User login
Cardiac Risks of Newer Psoriasis Biologics vs. TNF Inhibitors Compared
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The newer biologics — .
METHODOLOGY:
- In a retrospective cohort study, researchers conducted an emulated target trial analysis using data of 32,098 biologic-naive patients with psoriasis or PsA who were treated with one of the newer biologics (infliximab, adalimumab, etanercept, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, and tildrakizumab) from the TriNetX Research Network between 2014 and 2022.
- Patients received TNF inhibitors (n = 20,314), IL-17 inhibitors (n = 5073), IL-12/23 inhibitors (n = 3573), or IL-23 inhibitors (n = 3138).
- A propensity-matched analysis compared each class of newer biologics with TNF inhibitors, adjusting for demographics, comorbidities, and medication use.
- The primary outcomes were major adverse cardiovascular events (MACE; myocardial infarction and stroke) or venous thromboembolic events (VTE).
TAKEAWAY:
- Compared with patients who received TNF inhibitors, the risk for MACE was not significantly different between patients who received IL-17 inhibitors (incidence rate ratio [IRR], 1.14; 95% CI, 0.86-1.52), IL-12/23 inhibitors (IRR, 1.24; 95% CI, 0.84-1.78), or IL-23 inhibitors (IRR, 0.93; 95% CI, 0.61-1.38)
- The VTE risk was also not significantly different between patients who received IL-17 inhibitors (IRR, 1.12; 95% CI, 0.63-2.08), IL-12/23 inhibitors (IRR, 1.51; 95% CI, 0.73-3.19), or IL-23 inhibitors (IRR, 1.42; 95% CI, 0.64-3.25) compared with those who received TNF inhibitors.
- Subgroup analyses for psoriasis or psoriatic arthritis alone confirmed consistent findings.
- Patients with preexisting hyperlipidemia and diabetes mellitus showed lower risks for MACE and VTE with newer biologics compared with TNF inhibitors.
IN PRACTICE:
“No significant MACE and VTE risk differences were detected in patients with psoriasis or PsA between those receiving IL-17, IL-12/23, and IL-23 inhibitors and those with TNF inhibitors,” the authors concluded. These findings, they added “can be considered by physicians and patients when making treatment decisions” and also provide “evidence for future pharmacovigilance studies.”
SOURCE:
The study was led by Tai-Li Chen, MD, of the Department of Dermatology, Taipei Veterans General Hospital in Taipei, Taiwan. It was published online on December 27, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Study limitations included potential residual confounding factors, lack of information on disease severity, and inclusion of predominantly White individuals.
DISCLOSURES:
The study received support from Taipei Veterans General Hospital and Ministry of Science and Technology, Taiwan. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by uncontrolled hyperglycemia. Over the past few decades, its prevalence has steadily increased, now affecting approximately 10% of adults worldwide and ranking among the top 10 leading causes of death globally.1 The pathophysiology of T2DM involves persistent hyperglycemia that drives insulin resistance and a progressive decline in insulin production from the pancreas.2 Medical management of this condition aims to reduce blood glucose levels or enhance insulin production and sensitivity. Aside from lifestyle modifications, metformin is considered the first-line treatment for glycemic control according to the 2023 American Association of Clinical Endocrinology’s T2DM management algorithm.3 These updated guidelines stratify adjunct treatments by individualized glycemic targets and patient needs. For patients who are overweight or obese, glucagonlike peptide 1 (GLP-1) and dual GLP-1/ gastric inhibitory polypeptide (GIP) agonists are the preferred adjunct or second-line treatments.3
In this review, we highlight the dermatologic adverse effects and potential therapeutic benefits of metformin as well as GLP-1 and GLP-1/GIP agonists.
METFORMIN
Metformin is a biguanide agent used as a first-line treatment for T2DM because of its ability to reduce hepatic glucose production and increase peripheral tissue glucose uptake.4 In addition to its effects on glucose, metformin has been shown to have anti-inflammatory properties via inhibition of the nuclear factor κB and mammalian target of rapamycin (mTOR) pathways, leading to decreased production of cytokines associated with T helper (Th) 1 and Th17 cell responses, such as IL-17, interferon gamma (IFN-γ), and tumor necrosis factor α (TNF-α).5-7 These findings have spurred interest among clinicians in the potential use of metformin for inflammatory conditions, including dermatologic diseases such as psoriasis and hidradenitis suppurativa (HS).8
Adverse Effects
Metformin is administered orally and generally is well tolerated. The most common adverse effects include gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain.9 While cutaneous adverse effects are rare, multiple dermatologic adverse reactions to metformin have been reported,10,11 including leukocytoclastic vasculitis,11-13 fixed drug eruptions,14-17 drug rash with eosinophilia and systemic symptoms (DRESS) syndrome,18 and photosensitivity reactions.19 Leukocytoclastic vasculitis and DRESS syndrome typically develop within the first month following metformin initiation, while fixed drug eruption and photosensitivity reactions have more variable timing, occurring weeks to years after treatment initiation.12-19
Dermatologic Implications
Acanthosis Nigricans—Acanthosis nigricans (AN) is characterized by hyperpigmentation and velvety skin thickening, typically in intertriginous areas such as the back of the neck, axillae, and groin.20 It commonly is associated with insulin resistance and obesity.21-23 Treatments for AN primarily center around insulin sensitivity and weight loss,24,25 with some benefit observed from the use of keratolytic agents.26,27 Metformin may have utility in treating AN through its effects on insulin sensitivity and glycemic control. Multiple case reports have noted marked improvements in AN in patients with and without obesity with the addition of metformin to their existing treatment regimens in doses ranging from 500 mg to 1700 mg daily.28-30 However, an unblinded randomized controlled trial (RCT) comparing the efficacy of metformin (500 mg 3 times daily) with rosiglitazone (4 mg/d), another T2DM medication, on AN neck lesions in patients who were overweight and obese found no significant effects in lesion severity and only modest improvements in skin texture in both groups at 12 weeks following treatment initiation.31 Another RCT comparing metformin (500 mg twice daily) with a twice-daily capsule containing α-lipoic acid, biotin, chromium polynicotinate, and zinc sulfate, showed significant (P<.001) improvements in AN neck lesions in both groups after 12 weeks.32 According to Sung et al,8 longer duration of therapy (>6 months), higher doses (1700–2000 mg), and lower baseline weight were associated with higher efficacy of metformin for treatment of AN. Overall, the use of metformin as an adjunct treatment for AN, particularly in patients with underlying hyperglycemia, is supported in the literature, but further studies are needed to clarify dosing, duration of therapy, and patient populations that will benefit most from adding metformin to their treatment regimens.
Hirsutism—Hirsutism, which is characterized by excessive hair growth in androgen-dependent areas, can be challenging to treat. Metformin has been shown to reduce circulating insulin, luteinizing hormone, androstenedione, and testosterone, thus improving underlying hyperandrogenism, particularly in patients with polycystic ovary syndrome (PCOS).33-35 Although single studies evaluating the efficacy of metformin for treatment of hirsutism in patients with PCOS have shown potential benefits,36-38 meta-analyses showed no significant effects of metformin compared to placebo or oral contraceptives and decreased benefits compared to spironolactone and flutamide.39 Given these findings showing that metformin was no more effective than placebo or other treatments, the current Endocrine Society guidelines recommend against the use of metformin for hirsutism.39,40 There may be a role for metformin as an adjuvant therapy in certain populations (eg, patients with comorbid T2DM), although further studies stratifying risk factors such as body mass index and age are needed.41
Hidradenitis Suppurativa—Hidradenitis suppurativa is a follicular occlusive disease characterized by recurrent inflamed nodules leading to chronic dermal abscesses, fibrosis, and sinus tract formation primarily in intertriginous areas such as the axillae and groin.42 Medical management depends on disease severity but usually involves antibiotic treatment with adjunct therapies such as oral contraceptives, antiandrogenic medications (eg, spironolactone), biologic medications, and metformin.42 Preclinical and clinical data suggest that metformin can impact HS through metabolic and immunomodulatory mechanisms.5,42 Like many chronic inflammatory disorders, HS is associated with metabolic syndrome.43,44 A study evaluating insulin secretion after oral glucose tolerance testing showed increased insulin levels in patients with HS compared to controls (P=.02), with 60% (6/10) of patients with HS meeting criteria for insulin resistance. In addition, serum insulin levels in insulin-resistant patients with HS correlated with increased lesional skin mTOR gene expression at 30 (r=.80) and 60 (r=1.00) minutes, and mTOR was found to be upregulated in lesional and extralesional skin in patients with HS compared to healthy controls (P<.01).45 Insulin activates mTOR signaling, which mediates cell growth and survival, among other processes.46 Thus, metformin’s ability to increase insulin sensitivity and inhibit mTOR signaling could be beneficial in the setting of HS. Additionally, insulin and insulinlike growth factor 1 (IGF-1) increase androgen signaling, a process that has been implicated in HS.47
Metformin also may impact HS through its effects on testosterone and other hormones.48 A study evaluating peripheral blood mononuclear cells in patients with HS showed reduced IL-17, IFN-γ, TNF-α, and IL-6 levels in patients who were taking metformin (dose not reported) for longer than 6 months compared to patients who were not on metformin. Further analysis of ex vivo HS lesions cultured with metformin showed decreased IL-17, IFN-γ, TNF-α, and IL-8 expression in tissue, suggesting an antiinflammatory role of metformin in HS.5
Although there are no known RCTs assessing the efficacy of metformin in HS, existing clinical data are supportive of the use of metformin for refractory HS.49 Following a case report describing a patient with T2DM and stable HS while on metformin,50 several cohort studies have assessed the efficacy of metformin for the treatment of HS. A prospective study evaluating the efficacy of metformin monotherapy (starting dose of 500 mg/d, titrated to 500 mg 3 times daily) in patients with and without T2DM with HS refractory to other therapies found clinical improvement in 72% (18/25) of patients using the Sartorius Hidradenitis Suppurativa Score, improving from a mean (SD) score of 34.40 (12.46) to 26.76 (11.22) at 12 weeks (P=.0055,) and 22.39 (11.30) at 24 weeks (P=.0001). Additionally, 64% (16/25) of patients showed improved quality of life as evaluated by the Dermatology Life Quality Index (DLQI), which decreased from a mean (SD) score of 15.00 (4.96) to 10.08 (5.96)(P=.0017) at 12 weeks and 7.65 (7.12)(P=.000009) at 24 weeks on treatment.48 In a retrospective study of 53 patients with HS taking metformin started at 500 mg daily and increased to 500 mg twice daily after 2 weeks (when tolerated), 68% (36/53) showed some clinical response, with 19% (7/36) of those patients having achieved complete response to metformin monotherapy (defined as no active HS).51 Similarly, a retrospective study of pediatric patients with HS evaluating metformin (doses ranging from 500-2000 mg daily) as an adjunct therapy described a subset of patients with decreased frequency of HS flares with metformin.52 These studies emphasize the safety profile of metformin and support its current use as an adjunctive therapy for HS.
Acne Vulgaris—Acne vulgaris (AV) is a chronic inflammatory disorder affecting the pilosebaceous follicles.11 Similar to HS, AV has metabolic and hormonal influences that can be targeted by metformin.53 In AV, androgens lead to increased sebum production by binding to androgen receptors on sebocytes, which in turn attracts Cutibacterium acnes and promotes hyperkeratinization, inducing inflammation.54 Thus, the antiandrogenic effects of metformin may be beneficial for treatment of AV. Additionally, sebocytes express receptors for insulin and IGF-1, which can increase the size and number of sebocytes, as well as promote lipogenesis and inflammatory response, influencing sebum production.54 Serum levels for IGF-1 have been observed to be increased in patients with AV55 and reduced by metformin.56 A recent meta-analysis assessing the efficacy of metformin on AV indicated that 87% (13/15) of studies noted disease improvement on metformin, with 47% (7/15) of studies showing statistically significant (P<0.05) decreases in acne severity.57 Although most studies showed improvement, 47% (7/15) did not find significant differences between metformin and other interventions, indicating the availability of comparable treatment options. Overall, there has been a positive association between metformin use and acne improvement.57 However, it is important to note that most studies have focused on females with PCOS,57 and the main benefits of metformin in acne might be seen when managing comorbid conditions, particularly those associated with metabolic dysregulation and insulin resistance. Further studies are needed to determine the generalizability of prior results.
Psoriasis—Psoriasis is a chronic autoinflammatory disease characterized by epidermal hyperplasia with multiple cutaneous manifestations and potential for multiorgan involvement. Comorbid conditions include psoriatic arthritis, metabolic syndrome, and cardiovascular disease.58 Current treatment options depend on several factors (eg, disease severity, location of cutaneous lesions, comorbidities) and include topical, systemic, and phototherapy options, many of which target the immune system.58,59 A meta-analysis of 3 RCTs showed that metformin (500 mg/d or 1000 mg/d) was associated with significantly improved Psoriasis Area and Severity Index (PASI) 75% reductions (odds ratio [OR], 22.02; 95% CI, 2.12-228.49; P=.01) and 75% reductions in erythema, scaling, and induration (OR, 9.12; 95% CI, 2.13-39.02; P=.003) compared to placebo.60 In addition, an RCT evaluating the efficacy of metformin (1000 mg/d) or pioglitazone (30 mg/d) for 12 weeks in patients with psoriasis with metabolic syndrome found significant improvements in PASI75 (P=.001) and erythema, scaling, and induration (P=.016) scores as well as in Physician Global Assessment scores (P=.012) compared to placebo and no differences compared to pioglitazone.61 While current psoriasis management guidelines do not include metformin, its use may be worth consideration as an adjunct therapy in patients with psoriasis and comorbidities such as T2DM and metabolic syndrome.59 Metformin’s potential benefits in psoriasis may lie outside its metabolic influences and occur secondary to its immunomodulatory effects, including targeting of the Th17 axis or cytokine-specific pathways such as TNF-α, which are known to be involved in psoriasis pathogenesis.58
Central Centrifugal Cicatricial Alopecia—Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia characterized by chronic inflammation leading to permanent loss of hair follicles on the crown of the scalp.62 Current treatments include topical and intralesional corticosteroids, as well as oral antibiotics. In addition, therapies including the antimalarial hydroxychloroquine and immunosuppressants mycophenolate and cyclosporine are used in refractory disease.63,64 A case report described 2 patients with hair regrowth after 4 and 6 months of treatment with topical metformin 10% compounded in a proprietary transdermal vehicle.65 The authors speculated that metformin’s effects on CCCA could be attributed to its known agonistic effects on the adenosine monophosphate-activated protein kinase (AMPK) pathway with subsequent reduction in inflammation-induced fibrosis.65,66 Microarray67 and proteomic68 analysis have shown that AMPK is known to be downregulated in CCCA , making it an interesting therapeutic target in this disease. A recent retrospective case series demonstrated that 67% (8/12) of patients with refractory CCCA had symptomatic improvement, and 50% (6/12) showed hair regrowth after 6 months of low-dose (500 mg/d) oral metformin treatment.62 In addition, metformin therapy showed antifibrotic and anti-inflammatory effects when comparing scalp biopsies before and after treatment. Results showed decreased expression of fibrosisrelated genes (matrix metalloproteinase 7, collagen type IV á 1 chain), and gene set variation analysis showing reduced Th17 (P=.04) and increased AMPK signaling (P=.02) gene set expression.62 These findings are consistent with previous studies describing the upregulation of AMPK66 and downregulation of Th176 following metformin treatment. The immunomodulatory effects of metformin could be attributed to AMPK-mediated mTOR and NF-κB downregulation,62 although more studies are needed to understand these mechanisms and further explore the use of metformin in CCCA.
Skin Cancer—Metformin also has been evaluated in the setting of skin malignancies, including melanoma, squamous cell carcinoma, and basal cell carcinoma. Preclinical data suggest that metformin decreases cell viability in tumors through interactions with pathways involved in proinflammatory and prosurvival mechanisms such as NF-κB and mTOR.69,70 Additionally, given metformin’s inhibitory effects on oxidative phosphorylation, it has been postulated that it could be used to overcome treatment resistance driven by metabolic reprogramming.71,72 Most studies related to metformin and skin malignancies are still in preclinical stages; however, a meta-analysis of RCTs and cohort studies did not find significant associations between metformin use and skin cancer risk, although data trended toward a modest reduction in skin cancer among metformin users.73 A retrospective cohort study of melanoma in patients with T2DM taking metformin (250-2000 mg/d) found that the 5-year incidence of recurrence was lower in the metformin cohort compared to nonusers (43.8% vs 58.2%, respectively)(P=.002), and overall survival rates trended upward in the higher body mass index (>30) and melanoma stages 1 and 2 groups but did not reach statistical significance.74 In addition, a whole population casecontrol study in Iceland reported that metformin use at least 2 years before first-time basal cell carcinoma diagnosis was associated with a lower risk for disease (adjusted OR, 0.71; 95% CI, 0.61-0.83) with no significant dose-dependent differences; there were no notable effects on squamous cell carcinoma risk.75 Further preclinical and clinical data are needed to elucidate metformin’s effects on skin malignancies.
GLP-1 AND DUAL GLP-1/GIP AGONISTS
Glucagonlike peptide 1 and dual GLP-1/GIP agonists are emerging classes of medications currently approved as adjunct and second-line therapies for T2DM, particularly in patients who are overweight or obese as well as in those who are at risk for hypoglycemia.3 Currently approved GLP-1 agonists for T2DM include semaglutide, dulaglutide, exenatide, liraglutide, and lixisenatide, while tirzepatide is the only approved dual GLP-1/GIP agonist. Activating GLP-1 and GIP receptors stimulates insulin secretion and decreases glucagon production by the pancreas, thereby reducing blood glucose levels. Additionally, some of these medications are approved for obesity given their effects in delayed gastric emptying and increased satiety, among other factors.
Over the past few years, multiple case reports have described the associations between GLP-1 agonist use and improvement of dermatologic conditions, particularly those associated with T2DM and obesity, including HS and psoriasis.76,77 The mechanisms through which this occurs are not fully elucidated, although basic science and clinical studies have shown that GLP-1 agonists have immunomodulatory effects by reducing proinflammatory cytokines and altering immune cell populations.77-80 The numerous ongoing clinical trials and research studies will help further elucidate their benefits in other disease settings.81
Adverse Reactions
Most GLP-1 and GLP-1/GIP agonists are administered subcutaneously, and the most commonly reported cutaneous adverse effects are injection site reactions.82 Anaphylactic reactions to these medications also have been reported, although it is unclear if these were specific to the active ingredients or to injection excipients.83,84 A review of 33 cases of cutaneous reactions to GLP-1 agonists reported 11 (33%) dermal hypersensitivity reactions occurring as early as 4 weeks and as late as 3 years after treatment initiation. It also described 10 (30%) cases of eosinophilic panniculitis that developed within 3 weeks to 5 months of GLP-1 treatment, 3 (9%) cases of bullous pemphigoid that occurred within the first 2 months, 2 (6%) morbilliform drug eruptions that occurred within 5 weeks, 2 (6%) cases of angioedema that occurred 15 minutes to 2 weeks after treatment initiation, and 7 (21%) other isolated cutaneous reactions. Extended-release exenatide had the most reported reactions followed by liraglutide and subcutaneous semaglutide.85
In a different study, semaglutide use was most commonly associated with injection site reactions followed by alopecia, especially with oral administration. Unique cases of angioedema (2 days after injection), cutaneous hypersensitivity (within 10 months on treatment), bullous pemphigoid (within 2 months on treatment), eosinophilic fasciitis (within 2 weeks on treatment), and leukocytoclastic vasculitis (unclear timing), most of which resolved after discontinuation, also were reported.86 A recent case report linked semaglutide (0.5 mg/wk) to a case of drug-induced systemic lupus erythematosus that developed within 3 months of treatment initiation and described systemic lupus erythematosus–like symptoms in a subset of patients using this medication, namely females older than 60 years, within the first month of treatment.87 Hyperhidrosis was listed as a common adverse event in exenatide clinical trials, and various cases of panniculitis with exenatide use have been reported.82,88 Alopecia, mainly attributed to accelerated telogen effluvium secondary to rapid weight loss, also has been reported, although hair loss is not officially listed as an adverse effect of GLP-1 agonists, and reports are highly variable.89 Also secondary to weight loss, facial changes including sunken eyes, development of wrinkles, sagging jowls around the neck and jaw, and a hollowed appearance, among others, are recognized as undesirable adverse effects.90 Mansour et al90 described the potential challenges and considerations to these rising concerns associated with GLP1-agonist use.
Dermatologic Implications
Hidradenitis Suppurativa—Weight loss commonly is recommended as a lifestyle modification in the management of HS. Multiple reports have described clinical improvement of HS following weight loss with other medical interventions, such as dietary measures and bariatric surgery.91-94 Thus, it has been postulated that medically supported weight loss with GLP-1 agonists can help improve HS95; however, the data on the effectiveness of GLP-1 agonists on HS are still scarce and mostly have been reported in individual patients. One case report described a patient with improvements in their recalcitrant HS and DLQI score following weight loss on liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d).76 In addition, a recent case report described improvements in HS and DLQI score following concomitant tirzepatide (initial dose of 2.5 mg/0.5 mL weekly, titrated to 7.5 mg/0.5 mL weekly) and infliximab treatment.96 The off-label use of these medications for HS is debated, and further studies regarding the benefits of GLP-1 agonists on HS still are needed.
