User login
HR-positive breast cancer: Extended intermittent letrozole yields no survival benefit
Key clinical point: Extended intermittent and continuous letrozole yield similar disease-free survival in postmenopausal women with hormone receptor (HR)-positive breast cancer. Circulating estrogen levels were restored after letrozole interruption.
Major finding: There was no difference in 7-year disease-free survival between intermittent and continuous letrozole groups (P = 0.64). The median change in estradiol levels after letrozole interruption was 141% vs. 2.2% with continuous letrozole during the same duration.
Study details: The Study of Letrozole Extension (SOLE) trial was an open-label, phase 3 study of 4,884 postmenopausal women with HR-positive, lymph node-positive, operable breast cancer, randomly assigned to extended intermittent or continuous letrozole. The SOLE-EST sub study (n=104) evaluated changes in circulating estrogen levels.
Disclosures: SOLE study was supported by Novartis and the International Breast Cancer Study Group. SOLE-EST was partially funded by the Belgian Foundation Against Cancer, Breast Cancer Trials Australia and New Zealand, and Susan G. Komen for the Cure. The authors received research support, honoraria, nonfinancial support, advisory/consulting/travel fees, and/or salary from various sources.
Source: Jerusalem G et al. Ann Oncol. 2021 Aug 9 (in press). doi: 10.1016/j.annonc.2021.07.017.
Key clinical point: Extended intermittent and continuous letrozole yield similar disease-free survival in postmenopausal women with hormone receptor (HR)-positive breast cancer. Circulating estrogen levels were restored after letrozole interruption.
Major finding: There was no difference in 7-year disease-free survival between intermittent and continuous letrozole groups (P = 0.64). The median change in estradiol levels after letrozole interruption was 141% vs. 2.2% with continuous letrozole during the same duration.
Study details: The Study of Letrozole Extension (SOLE) trial was an open-label, phase 3 study of 4,884 postmenopausal women with HR-positive, lymph node-positive, operable breast cancer, randomly assigned to extended intermittent or continuous letrozole. The SOLE-EST sub study (n=104) evaluated changes in circulating estrogen levels.
Disclosures: SOLE study was supported by Novartis and the International Breast Cancer Study Group. SOLE-EST was partially funded by the Belgian Foundation Against Cancer, Breast Cancer Trials Australia and New Zealand, and Susan G. Komen for the Cure. The authors received research support, honoraria, nonfinancial support, advisory/consulting/travel fees, and/or salary from various sources.
Source: Jerusalem G et al. Ann Oncol. 2021 Aug 9 (in press). doi: 10.1016/j.annonc.2021.07.017.
Key clinical point: Extended intermittent and continuous letrozole yield similar disease-free survival in postmenopausal women with hormone receptor (HR)-positive breast cancer. Circulating estrogen levels were restored after letrozole interruption.
Major finding: There was no difference in 7-year disease-free survival between intermittent and continuous letrozole groups (P = 0.64). The median change in estradiol levels after letrozole interruption was 141% vs. 2.2% with continuous letrozole during the same duration.
Study details: The Study of Letrozole Extension (SOLE) trial was an open-label, phase 3 study of 4,884 postmenopausal women with HR-positive, lymph node-positive, operable breast cancer, randomly assigned to extended intermittent or continuous letrozole. The SOLE-EST sub study (n=104) evaluated changes in circulating estrogen levels.
Disclosures: SOLE study was supported by Novartis and the International Breast Cancer Study Group. SOLE-EST was partially funded by the Belgian Foundation Against Cancer, Breast Cancer Trials Australia and New Zealand, and Susan G. Komen for the Cure. The authors received research support, honoraria, nonfinancial support, advisory/consulting/travel fees, and/or salary from various sources.
Source: Jerusalem G et al. Ann Oncol. 2021 Aug 9 (in press). doi: 10.1016/j.annonc.2021.07.017.
Triple Negative Breast Cancer (TNBC): Adding ipatasertib to paclitaxel does not improve survival
Key clinical point: In patients with inoperable locally advanced/metastatic triple-negative breast cancer (TNBC), the overall survival benefit with add-on ipatasertib was not statistically significant but numerically longer.
Major finding: The median overall survival with ipatasertib plus paclitaxel vs placebo plus paclitaxel was 25.8 months vs. 16.9 months (hazard ratio, 0.80; 95% confidence interval, 0.50-1.28). The most common grade ≥3 adverse events in the ipatasertib vs placebo groups were diarrhea (23% vs. 0%) and neutropenia (10% vs. 2%).
