User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Doctors as trusted messengers
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
Not All Pulmonary Nodules in Smokers are Lung Cancer
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
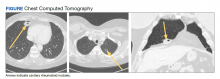
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
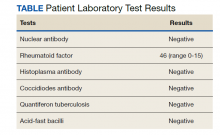
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
Identification of pulmonary nodules in older adults who smoke immediately brings concern for malignancy in the mind of clinicians. This is particularly the case in patients with significant smoking history. According to the National Cancer Institute in 2019, 12.9% of all new cancer cases were lung cancers.1 Screening for lung cancer, especially in patients with increased risk from smoking, is imperative to early detection and treatment. However, 20% of patients will be overdiagnosed by lung cancer-screening techniques.2 The rate of malignancy noted on a patient’s first screening computed tomography (CT) scan was between 3.7% and 5.5%.3
Rheumatoid arthritis (RA) is an autoimmune inflammatory condition that mainly affects the joints. Extraarticular manifestations can arise in various locations throughout the body, however. These manifestations are commonly observed in the skin, heart, and lungs.4 Prevalence of pulmonary rheumatoid nodules ranges from < 0.4% in radiologic studies to 32% in lung biopsies of patients with RA and nodules.5
Furthermore, there is a strong association between the risk of rheumatoid nodules in patients with positive serum rheumatoid factor (RF) and smoking history.6 Solitary pulmonary nodules in patients with RA can coexist with bronchogenic carcinoma, making their diagnosis more important.7
Case Presentation
A 54-year-old woman with a 30 pack-year smoking history and history of RA initially presented to the emergency department for cough and dyspnea for 5-day duration. Her initial diagnosis was bronchitis based on presenting symptom profile. A chest CT demonstrated 3 cavitary pulmonary nodules, 1 measuring 2.4 x 2.0 cm in the right middle lobe, and 2 additional nodules, measuring 1.8 x 1.4 and 1.5 x 1.4 in the left upper lobe (Figure). She had no improvement of symptoms after a 7-day course of doxycycline. The patient was taking methotrexate 15 mg weekly and golimumab 50 mg subcutaneously every 4 weeks as treatment for RA, prescribed by her rheumatologist.
Pulmonology was consulted and a positron emission tomography-CT (PET-CT) confirmed several cavitary pulmonary nodules involving both lungs with no suspicious fluorodeoxyglucose (FDG) uptake. The largest lesion was in the right middle lobe with FDG uptake of 1.9. Additional nodules were found in the left upper lobe, measuring 1.8 x 1.4 cm with FDG of 4.01, and in the left lung apex, measuring 1.5 x 1.4 cm with uptake of 3.53. CTguided percutaneous fine needle aspiration (PFNA) of the right middle lobe lung nodule demonstrated granuloma with central inflammatory debris. Grocott methenamine silver (GMS) stain was negative for fungal organism, acid-fast bacteria (AFB) stain was negative for acid-fast bacilli, and CD20 and CD3 immunostaining demonstrated mixed B- and T-cell populations. There was no evidence of atypia or malignancy. The biopsy demonstrated granuloma with central inflammatory debris on a background of densely fibrotic tissue and lympho-plasmatic inflammation. This finding confirmed the diagnosis of RA with pulmonary involvement.
Outpatient follow-up was established with a pulmonologist and rheumatologist. Methotrexate 15 mg weekly and golimumab subcutaneously 50 mg every 4 weeks were prescribed for the patient. The nodules are being monitored based on Fleischer guidelines with CT imaging 3 to 6 months following initial presentation. Further imaging will be considered at 18 to 24 months as well to further assess stability of the nodules and monitor for changes in size, shape, and necrosis. The patient also was encouraged to quit smoking. Her clinical course since the diagnosis has been stable.
Discussion
The differential diagnosis for new multiple pulmonary nodules on imaging studies is broad and includes infectious processes, such as tuberculosis, as well as other mycobacterial, fungal, and bacterial infections. Noninfectious causes of lung disease are an even broader category of consideration. Noninfectious pulmonary nodules differential includes sarcoidosis, granulomatous with polyangiitis, hypersensitivity pneumonitis, methotrexate drug reaction, pulmonary manifestations of systemic conditions, such as RA chronic granulomatous disease and malignancy.8 Bronchogenic carcinoma was suspected in this patient due to her smoking history. Squamous cell carcinoma was also considered as the lesion was cavitary. AFB and GMS stains were negative for fungi. Langerhans cell histiocytosis were considered but ruled out as these lesions contain larger numbers of eosinophils than described in the pathology report. Histoplasma and coccidiosis laboratory tests were obtained as the patient lived in a region endemic to both these fungi but were negative (Table). A diagnosis of rheumatoid nodule was made based on the clinical setting, typical radiographic, histopathology features, and negative cultures.
This case is unique due to the quality and location of the rheumatoid nodules within the lungs. Pulmonary manifestations of RA are usually subcutaneous or subpleural, solid, and peripherally located.9 This patient’s nodules were necrobiotic and located within the lung parenchyma. There was significant cavitation. These factors are atypical features of pulmonary RA.
Pulmonary RA can have many associated symptoms and remains an important factor in patient mortality. Estimates demonstrate that 10 to 20% of RA-related deaths are secondary to pulmonary manifestations.10 There are a wide array of symptoms and presentations to be aware of clinically. These symptoms are often nondescript, widely sensitive to many disease processes, and nonspecific to pulmonary RA. These symptoms include dyspnea, wheezing, and nonproductive cough.10 Bronchiectasis is a common symptom as well as small airway obstruction.10 Consolidated necrobiotic lesions are present in up to 20% of pulmonary RA cases.10 Generally these lesions are asymptomatic but can also be associated with pneumothorax, hemoptysis, and airway obstruction.10 Awareness of these symptoms is important for diagnosis and monitoring clinical improvement in patients.
Further workup is necessary to differentiate malignancy-related pulmonary nodules and other causes; if the index of suspicion is high for malignancy as in our case, the workup should be more aggressive. Biopsy is mandatory in such cases to rule out infections and malignancy, as it is highly sensitive and specific. The main problem hindering management is when a clinician fails to include this in their differential diagnosis. This further elucidates the importance of awareness of this diagnosis. Suspicious lesions in a proper clinical setting should be followed up by imaging studies and confirmatory histopathological diagnosis. Typical follow-up is 3 months after initial presentation to assess stability and possibly 18 to 24 months as well based on Fleischer guidelines.
Various treatment modalities have been tried as per literature, including tocilizumab and rituximab. 11,12 Our patient is currently being treated with golimumab based on outpatient rheumatologist recommendations.
Conclusions
This case demonstrates the importance of a careful workup to narrow a broad differential. Medical diagnosis of pulmonary nodules requires an in-depth workup, including clinical evaluation, laboratory and pulmonary functions tests, as well as various imaging studies.
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
1. Lung and Bronchus Cancer - Cancer Stat Facts. SEER. Accessed February 2, 2020. https://seer.cancer.gov /statfacts/html/lungb.html
2. Shaughnessy AF. One in Five Patients Overdiagnosed with Lung Cancer Screening. Am Fam Physician. 2014 Jul 15;90(2):112.
3. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369;910-919. doi:10.1056/NEJMoa1214726
4. Stamp LK, Cleland LG. Rheumatoid arthritis. In: Thompson LU, Ward WE, eds. Optimizing Women’s Health through Nutrition. CRC Press; 2008; 279-320.
5. Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770-777. doi:10.1164/arrd.1985.131.5.770
6. Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, Turesson C; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601-606. doi:10.1136/ard.2005.039172
7. Shenberger KN, Schned AR, Taylor TH. Rheumatoid disease and bronchogenic carcinoma—case report and review of the literature. J Rheumatol. 1984;11:226–228.
