User login
Ground-breaking therapy comes with distinct challenges
NEW YORK—Two chimeric antigen receptor (CAR) T-cell therapies—axicabtagene ciloleucel (Yescarta ®) and tisagenlecleucel (Kymriah™)—are already approved in B-cell lymphoma by the U.S. Food and Drug Administration.
A third, lisocabtagene maraleucel, will most likely be approved before too long.
Despite differences in their costimulatory molecules, persistence, efficacy, and toxicity profiles, they all have high overall response rates and a fall-out of response during the first 3 to 6 months.
Longer-term follow-up is necessary to determine whether CAR T-cell therapy is actually curative.
“But based on the way things are looking,” said Reem Karmali, MD, of Robert H. Lurie Comprehensive Cancer Center of Northwestern University, “it seems this might be a realistic expectation.”
“CAR T-cell therapy is clearly effective and has been a ground-breaking form of therapy,” she said, “but there seems to be two sides to the coin. There are a number of challenges that we face with CAR T-cell therapy.”
Dr. Karmali outlined those challenges in a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies.
Patient selection
One of the biggest challenges, according to Dr. Karmali, is patient selection.
First, patients must have an adequate hematopoietic reserve to ensure successful CAR T-cell manufacture.
Dr. Karmali referred to the JULIET study, in which 7% of patients failed the manufacturing process due to insufficient apheresis.
Second, the patient’s disease must be stable enough to make it through the time it takes to manufacturing the CAR product, which is typically 2 to 4 weeks.
Third, the patient’s overall health must be good enough to tolerate CAR T toxicities. "The patient needs good major organ function as well as preserved neurologic function,” she explained, “to withstand the unique toxicities that come with CAR T-cell therapy, specifically CRS [cytokine release syndrome] and neurotoxicity.”
Toxicities
The major toxicities are CRS and CAR‑T‑cell‑related encephalopathy syndrome (CRES).
Dr. Karmali pointed out there is also a theoretical risk of insertional oncogenesis from viral transduction used in manufacturing the T cells, and an off-tumor on target-effect that can result in B-cell aplasia and hypogammaglobulinemia.
The profiles of inflammatory cytokines and inflammation markers differ for each CAR construct and are driven in different ways. However, IL-6 is an important mediator for CRS and IL-6 receptor blockade is effective in managing the toxicity.
The drug of choice is tocilizumab, Dr. Karmali said, and for patients who are refractory to tocilizumab, siltuximab can be used.
“Steroids are extremely useful for CRS,” she added, “because they hold down inflammation and prevent immune activation.”
Steroids are also the mainstay for managing the neurotoxicity of CAR T-cell therapy because they help stabilize the blood-brain barrier.
“It’s important to make a note,” she said, “that there actually have been a number of analyses that have looked at the impact of using IL-6 receptor blockade and steroids on CAR T-cell expansion and persistence and there really doesn’t seem to be an impact.”
“So we really ought to use these quite liberally for grade 2 or higher toxicity without worrying about dampening the effect of CAR T-cell therapy,” she emphasized.
The Lee grading criteria for the management of CRS and the CTCAE 4.03 and CARTOX-10 for CRES provide guidance in assessing and managing the toxicities.
Future directions
Dr. Karmali outlined a few new directions to address the challenges with CAR T-cell therapy, such as switchable CARs that can be turned on or off and potentially improve safety; development of new constructs that may improve homing; improvement in persistence; use of combination and sequencing strategies; and improved antigen selection that may be effective with other lymphoproliferative diseases.
“A provocative question is whether CAR T-cell therapy can actually replace autologous stem cell transplant as second-line therapy,” she said. “This is actually being actively evaluated in a number of clinical trials including ZUMA-7 (NCT03391466).”
“I think another provocative question is whether CAR T-cell therapy can be used as consolidation in CR1 [first complete remission],” she added.
The rationale for using CAR Ts as either a replacement for autologous stem cell transplant or in CR1 is that there may be minimal residual disease present that would be enough to elicit a CAR T-cell effect, she explained.
“Ultimately, one envisions the following paradigm for the treatment of lymphomas across the board,” Dr. Karmali concluded.
“Specifically, chemotherapy with a targeted agent for rapid cytoreduction, followed by CAR T-cell consolidation in combination with either other cellular therapies or immunotherapy as a means of eradicating the minimal residual disease and ensuring a pathway to cure.”
NEW YORK—Two chimeric antigen receptor (CAR) T-cell therapies—axicabtagene ciloleucel (Yescarta ®) and tisagenlecleucel (Kymriah™)—are already approved in B-cell lymphoma by the U.S. Food and Drug Administration.
A third, lisocabtagene maraleucel, will most likely be approved before too long.
Despite differences in their costimulatory molecules, persistence, efficacy, and toxicity profiles, they all have high overall response rates and a fall-out of response during the first 3 to 6 months.
Longer-term follow-up is necessary to determine whether CAR T-cell therapy is actually curative.
“But based on the way things are looking,” said Reem Karmali, MD, of Robert H. Lurie Comprehensive Cancer Center of Northwestern University, “it seems this might be a realistic expectation.”
“CAR T-cell therapy is clearly effective and has been a ground-breaking form of therapy,” she said, “but there seems to be two sides to the coin. There are a number of challenges that we face with CAR T-cell therapy.”
Dr. Karmali outlined those challenges in a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies.
Patient selection
One of the biggest challenges, according to Dr. Karmali, is patient selection.
First, patients must have an adequate hematopoietic reserve to ensure successful CAR T-cell manufacture.
Dr. Karmali referred to the JULIET study, in which 7% of patients failed the manufacturing process due to insufficient apheresis.
Second, the patient’s disease must be stable enough to make it through the time it takes to manufacturing the CAR product, which is typically 2 to 4 weeks.
Third, the patient’s overall health must be good enough to tolerate CAR T toxicities. "The patient needs good major organ function as well as preserved neurologic function,” she explained, “to withstand the unique toxicities that come with CAR T-cell therapy, specifically CRS [cytokine release syndrome] and neurotoxicity.”
Toxicities
The major toxicities are CRS and CAR‑T‑cell‑related encephalopathy syndrome (CRES).
Dr. Karmali pointed out there is also a theoretical risk of insertional oncogenesis from viral transduction used in manufacturing the T cells, and an off-tumor on target-effect that can result in B-cell aplasia and hypogammaglobulinemia.
The profiles of inflammatory cytokines and inflammation markers differ for each CAR construct and are driven in different ways. However, IL-6 is an important mediator for CRS and IL-6 receptor blockade is effective in managing the toxicity.
The drug of choice is tocilizumab, Dr. Karmali said, and for patients who are refractory to tocilizumab, siltuximab can be used.
“Steroids are extremely useful for CRS,” she added, “because they hold down inflammation and prevent immune activation.”
Steroids are also the mainstay for managing the neurotoxicity of CAR T-cell therapy because they help stabilize the blood-brain barrier.
“It’s important to make a note,” she said, “that there actually have been a number of analyses that have looked at the impact of using IL-6 receptor blockade and steroids on CAR T-cell expansion and persistence and there really doesn’t seem to be an impact.”
“So we really ought to use these quite liberally for grade 2 or higher toxicity without worrying about dampening the effect of CAR T-cell therapy,” she emphasized.
The Lee grading criteria for the management of CRS and the CTCAE 4.03 and CARTOX-10 for CRES provide guidance in assessing and managing the toxicities.
Future directions
Dr. Karmali outlined a few new directions to address the challenges with CAR T-cell therapy, such as switchable CARs that can be turned on or off and potentially improve safety; development of new constructs that may improve homing; improvement in persistence; use of combination and sequencing strategies; and improved antigen selection that may be effective with other lymphoproliferative diseases.
“A provocative question is whether CAR T-cell therapy can actually replace autologous stem cell transplant as second-line therapy,” she said. “This is actually being actively evaluated in a number of clinical trials including ZUMA-7 (NCT03391466).”
“I think another provocative question is whether CAR T-cell therapy can be used as consolidation in CR1 [first complete remission],” she added.
The rationale for using CAR Ts as either a replacement for autologous stem cell transplant or in CR1 is that there may be minimal residual disease present that would be enough to elicit a CAR T-cell effect, she explained.
“Ultimately, one envisions the following paradigm for the treatment of lymphomas across the board,” Dr. Karmali concluded.
“Specifically, chemotherapy with a targeted agent for rapid cytoreduction, followed by CAR T-cell consolidation in combination with either other cellular therapies or immunotherapy as a means of eradicating the minimal residual disease and ensuring a pathway to cure.”
NEW YORK—Two chimeric antigen receptor (CAR) T-cell therapies—axicabtagene ciloleucel (Yescarta ®) and tisagenlecleucel (Kymriah™)—are already approved in B-cell lymphoma by the U.S. Food and Drug Administration.
A third, lisocabtagene maraleucel, will most likely be approved before too long.
Despite differences in their costimulatory molecules, persistence, efficacy, and toxicity profiles, they all have high overall response rates and a fall-out of response during the first 3 to 6 months.
Longer-term follow-up is necessary to determine whether CAR T-cell therapy is actually curative.
“But based on the way things are looking,” said Reem Karmali, MD, of Robert H. Lurie Comprehensive Cancer Center of Northwestern University, “it seems this might be a realistic expectation.”
