User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


More Side Effects With Local Therapies for Prostate Cancer
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Colorectal Cancer Risk Increasing Across Successive Birth Cohorts
Colorectal cancer (CRC) epidemiology is changing due to a birth cohort effect, also called birth cohort CRC — the observed phenomena of the rising risk for CRC across successive generations of people born in 1960 and later — according to a new narrative review.
Birth cohort CRC is associated with increasing rectal cancer (greater than colon cancer) diagnosis and distant-stage (greater than local-stage) CRC diagnosis, and a rising incidence of early-onset CRC (EOCRC), defined as occurring before age 50.
Recognizing this birth cohort effect could improve the understanding of CRC risk factors, etiology, mechanisms, as well as the public health consequences of rising rates.
“The changing epidemiology means that we need to redouble our efforts at optimizing early detection and prevention of colorectal cancer,” Samir Gupta, MD, the review’s lead author and professor of gastroenterology at the University of California, San Diego, California, told this news organization. Dr. Gupta serves as the co-lead for the cancer control program at Moores Cancer Center at UC San Diego Health.
This requires “being alert for potential red flag signs and symptoms of colorectal cancer, such as iron deficiency anemia and rectal bleeding, that are otherwise unexplained, including for those under age 45,” he said.
We also should make “sure that all people eligible for screening — at age 45 and older — have every opportunity to get screened for colorectal cancer,” Dr. Gupta added.
The review was published online in Clinical Gastroenterology and Hepatology.
Tracking Birth Cohort Trends
CRC rates have increased in the United States among people born since the early 1960s, the authors wrote.
Generation X (individuals born in 1965-1980) experienced an increase in EOCRC, and rates subsequently increased in this generation after age 50. Rates are 1.22-fold higher among people born in 1965-1969 and 1.58-fold higher among those born 1975-1979 than among people born in 1950-1954.
Now rates are also increasing across younger generations, particularly among Millennials (individuals born in 1981-1996) as they enter mid-adulthood. Incidence rates are 1.89-fold higher among people born in 1980-1984 and 2.98-fold higher among those born in 1990-1994 than among individuals born in 1950-1954.
These birth cohort effects are evident globally, despite differences in population age structures, screening programs, and diagnostic strategies around the world. Due to this ongoing trend, physicians anticipate that CRC rates will likely continue to increase as higher-risk birth cohorts become older, the authors wrote.
Notably, four important shifts in CRC incidence are apparent, they noted. First, rates are steadily increasing up to age 50 and plateauing after age 60. Rectal cancers are now predominant through ages 50-59. Rates of distant-stage disease have increased most rapidly among ages 30-49 and more slowly decreased among ages 60-79 compared with those of local-stage disease. In addition, the increasing rates of EOCRC have been observed across all racial and ethnic groups since the early 1990s.
These shifts led to major changes in the types of patients diagnosed with CRC now vs 30 years ago, with a higher proportion being patients younger than 60, as well as Black, Asian or Pacific Islander, American Indian/Alaska Native, and Hispanic patients.
The combination of age-related increases in CRC and birth cohort–related trends will likely lead to substantial increases in the number of people diagnosed with CRC in coming years, especially as Generation X patients move into their 50s and 60s, the authors wrote.
Research and Clinical Implications
Birth cohort CRC, including increasing EOCRC incidence, likely is driven by a range of influences, including demographic, lifestyle, early life, environmental, genetic, and somatic factors, as well as interactions among them, the authors noted. Examples within these broad categories include male sex, food insecurity, income inequality, diabetes, alcohol use, less healthy dietary patterns, in utero exposure to certain medications, and microbiome concerns such as early life antibiotic exposure or dysbiosis.
“From a research perspective, this means that we need to think about risk factors and mechanisms that are associated with birth cohorts, not just age at diagnosis,” Dr. Gupta said. “To date, most studies of changing epidemiology have not taken into account birth cohort, such as whether someone is Generation X or later versus pre-Baby Boomer.”
Although additional research is needed, the epidemiology changes have several immediate clinical implications, Dr. Gupta said. For those younger than 45, it is critical to raise awareness about the signs and symptoms of CRC, such as hematochezia, iron deficiency anemia, and unintentional weight loss, as well as family history.
For ages 45 and older, a major focus should be placed on increasing screening participation and follow-up after abnormal results, addressing disparities in screening participation, and optimizing screening quality.
In addition, as CRC incidence continues to increase, health systems and policymakers should ensure every patient has access to guideline-appropriate care and innovative clinical trials, the authors wrote. This access may be particularly important to address the increasing burden of rectal cancer, as treatment approaches rapidly evolve toward more effective therapies, such as neoadjuvant chemotherapy and radiation prior to surgery, and with less-morbid treatments on the horizon, they added.
‘An Interesting Concept’
“Birth cohort CRC is an interesting concept that allows people to think of their CRC risk according to their birth cohort in addition to age,” Shuji Ogino, MD, PhD, chief of the Molecular Pathological Epidemiology program at Brigham & Women’s Hospital, Boston, Massachusetts, told this news organization.
Dr. Ogino, who wasn’t involved with this study, serves as a member of the cancer immunology and cancer epidemiology programs at the Dana-Farber Harvard Cancer Center. In studies of EOCRC, he and colleagues have found various biogeographical and pathogenic trends across age groups.
“More research is needed to disentangle the complex etiologies of birth cohort CRC and early-onset CRC,” Dr. Ogino said. “Tumor cells and tissues have certain past and ongoing pathological marks, which we can detect to better understand birth cohort CRC and early-onset CRC.”
The study was funded by several National Institutes of Health/National Cancer Institute grants. Dr. Gupta disclosed consulting for Geneoscopy, Guardant Health, Universal Diagnostics, InterVenn Bio, and CellMax. Another author reported consulting for Freenome, Exact Sciences, Medtronic, and Geneoscopy. Dr. Ogino reported no relevant financial disclosures.
A version of this article appeared on Medscape.com .
Colorectal cancer (CRC) epidemiology is changing due to a birth cohort effect, also called birth cohort CRC — the observed phenomena of the rising risk for CRC across successive generations of people born in 1960 and later — according to a new narrative review.
Birth cohort CRC is associated with increasing rectal cancer (greater than colon cancer) diagnosis and distant-stage (greater than local-stage) CRC diagnosis, and a rising incidence of early-onset CRC (EOCRC), defined as occurring before age 50.
Recognizing this birth cohort effect could improve the understanding of CRC risk factors, etiology, mechanisms, as well as the public health consequences of rising rates.
“The changing epidemiology means that we need to redouble our efforts at optimizing early detection and prevention of colorectal cancer,” Samir Gupta, MD, the review’s lead author and professor of gastroenterology at the University of California, San Diego, California, told this news organization. Dr. Gupta serves as the co-lead for the cancer control program at Moores Cancer Center at UC San Diego Health.
This requires “being alert for potential red flag signs and symptoms of colorectal cancer, such as iron deficiency anemia and rectal bleeding, that are otherwise unexplained, including for those under age 45,” he said.
We also should make “sure that all people eligible for screening — at age 45 and older — have every opportunity to get screened for colorectal cancer,” Dr. Gupta added.
The review was published online in Clinical Gastroenterology and Hepatology.
Tracking Birth Cohort Trends
CRC rates have increased in the United States among people born since the early 1960s, the authors wrote.
Generation X (individuals born in 1965-1980) experienced an increase in EOCRC, and rates subsequently increased in this generation after age 50. Rates are 1.22-fold higher among people born in 1965-1969 and 1.58-fold higher among those born 1975-1979 than among people born in 1950-1954.
Now rates are also increasing across younger generations, particularly among Millennials (individuals born in 1981-1996) as they enter mid-adulthood. Incidence rates are 1.89-fold higher among people born in 1980-1984 and 2.98-fold higher among those born in 1990-1994 than among individuals born in 1950-1954.
