User login
History: Initial symptoms
Ms. F, age 39, presented with depression, severe anxiety, and disturbed sleep. She denied psychiatric or medical history, but reported that her depressive symptoms hampered her performance at work and led to work-related stress.
Ms. F’s Beck Depression Inventory (BDI) score at baseline was 21, indicating moderate depression. The psychiatrist diagnosed her as having generalized anxiety disorder and adjustment disorder with depressed mood.
The patient was started on paroxetine, 10 mg/d. Five weeks later, her anxiety had decreased significantly and her BDI score had dropped to 10, indicating normal mood. Both the patient and clinician decided at this point that Ms. F had reached remission. The patient demanded that paroxetine be stopped, saying that she “does not like medication.” The psychiatrist reluctantly agreed.
Eight weeks later, during a follow-up examination, Ms. F complained of severely depressed mood with frequent crying spells. She complained of fatigue, nausea, headaches, decreased appetite, and dizziness. Her work performance, which had improved during the paroxetine trial, was again compromised. Her BDI score was 32, indicating severe depression.
Was the psychiatrist justified in stopping paroxetine therapy after 5 weeks?
Dr. Fishbain’s observations
Premature paroxetine discontinuation cannot be ruled out as a cause for Ms. F’s relapse. Sood et al1 found that duration of antidepressant therapy beyond treatment guidelines correlated with longer times to relapse.
By contrast, Ms. F’s initial therapy duration fell short of the 6 to 8 weeks recommended by the American Psychiatric Association.2
Patients commonly cite adverse events as a reason for wanting to stop antidepressant therapy.3 Ms. F reported no adverse effects, however; she said only that she did “not like medication.” Despite her insistence to the contrary, antidepressant therapy probably should not have been stopped.
Further history: A painful discovery
After questioning, Ms. F told the psychiatrist that she had been involved in a motor vehicle accident 2 weeks before the follow-up visit and had since been suffering lower back and neck pain.
After more questioning, Mr. F revealed that the pain was disrupting her sleep. She was getting about 4 hours of fragmented sleep per night, resulting in lack of energy during the day. The pain made it hard for her to sit, further impairing her work performance.
Ms. F was restarted on paroxetine, 10 mg/d titrated across 6 weeks to 60 mg/d for her depression, and zolpidem, 10 mg at bedtime, to help her sleep. After 6 weeks, her BDI score improved to 20, and she was less labile. Her depressive symptoms persisted, however, as did her pain, fatigue, headaches, nausea, dizziness, and sleep disturbances.
What role did Ms. F’s pain play in her relapse? How can clinicians detect somatic symptoms and gauge their effect on mood?
Dr. Fishbain’s observations
Pain most likely caused Ms. F’s depression relapse. McBeth et al4 have demonstrated that pain can contribute to depression’s development. In another study,5 43.4% of subjects who met criteria for major depression reported painful symptoms. The presence of a chronic painful condition was also found to contribute to major depression.5
Nakao et al6 screened 2,215 outpatients who were referred with mind/body complaints. Patients who were diagnosed with major depression had significantly higher rates of fatigue (86% vs. 65%), insomnia (79% vs. 58%), nausea/vomiting (51% vs. 40%), and low-back pain (36% vs. 24%) than those who were not. Within the major depression group, somatic symptoms were more abundant in patients with severe depressive episodes than in those with mild depressive episodes (5.8 vs. 3.7, P < 0.05).
Depression prevalence appears to increase when somatic symptoms are considered in the diagnosis. Posse and Hallstrom7 used a two-stage design to screen for depression. In the first stage, depression prevalence was 1.8%. In the second stage, 62 patients with high somatic complaint scores were re-evaluated. Of this group, 41 were diagnosed with a major depressive disorder or dysthymia.
