User login
Cervical stenosis and difficult uterine and vaginal anatomy pose a challenge for the gynecologist who needs access to the cervix and uterus to evaluate pathology. Overcoming this hurdle requires a careful, considered approach to avoid the complications of dilation, such as laceration, creation of a false passage, uterine perforation, and failed procedures. Care and consideration also ensure a successful and comfortable procedure; save the patient a great deal of time and the higher expense of the operating room (OR); and avert the need for general anesthesia.
In this first Update on Minimally Invasive Surgery, I will:
- describe the continuing shift from the OR to office for many gynecologic procedures
- review recent data on cervical softening
- outline the components of mechanical dilation
- offer tips on pain relief.
Need for cervical access should not prohibit office-based procedures
Cervical access is critical to increase the percentage of procedures performed in the office setting. The office has long been the ideal environment for minor procedures such as endometrial biopsy, dilation and curettage, diagnostic hysteroscopy, hysterosonography, and insertion of an intrauterine device—but difficulty traversing the cervix has relegated many of these procedures to the OR.
Minor procedures such as tubal sterilization and endometrial ablation have begun to move from the outpatient environment into the office as well, upping the number of office procedures that require safe access to the endometrial cavity.
For example, hysteroscopic tubal occlusion (Essure) is performed transcervically, thereby eliminating all incisions and the need for general anesthesia. Approximately 50% of all Essure sterilization procedures performed in the United States today are done in an office, and that percentage is expected to rise to 60% in 2009.1
The smallest operative hysteroscopes that allow for placement of Essure coils have an outer-sheath diameter between 5 and 6 mm. Even with such small diameters, cervical dilation is sometimes needed.
Endometrial ablation offers women who have menorrhagia a minimally invasive option for treatment. Several FDA-approved devices are used safely in the office.2-5 Cervical dilation requirements for these devices range from 5 to 7.8 mm, making cervical access paramount (TABLE).
A number of measures, such as cervical softening and mechanical dilation, can ease dilation in an office setting so that a stenotic cervix no longer requires an OR for the procedure to be completed. Successful in-office cervical dilation also greatly reduces cost.
TABLE
Size of the instrument varies across endometrial ablation systems
| Instrument | Diameter | Instrument | Diameter |
|---|---|---|---|
| ThermaChoice (uterine balloon therapy) | 5 mm | NovaSure | 7.2 mm |
| Her Option (cryoablation therapy) | 5 mm | Hydro ThermAblator | 7.8 mm |
New data back efficacy of vaginal misoprostol for cervical softening
da Costa AR, Pinto-Neto AM, Amorim M, Paiva LH, Scavuzzi A, Schettini J. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Minim Invasive Gynecol. 2008;15:67–73.
Waddell G, Desindes S, Takser L, Bequchemin M, Bessett P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15:739–744.
Uckuyu A, Ozcimen E, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. J Minim Invasive Gynecol. 2008;15:472–475.
Valente EP, Amorim MM, da Costa AR, de Miranda VD. Vaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trial. J Minim Invasive Gynecol. 2008;15:452–458.
A synthetic analog of prostaglandin E1, misoprostol is thought to act on the extracellular matrix of the cervix, leading to water absorption, neutrophil collagenase release, and cervical softening. Smooth muscle is activated by the drug, especially in the uterus.
Pharmacokinetic studies suggest that the oral route of misoprostol has the shortest interval to peak serum concentration (within 30 minutes of ingestion), but that concentration declines within 1 hour. The vaginal route, on the other hand, has fewer side effects, with longer duration and approximately three times the bioavailability.6,7 Peak values are equal to those of orally administered misoprostol. They are attained at 60 minutes, then decline slowly, reaching 50% of peak values by 240 minutes. Serum concentration remains elevated, improving efficacy.
I recommend an interval of 4 to 12 hours between vaginal placement and the start of the procedure.
Vaginal route is clearly effective in premenopausal women
In premenopausal women, several recent randomized clinical trials show that vaginal misoprostol, administered before hysteroscopy, not only decreases pain and the force and amount of dilation needed, but also reduces complications of cervical dilation.8 As Uckuyu and colleagues observe, these findings are consistent in nulliparous women who have a history of cesarean delivery and who receive 400 μg of vaginal misoprostol 6 to 12 hours before hysteroscopy.
Several studies found no improvement in ease of dilation or operative time in menopausal women who received misoprostol before hysteroscopy.9,10 However, in their randomized, placebo-controlled trial, da Costa and colleagues found that women who received 200 μg of vaginal misoprostol 8 hours before hysteroscopy had a significant decrease in intraprocedural pain associated with cervical dilation.
Recommended protocol
For women at significant risk of cervical stenosis, give 400 μg of intravaginal misoprostol approximately 12 hours before the scheduled procedure. The patient should begin round-the-clock use of a nonsteroidal anti-inflammatory drug (NSAID) an hour before insertion of the misoprostol tablets.
Side effects of vaginal and oral misoprostol include occasional diarrhea, abdominal cramping, uterine bleeding, and pyrexia. These effects are usually mild and limited.8 Concomitant administration of NSAIDs reduces or eliminates these side effects.
