User login
Hirsutism causes significant anxiety and lack of self-esteem in women. Although it is itself a benign condition, it is often the sign of an underlying and possibly serious endocrine condition.
As we will discuss, the diagnosis begins with a detailed history and physical examination, with laboratory testing and imaging as needed to confirm or rule out underlying causes. Management begins with patient education and support and includes hair removal and drug treatment of any underlying metabolic derangement.
PREVALENCE AND IMPACT
Hirsutism is a common disorder of excess growth of terminal hair in an androgen-dependent male distribution in women, including the chin, upper lip, breasts, upper back, and abdomen.1 It affects 5% to 10% of women of reproductive age.1,2
Hirsutism should be differentiated from hypertrichosis, which can be hereditary or acquired, and which is defined as increased general hair growth in androgen-independent areas.1
Excess hair is cosmetically concerning for women and can significantly affect self-esteem. 3 Normal or acceptable hair growth depends on a woman’s ethnicity and her perception of familial, cultural, and societal norms for the quantity and distribution of hair. Mediterranean women generally have a medium amount of body and facial hair, whereas Asian women have a minimal amount.1,4,5
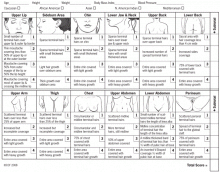
Hirsutism can be clinically graded according to the Ferriman-Gallwey scale2,6 and is defined as a Ferriman-Gallwey score of 8 or higher.1
HOW CIRCULATING ANDROGENS AFFECT HAIR FOLLICLES
In androgen-dependent areas, circulating androgens influence hair follicle characteristics. Androgens increase the size and diameter of the hair fibers in certain androgen-dependent sites, as seen in puberty with the transformation of vellus hairs (small, nonpigmented hairs) into terminal hairs (large, pigmented hairs) in the pubic and axillary regions in women, as well as the beard area in men.2,7 Interestingly, the same circulating androgens cause miniaturization of the susceptible hair follicles of the central scalp.7 The susceptibility of the hair follicle to the effects of the androgens may be genetically determined.7,8
Hirsutism is a sign of hyperandrogenism and increased action of androgens on hair follicles. In women, about half of circulating testosterone arises from the ovaries and adrenal glands; the rest originates from peripheral conversion of weaker androgens (such as androstenedione produced by the adrenals and ovaries) into testosterone.9 Dehydroepiandrosterone sulfate (DHEAS) originates mainly in the adrenal glands.9,10 Testosterone is converted to the more potent dihydrotestosterone (DHT) by type II 5-alpha reductase in the skin, which can then act on susceptible hair follicles.7,11 Therefore, hirsutism can be a consequence of endogenous androgen over-production from the ovaries or the adrenal glands (or both), of exposure to an exogenous source of androgen such as a drug, or of heightened hair follicle sensitivity and metabolism of normal circulating androgen levels (target end-organ dysfunction).1
‘IDIOPATHIC’ HIRSUTISM: A MISLEADING DIAGNOSIS
Many women with hirsutism are found to have polycystic ovary syndrome as the underlying cause, but hirsutism is also commonly labeled as idiopathic when it occurs without an obvious cause, eg, in women with regular menses and normal androgen levels and without features suspicious for other causes of hirsutism. 1,2,12,13 But while this term is commonly used,1,12 it may be misleading, especially if the diagnosis of idiopathic hirsutism is based on standard laboratory tests, which do not always detect androgen excess.2,13 Minor ovarian or adrenal functional hyperandrogenism,14 increased peripheral activity of 5-alpha reductase in the hair follicle, or abnormalities in the androgen receptor have been implicated in the pathogenesis of so-called idiopathic hirsutism. 2,15
HIRSUTISM AND POLYCYSTIC OVARY SYNDROME
Polycystic ovary syndrome, a metabolic syndrome, presents clinically with menstrual irregularities such as oligomenorrhea or amenorrhea, infertility, and signs of hyperandrogenism such as hirsutism, acne, or androgenetic alopecia.16,17 Metabolic disturbances including insulin resistance, impaired glucose tolerance, hyperlipidemia, and obesity (body mass index > 30 kg/m2) also can occur, thus increasing cardiovascular risk.16–18
The finding of polycystic ovaries is not required to make the diagnosis of polycystic ovary syndrome, and their presence does not prove the diagnosis.16,19 Gonadotropin-dependent functional ovarian hyperandrogenism is believed to cause this syndrome; however, mild adrenocorticotropic-dependent functional adrenal hyperandrogenism also is a feature in many cases. In rare cases, polycystic ovary syndrome presents with an isolated elevation of DHEAS.16,20
OTHER CONDITIONS OF EXCESS ANDROGEN
The syndrome of hyperandrogenism, insulin resistance, and acanthosis nigricans, abbreviated as HAIR-AN, is separate from polycystic ovary syndrome; it characterizes a group of inherited syndromes associated with severe metabolic abnormalities of insulin and glucose metabolism and with marked clinical signs of hyperandrogenism.12
The syndrome of seborrhea, acne, hirsutism, and acanthosis nigricans, abbreviated as SAHA, while not itself a diagnosis, is a clinical spectrum of dermatologic signs and symptoms also associated with hyperandrogenism. These are signs that may present with the HAIR-AN syndrome or with another cause of excess androgens, such as idiopathic, ovarian, adrenal, or hyperprolactinemic hyperandrogenism.21
Thyroid disease, hyperprolactinemia, acromegaly, Cushing syndrome, exogenous factors such as androgenic drugs, and nonclassical congenital adrenal hyperplasia can also produce hirsutism.12 In nonclassical congenital adrenal hyperplasia, which is typically caused by a deficiency of 21-hydroxylase, patients present with premature pubarche, hirsutism in the prepubertal years, and menstrual irregularities including primary amenorrhea.22,23
Important rare causes of hirsutism include benign and malignant androgen-secreting tumors of adrenal or ovarian origin. In such cases, hirsutism can have an acute onset or rapid progression and may be associated with features of virilization, such as deepened voice, increased muscle mass, androgenetic alopecia, clitoromegaly, and increased libido.12
A THOROUGH HISTORY IS CRITICAL TO DIAGNOSIS
A thorough medical history can provide important diagnostic clues in women with hirsutism. The clinician should elicit details about the onset and progression of the hair growth,12,15 previous treatments, and any cutaneous signs of hyperandrogenism, such as acne, seborrhea, acanthosis nigricans, or patterned hair loss.
Also important are the menstrual history and a history of infertility. Primary amenorrhea is defined as failure to menstruate by 16 years of age if secondary sexual characteristics have developed, or by 14 years of age if no secondary sexual characteristics have developed, and it can indicate nonclassical congenital adrenal hyperplasia.
The clinician should also try to determine if the patient has a history of galactorrhea or symptoms of virilization (eg, deepened voice, clitoromegaly, increased muscle mass); a family history of hirsutism, polycystic ovary syndrome, HAIR-AN syndrome, metabolic conditions such as type 2 diabetes mellitus, or cardiovascular disease12,15; or a history of symptoms of any condition known to produce hirsutism, such as Cushing disease, acromegaly, or a thyroid disorder. Also important is a drug history to determine if the patient has taken drugs such as androgens, anabolic steroids, or valproic acid (Depakote).20
THE PHYSICAL EXAMINATION
Another proposed predictor of hirsutism is that terminal hair on the chin or the lower abdomen (Ferriman-Gallwey score ≥ 2) is nearly 100% sensitive and 27% specific at predicting total-body hirsutism.24
As part of the physical examination, the clinician should also look for other cutaneous signs of hyperandrogenism, such as acne, androgenetic alopecia, and seborrhea. Acanthosis nigricans is a sign of insulin resistance. Height and weight should be measured and the body mass index calculated. Blood pressure should be recorded, as high blood pressure may be seen in Cushing syndrome and is an important cardiovascular risk factor. Signs of virilization should be identified. Indicators of Cushing disease such as striae, moon facies, fat redistribution, fragile skin, and proximal myopathy should be noted as well as signs of thyroid disease, such as textural skin changes, goiter, and hair loss. Expressible or spontaneous galactorrhea suggests hyperprolactinemia. Acromegaly is associated with coarse facies and enlarged hands and feet. Many of the endocrinopathies can be caused by a pituitary adenoma, which can manifest as a visual field defect, so visual fields should be examined.25 The examination should also exclude any palpable ovarian or adrenal mass.12
WHEN IS ADDITIONAL TESTING NEEDED?
The current Endocrine Society guidelines20 recommend obtaining an early-morning testosterone blood level in the following patients:
- Women with moderate or severe hirsutism
- Women with hirsutism of any degree with sudden onset or rapid progression, or accompanied by signs or symptoms suggesting malignancy or polycystic ovary syndrome: eg, menstrual irregularity, infertility, central obesity, clitoromegaly, or acanthosis nigricans.15,20
Testing androgen levels in mild, isolated hirsutism has not been proven to be useful or to alter management.20
Free testosterone level
An early-morning total or free testosterone level is the initial test in the laboratory evaluation of hirsutism.12,15 Additional specialized laboratory testing may be needed to determine the free testosterone level,15 as the free testosterone test is not available at all laboratories. A normal total testosterone level does not exclude hyperandrogenism but can suggest the diagnosis of idiopathic hirsutism.15
Further testing is needed if the total testosterone level is normal or only slightly elevated, or if there is a strong clinical suspicion of an underlying condition such as endocrinopathy or tumor. It is also useful in patients whose hirsutism responds poorly to medical treatments15 (see discussion below).
If the total testosterone level is elevated, if the hirsutism is moderate to severe, if there are associated symptoms, or if hirsutism is acute or progressive, a further endocrinologic workup is needed,15 possibly including measurement of free testosterone, sex hormone-binding globulin, DHEAS, and androstenedione.15 Free testosterone, unbound to sex hormone-binding globulin, is the biologically active fraction, with the levels of binding globulin increased by drugs such as oral contraceptives15 and decreased by high insulin levels in insulin resistance.25
Test in patients with mild hirsutism?
Although the guidelines suggest that no additional workup is necessary for women with mild hirsutism, we evaluate all patients with hirsutism and those with the SAHA clinical spectrum by measuring free and total testosterone and DHEAS. In our experience, even women with mild hirsutism with subtle symptoms and signs of hyperandrogenism and mild hirsutism often have elevated androgen levels.
Test in women with idiopathic hirsutism?
