User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Mortality differs by LVEF between women and men
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
FROM ESC HEART FAILURE 2020
Daily Recap: Feds seek COVID-19 info through app, hospitalists take on new roles
Here are the stories our MDedge editors across specialties think you need to know about today:
FDA seeks COVID-19 info through CURE ID
Federal health officials are asking clinicians to use the free CURE ID mobile app and web platform as a tool to collect information on the treatment of patients with COVID-19. CURE ID is an Internet-based data repository first developed in 2013 as a collaboration between the Food and Drug Administration and the National Center for Advancing Translational Sciences, part of the National Institutes of Health. It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. “By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” said Heather A. Stone, MPH, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.” Read more.
Hospitalists take on new roles in COVID era
Whether it’s working shifts in the ICU, caring for ventilator patients, or reporting to postanesthesia care units and post-acute or step-down units, hospitalists are stepping into a variety of new roles as part of their frontline response to the COVID-19 pandemic. Valerie Vaughn, MD, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.” Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care professionals, who volunteered to manage COVID-19 patients in the ICU and other hospital units. Read more.
COVID-19 recommendations for rheumatic disease treatment
The European League Against Rheumatism (EULAR) issued provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Contrary to earlier expectations, there is no indication that patients with rheumatic and musculoskeletal diseases have a higher risk of contracting the virus or have a worse course if they do, according to the task force that worked on the recommendations. The task force also pointed out that rheumatology drugs are being used to treat COVID-19 patients who don’t have rheumatic diseases, raising the possibility of a shortage of disease-modifying antirheumatic drugs. Read more.
Mental health visits are 19% of ED costs
Mental and substance use disorders represented 19% of all emergency department visits in 2017 and cost $14.6 billion, according to figures from the Agency for Healthcare Research and Quality. The most costly mental and substance use disorder diagnosis was anxiety and fear-related disorders, accounting for $5.6 billion worth of visits, following by depressive disorders and alcohol-related disorders. Read more.
Food deserts linked to health issues in pregnancy
Living in a neighborhood lacking adequate access to affordable, high-quality food is associated with a somewhat greater risk of developing pregnancy morbidity, according to an observational study. Researchers found that women who lived in a food desert had a 1.6 times greater odds of pregnancy comorbidity than if they did not. “An additional, albeit less obvious factor that may be unique to patients suffering disproportionately from obstetric morbidity is exposure to toxic elements,” the researchers reported in Obstetrics & Gynecology. “It has been shown in a previous study that low-income, predominantly black communities of pregnant women may suffer disproportionately from lead or arsenic exposure.” Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
FDA seeks COVID-19 info through CURE ID
Federal health officials are asking clinicians to use the free CURE ID mobile app and web platform as a tool to collect information on the treatment of patients with COVID-19. CURE ID is an Internet-based data repository first developed in 2013 as a collaboration between the Food and Drug Administration and the National Center for Advancing Translational Sciences, part of the National Institutes of Health. It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. “By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” said Heather A. Stone, MPH, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.” Read more.
Hospitalists take on new roles in COVID era
Whether it’s working shifts in the ICU, caring for ventilator patients, or reporting to postanesthesia care units and post-acute or step-down units, hospitalists are stepping into a variety of new roles as part of their frontline response to the COVID-19 pandemic. Valerie Vaughn, MD, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.” Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care professionals, who volunteered to manage COVID-19 patients in the ICU and other hospital units. Read more.
COVID-19 recommendations for rheumatic disease treatment
The European League Against Rheumatism (EULAR) issued provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Contrary to earlier expectations, there is no indication that patients with rheumatic and musculoskeletal diseases have a higher risk of contracting the virus or have a worse course if they do, according to the task force that worked on the recommendations. The task force also pointed out that rheumatology drugs are being used to treat COVID-19 patients who don’t have rheumatic diseases, raising the possibility of a shortage of disease-modifying antirheumatic drugs. Read more.
Mental health visits are 19% of ED costs
Mental and substance use disorders represented 19% of all emergency department visits in 2017 and cost $14.6 billion, according to figures from the Agency for Healthcare Research and Quality. The most costly mental and substance use disorder diagnosis was anxiety and fear-related disorders, accounting for $5.6 billion worth of visits, following by depressive disorders and alcohol-related disorders. Read more.
Food deserts linked to health issues in pregnancy
Living in a neighborhood lacking adequate access to affordable, high-quality food is associated with a somewhat greater risk of developing pregnancy morbidity, according to an observational study. Researchers found that women who lived in a food desert had a 1.6 times greater odds of pregnancy comorbidity than if they did not. “An additional, albeit less obvious factor that may be unique to patients suffering disproportionately from obstetric morbidity is exposure to toxic elements,” the researchers reported in Obstetrics & Gynecology. “It has been shown in a previous study that low-income, predominantly black communities of pregnant women may suffer disproportionately from lead or arsenic exposure.” Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
FDA seeks COVID-19 info through CURE ID
Federal health officials are asking clinicians to use the free CURE ID mobile app and web platform as a tool to collect information on the treatment of patients with COVID-19. CURE ID is an Internet-based data repository first developed in 2013 as a collaboration between the Food and Drug Administration and the National Center for Advancing Translational Sciences, part of the National Institutes of Health. It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. “By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” said Heather A. Stone, MPH, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.” Read more.
Hospitalists take on new roles in COVID era
Whether it’s working shifts in the ICU, caring for ventilator patients, or reporting to postanesthesia care units and post-acute or step-down units, hospitalists are stepping into a variety of new roles as part of their frontline response to the COVID-19 pandemic. Valerie Vaughn, MD, a hospitalist with Michigan Medicine and assistant professor of medicine at the University of Michigan in Ann Arbor, was doing research on how to reduce overuse of antibiotics in hospitals when the COVID-19 crisis hit and dramatically redefined her job. “We were afraid that we might have 3,000 to 5,000 hospitalized COVID patients by now, based on predictive modeling done while the pandemic was still growing exponentially,” she explained. Although Michigan continues to have high COVID-19 infection rates, centered on nearby Detroit, “things are a lot better today than they were 4 weeks ago.” Dr. Vaughn helped to mobilize a team of 25 hospitalists, along with other health care professionals, who volunteered to manage COVID-19 patients in the ICU and other hospital units. Read more.
COVID-19 recommendations for rheumatic disease treatment
The European League Against Rheumatism (EULAR) issued provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Contrary to earlier expectations, there is no indication that patients with rheumatic and musculoskeletal diseases have a higher risk of contracting the virus or have a worse course if they do, according to the task force that worked on the recommendations. The task force also pointed out that rheumatology drugs are being used to treat COVID-19 patients who don’t have rheumatic diseases, raising the possibility of a shortage of disease-modifying antirheumatic drugs. Read more.
Mental health visits are 19% of ED costs
Mental and substance use disorders represented 19% of all emergency department visits in 2017 and cost $14.6 billion, according to figures from the Agency for Healthcare Research and Quality. The most costly mental and substance use disorder diagnosis was anxiety and fear-related disorders, accounting for $5.6 billion worth of visits, following by depressive disorders and alcohol-related disorders. Read more.
Food deserts linked to health issues in pregnancy
Living in a neighborhood lacking adequate access to affordable, high-quality food is associated with a somewhat greater risk of developing pregnancy morbidity, according to an observational study. Researchers found that women who lived in a food desert had a 1.6 times greater odds of pregnancy comorbidity than if they did not. “An additional, albeit less obvious factor that may be unique to patients suffering disproportionately from obstetric morbidity is exposure to toxic elements,” the researchers reported in Obstetrics & Gynecology. “It has been shown in a previous study that low-income, predominantly black communities of pregnant women may suffer disproportionately from lead or arsenic exposure.” Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Clinicians urged to use CURE ID to report COVID-19 cases
in conjunction with ongoing clinical trial efforts.
“By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” Heather A. Stone, MPH, said during a June 9 webinar sponsored by the Food and Drug Administration. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.”
During the hour-long webinar, Ms. Stone, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research, demonstrated CURE ID, an Internet-based data repository first developed in 2013 as a collaboration between the FDA and the National Center for Advancing Translational Sciences, a part of the National Institutes of Health (NCATS/NIH). It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. The app can be downloaded for free at http://cure.ncats.io. It can also be downloaded from the Apple app store or the Google Play store by searching “CURE ID.”
According to Ms. Stone, the platform’s three main goals are to enhance the understanding of new uses of approved medical products, to facilitate clinical trials and drug development, and to serve as a resource for physicians to share information where no FDA-approved product (which has been proven to be safe and effective) exists for the new use. CURE ID enables users to report their own cases as well as read cases of neglected infectious diseases with no sufficient approved therapies from other clinicians around the world. “It also enables clinicians to engage directly with communities of disease experts around the world, breaking down geographic and specialty silos,” Ms. Stone said. “It also enables them to access information on approved therapies for each disease and as well on active clinical trials.”
To date, CURE-ID contains information on 325 infectious diseases, including 1,580 case reports and 18,907 clinical trials. Initial pilot priority diseases include COVID-19, mycetoma, atypical mycobacteria, drug-resistant gonorrhea, rare and resistant fungal infections, as well as multidrug resistant gram-negative bacteria.
As of June 9, COVID-19-related data on the platform includes 151 case reports that have been extracted from the published literature or entered by clinician users, 80 discussion posts, and links to 694 clinical trials, 303 journal articles, 212 news articles, and 34 events. A total of 65 repurposed drugs have been identified as potential treatments for the virus, including 15 drugs with 10 or more cases.