8. Mukhopadhyay S, Wilcox BE, Myers JL, et al. Pulmonary necrotizing granulomas of unknown cause clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813-824. doi:10.1378/chest.12-2113
9. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: Number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev. 2017;26(145):170012. Published 2017 Aug 9. doi:10.1183/16000617.0012-2017
10. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448. doi:10.1513/pats.200703-045MS
11. Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72(2):166-171. doi:10.1007/s00393-012-1054-0
12. Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford). 2012;51(6):1132-1134. doi:10.1093/rheumatology/ker455
More evidence ties some antipsychotics to increased breast cancer risk
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHOPHARMACOLOGY
Sacituzumab govitecan effective in Black mTNBC patients
, shows a prespecified analysis of ASCENT.
A heterogenous disease with few treatment options and poor outcomes, mTNBC has an incidence rate twice as high in Black as in White women.
Black women with mTNBC may also experience worse outcomes than other groups, with a greater risk of mortality related to disparities in access to health care and in income, delays in treatment, a higher prevalence of comorbidities, and differences in tumor biology.
Previously presented data from the phase 3 ASCENT trial showed that SG nearly doubled overall survival versus single-agent chemotherapy in pretreated women with mTNBC, with the benefit observed across patient subgroups.
Based on these findings, the Food and Drug Administration approved SG for patients with mTNBC who have received at least two prior chemotherapies, at least one of which is to have been given in the metastatic setting.
Now, an analysis of the ASCENT data in just over 60 Black women with mTNBC showed that they can expect to see their progression-free survival (PFS) improve by 56% and their overall survival increase by a nonsignificant 36% when given SG as opposed to single-agent chemotherapy.
The research (abstract P5-16-07) was presented at the San Antonio Breast Cancer Symposium on Dec. 10.
The team says that Black women with mTNBC “derived a similar clinical benefit” from SG versus chemotherapy to other women in the study, and had a “manageable” safety profile, which was “consistent with the full trial population.”
Consequently, SG “should be considered a treatment option for Black patients with mTNBC who have received ≥ 2 prior chemotherapies,” at least one of which having been given in the metastatic setting.
Lead researcher Lisa A. Carey, MD, told this news organiztion that it is “very important” to show that the drug works in Black patients, adding: “We know that certain drugs don’t perform so well and it’s also true that people of color are particularly affected by TNBC.”
She said there were “only 62” Black patients in ASCENT, “so if you look at the entire trial and make assumptions that the drug performs the same in all the subsets, then sometimes you’re wrong.”
Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research, UNC Lineberger Comprehensive Cancer Center, Chapel Hill, N.C., said there is “emerging interest” in racial disparities in cancer outcomes.
“Black patients have more trouble with access to care,” she said, noting that “in trial populations, [the outcomes] generally seem similar because the patients who go onto the trials tend to be those that can participate, but you never know until you look.”
Overall, Dr. Carey said the current results suggest that, “at least from the standpoint of the therapeutic implications of this drug – which is really a pretty remarkable drug in the overall study – it behaves very similarly in this group.”
Jennifer K. Litton, MD, vice president of clinical research at University of Texas MD Anderson Cancer Center, Houston, said: “We have known that minority patients, especially Black patients, have a higher rate of triple negative breast cancer and aggressive biologies, and have had worse breast cancer outcomes in many published series.”
She told this news organization that, “additionally, they are often underrepresented in breast cancer clinical trials.”
Dr. Litton said “the very favorable outcomes” reported in “this important subset of patients who participated in the ASCENT trial” confirm the use of SG in patients with mTNBC.
To examine the clinical outcomes of Black patients in the ASCENT study, the team conduced a prespecified analysis of participants self-reporting Black race who had been randomized to SG or single-agent chemotherapy of physician’s choice, including those with and without brain metastases.
Of the 529 patients enrolled to ASCENT, 62 (12%) were Black, of whom 28 were assigned to SG and 34 to single agent chemotherapy. The two groups were generally well balanced, although six patients in the chemotherapy arm had known brain metastases at baseline versus none of those given SG.
After a median treatment duration of 5.3 months with SG and 1.6 months for single-agent chemotherapy, there was a significant improvement in PFS with SG, at 5.4 months versus 2.2 months for chemotherapy, and a hazard ratio of 0.44 (P = .008).
There was also a nonsignificant improvement in overall survival with SG at 13.8 months versus 8.5 months for chemotherapy, and a hazard ratio of 0.64 (P = .159).
The objective response rate was 32% with SG versus 6% in patients given chemotherapy, while the median duration of response was 9.2 months in the SG arm and not evaluable for chemotherapy.
The researchers note that these efficacy findings were “consistent” with those seen in the full ASCENT study population.
In terms of safety, the most common treatment-related adverse events were neutropenia, seen in 64% of SG and 61% of chemotherapy patients, diarrhea in 64% and 13%, respectively, and fatigue, in 52% and 39%, respectively.
The most common grade ≥3 events were neutropenia, in 48% and 42% of SG and chemotherapy patients, respectively, followed by anemia, in 12% and 6%, respectively, leukopenia in 8% and 16%, respectively, and febrile neutropenia in 8% and 3%, respectively.
No treatment-related deaths occurred in either treatment arm.
Dose reduction due to treatment-emergent adverse events was recorded in 28% of patients receiving SG and 35% of those assigned to single-agent chemotherapy, and discontinuations occurred in 0% and 3%, respectively.
The study was sponsored by Gilead Sciences. Dr. Carey reports research funding from Sanofi, Novartis, Genentech/Roche, and GSK; spouse serves on the board of Falcon Therapeutics.
, shows a prespecified analysis of ASCENT.
A heterogenous disease with few treatment options and poor outcomes, mTNBC has an incidence rate twice as high in Black as in White women.
Black women with mTNBC may also experience worse outcomes than other groups, with a greater risk of mortality related to disparities in access to health care and in income, delays in treatment, a higher prevalence of comorbidities, and differences in tumor biology.
Previously presented data from the phase 3 ASCENT trial showed that SG nearly doubled overall survival versus single-agent chemotherapy in pretreated women with mTNBC, with the benefit observed across patient subgroups.
Based on these findings, the Food and Drug Administration approved SG for patients with mTNBC who have received at least two prior chemotherapies, at least one of which is to have been given in the metastatic setting.
Now, an analysis of the ASCENT data in just over 60 Black women with mTNBC showed that they can expect to see their progression-free survival (PFS) improve by 56% and their overall survival increase by a nonsignificant 36% when given SG as opposed to single-agent chemotherapy.
The research (abstract P5-16-07) was presented at the San Antonio Breast Cancer Symposium on Dec. 10.
The team says that Black women with mTNBC “derived a similar clinical benefit” from SG versus chemotherapy to other women in the study, and had a “manageable” safety profile, which was “consistent with the full trial population.”
Consequently, SG “should be considered a treatment option for Black patients with mTNBC who have received ≥ 2 prior chemotherapies,” at least one of which having been given in the metastatic setting.
Lead researcher Lisa A. Carey, MD, told this news organiztion that it is “very important” to show that the drug works in Black patients, adding: “We know that certain drugs don’t perform so well and it’s also true that people of color are particularly affected by TNBC.”
She said there were “only 62” Black patients in ASCENT, “so if you look at the entire trial and make assumptions that the drug performs the same in all the subsets, then sometimes you’re wrong.”
Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research, UNC Lineberger Comprehensive Cancer Center, Chapel Hill, N.C., said there is “emerging interest” in racial disparities in cancer outcomes.
“Black patients have more trouble with access to care,” she said, noting that “in trial populations, [the outcomes] generally seem similar because the patients who go onto the trials tend to be those that can participate, but you never know until you look.”
Overall, Dr. Carey said the current results suggest that, “at least from the standpoint of the therapeutic implications of this drug – which is really a pretty remarkable drug in the overall study – it behaves very similarly in this group.”
Jennifer K. Litton, MD, vice president of clinical research at University of Texas MD Anderson Cancer Center, Houston, said: “We have known that minority patients, especially Black patients, have a higher rate of triple negative breast cancer and aggressive biologies, and have had worse breast cancer outcomes in many published series.”
She told this news organization that, “additionally, they are often underrepresented in breast cancer clinical trials.”
