User login
Manage, don’t accept adverse ‘calming’ effect
Sedation is a frequent side effect of antipsychotics, especially at relatively high doses. Antipsychotics’ sedative effects can reduce agitation in acute psychosis and promote sleep in insomnia, but long-term sedation may:
- interfere with schizophrenia patients’ efforts to go to work or school or engage in normal socialization
- prevent improvement from psychosocial training, psychiatric rehabilitation, and other treatments.
This article discusses how to manage acute psychosis without oversedation and ways to address persistent sedation and chronic insomnia with less-sedating antipsychotics or adjunctive medications.
Neurobiology or psychopharmacology?
Many patients experience only mild, transient somnolence at the beginning of antipsychotic treatment, and most develop some tolerance to the sedating effects with continued administration. Others may have persistent daytime sedation that interferes with normal functioning.
Sedation is especially common in elderly patients receiving antipsychotics. Compared with younger patients, older patients receiving the same doses of the same medications become more heavily sedated for longer periods of time. The resulting sedation can impair arousal levels during the day and increase the risk of falls.
Sedation can occur with first-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs), but it is seen more commonly and tends to be more severe with low-potency FGAs than with SGAs. Clinical challenges come with:
- distinguishing between sedation and negative symptoms of schizophrenia such as avolition, amotivation, withdrawal, and anhedonia
- determining whether an individual’s cognitive impairment is related to the antipsychotic’s sedative properties.
Because the treatments are different, it is important to try to distinguish negative symptoms and/or cognitive impairment related to schizophrenia’s neurobiology from sedation related to the antipsychotic. Ask patients if they nap during the day or just lie around, and if they want to do things but can’t:
- If they want to do things but feel too tired, this likely is sedation caused by the antipsychotic. Treatment might be a dose reduction.
- If they are not interested in doing things, the likely cause is negative symptoms. Treatment might be a medication such as a selective serotonin reuptake inhibitor.
- If they want to do things but cannot organize themselves to be able to do them, this likely is cognitive impairment. Treatment might be cognitive training or remediation.
Efficacy and sedation
Antipsychotics are thought to exert their effect by antagonism of postsynaptic dopamine D2 and serotonin 5HT2A receptors and possibly other receptors in the brain. Four SGAs—risperidone, olanzapine, quetiapine, and ziprasidone—act as dopamine D2 and 5HT2A antagonists. Aripiprazole is a dopamine D2 partial agonist, serotonin 5HT1A partial agonist, and serotonin 5HT2A antagonist.1 Efficacy data comparing SGAs with each other and with FGAs vary, but all 5 of these SGAs have been shown to be effective antipsychotics.2-4 They also generally cause less sedation than FGAs.
Patients with acute exacerbation of psychosis often have insomnia and frequently report paranoia that “something” will happen to them while they sleep. When treating agitated patients, many clinicians consider calming effects and true antipsychotic effects to be one in the same, which is not correct. All available antipsychotics are, on average, equally effective in treating acute psychotic symptoms but vary considerably in the amount of sedation they produce. Studies of short-acting injectable SGAs, such as ziprasidone and aripiprazole, have shown that agitation and acute symptoms can be controlled without significant sedation.5,6
Antipsychotic effects are not immediate and historically were thought to occur over several weeks. Recent meta-analyses suggest, however, that some antipsychotic effects are evident within the first week of treatment.7,8 To avoid overmedication, therefore, it’s important to separate calming effects from antipsychotic effects.
Recommendations. Choose the initial antipsychotic based on its effectiveness in treating the underlying disease, rather than relying on side effects—such as sedation—to control disease manifestations. Without sedation, patients are better able to engage in therapy; participate in family, social, school, and work activities; and increase their chances of recovery.
Initiate the antipsychotic at or titrate to a reasonable, not overly high dose—such as:
- olanzapine, 10 to 20 mg/d
- risperidone, 3 to 6 mg/d
- ziprasidone, 100 to 140 mg/d
- quetiapine, 400 to 600 mg/d
- aripiprazole, 15 mg/d.
Continue the patient on that dose, and use a nonantipsychotic such as a benzodiazepine to help control insomnia, anxiety, and agitation. Two to 4 weeks is generally adequate, but some patients may need the adjunctive therapy for several months. If you initiate a more sedating antipsychotic acutely, switching to a less sedating agent when the patient is stable and the illness is in remission may support adherence and improve outcomes.
Dosages and sedation. Not all FGAs have the same sedative effect, nor do all SGAs (Table 1).9 In general, the high-milligram, low-potency FGAs—such as chlorpromazine—produce more sedation than the low-milligram, high-potency FGAs—such as haloperidol and fluphenazine.9 This principle tends to hold true for the SGAs as well. For example, the high-potency, low-dose SGA risperidone is less sedating than the lower-potency, high-dose SGAs quetiapine and clozapine.
Dose does not always determine sedation, however. Olanzapine, which is commonly dosed at 15 to 30 mg/d, is more sedating than ziprasidone, for which the usual range is 80 to 160 mg/d.3,10-12
Table 1
Antipsychotics’ potency, dosages, and sedative properties
| Medication | Relative potency (mg)* | Common dosage (mg/d) | Sedation |
|---|---|---|---|
| First-generation antipsychotics | |||
| Chlorpromazine | 100.0 | 300 to 600 | Moderate |
| Fluphenazine | 1.0 to 2.0 | 4 to 20 | Mild |
| Haloperidol | 2.0 | 5 to 20 | Mild |
| Second-generation antipsychotics | |||
| Aripiprazole | 7.5 | 15 to 30 | Mild |
| Clozapine | 50.0 | 250 to 500 | Marked |
| Olanzapine | 4.0 | 15 to 30 | Moderate |
| Quetiapine | 80.0 | 300 to 800 | Moderate |
| Risperidone | 1.0 | 2 to 6 | Mild |
| Ziprasidone | 20.0 | 80 to 160 | Mild |
| * Approximate dose equivalent to 100 mg of chlorpromazine | |||
| Source: Data from reference 9. | |||
Mechanism and sedation
The mechanisms of antipsychotics’ therapeutic and sedative properties appear to be different.13 The degree of sedation shows little relationship with the various antipsychotics’ potency at the dopamine D2 receptor, which suggests that dopamine D2 receptor antagonism is not involved in causing sedation.
Instead, the degree of sedation may be associated with each antipsychotic’s affinity for the histamine H1 receptor, which is highly variable (Table 2).1,14,15 In general:
- agents that are more potent histamine H1 antagonists—such as olanzapine and clozapine14—produce more sedation
- agents that are weaker H1 antagonists—such as risperidone, ziprasidone, and aripiprazole—produce less sedation.
Although dosage and affinity for histamine H1 receptors play important roles in the sedative effect of a medication, what ultimately determines sedative effect is the combination of histamine H1 affinity and the amount of drug reaching the histamine H1 receptors in the CNS.
For example, the SGA quetiapine—which has moderate affinity for histamine H1 receptors14—also has relatively low affinity for dopamine D2 receptors. Higher dosages of quetiapine are therefore required to produce antipsychotic effects compared with other SGAs—such as risperidone, ziprasidone, and aripiprazole—that have higher affinities for the dopamine D2 receptors.
Because of higher dosing, higher amounts of quetiapine are assumed to be reaching the histamine H1 receptors in the CNS. This is why quetiapine causes more sedation in clinical use than does risperidone, even though risperidone has greater affinity for the histamine H1 receptor.
