User login
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the Department of the Navy, or the naval services at large.
Introduction
An estimated 850,000 to 950,000 persons in the United States are living with human immunodeficiency virus (HIV), 280,000 of whom are unaware of their infection and another 43,000 of whom meet the definition of acquired immunodeficiency syndrome (AIDS) (www.cdc.gov). The use of highly active antiretroviral therapy (HAART) has produced significant declines in morbidity and mortality from AIDS. Compared with the first 2 decades of the HIV pandemic, the number of HIV-related hospital admissions has declined. However, recently, this rate of decline has markedly slowed (1-3). The reasons for this plateau are many including a steady number of admissions for complications related to HAART, treatment failures, and the overall increased prevalence of HIV infection. Not only will HIV-infected patients still frequently require admission to the hospital, but the complexity of their inpatient care will continue to increase with the advancements in multiple drug regimens, aging of the HIV-infected population, and the interaction of HIV infection with medical comorbidities, many of which are attributable to HAART.
The hospitalist caring for the inpatient with AIDS is presented with several challenges including not only the diagnosis and management of opportunistic infections, but also the complications of HAART. In this article we review the guidelines for the initiation and continuation of HAART in the hospital, review important clinical complications of antiretroviral therapy, and review conditions that may result in the hospitalization of AIDS patients.
Initiation of HAART in the Hospital
In those who do not have access to health care, the initial diagnosis of HIV infection frequently occurs during a hospitalization for an AIDS-defining illness. Initiation of antiretrovirals is contingent on several issues, including CD4 count, viral load, clinical status, likelihood of continued adherence, and the concurrent treatment of opportunistic infections (OIs). All patients with HIV infection and a CD4 count <200 cells/mm3 or an AIDS-defining illness should receive antiretroviral therapy. Controversy exists as to whether a patient admitted for the treatment of an opportunistic infection should begin antiretroviral therapy immediately, or whether this therapy should be deferred until after acute treatment of the OI. The potential detrimental effects of drug-drug interactions, the need for treatment interruptions, and drug-related toxicity between antiretrovirals and OI-specific therapy may support initiating HAART after control of an OI is achieved. Conversely, for some opportunistic infections, such as cryptosporidiosis, the use of HAART is essential for successful treatment of the infection.
An ongoing randomized controlled trial initiated within the Adult AIDS Clinical Trials Group (ACTG) comparing outcomes between patients who start HAART immediately after presentation with an acute OI and patients who start HAART at least 4 weeks after the OI has resolved should help identify the factors supporting early or delayed initiation of antiretrovirals (4).
Generally speaking, HAART can be administered by combining either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) with 2 nucleoside reverse transcriptase inhibitors (NRTIs). There are currently more than 20 FDA-approved antiretrovirals. Frequent updates on the guidelines for the use of antiretroviral agents in HIV-infected adults are available at www.AIDSinfo.nih.gov, and a discussion of this topic is beyond the scope of this review.
Continuation of HAART in the Hospital
In most cases, every effort should be made to minimize interruption of HAART during a hospitalization. Although some investigators are examining the virologic and immunologic safety of interrupting HAART as a treatment strategy, there are few data on viral replication, CD4 cell count decline, and rate of acquisition of new mutations in hospitalized patients who have unexpected treatment interruptions (5). The long half-life of some antiretrovirals promotes the emergence of resistance once HAART is stopped. For example, once NNRTIs are stopped, subtherapeutic levels remain in the plasma and cells for several days. HIV then replicates in a milieu that may select for resistance mutations.
Because zidovudine is the only antiretroviral available in a parenteral preparation, it is often difficult to continue HAART when a patient cannot take medications by mouth. Drugs given by the enteral route in a hospitalized patient may also be poorly absorbed, and few data exist on the absorption of antiretrovirals administered through a gastrostomy or jejunostomy tube (6).
Prescribing HAART in the Hospital
Antiretroviral prescribing errors occur frequently in the hospitalized AIDS patient. The most common errors include overdosing or underdosing, missing components of multidrug regimens, or missing critical drug-drug interactions (7). Underdosing may lead to resistance, and overdosing contributes to increased toxicity. In one report, prescribing errors occurred in 12% of admissions in the post-HAART era (1998) compared with 2% of admissions in the pre-HAART era (1996) (7). The NRTIs, including didanosine, emtricitabine, lamivudine, stavudine, and zidovudine, require decreased dosing in renal insufficiency. Tenofovir is not recommended for use if the creatine clearance is less than 60 mL/minute. Dosage adjustments in hepatic disease are recommended for amprenavir, fosamprenavir, delavirdine, efavirenz, and nevirapine.
Immune Reconstitution Syndrome
The widespread use of HAART has produced sustained suppression of HIV replication and recovery of CD4 cell counts. It also became evident that HAART resulted in not only a numerical increase in CD4 cells, but also in a functional immune recovery (8-10). This improved T-cell response to antigens results in adequate protection against specific opportunistic infections, allowing for discontinuation of primary and secondary prophylaxis in HIV-infected patients. Immune reconstitution syndrome (IRS), an inflammatory syndrome, is recognized as a potential complication that can occur days to months after starting HAART. The onset of IRS is characterized by a paradoxical worsening of clinical or laboratory parameters despite a favorable response in CD4 cell counts and the suppression of viral replication (9,11,12). IRS has been reported to occur in 10–25% of patients who receive HAART and more commonly in those whose CD4 cell counts are <50 cells/mm3 at the start of HAART (9,11). It is postulated that the inflammatory response is triggered by the recognition of antigens associated with ongoing infection or recognition of persisting antigens associated with past (nonreplicating) infections. Mycobacterial antigens, frequently implicated in IRS, are responsible for about one third of cases. Other antigens associated with IRS include cytomegalovirus and hepatitis B and C (11). In most circumstances, with the management of IRS, HAART should be continued, while specific antimicrobial therapy and steroids should be considered (10).
Medical Conditions that Should Prompt HIV Screening
There are several medical conditions that should prompt screening for HIV infection. Generally, anyone presenting with a fever of unknown etiology who is sexually active or had a blood transfusion prior to 1985 should be screened for HIV infection. Symptoms consistent with acute retroviral syndrome (fever, sore throat, malaise, and skin rash) may be more commonly recognized by clinicians now than previously, and this remains a “golden opportunity” to intervene. Frequently, acute retroviral syndrome will be attributed to Epstein-Barr virus; however, caution should be used in the diagnosis of mononucleosis in those other than teenage populations. It is recommended that all persons presenting with any sexually transmitted disease, unexplained generalized lymphadenopathy, oral candidiasis, or tuberculosis should also be tested. Other conditions where HIV infection should be considered include enigmatic pneumonia, acute hepatitis B infection, herpes zoster infection (particularly in younger, seemingly immunocompetent individuals), idiopathic thrombocytopenic purpura, and nephropathy of unknown cause.
Drug Interactions
Drug interactions are an important consideration in the treatment of HIV infection. Interactions between HAART and other drugs used for the treatment or prophylaxis of opportunistic infections along with those used for the treatment of drug-induced endocrinopathies (hyperlipidemia, diabetes mellitus) are virtually unavoidable. Drug interactions occur either because of drug metabolism or absorption. The multiple metabolic pathways of some drugs make it difficult to predict the outcome of drug interactions. All protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by the cytochrome P-450 enzyme system and each of these drugs may alter the metabolism of other antiretrovirals and concomitantly administered drugs (13,14). A decrease in trough plasma concentrations of the protease inhibitors to a level below the in vitro concentration required to inhibit replication of 50% of viral strains (IC50) may lead to development of resistance. Because nucleoside analogue reverse transcriptase inhibitors are primarily eliminated by the kidney, they do not interact with other drugs through the cytochrome P-450 system.
One noteworthy interaction that the clinician caring for HIV-infected patients should be aware of is the interaction of ribavirin with zidovudine. Ribavirin decreases the phosphorylation of zidovudine and stavudine in vitro, resulting in decreased concentrations of the active compound. HIV-infected patients who are coinfected with hepatitis C may be treated with regimens that include ribavirin, which may reduce the efficacy of zidovudine (15). Another important interaction is the effect of nevirapine or efavirenz on plasma methadone concentrations. Both drugs can decrease methadone plasma levels by 50%, and patients receiving chronic therapy may need increased methadone doses to prevent withdrawal symptoms (16).
Protease inhibitors are associated with numerous interactions including certain antiarrhythmics, sedatives, hypnotics, ergot derivatives, and several lipid-lowering agents (statins). Not only do protease inhibitors affect the metabolism of certain drugs, but also their own metabolism is altered by other inducers or inhibitors of cytochrome activity that can cause clinically important decreases in serum levels of protease inhibitors. One widely recognized interaction is that of rifampin, which may decrease levels of some protease inhibitors by 80%. The resulting low plasma concentrations may promote viral resistance and result in treatment failure. Patients being treated for tuberculosis, who are also receiving protease inhibitors should be treated with a four-drug regimen that includes rifabutin (at half dose) instead of rifampin. Updated guidelines for the use of rifabutin or rifampin in HIV-infected patients receiving antiretroviral agents have been reviewed recently (17).
Other potent inducers such as phenytoin, phenobarbital, and carbamazepine can cause similar reductions in serum levels of protease inhibitors. Azole antifungal drugs and macrolides also have important interactions that complicate both the treatment and prophylaxis of opportunistic infections.
Interactions that interfere with absorption can also affect plasma drug concentrations. For example, the absorption of fluconazole is unaffected by variations in gastric pH, while itraconazole and ketoconazole require an acidic environment for optimal absorption. The protease inhibitor, atazanavir, also requires a low pH for absorption and thus is contraindicated with the use of proton pump inhibitors; taking atazanavir with acidic beverages is not sufficient to overcome this (18).
New information about drug interactions becomes known on almost a daily basis in patients with HIV infection. The number of documented and theoretical interactions can become overwhelming to the clinician. Clinicians should suspect potential drug interactions in a patient who is failing therapy but who is adherent to HAART. Fortunately, there are extensive tables on Web sites (www.hivatis.org) and product information to aid in the recognition and management of drug interactions.
