User login
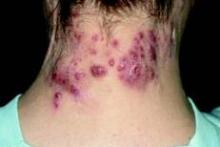
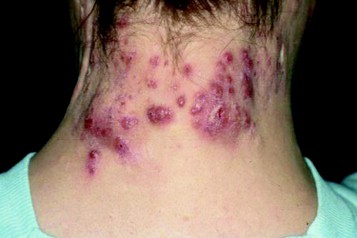
PRAGUE – A handful of drugs are backed by solid-quality evidence for the treatment of hidradenitis suppurativa, although to date no form of therapy is approved for this indication.
Topping the list are the tumor necrosis factor (TNF)-alpha inhibitors. They have a plausible mechanism of benefit in hidradenitis suppurativa (HS). They’ve also demonstrated effectiveness for this common inflammatory skin disease in randomized, placebo-controlled clinical trials, Dr. Gregor B. Jemec said at the annual congress of the European Academy of Dermatology and Venereology.
In addition, one older randomized trial suggests that topical clindamycin is effective, mainly in milder cases. The most promising antibiotic regimen, consisting of oral clindamycin at 300 mg twice daily plus rifampicin at 600 mg daily, has not been studied in a randomized trial. But several large, rigorous case series have demonstrated "dramatic improvement" in response to the combination, according to Dr. Jemec of the University of Copenhagen.
TNF Inhibitors
The discovery that anti-TNF therapy is effective for HS was a serendipitous finding that occurred when physicians noted marked improvement in the skin disease in a patient taking infliximab for Crohn’s disease. The biologic plausibility of the observed benefit is underscored by the fact that TNF levels are actually more elevated in patients with HS than in those with psoriasis, Dr. Jemec said.
Infliximab missed its primary efficacy end point of clear or almost clear in a 38-patient randomized trial, but it did hit several clinically important secondary end points, including pain reduction and improved quality of life (J. Am. Acad. Dermatol. 2010;62:205-17).
On the other hand, etanercept showed no benefit in a 20-patient pilot randomized trial (Arch. Dermatol. 2010;146:501-4).
By far the best studied TNF inhibitor in HS is adalimumab (Humira). It is the subject of two ongoing, pivotal phase III randomized, double-blind, placebo-controlled, multinational clinical trials aimed at winning adalimumab regulatory approval as the first-ever drug indicated for the treatment of HS.
Last year, Dr. Alexa B. Kimball of Harvard Medical School, Boston, presented the primary end point of a 16-week, randomized, double-blind phase II clinical trial involving 154 patients with HS who were placed on adalimumab 40 mg weekly, adalimumab 40 mg every other week, or placebo. At week 16, a Physician Global Assessment of clear, minimal, or mild disease with at least a 2-grade improvement over baseline was documented in 23.5% of patients on placebo, 21.2% of those on alternate-week adalimumab, and 49% of those on adalimumab 40 mg every week.
With a therapy that achieves clear or close to it in only half of treated patients, other end points take on added importance. At this year’s EADV (European Academy of Dermatology and Venereology) congress, Dr. Kimball and others presented several key secondary outcomes from the phase II study. One involved pain, a particularly prominent feature in HS. From a mean baseline pain score of 52 on a 0-100 visual analog scale, the weekly adalimumab group’s score dropped by a placebo-subtracted 15.8 points by week 2, 18.4 points at week 4, 11.7 points by week 8, 20.7 points at week 12, and by 11 points at week 20.
The minimum clinically important difference, predefined as at least a 15.4-point improvement in self-rated pain scores, occurred in 33% of the weekly adalimumab group, compared with 15% of patients on placebo at week 2. By week 12 this end point had been achieved in 63% of the weekly adalimumab group and 26% of the placebo group. Adalimumab every other week didn’t consistently improve pain relative to placebo, she reported.
Dr. Ulrich Mrowietz reported separately that the impaired work productivity that often affects patients with HS was significantly lessened by treatment with adalimumab 40 mg weekly. Total work productivity impairment as measured at week 16 by the Work Productivity and Activity Impairment Questionnaire improved by a mean of 15 points in the weekly adalimumab group while worsening by 2.7 points with placebo; the net 17.7-unit difference exceeded the minimum clinically important difference threshold of 16.2 points.
Patients assigned to weekly adalimumab also experienced significantly improved scores on the Dermatology Life Quality Index beginning on week 2 and lasting throughout the 16-week study. At week 16, for example, they enjoyed a mean 5.5-point improvement relative to baseline, compared with a 1.6-point improvement with placebo, according to Dr. Mrowietz of University Medical Center in Kiel, Germany.
