User login
Case
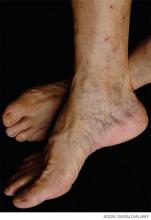
A 70-year-old woman with recently diagnosed pancreatic cancer is admitted with worsening abdominal pain and new right-lower-extremity edema. A CT scan of the abdomen and pelvis reveals occlusive IVC and common iliac thromboses. A right-common-femoral DVT appears on Doppler ultrasound. A CT angiogram of her chest is negative for pulmonary emboli. What is the appropriate treatment for this patient’s thromboembolic disease?
Overview
VTE in the setting of malignancy is common, and the incidence of VTE among cancer patients is increasing for unclear reasons.1,2,3 VTE occurs in an estimated 4% to 20% of all malignancy patients. Overall, cancer patients are at a sixfold increased risk of developing VTE compared with the general population, and these patients represent approximately 20% of all VTE cases.4,5
VTE is a leading cause of mortality among hospitalized cancer patients; evidence of VTE exists in up to 50% of cancer patients at autopsy.3,4 VTE complications lead to significant morbidity, including recurrent VTE, pulmonary hypertension, post-thrombophlebitic syndrome, bleeding, and decreased quality of life.6,7
The risk is greatest for hospitalized cancer patients and those receiving active chemotherapy, particularly with such antiangiogenic agents as thalidomide and bevacizumab.4,6 Certain malignancies are more frequently associated with VTE; these include gastrointestinal, gynecologic, brain, lung, renal, and hematologic cancers.4 Table 1 (p. 50) summarizes risk factors associated with VTE in cancer patients.4
Patients with malignancy are hypercoagulable, primarily because of tumor-related alterations in the coagulation cascade.8 Tumors contain cell-surface proteins (e.g., tissue factor and cancer procoagulant) that lead to activation of the clotting cascade via interactions with factors IX and/or X. In addition, tumor-released cytokines cause prothrombotic changes in the vascular endothelium.8
This hypercoagulability is exacerbated by cancer-associated treatment events, including immobility, central venous catheters, erythropoiesis-stimulating agents, and packed red-blood-cell and platelet transfusions.5,9
Cancer-related therapy complications further confound VTE management. Drug interactions, chemotherapy-induced thrombocytopenia, malnutrition, bleeding risk from surgery or tumor location, and liver dysfunction make safe management of cancer-associated VTE a challenge.
Review of the Data
Low-molecular-weight heparin (LMWH) vs. vitamin K antagonists (VKA): There is significant evidence that LMWH is the preferred pharmacologic therapy for the initial and long-term treatment of cancer-related VTE. The most convincing data were published as the CLOT trial, and is supported by several smaller studies.10 Recommendations for the preferred use of LMWH are consistent among several national and international guidelines.6,7
In the CLOT (Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial, six months of treatment with an oral VKA preceded by five to seven days of LMWH was compared with six months of treatment with LMWH (dalteparin). Patients with the diagnosis of acute symptomatic VTE were evaluated. After six months of treatment, 53 of 336 patients in the oral anticoagulant group had recurrent VTE, compared with 27 of 336 patients in the LMWH group—a relative risk reduction of 52% and an absolute risk reduction of 8% (P=0.002). Complication rates (major and minor bleeding) and overall mortality were similar between the two groups.7,10
Two smaller trials compared LMWHs with warfarin in the treatment of VTE in the setting of cancer. In one trial, seven of 67 enoxaparin-treated patients developed bleeding or recurrent VTE, compared with 15 of 71 warfarin-treated subjects; however, this reduction was not statistically significant (P=0.09).11 In another trial, seven of 100 tinzaparin-treated patients developed recurrent VTE, compared with 16 of 100 warfarin-treated patients (P=0.044).12
Thus, LMWH is the preferred therapeutic agent for both DVT and PE in the setting of malignancy.5 Even so, although the clinical evidence supports LMWH use in the treatment of cancer-associated VTE, it’s worth noting that many of the authors of the cited papers received compensation from large pharmaceutical companies. In addition, the CLOT trial was funded by Pharmacia, which supplied the study drug.
Initial treatment (5-10 days): The American Society of Clinical Oncology (ASCO) guidelines advocate the use of LMWH during the initial five to 10 days following VTE diagnosis. Four other cancer research organization guidelines, National Comprehensive Cancer Network (NCCN), the Italian Association of Medical Oncology (AIOM), the European Society of Medical Oncology (ESMO), and the French National Federation of the League of Centers Against Cancer (FNCLCC), echo this approach, with unfractionated heparin (UFH) or fondaparinux as additional initial treatment depending on the clinical situation—i.e., renal failure, heparin-induced thrombocytopenia (HIT), or bleeding risk.6
Table 2 (below) summarizes recommendations for VTE treatment in cancer patients from all five guideline panels.6
Long-term treatment and duration (3-6 months): All of the panels agree LMWH is the preferred agent for long-term therapy of cancer-related VTE. ASCO supports treatment with LMWH for at least six months; oral VKAs with a targeted INR of 2 to 3 are acceptable when LMWH is not feasible. NCCN, AIOM, ESMO, and FNCLCC recommend an LMWH treatment duration from three to six months (ASCO recommends at least six months).
