User login
The importance of formalized antimicrobial stewardship programs (ASPs) has gained recognition over the past 2 decades. The increasing requirements for ASP programs from national entities often outpace the staffing, technology, and analytic support needed to meet these demands.1,2 A multimodal approach to stewardship that includes education initiatives, audit-and-feedback methodology, and system support is effective in producing sustained change.3 However, this approach is resource intensive, and many ASPs must look outward for additional support.
Centralized ASP collaboratives and stewardship networks have been effective in positively impacting initiatives and outcomes through resource sharing.3-5 These collaboratives can take on multiple forms ranging from centralized education distribution to individual sites coming together to set goals and develop strategies to address common issues.5-8 Collaboratives can provide enhanced data analysis through data pooling, which may lead to shared dashboards or antibiotic use (AU) reports, allowing for robust benchmarking.5-7 Productivity at individual centers is often measured by AU and antimicrobial resistance (AMR) rates, but these measures alone do not fully capture the benefits of collaborative participation.
The US Department of Veterans Affairs (VA), similar to other large health care systems, is uniquely positioned to promote the development of ASP collaboratives due to the use of the same electronic health record system and infrastructure for data. This centralized data lends itself more readily to data dashboards and interfacility comparison. In turn, the identification of facilities that have outlying data for specific measures can lead to a collaborative effort to identify aberrant processes or facility-specific problems and identify, implement, and track the progress of appropriate solutions with less effort and resources.7 The VA has a national stewardship group, the Antimicrobial Stewardship Task Force (ASTF), that identifies and disseminates best practices and advocates for stewardship resources.
VA facilities are heterogeneous with regard to patient population, services, availability of specialists, and antibiotic resistance patterns.9 Therefore, clinical practice and needs vary. The ASTF has spearheaded the development of regional collaboratives, recognizing the potential benefit of smaller groups with shared leadership. The Veterans Integrated Services Networks (VISNs) are geographically demarcated regions that lend themselves well to coordination among member facilities due to similar populations, challenges, and opportunities. The Veterans Affairs Midsouth Healthcare Network (VISN 9) includes 5 facilities across Tennessee, Kentucky, Mississippi, Arkansas, Georgia, Virginia, and Indiana and serves about 293,000 veterans, ranging from 35,000 to 105,000 per facility.
A VISN 9 stewardship collaborative (as described by Buckel and colleagues in 2022) was established to enhance member facility ASPs through shared goal setting.6 Initially, the collaborative met quarterly; however, with increased participation and the onset of COVID-19, the collaborative evolved to meet burgeoning ASP needs. While intrafacility multidisciplinary ASP collaboration has been previously published, few publications on interfacility collaborations exist.3-6 To our knowledge, no previous publications have reported the impact of a VA ASP collaborative on the productivity and effectiveness of participating ASP facilities and the region. We aim to share the structure and processes of this ASP collaborative, demonstrate its impact through quantification of productivity, and aid others in developing similar collaboratives to further ASPs’ impact.
Methods
The regional VISN 9 ASP collaborative was formed in January 2020 to address common issues across facilities and optimize human capital and resources. The initial collaborative included ASP pharmacists but quickly expanded to include physicians and nurse practitioners. The collaborative is co-led by 2 members from different facilities that rotate.
In April 2021, clinical guidance and research/quality improvement (QI) subcommittees were created. The monthly research/QI subcommittee discusses current initiatives and barriers to ongoing research, adapt and disseminate successful interventions to other facilities, and develop new collaborative initiatives. The clinical guidance subcommittee creates and disseminates clinical expert recommendations regarding common issues or emerging needs.
Data Plan and Collection
To measure success and growth, we evaluated annual facility reports that convey the state of each facility’s ASP, outline its current initiatives and progress, highlight areas of need, and set a programmatic goal and strategy for the upcoming year. These reports, required by a VA directive, are submitted annually by each facility to local and VISN leadership and must address the following 7 areas: (1) ASP structure and fulfillment of national VA policy for ASP; (2) fulfillment of the Joint Commission ASP standards; (3) ASP metrics; (4) ASP activities and interventions; (5) ASP QI and research initiatives; (6) education; and (7) goals and priorities.