In women with idiopathic hirsutism, minor forms of functional ovarian and adrenal hyperandrogenism are believed to play a role and are thought to be undetectable with conventional testing.25 The gonadotropin-releasing hormone (GnRH) analogue stimulation test may uncover occult hyperandrogenism in this setting, but it is used as a research tool and does not currently have application in routine clinical practice.14
It is important to remember that some women with apparent idiopathic hirsutism and a history of regular menstrual cycles are actually oligo-ovulatory or anovulatory. In these instances, another diagnosis should be considered,13 and referral to an endocrinologist for further evaluation of ovulatory function is recommended.13
CURRENT USE OF DIAGNOSTIC IMAGING
When malignancy is suspected
A testosterone level above 200 ng/dL suggests an ovarian tumor, and a DHEAS level above 700 μg/dL suggests an adrenal tumor.26 However, not all tumors present with such high androgen levels, and sudden onset of hirsutism, rapid progression of hirsutism, or signs of virilization suggest a tumor.15 In such cases, transvaginal ultrasonography, computed tomography, or magnetic resonance imaging (MRI) of the abdomen can exclude an ovarian or adrenal tumor.
When polycystic ovary syndrome is suspected
The diagnosis of polycystic ovary syndrome is confirmed by two out of three criteria:
- Oligo-ovulation or anovulation
- Clinical or laboratory signs of hyperandrogenism
- Ultrasonographic evidence of polycystic ovaries, with exclusion of other causes of hyperandrogenism.
ADDITIONAL LABORATORY TESTING
Tests for polycystic ovary syndrome
Assessment of polycystic ovary syndrome involves transvaginal ultrasonography, but ultrasonographic evidence of a polycystic ovary is not necessary for the diagnosis.16 A fasting lipid profile and fasting serum glucose are recommended, and if the fasting serum glucose is normal, an oral glucose tolerance test is recommended. 17
Some have reported measuring the ratio of luteinizing hormone to follicle-stimulating hormone in the workup of polycystic ovary syndrome, and a ratio greater than 2 has been considered indicative but not diagnostic.16,25 The individual levels of luteinizing hormone, follicle-stimulating hormone, and estradiol are more important in the evaluation of infertility and ovulatory dysfunction. In patients with elevations of these hormones or with these symptoms, referral for infertility screening with an endocrinologist or gynecologist is recommended. 25
Additional testing and referral for Cushing syndrome, other conditions
Cushing syndrome can be tested for with a 24-hour urine cortisol, overnight low-dose dexamethasone suppression test, and late-night salivary cortisol.27,28 Referral to an endocrinologist for further testing can differentiate between corticotropin-dependent or corticotropin-independent Cushing syndrome.25 Cushing syndrome is often associated with hyperandrogenism, particularly in those cases caused by adrenal tumors.29
The prolactin level and the level of somatomedin C (insulin-like growth factor 1) can be used to rule out hyperprolactinemia and acromegaly, respectively.12 If Cushing syndrome, hyperprolactinemia, or acromegaly is diagnosed by endocrinologic testing, pituitary MRI should be performed.12,25
Referral to specialist centers with experience with these conditions is essential. Nonclassical congenital adrenal hyperplasia can be screened for by a serum 17-hydroxyprogesterone level measured in the follicular phase.12 Measurement of thyroid-stimulating hormone, free thyroxine, and thyroid peroxidase antibodies screens for thyroid disease.12 Hirsutism has been reported with the commencement of L-thyroxine therapy.30
THE PRINCIPLES OF TREATMENT
Patient education regarding the cause of hirsutism and reasonable treatment expectations and emotional support are important in the management of hirsutism. Also important is regular follow-up to measure and document the response to treatment; this can include repeating Ferriman-Gallwey scoring, taking photographs of affected areas, and retesting androgen levels after 3 to 6 months.12
Treatment must be continued for an ongoing effect, and most pharmacologic treatments can take up to 3 to 6 months to produce significant improvement.1
When an underlying condition is diagnosed, treatment of the condition is essential. Androgen-secreting tumors require surgical management.12 Cushing disease, hyperprolactinemia, and acromegaly should be clinically apparent from examination and testing, and appropriate referral and standard management should be instigated. Exogenous sources of androgen such as androgenic progestins or anabolic steroids should be discontinued. Lifestyle management is important, and weight loss in obese patients with polycystic ovary syndrome can improve hirsutism as well as mitigate cardiovascular risk factors.31
In classic congenital adrenal hyperplasia, glucocorticoid therapy manages both ovulation induction and hirsutism.20 However, in nonclassical congenital adrenal hyperplasia, glucocorticoid therapy supports ovulation induction, but hirsutism usually requires both systemic antiandrogen and hair removal.20
CURRENT OPTIONS FOR HAIR REMOVAL
The choice of method depends on patient preference, adverse effects, the degree of hirsutism, the level of distress, previous treatments, and cost.1,15,32
Self-care methods
Self-care methods offer only temporary reduction of excess hairs.
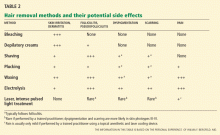
Plucking removes the entire hair, including the root, but it is painful and time-consuming, and it is only practical for areas where few hairs exist, such as on the face.1
Shaving is an easy, inexpensive, and painless choice for hair removal. Although a common belief is that shaving causes faster or thicker hair regrowth, shaving affects neither the diameter nor the rate of growth of the hair.32 Given its masculine association, shaving is not acceptable to most women except perhaps for use on the legs and axillae.1,32 Shaving can cause irritation, folliculitis, pseudofolliculitis, and infection.1
Waxing removes the entire hair. While it is more expensive than plucking, regrowth is slower, occurring over weeks. It is painful and can cause thermal burns, irritation, folliculitis, scarring, and postinflammatory dyspigmentation.1
Chemical depilatories, usually thioglycollic acid preparations, are inexpensive, painless, and easy to use. However, the resulting hair reduction is of short duration because the hair shafts are only removed at the level of the skin surface.1 They can also cause irritant dermatitis. 1
Bleaching with hydrogen peroxide is inexpensive and can camouflage dark facial hair, but it can also cause skin discoloration and irritation. 1
Clinic-based methods
Electrolysis often results in a permanent reduction in hair growth.1,32 A fine needle is placed into the hair follicle and an electrical current is applied. Each follicle is treated individually. 1,32 Best results are seen on darker hairs in patients with lighter skin, but it can be used on all skin types and hair colors.1,32
Electrolysis is operator-dependent, and there are US Food and Drug Administration (FDA) regulations regarding electrolysis techniques. It requires multiple treatments, and it is painful and can cause erythema, folliculitis, pseudofolliculitis, infection, scarring, and postinflammatory dyspigmentation.1,32 Some reports suggest that prior waxing and plucking of hairs damages the hair by twisting the hair shaft, making electrolysis more difficult.32
Laser treatment uses light of certain wavelengths to damage the hair follicles. While laser hair removal does not result in complete or persistent hair removal, it is more effective than shaving, waxing, and electrolysis, producing partial hair reduction for up to 6 months; the effect is enhanced with multiple treatments.33,34 The number of treatments required depends on the laser type and on the nature of the patient’s hair follicles.35
Laser systems for hair removal are of various wavelengths and also include intense pulsed light systems. The choice of system depends on the patient’s skin type and hair color. Women with fair skin and dark hair are ideal candidates; longer-wavelength lasers are preferred for darker or tanned skin types.
Adverse effects of laser hair removal include pain, erythema, burns, dyspigmentation, and scarring. Laser cooling devices can prevent or minimize some of these effects. Laser treatment has also been known to cause a paradoxical increase in hair growth.1,33,34
DRUG THERAPIES FOR HIRSUTISM
The drugs most commonly used for hirsutism are oral contraceptives (off-label use) and antiandrogenic drugs (off-label use). Topical eflornithine cream (Vaniqa) is FDA-approved for hirsutism but is less commonly used. Insulin sensitizers, GnRH analogues, and other drugs are occasionally used (off-label) to treat hirsutism.
Topical eflornithine cream
Topical eflornithine cream treats facial hirsutism by slowing the rate of hair growth; it does this by irreversibly inhibiting ornithine decarboxylase, an enzyme essential for hair growth.39,40 Studies showed that twice-daily application reduced unwanted facial hair in women after 24 weeks of treatment.39,40 Treatment must be continuous, since hair growth rapidly returns to the pretreatment rate by 8 weeks after discontinuing eflornithine.39,40 White women have been shown to respond better than black women.39 Adverse effects include a mild burning sensation, acne, pseudofolliculitis barbae, irritation, and allergic contact dermatitis.39,40 Improved outcomes have been suggested when eflornithine cream is combined with laser hair removal.41
Oral contraceptives
Oral contraceptives are commonly used off-label for the management of hirsutism.20 Oral contraceptives suppress the secretion of luteinizing hormone and, hence, the synthesis of ovarian androgen, thereby increasing levels of sex hormone-binding globulin and decreasing free plasma testosterone.1,20 Adrenal androgen production is also slightly reduced.20
Oral contraceptives usually combine a synthetic estrogen and a progestin. Certain progestins are more androgenic and should be avoided.1
For treating hirsutism, oral contraceptives should be used that contain low-androgenic progestins such as cyproterone acetate (not available in the United States), drosperinone (eg, in Yasmin), norgestimate (eg, in Ortho Tri-Cyclen), or desogestrel (eg, in Mircette).1,20
Side effects of oral contraceptives include breast tenderness, gastrointestinal upset, headache, loss of libido, hypertension, and the potential risk of venous thromboembolism.1,15,32,36
Antiandrogenic drugs
Several antiandrogenic drugs are used off-label to treat hirsutism.
Spironolactone (Aldactone), a competitive inhibitor of the androgen receptor and 5-alpha reductase activity,20 can be effective in the treatment of hirsutism. Monotherapy with spironolactone, without an oral contraceptive or other reliable form of contraception, is not recommended because of the teratogenic potential of all antiandrogens to feminize a developing male fetus.20 Thus, reliable contraception should be used in females of childbearing age when starting antiandrogen therapy.