Although you may sometimes find an incompletely dissolved tablet within the vagina, active medication usually has been absorbed, leaving the less soluble vehicle behind.
Frequent causes of stenosis include:
- Loop electrosurgical excision procedure, conization, and laser vaporization—In one series, 43% of cases of cervical stenosis resulted from one of these procedures, with a recurrence rate of 14%.11
- Scarring of the external os—Common in the parous cervix. Usually, only minimal dilation is needed; the remainder of the cervix is traversed easily.
- Narrow or closed external os—Common in menopausal women and increasingly common in nulligravid and nulliparous women as the rate of elective cesarean delivery rises. In these cases, both the endocervical canal and internal os are narrow, necessitating dilation through the entire length of the cervix.
- Genital atrophy—In postmenopausal women, cervical stenosis as a result of genital atrophy is associated with pain upon cervical dilation. The situation generally necessitates local anesthesia.12
When mechanical dilation is necessary, a few prerequisites can make a difference
By assessing each patient carefully, the gynecologist can customize the intervention. Accordingly, it is wise to have multiple types of dilators accessible to accommodate varying clinical needs and anatomic scenarios.
Begin by stabilizing the cervix. Use of a single-toothed tenaculum, placed on the anterior lip of the cervix, has several advantages. Countertraction against the dilating instrument can facilitate more controlled placement of the dilator, preventing perforation of the uterus. This maneuver is especially useful when the cervical canal and internal os are tight or resistant.
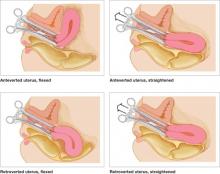
Use of a tenaculum when placing a hysteroscope produces a similar result and can add an element of safety to uterine access, especially when the uterus is significantly flexed (FIGURE 1).
FIGURE 1 Straighten a flexed uterus before dilating the cervix
When the uterus is flexed, creating acute angulation at the cervicouterine junction, it can be difficult to move the dilator through the internal os. By placing a tenaculum at the 12 o’clock position on the cervix of an anteverted uterus, or 6 o’clock on a retroverted uterus, and applying outward traction, you can straighten the distorted canal.
A local anesthetic can help
It is helpful to administer a local anesthetic before placing a penetrating instrument such as the tenaculum. Patients are usually grateful for the extra few minutes taken to ensure their comfort.
If the cervix is resistant, or the patient is uncomfortable, after initial attempts to dilate the cervix, consider placing a paracervical or intracervical stromal local anesthetic. Lidocaine 1% has a rapid onset of action, reaching peak effectiveness in just a few minutes, with a duration of approximately 60 minutes. Bupivacaine 0.25% has a slightly slower onset of action (8–10 minutes), but offers long duration—about 240 minutes.
Place 5 to 10 cc of local anesthetic paracervically at the 4 and 8 o’clock positions. The choice of anesthetic depends on the procedure. For example, a nulligravid patient may benefit from bupivacaine because cervical dilation can cause significant and prolonged cramping. Bupivacaine is more potent than lidocaine and, therefore, potentially more cardiotoxic, so caution is advised.
Used correctly, local anesthetic facilitates cervical dilation.
Types of dilators
Many instruments are available. Be familiar with the benefits and shortcomings of each to achieve successful cervical dilation in the office.
Lacrimal-duct dilators. These instruments have long been used by gynecologists for cervical access when the closed external os is no more than a tiny dimple. These ophthalmologic instruments come in diameters smaller than 1 mm and allow the closed menopausal cervix to be dilated enough to allow placement of more traditional instruments (FIGURE 2).
Traditional dilators. Tapered metal or plastic dilators facilitate access to a tightly stenotic external os (FIGURE 3). These instruments have a more symmetrical segment proximal to the tip to allow gentle, gradual dilation of the canal. However, when dilation is needed for the entire length of the cervix, as it frequently is in nulliparous women, the tapered end will not dilate the internal os unless it is passed far enough into the canal—but passing the instrument too far increases the risk of uterine perforation. In this scenario, a symmetric dilator may be a better option to achieve uniform diameter of the endocervical canal from external to internal os. Be cautious: These symmetric dilators sometimes require additional force.
When the entire length of the cervix needs to be dilated, it may be wise to use a symmetric dilator that is the same size as the tapered dilator before increasing the diameter, to reduce the risk of perforation.
FIGURE 2 A borrowed tool to unlock the external os
From the world of ophthalmology, a lacrimal-duct dilator facilitates dilation of the extremely stenotic cervix.
FIGURE 3 Tapered instruments allow gradual dilation
Tapered cervical dilators, like this disposable set, have a more symmetrical segment proximal to the tip.
When every dilator is too large
Occasionally, the external os is so completely closed that even a lacrimal-duct dilator cannot be placed. In this situation, administer 2 cc of local anesthetic in the region of the external os, then gently penetrate the os using the tip of a #11 scalpel blade. This technique generally allows quick and easy access to the endocervical canal. (Typically, the entire canal will not be stenotic and, once the external opening is created, easy dilation can be accomplished.)
Navigating a crooked cervical canal
When the cervical canal is distorted, sometimes even the most carefully selected dilator will fail.