Complications of HAART
Diabetes mellitus, hyperlipidemias, lipodystrophy, and insulin resistance are among the many complex metabolic abnormalities attributable to the use of HAART. For the most part, these complications are managed conservatively and usually do not mandate the discontinuation of HAART. Pancreatitis, hepatic steatosis, and lactic acidosis are wellrecognized complications of NRTIs. These are usually more acute and may result in hospitalization and necessitate the discontinuation of medications. Cessation of the offending agent (didanosine [ddI], stavudine [d4T], and zalcitabine [ddC) usually results in resolution of pancreatitis, but the episode may limit use of these agents in the future. Hepatic steatosis and lactic acidosis are rare but life-threatening adverse effects associated with the mitochondrial toxicity seen with the NRTIs. Symptoms usually develop insidiously with nausea, vomiting, abdominal pain, weight loss, or dyspnea and can progress rapidly to fatal lactic acidosis. Hepatomegaly, ascites, elevated liver associated enzymes, and an increased anion gap with lactic acidemia are usually present (19,20). Discontinuation of antiretroivirals is imperative.
There is an accumulating body of evidence that suggests that HIV-infected patients receiving HAART may be at risk for accelerated coronary disease (21). In addition, some cohort studies have reported an increased incidence of myocardial infarction (MI) following the introduction of HAART and the risk for MI rose progressively with the number of years on antiretroviral therapy (22,23). However, it is important to note that many traditional risk factors for coronary artery disease contribute more substantially to the risk for a cardiovascular event than does HAART. Therefore, aggressive modification of primary cardiac risk factors is warranted.
Hypersensitivity Drug Reactions
Drug hypersensitivity reactions are life-threatening reactions that result in a systemic illness that usually includes fever and maculopapular rash accompanied by constitutional symptoms (fatigue, myalgias, and arthralgias), visceral involvement (lymphadenopathy, mucositis, pneumonitis, myocarditis, hepatitis, and interstitial nephritis), and hematologic abnormalities (eosinophilia) (24).
Abacavir, an NRTI, is a relatively new antiretroviral agent used in many HAART regimens. Abacavir is associated with a hypersensitivity reaction, which can be fatal if abacavir use is continued despite the reaction, or if re-challenge with the drug takes place after the reaction (25). The overall incidence of this reaction appears to be around 4% (25). Prior antiretroviral experience and being of African descent are associated with a nearly 40% reduction in the risk of this hypersensitivity reaction, while patients of white race are at a significantly greater risk. CD4 cell counts do not appear to be significantly related to abacavir hypersensitivity (26). The exact metabolite that is likely responsible for abacavir hypersensitivity is unknown.
The most common symptoms of abacavir hypersensitivity reaction are fever, rash, nausea, vomiting, and abdominal pain. Occasionally, respiratory symptoms will be present and can mimic influenza. However, gastrointestinal symptoms are the most prominent complaints after fever and rash and help to distinguish between influenza and abacavir hypersensitivity. More than 90% of hypersensitivity reactions occur during the first 6 weeks of treatment, with a median time to development of 8 days. A fever that develops within a few weeks after the initiation of therapy with abacavir may be due to causes other than hypersensitivity. One of the most common situations is the simultaneous initiation of treatment with other drugs, such as trimethoprimsulfamethxazole, efavirenz, or nevirapine, all of which are associated with a higher incidence of hypersensitivity than abacavir (27,28). Symptoms may be sudden and worsen over a few days if abacavir is continued. Symptoms tend to improve in 48 hours after abacavir is discontinued. Supportive therapy includes intravenous hydration and withdrawal of abacavir as well as all other antiretrovirals. Early in the use of this medication, 20% of patients who were re-challenged with the drug experienced unanticipated life-threatening events manifesting as an anaphylactic or immediate type hypersensitivity reaction. Hypotension, renal insufficiency, and bronchospasm have resulted in death. Rechallenge symptoms have been seen with the first dose (29). A discussion of the potential for this hypersensitivity is warranted when prescribing this agent. In the United States, a patient information card warning of this hypersensitivity reaction is distributed to the patient with each bottle of abacavir. Prednisone does not prevent the development of hypersensitivity reaction.
Symptoms of toxicity from TMP-SMX are more likely to occur in the HIV infected than in patients without HIV infection. Fever and rash can occur in up to 50% of HIV-infected patients. The rash can be treatment limiting or severe in up to 20% of HIV-infected patients who receive it. Life-threatening reactions may occur, including fatal Stevens Johnson-type exfoliative skin reactions. Most toxicity in HIV-infected patients appears to be related to metabolites of the sulfamethoxazole component and decreased levels of glutathione. There have been reports of severe systemic reactions that resemble anaphylaxis or septic shock occurring in HIV-infected patients who are re-challenged with TMP-SMX after experiencing toxicity within the previous 6–8 weeks (30).
The NNRTIf nevirapine and efavirenz can cause a delayed hypersensitivity reaction similar to that seen with abacavir. Cutaneous involvement is a prominent component of both nevirapine and efavirenz hypersensitivity reaction, with rash more likely to occur with the use of nevirapine. In addition, female patients have a higher propensity of developing Stevens-Johnson syndrome and symptomatic hepatic events from nevirapine (28,31).
Laboratory Abnormalities Related to Drugs
Hyperbilirubinemia and Atazanavir and Indinavir
Atazanavir, a protease inhibitor, is metabolized by the liver via CYP3A and also inhibits both CTP3A and UGT1A1. UGT1A1 is required for conjugation of bilirubin and inhibition of this enzyme results in elevated levels of unconjugated bilirubin. This effect is similar to what is observed in Gilbert’s syndrome. Asymptomatic indirect hyperbilirubemia may be seen in up to 60% of patients receiving atazanavir. Total bilirubin levels may rise to greater than 5 mg/dL, and more than 17% of patients may experience jaundice (18). Concurrent elevations in hepatic serum transaminases should not be attributed to atazanavir and alternative etiologies for these elevations should be sought. This hyperbilirubinemia is reversible upon discontinuation of the atazanavir.
Similarly, but to a lesser degree, asymptomatic unconjugated hyperbilirubinemia (>2 mg/dL) has been reported in up to 14% of patients treated with indinavir. Elevated serum transaminases were seen in less than 1%.
Renal Abnormalities and Indinavir
Several renal syndromes have been associated with indinavir use, ranging from obstructive uropathy and acute renal failure to asymptomatic pyuria. The range of clinical syndromes is a consequence of indinavir crystals aggregating within or irritating the urinary tract (32). Symptomatic nephrolithiasis (indinavir crystallization) has been reported to affect up to 12% (range, 5–35 %) of patients who receive indinavir, while up to 67% of patients will have asymptomatic crystalluria. The cumulative frequency of nephrolithiasis events increases with increasing exposure to indinavir. Therapy may be continued or interrupted for a few days. Adequate hydration is necessary with the administration of indinavir. Indinavir associated pyuria is frequently associated with interstitial nephritis or urothelial inflammation. Discontinuation of indinavir will lead to resolution of urine abnormalities.
Elevated Mean Corpuscular Volume (MCV) and NRTIs
Elevation of MCV or macrocytosis occurs in more than 90% of patients treated with zidovudine, but is not correlated with the development of anemia. Macrocytosis (MCV values exceeding 110/fl) develops within 2 weeks following the initiation of zidovudine therapy, and its presence can be used as a marker for medical adherence. When anemia does occur it is associated with a dose related bone marrow toxicity manifested as a macrocytic anemia. Serum B12 and folate levels are normal. Stavudine use is also associated with macrocytosis, in non-zidovudine-containing regimens (33).
Drug Screens and Efavirenz
The use of efavirenz, a potent non-nucleoside reverse transcriptase inhibitor, can cause a false-positive urine drug screen for cannabinoid. Efavirenz does not bind to cannabinoid receptors. The false-positive test results are specific to the assay kit used (34).
Evaluation of the AIDS Patient with Fever
Fever is a common symptom in HIV-infected patients, the etiology of which can be identified in more than 80% of cases (35,36). The AIDS patient with fever poses a considerable challenge given that the expanded differential may include a wide range of OIs. The CD4 cell count remains a valuable predictor of risk for infection. Patients with CD4 cell counts greater than 500 cells/mm3 should be evaluated as if immunocompetent. Patients with CD4 cell counts between 200 and 500 cells/mm3 are at increased risk for upper and lower bacterial respiratory infections, tuberculosis, and sinusitis, but overall their risk for opportunistic infection is not increased. In patients with CD4 counts less than 200 cells/mm3, Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common cause of fever in those not receiving primary prophylaxis. As the CD4 cell count decreases below 100 cells/mm3, the risk for disseminated MAC, toxoplasmosis, CMV, disseminated fungal infections, and lymphoma should be considered possible causes of fever. HIV itself is usually not the cause of fever in patients with advanced immunosuppression (37).
In patients with CD4 counts >200 cells/mm3, the clinician can usually construct a laboratory and radiographic evaluation guided by symptoms, while in patients with severe immunosuppression a broader evaluation is required. A serum cryptococcal antigen should be obtained, as it has high sensitivity and specificity for both systemic disease as well as meningitis (38). Bacterial, mycobacterial, and fungal isolator blood cultures should be performed, as well as a urine culture, despite lack of symptoms. Urine AFB cultures can be added if there is a suspicion for tuberculosis. Sputum should be evaluated with gram stain, AFB smear, and culture, as well as PCP direct fluorescent antibody. If diarrhea is present, stool studies should include bacterial culture, ova and parasites evaluation, an assay for C. difficile, and Cryptosporidia and Giardia stool antigen assays. A serum LDH may be elevated in PCP, disseminated histoplasmosis, or lymphoma. A serum CMV antigen may be useful in the patient with fever and diarrhea, hepatitis, or retinitis.
A chest radiograph should be performed in all febrile AIDS patients. Chest films may be normal in 5–10% of HIV-infected patients with tuberculosis (TB). The typical radiographic appearance of Pneumocystis jiroveci pneumonia is a bilateral interstitial pattern characterized by reticular or ground-glass opacities. However, normal chest radiographs may be seen in one third of AIDS patients with active PCP. High-resolution computed tomography (HRCT) of the chest should be obtained if there is still a clinical suspicion for Pneumocystis. HRCT has been shown in several reports to have a 100% negative predictive value in the evaluation for PCP (39,40).
A lumbar puncture should be performed if the patient is symptomatic or if the serum cryptococcal antigen is reactive. A bone marrow biopsy and culture is also useful particularly in the evaluation of the patient with cytopenias. Bacterial, fungal, and AFB cultures may yield disseminated mycobacterial or fungal disease. Histopathologic evaluation may reveal granulomas with organisms or lymphoma. Bronchoscopy may be pursued in cases of high suspicion for TB or PCP.
Guidelines for the management of opportunistic infections associated with human immunodeficiency virus are available at www.AIDSinfo.nih.gov.
