User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Helping patients through a benzodiazepine taper
Benzodiazepines are one of the most commonly prescribed medication classes worldwide.1 Patients prescribed benzodiazepines who have no history of abuse or misuse may want to reduce or discontinue using these agents for various reasons, including adverse effects or wanting to reduce the number of medications they take. In this article, we offer strategies for creating an individualized taper plan, and describe additional nonpharmacologic interventions to help ensure that the taper is successful.
Formulating a taper plan
There is no gold-standard algorithm for tapering benzodiazepines.1,2 Even with a carefully designed plan, tapering can be challenging because approximately one-third of patients will experience difficulties such as withdrawal symptoms.1 Prior to creating a plan, carefully assess the patient’s history, including the type of benzodiazepine prescribed (short- or long-acting); the dose, dosing frequency, and duration of use; comorbid medical and psychiatric conditions; any previous experience with withdrawal symptoms; and psychosocial factors (eg, lifestyle and personality). Consider whether the patient can be safely tapered in an outpatient setting or will require hospitalization. Tapering designed to take place over several weeks or months tends to be more successful; however, patient-specific circumstances play a role in determining the duration of the taper.1,2
For the greatest chance of success, a benzodiazepine should not be reduced faster than 25% of the total daily dose per week.1 Consider which of the following pharmacologic approaches to benzodiazepine tapering might work best for your patient:
- Reduce the daily dose by one-eighth to one-tenth every 1 to 2 weeks over a 2- to 12-month period for patients with a physiological dependence.1
- Reduce the benzodiazepine dose by 10% to 25% every 2 weeks over a 4- to 8-week period.2
- Some guidelines have suggested converting the prescribed benzodiazepine to an equivalent dose of diazepam because of its long half-life, and then reducing the diazepam dose by one-eighth every 2 weeks.3
There is uncertainty in the medical literature about using a long-acting benzodiazepine to taper off a short-acting benzodiazepine, although this practice is generally clinically accepted.1,2 Similarly, there is no definitive evidence that supports using adjuvant medications to facilitate tapering.1,2
Nonpharmacologic interventions
Patients are more likely to have a successful taper if nonpharmacologic interventions are part of a comprehensive treatment plan.1
To help your patients through the challenges of a benzodiazepine taper:
- Validate their concerns, reassure them that you will support them throughout the taper, and provide information on additional resources for support.
- Provide education about the process of tapering and symptoms of withdrawal.
- Recommend therapies, such as cognitive-behavioral therapy or motivational interventions, that develop or enhance coping skills.
- Enlist the help of the patient’s family and friends for support and encouragement.
Despite some clinicians’ trepidation, 70% to 90% of patients can be successfully tapered off benzodiazepines by using an individualized approach that includes tailored tapering and nonpharmacologic interventions that provide benefits that persist after the patient completes the taper.1
1. Guina J, Merrill B. Benzodiazepines II: waking up on sedatives: providing optimal care when inheriting benzodiazepine prescriptions in transfer patients. J Clin Med. 2018;7(2):pii: E20. doi: 10.3390/jcm7020020.
2. Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. 2017;376(12):1147-1157.
3. Diaper AM, Law FD, Melichar JK. Pharmacological strategies for detoxification. Br J Clin Pharmacol. 2014;77(2):302-314.
Benzodiazepines are one of the most commonly prescribed medication classes worldwide.1 Patients prescribed benzodiazepines who have no history of abuse or misuse may want to reduce or discontinue using these agents for various reasons, including adverse effects or wanting to reduce the number of medications they take. In this article, we offer strategies for creating an individualized taper plan, and describe additional nonpharmacologic interventions to help ensure that the taper is successful.
Formulating a taper plan
There is no gold-standard algorithm for tapering benzodiazepines.1,2 Even with a carefully designed plan, tapering can be challenging because approximately one-third of patients will experience difficulties such as withdrawal symptoms.1 Prior to creating a plan, carefully assess the patient’s history, including the type of benzodiazepine prescribed (short- or long-acting); the dose, dosing frequency, and duration of use; comorbid medical and psychiatric conditions; any previous experience with withdrawal symptoms; and psychosocial factors (eg, lifestyle and personality). Consider whether the patient can be safely tapered in an outpatient setting or will require hospitalization. Tapering designed to take place over several weeks or months tends to be more successful; however, patient-specific circumstances play a role in determining the duration of the taper.1,2
For the greatest chance of success, a benzodiazepine should not be reduced faster than 25% of the total daily dose per week.1 Consider which of the following pharmacologic approaches to benzodiazepine tapering might work best for your patient:
- Reduce the daily dose by one-eighth to one-tenth every 1 to 2 weeks over a 2- to 12-month period for patients with a physiological dependence.1
- Reduce the benzodiazepine dose by 10% to 25% every 2 weeks over a 4- to 8-week period.2
- Some guidelines have suggested converting the prescribed benzodiazepine to an equivalent dose of diazepam because of its long half-life, and then reducing the diazepam dose by one-eighth every 2 weeks.3
There is uncertainty in the medical literature about using a long-acting benzodiazepine to taper off a short-acting benzodiazepine, although this practice is generally clinically accepted.1,2 Similarly, there is no definitive evidence that supports using adjuvant medications to facilitate tapering.1,2
Nonpharmacologic interventions
Patients are more likely to have a successful taper if nonpharmacologic interventions are part of a comprehensive treatment plan.1
To help your patients through the challenges of a benzodiazepine taper:
- Validate their concerns, reassure them that you will support them throughout the taper, and provide information on additional resources for support.
- Provide education about the process of tapering and symptoms of withdrawal.
- Recommend therapies, such as cognitive-behavioral therapy or motivational interventions, that develop or enhance coping skills.
- Enlist the help of the patient’s family and friends for support and encouragement.
Despite some clinicians’ trepidation, 70% to 90% of patients can be successfully tapered off benzodiazepines by using an individualized approach that includes tailored tapering and nonpharmacologic interventions that provide benefits that persist after the patient completes the taper.1
Benzodiazepines are one of the most commonly prescribed medication classes worldwide.1 Patients prescribed benzodiazepines who have no history of abuse or misuse may want to reduce or discontinue using these agents for various reasons, including adverse effects or wanting to reduce the number of medications they take. In this article, we offer strategies for creating an individualized taper plan, and describe additional nonpharmacologic interventions to help ensure that the taper is successful.
Formulating a taper plan
There is no gold-standard algorithm for tapering benzodiazepines.1,2 Even with a carefully designed plan, tapering can be challenging because approximately one-third of patients will experience difficulties such as withdrawal symptoms.1 Prior to creating a plan, carefully assess the patient’s history, including the type of benzodiazepine prescribed (short- or long-acting); the dose, dosing frequency, and duration of use; comorbid medical and psychiatric conditions; any previous experience with withdrawal symptoms; and psychosocial factors (eg, lifestyle and personality). Consider whether the patient can be safely tapered in an outpatient setting or will require hospitalization. Tapering designed to take place over several weeks or months tends to be more successful; however, patient-specific circumstances play a role in determining the duration of the taper.1,2
For the greatest chance of success, a benzodiazepine should not be reduced faster than 25% of the total daily dose per week.1 Consider which of the following pharmacologic approaches to benzodiazepine tapering might work best for your patient:
- Reduce the daily dose by one-eighth to one-tenth every 1 to 2 weeks over a 2- to 12-month period for patients with a physiological dependence.1
- Reduce the benzodiazepine dose by 10% to 25% every 2 weeks over a 4- to 8-week period.2
- Some guidelines have suggested converting the prescribed benzodiazepine to an equivalent dose of diazepam because of its long half-life, and then reducing the diazepam dose by one-eighth every 2 weeks.3
There is uncertainty in the medical literature about using a long-acting benzodiazepine to taper off a short-acting benzodiazepine, although this practice is generally clinically accepted.1,2 Similarly, there is no definitive evidence that supports using adjuvant medications to facilitate tapering.1,2
Nonpharmacologic interventions
Patients are more likely to have a successful taper if nonpharmacologic interventions are part of a comprehensive treatment plan.1
To help your patients through the challenges of a benzodiazepine taper:
- Validate their concerns, reassure them that you will support them throughout the taper, and provide information on additional resources for support.
- Provide education about the process of tapering and symptoms of withdrawal.
- Recommend therapies, such as cognitive-behavioral therapy or motivational interventions, that develop or enhance coping skills.
- Enlist the help of the patient’s family and friends for support and encouragement.
Despite some clinicians’ trepidation, 70% to 90% of patients can be successfully tapered off benzodiazepines by using an individualized approach that includes tailored tapering and nonpharmacologic interventions that provide benefits that persist after the patient completes the taper.1
1. Guina J, Merrill B. Benzodiazepines II: waking up on sedatives: providing optimal care when inheriting benzodiazepine prescriptions in transfer patients. J Clin Med. 2018;7(2):pii: E20. doi: 10.3390/jcm7020020.
2. Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. 2017;376(12):1147-1157.
3. Diaper AM, Law FD, Melichar JK. Pharmacological strategies for detoxification. Br J Clin Pharmacol. 2014;77(2):302-314.
1. Guina J, Merrill B. Benzodiazepines II: waking up on sedatives: providing optimal care when inheriting benzodiazepine prescriptions in transfer patients. J Clin Med. 2018;7(2):pii: E20. doi: 10.3390/jcm7020020.
2. Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. 2017;376(12):1147-1157.
3. Diaper AM, Law FD, Melichar JK. Pharmacological strategies for detoxification. Br J Clin Pharmacol. 2014;77(2):302-314.
A suicide attempt, or something else?
CASE Unexplained hypoglycemia
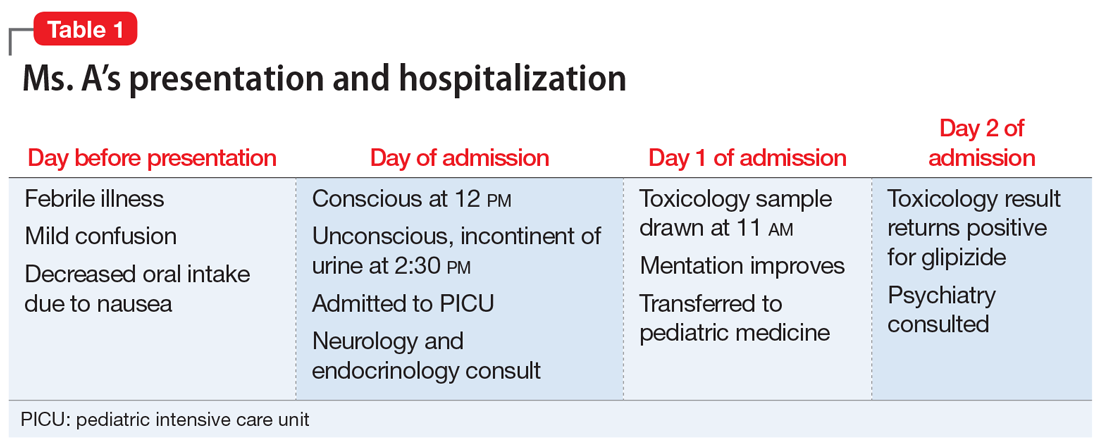
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).
A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
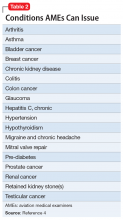
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).

A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
CASE Unexplained hypoglycemia
Ms. A, age 12, is brought to the emergency department (ED) via ambulance with altered mentation and life-threatening hypoglycemia for management of a hypoglycemic seizure. Earlier that day, Ms. A’s parents had found her unresponsive and incontinent of urine. In the ED, Ms. A is minimally responsive. Her blood glucose level measurements are in the range of 30 to 39 mg/dL (reference range: 70 to 99 mg/dL), despite having received IV dextrose first from paramedics, and then in the ED. Ms. A has no history of hypoglycemia or diabetes. Her parents say that the night before coming to the ED, Ms. A had experienced flu-like symptoms, including nausea, vomiting, and diarrhea, that continued overnight and resulted in minimal food intake for 24 hours (Table 1).