Table 2
Antipsychotics’ sedative effects by the numbers: Equilibrium dissociation constants at brain receptors*
| Receptors | |||
|---|---|---|---|
| Dopamine D2 | Serotonin 5-HT2A | Histamine H1 | |
| FGA | |||
| Haloperidol | 2.60 | 61.00 | 260.0 |
| SGAs | |||
| Aripiprazole | 0.34 | 3.40 | 61.00 |
| Clozapine | 210.00 | 2.60 | 3.10 |
| Olanzapine | 20.00 | 1.50 | 0.10 |
| Risperidone | 3.80 | 0.15 | 5.20 |
| Quetiapine | 770.00 | 31.00 | 19.00 |
| Ziprasidone | 2.60 | 0.12 | 4.60 |
| * Lower numbers are equivalent to higher receptor binding affinity. Each antipsychotic’s sedative effect is determined by histamine H1 affinity and the amount of drug that reaches H1 receptors in the CNS—which in turn is affected by the agent’s dosage and dopamine D2 receptor affinity. | |||
| FGA: first-generation antipsychotic; SGAs: second-generation antipsychotics | |||
| Source: References 1,14,15 | |||
Sleep patterns in mental illness
Sleep disturbances—including changes in sleep patterns, insomnia, and excessive sleeping—occur frequently in patients with psychiatric disorders. The sleep process itself (Table 3) is disrupted in patients with schizophrenia.
A study that examined sleep patterns in 40 patients with schizophrenia found longer sleep latency, more frequent arousals, and increased periods of wakefulness after sleep onset compared with controls without a psychiatric disorder. Ratings of sleep efficiency—ratio of sleep time to time in bed—were:
- 95% in the control group
- 78% in antipsychotic-naïve patients with schizophrenia
- 72% in patients with chronic schizophrenia.15
A study of sleep in 19 patients with schizophrenia and 13 nonpsychiatric controls16 found individuals with schizophrenia had:
- increased duration of stage 1 sleep
- decreased duration of stages 3 and 4 (slow-wave) sleep
- 83% total sleep efficiency, compared with 95% in nonpsychiatric controls.
Because of these differences in sleep patterns, patients with schizophrenia often experience inadequate sleep.
Antipsychotic effects on sleep patterns. Your choice of an antipsychotic also can affect the patient’s sleep. In a study of sleep measures in patients with schizophrenia treated with risperidone or haloperidol, Yamashita et al17 reported a significant difference in the time each group spent in slow-wave sleep (27% with risperidone vs 20% with haloperidol). The authors suggested that risperidone might lengthen the amount of slow-wave sleep because of its higher affinity for serotonin 5-HT2 receptors compared with haloperidol.
Salin-Pascual et al18 found that olanzapine improved total sleep time and sleep efficacy, reduced stage 1 sleep, and significantly enhanced stage 2 and slow-wave (delta) sleep.
Serotonin 5-HT2 receptors have been reported to be involved in controlling sleep quality.19 Similar to risperidone and olanzapine, the other SGAs also have a higher affinity than haloperidol for serotonin 5-HT2 receptors (Table 2). Thus, although antipsychotics’ sedative effects may adversely affect patients, SGAs may have the potential to improve sleep quality in individuals with schizophrenia. SGAs increase slow-wave sleep, and patients feel more rested after awakening.
Table 3
Normal sleep architecture, which can be disordered in schizophrenia
| Nonrapid eye movement (NREM) sleep | |
| Stage 1 | Drowsiness; represents transition between waking and sleeping |
| Stage 2 | Sleep deepens |
| Stages 3 and 4 | Deepest levels of NREM sleep subjects are most difficult to arouse (slow-wave or delta sleep) |
| Rapid eye movement (REM) sleep | |
| Vivid dreams; pulse and respiration rates are higher and more variable than during NREM sleep | |
| During the second half of the night, slow-wave sleep decreases compared with the first half of the night, but REM periods become more frequent and prolonged. | |
Managing excessive sedation
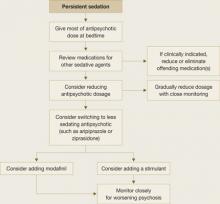
Take steps to minimize bothersome sedation in patients taking antipsychotics (Figure). To reduce daytime sedation, instruct the patient to take all or most of the antipsychotic dose at bedtime. Also rule out medical conditions that can produce fatigue and sedation, such as hypothyroidism, obstructive sleep apnea (OSA), and restless legs syndrome.
Figure
Managing sedation in patients with schizophrenia
Review the patient’s medication list to determine if other potentially sedating medications can be reduced or eliminated. Psychotropics that can cause sedation include:
- antidepressants such as the tricyclics and mirtazapine
- mood stabilizers (particularly valproic acid, but also carbamazepine, lithium, and lamotrigine).
Also consider gradually reducing the patient’s antipsychotic dose, and closely monitor for worsening of psychosis.
If sedation persists despite these interventions, consider switching the patient to a less sedating antipsychotic such as ziprasidone or aripiprazole. If these efforts also are ineffective, caffeine or off-label bupropion—75 to 100 mg once in the morning or up to twice daily—might help the patient feel more alert. Many patients taking antipsychotics drink several cups of coffee every morning to feel less sedated.
Stimulants. A consensus guideline on treating schizophrenia20 recommends prescribing amphetamine-related stimulants for patients who are persistently sedated, but this practice is highly controversial. Many stimulants increase dopamine release in the CNS, which theoretically can worsen psychosis. A clinician could be held liable for the actions of patients medicated with stimulants.
Modafinil is a nonamphetamine CNS stimulant approved for use in disorders of excessive sleep such as narcolepsy, OSA, shift work sleep disorder, and fatigue related to multiple sclerosis. Its mechanism of action in promoting wakefulness in these disorders is not fully understood; it may activate histaminergic projections in the frontal cortex from the tuberomammillary nucleus, which plays a major role in maintaining wakefulness.21
Modafinil, 200 mg in the morning, has been reported to reduce total sleep time without adverse effects in 3 patients experiencing sedation associated with antipsychotics.22 A later double-blind, placebo-controlled trial by Sevy et al23 found that modafinil and placebo were associated with similar, significant improvement in fatigue over time. Narendran24 reported a case in which modafinil might have exacerbated psychosis in a patient with schizophrenia who was taking 200 mg qid.
Managing chronic insomnia
Schizophrenia patients with chronic insomnia usually require education about appropriate sleep habits, combined with additional treatments.
Sleep hygiene. Instruct patients to:
- Wake up at the same time every day, regardless of when they went to sleep.
- Maintain a consistent bedtime.
- Exercise regularly, preferably in the late afternoon but not within 2 to 4 hours of bedtime.
- Perform relaxing activities before bed.
- Keep the bedroom quiet and cool (extreme temperatures compromise sleep).
- Do not watch the clock at night.
- Avoid caffeine and nicotine for at least 6 hours before bedtime.
- Drink alcohol only in moderation, and avoid use for at least 4 hours before bedtime.
- Avoid napping; it may interfere with the ability to fall asleep at night.
Medications. The consensus guideline on treating schizophrenia20 offers the option of switching the patient with chronic insomnia to one of the more sedating antipsychotics, such as olanzapine, quetiapine, or clozapine. Sedation alone should not be the reason to switch to clozapine, however.