Dr. Litton said “the very favorable outcomes” reported in “this important subset of patients who participated in the ASCENT trial” confirm the use of SG in patients with mTNBC.
To examine the clinical outcomes of Black patients in the ASCENT study, the team conduced a prespecified analysis of participants self-reporting Black race who had been randomized to SG or single-agent chemotherapy of physician’s choice, including those with and without brain metastases.
Of the 529 patients enrolled to ASCENT, 62 (12%) were Black, of whom 28 were assigned to SG and 34 to single agent chemotherapy. The two groups were generally well balanced, although six patients in the chemotherapy arm had known brain metastases at baseline versus none of those given SG.
After a median treatment duration of 5.3 months with SG and 1.6 months for single-agent chemotherapy, there was a significant improvement in PFS with SG, at 5.4 months versus 2.2 months for chemotherapy, and a hazard ratio of 0.44 (P = .008).
There was also a nonsignificant improvement in overall survival with SG at 13.8 months versus 8.5 months for chemotherapy, and a hazard ratio of 0.64 (P = .159).
The objective response rate was 32% with SG versus 6% in patients given chemotherapy, while the median duration of response was 9.2 months in the SG arm and not evaluable for chemotherapy.
The researchers note that these efficacy findings were “consistent” with those seen in the full ASCENT study population.
In terms of safety, the most common treatment-related adverse events were neutropenia, seen in 64% of SG and 61% of chemotherapy patients, diarrhea in 64% and 13%, respectively, and fatigue, in 52% and 39%, respectively.
The most common grade ≥3 events were neutropenia, in 48% and 42% of SG and chemotherapy patients, respectively, followed by anemia, in 12% and 6%, respectively, leukopenia in 8% and 16%, respectively, and febrile neutropenia in 8% and 3%, respectively.
No treatment-related deaths occurred in either treatment arm.
Dose reduction due to treatment-emergent adverse events was recorded in 28% of patients receiving SG and 35% of those assigned to single-agent chemotherapy, and discontinuations occurred in 0% and 3%, respectively.
The study was sponsored by Gilead Sciences. Dr. Carey reports research funding from Sanofi, Novartis, Genentech/Roche, and GSK; spouse serves on the board of Falcon Therapeutics.
, shows a prespecified analysis of ASCENT.
A heterogenous disease with few treatment options and poor outcomes, mTNBC has an incidence rate twice as high in Black as in White women.
Black women with mTNBC may also experience worse outcomes than other groups, with a greater risk of mortality related to disparities in access to health care and in income, delays in treatment, a higher prevalence of comorbidities, and differences in tumor biology.
Previously presented data from the phase 3 ASCENT trial showed that SG nearly doubled overall survival versus single-agent chemotherapy in pretreated women with mTNBC, with the benefit observed across patient subgroups.
Based on these findings, the Food and Drug Administration approved SG for patients with mTNBC who have received at least two prior chemotherapies, at least one of which is to have been given in the metastatic setting.
Now, an analysis of the ASCENT data in just over 60 Black women with mTNBC showed that they can expect to see their progression-free survival (PFS) improve by 56% and their overall survival increase by a nonsignificant 36% when given SG as opposed to single-agent chemotherapy.
The research (abstract P5-16-07) was presented at the San Antonio Breast Cancer Symposium on Dec. 10.
The team says that Black women with mTNBC “derived a similar clinical benefit” from SG versus chemotherapy to other women in the study, and had a “manageable” safety profile, which was “consistent with the full trial population.”
Consequently, SG “should be considered a treatment option for Black patients with mTNBC who have received ≥ 2 prior chemotherapies,” at least one of which having been given in the metastatic setting.
Lead researcher Lisa A. Carey, MD, told this news organiztion that it is “very important” to show that the drug works in Black patients, adding: “We know that certain drugs don’t perform so well and it’s also true that people of color are particularly affected by TNBC.”
She said there were “only 62” Black patients in ASCENT, “so if you look at the entire trial and make assumptions that the drug performs the same in all the subsets, then sometimes you’re wrong.”
Dr. Carey, the Richardson and Marilyn Jacobs Preyer Distinguished Professor in Breast Cancer Research, UNC Lineberger Comprehensive Cancer Center, Chapel Hill, N.C., said there is “emerging interest” in racial disparities in cancer outcomes.
“Black patients have more trouble with access to care,” she said, noting that “in trial populations, [the outcomes] generally seem similar because the patients who go onto the trials tend to be those that can participate, but you never know until you look.”
Overall, Dr. Carey said the current results suggest that, “at least from the standpoint of the therapeutic implications of this drug – which is really a pretty remarkable drug in the overall study – it behaves very similarly in this group.”
Jennifer K. Litton, MD, vice president of clinical research at University of Texas MD Anderson Cancer Center, Houston, said: “We have known that minority patients, especially Black patients, have a higher rate of triple negative breast cancer and aggressive biologies, and have had worse breast cancer outcomes in many published series.”
She told this news organization that, “additionally, they are often underrepresented in breast cancer clinical trials.”
Dr. Litton said “the very favorable outcomes” reported in “this important subset of patients who participated in the ASCENT trial” confirm the use of SG in patients with mTNBC.
To examine the clinical outcomes of Black patients in the ASCENT study, the team conduced a prespecified analysis of participants self-reporting Black race who had been randomized to SG or single-agent chemotherapy of physician’s choice, including those with and without brain metastases.
Of the 529 patients enrolled to ASCENT, 62 (12%) were Black, of whom 28 were assigned to SG and 34 to single agent chemotherapy. The two groups were generally well balanced, although six patients in the chemotherapy arm had known brain metastases at baseline versus none of those given SG.
After a median treatment duration of 5.3 months with SG and 1.6 months for single-agent chemotherapy, there was a significant improvement in PFS with SG, at 5.4 months versus 2.2 months for chemotherapy, and a hazard ratio of 0.44 (P = .008).
There was also a nonsignificant improvement in overall survival with SG at 13.8 months versus 8.5 months for chemotherapy, and a hazard ratio of 0.64 (P = .159).
The objective response rate was 32% with SG versus 6% in patients given chemotherapy, while the median duration of response was 9.2 months in the SG arm and not evaluable for chemotherapy.
The researchers note that these efficacy findings were “consistent” with those seen in the full ASCENT study population.
In terms of safety, the most common treatment-related adverse events were neutropenia, seen in 64% of SG and 61% of chemotherapy patients, diarrhea in 64% and 13%, respectively, and fatigue, in 52% and 39%, respectively.
The most common grade ≥3 events were neutropenia, in 48% and 42% of SG and chemotherapy patients, respectively, followed by anemia, in 12% and 6%, respectively, leukopenia in 8% and 16%, respectively, and febrile neutropenia in 8% and 3%, respectively.
No treatment-related deaths occurred in either treatment arm.
Dose reduction due to treatment-emergent adverse events was recorded in 28% of patients receiving SG and 35% of those assigned to single-agent chemotherapy, and discontinuations occurred in 0% and 3%, respectively.
The study was sponsored by Gilead Sciences. Dr. Carey reports research funding from Sanofi, Novartis, Genentech/Roche, and GSK; spouse serves on the board of Falcon Therapeutics.
FROM SABCS 2021
PD-L1 cutoff for pembrolizumab in mTNBC confirmed
recently presented at the San Antonio Breast Cancer Symposium.
Patients enrolled in KEYNOTE-355 – which is a phase 3, placebo-controlled trial of 847 patients – were stratified by CPS scores of at least 1 and at least 10, with the latter group in which adding pembrolizumab to chemotherapy was shown to significantly improve both overall survival and progression-free survival.
As it was unclear whether taking a more fine-grained approach would reveal specific CPS scores at which pembrolizumab would be beneficial, Javier Cortes, MD, PhD, International Breast Cancer Center, Barcelona, and colleagues divided the patients into four CPS levels: less than 1, 1-9, 10-19, and at least 20.
Patients with a CPS 10-19 and at least 20 given pembrolizumab alongside chemotherapy had an overall survival benefit of 29% and 28%, respectively, while the PFS improvement was 30% and 38%. In the CPS of less than 1 and 1-9 groups, there were no discernible benefits from adding the checkpoint inhibitor.