To standardize evaluation and accurately reflect ASP effort across heterogeneous reports, 4 core areas were identified from areas 1, 3, 4 and 5 listed previously. Area 2 was excluded for its similarity among all facilities, and areas 6 and 7 were excluded for significant differences in definitions and reporting across facilities.
The project team consisted of 5 members from the collaborative who initially discussed definitions and annual report review methodology. A subgroup was assigned to area 1 and another to areas 3, 4, and 5 for initial review and data extraction. Results were later reviewed to address discrepancies and finalize collation and presentation.
The impact of the collaborative on individual facilities was measured by both quantitative and qualitative measures. Quantitative measures included: (1) designated ASP pharmacy, physician, or advanced practice provider (APP) full-time equivalents (FTE) at each facility compared with the recommended FTE for facility size; (2) the number of inpatient and outpatient ASP AU metrics for each facility and the VISN total; (3) reported improvement in annual ASP metrics calculated as frequency of improved metrics for each facility and the VISN; (4) the number of QI or research initiatives for each facility and the VISN, which included clinical pathways and order sets; and (5) the number of initiatives published as either abstract or manuscript.10 Additionally, the number of collaborative efforts involving more than 1 facility was tracked. Qualitative data included categories of metrics and QI and research initiatives. Data were collected by year and facility. Facilities are labeled A to E throughout this article.
Along with facility annual ASP reports, facility and VISN AU trends for fiscal years (FY) 2019-2022 were collected from existing VA dashboards tracking AU in both acute respiratory infections (ARI) and in patients with COVID-19. Quantitative data included facility and VISN quarterly AU rates for ARI, extracted from the national VA dashboard. Facility and VISN AU rates in patients with COVID-19 were extracted from a dashboard developed by the VISN 9 ASP collaborative. The VISN 9 Institutional Review Board deemed this work QI and approval was waived.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
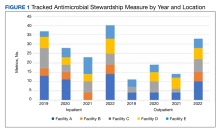
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
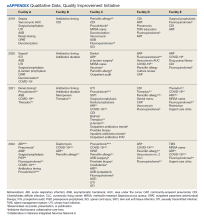
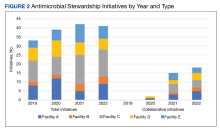
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.
Discussion
This study demonstrates the benefit of participating in a regional ASP collaborative for individual facilities and the region. Some products from the collaborative include the development of regionwide guidance for the use of antimicrobials in COVID-19, interfacility collaborative initiatives, a COVID-19 dashboard, improvement in metrics, and several publications. Importantly, this expansion occurred during the COVID-19 pandemic when many ASP members were spread thin. Moreover, despite 4 sites not meeting VA-recommended ASP staffing requirements for both pharmacists and physicians, productivity increased within the VISN as facilities worked together sharing local challenges and successful paths in removing ASP barriers. The collaborative shared QI strategies, advocated for technological support (ie, Theradoc and dashboards) to maximize available ASP human capital, standardized metric reporting, and made continued efforts sustainable.
Previous reports in the literature have found ASP collaboratives to be an effective model for long-term program growth.3 Two collaboratives found improved adherence to the Centers for Disease Control and Prevention core elements for ASP.4,5
Our findings highlight that ASP collaboratives can help answer the recent call to action from McGregor, Fitzpatrick, and Suda who advocated for ASPs to take the next steps in stewardship, which include standardization of evaluating metrics and the use of robust QI frameworks.11 Moving forward, an area for research could include a comparison of ASP collaborative infrastructures and productivity to identify optimal fit dependent on facility structure and setting. Parallel to our experience, other reports cite heterogeneous ASP metrics and a lack of benchmarking, spotlighting the need for standardization.8,11,12
Limitations
Using annual reports was a limitation for analyzing and reporting the full impact of the collaborative. Local facility-level discretion of content inclusion led to many facilities only reporting on the forefront of new initiatives that they had developed and may have led to the omission of other ongoing work. Further, time invested into the ASP regional collaborative was not captured within annual reports; therefore, the opportunity cost cannot be determined.