“This facilitates clinicians reporting their real-world experiences treating COVID-19 patients, when patients are unable to be enrolled in a clinical trial,” Ms. Stone said. “It includes an updated case report form tailored to COVID-19 and data fields that have been harmonized with other real-world data and clinical trial platforms.” She pointed out that voluntary submission of cases to CURE ID is not a substitute for filing information with regulatory and public health authorities, where required. The platform also enables data to be entered and adverse events to be automatically shared with the FDA’s MedWatch Adverse Reporting System.
Ms. Stone concluded the webinar by announcing the formation of a new private-public partnership between the Critical Path Institute and the FDA and NCATS/NIH known as the CURE Drug Repurposing Collaboratory. The effort will begin with a pilot project focused on furthering drug development for COVID-19 through use of the CURE ID platform. “The Collaboratory will demonstrate how data shared from clinicians in real-time can be used to inform ongoing and future clinical trials, and potentially drug labeling,” Ms. Stone said. She reported having no financial disclosures.
in conjunction with ongoing clinical trial efforts.
“By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” Heather A. Stone, MPH, said during a June 9 webinar sponsored by the Food and Drug Administration. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.”
During the hour-long webinar, Ms. Stone, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research, demonstrated CURE ID, an Internet-based data repository first developed in 2013 as a collaboration between the FDA and the National Center for Advancing Translational Sciences, a part of the National Institutes of Health (NCATS/NIH). It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. The app can be downloaded for free at http://cure.ncats.io. It can also be downloaded from the Apple app store or the Google Play store by searching “CURE ID.”
According to Ms. Stone, the platform’s three main goals are to enhance the understanding of new uses of approved medical products, to facilitate clinical trials and drug development, and to serve as a resource for physicians to share information where no FDA-approved product (which has been proven to be safe and effective) exists for the new use. CURE ID enables users to report their own cases as well as read cases of neglected infectious diseases with no sufficient approved therapies from other clinicians around the world. “It also enables clinicians to engage directly with communities of disease experts around the world, breaking down geographic and specialty silos,” Ms. Stone said. “It also enables them to access information on approved therapies for each disease and as well on active clinical trials.”
To date, CURE-ID contains information on 325 infectious diseases, including 1,580 case reports and 18,907 clinical trials. Initial pilot priority diseases include COVID-19, mycetoma, atypical mycobacteria, drug-resistant gonorrhea, rare and resistant fungal infections, as well as multidrug resistant gram-negative bacteria.
As of June 9, COVID-19-related data on the platform includes 151 case reports that have been extracted from the published literature or entered by clinician users, 80 discussion posts, and links to 694 clinical trials, 303 journal articles, 212 news articles, and 34 events. A total of 65 repurposed drugs have been identified as potential treatments for the virus, including 15 drugs with 10 or more cases.
“This facilitates clinicians reporting their real-world experiences treating COVID-19 patients, when patients are unable to be enrolled in a clinical trial,” Ms. Stone said. “It includes an updated case report form tailored to COVID-19 and data fields that have been harmonized with other real-world data and clinical trial platforms.” She pointed out that voluntary submission of cases to CURE ID is not a substitute for filing information with regulatory and public health authorities, where required. The platform also enables data to be entered and adverse events to be automatically shared with the FDA’s MedWatch Adverse Reporting System.
Ms. Stone concluded the webinar by announcing the formation of a new private-public partnership between the Critical Path Institute and the FDA and NCATS/NIH known as the CURE Drug Repurposing Collaboratory. The effort will begin with a pilot project focused on furthering drug development for COVID-19 through use of the CURE ID platform. “The Collaboratory will demonstrate how data shared from clinicians in real-time can be used to inform ongoing and future clinical trials, and potentially drug labeling,” Ms. Stone said. She reported having no financial disclosures.
in conjunction with ongoing clinical trial efforts.
“By utilizing the CURE ID platform now for COVID-19 case collection – in conjunction with data gathered from other registries, EHR systems, and clinical trials – data collected during an outbreak can be improved and coordinated,” Heather A. Stone, MPH, said during a June 9 webinar sponsored by the Food and Drug Administration. “This may allow us to find possible treatments to help ease this pandemic, and prepare us better to fight the next one.”
During the hour-long webinar, Ms. Stone, a health science policy analyst in the office of medical policy at the FDA’s Center for Drug Evaluation and Research, demonstrated CURE ID, an Internet-based data repository first developed in 2013 as a collaboration between the FDA and the National Center for Advancing Translational Sciences, a part of the National Institutes of Health (NCATS/NIH). It provides licensed clinicians worldwide with an opportunity to report novel uses of existing drugs for patients with difficult-to-treat infectious diseases, including COVID-19, through a website, a smartphone, or other mobile device. The app can be downloaded for free at http://cure.ncats.io. It can also be downloaded from the Apple app store or the Google Play store by searching “CURE ID.”
According to Ms. Stone, the platform’s three main goals are to enhance the understanding of new uses of approved medical products, to facilitate clinical trials and drug development, and to serve as a resource for physicians to share information where no FDA-approved product (which has been proven to be safe and effective) exists for the new use. CURE ID enables users to report their own cases as well as read cases of neglected infectious diseases with no sufficient approved therapies from other clinicians around the world. “It also enables clinicians to engage directly with communities of disease experts around the world, breaking down geographic and specialty silos,” Ms. Stone said. “It also enables them to access information on approved therapies for each disease and as well on active clinical trials.”
To date, CURE-ID contains information on 325 infectious diseases, including 1,580 case reports and 18,907 clinical trials. Initial pilot priority diseases include COVID-19, mycetoma, atypical mycobacteria, drug-resistant gonorrhea, rare and resistant fungal infections, as well as multidrug resistant gram-negative bacteria.
As of June 9, COVID-19-related data on the platform includes 151 case reports that have been extracted from the published literature or entered by clinician users, 80 discussion posts, and links to 694 clinical trials, 303 journal articles, 212 news articles, and 34 events. A total of 65 repurposed drugs have been identified as potential treatments for the virus, including 15 drugs with 10 or more cases.
“This facilitates clinicians reporting their real-world experiences treating COVID-19 patients, when patients are unable to be enrolled in a clinical trial,” Ms. Stone said. “It includes an updated case report form tailored to COVID-19 and data fields that have been harmonized with other real-world data and clinical trial platforms.” She pointed out that voluntary submission of cases to CURE ID is not a substitute for filing information with regulatory and public health authorities, where required. The platform also enables data to be entered and adverse events to be automatically shared with the FDA’s MedWatch Adverse Reporting System.
Ms. Stone concluded the webinar by announcing the formation of a new private-public partnership between the Critical Path Institute and the FDA and NCATS/NIH known as the CURE Drug Repurposing Collaboratory. The effort will begin with a pilot project focused on furthering drug development for COVID-19 through use of the CURE ID platform. “The Collaboratory will demonstrate how data shared from clinicians in real-time can be used to inform ongoing and future clinical trials, and potentially drug labeling,” Ms. Stone said. She reported having no financial disclosures.
EULAR’s COVID-19 recommendations offer no surprises
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
As might be expected, the “EULAR [European League Against Rheumatism] provisional recommendations for the management of rheumatic and musculoskeletal diseases [RMDs] in the context of SARS-CoV-2” concur with much of the guidance already released on how best to manage patients during the current pandemic.
Highlights of the five overarching principles are that, contrary to earlier expectations, “there is no indication that patients with RMDs have an additional, or have a higher, risk of contracting the virus, or that they fare a worse course” than the general population, said the task force convener Robert Landewé, MD, PhD, professor of rheumatology at the University of Amsterdam.
“The second pertinent highlight is that, when it comes to managerial discussions, whether or not to stop or to start treatment for RMDs, rheumatologists should definitely be involved,” Dr. Landewé said during a live session at the annual European Congress of Rheumatology, held online this year due to COVID-19. “In practice, something that happens very often is that immunosuppressive drugs are stopped by medical specialists involved in the care of COVID but without any expertise in treating patients with rheumatic diseases. We should try to avoid that situation.”
The third highlight, something many rheumatologists may already be well aware of, is that rheumatology drugs are being used to treat COVID-19 patients without RMDs and a shortage of disease-modifying antirheumatic drugs (DMARDs) agents is a real possibility. As such, the fifth overarching highlight states that the availability of both synthetic and biologic DMARDs is “a delicate societal responsibility” and that “the off-label use of DMARDs in COVID-19 outside the context of clinical trials should be discouraged.”
The EULAR recommendation are now published online in Annals of the Rheumatic Diseases and they are “what you could call an unprecedented set of recommendations,” Dr. Landewé said. “We have never done this before,” he added, referring to the speed and way in which they had to be put together, remotely, and with little scientific evidence currently available. “Three months ago we hadn’t even heard about the virus.”
From the first patient being identified in the Hubei province of China in November 2019, to the first U.S. patient in the state of Washington on Jan. 20, 2020, and to the first European patient identified a little over 10 days later, the COVID-19 pandemic has taken the world by storm. It was only declared a pandemic on March 11, 2020, however, and Dr. Landewé noted that the response to the pandemic had been very variable – some countries locking down their borders early, while others took their time to make an appropriate response, if at all.
The rheumatology community was particularly concerned, Dr. Landewé said, because people with autoimmune diseases who were taking immunosuppressant drugs might be at higher risk for becoming infected with SARS-CoV-2, and may be at higher risk than others for a worse disease course. Thankfully, that seems not to be the case according to data that are emerging from new registries that have been set up, including EULAR’s own COVID-19 registry.