Psoriasis—Similarly, several case reports have commented on the effects of GLP-1 agonists on psoriasis.97,98 An early study found GLP-1 receptors were expressed in psoriasis plaques but not in healthy skin and discussed that this could be due to immune infiltration in the plaques, providing a potential rationale for using anti-inflammatory GLP-1 agonists for psoriasis.99 Two prospective cohort studies observed improvements in PASI and DLQI scores in patients with psoriasis and T2DM after liraglutide treatment and noted important changes in immune cell populations.80,100 A recent RCT also found improvements in DLQI and PASI scores (P<.05) in patients with T2DM following liraglutide (1.8 mg/d) treatment, along with overall decreases in inflammatory cytokines, such as IL-23, IL-17, and TNF-α.77 However, another RCT in patients with obesity did not observe significant improvements in PASI and DLQI scores compared to placebo after 8 weeks of liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d) treatment. 99 Although these results could have been influenced by the short length of treatment compared to other studies, which observed participants for more than 10 weeks, they highlight the need for tailored studies considering the different comorbidities to identify patients who could benefit the most from these therapies.
Alopecia—Although some studies have reported increased rates of alopecia following GLP-1 agonist treatment, others have speculated about the potential role of these medications in treating hair loss through improved insulin sensitivity and scalp blood flow.86,89 For example, a case report described a patient with improvement in androgenetic alopecia within 6 months of tirzepatide monotherapy at 2.5 mg weekly for the first 3 months followed by an increased dose of 5 mg weekly.101 The authors described the role of insulin in increasing dihydrotestosterone levels, which leads to miniaturization of the dermal papilla of hair follicles and argued that improvement of insulin resistance could benefit hair loss. Further studies can help elucidate the role of these medications on alopecia.
FINAL THOUGHTS
Standard T2DM treatments including metformin and GLP-1 and GLP-1/GIP agonists exhibit metabolic, immunologic, and hormonal effects that should be explored in other disease contexts. We reviewed the current data on T2DM medications in dermatologic conditions to highlight the need for additional studies to better understand the role that these medications play across diverse patient populations. Type 2 diabetes mellitus is a common comorbidity in dermatology patients, and understanding the multifactorial effects of these medications can help optimize treatment strategies, especially in patients with coexisting dermatologic and metabolic diseases.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. doi:10.1038/nrendo.2017.151
- Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet. 2022;400: 1803-1820. doi:10.1016/s0140-6736(22)01655-5
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305-340. doi:10.1016/j.eprac.2023.02.001
- LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77-96. doi:10.1210/endrev/bnaa023
- Petrasca A, Hambly R, Kearney N, et al. Metformin has antiinflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br J Dermatol. 2023;189:730-740. doi:10.1093/bjd/ljad305
- Duan W, Ding Y, Yu X, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-2402.
- Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320:C873-C879. doi:10.1152 /ajpcell.00604.2020
- Sung CT, Chao T, Lee A, et al. Oral metformin for treating dermatological diseases: a systematic review. J Drugs Dermatol. 2020;19:713-720. doi:10.36849/jdd.2020.4874
- Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi:10.1016/j.phrs.2022.106114
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. Br Med J (Clin Res Ed). 1986;293:483. doi:10.1136/bmj.293.6545.483
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27:1329-1335. doi:10.1111/jdv.12116
- Czarnowicki T, Ramot Y, Ingber A, et al. Metformin-induced leukocytoclastic vasculitis: a case report. Am J Clin Dermatol. 2012;13:61-63. doi:10.2165/11593230-000000000-00000
- Ben Salem C, Hmouda H, Slim R, et al. Rare case of metformininduced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685-1687. doi:10.1345/aph.1H155
- Abtahi-Naeini B, Momen T, Amiri R, et al. Metformin-induced generalized bullous fixed-drug eruption with a positive dechallengerechallenge test: a case report and literature review. Case Rep Dermatol Med. 2023;2023:6353919. doi:10.1155/2023/6353919
- Al Masri D, Fleifel M, Hirbli K. Fixed drug eruption secondary to four anti-diabetic medications: an unusual case of polysensitivity. Cureus. 2021;13:E18599. doi:10.7759/cureus.18599
- Ramírez-Bellver JL, Lopez J, Macias E, et al. Metformin-induced generalized fixed drug eruption with cutaneous hemophagocytosis. Am J Dermatopathol. 2017;39:471-475. doi:10.1097/dad.0000000000000800
- Steber CJ, Perkins SL, Harris KB. Metformin-induced fixed-drug eruption confirmed by multiple exposures. Am J Case Rep. 2016;17:231-234. doi:10.12659/ajcr.896424
- Voore P, Odigwe C, Mirrakhimov AE, et al. DRESS syndrome following metformin administration: a case report and review of the literature. Am J Ther. 2016;23:E1970-E1973. doi:10.1097/mjt.0000000000000292
- Kastalli S, El Aïdli S, Chaabane A, et al. Photosensitivity induced by metformin: a report of 3 cases. Article in French. Tunis Med. 2009;87:703-705.
- Karadağ AS, You Y, Danarti R, et al. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36:48-53. doi:10.1016/j.clindermatol.2017.09.008
- Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202-208. doi:10.1370/afm.678
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79. doi:10.1177/000992289803700203
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Novotny R, Davis J, Butel J, et al. Effect of the Children’s Healthy Living Program on young child overweight, obesity, and acanthosis nigricans in the US-affiliated Pacific region: a randomized clinical trial. JAMA Netw Open. 2018;1:E183896. doi:10.1001/jamanetworkopen.2018.3896
- Romo A, Benavides S. Treatment options in insulin resistance obesityrelated acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094. doi:10.1345/aph.1K446
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 2021;32:837-842. doi:10.108 0/09546634.2019.1708855
- Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr Dermatol. 2019;36:330-334. doi:10.1111/pde.13799
- Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12-14. doi:10.1046 /j.1525-1470.2002.00013.x
- Walling HW, Messingham M, Myers LM, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677-681.
- Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and its response to metformin. Pediatr Dermatol. 2017;34:e281-e282. doi:10.1111/pde.13206
- Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5:884-889.
- Sett A, Pradhan S, Sancheti K, et al. Effectiveness and safety of metformin versus Canthex™ in patients with acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64:115-121. doi:10.4103/ijd.IJD_417_17
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2004;81:114-119. doi:10.1016 /j.fertnstert.2003.05.020
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17:51-56.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149-1154. doi:10.1016 /s0015-0282(00)00501-x
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221. doi:10.1530/eje.0.1470217
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123. doi:10.1210/jc.2003-030424
- Rezvanian H, Adibi N, Siavash M, et al. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218: 231-236. doi:10.1159/000187718
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-1142. doi:10.1210/jc.2007-2429
- Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257.
- Fraison E, Kostova E, Moran LJ, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi:10.1002/14651858.CD005552.pub3
- Hambly R, Kearney N, Hughes R, et al. Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. Int J Mol Sci. 2023;24:6969. doi:10.3390/ijms24086969
- Gold DA, Reeder VJ, Mahan MG, et al. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699-703. doi:10.1016/j.jaad.2013.11.014
- Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150: 1273-1280. doi:10.1001/jamadermatol.2014.1165
- Monfrecola G, Balato A, Caiazzo G, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631-1633. doi:10.1111/jdv.13233
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi:10.3390/nu9111176
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi:10.1111/j.1468-3083.2012.04668.x
- Tsentemeidou A, Vakirlis E, Papadimitriou I, et al. Metformin in hidradenitis suppurativa: is it worth pursuing further? Skin Appendage Disord. 2023;9:187-190. doi:10.1159/000529359
- Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920-921. doi:10.1111/j.1365-2230.2008.03121.x
- Jennings L, Hambly R, Hughes R, et al. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31:261-263. doi:10.1080/09546634 .2019.1592100
- Moussa C, Wadowski L, Price H, et al. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol. 2020;19:1231-1234. doi:10.36849/jdd.2020.5447
- Cho M, Woo YR, Cho SH, et al. Metformin: a potential treatment for acne, hidradenitis suppurativa and rosacea. Acta Derm Venereol. 2023;103:adv18392. doi:10.2340/actadv.v103.18392
- Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35:2298878. doi:10.1080/09546634.2023.2298878
- El-Tahlawi S, Ezzat Mohammad N, Mohamed El-Amir A, et al. Survivin and insulin-like growth factor-I: potential role in the pathogenesis of acne and post-acne scar. Scars Burn Heal. 2019;5:2059513118818031. doi:10.1177/2059513118818031
- Albalat W, Darwish H, Abd-Elaal WH, et al. The potential role of insulin-like growth factor 1 in acne vulgaris and its correlation with the clinical response before and after treatment with metformin. J Cosmet Dermatol. 2022;21:6209-6214. doi:10.1111/jocd.15210
- Nguyen S, Nguyen ML, Roberts WS, et al. The efficacy of metformin as a therapeutic agent in the treatment of acne vulgaris: a systematic review. Cureus. 2024;16:E56246. doi:10.7759/cureus.56246
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994. doi:10.1016 /s0140-6736(14)61909-7
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Huang Z, Li J, Chen H, et al. The efficacy of metformin for the treatment of psoriasis: a meta-analysis study. Postepy Dermatol Alergol. 2023;40:606-610. doi:10.5114/ada.2023.130524
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (topical treatment cohort). BMC Dermatol. 2016;16:12. doi:10.1186 /s12895-016-0049-y
- Bao A, Qadri A, Gadre A, et al. Low-dose metformin and profibrotic signature in central centrifugal cicatricial alopecia. JAMA Dermatol. 2024;E243062. doi:10.1001/jamadermatol.2024.3062
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j .jaad.2008.09.066
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j.jdcr.2019.12.008
- Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. doi:10.1016 /j.cmet.2014.09.018
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e1. doi:10.1016/j.jaad.2018.05.1257
- Gadre A, Dyson T, Jedrych J, et al. Proteomic profiling of central centrifugal cicatricial alopecia reveals role of humoral immune response pathway and metabolic dysregulation. JID Innov. 2024;4:100263. doi:10.1016/j.xjidi.2024.100263
- Chaudhary SC, Kurundkar D, Elmets CA, et al. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149-1156. doi:10.1111/j.1751-1097.2012.01165.x
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi:10.1038/cddis.2011.86
- Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
- Mascaraque M, Delgado-Wicke P, Nuevo-Tapioles C, et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel). 2020;12:668. doi:10.3390/cancers12030668
- Chang MS, Hartman RI, Xue J, et al. Risk of skin cancer associated with metformin use: a meta-analysis of randomized controlled trials and observational studies. Cancer Prev Res (Phila). 2021;14:77-84. doi:10.1158/1940-6207.Capr-20-0376
- Augustin RC, Huang Z, Ding F, et al. Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Front Oncol. 2023;13:1075823. doi:10.3389 /fonc.2023.1075823
- Adalsteinsson JA, Muzumdar S, Waldman R, et al. Metformin is associated with decreased risk of basal cell carcinoma: a whole-population casecontrol study from Iceland. J Am Acad Dermatol. 2021;85:56-61. doi:10.1016/j.jaad.2021.02.042
- Jennings L, Nestor L, Molloy O, et al. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol. 2017;177:858-859. doi:10.1111/bjd.15233
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33: 1428-1434. doi:10.1080/09546634.2020.1826392
- Karacabeyli D, Lacaille D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J Clin Rheumatol. 2024;30:26-31. doi:10.1097/rhu.0000000000001949
- Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107: 124-128. doi:10.1016/j.yexmp.2019.01.014
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal Υϛ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161. doi:10.1111/bjd.12886
- Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13:65. doi:10.3390/cells13010065
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230. doi:10.1900 /rds.2014.11.202
- He Z, Tabe AN, Rana S, et al. Tirzepatide-induced biphasic anaphylactic reaction: a case report. Cureus. 2023;15:e50112. doi:10.7759/cureus.50112
- Anthony MS, Aroda VR, Parlett LE, et al. Risk of anaphylaxis among new users of glp-1 receptor agonists: a cohort study. Diabetes Care. 2024;47:712-719. doi:10.2337/dc23-1911
- Salazar CE, Patil MK, Aihie O, et al. Rare cutaneous adverse reactions associated with GLP-1 agonists: a review of the published literature. Arch Dermatol Res. 2024;316:248. doi:10.1007/s00403-024-02969-3
- Tran MM, Mirza FN, Lee AC, et al. Dermatologic findings associated with semaglutide use: a scoping review. J Am Acad Dermatol. 2024;91:166-168. doi:10.1016/j.jaad.2024.03.021
- Castellanos V, Workneh H, Malik A, et al. Semaglutide-induced lupus erythematosus with multiorgan involvement. Cureus. 2024;16:E55324. doi:10.7759/cureus.55324
- Boccardi A, Shubrook JH. Cutaneous reactions to antidiabetic agents: a narrative review. Diabetology. 2022;3:97-107.
- Desai DD, Sikora M, Nohria A, et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63:1128-1130. doi:10.1111 /ijd.17246
- Mansour MR, Hannawa OM, Yaldo MM, et al. The rise of “Ozempic face”: analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J Plast Reconstr Aesthet Surg. 2024;96:225-227. doi:10.1016/j.bjps.2024.07.051
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64-72. doi:10.1177/1203475419874412
- Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol. 2016;30:895-896. doi:10.1111/jdv.13059
- Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol. 2014;39:315-7; quiz 317-8. doi:10.1111/ced.12269
- Mandour MO, Al-Musawi S, Idowu E, et al. Metabolic endoscopy and a simplified low-carbohydrate-high-dietary fiber template as novel treatments for hidradenitis suppurativa—a case series. JAAD Case Rep. 2023;34:23-26. doi:10.1016/j.jdcr.2023.01.035
- Henry T, Cahn B, Haber R, et al. Therapeutic potential of GLP-1 agonists for hidradenitis suppurativa. Int J Dermatol. 2023;62:1543-1544. doi:10.1111/ijd.16892
- Chan LJ, Kaur M, Kaffenberger BH. A case of recalcitrant hidradenitis suppurativa concomitantly treated with tirzepatide. JAAD Case Rep. 2024;52:101-102. doi:10.1016/j.jdcr.2024.02.023
- Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-00007. doi:10.1530 /edm-21-0007
- Buysschaert M, Tennstedt D, Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab. 2012;38:86-88. doi:10.1016/j.diabet.2011.11.004
- Faurschou A, Gyldenløve M, Rohde U, et al. Lack of effect of the glucagonlike peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J Eur Acad Dermatol Venereol. 2015;29:555-559. doi:10.1111/jdv.12629
- Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27:1440-1443. doi:10.1111/j.1468-3083.2012.04609.x
- Gordon ER, Musleh S, Bordone LA. Treatment of insulin resistance with tirzepatide leading to improvement of hair loss. JAAD Case Rep. 2024;50:123-125. doi:10.1016/j.jdcr.2024.06.001
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by uncontrolled hyperglycemia. Over the past few decades, its prevalence has steadily increased, now affecting approximately 10% of adults worldwide and ranking among the top 10 leading causes of death globally.1 The pathophysiology of T2DM involves persistent hyperglycemia that drives insulin resistance and a progressive decline in insulin production from the pancreas.2 Medical management of this condition aims to reduce blood glucose levels or enhance insulin production and sensitivity. Aside from lifestyle modifications, metformin is considered the first-line treatment for glycemic control according to the 2023 American Association of Clinical Endocrinology’s T2DM management algorithm.3 These updated guidelines stratify adjunct treatments by individualized glycemic targets and patient needs. For patients who are overweight or obese, glucagonlike peptide 1 (GLP-1) and dual GLP-1/ gastric inhibitory polypeptide (GIP) agonists are the preferred adjunct or second-line treatments.3
In this review, we highlight the dermatologic adverse effects and potential therapeutic benefits of metformin as well as GLP-1 and GLP-1/GIP agonists.
METFORMIN
Metformin is a biguanide agent used as a first-line treatment for T2DM because of its ability to reduce hepatic glucose production and increase peripheral tissue glucose uptake.4 In addition to its effects on glucose, metformin has been shown to have anti-inflammatory properties via inhibition of the nuclear factor κB and mammalian target of rapamycin (mTOR) pathways, leading to decreased production of cytokines associated with T helper (Th) 1 and Th17 cell responses, such as IL-17, interferon gamma (IFN-γ), and tumor necrosis factor α (TNF-α).5-7 These findings have spurred interest among clinicians in the potential use of metformin for inflammatory conditions, including dermatologic diseases such as psoriasis and hidradenitis suppurativa (HS).8
Adverse Effects
Metformin is administered orally and generally is well tolerated. The most common adverse effects include gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain.9 While cutaneous adverse effects are rare, multiple dermatologic adverse reactions to metformin have been reported,10,11 including leukocytoclastic vasculitis,11-13 fixed drug eruptions,14-17 drug rash with eosinophilia and systemic symptoms (DRESS) syndrome,18 and photosensitivity reactions.19 Leukocytoclastic vasculitis and DRESS syndrome typically develop within the first month following metformin initiation, while fixed drug eruption and photosensitivity reactions have more variable timing, occurring weeks to years after treatment initiation.12-19
Dermatologic Implications
Acanthosis Nigricans—Acanthosis nigricans (AN) is characterized by hyperpigmentation and velvety skin thickening, typically in intertriginous areas such as the back of the neck, axillae, and groin.20 It commonly is associated with insulin resistance and obesity.21-23 Treatments for AN primarily center around insulin sensitivity and weight loss,24,25 with some benefit observed from the use of keratolytic agents.26,27 Metformin may have utility in treating AN through its effects on insulin sensitivity and glycemic control. Multiple case reports have noted marked improvements in AN in patients with and without obesity with the addition of metformin to their existing treatment regimens in doses ranging from 500 mg to 1700 mg daily.28-30 However, an unblinded randomized controlled trial (RCT) comparing the efficacy of metformin (500 mg 3 times daily) with rosiglitazone (4 mg/d), another T2DM medication, on AN neck lesions in patients who were overweight and obese found no significant effects in lesion severity and only modest improvements in skin texture in both groups at 12 weeks following treatment initiation.31 Another RCT comparing metformin (500 mg twice daily) with a twice-daily capsule containing α-lipoic acid, biotin, chromium polynicotinate, and zinc sulfate, showed significant (P<.001) improvements in AN neck lesions in both groups after 12 weeks.32 According to Sung et al,8 longer duration of therapy (>6 months), higher doses (1700–2000 mg), and lower baseline weight were associated with higher efficacy of metformin for treatment of AN. Overall, the use of metformin as an adjunct treatment for AN, particularly in patients with underlying hyperglycemia, is supported in the literature, but further studies are needed to clarify dosing, duration of therapy, and patient populations that will benefit most from adding metformin to their treatment regimens.
Hirsutism—Hirsutism, which is characterized by excessive hair growth in androgen-dependent areas, can be challenging to treat. Metformin has been shown to reduce circulating insulin, luteinizing hormone, androstenedione, and testosterone, thus improving underlying hyperandrogenism, particularly in patients with polycystic ovary syndrome (PCOS).33-35 Although single studies evaluating the efficacy of metformin for treatment of hirsutism in patients with PCOS have shown potential benefits,36-38 meta-analyses showed no significant effects of metformin compared to placebo or oral contraceptives and decreased benefits compared to spironolactone and flutamide.39 Given these findings showing that metformin was no more effective than placebo or other treatments, the current Endocrine Society guidelines recommend against the use of metformin for hirsutism.39,40 There may be a role for metformin as an adjuvant therapy in certain populations (eg, patients with comorbid T2DM), although further studies stratifying risk factors such as body mass index and age are needed.41
Hidradenitis Suppurativa—Hidradenitis suppurativa is a follicular occlusive disease characterized by recurrent inflamed nodules leading to chronic dermal abscesses, fibrosis, and sinus tract formation primarily in intertriginous areas such as the axillae and groin.42 Medical management depends on disease severity but usually involves antibiotic treatment with adjunct therapies such as oral contraceptives, antiandrogenic medications (eg, spironolactone), biologic medications, and metformin.42 Preclinical and clinical data suggest that metformin can impact HS through metabolic and immunomodulatory mechanisms.5,42 Like many chronic inflammatory disorders, HS is associated with metabolic syndrome.43,44 A study evaluating insulin secretion after oral glucose tolerance testing showed increased insulin levels in patients with HS compared to controls (P=.02), with 60% (6/10) of patients with HS meeting criteria for insulin resistance. In addition, serum insulin levels in insulin-resistant patients with HS correlated with increased lesional skin mTOR gene expression at 30 (r=.80) and 60 (r=1.00) minutes, and mTOR was found to be upregulated in lesional and extralesional skin in patients with HS compared to healthy controls (P<.01).45 Insulin activates mTOR signaling, which mediates cell growth and survival, among other processes.46 Thus, metformin’s ability to increase insulin sensitivity and inhibit mTOR signaling could be beneficial in the setting of HS. Additionally, insulin and insulinlike growth factor 1 (IGF-1) increase androgen signaling, a process that has been implicated in HS.47
Metformin also may impact HS through its effects on testosterone and other hormones.48 A study evaluating peripheral blood mononuclear cells in patients with HS showed reduced IL-17, IFN-γ, TNF-α, and IL-6 levels in patients who were taking metformin (dose not reported) for longer than 6 months compared to patients who were not on metformin. Further analysis of ex vivo HS lesions cultured with metformin showed decreased IL-17, IFN-γ, TNF-α, and IL-8 expression in tissue, suggesting an antiinflammatory role of metformin in HS.5
Although there are no known RCTs assessing the efficacy of metformin in HS, existing clinical data are supportive of the use of metformin for refractory HS.49 Following a case report describing a patient with T2DM and stable HS while on metformin,50 several cohort studies have assessed the efficacy of metformin for the treatment of HS. A prospective study evaluating the efficacy of metformin monotherapy (starting dose of 500 mg/d, titrated to 500 mg 3 times daily) in patients with and without T2DM with HS refractory to other therapies found clinical improvement in 72% (18/25) of patients using the Sartorius Hidradenitis Suppurativa Score, improving from a mean (SD) score of 34.40 (12.46) to 26.76 (11.22) at 12 weeks (P=.0055,) and 22.39 (11.30) at 24 weeks (P=.0001). Additionally, 64% (16/25) of patients showed improved quality of life as evaluated by the Dermatology Life Quality Index (DLQI), which decreased from a mean (SD) score of 15.00 (4.96) to 10.08 (5.96)(P=.0017) at 12 weeks and 7.65 (7.12)(P=.000009) at 24 weeks on treatment.48 In a retrospective study of 53 patients with HS taking metformin started at 500 mg daily and increased to 500 mg twice daily after 2 weeks (when tolerated), 68% (36/53) showed some clinical response, with 19% (7/36) of those patients having achieved complete response to metformin monotherapy (defined as no active HS).51 Similarly, a retrospective study of pediatric patients with HS evaluating metformin (doses ranging from 500-2000 mg daily) as an adjunct therapy described a subset of patients with decreased frequency of HS flares with metformin.52 These studies emphasize the safety profile of metformin and support its current use as an adjunctive therapy for HS.