You could consider adding a bedtime sedative to the patient’s medications (Table 4). FDA-approved sedatives include nonbenzodiazepines such as zolpidem, zolpidem extended-release, zaleplon, or eszopiclone, and the melatonin receptor agonist ramelteon. Although not approved as sedatives, some antidepressants such as trazodone or mirtazapine and antihistamines such as diphenhydramine and hydroxyzine are used to promote sleep. Benzodiazepines can be helpful but require caution when prescribed to patients with comorbid substance abuse disorders.
Sedatives have been studied extensively in general populations with insomnia but not in patients receiving antipsychotics. Combining antipsychotics and sedatives can produce daytime drowsiness and sedation.
Table 4
Sedatives to treat insomnia in patients with schizophrenia
| Medication | Common bedtime dose range* |
|---|---|
| Benzodiazepines | |
| Estazolam | 1 to 2 mg |
| Flurazepam | 15 to 30 mg |
| Temazepam | 7.5 to 30 mg |
| Triazolam | 0.125 to 0.25 mg |
| Benzodiazepine agonists | |
| Eszopiclone | 2 to 3 mg |
| Zaleplon | 5 to 10 mg |
| Zolpidem | 5 to 10 mg |
| Zolpidem CR | 6.25 to 12.5 mg |
| Melatonin receptor agonist | |
| Ramelteon | 8 mg |
| H1 antihistamines | |
| Diphenhydramine | 25 to 50 mg |
| Hydroxyzine | 50 to 100 mg |
| Antidepressants | |
| Mirtazapine | 15 to 30 mg |
| Trazodone | 50 to 200 mg |
| * No dosage adjustment is required in this patient population | |
Related resources
- Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry 2004;6(suppl 2):3-7.
- Benca RM, Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv 2005;56(3):332-43.
Drug Brand Names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Haloperidol • Haldol
- Hydroxyzine • Vistaril, Atarax
- Lamotrigine • Lamictal
- Mirtazapine • Remeron
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Ramelteon • Rozerem
- Risperidone • Risperdal
- Temazepam • Restor
- Trazodone • Desyrel
- Triazolam • Halcion
- Valproic acid • Depakote
- Zaleplon • Sonata
- Ziprasidone • Geodon
- Zolpidem • Ambien, Ambien CR
Disclosure
Dr. Miller receives research support from Pfizer Inc. and has received honoraria from AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, and Pfizer Inc.
1. McQuade R, Burris KD, Jordan S, et al. Aripiprazole: a dopamine-serotonin system stabilizer [abstract no. P.3.W.080]. Int J Neuropsychopharmacol 2002;5(suppl 1):S176.-
2. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003;60(6):553-64.
3. Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. Can Med Assoc J 2005;172(13):1703-11.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
5. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacol (Berl) 2006;188(3):281-92.
6. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. Ziprasidone I.M. Study Group. J Clin Psychiatry 2000;61(12):933-41.
7. Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 2003;60(12):1228-35.
8. Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 2005;57(12):1543-9.
9. Jibson MD, Tandon R. An overview of antipsychotic medications. CNS News 2001;3:49-54.
10. Collaborative Working Group on Clinical Trial Evaluations. Treatment of special populations with the atypical antipsychotics. J Clin Psychiatry 1998;59(suppl 12):46-52.
11. El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database Syst Rev 2004(2);CD004578.-
12. Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinol 2003;28(suppl 1):9-26.
13. Tandon R. Safety and tolerability: how do newer generation ”atypical” antipsychotics compare? Psychiatr Q 2002;73(4):297-311.
14. Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 2000;68(1):29-39.
15. Tandon R, Shipley JE, Taylor S, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry 1992;49(3):185-94.
16. Benson KL, Zarcone VP Jr. Rapid eye movement sleep eye movements in schizophrenia and depression. Arch Gen Psychiatry 1993;50(6):474-82.
17. Yamashita H, Morinobu S, Yamawaki S, et al. Effect of risperidone on sleep in schizophrenia: a comparison with haloperidol. Psychiatry Res 2002;109(2):137-42.
18. Salin-Pascual RJ, Herrera-Estrella M, Galicia-Polo L, Laurrabaquio MR. Olanzapine acute administration in schizophrenic patients increases delta sleep and sleep efficiency. Biol Psychiatry 1999;46(1):141-3.
19. Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res 1986;378(1):164-8.
20. Expert Consensus Guideline Series Treatment of schizophrenia. J Clin Psychiatry 1999;60(suppl 11):1-80.
21. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 2000;20(22):8620-8.
22. Makela EH, Miller K, Cutlip WD, 2nd. Three case reports of modafinil use in treating sedation induced by antipsychotic medications. J Clin Psychiatry 2003;64(4):485-6.
23. Sevy S, Rosenthal MH, Alvir J, et al. Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry 2005;66(7):839-43.
24. Narendran R, Young CM, Valenti AM, et al. Is psychosis exacerbated by modafinil? Arch Gen Psychiatry 2002;59(3):292-3.
Manage, don’t accept adverse ‘calming’ effect
Sedation is a frequent side effect of antipsychotics, especially at relatively high doses. Antipsychotics’ sedative effects can reduce agitation in acute psychosis and promote sleep in insomnia, but long-term sedation may:
- interfere with schizophrenia patients’ efforts to go to work or school or engage in normal socialization
- prevent improvement from psychosocial training, psychiatric rehabilitation, and other treatments.
This article discusses how to manage acute psychosis without oversedation and ways to address persistent sedation and chronic insomnia with less-sedating antipsychotics or adjunctive medications.
Neurobiology or psychopharmacology?
Many patients experience only mild, transient somnolence at the beginning of antipsychotic treatment, and most develop some tolerance to the sedating effects with continued administration. Others may have persistent daytime sedation that interferes with normal functioning.
Sedation is especially common in elderly patients receiving antipsychotics. Compared with younger patients, older patients receiving the same doses of the same medications become more heavily sedated for longer periods of time. The resulting sedation can impair arousal levels during the day and increase the risk of falls.
Sedation can occur with first-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs), but it is seen more commonly and tends to be more severe with low-potency FGAs than with SGAs. Clinical challenges come with:
- distinguishing between sedation and negative symptoms of schizophrenia such as avolition, amotivation, withdrawal, and anhedonia
- determining whether an individual’s cognitive impairment is related to the antipsychotic’s sedative properties.
Because the treatments are different, it is important to try to distinguish negative symptoms and/or cognitive impairment related to schizophrenia’s neurobiology from sedation related to the antipsychotic. Ask patients if they nap during the day or just lie around, and if they want to do things but can’t:
- If they want to do things but feel too tired, this likely is sedation caused by the antipsychotic. Treatment might be a dose reduction.
- If they are not interested in doing things, the likely cause is negative symptoms. Treatment might be a medication such as a selective serotonin reuptake inhibitor.
- If they want to do things but cannot organize themselves to be able to do them, this likely is cognitive impairment. Treatment might be cognitive training or remediation.
Efficacy and sedation
Antipsychotics are thought to exert their effect by antagonism of postsynaptic dopamine D2 and serotonin 5HT2A receptors and possibly other receptors in the brain. Four SGAs—risperidone, olanzapine, quetiapine, and ziprasidone—act as dopamine D2 and 5HT2A antagonists. Aripiprazole is a dopamine D2 partial agonist, serotonin 5HT1A partial agonist, and serotonin 5HT2A antagonist.1 Efficacy data comparing SGAs with each other and with FGAs vary, but all 5 of these SGAs have been shown to be effective antipsychotics.2-4 They also generally cause less sedation than FGAs.