There are 13 recommendations that cover 4 themes: general measures and prevention of SARS-CoV-2 infection; the management of RMD patients during the pandemic; the management of RMD patients who have COVID-19; and the prevention of other pulmonary infections in RMD patients.
Highlighting the first three general recommendations, Dr. Landewé said: “Follow the regular guidelines in your country; if a patient with RMD does not have symptoms of COVID-19, simply continue RMD treatments,” albeit with a couple of exceptions.
The next four recommendation highlights are to avoid visits to the hospital or to the office; use remote monitoring via the telephone, for example; and if visits cannot be avoided, then take appropriate precautions. Finally, if you suspect a patient has COVID-19, do a test.
If patients test positive, then the next four recommendations cover what to do, such as continuing use of RMD treatments, but in the case of glucocorticoids this should be the lowest possible dose necessary. There is no consensus on what to do in cases of mild symptoms; the recommendation is to “decide on a case-by-case basis,” said Dr. Landewé. If a patient’s symptoms worsen, then “seek expert advice immediately and follow local treatment recommendations. The rheumatologist is not the expert to treat COVID-19,” he added. That responsibility lies with the pulmonologist, infectious disease specialist, or maybe the intensive care specialist, depending on local situations.
On the whole, the EULAR recommendations are pretty similar to those already released by the American College of Rheumatology, said Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha. The ACR recommendations are “slightly more prescriptive”, he suggested, with 25 final guidance statements. For example, general statements focused not only on the use of glucocorticoids, but also other medicines, such as antihypertensives.
“There’s really not a [lot of], I would say, major differences in the two efforts and that’s ... somewhat reassuring that we’re approaching the unknown from very different parts of the world, and driving in a very similar place,” commented Dr. Mikuls, who is a member of the ACR COVID-19 recommendations task force.
“I think one of the very important similarities that I would highlight is that, in the absence of known exposure, in the absence of COVID-19 infection, our panel felt very strongly about the importance of continuing rheumatic disease treatments,” Dr. Mikuls observed. The ACR guidelines also touch upon societal perspectives, including “some statements that were made very specific to lupus, and the use of antimalarials, given supply chain issues that we have encountered.”
Dr. Mikuls also said that the American recommendations emphasized that “you really have to manage active inflammatory rheumatic disease. Even in the context of the COVID-19 pandemic, given what we saw as the potential risk of unchecked inflammation and unchecked rheumatic disease.”
One notable difference, however, is that the European recommendations advise on immunizations and pneumonia prophylaxis, saying that all patients without COVID-19 symptoms should make sure they are up to date with any recommended vaccinations, “with a particular focus on pneumococcal and influenza vaccinations,” Dr. Landewé said.
Another difference is that the ACR recommendations are a living document and could potentially be updated monthly if the evidence arrives to allow that. In that sense, the American guidance is more agile, with EULAR expecting to update its recommendations every 3 months.
“The current evidence is extremely sparse and fragmented,” Dr. Landewé said. “We, as a task force are essentially flying blindly. We also have to cover many jurisdictions within Europe, with many conflicting opinions. So the last word to say is that updates are truly necessary, but we have to wait a while.”
SOURCE: Landewé RB et al. Ann Rheum Dis. 2020 Jun 5. doi: 10.1136/annrheumdis-2020-217877.
FROM THE EULAR 2020 E-CONGRESS
VICTORIA results deepen mystery of vericiguat in low-EF heart failure
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
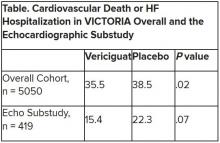
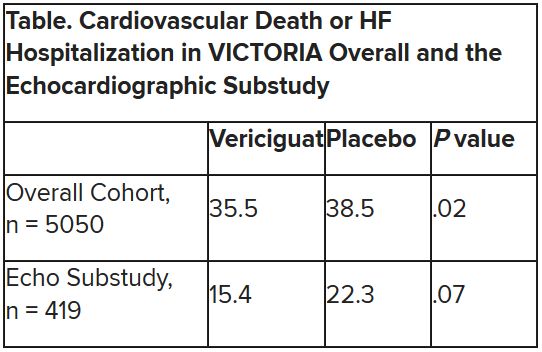
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
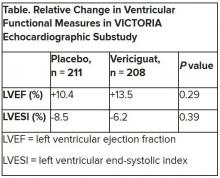
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2020
Mental health visits account for 19% of ED costs
Emergency department visits for mental and substance use disorders (MUSDs) cost $14.6 billion in 2017, representing 19% of the total for all ED visits that year, according to the Agency for Healthcare Quality and Research.
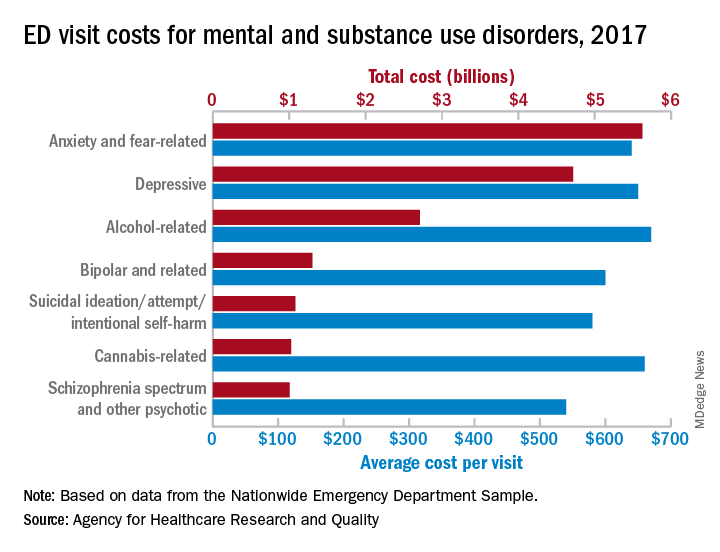
In terms of the total number of visits for MUSDs, 23.1 million, the proportion was slightly lower: 16% of all ED visits for the year, Zeynal Karaca, PhD, a senior economist with AHRQ, and Brian J. Moore, PhD, a senior research leader at IBM Watson Health, said in a recent statistical brief.
Put those figures together and the average visit for an MUSD diagnosis cost $630 and that is 19% higher than the average of $530 for all 145 million ED visits, they reported based on data from the Nationwide Emergency Department Sample.
The most costly MUSD diagnosis in 2017 was anxiety and fear-related disorders, with a total of $5.6 billion for ED visits, followed by depressive disorders at $4.7 billion and alcohol-related disorders at $2.7 billion. Some ED visits may involve more than one MUSD diagnosis, so the sum of all the individual diagnoses does not agree with the total for the entire MUSD category, the researchers noted.
On a per-visit basis, in 2017. [It was not included in the graph because it was 13th.] Other disorders with high per-visit costs were alcohol-related ($670), cannabis-related ($660), and depressive and stimulant-related (both with $650), Dr. Karaca and Dr. Moore said.
Patients with MUSDs who were routinely discharged after an ED visit in 2017 represented a much lower share of the total MUSD cost (68.0%), compared with the overall group of ED visitors (81.4%), but MUSD visits resulting in an inpatient admission made up a larger proportion of costs (19.0%), compared with all visits (9.5%), they said.
Costs between MUSD visits and all ED visits also differed by patient age. Visits by patients aged 0-9 years represented only 0.7% of MUSD-related ED costs but 5.6% of the overall cost, but the respective figures for those aged 45-64 were 36.2% for MUSD costs and 28.5% for the total ED cost, they reported.
SOURCE: Karaca Z and Moore BJ. HCUP Statistical Brief #257. May 12, 2020.
Emergency department visits for mental and substance use disorders (MUSDs) cost $14.6 billion in 2017, representing 19% of the total for all ED visits that year, according to the Agency for Healthcare Quality and Research.

In terms of the total number of visits for MUSDs, 23.1 million, the proportion was slightly lower: 16% of all ED visits for the year, Zeynal Karaca, PhD, a senior economist with AHRQ, and Brian J. Moore, PhD, a senior research leader at IBM Watson Health, said in a recent statistical brief.
Put those figures together and the average visit for an MUSD diagnosis cost $630 and that is 19% higher than the average of $530 for all 145 million ED visits, they reported based on data from the Nationwide Emergency Department Sample.
The most costly MUSD diagnosis in 2017 was anxiety and fear-related disorders, with a total of $5.6 billion for ED visits, followed by depressive disorders at $4.7 billion and alcohol-related disorders at $2.7 billion. Some ED visits may involve more than one MUSD diagnosis, so the sum of all the individual diagnoses does not agree with the total for the entire MUSD category, the researchers noted.
On a per-visit basis, in 2017. [It was not included in the graph because it was 13th.] Other disorders with high per-visit costs were alcohol-related ($670), cannabis-related ($660), and depressive and stimulant-related (both with $650), Dr. Karaca and Dr. Moore said.
Patients with MUSDs who were routinely discharged after an ED visit in 2017 represented a much lower share of the total MUSD cost (68.0%), compared with the overall group of ED visitors (81.4%), but MUSD visits resulting in an inpatient admission made up a larger proportion of costs (19.0%), compared with all visits (9.5%), they said.
Costs between MUSD visits and all ED visits also differed by patient age. Visits by patients aged 0-9 years represented only 0.7% of MUSD-related ED costs but 5.6% of the overall cost, but the respective figures for those aged 45-64 were 36.2% for MUSD costs and 28.5% for the total ED cost, they reported.