Acne Vulgaris—Acne vulgaris (AV) is a chronic inflammatory disorder affecting the pilosebaceous follicles.11 Similar to HS, AV has metabolic and hormonal influences that can be targeted by metformin.53 In AV, androgens lead to increased sebum production by binding to androgen receptors on sebocytes, which in turn attracts Cutibacterium acnes and promotes hyperkeratinization, inducing inflammation.54 Thus, the antiandrogenic effects of metformin may be beneficial for treatment of AV. Additionally, sebocytes express receptors for insulin and IGF-1, which can increase the size and number of sebocytes, as well as promote lipogenesis and inflammatory response, influencing sebum production.54 Serum levels for IGF-1 have been observed to be increased in patients with AV55 and reduced by metformin.56 A recent meta-analysis assessing the efficacy of metformin on AV indicated that 87% (13/15) of studies noted disease improvement on metformin, with 47% (7/15) of studies showing statistically significant (P<0.05) decreases in acne severity.57 Although most studies showed improvement, 47% (7/15) did not find significant differences between metformin and other interventions, indicating the availability of comparable treatment options. Overall, there has been a positive association between metformin use and acne improvement.57 However, it is important to note that most studies have focused on females with PCOS,57 and the main benefits of metformin in acne might be seen when managing comorbid conditions, particularly those associated with metabolic dysregulation and insulin resistance. Further studies are needed to determine the generalizability of prior results.
Psoriasis—Psoriasis is a chronic autoinflammatory disease characterized by epidermal hyperplasia with multiple cutaneous manifestations and potential for multiorgan involvement. Comorbid conditions include psoriatic arthritis, metabolic syndrome, and cardiovascular disease.58 Current treatment options depend on several factors (eg, disease severity, location of cutaneous lesions, comorbidities) and include topical, systemic, and phototherapy options, many of which target the immune system.58,59 A meta-analysis of 3 RCTs showed that metformin (500 mg/d or 1000 mg/d) was associated with significantly improved Psoriasis Area and Severity Index (PASI) 75% reductions (odds ratio [OR], 22.02; 95% CI, 2.12-228.49; P=.01) and 75% reductions in erythema, scaling, and induration (OR, 9.12; 95% CI, 2.13-39.02; P=.003) compared to placebo.60 In addition, an RCT evaluating the efficacy of metformin (1000 mg/d) or pioglitazone (30 mg/d) for 12 weeks in patients with psoriasis with metabolic syndrome found significant improvements in PASI75 (P=.001) and erythema, scaling, and induration (P=.016) scores as well as in Physician Global Assessment scores (P=.012) compared to placebo and no differences compared to pioglitazone.61 While current psoriasis management guidelines do not include metformin, its use may be worth consideration as an adjunct therapy in patients with psoriasis and comorbidities such as T2DM and metabolic syndrome.59 Metformin’s potential benefits in psoriasis may lie outside its metabolic influences and occur secondary to its immunomodulatory effects, including targeting of the Th17 axis or cytokine-specific pathways such as TNF-α, which are known to be involved in psoriasis pathogenesis.58
Central Centrifugal Cicatricial Alopecia—Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia characterized by chronic inflammation leading to permanent loss of hair follicles on the crown of the scalp.62 Current treatments include topical and intralesional corticosteroids, as well as oral antibiotics. In addition, therapies including the antimalarial hydroxychloroquine and immunosuppressants mycophenolate and cyclosporine are used in refractory disease.63,64 A case report described 2 patients with hair regrowth after 4 and 6 months of treatment with topical metformin 10% compounded in a proprietary transdermal vehicle.65 The authors speculated that metformin’s effects on CCCA could be attributed to its known agonistic effects on the adenosine monophosphate-activated protein kinase (AMPK) pathway with subsequent reduction in inflammation-induced fibrosis.65,66 Microarray67 and proteomic68 analysis have shown that AMPK is known to be downregulated in CCCA , making it an interesting therapeutic target in this disease. A recent retrospective case series demonstrated that 67% (8/12) of patients with refractory CCCA had symptomatic improvement, and 50% (6/12) showed hair regrowth after 6 months of low-dose (500 mg/d) oral metformin treatment.62 In addition, metformin therapy showed antifibrotic and anti-inflammatory effects when comparing scalp biopsies before and after treatment. Results showed decreased expression of fibrosisrelated genes (matrix metalloproteinase 7, collagen type IV á 1 chain), and gene set variation analysis showing reduced Th17 (P=.04) and increased AMPK signaling (P=.02) gene set expression.62 These findings are consistent with previous studies describing the upregulation of AMPK66 and downregulation of Th176 following metformin treatment. The immunomodulatory effects of metformin could be attributed to AMPK-mediated mTOR and NF-κB downregulation,62 although more studies are needed to understand these mechanisms and further explore the use of metformin in CCCA.
Skin Cancer—Metformin also has been evaluated in the setting of skin malignancies, including melanoma, squamous cell carcinoma, and basal cell carcinoma. Preclinical data suggest that metformin decreases cell viability in tumors through interactions with pathways involved in proinflammatory and prosurvival mechanisms such as NF-κB and mTOR.69,70 Additionally, given metformin’s inhibitory effects on oxidative phosphorylation, it has been postulated that it could be used to overcome treatment resistance driven by metabolic reprogramming.71,72 Most studies related to metformin and skin malignancies are still in preclinical stages; however, a meta-analysis of RCTs and cohort studies did not find significant associations between metformin use and skin cancer risk, although data trended toward a modest reduction in skin cancer among metformin users.73 A retrospective cohort study of melanoma in patients with T2DM taking metformin (250-2000 mg/d) found that the 5-year incidence of recurrence was lower in the metformin cohort compared to nonusers (43.8% vs 58.2%, respectively)(P=.002), and overall survival rates trended upward in the higher body mass index (>30) and melanoma stages 1 and 2 groups but did not reach statistical significance.74 In addition, a whole population casecontrol study in Iceland reported that metformin use at least 2 years before first-time basal cell carcinoma diagnosis was associated with a lower risk for disease (adjusted OR, 0.71; 95% CI, 0.61-0.83) with no significant dose-dependent differences; there were no notable effects on squamous cell carcinoma risk.75 Further preclinical and clinical data are needed to elucidate metformin’s effects on skin malignancies.
GLP-1 AND DUAL GLP-1/GIP AGONISTS
Glucagonlike peptide 1 and dual GLP-1/GIP agonists are emerging classes of medications currently approved as adjunct and second-line therapies for T2DM, particularly in patients who are overweight or obese as well as in those who are at risk for hypoglycemia.3 Currently approved GLP-1 agonists for T2DM include semaglutide, dulaglutide, exenatide, liraglutide, and lixisenatide, while tirzepatide is the only approved dual GLP-1/GIP agonist. Activating GLP-1 and GIP receptors stimulates insulin secretion and decreases glucagon production by the pancreas, thereby reducing blood glucose levels. Additionally, some of these medications are approved for obesity given their effects in delayed gastric emptying and increased satiety, among other factors.
Over the past few years, multiple case reports have described the associations between GLP-1 agonist use and improvement of dermatologic conditions, particularly those associated with T2DM and obesity, including HS and psoriasis.76,77 The mechanisms through which this occurs are not fully elucidated, although basic science and clinical studies have shown that GLP-1 agonists have immunomodulatory effects by reducing proinflammatory cytokines and altering immune cell populations.77-80 The numerous ongoing clinical trials and research studies will help further elucidate their benefits in other disease settings.81
Adverse Reactions
Most GLP-1 and GLP-1/GIP agonists are administered subcutaneously, and the most commonly reported cutaneous adverse effects are injection site reactions.82 Anaphylactic reactions to these medications also have been reported, although it is unclear if these were specific to the active ingredients or to injection excipients.83,84 A review of 33 cases of cutaneous reactions to GLP-1 agonists reported 11 (33%) dermal hypersensitivity reactions occurring as early as 4 weeks and as late as 3 years after treatment initiation. It also described 10 (30%) cases of eosinophilic panniculitis that developed within 3 weeks to 5 months of GLP-1 treatment, 3 (9%) cases of bullous pemphigoid that occurred within the first 2 months, 2 (6%) morbilliform drug eruptions that occurred within 5 weeks, 2 (6%) cases of angioedema that occurred 15 minutes to 2 weeks after treatment initiation, and 7 (21%) other isolated cutaneous reactions. Extended-release exenatide had the most reported reactions followed by liraglutide and subcutaneous semaglutide.85
In a different study, semaglutide use was most commonly associated with injection site reactions followed by alopecia, especially with oral administration. Unique cases of angioedema (2 days after injection), cutaneous hypersensitivity (within 10 months on treatment), bullous pemphigoid (within 2 months on treatment), eosinophilic fasciitis (within 2 weeks on treatment), and leukocytoclastic vasculitis (unclear timing), most of which resolved after discontinuation, also were reported.86 A recent case report linked semaglutide (0.5 mg/wk) to a case of drug-induced systemic lupus erythematosus that developed within 3 months of treatment initiation and described systemic lupus erythematosus–like symptoms in a subset of patients using this medication, namely females older than 60 years, within the first month of treatment.87 Hyperhidrosis was listed as a common adverse event in exenatide clinical trials, and various cases of panniculitis with exenatide use have been reported.82,88 Alopecia, mainly attributed to accelerated telogen effluvium secondary to rapid weight loss, also has been reported, although hair loss is not officially listed as an adverse effect of GLP-1 agonists, and reports are highly variable.89 Also secondary to weight loss, facial changes including sunken eyes, development of wrinkles, sagging jowls around the neck and jaw, and a hollowed appearance, among others, are recognized as undesirable adverse effects.90 Mansour et al90 described the potential challenges and considerations to these rising concerns associated with GLP1-agonist use.
Dermatologic Implications
Hidradenitis Suppurativa—Weight loss commonly is recommended as a lifestyle modification in the management of HS. Multiple reports have described clinical improvement of HS following weight loss with other medical interventions, such as dietary measures and bariatric surgery.91-94 Thus, it has been postulated that medically supported weight loss with GLP-1 agonists can help improve HS95; however, the data on the effectiveness of GLP-1 agonists on HS are still scarce and mostly have been reported in individual patients. One case report described a patient with improvements in their recalcitrant HS and DLQI score following weight loss on liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d).76 In addition, a recent case report described improvements in HS and DLQI score following concomitant tirzepatide (initial dose of 2.5 mg/0.5 mL weekly, titrated to 7.5 mg/0.5 mL weekly) and infliximab treatment.96 The off-label use of these medications for HS is debated, and further studies regarding the benefits of GLP-1 agonists on HS still are needed.
Psoriasis—Similarly, several case reports have commented on the effects of GLP-1 agonists on psoriasis.97,98 An early study found GLP-1 receptors were expressed in psoriasis plaques but not in healthy skin and discussed that this could be due to immune infiltration in the plaques, providing a potential rationale for using anti-inflammatory GLP-1 agonists for psoriasis.99 Two prospective cohort studies observed improvements in PASI and DLQI scores in patients with psoriasis and T2DM after liraglutide treatment and noted important changes in immune cell populations.80,100 A recent RCT also found improvements in DLQI and PASI scores (P<.05) in patients with T2DM following liraglutide (1.8 mg/d) treatment, along with overall decreases in inflammatory cytokines, such as IL-23, IL-17, and TNF-α.77 However, another RCT in patients with obesity did not observe significant improvements in PASI and DLQI scores compared to placebo after 8 weeks of liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d) treatment. 99 Although these results could have been influenced by the short length of treatment compared to other studies, which observed participants for more than 10 weeks, they highlight the need for tailored studies considering the different comorbidities to identify patients who could benefit the most from these therapies.
Alopecia—Although some studies have reported increased rates of alopecia following GLP-1 agonist treatment, others have speculated about the potential role of these medications in treating hair loss through improved insulin sensitivity and scalp blood flow.86,89 For example, a case report described a patient with improvement in androgenetic alopecia within 6 months of tirzepatide monotherapy at 2.5 mg weekly for the first 3 months followed by an increased dose of 5 mg weekly.101 The authors described the role of insulin in increasing dihydrotestosterone levels, which leads to miniaturization of the dermal papilla of hair follicles and argued that improvement of insulin resistance could benefit hair loss. Further studies can help elucidate the role of these medications on alopecia.
FINAL THOUGHTS
Standard T2DM treatments including metformin and GLP-1 and GLP-1/GIP agonists exhibit metabolic, immunologic, and hormonal effects that should be explored in other disease contexts. We reviewed the current data on T2DM medications in dermatologic conditions to highlight the need for additional studies to better understand the role that these medications play across diverse patient populations. Type 2 diabetes mellitus is a common comorbidity in dermatology patients, and understanding the multifactorial effects of these medications can help optimize treatment strategies, especially in patients with coexisting dermatologic and metabolic diseases.
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by uncontrolled hyperglycemia. Over the past few decades, its prevalence has steadily increased, now affecting approximately 10% of adults worldwide and ranking among the top 10 leading causes of death globally.1 The pathophysiology of T2DM involves persistent hyperglycemia that drives insulin resistance and a progressive decline in insulin production from the pancreas.2 Medical management of this condition aims to reduce blood glucose levels or enhance insulin production and sensitivity. Aside from lifestyle modifications, metformin is considered the first-line treatment for glycemic control according to the 2023 American Association of Clinical Endocrinology’s T2DM management algorithm.3 These updated guidelines stratify adjunct treatments by individualized glycemic targets and patient needs. For patients who are overweight or obese, glucagonlike peptide 1 (GLP-1) and dual GLP-1/ gastric inhibitory polypeptide (GIP) agonists are the preferred adjunct or second-line treatments.3
In this review, we highlight the dermatologic adverse effects and potential therapeutic benefits of metformin as well as GLP-1 and GLP-1/GIP agonists.
METFORMIN
Metformin is a biguanide agent used as a first-line treatment for T2DM because of its ability to reduce hepatic glucose production and increase peripheral tissue glucose uptake.4 In addition to its effects on glucose, metformin has been shown to have anti-inflammatory properties via inhibition of the nuclear factor κB and mammalian target of rapamycin (mTOR) pathways, leading to decreased production of cytokines associated with T helper (Th) 1 and Th17 cell responses, such as IL-17, interferon gamma (IFN-γ), and tumor necrosis factor α (TNF-α).5-7 These findings have spurred interest among clinicians in the potential use of metformin for inflammatory conditions, including dermatologic diseases such as psoriasis and hidradenitis suppurativa (HS).8
Adverse Effects
Metformin is administered orally and generally is well tolerated. The most common adverse effects include gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain.9 While cutaneous adverse effects are rare, multiple dermatologic adverse reactions to metformin have been reported,10,11 including leukocytoclastic vasculitis,11-13 fixed drug eruptions,14-17 drug rash with eosinophilia and systemic symptoms (DRESS) syndrome,18 and photosensitivity reactions.19 Leukocytoclastic vasculitis and DRESS syndrome typically develop within the first month following metformin initiation, while fixed drug eruption and photosensitivity reactions have more variable timing, occurring weeks to years after treatment initiation.12-19
Dermatologic Implications
Acanthosis Nigricans—Acanthosis nigricans (AN) is characterized by hyperpigmentation and velvety skin thickening, typically in intertriginous areas such as the back of the neck, axillae, and groin.20 It commonly is associated with insulin resistance and obesity.21-23 Treatments for AN primarily center around insulin sensitivity and weight loss,24,25 with some benefit observed from the use of keratolytic agents.26,27 Metformin may have utility in treating AN through its effects on insulin sensitivity and glycemic control. Multiple case reports have noted marked improvements in AN in patients with and without obesity with the addition of metformin to their existing treatment regimens in doses ranging from 500 mg to 1700 mg daily.28-30 However, an unblinded randomized controlled trial (RCT) comparing the efficacy of metformin (500 mg 3 times daily) with rosiglitazone (4 mg/d), another T2DM medication, on AN neck lesions in patients who were overweight and obese found no significant effects in lesion severity and only modest improvements in skin texture in both groups at 12 weeks following treatment initiation.31 Another RCT comparing metformin (500 mg twice daily) with a twice-daily capsule containing α-lipoic acid, biotin, chromium polynicotinate, and zinc sulfate, showed significant (P<.001) improvements in AN neck lesions in both groups after 12 weeks.32 According to Sung et al,8 longer duration of therapy (>6 months), higher doses (1700–2000 mg), and lower baseline weight were associated with higher efficacy of metformin for treatment of AN. Overall, the use of metformin as an adjunct treatment for AN, particularly in patients with underlying hyperglycemia, is supported in the literature, but further studies are needed to clarify dosing, duration of therapy, and patient populations that will benefit most from adding metformin to their treatment regimens.
Hirsutism—Hirsutism, which is characterized by excessive hair growth in androgen-dependent areas, can be challenging to treat. Metformin has been shown to reduce circulating insulin, luteinizing hormone, androstenedione, and testosterone, thus improving underlying hyperandrogenism, particularly in patients with polycystic ovary syndrome (PCOS).33-35 Although single studies evaluating the efficacy of metformin for treatment of hirsutism in patients with PCOS have shown potential benefits,36-38 meta-analyses showed no significant effects of metformin compared to placebo or oral contraceptives and decreased benefits compared to spironolactone and flutamide.39 Given these findings showing that metformin was no more effective than placebo or other treatments, the current Endocrine Society guidelines recommend against the use of metformin for hirsutism.39,40 There may be a role for metformin as an adjuvant therapy in certain populations (eg, patients with comorbid T2DM), although further studies stratifying risk factors such as body mass index and age are needed.41
Hidradenitis Suppurativa—Hidradenitis suppurativa is a follicular occlusive disease characterized by recurrent inflamed nodules leading to chronic dermal abscesses, fibrosis, and sinus tract formation primarily in intertriginous areas such as the axillae and groin.42 Medical management depends on disease severity but usually involves antibiotic treatment with adjunct therapies such as oral contraceptives, antiandrogenic medications (eg, spironolactone), biologic medications, and metformin.42 Preclinical and clinical data suggest that metformin can impact HS through metabolic and immunomodulatory mechanisms.5,42 Like many chronic inflammatory disorders, HS is associated with metabolic syndrome.43,44 A study evaluating insulin secretion after oral glucose tolerance testing showed increased insulin levels in patients with HS compared to controls (P=.02), with 60% (6/10) of patients with HS meeting criteria for insulin resistance. In addition, serum insulin levels in insulin-resistant patients with HS correlated with increased lesional skin mTOR gene expression at 30 (r=.80) and 60 (r=1.00) minutes, and mTOR was found to be upregulated in lesional and extralesional skin in patients with HS compared to healthy controls (P<.01).45 Insulin activates mTOR signaling, which mediates cell growth and survival, among other processes.46 Thus, metformin’s ability to increase insulin sensitivity and inhibit mTOR signaling could be beneficial in the setting of HS. Additionally, insulin and insulinlike growth factor 1 (IGF-1) increase androgen signaling, a process that has been implicated in HS.47
Metformin also may impact HS through its effects on testosterone and other hormones.48 A study evaluating peripheral blood mononuclear cells in patients with HS showed reduced IL-17, IFN-γ, TNF-α, and IL-6 levels in patients who were taking metformin (dose not reported) for longer than 6 months compared to patients who were not on metformin. Further analysis of ex vivo HS lesions cultured with metformin showed decreased IL-17, IFN-γ, TNF-α, and IL-8 expression in tissue, suggesting an antiinflammatory role of metformin in HS.5
Although there are no known RCTs assessing the efficacy of metformin in HS, existing clinical data are supportive of the use of metformin for refractory HS.49 Following a case report describing a patient with T2DM and stable HS while on metformin,50 several cohort studies have assessed the efficacy of metformin for the treatment of HS. A prospective study evaluating the efficacy of metformin monotherapy (starting dose of 500 mg/d, titrated to 500 mg 3 times daily) in patients with and without T2DM with HS refractory to other therapies found clinical improvement in 72% (18/25) of patients using the Sartorius Hidradenitis Suppurativa Score, improving from a mean (SD) score of 34.40 (12.46) to 26.76 (11.22) at 12 weeks (P=.0055,) and 22.39 (11.30) at 24 weeks (P=.0001). Additionally, 64% (16/25) of patients showed improved quality of life as evaluated by the Dermatology Life Quality Index (DLQI), which decreased from a mean (SD) score of 15.00 (4.96) to 10.08 (5.96)(P=.0017) at 12 weeks and 7.65 (7.12)(P=.000009) at 24 weeks on treatment.48 In a retrospective study of 53 patients with HS taking metformin started at 500 mg daily and increased to 500 mg twice daily after 2 weeks (when tolerated), 68% (36/53) showed some clinical response, with 19% (7/36) of those patients having achieved complete response to metformin monotherapy (defined as no active HS).51 Similarly, a retrospective study of pediatric patients with HS evaluating metformin (doses ranging from 500-2000 mg daily) as an adjunct therapy described a subset of patients with decreased frequency of HS flares with metformin.52 These studies emphasize the safety profile of metformin and support its current use as an adjunctive therapy for HS.