Study details: A multicenter, randomized, double-blind phase 2 study of 124 patients with inoperable locally advanced/metastatic TNBC who were randomly assigned to receive first-line paclitaxel with either ipatasertib or placebo.
Disclosures: This study was supported by F. Hoffmann-La Roche Ltd. The authors received honoraria, research funding, consultancy/advisory fees, travel/accommodation/expenses, and research funding. Some authors or their spouses were employed and/or held stocks and/or had ownership in companies.
Source: Dent R et al. Breast Cancer Res Treat. 2021;189:377-386. doi: 10.1007/s10549-021-06143-5.
Key clinical point: In patients with inoperable locally advanced/metastatic triple-negative breast cancer (TNBC), the overall survival benefit with add-on ipatasertib was not statistically significant but numerically longer.
Major finding: The median overall survival with ipatasertib plus paclitaxel vs placebo plus paclitaxel was 25.8 months vs. 16.9 months (hazard ratio, 0.80; 95% confidence interval, 0.50-1.28). The most common grade ≥3 adverse events in the ipatasertib vs placebo groups were diarrhea (23% vs. 0%) and neutropenia (10% vs. 2%).
Study details: A multicenter, randomized, double-blind phase 2 study of 124 patients with inoperable locally advanced/metastatic TNBC who were randomly assigned to receive first-line paclitaxel with either ipatasertib or placebo.
Disclosures: This study was supported by F. Hoffmann-La Roche Ltd. The authors received honoraria, research funding, consultancy/advisory fees, travel/accommodation/expenses, and research funding. Some authors or their spouses were employed and/or held stocks and/or had ownership in companies.
Source: Dent R et al. Breast Cancer Res Treat. 2021;189:377-386. doi: 10.1007/s10549-021-06143-5.
Key clinical point: In patients with inoperable locally advanced/metastatic triple-negative breast cancer (TNBC), the overall survival benefit with add-on ipatasertib was not statistically significant but numerically longer.
Major finding: The median overall survival with ipatasertib plus paclitaxel vs placebo plus paclitaxel was 25.8 months vs. 16.9 months (hazard ratio, 0.80; 95% confidence interval, 0.50-1.28). The most common grade ≥3 adverse events in the ipatasertib vs placebo groups were diarrhea (23% vs. 0%) and neutropenia (10% vs. 2%).
Study details: A multicenter, randomized, double-blind phase 2 study of 124 patients with inoperable locally advanced/metastatic TNBC who were randomly assigned to receive first-line paclitaxel with either ipatasertib or placebo.
Disclosures: This study was supported by F. Hoffmann-La Roche Ltd. The authors received honoraria, research funding, consultancy/advisory fees, travel/accommodation/expenses, and research funding. Some authors or their spouses were employed and/or held stocks and/or had ownership in companies.
Source: Dent R et al. Breast Cancer Res Treat. 2021;189:377-386. doi: 10.1007/s10549-021-06143-5.
HER2-negative breast cancer: Adjuvant celecoxib fails phase 3 trial
Key clinical point: Adjuvant celecoxib for 2 years vs placebo fails to extend survival in patients with human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Major finding: In patients who received celecoxib vs placebo, no significant difference was observed in the disease-free survival (P = 0.75) and the overall survival (P = 0.78). In the celecoxib vs. placebo group, similar rates of cardiac events (15% vs. 13%) and new primary non-breast cancers (4% vs 4%) were reported.
Study details: A phase 3, randomized, double-blind study of patients with HER2-negative breast cancer who were randomly assigned to adjuvant celecoxib for 2 years or placebo following complete resection.
Disclosures: The study was supported by Pfizer, Cancer Research UK, The Royal Marsden NHS Foundation Trust, and the Institute of Cancer Research, London. The authors reported receiving grants, research funding, personal fees, advisory fees, nonfinancial support, and/or honoraria and/or having patents outside this work.
Source: Coombes RC et al. JAMA Oncol. 2021 Jul 15 (in press). doi: 10.1001/jamaoncol.2021.2193.
Key clinical point: Adjuvant celecoxib for 2 years vs placebo fails to extend survival in patients with human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Major finding: In patients who received celecoxib vs placebo, no significant difference was observed in the disease-free survival (P = 0.75) and the overall survival (P = 0.78). In the celecoxib vs. placebo group, similar rates of cardiac events (15% vs. 13%) and new primary non-breast cancers (4% vs 4%) were reported.
Study details: A phase 3, randomized, double-blind study of patients with HER2-negative breast cancer who were randomly assigned to adjuvant celecoxib for 2 years or placebo following complete resection.
Disclosures: The study was supported by Pfizer, Cancer Research UK, The Royal Marsden NHS Foundation Trust, and the Institute of Cancer Research, London. The authors reported receiving grants, research funding, personal fees, advisory fees, nonfinancial support, and/or honoraria and/or having patents outside this work.
Source: Coombes RC et al. JAMA Oncol. 2021 Jul 15 (in press). doi: 10.1001/jamaoncol.2021.2193.
Key clinical point: Adjuvant celecoxib for 2 years vs placebo fails to extend survival in patients with human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Major finding: In patients who received celecoxib vs placebo, no significant difference was observed in the disease-free survival (P = 0.75) and the overall survival (P = 0.78). In the celecoxib vs. placebo group, similar rates of cardiac events (15% vs. 13%) and new primary non-breast cancers (4% vs 4%) were reported.
Study details: A phase 3, randomized, double-blind study of patients with HER2-negative breast cancer who were randomly assigned to adjuvant celecoxib for 2 years or placebo following complete resection.
Disclosures: The study was supported by Pfizer, Cancer Research UK, The Royal Marsden NHS Foundation Trust, and the Institute of Cancer Research, London. The authors reported receiving grants, research funding, personal fees, advisory fees, nonfinancial support, and/or honoraria and/or having patents outside this work.
Source: Coombes RC et al. JAMA Oncol. 2021 Jul 15 (in press). doi: 10.1001/jamaoncol.2021.2193.
HER2-positive breast cancer: Add-on trastuzumab lowers recurrence and mortality
Key clinical point: Adding trastuzumab to chemotherapy in patients with early-stage, epidermal growth hormone receptor 2 (HER2)-positive breast cancer lowers the rate of recurrence and mortality from breast cancer.
Major finding: Trastuzumab plus chemotherapy vs chemotherapy alone significantly reduced the rates of breast cancer recurrence (rate ratio [RR], 0.66; P < 0.0001) and death from breast cancer (RR, 0.67; P < 0.0001). The average absolute reduction in 10-year risk of recurrence was 9.0% (P < 0.0001) and in 10-year breast cancer mortality was 6.4% (P < 0.0001).
Study details: This was a meta-analysis of individual patient data from 7 randomized trials including 13,864 patients with early-stage, HER2-positive breast cancer who were randomly assigned to trastuzumab plus chemotherapy or chemotherapy alone.
Disclosures: The study was funded by Cancer Research UK and the UK Medical Research Council. The authors declared grants, contracts, consulting/speaker/advisory fees, patents, royalties, and support for attending meetings and travel. Dr. H Joensuu reported a leadership/fiduciary role and stock or stock options from Orion Pharma.
Source: Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Lancet Oncol. 2021;22(8):1139-1150. doi: 10.1016/S1470-2045(21)00288-6.
Key clinical point: Adding trastuzumab to chemotherapy in patients with early-stage, epidermal growth hormone receptor 2 (HER2)-positive breast cancer lowers the rate of recurrence and mortality from breast cancer.
Major finding: Trastuzumab plus chemotherapy vs chemotherapy alone significantly reduced the rates of breast cancer recurrence (rate ratio [RR], 0.66; P < 0.0001) and death from breast cancer (RR, 0.67; P < 0.0001). The average absolute reduction in 10-year risk of recurrence was 9.0% (P < 0.0001) and in 10-year breast cancer mortality was 6.4% (P < 0.0001).
Study details: This was a meta-analysis of individual patient data from 7 randomized trials including 13,864 patients with early-stage, HER2-positive breast cancer who were randomly assigned to trastuzumab plus chemotherapy or chemotherapy alone.
Disclosures: The study was funded by Cancer Research UK and the UK Medical Research Council. The authors declared grants, contracts, consulting/speaker/advisory fees, patents, royalties, and support for attending meetings and travel. Dr. H Joensuu reported a leadership/fiduciary role and stock or stock options from Orion Pharma.
Source: Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Lancet Oncol. 2021;22(8):1139-1150. doi: 10.1016/S1470-2045(21)00288-6.
Key clinical point: Adding trastuzumab to chemotherapy in patients with early-stage, epidermal growth hormone receptor 2 (HER2)-positive breast cancer lowers the rate of recurrence and mortality from breast cancer.
Major finding: Trastuzumab plus chemotherapy vs chemotherapy alone significantly reduced the rates of breast cancer recurrence (rate ratio [RR], 0.66; P < 0.0001) and death from breast cancer (RR, 0.67; P < 0.0001). The average absolute reduction in 10-year risk of recurrence was 9.0% (P < 0.0001) and in 10-year breast cancer mortality was 6.4% (P < 0.0001).
Study details: This was a meta-analysis of individual patient data from 7 randomized trials including 13,864 patients with early-stage, HER2-positive breast cancer who were randomly assigned to trastuzumab plus chemotherapy or chemotherapy alone.
Disclosures: The study was funded by Cancer Research UK and the UK Medical Research Council. The authors declared grants, contracts, consulting/speaker/advisory fees, patents, royalties, and support for attending meetings and travel. Dr. H Joensuu reported a leadership/fiduciary role and stock or stock options from Orion Pharma.
Source: Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Lancet Oncol. 2021;22(8):1139-1150. doi: 10.1016/S1470-2045(21)00288-6.
HR-positive breast cancer: Entinostat fails phase 3 trial
Key clinical point: Adding entinostat to exemestane does not improve survival in aromatase inhibitor (AI)-resistant advanced hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Major finding: There were no differences between the entinostat and placebo groups in median progression-free survival (3.3 months vs. 3.1 months; P = 0.30) and median overall survival (23.4 months vs. 21.7 months; P = 0.94). The most common grade 3-4 adverse events in the entinostat group were neutropenia (20%) and hypophosphatemia (14%).
Study details: This was a multicenter, randomized, double-blind, placebo-controlled phase 3 E2112 study of 608 patients with AI-resistant, HR-positive, HER2-negative breast cancer, randomly assigned to entinostat plus exemestane or placebo plus exemestane.
Disclosures: The study was supported by the National Cancer Institute of the National Institutes of Health. The authors reported receiving consulting/advisory/speaker fees, research funding, accommodation/travel/expenses, and royalties from and/or stock ownership and/or other relationship in companies or patents owned/filed.
Source: Connoly RM et al. J Clin Oncol. 2021 Aug 6 (in press). doi: 10.1200/JCO.21.00944.
Key clinical point: Adding entinostat to exemestane does not improve survival in aromatase inhibitor (AI)-resistant advanced hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Major finding: There were no differences between the entinostat and placebo groups in median progression-free survival (3.3 months vs. 3.1 months; P = 0.30) and median overall survival (23.4 months vs. 21.7 months; P = 0.94). The most common grade 3-4 adverse events in the entinostat group were neutropenia (20%) and hypophosphatemia (14%).
Study details: This was a multicenter, randomized, double-blind, placebo-controlled phase 3 E2112 study of 608 patients with AI-resistant, HR-positive, HER2-negative breast cancer, randomly assigned to entinostat plus exemestane or placebo plus exemestane.
Disclosures: The study was supported by the National Cancer Institute of the National Institutes of Health. The authors reported receiving consulting/advisory/speaker fees, research funding, accommodation/travel/expenses, and royalties from and/or stock ownership and/or other relationship in companies or patents owned/filed.
Source: Connoly RM et al. J Clin Oncol. 2021 Aug 6 (in press). doi: 10.1200/JCO.21.00944.
Key clinical point: Adding entinostat to exemestane does not improve survival in aromatase inhibitor (AI)-resistant advanced hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Major finding: There were no differences between the entinostat and placebo groups in median progression-free survival (3.3 months vs. 3.1 months; P = 0.30) and median overall survival (23.4 months vs. 21.7 months; P = 0.94). The most common grade 3-4 adverse events in the entinostat group were neutropenia (20%) and hypophosphatemia (14%).
Study details: This was a multicenter, randomized, double-blind, placebo-controlled phase 3 E2112 study of 608 patients with AI-resistant, HR-positive, HER2-negative breast cancer, randomly assigned to entinostat plus exemestane or placebo plus exemestane.
Disclosures: The study was supported by the National Cancer Institute of the National Institutes of Health. The authors reported receiving consulting/advisory/speaker fees, research funding, accommodation/travel/expenses, and royalties from and/or stock ownership and/or other relationship in companies or patents owned/filed.
Source: Connoly RM et al. J Clin Oncol. 2021 Aug 6 (in press). doi: 10.1200/JCO.21.00944.
Breast cancer: 10-year treatment extension with aromatase inhibitors yields no benefit
Key clinical point: A 10-year vs 7-year treatment with aromatase inhibitors (anastrozole) in patients with hormone receptor (HR)-positive breast cancer does not yield survival benefit but increases the risk for bone fracture.
Major finding: Anastrozole treatment for 10 years vs. 7 years was not associated with a significant difference in disease-free survival (P = 0.90). The risk of clinical bone fracture was higher with 10-year treatment (hazard ratio, 1.35; 95% confidence interval, 1.00-1.84).
