User login
Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
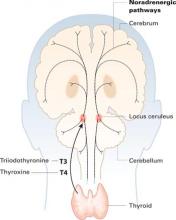
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.
Antidepressant boosters
Thyroid hormones have been used extensively to treat MDD since the 1950s, when researchers reported that T3 monotherapy was efficacious for treating depression. These early studies had important methodologic limitations, including open designs and poorly defined diagnostic criteria and response.
For MDD, the most extensively researched uses of thyroid hormones are to augment therapy for antidepressant nonresponders and to accelerate partial response to antidepressants.
Tricyclics. Open studies primarily among outpatients in the 1970s and ‘80s suggested that thyroid hormones are a valid augmentation strategy for nonresponders to tricyclic antidepressants. Most—but not all—reported response rates >50% with T3 dosages of 20 to 50 mcg/d.22,23
Compared with outpatient studies, however, an open trial of T3 augmentation by Birkenhager et al24 found no evidence of efficacy in 14 severely depressed inpatients who had not responded to 6 weeks of tricyclics. These patients—mainly with melancholic and/or psychotic depression—showed greater response to a monoamine oxidase inhibitor (MAOI) or electroconvulsive therapy than to thyroid hormone.
Three of five double-blind controlled studies of thyroid hormone augmentation of tricyclics reported response rates of 50 to 65% (Table).14,18,25 Earlier controlled studies, including two negative studies26,27 had important methodologic limitations: all included few subjects (≤16), and two studies included both unipolar and bipolar patients. Joffe et al partially addressed these problems with two randomized, double-blind studies that were controlled with placebo or T4:
- In a 3-week trial, T3 was more effective than T4 when added to imipramine or desipramine.14
- In a 2-week trial, T3 was significantly more effective than placebo when added to imipramine or desipramine.25
In the latter study, T3 and lithium augmentation appeared equally effective. Conversely, T4 was more effective than lithium as the first augmentation strategy in a double-blind, crossover study by Spoov and Lahdelma.28
In conclusion, depressed patients given T3 with tricyclic antidepressants may be twice as likely as controls to respond to treatment, according to a meta-analysis of eight studies totaling 292 patients. This analysis by Aronson et al29 supports T3 augmentation while addressing the surveyed studies’ limitations.
Table
Treatment-resistant MDD: Controlled studies of thyroid hormone augmentation
| Study authors/design | Initial therapy | Augmentation | Results |
|---|---|---|---|
| Steiner et al, 1978 Randomized double-blind; 8 patients* | Several TCAs (6 weeks) | T3, 25 mcg/d, or placebo (35 days) | T3: 75% responders (3/4) Placebo: 75% (3/4) |
| Goodwin et al, 1982 Double-blind, mirror design; 12 patients* | Desipramine or imipramine (4 weeks) | T3, 25 to 50 mcg/d, or placebo (21 days) | T3: 33% responders (4/12) Placebo: 0% (0/6) |
| Gitlin et al, 1987 Double-blind with crossover; 16 patients | Imipramine (4 weeks) | T3, 25 mcg/d, or placebo (2 weeks); crossover (2 weeks) | No difference between T3 and placebo |
| Joffe and Singer, 1990 Randomized double-blind; 38 patients | Desipramine or imipramine (4 weeks) | T3, 37.5 mcg/d, or T4, 150 mcg/d (21 days) | T3: 53% responders (9/17) T4: 19% (4/21) |
| Joffe et al, 1993 Randomized double-blind; 33 patients | Desipramine or imipramine (5 weeks) | T3, 37.5 mcg/d, or placebo (14 days) | T3: 59% responders (10/17) Placebo: 19% (3/16) |
| Sopov and Lahdelma, 1998 Randomized double-blind with crossover; 22 patients* | TCA, MAOI antidepressants | T4, 200 mcg/d, or lithium, 500 mg/d, (4 weeks); then crossover (4 weeks) | T4: 64% responders (7/11) Lithium: 18% responders (2/11) |
| *Included patients with unipolar or bipolar depression | |||
| MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; T3: triiodothyronine; T4: thyroxine; TCA: tricyclic antidepressant. | |||
MAO inhibitors. One small report has addressed the efficacy of thyroid hormones as adjuvants to MAOIs.30 In two patients, adding T3 to phenelzine enhanced the antidepressant response.
High-dose T4. Two open studies of patients with treatment-resistant bipolar or unipolar depression12,13 have examined the efficacy of high-dose T4 augmentation. These patients were taking a variety of antidepressants, including TCAs and SSRIs.
- In the study by Baurer et al,12 clinical remission (Hamilton Depression Scale [HAM-D] score ≤10) occurred in 4 of 5 patients with severe treatment-resistant unipolar depression who received adjunctive T4, mean 482±72 mcg/d, with antidepressants.
- In the study by Rudas et al,13 clinical remission (HAM-D ≤9) occurred in 7 of 9 patients with treatment-resistant MDD after T4, 150 to 300 mcg/d, was added to their antidepressant therapy. Side effects may limit this high-dose strategy, however, because 2 of the patients dropped out with thyrotoxicosis symptoms.
