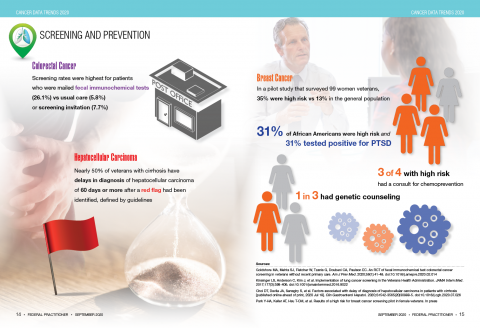
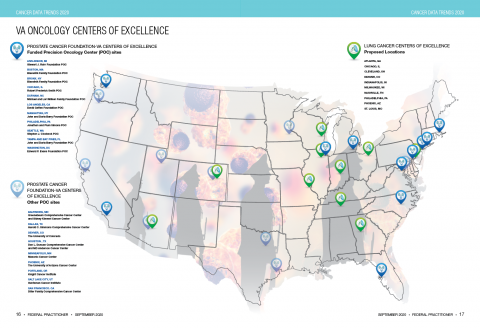
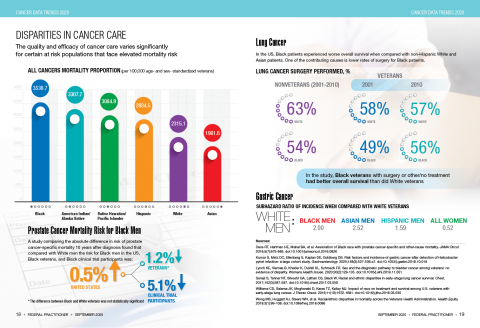
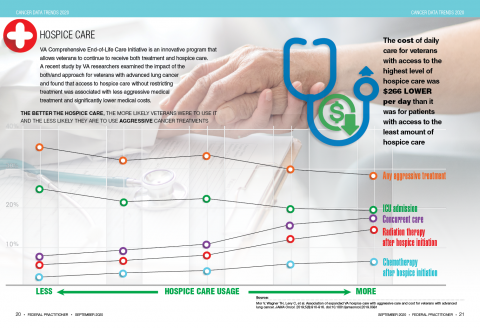
User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


COVID-19 prompts ‘democratization’ of cancer trials
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
FROM AACR: COVID-19 and Cancer
COVID-19 and Blood Clots: Inside the Battle to Save Patients
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
VA Looks to Increase Real-World Impact of Clinical Research
The US Department of Veterans Affairs (VA) is embracing clinical trials with a focus on oncology, and patients will benefit from new priorities and programs, VA officials reported at the Association of VA Hematology/Oncology (AVAHO) virtual meeting. “The whole model is one that is far more proactive,” said Carolyn Clancy, MD, Under Secretary for Health for Discovery, Education, and Affiliate Networks.
According to Clancy, the department’s top research priority is to increase veteran access to high-quality clinical trials. “Priority number 2 is increasing the real-world impact of VA research,” she said. “Our commitment to veterans and the taxpayers is to reverse and shorten the [research-to-implementation] timeline. And the third priority is to put VA data to work for veterans, not just through people who work in VA and Veterans Health Administration, but through other researchers who can have access to them.”
To meet these goals, VA is engaging in multiple research programs and collaborations. Rachel B. Ramoni, DMD, ScD, the VA chief research and development officer, highlighted a number of the projects in a separate AVAHO meeting presentation, including:
- The National Cancer Institute and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE), an interagency collaboration between the VA and the National Cancer Institute (NCI). This program established a network of sites to help enrolled veterans take part in NCI-supported clinical trials. “It really got up and running in 2018, and I’m proud to say that over 250 veterans have been enrolled, and enrollment exceeds that at non-NAVIGATE sites,” Ramoni reported. “Clearly, the additional support that these sites are getting is really helping to achieve the outcome of getting more veterans access to these trials.” However, she said, some areas of the nation aren’t yet covered by the program.
- The Precision Oncology Program for Cancer of the Prostate (POPCaP), established through a partnership with the Prostate Cancer Foundation. The foundation provided a $50 million investment. “This program ensures that veterans, no matter where they are, get best-in-class prostate cancer care,” Ramoni explained. “The initial focus was ensuring that men get sequencing if they have metastatic prostate cancer, and that they get access to clinical trials. The really distinguishing factor about POPCaP is that it has built a vibrant community of clinicians, researchers and program offices. The whole is much greater than the sum of its parts.” More POPCaP hubs are in development, she said.