These birth cohort effects are evident globally, despite differences in population age structures, screening programs, and diagnostic strategies around the world. Due to this ongoing trend, physicians anticipate that CRC rates will likely continue to increase as higher-risk birth cohorts become older, the authors wrote.
Notably, four important shifts in CRC incidence are apparent, they noted. First, rates are steadily increasing up to age 50 and plateauing after age 60. Rectal cancers are now predominant through ages 50-59. Rates of distant-stage disease have increased most rapidly among ages 30-49 and more slowly decreased among ages 60-79 compared with those of local-stage disease. In addition, the increasing rates of EOCRC have been observed across all racial and ethnic groups since the early 1990s.
These shifts led to major changes in the types of patients diagnosed with CRC now vs 30 years ago, with a higher proportion being patients younger than 60, as well as Black, Asian or Pacific Islander, American Indian/Alaska Native, and Hispanic patients.
The combination of age-related increases in CRC and birth cohort–related trends will likely lead to substantial increases in the number of people diagnosed with CRC in coming years, especially as Generation X patients move into their 50s and 60s, the authors wrote.
Research and Clinical Implications
Birth cohort CRC, including increasing EOCRC incidence, likely is driven by a range of influences, including demographic, lifestyle, early life, environmental, genetic, and somatic factors, as well as interactions among them, the authors noted. Examples within these broad categories include male sex, food insecurity, income inequality, diabetes, alcohol use, less healthy dietary patterns, in utero exposure to certain medications, and microbiome concerns such as early life antibiotic exposure or dysbiosis.
“From a research perspective, this means that we need to think about risk factors and mechanisms that are associated with birth cohorts, not just age at diagnosis,” Dr. Gupta said. “To date, most studies of changing epidemiology have not taken into account birth cohort, such as whether someone is Generation X or later versus pre-Baby Boomer.”
Although additional research is needed, the epidemiology changes have several immediate clinical implications, Dr. Gupta said. For those younger than 45, it is critical to raise awareness about the signs and symptoms of CRC, such as hematochezia, iron deficiency anemia, and unintentional weight loss, as well as family history.
For ages 45 and older, a major focus should be placed on increasing screening participation and follow-up after abnormal results, addressing disparities in screening participation, and optimizing screening quality.
In addition, as CRC incidence continues to increase, health systems and policymakers should ensure every patient has access to guideline-appropriate care and innovative clinical trials, the authors wrote. This access may be particularly important to address the increasing burden of rectal cancer, as treatment approaches rapidly evolve toward more effective therapies, such as neoadjuvant chemotherapy and radiation prior to surgery, and with less-morbid treatments on the horizon, they added.
‘An Interesting Concept’
“Birth cohort CRC is an interesting concept that allows people to think of their CRC risk according to their birth cohort in addition to age,” Shuji Ogino, MD, PhD, chief of the Molecular Pathological Epidemiology program at Brigham & Women’s Hospital, Boston, Massachusetts, told this news organization.
Dr. Ogino, who wasn’t involved with this study, serves as a member of the cancer immunology and cancer epidemiology programs at the Dana-Farber Harvard Cancer Center. In studies of EOCRC, he and colleagues have found various biogeographical and pathogenic trends across age groups.
“More research is needed to disentangle the complex etiologies of birth cohort CRC and early-onset CRC,” Dr. Ogino said. “Tumor cells and tissues have certain past and ongoing pathological marks, which we can detect to better understand birth cohort CRC and early-onset CRC.”
The study was funded by several National Institutes of Health/National Cancer Institute grants. Dr. Gupta disclosed consulting for Geneoscopy, Guardant Health, Universal Diagnostics, InterVenn Bio, and CellMax. Another author reported consulting for Freenome, Exact Sciences, Medtronic, and Geneoscopy. Dr. Ogino reported no relevant financial disclosures.
A version of this article appeared on Medscape.com .
Colorectal cancer (CRC) epidemiology is changing due to a birth cohort effect, also called birth cohort CRC — the observed phenomena of the rising risk for CRC across successive generations of people born in 1960 and later — according to a new narrative review.
Birth cohort CRC is associated with increasing rectal cancer (greater than colon cancer) diagnosis and distant-stage (greater than local-stage) CRC diagnosis, and a rising incidence of early-onset CRC (EOCRC), defined as occurring before age 50.
Recognizing this birth cohort effect could improve the understanding of CRC risk factors, etiology, mechanisms, as well as the public health consequences of rising rates.
“The changing epidemiology means that we need to redouble our efforts at optimizing early detection and prevention of colorectal cancer,” Samir Gupta, MD, the review’s lead author and professor of gastroenterology at the University of California, San Diego, California, told this news organization. Dr. Gupta serves as the co-lead for the cancer control program at Moores Cancer Center at UC San Diego Health.
This requires “being alert for potential red flag signs and symptoms of colorectal cancer, such as iron deficiency anemia and rectal bleeding, that are otherwise unexplained, including for those under age 45,” he said.
We also should make “sure that all people eligible for screening — at age 45 and older — have every opportunity to get screened for colorectal cancer,” Dr. Gupta added.
The review was published online in Clinical Gastroenterology and Hepatology.
Tracking Birth Cohort Trends
CRC rates have increased in the United States among people born since the early 1960s, the authors wrote.
Generation X (individuals born in 1965-1980) experienced an increase in EOCRC, and rates subsequently increased in this generation after age 50. Rates are 1.22-fold higher among people born in 1965-1969 and 1.58-fold higher among those born 1975-1979 than among people born in 1950-1954.
Now rates are also increasing across younger generations, particularly among Millennials (individuals born in 1981-1996) as they enter mid-adulthood. Incidence rates are 1.89-fold higher among people born in 1980-1984 and 2.98-fold higher among those born in 1990-1994 than among individuals born in 1950-1954.
These birth cohort effects are evident globally, despite differences in population age structures, screening programs, and diagnostic strategies around the world. Due to this ongoing trend, physicians anticipate that CRC rates will likely continue to increase as higher-risk birth cohorts become older, the authors wrote.
Notably, four important shifts in CRC incidence are apparent, they noted. First, rates are steadily increasing up to age 50 and plateauing after age 60. Rectal cancers are now predominant through ages 50-59. Rates of distant-stage disease have increased most rapidly among ages 30-49 and more slowly decreased among ages 60-79 compared with those of local-stage disease. In addition, the increasing rates of EOCRC have been observed across all racial and ethnic groups since the early 1990s.
These shifts led to major changes in the types of patients diagnosed with CRC now vs 30 years ago, with a higher proportion being patients younger than 60, as well as Black, Asian or Pacific Islander, American Indian/Alaska Native, and Hispanic patients.
The combination of age-related increases in CRC and birth cohort–related trends will likely lead to substantial increases in the number of people diagnosed with CRC in coming years, especially as Generation X patients move into their 50s and 60s, the authors wrote.
Research and Clinical Implications
Birth cohort CRC, including increasing EOCRC incidence, likely is driven by a range of influences, including demographic, lifestyle, early life, environmental, genetic, and somatic factors, as well as interactions among them, the authors noted. Examples within these broad categories include male sex, food insecurity, income inequality, diabetes, alcohol use, less healthy dietary patterns, in utero exposure to certain medications, and microbiome concerns such as early life antibiotic exposure or dysbiosis.
“From a research perspective, this means that we need to think about risk factors and mechanisms that are associated with birth cohorts, not just age at diagnosis,” Dr. Gupta said. “To date, most studies of changing epidemiology have not taken into account birth cohort, such as whether someone is Generation X or later versus pre-Baby Boomer.”
Although additional research is needed, the epidemiology changes have several immediate clinical implications, Dr. Gupta said. For those younger than 45, it is critical to raise awareness about the signs and symptoms of CRC, such as hematochezia, iron deficiency anemia, and unintentional weight loss, as well as family history.
For ages 45 and older, a major focus should be placed on increasing screening participation and follow-up after abnormal results, addressing disparities in screening participation, and optimizing screening quality.