SSRIs. Three open trials to date have investigated using thyroid hormones to augment SSRIs in treatment-resistant MDD. In a prospective study by Agid and Lerer,31 10 of 25 (40%) patients who did not respond to SSRI treatment did so after T3 was added. No men improved, however, which led the authors to suggest that men and women might respond differently to T3 augmentation of SSRIs.
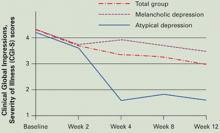
In our study, 7 of 20 patients (35%) with MDD who did not respond to 8 weeks of SSRI therapy did so when we added T3, 50 mcg/d, for 4 weeks (Figure). Response rates were high (5/5, 100%) in patients with atypical features by DSMIV criteria and low (1/8, 12.5%) in those with melancholic features.32
Abraham et al33 added T3, 50 mcg/d, to the regimens of 12 patients with MDD who did not respond to SSRIs alone. One patient dropped out with side effects. After 4 weeks of T3 augmentation, 5 patients (42%) showed 50% or greater improvement in HAM-D scores from baseline.
Figure T3 augmentation of SSRIs in 20 patients with resistant major depressive disorder
Open T3 augmentation, 50 mcg/d, given to 20 nonresponders to 8 weeks of selective serotonin reuptake inhibitors (SSRIs) improved baseline CGI-S scores significantly (P=0.006) at 4 weeks in those with atypical depression and modestly (P>0.05) in those with melancholic depression.
Source: Reference 32Antidepressant accelerators. Five of seven early double-blind, controlled studies indicated that adding small doses of thyroid hormones at the beginning of antidepressant treatment accelerated treatment response. All were limited by small sample sizes and other methodologic problems. A more-recent meta-analysis of six studies totalling 125 patients by Altshuler et al34 found:
- T3 was significantly more effective than placebo in accelerating clinical response to tricyclics
- the acceleration effect was more pronounced for women than for men.
Clinical recommendations
Thyroid hormones can be useful to augment and accelerate treatment of MDD. Evidence strongly supports their use with tricyclic antidepressants and suggests they also can be effective adjuvants for patients who do not respond to SSRIs.
Either T3 (up to 50 mcg/d) or T4 (up to 150 mcg/d) can be used as augmentation. T3’s antidepressant properties are considered more effective than those of T4, but the only head-to-head study supporting this conclusion was small (38 patients).14 Some T4 augmentation studies used very high dosages (300 to 600 mcg/d),12 which increase the risk of acute overdose.
Start T3 augmentation at 25 mcg/d and increase, if tolerated, to 50 mcg/d after 1 week. Measure baseline serum thyroid-stimulating hormone (TSH), and do not treat patients with TSH <0.5 mIU/L). Baseline TSH, T4, or T3 levels do not predict response to T3 augmentation in euthyroid MDD patients.32
Common side effects. Adjuvant T3, 25 to 50 mcg/d, was well-tolerated in our study of 20 patients also taking SSRIs:
- 2 (10%) experienced fatigue and diaphoresis
- 1 each (5%) had tremor, dry mouth, headaches, muscle aches, and vivid dreams.32
We saw no significant changes in blood pressure, but heart rates increased significantly in our 4-week study—from 76±12 bpm (range 60 to 96) to 82±9 bpm (range 66 to 96). Thus, T3 augmentation may not be indicated for patients with coronary artery disease or chronic heart failure. Patients’ weight decreased an average 2.5±6.6 lbs (range –20 to +7).
Thyroid hormones may cause hypoglycemia and change insulin requirements in patients with diabetes. High doses of T3 or T4 may be associated with hyperthyroidism, weight loss, nervousness, sweating, tachycardia, insomnia, heat intolerance, menstrual irregularities, palpitations, psychosis, or fever. Discontinue treatment if these symptoms develop.
Onset of antidepressant effect. Assess patient response in 4 to 6 weeks, whether using augmentation to address antidepressant nonresponse14,25 or to accelerate response.33 If you detect only partial improvement, studies support continuing treatment up to 8 weeks.
Treatment duration. No guidelines exist on how long to continue thyroid hormones after the initial antidepressant response. TSH levels become suppressed (TSH <0.1 mIU/L) after 4 weeks of T3, 50 mcg/d, in patients with normal baseline thyroid function.32 This suggests thyroid hormone’s booster effect is self limited, and augmentation may not need to continue after 2 to 3 months—even in responders.
T3 augmentation at 25 mcg/d can be discontinued immediately. For 50 to 75 mcg/d, taper across 1 to 2 weeks. The hypothalamic-pituitary-thyroid axis returns to normal function 6 to 8 weeks after T3 augmentation is stopped.
In this model, thyroid hormone augmentation can be used to boost antidepressant efficacy several months at a time. If effective, the same strategy could be tried again for subsequent MDD episodes.
Related resources
- DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol 2006;20(3):11-18.