- PATCH (Prostate Cancer Analysis for Therapy Choice), a program funded by the VA and the Prostate Cancer Foundation. “The whole purpose of PATCH is to create this network of sites to systematically go through different clinical trials that are biomarker-driven,” Ramoni said. “One of the great things about PATCH is that it’s leveraging the genetics databases to help proactively identify men who might qualify for these trials and to find them wherever they might be across the system so they have access to these trials.” She also praised the program’s commitment to collaboration and mentorship. “If you’re new to putting together clinical trials concepts or to submitting merit proposals to VA for funding, PATCH is a great place to get into a community that’s supportive and wants to help you succeed.”
- The VA Phenomics Library. This library, based at the Boston VA Medical Center, focuses on improving the analysis of “messy” electronic health record data, Ramoni noted. “There are automated algorithms that go through and help you clean up that data to make sense of it,” she said. “The problem is that it’s really been an every-person-for-himself-or-herself system. Each researcher who needed these phenotypes was creating his or her own.” The Phenomics Library will promote sharing “so there’s not going to be as much wasted time duplicating effort,” she said. “By the end of fiscal year 2021, we will have over 1,000 curated phenotypes in there. We hope that will be a great resource for the oncology community as well as many other communities.”
- Access to Clinical Trials (ACT) for Veterans. “This program, which began a couple of years ago, has really succeeded,” Ramoni said. “We were focusing on decreasing the time it takes to start up multi-site industry trials. When we got started with ACT, it was taking over 200 days to get started. And now, just a couple years later, we are well under 100 days, which is within industry standards.” Also, she said, the VA established a Partnered Research Program office, “which serves to interact with our industry partners and really guide them through the VA system, which can be complex if you’re approaching it for the first time.”
In a separate presentation, Krissa Caroff, MS, CPC, program manager of the Partnered Research Program, said it had quickened the process of implementing clinical trials by tackling roadblocks such as the need for multiple master agreements to be signed. Central coordination has been key, she said, “and we are working closely to ensure that we when have a multisite trial, all the VA sites are utilizing the same single IRB [institutional review board]. We’ve also identified the critical information that we need to collect from industry in order for us to evaluate a trial.”
What’s next? “We really are going to be focusing on oncology trials,” Ramoni insisted. “This is a high priority for us.” She added: “Please share your feedback and experiences with us. And also please communicate amongst your colleagues within your organization to explain how we’re standardizing things within VA.”
The speakers reported no relevant disclosures.
The US Department of Veterans Affairs (VA) is embracing clinical trials with a focus on oncology, and patients will benefit from new priorities and programs, VA officials reported at the Association of VA Hematology/Oncology (AVAHO) virtual meeting. “The whole model is one that is far more proactive,” said Carolyn Clancy, MD, Under Secretary for Health for Discovery, Education, and Affiliate Networks.
According to Clancy, the department’s top research priority is to increase veteran access to high-quality clinical trials. “Priority number 2 is increasing the real-world impact of VA research,” she said. “Our commitment to veterans and the taxpayers is to reverse and shorten the [research-to-implementation] timeline. And the third priority is to put VA data to work for veterans, not just through people who work in VA and Veterans Health Administration, but through other researchers who can have access to them.”
To meet these goals, VA is engaging in multiple research programs and collaborations. Rachel B. Ramoni, DMD, ScD, the VA chief research and development officer, highlighted a number of the projects in a separate AVAHO meeting presentation, including:
- The National Cancer Institute and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE), an interagency collaboration between the VA and the National Cancer Institute (NCI). This program established a network of sites to help enrolled veterans take part in NCI-supported clinical trials. “It really got up and running in 2018, and I’m proud to say that over 250 veterans have been enrolled, and enrollment exceeds that at non-NAVIGATE sites,” Ramoni reported. “Clearly, the additional support that these sites are getting is really helping to achieve the outcome of getting more veterans access to these trials.” However, she said, some areas of the nation aren’t yet covered by the program.
