User login
Topical ingenol mebutate gel effectively treated actinic keratoses when applied to the face, scalp, trunk, or extremities for 2-3 days, according to a report in the March 15 issue of the New England Journal of Medicine.
Compared with existing therapies for actinic keratoses, the chief advantage of ingenol mebutate gel is the short exposure time, reported Dr. Mark Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York, and his associates. This allows for relatively rapid resolution of local reactions, and it will likely improve adherence to treatment, which in turn should improve the therapy’s effectiveness, they noted.
"Many patients find it difficult to adhere to the currently available regimens of topical treatment that last for periods of 1-4 months, which may result in ‘real-world’ effectiveness lower than that achieved in supervised and patient-compensated clinical trials," Dr. Lebwohl and his colleagues wrote.
The researchers evaluated the safety and effectiveness of ingenol mebutate gel compared with a placebo in a manufacturer-sponsored, randomized, double-blind trial of 1,005 patients treated at four medical centers. The majority of the patients had Fitzpatrick type I or II skin, and their mean age was 65 years.
Approximately half of the study patients had a history of skin cancer, and 75% had already had their keratosis treated with cryotherapy, imiquimod, or topical fluorouracil.
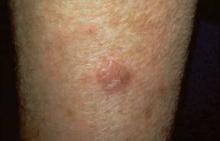
In each patient, a 25-cm contiguous field containing at least four to eight clinically typical and discrete actinic keratoses was selected for treatment. The study patients were divided into two groups according to the location of the treated area: on the head (face or scalp), and on the body (trunk or extremities).
Those with facial or scalp lesions were randomly assigned to self-apply either 0.015% active gel (277 patients) or placebo gel (270 patients) to the area once daily for 3 consecutive days. Those with trunk or extremity lesions were randomly assigned to self-apply 0.05% active gel (226 patients) or placebo gel (232 patients) to the area once daily for 2 consecutive days.
The primary end point was complete clearance of all clinically visible actinic keratoses in the treatment area on day 57.
Among patients with facial or scalp lesions, 42.2% of those who used active gel reached this end point, compared with only 3.7% of those who used placebo gel. Among patients with trunk or extremity lesions, 34.1% who used active gel reached this end point, compared with only 4.7% of those who used placebo gel.
In addition, patients with face or scalp lesions that were treated with ingenol mebutate showed a median reduction of 83% in the number of actinic keratoses, compared with a 0% reduction with placebo gel. "We calculated that the number of patients who needed to be treated with ingenol mebutate to obtain complete clearance in one patient was 2.6," the investigators reported (N. Engl. J. Med. 2012;366:1010-19).
Patients with trunk or extremity lesions treated with ingenol mebutate showed a median reduction of 75% in the number of actinic keratoses, compared with a 0% reduction with placebo gel. "We calculated that the number of patients who would need to be treated with ingenol mebutate to obtain complete clearance in one patient was 3.4," the researchers noted.
A secondary end point was partial (75% or more) clearance in the number of clinically visible actinic keratoses in the treatment area on day 57.
Among patients with facial or scalp lesions, 63.9% of those who used active gel reached this end point, compared with only 7.4% of those who used placebo gel. Among patients with trunk or extremity lesions, 49.1% of those who used active gel reached this end point, compared with only 6.9% of those who used placebo gel.
Patients who used ingenol mebutate showed minimal scarring or change in pigmentation. Local reactions such as erythema, crusting, swelling, vesiculation, or pustulation were common, but were mild to moderate in intensity; they peaked within a few days of treatment and resolved rapidly afterward, with no sequelae. One patient developed eye pain, burning, and periorbital edema related to ingenol mebutate and dropped out of the study, Dr. Lebwohl and his associates noted.
"Future studies are needed to assess the risks and benefits of treating larger areas of skin, using multiple treatments in the same area, and using combination therapies," they added.
This study was funded by LEO Pharma, maker of ingenol mebutate. Dr. Lebwohl reported ties to LEO Pharma, Graceway, PharmaDerm, and Peplin LTD.
Topical ingenol mebutate gel effectively treated actinic keratoses when applied to the face, scalp, trunk, or extremities for 2-3 days, according to a report in the March 15 issue of the New England Journal of Medicine.
