User login
Team reports success with mobile platelet collection
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
FDA issues draft guidance on MRD
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
The U.S. Food and Drug Administration (FDA) has issued a draft guidance on the use of minimal residual disease (MRD) assessment in trials of patients with hematologic malignancies.
The FDA said it developed this guidance to assist sponsors who are planning to use MRD as a biomarker in clinical trials conducted under an investigational new drug application or to support FDA approval of products intended to treat hematologic malignancies.
“As a result of important workshops where we’ve heard from stakeholders and an analysis of marketing applications showing inconsistent quality of MRD data, the FDA identified a need to provide sponsors with guidance on the use of MRD as a biomarker in regulatory submissions,” said FDA Commissioner Scott Gottlieb, MD.
The guidance explains how MRD might be used in clinical trials, highlights considerations for MRD assessment that are specific to certain hematologic malignancies, and lists requirements for regulatory submissions that utilize MRD.
The full document, “Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment,” is available for download from the FDA website.
How MRD can be used
The guidance notes that MRD could potentially be used as a biomarker in clinical trials, specifically, as a diagnostic, prognostic, predictive, efficacy-response, or monitoring biomarker.
MRD could also be used as a surrogate endpoint, and there are two mechanisms for obtaining FDA feedback on the use of a novel surrogate endpoint to support approval of a product:
- The drug development tool qualification process
- Discussions with the specific Center for Drug Evaluation and Research or Center for Biologics Evaluation and Research review division.
Furthermore, a sponsor can use MRD “to select patients at high risk or to enrich the trial population,” according to the guidance.
Disease specifics
The guidance also details specific considerations for MRD assessment in individual hematologic malignancies. For example:
- In acute lymphoblastic leukemia, a patient with an MRD level of 0.1% or more in first or second complete remission has a high risk of relapse.
- In trials of acute myeloid leukemia, the sponsor should provide data showing that the marker selected to assess MRD “reflects the leukemia and not underlying clonal hematopoiesis.”
- Patients with low-risk acute promyelocytic leukemia who achieve MRD negativity after arsenic/tretinoin-based therapy are generally considered cured.
- In chronic lymphocytic leukemia, MRD can be assessed in the peripheral blood or bone marrow, but the sample source should remain the same throughout a trial.
- In chronic myeloid leukemia, MRD can be used to select and monitor patients who are eligible to discontinue treatment with tyrosine kinase inhibitors.
- In multiple myeloma, imaging techniques may be combined with MRD assessment of the bone marrow to assess patient response to treatment.
Types of technology
The guidance lists the four general technologies used for MRD assessment in hematologic malignancies:
- Multiparametric flow cytometry
- Next-generation sequencing
- Quantitative reverse transcription polymerase chain reaction of specific gene fusions
- Allele-specific oligonucleotide polymerase chain reaction.
The FDA said it does not have a preference as to which technology is used in a trial. However, the sponsor must pre-specify the technology used and should utilize the same technology throughout a trial.
The FDA also said it “does not foresee the need for co-development of an MRD assay with a drug product.” However, the assay must be analytically valid for results important to the trial, and MRD assessment must be a clinically valid biomarker in the context in which it’s used.
If the MRD assay used is not FDA-cleared or -approved, additional information about the assay must be provided to the FDA.
Questions surround MRD assessment in MM
DUBROVNIK, CROATIA—Clinical trials are needed to answer the many questions related to minimal residual disease (MRD) assessment in multiple myeloma (MM), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
MM patients are increasingly assessed for MRD, which is a strong prognostic factor and surrogate for overall survival, according to Toni Valković, MD, PhD, of University Hospital Center Rijeka in Croatia.
However, Dr. Valković said MRD assessment has not become a part of routine clinical practice, perhaps because we haven’t determined the best way to utilize MRD assessment in MM patients.
The optimal sensitivity threshold, technique, and timing of MRD assessment are not known, and it isn’t clear how MRD should be used to guide treatment.
What we know
Dr. Valković cited studies showing that MRD negativity is associated with superior survival in MM1, and, when MRD negativity is achieved, high-risk cytogenetics, age, and previous treatment regimens appear to have no further impact on prognosis.2
Dr. Valković went on to explain the benefits and detriments of multiparametric flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment.3
NGS requires a baseline patient sample, but MFC does not. More cells are required with MFC than with NGS (>5 million vs. <1 million).
With MFC, samples must be processed within 24 to 48 hours, whereas, with NGS, fresh or stored samples can be used. MFC can be done in a few hours, while NGS can take several days.
Despite these differences, both methods provide similar levels of sensitivity for detecting MRD (≥1 in 105).
Dr. Valković also noted that MRD should be evaluated outside the bone marrow as well, which can be done with positron emission tomography-computed tomography (PET-CT).
Research has shown that patients who are MRD-negative according to both MFC and PET-CT have better outcomes than patients who are MRD-positive by MFC, PET-CT, or both.4
What we don’t know
Though he compared MFC and NGS, Dr. Valković said we don’t know the optimal technique for assessing MRD in the bone marrow.
Another uncertainty is the optimal sensitivity threshold. In the POLLUX study5, researchers found that a threshold of 10-4 resulted in lots of patients with MRD negativity, but some of these were false-negatives.
So although 10-4 proved inaccurate, it isn’t clear if the optimal threshold is 10-5 or 10-6, Dr. Valković said.
Likewise, it isn’t clear if PET-CT is the optimal method for evaluating MRD outside the bone marrow.
In a study published in 2017, PET produced false-negatives in MM patients.6 In 11% of patients (26/227), there was evidence of disease with diffusion-weighted magnetic resonance imaging with background signal suppression, but there was no apparent disease with PET. The researchers said low expression of hexokinase-2 was associated with a false-negative PET result.
Finally, Dr. Valković said we don’t know how best to use MRD to tailor therapy in MM patients. He posed the following questions:
- If patients don’t achieve MRD negativity, should they continue on the therapy?
- If MRD-negative patients become MRD-positive, should they begin therapy immediately, or should treatment be put off until a biochemical or clinical relapse?
- Should MRD status be used to determine the number of treatment cycles a patient receives, the timing of transplant, or when to begin and end maintenance therapy?