- The Precision Oncology Program for Cancer of the Prostate (POPCaP), established through a partnership with the Prostate Cancer Foundation. The foundation provided a $50 million investment. “This program ensures that veterans, no matter where they are, get best-in-class prostate cancer care,” Ramoni explained. “The initial focus was ensuring that men get sequencing if they have metastatic prostate cancer, and that they get access to clinical trials. The really distinguishing factor about POPCaP is that it has built a vibrant community of clinicians, researchers and program offices. The whole is much greater than the sum of its parts.” More POPCaP hubs are in development, she said.
- PATCH (Prostate Cancer Analysis for Therapy Choice), a program funded by the VA and the Prostate Cancer Foundation. “The whole purpose of PATCH is to create this network of sites to systematically go through different clinical trials that are biomarker-driven,” Ramoni said. “One of the great things about PATCH is that it’s leveraging the genetics databases to help proactively identify men who might qualify for these trials and to find them wherever they might be across the system so they have access to these trials.” She also praised the program’s commitment to collaboration and mentorship. “If you’re new to putting together clinical trials concepts or to submitting merit proposals to VA for funding, PATCH is a great place to get into a community that’s supportive and wants to help you succeed.”
- The VA Phenomics Library. This library, based at the Boston VA Medical Center, focuses on improving the analysis of “messy” electronic health record data, Ramoni noted. “There are automated algorithms that go through and help you clean up that data to make sense of it,” she said. “The problem is that it’s really been an every-person-for-himself-or-herself system. Each researcher who needed these phenotypes was creating his or her own.” The Phenomics Library will promote sharing “so there’s not going to be as much wasted time duplicating effort,” she said. “By the end of fiscal year 2021, we will have over 1,000 curated phenotypes in there. We hope that will be a great resource for the oncology community as well as many other communities.”
- Access to Clinical Trials (ACT) for Veterans. “This program, which began a couple of years ago, has really succeeded,” Ramoni said. “We were focusing on decreasing the time it takes to start up multi-site industry trials. When we got started with ACT, it was taking over 200 days to get started. And now, just a couple years later, we are well under 100 days, which is within industry standards.” Also, she said, the VA established a Partnered Research Program office, “which serves to interact with our industry partners and really guide them through the VA system, which can be complex if you’re approaching it for the first time.”
In a separate presentation, Krissa Caroff, MS, CPC, program manager of the Partnered Research Program, said it had quickened the process of implementing clinical trials by tackling roadblocks such as the need for multiple master agreements to be signed. Central coordination has been key, she said, “and we are working closely to ensure that we when have a multisite trial, all the VA sites are utilizing the same single IRB [institutional review board]. We’ve also identified the critical information that we need to collect from industry in order for us to evaluate a trial.”
What’s next? “We really are going to be focusing on oncology trials,” Ramoni insisted. “This is a high priority for us.” She added: “Please share your feedback and experiences with us. And also please communicate amongst your colleagues within your organization to explain how we’re standardizing things within VA.”
The speakers reported no relevant disclosures.
The US Department of Veterans Affairs (VA) is embracing clinical trials with a focus on oncology, and patients will benefit from new priorities and programs, VA officials reported at the Association of VA Hematology/Oncology (AVAHO) virtual meeting. “The whole model is one that is far more proactive,” said Carolyn Clancy, MD, Under Secretary for Health for Discovery, Education, and Affiliate Networks.
According to Clancy, the department’s top research priority is to increase veteran access to high-quality clinical trials. “Priority number 2 is increasing the real-world impact of VA research,” she said. “Our commitment to veterans and the taxpayers is to reverse and shorten the [research-to-implementation] timeline. And the third priority is to put VA data to work for veterans, not just through people who work in VA and Veterans Health Administration, but through other researchers who can have access to them.”
To meet these goals, VA is engaging in multiple research programs and collaborations. Rachel B. Ramoni, DMD, ScD, the VA chief research and development officer, highlighted a number of the projects in a separate AVAHO meeting presentation, including:
- The National Cancer Institute and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE), an interagency collaboration between the VA and the National Cancer Institute (NCI). This program established a network of sites to help enrolled veterans take part in NCI-supported clinical trials. “It really got up and running in 2018, and I’m proud to say that over 250 veterans have been enrolled, and enrollment exceeds that at non-NAVIGATE sites,” Ramoni reported. “Clearly, the additional support that these sites are getting is really helping to achieve the outcome of getting more veterans access to these trials.” However, she said, some areas of the nation aren’t yet covered by the program.