Acne Vulgaris—Acne vulgaris (AV) is a chronic inflammatory disorder affecting the pilosebaceous follicles.11 Similar to HS, AV has metabolic and hormonal influences that can be targeted by metformin.53 In AV, androgens lead to increased sebum production by binding to androgen receptors on sebocytes, which in turn attracts Cutibacterium acnes and promotes hyperkeratinization, inducing inflammation.54 Thus, the antiandrogenic effects of metformin may be beneficial for treatment of AV. Additionally, sebocytes express receptors for insulin and IGF-1, which can increase the size and number of sebocytes, as well as promote lipogenesis and inflammatory response, influencing sebum production.54 Serum levels for IGF-1 have been observed to be increased in patients with AV55 and reduced by metformin.56 A recent meta-analysis assessing the efficacy of metformin on AV indicated that 87% (13/15) of studies noted disease improvement on metformin, with 47% (7/15) of studies showing statistically significant (P<0.05) decreases in acne severity.57 Although most studies showed improvement, 47% (7/15) did not find significant differences between metformin and other interventions, indicating the availability of comparable treatment options. Overall, there has been a positive association between metformin use and acne improvement.57 However, it is important to note that most studies have focused on females with PCOS,57 and the main benefits of metformin in acne might be seen when managing comorbid conditions, particularly those associated with metabolic dysregulation and insulin resistance. Further studies are needed to determine the generalizability of prior results.
Psoriasis—Psoriasis is a chronic autoinflammatory disease characterized by epidermal hyperplasia with multiple cutaneous manifestations and potential for multiorgan involvement. Comorbid conditions include psoriatic arthritis, metabolic syndrome, and cardiovascular disease.58 Current treatment options depend on several factors (eg, disease severity, location of cutaneous lesions, comorbidities) and include topical, systemic, and phototherapy options, many of which target the immune system.58,59 A meta-analysis of 3 RCTs showed that metformin (500 mg/d or 1000 mg/d) was associated with significantly improved Psoriasis Area and Severity Index (PASI) 75% reductions (odds ratio [OR], 22.02; 95% CI, 2.12-228.49; P=.01) and 75% reductions in erythema, scaling, and induration (OR, 9.12; 95% CI, 2.13-39.02; P=.003) compared to placebo.60 In addition, an RCT evaluating the efficacy of metformin (1000 mg/d) or pioglitazone (30 mg/d) for 12 weeks in patients with psoriasis with metabolic syndrome found significant improvements in PASI75 (P=.001) and erythema, scaling, and induration (P=.016) scores as well as in Physician Global Assessment scores (P=.012) compared to placebo and no differences compared to pioglitazone.61 While current psoriasis management guidelines do not include metformin, its use may be worth consideration as an adjunct therapy in patients with psoriasis and comorbidities such as T2DM and metabolic syndrome.59 Metformin’s potential benefits in psoriasis may lie outside its metabolic influences and occur secondary to its immunomodulatory effects, including targeting of the Th17 axis or cytokine-specific pathways such as TNF-α, which are known to be involved in psoriasis pathogenesis.58
Central Centrifugal Cicatricial Alopecia—Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia characterized by chronic inflammation leading to permanent loss of hair follicles on the crown of the scalp.62 Current treatments include topical and intralesional corticosteroids, as well as oral antibiotics. In addition, therapies including the antimalarial hydroxychloroquine and immunosuppressants mycophenolate and cyclosporine are used in refractory disease.63,64 A case report described 2 patients with hair regrowth after 4 and 6 months of treatment with topical metformin 10% compounded in a proprietary transdermal vehicle.65 The authors speculated that metformin’s effects on CCCA could be attributed to its known agonistic effects on the adenosine monophosphate-activated protein kinase (AMPK) pathway with subsequent reduction in inflammation-induced fibrosis.65,66 Microarray67 and proteomic68 analysis have shown that AMPK is known to be downregulated in CCCA , making it an interesting therapeutic target in this disease. A recent retrospective case series demonstrated that 67% (8/12) of patients with refractory CCCA had symptomatic improvement, and 50% (6/12) showed hair regrowth after 6 months of low-dose (500 mg/d) oral metformin treatment.62 In addition, metformin therapy showed antifibrotic and anti-inflammatory effects when comparing scalp biopsies before and after treatment. Results showed decreased expression of fibrosisrelated genes (matrix metalloproteinase 7, collagen type IV á 1 chain), and gene set variation analysis showing reduced Th17 (P=.04) and increased AMPK signaling (P=.02) gene set expression.62 These findings are consistent with previous studies describing the upregulation of AMPK66 and downregulation of Th176 following metformin treatment. The immunomodulatory effects of metformin could be attributed to AMPK-mediated mTOR and NF-κB downregulation,62 although more studies are needed to understand these mechanisms and further explore the use of metformin in CCCA.
Skin Cancer—Metformin also has been evaluated in the setting of skin malignancies, including melanoma, squamous cell carcinoma, and basal cell carcinoma. Preclinical data suggest that metformin decreases cell viability in tumors through interactions with pathways involved in proinflammatory and prosurvival mechanisms such as NF-κB and mTOR.69,70 Additionally, given metformin’s inhibitory effects on oxidative phosphorylation, it has been postulated that it could be used to overcome treatment resistance driven by metabolic reprogramming.71,72 Most studies related to metformin and skin malignancies are still in preclinical stages; however, a meta-analysis of RCTs and cohort studies did not find significant associations between metformin use and skin cancer risk, although data trended toward a modest reduction in skin cancer among metformin users.73 A retrospective cohort study of melanoma in patients with T2DM taking metformin (250-2000 mg/d) found that the 5-year incidence of recurrence was lower in the metformin cohort compared to nonusers (43.8% vs 58.2%, respectively)(P=.002), and overall survival rates trended upward in the higher body mass index (>30) and melanoma stages 1 and 2 groups but did not reach statistical significance.74 In addition, a whole population casecontrol study in Iceland reported that metformin use at least 2 years before first-time basal cell carcinoma diagnosis was associated with a lower risk for disease (adjusted OR, 0.71; 95% CI, 0.61-0.83) with no significant dose-dependent differences; there were no notable effects on squamous cell carcinoma risk.75 Further preclinical and clinical data are needed to elucidate metformin’s effects on skin malignancies.
GLP-1 AND DUAL GLP-1/GIP AGONISTS
Glucagonlike peptide 1 and dual GLP-1/GIP agonists are emerging classes of medications currently approved as adjunct and second-line therapies for T2DM, particularly in patients who are overweight or obese as well as in those who are at risk for hypoglycemia.3 Currently approved GLP-1 agonists for T2DM include semaglutide, dulaglutide, exenatide, liraglutide, and lixisenatide, while tirzepatide is the only approved dual GLP-1/GIP agonist. Activating GLP-1 and GIP receptors stimulates insulin secretion and decreases glucagon production by the pancreas, thereby reducing blood glucose levels. Additionally, some of these medications are approved for obesity given their effects in delayed gastric emptying and increased satiety, among other factors.
Over the past few years, multiple case reports have described the associations between GLP-1 agonist use and improvement of dermatologic conditions, particularly those associated with T2DM and obesity, including HS and psoriasis.76,77 The mechanisms through which this occurs are not fully elucidated, although basic science and clinical studies have shown that GLP-1 agonists have immunomodulatory effects by reducing proinflammatory cytokines and altering immune cell populations.77-80 The numerous ongoing clinical trials and research studies will help further elucidate their benefits in other disease settings.81
Adverse Reactions
Most GLP-1 and GLP-1/GIP agonists are administered subcutaneously, and the most commonly reported cutaneous adverse effects are injection site reactions.82 Anaphylactic reactions to these medications also have been reported, although it is unclear if these were specific to the active ingredients or to injection excipients.83,84 A review of 33 cases of cutaneous reactions to GLP-1 agonists reported 11 (33%) dermal hypersensitivity reactions occurring as early as 4 weeks and as late as 3 years after treatment initiation. It also described 10 (30%) cases of eosinophilic panniculitis that developed within 3 weeks to 5 months of GLP-1 treatment, 3 (9%) cases of bullous pemphigoid that occurred within the first 2 months, 2 (6%) morbilliform drug eruptions that occurred within 5 weeks, 2 (6%) cases of angioedema that occurred 15 minutes to 2 weeks after treatment initiation, and 7 (21%) other isolated cutaneous reactions. Extended-release exenatide had the most reported reactions followed by liraglutide and subcutaneous semaglutide.85
In a different study, semaglutide use was most commonly associated with injection site reactions followed by alopecia, especially with oral administration. Unique cases of angioedema (2 days after injection), cutaneous hypersensitivity (within 10 months on treatment), bullous pemphigoid (within 2 months on treatment), eosinophilic fasciitis (within 2 weeks on treatment), and leukocytoclastic vasculitis (unclear timing), most of which resolved after discontinuation, also were reported.86 A recent case report linked semaglutide (0.5 mg/wk) to a case of drug-induced systemic lupus erythematosus that developed within 3 months of treatment initiation and described systemic lupus erythematosus–like symptoms in a subset of patients using this medication, namely females older than 60 years, within the first month of treatment.87 Hyperhidrosis was listed as a common adverse event in exenatide clinical trials, and various cases of panniculitis with exenatide use have been reported.82,88 Alopecia, mainly attributed to accelerated telogen effluvium secondary to rapid weight loss, also has been reported, although hair loss is not officially listed as an adverse effect of GLP-1 agonists, and reports are highly variable.89 Also secondary to weight loss, facial changes including sunken eyes, development of wrinkles, sagging jowls around the neck and jaw, and a hollowed appearance, among others, are recognized as undesirable adverse effects.90 Mansour et al90 described the potential challenges and considerations to these rising concerns associated with GLP1-agonist use.
Dermatologic Implications
Hidradenitis Suppurativa—Weight loss commonly is recommended as a lifestyle modification in the management of HS. Multiple reports have described clinical improvement of HS following weight loss with other medical interventions, such as dietary measures and bariatric surgery.91-94 Thus, it has been postulated that medically supported weight loss with GLP-1 agonists can help improve HS95; however, the data on the effectiveness of GLP-1 agonists on HS are still scarce and mostly have been reported in individual patients. One case report described a patient with improvements in their recalcitrant HS and DLQI score following weight loss on liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d).76 In addition, a recent case report described improvements in HS and DLQI score following concomitant tirzepatide (initial dose of 2.5 mg/0.5 mL weekly, titrated to 7.5 mg/0.5 mL weekly) and infliximab treatment.96 The off-label use of these medications for HS is debated, and further studies regarding the benefits of GLP-1 agonists on HS still are needed.
Psoriasis—Similarly, several case reports have commented on the effects of GLP-1 agonists on psoriasis.97,98 An early study found GLP-1 receptors were expressed in psoriasis plaques but not in healthy skin and discussed that this could be due to immune infiltration in the plaques, providing a potential rationale for using anti-inflammatory GLP-1 agonists for psoriasis.99 Two prospective cohort studies observed improvements in PASI and DLQI scores in patients with psoriasis and T2DM after liraglutide treatment and noted important changes in immune cell populations.80,100 A recent RCT also found improvements in DLQI and PASI scores (P<.05) in patients with T2DM following liraglutide (1.8 mg/d) treatment, along with overall decreases in inflammatory cytokines, such as IL-23, IL-17, and TNF-α.77 However, another RCT in patients with obesity did not observe significant improvements in PASI and DLQI scores compared to placebo after 8 weeks of liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d) treatment. 99 Although these results could have been influenced by the short length of treatment compared to other studies, which observed participants for more than 10 weeks, they highlight the need for tailored studies considering the different comorbidities to identify patients who could benefit the most from these therapies.
Alopecia—Although some studies have reported increased rates of alopecia following GLP-1 agonist treatment, others have speculated about the potential role of these medications in treating hair loss through improved insulin sensitivity and scalp blood flow.86,89 For example, a case report described a patient with improvement in androgenetic alopecia within 6 months of tirzepatide monotherapy at 2.5 mg weekly for the first 3 months followed by an increased dose of 5 mg weekly.101 The authors described the role of insulin in increasing dihydrotestosterone levels, which leads to miniaturization of the dermal papilla of hair follicles and argued that improvement of insulin resistance could benefit hair loss. Further studies can help elucidate the role of these medications on alopecia.
FINAL THOUGHTS
Standard T2DM treatments including metformin and GLP-1 and GLP-1/GIP agonists exhibit metabolic, immunologic, and hormonal effects that should be explored in other disease contexts. We reviewed the current data on T2DM medications in dermatologic conditions to highlight the need for additional studies to better understand the role that these medications play across diverse patient populations. Type 2 diabetes mellitus is a common comorbidity in dermatology patients, and understanding the multifactorial effects of these medications can help optimize treatment strategies, especially in patients with coexisting dermatologic and metabolic diseases.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. doi:10.1038/nrendo.2017.151
- Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet. 2022;400: 1803-1820. doi:10.1016/s0140-6736(22)01655-5
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305-340. doi:10.1016/j.eprac.2023.02.001
- LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77-96. doi:10.1210/endrev/bnaa023
- Petrasca A, Hambly R, Kearney N, et al. Metformin has antiinflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br J Dermatol. 2023;189:730-740. doi:10.1093/bjd/ljad305
- Duan W, Ding Y, Yu X, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-2402.
- Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320:C873-C879. doi:10.1152 /ajpcell.00604.2020
- Sung CT, Chao T, Lee A, et al. Oral metformin for treating dermatological diseases: a systematic review. J Drugs Dermatol. 2020;19:713-720. doi:10.36849/jdd.2020.4874
- Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi:10.1016/j.phrs.2022.106114
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. Br Med J (Clin Res Ed). 1986;293:483. doi:10.1136/bmj.293.6545.483
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27:1329-1335. doi:10.1111/jdv.12116
- Czarnowicki T, Ramot Y, Ingber A, et al. Metformin-induced leukocytoclastic vasculitis: a case report. Am J Clin Dermatol. 2012;13:61-63. doi:10.2165/11593230-000000000-00000
- Ben Salem C, Hmouda H, Slim R, et al. Rare case of metformininduced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685-1687. doi:10.1345/aph.1H155
- Abtahi-Naeini B, Momen T, Amiri R, et al. Metformin-induced generalized bullous fixed-drug eruption with a positive dechallengerechallenge test: a case report and literature review. Case Rep Dermatol Med. 2023;2023:6353919. doi:10.1155/2023/6353919
- Al Masri D, Fleifel M, Hirbli K. Fixed drug eruption secondary to four anti-diabetic medications: an unusual case of polysensitivity. Cureus. 2021;13:E18599. doi:10.7759/cureus.18599
- Ramírez-Bellver JL, Lopez J, Macias E, et al. Metformin-induced generalized fixed drug eruption with cutaneous hemophagocytosis. Am J Dermatopathol. 2017;39:471-475. doi:10.1097/dad.0000000000000800
- Steber CJ, Perkins SL, Harris KB. Metformin-induced fixed-drug eruption confirmed by multiple exposures. Am J Case Rep. 2016;17:231-234. doi:10.12659/ajcr.896424
- Voore P, Odigwe C, Mirrakhimov AE, et al. DRESS syndrome following metformin administration: a case report and review of the literature. Am J Ther. 2016;23:E1970-E1973. doi:10.1097/mjt.0000000000000292
- Kastalli S, El Aïdli S, Chaabane A, et al. Photosensitivity induced by metformin: a report of 3 cases. Article in French. Tunis Med. 2009;87:703-705.
- Karadağ AS, You Y, Danarti R, et al. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36:48-53. doi:10.1016/j.clindermatol.2017.09.008
- Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202-208. doi:10.1370/afm.678
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79. doi:10.1177/000992289803700203
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Novotny R, Davis J, Butel J, et al. Effect of the Children’s Healthy Living Program on young child overweight, obesity, and acanthosis nigricans in the US-affiliated Pacific region: a randomized clinical trial. JAMA Netw Open. 2018;1:E183896. doi:10.1001/jamanetworkopen.2018.3896
- Romo A, Benavides S. Treatment options in insulin resistance obesityrelated acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094. doi:10.1345/aph.1K446
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 2021;32:837-842. doi:10.108 0/09546634.2019.1708855
- Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr Dermatol. 2019;36:330-334. doi:10.1111/pde.13799
- Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12-14. doi:10.1046 /j.1525-1470.2002.00013.x
- Walling HW, Messingham M, Myers LM, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677-681.
- Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and its response to metformin. Pediatr Dermatol. 2017;34:e281-e282. doi:10.1111/pde.13206
- Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5:884-889.
- Sett A, Pradhan S, Sancheti K, et al. Effectiveness and safety of metformin versus Canthex™ in patients with acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64:115-121. doi:10.4103/ijd.IJD_417_17
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2004;81:114-119. doi:10.1016 /j.fertnstert.2003.05.020
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17:51-56.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149-1154. doi:10.1016 /s0015-0282(00)00501-x
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221. doi:10.1530/eje.0.1470217
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123. doi:10.1210/jc.2003-030424
- Rezvanian H, Adibi N, Siavash M, et al. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218: 231-236. doi:10.1159/000187718
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-1142. doi:10.1210/jc.2007-2429
- Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257.
