User login
Inhibitor receives orphan designation for ITP
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to PRN1008 for the treatment of patients with immune thrombocytopenia (ITP).
PRN1008 is an oral, reversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor being developed by Principia Biopharma, Inc.
Principia is conducting a phase 1/2 trial (NCT03395210) to evaluate the safety and efficacy of PRN1008 in patients with ITP.
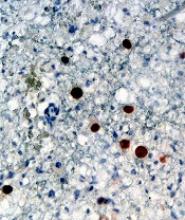
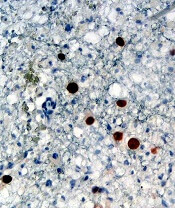
Results of preclinical research with PRN1008 in ITP were presented at the 2017 ASH Annual Meeting.
There, researchers reported that PRN1008 significantly reduced platelet loss in a mouse model of ITP.
The team found the BTK inhibitor could diminish platelet loss in two ways:
- By reducing platelet destruction via inhibition of autoantibody/FcγR signaling in splenic macrophages
- By reducing autoantibody generation through inhibition of B-cell activation and maturation.
The researchers also assessed the effects of PRN1008 and ibrutinib on platelet function in samples from healthy volunteers and ITP patients.
Samples were treated with one of the two BTK inhibitors, and platelet aggregation was induced by platelet agonists.
Unlike ibrutinib, PRN1008 did not impact platelet aggregation in healthy volunteer or ITP patient samples.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to PRN1008 for the treatment of patients with immune thrombocytopenia (ITP).
PRN1008 is an oral, reversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor being developed by Principia Biopharma, Inc.
Principia is conducting a phase 1/2 trial (NCT03395210) to evaluate the safety and efficacy of PRN1008 in patients with ITP.
Results of preclinical research with PRN1008 in ITP were presented at the 2017 ASH Annual Meeting.
There, researchers reported that PRN1008 significantly reduced platelet loss in a mouse model of ITP.
The team found the BTK inhibitor could diminish platelet loss in two ways:
- By reducing platelet destruction via inhibition of autoantibody/FcγR signaling in splenic macrophages
- By reducing autoantibody generation through inhibition of B-cell activation and maturation.
The researchers also assessed the effects of PRN1008 and ibrutinib on platelet function in samples from healthy volunteers and ITP patients.
Samples were treated with one of the two BTK inhibitors, and platelet aggregation was induced by platelet agonists.
Unlike ibrutinib, PRN1008 did not impact platelet aggregation in healthy volunteer or ITP patient samples.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to PRN1008 for the treatment of patients with immune thrombocytopenia (ITP).
PRN1008 is an oral, reversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor being developed by Principia Biopharma, Inc.
Principia is conducting a phase 1/2 trial (NCT03395210) to evaluate the safety and efficacy of PRN1008 in patients with ITP.
Results of preclinical research with PRN1008 in ITP were presented at the 2017 ASH Annual Meeting.
There, researchers reported that PRN1008 significantly reduced platelet loss in a mouse model of ITP.
The team found the BTK inhibitor could diminish platelet loss in two ways:
- By reducing platelet destruction via inhibition of autoantibody/FcγR signaling in splenic macrophages
- By reducing autoantibody generation through inhibition of B-cell activation and maturation.
The researchers also assessed the effects of PRN1008 and ibrutinib on platelet function in samples from healthy volunteers and ITP patients.
Samples were treated with one of the two BTK inhibitors, and platelet aggregation was induced by platelet agonists.
Unlike ibrutinib, PRN1008 did not impact platelet aggregation in healthy volunteer or ITP patient samples.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
CHMP recommends change for eptacog alfa
The European Medicine’s Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a change to the terms of the marketing authorization for the recombinant factor VIIa product eptacog alfa (NovoSeven).
The recommendation is to expand the approved use of eptacog alfa in patients with Glanzmann’s thrombasthenia.
Eptacog alfa is already approved by the European Commission (EC) for use in patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen who have past or present refractoriness to platelet transfusions.
Now, the CHMP has recommended expanding the use of eptacog alfa to include situations in which patients are not refractory to platelet transfusions but platelets are not readily available.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
If the EC follows the CHMP’s recommendation for eptacog alfa, the product will be approved for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- Patients with congenital hemophilia with inhibitors to coagulation factors VIII or IX > 5 Bethesda units
- Patients with congenital hemophilia who are expected to have a high anamnestic response to factor VIII or factor IX administration
- Patients with acquired hemophilia
- Patients with congenital FVII deficiency
- Patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen and past or present refractoriness to platelet transfusions, or where platelets are not readily available.
The European Medicine’s Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a change to the terms of the marketing authorization for the recombinant factor VIIa product eptacog alfa (NovoSeven).
The recommendation is to expand the approved use of eptacog alfa in patients with Glanzmann’s thrombasthenia.
Eptacog alfa is already approved by the European Commission (EC) for use in patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen who have past or present refractoriness to platelet transfusions.