All of the panels, however, advocate indefinite duration of anticoagulation in patients with such continuing risk factors as active (metastatic, recurrent, or persistent) cancer or chemotherapy treatment.6 AIOM and ESMO specify indefinite LMWH, whereas ASCO does not specify oral VKA or LMWH use. Given the difficulties of managing VKA in the setting of advanced malignancy or chemotherapy, LMWH is the most practical choice, if it’s available and isn’t cost-prohibitive.
LMWH availability: Dalteparin, tinzaparin, and enoxaparin are LMWHs currently available in the U.S. for VTE treatment. Dalteparin is the only FDA-approved LMWH drug for long-term use to treat cancer-associated VTE, although enoxaparin and tinzaparin are used in practice. All three are supplied in prefilled syringes, which make self-administration easier and significantly reduce the risk of dosage error.6
LMWH vs. UFH: LMWH is safer and more efficacious than UFH. A summary of 22 clinical trials (including cancer patients and noncancer patients) by a Cochrane systematic review showed that treatment with LMWH in comparison with UFH results in decreased recurrent VTE, bleeding, mortality, HIT, and osteoporosis.7,13
Other agents: Fondaparinux is a factor Xa inhibitor rarely associated with heparin-induced thrombocytopenia (HIT), which makes it an attractive alternate anticoagulant for HIT patients. Its use in comparison with other parenteral anticoagulants has not been established in patients with malignancy.
Fondaparinux is FDA-approved for initial VTE treatment, and is used in practice as an alternative prophylactic anticoagulant for patients with HIT history or heparin allergy.7 However, no randomized clinical trials have evaluated its efficacy in patients with HIT.
Several new oral anticoagulants, including direct thrombin and factor Xa inhibitors, are being investigated; efficacy has not been established in cancer patients.7
Inferior vena cava (IVC) filters: Limited data surround the use of IVC filters, and most consensus guidelines recommend their use only in specific settings. One randomized controlled study (not limited to patients with cancer) showed no difference in overall short-term or long-term survival among patients receiving IVC filters for VTE treatment. However, there was a decrease in symptomatic PE in the filter group 12 days after placement. The tradeoff was significantly more recurrent DVTs at two years’ followup. The authors concluded that systematic use of IVC filters is not recommended.14
Because all of the study participants in this trial were receiving concomitant anticoagulation, it is difficult to generalize results to patients who have contraindications to anticoagulation. Nonetheless, ASCO, NCCN, and several other guideline panels recommend IVC filters only for patients with contraindications to anticoagulation or failure of long-term anticoagulation. NCCN broadens these recommendations for IVC filter placement to include patients with severe cardiac or pulmonary dysfunction.6 However, this recommendation is controversial, given the lack of supporting data, cost, and invasiveness of the procedure.7
All patients with IVC filters without contraindication to anticoagulation should be anticoagulated.9
Prophylaxis: The importance of VTE prophylaxis in all hospitalized patients, including patients with cancer, is under close scrutiny in an attempt to improve patient safety and quality. The Centers for Medicare & Medicaid Services (CMS), in conjunction with the Joint Commission, has added VTE to its list of conditions that are reasonably preventable, and hospitals will no longer be reimbursed for the cost of treatment if acquired during a hospital stay. This rule currently is in effect for knee and hip replacements.15
The need for improvement in aggressive and appropriate prophylaxis of hospitalized patients was demonstrated by a single study in which just one-third of hospitalized patients received appropriate prophylaxis, according to American College of Chest Physicians (ACCP) guidelines.16
Despite a lack of robust data regarding VTE prophylaxis in patients with malignancy, strong data exist for VTE prophylaxis in hospitalized patients. All patients with cancer, and without contraindications to anticoagulation, should receive pharmacologic VTE prophylaxis while hospitalized. In the absence of supporting data, guidelines do not suggest VTE prophylaxis for ambulatory patients with cancer, except in certain multiple myeloma patients.6
Intracranial malignancies: Patients with intracranial malignancies are at risk for VTE, and the presence of intracranial tumors or metastases are not absolute contraindications to anticoagulation. However, data are sparse regarding VTE therapy in this subgroup. Active intracranial bleeding is an absolute contraindication to anticoagulation, and it should be avoided in patients with recent intracranial surgery or thrombocytopenia (e.g., platelet count < 50 x 109).4 In general, the presence of intracranial lesions without high-risk features should not deter the use of prophylactic anticoagulation.