Evaluation of the AIDS Patient with Focal Neurological Disease
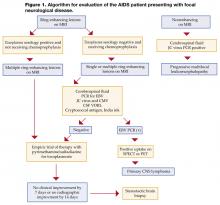
Toxoplasma encephalitis (TE) may be distinguished from primary CNS lymphoma without a brain biopsy. TE is caused by reactivation of latent infection by the protozoan Toxoplasma gondii. Almost 90% of patients have CD4 counts less than 200 cells/mm3 and 75% have CD4 counts less than 100 cells/mm3. Serum anti-Toxoplasma IgG antibodies are detected in more than 90% of patients with TE. Lesions on CT or MRI (a more sensitive modality) are typically multiple ring-enhancing lesions with a predilection for the basal ganglia. An emipiric trial of therapy is recommended, and a response confirms a diagnosis in the patient who has a positive Toxoplasma antibody and is not receiving trimethoprim-sulfamethoxazole prophylaxis. A lumbar puncture in this setting is not necessary and maybe ill advised if cerebral edema is present (Figure 1, page 24).
Primary CNS lymphoma (PCNSL) has a similar radiographic appearance as TE. Solitary lesions are more frequent in PCNSL. Positron emission tomography (PET) and single photon emission CT (SPECT) are useful adjunctive imaging modalities as they are positive in PCNSL due to the increased metabolic activity of the tumor. Cytologic analysis of the CSF may show lymphomatous cells. Epstein-Barr virus (EBV) DNA is uniformly detected in PCNSL in AIDS patients and detection of EBV DNA by PCR on the CSF has a sensitivity of 90–100% and a specificity of 87–98% for the diagnosis of PCNSL (41,42). Combining SPECT imaging and EBV PCR provides 100% sensitivity and 100% negative predictive value in the evaluation of AIDS-related primary CNS lymphoma (43), obviating the need for brain biopsy.
Progressive multifocal leukoencephalopathy (PML) is characterized radiographically by multiple and bilateral hypodense lesions of the white matter without mass effect or enhancement on CT. MRI demonstrates areas of hypointensity on T1-weighted images and increased intensity on T2-weighted images. JC virus DNA can be detected by PCR of CSF or brain tissue with sensitivity of approximately 80% and specificity of 95%. Because of the high positive predictive value of a positive PCR, a patient with AIDS who also has a compatible MRI can be diagnosed with PML (44,45).
Other focal neurologic disease seen in AIDS patients includes cryptococcomas, tuberculomas, CMV encephalitis, neurosyphilis, Nocardia and Aspergillus infection, and bacterial brain abscesses (46).
Conclusion
As survival of the HIV-infected population improves, more patients may require hospitalization for HAART treatment failures or complications attributed to antiretroviral therapy. The hospitalist should be familiar with the complications of antiretroviral agents, the interactions between HAART and medications used to treat opportunistic infections, and medical conditions induced by HAART. Evaluation of the HIV-infected patient presenting with fever can be based on the CD4 cell count, which predicts risk for opportunistic infections. Finally, using combined diagnostic approaches along with modern imaging and laboratory assays may preclude the need for more invasive procedures in the HIV-infected hospitalized patient.
Dr. Decker may be reached at [email protected].
References
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531-2.
- Fleishman JA, Hellinger FJ. Trends in HIV-related inpatient admissions from 1993 to 1997: a seven-state study. J Acquir Immune Defic Syndr. 2001;28:73-80.
- Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996-2000: a 7-state study. J Acquir Immune Defic Syndr. 2003;34:102-10.
- Powderly WG. Should patients with an acute opportunistic infection receive antiretroviral therapy immediately? Advanced Studies in Medicine. 2005;5:S111-S116.
- Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220-6.
- Soni N, Pozniak A. Continuing HIV therapy in the ICU. Crit Care. 2001;5:247-8.
- Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833-8.
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112-6.
- Cooney EL. Clinical indicators of immune restoration following highly active antiretroviral therapy. Clin Infect Dis. 2002;34:224-33.
- Hirsch HH, Kaufmann G, Sendi P, BaĴegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159-66.
- Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882-92.
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43-9.
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-96.
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289-304.
- Sim SM, Hoggard PG, Sales SD, Phiboonbanakit D, Hart CA, Back DJ. Effect of ribavirin on zidovudine efficacy and toxicity in vitro: a concentration-dependent interaction. AIDS Res Hum Retroviruses. 1998;14:1661-7.
- Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213-7.
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;49:185-9.
- Havlir DV, O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599-604.
- Sheng WH, Hsieh SM, Lee SC, et al. Fatal lactic acidosis associated with highly active antiretroviral therapy in patients with advanced human immunodeficiency virus infection in Taiwan. Int J STD AIDS. 2004;15:249-53.
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-62;quiz 563-4.
- Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958.
- Klein D, Hurley LB, Sidney S. Cardiovascular Disease and HIV Infection. N Engl J Med. 2003;349:1869-70; author reply 1869-70.
- Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
- Anderson JA, Adkinson NF Jr. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891-9.
- Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis. 2002;34:1137-42.
- Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227-8.
- Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-2.
- Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with abacavir. AIDS. 1999;13:999-1000.
- Martin GJ, Paparello SF, Decker CF. A severe systemic reaction to trimethoprim-sulfamethoxazole in a patient infected with the human immunodeficiency virus. Clin Infect Dis. 1993;16:175-6.
- Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124-9.
- Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122-8.
- Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160-3.
- Gottesman L. Sustiva may cause false positive on marijuana test. WORLD. 1999:7.
- Sepkowitz KA, Telzak EE, Carrow M, Armstrong D. Fever among outpatients with advanced human immunodeficiency virus infection. Arch Intern Med. 1993;153: 1909-12.
- Barat LM, Gunn JE, Steger KA, Perkins CJ, Craven DE. Causes of fever in patients infected with human immunodeficiency virus who were admitted to Boston City Hospital. Clin Infect Dis. 1996;23:320-8.
- Sepkowitz KA. FUO and AIDS. Curr Clin Top Infect Dis. 1999;19:1-15.
- Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65-71.
- Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689-93.
- Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967-75.
- MacMahon EM, Glass JD, Hayward SD, et al. Association of Epstein-Barr virus with primary central nervous system lymphoma in AIDS. AIDS Res Hum Retroviruses. 1992;8:740-2.
- Rao CR, Jain K, Bhatia K, Laksmaiah KC, Shankar SK. Association of primary central nervous system lymphomas with the Epstein-Barr virus. Neurol India. 2003;51:237-40.
- Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554-60.
- Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896-906.
- Weber T. Cerebrospinal fluid analysis for the diagnosis of human immunodeficiency virus-related neurologic diseases. Semin Neurol. 1999;19:223-33.
- Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103-15.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the Department of the Navy, or the naval services at large.
Introduction
An estimated 850,000 to 950,000 persons in the United States are living with human immunodeficiency virus (HIV), 280,000 of whom are unaware of their infection and another 43,000 of whom meet the definition of acquired immunodeficiency syndrome (AIDS) (www.cdc.gov). The use of highly active antiretroviral therapy (HAART) has produced significant declines in morbidity and mortality from AIDS. Compared with the first 2 decades of the HIV pandemic, the number of HIV-related hospital admissions has declined. However, recently, this rate of decline has markedly slowed (1-3). The reasons for this plateau are many including a steady number of admissions for complications related to HAART, treatment failures, and the overall increased prevalence of HIV infection. Not only will HIV-infected patients still frequently require admission to the hospital, but the complexity of their inpatient care will continue to increase with the advancements in multiple drug regimens, aging of the HIV-infected population, and the interaction of HIV infection with medical comorbidities, many of which are attributable to HAART.
The hospitalist caring for the inpatient with AIDS is presented with several challenges including not only the diagnosis and management of opportunistic infections, but also the complications of HAART. In this article we review the guidelines for the initiation and continuation of HAART in the hospital, review important clinical complications of antiretroviral therapy, and review conditions that may result in the hospitalization of AIDS patients.
Initiation of HAART in the Hospital
In those who do not have access to health care, the initial diagnosis of HIV infection frequently occurs during a hospitalization for an AIDS-defining illness. Initiation of antiretrovirals is contingent on several issues, including CD4 count, viral load, clinical status, likelihood of continued adherence, and the concurrent treatment of opportunistic infections (OIs). All patients with HIV infection and a CD4 count <200 cells/mm3 or an AIDS-defining illness should receive antiretroviral therapy. Controversy exists as to whether a patient admitted for the treatment of an opportunistic infection should begin antiretroviral therapy immediately, or whether this therapy should be deferred until after acute treatment of the OI. The potential detrimental effects of drug-drug interactions, the need for treatment interruptions, and drug-related toxicity between antiretrovirals and OI-specific therapy may support initiating HAART after control of an OI is achieved. Conversely, for some opportunistic infections, such as cryptosporidiosis, the use of HAART is essential for successful treatment of the infection.
An ongoing randomized controlled trial initiated within the Adult AIDS Clinical Trials Group (ACTG) comparing outcomes between patients who start HAART immediately after presentation with an acute OI and patients who start HAART at least 4 weeks after the OI has resolved should help identify the factors supporting early or delayed initiation of antiretrovirals (4).
Generally speaking, HAART can be administered by combining either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) with 2 nucleoside reverse transcriptase inhibitors (NRTIs). There are currently more than 20 FDA-approved antiretrovirals. Frequent updates on the guidelines for the use of antiretroviral agents in HIV-infected adults are available at www.AIDSinfo.nih.gov, and a discussion of this topic is beyond the scope of this review.
Continuation of HAART in the Hospital
In most cases, every effort should be made to minimize interruption of HAART during a hospitalization. Although some investigators are examining the virologic and immunologic safety of interrupting HAART as a treatment strategy, there are few data on viral replication, CD4 cell count decline, and rate of acquisition of new mutations in hospitalized patients who have unexpected treatment interruptions (5). The long half-life of some antiretrovirals promotes the emergence of resistance once HAART is stopped. For example, once NNRTIs are stopped, subtherapeutic levels remain in the plasma and cells for several days. HIV then replicates in a milieu that may select for resistance mutations.
Because zidovudine is the only antiretroviral available in a parenteral preparation, it is often difficult to continue HAART when a patient cannot take medications by mouth. Drugs given by the enteral route in a hospitalized patient may also be poorly absorbed, and few data exist on the absorption of antiretrovirals administered through a gastrostomy or jejunostomy tube (6).