Now, the CHMP has recommended expanding the use of eptacog alfa to include situations in which patients are not refractory to platelet transfusions but platelets are not readily available.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
If the EC follows the CHMP’s recommendation for eptacog alfa, the product will be approved for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- Patients with congenital hemophilia with inhibitors to coagulation factors VIII or IX > 5 Bethesda units
- Patients with congenital hemophilia who are expected to have a high anamnestic response to factor VIII or factor IX administration
- Patients with acquired hemophilia
- Patients with congenital FVII deficiency
- Patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen and past or present refractoriness to platelet transfusions, or where platelets are not readily available.
The European Medicine’s Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a change to the terms of the marketing authorization for the recombinant factor VIIa product eptacog alfa (NovoSeven).
The recommendation is to expand the approved use of eptacog alfa in patients with Glanzmann’s thrombasthenia.
Eptacog alfa is already approved by the European Commission (EC) for use in patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen who have past or present refractoriness to platelet transfusions.
Now, the CHMP has recommended expanding the use of eptacog alfa to include situations in which patients are not refractory to platelet transfusions but platelets are not readily available.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
If the EC follows the CHMP’s recommendation for eptacog alfa, the product will be approved for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- Patients with congenital hemophilia with inhibitors to coagulation factors VIII or IX > 5 Bethesda units
- Patients with congenital hemophilia who are expected to have a high anamnestic response to factor VIII or factor IX administration
- Patients with acquired hemophilia
- Patients with congenital FVII deficiency
- Patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen and past or present refractoriness to platelet transfusions, or where platelets are not readily available.
Study supports sequencing in kids with cancer
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
‘Intense’ end-of-life care may be common in HSCT recipients
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Patients who die within a year of allogeneic hematopoietic stem cell transplant (HSCT) tend to receive “medically intense” end-of-life care, an analysis suggests.
Researchers studied more than 2,000 patients who died within a year of allogeneic HSCT and found that a majority of the patients died in the hospital, and about half of them were admitted to the intensive care unit (ICU).
However, patient age, underlying diagnosis, and other factors influenced the likelihood of receiving intense end-of-life care.
For example, patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) were less likely than patients with acute lymphoblastic leukemia (ALL) to receive medically intense care.
Emily Johnston, MD, of the University of Alabama at Birmingham, and her colleagues reported these findings in the Journal of Clinical Oncology.
The researchers studied 2,135 patients in California who underwent inpatient HSCT and died within a year of the transplant (not as a result of peripartum events or trauma) between 2000 and 2013.
Fifty-three percent of the patients received some type of medically intense intervention, and 57% had at least two types of intense interventions.
Eighty-three percent of patients died in hospital, and 43% spent all of their last 30 days in the hospital.
Forty-nine percent of patients were admitted to the ICU, 45% were intubated, 22% underwent hemodialysis, and 8% received cardiopulmonary resuscitation.
Factors associated with intense care
The researchers said receipt of a medically intense intervention varied by age at death, underlying diagnosis, year of HSCT, location of care, and comorbidities. However, use of intense interventions did not vary according to sex, race/ethnicity, insurance type, or income.
Compared to patients age 60 and older, patients in the following age groups were more likely to receive medically intense interventions:
- Ages 15 to 21—odds ratio (OR)=2.6 (P<0.001)
- Ages 30 to 39—OR=1.8 (P<0.01)
- Ages 40 to 49—OR=1.4 (P<0.05).
Patients with comorbidities were more likely to receive intense interventions as well. The OR was 1.6 (P<0.01) for patients with one comorbidity and 2.5 (P<0.001) for patients with two or more comorbidities.
Patients with AML or MDS were less likely than patients with ALL to receive a medically intense intervention—OR=0.7 (P<0.05).
Patients who were transplanted between 2000 and 2004 were less likely to receive an intense intervention than patients transplanted between 2010 and 2013—OR=0.7 (P<0.01).
Patients who changed hospitals between HSCT and death were less likely to receive an intense intervention than patients who stayed at the same hospital. The OR was 0.3 if they transferred to a community hospital and 0.4 if they transferred to a specialty hospital (P<0.001 for both).
Patients living in rural areas were less likely than urban patients to receive a medically intense intervention—OR=0.6 (P<0.05).
“From our data, we understand there is a correlation with high-intensity end-of-life care in patients who die within one year after receiving a stem cell transplant, but we are still unsure if that was the care they wanted,” Dr. Johnston said.
“The findings suggest that, as oncologists, we need to start having end-of-life care conversations earlier with patients to determine if a high-intensity treatment plan is consistent with their goals or if a lower-intensity treatment plan is best. It’s not a one-size-fits-all approach in end-of-life care.”
This research was supported by Stanford University. One study author reported relationships with Corvus Pharmaceuticals, Shire Pharmaceuticals, and Adaptive Biotechnologies. All other authors reported no conflicts.
Adoptive T-cell therapy treats PML
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Dataset could reveal better therapies for AML
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
Researchers have released a dataset detailing the molecular makeup of tumor cells from more than 500 patients with acute myeloid leukemia (AML).