Thrombocytopenia: Cancer patients frequently have chemotherapy-induced cytopenias, including thrombocytopenia. There is a paucity of data regarding anticoagulation in the setting of thrombocytopenia. In a recent review of VTE in the setting of cancer, Lee suggests dose reduction of LMWH by half in patients with platelet counts between 20 and 50 x 109/L.7 At our institution, hematologists suggest halving treatment-dose LMWH at platelet counts between 30 and 50 x 109/L, and discontinuing pharmacologic prophylaxis at platelet counts of less than 50 x 109/L—although there are little data to support these recommendations. Anticoagulation should be discontinued in patients with platelet counts below 20-30 x 109/L, as the risk of bleeding becomes too high.7
Thrombolytic therapy: Catheter-directed thrombolysis or systemic thrombolytic therapy might be appropriate treatment options for acute VTE. Presence of extensive proximal DVT, symptom duration of less than two weeks, life expectancy of greater than one year, good functional status, and low bleeding risk are prerequisites. Anticoagulation recommendations do not change following thrombolytic therapy.17
In the setting of PE, hemodynamic instability is the primary indication for thrombolysis. ACCP does not recommend thrombolytic therapy for the majority of VTE patients.17
Back to the Case
Our patient’s lower-extremity ultrasound and CT of the abdomen and pelvis both revealed extensive clot burden, which likely is related to her underlying pancreatic cancer. She should be started on an LMWH. Given the low likelihood of cure from her cancer, anticoagulation should be continued indefinitely, and an LMWH should be given for at least the first six months. Whether she should be switched to an oral VKA after six months depends on several factors: cost considerations, nutritional status, chemotherapy, presence of cytopenias, and bleeding risk.
IVC filter placement is not indicated at this time, as the patient does not have any contraindications to anticoagulation, has not failed anticoagulation, and does not have impending cardiac or pulmonary compromise. She is not a candidate for thrombolytic therapy due to her poor functional status and limited life expectancy.
Bottom Line
Cancer-associated VTE is increasingly common, and optimally is treated with LMWH for at least six months—indefinitely in the setting of active cancer or treatment. IVC filters have limited and specific indications in the setting of cancer-associated VTE, and should be reserved for situations when anticoagulation is contraindicated. TH
Dr. Weaver is an instructor in the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago. Dr. Barsuk is an assistant professor in the division of hospital medicine at Feinberg.
References
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10): 2339-2346.
- Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olsen RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006(1);119:60-68.
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634.
- Lyman GA, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490-5505.
- Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27(29):4821-4826.
- Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27(29):4919-4926.
- Lee, AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27(29):4895-4901.
- Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27(29):4902-4911.
- Prandoni, P. How I treat venous thromboembolism in patients with cancer. Blood. 2005;106(13):4027-4033.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729-1735.
- Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062-1072.
- Van Dongen CJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004:18(4);CD001100.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7): 409-415.
- Amin AN, Deitelzweig SB. Optimizing the prevention of venous thromboembolism: recent quality initiatives and strategies to drive improvement. Jt Comm J Qual Patient Saf. 2009;35(11):558-564.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost. 2007;5(8):1610-1616.
- Hirsch J, Guyatt G, Albers W, et al. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed.). Chest. 2008:133(6 Suppl);71S-105S.
Case
A 70-year-old woman with recently diagnosed pancreatic cancer is admitted with worsening abdominal pain and new right-lower-extremity edema. A CT scan of the abdomen and pelvis reveals occlusive IVC and common iliac thromboses. A right-common-femoral DVT appears on Doppler ultrasound. A CT angiogram of her chest is negative for pulmonary emboli. What is the appropriate treatment for this patient’s thromboembolic disease?
Overview
VTE in the setting of malignancy is common, and the incidence of VTE among cancer patients is increasing for unclear reasons.1,2,3 VTE occurs in an estimated 4% to 20% of all malignancy patients. Overall, cancer patients are at a sixfold increased risk of developing VTE compared with the general population, and these patients represent approximately 20% of all VTE cases.4,5
VTE is a leading cause of mortality among hospitalized cancer patients; evidence of VTE exists in up to 50% of cancer patients at autopsy.3,4 VTE complications lead to significant morbidity, including recurrent VTE, pulmonary hypertension, post-thrombophlebitic syndrome, bleeding, and decreased quality of life.6,7
The risk is greatest for hospitalized cancer patients and those receiving active chemotherapy, particularly with such antiangiogenic agents as thalidomide and bevacizumab.4,6 Certain malignancies are more frequently associated with VTE; these include gastrointestinal, gynecologic, brain, lung, renal, and hematologic cancers.4 Table 1 (p. 50) summarizes risk factors associated with VTE in cancer patients.4
Patients with malignancy are hypercoagulable, primarily because of tumor-related alterations in the coagulation cascade.8 Tumors contain cell-surface proteins (e.g., tissue factor and cancer procoagulant) that lead to activation of the clotting cascade via interactions with factors IX and/or X. In addition, tumor-released cytokines cause prothrombotic changes in the vascular endothelium.8
This hypercoagulability is exacerbated by cancer-associated treatment events, including immobility, central venous catheters, erythropoiesis-stimulating agents, and packed red-blood-cell and platelet transfusions.5,9
Cancer-related therapy complications further confound VTE management. Drug interactions, chemotherapy-induced thrombocytopenia, malnutrition, bleeding risk from surgery or tumor location, and liver dysfunction make safe management of cancer-associated VTE a challenge.