Patients with acute exacerbation of psychosis often have insomnia and frequently report paranoia that “something” will happen to them while they sleep. When treating agitated patients, many clinicians consider calming effects and true antipsychotic effects to be one in the same, which is not correct. All available antipsychotics are, on average, equally effective in treating acute psychotic symptoms but vary considerably in the amount of sedation they produce. Studies of short-acting injectable SGAs, such as ziprasidone and aripiprazole, have shown that agitation and acute symptoms can be controlled without significant sedation.5,6
Antipsychotic effects are not immediate and historically were thought to occur over several weeks. Recent meta-analyses suggest, however, that some antipsychotic effects are evident within the first week of treatment.7,8 To avoid overmedication, therefore, it’s important to separate calming effects from antipsychotic effects.
Recommendations. Choose the initial antipsychotic based on its effectiveness in treating the underlying disease, rather than relying on side effects—such as sedation—to control disease manifestations. Without sedation, patients are better able to engage in therapy; participate in family, social, school, and work activities; and increase their chances of recovery.
Initiate the antipsychotic at or titrate to a reasonable, not overly high dose—such as:
- olanzapine, 10 to 20 mg/d
- risperidone, 3 to 6 mg/d
- ziprasidone, 100 to 140 mg/d
- quetiapine, 400 to 600 mg/d
- aripiprazole, 15 mg/d.
Continue the patient on that dose, and use a nonantipsychotic such as a benzodiazepine to help control insomnia, anxiety, and agitation. Two to 4 weeks is generally adequate, but some patients may need the adjunctive therapy for several months. If you initiate a more sedating antipsychotic acutely, switching to a less sedating agent when the patient is stable and the illness is in remission may support adherence and improve outcomes.
Dosages and sedation. Not all FGAs have the same sedative effect, nor do all SGAs (Table 1).9 In general, the high-milligram, low-potency FGAs—such as chlorpromazine—produce more sedation than the low-milligram, high-potency FGAs—such as haloperidol and fluphenazine.9 This principle tends to hold true for the SGAs as well. For example, the high-potency, low-dose SGA risperidone is less sedating than the lower-potency, high-dose SGAs quetiapine and clozapine.
Dose does not always determine sedation, however. Olanzapine, which is commonly dosed at 15 to 30 mg/d, is more sedating than ziprasidone, for which the usual range is 80 to 160 mg/d.3,10-12
Table 1
Antipsychotics’ potency, dosages, and sedative properties
| Medication | Relative potency (mg)* | Common dosage (mg/d) | Sedation |
|---|---|---|---|
| First-generation antipsychotics | |||
| Chlorpromazine | 100.0 | 300 to 600 | Moderate |
| Fluphenazine | 1.0 to 2.0 | 4 to 20 | Mild |
| Haloperidol | 2.0 | 5 to 20 | Mild |
| Second-generation antipsychotics | |||
| Aripiprazole | 7.5 | 15 to 30 | Mild |
| Clozapine | 50.0 | 250 to 500 | Marked |
| Olanzapine | 4.0 | 15 to 30 | Moderate |
| Quetiapine | 80.0 | 300 to 800 | Moderate |
| Risperidone | 1.0 | 2 to 6 | Mild |
| Ziprasidone | 20.0 | 80 to 160 | Mild |
| * Approximate dose equivalent to 100 mg of chlorpromazine | |||
| Source: Data from reference 9. | |||
Mechanism and sedation
The mechanisms of antipsychotics’ therapeutic and sedative properties appear to be different.13 The degree of sedation shows little relationship with the various antipsychotics’ potency at the dopamine D2 receptor, which suggests that dopamine D2 receptor antagonism is not involved in causing sedation.
Instead, the degree of sedation may be associated with each antipsychotic’s affinity for the histamine H1 receptor, which is highly variable (Table 2).1,14,15 In general:
- agents that are more potent histamine H1 antagonists—such as olanzapine and clozapine14—produce more sedation
- agents that are weaker H1 antagonists—such as risperidone, ziprasidone, and aripiprazole—produce less sedation.
Although dosage and affinity for histamine H1 receptors play important roles in the sedative effect of a medication, what ultimately determines sedative effect is the combination of histamine H1 affinity and the amount of drug reaching the histamine H1 receptors in the CNS.
For example, the SGA quetiapine—which has moderate affinity for histamine H1 receptors14—also has relatively low affinity for dopamine D2 receptors. Higher dosages of quetiapine are therefore required to produce antipsychotic effects compared with other SGAs—such as risperidone, ziprasidone, and aripiprazole—that have higher affinities for the dopamine D2 receptors.
Because of higher dosing, higher amounts of quetiapine are assumed to be reaching the histamine H1 receptors in the CNS. This is why quetiapine causes more sedation in clinical use than does risperidone, even though risperidone has greater affinity for the histamine H1 receptor.
Table 2
Antipsychotics’ sedative effects by the numbers: Equilibrium dissociation constants at brain receptors*
| Receptors | |||
|---|---|---|---|
| Dopamine D2 | Serotonin 5-HT2A | Histamine H1 | |
| FGA | |||
| Haloperidol | 2.60 | 61.00 | 260.0 |
| SGAs | |||
| Aripiprazole | 0.34 | 3.40 | 61.00 |
| Clozapine | 210.00 | 2.60 | 3.10 |
| Olanzapine | 20.00 | 1.50 | 0.10 |
| Risperidone | 3.80 | 0.15 | 5.20 |
| Quetiapine | 770.00 | 31.00 | 19.00 |
| Ziprasidone | 2.60 | 0.12 | 4.60 |
| * Lower numbers are equivalent to higher receptor binding affinity. Each antipsychotic’s sedative effect is determined by histamine H1 affinity and the amount of drug that reaches H1 receptors in the CNS—which in turn is affected by the agent’s dosage and dopamine D2 receptor affinity. | |||
| FGA: first-generation antipsychotic; SGAs: second-generation antipsychotics | |||
| Source: References 1,14,15 | |||
Sleep patterns in mental illness
Sleep disturbances—including changes in sleep patterns, insomnia, and excessive sleeping—occur frequently in patients with psychiatric disorders. The sleep process itself (Table 3) is disrupted in patients with schizophrenia.
A study that examined sleep patterns in 40 patients with schizophrenia found longer sleep latency, more frequent arousals, and increased periods of wakefulness after sleep onset compared with controls without a psychiatric disorder. Ratings of sleep efficiency—ratio of sleep time to time in bed—were:
- 95% in the control group
- 78% in antipsychotic-naïve patients with schizophrenia
- 72% in patients with chronic schizophrenia.15
A study of sleep in 19 patients with schizophrenia and 13 nonpsychiatric controls16 found individuals with schizophrenia had:
- increased duration of stage 1 sleep
- decreased duration of stages 3 and 4 (slow-wave) sleep
- 83% total sleep efficiency, compared with 95% in nonpsychiatric controls.
Because of these differences in sleep patterns, patients with schizophrenia often experience inadequate sleep.