The team discovered mutations not previously observed in AML and found associations between mutations and responses to certain therapies.
For instance, AML cases with FLT3, NPM1, and DNMT3A mutations proved sensitive to the BTK inhibitor ibrutinib.
The researchers described their findings in Nature.
The team also made their dataset available via Vizome, an online data viewer. Other researchers can use Vizome to find out which targeted therapies might be most effective against specific subsets of AML cells.
“People can get online, search our database, and very quickly get answers to ‘Is this a good drug?’ or ‘Is there a patient population my drug can work in?’” said study author Brian Druker, MD, of Oregon Health & Science University (OHSU) in Portland, Oregon.
Newly identified mutations
For this study, part of the Beat AML initiative, Dr. Druker and his colleagues performed whole-exome and RNA sequencing on 672 samples from 562 AML patients.
The team identified mutations in 11 genes that were called in 1% or more of patients in this dataset but had not been observed in previous AML sequencing studies. The genes were:
- CUB and Sushi multiple domains 2 (CSMD2)
- NAC alpha domain containing (NACAD)
- Teneurin transmembrane protein 2 (TENM2)
- Aggrecan (ACAN)
- ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7)
- Immunoglobulin-like and fibronectin type III domain containing 1 (IGFN1)
- Neurobeachin-like 2 (NBEAL2)
- Poly(U) binding splicing factor 60 (PUF60)
- Zinc-finger protein 687 (ZNF687)
- Cadherin EGF LAG sevenpass G-type receptor 2 (CELSR2)
- Glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B).
Testing therapies
The researchers also assessed how AML cells from 409 of the patient samples responded to each of 122 targeted therapies.
The team found that mutations in TP53, ASXL1, NRAS, and KRAS caused “a broad pattern of drug resistance.”
However, cases with TP53 mutations were sensitive to elesclomol (a drug that targets cancer cell metabolism), cases with ASXL1 mutations were sensitive to the HDAC inhibitor panobinostat, and cases with KRAS/NRAS mutations were sensitive to MAPK inhibitors (with NRAS-mutated cases demonstrating greater sensitivity).
The researchers also found that IDH2 mutations “conferred sensitivity to a broad spectrum of drugs,” but IDH1 mutations were associated with resistance to most drugs.
As previously mentioned, the researchers found a significant association between mutations in FLT3, NPM1, and DNMT3A and sensitivity to ibrutinib. However, the team found that cases with DNMT3A mutations alone or mutations in DNMT3A and FLT3 were not significantly different from cases with wild-type genes.
On the other hand, cases with FLT3-ITD alone or any combination with a mutation in NPM1 (including mutations in all three genes) were significantly more sensitive to ibrutinib than cases with wild-type genes.
Cases with FLT3-ITD and mutations in NPM1 were sensitive to another kinase inhibitor, entospletinib, as well.
The researchers also found that mutations in both BCOR and RUNX1 correlated with increased sensitivity to four JAK inhibitors—momelotinib, ruxolitinib, tofacitinib, and JAK inhibitor I.
However, cases with BCOR mutations alone or mutations in BCOR and DNMT3A or SRSF2 showed no difference in sensitivity to the JAK inhibitors from cases with wild-type genes.
Next steps
“We’re just starting to scratch the surface of what we can do when we analyze the data,” Dr. Druker said. “The real power comes when you start to integrate all that data. You can analyze what drug worked and why it worked.”
In fact, the researchers are already developing and initiating clinical trials to test hypotheses generated by this study.
“You can start to sense some momentum building with new, better therapeutics for AML patients, and, hopefully, this dataset will help fuel that momentum even further,” said study author Jeff Tyner, PhD, of the OHSU School of Medicine.
“We want to parlay this information into clinical trials as much as we can, and we also want the broader community to use this dataset to accelerate their own work.”
Funding for the current study was provided by grants from The Leukemia & Lymphoma Society, the National Cancer Institute, the National Library of Medicine, and other groups.
BTK inhibitor shows early promise for WM
NEW YORK—The BTK inhibitor zanubrutinib has demonstrated “robust activity” and “good tolerability” in patients with Waldenström’s macroglobulinemia (WM), according to an investigator.
In a phase 1 trial, zanubrutinib produced an overall response rate (ORR) of 92%, and the estimated 12-month progression-free survival (PFS) rate was 89%.
Most adverse events (AEs) in this trial were grade 1 or 2 in severity, although the incidence of serious AEs was 42%.
Constantine Tam, MD, of the Peter MacCallum Cancer Center in Victoria, Australia, presented these results at the 10th International Workshop on Waldenström’s Macroglobulinemia.
The trial is sponsored by BeiGene, Ltd., the company developing zanubrutinib.
The trial (NCT02343120) includes patients with WM and other B-cell malignancies. As of July 24, 2018, 77 patients with treatment-naïve or relapsed/refractory WM had been enrolled.
Seventy-three patients were evaluable for efficacy in this analysis, and the median follow-up time was 22.5 months (range, 4.1-43.9).
At the time of the data cutoff, 62 patients remained on study treatment. Four patients (3%) discontinued treatment due to disease progression, and one patient remains on treatment post-progression.
Efficacy
The median time to response was 85 days (range, 55-749).
The ORR was 92% (67/73), and the major response rate (MRR) was 82%. Forty-one percent of patients achieved a very good partial response (VGPR), defined as a greater than 90% reduction in baseline immunoglobulin M (IgM) levels and improvement of extramedullary disease by computed tomography.
The median IgM decreased from 32.7 g/L (range, 5.3-91.9) at baseline to 8.2 g/L (range, 0.3-57.8). The median hemoglobin increased from 8.85 g/dL (range, 6.3-9.8) to 13.4 g/dL (range, 7.7-17.0) among 32 patients with hemoglobin less than 10 g/dL at baseline.
MYD88 genotype was known in 63 patients. In the subset known to have the MYD88L265P mutation (n=54), the ORR was 94%, the MRR was 89%, and the VGPR rate was 46%.
In the nine patients known to have wild-type MYD88 (a genotype that, historically, has had sub-optimal response to BTK inhibition), the ORR was 89%, the MRR was 67%, and the VGPR rate was 22%.
The 12-month PFS was estimated to be 89%, and the median PFS had not been reached.
Safety
The most frequent AEs of any attribution were petechiae/purpura/contusion (43%), upper respiratory tract infection (42%), cough (17%), diarrhea (17%), constipation (16%), back pain (16%), and headache (16%).
Grade 3-4 AEs of any attribution reported in three or more patients included neutropenia (9%), anemia (7%), hypertension (5%), basal cell carcinoma (5%), renal and urinary disorders (4%), and pneumonia (4%).
Serious AEs were seen in 32 patients (42%). Events in five patients (7%) were considered possibly related to zanubrutinib treatment—febrile neutropenia, colitis, atrial fibrillation, hemothorax, and pneumonia.
Nine patients (12%) discontinued study treatment due to AEs, but all of these events were considered unrelated to treatment. The AEs (n=1 for each) included abdominal sepsis (fatal), gastric adenocarcinoma (fatal), septic shoulder, worsening bronchiectasis, scedosporium infection, prostate adenocarcinoma, metastatic neuroendocrine carcinoma, acute myeloid leukemia, and breast cancer.
Atrial fibrillation/flutter occurred in four patients (5%), and major hemorrhage was observed in two patients (3%).
“We are encouraged that additional data on zanubrutinib in patients with WM confirms the initially reported experience, with consistent demonstration of robust activity and good tolerability,” Dr. Tam said.
“We are hopeful that zanubrutinib, if approved, could potentially provide an important new treatment option to patients with WM and other hematologic malignancies.”
Dr. Tam reported financial relationships with BeiGene and other companies.
NEW YORK—The BTK inhibitor zanubrutinib has demonstrated “robust activity” and “good tolerability” in patients with Waldenström’s macroglobulinemia (WM), according to an investigator.
In a phase 1 trial, zanubrutinib produced an overall response rate (ORR) of 92%, and the estimated 12-month progression-free survival (PFS) rate was 89%.
Most adverse events (AEs) in this trial were grade 1 or 2 in severity, although the incidence of serious AEs was 42%.
Constantine Tam, MD, of the Peter MacCallum Cancer Center in Victoria, Australia, presented these results at the 10th International Workshop on Waldenström’s Macroglobulinemia.
The trial is sponsored by BeiGene, Ltd., the company developing zanubrutinib.
The trial (NCT02343120) includes patients with WM and other B-cell malignancies. As of July 24, 2018, 77 patients with treatment-naïve or relapsed/refractory WM had been enrolled.
Seventy-three patients were evaluable for efficacy in this analysis, and the median follow-up time was 22.5 months (range, 4.1-43.9).
At the time of the data cutoff, 62 patients remained on study treatment. Four patients (3%) discontinued treatment due to disease progression, and one patient remains on treatment post-progression.
Efficacy
The median time to response was 85 days (range, 55-749).
The ORR was 92% (67/73), and the major response rate (MRR) was 82%. Forty-one percent of patients achieved a very good partial response (VGPR), defined as a greater than 90% reduction in baseline immunoglobulin M (IgM) levels and improvement of extramedullary disease by computed tomography.
The median IgM decreased from 32.7 g/L (range, 5.3-91.9) at baseline to 8.2 g/L (range, 0.3-57.8). The median hemoglobin increased from 8.85 g/dL (range, 6.3-9.8) to 13.4 g/dL (range, 7.7-17.0) among 32 patients with hemoglobin less than 10 g/dL at baseline.
MYD88 genotype was known in 63 patients. In the subset known to have the MYD88L265P mutation (n=54), the ORR was 94%, the MRR was 89%, and the VGPR rate was 46%.
In the nine patients known to have wild-type MYD88 (a genotype that, historically, has had sub-optimal response to BTK inhibition), the ORR was 89%, the MRR was 67%, and the VGPR rate was 22%.
The 12-month PFS was estimated to be 89%, and the median PFS had not been reached.
Safety
The most frequent AEs of any attribution were petechiae/purpura/contusion (43%), upper respiratory tract infection (42%), cough (17%), diarrhea (17%), constipation (16%), back pain (16%), and headache (16%).
Grade 3-4 AEs of any attribution reported in three or more patients included neutropenia (9%), anemia (7%), hypertension (5%), basal cell carcinoma (5%), renal and urinary disorders (4%), and pneumonia (4%).
Serious AEs were seen in 32 patients (42%). Events in five patients (7%) were considered possibly related to zanubrutinib treatment—febrile neutropenia, colitis, atrial fibrillation, hemothorax, and pneumonia.
Nine patients (12%) discontinued study treatment due to AEs, but all of these events were considered unrelated to treatment. The AEs (n=1 for each) included abdominal sepsis (fatal), gastric adenocarcinoma (fatal), septic shoulder, worsening bronchiectasis, scedosporium infection, prostate adenocarcinoma, metastatic neuroendocrine carcinoma, acute myeloid leukemia, and breast cancer.
Atrial fibrillation/flutter occurred in four patients (5%), and major hemorrhage was observed in two patients (3%).
“We are encouraged that additional data on zanubrutinib in patients with WM confirms the initially reported experience, with consistent demonstration of robust activity and good tolerability,” Dr. Tam said.
“We are hopeful that zanubrutinib, if approved, could potentially provide an important new treatment option to patients with WM and other hematologic malignancies.”
Dr. Tam reported financial relationships with BeiGene and other companies.
NEW YORK—The BTK inhibitor zanubrutinib has demonstrated “robust activity” and “good tolerability” in patients with Waldenström’s macroglobulinemia (WM), according to an investigator.
In a phase 1 trial, zanubrutinib produced an overall response rate (ORR) of 92%, and the estimated 12-month progression-free survival (PFS) rate was 89%.
Most adverse events (AEs) in this trial were grade 1 or 2 in severity, although the incidence of serious AEs was 42%.
Constantine Tam, MD, of the Peter MacCallum Cancer Center in Victoria, Australia, presented these results at the 10th International Workshop on Waldenström’s Macroglobulinemia.
The trial is sponsored by BeiGene, Ltd., the company developing zanubrutinib.
The trial (NCT02343120) includes patients with WM and other B-cell malignancies. As of July 24, 2018, 77 patients with treatment-naïve or relapsed/refractory WM had been enrolled.
Seventy-three patients were evaluable for efficacy in this analysis, and the median follow-up time was 22.5 months (range, 4.1-43.9).
At the time of the data cutoff, 62 patients remained on study treatment. Four patients (3%) discontinued treatment due to disease progression, and one patient remains on treatment post-progression.
Efficacy
The median time to response was 85 days (range, 55-749).
The ORR was 92% (67/73), and the major response rate (MRR) was 82%. Forty-one percent of patients achieved a very good partial response (VGPR), defined as a greater than 90% reduction in baseline immunoglobulin M (IgM) levels and improvement of extramedullary disease by computed tomography.
The median IgM decreased from 32.7 g/L (range, 5.3-91.9) at baseline to 8.2 g/L (range, 0.3-57.8). The median hemoglobin increased from 8.85 g/dL (range, 6.3-9.8) to 13.4 g/dL (range, 7.7-17.0) among 32 patients with hemoglobin less than 10 g/dL at baseline.
MYD88 genotype was known in 63 patients. In the subset known to have the MYD88L265P mutation (n=54), the ORR was 94%, the MRR was 89%, and the VGPR rate was 46%.
In the nine patients known to have wild-type MYD88 (a genotype that, historically, has had sub-optimal response to BTK inhibition), the ORR was 89%, the MRR was 67%, and the VGPR rate was 22%.
The 12-month PFS was estimated to be 89%, and the median PFS had not been reached.
Safety
The most frequent AEs of any attribution were petechiae/purpura/contusion (43%), upper respiratory tract infection (42%), cough (17%), diarrhea (17%), constipation (16%), back pain (16%), and headache (16%).
Grade 3-4 AEs of any attribution reported in three or more patients included neutropenia (9%), anemia (7%), hypertension (5%), basal cell carcinoma (5%), renal and urinary disorders (4%), and pneumonia (4%).
Serious AEs were seen in 32 patients (42%). Events in five patients (7%) were considered possibly related to zanubrutinib treatment—febrile neutropenia, colitis, atrial fibrillation, hemothorax, and pneumonia.
Nine patients (12%) discontinued study treatment due to AEs, but all of these events were considered unrelated to treatment. The AEs (n=1 for each) included abdominal sepsis (fatal), gastric adenocarcinoma (fatal), septic shoulder, worsening bronchiectasis, scedosporium infection, prostate adenocarcinoma, metastatic neuroendocrine carcinoma, acute myeloid leukemia, and breast cancer.
Atrial fibrillation/flutter occurred in four patients (5%), and major hemorrhage was observed in two patients (3%).
“We are encouraged that additional data on zanubrutinib in patients with WM confirms the initially reported experience, with consistent demonstration of robust activity and good tolerability,” Dr. Tam said.
“We are hopeful that zanubrutinib, if approved, could potentially provide an important new treatment option to patients with WM and other hematologic malignancies.”
Dr. Tam reported financial relationships with BeiGene and other companies.
sNDA gets priority review for CLL/SLL
The U.S. Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®).
With this sNDA, Pharmacyclics LLC (an AbbVie company) and Janssen Biotech, Inc., are seeking approval for ibrutinib in combination with obinutuzumab (Gazyva®) in previously untreated adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
Ibrutinib is already FDA-approved as monotherapy for adults with CLL/SLL (previously treated or untreated), with and without 17p deletion. Ibrutinib is also approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
Obinutuzumab is FDA-approved for use in combination with chlorambucil to treat previously untreated CLL.
The sNDA for ibrutinib in combination with obinutuzumab is based on results from the phase 3 iLLUMINATE trial (NCT02264574).
The trial is a comparison of ibrutinib plus obinutuzumab and chlorambucil plus obinutuzumab in patients with previously untreated CLL/SLL.
In May, AbbVie announced that the trial’s primary endpoint was met. Specifically, ibrutinib plus obinutuzumab was associated with significantly longer progression-free survival than chlorambucil plus obinutuzumab.
Data from the trial have not been released. Pharmacyclics and Janssen said they plan to present the data in a future publication or at a medical congress.
The U.S. Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®).
With this sNDA, Pharmacyclics LLC (an AbbVie company) and Janssen Biotech, Inc., are seeking approval for ibrutinib in combination with obinutuzumab (Gazyva®) in previously untreated adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
Ibrutinib is already FDA-approved as monotherapy for adults with CLL/SLL (previously treated or untreated), with and without 17p deletion. Ibrutinib is also approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
Obinutuzumab is FDA-approved for use in combination with chlorambucil to treat previously untreated CLL.
The sNDA for ibrutinib in combination with obinutuzumab is based on results from the phase 3 iLLUMINATE trial (NCT02264574).
The trial is a comparison of ibrutinib plus obinutuzumab and chlorambucil plus obinutuzumab in patients with previously untreated CLL/SLL.
In May, AbbVie announced that the trial’s primary endpoint was met. Specifically, ibrutinib plus obinutuzumab was associated with significantly longer progression-free survival than chlorambucil plus obinutuzumab.
Data from the trial have not been released. Pharmacyclics and Janssen said they plan to present the data in a future publication or at a medical congress.
The U.S. Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®).
With this sNDA, Pharmacyclics LLC (an AbbVie company) and Janssen Biotech, Inc., are seeking approval for ibrutinib in combination with obinutuzumab (Gazyva®) in previously untreated adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency intends to take action on a priority review application within 6 months of receiving it rather than the standard 10 months.
Ibrutinib is already FDA-approved as monotherapy for adults with CLL/SLL (previously treated or untreated), with and without 17p deletion. Ibrutinib is also approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
Obinutuzumab is FDA-approved for use in combination with chlorambucil to treat previously untreated CLL.
The sNDA for ibrutinib in combination with obinutuzumab is based on results from the phase 3 iLLUMINATE trial (NCT02264574).
The trial is a comparison of ibrutinib plus obinutuzumab and chlorambucil plus obinutuzumab in patients with previously untreated CLL/SLL.
In May, AbbVie announced that the trial’s primary endpoint was met. Specifically, ibrutinib plus obinutuzumab was associated with significantly longer progression-free survival than chlorambucil plus obinutuzumab.
Data from the trial have not been released. Pharmacyclics and Janssen said they plan to present the data in a future publication or at a medical congress.
Transfusion errors more common in kids than adults, study suggests
BOSTON—Even the most vigilant hospitals experience transfusion errors and problems with blood storage, according to researchers.
A review of data from 32 U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems than adult transfusions, with errors differing by age group.
The most common error in the pediatric population was failure to follow protocol, and the most common error in the adult population was that scheduled transfusions were not performed.
Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital in New York, described these findings at AABB 2018 (abstract QT4).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues reviewed data on events reported by three children’s hospitals and 29 adult hospitals. Events were reported to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
The researchers identified a total of 1,806 reports associated with approximately 1,088,884 transfusions.
There were 249 reports associated with 99,064 pediatric transfusions and 1,577 reports associated with 989,820 adult transfusions. So the reporting rate was 251 per 100,000 transfusions for the pediatric population and 157 per 100,000 transfusions for the adult population (P<0.001).
The most common error for pediatric patients—failure to follow the transfusion protocol—made up 31% of the pediatric errors.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West,” Dr. Vossoughi said. “People say, ‘Well, I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas, on the adult side, [clinicians] seem to be much less likely to just deviate from the protocol.”
Among adults, the most common error was “transfusion not performed,” which accounted for 43% of the adult errors. Dr. Vossoughi said transfusions may be skipped due to a bungled patient hand-off during a shift change or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC [complete blood count] and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
She and her colleagues also found that 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
“It’s very common for blood banks to find platelets in the refrigerator,” Dr. Vossoughi said. “It doesn’t matter how old you are or what type of hospital you’re at. Everyone’s putting platelets in the fridge.”
Dr. Vossoughi and her colleagues believe these findings could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow or make the checklist less cumbersome, and then maybe they’ll follow them more often,” Dr. Vossoughi said.
This research was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The researchers reported no conflicts of interest.
BOSTON—Even the most vigilant hospitals experience transfusion errors and problems with blood storage, according to researchers.
A review of data from 32 U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems than adult transfusions, with errors differing by age group.
The most common error in the pediatric population was failure to follow protocol, and the most common error in the adult population was that scheduled transfusions were not performed.
Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital in New York, described these findings at AABB 2018 (abstract QT4).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues reviewed data on events reported by three children’s hospitals and 29 adult hospitals. Events were reported to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
The researchers identified a total of 1,806 reports associated with approximately 1,088,884 transfusions.
There were 249 reports associated with 99,064 pediatric transfusions and 1,577 reports associated with 989,820 adult transfusions. So the reporting rate was 251 per 100,000 transfusions for the pediatric population and 157 per 100,000 transfusions for the adult population (P<0.001).
The most common error for pediatric patients—failure to follow the transfusion protocol—made up 31% of the pediatric errors.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West,” Dr. Vossoughi said. “People say, ‘Well, I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas, on the adult side, [clinicians] seem to be much less likely to just deviate from the protocol.”
Among adults, the most common error was “transfusion not performed,” which accounted for 43% of the adult errors. Dr. Vossoughi said transfusions may be skipped due to a bungled patient hand-off during a shift change or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC [complete blood count] and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
She and her colleagues also found that 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
“It’s very common for blood banks to find platelets in the refrigerator,” Dr. Vossoughi said. “It doesn’t matter how old you are or what type of hospital you’re at. Everyone’s putting platelets in the fridge.”
Dr. Vossoughi and her colleagues believe these findings could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow or make the checklist less cumbersome, and then maybe they’ll follow them more often,” Dr. Vossoughi said.
This research was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The researchers reported no conflicts of interest.
BOSTON—Even the most vigilant hospitals experience transfusion errors and problems with blood storage, according to researchers.
A review of data from 32 U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems than adult transfusions, with errors differing by age group.
The most common error in the pediatric population was failure to follow protocol, and the most common error in the adult population was that scheduled transfusions were not performed.
Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital in New York, described these findings at AABB 2018 (abstract QT4).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues reviewed data on events reported by three children’s hospitals and 29 adult hospitals. Events were reported to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
The researchers identified a total of 1,806 reports associated with approximately 1,088,884 transfusions.
There were 249 reports associated with 99,064 pediatric transfusions and 1,577 reports associated with 989,820 adult transfusions. So the reporting rate was 251 per 100,000 transfusions for the pediatric population and 157 per 100,000 transfusions for the adult population (P<0.001).
The most common error for pediatric patients—failure to follow the transfusion protocol—made up 31% of the pediatric errors.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West,” Dr. Vossoughi said. “People say, ‘Well, I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas, on the adult side, [clinicians] seem to be much less likely to just deviate from the protocol.”
Among adults, the most common error was “transfusion not performed,” which accounted for 43% of the adult errors. Dr. Vossoughi said transfusions may be skipped due to a bungled patient hand-off during a shift change or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC [complete blood count] and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
She and her colleagues also found that 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
“It’s very common for blood banks to find platelets in the refrigerator,” Dr. Vossoughi said. “It doesn’t matter how old you are or what type of hospital you’re at. Everyone’s putting platelets in the fridge.”
Dr. Vossoughi and her colleagues believe these findings could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow or make the checklist less cumbersome, and then maybe they’ll follow them more often,” Dr. Vossoughi said.
This research was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The researchers reported no conflicts of interest.
Understanding the role of HSCT in PTCL
DUBROVNIK, CROATIA—Hematopoietic stem cell transplant (HSCT) can be hit-or-miss in patients with peripheral T-cell lymphomas (PTCLs), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Ali Bazarbachi, MD, PhD, of the American University of Beirut in Lebanon, noted that the success of HSCT varies according to the subtype of PTCL and the type of transplant.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL-not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with non-localized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Dr. Bazarbachi began his presentation by pointing out that patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable—localized ENKTL and ALK-positive ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
HSCT in PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 20121, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival—compared to previous results with CHOP2—in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma.
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients—those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study3 showed improved survival in patients who received consolidation with auto- or allo-HSCT, as compared to patients who did not receive a transplant.
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Dr. Bazarbachi noted that allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL.
However, chemosensitive patients who have relapsed should only receive auto-HSCT if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
HSCT in ATLL
Dr. Bazarbachi noted that ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative.4,5 It is most effective in patients who have achieved a complete or partial response to induction.
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time—as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 20145, all four ATLL patients who underwent auto-HSCT “rapidly” died.
HSCT in ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients6, and it is most effective when patients have achieved a complete response to induction.
Allo-HSCT is also an option for frontline consolidation in patients with non-localized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should only receive auto-HSCT if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, non-localized ENKTL, nasal type should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
He did not declare any conflicts of interest.
1. d’Amore F et al. J Clin Oncol. 2012 Sep 1;30(25):3093-9. doi: 10.1200/JCO.2011.40.2719
2. AbouYabis AN et al. ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924
3. Park SI et al. Blood 2017 130:342
4. Ishida T et al. Blood 2012 Aug 23;120(8):1734-41. doi: 10.1182/blood-2012-03-414490
5. Bazarbachi A et al. Bone Marrow Transplant. 2014 Oct;49(10):1266-8. doi: 10.1038/bmt.2014.143
6. Lee J et al. Biol Blood Marrow Transplant. 2008 Dec;14(12):1356-64. doi: 10.1016/j.bbmt.2008.09.014
DUBROVNIK, CROATIA—Hematopoietic stem cell transplant (HSCT) can be hit-or-miss in patients with peripheral T-cell lymphomas (PTCLs), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Ali Bazarbachi, MD, PhD, of the American University of Beirut in Lebanon, noted that the success of HSCT varies according to the subtype of PTCL and the type of transplant.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL-not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with non-localized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Dr. Bazarbachi began his presentation by pointing out that patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable—localized ENKTL and ALK-positive ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
HSCT in PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 20121, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival—compared to previous results with CHOP2—in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma.
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients—those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study3 showed improved survival in patients who received consolidation with auto- or allo-HSCT, as compared to patients who did not receive a transplant.
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Dr. Bazarbachi noted that allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL.
However, chemosensitive patients who have relapsed should only receive auto-HSCT if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
HSCT in ATLL
Dr. Bazarbachi noted that ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative.4,5 It is most effective in patients who have achieved a complete or partial response to induction.
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time—as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 20145, all four ATLL patients who underwent auto-HSCT “rapidly” died.
HSCT in ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients6, and it is most effective when patients have achieved a complete response to induction.
Allo-HSCT is also an option for frontline consolidation in patients with non-localized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should only receive auto-HSCT if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, non-localized ENKTL, nasal type should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
He did not declare any conflicts of interest.
1. d’Amore F et al. J Clin Oncol. 2012 Sep 1;30(25):3093-9. doi: 10.1200/JCO.2011.40.2719
2. AbouYabis AN et al. ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924
3. Park SI et al. Blood 2017 130:342
4. Ishida T et al. Blood 2012 Aug 23;120(8):1734-41. doi: 10.1182/blood-2012-03-414490
5. Bazarbachi A et al. Bone Marrow Transplant. 2014 Oct;49(10):1266-8. doi: 10.1038/bmt.2014.143
6. Lee J et al. Biol Blood Marrow Transplant. 2008 Dec;14(12):1356-64. doi: 10.1016/j.bbmt.2008.09.014
DUBROVNIK, CROATIA—Hematopoietic stem cell transplant (HSCT) can be hit-or-miss in patients with peripheral T-cell lymphomas (PTCLs), according to a speaker at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Ali Bazarbachi, MD, PhD, of the American University of Beirut in Lebanon, noted that the success of HSCT varies according to the subtype of PTCL and the type of transplant.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL-not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with non-localized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Dr. Bazarbachi began his presentation by pointing out that patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable—localized ENKTL and ALK-positive ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
HSCT in PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 20121, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival—compared to previous results with CHOP2—in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma.
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients—those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study3 showed improved survival in patients who received consolidation with auto- or allo-HSCT, as compared to patients who did not receive a transplant.
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Dr. Bazarbachi noted that allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL.
However, chemosensitive patients who have relapsed should only receive auto-HSCT if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
HSCT in ATLL
Dr. Bazarbachi noted that ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative.4,5 It is most effective in patients who have achieved a complete or partial response to induction.
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time—as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 20145, all four ATLL patients who underwent auto-HSCT “rapidly” died.
HSCT in ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients6, and it is most effective when patients have achieved a complete response to induction.
Allo-HSCT is also an option for frontline consolidation in patients with non-localized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should only receive auto-HSCT if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, non-localized ENKTL, nasal type should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
He did not declare any conflicts of interest.
1. d’Amore F et al. J Clin Oncol. 2012 Sep 1;30(25):3093-9. doi: 10.1200/JCO.2011.40.2719
2. AbouYabis AN et al. ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924
3. Park SI et al. Blood 2017 130:342
4. Ishida T et al. Blood 2012 Aug 23;120(8):1734-41. doi: 10.1182/blood-2012-03-414490
5. Bazarbachi A et al. Bone Marrow Transplant. 2014 Oct;49(10):1266-8. doi: 10.1038/bmt.2014.143
6. Lee J et al. Biol Blood Marrow Transplant. 2008 Dec;14(12):1356-64. doi: 10.1016/j.bbmt.2008.09.014