Patients with continued pain after depression treatment are at high risk for depression recurrence.8 Diffuseness of pain and extent to which it interferes with daily activities strongly predict depression.9
Diagnostic challenges. Patients with depression often present to their primary care physicians with somatic rather than behavioral symptoms, making it hard for the family doctor to diagnose depression.10 By contrast, when presenting to a psychiatrist, depressed patients tend to discuss their emotional symptoms but not their physical complaints.11 This is because patients often:
- attribute physical symptoms to an unrelated medical illness
- consider aches and pains a normal part of aging
- or are not aware that psychiatrists can treat physical symptoms.11
Psychiatrists in the past have emphasized emotional symptoms while barely addressing physical symptoms. This trend is changing, however, as the link between physical pain and depression has become clearer.
Be sure to include chronic pain or other somatic symptoms in the systems review. Screening tools such as the Visual Analogue Scale can measure pain intensity, while the Cornell Medical Index can uncover somatic symptoms. No all-inclusive tool exists to help detect depression-related somatic symptoms. however.
Should the psychiatrist continue to address Ms. F’s depressive symptoms, or should the focus shift to her somatic symptoms?
Dr. Fishbain’s observations
Patients who do not respond to depression treatment (ie, achieve >50% symptom reduction), or who respond without achieving remission, usually have residual physical symptoms—often fatigue, sleep disturbance, decreased appetite, anxiety, sexual dysfunction, and/or pain.12-14 Severe pain and other somatic symptoms are likely prolonging Ms. F’s depression, despite increased paroxetine dosages.
Paykel et al8 have associated residual depression symptoms with early relapse of depression. In their study, 94% of depressed patients with lingering depressive symptoms had mild to moderate physical symptoms. By contrast, degree of physical symptom improvement has been shown to correlate with likelihood of depression remission.15
Although emotional symptoms improve with antidepressants,16 some evidence17 indicates that physical symptoms associated with depression may be less responsive.
Also, because many psychiatrists have been taught to track emotional symptoms and only some physical symptoms, somatic symptoms of depression often are not targeted for treatment.17 Lack of rating scales to track somatic symptoms compounds this problem.17
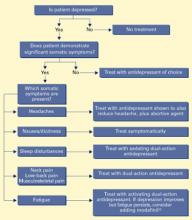
Psychiatrists need to target both the physical and emotional symptoms of depression. When pain prolongs depression, it should be the primary target of antidepressant drug therapy (Algorithm).
To date, several meta-analyses18-20 have demonstrated that antidepressants have a separate analgesic effect on all forms of chronic pain. Evidence21,22 also indicates that the dualaction antidepressants—such as amitriptyline, bupropion, venlafaxine, and (awaiting FDA approval) duloxetine—have a more-consistent analgesic effect than do the serotonin reuptake inhibitors.
The analgesic effects of bupropion, duloxetine, and venlafaxine have not been compared with those of tricyclics or other older antidepressants. If one of the newer dualaction antidepressants does not reduce somatic conditions or produce an adequate response, consider switching to a tricyclic.
Treatment: No pain, some gain
Another psychiatrist who specializes in pain medicine targeted some of Ms. F’s somatic symptoms with antidepressants. Paroxetine and zolpidem were discontinued and the patient was started on:
- venlafaxine, 37.5 mg bid, titrated to 225 mg/d across 2 weeks. Because of its activating properties, venlafaxine was chosen to address Ms. F’s pain and daytime fatigue.
- amitriptyline, 50 mg at bedtime nightly, to promote sleep
- prochlorperazine, 10 mg as needed, and meclizine, 25 mg as needed, to treat her nausea and dizziness, respectively.
Algorithm Suggested drug treatment of depression with somatic symptoms
Ms. F also was advised to take an abortive migraine compound (Midrin, 2 tablets at headache onset and 1 additional tablet every half-hour as needed, maximum 5 tablets per day). Mr. F’s primary care physician also referred her to a physical therapist to treat her neck and low-back pain; workup revealed no surgically treatable problem.
Four weeks later, Ms. F reported that her somatic symptoms significantly improved and that she was sleeping nearly 8 hours per night. Her BDI score was 12, indicating normal mood. She was functioning much more effectively at work and could once again routinely perform her daily activities.
Ms. F continued her medication regimen for 8 months, after which she was lost to follow-up. At her most recent visit, her depression remained in remission. Her pain persisted, though at a lower intensity.
Related resources
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13:116-37.