Antipsychotic effects on sleep patterns. Your choice of an antipsychotic also can affect the patient’s sleep. In a study of sleep measures in patients with schizophrenia treated with risperidone or haloperidol, Yamashita et al17 reported a significant difference in the time each group spent in slow-wave sleep (27% with risperidone vs 20% with haloperidol). The authors suggested that risperidone might lengthen the amount of slow-wave sleep because of its higher affinity for serotonin 5-HT2 receptors compared with haloperidol.
Salin-Pascual et al18 found that olanzapine improved total sleep time and sleep efficacy, reduced stage 1 sleep, and significantly enhanced stage 2 and slow-wave (delta) sleep.
Serotonin 5-HT2 receptors have been reported to be involved in controlling sleep quality.19 Similar to risperidone and olanzapine, the other SGAs also have a higher affinity than haloperidol for serotonin 5-HT2 receptors (Table 2). Thus, although antipsychotics’ sedative effects may adversely affect patients, SGAs may have the potential to improve sleep quality in individuals with schizophrenia. SGAs increase slow-wave sleep, and patients feel more rested after awakening.
Table 3
Normal sleep architecture, which can be disordered in schizophrenia
| Nonrapid eye movement (NREM) sleep | |
| Stage 1 | Drowsiness; represents transition between waking and sleeping |
| Stage 2 | Sleep deepens |
| Stages 3 and 4 | Deepest levels of NREM sleep subjects are most difficult to arouse (slow-wave or delta sleep) |
| Rapid eye movement (REM) sleep | |
| Vivid dreams; pulse and respiration rates are higher and more variable than during NREM sleep | |
| During the second half of the night, slow-wave sleep decreases compared with the first half of the night, but REM periods become more frequent and prolonged. | |
Managing excessive sedation
Take steps to minimize bothersome sedation in patients taking antipsychotics (Figure). To reduce daytime sedation, instruct the patient to take all or most of the antipsychotic dose at bedtime. Also rule out medical conditions that can produce fatigue and sedation, such as hypothyroidism, obstructive sleep apnea (OSA), and restless legs syndrome.
Figure
Managing sedation in patients with schizophrenia
Review the patient’s medication list to determine if other potentially sedating medications can be reduced or eliminated. Psychotropics that can cause sedation include:
- antidepressants such as the tricyclics and mirtazapine
- mood stabilizers (particularly valproic acid, but also carbamazepine, lithium, and lamotrigine).
Also consider gradually reducing the patient’s antipsychotic dose, and closely monitor for worsening of psychosis.
If sedation persists despite these interventions, consider switching the patient to a less sedating antipsychotic such as ziprasidone or aripiprazole. If these efforts also are ineffective, caffeine or off-label bupropion—75 to 100 mg once in the morning or up to twice daily—might help the patient feel more alert. Many patients taking antipsychotics drink several cups of coffee every morning to feel less sedated.
Stimulants. A consensus guideline on treating schizophrenia20 recommends prescribing amphetamine-related stimulants for patients who are persistently sedated, but this practice is highly controversial. Many stimulants increase dopamine release in the CNS, which theoretically can worsen psychosis. A clinician could be held liable for the actions of patients medicated with stimulants.
Modafinil is a nonamphetamine CNS stimulant approved for use in disorders of excessive sleep such as narcolepsy, OSA, shift work sleep disorder, and fatigue related to multiple sclerosis. Its mechanism of action in promoting wakefulness in these disorders is not fully understood; it may activate histaminergic projections in the frontal cortex from the tuberomammillary nucleus, which plays a major role in maintaining wakefulness.21
Modafinil, 200 mg in the morning, has been reported to reduce total sleep time without adverse effects in 3 patients experiencing sedation associated with antipsychotics.22 A later double-blind, placebo-controlled trial by Sevy et al23 found that modafinil and placebo were associated with similar, significant improvement in fatigue over time. Narendran24 reported a case in which modafinil might have exacerbated psychosis in a patient with schizophrenia who was taking 200 mg qid.
Managing chronic insomnia
Schizophrenia patients with chronic insomnia usually require education about appropriate sleep habits, combined with additional treatments.
Sleep hygiene. Instruct patients to:
- Wake up at the same time every day, regardless of when they went to sleep.
- Maintain a consistent bedtime.
- Exercise regularly, preferably in the late afternoon but not within 2 to 4 hours of bedtime.
- Perform relaxing activities before bed.
- Keep the bedroom quiet and cool (extreme temperatures compromise sleep).
- Do not watch the clock at night.
- Avoid caffeine and nicotine for at least 6 hours before bedtime.
- Drink alcohol only in moderation, and avoid use for at least 4 hours before bedtime.
- Avoid napping; it may interfere with the ability to fall asleep at night.
Medications. The consensus guideline on treating schizophrenia20 offers the option of switching the patient with chronic insomnia to one of the more sedating antipsychotics, such as olanzapine, quetiapine, or clozapine. Sedation alone should not be the reason to switch to clozapine, however.
You could consider adding a bedtime sedative to the patient’s medications (Table 4). FDA-approved sedatives include nonbenzodiazepines such as zolpidem, zolpidem extended-release, zaleplon, or eszopiclone, and the melatonin receptor agonist ramelteon. Although not approved as sedatives, some antidepressants such as trazodone or mirtazapine and antihistamines such as diphenhydramine and hydroxyzine are used to promote sleep. Benzodiazepines can be helpful but require caution when prescribed to patients with comorbid substance abuse disorders.
Sedatives have been studied extensively in general populations with insomnia but not in patients receiving antipsychotics. Combining antipsychotics and sedatives can produce daytime drowsiness and sedation.
Table 4
Sedatives to treat insomnia in patients with schizophrenia
| Medication | Common bedtime dose range* |
|---|---|
| Benzodiazepines | |
| Estazolam | 1 to 2 mg |
| Flurazepam | 15 to 30 mg |
| Temazepam | 7.5 to 30 mg |
| Triazolam | 0.125 to 0.25 mg |
| Benzodiazepine agonists | |
| Eszopiclone | 2 to 3 mg |
| Zaleplon | 5 to 10 mg |
| Zolpidem | 5 to 10 mg |
| Zolpidem CR | 6.25 to 12.5 mg |
| Melatonin receptor agonist | |
| Ramelteon | 8 mg |
| H1 antihistamines | |
| Diphenhydramine | 25 to 50 mg |
| Hydroxyzine | 50 to 100 mg |
| Antidepressants | |
| Mirtazapine | 15 to 30 mg |
| Trazodone | 50 to 200 mg |
| * No dosage adjustment is required in this patient population | |
Related resources
- Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry 2004;6(suppl 2):3-7.
- Benca RM, Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv 2005;56(3):332-43.
Drug Brand Names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Haloperidol • Haldol
- Hydroxyzine • Vistaril, Atarax
- Lamotrigine • Lamictal
- Mirtazapine • Remeron
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Ramelteon • Rozerem
- Risperidone • Risperdal
- Temazepam • Restor
- Trazodone • Desyrel
- Triazolam • Halcion
- Valproic acid • Depakote
- Zaleplon • Sonata
- Ziprasidone • Geodon
- Zolpidem • Ambien, Ambien CR
Disclosure
Dr. Miller receives research support from Pfizer Inc. and has received honoraria from AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, and Pfizer Inc.
