User login
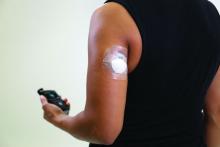
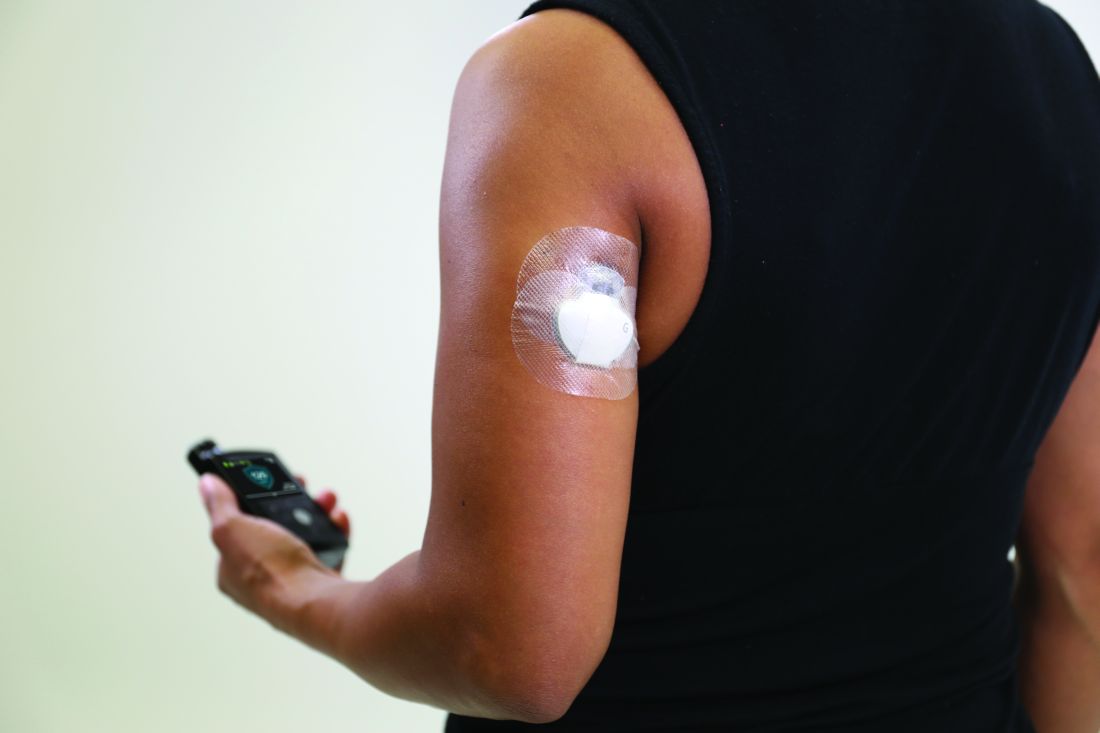
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”