“Given the similar outcomes in the CPS 10-19 and the CPS ≥20 subgroups, a CPS of 10 or more is a reasonable cutoff to define the population of patients with metastatic TNBC that might have benefit from the addition of pembrolizumab to chemotherapy,” Dr. Cortes said. “In my opinion, these results provide further support for pembrolizumab in combination with chemotherapy as a good option, maybe a standard of care for some patients ... with local recurrent unresectable or metastatic TNBC whose tumors express PD-1 CPS ≥10.”
Invited discussant Hope S. Rugo, MD, said the study demonstrates that PD-L1 CPS of at least 10 is “clearly the optimal cutoff for differentiating benefit from pembrolizumab” and confirms the combination with chemotherapy as a “standard of care in this population”.
However, there are a number of outstanding questions in the metastatic setting, she said, including the test used to determine PD-L1 expression.
“Clearly the test that you order should be matched to the planned checkpoint inhibitor, and we look forward to additional data” on the relative overlap of the assays used in both the current study and in KEYNOTE-522.
However, IMpassion130 showed there is “incomplete overlap in terms of the two antibodies and tests that have been used to define PD-L1 positivity in breast cancer,” said Dr. Rugo, professor of medicine in hematology and oncology at the University of California, San Francisco.
“For excellent responders, can chemotherapy and eventually immunotherapy be discontinued, and when is it optimal? How long should we be continuing the combination and how long should we continue the checkpoint inhibitor alone?” she asked.
“Certainly in my own clinical practice,” Dr. Rugo explained, “in those excellent responders, it’s difficult to know when to stop the checkpoint inhibitor, but sometimes toxicity tells us the answer to that question. At some point, we need to stop therapy and understand what happens to those patients.”
She said that only 38% of patients in the current study benefited from pembrolizumab. “How can we amplify the immune response in those patients who do not have PD-L1–positive disease to further extend this benefit, and can we extend the efficacy to other subtypes? There are ongoing studies evaluating this question,” Dr. Rugo said.
Dr. Cortes said that KEYNOTE-355 showed the addition of pembrolizumab to chemotherapy led to clinically meaningful improvements in both PFS and overall survival versus chemotherapy alone in the first-line treatment of mTNBC.
However, that benefit was seen only in patients with a PD-L1 CPS of at least 10, while there was no statistically significant improvement in either PFS or overall survival in those with a CPS of at least 1.
He explained that 847 patients with previously untreated locally recurrent or metastatic TNBC, or those who had been treated at least 6 months prior to disease recurrence, were randomized 2:1 to pembrolizumab or placebo plus chemotherapy.
For the current analysis, they substratified patients by PD-L1 CPS into less than 1, which accounted for 24.9% of patients; 1-9, seen in 36.2%-38.4%; 10-19, accounting for 13.9%-14.1%; and at least 20, seen in 22.8%-24.7% of patients.
Dr. Cortes said the overall survival rate among patients with CPS of at least 10 was 70.5% for patients treated with pembrolizumab plus chemotherapy versus 81.6% for those assigned to placebo, at a significant hazard ratio of 0.73 (P = .0093).
Among patients with CPS of at least 1, the overall survival rate was 79.1% with pembrolizumab plus chemotherapy and 83.9% in those given placebo, at a nonsignificant hazard ratio of 0.86. This translated into an HR of 0.89 in the intention-to-treat analysis.
Turning to the novel subgroups, Dr. Cortes showed that the HR for overall survival for pembrolizumab versus placebo was nonsignificant in patients with CPS of at least 1, at 0.97, and in those with CPS 1-9, at 1.09.
However, the HRs were markedly improved in patients with CPD 10-19, at 0.71, and in those with CPS of at least 20, at 0.72, showing that the “relative benefit of adding pembrolizumab to chemotherapy was pretty much the same ... suggesting that CPS ≥10 could be a reasonable cutoff.”
In both of these groups, there was a sustained separation in the overall survival curves starting at around 10 months.
Turning to the PFS results, Dr Cortes said the event-free rate was 65.5% with the addition of pembrolizumab to chemotherapy in patients with PD-L1 CPS of at least 10, while those given placebo had a rate of 78.6%, at an HR of 0.66.
In patients with PD-L1 CPS of at least 1, the HR was 0.75, or 0.82 in the intention-to-treat analysis.
“As with overall survival,” he said, there was a “trend toward improved efficacy with PD-L1 enrichment with the addition of pembrolizumab to chemotherapy, although the PFS benefit in the pembro arm was slightly greater in the CPS ≥20 subgroup, compared to the CPS 10-19 subgroup.”
However, they highlighted that the difference was “small and the confidence intervals clearly overlapped.”
Why does PD-L1 expression play a role in response to pembrolizumab in mTNBC, but not in the early disease setting as seen in KEYNOTE-522?
“This is a question we have raised many, many times and have had many debates on,” Dr. Cortes said. “They are two completely different populations with the early breast cancer setting completely different to that in metastatic disease. Maybe the microenvironment plays a different role there, maybe we have to explore more in detail other biomarkers. I also think that different drugs were used in the neoadjuvant setting. We still have many unanswered questions.”
Dr. Rugo suggested that previous studies have given some clues to these questions with reductions in PD-L1 expression and tumor-infiltrating leukocytes observed between primary and metastatic disease.
The immune differences between primary and metastatic disease lead to immune escape, she said, adding: “This is clearly complicated by mutational complexity under the pressure of treatment.”
The study was funded by Merck Sharp and Dohme. Dr. Cortes and Dr. Rugo reported relationships with numerous pharmaceutical companies.
recently presented at the San Antonio Breast Cancer Symposium.
Patients enrolled in KEYNOTE-355 – which is a phase 3, placebo-controlled trial of 847 patients – were stratified by CPS scores of at least 1 and at least 10, with the latter group in which adding pembrolizumab to chemotherapy was shown to significantly improve both overall survival and progression-free survival.
As it was unclear whether taking a more fine-grained approach would reveal specific CPS scores at which pembrolizumab would be beneficial, Javier Cortes, MD, PhD, International Breast Cancer Center, Barcelona, and colleagues divided the patients into four CPS levels: less than 1, 1-9, 10-19, and at least 20.
Patients with a CPS 10-19 and at least 20 given pembrolizumab alongside chemotherapy had an overall survival benefit of 29% and 28%, respectively, while the PFS improvement was 30% and 38%. In the CPS of less than 1 and 1-9 groups, there were no discernible benefits from adding the checkpoint inhibitor.
“Given the similar outcomes in the CPS 10-19 and the CPS ≥20 subgroups, a CPS of 10 or more is a reasonable cutoff to define the population of patients with metastatic TNBC that might have benefit from the addition of pembrolizumab to chemotherapy,” Dr. Cortes said. “In my opinion, these results provide further support for pembrolizumab in combination with chemotherapy as a good option, maybe a standard of care for some patients ... with local recurrent unresectable or metastatic TNBC whose tumors express PD-1 CPS ≥10.”
Invited discussant Hope S. Rugo, MD, said the study demonstrates that PD-L1 CPS of at least 10 is “clearly the optimal cutoff for differentiating benefit from pembrolizumab” and confirms the combination with chemotherapy as a “standard of care in this population”.
However, there are a number of outstanding questions in the metastatic setting, she said, including the test used to determine PD-L1 expression.
“Clearly the test that you order should be matched to the planned checkpoint inhibitor, and we look forward to additional data” on the relative overlap of the assays used in both the current study and in KEYNOTE-522.
However, IMpassion130 showed there is “incomplete overlap in terms of the two antibodies and tests that have been used to define PD-L1 positivity in breast cancer,” said Dr. Rugo, professor of medicine in hematology and oncology at the University of California, San Francisco.
“For excellent responders, can chemotherapy and eventually immunotherapy be discontinued, and when is it optimal? How long should we be continuing the combination and how long should we continue the checkpoint inhibitor alone?” she asked.