A physical exam demonstrates left-sided weakness of face, arm, and leg, rightward gaze, and left-sided neglect. However, the results of CT angiography and an MRI of the brain rule out a stroke. An EEG shows right hemispheric slowing consistent with postictal paralysis, but no ongoing seizure activity. Ms. A is transferred to the pediatric intensive care unit (PICU).
Although Ms. A has no psychiatric diagnoses, she has a history of depressive symptoms, self-harm by cutting, and a suicide attempt by ingestion of an over-the-counter (OTC) medication 1 year ago. She had reported the suicide attempt to her parents several months after the fact, and asked them to find her a therapist, which her parents arranged. She also has a history of asthma, which is well-controlled with montelukast, 5 mg/d.
EVALUATION Elevated insulin levels
Subsequent investigations for organic causes of hypoglycemia are negative for adrenal insufficiency, fatty acid oxidation defect, and sepsis. Blood results demonstrate significantly elevated insulin levels of 92.4 mcIU/mL (reference range: 2.6 to 24.9 mcIU/mL) and a C-peptide level of 9.5 ng/mL (reference range: 1.1 to 4.4 ng/mL).
On Day 1 of admission to the PICU, Ms. A’s blood glucose level normalizes, and her mentation improves. Her parents report that one of them has diabetes and takes oral hypoglycemic agents at home, including glipizide immediate release (IR) tablets, 10 mg, and long-acting insulin glargine. The treatment team suspects that Ms. A may have ingested one or both of these agents, and orders a toxicologic screening for oral hypoglycemic agents.
On Day 2, the toxicology results are returned and are positive for glipizide, which Ms. A had not been prescribed. Ms. A states that she had taken only her montelukast tablet on the day of admission and adamantly denies deliberately ingesting her parent’s diabetes medications. Her parents check the home medications and state there are no missing glipizide IR tablets or insulin vials. They also report that Ms. A had no access to extended-release glipizide.
The treatment team discuss Ms. A’s clinical condition and toxicology results with the pediatric endocrinology team. The endocrinology team states that with no history of hypoglycemic episodes, it is unlikely that Ms. A had an endogenous etiology that would present so catastrophically. In their experience, inexplicable hypoglycemia in a healthy individual who lives in a household with someone who has diabetes is due to ingestion of a hypoglycemic agent until proven otherwise.
[polldaddy:10252689]
Continue to: The authors' observations
The authors’ observations
In the context of Ms. A’s prior suicide attempt and history of self-harm, the pediatric team was concerned that her presentation was consistent with a suicide attempt and consulted the psychiatry service.
Glipizide is a second-generation sulfonylurea used to treat type 2 diabetes. It lowers blood glucose by stimulating pancreatic insulin secretion. It is a rare drug of overdose.1 Although pediatric glipizide overdoses have been documented, there are currently no pediatric or adolescent glipizide pharmacokinetic studies in the literature.1-4 In adults, the immediate-release formulation has 100% oral bioavailability, with a maximum plasma concentration (Tmax) of approximately 2 hours.5 The half-life typically ranges from 4 to 6 hours in adults.6 Patients who do not have diabetes are much more susceptible to the hypoglycemic effects of glipizide because the medication simulates their fully functional pancreas to produce a vigorous insulin response.
Ms. A’s significantly elevated insulin was consistent with normal glipizide effects in a healthy child, while the elevated C-peptide was consistent with insulin being endogenously produced, which ruled out ingestion of her parent’s insulin. Importantly, the pediatric endocrinology team noted that, in their experience, a single 5- to 10-mg dose of glipizide IR was sufficient to lower blood glucose levels to the low 30s mg/dL in the context of a functional pancreas, which suggested that Ms. A might have accidentally ingested a single glipizide IR tablet, and might be telling the truth when she denies deliberately ingesting it to hurt herself.
The clinical value of pharmacokinetics
The screen of Ms. A’s toxicology sample detected glipizide. The laboratory used a semi-quantitative serum screen of several hypoglycemic agents. A positive result for each agent is based on a quantitative cut-off value, which is 3 ng/mL for glipizide. The clinical chemist on call was asked to assist in interpreting the results. The serum specimen collected on Day 1 had a significantly positive glipizide result of 86 to 130 ng/mL. The maximum effective glipizide concentration for adult patients with diabetes is 100 ng/mL.7 Thus, the glipizide level of 86 to 130 ng/mL (20.5 hours after initial symptoms) is consistent with the clinical presentation of persistent hypoglycemia requiring ongoing glucose replacement therapy.
Due to the lack of pediatric pharmacokinetic data for glipizide and only a single serum measurement, it is not possible to estimate the glipizide concentration at the time of maximal symptoms (loss of consciousness at 2:30
Continue to: Clinicians need to be aware that...
Clinicians need to be aware that although hypoglycemia usually presents rapidly, in children glipizide IR can rarely cause delayed hypoglycemia up to 16 hours after ingestion,2 and a delay of 45 hours was reported in a case of ingestion of extended-release glipizide.8 Hypoglycemia can last up to nearly 24 hours and is exacerbated if the patient has not eaten.1,2 Importantly, Ms. A’s parents reported that she had no access to extended-release glipizide. When detailed pharmacokinetic data are not available, the information provided by the patient and parents becomes extremely important, especially in distinguishing between single and multiple overdoses prior to presentation, or co-ingestions, or decreased food intake that could exacerbate hypoglycemia.
EVALUATION Safety assessment
On Day 2, Ms. A and her parents are interviewed separately, and they all are consistent in their recollection that Ms. A had been feverish with flu-like symptoms throughout the night before coming to the ED, and had still seemed mildly confused on the morning of admission.
During the interview, her parents wonder when Ms. A took her daily dose of a single montelukast tablet for asthma, and whether she had accidentally confused it with their glipizide. They report that on the morning of admission, both the glipizide and montelukast medication vials were in the same room. The vials are the same color, the same size, and labeled from the same pharmacy, and contain white, scored, round tablets that look very similar.
During the interview, Ms. A consistently denies having thoughts of hurting or killing herself on the day of admission or before that.
[polldaddy:10252690]
Continue to: The authors' observations
The authors’ observations
This case was ultimately an accidental ingestion of glipizide, rather than a suicide attempt. The initial suspicion for a suicide attempt had been reasonable in the context of Ms. A’s depressive symptoms, remote history of a prior suicide attempt by ingesting an OTC medication, and toxicologic evidence of ingesting a drug not prescribed to her. Additionally, because of the life-threatening presentation, it was easy to make the erroneous assumption that the ingestion of glipizide must have involved many tablets, and thus must have been deliberate. However, through multidisciplinary teamwork, we were able to demonstrate that this was likely an accidental ingestion by a patient who had an acute febrile illness. Her illness had caused confusion, which contributed to the accidental ingestion, and also caused reduced food intake, which enhanced the hypoglycemic effects of glipizide. Additionally, a lack of awareness of medication safety in the home had facilitated the confusion between the two medication vials.
A single tablet of glipizide IR is sufficient to produce profound clinical effects that could be mistaken by medical and psychiatric teams for a much larger and/or deliberate overdose, especially in patients with a psychiatric history. The inappropriate psychiatric hospitalization of a patient, especially a child, who has been mistakenly diagnosed as having attempted suicide, can have negative therapeutic consequences (Table 2). A psychiatric admission would have been misguided if it attempted to address safety and reduce suicidality when no such concerns were present. Additionally, it could have damaged relationships with the patient and the family, especially in a child who had historically not sought psychiatric care despite depressive symptoms and a previous suicide attempt. When assessing for suicidality, consider accidental ingestion in the differential and use specialty expertise and confirmatory testing in the evaluation, taking the pharmacokinetics of the suspected agent into account.
OUTCOME Outpatient treatment
Ms. A’s neurologic symptoms resolve within 24 hours of admission. She is offered psychiatric inpatient hospitalization to address her depressive symptoms; however, her parents prefer that she receive outpatient care. Ms. A’s parents also state that after Ms. A’s admission, they locked up all household medications and will be more mindful with medication in the home. Because her parents are arranging appropriate outpatient treatment for Ms. A’s depression and maintenance of her safety, an involuntary hospitalization is not deemed necessary.
On Day 2, Ms. A is eating normally, her blood glucose levels remain stable, and she is discharged home.
Bottom Line
Oral hypoglycemic agents can cause life-threatening syndromes in healthy patients and can clinically mimic large, intentional overdoses. Clinicians must be aware of the differential of accidental ingestion when assessing for suicidality, and can use toxicology results in their assessment.
Related Resources
- Kidemergencies.com. Emergencies: One pill can kill. http://kidemergencies.com/onepill1.html.
- Safe Kids Worldwide. Medication safety. https://www.safekids.org/medicinesafety.
- American Association of Poison Control Centers. http://www.aapcc.org/.
Drug Brand Names
Glipizide • Glucotrol
Insulin glargine • Lantus
Montelukast • Singulair
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
1. Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131(1):141-146.
2. Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34(3):267-270.
3. Borowski H, Caraccio T, Mofenson H. Sulfonylurea ingestion in children: is an 8-hour observation period sufficient? J Pediatr. 1998;133(4):584-585.
4. Little GL, Boniface KS. Are one or two dangerous? Sulfony-lurea exposure in toddlers. J Emerg Med. 2005;28(3):305-310.
5. Huupponen R, Seppala P, Iisalo E. Glipizide pharma-cokinetics and response in diabetics. Int J Clin Pharmacol Ther Toxicol. 1982;20(9):417-422.
6. Baselt RC. Disposition of toxic drugs and chemicals in man. 10th ed. Seal Beach, California: Biomedical Publications; 2014.
7. Simonson DC, Kourides IA, Feinglos M, et al; the Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597-606.
8. Pelavin PI, Abramson E, Pon S, et al. Extended-release glipizide overdose presenting with delayed hypoglycemia and treated with subcutaneous octreotide. J Pediatr Endocrinol Metab. 2009;22(2):171-175.
Motherhood and the working psychiatrist
Raising a child is difficult. For working professional women, including doctors, that difficulty extends beyond bottles, bath time, and burping; it impacts day-to-day physiological function, time management, and emotional well-being.
The 1950s upheld a family model with traditional gender roles. By 1960, the family portrait of a breadwinner father and a stay-at-home mother with one or more children comprised 62% of American households.1 Precipitous changes occurred over the next decades as the housing market soared, education costs increased, and divorce rates rose. The 1980s ushered the arrival of women’s power suits and the notion of women “having it all.”1
Fast-forward to modern times. Medicine is changing, too. Women are slowly but surely starting to rise in this once male-led field. In 2017, for the first time more women than men enrolled in medical schools in the United States.2 In a 2015 report, the Association of American Medical Colleges found that 57% of residents who were pursuing psychiatry were women.3 And the median age of women applying to medical school who enrolled in 2017 or 2018 was 23 years.4
Choosing to parent as a physician poses challenges for women and men alike. As the rates of women in medicine and psychiatry are increasing, this article focuses on unique obstacles faced by mothers and aims to:
- explore the dueling duties of mothers who practice medicine
- consider the dilemma women face when returning to the workforce during the postpartum period
- discuss options for enhanced recognition and care of maternal and child well-being.
Duty: Being both parent and physician
The working psychiatrist mother has a duty to her patients and profession—not to mention a duty to her child. The demands are endless on both sides. No matter what stage of her professional career (medical school, residency, fellowship, or beyond) she chooses to begin motherhood, the responsibilities and expectations can be overwhelming. Doctor appointments, nausea and vomiting, fatigue, discomfort, and stress do not fit well within a schedule of intensive studying, working 24-hour shifts, navigating complex schedules, treating patients, and sorting out the financial heft of loan repayment, home ownership, contract negotiation, or relocation.5
Psychiatry carries a notable dichotomy of lecturing at length on the importance of maternal-infant attachment. John Bowlby argued that a child’s attachment to the mother is instinctual and primary, noting that early loss creates true mourning due to the primal ties of child to mother.6 Bowlby also asserted that personality development and psychopathology are rooted in the concept of attachment and the emotional security built through early childhood experiences.6
Continue to: Dr. Donald Winnnicott introduced the concept of...
Dr. Donald Winnicott introduced the concept of a “good-enough” mother in 1953.7 Today, although Winnicott’s teachings are explored in psychiatry training programs and practice, his concept does not resonate with many working mothers. Most physicians strive for perfection while struggling to balance their personal and professional lives.7
It’s no wonder that tales abound of female physicians being praised for their ability to take on grueling shifts up to their due date, forego lunch to pump breast milk, or cover shifts beyond child daycare closing times. This raises an interesting dilemma: Is the primary goal the efficiency of promoting commerce, patient numbers, and the workings of the health care system? Or is it the wellness of expecting mothers and the development and attachment of an infant to the parent? Is the goal to slowly and carefully craft our next generation of young humans? Or is there a way to “have it all”?
Dilemma: Misperceptions after returning to work
As they regain control of their bodies, sleep, and overall health, women who return to work during the postpartum period battle a myriad of misperceptions along with the logistical hurdles of breast-feeding. In a study of surgical residencies, 61% of program directors reported that female trainees’ work was negatively affected by becoming parents.8 But other evidence suggests there is a disparity between perception and reality.
As a result, misperceptions can negatively affect maternity leave or lactation time. Women often rightfully fear they may be viewed as taking leisure time or making convenient excuses to shirk responsibility, rather than focusing on the necessities for recovery, care, and bonding. Such pressures can lead to burnout and resentment. The struggle with breast-feeding is pervasive across all medical specialties. In a 2018 survey of 347 women who had children during surgical residency, 39% of respondents strongly considered leaving their training, 95.6% indicated that breast-feeding was important to them, and 58.1% stopped breast-feeding earlier than desired due to challenges faced in the workplace, such as poor access to lactation facilities and difficulty leaving the operating room to express milk.11
The American Academy of Pediatrics (AAP) recommends exclusive breast-feeding through 6 months of the postpartum period, and continued breast-feeding until the infant is at least 12 months old. Breast-feeding confers benefits to both the infant and mother, including positive impacts on the child’s cognitive development and health into adulthood, as well as higher productivity and lower absenteeism for breast-feeding mothers.12 By 2009, only 23 states had adopted laws to encourage breast-feeding in the workplace. In 2010, the United States government enacted the “reasonable break time” provision in Section 4207 of the Patient Protection and Affordable Care Act (ACA), which requires all employers to provide a period of time and private space other than a bathroom in which female employees can express milk for a child up to age 1.12
Continue to: In 2016...
In 2016, a follow-up national survey of employed women explored workplace changes after the ACA, and noted that only 40% of women had access to both break time and a private space for lactation.13 If the goal is to give working women a true choice of whether to continue breast-feeding after returning to work, these mothers need to be provided with the proper social and structural supports in order to allow for that personal decision.14
Discussion: Barriers to change
Breast-feeding, it has been argued, is the most enduring investment in women’s physical, cognitive, and social capacities, and provides protection for children against death, disease, and poverty.15 Research has shown that breast-feeding every child until age 1 would yield medical benefits, including fewer infections, increased intelligence in children, protection against breast cancer in mothers, and economic savings of $300 billion for the United States.15
We are no longer in the 1950s, but modern times still present challenges for mothers who are working as physicians. Although the AAP recommends that new parents receive 12 weeks leave from work, policies for faculty at the 12 top medical schools in the United States offer new mothers only approximately 2 months of paid leave.16 There also are problems of inconsistency among approaches to parenthood in graduate medical education (GME) training, different specialty clinical requirements, and different residency training programs. These factors all contribute to negative attitudes towards parenthood.17
We know the barriers for women.18 With more women entering the medical profession, we need to continue finding creative and workable solutions as these problems become more pressing.19 In a 2018 Time article, Lily Rothman wrote, “you can’t talk about breastfeeding in the United States without pointing out that every other wealthy country has found a way to accommodate breastfeeding mothers, and usually in the form of lengthy paid maternity leave.”20 However, maternity leave in the United States today dictates that mothers return to work while their children would still benefit from nursing.21
When it comes to GME and medical institutions, programs could look at barriers such as lack of accommodations for trainees who are pregnant or have young children. Addressing these barriers could include making private lactation rooms available and instituting flexible scheduling. It would be best if scheduling accommodations and policies were established by an institution’s administration, rather than leaving coverage up to the students or residents. Going further, institutions could consider offering flexible maternity leave and work schedules, allowing breaks for those who are breast-feeding, and creating lactation facilities.22 This could take the form of a breast-feeding support program that fits available budget resources.23
Continue to: Psychiatrists frequently discuss...
Psychiatrists frequently discuss Winnicott’s “good-enough mother” concept, with the mother transitioning from focusing on her baby’s needs to her own sense of personhood that is unable to respond to her baby’s every wish.6 This concept was established well before the shifting demographics of the nuclear family, the short maternity leaves and early returns to work, early separation of one’s infants to childcare settings, and experiences with pumped lactation milk that working mothers experience today. Is it any wonder childbearing female psychiatrists face a special kind of working-mother guilt?
1. Collins G. When everything changed: the amazing journey of American women from 1960 to the present. New York, NY: Little, Brown and Company; 2009;271, 301.
2. AAMCNews. More women than men enrolled in U.S. medical schools in 2017. Association of American Medical Colleges. https://news.aamc.org/press-releases/article/applicant-enrollment-2017/. Published December 18, 2017. Accessed November 21, 2018.
3. Vassar L. How medical specialties vary by gender. American Medical Association. https://wire.ama-assn.org/education/how-medical-specialties-vary-gender. Published February 18, 2015. Accessed November 21, 2018.
4. Association of American Medical Colleges. Table A-6: age of applicants to U.S. medical schools at anticipated matriculation by sex and race/ethnicity, 2014-2015 through 2017-2018. https://www.aamc.org/download/321468/data/factstablea6.pdf. Published November 30, 2017. Accessed February 7, 2019.
5. Jones V. Best time to have a baby as a physician? It depends. Doximity. https://opmed.doximity.com/articles/the-best-time-to-have-a-baby-as-a-physician-it-depends-c8064a92156c. Published September 11, 2017. Accessed November 21, 2018.
6. Mitchell SA, Black MJ. The British object relations school: W.R.D. Fairbairn and D.W. Winnicott. In: Freud and beyond: a history of modern psychoanalytic thought. New York, NY: Basic Books; 1995:125-126, 137.
7. Ratnapalan S, Batty H. To be good enough. Can Fam Physician. 2009;55(3):239-242.
8. Sandler BJ, Tackett JJ, Longo WE, et al. Pregnancy and parenthood among surgery residents: results of the first nationwide survey of general surgery residency program Directors. J Am Coll Surg. 2016;222(6):1090-1096.
9. Kmec JA. Are motherhood penalties and fatherhood bonuses warranted? Comparing pro-work behaviors and conditions of mothers, fathers, and non-parents. Social Science Research. 2011;40(2):444-459.
10. Hampton R. Working moms don’t deserve the blame for unfair work expectations. Slate. https://slate.com/human-interest/2018/05/working-moms-dont-deserve-blame-for-unfair-work-expectations.html. Published May 18, 2018. Accessed November 25, 2018.
11. Rangel EL, Smink DS, Castillo-Angeles M, et al. Pregnancy and motherhood during surgical training. JAMA Surgery. 2018;153(7):644-652.
12. Murtagh L, Moulton AD. Working mothers, breastfeeding, and the law. Am J Public Health. 2011;101(2):217-223.
13. Kozhimannil KB, Jou J, Gjerdingen DK, et al. Access to workplace accommodations to support breastfeeding after passage of the Affordable Care Act. Womens Health Issues. 2016;26(1):6-13.
14. Dinour LM, Bai YK. Breastfeeding: the illusion of choice. Womens Health Issues. 2016;26(5):479-482.
15. Hansen K. Breastfeeding: a smart investment in people and in economies. Lancet. 2016;387(10017):416.
16. Greenfield R. Even America’s top doctors aren’t getting the parental leave doctors recommend. Bloomberg. https://www.bloomberg.com/news/articles/2018-02-13/even-america-s-top-doctors-aren-t-getting-the-parental-leave-doctors-recommend. Published February 13, 2018. Accessed November 21, 2018.
17. Humphries LS, Lyon S, Garza R, et al. Parental leave policies in graduate medical education: a systematic review. American J Surg. 2017;214(4):634-639.
18. Raju TNK. Continued barriers for breast-feeding in public and the workplace. J Pediatr. 2006;148(5):677-679.
19. Stewart DE, Robinson GE. Combining motherhood with psychiatric training and practice. Can J Psychiatry. 1985;30(1):28-34.
20. Rothman L. D esperate women, desperate doctors and the surprising history behind the breastfeeding debate. Time. http://time.com/5353068/breastfeeding-debate-history/. Published July 31, 2018. Accessed November 21, 2018.
21. Livingston G. Among 41 nations, U.S. is the outlier when it comes to paid parental leave. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/09/26/u-s-lacks-mandated-paid-parental-leave/. Published September 26, 2016. Accessed November 21, 2018.
22. McCluskey PD. Long hours, short leaves force moms to reconsider jobs as surgeons. Boston Globe. https://www.bostonglobe.com/metro/2018/03/21/new-survey-says-female-surgical-residents-struggle-balance-training-motherhood/2ENQU1aPZmIJYy20iaRlLL/story.html. Published March 21, 2018. Accessed November 21, 2018.
23. Dinour LM, Szaro JM. Employer-based programs to support breastfeeding among working mothers: a systematic review. Breastfeeding Med. 2017;12:131-141.
Raising a child is difficult. For working professional women, including doctors, that difficulty extends beyond bottles, bath time, and burping; it impacts day-to-day physiological function, time management, and emotional well-being.
The 1950s upheld a family model with traditional gender roles. By 1960, the family portrait of a breadwinner father and a stay-at-home mother with one or more children comprised 62% of American households.1 Precipitous changes occurred over the next decades as the housing market soared, education costs increased, and divorce rates rose. The 1980s ushered the arrival of women’s power suits and the notion of women “having it all.”1
Fast-forward to modern times. Medicine is changing, too. Women are slowly but surely starting to rise in this once male-led field. In 2017, for the first time more women than men enrolled in medical schools in the United States.2 In a 2015 report, the Association of American Medical Colleges found that 57% of residents who were pursuing psychiatry were women.3 And the median age of women applying to medical school who enrolled in 2017 or 2018 was 23 years.4
Choosing to parent as a physician poses challenges for women and men alike. As the rates of women in medicine and psychiatry are increasing, this article focuses on unique obstacles faced by mothers and aims to:
- explore the dueling duties of mothers who practice medicine
- consider the dilemma women face when returning to the workforce during the postpartum period
- discuss options for enhanced recognition and care of maternal and child well-being.
Duty: Being both parent and physician
The working psychiatrist mother has a duty to her patients and profession—not to mention a duty to her child. The demands are endless on both sides. No matter what stage of her professional career (medical school, residency, fellowship, or beyond) she chooses to begin motherhood, the responsibilities and expectations can be overwhelming. Doctor appointments, nausea and vomiting, fatigue, discomfort, and stress do not fit well within a schedule of intensive studying, working 24-hour shifts, navigating complex schedules, treating patients, and sorting out the financial heft of loan repayment, home ownership, contract negotiation, or relocation.5
Psychiatry carries a notable dichotomy of lecturing at length on the importance of maternal-infant attachment. John Bowlby argued that a child’s attachment to the mother is instinctual and primary, noting that early loss creates true mourning due to the primal ties of child to mother.6 Bowlby also asserted that personality development and psychopathology are rooted in the concept of attachment and the emotional security built through early childhood experiences.6
Continue to: Dr. Donald Winnnicott introduced the concept of...
Dr. Donald Winnicott introduced the concept of a “good-enough” mother in 1953.7 Today, although Winnicott’s teachings are explored in psychiatry training programs and practice, his concept does not resonate with many working mothers. Most physicians strive for perfection while struggling to balance their personal and professional lives.7
It’s no wonder that tales abound of female physicians being praised for their ability to take on grueling shifts up to their due date, forego lunch to pump breast milk, or cover shifts beyond child daycare closing times. This raises an interesting dilemma: Is the primary goal the efficiency of promoting commerce, patient numbers, and the workings of the health care system? Or is it the wellness of expecting mothers and the development and attachment of an infant to the parent? Is the goal to slowly and carefully craft our next generation of young humans? Or is there a way to “have it all”?
Dilemma: Misperceptions after returning to work
As they regain control of their bodies, sleep, and overall health, women who return to work during the postpartum period battle a myriad of misperceptions along with the logistical hurdles of breast-feeding. In a study of surgical residencies, 61% of program directors reported that female trainees’ work was negatively affected by becoming parents.8 But other evidence suggests there is a disparity between perception and reality.
As a result, misperceptions can negatively affect maternity leave or lactation time. Women often rightfully fear they may be viewed as taking leisure time or making convenient excuses to shirk responsibility, rather than focusing on the necessities for recovery, care, and bonding. Such pressures can lead to burnout and resentment. The struggle with breast-feeding is pervasive across all medical specialties. In a 2018 survey of 347 women who had children during surgical residency, 39% of respondents strongly considered leaving their training, 95.6% indicated that breast-feeding was important to them, and 58.1% stopped breast-feeding earlier than desired due to challenges faced in the workplace, such as poor access to lactation facilities and difficulty leaving the operating room to express milk.11
The American Academy of Pediatrics (AAP) recommends exclusive breast-feeding through 6 months of the postpartum period, and continued breast-feeding until the infant is at least 12 months old. Breast-feeding confers benefits to both the infant and mother, including positive impacts on the child’s cognitive development and health into adulthood, as well as higher productivity and lower absenteeism for breast-feeding mothers.12 By 2009, only 23 states had adopted laws to encourage breast-feeding in the workplace. In 2010, the United States government enacted the “reasonable break time” provision in Section 4207 of the Patient Protection and Affordable Care Act (ACA), which requires all employers to provide a period of time and private space other than a bathroom in which female employees can express milk for a child up to age 1.12
Continue to: In 2016...
In 2016, a follow-up national survey of employed women explored workplace changes after the ACA, and noted that only 40% of women had access to both break time and a private space for lactation.13 If the goal is to give working women a true choice of whether to continue breast-feeding after returning to work, these mothers need to be provided with the proper social and structural supports in order to allow for that personal decision.14
Discussion: Barriers to change
Breast-feeding, it has been argued, is the most enduring investment in women’s physical, cognitive, and social capacities, and provides protection for children against death, disease, and poverty.15 Research has shown that breast-feeding every child until age 1 would yield medical benefits, including fewer infections, increased intelligence in children, protection against breast cancer in mothers, and economic savings of $300 billion for the United States.15
We are no longer in the 1950s, but modern times still present challenges for mothers who are working as physicians. Although the AAP recommends that new parents receive 12 weeks leave from work, policies for faculty at the 12 top medical schools in the United States offer new mothers only approximately 2 months of paid leave.16 There also are problems of inconsistency among approaches to parenthood in graduate medical education (GME) training, different specialty clinical requirements, and different residency training programs. These factors all contribute to negative attitudes towards parenthood.17
We know the barriers for women.18 With more women entering the medical profession, we need to continue finding creative and workable solutions as these problems become more pressing.19 In a 2018 Time article, Lily Rothman wrote, “you can’t talk about breastfeeding in the United States without pointing out that every other wealthy country has found a way to accommodate breastfeeding mothers, and usually in the form of lengthy paid maternity leave.”20 However, maternity leave in the United States today dictates that mothers return to work while their children would still benefit from nursing.21
When it comes to GME and medical institutions, programs could look at barriers such as lack of accommodations for trainees who are pregnant or have young children. Addressing these barriers could include making private lactation rooms available and instituting flexible scheduling. It would be best if scheduling accommodations and policies were established by an institution’s administration, rather than leaving coverage up to the students or residents. Going further, institutions could consider offering flexible maternity leave and work schedules, allowing breaks for those who are breast-feeding, and creating lactation facilities.22 This could take the form of a breast-feeding support program that fits available budget resources.23
Continue to: Psychiatrists frequently discuss...
Psychiatrists frequently discuss Winnicott’s “good-enough mother” concept, with the mother transitioning from focusing on her baby’s needs to her own sense of personhood that is unable to respond to her baby’s every wish.6 This concept was established well before the shifting demographics of the nuclear family, the short maternity leaves and early returns to work, early separation of one’s infants to childcare settings, and experiences with pumped lactation milk that working mothers experience today. Is it any wonder childbearing female psychiatrists face a special kind of working-mother guilt?
Raising a child is difficult. For working professional women, including doctors, that difficulty extends beyond bottles, bath time, and burping; it impacts day-to-day physiological function, time management, and emotional well-being.
The 1950s upheld a family model with traditional gender roles. By 1960, the family portrait of a breadwinner father and a stay-at-home mother with one or more children comprised 62% of American households.1 Precipitous changes occurred over the next decades as the housing market soared, education costs increased, and divorce rates rose. The 1980s ushered the arrival of women’s power suits and the notion of women “having it all.”1
Fast-forward to modern times. Medicine is changing, too. Women are slowly but surely starting to rise in this once male-led field. In 2017, for the first time more women than men enrolled in medical schools in the United States.2 In a 2015 report, the Association of American Medical Colleges found that 57% of residents who were pursuing psychiatry were women.3 And the median age of women applying to medical school who enrolled in 2017 or 2018 was 23 years.4
Choosing to parent as a physician poses challenges for women and men alike. As the rates of women in medicine and psychiatry are increasing, this article focuses on unique obstacles faced by mothers and aims to:
- explore the dueling duties of mothers who practice medicine
- consider the dilemma women face when returning to the workforce during the postpartum period
- discuss options for enhanced recognition and care of maternal and child well-being.
Duty: Being both parent and physician
The working psychiatrist mother has a duty to her patients and profession—not to mention a duty to her child. The demands are endless on both sides. No matter what stage of her professional career (medical school, residency, fellowship, or beyond) she chooses to begin motherhood, the responsibilities and expectations can be overwhelming. Doctor appointments, nausea and vomiting, fatigue, discomfort, and stress do not fit well within a schedule of intensive studying, working 24-hour shifts, navigating complex schedules, treating patients, and sorting out the financial heft of loan repayment, home ownership, contract negotiation, or relocation.5
Psychiatry carries a notable dichotomy of lecturing at length on the importance of maternal-infant attachment. John Bowlby argued that a child’s attachment to the mother is instinctual and primary, noting that early loss creates true mourning due to the primal ties of child to mother.6 Bowlby also asserted that personality development and psychopathology are rooted in the concept of attachment and the emotional security built through early childhood experiences.6
Continue to: Dr. Donald Winnnicott introduced the concept of...
Dr. Donald Winnicott introduced the concept of a “good-enough” mother in 1953.7 Today, although Winnicott’s teachings are explored in psychiatry training programs and practice, his concept does not resonate with many working mothers. Most physicians strive for perfection while struggling to balance their personal and professional lives.7
It’s no wonder that tales abound of female physicians being praised for their ability to take on grueling shifts up to their due date, forego lunch to pump breast milk, or cover shifts beyond child daycare closing times. This raises an interesting dilemma: Is the primary goal the efficiency of promoting commerce, patient numbers, and the workings of the health care system? Or is it the wellness of expecting mothers and the development and attachment of an infant to the parent? Is the goal to slowly and carefully craft our next generation of young humans? Or is there a way to “have it all”?
Dilemma: Misperceptions after returning to work
As they regain control of their bodies, sleep, and overall health, women who return to work during the postpartum period battle a myriad of misperceptions along with the logistical hurdles of breast-feeding. In a study of surgical residencies, 61% of program directors reported that female trainees’ work was negatively affected by becoming parents.8 But other evidence suggests there is a disparity between perception and reality.
As a result, misperceptions can negatively affect maternity leave or lactation time. Women often rightfully fear they may be viewed as taking leisure time or making convenient excuses to shirk responsibility, rather than focusing on the necessities for recovery, care, and bonding. Such pressures can lead to burnout and resentment. The struggle with breast-feeding is pervasive across all medical specialties. In a 2018 survey of 347 women who had children during surgical residency, 39% of respondents strongly considered leaving their training, 95.6% indicated that breast-feeding was important to them, and 58.1% stopped breast-feeding earlier than desired due to challenges faced in the workplace, such as poor access to lactation facilities and difficulty leaving the operating room to express milk.11
The American Academy of Pediatrics (AAP) recommends exclusive breast-feeding through 6 months of the postpartum period, and continued breast-feeding until the infant is at least 12 months old. Breast-feeding confers benefits to both the infant and mother, including positive impacts on the child’s cognitive development and health into adulthood, as well as higher productivity and lower absenteeism for breast-feeding mothers.12 By 2009, only 23 states had adopted laws to encourage breast-feeding in the workplace. In 2010, the United States government enacted the “reasonable break time” provision in Section 4207 of the Patient Protection and Affordable Care Act (ACA), which requires all employers to provide a period of time and private space other than a bathroom in which female employees can express milk for a child up to age 1.12
Continue to: In 2016...
In 2016, a follow-up national survey of employed women explored workplace changes after the ACA, and noted that only 40% of women had access to both break time and a private space for lactation.13 If the goal is to give working women a true choice of whether to continue breast-feeding after returning to work, these mothers need to be provided with the proper social and structural supports in order to allow for that personal decision.14
Discussion: Barriers to change
Breast-feeding, it has been argued, is the most enduring investment in women’s physical, cognitive, and social capacities, and provides protection for children against death, disease, and poverty.15 Research has shown that breast-feeding every child until age 1 would yield medical benefits, including fewer infections, increased intelligence in children, protection against breast cancer in mothers, and economic savings of $300 billion for the United States.15
We are no longer in the 1950s, but modern times still present challenges for mothers who are working as physicians. Although the AAP recommends that new parents receive 12 weeks leave from work, policies for faculty at the 12 top medical schools in the United States offer new mothers only approximately 2 months of paid leave.16 There also are problems of inconsistency among approaches to parenthood in graduate medical education (GME) training, different specialty clinical requirements, and different residency training programs. These factors all contribute to negative attitudes towards parenthood.17
We know the barriers for women.18 With more women entering the medical profession, we need to continue finding creative and workable solutions as these problems become more pressing.19 In a 2018 Time article, Lily Rothman wrote, “you can’t talk about breastfeeding in the United States without pointing out that every other wealthy country has found a way to accommodate breastfeeding mothers, and usually in the form of lengthy paid maternity leave.”20 However, maternity leave in the United States today dictates that mothers return to work while their children would still benefit from nursing.21
When it comes to GME and medical institutions, programs could look at barriers such as lack of accommodations for trainees who are pregnant or have young children. Addressing these barriers could include making private lactation rooms available and instituting flexible scheduling. It would be best if scheduling accommodations and policies were established by an institution’s administration, rather than leaving coverage up to the students or residents. Going further, institutions could consider offering flexible maternity leave and work schedules, allowing breaks for those who are breast-feeding, and creating lactation facilities.22 This could take the form of a breast-feeding support program that fits available budget resources.23
Continue to: Psychiatrists frequently discuss...
Psychiatrists frequently discuss Winnicott’s “good-enough mother” concept, with the mother transitioning from focusing on her baby’s needs to her own sense of personhood that is unable to respond to her baby’s every wish.6 This concept was established well before the shifting demographics of the nuclear family, the short maternity leaves and early returns to work, early separation of one’s infants to childcare settings, and experiences with pumped lactation milk that working mothers experience today. Is it any wonder childbearing female psychiatrists face a special kind of working-mother guilt?
1. Collins G. When everything changed: the amazing journey of American women from 1960 to the present. New York, NY: Little, Brown and Company; 2009;271, 301.
2. AAMCNews. More women than men enrolled in U.S. medical schools in 2017. Association of American Medical Colleges. https://news.aamc.org/press-releases/article/applicant-enrollment-2017/. Published December 18, 2017. Accessed November 21, 2018.
3. Vassar L. How medical specialties vary by gender. American Medical Association. https://wire.ama-assn.org/education/how-medical-specialties-vary-gender. Published February 18, 2015. Accessed November 21, 2018.
4. Association of American Medical Colleges. Table A-6: age of applicants to U.S. medical schools at anticipated matriculation by sex and race/ethnicity, 2014-2015 through 2017-2018. https://www.aamc.org/download/321468/data/factstablea6.pdf. Published November 30, 2017. Accessed February 7, 2019.
5. Jones V. Best time to have a baby as a physician? It depends. Doximity. https://opmed.doximity.com/articles/the-best-time-to-have-a-baby-as-a-physician-it-depends-c8064a92156c. Published September 11, 2017. Accessed November 21, 2018.
6. Mitchell SA, Black MJ. The British object relations school: W.R.D. Fairbairn and D.W. Winnicott. In: Freud and beyond: a history of modern psychoanalytic thought. New York, NY: Basic Books; 1995:125-126, 137.
7. Ratnapalan S, Batty H. To be good enough. Can Fam Physician. 2009;55(3):239-242.
8. Sandler BJ, Tackett JJ, Longo WE, et al. Pregnancy and parenthood among surgery residents: results of the first nationwide survey of general surgery residency program Directors. J Am Coll Surg. 2016;222(6):1090-1096.
9. Kmec JA. Are motherhood penalties and fatherhood bonuses warranted? Comparing pro-work behaviors and conditions of mothers, fathers, and non-parents. Social Science Research. 2011;40(2):444-459.
10. Hampton R. Working moms don’t deserve the blame for unfair work expectations. Slate. https://slate.com/human-interest/2018/05/working-moms-dont-deserve-blame-for-unfair-work-expectations.html. Published May 18, 2018. Accessed November 25, 2018.
11. Rangel EL, Smink DS, Castillo-Angeles M, et al. Pregnancy and motherhood during surgical training. JAMA Surgery. 2018;153(7):644-652.
12. Murtagh L, Moulton AD. Working mothers, breastfeeding, and the law. Am J Public Health. 2011;101(2):217-223.
13. Kozhimannil KB, Jou J, Gjerdingen DK, et al. Access to workplace accommodations to support breastfeeding after passage of the Affordable Care Act. Womens Health Issues. 2016;26(1):6-13.
14. Dinour LM, Bai YK. Breastfeeding: the illusion of choice. Womens Health Issues. 2016;26(5):479-482.
15. Hansen K. Breastfeeding: a smart investment in people and in economies. Lancet. 2016;387(10017):416.
16. Greenfield R. Even America’s top doctors aren’t getting the parental leave doctors recommend. Bloomberg. https://www.bloomberg.com/news/articles/2018-02-13/even-america-s-top-doctors-aren-t-getting-the-parental-leave-doctors-recommend. Published February 13, 2018. Accessed November 21, 2018.
17. Humphries LS, Lyon S, Garza R, et al. Parental leave policies in graduate medical education: a systematic review. American J Surg. 2017;214(4):634-639.
18. Raju TNK. Continued barriers for breast-feeding in public and the workplace. J Pediatr. 2006;148(5):677-679.
19. Stewart DE, Robinson GE. Combining motherhood with psychiatric training and practice. Can J Psychiatry. 1985;30(1):28-34.
20. Rothman L. D esperate women, desperate doctors and the surprising history behind the breastfeeding debate. Time. http://time.com/5353068/breastfeeding-debate-history/. Published July 31, 2018. Accessed November 21, 2018.
21. Livingston G. Among 41 nations, U.S. is the outlier when it comes to paid parental leave. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/09/26/u-s-lacks-mandated-paid-parental-leave/. Published September 26, 2016. Accessed November 21, 2018.
22. McCluskey PD. Long hours, short leaves force moms to reconsider jobs as surgeons. Boston Globe. https://www.bostonglobe.com/metro/2018/03/21/new-survey-says-female-surgical-residents-struggle-balance-training-motherhood/2ENQU1aPZmIJYy20iaRlLL/story.html. Published March 21, 2018. Accessed November 21, 2018.
23. Dinour LM, Szaro JM. Employer-based programs to support breastfeeding among working mothers: a systematic review. Breastfeeding Med. 2017;12:131-141.
1. Collins G. When everything changed: the amazing journey of American women from 1960 to the present. New York, NY: Little, Brown and Company; 2009;271, 301.
2. AAMCNews. More women than men enrolled in U.S. medical schools in 2017. Association of American Medical Colleges. https://news.aamc.org/press-releases/article/applicant-enrollment-2017/. Published December 18, 2017. Accessed November 21, 2018.
3. Vassar L. How medical specialties vary by gender. American Medical Association. https://wire.ama-assn.org/education/how-medical-specialties-vary-gender. Published February 18, 2015. Accessed November 21, 2018.
4. Association of American Medical Colleges. Table A-6: age of applicants to U.S. medical schools at anticipated matriculation by sex and race/ethnicity, 2014-2015 through 2017-2018. https://www.aamc.org/download/321468/data/factstablea6.pdf. Published November 30, 2017. Accessed February 7, 2019.
5. Jones V. Best time to have a baby as a physician? It depends. Doximity. https://opmed.doximity.com/articles/the-best-time-to-have-a-baby-as-a-physician-it-depends-c8064a92156c. Published September 11, 2017. Accessed November 21, 2018.
6. Mitchell SA, Black MJ. The British object relations school: W.R.D. Fairbairn and D.W. Winnicott. In: Freud and beyond: a history of modern psychoanalytic thought. New York, NY: Basic Books; 1995:125-126, 137.
7. Ratnapalan S, Batty H. To be good enough. Can Fam Physician. 2009;55(3):239-242.
8. Sandler BJ, Tackett JJ, Longo WE, et al. Pregnancy and parenthood among surgery residents: results of the first nationwide survey of general surgery residency program Directors. J Am Coll Surg. 2016;222(6):1090-1096.
9. Kmec JA. Are motherhood penalties and fatherhood bonuses warranted? Comparing pro-work behaviors and conditions of mothers, fathers, and non-parents. Social Science Research. 2011;40(2):444-459.
10. Hampton R. Working moms don’t deserve the blame for unfair work expectations. Slate. https://slate.com/human-interest/2018/05/working-moms-dont-deserve-blame-for-unfair-work-expectations.html. Published May 18, 2018. Accessed November 25, 2018.
11. Rangel EL, Smink DS, Castillo-Angeles M, et al. Pregnancy and motherhood during surgical training. JAMA Surgery. 2018;153(7):644-652.
12. Murtagh L, Moulton AD. Working mothers, breastfeeding, and the law. Am J Public Health. 2011;101(2):217-223.
13. Kozhimannil KB, Jou J, Gjerdingen DK, et al. Access to workplace accommodations to support breastfeeding after passage of the Affordable Care Act. Womens Health Issues. 2016;26(1):6-13.
14. Dinour LM, Bai YK. Breastfeeding: the illusion of choice. Womens Health Issues. 2016;26(5):479-482.
15. Hansen K. Breastfeeding: a smart investment in people and in economies. Lancet. 2016;387(10017):416.
16. Greenfield R. Even America’s top doctors aren’t getting the parental leave doctors recommend. Bloomberg. https://www.bloomberg.com/news/articles/2018-02-13/even-america-s-top-doctors-aren-t-getting-the-parental-leave-doctors-recommend. Published February 13, 2018. Accessed November 21, 2018.
17. Humphries LS, Lyon S, Garza R, et al. Parental leave policies in graduate medical education: a systematic review. American J Surg. 2017;214(4):634-639.
18. Raju TNK. Continued barriers for breast-feeding in public and the workplace. J Pediatr. 2006;148(5):677-679.
19. Stewart DE, Robinson GE. Combining motherhood with psychiatric training and practice. Can J Psychiatry. 1985;30(1):28-34.
20. Rothman L. D esperate women, desperate doctors and the surprising history behind the breastfeeding debate. Time. http://time.com/5353068/breastfeeding-debate-history/. Published July 31, 2018. Accessed November 21, 2018.
21. Livingston G. Among 41 nations, U.S. is the outlier when it comes to paid parental leave. Pew Research Center. http://www.pewresearch.org/fact-tank/2016/09/26/u-s-lacks-mandated-paid-parental-leave/. Published September 26, 2016. Accessed November 21, 2018.
22. McCluskey PD. Long hours, short leaves force moms to reconsider jobs as surgeons. Boston Globe. https://www.bostonglobe.com/metro/2018/03/21/new-survey-says-female-surgical-residents-struggle-balance-training-motherhood/2ENQU1aPZmIJYy20iaRlLL/story.html. Published March 21, 2018. Accessed November 21, 2018.
23. Dinour LM, Szaro JM. Employer-based programs to support breastfeeding among working mothers: a systematic review. Breastfeeding Med. 2017;12:131-141.
Management of treatment-resistant depression: A review of 3 studies
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.
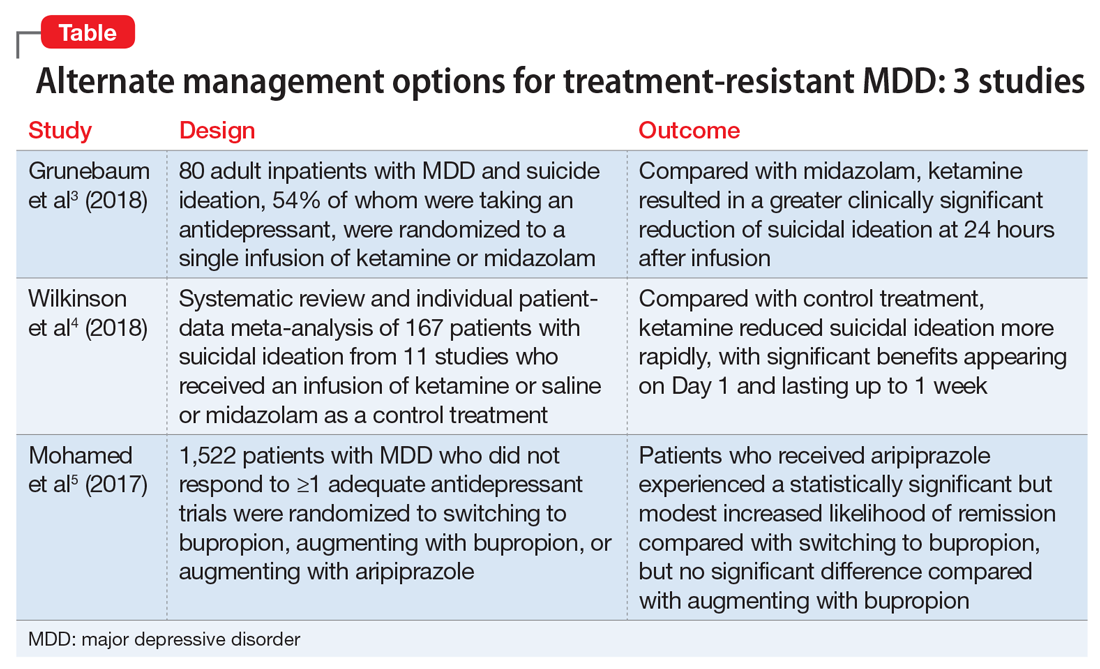
The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.