Review of the Data
Low-molecular-weight heparin (LMWH) vs. vitamin K antagonists (VKA): There is significant evidence that LMWH is the preferred pharmacologic therapy for the initial and long-term treatment of cancer-related VTE. The most convincing data were published as the CLOT trial, and is supported by several smaller studies.10 Recommendations for the preferred use of LMWH are consistent among several national and international guidelines.6,7
In the CLOT (Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial, six months of treatment with an oral VKA preceded by five to seven days of LMWH was compared with six months of treatment with LMWH (dalteparin). Patients with the diagnosis of acute symptomatic VTE were evaluated. After six months of treatment, 53 of 336 patients in the oral anticoagulant group had recurrent VTE, compared with 27 of 336 patients in the LMWH group—a relative risk reduction of 52% and an absolute risk reduction of 8% (P=0.002). Complication rates (major and minor bleeding) and overall mortality were similar between the two groups.7,10
Two smaller trials compared LMWHs with warfarin in the treatment of VTE in the setting of cancer. In one trial, seven of 67 enoxaparin-treated patients developed bleeding or recurrent VTE, compared with 15 of 71 warfarin-treated subjects; however, this reduction was not statistically significant (P=0.09).11 In another trial, seven of 100 tinzaparin-treated patients developed recurrent VTE, compared with 16 of 100 warfarin-treated patients (P=0.044).12
Thus, LMWH is the preferred therapeutic agent for both DVT and PE in the setting of malignancy.5 Even so, although the clinical evidence supports LMWH use in the treatment of cancer-associated VTE, it’s worth noting that many of the authors of the cited papers received compensation from large pharmaceutical companies. In addition, the CLOT trial was funded by Pharmacia, which supplied the study drug.
Initial treatment (5-10 days): The American Society of Clinical Oncology (ASCO) guidelines advocate the use of LMWH during the initial five to 10 days following VTE diagnosis. Four other cancer research organization guidelines, National Comprehensive Cancer Network (NCCN), the Italian Association of Medical Oncology (AIOM), the European Society of Medical Oncology (ESMO), and the French National Federation of the League of Centers Against Cancer (FNCLCC), echo this approach, with unfractionated heparin (UFH) or fondaparinux as additional initial treatment depending on the clinical situation—i.e., renal failure, heparin-induced thrombocytopenia (HIT), or bleeding risk.6
Table 2 (below) summarizes recommendations for VTE treatment in cancer patients from all five guideline panels.6
Long-term treatment and duration (3-6 months): All of the panels agree LMWH is the preferred agent for long-term therapy of cancer-related VTE. ASCO supports treatment with LMWH for at least six months; oral VKAs with a targeted INR of 2 to 3 are acceptable when LMWH is not feasible. NCCN, AIOM, ESMO, and FNCLCC recommend an LMWH treatment duration from three to six months (ASCO recommends at least six months).
All of the panels, however, advocate indefinite duration of anticoagulation in patients with such continuing risk factors as active (metastatic, recurrent, or persistent) cancer or chemotherapy treatment.6 AIOM and ESMO specify indefinite LMWH, whereas ASCO does not specify oral VKA or LMWH use. Given the difficulties of managing VKA in the setting of advanced malignancy or chemotherapy, LMWH is the most practical choice, if it’s available and isn’t cost-prohibitive.
LMWH availability: Dalteparin, tinzaparin, and enoxaparin are LMWHs currently available in the U.S. for VTE treatment. Dalteparin is the only FDA-approved LMWH drug for long-term use to treat cancer-associated VTE, although enoxaparin and tinzaparin are used in practice. All three are supplied in prefilled syringes, which make self-administration easier and significantly reduce the risk of dosage error.6
LMWH vs. UFH: LMWH is safer and more efficacious than UFH. A summary of 22 clinical trials (including cancer patients and noncancer patients) by a Cochrane systematic review showed that treatment with LMWH in comparison with UFH results in decreased recurrent VTE, bleeding, mortality, HIT, and osteoporosis.7,13
Other agents: Fondaparinux is a factor Xa inhibitor rarely associated with heparin-induced thrombocytopenia (HIT), which makes it an attractive alternate anticoagulant for HIT patients. Its use in comparison with other parenteral anticoagulants has not been established in patients with malignancy.
