User login
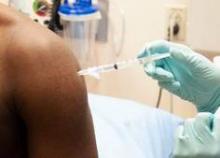
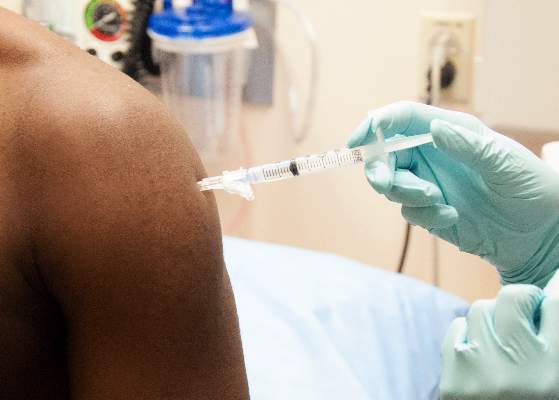
A top scientist with the World Health Organization says that the two leading Ebola vaccine candidates were close to being tested in healthy volunteers, and that if an ideal dosage can be successfully established in the coming weeks, clinical efficacy testing could begin in Africa in January.
Marie-Paule Kieny, Ph.D., WHO’s assistant director-general for health systems and innovation, told reporters at a press conference Oct. 21 that the two experimental live attenuated vaccines, one developed by GlaxoSmithKline and the other by the Canadian government, would be tested in hundreds of healthy volunteers, most of them in Europe, starting in the next 2 weeks. This round of testing involves a range of doses to determine the ideal dose for safety and immunogenicity.
Some 800 vials of the Canadian vaccine have been sent to WHO’s offices in Geneva for distribution, Dr. Kieny said, and will be forwarded to the testing sites in Germany and Switzerland within days, along with sites in Kenya and Gabon.
With both vaccines, “we should know dose levels by December,” Dr. Kieny said, “and these data will be absolutely crucial to allow decision-making in determining what dose level should go in efficacy testing in Africa allowing for trials of the vaccines in Africa to begin as soon as January.”
Clinical efficacy testing would most likely begin with people at highest risk: health care workers, burial teams, family members, and contacts of known Ebola cases. “These possible targets are being discussed now,” Dr. Kieny said.
Dr. Kieny also noted that there were additional vaccine candidates in advanced stages of development in the United States and Russia.
One Ebola treatment strategy currently being investigated involves transfusions of blood and serum of convalescent patients. In Liberia, progress in collecting and processing blood “is moving quickly,” Dr. Kieny reported. WHO is also in discussions with facilities in Guinea and Sierra Leone, the other two countries where Ebola transmission is intense, to set up blood-processing centers.
On the drug front, Dr. Kieny said that an antiviral agent developed in Japan is about to undergo efficacy testing in Guinea, which would represent the first formal clinical drug trial in this Ebola outbreak, though experimental agents have been used ad-hoc to date.
A top scientist with the World Health Organization says that the two leading Ebola vaccine candidates were close to being tested in healthy volunteers, and that if an ideal dosage can be successfully established in the coming weeks, clinical efficacy testing could begin in Africa in January.
Marie-Paule Kieny, Ph.D., WHO’s assistant director-general for health systems and innovation, told reporters at a press conference Oct. 21 that the two experimental live attenuated vaccines, one developed by GlaxoSmithKline and the other by the Canadian government, would be tested in hundreds of healthy volunteers, most of them in Europe, starting in the next 2 weeks. This round of testing involves a range of doses to determine the ideal dose for safety and immunogenicity.
Some 800 vials of the Canadian vaccine have been sent to WHO’s offices in Geneva for distribution, Dr. Kieny said, and will be forwarded to the testing sites in Germany and Switzerland within days, along with sites in Kenya and Gabon.
With both vaccines, “we should know dose levels by December,” Dr. Kieny said, “and these data will be absolutely crucial to allow decision-making in determining what dose level should go in efficacy testing in Africa allowing for trials of the vaccines in Africa to begin as soon as January.”
Clinical efficacy testing would most likely begin with people at highest risk: health care workers, burial teams, family members, and contacts of known Ebola cases. “These possible targets are being discussed now,” Dr. Kieny said.
Dr. Kieny also noted that there were additional vaccine candidates in advanced stages of development in the United States and Russia.
One Ebola treatment strategy currently being investigated involves transfusions of blood and serum of convalescent patients. In Liberia, progress in collecting and processing blood “is moving quickly,” Dr. Kieny reported. WHO is also in discussions with facilities in Guinea and Sierra Leone, the other two countries where Ebola transmission is intense, to set up blood-processing centers.
On the drug front, Dr. Kieny said that an antiviral agent developed in Japan is about to undergo efficacy testing in Guinea, which would represent the first formal clinical drug trial in this Ebola outbreak, though experimental agents have been used ad-hoc to date.
A top scientist with the World Health Organization says that the two leading Ebola vaccine candidates were close to being tested in healthy volunteers, and that if an ideal dosage can be successfully established in the coming weeks, clinical efficacy testing could begin in Africa in January.
Marie-Paule Kieny, Ph.D., WHO’s assistant director-general for health systems and innovation, told reporters at a press conference Oct. 21 that the two experimental live attenuated vaccines, one developed by GlaxoSmithKline and the other by the Canadian government, would be tested in hundreds of healthy volunteers, most of them in Europe, starting in the next 2 weeks. This round of testing involves a range of doses to determine the ideal dose for safety and immunogenicity.
Some 800 vials of the Canadian vaccine have been sent to WHO’s offices in Geneva for distribution, Dr. Kieny said, and will be forwarded to the testing sites in Germany and Switzerland within days, along with sites in Kenya and Gabon.
With both vaccines, “we should know dose levels by December,” Dr. Kieny said, “and these data will be absolutely crucial to allow decision-making in determining what dose level should go in efficacy testing in Africa allowing for trials of the vaccines in Africa to begin as soon as January.”
Clinical efficacy testing would most likely begin with people at highest risk: health care workers, burial teams, family members, and contacts of known Ebola cases. “These possible targets are being discussed now,” Dr. Kieny said.
Dr. Kieny also noted that there were additional vaccine candidates in advanced stages of development in the United States and Russia.
One Ebola treatment strategy currently being investigated involves transfusions of blood and serum of convalescent patients. In Liberia, progress in collecting and processing blood “is moving quickly,” Dr. Kieny reported. WHO is also in discussions with facilities in Guinea and Sierra Leone, the other two countries where Ebola transmission is intense, to set up blood-processing centers.
On the drug front, Dr. Kieny said that an antiviral agent developed in Japan is about to undergo efficacy testing in Guinea, which would represent the first formal clinical drug trial in this Ebola outbreak, though experimental agents have been used ad-hoc to date.