User login
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
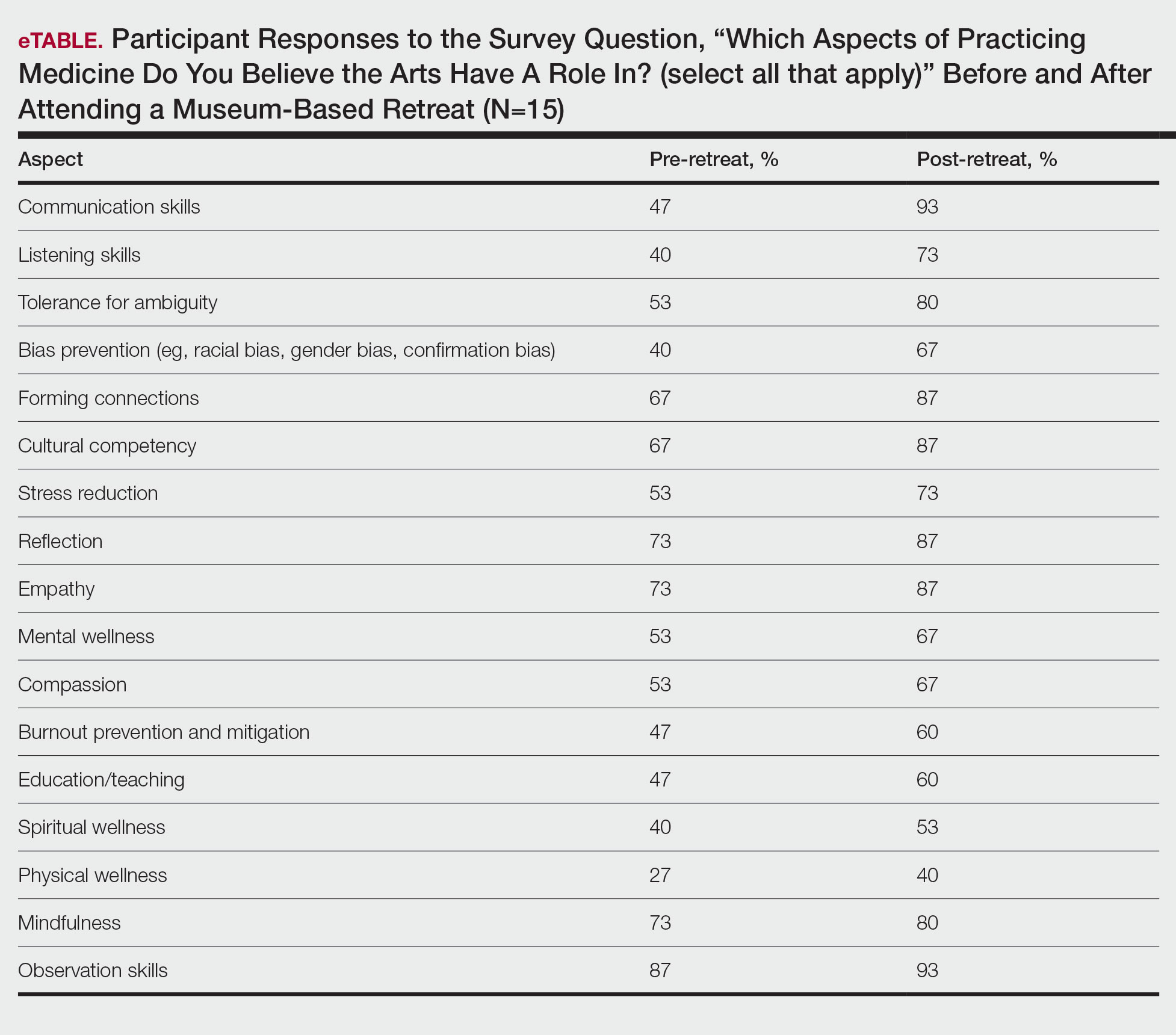
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).

Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).


Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Prior research has demonstrated that museum-based programming decreases resident burnout and depersonalization.1 A partnership between the Museum of Fine Arts Boston and the Harvard Combined Dermatology Residency Program was well received by residents and resulted in improvement of their observational skills.2 The impact of museum-based programming on the clinical practice skills and well-being of Duke dermatology residents and faculty has not been previously assessed.
In this study, our objective was to evaluate the impact of a 3-part museum-based arts retreat on arts appreciation, clinical practice skills, and well-being among dermatology resident and faculty participants. Surveys administered before and after the retreat were used to assess the value that participants attributed to the arts in various areas of clinical practice.
Methods
A 3-part museum-based retreat held on February 7, 2024, was developed with a Nasher Museum of Art (Durham, North Carolina) curator (E.R.). Part 1 was a personal response tour in which 15 residents and 3 faculty members were given individualized prompts and asked to identify an art piece in the museum that encapsulated their response; they then were asked to explain to the group why they chose that particular piece. Participants were given 10 minutes to explore the museum galleries to choose their piece, followed by 15 minutes to share their selected work in groups of 3 to 4.
Part 2 encompassed visual-thinking strategies, a research-based method that uses art to teach visual literacy, thinking, and communication skills.2 Using this method, facilitators follow a specific protocol to guide participants in the exploration of an art piece through sharing observations and interpretations.4 Participants were divided into 2 groups led by trained museum educators (including E.R.) to analyze and ascribe meaning to a chosen art piece. Three questions were asked: What’s going on in this picture? What do you see that makes you say that? What else can we find?
Part 3 involved back-to-back drawing, in which participants were paired up and tasked with recreating an art piece in the museum based solely on their partner’s verbal description. In each pair, both participants took turns as the describer and the drawer.
After each part of the retreat, 5 to 10 minutes were dedicated to debriefing in small groups about how each activity may connect to the role of a clinician. A total of 15 participants completed pre- and post-retreat surveys to assess the value they attributed to the arts and identify in which aspects of clinical practice they believe the arts play a role.
Results
Seventy-three percent of participants (11/15) found the museum-based retreat “extremely useful” or “very useful.” There was a 20% increase in those who attributed at least moderate value to the arts as a clinician after compared to before the retreat (13/15 [87%] vs 8/15 [53%]), and 100% of the participants desired to participate in future arts-based programming. Following the retreat, a greater percentage of participants believed the arts have a role in the following aspects of clinical practice: education, observation, listening, communication, empathy, compassion, forming connections, cultural sensitivity, tolerance for ambiguity, reflection, mindfulness, stress reduction, preventing burnout, bias prevention, mental wellness, spiritual wellness, and physical wellness (eTable). Qualitative feedback compiled from the participants’ responses to survey questions following the retreat about their thoughts on each activity and overall feedback was used to create a word cloud (eFigure).


Comment
The importance of arts and humanities integration into medical education previously has been described.5 Our survey results suggest that museum-based programming increases dermatology resident and faculty appreciation for the arts and encourages participation in future arts-based programming. Our results also demonstrate that arts-based programming positively impacts important resident competencies in the practice of medicine including tolerance for ambiguity, bias prevention, and cultural competency, and that the incorporation of arts-based programming can enhance residents’ well-being (physical, mental, and spiritual) as well as their ability to be better clinicians by addressing skills in communication, listening, and observation. The structure of our 3-part museum-based retreat offers practical implementation strategies for integrating the humanities into dermatology residency curricula and easily can be modified to meet the needs of different dermatology residency programs.
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Orr AR, Moghbeli N, Swain A, et al. The Fostering Resilience through Art in Medical Education (FRAME) workshop: a partnership with the Philadelphia Museum of Art. Adv Med Educ Pract. 2019;10:361-369. doi:10.2147/AMEP.S194575
Zimmermann C, Huang JT, Buzney EA. Refining the eye: dermatology and visual literacy. J Museum Ed. 2016;41:116-122.
Yenawine P. Visual Thinking Strategies: Using Art to Deepen Learning Across School Disciplines. Harvard Education Press; 2013.
Hailey D, Miller A, Yenawine P. Understanding visual literacy: the visual thinking strategies approach. In: Baylen DM, D’Alba A. Essentials of Teaching and Integrating Visual and Media Literacy: Visualizing Learning. Springer Cham; 2015:49-73. doi:10.1007/978-3-319-05837-5
Howley L, Gaufberg E, King BE. The Fundamental Role of the Arts and Humanities in Medical Education. Association of American Medical Colleges; 2020. Accessed December 18, 2025. https://store.aamc.org/the-fundamental-role-of-the-arts-and-humanities-in-medical-education.html
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Impact of a Museum-Based Retreat on the Clinical Skills and Well-Being of Dermatology Residents and Faculty
Practice Points
- Arts-based programming positively impacts resident competencies that are important to the practice of medicine.
- Incorporating arts-based programming in the dermatology residency curriculum can enhance resident well-being and the ability to be better clinicians.
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).
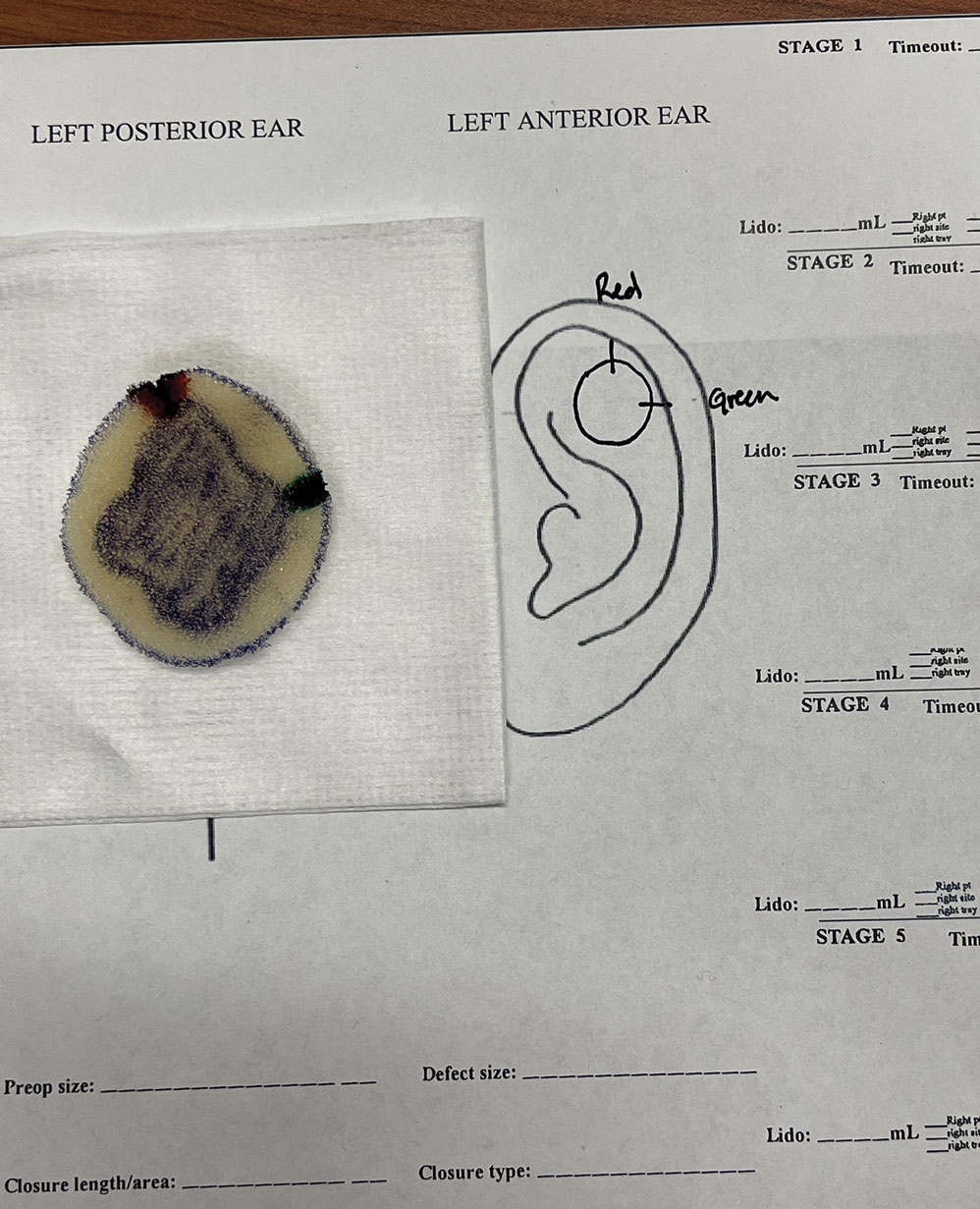
The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
Practice Gap
Tissue processing and complete margin assessment in Mohs micrographic surgery (MMS) are challenging concepts for residents, yet they are essential components of the dermatology residency curriculum. We propose a hands-on active teaching method using craft foam blocks to help residents master these techniques. Prior educational tools have included instructional videos1 as well as the peanut butter–cup and cantaloupe analogies.2,3 Specifically, our method utilizes inexpensive, readily available supplies that allow for repeated practice in a low-stakes environment without limitation of resources. This method provides an immersive, hands-on experience that allows residents to perform multiple practice excisions and simulate positive peripheral or deep margins, unlike tools that offer only fixed-depth or purely visual representations. Additionally, our learning model uniquely enables residents to flatten the simulated tissue, providing a clearer understanding of how a 3-dimensional specimen is transformed on a slide during histologic preparation. This step is particularly important, as tissue architecture can shift during processing, making it one of the most difficult concepts to grasp without hands-on experience. Having a multitude of teaching methods is crucial to accommodate various learning styles, and active learning has been shown to enhance retention for dermatology residents.4
The Technique
Residents use simple art supplies (including craft foam blocks and ink) and inexpensive, readily available surgical tools to simulate MMS (Table)(Figure 1). If desired, the resident can follow along with the comprehensive, stepwise textbook description of MMS, outlined by Benedetto et al5 to contextualize this hands-on exercise within a standardized didactic framework.