- Massachusetts General Hospital Psychiatric Academy (including web-casts on treatment-resistant depression. www.mghcme.com
Drug brand names
- Desipramine • Norpramin
- Imipramine • Tofranil
- Levothyroxine (T4) • Levoxyl, Levothroid, Synthroid, others
- Liothyronine (synthetic T3) • Cytomel
Disclosures
The author receives research support from Aspect Medical Systems, Forest Laboratories, and Janssen Pharmaceutica; is a consultant to Pfizer, Inc., and Forest Laboratories; and a speaker for Eli Lilly and Co., Pfizer, Inc., and Cephalon.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200.
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
3. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3.
4. Nierenberg AA, Papakostas GI, Petersen T, et al. Nortriptyline for treatment-resistant depression. J Clin Psychiatry 2003;64(1):35-9.
5. Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience 1996;74:897-915.
6. Cheng LY, Outterbridge LV, Covatta ND, et al. Film autoradiography identifies unique features of [125I]3,3’5’-(reverse) triiodothyronine transport from blood to brain. J Neurophysiol 1994;72:380-91.
7. Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3’,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 1999;93:943-54.
8. Howland RH. Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993;54:47-54.
9. Joffe RT. Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 1999;45:1053-5.
10. Iosifescu DV, Howarth S, Alpert JE, et al. T3 blood levels and treatment outcome in depression. Int J Psychiatry Med 2001;31:367-73.
11. Joffe RT, Roy-Byrne PP, Udhe TW, Post RM. Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984;19:1685-91.
12. Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998;18:444-55.
13. Rudas S, Schmitz M, Pichler P, Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol Psychiatry 1999;45:229-33.
14. Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990;32:241-51.
15. Sandrini M, Vitale G, Vergoni AV, et al. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci 1996;58:1551-9.
16. Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999;288:81-7.
17. Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9.
18. Goodwin FK, Prange AJ, Post RM, et al. Potentiation of antidepressant effects by l-triiodothyronine in tricyclic nonresponders. Am J Psychiatry 1982;139:34-8.
19. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001;62(suppl 15):12-7.
20. Smith CD, Ain KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 1995;345:619-20.
21. Kato T, Murashita J, Shioiri T, et al. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417-22.
22. Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry 1970;126:1667-9.
23. Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression: I. An open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989;50:385-8.
24. Birkenhager TK, Vegt M, Nolen WA. An open study of triiodothyronine augmentation of tricyclic antidepressants in inpatients with refractory depression. Pharmacopsychiatry 1997;30:23-6.
25. Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-93.
26. Steiner M, Radwan M, Elizur A, et al. Failure of l-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 1978;23:655-9.
27. Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord 1987;13:267-72.
28. Spoov J, Lahdelma L. Should thyroid augmentation precede lithium augmentation—a pilot study. J Affect Disord 1998;49:235-9.
29. Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996;53:842-8.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry 1988;49:409-10.
31. Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol 2003;6(1):41-9.
32. Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66:1038-42.
33. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006;91(2-3):211-15.
34. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001;158:1617-22.
Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.
Antidepressant boosters
Thyroid hormones have been used extensively to treat MDD since the 1950s, when researchers reported that T3 monotherapy was efficacious for treating depression. These early studies had important methodologic limitations, including open designs and poorly defined diagnostic criteria and response.
For MDD, the most extensively researched uses of thyroid hormones are to augment therapy for antidepressant nonresponders and to accelerate partial response to antidepressants.
Tricyclics. Open studies primarily among outpatients in the 1970s and ‘80s suggested that thyroid hormones are a valid augmentation strategy for nonresponders to tricyclic antidepressants. Most—but not all—reported response rates >50% with T3 dosages of 20 to 50 mcg/d.22,23
Compared with outpatient studies, however, an open trial of T3 augmentation by Birkenhager et al24 found no evidence of efficacy in 14 severely depressed inpatients who had not responded to 6 weeks of tricyclics. These patients—mainly with melancholic and/or psychotic depression—showed greater response to a monoamine oxidase inhibitor (MAOI) or electroconvulsive therapy than to thyroid hormone.
Three of five double-blind controlled studies of thyroid hormone augmentation of tricyclics reported response rates of 50 to 65% (Table).14,18,25 Earlier controlled studies, including two negative studies26,27 had important methodologic limitations: all included few subjects (≤16), and two studies included both unipolar and bipolar patients. Joffe et al partially addressed these problems with two randomized, double-blind studies that were controlled with placebo or T4:
- In a 3-week trial, T3 was more effective than T4 when added to imipramine or desipramine.14
- In a 2-week trial, T3 was significantly more effective than placebo when added to imipramine or desipramine.25
In the latter study, T3 and lithium augmentation appeared equally effective. Conversely, T4 was more effective than lithium as the first augmentation strategy in a double-blind, crossover study by Spoov and Lahdelma.28
In conclusion, depressed patients given T3 with tricyclic antidepressants may be twice as likely as controls to respond to treatment, according to a meta-analysis of eight studies totaling 292 patients. This analysis by Aronson et al29 supports T3 augmentation while addressing the surveyed studies’ limitations.