The dosage of spironolactone for hirsutism is usually 100 mg to 200 mg daily.1,20 Hyperkalemia, polyuria, postural hypotension, irregular menses, and liver abnormalities are among the possible adverse effects (Table 3). Spironolactone was found to be tumorigenic in animal studies, although this has unknown relevance in humans.36
Cyproterone, an antiandrogen not available in the United States,42 competitively inhibits the androgen receptor and 5-alpha-reductase activity.1,20,36 It can be used for only the first 10 days of the menstrual cycle (50-mg or 100-mg dose) with an oral contraceptive pill, or in a low dose in a combined oral contraceptive pill (Diane-35 in Canada and the United Kingdom).1
Side effects are similar to those of oral contraceptives and include fatigue, mood change, risk of venous thromboembolism, and decreased libido.1,15,36 Importantly, in woman of childbearing age, there is the potential risk of feminization of a male fetus, so reliable contraception must be used.15,36
Flutamide, an investigational antiandrogen, has shown promise in the treatment of hirsutism.20 Flutamide is a nonsteroidal competitive inhibitor of androgen receptor binding. It carries a significant risk of hepatotoxicity. 1,15
Finasteride (Propecia) 1 mg is only occasionally used in the treatment of hirsutism (off-label usage). It inhibits type II 5-alphareductase to suppress dihydrotestosterone levels. 32 It carries a risk of gastrointestinal disturbance, decreased libido, hepatotoxicity, and feminization of a male fetus (pregnancy category X), so reliable contraception is required in all females of childbearing age, as with all antiandrogens1 (Table 3).
Dutasteride (Avodart), a type I and II 5-alpha-reductase inhibitor, has not been studied for the treatment of hirsutism (pregnancy category X).
Insulin sensitizers
Metformin (Glucophage) and other insulin sensitizers are less effective than antiandrogens at reducing hirsutism.20,38 However, metformin is effective at inducing ovulation in patients with polycystic ovary syndrome.38 Gastrointestinal upset is a common side effect; lactic acidosis is a serious but rare adverse effect.1
Gonadotropin-releasing hormone analogues
GnRH analogues are an option only if oral contraceptives and antiandrogen drugs are unsuccessful in patients with severe hyperandrogenism. 20 They suppress secretion of luteinizing hormone and the synthesis of ovarian androgen.1,20 These drugs are given as monthly intramuscular injections, usually with some form of estrogen-progestin replacement, since GnRH analogues cause estrogen levels to fall to menopausal levels.1
Side effects include signs and symptoms of menopause including hot flushes, atrophic vaginitis, and osteoporosis.1,15 These drugs completely inhibit ovulation, and some endocrinologists and gynecologists do not suggest further contraception in women of childbearing years for this reason. However, GnRH analogues are not approved as a contraceptive and are pregnancy category X.
Other drugs
Other drugs with antiandrogen activity include cimetidine and ketoconazole.12 Cimetidine (Tagamet) is not effective for the treatment of hirsutism, and ketoconazole (Nizoral) is associated with significant risk for adrenocortical suppression12 and hepatotoxicity in addition to multiple drug interactions, given its effect on the hepatic P450 enzyme system.
Acknowledgment: Many thanks to Rebecca Tung, MD, dermatologic surgeon, Cleveland Clinic, for her advice on lasers.
- Mofid A, Seyyed Alinaghi SA, Zandieh S, Yazdani T. Hirsutism. Int J Clin Pract 2008; 62:433–443.
- Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev 2000; 21:347–362.
- Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv 2006; 61:723–732.
- Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol 2001; 41:202–206.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999; 84:4006–4011.
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 21:1440–1447.
- Messenger AG. The control of hair growth: an overview. J Invest Dermatol 1993; 101(suppl 1):4S–9S.
- Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc 2005; 10:205–208.
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 1986; 15:213–228.
- Braunstein GD. Testis. In:Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology. 8th ed. New York: McGraw-Hill, 2007.
- Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000; 21:363–392.
- Practice Committee of the American Society for Reproductive Medicine. The evaluation and treatment of androgen excess. Fertil Steril 2006; 86(suppl 5):S241–S247.
- Azziz R, Waggoner WT, Ochoa T, Knochenhauer ES, Boots LR. Idiopathic hirsutism: an uncommon cause of hirsutism in Alabama. Fertil Steril 1998; 70:274–278.
- Rossi R, Tauchmanovà L, Luciano A, et al. Functional hyperandrogenism detected by corticotropin and GnRH-analogue stimulation tests in women affected by apparently idiopathic hirsutism. J Endocrinol Invest 2001; 24:491–498.
- Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med 2005; 353:2578–2588.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19:41–47.
- Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007; 92:4546–4556.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365:1415–1428.
- Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril 2005; 83:1343–1346.
- Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. http://www.endo-society.org/guidelines/final/upload/Hirsutism_Guideline.pdf. Accessed March 30, 2010.
- Orfanos CE, Adler YD, Zouboulis CC. The SAHA syndrome. Horm Res 2000; 54:251–258.
- New MI. Extensive clinical experience: nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab 2006; 91:4205–4214.
- Kohn B, Levine LS, Pollack MS, et al. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 1982; 55:817–827.
- Knochenhauer ES, Hines G, Conway-Myers BA, Azziz R. Examination of the chin or lower abdomen only for the prediction of hirsutism. Fertil Steril 2000; 74:980–983.
- Somani N, Harrison S, Bergfeld WF. The clinical evaluation of hirsutism. Dermatol Ther 2008; 21:376–391.
- Waggoner W, Boots LR, Azziz R. Total testosterone and DHEAS levels as predictors of androgen-secreting neoplasms: a populational study. Gynecol Endocrinol 1999; 13:394–400.
- Crapo L. Cushing’s syndrome: a review of diagnostic tests. Metabolism 1979; 28:955–977.
- Blethen SL, Chasalow FI. Overnight dexamethasone suppression test: normal responses and the diagnosis of Cushing’s syndrome. Steroids 1989; 54:185–193.
- Bertagna C, Orth DN. Clinical and laboratory findings and results of therapy in 58 patients with adrenocortical tumors admitted to a single medical center (1951 to 1978). Am J Med 1981; 71:855–875.
- Kologlu S, Baskal N, Kologlu LB, Laleli Y, Tuccar E. Hirsutism due to the treatment with L-thyroxine in patients with thyroid pathology. Endocrinologie 1988; 26:179–185.
- Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 2006; 91:3970–3980.
- Dawber RP. Guidance for the management of hirsutism. Curr Med Res Opin 2005; 21:1227–1234.
- Haedersdal M, Wulf HC. Evidence based review of hair removal using lasers and light sources. J Eur Acad Dermatol Venereol 2006; 20:9–20.
- Sadighha A, Mohaghegh Zahed G. Meta-analysis of hair removal laser trials. Lasers Med Sci 2009; 24:21–25.
- Casey AS, Goldberg D. Guidelines for laser hair removal. J Cosmet Laser Ther 2008; 10:24–33.
- Wakelin SH, Maibach HI, editors. Handbook of Systemic Drug Treatment in Dermatology. London: Manson Publishing Ltd, 2004.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1153–1160.
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1135–1142.
- Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol 2001; 2:197–201.
- Wolf JE, Shander D, Huber F, et al; Eflornithine HCl Study Group. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dematol 2007; 46:94–98.
- Hamzavi I, Tan E, Shapiro J, Lui H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol 2007; 57:54–59.
- Van der Spuy ZM, le Roux PA. Cyproterone acetate for hirsutism. Cochrane Database Syst Rev 2003; 4:CD001125.
Hirsutism causes significant anxiety and lack of self-esteem in women. Although it is itself a benign condition, it is often the sign of an underlying and possibly serious endocrine condition.
As we will discuss, the diagnosis begins with a detailed history and physical examination, with laboratory testing and imaging as needed to confirm or rule out underlying causes. Management begins with patient education and support and includes hair removal and drug treatment of any underlying metabolic derangement.
PREVALENCE AND IMPACT
Hirsutism is a common disorder of excess growth of terminal hair in an androgen-dependent male distribution in women, including the chin, upper lip, breasts, upper back, and abdomen.1 It affects 5% to 10% of women of reproductive age.1,2
Hirsutism should be differentiated from hypertrichosis, which can be hereditary or acquired, and which is defined as increased general hair growth in androgen-independent areas.1
Excess hair is cosmetically concerning for women and can significantly affect self-esteem. 3 Normal or acceptable hair growth depends on a woman’s ethnicity and her perception of familial, cultural, and societal norms for the quantity and distribution of hair. Mediterranean women generally have a medium amount of body and facial hair, whereas Asian women have a minimal amount.1,4,5
Hirsutism can be clinically graded according to the Ferriman-Gallwey scale2,6 and is defined as a Ferriman-Gallwey score of 8 or higher.1
HOW CIRCULATING ANDROGENS AFFECT HAIR FOLLICLES
In androgen-dependent areas, circulating androgens influence hair follicle characteristics. Androgens increase the size and diameter of the hair fibers in certain androgen-dependent sites, as seen in puberty with the transformation of vellus hairs (small, nonpigmented hairs) into terminal hairs (large, pigmented hairs) in the pubic and axillary regions in women, as well as the beard area in men.2,7 Interestingly, the same circulating androgens cause miniaturization of the susceptible hair follicles of the central scalp.7 The susceptibility of the hair follicle to the effects of the androgens may be genetically determined.7,8
Hirsutism is a sign of hyperandrogenism and increased action of androgens on hair follicles. In women, about half of circulating testosterone arises from the ovaries and adrenal glands; the rest originates from peripheral conversion of weaker androgens (such as androstenedione produced by the adrenals and ovaries) into testosterone.9 Dehydroepiandrosterone sulfate (DHEAS) originates mainly in the adrenal glands.9,10 Testosterone is converted to the more potent dihydrotestosterone (DHT) by type II 5-alpha reductase in the skin, which can then act on susceptible hair follicles.7,11 Therefore, hirsutism can be a consequence of endogenous androgen over-production from the ovaries or the adrenal glands (or both), of exposure to an exogenous source of androgen such as a drug, or of heightened hair follicle sensitivity and metabolism of normal circulating androgen levels (target end-organ dysfunction).1
‘IDIOPATHIC’ HIRSUTISM: A MISLEADING DIAGNOSIS
Many women with hirsutism are found to have polycystic ovary syndrome as the underlying cause, but hirsutism is also commonly labeled as idiopathic when it occurs without an obvious cause, eg, in women with regular menses and normal androgen levels and without features suspicious for other causes of hirsutism. 1,2,12,13 But while this term is commonly used,1,12 it may be misleading, especially if the diagnosis of idiopathic hirsutism is based on standard laboratory tests, which do not always detect androgen excess.2,13 Minor ovarian or adrenal functional hyperandrogenism,14 increased peripheral activity of 5-alpha reductase in the hair follicle, or abnormalities in the androgen receptor have been implicated in the pathogenesis of so-called idiopathic hirsutism. 2,15
HIRSUTISM AND POLYCYSTIC OVARY SYNDROME
Polycystic ovary syndrome, a metabolic syndrome, presents clinically with menstrual irregularities such as oligomenorrhea or amenorrhea, infertility, and signs of hyperandrogenism such as hirsutism, acne, or androgenetic alopecia.16,17 Metabolic disturbances including insulin resistance, impaired glucose tolerance, hyperlipidemia, and obesity (body mass index > 30 kg/m2) also can occur, thus increasing cardiovascular risk.16–18
The finding of polycystic ovaries is not required to make the diagnosis of polycystic ovary syndrome, and their presence does not prove the diagnosis.16,19 Gonadotropin-dependent functional ovarian hyperandrogenism is believed to cause this syndrome; however, mild adrenocorticotropic-dependent functional adrenal hyperandrogenism also is a feature in many cases. In rare cases, polycystic ovary syndrome presents with an isolated elevation of DHEAS.16,20
OTHER CONDITIONS OF EXCESS ANDROGEN
The syndrome of hyperandrogenism, insulin resistance, and acanthosis nigricans, abbreviated as HAIR-AN, is separate from polycystic ovary syndrome; it characterizes a group of inherited syndromes associated with severe metabolic abnormalities of insulin and glucose metabolism and with marked clinical signs of hyperandrogenism.12
The syndrome of seborrhea, acne, hirsutism, and acanthosis nigricans, abbreviated as SAHA, while not itself a diagnosis, is a clinical spectrum of dermatologic signs and symptoms also associated with hyperandrogenism. These are signs that may present with the HAIR-AN syndrome or with another cause of excess androgens, such as idiopathic, ovarian, adrenal, or hyperprolactinemic hyperandrogenism.21
Thyroid disease, hyperprolactinemia, acromegaly, Cushing syndrome, exogenous factors such as androgenic drugs, and nonclassical congenital adrenal hyperplasia can also produce hirsutism.12 In nonclassical congenital adrenal hyperplasia, which is typically caused by a deficiency of 21-hydroxylase, patients present with premature pubarche, hirsutism in the prepubertal years, and menstrual irregularities including primary amenorrhea.22,23
Important rare causes of hirsutism include benign and malignant androgen-secreting tumors of adrenal or ovarian origin. In such cases, hirsutism can have an acute onset or rapid progression and may be associated with features of virilization, such as deepened voice, increased muscle mass, androgenetic alopecia, clitoromegaly, and increased libido.12
A THOROUGH HISTORY IS CRITICAL TO DIAGNOSIS
A thorough medical history can provide important diagnostic clues in women with hirsutism. The clinician should elicit details about the onset and progression of the hair growth,12,15 previous treatments, and any cutaneous signs of hyperandrogenism, such as acne, seborrhea, acanthosis nigricans, or patterned hair loss.