The foam block, which represents patient tissue, serves as the specimen. The resident begins by freehand drawing a simulated cutaneous tumor directly onto the foam using a surgical marking pen. At this point, the instructor discusses the advantages and limitations of tumor debulking with a sharp blade or curette. Residents then mark appropriate margins (1-3 mm) of normal-appearing “epidermis” on the foam block and add hash marks for orientation. This is another opportunity for the instructor to discuss common methods for marking tissue in vivo and to review situations when larger or smaller margins might be appropriate.
Next, the resident removes the first layer of simulated tissue using a disposable #15 blade scalpel at a 45° angle circumferentially and deep around the representative tumor. The resident also may use scissors and tissue forceps to remove the representative tumor. Next, the excised foam layer (the simulated “specimen”) is transferred to gauze. To demonstrate a positive margin, the resident or instructor marks the deep or peripheral foam block with a surgical marking pen, indicating residual tumor (Figure 2). This allows for multiple sequential layers of foam to be removed, demonstrating successive stages of MMS.

An inkwell holds different colors of washable paint to simulate tissue inking. After excision, the resident uses cotton-tipped applicators to apply different paint colors to the edges of the excised foam specimen at designated orientation points (eg, 3 o'clock and 12 o'clock). The resident then records the location of the excised sample by hand-drawing it on a printable Mohs map, labeling the corresponding paint colors to indicate orientation (Figure 3).

The resident then places the specimen between 2 plastic page protectors mimicking a glass slide and cover slip. Clear tape can be used to help flatten the specimen (Figure 4). The tissue is compressed between the page protector so that the simulated epidermis, dermis, and subcutaneous fat are all in the same plane. At this stage, the instructor may discuss the use of relaxing incisions, especially for deeper tissue specimens or when excision at a 45° bevel is not achieved.5 The view from the underside of the page protector reveals 100% of the specimen’s margin and mimics the first cut off the tissue block. The resident can visualize the complete circumferential, peripheral, and deep margins and can easily identify any positive margins. At this point, the exercise can conclude, or the resident can explore further stages for positive margins, bisected specimens, or other tissue preparation variations.

Practice Implications
By individually designing and removing a representative tumor with margins, creating hash marks, and preparing a tissue specimen for histologic analysis, our interactive teaching method provides dermatology residents with a relatively simple, effective, and active learning experience for MMS outside the surgical setting. Using a piece of craft foam allows the representative tissue to be manipulated and flattened, similar to cutaneous tissue. This method was implemented and refined across 3 separate teaching sessions held by teaching faculty (E.I.P and E.B.W.) at the San Antonio Uniformed Services Health Education Consortium Dermatology Residency Program (San Antonio, Texas). This method has consistently generated strong resident engagement and prompted insightful questions and discussions. Program directors at other residency programs can readily incorporate this method in their surgical curriculum by allocating a brief didactic period to the exercise and facilitating the discussion with a dermatologic surgeon. Its simplicity, low cost, and effectiveness make the foam block model an easily adoptable teaching tool for dermatology residency programs seeking to provide a comprehensive, hands-on understanding of MMS.
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
- McNeil E, Reich H, Hurliman E. Educational video improves dermatology residents’ understanding of Mohs micrographic surgery: a surveybased matched cohort study. J Am Acad Dermatol. 2020;83:926-927. doi:10.1016/j.jaad.2020.01.013
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744. doi:10.1001 /jamadermatol.2017.0614
- Vassantachart JM, Guccione J, Seeburger J. Clinical pearl: Mohs cantaloupe analogy for the dermatology resident. Cutis. 2018; 102:65-66.
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425. doi:10.1080/01421590801946988
- Benedetto PX, Poblete-Lopez C. Mohs micrographic surgery technique. Dermatol Clinics. 2011;29:141-151. doi:10.1016/j.det.2011.02.002
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Interactive Approach to Teaching Mohs Micrographic Surgery to Dermatology Residents
Mobile Tender Papule on the Scalp
Mobile Tender Papule on the Scalp
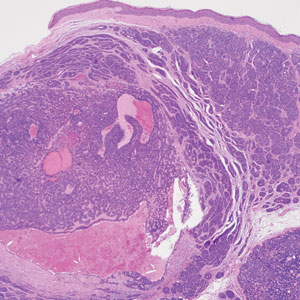
The Diagnosis: Spiradenocylindroma
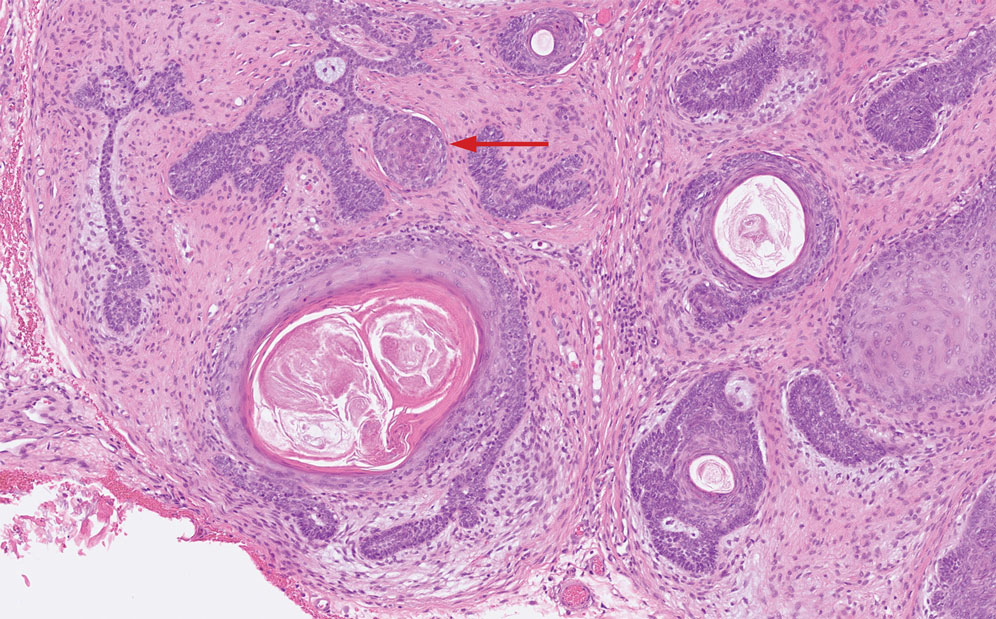
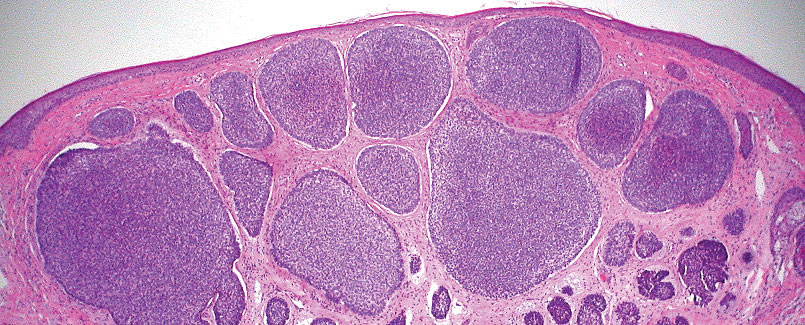
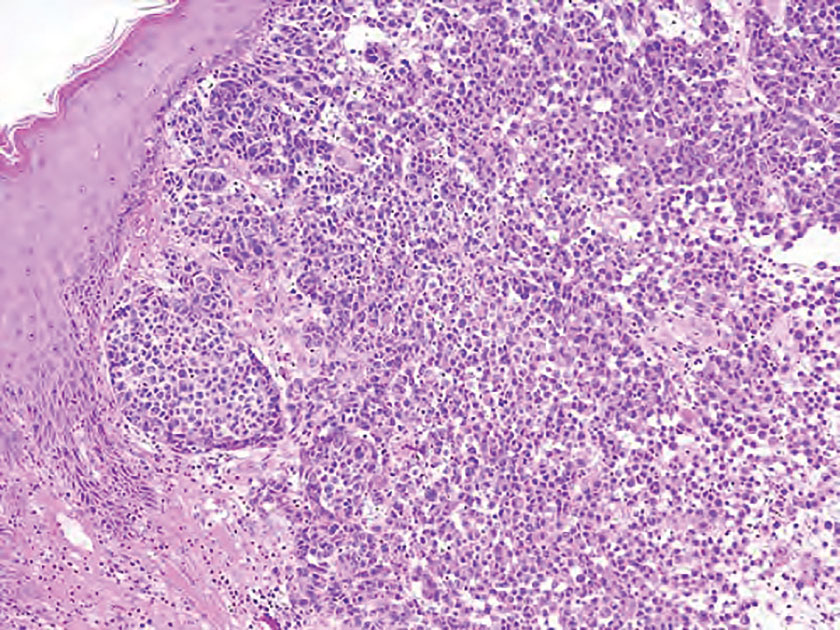
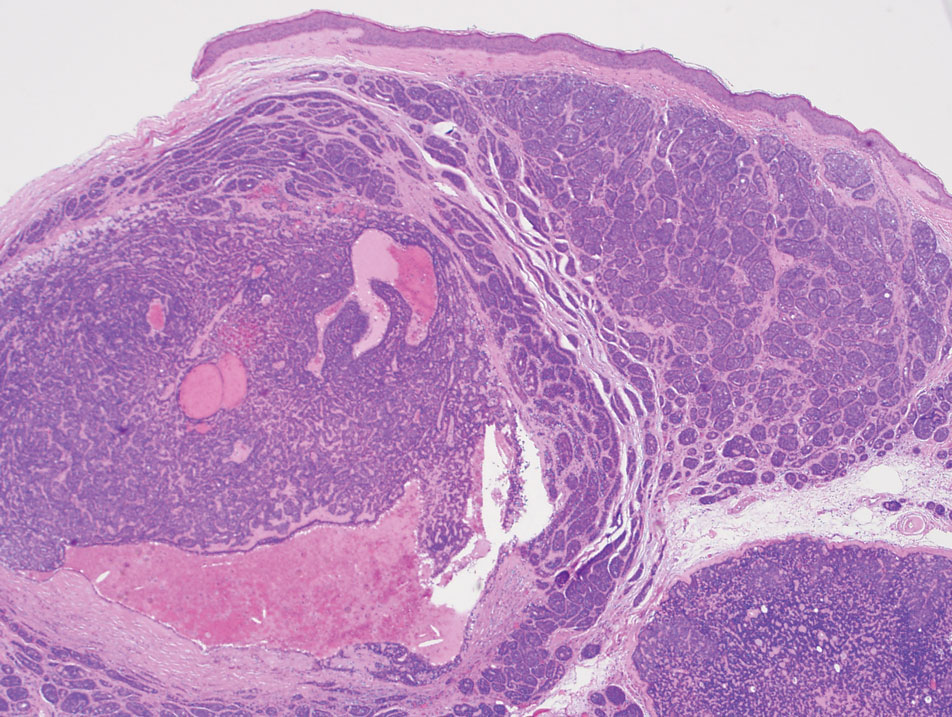
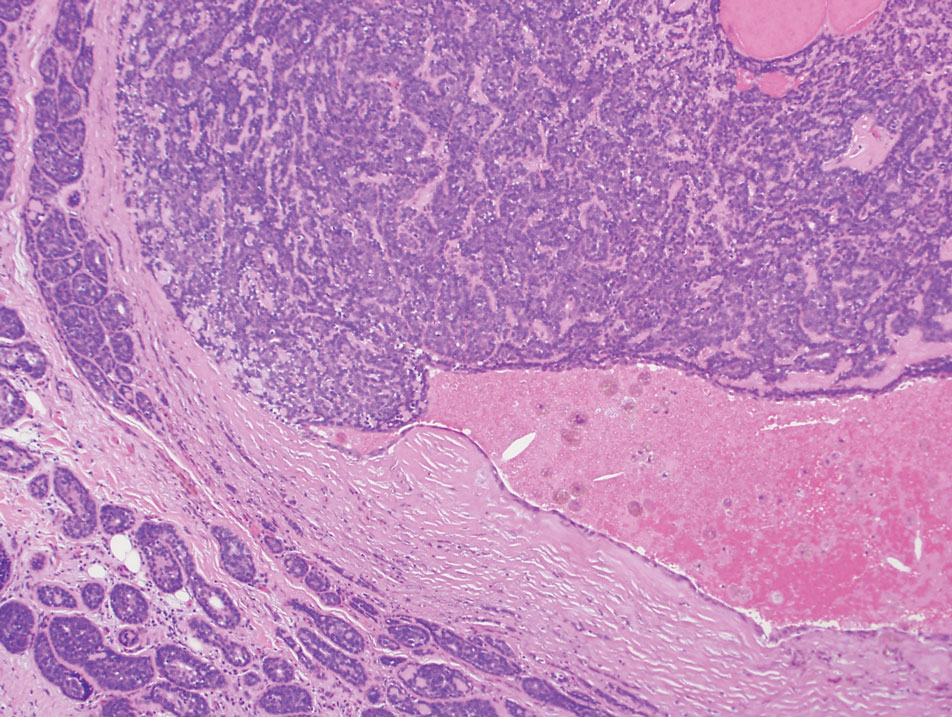
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.
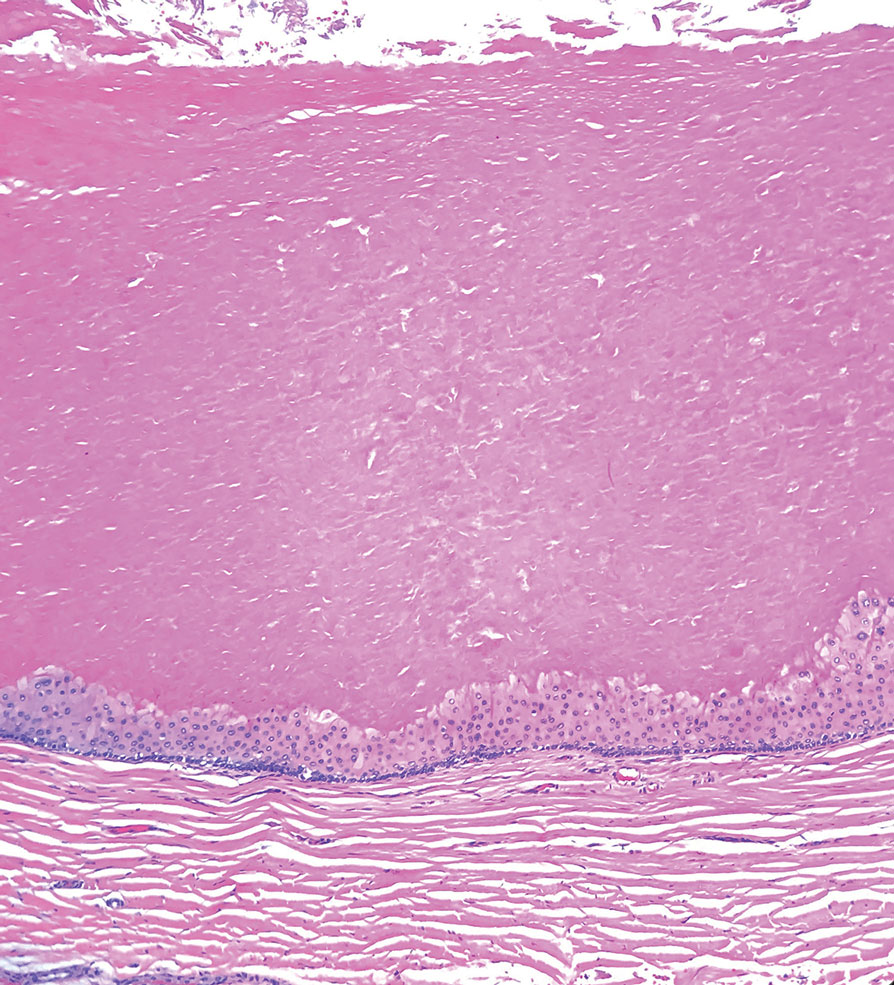
Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.
Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15
Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.
- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.

Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.

Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15

Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.

The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.

Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.

Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15

Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.

- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
Mobile Tender Papule on the Scalp
Mobile Tender Papule on the Scalp
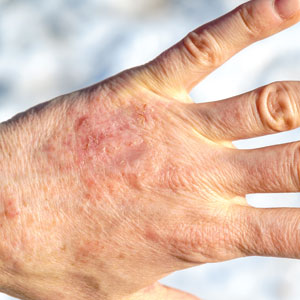
A 73-year-old man presented to the plastic surgery department with a single, progressively enlarging nodule on the scalp of 1 year’s duration. Dermatologic examination revealed a 0.8-cm, soft, mobile, gray-blue, dome-shaped papule on the left postauricular scalp that was tender to palpation. There was no central punctum, and the patient denied any history of drainage or odor. He had no personal or family history of similar lesions. An excisional biopsy of the papule was performed.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
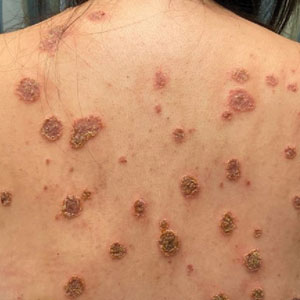
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
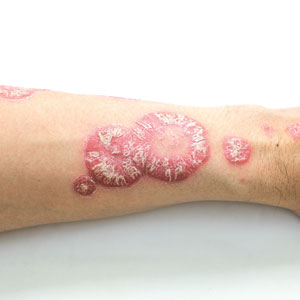
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.
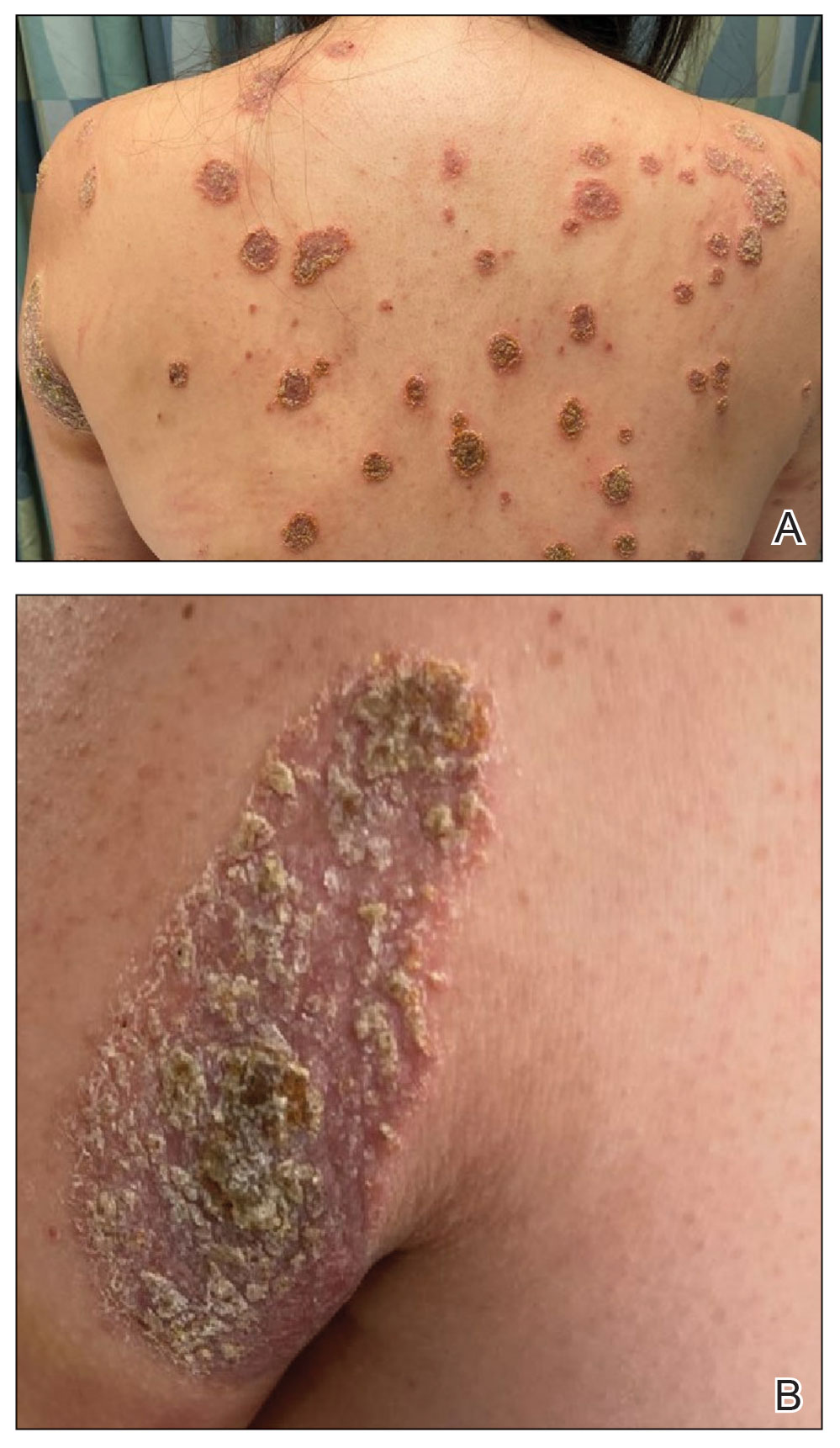
Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
Practice Points
- Consider a rupioid id reaction when a patient presents with lesions featuring scale that is dirty appearing and resembles an oyster shell.
- Recognize that exuberant id reactions can manifest with peripheral eosinophilia; its presence should not lead you to automatically rule out an id reaction in favor of other eosinophilic eruptions.
- Focus on uncovering the source of an id reaction (eg, contactants, infections, bites); resolving the primary insult is essential for rapid clearance of even dramatic rupioid eruptions.
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.
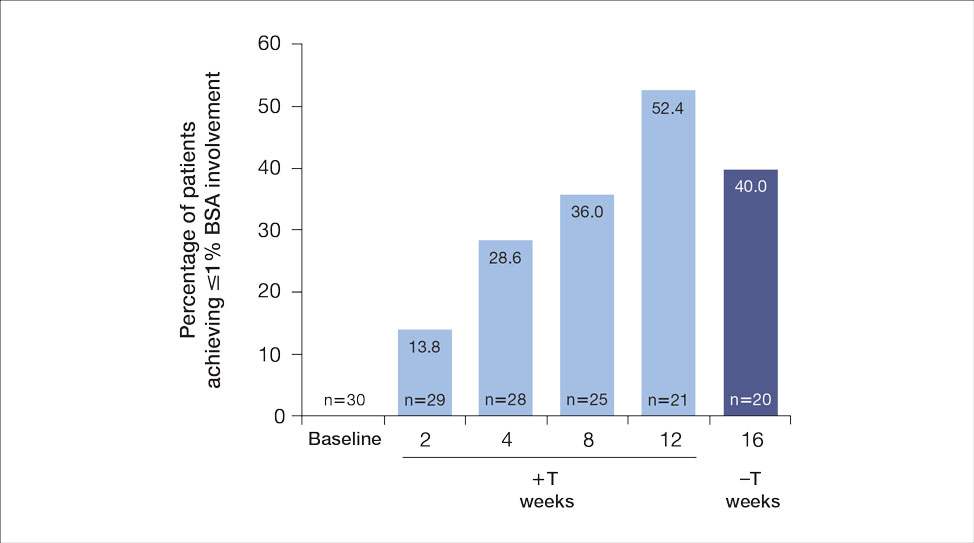
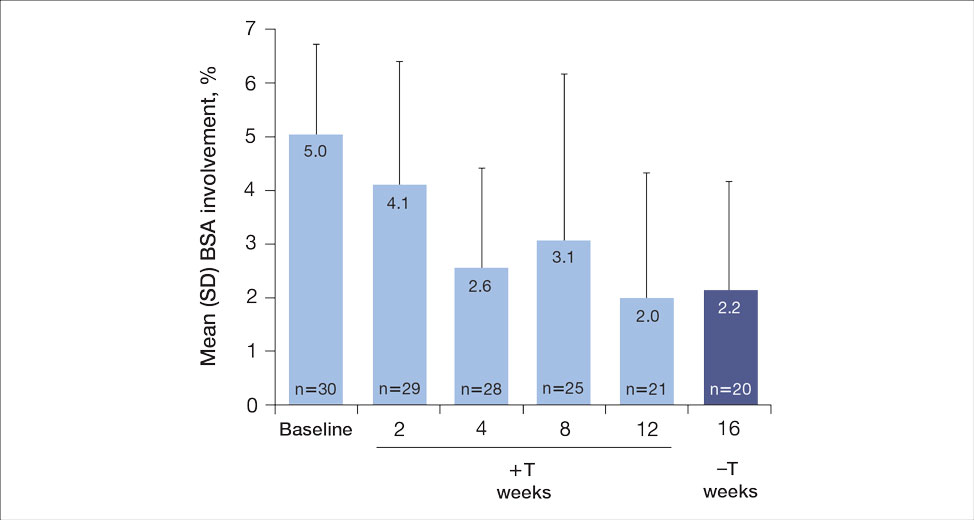
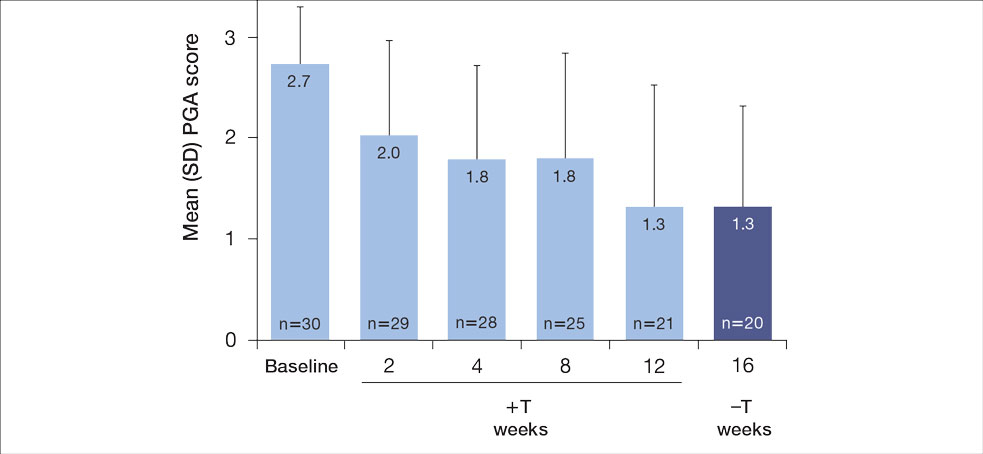
After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.
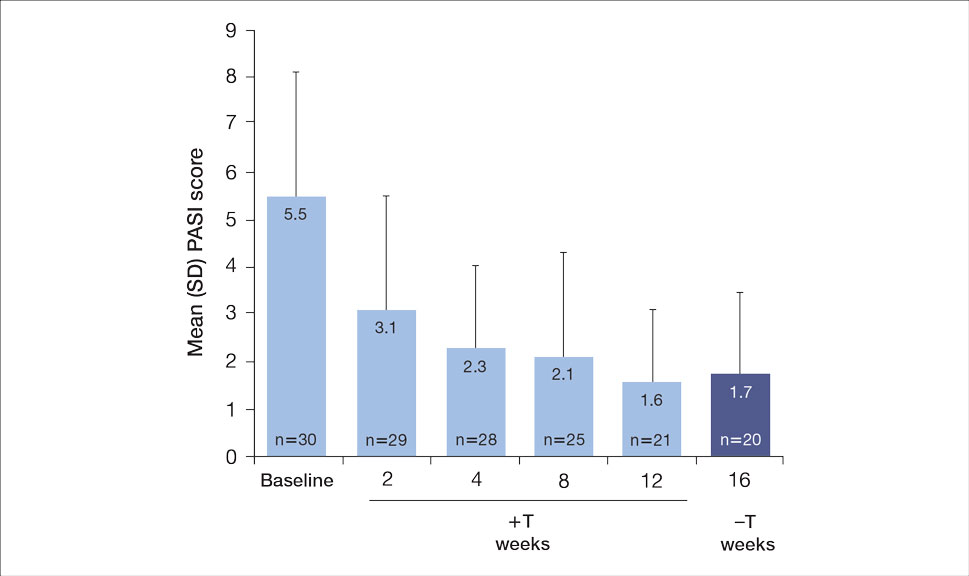
Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.
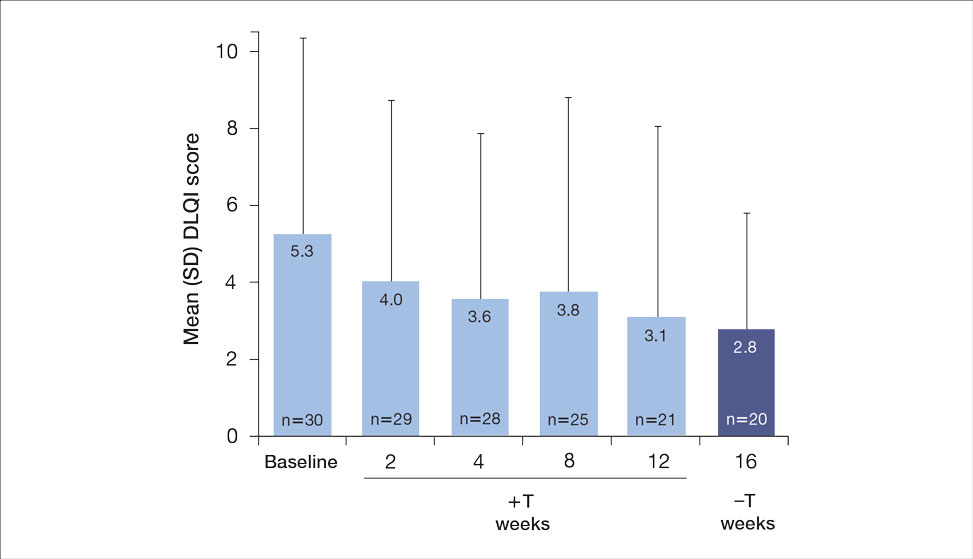

A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.
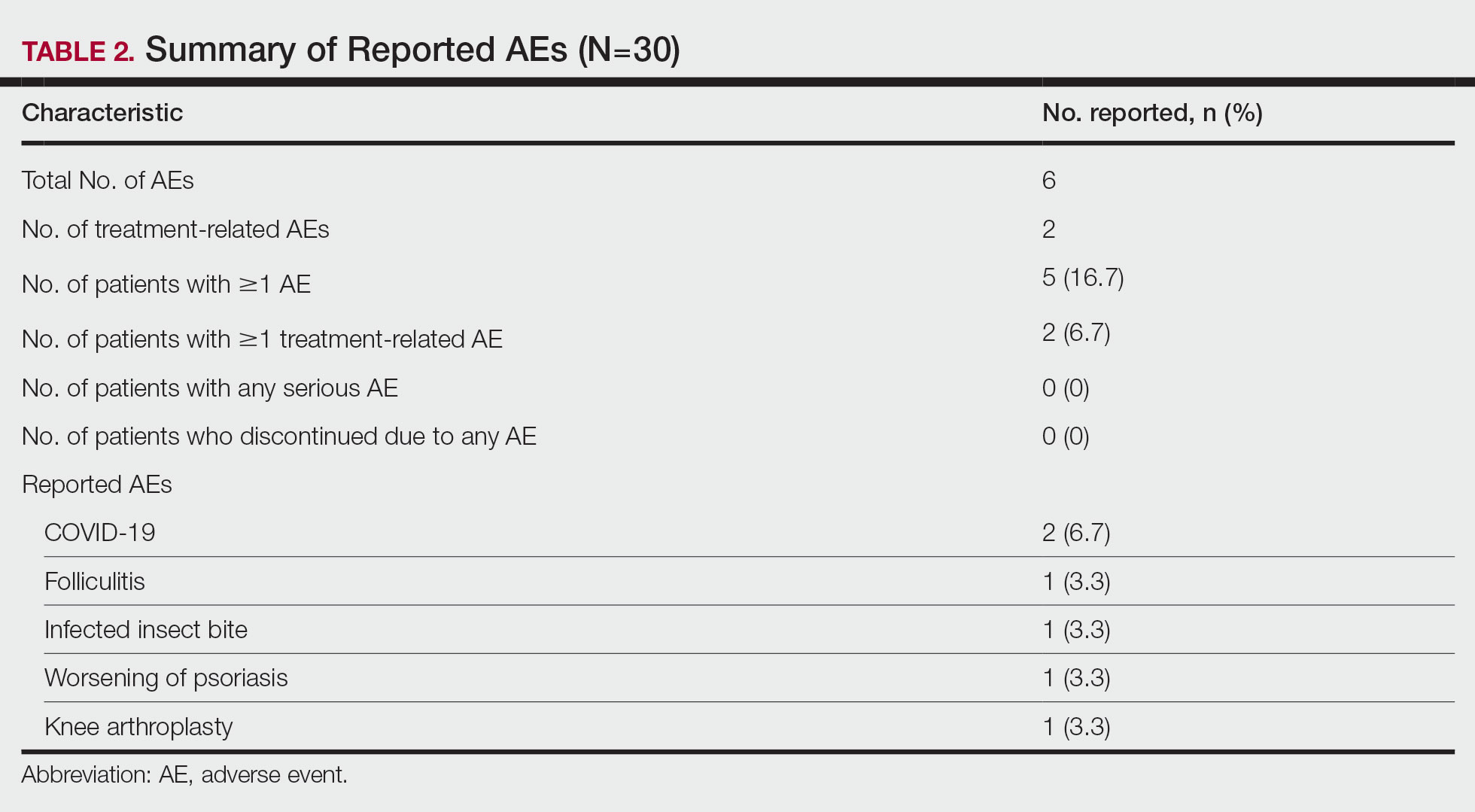
Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Practice Points
- Patients with moderate to severe psoriasis do not always reach treatment goals with biologic therapy alone.
- Adjunctive use of nonsteroidal tapinarof cream 1% may enhance the effects of ongoing biologic therapy in patients with moderate to severe plaque psoriasis, possibly avoiding the need to switch to another biologic.
- Patients with moderate to severe plaque psoriasis who are not adequately responding to biologics may benefit from adding tapinarof cream 1% to their current regimen.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).
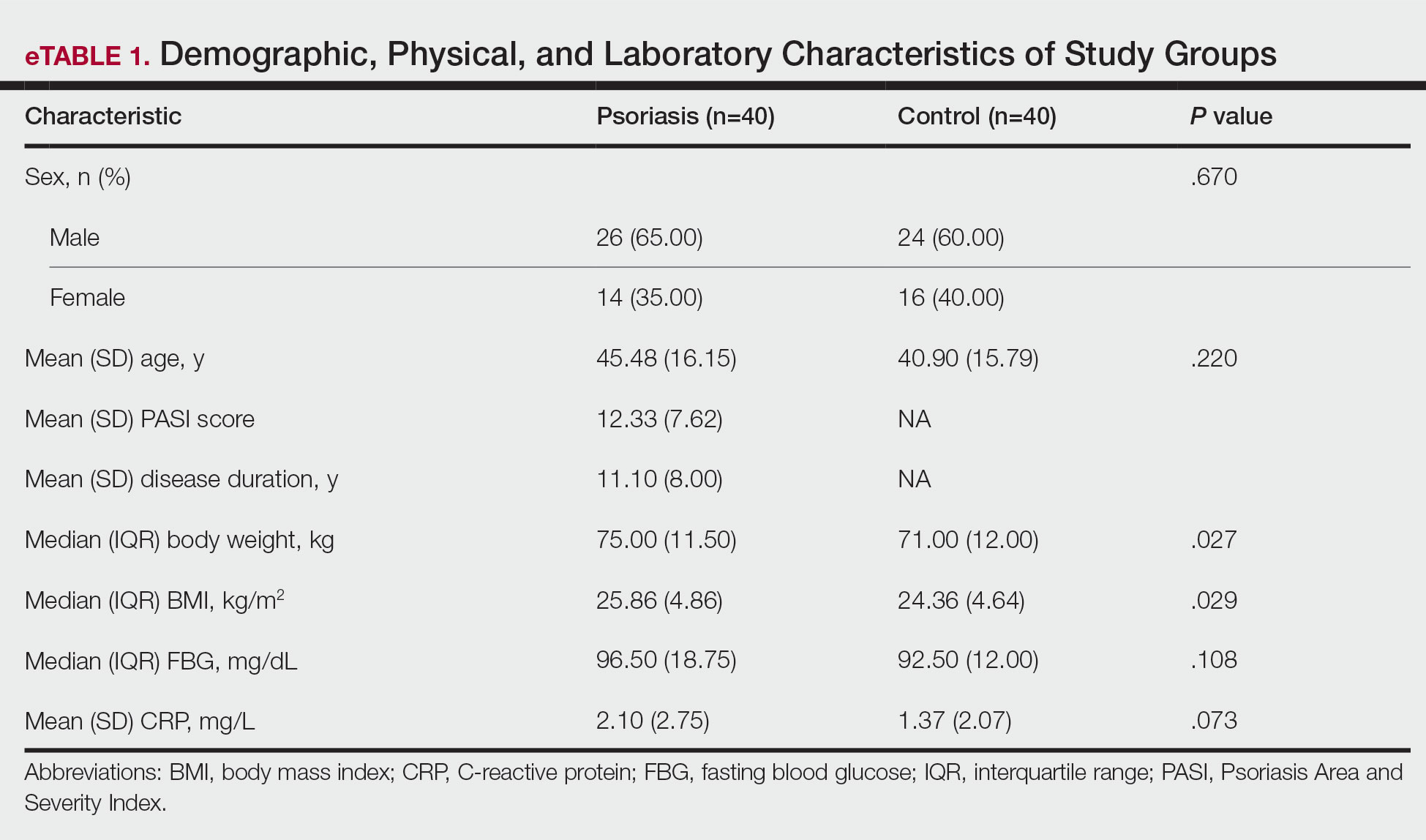
The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).
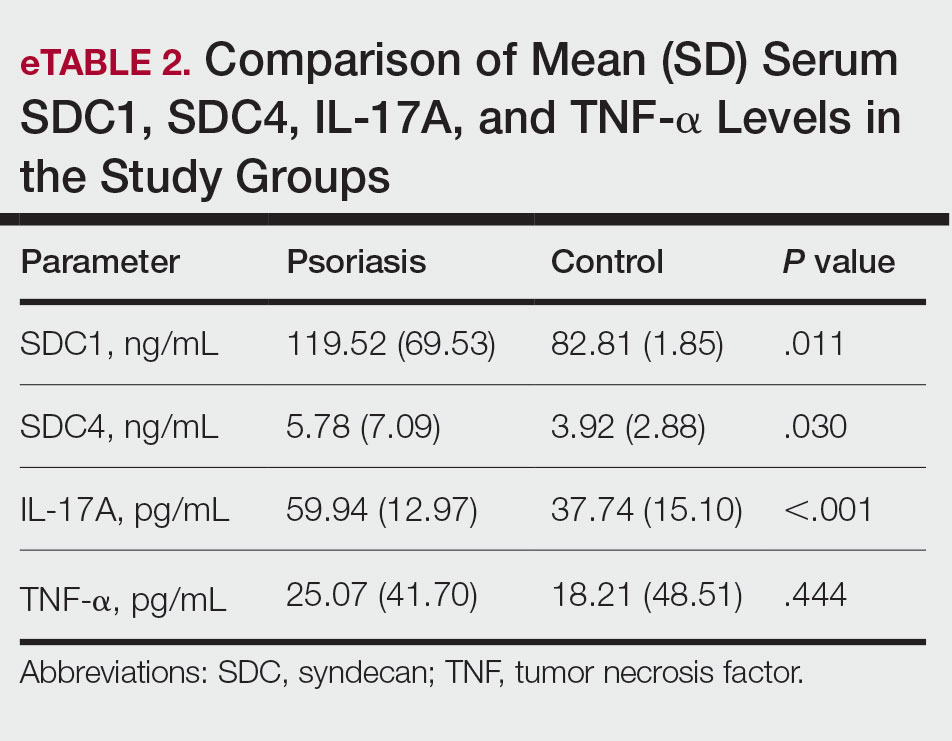
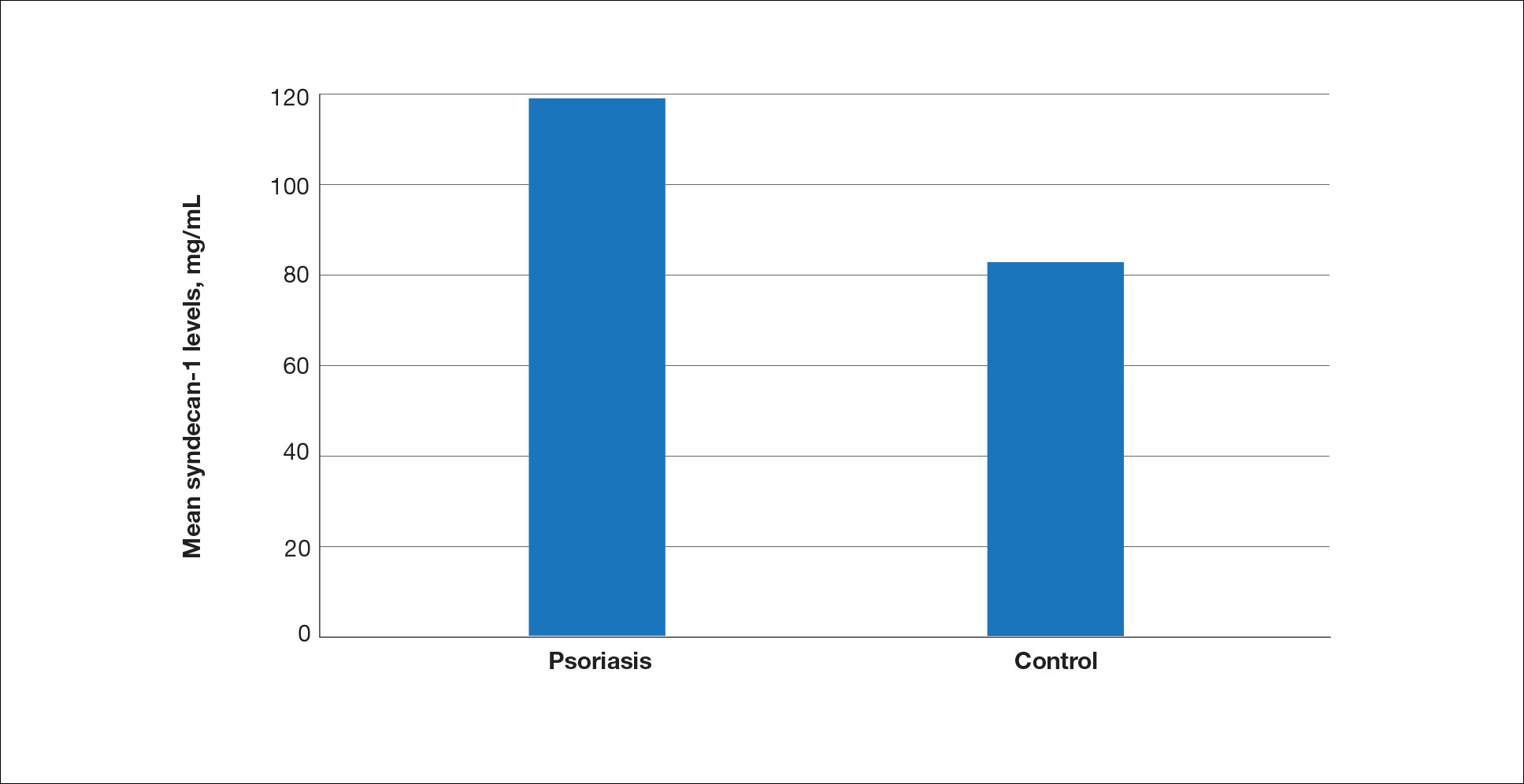
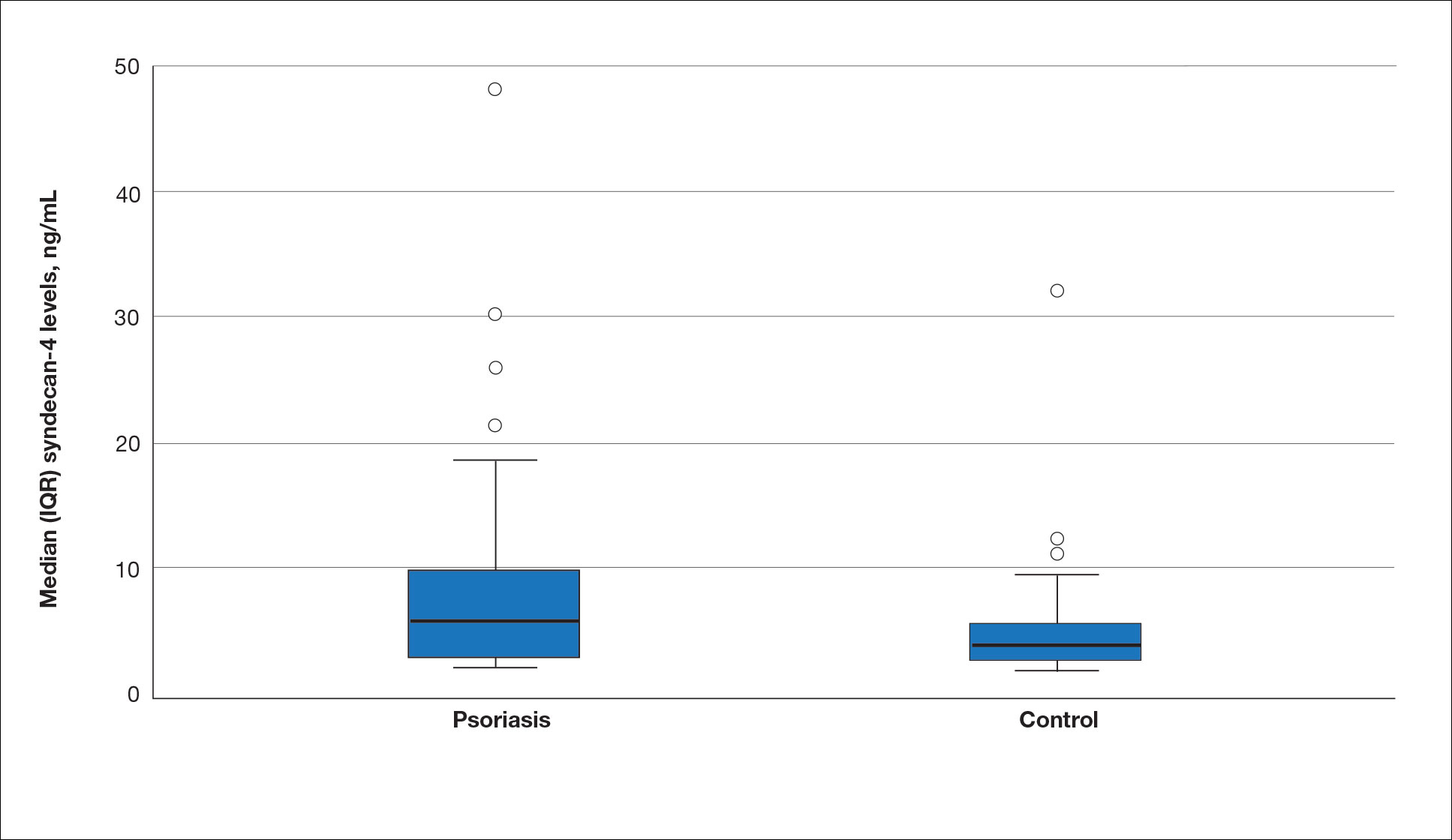
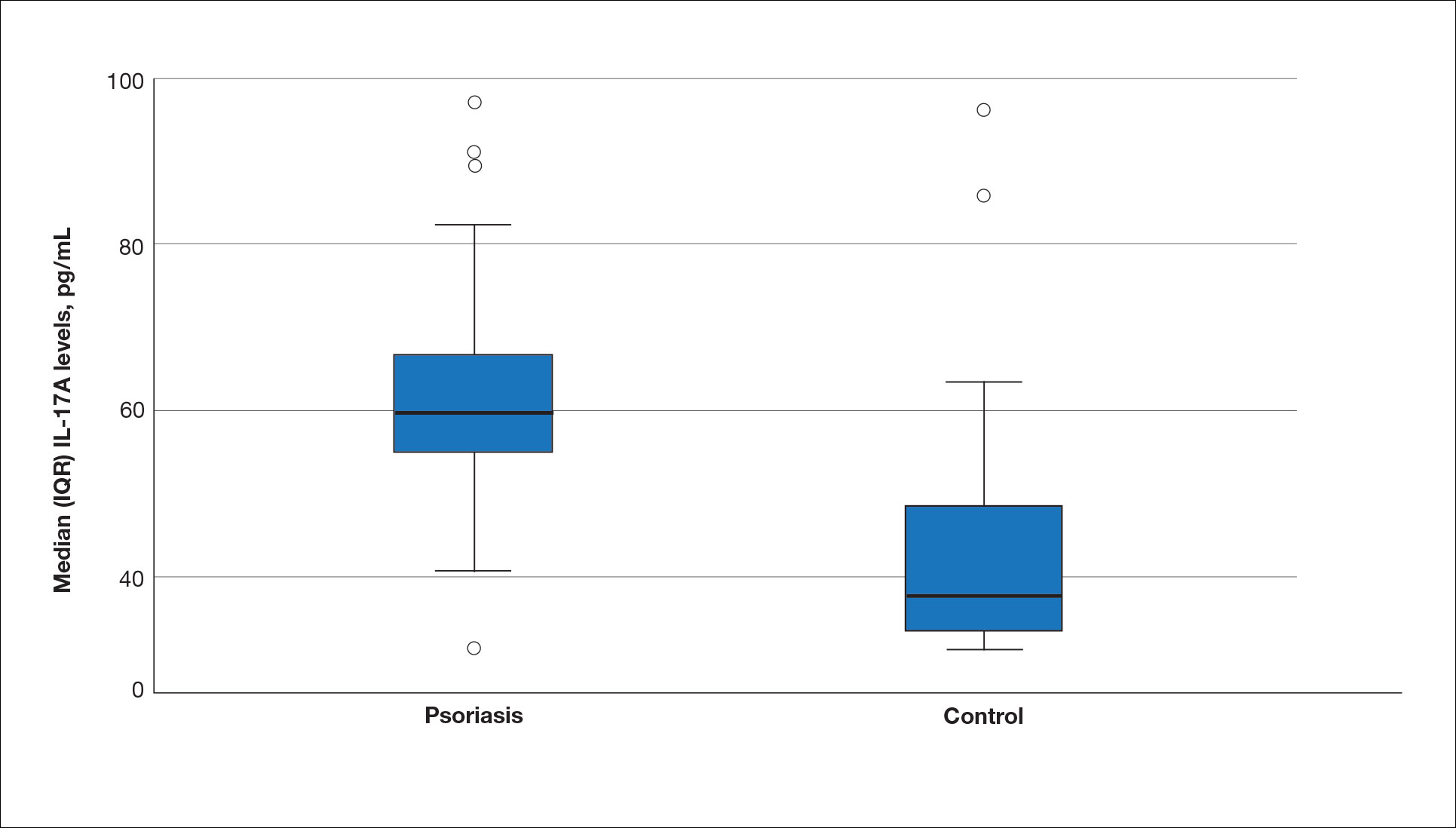
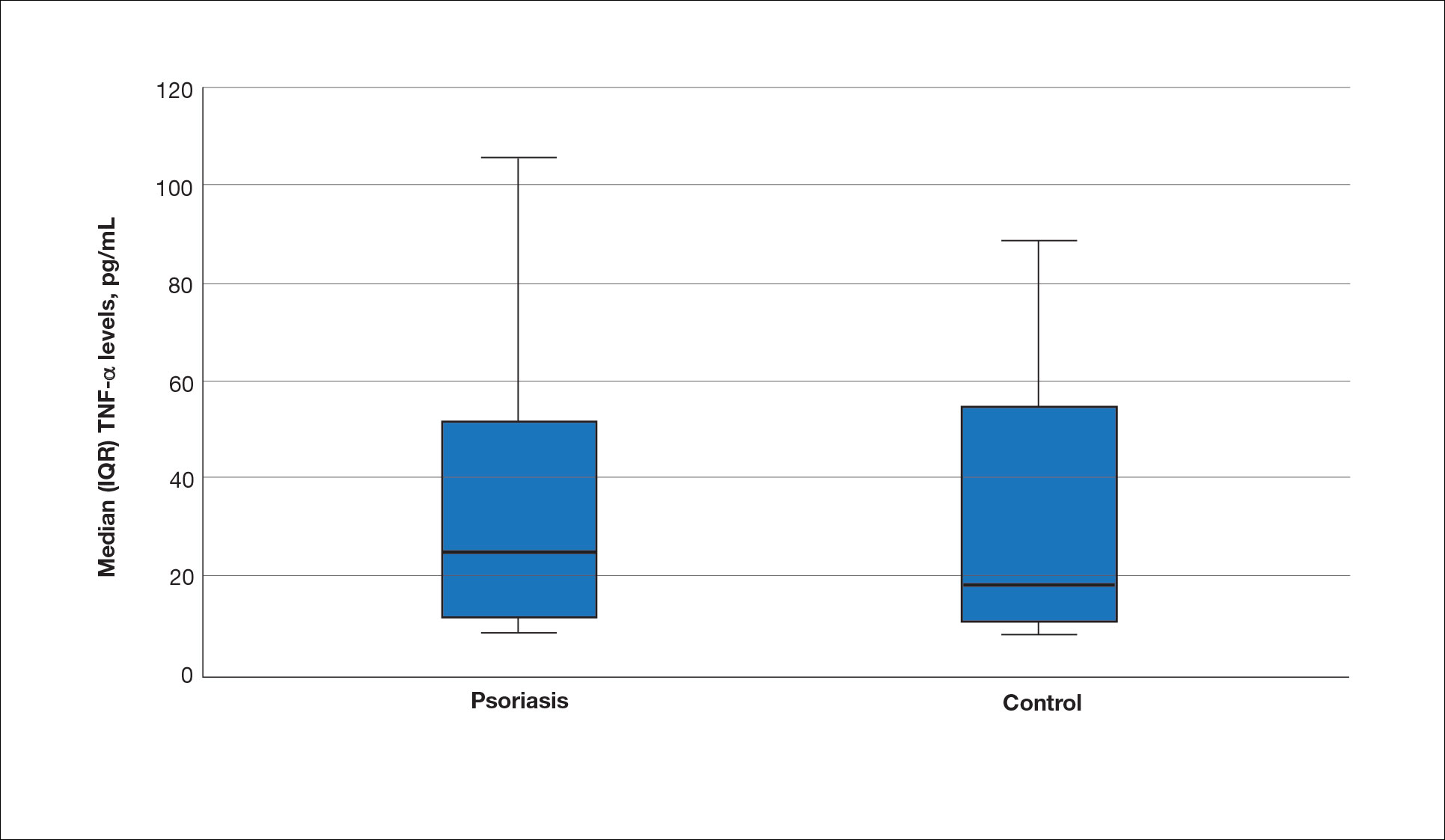
A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).
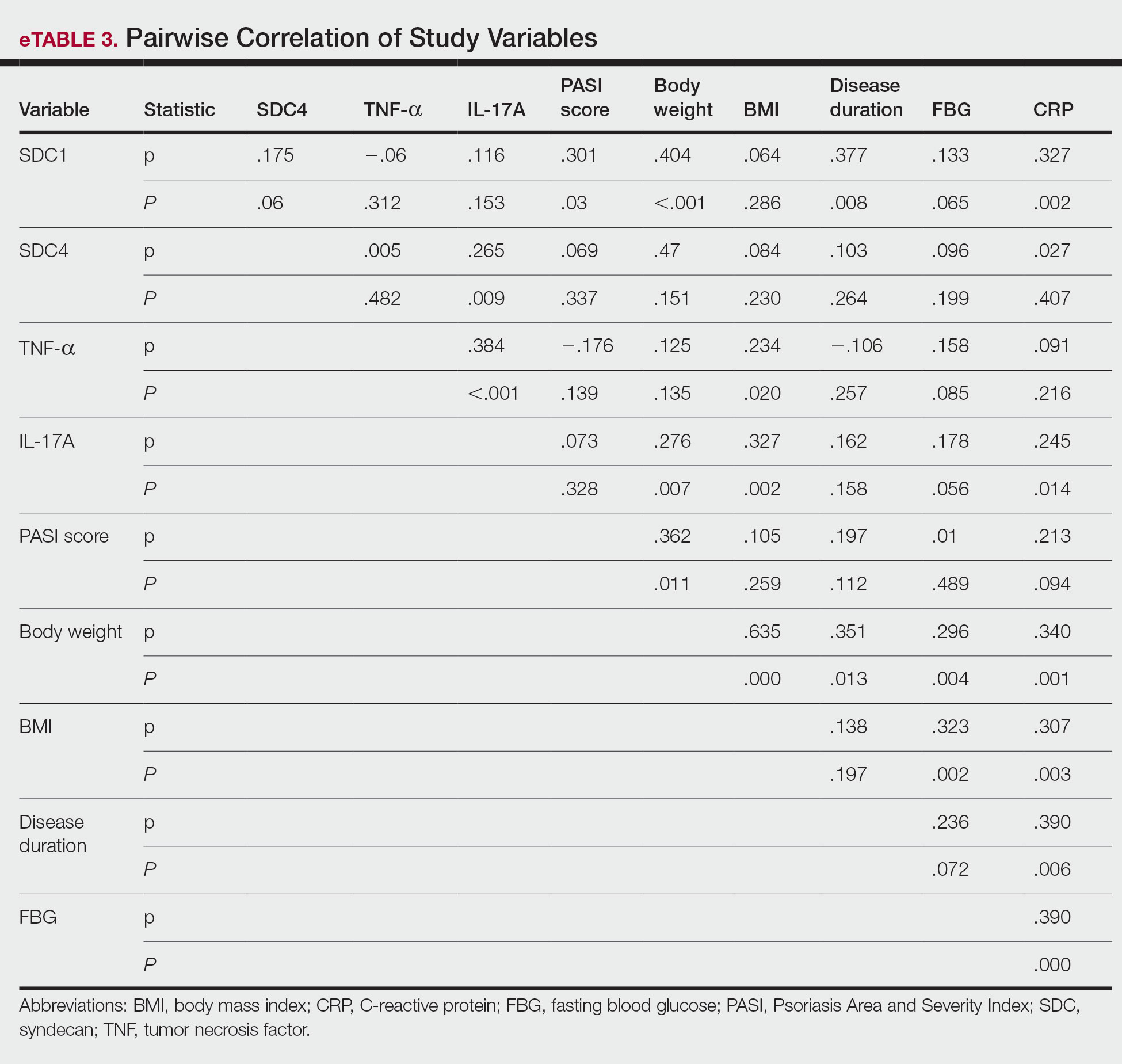
Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).
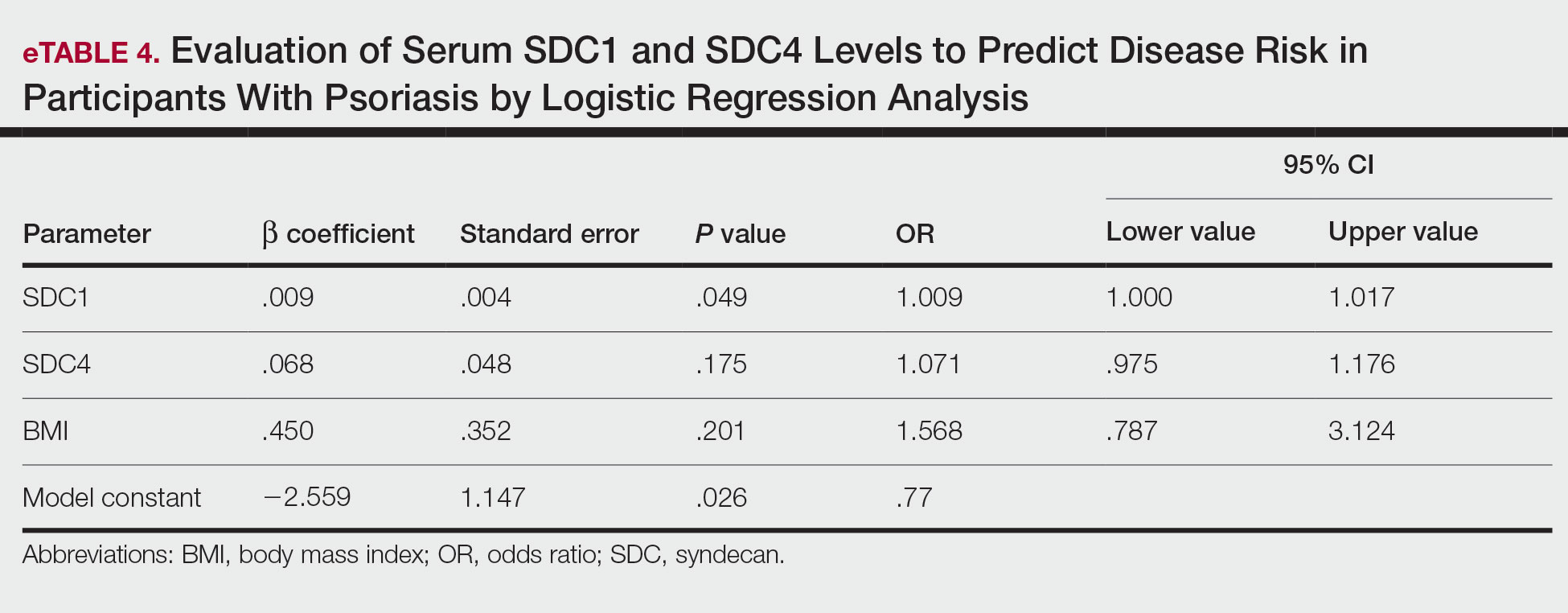
Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
PRACTICE POINTS
- Improved understanding of psoriasis pathogenesis has enabled the development of targeted treatments, although the mediators driving the disease have not yet been fully identified.
- Based on the findings of this study and existing literature, we suggest that syndecan-1 and syndecan-4 may play a role in the pathogenesis of psoriasis; however, further studies are needed to elucidate their precise mechanisms of action.
Antibiotic Stewardship in Acne: Practical Tips From Dr. Lorraine L. Rosamilia
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.
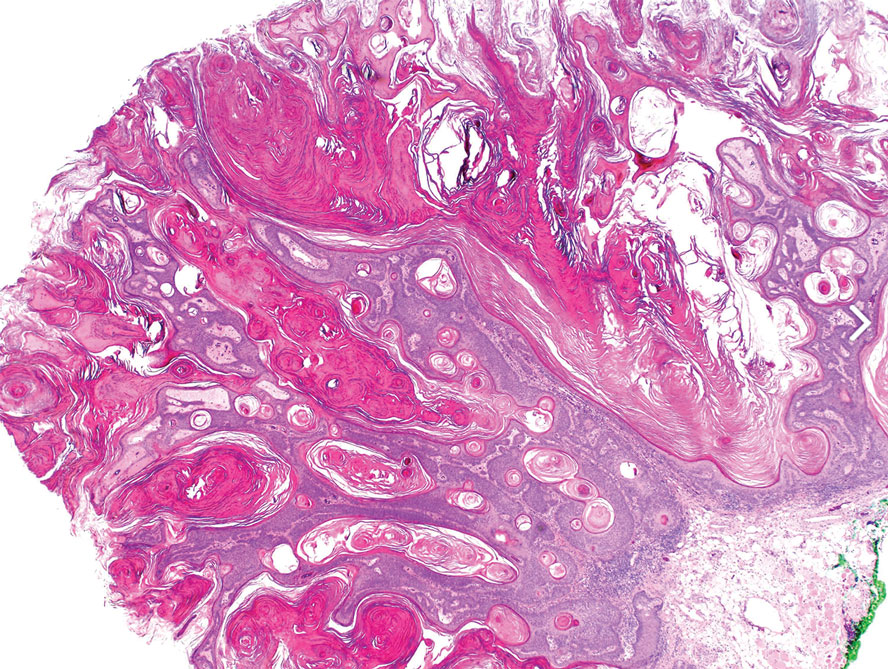
Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
A 33-year-old man with moderately to deeply pigmented skin presented to the dermatology department with a dark-brown macule in the periumbilical area of more than 1 year’s duration. The patient was otherwise healthy and reported no personal or family history of atypical nevi, nonmelanoma skin cancer, or melanoma. Dermoscopy of the lesion showed a dark brown macule less than 2 mm in diameter with a starburst like pattern and a blue-hued border. A shave biopsy of the lesion was performed.
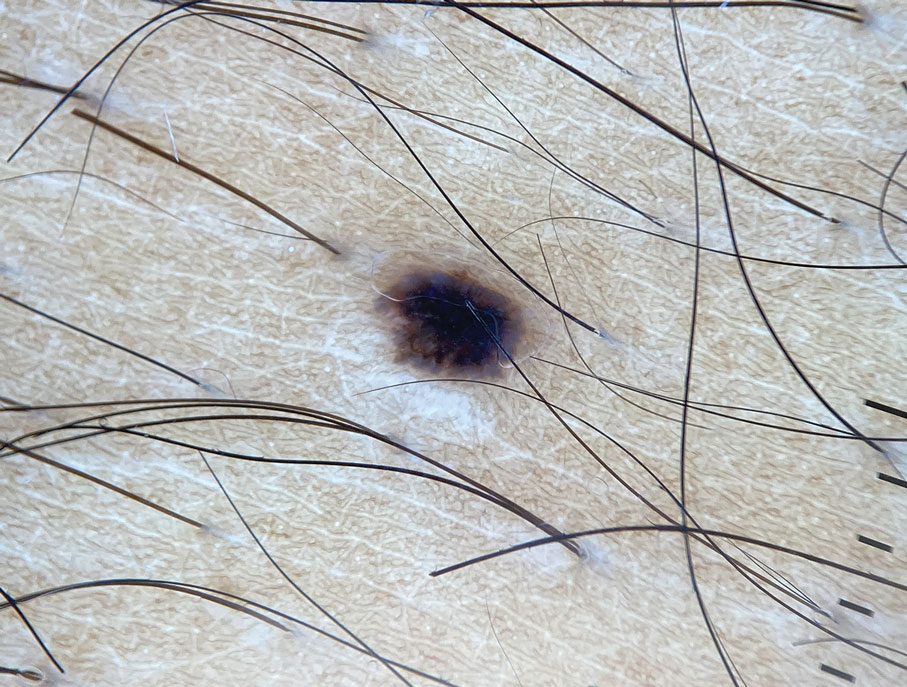
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24
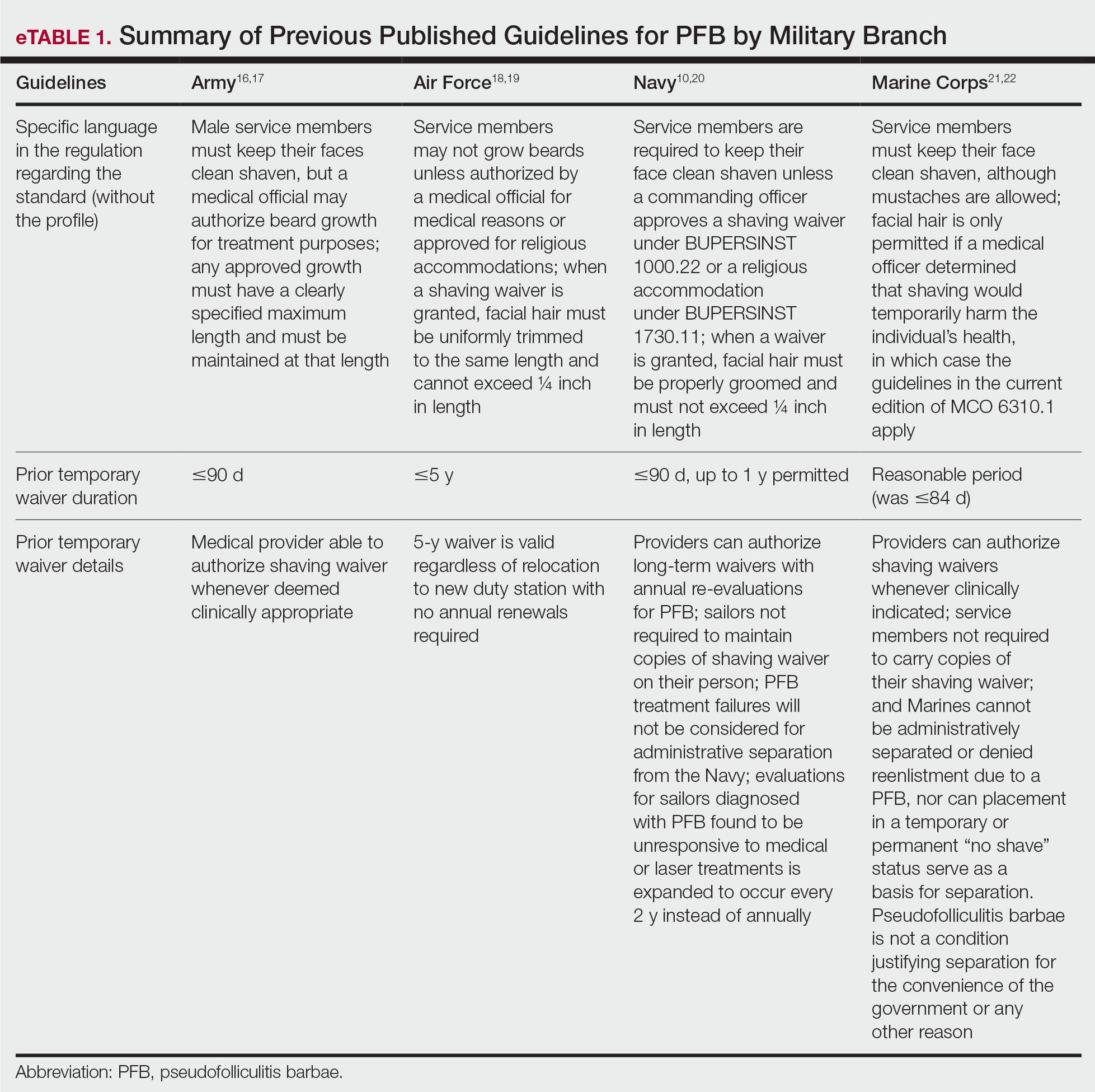
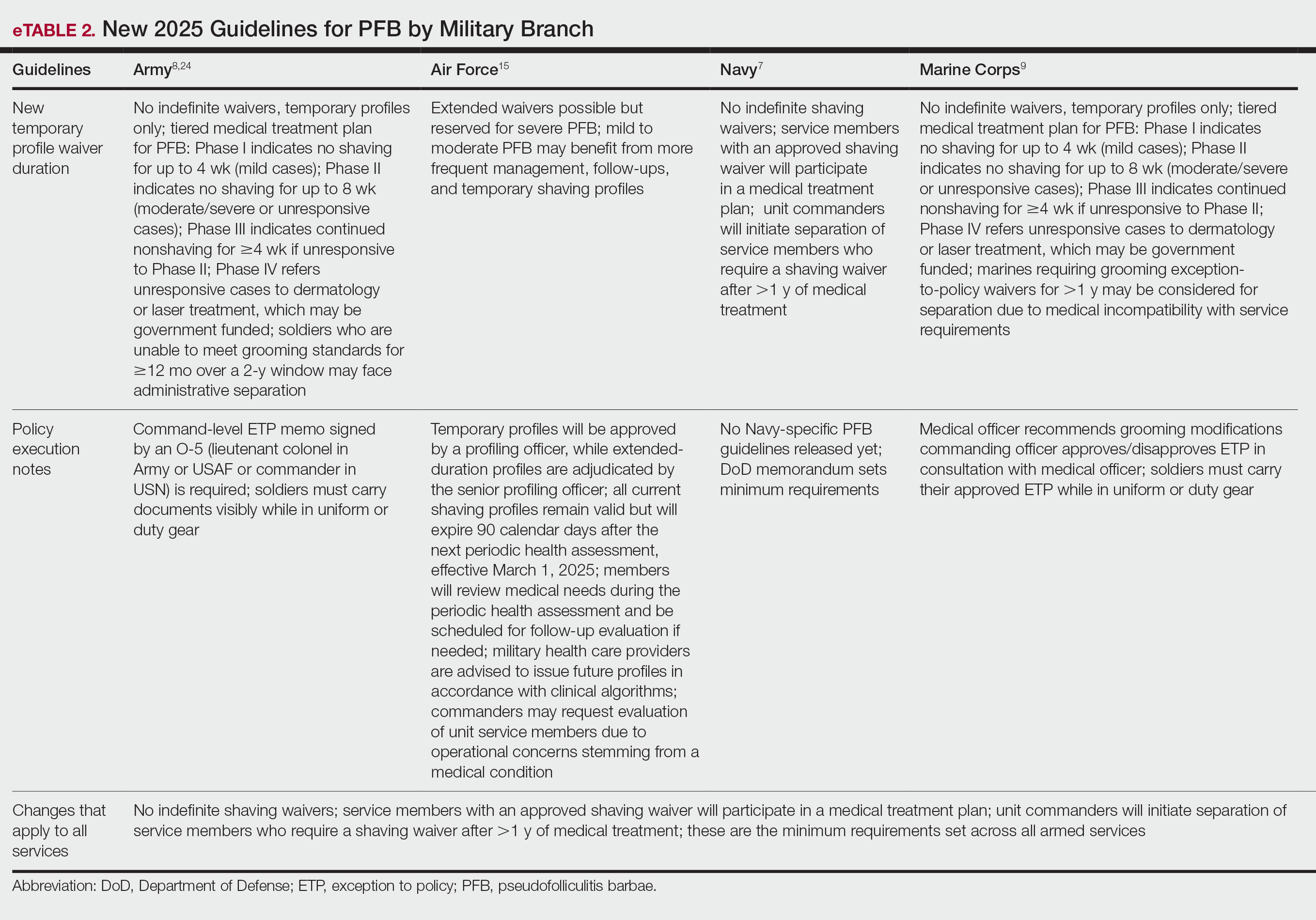
The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24


The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24


The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Practice Points
- Revised US Department of Defense grooming policies eliminate permanent shaving waivers and limit medical waivers for pseudofolliculitis barbae (PFB) to no more than 1 year, after which administrative separation may be initiated if grooming standards cannot be met.
- These changes impose increased administrative and clinical demands on service members, military medical personnel, and commanders, requiring recurrent evaluation, documentation, and approvals for temporary shaving waivers.
- Civilian dermatologists should be aware of these policy changes and their potential career implications to appropriately counsel active-duty personnel and prospective military recruits.
- Laser hair removal may see increased utilization as a treatment option for service members for whom conservative management fails.
Noncompete Agreements and Their Impact on the Medical Landscape
In April 2024, the Federal Trade Commission (FTC) issued a nationwide rule to ban most employee noncompete agreements, including many used in health care1; however, that rule never took effect. In August 2024, a federal district court ruled that the FTC had exceeded its statutory authority and blocked the ban,2 and subsequent litigation and agency actions followed. On September 5, 2025, the FTC formally moved to accede to vacatur—in other words, it will not enforce the rule and backed away from defending it on appeal.3 As of December 2025, there is no active federal ban on physician noncompetes. The obligations of the physician employee are dictated by state law and the precise language of the contract that is signed.
In this article, we discuss the historical origins of noncompetes, employer and physician perspectives, and the downstream consequences for patient continuity, access, and health care costs.
Background
The concept of noncompete agreements is not new—this legal principle dates back several centuries, but it was not until several hundred years later, between the 1950s and 1980s, that noncompete agreements became routine in physician contracts. This trend emerged, at least in part, from the growing commoditization of medicine, the expansion of hospital infrastructure, and the rise of physicians employed by entities rather than owning a private practice. Medical practices, hospitals, and increasingly large private groups began using noncompete agreements to prevent physicians from leaving and establishing competing practices nearby. Since then, noncompetes have remained a contentious issue within both the legal system and the broader physician-employer relationship.
Employer vs Employee Perspective
From the employer’s perspective, health care systems and medical groups argue that noncompete agreements are necessary to protect legitimate business interests, citing physician training, established patient relationships, and proprietary information gained from employment with that entity as supporting reasons. Additionally, employers maintain that recouping the cost of recruitment and onboarding investments as well as sustaining continuity of care within the organization should take precedence. On occasion, health care systems will invest time and financial resources in recruiting physicians, provide administrative and clinical support, and integrate new employees into established referral pathways and patient populations. In this view, noncompetes serve as a tool to ensure stability within the health care system, discouraging abrupt departures that could fracture patient care or lead to unfair competition using institutional resources. While these arguments hold merit in certain cases, many physicians do not receive employer-funded education or training beyond what is required in residency and fellowship. As a result, the financial justifications for noncompetes often are overstated; on the contrary, the cost of a “buy-out” or the financial barrier imposed by a noncompete clause can amount to a considerable portion of a physician’s annual salary—sometimes multiple times that amount—creating an imbalance that favors the employer and limits professional mobility.
When a physician is prohibited from practicing in a specific area after leaving an employer, a complex web of adverse consequences can arise, impacting both the physician and the patients they serve. Physician mobility and career choice become restricted, effectively constraining the physicians’ livelihood and ability to provide for themselves and their dependents; in single-earner physician families, this can have devastating financial consequences. These limitations contribute to growing burnout and dissatisfaction within the medical profession, which already is facing unprecedented levels of stress and physician workforce shortages.4
Effect on Patients
When a physician is forced to relocate to a new geographic region because of a noncompete clause, their patients can experience substantial disruptions in care. Access to medical services may be affected, leading to longer wait-times and fewer available appointments, especially in areas that already have a shortage of providers. Patients may lose longstanding relationships with doctors who know their medical histories, which can interrupt treatment plans and increase the risk of complications. Those with chronic illnesses, complex conditions, or time-sensitive treatments are particularly vulnerable to adverse outcomes. Many patients must travel farther—sometimes out of their insurance network—to find replacement care, increasing both financial and logistical burdens. These abrupt transitions also can raise health care costs due to emergency department use, inefficient handoffs, and higher incidence of morbidity/mortality.5 Noncompete restrictions often prevent physicians from informing patients where they are relocating, creating confusion and fragmentation of care. As a result, trust in the health care system may decline when patients perceive that business agreements are being prioritized above their wellbeing. The impact may be even more severe in rural or underserved communities where alternative providers are scarce.
Final Thoughts
In recent years, noncompete agreements in health care have come under intensified scrutiny for their potential to stifle physician mobility, reduce competition, and inflate health care costs by limiting where and how physicians can practice. The trajectory of noncompetes in physician employment reflects broader shifts in how medicine is structured and delivered in the United States. In the latter half of the 20th century, what began as a centuries-old legal concept became a standard feature of physician employment contracts. That evolution largely was driven by the corporatization of medicine and large hospital group/private equity employment of physicians. As these agreements proliferated, public policy questions emerged: What does restricting a physician’s mobility do to patient access? To competition in provider markets? To the cost and availability of care? To the current epidemic of physician burnout?
These questions moved from the legal sidelines to center stage in the 2020s, when the FTC sought to tackle noncompetes across the entire economy—physicians included—on the theory they suppressed labor mobility, entrepreneurship, and competition. In February 2020, the American Medical Association submitted comments to the FTC on the utility of noncompete agreements in employee contracts stating that they restrict competition, can disrupt continuity of care, and may limit access to care.6 Although the FTC’s regulatory attempt in April 2024 provoked strong policy signals, it was challenged and ultimately blocked. Rather than a clear federal prohibition, the outcome is a more incremental state-based shift in rules governing physician noncompetes. For physicians today, this means more awareness and more leverage, but also more complexity. Whether a noncompete will be enforceable depends heavily on the state, the wording of the contract, the structure of the employer, and the specialty. From a negotiation standpoint, physicians need more guidance and awareness on the exact ramifications of their employee contract. For newly minted physicians, many of whom enter the workforce with considerable training debt, the priority often is securing employment to work toward financial stability, building a family, or both; however, all physicians should press for shorter durations, tighter geographic limits, narrower scopes of service, clear buy-out options, and explicit patient-continuity protections. Better yet, physicians can exercise the right of refusal to any noncompete clause at all. Becoming involved with a local medical organization or foundation can provide immense support, both in reviewing contracts as well as learning how to become advocates for physicians in this environment. As more physicians stand together to protect both practice autonomy and the right to quality care, we all become closer to rediscovering the beauty and fulfillment in the purest form of medicine.
- Federal Trade Commission. FTC announces rule banning noncompetes. April 23, 2024. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes
- US Chamber of Commerce. Ryan LLC v FTC. August 20, 2024. Accessed December 1, 2025. https://www.uschamber.com/cases/antitrust-and-competition-law/ryan-llc-v.-ftc
- Federal Trade Commission. Federal Trade Commission files to accede to vacatur of non-compete clause rule. September 5, 2025. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2025/09/federal-trade-commission-files-accede-vacatur-non-compete-clause-rule
- Marshall JJ, Ashwath ML, Jefferies JL, et al. Restrictive covenants and noncompete clauses for physicians. JACC Adv. 2023;2:100547.
- Sabety A. The value of relationships in healthcare. J Publich Economics. 2023;225:104927.
- American Medical Association. AMA provides comment to FTC on non-compete agreements. National Advocacy Update. February 14, 2020. Accessed November 25, 2025. https://www.ama-assn.org/health-care-advocacy/advocacy-update/feb-14-2020-national-advocacy-update
In April 2024, the Federal Trade Commission (FTC) issued a nationwide rule to ban most employee noncompete agreements, including many used in health care1; however, that rule never took effect. In August 2024, a federal district court ruled that the FTC had exceeded its statutory authority and blocked the ban,2 and subsequent litigation and agency actions followed. On September 5, 2025, the FTC formally moved to accede to vacatur—in other words, it will not enforce the rule and backed away from defending it on appeal.3 As of December 2025, there is no active federal ban on physician noncompetes. The obligations of the physician employee are dictated by state law and the precise language of the contract that is signed.
In this article, we discuss the historical origins of noncompetes, employer and physician perspectives, and the downstream consequences for patient continuity, access, and health care costs.
Background
The concept of noncompete agreements is not new—this legal principle dates back several centuries, but it was not until several hundred years later, between the 1950s and 1980s, that noncompete agreements became routine in physician contracts. This trend emerged, at least in part, from the growing commoditization of medicine, the expansion of hospital infrastructure, and the rise of physicians employed by entities rather than owning a private practice. Medical practices, hospitals, and increasingly large private groups began using noncompete agreements to prevent physicians from leaving and establishing competing practices nearby. Since then, noncompetes have remained a contentious issue within both the legal system and the broader physician-employer relationship.
Employer vs Employee Perspective
From the employer’s perspective, health care systems and medical groups argue that noncompete agreements are necessary to protect legitimate business interests, citing physician training, established patient relationships, and proprietary information gained from employment with that entity as supporting reasons. Additionally, employers maintain that recouping the cost of recruitment and onboarding investments as well as sustaining continuity of care within the organization should take precedence. On occasion, health care systems will invest time and financial resources in recruiting physicians, provide administrative and clinical support, and integrate new employees into established referral pathways and patient populations. In this view, noncompetes serve as a tool to ensure stability within the health care system, discouraging abrupt departures that could fracture patient care or lead to unfair competition using institutional resources. While these arguments hold merit in certain cases, many physicians do not receive employer-funded education or training beyond what is required in residency and fellowship. As a result, the financial justifications for noncompetes often are overstated; on the contrary, the cost of a “buy-out” or the financial barrier imposed by a noncompete clause can amount to a considerable portion of a physician’s annual salary—sometimes multiple times that amount—creating an imbalance that favors the employer and limits professional mobility.
When a physician is prohibited from practicing in a specific area after leaving an employer, a complex web of adverse consequences can arise, impacting both the physician and the patients they serve. Physician mobility and career choice become restricted, effectively constraining the physicians’ livelihood and ability to provide for themselves and their dependents; in single-earner physician families, this can have devastating financial consequences. These limitations contribute to growing burnout and dissatisfaction within the medical profession, which already is facing unprecedented levels of stress and physician workforce shortages.4
Effect on Patients
When a physician is forced to relocate to a new geographic region because of a noncompete clause, their patients can experience substantial disruptions in care. Access to medical services may be affected, leading to longer wait-times and fewer available appointments, especially in areas that already have a shortage of providers. Patients may lose longstanding relationships with doctors who know their medical histories, which can interrupt treatment plans and increase the risk of complications. Those with chronic illnesses, complex conditions, or time-sensitive treatments are particularly vulnerable to adverse outcomes. Many patients must travel farther—sometimes out of their insurance network—to find replacement care, increasing both financial and logistical burdens. These abrupt transitions also can raise health care costs due to emergency department use, inefficient handoffs, and higher incidence of morbidity/mortality.5 Noncompete restrictions often prevent physicians from informing patients where they are relocating, creating confusion and fragmentation of care. As a result, trust in the health care system may decline when patients perceive that business agreements are being prioritized above their wellbeing. The impact may be even more severe in rural or underserved communities where alternative providers are scarce.
Final Thoughts
In recent years, noncompete agreements in health care have come under intensified scrutiny for their potential to stifle physician mobility, reduce competition, and inflate health care costs by limiting where and how physicians can practice. The trajectory of noncompetes in physician employment reflects broader shifts in how medicine is structured and delivered in the United States. In the latter half of the 20th century, what began as a centuries-old legal concept became a standard feature of physician employment contracts. That evolution largely was driven by the corporatization of medicine and large hospital group/private equity employment of physicians. As these agreements proliferated, public policy questions emerged: What does restricting a physician’s mobility do to patient access? To competition in provider markets? To the cost and availability of care? To the current epidemic of physician burnout?
These questions moved from the legal sidelines to center stage in the 2020s, when the FTC sought to tackle noncompetes across the entire economy—physicians included—on the theory they suppressed labor mobility, entrepreneurship, and competition. In February 2020, the American Medical Association submitted comments to the FTC on the utility of noncompete agreements in employee contracts stating that they restrict competition, can disrupt continuity of care, and may limit access to care.6 Although the FTC’s regulatory attempt in April 2024 provoked strong policy signals, it was challenged and ultimately blocked. Rather than a clear federal prohibition, the outcome is a more incremental state-based shift in rules governing physician noncompetes. For physicians today, this means more awareness and more leverage, but also more complexity. Whether a noncompete will be enforceable depends heavily on the state, the wording of the contract, the structure of the employer, and the specialty. From a negotiation standpoint, physicians need more guidance and awareness on the exact ramifications of their employee contract. For newly minted physicians, many of whom enter the workforce with considerable training debt, the priority often is securing employment to work toward financial stability, building a family, or both; however, all physicians should press for shorter durations, tighter geographic limits, narrower scopes of service, clear buy-out options, and explicit patient-continuity protections. Better yet, physicians can exercise the right of refusal to any noncompete clause at all. Becoming involved with a local medical organization or foundation can provide immense support, both in reviewing contracts as well as learning how to become advocates for physicians in this environment. As more physicians stand together to protect both practice autonomy and the right to quality care, we all become closer to rediscovering the beauty and fulfillment in the purest form of medicine.
In April 2024, the Federal Trade Commission (FTC) issued a nationwide rule to ban most employee noncompete agreements, including many used in health care1; however, that rule never took effect. In August 2024, a federal district court ruled that the FTC had exceeded its statutory authority and blocked the ban,2 and subsequent litigation and agency actions followed. On September 5, 2025, the FTC formally moved to accede to vacatur—in other words, it will not enforce the rule and backed away from defending it on appeal.3 As of December 2025, there is no active federal ban on physician noncompetes. The obligations of the physician employee are dictated by state law and the precise language of the contract that is signed.
In this article, we discuss the historical origins of noncompetes, employer and physician perspectives, and the downstream consequences for patient continuity, access, and health care costs.
Background
The concept of noncompete agreements is not new—this legal principle dates back several centuries, but it was not until several hundred years later, between the 1950s and 1980s, that noncompete agreements became routine in physician contracts. This trend emerged, at least in part, from the growing commoditization of medicine, the expansion of hospital infrastructure, and the rise of physicians employed by entities rather than owning a private practice. Medical practices, hospitals, and increasingly large private groups began using noncompete agreements to prevent physicians from leaving and establishing competing practices nearby. Since then, noncompetes have remained a contentious issue within both the legal system and the broader physician-employer relationship.
Employer vs Employee Perspective
From the employer’s perspective, health care systems and medical groups argue that noncompete agreements are necessary to protect legitimate business interests, citing physician training, established patient relationships, and proprietary information gained from employment with that entity as supporting reasons. Additionally, employers maintain that recouping the cost of recruitment and onboarding investments as well as sustaining continuity of care within the organization should take precedence. On occasion, health care systems will invest time and financial resources in recruiting physicians, provide administrative and clinical support, and integrate new employees into established referral pathways and patient populations. In this view, noncompetes serve as a tool to ensure stability within the health care system, discouraging abrupt departures that could fracture patient care or lead to unfair competition using institutional resources. While these arguments hold merit in certain cases, many physicians do not receive employer-funded education or training beyond what is required in residency and fellowship. As a result, the financial justifications for noncompetes often are overstated; on the contrary, the cost of a “buy-out” or the financial barrier imposed by a noncompete clause can amount to a considerable portion of a physician’s annual salary—sometimes multiple times that amount—creating an imbalance that favors the employer and limits professional mobility.
When a physician is prohibited from practicing in a specific area after leaving an employer, a complex web of adverse consequences can arise, impacting both the physician and the patients they serve. Physician mobility and career choice become restricted, effectively constraining the physicians’ livelihood and ability to provide for themselves and their dependents; in single-earner physician families, this can have devastating financial consequences. These limitations contribute to growing burnout and dissatisfaction within the medical profession, which already is facing unprecedented levels of stress and physician workforce shortages.4
Effect on Patients
When a physician is forced to relocate to a new geographic region because of a noncompete clause, their patients can experience substantial disruptions in care. Access to medical services may be affected, leading to longer wait-times and fewer available appointments, especially in areas that already have a shortage of providers. Patients may lose longstanding relationships with doctors who know their medical histories, which can interrupt treatment plans and increase the risk of complications. Those with chronic illnesses, complex conditions, or time-sensitive treatments are particularly vulnerable to adverse outcomes. Many patients must travel farther—sometimes out of their insurance network—to find replacement care, increasing both financial and logistical burdens. These abrupt transitions also can raise health care costs due to emergency department use, inefficient handoffs, and higher incidence of morbidity/mortality.5 Noncompete restrictions often prevent physicians from informing patients where they are relocating, creating confusion and fragmentation of care. As a result, trust in the health care system may decline when patients perceive that business agreements are being prioritized above their wellbeing. The impact may be even more severe in rural or underserved communities where alternative providers are scarce.
Final Thoughts
In recent years, noncompete agreements in health care have come under intensified scrutiny for their potential to stifle physician mobility, reduce competition, and inflate health care costs by limiting where and how physicians can practice. The trajectory of noncompetes in physician employment reflects broader shifts in how medicine is structured and delivered in the United States. In the latter half of the 20th century, what began as a centuries-old legal concept became a standard feature of physician employment contracts. That evolution largely was driven by the corporatization of medicine and large hospital group/private equity employment of physicians. As these agreements proliferated, public policy questions emerged: What does restricting a physician’s mobility do to patient access? To competition in provider markets? To the cost and availability of care? To the current epidemic of physician burnout?
These questions moved from the legal sidelines to center stage in the 2020s, when the FTC sought to tackle noncompetes across the entire economy—physicians included—on the theory they suppressed labor mobility, entrepreneurship, and competition. In February 2020, the American Medical Association submitted comments to the FTC on the utility of noncompete agreements in employee contracts stating that they restrict competition, can disrupt continuity of care, and may limit access to care.6 Although the FTC’s regulatory attempt in April 2024 provoked strong policy signals, it was challenged and ultimately blocked. Rather than a clear federal prohibition, the outcome is a more incremental state-based shift in rules governing physician noncompetes. For physicians today, this means more awareness and more leverage, but also more complexity. Whether a noncompete will be enforceable depends heavily on the state, the wording of the contract, the structure of the employer, and the specialty. From a negotiation standpoint, physicians need more guidance and awareness on the exact ramifications of their employee contract. For newly minted physicians, many of whom enter the workforce with considerable training debt, the priority often is securing employment to work toward financial stability, building a family, or both; however, all physicians should press for shorter durations, tighter geographic limits, narrower scopes of service, clear buy-out options, and explicit patient-continuity protections. Better yet, physicians can exercise the right of refusal to any noncompete clause at all. Becoming involved with a local medical organization or foundation can provide immense support, both in reviewing contracts as well as learning how to become advocates for physicians in this environment. As more physicians stand together to protect both practice autonomy and the right to quality care, we all become closer to rediscovering the beauty and fulfillment in the purest form of medicine.
- Federal Trade Commission. FTC announces rule banning noncompetes. April 23, 2024. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes
- US Chamber of Commerce. Ryan LLC v FTC. August 20, 2024. Accessed December 1, 2025. https://www.uschamber.com/cases/antitrust-and-competition-law/ryan-llc-v.-ftc
- Federal Trade Commission. Federal Trade Commission files to accede to vacatur of non-compete clause rule. September 5, 2025. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2025/09/federal-trade-commission-files-accede-vacatur-non-compete-clause-rule
- Marshall JJ, Ashwath ML, Jefferies JL, et al. Restrictive covenants and noncompete clauses for physicians. JACC Adv. 2023;2:100547.
- Sabety A. The value of relationships in healthcare. J Publich Economics. 2023;225:104927.
- American Medical Association. AMA provides comment to FTC on non-compete agreements. National Advocacy Update. February 14, 2020. Accessed November 25, 2025. https://www.ama-assn.org/health-care-advocacy/advocacy-update/feb-14-2020-national-advocacy-update
- Federal Trade Commission. FTC announces rule banning noncompetes. April 23, 2024. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes
- US Chamber of Commerce. Ryan LLC v FTC. August 20, 2024. Accessed December 1, 2025. https://www.uschamber.com/cases/antitrust-and-competition-law/ryan-llc-v.-ftc
- Federal Trade Commission. Federal Trade Commission files to accede to vacatur of non-compete clause rule. September 5, 2025. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2025/09/federal-trade-commission-files-accede-vacatur-non-compete-clause-rule
- Marshall JJ, Ashwath ML, Jefferies JL, et al. Restrictive covenants and noncompete clauses for physicians. JACC Adv. 2023;2:100547.
- Sabety A. The value of relationships in healthcare. J Publich Economics. 2023;225:104927.
- American Medical Association. AMA provides comment to FTC on non-compete agreements. National Advocacy Update. February 14, 2020. Accessed November 25, 2025. https://www.ama-assn.org/health-care-advocacy/advocacy-update/feb-14-2020-national-advocacy-update
PRACTICE POINTS
- There is no active federal ban on physician noncompete agreements as of late 2025.
- Physician noncompetes have expanded alongside the corporatization of medicine but raise serious concerns about physician mobility, burnout, workforce shortages, and patient access to care, particularly in underserved areas.
- Physicians should critically evaluate noncompetes prior to signing an agreement, advocating for narrower limits or refusal altogether to protect professional autonomy, continuity of care, and patient welfare.