Drug brand names
- Amitriptyline • Elavil
- Duloxetine • Cymbalta
- Meclizine • Antivert
- Modafinil • Provigil
- Paroxetine • Paxil
- Prochlorperazine • Compazine
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
Dr. Fishbain is a consultant to Eli Lilly and Co. and a speaker for Purdue Pharmaceuticals.
1. Sood N, Treglia M, Obenchain RL, et al. Determinants of antidepressant outcome. Am J Manag Care 2000;6:1327-36.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(suppl 4):1-45.
3. Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care 1996;33:67-74.
4. McBeth J, MacFarlane G, Silman A. Does chronic pain predict future psychological distress? Pain 2002;96:239-45.
5. Ohayon M, Schatzberg A. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry 2003;60:39-47.
6. Nakao M, Yamanaka G, Kuboki T. Major depression and somatic symptoms in a mind/body medicine clinic. Psychopathology 2001;34:230-5.
7. Posse M, Hallstrom T. Depressive disorders among somatizing patients in primary health care. Acta Psychiatr Scand 1998;98:187-92.
8. Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995;25:1171-80.
9. Von Korff M, Simon G. The relationship between pain and depression. Br J Psychiatry 1996;168:101-8.
10. Goldberg DP, Bridges K. Somatic presentations of psychiatric illness in primary care setting. J Psychosom Res 1988;32:137-44.
11. Sartorius N. Physical symptoms of depression as a public health concern. J Clinical Psych 2003;64(suppl 7):3-4.
12. Keller MB. Long-term treatment strategies in affective disorders. Psychopharmacol Bull 2002;36(suppl 2):36-48.
13. Keller MB, Berndt ER. Depression treatment: a lifelong commitment? Psychopharmacol Bull 2002;36(suppl 2):133-41.
14. Segal Z, Vincent P, Levitt A. Efficacy of combined, sequential and crossover psychotherapy and pharmacotherapy in improving outcomes in depression. J Psychiatry Neurosci 2002;27:281-90.
15. Denninger JW, Henderson PO, Fallis K. The relationship between somatic symptoms and depression (presentation). Philadelphia, PA: American Psychiatric Association annual meeting, 2002.
16. Worthington J, Fava M, Davidson K, et al. Patterns of improvement in depressive symptoms with fluoxetine treatment. Psychopharmacol Bull 1995;31:223-6.
17. Fava M. Somatic symptoms, depression, and antidepressant treatment. J Clin Psychiatry 2002;63:4-305-7.
18. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med 1998;60:503-9.
19. O’Malley PG, Belden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med 2000;15:659-66.
20. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002;162:19-24.
21. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med 2000;32:305-16.
22. Fishbain DA, Cutler R, Rosomoff HL, et al. Evidence-based data from animal and human experimental studies on pain relief with antidepressants: a structured review. Pain Med 2000;1:310-16.
History: Initial symptoms
Ms. F, age 39, presented with depression, severe anxiety, and disturbed sleep. She denied psychiatric or medical history, but reported that her depressive symptoms hampered her performance at work and led to work-related stress.
Ms. F’s Beck Depression Inventory (BDI) score at baseline was 21, indicating moderate depression. The psychiatrist diagnosed her as having generalized anxiety disorder and adjustment disorder with depressed mood.
The patient was started on paroxetine, 10 mg/d. Five weeks later, her anxiety had decreased significantly and her BDI score had dropped to 10, indicating normal mood. Both the patient and clinician decided at this point that Ms. F had reached remission. The patient demanded that paroxetine be stopped, saying that she “does not like medication.” The psychiatrist reluctantly agreed.
Eight weeks later, during a follow-up examination, Ms. F complained of severely depressed mood with frequent crying spells. She complained of fatigue, nausea, headaches, decreased appetite, and dizziness. Her work performance, which had improved during the paroxetine trial, was again compromised. Her BDI score was 32, indicating severe depression.
Was the psychiatrist justified in stopping paroxetine therapy after 5 weeks?
Dr. Fishbain’s observations
Premature paroxetine discontinuation cannot be ruled out as a cause for Ms. F’s relapse. Sood et al1 found that duration of antidepressant therapy beyond treatment guidelines correlated with longer times to relapse.