Conclusions
The VA has an advantage that many private health care facilities do not: the ability to work across systems to ease the burden of duplicative work and more readily disseminate effective strategies. The regional ASP collaborative bred innovation and the tearing down of silos. The implementation of the collaborative aided in robust QI infrastructure, standardization of reporting and metrics, and greater support through facility alignments with regional guidance. ASP interfacility collaboratives provide a sustainable solution in a resource-limited landscape.
Acknowledgments
This work was made possible by the resources provided through the Antimicrobial Stewardship Programs in the Veterans Integrated Services Network (VISN) 9.
1. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103-108. doi:10.1016/j.ijid.2021.10.001
2. Emberger J, Tassone D, Stevens MP, Markley JD. The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep. 2018;20(9):31. doi:10.1007/s11908-018-0637-6
3. Moehring RW, Yarrington ME, Davis AE, et al. Effects of a collaborative, community hospital network for antimicrobial stewardship program implementation. Clin Infect Dis. 2021;73(9):1656-1663. doi:10.1093/cid/ciab356
4. Logan AY, Williamson JE, Reinke EK, Jarrett SW, Boger MS, Davidson LE. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Jt Comm J Qual Patient Saf. 2019;45(9):591-599. doi:10.1016/j.jcjq.2019.03.002
5. Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6):e20190589. doi:10.1542/peds.2019-0589
6. Buckel WR, Stenehjem EA, Hersh AL, Hyun DY, Zetts RM. Harnessing the power of health systems and networks for antimicrobial stewardship. Clin Infect Dis. 2022;75(11):2038-2044. doi:10.1093/cid/ciac515
7. Graber CJ, Jones MM, Goetz MB, et al. Decreases in antimicrobial use associated with multihospital implementation of electronic antimicrobial stewardship tools. Clin Infect Dis. 2020;71(5):1168-1176. doi:10.1093/cid/ciz941
8. Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi:10.1017/ice.2016.328
9. US Department of Veterans Affairs. About VHA. 2022. Updated September 7, 2023. Accessed November 7, 2023. https://www.va.gov/health/aboutVHA.asp
10. Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Health Syst Pharm. 2017;74(21):1785-1790. doi:10.2146/ajhp160825
11. McGregor JC, Fitzpatrick MA, Suda KJ. Expanding antimicrobial stewardship through quality improvement. JAMA Netw Open. 2021;4(2):e211072. doi:10.1001/jamanetworkopen.2021.1072
12. Newland JG, Gerber JS, Kronman MP, et al. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): a quality improvement collaborative. J Pediatr Infect Dis Soc. 2018;7(2):124-128. doi:10.1093/jpids/pix020
The importance of formalized antimicrobial stewardship programs (ASPs) has gained recognition over the past 2 decades. The increasing requirements for ASP programs from national entities often outpace the staffing, technology, and analytic support needed to meet these demands.1,2 A multimodal approach to stewardship that includes education initiatives, audit-and-feedback methodology, and system support is effective in producing sustained change.3 However, this approach is resource intensive, and many ASPs must look outward for additional support.
Centralized ASP collaboratives and stewardship networks have been effective in positively impacting initiatives and outcomes through resource sharing.3-5 These collaboratives can take on multiple forms ranging from centralized education distribution to individual sites coming together to set goals and develop strategies to address common issues.5-8 Collaboratives can provide enhanced data analysis through data pooling, which may lead to shared dashboards or antibiotic use (AU) reports, allowing for robust benchmarking.5-7 Productivity at individual centers is often measured by AU and antimicrobial resistance (AMR) rates, but these measures alone do not fully capture the benefits of collaborative participation.