Table
Treatment-resistant MDD: Controlled studies of thyroid hormone augmentation
| Study authors/design | Initial therapy | Augmentation | Results |
|---|---|---|---|
| Steiner et al, 1978 Randomized double-blind; 8 patients* | Several TCAs (6 weeks) | T3, 25 mcg/d, or placebo (35 days) | T3: 75% responders (3/4) Placebo: 75% (3/4) |
| Goodwin et al, 1982 Double-blind, mirror design; 12 patients* | Desipramine or imipramine (4 weeks) | T3, 25 to 50 mcg/d, or placebo (21 days) | T3: 33% responders (4/12) Placebo: 0% (0/6) |
| Gitlin et al, 1987 Double-blind with crossover; 16 patients | Imipramine (4 weeks) | T3, 25 mcg/d, or placebo (2 weeks); crossover (2 weeks) | No difference between T3 and placebo |
| Joffe and Singer, 1990 Randomized double-blind; 38 patients | Desipramine or imipramine (4 weeks) | T3, 37.5 mcg/d, or T4, 150 mcg/d (21 days) | T3: 53% responders (9/17) T4: 19% (4/21) |
| Joffe et al, 1993 Randomized double-blind; 33 patients | Desipramine or imipramine (5 weeks) | T3, 37.5 mcg/d, or placebo (14 days) | T3: 59% responders (10/17) Placebo: 19% (3/16) |
| Sopov and Lahdelma, 1998 Randomized double-blind with crossover; 22 patients* | TCA, MAOI antidepressants | T4, 200 mcg/d, or lithium, 500 mg/d, (4 weeks); then crossover (4 weeks) | T4: 64% responders (7/11) Lithium: 18% responders (2/11) |
| *Included patients with unipolar or bipolar depression | |||
| MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; T3: triiodothyronine; T4: thyroxine; TCA: tricyclic antidepressant. | |||
MAO inhibitors. One small report has addressed the efficacy of thyroid hormones as adjuvants to MAOIs.30 In two patients, adding T3 to phenelzine enhanced the antidepressant response.
High-dose T4. Two open studies of patients with treatment-resistant bipolar or unipolar depression12,13 have examined the efficacy of high-dose T4 augmentation. These patients were taking a variety of antidepressants, including TCAs and SSRIs.
- In the study by Baurer et al,12 clinical remission (Hamilton Depression Scale [HAM-D] score ≤10) occurred in 4 of 5 patients with severe treatment-resistant unipolar depression who received adjunctive T4, mean 482±72 mcg/d, with antidepressants.
- In the study by Rudas et al,13 clinical remission (HAM-D ≤9) occurred in 7 of 9 patients with treatment-resistant MDD after T4, 150 to 300 mcg/d, was added to their antidepressant therapy. Side effects may limit this high-dose strategy, however, because 2 of the patients dropped out with thyrotoxicosis symptoms.
SSRIs. Three open trials to date have investigated using thyroid hormones to augment SSRIs in treatment-resistant MDD. In a prospective study by Agid and Lerer,31 10 of 25 (40%) patients who did not respond to SSRI treatment did so after T3 was added. No men improved, however, which led the authors to suggest that men and women might respond differently to T3 augmentation of SSRIs.
In our study, 7 of 20 patients (35%) with MDD who did not respond to 8 weeks of SSRI therapy did so when we added T3, 50 mcg/d, for 4 weeks (Figure). Response rates were high (5/5, 100%) in patients with atypical features by DSMIV criteria and low (1/8, 12.5%) in those with melancholic features.32
Abraham et al33 added T3, 50 mcg/d, to the regimens of 12 patients with MDD who did not respond to SSRIs alone. One patient dropped out with side effects. After 4 weeks of T3 augmentation, 5 patients (42%) showed 50% or greater improvement in HAM-D scores from baseline.
Figure T3 augmentation of SSRIs in 20 patients with resistant major depressive disorder
Open T3 augmentation, 50 mcg/d, given to 20 nonresponders to 8 weeks of selective serotonin reuptake inhibitors (SSRIs) improved baseline CGI-S scores significantly (P=0.006) at 4 weeks in those with atypical depression and modestly (P>0.05) in those with melancholic depression.
Source: Reference 32Antidepressant accelerators. Five of seven early double-blind, controlled studies indicated that adding small doses of thyroid hormones at the beginning of antidepressant treatment accelerated treatment response. All were limited by small sample sizes and other methodologic problems. A more-recent meta-analysis of six studies totalling 125 patients by Altshuler et al34 found:
- T3 was significantly more effective than placebo in accelerating clinical response to tricyclics
- the acceleration effect was more pronounced for women than for men.
Clinical recommendations
Thyroid hormones can be useful to augment and accelerate treatment of MDD. Evidence strongly supports their use with tricyclic antidepressants and suggests they also can be effective adjuvants for patients who do not respond to SSRIs.
Either T3 (up to 50 mcg/d) or T4 (up to 150 mcg/d) can be used as augmentation. T3’s antidepressant properties are considered more effective than those of T4, but the only head-to-head study supporting this conclusion was small (38 patients).14 Some T4 augmentation studies used very high dosages (300 to 600 mcg/d),12 which increase the risk of acute overdose.
