to address five clinical questions on the management of AKs in adults. The questions were: What are the efficacy, effectiveness, and adverse effects of surgical and chemical peel treatments for AK; of topically applied agents for AK; of energy devices and other miscellaneous treatments for AK; and of combination therapy for the treatment of AK? And what are the special considerations to be taken into account when treating AK in immunocompromised individuals?
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
“As a participant in the work group, I was impressed by the level of care and detail and the involvement of relevant stakeholders, including a patient advocate, as well as having the draft guidelines go out to the AAD membership at large, and evaluating every comment that came in,” Maryam Asgari, MD, MPH, professor of dermatology at Harvard University, Boston, said in an interview. “The academy sought stakeholder and leadership input in revising and revamping the guidelines. The AAD also made sure the work group had minimal conflicts of interest by requiring that the majority of experts convened did not have relevant financial conflicts of interest. That might not be the case in a publication such as a systematic review, where no threshold for financial conflict of interest for coauthorship is set.”
Of the 18 recommendations the work group made for patients with AKs, only four were ranked as “strong” based on the evidence reviewed, while the rest were ranked as “conditional.”
The strong recommendations include the use of UV protection, field treatment with 5-FU, field treatment with imiquimod, and the use of cryosurgery.
The first four conditional recommendations for patients with AKs include the use of diclofenac, treatment with cryosurgery over CO2 laser ablation, aminolevulinic acid (ALA)–red-light PDT, and 1- to 4-hour 5-ALA incubation time to enhance complete clearance with red-light PDT. The work group also conditionally recommends ALA-daylight PDT as less painful than but equally effective as ALA–red-light PDT.
In the clinical experience of Catherine M. DiGiorgio, MD, who was not involved in the guidelines, daylight PDT with ALA is a viable, cost-effective option. “Patients can come into the office, apply the ALA and then they go outside for 2 hours – not in direct sunlight but in a shady area,” Dr. DiGiorgio, a dermatologist who practices at the Boston Center for Facial Rejuvenation, said in an interview. “That’s a cost-effective treatment for patients who perhaps can’t afford some of the chemotherapy creams. I don’t think we’ve adopted ALA-daylight PDT here in the U.S. very much.”
The work group noted that topical 1% tirbanibulin ointment, a novel microtubule inhibitor, was approved for treatment of AKs on the face and scalp by the Food and Drug Administration after the guidelines had been put together.
Several trials of combination therapy were included in the review of evidence, prompting several recommendations. For example, the work group conditionally recommends combined 5-FU cream and cryosurgery over cryosurgery alone, based on moderate-quality evidence and conditionally recommends combined imiquimod and cryosurgery over cryosurgery alone based on low-quality evidence. In addition, the work group conditionally recommends against the use of 3% diclofenac in addition to cryosurgery, favoring cryosurgery alone based on low-quality evidence, and conditionally recommends against the use of imiquimod typically after ALA–blue-light PDT, based on moderate-quality data.
“The additional treatment with imiquimod was thought to add both expense and burden to the patient, which negates much of the perceived convenience of using PDT as a stand-alone treatment modality and which is not mitigated by the modest increase in lesion reduction,” the authors wrote.
The guidelines emphasize the importance of shared decision-making between patients and clinicians on the choice of therapy, a point that resonates with Dr. DiGiorgio. Success of a treatment can depend on whether a patient is willing to go through with it, she said. “Some patients don’t want to do a therapeutic topical like 5-FU. They prefer to come in and have cryotherapy done. Others prefer to not come in and have the cream at home and treat themselves.”
Assembling the guidelines exposed certain gaps in research, according to the work group. Of the 18 recommendations, seven were based on low-quality evidence, and there were not enough data to make guidelines for the treatment of AKs in immunocompromised individuals.
“I can’t tell you the number of times we in the committee sat back and said, ‘we need to have a randomized trial that looks at this, or compares this to that head on,’” Dr. Asgari said. Such limitations “give researchers direction for where the areas of study need to go to help us answer some of these management conundrums.”
She added that the new guidelines “give clinicians a leg to stand on” when an insurer pushes back on a recommended treatment for AK. “It gives you a way to have dialogue with insurers if you’re prescribing some of these treatments.”
The guidelines authors write that there is “strong theoretic rationale for the treatment of AK to prevent skin cancers” but acknowledge that only a few studies in the review “report the incidence of skin cancer as an outcome measure or have sufficient follow-up to viably measure carcinoma development.” In addition, “more long-term research is needed to validate our current understanding of skin cancer progression from AKs to keratinocyte carcinoma.”
Dr. DiGiorgio thinks about this differently. “I think treatment of AKs does prevent skin cancers,” she said. “We call them precancers as we’re treating our patients because we know a certain percentage of them can develop into skin cancers over time.”
The study was funded by internal funds from the AAD. Dr. Asgari disclosed that she serves as an investigator for Pfizer. Several of the other authors reported having financial disclosures.
Dr. DiGiorgio reported having no financial disclosures.
FROM JAAD
Pandemic took a cut of cosmetic procedures in 2020
pandemic, according to the American Society of Plastic Surgeons.
There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
Rituximab’s serious infection risk in ANCA-vasculitis allayed by antibiotic use
The serious infection risk associated with rituximab treatment for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is high but can be offset by co-prescribing co-trimoxazole, data from a single-center, retrospective study reaffirm.
Over the course of a 3-year study period, 14 (28%) of 50 patients with AAV treated with rituximab experienced at latest one severe infection defined as a grade 3 or higher event. The incidence of severe infections was 15.4 per 100 person-years.
However, a lower rate of infections was seen in patients who had been co-prescribed co-trimoxazole (trimethoprim and sulfamethoxazole), Francesco Dernie, a fifth-year medical student at the University of Oxford (England), reported at the British Society for Rheumatology annual conference.
“In the case of rituximab, the depletion of B cells and associated immune suppression is a double-edged sword, allowing effective disease control, but also leaving the body vulnerable to opportunistic and severe infections,” Mr. Dernie said at the meeting.
Of the patients who developed a severe infection on rituximab, just 7% had been treated with co-trimoxazole. In comparison, 44% of those who did not get a severe infection had received co-trimoxazole. Multivariate analysis confirmed that co-trimoxazole use was an influencing factor, with an odds ratio (OR) of 0.096 (95% confidence interval, 0.009–0.996; P = .05).
Another finding was that patients with low immunoglobulin G levels (less than 6 g/L) were more likely to develop a severe infection than were those with higher IgG levels. Indeed, the OR for hypogammaglobulinemia and the risk for infection was 8.782 (95% CI, 1.19–64.6; P = .033).
“Our results support the monitoring of IgG levels to identify patients who may be more susceptible to infection, as well as the prescription of prophylactic co-trimoxazole to reduce overall severe infection risk,” Mr. Dernie and associates concluded in their abstract.
It’s a “really important message around co-trimoxazole,” observed Neil Basu, MBChB, a clinical senior lecturer and honorary consultant at the Institute of Infection, Immunity & Inflammation, University of Glasgow (Scotland).
“It still frustrates me when I see that patients haven’t received that while receiving rituximab. Of course, co-trimoxazole can have its problems,” said Dr. Basu, who was not involved in the study. “It’s not uncommon for patients to develop reactions or be intolerant to the drug.”
Raashid Luqmani, DM, a senior coauthor of the work and professor of rheumatology at the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Science, University of Oxford, said: “The tolerance of co-trimoxazole has been remarkably good in this cohort.” If there was a problem with using co-trimoxazole, then “our standard would be to go with trimethoprim alone as the next in line and follow that with inhaled pentamidine. So, it’s kind of following what we would all generally do,” Dr. Luqmani said.
These data add further support for coprescribing antibiotic treatment with rituximab, he suggested.
“Worry about infection, worry about it a lot; not just worry about it, do something about it,” Dr. Luqmani said, and co-trimoxazole “is probably an effective means to do something about it.”
Study details
To look at the characteristics of and risk factors for serious infections associated with rituximab use in AAV, Mr. Dernie and associates retrospectively examined the electronic records of patients who had been treated between August 2016 and August 2019. Follow-up was until August 2020.
Of the 50 patients identified, nearly half (48%) were men. The average age was 60 years, ranging from 25 to 90 years. Most (n = 36; 72%) patients had a diagnosis of granulomatosis with polyangiitis, while another 2 (4%) had microscopic polyangiitis, 1 (2%) had eosinophilic granulomatosis with polyangiitis, and 11 (22%) had an overlapping type of vasculitis or undefined AAV.
Of the 18 severe infection events recorded, most (56%) involved the respiratory tract. Less than one-third (28%) were sepsis or neutropenic sepsis events, and there was one case each (6%) of cellulitis, complicated urinary tract infection, and recurrent wound infection.
There were “small numbers of individual comorbidities that were not sufficient to enter into our regression analysis,” Mr. Dernie noted. “It’s likely that comorbid conditions such as COPD [chronic obstructive pulmonary disease] also contribute to an individual’s risk of developing severe infections, and thus should factor into their individualized management.”
Mr. Dernie acknowledged in discussion: “One of the limitations of the study was we just looked at patients in a time when they were receiving rituximab, so they may have historically been exposed to other treatment options.” However, he added, “they weren’t having any other major DMARDs or immunosuppressive treatments at the time.”