- Fraison E, Kostova E, Moran LJ, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi:10.1002/14651858.CD005552.pub3
- Hambly R, Kearney N, Hughes R, et al. Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. Int J Mol Sci. 2023;24:6969. doi:10.3390/ijms24086969
- Gold DA, Reeder VJ, Mahan MG, et al. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699-703. doi:10.1016/j.jaad.2013.11.014
- Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150: 1273-1280. doi:10.1001/jamadermatol.2014.1165
- Monfrecola G, Balato A, Caiazzo G, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631-1633. doi:10.1111/jdv.13233
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi:10.3390/nu9111176
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi:10.1111/j.1468-3083.2012.04668.x
- Tsentemeidou A, Vakirlis E, Papadimitriou I, et al. Metformin in hidradenitis suppurativa: is it worth pursuing further? Skin Appendage Disord. 2023;9:187-190. doi:10.1159/000529359
- Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920-921. doi:10.1111/j.1365-2230.2008.03121.x
- Jennings L, Hambly R, Hughes R, et al. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31:261-263. doi:10.1080/09546634 .2019.1592100
- Moussa C, Wadowski L, Price H, et al. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol. 2020;19:1231-1234. doi:10.36849/jdd.2020.5447
- Cho M, Woo YR, Cho SH, et al. Metformin: a potential treatment for acne, hidradenitis suppurativa and rosacea. Acta Derm Venereol. 2023;103:adv18392. doi:10.2340/actadv.v103.18392
- Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35:2298878. doi:10.1080/09546634.2023.2298878
- El-Tahlawi S, Ezzat Mohammad N, Mohamed El-Amir A, et al. Survivin and insulin-like growth factor-I: potential role in the pathogenesis of acne and post-acne scar. Scars Burn Heal. 2019;5:2059513118818031. doi:10.1177/2059513118818031
- Albalat W, Darwish H, Abd-Elaal WH, et al. The potential role of insulin-like growth factor 1 in acne vulgaris and its correlation with the clinical response before and after treatment with metformin. J Cosmet Dermatol. 2022;21:6209-6214. doi:10.1111/jocd.15210
- Nguyen S, Nguyen ML, Roberts WS, et al. The efficacy of metformin as a therapeutic agent in the treatment of acne vulgaris: a systematic review. Cureus. 2024;16:E56246. doi:10.7759/cureus.56246
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994. doi:10.1016 /s0140-6736(14)61909-7
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Huang Z, Li J, Chen H, et al. The efficacy of metformin for the treatment of psoriasis: a meta-analysis study. Postepy Dermatol Alergol. 2023;40:606-610. doi:10.5114/ada.2023.130524
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (topical treatment cohort). BMC Dermatol. 2016;16:12. doi:10.1186 /s12895-016-0049-y
- Bao A, Qadri A, Gadre A, et al. Low-dose metformin and profibrotic signature in central centrifugal cicatricial alopecia. JAMA Dermatol. 2024;E243062. doi:10.1001/jamadermatol.2024.3062
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j .jaad.2008.09.066
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j.jdcr.2019.12.008
- Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. doi:10.1016 /j.cmet.2014.09.018
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e1. doi:10.1016/j.jaad.2018.05.1257
- Gadre A, Dyson T, Jedrych J, et al. Proteomic profiling of central centrifugal cicatricial alopecia reveals role of humoral immune response pathway and metabolic dysregulation. JID Innov. 2024;4:100263. doi:10.1016/j.xjidi.2024.100263
- Chaudhary SC, Kurundkar D, Elmets CA, et al. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149-1156. doi:10.1111/j.1751-1097.2012.01165.x
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi:10.1038/cddis.2011.86
- Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
- Mascaraque M, Delgado-Wicke P, Nuevo-Tapioles C, et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel). 2020;12:668. doi:10.3390/cancers12030668
- Chang MS, Hartman RI, Xue J, et al. Risk of skin cancer associated with metformin use: a meta-analysis of randomized controlled trials and observational studies. Cancer Prev Res (Phila). 2021;14:77-84. doi:10.1158/1940-6207.Capr-20-0376
- Augustin RC, Huang Z, Ding F, et al. Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Front Oncol. 2023;13:1075823. doi:10.3389 /fonc.2023.1075823
- Adalsteinsson JA, Muzumdar S, Waldman R, et al. Metformin is associated with decreased risk of basal cell carcinoma: a whole-population casecontrol study from Iceland. J Am Acad Dermatol. 2021;85:56-61. doi:10.1016/j.jaad.2021.02.042
- Jennings L, Nestor L, Molloy O, et al. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol. 2017;177:858-859. doi:10.1111/bjd.15233
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33: 1428-1434. doi:10.1080/09546634.2020.1826392
- Karacabeyli D, Lacaille D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J Clin Rheumatol. 2024;30:26-31. doi:10.1097/rhu.0000000000001949
- Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107: 124-128. doi:10.1016/j.yexmp.2019.01.014
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal Υϛ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161. doi:10.1111/bjd.12886
- Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13:65. doi:10.3390/cells13010065
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230. doi:10.1900 /rds.2014.11.202
- He Z, Tabe AN, Rana S, et al. Tirzepatide-induced biphasic anaphylactic reaction: a case report. Cureus. 2023;15:e50112. doi:10.7759/cureus.50112
- Anthony MS, Aroda VR, Parlett LE, et al. Risk of anaphylaxis among new users of glp-1 receptor agonists: a cohort study. Diabetes Care. 2024;47:712-719. doi:10.2337/dc23-1911
- Salazar CE, Patil MK, Aihie O, et al. Rare cutaneous adverse reactions associated with GLP-1 agonists: a review of the published literature. Arch Dermatol Res. 2024;316:248. doi:10.1007/s00403-024-02969-3
- Tran MM, Mirza FN, Lee AC, et al. Dermatologic findings associated with semaglutide use: a scoping review. J Am Acad Dermatol. 2024;91:166-168. doi:10.1016/j.jaad.2024.03.021
- Castellanos V, Workneh H, Malik A, et al. Semaglutide-induced lupus erythematosus with multiorgan involvement. Cureus. 2024;16:E55324. doi:10.7759/cureus.55324
- Boccardi A, Shubrook JH. Cutaneous reactions to antidiabetic agents: a narrative review. Diabetology. 2022;3:97-107.
- Desai DD, Sikora M, Nohria A, et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63:1128-1130. doi:10.1111 /ijd.17246
- Mansour MR, Hannawa OM, Yaldo MM, et al. The rise of “Ozempic face”: analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J Plast Reconstr Aesthet Surg. 2024;96:225-227. doi:10.1016/j.bjps.2024.07.051
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64-72. doi:10.1177/1203475419874412
- Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol. 2016;30:895-896. doi:10.1111/jdv.13059
- Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol. 2014;39:315-7; quiz 317-8. doi:10.1111/ced.12269
- Mandour MO, Al-Musawi S, Idowu E, et al. Metabolic endoscopy and a simplified low-carbohydrate-high-dietary fiber template as novel treatments for hidradenitis suppurativa—a case series. JAAD Case Rep. 2023;34:23-26. doi:10.1016/j.jdcr.2023.01.035
- Henry T, Cahn B, Haber R, et al. Therapeutic potential of GLP-1 agonists for hidradenitis suppurativa. Int J Dermatol. 2023;62:1543-1544. doi:10.1111/ijd.16892
- Chan LJ, Kaur M, Kaffenberger BH. A case of recalcitrant hidradenitis suppurativa concomitantly treated with tirzepatide. JAAD Case Rep. 2024;52:101-102. doi:10.1016/j.jdcr.2024.02.023
- Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-00007. doi:10.1530 /edm-21-0007
- Buysschaert M, Tennstedt D, Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab. 2012;38:86-88. doi:10.1016/j.diabet.2011.11.004
- Faurschou A, Gyldenløve M, Rohde U, et al. Lack of effect of the glucagonlike peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J Eur Acad Dermatol Venereol. 2015;29:555-559. doi:10.1111/jdv.12629
- Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27:1440-1443. doi:10.1111/j.1468-3083.2012.04609.x
- Gordon ER, Musleh S, Bordone LA. Treatment of insulin resistance with tirzepatide leading to improvement of hair loss. JAAD Case Rep. 2024;50:123-125. doi:10.1016/j.jdcr.2024.06.001
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. doi:10.1038/nrendo.2017.151
- Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet. 2022;400: 1803-1820. doi:10.1016/s0140-6736(22)01655-5
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305-340. doi:10.1016/j.eprac.2023.02.001
- LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77-96. doi:10.1210/endrev/bnaa023
- Petrasca A, Hambly R, Kearney N, et al. Metformin has antiinflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br J Dermatol. 2023;189:730-740. doi:10.1093/bjd/ljad305
- Duan W, Ding Y, Yu X, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-2402.
- Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320:C873-C879. doi:10.1152 /ajpcell.00604.2020
- Sung CT, Chao T, Lee A, et al. Oral metformin for treating dermatological diseases: a systematic review. J Drugs Dermatol. 2020;19:713-720. doi:10.36849/jdd.2020.4874
- Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi:10.1016/j.phrs.2022.106114
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. Br Med J (Clin Res Ed). 1986;293:483. doi:10.1136/bmj.293.6545.483
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27:1329-1335. doi:10.1111/jdv.12116
- Czarnowicki T, Ramot Y, Ingber A, et al. Metformin-induced leukocytoclastic vasculitis: a case report. Am J Clin Dermatol. 2012;13:61-63. doi:10.2165/11593230-000000000-00000
- Ben Salem C, Hmouda H, Slim R, et al. Rare case of metformininduced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685-1687. doi:10.1345/aph.1H155
- Abtahi-Naeini B, Momen T, Amiri R, et al. Metformin-induced generalized bullous fixed-drug eruption with a positive dechallengerechallenge test: a case report and literature review. Case Rep Dermatol Med. 2023;2023:6353919. doi:10.1155/2023/6353919
- Al Masri D, Fleifel M, Hirbli K. Fixed drug eruption secondary to four anti-diabetic medications: an unusual case of polysensitivity. Cureus. 2021;13:E18599. doi:10.7759/cureus.18599
- Ramírez-Bellver JL, Lopez J, Macias E, et al. Metformin-induced generalized fixed drug eruption with cutaneous hemophagocytosis. Am J Dermatopathol. 2017;39:471-475. doi:10.1097/dad.0000000000000800
- Steber CJ, Perkins SL, Harris KB. Metformin-induced fixed-drug eruption confirmed by multiple exposures. Am J Case Rep. 2016;17:231-234. doi:10.12659/ajcr.896424
- Voore P, Odigwe C, Mirrakhimov AE, et al. DRESS syndrome following metformin administration: a case report and review of the literature. Am J Ther. 2016;23:E1970-E1973. doi:10.1097/mjt.0000000000000292
- Kastalli S, El Aïdli S, Chaabane A, et al. Photosensitivity induced by metformin: a report of 3 cases. Article in French. Tunis Med. 2009;87:703-705.
- Karadağ AS, You Y, Danarti R, et al. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36:48-53. doi:10.1016/j.clindermatol.2017.09.008
- Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202-208. doi:10.1370/afm.678
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79. doi:10.1177/000992289803700203
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Novotny R, Davis J, Butel J, et al. Effect of the Children’s Healthy Living Program on young child overweight, obesity, and acanthosis nigricans in the US-affiliated Pacific region: a randomized clinical trial. JAMA Netw Open. 2018;1:E183896. doi:10.1001/jamanetworkopen.2018.3896
- Romo A, Benavides S. Treatment options in insulin resistance obesityrelated acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094. doi:10.1345/aph.1K446
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 2021;32:837-842. doi:10.108 0/09546634.2019.1708855
- Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr Dermatol. 2019;36:330-334. doi:10.1111/pde.13799
- Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12-14. doi:10.1046 /j.1525-1470.2002.00013.x
- Walling HW, Messingham M, Myers LM, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677-681.
- Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and its response to metformin. Pediatr Dermatol. 2017;34:e281-e282. doi:10.1111/pde.13206
- Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5:884-889.
- Sett A, Pradhan S, Sancheti K, et al. Effectiveness and safety of metformin versus Canthex™ in patients with acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64:115-121. doi:10.4103/ijd.IJD_417_17
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2004;81:114-119. doi:10.1016 /j.fertnstert.2003.05.020
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17:51-56.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149-1154. doi:10.1016 /s0015-0282(00)00501-x
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221. doi:10.1530/eje.0.1470217
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123. doi:10.1210/jc.2003-030424
- Rezvanian H, Adibi N, Siavash M, et al. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218: 231-236. doi:10.1159/000187718
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-1142. doi:10.1210/jc.2007-2429
- Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257.
- Fraison E, Kostova E, Moran LJ, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi:10.1002/14651858.CD005552.pub3
- Hambly R, Kearney N, Hughes R, et al. Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. Int J Mol Sci. 2023;24:6969. doi:10.3390/ijms24086969
- Gold DA, Reeder VJ, Mahan MG, et al. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699-703. doi:10.1016/j.jaad.2013.11.014
- Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150: 1273-1280. doi:10.1001/jamadermatol.2014.1165
- Monfrecola G, Balato A, Caiazzo G, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631-1633. doi:10.1111/jdv.13233
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi:10.3390/nu9111176
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi:10.1111/j.1468-3083.2012.04668.x
- Tsentemeidou A, Vakirlis E, Papadimitriou I, et al. Metformin in hidradenitis suppurativa: is it worth pursuing further? Skin Appendage Disord. 2023;9:187-190. doi:10.1159/000529359
- Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920-921. doi:10.1111/j.1365-2230.2008.03121.x
- Jennings L, Hambly R, Hughes R, et al. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31:261-263. doi:10.1080/09546634 .2019.1592100
- Moussa C, Wadowski L, Price H, et al. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol. 2020;19:1231-1234. doi:10.36849/jdd.2020.5447
- Cho M, Woo YR, Cho SH, et al. Metformin: a potential treatment for acne, hidradenitis suppurativa and rosacea. Acta Derm Venereol. 2023;103:adv18392. doi:10.2340/actadv.v103.18392
- Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35:2298878. doi:10.1080/09546634.2023.2298878
- El-Tahlawi S, Ezzat Mohammad N, Mohamed El-Amir A, et al. Survivin and insulin-like growth factor-I: potential role in the pathogenesis of acne and post-acne scar. Scars Burn Heal. 2019;5:2059513118818031. doi:10.1177/2059513118818031
- Albalat W, Darwish H, Abd-Elaal WH, et al. The potential role of insulin-like growth factor 1 in acne vulgaris and its correlation with the clinical response before and after treatment with metformin. J Cosmet Dermatol. 2022;21:6209-6214. doi:10.1111/jocd.15210
- Nguyen S, Nguyen ML, Roberts WS, et al. The efficacy of metformin as a therapeutic agent in the treatment of acne vulgaris: a systematic review. Cureus. 2024;16:E56246. doi:10.7759/cureus.56246
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994. doi:10.1016 /s0140-6736(14)61909-7
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Huang Z, Li J, Chen H, et al. The efficacy of metformin for the treatment of psoriasis: a meta-analysis study. Postepy Dermatol Alergol. 2023;40:606-610. doi:10.5114/ada.2023.130524
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (topical treatment cohort). BMC Dermatol. 2016;16:12. doi:10.1186 /s12895-016-0049-y
- Bao A, Qadri A, Gadre A, et al. Low-dose metformin and profibrotic signature in central centrifugal cicatricial alopecia. JAMA Dermatol. 2024;E243062. doi:10.1001/jamadermatol.2024.3062
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j .jaad.2008.09.066
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j.jdcr.2019.12.008
- Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. doi:10.1016 /j.cmet.2014.09.018
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e1. doi:10.1016/j.jaad.2018.05.1257
- Gadre A, Dyson T, Jedrych J, et al. Proteomic profiling of central centrifugal cicatricial alopecia reveals role of humoral immune response pathway and metabolic dysregulation. JID Innov. 2024;4:100263. doi:10.1016/j.xjidi.2024.100263
- Chaudhary SC, Kurundkar D, Elmets CA, et al. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149-1156. doi:10.1111/j.1751-1097.2012.01165.x
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi:10.1038/cddis.2011.86
- Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
- Mascaraque M, Delgado-Wicke P, Nuevo-Tapioles C, et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel). 2020;12:668. doi:10.3390/cancers12030668
- Chang MS, Hartman RI, Xue J, et al. Risk of skin cancer associated with metformin use: a meta-analysis of randomized controlled trials and observational studies. Cancer Prev Res (Phila). 2021;14:77-84. doi:10.1158/1940-6207.Capr-20-0376
- Augustin RC, Huang Z, Ding F, et al. Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Front Oncol. 2023;13:1075823. doi:10.3389 /fonc.2023.1075823
- Adalsteinsson JA, Muzumdar S, Waldman R, et al. Metformin is associated with decreased risk of basal cell carcinoma: a whole-population casecontrol study from Iceland. J Am Acad Dermatol. 2021;85:56-61. doi:10.1016/j.jaad.2021.02.042
- Jennings L, Nestor L, Molloy O, et al. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol. 2017;177:858-859. doi:10.1111/bjd.15233
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33: 1428-1434. doi:10.1080/09546634.2020.1826392
- Karacabeyli D, Lacaille D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J Clin Rheumatol. 2024;30:26-31. doi:10.1097/rhu.0000000000001949
- Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107: 124-128. doi:10.1016/j.yexmp.2019.01.014
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal Υϛ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161. doi:10.1111/bjd.12886
- Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13:65. doi:10.3390/cells13010065
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230. doi:10.1900 /rds.2014.11.202
- He Z, Tabe AN, Rana S, et al. Tirzepatide-induced biphasic anaphylactic reaction: a case report. Cureus. 2023;15:e50112. doi:10.7759/cureus.50112
- Anthony MS, Aroda VR, Parlett LE, et al. Risk of anaphylaxis among new users of glp-1 receptor agonists: a cohort study. Diabetes Care. 2024;47:712-719. doi:10.2337/dc23-1911
- Salazar CE, Patil MK, Aihie O, et al. Rare cutaneous adverse reactions associated with GLP-1 agonists: a review of the published literature. Arch Dermatol Res. 2024;316:248. doi:10.1007/s00403-024-02969-3
- Tran MM, Mirza FN, Lee AC, et al. Dermatologic findings associated with semaglutide use: a scoping review. J Am Acad Dermatol. 2024;91:166-168. doi:10.1016/j.jaad.2024.03.021
- Castellanos V, Workneh H, Malik A, et al. Semaglutide-induced lupus erythematosus with multiorgan involvement. Cureus. 2024;16:E55324. doi:10.7759/cureus.55324
- Boccardi A, Shubrook JH. Cutaneous reactions to antidiabetic agents: a narrative review. Diabetology. 2022;3:97-107.
- Desai DD, Sikora M, Nohria A, et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63:1128-1130. doi:10.1111 /ijd.17246
- Mansour MR, Hannawa OM, Yaldo MM, et al. The rise of “Ozempic face”: analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J Plast Reconstr Aesthet Surg. 2024;96:225-227. doi:10.1016/j.bjps.2024.07.051
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64-72. doi:10.1177/1203475419874412
- Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol. 2016;30:895-896. doi:10.1111/jdv.13059
- Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol. 2014;39:315-7; quiz 317-8. doi:10.1111/ced.12269
- Mandour MO, Al-Musawi S, Idowu E, et al. Metabolic endoscopy and a simplified low-carbohydrate-high-dietary fiber template as novel treatments for hidradenitis suppurativa—a case series. JAAD Case Rep. 2023;34:23-26. doi:10.1016/j.jdcr.2023.01.035
- Henry T, Cahn B, Haber R, et al. Therapeutic potential of GLP-1 agonists for hidradenitis suppurativa. Int J Dermatol. 2023;62:1543-1544. doi:10.1111/ijd.16892
- Chan LJ, Kaur M, Kaffenberger BH. A case of recalcitrant hidradenitis suppurativa concomitantly treated with tirzepatide. JAAD Case Rep. 2024;52:101-102. doi:10.1016/j.jdcr.2024.02.023
- Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-00007. doi:10.1530 /edm-21-0007
- Buysschaert M, Tennstedt D, Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab. 2012;38:86-88. doi:10.1016/j.diabet.2011.11.004
- Faurschou A, Gyldenløve M, Rohde U, et al. Lack of effect of the glucagonlike peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J Eur Acad Dermatol Venereol. 2015;29:555-559. doi:10.1111/jdv.12629
- Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27:1440-1443. doi:10.1111/j.1468-3083.2012.04609.x
- Gordon ER, Musleh S, Bordone LA. Treatment of insulin resistance with tirzepatide leading to improvement of hair loss. JAAD Case Rep. 2024;50:123-125. doi:10.1016/j.jdcr.2024.06.001
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
PRACTICE POINTS
- Type 2 diabetes mellitus (T2DM) is highly prevalent in patients with various dermatologic conditions; therefore, it is important for dermatologists to understand the adverse effects of T2DM medications to optimize treatment strategies.
- In addition to glycemic control and management, the hormonal and immunologic effects of T2DM medications can be leveraged to treat dermatologic conditions, particularly those associated with metabolic dysregulation.
Combined Clinics, Personalized Medicine for Psoriatic Disease Face Barriers
NEW YORK CITY — The idea of having dermatologists and rheumatologists under one roof to see patients with psoriasis prone to psoriatic arthritis (PsA) — a concept known as combined clinics — has been around for more than a decade, and the idea of personalized medicine for these patients even longer than that, yet both approaches to care have encountered a host of obstacles, a longtime research rheumatologist said.
“It’s important that we work together, but there is a problem in terms of staffing — managing the meetings with patients together — and in the states in particular it’s a matter of who’s charging for what,” Dafna Gladman, MD, a rheumatologist at the University of Toronto, in Ontario, Canada, told attendees at the annual New York University (NYU) Langone Advanced Seminar in Psoriasis and Psoriatic Arthritis. Her institution has one of the 44 worldwide combined clinics registered in the Psoriasis & Psoriatic Arthritis Clinics Multicenter Advancement Network (PPACMAN), of which Gladman is an advisory board member.
Barriers to Combined Clinics
“Some of the barriers are physical in the sense that, for the dermatologists and rheumatologists to work at the same time, you need the right space, and in many places, you just don’t have the space to have the two specialists sitting at the same time,” Gladman told Medscape Medical News.
Some centers get around this by having the dermatology and rheumatology clinics next to or near each other. “So these two specialists are close enough to be able to go from room to room,” she added.
Another challenge facing combined clinics lies in the nature of how dermatologists and rheumatologists see patients. “The dermatologist sees patients a lot faster than the rheumatologist, so if the dermatologist and rheumatologist are sitting together, the dermatologist may not see as many patients as they would otherwise and therefore may not get reimbursed properly,” Gladman said.
To overcome these challenges, different models have emerged, Gladman said. If space allows, the ideal model is to have both specialties in one clinic, she said, while compensating for the different pace at which dermatologists and rheumatologists see patients.
The other model is to locate the two clinics close enough so that a person with suspected PsA can get to the rheumatology clinic soon after their dermatologic consult, or the rheumatologist can go to the dermatology clinic, Gladman said. Or the situation may be reversed when the rheumatologist needs a dermatology consult, she added.
When that’s not possible, a virtual visit may be the solution, Gladman said. She noted that PPACMAN offers ways to overcome the challenges of running a combined clinic.
Whatever combined clinic model a center chooses, clinicians must be mindful of preventing patients from falling through the cracks, Gladman said.
“When you treat patients separately, the patient sees the rheumatologist, and the rheumatologist wants to do one thing; then they go to the dermatologist and the dermatologist wants to do another thing, and the patient doesn’t do anything because they don’t know what to choose,” she said.
The combined clinic allows the patient to get the opinions of both specialists and avoid the uncertainty about the course of treatment, Gladman added.
Some combined clinics may also house other specialists, such as gastroenterologists, cardiologists, and nurse practitioners, noted Jose U. Scher, MD, director of the Arthritis Clinic and Psoriatic Arthritis Center at NYU Langone Health in New York City. Such centers are typically in academic centers “given challenges with space, scheduling, and reimbursement,” he told Medscape Medical News. NYU has a PPACMAN-registered combined clinic.
Regardless of how combined clinics are organized, Scher said, “We have found that the most important aspect of combined clinics is the open communication and integration of care between and amongst specialists and patients.”