Start T3 augmentation at 25 mcg/d and increase, if tolerated, to 50 mcg/d after 1 week. Measure baseline serum thyroid-stimulating hormone (TSH), and do not treat patients with TSH <0.5 mIU/L). Baseline TSH, T4, or T3 levels do not predict response to T3 augmentation in euthyroid MDD patients.32
Common side effects. Adjuvant T3, 25 to 50 mcg/d, was well-tolerated in our study of 20 patients also taking SSRIs:
- 2 (10%) experienced fatigue and diaphoresis
- 1 each (5%) had tremor, dry mouth, headaches, muscle aches, and vivid dreams.32
We saw no significant changes in blood pressure, but heart rates increased significantly in our 4-week study—from 76±12 bpm (range 60 to 96) to 82±9 bpm (range 66 to 96). Thus, T3 augmentation may not be indicated for patients with coronary artery disease or chronic heart failure. Patients’ weight decreased an average 2.5±6.6 lbs (range –20 to +7).
Thyroid hormones may cause hypoglycemia and change insulin requirements in patients with diabetes. High doses of T3 or T4 may be associated with hyperthyroidism, weight loss, nervousness, sweating, tachycardia, insomnia, heat intolerance, menstrual irregularities, palpitations, psychosis, or fever. Discontinue treatment if these symptoms develop.
Onset of antidepressant effect. Assess patient response in 4 to 6 weeks, whether using augmentation to address antidepressant nonresponse14,25 or to accelerate response.33 If you detect only partial improvement, studies support continuing treatment up to 8 weeks.
Treatment duration. No guidelines exist on how long to continue thyroid hormones after the initial antidepressant response. TSH levels become suppressed (TSH <0.1 mIU/L) after 4 weeks of T3, 50 mcg/d, in patients with normal baseline thyroid function.32 This suggests thyroid hormone’s booster effect is self limited, and augmentation may not need to continue after 2 to 3 months—even in responders.
T3 augmentation at 25 mcg/d can be discontinued immediately. For 50 to 75 mcg/d, taper across 1 to 2 weeks. The hypothalamic-pituitary-thyroid axis returns to normal function 6 to 8 weeks after T3 augmentation is stopped.
In this model, thyroid hormone augmentation can be used to boost antidepressant efficacy several months at a time. If effective, the same strategy could be tried again for subsequent MDD episodes.
Related resources
- DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol 2006;20(3):11-18.
- Massachusetts General Hospital Psychiatric Academy (including web-casts on treatment-resistant depression. www.mghcme.com
Drug brand names
- Desipramine • Norpramin
- Imipramine • Tofranil
- Levothyroxine (T4) • Levoxyl, Levothroid, Synthroid, others
- Liothyronine (synthetic T3) • Cytomel
Disclosures
The author receives research support from Aspect Medical Systems, Forest Laboratories, and Janssen Pharmaceutica; is a consultant to Pfizer, Inc., and Forest Laboratories; and a speaker for Eli Lilly and Co., Pfizer, Inc., and Cephalon.
Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.
Antidepressant boosters
Thyroid hormones have been used extensively to treat MDD since the 1950s, when researchers reported that T3 monotherapy was efficacious for treating depression. These early studies had important methodologic limitations, including open designs and poorly defined diagnostic criteria and response.
For MDD, the most extensively researched uses of thyroid hormones are to augment therapy for antidepressant nonresponders and to accelerate partial response to antidepressants.
Tricyclics. Open studies primarily among outpatients in the 1970s and ‘80s suggested that thyroid hormones are a valid augmentation strategy for nonresponders to tricyclic antidepressants. Most—but not all—reported response rates >50% with T3 dosages of 20 to 50 mcg/d.22,23
Compared with outpatient studies, however, an open trial of T3 augmentation by Birkenhager et al24 found no evidence of efficacy in 14 severely depressed inpatients who had not responded to 6 weeks of tricyclics. These patients—mainly with melancholic and/or psychotic depression—showed greater response to a monoamine oxidase inhibitor (MAOI) or electroconvulsive therapy than to thyroid hormone.
Three of five double-blind controlled studies of thyroid hormone augmentation of tricyclics reported response rates of 50 to 65% (Table).14,18,25 Earlier controlled studies, including two negative studies26,27 had important methodologic limitations: all included few subjects (≤16), and two studies included both unipolar and bipolar patients. Joffe et al partially addressed these problems with two randomized, double-blind studies that were controlled with placebo or T4:
- In a 3-week trial, T3 was more effective than T4 when added to imipramine or desipramine.14
- In a 2-week trial, T3 was significantly more effective than placebo when added to imipramine or desipramine.25
In the latter study, T3 and lithium augmentation appeared equally effective. Conversely, T4 was more effective than lithium as the first augmentation strategy in a double-blind, crossover study by Spoov and Lahdelma.28
In conclusion, depressed patients given T3 with tricyclic antidepressants may be twice as likely as controls to respond to treatment, according to a meta-analysis of eight studies totaling 292 patients. This analysis by Aronson et al29 supports T3 augmentation while addressing the surveyed studies’ limitations.