“Certainly in my own clinical practice,” Dr. Rugo explained, “in those excellent responders, it’s difficult to know when to stop the checkpoint inhibitor, but sometimes toxicity tells us the answer to that question. At some point, we need to stop therapy and understand what happens to those patients.”
She said that only 38% of patients in the current study benefited from pembrolizumab. “How can we amplify the immune response in those patients who do not have PD-L1–positive disease to further extend this benefit, and can we extend the efficacy to other subtypes? There are ongoing studies evaluating this question,” Dr. Rugo said.
Dr. Cortes said that KEYNOTE-355 showed the addition of pembrolizumab to chemotherapy led to clinically meaningful improvements in both PFS and overall survival versus chemotherapy alone in the first-line treatment of mTNBC.
However, that benefit was seen only in patients with a PD-L1 CPS of at least 10, while there was no statistically significant improvement in either PFS or overall survival in those with a CPS of at least 1.
He explained that 847 patients with previously untreated locally recurrent or metastatic TNBC, or those who had been treated at least 6 months prior to disease recurrence, were randomized 2:1 to pembrolizumab or placebo plus chemotherapy.
For the current analysis, they substratified patients by PD-L1 CPS into less than 1, which accounted for 24.9% of patients; 1-9, seen in 36.2%-38.4%; 10-19, accounting for 13.9%-14.1%; and at least 20, seen in 22.8%-24.7% of patients.
Dr. Cortes said the overall survival rate among patients with CPS of at least 10 was 70.5% for patients treated with pembrolizumab plus chemotherapy versus 81.6% for those assigned to placebo, at a significant hazard ratio of 0.73 (P = .0093).
Among patients with CPS of at least 1, the overall survival rate was 79.1% with pembrolizumab plus chemotherapy and 83.9% in those given placebo, at a nonsignificant hazard ratio of 0.86. This translated into an HR of 0.89 in the intention-to-treat analysis.
Turning to the novel subgroups, Dr. Cortes showed that the HR for overall survival for pembrolizumab versus placebo was nonsignificant in patients with CPS of at least 1, at 0.97, and in those with CPS 1-9, at 1.09.
However, the HRs were markedly improved in patients with CPD 10-19, at 0.71, and in those with CPS of at least 20, at 0.72, showing that the “relative benefit of adding pembrolizumab to chemotherapy was pretty much the same ... suggesting that CPS ≥10 could be a reasonable cutoff.”
In both of these groups, there was a sustained separation in the overall survival curves starting at around 10 months.
Turning to the PFS results, Dr Cortes said the event-free rate was 65.5% with the addition of pembrolizumab to chemotherapy in patients with PD-L1 CPS of at least 10, while those given placebo had a rate of 78.6%, at an HR of 0.66.
In patients with PD-L1 CPS of at least 1, the HR was 0.75, or 0.82 in the intention-to-treat analysis.
“As with overall survival,” he said, there was a “trend toward improved efficacy with PD-L1 enrichment with the addition of pembrolizumab to chemotherapy, although the PFS benefit in the pembro arm was slightly greater in the CPS ≥20 subgroup, compared to the CPS 10-19 subgroup.”
However, they highlighted that the difference was “small and the confidence intervals clearly overlapped.”
Why does PD-L1 expression play a role in response to pembrolizumab in mTNBC, but not in the early disease setting as seen in KEYNOTE-522?
“This is a question we have raised many, many times and have had many debates on,” Dr. Cortes said. “They are two completely different populations with the early breast cancer setting completely different to that in metastatic disease. Maybe the microenvironment plays a different role there, maybe we have to explore more in detail other biomarkers. I also think that different drugs were used in the neoadjuvant setting. We still have many unanswered questions.”
Dr. Rugo suggested that previous studies have given some clues to these questions with reductions in PD-L1 expression and tumor-infiltrating leukocytes observed between primary and metastatic disease.
The immune differences between primary and metastatic disease lead to immune escape, she said, adding: “This is clearly complicated by mutational complexity under the pressure of treatment.”
The study was funded by Merck Sharp and Dohme. Dr. Cortes and Dr. Rugo reported relationships with numerous pharmaceutical companies.
recently presented at the San Antonio Breast Cancer Symposium.
Patients enrolled in KEYNOTE-355 – which is a phase 3, placebo-controlled trial of 847 patients – were stratified by CPS scores of at least 1 and at least 10, with the latter group in which adding pembrolizumab to chemotherapy was shown to significantly improve both overall survival and progression-free survival.
As it was unclear whether taking a more fine-grained approach would reveal specific CPS scores at which pembrolizumab would be beneficial, Javier Cortes, MD, PhD, International Breast Cancer Center, Barcelona, and colleagues divided the patients into four CPS levels: less than 1, 1-9, 10-19, and at least 20.
Patients with a CPS 10-19 and at least 20 given pembrolizumab alongside chemotherapy had an overall survival benefit of 29% and 28%, respectively, while the PFS improvement was 30% and 38%. In the CPS of less than 1 and 1-9 groups, there were no discernible benefits from adding the checkpoint inhibitor.
“Given the similar outcomes in the CPS 10-19 and the CPS ≥20 subgroups, a CPS of 10 or more is a reasonable cutoff to define the population of patients with metastatic TNBC that might have benefit from the addition of pembrolizumab to chemotherapy,” Dr. Cortes said. “In my opinion, these results provide further support for pembrolizumab in combination with chemotherapy as a good option, maybe a standard of care for some patients ... with local recurrent unresectable or metastatic TNBC whose tumors express PD-1 CPS ≥10.”
Invited discussant Hope S. Rugo, MD, said the study demonstrates that PD-L1 CPS of at least 10 is “clearly the optimal cutoff for differentiating benefit from pembrolizumab” and confirms the combination with chemotherapy as a “standard of care in this population”.
However, there are a number of outstanding questions in the metastatic setting, she said, including the test used to determine PD-L1 expression.
“Clearly the test that you order should be matched to the planned checkpoint inhibitor, and we look forward to additional data” on the relative overlap of the assays used in both the current study and in KEYNOTE-522.
However, IMpassion130 showed there is “incomplete overlap in terms of the two antibodies and tests that have been used to define PD-L1 positivity in breast cancer,” said Dr. Rugo, professor of medicine in hematology and oncology at the University of California, San Francisco.
“For excellent responders, can chemotherapy and eventually immunotherapy be discontinued, and when is it optimal? How long should we be continuing the combination and how long should we continue the checkpoint inhibitor alone?” she asked.
“Certainly in my own clinical practice,” Dr. Rugo explained, “in those excellent responders, it’s difficult to know when to stop the checkpoint inhibitor, but sometimes toxicity tells us the answer to that question. At some point, we need to stop therapy and understand what happens to those patients.”
She said that only 38% of patients in the current study benefited from pembrolizumab. “How can we amplify the immune response in those patients who do not have PD-L1–positive disease to further extend this benefit, and can we extend the efficacy to other subtypes? There are ongoing studies evaluating this question,” Dr. Rugo said.
Dr. Cortes said that KEYNOTE-355 showed the addition of pembrolizumab to chemotherapy led to clinically meaningful improvements in both PFS and overall survival versus chemotherapy alone in the first-line treatment of mTNBC.
However, that benefit was seen only in patients with a PD-L1 CPS of at least 10, while there was no statistically significant improvement in either PFS or overall survival in those with a CPS of at least 1.
He explained that 847 patients with previously untreated locally recurrent or metastatic TNBC, or those who had been treated at least 6 months prior to disease recurrence, were randomized 2:1 to pembrolizumab or placebo plus chemotherapy.
For the current analysis, they substratified patients by PD-L1 CPS into less than 1, which accounted for 24.9% of patients; 1-9, seen in 36.2%-38.4%; 10-19, accounting for 13.9%-14.1%; and at least 20, seen in 22.8%-24.7% of patients.
Dr. Cortes said the overall survival rate among patients with CPS of at least 10 was 70.5% for patients treated with pembrolizumab plus chemotherapy versus 81.6% for those assigned to placebo, at a significant hazard ratio of 0.73 (P = .0093).