“There are a lot of issues and unanswered questions related to the optimal techniques for the evaluation of MRD and their sensitivity, the timing for MRD assessment during and after therapy, and its role in the treatment decisions, which should be answered in future clinical studies,” Dr. Valković concluded.
He did not declare any conflicts of interest.
1. Munshi NC et al. JAMA Oncol. 2017;3(1):28-35. doi:10.1001/jamaoncol.2016.3160
2. Paiva B et al. Blood. 2016 Jun 23;127(25):3165-74. doi: 10.1182/blood-2016-03-705319
3. Kumar S et al. Lancet Oncol. 2016; 17 (8):e328-46 doi: https://doi.org/10.1016/S1470-2045(16)30206-6
4. Fernandez RA et al. Blood. 2017; 130:3098
5. Dimopoulos MA et al. Haematologica. 2018 Sep 20. pii: haematol.2018.194282. doi: 10.3324/haematol.2018.194282
6. Rasche L et al. Blood. 2017 Jul 6;130(1):30-34. doi: 10.1182/blood-2017-03-774422
DUBROVNIK, CROATIA—Clinical trials are needed to answer the many questions related to minimal residual disease (MRD) assessment in multiple myeloma (MM), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
MM patients are increasingly assessed for MRD, which is a strong prognostic factor and surrogate for overall survival, according to Toni Valković, MD, PhD, of University Hospital Center Rijeka in Croatia.
However, Dr. Valković said MRD assessment has not become a part of routine clinical practice, perhaps because we haven’t determined the best way to utilize MRD assessment in MM patients.
The optimal sensitivity threshold, technique, and timing of MRD assessment are not known, and it isn’t clear how MRD should be used to guide treatment.
What we know
Dr. Valković cited studies showing that MRD negativity is associated with superior survival in MM1, and, when MRD negativity is achieved, high-risk cytogenetics, age, and previous treatment regimens appear to have no further impact on prognosis.2
Dr. Valković went on to explain the benefits and detriments of multiparametric flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment.3
NGS requires a baseline patient sample, but MFC does not. More cells are required with MFC than with NGS (>5 million vs. <1 million).
With MFC, samples must be processed within 24 to 48 hours, whereas, with NGS, fresh or stored samples can be used. MFC can be done in a few hours, while NGS can take several days.
Despite these differences, both methods provide similar levels of sensitivity for detecting MRD (≥1 in 105).
Dr. Valković also noted that MRD should be evaluated outside the bone marrow as well, which can be done with positron emission tomography-computed tomography (PET-CT).
Research has shown that patients who are MRD-negative according to both MFC and PET-CT have better outcomes than patients who are MRD-positive by MFC, PET-CT, or both.4
What we don’t know
Though he compared MFC and NGS, Dr. Valković said we don’t know the optimal technique for assessing MRD in the bone marrow.
Another uncertainty is the optimal sensitivity threshold. In the POLLUX study5, researchers found that a threshold of 10-4 resulted in lots of patients with MRD negativity, but some of these were false-negatives.
So although 10-4 proved inaccurate, it isn’t clear if the optimal threshold is 10-5 or 10-6, Dr. Valković said.
Likewise, it isn’t clear if PET-CT is the optimal method for evaluating MRD outside the bone marrow.
In a study published in 2017, PET produced false-negatives in MM patients.6 In 11% of patients (26/227), there was evidence of disease with diffusion-weighted magnetic resonance imaging with background signal suppression, but there was no apparent disease with PET. The researchers said low expression of hexokinase-2 was associated with a false-negative PET result.
Finally, Dr. Valković said we don’t know how best to use MRD to tailor therapy in MM patients. He posed the following questions:
- If patients don’t achieve MRD negativity, should they continue on the therapy?
- If MRD-negative patients become MRD-positive, should they begin therapy immediately, or should treatment be put off until a biochemical or clinical relapse?
- Should MRD status be used to determine the number of treatment cycles a patient receives, the timing of transplant, or when to begin and end maintenance therapy?
“There are a lot of issues and unanswered questions related to the optimal techniques for the evaluation of MRD and their sensitivity, the timing for MRD assessment during and after therapy, and its role in the treatment decisions, which should be answered in future clinical studies,” Dr. Valković concluded.
He did not declare any conflicts of interest.
1. Munshi NC et al. JAMA Oncol. 2017;3(1):28-35. doi:10.1001/jamaoncol.2016.3160
2. Paiva B et al. Blood. 2016 Jun 23;127(25):3165-74. doi: 10.1182/blood-2016-03-705319
3. Kumar S et al. Lancet Oncol. 2016; 17 (8):e328-46 doi: https://doi.org/10.1016/S1470-2045(16)30206-6
4. Fernandez RA et al. Blood. 2017; 130:3098
5. Dimopoulos MA et al. Haematologica. 2018 Sep 20. pii: haematol.2018.194282. doi: 10.3324/haematol.2018.194282
6. Rasche L et al. Blood. 2017 Jul 6;130(1):30-34. doi: 10.1182/blood-2017-03-774422
DUBROVNIK, CROATIA—Clinical trials are needed to answer the many questions related to minimal residual disease (MRD) assessment in multiple myeloma (MM), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
MM patients are increasingly assessed for MRD, which is a strong prognostic factor and surrogate for overall survival, according to Toni Valković, MD, PhD, of University Hospital Center Rijeka in Croatia.
However, Dr. Valković said MRD assessment has not become a part of routine clinical practice, perhaps because we haven’t determined the best way to utilize MRD assessment in MM patients.
The optimal sensitivity threshold, technique, and timing of MRD assessment are not known, and it isn’t clear how MRD should be used to guide treatment.
What we know
Dr. Valković cited studies showing that MRD negativity is associated with superior survival in MM1, and, when MRD negativity is achieved, high-risk cytogenetics, age, and previous treatment regimens appear to have no further impact on prognosis.2
Dr. Valković went on to explain the benefits and detriments of multiparametric flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment.3
NGS requires a baseline patient sample, but MFC does not. More cells are required with MFC than with NGS (>5 million vs. <1 million).
With MFC, samples must be processed within 24 to 48 hours, whereas, with NGS, fresh or stored samples can be used. MFC can be done in a few hours, while NGS can take several days.
Despite these differences, both methods provide similar levels of sensitivity for detecting MRD (≥1 in 105).