Why?
Because the diameter of the cervix is not the obstacle.
When a straight—or even partially curved—dilator cannot traverse the tortuous path of a distorted canal, the small flexible hysteroscope may offer a solution, allowing identification of the anatomic obstruction and visualization of the course of the cervical canal. Prior visualization of the canal enhances proprioception with the dilating instrument and improves the likelihood of safe uterine access.
The use of concomitant ultrasonography may also be helpful.11
From the standpoint of a payer, obtaining surgical access to the site of a procedure is included in the procedure payment. This is generally the rule for surgical access on the day of the procedure. Some payers reimburse separately, however, when access (specifically, dilation of the cervix) is performed a day, or few days, before the procedure because of an anatomic problem—such as the cervical stenosis discussed in the main “Update” article.
What are your options?
You have several coding options available for dilation of the cervix, depending on the approach you take:
- When you’ve given oral misoprostol, bill the visit at which you prescribed the drug.
- When you’ve inserted misoprostol vaginally, report code 59200 [insertion of cervical dilator (e.g., laminaria, prostaglandin) (separate procedure)].
The fact that 59200 is found in the “Maternity Care and Delivery” chapter of the CPT does not limit its use to obstetric cases. Because this code has a zero-day global period, you are not considered to be in the postoperative period of the first procedure when the surgical procedure is performed the next day (or even longer afterward), and the two procedures will not be bundled.
Another method of cervical dilation is represented by code 57800 [Dilation of cervical canal, instrumental (separate procedure)], which also has a zero-day global period.
The diagnosis code must support the service
That’s true whichever technique you choose. In this case, the diagnosis code will be either:
- 622.4 (Stricture and stenosis of cervix) or
- 752.49 (Other anomalies of cervix, vagina, and external female genitalia)—when the stenosis is the result of a congenital condition.—
MELANIE WITT, RN, CPC, COBGC, MA
1. Essure Earnings Report Fourth Quarter 2008. Mountain View, Calif: Conceptus Inc.
2. Farrugia M, Hussain S. Hysteroscopic endometrial ablation using the Hydro ThermAblator in an outpatient hysteroscopy clinic: feasibility and acceptability. J Minim Invasive Gynecol. 2006;13:178-182.
3. Fernandez H, Capella S, Audiebert F. Uterine thermal balloon therapy under local anesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511-2514.
4. Bertrand J. Use of NovaSure endometrial ablation system in an office setting environment. J Minim Invasive Gynecol. 2005;12(5 Suppl):14.-
5. Roy K, Whiteside D, Manjon J, et al. Assessment of procedure tolerability during Her Option endometrial cryoablation in an outpatient setting. J Minim Invasive Gynecol. 2005;12(5 Suppl):87.-
6. Khan RU, El-Rafeay H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866-869.
7. Zieman M, Fong S, Benowitz N, Bankster D, Darney P. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
8. Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94:427-430.
9. Fung TM, Lam MW, Wong SF, Ho LC. A randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. BJOG. 2002;119:561-565.
10. Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in postmenopausal women. Hum Reprod. 2001;16:1486-1488.
11. Valle RF, Sankpal R, Marlow J, Cohen L. Cervical stenosis: a challenging clinical entity. J Gynecol Surg. 2002;18:129-143.
12. Perez-Medina T, Bajo MJ, Martinez-Cortes L, Castellanos P, Perez de Avila I. Six thousand office diagnostic-operative hysteroscopies. Int J Obstet Gynecol. 2000;71:33-38.
Cervical stenosis and difficult uterine and vaginal anatomy pose a challenge for the gynecologist who needs access to the cervix and uterus to evaluate pathology. Overcoming this hurdle requires a careful, considered approach to avoid the complications of dilation, such as laceration, creation of a false passage, uterine perforation, and failed procedures. Care and consideration also ensure a successful and comfortable procedure; save the patient a great deal of time and the higher expense of the operating room (OR); and avert the need for general anesthesia.
In this first Update on Minimally Invasive Surgery, I will:
- describe the continuing shift from the OR to office for many gynecologic procedures
- review recent data on cervical softening
- outline the components of mechanical dilation
- offer tips on pain relief.
Need for cervical access should not prohibit office-based procedures
Cervical access is critical to increase the percentage of procedures performed in the office setting. The office has long been the ideal environment for minor procedures such as endometrial biopsy, dilation and curettage, diagnostic hysteroscopy, hysterosonography, and insertion of an intrauterine device—but difficulty traversing the cervix has relegated many of these procedures to the OR.
Minor procedures such as tubal sterilization and endometrial ablation have begun to move from the outpatient environment into the office as well, upping the number of office procedures that require safe access to the endometrial cavity.
For example, hysteroscopic tubal occlusion (Essure) is performed transcervically, thereby eliminating all incisions and the need for general anesthesia. Approximately 50% of all Essure sterilization procedures performed in the United States today are done in an office, and that percentage is expected to rise to 60% in 2009.1
The smallest operative hysteroscopes that allow for placement of Essure coils have an outer-sheath diameter between 5 and 6 mm. Even with such small diameters, cervical dilation is sometimes needed.