In addition, as CRC incidence continues to increase, health systems and policymakers should ensure every patient has access to guideline-appropriate care and innovative clinical trials, the authors wrote. This access may be particularly important to address the increasing burden of rectal cancer, as treatment approaches rapidly evolve toward more effective therapies, such as neoadjuvant chemotherapy and radiation prior to surgery, and with less-morbid treatments on the horizon, they added.
‘An Interesting Concept’
“Birth cohort CRC is an interesting concept that allows people to think of their CRC risk according to their birth cohort in addition to age,” Shuji Ogino, MD, PhD, chief of the Molecular Pathological Epidemiology program at Brigham & Women’s Hospital, Boston, Massachusetts, told this news organization.
Dr. Ogino, who wasn’t involved with this study, serves as a member of the cancer immunology and cancer epidemiology programs at the Dana-Farber Harvard Cancer Center. In studies of EOCRC, he and colleagues have found various biogeographical and pathogenic trends across age groups.
“More research is needed to disentangle the complex etiologies of birth cohort CRC and early-onset CRC,” Dr. Ogino said. “Tumor cells and tissues have certain past and ongoing pathological marks, which we can detect to better understand birth cohort CRC and early-onset CRC.”
The study was funded by several National Institutes of Health/National Cancer Institute grants. Dr. Gupta disclosed consulting for Geneoscopy, Guardant Health, Universal Diagnostics, InterVenn Bio, and CellMax. Another author reported consulting for Freenome, Exact Sciences, Medtronic, and Geneoscopy. Dr. Ogino reported no relevant financial disclosures.
A version of this article appeared on Medscape.com .
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
New Guidelines: Start PSA Screening Earlier in Black Men
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, baseline PSA testing in Black men should begin at age 40-45, sooner than current guidelines recommend, and should be followed by regular screening intervals, preferably annually, at least until age 70, a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues wrote. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Taskforce recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” Dr. Oh added.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Dr. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, baseline PSA testing in Black men should begin at age 40-45, sooner than current guidelines recommend, and should be followed by regular screening intervals, preferably annually, at least until age 70, a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues wrote. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Taskforce recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” Dr. Oh added.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Dr. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, baseline PSA testing in Black men should begin at age 40-45, sooner than current guidelines recommend, and should be followed by regular screening intervals, preferably annually, at least until age 70, a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues wrote. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Taskforce recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” Dr. Oh added.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Dr. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
FROM ASCO GU 2024
CT Poses Risk for Malignant Hematopathies Among Children
More than a million European children undergo a CT scan each year. Ionizing radiation at moderate (> 100 mGy) to high (> 1 Gy) doses is a recognized risk factor for malignant hematopathies. The risk associated with exposure to low doses (< 100 mGy), typically delivered during a CT scan in children or adolescents, is unknown.
Previous studies assessed the risk for malignant hematopathies related to ionizing radiation from CT scans in young patients. Some showed an increased risk for leukemia with repeated scans, but confounding factors resulted in a lack of statistical power or biases in some cases. The EPI-CT study, coordinated by the International Agency for Research on Cancer, aimed to evaluate the cancer risk among children and adolescents after exposure to low doses of ionizing radiation during CT scans.
A European Cohort
A recent article presents an assessment of observed malignant hematopathies following CT scan. The authors followed a multinational European cohort of 948,174 patients who had a CT scan before age 22 years. Ionizing radiation doses to the bone marrow were evaluated based on the scanned body region, patient characteristics, scan year, and the technical parameters of the machine. The analysis involved 876,771 patients who underwent 1,331,896 scans (an average of 1.52 per patient) and were followed for at least 2 years after the first scan.
In total, 790 malignant hematopathies were diagnosed, including 578 lymphoid hematopathies and 203 myeloid hematopathies and acute leukemias. The average follow-up period was 7.8 years. At the time of diagnosis, 51% of patients were under the age of 20 years, and 88.5% were under the age of 30 years. There was an association between cumulative dose and the observed malignant hematopathy, with an observed rate of 1.96 per 100 mGy (790 cases).
This rate corresponds to a 16% increased rate per scan (for a dose observed per scan of 8 mGy). A higher rate for any type of malignant hematopathy was observed for doses > 10 mGy, with an observed rate of 2.66 for doses > 50 mGy, compared with doses < 5 mGy.
The rate of malignant hematopathy increased with older age at the time of radiation exposure, particularly for lymphoid observations. The rate in the 5- to 9-year age group and the > 10-year age group was, respectively, two times and three to four times higher than that in the < 5-year age group. The rate decreased over time, with the highest observed rate between 2 and 5 years after ionizing radiation exposure and the lowest after 10 years.
CT Scans Must Be Warranted
This study, which involved nearly a million patients, has higher statistical power than previous studies, despite missing or approximate data (including that related to actually delivered doses). An association was shown between cumulative dose to the bone marrow and the risk of developing malignant hematopathy, both lymphoid and myeloid, with an increased risk even at low doses (10-15 mGy).
The results suggest that for every 10,000 children examined today (with a dose per scan of 8 mGy), 1-2 could develop a radiation-related malignant hematopathy in the next 12 years (1.4 cases). This study confirms the higher risk for cancer at low radiation doses and emphasizes the importance of justifying each pediatric CT scan and optimizing delivered doses. It is important to recall that an MRI or ultrasound can sometimes be an adequate substitute for a CT scan.
This article was translated from JIM , which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com .
More than a million European children undergo a CT scan each year. Ionizing radiation at moderate (> 100 mGy) to high (> 1 Gy) doses is a recognized risk factor for malignant hematopathies. The risk associated with exposure to low doses (< 100 mGy), typically delivered during a CT scan in children or adolescents, is unknown.
Previous studies assessed the risk for malignant hematopathies related to ionizing radiation from CT scans in young patients. Some showed an increased risk for leukemia with repeated scans, but confounding factors resulted in a lack of statistical power or biases in some cases. The EPI-CT study, coordinated by the International Agency for Research on Cancer, aimed to evaluate the cancer risk among children and adolescents after exposure to low doses of ionizing radiation during CT scans.
A European Cohort
A recent article presents an assessment of observed malignant hematopathies following CT scan. The authors followed a multinational European cohort of 948,174 patients who had a CT scan before age 22 years. Ionizing radiation doses to the bone marrow were evaluated based on the scanned body region, patient characteristics, scan year, and the technical parameters of the machine. The analysis involved 876,771 patients who underwent 1,331,896 scans (an average of 1.52 per patient) and were followed for at least 2 years after the first scan.
In total, 790 malignant hematopathies were diagnosed, including 578 lymphoid hematopathies and 203 myeloid hematopathies and acute leukemias. The average follow-up period was 7.8 years. At the time of diagnosis, 51% of patients were under the age of 20 years, and 88.5% were under the age of 30 years. There was an association between cumulative dose and the observed malignant hematopathy, with an observed rate of 1.96 per 100 mGy (790 cases).
This rate corresponds to a 16% increased rate per scan (for a dose observed per scan of 8 mGy). A higher rate for any type of malignant hematopathy was observed for doses > 10 mGy, with an observed rate of 2.66 for doses > 50 mGy, compared with doses < 5 mGy.
The rate of malignant hematopathy increased with older age at the time of radiation exposure, particularly for lymphoid observations. The rate in the 5- to 9-year age group and the > 10-year age group was, respectively, two times and three to four times higher than that in the < 5-year age group. The rate decreased over time, with the highest observed rate between 2 and 5 years after ionizing radiation exposure and the lowest after 10 years.
CT Scans Must Be Warranted
This study, which involved nearly a million patients, has higher statistical power than previous studies, despite missing or approximate data (including that related to actually delivered doses). An association was shown between cumulative dose to the bone marrow and the risk of developing malignant hematopathy, both lymphoid and myeloid, with an increased risk even at low doses (10-15 mGy).