SOURCE: Karaca Z and Moore BJ. HCUP Statistical Brief #257. May 12, 2020.
Emergency department visits for mental and substance use disorders (MUSDs) cost $14.6 billion in 2017, representing 19% of the total for all ED visits that year, according to the Agency for Healthcare Quality and Research.

In terms of the total number of visits for MUSDs, 23.1 million, the proportion was slightly lower: 16% of all ED visits for the year, Zeynal Karaca, PhD, a senior economist with AHRQ, and Brian J. Moore, PhD, a senior research leader at IBM Watson Health, said in a recent statistical brief.
Put those figures together and the average visit for an MUSD diagnosis cost $630 and that is 19% higher than the average of $530 for all 145 million ED visits, they reported based on data from the Nationwide Emergency Department Sample.
The most costly MUSD diagnosis in 2017 was anxiety and fear-related disorders, with a total of $5.6 billion for ED visits, followed by depressive disorders at $4.7 billion and alcohol-related disorders at $2.7 billion. Some ED visits may involve more than one MUSD diagnosis, so the sum of all the individual diagnoses does not agree with the total for the entire MUSD category, the researchers noted.
On a per-visit basis, in 2017. [It was not included in the graph because it was 13th.] Other disorders with high per-visit costs were alcohol-related ($670), cannabis-related ($660), and depressive and stimulant-related (both with $650), Dr. Karaca and Dr. Moore said.
Patients with MUSDs who were routinely discharged after an ED visit in 2017 represented a much lower share of the total MUSD cost (68.0%), compared with the overall group of ED visitors (81.4%), but MUSD visits resulting in an inpatient admission made up a larger proportion of costs (19.0%), compared with all visits (9.5%), they said.
Costs between MUSD visits and all ED visits also differed by patient age. Visits by patients aged 0-9 years represented only 0.7% of MUSD-related ED costs but 5.6% of the overall cost, but the respective figures for those aged 45-64 were 36.2% for MUSD costs and 28.5% for the total ED cost, they reported.
SOURCE: Karaca Z and Moore BJ. HCUP Statistical Brief #257. May 12, 2020.
COVID-19: Where doctors can get help for emotional distress
Nisha Mehta, MD, said her phone has been ringing with calls from tearful and shaken physicians who are distressed and unsettled about their work and home situation and don’t know what to do.
What’s more, many frontline physicians are living apart from family to protect them from infection. “So many physicians have called me crying. ... They can’t even come home and get a hug,” Dr. Mehta said. “What I’m hearing from a lot of people who are in New York and New Jersey is not just that they go to work all day and it’s this exhausting process throughout the entire day, not only physically but also emotionally.”
Physician burnout has held a steady spotlight since long before the COVID-19 crisis began, Dr. Mehta said. “The reason for that is multifold, but in part, it’s hard for physicians to find an appropriate way to be able to process a lot of the emotions related to their work,” she said. “A lot of that brews below the surface, but COVID-19 has really brought many of these issues above that surface.”
Frustrated that governments weren’t doing enough to support health care workers during the pandemic, Dr. Mehta, a radiologist in Charlotte, N.C., decided there needed to be change. On April 4, Dr. Mehta and two physician colleagues submitted to Congress the COVID-19 Pandemic Physician Protection Act, which ensures, among other provisions, mental health coverage for health care workers. An accompanying petition on change.org had received nearly 300,000 signatures as of May 29.
Don’t suffer in silence
A career in medicine comes with immense stress in the best of times, she notes, and managing a pandemic in an already strained system has taken those challenges to newer heights. “We need better support structures at baseline for physician mental health,” said Dr. Mehta.
“That’s something we’ve always been lacking because it’s been against the culture of medicine for so long to say, ‘I’m having a hard time.’ ”
If you’re hurting, the first thing to recognize is that you are not alone in facing these challenges. This is true with respect not only to medical care but also to all of the family, financial, and business concerns physicians are currently facing. “Having all of those things hanging over your head is a lot. We’ve got to find ways to help each other out,” Dr. Mehta said.
Where to find support
Fortunately, the medical community has created several pathways to help its own.
The following list represents a cross-section of opportunities for caregivers to receive care for themselves.
Crisis hotlines
- Physician Support Line. This free and confidential hotline was launched on March 30 by Mona Masood, DO, a Philadelphia-area psychiatrist and moderator of a Facebook forum called the COVID-19 Physicians Group. The PSL is run by more than 600 volunteer psychiatrists who take calls from U.S. physicians 7 days a week from 8:00 a.m. to 1:00 a.m., with no appointment necessary. The toll-free number is 888-409-0141.
- For the Frontlines. This 24/7 help line provides free crisis counseling for frontline workers. They can text FRONTLINE to 741741 in the United States (support is also available for residents of Canada, Ireland, and the United Kingdom).
Resources from professional groups
- Action Collaborative on Clinician Well-Being and Resilience. Created by the National Academy of Medicine in 2017, the Action Collaborative comprises more than 60 organizations committed to reversing trends in clinician burnout. In response to the pandemic, the group has compiled a list of strategies and resources to support the health and well-being of clinicians who are providing healthcare during the COVID-19 outbreak.
- American Medical Association. The AMA has created a resource center dedicated to providing care for caregivers during the COVID-19 pandemic. The website includes specific guidance for managing mental health during the pandemic.
- American College of Physicians. The professional society of internal medicine physicians has created a comprehensive guide for physicians specific to COVID-19, with a section dedicated to clinician well-being that includes information about hotlines, counseling services, grief support, and more.
- American Hospital Association. The AHA’s website now includes regularly updated resources for healthcare clinicians and staff, as well as a special section dedicated to protecting and enabling healthcare workers in the midst of the pandemic.
Virtual psychological counseling
Not unlike the way telemedicine has allowed some physicians to keep seeing their patients, many modalities enable participation in therapy through video, chat, phone call, or any combination thereof. Look for a service that is convenient, flexible, and HIPAA compliant.
Traditional in-office mental health therapy has quickly moved to telemedicine. Many if not most insurers that cover counseling visits are paying for telepsychiatry or telecounseling. If you don’t know of an appropriate therapist, check the American Psychiatric Association or its state chapters; the American Psychological Association; or look for a licensed mental health counselor.
Because financial constraints are a potential barrier to therapy, Project Parachute, in cooperation with Eleos Health, has organized a cadre of therapists willing to provide pro bono online therapy for health care workers. The amount of free therapy provided to qualified frontline workers is up to the individual therapists. Discuss these parameters with your therapists up front.
Similar services are offered from companies such as Talkspace and BetterHelp on a subscription basis. These services are typically less expensive than in-person sessions. Ask about discounts for healthcare workers. Talkspace, for example, announced in March, “Effective immediately, healthcare workers across the country can get access to a free month of our...online therapy that includes unlimited text, video, and audio messaging with a licensed therapist.”
Online support groups and social media
For more on-demand peer support, look for groups such as the COR Sharing Circle for Healthcare Workers on Facebook. The site’s search engine can point users to plenty of other groups, many of which are closed (meaning posts are visible to members only).
Dr. Mehta hosts her own Facebook group called Physician Community. “I would like to think (and genuinely feel) that we’ve been doing a great job of supporting each other there with daily threads on challenges, treatments, pick-me-ups, vent posts, advocacy, and more,” she said.
For anyone in need, PeerRxMed is a free, peer-to-peer program for physicians and other health care workers that is designed to provide support, connection, encouragement, resources, and skill-building to optimize well-being.
For those craving spiritual comfort during this crisis, a number of churches have begun offering that experience virtually, too. First Unitarian Church of Worcester, Massachusetts, for example, offers weekly services via YouTube. Similar online programming is being offered from all sorts of organizations across denominations.
Apps
For DIY or on-the-spot coping support, apps can help physicians get through the day. Apps and websites that offer guided meditations and other relaxation tools include Headspace, Calm, and Insight Timer. Before downloading, look for special discounts and promotions for healthcare workers.
Additionally, COVID Coach is a free, secure app designed by the U.S. Department of Veterans Affairs that includes tools to help you cope with stress and stay well, safe, healthy, and connected. It also offers advice on navigating parenting, care giving, and working from home while social distancing, quarantined, or sheltering in place.
For practicing daily gratitude, Delightful Journal is a free app that offers journaling prompts, themes, reminders, and unlimited private space to record one’s thoughts.
Adopt a ritual
Although self-care for physicians is more crucial now than ever, it can look different for every individual. Along the same lines as keeping a journal, wellness experts often recommend beginning a “gratitude practice” to help provide solace and perspective.
Tweak and personalize these activities to suit your own needs, but be sure to use them even when you’re feeling well, said Mohana Karlekar, MD, medical director of palliative care and assistant professor at Vanderbilt University Medical Center, Nashville, Tenn.
One exercise she recommends is known as Three Good Things. “Every day, at the end of the day, think about three good things that have happened,” she explained. “You can always find the joys. And the joys don’t have to be enormous. There is joy – there is hope – in everything,” Dr. Karlekar said.
A version of this article originally appeared on Medscape.com.
Nisha Mehta, MD, said her phone has been ringing with calls from tearful and shaken physicians who are distressed and unsettled about their work and home situation and don’t know what to do.
What’s more, many frontline physicians are living apart from family to protect them from infection. “So many physicians have called me crying. ... They can’t even come home and get a hug,” Dr. Mehta said. “What I’m hearing from a lot of people who are in New York and New Jersey is not just that they go to work all day and it’s this exhausting process throughout the entire day, not only physically but also emotionally.”
