User login
PET Radiotracer Identifies Glioma Treatment Response
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY OF NUCLEAR MEDICINE AND MOLECULAR IMAGING
Major Finding: The 17 patients identified as responders to bevacizumab by 18F-FDOPA PET after 2 weeks of treatment survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders.
Data Source: This pilot study involved 28 patients with recurrent high-grade gliomas who underwent MRI and 18F-FDOPA PET at baseline and after 2 and 6 weeks.
Disclosures: The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
Amyloid Imaging Studies Track Dementia Development
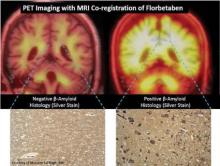
Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Those results, reported in two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging in Miami, showed that the tracers could be used to predict progression to Alzheimer’s in 66%-75% of those with elevated binding of the agents to beta-amyloid plaques in the brains of individuals with mild cognitive impairment (MCI). In cases where an individual tested negative for elevated beta-amyloid binding, fewer than 20% progressed to another type of dementia.
Results such as these show that detecting beta-amyloid burden in the brain "can help lead to diagnosis of Alzheimer’s disease when a patient has mild symptoms rather than wait until they have established dementia as is the current clinical practice. This may have important benefits for the patient, for their family, and for society," said Dr. Christopher Rowe, the lead investigator on the PiB study and senior investigator on the florbetaben study.
The PiB study, called the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, included 92 patients with MCI. At baseline, Dr. Rowe and his colleagues detected high PiB binding in 65% of MCI patients. After 3 years, 66% of MCI patients who had a positive scan for high beta-amyloid burden at baseline had been diagnosed with Alzheimer’s, compared with only 7% of MCI patients with a negative scan.
Other studies of PiB out to 6 years of follow-up have shown that patients accrue beta-amyloid at "an incredibly slow" rate of about 2% per year. "If you have a negative scan, you can be reassured that you’re not going to have Alzheimer’s disease for at least the next 10 years," Dr. Rowe said at a press conference at the meeting.
Because the half-life of PiB is only 20 minutes, it is not suitable for clinical use. The 2-hour half-life of florbetaben and other 18F amyloid radiotracers make them much more cost effective for clinical use, noted Dr. Rowe, director of the department of nuclear medicine and the center for PET and a consultant neurologist to the memory disorders clinic at the Austin Hospital in Melbourne, Australia.
The florbetaben study involved 45 patients with MCI who received PET imaging with the compound. At baseline, 53% had a high level of neocortical binding, and binding increased 3% after 2 years in those with already high levels. Overall, 75% of patients with elevated florbetaben binding progressed to Alzheimer’s disease, whereas 19% of those with a low level of binding progressed to other kinds of dementias.
MR imaging in the same individuals indicated that 53% of patients with hippocampal atrophy at baseline had progressed to Alzheimer’s. When the combination of high florbetaben binding and hippocampal atrophy was present, 80% had progressed to Alzheimer’s after 2 years.
"We don’t say that everybody who’s got Alzheimer’s disease should have these scans because I don’t think that would be cost effective. But in selected scenarios, when they’ve seen a memory specialist, I think these can be very useful clinically," Dr. Rowe said.
He said amyloid imaging agents such as florbetaben have two potential uses:
• In patients with established dementia when there is uncertainty about whether the patient has Alzheimer’s disease or frontotemporal dementia. He noted that this has therapeutic implications because some of the medications used to treat Alzheimer’s, such as cholinesterase inhibitors, can make behavioral symptoms worse in frontotemporal dementia.
• In patients with MCI who have seen a specialist who is not sure what the cause of the symptoms might be and wants to see if it might be Alzheimer’s instead of waiting years for dementia to develop. Because about 40% with MCI do not go on to develop dementia, amyloid imaging studies would be reassuring to those patients, Dr. Rowe said.
Guidelines from the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging (which recently changed its name from the Society of Nuclear Medicine) will soon be available on the appropriate use of the imaging agents, Dr. Rowe said in an interview.
He disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.
Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Those results, reported in two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging in Miami, showed that the tracers could be used to predict progression to Alzheimer’s in 66%-75% of those with elevated binding of the agents to beta-amyloid plaques in the brains of individuals with mild cognitive impairment (MCI). In cases where an individual tested negative for elevated beta-amyloid binding, fewer than 20% progressed to another type of dementia.
Results such as these show that detecting beta-amyloid burden in the brain "can help lead to diagnosis of Alzheimer’s disease when a patient has mild symptoms rather than wait until they have established dementia as is the current clinical practice. This may have important benefits for the patient, for their family, and for society," said Dr. Christopher Rowe, the lead investigator on the PiB study and senior investigator on the florbetaben study.
The PiB study, called the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, included 92 patients with MCI. At baseline, Dr. Rowe and his colleagues detected high PiB binding in 65% of MCI patients. After 3 years, 66% of MCI patients who had a positive scan for high beta-amyloid burden at baseline had been diagnosed with Alzheimer’s, compared with only 7% of MCI patients with a negative scan.
Other studies of PiB out to 6 years of follow-up have shown that patients accrue beta-amyloid at "an incredibly slow" rate of about 2% per year. "If you have a negative scan, you can be reassured that you’re not going to have Alzheimer’s disease for at least the next 10 years," Dr. Rowe said at a press conference at the meeting.
Because the half-life of PiB is only 20 minutes, it is not suitable for clinical use. The 2-hour half-life of florbetaben and other 18F amyloid radiotracers make them much more cost effective for clinical use, noted Dr. Rowe, director of the department of nuclear medicine and the center for PET and a consultant neurologist to the memory disorders clinic at the Austin Hospital in Melbourne, Australia.