By contrast, Ms. F’s initial therapy duration fell short of the 6 to 8 weeks recommended by the American Psychiatric Association.2
Patients commonly cite adverse events as a reason for wanting to stop antidepressant therapy.3 Ms. F reported no adverse effects, however; she said only that she did “not like medication.” Despite her insistence to the contrary, antidepressant therapy probably should not have been stopped.
Further history: A painful discovery
After questioning, Ms. F told the psychiatrist that she had been involved in a motor vehicle accident 2 weeks before the follow-up visit and had since been suffering lower back and neck pain.
After more questioning, Mr. F revealed that the pain was disrupting her sleep. She was getting about 4 hours of fragmented sleep per night, resulting in lack of energy during the day. The pain made it hard for her to sit, further impairing her work performance.
Ms. F was restarted on paroxetine, 10 mg/d titrated across 6 weeks to 60 mg/d for her depression, and zolpidem, 10 mg at bedtime, to help her sleep. After 6 weeks, her BDI score improved to 20, and she was less labile. Her depressive symptoms persisted, however, as did her pain, fatigue, headaches, nausea, dizziness, and sleep disturbances.
What role did Ms. F’s pain play in her relapse? How can clinicians detect somatic symptoms and gauge their effect on mood?
Dr. Fishbain’s observations
Pain most likely caused Ms. F’s depression relapse. McBeth et al4 have demonstrated that pain can contribute to depression’s development. In another study,5 43.4% of subjects who met criteria for major depression reported painful symptoms. The presence of a chronic painful condition was also found to contribute to major depression.5
Nakao et al6 screened 2,215 outpatients who were referred with mind/body complaints. Patients who were diagnosed with major depression had significantly higher rates of fatigue (86% vs. 65%), insomnia (79% vs. 58%), nausea/vomiting (51% vs. 40%), and low-back pain (36% vs. 24%) than those who were not. Within the major depression group, somatic symptoms were more abundant in patients with severe depressive episodes than in those with mild depressive episodes (5.8 vs. 3.7, P < 0.05).
Depression prevalence appears to increase when somatic symptoms are considered in the diagnosis. Posse and Hallstrom7 used a two-stage design to screen for depression. In the first stage, depression prevalence was 1.8%. In the second stage, 62 patients with high somatic complaint scores were re-evaluated. Of this group, 41 were diagnosed with a major depressive disorder or dysthymia.
Patients with continued pain after depression treatment are at high risk for depression recurrence.8 Diffuseness of pain and extent to which it interferes with daily activities strongly predict depression.9
Diagnostic challenges. Patients with depression often present to their primary care physicians with somatic rather than behavioral symptoms, making it hard for the family doctor to diagnose depression.10 By contrast, when presenting to a psychiatrist, depressed patients tend to discuss their emotional symptoms but not their physical complaints.11 This is because patients often:
- attribute physical symptoms to an unrelated medical illness
- consider aches and pains a normal part of aging
- or are not aware that psychiatrists can treat physical symptoms.11
Psychiatrists in the past have emphasized emotional symptoms while barely addressing physical symptoms. This trend is changing, however, as the link between physical pain and depression has become clearer.
Be sure to include chronic pain or other somatic symptoms in the systems review. Screening tools such as the Visual Analogue Scale can measure pain intensity, while the Cornell Medical Index can uncover somatic symptoms. No all-inclusive tool exists to help detect depression-related somatic symptoms. however.
Should the psychiatrist continue to address Ms. F’s depressive symptoms, or should the focus shift to her somatic symptoms?
Dr. Fishbain’s observations
Patients who do not respond to depression treatment (ie, achieve >50% symptom reduction), or who respond without achieving remission, usually have residual physical symptoms—often fatigue, sleep disturbance, decreased appetite, anxiety, sexual dysfunction, and/or pain.12-14 Severe pain and other somatic symptoms are likely prolonging Ms. F’s depression, despite increased paroxetine dosages.