Prescribing HAART in the Hospital
Antiretroviral prescribing errors occur frequently in the hospitalized AIDS patient. The most common errors include overdosing or underdosing, missing components of multidrug regimens, or missing critical drug-drug interactions (7). Underdosing may lead to resistance, and overdosing contributes to increased toxicity. In one report, prescribing errors occurred in 12% of admissions in the post-HAART era (1998) compared with 2% of admissions in the pre-HAART era (1996) (7). The NRTIs, including didanosine, emtricitabine, lamivudine, stavudine, and zidovudine, require decreased dosing in renal insufficiency. Tenofovir is not recommended for use if the creatine clearance is less than 60 mL/minute. Dosage adjustments in hepatic disease are recommended for amprenavir, fosamprenavir, delavirdine, efavirenz, and nevirapine.
Immune Reconstitution Syndrome
The widespread use of HAART has produced sustained suppression of HIV replication and recovery of CD4 cell counts. It also became evident that HAART resulted in not only a numerical increase in CD4 cells, but also in a functional immune recovery (8-10). This improved T-cell response to antigens results in adequate protection against specific opportunistic infections, allowing for discontinuation of primary and secondary prophylaxis in HIV-infected patients. Immune reconstitution syndrome (IRS), an inflammatory syndrome, is recognized as a potential complication that can occur days to months after starting HAART. The onset of IRS is characterized by a paradoxical worsening of clinical or laboratory parameters despite a favorable response in CD4 cell counts and the suppression of viral replication (9,11,12). IRS has been reported to occur in 10–25% of patients who receive HAART and more commonly in those whose CD4 cell counts are <50 cells/mm3 at the start of HAART (9,11). It is postulated that the inflammatory response is triggered by the recognition of antigens associated with ongoing infection or recognition of persisting antigens associated with past (nonreplicating) infections. Mycobacterial antigens, frequently implicated in IRS, are responsible for about one third of cases. Other antigens associated with IRS include cytomegalovirus and hepatitis B and C (11). In most circumstances, with the management of IRS, HAART should be continued, while specific antimicrobial therapy and steroids should be considered (10).
Medical Conditions that Should Prompt HIV Screening
There are several medical conditions that should prompt screening for HIV infection. Generally, anyone presenting with a fever of unknown etiology who is sexually active or had a blood transfusion prior to 1985 should be screened for HIV infection. Symptoms consistent with acute retroviral syndrome (fever, sore throat, malaise, and skin rash) may be more commonly recognized by clinicians now than previously, and this remains a “golden opportunity” to intervene. Frequently, acute retroviral syndrome will be attributed to Epstein-Barr virus; however, caution should be used in the diagnosis of mononucleosis in those other than teenage populations. It is recommended that all persons presenting with any sexually transmitted disease, unexplained generalized lymphadenopathy, oral candidiasis, or tuberculosis should also be tested. Other conditions where HIV infection should be considered include enigmatic pneumonia, acute hepatitis B infection, herpes zoster infection (particularly in younger, seemingly immunocompetent individuals), idiopathic thrombocytopenic purpura, and nephropathy of unknown cause.
Drug Interactions
Drug interactions are an important consideration in the treatment of HIV infection. Interactions between HAART and other drugs used for the treatment or prophylaxis of opportunistic infections along with those used for the treatment of drug-induced endocrinopathies (hyperlipidemia, diabetes mellitus) are virtually unavoidable. Drug interactions occur either because of drug metabolism or absorption. The multiple metabolic pathways of some drugs make it difficult to predict the outcome of drug interactions. All protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by the cytochrome P-450 enzyme system and each of these drugs may alter the metabolism of other antiretrovirals and concomitantly administered drugs (13,14). A decrease in trough plasma concentrations of the protease inhibitors to a level below the in vitro concentration required to inhibit replication of 50% of viral strains (IC50) may lead to development of resistance. Because nucleoside analogue reverse transcriptase inhibitors are primarily eliminated by the kidney, they do not interact with other drugs through the cytochrome P-450 system.
One noteworthy interaction that the clinician caring for HIV-infected patients should be aware of is the interaction of ribavirin with zidovudine. Ribavirin decreases the phosphorylation of zidovudine and stavudine in vitro, resulting in decreased concentrations of the active compound. HIV-infected patients who are coinfected with hepatitis C may be treated with regimens that include ribavirin, which may reduce the efficacy of zidovudine (15). Another important interaction is the effect of nevirapine or efavirenz on plasma methadone concentrations. Both drugs can decrease methadone plasma levels by 50%, and patients receiving chronic therapy may need increased methadone doses to prevent withdrawal symptoms (16).
Protease inhibitors are associated with numerous interactions including certain antiarrhythmics, sedatives, hypnotics, ergot derivatives, and several lipid-lowering agents (statins). Not only do protease inhibitors affect the metabolism of certain drugs, but also their own metabolism is altered by other inducers or inhibitors of cytochrome activity that can cause clinically important decreases in serum levels of protease inhibitors. One widely recognized interaction is that of rifampin, which may decrease levels of some protease inhibitors by 80%. The resulting low plasma concentrations may promote viral resistance and result in treatment failure. Patients being treated for tuberculosis, who are also receiving protease inhibitors should be treated with a four-drug regimen that includes rifabutin (at half dose) instead of rifampin. Updated guidelines for the use of rifabutin or rifampin in HIV-infected patients receiving antiretroviral agents have been reviewed recently (17).
Other potent inducers such as phenytoin, phenobarbital, and carbamazepine can cause similar reductions in serum levels of protease inhibitors. Azole antifungal drugs and macrolides also have important interactions that complicate both the treatment and prophylaxis of opportunistic infections.
Interactions that interfere with absorption can also affect plasma drug concentrations. For example, the absorption of fluconazole is unaffected by variations in gastric pH, while itraconazole and ketoconazole require an acidic environment for optimal absorption. The protease inhibitor, atazanavir, also requires a low pH for absorption and thus is contraindicated with the use of proton pump inhibitors; taking atazanavir with acidic beverages is not sufficient to overcome this (18).
New information about drug interactions becomes known on almost a daily basis in patients with HIV infection. The number of documented and theoretical interactions can become overwhelming to the clinician. Clinicians should suspect potential drug interactions in a patient who is failing therapy but who is adherent to HAART. Fortunately, there are extensive tables on Web sites (www.hivatis.org) and product information to aid in the recognition and management of drug interactions.
Complications of HAART
Diabetes mellitus, hyperlipidemias, lipodystrophy, and insulin resistance are among the many complex metabolic abnormalities attributable to the use of HAART. For the most part, these complications are managed conservatively and usually do not mandate the discontinuation of HAART. Pancreatitis, hepatic steatosis, and lactic acidosis are wellrecognized complications of NRTIs. These are usually more acute and may result in hospitalization and necessitate the discontinuation of medications. Cessation of the offending agent (didanosine [ddI], stavudine [d4T], and zalcitabine [ddC) usually results in resolution of pancreatitis, but the episode may limit use of these agents in the future. Hepatic steatosis and lactic acidosis are rare but life-threatening adverse effects associated with the mitochondrial toxicity seen with the NRTIs. Symptoms usually develop insidiously with nausea, vomiting, abdominal pain, weight loss, or dyspnea and can progress rapidly to fatal lactic acidosis. Hepatomegaly, ascites, elevated liver associated enzymes, and an increased anion gap with lactic acidemia are usually present (19,20). Discontinuation of antiretroivirals is imperative.
There is an accumulating body of evidence that suggests that HIV-infected patients receiving HAART may be at risk for accelerated coronary disease (21). In addition, some cohort studies have reported an increased incidence of myocardial infarction (MI) following the introduction of HAART and the risk for MI rose progressively with the number of years on antiretroviral therapy (22,23). However, it is important to note that many traditional risk factors for coronary artery disease contribute more substantially to the risk for a cardiovascular event than does HAART. Therefore, aggressive modification of primary cardiac risk factors is warranted.
Hypersensitivity Drug Reactions
Drug hypersensitivity reactions are life-threatening reactions that result in a systemic illness that usually includes fever and maculopapular rash accompanied by constitutional symptoms (fatigue, myalgias, and arthralgias), visceral involvement (lymphadenopathy, mucositis, pneumonitis, myocarditis, hepatitis, and interstitial nephritis), and hematologic abnormalities (eosinophilia) (24).
Abacavir, an NRTI, is a relatively new antiretroviral agent used in many HAART regimens. Abacavir is associated with a hypersensitivity reaction, which can be fatal if abacavir use is continued despite the reaction, or if re-challenge with the drug takes place after the reaction (25). The overall incidence of this reaction appears to be around 4% (25). Prior antiretroviral experience and being of African descent are associated with a nearly 40% reduction in the risk of this hypersensitivity reaction, while patients of white race are at a significantly greater risk. CD4 cell counts do not appear to be significantly related to abacavir hypersensitivity (26). The exact metabolite that is likely responsible for abacavir hypersensitivity is unknown.
The most common symptoms of abacavir hypersensitivity reaction are fever, rash, nausea, vomiting, and abdominal pain. Occasionally, respiratory symptoms will be present and can mimic influenza. However, gastrointestinal symptoms are the most prominent complaints after fever and rash and help to distinguish between influenza and abacavir hypersensitivity. More than 90% of hypersensitivity reactions occur during the first 6 weeks of treatment, with a median time to development of 8 days. A fever that develops within a few weeks after the initiation of therapy with abacavir may be due to causes other than hypersensitivity. One of the most common situations is the simultaneous initiation of treatment with other drugs, such as trimethoprimsulfamethxazole, efavirenz, or nevirapine, all of which are associated with a higher incidence of hypersensitivity than abacavir (27,28). Symptoms may be sudden and worsen over a few days if abacavir is continued. Symptoms tend to improve in 48 hours after abacavir is discontinued. Supportive therapy includes intravenous hydration and withdrawal of abacavir as well as all other antiretrovirals. Early in the use of this medication, 20% of patients who were re-challenged with the drug experienced unanticipated life-threatening events manifesting as an anaphylactic or immediate type hypersensitivity reaction. Hypotension, renal insufficiency, and bronchospasm have resulted in death. Rechallenge symptoms have been seen with the first dose (29). A discussion of the potential for this hypersensitivity is warranted when prescribing this agent. In the United States, a patient information card warning of this hypersensitivity reaction is distributed to the patient with each bottle of abacavir. Prednisone does not prevent the development of hypersensitivity reaction.