Among patients with CPS of at least 1, the overall survival rate was 79.1% with pembrolizumab plus chemotherapy and 83.9% in those given placebo, at a nonsignificant hazard ratio of 0.86. This translated into an HR of 0.89 in the intention-to-treat analysis.
Turning to the novel subgroups, Dr. Cortes showed that the HR for overall survival for pembrolizumab versus placebo was nonsignificant in patients with CPS of at least 1, at 0.97, and in those with CPS 1-9, at 1.09.
However, the HRs were markedly improved in patients with CPD 10-19, at 0.71, and in those with CPS of at least 20, at 0.72, showing that the “relative benefit of adding pembrolizumab to chemotherapy was pretty much the same ... suggesting that CPS ≥10 could be a reasonable cutoff.”
In both of these groups, there was a sustained separation in the overall survival curves starting at around 10 months.
Turning to the PFS results, Dr Cortes said the event-free rate was 65.5% with the addition of pembrolizumab to chemotherapy in patients with PD-L1 CPS of at least 10, while those given placebo had a rate of 78.6%, at an HR of 0.66.
In patients with PD-L1 CPS of at least 1, the HR was 0.75, or 0.82 in the intention-to-treat analysis.
“As with overall survival,” he said, there was a “trend toward improved efficacy with PD-L1 enrichment with the addition of pembrolizumab to chemotherapy, although the PFS benefit in the pembro arm was slightly greater in the CPS ≥20 subgroup, compared to the CPS 10-19 subgroup.”
However, they highlighted that the difference was “small and the confidence intervals clearly overlapped.”
Why does PD-L1 expression play a role in response to pembrolizumab in mTNBC, but not in the early disease setting as seen in KEYNOTE-522?
“This is a question we have raised many, many times and have had many debates on,” Dr. Cortes said. “They are two completely different populations with the early breast cancer setting completely different to that in metastatic disease. Maybe the microenvironment plays a different role there, maybe we have to explore more in detail other biomarkers. I also think that different drugs were used in the neoadjuvant setting. We still have many unanswered questions.”
Dr. Rugo suggested that previous studies have given some clues to these questions with reductions in PD-L1 expression and tumor-infiltrating leukocytes observed between primary and metastatic disease.
The immune differences between primary and metastatic disease lead to immune escape, she said, adding: “This is clearly complicated by mutational complexity under the pressure of treatment.”
The study was funded by Merck Sharp and Dohme. Dr. Cortes and Dr. Rugo reported relationships with numerous pharmaceutical companies.
FROM SABCS 2021
NHL: As a second-line treatment in phase 3 trial, tisa-cel disappoints
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
FROM ASH 2021
TKI/BiTE combo extends survival of older patients with Ph+ALL
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2021
Isatuximab added to RVd boosts response in new myeloma
ATLANTA -
The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
Now it has shown benefit in patients who have been newly diagnosed with the disease. When isatuximab was added onto a usual triplet therapy for myeloma, it increased the likelihood that patients would be negative for minimal residual disease (MRD) at the end of the induction phase of treatment, thereby increasing their chances for a successful autologous stem cell transplant (ASCT).
The new results come from the GMMG-HD7 trial, in which all patients were treated with the triplet combination of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd).
Some patients, after randomization, also received isatuximab, and in this group, the MRD-negativity rate was 50.1% at the end of induction therapy compared with 35.6% for patients treated with RVd alone.
Patients who are MRD-negative at the time of ASCT have significantly better outcomes than patients who remain MRD-positive.
“Isa-RVd is the first regimen to demonstrate significant MRD-negativity benefit at the end of induction versus RVd in a phase 3 trial,” reported Hartmut Goldschmidt, MD, from University Hospital Heidelberg, Germany.
“The benefits of the addition of Isa to RVd versus RVd regarding MRD negativity after induction therapy was consistent in all subgroups,” he added.
Dr. Goldschmidt spoke at a press briefing prior to his presentation of the data here at the annual meeting of the American Society of Hematology (ASH).
“I think that these data are encouraging, but they are preliminary, and we need mature data to be absolutely certain about whether this presents a major advance in treatment,” commented Ravi Vij, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis. Dr. Vij was not involved in the study.
“We know that for transplant-eligible patients, for whom this trial was conducted, the field is moving toward giving four drugs for induction,” he said in an interview with this news organization.
He noted that the combination of RVd with the other currently available anti-CD38 antibody, daratumumab (Darzalex), was approved for this indication in the United States in Jan. 2021.
Dr. Vij said that isatuximab has been slow to catch on in the United States both because it was approved after clinicians had already become familiar with daratumumab and because it is given intravenously, compared with subcutaneous administration of the latest formulation of daratumumab.
“Whereas isatuximab can take an hour-and-a-half with each infusion, daratumumab takes 5 minutes for an injection and the patient is out of there, so it is convenient both for the patient and the treating institution,” he said.
MRD vs. CR?
Dr. Goldschmidt was asked during the briefing about whether MRD-negativity or complete response rates are better predictors of progression-free survival (PFS). He replied that with current standardized sequencing techniques and sensitivity down to 10-6, “it’s a big benefit to analyze MRD negativity, and there is ongoing discussion between colleagues from the myeloma group with the Food and Drug Administration about how we can merge the data and predict PFS and overall survival.”
Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer, Vancouver, who moderated the briefing, commented that “we’re desperately looking for surrogate markers to speed up answers to clinical trials, and I think MRD in myeloma is quickly becoming a very important surrogate marker.”
GMMG-7 results
For their trial, Dr. Goldschmidt and colleagues enrolled 662 patients with newly diagnosed multiple myeloma who were candidates for high-dose therapy and ASCT and after stratification by revised International Staging System (r-ISS) criteria, randomly assigned them six three-week cycles of induction therapy with Isa-RVd or RVd alone.
Following ASCT, patients were again randomized to maintenance with either isatuximab plus lenalidomide or lenalidomide alone.
As noted before, MRD rates at the end of induction were 50.1% with Isa-RVd versus 35.6% with RVd alone, translating to a hazard ratio favoring the four-drug combination of 1.83 (P < .001).
Treatment with Isa-RVd was the only significant predictor for the likelihood of MRD negativity in a multivariate analysis controlling for treatment group, r-ISS status, performance status, renal impairment, age, and sex.
Although the rate of complete responses at the end of induction was similar between the treatment groups, the rate of very good partial response or better was higher with the isatuximab-containing combination (77.3% vs. 60.5%; P < .001).
The respective rates of disease progression at the end of induction in the Isa-RVd and RVd groups were 1.5% versus 4.0%.
The rates of adverse events were generally similar between the groups, except a higher proportion of patients had leukocytopenia or neutropenia in the Isa-RVd than the RVdgroup (26.4% vs. 9.1%). There were four deaths in the Isa-RVd group and eight in the RVd group. Most of the deaths were attributable to disease progression or COVID-19, said Dr. Goldschmidt.
The study was funded by Sanofi. Dr. Goldschmidt has disclosed honoraria and research grants from Sanofi and others. Dr. Vij has disclosed honoraria or advisory board activities from various companies, including Sanofi. Dr. Sehn is a consultant for and has received honoraria from various companies, not including Sanofi.
A version of this article first appeared on Medscape.com.
ATLANTA -
The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
Now it has shown benefit in patients who have been newly diagnosed with the disease. When isatuximab was added onto a usual triplet therapy for myeloma, it increased the likelihood that patients would be negative for minimal residual disease (MRD) at the end of the induction phase of treatment, thereby increasing their chances for a successful autologous stem cell transplant (ASCT).
The new results come from the GMMG-HD7 trial, in which all patients were treated with the triplet combination of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd).
Some patients, after randomization, also received isatuximab, and in this group, the MRD-negativity rate was 50.1% at the end of induction therapy compared with 35.6% for patients treated with RVd alone.
Patients who are MRD-negative at the time of ASCT have significantly better outcomes than patients who remain MRD-positive.