Compared with existing therapies for actinic keratoses, the chief advantage of ingenol mebutate gel is the short exposure time, reported Dr. Mark Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York, and his associates. This allows for relatively rapid resolution of local reactions, and it will likely improve adherence to treatment, which in turn should improve the therapy’s effectiveness, they noted.
"Many patients find it difficult to adhere to the currently available regimens of topical treatment that last for periods of 1-4 months, which may result in ‘real-world’ effectiveness lower than that achieved in supervised and patient-compensated clinical trials," Dr. Lebwohl and his colleagues wrote.
The researchers evaluated the safety and effectiveness of ingenol mebutate gel compared with a placebo in a manufacturer-sponsored, randomized, double-blind trial of 1,005 patients treated at four medical centers. The majority of the patients had Fitzpatrick type I or II skin, and their mean age was 65 years.
Approximately half of the study patients had a history of skin cancer, and 75% had already had their keratosis treated with cryotherapy, imiquimod, or topical fluorouracil.
In each patient, a 25-cm contiguous field containing at least four to eight clinically typical and discrete actinic keratoses was selected for treatment. The study patients were divided into two groups according to the location of the treated area: on the head (face or scalp), and on the body (trunk or extremities).
Those with facial or scalp lesions were randomly assigned to self-apply either 0.015% active gel (277 patients) or placebo gel (270 patients) to the area once daily for 3 consecutive days. Those with trunk or extremity lesions were randomly assigned to self-apply 0.05% active gel (226 patients) or placebo gel (232 patients) to the area once daily for 2 consecutive days.
The primary end point was complete clearance of all clinically visible actinic keratoses in the treatment area on day 57.
Among patients with facial or scalp lesions, 42.2% of those who used active gel reached this end point, compared with only 3.7% of those who used placebo gel. Among patients with trunk or extremity lesions, 34.1% who used active gel reached this end point, compared with only 4.7% of those who used placebo gel.
In addition, patients with face or scalp lesions that were treated with ingenol mebutate showed a median reduction of 83% in the number of actinic keratoses, compared with a 0% reduction with placebo gel. "We calculated that the number of patients who needed to be treated with ingenol mebutate to obtain complete clearance in one patient was 2.6," the investigators reported (N. Engl. J. Med. 2012;366:1010-19).
Patients with trunk or extremity lesions treated with ingenol mebutate showed a median reduction of 75% in the number of actinic keratoses, compared with a 0% reduction with placebo gel. "We calculated that the number of patients who would need to be treated with ingenol mebutate to obtain complete clearance in one patient was 3.4," the researchers noted.
A secondary end point was partial (75% or more) clearance in the number of clinically visible actinic keratoses in the treatment area on day 57.
Among patients with facial or scalp lesions, 63.9% of those who used active gel reached this end point, compared with only 7.4% of those who used placebo gel. Among patients with trunk or extremity lesions, 49.1% of those who used active gel reached this end point, compared with only 6.9% of those who used placebo gel.
Patients who used ingenol mebutate showed minimal scarring or change in pigmentation. Local reactions such as erythema, crusting, swelling, vesiculation, or pustulation were common, but were mild to moderate in intensity; they peaked within a few days of treatment and resolved rapidly afterward, with no sequelae. One patient developed eye pain, burning, and periorbital edema related to ingenol mebutate and dropped out of the study, Dr. Lebwohl and his associates noted.
"Future studies are needed to assess the risks and benefits of treating larger areas of skin, using multiple treatments in the same area, and using combination therapies," they added.
This study was funded by LEO Pharma, maker of ingenol mebutate. Dr. Lebwohl reported ties to LEO Pharma, Graceway, PharmaDerm, and Peplin LTD.
Topical ingenol mebutate gel effectively treated actinic keratoses when applied to the face, scalp, trunk, or extremities for 2-3 days, according to a report in the March 15 issue of the New England Journal of Medicine.
Compared with existing therapies for actinic keratoses, the chief advantage of ingenol mebutate gel is the short exposure time, reported Dr. Mark Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York, and his associates. This allows for relatively rapid resolution of local reactions, and it will likely improve adherence to treatment, which in turn should improve the therapy’s effectiveness, they noted.