Physician burnout has held a steady spotlight since long before the COVID-19 crisis began, Dr. Mehta said. “The reason for that is multifold, but in part, it’s hard for physicians to find an appropriate way to be able to process a lot of the emotions related to their work,” she said. “A lot of that brews below the surface, but COVID-19 has really brought many of these issues above that surface.”
Frustrated that governments weren’t doing enough to support health care workers during the pandemic, Dr. Mehta, a radiologist in Charlotte, N.C., decided there needed to be change. On April 4, Dr. Mehta and two physician colleagues submitted to Congress the COVID-19 Pandemic Physician Protection Act, which ensures, among other provisions, mental health coverage for health care workers. An accompanying petition on change.org had received nearly 300,000 signatures as of May 29.
Don’t suffer in silence
A career in medicine comes with immense stress in the best of times, she notes, and managing a pandemic in an already strained system has taken those challenges to newer heights. “We need better support structures at baseline for physician mental health,” said Dr. Mehta.
“That’s something we’ve always been lacking because it’s been against the culture of medicine for so long to say, ‘I’m having a hard time.’ ”
If you’re hurting, the first thing to recognize is that you are not alone in facing these challenges. This is true with respect not only to medical care but also to all of the family, financial, and business concerns physicians are currently facing. “Having all of those things hanging over your head is a lot. We’ve got to find ways to help each other out,” Dr. Mehta said.
Where to find support
Fortunately, the medical community has created several pathways to help its own.
The following list represents a cross-section of opportunities for caregivers to receive care for themselves.
Crisis hotlines
- Physician Support Line. This free and confidential hotline was launched on March 30 by Mona Masood, DO, a Philadelphia-area psychiatrist and moderator of a Facebook forum called the COVID-19 Physicians Group. The PSL is run by more than 600 volunteer psychiatrists who take calls from U.S. physicians 7 days a week from 8:00 a.m. to 1:00 a.m., with no appointment necessary. The toll-free number is 888-409-0141.
- For the Frontlines. This 24/7 help line provides free crisis counseling for frontline workers. They can text FRONTLINE to 741741 in the United States (support is also available for residents of Canada, Ireland, and the United Kingdom).
Resources from professional groups
- Action Collaborative on Clinician Well-Being and Resilience. Created by the National Academy of Medicine in 2017, the Action Collaborative comprises more than 60 organizations committed to reversing trends in clinician burnout. In response to the pandemic, the group has compiled a list of strategies and resources to support the health and well-being of clinicians who are providing healthcare during the COVID-19 outbreak.
- American Medical Association. The AMA has created a resource center dedicated to providing care for caregivers during the COVID-19 pandemic. The website includes specific guidance for managing mental health during the pandemic.
- American College of Physicians. The professional society of internal medicine physicians has created a comprehensive guide for physicians specific to COVID-19, with a section dedicated to clinician well-being that includes information about hotlines, counseling services, grief support, and more.
- American Hospital Association. The AHA’s website now includes regularly updated resources for healthcare clinicians and staff, as well as a special section dedicated to protecting and enabling healthcare workers in the midst of the pandemic.
Virtual psychological counseling
Not unlike the way telemedicine has allowed some physicians to keep seeing their patients, many modalities enable participation in therapy through video, chat, phone call, or any combination thereof. Look for a service that is convenient, flexible, and HIPAA compliant.
Traditional in-office mental health therapy has quickly moved to telemedicine. Many if not most insurers that cover counseling visits are paying for telepsychiatry or telecounseling. If you don’t know of an appropriate therapist, check the American Psychiatric Association or its state chapters; the American Psychological Association; or look for a licensed mental health counselor.
Because financial constraints are a potential barrier to therapy, Project Parachute, in cooperation with Eleos Health, has organized a cadre of therapists willing to provide pro bono online therapy for health care workers. The amount of free therapy provided to qualified frontline workers is up to the individual therapists. Discuss these parameters with your therapists up front.
Similar services are offered from companies such as Talkspace and BetterHelp on a subscription basis. These services are typically less expensive than in-person sessions. Ask about discounts for healthcare workers. Talkspace, for example, announced in March, “Effective immediately, healthcare workers across the country can get access to a free month of our...online therapy that includes unlimited text, video, and audio messaging with a licensed therapist.”
Online support groups and social media
For more on-demand peer support, look for groups such as the COR Sharing Circle for Healthcare Workers on Facebook. The site’s search engine can point users to plenty of other groups, many of which are closed (meaning posts are visible to members only).
Dr. Mehta hosts her own Facebook group called Physician Community. “I would like to think (and genuinely feel) that we’ve been doing a great job of supporting each other there with daily threads on challenges, treatments, pick-me-ups, vent posts, advocacy, and more,” she said.
For anyone in need, PeerRxMed is a free, peer-to-peer program for physicians and other health care workers that is designed to provide support, connection, encouragement, resources, and skill-building to optimize well-being.
For those craving spiritual comfort during this crisis, a number of churches have begun offering that experience virtually, too. First Unitarian Church of Worcester, Massachusetts, for example, offers weekly services via YouTube. Similar online programming is being offered from all sorts of organizations across denominations.
Apps
For DIY or on-the-spot coping support, apps can help physicians get through the day. Apps and websites that offer guided meditations and other relaxation tools include Headspace, Calm, and Insight Timer. Before downloading, look for special discounts and promotions for healthcare workers.
Additionally, COVID Coach is a free, secure app designed by the U.S. Department of Veterans Affairs that includes tools to help you cope with stress and stay well, safe, healthy, and connected. It also offers advice on navigating parenting, care giving, and working from home while social distancing, quarantined, or sheltering in place.
For practicing daily gratitude, Delightful Journal is a free app that offers journaling prompts, themes, reminders, and unlimited private space to record one’s thoughts.
Adopt a ritual
Although self-care for physicians is more crucial now than ever, it can look different for every individual. Along the same lines as keeping a journal, wellness experts often recommend beginning a “gratitude practice” to help provide solace and perspective.
Tweak and personalize these activities to suit your own needs, but be sure to use them even when you’re feeling well, said Mohana Karlekar, MD, medical director of palliative care and assistant professor at Vanderbilt University Medical Center, Nashville, Tenn.
One exercise she recommends is known as Three Good Things. “Every day, at the end of the day, think about three good things that have happened,” she explained. “You can always find the joys. And the joys don’t have to be enormous. There is joy – there is hope – in everything,” Dr. Karlekar said.
A version of this article originally appeared on Medscape.com.
Nisha Mehta, MD, said her phone has been ringing with calls from tearful and shaken physicians who are distressed and unsettled about their work and home situation and don’t know what to do.
What’s more, many frontline physicians are living apart from family to protect them from infection. “So many physicians have called me crying. ... They can’t even come home and get a hug,” Dr. Mehta said. “What I’m hearing from a lot of people who are in New York and New Jersey is not just that they go to work all day and it’s this exhausting process throughout the entire day, not only physically but also emotionally.”
Physician burnout has held a steady spotlight since long before the COVID-19 crisis began, Dr. Mehta said. “The reason for that is multifold, but in part, it’s hard for physicians to find an appropriate way to be able to process a lot of the emotions related to their work,” she said. “A lot of that brews below the surface, but COVID-19 has really brought many of these issues above that surface.”
Frustrated that governments weren’t doing enough to support health care workers during the pandemic, Dr. Mehta, a radiologist in Charlotte, N.C., decided there needed to be change. On April 4, Dr. Mehta and two physician colleagues submitted to Congress the COVID-19 Pandemic Physician Protection Act, which ensures, among other provisions, mental health coverage for health care workers. An accompanying petition on change.org had received nearly 300,000 signatures as of May 29.
Don’t suffer in silence
A career in medicine comes with immense stress in the best of times, she notes, and managing a pandemic in an already strained system has taken those challenges to newer heights. “We need better support structures at baseline for physician mental health,” said Dr. Mehta.
“That’s something we’ve always been lacking because it’s been against the culture of medicine for so long to say, ‘I’m having a hard time.’ ”
If you’re hurting, the first thing to recognize is that you are not alone in facing these challenges. This is true with respect not only to medical care but also to all of the family, financial, and business concerns physicians are currently facing. “Having all of those things hanging over your head is a lot. We’ve got to find ways to help each other out,” Dr. Mehta said.
Where to find support
Fortunately, the medical community has created several pathways to help its own.
The following list represents a cross-section of opportunities for caregivers to receive care for themselves.
Crisis hotlines
- Physician Support Line. This free and confidential hotline was launched on March 30 by Mona Masood, DO, a Philadelphia-area psychiatrist and moderator of a Facebook forum called the COVID-19 Physicians Group. The PSL is run by more than 600 volunteer psychiatrists who take calls from U.S. physicians 7 days a week from 8:00 a.m. to 1:00 a.m., with no appointment necessary. The toll-free number is 888-409-0141.
- For the Frontlines. This 24/7 help line provides free crisis counseling for frontline workers. They can text FRONTLINE to 741741 in the United States (support is also available for residents of Canada, Ireland, and the United Kingdom).
Resources from professional groups
- Action Collaborative on Clinician Well-Being and Resilience. Created by the National Academy of Medicine in 2017, the Action Collaborative comprises more than 60 organizations committed to reversing trends in clinician burnout. In response to the pandemic, the group has compiled a list of strategies and resources to support the health and well-being of clinicians who are providing healthcare during the COVID-19 outbreak.