The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
An estimated 7.1% of the adults in United States had a major depressive episode in 2017, and this prevalence has been trending upward over the past few years.1 The prevalence is even higher in adults between age 18 and 25 (13.1%).1 Like other psychiatric diagnoses, major depressive disorder (MDD) has a significant impact on productivity as well as daily functioning. Only one-third of patients with MDD achieve remission on the first antidepressant medication.2 This leaves an estimated 11.47 million people in the United States in need of an alternate regimen for management of their depressive episode.
The data on evidence-based biologic treatments for treatment-resistant depression are limited (other than for electroconvulsive therapy). Pharmacologic options include switching to a different medication, combining medications, and augmentation strategies or novel approaches such as ketamine and related agents. Here we summarize the findings from 3 recent studies that investigate alternate management options for MDD.
Ketamine: Randomized controlled trial
Traditional antidepressants may reduce suicidal ideation by improving depressive symptoms, but this effect may take weeks. Ketamine, an N-methyl-
_
1. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
Grunebaum et al3 evaluated the acute effect of adjunctive subanesthetic IV ketamine on clinically significant suicidal ideation in patients with MDD, with a comparison arm that received an infusion of midazolam.
Study design
- 80 inpatients (age 18 to 65 years) with MDD who had a score ≥16 on the Hamilton Depression Rating Scale (HAM-D) and a score ≥4 on the Scale for Suicidal Ideation (SSI). Approximately one-half (54%) were taking an antidepressant
- Patients were randomly assigned to IV racemic ketamine hydrochloride, .5 mg/kg, or IV midazolam, .02 mg/kg, both administered in 100 mL normal saline over 40 minutes.
Outcomes
- Scale for Suicidal Ideation scores were assessed at screening, before infusion, 230 minutes after infusion, 24 hours after infusion, and after 1 to 6 weeks of follow-up. The average SSI score on Day 1 was 4.96 points lower in the ketamine group compared with the midazolam group. The proportion of responders (defined as patients who experienced a 50% reduction in SSI score) on Day 1 was 55% for patients in the ketamine group compared with 30% in the midazolam group.
Conclusion
- Compared with midazolam, ketamine produced a greater clinically meaningful reduction in suicidal ideation 24 hours after infusion.
Apart from the primary outcome of reduction in suicidal ideation, greater reductions were also found in overall mood disturbance, depression subscale, and fatigue subscale scores as assessed on the Profile of Mood States (POMS). Although the study noted improvement in depression scores, the proportion of responders on Day 1 in depression scales, including HAM-D and the self-rated Beck Depression Inventory, fell short of statistical significance. Overall, compared with the midazolam infusion, a single adjunctive subanesthetic ketamine infusion was associated with a greater clinically significant reduction in suicidal ideation on Day 1.
Continue to: Ketamine
Ketamine: Review and meta-analysis
Wilkinson et al4 conducted a systematic review and individual participant data meta-analysis of 11 similar comparison intervention studies examining the effects of ketamine in reducing suicidal thoughts.
2. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
Study design
- Review of 11 studies of a single dose of IV ketamine for treatment of any psychiatric disorder. Only comparison intervention trials using saline placebo or midazolam were included:
- Individual patient-level data of 298 patients were obtained from 10 of the 11 trials. Analysis was performed on 167 patients who had suicidal ideation at baseline.
- Results were assessed by clinician-administered rating scales.
Outcomes
- Ketamine reduced suicidal ideation more rapidly compared with control infusions as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) and HAM-D, with significant benefits appearing on Day 1 and extending up to Day 7. The mean MADRS score in the ketamine group decreased to 19.5 from 33.8 within 1 day of infusion, compared with a reduction to 29.2 from 32.9 in the control groups.
- The number needed to treat to be free of suicidal ideation for ketamine (compared with control) was 3.1 to 4.0 for all time points in the first week after infusion.
Conclusion
- This meta-analysis provided evidence from the largest sample to date (N = 298) that ketamine reduces suicidal ideation partially independently of mood symptoms.
While the anti-suicidal effects of ketamine appear to be robust in the above studies, the possibility of rebound suicidal ideation remains in the weeks or months following exposure. Also, these studies only prove a reduction in suicidal ideation; reduction in suicidal behavior was not studied. Nevertheless, ketamine holds considerable promise as a potential rapid-acting agent in patients at risk of suicide.
Continue to: Strategies for augmentation or switching
Strategies for augmentation or switching
Only one-third of the patients with depression achieve remission on the first antidepressant medication. The American Psychiatric Association’s current management guidelines2 for patients who do not respond to the first-choice antidepressant include multiple options. Switching strategies recommended in these guidelines include changing to an antidepressant of the same class, or to one from a different class (eg, from a selective serotonin reuptake inhibitor [SSRI] to a serotonin-norepinephrine reuptake inhibitor, or from an SSRI to a tricyclic antidepressant). Augmentation strategies include augmenting with a non-monoamine oxidase inhibitor antidepressant from a different class, lithium, thyroid hormone, or an atypical antipsychotic.
The VAST-D trial5 evaluated the relative effectiveness and safety of 3 common treatments for treatment-resistant MDD:
- switching to bupropion
- augmenting the current treatment with bupropion
- augmenting the current treatment with the second-generation antipsychotic aripiprazole.
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Study design
- A multi-site, randomized, single-blind, parallel-assignment trial of 1,522 patients at 35 US Veteran Health Administration medical centers with nonpsychotic MDD with a suboptimal response to at least one antidepressant (defined as a score of ≥16 on the Quick Inventory Depressive Symptomatology-Clinician Rated questionnaire [QIDS-C16]).
- Participants were randomly assigned to 1 of 3 groups: switching to bupropion (n = 511), augmenting with bupropion (n = 506), or augmenting with aripiprazole (n = 505).
- The primary outcome was remission (defined as a QIDS-C16 score ≤5 at 2 consecutively scheduled follow-up visits). Secondary outcome was a reduction in QIDS-C16 score by ≥50%, or a Clinical Global Impression (CGI) Improvement scale score of 1 (very much improved) or 2 (much improved).
Outcomes
- The aripiprazole group showed a modest, statistically significant remission rate (28.9%) compared with the bupropion switch group (22.3%), but did not show any statistically significant difference compared with the bupropion augmentation group.
- For the secondary outcome, there was a significantly higher response rate in the aripiprazole group (74.3%) compared with the bupropion switch group (62.4%) and bupropion augmentation group (65.6%). Response measured by the CGI– Improvement scale score also favored the aripiprazole group (79%) compared with the bupropion switch group (70%) and bupropion augmentation group (74%).
Continue to: Conclusion
Conclusion
- Overall, the study found a statistically significant but modest increased likelihood of remission during 12 weeks of augmentation treatment with aripiprazole, compared with switching to bupropion monotherapy.

