User login
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
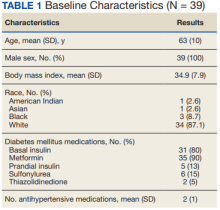
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
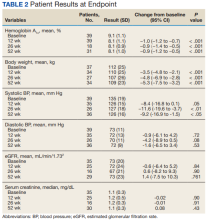
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
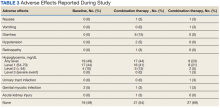
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8