“CAR T-cell therapy is clearly effective and has been a ground-breaking form of therapy,” she said, “but there seems to be two sides to the coin. There are a number of challenges that we face with CAR T-cell therapy.”
Dr. Karmali outlined those challenges in a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies.
Patient selection
One of the biggest challenges, according to Dr. Karmali, is patient selection.
First, patients must have an adequate hematopoietic reserve to ensure successful CAR T-cell manufacture.
Dr. Karmali referred to the JULIET study, in which 7% of patients failed the manufacturing process due to insufficient apheresis.
Second, the patient’s disease must be stable enough to make it through the time it takes to manufacturing the CAR product, which is typically 2 to 4 weeks.
Third, the patient’s overall health must be good enough to tolerate CAR T toxicities. "The patient needs good major organ function as well as preserved neurologic function,” she explained, “to withstand the unique toxicities that come with CAR T-cell therapy, specifically CRS [cytokine release syndrome] and neurotoxicity.”
Toxicities
The major toxicities are CRS and CAR‑T‑cell‑related encephalopathy syndrome (CRES).
Dr. Karmali pointed out there is also a theoretical risk of insertional oncogenesis from viral transduction used in manufacturing the T cells, and an off-tumor on target-effect that can result in B-cell aplasia and hypogammaglobulinemia.
The profiles of inflammatory cytokines and inflammation markers differ for each CAR construct and are driven in different ways. However, IL-6 is an important mediator for CRS and IL-6 receptor blockade is effective in managing the toxicity.
The drug of choice is tocilizumab, Dr. Karmali said, and for patients who are refractory to tocilizumab, siltuximab can be used.
“Steroids are extremely useful for CRS,” she added, “because they hold down inflammation and prevent immune activation.”
Steroids are also the mainstay for managing the neurotoxicity of CAR T-cell therapy because they help stabilize the blood-brain barrier.
“It’s important to make a note,” she said, “that there actually have been a number of analyses that have looked at the impact of using IL-6 receptor blockade and steroids on CAR T-cell expansion and persistence and there really doesn’t seem to be an impact.”
“So we really ought to use these quite liberally for grade 2 or higher toxicity without worrying about dampening the effect of CAR T-cell therapy,” she emphasized.
The Lee grading criteria for the management of CRS and the CTCAE 4.03 and CARTOX-10 for CRES provide guidance in assessing and managing the toxicities.
Future directions
Dr. Karmali outlined a few new directions to address the challenges with CAR T-cell therapy, such as switchable CARs that can be turned on or off and potentially improve safety; development of new constructs that may improve homing; improvement in persistence; use of combination and sequencing strategies; and improved antigen selection that may be effective with other lymphoproliferative diseases.
“A provocative question is whether CAR T-cell therapy can actually replace autologous stem cell transplant as second-line therapy,” she said. “This is actually being actively evaluated in a number of clinical trials including ZUMA-7 (NCT03391466).”
“I think another provocative question is whether CAR T-cell therapy can be used as consolidation in CR1 [first complete remission],” she added.
The rationale for using CAR Ts as either a replacement for autologous stem cell transplant or in CR1 is that there may be minimal residual disease present that would be enough to elicit a CAR T-cell effect, she explained.
“Ultimately, one envisions the following paradigm for the treatment of lymphomas across the board,” Dr. Karmali concluded.
“Specifically, chemotherapy with a targeted agent for rapid cytoreduction, followed by CAR T-cell consolidation in combination with either other cellular therapies or immunotherapy as a means of eradicating the minimal residual disease and ensuring a pathway to cure.”
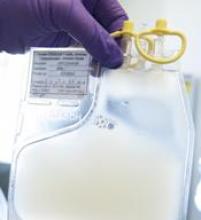
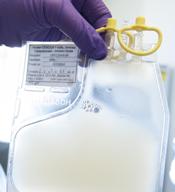
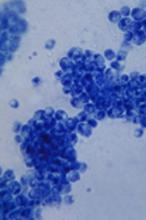
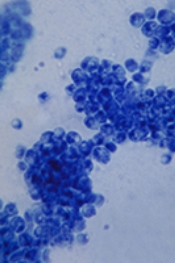
Single leukemic cell can contaminate CAR T-cell product
Investigators report that a single leukemic cell unintentionally engineered into the chimeric antigen receptor (CAR) T-cell product can mask it from recognition and confer resistance to CAR T-cell therapy.
They described the case of a 20-year-old male who received the anti-CD19 CAR tisagenlecleucel (Kymriah) and relapsed at day 252 after the infusion.
The transduction of a leukemic cell during manufacture of the CAR T-cell product “is a rare event,” they wrote, and indicated that “this is the only case out of 369 patients reported worldwide at the time of publication.”
Lead author Marco Ruella, MD, of the University of Pennsylvania, and colleagues described the case in a Brief Communication published in Nature Medicine.
"In this case,” Dr. Ruella said, “we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells."
The patient had B-cell acute lymphoblastic leukemia (B-ALL) and relapsed three times after chemotherapy and a cord blood transplant before enrolling in the phase 1 trial of CTL019 (NCT 01626495).
The investigators reported that the infused CAR cells “displayed the typical pattern of in vivo engraftment and expansion.” At day 28 after the infusion, the patient was in complete remission.
But by day 252, he experienced a second expansion of CAR cells that did not correspond to the re-expansion of CAR+ T cells.
At day 261, the patient relapsed with more than 90% CD10+CD19- leukemic cells in the bone marrow and circulating blasts. The cells were CAR-transduced B-cell leukemia (CARB) cells.
The CARB cells continued to expand, and the patient died of progressive leukemia.
The investigators tracked the origin of the CARB cells by analyzing the relapsed CAR19+ cells using next-generation sequencing.
They hypothesized that the CAR19+ leukemia relapse occurred through lentiviral transduction during the manufacturing process, since they detected no replication-competent lentivirus when testing the patient’s peripheral blood at numerous time points after CTL019 infusion.
Further analysis confirmed the CARB cells were a byproduct made during CTL019 cell manufacturing.
To confirm that the leukemia relapse originated from a single clone, the investigators expanded in mice blast cells detected in the patient at month 9. Nine of 71 cells analyzed were positive for vector-host junctions. This confirmed that the relapsed cells originated from a single blast clone.
The investigators also excluded other possible reasons for the loss of CD19, including mutations, splicing variants, and structural alteration of the B-cell receptor complex.
They found that expression of the CAR in cis on B-ALL blasts masked the CAR target epitope.
The investigators concluded that their results “provide a direct confirmation of the cancer stem cell hypothesis in humans, given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo.”
They called for improved manufacturing technologies that can eliminate contamination by residual tumor cells from engineered T cells.
Interestingly, this case developed not long after a case that showed essentially the opposite situation—a patient with chronic lymphocytic leukemia went into remission because of a single CAR T cell that reproduced and fought off the disease.
Investigators report that a single leukemic cell unintentionally engineered into the chimeric antigen receptor (CAR) T-cell product can mask it from recognition and confer resistance to CAR T-cell therapy.
They described the case of a 20-year-old male who received the anti-CD19 CAR tisagenlecleucel (Kymriah) and relapsed at day 252 after the infusion.
The transduction of a leukemic cell during manufacture of the CAR T-cell product “is a rare event,” they wrote, and indicated that “this is the only case out of 369 patients reported worldwide at the time of publication.”
Lead author Marco Ruella, MD, of the University of Pennsylvania, and colleagues described the case in a Brief Communication published in Nature Medicine.
"In this case,” Dr. Ruella said, “we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells."
The patient had B-cell acute lymphoblastic leukemia (B-ALL) and relapsed three times after chemotherapy and a cord blood transplant before enrolling in the phase 1 trial of CTL019 (NCT 01626495).
The investigators reported that the infused CAR cells “displayed the typical pattern of in vivo engraftment and expansion.” At day 28 after the infusion, the patient was in complete remission.
But by day 252, he experienced a second expansion of CAR cells that did not correspond to the re-expansion of CAR+ T cells.
At day 261, the patient relapsed with more than 90% CD10+CD19- leukemic cells in the bone marrow and circulating blasts. The cells were CAR-transduced B-cell leukemia (CARB) cells.
The CARB cells continued to expand, and the patient died of progressive leukemia.
The investigators tracked the origin of the CARB cells by analyzing the relapsed CAR19+ cells using next-generation sequencing.
They hypothesized that the CAR19+ leukemia relapse occurred through lentiviral transduction during the manufacturing process, since they detected no replication-competent lentivirus when testing the patient’s peripheral blood at numerous time points after CTL019 infusion.
Further analysis confirmed the CARB cells were a byproduct made during CTL019 cell manufacturing.
To confirm that the leukemia relapse originated from a single clone, the investigators expanded in mice blast cells detected in the patient at month 9. Nine of 71 cells analyzed were positive for vector-host junctions. This confirmed that the relapsed cells originated from a single blast clone.
The investigators also excluded other possible reasons for the loss of CD19, including mutations, splicing variants, and structural alteration of the B-cell receptor complex.
They found that expression of the CAR in cis on B-ALL blasts masked the CAR target epitope.
The investigators concluded that their results “provide a direct confirmation of the cancer stem cell hypothesis in humans, given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo.”
They called for improved manufacturing technologies that can eliminate contamination by residual tumor cells from engineered T cells.
Interestingly, this case developed not long after a case that showed essentially the opposite situation—a patient with chronic lymphocytic leukemia went into remission because of a single CAR T cell that reproduced and fought off the disease.