The studies discussed here, which are summarized in the Table,3-5 provide some potential avenues for research into interventions for patients who are acutely suicidal and those with treatment-resistant depression. Further research into long-term outcomes and adverse effects of ketamine use for suicidality in patients with depression is needed. The VAST-D trial suggests a need for further exploration into the efficacy of augmentation with second-generation antipsychotics for treatment-resistant depression.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
1. Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2017 National Survey on Drug Use and Health (NSDUH). https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed November 12, 2018.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published 2010. Accessed November 12, 2018.
3. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175(4):327-335.
4. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
5. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
Career Choices: Addiction psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, talked with Cornel Stanciu, MD. Dr. Stanciu is an addiction psychiatrist at Dartmouth’s Geisel School of Medicine, where he is an Assistant Professor, and serves as the Director of Addiction Services at New Hampshire Hospital. He provides support to clinicians managing patients with addictive disorders in a multitude of settings, and also assists with policy making and delivery of addiction care at the state level. He is also the author of Deciphering the Addicted Brain, a guide to help families and the general public better understand addictive disorders.
Dr. Ahmed: What attracted you to pursue subspecialty training in addictive disorders?
Dr. Stanciu: In the early stages of my training, I frequently encountered individuals with medical and mental health disorders whose treatment was impacted by underlying substance use. I soon came to realize any attempts at (for example) managing hypertension in someone with cocaine use disorder, or managing schizophrenia in someone with ongoing cannabis use, were futile. Almost all of my patients receiving treatment for mental health disorders were dependent on tobacco or other substances, and most were interested in cessation. Through mentorship from addiction-trained residency faculty members, I was able to get a taste of the neurobiologic complexities of the disease, something that left me with a desire to develop a deeper understanding of the disease process. Witnessing strikingly positive outcomes with implementation of evidence-based treatment modalities further solidified my path to subspecialty training. Even during that early phase, because I expressed interest in managing these conditions, I was immediately put in a position to share and disseminate any newly acquired knowledge to other specialties as well as the public.
Dr. Ahmed: Could one manage addictive disorders with just general psychiatry training, and what are the differences between the different paths to certification that a resident could undertake?
Dr. Stanciu: Addictive disorders fall under the general umbrella of psychiatric care. Most individuals with these disorders exhibit some degree of mental illness. Medical school curriculum offers on average 2 hours of addiction-related didactics during 4 years. General psychiatry training programs vary significantly in the type of exposure to addiction—some residencies have an affiliated addiction fellowship, others have addiction-trained psychiatrists on staff, but most have none. Ultimately, there is great variability in the degree of comfort in working with individuals with addictive disorders post-residency. Being able to prescribe medications for the treatment of addictive disorders is very different from being familiar with the latest evidence-based recommendations and guidelines; the latter is
Addiction medicine is a fairly new route initially intended to allow non-psychiatric specialties access to addictive disorders training and certification. This is offered through the American Board of Preventive Medicine. There are currently 2 routes to sitting for the exam: through completion of a 1-year addiction medicine fellowship, or through the “practice pathway” still available until 2020. To be eligible for the latter, individuals must provide documentation of clinical experience post-residency, which is quantified as number of hours spent treating patients with addictions, plus any additional courses or training, and must be endorsed by a certified addictionologist.
Continue to: What was your fellowship experience link...
Dr. Ahmed: What was your fellowship experience like, and what should one consider when choosing a program?
Dr. Stanciu: I completed my fellowship training through Dartmouth’s Geisel School of Medicine, and the experience was tremendously valuable. In evaluating programs, one of the starting points is whether you have interest in a formal research track, because several programs include an optional year for that. Most programs tend to provide exposure to the Veterans Affairs system. The 1 year should provide you with broad exposure to all possible settings, all addictive disorders and patient populations, and all treatment modalities, in addition to rigorous didactic sessions. The ideal program should include rotations through methadone treatment centers, intensive outpatient programs, pain and interdisciplinary clinics, detoxification units, and centers for treatment of adolescent and young adults, as well as general medical settings and infectious disease clinics. There should also be close collaboration with psychologists who can provide training in evidence-based therapeutic modalities. During this year, it is vital to expand your knowledge of the ethical and legal regulations of treatment programs, state and federal requirements, insurance complexities, and requirements for privacy and protection of health information. The size of these programs can vary significantly, which may limit the one-on-one time devoted to your training, which is something I personally valued. My faculty was very supportive of academic endeavors, providing guidance, funding, and encouragement for attending and presenting at conferences, publishing papers, and other academic pursuits. Additionally, faculty should be current with emerging literature and willing to develop or implement new protocols and evaluate new pharmacologic therapies.
Dr. Ahmed: What are some of the career options and work settings for addiction psychiatrists?
Dr. Stanciu: Addiction psychiatrists work in numerous settings and various capacities. They can provide subspecialty care directly by seeing patients in outpatient clinics or inpatient addiction treatment centers for detoxification or rehabilitation, or they can work with dual-diagnosis populations in inpatient units. The expansion of telemedicine also holds promise for a role through virtual services. Indirectly, they can serve as a resource for expertise in the field through consultations in medical and psychiatric settings, or through policy making by working with the legislature and public health departments. Additionally, they can help create and integrate new knowledge into practice and educate future generations of physicians and the public.
Dr. Ahmed: What are some of the prevalent disorders and reasons for consultation that you encounter in your daily practice?
Continue to: Dr. Stanciu's response...
Dr. Stanciu: This can vary significantly depending on the setting, geographical region, and demographics of the population. My main non-administrative responsibilities are primarily consultative assisting clinicians at a 200-bed psychiatric hospital to address co-occurring addictive disorders. In short-term units, I am primarily asked to provide input on issues related to various toxidromes and withdrawals and the use of relapse prevention medications for alcohol use disorders as well as the use of buprenorphine or other forms of medication-assisted treatment. I work closely with licensed drug and alcohol counselors in implementing brief interventions as well as facilitating outpatient treatment referrals. Clinicians in longer term units may consult on issues related to pain management in individuals who have addictive disorders, the use of evidence-based pharmacologic agents to address cravings, or the use of relapse prevention medications for someone close to discharge. In terms of specific drugs of abuse, although opioids have recently received a tremendous amount of attention due to the visible costs through overdose deaths, the magnitude of individuals who are losing years of quality life through the use of alcohol and tobacco is significant, and hence this is a large portion of the conditions I encounter. I have also seen an abundance of marijuana use due to decreased perception of harm and increased access.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Stanciu: Historically, funding for services has been an issue for clinicians working primarily with addictive disorders from the standpoint of reimbursement, patient access to evidence-based pharmacotherapy, and ability to collaborate with existing levels of care. In recent years, federal funding and policies have changed this, and after numerous studies have found increased cost savings, commercial insurances are providing coverage. A significant challenge also has been public stigma and dealing with a condition that is relapsing-remitting, poorly understood by other specialties and the general public, and sometimes labeled as a defect of character. Several efforts in education have lessened this; however, the impact still takes a toll on patients, who may feel ashamed of their disorder and sometimes are hesitant to take medications because they may believe that they are not “clean” if they depend on a medication for remission. Lastly, recent changes in marijuana policies make conversations about this drug quite difficult because patients often view it as harmless, and the laws governing legality and indications for therapeutic use are slightly ahead of the evidence.
Dr. Ahmed: In what direction do you believe the subspecialty is headed?
Dr. Stanciu: Currently, there are approximately 1,000 certified addiction psychiatrists for the 45 million Americans who have addictive disorders. Smoking and other forms of tobacco use pose significant threats to the 2020 Healthy People Tobacco Use objectives. There is a significant demand for addictionologists in both public and private sectors. As with mental health, demand exceeds supply, and efforts are underway to expand downstream education and increase access to specialists. Several federal laws have been put in place to remove barriers and expand access to care and have paved the way to a brighter future. One is the Affordable Care Act, which requires all insurances including Medicaid to cover the cost of treatment. Second is the Mental Health Parity and Addiction Equity Act, which ensures that the duration and dollar amount of coverage for substance use disorders is comparable to that of medical and surgical care.
Continue to: Another exciting possibility...
Another exciting possibility comes from the world of pharmaceuticals. Some medications have modest efficacy for addressing addictive disorders; however, historically these have been poorly utilized. Enhanced understanding of the neurobiology combined with increased insurance reimbursement should prompt research and new drug development. Some promising agents are already in the pipeline. Research into molecular and gene therapy as a way to better individualize care is also underway.
Going forward, I think we will also encounter a different landscape of drugs. Synthetic agents are emerging and increasing in popularity. Alarmingly, public perception of harm is decreasing. When it comes to cannabis use, I see a rise in pathologic use and the ramifications of this will have a drastic impact, particularly on patients with mental health conditions. We will need to undertake better efforts in monitoring, staying updated, and providing public education campaigns.
Dr. Ahmed: What advice do you have for trainees contemplating subspecialty training in addiction psychiatry?
Dr. Stanciu: I cannot emphasize enough the importance of mentorship. The American Academy of Addiction Psychiatry has a robust system for connecting mentees with mentors at all stages in their careers. This can be extremely helpful, especially in situations where the residency program does not have addiction-trained faculty or rotations through treatment centers. Joining such an organization also grants you access to resources that can help further your enthusiasm. Those interested should also familiarize themselves with currently available pharmacotherapeutic treatments that have evidence supporting efficacy for various addictive disorders, and begin to incorporate these medications into general mental health practice, along with attempts at motivational interviewing. For example, begin discussing naltrexone with patients who have comorbid alcohol use disorders and are interested in reducing their drinking; and varenicline with patients who smoke and are interested in quitting. The outcomes should automatically elicit an interest in pursuing further training in the field!
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, talked with Cornel Stanciu, MD. Dr. Stanciu is an addiction psychiatrist at Dartmouth’s Geisel School of Medicine, where he is an Assistant Professor, and serves as the Director of Addiction Services at New Hampshire Hospital. He provides support to clinicians managing patients with addictive disorders in a multitude of settings, and also assists with policy making and delivery of addiction care at the state level. He is also the author of Deciphering the Addicted Brain, a guide to help families and the general public better understand addictive disorders.
Dr. Ahmed: What attracted you to pursue subspecialty training in addictive disorders?
Dr. Stanciu: In the early stages of my training, I frequently encountered individuals with medical and mental health disorders whose treatment was impacted by underlying substance use. I soon came to realize any attempts at (for example) managing hypertension in someone with cocaine use disorder, or managing schizophrenia in someone with ongoing cannabis use, were futile. Almost all of my patients receiving treatment for mental health disorders were dependent on tobacco or other substances, and most were interested in cessation. Through mentorship from addiction-trained residency faculty members, I was able to get a taste of the neurobiologic complexities of the disease, something that left me with a desire to develop a deeper understanding of the disease process. Witnessing strikingly positive outcomes with implementation of evidence-based treatment modalities further solidified my path to subspecialty training. Even during that early phase, because I expressed interest in managing these conditions, I was immediately put in a position to share and disseminate any newly acquired knowledge to other specialties as well as the public.
Dr. Ahmed: Could one manage addictive disorders with just general psychiatry training, and what are the differences between the different paths to certification that a resident could undertake?
Dr. Stanciu: Addictive disorders fall under the general umbrella of psychiatric care. Most individuals with these disorders exhibit some degree of mental illness. Medical school curriculum offers on average 2 hours of addiction-related didactics during 4 years. General psychiatry training programs vary significantly in the type of exposure to addiction—some residencies have an affiliated addiction fellowship, others have addiction-trained psychiatrists on staff, but most have none. Ultimately, there is great variability in the degree of comfort in working with individuals with addictive disorders post-residency. Being able to prescribe medications for the treatment of addictive disorders is very different from being familiar with the latest evidence-based recommendations and guidelines; the latter is
Addiction medicine is a fairly new route initially intended to allow non-psychiatric specialties access to addictive disorders training and certification. This is offered through the American Board of Preventive Medicine. There are currently 2 routes to sitting for the exam: through completion of a 1-year addiction medicine fellowship, or through the “practice pathway” still available until 2020. To be eligible for the latter, individuals must provide documentation of clinical experience post-residency, which is quantified as number of hours spent treating patients with addictions, plus any additional courses or training, and must be endorsed by a certified addictionologist.
Continue to: What was your fellowship experience link...
Dr. Ahmed: What was your fellowship experience like, and what should one consider when choosing a program?
Dr. Stanciu: I completed my fellowship training through Dartmouth’s Geisel School of Medicine, and the experience was tremendously valuable. In evaluating programs, one of the starting points is whether you have interest in a formal research track, because several programs include an optional year for that. Most programs tend to provide exposure to the Veterans Affairs system. The 1 year should provide you with broad exposure to all possible settings, all addictive disorders and patient populations, and all treatment modalities, in addition to rigorous didactic sessions. The ideal program should include rotations through methadone treatment centers, intensive outpatient programs, pain and interdisciplinary clinics, detoxification units, and centers for treatment of adolescent and young adults, as well as general medical settings and infectious disease clinics. There should also be close collaboration with psychologists who can provide training in evidence-based therapeutic modalities. During this year, it is vital to expand your knowledge of the ethical and legal regulations of treatment programs, state and federal requirements, insurance complexities, and requirements for privacy and protection of health information. The size of these programs can vary significantly, which may limit the one-on-one time devoted to your training, which is something I personally valued. My faculty was very supportive of academic endeavors, providing guidance, funding, and encouragement for attending and presenting at conferences, publishing papers, and other academic pursuits. Additionally, faculty should be current with emerging literature and willing to develop or implement new protocols and evaluate new pharmacologic therapies.
Dr. Ahmed: What are some of the career options and work settings for addiction psychiatrists?
Dr. Stanciu: Addiction psychiatrists work in numerous settings and various capacities. They can provide subspecialty care directly by seeing patients in outpatient clinics or inpatient addiction treatment centers for detoxification or rehabilitation, or they can work with dual-diagnosis populations in inpatient units. The expansion of telemedicine also holds promise for a role through virtual services. Indirectly, they can serve as a resource for expertise in the field through consultations in medical and psychiatric settings, or through policy making by working with the legislature and public health departments. Additionally, they can help create and integrate new knowledge into practice and educate future generations of physicians and the public.
Dr. Ahmed: What are some of the prevalent disorders and reasons for consultation that you encounter in your daily practice?
Continue to: Dr. Stanciu's response...
Dr. Stanciu: This can vary significantly depending on the setting, geographical region, and demographics of the population. My main non-administrative responsibilities are primarily consultative assisting clinicians at a 200-bed psychiatric hospital to address co-occurring addictive disorders. In short-term units, I am primarily asked to provide input on issues related to various toxidromes and withdrawals and the use of relapse prevention medications for alcohol use disorders as well as the use of buprenorphine or other forms of medication-assisted treatment. I work closely with licensed drug and alcohol counselors in implementing brief interventions as well as facilitating outpatient treatment referrals. Clinicians in longer term units may consult on issues related to pain management in individuals who have addictive disorders, the use of evidence-based pharmacologic agents to address cravings, or the use of relapse prevention medications for someone close to discharge. In terms of specific drugs of abuse, although opioids have recently received a tremendous amount of attention due to the visible costs through overdose deaths, the magnitude of individuals who are losing years of quality life through the use of alcohol and tobacco is significant, and hence this is a large portion of the conditions I encounter. I have also seen an abundance of marijuana use due to decreased perception of harm and increased access.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Stanciu: Historically, funding for services has been an issue for clinicians working primarily with addictive disorders from the standpoint of reimbursement, patient access to evidence-based pharmacotherapy, and ability to collaborate with existing levels of care. In recent years, federal funding and policies have changed this, and after numerous studies have found increased cost savings, commercial insurances are providing coverage. A significant challenge also has been public stigma and dealing with a condition that is relapsing-remitting, poorly understood by other specialties and the general public, and sometimes labeled as a defect of character. Several efforts in education have lessened this; however, the impact still takes a toll on patients, who may feel ashamed of their disorder and sometimes are hesitant to take medications because they may believe that they are not “clean” if they depend on a medication for remission. Lastly, recent changes in marijuana policies make conversations about this drug quite difficult because patients often view it as harmless, and the laws governing legality and indications for therapeutic use are slightly ahead of the evidence.
Dr. Ahmed: In what direction do you believe the subspecialty is headed?
Dr. Stanciu: Currently, there are approximately 1,000 certified addiction psychiatrists for the 45 million Americans who have addictive disorders. Smoking and other forms of tobacco use pose significant threats to the 2020 Healthy People Tobacco Use objectives. There is a significant demand for addictionologists in both public and private sectors. As with mental health, demand exceeds supply, and efforts are underway to expand downstream education and increase access to specialists. Several federal laws have been put in place to remove barriers and expand access to care and have paved the way to a brighter future. One is the Affordable Care Act, which requires all insurances including Medicaid to cover the cost of treatment. Second is the Mental Health Parity and Addiction Equity Act, which ensures that the duration and dollar amount of coverage for substance use disorders is comparable to that of medical and surgical care.
Continue to: Another exciting possibility...
Another exciting possibility comes from the world of pharmaceuticals. Some medications have modest efficacy for addressing addictive disorders; however, historically these have been poorly utilized. Enhanced understanding of the neurobiology combined with increased insurance reimbursement should prompt research and new drug development. Some promising agents are already in the pipeline. Research into molecular and gene therapy as a way to better individualize care is also underway.
Going forward, I think we will also encounter a different landscape of drugs. Synthetic agents are emerging and increasing in popularity. Alarmingly, public perception of harm is decreasing. When it comes to cannabis use, I see a rise in pathologic use and the ramifications of this will have a drastic impact, particularly on patients with mental health conditions. We will need to undertake better efforts in monitoring, staying updated, and providing public education campaigns.
Dr. Ahmed: What advice do you have for trainees contemplating subspecialty training in addiction psychiatry?
Dr. Stanciu: I cannot emphasize enough the importance of mentorship. The American Academy of Addiction Psychiatry has a robust system for connecting mentees with mentors at all stages in their careers. This can be extremely helpful, especially in situations where the residency program does not have addiction-trained faculty or rotations through treatment centers. Joining such an organization also grants you access to resources that can help further your enthusiasm. Those interested should also familiarize themselves with currently available pharmacotherapeutic treatments that have evidence supporting efficacy for various addictive disorders, and begin to incorporate these medications into general mental health practice, along with attempts at motivational interviewing. For example, begin discussing naltrexone with patients who have comorbid alcohol use disorders and are interested in reducing their drinking; and varenicline with patients who smoke and are interested in quitting. The outcomes should automatically elicit an interest in pursuing further training in the field!
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, talked with Cornel Stanciu, MD. Dr. Stanciu is an addiction psychiatrist at Dartmouth’s Geisel School of Medicine, where he is an Assistant Professor, and serves as the Director of Addiction Services at New Hampshire Hospital. He provides support to clinicians managing patients with addictive disorders in a multitude of settings, and also assists with policy making and delivery of addiction care at the state level. He is also the author of Deciphering the Addicted Brain, a guide to help families and the general public better understand addictive disorders.
Dr. Ahmed: What attracted you to pursue subspecialty training in addictive disorders?
Dr. Stanciu: In the early stages of my training, I frequently encountered individuals with medical and mental health disorders whose treatment was impacted by underlying substance use. I soon came to realize any attempts at (for example) managing hypertension in someone with cocaine use disorder, or managing schizophrenia in someone with ongoing cannabis use, were futile. Almost all of my patients receiving treatment for mental health disorders were dependent on tobacco or other substances, and most were interested in cessation. Through mentorship from addiction-trained residency faculty members, I was able to get a taste of the neurobiologic complexities of the disease, something that left me with a desire to develop a deeper understanding of the disease process. Witnessing strikingly positive outcomes with implementation of evidence-based treatment modalities further solidified my path to subspecialty training. Even during that early phase, because I expressed interest in managing these conditions, I was immediately put in a position to share and disseminate any newly acquired knowledge to other specialties as well as the public.
Dr. Ahmed: Could one manage addictive disorders with just general psychiatry training, and what are the differences between the different paths to certification that a resident could undertake?
Dr. Stanciu: Addictive disorders fall under the general umbrella of psychiatric care. Most individuals with these disorders exhibit some degree of mental illness. Medical school curriculum offers on average 2 hours of addiction-related didactics during 4 years. General psychiatry training programs vary significantly in the type of exposure to addiction—some residencies have an affiliated addiction fellowship, others have addiction-trained psychiatrists on staff, but most have none. Ultimately, there is great variability in the degree of comfort in working with individuals with addictive disorders post-residency. Being able to prescribe medications for the treatment of addictive disorders is very different from being familiar with the latest evidence-based recommendations and guidelines; the latter is
Addiction medicine is a fairly new route initially intended to allow non-psychiatric specialties access to addictive disorders training and certification. This is offered through the American Board of Preventive Medicine. There are currently 2 routes to sitting for the exam: through completion of a 1-year addiction medicine fellowship, or through the “practice pathway” still available until 2020. To be eligible for the latter, individuals must provide documentation of clinical experience post-residency, which is quantified as number of hours spent treating patients with addictions, plus any additional courses or training, and must be endorsed by a certified addictionologist.
Continue to: What was your fellowship experience link...
Dr. Ahmed: What was your fellowship experience like, and what should one consider when choosing a program?
Dr. Stanciu: I completed my fellowship training through Dartmouth’s Geisel School of Medicine, and the experience was tremendously valuable. In evaluating programs, one of the starting points is whether you have interest in a formal research track, because several programs include an optional year for that. Most programs tend to provide exposure to the Veterans Affairs system. The 1 year should provide you with broad exposure to all possible settings, all addictive disorders and patient populations, and all treatment modalities, in addition to rigorous didactic sessions. The ideal program should include rotations through methadone treatment centers, intensive outpatient programs, pain and interdisciplinary clinics, detoxification units, and centers for treatment of adolescent and young adults, as well as general medical settings and infectious disease clinics. There should also be close collaboration with psychologists who can provide training in evidence-based therapeutic modalities. During this year, it is vital to expand your knowledge of the ethical and legal regulations of treatment programs, state and federal requirements, insurance complexities, and requirements for privacy and protection of health information. The size of these programs can vary significantly, which may limit the one-on-one time devoted to your training, which is something I personally valued. My faculty was very supportive of academic endeavors, providing guidance, funding, and encouragement for attending and presenting at conferences, publishing papers, and other academic pursuits. Additionally, faculty should be current with emerging literature and willing to develop or implement new protocols and evaluate new pharmacologic therapies.
Dr. Ahmed: What are some of the career options and work settings for addiction psychiatrists?
Dr. Stanciu: Addiction psychiatrists work in numerous settings and various capacities. They can provide subspecialty care directly by seeing patients in outpatient clinics or inpatient addiction treatment centers for detoxification or rehabilitation, or they can work with dual-diagnosis populations in inpatient units. The expansion of telemedicine also holds promise for a role through virtual services. Indirectly, they can serve as a resource for expertise in the field through consultations in medical and psychiatric settings, or through policy making by working with the legislature and public health departments. Additionally, they can help create and integrate new knowledge into practice and educate future generations of physicians and the public.
Dr. Ahmed: What are some of the prevalent disorders and reasons for consultation that you encounter in your daily practice?
Continue to: Dr. Stanciu's response...
Dr. Stanciu: This can vary significantly depending on the setting, geographical region, and demographics of the population. My main non-administrative responsibilities are primarily consultative assisting clinicians at a 200-bed psychiatric hospital to address co-occurring addictive disorders. In short-term units, I am primarily asked to provide input on issues related to various toxidromes and withdrawals and the use of relapse prevention medications for alcohol use disorders as well as the use of buprenorphine or other forms of medication-assisted treatment. I work closely with licensed drug and alcohol counselors in implementing brief interventions as well as facilitating outpatient treatment referrals. Clinicians in longer term units may consult on issues related to pain management in individuals who have addictive disorders, the use of evidence-based pharmacologic agents to address cravings, or the use of relapse prevention medications for someone close to discharge. In terms of specific drugs of abuse, although opioids have recently received a tremendous amount of attention due to the visible costs through overdose deaths, the magnitude of individuals who are losing years of quality life through the use of alcohol and tobacco is significant, and hence this is a large portion of the conditions I encounter. I have also seen an abundance of marijuana use due to decreased perception of harm and increased access.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Stanciu: Historically, funding for services has been an issue for clinicians working primarily with addictive disorders from the standpoint of reimbursement, patient access to evidence-based pharmacotherapy, and ability to collaborate with existing levels of care. In recent years, federal funding and policies have changed this, and after numerous studies have found increased cost savings, commercial insurances are providing coverage. A significant challenge also has been public stigma and dealing with a condition that is relapsing-remitting, poorly understood by other specialties and the general public, and sometimes labeled as a defect of character. Several efforts in education have lessened this; however, the impact still takes a toll on patients, who may feel ashamed of their disorder and sometimes are hesitant to take medications because they may believe that they are not “clean” if they depend on a medication for remission. Lastly, recent changes in marijuana policies make conversations about this drug quite difficult because patients often view it as harmless, and the laws governing legality and indications for therapeutic use are slightly ahead of the evidence.
Dr. Ahmed: In what direction do you believe the subspecialty is headed?
Dr. Stanciu: Currently, there are approximately 1,000 certified addiction psychiatrists for the 45 million Americans who have addictive disorders. Smoking and other forms of tobacco use pose significant threats to the 2020 Healthy People Tobacco Use objectives. There is a significant demand for addictionologists in both public and private sectors. As with mental health, demand exceeds supply, and efforts are underway to expand downstream education and increase access to specialists. Several federal laws have been put in place to remove barriers and expand access to care and have paved the way to a brighter future. One is the Affordable Care Act, which requires all insurances including Medicaid to cover the cost of treatment. Second is the Mental Health Parity and Addiction Equity Act, which ensures that the duration and dollar amount of coverage for substance use disorders is comparable to that of medical and surgical care.
Continue to: Another exciting possibility...
Another exciting possibility comes from the world of pharmaceuticals. Some medications have modest efficacy for addressing addictive disorders; however, historically these have been poorly utilized. Enhanced understanding of the neurobiology combined with increased insurance reimbursement should prompt research and new drug development. Some promising agents are already in the pipeline. Research into molecular and gene therapy as a way to better individualize care is also underway.
Going forward, I think we will also encounter a different landscape of drugs. Synthetic agents are emerging and increasing in popularity. Alarmingly, public perception of harm is decreasing. When it comes to cannabis use, I see a rise in pathologic use and the ramifications of this will have a drastic impact, particularly on patients with mental health conditions. We will need to undertake better efforts in monitoring, staying updated, and providing public education campaigns.
Dr. Ahmed: What advice do you have for trainees contemplating subspecialty training in addiction psychiatry?
Dr. Stanciu: I cannot emphasize enough the importance of mentorship. The American Academy of Addiction Psychiatry has a robust system for connecting mentees with mentors at all stages in their careers. This can be extremely helpful, especially in situations where the residency program does not have addiction-trained faculty or rotations through treatment centers. Joining such an organization also grants you access to resources that can help further your enthusiasm. Those interested should also familiarize themselves with currently available pharmacotherapeutic treatments that have evidence supporting efficacy for various addictive disorders, and begin to incorporate these medications into general mental health practice, along with attempts at motivational interviewing. For example, begin discussing naltrexone with patients who have comorbid alcohol use disorders and are interested in reducing their drinking; and varenicline with patients who smoke and are interested in quitting. The outcomes should automatically elicit an interest in pursuing further training in the field!
Shining a spotlight on physician well-being, more
Shining a spotlight on physician well-being
In “Physician impairment”1 (C
Dr. Farrell’s article does not acknowledge the rule of law. I do not understand why anyone wanting to help physicians would not want them to be aware of their employment rights under the ADA or advise that their ADA rights protect them from unwarranted medical inquiries and referrals to physician health programs (PHPs) or other entities for evaluation.
Dr. Farrell also claims that burnout, poor well-being, and mental disorders cause medical errors and low quality of patient care, but there are many reasons to doubt that this is the case.3,4 Readers should be wary of medical journal articles that cover topics related to physician well-being. Articles related to PHPs, in particular, typically paint an overly rosy picture of the effectiveness of these programs and fail to note important problematic aspects.5,6
Nicholas D. Lawson, MD
Georgetown University Law Center
Washington, DC
References
1. Lawson ND. Physician impairment. Current Psychiatry. 2017;16(10):8.
2. Farrell H. Physician impairment: a need for prevention. Current Psychiatry. 2018;17(9):41-44.
3. Lawson ND. Burnout is not associated with increased medical errors. Mayo Clin Proc. 2018;93(11):1683.
4. Tyssen R. What is the level of burnout that impairs functioning? J Intern Med. 2018;283(6):594-596.
5. Lawson ND, Boyd JW. Flaws in the methods and reporting of physician health program outcome studies. Gen Hosp Psychiatry. 2018;54:65-66.
6. Lawson ND, Boyd JW. Physician health program outcome data should be viewed with caution. Judges J. 2018;57(4):36.
The author responds
I thank Dr. Lawson for his interest in my article. In this extremely challenging work that we do as psychiatrists, which can sometimes be quite isolating, there is a long continuum of experience, reward, and challenge. Dr. Lawson’s research and publication on the topic of physician’s health issues are very much respected and appreciated. In fact, I see no conflict between Dr. Lawson’s letter and my 2018 column on the prevention of impairment.
Given the extensive continuum of our work, my article on physician’s health issues sought to shine a bright spotlight solely on the topic of prevention. As colleagues, there is significant value in supporting rather than reporting one another. Awareness of and sensitivity to physician vulnerability, early detection, and prevention will hopefully continue to gain traction in the future.
By putting the focus on proactively helping colleagues, my hope is that my article will spark an ongoing conversation about how we can work collaboratively to make well-being a priority.
Dr. Lawson’s thoughtful letter is much appreciated because it continues the discussion by shining a spotlight further down the continuum. He focuses on the aftermath of impairment and aptly points out the complications in reporting, confusion about duty, and the protections provided by the ADA. Also, I support Dr. Lawson’s cautions regarding PHPs—all the more reason to join together in shifting the dialogue from management of a crisis to prevention of it.
Helen M. Farrell, MD
Lecturer
Harvard Medical School
Psychiatrist
Beth Israel Deaconess Medical Center
Boston, Massachusetts
Continue to: Neuropolitics
Neuropolitics: Psychiatrists’ responsibility
Regarding Dr. Nasrallah’s editorial “Neuropolitics in the age of extremism: Brain regions involved in hatred” (C
I agree with Dr. Nasrallah that “even the most skillful psychiatrists” cannot “repair a nation caught up in poisonous emotional turmoil”—at least not by employing clinical skills alone. But that doesn’t mean we shouldn’t try, and the American Psychiatric Association (APA) ethics code (Sections 1.2, 3, and 7) compels us to speak out when our patients or the public are being harmed by public policy.1 We are much more likely to have an impact when we speak with one voice, as is the case with professional medical organizations such as the APA. In December 2018, APA President Dr. Altha J. Stewart issued a call to action addressing “the current climate of hateful and divisive rhetoric that leads to senseless violence and tragic loss of life,” stating “… we members must speak out, use our specialized training and expertise for the public’s benefit, and apply it to not only healing, but also preventing psychological trauma and senseless tragedies.”2
James L. Fleming, MD
Psychiatric Medical Care
Lee’s Summit, Missouri
References
1. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry, 2013 edition. https://www.psychiatry.org/psychiatrists/practice/ethics. Published 2013. Accessed February 5, 2019.
2. Stewart A, Pozios, V. Forget about staying in our lane: let’s connect the dots. American Psychiatric Association Publishing. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a20. Published December 3, 2018. Accessed February 3, 2019.
Shining a spotlight on physician well-being
In “Physician impairment”1 (C
Dr. Farrell’s article does not acknowledge the rule of law. I do not understand why anyone wanting to help physicians would not want them to be aware of their employment rights under the ADA or advise that their ADA rights protect them from unwarranted medical inquiries and referrals to physician health programs (PHPs) or other entities for evaluation.
Dr. Farrell also claims that burnout, poor well-being, and mental disorders cause medical errors and low quality of patient care, but there are many reasons to doubt that this is the case.3,4 Readers should be wary of medical journal articles that cover topics related to physician well-being. Articles related to PHPs, in particular, typically paint an overly rosy picture of the effectiveness of these programs and fail to note important problematic aspects.5,6
Nicholas D. Lawson, MD
Georgetown University Law Center
Washington, DC
References
1. Lawson ND. Physician impairment. Current Psychiatry. 2017;16(10):8.
2. Farrell H. Physician impairment: a need for prevention. Current Psychiatry. 2018;17(9):41-44.
3. Lawson ND. Burnout is not associated with increased medical errors. Mayo Clin Proc. 2018;93(11):1683.
4. Tyssen R. What is the level of burnout that impairs functioning? J Intern Med. 2018;283(6):594-596.
5. Lawson ND, Boyd JW. Flaws in the methods and reporting of physician health program outcome studies. Gen Hosp Psychiatry. 2018;54:65-66.
6. Lawson ND, Boyd JW. Physician health program outcome data should be viewed with caution. Judges J. 2018;57(4):36.
The author responds
I thank Dr. Lawson for his interest in my article. In this extremely challenging work that we do as psychiatrists, which can sometimes be quite isolating, there is a long continuum of experience, reward, and challenge. Dr. Lawson’s research and publication on the topic of physician’s health issues are very much respected and appreciated. In fact, I see no conflict between Dr. Lawson’s letter and my 2018 column on the prevention of impairment.
Given the extensive continuum of our work, my article on physician’s health issues sought to shine a bright spotlight solely on the topic of prevention. As colleagues, there is significant value in supporting rather than reporting one another. Awareness of and sensitivity to physician vulnerability, early detection, and prevention will hopefully continue to gain traction in the future.
By putting the focus on proactively helping colleagues, my hope is that my article will spark an ongoing conversation about how we can work collaboratively to make well-being a priority.
Dr. Lawson’s thoughtful letter is much appreciated because it continues the discussion by shining a spotlight further down the continuum. He focuses on the aftermath of impairment and aptly points out the complications in reporting, confusion about duty, and the protections provided by the ADA. Also, I support Dr. Lawson’s cautions regarding PHPs—all the more reason to join together in shifting the dialogue from management of a crisis to prevention of it.
Helen M. Farrell, MD
Lecturer
Harvard Medical School
Psychiatrist
Beth Israel Deaconess Medical Center
Boston, Massachusetts
Continue to: Neuropolitics
Neuropolitics: Psychiatrists’ responsibility
Regarding Dr. Nasrallah’s editorial “Neuropolitics in the age of extremism: Brain regions involved in hatred” (C
I agree with Dr. Nasrallah that “even the most skillful psychiatrists” cannot “repair a nation caught up in poisonous emotional turmoil”—at least not by employing clinical skills alone. But that doesn’t mean we shouldn’t try, and the American Psychiatric Association (APA) ethics code (Sections 1.2, 3, and 7) compels us to speak out when our patients or the public are being harmed by public policy.1 We are much more likely to have an impact when we speak with one voice, as is the case with professional medical organizations such as the APA. In December 2018, APA President Dr. Altha J. Stewart issued a call to action addressing “the current climate of hateful and divisive rhetoric that leads to senseless violence and tragic loss of life,” stating “… we members must speak out, use our specialized training and expertise for the public’s benefit, and apply it to not only healing, but also preventing psychological trauma and senseless tragedies.”2
James L. Fleming, MD
Psychiatric Medical Care
Lee’s Summit, Missouri
References
1. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry, 2013 edition. https://www.psychiatry.org/psychiatrists/practice/ethics. Published 2013. Accessed February 5, 2019.
2. Stewart A, Pozios, V. Forget about staying in our lane: let’s connect the dots. American Psychiatric Association Publishing. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a20. Published December 3, 2018. Accessed February 3, 2019.
Shining a spotlight on physician well-being
In “Physician impairment”1 (C
Dr. Farrell’s article does not acknowledge the rule of law. I do not understand why anyone wanting to help physicians would not want them to be aware of their employment rights under the ADA or advise that their ADA rights protect them from unwarranted medical inquiries and referrals to physician health programs (PHPs) or other entities for evaluation.
Dr. Farrell also claims that burnout, poor well-being, and mental disorders cause medical errors and low quality of patient care, but there are many reasons to doubt that this is the case.3,4 Readers should be wary of medical journal articles that cover topics related to physician well-being. Articles related to PHPs, in particular, typically paint an overly rosy picture of the effectiveness of these programs and fail to note important problematic aspects.5,6
Nicholas D. Lawson, MD
Georgetown University Law Center
Washington, DC
References
1. Lawson ND. Physician impairment. Current Psychiatry. 2017;16(10):8.
2. Farrell H. Physician impairment: a need for prevention. Current Psychiatry. 2018;17(9):41-44.
3. Lawson ND. Burnout is not associated with increased medical errors. Mayo Clin Proc. 2018;93(11):1683.
4. Tyssen R. What is the level of burnout that impairs functioning? J Intern Med. 2018;283(6):594-596.
5. Lawson ND, Boyd JW. Flaws in the methods and reporting of physician health program outcome studies. Gen Hosp Psychiatry. 2018;54:65-66.
6. Lawson ND, Boyd JW. Physician health program outcome data should be viewed with caution. Judges J. 2018;57(4):36.
The author responds
I thank Dr. Lawson for his interest in my article. In this extremely challenging work that we do as psychiatrists, which can sometimes be quite isolating, there is a long continuum of experience, reward, and challenge. Dr. Lawson’s research and publication on the topic of physician’s health issues are very much respected and appreciated. In fact, I see no conflict between Dr. Lawson’s letter and my 2018 column on the prevention of impairment.
Given the extensive continuum of our work, my article on physician’s health issues sought to shine a bright spotlight solely on the topic of prevention. As colleagues, there is significant value in supporting rather than reporting one another. Awareness of and sensitivity to physician vulnerability, early detection, and prevention will hopefully continue to gain traction in the future.
By putting the focus on proactively helping colleagues, my hope is that my article will spark an ongoing conversation about how we can work collaboratively to make well-being a priority.
Dr. Lawson’s thoughtful letter is much appreciated because it continues the discussion by shining a spotlight further down the continuum. He focuses on the aftermath of impairment and aptly points out the complications in reporting, confusion about duty, and the protections provided by the ADA. Also, I support Dr. Lawson’s cautions regarding PHPs—all the more reason to join together in shifting the dialogue from management of a crisis to prevention of it.
Helen M. Farrell, MD
Lecturer
Harvard Medical School
Psychiatrist
Beth Israel Deaconess Medical Center
Boston, Massachusetts
Continue to: Neuropolitics
Neuropolitics: Psychiatrists’ responsibility
Regarding Dr. Nasrallah’s editorial “Neuropolitics in the age of extremism: Brain regions involved in hatred” (C
I agree with Dr. Nasrallah that “even the most skillful psychiatrists” cannot “repair a nation caught up in poisonous emotional turmoil”—at least not by employing clinical skills alone. But that doesn’t mean we shouldn’t try, and the American Psychiatric Association (APA) ethics code (Sections 1.2, 3, and 7) compels us to speak out when our patients or the public are being harmed by public policy.1 We are much more likely to have an impact when we speak with one voice, as is the case with professional medical organizations such as the APA. In December 2018, APA President Dr. Altha J. Stewart issued a call to action addressing “the current climate of hateful and divisive rhetoric that leads to senseless violence and tragic loss of life,” stating “… we members must speak out, use our specialized training and expertise for the public’s benefit, and apply it to not only healing, but also preventing psychological trauma and senseless tragedies.”2
James L. Fleming, MD
Psychiatric Medical Care
Lee’s Summit, Missouri
References
1. American Psychiatric Association. The principles of medical ethics with annotations especially applicable to psychiatry, 2013 edition. https://www.psychiatry.org/psychiatrists/practice/ethics. Published 2013. Accessed February 5, 2019.
2. Stewart A, Pozios, V. Forget about staying in our lane: let’s connect the dots. American Psychiatric Association Publishing. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a20. Published December 3, 2018. Accessed February 3, 2019.
Psychiatry and neurology: Sister neuroscience specialties with different approaches to the brain
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.
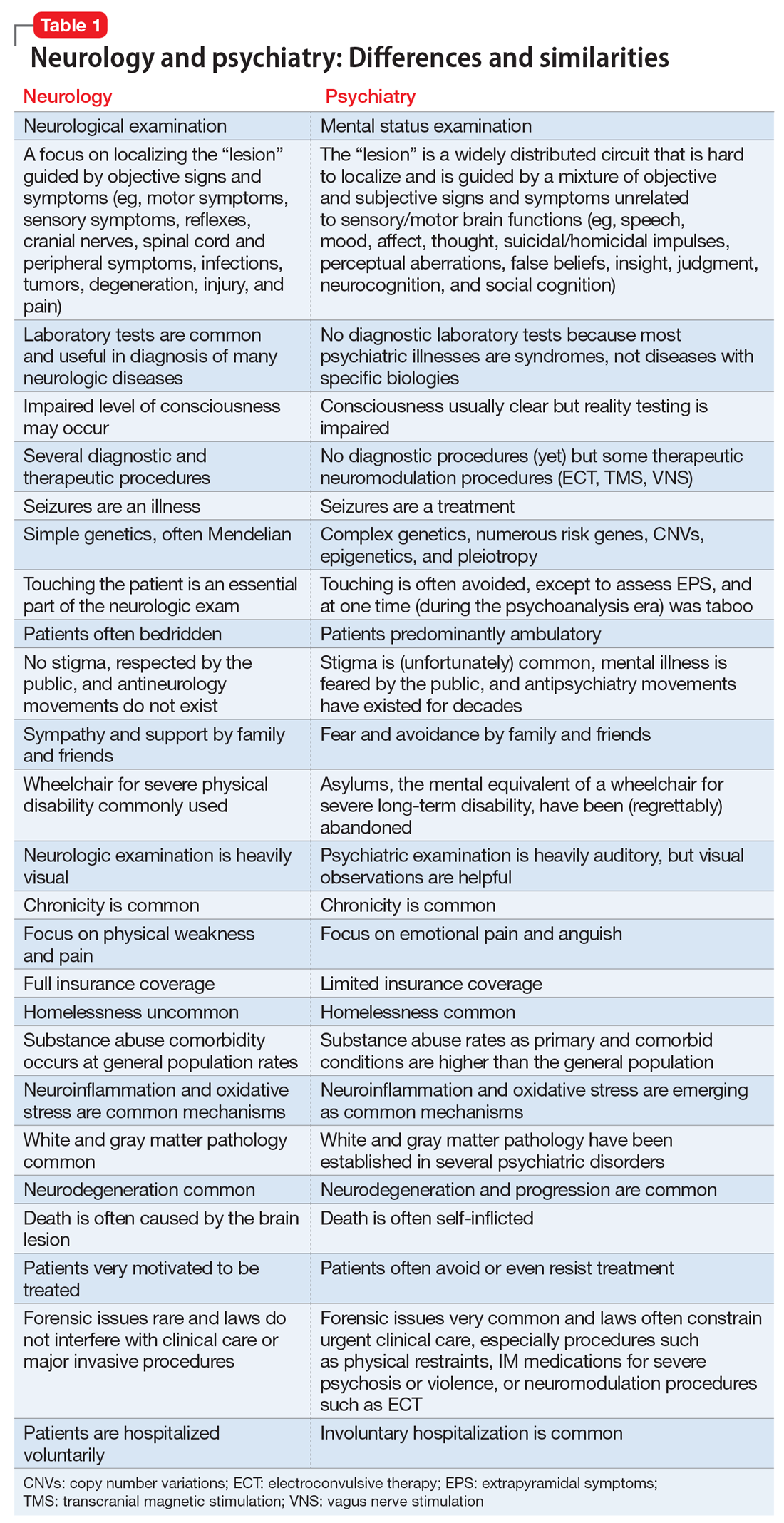
Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.

Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: [email protected].
Neurologists and psychiatrists diagnose and treat disorders of the brain’s hardware and software, respectively. The brain is a physically tangible structure, while its mind is virtual and intangible.
Not surprisingly, neurology and psychiatry have very different approaches to the assessment and treatment of brain and mind disorders. It reminds me of ophthalmology, where some of the faculty focus on the hardware of the eye (cornea, lens, and retina) while others focus on the major function of the eye—vision. Similarly, the mind is the major function of the brain.
Clinical neuroscience represents the shared foundational underpinnings of neurologists and psychiatrists, but their management of brain and mind disorders is understandably quite different, albeit with the same final goal: to repair and restore the structure and function of this divinely complex organ, the command and control center of the human soul and behavior.
In Table 1, I compare and contrast the clinical approaches of these 2 sister clinical neuroscience specialties, beyond the shared standard medical templates of history of present illness, medical history, social history, family history, review of systems, and physical examination.

Despite those many differences in assessing and treating neurologic vs psychiatric disorders of the brain, there is an indisputable fact: Every neurologic disorder is associated with psychiatric manifestations, and every psychiatric illness is associated with neurologic symptoms. The brain is the most complex structure in the universe; its development requires the expression of 50% of the human genome, and its major task is to generate a mind that enables every human being to navigate the biopsychosocial imperatives of life. Any brain lesion, regardless of size and location, will disrupt the integrity of the mind in one way or another, such as speaking, thinking, fantasizing, arguing, understanding, feeling, remembering, plotting, enjoying, socializing, or courting. The bottom line is that every patient with a brain/mind disorder should ideally receive both neurologic and psychiatric evaluation, and the requisite dual interventions as necessary.1 If the focus is exclusively on either the brain or the mind, clinical and functional outcomes for the patient will be suboptimal.
Neuropsychiatrists and behavioral neurologists represent excellent bridges across these 2 sister specialties. There are twice as many psychiatrists as neurologists, but very few neuropsychiatrists or behavioral neurologists. The American Board of Psychiatry and Neurology (ABPN) has approved several board certifications for both specialties, and several subspecialties as well (Table 2). When will the ABPN approve neuropsychiatry and behavioral neurology as subspecialties, to facilitate the integration of the brain and the mind,2 and to bridge the chasm between disorders of the brain and mind?
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
1. Nasrallah HA. Toward the era of transformational neuropsychiatry. Asian J Psychiatr. 2015;17:140-141.
2. Nasrallah HA. Reintegrating psychiatry and neurology is long overdue: Part 1. April 30, 2014. https://www.cmeinstitute.com/pages/lets-talk.aspx?bid=72. Accessed February 11, 2019.
Aerospace medicine and psychiatry
As part of my psychiatry residency training, I had the privilege to work with and learn from an aerospace psychiatrist. Aerospace medicine is a branch of preventive and occupational medicine in which aviators (pilots, aircrew, or astronauts) are subject to evaluation/treatment. The goal is to assess physical and mental health factors to mitigate risks, protect public safety, and ensure the aviators’ well-being.1,2 Aerospace psychiatry is a highly specialized area in which practitioners are trained to perform specific evaluations. In this article, I review those evaluations for those looking to gain insight into the field.
Aviation medical examination
Under Title 14 of the Code of Federal Regulations, the Federal Aviation Administration (FAA) requires aviators to be evaluated for medical certification by undergoing an aviation medical exam.2 In order to be deemed “fit for duty,” aviators must meet strict physical and mental health standards set by the FAA. The extent of these standards varies by the class of licensure (Table 13). Aviation medical exams are performed by any physician who has been designated by the FAA and completed the appropriate FAA aviation medical examiner (AME) training. Aviators who meet the medical standards for their licensure class are recommended for medical certification. If the AME brings up further questions due to the limits of the examination and/or a lack of medical records, the certification will likely be deferred pending further evaluation by an FAA-approved medical specialist and/or the receipt of additional medical records. Questions about a possible psychiatric diagnosis/history or substance use disorder will lead to referral to a psychiatrist familiar with aviation standards for further evaluation.
_
Special issuances and Conditions AMEs Can Issue
There are 15 disqualifying conditions for medical certification (Table 13). However, a special issuance of a medical certification may be granted if the aviator shows to the satisfaction of the aviation medical examiner that the duties of the licensure class can be performed without endangering the public safety and that the condition is deemed stable. This may be shown through additional medical evaluations/tests and/or records.
There are certain medical conditions for which an AME can issue a medical certificate without further review from other specialists; thus, an AME can review and follow the Conditions AMEs Can Issue (CACI) worksheet to recommend medical certification (Table 24). The CACI guidelines and worksheets are updated by the FAA regularly to ensure aviators’ health and minimize public risk.
Psychiatric & Psychological Evaluation
Aviators may be referred for Psychiatric and Psychological Evaluation (P&P) if an AME discovers additional concerns about psychiatric and neurocognitive disorders. These cases are not clear-cut. An example would be an aviator who was receiving a psychotropic medication in the past and reported past heavy alcohol use. The P&P includes a thorough psychiatric evaluation by an aerospace psychiatrist and extensive psychological testing by an aerospace psychologist. These clinicians also review collateral information and past medical/AME records. Aviators may be recommended for medical certification with special issuance or may be denied medical certification as a result of these examinations.
Human Intervention Motivation Study program
The Human Intervention Motivation Study (HIMS) program was established to provide an avenue whereby commercial pilots with active substance use disorders can be identified, treated, and successfully returned to active flight status.5 The goal of the HIMS program is to save lives and careers while enhancing flight safety. Physicians trained in HIMS evaluations follow the multifactorial addiction disease model. This evaluation is used to identify active substance use and initiate treatment, and to maintain sobriety and monitor aftercare adherence.
1. Bor R, Hubbard T. Aviation mental health: psychological implications for air transportation. Hampshire, England: Ashgate Publishing Limited; 2006.
2. US Department of Transportation Federal Aviation Administration. Medical certification. https://www.faa.gov/licenses_certificates/medical_certification/. Updated February 1, 2019. Accessed February 19, 2019.
3. US Department of Transportation Federal Aviation Administration. Summary of medical standards. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/media/synopsis.pdf. Revised April 3, 2006. Accessed October 7, 2018.
4. US Department of Transportation Federal Aviation Administration. Guide for aviation medical examiners: CACI conditions. Revised April 3, 2006. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/certification_ws/. Accessed October 8, 2018.
5. HIMS. About HIMS. http://www.himsprogram.com/Home/About. Accessed February 6, 2019.
As part of my psychiatry residency training, I had the privilege to work with and learn from an aerospace psychiatrist. Aerospace medicine is a branch of preventive and occupational medicine in which aviators (pilots, aircrew, or astronauts) are subject to evaluation/treatment. The goal is to assess physical and mental health factors to mitigate risks, protect public safety, and ensure the aviators’ well-being.1,2 Aerospace psychiatry is a highly specialized area in which practitioners are trained to perform specific evaluations. In this article, I review those evaluations for those looking to gain insight into the field.
Aviation medical examination
Under Title 14 of the Code of Federal Regulations, the Federal Aviation Administration (FAA) requires aviators to be evaluated for medical certification by undergoing an aviation medical exam.2 In order to be deemed “fit for duty,” aviators must meet strict physical and mental health standards set by the FAA. The extent of these standards varies by the class of licensure (Table 13). Aviation medical exams are performed by any physician who has been designated by the FAA and completed the appropriate FAA aviation medical examiner (AME) training. Aviators who meet the medical standards for their licensure class are recommended for medical certification. If the AME brings up further questions due to the limits of the examination and/or a lack of medical records, the certification will likely be deferred pending further evaluation by an FAA-approved medical specialist and/or the receipt of additional medical records. Questions about a possible psychiatric diagnosis/history or substance use disorder will lead to referral to a psychiatrist familiar with aviation standards for further evaluation.