The Potential of Personalized Medicine
“Personalized medicine is where we need to get to,” Gladman told seminar attendees. She said she had hoped it would be further along by now and be more integrated into the care of patients with psoriasis and PsA. “The idea is to identify psoriasis patients that are destined to develop psoriatic arthritis,” she said.
Besides that, identifying biomarkers is key to advancing personalized medicine for psoriasis, Gladman noted.
“In the skin, it’s easy; even the patient can assess their psoriasis,” she said. “But in the joints, it’s very difficult, so it would be nice to have some kind of biomarker, whether it’s the blood or an imaging modality. We want to identify the biomarkers for drug response or lack thereof so we know what drugs would be appropriate for the individual patient, and therefore, we can provide the right drug for the right person and fortunately at the right time.”
In explaining why personalized medicine isn’t further along in dermatology and rheumatology, Gladman told Medscape Medical News, “It’s a matter of finding the right things; we haven’t solved the mystery.” She cited a previous discussion at the seminar about the pathogenesis of PsA. “One person thinks it’s the bone marrow and another thinks it’s the T cells, so we haven’t quite put it all together to have a definitive answer.”
Personalized medicine in psoriasis and PsA is a “key unmet need,” Scher said. “Multiomics” — a biological analysis approach that uses multiple “omes,” such as the genome and microbiome — digital features, and wearables “can unlock novel diagnostic and therapeutic pathways that are desperately needed to enhance clinical response in PsA,” he said.
Also emerging are humanized animal models for laboratory research, which Scher called “potentially very useful tools to personalize approaches to PsA pathogenesis and treatment.”
Gladman disclosed financial relationships with AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Scher had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — The idea of having dermatologists and rheumatologists under one roof to see patients with psoriasis prone to psoriatic arthritis (PsA) — a concept known as combined clinics — has been around for more than a decade, and the idea of personalized medicine for these patients even longer than that, yet both approaches to care have encountered a host of obstacles, a longtime research rheumatologist said.
“It’s important that we work together, but there is a problem in terms of staffing — managing the meetings with patients together — and in the states in particular it’s a matter of who’s charging for what,” Dafna Gladman, MD, a rheumatologist at the University of Toronto, in Ontario, Canada, told attendees at the annual New York University (NYU) Langone Advanced Seminar in Psoriasis and Psoriatic Arthritis. Her institution has one of the 44 worldwide combined clinics registered in the Psoriasis & Psoriatic Arthritis Clinics Multicenter Advancement Network (PPACMAN), of which Gladman is an advisory board member.
Barriers to Combined Clinics
“Some of the barriers are physical in the sense that, for the dermatologists and rheumatologists to work at the same time, you need the right space, and in many places, you just don’t have the space to have the two specialists sitting at the same time,” Gladman told Medscape Medical News.
Some centers get around this by having the dermatology and rheumatology clinics next to or near each other. “So these two specialists are close enough to be able to go from room to room,” she added.
Another challenge facing combined clinics lies in the nature of how dermatologists and rheumatologists see patients. “The dermatologist sees patients a lot faster than the rheumatologist, so if the dermatologist and rheumatologist are sitting together, the dermatologist may not see as many patients as they would otherwise and therefore may not get reimbursed properly,” Gladman said.
To overcome these challenges, different models have emerged, Gladman said. If space allows, the ideal model is to have both specialties in one clinic, she said, while compensating for the different pace at which dermatologists and rheumatologists see patients.
The other model is to locate the two clinics close enough so that a person with suspected PsA can get to the rheumatology clinic soon after their dermatologic consult, or the rheumatologist can go to the dermatology clinic, Gladman said. Or the situation may be reversed when the rheumatologist needs a dermatology consult, she added.
When that’s not possible, a virtual visit may be the solution, Gladman said. She noted that PPACMAN offers ways to overcome the challenges of running a combined clinic.
Whatever combined clinic model a center chooses, clinicians must be mindful of preventing patients from falling through the cracks, Gladman said.
“When you treat patients separately, the patient sees the rheumatologist, and the rheumatologist wants to do one thing; then they go to the dermatologist and the dermatologist wants to do another thing, and the patient doesn’t do anything because they don’t know what to choose,” she said.
The combined clinic allows the patient to get the opinions of both specialists and avoid the uncertainty about the course of treatment, Gladman added.
Some combined clinics may also house other specialists, such as gastroenterologists, cardiologists, and nurse practitioners, noted Jose U. Scher, MD, director of the Arthritis Clinic and Psoriatic Arthritis Center at NYU Langone Health in New York City. Such centers are typically in academic centers “given challenges with space, scheduling, and reimbursement,” he told Medscape Medical News. NYU has a PPACMAN-registered combined clinic.
Regardless of how combined clinics are organized, Scher said, “We have found that the most important aspect of combined clinics is the open communication and integration of care between and amongst specialists and patients.”
The Potential of Personalized Medicine
“Personalized medicine is where we need to get to,” Gladman told seminar attendees. She said she had hoped it would be further along by now and be more integrated into the care of patients with psoriasis and PsA. “The idea is to identify psoriasis patients that are destined to develop psoriatic arthritis,” she said.
Besides that, identifying biomarkers is key to advancing personalized medicine for psoriasis, Gladman noted.
“In the skin, it’s easy; even the patient can assess their psoriasis,” she said. “But in the joints, it’s very difficult, so it would be nice to have some kind of biomarker, whether it’s the blood or an imaging modality. We want to identify the biomarkers for drug response or lack thereof so we know what drugs would be appropriate for the individual patient, and therefore, we can provide the right drug for the right person and fortunately at the right time.”
In explaining why personalized medicine isn’t further along in dermatology and rheumatology, Gladman told Medscape Medical News, “It’s a matter of finding the right things; we haven’t solved the mystery.” She cited a previous discussion at the seminar about the pathogenesis of PsA. “One person thinks it’s the bone marrow and another thinks it’s the T cells, so we haven’t quite put it all together to have a definitive answer.”
Personalized medicine in psoriasis and PsA is a “key unmet need,” Scher said. “Multiomics” — a biological analysis approach that uses multiple “omes,” such as the genome and microbiome — digital features, and wearables “can unlock novel diagnostic and therapeutic pathways that are desperately needed to enhance clinical response in PsA,” he said.
Also emerging are humanized animal models for laboratory research, which Scher called “potentially very useful tools to personalize approaches to PsA pathogenesis and treatment.”
Gladman disclosed financial relationships with AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Scher had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — The idea of having dermatologists and rheumatologists under one roof to see patients with psoriasis prone to psoriatic arthritis (PsA) — a concept known as combined clinics — has been around for more than a decade, and the idea of personalized medicine for these patients even longer than that, yet both approaches to care have encountered a host of obstacles, a longtime research rheumatologist said.
“It’s important that we work together, but there is a problem in terms of staffing — managing the meetings with patients together — and in the states in particular it’s a matter of who’s charging for what,” Dafna Gladman, MD, a rheumatologist at the University of Toronto, in Ontario, Canada, told attendees at the annual New York University (NYU) Langone Advanced Seminar in Psoriasis and Psoriatic Arthritis. Her institution has one of the 44 worldwide combined clinics registered in the Psoriasis & Psoriatic Arthritis Clinics Multicenter Advancement Network (PPACMAN), of which Gladman is an advisory board member.
Barriers to Combined Clinics
“Some of the barriers are physical in the sense that, for the dermatologists and rheumatologists to work at the same time, you need the right space, and in many places, you just don’t have the space to have the two specialists sitting at the same time,” Gladman told Medscape Medical News.
Some centers get around this by having the dermatology and rheumatology clinics next to or near each other. “So these two specialists are close enough to be able to go from room to room,” she added.
Another challenge facing combined clinics lies in the nature of how dermatologists and rheumatologists see patients. “The dermatologist sees patients a lot faster than the rheumatologist, so if the dermatologist and rheumatologist are sitting together, the dermatologist may not see as many patients as they would otherwise and therefore may not get reimbursed properly,” Gladman said.
To overcome these challenges, different models have emerged, Gladman said. If space allows, the ideal model is to have both specialties in one clinic, she said, while compensating for the different pace at which dermatologists and rheumatologists see patients.
The other model is to locate the two clinics close enough so that a person with suspected PsA can get to the rheumatology clinic soon after their dermatologic consult, or the rheumatologist can go to the dermatology clinic, Gladman said. Or the situation may be reversed when the rheumatologist needs a dermatology consult, she added.
When that’s not possible, a virtual visit may be the solution, Gladman said. She noted that PPACMAN offers ways to overcome the challenges of running a combined clinic.
Whatever combined clinic model a center chooses, clinicians must be mindful of preventing patients from falling through the cracks, Gladman said.
“When you treat patients separately, the patient sees the rheumatologist, and the rheumatologist wants to do one thing; then they go to the dermatologist and the dermatologist wants to do another thing, and the patient doesn’t do anything because they don’t know what to choose,” she said.
The combined clinic allows the patient to get the opinions of both specialists and avoid the uncertainty about the course of treatment, Gladman added.
Some combined clinics may also house other specialists, such as gastroenterologists, cardiologists, and nurse practitioners, noted Jose U. Scher, MD, director of the Arthritis Clinic and Psoriatic Arthritis Center at NYU Langone Health in New York City. Such centers are typically in academic centers “given challenges with space, scheduling, and reimbursement,” he told Medscape Medical News. NYU has a PPACMAN-registered combined clinic.
Regardless of how combined clinics are organized, Scher said, “We have found that the most important aspect of combined clinics is the open communication and integration of care between and amongst specialists and patients.”
The Potential of Personalized Medicine
“Personalized medicine is where we need to get to,” Gladman told seminar attendees. She said she had hoped it would be further along by now and be more integrated into the care of patients with psoriasis and PsA. “The idea is to identify psoriasis patients that are destined to develop psoriatic arthritis,” she said.
Besides that, identifying biomarkers is key to advancing personalized medicine for psoriasis, Gladman noted.
“In the skin, it’s easy; even the patient can assess their psoriasis,” she said. “But in the joints, it’s very difficult, so it would be nice to have some kind of biomarker, whether it’s the blood or an imaging modality. We want to identify the biomarkers for drug response or lack thereof so we know what drugs would be appropriate for the individual patient, and therefore, we can provide the right drug for the right person and fortunately at the right time.”
In explaining why personalized medicine isn’t further along in dermatology and rheumatology, Gladman told Medscape Medical News, “It’s a matter of finding the right things; we haven’t solved the mystery.” She cited a previous discussion at the seminar about the pathogenesis of PsA. “One person thinks it’s the bone marrow and another thinks it’s the T cells, so we haven’t quite put it all together to have a definitive answer.”
Personalized medicine in psoriasis and PsA is a “key unmet need,” Scher said. “Multiomics” — a biological analysis approach that uses multiple “omes,” such as the genome and microbiome — digital features, and wearables “can unlock novel diagnostic and therapeutic pathways that are desperately needed to enhance clinical response in PsA,” he said.
Also emerging are humanized animal models for laboratory research, which Scher called “potentially very useful tools to personalize approaches to PsA pathogenesis and treatment.”
Gladman disclosed financial relationships with AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Scher had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA Approves Ustekinumab Biosimilar Steqeyma, the Seventh of Its Kind
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved ustekinumab-stba (Steqeyma) as a biosimilar to the interleukin-12 and -23 inhibitor ustekinumab (Stelara) for the treatment of adults with active Crohn’s disease or ulcerative colitis and for both children aged ≥ 6 years and adults with moderate to severe plaque psoriasis or active psoriatic arthritis.
This is the seventh ustekinumab biosimilar approved by the FDA. The biosimilar, developed by Celltrion, has a license entry date in February 2025 as part of the settlement and license agreement with the manufacturer of the reference biologic, Johnson & Johnson.
Ustekinumab-stba will be available in two formulations: A subcutaneous injection in two strengths — a 45 mg/0.5 mL or 90 mg/1 mL solution in a single-dose, prefilled syringe — and an intravenous infusion of a 130 mg/26 mL (5 mg/mL) solution in a single-dose vial.
“The approval of Steqeyma reflects Celltrion’s continued investment in providing treatment options to patients diagnosed with ulcerative colitis, Crohn’s disease, psoriasis, and psoriatic arthritis,” said Thomas Nusbickel, Chief Commercial Officer at Celltrion USA, Jersey City, New Jersey, in a press release.
The FDA has previously approved the company’s adalimumab biosimilar Yuflyma and its infliximab biosimilar Zymfentra.
The full prescribing information for ustekinumab-stba is available here.
A version of this article first appeared on Medscape.com.
Optimal Exercise Levels for Dermatology Patients With Psoriasis
Optimal Exercise Levels for Dermatology Patients With Psoriasis
There is a direct link between psoriasis and metabolic conditions such as diabetes mellitus and obesity.1 Exercise of varied intensity in patients with chronic inflammatory and metabolic conditions can help improve quality of life and severity of disease; however, there has not been a clear consensus on the recommended duration and types of exercise that are most advantageous.1-5 We reviewed the literature to identify physical and mental health impacts of exercise on patients with psoriasis, and we present the recommended duration and types of exercise that are most impactful for these patients.
One indicator of the link between psoriasis and exercise is the level of peroxisome proliferator activated receptor gamma coactivator-1 α (PGC-1α) in muscle cells.2 This marker reduces inflammation. When levels are low in muscle cells, an induction occurs that leads to systemic or local inflammation; however, skeletal muscle PGC-1α levels increase following exercise, indicating reduced inflammation.2 The level of PGC-1α is measured through muscle biopsy and polymerase chain reaction.6 Another indicator of the correlation between exercise and inflammation is lipoprotein-associated phospholipase A2, which is produced by inflammatory cells and has a correlation with cardiovascular disease. Exercise reduces lipoprotein-associated phospholipase A2 levels, and a sedentary lifestyle correlates with increased levels of this marker.3 Lipoprotein-associated phospholipase A2 is measured through an enzyme-linked immunosorbent assay of the blood, with levels around 200 ng/mL considered high.7 Patients with psoriasis are 30% less likely to participate in physical activity compared to patients without psoriasis, which can be attributed to psychosocial impairment and other factors. Sedentary lifestyle is associated with new or worsening metabolic disease and prevalence of psoriatic lesions.1
A metabolic equivalent task score is a classification system that measures the rate of the body’s oxygen uptake for any given activity.4 A score of 20.9 or more metabolic equivalent task hours of vigorous exercise per week—equal to 105 minutes of running or 180 minutes of swimming or playing tennis—is linked with a 25% to 30% risk reduction of psoriasis in women.4 Therefore, we recommend 30 minutes of exercise 4 to 5 times per week for women. These periods of exercise should consist mainly of activities that will not cause psoriasis flares due to excessive sweating, skin trauma, or prolonged sun exposure.5 Walking, yoga, and bike riding all could be good exercise options for those with psoriasis. The National Psoriasis Foundation offers guidance on physical activity in patients with psoriasis or psoriatic arthritis.8 Psoriasis has apparent physical and psychosocial impacts on patients that can be prevented and improved through the exercise recommendations presented in this article. Dermatologists should use these recommendations to address psoriasis in their everyday practice.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153. doi:10.1111/1346-8138.12721
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454: 463-469. doi:10.1038/nature07206
- Clark K, Sharp S, Womack CJ, et al. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;32:1703-1710. doi:10.1016/j.numecd.2022.04.023
- Yeh C, Flatley E, Elkattawy O, et al. Exercise in dermatology: exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. doi:10.1016/j.jaad.2021.07.023
- Sheppard R, Gan WK, Onambele-Pearson GL, et al. Developing an aerobic exercise intervention for patients with psoriasis to support lifestyle behaviour change and improve health outcomes. Clin Exp Dermatol. 2023;48:5-11. doi:10.1093/ced/llac008
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904.023
- National Psoriasis Foundation. Active and mindful lifestyles. https://www.psoriasis.org/active-and-mindful-lifestyles/
There is a direct link between psoriasis and metabolic conditions such as diabetes mellitus and obesity.1 Exercise of varied intensity in patients with chronic inflammatory and metabolic conditions can help improve quality of life and severity of disease; however, there has not been a clear consensus on the recommended duration and types of exercise that are most advantageous.1-5 We reviewed the literature to identify physical and mental health impacts of exercise on patients with psoriasis, and we present the recommended duration and types of exercise that are most impactful for these patients.
One indicator of the link between psoriasis and exercise is the level of peroxisome proliferator activated receptor gamma coactivator-1 α (PGC-1α) in muscle cells.2 This marker reduces inflammation. When levels are low in muscle cells, an induction occurs that leads to systemic or local inflammation; however, skeletal muscle PGC-1α levels increase following exercise, indicating reduced inflammation.2 The level of PGC-1α is measured through muscle biopsy and polymerase chain reaction.6 Another indicator of the correlation between exercise and inflammation is lipoprotein-associated phospholipase A2, which is produced by inflammatory cells and has a correlation with cardiovascular disease. Exercise reduces lipoprotein-associated phospholipase A2 levels, and a sedentary lifestyle correlates with increased levels of this marker.3 Lipoprotein-associated phospholipase A2 is measured through an enzyme-linked immunosorbent assay of the blood, with levels around 200 ng/mL considered high.7 Patients with psoriasis are 30% less likely to participate in physical activity compared to patients without psoriasis, which can be attributed to psychosocial impairment and other factors. Sedentary lifestyle is associated with new or worsening metabolic disease and prevalence of psoriatic lesions.1
A metabolic equivalent task score is a classification system that measures the rate of the body’s oxygen uptake for any given activity.4 A score of 20.9 or more metabolic equivalent task hours of vigorous exercise per week—equal to 105 minutes of running or 180 minutes of swimming or playing tennis—is linked with a 25% to 30% risk reduction of psoriasis in women.4 Therefore, we recommend 30 minutes of exercise 4 to 5 times per week for women. These periods of exercise should consist mainly of activities that will not cause psoriasis flares due to excessive sweating, skin trauma, or prolonged sun exposure.5 Walking, yoga, and bike riding all could be good exercise options for those with psoriasis. The National Psoriasis Foundation offers guidance on physical activity in patients with psoriasis or psoriatic arthritis.8 Psoriasis has apparent physical and psychosocial impacts on patients that can be prevented and improved through the exercise recommendations presented in this article. Dermatologists should use these recommendations to address psoriasis in their everyday practice.
There is a direct link between psoriasis and metabolic conditions such as diabetes mellitus and obesity.1 Exercise of varied intensity in patients with chronic inflammatory and metabolic conditions can help improve quality of life and severity of disease; however, there has not been a clear consensus on the recommended duration and types of exercise that are most advantageous.1-5 We reviewed the literature to identify physical and mental health impacts of exercise on patients with psoriasis, and we present the recommended duration and types of exercise that are most impactful for these patients.
One indicator of the link between psoriasis and exercise is the level of peroxisome proliferator activated receptor gamma coactivator-1 α (PGC-1α) in muscle cells.2 This marker reduces inflammation. When levels are low in muscle cells, an induction occurs that leads to systemic or local inflammation; however, skeletal muscle PGC-1α levels increase following exercise, indicating reduced inflammation.2 The level of PGC-1α is measured through muscle biopsy and polymerase chain reaction.6 Another indicator of the correlation between exercise and inflammation is lipoprotein-associated phospholipase A2, which is produced by inflammatory cells and has a correlation with cardiovascular disease. Exercise reduces lipoprotein-associated phospholipase A2 levels, and a sedentary lifestyle correlates with increased levels of this marker.3 Lipoprotein-associated phospholipase A2 is measured through an enzyme-linked immunosorbent assay of the blood, with levels around 200 ng/mL considered high.7 Patients with psoriasis are 30% less likely to participate in physical activity compared to patients without psoriasis, which can be attributed to psychosocial impairment and other factors. Sedentary lifestyle is associated with new or worsening metabolic disease and prevalence of psoriatic lesions.1
A metabolic equivalent task score is a classification system that measures the rate of the body’s oxygen uptake for any given activity.4 A score of 20.9 or more metabolic equivalent task hours of vigorous exercise per week—equal to 105 minutes of running or 180 minutes of swimming or playing tennis—is linked with a 25% to 30% risk reduction of psoriasis in women.4 Therefore, we recommend 30 minutes of exercise 4 to 5 times per week for women. These periods of exercise should consist mainly of activities that will not cause psoriasis flares due to excessive sweating, skin trauma, or prolonged sun exposure.5 Walking, yoga, and bike riding all could be good exercise options for those with psoriasis. The National Psoriasis Foundation offers guidance on physical activity in patients with psoriasis or psoriatic arthritis.8 Psoriasis has apparent physical and psychosocial impacts on patients that can be prevented and improved through the exercise recommendations presented in this article. Dermatologists should use these recommendations to address psoriasis in their everyday practice.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153. doi:10.1111/1346-8138.12721
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454: 463-469. doi:10.1038/nature07206
- Clark K, Sharp S, Womack CJ, et al. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;32:1703-1710. doi:10.1016/j.numecd.2022.04.023
- Yeh C, Flatley E, Elkattawy O, et al. Exercise in dermatology: exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. doi:10.1016/j.jaad.2021.07.023
- Sheppard R, Gan WK, Onambele-Pearson GL, et al. Developing an aerobic exercise intervention for patients with psoriasis to support lifestyle behaviour change and improve health outcomes. Clin Exp Dermatol. 2023;48:5-11. doi:10.1093/ced/llac008
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904.023
- National Psoriasis Foundation. Active and mindful lifestyles. https://www.psoriasis.org/active-and-mindful-lifestyles/
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153. doi:10.1111/1346-8138.12721
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454: 463-469. doi:10.1038/nature07206
- Clark K, Sharp S, Womack CJ, et al. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;32:1703-1710. doi:10.1016/j.numecd.2022.04.023
- Yeh C, Flatley E, Elkattawy O, et al. Exercise in dermatology: exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. doi:10.1016/j.jaad.2021.07.023
- Sheppard R, Gan WK, Onambele-Pearson GL, et al. Developing an aerobic exercise intervention for patients with psoriasis to support lifestyle behaviour change and improve health outcomes. Clin Exp Dermatol. 2023;48:5-11. doi:10.1093/ced/llac008
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904.023
- National Psoriasis Foundation. Active and mindful lifestyles. https://www.psoriasis.org/active-and-mindful-lifestyles/
Optimal Exercise Levels for Dermatology Patients With Psoriasis
Optimal Exercise Levels for Dermatology Patients With Psoriasis
PRACTICE POINTS
- Patients with psoriasis should exercise for less time (~30 min) more frequently (4–5 times per week).