Oral Antibiotics
Dr. Jemec highlighted as particularly impressive a retrospective French case series of 116 patients with severe HS treated using the combination of clindamycin 300 mg twice daily and rifampicin 600 mg daily. Severity scores decreased as a result by 50%. Seven percent of patients dropped the therapy due to diarrhea (Dermatology 2009;219:148-54).
Two smaller published case series have boosted to 164 the total number of HS patients treated with similarly "dramatic" results, noted Dr. Jemec. He was an investigator in one of these studies, in which 10 weeks on the antibiotic combination resulted in at least partial improvement in 28 of 34 patients and total remission in 16 (47%). Eight of the 16 relapsed after a mean of 5 months (Dermatology 2009;219:143-7).
Another approach has involved an intensive oral regimen of broad-spectrum antibiotics consisting of rifampin at 10 mg/kg once daily, moxifloxacin at 400 mg/day, and metronidazole at 500 mg three times daily. In a series of 28 consecutive patients with long-standing refractory HS, French investigators prescribed this regimen for 6-12 weeks before switching to consolidation therapy with rifampin and moxifloxacin for 6 more weeks. Complete remission was achieved in 16 of the 28 patients (Dermatology 2011;222:49-58).
"This therapy has yielded remarkably good results in terms of clearance and maintenance of clearance," commented Dr. Jemec.
The main side effects were diarrhea, a complaint in nearly two-thirds of patients, and vaginal candidiasis, which occurred in one-third of treated women.
Topical Clindamycin
Dr. Jemec was a coinvestigator in an early double-blind, 46-patient study that established topical clindamycin as being as effective as oral tetracycline in patients with mild disease (J. Am. Acad. Dermatol. 1998;39:971-4).
Beyond this point, the strength of evidence for proposed HS therapies tails off considerably. But Dr. Jemec deemed a couple of agents worthy of mention on the basis of intriguing yet less than persuasive supporting data:
• Acitretin. In a case series of 12 HS patients treated for 9-12 months at the relatively high dose of 0.6 mg/kg per day of acitretin, with stringent 4-year follow-up, all patients went into remission. In eight patients, the remission lasted for at least 1 year, including a single patient who has been in remission for 4 years and counting (Br. J. Dermatol. 2011;164:170-5). "Remarkable," Dr. Jemec commented.
• Dapsone. This drug is frequently reported anecdotally as being useful in HS, typically with an n-of-1 experience. Dr. Jemec and his coworkers recently published a case series of 24 treated patients, 9 of whom (38%) showed improvement (Dermatology 2011;222:342-6). None of the four severely affected patients improved, however. It was Dr. Jemec’s impression that the degree of improvement was less than he’s seen with combined oral clindamycin and rifampicin. He’d like to see a head-to-head comparison before reaching a final judgment as to a possible role for dapsone.
Dr. Jemec has received research grants from and serves as a consultant to Abbott Laboratories, which is developing adalimumab as a treatment for HS, as well as a dozen other pharmaceutical companies. Dr. Mrowietz is a consultant to Abbott, which funded his study, and to other dermatologic pharmaceutical companies.
PRAGUE – A handful of drugs are backed by solid-quality evidence for the treatment of hidradenitis suppurativa, although to date no form of therapy is approved for this indication.
Topping the list are the tumor necrosis factor (TNF)-alpha inhibitors. They have a plausible mechanism of benefit in hidradenitis suppurativa (HS). They’ve also demonstrated effectiveness for this common inflammatory skin disease in randomized, placebo-controlled clinical trials, Dr. Gregor B. Jemec said at the annual congress of the European Academy of Dermatology and Venereology.
In addition, one older randomized trial suggests that topical clindamycin is effective, mainly in milder cases. The most promising antibiotic regimen, consisting of oral clindamycin at 300 mg twice daily plus rifampicin at 600 mg daily, has not been studied in a randomized trial. But several large, rigorous case series have demonstrated "dramatic improvement" in response to the combination, according to Dr. Jemec of the University of Copenhagen.
TNF Inhibitors
The discovery that anti-TNF therapy is effective for HS was a serendipitous finding that occurred when physicians noted marked improvement in the skin disease in a patient taking infliximab for Crohn’s disease. The biologic plausibility of the observed benefit is underscored by the fact that TNF levels are actually more elevated in patients with HS than in those with psoriasis, Dr. Jemec said.