The results suggest that for every 10,000 children examined today (with a dose per scan of 8 mGy), 1-2 could develop a radiation-related malignant hematopathy in the next 12 years (1.4 cases). This study confirms the higher risk for cancer at low radiation doses and emphasizes the importance of justifying each pediatric CT scan and optimizing delivered doses. It is important to recall that an MRI or ultrasound can sometimes be an adequate substitute for a CT scan.
This article was translated from JIM , which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com .
More than a million European children undergo a CT scan each year. Ionizing radiation at moderate (> 100 mGy) to high (> 1 Gy) doses is a recognized risk factor for malignant hematopathies. The risk associated with exposure to low doses (< 100 mGy), typically delivered during a CT scan in children or adolescents, is unknown.
Previous studies assessed the risk for malignant hematopathies related to ionizing radiation from CT scans in young patients. Some showed an increased risk for leukemia with repeated scans, but confounding factors resulted in a lack of statistical power or biases in some cases. The EPI-CT study, coordinated by the International Agency for Research on Cancer, aimed to evaluate the cancer risk among children and adolescents after exposure to low doses of ionizing radiation during CT scans.
A European Cohort
A recent article presents an assessment of observed malignant hematopathies following CT scan. The authors followed a multinational European cohort of 948,174 patients who had a CT scan before age 22 years. Ionizing radiation doses to the bone marrow were evaluated based on the scanned body region, patient characteristics, scan year, and the technical parameters of the machine. The analysis involved 876,771 patients who underwent 1,331,896 scans (an average of 1.52 per patient) and were followed for at least 2 years after the first scan.
In total, 790 malignant hematopathies were diagnosed, including 578 lymphoid hematopathies and 203 myeloid hematopathies and acute leukemias. The average follow-up period was 7.8 years. At the time of diagnosis, 51% of patients were under the age of 20 years, and 88.5% were under the age of 30 years. There was an association between cumulative dose and the observed malignant hematopathy, with an observed rate of 1.96 per 100 mGy (790 cases).
This rate corresponds to a 16% increased rate per scan (for a dose observed per scan of 8 mGy). A higher rate for any type of malignant hematopathy was observed for doses > 10 mGy, with an observed rate of 2.66 for doses > 50 mGy, compared with doses < 5 mGy.
The rate of malignant hematopathy increased with older age at the time of radiation exposure, particularly for lymphoid observations. The rate in the 5- to 9-year age group and the > 10-year age group was, respectively, two times and three to four times higher than that in the < 5-year age group. The rate decreased over time, with the highest observed rate between 2 and 5 years after ionizing radiation exposure and the lowest after 10 years.
CT Scans Must Be Warranted
This study, which involved nearly a million patients, has higher statistical power than previous studies, despite missing or approximate data (including that related to actually delivered doses). An association was shown between cumulative dose to the bone marrow and the risk of developing malignant hematopathy, both lymphoid and myeloid, with an increased risk even at low doses (10-15 mGy).
The results suggest that for every 10,000 children examined today (with a dose per scan of 8 mGy), 1-2 could develop a radiation-related malignant hematopathy in the next 12 years (1.4 cases). This study confirms the higher risk for cancer at low radiation doses and emphasizes the importance of justifying each pediatric CT scan and optimizing delivered doses. It is important to recall that an MRI or ultrasound can sometimes be an adequate substitute for a CT scan.
This article was translated from JIM , which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com .
Stockholm3 Prostate Test Bests PSA for Prostate Cancer Risk in North America
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
FROM ASCO GU 2024
More Young Women Being Diagnosed With Breast Cancer Than Ever Before
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.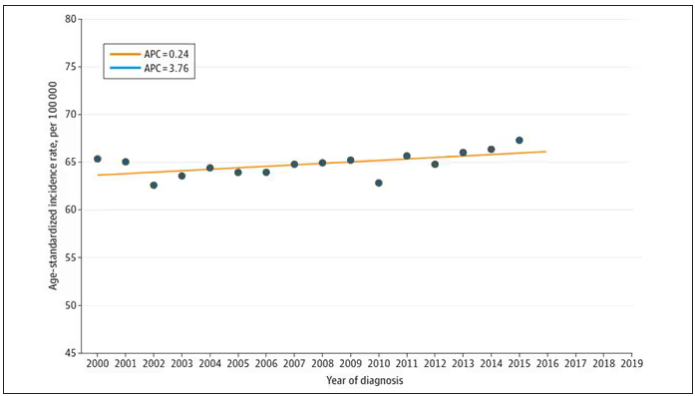
And then this happened: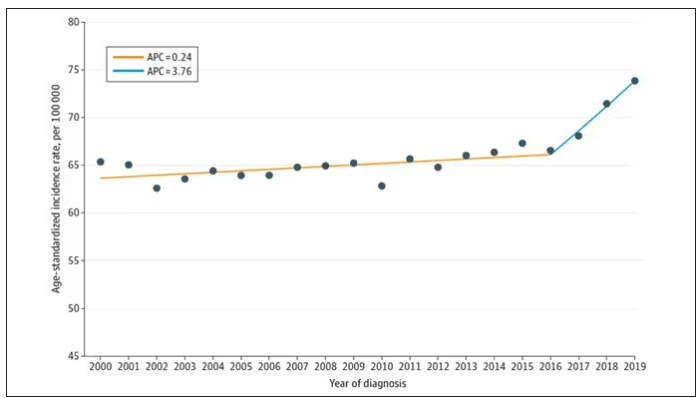
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.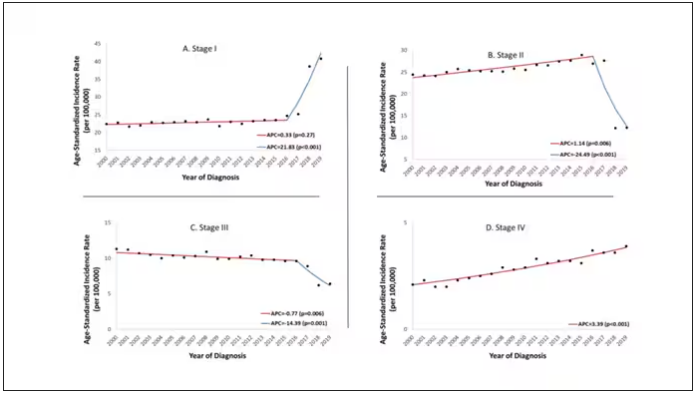
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.
And then this happened:
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.
And then this happened:
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Chemo-Free Maintenance Strategies May Boost Survival in TNBC
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
Treatment Sequence May Impact Pancreatic Cancer Survival
TOPLINE:
METHODOLOGY:
- Despite therapeutic advances, survival among patients with unresectable and/or metastatic pancreatic ductal adenocarcinoma has not markedly improved in recent years.
- In the current analysis, researchers evaluated whether treatment sequence could affect survival outcomes in this patient population.
- To this end , researchers conducted a single institution, retrospective analysis of patients who received different lines of treatment between January 2015 and December 2021.
- The most common first-line therapy was nab-paclitaxel plus S-1 (58%), followed by FOLFIRINOX (10%), nab-paclitaxel plus gemcitabine (8%), gemcitabine alone (7%), gemcitabine plus oxaliplatin (6%); second-line therapies, in order of frequency, included gemcitabine combination therapy (48%), nab-paclitaxel combination therapy (19%), FOLFIRINOX (10%), and gemcitabine alone (7%); third-line treatments consisted of FOLFIRINOX (31%), irinotecan or oxaliplatin combination therapy (23%), immunotherapy (19%), and gemcitabine combination therapy (10%).
TAKEAWAY:
- Overall, progression occurred in 90% of patients, and the median overall survival was 12.0 months, with only 48% of patients able to start a third-line therapy.
- The researchers focused on three common therapy sequences: nab-paclitaxel plus gemcitabine or nab-paclitaxel combination therapy as first-line and FOLFIRINOX as second-line (line A); nab-paclitaxel combination therapy to gemcitabine combination therapy to FOLFIRINOX (line B); and nab-paclitaxel combination therapy, to gemcitabine combination therapy, to oxaliplatin or irinotecan combination therapy (line C).
- Overall, the researchers observed a median overall survival of 14 months among patients receiving line A and C sequences and 18 months with line B.
- Patients receiving line B therapy demonstrated a 52% lower risk for death compared with those receiving line A treatment (hazard ratio [HR], 0.48; P = .018) and a 75% reduced risk for death compared with those on the line C sequence (HR, 0.25; P = .040).
IN PRACTICE:
“Our study provides real-world evidence for the effectiveness of different treatment sequences and underscores the [impact of] treatment sequences on survival outcome when considering the entire management in advanced pancreatic ductal adenocarcinoma,” the authors concluded.
SOURCE:
The study, led by Guanghai Dai, MD, from the Chinese People’s Liberation Army General Hospital, Beijing, was published in BMC Cancer on January 12, 2024.
LIMITATIONS:
The study was a single-center, retrospective analysis.
DISCLOSURES:
The paper was funded by Beijing natural science foundation. The authors did not declare any relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Despite therapeutic advances, survival among patients with unresectable and/or metastatic pancreatic ductal adenocarcinoma has not markedly improved in recent years.
- In the current analysis, researchers evaluated whether treatment sequence could affect survival outcomes in this patient population.
- To this end , researchers conducted a single institution, retrospective analysis of patients who received different lines of treatment between January 2015 and December 2021.
- The most common first-line therapy was nab-paclitaxel plus S-1 (58%), followed by FOLFIRINOX (10%), nab-paclitaxel plus gemcitabine (8%), gemcitabine alone (7%), gemcitabine plus oxaliplatin (6%); second-line therapies, in order of frequency, included gemcitabine combination therapy (48%), nab-paclitaxel combination therapy (19%), FOLFIRINOX (10%), and gemcitabine alone (7%); third-line treatments consisted of FOLFIRINOX (31%), irinotecan or oxaliplatin combination therapy (23%), immunotherapy (19%), and gemcitabine combination therapy (10%).
TAKEAWAY:
- Overall, progression occurred in 90% of patients, and the median overall survival was 12.0 months, with only 48% of patients able to start a third-line therapy.
- The researchers focused on three common therapy sequences: nab-paclitaxel plus gemcitabine or nab-paclitaxel combination therapy as first-line and FOLFIRINOX as second-line (line A); nab-paclitaxel combination therapy to gemcitabine combination therapy to FOLFIRINOX (line B); and nab-paclitaxel combination therapy, to gemcitabine combination therapy, to oxaliplatin or irinotecan combination therapy (line C).
- Overall, the researchers observed a median overall survival of 14 months among patients receiving line A and C sequences and 18 months with line B.
- Patients receiving line B therapy demonstrated a 52% lower risk for death compared with those receiving line A treatment (hazard ratio [HR], 0.48; P = .018) and a 75% reduced risk for death compared with those on the line C sequence (HR, 0.25; P = .040).
IN PRACTICE:
“Our study provides real-world evidence for the effectiveness of different treatment sequences and underscores the [impact of] treatment sequences on survival outcome when considering the entire management in advanced pancreatic ductal adenocarcinoma,” the authors concluded.
SOURCE:
The study, led by Guanghai Dai, MD, from the Chinese People’s Liberation Army General Hospital, Beijing, was published in BMC Cancer on January 12, 2024.
LIMITATIONS:
The study was a single-center, retrospective analysis.
DISCLOSURES:
The paper was funded by Beijing natural science foundation. The authors did not declare any relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Despite therapeutic advances, survival among patients with unresectable and/or metastatic pancreatic ductal adenocarcinoma has not markedly improved in recent years.
- In the current analysis, researchers evaluated whether treatment sequence could affect survival outcomes in this patient population.
- To this end , researchers conducted a single institution, retrospective analysis of patients who received different lines of treatment between January 2015 and December 2021.
- The most common first-line therapy was nab-paclitaxel plus S-1 (58%), followed by FOLFIRINOX (10%), nab-paclitaxel plus gemcitabine (8%), gemcitabine alone (7%), gemcitabine plus oxaliplatin (6%); second-line therapies, in order of frequency, included gemcitabine combination therapy (48%), nab-paclitaxel combination therapy (19%), FOLFIRINOX (10%), and gemcitabine alone (7%); third-line treatments consisted of FOLFIRINOX (31%), irinotecan or oxaliplatin combination therapy (23%), immunotherapy (19%), and gemcitabine combination therapy (10%).
TAKEAWAY:
- Overall, progression occurred in 90% of patients, and the median overall survival was 12.0 months, with only 48% of patients able to start a third-line therapy.
- The researchers focused on three common therapy sequences: nab-paclitaxel plus gemcitabine or nab-paclitaxel combination therapy as first-line and FOLFIRINOX as second-line (line A); nab-paclitaxel combination therapy to gemcitabine combination therapy to FOLFIRINOX (line B); and nab-paclitaxel combination therapy, to gemcitabine combination therapy, to oxaliplatin or irinotecan combination therapy (line C).
- Overall, the researchers observed a median overall survival of 14 months among patients receiving line A and C sequences and 18 months with line B.
- Patients receiving line B therapy demonstrated a 52% lower risk for death compared with those receiving line A treatment (hazard ratio [HR], 0.48; P = .018) and a 75% reduced risk for death compared with those on the line C sequence (HR, 0.25; P = .040).
IN PRACTICE:
“Our study provides real-world evidence for the effectiveness of different treatment sequences and underscores the [impact of] treatment sequences on survival outcome when considering the entire management in advanced pancreatic ductal adenocarcinoma,” the authors concluded.
SOURCE:
The study, led by Guanghai Dai, MD, from the Chinese People’s Liberation Army General Hospital, Beijing, was published in BMC Cancer on January 12, 2024.
LIMITATIONS:
The study was a single-center, retrospective analysis.
DISCLOSURES:
The paper was funded by Beijing natural science foundation. The authors did not declare any relevant financial relationships.
A version of this article appeared on Medscape.com.
ALL: When Should MRD Trigger Stem Cell Transplants?
Allogeneic hematopoietic stem cell transplants (HSCT) are still part of the hematology armamentarium for relapsed/refractory (R/R) patients with Ph-negative ALL who are MRD positive. However, when asked about the best treatment strategy for patients who are MRD-negative, hematologist Mark R. Litzow, MD, of the Mayo Clinic in Rochester, Minnesota, said in an interview, “There is no firm consensus about that.”
Discussing how medicine has evolved over the past 20 to 30 years, Dr. Litzow recalled that HSCT used to be standard treatment for adult patients with ALL. “We felt that in most instances, chemotherapy alone was not going to be effective in curing them. A vast majority would relapse,” he said. Nowadays, however, specialists differ on the use of HSCT in patients with Ph-negative, MRD-negative ALL.
A pair of commentaries in the January issue of The Lancet Hematology tackle this topic from different perspectives. On one hand, hematologist Patrice Chevallier, MD, of the University of Nantes in France, argues that for such patients, HSCT “remains a valid option,”and MRD status shouldn’t be the sole factor used for a decision.
However, hematologist Nicolas Boissel, MD, PhD, of Paris Cité University, contends that detectable early MRD is the “only robust predictor” of HSCT benefit in patients under 60 with Ph-negative ALL, and it has “unproven” benefit in older patients.
As Dr. Chevallier notes, “allogeneic HSCT is indicated in patients defined as having a high risk of relapse. Currently, a high level of residual leukemic cells after treatment is recognized as the strongest, and sometimes sole, criterion defining high-risk patients.”
As first- and second-line therapy in pediatric patients and as first-line therapy in adults, the “rule” is to offer HSCT to MRD-positive patients but not MRD-negative ones, he writes. “In older patients and those who are relapsed or refractory, the recent demonstration of efficient immunotherapies and cell therapies has launched the debate on the role of MRD status and the question of whether or not to transplant patients who are MRD-negative in both settings.”