Also important are the menstrual history and a history of infertility. Primary amenorrhea is defined as failure to menstruate by 16 years of age if secondary sexual characteristics have developed, or by 14 years of age if no secondary sexual characteristics have developed, and it can indicate nonclassical congenital adrenal hyperplasia.
The clinician should also try to determine if the patient has a history of galactorrhea or symptoms of virilization (eg, deepened voice, clitoromegaly, increased muscle mass); a family history of hirsutism, polycystic ovary syndrome, HAIR-AN syndrome, metabolic conditions such as type 2 diabetes mellitus, or cardiovascular disease12,15; or a history of symptoms of any condition known to produce hirsutism, such as Cushing disease, acromegaly, or a thyroid disorder. Also important is a drug history to determine if the patient has taken drugs such as androgens, anabolic steroids, or valproic acid (Depakote).20
THE PHYSICAL EXAMINATION
Another proposed predictor of hirsutism is that terminal hair on the chin or the lower abdomen (Ferriman-Gallwey score ≥ 2) is nearly 100% sensitive and 27% specific at predicting total-body hirsutism.24
As part of the physical examination, the clinician should also look for other cutaneous signs of hyperandrogenism, such as acne, androgenetic alopecia, and seborrhea. Acanthosis nigricans is a sign of insulin resistance. Height and weight should be measured and the body mass index calculated. Blood pressure should be recorded, as high blood pressure may be seen in Cushing syndrome and is an important cardiovascular risk factor. Signs of virilization should be identified. Indicators of Cushing disease such as striae, moon facies, fat redistribution, fragile skin, and proximal myopathy should be noted as well as signs of thyroid disease, such as textural skin changes, goiter, and hair loss. Expressible or spontaneous galactorrhea suggests hyperprolactinemia. Acromegaly is associated with coarse facies and enlarged hands and feet. Many of the endocrinopathies can be caused by a pituitary adenoma, which can manifest as a visual field defect, so visual fields should be examined.25 The examination should also exclude any palpable ovarian or adrenal mass.12
WHEN IS ADDITIONAL TESTING NEEDED?
The current Endocrine Society guidelines20 recommend obtaining an early-morning testosterone blood level in the following patients:
- Women with moderate or severe hirsutism
- Women with hirsutism of any degree with sudden onset or rapid progression, or accompanied by signs or symptoms suggesting malignancy or polycystic ovary syndrome: eg, menstrual irregularity, infertility, central obesity, clitoromegaly, or acanthosis nigricans.15,20
Testing androgen levels in mild, isolated hirsutism has not been proven to be useful or to alter management.20
Free testosterone level
An early-morning total or free testosterone level is the initial test in the laboratory evaluation of hirsutism.12,15 Additional specialized laboratory testing may be needed to determine the free testosterone level,15 as the free testosterone test is not available at all laboratories. A normal total testosterone level does not exclude hyperandrogenism but can suggest the diagnosis of idiopathic hirsutism.15
Further testing is needed if the total testosterone level is normal or only slightly elevated, or if there is a strong clinical suspicion of an underlying condition such as endocrinopathy or tumor. It is also useful in patients whose hirsutism responds poorly to medical treatments15 (see discussion below).
If the total testosterone level is elevated, if the hirsutism is moderate to severe, if there are associated symptoms, or if hirsutism is acute or progressive, a further endocrinologic workup is needed,15 possibly including measurement of free testosterone, sex hormone-binding globulin, DHEAS, and androstenedione.15 Free testosterone, unbound to sex hormone-binding globulin, is the biologically active fraction, with the levels of binding globulin increased by drugs such as oral contraceptives15 and decreased by high insulin levels in insulin resistance.25
Test in patients with mild hirsutism?
Although the guidelines suggest that no additional workup is necessary for women with mild hirsutism, we evaluate all patients with hirsutism and those with the SAHA clinical spectrum by measuring free and total testosterone and DHEAS. In our experience, even women with mild hirsutism with subtle symptoms and signs of hyperandrogenism and mild hirsutism often have elevated androgen levels.
Test in women with idiopathic hirsutism?
In women with idiopathic hirsutism, minor forms of functional ovarian and adrenal hyperandrogenism are believed to play a role and are thought to be undetectable with conventional testing.25 The gonadotropin-releasing hormone (GnRH) analogue stimulation test may uncover occult hyperandrogenism in this setting, but it is used as a research tool and does not currently have application in routine clinical practice.14
It is important to remember that some women with apparent idiopathic hirsutism and a history of regular menstrual cycles are actually oligo-ovulatory or anovulatory. In these instances, another diagnosis should be considered,13 and referral to an endocrinologist for further evaluation of ovulatory function is recommended.13
CURRENT USE OF DIAGNOSTIC IMAGING
When malignancy is suspected
A testosterone level above 200 ng/dL suggests an ovarian tumor, and a DHEAS level above 700 μg/dL suggests an adrenal tumor.26 However, not all tumors present with such high androgen levels, and sudden onset of hirsutism, rapid progression of hirsutism, or signs of virilization suggest a tumor.15 In such cases, transvaginal ultrasonography, computed tomography, or magnetic resonance imaging (MRI) of the abdomen can exclude an ovarian or adrenal tumor.
When polycystic ovary syndrome is suspected
The diagnosis of polycystic ovary syndrome is confirmed by two out of three criteria:
- Oligo-ovulation or anovulation
- Clinical or laboratory signs of hyperandrogenism
- Ultrasonographic evidence of polycystic ovaries, with exclusion of other causes of hyperandrogenism.
ADDITIONAL LABORATORY TESTING
Tests for polycystic ovary syndrome
Assessment of polycystic ovary syndrome involves transvaginal ultrasonography, but ultrasonographic evidence of a polycystic ovary is not necessary for the diagnosis.16 A fasting lipid profile and fasting serum glucose are recommended, and if the fasting serum glucose is normal, an oral glucose tolerance test is recommended. 17
Some have reported measuring the ratio of luteinizing hormone to follicle-stimulating hormone in the workup of polycystic ovary syndrome, and a ratio greater than 2 has been considered indicative but not diagnostic.16,25 The individual levels of luteinizing hormone, follicle-stimulating hormone, and estradiol are more important in the evaluation of infertility and ovulatory dysfunction. In patients with elevations of these hormones or with these symptoms, referral for infertility screening with an endocrinologist or gynecologist is recommended. 25
Additional testing and referral for Cushing syndrome, other conditions
Cushing syndrome can be tested for with a 24-hour urine cortisol, overnight low-dose dexamethasone suppression test, and late-night salivary cortisol.27,28 Referral to an endocrinologist for further testing can differentiate between corticotropin-dependent or corticotropin-independent Cushing syndrome.25 Cushing syndrome is often associated with hyperandrogenism, particularly in those cases caused by adrenal tumors.29
The prolactin level and the level of somatomedin C (insulin-like growth factor 1) can be used to rule out hyperprolactinemia and acromegaly, respectively.12 If Cushing syndrome, hyperprolactinemia, or acromegaly is diagnosed by endocrinologic testing, pituitary MRI should be performed.12,25
Referral to specialist centers with experience with these conditions is essential. Nonclassical congenital adrenal hyperplasia can be screened for by a serum 17-hydroxyprogesterone level measured in the follicular phase.12 Measurement of thyroid-stimulating hormone, free thyroxine, and thyroid peroxidase antibodies screens for thyroid disease.12 Hirsutism has been reported with the commencement of L-thyroxine therapy.30
THE PRINCIPLES OF TREATMENT
Patient education regarding the cause of hirsutism and reasonable treatment expectations and emotional support are important in the management of hirsutism. Also important is regular follow-up to measure and document the response to treatment; this can include repeating Ferriman-Gallwey scoring, taking photographs of affected areas, and retesting androgen levels after 3 to 6 months.12
Treatment must be continued for an ongoing effect, and most pharmacologic treatments can take up to 3 to 6 months to produce significant improvement.1
When an underlying condition is diagnosed, treatment of the condition is essential. Androgen-secreting tumors require surgical management.12 Cushing disease, hyperprolactinemia, and acromegaly should be clinically apparent from examination and testing, and appropriate referral and standard management should be instigated. Exogenous sources of androgen such as androgenic progestins or anabolic steroids should be discontinued. Lifestyle management is important, and weight loss in obese patients with polycystic ovary syndrome can improve hirsutism as well as mitigate cardiovascular risk factors.31
In classic congenital adrenal hyperplasia, glucocorticoid therapy manages both ovulation induction and hirsutism.20 However, in nonclassical congenital adrenal hyperplasia, glucocorticoid therapy supports ovulation induction, but hirsutism usually requires both systemic antiandrogen and hair removal.20
CURRENT OPTIONS FOR HAIR REMOVAL
The choice of method depends on patient preference, adverse effects, the degree of hirsutism, the level of distress, previous treatments, and cost.1,15,32
Self-care methods
Self-care methods offer only temporary reduction of excess hairs.