Infliximab missed its primary efficacy end point of clear or almost clear in a 38-patient randomized trial, but it did hit several clinically important secondary end points, including pain reduction and improved quality of life (J. Am. Acad. Dermatol. 2010;62:205-17).
On the other hand, etanercept showed no benefit in a 20-patient pilot randomized trial (Arch. Dermatol. 2010;146:501-4).
By far the best studied TNF inhibitor in HS is adalimumab (Humira). It is the subject of two ongoing, pivotal phase III randomized, double-blind, placebo-controlled, multinational clinical trials aimed at winning adalimumab regulatory approval as the first-ever drug indicated for the treatment of HS.
Last year, Dr. Alexa B. Kimball of Harvard Medical School, Boston, presented the primary end point of a 16-week, randomized, double-blind phase II clinical trial involving 154 patients with HS who were placed on adalimumab 40 mg weekly, adalimumab 40 mg every other week, or placebo. At week 16, a Physician Global Assessment of clear, minimal, or mild disease with at least a 2-grade improvement over baseline was documented in 23.5% of patients on placebo, 21.2% of those on alternate-week adalimumab, and 49% of those on adalimumab 40 mg every week.
With a therapy that achieves clear or close to it in only half of treated patients, other end points take on added importance. At this year’s EADV (European Academy of Dermatology and Venereology) congress, Dr. Kimball and others presented several key secondary outcomes from the phase II study. One involved pain, a particularly prominent feature in HS. From a mean baseline pain score of 52 on a 0-100 visual analog scale, the weekly adalimumab group’s score dropped by a placebo-subtracted 15.8 points by week 2, 18.4 points at week 4, 11.7 points by week 8, 20.7 points at week 12, and by 11 points at week 20.
The minimum clinically important difference, predefined as at least a 15.4-point improvement in self-rated pain scores, occurred in 33% of the weekly adalimumab group, compared with 15% of patients on placebo at week 2. By week 12 this end point had been achieved in 63% of the weekly adalimumab group and 26% of the placebo group. Adalimumab every other week didn’t consistently improve pain relative to placebo, she reported.
Dr. Ulrich Mrowietz reported separately that the impaired work productivity that often affects patients with HS was significantly lessened by treatment with adalimumab 40 mg weekly. Total work productivity impairment as measured at week 16 by the Work Productivity and Activity Impairment Questionnaire improved by a mean of 15 points in the weekly adalimumab group while worsening by 2.7 points with placebo; the net 17.7-unit difference exceeded the minimum clinically important difference threshold of 16.2 points.
Patients assigned to weekly adalimumab also experienced significantly improved scores on the Dermatology Life Quality Index beginning on week 2 and lasting throughout the 16-week study. At week 16, for example, they enjoyed a mean 5.5-point improvement relative to baseline, compared with a 1.6-point improvement with placebo, according to Dr. Mrowietz of University Medical Center in Kiel, Germany.
Oral Antibiotics
Dr. Jemec highlighted as particularly impressive a retrospective French case series of 116 patients with severe HS treated using the combination of clindamycin 300 mg twice daily and rifampicin 600 mg daily. Severity scores decreased as a result by 50%. Seven percent of patients dropped the therapy due to diarrhea (Dermatology 2009;219:148-54).
Two smaller published case series have boosted to 164 the total number of HS patients treated with similarly "dramatic" results, noted Dr. Jemec. He was an investigator in one of these studies, in which 10 weeks on the antibiotic combination resulted in at least partial improvement in 28 of 34 patients and total remission in 16 (47%). Eight of the 16 relapsed after a mean of 5 months (Dermatology 2009;219:143-7).
Another approach has involved an intensive oral regimen of broad-spectrum antibiotics consisting of rifampin at 10 mg/kg once daily, moxifloxacin at 400 mg/day, and metronidazole at 500 mg three times daily. In a series of 28 consecutive patients with long-standing refractory HS, French investigators prescribed this regimen for 6-12 weeks before switching to consolidation therapy with rifampin and moxifloxacin for 6 more weeks. Complete remission was achieved in 16 of the 28 patients (Dermatology 2011;222:49-58).
"This therapy has yielded remarkably good results in terms of clearance and maintenance of clearance," commented Dr. Jemec.
The main side effects were diarrhea, a complaint in nearly two-thirds of patients, and vaginal candidiasis, which occurred in one-third of treated women.