Table
Treatment-resistant MDD: Controlled studies of thyroid hormone augmentation
| Study authors/design | Initial therapy | Augmentation | Results |
|---|---|---|---|
| Steiner et al, 1978 Randomized double-blind; 8 patients* | Several TCAs (6 weeks) | T3, 25 mcg/d, or placebo (35 days) | T3: 75% responders (3/4) Placebo: 75% (3/4) |
| Goodwin et al, 1982 Double-blind, mirror design; 12 patients* | Desipramine or imipramine (4 weeks) | T3, 25 to 50 mcg/d, or placebo (21 days) | T3: 33% responders (4/12) Placebo: 0% (0/6) |
| Gitlin et al, 1987 Double-blind with crossover; 16 patients | Imipramine (4 weeks) | T3, 25 mcg/d, or placebo (2 weeks); crossover (2 weeks) | No difference between T3 and placebo |
| Joffe and Singer, 1990 Randomized double-blind; 38 patients | Desipramine or imipramine (4 weeks) | T3, 37.5 mcg/d, or T4, 150 mcg/d (21 days) | T3: 53% responders (9/17) T4: 19% (4/21) |
| Joffe et al, 1993 Randomized double-blind; 33 patients | Desipramine or imipramine (5 weeks) | T3, 37.5 mcg/d, or placebo (14 days) | T3: 59% responders (10/17) Placebo: 19% (3/16) |
| Sopov and Lahdelma, 1998 Randomized double-blind with crossover; 22 patients* | TCA, MAOI antidepressants | T4, 200 mcg/d, or lithium, 500 mg/d, (4 weeks); then crossover (4 weeks) | T4: 64% responders (7/11) Lithium: 18% responders (2/11) |
| *Included patients with unipolar or bipolar depression | |||
| MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; T3: triiodothyronine; T4: thyroxine; TCA: tricyclic antidepressant. | |||
MAO inhibitors. One small report has addressed the efficacy of thyroid hormones as adjuvants to MAOIs.30 In two patients, adding T3 to phenelzine enhanced the antidepressant response.
High-dose T4. Two open studies of patients with treatment-resistant bipolar or unipolar depression12,13 have examined the efficacy of high-dose T4 augmentation. These patients were taking a variety of antidepressants, including TCAs and SSRIs.
- In the study by Baurer et al,12 clinical remission (Hamilton Depression Scale [HAM-D] score ≤10) occurred in 4 of 5 patients with severe treatment-resistant unipolar depression who received adjunctive T4, mean 482±72 mcg/d, with antidepressants.
- In the study by Rudas et al,13 clinical remission (HAM-D ≤9) occurred in 7 of 9 patients with treatment-resistant MDD after T4, 150 to 300 mcg/d, was added to their antidepressant therapy. Side effects may limit this high-dose strategy, however, because 2 of the patients dropped out with thyrotoxicosis symptoms.
SSRIs. Three open trials to date have investigated using thyroid hormones to augment SSRIs in treatment-resistant MDD. In a prospective study by Agid and Lerer,31 10 of 25 (40%) patients who did not respond to SSRI treatment did so after T3 was added. No men improved, however, which led the authors to suggest that men and women might respond differently to T3 augmentation of SSRIs.
In our study, 7 of 20 patients (35%) with MDD who did not respond to 8 weeks of SSRI therapy did so when we added T3, 50 mcg/d, for 4 weeks (Figure). Response rates were high (5/5, 100%) in patients with atypical features by DSMIV criteria and low (1/8, 12.5%) in those with melancholic features.32
Abraham et al33 added T3, 50 mcg/d, to the regimens of 12 patients with MDD who did not respond to SSRIs alone. One patient dropped out with side effects. After 4 weeks of T3 augmentation, 5 patients (42%) showed 50% or greater improvement in HAM-D scores from baseline.
Figure T3 augmentation of SSRIs in 20 patients with resistant major depressive disorder
Open T3 augmentation, 50 mcg/d, given to 20 nonresponders to 8 weeks of selective serotonin reuptake inhibitors (SSRIs) improved baseline CGI-S scores significantly (P=0.006) at 4 weeks in those with atypical depression and modestly (P>0.05) in those with melancholic depression.
Source: Reference 32Antidepressant accelerators. Five of seven early double-blind, controlled studies indicated that adding small doses of thyroid hormones at the beginning of antidepressant treatment accelerated treatment response. All were limited by small sample sizes and other methodologic problems. A more-recent meta-analysis of six studies totalling 125 patients by Altshuler et al34 found:
- T3 was significantly more effective than placebo in accelerating clinical response to tricyclics
- the acceleration effect was more pronounced for women than for men.
Clinical recommendations
Thyroid hormones can be useful to augment and accelerate treatment of MDD. Evidence strongly supports their use with tricyclic antidepressants and suggests they also can be effective adjuvants for patients who do not respond to SSRIs.