- American Medical Association. The AMA has created a resource center dedicated to providing care for caregivers during the COVID-19 pandemic. The website includes specific guidance for managing mental health during the pandemic.
- American College of Physicians. The professional society of internal medicine physicians has created a comprehensive guide for physicians specific to COVID-19, with a section dedicated to clinician well-being that includes information about hotlines, counseling services, grief support, and more.
- American Hospital Association. The AHA’s website now includes regularly updated resources for healthcare clinicians and staff, as well as a special section dedicated to protecting and enabling healthcare workers in the midst of the pandemic.
Virtual psychological counseling
Not unlike the way telemedicine has allowed some physicians to keep seeing their patients, many modalities enable participation in therapy through video, chat, phone call, or any combination thereof. Look for a service that is convenient, flexible, and HIPAA compliant.
Traditional in-office mental health therapy has quickly moved to telemedicine. Many if not most insurers that cover counseling visits are paying for telepsychiatry or telecounseling. If you don’t know of an appropriate therapist, check the American Psychiatric Association or its state chapters; the American Psychological Association; or look for a licensed mental health counselor.
Because financial constraints are a potential barrier to therapy, Project Parachute, in cooperation with Eleos Health, has organized a cadre of therapists willing to provide pro bono online therapy for health care workers. The amount of free therapy provided to qualified frontline workers is up to the individual therapists. Discuss these parameters with your therapists up front.
Similar services are offered from companies such as Talkspace and BetterHelp on a subscription basis. These services are typically less expensive than in-person sessions. Ask about discounts for healthcare workers. Talkspace, for example, announced in March, “Effective immediately, healthcare workers across the country can get access to a free month of our...online therapy that includes unlimited text, video, and audio messaging with a licensed therapist.”
Online support groups and social media
For more on-demand peer support, look for groups such as the COR Sharing Circle for Healthcare Workers on Facebook. The site’s search engine can point users to plenty of other groups, many of which are closed (meaning posts are visible to members only).
Dr. Mehta hosts her own Facebook group called Physician Community. “I would like to think (and genuinely feel) that we’ve been doing a great job of supporting each other there with daily threads on challenges, treatments, pick-me-ups, vent posts, advocacy, and more,” she said.
For anyone in need, PeerRxMed is a free, peer-to-peer program for physicians and other health care workers that is designed to provide support, connection, encouragement, resources, and skill-building to optimize well-being.
For those craving spiritual comfort during this crisis, a number of churches have begun offering that experience virtually, too. First Unitarian Church of Worcester, Massachusetts, for example, offers weekly services via YouTube. Similar online programming is being offered from all sorts of organizations across denominations.
Apps
For DIY or on-the-spot coping support, apps can help physicians get through the day. Apps and websites that offer guided meditations and other relaxation tools include Headspace, Calm, and Insight Timer. Before downloading, look for special discounts and promotions for healthcare workers.
Additionally, COVID Coach is a free, secure app designed by the U.S. Department of Veterans Affairs that includes tools to help you cope with stress and stay well, safe, healthy, and connected. It also offers advice on navigating parenting, care giving, and working from home while social distancing, quarantined, or sheltering in place.
For practicing daily gratitude, Delightful Journal is a free app that offers journaling prompts, themes, reminders, and unlimited private space to record one’s thoughts.
Adopt a ritual
Although self-care for physicians is more crucial now than ever, it can look different for every individual. Along the same lines as keeping a journal, wellness experts often recommend beginning a “gratitude practice” to help provide solace and perspective.
Tweak and personalize these activities to suit your own needs, but be sure to use them even when you’re feeling well, said Mohana Karlekar, MD, medical director of palliative care and assistant professor at Vanderbilt University Medical Center, Nashville, Tenn.
One exercise she recommends is known as Three Good Things. “Every day, at the end of the day, think about three good things that have happened,” she explained. “You can always find the joys. And the joys don’t have to be enormous. There is joy – there is hope – in everything,” Dr. Karlekar said.
A version of this article originally appeared on Medscape.com.
Money worries during COVID-19? Six tips to keep your finances afloat
Even before Atlanta had an official shelter-in-place order, patients at the private plastic surgery practice of Nicholas Jones, MD, began canceling and rescheduled planned procedures.
After a few weeks, Dr. Jones, aged 40 years, stopped seeing patients entirely, but as a self-employed independent contractor, that means he’d lost most of his income. Dr. Jones still makes some money via a wound care job at a local nursing home, but he’s concerned that job may also be eliminated.
“I’m not hurting yet,” he said. “But I’m preparing for the worst possible scenario.”
In preparation, he and his fiancé have cut back on extraneous expenses like Uber Eats, magazine subscriptions, and streaming music services. Even though he has a 6-month emergency fund, Jones has reached out to utility companies, mortgage lenders, and student loan servicers to find out about any programs they offer to people who’ve suffered financially from the coronavirus crisis.
He’s also considered traveling to one of the COVID-19 epicenters – he has family in New Orleans and Chicago – to work in a hospital there. Jones has trauma experience and is double-boarded in general and plastic surgery.
“I could provide relief to those in need and also float through this troubled time with some financial relief,” he said.
Whereas much of the world’s attention has been on physicians who are on the front line and working around the clock in hospitals to help COVID-19 patients, thousands of other physicians are experiencing the opposite phenomenon – a slowdown or even stoppage of work (and income) altogether.
Even among those practices that remain open, the number of patients has declined as people avoid going to the office unless they absolutely have to.
At the same time, doctors in two-income households may have a spouse experiencing a job loss or income decline. Nearly 10 million Americans applied for unemployment benefits in the last 2 weeks of March, the largest number on record.
Still, while there’s uncertainty around how long the coronavirus crisis will last, experts agree that at some point America will return to a “new normal” and business operations will begin to reopen. For physicians experiencing a reduction in income who, like Jones, have an emergency fund with a few months’ worth of expenses, now’s the time to tap into it. (Or if you still have income, now’s the time to focus on growing that emergency fund to give yourself an even bigger safety net.)
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here’s how to stay afloat in the near term:
Cut back on expenses
Some household spending has naturally tapered off for many families because social distancing restrictions reduce spending on eating out, travel, and other leisure activities. But this is also an opportunity to look for other ways to reduce spending. Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need.
Some gyms are not allowing membership termination right now, but it pays to ask. If a service you’re not using won’t facilitate the cancellation, call your credit card company to dispute and stop the charges, and report them to the Better Business Bureau.
You should also stop contributing to nonemergency savings accounts such as your retirement fund or your children’s college funds.
“A lot of people are hesitant to stop their automatic savings if they’ve been maxing out their 401(k) contribution or 529 accounts,” says Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “But if you’re thinking long term, the reality is that missing a couple of months won’t make or break a plan. Cutting back on the amount you’re saving in the short term will increase your cash flow and is a good way to make ends meet.”
Take advantage of regulatory changes
Although many physicians won’t qualify for direct payments via the Coronavirus Aid Relief and Economic Security (CARES) Act (the $1,200 payments to individuals start phasing out once income hits $75,000 and disappear entirely for those making more than $99,000), there are other provisions in the stimulus bill that may help physicians. The bill, for example, boosts state unemployment payments by $600 per week for the next 4 months, meaning qualified workers could receive an average of nearly $1,000 per week, depending on their state, and there are new provisions providing unemployment payments to self-employed and contract workers.
The CARES Act also includes a break for federal student loan holders. Under that rule, you can skip your payments through September without incurring additional interest. Physicians in the loan forgiveness program will still get credit for payments skipped during this program.
Separately, the IRS has extended the tax deadline from April 15 to July 15, which means not only do you not have to file your taxes until then, you also don’t have to pay any taxes you owe until mid-July. The deadline for first quarter estimated tax payments has also moved to July 15. (If you’re expecting a refund, however, you should file ASAP, since the IRS will typically issue those within a few weeks of receiving your returns.)
Tap your home equity – if you’re planning to stay put
If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Interest rates have fallen recently amid economic turbulence, so if you haven’t refinanced recently you may be able to shave your monthly payment. If you need cash, a cash-out refinance, home equity line of credit, or a reverse mortgage (available if you’re over age 62) are among the lowest-cost ways to borrow.
“With interest rates so low, there can be a lot of benefit to refinancing and leveraging your house, especially if you’re planning to stay there,” says Jamie Hopkins, a director at the Carson Group. “The challenge is if you’re planning to move in the next few years. There’s a real risk that the housing market could go down in the next couple of years, and if you’re planning to sell, there’s a risk that you might not get back what you borrowed.”
Communicate early with your bank or landlord
If you don’t have the income to refinance, and you think you’re going to run into trouble making your housing payment, you should let your bank or landlord know as soon as possible. The CARES Act allows homeowners with federally backed mortgages to obtain a 180-day postponement of mortgage payments because of COVID-19 financial hardship, with the potential to extend for another 180 days. It also bans eviction by landlords with federal mortgages for 120 days.
Even if you don’t have a federally backed mortgage, you should still get in touch with your lender. Many mortgage servicers have their own forbearance programs for borrowers who can prove a temporary financial hardship. (Some banks are also waiving fees on early withdrawals on CDs and giving cardholders a reprieve on credit card payments.) Commercial landlords are also working with struggling tenants, so you may also be able to get some relief on your office lease as well.
“All of the lenders are setting up helplines for people affected,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “The best thing you can do is contact them right away if you think that you’re going to have a problem vs. just letting the bills go.”
Consider retirement account withdrawals
Standard personal finance advice holds that you should exhaust all other options before pulling money out of your retirement account because of the high penalties for early withdrawals and because money removed from retirement accounts is no longer compounding over time.