Topical Clindamycin
Dr. Jemec was a coinvestigator in an early double-blind, 46-patient study that established topical clindamycin as being as effective as oral tetracycline in patients with mild disease (J. Am. Acad. Dermatol. 1998;39:971-4).
Beyond this point, the strength of evidence for proposed HS therapies tails off considerably. But Dr. Jemec deemed a couple of agents worthy of mention on the basis of intriguing yet less than persuasive supporting data:
• Acitretin. In a case series of 12 HS patients treated for 9-12 months at the relatively high dose of 0.6 mg/kg per day of acitretin, with stringent 4-year follow-up, all patients went into remission. In eight patients, the remission lasted for at least 1 year, including a single patient who has been in remission for 4 years and counting (Br. J. Dermatol. 2011;164:170-5). "Remarkable," Dr. Jemec commented.
• Dapsone. This drug is frequently reported anecdotally as being useful in HS, typically with an n-of-1 experience. Dr. Jemec and his coworkers recently published a case series of 24 treated patients, 9 of whom (38%) showed improvement (Dermatology 2011;222:342-6). None of the four severely affected patients improved, however. It was Dr. Jemec’s impression that the degree of improvement was less than he’s seen with combined oral clindamycin and rifampicin. He’d like to see a head-to-head comparison before reaching a final judgment as to a possible role for dapsone.
Dr. Jemec has received research grants from and serves as a consultant to Abbott Laboratories, which is developing adalimumab as a treatment for HS, as well as a dozen other pharmaceutical companies. Dr. Mrowietz is a consultant to Abbott, which funded his study, and to other dermatologic pharmaceutical companies.
PRAGUE – A handful of drugs are backed by solid-quality evidence for the treatment of hidradenitis suppurativa, although to date no form of therapy is approved for this indication.
Topping the list are the tumor necrosis factor (TNF)-alpha inhibitors. They have a plausible mechanism of benefit in hidradenitis suppurativa (HS). They’ve also demonstrated effectiveness for this common inflammatory skin disease in randomized, placebo-controlled clinical trials, Dr. Gregor B. Jemec said at the annual congress of the European Academy of Dermatology and Venereology.
In addition, one older randomized trial suggests that topical clindamycin is effective, mainly in milder cases. The most promising antibiotic regimen, consisting of oral clindamycin at 300 mg twice daily plus rifampicin at 600 mg daily, has not been studied in a randomized trial. But several large, rigorous case series have demonstrated "dramatic improvement" in response to the combination, according to Dr. Jemec of the University of Copenhagen.
TNF Inhibitors
The discovery that anti-TNF therapy is effective for HS was a serendipitous finding that occurred when physicians noted marked improvement in the skin disease in a patient taking infliximab for Crohn’s disease. The biologic plausibility of the observed benefit is underscored by the fact that TNF levels are actually more elevated in patients with HS than in those with psoriasis, Dr. Jemec said.
Infliximab missed its primary efficacy end point of clear or almost clear in a 38-patient randomized trial, but it did hit several clinically important secondary end points, including pain reduction and improved quality of life (J. Am. Acad. Dermatol. 2010;62:205-17).
On the other hand, etanercept showed no benefit in a 20-patient pilot randomized trial (Arch. Dermatol. 2010;146:501-4).
By far the best studied TNF inhibitor in HS is adalimumab (Humira). It is the subject of two ongoing, pivotal phase III randomized, double-blind, placebo-controlled, multinational clinical trials aimed at winning adalimumab regulatory approval as the first-ever drug indicated for the treatment of HS.
Last year, Dr. Alexa B. Kimball of Harvard Medical School, Boston, presented the primary end point of a 16-week, randomized, double-blind phase II clinical trial involving 154 patients with HS who were placed on adalimumab 40 mg weekly, adalimumab 40 mg every other week, or placebo. At week 16, a Physician Global Assessment of clear, minimal, or mild disease with at least a 2-grade improvement over baseline was documented in 23.5% of patients on placebo, 21.2% of those on alternate-week adalimumab, and 49% of those on adalimumab 40 mg every week.
With a therapy that achieves clear or close to it in only half of treated patients, other end points take on added importance. At this year’s EADV (European Academy of Dermatology and Venereology) congress, Dr. Kimball and others presented several key secondary outcomes from the phase II study. One involved pain, a particularly prominent feature in HS. From a mean baseline pain score of 52 on a 0-100 visual analog scale, the weekly adalimumab group’s score dropped by a placebo-subtracted 15.8 points by week 2, 18.4 points at week 4, 11.7 points by week 8, 20.7 points at week 12, and by 11 points at week 20.