Manage, don’t accept adverse ‘calming’ effect
Sedation is a frequent side effect of antipsychotics, especially at relatively high doses. Antipsychotics’ sedative effects can reduce agitation in acute psychosis and promote sleep in insomnia, but long-term sedation may:
- interfere with schizophrenia patients’ efforts to go to work or school or engage in normal socialization
- prevent improvement from psychosocial training, psychiatric rehabilitation, and other treatments.
This article discusses how to manage acute psychosis without oversedation and ways to address persistent sedation and chronic insomnia with less-sedating antipsychotics or adjunctive medications.
Neurobiology or psychopharmacology?
Many patients experience only mild, transient somnolence at the beginning of antipsychotic treatment, and most develop some tolerance to the sedating effects with continued administration. Others may have persistent daytime sedation that interferes with normal functioning.
Sedation is especially common in elderly patients receiving antipsychotics. Compared with younger patients, older patients receiving the same doses of the same medications become more heavily sedated for longer periods of time. The resulting sedation can impair arousal levels during the day and increase the risk of falls.
Sedation can occur with first-generation antipsychotics (FGAs) and second-generation antipsychotics (SGAs), but it is seen more commonly and tends to be more severe with low-potency FGAs than with SGAs. Clinical challenges come with:
- distinguishing between sedation and negative symptoms of schizophrenia such as avolition, amotivation, withdrawal, and anhedonia
- determining whether an individual’s cognitive impairment is related to the antipsychotic’s sedative properties.
Because the treatments are different, it is important to try to distinguish negative symptoms and/or cognitive impairment related to schizophrenia’s neurobiology from sedation related to the antipsychotic. Ask patients if they nap during the day or just lie around, and if they want to do things but can’t:
- If they want to do things but feel too tired, this likely is sedation caused by the antipsychotic. Treatment might be a dose reduction.
- If they are not interested in doing things, the likely cause is negative symptoms. Treatment might be a medication such as a selective serotonin reuptake inhibitor.
- If they want to do things but cannot organize themselves to be able to do them, this likely is cognitive impairment. Treatment might be cognitive training or remediation.
Efficacy and sedation
Antipsychotics are thought to exert their effect by antagonism of postsynaptic dopamine D2 and serotonin 5HT2A receptors and possibly other receptors in the brain. Four SGAs—risperidone, olanzapine, quetiapine, and ziprasidone—act as dopamine D2 and 5HT2A antagonists. Aripiprazole is a dopamine D2 partial agonist, serotonin 5HT1A partial agonist, and serotonin 5HT2A antagonist.1 Efficacy data comparing SGAs with each other and with FGAs vary, but all 5 of these SGAs have been shown to be effective antipsychotics.2-4 They also generally cause less sedation than FGAs.
Patients with acute exacerbation of psychosis often have insomnia and frequently report paranoia that “something” will happen to them while they sleep. When treating agitated patients, many clinicians consider calming effects and true antipsychotic effects to be one in the same, which is not correct. All available antipsychotics are, on average, equally effective in treating acute psychotic symptoms but vary considerably in the amount of sedation they produce. Studies of short-acting injectable SGAs, such as ziprasidone and aripiprazole, have shown that agitation and acute symptoms can be controlled without significant sedation.5,6
Antipsychotic effects are not immediate and historically were thought to occur over several weeks. Recent meta-analyses suggest, however, that some antipsychotic effects are evident within the first week of treatment.7,8 To avoid overmedication, therefore, it’s important to separate calming effects from antipsychotic effects.
Recommendations. Choose the initial antipsychotic based on its effectiveness in treating the underlying disease, rather than relying on side effects—such as sedation—to control disease manifestations. Without sedation, patients are better able to engage in therapy; participate in family, social, school, and work activities; and increase their chances of recovery.
Initiate the antipsychotic at or titrate to a reasonable, not overly high dose—such as:
- olanzapine, 10 to 20 mg/d
- risperidone, 3 to 6 mg/d
- ziprasidone, 100 to 140 mg/d
- quetiapine, 400 to 600 mg/d
- aripiprazole, 15 mg/d.
Continue the patient on that dose, and use a nonantipsychotic such as a benzodiazepine to help control insomnia, anxiety, and agitation. Two to 4 weeks is generally adequate, but some patients may need the adjunctive therapy for several months. If you initiate a more sedating antipsychotic acutely, switching to a less sedating agent when the patient is stable and the illness is in remission may support adherence and improve outcomes.
Dosages and sedation. Not all FGAs have the same sedative effect, nor do all SGAs (Table 1).9 In general, the high-milligram, low-potency FGAs—such as chlorpromazine—produce more sedation than the low-milligram, high-potency FGAs—such as haloperidol and fluphenazine.9 This principle tends to hold true for the SGAs as well. For example, the high-potency, low-dose SGA risperidone is less sedating than the lower-potency, high-dose SGAs quetiapine and clozapine.
Dose does not always determine sedation, however. Olanzapine, which is commonly dosed at 15 to 30 mg/d, is more sedating than ziprasidone, for which the usual range is 80 to 160 mg/d.3,10-12
Table 1
Antipsychotics’ potency, dosages, and sedative properties
| Medication | Relative potency (mg)* | Common dosage (mg/d) | Sedation |
|---|---|---|---|
| First-generation antipsychotics | |||
| Chlorpromazine | 100.0 | 300 to 600 | Moderate |
| Fluphenazine | 1.0 to 2.0 | 4 to 20 | Mild |
| Haloperidol | 2.0 | 5 to 20 | Mild |
| Second-generation antipsychotics | |||
| Aripiprazole | 7.5 | 15 to 30 | Mild |
| Clozapine | 50.0 | 250 to 500 | Marked |
| Olanzapine | 4.0 | 15 to 30 | Moderate |
| Quetiapine | 80.0 | 300 to 800 | Moderate |
| Risperidone | 1.0 | 2 to 6 | Mild |
| Ziprasidone | 20.0 | 80 to 160 | Mild |
| * Approximate dose equivalent to 100 mg of chlorpromazine | |||
| Source: Data from reference 9. | |||
Mechanism and sedation
The mechanisms of antipsychotics’ therapeutic and sedative properties appear to be different.13 The degree of sedation shows little relationship with the various antipsychotics’ potency at the dopamine D2 receptor, which suggests that dopamine D2 receptor antagonism is not involved in causing sedation.
Instead, the degree of sedation may be associated with each antipsychotic’s affinity for the histamine H1 receptor, which is highly variable (Table 2).1,14,15 In general:
- agents that are more potent histamine H1 antagonists—such as olanzapine and clozapine14—produce more sedation
- agents that are weaker H1 antagonists—such as risperidone, ziprasidone, and aripiprazole—produce less sedation.
Although dosage and affinity for histamine H1 receptors play important roles in the sedative effect of a medication, what ultimately determines sedative effect is the combination of histamine H1 affinity and the amount of drug reaching the histamine H1 receptors in the CNS.
For example, the SGA quetiapine—which has moderate affinity for histamine H1 receptors14—also has relatively low affinity for dopamine D2 receptors. Higher dosages of quetiapine are therefore required to produce antipsychotic effects compared with other SGAs—such as risperidone, ziprasidone, and aripiprazole—that have higher affinities for the dopamine D2 receptors.
Because of higher dosing, higher amounts of quetiapine are assumed to be reaching the histamine H1 receptors in the CNS. This is why quetiapine causes more sedation in clinical use than does risperidone, even though risperidone has greater affinity for the histamine H1 receptor.