Dr. Valković also noted that MRD should be evaluated outside the bone marrow as well, which can be done with positron emission tomography-computed tomography (PET-CT).
Research has shown that patients who are MRD-negative according to both MFC and PET-CT have better outcomes than patients who are MRD-positive by MFC, PET-CT, or both.4
What we don’t know
Though he compared MFC and NGS, Dr. Valković said we don’t know the optimal technique for assessing MRD in the bone marrow.
Another uncertainty is the optimal sensitivity threshold. In the POLLUX study5, researchers found that a threshold of 10-4 resulted in lots of patients with MRD negativity, but some of these were false-negatives.
So although 10-4 proved inaccurate, it isn’t clear if the optimal threshold is 10-5 or 10-6, Dr. Valković said.
Likewise, it isn’t clear if PET-CT is the optimal method for evaluating MRD outside the bone marrow.
In a study published in 2017, PET produced false-negatives in MM patients.6 In 11% of patients (26/227), there was evidence of disease with diffusion-weighted magnetic resonance imaging with background signal suppression, but there was no apparent disease with PET. The researchers said low expression of hexokinase-2 was associated with a false-negative PET result.
Finally, Dr. Valković said we don’t know how best to use MRD to tailor therapy in MM patients. He posed the following questions:
- If patients don’t achieve MRD negativity, should they continue on the therapy?
- If MRD-negative patients become MRD-positive, should they begin therapy immediately, or should treatment be put off until a biochemical or clinical relapse?
- Should MRD status be used to determine the number of treatment cycles a patient receives, the timing of transplant, or when to begin and end maintenance therapy?
“There are a lot of issues and unanswered questions related to the optimal techniques for the evaluation of MRD and their sensitivity, the timing for MRD assessment during and after therapy, and its role in the treatment decisions, which should be answered in future clinical studies,” Dr. Valković concluded.
He did not declare any conflicts of interest.
1. Munshi NC et al. JAMA Oncol. 2017;3(1):28-35. doi:10.1001/jamaoncol.2016.3160
2. Paiva B et al. Blood. 2016 Jun 23;127(25):3165-74. doi: 10.1182/blood-2016-03-705319
3. Kumar S et al. Lancet Oncol. 2016; 17 (8):e328-46 doi: https://doi.org/10.1016/S1470-2045(16)30206-6
4. Fernandez RA et al. Blood. 2017; 130:3098
5. Dimopoulos MA et al. Haematologica. 2018 Sep 20. pii: haematol.2018.194282. doi: 10.3324/haematol.2018.194282
6. Rasche L et al. Blood. 2017 Jul 6;130(1):30-34. doi: 10.1182/blood-2017-03-774422
CTPA overused in veterans with suspected PE
SAN ANTONIO—The recommended approach to evaluating suspected pulmonary embolism (PE) is “greatly underutilized” in the Veterans Health Administration system, according to a speaker at CHEST 2018.
A survey showed that, contrary to guideline recommendations, most Veterans Affairs sites did not require incorporation of a clinical decision rule (CDR) and D-dimer prior to the ordering of computed tomographic pulmonary angiography (CTPA) for suspected PE.
Therefore, CTPA was overused.
Nancy Hsu, MD, a pulmonologist in Los Angeles, California, discussed this finding at the meeting.
She noted that CTPA has become the imaging modality of choice for evaluating suspected PE, but it is overused and potentially avoidable in one-third of cases.
“In the 10 years following the advent of CTPA use, there was a 14-fold increase in usage, but there was no change in mortality,” Dr. Hsu said. “This is consistent with overdiagnosis.”
Indiscriminate use of CTPA results in unnecessary and avoidable radiation exposure, contrast-related reactions, and treatment-related bleeding, Dr. Hsu noted.
She and a colleague discovered CTPA overuse in the Veterans Health Administration system by conducting a survey of stakeholders at 18 Veterans Integrated Service Networks and 143 medical centers.
A total of 120 fully completed questionnaires were analyzed. Most respondents (63%) were chief physicians, and 80% had 11 or more years of experience.
Most respondents (85%) said CDR with or without D-dimer was not required before ordering CTPA. Less than 7% of respondents said they required both CDR and D-dimer before CTPA.
The biggest barrier to optimal practice may be the fear of having a patient who “falls through the cracks” based on false-negative CDR and D-dimer data, according to Dr. Hsu.
On the other hand, judicious use of CTPA likely avoids negative sequelae related to radiation, contrast exposure, and treatment-related bleeding, she said.
Dr. Hsu and her colleague, Guy Soo Hoo, MD, said they had no relationships relevant to this research.
SAN ANTONIO—The recommended approach to evaluating suspected pulmonary embolism (PE) is “greatly underutilized” in the Veterans Health Administration system, according to a speaker at CHEST 2018.
A survey showed that, contrary to guideline recommendations, most Veterans Affairs sites did not require incorporation of a clinical decision rule (CDR) and D-dimer prior to the ordering of computed tomographic pulmonary angiography (CTPA) for suspected PE.
Therefore, CTPA was overused.
Nancy Hsu, MD, a pulmonologist in Los Angeles, California, discussed this finding at the meeting.
She noted that CTPA has become the imaging modality of choice for evaluating suspected PE, but it is overused and potentially avoidable in one-third of cases.
“In the 10 years following the advent of CTPA use, there was a 14-fold increase in usage, but there was no change in mortality,” Dr. Hsu said. “This is consistent with overdiagnosis.”
Indiscriminate use of CTPA results in unnecessary and avoidable radiation exposure, contrast-related reactions, and treatment-related bleeding, Dr. Hsu noted.
She and a colleague discovered CTPA overuse in the Veterans Health Administration system by conducting a survey of stakeholders at 18 Veterans Integrated Service Networks and 143 medical centers.
A total of 120 fully completed questionnaires were analyzed. Most respondents (63%) were chief physicians, and 80% had 11 or more years of experience.
Most respondents (85%) said CDR with or without D-dimer was not required before ordering CTPA. Less than 7% of respondents said they required both CDR and D-dimer before CTPA.
The biggest barrier to optimal practice may be the fear of having a patient who “falls through the cracks” based on false-negative CDR and D-dimer data, according to Dr. Hsu.