Dr. Chevallier notes that “there is no standard definition of an MRD-negative status,” and the best timing for evaluation is unknown. Further, he adds, a “variable proportion of MRD-negative patients still relapse after treatment — up to 25% of patients who respond early and more than 50% of patients who respond late.”
He also points out that there’s an 80% chance that patients will convert from MRD negative to MRD positive after blinatumomab therapy, and he highlights the low long-term survival rate (20%) after brexucabtagene autoleucel (Tecartus), a CAR T-cell therapy.
As for older patients, Dr. Chevallier observes that improved chemo-immunotherapy and conditioning regimens could spark a rethinking of the feasibility of HSCT. However, for now, in those patients, “MRD is not decisional, and allogeneic HSCT is not a routine practice,” he writes.
In his commentary, Dr. Boissel points out that there have been no controlled studies of HSCT in the first-remission setting, although he writes that some data suggests that HSCT may be helpful for patients in high-risk genetic subgroups, regardless of MRD status. On the other hand, “converging observations suggest no benefit of HSCT in MRD-positive patients treated with blinatumomab in the front-line setting.”
If MRD monitoring is unavailable, Dr. Boissel adds, “it seems reasonable to use early blast clearance or other baseline high-risk features to indicate HSCT.”
How can hematologists make the best decision about HSCT?
In an interview, City of Hope Medical Center (Duarte, California) hematologist-oncologist Ibrahim T. Aldoss, MD, said that chemotherapy — with or without immunotherapy — can often be enough to treat younger patients without high-risk genetic factors. “Potentially, these patients can be spared from transplants,” he said, although patients with resistant MRD “clearly need transplants.”
The risks of transplants are significant, he noted. While they can reduce the risk of relapse, the risk of dying during remission is higher vs chemotherapy. “So you have to balance the risks that you’re willing to take,” he said, keeping in mind that some patients can be cured with chemotherapy.
In addition, Dr. Aldoss said, acute graft-versus-host disease in the first few months after transplant can become chronic. “Many years later, patients can be struggling to where it actually impacts their daily activity. And unfortunately, patients can die from it.”
In the big picture, “you cannot have a generalized statement about whether you shouldn’t do transplants in every MRD-negative patient,” he said. However, “if you do achieve MRD negativity, most patients likely don’t need transplants.”
The Mayo Clinic’s Dr. Litzow urged colleagues to consider several factors when making decisions. Do patients have a high level of comorbidities that would raise the risk of death from HSCT? He noted that there’s nearly a 20% risk of death from HSCT, and comorbidities can boost the risk to 40%-50%.
Also, does the patient have a suitable donor? While advances have boosted the number of eligible donors, he said, “not everybody has an ideal donor.”
If a patient is MRD-negative but not a good candidate for a transplant, Dr. Litzow said consolidation therapy followed by maintenance therapy may be indicated. “Continue to check their bone marrow and their blood periodically as they’re going through treatment and reassess their MRD status to make sure they’re staying negative. If they turn MRD-positive during the course of their therapy, then we have to step back and rethink the role of transplant.”
As for cost, Dr. Litzow points out that HSCT is very expensive, although ALL is an accepted indication for HSCT. However, “if someone doesn’t have medical insurance, then it can be difficult to consider them having a transplant.”
What’s next? In his commentary, Dr. Boissel writes that his team aims to study whether HSCT is helpful in patients with high-risk B-cell ALL “who reach MRD negativity after a consolidation phase including blinatumomab.”
Dr. Aldoss discloses relationships with Amgen, Kite, Pfizer, Jazz, AbbVie, Sobi, Agios, Autolus, and MacroGenics. Dr. Litzow reports ties with Amgen. Dr. Boissel declares relationships with Amgen, Pfizer, Novartis, and Servier. Dr. Chevallier has no disclosures.
Allogeneic hematopoietic stem cell transplants (HSCT) are still part of the hematology armamentarium for relapsed/refractory (R/R) patients with Ph-negative ALL who are MRD positive. However, when asked about the best treatment strategy for patients who are MRD-negative, hematologist Mark R. Litzow, MD, of the Mayo Clinic in Rochester, Minnesota, said in an interview, “There is no firm consensus about that.”
Discussing how medicine has evolved over the past 20 to 30 years, Dr. Litzow recalled that HSCT used to be standard treatment for adult patients with ALL. “We felt that in most instances, chemotherapy alone was not going to be effective in curing them. A vast majority would relapse,” he said. Nowadays, however, specialists differ on the use of HSCT in patients with Ph-negative, MRD-negative ALL.
A pair of commentaries in the January issue of The Lancet Hematology tackle this topic from different perspectives. On one hand, hematologist Patrice Chevallier, MD, of the University of Nantes in France, argues that for such patients, HSCT “remains a valid option,”and MRD status shouldn’t be the sole factor used for a decision.
However, hematologist Nicolas Boissel, MD, PhD, of Paris Cité University, contends that detectable early MRD is the “only robust predictor” of HSCT benefit in patients under 60 with Ph-negative ALL, and it has “unproven” benefit in older patients.
As Dr. Chevallier notes, “allogeneic HSCT is indicated in patients defined as having a high risk of relapse. Currently, a high level of residual leukemic cells after treatment is recognized as the strongest, and sometimes sole, criterion defining high-risk patients.”
As first- and second-line therapy in pediatric patients and as first-line therapy in adults, the “rule” is to offer HSCT to MRD-positive patients but not MRD-negative ones, he writes. “In older patients and those who are relapsed or refractory, the recent demonstration of efficient immunotherapies and cell therapies has launched the debate on the role of MRD status and the question of whether or not to transplant patients who are MRD-negative in both settings.”
Dr. Chevallier notes that “there is no standard definition of an MRD-negative status,” and the best timing for evaluation is unknown. Further, he adds, a “variable proportion of MRD-negative patients still relapse after treatment — up to 25% of patients who respond early and more than 50% of patients who respond late.”
He also points out that there’s an 80% chance that patients will convert from MRD negative to MRD positive after blinatumomab therapy, and he highlights the low long-term survival rate (20%) after brexucabtagene autoleucel (Tecartus), a CAR T-cell therapy.
As for older patients, Dr. Chevallier observes that improved chemo-immunotherapy and conditioning regimens could spark a rethinking of the feasibility of HSCT. However, for now, in those patients, “MRD is not decisional, and allogeneic HSCT is not a routine practice,” he writes.
In his commentary, Dr. Boissel points out that there have been no controlled studies of HSCT in the first-remission setting, although he writes that some data suggests that HSCT may be helpful for patients in high-risk genetic subgroups, regardless of MRD status. On the other hand, “converging observations suggest no benefit of HSCT in MRD-positive patients treated with blinatumomab in the front-line setting.”
If MRD monitoring is unavailable, Dr. Boissel adds, “it seems reasonable to use early blast clearance or other baseline high-risk features to indicate HSCT.”
How can hematologists make the best decision about HSCT?
In an interview, City of Hope Medical Center (Duarte, California) hematologist-oncologist Ibrahim T. Aldoss, MD, said that chemotherapy — with or without immunotherapy — can often be enough to treat younger patients without high-risk genetic factors. “Potentially, these patients can be spared from transplants,” he said, although patients with resistant MRD “clearly need transplants.”
The risks of transplants are significant, he noted. While they can reduce the risk of relapse, the risk of dying during remission is higher vs chemotherapy. “So you have to balance the risks that you’re willing to take,” he said, keeping in mind that some patients can be cured with chemotherapy.
In addition, Dr. Aldoss said, acute graft-versus-host disease in the first few months after transplant can become chronic. “Many years later, patients can be struggling to where it actually impacts their daily activity. And unfortunately, patients can die from it.”
In the big picture, “you cannot have a generalized statement about whether you shouldn’t do transplants in every MRD-negative patient,” he said. However, “if you do achieve MRD negativity, most patients likely don’t need transplants.”