Table 2
Antipsychotics’ sedative effects by the numbers: Equilibrium dissociation constants at brain receptors*
| Receptors | |||
|---|---|---|---|
| Dopamine D2 | Serotonin 5-HT2A | Histamine H1 | |
| FGA | |||
| Haloperidol | 2.60 | 61.00 | 260.0 |
| SGAs | |||
| Aripiprazole | 0.34 | 3.40 | 61.00 |
| Clozapine | 210.00 | 2.60 | 3.10 |
| Olanzapine | 20.00 | 1.50 | 0.10 |
| Risperidone | 3.80 | 0.15 | 5.20 |
| Quetiapine | 770.00 | 31.00 | 19.00 |
| Ziprasidone | 2.60 | 0.12 | 4.60 |
| * Lower numbers are equivalent to higher receptor binding affinity. Each antipsychotic’s sedative effect is determined by histamine H1 affinity and the amount of drug that reaches H1 receptors in the CNS—which in turn is affected by the agent’s dosage and dopamine D2 receptor affinity. | |||
| FGA: first-generation antipsychotic; SGAs: second-generation antipsychotics | |||
| Source: References 1,14,15 | |||
Sleep patterns in mental illness
Sleep disturbances—including changes in sleep patterns, insomnia, and excessive sleeping—occur frequently in patients with psychiatric disorders. The sleep process itself (Table 3) is disrupted in patients with schizophrenia.
A study that examined sleep patterns in 40 patients with schizophrenia found longer sleep latency, more frequent arousals, and increased periods of wakefulness after sleep onset compared with controls without a psychiatric disorder. Ratings of sleep efficiency—ratio of sleep time to time in bed—were:
- 95% in the control group
- 78% in antipsychotic-naïve patients with schizophrenia
- 72% in patients with chronic schizophrenia.15
A study of sleep in 19 patients with schizophrenia and 13 nonpsychiatric controls16 found individuals with schizophrenia had:
- increased duration of stage 1 sleep
- decreased duration of stages 3 and 4 (slow-wave) sleep
- 83% total sleep efficiency, compared with 95% in nonpsychiatric controls.
Because of these differences in sleep patterns, patients with schizophrenia often experience inadequate sleep.
Antipsychotic effects on sleep patterns. Your choice of an antipsychotic also can affect the patient’s sleep. In a study of sleep measures in patients with schizophrenia treated with risperidone or haloperidol, Yamashita et al17 reported a significant difference in the time each group spent in slow-wave sleep (27% with risperidone vs 20% with haloperidol). The authors suggested that risperidone might lengthen the amount of slow-wave sleep because of its higher affinity for serotonin 5-HT2 receptors compared with haloperidol.
Salin-Pascual et al18 found that olanzapine improved total sleep time and sleep efficacy, reduced stage 1 sleep, and significantly enhanced stage 2 and slow-wave (delta) sleep.
Serotonin 5-HT2 receptors have been reported to be involved in controlling sleep quality.19 Similar to risperidone and olanzapine, the other SGAs also have a higher affinity than haloperidol for serotonin 5-HT2 receptors (Table 2). Thus, although antipsychotics’ sedative effects may adversely affect patients, SGAs may have the potential to improve sleep quality in individuals with schizophrenia. SGAs increase slow-wave sleep, and patients feel more rested after awakening.
Table 3
Normal sleep architecture, which can be disordered in schizophrenia
| Nonrapid eye movement (NREM) sleep | |
| Stage 1 | Drowsiness; represents transition between waking and sleeping |
| Stage 2 | Sleep deepens |
| Stages 3 and 4 | Deepest levels of NREM sleep subjects are most difficult to arouse (slow-wave or delta sleep) |
| Rapid eye movement (REM) sleep | |
| Vivid dreams; pulse and respiration rates are higher and more variable than during NREM sleep | |
| During the second half of the night, slow-wave sleep decreases compared with the first half of the night, but REM periods become more frequent and prolonged. | |
Managing excessive sedation
Take steps to minimize bothersome sedation in patients taking antipsychotics (Figure). To reduce daytime sedation, instruct the patient to take all or most of the antipsychotic dose at bedtime. Also rule out medical conditions that can produce fatigue and sedation, such as hypothyroidism, obstructive sleep apnea (OSA), and restless legs syndrome.
Figure
Managing sedation in patients with schizophrenia
Review the patient’s medication list to determine if other potentially sedating medications can be reduced or eliminated. Psychotropics that can cause sedation include:
- antidepressants such as the tricyclics and mirtazapine
- mood stabilizers (particularly valproic acid, but also carbamazepine, lithium, and lamotrigine).
Also consider gradually reducing the patient’s antipsychotic dose, and closely monitor for worsening of psychosis.
If sedation persists despite these interventions, consider switching the patient to a less sedating antipsychotic such as ziprasidone or aripiprazole. If these efforts also are ineffective, caffeine or off-label bupropion—75 to 100 mg once in the morning or up to twice daily—might help the patient feel more alert. Many patients taking antipsychotics drink several cups of coffee every morning to feel less sedated.
Stimulants. A consensus guideline on treating schizophrenia20 recommends prescribing amphetamine-related stimulants for patients who are persistently sedated, but this practice is highly controversial. Many stimulants increase dopamine release in the CNS, which theoretically can worsen psychosis. A clinician could be held liable for the actions of patients medicated with stimulants.
Modafinil is a nonamphetamine CNS stimulant approved for use in disorders of excessive sleep such as narcolepsy, OSA, shift work sleep disorder, and fatigue related to multiple sclerosis. Its mechanism of action in promoting wakefulness in these disorders is not fully understood; it may activate histaminergic projections in the frontal cortex from the tuberomammillary nucleus, which plays a major role in maintaining wakefulness.21
Modafinil, 200 mg in the morning, has been reported to reduce total sleep time without adverse effects in 3 patients experiencing sedation associated with antipsychotics.22 A later double-blind, placebo-controlled trial by Sevy et al23 found that modafinil and placebo were associated with similar, significant improvement in fatigue over time. Narendran24 reported a case in which modafinil might have exacerbated psychosis in a patient with schizophrenia who was taking 200 mg qid.
Managing chronic insomnia
Schizophrenia patients with chronic insomnia usually require education about appropriate sleep habits, combined with additional treatments.
Sleep hygiene. Instruct patients to:
- Wake up at the same time every day, regardless of when they went to sleep.
- Maintain a consistent bedtime.
- Exercise regularly, preferably in the late afternoon but not within 2 to 4 hours of bedtime.
- Perform relaxing activities before bed.
- Keep the bedroom quiet and cool (extreme temperatures compromise sleep).
- Do not watch the clock at night.
- Avoid caffeine and nicotine for at least 6 hours before bedtime.
- Drink alcohol only in moderation, and avoid use for at least 4 hours before bedtime.
- Avoid napping; it may interfere with the ability to fall asleep at night.
Medications. The consensus guideline on treating schizophrenia20 offers the option of switching the patient with chronic insomnia to one of the more sedating antipsychotics, such as olanzapine, quetiapine, or clozapine. Sedation alone should not be the reason to switch to clozapine, however.
You could consider adding a bedtime sedative to the patient’s medications (Table 4). FDA-approved sedatives include nonbenzodiazepines such as zolpidem, zolpidem extended-release, zaleplon, or eszopiclone, and the melatonin receptor agonist ramelteon. Although not approved as sedatives, some antidepressants such as trazodone or mirtazapine and antihistamines such as diphenhydramine and hydroxyzine are used to promote sleep. Benzodiazepines can be helpful but require caution when prescribed to patients with comorbid substance abuse disorders.