“Isa-RVd is the first regimen to demonstrate significant MRD-negativity benefit at the end of induction versus RVd in a phase 3 trial,” reported Hartmut Goldschmidt, MD, from University Hospital Heidelberg, Germany.
“The benefits of the addition of Isa to RVd versus RVd regarding MRD negativity after induction therapy was consistent in all subgroups,” he added.
Dr. Goldschmidt spoke at a press briefing prior to his presentation of the data here at the annual meeting of the American Society of Hematology (ASH).
“I think that these data are encouraging, but they are preliminary, and we need mature data to be absolutely certain about whether this presents a major advance in treatment,” commented Ravi Vij, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis. Dr. Vij was not involved in the study.
“We know that for transplant-eligible patients, for whom this trial was conducted, the field is moving toward giving four drugs for induction,” he said in an interview with this news organization.
He noted that the combination of RVd with the other currently available anti-CD38 antibody, daratumumab (Darzalex), was approved for this indication in the United States in Jan. 2021.
Dr. Vij said that isatuximab has been slow to catch on in the United States both because it was approved after clinicians had already become familiar with daratumumab and because it is given intravenously, compared with subcutaneous administration of the latest formulation of daratumumab.
“Whereas isatuximab can take an hour-and-a-half with each infusion, daratumumab takes 5 minutes for an injection and the patient is out of there, so it is convenient both for the patient and the treating institution,” he said.
MRD vs. CR?
Dr. Goldschmidt was asked during the briefing about whether MRD-negativity or complete response rates are better predictors of progression-free survival (PFS). He replied that with current standardized sequencing techniques and sensitivity down to 10-6, “it’s a big benefit to analyze MRD negativity, and there is ongoing discussion between colleagues from the myeloma group with the Food and Drug Administration about how we can merge the data and predict PFS and overall survival.”
Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer, Vancouver, who moderated the briefing, commented that “we’re desperately looking for surrogate markers to speed up answers to clinical trials, and I think MRD in myeloma is quickly becoming a very important surrogate marker.”
GMMG-7 results
For their trial, Dr. Goldschmidt and colleagues enrolled 662 patients with newly diagnosed multiple myeloma who were candidates for high-dose therapy and ASCT and after stratification by revised International Staging System (r-ISS) criteria, randomly assigned them six three-week cycles of induction therapy with Isa-RVd or RVd alone.
Following ASCT, patients were again randomized to maintenance with either isatuximab plus lenalidomide or lenalidomide alone.
As noted before, MRD rates at the end of induction were 50.1% with Isa-RVd versus 35.6% with RVd alone, translating to a hazard ratio favoring the four-drug combination of 1.83 (P < .001).
Treatment with Isa-RVd was the only significant predictor for the likelihood of MRD negativity in a multivariate analysis controlling for treatment group, r-ISS status, performance status, renal impairment, age, and sex.
Although the rate of complete responses at the end of induction was similar between the treatment groups, the rate of very good partial response or better was higher with the isatuximab-containing combination (77.3% vs. 60.5%; P < .001).
The respective rates of disease progression at the end of induction in the Isa-RVd and RVd groups were 1.5% versus 4.0%.
The rates of adverse events were generally similar between the groups, except a higher proportion of patients had leukocytopenia or neutropenia in the Isa-RVd than the RVdgroup (26.4% vs. 9.1%). There were four deaths in the Isa-RVd group and eight in the RVd group. Most of the deaths were attributable to disease progression or COVID-19, said Dr. Goldschmidt.
The study was funded by Sanofi. Dr. Goldschmidt has disclosed honoraria and research grants from Sanofi and others. Dr. Vij has disclosed honoraria or advisory board activities from various companies, including Sanofi. Dr. Sehn is a consultant for and has received honoraria from various companies, not including Sanofi.
A version of this article first appeared on Medscape.com.
ATLANTA -
The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
Now it has shown benefit in patients who have been newly diagnosed with the disease. When isatuximab was added onto a usual triplet therapy for myeloma, it increased the likelihood that patients would be negative for minimal residual disease (MRD) at the end of the induction phase of treatment, thereby increasing their chances for a successful autologous stem cell transplant (ASCT).
The new results come from the GMMG-HD7 trial, in which all patients were treated with the triplet combination of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd).
Some patients, after randomization, also received isatuximab, and in this group, the MRD-negativity rate was 50.1% at the end of induction therapy compared with 35.6% for patients treated with RVd alone.
Patients who are MRD-negative at the time of ASCT have significantly better outcomes than patients who remain MRD-positive.
“Isa-RVd is the first regimen to demonstrate significant MRD-negativity benefit at the end of induction versus RVd in a phase 3 trial,” reported Hartmut Goldschmidt, MD, from University Hospital Heidelberg, Germany.
“The benefits of the addition of Isa to RVd versus RVd regarding MRD negativity after induction therapy was consistent in all subgroups,” he added.
Dr. Goldschmidt spoke at a press briefing prior to his presentation of the data here at the annual meeting of the American Society of Hematology (ASH).
“I think that these data are encouraging, but they are preliminary, and we need mature data to be absolutely certain about whether this presents a major advance in treatment,” commented Ravi Vij, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis. Dr. Vij was not involved in the study.
“We know that for transplant-eligible patients, for whom this trial was conducted, the field is moving toward giving four drugs for induction,” he said in an interview with this news organization.
He noted that the combination of RVd with the other currently available anti-CD38 antibody, daratumumab (Darzalex), was approved for this indication in the United States in Jan. 2021.
Dr. Vij said that isatuximab has been slow to catch on in the United States both because it was approved after clinicians had already become familiar with daratumumab and because it is given intravenously, compared with subcutaneous administration of the latest formulation of daratumumab.
“Whereas isatuximab can take an hour-and-a-half with each infusion, daratumumab takes 5 minutes for an injection and the patient is out of there, so it is convenient both for the patient and the treating institution,” he said.
MRD vs. CR?
Dr. Goldschmidt was asked during the briefing about whether MRD-negativity or complete response rates are better predictors of progression-free survival (PFS). He replied that with current standardized sequencing techniques and sensitivity down to 10-6, “it’s a big benefit to analyze MRD negativity, and there is ongoing discussion between colleagues from the myeloma group with the Food and Drug Administration about how we can merge the data and predict PFS and overall survival.”
Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer, Vancouver, who moderated the briefing, commented that “we’re desperately looking for surrogate markers to speed up answers to clinical trials, and I think MRD in myeloma is quickly becoming a very important surrogate marker.”
GMMG-7 results
For their trial, Dr. Goldschmidt and colleagues enrolled 662 patients with newly diagnosed multiple myeloma who were candidates for high-dose therapy and ASCT and after stratification by revised International Staging System (r-ISS) criteria, randomly assigned them six three-week cycles of induction therapy with Isa-RVd or RVd alone.
Following ASCT, patients were again randomized to maintenance with either isatuximab plus lenalidomide or lenalidomide alone.
As noted before, MRD rates at the end of induction were 50.1% with Isa-RVd versus 35.6% with RVd alone, translating to a hazard ratio favoring the four-drug combination of 1.83 (P < .001).
Treatment with Isa-RVd was the only significant predictor for the likelihood of MRD negativity in a multivariate analysis controlling for treatment group, r-ISS status, performance status, renal impairment, age, and sex.
Although the rate of complete responses at the end of induction was similar between the treatment groups, the rate of very good partial response or better was higher with the isatuximab-containing combination (77.3% vs. 60.5%; P < .001).
The respective rates of disease progression at the end of induction in the Isa-RVd and RVd groups were 1.5% versus 4.0%.
The rates of adverse events were generally similar between the groups, except a higher proportion of patients had leukocytopenia or neutropenia in the Isa-RVd than the RVdgroup (26.4% vs. 9.1%). There were four deaths in the Isa-RVd group and eight in the RVd group. Most of the deaths were attributable to disease progression or COVID-19, said Dr. Goldschmidt.
