User login
Rare frameshift mutations in the NAF1 gene were discovered to cause a telomere-shortening syndrome which, among other adverse effects, predisposes carriers to develop pulmonary fibrosis (PF) and emphysema, according to a report published in Science Translational Medicine.
“Our findings here ... highlight how telomere shortening is a relevant mechanism for PF-emphysema susceptibility in a subset of patients beyond those with mutations in the telomerase core components. It is thus possible that efforts to reverse the telomere defect, or other regenerative approaches, will influence the natural history of these progressive pathologies in patients with telomere-mediated lung disease,” said Susan E. Stanley, an MD-PhD candidate in the department of oncology, Johns Hopkins University, Baltimore, and her associates.
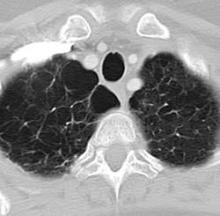
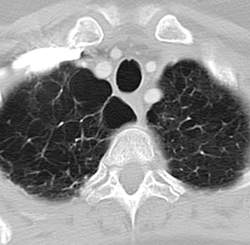
Pulmonary fibrosis and emphysema cluster in some families, but the genetic basis of such cases is poorly understood. Both PF and emphysema have been linked to premature aging of lung tissue and to abnormalities in the maintenance of telomere length. In addition, at least half of patients with familial and sporadic PF, and many with emphysema, have the clinical features of a short-telomere syndrome, including bone marrow failure/myelodysplastic syndrome, liver disease, and infertility.
The diagnosis of a short-telomere syndrome, as opposed to isolated PF-emphysema, is essential for appropriate treatment because if the defect is systemic, patients will “show exquisite sensitivity to otherwise tolerated medications and procedures, especially in the setting of lung transplantation,” the investigators said (Sci Transl Med. 2016;8:351ra107).
To explore the genetic basis of familial PF-emphysema, the researchers performed a series of studies, beginning with whole-genome sequencing on peripheral blood samples from five unrelated probands in familial PF-emphysema pedigrees. These participants had abnormally short telomeres and extrapulmonary features of short-telomere syndrome. Three of them who had low levels of the telomerase RNA component TR were selected for a candidate gene search, which revealed the NAF1 mutations.
The mutations were then found to be present in 2 of 30 (7%) affected members of a prevalence cohort but in none of 134 unaffected control subjects (0%), and in none of 9,006 samples from a public database of unaffected people (0%). Further genetic laboratory and mouse studies were performed to link the mutations with specific pathologies and to trace their functional effects. Their results led the researchers to conclude that these rare NAF1 variants interfere with RNA biogenesis, causing short telomeres resulting in lung disease and other abnormalities.
This work was supported by the National Institutes of Health, the Commonwealth Foundation, and the American Cancer Society. Ms. Stanley and her associates reported having no relevant financial disclosures.
Rare frameshift mutations in the NAF1 gene were discovered to cause a telomere-shortening syndrome which, among other adverse effects, predisposes carriers to develop pulmonary fibrosis (PF) and emphysema, according to a report published in Science Translational Medicine.
“Our findings here ... highlight how telomere shortening is a relevant mechanism for PF-emphysema susceptibility in a subset of patients beyond those with mutations in the telomerase core components. It is thus possible that efforts to reverse the telomere defect, or other regenerative approaches, will influence the natural history of these progressive pathologies in patients with telomere-mediated lung disease,” said Susan E. Stanley, an MD-PhD candidate in the department of oncology, Johns Hopkins University, Baltimore, and her associates.
Pulmonary fibrosis and emphysema cluster in some families, but the genetic basis of such cases is poorly understood. Both PF and emphysema have been linked to premature aging of lung tissue and to abnormalities in the maintenance of telomere length. In addition, at least half of patients with familial and sporadic PF, and many with emphysema, have the clinical features of a short-telomere syndrome, including bone marrow failure/myelodysplastic syndrome, liver disease, and infertility.
The diagnosis of a short-telomere syndrome, as opposed to isolated PF-emphysema, is essential for appropriate treatment because if the defect is systemic, patients will “show exquisite sensitivity to otherwise tolerated medications and procedures, especially in the setting of lung transplantation,” the investigators said (Sci Transl Med. 2016;8:351ra107).