Dr. Luqmani observed: “If you look at Francesco’s data on the hypogammaglobulinemia at the start of rituximab, that probably gives you a good idea of just how immunosuppressed these patients were already before we got to this point.”
Dr. Luqmani added: “I suspect that’s in keeping with a lot of other centers that have started using rituximab an awful lot for patients who previously had episodes of vasculitis treated with other disease-modifying therapies, particularly cyclophosphamide.”
But for how long should co-trimoxazole be given after the last rituximab dose? asked the chair of the session, Richard Watts, DM, of Norwich (England) Medical School. These data are purely observational, so it’s not possible to say, Mr. Dernie noted: “The patients that we included as having co-trimoxazole seem to be on it more or less consistently, permanently,” he said.
What about the best dose? “It’s a tricky one,” Dr. Luqmani said, as “we not only use co-trimoxazole for prophylaxis, but we often also want to use it for treatment of the vasculitis itself.”
It’s very likely that there was a mix of patients in the analysis that had received co-trimoxazole as either a treatment or prophylaxis, which means different doses, he said.
“It might be interesting to know whether there was a difference” between doses used and the prevention of infection, added Dr. Luqmani, “but I suspect the numbers are too small to tell.”
Mr. Dernie, Dr. Luqmani, and the other coauthors had no disclosures.
The serious infection risk associated with rituximab treatment for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is high but can be offset by co-prescribing co-trimoxazole, data from a single-center, retrospective study reaffirm.
Over the course of a 3-year study period, 14 (28%) of 50 patients with AAV treated with rituximab experienced at latest one severe infection defined as a grade 3 or higher event. The incidence of severe infections was 15.4 per 100 person-years.
However, a lower rate of infections was seen in patients who had been co-prescribed co-trimoxazole (trimethoprim and sulfamethoxazole), Francesco Dernie, a fifth-year medical student at the University of Oxford (England), reported at the British Society for Rheumatology annual conference.
“In the case of rituximab, the depletion of B cells and associated immune suppression is a double-edged sword, allowing effective disease control, but also leaving the body vulnerable to opportunistic and severe infections,” Mr. Dernie said at the meeting.
Of the patients who developed a severe infection on rituximab, just 7% had been treated with co-trimoxazole. In comparison, 44% of those who did not get a severe infection had received co-trimoxazole. Multivariate analysis confirmed that co-trimoxazole use was an influencing factor, with an odds ratio (OR) of 0.096 (95% confidence interval, 0.009–0.996; P = .05).
Another finding was that patients with low immunoglobulin G levels (less than 6 g/L) were more likely to develop a severe infection than were those with higher IgG levels. Indeed, the OR for hypogammaglobulinemia and the risk for infection was 8.782 (95% CI, 1.19–64.6; P = .033).
“Our results support the monitoring of IgG levels to identify patients who may be more susceptible to infection, as well as the prescription of prophylactic co-trimoxazole to reduce overall severe infection risk,” Mr. Dernie and associates concluded in their abstract.
It’s a “really important message around co-trimoxazole,” observed Neil Basu, MBChB, a clinical senior lecturer and honorary consultant at the Institute of Infection, Immunity & Inflammation, University of Glasgow (Scotland).
“It still frustrates me when I see that patients haven’t received that while receiving rituximab. Of course, co-trimoxazole can have its problems,” said Dr. Basu, who was not involved in the study. “It’s not uncommon for patients to develop reactions or be intolerant to the drug.”
Raashid Luqmani, DM, a senior coauthor of the work and professor of rheumatology at the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Science, University of Oxford, said: “The tolerance of co-trimoxazole has been remarkably good in this cohort.” If there was a problem with using co-trimoxazole, then “our standard would be to go with trimethoprim alone as the next in line and follow that with inhaled pentamidine. So, it’s kind of following what we would all generally do,” Dr. Luqmani said.
These data add further support for coprescribing antibiotic treatment with rituximab, he suggested.
“Worry about infection, worry about it a lot; not just worry about it, do something about it,” Dr. Luqmani said, and co-trimoxazole “is probably an effective means to do something about it.”
Study details
To look at the characteristics of and risk factors for serious infections associated with rituximab use in AAV, Mr. Dernie and associates retrospectively examined the electronic records of patients who had been treated between August 2016 and August 2019. Follow-up was until August 2020.
Of the 50 patients identified, nearly half (48%) were men. The average age was 60 years, ranging from 25 to 90 years. Most (n = 36; 72%) patients had a diagnosis of granulomatosis with polyangiitis, while another 2 (4%) had microscopic polyangiitis, 1 (2%) had eosinophilic granulomatosis with polyangiitis, and 11 (22%) had an overlapping type of vasculitis or undefined AAV.
Of the 18 severe infection events recorded, most (56%) involved the respiratory tract. Less than one-third (28%) were sepsis or neutropenic sepsis events, and there was one case each (6%) of cellulitis, complicated urinary tract infection, and recurrent wound infection.
There were “small numbers of individual comorbidities that were not sufficient to enter into our regression analysis,” Mr. Dernie noted. “It’s likely that comorbid conditions such as COPD [chronic obstructive pulmonary disease] also contribute to an individual’s risk of developing severe infections, and thus should factor into their individualized management.”
Mr. Dernie acknowledged in discussion: “One of the limitations of the study was we just looked at patients in a time when they were receiving rituximab, so they may have historically been exposed to other treatment options.” However, he added, “they weren’t having any other major DMARDs or immunosuppressive treatments at the time.”
Dr. Luqmani observed: “If you look at Francesco’s data on the hypogammaglobulinemia at the start of rituximab, that probably gives you a good idea of just how immunosuppressed these patients were already before we got to this point.”
Dr. Luqmani added: “I suspect that’s in keeping with a lot of other centers that have started using rituximab an awful lot for patients who previously had episodes of vasculitis treated with other disease-modifying therapies, particularly cyclophosphamide.”
But for how long should co-trimoxazole be given after the last rituximab dose? asked the chair of the session, Richard Watts, DM, of Norwich (England) Medical School. These data are purely observational, so it’s not possible to say, Mr. Dernie noted: “The patients that we included as having co-trimoxazole seem to be on it more or less consistently, permanently,” he said.
What about the best dose? “It’s a tricky one,” Dr. Luqmani said, as “we not only use co-trimoxazole for prophylaxis, but we often also want to use it for treatment of the vasculitis itself.”
It’s very likely that there was a mix of patients in the analysis that had received co-trimoxazole as either a treatment or prophylaxis, which means different doses, he said.
“It might be interesting to know whether there was a difference” between doses used and the prevention of infection, added Dr. Luqmani, “but I suspect the numbers are too small to tell.”
Mr. Dernie, Dr. Luqmani, and the other coauthors had no disclosures.
The serious infection risk associated with rituximab treatment for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is high but can be offset by co-prescribing co-trimoxazole, data from a single-center, retrospective study reaffirm.
Over the course of a 3-year study period, 14 (28%) of 50 patients with AAV treated with rituximab experienced at latest one severe infection defined as a grade 3 or higher event. The incidence of severe infections was 15.4 per 100 person-years.
However, a lower rate of infections was seen in patients who had been co-prescribed co-trimoxazole (trimethoprim and sulfamethoxazole), Francesco Dernie, a fifth-year medical student at the University of Oxford (England), reported at the British Society for Rheumatology annual conference.
“In the case of rituximab, the depletion of B cells and associated immune suppression is a double-edged sword, allowing effective disease control, but also leaving the body vulnerable to opportunistic and severe infections,” Mr. Dernie said at the meeting.
Of the patients who developed a severe infection on rituximab, just 7% had been treated with co-trimoxazole. In comparison, 44% of those who did not get a severe infection had received co-trimoxazole. Multivariate analysis confirmed that co-trimoxazole use was an influencing factor, with an odds ratio (OR) of 0.096 (95% confidence interval, 0.009–0.996; P = .05).
Another finding was that patients with low immunoglobulin G levels (less than 6 g/L) were more likely to develop a severe infection than were those with higher IgG levels. Indeed, the OR for hypogammaglobulinemia and the risk for infection was 8.782 (95% CI, 1.19–64.6; P = .033).
“Our results support the monitoring of IgG levels to identify patients who may be more susceptible to infection, as well as the prescription of prophylactic co-trimoxazole to reduce overall severe infection risk,” Mr. Dernie and associates concluded in their abstract.
It’s a “really important message around co-trimoxazole,” observed Neil Basu, MBChB, a clinical senior lecturer and honorary consultant at the Institute of Infection, Immunity & Inflammation, University of Glasgow (Scotland).
“It still frustrates me when I see that patients haven’t received that while receiving rituximab. Of course, co-trimoxazole can have its problems,” said Dr. Basu, who was not involved in the study. “It’s not uncommon for patients to develop reactions or be intolerant to the drug.”
Raashid Luqmani, DM, a senior coauthor of the work and professor of rheumatology at the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Science, University of Oxford, said: “The tolerance of co-trimoxazole has been remarkably good in this cohort.” If there was a problem with using co-trimoxazole, then “our standard would be to go with trimethoprim alone as the next in line and follow that with inhaled pentamidine. So, it’s kind of following what we would all generally do,” Dr. Luqmani said.