On the other hand, judicious use of CTPA likely avoids negative sequelae related to radiation, contrast exposure, and treatment-related bleeding, she said.
Dr. Hsu and her colleague, Guy Soo Hoo, MD, said they had no relationships relevant to this research.
SAN ANTONIO—The recommended approach to evaluating suspected pulmonary embolism (PE) is “greatly underutilized” in the Veterans Health Administration system, according to a speaker at CHEST 2018.
A survey showed that, contrary to guideline recommendations, most Veterans Affairs sites did not require incorporation of a clinical decision rule (CDR) and D-dimer prior to the ordering of computed tomographic pulmonary angiography (CTPA) for suspected PE.
Therefore, CTPA was overused.
Nancy Hsu, MD, a pulmonologist in Los Angeles, California, discussed this finding at the meeting.
She noted that CTPA has become the imaging modality of choice for evaluating suspected PE, but it is overused and potentially avoidable in one-third of cases.
“In the 10 years following the advent of CTPA use, there was a 14-fold increase in usage, but there was no change in mortality,” Dr. Hsu said. “This is consistent with overdiagnosis.”
Indiscriminate use of CTPA results in unnecessary and avoidable radiation exposure, contrast-related reactions, and treatment-related bleeding, Dr. Hsu noted.
She and a colleague discovered CTPA overuse in the Veterans Health Administration system by conducting a survey of stakeholders at 18 Veterans Integrated Service Networks and 143 medical centers.
A total of 120 fully completed questionnaires were analyzed. Most respondents (63%) were chief physicians, and 80% had 11 or more years of experience.
Most respondents (85%) said CDR with or without D-dimer was not required before ordering CTPA. Less than 7% of respondents said they required both CDR and D-dimer before CTPA.
The biggest barrier to optimal practice may be the fear of having a patient who “falls through the cracks” based on false-negative CDR and D-dimer data, according to Dr. Hsu.
On the other hand, judicious use of CTPA likely avoids negative sequelae related to radiation, contrast exposure, and treatment-related bleeding, she said.
Dr. Hsu and her colleague, Guy Soo Hoo, MD, said they had no relationships relevant to this research.
Drones can deliver blood products, but hurdles remain
BOSTON—Drone-delivered blood products may be coming soon to a hospital near you, experts said at AABB 2018.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries,” Kenney said. “That’s almost 20,000 units of blood.”
One-third of all deliveries are needed for urgent, life-saving interventions, he added.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, according to Kenney.
How it works
Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section.
The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products, which can be done by text message or via WhatsApp, a free messaging and voiceover IP calling service.
After the order was placed, Zipline was able to deliver blood products using multiple drone launches over the course of 90 minutes. The deliveries consisted of 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15 to 25 minutes by air, Kenney said.
The drones—more formally known as “unmanned aerial vehicles”—fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters, and drop the package, which has a parachute attached.
Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Kenney said.
“The whole process is 100% autonomous,” he noted. “The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package.”
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are significantly cheaper than ground-based services for local delivery, according to Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold cited statistics suggesting the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more. However, drone delivery could be as cheap as 5 cents per mile.
Barriers to drone delivery
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, according to Dr. Eastvold.
He described the hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters in the United States.
Dr. Eastvold said the barriers to adoption of drone-based delivery systems include differences in state laws about when, where, and how drones can be used and who can operate them as well as Federal Aviation Administration (FAA) airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation for most drone operators, meaning the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery.
In 2015, Johns Hopkins researchers reported that transporting blood samples on hobby-sized drones did not affect the results of common and routine blood tests.
In 2016, the researchers showed that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
In 2017, the researchers demonstrated that a drone could deliver blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Kenney said his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON—Drone-delivered blood products may be coming soon to a hospital near you, experts said at AABB 2018.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries,” Kenney said. “That’s almost 20,000 units of blood.”
One-third of all deliveries are needed for urgent, life-saving interventions, he added.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, according to Kenney.
How it works
Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section.
The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products, which can be done by text message or via WhatsApp, a free messaging and voiceover IP calling service.
After the order was placed, Zipline was able to deliver blood products using multiple drone launches over the course of 90 minutes. The deliveries consisted of 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15 to 25 minutes by air, Kenney said.
The drones—more formally known as “unmanned aerial vehicles”—fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters, and drop the package, which has a parachute attached.
Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Kenney said.
“The whole process is 100% autonomous,” he noted. “The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package.”
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are significantly cheaper than ground-based services for local delivery, according to Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold cited statistics suggesting the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more. However, drone delivery could be as cheap as 5 cents per mile.
Barriers to drone delivery
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, according to Dr. Eastvold.
He described the hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters in the United States.
Dr. Eastvold said the barriers to adoption of drone-based delivery systems include differences in state laws about when, where, and how drones can be used and who can operate them as well as Federal Aviation Administration (FAA) airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation for most drone operators, meaning the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery.
In 2015, Johns Hopkins researchers reported that transporting blood samples on hobby-sized drones did not affect the results of common and routine blood tests.
In 2016, the researchers showed that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
In 2017, the researchers demonstrated that a drone could deliver blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Kenney said his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON—Drone-delivered blood products may be coming soon to a hospital near you, experts said at AABB 2018.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries,” Kenney said. “That’s almost 20,000 units of blood.”
One-third of all deliveries are needed for urgent, life-saving interventions, he added.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, according to Kenney.
How it works
Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section.
The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products, which can be done by text message or via WhatsApp, a free messaging and voiceover IP calling service.
After the order was placed, Zipline was able to deliver blood products using multiple drone launches over the course of 90 minutes. The deliveries consisted of 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15 to 25 minutes by air, Kenney said.
The drones—more formally known as “unmanned aerial vehicles”—fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters, and drop the package, which has a parachute attached.
Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Kenney said.
“The whole process is 100% autonomous,” he noted. “The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package.”
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are significantly cheaper than ground-based services for local delivery, according to Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold cited statistics suggesting the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more. However, drone delivery could be as cheap as 5 cents per mile.
Barriers to drone delivery
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, according to Dr. Eastvold.
He described the hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters in the United States.
Dr. Eastvold said the barriers to adoption of drone-based delivery systems include differences in state laws about when, where, and how drones can be used and who can operate them as well as Federal Aviation Administration (FAA) airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation for most drone operators, meaning the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery.