_
Special issuances and Conditions AMEs Can Issue
There are 15 disqualifying conditions for medical certification (Table 13). However, a special issuance of a medical certification may be granted if the aviator shows to the satisfaction of the aviation medical examiner that the duties of the licensure class can be performed without endangering the public safety and that the condition is deemed stable. This may be shown through additional medical evaluations/tests and/or records.
There are certain medical conditions for which an AME can issue a medical certificate without further review from other specialists; thus, an AME can review and follow the Conditions AMEs Can Issue (CACI) worksheet to recommend medical certification (Table 24). The CACI guidelines and worksheets are updated by the FAA regularly to ensure aviators’ health and minimize public risk.
Psychiatric & Psychological Evaluation
Aviators may be referred for Psychiatric and Psychological Evaluation (P&P) if an AME discovers additional concerns about psychiatric and neurocognitive disorders. These cases are not clear-cut. An example would be an aviator who was receiving a psychotropic medication in the past and reported past heavy alcohol use. The P&P includes a thorough psychiatric evaluation by an aerospace psychiatrist and extensive psychological testing by an aerospace psychologist. These clinicians also review collateral information and past medical/AME records. Aviators may be recommended for medical certification with special issuance or may be denied medical certification as a result of these examinations.
Human Intervention Motivation Study program
The Human Intervention Motivation Study (HIMS) program was established to provide an avenue whereby commercial pilots with active substance use disorders can be identified, treated, and successfully returned to active flight status.5 The goal of the HIMS program is to save lives and careers while enhancing flight safety. Physicians trained in HIMS evaluations follow the multifactorial addiction disease model. This evaluation is used to identify active substance use and initiate treatment, and to maintain sobriety and monitor aftercare adherence.
As part of my psychiatry residency training, I had the privilege to work with and learn from an aerospace psychiatrist. Aerospace medicine is a branch of preventive and occupational medicine in which aviators (pilots, aircrew, or astronauts) are subject to evaluation/treatment. The goal is to assess physical and mental health factors to mitigate risks, protect public safety, and ensure the aviators’ well-being.1,2 Aerospace psychiatry is a highly specialized area in which practitioners are trained to perform specific evaluations. In this article, I review those evaluations for those looking to gain insight into the field.
Aviation medical examination
Under Title 14 of the Code of Federal Regulations, the Federal Aviation Administration (FAA) requires aviators to be evaluated for medical certification by undergoing an aviation medical exam.2 In order to be deemed “fit for duty,” aviators must meet strict physical and mental health standards set by the FAA. The extent of these standards varies by the class of licensure (Table 13). Aviation medical exams are performed by any physician who has been designated by the FAA and completed the appropriate FAA aviation medical examiner (AME) training. Aviators who meet the medical standards for their licensure class are recommended for medical certification. If the AME brings up further questions due to the limits of the examination and/or a lack of medical records, the certification will likely be deferred pending further evaluation by an FAA-approved medical specialist and/or the receipt of additional medical records. Questions about a possible psychiatric diagnosis/history or substance use disorder will lead to referral to a psychiatrist familiar with aviation standards for further evaluation.