The study was funded by Sanofi. Dr. Goldschmidt has disclosed honoraria and research grants from Sanofi and others. Dr. Vij has disclosed honoraria or advisory board activities from various companies, including Sanofi. Dr. Sehn is a consultant for and has received honoraria from various companies, not including Sanofi.
A version of this article first appeared on Medscape.com.
AT ASH 2021
Antibiotic use associated with triple-negative breast cancer mortality
SAN ANTONIO –
The study was recently presented at the San Antonio Breast Cancer Symposium by Julia D. Ransohoff, MD, of Stanford (Calif.) University.
Gut-associated lymphoid tissues are the largest component of the immune system. They influence both local and systemic immune responses, but the use of antimicrobials can decrease circulating and tumor-infiltrating lymphocytes that effect the immune repertoire and in turn, the survival of women with triple-negative breast cancer.
Dr. Ransohoff and colleagues hypothesized that increasing antimicrobial exposure in the presence of time-varying absolute lymphocyte counts may lead to higher overall and breast cancer–specific mortality. Their analysis is based on data from the population-based Surveillance, Epidemiology, and End Results registry and electronic medical records from Stanford University and Sutter Health. It included 772 women who were treated for triple-negative breast cancer between 2000 and 2014. The women were followed for an average of 104 months.
In an earlier analysis of this same group, Dr. Ransohoff found that higher minimum absolute lymphocyte counts were associated with lower overall mortality (hazard ratio, 0.23; 95% confidence interval, 0.16-0.35) and breast cancer mortality (HR, 0.19; 95% CI, 0.11-0.34) The association between higher peripheral lymphocyte counts and tumor-infiltrating lymphocytes was significant.
In the analysis of relationships between antibiotic use and mortality, 85% of women (n = 654) were prescribed antibiotics after having been diagnosed with triple-negative breast cancer. The death rate among patients who were prescribed antibiotics was 23% (153/654), compared with 20% (24/118) among the patients who were not treated with antibiotics (which accounts for 15% of the entire group).
For total antibiotic exposure, the HR for overall mortality was 1.06 (95% CI, 1.03-1.09; P < .001) and 1.07 for breast cancer–specific mortality (95% CI, 1.04-1.10; P < .001). For unique antibiotic exposure (not counting repeat prescriptions of the same antibiotic), the HR for overall mortality was 1.17 (95% CI, 1.12-1.22; P < .001) and 1.18 for breast cancer–specific mortality (95% CI, 1.12-1.24; P < .001).
“These were all statistically significant associations derived from a statistical model that takes into account baseline patient characteristics, so the reported hazard ratios, to the best of our ability, represent the risk of death associated with antibiotic use adjusted for other baseline covariates. We’ve attempted to account for differences at baseline that may indicate patients are sicker, and so the reported risk represents mortality related with antibiotic exposure,” Dr. Ransohoff said.
Elucidating the role of the microbiome in mediating absolute lymphocyte counts and immune response may inform interventions to reduce triple-negative mortality, she said.
SAN ANTONIO –
The study was recently presented at the San Antonio Breast Cancer Symposium by Julia D. Ransohoff, MD, of Stanford (Calif.) University.
Gut-associated lymphoid tissues are the largest component of the immune system. They influence both local and systemic immune responses, but the use of antimicrobials can decrease circulating and tumor-infiltrating lymphocytes that effect the immune repertoire and in turn, the survival of women with triple-negative breast cancer.
Dr. Ransohoff and colleagues hypothesized that increasing antimicrobial exposure in the presence of time-varying absolute lymphocyte counts may lead to higher overall and breast cancer–specific mortality. Their analysis is based on data from the population-based Surveillance, Epidemiology, and End Results registry and electronic medical records from Stanford University and Sutter Health. It included 772 women who were treated for triple-negative breast cancer between 2000 and 2014. The women were followed for an average of 104 months.
In an earlier analysis of this same group, Dr. Ransohoff found that higher minimum absolute lymphocyte counts were associated with lower overall mortality (hazard ratio, 0.23; 95% confidence interval, 0.16-0.35) and breast cancer mortality (HR, 0.19; 95% CI, 0.11-0.34) The association between higher peripheral lymphocyte counts and tumor-infiltrating lymphocytes was significant.
In the analysis of relationships between antibiotic use and mortality, 85% of women (n = 654) were prescribed antibiotics after having been diagnosed with triple-negative breast cancer. The death rate among patients who were prescribed antibiotics was 23% (153/654), compared with 20% (24/118) among the patients who were not treated with antibiotics (which accounts for 15% of the entire group).
For total antibiotic exposure, the HR for overall mortality was 1.06 (95% CI, 1.03-1.09; P < .001) and 1.07 for breast cancer–specific mortality (95% CI, 1.04-1.10; P < .001). For unique antibiotic exposure (not counting repeat prescriptions of the same antibiotic), the HR for overall mortality was 1.17 (95% CI, 1.12-1.22; P < .001) and 1.18 for breast cancer–specific mortality (95% CI, 1.12-1.24; P < .001).
“These were all statistically significant associations derived from a statistical model that takes into account baseline patient characteristics, so the reported hazard ratios, to the best of our ability, represent the risk of death associated with antibiotic use adjusted for other baseline covariates. We’ve attempted to account for differences at baseline that may indicate patients are sicker, and so the reported risk represents mortality related with antibiotic exposure,” Dr. Ransohoff said.
Elucidating the role of the microbiome in mediating absolute lymphocyte counts and immune response may inform interventions to reduce triple-negative mortality, she said.
SAN ANTONIO –
The study was recently presented at the San Antonio Breast Cancer Symposium by Julia D. Ransohoff, MD, of Stanford (Calif.) University.
Gut-associated lymphoid tissues are the largest component of the immune system. They influence both local and systemic immune responses, but the use of antimicrobials can decrease circulating and tumor-infiltrating lymphocytes that effect the immune repertoire and in turn, the survival of women with triple-negative breast cancer.
Dr. Ransohoff and colleagues hypothesized that increasing antimicrobial exposure in the presence of time-varying absolute lymphocyte counts may lead to higher overall and breast cancer–specific mortality. Their analysis is based on data from the population-based Surveillance, Epidemiology, and End Results registry and electronic medical records from Stanford University and Sutter Health. It included 772 women who were treated for triple-negative breast cancer between 2000 and 2014. The women were followed for an average of 104 months.
In an earlier analysis of this same group, Dr. Ransohoff found that higher minimum absolute lymphocyte counts were associated with lower overall mortality (hazard ratio, 0.23; 95% confidence interval, 0.16-0.35) and breast cancer mortality (HR, 0.19; 95% CI, 0.11-0.34) The association between higher peripheral lymphocyte counts and tumor-infiltrating lymphocytes was significant.
In the analysis of relationships between antibiotic use and mortality, 85% of women (n = 654) were prescribed antibiotics after having been diagnosed with triple-negative breast cancer. The death rate among patients who were prescribed antibiotics was 23% (153/654), compared with 20% (24/118) among the patients who were not treated with antibiotics (which accounts for 15% of the entire group).
For total antibiotic exposure, the HR for overall mortality was 1.06 (95% CI, 1.03-1.09; P < .001) and 1.07 for breast cancer–specific mortality (95% CI, 1.04-1.10; P < .001). For unique antibiotic exposure (not counting repeat prescriptions of the same antibiotic), the HR for overall mortality was 1.17 (95% CI, 1.12-1.22; P < .001) and 1.18 for breast cancer–specific mortality (95% CI, 1.12-1.24; P < .001).
“These were all statistically significant associations derived from a statistical model that takes into account baseline patient characteristics, so the reported hazard ratios, to the best of our ability, represent the risk of death associated with antibiotic use adjusted for other baseline covariates. We’ve attempted to account for differences at baseline that may indicate patients are sicker, and so the reported risk represents mortality related with antibiotic exposure,” Dr. Ransohoff said.
Elucidating the role of the microbiome in mediating absolute lymphocyte counts and immune response may inform interventions to reduce triple-negative mortality, she said.
AT SABCS 2021
Women struggle with benzodiazepine addiction post chemotherapy treatment
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
AT SABCS 2021