Paykel et al8 have associated residual depression symptoms with early relapse of depression. In their study, 94% of depressed patients with lingering depressive symptoms had mild to moderate physical symptoms. By contrast, degree of physical symptom improvement has been shown to correlate with likelihood of depression remission.15
Although emotional symptoms improve with antidepressants,16 some evidence17 indicates that physical symptoms associated with depression may be less responsive.
Also, because many psychiatrists have been taught to track emotional symptoms and only some physical symptoms, somatic symptoms of depression often are not targeted for treatment.17 Lack of rating scales to track somatic symptoms compounds this problem.17
Psychiatrists need to target both the physical and emotional symptoms of depression. When pain prolongs depression, it should be the primary target of antidepressant drug therapy (Algorithm).
To date, several meta-analyses18-20 have demonstrated that antidepressants have a separate analgesic effect on all forms of chronic pain. Evidence21,22 also indicates that the dualaction antidepressants—such as amitriptyline, bupropion, venlafaxine, and (awaiting FDA approval) duloxetine—have a more-consistent analgesic effect than do the serotonin reuptake inhibitors.
The analgesic effects of bupropion, duloxetine, and venlafaxine have not been compared with those of tricyclics or other older antidepressants. If one of the newer dualaction antidepressants does not reduce somatic conditions or produce an adequate response, consider switching to a tricyclic.
Treatment: No pain, some gain
Another psychiatrist who specializes in pain medicine targeted some of Ms. F’s somatic symptoms with antidepressants. Paroxetine and zolpidem were discontinued and the patient was started on:
- venlafaxine, 37.5 mg bid, titrated to 225 mg/d across 2 weeks. Because of its activating properties, venlafaxine was chosen to address Ms. F’s pain and daytime fatigue.
- amitriptyline, 50 mg at bedtime nightly, to promote sleep
- prochlorperazine, 10 mg as needed, and meclizine, 25 mg as needed, to treat her nausea and dizziness, respectively.
Algorithm Suggested drug treatment of depression with somatic symptoms
Ms. F also was advised to take an abortive migraine compound (Midrin, 2 tablets at headache onset and 1 additional tablet every half-hour as needed, maximum 5 tablets per day). Mr. F’s primary care physician also referred her to a physical therapist to treat her neck and low-back pain; workup revealed no surgically treatable problem.
Four weeks later, Ms. F reported that her somatic symptoms significantly improved and that she was sleeping nearly 8 hours per night. Her BDI score was 12, indicating normal mood. She was functioning much more effectively at work and could once again routinely perform her daily activities.
Ms. F continued her medication regimen for 8 months, after which she was lost to follow-up. At her most recent visit, her depression remained in remission. Her pain persisted, though at a lower intensity.
Related resources
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13:116-37.
Drug brand names
- Amitriptyline • Elavil
- Duloxetine • Cymbalta
- Meclizine • Antivert
- Modafinil • Provigil
- Paroxetine • Paxil
- Prochlorperazine • Compazine
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
Dr. Fishbain is a consultant to Eli Lilly and Co. and a speaker for Purdue Pharmaceuticals.
History: Initial symptoms
Ms. F, age 39, presented with depression, severe anxiety, and disturbed sleep. She denied psychiatric or medical history, but reported that her depressive symptoms hampered her performance at work and led to work-related stress.
Ms. F’s Beck Depression Inventory (BDI) score at baseline was 21, indicating moderate depression. The psychiatrist diagnosed her as having generalized anxiety disorder and adjustment disorder with depressed mood.
The patient was started on paroxetine, 10 mg/d. Five weeks later, her anxiety had decreased significantly and her BDI score had dropped to 10, indicating normal mood. Both the patient and clinician decided at this point that Ms. F had reached remission. The patient demanded that paroxetine be stopped, saying that she “does not like medication.” The psychiatrist reluctantly agreed.
Eight weeks later, during a follow-up examination, Ms. F complained of severely depressed mood with frequent crying spells. She complained of fatigue, nausea, headaches, decreased appetite, and dizziness. Her work performance, which had improved during the paroxetine trial, was again compromised. Her BDI score was 32, indicating severe depression.
Was the psychiatrist justified in stopping paroxetine therapy after 5 weeks?