Investigators report that a single leukemic cell unintentionally engineered into the chimeric antigen receptor (CAR) T-cell product can mask it from recognition and confer resistance to CAR T-cell therapy.
They described the case of a 20-year-old male who received the anti-CD19 CAR tisagenlecleucel (Kymriah) and relapsed at day 252 after the infusion.
The transduction of a leukemic cell during manufacture of the CAR T-cell product “is a rare event,” they wrote, and indicated that “this is the only case out of 369 patients reported worldwide at the time of publication.”
Lead author Marco Ruella, MD, of the University of Pennsylvania, and colleagues described the case in a Brief Communication published in Nature Medicine.
"In this case,” Dr. Ruella said, “we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells."
The patient had B-cell acute lymphoblastic leukemia (B-ALL) and relapsed three times after chemotherapy and a cord blood transplant before enrolling in the phase 1 trial of CTL019 (NCT 01626495).
The investigators reported that the infused CAR cells “displayed the typical pattern of in vivo engraftment and expansion.” At day 28 after the infusion, the patient was in complete remission.
But by day 252, he experienced a second expansion of CAR cells that did not correspond to the re-expansion of CAR+ T cells.
At day 261, the patient relapsed with more than 90% CD10+CD19- leukemic cells in the bone marrow and circulating blasts. The cells were CAR-transduced B-cell leukemia (CARB) cells.
The CARB cells continued to expand, and the patient died of progressive leukemia.
The investigators tracked the origin of the CARB cells by analyzing the relapsed CAR19+ cells using next-generation sequencing.
They hypothesized that the CAR19+ leukemia relapse occurred through lentiviral transduction during the manufacturing process, since they detected no replication-competent lentivirus when testing the patient’s peripheral blood at numerous time points after CTL019 infusion.
Further analysis confirmed the CARB cells were a byproduct made during CTL019 cell manufacturing.
To confirm that the leukemia relapse originated from a single clone, the investigators expanded in mice blast cells detected in the patient at month 9. Nine of 71 cells analyzed were positive for vector-host junctions. This confirmed that the relapsed cells originated from a single blast clone.
The investigators also excluded other possible reasons for the loss of CD19, including mutations, splicing variants, and structural alteration of the B-cell receptor complex.
They found that expression of the CAR in cis on B-ALL blasts masked the CAR target epitope.
The investigators concluded that their results “provide a direct confirmation of the cancer stem cell hypothesis in humans, given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo.”
They called for improved manufacturing technologies that can eliminate contamination by residual tumor cells from engineered T cells.
Interestingly, this case developed not long after a case that showed essentially the opposite situation—a patient with chronic lymphocytic leukemia went into remission because of a single CAR T cell that reproduced and fought off the disease.
Two immunologists receive Nobel Prize in medicine
Two immunologists have been awarded the Nobel Prize in Physiology or Medicine for discoveries that represent a “paradigmatic shift in the fight against cancer,” the Nobel committee said.
James P. Allison, PhD, of MD Anderson Cancer Center, and Tasuku Honjo, MD, PhD, of Kyoto University, shared the prize for their discovery of cancer therapies that work by inhibiting negative immune regulation.
Dr. Allison studied the protein CTLA-4 found on T cells, which acts as a T-cell brake, and Dr. Honjo discovered a protein on immune cells called PD-1 that also acts as a T-cell brake.
In addition to sharing the honor, the scientists will split the 9 million Swedish kronor ($1.01 million) that comes with the prize.
Drs. Allison and Honjo, working in parallel, pursued different strategies for inhibiting the brakes on the immune system. Both strategies produced effective checkpoint inhibitors in the treatment of cancer.
James P. Allison
Dr. Allison was one of several scientists during the 1990s who noticed that CTLA-4 functions as a brake on T cells. Unlike other scientists, however, he set out to investigate whether blocking CTLA-4 with an antibody he had already developed could release the brake on the immune system.
The antibody had “spectacular” effects in curing mice with cancer. Despite little interest from the pharmaceutical industry, Dr. Allison continued efforts to develop the antibody therapy for humans.
The antibody turned out to be ipilimumab, which was approved in 2011 by the U.S. Food and Drug Administration (FDA) for the treatment of advanced melanoma.
Tasuko Honjo
A few years prior to Dr. Allison’s finding, Dr. Honjo discovered PD-1 and set out to determine its function. PD-1 also operates as a T-cell brake, but it uses a different mechanism than does CTLA-4.
Dr. Honjo and others demonstrated in animal experiments that PD-1 blockade could be an effective anticancer therapy. Over the years he demonstrated the efficacy of targeting PD-1 in different types of human cancers.
The first two PD-1 checkpoint inhibitors—pembrolizumab and nivolumab—were approved by the FDA in 2014 for the treatment of melanoma.
Nivolumab is also approved to treat classical Hodgkin lymphoma (HL), non-small cell lung cancer (NSCLC), small cell lung cancer, squamous cell carcinoma of the head and neck, colorectal cancer, hepatocellular carcinoma, renal cell carcinoma, urothelial carcinoma, and microsatellite instability-high or mismatch repair deficient colorectal cancer.
Pembrolizumab is also approved to treat primary mediastinal large B-cell lymphoma, advanced NSCLC, classical HL, advanced gastric cancer, advanced cervical cancer, head and neck squamous cell cancer, advanced urothelial bladder cancer, and microsatellite instability-high cancer.
And targeting both CTLA-4 and PD-1 in combination therapy together may prove to be even more effective in eliminating cancer cells than either strategy alone, as is being demonstrated in patients with melanoma.
The Nobel organization wrote in a press release, “Checkpoint therapy has now revolutionized cancer treatment and has fundamentally changed the way we view how cancer can be managed.”
Two immunologists have been awarded the Nobel Prize in Physiology or Medicine for discoveries that represent a “paradigmatic shift in the fight against cancer,” the Nobel committee said.
James P. Allison, PhD, of MD Anderson Cancer Center, and Tasuku Honjo, MD, PhD, of Kyoto University, shared the prize for their discovery of cancer therapies that work by inhibiting negative immune regulation.
Dr. Allison studied the protein CTLA-4 found on T cells, which acts as a T-cell brake, and Dr. Honjo discovered a protein on immune cells called PD-1 that also acts as a T-cell brake.
In addition to sharing the honor, the scientists will split the 9 million Swedish kronor ($1.01 million) that comes with the prize.
Drs. Allison and Honjo, working in parallel, pursued different strategies for inhibiting the brakes on the immune system. Both strategies produced effective checkpoint inhibitors in the treatment of cancer.
James P. Allison
Dr. Allison was one of several scientists during the 1990s who noticed that CTLA-4 functions as a brake on T cells. Unlike other scientists, however, he set out to investigate whether blocking CTLA-4 with an antibody he had already developed could release the brake on the immune system.
The antibody had “spectacular” effects in curing mice with cancer. Despite little interest from the pharmaceutical industry, Dr. Allison continued efforts to develop the antibody therapy for humans.
The antibody turned out to be ipilimumab, which was approved in 2011 by the U.S. Food and Drug Administration (FDA) for the treatment of advanced melanoma.
Tasuko Honjo
A few years prior to Dr. Allison’s finding, Dr. Honjo discovered PD-1 and set out to determine its function. PD-1 also operates as a T-cell brake, but it uses a different mechanism than does CTLA-4.
Dr. Honjo and others demonstrated in animal experiments that PD-1 blockade could be an effective anticancer therapy. Over the years he demonstrated the efficacy of targeting PD-1 in different types of human cancers.
The first two PD-1 checkpoint inhibitors—pembrolizumab and nivolumab—were approved by the FDA in 2014 for the treatment of melanoma.
Nivolumab is also approved to treat classical Hodgkin lymphoma (HL), non-small cell lung cancer (NSCLC), small cell lung cancer, squamous cell carcinoma of the head and neck, colorectal cancer, hepatocellular carcinoma, renal cell carcinoma, urothelial carcinoma, and microsatellite instability-high or mismatch repair deficient colorectal cancer.
Pembrolizumab is also approved to treat primary mediastinal large B-cell lymphoma, advanced NSCLC, classical HL, advanced gastric cancer, advanced cervical cancer, head and neck squamous cell cancer, advanced urothelial bladder cancer, and microsatellite instability-high cancer.
And targeting both CTLA-4 and PD-1 in combination therapy together may prove to be even more effective in eliminating cancer cells than either strategy alone, as is being demonstrated in patients with melanoma.
The Nobel organization wrote in a press release, “Checkpoint therapy has now revolutionized cancer treatment and has fundamentally changed the way we view how cancer can be managed.”
Two immunologists have been awarded the Nobel Prize in Physiology or Medicine for discoveries that represent a “paradigmatic shift in the fight against cancer,” the Nobel committee said.
James P. Allison, PhD, of MD Anderson Cancer Center, and Tasuku Honjo, MD, PhD, of Kyoto University, shared the prize for their discovery of cancer therapies that work by inhibiting negative immune regulation.
Dr. Allison studied the protein CTLA-4 found on T cells, which acts as a T-cell brake, and Dr. Honjo discovered a protein on immune cells called PD-1 that also acts as a T-cell brake.
In addition to sharing the honor, the scientists will split the 9 million Swedish kronor ($1.01 million) that comes with the prize.