To explore the genetic basis of familial PF-emphysema, the researchers performed a series of studies, beginning with whole-genome sequencing on peripheral blood samples from five unrelated probands in familial PF-emphysema pedigrees. These participants had abnormally short telomeres and extrapulmonary features of short-telomere syndrome. Three of them who had low levels of the telomerase RNA component TR were selected for a candidate gene search, which revealed the NAF1 mutations.
The mutations were then found to be present in 2 of 30 (7%) affected members of a prevalence cohort but in none of 134 unaffected control subjects (0%), and in none of 9,006 samples from a public database of unaffected people (0%). Further genetic laboratory and mouse studies were performed to link the mutations with specific pathologies and to trace their functional effects. Their results led the researchers to conclude that these rare NAF1 variants interfere with RNA biogenesis, causing short telomeres resulting in lung disease and other abnormalities.
This work was supported by the National Institutes of Health, the Commonwealth Foundation, and the American Cancer Society. Ms. Stanley and her associates reported having no relevant financial disclosures.
Rare frameshift mutations in the NAF1 gene were discovered to cause a telomere-shortening syndrome which, among other adverse effects, predisposes carriers to develop pulmonary fibrosis (PF) and emphysema, according to a report published in Science Translational Medicine.
“Our findings here ... highlight how telomere shortening is a relevant mechanism for PF-emphysema susceptibility in a subset of patients beyond those with mutations in the telomerase core components. It is thus possible that efforts to reverse the telomere defect, or other regenerative approaches, will influence the natural history of these progressive pathologies in patients with telomere-mediated lung disease,” said Susan E. Stanley, an MD-PhD candidate in the department of oncology, Johns Hopkins University, Baltimore, and her associates.
Pulmonary fibrosis and emphysema cluster in some families, but the genetic basis of such cases is poorly understood. Both PF and emphysema have been linked to premature aging of lung tissue and to abnormalities in the maintenance of telomere length. In addition, at least half of patients with familial and sporadic PF, and many with emphysema, have the clinical features of a short-telomere syndrome, including bone marrow failure/myelodysplastic syndrome, liver disease, and infertility.
The diagnosis of a short-telomere syndrome, as opposed to isolated PF-emphysema, is essential for appropriate treatment because if the defect is systemic, patients will “show exquisite sensitivity to otherwise tolerated medications and procedures, especially in the setting of lung transplantation,” the investigators said (Sci Transl Med. 2016;8:351ra107).
To explore the genetic basis of familial PF-emphysema, the researchers performed a series of studies, beginning with whole-genome sequencing on peripheral blood samples from five unrelated probands in familial PF-emphysema pedigrees. These participants had abnormally short telomeres and extrapulmonary features of short-telomere syndrome. Three of them who had low levels of the telomerase RNA component TR were selected for a candidate gene search, which revealed the NAF1 mutations.
The mutations were then found to be present in 2 of 30 (7%) affected members of a prevalence cohort but in none of 134 unaffected control subjects (0%), and in none of 9,006 samples from a public database of unaffected people (0%). Further genetic laboratory and mouse studies were performed to link the mutations with specific pathologies and to trace their functional effects. Their results led the researchers to conclude that these rare NAF1 variants interfere with RNA biogenesis, causing short telomeres resulting in lung disease and other abnormalities.
This work was supported by the National Institutes of Health, the Commonwealth Foundation, and the American Cancer Society. Ms. Stanley and her associates reported having no relevant financial disclosures.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Certain rare mutations in the NAF1 gene were discovered to predispose carriers to develop pulmonary fibrosis and emphysema.
Major finding: The rare NAF1 mutations were detected in 2 of 30 (7%) family members in an affected pedigree but in 0 of 134 controls.
Data source: A series of genetic sequencing and other studies involving five affected probands, 30 unrelated but affected patients, and 134 control subjects.
Disclosures: This work was supported by the National Institutes of Health, the Commonwealth Foundation, and the American Cancer Society. Ms. Stanley and her associates reported having no relevant financial disclosures.