Endometrial ablation offers women who have menorrhagia a minimally invasive option for treatment. Several FDA-approved devices are used safely in the office.2-5 Cervical dilation requirements for these devices range from 5 to 7.8 mm, making cervical access paramount (TABLE).
A number of measures, such as cervical softening and mechanical dilation, can ease dilation in an office setting so that a stenotic cervix no longer requires an OR for the procedure to be completed. Successful in-office cervical dilation also greatly reduces cost.
TABLE
Size of the instrument varies across endometrial ablation systems
| Instrument | Diameter | Instrument | Diameter |
|---|---|---|---|
| ThermaChoice (uterine balloon therapy) | 5 mm | NovaSure | 7.2 mm |
| Her Option (cryoablation therapy) | 5 mm | Hydro ThermAblator | 7.8 mm |
New data back efficacy of vaginal misoprostol for cervical softening
da Costa AR, Pinto-Neto AM, Amorim M, Paiva LH, Scavuzzi A, Schettini J. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Minim Invasive Gynecol. 2008;15:67–73.
Waddell G, Desindes S, Takser L, Bequchemin M, Bessett P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15:739–744.
Uckuyu A, Ozcimen E, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. J Minim Invasive Gynecol. 2008;15:472–475.
Valente EP, Amorim MM, da Costa AR, de Miranda VD. Vaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trial. J Minim Invasive Gynecol. 2008;15:452–458.
A synthetic analog of prostaglandin E1, misoprostol is thought to act on the extracellular matrix of the cervix, leading to water absorption, neutrophil collagenase release, and cervical softening. Smooth muscle is activated by the drug, especially in the uterus.
Pharmacokinetic studies suggest that the oral route of misoprostol has the shortest interval to peak serum concentration (within 30 minutes of ingestion), but that concentration declines within 1 hour. The vaginal route, on the other hand, has fewer side effects, with longer duration and approximately three times the bioavailability.6,7 Peak values are equal to those of orally administered misoprostol. They are attained at 60 minutes, then decline slowly, reaching 50% of peak values by 240 minutes. Serum concentration remains elevated, improving efficacy.
I recommend an interval of 4 to 12 hours between vaginal placement and the start of the procedure.
Vaginal route is clearly effective in premenopausal women
In premenopausal women, several recent randomized clinical trials show that vaginal misoprostol, administered before hysteroscopy, not only decreases pain and the force and amount of dilation needed, but also reduces complications of cervical dilation.8 As Uckuyu and colleagues observe, these findings are consistent in nulliparous women who have a history of cesarean delivery and who receive 400 μg of vaginal misoprostol 6 to 12 hours before hysteroscopy.
Several studies found no improvement in ease of dilation or operative time in menopausal women who received misoprostol before hysteroscopy.9,10 However, in their randomized, placebo-controlled trial, da Costa and colleagues found that women who received 200 μg of vaginal misoprostol 8 hours before hysteroscopy had a significant decrease in intraprocedural pain associated with cervical dilation.
Recommended protocol
For women at significant risk of cervical stenosis, give 400 μg of intravaginal misoprostol approximately 12 hours before the scheduled procedure. The patient should begin round-the-clock use of a nonsteroidal anti-inflammatory drug (NSAID) an hour before insertion of the misoprostol tablets.
Side effects of vaginal and oral misoprostol include occasional diarrhea, abdominal cramping, uterine bleeding, and pyrexia. These effects are usually mild and limited.8 Concomitant administration of NSAIDs reduces or eliminates these side effects.
Although you may sometimes find an incompletely dissolved tablet within the vagina, active medication usually has been absorbed, leaving the less soluble vehicle behind.
Frequent causes of stenosis include:
- Loop electrosurgical excision procedure, conization, and laser vaporization—In one series, 43% of cases of cervical stenosis resulted from one of these procedures, with a recurrence rate of 14%.11
- Scarring of the external os—Common in the parous cervix. Usually, only minimal dilation is needed; the remainder of the cervix is traversed easily.
- Narrow or closed external os—Common in menopausal women and increasingly common in nulligravid and nulliparous women as the rate of elective cesarean delivery rises. In these cases, both the endocervical canal and internal os are narrow, necessitating dilation through the entire length of the cervix.
- Genital atrophy—In postmenopausal women, cervical stenosis as a result of genital atrophy is associated with pain upon cervical dilation. The situation generally necessitates local anesthesia.12
When mechanical dilation is necessary, a few prerequisites can make a difference
By assessing each patient carefully, the gynecologist can customize the intervention. Accordingly, it is wise to have multiple types of dilators accessible to accommodate varying clinical needs and anatomic scenarios.
Begin by stabilizing the cervix. Use of a single-toothed tenaculum, placed on the anterior lip of the cervix, has several advantages. Countertraction against the dilating instrument can facilitate more controlled placement of the dilator, preventing perforation of the uterus. This maneuver is especially useful when the cervical canal and internal os are tight or resistant.
Use of a tenaculum when placing a hysteroscope produces a similar result and can add an element of safety to uterine access, especially when the uterus is significantly flexed (FIGURE 1).