Either T3 (up to 50 mcg/d) or T4 (up to 150 mcg/d) can be used as augmentation. T3’s antidepressant properties are considered more effective than those of T4, but the only head-to-head study supporting this conclusion was small (38 patients).14 Some T4 augmentation studies used very high dosages (300 to 600 mcg/d),12 which increase the risk of acute overdose.
Start T3 augmentation at 25 mcg/d and increase, if tolerated, to 50 mcg/d after 1 week. Measure baseline serum thyroid-stimulating hormone (TSH), and do not treat patients with TSH <0.5 mIU/L). Baseline TSH, T4, or T3 levels do not predict response to T3 augmentation in euthyroid MDD patients.32
Common side effects. Adjuvant T3, 25 to 50 mcg/d, was well-tolerated in our study of 20 patients also taking SSRIs:
- 2 (10%) experienced fatigue and diaphoresis
- 1 each (5%) had tremor, dry mouth, headaches, muscle aches, and vivid dreams.32
We saw no significant changes in blood pressure, but heart rates increased significantly in our 4-week study—from 76±12 bpm (range 60 to 96) to 82±9 bpm (range 66 to 96). Thus, T3 augmentation may not be indicated for patients with coronary artery disease or chronic heart failure. Patients’ weight decreased an average 2.5±6.6 lbs (range –20 to +7).
Thyroid hormones may cause hypoglycemia and change insulin requirements in patients with diabetes. High doses of T3 or T4 may be associated with hyperthyroidism, weight loss, nervousness, sweating, tachycardia, insomnia, heat intolerance, menstrual irregularities, palpitations, psychosis, or fever. Discontinue treatment if these symptoms develop.
Onset of antidepressant effect. Assess patient response in 4 to 6 weeks, whether using augmentation to address antidepressant nonresponse14,25 or to accelerate response.33 If you detect only partial improvement, studies support continuing treatment up to 8 weeks.
Treatment duration. No guidelines exist on how long to continue thyroid hormones after the initial antidepressant response. TSH levels become suppressed (TSH <0.1 mIU/L) after 4 weeks of T3, 50 mcg/d, in patients with normal baseline thyroid function.32 This suggests thyroid hormone’s booster effect is self limited, and augmentation may not need to continue after 2 to 3 months—even in responders.
T3 augmentation at 25 mcg/d can be discontinued immediately. For 50 to 75 mcg/d, taper across 1 to 2 weeks. The hypothalamic-pituitary-thyroid axis returns to normal function 6 to 8 weeks after T3 augmentation is stopped.
In this model, thyroid hormone augmentation can be used to boost antidepressant efficacy several months at a time. If effective, the same strategy could be tried again for subsequent MDD episodes.
Related resources
- DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol 2006;20(3):11-18.
- Massachusetts General Hospital Psychiatric Academy (including web-casts on treatment-resistant depression. www.mghcme.com
Drug brand names
- Desipramine • Norpramin
- Imipramine • Tofranil
- Levothyroxine (T4) • Levoxyl, Levothroid, Synthroid, others
- Liothyronine (synthetic T3) • Cytomel
Disclosures
The author receives research support from Aspect Medical Systems, Forest Laboratories, and Janssen Pharmaceutica; is a consultant to Pfizer, Inc., and Forest Laboratories; and a speaker for Eli Lilly and Co., Pfizer, Inc., and Cephalon.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200.
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
3. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3.
4. Nierenberg AA, Papakostas GI, Petersen T, et al. Nortriptyline for treatment-resistant depression. J Clin Psychiatry 2003;64(1):35-9.
5. Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience 1996;74:897-915.
6. Cheng LY, Outterbridge LV, Covatta ND, et al. Film autoradiography identifies unique features of [125I]3,3’5’-(reverse) triiodothyronine transport from blood to brain. J Neurophysiol 1994;72:380-91.
7. Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3’,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 1999;93:943-54.
8. Howland RH. Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993;54:47-54.
9. Joffe RT. Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 1999;45:1053-5.
10. Iosifescu DV, Howarth S, Alpert JE, et al. T3 blood levels and treatment outcome in depression. Int J Psychiatry Med 2001;31:367-73.
11. Joffe RT, Roy-Byrne PP, Udhe TW, Post RM. Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984;19:1685-91.
12. Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998;18:444-55.
13. Rudas S, Schmitz M, Pichler P, Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol Psychiatry 1999;45:229-33.
14. Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990;32:241-51.
15. Sandrini M, Vitale G, Vergoni AV, et al. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci 1996;58:1551-9.
16. Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999;288:81-7.
17. Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9.
18. Goodwin FK, Prange AJ, Post RM, et al. Potentiation of antidepressant effects by l-triiodothyronine in tricyclic nonresponders. Am J Psychiatry 1982;139:34-8.
19. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001;62(suppl 15):12-7.
20. Smith CD, Ain KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 1995;345:619-20.
21. Kato T, Murashita J, Shioiri T, et al. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417-22.
22. Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry 1970;126:1667-9.
23. Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression: I. An open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989;50:385-8.