In 2015, Johns Hopkins researchers reported that transporting blood samples on hobby-sized drones did not affect the results of common and routine blood tests.
In 2016, the researchers showed that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
In 2017, the researchers demonstrated that a drone could deliver blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Kenney said his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
Obese PE patients have lower risk of death
SAN ANTONIO—Patients with pulmonary embolism (PE) have a lower mortality risk if they are obese, according to a retrospective analysis of nearly 2 million PE discharges.
The obese patients had a lower mortality risk despite receiving more thrombolytics and mechanical intubation, said study investigator Zubair Khan, MD, of the University of Toledo Medical Center in Ohio.
“Surprisingly, the mortality of PE was significantly less in obese patients,” Dr. Khan said. “When we initiated the study, we did not expect this result.”
Dr. Khan discussed this result in a presentation at CHEST 2018.
Dr. Khan noted that the association between obesity and lower mortality, sometimes called the “obesity paradox,” has been observed in studies of other chronic health conditions, including stable heart failure, coronary artery disease, unstable angina, myocardial infarction, and also in some PE studies.
His team’s study, conducted using the National Inpatient Sample database, included adults with a primary discharge diagnosis of PE between 2002 and 2014. The researchers included 1,959,018 PE discharges, of which 312,770 (16%) had an underlying obesity diagnosis.
Obese PE patients had more risk factors and more severe disease but an overall mortality of 2.2%, compared with 3.7% in PE patients without obesity (P<0.001), Dr. Khan reported.
Hypertension was significantly more prevalent in the obese PE patients (65% vs. 50.5%; P<0.001), as was chronic lung disease and chronic liver disease.
Obese patients more often received thrombolytics (3.6% vs. 1.9%; P<0.001) and mechanical ventilation (5.8% vs. 4%; P<0.001), and they more frequently had cardiogenic shock (0.65% vs. 0.45%; P<0.001).
The obese PE patients were more often female, black, and younger than 65 years of age.
Notably, the prevalence of obesity in PE patients more than doubled over the course of the study period, from 10.2% in 2002 to 22.6% in 2014.
The lower mortality in obese patients might be explained by increased levels of endocannabinoids, which have shown protective effects in rat and mouse studies, Dr. Khan said.
“I think it’s a rich area for more and further research, especially in basic science,” he added.
Dr. Khan and his coauthors said they had no relationships relevant to the study.
SAN ANTONIO—Patients with pulmonary embolism (PE) have a lower mortality risk if they are obese, according to a retrospective analysis of nearly 2 million PE discharges.
The obese patients had a lower mortality risk despite receiving more thrombolytics and mechanical intubation, said study investigator Zubair Khan, MD, of the University of Toledo Medical Center in Ohio.
“Surprisingly, the mortality of PE was significantly less in obese patients,” Dr. Khan said. “When we initiated the study, we did not expect this result.”
Dr. Khan discussed this result in a presentation at CHEST 2018.
Dr. Khan noted that the association between obesity and lower mortality, sometimes called the “obesity paradox,” has been observed in studies of other chronic health conditions, including stable heart failure, coronary artery disease, unstable angina, myocardial infarction, and also in some PE studies.
His team’s study, conducted using the National Inpatient Sample database, included adults with a primary discharge diagnosis of PE between 2002 and 2014. The researchers included 1,959,018 PE discharges, of which 312,770 (16%) had an underlying obesity diagnosis.
Obese PE patients had more risk factors and more severe disease but an overall mortality of 2.2%, compared with 3.7% in PE patients without obesity (P<0.001), Dr. Khan reported.
Hypertension was significantly more prevalent in the obese PE patients (65% vs. 50.5%; P<0.001), as was chronic lung disease and chronic liver disease.
Obese patients more often received thrombolytics (3.6% vs. 1.9%; P<0.001) and mechanical ventilation (5.8% vs. 4%; P<0.001), and they more frequently had cardiogenic shock (0.65% vs. 0.45%; P<0.001).
The obese PE patients were more often female, black, and younger than 65 years of age.
Notably, the prevalence of obesity in PE patients more than doubled over the course of the study period, from 10.2% in 2002 to 22.6% in 2014.
The lower mortality in obese patients might be explained by increased levels of endocannabinoids, which have shown protective effects in rat and mouse studies, Dr. Khan said.
“I think it’s a rich area for more and further research, especially in basic science,” he added.
Dr. Khan and his coauthors said they had no relationships relevant to the study.
SAN ANTONIO—Patients with pulmonary embolism (PE) have a lower mortality risk if they are obese, according to a retrospective analysis of nearly 2 million PE discharges.
The obese patients had a lower mortality risk despite receiving more thrombolytics and mechanical intubation, said study investigator Zubair Khan, MD, of the University of Toledo Medical Center in Ohio.
“Surprisingly, the mortality of PE was significantly less in obese patients,” Dr. Khan said. “When we initiated the study, we did not expect this result.”
Dr. Khan discussed this result in a presentation at CHEST 2018.
Dr. Khan noted that the association between obesity and lower mortality, sometimes called the “obesity paradox,” has been observed in studies of other chronic health conditions, including stable heart failure, coronary artery disease, unstable angina, myocardial infarction, and also in some PE studies.
His team’s study, conducted using the National Inpatient Sample database, included adults with a primary discharge diagnosis of PE between 2002 and 2014. The researchers included 1,959,018 PE discharges, of which 312,770 (16%) had an underlying obesity diagnosis.
Obese PE patients had more risk factors and more severe disease but an overall mortality of 2.2%, compared with 3.7% in PE patients without obesity (P<0.001), Dr. Khan reported.
Hypertension was significantly more prevalent in the obese PE patients (65% vs. 50.5%; P<0.001), as was chronic lung disease and chronic liver disease.
Obese patients more often received thrombolytics (3.6% vs. 1.9%; P<0.001) and mechanical ventilation (5.8% vs. 4%; P<0.001), and they more frequently had cardiogenic shock (0.65% vs. 0.45%; P<0.001).
The obese PE patients were more often female, black, and younger than 65 years of age.
Notably, the prevalence of obesity in PE patients more than doubled over the course of the study period, from 10.2% in 2002 to 22.6% in 2014.
The lower mortality in obese patients might be explained by increased levels of endocannabinoids, which have shown protective effects in rat and mouse studies, Dr. Khan said.
“I think it’s a rich area for more and further research, especially in basic science,” he added.
Dr. Khan and his coauthors said they had no relationships relevant to the study.
STRO-001 receives orphan designation for MM
The U.S. Food and Drug Administration (FDA) has granted orphan designation to STRO-001 for the treatment of multiple myeloma (MM).
STRO-001 is an antibody-drug conjugate targeting CD74, a protein highly expressed in MM and other B-cell malignancies.
Sutro Biopharma, Inc., is currently studying STRO-001 in a phase 1 trial enrolling separate dose-escalation cohorts for MM and B-cell lymphoma.
Preclinical research of STRO-001 in MM was presented at the 2017 ASH Annual Meeting.
Researchers examined bone marrow samples from MM patients and detected CD74 expression in 35 of the 36 samples, including specimens from patients who were treatment-naïve and patients who had been heavily pretreated with chemotherapy and stem cell transplant.
The researchers then found that STRO-001 demonstrated cytotoxicity in MM cell lines.
STRO-001 also reduced tumor burden in two disseminated xenograft models (ARP-1 and MM.1S) and prolonged survival in one of them (MM.1S).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan designation to STRO-001 for the treatment of multiple myeloma (MM).
STRO-001 is an antibody-drug conjugate targeting CD74, a protein highly expressed in MM and other B-cell malignancies.
Sutro Biopharma, Inc., is currently studying STRO-001 in a phase 1 trial enrolling separate dose-escalation cohorts for MM and B-cell lymphoma.
Preclinical research of STRO-001 in MM was presented at the 2017 ASH Annual Meeting.
Researchers examined bone marrow samples from MM patients and detected CD74 expression in 35 of the 36 samples, including specimens from patients who were treatment-naïve and patients who had been heavily pretreated with chemotherapy and stem cell transplant.
The researchers then found that STRO-001 demonstrated cytotoxicity in MM cell lines.
STRO-001 also reduced tumor burden in two disseminated xenograft models (ARP-1 and MM.1S) and prolonged survival in one of them (MM.1S).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan designation to STRO-001 for the treatment of multiple myeloma (MM).
STRO-001 is an antibody-drug conjugate targeting CD74, a protein highly expressed in MM and other B-cell malignancies.
Sutro Biopharma, Inc., is currently studying STRO-001 in a phase 1 trial enrolling separate dose-escalation cohorts for MM and B-cell lymphoma.
Preclinical research of STRO-001 in MM was presented at the 2017 ASH Annual Meeting.
Researchers examined bone marrow samples from MM patients and detected CD74 expression in 35 of the 36 samples, including specimens from patients who were treatment-naïve and patients who had been heavily pretreated with chemotherapy and stem cell transplant.
The researchers then found that STRO-001 demonstrated cytotoxicity in MM cell lines.
STRO-001 also reduced tumor burden in two disseminated xenograft models (ARP-1 and MM.1S) and prolonged survival in one of them (MM.1S).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
FDA approves test to determine blood compatibility
The U.S. Food and Drug Administration (FDA) has approved ID CORE XT, a molecular-based assay used to determine blood donor and recipient compatibility.
ID CORE XT is a qualitative, polymerase chain reaction-based and hybridization-based genotyping test.
It is used for the simultaneous identification of multiple alleles encoding human erythrocyte antigens in genomic DNA extracted from whole blood specimens collected in ethylenediaminetetraacetic acid.
The test genotypes 29 polymorphisms determining 37 human erythrocyte antigen phenotypes of 10 blood group systems—Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright, and Lutheran.
ID CORE XT is the second molecular assay approved by the FDA for use in transfusion medicine and the first to report genotypes as final results.
The approval of ID CORE XT was granted to Progenika Biopharma S.A., a Grifols company.
According to Progenika, ID CORE XT will benefit patients who require ongoing transfusions, such as individuals with hemoglobinopathies.
ID CORE XT can also be used for cancer patients who require more thorough blood typing, patients with warm autoimmune hemolytic anemia, and patients taking daratumumab.
“The approval of the ID CORE XT test can streamline blood compatibility testing and provides an additional alternative to testing blood with antisera,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
In a study published in Blood Transfusion this year, typing results with ID CORE XT were similar to results obtained with serology and molecular methods.
Researchers said there was 100% agreement between the positive results predicted by ID CORE XT and results obtained via serology (100% sensitivity).
For negative results, there was one discrepancy for E antigen (99.9% agreement) and 33 discrepancies for Fyb antigen (95.5% agreement). However, additional testing suggested that serology produced 34 false-negatives.
Both positive and negative results with ID CORE XT were in full agreement with results obtained via molecular methods (100% sensitivity and specificity).
The U.S. Food and Drug Administration (FDA) has approved ID CORE XT, a molecular-based assay used to determine blood donor and recipient compatibility.
ID CORE XT is a qualitative, polymerase chain reaction-based and hybridization-based genotyping test.
It is used for the simultaneous identification of multiple alleles encoding human erythrocyte antigens in genomic DNA extracted from whole blood specimens collected in ethylenediaminetetraacetic acid.
The test genotypes 29 polymorphisms determining 37 human erythrocyte antigen phenotypes of 10 blood group systems—Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright, and Lutheran.
ID CORE XT is the second molecular assay approved by the FDA for use in transfusion medicine and the first to report genotypes as final results.
The approval of ID CORE XT was granted to Progenika Biopharma S.A., a Grifols company.
According to Progenika, ID CORE XT will benefit patients who require ongoing transfusions, such as individuals with hemoglobinopathies.
ID CORE XT can also be used for cancer patients who require more thorough blood typing, patients with warm autoimmune hemolytic anemia, and patients taking daratumumab.
“The approval of the ID CORE XT test can streamline blood compatibility testing and provides an additional alternative to testing blood with antisera,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
In a study published in Blood Transfusion this year, typing results with ID CORE XT were similar to results obtained with serology and molecular methods.
Researchers said there was 100% agreement between the positive results predicted by ID CORE XT and results obtained via serology (100% sensitivity).
For negative results, there was one discrepancy for E antigen (99.9% agreement) and 33 discrepancies for Fyb antigen (95.5% agreement). However, additional testing suggested that serology produced 34 false-negatives.