These data add further support for coprescribing antibiotic treatment with rituximab, he suggested.
“Worry about infection, worry about it a lot; not just worry about it, do something about it,” Dr. Luqmani said, and co-trimoxazole “is probably an effective means to do something about it.”
Study details
To look at the characteristics of and risk factors for serious infections associated with rituximab use in AAV, Mr. Dernie and associates retrospectively examined the electronic records of patients who had been treated between August 2016 and August 2019. Follow-up was until August 2020.
Of the 50 patients identified, nearly half (48%) were men. The average age was 60 years, ranging from 25 to 90 years. Most (n = 36; 72%) patients had a diagnosis of granulomatosis with polyangiitis, while another 2 (4%) had microscopic polyangiitis, 1 (2%) had eosinophilic granulomatosis with polyangiitis, and 11 (22%) had an overlapping type of vasculitis or undefined AAV.
Of the 18 severe infection events recorded, most (56%) involved the respiratory tract. Less than one-third (28%) were sepsis or neutropenic sepsis events, and there was one case each (6%) of cellulitis, complicated urinary tract infection, and recurrent wound infection.
There were “small numbers of individual comorbidities that were not sufficient to enter into our regression analysis,” Mr. Dernie noted. “It’s likely that comorbid conditions such as COPD [chronic obstructive pulmonary disease] also contribute to an individual’s risk of developing severe infections, and thus should factor into their individualized management.”
Mr. Dernie acknowledged in discussion: “One of the limitations of the study was we just looked at patients in a time when they were receiving rituximab, so they may have historically been exposed to other treatment options.” However, he added, “they weren’t having any other major DMARDs or immunosuppressive treatments at the time.”
Dr. Luqmani observed: “If you look at Francesco’s data on the hypogammaglobulinemia at the start of rituximab, that probably gives you a good idea of just how immunosuppressed these patients were already before we got to this point.”
Dr. Luqmani added: “I suspect that’s in keeping with a lot of other centers that have started using rituximab an awful lot for patients who previously had episodes of vasculitis treated with other disease-modifying therapies, particularly cyclophosphamide.”
But for how long should co-trimoxazole be given after the last rituximab dose? asked the chair of the session, Richard Watts, DM, of Norwich (England) Medical School. These data are purely observational, so it’s not possible to say, Mr. Dernie noted: “The patients that we included as having co-trimoxazole seem to be on it more or less consistently, permanently,” he said.
What about the best dose? “It’s a tricky one,” Dr. Luqmani said, as “we not only use co-trimoxazole for prophylaxis, but we often also want to use it for treatment of the vasculitis itself.”
It’s very likely that there was a mix of patients in the analysis that had received co-trimoxazole as either a treatment or prophylaxis, which means different doses, he said.
“It might be interesting to know whether there was a difference” between doses used and the prevention of infection, added Dr. Luqmani, “but I suspect the numbers are too small to tell.”
Mr. Dernie, Dr. Luqmani, and the other coauthors had no disclosures.
FROM BSR 2021
Insomnia? Referral, drugs not usually needed
Too often, medications are the treatment of choice, and when used long term they can perpetuate a problematic cycle, said Dr. Lettieri, professor in pulmonary, critical care, and sleep medicine at Johns Hopkins University, Baltimore.
However, medications alone won’t work without other behavior modifications and they come with potential side effects, he said in his talk. Prescription medications typically don’t treat the cause of the insomnia, just the symptoms.
“In the 15 years I’ve been practicing sleep medicine, I can honestly say I only have a handful of patients that I treat with long-term pharmacotherapy,” Dr. Lettieri said.
He said he typically uses pharmacotherapy only when conservative measures have failed or to help jump-start patients to behavior modifications.
Restricted sleep is a good place to start for chronic insomnia, he continued.
Physicians should ask patients the latest time they can wake up to make it to school, work, etc. If that time is 6 a.m., the goal is to move bedtime back to 10 p.m.–11 p.m. If the patient, however, is unable to sleep until 12:30 a.m., move bedtime there, he said.
Though the 5.5-hour window is not ideal, it’s better to get into bed when ready for sleep. From there, try to get the patient to move bedtime back 15 minutes each week as they train themselves to fall asleep earlier, he said.
“I promise you this works in the majority of patients and doesn’t require any medication. You can also accomplish this with one or two office visits, so it is not a huge drain on resources,” he said.
Sleep specialists in short supply
Cognitive-behavioral therapy (CBT) is “without question the best way to treat chronic insomnia and it’s recommended as first-line therapy by all published guidelines,” Dr. Lettieri said.
He defined chronic insomnia as happening most nights over at least 3 months. It affects twice as many women as men.
CBT offers a formalized way of changing sleep patterns with the help of an expert in sleep behavior disorders. It combines cognitive therapies with education about sleep and stimulus control and uses techniques such as mindfulness and relaxation.
However, most programs take 4-8 sessions with a sleep medicine provider and are usually not covered by insurance. In addition, the number of insomnia specialists is not nearly adequate to meet demand, he added.
Online and mobile-platform CBT programs are widely effective, Dr. Lettieri said. Many are free and all are convenient for patients to use. He said many of his patients use Sleepio, but many other online programs are effective.
“You can provide sufficient therapy for many of your patients and reserve CBT for patients who can’t be fixed with more conservative measures,” he said.
Insomnia among older patients
Interest in helping older patients with insomnia dominated the chat session associated with the talk.
Insomnia increases with age and older patients have often been using prescription or over-the-counter sleep aids for decades.
Additionally, “insomnia is the second-most common reason why people get admitted to long-term care facilities, second only to urinary incontinence,” Dr. Lettieri said.
If physicians use medications with older patients, he said, extra caution is needed. Older people have more neurocognitive impairments than younger adults and may already be taking several other medications. Sleep medications may come with longer elimination half-lives. Polypharmacy may increase risk for falls and have other consequences.
“If you have to go to a medication, try something simple like melatonin,” he said, adding that it should be pharmaceutical grade and extended release.
Also, bright lights during the day, movement throughout the day, and dim lights closer to bedtime are especially important for the elderly, Dr. Lettieri said.
Andrew Corr, MD, a geriatric specialist in primary care with the Riverside (Calif.) Medical Clinic, said in an interview the main message he will take back to his physician group is more CBT and less medication.
He said that, although he has long known CBT is the top first-line treatment, it is difficult to find experts in his area who are trained to do CBT for insomnia, so he was glad to hear online programs and self-directed reading are typically effective.
He also said there’s a common misperception that there’s no harm in prescribing medications such as trazodone (Desyrel), an antidepressant commonly used off label as a sleep aid.
Dr. Lettieri’s talk highlighted his recommendation against using trazodone for sleep. “Despite several recommendations against its use for insomnia, it is still commonly prescribed. You just shouldn’t use it for insomnia,” Dr. Lettieri said.
“It has no measurable effect in a third of patients and at least unacceptable side effects in another third. Right off the bat, it’s not efficacious in two thirds of patients.”
Additionally, priapism, a prolonged erection, has been associated with trazodone, Dr. Lettieri said, “and I have literally never met a patient on trazodone who was counseled about this.”
Trazodone also has a black box warning from the Food and Drug Administration warning about increased risk for suicidal thoughts.
Dr. Lettieri and Dr. Corr disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too often, medications are the treatment of choice, and when used long term they can perpetuate a problematic cycle, said Dr. Lettieri, professor in pulmonary, critical care, and sleep medicine at Johns Hopkins University, Baltimore.
However, medications alone won’t work without other behavior modifications and they come with potential side effects, he said in his talk. Prescription medications typically don’t treat the cause of the insomnia, just the symptoms.
“In the 15 years I’ve been practicing sleep medicine, I can honestly say I only have a handful of patients that I treat with long-term pharmacotherapy,” Dr. Lettieri said.
He said he typically uses pharmacotherapy only when conservative measures have failed or to help jump-start patients to behavior modifications.
Restricted sleep is a good place to start for chronic insomnia, he continued.
Physicians should ask patients the latest time they can wake up to make it to school, work, etc. If that time is 6 a.m., the goal is to move bedtime back to 10 p.m.–11 p.m. If the patient, however, is unable to sleep until 12:30 a.m., move bedtime there, he said.
Though the 5.5-hour window is not ideal, it’s better to get into bed when ready for sleep. From there, try to get the patient to move bedtime back 15 minutes each week as they train themselves to fall asleep earlier, he said.
“I promise you this works in the majority of patients and doesn’t require any medication. You can also accomplish this with one or two office visits, so it is not a huge drain on resources,” he said.
Sleep specialists in short supply
Cognitive-behavioral therapy (CBT) is “without question the best way to treat chronic insomnia and it’s recommended as first-line therapy by all published guidelines,” Dr. Lettieri said.
He defined chronic insomnia as happening most nights over at least 3 months. It affects twice as many women as men.
CBT offers a formalized way of changing sleep patterns with the help of an expert in sleep behavior disorders. It combines cognitive therapies with education about sleep and stimulus control and uses techniques such as mindfulness and relaxation.