Dr. Fishbain’s observations
Premature paroxetine discontinuation cannot be ruled out as a cause for Ms. F’s relapse. Sood et al1 found that duration of antidepressant therapy beyond treatment guidelines correlated with longer times to relapse.
By contrast, Ms. F’s initial therapy duration fell short of the 6 to 8 weeks recommended by the American Psychiatric Association.2
Patients commonly cite adverse events as a reason for wanting to stop antidepressant therapy.3 Ms. F reported no adverse effects, however; she said only that she did “not like medication.” Despite her insistence to the contrary, antidepressant therapy probably should not have been stopped.
Further history: A painful discovery
After questioning, Ms. F told the psychiatrist that she had been involved in a motor vehicle accident 2 weeks before the follow-up visit and had since been suffering lower back and neck pain.
After more questioning, Mr. F revealed that the pain was disrupting her sleep. She was getting about 4 hours of fragmented sleep per night, resulting in lack of energy during the day. The pain made it hard for her to sit, further impairing her work performance.
Ms. F was restarted on paroxetine, 10 mg/d titrated across 6 weeks to 60 mg/d for her depression, and zolpidem, 10 mg at bedtime, to help her sleep. After 6 weeks, her BDI score improved to 20, and she was less labile. Her depressive symptoms persisted, however, as did her pain, fatigue, headaches, nausea, dizziness, and sleep disturbances.
What role did Ms. F’s pain play in her relapse? How can clinicians detect somatic symptoms and gauge their effect on mood?
Dr. Fishbain’s observations
Pain most likely caused Ms. F’s depression relapse. McBeth et al4 have demonstrated that pain can contribute to depression’s development. In another study,5 43.4% of subjects who met criteria for major depression reported painful symptoms. The presence of a chronic painful condition was also found to contribute to major depression.5
Nakao et al6 screened 2,215 outpatients who were referred with mind/body complaints. Patients who were diagnosed with major depression had significantly higher rates of fatigue (86% vs. 65%), insomnia (79% vs. 58%), nausea/vomiting (51% vs. 40%), and low-back pain (36% vs. 24%) than those who were not. Within the major depression group, somatic symptoms were more abundant in patients with severe depressive episodes than in those with mild depressive episodes (5.8 vs. 3.7, P < 0.05).
Depression prevalence appears to increase when somatic symptoms are considered in the diagnosis. Posse and Hallstrom7 used a two-stage design to screen for depression. In the first stage, depression prevalence was 1.8%. In the second stage, 62 patients with high somatic complaint scores were re-evaluated. Of this group, 41 were diagnosed with a major depressive disorder or dysthymia.
Patients with continued pain after depression treatment are at high risk for depression recurrence.8 Diffuseness of pain and extent to which it interferes with daily activities strongly predict depression.9
Diagnostic challenges. Patients with depression often present to their primary care physicians with somatic rather than behavioral symptoms, making it hard for the family doctor to diagnose depression.10 By contrast, when presenting to a psychiatrist, depressed patients tend to discuss their emotional symptoms but not their physical complaints.11 This is because patients often:
- attribute physical symptoms to an unrelated medical illness
- consider aches and pains a normal part of aging
- or are not aware that psychiatrists can treat physical symptoms.11
Psychiatrists in the past have emphasized emotional symptoms while barely addressing physical symptoms. This trend is changing, however, as the link between physical pain and depression has become clearer.
Be sure to include chronic pain or other somatic symptoms in the systems review. Screening tools such as the Visual Analogue Scale can measure pain intensity, while the Cornell Medical Index can uncover somatic symptoms. No all-inclusive tool exists to help detect depression-related somatic symptoms. however.
Should the psychiatrist continue to address Ms. F’s depressive symptoms, or should the focus shift to her somatic symptoms?
Dr. Fishbain’s observations
Patients who do not respond to depression treatment (ie, achieve >50% symptom reduction), or who respond without achieving remission, usually have residual physical symptoms—often fatigue, sleep disturbance, decreased appetite, anxiety, sexual dysfunction, and/or pain.12-14 Severe pain and other somatic symptoms are likely prolonging Ms. F’s depression, despite increased paroxetine dosages.