"Many patients find it difficult to adhere to the currently available regimens of topical treatment that last for periods of 1-4 months, which may result in ‘real-world’ effectiveness lower than that achieved in supervised and patient-compensated clinical trials," Dr. Lebwohl and his colleagues wrote.
The researchers evaluated the safety and effectiveness of ingenol mebutate gel compared with a placebo in a manufacturer-sponsored, randomized, double-blind trial of 1,005 patients treated at four medical centers. The majority of the patients had Fitzpatrick type I or II skin, and their mean age was 65 years.
Approximately half of the study patients had a history of skin cancer, and 75% had already had their keratosis treated with cryotherapy, imiquimod, or topical fluorouracil.
In each patient, a 25-cm contiguous field containing at least four to eight clinically typical and discrete actinic keratoses was selected for treatment. The study patients were divided into two groups according to the location of the treated area: on the head (face or scalp), and on the body (trunk or extremities).
Those with facial or scalp lesions were randomly assigned to self-apply either 0.015% active gel (277 patients) or placebo gel (270 patients) to the area once daily for 3 consecutive days. Those with trunk or extremity lesions were randomly assigned to self-apply 0.05% active gel (226 patients) or placebo gel (232 patients) to the area once daily for 2 consecutive days.
The primary end point was complete clearance of all clinically visible actinic keratoses in the treatment area on day 57.
Among patients with facial or scalp lesions, 42.2% of those who used active gel reached this end point, compared with only 3.7% of those who used placebo gel. Among patients with trunk or extremity lesions, 34.1% who used active gel reached this end point, compared with only 4.7% of those who used placebo gel.
In addition, patients with face or scalp lesions that were treated with ingenol mebutate showed a median reduction of 83% in the number of actinic keratoses, compared with a 0% reduction with placebo gel. "We calculated that the number of patients who needed to be treated with ingenol mebutate to obtain complete clearance in one patient was 2.6," the investigators reported (N. Engl. J. Med. 2012;366:1010-19).
Patients with trunk or extremity lesions treated with ingenol mebutate showed a median reduction of 75% in the number of actinic keratoses, compared with a 0% reduction with placebo gel. "We calculated that the number of patients who would need to be treated with ingenol mebutate to obtain complete clearance in one patient was 3.4," the researchers noted.
A secondary end point was partial (75% or more) clearance in the number of clinically visible actinic keratoses in the treatment area on day 57.
Among patients with facial or scalp lesions, 63.9% of those who used active gel reached this end point, compared with only 7.4% of those who used placebo gel. Among patients with trunk or extremity lesions, 49.1% of those who used active gel reached this end point, compared with only 6.9% of those who used placebo gel.
Patients who used ingenol mebutate showed minimal scarring or change in pigmentation. Local reactions such as erythema, crusting, swelling, vesiculation, or pustulation were common, but were mild to moderate in intensity; they peaked within a few days of treatment and resolved rapidly afterward, with no sequelae. One patient developed eye pain, burning, and periorbital edema related to ingenol mebutate and dropped out of the study, Dr. Lebwohl and his associates noted.
"Future studies are needed to assess the risks and benefits of treating larger areas of skin, using multiple treatments in the same area, and using combination therapies," they added.
This study was funded by LEO Pharma, maker of ingenol mebutate. Dr. Lebwohl reported ties to LEO Pharma, Graceway, PharmaDerm, and Peplin LTD.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: In patients with facial or scalp lesions, 42.2% who used ingenol mebutate gel achieved complete clearance at 2 months, compared with only 3.7% of those who used placebo gel; in patients with trunk or extremity lesions, 34.1% who used active gel achieved complete clearance, compared with only 4.7% of those who used placebo gel.
Data Source: Data are from a manufacturer-sponsored, randomized, double-blind trial of 1,005 patients treated at four medical centers.
Disclosures: This study was funded by LEO Pharma, maker of ingenol mebutate. Dr. Lebwohl reported ties to LEO Pharma, Graceway, PharmaDerm, and Peplin LTD.