However, most programs take 4-8 sessions with a sleep medicine provider and are usually not covered by insurance. In addition, the number of insomnia specialists is not nearly adequate to meet demand, he added.
Online and mobile-platform CBT programs are widely effective, Dr. Lettieri said. Many are free and all are convenient for patients to use. He said many of his patients use Sleepio, but many other online programs are effective.
“You can provide sufficient therapy for many of your patients and reserve CBT for patients who can’t be fixed with more conservative measures,” he said.
Insomnia among older patients
Interest in helping older patients with insomnia dominated the chat session associated with the talk.
Insomnia increases with age and older patients have often been using prescription or over-the-counter sleep aids for decades.
Additionally, “insomnia is the second-most common reason why people get admitted to long-term care facilities, second only to urinary incontinence,” Dr. Lettieri said.
If physicians use medications with older patients, he said, extra caution is needed. Older people have more neurocognitive impairments than younger adults and may already be taking several other medications. Sleep medications may come with longer elimination half-lives. Polypharmacy may increase risk for falls and have other consequences.
“If you have to go to a medication, try something simple like melatonin,” he said, adding that it should be pharmaceutical grade and extended release.
Also, bright lights during the day, movement throughout the day, and dim lights closer to bedtime are especially important for the elderly, Dr. Lettieri said.
Andrew Corr, MD, a geriatric specialist in primary care with the Riverside (Calif.) Medical Clinic, said in an interview the main message he will take back to his physician group is more CBT and less medication.
He said that, although he has long known CBT is the top first-line treatment, it is difficult to find experts in his area who are trained to do CBT for insomnia, so he was glad to hear online programs and self-directed reading are typically effective.
He also said there’s a common misperception that there’s no harm in prescribing medications such as trazodone (Desyrel), an antidepressant commonly used off label as a sleep aid.
Dr. Lettieri’s talk highlighted his recommendation against using trazodone for sleep. “Despite several recommendations against its use for insomnia, it is still commonly prescribed. You just shouldn’t use it for insomnia,” Dr. Lettieri said.
“It has no measurable effect in a third of patients and at least unacceptable side effects in another third. Right off the bat, it’s not efficacious in two thirds of patients.”
Additionally, priapism, a prolonged erection, has been associated with trazodone, Dr. Lettieri said, “and I have literally never met a patient on trazodone who was counseled about this.”
Trazodone also has a black box warning from the Food and Drug Administration warning about increased risk for suicidal thoughts.
Dr. Lettieri and Dr. Corr disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too often, medications are the treatment of choice, and when used long term they can perpetuate a problematic cycle, said Dr. Lettieri, professor in pulmonary, critical care, and sleep medicine at Johns Hopkins University, Baltimore.
However, medications alone won’t work without other behavior modifications and they come with potential side effects, he said in his talk. Prescription medications typically don’t treat the cause of the insomnia, just the symptoms.
“In the 15 years I’ve been practicing sleep medicine, I can honestly say I only have a handful of patients that I treat with long-term pharmacotherapy,” Dr. Lettieri said.
He said he typically uses pharmacotherapy only when conservative measures have failed or to help jump-start patients to behavior modifications.
Restricted sleep is a good place to start for chronic insomnia, he continued.
Physicians should ask patients the latest time they can wake up to make it to school, work, etc. If that time is 6 a.m., the goal is to move bedtime back to 10 p.m.–11 p.m. If the patient, however, is unable to sleep until 12:30 a.m., move bedtime there, he said.
Though the 5.5-hour window is not ideal, it’s better to get into bed when ready for sleep. From there, try to get the patient to move bedtime back 15 minutes each week as they train themselves to fall asleep earlier, he said.
“I promise you this works in the majority of patients and doesn’t require any medication. You can also accomplish this with one or two office visits, so it is not a huge drain on resources,” he said.
Sleep specialists in short supply
Cognitive-behavioral therapy (CBT) is “without question the best way to treat chronic insomnia and it’s recommended as first-line therapy by all published guidelines,” Dr. Lettieri said.
He defined chronic insomnia as happening most nights over at least 3 months. It affects twice as many women as men.
CBT offers a formalized way of changing sleep patterns with the help of an expert in sleep behavior disorders. It combines cognitive therapies with education about sleep and stimulus control and uses techniques such as mindfulness and relaxation.
However, most programs take 4-8 sessions with a sleep medicine provider and are usually not covered by insurance. In addition, the number of insomnia specialists is not nearly adequate to meet demand, he added.
Online and mobile-platform CBT programs are widely effective, Dr. Lettieri said. Many are free and all are convenient for patients to use. He said many of his patients use Sleepio, but many other online programs are effective.
“You can provide sufficient therapy for many of your patients and reserve CBT for patients who can’t be fixed with more conservative measures,” he said.
Insomnia among older patients
Interest in helping older patients with insomnia dominated the chat session associated with the talk.
Insomnia increases with age and older patients have often been using prescription or over-the-counter sleep aids for decades.
Additionally, “insomnia is the second-most common reason why people get admitted to long-term care facilities, second only to urinary incontinence,” Dr. Lettieri said.
If physicians use medications with older patients, he said, extra caution is needed. Older people have more neurocognitive impairments than younger adults and may already be taking several other medications. Sleep medications may come with longer elimination half-lives. Polypharmacy may increase risk for falls and have other consequences.
“If you have to go to a medication, try something simple like melatonin,” he said, adding that it should be pharmaceutical grade and extended release.
Also, bright lights during the day, movement throughout the day, and dim lights closer to bedtime are especially important for the elderly, Dr. Lettieri said.
Andrew Corr, MD, a geriatric specialist in primary care with the Riverside (Calif.) Medical Clinic, said in an interview the main message he will take back to his physician group is more CBT and less medication.
He said that, although he has long known CBT is the top first-line treatment, it is difficult to find experts in his area who are trained to do CBT for insomnia, so he was glad to hear online programs and self-directed reading are typically effective.
He also said there’s a common misperception that there’s no harm in prescribing medications such as trazodone (Desyrel), an antidepressant commonly used off label as a sleep aid.
Dr. Lettieri’s talk highlighted his recommendation against using trazodone for sleep. “Despite several recommendations against its use for insomnia, it is still commonly prescribed. You just shouldn’t use it for insomnia,” Dr. Lettieri said.
“It has no measurable effect in a third of patients and at least unacceptable side effects in another third. Right off the bat, it’s not efficacious in two thirds of patients.”
Additionally, priapism, a prolonged erection, has been associated with trazodone, Dr. Lettieri said, “and I have literally never met a patient on trazodone who was counseled about this.”
Trazodone also has a black box warning from the Food and Drug Administration warning about increased risk for suicidal thoughts.
Dr. Lettieri and Dr. Corr disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2021
How to utilize the updated PHM Core Competencies
Converge 2021 session
Making The Pediatric Hospital Medicine Core Competencies Work for You
Presenters
Erin Fisher, MD, MHM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sofia Teferi, MD, SFHM, FAAP
Session summary
The Pediatric Hospital Medicine (PHM) Core Competencies were originally published in the Journal of Hospital Medicine in 2010, and created a framework for graduate and continuing medical education, reflecting the roles and expectations for all pediatric hospitalists in the United States. Since that time, the field of PHM, scope of practice, and roles of hospitalists has evolved, making a substantial update to this dossier necessary.
The 2020 PHM Core Competencies consist of four sections, including common clinical diagnoses and conditions, specialized services, core skills, and the health care system. The four topics are covered in 66 chapters, which were updated or created for the present version.
The Core Competencies have many practical applications, including teaching or curriculum development, which may be used by trainees as well as PHM providers. The speakers gave real-world examples of the competencies’ application to evaluations, and the continuum of knowledge, skills, attitudes, and system implementation in the development of a trainee from student to practicing hospitalist. Trainees’ knowledge gaps can be identified using the competencies, and utilization of the provided compendium will help identify sources that can aid in teaching.
Professional development is an excellent way to utilize the Core Competencies. Division directors may identify a needed area for improvement and the competencies can serve as a road map for establishing goals, plan development, and analysis of results of the intervention. They are also a great resource for PHM board prep. Although the competencies were not developed specifically for the PHM boards, they do contain all 13 PHM content domains set forth by the American Board of Pediatrics for PHM.
The Core Competencies can also be used to justify service line needs and resources in discussions with administration. For instance, if one is a pediatric hospitalist at a community hospital and asked to take over the newborn nursery, the competencies can be used to get buy-in from the group, as a guide for additional training, to provide a framework for development of practice pathways, and to request resources needed.
The Pediatric Core Competencies are a great resource for pediatric hospitalists and group leaders with many uses, from board preparation to education and professional development. They provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Key takeaways
- Given a change in scope of practice of pediatric hospitalists over the past 10 years, the PHM Core Competencies were updated and published in the Journal of Hospital Medicine in 2020.
- The Core Competencies have many practical applications including education, curriculum development, professional development, and PHM board preparation.
- The Core Competencies provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Dr. Schwenk is a pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., where he serves as a medical director of inpatient services. He is an associate professor of pediatrics at the University of Louisville School of Medicine. He is a Senior Fellow of Hospital Medicine and has served on the executive council of the Pediatrics Special Interest Group and the Annual Meeting Committee for SHM Converge.
Converge 2021 session
Making The Pediatric Hospital Medicine Core Competencies Work for You
Presenters
Erin Fisher, MD, MHM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sofia Teferi, MD, SFHM, FAAP
Session summary
The Pediatric Hospital Medicine (PHM) Core Competencies were originally published in the Journal of Hospital Medicine in 2010, and created a framework for graduate and continuing medical education, reflecting the roles and expectations for all pediatric hospitalists in the United States. Since that time, the field of PHM, scope of practice, and roles of hospitalists has evolved, making a substantial update to this dossier necessary.
The 2020 PHM Core Competencies consist of four sections, including common clinical diagnoses and conditions, specialized services, core skills, and the health care system. The four topics are covered in 66 chapters, which were updated or created for the present version.
The Core Competencies have many practical applications, including teaching or curriculum development, which may be used by trainees as well as PHM providers. The speakers gave real-world examples of the competencies’ application to evaluations, and the continuum of knowledge, skills, attitudes, and system implementation in the development of a trainee from student to practicing hospitalist. Trainees’ knowledge gaps can be identified using the competencies, and utilization of the provided compendium will help identify sources that can aid in teaching.
Professional development is an excellent way to utilize the Core Competencies. Division directors may identify a needed area for improvement and the competencies can serve as a road map for establishing goals, plan development, and analysis of results of the intervention. They are also a great resource for PHM board prep. Although the competencies were not developed specifically for the PHM boards, they do contain all 13 PHM content domains set forth by the American Board of Pediatrics for PHM.
The Core Competencies can also be used to justify service line needs and resources in discussions with administration. For instance, if one is a pediatric hospitalist at a community hospital and asked to take over the newborn nursery, the competencies can be used to get buy-in from the group, as a guide for additional training, to provide a framework for development of practice pathways, and to request resources needed.
The Pediatric Core Competencies are a great resource for pediatric hospitalists and group leaders with many uses, from board preparation to education and professional development. They provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Key takeaways
- Given a change in scope of practice of pediatric hospitalists over the past 10 years, the PHM Core Competencies were updated and published in the Journal of Hospital Medicine in 2020.
- The Core Competencies have many practical applications including education, curriculum development, professional development, and PHM board preparation.
- The Core Competencies provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Dr. Schwenk is a pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., where he serves as a medical director of inpatient services. He is an associate professor of pediatrics at the University of Louisville School of Medicine. He is a Senior Fellow of Hospital Medicine and has served on the executive council of the Pediatrics Special Interest Group and the Annual Meeting Committee for SHM Converge.
Converge 2021 session
Making The Pediatric Hospital Medicine Core Competencies Work for You
Presenters
Erin Fisher, MD, MHM, FAAP; Sandra Gage, MD, PhD, SFHM, FAAP; Jennifer Maniscalco, MD, MPH, MAcM, FAAP; Sofia Teferi, MD, SFHM, FAAP
Session summary
The Pediatric Hospital Medicine (PHM) Core Competencies were originally published in the Journal of Hospital Medicine in 2010, and created a framework for graduate and continuing medical education, reflecting the roles and expectations for all pediatric hospitalists in the United States. Since that time, the field of PHM, scope of practice, and roles of hospitalists has evolved, making a substantial update to this dossier necessary.
The 2020 PHM Core Competencies consist of four sections, including common clinical diagnoses and conditions, specialized services, core skills, and the health care system. The four topics are covered in 66 chapters, which were updated or created for the present version.
The Core Competencies have many practical applications, including teaching or curriculum development, which may be used by trainees as well as PHM providers. The speakers gave real-world examples of the competencies’ application to evaluations, and the continuum of knowledge, skills, attitudes, and system implementation in the development of a trainee from student to practicing hospitalist. Trainees’ knowledge gaps can be identified using the competencies, and utilization of the provided compendium will help identify sources that can aid in teaching.
Professional development is an excellent way to utilize the Core Competencies. Division directors may identify a needed area for improvement and the competencies can serve as a road map for establishing goals, plan development, and analysis of results of the intervention. They are also a great resource for PHM board prep. Although the competencies were not developed specifically for the PHM boards, they do contain all 13 PHM content domains set forth by the American Board of Pediatrics for PHM.
The Core Competencies can also be used to justify service line needs and resources in discussions with administration. For instance, if one is a pediatric hospitalist at a community hospital and asked to take over the newborn nursery, the competencies can be used to get buy-in from the group, as a guide for additional training, to provide a framework for development of practice pathways, and to request resources needed.
The Pediatric Core Competencies are a great resource for pediatric hospitalists and group leaders with many uses, from board preparation to education and professional development. They provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Key takeaways
- Given a change in scope of practice of pediatric hospitalists over the past 10 years, the PHM Core Competencies were updated and published in the Journal of Hospital Medicine in 2020.
- The Core Competencies have many practical applications including education, curriculum development, professional development, and PHM board preparation.
- The Core Competencies provide a framework for improvement of knowledge, skills, and attitudes within an organization.
Dr. Schwenk is a pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., where he serves as a medical director of inpatient services. He is an associate professor of pediatrics at the University of Louisville School of Medicine. He is a Senior Fellow of Hospital Medicine and has served on the executive council of the Pediatrics Special Interest Group and the Annual Meeting Committee for SHM Converge.
FROM SHM CONVERGE 2021
VARC-3 sets goalpost for future aortic valve trials
The newly updated Valve Academic Research Consortium 3 (VARC-3) definitions and endpoints proposed for transcatheter and surgical aortic valve replacement (TAVR/SAVR) research aim to add more granularity and a patient focus to a rapidly evolving field, the authors say.
Work began in 2016 to update definitions in the document to be more contemporary, as TAVR matured over the last 10 years to include younger, lower-risk patients and began moving to long-term outcomes, lead author Philippe Généreux, MD, said in an interview.
“The main change in VARC-3 is really that we tried to define not only procedural outcome, both for TAVR and aortic valve replacement performed by surgery, but also more the long-term outcomes mainly based on the patient – so quality of life, bioprosthetic valve failure, how do we define a valve failure, and also the need for rehospitalization,” he said.
However, soon after the VARC-3 document was published on April 19, 2021, in the European Heart Journal and Journal of the American College of Cardiology, surgeons took to social media to highlight the writing committee’s financial ties to industry and to suggest some definitions were shaped to favor transcatheter approaches.
“There’s no doubt that the coauthors who participated in these guidelines are experts; nobody would argue about that but what we can argue, and I’m 100% sure about, is that we have experts outside the payroll of industry who are excellent and can be part of this guideline drafting in an unbiased way,” Victor Dayan, MD, adjunct professor of cardiac surgery, National Institute of Cardiac Surgery, Montevideo, Uruguay, said in an interview.
Although the American College of Physicians recommends guideline committee members with moderate- or high-level conflicts of interest recuse themselves from authorship, he noted that one author has received more than $2 million in fees from industry in the past 4-5 years.
In all, 20 of 23 authors were involved in PARTNER, SURTAVI, and PORTICO, and several also write clinical guidelines for the American College of Cardiology and American Heart Association. “So we have the same authors that are judge, jury, and attorney for these issues,” Dr. Dayan said.
In a comment, J. Rafael Sádaba, MD, PhD, interim secretary general for the European Association for Cardio-Thoracic Surgery, pointed out that only three committee members are surgeons and that author disclosures took up nearly a full page of the document. “Surely they would be able to find very capable physicians with far less conflicts of interest.”
Dr. Sádaba said the question to him is why professional societies like ACC and AHA don’t define the endpoints for the clinical trials that will inform their guidelines.
“One could say these people are there because they’re good scientists, trialists, but one at least has to ask why is this happening. Why are these people setting the rules for the trials they’re running?” said Dr. Sádaba, of the Royal Navarre Hospital, Pamplona, Spain.
Dr. Généreux dismissed the Twitter comments as coming from a handful of people who engage in conspiracy theories. The VARC-3 document, he said, was created with input from 75 experts, including Food and Drug Administration officials, and the final document was reviewed by the FDA and underwent rigorous peer review prior to publication.
“The question is: do you believe there is bias when people are involved in studies driven by the industry? Well, this is where we derive our science in this field,” he said. “We are very transparent and disclose our conflicts of interest [COI].”
Commenting further, Dr. Généreux added, “this was a very well-balanced group and to imply that because we work with industry, we don’t have the best interest of the patient in mind is wrong.”
Editor in chief of the EHJ, Filippo Crea, MD, PhD, Catholic University, Rome, said in an comment that “it is not surprising that most of the authors have experience in TAVR trials. All of the authors have carefully disclosed their COIs.”
He noted that the EHJ and JACC copublished the first VARC consensus in 2011, VARC-2 1 year later, and that VARC-3 was reviewed by four external reviewers and two editors and was accepted for publication after two revisions.
Asked about a shot on social media that the EHJ had long ago “sold its soul” to be the scientific “arm” of industry, Dr. Crea said allegations need to be substantiated by facts.
“The wide adoption of VARC definitions implies that they have been well accepted by the scientific community and that they have stood the test of time,” Dr. Crea said. “EHJ has a history of publishing high-quality science. We welcome robust arguments that may challenge previously published work. Readers who perceive gaps are encouraged to provide a detailed challenge and engage with the journal.”
Defining hospitalizations
One of the surgeons’ biggest concerns is that VARC-3 now defines hospitalization or rehospitalization as “any admission after the index hospitalization or study enrollment” for at least 24 hours, including an ED stay.
VARC-2 and SURTAVI defined hospitalizations as those for valve-related symptoms or worsening heart failure, whereas the newly reformulated definition of hospitalization was part of the main composite endpoint in the PARTNER-3 trial, along with stroke and mortality, that drove the superiority of TAVR over SAVR at 1 year for low-risk patients, Dr. Dayan noted.
“It’s not uncommon for patients who have cardiac surgery to come back for issues related to wound healing or mild pulmonary edema for a day or 2, and if you include these hospitalizations in the primary endpoint, it will dilute the real benefit of SAVR versus TAVR, which is mortality and stroke,” he added.
In choosing the broader definition, Dr. Généreux said they borrowed from heart failure studies that take a granular approach and account for every hospitalization, be it for a medication change or adjustment. “We cannot pick and choose which hospitalization we are going to consider or ignore.”
VARC-3 proposes criteria for identifying and diagnosing hypoattenuated leaflet thickening (HALT) and reduced leaflet motion and features a detailed chart of the new classification scheme for bioprosthetic valve dysfunction and failure.
Bioprosthetic valve dysfunction includes structural valve deterioration, nonstructural valve dysfunction (including abnormalities not intrinsic to the valve such as paravalvular regurgitation or prosthesis-patient mismatch), thrombosis, and endocarditis. VARC-3 proposes a five-class grading system for paravalvular regurgitation (mild, mild-moderate, moderate, moderate-severe, severe).
The document updates what the authors called a “previously vague definition” of valve thrombosis proposed in 2011 to now include “clinically significant” prosthetic valve thrombosis. This requires clinical sequelae of a thromboembolic event (stroke, transient ischemic attack, retinal occlusion, or other evidence of thromboembolism) or worsening valve stenosis/regurgitation and either hemodynamic valve deterioration stage 2 or 3 or confirmatory imaging (CT evidence of HALT or transesophageal echocardiographic findings). In the absence of symptoms/clinical sequelae, valve thrombosis (subclinical) can be diagnosed if there is hemodynamic valve deterioration stage 3 and confirmatory imaging.
Bioprosthetic valve failure is divided into three stages, with stage 1 taking into account clinical factors along with valve dysfunction, stage 2 being reintervention, and stage 3 being valve-related death.
“For us, bioprosthesis valve failure is not only the need for reintervention, but it’s also mortality, it’s also a significant increase in gradient or the occurrence of paravalvular leak,” said Dr. Généreux, of the Morristown (N.J.) Medical Center. “So it’s much more clinical.”
Stroke, myocardial infarction
VARC-3 provides detailed definitions of neurologic events and, in an attempt to harmonize with the Neurologic Academic Research Consortium, recommends combining assessment of neurologic symptoms with tissue-based criteria (pathology or neuroimaging, ideally diffusion-weighted MRI) to define stroke and other central nervous system injury.
It also recommends that assessment be performed 30-90 days after a neurologic event and that assessment of neurologic deficits for cerebral embolic protection trials be performed by a neurologist.
VARC-3 endorses the fourth Universal Definition of Myocardial Infarction for MI types 1-3, 4B, and 4C.
For periprocedural MI after percutaneous coronary intervention (PCI), coronary bypass graft surgery, TAVR, and SAVR, however, it endorses the modified Society for Cardiovascular Angiography and Interventions and Academic Research Consortium-2 definition, which uses troponin or creatine kinase-MB thresholds.
“Given that most current and future studies related to AVR strategies will involve long-term follow-up, with patients frequently suffering from coronary artery disease, VARC-3 believes that these definitions will allow the most appropriate characterization and classification of types of MI occurring in this population,” the committee wrote.
The decision comes after last year’s controversy surrounding the Abbott-sponsored EXCEL trial, which used a modified version of the SCAI definition for periprocedural MI as part of its primary composite endpoint of death, stroke, and MI.
Initial reports showed nearly twice the rate of periprocedural MI with cardiac surgery as with PCI, but after a BBC investigation involving leaked data and an onslaught of criticism from surgeons, later results using the third universal definition showed surgery had the advantage.
The debacle frayed relations between surgeons and interventionalists and prompted EACTS to withdraw its support for treatment recommendations for left main coronary artery disease.
Dr. Dayan applauded VARC-3 for incorporating more detailed information on stroke and neurologic events, but said the use of the SCAI definition in the final published document is in “total disregard” to the controversy generated among surgeons and interventionalists.
“The main concern for surgeons is defining periprocedural MI just by biochemical definitions, without any additional criteria like ECG, angiographic,” he said. “This is totally new and goes against what surgeons have been advocating for years around EXCEL.”
Dr. Sádaba was troubled by the definitions of MI and hospitalization, but also questioned other changes, like lumping vascular complications together with access-related complications. “The sense is a lot of what they’ve put here favors one type of intervention over the other.”
Dr. Généreux reported receiving consultant fees from Abbott Vascular, Abiomed, Boston Scientific, Cardinal Health, Cardiovascular System, Edwards Lifesciences, Medtronic, Opsens, Siemens, SoundBite Medical Solutions, Sig.Num, Saranas, Teleflex, Tryton Medical, and has equity in Pi-Cardia, Sig.Num, SoundBite Medical Solutions, Saranas, and Puzzle Medical. Dr. Crea reported receiving personal fees from Novartis, Bristol-Myers Squibb, Amgen, and AstraZeneca, and is a member of the advisory board of GlyCardial Diagnostics. Dr. Dayan and Dr. Sádaba reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The newly updated Valve Academic Research Consortium 3 (VARC-3) definitions and endpoints proposed for transcatheter and surgical aortic valve replacement (TAVR/SAVR) research aim to add more granularity and a patient focus to a rapidly evolving field, the authors say.
Work began in 2016 to update definitions in the document to be more contemporary, as TAVR matured over the last 10 years to include younger, lower-risk patients and began moving to long-term outcomes, lead author Philippe Généreux, MD, said in an interview.
“The main change in VARC-3 is really that we tried to define not only procedural outcome, both for TAVR and aortic valve replacement performed by surgery, but also more the long-term outcomes mainly based on the patient – so quality of life, bioprosthetic valve failure, how do we define a valve failure, and also the need for rehospitalization,” he said.
However, soon after the VARC-3 document was published on April 19, 2021, in the European Heart Journal and Journal of the American College of Cardiology, surgeons took to social media to highlight the writing committee’s financial ties to industry and to suggest some definitions were shaped to favor transcatheter approaches.
“There’s no doubt that the coauthors who participated in these guidelines are experts; nobody would argue about that but what we can argue, and I’m 100% sure about, is that we have experts outside the payroll of industry who are excellent and can be part of this guideline drafting in an unbiased way,” Victor Dayan, MD, adjunct professor of cardiac surgery, National Institute of Cardiac Surgery, Montevideo, Uruguay, said in an interview.
Although the American College of Physicians recommends guideline committee members with moderate- or high-level conflicts of interest recuse themselves from authorship, he noted that one author has received more than $2 million in fees from industry in the past 4-5 years.
In all, 20 of 23 authors were involved in PARTNER, SURTAVI, and PORTICO, and several also write clinical guidelines for the American College of Cardiology and American Heart Association. “So we have the same authors that are judge, jury, and attorney for these issues,” Dr. Dayan said.
In a comment, J. Rafael Sádaba, MD, PhD, interim secretary general for the European Association for Cardio-Thoracic Surgery, pointed out that only three committee members are surgeons and that author disclosures took up nearly a full page of the document. “Surely they would be able to find very capable physicians with far less conflicts of interest.”
Dr. Sádaba said the question to him is why professional societies like ACC and AHA don’t define the endpoints for the clinical trials that will inform their guidelines.
“One could say these people are there because they’re good scientists, trialists, but one at least has to ask why is this happening. Why are these people setting the rules for the trials they’re running?” said Dr. Sádaba, of the Royal Navarre Hospital, Pamplona, Spain.
Dr. Généreux dismissed the Twitter comments as coming from a handful of people who engage in conspiracy theories. The VARC-3 document, he said, was created with input from 75 experts, including Food and Drug Administration officials, and the final document was reviewed by the FDA and underwent rigorous peer review prior to publication.
“The question is: do you believe there is bias when people are involved in studies driven by the industry? Well, this is where we derive our science in this field,” he said. “We are very transparent and disclose our conflicts of interest [COI].”
Commenting further, Dr. Généreux added, “this was a very well-balanced group and to imply that because we work with industry, we don’t have the best interest of the patient in mind is wrong.”
Editor in chief of the EHJ, Filippo Crea, MD, PhD, Catholic University, Rome, said in an comment that “it is not surprising that most of the authors have experience in TAVR trials. All of the authors have carefully disclosed their COIs.”
He noted that the EHJ and JACC copublished the first VARC consensus in 2011, VARC-2 1 year later, and that VARC-3 was reviewed by four external reviewers and two editors and was accepted for publication after two revisions.
Asked about a shot on social media that the EHJ had long ago “sold its soul” to be the scientific “arm” of industry, Dr. Crea said allegations need to be substantiated by facts.
“The wide adoption of VARC definitions implies that they have been well accepted by the scientific community and that they have stood the test of time,” Dr. Crea said. “EHJ has a history of publishing high-quality science. We welcome robust arguments that may challenge previously published work. Readers who perceive gaps are encouraged to provide a detailed challenge and engage with the journal.”
Defining hospitalizations
One of the surgeons’ biggest concerns is that VARC-3 now defines hospitalization or rehospitalization as “any admission after the index hospitalization or study enrollment” for at least 24 hours, including an ED stay.
VARC-2 and SURTAVI defined hospitalizations as those for valve-related symptoms or worsening heart failure, whereas the newly reformulated definition of hospitalization was part of the main composite endpoint in the PARTNER-3 trial, along with stroke and mortality, that drove the superiority of TAVR over SAVR at 1 year for low-risk patients, Dr. Dayan noted.
“It’s not uncommon for patients who have cardiac surgery to come back for issues related to wound healing or mild pulmonary edema for a day or 2, and if you include these hospitalizations in the primary endpoint, it will dilute the real benefit of SAVR versus TAVR, which is mortality and stroke,” he added.
In choosing the broader definition, Dr. Généreux said they borrowed from heart failure studies that take a granular approach and account for every hospitalization, be it for a medication change or adjustment. “We cannot pick and choose which hospitalization we are going to consider or ignore.”
VARC-3 proposes criteria for identifying and diagnosing hypoattenuated leaflet thickening (HALT) and reduced leaflet motion and features a detailed chart of the new classification scheme for bioprosthetic valve dysfunction and failure.
Bioprosthetic valve dysfunction includes structural valve deterioration, nonstructural valve dysfunction (including abnormalities not intrinsic to the valve such as paravalvular regurgitation or prosthesis-patient mismatch), thrombosis, and endocarditis. VARC-3 proposes a five-class grading system for paravalvular regurgitation (mild, mild-moderate, moderate, moderate-severe, severe).
The document updates what the authors called a “previously vague definition” of valve thrombosis proposed in 2011 to now include “clinically significant” prosthetic valve thrombosis. This requires clinical sequelae of a thromboembolic event (stroke, transient ischemic attack, retinal occlusion, or other evidence of thromboembolism) or worsening valve stenosis/regurgitation and either hemodynamic valve deterioration stage 2 or 3 or confirmatory imaging (CT evidence of HALT or transesophageal echocardiographic findings). In the absence of symptoms/clinical sequelae, valve thrombosis (subclinical) can be diagnosed if there is hemodynamic valve deterioration stage 3 and confirmatory imaging.
Bioprosthetic valve failure is divided into three stages, with stage 1 taking into account clinical factors along with valve dysfunction, stage 2 being reintervention, and stage 3 being valve-related death.
“For us, bioprosthesis valve failure is not only the need for reintervention, but it’s also mortality, it’s also a significant increase in gradient or the occurrence of paravalvular leak,” said Dr. Généreux, of the Morristown (N.J.) Medical Center. “So it’s much more clinical.”
Stroke, myocardial infarction
VARC-3 provides detailed definitions of neurologic events and, in an attempt to harmonize with the Neurologic Academic Research Consortium, recommends combining assessment of neurologic symptoms with tissue-based criteria (pathology or neuroimaging, ideally diffusion-weighted MRI) to define stroke and other central nervous system injury.
It also recommends that assessment be performed 30-90 days after a neurologic event and that assessment of neurologic deficits for cerebral embolic protection trials be performed by a neurologist.
VARC-3 endorses the fourth Universal Definition of Myocardial Infarction for MI types 1-3, 4B, and 4C.
For periprocedural MI after percutaneous coronary intervention (PCI), coronary bypass graft surgery, TAVR, and SAVR, however, it endorses the modified Society for Cardiovascular Angiography and Interventions and Academic Research Consortium-2 definition, which uses troponin or creatine kinase-MB thresholds.
“Given that most current and future studies related to AVR strategies will involve long-term follow-up, with patients frequently suffering from coronary artery disease, VARC-3 believes that these definitions will allow the most appropriate characterization and classification of types of MI occurring in this population,” the committee wrote.
The decision comes after last year’s controversy surrounding the Abbott-sponsored EXCEL trial, which used a modified version of the SCAI definition for periprocedural MI as part of its primary composite endpoint of death, stroke, and MI.
Initial reports showed nearly twice the rate of periprocedural MI with cardiac surgery as with PCI, but after a BBC investigation involving leaked data and an onslaught of criticism from surgeons, later results using the third universal definition showed surgery had the advantage.
The debacle frayed relations between surgeons and interventionalists and prompted EACTS to withdraw its support for treatment recommendations for left main coronary artery disease.
Dr. Dayan applauded VARC-3 for incorporating more detailed information on stroke and neurologic events, but said the use of the SCAI definition in the final published document is in “total disregard” to the controversy generated among surgeons and interventionalists.
“The main concern for surgeons is defining periprocedural MI just by biochemical definitions, without any additional criteria like ECG, angiographic,” he said. “This is totally new and goes against what surgeons have been advocating for years around EXCEL.”
Dr. Sádaba was troubled by the definitions of MI and hospitalization, but also questioned other changes, like lumping vascular complications together with access-related complications. “The sense is a lot of what they’ve put here favors one type of intervention over the other.”
Dr. Généreux reported receiving consultant fees from Abbott Vascular, Abiomed, Boston Scientific, Cardinal Health, Cardiovascular System, Edwards Lifesciences, Medtronic, Opsens, Siemens, SoundBite Medical Solutions, Sig.Num, Saranas, Teleflex, Tryton Medical, and has equity in Pi-Cardia, Sig.Num, SoundBite Medical Solutions, Saranas, and Puzzle Medical. Dr. Crea reported receiving personal fees from Novartis, Bristol-Myers Squibb, Amgen, and AstraZeneca, and is a member of the advisory board of GlyCardial Diagnostics. Dr. Dayan and Dr. Sádaba reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The newly updated Valve Academic Research Consortium 3 (VARC-3) definitions and endpoints proposed for transcatheter and surgical aortic valve replacement (TAVR/SAVR) research aim to add more granularity and a patient focus to a rapidly evolving field, the authors say.
Work began in 2016 to update definitions in the document to be more contemporary, as TAVR matured over the last 10 years to include younger, lower-risk patients and began moving to long-term outcomes, lead author Philippe Généreux, MD, said in an interview.
“The main change in VARC-3 is really that we tried to define not only procedural outcome, both for TAVR and aortic valve replacement performed by surgery, but also more the long-term outcomes mainly based on the patient – so quality of life, bioprosthetic valve failure, how do we define a valve failure, and also the need for rehospitalization,” he said.
However, soon after the VARC-3 document was published on April 19, 2021, in the European Heart Journal and Journal of the American College of Cardiology, surgeons took to social media to highlight the writing committee’s financial ties to industry and to suggest some definitions were shaped to favor transcatheter approaches.
“There’s no doubt that the coauthors who participated in these guidelines are experts; nobody would argue about that but what we can argue, and I’m 100% sure about, is that we have experts outside the payroll of industry who are excellent and can be part of this guideline drafting in an unbiased way,” Victor Dayan, MD, adjunct professor of cardiac surgery, National Institute of Cardiac Surgery, Montevideo, Uruguay, said in an interview.
Although the American College of Physicians recommends guideline committee members with moderate- or high-level conflicts of interest recuse themselves from authorship, he noted that one author has received more than $2 million in fees from industry in the past 4-5 years.
In all, 20 of 23 authors were involved in PARTNER, SURTAVI, and PORTICO, and several also write clinical guidelines for the American College of Cardiology and American Heart Association. “So we have the same authors that are judge, jury, and attorney for these issues,” Dr. Dayan said.
In a comment, J. Rafael Sádaba, MD, PhD, interim secretary general for the European Association for Cardio-Thoracic Surgery, pointed out that only three committee members are surgeons and that author disclosures took up nearly a full page of the document. “Surely they would be able to find very capable physicians with far less conflicts of interest.”
Dr. Sádaba said the question to him is why professional societies like ACC and AHA don’t define the endpoints for the clinical trials that will inform their guidelines.
“One could say these people are there because they’re good scientists, trialists, but one at least has to ask why is this happening. Why are these people setting the rules for the trials they’re running?” said Dr. Sádaba, of the Royal Navarre Hospital, Pamplona, Spain.
Dr. Généreux dismissed the Twitter comments as coming from a handful of people who engage in conspiracy theories. The VARC-3 document, he said, was created with input from 75 experts, including Food and Drug Administration officials, and the final document was reviewed by the FDA and underwent rigorous peer review prior to publication.
“The question is: do you believe there is bias when people are involved in studies driven by the industry? Well, this is where we derive our science in this field,” he said. “We are very transparent and disclose our conflicts of interest [COI].”
Commenting further, Dr. Généreux added, “this was a very well-balanced group and to imply that because we work with industry, we don’t have the best interest of the patient in mind is wrong.”
Editor in chief of the EHJ, Filippo Crea, MD, PhD, Catholic University, Rome, said in an comment that “it is not surprising that most of the authors have experience in TAVR trials. All of the authors have carefully disclosed their COIs.”
He noted that the EHJ and JACC copublished the first VARC consensus in 2011, VARC-2 1 year later, and that VARC-3 was reviewed by four external reviewers and two editors and was accepted for publication after two revisions.
Asked about a shot on social media that the EHJ had long ago “sold its soul” to be the scientific “arm” of industry, Dr. Crea said allegations need to be substantiated by facts.
“The wide adoption of VARC definitions implies that they have been well accepted by the scientific community and that they have stood the test of time,” Dr. Crea said. “EHJ has a history of publishing high-quality science. We welcome robust arguments that may challenge previously published work. Readers who perceive gaps are encouraged to provide a detailed challenge and engage with the journal.”
Defining hospitalizations
One of the surgeons’ biggest concerns is that VARC-3 now defines hospitalization or rehospitalization as “any admission after the index hospitalization or study enrollment” for at least 24 hours, including an ED stay.
VARC-2 and SURTAVI defined hospitalizations as those for valve-related symptoms or worsening heart failure, whereas the newly reformulated definition of hospitalization was part of the main composite endpoint in the PARTNER-3 trial, along with stroke and mortality, that drove the superiority of TAVR over SAVR at 1 year for low-risk patients, Dr. Dayan noted.
“It’s not uncommon for patients who have cardiac surgery to come back for issues related to wound healing or mild pulmonary edema for a day or 2, and if you include these hospitalizations in the primary endpoint, it will dilute the real benefit of SAVR versus TAVR, which is mortality and stroke,” he added.
In choosing the broader definition, Dr. Généreux said they borrowed from heart failure studies that take a granular approach and account for every hospitalization, be it for a medication change or adjustment. “We cannot pick and choose which hospitalization we are going to consider or ignore.”
VARC-3 proposes criteria for identifying and diagnosing hypoattenuated leaflet thickening (HALT) and reduced leaflet motion and features a detailed chart of the new classification scheme for bioprosthetic valve dysfunction and failure.
Bioprosthetic valve dysfunction includes structural valve deterioration, nonstructural valve dysfunction (including abnormalities not intrinsic to the valve such as paravalvular regurgitation or prosthesis-patient mismatch), thrombosis, and endocarditis. VARC-3 proposes a five-class grading system for paravalvular regurgitation (mild, mild-moderate, moderate, moderate-severe, severe).
The document updates what the authors called a “previously vague definition” of valve thrombosis proposed in 2011 to now include “clinically significant” prosthetic valve thrombosis. This requires clinical sequelae of a thromboembolic event (stroke, transient ischemic attack, retinal occlusion, or other evidence of thromboembolism) or worsening valve stenosis/regurgitation and either hemodynamic valve deterioration stage 2 or 3 or confirmatory imaging (CT evidence of HALT or transesophageal echocardiographic findings). In the absence of symptoms/clinical sequelae, valve thrombosis (subclinical) can be diagnosed if there is hemodynamic valve deterioration stage 3 and confirmatory imaging.
Bioprosthetic valve failure is divided into three stages, with stage 1 taking into account clinical factors along with valve dysfunction, stage 2 being reintervention, and stage 3 being valve-related death.
“For us, bioprosthesis valve failure is not only the need for reintervention, but it’s also mortality, it’s also a significant increase in gradient or the occurrence of paravalvular leak,” said Dr. Généreux, of the Morristown (N.J.) Medical Center. “So it’s much more clinical.”
Stroke, myocardial infarction
VARC-3 provides detailed definitions of neurologic events and, in an attempt to harmonize with the Neurologic Academic Research Consortium, recommends combining assessment of neurologic symptoms with tissue-based criteria (pathology or neuroimaging, ideally diffusion-weighted MRI) to define stroke and other central nervous system injury.
It also recommends that assessment be performed 30-90 days after a neurologic event and that assessment of neurologic deficits for cerebral embolic protection trials be performed by a neurologist.
VARC-3 endorses the fourth Universal Definition of Myocardial Infarction for MI types 1-3, 4B, and 4C.
For periprocedural MI after percutaneous coronary intervention (PCI), coronary bypass graft surgery, TAVR, and SAVR, however, it endorses the modified Society for Cardiovascular Angiography and Interventions and Academic Research Consortium-2 definition, which uses troponin or creatine kinase-MB thresholds.
“Given that most current and future studies related to AVR strategies will involve long-term follow-up, with patients frequently suffering from coronary artery disease, VARC-3 believes that these definitions will allow the most appropriate characterization and classification of types of MI occurring in this population,” the committee wrote.
The decision comes after last year’s controversy surrounding the Abbott-sponsored EXCEL trial, which used a modified version of the SCAI definition for periprocedural MI as part of its primary composite endpoint of death, stroke, and MI.
Initial reports showed nearly twice the rate of periprocedural MI with cardiac surgery as with PCI, but after a BBC investigation involving leaked data and an onslaught of criticism from surgeons, later results using the third universal definition showed surgery had the advantage.
The debacle frayed relations between surgeons and interventionalists and prompted EACTS to withdraw its support for treatment recommendations for left main coronary artery disease.
Dr. Dayan applauded VARC-3 for incorporating more detailed information on stroke and neurologic events, but said the use of the SCAI definition in the final published document is in “total disregard” to the controversy generated among surgeons and interventionalists.
“The main concern for surgeons is defining periprocedural MI just by biochemical definitions, without any additional criteria like ECG, angiographic,” he said. “This is totally new and goes against what surgeons have been advocating for years around EXCEL.”
Dr. Sádaba was troubled by the definitions of MI and hospitalization, but also questioned other changes, like lumping vascular complications together with access-related complications. “The sense is a lot of what they’ve put here favors one type of intervention over the other.”
Dr. Généreux reported receiving consultant fees from Abbott Vascular, Abiomed, Boston Scientific, Cardinal Health, Cardiovascular System, Edwards Lifesciences, Medtronic, Opsens, Siemens, SoundBite Medical Solutions, Sig.Num, Saranas, Teleflex, Tryton Medical, and has equity in Pi-Cardia, Sig.Num, SoundBite Medical Solutions, Saranas, and Puzzle Medical. Dr. Crea reported receiving personal fees from Novartis, Bristol-Myers Squibb, Amgen, and AstraZeneca, and is a member of the advisory board of GlyCardial Diagnostics. Dr. Dayan and Dr. Sádaba reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Eptinezumab safe and effective for treatment of migraine
Key clinical point: Eptinezumab, particularly at a dosage of 300 mg, has significant efficacy and an acceptable safety profile for treatment of migraine.
Major finding: Eptinezumab significantly reduced the mean monthly migraine days (MMDs) compared with placebo at week 12 at a dosage of 30 mg (change in MMDs, −0.29; P = .0001), 100 mg (change in MMDs, −0.31; P less than .00001), and 300 mg (change in MMDs, −0.41; P less than .00001). Treatment-emergent adverse events were not significantly different between eptinezumab and placebo.
Study details: This was a meta-analysis of 4 randomized controlled trials including 2,739 patients with migraine.
Disclosures: This work was supported by the Suzhou Health Talents Training Project. The authors declared no competing interests.
Source: Yan Z et al. J Headache Pain. 2021 Mar 6. doi: 10.1186/s10194-021-01220-y.
Key clinical point: Eptinezumab, particularly at a dosage of 300 mg, has significant efficacy and an acceptable safety profile for treatment of migraine.
Major finding: Eptinezumab significantly reduced the mean monthly migraine days (MMDs) compared with placebo at week 12 at a dosage of 30 mg (change in MMDs, −0.29; P = .0001), 100 mg (change in MMDs, −0.31; P less than .00001), and 300 mg (change in MMDs, −0.41; P less than .00001). Treatment-emergent adverse events were not significantly different between eptinezumab and placebo.
Study details: This was a meta-analysis of 4 randomized controlled trials including 2,739 patients with migraine.
Disclosures: This work was supported by the Suzhou Health Talents Training Project. The authors declared no competing interests.
Source: Yan Z et al. J Headache Pain. 2021 Mar 6. doi: 10.1186/s10194-021-01220-y.
Key clinical point: Eptinezumab, particularly at a dosage of 300 mg, has significant efficacy and an acceptable safety profile for treatment of migraine.
Major finding: Eptinezumab significantly reduced the mean monthly migraine days (MMDs) compared with placebo at week 12 at a dosage of 30 mg (change in MMDs, −0.29; P = .0001), 100 mg (change in MMDs, −0.31; P less than .00001), and 300 mg (change in MMDs, −0.41; P less than .00001). Treatment-emergent adverse events were not significantly different between eptinezumab and placebo.
Study details: This was a meta-analysis of 4 randomized controlled trials including 2,739 patients with migraine.
Disclosures: This work was supported by the Suzhou Health Talents Training Project. The authors declared no competing interests.
Source: Yan Z et al. J Headache Pain. 2021 Mar 6. doi: 10.1186/s10194-021-01220-y.