Plucking removes the entire hair, including the root, but it is painful and time-consuming, and it is only practical for areas where few hairs exist, such as on the face.1
Shaving is an easy, inexpensive, and painless choice for hair removal. Although a common belief is that shaving causes faster or thicker hair regrowth, shaving affects neither the diameter nor the rate of growth of the hair.32 Given its masculine association, shaving is not acceptable to most women except perhaps for use on the legs and axillae.1,32 Shaving can cause irritation, folliculitis, pseudofolliculitis, and infection.1
Waxing removes the entire hair. While it is more expensive than plucking, regrowth is slower, occurring over weeks. It is painful and can cause thermal burns, irritation, folliculitis, scarring, and postinflammatory dyspigmentation.1
Chemical depilatories, usually thioglycollic acid preparations, are inexpensive, painless, and easy to use. However, the resulting hair reduction is of short duration because the hair shafts are only removed at the level of the skin surface.1 They can also cause irritant dermatitis. 1
Bleaching with hydrogen peroxide is inexpensive and can camouflage dark facial hair, but it can also cause skin discoloration and irritation. 1
Clinic-based methods
Electrolysis often results in a permanent reduction in hair growth.1,32 A fine needle is placed into the hair follicle and an electrical current is applied. Each follicle is treated individually. 1,32 Best results are seen on darker hairs in patients with lighter skin, but it can be used on all skin types and hair colors.1,32
Electrolysis is operator-dependent, and there are US Food and Drug Administration (FDA) regulations regarding electrolysis techniques. It requires multiple treatments, and it is painful and can cause erythema, folliculitis, pseudofolliculitis, infection, scarring, and postinflammatory dyspigmentation.1,32 Some reports suggest that prior waxing and plucking of hairs damages the hair by twisting the hair shaft, making electrolysis more difficult.32
Laser treatment uses light of certain wavelengths to damage the hair follicles. While laser hair removal does not result in complete or persistent hair removal, it is more effective than shaving, waxing, and electrolysis, producing partial hair reduction for up to 6 months; the effect is enhanced with multiple treatments.33,34 The number of treatments required depends on the laser type and on the nature of the patient’s hair follicles.35
Laser systems for hair removal are of various wavelengths and also include intense pulsed light systems. The choice of system depends on the patient’s skin type and hair color. Women with fair skin and dark hair are ideal candidates; longer-wavelength lasers are preferred for darker or tanned skin types.
Adverse effects of laser hair removal include pain, erythema, burns, dyspigmentation, and scarring. Laser cooling devices can prevent or minimize some of these effects. Laser treatment has also been known to cause a paradoxical increase in hair growth.1,33,34
DRUG THERAPIES FOR HIRSUTISM
The drugs most commonly used for hirsutism are oral contraceptives (off-label use) and antiandrogenic drugs (off-label use). Topical eflornithine cream (Vaniqa) is FDA-approved for hirsutism but is less commonly used. Insulin sensitizers, GnRH analogues, and other drugs are occasionally used (off-label) to treat hirsutism.
Topical eflornithine cream
Topical eflornithine cream treats facial hirsutism by slowing the rate of hair growth; it does this by irreversibly inhibiting ornithine decarboxylase, an enzyme essential for hair growth.39,40 Studies showed that twice-daily application reduced unwanted facial hair in women after 24 weeks of treatment.39,40 Treatment must be continuous, since hair growth rapidly returns to the pretreatment rate by 8 weeks after discontinuing eflornithine.39,40 White women have been shown to respond better than black women.39 Adverse effects include a mild burning sensation, acne, pseudofolliculitis barbae, irritation, and allergic contact dermatitis.39,40 Improved outcomes have been suggested when eflornithine cream is combined with laser hair removal.41
Oral contraceptives
Oral contraceptives are commonly used off-label for the management of hirsutism.20 Oral contraceptives suppress the secretion of luteinizing hormone and, hence, the synthesis of ovarian androgen, thereby increasing levels of sex hormone-binding globulin and decreasing free plasma testosterone.1,20 Adrenal androgen production is also slightly reduced.20
Oral contraceptives usually combine a synthetic estrogen and a progestin. Certain progestins are more androgenic and should be avoided.1
For treating hirsutism, oral contraceptives should be used that contain low-androgenic progestins such as cyproterone acetate (not available in the United States), drosperinone (eg, in Yasmin), norgestimate (eg, in Ortho Tri-Cyclen), or desogestrel (eg, in Mircette).1,20
Side effects of oral contraceptives include breast tenderness, gastrointestinal upset, headache, loss of libido, hypertension, and the potential risk of venous thromboembolism.1,15,32,36
Antiandrogenic drugs
Several antiandrogenic drugs are used off-label to treat hirsutism.
Spironolactone (Aldactone), a competitive inhibitor of the androgen receptor and 5-alpha reductase activity,20 can be effective in the treatment of hirsutism. Monotherapy with spironolactone, without an oral contraceptive or other reliable form of contraception, is not recommended because of the teratogenic potential of all antiandrogens to feminize a developing male fetus.20 Thus, reliable contraception should be used in females of childbearing age when starting antiandrogen therapy.
The dosage of spironolactone for hirsutism is usually 100 mg to 200 mg daily.1,20 Hyperkalemia, polyuria, postural hypotension, irregular menses, and liver abnormalities are among the possible adverse effects (Table 3). Spironolactone was found to be tumorigenic in animal studies, although this has unknown relevance in humans.36
Cyproterone, an antiandrogen not available in the United States,42 competitively inhibits the androgen receptor and 5-alpha-reductase activity.1,20,36 It can be used for only the first 10 days of the menstrual cycle (50-mg or 100-mg dose) with an oral contraceptive pill, or in a low dose in a combined oral contraceptive pill (Diane-35 in Canada and the United Kingdom).1
Side effects are similar to those of oral contraceptives and include fatigue, mood change, risk of venous thromboembolism, and decreased libido.1,15,36 Importantly, in woman of childbearing age, there is the potential risk of feminization of a male fetus, so reliable contraception must be used.15,36
Flutamide, an investigational antiandrogen, has shown promise in the treatment of hirsutism.20 Flutamide is a nonsteroidal competitive inhibitor of androgen receptor binding. It carries a significant risk of hepatotoxicity. 1,15
Finasteride (Propecia) 1 mg is only occasionally used in the treatment of hirsutism (off-label usage). It inhibits type II 5-alphareductase to suppress dihydrotestosterone levels. 32 It carries a risk of gastrointestinal disturbance, decreased libido, hepatotoxicity, and feminization of a male fetus (pregnancy category X), so reliable contraception is required in all females of childbearing age, as with all antiandrogens1 (Table 3).
Dutasteride (Avodart), a type I and II 5-alpha-reductase inhibitor, has not been studied for the treatment of hirsutism (pregnancy category X).
Insulin sensitizers
Metformin (Glucophage) and other insulin sensitizers are less effective than antiandrogens at reducing hirsutism.20,38 However, metformin is effective at inducing ovulation in patients with polycystic ovary syndrome.38 Gastrointestinal upset is a common side effect; lactic acidosis is a serious but rare adverse effect.1
Gonadotropin-releasing hormone analogues
GnRH analogues are an option only if oral contraceptives and antiandrogen drugs are unsuccessful in patients with severe hyperandrogenism. 20 They suppress secretion of luteinizing hormone and the synthesis of ovarian androgen.1,20 These drugs are given as monthly intramuscular injections, usually with some form of estrogen-progestin replacement, since GnRH analogues cause estrogen levels to fall to menopausal levels.1
Side effects include signs and symptoms of menopause including hot flushes, atrophic vaginitis, and osteoporosis.1,15 These drugs completely inhibit ovulation, and some endocrinologists and gynecologists do not suggest further contraception in women of childbearing years for this reason. However, GnRH analogues are not approved as a contraceptive and are pregnancy category X.
Other drugs
Other drugs with antiandrogen activity include cimetidine and ketoconazole.12 Cimetidine (Tagamet) is not effective for the treatment of hirsutism, and ketoconazole (Nizoral) is associated with significant risk for adrenocortical suppression12 and hepatotoxicity in addition to multiple drug interactions, given its effect on the hepatic P450 enzyme system.
Acknowledgment: Many thanks to Rebecca Tung, MD, dermatologic surgeon, Cleveland Clinic, for her advice on lasers.
Hirsutism causes significant anxiety and lack of self-esteem in women. Although it is itself a benign condition, it is often the sign of an underlying and possibly serious endocrine condition.
As we will discuss, the diagnosis begins with a detailed history and physical examination, with laboratory testing and imaging as needed to confirm or rule out underlying causes. Management begins with patient education and support and includes hair removal and drug treatment of any underlying metabolic derangement.