Symptoms of toxicity from TMP-SMX are more likely to occur in the HIV infected than in patients without HIV infection. Fever and rash can occur in up to 50% of HIV-infected patients. The rash can be treatment limiting or severe in up to 20% of HIV-infected patients who receive it. Life-threatening reactions may occur, including fatal Stevens Johnson-type exfoliative skin reactions. Most toxicity in HIV-infected patients appears to be related to metabolites of the sulfamethoxazole component and decreased levels of glutathione. There have been reports of severe systemic reactions that resemble anaphylaxis or septic shock occurring in HIV-infected patients who are re-challenged with TMP-SMX after experiencing toxicity within the previous 6–8 weeks (30).
The NNRTIf nevirapine and efavirenz can cause a delayed hypersensitivity reaction similar to that seen with abacavir. Cutaneous involvement is a prominent component of both nevirapine and efavirenz hypersensitivity reaction, with rash more likely to occur with the use of nevirapine. In addition, female patients have a higher propensity of developing Stevens-Johnson syndrome and symptomatic hepatic events from nevirapine (28,31).
Laboratory Abnormalities Related to Drugs
Hyperbilirubinemia and Atazanavir and Indinavir
Atazanavir, a protease inhibitor, is metabolized by the liver via CYP3A and also inhibits both CTP3A and UGT1A1. UGT1A1 is required for conjugation of bilirubin and inhibition of this enzyme results in elevated levels of unconjugated bilirubin. This effect is similar to what is observed in Gilbert’s syndrome. Asymptomatic indirect hyperbilirubemia may be seen in up to 60% of patients receiving atazanavir. Total bilirubin levels may rise to greater than 5 mg/dL, and more than 17% of patients may experience jaundice (18). Concurrent elevations in hepatic serum transaminases should not be attributed to atazanavir and alternative etiologies for these elevations should be sought. This hyperbilirubinemia is reversible upon discontinuation of the atazanavir.
Similarly, but to a lesser degree, asymptomatic unconjugated hyperbilirubinemia (>2 mg/dL) has been reported in up to 14% of patients treated with indinavir. Elevated serum transaminases were seen in less than 1%.
Renal Abnormalities and Indinavir
Several renal syndromes have been associated with indinavir use, ranging from obstructive uropathy and acute renal failure to asymptomatic pyuria. The range of clinical syndromes is a consequence of indinavir crystals aggregating within or irritating the urinary tract (32). Symptomatic nephrolithiasis (indinavir crystallization) has been reported to affect up to 12% (range, 5–35 %) of patients who receive indinavir, while up to 67% of patients will have asymptomatic crystalluria. The cumulative frequency of nephrolithiasis events increases with increasing exposure to indinavir. Therapy may be continued or interrupted for a few days. Adequate hydration is necessary with the administration of indinavir. Indinavir associated pyuria is frequently associated with interstitial nephritis or urothelial inflammation. Discontinuation of indinavir will lead to resolution of urine abnormalities.
Elevated Mean Corpuscular Volume (MCV) and NRTIs
Elevation of MCV or macrocytosis occurs in more than 90% of patients treated with zidovudine, but is not correlated with the development of anemia. Macrocytosis (MCV values exceeding 110/fl) develops within 2 weeks following the initiation of zidovudine therapy, and its presence can be used as a marker for medical adherence. When anemia does occur it is associated with a dose related bone marrow toxicity manifested as a macrocytic anemia. Serum B12 and folate levels are normal. Stavudine use is also associated with macrocytosis, in non-zidovudine-containing regimens (33).
Drug Screens and Efavirenz
The use of efavirenz, a potent non-nucleoside reverse transcriptase inhibitor, can cause a false-positive urine drug screen for cannabinoid. Efavirenz does not bind to cannabinoid receptors. The false-positive test results are specific to the assay kit used (34).
Evaluation of the AIDS Patient with Fever
Fever is a common symptom in HIV-infected patients, the etiology of which can be identified in more than 80% of cases (35,36). The AIDS patient with fever poses a considerable challenge given that the expanded differential may include a wide range of OIs. The CD4 cell count remains a valuable predictor of risk for infection. Patients with CD4 cell counts greater than 500 cells/mm3 should be evaluated as if immunocompetent. Patients with CD4 cell counts between 200 and 500 cells/mm3 are at increased risk for upper and lower bacterial respiratory infections, tuberculosis, and sinusitis, but overall their risk for opportunistic infection is not increased. In patients with CD4 counts less than 200 cells/mm3, Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common cause of fever in those not receiving primary prophylaxis. As the CD4 cell count decreases below 100 cells/mm3, the risk for disseminated MAC, toxoplasmosis, CMV, disseminated fungal infections, and lymphoma should be considered possible causes of fever. HIV itself is usually not the cause of fever in patients with advanced immunosuppression (37).
In patients with CD4 counts >200 cells/mm3, the clinician can usually construct a laboratory and radiographic evaluation guided by symptoms, while in patients with severe immunosuppression a broader evaluation is required. A serum cryptococcal antigen should be obtained, as it has high sensitivity and specificity for both systemic disease as well as meningitis (38). Bacterial, mycobacterial, and fungal isolator blood cultures should be performed, as well as a urine culture, despite lack of symptoms. Urine AFB cultures can be added if there is a suspicion for tuberculosis. Sputum should be evaluated with gram stain, AFB smear, and culture, as well as PCP direct fluorescent antibody. If diarrhea is present, stool studies should include bacterial culture, ova and parasites evaluation, an assay for C. difficile, and Cryptosporidia and Giardia stool antigen assays. A serum LDH may be elevated in PCP, disseminated histoplasmosis, or lymphoma. A serum CMV antigen may be useful in the patient with fever and diarrhea, hepatitis, or retinitis.
A chest radiograph should be performed in all febrile AIDS patients. Chest films may be normal in 5–10% of HIV-infected patients with tuberculosis (TB). The typical radiographic appearance of Pneumocystis jiroveci pneumonia is a bilateral interstitial pattern characterized by reticular or ground-glass opacities. However, normal chest radiographs may be seen in one third of AIDS patients with active PCP. High-resolution computed tomography (HRCT) of the chest should be obtained if there is still a clinical suspicion for Pneumocystis. HRCT has been shown in several reports to have a 100% negative predictive value in the evaluation for PCP (39,40).
A lumbar puncture should be performed if the patient is symptomatic or if the serum cryptococcal antigen is reactive. A bone marrow biopsy and culture is also useful particularly in the evaluation of the patient with cytopenias. Bacterial, fungal, and AFB cultures may yield disseminated mycobacterial or fungal disease. Histopathologic evaluation may reveal granulomas with organisms or lymphoma. Bronchoscopy may be pursued in cases of high suspicion for TB or PCP.
Guidelines for the management of opportunistic infections associated with human immunodeficiency virus are available at www.AIDSinfo.nih.gov.
Evaluation of the AIDS Patient with Focal Neurological Disease
Toxoplasma encephalitis (TE) may be distinguished from primary CNS lymphoma without a brain biopsy. TE is caused by reactivation of latent infection by the protozoan Toxoplasma gondii. Almost 90% of patients have CD4 counts less than 200 cells/mm3 and 75% have CD4 counts less than 100 cells/mm3. Serum anti-Toxoplasma IgG antibodies are detected in more than 90% of patients with TE. Lesions on CT or MRI (a more sensitive modality) are typically multiple ring-enhancing lesions with a predilection for the basal ganglia. An emipiric trial of therapy is recommended, and a response confirms a diagnosis in the patient who has a positive Toxoplasma antibody and is not receiving trimethoprim-sulfamethoxazole prophylaxis. A lumbar puncture in this setting is not necessary and maybe ill advised if cerebral edema is present (Figure 1, page 24).
Primary CNS lymphoma (PCNSL) has a similar radiographic appearance as TE. Solitary lesions are more frequent in PCNSL. Positron emission tomography (PET) and single photon emission CT (SPECT) are useful adjunctive imaging modalities as they are positive in PCNSL due to the increased metabolic activity of the tumor. Cytologic analysis of the CSF may show lymphomatous cells. Epstein-Barr virus (EBV) DNA is uniformly detected in PCNSL in AIDS patients and detection of EBV DNA by PCR on the CSF has a sensitivity of 90–100% and a specificity of 87–98% for the diagnosis of PCNSL (41,42). Combining SPECT imaging and EBV PCR provides 100% sensitivity and 100% negative predictive value in the evaluation of AIDS-related primary CNS lymphoma (43), obviating the need for brain biopsy.
Progressive multifocal leukoencephalopathy (PML) is characterized radiographically by multiple and bilateral hypodense lesions of the white matter without mass effect or enhancement on CT. MRI demonstrates areas of hypointensity on T1-weighted images and increased intensity on T2-weighted images. JC virus DNA can be detected by PCR of CSF or brain tissue with sensitivity of approximately 80% and specificity of 95%. Because of the high positive predictive value of a positive PCR, a patient with AIDS who also has a compatible MRI can be diagnosed with PML (44,45).
Other focal neurologic disease seen in AIDS patients includes cryptococcomas, tuberculomas, CMV encephalitis, neurosyphilis, Nocardia and Aspergillus infection, and bacterial brain abscesses (46).
Conclusion
As survival of the HIV-infected population improves, more patients may require hospitalization for HAART treatment failures or complications attributed to antiretroviral therapy. The hospitalist should be familiar with the complications of antiretroviral agents, the interactions between HAART and medications used to treat opportunistic infections, and medical conditions induced by HAART. Evaluation of the HIV-infected patient presenting with fever can be based on the CD4 cell count, which predicts risk for opportunistic infections. Finally, using combined diagnostic approaches along with modern imaging and laboratory assays may preclude the need for more invasive procedures in the HIV-infected hospitalized patient.
Dr. Decker may be reached at [email protected].
References
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531-2.
- Fleishman JA, Hellinger FJ. Trends in HIV-related inpatient admissions from 1993 to 1997: a seven-state study. J Acquir Immune Defic Syndr. 2001;28:73-80.
- Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996-2000: a 7-state study. J Acquir Immune Defic Syndr. 2003;34:102-10.
- Powderly WG. Should patients with an acute opportunistic infection receive antiretroviral therapy immediately? Advanced Studies in Medicine. 2005;5:S111-S116.
- Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220-6.
- Soni N, Pozniak A. Continuing HIV therapy in the ICU. Crit Care. 2001;5:247-8.
- Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833-8.
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112-6.
- Cooney EL. Clinical indicators of immune restoration following highly active antiretroviral therapy. Clin Infect Dis. 2002;34:224-33.
- Hirsch HH, Kaufmann G, Sendi P, BaĴegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159-66.
- Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882-92.
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43-9.
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-96.
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289-304.
- Sim SM, Hoggard PG, Sales SD, Phiboonbanakit D, Hart CA, Back DJ. Effect of ribavirin on zidovudine efficacy and toxicity in vitro: a concentration-dependent interaction. AIDS Res Hum Retroviruses. 1998;14:1661-7.
- Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213-7.
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;49:185-9.
- Havlir DV, O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599-604.
- Sheng WH, Hsieh SM, Lee SC, et al. Fatal lactic acidosis associated with highly active antiretroviral therapy in patients with advanced human immunodeficiency virus infection in Taiwan. Int J STD AIDS. 2004;15:249-53.
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-62;quiz 563-4.
- Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958.
- Klein D, Hurley LB, Sidney S. Cardiovascular Disease and HIV Infection. N Engl J Med. 2003;349:1869-70; author reply 1869-70.
- Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
- Anderson JA, Adkinson NF Jr. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891-9.
- Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis. 2002;34:1137-42.
- Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227-8.
- Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-2.
- Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with abacavir. AIDS. 1999;13:999-1000.
- Martin GJ, Paparello SF, Decker CF. A severe systemic reaction to trimethoprim-sulfamethoxazole in a patient infected with the human immunodeficiency virus. Clin Infect Dis. 1993;16:175-6.
- Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124-9.
- Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122-8.
- Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160-3.
- Gottesman L. Sustiva may cause false positive on marijuana test. WORLD. 1999:7.
- Sepkowitz KA, Telzak EE, Carrow M, Armstrong D. Fever among outpatients with advanced human immunodeficiency virus infection. Arch Intern Med. 1993;153: 1909-12.
- Barat LM, Gunn JE, Steger KA, Perkins CJ, Craven DE. Causes of fever in patients infected with human immunodeficiency virus who were admitted to Boston City Hospital. Clin Infect Dis. 1996;23:320-8.
- Sepkowitz KA. FUO and AIDS. Curr Clin Top Infect Dis. 1999;19:1-15.
- Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65-71.
- Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689-93.
- Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967-75.
- MacMahon EM, Glass JD, Hayward SD, et al. Association of Epstein-Barr virus with primary central nervous system lymphoma in AIDS. AIDS Res Hum Retroviruses. 1992;8:740-2.
- Rao CR, Jain K, Bhatia K, Laksmaiah KC, Shankar SK. Association of primary central nervous system lymphomas with the Epstein-Barr virus. Neurol India. 2003;51:237-40.
- Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554-60.
- Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896-906.
- Weber T. Cerebrospinal fluid analysis for the diagnosis of human immunodeficiency virus-related neurologic diseases. Semin Neurol. 1999;19:223-33.
- Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103-15.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Department of Defense, the Department of the Navy, or the naval services at large.
Introduction
An estimated 850,000 to 950,000 persons in the United States are living with human immunodeficiency virus (HIV), 280,000 of whom are unaware of their infection and another 43,000 of whom meet the definition of acquired immunodeficiency syndrome (AIDS) (www.cdc.gov). The use of highly active antiretroviral therapy (HAART) has produced significant declines in morbidity and mortality from AIDS. Compared with the first 2 decades of the HIV pandemic, the number of HIV-related hospital admissions has declined. However, recently, this rate of decline has markedly slowed (1-3). The reasons for this plateau are many including a steady number of admissions for complications related to HAART, treatment failures, and the overall increased prevalence of HIV infection. Not only will HIV-infected patients still frequently require admission to the hospital, but the complexity of their inpatient care will continue to increase with the advancements in multiple drug regimens, aging of the HIV-infected population, and the interaction of HIV infection with medical comorbidities, many of which are attributable to HAART.
The hospitalist caring for the inpatient with AIDS is presented with several challenges including not only the diagnosis and management of opportunistic infections, but also the complications of HAART. In this article we review the guidelines for the initiation and continuation of HAART in the hospital, review important clinical complications of antiretroviral therapy, and review conditions that may result in the hospitalization of AIDS patients.
Initiation of HAART in the Hospital
In those who do not have access to health care, the initial diagnosis of HIV infection frequently occurs during a hospitalization for an AIDS-defining illness. Initiation of antiretrovirals is contingent on several issues, including CD4 count, viral load, clinical status, likelihood of continued adherence, and the concurrent treatment of opportunistic infections (OIs). All patients with HIV infection and a CD4 count <200 cells/mm3 or an AIDS-defining illness should receive antiretroviral therapy. Controversy exists as to whether a patient admitted for the treatment of an opportunistic infection should begin antiretroviral therapy immediately, or whether this therapy should be deferred until after acute treatment of the OI. The potential detrimental effects of drug-drug interactions, the need for treatment interruptions, and drug-related toxicity between antiretrovirals and OI-specific therapy may support initiating HAART after control of an OI is achieved. Conversely, for some opportunistic infections, such as cryptosporidiosis, the use of HAART is essential for successful treatment of the infection.
An ongoing randomized controlled trial initiated within the Adult AIDS Clinical Trials Group (ACTG) comparing outcomes between patients who start HAART immediately after presentation with an acute OI and patients who start HAART at least 4 weeks after the OI has resolved should help identify the factors supporting early or delayed initiation of antiretrovirals (4).
Generally speaking, HAART can be administered by combining either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) with 2 nucleoside reverse transcriptase inhibitors (NRTIs). There are currently more than 20 FDA-approved antiretrovirals. Frequent updates on the guidelines for the use of antiretroviral agents in HIV-infected adults are available at www.AIDSinfo.nih.gov, and a discussion of this topic is beyond the scope of this review.
Continuation of HAART in the Hospital
In most cases, every effort should be made to minimize interruption of HAART during a hospitalization. Although some investigators are examining the virologic and immunologic safety of interrupting HAART as a treatment strategy, there are few data on viral replication, CD4 cell count decline, and rate of acquisition of new mutations in hospitalized patients who have unexpected treatment interruptions (5). The long half-life of some antiretrovirals promotes the emergence of resistance once HAART is stopped. For example, once NNRTIs are stopped, subtherapeutic levels remain in the plasma and cells for several days. HIV then replicates in a milieu that may select for resistance mutations.
Because zidovudine is the only antiretroviral available in a parenteral preparation, it is often difficult to continue HAART when a patient cannot take medications by mouth. Drugs given by the enteral route in a hospitalized patient may also be poorly absorbed, and few data exist on the absorption of antiretrovirals administered through a gastrostomy or jejunostomy tube (6).
Prescribing HAART in the Hospital
Antiretroviral prescribing errors occur frequently in the hospitalized AIDS patient. The most common errors include overdosing or underdosing, missing components of multidrug regimens, or missing critical drug-drug interactions (7). Underdosing may lead to resistance, and overdosing contributes to increased toxicity. In one report, prescribing errors occurred in 12% of admissions in the post-HAART era (1998) compared with 2% of admissions in the pre-HAART era (1996) (7). The NRTIs, including didanosine, emtricitabine, lamivudine, stavudine, and zidovudine, require decreased dosing in renal insufficiency. Tenofovir is not recommended for use if the creatine clearance is less than 60 mL/minute. Dosage adjustments in hepatic disease are recommended for amprenavir, fosamprenavir, delavirdine, efavirenz, and nevirapine.
Immune Reconstitution Syndrome
The widespread use of HAART has produced sustained suppression of HIV replication and recovery of CD4 cell counts. It also became evident that HAART resulted in not only a numerical increase in CD4 cells, but also in a functional immune recovery (8-10). This improved T-cell response to antigens results in adequate protection against specific opportunistic infections, allowing for discontinuation of primary and secondary prophylaxis in HIV-infected patients. Immune reconstitution syndrome (IRS), an inflammatory syndrome, is recognized as a potential complication that can occur days to months after starting HAART. The onset of IRS is characterized by a paradoxical worsening of clinical or laboratory parameters despite a favorable response in CD4 cell counts and the suppression of viral replication (9,11,12). IRS has been reported to occur in 10–25% of patients who receive HAART and more commonly in those whose CD4 cell counts are <50 cells/mm3 at the start of HAART (9,11). It is postulated that the inflammatory response is triggered by the recognition of antigens associated with ongoing infection or recognition of persisting antigens associated with past (nonreplicating) infections. Mycobacterial antigens, frequently implicated in IRS, are responsible for about one third of cases. Other antigens associated with IRS include cytomegalovirus and hepatitis B and C (11). In most circumstances, with the management of IRS, HAART should be continued, while specific antimicrobial therapy and steroids should be considered (10).
Medical Conditions that Should Prompt HIV Screening
There are several medical conditions that should prompt screening for HIV infection. Generally, anyone presenting with a fever of unknown etiology who is sexually active or had a blood transfusion prior to 1985 should be screened for HIV infection. Symptoms consistent with acute retroviral syndrome (fever, sore throat, malaise, and skin rash) may be more commonly recognized by clinicians now than previously, and this remains a “golden opportunity” to intervene. Frequently, acute retroviral syndrome will be attributed to Epstein-Barr virus; however, caution should be used in the diagnosis of mononucleosis in those other than teenage populations. It is recommended that all persons presenting with any sexually transmitted disease, unexplained generalized lymphadenopathy, oral candidiasis, or tuberculosis should also be tested. Other conditions where HIV infection should be considered include enigmatic pneumonia, acute hepatitis B infection, herpes zoster infection (particularly in younger, seemingly immunocompetent individuals), idiopathic thrombocytopenic purpura, and nephropathy of unknown cause.
Drug Interactions
Drug interactions are an important consideration in the treatment of HIV infection. Interactions between HAART and other drugs used for the treatment or prophylaxis of opportunistic infections along with those used for the treatment of drug-induced endocrinopathies (hyperlipidemia, diabetes mellitus) are virtually unavoidable. Drug interactions occur either because of drug metabolism or absorption. The multiple metabolic pathways of some drugs make it difficult to predict the outcome of drug interactions. All protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by the cytochrome P-450 enzyme system and each of these drugs may alter the metabolism of other antiretrovirals and concomitantly administered drugs (13,14). A decrease in trough plasma concentrations of the protease inhibitors to a level below the in vitro concentration required to inhibit replication of 50% of viral strains (IC50) may lead to development of resistance. Because nucleoside analogue reverse transcriptase inhibitors are primarily eliminated by the kidney, they do not interact with other drugs through the cytochrome P-450 system.