Still, the CARES act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. You’ll owe ordinary income taxes on the withdrawal, but you have 3 years to pay them or to return the money to your retirement account.
“That’s a great relief provision, especially for higher-income physicians who might have a higher 401(k) balance,” said Jamie Hopkins.
Be smart about credit cards
Although using credit cards that you can’t pay off every month is typically an expensive way to access money, getting a new card with a low or zero percent introductory rate is a short-term strategy to consider when you’ve exhausted other options. If you have good credit, you may be able to qualify for a credit card with a 0% introductory interest rate on new transactions. Pay close attention to the fine print, including the cap on the balance you can carry without interest and whether you’ll be required to make minimum payments.
The average 0% credit card offer is for 11 months, but there are some cards that can extend the offer for up to a year-and-a-half. If you choose to use this strategy, you’ll need a plan to pay off the entire balance before the introductory period ends. If there’s a balance remaining once the rate resets, you may end up owing deferred interest on it.
The financial ramifications of the coronavirus can feel overwhelming, but it’s important not to panic. While it remains unclear how long the current crisis will last, making some smart money moves to preserve your cash in the meantime can help you stay afloat.
A version of this article originally appeared on Medscape.com.
Even before Atlanta had an official shelter-in-place order, patients at the private plastic surgery practice of Nicholas Jones, MD, began canceling and rescheduled planned procedures.
After a few weeks, Dr. Jones, aged 40 years, stopped seeing patients entirely, but as a self-employed independent contractor, that means he’d lost most of his income. Dr. Jones still makes some money via a wound care job at a local nursing home, but he’s concerned that job may also be eliminated.
“I’m not hurting yet,” he said. “But I’m preparing for the worst possible scenario.”
In preparation, he and his fiancé have cut back on extraneous expenses like Uber Eats, magazine subscriptions, and streaming music services. Even though he has a 6-month emergency fund, Jones has reached out to utility companies, mortgage lenders, and student loan servicers to find out about any programs they offer to people who’ve suffered financially from the coronavirus crisis.
He’s also considered traveling to one of the COVID-19 epicenters – he has family in New Orleans and Chicago – to work in a hospital there. Jones has trauma experience and is double-boarded in general and plastic surgery.
“I could provide relief to those in need and also float through this troubled time with some financial relief,” he said.
Whereas much of the world’s attention has been on physicians who are on the front line and working around the clock in hospitals to help COVID-19 patients, thousands of other physicians are experiencing the opposite phenomenon – a slowdown or even stoppage of work (and income) altogether.
Even among those practices that remain open, the number of patients has declined as people avoid going to the office unless they absolutely have to.
At the same time, doctors in two-income households may have a spouse experiencing a job loss or income decline. Nearly 10 million Americans applied for unemployment benefits in the last 2 weeks of March, the largest number on record.
Still, while there’s uncertainty around how long the coronavirus crisis will last, experts agree that at some point America will return to a “new normal” and business operations will begin to reopen. For physicians experiencing a reduction in income who, like Jones, have an emergency fund with a few months’ worth of expenses, now’s the time to tap into it. (Or if you still have income, now’s the time to focus on growing that emergency fund to give yourself an even bigger safety net.)
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here’s how to stay afloat in the near term:
Cut back on expenses
Some household spending has naturally tapered off for many families because social distancing restrictions reduce spending on eating out, travel, and other leisure activities. But this is also an opportunity to look for other ways to reduce spending. Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need.
Some gyms are not allowing membership termination right now, but it pays to ask. If a service you’re not using won’t facilitate the cancellation, call your credit card company to dispute and stop the charges, and report them to the Better Business Bureau.
You should also stop contributing to nonemergency savings accounts such as your retirement fund or your children’s college funds.
“A lot of people are hesitant to stop their automatic savings if they’ve been maxing out their 401(k) contribution or 529 accounts,” says Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “But if you’re thinking long term, the reality is that missing a couple of months won’t make or break a plan. Cutting back on the amount you’re saving in the short term will increase your cash flow and is a good way to make ends meet.”
Take advantage of regulatory changes
Although many physicians won’t qualify for direct payments via the Coronavirus Aid Relief and Economic Security (CARES) Act (the $1,200 payments to individuals start phasing out once income hits $75,000 and disappear entirely for those making more than $99,000), there are other provisions in the stimulus bill that may help physicians. The bill, for example, boosts state unemployment payments by $600 per week for the next 4 months, meaning qualified workers could receive an average of nearly $1,000 per week, depending on their state, and there are new provisions providing unemployment payments to self-employed and contract workers.
The CARES Act also includes a break for federal student loan holders. Under that rule, you can skip your payments through September without incurring additional interest. Physicians in the loan forgiveness program will still get credit for payments skipped during this program.
Separately, the IRS has extended the tax deadline from April 15 to July 15, which means not only do you not have to file your taxes until then, you also don’t have to pay any taxes you owe until mid-July. The deadline for first quarter estimated tax payments has also moved to July 15. (If you’re expecting a refund, however, you should file ASAP, since the IRS will typically issue those within a few weeks of receiving your returns.)
Tap your home equity – if you’re planning to stay put
If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Interest rates have fallen recently amid economic turbulence, so if you haven’t refinanced recently you may be able to shave your monthly payment. If you need cash, a cash-out refinance, home equity line of credit, or a reverse mortgage (available if you’re over age 62) are among the lowest-cost ways to borrow.
“With interest rates so low, there can be a lot of benefit to refinancing and leveraging your house, especially if you’re planning to stay there,” says Jamie Hopkins, a director at the Carson Group. “The challenge is if you’re planning to move in the next few years. There’s a real risk that the housing market could go down in the next couple of years, and if you’re planning to sell, there’s a risk that you might not get back what you borrowed.”
Communicate early with your bank or landlord
If you don’t have the income to refinance, and you think you’re going to run into trouble making your housing payment, you should let your bank or landlord know as soon as possible. The CARES Act allows homeowners with federally backed mortgages to obtain a 180-day postponement of mortgage payments because of COVID-19 financial hardship, with the potential to extend for another 180 days. It also bans eviction by landlords with federal mortgages for 120 days.
Even if you don’t have a federally backed mortgage, you should still get in touch with your lender. Many mortgage servicers have their own forbearance programs for borrowers who can prove a temporary financial hardship. (Some banks are also waiving fees on early withdrawals on CDs and giving cardholders a reprieve on credit card payments.) Commercial landlords are also working with struggling tenants, so you may also be able to get some relief on your office lease as well.
“All of the lenders are setting up helplines for people affected,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “The best thing you can do is contact them right away if you think that you’re going to have a problem vs. just letting the bills go.”
Consider retirement account withdrawals
Standard personal finance advice holds that you should exhaust all other options before pulling money out of your retirement account because of the high penalties for early withdrawals and because money removed from retirement accounts is no longer compounding over time.
Still, the CARES act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. You’ll owe ordinary income taxes on the withdrawal, but you have 3 years to pay them or to return the money to your retirement account.
“That’s a great relief provision, especially for higher-income physicians who might have a higher 401(k) balance,” said Jamie Hopkins.
Be smart about credit cards
Although using credit cards that you can’t pay off every month is typically an expensive way to access money, getting a new card with a low or zero percent introductory rate is a short-term strategy to consider when you’ve exhausted other options. If you have good credit, you may be able to qualify for a credit card with a 0% introductory interest rate on new transactions. Pay close attention to the fine print, including the cap on the balance you can carry without interest and whether you’ll be required to make minimum payments.
The average 0% credit card offer is for 11 months, but there are some cards that can extend the offer for up to a year-and-a-half. If you choose to use this strategy, you’ll need a plan to pay off the entire balance before the introductory period ends. If there’s a balance remaining once the rate resets, you may end up owing deferred interest on it.
The financial ramifications of the coronavirus can feel overwhelming, but it’s important not to panic. While it remains unclear how long the current crisis will last, making some smart money moves to preserve your cash in the meantime can help you stay afloat.
A version of this article originally appeared on Medscape.com.
Even before Atlanta had an official shelter-in-place order, patients at the private plastic surgery practice of Nicholas Jones, MD, began canceling and rescheduled planned procedures.
After a few weeks, Dr. Jones, aged 40 years, stopped seeing patients entirely, but as a self-employed independent contractor, that means he’d lost most of his income. Dr. Jones still makes some money via a wound care job at a local nursing home, but he’s concerned that job may also be eliminated.
“I’m not hurting yet,” he said. “But I’m preparing for the worst possible scenario.”
In preparation, he and his fiancé have cut back on extraneous expenses like Uber Eats, magazine subscriptions, and streaming music services. Even though he has a 6-month emergency fund, Jones has reached out to utility companies, mortgage lenders, and student loan servicers to find out about any programs they offer to people who’ve suffered financially from the coronavirus crisis.
He’s also considered traveling to one of the COVID-19 epicenters – he has family in New Orleans and Chicago – to work in a hospital there. Jones has trauma experience and is double-boarded in general and plastic surgery.
“I could provide relief to those in need and also float through this troubled time with some financial relief,” he said.
Whereas much of the world’s attention has been on physicians who are on the front line and working around the clock in hospitals to help COVID-19 patients, thousands of other physicians are experiencing the opposite phenomenon – a slowdown or even stoppage of work (and income) altogether.
Even among those practices that remain open, the number of patients has declined as people avoid going to the office unless they absolutely have to.