PREVALENCE AND IMPACT
Hirsutism is a common disorder of excess growth of terminal hair in an androgen-dependent male distribution in women, including the chin, upper lip, breasts, upper back, and abdomen.1 It affects 5% to 10% of women of reproductive age.1,2
Hirsutism should be differentiated from hypertrichosis, which can be hereditary or acquired, and which is defined as increased general hair growth in androgen-independent areas.1
Excess hair is cosmetically concerning for women and can significantly affect self-esteem. 3 Normal or acceptable hair growth depends on a woman’s ethnicity and her perception of familial, cultural, and societal norms for the quantity and distribution of hair. Mediterranean women generally have a medium amount of body and facial hair, whereas Asian women have a minimal amount.1,4,5
Hirsutism can be clinically graded according to the Ferriman-Gallwey scale2,6 and is defined as a Ferriman-Gallwey score of 8 or higher.1
HOW CIRCULATING ANDROGENS AFFECT HAIR FOLLICLES
In androgen-dependent areas, circulating androgens influence hair follicle characteristics. Androgens increase the size and diameter of the hair fibers in certain androgen-dependent sites, as seen in puberty with the transformation of vellus hairs (small, nonpigmented hairs) into terminal hairs (large, pigmented hairs) in the pubic and axillary regions in women, as well as the beard area in men.2,7 Interestingly, the same circulating androgens cause miniaturization of the susceptible hair follicles of the central scalp.7 The susceptibility of the hair follicle to the effects of the androgens may be genetically determined.7,8
Hirsutism is a sign of hyperandrogenism and increased action of androgens on hair follicles. In women, about half of circulating testosterone arises from the ovaries and adrenal glands; the rest originates from peripheral conversion of weaker androgens (such as androstenedione produced by the adrenals and ovaries) into testosterone.9 Dehydroepiandrosterone sulfate (DHEAS) originates mainly in the adrenal glands.9,10 Testosterone is converted to the more potent dihydrotestosterone (DHT) by type II 5-alpha reductase in the skin, which can then act on susceptible hair follicles.7,11 Therefore, hirsutism can be a consequence of endogenous androgen over-production from the ovaries or the adrenal glands (or both), of exposure to an exogenous source of androgen such as a drug, or of heightened hair follicle sensitivity and metabolism of normal circulating androgen levels (target end-organ dysfunction).1
‘IDIOPATHIC’ HIRSUTISM: A MISLEADING DIAGNOSIS
Many women with hirsutism are found to have polycystic ovary syndrome as the underlying cause, but hirsutism is also commonly labeled as idiopathic when it occurs without an obvious cause, eg, in women with regular menses and normal androgen levels and without features suspicious for other causes of hirsutism. 1,2,12,13 But while this term is commonly used,1,12 it may be misleading, especially if the diagnosis of idiopathic hirsutism is based on standard laboratory tests, which do not always detect androgen excess.2,13 Minor ovarian or adrenal functional hyperandrogenism,14 increased peripheral activity of 5-alpha reductase in the hair follicle, or abnormalities in the androgen receptor have been implicated in the pathogenesis of so-called idiopathic hirsutism. 2,15
HIRSUTISM AND POLYCYSTIC OVARY SYNDROME
Polycystic ovary syndrome, a metabolic syndrome, presents clinically with menstrual irregularities such as oligomenorrhea or amenorrhea, infertility, and signs of hyperandrogenism such as hirsutism, acne, or androgenetic alopecia.16,17 Metabolic disturbances including insulin resistance, impaired glucose tolerance, hyperlipidemia, and obesity (body mass index > 30 kg/m2) also can occur, thus increasing cardiovascular risk.16–18
The finding of polycystic ovaries is not required to make the diagnosis of polycystic ovary syndrome, and their presence does not prove the diagnosis.16,19 Gonadotropin-dependent functional ovarian hyperandrogenism is believed to cause this syndrome; however, mild adrenocorticotropic-dependent functional adrenal hyperandrogenism also is a feature in many cases. In rare cases, polycystic ovary syndrome presents with an isolated elevation of DHEAS.16,20
OTHER CONDITIONS OF EXCESS ANDROGEN
The syndrome of hyperandrogenism, insulin resistance, and acanthosis nigricans, abbreviated as HAIR-AN, is separate from polycystic ovary syndrome; it characterizes a group of inherited syndromes associated with severe metabolic abnormalities of insulin and glucose metabolism and with marked clinical signs of hyperandrogenism.12
The syndrome of seborrhea, acne, hirsutism, and acanthosis nigricans, abbreviated as SAHA, while not itself a diagnosis, is a clinical spectrum of dermatologic signs and symptoms also associated with hyperandrogenism. These are signs that may present with the HAIR-AN syndrome or with another cause of excess androgens, such as idiopathic, ovarian, adrenal, or hyperprolactinemic hyperandrogenism.21
Thyroid disease, hyperprolactinemia, acromegaly, Cushing syndrome, exogenous factors such as androgenic drugs, and nonclassical congenital adrenal hyperplasia can also produce hirsutism.12 In nonclassical congenital adrenal hyperplasia, which is typically caused by a deficiency of 21-hydroxylase, patients present with premature pubarche, hirsutism in the prepubertal years, and menstrual irregularities including primary amenorrhea.22,23
Important rare causes of hirsutism include benign and malignant androgen-secreting tumors of adrenal or ovarian origin. In such cases, hirsutism can have an acute onset or rapid progression and may be associated with features of virilization, such as deepened voice, increased muscle mass, androgenetic alopecia, clitoromegaly, and increased libido.12
A THOROUGH HISTORY IS CRITICAL TO DIAGNOSIS
A thorough medical history can provide important diagnostic clues in women with hirsutism. The clinician should elicit details about the onset and progression of the hair growth,12,15 previous treatments, and any cutaneous signs of hyperandrogenism, such as acne, seborrhea, acanthosis nigricans, or patterned hair loss.
Also important are the menstrual history and a history of infertility. Primary amenorrhea is defined as failure to menstruate by 16 years of age if secondary sexual characteristics have developed, or by 14 years of age if no secondary sexual characteristics have developed, and it can indicate nonclassical congenital adrenal hyperplasia.
The clinician should also try to determine if the patient has a history of galactorrhea or symptoms of virilization (eg, deepened voice, clitoromegaly, increased muscle mass); a family history of hirsutism, polycystic ovary syndrome, HAIR-AN syndrome, metabolic conditions such as type 2 diabetes mellitus, or cardiovascular disease12,15; or a history of symptoms of any condition known to produce hirsutism, such as Cushing disease, acromegaly, or a thyroid disorder. Also important is a drug history to determine if the patient has taken drugs such as androgens, anabolic steroids, or valproic acid (Depakote).20
THE PHYSICAL EXAMINATION
Another proposed predictor of hirsutism is that terminal hair on the chin or the lower abdomen (Ferriman-Gallwey score ≥ 2) is nearly 100% sensitive and 27% specific at predicting total-body hirsutism.24
As part of the physical examination, the clinician should also look for other cutaneous signs of hyperandrogenism, such as acne, androgenetic alopecia, and seborrhea. Acanthosis nigricans is a sign of insulin resistance. Height and weight should be measured and the body mass index calculated. Blood pressure should be recorded, as high blood pressure may be seen in Cushing syndrome and is an important cardiovascular risk factor. Signs of virilization should be identified. Indicators of Cushing disease such as striae, moon facies, fat redistribution, fragile skin, and proximal myopathy should be noted as well as signs of thyroid disease, such as textural skin changes, goiter, and hair loss. Expressible or spontaneous galactorrhea suggests hyperprolactinemia. Acromegaly is associated with coarse facies and enlarged hands and feet. Many of the endocrinopathies can be caused by a pituitary adenoma, which can manifest as a visual field defect, so visual fields should be examined.25 The examination should also exclude any palpable ovarian or adrenal mass.12
WHEN IS ADDITIONAL TESTING NEEDED?
The current Endocrine Society guidelines20 recommend obtaining an early-morning testosterone blood level in the following patients:
- Women with moderate or severe hirsutism
- Women with hirsutism of any degree with sudden onset or rapid progression, or accompanied by signs or symptoms suggesting malignancy or polycystic ovary syndrome: eg, menstrual irregularity, infertility, central obesity, clitoromegaly, or acanthosis nigricans.15,20
Testing androgen levels in mild, isolated hirsutism has not been proven to be useful or to alter management.20
Free testosterone level
An early-morning total or free testosterone level is the initial test in the laboratory evaluation of hirsutism.12,15 Additional specialized laboratory testing may be needed to determine the free testosterone level,15 as the free testosterone test is not available at all laboratories. A normal total testosterone level does not exclude hyperandrogenism but can suggest the diagnosis of idiopathic hirsutism.15
Further testing is needed if the total testosterone level is normal or only slightly elevated, or if there is a strong clinical suspicion of an underlying condition such as endocrinopathy or tumor. It is also useful in patients whose hirsutism responds poorly to medical treatments15 (see discussion below).
If the total testosterone level is elevated, if the hirsutism is moderate to severe, if there are associated symptoms, or if hirsutism is acute or progressive, a further endocrinologic workup is needed,15 possibly including measurement of free testosterone, sex hormone-binding globulin, DHEAS, and androstenedione.15 Free testosterone, unbound to sex hormone-binding globulin, is the biologically active fraction, with the levels of binding globulin increased by drugs such as oral contraceptives15 and decreased by high insulin levels in insulin resistance.25
Test in patients with mild hirsutism?
Although the guidelines suggest that no additional workup is necessary for women with mild hirsutism, we evaluate all patients with hirsutism and those with the SAHA clinical spectrum by measuring free and total testosterone and DHEAS. In our experience, even women with mild hirsutism with subtle symptoms and signs of hyperandrogenism and mild hirsutism often have elevated androgen levels.
Test in women with idiopathic hirsutism?
In women with idiopathic hirsutism, minor forms of functional ovarian and adrenal hyperandrogenism are believed to play a role and are thought to be undetectable with conventional testing.25 The gonadotropin-releasing hormone (GnRH) analogue stimulation test may uncover occult hyperandrogenism in this setting, but it is used as a research tool and does not currently have application in routine clinical practice.14
It is important to remember that some women with apparent idiopathic hirsutism and a history of regular menstrual cycles are actually oligo-ovulatory or anovulatory. In these instances, another diagnosis should be considered,13 and referral to an endocrinologist for further evaluation of ovulatory function is recommended.13
CURRENT USE OF DIAGNOSTIC IMAGING
When malignancy is suspected
A testosterone level above 200 ng/dL suggests an ovarian tumor, and a DHEAS level above 700 μg/dL suggests an adrenal tumor.26 However, not all tumors present with such high androgen levels, and sudden onset of hirsutism, rapid progression of hirsutism, or signs of virilization suggest a tumor.15 In such cases, transvaginal ultrasonography, computed tomography, or magnetic resonance imaging (MRI) of the abdomen can exclude an ovarian or adrenal tumor.
When polycystic ovary syndrome is suspected
The diagnosis of polycystic ovary syndrome is confirmed by two out of three criteria:
- Oligo-ovulation or anovulation
- Clinical or laboratory signs of hyperandrogenism
- Ultrasonographic evidence of polycystic ovaries, with exclusion of other causes of hyperandrogenism.