- Exercise that involves excessive sweating should be avoided; recommended types of exercise for patients with psoriasis include walking, yoga, and bike riding.
- Physicians should educate patients on the processes behind psoriasis and direct them to the National Psoriasis Foundation’s website when needed.
Geriatric Dermatology: Q&A With Daniel C. Butler, MD
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
PRACTICE POINTS
- Nail psoriasis can masquerade as other dermatologic conditions, including squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
- Nail psoriasis can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.
- Deucravacitinib, an oral tyrosine kinase 2 inhibitor approved for the treatment of plaque psoriasis, has shown promise as an effective treatment for nail psoriasis in cases that are refractory to standard therapies.
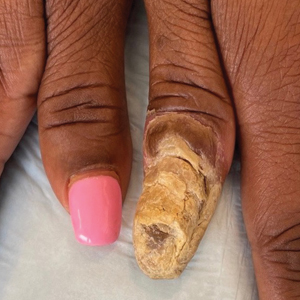
Adalimumab for Psoriasis: Study Compares Biosimilars Vs. Originator
TOPLINE:
rate than those who remained on Humira.
METHODOLOGY:
- Researchers conducted a cohort study using data on patients with psoriasis who were treated with adalimumab, a tumor necrosis factor alpha inhibitor used to treat moderate to severe psoriasis, from the French National Health Data System, British Association of Dermatologists Biologics and Immunomodulators Register, and Spanish Registry of Systemic Therapy in Psoriasis.
- The analysis included 7387 adalimumab-naive patients who were new users of an adalimumab biosimilar and 3654 patients (switchers) who switched from Humira to a biosimilar. Patients were matched and compared with patients receiving Humira.
- Co-primary outcomes of the study were drug discontinuation and serious adverse events.
- Researchers assessed the following adalimumab biosimilar brands: Amgevita, Imraldi, Hyrimoz, Idacio, and Hulio.
TAKEAWAY:
- All-cause drug discontinuation rates were similar between new users of biosimilars and Humira new users (hazard ratio [HR], 0.99; 95% CI, 0.94-1.04).
- Discontinuation rates were higher among those who switched from Humira to a biosimilar (HR, 1.35; 95% CI, 1.19-1.52) than among those who stayed on Humira. Switching to Amgevita (HR, 1.25; 95% CI, 1.13-1.27), Imraldi (HR, 1.53; 95% CI, 1.33-1.76), and Hyrimoz (HR, 1.80; 95% CI, 1.29-2.52) was associated with higher discontinuation rates.
- Serious adverse events were not significantly different between new users of Humira and biosimilar new users (incidence rate ratio [IRR], 0.91; 95% CI, 0.80-1.05), and between patients who switched from a biosimilar to Humira and those who stayed on Humira (IRR, 0.92; 95% CI, 0.83-1.01).
- No significant differences in discontinuation because of ineffectiveness were found between biosimilar and Humira new users (HR, 0.97; 95% CI, 0.88-1.08). Discontinuation because of adverse events was also comparable for all biosimilars among new users, except for Hyrimoz (HR, 0.54; 95% CI, 0.35-0.85), which showed fewer discontinuations than Humira.
IN PRACTICE:
“This study found comparable drug survival and safety between adalimumab biosimilars and Humira in adalimumab-naive patients, supporting the use of biosimilars as viable alternatives for new patients,” the authors wrote. However, noting that discontinuation was more likely among those who switched from Humira to a biosimilar, they added: “Changes in treatment response, skin or injection site reactions, and nocebo effects may contribute to treatment discontinuation post-switch. Thus, patients who switch from Humira to biosimilars may require closer monitoring and support to alleviate these challenges.”
SOURCE:
The study was led by Duc Binh Phan, Dermatology Centre, Northern Care Alliance NHS Foundation Trust in Manchester, England. It was published online in The British Journal of Dermatology.
LIMITATIONS:
Unmeasured factors including psychological perceptions, regional policies, and drug availability could influence drug survival, making the results not fully reflective of treatment effectiveness or safety. Most Humira users in registries were enrolled before biosimilars became available, making it impractical to match new users on the basis of treatment initiation years. Additionally, reasons for discontinuation were not available in the French National Health Data System.
DISCLOSURES:
In the United Kingdom, the research was funded by the Psoriasis Association PhD studentship and supported by the NIHR Manchester Biomedical Research Centre. In France, the authors are employees of the French National Health Insurance, the French National Agency for the Safety of Medicines and Health Products, and the Assistance Publique — Hôpitaux de Paris and received no funding. The authors reported receiving consulting and speaker fees and clinical trial sponsorship from various pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
rate than those who remained on Humira.
METHODOLOGY:
- Researchers conducted a cohort study using data on patients with psoriasis who were treated with adalimumab, a tumor necrosis factor alpha inhibitor used to treat moderate to severe psoriasis, from the French National Health Data System, British Association of Dermatologists Biologics and Immunomodulators Register, and Spanish Registry of Systemic Therapy in Psoriasis.
- The analysis included 7387 adalimumab-naive patients who were new users of an adalimumab biosimilar and 3654 patients (switchers) who switched from Humira to a biosimilar. Patients were matched and compared with patients receiving Humira.
- Co-primary outcomes of the study were drug discontinuation and serious adverse events.
- Researchers assessed the following adalimumab biosimilar brands: Amgevita, Imraldi, Hyrimoz, Idacio, and Hulio.
TAKEAWAY:
- All-cause drug discontinuation rates were similar between new users of biosimilars and Humira new users (hazard ratio [HR], 0.99; 95% CI, 0.94-1.04).
- Discontinuation rates were higher among those who switched from Humira to a biosimilar (HR, 1.35; 95% CI, 1.19-1.52) than among those who stayed on Humira. Switching to Amgevita (HR, 1.25; 95% CI, 1.13-1.27), Imraldi (HR, 1.53; 95% CI, 1.33-1.76), and Hyrimoz (HR, 1.80; 95% CI, 1.29-2.52) was associated with higher discontinuation rates.
- Serious adverse events were not significantly different between new users of Humira and biosimilar new users (incidence rate ratio [IRR], 0.91; 95% CI, 0.80-1.05), and between patients who switched from a biosimilar to Humira and those who stayed on Humira (IRR, 0.92; 95% CI, 0.83-1.01).
- No significant differences in discontinuation because of ineffectiveness were found between biosimilar and Humira new users (HR, 0.97; 95% CI, 0.88-1.08). Discontinuation because of adverse events was also comparable for all biosimilars among new users, except for Hyrimoz (HR, 0.54; 95% CI, 0.35-0.85), which showed fewer discontinuations than Humira.
IN PRACTICE:
“This study found comparable drug survival and safety between adalimumab biosimilars and Humira in adalimumab-naive patients, supporting the use of biosimilars as viable alternatives for new patients,” the authors wrote. However, noting that discontinuation was more likely among those who switched from Humira to a biosimilar, they added: “Changes in treatment response, skin or injection site reactions, and nocebo effects may contribute to treatment discontinuation post-switch. Thus, patients who switch from Humira to biosimilars may require closer monitoring and support to alleviate these challenges.”
SOURCE:
The study was led by Duc Binh Phan, Dermatology Centre, Northern Care Alliance NHS Foundation Trust in Manchester, England. It was published online in The British Journal of Dermatology.
LIMITATIONS:
Unmeasured factors including psychological perceptions, regional policies, and drug availability could influence drug survival, making the results not fully reflective of treatment effectiveness or safety. Most Humira users in registries were enrolled before biosimilars became available, making it impractical to match new users on the basis of treatment initiation years. Additionally, reasons for discontinuation were not available in the French National Health Data System.
DISCLOSURES:
In the United Kingdom, the research was funded by the Psoriasis Association PhD studentship and supported by the NIHR Manchester Biomedical Research Centre. In France, the authors are employees of the French National Health Insurance, the French National Agency for the Safety of Medicines and Health Products, and the Assistance Publique — Hôpitaux de Paris and received no funding. The authors reported receiving consulting and speaker fees and clinical trial sponsorship from various pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
rate than those who remained on Humira.
METHODOLOGY:
- Researchers conducted a cohort study using data on patients with psoriasis who were treated with adalimumab, a tumor necrosis factor alpha inhibitor used to treat moderate to severe psoriasis, from the French National Health Data System, British Association of Dermatologists Biologics and Immunomodulators Register, and Spanish Registry of Systemic Therapy in Psoriasis.
- The analysis included 7387 adalimumab-naive patients who were new users of an adalimumab biosimilar and 3654 patients (switchers) who switched from Humira to a biosimilar. Patients were matched and compared with patients receiving Humira.
- Co-primary outcomes of the study were drug discontinuation and serious adverse events.
- Researchers assessed the following adalimumab biosimilar brands: Amgevita, Imraldi, Hyrimoz, Idacio, and Hulio.
TAKEAWAY:
- All-cause drug discontinuation rates were similar between new users of biosimilars and Humira new users (hazard ratio [HR], 0.99; 95% CI, 0.94-1.04).
- Discontinuation rates were higher among those who switched from Humira to a biosimilar (HR, 1.35; 95% CI, 1.19-1.52) than among those who stayed on Humira. Switching to Amgevita (HR, 1.25; 95% CI, 1.13-1.27), Imraldi (HR, 1.53; 95% CI, 1.33-1.76), and Hyrimoz (HR, 1.80; 95% CI, 1.29-2.52) was associated with higher discontinuation rates.
- Serious adverse events were not significantly different between new users of Humira and biosimilar new users (incidence rate ratio [IRR], 0.91; 95% CI, 0.80-1.05), and between patients who switched from a biosimilar to Humira and those who stayed on Humira (IRR, 0.92; 95% CI, 0.83-1.01).
- No significant differences in discontinuation because of ineffectiveness were found between biosimilar and Humira new users (HR, 0.97; 95% CI, 0.88-1.08). Discontinuation because of adverse events was also comparable for all biosimilars among new users, except for Hyrimoz (HR, 0.54; 95% CI, 0.35-0.85), which showed fewer discontinuations than Humira.
IN PRACTICE:
“This study found comparable drug survival and safety between adalimumab biosimilars and Humira in adalimumab-naive patients, supporting the use of biosimilars as viable alternatives for new patients,” the authors wrote. However, noting that discontinuation was more likely among those who switched from Humira to a biosimilar, they added: “Changes in treatment response, skin or injection site reactions, and nocebo effects may contribute to treatment discontinuation post-switch. Thus, patients who switch from Humira to biosimilars may require closer monitoring and support to alleviate these challenges.”
SOURCE:
The study was led by Duc Binh Phan, Dermatology Centre, Northern Care Alliance NHS Foundation Trust in Manchester, England. It was published online in The British Journal of Dermatology.
LIMITATIONS:
Unmeasured factors including psychological perceptions, regional policies, and drug availability could influence drug survival, making the results not fully reflective of treatment effectiveness or safety. Most Humira users in registries were enrolled before biosimilars became available, making it impractical to match new users on the basis of treatment initiation years. Additionally, reasons for discontinuation were not available in the French National Health Data System.
DISCLOSURES:
In the United Kingdom, the research was funded by the Psoriasis Association PhD studentship and supported by the NIHR Manchester Biomedical Research Centre. In France, the authors are employees of the French National Health Insurance, the French National Agency for the Safety of Medicines and Health Products, and the Assistance Publique — Hôpitaux de Paris and received no funding. The authors reported receiving consulting and speaker fees and clinical trial sponsorship from various pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Post COVID-19, Long-term Risk for Autoimmune, Autoinflammatory Skin Disorders Increased, Study Finds
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Updated Guidance for Psoriatic Arthritis Ultrasound Comes at Time of Growing Use, New Technology
WASHINGTON — New draft guidance on the use of musculoskeletal ultrasound (MSUS) for diagnosis, monitoring, and prognosis of psoriatic arthritis was presented at the American College of Rheumatology (ACR) 2024 Annual Meeting. The new recommendations, intended to update 2012 guidance on rheumatologic use of MSUS, will go through another round of expert committee voting before being finalized and published.
“Even in the last 12 years, we’ve seen substantive advances, and there’s been significant improvements in musculoskeletal ultrasound technology,” Veena K. Ranganath, MD, professor of clinical medicine at the University of California, Los Angeles, and director of their Rheumatology Fellowship Musculoskeletal Ultrasound Training Program, told attendees. She noted that more than 30,000 articles on MSUS and arthritis have been published since the 2012 guidance. “We’ve seen mastery in teaching and really a wide distribution of this education to the next generation of rheumatologists, and this has led to significant increases in the use of musculoskeletal ultrasound in clinical practices.”
She also noted there have been significant improvements in therapeutic agents and strategies in psoriatic arthritis medications and that differences in today’s patients compared with those of a decade ago have influenced clinical questions related to the use of MSUS in rheumatology.
To develop the guidelines, a committee identified key domains and relevant clinical questions for ultrasonography using the PICO model (patient/population, intervention, comparison, and outcomes). A review of the literature published since 1993 in PubMed, Embase, and the Cochrane Database provided the evidence base, and a committee of 11 experts voted on the strength of the evidence for 22 statements. They rejected two that lacked consensus, and another round of voting will occur before the guidance is published.
Michael Stein, MD, assistant professor of medicine in rheumatology at McGill University in Montreal, Quebec, Canada, who was not involved in the guidance development, said he hopes and expects this new guidance will help persuade more clinicians to recognize the value of using MSUS in their practice.
“Number one, it’ll highlight the huge amount of data that exist that support using this technology for managing these groups of patients, among others, and I think it’ll also highlight the enormous number of questions that still exist that will hopefully be answered in the future, promoting new research,” Stein told this news organization.
“I do think it does allow people who are not comfortable with technology to adopt technology in a very gradual way and make it less threatening,” Stein added.
“Ultrasound is becoming part of the landscape, and so increasingly, we’re trying to promote it as being part of the standard of care, or at least an adjunct to care. I commend the committee for doing all this amazing work.”
Predicting and Diagnosing Early Psoriatic Arthritis
Catherine J. Bakewell, MD, a rheumatologist at Intermountain Health in Salt Lake City, Utah, reviewed the committee’s statements, starting with strong consensus that MSUS can help with diagnosing early psoriatic arthritis. Evidence has shown that patients with psoriasis who have subclinical synovitis, enthesitis, and other features have gone on to develop psoriatic arthritis, and researchers have documented the transition with ultrasonography.
“We can use it to enhance our CASPAR classification criteria” by using ultrasound to change how clinicians apply the classification criteria, Bakewell said. “For example, in order to go through those classification criteria, a patient has to have confirmed inflammatory articular disease, either the joint synthesis or spine, and ultrasound can help clarify that state for us.”
She also noted the potential for ultrasonography to help as a screening tool because studies have suggested that dermatologists’ use of handheld ultrasound transducers can help in screening appropriate patients to refer to rheumatologists.
Patients with psoriasis being evaluated for a potential early psoriatic arthritis diagnosis should undergo MSUS of the bilateral quadriceps tendon, patellar ligament, Achilles tendon, and plantar fascia entheses at a minimum, per moderate consensus.
“This truly is just designed to be the highest bang for your buck. This is designed for clinicians in practice,” Bakewell said. She noted criticism about the exclusion of upper extremities — something that will be discussed in the future published paper — but one reason that was excluded is because common findings have occurred in healthy individuals in some areas.
Moderate consensus also supported reliance on entheseal features — including hypoechogenicity, thickening, Doppler signal, bone erosions, enthesophytes/calcifications, and bursal enlargement — to support a diagnosis. Interpretation of entheseal changes in patients with psoriasis should take into account characteristics such as age, body mass index (BMI), and biomechanical stress.
“There are numerous articles already existing pointing out that people who are over the age of 50 with a BMI over 30 kg/m2 or who have higher levels of biomechanical stress will score more highly on endocytoscoring systems, even in the absence of an underlying disorder,” Bakewell said. Among the mitigating strategies proposed in the literature are to have at least three positive sites to qualify for an indication or to look at the specificity of each elementary lesion. “Whatever mitigating strategy the clinician chooses to use, they need to bear in mind some of these features are not exclusive to spondyloarthritis,” she said. “It has to be taken in the clinical context.”
Scanning the hand, wrist, foot, and relevant symptomatic joints with MSUS to diagnose early psoriatic arthritis in patients with psoriasis received strong consensus. Intracapsular findings of synovitis and erosions may help support an early diagnosis in patients with psoriasis. “These are not obviously specific to psoriatic arthritis but support the diagnosis” with moderate consensus, Bakewell said. “The more specific findings are these extracapsular findings — which did attain a strong level of consensus — which are enthesitis, tenosynovitis, and dactylitis, all supporting that diagnosis of early psoriatic arthritis.”
For patients with psoriatic arthritis, the cutoff for defining a positive joint received moderate consensus for grayscale (GS) of at least 2 or at least 1 with power Doppler (PD) of at least 1.
Strong consensus supported confirming the presence of dactylitis in patients with psoriasis or psoriatic arthritis through a combination of features including tenosynovitis, subcutaneous edema, soft tissue thickening, synovitis, paratenonitis, and pulley thickening.
“I will also note that enthesitis is missing from this definition of dactylitis,” Bakewell said. “It is, however, a feature that is detectable with those higher-frequency transducers, but this is a relatively early area of research and did not make it into this guidance statement.”
Moderate consensus supported determination of an increased risk of radiographic erosions in patients with a dactylitis PD score of at least 1.
“We know as far back as 2005, Brockbank et al taught us that the dactylitic digit is associated with radiographic erosion in that particular digit,” Bakewell said. “Flash forward all the way to 2021: Dubash et al published the paper, ‘Dactylitis is an indicator of a more severe phenotype independently associated with greater swollen joint counts, C-reactive protein, ultrasound synovitis, and erosive damage,’ showing us that this is more than just that particular digit. It is a more severe phenotype, and very minimal Doppler signal, just 1+, is associated with erosive damage.”
Progression of Psoriatic Arthritis and Shared Decision-Making
Strong consensus existed for all statements related to progression of psoriatic arthritis and the role of MSUS in shared decision-making. The first is that synovitis and enthesitis in MSUS can predict radiographic progression and worsening of patient-related outcomes. Second, sonographic features — including increased Doppler signal in synovitis, enthesitis, and tenosynovitis — and presence of bone erosions and dactylitis can help inform decisions regarding therapy escalation.
“This is the first treatment management–specific statement we have made, but we feel this to be justified because each of these ultrasonographic features is associated with overall inflammatory burden and worse outcomes, be it health assessment questionnaires, disability index, or patient-reported outcomes to harder endpoints, such as radiographic erosions or relapse of clinical remission,” Bakewell said.
Finally, MSUS can help inform patients of their disease activity to assist in shared decision-making regarding escalation or de-escalation of therapy.
“We’ve all had this in our practices. You’ve had the patient in front of you who is very inflamed, and they say, ‘Doctor, can’t I please use doTERRA oils? Do I really need to go on one of these toxic drugs? I’ve read the package insert,’” Bakewell said. “Aside from having that conversation about the relative risk–benefit of any individual medication that you recommend, it’s helpful to put the ultrasound transducer on the patient, show them the fire of the Doppler, show them the erosion, show them the damage that is being done. It comes to life for them, especially if they’re not suffering that much with pain or stiffness.”
Bakewell also addressed patients at the other end of the pain spectrum who are suffering more. “You’ve also probably had the patient with psoriatic arthritis and fibromyalgia who comes in and tells you, ‘Doctor, my psoriatic arthritis has been terrible. I’m flaring. I need more immune-suppressing medication,’” she said. “Their exam looks pretty good, and it’s helpful to put that transducer on them and show them the absence of Doppler signal, show them that you’re taking them very seriously. You didn’t just squeeze them and say they’re fine, but you looked more deeply. You looked underneath the skin, and that helps with that patient–provider understanding and communication. I use this every day.”
Clarifying Disease State and Defining Remission
As with patients with psoriasis undergoing evaluation, there was strong consensus for interpreting entheseal changes in psoriatic arthritis in the context of patient characteristics such as age, BMI, and biomechanical stress.
There was moderate consensus for confirming psoriatic arthritis flare with MSUS. Bakewell noted that many have seen in their practices how physical exams can be misleading, such as when a patient appears clinically normal but has ongoing synovitis, or on the flip side, the patient has a swollen joint but nothing is lighting up with Doppler on the ultrasound.
