User login
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
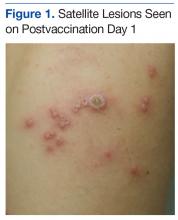
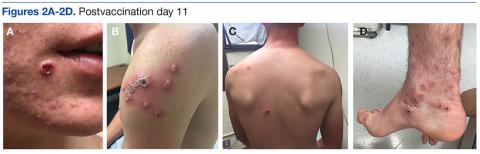
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
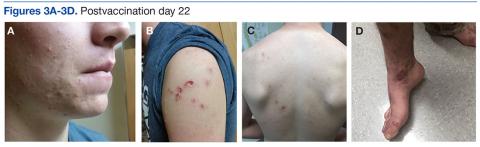
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.