The US Department of Veterans Affairs (VA), similar to other large health care systems, is uniquely positioned to promote the development of ASP collaboratives due to the use of the same electronic health record system and infrastructure for data. This centralized data lends itself more readily to data dashboards and interfacility comparison. In turn, the identification of facilities that have outlying data for specific measures can lead to a collaborative effort to identify aberrant processes or facility-specific problems and identify, implement, and track the progress of appropriate solutions with less effort and resources.7 The VA has a national stewardship group, the Antimicrobial Stewardship Task Force (ASTF), that identifies and disseminates best practices and advocates for stewardship resources.
VA facilities are heterogeneous with regard to patient population, services, availability of specialists, and antibiotic resistance patterns.9 Therefore, clinical practice and needs vary. The ASTF has spearheaded the development of regional collaboratives, recognizing the potential benefit of smaller groups with shared leadership. The Veterans Integrated Services Networks (VISNs) are geographically demarcated regions that lend themselves well to coordination among member facilities due to similar populations, challenges, and opportunities. The Veterans Affairs Midsouth Healthcare Network (VISN 9) includes 5 facilities across Tennessee, Kentucky, Mississippi, Arkansas, Georgia, Virginia, and Indiana and serves about 293,000 veterans, ranging from 35,000 to 105,000 per facility.
A VISN 9 stewardship collaborative (as described by Buckel and colleagues in 2022) was established to enhance member facility ASPs through shared goal setting.6 Initially, the collaborative met quarterly; however, with increased participation and the onset of COVID-19, the collaborative evolved to meet burgeoning ASP needs. While intrafacility multidisciplinary ASP collaboration has been previously published, few publications on interfacility collaborations exist.3-6 To our knowledge, no previous publications have reported the impact of a VA ASP collaborative on the productivity and effectiveness of participating ASP facilities and the region. We aim to share the structure and processes of this ASP collaborative, demonstrate its impact through quantification of productivity, and aid others in developing similar collaboratives to further ASPs’ impact.
Methods
The regional VISN 9 ASP collaborative was formed in January 2020 to address common issues across facilities and optimize human capital and resources. The initial collaborative included ASP pharmacists but quickly expanded to include physicians and nurse practitioners. The collaborative is co-led by 2 members from different facilities that rotate.
In April 2021, clinical guidance and research/quality improvement (QI) subcommittees were created. The monthly research/QI subcommittee discusses current initiatives and barriers to ongoing research, adapt and disseminate successful interventions to other facilities, and develop new collaborative initiatives. The clinical guidance subcommittee creates and disseminates clinical expert recommendations regarding common issues or emerging needs.
Data Plan and Collection
To measure success and growth, we evaluated annual facility reports that convey the state of each facility’s ASP, outline its current initiatives and progress, highlight areas of need, and set a programmatic goal and strategy for the upcoming year. These reports, required by a VA directive, are submitted annually by each facility to local and VISN leadership and must address the following 7 areas: (1) ASP structure and fulfillment of national VA policy for ASP; (2) fulfillment of the Joint Commission ASP standards; (3) ASP metrics; (4) ASP activities and interventions; (5) ASP QI and research initiatives; (6) education; and (7) goals and priorities.
To standardize evaluation and accurately reflect ASP effort across heterogeneous reports, 4 core areas were identified from areas 1, 3, 4 and 5 listed previously. Area 2 was excluded for its similarity among all facilities, and areas 6 and 7 were excluded for significant differences in definitions and reporting across facilities.
The project team consisted of 5 members from the collaborative who initially discussed definitions and annual report review methodology. A subgroup was assigned to area 1 and another to areas 3, 4, and 5 for initial review and data extraction. Results were later reviewed to address discrepancies and finalize collation and presentation.