All of the statements on MSUS for remission received moderate consensus. These included defining MSUS remission as a PD score of 0 in entheses and synovial tissues and defining ultrasonographic remission as a total PD ultrasound score of 0, summing all analyzed joints and entheses, at a single given time point.
When using MSUS to evaluate for remission, it’s reasonable to screen the lower-extremity entheses, wrists, metacarpophalangeal joints, interphalangeal hand joints, metatarsophalangeal joints, and relevant symptomatic joints. The inflammatory features to evaluate to confirm ultrasound-defined remission include PD enthesitis, GS and PD synovitis, tenosynovitis, and dactylitis. Finally, for those in remission, subclinical inflammation detected by MSUS likely predicts a higher rate of flare.
During the discussion, Bakewell reiterated that MSUS should be regarded as a tool for patient subsets who can benefit from its use, rather than being used routinely across large patient groups without a clear purpose. “It’s used to answer a question,” she said. “If you’re going to demonstrate the efficacy of a tool, you have to use it appropriately, aka when there’s a question. We don’t need to ultrasound every patient every visit.”
No external funding for the development of the guidance was noted. Ranganath has reported receiving research support from Bristol Myers Squibb and Mallinckrodt. Bakewell has reported receiving speaking/consulting fees from AbbVie, UCB, Lilly, Janssen, Novartis, Sanofi/Regeneron/Genzyme, and Pfizer. Stein had no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — New draft guidance on the use of musculoskeletal ultrasound (MSUS) for diagnosis, monitoring, and prognosis of psoriatic arthritis was presented at the American College of Rheumatology (ACR) 2024 Annual Meeting. The new recommendations, intended to update 2012 guidance on rheumatologic use of MSUS, will go through another round of expert committee voting before being finalized and published.
“Even in the last 12 years, we’ve seen substantive advances, and there’s been significant improvements in musculoskeletal ultrasound technology,” Veena K. Ranganath, MD, professor of clinical medicine at the University of California, Los Angeles, and director of their Rheumatology Fellowship Musculoskeletal Ultrasound Training Program, told attendees. She noted that more than 30,000 articles on MSUS and arthritis have been published since the 2012 guidance. “We’ve seen mastery in teaching and really a wide distribution of this education to the next generation of rheumatologists, and this has led to significant increases in the use of musculoskeletal ultrasound in clinical practices.”
She also noted there have been significant improvements in therapeutic agents and strategies in psoriatic arthritis medications and that differences in today’s patients compared with those of a decade ago have influenced clinical questions related to the use of MSUS in rheumatology.
To develop the guidelines, a committee identified key domains and relevant clinical questions for ultrasonography using the PICO model (patient/population, intervention, comparison, and outcomes). A review of the literature published since 1993 in PubMed, Embase, and the Cochrane Database provided the evidence base, and a committee of 11 experts voted on the strength of the evidence for 22 statements. They rejected two that lacked consensus, and another round of voting will occur before the guidance is published.
Michael Stein, MD, assistant professor of medicine in rheumatology at McGill University in Montreal, Quebec, Canada, who was not involved in the guidance development, said he hopes and expects this new guidance will help persuade more clinicians to recognize the value of using MSUS in their practice.
“Number one, it’ll highlight the huge amount of data that exist that support using this technology for managing these groups of patients, among others, and I think it’ll also highlight the enormous number of questions that still exist that will hopefully be answered in the future, promoting new research,” Stein told this news organization.
“I do think it does allow people who are not comfortable with technology to adopt technology in a very gradual way and make it less threatening,” Stein added.
“Ultrasound is becoming part of the landscape, and so increasingly, we’re trying to promote it as being part of the standard of care, or at least an adjunct to care. I commend the committee for doing all this amazing work.”
Predicting and Diagnosing Early Psoriatic Arthritis
Catherine J. Bakewell, MD, a rheumatologist at Intermountain Health in Salt Lake City, Utah, reviewed the committee’s statements, starting with strong consensus that MSUS can help with diagnosing early psoriatic arthritis. Evidence has shown that patients with psoriasis who have subclinical synovitis, enthesitis, and other features have gone on to develop psoriatic arthritis, and researchers have documented the transition with ultrasonography.
“We can use it to enhance our CASPAR classification criteria” by using ultrasound to change how clinicians apply the classification criteria, Bakewell said. “For example, in order to go through those classification criteria, a patient has to have confirmed inflammatory articular disease, either the joint synthesis or spine, and ultrasound can help clarify that state for us.”
She also noted the potential for ultrasonography to help as a screening tool because studies have suggested that dermatologists’ use of handheld ultrasound transducers can help in screening appropriate patients to refer to rheumatologists.
Patients with psoriasis being evaluated for a potential early psoriatic arthritis diagnosis should undergo MSUS of the bilateral quadriceps tendon, patellar ligament, Achilles tendon, and plantar fascia entheses at a minimum, per moderate consensus.
“This truly is just designed to be the highest bang for your buck. This is designed for clinicians in practice,” Bakewell said. She noted criticism about the exclusion of upper extremities — something that will be discussed in the future published paper — but one reason that was excluded is because common findings have occurred in healthy individuals in some areas.
Moderate consensus also supported reliance on entheseal features — including hypoechogenicity, thickening, Doppler signal, bone erosions, enthesophytes/calcifications, and bursal enlargement — to support a diagnosis. Interpretation of entheseal changes in patients with psoriasis should take into account characteristics such as age, body mass index (BMI), and biomechanical stress.
“There are numerous articles already existing pointing out that people who are over the age of 50 with a BMI over 30 kg/m2 or who have higher levels of biomechanical stress will score more highly on endocytoscoring systems, even in the absence of an underlying disorder,” Bakewell said. Among the mitigating strategies proposed in the literature are to have at least three positive sites to qualify for an indication or to look at the specificity of each elementary lesion. “Whatever mitigating strategy the clinician chooses to use, they need to bear in mind some of these features are not exclusive to spondyloarthritis,” she said. “It has to be taken in the clinical context.”
Scanning the hand, wrist, foot, and relevant symptomatic joints with MSUS to diagnose early psoriatic arthritis in patients with psoriasis received strong consensus. Intracapsular findings of synovitis and erosions may help support an early diagnosis in patients with psoriasis. “These are not obviously specific to psoriatic arthritis but support the diagnosis” with moderate consensus, Bakewell said. “The more specific findings are these extracapsular findings — which did attain a strong level of consensus — which are enthesitis, tenosynovitis, and dactylitis, all supporting that diagnosis of early psoriatic arthritis.”
For patients with psoriatic arthritis, the cutoff for defining a positive joint received moderate consensus for grayscale (GS) of at least 2 or at least 1 with power Doppler (PD) of at least 1.
Strong consensus supported confirming the presence of dactylitis in patients with psoriasis or psoriatic arthritis through a combination of features including tenosynovitis, subcutaneous edema, soft tissue thickening, synovitis, paratenonitis, and pulley thickening.
“I will also note that enthesitis is missing from this definition of dactylitis,” Bakewell said. “It is, however, a feature that is detectable with those higher-frequency transducers, but this is a relatively early area of research and did not make it into this guidance statement.”
Moderate consensus supported determination of an increased risk of radiographic erosions in patients with a dactylitis PD score of at least 1.
“We know as far back as 2005, Brockbank et al taught us that the dactylitic digit is associated with radiographic erosion in that particular digit,” Bakewell said. “Flash forward all the way to 2021: Dubash et al published the paper, ‘Dactylitis is an indicator of a more severe phenotype independently associated with greater swollen joint counts, C-reactive protein, ultrasound synovitis, and erosive damage,’ showing us that this is more than just that particular digit. It is a more severe phenotype, and very minimal Doppler signal, just 1+, is associated with erosive damage.”
Progression of Psoriatic Arthritis and Shared Decision-Making
Strong consensus existed for all statements related to progression of psoriatic arthritis and the role of MSUS in shared decision-making. The first is that synovitis and enthesitis in MSUS can predict radiographic progression and worsening of patient-related outcomes. Second, sonographic features — including increased Doppler signal in synovitis, enthesitis, and tenosynovitis — and presence of bone erosions and dactylitis can help inform decisions regarding therapy escalation.
“This is the first treatment management–specific statement we have made, but we feel this to be justified because each of these ultrasonographic features is associated with overall inflammatory burden and worse outcomes, be it health assessment questionnaires, disability index, or patient-reported outcomes to harder endpoints, such as radiographic erosions or relapse of clinical remission,” Bakewell said.
Finally, MSUS can help inform patients of their disease activity to assist in shared decision-making regarding escalation or de-escalation of therapy.
“We’ve all had this in our practices. You’ve had the patient in front of you who is very inflamed, and they say, ‘Doctor, can’t I please use doTERRA oils? Do I really need to go on one of these toxic drugs? I’ve read the package insert,’” Bakewell said. “Aside from having that conversation about the relative risk–benefit of any individual medication that you recommend, it’s helpful to put the ultrasound transducer on the patient, show them the fire of the Doppler, show them the erosion, show them the damage that is being done. It comes to life for them, especially if they’re not suffering that much with pain or stiffness.”
Bakewell also addressed patients at the other end of the pain spectrum who are suffering more. “You’ve also probably had the patient with psoriatic arthritis and fibromyalgia who comes in and tells you, ‘Doctor, my psoriatic arthritis has been terrible. I’m flaring. I need more immune-suppressing medication,’” she said. “Their exam looks pretty good, and it’s helpful to put that transducer on them and show them the absence of Doppler signal, show them that you’re taking them very seriously. You didn’t just squeeze them and say they’re fine, but you looked more deeply. You looked underneath the skin, and that helps with that patient–provider understanding and communication. I use this every day.”
Clarifying Disease State and Defining Remission
As with patients with psoriasis undergoing evaluation, there was strong consensus for interpreting entheseal changes in psoriatic arthritis in the context of patient characteristics such as age, BMI, and biomechanical stress.
There was moderate consensus for confirming psoriatic arthritis flare with MSUS. Bakewell noted that many have seen in their practices how physical exams can be misleading, such as when a patient appears clinically normal but has ongoing synovitis, or on the flip side, the patient has a swollen joint but nothing is lighting up with Doppler on the ultrasound.
All of the statements on MSUS for remission received moderate consensus. These included defining MSUS remission as a PD score of 0 in entheses and synovial tissues and defining ultrasonographic remission as a total PD ultrasound score of 0, summing all analyzed joints and entheses, at a single given time point.
When using MSUS to evaluate for remission, it’s reasonable to screen the lower-extremity entheses, wrists, metacarpophalangeal joints, interphalangeal hand joints, metatarsophalangeal joints, and relevant symptomatic joints. The inflammatory features to evaluate to confirm ultrasound-defined remission include PD enthesitis, GS and PD synovitis, tenosynovitis, and dactylitis. Finally, for those in remission, subclinical inflammation detected by MSUS likely predicts a higher rate of flare.
During the discussion, Bakewell reiterated that MSUS should be regarded as a tool for patient subsets who can benefit from its use, rather than being used routinely across large patient groups without a clear purpose. “It’s used to answer a question,” she said. “If you’re going to demonstrate the efficacy of a tool, you have to use it appropriately, aka when there’s a question. We don’t need to ultrasound every patient every visit.”
No external funding for the development of the guidance was noted. Ranganath has reported receiving research support from Bristol Myers Squibb and Mallinckrodt. Bakewell has reported receiving speaking/consulting fees from AbbVie, UCB, Lilly, Janssen, Novartis, Sanofi/Regeneron/Genzyme, and Pfizer. Stein had no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — New draft guidance on the use of musculoskeletal ultrasound (MSUS) for diagnosis, monitoring, and prognosis of psoriatic arthritis was presented at the American College of Rheumatology (ACR) 2024 Annual Meeting. The new recommendations, intended to update 2012 guidance on rheumatologic use of MSUS, will go through another round of expert committee voting before being finalized and published.
“Even in the last 12 years, we’ve seen substantive advances, and there’s been significant improvements in musculoskeletal ultrasound technology,” Veena K. Ranganath, MD, professor of clinical medicine at the University of California, Los Angeles, and director of their Rheumatology Fellowship Musculoskeletal Ultrasound Training Program, told attendees. She noted that more than 30,000 articles on MSUS and arthritis have been published since the 2012 guidance. “We’ve seen mastery in teaching and really a wide distribution of this education to the next generation of rheumatologists, and this has led to significant increases in the use of musculoskeletal ultrasound in clinical practices.”
She also noted there have been significant improvements in therapeutic agents and strategies in psoriatic arthritis medications and that differences in today’s patients compared with those of a decade ago have influenced clinical questions related to the use of MSUS in rheumatology.
To develop the guidelines, a committee identified key domains and relevant clinical questions for ultrasonography using the PICO model (patient/population, intervention, comparison, and outcomes). A review of the literature published since 1993 in PubMed, Embase, and the Cochrane Database provided the evidence base, and a committee of 11 experts voted on the strength of the evidence for 22 statements. They rejected two that lacked consensus, and another round of voting will occur before the guidance is published.
Michael Stein, MD, assistant professor of medicine in rheumatology at McGill University in Montreal, Quebec, Canada, who was not involved in the guidance development, said he hopes and expects this new guidance will help persuade more clinicians to recognize the value of using MSUS in their practice.
“Number one, it’ll highlight the huge amount of data that exist that support using this technology for managing these groups of patients, among others, and I think it’ll also highlight the enormous number of questions that still exist that will hopefully be answered in the future, promoting new research,” Stein told this news organization.
“I do think it does allow people who are not comfortable with technology to adopt technology in a very gradual way and make it less threatening,” Stein added.
“Ultrasound is becoming part of the landscape, and so increasingly, we’re trying to promote it as being part of the standard of care, or at least an adjunct to care. I commend the committee for doing all this amazing work.”
Predicting and Diagnosing Early Psoriatic Arthritis
Catherine J. Bakewell, MD, a rheumatologist at Intermountain Health in Salt Lake City, Utah, reviewed the committee’s statements, starting with strong consensus that MSUS can help with diagnosing early psoriatic arthritis. Evidence has shown that patients with psoriasis who have subclinical synovitis, enthesitis, and other features have gone on to develop psoriatic arthritis, and researchers have documented the transition with ultrasonography.
“We can use it to enhance our CASPAR classification criteria” by using ultrasound to change how clinicians apply the classification criteria, Bakewell said. “For example, in order to go through those classification criteria, a patient has to have confirmed inflammatory articular disease, either the joint synthesis or spine, and ultrasound can help clarify that state for us.”
She also noted the potential for ultrasonography to help as a screening tool because studies have suggested that dermatologists’ use of handheld ultrasound transducers can help in screening appropriate patients to refer to rheumatologists.
Patients with psoriasis being evaluated for a potential early psoriatic arthritis diagnosis should undergo MSUS of the bilateral quadriceps tendon, patellar ligament, Achilles tendon, and plantar fascia entheses at a minimum, per moderate consensus.
“This truly is just designed to be the highest bang for your buck. This is designed for clinicians in practice,” Bakewell said. She noted criticism about the exclusion of upper extremities — something that will be discussed in the future published paper — but one reason that was excluded is because common findings have occurred in healthy individuals in some areas.
Moderate consensus also supported reliance on entheseal features — including hypoechogenicity, thickening, Doppler signal, bone erosions, enthesophytes/calcifications, and bursal enlargement — to support a diagnosis. Interpretation of entheseal changes in patients with psoriasis should take into account characteristics such as age, body mass index (BMI), and biomechanical stress.
“There are numerous articles already existing pointing out that people who are over the age of 50 with a BMI over 30 kg/m2 or who have higher levels of biomechanical stress will score more highly on endocytoscoring systems, even in the absence of an underlying disorder,” Bakewell said. Among the mitigating strategies proposed in the literature are to have at least three positive sites to qualify for an indication or to look at the specificity of each elementary lesion. “Whatever mitigating strategy the clinician chooses to use, they need to bear in mind some of these features are not exclusive to spondyloarthritis,” she said. “It has to be taken in the clinical context.”
Scanning the hand, wrist, foot, and relevant symptomatic joints with MSUS to diagnose early psoriatic arthritis in patients with psoriasis received strong consensus. Intracapsular findings of synovitis and erosions may help support an early diagnosis in patients with psoriasis. “These are not obviously specific to psoriatic arthritis but support the diagnosis” with moderate consensus, Bakewell said. “The more specific findings are these extracapsular findings — which did attain a strong level of consensus — which are enthesitis, tenosynovitis, and dactylitis, all supporting that diagnosis of early psoriatic arthritis.”
For patients with psoriatic arthritis, the cutoff for defining a positive joint received moderate consensus for grayscale (GS) of at least 2 or at least 1 with power Doppler (PD) of at least 1.
Strong consensus supported confirming the presence of dactylitis in patients with psoriasis or psoriatic arthritis through a combination of features including tenosynovitis, subcutaneous edema, soft tissue thickening, synovitis, paratenonitis, and pulley thickening.
“I will also note that enthesitis is missing from this definition of dactylitis,” Bakewell said. “It is, however, a feature that is detectable with those higher-frequency transducers, but this is a relatively early area of research and did not make it into this guidance statement.”
Moderate consensus supported determination of an increased risk of radiographic erosions in patients with a dactylitis PD score of at least 1.
“We know as far back as 2005, Brockbank et al taught us that the dactylitic digit is associated with radiographic erosion in that particular digit,” Bakewell said. “Flash forward all the way to 2021: Dubash et al published the paper, ‘Dactylitis is an indicator of a more severe phenotype independently associated with greater swollen joint counts, C-reactive protein, ultrasound synovitis, and erosive damage,’ showing us that this is more than just that particular digit. It is a more severe phenotype, and very minimal Doppler signal, just 1+, is associated with erosive damage.”
Progression of Psoriatic Arthritis and Shared Decision-Making
Strong consensus existed for all statements related to progression of psoriatic arthritis and the role of MSUS in shared decision-making. The first is that synovitis and enthesitis in MSUS can predict radiographic progression and worsening of patient-related outcomes. Second, sonographic features — including increased Doppler signal in synovitis, enthesitis, and tenosynovitis — and presence of bone erosions and dactylitis can help inform decisions regarding therapy escalation.
“This is the first treatment management–specific statement we have made, but we feel this to be justified because each of these ultrasonographic features is associated with overall inflammatory burden and worse outcomes, be it health assessment questionnaires, disability index, or patient-reported outcomes to harder endpoints, such as radiographic erosions or relapse of clinical remission,” Bakewell said.
Finally, MSUS can help inform patients of their disease activity to assist in shared decision-making regarding escalation or de-escalation of therapy.
“We’ve all had this in our practices. You’ve had the patient in front of you who is very inflamed, and they say, ‘Doctor, can’t I please use doTERRA oils? Do I really need to go on one of these toxic drugs? I’ve read the package insert,’” Bakewell said. “Aside from having that conversation about the relative risk–benefit of any individual medication that you recommend, it’s helpful to put the ultrasound transducer on the patient, show them the fire of the Doppler, show them the erosion, show them the damage that is being done. It comes to life for them, especially if they’re not suffering that much with pain or stiffness.”
Bakewell also addressed patients at the other end of the pain spectrum who are suffering more. “You’ve also probably had the patient with psoriatic arthritis and fibromyalgia who comes in and tells you, ‘Doctor, my psoriatic arthritis has been terrible. I’m flaring. I need more immune-suppressing medication,’” she said. “Their exam looks pretty good, and it’s helpful to put that transducer on them and show them the absence of Doppler signal, show them that you’re taking them very seriously. You didn’t just squeeze them and say they’re fine, but you looked more deeply. You looked underneath the skin, and that helps with that patient–provider understanding and communication. I use this every day.”
Clarifying Disease State and Defining Remission
As with patients with psoriasis undergoing evaluation, there was strong consensus for interpreting entheseal changes in psoriatic arthritis in the context of patient characteristics such as age, BMI, and biomechanical stress.
There was moderate consensus for confirming psoriatic arthritis flare with MSUS. Bakewell noted that many have seen in their practices how physical exams can be misleading, such as when a patient appears clinically normal but has ongoing synovitis, or on the flip side, the patient has a swollen joint but nothing is lighting up with Doppler on the ultrasound.
All of the statements on MSUS for remission received moderate consensus. These included defining MSUS remission as a PD score of 0 in entheses and synovial tissues and defining ultrasonographic remission as a total PD ultrasound score of 0, summing all analyzed joints and entheses, at a single given time point.
When using MSUS to evaluate for remission, it’s reasonable to screen the lower-extremity entheses, wrists, metacarpophalangeal joints, interphalangeal hand joints, metatarsophalangeal joints, and relevant symptomatic joints. The inflammatory features to evaluate to confirm ultrasound-defined remission include PD enthesitis, GS and PD synovitis, tenosynovitis, and dactylitis. Finally, for those in remission, subclinical inflammation detected by MSUS likely predicts a higher rate of flare.
During the discussion, Bakewell reiterated that MSUS should be regarded as a tool for patient subsets who can benefit from its use, rather than being used routinely across large patient groups without a clear purpose. “It’s used to answer a question,” she said. “If you’re going to demonstrate the efficacy of a tool, you have to use it appropriately, aka when there’s a question. We don’t need to ultrasound every patient every visit.”
No external funding for the development of the guidance was noted. Ranganath has reported receiving research support from Bristol Myers Squibb and Mallinckrodt. Bakewell has reported receiving speaking/consulting fees from AbbVie, UCB, Lilly, Janssen, Novartis, Sanofi/Regeneron/Genzyme, and Pfizer. Stein had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACR 2024