ADDITIONAL LABORATORY TESTING
Tests for polycystic ovary syndrome
Assessment of polycystic ovary syndrome involves transvaginal ultrasonography, but ultrasonographic evidence of a polycystic ovary is not necessary for the diagnosis.16 A fasting lipid profile and fasting serum glucose are recommended, and if the fasting serum glucose is normal, an oral glucose tolerance test is recommended. 17
Some have reported measuring the ratio of luteinizing hormone to follicle-stimulating hormone in the workup of polycystic ovary syndrome, and a ratio greater than 2 has been considered indicative but not diagnostic.16,25 The individual levels of luteinizing hormone, follicle-stimulating hormone, and estradiol are more important in the evaluation of infertility and ovulatory dysfunction. In patients with elevations of these hormones or with these symptoms, referral for infertility screening with an endocrinologist or gynecologist is recommended. 25
Additional testing and referral for Cushing syndrome, other conditions
Cushing syndrome can be tested for with a 24-hour urine cortisol, overnight low-dose dexamethasone suppression test, and late-night salivary cortisol.27,28 Referral to an endocrinologist for further testing can differentiate between corticotropin-dependent or corticotropin-independent Cushing syndrome.25 Cushing syndrome is often associated with hyperandrogenism, particularly in those cases caused by adrenal tumors.29
The prolactin level and the level of somatomedin C (insulin-like growth factor 1) can be used to rule out hyperprolactinemia and acromegaly, respectively.12 If Cushing syndrome, hyperprolactinemia, or acromegaly is diagnosed by endocrinologic testing, pituitary MRI should be performed.12,25
Referral to specialist centers with experience with these conditions is essential. Nonclassical congenital adrenal hyperplasia can be screened for by a serum 17-hydroxyprogesterone level measured in the follicular phase.12 Measurement of thyroid-stimulating hormone, free thyroxine, and thyroid peroxidase antibodies screens for thyroid disease.12 Hirsutism has been reported with the commencement of L-thyroxine therapy.30
THE PRINCIPLES OF TREATMENT
Patient education regarding the cause of hirsutism and reasonable treatment expectations and emotional support are important in the management of hirsutism. Also important is regular follow-up to measure and document the response to treatment; this can include repeating Ferriman-Gallwey scoring, taking photographs of affected areas, and retesting androgen levels after 3 to 6 months.12
Treatment must be continued for an ongoing effect, and most pharmacologic treatments can take up to 3 to 6 months to produce significant improvement.1
When an underlying condition is diagnosed, treatment of the condition is essential. Androgen-secreting tumors require surgical management.12 Cushing disease, hyperprolactinemia, and acromegaly should be clinically apparent from examination and testing, and appropriate referral and standard management should be instigated. Exogenous sources of androgen such as androgenic progestins or anabolic steroids should be discontinued. Lifestyle management is important, and weight loss in obese patients with polycystic ovary syndrome can improve hirsutism as well as mitigate cardiovascular risk factors.31
In classic congenital adrenal hyperplasia, glucocorticoid therapy manages both ovulation induction and hirsutism.20 However, in nonclassical congenital adrenal hyperplasia, glucocorticoid therapy supports ovulation induction, but hirsutism usually requires both systemic antiandrogen and hair removal.20
CURRENT OPTIONS FOR HAIR REMOVAL
The choice of method depends on patient preference, adverse effects, the degree of hirsutism, the level of distress, previous treatments, and cost.1,15,32
Self-care methods
Self-care methods offer only temporary reduction of excess hairs.
Plucking removes the entire hair, including the root, but it is painful and time-consuming, and it is only practical for areas where few hairs exist, such as on the face.1
Shaving is an easy, inexpensive, and painless choice for hair removal. Although a common belief is that shaving causes faster or thicker hair regrowth, shaving affects neither the diameter nor the rate of growth of the hair.32 Given its masculine association, shaving is not acceptable to most women except perhaps for use on the legs and axillae.1,32 Shaving can cause irritation, folliculitis, pseudofolliculitis, and infection.1
Waxing removes the entire hair. While it is more expensive than plucking, regrowth is slower, occurring over weeks. It is painful and can cause thermal burns, irritation, folliculitis, scarring, and postinflammatory dyspigmentation.1
Chemical depilatories, usually thioglycollic acid preparations, are inexpensive, painless, and easy to use. However, the resulting hair reduction is of short duration because the hair shafts are only removed at the level of the skin surface.1 They can also cause irritant dermatitis. 1
Bleaching with hydrogen peroxide is inexpensive and can camouflage dark facial hair, but it can also cause skin discoloration and irritation. 1
Clinic-based methods
Electrolysis often results in a permanent reduction in hair growth.1,32 A fine needle is placed into the hair follicle and an electrical current is applied. Each follicle is treated individually. 1,32 Best results are seen on darker hairs in patients with lighter skin, but it can be used on all skin types and hair colors.1,32
Electrolysis is operator-dependent, and there are US Food and Drug Administration (FDA) regulations regarding electrolysis techniques. It requires multiple treatments, and it is painful and can cause erythema, folliculitis, pseudofolliculitis, infection, scarring, and postinflammatory dyspigmentation.1,32 Some reports suggest that prior waxing and plucking of hairs damages the hair by twisting the hair shaft, making electrolysis more difficult.32
Laser treatment uses light of certain wavelengths to damage the hair follicles. While laser hair removal does not result in complete or persistent hair removal, it is more effective than shaving, waxing, and electrolysis, producing partial hair reduction for up to 6 months; the effect is enhanced with multiple treatments.33,34 The number of treatments required depends on the laser type and on the nature of the patient’s hair follicles.35
Laser systems for hair removal are of various wavelengths and also include intense pulsed light systems. The choice of system depends on the patient’s skin type and hair color. Women with fair skin and dark hair are ideal candidates; longer-wavelength lasers are preferred for darker or tanned skin types.
Adverse effects of laser hair removal include pain, erythema, burns, dyspigmentation, and scarring. Laser cooling devices can prevent or minimize some of these effects. Laser treatment has also been known to cause a paradoxical increase in hair growth.1,33,34
DRUG THERAPIES FOR HIRSUTISM
The drugs most commonly used for hirsutism are oral contraceptives (off-label use) and antiandrogenic drugs (off-label use). Topical eflornithine cream (Vaniqa) is FDA-approved for hirsutism but is less commonly used. Insulin sensitizers, GnRH analogues, and other drugs are occasionally used (off-label) to treat hirsutism.
Topical eflornithine cream
Topical eflornithine cream treats facial hirsutism by slowing the rate of hair growth; it does this by irreversibly inhibiting ornithine decarboxylase, an enzyme essential for hair growth.39,40 Studies showed that twice-daily application reduced unwanted facial hair in women after 24 weeks of treatment.39,40 Treatment must be continuous, since hair growth rapidly returns to the pretreatment rate by 8 weeks after discontinuing eflornithine.39,40 White women have been shown to respond better than black women.39 Adverse effects include a mild burning sensation, acne, pseudofolliculitis barbae, irritation, and allergic contact dermatitis.39,40 Improved outcomes have been suggested when eflornithine cream is combined with laser hair removal.41
Oral contraceptives
Oral contraceptives are commonly used off-label for the management of hirsutism.20 Oral contraceptives suppress the secretion of luteinizing hormone and, hence, the synthesis of ovarian androgen, thereby increasing levels of sex hormone-binding globulin and decreasing free plasma testosterone.1,20 Adrenal androgen production is also slightly reduced.20
Oral contraceptives usually combine a synthetic estrogen and a progestin. Certain progestins are more androgenic and should be avoided.1
For treating hirsutism, oral contraceptives should be used that contain low-androgenic progestins such as cyproterone acetate (not available in the United States), drosperinone (eg, in Yasmin), norgestimate (eg, in Ortho Tri-Cyclen), or desogestrel (eg, in Mircette).1,20
Side effects of oral contraceptives include breast tenderness, gastrointestinal upset, headache, loss of libido, hypertension, and the potential risk of venous thromboembolism.1,15,32,36
Antiandrogenic drugs
Several antiandrogenic drugs are used off-label to treat hirsutism.
Spironolactone (Aldactone), a competitive inhibitor of the androgen receptor and 5-alpha reductase activity,20 can be effective in the treatment of hirsutism. Monotherapy with spironolactone, without an oral contraceptive or other reliable form of contraception, is not recommended because of the teratogenic potential of all antiandrogens to feminize a developing male fetus.20 Thus, reliable contraception should be used in females of childbearing age when starting antiandrogen therapy.
The dosage of spironolactone for hirsutism is usually 100 mg to 200 mg daily.1,20 Hyperkalemia, polyuria, postural hypotension, irregular menses, and liver abnormalities are among the possible adverse effects (Table 3). Spironolactone was found to be tumorigenic in animal studies, although this has unknown relevance in humans.36
Cyproterone, an antiandrogen not available in the United States,42 competitively inhibits the androgen receptor and 5-alpha-reductase activity.1,20,36 It can be used for only the first 10 days of the menstrual cycle (50-mg or 100-mg dose) with an oral contraceptive pill, or in a low dose in a combined oral contraceptive pill (Diane-35 in Canada and the United Kingdom).1
Side effects are similar to those of oral contraceptives and include fatigue, mood change, risk of venous thromboembolism, and decreased libido.1,15,36 Importantly, in woman of childbearing age, there is the potential risk of feminization of a male fetus, so reliable contraception must be used.15,36
Flutamide, an investigational antiandrogen, has shown promise in the treatment of hirsutism.20 Flutamide is a nonsteroidal competitive inhibitor of androgen receptor binding. It carries a significant risk of hepatotoxicity. 1,15
Finasteride (Propecia) 1 mg is only occasionally used in the treatment of hirsutism (off-label usage). It inhibits type II 5-alphareductase to suppress dihydrotestosterone levels. 32 It carries a risk of gastrointestinal disturbance, decreased libido, hepatotoxicity, and feminization of a male fetus (pregnancy category X), so reliable contraception is required in all females of childbearing age, as with all antiandrogens1 (Table 3).
Dutasteride (Avodart), a type I and II 5-alpha-reductase inhibitor, has not been studied for the treatment of hirsutism (pregnancy category X).