The impact of the collaborative on individual facilities was measured by both quantitative and qualitative measures. Quantitative measures included: (1) designated ASP pharmacy, physician, or advanced practice provider (APP) full-time equivalents (FTE) at each facility compared with the recommended FTE for facility size; (2) the number of inpatient and outpatient ASP AU metrics for each facility and the VISN total; (3) reported improvement in annual ASP metrics calculated as frequency of improved metrics for each facility and the VISN; (4) the number of QI or research initiatives for each facility and the VISN, which included clinical pathways and order sets; and (5) the number of initiatives published as either abstract or manuscript.10 Additionally, the number of collaborative efforts involving more than 1 facility was tracked. Qualitative data included categories of metrics and QI and research initiatives. Data were collected by year and facility. Facilities are labeled A to E throughout this article.
Along with facility annual ASP reports, facility and VISN AU trends for fiscal years (FY) 2019-2022 were collected from existing VA dashboards tracking AU in both acute respiratory infections (ARI) and in patients with COVID-19. Quantitative data included facility and VISN quarterly AU rates for ARI, extracted from the national VA dashboard. Facility and VISN AU rates in patients with COVID-19 were extracted from a dashboard developed by the VISN 9 ASP collaborative. The VISN 9 Institutional Review Board deemed this work QI and approval was waived.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.
Discussion
This study demonstrates the benefit of participating in a regional ASP collaborative for individual facilities and the region. Some products from the collaborative include the development of regionwide guidance for the use of antimicrobials in COVID-19, interfacility collaborative initiatives, a COVID-19 dashboard, improvement in metrics, and several publications. Importantly, this expansion occurred during the COVID-19 pandemic when many ASP members were spread thin. Moreover, despite 4 sites not meeting VA-recommended ASP staffing requirements for both pharmacists and physicians, productivity increased within the VISN as facilities worked together sharing local challenges and successful paths in removing ASP barriers. The collaborative shared QI strategies, advocated for technological support (ie, Theradoc and dashboards) to maximize available ASP human capital, standardized metric reporting, and made continued efforts sustainable.
Previous reports in the literature have found ASP collaboratives to be an effective model for long-term program growth.3 Two collaboratives found improved adherence to the Centers for Disease Control and Prevention core elements for ASP.4,5
Our findings highlight that ASP collaboratives can help answer the recent call to action from McGregor, Fitzpatrick, and Suda who advocated for ASPs to take the next steps in stewardship, which include standardization of evaluating metrics and the use of robust QI frameworks.11 Moving forward, an area for research could include a comparison of ASP collaborative infrastructures and productivity to identify optimal fit dependent on facility structure and setting. Parallel to our experience, other reports cite heterogeneous ASP metrics and a lack of benchmarking, spotlighting the need for standardization.8,11,12
Limitations
Using annual reports was a limitation for analyzing and reporting the full impact of the collaborative. Local facility-level discretion of content inclusion led to many facilities only reporting on the forefront of new initiatives that they had developed and may have led to the omission of other ongoing work. Further, time invested into the ASP regional collaborative was not captured within annual reports; therefore, the opportunity cost cannot be determined.
Conclusions
The VA has an advantage that many private health care facilities do not: the ability to work across systems to ease the burden of duplicative work and more readily disseminate effective strategies. The regional ASP collaborative bred innovation and the tearing down of silos. The implementation of the collaborative aided in robust QI infrastructure, standardization of reporting and metrics, and greater support through facility alignments with regional guidance. ASP interfacility collaboratives provide a sustainable solution in a resource-limited landscape.
Acknowledgments
This work was made possible by the resources provided through the Antimicrobial Stewardship Programs in the Veterans Integrated Services Network (VISN) 9.
The importance of formalized antimicrobial stewardship programs (ASPs) has gained recognition over the past 2 decades. The increasing requirements for ASP programs from national entities often outpace the staffing, technology, and analytic support needed to meet these demands.1,2 A multimodal approach to stewardship that includes education initiatives, audit-and-feedback methodology, and system support is effective in producing sustained change.3 However, this approach is resource intensive, and many ASPs must look outward for additional support.
Centralized ASP collaboratives and stewardship networks have been effective in positively impacting initiatives and outcomes through resource sharing.3-5 These collaboratives can take on multiple forms ranging from centralized education distribution to individual sites coming together to set goals and develop strategies to address common issues.5-8 Collaboratives can provide enhanced data analysis through data pooling, which may lead to shared dashboards or antibiotic use (AU) reports, allowing for robust benchmarking.5-7 Productivity at individual centers is often measured by AU and antimicrobial resistance (AMR) rates, but these measures alone do not fully capture the benefits of collaborative participation.
The US Department of Veterans Affairs (VA), similar to other large health care systems, is uniquely positioned to promote the development of ASP collaboratives due to the use of the same electronic health record system and infrastructure for data. This centralized data lends itself more readily to data dashboards and interfacility comparison. In turn, the identification of facilities that have outlying data for specific measures can lead to a collaborative effort to identify aberrant processes or facility-specific problems and identify, implement, and track the progress of appropriate solutions with less effort and resources.7 The VA has a national stewardship group, the Antimicrobial Stewardship Task Force (ASTF), that identifies and disseminates best practices and advocates for stewardship resources.
VA facilities are heterogeneous with regard to patient population, services, availability of specialists, and antibiotic resistance patterns.9 Therefore, clinical practice and needs vary. The ASTF has spearheaded the development of regional collaboratives, recognizing the potential benefit of smaller groups with shared leadership. The Veterans Integrated Services Networks (VISNs) are geographically demarcated regions that lend themselves well to coordination among member facilities due to similar populations, challenges, and opportunities. The Veterans Affairs Midsouth Healthcare Network (VISN 9) includes 5 facilities across Tennessee, Kentucky, Mississippi, Arkansas, Georgia, Virginia, and Indiana and serves about 293,000 veterans, ranging from 35,000 to 105,000 per facility.
A VISN 9 stewardship collaborative (as described by Buckel and colleagues in 2022) was established to enhance member facility ASPs through shared goal setting.6 Initially, the collaborative met quarterly; however, with increased participation and the onset of COVID-19, the collaborative evolved to meet burgeoning ASP needs. While intrafacility multidisciplinary ASP collaboration has been previously published, few publications on interfacility collaborations exist.3-6 To our knowledge, no previous publications have reported the impact of a VA ASP collaborative on the productivity and effectiveness of participating ASP facilities and the region. We aim to share the structure and processes of this ASP collaborative, demonstrate its impact through quantification of productivity, and aid others in developing similar collaboratives to further ASPs’ impact.
Methods
The regional VISN 9 ASP collaborative was formed in January 2020 to address common issues across facilities and optimize human capital and resources. The initial collaborative included ASP pharmacists but quickly expanded to include physicians and nurse practitioners. The collaborative is co-led by 2 members from different facilities that rotate.
In April 2021, clinical guidance and research/quality improvement (QI) subcommittees were created. The monthly research/QI subcommittee discusses current initiatives and barriers to ongoing research, adapt and disseminate successful interventions to other facilities, and develop new collaborative initiatives. The clinical guidance subcommittee creates and disseminates clinical expert recommendations regarding common issues or emerging needs.
Data Plan and Collection
To measure success and growth, we evaluated annual facility reports that convey the state of each facility’s ASP, outline its current initiatives and progress, highlight areas of need, and set a programmatic goal and strategy for the upcoming year. These reports, required by a VA directive, are submitted annually by each facility to local and VISN leadership and must address the following 7 areas: (1) ASP structure and fulfillment of national VA policy for ASP; (2) fulfillment of the Joint Commission ASP standards; (3) ASP metrics; (4) ASP activities and interventions; (5) ASP QI and research initiatives; (6) education; and (7) goals and priorities.
To standardize evaluation and accurately reflect ASP effort across heterogeneous reports, 4 core areas were identified from areas 1, 3, 4 and 5 listed previously. Area 2 was excluded for its similarity among all facilities, and areas 6 and 7 were excluded for significant differences in definitions and reporting across facilities.
The project team consisted of 5 members from the collaborative who initially discussed definitions and annual report review methodology. A subgroup was assigned to area 1 and another to areas 3, 4, and 5 for initial review and data extraction. Results were later reviewed to address discrepancies and finalize collation and presentation.