Paykel et al8 have associated residual depression symptoms with early relapse of depression. In their study, 94% of depressed patients with lingering depressive symptoms had mild to moderate physical symptoms. By contrast, degree of physical symptom improvement has been shown to correlate with likelihood of depression remission.15
Although emotional symptoms improve with antidepressants,16 some evidence17 indicates that physical symptoms associated with depression may be less responsive.
Also, because many psychiatrists have been taught to track emotional symptoms and only some physical symptoms, somatic symptoms of depression often are not targeted for treatment.17 Lack of rating scales to track somatic symptoms compounds this problem.17
Psychiatrists need to target both the physical and emotional symptoms of depression. When pain prolongs depression, it should be the primary target of antidepressant drug therapy (Algorithm).
To date, several meta-analyses18-20 have demonstrated that antidepressants have a separate analgesic effect on all forms of chronic pain. Evidence21,22 also indicates that the dualaction antidepressants—such as amitriptyline, bupropion, venlafaxine, and (awaiting FDA approval) duloxetine—have a more-consistent analgesic effect than do the serotonin reuptake inhibitors.
The analgesic effects of bupropion, duloxetine, and venlafaxine have not been compared with those of tricyclics or other older antidepressants. If one of the newer dualaction antidepressants does not reduce somatic conditions or produce an adequate response, consider switching to a tricyclic.
Treatment: No pain, some gain
Another psychiatrist who specializes in pain medicine targeted some of Ms. F’s somatic symptoms with antidepressants. Paroxetine and zolpidem were discontinued and the patient was started on:
- venlafaxine, 37.5 mg bid, titrated to 225 mg/d across 2 weeks. Because of its activating properties, venlafaxine was chosen to address Ms. F’s pain and daytime fatigue.
- amitriptyline, 50 mg at bedtime nightly, to promote sleep
- prochlorperazine, 10 mg as needed, and meclizine, 25 mg as needed, to treat her nausea and dizziness, respectively.
Algorithm Suggested drug treatment of depression with somatic symptoms
Ms. F also was advised to take an abortive migraine compound (Midrin, 2 tablets at headache onset and 1 additional tablet every half-hour as needed, maximum 5 tablets per day). Mr. F’s primary care physician also referred her to a physical therapist to treat her neck and low-back pain; workup revealed no surgically treatable problem.
Four weeks later, Ms. F reported that her somatic symptoms significantly improved and that she was sleeping nearly 8 hours per night. Her BDI score was 12, indicating normal mood. She was functioning much more effectively at work and could once again routinely perform her daily activities.
Ms. F continued her medication regimen for 8 months, after which she was lost to follow-up. At her most recent visit, her depression remained in remission. Her pain persisted, though at a lower intensity.
Related resources
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13:116-37.
Drug brand names
- Amitriptyline • Elavil
- Duloxetine • Cymbalta
- Meclizine • Antivert
- Modafinil • Provigil
- Paroxetine • Paxil
- Prochlorperazine • Compazine
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
Dr. Fishbain is a consultant to Eli Lilly and Co. and a speaker for Purdue Pharmaceuticals.
1. Sood N, Treglia M, Obenchain RL, et al. Determinants of antidepressant outcome. Am J Manag Care 2000;6:1327-36.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(suppl 4):1-45.
3. Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care 1996;33:67-74.
4. McBeth J, MacFarlane G, Silman A. Does chronic pain predict future psychological distress? Pain 2002;96:239-45.
5. Ohayon M, Schatzberg A. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry 2003;60:39-47.
6. Nakao M, Yamanaka G, Kuboki T. Major depression and somatic symptoms in a mind/body medicine clinic. Psychopathology 2001;34:230-5.
7. Posse M, Hallstrom T. Depressive disorders among somatizing patients in primary health care. Acta Psychiatr Scand 1998;98:187-92.
8. Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995;25:1171-80.
9. Von Korff M, Simon G. The relationship between pain and depression. Br J Psychiatry 1996;168:101-8.
10. Goldberg DP, Bridges K. Somatic presentations of psychiatric illness in primary care setting. J Psychosom Res 1988;32:137-44.