Drs. Allison and Honjo, working in parallel, pursued different strategies for inhibiting the brakes on the immune system. Both strategies produced effective checkpoint inhibitors in the treatment of cancer.
James P. Allison
Dr. Allison was one of several scientists during the 1990s who noticed that CTLA-4 functions as a brake on T cells. Unlike other scientists, however, he set out to investigate whether blocking CTLA-4 with an antibody he had already developed could release the brake on the immune system.
The antibody had “spectacular” effects in curing mice with cancer. Despite little interest from the pharmaceutical industry, Dr. Allison continued efforts to develop the antibody therapy for humans.
The antibody turned out to be ipilimumab, which was approved in 2011 by the U.S. Food and Drug Administration (FDA) for the treatment of advanced melanoma.
Tasuko Honjo
A few years prior to Dr. Allison’s finding, Dr. Honjo discovered PD-1 and set out to determine its function. PD-1 also operates as a T-cell brake, but it uses a different mechanism than does CTLA-4.
Dr. Honjo and others demonstrated in animal experiments that PD-1 blockade could be an effective anticancer therapy. Over the years he demonstrated the efficacy of targeting PD-1 in different types of human cancers.
The first two PD-1 checkpoint inhibitors—pembrolizumab and nivolumab—were approved by the FDA in 2014 for the treatment of melanoma.
Nivolumab is also approved to treat classical Hodgkin lymphoma (HL), non-small cell lung cancer (NSCLC), small cell lung cancer, squamous cell carcinoma of the head and neck, colorectal cancer, hepatocellular carcinoma, renal cell carcinoma, urothelial carcinoma, and microsatellite instability-high or mismatch repair deficient colorectal cancer.
Pembrolizumab is also approved to treat primary mediastinal large B-cell lymphoma, advanced NSCLC, classical HL, advanced gastric cancer, advanced cervical cancer, head and neck squamous cell cancer, advanced urothelial bladder cancer, and microsatellite instability-high cancer.
And targeting both CTLA-4 and PD-1 in combination therapy together may prove to be even more effective in eliminating cancer cells than either strategy alone, as is being demonstrated in patients with melanoma.
The Nobel organization wrote in a press release, “Checkpoint therapy has now revolutionized cancer treatment and has fundamentally changed the way we view how cancer can be managed.”
BCMA-targeted platforms could alter MM therapy
New York—Three novel treatment strategies that target B-cell maturation antigen (BCMA) are showing promise in recent multiple myeloma (MM) clinical trials, according to Shaji K. Kumar, MD, of Mayo Clinic Cancer Center in Rochester, Minnesota.
The strategies include B-cell maturation antigen (BCMA) antibody-drug conjugate, BCMA-specific chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs).
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these 3 platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar told attendees at the NCCN 13th Annual Congress: Hematologic Malignancies.
BCMA is required for plasma cell survival and is broadly expressed on malignant plasma cells.
BCMA antibody-drug conjugate
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent. It produced an overall response rate of 67% at the 2 highest dose levels in 9 MM patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, he said, including in an upcoming phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone in patients with relapsed or refractory disease.
BCMA-specific CAR T-cell therapy
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma, according to Dr. Kumar, involves bb2121. bb2121 showed durable clinical responses in heavily pretreated patients, according to an ASH 2017 presentation.
“The overall response rate is quite significant,” Dr. Kumar said. He related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the responses were lasting, he said, with 5 patients in ongoing response for more than a year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar added.
Another novel CAR T-cell product, LCAR-B38M, has demonstrated promising results. LCAR-B38M principally targets BCMA and has led to a significant number of patients achieving stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and different costimulatory molecules are currently in clinical trials, Dr. Kumar said.
BiTEs
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short timeframe, so having an approach that is off-the-shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies to watch that are under investigation include AMG 420 and PF-06863135, he said.
New York—Three novel treatment strategies that target B-cell maturation antigen (BCMA) are showing promise in recent multiple myeloma (MM) clinical trials, according to Shaji K. Kumar, MD, of Mayo Clinic Cancer Center in Rochester, Minnesota.
The strategies include B-cell maturation antigen (BCMA) antibody-drug conjugate, BCMA-specific chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs).
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these 3 platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar told attendees at the NCCN 13th Annual Congress: Hematologic Malignancies.
BCMA is required for plasma cell survival and is broadly expressed on malignant plasma cells.
BCMA antibody-drug conjugate
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent. It produced an overall response rate of 67% at the 2 highest dose levels in 9 MM patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, he said, including in an upcoming phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone in patients with relapsed or refractory disease.
BCMA-specific CAR T-cell therapy
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma, according to Dr. Kumar, involves bb2121. bb2121 showed durable clinical responses in heavily pretreated patients, according to an ASH 2017 presentation.
“The overall response rate is quite significant,” Dr. Kumar said. He related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the responses were lasting, he said, with 5 patients in ongoing response for more than a year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar added.
Another novel CAR T-cell product, LCAR-B38M, has demonstrated promising results. LCAR-B38M principally targets BCMA and has led to a significant number of patients achieving stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and different costimulatory molecules are currently in clinical trials, Dr. Kumar said.
BiTEs
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short timeframe, so having an approach that is off-the-shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies to watch that are under investigation include AMG 420 and PF-06863135, he said.
New York—Three novel treatment strategies that target B-cell maturation antigen (BCMA) are showing promise in recent multiple myeloma (MM) clinical trials, according to Shaji K. Kumar, MD, of Mayo Clinic Cancer Center in Rochester, Minnesota.
The strategies include B-cell maturation antigen (BCMA) antibody-drug conjugate, BCMA-specific chimeric antigen receptor (CAR) T-cell therapies, and bispecific T-cell engagers (BiTEs).
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these 3 platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar told attendees at the NCCN 13th Annual Congress: Hematologic Malignancies.
BCMA is required for plasma cell survival and is broadly expressed on malignant plasma cells.
BCMA antibody-drug conjugate
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent. It produced an overall response rate of 67% at the 2 highest dose levels in 9 MM patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, he said, including in an upcoming phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone in patients with relapsed or refractory disease.
BCMA-specific CAR T-cell therapy
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma, according to Dr. Kumar, involves bb2121. bb2121 showed durable clinical responses in heavily pretreated patients, according to an ASH 2017 presentation.
“The overall response rate is quite significant,” Dr. Kumar said. He related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the responses were lasting, he said, with 5 patients in ongoing response for more than a year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar added.
Another novel CAR T-cell product, LCAR-B38M, has demonstrated promising results. LCAR-B38M principally targets BCMA and has led to a significant number of patients achieving stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and different costimulatory molecules are currently in clinical trials, Dr. Kumar said.
BiTEs
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short timeframe, so having an approach that is off-the-shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies to watch that are under investigation include AMG 420 and PF-06863135, he said.
Brentuximab improves survival in older HL patients
Older patients with untreated Hodgkin lymphoma (HL) can achieve significantly improved survival by adding brentuximab vedotin to their treatment before and after standard chemotherapy, a recent study found.
In patients with low comorbidity scores, responses were even more robust, reported lead author Andrew M. Evens, DO, of the Rutgers Cancer Institute of New Jersey, and colleagues.
“Causes of poor outcomes for older patients with HL are not fully understood but have been attributed to a combination of factors, including presence of comorbidities, poorer performance status, disease and biological differences, inability to tolerate chemotherapy at the full dose, and increased treatment-related toxicities,” the authors wrote in the Journal of Clinical Oncology.
The primary goal of the study was to improve outcomes for untreated, older patients, a group that’s historically been a difficult-to-treat patient population.
The phase 2 trial included 48 HL patients with a median age of 69 (range, 60 – 88).
All patients underwent geriatric assessment for comorbidities and loss of activities of daily living.
Treatment consisted of two doses of brentuximab followed by six cycles of doxorubicin, vinblastine, and dacarbazine (AVD), then four more doses of brentuximab (consolidation doses).
The primary endpoint was complete remission at completion of AVD.
Secondary outcomes included overall response rate, 2-year progression-free survival, 2-year overall survival, and safety.
Just over half the patients (52%) completed all cycles of therapy, and almost three quarters (73%) received at least one consolidation dose of brentuximab.
Among the first 23 evaluable patients, both the complete remission rate and overall response rate were 96%. Intention-to-treat survival rates for all 48 patients were 84% for 2-year progression-free survival and 93% for 2-year overall survival.
Historical 2-year progression-free survival rates in similar older patients is poor, at 50%, so the progression-free survival rate of 84% in this study represents a significant improvement.
Of note, patients with fewer comorbidities and without loss of instrumental activities of daily living showed more robust responses.
Patients with Cumulative Illness Rating Scale for Geriatrics (CIRS-G) comorbidity scores of less than 10 had a 2-year progression-free survival rate of 100% versus 45% for those with higher scores.
Similarly, patients without loss of instrumental activities achieved a progression-free survival rate of 94% versus 25% for those who had lost some instrumental activities.
Grade 3 or 4 adverse events occurred in 42% of patients, with neutropenia being the most common (44%).
“This study represents among the best-reported outcomes to date for untreated older patients with HL,” the investigators concluded.
Seattle Genetics supported the investigator-initiated trial.
Older patients with untreated Hodgkin lymphoma (HL) can achieve significantly improved survival by adding brentuximab vedotin to their treatment before and after standard chemotherapy, a recent study found.