_
Special issuances and Conditions AMEs Can Issue
There are 15 disqualifying conditions for medical certification (Table 13). However, a special issuance of a medical certification may be granted if the aviator shows to the satisfaction of the aviation medical examiner that the duties of the licensure class can be performed without endangering the public safety and that the condition is deemed stable. This may be shown through additional medical evaluations/tests and/or records.
There are certain medical conditions for which an AME can issue a medical certificate without further review from other specialists; thus, an AME can review and follow the Conditions AMEs Can Issue (CACI) worksheet to recommend medical certification (Table 24). The CACI guidelines and worksheets are updated by the FAA regularly to ensure aviators’ health and minimize public risk.
Psychiatric & Psychological Evaluation
Aviators may be referred for Psychiatric and Psychological Evaluation (P&P) if an AME discovers additional concerns about psychiatric and neurocognitive disorders. These cases are not clear-cut. An example would be an aviator who was receiving a psychotropic medication in the past and reported past heavy alcohol use. The P&P includes a thorough psychiatric evaluation by an aerospace psychiatrist and extensive psychological testing by an aerospace psychologist. These clinicians also review collateral information and past medical/AME records. Aviators may be recommended for medical certification with special issuance or may be denied medical certification as a result of these examinations.
Human Intervention Motivation Study program
The Human Intervention Motivation Study (HIMS) program was established to provide an avenue whereby commercial pilots with active substance use disorders can be identified, treated, and successfully returned to active flight status.5 The goal of the HIMS program is to save lives and careers while enhancing flight safety. Physicians trained in HIMS evaluations follow the multifactorial addiction disease model. This evaluation is used to identify active substance use and initiate treatment, and to maintain sobriety and monitor aftercare adherence.
1. Bor R, Hubbard T. Aviation mental health: psychological implications for air transportation. Hampshire, England: Ashgate Publishing Limited; 2006.
2. US Department of Transportation Federal Aviation Administration. Medical certification. https://www.faa.gov/licenses_certificates/medical_certification/. Updated February 1, 2019. Accessed February 19, 2019.
3. US Department of Transportation Federal Aviation Administration. Summary of medical standards. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/media/synopsis.pdf. Revised April 3, 2006. Accessed October 7, 2018.
4. US Department of Transportation Federal Aviation Administration. Guide for aviation medical examiners: CACI conditions. Revised April 3, 2006. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/certification_ws/. Accessed October 8, 2018.
5. HIMS. About HIMS. http://www.himsprogram.com/Home/About. Accessed February 6, 2019.
1. Bor R, Hubbard T. Aviation mental health: psychological implications for air transportation. Hampshire, England: Ashgate Publishing Limited; 2006.
2. US Department of Transportation Federal Aviation Administration. Medical certification. https://www.faa.gov/licenses_certificates/medical_certification/. Updated February 1, 2019. Accessed February 19, 2019.
3. US Department of Transportation Federal Aviation Administration. Summary of medical standards. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/media/synopsis.pdf. Revised April 3, 2006. Accessed October 7, 2018.
4. US Department of Transportation Federal Aviation Administration. Guide for aviation medical examiners: CACI conditions. Revised April 3, 2006. https://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/certification_ws/. Accessed October 8, 2018.
5. HIMS. About HIMS. http://www.himsprogram.com/Home/About. Accessed February 6, 2019.
Antipsychotics and seizures: What are the risks?
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40

Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
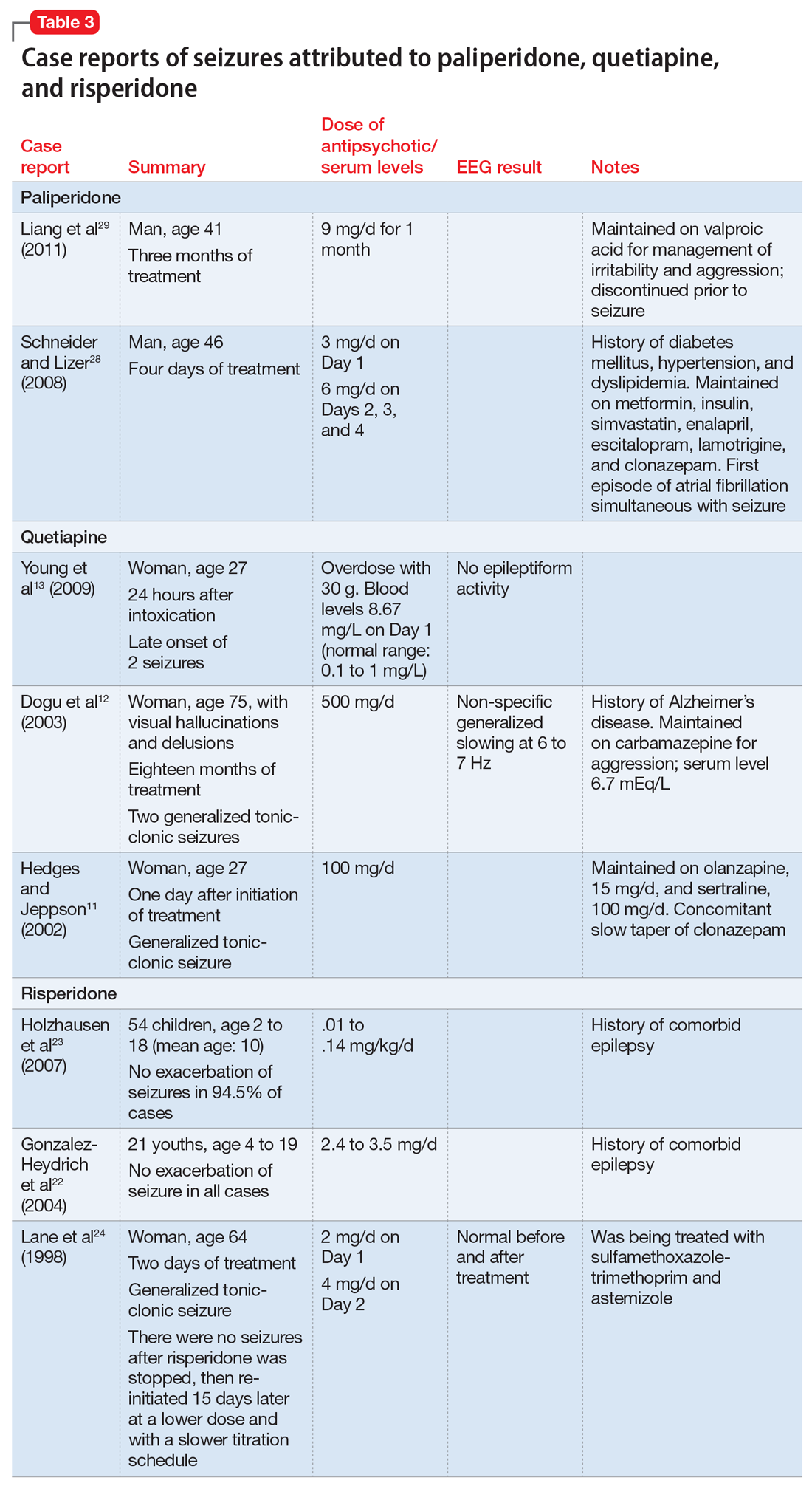
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75

Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).

A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40

Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).

Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75

Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).

A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40

Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).

Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75

Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
What’s new in transcranial magnetic stimulation
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
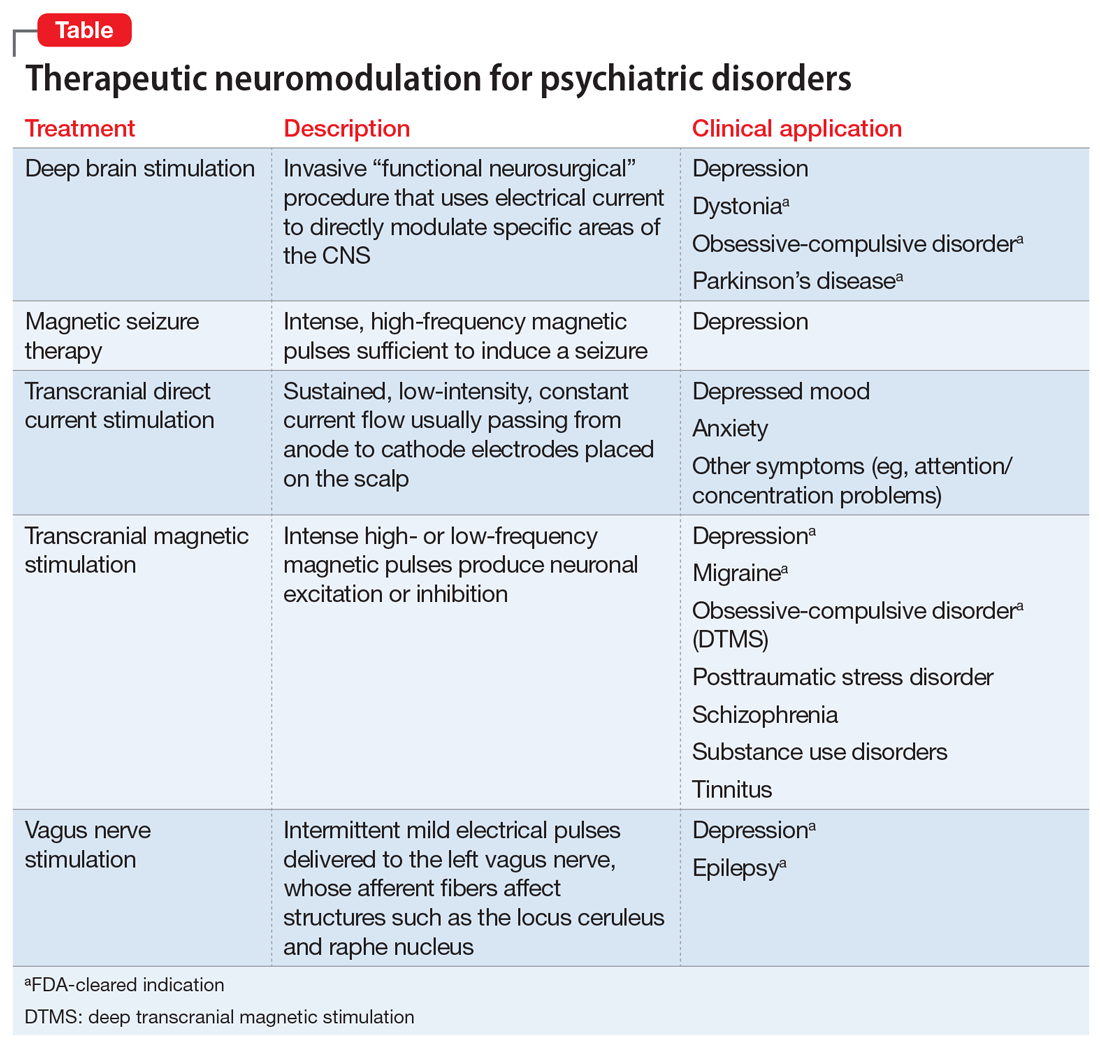
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.

Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.

Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.