Fondaparinux is FDA-approved for initial VTE treatment, and is used in practice as an alternative prophylactic anticoagulant for patients with HIT history or heparin allergy.7 However, no randomized clinical trials have evaluated its efficacy in patients with HIT.
Several new oral anticoagulants, including direct thrombin and factor Xa inhibitors, are being investigated; efficacy has not been established in cancer patients.7
Inferior vena cava (IVC) filters: Limited data surround the use of IVC filters, and most consensus guidelines recommend their use only in specific settings. One randomized controlled study (not limited to patients with cancer) showed no difference in overall short-term or long-term survival among patients receiving IVC filters for VTE treatment. However, there was a decrease in symptomatic PE in the filter group 12 days after placement. The tradeoff was significantly more recurrent DVTs at two years’ followup. The authors concluded that systematic use of IVC filters is not recommended.14
Because all of the study participants in this trial were receiving concomitant anticoagulation, it is difficult to generalize results to patients who have contraindications to anticoagulation. Nonetheless, ASCO, NCCN, and several other guideline panels recommend IVC filters only for patients with contraindications to anticoagulation or failure of long-term anticoagulation. NCCN broadens these recommendations for IVC filter placement to include patients with severe cardiac or pulmonary dysfunction.6 However, this recommendation is controversial, given the lack of supporting data, cost, and invasiveness of the procedure.7
All patients with IVC filters without contraindication to anticoagulation should be anticoagulated.9
Prophylaxis: The importance of VTE prophylaxis in all hospitalized patients, including patients with cancer, is under close scrutiny in an attempt to improve patient safety and quality. The Centers for Medicare & Medicaid Services (CMS), in conjunction with the Joint Commission, has added VTE to its list of conditions that are reasonably preventable, and hospitals will no longer be reimbursed for the cost of treatment if acquired during a hospital stay. This rule currently is in effect for knee and hip replacements.15
The need for improvement in aggressive and appropriate prophylaxis of hospitalized patients was demonstrated by a single study in which just one-third of hospitalized patients received appropriate prophylaxis, according to American College of Chest Physicians (ACCP) guidelines.16
Despite a lack of robust data regarding VTE prophylaxis in patients with malignancy, strong data exist for VTE prophylaxis in hospitalized patients. All patients with cancer, and without contraindications to anticoagulation, should receive pharmacologic VTE prophylaxis while hospitalized. In the absence of supporting data, guidelines do not suggest VTE prophylaxis for ambulatory patients with cancer, except in certain multiple myeloma patients.6
Intracranial malignancies: Patients with intracranial malignancies are at risk for VTE, and the presence of intracranial tumors or metastases are not absolute contraindications to anticoagulation. However, data are sparse regarding VTE therapy in this subgroup. Active intracranial bleeding is an absolute contraindication to anticoagulation, and it should be avoided in patients with recent intracranial surgery or thrombocytopenia (e.g., platelet count < 50 x 109).4 In general, the presence of intracranial lesions without high-risk features should not deter the use of prophylactic anticoagulation.
Thrombocytopenia: Cancer patients frequently have chemotherapy-induced cytopenias, including thrombocytopenia. There is a paucity of data regarding anticoagulation in the setting of thrombocytopenia. In a recent review of VTE in the setting of cancer, Lee suggests dose reduction of LMWH by half in patients with platelet counts between 20 and 50 x 109/L.7 At our institution, hematologists suggest halving treatment-dose LMWH at platelet counts between 30 and 50 x 109/L, and discontinuing pharmacologic prophylaxis at platelet counts of less than 50 x 109/L—although there are little data to support these recommendations. Anticoagulation should be discontinued in patients with platelet counts below 20-30 x 109/L, as the risk of bleeding becomes too high.7
Thrombolytic therapy: Catheter-directed thrombolysis or systemic thrombolytic therapy might be appropriate treatment options for acute VTE. Presence of extensive proximal DVT, symptom duration of less than two weeks, life expectancy of greater than one year, good functional status, and low bleeding risk are prerequisites. Anticoagulation recommendations do not change following thrombolytic therapy.17
In the setting of PE, hemodynamic instability is the primary indication for thrombolysis. ACCP does not recommend thrombolytic therapy for the majority of VTE patients.17
Back to the Case
Our patient’s lower-extremity ultrasound and CT of the abdomen and pelvis both revealed extensive clot burden, which likely is related to her underlying pancreatic cancer. She should be started on an LMWH. Given the low likelihood of cure from her cancer, anticoagulation should be continued indefinitely, and an LMWH should be given for at least the first six months. Whether she should be switched to an oral VKA after six months depends on several factors: cost considerations, nutritional status, chemotherapy, presence of cytopenias, and bleeding risk.
IVC filter placement is not indicated at this time, as the patient does not have any contraindications to anticoagulation, has not failed anticoagulation, and does not have impending cardiac or pulmonary compromise. She is not a candidate for thrombolytic therapy due to her poor functional status and limited life expectancy.
Bottom Line
Cancer-associated VTE is increasingly common, and optimally is treated with LMWH for at least six months—indefinitely in the setting of active cancer or treatment. IVC filters have limited and specific indications in the setting of cancer-associated VTE, and should be reserved for situations when anticoagulation is contraindicated. TH
Dr. Weaver is an instructor in the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago. Dr. Barsuk is an assistant professor in the division of hospital medicine at Feinberg.
References
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10): 2339-2346.
- Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olsen RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006(1);119:60-68.
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634.
- Lyman GA, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490-5505.
- Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27(29):4821-4826.
- Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27(29):4919-4926.
- Lee, AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27(29):4895-4901.
- Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27(29):4902-4911.
- Prandoni, P. How I treat venous thromboembolism in patients with cancer. Blood. 2005;106(13):4027-4033.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729-1735.
- Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062-1072.
- Van Dongen CJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004:18(4);CD001100.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7): 409-415.
- Amin AN, Deitelzweig SB. Optimizing the prevention of venous thromboembolism: recent quality initiatives and strategies to drive improvement. Jt Comm J Qual Patient Saf. 2009;35(11):558-564.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost. 2007;5(8):1610-1616.
- Hirsch J, Guyatt G, Albers W, et al. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed.). Chest. 2008:133(6 Suppl);71S-105S.
Case
A 70-year-old woman with recently diagnosed pancreatic cancer is admitted with worsening abdominal pain and new right-lower-extremity edema. A CT scan of the abdomen and pelvis reveals occlusive IVC and common iliac thromboses. A right-common-femoral DVT appears on Doppler ultrasound. A CT angiogram of her chest is negative for pulmonary emboli. What is the appropriate treatment for this patient’s thromboembolic disease?
Overview
VTE in the setting of malignancy is common, and the incidence of VTE among cancer patients is increasing for unclear reasons.1,2,3 VTE occurs in an estimated 4% to 20% of all malignancy patients. Overall, cancer patients are at a sixfold increased risk of developing VTE compared with the general population, and these patients represent approximately 20% of all VTE cases.4,5
VTE is a leading cause of mortality among hospitalized cancer patients; evidence of VTE exists in up to 50% of cancer patients at autopsy.3,4 VTE complications lead to significant morbidity, including recurrent VTE, pulmonary hypertension, post-thrombophlebitic syndrome, bleeding, and decreased quality of life.6,7
The risk is greatest for hospitalized cancer patients and those receiving active chemotherapy, particularly with such antiangiogenic agents as thalidomide and bevacizumab.4,6 Certain malignancies are more frequently associated with VTE; these include gastrointestinal, gynecologic, brain, lung, renal, and hematologic cancers.4 Table 1 (p. 50) summarizes risk factors associated with VTE in cancer patients.4
Patients with malignancy are hypercoagulable, primarily because of tumor-related alterations in the coagulation cascade.8 Tumors contain cell-surface proteins (e.g., tissue factor and cancer procoagulant) that lead to activation of the clotting cascade via interactions with factors IX and/or X. In addition, tumor-released cytokines cause prothrombotic changes in the vascular endothelium.8
This hypercoagulability is exacerbated by cancer-associated treatment events, including immobility, central venous catheters, erythropoiesis-stimulating agents, and packed red-blood-cell and platelet transfusions.5,9
Cancer-related therapy complications further confound VTE management. Drug interactions, chemotherapy-induced thrombocytopenia, malnutrition, bleeding risk from surgery or tumor location, and liver dysfunction make safe management of cancer-associated VTE a challenge.
Review of the Data
Low-molecular-weight heparin (LMWH) vs. vitamin K antagonists (VKA): There is significant evidence that LMWH is the preferred pharmacologic therapy for the initial and long-term treatment of cancer-related VTE. The most convincing data were published as the CLOT trial, and is supported by several smaller studies.10 Recommendations for the preferred use of LMWH are consistent among several national and international guidelines.6,7
In the CLOT (Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial, six months of treatment with an oral VKA preceded by five to seven days of LMWH was compared with six months of treatment with LMWH (dalteparin). Patients with the diagnosis of acute symptomatic VTE were evaluated. After six months of treatment, 53 of 336 patients in the oral anticoagulant group had recurrent VTE, compared with 27 of 336 patients in the LMWH group—a relative risk reduction of 52% and an absolute risk reduction of 8% (P=0.002). Complication rates (major and minor bleeding) and overall mortality were similar between the two groups.7,10
Two smaller trials compared LMWHs with warfarin in the treatment of VTE in the setting of cancer. In one trial, seven of 67 enoxaparin-treated patients developed bleeding or recurrent VTE, compared with 15 of 71 warfarin-treated subjects; however, this reduction was not statistically significant (P=0.09).11 In another trial, seven of 100 tinzaparin-treated patients developed recurrent VTE, compared with 16 of 100 warfarin-treated patients (P=0.044).12
Thus, LMWH is the preferred therapeutic agent for both DVT and PE in the setting of malignancy.5 Even so, although the clinical evidence supports LMWH use in the treatment of cancer-associated VTE, it’s worth noting that many of the authors of the cited papers received compensation from large pharmaceutical companies. In addition, the CLOT trial was funded by Pharmacia, which supplied the study drug.
Initial treatment (5-10 days): The American Society of Clinical Oncology (ASCO) guidelines advocate the use of LMWH during the initial five to 10 days following VTE diagnosis. Four other cancer research organization guidelines, National Comprehensive Cancer Network (NCCN), the Italian Association of Medical Oncology (AIOM), the European Society of Medical Oncology (ESMO), and the French National Federation of the League of Centers Against Cancer (FNCLCC), echo this approach, with unfractionated heparin (UFH) or fondaparinux as additional initial treatment depending on the clinical situation—i.e., renal failure, heparin-induced thrombocytopenia (HIT), or bleeding risk.6
Table 2 (below) summarizes recommendations for VTE treatment in cancer patients from all five guideline panels.6
Long-term treatment and duration (3-6 months): All of the panels agree LMWH is the preferred agent for long-term therapy of cancer-related VTE. ASCO supports treatment with LMWH for at least six months; oral VKAs with a targeted INR of 2 to 3 are acceptable when LMWH is not feasible. NCCN, AIOM, ESMO, and FNCLCC recommend an LMWH treatment duration from three to six months (ASCO recommends at least six months).
All of the panels, however, advocate indefinite duration of anticoagulation in patients with such continuing risk factors as active (metastatic, recurrent, or persistent) cancer or chemotherapy treatment.6 AIOM and ESMO specify indefinite LMWH, whereas ASCO does not specify oral VKA or LMWH use. Given the difficulties of managing VKA in the setting of advanced malignancy or chemotherapy, LMWH is the most practical choice, if it’s available and isn’t cost-prohibitive.
LMWH availability: Dalteparin, tinzaparin, and enoxaparin are LMWHs currently available in the U.S. for VTE treatment. Dalteparin is the only FDA-approved LMWH drug for long-term use to treat cancer-associated VTE, although enoxaparin and tinzaparin are used in practice. All three are supplied in prefilled syringes, which make self-administration easier and significantly reduce the risk of dosage error.6
LMWH vs. UFH: LMWH is safer and more efficacious than UFH. A summary of 22 clinical trials (including cancer patients and noncancer patients) by a Cochrane systematic review showed that treatment with LMWH in comparison with UFH results in decreased recurrent VTE, bleeding, mortality, HIT, and osteoporosis.7,13
Other agents: Fondaparinux is a factor Xa inhibitor rarely associated with heparin-induced thrombocytopenia (HIT), which makes it an attractive alternate anticoagulant for HIT patients. Its use in comparison with other parenteral anticoagulants has not been established in patients with malignancy.
Fondaparinux is FDA-approved for initial VTE treatment, and is used in practice as an alternative prophylactic anticoagulant for patients with HIT history or heparin allergy.7 However, no randomized clinical trials have evaluated its efficacy in patients with HIT.