FIGURE 1 Straighten a flexed uterus before dilating the cervix
When the uterus is flexed, creating acute angulation at the cervicouterine junction, it can be difficult to move the dilator through the internal os. By placing a tenaculum at the 12 o’clock position on the cervix of an anteverted uterus, or 6 o’clock on a retroverted uterus, and applying outward traction, you can straighten the distorted canal.
A local anesthetic can help
It is helpful to administer a local anesthetic before placing a penetrating instrument such as the tenaculum. Patients are usually grateful for the extra few minutes taken to ensure their comfort.
If the cervix is resistant, or the patient is uncomfortable, after initial attempts to dilate the cervix, consider placing a paracervical or intracervical stromal local anesthetic. Lidocaine 1% has a rapid onset of action, reaching peak effectiveness in just a few minutes, with a duration of approximately 60 minutes. Bupivacaine 0.25% has a slightly slower onset of action (8–10 minutes), but offers long duration—about 240 minutes.
Place 5 to 10 cc of local anesthetic paracervically at the 4 and 8 o’clock positions. The choice of anesthetic depends on the procedure. For example, a nulligravid patient may benefit from bupivacaine because cervical dilation can cause significant and prolonged cramping. Bupivacaine is more potent than lidocaine and, therefore, potentially more cardiotoxic, so caution is advised.
Used correctly, local anesthetic facilitates cervical dilation.
Types of dilators
Many instruments are available. Be familiar with the benefits and shortcomings of each to achieve successful cervical dilation in the office.
Lacrimal-duct dilators. These instruments have long been used by gynecologists for cervical access when the closed external os is no more than a tiny dimple. These ophthalmologic instruments come in diameters smaller than 1 mm and allow the closed menopausal cervix to be dilated enough to allow placement of more traditional instruments (FIGURE 2).
Traditional dilators. Tapered metal or plastic dilators facilitate access to a tightly stenotic external os (FIGURE 3). These instruments have a more symmetrical segment proximal to the tip to allow gentle, gradual dilation of the canal. However, when dilation is needed for the entire length of the cervix, as it frequently is in nulliparous women, the tapered end will not dilate the internal os unless it is passed far enough into the canal—but passing the instrument too far increases the risk of uterine perforation. In this scenario, a symmetric dilator may be a better option to achieve uniform diameter of the endocervical canal from external to internal os. Be cautious: These symmetric dilators sometimes require additional force.
When the entire length of the cervix needs to be dilated, it may be wise to use a symmetric dilator that is the same size as the tapered dilator before increasing the diameter, to reduce the risk of perforation.
FIGURE 2 A borrowed tool to unlock the external os
From the world of ophthalmology, a lacrimal-duct dilator facilitates dilation of the extremely stenotic cervix.
FIGURE 3 Tapered instruments allow gradual dilation
Tapered cervical dilators, like this disposable set, have a more symmetrical segment proximal to the tip.
When every dilator is too large
Occasionally, the external os is so completely closed that even a lacrimal-duct dilator cannot be placed. In this situation, administer 2 cc of local anesthetic in the region of the external os, then gently penetrate the os using the tip of a #11 scalpel blade. This technique generally allows quick and easy access to the endocervical canal. (Typically, the entire canal will not be stenotic and, once the external opening is created, easy dilation can be accomplished.)
Navigating a crooked cervical canal
When the cervical canal is distorted, sometimes even the most carefully selected dilator will fail.
Why?
Because the diameter of the cervix is not the obstacle.
When a straight—or even partially curved—dilator cannot traverse the tortuous path of a distorted canal, the small flexible hysteroscope may offer a solution, allowing identification of the anatomic obstruction and visualization of the course of the cervical canal. Prior visualization of the canal enhances proprioception with the dilating instrument and improves the likelihood of safe uterine access.
The use of concomitant ultrasonography may also be helpful.11
From the standpoint of a payer, obtaining surgical access to the site of a procedure is included in the procedure payment. This is generally the rule for surgical access on the day of the procedure. Some payers reimburse separately, however, when access (specifically, dilation of the cervix) is performed a day, or few days, before the procedure because of an anatomic problem—such as the cervical stenosis discussed in the main “Update” article.
What are your options?
You have several coding options available for dilation of the cervix, depending on the approach you take:
- When you’ve given oral misoprostol, bill the visit at which you prescribed the drug.
- When you’ve inserted misoprostol vaginally, report code 59200 [insertion of cervical dilator (e.g., laminaria, prostaglandin) (separate procedure)].
The fact that 59200 is found in the “Maternity Care and Delivery” chapter of the CPT does not limit its use to obstetric cases. Because this code has a zero-day global period, you are not considered to be in the postoperative period of the first procedure when the surgical procedure is performed the next day (or even longer afterward), and the two procedures will not be bundled.
Another method of cervical dilation is represented by code 57800 [Dilation of cervical canal, instrumental (separate procedure)], which also has a zero-day global period.