- The Precision Oncology Program for Cancer of the Prostate (POPCaP), established through a partnership with the Prostate Cancer Foundation. The foundation provided a $50 million investment. “This program ensures that veterans, no matter where they are, get best-in-class prostate cancer care,” Ramoni explained. “The initial focus was ensuring that men get sequencing if they have metastatic prostate cancer, and that they get access to clinical trials. The really distinguishing factor about POPCaP is that it has built a vibrant community of clinicians, researchers and program offices. The whole is much greater than the sum of its parts.” More POPCaP hubs are in development, she said.
- PATCH (Prostate Cancer Analysis for Therapy Choice), a program funded by the VA and the Prostate Cancer Foundation. “The whole purpose of PATCH is to create this network of sites to systematically go through different clinical trials that are biomarker-driven,” Ramoni said. “One of the great things about PATCH is that it’s leveraging the genetics databases to help proactively identify men who might qualify for these trials and to find them wherever they might be across the system so they have access to these trials.” She also praised the program’s commitment to collaboration and mentorship. “If you’re new to putting together clinical trials concepts or to submitting merit proposals to VA for funding, PATCH is a great place to get into a community that’s supportive and wants to help you succeed.”
- The VA Phenomics Library. This library, based at the Boston VA Medical Center, focuses on improving the analysis of “messy” electronic health record data, Ramoni noted. “There are automated algorithms that go through and help you clean up that data to make sense of it,” she said. “The problem is that it’s really been an every-person-for-himself-or-herself system. Each researcher who needed these phenotypes was creating his or her own.” The Phenomics Library will promote sharing “so there’s not going to be as much wasted time duplicating effort,” she said. “By the end of fiscal year 2021, we will have over 1,000 curated phenotypes in there. We hope that will be a great resource for the oncology community as well as many other communities.”
- Access to Clinical Trials (ACT) for Veterans. “This program, which began a couple of years ago, has really succeeded,” Ramoni said. “We were focusing on decreasing the time it takes to start up multi-site industry trials. When we got started with ACT, it was taking over 200 days to get started. And now, just a couple years later, we are well under 100 days, which is within industry standards.” Also, she said, the VA established a Partnered Research Program office, “which serves to interact with our industry partners and really guide them through the VA system, which can be complex if you’re approaching it for the first time.”
In a separate presentation, Krissa Caroff, MS, CPC, program manager of the Partnered Research Program, said it had quickened the process of implementing clinical trials by tackling roadblocks such as the need for multiple master agreements to be signed. Central coordination has been key, she said, “and we are working closely to ensure that we when have a multisite trial, all the VA sites are utilizing the same single IRB [institutional review board]. We’ve also identified the critical information that we need to collect from industry in order for us to evaluate a trial.”
What’s next? “We really are going to be focusing on oncology trials,” Ramoni insisted. “This is a high priority for us.” She added: “Please share your feedback and experiences with us. And also please communicate amongst your colleagues within your organization to explain how we’re standardizing things within VA.”
The speakers reported no relevant disclosures.
2020 Cancer Data Trends
August 2020 Advances in Precision Oncology
Click here to access August 2020 Advances in Precision Oncology
Table of Contents
- Foreword
- Introduction: Precision Oncology Changes the Game for VA Health Care
- VA National Precision Oncology Program
- Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model to Advance Treatment and Care of Invasive Cancers
- The Precision Oncology Program for Cancer of the Prostate Network: A VA-Prostate Cancer Foundation Alliance
- Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology
- Strategic Initiatives for Veterans With Lung Cancer
- Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities

Click here to access August 2020 Advances in Precision Oncology
Table of Contents
- Foreword
- Introduction: Precision Oncology Changes the Game for VA Health Care
- VA National Precision Oncology Program
- Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model to Advance Treatment and Care of Invasive Cancers
- The Precision Oncology Program for Cancer of the Prostate Network: A VA-Prostate Cancer Foundation Alliance
- Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology
- Strategic Initiatives for Veterans With Lung Cancer
- Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities

Click here to access August 2020 Advances in Precision Oncology
Table of Contents
- Foreword
- Introduction: Precision Oncology Changes the Game for VA Health Care
- VA National Precision Oncology Program
- Prostate Cancer Foundation-Department of Veterans Affairs Partnership: A Model to Advance Treatment and Care of Invasive Cancers
- The Precision Oncology Program for Cancer of the Prostate Network: A VA-Prostate Cancer Foundation Alliance
- Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology
- Strategic Initiatives for Veterans With Lung Cancer
- Integrating Germline Genetics Into Precision Oncology Practice in the Veterans Health Administration: Challenges and Opportunities

Statins linked to improved survival in multiple myeloma
Statin use was associated with an overall reduction of the risk of death in multiple myeloma (MM) patients, according to a report published in Clinical Lymphoma, Myeloma & Leukemia.