11. Sartorius N. Physical symptoms of depression as a public health concern. J Clinical Psych 2003;64(suppl 7):3-4.
12. Keller MB. Long-term treatment strategies in affective disorders. Psychopharmacol Bull 2002;36(suppl 2):36-48.
13. Keller MB, Berndt ER. Depression treatment: a lifelong commitment? Psychopharmacol Bull 2002;36(suppl 2):133-41.
14. Segal Z, Vincent P, Levitt A. Efficacy of combined, sequential and crossover psychotherapy and pharmacotherapy in improving outcomes in depression. J Psychiatry Neurosci 2002;27:281-90.
15. Denninger JW, Henderson PO, Fallis K. The relationship between somatic symptoms and depression (presentation). Philadelphia, PA: American Psychiatric Association annual meeting, 2002.
16. Worthington J, Fava M, Davidson K, et al. Patterns of improvement in depressive symptoms with fluoxetine treatment. Psychopharmacol Bull 1995;31:223-6.
17. Fava M. Somatic symptoms, depression, and antidepressant treatment. J Clin Psychiatry 2002;63:4-305-7.
18. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med 1998;60:503-9.
19. O’Malley PG, Belden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med 2000;15:659-66.
20. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002;162:19-24.
21. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med 2000;32:305-16.
22. Fishbain DA, Cutler R, Rosomoff HL, et al. Evidence-based data from animal and human experimental studies on pain relief with antidepressants: a structured review. Pain Med 2000;1:310-16.
1. Sood N, Treglia M, Obenchain RL, et al. Determinants of antidepressant outcome. Am J Manag Care 2000;6:1327-36.
2. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(suppl 4):1-45.
3. Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care 1996;33:67-74.
4. McBeth J, MacFarlane G, Silman A. Does chronic pain predict future psychological distress? Pain 2002;96:239-45.
5. Ohayon M, Schatzberg A. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry 2003;60:39-47.
6. Nakao M, Yamanaka G, Kuboki T. Major depression and somatic symptoms in a mind/body medicine clinic. Psychopathology 2001;34:230-5.
7. Posse M, Hallstrom T. Depressive disorders among somatizing patients in primary health care. Acta Psychiatr Scand 1998;98:187-92.
8. Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995;25:1171-80.
9. Von Korff M, Simon G. The relationship between pain and depression. Br J Psychiatry 1996;168:101-8.
10. Goldberg DP, Bridges K. Somatic presentations of psychiatric illness in primary care setting. J Psychosom Res 1988;32:137-44.
11. Sartorius N. Physical symptoms of depression as a public health concern. J Clinical Psych 2003;64(suppl 7):3-4.
12. Keller MB. Long-term treatment strategies in affective disorders. Psychopharmacol Bull 2002;36(suppl 2):36-48.
13. Keller MB, Berndt ER. Depression treatment: a lifelong commitment? Psychopharmacol Bull 2002;36(suppl 2):133-41.
14. Segal Z, Vincent P, Levitt A. Efficacy of combined, sequential and crossover psychotherapy and pharmacotherapy in improving outcomes in depression. J Psychiatry Neurosci 2002;27:281-90.
15. Denninger JW, Henderson PO, Fallis K. The relationship between somatic symptoms and depression (presentation). Philadelphia, PA: American Psychiatric Association annual meeting, 2002.
16. Worthington J, Fava M, Davidson K, et al. Patterns of improvement in depressive symptoms with fluoxetine treatment. Psychopharmacol Bull 1995;31:223-6.
17. Fava M. Somatic symptoms, depression, and antidepressant treatment. J Clin Psychiatry 2002;63:4-305-7.
18. Fishbain DA, Cutler RB, Rosomoff HL, et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosom Med 1998;60:503-9.
19. O’Malley PG, Belden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med 2000;15:659-66.
20. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002;162:19-24.
21. Fishbain DA. Evidence-based data on pain relief with antidepressants. Ann Med 2000;32:305-16.
22. Fishbain DA, Cutler R, Rosomoff HL, et al. Evidence-based data from animal and human experimental studies on pain relief with antidepressants: a structured review. Pain Med 2000;1:310-16.