Both positive and negative results with ID CORE XT were in full agreement with results obtained via molecular methods (100% sensitivity and specificity).
The U.S. Food and Drug Administration (FDA) has approved ID CORE XT, a molecular-based assay used to determine blood donor and recipient compatibility.
ID CORE XT is a qualitative, polymerase chain reaction-based and hybridization-based genotyping test.
It is used for the simultaneous identification of multiple alleles encoding human erythrocyte antigens in genomic DNA extracted from whole blood specimens collected in ethylenediaminetetraacetic acid.
The test genotypes 29 polymorphisms determining 37 human erythrocyte antigen phenotypes of 10 blood group systems—Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright, and Lutheran.
ID CORE XT is the second molecular assay approved by the FDA for use in transfusion medicine and the first to report genotypes as final results.
The approval of ID CORE XT was granted to Progenika Biopharma S.A., a Grifols company.
According to Progenika, ID CORE XT will benefit patients who require ongoing transfusions, such as individuals with hemoglobinopathies.
ID CORE XT can also be used for cancer patients who require more thorough blood typing, patients with warm autoimmune hemolytic anemia, and patients taking daratumumab.
“The approval of the ID CORE XT test can streamline blood compatibility testing and provides an additional alternative to testing blood with antisera,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
In a study published in Blood Transfusion this year, typing results with ID CORE XT were similar to results obtained with serology and molecular methods.
Researchers said there was 100% agreement between the positive results predicted by ID CORE XT and results obtained via serology (100% sensitivity).
For negative results, there was one discrepancy for E antigen (99.9% agreement) and 33 discrepancies for Fyb antigen (95.5% agreement). However, additional testing suggested that serology produced 34 false-negatives.
Both positive and negative results with ID CORE XT were in full agreement with results obtained via molecular methods (100% sensitivity and specificity).
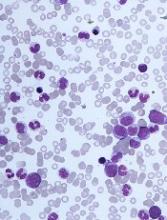
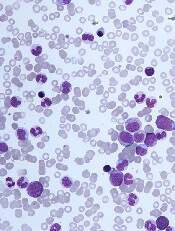
The challenges of diagnosing CMML
DUBROVNIK, CROATIA—Diagnosing chronic myelomonocytic leukemia (CMML) remains a challenge in 2018, according to a presentation at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Duraković, MD, PhD, of the University Hospital Zagreb in Croatia.
However, Dr. Duraković said there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML).
Furthermore, studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
Dr. Duraković began her presentation with an overview of the 2016 WHO classification of CMML (Blood 2016 127:2391-2405).
According to the WHO, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L) with monocytes accounting for 10% of the white blood cell count
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement
- They have fewer than 20% blasts in the blood and bone marrow
- They have dysplasia in one or more myeloid lineages
- If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present.
Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Duraković said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions such as pregnancy.
However, Dr. Duraković pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“[T]here are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Duraković said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Distinguishing CMML from other conditions
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Duraković said. “There are dysplastic features that are present in CMML . . . but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Duraković noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She went on to explain that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Duraković also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Duraković said.
She also noted that it can be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Duraković explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Duraković noted.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Duraković said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Duraković said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes—SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood 2012 120:3080-3088).
However, Dr. Duraković noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML—monocyte subset distribution analysis (Blood 2015 125(23): 3618–3626).
For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−)
- Intermediate/MO2 (CD14bright/CD16+)
- Non-classical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94.0%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis.
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML (Am J Clin Pathol 2018 150(4):293-302).
This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes.
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Duraković said.
She added that the technique can be implemented in clinical practice using the HematoflowTM solution (Cytometry B Clin Cytom 2018 94(5):658-661).
Dr. Duraković did not report any conflicts of interest.
DUBROVNIK, CROATIA—Diagnosing chronic myelomonocytic leukemia (CMML) remains a challenge in 2018, according to a presentation at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Duraković, MD, PhD, of the University Hospital Zagreb in Croatia.
However, Dr. Duraković said there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML).
Furthermore, studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
Dr. Duraković began her presentation with an overview of the 2016 WHO classification of CMML (Blood 2016 127:2391-2405).
According to the WHO, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L) with monocytes accounting for 10% of the white blood cell count
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement
- They have fewer than 20% blasts in the blood and bone marrow
- They have dysplasia in one or more myeloid lineages
- If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present.
Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Duraković said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions such as pregnancy.
However, Dr. Duraković pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“[T]here are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Duraković said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Distinguishing CMML from other conditions
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Duraković said. “There are dysplastic features that are present in CMML . . . but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Duraković noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She went on to explain that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Duraković also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Duraković said.
She also noted that it can be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Duraković explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Duraković noted.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Duraković said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Duraković said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes—SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood 2012 120:3080-3088).
However, Dr. Duraković noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML—monocyte subset distribution analysis (Blood 2015 125(23): 3618–3626).
For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−)
- Intermediate/MO2 (CD14bright/CD16+)
- Non-classical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94.0%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis.
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML (Am J Clin Pathol 2018 150(4):293-302).
This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes.
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Duraković said.
She added that the technique can be implemented in clinical practice using the HematoflowTM solution (Cytometry B Clin Cytom 2018 94(5):658-661).
Dr. Duraković did not report any conflicts of interest.
DUBROVNIK, CROATIA—Diagnosing chronic myelomonocytic leukemia (CMML) remains a challenge in 2018, according to a presentation at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Duraković, MD, PhD, of the University Hospital Zagreb in Croatia.
However, Dr. Duraković said there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML).
Furthermore, studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
Dr. Duraković began her presentation with an overview of the 2016 WHO classification of CMML (Blood 2016 127:2391-2405).
According to the WHO, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L) with monocytes accounting for 10% of the white blood cell count
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement
- They have fewer than 20% blasts in the blood and bone marrow
- They have dysplasia in one or more myeloid lineages
- If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present.
Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Duraković said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions such as pregnancy.
However, Dr. Duraković pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“[T]here are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Duraković said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Distinguishing CMML from other conditions
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Duraković said. “There are dysplastic features that are present in CMML . . . but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Duraković noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She went on to explain that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Duraković also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Duraković said.