Several new oral anticoagulants, including direct thrombin and factor Xa inhibitors, are being investigated; efficacy has not been established in cancer patients.7
Inferior vena cava (IVC) filters: Limited data surround the use of IVC filters, and most consensus guidelines recommend their use only in specific settings. One randomized controlled study (not limited to patients with cancer) showed no difference in overall short-term or long-term survival among patients receiving IVC filters for VTE treatment. However, there was a decrease in symptomatic PE in the filter group 12 days after placement. The tradeoff was significantly more recurrent DVTs at two years’ followup. The authors concluded that systematic use of IVC filters is not recommended.14
Because all of the study participants in this trial were receiving concomitant anticoagulation, it is difficult to generalize results to patients who have contraindications to anticoagulation. Nonetheless, ASCO, NCCN, and several other guideline panels recommend IVC filters only for patients with contraindications to anticoagulation or failure of long-term anticoagulation. NCCN broadens these recommendations for IVC filter placement to include patients with severe cardiac or pulmonary dysfunction.6 However, this recommendation is controversial, given the lack of supporting data, cost, and invasiveness of the procedure.7
All patients with IVC filters without contraindication to anticoagulation should be anticoagulated.9
Prophylaxis: The importance of VTE prophylaxis in all hospitalized patients, including patients with cancer, is under close scrutiny in an attempt to improve patient safety and quality. The Centers for Medicare & Medicaid Services (CMS), in conjunction with the Joint Commission, has added VTE to its list of conditions that are reasonably preventable, and hospitals will no longer be reimbursed for the cost of treatment if acquired during a hospital stay. This rule currently is in effect for knee and hip replacements.15
The need for improvement in aggressive and appropriate prophylaxis of hospitalized patients was demonstrated by a single study in which just one-third of hospitalized patients received appropriate prophylaxis, according to American College of Chest Physicians (ACCP) guidelines.16
Despite a lack of robust data regarding VTE prophylaxis in patients with malignancy, strong data exist for VTE prophylaxis in hospitalized patients. All patients with cancer, and without contraindications to anticoagulation, should receive pharmacologic VTE prophylaxis while hospitalized. In the absence of supporting data, guidelines do not suggest VTE prophylaxis for ambulatory patients with cancer, except in certain multiple myeloma patients.6
Intracranial malignancies: Patients with intracranial malignancies are at risk for VTE, and the presence of intracranial tumors or metastases are not absolute contraindications to anticoagulation. However, data are sparse regarding VTE therapy in this subgroup. Active intracranial bleeding is an absolute contraindication to anticoagulation, and it should be avoided in patients with recent intracranial surgery or thrombocytopenia (e.g., platelet count < 50 x 109).4 In general, the presence of intracranial lesions without high-risk features should not deter the use of prophylactic anticoagulation.
Thrombocytopenia: Cancer patients frequently have chemotherapy-induced cytopenias, including thrombocytopenia. There is a paucity of data regarding anticoagulation in the setting of thrombocytopenia. In a recent review of VTE in the setting of cancer, Lee suggests dose reduction of LMWH by half in patients with platelet counts between 20 and 50 x 109/L.7 At our institution, hematologists suggest halving treatment-dose LMWH at platelet counts between 30 and 50 x 109/L, and discontinuing pharmacologic prophylaxis at platelet counts of less than 50 x 109/L—although there are little data to support these recommendations. Anticoagulation should be discontinued in patients with platelet counts below 20-30 x 109/L, as the risk of bleeding becomes too high.7
Thrombolytic therapy: Catheter-directed thrombolysis or systemic thrombolytic therapy might be appropriate treatment options for acute VTE. Presence of extensive proximal DVT, symptom duration of less than two weeks, life expectancy of greater than one year, good functional status, and low bleeding risk are prerequisites. Anticoagulation recommendations do not change following thrombolytic therapy.17
In the setting of PE, hemodynamic instability is the primary indication for thrombolysis. ACCP does not recommend thrombolytic therapy for the majority of VTE patients.17
Back to the Case
Our patient’s lower-extremity ultrasound and CT of the abdomen and pelvis both revealed extensive clot burden, which likely is related to her underlying pancreatic cancer. She should be started on an LMWH. Given the low likelihood of cure from her cancer, anticoagulation should be continued indefinitely, and an LMWH should be given for at least the first six months. Whether she should be switched to an oral VKA after six months depends on several factors: cost considerations, nutritional status, chemotherapy, presence of cytopenias, and bleeding risk.
IVC filter placement is not indicated at this time, as the patient does not have any contraindications to anticoagulation, has not failed anticoagulation, and does not have impending cardiac or pulmonary compromise. She is not a candidate for thrombolytic therapy due to her poor functional status and limited life expectancy.
Bottom Line
Cancer-associated VTE is increasingly common, and optimally is treated with LMWH for at least six months—indefinitely in the setting of active cancer or treatment. IVC filters have limited and specific indications in the setting of cancer-associated VTE, and should be reserved for situations when anticoagulation is contraindicated. TH
Dr. Weaver is an instructor in the division of hospital medicine at Northwestern University’s Feinberg School of Medicine in Chicago. Dr. Barsuk is an assistant professor in the division of hospital medicine at Feinberg.
References
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10): 2339-2346.
- Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olsen RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006(1);119:60-68.
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634.
- Lyman GA, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490-5505.
- Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009;27(29):4821-4826.
- Khorana AA, Streiff MB, Farge D, et al. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27(29):4919-4926.
- Lee, AY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27(29):4895-4901.
- Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27(29):4902-4911.
- Prandoni, P. How I treat venous thromboembolism in patients with cancer. Blood. 2005;106(13):4027-4033.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729-1735.
- Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062-1072.
- Van Dongen CJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004:18(4);CD001100.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7): 409-415.
- Amin AN, Deitelzweig SB. Optimizing the prevention of venous thromboembolism: recent quality initiatives and strategies to drive improvement. Jt Comm J Qual Patient Saf. 2009;35(11):558-564.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost. 2007;5(8):1610-1616.
- Hirsch J, Guyatt G, Albers W, et al. Executive summary: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed.). Chest. 2008:133(6 Suppl);71S-105S.