The diagnosis code must support the service
That’s true whichever technique you choose. In this case, the diagnosis code will be either:
- 622.4 (Stricture and stenosis of cervix) or
- 752.49 (Other anomalies of cervix, vagina, and external female genitalia)—when the stenosis is the result of a congenital condition.—
MELANIE WITT, RN, CPC, COBGC, MA
Cervical stenosis and difficult uterine and vaginal anatomy pose a challenge for the gynecologist who needs access to the cervix and uterus to evaluate pathology. Overcoming this hurdle requires a careful, considered approach to avoid the complications of dilation, such as laceration, creation of a false passage, uterine perforation, and failed procedures. Care and consideration also ensure a successful and comfortable procedure; save the patient a great deal of time and the higher expense of the operating room (OR); and avert the need for general anesthesia.
In this first Update on Minimally Invasive Surgery, I will:
- describe the continuing shift from the OR to office for many gynecologic procedures
- review recent data on cervical softening
- outline the components of mechanical dilation
- offer tips on pain relief.
Need for cervical access should not prohibit office-based procedures
Cervical access is critical to increase the percentage of procedures performed in the office setting. The office has long been the ideal environment for minor procedures such as endometrial biopsy, dilation and curettage, diagnostic hysteroscopy, hysterosonography, and insertion of an intrauterine device—but difficulty traversing the cervix has relegated many of these procedures to the OR.
Minor procedures such as tubal sterilization and endometrial ablation have begun to move from the outpatient environment into the office as well, upping the number of office procedures that require safe access to the endometrial cavity.
For example, hysteroscopic tubal occlusion (Essure) is performed transcervically, thereby eliminating all incisions and the need for general anesthesia. Approximately 50% of all Essure sterilization procedures performed in the United States today are done in an office, and that percentage is expected to rise to 60% in 2009.1
The smallest operative hysteroscopes that allow for placement of Essure coils have an outer-sheath diameter between 5 and 6 mm. Even with such small diameters, cervical dilation is sometimes needed.
Endometrial ablation offers women who have menorrhagia a minimally invasive option for treatment. Several FDA-approved devices are used safely in the office.2-5 Cervical dilation requirements for these devices range from 5 to 7.8 mm, making cervical access paramount (TABLE).
A number of measures, such as cervical softening and mechanical dilation, can ease dilation in an office setting so that a stenotic cervix no longer requires an OR for the procedure to be completed. Successful in-office cervical dilation also greatly reduces cost.
TABLE
Size of the instrument varies across endometrial ablation systems
| Instrument | Diameter | Instrument | Diameter |
|---|---|---|---|
| ThermaChoice (uterine balloon therapy) | 5 mm | NovaSure | 7.2 mm |
| Her Option (cryoablation therapy) | 5 mm | Hydro ThermAblator | 7.8 mm |
New data back efficacy of vaginal misoprostol for cervical softening
da Costa AR, Pinto-Neto AM, Amorim M, Paiva LH, Scavuzzi A, Schettini J. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Minim Invasive Gynecol. 2008;15:67–73.
Waddell G, Desindes S, Takser L, Bequchemin M, Bessett P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15:739–744.
Uckuyu A, Ozcimen E, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. J Minim Invasive Gynecol. 2008;15:472–475.
Valente EP, Amorim MM, da Costa AR, de Miranda VD. Vaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trial. J Minim Invasive Gynecol. 2008;15:452–458.
A synthetic analog of prostaglandin E1, misoprostol is thought to act on the extracellular matrix of the cervix, leading to water absorption, neutrophil collagenase release, and cervical softening. Smooth muscle is activated by the drug, especially in the uterus.
Pharmacokinetic studies suggest that the oral route of misoprostol has the shortest interval to peak serum concentration (within 30 minutes of ingestion), but that concentration declines within 1 hour. The vaginal route, on the other hand, has fewer side effects, with longer duration and approximately three times the bioavailability.6,7 Peak values are equal to those of orally administered misoprostol. They are attained at 60 minutes, then decline slowly, reaching 50% of peak values by 240 minutes. Serum concentration remains elevated, improving efficacy.
I recommend an interval of 4 to 12 hours between vaginal placement and the start of the procedure.
Vaginal route is clearly effective in premenopausal women
In premenopausal women, several recent randomized clinical trials show that vaginal misoprostol, administered before hysteroscopy, not only decreases pain and the force and amount of dilation needed, but also reduces complications of cervical dilation.8 As Uckuyu and colleagues observe, these findings are consistent in nulliparous women who have a history of cesarean delivery and who receive 400 μg of vaginal misoprostol 6 to 12 hours before hysteroscopy.
Several studies found no improvement in ease of dilation or operative time in menopausal women who received misoprostol before hysteroscopy.9,10 However, in their randomized, placebo-controlled trial, da Costa and colleagues found that women who received 200 μg of vaginal misoprostol 8 hours before hysteroscopy had a significant decrease in intraprocedural pain associated with cervical dilation.
Recommended protocol
For women at significant risk of cervical stenosis, give 400 μg of intravaginal misoprostol approximately 12 hours before the scheduled procedure. The patient should begin round-the-clock use of a nonsteroidal anti-inflammatory drug (NSAID) an hour before insertion of the misoprostol tablets.