One noteworthy interaction that the clinician caring for HIV-infected patients should be aware of is the interaction of ribavirin with zidovudine. Ribavirin decreases the phosphorylation of zidovudine and stavudine in vitro, resulting in decreased concentrations of the active compound. HIV-infected patients who are coinfected with hepatitis C may be treated with regimens that include ribavirin, which may reduce the efficacy of zidovudine (15). Another important interaction is the effect of nevirapine or efavirenz on plasma methadone concentrations. Both drugs can decrease methadone plasma levels by 50%, and patients receiving chronic therapy may need increased methadone doses to prevent withdrawal symptoms (16).
Protease inhibitors are associated with numerous interactions including certain antiarrhythmics, sedatives, hypnotics, ergot derivatives, and several lipid-lowering agents (statins). Not only do protease inhibitors affect the metabolism of certain drugs, but also their own metabolism is altered by other inducers or inhibitors of cytochrome activity that can cause clinically important decreases in serum levels of protease inhibitors. One widely recognized interaction is that of rifampin, which may decrease levels of some protease inhibitors by 80%. The resulting low plasma concentrations may promote viral resistance and result in treatment failure. Patients being treated for tuberculosis, who are also receiving protease inhibitors should be treated with a four-drug regimen that includes rifabutin (at half dose) instead of rifampin. Updated guidelines for the use of rifabutin or rifampin in HIV-infected patients receiving antiretroviral agents have been reviewed recently (17).
Other potent inducers such as phenytoin, phenobarbital, and carbamazepine can cause similar reductions in serum levels of protease inhibitors. Azole antifungal drugs and macrolides also have important interactions that complicate both the treatment and prophylaxis of opportunistic infections.
Interactions that interfere with absorption can also affect plasma drug concentrations. For example, the absorption of fluconazole is unaffected by variations in gastric pH, while itraconazole and ketoconazole require an acidic environment for optimal absorption. The protease inhibitor, atazanavir, also requires a low pH for absorption and thus is contraindicated with the use of proton pump inhibitors; taking atazanavir with acidic beverages is not sufficient to overcome this (18).
New information about drug interactions becomes known on almost a daily basis in patients with HIV infection. The number of documented and theoretical interactions can become overwhelming to the clinician. Clinicians should suspect potential drug interactions in a patient who is failing therapy but who is adherent to HAART. Fortunately, there are extensive tables on Web sites (www.hivatis.org) and product information to aid in the recognition and management of drug interactions.
Complications of HAART
Diabetes mellitus, hyperlipidemias, lipodystrophy, and insulin resistance are among the many complex metabolic abnormalities attributable to the use of HAART. For the most part, these complications are managed conservatively and usually do not mandate the discontinuation of HAART. Pancreatitis, hepatic steatosis, and lactic acidosis are wellrecognized complications of NRTIs. These are usually more acute and may result in hospitalization and necessitate the discontinuation of medications. Cessation of the offending agent (didanosine [ddI], stavudine [d4T], and zalcitabine [ddC) usually results in resolution of pancreatitis, but the episode may limit use of these agents in the future. Hepatic steatosis and lactic acidosis are rare but life-threatening adverse effects associated with the mitochondrial toxicity seen with the NRTIs. Symptoms usually develop insidiously with nausea, vomiting, abdominal pain, weight loss, or dyspnea and can progress rapidly to fatal lactic acidosis. Hepatomegaly, ascites, elevated liver associated enzymes, and an increased anion gap with lactic acidemia are usually present (19,20). Discontinuation of antiretroivirals is imperative.
There is an accumulating body of evidence that suggests that HIV-infected patients receiving HAART may be at risk for accelerated coronary disease (21). In addition, some cohort studies have reported an increased incidence of myocardial infarction (MI) following the introduction of HAART and the risk for MI rose progressively with the number of years on antiretroviral therapy (22,23). However, it is important to note that many traditional risk factors for coronary artery disease contribute more substantially to the risk for a cardiovascular event than does HAART. Therefore, aggressive modification of primary cardiac risk factors is warranted.
Hypersensitivity Drug Reactions
Drug hypersensitivity reactions are life-threatening reactions that result in a systemic illness that usually includes fever and maculopapular rash accompanied by constitutional symptoms (fatigue, myalgias, and arthralgias), visceral involvement (lymphadenopathy, mucositis, pneumonitis, myocarditis, hepatitis, and interstitial nephritis), and hematologic abnormalities (eosinophilia) (24).
Abacavir, an NRTI, is a relatively new antiretroviral agent used in many HAART regimens. Abacavir is associated with a hypersensitivity reaction, which can be fatal if abacavir use is continued despite the reaction, or if re-challenge with the drug takes place after the reaction (25). The overall incidence of this reaction appears to be around 4% (25). Prior antiretroviral experience and being of African descent are associated with a nearly 40% reduction in the risk of this hypersensitivity reaction, while patients of white race are at a significantly greater risk. CD4 cell counts do not appear to be significantly related to abacavir hypersensitivity (26). The exact metabolite that is likely responsible for abacavir hypersensitivity is unknown.
The most common symptoms of abacavir hypersensitivity reaction are fever, rash, nausea, vomiting, and abdominal pain. Occasionally, respiratory symptoms will be present and can mimic influenza. However, gastrointestinal symptoms are the most prominent complaints after fever and rash and help to distinguish between influenza and abacavir hypersensitivity. More than 90% of hypersensitivity reactions occur during the first 6 weeks of treatment, with a median time to development of 8 days. A fever that develops within a few weeks after the initiation of therapy with abacavir may be due to causes other than hypersensitivity. One of the most common situations is the simultaneous initiation of treatment with other drugs, such as trimethoprimsulfamethxazole, efavirenz, or nevirapine, all of which are associated with a higher incidence of hypersensitivity than abacavir (27,28). Symptoms may be sudden and worsen over a few days if abacavir is continued. Symptoms tend to improve in 48 hours after abacavir is discontinued. Supportive therapy includes intravenous hydration and withdrawal of abacavir as well as all other antiretrovirals. Early in the use of this medication, 20% of patients who were re-challenged with the drug experienced unanticipated life-threatening events manifesting as an anaphylactic or immediate type hypersensitivity reaction. Hypotension, renal insufficiency, and bronchospasm have resulted in death. Rechallenge symptoms have been seen with the first dose (29). A discussion of the potential for this hypersensitivity is warranted when prescribing this agent. In the United States, a patient information card warning of this hypersensitivity reaction is distributed to the patient with each bottle of abacavir. Prednisone does not prevent the development of hypersensitivity reaction.
Symptoms of toxicity from TMP-SMX are more likely to occur in the HIV infected than in patients without HIV infection. Fever and rash can occur in up to 50% of HIV-infected patients. The rash can be treatment limiting or severe in up to 20% of HIV-infected patients who receive it. Life-threatening reactions may occur, including fatal Stevens Johnson-type exfoliative skin reactions. Most toxicity in HIV-infected patients appears to be related to metabolites of the sulfamethoxazole component and decreased levels of glutathione. There have been reports of severe systemic reactions that resemble anaphylaxis or septic shock occurring in HIV-infected patients who are re-challenged with TMP-SMX after experiencing toxicity within the previous 6–8 weeks (30).
The NNRTIf nevirapine and efavirenz can cause a delayed hypersensitivity reaction similar to that seen with abacavir. Cutaneous involvement is a prominent component of both nevirapine and efavirenz hypersensitivity reaction, with rash more likely to occur with the use of nevirapine. In addition, female patients have a higher propensity of developing Stevens-Johnson syndrome and symptomatic hepatic events from nevirapine (28,31).
Laboratory Abnormalities Related to Drugs
Hyperbilirubinemia and Atazanavir and Indinavir
Atazanavir, a protease inhibitor, is metabolized by the liver via CYP3A and also inhibits both CTP3A and UGT1A1. UGT1A1 is required for conjugation of bilirubin and inhibition of this enzyme results in elevated levels of unconjugated bilirubin. This effect is similar to what is observed in Gilbert’s syndrome. Asymptomatic indirect hyperbilirubemia may be seen in up to 60% of patients receiving atazanavir. Total bilirubin levels may rise to greater than 5 mg/dL, and more than 17% of patients may experience jaundice (18). Concurrent elevations in hepatic serum transaminases should not be attributed to atazanavir and alternative etiologies for these elevations should be sought. This hyperbilirubinemia is reversible upon discontinuation of the atazanavir.
Similarly, but to a lesser degree, asymptomatic unconjugated hyperbilirubinemia (>2 mg/dL) has been reported in up to 14% of patients treated with indinavir. Elevated serum transaminases were seen in less than 1%.
Renal Abnormalities and Indinavir
Several renal syndromes have been associated with indinavir use, ranging from obstructive uropathy and acute renal failure to asymptomatic pyuria. The range of clinical syndromes is a consequence of indinavir crystals aggregating within or irritating the urinary tract (32). Symptomatic nephrolithiasis (indinavir crystallization) has been reported to affect up to 12% (range, 5–35 %) of patients who receive indinavir, while up to 67% of patients will have asymptomatic crystalluria. The cumulative frequency of nephrolithiasis events increases with increasing exposure to indinavir. Therapy may be continued or interrupted for a few days. Adequate hydration is necessary with the administration of indinavir. Indinavir associated pyuria is frequently associated with interstitial nephritis or urothelial inflammation. Discontinuation of indinavir will lead to resolution of urine abnormalities.
Elevated Mean Corpuscular Volume (MCV) and NRTIs
Elevation of MCV or macrocytosis occurs in more than 90% of patients treated with zidovudine, but is not correlated with the development of anemia. Macrocytosis (MCV values exceeding 110/fl) develops within 2 weeks following the initiation of zidovudine therapy, and its presence can be used as a marker for medical adherence. When anemia does occur it is associated with a dose related bone marrow toxicity manifested as a macrocytic anemia. Serum B12 and folate levels are normal. Stavudine use is also associated with macrocytosis, in non-zidovudine-containing regimens (33).
Drug Screens and Efavirenz
The use of efavirenz, a potent non-nucleoside reverse transcriptase inhibitor, can cause a false-positive urine drug screen for cannabinoid. Efavirenz does not bind to cannabinoid receptors. The false-positive test results are specific to the assay kit used (34).