The impact of the collaborative on individual facilities was measured by both quantitative and qualitative measures. Quantitative measures included: (1) designated ASP pharmacy, physician, or advanced practice provider (APP) full-time equivalents (FTE) at each facility compared with the recommended FTE for facility size; (2) the number of inpatient and outpatient ASP AU metrics for each facility and the VISN total; (3) reported improvement in annual ASP metrics calculated as frequency of improved metrics for each facility and the VISN; (4) the number of QI or research initiatives for each facility and the VISN, which included clinical pathways and order sets; and (5) the number of initiatives published as either abstract or manuscript.10 Additionally, the number of collaborative efforts involving more than 1 facility was tracked. Qualitative data included categories of metrics and QI and research initiatives. Data were collected by year and facility. Facilities are labeled A to E throughout this article.
Along with facility annual ASP reports, facility and VISN AU trends for fiscal years (FY) 2019-2022 were collected from existing VA dashboards tracking AU in both acute respiratory infections (ARI) and in patients with COVID-19. Quantitative data included facility and VISN quarterly AU rates for ARI, extracted from the national VA dashboard. Facility and VISN AU rates in patients with COVID-19 were extracted from a dashboard developed by the VISN 9 ASP collaborative. The VISN 9 Institutional Review Board deemed this work QI and approval was waived.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.
Discussion
This study demonstrates the benefit of participating in a regional ASP collaborative for individual facilities and the region. Some products from the collaborative include the development of regionwide guidance for the use of antimicrobials in COVID-19, interfacility collaborative initiatives, a COVID-19 dashboard, improvement in metrics, and several publications. Importantly, this expansion occurred during the COVID-19 pandemic when many ASP members were spread thin. Moreover, despite 4 sites not meeting VA-recommended ASP staffing requirements for both pharmacists and physicians, productivity increased within the VISN as facilities worked together sharing local challenges and successful paths in removing ASP barriers. The collaborative shared QI strategies, advocated for technological support (ie, Theradoc and dashboards) to maximize available ASP human capital, standardized metric reporting, and made continued efforts sustainable.
Previous reports in the literature have found ASP collaboratives to be an effective model for long-term program growth.3 Two collaboratives found improved adherence to the Centers for Disease Control and Prevention core elements for ASP.4,5
Our findings highlight that ASP collaboratives can help answer the recent call to action from McGregor, Fitzpatrick, and Suda who advocated for ASPs to take the next steps in stewardship, which include standardization of evaluating metrics and the use of robust QI frameworks.11 Moving forward, an area for research could include a comparison of ASP collaborative infrastructures and productivity to identify optimal fit dependent on facility structure and setting. Parallel to our experience, other reports cite heterogeneous ASP metrics and a lack of benchmarking, spotlighting the need for standardization.8,11,12
Limitations
Using annual reports was a limitation for analyzing and reporting the full impact of the collaborative. Local facility-level discretion of content inclusion led to many facilities only reporting on the forefront of new initiatives that they had developed and may have led to the omission of other ongoing work. Further, time invested into the ASP regional collaborative was not captured within annual reports; therefore, the opportunity cost cannot be determined.
Conclusions
The VA has an advantage that many private health care facilities do not: the ability to work across systems to ease the burden of duplicative work and more readily disseminate effective strategies. The regional ASP collaborative bred innovation and the tearing down of silos. The implementation of the collaborative aided in robust QI infrastructure, standardization of reporting and metrics, and greater support through facility alignments with regional guidance. ASP interfacility collaboratives provide a sustainable solution in a resource-limited landscape.
Acknowledgments
This work was made possible by the resources provided through the Antimicrobial Stewardship Programs in the Veterans Integrated Services Network (VISN) 9.
1. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103-108. doi:10.1016/j.ijid.2021.10.001
2. Emberger J, Tassone D, Stevens MP, Markley JD. The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep. 2018;20(9):31. doi:10.1007/s11908-018-0637-6
3. Moehring RW, Yarrington ME, Davis AE, et al. Effects of a collaborative, community hospital network for antimicrobial stewardship program implementation. Clin Infect Dis. 2021;73(9):1656-1663. doi:10.1093/cid/ciab356
4. Logan AY, Williamson JE, Reinke EK, Jarrett SW, Boger MS, Davidson LE. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Jt Comm J Qual Patient Saf. 2019;45(9):591-599. doi:10.1016/j.jcjq.2019.03.002
5. Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6):e20190589. doi:10.1542/peds.2019-0589
6. Buckel WR, Stenehjem EA, Hersh AL, Hyun DY, Zetts RM. Harnessing the power of health systems and networks for antimicrobial stewardship. Clin Infect Dis. 2022;75(11):2038-2044. doi:10.1093/cid/ciac515
7. Graber CJ, Jones MM, Goetz MB, et al. Decreases in antimicrobial use associated with multihospital implementation of electronic antimicrobial stewardship tools. Clin Infect Dis. 2020;71(5):1168-1176. doi:10.1093/cid/ciz941
8. Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi:10.1017/ice.2016.328
9. US Department of Veterans Affairs. About VHA. 2022. Updated September 7, 2023. Accessed November 7, 2023. https://www.va.gov/health/aboutVHA.asp
10. Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Health Syst Pharm. 2017;74(21):1785-1790. doi:10.2146/ajhp160825
11. McGregor JC, Fitzpatrick MA, Suda KJ. Expanding antimicrobial stewardship through quality improvement. JAMA Netw Open. 2021;4(2):e211072. doi:10.1001/jamanetworkopen.2021.1072
12. Newland JG, Gerber JS, Kronman MP, et al. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): a quality improvement collaborative. J Pediatr Infect Dis Soc. 2018;7(2):124-128. doi:10.1093/jpids/pix020
1. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103-108. doi:10.1016/j.ijid.2021.10.001
2. Emberger J, Tassone D, Stevens MP, Markley JD. The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep. 2018;20(9):31. doi:10.1007/s11908-018-0637-6
3. Moehring RW, Yarrington ME, Davis AE, et al. Effects of a collaborative, community hospital network for antimicrobial stewardship program implementation. Clin Infect Dis. 2021;73(9):1656-1663. doi:10.1093/cid/ciab356
4. Logan AY, Williamson JE, Reinke EK, Jarrett SW, Boger MS, Davidson LE. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Jt Comm J Qual Patient Saf. 2019;45(9):591-599. doi:10.1016/j.jcjq.2019.03.002
5. Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6):e20190589. doi:10.1542/peds.2019-0589
6. Buckel WR, Stenehjem EA, Hersh AL, Hyun DY, Zetts RM. Harnessing the power of health systems and networks for antimicrobial stewardship. Clin Infect Dis. 2022;75(11):2038-2044. doi:10.1093/cid/ciac515
7. Graber CJ, Jones MM, Goetz MB, et al. Decreases in antimicrobial use associated with multihospital implementation of electronic antimicrobial stewardship tools. Clin Infect Dis. 2020;71(5):1168-1176. doi:10.1093/cid/ciz941
8. Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi:10.1017/ice.2016.328
9. US Department of Veterans Affairs. About VHA. 2022. Updated September 7, 2023. Accessed November 7, 2023. https://www.va.gov/health/aboutVHA.asp
10. Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Health Syst Pharm. 2017;74(21):1785-1790. doi:10.2146/ajhp160825
11. McGregor JC, Fitzpatrick MA, Suda KJ. Expanding antimicrobial stewardship through quality improvement. JAMA Netw Open. 2021;4(2):e211072. doi:10.1001/jamanetworkopen.2021.1072
12. Newland JG, Gerber JS, Kronman MP, et al. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): a quality improvement collaborative. J Pediatr Infect Dis Soc. 2018;7(2):124-128. doi:10.1093/jpids/pix020