Side effects of vaginal and oral misoprostol include occasional diarrhea, abdominal cramping, uterine bleeding, and pyrexia. These effects are usually mild and limited.8 Concomitant administration of NSAIDs reduces or eliminates these side effects.
Although you may sometimes find an incompletely dissolved tablet within the vagina, active medication usually has been absorbed, leaving the less soluble vehicle behind.
Frequent causes of stenosis include:
- Loop electrosurgical excision procedure, conization, and laser vaporization—In one series, 43% of cases of cervical stenosis resulted from one of these procedures, with a recurrence rate of 14%.11
- Scarring of the external os—Common in the parous cervix. Usually, only minimal dilation is needed; the remainder of the cervix is traversed easily.
- Narrow or closed external os—Common in menopausal women and increasingly common in nulligravid and nulliparous women as the rate of elective cesarean delivery rises. In these cases, both the endocervical canal and internal os are narrow, necessitating dilation through the entire length of the cervix.
- Genital atrophy—In postmenopausal women, cervical stenosis as a result of genital atrophy is associated with pain upon cervical dilation. The situation generally necessitates local anesthesia.12
When mechanical dilation is necessary, a few prerequisites can make a difference
By assessing each patient carefully, the gynecologist can customize the intervention. Accordingly, it is wise to have multiple types of dilators accessible to accommodate varying clinical needs and anatomic scenarios.
Begin by stabilizing the cervix. Use of a single-toothed tenaculum, placed on the anterior lip of the cervix, has several advantages. Countertraction against the dilating instrument can facilitate more controlled placement of the dilator, preventing perforation of the uterus. This maneuver is especially useful when the cervical canal and internal os are tight or resistant.
Use of a tenaculum when placing a hysteroscope produces a similar result and can add an element of safety to uterine access, especially when the uterus is significantly flexed (FIGURE 1).
FIGURE 1 Straighten a flexed uterus before dilating the cervix
When the uterus is flexed, creating acute angulation at the cervicouterine junction, it can be difficult to move the dilator through the internal os. By placing a tenaculum at the 12 o’clock position on the cervix of an anteverted uterus, or 6 o’clock on a retroverted uterus, and applying outward traction, you can straighten the distorted canal.
A local anesthetic can help
It is helpful to administer a local anesthetic before placing a penetrating instrument such as the tenaculum. Patients are usually grateful for the extra few minutes taken to ensure their comfort.
If the cervix is resistant, or the patient is uncomfortable, after initial attempts to dilate the cervix, consider placing a paracervical or intracervical stromal local anesthetic. Lidocaine 1% has a rapid onset of action, reaching peak effectiveness in just a few minutes, with a duration of approximately 60 minutes. Bupivacaine 0.25% has a slightly slower onset of action (8–10 minutes), but offers long duration—about 240 minutes.
Place 5 to 10 cc of local anesthetic paracervically at the 4 and 8 o’clock positions. The choice of anesthetic depends on the procedure. For example, a nulligravid patient may benefit from bupivacaine because cervical dilation can cause significant and prolonged cramping. Bupivacaine is more potent than lidocaine and, therefore, potentially more cardiotoxic, so caution is advised.
Used correctly, local anesthetic facilitates cervical dilation.
Types of dilators
Many instruments are available. Be familiar with the benefits and shortcomings of each to achieve successful cervical dilation in the office.
Lacrimal-duct dilators. These instruments have long been used by gynecologists for cervical access when the closed external os is no more than a tiny dimple. These ophthalmologic instruments come in diameters smaller than 1 mm and allow the closed menopausal cervix to be dilated enough to allow placement of more traditional instruments (FIGURE 2).
Traditional dilators. Tapered metal or plastic dilators facilitate access to a tightly stenotic external os (FIGURE 3). These instruments have a more symmetrical segment proximal to the tip to allow gentle, gradual dilation of the canal. However, when dilation is needed for the entire length of the cervix, as it frequently is in nulliparous women, the tapered end will not dilate the internal os unless it is passed far enough into the canal—but passing the instrument too far increases the risk of uterine perforation. In this scenario, a symmetric dilator may be a better option to achieve uniform diameter of the endocervical canal from external to internal os. Be cautious: These symmetric dilators sometimes require additional force.
When the entire length of the cervix needs to be dilated, it may be wise to use a symmetric dilator that is the same size as the tapered dilator before increasing the diameter, to reduce the risk of perforation.
FIGURE 2 A borrowed tool to unlock the external os
From the world of ophthalmology, a lacrimal-duct dilator facilitates dilation of the extremely stenotic cervix.
FIGURE 3 Tapered instruments allow gradual dilation
Tapered cervical dilators, like this disposable set, have a more symmetrical segment proximal to the tip.
When every dilator is too large
Occasionally, the external os is so completely closed that even a lacrimal-duct dilator cannot be placed. In this situation, administer 2 cc of local anesthetic in the region of the external os, then gently penetrate the os using the tip of a #11 scalpel blade. This technique generally allows quick and easy access to the endocervical canal. (Typically, the entire canal will not be stenotic and, once the external opening is created, easy dilation can be accomplished.)
Navigating a crooked cervical canal
When the cervical canal is distorted, sometimes even the most carefully selected dilator will fail.
Why?
Because the diameter of the cervix is not the obstacle.
When a straight—or even partially curved—dilator cannot traverse the tortuous path of a distorted canal, the small flexible hysteroscope may offer a solution, allowing identification of the anatomic obstruction and visualization of the course of the cervical canal. Prior visualization of the canal enhances proprioception with the dilating instrument and improves the likelihood of safe uterine access.
The use of concomitant ultrasonography may also be helpful.11
From the standpoint of a payer, obtaining surgical access to the site of a procedure is included in the procedure payment. This is generally the rule for surgical access on the day of the procedure. Some payers reimburse separately, however, when access (specifically, dilation of the cervix) is performed a day, or few days, before the procedure because of an anatomic problem—such as the cervical stenosis discussed in the main “Update” article.
What are your options?
You have several coding options available for dilation of the cervix, depending on the approach you take:
- When you’ve given oral misoprostol, bill the visit at which you prescribed the drug.
- When you’ve inserted misoprostol vaginally, report code 59200 [insertion of cervical dilator (e.g., laminaria, prostaglandin) (separate procedure)].
The fact that 59200 is found in the “Maternity Care and Delivery” chapter of the CPT does not limit its use to obstetric cases. Because this code has a zero-day global period, you are not considered to be in the postoperative period of the first procedure when the surgical procedure is performed the next day (or even longer afterward), and the two procedures will not be bundled.
Another method of cervical dilation is represented by code 57800 [Dilation of cervical canal, instrumental (separate procedure)], which also has a zero-day global period.
The diagnosis code must support the service
That’s true whichever technique you choose. In this case, the diagnosis code will be either:
- 622.4 (Stricture and stenosis of cervix) or
- 752.49 (Other anomalies of cervix, vagina, and external female genitalia)—when the stenosis is the result of a congenital condition.—
MELANIE WITT, RN, CPC, COBGC, MA
1. Essure Earnings Report Fourth Quarter 2008. Mountain View, Calif: Conceptus Inc.
2. Farrugia M, Hussain S. Hysteroscopic endometrial ablation using the Hydro ThermAblator in an outpatient hysteroscopy clinic: feasibility and acceptability. J Minim Invasive Gynecol. 2006;13:178-182.
3. Fernandez H, Capella S, Audiebert F. Uterine thermal balloon therapy under local anesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511-2514.
4. Bertrand J. Use of NovaSure endometrial ablation system in an office setting environment. J Minim Invasive Gynecol. 2005;12(5 Suppl):14.-
5. Roy K, Whiteside D, Manjon J, et al. Assessment of procedure tolerability during Her Option endometrial cryoablation in an outpatient setting. J Minim Invasive Gynecol. 2005;12(5 Suppl):87.-
6. Khan RU, El-Rafeay H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866-869.
7. Zieman M, Fong S, Benowitz N, Bankster D, Darney P. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
8. Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94:427-430.
9. Fung TM, Lam MW, Wong SF, Ho LC. A randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. BJOG. 2002;119:561-565.
10. Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in postmenopausal women. Hum Reprod. 2001;16:1486-1488.
11. Valle RF, Sankpal R, Marlow J, Cohen L. Cervical stenosis: a challenging clinical entity. J Gynecol Surg. 2002;18:129-143.
12. Perez-Medina T, Bajo MJ, Martinez-Cortes L, Castellanos P, Perez de Avila I. Six thousand office diagnostic-operative hysteroscopies. Int J Obstet Gynecol. 2000;71:33-38.
1. Essure Earnings Report Fourth Quarter 2008. Mountain View, Calif: Conceptus Inc.
2. Farrugia M, Hussain S. Hysteroscopic endometrial ablation using the Hydro ThermAblator in an outpatient hysteroscopy clinic: feasibility and acceptability. J Minim Invasive Gynecol. 2006;13:178-182.
3. Fernandez H, Capella S, Audiebert F. Uterine thermal balloon therapy under local anesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511-2514.
4. Bertrand J. Use of NovaSure endometrial ablation system in an office setting environment. J Minim Invasive Gynecol. 2005;12(5 Suppl):14.-
5. Roy K, Whiteside D, Manjon J, et al. Assessment of procedure tolerability during Her Option endometrial cryoablation in an outpatient setting. J Minim Invasive Gynecol. 2005;12(5 Suppl):87.-
6. Khan RU, El-Rafeay H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866-869.
7. Zieman M, Fong S, Benowitz N, Bankster D, Darney P. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
8. Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94:427-430.
9. Fung TM, Lam MW, Wong SF, Ho LC. A randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. BJOG. 2002;119:561-565.
10. Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in postmenopausal women. Hum Reprod. 2001;16:1486-1488.
11. Valle RF, Sankpal R, Marlow J, Cohen L. Cervical stenosis: a challenging clinical entity. J Gynecol Surg. 2002;18:129-143.
12. Perez-Medina T, Bajo MJ, Martinez-Cortes L, Castellanos P, Perez de Avila I. Six thousand office diagnostic-operative hysteroscopies. Int J Obstet Gynecol. 2000;71:33-38.