The Mayo Clinic’s Dr. Litzow urged colleagues to consider several factors when making decisions. Do patients have a high level of comorbidities that would raise the risk of death from HSCT? He noted that there’s nearly a 20% risk of death from HSCT, and comorbidities can boost the risk to 40%-50%.
Also, does the patient have a suitable donor? While advances have boosted the number of eligible donors, he said, “not everybody has an ideal donor.”
If a patient is MRD-negative but not a good candidate for a transplant, Dr. Litzow said consolidation therapy followed by maintenance therapy may be indicated. “Continue to check their bone marrow and their blood periodically as they’re going through treatment and reassess their MRD status to make sure they’re staying negative. If they turn MRD-positive during the course of their therapy, then we have to step back and rethink the role of transplant.”
As for cost, Dr. Litzow points out that HSCT is very expensive, although ALL is an accepted indication for HSCT. However, “if someone doesn’t have medical insurance, then it can be difficult to consider them having a transplant.”
What’s next? In his commentary, Dr. Boissel writes that his team aims to study whether HSCT is helpful in patients with high-risk B-cell ALL “who reach MRD negativity after a consolidation phase including blinatumomab.”
Dr. Aldoss discloses relationships with Amgen, Kite, Pfizer, Jazz, AbbVie, Sobi, Agios, Autolus, and MacroGenics. Dr. Litzow reports ties with Amgen. Dr. Boissel declares relationships with Amgen, Pfizer, Novartis, and Servier. Dr. Chevallier has no disclosures.
Allogeneic hematopoietic stem cell transplants (HSCT) are still part of the hematology armamentarium for relapsed/refractory (R/R) patients with Ph-negative ALL who are MRD positive. However, when asked about the best treatment strategy for patients who are MRD-negative, hematologist Mark R. Litzow, MD, of the Mayo Clinic in Rochester, Minnesota, said in an interview, “There is no firm consensus about that.”
Discussing how medicine has evolved over the past 20 to 30 years, Dr. Litzow recalled that HSCT used to be standard treatment for adult patients with ALL. “We felt that in most instances, chemotherapy alone was not going to be effective in curing them. A vast majority would relapse,” he said. Nowadays, however, specialists differ on the use of HSCT in patients with Ph-negative, MRD-negative ALL.
A pair of commentaries in the January issue of The Lancet Hematology tackle this topic from different perspectives. On one hand, hematologist Patrice Chevallier, MD, of the University of Nantes in France, argues that for such patients, HSCT “remains a valid option,”and MRD status shouldn’t be the sole factor used for a decision.
However, hematologist Nicolas Boissel, MD, PhD, of Paris Cité University, contends that detectable early MRD is the “only robust predictor” of HSCT benefit in patients under 60 with Ph-negative ALL, and it has “unproven” benefit in older patients.
As Dr. Chevallier notes, “allogeneic HSCT is indicated in patients defined as having a high risk of relapse. Currently, a high level of residual leukemic cells after treatment is recognized as the strongest, and sometimes sole, criterion defining high-risk patients.”
As first- and second-line therapy in pediatric patients and as first-line therapy in adults, the “rule” is to offer HSCT to MRD-positive patients but not MRD-negative ones, he writes. “In older patients and those who are relapsed or refractory, the recent demonstration of efficient immunotherapies and cell therapies has launched the debate on the role of MRD status and the question of whether or not to transplant patients who are MRD-negative in both settings.”
Dr. Chevallier notes that “there is no standard definition of an MRD-negative status,” and the best timing for evaluation is unknown. Further, he adds, a “variable proportion of MRD-negative patients still relapse after treatment — up to 25% of patients who respond early and more than 50% of patients who respond late.”
He also points out that there’s an 80% chance that patients will convert from MRD negative to MRD positive after blinatumomab therapy, and he highlights the low long-term survival rate (20%) after brexucabtagene autoleucel (Tecartus), a CAR T-cell therapy.
As for older patients, Dr. Chevallier observes that improved chemo-immunotherapy and conditioning regimens could spark a rethinking of the feasibility of HSCT. However, for now, in those patients, “MRD is not decisional, and allogeneic HSCT is not a routine practice,” he writes.
In his commentary, Dr. Boissel points out that there have been no controlled studies of HSCT in the first-remission setting, although he writes that some data suggests that HSCT may be helpful for patients in high-risk genetic subgroups, regardless of MRD status. On the other hand, “converging observations suggest no benefit of HSCT in MRD-positive patients treated with blinatumomab in the front-line setting.”
If MRD monitoring is unavailable, Dr. Boissel adds, “it seems reasonable to use early blast clearance or other baseline high-risk features to indicate HSCT.”
How can hematologists make the best decision about HSCT?
In an interview, City of Hope Medical Center (Duarte, California) hematologist-oncologist Ibrahim T. Aldoss, MD, said that chemotherapy — with or without immunotherapy — can often be enough to treat younger patients without high-risk genetic factors. “Potentially, these patients can be spared from transplants,” he said, although patients with resistant MRD “clearly need transplants.”
The risks of transplants are significant, he noted. While they can reduce the risk of relapse, the risk of dying during remission is higher vs chemotherapy. “So you have to balance the risks that you’re willing to take,” he said, keeping in mind that some patients can be cured with chemotherapy.
In addition, Dr. Aldoss said, acute graft-versus-host disease in the first few months after transplant can become chronic. “Many years later, patients can be struggling to where it actually impacts their daily activity. And unfortunately, patients can die from it.”
In the big picture, “you cannot have a generalized statement about whether you shouldn’t do transplants in every MRD-negative patient,” he said. However, “if you do achieve MRD negativity, most patients likely don’t need transplants.”
The Mayo Clinic’s Dr. Litzow urged colleagues to consider several factors when making decisions. Do patients have a high level of comorbidities that would raise the risk of death from HSCT? He noted that there’s nearly a 20% risk of death from HSCT, and comorbidities can boost the risk to 40%-50%.
Also, does the patient have a suitable donor? While advances have boosted the number of eligible donors, he said, “not everybody has an ideal donor.”
If a patient is MRD-negative but not a good candidate for a transplant, Dr. Litzow said consolidation therapy followed by maintenance therapy may be indicated. “Continue to check their bone marrow and their blood periodically as they’re going through treatment and reassess their MRD status to make sure they’re staying negative. If they turn MRD-positive during the course of their therapy, then we have to step back and rethink the role of transplant.”
As for cost, Dr. Litzow points out that HSCT is very expensive, although ALL is an accepted indication for HSCT. However, “if someone doesn’t have medical insurance, then it can be difficult to consider them having a transplant.”
What’s next? In his commentary, Dr. Boissel writes that his team aims to study whether HSCT is helpful in patients with high-risk B-cell ALL “who reach MRD negativity after a consolidation phase including blinatumomab.”
Dr. Aldoss discloses relationships with Amgen, Kite, Pfizer, Jazz, AbbVie, Sobi, Agios, Autolus, and MacroGenics. Dr. Litzow reports ties with Amgen. Dr. Boissel declares relationships with Amgen, Pfizer, Novartis, and Servier. Dr. Chevallier has no disclosures.
Two-Step Strategy Improves Early-Stage Ovarian Cancer Detection
TOPLINE:
a new analysis with a 21-year follow-up found.
METHODOLOGY:
- Detecting ovarian cancer at stage I or II could significantly reduce ovarian cancer-related deaths, but only 25%-30% of patients are diagnosed at an early stage.
- In this single-arm prospective analysis, 7,856 healthy postmenopausal women received annual screening for ovarian cancer between 2011 and 2022. Screening involved an annual blood test to detect levels of cancer antigen 125 and track these levels over time.
- Investigators used the Risk of Ovarian Cancer Algorithm (ROCA) to determine whether ovarian cancer risk was normal, intermediate, or high. Those with elevated ROCA scores were referred for transvaginal sonography; those with intermediate scores received follow-up blood tests every 3 months.