The florbetaben study involved 45 patients with MCI who received PET imaging with the compound. At baseline, 53% had a high level of neocortical binding, and binding increased 3% after 2 years in those with already high levels. Overall, 75% of patients with elevated florbetaben binding progressed to Alzheimer’s disease, whereas 19% of those with a low level of binding progressed to other kinds of dementias.
MR imaging in the same individuals indicated that 53% of patients with hippocampal atrophy at baseline had progressed to Alzheimer’s. When the combination of high florbetaben binding and hippocampal atrophy was present, 80% had progressed to Alzheimer’s after 2 years.
"We don’t say that everybody who’s got Alzheimer’s disease should have these scans because I don’t think that would be cost effective. But in selected scenarios, when they’ve seen a memory specialist, I think these can be very useful clinically," Dr. Rowe said.
He said amyloid imaging agents such as florbetaben have two potential uses:
• In patients with established dementia when there is uncertainty about whether the patient has Alzheimer’s disease or frontotemporal dementia. He noted that this has therapeutic implications because some of the medications used to treat Alzheimer’s, such as cholinesterase inhibitors, can make behavioral symptoms worse in frontotemporal dementia.
• In patients with MCI who have seen a specialist who is not sure what the cause of the symptoms might be and wants to see if it might be Alzheimer’s instead of waiting years for dementia to develop. Because about 40% with MCI do not go on to develop dementia, amyloid imaging studies would be reassuring to those patients, Dr. Rowe said.
Guidelines from the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging (which recently changed its name from the Society of Nuclear Medicine) will soon be available on the appropriate use of the imaging agents, Dr. Rowe said in an interview.
He disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.
Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Those results, reported in two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging in Miami, showed that the tracers could be used to predict progression to Alzheimer’s in 66%-75% of those with elevated binding of the agents to beta-amyloid plaques in the brains of individuals with mild cognitive impairment (MCI). In cases where an individual tested negative for elevated beta-amyloid binding, fewer than 20% progressed to another type of dementia.
Results such as these show that detecting beta-amyloid burden in the brain "can help lead to diagnosis of Alzheimer’s disease when a patient has mild symptoms rather than wait until they have established dementia as is the current clinical practice. This may have important benefits for the patient, for their family, and for society," said Dr. Christopher Rowe, the lead investigator on the PiB study and senior investigator on the florbetaben study.
The PiB study, called the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing, included 92 patients with MCI. At baseline, Dr. Rowe and his colleagues detected high PiB binding in 65% of MCI patients. After 3 years, 66% of MCI patients who had a positive scan for high beta-amyloid burden at baseline had been diagnosed with Alzheimer’s, compared with only 7% of MCI patients with a negative scan.
Other studies of PiB out to 6 years of follow-up have shown that patients accrue beta-amyloid at "an incredibly slow" rate of about 2% per year. "If you have a negative scan, you can be reassured that you’re not going to have Alzheimer’s disease for at least the next 10 years," Dr. Rowe said at a press conference at the meeting.
Because the half-life of PiB is only 20 minutes, it is not suitable for clinical use. The 2-hour half-life of florbetaben and other 18F amyloid radiotracers make them much more cost effective for clinical use, noted Dr. Rowe, director of the department of nuclear medicine and the center for PET and a consultant neurologist to the memory disorders clinic at the Austin Hospital in Melbourne, Australia.
The florbetaben study involved 45 patients with MCI who received PET imaging with the compound. At baseline, 53% had a high level of neocortical binding, and binding increased 3% after 2 years in those with already high levels. Overall, 75% of patients with elevated florbetaben binding progressed to Alzheimer’s disease, whereas 19% of those with a low level of binding progressed to other kinds of dementias.
MR imaging in the same individuals indicated that 53% of patients with hippocampal atrophy at baseline had progressed to Alzheimer’s. When the combination of high florbetaben binding and hippocampal atrophy was present, 80% had progressed to Alzheimer’s after 2 years.
"We don’t say that everybody who’s got Alzheimer’s disease should have these scans because I don’t think that would be cost effective. But in selected scenarios, when they’ve seen a memory specialist, I think these can be very useful clinically," Dr. Rowe said.
He said amyloid imaging agents such as florbetaben have two potential uses:
• In patients with established dementia when there is uncertainty about whether the patient has Alzheimer’s disease or frontotemporal dementia. He noted that this has therapeutic implications because some of the medications used to treat Alzheimer’s, such as cholinesterase inhibitors, can make behavioral symptoms worse in frontotemporal dementia.
• In patients with MCI who have seen a specialist who is not sure what the cause of the symptoms might be and wants to see if it might be Alzheimer’s instead of waiting years for dementia to develop. Because about 40% with MCI do not go on to develop dementia, amyloid imaging studies would be reassuring to those patients, Dr. Rowe said.
Guidelines from the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging (which recently changed its name from the Society of Nuclear Medicine) will soon be available on the appropriate use of the imaging agents, Dr. Rowe said in an interview.
He disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.
FROM THE ANNUAL MEETING OF THE SOCIETY OF NUCLEAR MEDICINE AND MOLECULAR IMAGING
Major Finding: Longitudinal tracking of the deposition of beta-amyloid over a period of 2-3 years with the use of PET imaging radiotracers in patients with mild cognitive impairment can help predict progression to Alzheimer’s disease or reliably rule it out as a diagnosis.
Data Source: Data were taken from two studies of the investigational agents 11C-Pittsburgh compound B (PiB) and 18F-florbetaben.
Disclosures: Dr. Rowe disclosed that he has served as an investigator on studies for many of the companies developing amyloid imaging products, including Avid Radiopharmaceuticals, Bayer Schering Pharma, GE Healthcare, and AstraZeneca. He has also received payments for consulting for Bayer and GE.