At the same time, doctors in two-income households may have a spouse experiencing a job loss or income decline. Nearly 10 million Americans applied for unemployment benefits in the last 2 weeks of March, the largest number on record.
Still, while there’s uncertainty around how long the coronavirus crisis will last, experts agree that at some point America will return to a “new normal” and business operations will begin to reopen. For physicians experiencing a reduction in income who, like Jones, have an emergency fund with a few months’ worth of expenses, now’s the time to tap into it. (Or if you still have income, now’s the time to focus on growing that emergency fund to give yourself an even bigger safety net.)
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here’s how to stay afloat in the near term:
Cut back on expenses
Some household spending has naturally tapered off for many families because social distancing restrictions reduce spending on eating out, travel, and other leisure activities. But this is also an opportunity to look for other ways to reduce spending. Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need.
Some gyms are not allowing membership termination right now, but it pays to ask. If a service you’re not using won’t facilitate the cancellation, call your credit card company to dispute and stop the charges, and report them to the Better Business Bureau.
You should also stop contributing to nonemergency savings accounts such as your retirement fund or your children’s college funds.
“A lot of people are hesitant to stop their automatic savings if they’ve been maxing out their 401(k) contribution or 529 accounts,” says Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “But if you’re thinking long term, the reality is that missing a couple of months won’t make or break a plan. Cutting back on the amount you’re saving in the short term will increase your cash flow and is a good way to make ends meet.”
Take advantage of regulatory changes
Although many physicians won’t qualify for direct payments via the Coronavirus Aid Relief and Economic Security (CARES) Act (the $1,200 payments to individuals start phasing out once income hits $75,000 and disappear entirely for those making more than $99,000), there are other provisions in the stimulus bill that may help physicians. The bill, for example, boosts state unemployment payments by $600 per week for the next 4 months, meaning qualified workers could receive an average of nearly $1,000 per week, depending on their state, and there are new provisions providing unemployment payments to self-employed and contract workers.
The CARES Act also includes a break for federal student loan holders. Under that rule, you can skip your payments through September without incurring additional interest. Physicians in the loan forgiveness program will still get credit for payments skipped during this program.
Separately, the IRS has extended the tax deadline from April 15 to July 15, which means not only do you not have to file your taxes until then, you also don’t have to pay any taxes you owe until mid-July. The deadline for first quarter estimated tax payments has also moved to July 15. (If you’re expecting a refund, however, you should file ASAP, since the IRS will typically issue those within a few weeks of receiving your returns.)
Tap your home equity – if you’re planning to stay put
If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Interest rates have fallen recently amid economic turbulence, so if you haven’t refinanced recently you may be able to shave your monthly payment. If you need cash, a cash-out refinance, home equity line of credit, or a reverse mortgage (available if you’re over age 62) are among the lowest-cost ways to borrow.
“With interest rates so low, there can be a lot of benefit to refinancing and leveraging your house, especially if you’re planning to stay there,” says Jamie Hopkins, a director at the Carson Group. “The challenge is if you’re planning to move in the next few years. There’s a real risk that the housing market could go down in the next couple of years, and if you’re planning to sell, there’s a risk that you might not get back what you borrowed.”
Communicate early with your bank or landlord
If you don’t have the income to refinance, and you think you’re going to run into trouble making your housing payment, you should let your bank or landlord know as soon as possible. The CARES Act allows homeowners with federally backed mortgages to obtain a 180-day postponement of mortgage payments because of COVID-19 financial hardship, with the potential to extend for another 180 days. It also bans eviction by landlords with federal mortgages for 120 days.
Even if you don’t have a federally backed mortgage, you should still get in touch with your lender. Many mortgage servicers have their own forbearance programs for borrowers who can prove a temporary financial hardship. (Some banks are also waiving fees on early withdrawals on CDs and giving cardholders a reprieve on credit card payments.) Commercial landlords are also working with struggling tenants, so you may also be able to get some relief on your office lease as well.
“All of the lenders are setting up helplines for people affected,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “The best thing you can do is contact them right away if you think that you’re going to have a problem vs. just letting the bills go.”
Consider retirement account withdrawals
Standard personal finance advice holds that you should exhaust all other options before pulling money out of your retirement account because of the high penalties for early withdrawals and because money removed from retirement accounts is no longer compounding over time.
Still, the CARES act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. You’ll owe ordinary income taxes on the withdrawal, but you have 3 years to pay them or to return the money to your retirement account.
“That’s a great relief provision, especially for higher-income physicians who might have a higher 401(k) balance,” said Jamie Hopkins.
Be smart about credit cards
Although using credit cards that you can’t pay off every month is typically an expensive way to access money, getting a new card with a low or zero percent introductory rate is a short-term strategy to consider when you’ve exhausted other options. If you have good credit, you may be able to qualify for a credit card with a 0% introductory interest rate on new transactions. Pay close attention to the fine print, including the cap on the balance you can carry without interest and whether you’ll be required to make minimum payments.
The average 0% credit card offer is for 11 months, but there are some cards that can extend the offer for up to a year-and-a-half. If you choose to use this strategy, you’ll need a plan to pay off the entire balance before the introductory period ends. If there’s a balance remaining once the rate resets, you may end up owing deferred interest on it.
The financial ramifications of the coronavirus can feel overwhelming, but it’s important not to panic. While it remains unclear how long the current crisis will last, making some smart money moves to preserve your cash in the meantime can help you stay afloat.
A version of this article originally appeared on Medscape.com.
Daily Recap: How to stay afloat financially during COVID-19, more bad news on e-cigs
Here are the stories our MDedge editors across specialties think you need to know about today:
Tips to keep your finances healthy during COVID-19
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here are some tips on how to stay afloat in the near term. Cut back on expenses: Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need. Tap your home equity: If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Consider retirement account withdrawals: the CARES Act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. Read more.
Nursing homes overhaul infection control
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support. “Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton. Experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following: “Infection preventionists” to lead improvements in emergency preparedness and infection prevention and control, well-qualified and engaged medical directors, a survey/inspection process that focuses on education, and more resources and attention to structural reform. Read more.
WHO backtracks on asymptomatic SARS-CoV-2 transmission
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir on June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said. But on June 9 – following a day of criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.” Physicians and public health experts slammed the initial comments, saying that they created confusion. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove’s initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is “not correct.” Read more.
E-cigs linked to smoking relapse
The use of electronic nicotine delivery systems is associated with increased risk of cigarette smoking relapse among former smokers, results from a large longitudinal cohort study demonstrated. The findings come from a survey of adult former smokers who participated in the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). Adjusted hazard ratio (AHR) analysis revealed that the use of electronic nicotine delivery systems was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was similarly associated with a significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82). “For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open. Read more.
Formula feeding leads to early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics. The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). “Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Tips to keep your finances healthy during COVID-19
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here are some tips on how to stay afloat in the near term. Cut back on expenses: Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need. Tap your home equity: If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Consider retirement account withdrawals: the CARES Act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. Read more.
Nursing homes overhaul infection control
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support. “Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton. Experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following: “Infection preventionists” to lead improvements in emergency preparedness and infection prevention and control, well-qualified and engaged medical directors, a survey/inspection process that focuses on education, and more resources and attention to structural reform. Read more.
WHO backtracks on asymptomatic SARS-CoV-2 transmission
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir on June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said. But on June 9 – following a day of criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.” Physicians and public health experts slammed the initial comments, saying that they created confusion. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove’s initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is “not correct.” Read more.
E-cigs linked to smoking relapse
The use of electronic nicotine delivery systems is associated with increased risk of cigarette smoking relapse among former smokers, results from a large longitudinal cohort study demonstrated. The findings come from a survey of adult former smokers who participated in the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). Adjusted hazard ratio (AHR) analysis revealed that the use of electronic nicotine delivery systems was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was similarly associated with a significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82). “For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open. Read more.
Formula feeding leads to early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics. The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). “Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Tips to keep your finances healthy during COVID-19
If you’re among the more than half of Americans with less than 6 months of expenses saved for a rainy day, here are some tips on how to stay afloat in the near term. Cut back on expenses: Look through your credit card bills to see whether there are recurring payments you can cut, such as a payment to a gym that’s temporarily closed or a monthly subscription box that you don’t need. Tap your home equity: If you have good credit and still have some income, you might consider refinancing your home mortgage or opening a home equity line of credit. Consider retirement account withdrawals: the CARES Act has provisions making it less financially onerous to pull money from your retirement accounts. Under the new law, you can take a distribution of up to $100,000 from your IRA or 401(k) without having to pay the 10% early withdrawal penalty. Read more.
Nursing homes overhaul infection control
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support. “Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton. Experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following: “Infection preventionists” to lead improvements in emergency preparedness and infection prevention and control, well-qualified and engaged medical directors, a survey/inspection process that focuses on education, and more resources and attention to structural reform. Read more.
WHO backtracks on asymptomatic SARS-CoV-2 transmission
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir on June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said. But on June 9 – following a day of criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.” Physicians and public health experts slammed the initial comments, saying that they created confusion. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove’s initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is “not correct.” Read more.
E-cigs linked to smoking relapse
The use of electronic nicotine delivery systems is associated with increased risk of cigarette smoking relapse among former smokers, results from a large longitudinal cohort study demonstrated. The findings come from a survey of adult former smokers who participated in the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). Adjusted hazard ratio (AHR) analysis revealed that the use of electronic nicotine delivery systems was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was similarly associated with a significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82). “For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open. Read more.
Formula feeding leads to early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics. The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). “Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Kids with food allergies the newest victims of COVID-19?
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.