- Overall, 92.3% of women were normal risk, 5.7% were intermediate, and 2% were high risk and recommended for transvaginal sonography.
TAKEAWAY:
- Most women (95.5%) referred for transvaginal ultrasound had one. Of these ultrasounds, most (90%) were negative or revealed benign findings, 5.2% required a repeat ultrasound, and 4.8% (34 patients) showed suspicious findings.
- Of 34 patients with suspicious findings and recommended for surgery, 15 had ovarian cancer and two had borderline tumors, indicating a positive predictive value of 50% (17 of 34 patients) for ovarian cancer. Of these 17 patients, 12 (70.6%) had stage I or II disease.
- Following abnormal ROCA results, seven other women were diagnosed with endometrial tumors (six of which were stage I), indicating a positive predictive value of 74% (25 of 34) for any cancer.
- The specificity for elevated risk ROCA prompting ultrasound was 98%, and the specificity of the ROCA and ultrasound prompting surgery was 99.8%. The sensitivity for detecting ovarian and borderline cancer was 74% (17 of 23).
IN PRACTICE:
“Remarkably, 70% of ovarian cancers detected by the ROCA” were early stage,” the authors concluded. Although the trial was not powered to detect reduced mortality, the high specificity, positive predictive value, and shift to identifying earlier-stage cancers “support further development of this strategy,” the investigators said.
LIMITATIONS:
This trial was not powered to detect mortality benefit. Six ovarian cancers and borderline tumors were missed. Only 80% of ovarian cancers express cancer antigen 125, potentially limiting the sensitivity of the algorithm.
SOURCE:
This study, led by Chae Young Han from the University of Texas MD Anderson Cancer Center, Houston, was published online on January 12 in the Journal of Clinical Oncology.
DISCLOSURES:
This study was supported by funds from the NCI Early Detection Research Network, the MD Anderson Ovarian SPOREs, the National Cancer Institute, the Department of Health and Human Services, and others. The authors reported receiving research funding, grants, consulting, and personal fees from various companies, including Curio Science, Fujirebio Diagnostics, GlaxoSmithKline, AstraZeneca, and Genentech.
A version of this article appeared on Medscape.com.
TOPLINE:
a new analysis with a 21-year follow-up found.
METHODOLOGY:
- Detecting ovarian cancer at stage I or II could significantly reduce ovarian cancer-related deaths, but only 25%-30% of patients are diagnosed at an early stage.
- In this single-arm prospective analysis, 7,856 healthy postmenopausal women received annual screening for ovarian cancer between 2011 and 2022. Screening involved an annual blood test to detect levels of cancer antigen 125 and track these levels over time.
- Investigators used the Risk of Ovarian Cancer Algorithm (ROCA) to determine whether ovarian cancer risk was normal, intermediate, or high. Those with elevated ROCA scores were referred for transvaginal sonography; those with intermediate scores received follow-up blood tests every 3 months.
- Overall, 92.3% of women were normal risk, 5.7% were intermediate, and 2% were high risk and recommended for transvaginal sonography.
TAKEAWAY:
- Most women (95.5%) referred for transvaginal ultrasound had one. Of these ultrasounds, most (90%) were negative or revealed benign findings, 5.2% required a repeat ultrasound, and 4.8% (34 patients) showed suspicious findings.
- Of 34 patients with suspicious findings and recommended for surgery, 15 had ovarian cancer and two had borderline tumors, indicating a positive predictive value of 50% (17 of 34 patients) for ovarian cancer. Of these 17 patients, 12 (70.6%) had stage I or II disease.
- Following abnormal ROCA results, seven other women were diagnosed with endometrial tumors (six of which were stage I), indicating a positive predictive value of 74% (25 of 34) for any cancer.
- The specificity for elevated risk ROCA prompting ultrasound was 98%, and the specificity of the ROCA and ultrasound prompting surgery was 99.8%. The sensitivity for detecting ovarian and borderline cancer was 74% (17 of 23).
IN PRACTICE:
“Remarkably, 70% of ovarian cancers detected by the ROCA” were early stage,” the authors concluded. Although the trial was not powered to detect reduced mortality, the high specificity, positive predictive value, and shift to identifying earlier-stage cancers “support further development of this strategy,” the investigators said.
LIMITATIONS:
This trial was not powered to detect mortality benefit. Six ovarian cancers and borderline tumors were missed. Only 80% of ovarian cancers express cancer antigen 125, potentially limiting the sensitivity of the algorithm.
SOURCE:
This study, led by Chae Young Han from the University of Texas MD Anderson Cancer Center, Houston, was published online on January 12 in the Journal of Clinical Oncology.
DISCLOSURES:
This study was supported by funds from the NCI Early Detection Research Network, the MD Anderson Ovarian SPOREs, the National Cancer Institute, the Department of Health and Human Services, and others. The authors reported receiving research funding, grants, consulting, and personal fees from various companies, including Curio Science, Fujirebio Diagnostics, GlaxoSmithKline, AstraZeneca, and Genentech.
A version of this article appeared on Medscape.com.
TOPLINE:
a new analysis with a 21-year follow-up found.
METHODOLOGY:
- Detecting ovarian cancer at stage I or II could significantly reduce ovarian cancer-related deaths, but only 25%-30% of patients are diagnosed at an early stage.
- In this single-arm prospective analysis, 7,856 healthy postmenopausal women received annual screening for ovarian cancer between 2011 and 2022. Screening involved an annual blood test to detect levels of cancer antigen 125 and track these levels over time.
- Investigators used the Risk of Ovarian Cancer Algorithm (ROCA) to determine whether ovarian cancer risk was normal, intermediate, or high. Those with elevated ROCA scores were referred for transvaginal sonography; those with intermediate scores received follow-up blood tests every 3 months.
- Overall, 92.3% of women were normal risk, 5.7% were intermediate, and 2% were high risk and recommended for transvaginal sonography.
TAKEAWAY:
- Most women (95.5%) referred for transvaginal ultrasound had one. Of these ultrasounds, most (90%) were negative or revealed benign findings, 5.2% required a repeat ultrasound, and 4.8% (34 patients) showed suspicious findings.
- Of 34 patients with suspicious findings and recommended for surgery, 15 had ovarian cancer and two had borderline tumors, indicating a positive predictive value of 50% (17 of 34 patients) for ovarian cancer. Of these 17 patients, 12 (70.6%) had stage I or II disease.
- Following abnormal ROCA results, seven other women were diagnosed with endometrial tumors (six of which were stage I), indicating a positive predictive value of 74% (25 of 34) for any cancer.
- The specificity for elevated risk ROCA prompting ultrasound was 98%, and the specificity of the ROCA and ultrasound prompting surgery was 99.8%. The sensitivity for detecting ovarian and borderline cancer was 74% (17 of 23).
IN PRACTICE:
“Remarkably, 70% of ovarian cancers detected by the ROCA” were early stage,” the authors concluded. Although the trial was not powered to detect reduced mortality, the high specificity, positive predictive value, and shift to identifying earlier-stage cancers “support further development of this strategy,” the investigators said.
LIMITATIONS:
This trial was not powered to detect mortality benefit. Six ovarian cancers and borderline tumors were missed. Only 80% of ovarian cancers express cancer antigen 125, potentially limiting the sensitivity of the algorithm.
SOURCE:
This study, led by Chae Young Han from the University of Texas MD Anderson Cancer Center, Houston, was published online on January 12 in the Journal of Clinical Oncology.
DISCLOSURES:
This study was supported by funds from the NCI Early Detection Research Network, the MD Anderson Ovarian SPOREs, the National Cancer Institute, the Department of Health and Human Services, and others. The authors reported receiving research funding, grants, consulting, and personal fees from various companies, including Curio Science, Fujirebio Diagnostics, GlaxoSmithKline, AstraZeneca, and Genentech.
A version of this article appeared on Medscape.com.