Statins maintained their benefit in patients with multiple myeloma treated with modern-day chemotherapy regimens based on novel agents, but the benefit is less pronounced, reported Amber Afzal, MD, Washington University, St Louis, and colleagues.
Dr. Afzal and colleagues assessed results from 5,922 patients who were diagnosed with multiple myeloma within the study period between 2007 and 2013. The association of statins with mortality in patients with MM was determined using multivariate Cox proportional hazards regression analysis, and a subanalysis was also performed to investigate the effect of statins on mortality in those patients treated with novel agents.
Mortality reduction seen
The study found that the use of statins was associated with a 21% reduction in risk of death (adjusted hazard ratio,] 0.79; 95% confidence interval, 0.74-0.84) among all patients with MM. Among the patents treated with novel agents (n = 3,603), statins reduced mortality by 10% (aHR, 0.90; 95% CI, 0.83-0.98).
“Our current study is the first one to support the survival benefit of statins in patients with myeloma treated with modern-day regimens based on novel agents, although it appears the benefit may not be as pronounced. Therefore, as myeloma regimens become more effective, the benefits of statins may diminish,” the researchers concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Afzal A et al. Clin Lymphoma Myeloma Leuk. 2020 Jul 16. doi: 10.1016/j.clml.2020.07.003.
Statin use was associated with an overall reduction of the risk of death in multiple myeloma (MM) patients, according to a report published in Clinical Lymphoma, Myeloma & Leukemia.
Statins maintained their benefit in patients with multiple myeloma treated with modern-day chemotherapy regimens based on novel agents, but the benefit is less pronounced, reported Amber Afzal, MD, Washington University, St Louis, and colleagues.
Dr. Afzal and colleagues assessed results from 5,922 patients who were diagnosed with multiple myeloma within the study period between 2007 and 2013. The association of statins with mortality in patients with MM was determined using multivariate Cox proportional hazards regression analysis, and a subanalysis was also performed to investigate the effect of statins on mortality in those patients treated with novel agents.
Mortality reduction seen
The study found that the use of statins was associated with a 21% reduction in risk of death (adjusted hazard ratio,] 0.79; 95% confidence interval, 0.74-0.84) among all patients with MM. Among the patents treated with novel agents (n = 3,603), statins reduced mortality by 10% (aHR, 0.90; 95% CI, 0.83-0.98).
“Our current study is the first one to support the survival benefit of statins in patients with myeloma treated with modern-day regimens based on novel agents, although it appears the benefit may not be as pronounced. Therefore, as myeloma regimens become more effective, the benefits of statins may diminish,” the researchers concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Afzal A et al. Clin Lymphoma Myeloma Leuk. 2020 Jul 16. doi: 10.1016/j.clml.2020.07.003.
Statin use was associated with an overall reduction of the risk of death in multiple myeloma (MM) patients, according to a report published in Clinical Lymphoma, Myeloma & Leukemia.
Statins maintained their benefit in patients with multiple myeloma treated with modern-day chemotherapy regimens based on novel agents, but the benefit is less pronounced, reported Amber Afzal, MD, Washington University, St Louis, and colleagues.
Dr. Afzal and colleagues assessed results from 5,922 patients who were diagnosed with multiple myeloma within the study period between 2007 and 2013. The association of statins with mortality in patients with MM was determined using multivariate Cox proportional hazards regression analysis, and a subanalysis was also performed to investigate the effect of statins on mortality in those patients treated with novel agents.
Mortality reduction seen
The study found that the use of statins was associated with a 21% reduction in risk of death (adjusted hazard ratio,] 0.79; 95% confidence interval, 0.74-0.84) among all patients with MM. Among the patents treated with novel agents (n = 3,603), statins reduced mortality by 10% (aHR, 0.90; 95% CI, 0.83-0.98).
“Our current study is the first one to support the survival benefit of statins in patients with myeloma treated with modern-day regimens based on novel agents, although it appears the benefit may not be as pronounced. Therefore, as myeloma regimens become more effective, the benefits of statins may diminish,” the researchers concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Afzal A et al. Clin Lymphoma Myeloma Leuk. 2020 Jul 16. doi: 10.1016/j.clml.2020.07.003.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Abstracts Presented at the 2020 AVAHO Annual Meeting (Digital Edition)
Study supports multigene panel testing for all breast cancer patients with second primary cancers
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
according to a paper published in JCO Precision Oncology.
The authors noted that women with breast cancer have a 4%-16% lifetime risk of a second primary cancer. However, it hasn’t been clear if mutations in genes other than BRCA1/2 are enriched in patients with multiple primary cancers.
“Surprisingly few papers have focused on genetic evaluation of patients with multiple primary cancers,” senior author Katherine L. Nathanson, MD, of the University of Pennsylvania in Philadelphia, said in an interview.
“Ours is one of the first studies to look closely at this issue. We know from clinical experience that these patients are more likely to have more than one genetic mutation,” she added.
For their study, Dr. Nathanson and colleagues identified pathogenic germline mutations in 17 cancer susceptibility genes in patients with BRCA1/2-negative breast cancer in two cohorts.
Cohort 1 consisted of 1,000 high-risk breast cancer patients – 551 with multiple primary cancers and 449 with a single breast cancer.
Cohort 2 included 1,804 familial breast cancer patients – 340 with multiple primaries and 1,464 with a single breast cancer.
The researchers assessed mutations in these cohorts and compared them with mutations in a control data set.
Mutation rates and age
Pathogenic mutation rates were higher in both cohorts in patients with multiple primaries as compared with patients with single primaries.
In cohort 1, the overall panel positive rate was 8.53% in the multiple-primaries group and 4.90% in the single-primary group (P = .024).
In cohort 2, the overall panel positive rate was 7.06% in the multiple-primaries group and 4.23% in the single-primary group (P = .034).
In both cohorts, younger age at first breast cancer was associated with higher mutation rates. However, the age at onset of cancers other than breast cancer was not related to mutation rate.
“Regardless of age, mutations in genes other than BRCA1/2 are found in at least 5% of patients with breast cancer and another primary cancer, with up to 25% in patients with their first breast cancer at age 30 years,” Dr. Nathanson said. “This supports the need for multigene panel testing in all patients with breast cancer and another primary cancer.”
“Once a woman has multiple primaries with breast cancer, it doesn’t matter what her family history is, she is more likely to be at risk,” Dr. Nathanson added.
Genetic susceptibility
The researchers also identified genes associated with multiple primary cancers. TP53 and MSH6 mutations were significantly enriched in patients with multiple primaries but not single primaries. ATM and PALB2 mutations were significantly enriched in both groups when compared with controls.
The researchers noted that high-penetrance cancer genes were responsible for higher mutation rates in the cohort enriched for early-onset breast cancer and non–breast cancer second primaries. Moderate-penetrance cancer genes were responsible for the higher mutation rates in the cohort enriched for familial breast cancer and second breast cancer primaries.
“In multiple primary cancers, we found additional genes with moderate penetrance and some genes with high penetrance associated with TP53 and Lynch syndrome,” Dr. Nathanson said.
Cancer prevention and screening
The results of this study could lead to better implementation of cancer prevention and screening strategies, according to the researchers.
“As we look at guidelines in development and NCCN recommendations, our data suggest that age should not be part of the criteria for genetic testing in patients who have more than one primary cancer. These patients are at high risk and should be recommended for screening,” Dr. Nathanson said.
“If you see a patient with multiple primary cancers, refer for genetic testing. Age does not matter,” she reiterated.
Future research will look at potentially missing mutations.
“With targeted sequencing, structurally rearranged genes might be missed for those at risk. We will try to identify cancer susceptibility genes and define the true risk of penetrance of these genes in the general population,” Dr. Nathanson said.
This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Dr. Nathanson disclosed no conflicts of interest. Other authors disclosed relationships with a range of companies, all listed in the paper.
SOURCE: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
FROM JCO PRECISION ONCOLOGY
Five reasons why medical meetings will never be the same
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
In the wake of the COVID-19 pandemic, the virtual medical meeting is now the norm. And while it’s admirable that key data are being disseminated (often for free), there is no escaping the fact that it is a fundamentally different and lesser experience.