24. Birkenhager TK, Vegt M, Nolen WA. An open study of triiodothyronine augmentation of tricyclic antidepressants in inpatients with refractory depression. Pharmacopsychiatry 1997;30:23-6.
25. Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-93.
26. Steiner M, Radwan M, Elizur A, et al. Failure of l-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 1978;23:655-9.
27. Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord 1987;13:267-72.
28. Spoov J, Lahdelma L. Should thyroid augmentation precede lithium augmentation—a pilot study. J Affect Disord 1998;49:235-9.
29. Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996;53:842-8.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry 1988;49:409-10.
31. Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol 2003;6(1):41-9.
32. Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66:1038-42.
33. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006;91(2-3):211-15.
34. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001;158:1617-22.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996;19:179-200.
2. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
3. Nierenberg AA, McLean NE, Alpert JE, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3.
4. Nierenberg AA, Papakostas GI, Petersen T, et al. Nortriptyline for treatment-resistant depression. J Clin Psychiatry 2003;64(1):35-9.
5. Rozanov CB, Dratman MB. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience 1996;74:897-915.
6. Cheng LY, Outterbridge LV, Covatta ND, et al. Film autoradiography identifies unique features of [125I]3,3’5’-(reverse) triiodothyronine transport from blood to brain. J Neurophysiol 1994;72:380-91.
7. Gordon JT, Kaminski DM, Rozanov CB, Dratman MB. Evidence that 3,3’,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience 1999;93:943-54.
8. Howland RH. Thyroid dysfunction in refractory depression: implications for pathophysiology and treatment. J Clin Psychiatry 1993;54:47-54.
9. Joffe RT. Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 1999;45:1053-5.
10. Iosifescu DV, Howarth S, Alpert JE, et al. T3 blood levels and treatment outcome in depression. Int J Psychiatry Med 2001;31:367-73.
11. Joffe RT, Roy-Byrne PP, Udhe TW, Post RM. Thyroid function and affective illness: a reappraisal. Biol Psychiatry 1984;19:1685-91.
12. Bauer M, Hellweg R, Graf KJ, Baumgartner A. Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 1998;18:444-55.
13. Rudas S, Schmitz M, Pichler P, Baumgartner A. Treatment of refractory chronic depression and dysthymia with high-dose thyroxine. Biol Psychiatry 1999;45:229-33.
14. Joffe RT, Singer W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 1990;32:241-51.
15. Sandrini M, Vitale G, Vergoni AV, et al. Effect of acute and chronic treatment with triiodothyronine on serotonin levels and serotonergic receptor subtypes in the rat brain. Life Sci 1996;58:1551-9.
16. Gur E, Lerer B, Newman ME. Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999;288:81-7.
17. Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9.
18. Goodwin FK, Prange AJ, Post RM, et al. Potentiation of antidepressant effects by l-triiodothyronine in tricyclic nonresponders. Am J Psychiatry 1982;139:34-8.
19. Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001;62(suppl 15):12-7.
20. Smith CD, Ain KB. Brain metabolism in hypothyroidism studied with 31P magnetic-resonance spectroscopy. Lancet 1995;345:619-20.
21. Kato T, Murashita J, Shioiri T, et al. Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996;8:417-22.
22. Earle BV. Thyroid hormone and tricyclic antidepressants in resistant depressions. Am J Psychiatry 1970;126:1667-9.
23. Thase ME, Kupfer DJ, Jarrett DB. Treatment of imipramine-resistant recurrent depression: I. An open clinical trial of adjunctive l-triiodothyronine. J Clin Psychiatry 1989;50:385-8.
24. Birkenhager TK, Vegt M, Nolen WA. An open study of triiodothyronine augmentation of tricyclic antidepressants in inpatients with refractory depression. Pharmacopsychiatry 1997;30:23-6.
25. Joffe RT, Singer W, Levitt AJ, MacDonald C. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 1993;50:387-93.
26. Steiner M, Radwan M, Elizur A, et al. Failure of l-triiodothyronine to potentiate tricyclic antidepressant response. Curr Ther Res 1978;23:655-9.
27. Gitlin MJ, Weiner H, Fairbanks L, et al. Failure of T3 to potentiate tricyclic antidepressant response. J Affective Disord 1987;13:267-72.
28. Spoov J, Lahdelma L. Should thyroid augmentation precede lithium augmentation—a pilot study. J Affect Disord 1998;49:235-9.
29. Aronson R, Offman HJ, Joffe RT, Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 1996;53:842-8.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry 1988;49:409-10.
31. Agid O, Lerer B. Algorithm-based treatment of major depression in an outpatient clinic: clinical correlates of response to a specific serotonin reuptake inhibitor and to triiodothyronine augmentation. Int J Neuropsychopharmacol 2003;6(1):41-9.
32. Iosifescu DV, Nierenberg AA, Mischoulon D, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. J Clin Psychiatry 2005;66:1038-42.
33. Abraham G, Milev R, Stuart Lawson J. T3 augmentation of SSRI resistant depression. J Affect Disord 2006;91(2-3):211-15.
34. Altshuler LL, Bauer M, Frye MA, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 2001;158:1617-22.