Insulin sensitizers
Metformin (Glucophage) and other insulin sensitizers are less effective than antiandrogens at reducing hirsutism.20,38 However, metformin is effective at inducing ovulation in patients with polycystic ovary syndrome.38 Gastrointestinal upset is a common side effect; lactic acidosis is a serious but rare adverse effect.1
Gonadotropin-releasing hormone analogues
GnRH analogues are an option only if oral contraceptives and antiandrogen drugs are unsuccessful in patients with severe hyperandrogenism. 20 They suppress secretion of luteinizing hormone and the synthesis of ovarian androgen.1,20 These drugs are given as monthly intramuscular injections, usually with some form of estrogen-progestin replacement, since GnRH analogues cause estrogen levels to fall to menopausal levels.1
Side effects include signs and symptoms of menopause including hot flushes, atrophic vaginitis, and osteoporosis.1,15 These drugs completely inhibit ovulation, and some endocrinologists and gynecologists do not suggest further contraception in women of childbearing years for this reason. However, GnRH analogues are not approved as a contraceptive and are pregnancy category X.
Other drugs
Other drugs with antiandrogen activity include cimetidine and ketoconazole.12 Cimetidine (Tagamet) is not effective for the treatment of hirsutism, and ketoconazole (Nizoral) is associated with significant risk for adrenocortical suppression12 and hepatotoxicity in addition to multiple drug interactions, given its effect on the hepatic P450 enzyme system.
Acknowledgment: Many thanks to Rebecca Tung, MD, dermatologic surgeon, Cleveland Clinic, for her advice on lasers.
- Mofid A, Seyyed Alinaghi SA, Zandieh S, Yazdani T. Hirsutism. Int J Clin Pract 2008; 62:433–443.
- Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev 2000; 21:347–362.
- Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv 2006; 61:723–732.
- Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol 2001; 41:202–206.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999; 84:4006–4011.
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 21:1440–1447.
- Messenger AG. The control of hair growth: an overview. J Invest Dermatol 1993; 101(suppl 1):4S–9S.
- Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc 2005; 10:205–208.
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 1986; 15:213–228.
- Braunstein GD. Testis. In:Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology. 8th ed. New York: McGraw-Hill, 2007.
- Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000; 21:363–392.
- Practice Committee of the American Society for Reproductive Medicine. The evaluation and treatment of androgen excess. Fertil Steril 2006; 86(suppl 5):S241–S247.
- Azziz R, Waggoner WT, Ochoa T, Knochenhauer ES, Boots LR. Idiopathic hirsutism: an uncommon cause of hirsutism in Alabama. Fertil Steril 1998; 70:274–278.
- Rossi R, Tauchmanovà L, Luciano A, et al. Functional hyperandrogenism detected by corticotropin and GnRH-analogue stimulation tests in women affected by apparently idiopathic hirsutism. J Endocrinol Invest 2001; 24:491–498.
- Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med 2005; 353:2578–2588.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19:41–47.
- Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007; 92:4546–4556.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365:1415–1428.
- Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril 2005; 83:1343–1346.
- Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. http://www.endo-society.org/guidelines/final/upload/Hirsutism_Guideline.pdf. Accessed March 30, 2010.
- Orfanos CE, Adler YD, Zouboulis CC. The SAHA syndrome. Horm Res 2000; 54:251–258.
- New MI. Extensive clinical experience: nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab 2006; 91:4205–4214.
- Kohn B, Levine LS, Pollack MS, et al. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 1982; 55:817–827.
- Knochenhauer ES, Hines G, Conway-Myers BA, Azziz R. Examination of the chin or lower abdomen only for the prediction of hirsutism. Fertil Steril 2000; 74:980–983.
- Somani N, Harrison S, Bergfeld WF. The clinical evaluation of hirsutism. Dermatol Ther 2008; 21:376–391.
- Waggoner W, Boots LR, Azziz R. Total testosterone and DHEAS levels as predictors of androgen-secreting neoplasms: a populational study. Gynecol Endocrinol 1999; 13:394–400.
- Crapo L. Cushing’s syndrome: a review of diagnostic tests. Metabolism 1979; 28:955–977.
- Blethen SL, Chasalow FI. Overnight dexamethasone suppression test: normal responses and the diagnosis of Cushing’s syndrome. Steroids 1989; 54:185–193.
- Bertagna C, Orth DN. Clinical and laboratory findings and results of therapy in 58 patients with adrenocortical tumors admitted to a single medical center (1951 to 1978). Am J Med 1981; 71:855–875.
- Kologlu S, Baskal N, Kologlu LB, Laleli Y, Tuccar E. Hirsutism due to the treatment with L-thyroxine in patients with thyroid pathology. Endocrinologie 1988; 26:179–185.
- Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 2006; 91:3970–3980.
- Dawber RP. Guidance for the management of hirsutism. Curr Med Res Opin 2005; 21:1227–1234.
- Haedersdal M, Wulf HC. Evidence based review of hair removal using lasers and light sources. J Eur Acad Dermatol Venereol 2006; 20:9–20.
- Sadighha A, Mohaghegh Zahed G. Meta-analysis of hair removal laser trials. Lasers Med Sci 2009; 24:21–25.
- Casey AS, Goldberg D. Guidelines for laser hair removal. J Cosmet Laser Ther 2008; 10:24–33.
- Wakelin SH, Maibach HI, editors. Handbook of Systemic Drug Treatment in Dermatology. London: Manson Publishing Ltd, 2004.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1153–1160.
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1135–1142.
- Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol 2001; 2:197–201.
- Wolf JE, Shander D, Huber F, et al; Eflornithine HCl Study Group. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dematol 2007; 46:94–98.
- Hamzavi I, Tan E, Shapiro J, Lui H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol 2007; 57:54–59.
- Van der Spuy ZM, le Roux PA. Cyproterone acetate for hirsutism. Cochrane Database Syst Rev 2003; 4:CD001125.
- Mofid A, Seyyed Alinaghi SA, Zandieh S, Yazdani T. Hirsutism. Int J Clin Pract 2008; 62:433–443.
- Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev 2000; 21:347–362.
- Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv 2006; 61:723–732.
- Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol 2001; 41:202–206.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999; 84:4006–4011.
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 21:1440–1447.
- Messenger AG. The control of hair growth: an overview. J Invest Dermatol 1993; 101(suppl 1):4S–9S.
- Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc 2005; 10:205–208.
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 1986; 15:213–228.
- Braunstein GD. Testis. In:Gardner DG, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology. 8th ed. New York: McGraw-Hill, 2007.
- Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000; 21:363–392.
- Practice Committee of the American Society for Reproductive Medicine. The evaluation and treatment of androgen excess. Fertil Steril 2006; 86(suppl 5):S241–S247.
- Azziz R, Waggoner WT, Ochoa T, Knochenhauer ES, Boots LR. Idiopathic hirsutism: an uncommon cause of hirsutism in Alabama. Fertil Steril 1998; 70:274–278.
- Rossi R, Tauchmanovà L, Luciano A, et al. Functional hyperandrogenism detected by corticotropin and GnRH-analogue stimulation tests in women affected by apparently idiopathic hirsutism. J Endocrinol Invest 2001; 24:491–498.
- Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med 2005; 353:2578–2588.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19:41–47.
- Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007; 92:4546–4556.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365:1415–1428.
- Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril 2005; 83:1343–1346.
- Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. http://www.endo-society.org/guidelines/final/upload/Hirsutism_Guideline.pdf. Accessed March 30, 2010.
- Orfanos CE, Adler YD, Zouboulis CC. The SAHA syndrome. Horm Res 2000; 54:251–258.
- New MI. Extensive clinical experience: nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab 2006; 91:4205–4214.
- Kohn B, Levine LS, Pollack MS, et al. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 1982; 55:817–827.
- Knochenhauer ES, Hines G, Conway-Myers BA, Azziz R. Examination of the chin or lower abdomen only for the prediction of hirsutism. Fertil Steril 2000; 74:980–983.
- Somani N, Harrison S, Bergfeld WF. The clinical evaluation of hirsutism. Dermatol Ther 2008; 21:376–391.
- Waggoner W, Boots LR, Azziz R. Total testosterone and DHEAS levels as predictors of androgen-secreting neoplasms: a populational study. Gynecol Endocrinol 1999; 13:394–400.
- Crapo L. Cushing’s syndrome: a review of diagnostic tests. Metabolism 1979; 28:955–977.
- Blethen SL, Chasalow FI. Overnight dexamethasone suppression test: normal responses and the diagnosis of Cushing’s syndrome. Steroids 1989; 54:185–193.
- Bertagna C, Orth DN. Clinical and laboratory findings and results of therapy in 58 patients with adrenocortical tumors admitted to a single medical center (1951 to 1978). Am J Med 1981; 71:855–875.
- Kologlu S, Baskal N, Kologlu LB, Laleli Y, Tuccar E. Hirsutism due to the treatment with L-thyroxine in patients with thyroid pathology. Endocrinologie 1988; 26:179–185.
- Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 2006; 91:3970–3980.
- Dawber RP. Guidance for the management of hirsutism. Curr Med Res Opin 2005; 21:1227–1234.
- Haedersdal M, Wulf HC. Evidence based review of hair removal using lasers and light sources. J Eur Acad Dermatol Venereol 2006; 20:9–20.
- Sadighha A, Mohaghegh Zahed G. Meta-analysis of hair removal laser trials. Lasers Med Sci 2009; 24:21–25.
- Casey AS, Goldberg D. Guidelines for laser hair removal. J Cosmet Laser Ther 2008; 10:24–33.
- Wakelin SH, Maibach HI, editors. Handbook of Systemic Drug Treatment in Dermatology. London: Manson Publishing Ltd, 2004.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1153–1160.
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1135–1142.
- Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol 2001; 2:197–201.
- Wolf JE, Shander D, Huber F, et al; Eflornithine HCl Study Group. Randomized, double-blind clinical evaluation of the efficacy and safety of topical eflornithine HCl 13.9% cream in the treatment of women with facial hair. Int J Dematol 2007; 46:94–98.
- Hamzavi I, Tan E, Shapiro J, Lui H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J Am Acad Dermatol 2007; 57:54–59.
- Van der Spuy ZM, le Roux PA. Cyproterone acetate for hirsutism. Cochrane Database Syst Rev 2003; 4:CD001125.
KEY POINTS
- The finding of polycystic ovaries is not required for the diagnosis of polycystic ovary syndrome, nor does their presence prove the diagnosis. Gonadotropin-dependent functional ovarian hyperandrogenism is believed to cause this syndrome; however, mild adrenocorticotropic-dependent functional adrenal hyperandrogenism also is a feature in many cases.
- Even women with mild hirsutism with subtle symptoms and signs of hyperandrogenism can have elevated androgen levels, and thus, they deserve a laboratory evaluation.
- Laser treatment does not result in complete, permanent hair reduction, but it is more effective than shaving, waxing, and electrolysis, producing partial hair reduction for up to 6 months.