In patients with low comorbidity scores, responses were even more robust, reported lead author Andrew M. Evens, DO, of the Rutgers Cancer Institute of New Jersey, and colleagues.
“Causes of poor outcomes for older patients with HL are not fully understood but have been attributed to a combination of factors, including presence of comorbidities, poorer performance status, disease and biological differences, inability to tolerate chemotherapy at the full dose, and increased treatment-related toxicities,” the authors wrote in the Journal of Clinical Oncology.
The primary goal of the study was to improve outcomes for untreated, older patients, a group that’s historically been a difficult-to-treat patient population.
The phase 2 trial included 48 HL patients with a median age of 69 (range, 60 – 88).
All patients underwent geriatric assessment for comorbidities and loss of activities of daily living.
Treatment consisted of two doses of brentuximab followed by six cycles of doxorubicin, vinblastine, and dacarbazine (AVD), then four more doses of brentuximab (consolidation doses).
The primary endpoint was complete remission at completion of AVD.
Secondary outcomes included overall response rate, 2-year progression-free survival, 2-year overall survival, and safety.
Just over half the patients (52%) completed all cycles of therapy, and almost three quarters (73%) received at least one consolidation dose of brentuximab.
Among the first 23 evaluable patients, both the complete remission rate and overall response rate were 96%. Intention-to-treat survival rates for all 48 patients were 84% for 2-year progression-free survival and 93% for 2-year overall survival.
Historical 2-year progression-free survival rates in similar older patients is poor, at 50%, so the progression-free survival rate of 84% in this study represents a significant improvement.
Of note, patients with fewer comorbidities and without loss of instrumental activities of daily living showed more robust responses.
Patients with Cumulative Illness Rating Scale for Geriatrics (CIRS-G) comorbidity scores of less than 10 had a 2-year progression-free survival rate of 100% versus 45% for those with higher scores.
Similarly, patients without loss of instrumental activities achieved a progression-free survival rate of 94% versus 25% for those who had lost some instrumental activities.
Grade 3 or 4 adverse events occurred in 42% of patients, with neutropenia being the most common (44%).
“This study represents among the best-reported outcomes to date for untreated older patients with HL,” the investigators concluded.
Seattle Genetics supported the investigator-initiated trial.
Older patients with untreated Hodgkin lymphoma (HL) can achieve significantly improved survival by adding brentuximab vedotin to their treatment before and after standard chemotherapy, a recent study found.
In patients with low comorbidity scores, responses were even more robust, reported lead author Andrew M. Evens, DO, of the Rutgers Cancer Institute of New Jersey, and colleagues.
“Causes of poor outcomes for older patients with HL are not fully understood but have been attributed to a combination of factors, including presence of comorbidities, poorer performance status, disease and biological differences, inability to tolerate chemotherapy at the full dose, and increased treatment-related toxicities,” the authors wrote in the Journal of Clinical Oncology.
The primary goal of the study was to improve outcomes for untreated, older patients, a group that’s historically been a difficult-to-treat patient population.
The phase 2 trial included 48 HL patients with a median age of 69 (range, 60 – 88).
All patients underwent geriatric assessment for comorbidities and loss of activities of daily living.
Treatment consisted of two doses of brentuximab followed by six cycles of doxorubicin, vinblastine, and dacarbazine (AVD), then four more doses of brentuximab (consolidation doses).
The primary endpoint was complete remission at completion of AVD.
Secondary outcomes included overall response rate, 2-year progression-free survival, 2-year overall survival, and safety.
Just over half the patients (52%) completed all cycles of therapy, and almost three quarters (73%) received at least one consolidation dose of brentuximab.
Among the first 23 evaluable patients, both the complete remission rate and overall response rate were 96%. Intention-to-treat survival rates for all 48 patients were 84% for 2-year progression-free survival and 93% for 2-year overall survival.
Historical 2-year progression-free survival rates in similar older patients is poor, at 50%, so the progression-free survival rate of 84% in this study represents a significant improvement.
Of note, patients with fewer comorbidities and without loss of instrumental activities of daily living showed more robust responses.
Patients with Cumulative Illness Rating Scale for Geriatrics (CIRS-G) comorbidity scores of less than 10 had a 2-year progression-free survival rate of 100% versus 45% for those with higher scores.
Similarly, patients without loss of instrumental activities achieved a progression-free survival rate of 94% versus 25% for those who had lost some instrumental activities.
Grade 3 or 4 adverse events occurred in 42% of patients, with neutropenia being the most common (44%).
“This study represents among the best-reported outcomes to date for untreated older patients with HL,” the investigators concluded.
Seattle Genetics supported the investigator-initiated trial.
First NGS assay approved for MRD detection in ALL or MM
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
Auto-FMT restores gut microbiota after HSCT
A randomized, controlled study of 25 patients has demonstrated that an autologous fecal microbiota transplant (auto-FMT) can restore beneficial gut bacteria depleted during allogeneic hematopoietic stem cell transplant (auto-HSCT), according to researchers.
Antibiotics given to prevent and treat bacterial infections during HSCT can also destroy patients’ beneficial intestinal bacteria, increasing their risk for infections and graft-versus-host disease.
The researchers reported that auto-FMT is a safe and effective way to help replenish the beneficial bacteria to near baseline levels within days.
Study patients receiving HSCT at Memorial Sloan Kettering Cancer Center (MSKCC) in New York provided fecal samples collected before transplant conditioning, which were frozen and stored before undergoing transplant.
Between 1 and 5 weeks, when physicians could confirm that patients’ stem cells had engrafted, they collected another fecal sample from patients and randomized the first 25 who lacked known beneficial bacteria to one of two groups.
Eleven control patients received standard of care without fecal transplant and 14 received auto-FMT by enema.
Researchers followed the subjects for 1 year after randomization, with fecal samples collected during this time.
Eleven of the 14 patients (79%) in the auto-FMT group recovered 75% or more of their initial good gut bacteria within days. This helped restore their digestive, immune, and other essential functions.
The beneficial microbial groups restored included Lachnospiraceae (family), Ruminococcaceae (family), and Bacteroidetes (phylum).
Patients in the standard care group took many weeks to replenish the beneficial bacteria destroyed during antibiotic treatment. Only 3 of 11 (27%) control patients had regained 75% or more of the microbiota in their initial fecal sample.
The investigators acknowledge a few study limitations, including that it was conducted at a single institution. Therefore, they say, the findings may not apply to patients undergoing allo-HSCT at other institutions.
In addition, auto-FMT patients may have been treated previously for cancer and depleted of some good bacteria as a result of prior antibiotic therapy.
Nevertheless, the investigators believe their study demonstrated the potential of auto-FMT as a clinical intervention, “thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” they wrote.
Lead author Ying Taur, MD, MPH, of MSKCC, and colleagues published their paper in Science Translational Medicine.
The trial, supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, is ongoing and continues to accrue patients (NCT02269150).
“This important study suggests that clinical intervention using auto-FMT can safely reverse the disruptive effects of broad-spectrum antibiotic treatment,” said Anthony S. Fauci, MD, director of NIAID.
“If validated in larger studies, this approach may prove to be a relatively simple way to quickly restore a person’s healthy microbiome following intensive antimicrobial therapy.”
A randomized, controlled study of 25 patients has demonstrated that an autologous fecal microbiota transplant (auto-FMT) can restore beneficial gut bacteria depleted during allogeneic hematopoietic stem cell transplant (auto-HSCT), according to researchers.
Antibiotics given to prevent and treat bacterial infections during HSCT can also destroy patients’ beneficial intestinal bacteria, increasing their risk for infections and graft-versus-host disease.
The researchers reported that auto-FMT is a safe and effective way to help replenish the beneficial bacteria to near baseline levels within days.
Study patients receiving HSCT at Memorial Sloan Kettering Cancer Center (MSKCC) in New York provided fecal samples collected before transplant conditioning, which were frozen and stored before undergoing transplant.
Between 1 and 5 weeks, when physicians could confirm that patients’ stem cells had engrafted, they collected another fecal sample from patients and randomized the first 25 who lacked known beneficial bacteria to one of two groups.
Eleven control patients received standard of care without fecal transplant and 14 received auto-FMT by enema.
Researchers followed the subjects for 1 year after randomization, with fecal samples collected during this time.
Eleven of the 14 patients (79%) in the auto-FMT group recovered 75% or more of their initial good gut bacteria within days. This helped restore their digestive, immune, and other essential functions.
The beneficial microbial groups restored included Lachnospiraceae (family), Ruminococcaceae (family), and Bacteroidetes (phylum).
Patients in the standard care group took many weeks to replenish the beneficial bacteria destroyed during antibiotic treatment. Only 3 of 11 (27%) control patients had regained 75% or more of the microbiota in their initial fecal sample.
The investigators acknowledge a few study limitations, including that it was conducted at a single institution. Therefore, they say, the findings may not apply to patients undergoing allo-HSCT at other institutions.
In addition, auto-FMT patients may have been treated previously for cancer and depleted of some good bacteria as a result of prior antibiotic therapy.
Nevertheless, the investigators believe their study demonstrated the potential of auto-FMT as a clinical intervention, “thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” they wrote.
Lead author Ying Taur, MD, MPH, of MSKCC, and colleagues published their paper in Science Translational Medicine.
The trial, supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, is ongoing and continues to accrue patients (NCT02269150).