She also noted that it can be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Duraković explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Duraković noted.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Duraković said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Duraković said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes—SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood 2012 120:3080-3088).
However, Dr. Duraković noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML—monocyte subset distribution analysis (Blood 2015 125(23): 3618–3626).
For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−)
- Intermediate/MO2 (CD14bright/CD16+)
- Non-classical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94.0%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis.
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML (Am J Clin Pathol 2018 150(4):293-302).
This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes.
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Duraković said.
She added that the technique can be implemented in clinical practice using the HematoflowTM solution (Cytometry B Clin Cytom 2018 94(5):658-661).
Dr. Duraković did not report any conflicts of interest.
Crizanlizumab appears effective across subgroups
Crizanlizumab can reduce vaso-occlusive crises (VOCs) across subgroups of patients with sickle cell disease (SCD), according to a post-hoc analysis of the phase 2 SUSTAIN trial.
Researchers found crizanlizumab was more effective than placebo at delaying time to first VOC and eliminating crises in patients who had numerous previous crises, exhibited the HbSS genotype, or were taking concomitant hydroxyurea.
Abdullah Kutlar, MD, of the Medical College of Georgia in Augusta, and his colleagues reported these findings in the American Journal of Hematology.
The phase 2 SUSTAIN trial previously showed that crizanlizumab—a humanized, anti–P-selectin monoclonal antibody—reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months.
Additionally, a subgroup analysis showed there was a lower frequency of VOCs with crizanlizumab at 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post-hoc analysis took a deeper look at these observations across the same subgroups. Specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events in 132 patients.
Crizanlizumab eliminated VOCs about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (5 to 10 VOCs in the year prior)—28.0% and 4.2%, respectively.
Crizanlizumab eliminated VOCs about twice as often as placebo in patients with the HbSS genotype—31.9% and 17.0%, respectively—and in patients who were using concomitant hydroxyurea—33.3% and 17.5%, respectively.
Further analysis showed that crizanlizumab delayed time to first VOC across all subgroups.
In patients with the HbSS genotype, the time to first VOC was 4.07 months with crizanlizumab and 1.12 months with placebo.
In patients with a higher frequency of previous VOCs, the time to first on-study VOC was 2.43 months with crizanlizumab and 1.03 months with placebo.
In patients taking hydroxyurea, the time to first VOC was 2.43 months with crizanlizumab and 1.45 months with placebo.
Safety was comparable across subgroups.
This study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Crizanlizumab can reduce vaso-occlusive crises (VOCs) across subgroups of patients with sickle cell disease (SCD), according to a post-hoc analysis of the phase 2 SUSTAIN trial.
Researchers found crizanlizumab was more effective than placebo at delaying time to first VOC and eliminating crises in patients who had numerous previous crises, exhibited the HbSS genotype, or were taking concomitant hydroxyurea.
Abdullah Kutlar, MD, of the Medical College of Georgia in Augusta, and his colleagues reported these findings in the American Journal of Hematology.
The phase 2 SUSTAIN trial previously showed that crizanlizumab—a humanized, anti–P-selectin monoclonal antibody—reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months.
Additionally, a subgroup analysis showed there was a lower frequency of VOCs with crizanlizumab at 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post-hoc analysis took a deeper look at these observations across the same subgroups. Specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events in 132 patients.
Crizanlizumab eliminated VOCs about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (5 to 10 VOCs in the year prior)—28.0% and 4.2%, respectively.
Crizanlizumab eliminated VOCs about twice as often as placebo in patients with the HbSS genotype—31.9% and 17.0%, respectively—and in patients who were using concomitant hydroxyurea—33.3% and 17.5%, respectively.
Further analysis showed that crizanlizumab delayed time to first VOC across all subgroups.
In patients with the HbSS genotype, the time to first VOC was 4.07 months with crizanlizumab and 1.12 months with placebo.
In patients with a higher frequency of previous VOCs, the time to first on-study VOC was 2.43 months with crizanlizumab and 1.03 months with placebo.
In patients taking hydroxyurea, the time to first VOC was 2.43 months with crizanlizumab and 1.45 months with placebo.
Safety was comparable across subgroups.
This study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.
Crizanlizumab can reduce vaso-occlusive crises (VOCs) across subgroups of patients with sickle cell disease (SCD), according to a post-hoc analysis of the phase 2 SUSTAIN trial.
Researchers found crizanlizumab was more effective than placebo at delaying time to first VOC and eliminating crises in patients who had numerous previous crises, exhibited the HbSS genotype, or were taking concomitant hydroxyurea.
Abdullah Kutlar, MD, of the Medical College of Georgia in Augusta, and his colleagues reported these findings in the American Journal of Hematology.
The phase 2 SUSTAIN trial previously showed that crizanlizumab—a humanized, anti–P-selectin monoclonal antibody—reduced the frequency of VOCs by 45% and delayed time to first crisis by about 3 months.
Additionally, a subgroup analysis showed there was a lower frequency of VOCs with crizanlizumab at 5 mg/kg, compared with placebo, regardless of the number of prior VOCs, concomitant hydroxyurea use, or the SCD genotype.
The present post-hoc analysis took a deeper look at these observations across the same subgroups. Specifically, the investigators assessed elimination of VOCs, time to first crisis, and adverse events in 132 patients.
Crizanlizumab eliminated VOCs about seven times more frequently than did placebo in patients who had a high frequency of VOCs before the study (5 to 10 VOCs in the year prior)—28.0% and 4.2%, respectively.
Crizanlizumab eliminated VOCs about twice as often as placebo in patients with the HbSS genotype—31.9% and 17.0%, respectively—and in patients who were using concomitant hydroxyurea—33.3% and 17.5%, respectively.
Further analysis showed that crizanlizumab delayed time to first VOC across all subgroups.
In patients with the HbSS genotype, the time to first VOC was 4.07 months with crizanlizumab and 1.12 months with placebo.
In patients with a higher frequency of previous VOCs, the time to first on-study VOC was 2.43 months with crizanlizumab and 1.03 months with placebo.
In patients taking hydroxyurea, the time to first VOC was 2.43 months with crizanlizumab and 1.45 months with placebo.
Safety was comparable across subgroups.
This study was sponsored by Novartis. The authors reported financial relationships with Novartis, Bluebird Bio, AstraZeneca, and others.