Evaluation of the AIDS Patient with Fever
Fever is a common symptom in HIV-infected patients, the etiology of which can be identified in more than 80% of cases (35,36). The AIDS patient with fever poses a considerable challenge given that the expanded differential may include a wide range of OIs. The CD4 cell count remains a valuable predictor of risk for infection. Patients with CD4 cell counts greater than 500 cells/mm3 should be evaluated as if immunocompetent. Patients with CD4 cell counts between 200 and 500 cells/mm3 are at increased risk for upper and lower bacterial respiratory infections, tuberculosis, and sinusitis, but overall their risk for opportunistic infection is not increased. In patients with CD4 counts less than 200 cells/mm3, Pneumocystis jiroveci pneumonia, formerly known as Pneumocystis carinii pneumonia, is the most common cause of fever in those not receiving primary prophylaxis. As the CD4 cell count decreases below 100 cells/mm3, the risk for disseminated MAC, toxoplasmosis, CMV, disseminated fungal infections, and lymphoma should be considered possible causes of fever. HIV itself is usually not the cause of fever in patients with advanced immunosuppression (37).
In patients with CD4 counts >200 cells/mm3, the clinician can usually construct a laboratory and radiographic evaluation guided by symptoms, while in patients with severe immunosuppression a broader evaluation is required. A serum cryptococcal antigen should be obtained, as it has high sensitivity and specificity for both systemic disease as well as meningitis (38). Bacterial, mycobacterial, and fungal isolator blood cultures should be performed, as well as a urine culture, despite lack of symptoms. Urine AFB cultures can be added if there is a suspicion for tuberculosis. Sputum should be evaluated with gram stain, AFB smear, and culture, as well as PCP direct fluorescent antibody. If diarrhea is present, stool studies should include bacterial culture, ova and parasites evaluation, an assay for C. difficile, and Cryptosporidia and Giardia stool antigen assays. A serum LDH may be elevated in PCP, disseminated histoplasmosis, or lymphoma. A serum CMV antigen may be useful in the patient with fever and diarrhea, hepatitis, or retinitis.
A chest radiograph should be performed in all febrile AIDS patients. Chest films may be normal in 5–10% of HIV-infected patients with tuberculosis (TB). The typical radiographic appearance of Pneumocystis jiroveci pneumonia is a bilateral interstitial pattern characterized by reticular or ground-glass opacities. However, normal chest radiographs may be seen in one third of AIDS patients with active PCP. High-resolution computed tomography (HRCT) of the chest should be obtained if there is still a clinical suspicion for Pneumocystis. HRCT has been shown in several reports to have a 100% negative predictive value in the evaluation for PCP (39,40).
A lumbar puncture should be performed if the patient is symptomatic or if the serum cryptococcal antigen is reactive. A bone marrow biopsy and culture is also useful particularly in the evaluation of the patient with cytopenias. Bacterial, fungal, and AFB cultures may yield disseminated mycobacterial or fungal disease. Histopathologic evaluation may reveal granulomas with organisms or lymphoma. Bronchoscopy may be pursued in cases of high suspicion for TB or PCP.
Guidelines for the management of opportunistic infections associated with human immunodeficiency virus are available at www.AIDSinfo.nih.gov.
Evaluation of the AIDS Patient with Focal Neurological Disease
Toxoplasma encephalitis (TE) may be distinguished from primary CNS lymphoma without a brain biopsy. TE is caused by reactivation of latent infection by the protozoan Toxoplasma gondii. Almost 90% of patients have CD4 counts less than 200 cells/mm3 and 75% have CD4 counts less than 100 cells/mm3. Serum anti-Toxoplasma IgG antibodies are detected in more than 90% of patients with TE. Lesions on CT or MRI (a more sensitive modality) are typically multiple ring-enhancing lesions with a predilection for the basal ganglia. An emipiric trial of therapy is recommended, and a response confirms a diagnosis in the patient who has a positive Toxoplasma antibody and is not receiving trimethoprim-sulfamethoxazole prophylaxis. A lumbar puncture in this setting is not necessary and maybe ill advised if cerebral edema is present (Figure 1, page 24).
Primary CNS lymphoma (PCNSL) has a similar radiographic appearance as TE. Solitary lesions are more frequent in PCNSL. Positron emission tomography (PET) and single photon emission CT (SPECT) are useful adjunctive imaging modalities as they are positive in PCNSL due to the increased metabolic activity of the tumor. Cytologic analysis of the CSF may show lymphomatous cells. Epstein-Barr virus (EBV) DNA is uniformly detected in PCNSL in AIDS patients and detection of EBV DNA by PCR on the CSF has a sensitivity of 90–100% and a specificity of 87–98% for the diagnosis of PCNSL (41,42). Combining SPECT imaging and EBV PCR provides 100% sensitivity and 100% negative predictive value in the evaluation of AIDS-related primary CNS lymphoma (43), obviating the need for brain biopsy.
Progressive multifocal leukoencephalopathy (PML) is characterized radiographically by multiple and bilateral hypodense lesions of the white matter without mass effect or enhancement on CT. MRI demonstrates areas of hypointensity on T1-weighted images and increased intensity on T2-weighted images. JC virus DNA can be detected by PCR of CSF or brain tissue with sensitivity of approximately 80% and specificity of 95%. Because of the high positive predictive value of a positive PCR, a patient with AIDS who also has a compatible MRI can be diagnosed with PML (44,45).
Other focal neurologic disease seen in AIDS patients includes cryptococcomas, tuberculomas, CMV encephalitis, neurosyphilis, Nocardia and Aspergillus infection, and bacterial brain abscesses (46).
Conclusion
As survival of the HIV-infected population improves, more patients may require hospitalization for HAART treatment failures or complications attributed to antiretroviral therapy. The hospitalist should be familiar with the complications of antiretroviral agents, the interactions between HAART and medications used to treat opportunistic infections, and medical conditions induced by HAART. Evaluation of the HIV-infected patient presenting with fever can be based on the CD4 cell count, which predicts risk for opportunistic infections. Finally, using combined diagnostic approaches along with modern imaging and laboratory assays may preclude the need for more invasive procedures in the HIV-infected hospitalized patient.
Dr. Decker may be reached at [email protected].
References
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531-2.
- Fleishman JA, Hellinger FJ. Trends in HIV-related inpatient admissions from 1993 to 1997: a seven-state study. J Acquir Immune Defic Syndr. 2001;28:73-80.
- Fleishman JA, Hellinger FH. Recent trends in HIV-related inpatient admissions 1996-2000: a 7-state study. J Acquir Immune Defic Syndr. 2003;34:102-10.
- Powderly WG. Should patients with an acute opportunistic infection receive antiretroviral therapy immediately? Advanced Studies in Medicine. 2005;5:S111-S116.
- Fagard C, Oxenius A, Gunthard H, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220-6.
- Soni N, Pozniak A. Continuing HIV therapy in the ICU. Crit Care. 2001;5:247-8.
- Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833-8.
- Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112-6.
- Cooney EL. Clinical indicators of immune restoration following highly active antiretroviral therapy. Clin Infect Dis. 2002;34:224-33.
- Hirsch HH, Kaufmann G, Sendi P, BaĴegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis. 2004;38:1159-66.
- Cheng VC, Yuen KY, Chan WM, Wong SS, Ma ES, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882-92.
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43-9.
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984-96.
- Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289-304.
- Sim SM, Hoggard PG, Sales SD, Phiboonbanakit D, Hart CA, Back DJ. Effect of ribavirin on zidovudine efficacy and toxicity in vitro: a concentration-dependent interaction. AIDS Res Hum Retroviruses. 1998;14:1661-7.
- Clarke SM, Mulcahy FM, Tjia J, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51:213-7.
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Morb Mortal Wkly Rep. 2000;49:185-9.
- Havlir DV, O’Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004;38:1599-604.
- Sheng WH, Hsieh SM, Lee SC, et al. Fatal lactic acidosis associated with highly active antiretroviral therapy in patients with advanced human immunodeficiency virus infection in Taiwan. Int J STD AIDS. 2004;15:249-53.
- Herman JS, Easterbrook PJ. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555-62;quiz 563-4.
- Behrens G, Schmidt H, Meyer D, Stoll M, Schmidt RE. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1958.
- Klein D, Hurley LB, Sidney S. Cardiovascular Disease and HIV Infection. N Engl J Med. 2003;349:1869-70; author reply 1869-70.
- Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003.
- Anderson JA, Adkinson NF Jr. Allergic reactions to drugs and biologic agents. JAMA. 1987;258:2891-9.
- Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis. 2002;34:1137-42.
- Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227-8.
- Bourezane Y, Salard D, Hoen B, Vandel S, Drobacheff C, Laurent R. DRESS (drug rash with eosinophilia and systemic symptoms) syndrome associated with nevirapine therapy. Clin Infect Dis. 1998;27:1321-2.
- Walensky RP, Goldberg JH, Daily JP. Anaphylaxis after rechallenge with abacavir. AIDS. 1999;13:999-1000.
- Martin GJ, Paparello SF, Decker CF. A severe systemic reaction to trimethoprim-sulfamethoxazole in a patient infected with the human immunodeficiency virus. Clin Infect Dis. 1993;16:175-6.
- Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124-9.
- Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122-8.
- Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine. Swiss HIV Cohort Study. J Infect. 2000;40:160-3.
- Gottesman L. Sustiva may cause false positive on marijuana test. WORLD. 1999:7.
- Sepkowitz KA, Telzak EE, Carrow M, Armstrong D. Fever among outpatients with advanced human immunodeficiency virus infection. Arch Intern Med. 1993;153: 1909-12.
- Barat LM, Gunn JE, Steger KA, Perkins CJ, Craven DE. Causes of fever in patients infected with human immunodeficiency virus who were admitted to Boston City Hospital. Clin Infect Dis. 1996;23:320-8.
- Sepkowitz KA. FUO and AIDS. Curr Clin Top Infect Dis. 1999;19:1-15.
- Asawavichienjinda T, Sitthi-Amorn C, Tanyanont V. Serum cyrptococcal antigen: diagnostic value in the diagnosis of AIDS-related cryptococcal meningitis. J Med Assoc Thai. 1999;82:65-71.
- Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51:689-93.
- Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967-75.
- MacMahon EM, Glass JD, Hayward SD, et al. Association of Epstein-Barr virus with primary central nervous system lymphoma in AIDS. AIDS Res Hum Retroviruses. 1992;8:740-2.
- Rao CR, Jain K, Bhatia K, Laksmaiah KC, Shankar SK. Association of primary central nervous system lymphomas with the Epstein-Barr virus. Neurol India. 2003;51:237-40.
- Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554-60.
- Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999;20:1896-906.
- Weber T. Cerebrospinal fluid analysis for the diagnosis of human immunodeficiency virus-related neurologic diseases. Semin Neurol. 1999;19:223-33.
- Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:103-15.