“This important study suggests that clinical intervention using auto-FMT can safely reverse the disruptive effects of broad-spectrum antibiotic treatment,” said Anthony S. Fauci, MD, director of NIAID.
“If validated in larger studies, this approach may prove to be a relatively simple way to quickly restore a person’s healthy microbiome following intensive antimicrobial therapy.”
A randomized, controlled study of 25 patients has demonstrated that an autologous fecal microbiota transplant (auto-FMT) can restore beneficial gut bacteria depleted during allogeneic hematopoietic stem cell transplant (auto-HSCT), according to researchers.
Antibiotics given to prevent and treat bacterial infections during HSCT can also destroy patients’ beneficial intestinal bacteria, increasing their risk for infections and graft-versus-host disease.
The researchers reported that auto-FMT is a safe and effective way to help replenish the beneficial bacteria to near baseline levels within days.
Study patients receiving HSCT at Memorial Sloan Kettering Cancer Center (MSKCC) in New York provided fecal samples collected before transplant conditioning, which were frozen and stored before undergoing transplant.
Between 1 and 5 weeks, when physicians could confirm that patients’ stem cells had engrafted, they collected another fecal sample from patients and randomized the first 25 who lacked known beneficial bacteria to one of two groups.
Eleven control patients received standard of care without fecal transplant and 14 received auto-FMT by enema.
Researchers followed the subjects for 1 year after randomization, with fecal samples collected during this time.
Eleven of the 14 patients (79%) in the auto-FMT group recovered 75% or more of their initial good gut bacteria within days. This helped restore their digestive, immune, and other essential functions.
The beneficial microbial groups restored included Lachnospiraceae (family), Ruminococcaceae (family), and Bacteroidetes (phylum).
Patients in the standard care group took many weeks to replenish the beneficial bacteria destroyed during antibiotic treatment. Only 3 of 11 (27%) control patients had regained 75% or more of the microbiota in their initial fecal sample.
The investigators acknowledge a few study limitations, including that it was conducted at a single institution. Therefore, they say, the findings may not apply to patients undergoing allo-HSCT at other institutions.
In addition, auto-FMT patients may have been treated previously for cancer and depleted of some good bacteria as a result of prior antibiotic therapy.
Nevertheless, the investigators believe their study demonstrated the potential of auto-FMT as a clinical intervention, “thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” they wrote.
Lead author Ying Taur, MD, MPH, of MSKCC, and colleagues published their paper in Science Translational Medicine.
The trial, supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, is ongoing and continues to accrue patients (NCT02269150).
“This important study suggests that clinical intervention using auto-FMT can safely reverse the disruptive effects of broad-spectrum antibiotic treatment,” said Anthony S. Fauci, MD, director of NIAID.
“If validated in larger studies, this approach may prove to be a relatively simple way to quickly restore a person’s healthy microbiome following intensive antimicrobial therapy.”
FDA grants rezafungin QIDP and fast track designations
The U.S. Food and Drug Administration (FDA) has granted Qualified Infectious Disease Product (QIDP) and fast track designations for rezafungin, an injection for the prevention of invasive fungal infections in adults undergoing allogeneic bone marrow transplantation.
Rezafungin is a novel antifungal echinocandin being developed by Cidara Therapeutics to treat candidemia and invasive candidiasis and for prophylaxis of invasive fungal infections due to Candida, Aspergillus, and Pneumocystis.
These pathogens typically affect patients with compromised immune systems, such as patients undergoing organ, bone marrow transplants, or chemotherapy.
Rezafungin is administered as a once-weekly, high-exposure therapy. According to Cidara, no one agent is approved to prevent infections caused by these pathogens. Current prophylaxis regimens often require multiple antifungal drugs, causing safety and tolerability issues.
The QIDP designation offers incentives for the development of new antifungal and antibacterial drugs, including fast track, priority review and, if approved by the FDA, eligibility for an additional five years of marketing exclusivity.
Fast track designation enables more frequent interactions with the FDA review team to expedite drug development.
For QIDP designation, a drug candidate must treat serious or life-threatening infections, particularly those caused by bacteria and fungi resistant to treatment or by resistant pathogens identified by the FDA.
Rezafungin acetate, formerly known as CD101 IV, met its primary safety and efficacy objectives in the phase 2 STRIVE trial, providing support for the company to initiate phase 3 trials.
The phase 2 trial compared rezafungin to caspofungin in patients with candidemia and/or invasive candidiasis.
Investigators enrolled 92 patients in the microbial intent-to-treat population.
Patients were randomized to one of two dose groups of rezafungin or to the caspofungin arm.
One hundred percent of patients with invasive candidiasis responded to the rezafungin 400 mg first-week dose followed by 200 mg once weekly up to four weeks, compared to 33% of patients who responded to daily caspofungin.
The company reported no concerning adverse event trends.
Cidara is initiating phase 3 pivotal trials of rezafungin in the treatment of candidemia and invasive candidiasis and the prophylaxis of invasive fungal infections.
The U.S. Food and Drug Administration (FDA) has granted Qualified Infectious Disease Product (QIDP) and fast track designations for rezafungin, an injection for the prevention of invasive fungal infections in adults undergoing allogeneic bone marrow transplantation.
Rezafungin is a novel antifungal echinocandin being developed by Cidara Therapeutics to treat candidemia and invasive candidiasis and for prophylaxis of invasive fungal infections due to Candida, Aspergillus, and Pneumocystis.
These pathogens typically affect patients with compromised immune systems, such as patients undergoing organ, bone marrow transplants, or chemotherapy.
Rezafungin is administered as a once-weekly, high-exposure therapy. According to Cidara, no one agent is approved to prevent infections caused by these pathogens. Current prophylaxis regimens often require multiple antifungal drugs, causing safety and tolerability issues.
The QIDP designation offers incentives for the development of new antifungal and antibacterial drugs, including fast track, priority review and, if approved by the FDA, eligibility for an additional five years of marketing exclusivity.
Fast track designation enables more frequent interactions with the FDA review team to expedite drug development.
For QIDP designation, a drug candidate must treat serious or life-threatening infections, particularly those caused by bacteria and fungi resistant to treatment or by resistant pathogens identified by the FDA.
Rezafungin acetate, formerly known as CD101 IV, met its primary safety and efficacy objectives in the phase 2 STRIVE trial, providing support for the company to initiate phase 3 trials.
The phase 2 trial compared rezafungin to caspofungin in patients with candidemia and/or invasive candidiasis.
Investigators enrolled 92 patients in the microbial intent-to-treat population.
Patients were randomized to one of two dose groups of rezafungin or to the caspofungin arm.
One hundred percent of patients with invasive candidiasis responded to the rezafungin 400 mg first-week dose followed by 200 mg once weekly up to four weeks, compared to 33% of patients who responded to daily caspofungin.
The company reported no concerning adverse event trends.
Cidara is initiating phase 3 pivotal trials of rezafungin in the treatment of candidemia and invasive candidiasis and the prophylaxis of invasive fungal infections.
The U.S. Food and Drug Administration (FDA) has granted Qualified Infectious Disease Product (QIDP) and fast track designations for rezafungin, an injection for the prevention of invasive fungal infections in adults undergoing allogeneic bone marrow transplantation.
Rezafungin is a novel antifungal echinocandin being developed by Cidara Therapeutics to treat candidemia and invasive candidiasis and for prophylaxis of invasive fungal infections due to Candida, Aspergillus, and Pneumocystis.
These pathogens typically affect patients with compromised immune systems, such as patients undergoing organ, bone marrow transplants, or chemotherapy.
Rezafungin is administered as a once-weekly, high-exposure therapy. According to Cidara, no one agent is approved to prevent infections caused by these pathogens. Current prophylaxis regimens often require multiple antifungal drugs, causing safety and tolerability issues.
The QIDP designation offers incentives for the development of new antifungal and antibacterial drugs, including fast track, priority review and, if approved by the FDA, eligibility for an additional five years of marketing exclusivity.
Fast track designation enables more frequent interactions with the FDA review team to expedite drug development.
For QIDP designation, a drug candidate must treat serious or life-threatening infections, particularly those caused by bacteria and fungi resistant to treatment or by resistant pathogens identified by the FDA.
Rezafungin acetate, formerly known as CD101 IV, met its primary safety and efficacy objectives in the phase 2 STRIVE trial, providing support for the company to initiate phase 3 trials.
The phase 2 trial compared rezafungin to caspofungin in patients with candidemia and/or invasive candidiasis.
Investigators enrolled 92 patients in the microbial intent-to-treat population.
Patients were randomized to one of two dose groups of rezafungin or to the caspofungin arm.
One hundred percent of patients with invasive candidiasis responded to the rezafungin 400 mg first-week dose followed by 200 mg once weekly up to four weeks, compared to 33% of patients who responded to daily caspofungin.
The company reported no concerning adverse event trends.
Cidara is initiating phase 3 pivotal trials of rezafungin in the treatment of candidemia and invasive candidiasis and the prophylaxis of invasive fungal infections.
Factors that may drive relapse in AYAs with ALL
New research suggests race, clinical trial participation, and treatment duration may influence the risk of relapse in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL).
The study showed that AYAs with ALL were significantly more likely to relapse than pediatric ALL patients.
Among AYAs, the risk of on-therapy relapse was higher for non-white patients and those who did not participate in clinical trials. The risk of relapse after therapy was higher for AYAs with a shorter treatment duration.