Sedatives have been studied extensively in general populations with insomnia but not in patients receiving antipsychotics. Combining antipsychotics and sedatives can produce daytime drowsiness and sedation.
Table 4
Sedatives to treat insomnia in patients with schizophrenia
| Medication | Common bedtime dose range* |
|---|---|
| Benzodiazepines | |
| Estazolam | 1 to 2 mg |
| Flurazepam | 15 to 30 mg |
| Temazepam | 7.5 to 30 mg |
| Triazolam | 0.125 to 0.25 mg |
| Benzodiazepine agonists | |
| Eszopiclone | 2 to 3 mg |
| Zaleplon | 5 to 10 mg |
| Zolpidem | 5 to 10 mg |
| Zolpidem CR | 6.25 to 12.5 mg |
| Melatonin receptor agonist | |
| Ramelteon | 8 mg |
| H1 antihistamines | |
| Diphenhydramine | 25 to 50 mg |
| Hydroxyzine | 50 to 100 mg |
| Antidepressants | |
| Mirtazapine | 15 to 30 mg |
| Trazodone | 50 to 200 mg |
| * No dosage adjustment is required in this patient population | |
Related resources
- Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry 2004;6(suppl 2):3-7.
- Benca RM, Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv 2005;56(3):332-43.
Drug Brand Names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Estazolam • ProSom
- Eszopiclone • Lunesta
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Haloperidol • Haldol
- Hydroxyzine • Vistaril, Atarax
- Lamotrigine • Lamictal
- Mirtazapine • Remeron
- Modafinil • Provigil
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Ramelteon • Rozerem
- Risperidone • Risperdal
- Temazepam • Restor
- Trazodone • Desyrel
- Triazolam • Halcion
- Valproic acid • Depakote
- Zaleplon • Sonata
- Ziprasidone • Geodon
- Zolpidem • Ambien, Ambien CR
Disclosure
Dr. Miller receives research support from Pfizer Inc. and has received honoraria from AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, and Pfizer Inc.
1. McQuade R, Burris KD, Jordan S, et al. Aripiprazole: a dopamine-serotonin system stabilizer [abstract no. P.3.W.080]. Int J Neuropsychopharmacol 2002;5(suppl 1):S176.-
2. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003;60(6):553-64.
3. Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. Can Med Assoc J 2005;172(13):1703-11.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
5. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacol (Berl) 2006;188(3):281-92.
6. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. Ziprasidone I.M. Study Group. J Clin Psychiatry 2000;61(12):933-41.
7. Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 2003;60(12):1228-35.
8. Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 2005;57(12):1543-9.
9. Jibson MD, Tandon R. An overview of antipsychotic medications. CNS News 2001;3:49-54.
10. Collaborative Working Group on Clinical Trial Evaluations. Treatment of special populations with the atypical antipsychotics. J Clin Psychiatry 1998;59(suppl 12):46-52.
11. El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database Syst Rev 2004(2);CD004578.-
12. Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinol 2003;28(suppl 1):9-26.
13. Tandon R. Safety and tolerability: how do newer generation ”atypical” antipsychotics compare? Psychiatr Q 2002;73(4):297-311.
14. Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 2000;68(1):29-39.
15. Tandon R, Shipley JE, Taylor S, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry 1992;49(3):185-94.
16. Benson KL, Zarcone VP Jr. Rapid eye movement sleep eye movements in schizophrenia and depression. Arch Gen Psychiatry 1993;50(6):474-82.
17. Yamashita H, Morinobu S, Yamawaki S, et al. Effect of risperidone on sleep in schizophrenia: a comparison with haloperidol. Psychiatry Res 2002;109(2):137-42.
18. Salin-Pascual RJ, Herrera-Estrella M, Galicia-Polo L, Laurrabaquio MR. Olanzapine acute administration in schizophrenic patients increases delta sleep and sleep efficiency. Biol Psychiatry 1999;46(1):141-3.
19. Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res 1986;378(1):164-8.
20. Expert Consensus Guideline Series Treatment of schizophrenia. J Clin Psychiatry 1999;60(suppl 11):1-80.
21. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 2000;20(22):8620-8.
22. Makela EH, Miller K, Cutlip WD, 2nd. Three case reports of modafinil use in treating sedation induced by antipsychotic medications. J Clin Psychiatry 2003;64(4):485-6.
23. Sevy S, Rosenthal MH, Alvir J, et al. Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry 2005;66(7):839-43.
24. Narendran R, Young CM, Valenti AM, et al. Is psychosis exacerbated by modafinil? Arch Gen Psychiatry 2002;59(3):292-3.
1. McQuade R, Burris KD, Jordan S, et al. Aripiprazole: a dopamine-serotonin system stabilizer [abstract no. P.3.W.080]. Int J Neuropsychopharmacol 2002;5(suppl 1):S176.-
2. Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003;60(6):553-64.
3. Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. Can Med Assoc J 2005;172(13):1703-11.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
5. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacol (Berl) 2006;188(3):281-92.
6. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. Ziprasidone I.M. Study Group. J Clin Psychiatry 2000;61(12):933-41.
7. Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 2003;60(12):1228-35.
8. Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 2005;57(12):1543-9.
9. Jibson MD, Tandon R. An overview of antipsychotic medications. CNS News 2001;3:49-54.
10. Collaborative Working Group on Clinical Trial Evaluations. Treatment of special populations with the atypical antipsychotics. J Clin Psychiatry 1998;59(suppl 12):46-52.
11. El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database Syst Rev 2004(2);CD004578.-
12. Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinol 2003;28(suppl 1):9-26.
13. Tandon R. Safety and tolerability: how do newer generation ”atypical” antipsychotics compare? Psychiatr Q 2002;73(4):297-311.
14. Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 2000;68(1):29-39.
15. Tandon R, Shipley JE, Taylor S, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry 1992;49(3):185-94.
16. Benson KL, Zarcone VP Jr. Rapid eye movement sleep eye movements in schizophrenia and depression. Arch Gen Psychiatry 1993;50(6):474-82.
17. Yamashita H, Morinobu S, Yamawaki S, et al. Effect of risperidone on sleep in schizophrenia: a comparison with haloperidol. Psychiatry Res 2002;109(2):137-42.
18. Salin-Pascual RJ, Herrera-Estrella M, Galicia-Polo L, Laurrabaquio MR. Olanzapine acute administration in schizophrenic patients increases delta sleep and sleep efficiency. Biol Psychiatry 1999;46(1):141-3.
19. Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res 1986;378(1):164-8.
20. Expert Consensus Guideline Series Treatment of schizophrenia. J Clin Psychiatry 1999;60(suppl 11):1-80.
21. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 2000;20(22):8620-8.
22. Makela EH, Miller K, Cutlip WD, 2nd. Three case reports of modafinil use in treating sedation induced by antipsychotic medications. J Clin Psychiatry 2003;64(4):485-6.
23. Sevy S, Rosenthal MH, Alvir J, et al. Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry 2005;66(7):839-43.
24. Narendran R, Young CM, Valenti AM, et al. Is psychosis exacerbated by modafinil? Arch Gen Psychiatry 2002;59(3):292-3.