Watching from home, most of us split our attention between live streams of the meeting and work and family obligations. There is far less urgency when early live presentations are recorded and can be viewed later.
In terms of discussing the data, Twitter may offer broader participation than a live meeting, yet only a small number of attendees actively engage online.
And the exhibit halls for these online meetings? With neither free coffee nor company-branded tchotchkes, I expect that they have virtual tumbleweeds blowing through and crickets chirping.
Even still, the virtual meeting experience, while inferior to the live one, is a tremendous advance. It should never be banished as a historical footnote but rather should remain an option. It’s analogous to watching the Super Bowl at home: Obviously, it’s not the same as being there, but it’s a terrific alternative. Like telemedicine, this pandemic has provided a critical proof of concept that there is a better model.
Reshaping the medical meeting
Let’s consider five reasons why medical meetings should be permanently reshaped by this pandemic.
This pandemic isn’t going away in 2020. While nearly every country has done a far better job than the United States of containing COVID-19 thus far, outbreaks remain a problem wherever crowds assemble. You’d be hard-pressed to devise a setting more conducive to mass spread than a conference of 20,000 attendees from all over the world sitting alongside each other cheek to jowl for 5 days. Worse yet is the thought of them returning home and infecting their patients, families, and friends. What medical society wants to be remembered for creating a COVID-19 superspreader event? Professional medical societies will need to offer this option as the safest alternative moving forward.
Virtual learning still conveys the most important content. Despite the many social benefits of a live meeting, its core purpose is to disseminate new research and current and emerging treatment options. Virtual meetings have proven that this format can effectively deliver the content, and not as a secondary offering but as the sole platform in real time.
Virtual learning levels the playing field. Traveling to attend conferences typically costs thousands of dollars, accounting for the registration fees, inflated hotel rates, ground transportation, and meals out for days on end. Most meetings also demand several days away from our work and families, forcing many of us to work extra in the days before we leave and upon our return. Parents and those with commitments at home also face special challenges. For international participants, the financial and time costs are even greater. A virtual meeting helps overcome these hurdles and erases barriers that have long precluded many from attending a conference.
Virtual learning is efficient and comfortable. Virtual meetings over the past 6 months have given us a glimpse of an astonishingly more efficient form. If the content seems of a lower magnitude without the fanfare of a live conference, it is in part because so much of a live meeting is spent walking a mile between session rooms, waiting in concession or taxi lines, sitting in traffic between venues, or simply waiting for a session to begin. All of that has been replaced with time that you can use productively in between video sessions viewed either live or on demand. And with a virtual meeting, you can comfortably watch the sessions. There’s no need to stand along the back wall of an overcrowded room or step over 10 people to squeeze into an open middle seat. You can be focused, rather than having an end-of-day presentation wash over you as your eyes cross because you’ve been running around for the past 12 hours.
Virtual learning and social media will only improve. While virtual meetings unquestionably have limitations, it’s important to acknowledge that the successes thus far still represent only the earliest forays into this endeavor. In-person meetings evolved to their present form over centuries. In contrast, virtual meetings are being cobbled together within a few weeks or months. They can only be expected to improve as presenters adapt their skills to the online audience and new tools improve virtual discussions.
I am not implying that live meetings will or should be replaced by virtual ones. We still need that experience of trainees and experts presenting to a live audience and discussing the results together, all while sharing the energy of the moment. But there should be room for both a live conference and a virtual version.
Practically speaking, it is unclear whether professional societies could forgo the revenue they receive from registration fees, meeting sponsorships, and corporate exhibits. Yet, there are certainly ways to obtain sponsorship revenue for a virtual program. Even if the virtual version of a conference costs far less than attending in person, there is plenty of room between that number and free. It costs remarkably little for a professional society to share its content, and virtual offerings further the mission of distributing this content broadly.
We should not rush to return to the previous status quo. Despite their limitations, virtual meetings have brought a new, higher standard of access and efficiency for sharing important new data and treatment options in medicine.
H. Jack West, MD, associate clinical professor and executive director of employer services at City of Hope Comprehensive Cancer Center in Duarte, Calif., regularly comments on lung cancer for Medscape. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing education programs and other educational programs, including hosting the audio podcast West Wind.
This article first appeared on Medscape.com.
First U.S. trial to test aerosolized chemotherapy in advanced cancers
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.
The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.