The minimum clinically important difference, predefined as at least a 15.4-point improvement in self-rated pain scores, occurred in 33% of the weekly adalimumab group, compared with 15% of patients on placebo at week 2. By week 12 this end point had been achieved in 63% of the weekly adalimumab group and 26% of the placebo group. Adalimumab every other week didn’t consistently improve pain relative to placebo, she reported.
Dr. Ulrich Mrowietz reported separately that the impaired work productivity that often affects patients with HS was significantly lessened by treatment with adalimumab 40 mg weekly. Total work productivity impairment as measured at week 16 by the Work Productivity and Activity Impairment Questionnaire improved by a mean of 15 points in the weekly adalimumab group while worsening by 2.7 points with placebo; the net 17.7-unit difference exceeded the minimum clinically important difference threshold of 16.2 points.
Patients assigned to weekly adalimumab also experienced significantly improved scores on the Dermatology Life Quality Index beginning on week 2 and lasting throughout the 16-week study. At week 16, for example, they enjoyed a mean 5.5-point improvement relative to baseline, compared with a 1.6-point improvement with placebo, according to Dr. Mrowietz of University Medical Center in Kiel, Germany.
Oral Antibiotics
Dr. Jemec highlighted as particularly impressive a retrospective French case series of 116 patients with severe HS treated using the combination of clindamycin 300 mg twice daily and rifampicin 600 mg daily. Severity scores decreased as a result by 50%. Seven percent of patients dropped the therapy due to diarrhea (Dermatology 2009;219:148-54).
Two smaller published case series have boosted to 164 the total number of HS patients treated with similarly "dramatic" results, noted Dr. Jemec. He was an investigator in one of these studies, in which 10 weeks on the antibiotic combination resulted in at least partial improvement in 28 of 34 patients and total remission in 16 (47%). Eight of the 16 relapsed after a mean of 5 months (Dermatology 2009;219:143-7).
Another approach has involved an intensive oral regimen of broad-spectrum antibiotics consisting of rifampin at 10 mg/kg once daily, moxifloxacin at 400 mg/day, and metronidazole at 500 mg three times daily. In a series of 28 consecutive patients with long-standing refractory HS, French investigators prescribed this regimen for 6-12 weeks before switching to consolidation therapy with rifampin and moxifloxacin for 6 more weeks. Complete remission was achieved in 16 of the 28 patients (Dermatology 2011;222:49-58).
"This therapy has yielded remarkably good results in terms of clearance and maintenance of clearance," commented Dr. Jemec.
The main side effects were diarrhea, a complaint in nearly two-thirds of patients, and vaginal candidiasis, which occurred in one-third of treated women.
Topical Clindamycin
Dr. Jemec was a coinvestigator in an early double-blind, 46-patient study that established topical clindamycin as being as effective as oral tetracycline in patients with mild disease (J. Am. Acad. Dermatol. 1998;39:971-4).
Beyond this point, the strength of evidence for proposed HS therapies tails off considerably. But Dr. Jemec deemed a couple of agents worthy of mention on the basis of intriguing yet less than persuasive supporting data:
• Acitretin. In a case series of 12 HS patients treated for 9-12 months at the relatively high dose of 0.6 mg/kg per day of acitretin, with stringent 4-year follow-up, all patients went into remission. In eight patients, the remission lasted for at least 1 year, including a single patient who has been in remission for 4 years and counting (Br. J. Dermatol. 2011;164:170-5). "Remarkable," Dr. Jemec commented.
• Dapsone. This drug is frequently reported anecdotally as being useful in HS, typically with an n-of-1 experience. Dr. Jemec and his coworkers recently published a case series of 24 treated patients, 9 of whom (38%) showed improvement (Dermatology 2011;222:342-6). None of the four severely affected patients improved, however. It was Dr. Jemec’s impression that the degree of improvement was less than he’s seen with combined oral clindamycin and rifampicin. He’d like to see a head-to-head comparison before reaching a final judgment as to a possible role for dapsone.
Dr. Jemec has received research grants from and serves as a consultant to Abbott Laboratories, which is developing adalimumab as a treatment for HS, as well as a dozen other pharmaceutical companies. Dr. Mrowietz is a consultant to Abbott, which funded his study, and to other dermatologic pharmaceutical companies.
EXPERT OPINION FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY