User login
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown.
The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
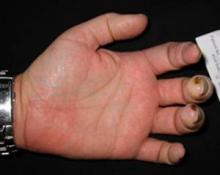
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo.
Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline.
The study's primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. "Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers," they stated. "This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect."
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, "the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline."
The reduction of new digital ulcers in bosentan-treated patients "did not translate into a smaller ulcer burden, as was seen in the previous study," the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote.
Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing "the need for continual monitoring of liver function with this treatment," the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc "may be challenged," the authors wrote. "In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up." However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment "may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers]."
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown.
The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo.
Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline.
The study's primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. "Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers," they stated. "This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect."
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, "the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline."
The reduction of new digital ulcers in bosentan-treated patients "did not translate into a smaller ulcer burden, as was seen in the previous study," the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote.
Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing "the need for continual monitoring of liver function with this treatment," the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc "may be challenged," the authors wrote. "In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up." However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment "may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers]."
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Treatment with bosentan reduced the occurrence of new ischemic digital ulcers in patients with systemic sclerosis, but did not hasten healing of existing ulcers, findings from a study have shown.
The dual endothelin receptor antagonist was associated with a 30% reduction in the number of new digital ulcers in a double-blind, placebo-controlled trial reported online in the Annals of the Rheumatic Diseases.
In the United States, the Food and Drug Administration has approved bosentan (Tracleer) for the treatment of pulmonary artery hypertension (PAH). Both PAH and digital ulcers are complications of systemic sclerosis (SSc). In Europe, bosentan is approved for pulmonary artery hypertension.
As a follow-up to a previous study of 122 SSc patients in whom bosentan treatment was associated with a 48% reduction, compared with placebo, in the mean number of new digital ulcers but no differences in healing after 16 weeks of treatment, Dr. Marco Matucci-Cerinic, professor of rheumatology and medicine at the University of Florence (Italy) and colleagues designed the current trial to evaluate the effects of the drug in a larger population of patients over a longer period of time.
Toward this end, the investigators enrolled 188 SSc patients with at least one active digital ulcer (the cardinal ulcer) to receive 62.5 mg of bosentan twice daily for 4 weeks and 125 mg twice daily for 20 weeks, or matching placebo.
Patients in both groups were at least 18 years old at the time of enrollment (between October 2003 and May 2005) and were well matched with respect to demographics, baseline disease characteristics, and concomitant treatment for digital ulcers at baseline.
The study's primary end points were the number of new digital ulcers and the time to healing of the cardinal ulcer; the secondary end points were pain, disability, and safety, the authors wrote (Ann. Rheum. Dis. 2010 Aug. 30 [doi:10.1136/ard.2010.130658]).
After 24 weeks, the mean total number of digital ulcers per patient was similar in the two groups, but the mean number of new digital ulcers was 1.9 in the treatment group and 2.7 in the placebo group, the authors reported. "Fewer new [digital ulcers] were observed with bosentan than placebo in all subgroups except among current smokers," they stated. "This included subgroups of limited and diffuse [SSc], with no difference between the two subgroups in the treatment effect."
In post hoc analysis, more pronounced reductions were seen in patients who had multiple digital ulcers, the authors observed. Specifically, they wrote, "the reduction of new [digital ulcers] appeared to be greater in patients with at least four [digital ulcers] at baseline."
The reduction of new digital ulcers in bosentan-treated patients "did not translate into a smaller ulcer burden, as was seen in the previous study," the authors stated, noting that this could be attributed to the fact that 38% of patients in the previous study did not have an active digital ulcer at baseline and had fewer digital ulcers to heal, possibly giving more weight to prevention in the reduction of overall ulcer burden.
In terms of healing, there was no difference in the time to healing of the cardinal ulcer between bosentan treatment and placebo. At 24 weeks, more than half of the patients in both groups had healing of the cardinal ulcer that was maintained for a minimum of 12 weeks, the authors wrote.
Additionally, no treatment effects were observed in patient-rated measures of overall hand pain and pain of the cardinal ulcer, as assessed by visual analog scales. Similarly, there were no significant differences in the changes from baseline in the Health Assessment Questionnaire–Disability Index and hand disability index at week 24, they reported.
The fact that the reduction in new ulcers was not associated with decreased pain or disability, relative to placebo, could be explained by the similar number of digital ulcers between treatment groups, or a lack of sensitivity to change and discriminative value in the current instruments that are used to assess hand function in systemic sclerosis, the authors hypothesized. It might also be indicative of the fact that bosentan treatment does not improve pain and disability in spite of the reduction of new digital ulcers, they wrote.
Safety and tolerability assessments showed that serious adverse events occurred in 9.4% and 16.7%, respectively, of patients in the bosentan and placebo groups, and that similar proportions of patients in both groups experienced at least one adverse event, the authors reported. More treatment vs. placebo patients experienced peripheral edema and events denoting elevated aminotransferases.
Laboratory analyses showed increases in aminotransferases in 10.5% of the bosentan patients, compared with 1.1% of the placebo patients, reinforcing "the need for continual monitoring of liver function with this treatment," the authors wrote.
The clinical utility of bosentan treatment for digital ulcer prevention in SSc "may be challenged," the authors wrote. "In a patient encountered with a single [digital ulcer], initiation of bosentan would not be expected to facilitate healing, and at least 66% of all bosentan-treated subjects would develop at least one additional [digital ulcer] over 6 months of follow-up." However, they noted, bosentan treatment offers greater potential benefit to patients who present with multiple digital ulcers. In this regard, the treatment "may be a useful adjunct in the management of patients with [SSc] and recurrent [digital ulcers]."
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.
Major Finding: Treatment with bosentan reduced the occurrence of new digital ulcers in SSc patients who had multiple ulcers at treatment initiation by 30%.
Data Source: A randomized, double-blind, placebo-controlled study comprising 188 systemic sclerosis patients.
Disclosures: This study was funded by Actelion Pharmaceuticals. The authors disclosed financial relationships with Actelion, Pfizer, GlaxoSmithKline Beecham, Encysive, Genzyme, Aspreva, Biovitrum, DiGNA, Gilead, MediQuest, Centocor, FibroGen, Bristol-Myers Squibb, Lilly, and United Therapeutics.