Julie A. Wolfson, MD, of University of Alabama at Birmingham, and her colleagues reported these findings in Cancer Epidemiology, Biomarkers & Prevention.
The researchers conducted this study to investigate why AYAs with ALL have not experienced the same improvement in survival rates as pediatric patients with ALL.
“Patients diagnosed between the ages of 15 and 39 simply have not seen the same improvement as those in other age groups,” Dr. Wolfson said. “In this study, we examined factors related to health care delivery and treatment to increase our understanding of why they experience poorer outcomes.”
The researchers retrospectively studied ALL patients diagnosed between ages 1 and 39 and treated at a single center between 1990 and 2010.
Ninety-one patients were children (ages 1 to 14), and 93 were AYAs (ages 15 to 39).
The researchers assessed variables including demographics, insurance status, participation in clinical trials, duration of treatment, and whether the patients had been treated with pediatric-inspired or adult-inspired regimens. Using Kaplan-Meier survival analysis, the researchers calculated the risk of relapse.
Results
As previous research indicated, children with ALL had superior relapse-free survival compared to AYAs.
The 5-year relapse-free survival rate was 74% in children, 29% in younger AYAs (ages 15 to 21), and 32% in older AYAs (ages 22-39). The difference between children and AYAs was statistically significant (P<0.0001), but the difference between younger and older AYAs was not (P=0.6).
Forty-eight percent of AYAs relapsed while on therapy, compared with 17% of children (P<0.001).
In a multivariable analysis adjusted for clinical prognosticators, health care delivery, and treatment, the risk of on-therapy relapse was more than 10 times higher among AYAs than children (hazard ratio [HR], 10.5; P=0.004).
Among AYAs, the strongest predictors of on-therapy relapse were race and enrollment in clinical trials.
Non-white patients were more than twice as likely to relapse as white patients (HR, 2.2; P=0.05), and patients who were not enrolled in clinical trials were more than twice as likely to relapse as trial participants (HR, 2.6, P=0.04).
Dr. Wolfson said this finding adds to evidence suggesting AYA patients should be encouraged to participate in clinical trials.
“It is possible that patients sometimes benefit from being enrolled on a clinical trial not only because the therapy itself is providing a benefit, but also because it is a protocolized, regulated approach that requires patients to stay on course and not take breaks,” she said.
After the completion of therapy, 47% of AYAs suffered a relapse, compared to 13% of children (P<0.0001).
In a multivariable analysis, the risk of relapse after therapy was more than seven times higher among AYAs than children (HR, 7.7; P<0.001).
Among AYAs who relapsed after therapy, the most significant factor associated with relapse was the duration of treatment.
For each additional month of consolidation therapy, there was a 20% lower risk of relapse (HR, 0.8; P=0.03). And for each additional month of maintenance, there was a 30% lower risk of relapse (HR, 0.7; P<0.001).
Dr. Wolfson noted that a range of factors may affect the duration of treatment. For example, the AYA population is more likely to be uninsured or underinsured, which can make them more likely to stop treatment or miss appointments.
Finally, Dr. Wolfson acknowledged that this study had limitations, primarily its single-institution approach and its limited sample size.
This study was funded by the National Institutes of Health, the St. Baldrick’s Scholar Career Development Award, and the Concern Foundation. The authors declared no conflicts of interest.
New research suggests race, clinical trial participation, and treatment duration may influence the risk of relapse in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL).
The study showed that AYAs with ALL were significantly more likely to relapse than pediatric ALL patients.
Among AYAs, the risk of on-therapy relapse was higher for non-white patients and those who did not participate in clinical trials. The risk of relapse after therapy was higher for AYAs with a shorter treatment duration.
Julie A. Wolfson, MD, of University of Alabama at Birmingham, and her colleagues reported these findings in Cancer Epidemiology, Biomarkers & Prevention.
The researchers conducted this study to investigate why AYAs with ALL have not experienced the same improvement in survival rates as pediatric patients with ALL.
“Patients diagnosed between the ages of 15 and 39 simply have not seen the same improvement as those in other age groups,” Dr. Wolfson said. “In this study, we examined factors related to health care delivery and treatment to increase our understanding of why they experience poorer outcomes.”
The researchers retrospectively studied ALL patients diagnosed between ages 1 and 39 and treated at a single center between 1990 and 2010.
Ninety-one patients were children (ages 1 to 14), and 93 were AYAs (ages 15 to 39).
The researchers assessed variables including demographics, insurance status, participation in clinical trials, duration of treatment, and whether the patients had been treated with pediatric-inspired or adult-inspired regimens. Using Kaplan-Meier survival analysis, the researchers calculated the risk of relapse.
Results
As previous research indicated, children with ALL had superior relapse-free survival compared to AYAs.
The 5-year relapse-free survival rate was 74% in children, 29% in younger AYAs (ages 15 to 21), and 32% in older AYAs (ages 22-39). The difference between children and AYAs was statistically significant (P<0.0001), but the difference between younger and older AYAs was not (P=0.6).
Forty-eight percent of AYAs relapsed while on therapy, compared with 17% of children (P<0.001).
In a multivariable analysis adjusted for clinical prognosticators, health care delivery, and treatment, the risk of on-therapy relapse was more than 10 times higher among AYAs than children (hazard ratio [HR], 10.5; P=0.004).
Among AYAs, the strongest predictors of on-therapy relapse were race and enrollment in clinical trials.
Non-white patients were more than twice as likely to relapse as white patients (HR, 2.2; P=0.05), and patients who were not enrolled in clinical trials were more than twice as likely to relapse as trial participants (HR, 2.6, P=0.04).
Dr. Wolfson said this finding adds to evidence suggesting AYA patients should be encouraged to participate in clinical trials.
“It is possible that patients sometimes benefit from being enrolled on a clinical trial not only because the therapy itself is providing a benefit, but also because it is a protocolized, regulated approach that requires patients to stay on course and not take breaks,” she said.
After the completion of therapy, 47% of AYAs suffered a relapse, compared to 13% of children (P<0.0001).
In a multivariable analysis, the risk of relapse after therapy was more than seven times higher among AYAs than children (HR, 7.7; P<0.001).
Among AYAs who relapsed after therapy, the most significant factor associated with relapse was the duration of treatment.
For each additional month of consolidation therapy, there was a 20% lower risk of relapse (HR, 0.8; P=0.03). And for each additional month of maintenance, there was a 30% lower risk of relapse (HR, 0.7; P<0.001).
Dr. Wolfson noted that a range of factors may affect the duration of treatment. For example, the AYA population is more likely to be uninsured or underinsured, which can make them more likely to stop treatment or miss appointments.
Finally, Dr. Wolfson acknowledged that this study had limitations, primarily its single-institution approach and its limited sample size.
This study was funded by the National Institutes of Health, the St. Baldrick’s Scholar Career Development Award, and the Concern Foundation. The authors declared no conflicts of interest.
New research suggests race, clinical trial participation, and treatment duration may influence the risk of relapse in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL).
The study showed that AYAs with ALL were significantly more likely to relapse than pediatric ALL patients.
Among AYAs, the risk of on-therapy relapse was higher for non-white patients and those who did not participate in clinical trials. The risk of relapse after therapy was higher for AYAs with a shorter treatment duration.
Julie A. Wolfson, MD, of University of Alabama at Birmingham, and her colleagues reported these findings in Cancer Epidemiology, Biomarkers & Prevention.
The researchers conducted this study to investigate why AYAs with ALL have not experienced the same improvement in survival rates as pediatric patients with ALL.
“Patients diagnosed between the ages of 15 and 39 simply have not seen the same improvement as those in other age groups,” Dr. Wolfson said. “In this study, we examined factors related to health care delivery and treatment to increase our understanding of why they experience poorer outcomes.”
The researchers retrospectively studied ALL patients diagnosed between ages 1 and 39 and treated at a single center between 1990 and 2010.
Ninety-one patients were children (ages 1 to 14), and 93 were AYAs (ages 15 to 39).
The researchers assessed variables including demographics, insurance status, participation in clinical trials, duration of treatment, and whether the patients had been treated with pediatric-inspired or adult-inspired regimens. Using Kaplan-Meier survival analysis, the researchers calculated the risk of relapse.
Results
As previous research indicated, children with ALL had superior relapse-free survival compared to AYAs.
The 5-year relapse-free survival rate was 74% in children, 29% in younger AYAs (ages 15 to 21), and 32% in older AYAs (ages 22-39). The difference between children and AYAs was statistically significant (P<0.0001), but the difference between younger and older AYAs was not (P=0.6).
Forty-eight percent of AYAs relapsed while on therapy, compared with 17% of children (P<0.001).
In a multivariable analysis adjusted for clinical prognosticators, health care delivery, and treatment, the risk of on-therapy relapse was more than 10 times higher among AYAs than children (hazard ratio [HR], 10.5; P=0.004).
Among AYAs, the strongest predictors of on-therapy relapse were race and enrollment in clinical trials.
Non-white patients were more than twice as likely to relapse as white patients (HR, 2.2; P=0.05), and patients who were not enrolled in clinical trials were more than twice as likely to relapse as trial participants (HR, 2.6, P=0.04).
Dr. Wolfson said this finding adds to evidence suggesting AYA patients should be encouraged to participate in clinical trials.
“It is possible that patients sometimes benefit from being enrolled on a clinical trial not only because the therapy itself is providing a benefit, but also because it is a protocolized, regulated approach that requires patients to stay on course and not take breaks,” she said.
After the completion of therapy, 47% of AYAs suffered a relapse, compared to 13% of children (P<0.0001).
In a multivariable analysis, the risk of relapse after therapy was more than seven times higher among AYAs than children (HR, 7.7; P<0.001).
Among AYAs who relapsed after therapy, the most significant factor associated with relapse was the duration of treatment.
For each additional month of consolidation therapy, there was a 20% lower risk of relapse (HR, 0.8; P=0.03). And for each additional month of maintenance, there was a 30% lower risk of relapse (HR, 0.7; P<0.001).
Dr. Wolfson noted that a range of factors may affect the duration of treatment. For example, the AYA population is more likely to be uninsured or underinsured, which can make them more likely to stop treatment or miss appointments.
Finally, Dr. Wolfson acknowledged that this study had limitations, primarily its single-institution approach and its limited sample size.
This study was funded by the National Institutes of Health, the St. Baldrick’s Scholar Career Development Award, and the Concern Foundation. The authors declared no conflicts of interest.
Study supports immediate compression after DVT
Immediate compression therapy provides a “clear benefit” for patients with deep vein thrombosis (DVT), according to the senior author of a study published in Blood.
In this prospective study, patients who started compression therapy within the first 24 hours of DVT diagnosis were 20% less likely to develop residual vein occlusion (RVO) than patients who did not start compression therapy in the acute phase.
Furthermore, patients who did not develop RVO were 8% less likely to have post-thrombotic syndrome (PTS) than patients with RVO.
“We found little reason for those treating DVT not to use compression therapy as a prevention measure against future complications,” said study author Arina J. ten Cate-Hoek, MD, PhD, of Maastricht University in the Netherlands.
“Although the use of compression stockings after DVT is routine across much of Europe, it is less common in the United States, where guidelines emphasize compression primarily for patients who complain of ongoing symptoms.”
Dr. ten Cate-Hoek and her colleagues studied 592 adults with DVT who were treated in 10 centers across the Netherlands.
All patients were treated with anticoagulants, largely vitamin K antagonists (80.6%) but also direct oral anticoagulants (3.0%), investigational anticoagulants (11.1%), and low-molecular-weight heparin (4.4%).
Within 24 hours of DVT diagnosis, most patients received compression therapy (pressure 35 mmHg) with either multilayer compression bandaging (62.3%) or compression hosiery (25.5%). A minority of patients did not receive compression in the acute phase (12.2%).
The average time from DVT diagnosis to RVO assessment was 5.3 months.
The incidence of RVO was significantly lower in patients who received immediate compression than in those who did not—46.3% and 66.7%, respectively (odds ratio [OR], 0.46; P=0.005).
In addition, PTS was more common among patients with RVO.
At 6 months, the incidence of PTS was 55.7% among patients with RVO and 44.3% among patients without RVO (OR, 0.66; P=0.029). At 24 months, the incidence of PTS was 54.0% and 46.0%, respectively (OR, 0.65; P=0.013).
The researchers said there was no significant association between RVO and recurrent DVT or pulmonary embolism.
Among the 30 patients with DVT recurrence, 60% had RVO and 40% did not (OR, 0.82; P=0.263).
Among the 19 patients who had recurrent pulmonary embolism, 52.6% had RVO and 47.7% did not (OR, 0.95; P=0.805).
Dr. ten Cate-Hoek noted that compression therapy is thought to improve blood flow by reducing the diameter of veins so that blood is pushed through them more forcefully, which helps to clear clot material.
“I think we can infer from our findings that this improved blood flow certainly helps prevent complications like residual vein occlusion and post-thrombotic syndrome after DVT,” she said.
“Given these outcomes, and that compression stockings are fairly easy to self-administer, relatively inexpensive, and minimally intrusive, compression therapy offers a clear benefit for all patients with DVT.”
Immediate compression therapy provides a “clear benefit” for patients with deep vein thrombosis (DVT), according to the senior author of a study published in Blood.
In this prospective study, patients who started compression therapy within the first 24 hours of DVT diagnosis were 20% less likely to develop residual vein occlusion (RVO) than patients who did not start compression therapy in the acute phase.
Furthermore, patients who did not develop RVO were 8% less likely to have post-thrombotic syndrome (PTS) than patients with RVO.
“We found little reason for those treating DVT not to use compression therapy as a prevention measure against future complications,” said study author Arina J. ten Cate-Hoek, MD, PhD, of Maastricht University in the Netherlands.
“Although the use of compression stockings after DVT is routine across much of Europe, it is less common in the United States, where guidelines emphasize compression primarily for patients who complain of ongoing symptoms.”
Dr. ten Cate-Hoek and her colleagues studied 592 adults with DVT who were treated in 10 centers across the Netherlands.
All patients were treated with anticoagulants, largely vitamin K antagonists (80.6%) but also direct oral anticoagulants (3.0%), investigational anticoagulants (11.1%), and low-molecular-weight heparin (4.4%).
Within 24 hours of DVT diagnosis, most patients received compression therapy (pressure 35 mmHg) with either multilayer compression bandaging (62.3%) or compression hosiery (25.5%). A minority of patients did not receive compression in the acute phase (12.2%).
The average time from DVT diagnosis to RVO assessment was 5.3 months.
The incidence of RVO was significantly lower in patients who received immediate compression than in those who did not—46.3% and 66.7%, respectively (odds ratio [OR], 0.46; P=0.005).
In addition, PTS was more common among patients with RVO.
At 6 months, the incidence of PTS was 55.7% among patients with RVO and 44.3% among patients without RVO (OR, 0.66; P=0.029). At 24 months, the incidence of PTS was 54.0% and 46.0%, respectively (OR, 0.65; P=0.013).
The researchers said there was no significant association between RVO and recurrent DVT or pulmonary embolism.
Among the 30 patients with DVT recurrence, 60% had RVO and 40% did not (OR, 0.82; P=0.263).
Among the 19 patients who had recurrent pulmonary embolism, 52.6% had RVO and 47.7% did not (OR, 0.95; P=0.805).
Dr. ten Cate-Hoek noted that compression therapy is thought to improve blood flow by reducing the diameter of veins so that blood is pushed through them more forcefully, which helps to clear clot material.
“I think we can infer from our findings that this improved blood flow certainly helps prevent complications like residual vein occlusion and post-thrombotic syndrome after DVT,” she said.
“Given these outcomes, and that compression stockings are fairly easy to self-administer, relatively inexpensive, and minimally intrusive, compression therapy offers a clear benefit for all patients with DVT.”
Immediate compression therapy provides a “clear benefit” for patients with deep vein thrombosis (DVT), according to the senior author of a study published in Blood.
In this prospective study, patients who started compression therapy within the first 24 hours of DVT diagnosis were 20% less likely to develop residual vein occlusion (RVO) than patients who did not start compression therapy in the acute phase.
Furthermore, patients who did not develop RVO were 8% less likely to have post-thrombotic syndrome (PTS) than patients with RVO.
“We found little reason for those treating DVT not to use compression therapy as a prevention measure against future complications,” said study author Arina J. ten Cate-Hoek, MD, PhD, of Maastricht University in the Netherlands.
“Although the use of compression stockings after DVT is routine across much of Europe, it is less common in the United States, where guidelines emphasize compression primarily for patients who complain of ongoing symptoms.”
Dr. ten Cate-Hoek and her colleagues studied 592 adults with DVT who were treated in 10 centers across the Netherlands.
All patients were treated with anticoagulants, largely vitamin K antagonists (80.6%) but also direct oral anticoagulants (3.0%), investigational anticoagulants (11.1%), and low-molecular-weight heparin (4.4%).
Within 24 hours of DVT diagnosis, most patients received compression therapy (pressure 35 mmHg) with either multilayer compression bandaging (62.3%) or compression hosiery (25.5%). A minority of patients did not receive compression in the acute phase (12.2%).
The average time from DVT diagnosis to RVO assessment was 5.3 months.
The incidence of RVO was significantly lower in patients who received immediate compression than in those who did not—46.3% and 66.7%, respectively (odds ratio [OR], 0.46; P=0.005).
In addition, PTS was more common among patients with RVO.
At 6 months, the incidence of PTS was 55.7% among patients with RVO and 44.3% among patients without RVO (OR, 0.66; P=0.029). At 24 months, the incidence of PTS was 54.0% and 46.0%, respectively (OR, 0.65; P=0.013).
The researchers said there was no significant association between RVO and recurrent DVT or pulmonary embolism.
Among the 30 patients with DVT recurrence, 60% had RVO and 40% did not (OR, 0.82; P=0.263).
Among the 19 patients who had recurrent pulmonary embolism, 52.6% had RVO and 47.7% did not (OR, 0.95; P=0.805).
Dr. ten Cate-Hoek noted that compression therapy is thought to improve blood flow by reducing the diameter of veins so that blood is pushed through them more forcefully, which helps to clear clot material.
“I think we can infer from our findings that this improved blood flow certainly helps prevent complications like residual vein occlusion and post-thrombotic syndrome after DVT,” she said.
“Given these outcomes, and that compression stockings are fairly easy to self-administer, relatively inexpensive, and minimally intrusive, compression therapy offers a clear benefit for all patients with DVT.”