Study details: The phase 3 Secondary Adjuvant Long Term Study With Arimidex (SALSA) trial studied 3,484 postmenopausal women with HR-positive breast cancer who had received anastrozole for 5 years and were randomly assigned to therapy extension by 2 years (for a total of 7 years) or 5 years (for a total of 10 years).
Disclosures: This study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. The authors received grants, honoraria, personal/lecture/advisory/consulting/speaker fees, and/or travel/accommodation/expenses outside this work.
Source: Gnant M et al. New Engl J Med. 2021;385:395-405. doi: 10.1056/NEJMoa2104162.
Key clinical point: A 10-year vs 7-year treatment with aromatase inhibitors (anastrozole) in patients with hormone receptor (HR)-positive breast cancer does not yield survival benefit but increases the risk for bone fracture.
Major finding: Anastrozole treatment for 10 years vs. 7 years was not associated with a significant difference in disease-free survival (P = 0.90). The risk of clinical bone fracture was higher with 10-year treatment (hazard ratio, 1.35; 95% confidence interval, 1.00-1.84).
Study details: The phase 3 Secondary Adjuvant Long Term Study With Arimidex (SALSA) trial studied 3,484 postmenopausal women with HR-positive breast cancer who had received anastrozole for 5 years and were randomly assigned to therapy extension by 2 years (for a total of 7 years) or 5 years (for a total of 10 years).
Disclosures: This study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. The authors received grants, honoraria, personal/lecture/advisory/consulting/speaker fees, and/or travel/accommodation/expenses outside this work.
Source: Gnant M et al. New Engl J Med. 2021;385:395-405. doi: 10.1056/NEJMoa2104162.
Key clinical point: A 10-year vs 7-year treatment with aromatase inhibitors (anastrozole) in patients with hormone receptor (HR)-positive breast cancer does not yield survival benefit but increases the risk for bone fracture.
Major finding: Anastrozole treatment for 10 years vs. 7 years was not associated with a significant difference in disease-free survival (P = 0.90). The risk of clinical bone fracture was higher with 10-year treatment (hazard ratio, 1.35; 95% confidence interval, 1.00-1.84).
Study details: The phase 3 Secondary Adjuvant Long Term Study With Arimidex (SALSA) trial studied 3,484 postmenopausal women with HR-positive breast cancer who had received anastrozole for 5 years and were randomly assigned to therapy extension by 2 years (for a total of 7 years) or 5 years (for a total of 10 years).
Disclosures: This study was supported by AstraZeneca and the Austrian Breast and Colorectal Cancer Study Group. The authors received grants, honoraria, personal/lecture/advisory/consulting/speaker fees, and/or travel/accommodation/expenses outside this work.
Source: Gnant M et al. New Engl J Med. 2021;385:395-405. doi: 10.1056/NEJMoa2104162.
Neuropsychiatry affects pediatric OCD treatment
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
FROM FOCUS ON NEUROPSYCHIATRY 2021
EDs saw more benzodiazepine overdoses, but fewer patients overall, in 2020
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.
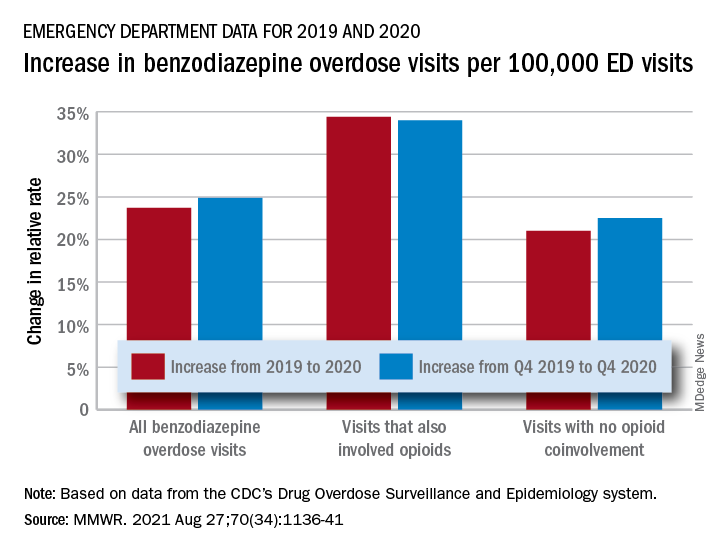
The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
FROM MMWR
Reassuring data on long-term outcomes among kids with MIS-C
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID symptoms can persist for more than 1 year, study shows
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.

